Clinical
Nutritional Genomics
Nutrition professionals have long been intrigued and puzzled by the fact that one person can be lean, yet that person’s identical twin can be overweight; that the Pima Indians living in northern Mexico are lean, but their genetic counterparts in the American southwest struggle with a high prevalence of obesity and type 2 diabetes mellitus (T2DM); and that a low-fat diet can improve blood lipid levels in many people, but not in everyone. Although our genetic makeup sets the stage, environmental factors such as nutrition and other lifestyle choices determine who among the susceptible actually develops a disease. The interactions among genes, diet, lifestyle factors, and their influence on health and disease are the focus of nutritional genomics.
Genetic research is rapidly clarifying how variations are correlated with dysfunction and disease. This appreciation for the central role of genes is having a significant effect on the way health is viewed. As the details of the connections among genes, their protein products, and disease unfold, the focus of the health care system is shifting. During the past 50 years the focus has been on treating overt disease, and physicians have had increasingly sophisticated drugs and technologies available to meet this challenge. However, with the understanding that disease is genetically based but environmentally influenced, the focus is now on targeted intervention and prevention. Although the first applications of this changed focus in health care involved the medical and pharmaceutical aspects of acute care, nutrition therapy is expected to figure prominently as a cornerstone of preventive care and in the management of the chronic, diet- and lifestyle-related diseases.
Genetic research is helping to clarify the pathogenesis of disease with the influence of bioactive components in food. From these advances will come diagnostic tests and assessments of disease susceptibility that, coupled with genetic testing and family history analysis, will allow health care professionals to predict those at risk for particular disorders. Nutrition can mitigate the harmful effects of many genetic changes that predispose to disease, from supplying missing metabolites to altering gene expression.
Nutritional genomics can also offer effective approaches for preventing disease. By analyzing individual genotypes prenatally or at birth, disease susceptibilities will be known from an early age and can be factored into the nutrition and lifestyle choices made throughout life. Armed with extensive knowledge of one’s genetic makeup (genotype) and how to make lifestyle choices that support that genotype, humans will have the option to live to their full genetic potential throughout a healthy, active life.
The nutrition profession is pivotal in this new era of health promotion and disease prevention. The role includes assessing disease susceptibilities, then recommending preventive therapy and lifestyle approaches. Increasingly, genotyping must be incorporated into the nutrition assessment and recommendations customized to the genetic uniqueness of individuals.
The Human Genome Project
The Human Genome Project has been the impetus for this fundamental shift to integrating genetic principles into health care. This project, completed in 2003, was a multinational effort to identify each of the nucleotides in the deoxyribonucleic acid (DNA) that makes up the genetic material (genome) of human beings. Currently the focus is on (1) cataloging the number of genes present in human DNA; (2) identifying the protein encoded within each gene and understanding its function (proteomics); (3) associating variations in genes with specific diseases; (4) understanding the way genes, proteins, and environmental factors interact to cause the functional changes that result in disease; (5) identifying metabolites that are useful in monitoring health status (metabolomics); and (6) understanding epigenetics (changes in single genes caused by environmental factors in utero, from chemicals or diet or aging) and epigenomics (population-specific gene changes) and their implications for human development and health. These efforts will lead to an understanding of effective approaches for restoring health and preventing disease.
Additional goals include sequencing the genomes of other organisms that are used as model systems in the laboratory to explore the molecular basis of disease, addressing the ethical, legal, and social implications of genetic research, developing genetic technologies useful for clinical applications, educating genetic scientists and clinicians, and integrating the results of genetic research into clinical practice. Sophisticated computer technology that can handle the vast amount of data is the backbone of the field of bioinformatics.
Clinical Applications
Much of the knowledge and many of the technologic advances gained from the Human Genome Project have clinical applications. Knowing the gene associated with a particular disease and the gene’s DNA sequence, its protein product, and the function of the protein in promoting health or illness provides the basis for diagnostic assays and effective interventions. For example, tumors that appear identical physically can be distinguished by their genetic profiles. This distinction is important for effective therapy because different types of tumors respond to different therapeutic approaches. Not only can such assays be used to definitively reach a diagnosis, they also can be used to detect dysfunction in those without symptoms, which allows interventions to be initiated before the symptoms of a disease become apparent.
Similarly, the information gained has been pivotal in developing diagnostic assays to determine genetic variations in drug-metabolizing enzymes (pharmacogenomics). Each human being has the same basic set of drug-metabolizing enzymes, but the genes and the resulting enzyme functions can vary. One drug may have the intended effects on one person, be ineffective for another, and actually be harmful to a third. The ability to assess an individual’s major drug-metabolizing gene variants helps the physician select the right drug and dosage. Like drugs, food requires enzymatic processes to be digested, absorbed, and used by the body’s cells. The ability to tailor food to the genetic makeup of individuals—the science of nutritional genomics—is expected to be an important application of genetic research, similar in concept to pharmacogenomics.
Genotype And Nutrition Assessment
The application expected to have the most dramatic effect on clinical nutrition professionals is the ability to associate a unique genotype with that person’s susceptibility to particular diseases. This advance is an important enhancement in the nutrition assessment, diagnosis, and intervention phases of the nutrition care process. As understanding of how genotype influences the ability to function within a particular environment and how environmental factors influence gene expression, nutrition protocols will be developed. Specific counseling and nutrient recommendations will be guided by the client’s genetic profile.
Nutrition professionals must be able to translate client genotypes to develop appropriate interventions. If nutrition professionals are going to be prepared for the era of genomic-directed health care, they must build a foundation in genetics, biochemistry, molecular biology, metabolism, and other foundational sciences of 21st century nutrition (Milner, 2008; Panagiotou and Nielsen, 2009; Stover and Caudill, 2008).
Genetic Fundamentals
The reader must have a basic understanding of DNA as the genetic material for chromosomal and molecular genetics. Among the key concepts at the chromosomal level are the packaging of DNA into chromosomes within the nucleus, the processes of meiosis and mitosis, autosomal and sex-linked inheritance, linkage and the mapping of genes, and chromosomal mutation and its consequences. At the molecular level, key concepts include (1) information stored within DNA must be decoded and converted into proteins through the processes of transcription, posttranscriptional processing, translation, and posttranslational processing; (2) genes have a regulatory region with response elements, transcription factors, promoters, and a coding region with exons and introns; and (3) genetic variations within the human genome affect an individual’s phenotype, including disease susceptibility.
In addition to the information contained in the nucleotide sequence of DNA (the “DNA code”), there are two other sources of information: the epigenetic code and the environmental factors to which cells are exposed. Epigenetics is nature’s “pen-and-pencil set” (Gosden and Feinberg, 2007). Acetyl or methyl groups covalently attached to histone proteins associated with DNA or to the DNA itself, respectively, affect whether DNA is accessible for decoding. These groups can be added and removed as needed, and are influenced by diet. Nutrition counselors will have a great opportunity to affect this. (Kauwell, 2008.)
Molecules in the environment such as traditional nutrients, phytochemicals, toxins, hormones, and drugs communicate information as to the state of the environment and ultimately influence when and whether particular genes are expressed. The science of nutritional genomics encompasses all of these types of interactions between diet and lifestyle factors and DNA and their influence on health outcomes. Figures 5-1 through 5-5 review these fundamental genetic principles.
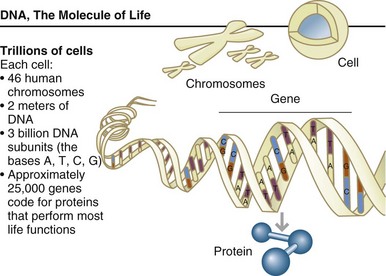
FIGURE 5-1 Cells are the fundamental working units of every living system. All the instructions needed to direct their activities are contained within the chemical deoxyribonucleic acid. (From U.S. Department of Energy, Human Genome Program: www.ornl.gov/hgmis.)
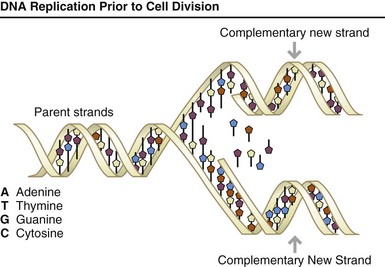
FIGURE 5-2 Each time a cell divides into two daughter cells, its full genome is duplicated; for humans and other complex organisms, this duplication occurs in the nucleus. During cell division the deoxyribonucleic acid (DNA) molecule unwinds, and the weak bonds between the base pairs break, allowing the strands to separate. Each strand directs the synthesis of a complementary new strand, with free nucleotides matching up with their complementary bases on each of the separated strands. Strict base-pairing rules are adhered to (i.e., adenine pairs only with thymine [an A-T pair] and cytosine with guanine [a C-G pair]). Each daughter cell receives one old and one new DNA strand. The cells’ adherence to these base-pairing rules ensures that the new strand is an exact copy of the old one. This minimizes the incidence of errors (mutations) that may greatly affect the resulting organism or its offspring. (From U.S. Department of Energy, Human Genome Program: www.ornl.gov/hgmis.)
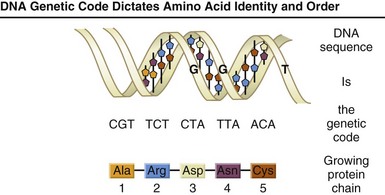
FIGURE 5-3 All living organisms are composed largely of proteins. Proteins are large, complex molecules made up of long chains of subunits called amino acids. Twenty different kinds of amino acids are usually found in proteins. Within the gene, each specific sequence of three deoxyribonucleic acid bases (codons) directs the cells protein-synthesizing machinery to add specific amino acids. For example, the base sequence ATG codes for the amino acid methionine. Because three bases code for one amino acid, the protein coded by an average-sized gene (3000 bp) will contain 1000 amino acids. The genetic code is thus a series of codons that specify which amino acids are required to make up specific proteins.
A, Adenine; bp, base pairs; G, guanine; T, thymine. (From U.S. Department of Energy, Human Genome Program: www.ornl.gov/hgmis.)
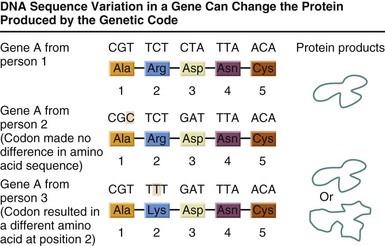
FIGURE 5-4 Some variations in a person’s genetic code will have no effect on the protein that is produced; others can lead to disease or an increased susceptibility to a disease. (From U.S. Department of Energy, Human Genome Program: www.ornl.gov/hgmis.)
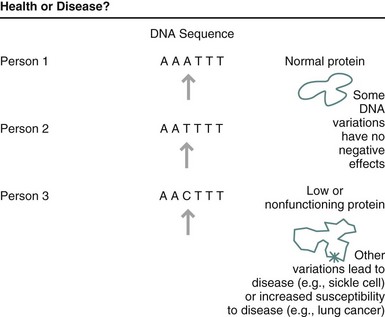
FIGURE 5-5 It is estimated that human beings differ from each other in only 0.1% of the total sequence of nucleotides that compose deoxyribonucleic acid. These variations in genetic information are thought to be the basis for the physical and functional differences between individuals. (From U.S. Department of Energy, Human Genome Program: www.ornl.gov/hgmis.)
Genetics and Genomics: Nutritional Genomics, Nutrigenetics, and Nutrigenomics
Genetics is the science of inheritance. Historically genetics focused on identifying the mechanisms by which traits were passed from parent to child, such as eye or hair color, and certain rare diseases that were inherited from generation to generation. Originally, genetic diseases were in a separate category of disease. Today scientists realize that, directly or indirectly, all disease is connected to the information in the genes.
The science of genetics has significantly expanded in scope and includes the whole set of genetic information in an organism—its genome—and the interactions of the various genes and their protein products with each other and the environment. Genomics more accurately describes this complex, interactive situation. Whereas genetics was initially concerned with diseases that arose from a change in a single gene, genomics is more concerned with today’s chronic diseases that result from the interaction between gene variants and factors in the environment. Nutritional genomics focuses on diet- and lifestyle-related disorders that result from these interactions. Nutritional genomics is the field itself, and includes nutrigenetics, nutrigenomics, and epigenetics or epigenomics.
Nutrigenetics concerns how an individual’s particular genetic variations affect function. For example, an individual with a particular variant in the 5,10-methylenetetrahydrofolate reductase (MTHFR) gene is likely to require a more bioavailable form of folate for optimal health. Nutrigenomics is the study of the influence of specific environmental factors on changes in the expression of particular genes. Epigenetics and epigenomics provide another influence on outcomes by controlling whether genes can be expressed, which in turn determines whether nutrigenetic or nutrigenomic influences can occur. Diet and other lifestyle choices should be geared toward the particular variants of each individual.
Genetic Basics
Deoxyribonucleic acid (DNA) is the genetic material of all living organisms. The molecule is a double helix consisting of two strands of nucleotide subunits held together by hydrogen bonds. Each subunit contains the sugar deoxyribose, the mineral phosphorus, and one of four nitrogenous bases: adenine (A), thymine (T), guanine (G), or cytosine (C). The nucleotides are arranged side by side, and it is this linear arrangement that determines the particular information encoded in a stretch of DNA.
Approximately 3 billion nucleotides make up the human genome, which is housed in the nucleus of cells. A gene is a sequence of nucleotides that encodes the information for synthesizing a protein. The human genome contains approximately 20,000 to 25,000 genes, which is only 2% of the total genome (Human Genome Project, 2010). Long stretches of nucleotides are often found between one gene and the next along the chromosome. Such sequences are called intervening sequences and compose the majority of the DNA in humans. These sequences do not code for proteins, but they are not “junk DNA.” Instead, they perform structural and regulatory functions, such as controlling when, where, and how much of a protein is produced.
To be useful to the cells, information in the DNA must first be decoded and translated into proteins, which perform the work of the organism at the cellular level. Information decoding occurs in two steps: (1) the process of transcription, during which the enzyme ribonucleic acid polymerase (RNA polymerase) converts DNA into an intermediate molecule (messenger RNA [mRNA]) and (2) a subsequent translation step in which the information encoded within the mRNA directs the assembly of amino acids into the protein molecule according to a universal genetic code. Genes have a common structure, with a promoter region where the binding of the RNA polymerase is controlled, which in turn controls transcription; and a coding (informational) region where the RNA polymerase transcribes the DNA into mRNA. Within the coding region are sequences of nucleotides (exons) that correspond to the order of the amino acids in the gene’s protein product. The coding region also contains introns (sequences that are interspersed between exons and do not code for amino acids).
Following transcription, the mRNA must be processed (posttranscriptional processing) so that the introns are removed before the protein is synthesized. At this point, each set of three nucleotides in the transcribed and processed exon makes up a codon, which in turn specifies a particular amino acid and its position within the protein. Some proteins need further posttranslational processing before they are active, such as occurs with glycoproteins, proenzymes, and prohormones that must be cleaved or enzymatically processed before becoming active.
Prior to (“upstream from”) the promoter region is the regulatory region, where control of transcription takes place. Within this region are response elements, DNA sequences that serve as binding sites for regulatory proteins such as transcription factors and their bound ligands. The binding of transcription factors triggers the recruitment of additional proteins to form a protein complex that in turn changes the expression of that gene by changing the conformation of the promoter region, increasing or decreasing the ability of RNA polymerase to attach and transcribe (express) the gene. The array of response elements within the promoter region can be quite complex, allowing for the binding of multiple transcription factors that in turn fine-tune the control of gene expression. It is through the binding of transcription factors to response elements that environmental factors such as the bioactive components in food essentially “talk” to a gene, conveying information that more or less of its protein product is needed.
The proteins coded for by the genes provide the metabolic machinery for the cells, such as enzymes, receptors, transporters, antibodies, hormones, and communicators. Changes within a gene can alter the amino acid sequence of the DNA protein. Such changes are called mutations, which historically have been associated with the concept of severely impairing the function of that protein and creating dysfunction within the cells and, ultimately, the organism. A single nucleotide change may be all that is needed to cause a debilitating disease. For example, in those with sickle cell disease a single nucleotide change causes a single amino acid change in the hemoglobin molecule, resulting in severe anemia (see Chapter 33).
Changes in the DNA are the basis for evolution; thus clearly not all mutations are harmful. Some changes actually improve function, and many silent mutations have no effect. The effect of the mutation on the functioning of the encoded protein is what determines the outcome, from debilitating disease to no effect at all. All changes to the DNA are technically mutations. However, at this point in the development of genomics, the term mutation tends to be applied to those changes that sufficiently influence function such that a measurable outcome results. In contrast, the term genetic variation (or gene variant) is reserved for those mutations with an effect on function that is not strong enough to lead to a disease or other measurable outcome by itself. Nutritional genomics is primarily concerned with those variations that interact with environmental factors.
Thus a gene can exist in slightly different forms as a result of a seemingly minor change, such as a substitution of a single nucleotide with another (e.g., guanine can replace cytosine). The term for the different forms of a gene is an allele or polymorphism. As a result, genes have protein products with differing amino acid sequences (isoforms) and often different functions. Polymorphism (allelism) is an important concept because it explains why human beings, although 99.9% alike genetically, are distinctively different. The 0.1% difference is sufficient to explain the obvious physical variations among humans. It is also the basis for more subtle differences that may not be readily observable, such as in the functional ability of a key metabolic enzyme to catalyze its characteristic reaction. Such variations are thought to underlie many of the inconsistencies that are observed in therapeutic outcomes and in nutritional intervention research.
The single nucleotide polymorphism (SNP) is the structural variant best studied to date. However, ongoing analysis of the human genome suggests that other structural variations may also play an important role in the genotypic and phenotypic variation among humans (Feuk et al., 2006). Loss or gain of nucleotides, duplication of nucleotide sequences, and copy number variants also have important consequences.
Understanding the prevalence and significance of genetic variation is a primary focus of 21st-century nutrition, which represents a major departure from nutrition research and therapy to date. Each person is susceptible to a different set of diseases, handles environmental toxins differently, metabolizes molecules somewhat differently, and has slightly unique nutritional requirements. These exciting discoveries are revolutionizing the way people think about the clinical aspects of medicine, pharmacology, and nutrition. Personalized therapy using individualized dietary requirements will be the practice of future dietitians.
Modes of Inheritance
Traits are transmitted from one generation to the next in three ways: Mendelian inheritance, mitochondrial inheritance, and epigenetic inheritance.
Mendelian Inheritance
Each cell’s nucleus contains a complete set of genetic material (genome), divided among 22 pairs of chromosomes (called autosomes) and 2 sex chromosomes for a total of 46 chromosomes. During cell division (mitosis) all 46 chromosomes are duplicated and distributed to each new cell. During meiosis, one member of each of the autosome and sex chromosome pairs is distributed to each egg or sperm; the full set of 46 chromosomes is then restored upon fertilization.
Because genes are carried on chromosomes, the rules governing the distribution of chromosomes during mitosis and meiosis govern the distribution of genes and any changes (mutations, variations) they contain. These rules describe the Mendelian inheritance of a gene, named after Gregor Mendel, who first deduced that the inheritance of traits was governed by a predictable set of rules. It is possible to track a mutation through multiple generations by knowing these rules of inheritance. This transmission is typically depicted as a pedigree and can be used to predict the probability of a genetic change being inherited by a particular family member. When the change causes a disease, a pedigree can be helpful in predicting the probability that another family member will inherit the disease. The Family History Initiative, implemented by the U.S. Surgeon General, helps people construct their family pedigree.
Mendelian transmission can be autosomal or sex-linked, dominant or recessive. There are five classic modes of Mendelian inheritance: autosomal dominant, autosomal recessive, X-linked dominant, X-linked recessive, and Y-linked. An individual’s genotype obeys the laws of inheritance, but the phenotype (the measurable expression of the genotype) may not. Each gene in an individual is present in two copies (alleles), one on each chromosome. When the alleles are the same (either both are the common or usual version or both are the mutant or variant form), the individual is said to be homozygous. If the alleles are different, the individual is heterozygous (also called a carrier).
Dominance and recessiveness refer to whether a trait is expressed in a heterozygous individual that has one common allele and one variant allele. If a trait is expressed when only a single copy of a variant allele is present, the allele is said to be dominant (i.e., the phenotype of the variant allele is the predominant one). Alleles that do not dominate the genotype when only a single copy is present are called recessive. The variant allele is present in the genome but the trait is not expressed unless two copies of the variant allele are present.
Further confounding the nomenclature is the concept of penetrance. Even when a pedigree suggests that a gene is present that should lead to the individual displaying a certain phenotype, the disease may not be evident. Such a gene is said to have reduced penetrance, meaning that not everyone who has the gene expresses it in a measurable form. An interesting side note is that “measurable form” very much depends on what is able to be measured. Many alleles that were thought to be recessive 50 years ago can be detected today as the result of new and more sensitive technologies. Penetrance is of interest to nutrition professionals because it reflects the inability of a genetic variation to impair function and cause disease unless the individual is exposed to specific environmental triggers, such as diet and lifestyle factors. Modifying these factors can potentially improve outcomes for those with such variants. Terminology may change as understanding advances in the field.
Mitochondrial Inheritance
In addition to genetic material in the nucleus, the mitochondria in each cell also contain DNA that codes for a limited number of proteins. The majority of these genes are involved in maintenance of the mitochondrion and its energy-producing activities. As with nuclear DNA, changes in mitochondrial DNA (mtDNA) can lead to disease. Traits resulting from mitochondrial genes have a characteristic inheritance pattern; they are non-Mendelian because mitochondria and their genetic material typically pass from mother to child, called mitochondrial or maternal inheritance. This biologic principle has become the basis for anthropologic studies that trace lineage and population migration patterns through the centuries. It also has provided a way to trace familial diseases caused by changes in mtDNA. However, as with other biologic processes, occasional mistakes occur; reports exist of some mtDNA being passed from father to child.
Epigenetic Inheritance, Genomic Imprinting
Epigenetic inheritance illustrates another mechanism by which genetic information is passed between generations. Epigenetics provides an additional set of instructions beyond that contained in the DNA nucleotide sequence. It affects gene expression but does not change the nucleotide sequence itself (van der Maarel, 2008; Villagra et al., 2010). At least three mechanisms are involved: histone modification, DNA modification, and RNA interference (RNAi).
Histones are proteins associated with DNA. Units of histones form a scaffolding around which DNA is wrapped to create the nucleosome, similar to thread wrapped around a spool. Similar in concept to condensing data on a hard drive, this mechanism helps to fit the large amount of DNA into the small space of the nucleus. When DNA is condensed, it is not available for transcribing into mRNA. The attachment and removal of acetyl groups is an important mechanism for controlling whether DNA is relaxed and available for transcription to proceed or condensed and closed to transcription, respectively.
Similarly, DNA itself can be modified by the covalent attachment and removal of functional groups, such as methyl groups. Methylation takes place at cytosine residues that occur within CpG islands found near a gene’s promoter region. CpG islands (the p refers to the phosphodiester bond between (C) cytosine and (G) guanine nucleotides) are DNA sequences enriched in cytosine and guanine that, when methylated, interfere with transcription and therefore gene expression. In general, methylation silences gene expression and demethylation promotes gene expression.
DNA methylation and histone modification can contribute to genomic imprinting and affect gene expression. Genomic imprinting is an unusual phenomenon in which only one of the two alleles of a gene is expressed, either the allele contributed by the mother or by the father. If each allele contains a different mutation that leads to a measurable phenotype, the individual’s phenotype will differ depending on whether the mother’s or the father’s allele is the one expressed. Prader-Willi syndrome and Angelman syndrome provide examples of genomic imprinting; they involve DNA on chromosome 15. When the father’s allele is expressed, the child develops Prader-Willi syndrome. When the mother’s allele is expressed, the child develops Angelman syndrome. Both syndromes are characterized by intellectual disabilities, but Prader-Willi individuals also experience a lack of perception of satiety, which leads to overeating and morbid obesity. The suspected underlying basis for the phenotypic differences is the different pattern of epigenetic markings (either histone acetylation or DNA methylation) between the two parents rather than differences in the DNA sequence itself. Genomic imprinting has important clinical implications (Butler, 2009; Das et al., 2009).
The third mechanism, RNA interference (RNAi), is a posttranscriptional mechanism whereby short pieces of single-stranded RNA (21-23 nucleotides) attach to DNA or mRNA. Attaching to mRNA interferes with gene expression by preventing translation of the gene into its encoded protein. Attaching to DNA leads to silencing of whole regions of chromosomes, a phenomenon called epigenetic gene silencing, which is the basis for X-inactivation in mammalian females in which one of the two X-chromosomes is silenced. In this way the amount of information contributed by the X-chromosome is equalized between females and males, the latter having only a single X-chromosome (Kloc and Martienssen, 2008; Suzuki and Kelleher, 2009).
Epigenetics is of interest to nutrition professionals because diet has been found to influence at least one epigenetic mechanism, DNA methylation, and the effects can be inherited. The mouse has been used as a mammalian model system for dissecting this complex process. In a landmark study by Waterland and Jirtle (2003), a strain of mice with a mutation in the agouti gene was used. The wild-type (normal) agouti allele causes the mouse’s coat color to be brown. The Avy mutation (agouti viable yellow allele) causes the coat color to be yellow and, because this allele is dominant, all mice with at least one copy of Avy have the potential to develop the yellow coat color. The researchers bred genetically identical female mice with brown coats (two copies of the normal agouti allele) with genetically identical males that had two copies of the Avy mutation and had yellow coats. On a standard mouse chow diet, the coat color of the mothers would be brown, that of the fathers would be yellow, and the coat color of the offspring, who have one agouti allele and one Avy allele, would be yellow because the Avy allele is dominant. In this study, half of the females were fed the usual diet and half were fed a methyl-rich diet in which methyl donors such as folate, vitamin B12, choline, and betaine were added to the diet. Most of the unsupplemented mothers had offspring with yellow coats. Most of the offspring from the mothers on the methyl-rich diet, however, had a mottled coat with a mix of brown and yellow (called pseudo-agouti). Clearly, the mother’s diet affected the coat color of the offspring and this effect persisted into adulthood. There was a correlation between mottled coat and degree of methylation of the agouti gene, suggesting that the methyl-rich diet led to epigenetic silencing of the Avy allele.
Furthermore, this effect of diet could be inherited. Cropley and associates (2006) found that feeding the females of the “grandmother” generation a methyl-rich diet but not enriching the daughter offspring’s diet with methyl donors still produced a number of offspring with mottled brown coats, suggesting that the effect the diet had on coat color could be transmitted between generations. Diet and possibly other environmental factors may have a transgenerational effect through their influence on epigenetic “markings” that affect gene expression without altering the DNA sequence. This type of gene-diet epigenetic mechanism could explain why identical twins, although having the exact same genotype, typically do not have identical phenotypes.
Inheritance and Disease
Changes to the genetic material, whether to the chromosomal DNA, mtDNA, or even a single nucleotide, have the potential to alter one or more proteins that may be critical to the operation of the cells, tissues, and organs of the body. There are important consequences from changes to the genetic material at each of these levels.
Disease at the Chromosomal Level
Change in the number of chromosomes, or the arrangement of the DNA within a chromosome, is almost always detrimental or fatal to the individual. Chromosomal disorders are detected by means of a karyotype, a visualization of all the chromosomes in picture form. An example of a nonfatal chromosomal abnormality is trisomy 21 (Down syndrome), which results from an addition to chromosome 21.
Some syndromes are caused by the loss of a portion of a chromosome (a partial deletion). In Beckwith-Wiedemann syndrome (a chromosome 11 deletion), changes are characterized by organ overgrowth, including an oversized tongue, which leads to feeding difficulties and hypoglycemia. Nutrition professionals play an important role in the therapy of those with chromosomal disorders because these individuals often have oral-motor problems that affect their nutritional status and cause growth problems in early life. Later in development, obesity may become an issue, and nutrition therapy is helpful in controlling weight, diabetes, and cardiovascular complications. In people with such abnormalities, varying degrees of mental retardation often complicate therapy. A knowledgeable nutrition professional can mitigate the detrimental effects of these disorders on nutritional status (see Chapter 45).
Disease at the Mitochondrial Level
Mitochondria are subcellular organelles that are thought to have originated from bacterium; they function primarily to produce adenosine triphosphate. Human mtDNA codes for 13 proteins, 2 ribosomal RNAs, and 22 transfer RNAs to synthesize these proteins; the remainder of the proteins are coded for by nuclear DNA. In contrast to nuclear DNA, mtDNA is small (16,569 base pairs), circular, and exists in hundreds to thousands of copies in each mitochondrion. As noted earlier, mtDNA is passed from the mother to her offspring.
Not surprisingly, alterations in mtDNA are typically degenerative and primarily affect tissues with a high demand for oxidative phosphorylation. They also have varied clinical manifestations because of the multiple copies of mtDNA, not all of which may contain the genetic change. Mutations in mtDNA can manifest at any age and include neurologic diseases, cardiomyopathies, and skeletal myopathies (MITOMAP, 2009). For example, Wolfram syndrome, a form of diabetes with associated deafness, was one of the earliest disorders to be traced to mtDNA. More than 60 diseases that result from changes in mtDNA have been identified thus far (Tuppen et al., 2009.)
Disease at the Molecular Level
The majority of disease conditions associated with nutritional genomics involve changes at the molecular level. Changes to the DNA typically involve a single nucleotide change or several nucleotides within a single gene through substitutions, additions or deletions. In addition, larger-scale changes involving the deletion or addition of multiple nucleotides can also occur in the regulatory or protein coding regions of a gene. Alterations in the regulatory region may increase or decrease the quantity of protein produced or alter the ability of the gene to respond to environmental signals. Alterations in the coding region may affect the amino acid sequence of the protein, which in turn can affect the conformation and function of the protein and thereby the functioning of the organism. Because the vast majority of human genes reside on nuclear chromosomes, gene variations are transmitted according to Mendelian inheritance and are subject to modification from epigenetic markings.
Autosomal dominant single-gene disorders that have nutritional implications include several that may result in oral-motor problems, growth problems, susceptibility to weight gain, and difficulties with constipation. Examples include Albright hereditary osteodystrophy, which commonly results in dental problems, obesity, hypocalcemia, and hyperphosphatemia; chondrodysplasias, which often result in oral-motor problems and obesity; and Marfan syndrome, which promotes cardiac disease, excessive growth, and increased nutritional needs. Familial hypercholesterolemia results in a defective low-density lipoprotein (LDL) receptor, elevated levels of cholesterol, and susceptibility to atherosclerosis.
Autosomal recessive disorders are much more common and include metabolic disorders of amino acid, carbohydrate, and lipid metabolism. Traditionally these disorders were detected because the mutation had a detrimental effect on the newborn infant that led to serious developmental consequences or death. These disorders were heritable, ultimately associated with a particular mutation, and designated inborn errors of metabolism (IEM).
IEM disorders are the earliest known examples of nutritional genomics, and dietary modification is the primary treatment modality (see Chapter 44). A brief overview of IEM from a genetic perspective is included here to emphasize the important role of the nutrition professional in restoring health to these individuals and to contrast the IEM with chronic disorders that result from the same type of genetic change but that affect function less severely.
A classic example of an IEM of amino acid metabolism is phenylketonuria (PKU). PKU results from a mutation in the gene coding for the enzyme phenylalanine hydroxylase, leading to an inability to convert phenylalanine to tyrosine. Lifelong dietary restriction of phenylalanine enables individuals with PKU to live into adulthood and enjoy a quality life. In maple syrup urine disease, the metabolic defect is branched-chain alpha-keto acid decarboxylase, an enzyme complex encoded by six genes. A mutation in any one of these genes can result in accumulation of alpha-keto acids in the urine, which produces an odor similar to maple syrup. Failure to limit branched-chain amino acid intake can lead to mental retardation, seizures, and death.
Hereditary fructose intolerance is an example of an autosomal recessive IEM of carbohydrate metabolism. A mutation in the gene encoding aldolase B (fructose-1,6-biphosphate aldolase) impairs the catalytic activity of the enzyme and prevents fructose from being converted to glucose. Breast-fed infants are typically asymptomatic until fruit is added to the diet. Nutrition therapy involves the elimination of fructose and the fructose-containing disaccharide sucrose.
Autosomal recessive disorders of lipid metabolism include the deficiency of medium-chain acyl-coenzyme A (acyl-CoA) dehydrogenase, which prevents medium-chain fatty acids from being oxidized to provide energy during periods of fasting. Nutrition therapy focuses on preventing the accumulation of toxic fatty acid intermediates that, when not controlled, can lead to death (Isaacs and Zand, 2007). Recent guidelines for expanded newborn screening for IEM in the United States use tandem mass spectrometry and provide information on approximately 40 diseases (Dietzen et al, 2009).
The X-linked dominant fragile X syndrome also affects nutritional status. Fragile X syndrome is characterized by developmental delays, mental impairment, and behavioral problems. The lesion occurs within the FMR1 gene on the X chromosome in which a CGG segment is repeated more times than normal. The multiple repeats of this trinucleotide make the X chromosome susceptible to breakage.
X-linked recessive conditions include nephrogenic diabetes insipidus, adrenoleukodystrophy, and Duchenne muscular dystrophy (DMD) disorders. Individuals with X-linked recessive nephrogenic diabetes insipidus are unable to concentrate urine and exhibit polyuria and polydipsia. This disorder is usually detected in infancy and can manifest as dehydration, poor feeding, vomiting, and failure to thrive. X-linked recessive adrenoleukodystrophy results from a defect in the enzyme that degrades long-chain fatty acids. These fats accumulate and lead to brain and adrenal dysfunction and ultimately motor dysfunction. X-linked recessive DMD is characterized by fatty infiltration of muscles and extreme muscle wasting. Children are typically confined to a wheelchair by the time they reach their teens and need assistance with feeding.
Y-linked inheritance disorders involve male sex determination and physiologic “housekeeping functions.” To date no nutrition-related disorders have been conclusively assigned to the Y chromosome.
In summary, any gene can potentially undergo mutation, which can affect the function of its protein and the health of the individual. Its location within the nuclear or mtDNA determines the mode of inheritance.
Genetic Technologies
Progressing beyond knowing the chromosomal location of a disease trait to associating the disease with a particular mutation and understanding its functional consequences has required the development of sophisticated molecular genetic technologies. One of the most critical technologic advances occurred in the early 1970s with the introduction of recombinant DNA technology, which allowed major progress in terms of studying genes, their functions, and the regulation of their expression. Using bacteria-derived restriction endonucleases (restriction enzymes), researchers could cut the DNA in precise, reproducible locations along the nucleotide chain, isolate the fragments and, using polymerase chain reaction (PCR) technology, make unlimited copies of the DNA for various applications. This basic approach has been the cornerstone of many routine techniques, such as genetic engineering and the production of therapeutic proteins such as insulin and growth hormone as well as new genetic strains of crops and food for animals.
Recombinant DNA technology paved the way for DNA sequencing, which is used to identify the sequence of nucleotides within a gene, pinpoint the exact location of any change, and identify each of the nucleotides in an individual’s genome. A recent improvement to DNA sequencing, whole exome capture, promises to be an efficient way to identify the DNA sequences that constitute genes (Choi et al., 2009). Recombinant DNA is also the basis for detecting variations in DNA sequences that can be used to identify individuals for forensic and paternity purposes and to predict disease susceptibilities. Another important application is gene therapy, by which a corrected gene sequence can be introduced into the cells of an individual with a disease-causing mutation.
One of the outgrowths of these earlier technologies is microarray technology. Microarrays, also called DNA “chips,” are used to determine which genes are expressed at a particular time under particular conditions, such as during the different developmental stages. They can also be used to determine which genes are turned on (or off) in response to environmental factors, such as nutrients. A useful clinical application is the comparison of gene expression between normal and diseased cells, with important implications in cancer.
Another type of genetic technology involves interfering with a gene’s expression to determine the function of that gene and its encoded protein. The concept was originally exploited in model systems involving transgenic animals, particularly the laboratory mouse (“knockout mouse”). Because the mouse and human share many of the same genes, the ability to manipulate the genetic material of the mouse and examine the effect on metabolism and physiologic function has been valuable for understanding humans.
In the knockout mouse, a gene is altered (“knocked out”) so that the normal protein is no longer made. Alternatively, a gene can be altered so that it expresses too much or too little of its product. Regulatory sequences can be altered so that a gene no longer responds appropriately to environmental signals. In these ways the normal function of a gene can be determined, the effects of over-expressing or under-expressing a gene can be studied, and details of the communication process between signals outside the organism and the genetic material inside the organism can be determined. Transgenic mice are particularly valuable for studying gene-diet interactions. A recent application of this concept involves RNAi. Short sequences of RNA bind to mRNA and interfere with translation of the mRNA into protein (“knock down”). By measuring the outcome of a decrease in a particular protein, researchers can gain insight into the role of the protein and its contribution to the organism’s function.
Genetics And Nutrition Therapy
Chromosomal or single-gene mutations alter nutrition status and illustrate the importance of nutrition therapy. The rapid development of molecular nutrition and nutritional genomics expands the role of the nutrition professional beyond rare disorders and into more prevalent chronic diseases such as cardiovascular disease (CVD), cancer, diabetes, inflammatory disorders, osteoporosis, and even obesity.
Progress in identifying gene variants associated with particular chronic disorders and understanding the interaction of bioactive food components with these variants requires that nutrition professionals be able to interpret genetic screening information and integrate the findings into their services. Nutrition professionals will be needed at an advanced practice level to assist individuals in making gene-directed diet and lifestyle choices (DeBusk, 2009; DeBusk and Joffe, 2006; Jones et al., 2010).
Nutritional genomics is unique in its focus on how the interactions between genetic variations and environmental factors influence the genetic potential of individuals and populations, the “gene x environment” (GxE) premise (Ordovas and Tai, 2008). Here, environment broadly encompasses the typical toxins to which humans are exposed, as well as diet and lifestyle choices that also influence genetic potential. Nutrigenetics is concerned with how an individual’s unique set of genetic variations affects the ability to function optimally in a particular environment. Nutrigenomics identifies how the environment affects gene expression.
Nutrigenetic Influences on Health and Disease
The interplay between nutrition and genetics varies from being straightforward to being highly complex. The most straightforward is the direct correlation between a faulty gene, a defective protein, a deficient level of a metabolite, and a resultant disease state that is passed on through Mendelian inheritance and is responsive to nutrition therapy. The IEM are good examples of such interactions and have been referred to as genetic diseases. As our understanding of disease at the molecular level has become more sophisticated, this terminology is no longer appropriate. The IEM are characterized as rare mutations that result in protein dysfunction that leads to metabolic disorders. The distinguishing characteristic is the rare occurrence of these particular mutations.
All humans have mutations that result in protein dysfunction that leads to metabolic disease. The human species requires certain amino acids, fatty acids, vitamins, and mineral, and there are mutations that limit the ability to synthesize these important nutrients. The diet must supply them to prevent dysfunction and disease. For example, humans lack the gene for the enzyme gulonolactone oxidase and cannot synthesize vitamin C. If dietary vitamin C intake is below needed levels, individuals are at risk for developing scurvy, which can be fatal.
New is the understanding of the genetic basis for nutrient requirements, the realization that nutrition therapy can circumvent genetic limitations by supplying the missing nutrients, and that each individual may require a different level of nutrient because of his or her particular set of genetic variations. More than 50 metabolic reactions that involve enzymes with decreased affinities for their cofactors and that require high levels of a nutrient to restore function have been identified. Many of the supplementation levels are well in excess of the usual recommended nutrient levels, which highlights the importance of remembering that each individual is genetically unique and has distinct metabolic needs.
Although generalized guidelines for recommended nutrient levels are helpful, individuals may have genetic variations that require them to consume significantly more or less of certain nutrients than the general recommendation. Nutritional genomics has changed the thinking about global dietary recommended intakes, from an age- and sex-related orientation to incorporating nutrigenetic makeup and its influence on protein function (Stover, 2006). Nutrition therapy, then, is a critical tool for compensating for changes in the DNA that can lead to increased risk of disease.
The inborn error of amino acid metabolism, classic homocystinuria, is of interest because it led to the realization that an elevated blood level of homocysteine is an independent risk factor for CVD. A defect in the vitamin B6–requiring enzyme cystathionine beta-synthase prevents the conversion of homocysteine to cystathionine. Homocysteine accumulates, promotes atherosclerosis, and forms the dipeptide homocystine, which leads to abnormal collagen crosslinking and osteoporosis. Nutrition therapy is multipronged, depending on the specific genetic defect. Some individuals have an enzyme defect that requires a high concentration of the vitamin B6 cofactor for activity. Others are not responsive to B6 and need a combination of folate, vitamin B12, choline, and betaine to convert homocysteine to methionine. Others must limit their methionine intake. At least three forms of homocystinuria exist, each requiring a different nutritional approach. The ability to use genetic analysis to distinguish these similar disorders has been a useful technologic advance (see Chapters 6 and 33).
Genetic variation in the MTHFR gene provides an excellent example of nutrigenetics as well as how genetic variation can influence nutrient requirements. This gene codes for the enzyme 5,10-methyltetrahydrofolate reductase that produces the biologically active form of folate (5-methyltetrahydrofolate). Folate is essential for the conversion of homocysteine to S-adenosylmethionine, a critical methyl donor to numerous metabolic reactions, including those involved in synthesizing nucleic acids (see Chapter 33). A common variation in the MTHFR gene is the 677C>T gene variant, which involves substitution of thymine (T) for cytosine (C) at nucleotide position 677 within the coding region of the MTHFR gene. The resultant enzyme has reduced activity, which leads to decreased production of active folate and accumulation of homocysteine. In addition to the increased risk of CVD, elevated serum homocysteine increases the risk of neural tube defects in developing fetuses. As a result of these risks, in the United States cereal grains are now fortified with folic acid to ensure adequate levels in women of childbearing age (see Chapter 16).
Studies indicate that homocysteine levels can be lowered through supplementation with one or more of the B vitamins folate, B2, B6, and B12 (Albert et al., 2008; Ebbing et al., 2008; Shidfar et al., 2009; Varela-Moreiras et al., 2009). The genotype of the individual is an important factor in this response, supporting the need for tailoring nutrient recommendations accordingly.
Disease-causing changes can also occur in genes coding for other types of proteins such as transport proteins, structural proteins, membrane receptors, hormones, and transcription factors. Mutations that increase the transport of iron (hereditary hemochromatosis) or copper (Wilson disease) to higher-than-normal levels have nutritional implications (see Chapter 30). Mutations in vitamin D receptors are not only associated with deleterious effects on bone health, but throughout the body because vitamin D is a hormone involved in hundreds of metabolic and regulatory processes. Changes in the gene coding for insulin can result in structural changes in the insulin hormone and lead to dysglycemia, as can mutations in the insulin receptor. Many proteins such as kinases, cytokines, and transcription factors that are involved in critical signaling cascades are subject to mutational changes, altered activities, and health consequences.
Nutrigenomic Influences on Health and Disease
In addition to compensating for metabolic limitations, nutrients and other bioactive components in food can influence gene expression. This ability has long been known from studies with lower organisms, such as is seen with the lac and trp operons of bacteria. In these situations the organism “senses” the presence of a nutrient in its external environment and alters its gene expression accordingly. In the case of lactose, the proteins required to use lactose as an energy source are induced by transcriptional regulation of the genes that code for the lactose transport system and for the enzyme that initially metabolizes lactose. The opposite occurs when tryptophan is present in the environment: the organism inhibits the endogenous biosynthesis of tryptophan by inhibiting transcription of the genes that encode tryptophan biosynthetic proteins. GxE interactions, such as monitoring and responding to environmental signals by changing gene expression, are fundamental processes of living systems, allowing them to use resources efficiently.
Higher organisms such as humans have similar mechanisms by which they monitor the environment that bathes their cells and alter cellular or molecular activities as needed. An example is the response of cells to the presence of glucose. Insulin is secreted and binds to its receptor on the surface of skeletal muscle cells and initiates a stepwise biochemical signaling cascade (signal transduction). Signaling results in the translocation of glucose transporter type 4 (GLUT4), a receptor involved in glucose entry into cells, from the interior of the cell to the cell surface. Exercise also promotes the translocation of GLUT4, which is helpful in controlling blood sugar levels. A drop in blood sugar levels triggers the release of epinephrine and glucagon that, in turn, bind to cell surface receptors in the liver and skeletal muscle and, through signal transduction, stimulate glycogen breakdown to glucose to restore blood sugar levels.
Nutrients and other bioactive food components can also serve as ligands, molecules that bind to specific nucleotide sequences (response elements) within a gene’s regulatory region. Binding results in a change in gene expression through the regulation of transcription. Examples of such food components are the polyunsaturated ω-3 fatty acids. These fats decrease inflammation. They serve as precursors for the synthesis of antiinflammatory eicosanoids and decrease the expression of genes that lead to the production of inflammatory cytokines, such as tumor necrosis factor–alpha and the interleukin-1 genes (Calder, 2009).
The ω-3 and ω-6 fatty acids have also been found to serve as ligands for the peroxisome proliferator-activated receptor (PPAR) family of transcription factors. The PPARs function as lipid sensors and regulate lipid and lipoprotein metabolism, glucose homeostasis, adipocyte proliferation and differentiation, and the formation of foam cells from monocytes during atherogenic plaque formation. They are important components in the sequence of events by which a high-fat diet promotes insulin resistance and obesity (Christodoulides and Vidal-Puig, 2009).
To influence the expression of the genes under its control, a PPAR transcription factor must complex with a second transcription factor, the retinoic X receptor (RXR). Each has its ligand attached—polyunsaturated fatty acid and retinoic acid (vitamin A derivative), respectively. The PPAR-RXR complex can then bind to the appropriate response element within the regulatory region of a gene under its control. Binding results in a conformational change in the structure of the DNA molecule that allows RNA polymerase to bind and transcribe the PPAR-regulated genes, leading to a host of lipogenic and proinflammatory activities. A large number of transcription factors have been identified and the mechanisms of action are under investigation.
The bioactive components that serve as ligands for these transcription factors are either provided by the diet or made endogenously, such as ω-3 and ω-6 fatty acids, cholesterol, steroid hormones, bile acids, xenobiotics (foreign chemicals, or “new-to-nature” molecules), the active form of vitamin D, and numerous phytonutrients, to name just a few (Wise, 2008). In all cases these bioactives must communicate their presence to the DNA sequestered within the nucleus. Depending on their size and lipid solubility, some bioactives can penetrate the various membrane barriers and interact directly with the DNA, as in the fatty acid example discussed previously. Others, including phytochemicals found in the cruciferous vegetables, may not be able to cross the cell membrane and will instead interact with a receptor on the cell surface and set into motion the cascade of signal transduction events that results in a transcription factor being translocated to the nucleus. See Focus On: Phytochemical and Bioactive Food Components.
Identification of the genetic and biochemical mechanisms underlying health and disease provides the basis for developing individualized intervention and prevention strategies. In the case of the ω-3 fatty acids, researchers are actively seeking conditions under which dietary ω-3s can be used to decrease inflammation and increase insulin sensitivity. An understanding of the mechanisms by which gene expression is controlled is also helpful in developing drugs that can target various aspects, including gene expression. For example, the thiazolidinedione class of antidiabetic drugs targets the PPAR mechanism described previously to improve insulin sensitivity.
Identifying bioactive components in fruits, vegetables, and whole grains that are responsible for positive health effects and the mechanisms by which they influence gene expression is of considerable interest. Small-molecular-weight lipophilic molecules can penetrate the cellular and nuclear membranes and serve as ligands for transcription factors that control gene expression. Depending on the gene and the particular bioactive, expression may be turned on or off or increased or decreased in magnitude in keeping with the information received. Examples include resveratrol from purple grape skins; along with a large number of flavonoids such as the catechins found in tea, dark chocolate, and onions and the isoflavones genistein and daidzein from soy.
For bioactive phytochemicals that are too large or too hydrophilic to penetrate the cell’s membrane barriers, communication occurs by means of signal transduction. The bioactive interacts with a receptor protein at the cell surface and initiates a cascade of biochemical reactions that ultimately results in one or more transcription factors interacting with DNA and modulating gene expression. Examples of this type of indirect communication are seen with the organosulfur compounds such as sulforaphane and other glucosinolates from the cabbage family vegetables. As a result of the signaling pathway, transcription factors (e.g., nrf) are activated and increase transcription of the glutathione-S-transferases needed for phase II detoxification, which helps to protect against cancer. Flavonoids such as naringenin found in citrus fruits and quercetin from onions and apples activate signaling pathways, leading to increased apoptosis of cancer cells.
It can be challenging to communicate the specifics of phytochemicals to consumers because they do not think about the bioactive substances in the food they eat. Attempts have been made to simplify the message, such as focusing on thinking of food in terms of its dominant color and understanding that each color contributes different valuable phytochemicals. For example, eating one to two servings from a wide variety of fruits, vegetables, legumes, grains, nuts, and seeds within the red, orange, green, purple, and white color categories daily will supply a variety of healthy phytochemicals. Individuals with particular disease susceptibilities or environmental challenges should increase the number of servings within a particular category to meet their specific health needs. Practitioners can provide a valuable service by translating these research findings into practical food solutions for consumers (Keijr et al., 2010; Kim et al., 2009).
Epigenetic Influences on Health and Disease
Epigenetics, including gene silencing and genomic imprinting, is under active investigation presently (Butler, 2009; Mathers, 2008; Waterland, 2009). Inappropriate gene expression can have serious consequences. During development, for example, specific genes are turned on or off with precise timing. Alterations in that timing can impair fetal development and may cause death. Cancer is another example. Certain genes (oncogenes) promote uncontrolled cell growth; others (tumor suppressor genes) help to put the brakes on such growth. Inappropriate methylation of these genes can result in oncogenes being expressed when they should be silent and tumor suppressor genes being silenced when they would normally be expressed. Either situation can promote uncontrolled cell growth and the development of cancer.
As the significance of epigenetics and the critical nature of epigenetic reprogramming during germ cell development and early embryogenesis is increasingly appreciated, researchers have begun to ask how reproductive technologies such as in vitro fertilization might affect fetal development (Dupont et al., 2009; Grace et al., 2009; Swanson et al., 2009).
Nutritional Genomics and Chronic Disease
Chronic disorders (e.g., CVD, cancer, diabetes, osteoporosis, inflammatory disorders) are typically more complex than single-gene disorders in which the change in the DNA is known, the abnormal protein can be identified and analyzed, and the resulting phenotype is clearly defined. Multiple genes, each with multiple variations, contribute in small ways to the overall chronic condition rather than a single variant having a dramatic effect. The genes involved with chronic disease are influenced by environmental factors in addition to the genetic variation. An individual might have gene variants that predispose to a particular chronic disorder, but the disorder may or may not develop.
Genetic Variability
Given the genetic variability among individuals in a population, the high degree of variability in client response to nutrition therapy should not be surprising. Although a change in a gene—including diet- and lifestyle-related genes—can affect function severely enough to cause disease outright, the majority of these genes appear to affect the magnitude of response and do not pose a life-threatening situation. They confer an increased susceptibility. Many of these variants are responsive to diet and other lifestyle parameters, providing the opportunity to minimize their effect through informed lifestyle choices.
The major focus of nutritional genomics research is on identifying (1) gene-disease associations, (2) the dietary components that influence these associations, (3) the mechanisms by which dietary components exert their effects, and (4) the genotypes that benefit most from particular diet and lifestyle choices. The practical applications of this research include a new set of tools that nutrition professionals can use. The growing body of knowledge will support strategies for disease prevention and intervention that are specifically targeted to the underlying mechanisms.
The following section takes a brief look at some of the key diet-related genes, their known variants, and how these variants affect a person’s response to diet. Chronic disease involves complex interactions among genes and bioactive food components, and unraveling the details will require population and intervention studies large enough to have the statistical power needed to draw meaningful conclusions.
Cardiovascular Disease
CVD remains the number one disease plaguing developed countries. Not surprisingly, a major focus of nutritional genomics has been to identify gene-diet associations for CVD and to study the influence of diet and exercise parameters in managing and preventing this chronic disease. Nutrition professionals who work with clients with dyslipidemia know firsthand the high degree of individual variability of responses to standard dietary interventions. These therapies are used primarily to lower elevated blood levels of LDL cholesterol (LDL-C), raise high-density lipoprotein cholesterol (HDL-C), and lower triglycerides (TGs). The standard approach is a diet low in saturated fat, with increased content of polyunsaturated fats (PUFAs). Response across a population varies, ranging from reduced LDL-C levels and TGs in some to decreased HDL-C levels or elevated TGs in others. Furthermore, some have had their LDL-C levels respond dramatically to dietary oat bran and other soluble fibers, whereas others have had more modest responses. In some a low-fat diet has caused a shift to a lipid pattern that is more atherogenic than the original. Genotype is an important factor; dietary interventions must be matched to genotypes to accomplish the intended lipid-lowering response.
A number of contributing genes have already been identified and include those involved with postprandial lipoprotein and TG response, homocysteine metabolism, hypertension, blood-clotting, and inflammation (Lovegrove and Gitau, 2008; Minihane, 2009). Gene-diet interactions are noted for those that code for apolipoprotein E (APOE), apolipoprotein A-1 (APOA1), cholesterol esteryl transport protein (CETP), hepatic lipase (LIPC), lipoxygenase-5 (ALOX5), MTHFR, angiotensinogen (AGT), angiotensin-converting enzyme (ACE), the interleukin-1 family (IL1), interleukin-6 (IL6), and tumor necrosis factor-alpha (TNF). Five new SNPs that appear to be common among multiple populations have been identified from the Atherosclerosis Risk in Communities Study by using the genome-wide association study technique (Bressler et al., 2010).
Data from the Framingham Study provided an early example of how knowing the client’s genetic variants could be helpful in developing effective nutritional interventions. The APOA1 gene codes for apolipoprotein A-1, the primary protein in HDL (see Chapters 15 and 34). One variant that is diet-related is −75G>A, in which guanine has been replaced with an adenine at position 75 within the regulatory region of the APOA1 gene (the “−” sign indicates the site for the variation comes prior to the first nucleotide—position “0”—of the gene’s coding region). In women with two copies of the more common G allele, increasing dietary PUFA was accompanied by declining HDL levels. However, in women with at least one copy of the A allele, increasing PUFA concentrations increased HDL levels. Manipulating PUFA levels will have different effects on HDL levels, depending on which variant an individual has and how many copies.
A sampling of other diet- and lifestyle-responsive gene variants that have implications for nutrition therapy related to preventing and treating CVD include APOE gene variants and the responses to dietary fat, soluble fiber, and alcohol (Corella and Ordovas, 2005); CETP variants and effects on HDL levels, lipid-modifying response to statin drugs, and response of lipid parameters to physical activity (Ayyobi et al., 2005); APOE, CETP, and APOA-IV gene variants and low HDL levels (Miltiadous et al., 2005); effect of LIPC gene variants on HDL levels and modification by saturated fat (Zhang et al., 2005); APOA2, dietary fat, and body mass index (Corella et al., 2009); and APOA5 and TGs (Lai et al., 2006; Tai and Ordovas, 2008). The possibilities for nutritional intervention are endless (Afman and Müller, 2006).
CVD is also an inflammatory disorder (Rocha and Libby, 2009), and variants of TNF, IL1, and IL6 are being investigated for their effect on CVD susceptibility. Knowing the genotype of clients provides additional important information as to how they are likely to respond to particular dietary interventions (Ordovas, 2007).
Inflammatory Disorders
Inflammation is now recognized as an underlying factor in chronic disorders, from heart disease to cancer to diabetes to obesity to more traditional inflammatory disorders such as arthritis and the inflammatory bowel disorders. Inflammation is a normal and desirable response to insult by the body. Typically, inflammation is an acute phase response; once the threat has passed, inflammation subsides and healing ensues. Certain genetic variations predispose individuals to be proinflammatory, making them more reactive to inflammatory triggers and extending the inflammation phase so that inflammation becomes a chronic state. The regular assault of proinflammatory mediators such as the cytokines and eicosanoids on tissues leads to oxidative stress and cellular degeneration rather than the healing that is characteristic of the acute phase.
Among the genes known to be of particular importance to the inflammatory response are IL1, which encodes the interleukin-1β cytokine (also known as IL-1F2), IL6 (encoding the interleukin-6 cytokine), and TNF. Variants in each of these genes have been discovered that increase the susceptibility of humans to be in a proinflammatory state, which in turn increases the risk of developing one or more chronic disorders. Certain diet and lifestyle approaches can minimize susceptibility and dampen existing inflammation (Massaro et al., 2008). Examples include the inclusion of fish and foods that contain ω-3 fatty acids and plant foods rich in various polyphenols.
Immune Health and Cancer
The relationship of gene variants and gene-diet interactions to immune health and cancer is of considerable interest to researchers around the world (Milner, 2008; Trottier et al., 2010; Villagra et al., 2010). One of the key mechanisms by which the body protects against cancer is detoxification, the process of neutralizing potentially harmful molecules. Among the better-characterized genes involved in various aspects of detoxification are the cytochrome P450 isozymes (CYPs), glutathione S-transferases (GSTs), and superoxide dismutases (SOD1, SOD2, SOD3). The CYP and GST genes are part of the phase I and phase II detoxification system, respectively, found in the liver and the gut. The SOD genes code for proteins that dismantle the reactive oxygen species superoxide. Each of these genes has nutritional implications, and variants have been identified that result in decreased detoxification. Nutritional genomics provides the basis for directing nutrition therapy to protect against cancer by augmenting endogenous detoxification activity.
Epidemiologic studies have suggested that consuming plant foods is cancer-protective. Numerous dietary factors play a role in protecting against cancer. Examples include curcumin from the spice turmeric (Aggarwal and Shishodia, 2006; Surh and Chun, 2007; Trottier et al., 2009), resveratrol from purple grape skins (Athar et al., 2009; Udenigwe et al., 2008), glucosinolates in cruciferous vegetables (Ambrosone and Tang, 2009), epigallocatechin gallate catechins from green tea (Na and Surh, 2008), isoflavones from soybeans (Steiner et al., 2008), folic acid (Ebbing et al., 2009; Fife et al., 2009; Oaks et al., 2009), and vitamin D (van der Rhee et al., 2009). Numerous labs are investigating the underlying mechanisms by which these phytochemicals exert their protective effects (Surh et al., 2008; Tan and Spivack, 2009).
Blood Sugar Regulation
Glucose is the preferred source of energy for the body’s cells. Accordingly, blood glucose levels are carefully controlled through an intricate system of checks and balances. When glucose levels are higher than normal (hyperglycemia), the hormone insulin is secreted from the beta-cells of the pancreas, glucose is taken up by the cells, and a normal blood sugar level (euglycemia) is restored. When blood glucose levels fall (hypoglycemia), the hormone glucagon is secreted by the liver, glycogen is hydrolyzed to glucose, and euglycemia is again restored. When this process goes awry, the stage is set for the chronic conditions of insulin resistance, metabolic syndrome, and, ultimately, T2DM.
Identification of gene variants that lead to T2DM would allow individuals with this susceptibility to be identified early in the lifespan so that intervention could be initiated. A few rare mutations have been associated with the development of T2DM, but they do not explain the high prevalence of the disease. It is likely that multiple gene variants contribute to the development of this condition. One promising variant is the transcription factor 7-like 2 (TCF7L2) identified by Grant and colleagues (2006). The variant occurs frequently within multiple populations. Evidence suggests the gene is involved in insulin secretion from pancreatic beta-cells (Villareal et al, 2010).
Bone Mineralization and Maintenance
Healthy bone tissue depends on a balance between the action of the osteoblasts that synthesize new bone tissue and resorption by osteoclasts. Important components in this dynamic balance are vitamin D, calcium, other nutrients, and hormones such as parathyroid hormone and estrogen. When resorption predominates, bones become fragile, are subject to fracture, and osteoporosis results. Osteoporosis can occur in men and women as they age; it is prevalent among older postmenopausal women.
Numerous genes and their protein products are involved in the overall process. Variants are being investigated, including the VDR gene, which codes for the vitamin D receptor present on the surface of many cell types. Vitamin D has multiple roles in metabolism, but its control of the absorption of dietary calcium from the digestive tract truly affects bone health. Four VDR variants have been studied over several years (ApaI, BsmI, FokI, and TaqI), but no clear association has emerged (Gennari et al., 2009). Two of the more promising variants at this time are COL1A1, which encodes the major protein in bone (α1 peptide of Type 1 collagen) and LRP5, which encodes a protein that forms a cell surface receptor and activates the signaling pathway. Further research is imperative.
Weight Management
The ability to maintain a healthy weight is another challenge of modern society. As with the other chronic disorders, the regulation of body weight is a complex process and offers multiple points at which a gene variant can give rise to an impaired protein that, when combined with the appropriate environmental trigger, promotes body fat storage. Similar to T2DM, variations in single genes have been associated with excess weight, but these genetic changes are not likely the basis for the rapid rise in prevalence of excess body weight during the past several generations (Hetherington and Cecil, 2010).
A variant in the FTO gene has been identified and found to occur frequently among multiple populations (Chu et al, 2008; Dina et al., 2007). An SNP in the FTO gene is associated with increased risk of obesity, and the effect was directly correlated with the number of copies of the SNP. That is, those with one copy of the risk allele weighed more than those with no copies, and those with two copies weighed the most of the three groups (Frayling et al., 2007). In 2009, two large genome-wide association studies found the FTO variant to be associated with BMI (Thorleifsson et al., 2009; Willer et al., 2009). The mechanism of action of the FTO gene is not yet known. A number of other gene variants have been implicated in weight management, including ADRB3, FABP2, POMC, and PPARG, but none so dramatically as FTO. The FTO variant may also increase risk for T2DM and CVD through its effect on susceptibility to increased body fat.
Adipose tissue is dynamic tissue that is highly vascularized and produces hormones, inflammatory peptides (cytokines), and new adipocytes in addition to storing excess calories as TGs and hydrolyzing them as energy is needed. The multistep process of transporting free fatty acids into the adipocyte, esterifying them into TGs, and mobilizing the TGs potentially provides many proteins that could be affected by genetic variation such that fat is stored more readily or mobilized more slowly than normal. Adipocytes have cell surface receptors that respond to various environmental factors, such as catecholamines produced during exercise, to mobilize stored fat. An example is the receptor encoded by the ADRB2 gene, which has a greater propensity to store dietary fat as body fat. Individuals with either of these variants will find it more challenging to maintain a healthy weight and may need to restrict their dietary fat intake or engage in regular, vigorous exercise to achieve and maintain a healthy weight.
Other Chronic Diseases
Candidate genes, gene variants, and diet-gene interactions are being investigated for many chronic disorders. Populations differ in the types and frequencies of the gene variants; dietary approaches that are most appropriate will vary accordingly. As gene variants and their health implications are identified, attention is also being paid to examining the frequency of particular variants among populations.
Ethical, Legal, And Social Implications
If nutritional genomics is to realize its potential as a valuable tool, genetic testing is an essential component of identifying the variations in each individual. Such testing is not, however, without controversy. Consumers worry that genetic testing for any purpose could be used against them, primarily to deny insurance coverage or employment; they are particularly uncomfortable with insurers and employers having access to their personal genetic information (Genetics & Public Policy Center, 2010.)
Although theoretically possible, according to existing case law such discrimination has rarely occurred. Furthermore, identifying gene variants that increase susceptibility to diet- and lifestyle-related diseases that can be addressed through readily available diet and lifestyle measures may also lead to legal debate. Many legislators and legal experts believe the Americans with Disabilities Act sufficiently protects against discrimination, but, as an added measure of protection, the Genetic Information Nondiscrimination Act (GINA) was passed by Congress and went into effect on November 21, 2009. GINA defines genetic testing and genetic information, bans discrimination based on genetic information, and penalizes those who violate the provisions of this law. Consumers and health care professionals can feel comfortable in adopting this new service.
Consumers and health care professionals should ask critical questions prior to consent for genetic testing. The laboratory itself should have the appropriate credentials and state licensing, if required (at a minimum, Clinical Laboratory Improvement Amendment (CLIA) certification according to the CLIA of 1988) and should have an appropriately credentialed health professional available for assistance in interpreting the test results. The laboratory should have written, readily available policies concerning how it will protect the privacy of the individual being tested and whether the DNA sample will be retained by the lab or destroyed following testing. Transparency in each of these areas will increase consumer comfort.
A second concern on the part of consumers and health care professionals is that nutritional genomics is elitist by nature, in that only the wealthy will be able to benefit. At this early stage in its development, the cost of nutritional genomics testing precludes its use as a public health measure and effectively restricts access to those with sufficient disposable income. However, like any other new technology, as the sales volume increases, the cost will decrease.
Numerous additional issues need to be thoroughly discussed in the course of integrating genetic technologies into health care. Box 5-1 lists key questions to be answered in the development of nutritional genomics practices. These and other ethical, legal, and social issues relating to nutritional genomics in particular have been explored. Authors address ethical treatment of research participants (Bergman et al., 2008), consumer versus health care professional testing (Foster and Sharp, 2008; Royal et al, 2010); the capacity gap in terms of health care professionals trained in nutritional genomics (Farrell, 2009); and various other aspects of the ethical, legal, and social implications of nutritional genomics (Reilly and DeBusk, 2008; Ries and Castle, 2008).
Center for Nutritional Genomics
Ethical, Legal, and Social Issues
http://www.ornl.gov/sci/techresources/Human_Genome/elsi/elsi.shtml
http://www.hhs.gov/familyhistory
http://www.genome.gov/Education/
http://www.nchpeg.org/core/Core_Comps_English_2007.pdf
Genetic Information Nondiscrimination Act (GINA)
www.gpo.gov/fdsys/pkg/PLAW-110publ233/pdf/PLAW-110publ233.pdf
www.ornl.gov/TechResources/Human_Genome/glossary
www.ornl.gov/hgmis/project/info.html
http://www.nugo.org/everyone/28182
References
Afman, L, Müller, M. Nutrigenomics: from molecular nutrition to the prevention of disease. J Am Diet Assoc. 2006;106:569.
Aggarwal, BB, Shishodia, S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397.
Albert, CM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299:2027.
Ambrosone, CB, Tang, L. Cruciferous vegetable intake and cancer prevention: role of nutrigenetics. Cancer Prev Res (Phila Pa). 2009;2:298.
Athar, M, et al. Multiple molecular targets of resveratrol: anti-carcinogenic mechanisms. Arch Biochem Biophys. 2009;486:95.
Ayyobi, AF, et al. Cholesterol ester transfer protein (CETP) Taq1B polymorphism influences the effect of a standardized cardiac rehabilitation program on lipid risk markers. Atherosclerosis. 2005;181:363.
Bergman, MM, et al. Bioethical considerations for human nutrigenomics. Annu Rev Nutr. 2008;28:447.
Bressler, J, et al. Genetic variants identified in a European genome-wide association study that were found to predict incident coronary heart disease in the atherosclerosis risk in communities study. Am J Epidemiol. 2010;171:14.
Butler, MG. Genomic imprinting disorders in humans: a mini-review. J Assist Reprod Genet. Oct 21, 2009. [Epub ahead of print.].
Calder, PC. The 2008 ESPEN Sir David Cuthbertson lecture: fatty acids and inflammation—From the membrane to the nucleus and from the laboratory bench to the clinic. Clin Nutr. 2009. [Epub ahead of print.].
Choi, M, et al. Genetic diagnosis by whole exome capture and massively parallel DNA sequencing. Proc Natl Acad Sci U S A. 2009;106:19096.
Christodoulides, C, Vidal-Puig, A. PPARS and adipocyte function. Mol Cell Endocrinol. 2010;318:61.
Chu, X, et al. Association of morbid obesity with FTO and INSIG2 allelic variants. Arch Surg. 2008;143:235.
Cropley, JE, et al. Germ-line epigenetic modification of the murine Avy allele by nutritional supplementation. Proc Natl Acad Sci USA. 2006;103:17308.
Corella, D, et al. APOA2, dietary fat, and body mass index: replication of a gene-diet interaction in 3 independent populations. Arch Intern Med. 2009;169(20):1897.
Corella, D, Ordovas, JM. Single nucleotide polymorphisms that influence lipid metabolism: interaction with dietary factors. Annu Rev Nutr. 2005;25:341.
Das, R, et al. Imprinting evolution and human health. Mamm Genome. 2009;20:563.
DeBusk, R. Diet-related disease, nutritional genomics, and food and nutrition professionals. J Am Diet Assoc. 2009;109:410.
DeBusk, R, Joffe, Y. It’s not just your genes!. San Diego: BKDR; 2006.
Dietzen, DJ, et al. National academy of clinical biochemistry laboratory medicine practice guidelines: follow-up testing for metabolic disease identified by expanded newborn screening using tandem mass spectrometry: executive summary. Clin Chem. 2009;55:1615.
Dina, C, et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet. 2007;39:724.
Dupont, C, et al. Epigenetics: definition, mechanisms and clinical perspective. Semin Reprod Med. 2009;27:351.
Ebbing, M, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA. 2009;302:2119.
Ebbing, M, et al. Mortality and cardiovascular events in patients treated with homocysteine-lowering B vitamins after coronary angiography: a randomized controlled trial. JAMA. 2008;300:795.
Farrell, J. Health care provider capacity in nutrition and genetics—a Canadian case study. In: Castle D, Ries N, eds. Nutrition and genomics: issues of ethics, law, regulation and communication. Toronto: Elsevier, 2009.
Feuk, L, et al. Structural variation in the human genome. Nat Rev Genet. 2006;7:85.
Fife, J, et al. folic acid supplementation and colorectal cancer risk: a meta-analysis. Colorectal Dis. Oct 27, 2009. [Epub ahead of print.].
Foster, MW, Sharp, RR. Out of sequence: how consumer genomics could displace clinical genetics. Nat Rev Genet. 2008;9:419.
Frayling, TM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889.
Gennari, L, et al. Update on the pharmacogenetics of the vitamin D receptor and osteoporosis. Pharmacogenomics. 2009;10:417.
Genetics & Public Policy Center, Johns Hopkins University. http://www.dnapolicy.org/images/reportpdfs/GINAPublic_Opinion_Genetic_Information_Discrimination.pdf [Accessed Jan 22, 2010].
Gosden, RG, Feinberg, AP. Genetics and epigenetics—nature’s pen-and-pencil set. N Engl J Med. 2007;356:731.
Grace, KS, Sinclair, KD. Assisted reproductive technology, epigenetics, and long-term health: a developmental time bomb still ticking. Semin Reprod Med. 2009;27:409.
Grant, SF, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38:320.
Hetherington, MM, Cecil, JE. Gene-environment interactions in obesity. Forum Nutr. 2010;63:195.
Human Genome Project. www.ornl.gov/hgmis/project/info.html [Accessed Jan 22, 2010].
Isaacs, JS, Zand, DJ. Single-gene autosomal recessive disorders and Prader-Willi syndrome: an update for food and nutrition professionals. J Am Diet Assoc. 2007;107:466.
Jones, DS, et al. 21st century medicine: a new model for medical education and practice. http://www.functionalmedicine.org. [Accessed Jan 2, 2010].
Kauwell, GP. Epigenetics: what it is and how it can affect dietetics practice. J Am Diet Assoc. 2008;108:1056.
Keijr, J, et al. Bioactive food components, cancer cell growth limitation and reversal of glycolytic metabolism. Biochim Biophys Acta. 2011;1807:697.
Kim, YS, et al. Bioactive food components, inflammatory targets, and cancer prevention. Cancer Prev Res (Phila Pa). 2009;2:200.
Kloc, A, Martienssen, R. RNAi, heterochromatin and the cell cycle. Trends Genet. 2008;24:51.
Lai, CQ, et al. Dietary intake of ω-6 fatty acids modulates effect of apolipoprotein A5 gene on plasma fasting triglycerides, remnant lipoprotein concentrations, and lipoprotein particle size: the Framingham Heart Study. Circulation. 2006;113:2062.
Lovegrove, JA, Gitau, R. Personalized nutrition for the prevention of cardiovascular disease: a future perspective. J Hum Nutr Diet. 2008;21:306.
Massaro, M, et al. ω-3 fatty acids, inflammation and angiogenesis: nutrigenomics effects as an explanation for anti-atherogenic and anti-inflammatory effects of fish and fish oils. J Nutrigenet Nutrigenomics. 2008;1:4.
Mathers, JC. Session 2: Personalised nutrition. Epigenomics: a basis for understanding individual differences? Proc Nutr Soc. 2008;67:390.
Milner, JA. Nutrition and cancer: essential elements for a roadmap. Cancer Lett. 2008;269:189.
Miltiadous, G, et al. Gene polymorphisms affecting HDL-cholesterol levels in the normolipidemic population. Nutr Metab Cardiovas Dis. 2005;15:219.
Minihane, AM. Nutrient gene interactions in lipid metabolism. Curr Opin Clin Nutr Metab Care. 2009;12:357.
MITOMAP. map of the mitochondrial genome. http://www.mitomap.org/. [Accessed 2009].
Na, HK, Surh, YJ. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenols EGCG. Food Chem Toxicol. 2008;46:1271.
Oaks, BM, et al. Folate intake, post-folic acid grain fortification, and pancreatic cancer risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am J Clin Nutr. December 2009. [Epub ahead of print.].
Ordovas, J. Diet/genetic interactions and their effects on inflammatory markers. Nutr Rev. 2007;65(12 Pt 2):S203.
Ordovas, JM, Tai, ES. Why study gene-environment interactions? Curr Opin Lipidol. 2008;19:158.
Panagiotou, G, Nielsen, J. Nutritional systems biology: definitions and approaches. Annu Rev Nutr. 2009;29:329.
Reilly, PR, DeBusk, RM. Ethical and legal issues in nutritional genomics. J Am Diet Assoc. 2008;108:36.
Ries, NM, Castle, D. Nutrigenomics and ethics interface: direct-to-consumer services and commercial aspects. OMICS. 2008;12:245.
Rocha, VZ, Libby, P. Obesity, inflammation and atherosclerosis. Nat Rev Cardiol. 2009;6:399.
Royal, CD, et al. Inferring genetic ancestry: opportunities, challenges, and implications. Am J Hum Genet. 2010;86:661.
Shidfar, F, et al. Effect of folate supplementation on serum homocysteine and plasma total antioxidant capacity in hypercholesterolemic adults under lovastatin treatment: a double-blind randomized controlled clinical trial. Arch Med Res. 2009;40:380.
Steiner, C, et al. Isoflavones and the prevention of breast and prostate cancer: new perspectives opened by nutrigenomics. Br J Nutr. 2008;99:ES78.
Stover, PJ. Influence of human genetic variation on nutritional requirements. Am J Clin Nutr. 2006;83:436S.
Stover, PJ, Caudill, MA. Genetic and epigenetic contributions to human nutrition and health: managing genome-diet interactions. J Am Diet Assoc. 2008;108:1480.
Surh, YJ, Chun, KS. Cancer chemopreventive effects of curcumin. Adv Exp Med Biol. 2007;595:149.
Surh, YJ, et al. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008;74:1526.
Suzuki, K, Kelleher, AD. Transcriptional regulation by promoter targeted RNAs. Curr Top Med Chem. 2009;9:1079.
Swanson, JM, et al. Developmental origins of health and disease: environmental exposures. Semin Reprod Med. 2009;27:391.
Tai, ES, Ordovas, JM. Clinical significance of apolipoprotein A5. Curr Opin Lipidol. 2008;19:349.
Tan, XL, Spivack, SD. Dietary chemoprevention strategies for induction of phase II xenobiotic-metabolizing enzymes in lung carcinogenesis: a review. Lung Cancer. 2009;65:129.
Thorleifsson, G, et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet. 2009;41:18.
Trottier, G, et al. Nutraceuticals and prostate cancer prevention: a current review. Nat Rev Urol. Dec 8, 2009. [Epub ahead of print.].
Tuppen, HA, et al. Mitochondrial DNA mutations and human disease. Biochim Biophys Acta. 2009. [Epub ahead of print.].
Udenigwe, CC, et al. Potential of resveratrol in anticancer and anti-inflammatory therapy. Nutr Rev. 2008;66:445.
Van der Maarel, SM. Epigenetic mechanisms in health and disease. Ann Rheum Dis. 2008;67(Suppl 3):iii97.
Van der Rhee, H, et al. Sunlight, vitamin D and the prevention of cancer: a systematic review of epidemiological studies. Eur J Cancer Prev. 2009;18:458.
Varela-Moreiras, G, et al. Cobalamin, folic acid, and homocysteine. Nutr Rev. 2009;67(Suppl 1):S69.
Villagra, A, et al. Histone deacetylases and the immunological network: implications in cancer and Inflammation. Oncogene. 2010;29:157.
Villareal, DT, et al. TCF7L2 variant rs7903146 affects the risk of type 2 diabetes by modulating incretin action. Diabetes. 2010;59:479.
Waterland, RA. Is epigenetics an important link between early life events and adult disease? Horm Res. 2009;71:13S.
Waterland, RA, Jirtle, RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293.
Willer, CJ, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009;41:24.
Wise, A. Transcriptional switches in the control of macronutrient metabolism. Nutr Rev. 2008;66:321.
Zhang, C, et al. Interactions between the −514C>T polymorphism of the hepatic lipase gene and lifestyle factors in relation to HDL concentrations among U.S. diabetic men. Am J Clin Nutr. 2005;81:1429.