Balance and Imbalance of Body Fluids
Chapter Outline
Disturbances of Fluid and Electrolyte Balance,
Disturbances of Acid-Base Balance,
Nursing Responsibilities in Fluid and Electrolyte Disturbances,
http://evolve.elsevier.com/wong/ncic
Alternative Feeding Techniques, Ch. 27
Burns, Ch. 29
The Child with Gastrointestinal Dysfunction, Ch. 33
The Child with Renal Dysfunction, Ch. 30
Collection of Specimens, Ch. 27
Diarrhea, Ch. 29
Family-Centered Home Care, Ch. 25
Intravenous Administration (of Medication), Ch. 27
Oral Hygiene, Ch. 27
Pain Management, Ch. 7
Preparation for Diagnostic and Therapeutic Procedures, Ch. 27
Shock States, Ch. 29
Vomiting, Ch. 29
Distribution of Body Fluids
The distribution of body fluids, or total body water (TBW), involves the presence of intracellular fluid (ICF) and extracellular fluid (ECF). Water is the major constituent of body tissues, and the TBW in an individual ranges from 45% (in late adolescence) to 75% (in term newborn) of total body weight.
The ICF refers to the fluid contained within the cells, whereas the ECF is the fluid outside the cells. The ECF is further broken down into several components: intravascular (contained within the blood vessels), interstitial (surrounding the cell; the location of most ECF), and transcellular (contained within specialized body cavities such as cerebrospinal, synovial, and pleural fluid). In the newborn about 50% of the body fluid is contained within the ECF, whereas 30% of the toddler’s body fluid is contained within the ECF.
Body water is important in body function not only because of its abundance but also because it is the medium in which body solutes are dissolved and all metabolic reactions take place. Because even small alterations in fluid composition affect these metabolic processes, precise regulation of the volume and composition of the fluid is essential. In healthy individuals, body water remains singularly constant, but marked alterations in either its volume or distribution, which occur in many disease states, can produce severely damaging physiologic consequences.
Water Balance
Under normal conditions the amount of water ingested closely approximates the amount of urine excreted in a 24-hour period, and the water in food and from oxidation approximates the amount lost in feces and through evaporation. In this way, the body maintains equilibrium.
Mechanisms of Fluid Movement
Water is retained in the body in a relatively constant amount and, with few exceptions, is freely exchangeable among all body fluid compartments. The proximity of the extravascular compartment to the cells allows for continuous change in volume and distribution of fluids, largely determined by solutes (especially sodium) and physical forces (Fig. 28-1). Transport mechanisms are the basis for all activity within the cells, and because the cells have limited ability to store materials, movement in and out of cells must be rapid. Internal control mechanisms are responsible for distribution and maintenance of fluid balance (Box 28-1).
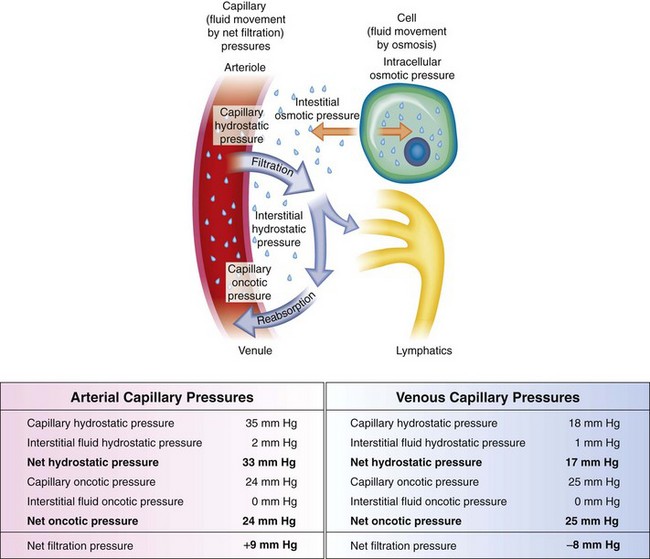
Fig. 28-1 Capillary filtration forces. Water, electrolytes, and small molecules exchange freely between the vascular compartment and the interstitial space at the site of capillaries and small venules. The rate and amount of exchange are driven by the physical forces of hydrostatic and oncotic pressures and the permeability and surface area of the capillary membranes. The two opposing hydrostatic pressures are capillary hydrostatic pressure and interstitial hydrostatic pressure. The two opposing oncotic pressures are capillary oncotic pressure and interstitial oncotic pressure. The forces that favor filtration from the capillary are capillary hydrostatic pressure and interstitial oncotic pressure, and the forces that oppose filtration are capillary oncotic pressure and interstitial hydrostatic pressure. The sum of their effects is known as net filtration pressure. In the example of normal exchange above, a small amount of fluid moves to the lymph vessels, which accounts for the net filtration difference between the arterial and venous ends of the capillary. (From McCance K, Huether S: Pathophysiology: the biological basis for disease in adults and children, ed 6, St Louis, 2010, Mosby.)
Maintaining Water Balance
Maintenance water requirement is the volume of water needed to replace obligatory fluid loss such as that from insensible water loss (through the skin and respiratory tract), evaporative water loss, and losses through urine and stool formation. The amount and type of these losses may be altered by disease states such as fever (with increased sweating), diarrhea, gastric suction, and pooling of body fluids in a body space (often referred to as third spacing).
Nurses should be alert for altered fluid requirements in various conditions:
Basal maintenance calculations for required body water are based on the body’s requirements for water in a normometabolic state, at rest; estimated fluid requirements are then increased or decreased from these parameters based on increased or decreased water losses, such as with elevated body temperature or congestive heart failure. Daily maintenance fluid requirements are listed in Table 28-1.
TABLE 28-1
DAILY MAINTENANCE FLUID REQUIREMENTS*
| BODY WEIGHT | AMOUNT OF FLUID PER DAY |
| 1-10 kg | 100 ml/kg |
| 11-20 kg | 1000 ml plus 50 ml/kg for each kg >10 kg |
| >20 kg | 1500 ml plus 20 ml/kg for each kg >20 kg |
Maintenance fluids contain both water and electrolytes and can be estimated from the child’s age, body weight, degree of activity, and body temperature. Basal metabolic rate (BMR) is derived from standard tables and adjusted for the child’s activity, temperature, and disease state. For example, for afebrile patients at rest, the maintenance water requirement is approximately 100 ml for each 100 kcal expended. Children with fluid losses or other alterations require adjustment of these basic needs to accommodate abnormal losses of both water and electrolytes as a result of a disease state. For example, insensible losses increase when basal expenditure increases by fever or hypermetabolic states. Hypometabolic states, such as hypothyroidism and hypothermia, decrease the BMR.
Changes in Fluid Volume Related to Growth
The percentage of TBW varies among individuals and in adults and older children is related primarily to the amount of body fat. Consequently, females, who have more body fat than males, and obese persons tend to have less water content in relation to weight.
The fetus is composed primarily of water, with little tissue substance. As the organism grows and develops, a progressive decrease occurs in TBW, with the fastest rate of decline taking place during fetal life. The changes in water content and distribution that occur with age reflect the changes that take place in the relative amounts of bone, muscle, and fat making up the body. At maturity the percentage of TBW is somewhat higher in the male than in the female and is probably a result of the differences in body composition, particularly fat and muscle content (Fig. 28-2).
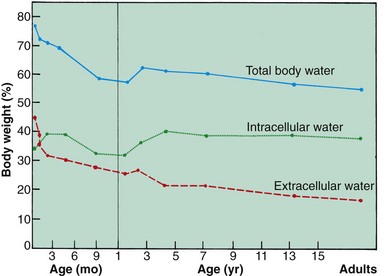
Fig. 28-2 Changes in total body water, intracellular water, and extracellular water in percentages of body weight. (Based on data from Friis-Hansen B: Body water compartments in children: changes during growth and related changes in body composition, Pediatrics 28:169-181, 1961.)
Another important aspect of growth change as it corresponds to water distribution is related to the ICF and ECF compartments. In the fetus and prematurely born infant, the largest proportion of body water is contained in the ECF compartment. As growth and development proceed, the proportion within this fluid compartment decreases as the ICF and cell solids increase. The ECF diminishes rapidly from approximately 40% of body weight at birth to less than 30% at 1 year of age. The different effects on males and females become apparent at puberty.
Water Balance in Infants
Because of several characteristics, infants and young children have a greater need for water and are more vulnerable to alterations in fluid and electrolyte balance. Compared with older children and adults, they have a greater fluid intake and output relative to size. Water and electrolyte disturbances occur more frequently and more rapidly, and children adjust less promptly to these alterations.
The fluid compartments in the infant vary significantly from those in the adult, primarily because of an expanded extracellular compartment. The ECF compartment constitutes more than half of the TBW at birth and has a greater relative content of extracellular sodium and chloride. The infant loses a large amount of fluid in the first few days after birth and still maintains a larger amount of ECF than the adult until about 2 to 3 years of age. This contributes to greater and more rapid water loss during this age period.
Fluid losses create compartment deficits that reflect the duration of dehydration. In general, approximately 60% of fluid is lost from the ECF, and the remaining 40% comes from the ICF. The amount of fluid lost from the ECF increases with acute illness and decreases with chronic loss.
Fluid losses may be divided into insensible, urinary, and fecal losses and vary with the patient’s age. Approximately two thirds of insensible losses occur through the skin, and the remaining one third is lost through the respiratory tract. Environmental heat and humidity, skin integrity, body temperature, and respiratory rate all influence insensible fluid loss. Infants and children have a much greater tendency to become highly febrile than do adults. Fever increases insensible water loss by approximately 7 ml/kg/24 hr for each 1° F rise in temperature above 37.2° C (99° F). Fever and increased surface area relative to volume both contribute to greater insensible fluid losses in young patients.
Body Surface Area: The infant’s relatively greater body surface area (BSA) allows larger quantities of fluid to be lost through the skin. It is estimated that the BSA of the premature neonate is five times more, and that of the newborn is two or three times more, than that of the older child or adult. The proportionately longer gastrointestinal tract in infancy is also a source of relatively greater fluid loss, especially from diarrhea.
Metabolic Rate: The rate of metabolism in infancy is significantly higher than in adulthood because of the larger BSA in relation to the mass of active tissue. Consequently, infants have a greater production of metabolic wastes that the kidneys must excrete. Any condition that increases metabolism causes greater heat production, with its concomitant insensible fluid loss and an increased need for water for excretion. The BMR in infants and children is higher to support cellular and tissue growth.
Kidney Function: The infant’s kidneys are functionally immature at birth and are therefore inefficient in excreting waste products of metabolism. Of particular importance for fluid balance is the inability of the infant’s kidneys to concentrate or dilute urine, to conserve or excrete sodium, or to acidify urine. Therefore the infant is less able to handle large quantities of solute-free water than is the older child and is more likely to become dehydrated when given concentrated formulas or overhydrated when given excessive free water or dilute formula.
Fluid Requirements: As a result of these characteristics, infants ingest and excrete a greater amount of fluid per kilogram of body weight than do older children. Because electrolytes are excreted with water and the infant has limited ability for conservation, maintenance requirements include both water and electrolytes. The daily exchange of ECF in the infant is much greater than that of older children, which leaves the infant little fluid volume reserve in dehydrated states. Fluid requirements depend on hydration status, size, environmental factors, and underlying disease.
Disturbances of Fluid and Electrolyte Balance
Disturbances of fluids and their solute concentration are closely interrelated. Alterations in fluid volume affect the electrolyte component, and changes in electrolyte concentration influence fluid movement. Because intracellular water and electrolytes move to and from the ECF compartment, any imbalance in the ICF is reflected by an imbalance in the ECF. Disturbances in the ECF involve either an excess or a deficit of fluid or electrolytes. Of these, fluid loss occurs more frequently.
Depletion of ECF, usually caused by gastroenteritis, is one of the most common problems encountered in infants and children. (See Chapter 29.) Until modern techniques for fluid replacement were perfected, gastroenteritis was one of the chief causes of infant mortality. Fluid and electrolyte problems related to specific diseases and their management are discussed throughout the book where appropriate. The major fluid disturbances, their usual causes, and clinical manifestations are listed in Table 28-2; the most common fluid disturbances, dehydration and edema, are elaborated further in the following sections. Problems of fluid and electrolyte disturbance always involve both water and electrolytes; therefore replacement includes administration of both, calculated on the basis of ongoing processes and laboratory serum electrolyte values.
TABLE 28-2
DISTURBANCES OF FLUID AND ELECTROLYTE BALANCE
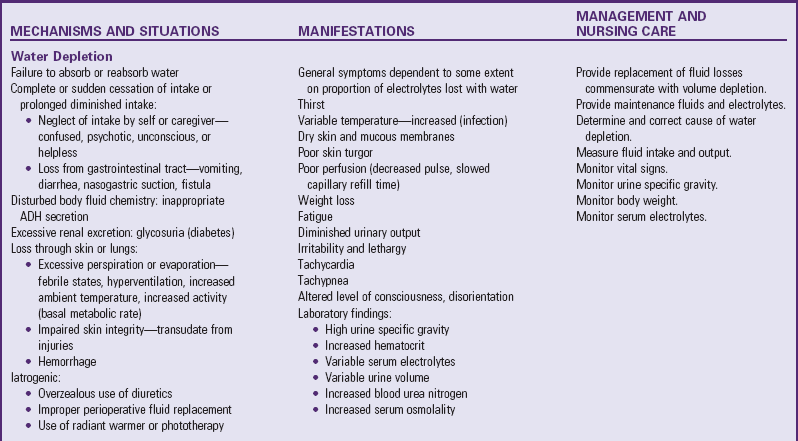
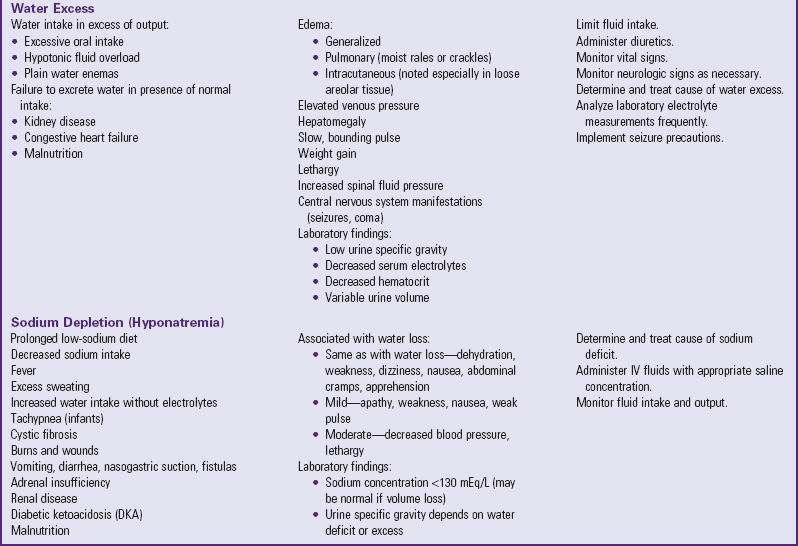
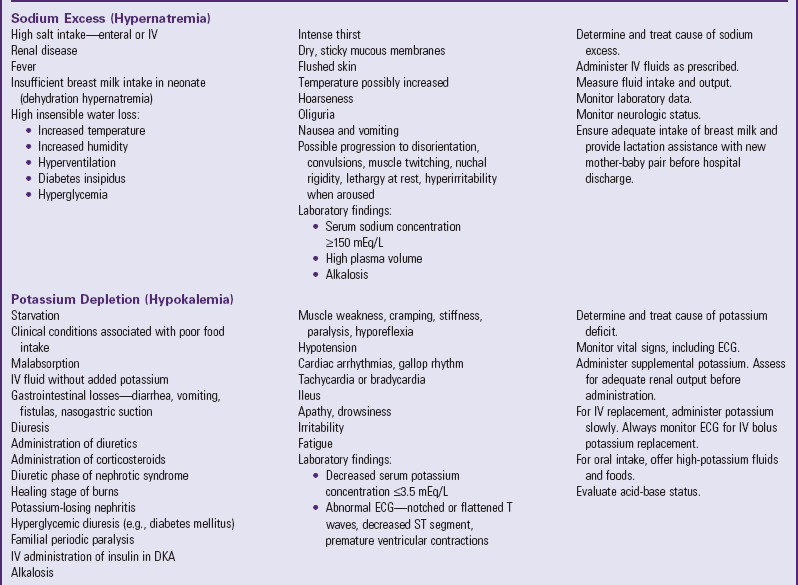
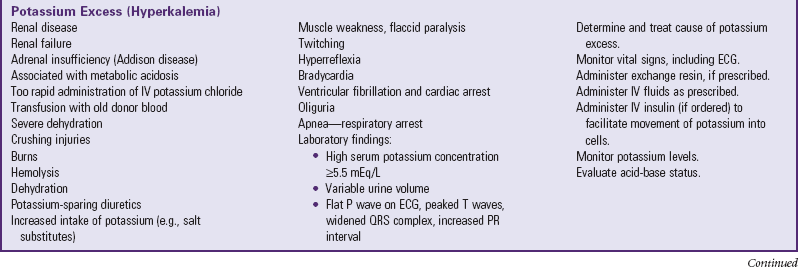

ADH, Antidiuretic hormone; ECG, electrocardiogram; IV, intravenous.
In problems that involve alterations in the amount and composition of body fluid compartments, nurses consider many factors when planning management (Box 28-2). The following discussion is concerned with the general concepts of two common fluid volume disturbances, dehydration and edema, which are features of a variety of conditions. Specific disorders are discussed in Chapters 29 and 30 and elsewhere in the book where appropriate.
Dehydration
![]() Dehydration is a common body fluid disturbance encountered in the nursing care of infants and children; it occurs whenever the total output of fluid exceeds the total intake, regardless of the underlying cause. Although dehydration can result from lack of oral intake (especially in elevated environmental temperatures), more often it is a result of abnormal losses, such as those that occur in vomiting or diarrhea, when oral intake only partially compensates for the abnormal losses. Other significant causes of dehydration are diabetic ketoacidosis and extensive burns.
Dehydration is a common body fluid disturbance encountered in the nursing care of infants and children; it occurs whenever the total output of fluid exceeds the total intake, regardless of the underlying cause. Although dehydration can result from lack of oral intake (especially in elevated environmental temperatures), more often it is a result of abnormal losses, such as those that occur in vomiting or diarrhea, when oral intake only partially compensates for the abnormal losses. Other significant causes of dehydration are diabetic ketoacidosis and extensive burns.
![]() Critical Thinking Case Study—Dehydration
Critical Thinking Case Study—Dehydration
In early dehydration (during the first 2 days), fluid loss is derived from both the ECF and the ICF because the increased osmolality of the diminished ECF volume causes fluid from the ICF compartment to move into the ECF compartment. As dehydration becomes chronic, the cellular losses become greater.
Types of Dehydration
Because sodium is the primary osmotic force that controls fluid movement between the major fluid compartments, dehydration is often described according to plasma sodium concentrations (i.e., isonatremic, hyponatremic, or hypernatremic). Other osmotic forces, however, such as glucose in diabetic ketoacidosis and protein in nephrotic syndrome, may also play a dominant role. Consequently, dehydration is conventionally classified as isotonic, hypotonic, or hypertonic.
Isotonic (isosmotic or isonatremic) dehydration occurs in conditions in which electrolyte and water deficits are present in approximately balanced proportions. This is the primary form of dehydration occurring in children. The observable fluid losses are not necessarily isotonic, but losses from other avenues make adjustments so that the sum of all losses, or the net loss, is isotonic. Because no osmotic force is present to cause a redistribution of water between the ICF and ECF, the major loss is sustained from the ECF compartment. This significantly reduces the plasma volume and thus the circulating blood volume, with its effect on the skin, muscles, and kidneys. Shock is the greatest threat to life in isotonic dehydration, and the child with isotonic dehydration displays symptoms characteristic of hypovolemic shock. Plasma sodium remains within normal limits, between 130 and 150 mEq/L (Huether, 2010).
Hypotonic (hyposmotic or hyponatremic) dehydration occurs when the electrolyte deficit exceeds the water deficit. Because ICF is more concentrated than ECF in hypotonic dehydration, water transfers from the ECF to the ICF to establish osmotic equilibrium. This movement further increases the ECF volume loss, and shock is a frequent result. Because there is a greater proportional loss of ECF in hypotonic dehydration, the physical signs tend to be more severe with smaller fluid losses than in isotonic or hypertonic dehydration. Plasma sodium concentrations are typically less than 130 mEq/L (Huether, 2010).
Hypertonic (hyperosmotic or hypernatremic) dehydration results from water loss in excess of electrolyte loss and is usually caused by a proportionately larger loss of water or a larger intake of electrolytes. This type of dehydration is the most dangerous and requires much more specific fluid therapy. This sometimes occurs in infants with diarrhea who are given fluids by mouth that contain large amounts of solute or in children receiving high-protein nasogastric tube feedings that place an excessive solute load on the kidneys. In hypertonic dehydration, fluid shifts from the lesser concentration of the ICF to the ECF. Plasma sodium concentration is greater than 150 mEq/L (Huether, 2010).
Because the ECF volume is proportionately larger, hypertonic dehydration consists of a greater degree of water loss for the same intensity of physical signs. Shock is less apparent in hypotonic dehydration. However, neurologic disturbances, such as seizures, are more likely to occur. Cerebral changes are serious and may result in permanent damage. These include disturbance of consciousness, poor ability to focus attention, lethargy, increased muscle tone with hyperreflexia, and hyperirritability to stimuli (tactile, auditory, bright lights).
Degree of Dehydration
Diagnosis of the type and degree of dehydration is necessary to develop an effective plan of therapy. The degree of dehydration has been described as a percentage of body weight dehydrated: mild—less than 3% in older children or less than 5% in infants; moderate—5% to 10% in infants and 3% to 6% in older children; and severe—more than 10% in infants and more than 6% in older children (Greenbaum, 2007). Water constitutes only 60% to 70% of the infant’s weight. However, adipose tissue contains little water and is highly variable in individual infants and children. A more accurate means of describing dehydration is to reflect acute loss (time frame of ≤48 hours) in milliliters per kilogram of body weight. For example, a loss of 50 ml/kg is considered to be a mild fluid loss, whereas a loss of 100 ml/kg produces severe dehydration.
Clinical signs provide clues to the extent of dehydration (Table 28-3). The earliest detectable sign is usually tachycardia, followed by dry skin and mucous membranes, sunken fontanels, signs of circulatory failure (coolness and mottling of extremities), loss of skin elasticity, and prolonged capillary filling time (see Table 28-4 for clinical manifestations of dehydration and Fig. 28-3 for signs of dehydration).
TABLE 28-3
EVALUATING EXTENT OF DEHYDRATION
*These signs are less prominent in patients who have hypernatremia.
Data from Jospe N, Forbes G: Fluids and electrolytes—clinical aspects, Pediatr Rev 17(11):395-403, 1996; and Steiner MJ, DeWalt DA, Byerly JS: Is this child dehydrated? JAMA 291(22):2746-2754, 2004.
Compensatory mechanisms attempt to maintain fluid volume by adjusting to these losses. Interstitial fluid moves into the vascular compartment to maintain the blood volume in response to hemoconcentration and hypovolemia, and vasoconstriction of peripheral arterioles helps maintain pumping pressure. When fluid losses exceed the body’s ability to sustain blood volume and blood pressure, circulation is seriously compromised and the blood pressure falls. This results in tissue hypoxia with accumulation of lactic acid, pyruvate, and other acid metabolites, which contribute to the development of metabolic acidosis.
Renal compensation is impaired by reduced blood flow through the kidneys, and little urine is formed. Increased serum osmolality stimulates the secretion of antidiuretic hormone (ADH) to conserve fluid and initiates the renin-angiotensin mechanisms in the kidney, causing further vasoconstriction. Aldosterone is released to promote sodium retention and conserve water in the kidneys. If dehydration increases in severity, urine formation is greatly diminished and metabolites and hydrogen ions that are normally excreted by this route are retained.
Shock, a common manifestation of severe depletion of ECF volume, is preceded by tachycardia and signs of poor perfusion and tissue oxygenation (by pulse oximeter readings). Peripheral circulation is poor as a result of reduced blood volume; therefore the skin is cool and mottled, with decreased capillary filling after blanching. Impaired kidney circulation often leads to oliguria and azotemia. Although low blood pressure may accompany other symptoms of shock, in infants and young children it is usually a late sign and may herald the onset of cardiovascular collapse.
Diagnostic Evaluation
To initiate a therapeutic plan, several factors must be determined:
• The degree of dehydration based on physical assessment
• The type of dehydration based on the pathophysiology of the specific illness responsible for the dehydrated state
• Specific physical signs other than general signs
• Initial plasma sodium concentrations
• Serum bicarbonate concentration
• Any associated electrolyte (especially serum potassium) and acid-base imbalances (as indicated).
Initial and regular, ongoing evaluations assess the patient’s progress toward equilibrium and the effectiveness of therapy.
In the examination of an infant or younger child, one of the most important determinants of the extent of dehydration is the weight, since this can assist in determining the percentage of total body fluid lost; however, since the preillness weight is often unknown, clinical manifestations must be evaluated (see Research Focus box). Important clinical manifestations include changing sensorium (irritability to lethargy); decreased response to stimuli; integumentary changes (decreased elasticity and turgor); prolonged capillary refill; increased heart rate; sunken eyes; and, in infants, sunken fontanels. Using multiple predictors increases the sensitivity of assessing the fluid deficit, and early studies have shown a reasonably high degree of agreement between experienced observers in assessment of the level of dehydration. Objective signs of dehydration are present at a fluid deficit of less than 5%.
Laboratory data are said to be useful only when results are significantly abnormal (Emond, 2009). Urine specific gravity, urine ketones, and urinary output during rehydration are reportedly unreliable assessments for determining dehydration in children (Steiner, Nager, and Wang, 2007).
Therapeutic Management
Medical management is directed at correcting the fluid imbalance and treating the underlying cause. When the child is alert, awake, and not in danger, correction of dehydration may be attempted with oral fluid administration. Most cases of dehydration are mild and can be managed at home by this method. Several commercial rehydration fluids are available for use (see Table 29-2). Oral rehydration management consists of replacement of fluid loss over 4 to 6 hours, replacement of continuing losses, and provision for maintenance fluid requirements. In general, the mildly dehydrated child may be given 50 ml/kg of oral rehydration solution (ORS), whereas the child with moderate dehydration may be given 100 ml/kg of ORS. The child with fluid losses from diarrhea may be given 10 ml/kg for each stool (Greenbaum, 2007). Amounts and rates are determined from body weight and severity of dehydration and are increased if rehydration is incomplete or if excess losses continue, until the child is well hydrated and the basic problem is under control.
The child may not be thirsty even though dehydrated and may refuse oral fluids initially for fear of continued emesis (if occurring) or because of decreased strength, oral stomatitis, or thrush. In such children rehydration may proceed by administering 2 to 5 ml of ORS by a syringe or small medication cup every 2 to 3 minutes until the child is able to tolerate larger amounts; if the child has emesis, administering small amounts (5 to 10 ml) of ORS every 5 minutes or so may help overcome fluid deficit, and the emesis will often lessen over time (Greenbaum, 2007). Oral administration of ondansetron (Zofran) to children with acute gastroenteritis and vomiting may reduce emesis and increase time to oral rehydration, thus preventing intravenous (IV) therapy (DeCamp, Byerly, Doshi, et al, 2008; Freedman, Adler, Seshadri, et al, 2006; Roslund, Hepps, and McQuillen, 2008). Oral rehydration therapy (ORT) is effective for treating mild or moderate dehydration in children, is less expensive, and involves fewer complications than therapy (American Academy of Pediatrics, 2009; Spandorfer, Alessandrini, Joffe, et al, 2005). (See Diarrhea, Chapter 29, for a complete discussion of fluid replacement therapy for dehydration.)
Parenteral Fluid Therapy: Parenteral fluid therapy is initiated whenever the child is unable to ingest sufficient amounts of fluid and electrolytes to (1) meet ongoing daily physiologic losses, (2) replace previous deficits, and (3) replace ongoing abnormal losses. Patients who usually require IV fluids are those with severe dehydration, those with uncontrollable vomiting, those who are unable to drink for any reason (e.g., extreme fatigue, coma), or those with severe gastric distention.
Because dehydration constitutes a great threat to life, the first priority is the restoration of circulation by rapid expansion of the ECF volume to treat or prevent shock. IV administration of fluid begins immediately, although the exact nature of the dehydration and the serum electrolyte values are not known. The solution selected is based on what is known regarding the probable type and cause of the dehydration. This usually involves an isotonic solution such as 0.9% sodium chloride or lactated Ringer, both of which are close to the body’s serum osmolality of 285 to 300 mOsm/kg and do not contain dextrose (which is contraindicated in the early treatment stages of diabetic ketoacidosis).
Parenteral rehydration therapy has three phases. The initial therapy is used to expand ECF volume quickly and to improve circulatory and renal function (Greenbaum, 2007). During initial therapy, an isotonic electrolyte solution is used at a rate of 20 ml/kg, given as an IV bolus over 20 minutes and repeated as necessary after assessment of the child’s response to therapy (Ford, 2009; Friedman, 2009). Subsequent therapy is used to replace deficits, meet maintenance water and electrolyte requirements, and catch up with ongoing losses. Water and sodium requirements for the deficit, maintenance, and ongoing losses are calculated at 8-hour intervals, taking into consideration the amount of fluids given with the initial boluses and the amount administered during the first 24-hour period. With improved circulation during this phase, water and electrolyte deficits can be evaluated, and acid-base status can be corrected either directly through the administration of fluids or indirectly through improved renal function. Potassium is withheld until kidney function is restored and assessed and circulation has improved.
The final phase of therapy allows the patient to return to normal and begin oral feedings, with a gradual correction of total body deficits. The potassium loss in ICF is replaced slowly by way of the ECF. The body fat and protein stores are replaced through diet. If the child is unable to eat or if feeding aggravates a chronic condition, IV maintenance fluids are provided.
Although the initial phase of fluid replacement is rapid in both isotonic and hypotonic dehydration, it is contraindicated in hypertonic dehydration because of the risk of water intoxication, especially in the brain cells, specifically the central pontine cells. Central pontine myelinolysis may occur with an overcorrection of fluid deficit and an overly rapid correction of serum sodium concentration (Greenbaum, 2007). There is an apparent lag time for sodium to reach a steady state when diffusing in and out of brain cells, whereas water diffuses almost instantaneously. Consequently, rapid administration of fluid will cause equally rapid diffusion of water into the dehydrated brain cells, causing marked cerebral edema. Because ECF volume is maintained relatively well in hypertonic as opposed to the other types of dehydration, shock is not a usual manifestation.
Water Intoxication
Water intoxication, or water overload, is observed less often than dehydration. However, it is important that nurses and others who care for children be alert to this possibility in certain situations. Children who ingest excessive amounts of electrolyte-free water develop a concurrent decrease in serum sodium accompanied by central nervous system (CNS) symptoms. There is a large urinary output and, because water moves into the brain more rapidly than sodium moves out, the child may also exhibit irritability, somnolence, headache, vomiting, diarrhea, or generalized seizures. The affected child usually appears well hydrated but may be edematous or even dehydrated.
Fluid intoxication can occur during acute IV water overloading, too rapid dialysis, tap water enemas, feeding of incorrectly mixed formula, or excess water ingestion, or with too rapid reduction of glucose levels in diabetic ketoacidosis (Metheny, 2000; Greenbaum, 2007). Patients with CNS infections occasionally retain excessive amounts of water. Administration of inappropriate hypotonic solutions (e.g., 0.45% sodium chloride) may cause a rapid reduction in sodium and result in symptoms of water overload.
Infants are especially vulnerable to fluid overload. Their thirst mechanism is not well developed; therefore they are unable to “turn off” fluid intake appropriately. A decreased glomerular filtration rate does not allow for repeated excretion of a water load, and ADH levels may not be maximally reduced. Consequently, infants are unable to excrete a water overload effectively.
Administration of inappropriately prepared formula is one of the more common causes of water intoxication in infants (Greenbaum, 2007; Metheny, 2000). Families who cannot afford to buy enough formula may dilute the formula to increase the volume or even substitute water for the formula. A family may run out of formula and dilute the remaining amount to make it last until they are able to purchase more. In addition, water is sometimes used for pacification. Water intoxication can also occur in infants who receive overly vigorous hydration during a febrile illness.
A number of clinicians have reported water intoxication in infants after swimming lessons (Fann, 1998; Metheny, 2000). Although they hold their breath, some infants apparently swallow a large amount of water during repeated submersion. Anticipatory guidance to parents should include a discussion of swimming instruction and advice to stop a lesson if the child swallows unusual amounts of water or exhibit any symptoms of hyponatremia.
Edema
Edema represents an abnormal accumulation of fluid within the interstitial tissue and subsequent tissue expansion and develops when a defect in the normal cardiovascular circulation or a failure in the lymphatic drainage to remove the increased amounts occurs. The processes responsible for fluid removal include venous hydrostatic pressure, oncotic pressure of intravascular and interstitial spaces, an intact semipermeable capillary wall, tissue tension, and lymphatic flow.
Mechanisms of Edema Formation
A defect of any of the homeostatic mechanisms maintaining fluid balance can cause accumulation of interstitial fluid. Disequilibrium results from anything that (1) alters the retention of sodium, such as renal disease or hormonal influences; (2) affects the formation or destruction of plasma proteins, such as starvation or liver disease; or (3) alters membrane permeability, such as minimal change nephrotic syndrome or trauma.
Edema may be localized to a small or large area, such as that occurring in urticaria, infection, and pulmonary congestion, or it can be generalized, as in the hypoproteinemia of the nephrotic syndrome and starvation. A severe, generalized accumulation of great amounts of fluid in all body tissues is termed anasarca.
Increased Venous Pressure: The colloidal osmotic pressure of the plasma proteins draws fluid back into the vascular system as long as this force is greater than the venous hydrostatic pressure. However, when the venous pressure increases, fluid tends to be retained in the interstitial spaces. This can occur when an individual remains in the same position for a long time, such as swollen ankles and feet after standing or sitting for long periods. Constrictive dressings or restraints applied too tightly to extremities will obstruct venous return, increase venous and capillary pressure, and cause edema. The most graphic pathologic illustrations are pulmonary edema caused by pulmonary circulation overload in cardiac defects with a left-to-right shunt and ascites caused by portal hypertension. Edema from any cause is increased in dependent areas because of this added factor of increased venous hydrostatic pressure and the gravitational effects in these areas.
Capillary Permeability: Damage to capillary walls or alteration in their permeability permits exudation of plasma protein into the interstitial space. Most often this occurs as local edema, such as that manifested in inflammatory and hypersensitivity reactions. Capillary damage from burns allows extensive exudation of protein-rich fluid into the interstitial spaces to compound edema formation.
Diminished Plasma Proteins: A fall in plasma protein levels hampers the osmotic pull back into the vessels. Consequently, fluid remains in the interstitial spaces. Although other factors play a role, such as hydrostatic pressure of both the arterial vascular system and the tissues and sodium concentration, significantly low protein levels (<4.5 mg/dl) are associated with edema. Examples of this are the massive albumin losses of the minimal change nephrotic syndrome, diminished serum protein from insufficient dietary protein, and (sometimes) hemodilution of plasma proteins from IV fluid administration in chronic dehydration.
Lymphatic Obstruction: Obstruction of lymph flow creates edema high in protein content. This occurs infrequently in childhood but can result from trauma to the lymphatic glands or from removal of lymph nodes.
Tissue Tension: Tissue hydrostatic pressure is ordinarily of little consequence. However, it plays a significant role in determining distribution of edema fluid in certain pathologic conditions. Loose tissues allow a greater amount of fluid accumulation than tissues that are tightly bound by dense fibrous bands in which tissue pressure rapidly increases to limit further extravasation of fluid. Edema appears earlier and more readily in loose structures such as those in the periorbital and genital tissues. The alveolar structure of lung tissue is probably a contributing factor in pulmonary edema, as well as in increased hydrostatic pressure in the pulmonary vessels.
Other Factors in Edema Formation: Any factor that causes sodium retention by the kidneys will produce or augment edema formation. This includes stimulation of the renin-angiotensin-aldosterone mechanisms for sodium reabsorption created by the diminished plasma volume in edema, which resulted from primary causes. The salt-retaining property of steroids is responsible for the edema associated with their administration.
Several types of edema exist, all of which can provide a palpable swelling of the interstitial space that is either localized or generalized. These include:
• Peripheral edema, or localized or generalized palpable swelling of the interstitial space
• Ascites, or the accumulation of fluid in the abdominal cavity (usually associated with renal or liver abnormalities)
• Pulmonary edema, which occurs when interstitial volume increases
• Cerebral edema, which is a particularly threatening form of edema caused by trauma, infection, or other etiologic factors, including vascular overload or injudicious IV administration of hypotonic solutions
• Overall fluid gain, especially seen in patients with kidney disease
Assessment
Generalized edema resulting from any of the above types is manifested by swelling in the extremities, face, perineum, and torso. Loss of normal skin creases may be assessed. Daily weights are more sensitive indicators of water gain or loss and should be obtained. Abdominal girth measurement changes may also be an indicator of edema in children. Pitting edema may occur and can be assessed by pressing the fingertip against a bony prominence for 5 seconds. If the tissue rebounds immediately on removing the finger, the patient does not have pitting edema. A quick way to determine the severity is to measure the degree of pitting edema (Fig. 28-4).
Therapeutic Management
The primary goal in the management of edema is treatment of the underlying disease process, which is discussed elsewhere in relation to the specific disorder. However, an essential aspect in the management of any fluid overload is early recognition, in which nurses play a vital role. The management of edema is discussed throughout the text with specific conditions.
Disturbances of Acid-Base Balance
The body’s ability to regulate acid-base status is one of the most crucial physiologic functions. Many disease states, such as diarrhea, vomiting, or febrile conditions, are complicated by disturbances in the acid-base balance, which are often more hazardous to the child’s survival than the primary disease process. Sometimes simply providing adequate hydration, replacing electrolytes, and correcting acid-base disturbances are all that is needed to sustain an infant or child until the primary disorder has stabilized.
Acid-Base Imbalance
A disturbance of acid-base equilibrium in the direction of acidosis or alkalosis may come about in a variety of ways. However, simply stated, acidosis (acidemia) results from either accumulation of acid or loss of base, and alkalosis (alkalemia) results from either accumulation of base or loss of acid.
Hydrogen Ion Concentration
The pH represents the concentration of hydrogen (H+) in solution and indicates only whether the imbalance is more acidic or more alkaline. It does not reflect the nature of the imbalance (i.e., whether it is of metabolic or respiratory origin). Body metabolism affects primarily the base bicarbonate (HCO3−); therefore alterations in the concentration of bicarbonate are termed metabolic disturbances of acid-base balance. Also, because the amount of carbon dioxide (CO2) exhaled through the lungs affects the carbonic acid (H2CO3), changes in carbonic acid concentration are referred to as respiratory disturbances. Consequently, the simple disturbances (those with a single primary cause) are categorized as metabolic acidosis or alkalosis and respiratory acidosis or alkalosis (Greenbaum, 2007).
It is also significant that the major signs and symptoms of hydrogen ion imbalances (acidosis and alkalosis) reflect CNS involvement. Depression of the CNS, manifested by lethargy, diminished mental capacity, delirium, stupor, and coma, is observed in acidosis of either metabolic or respiratory origin. On the other hand, alkalosis produces clinical manifestations of nervous system stimulation and excitement, including overexcitability, nervousness, tingling sensations, and tetany that may progress to seizures. Persons with epilepsy are particularly susceptible to seizures, which can be precipitated by hyperventilation.
It is also important to note that eventually all body systems become dysfunctional if the “normal” limits of pH are violated for long. The extent and severity of signs and symptoms depend on the length of time the imbalance has existed and the magnitude or degree of the deviation from normal. A rapid, severe imbalance will seriously compromise the compensatory mechanisms to the point where it is incompatible with life, whereas the body will be able to compensate adequately for a mild, gradual distortion and produce few if any observable signs or symptoms.
Compensatory Mechanisms
Respiratory regulation in acid-base balance involves carbon dioxide regulation; that is, the rate and depth of alveolar ventilation determine the concentration of carbon dioxide that is eliminated or retained. Renal processes, however, involve the regulation of bicarbonate via reabsorption, regeneration, and secretion of hydrogen ions. When the fundamental acid-base ratio is altered for any reason, the body attempts to correct the deviation. In a simple disturbance, a single primary factor affects one component of the acid-base pair and is usually accompanied by a compensatory or secondary change in the component that is not primarily affected. For example, increased formation of metabolic acid rapidly reduces the bicarbonate in the formation of carbonic acid. The respiratory mechanism immediately attempts to compensate for the imbalance by eliminating the carbonic acid through exhaled carbon dioxide and water. The imbalance is corrected when the kidneys excrete hydrogen and ammonium ions in exchange for reabsorbed sodium bicarbonate.
When the secondary changes (the hyperventilation and renal excretion of hydrogen ions in the preceding example) succeed in preventing a distortion of the acid-base ratio and the pH is restored to normal, the disturbance is described as compensated. The uncompensated state exists when there is no compensatory effect and the pH remains uncorrected. The imbalance is said to be corrected when physiologic mechanisms fully correct the primary abnormality. Mixed acid-base imbalances may also occur in diseases states, and the patient will manifest two simultaneous acid-base imbalances rather than a single imbalance. It is not within the scope of this text to discuss the many variations of mixed acid-base imbalances; the reader is referred to other published sources for such material (Huether, 2010; Curley and Moloney-Harmon, 2001) (see also Table 28-5).
Laboratory Measurements
Several laboratory tests are employed to assess the nature and extent of acid-base disturbances. The importance of these data is readily apparent when a clinical observation such as hyperventilation can represent either the primary factor in respiratory alkalosis or a secondary or compensatory factor in metabolic acidosis. The laboratory tests of value in the assessment of acid-base status are outlined in Table 28-6. To determine the acid-base status, three variables—the respiratory component (Pco2), the metabolic component (arterial bicarbonate or serum carbon dioxide [HCO3−]), and the serum pH—must be determined. In addition, the anion gap (AG) may be useful in determining the cause and extent of metabolic acidosis; therefore serum chemistry is obtained as well. Measurement of any two variables (Pco2, pH, HCO3−) will allow computation of the third using the Henderson-Hasselbach equation. A summary of relationships between these and other variables is outlined in Table 28-7.
TABLE 28-6
LABORATORY TESTS EMPLOYED IN ASSESSMENT OF ACID-BASE STATUS
*Data from Kliegman RM, Behrman RE, Jenson HB, et al, editors: Nelson textbook of pediatrics, ed 18, Philadelphia, 2007, Saunders.
†Huether SE: The cellular environment: fluids and electrolytes, acids and bases. In McCance KL, Huether SE, Brashers VL, et al, editors: Pathophysiology: the biologic basis for disease in adults and children, ed 6, St Louis, 2010, Mosby.
Associated Disturbances in Acid-Base Balance
Physiologic functions of the body take place optimally when the pH is maintained within a normal range. The disequilibrium created by moderately altered pH can produce disordered function of physiologic and enzyme systems, but great divergences are incompatible with life. In addition, electrolyte shifts that take place in response to changes in pH alter the electrolyte concentration in the fluid compartments to disturb the normal concentrations. For example, cell membrane permeability is affected by changes in pH. A lowered pH allows potassium (K+) to move from the ICF to the ECF. Serum potassium levels increase with acidosis and decrease with alkalosis.
Serum Potassium: One of the disturbances that complicate both fluid losses and acid-base imbalance is an alteration of potassium levels. During dehydration, fluid moves out of the ICF compartment into the ECF compartment in an attempt to balance the fluid losses. In doing so, potassium also moves out, creating a total body potassium depletion. Because renal function is drastically reduced in dehydration, normal excretion of potassium does not take place. This causes elevated serum levels that can produce all the signs and symptoms of hyperkalemia. During rapid rehydration therapy for gastrointestinal losses and diabetic ketoacidosis, the ECF potassium moves back into the ICF compartment, thereby posing the risk of hypokalemia unless there is an anticipated replacement. However, potassium is not replaced until the ICF is sufficient to restore adequate renal function.
Serum Calcium: Disturbed ECF calcium (Ca++) levels may occur in various types of dehydration. Usually the disturbance is in the form of reduced serum calcium levels, especially where there is a concomitant potassium loss. Although hypocalcemia is a common finding, it rarely reaches a point of tetany in current practice, which includes adequate replacement of potassium losses. Immediate effects of calcium imbalance associated with acidosis or alkalosis are tetany of metabolic alkalosis; long-term effects of chronic acidosis are related to bone resorption from renal disturbances.
Oxygen Combination: The capacity of oxygen to combine with hemoglobin is also affected by changes in pH. The affinity of hemoglobin for oxygen decreases with a decrease in pH so that, in a state of acidosis, less oxygen will be picked up by the hemoglobin as blood travels through the lungs. However, oxygen is more easily released to the tissues when the pH is lowered. The opposite effects operate during an increase in pH.
Blood Flow: Changes in pH alter blood flow in various areas. Pulmonary circulation constricts in acidosis, whereas decreased pH (acidosis) causes vasodilation in systemic vessels. This has distinct implications when caring for the newborn infant who is experiencing difficulty in making an effective cardiopulmonary transition to extrauterine life. (See Persistent Pulmonary Hypertension of the Newborn, Chapter 10.)
Respiratory Acidosis
Respiratory acidosis results from diminished or inadequate pulmonary ventilation that causes an elevation in plasma Pco2 and thus an increased concentration of dissolved carbonic acid, which leads to elevated carbonic acid and hydrogen ion concentration. Conditions that produce respiratory acidosis can originate at three levels in the respiratory system and result in inadequate gas exchange (Box 28-3).
Compensation is mediated through the kidneys, which are stimulated to conserve and thus increase the plasma bicarbonate concentration and to excrete hydrogen ions. Laboratory findings in respiratory acidosis include elevated plasma bicarbonate concentration.
The treatment of respiratory acidosis is aimed at correcting the underlying cause and improving gas exchange at the alveolar level to provide more efficient removal of carbon dioxide. Oxygen therapy is usually indicated, as well as mechanical ventilation as the condition warrants. Administration of buffers such as sodium bicarbonate to reduce hydrogen ion concentration is usually not indicated, since it can result in fluid volume excess by causing an osmolar fluid shift from the blood to the intravascular space, which would only further compromise respiratory function and aggravate the acidosis. In children with chronic metabolic acidosis, oral sodium bicarbonate may be administered (Greenbaum, 2007). IV sodium bicarbonate may be administered in acute cases of metabolic acidosis as a bolus push or titrated in a continuous infusion solution. However, the administration of IV sodium bicarbonate may be harmful and cause more problems, especially in neonates, prompting some clinicians to advocate for the cessation of this practice in neonatal resuscitation (Aschner and Poland, 2008). In preterm infants rapid volume expansion with sodium bicarbonate may increase intravascular volume with subsequent periventricular hemorrhage. Any patient receiving IV sodium bicarbonate is at risk for hypernatremia if sodium intake from other sources is not carefully monitored. Because sodium bicarbonate produces carbon dioxide as it is metabolized, patients who are not being effectively ventilated may actually develop a more severe respiratory acidosis as Pco2 accumulates.
Respiratory Alkalosis
Conversely, respiratory alkalosis is caused by a primary increase in the rate and depth of pulmonary ventilation, resulting in unusually large amounts of carbon dioxide being exhaled, or “blown off.” This reduces the plasma Pco2 and raises the pH. Metabolic compensation for a respiratory alkalosis, usually performed by the kidneys, is more gradual and may occur over a period of days; thus the pH and the bicarbonate level may remain normal (Greenbaum, 2007). Box 28-4 lists conditions that stimulate the respiratory center to produce hyperventilation.
A frequent cause of hyperventilation in children is voluntary hyperventilation before underwater swimming. The condition may also be seen in children with an anxiety attack and subsequent hyperventilation. It is also a consideration in the care of persons having mechanical ventilation, extracorporeal membrane oxygenation (ECMO), and hemodialysis (Greenbaum, 2007). Incorrectly set mechanical ventilators can cause respiratory rates and tidal volumes in excess of physiologic needs.
Compensation of respiratory alkalosis takes place in the kidneys and consists of excretion of carbonic acid in association with sodium (Na+) and potassium to conserve hydrogen. Laboratory findings include elevated plasma pH (>7.45), depressed plasma carbonic acid concentration (<23 mEq/L in older children, <20 mEq/L in young children), and lowered Pco2 (<35 mm Hg).
Treatment of respiratory alkalosis consists of correction of the underlying cause and prevention of lost anions and the associated potassium deficit. Rebreathing carbon dioxide slows respirations and provides rapid relief, as does oxygen therapy.
Metabolic Acidosis
Metabolic acidosis is a lowered plasma pH caused by any process that reduces the bicarbonate concentration. Metabolic acidosis can be produced by the gain of nonvolatile acids or the loss of bicarbonate. Strong acid is gained, and bicarbonate is lost by several specific mechanisms and routes (Box 28-5).
Compensation of metabolic acidosis is respiratory, with alveolar hyperventilation occurring immediately as the decrease in pH is sensed by the respiratory center. Strong acids are immediately buffered to generate the weaker carbonic acid, which the respiratory system attempts to eliminate through increased alveolar ventilation. In this respiratory effort the breathing is deep and rapid—the Kussmaul or air-hunger type of respirations. Bicarbonate conservation and excretion by the kidneys is a slower mechanism. Laboratory findings of uncompensated metabolic acidosis include lowered plasma pH (<7.35) and diminished plasma bicarbonate concentration.
The plasma AG may be helpful in the evaluation of patients with metabolic acidosis. The AG reflects the difference between the measured cation sodium and the anions (also measured) of chloride and bicarbonate (Greenbaum, 2007). Two diagnostic groups exist: those with a normal AG or those with an increased (high) AG. The formula for calculating the anion gap is as follows:
The normal AG is 4 to 11 mEq/L (Greenbaum, 2007). With a high AG there is an increase in the number of unmeasured ions (potassium, magnesium, calcium); conditions with a high AG include diabetic ketoacidosis, other ketoacidoses (starvation [e.g., disordered eating], alcoholic ketoacidosis), lactic acidosis, kidney failure (may also be mixed high and normal), some inborn errors of metabolism, and poisonings (ethylene glycol intoxication, salicylate intoxication, methyl alcohol intoxication). Diarrhea, renal tubular acidosis, acetazolamide ingestion, biliary or pancreatic fistulas, and excessive administration of isotonic saline or ammonium chloride are examples of conditions that are seen with a normal AG (Metheny, 2000; Greenbaum, 2007). In mild cases of metabolic acidosis and with certain conditions, the AG is not as helpful as other laboratory determinations and a comprehensive history and physical examination (Greenbaum, 2007).
Treatment is directed at correcting the basic deficit and replacing the excessive losses of bicarbonate with sodium or potassium bicarbonate or sodium lactate.
Metabolic Alkalosis
Metabolic alkalosis is represented by an elevated plasma pH that occurs when there is a reduction in hydrogen ion concentration and an excess of bicarbonate. This can be caused by a gain in base or a loss of acid (Box 28-6).
Compensation in metabolic alkalosis theoretically should be respiratory; however, such compensation is irregular and unpredictable. In addition, renal correction is complicated by losses of sodium, potassium, and chloride, which are lost in conditions such as hypertrophic pyloric stenosis through vomiting. The kidneys attempt to conserve the sodium and potassium concentration at the expense of hydrogen concentration and acid-base balance. Laboratory findings include elevated urine pH, elevated plasma pH, elevated plasma bicarbonate, and, if in conjunction with chloride deficit, reduced chloride concentration. Treatment of metabolic alkalosis is aimed at preventing further losses of acid and replacing lost electrolytes.
Nursing Responsibilities in Fluid and Electrolyte Disturbances
Nursing observation and intervention are essential to the detection and therapeutic management of disturbances in fluid and electrolyte balance. Imbalances may be precipitated by a variety of circumstances, and the balance may be so precarious, especially in newborns and infants, that changes can take place in a very short time. Therefore an important nursing responsibility is anticipation and perceptive observation for any signs of imbalance, particularly in those situations and conditions in which imbalance is likely to occur. Conditions in which changes can develop with surprising rapidity in young children include diarrhea; vomiting; sweating; fever; disorders such as type 1 diabetes, renal disease, and cardiac anomalies; administration of certain drugs such as diuretics and steroids; and trauma, such as major surgery, burns, and other extensive injury. Preterm infants with respiratory distress syndrome and other pulmonary conditions may exhibit acid-base imbalances. In such infants compensatory mechanisms are immature, and the child may not survive without prompt intervention. (See also Chapter 10.)
Nurses must be comfortable with equipment used to deliver fluids to infants and children and be familiar with the information and techniques for physical assessment of each age-group. An understanding of normal serum chemistry levels provides additional data on which to base assessments and interventions and to validate observations. Data that are helpful in assessment related to fluid and electrolyte balance include the proposed treatment plan, including medications and fluid therapies, laboratory reports, history of illness, and records of fluid intake and output (I&O). An important nursing role is teaching parents to recognize early signs of dehydration.
Assessment
Whether the child is at home, in the practitioner’s office or clinic, or in the hospital, nursing assessment is an essential part of the nursing care plan. The assessment of suspected or potential fluid and electrolyte disturbance begins with the observation of general appearance. Ill children usually have drawn expressions, have dry mucous membranes and lips, and “look sick.” Loss of appetite is one of the first behaviors observed in most childhood illnesses, and the infant’s or child’s activity level is diminished from baseline or usual activities. The cry of an ill infant is less vigorous, often whining, and higher pitched than usual. The child is irritable, seeks the parent’s comfort and attention, and displays purposeless movements and inappropriate responses to people and familiar objects. In some cases the child may not protest advances by the health care worker and procedures such as taking vital signs or starting an IV infusion. These are signs that the child truly feels bad and that the condition is serious and immediate intervention is necessary. As the child’s illness and level of dehydration become more severe, irritability progresses to lethargy and even unconsciousness.
History
The nurse can obtain much of the information regarding the child’s behavior from the parent or primary caregiver. In addition to initial observations, a good history is extremely valuable to the assessment. The amount and type of fluid I&O (especially abnormal output) are important. An accurate estimate of fluid losses is beyond the capacity of history givers, but rough estimates of excessive fluid losses or diminished output can usually be obtained from information such as the number and consistency of stools the child has passed in the past 24 hours, the number of times the child voided, and the type and amount of food and fluid ingested or vomited. For an infant, ask about the number of wet diapers in the past 24 hours. Parents frequently omit this information from their discussion with the health professional. They tell how much has been taken but not how much was excreted unless asked specifically. Having the parents estimate the amount of urine in the diaper at each void is of little value because of the absorbent diaper material, which pulls fluids away from the child’s skin.
Both the type and the amount of intake provide valuable information. The quality and quantity can be determined—is intake sustained, excessive, or curtailed? Loss early in diarrheal illness progresses rapidly, and the water losses can exceed sodium losses, leading to hypernatremia. Hypernatremic dehydration indicates a significant interference with water intake. Also important is a history of normal or increased intake of an unusual fluid, such as one containing sucrose, tea, juice, athletic hydration fluid (e.g., Gatorade), an alternative home remedy fluid, or other solute-containing fluids, which can contribute to hyponatremic dehydration in the face of abnormal losses.
A history of gradual weight gain and observations of any puffiness, especially in areas with less dense tissues (periorbital, scrotal), or “clothes fitting tighter” offer early clues to edema. A history of excessive water intake, especially when associated with diminished output, is important in assessing edema and water intoxication.
Clinical Observations
Fever and infection can also produce tachycardia, the earliest manifestation of dehydration. Therefore these are considered in the assessment of dehydration. Dry skin and mucous membranes (oral) usually appear early. A sunken fontanel is a useful observation if the status of the fontanel is known when the infant is healthy. Signs of circulatory failure usually indicate severe dehydration, since compensatory mechanisms are able to sustain blood pressure in the low normal range for some time. Loss of skin elasticity, generally manifested in children less than 2 years of age, is measured by the time it takes for pinched abdominal skin to recoil. This sign is also observed in undernourished children. Also, in hypertonic dehydration the skin has a smooth, velvety feel before it develops disturbed elasticity.
Assess capillary filling time by pinching a toe or a thumb or lightly pressing the abdominal skin and estimating the time it takes for the blood to return. Capillary filling time in mild dehydration is less than 2 seconds, increasing to more than 4 seconds in severe dehydration. The technique is effective in children of all ages. However, it can be altered in the presence of heart failure, which affects circulation time, and hypertonic dehydration, in which fluid loss is primarily intracellular. Additional clinical signs observed in children with dehydration include cool mottled extremities, sunken eyes, tachypnea, and changes in sensorium.
When caring for the ill child, assess vital signs as often as every 15 to 30 minutes, and record weight frequently during the initial phase of therapy. It is important to use the same scale each time the child is weighed and to predetermine the weight of any equipment or devices that must remain attached during the weighing process, including arm boards, and any clothing the child might be wearing. Take routine weights at the same time each day.
Intake and Output Measurement
One of the nurse’s most important roles in fluid and electrolyte disturbance is related to I&O. Accurate measurements are essential to the assessment of fluid balance. Measurements from all sources—including gastrointestinal and parenteral I&O from urine, stools, vomitus, fistulas, nasogastric suction, sweat, and drainage from wounds—must be taken into consideration. Although the practitioner usually indicates when I&O are to be recorded, it is a nursing responsibility to keep an accurate I&O record on certain children, including those:
• With severe thermal burns or injuries
• With renal disease or damage
• With congestive heart failure
Infants or small children who are unable to use a bedpan or those who have bowel movements with every voiding will require the application of a collecting device. (See Urine Specimens, Chapter 27.) Collecting bags may not be suitable for all infants (e.g., preterm and other infants whose fragile skin does not tolerate some types of self-adhesive appliances). If collecting bags are not used, wet diapers or pads are carefully weighed to ascertain the amount of fluid lost. This includes liquid stool, urine, vomitus, and other losses. The volume of fluid in milliliters is approximately equivalent to the weight of the fluid measured in grams. The specific gravity as a measure of osmolality is determined with a refractometer or urine dipsticks and assists in assessing the degree of hydration.
Disadvantages of the weighed diaper method of fluid measurement include (1) inability to differentiate one type of loss from another because of admixture (liquid stool versus urine); (2) loss of urine or liquid stool from leakage or evaporation, especially if the infant is under a radiant warmer; and (3) additional fluid in the diaper (superabsorbent disposable type) from absorption of atmospheric moisture (high-humidity incubators). Evaporative losses render measurements inaccurate unless the diaper is weighed and measured for specific gravity at least every 30 minutes when critical values are needed. Evaporative losses are greater in very low–birth-weight and extremely low–birth-weight infants, those under radiant warmers, and those being treated with phototherapy. However, research indicates that accurate specific gravity measurements can be made for up to 2 hours on urine obtained from a diaper that has been removed from an infant, folded, and stored in a utility room (Kee and Paulanka, 2000; Metheny, 2000).
It is important to measure and record all intake, oral and parenteral, as well as output from all sources, including urine, stool, emesis, drainage tubes, fistulas, and wounds from which appreciable amounts of fluid are lost. At home, advise parents to observe the number of times and how much the child voids. The newborn may be expected to void at least once in the first 24 hours, two or three times in the second 24 hours of life, three or four times in the third and fourth days of life, and a minimum of five or six times by the fifth and sixth days; if intake is adequate, the infant 5 to 6 days old and older may be expected to have a minimum of six to eight voidings per day (American Academy of Pediatrics, 2009). Infants younger than 1 year of age may void every 1 to 2 hours; toddlers urinate approximately every 3 hours. As children get older, they void less frequently. Instruct the parents to notify the nurse or clinician if the child appears to be voiding an insufficient amount or persistently losing fluid through vomiting or diarrhea.
Oral Fluid Intake
Under ordinary circumstances an adequate oral intake is no problem in children who are able to respond to thirst cues. Hydration becomes a nursing problem when infants or children are unable to take in fluids by mouth because of illness or because fatigue or discomfort makes them reluctant to swallow. Children with elevated temperatures, continued gastrointestinal losses, labile type 1 diabetes, or cystic fibrosis are especially prone to dehydration. Occasionally dehydration caused by inadequate breast milk intake has been observed in breast-fed infants in the first few weeks of life.
ORT is recommended for mild to moderate dehydration. An ORS containing 75 to 90 mMol sodium and 111 to 139 mMol glucose (e.g., World Health Organization solution, Pedialyte RS, Rehydralyte) is most commonly recommended for the first 4 to 6 hours. If this is tolerated, then oral fluids containing 30 to 60 mMol sodium and 111 to 139 mMol glucose (e.g., Pedialyte, Lytren, Resol, Infalyte) can be given for the next 18 to 24 hours at a dose of 45 to 60 ml/kg (1 to 2 oz) divided into frequent feedings consisting of 90 to 120 ml (3 to 4 oz) for young children. Older children can be given 30 to 60 ml (1 to 2 oz) every hour.
The American Academy of Pediatrics (2009) does not advocate withholding food and fluids for 24 hours after the onset of diarrhea, or administering the BRAT diet (bananas, rice, applesauce, and toast), which is low in protein and electrolytes and high in carbohydrates. Breast-fed infants should continue breast-feeding, provided that milk supply is adequate for hydration; once the older infant or child has successfully achieved rehydration, he or she may consume a regular diet, avoiding fat (no French fries!!), high sugar drinks, soda, Jell-O, or rice water (American Academy of Pediatrics, 2009; Wade, 2010). Flavored frozen Popsicles that are low in sucrose (≤5%) may be offered; these often contain 30 to 45 ml of fluid and are enticing to the child who is being rehydrated. Encourage the child to eat as little as desired and, after a given trial period, offer a second Popsicle. An antiemetic such as ondansetron (also available in oral dissolving tablet) may be given (as ordered), then followed in 20 to 30 minutes with an oral fluid challenge (the Popsicle). A lactation specialist should be consulted for assistance if ineffective latch-on in the breast-fed infant is part of the intake problem.
Persuading a reluctant child to drink fluids can be a nursing challenge and is not uncommon in the care of infants and children. Older children often respond to the challenge of meeting a specific goal for fluid intake (or deprivation) and can be active participants in planning an intake schedule. Contracts and rewards are effective strategies. However, young children require more creative tactics. Suggestions for encouraging children to drink fluids are discussed in Chapter 27. (See Chapter 27 for a discussion of nasogastric alimentation.)
The Child Who Is NPO
Infants or children who are unable or not permitted to take fluids by mouth (NPO) have special needs. To ensure that they do not receive fluids, place a sign in some obvious place, such as over their beds or pinned to their shirts, to alert others to the NPO status. Remove fluids from the bedside to reduce the temptation.
Oral hygiene, a part of routine hygienic care, is especially important when fluids are restricted or withheld. (See Chapter 27.) For young children who cannot brush their teeth or rinse their mouth without swallowing fluid, clean the mouth and teeth and keep it moist by swabbing with saline-moistened gauze. Judicious administration of ice chips provides moist, cool relief (if permitted by the practitioner). To meet the need to suck, provide infants a safe commercial pacifier.
The child on restricted fluids provides an equal challenge. Having fluids limited is often more difficult for the child than being NPO, especially when IV fluids are also eliminated. To make certain the child does not drink the entire amount allowed early in the day, the daily allotment is calculated to provide fluids at periodic intervals throughout the child’s waking hours. Serving the fluids in small containers gives the illusion of larger servings. No extra liquid is left at the bedside.
Parenteral Fluid Therapy
Before beginning an IV infusion, the nurse performs several preparatory activities. All needed equipment is gathered so that the operator can proceed without interruption. More important, the child and the family must be prepared for this stressful procedure.
Solution and Equipment: The composition of the IV solution is based on patient history and the diagnosis, or the type of fluid volume deficit being treated, and is selected on the basis of tonicity (osmolarity) and electrolyte content. A solution that is isotonic has the same osmolality, or tonicity, as body fluids such as plasma. A hypertonic solution is one that has a greater concentration of solutes than plasma; a hypotonic solution has a lower concentration. Examples of isotonic solutions are 0.9% normal saline solutions, lactated Ringer solution, and 5% dextrose in water; 10% glucose in water is a hypertonic solution; plain water (without electrolytes) and a solution with 0.2% sodium are hypotonic solutions. Although it is larger, one molecule of glucose has only half the osmolality of one molecule of sodium chloride (NaCl) because the sodium chloride ionizes in solution into two particles, the sodium and the chloride ions. Thus one molecule of sodium chloride exerts twice the osmotic pressure of one molecule of glucose.
Most common pediatric maintenance solutions include a combination of dextrose (usually 5% or 10%) and sodium chloride (usually 0.22% to 0.45%). The hypotonic solution is necessary for children, since their daily turnover of free water exceeds that of adults. Because infants and young children are subject to rapid fluid shifts, any IV solution given to them should contain at least 0.2% sodium chloride to prevent brain edema, a disorder to which they are susceptible if given plain water. Glucose is rapidly metabolized; therefore the osmolality of 5% glucose is further diminished.
To avoid infusing too much of the IV solution, the volume of the solution container should be based on the child’s age, size, and 24-hour volume needs. For infants and small children it is best to place 3 to 4 hours of required maintenance IV solution in a small container such as a graded buretrol to avoid fluid overload in the event of equipment failure (runaway infusion). Solution containers (usually a plastic bag) containing 250 to 500 ml are commonly used in infants and small children, as opposed to the 1000-ml containers used in adolescents and adults.
For most IV infusions in children, an over-the-needle 24- to 22-gauge catheter may be used if therapy will last less than 5 days. The smallest gauge and shortest length catheter that will accommodate the prescribed therapy should be chosen for the placement of a peripheral IV (PIV) line. The length of the catheter may be directly related to infection or embolus formation—the shorter the catheter, the fewer the complications. The gauge of the catheter should maintain adequate flow of the infusate into the cannulated vein while allowing adequate blood flow around the catheter walls to promote proper hemodilution of the infusate. Because stainless steel needles tend to dislodge and infiltrate more frequently than catheters, limit the use of these to short-term or single-dose administration.
The goal of IV therapy is to deliver the prescribed fluids or medications without complications. Determining the best catheter for the patient early in the therapy provides the best chance of avoiding catheter-related complications. As the length of therapy increases, explore decisions regarding the type of infusion device (short peripheral, midline, peripherally inserted central catheter [PICC], or central venous catheter).
The Infusion Nurses Society (2006) supports the use of chlorhexidine gluconate, or, povidone-iodine, as preferred antiseptics for cleaning the site before initiating a PIV. Several clinical trials in adults have demonstrated enhanced skin antisepsis when using chlorhexidine-containing products (Crosby and Mares, 2001; Milstone, Passaretti, and Perl, 2008). A number of published studies have demonstrated that 2% aqueous chlorhexidine is superior to 10% povidone-iodine and alcohol-based products for preventing catheter-related bacteremia in children (Kline, 2005). U.S. Food and Drug Administration approval for patients above the age of 2 months has led to the introduction of one such product, ChloraPrep. It is a sterile applicator composed of 2% chlorhexidine gluconate and 70% isopropyl alcohol. Researchers have demonstrated the safety and efficacy of 0.5% chlorhexidine scrub versus povidone-iodine as a skin disinfectant in a neonatal intensive care unit (Linder, Prince, Barzilai, et al, 2004). Chlorhexidine gluconate appears to be a safe and effective skin antiseptic solution for central venous catheter site care in children; however, several researchers (Carson, 2004; Lee and Johnston, 2005) point to conflicting evidence with regards to the most effective concentration of chlorhexidine (2% or 0.5 %) for preventing central venous catheter–related bloodstream infection in children, and the overall safety of the antiseptic solution in neonates and preterm infants has yet to be established.
Suggested equipment for starting a PIV includes:
• Gloves (if latex, check for patient latex sensitivity)
• Skin antiseptic (chlorhexidine, alcohol, or povidone-iodine)
• Buffered lidocaine, LMX4 (4% liposomal lidocaine), or EMLA (a eutectic mix of lidocaine and prilocaine) to anesthetize the area
• A tourniquet (again, check for patient latex sensitivity)
• Rolled towels or small blankets for maintaining position of head or extremity
• Tape (or dressing and bacteriostatic ointment as required by hospital policy)
• Sterile transparent occlusive dressing
• A T or J connector (an extension tube that decreases tension and movement of the catheter hub at the site, provides a port for piggyback medications, and makes changing the dressing and tubing easier)
• Blood collection tubes and syringes for collecting blood (blood samples should be collected at the time of IV insertion whenever possible to avoid an additional needlestick)
• Prefilled normal saline syringes to test patency of IV site before attaching the IV fluids
• A protection device to protect the IV site after insertion
The prescribed solution is flushed or primed through the T or J connector (if blood samples will not be collected), tubing, filter, and infusion pump in advance, ready to connect to the catheter hub after insertion of the IV catheter. A sharps container should be within reach if the IV catheter needle does not retract into a safety shield after the catheter is in place.
Safety Catheters and Needleless Systems: One of the main causes for change in IV therapy is the concern about needlestick injuries. To provide safer care for the patient and health care worker, and to comply with Occupational Safety and Health Administration standards and the Needlestick Safety and Prevention Act of 2001 (Regulation 1910.1030; available at www.osha.gov), manufacturers have developed safety catheters and needleless IV systems (Marini, Giangregorio, and Kraskinski, 2004).
Over-the-needle IV catheters with hollow-bore needles carry a high risk for transmission of blood-borne pathogens from needlestick injuries (Whitby, McLaws, and Slater, 2008). A number of safety catheters are currently used to prevent accidental needlesticks with over-the-needle IV catheters (Marini, Giangregorio, and Kraskinski, 2004). Needleless IV systems, which are designed to prevent needlestick injuries during administration of IV push medications and IV piggyback medications, may vary from manufacturer to manufacturer, but the concept is essentially the same. Some needleless systems are universal, whereas others require complete use of the entire IV delivery system for compatibility. Needleless IV systems rely on prepierced septa that are accessed by blunted plastic cannulas or systems that use valves that open and close a fluid path when activated by insertion of a syringe.
Blunt plastic cannulas and preslit injection port sites found in Interlink IV access systems (Fig. 28-5) eliminate the need for steel needles and conventional injection port sites but remain accessible to hypodermic needle use or access, a drawback except in emergent situations. Systems that do not permit needle access enhance safety by preventing health care workers from attempting to use needles; however, such systems are limited by the lack of needle access (interchangeability between needleless and needle insertion systems), especially in emergency situations. A syringe with a blue spike is available to access a single-dose vial (see Fig. 28-5, A). The preslit injection port sites are identified by a white ring surrounding the port; this ring alerts users that the system is needleless (see Fig. 28-5, B). Syringes are available with the blunt plastic cannula for accessing these sites (see Fig. 28-5, C). A lever lock (see Fig. 28-5, D) or threaded lock cannula (see Fig. 28-5, E) attaches to an IV line, IV Y site, or peripheral intermittent infusion device. A preslit universal vial adapter (not pictured) provides access to standard multiple-dose vials, and syringe cannulas are then used to access the adapter.
Infusion Pumps: Several modifications are made in equipment used for IV infusion for children. A gravity drainage apparatus for children is much the same as that for adults except that it is designed to deliver a reduced drop size (60 drops/ml) and contains a calibrated volume control chamber (e.g., a buretrol or soluset) that regulates the amount of fluid that can be infused. A microdropper facilitates calculation of flow rate, since a prescribed number of milliliters per hour equals the number of drops per minute. For example, if the solution is to infuse at a rate of 30 ml/hr, the infusion is regulated to deliver 30 drops/min.
A variety of infusion pumps are available. The IV solution is refillable from the bag, bottle, or soluset above or contained in a syringe pump to minimize the possibility of overloading the circulation. Infusion pumps are recommended in infants and small children because they can accurately infuse fluids (especially the syringe pumps, which infuse very small amounts of fluids) and accurately provide the prescribed amount of IV solution. It is an important nursing responsibility to understand and follow manufacturer’s directions for use, calculate the amount to be infused in a given time, set the infusion rate, and monitor the apparatus frequently (at least every 1 to 2 hours) to make certain that the desired rate is maintained, the integrity of the system remains intact, the site remains intact (free of redness, edema, infiltration, or irritation), and the infusion does not stop.
Continuous infusion pumps, although convenient and efficient, are not without risks. Overreliance on the machine’s accuracy can cause either too much or too little fluid to be infused; therefore its use does not eliminate careful periodic assessment by the nurse. Excess pressure can build up if the machine is set at a rate faster than the vein is able to accommodate (or continues to pump when the needle or cannula is out of the vessel lumen). This is especially true in very small infants. Regardless of the device used, a thorough understanding of the apparatus is essential for safe fluid administration.
Intraosseous Infusion
Situations may occur in which rapid establishment of systemic access is vital and venous access is complicated by peripheral circulatory collapse, hypovolemic shock (secondary to vomiting or diarrhea, burns, or trauma), cardiopulmonary arrest, or other conditions (de Caen, Reis, and Bhutta, 2008). It is recommended that intraosseous access be obtained if venous access cannot be readily achieved in a pediatric resuscitation (de Caen, Reis, and Bhutta, 2008; Hazinski, Zaritsky, Nadkarni, et al, 2002). Intraosseous infusion provides a rapid, safe, and lifesaving alternate route for administration of fluids and medications until intravascular access is possible, especially in children 6 years old or younger. Health care providers, including physicians, nurses, and paramedics, can secure intraosseous cannulation within 30 to 60 seconds. Some hospitals recommend pediatric advanced life support training before performing this procedure. This procedure is usually reserved for children who are unconscious or for those receiving analgesia, since the procedure is painful. Local anesthesia should be used for a semiconscious patient.
A large-bore rigid needle such as a bone marrow aspiration needle (e.g., Jamshidi) or an intraosseous needle (e.g., Cook) is inserted into the medullary cavity of a long bone. The anteromedial aspect of the tibia—1 to 3 cm (0.4 to 1.2 inch) below the tibial tuberosity—is the preferred site for children of all ages because it is flat and has a large marrow cavity. In newborns the distal third of the femur may be used. The distal tibia is an alternative site.
Observe the extremity closely for swelling or oozing of fluid at the insertion site. Give particular attention to the dependent tissue of the leg. Extravasation of fluid from the bone marrow may be hidden under the leg. Check for swelling of the entire lower leg when the intraosseous bone marrow needle is in the tibia or ankle, and check the entire upper leg when the intraosseous needle is in the femur. Compartment syndrome has resulted from an infiltrated intraosseous line. Other complications, although rare, include fractures, skin necrosis, osteomyelitis, and cellulitis (de Caen, Reis, and Bhutta, 2008; Fiorito, Mirza, Doran, et al, 2005).
Once the bone marrow needle is in place, the needle should stand alone and feel secure. Tape and gauze are used to secure the needle to the leg. Gauze should be built up around the needle to provide support and prevent trauma or dislodgment. Drugs may be pushed and fluids delivered via an infusion pump. The intraosseous line may be discontinued after IV access has been achieved.
Preparing the Child and Parents
Children of any age are anxious and fearful of injections, and unless the IV infusion is implemented as an emergency procedure, there will be time to prepare them. (See Preparation for Diagnostic and Therapeutic Procedures, Chapter 27.) Many children have never undergone the procedure, and those who have will remember the experience. Using an age-appropriate developmental approach, the nurse can ask them what they think about the procedure and why it is needed for them specifically. Children’s perceptions of the anticipated experience reveal any misconceptions that need to be clarified and help the nurse prepare them for what to expect. In addition, children’s observations provide some insight into how to cope with their reactions during the insertion procedure and throughout the course of the IV therapy. For children who have repeated venipunctures, it is helpful to ask them or their parents which vein has been successfully accessed in the past.
Play, always an excellent stress reduction technique, can be employed during the preparation process. Allowing children to handle the equipment and to “start” an IV infusion on a toy animal or doll helps familiarize them with the frightening aspects of the procedure. In some instances it may be helpful to introduce a child to another child who is coping well in the same situation.
It is best to arrange for a quiet, private setting for the child during the insertion. Avoid “safe places,” such as the playroom or the child’s hospital room when possible. The assurance of privacy relieves the child of some anxieties concerning loss of control in front of others. It also avoids subjecting other children to the potentially stress-provoking scene. Provide the child with some distracting activity, such as those described for injections, and perhaps allow them to “help” by holding supplies such as a gauze square, helping to clean the site with an antiseptic, and assisting in taping the site after the procedure.
Children usually cooperate better and feel more in command if they are allowed to sit up during the process, although this may not be possible even in some older, normally cooperative children. Toddlers and young children can be held on a parent’s lap, with the child’s legs tucked between the parent’s legs, and the child’s arm (not being used for the venipuncture) behind the parent. A hug should both restrain the child and provide comfort. The torso of the patient is held against the parent with the same hug. It is a mistake to assume that children will not lose control even after they promise to cooperate. It is wise to have ample assistance available in the event that a child cannot control anxiety. The child need not be restrained until necessary, but the assistant should be prepared to grasp a child gently but firmly during the insertion. Explaining to children what is being done during each step of the procedure and how they can participate helps obtain their cooperation and reduce their stress. Make every effort to reduce the pain of the needle insertion (see Atraumatic Care box).
Inform parents about the procedure, including the reasons for the procedure, how long the catheter must remain in place, and what they can expect during and after the insertion. Offer them the option of remaining with their child or leaving. Encourage parents who remain to kiss, hug, and distract the child during the procedure.
The Procedure
The site selected for PIV infusion depends on accessibility and convenience. Although it is possible to use any accessible vein in older children, consider the child’s developmental, cognitive, and mobility needs when selecting a site. Whenever possible, it is best to avoid the child’s favored hand to reduce the disability related to the procedure. Choose a site that restricts the child’s movements as little as possible; a site over a joint in an extremity is avoided as much as possible. An older child can help select the site and thereby maintain some measure of control.
For veins in the extremities, it is best to start with the most distal sites. If the vein is damaged, using distal sites initially preserves access to the vein in proximal sites. A scalp vein or a superficial vein of the wrist may also be used if larger veins are not accessible (Fig. 28-6). Avoid arteries for PIV therapy.
Most infants have one or two possible IV sites on each hand, arm, and foot and four to eight sites on the scalp. Because insertion is easy, scalp veins are sometimes used for IV therapy in infants less than 9 months of age but should be used only when attempts at other sites have failed. The temporal and forehead areas are suitable and do not interfere with side-to-side head movements. The use of a scalp vein site may require removing hair around the site to better visualize the vein and provide a smoother surface on which to tape the tubing. Clipping off a portion of the infant’s hair is upsetting to parents; therefore always tell them what to expect and reassure them that the hair will grow in again rapidly (save the hair, since parents often wish to keep it). Remove as little hair as possible directly over the insertion site and taping surface. To avoid microabrasions, do not shave the site, which increases the potential for introduction of microorganisms into the vascular system (Infusion Nurses Society, 2006). A rubber band slipped onto the head from brow to occiput will usually suffice as a tourniquet, although if the vessel is visible, a tourniquet may not be necessary in some infants.
An assistant should carefully restrain the extremity or head to facilitate venipuncture and minimize trauma from the child’s inadvertent movement. (See Chapter 27 for additional restraining methods.) For a scalp site it is helpful to visualize the way in which the needle will be secured after insertion.
Locating an extremity vein may be difficult because the veins are smaller and children have a significant amount of subcutaneous fat. When veins are not readily visible, applying a warm compress to the site, running warm water over the extremity, or holding the limb in a dependent position below body level will help fill the veins for better visualization. Gentle tapping sometimes causes the veins to stand out. A flashlight held against the skin below the intended site sometimes assists in locating vessels. A commercial vein transilluminator is often helpful in locating veins and assessing the depth and patency of the vessels but it requires an extra set of hands in infants and smaller children. If these measures do not help, a tourniquet applied with light pressure medial to the site may be needed. Although the tourniquet makes the veins more visible and provides a more rapid blood return, the added venous pressure may cause fragile veins to “blow” when punctured, producing a hematoma.
Before beginning the procedure, the nurse should prepare the materials needed to secure the IV. Tape should be precut and easily reached. All other necessary equipment should be set up in an orderly fashion, allowing the venipuncture to be performed in a timely manner.
The needle or catheter must be placed in the direction of the blood flow, which creates no problem when an extremity is used. Scalp veins are easy to visualize but difficult to assess and may actually be an artery. Therefore palpation for a pulse on scalp sites is recommended but again may be difficult because the veins may be hidden within the suture lines. In general, the venous blood flows from the top of the head toward the neck, so point the catheter downward toward the heart. To test the direction before insertion, place a forefinger on the vein at the site chosen for venipuncture. While gently pressing the vein, use a second finger to “strip” the vein in the direction of the top of the head. Release the pressure from the second finger. If the vein fills distal to the compressing finger, the direction of flow is toward the stationary finger.
Securing a Peripheral Intravenous Line
To maintain the integrity of the IV line, adequate protection of the site is required (Box 28-7). Firmly secure the catheter hub at the puncture site with a transparent dressing or clear, nonallergenic tape. Transparent dressings are ideal because the insertion site is easy to observe. Use minimum tape at the puncture site and on about 2.5 to 5 cm (1 to 2 inches) of skin beyond the site to avoid obscuring the insertion site for early detection of infiltration.
Apply a sterile dressing such as a transparent semipermeable membrane directly over the catheter insertion site to protect the infusion site (Infusion Nurses Society, 2006). Consider easy access to the IV site for frequent (hourly) assessments (Infusion Nurses Society, 2006). Improvised plastic cups that are cut in half with the ridged edges covered with tape should not be used, since they have injured patients. A commercial site protector, I.V. House, is available in different sizes (Fig. 28-7). Its ventilation holes prevent moisture from accumulating under the dome. This device is designed to protect the IV site and allows for visibility of the site. The device also minimizes use of padded boards, splints, or other restraints and tape and maintains skin integrity. The connector tubing or extension tubing can be looped to make it small enough to fit under the protective cover to prevent accidental snagging of the catheter. It is important to safely secure the IV tubing to prevent infants and children from becoming entangled in the tubing or from accidentally pulling the catheter or needle out. This securement also eliminates movement of the catheter hub at the insertion site (mechanical manipulation). Apply a colorful and interesting sticker to the protective device to add a positive note to the procedure.
Finger or toe areas are left unoccluded by dressings or tape to allow for assessment of circulation. The thumb should not be immobilized because of the danger of contractures with limited movement later on. An extremity should never be encircled with tape, since this may impede necessary blood flow to and from the extremity. The use of roll gauze, self-adhering stretch bandages, and Ace bandages can cause the same constriction and hide signs of infiltration.
Traditionally, padded boards or splints were used to partially immobilize the IV site. When metal needles were inserted into the vein, padded boards or splints and restraints were appropriate to prevent the sharp end from puncturing the vessel, especially at a joint. With the more recent use of soft, pliable catheters, arm or leg boards may not be necessary and have several disadvantages. They obscure the IV site, can constrict the extremity, may excoriate the underlying tissue and promote infection, can cause joint contracture, restrict useful movement of the extremity, and are uncomfortable. Unfortunately, no research has been conducted to demonstrate their proposed benefit of increasing dwell time (patency of the IV line). Adequate taping and protection with a commercial device should eliminate the need for padded boards in most circumstances. A number of elbow restraint or protective arm restraint devices are available that prevent the child from bending the arm or pulling at the IV site on the arm; these are made of cloth and a semi-rigid mold with Velcro straps or ties so the device may be adjusted to the child’s limb size and does not impair circulation. (See Chapter 27.) Older children who are alert and cooperative can usually be trusted to protect the IV site.
Immobilization is intolerable to the naturally active child, and the nurse should make every effort to reduce the use of restraints. To relieve the stress of immobilization, frequent removal of the restraints (if used at all) allows the child to move the extremities. Whenever possible, the infant or child is held and cuddled to help meet emotional needs during this trying time (Fig. 28-8). Range-of-motion exercises are employed for infants and children who are too ill or unable to move their extremities, but others should be encouraged to move their arms and legs. Most infants or small children instinctively move their extremities when released. If not, a toy or other stimulus can provide incentive.
Fig. 28-8 Intravenous infusion, as well as other equipment, does not prevent infant from being picked up and cuddled.
Appropriate documentation of the procedure, type of cannula used, patient tolerance, and status of the skin site is an important nursing intervention (Box 28-8).
Removal of a Peripheral Intravenous Line
When it comes time to discontinue an IV infusion, many children are distressed by the thought of catheter removal. Therefore they need a careful explanation of the process and suggestions for helping. Encouraging children to remove or help remove the tape from the site provides them with a measure of control and often fosters their cooperation. The procedure consists of turning off any pump apparatus, occluding the IV tubing, removing the tape, pulling the catheter out of the vessel in the opposite direction of insertion, and exerting firm pressure at the site after the catheter is removed until bleeding stops. (It is painful to apply pressure on the catheter while pulling it out.) Place a small dry dressing (adhesive bandage strip) over the puncture site. The use of adhesive removal pads can decrease the pain of tape removal, but the skin should be washed off after use to avoid irritation. An adhesive removal agent should be used with caution in some preterm infants and children with compromised skin because absorption is variable and toxicity may occur. To remove transparent dressings (e.g., OpSite or Tegaderm), pull the opposing edges parallel to the skin to loosen the bond. If a catheter was used for the IV infusion, inspect the tip to make certain the catheter is intact and no portion remains in the vein.
Removal of the IV line, especially the tape, is another painful and frightening experience for the child. Consider the child’s age, developmental and neurologic status, and predictability (how the child responds to painful treatments) when determining the need for assistance to maintain safety with this procedure. Manual removal of tape is the preferred method. Only if absolutely necessary should a small cut be made in the tape, using bandage scissors, to facilitate its removal. Before cutting the tape:
• Ensure that all digits are visible.
• Remove any barrier that hinders visibility, such as a protective covering.
• Protect the child’s skin and digits by sliding own finger(s) between the tape and the child’s skin so that the scissors do not touch the patient.
• Cut the tape on the medial aspect (thumb side) of the extremity.
Complications
The same precautions regarding maintenance of asepsis, prevention of infection, and observation for infiltration are carried out with patients of any age. However, infiltration is often more difficult to detect in infants and small children than in adults. The increased amount of subcutaneous fat and the amount of tape used to secure the catheter hub obscure the signs of early infiltration. When the fluid appears to be infusing too slowly or ceases, the usual assessment for obstruction within the apparatus (i.e., kinks; screw clamps; shutoff valve; and positioning interference, such as a bent elbow or wrist) often locates the difficulty. When these actions fail to detect the problem, it may be necessary to carefully remove some of the tape and other material that obscure a clear view of the venipuncture site. Examine dependent areas, such as the palm and undersides of the extremity or the occiput and behind the ears.
Whenever possible, place the IV infusion in an extremity to which the identification band (or bracelet) is not attached. Serious circulatory impairment can result from infiltrated solution distal to the band, which acts as a tourniquet preventing adequate venous return. To check for return blood flow through the catheter, lower the solution bag below the level of the infusion site. A good blood return, or lack thereof, is not always an indicator of infiltration in small infants. Flushing the catheter and observing the site for discoloration (i.e., blanching or redness), pain, tenderness, and edema or noting any exudate or drainage and increase in skin or basal temperatures is an appropriate assessment of the IV site. If the tubing is connected to an infusion pump, it must be removed from the pump before lowering.
IV therapy in pediatrics tends to be difficult to maintain because of mechanical and physical factors that tend to shorten dwell times. Such factors include vascular trauma resulting from PIV device selection (gauge and length of the catheter), the insertion site, the catheter dwell time, the size of the vessel, vessel fragility, and pressure of the infusion pump (determined in PSI, or pounds per square inch). Different infusion pumps have preset pressures for infusion, delivery, and occlusion; therefore the pump’s occlusion alarm is not entirely reliable for detecting infiltration. The patient’s activity level, operator skill and insertion technique, forceful administration of boluses of fluid, type of infusion solution, antibiotics and other medications being infused, and infusion of irritants or vesicants through a small vessel are also factors. These factors cause infiltration and extravasation injuries, which are reported with relative frequency (Fig. 28-9).
Infiltration is defined as inadvertent administration of a nonvesicant parenteral solution or medication into surrounding tissue as a result of catheter dislodgment (Infusion Nurses Society, 2006). Extravasation is defined as inadvertent administration of vesicant solution or medication into surrounding tissue as a result of catheter dislodgment (Infusion Nurses Society, 2006). A vesicant or sclerosing agent causes varying degrees of cellular damage when even minute amounts escape into surrounding tissue. A scale that provides a uniform standard for measuring the degree of infiltration is available from the Infusion Nurses Society*; the characteristics of infiltration include the degree of skin discoloration, blanching, edema, the amount of pain, and warmth or coolness of the area (Infusion Nurses Society, 2006).
Phlebitis, or inflammation of the vessel wall, may also develop in children who require IV therapy. Lamagna and MacPhee (2004) describe three types of phlebitis: mechanical (caused by rapid infusion rate, manipulation of the IV), chemical (caused by medications such as nafcillin, amphotericin B), and bacterial (caused by staphylococcal organisms). The initial sign of phlebitis is erythema (redness) at the insertion site. Pain may or may not be present (Lamagna and MacPhee, 2004).
Standardized IV site assessment tools, such as the one used at Children’s Hospital of Boston, assist in detection of infiltration, extravasation, and phlebitis in children so early intervention can prevent vessel and skin damage (Lamagna and MacPhee, 2004). Treatment of an infiltration or extravasation varies according to the type of infusate (see Nursing Care Guidelines box).
The following guidelines should be considered when starting a pediatric PIV:
• Avoid reinserting a stylet into the catheter. This can damage the catheter and cause catheter fragment embolus.
• If IV insertion is unsuccessful, obtain a new catheter for the subsequent attempts.
• Some parents count the number of times the catheter is moved in, out, and around the area of insertion while trying to locate the vein (probing) as sticks their child receives as opposed to counting the number of IV insertion attempts. Limit the amount of probing because it is painful.
• When setting up the supplies for the IV insertion, use caution in determining where to affix the precut tape. Keep in mind that the precut tape should be affixed to a clean surface.
PIV catheters are the most commonly used intravascular devices. Heavy cutaneous colonization of the insertion site is the single most important predictor of catheter-related infection with all types of short-term, percutaneously inserted catheters. Phlebitis, largely a mechanical rather than infectious process, remains the most important complication associated with the use of peripheral venous catheters (see Community Focus box).
Proper education of the patient and family regarding signs and symptoms of an infected site can help prevent infections from going unnoticed. When an IV infusion continues for several days, the tubing and solution administration set is changed at regular intervals according to hospital policy or at least every 72 hours for a continuous infusion and at least every 24 hours for an intermittent infusion (Infusion Nurses Society, 2006). Gillies, O’Riordan, Wallen, and colleagues (2004) recommend changing IV administration solution sets and tubing every 72 hours to decrease the probability of infection. Lipid-containing parenteral nutrition (PN) fluids such as three-in-one solutions should be changed after 24 hours (Gillies, O’Riordan, Wallen, et al, 2005; Infusion Nurses Society, 2006), whereas a 12-hour limit is recommended for pure intralipid infusions (O’Grady, Alexander, Dellinger, et al, 2002). The dressing can be left in place for the duration of the IV infusion unless the integrity has been compromised. To ensure that the solution and IV equipment is changed regularly, it is labeled with the date and time that the new bag and tubing are attached. Any signs of inflammation, such as redness or pain, are reported immediately. This usually requires removing the infusion and restarting it at another site or administering the medication by another route.
Prevention of insertion site infection can be decreased by strict adherence to the following guidelines:
• Practice good hand washing before starting an IV infusion.
• Cleanse the skin with an appropriate antiseptic before catheter insertion.
• When cleansing the insertion site, use a circular motion starting from the center and working outward.
• Allow the antiseptic to dry for 30 to 60 seconds before inserting the catheter.
• Avoid palpating the insertion site after the skin has been cleansed with the antiseptic.
Venous Access Devices
Venous access devices (VADs) have several different characteristics. The practitioner has to consider the best type of catheter for the individual patient’s needs. Factors that can influence the decision include the reason for placement of the catheter (diagnosis), patient age, length of therapy, risk to the patient in placement of the catheter, and availability of resources to assist the family in maintaining the catheter.
Central catheters can be categorized in three types:
1. Short-term or nontunneled catheters (subclavian, femoral, and jugular)
2. PICCs (Box 28-9)
3. Long-term, tunneled catheters and implanted ports (Table 28-8)
Short-term or nontunneled catheters are used in acute care, emergency, and intensive care units. These catheters are made of polyurethane and are placed in large veins such as the subclavian, femoral, or jugular. Take a chest radiograph to verify placement of the catheter tip before administration of fluids or medications. The other types are discussed in the following sections.
Peripheral Intermittent Infusion Device
The peripheral lock, also known as an intermittent infusion device or saline or heparin lock, is an alternative for a keep-open infusion when extended access to a vein is required without the need for continuous fluid. It is most frequently employed for intermittent infusion of medication into a peripheral venous route. A short, flexible catheter is used as the lock device, and a site is selected where there will be minimum movement, such as the forearm. The catheter is inserted and secured in the same manner as any IV infusion device, but the hub on the proximal end is occluded with a stopper or injection cap.
The type of device used may vary, and the care and use of the peripheral lock are carried out according to the specific protocol of the institution or unit. However, the general concept is the same. The catheter remains in place and is flushed with saline or heparin (1 : 10 units/ml) after infusion of the medication. The flush solution prevents blood from clotting in the device between infusions. Because heparin is incompatible with many drugs, the peripheral lock must also be flushed with saline before and after administering medication. There are conflicting data regarding the use of saline flush versus a heparinized saline flush and the dwell time of peripheral locks in children (see Evidence-Based Practice box). Some children are discharged with a peripheral lock in place in order to continue receiving medications without hospitalization; this is usually reserved for children who require medications on a short-term basis and are referred to a home-based infusion company. Those with chronic illnesses who require repeated blood sampling or medications, long-term chemotherapy, or frequent hyperalimentation or antibiotic therapy are best managed with a long-term central VAD.
There is controversy concerning the need to flush with heparin in any VAD, peripheral or central. A more important issue is the technique of flushing. The use of the turbulent-flow flush has proven successful in preventing clot formation in the device. It is described as the forward flushing motion on the syringe with a flush-stop-flush-stop technique. This causes a swirling and vigorous fluid movement that clears the catheter better than the continuous flush motion most commonly used. This procedure is combined with the positive pressure technique. As the flush is completed, hold the syringe stopper down, clamp the catheter, then remove the syringe. This prevents blood from backing into the tip of the catheter.
Peripherally Inserted Central Catheters
PICCs can be used for short-term to moderate-length therapy (see Box 28-9). These catheters consist of silicone or polymer material and are placed by specially trained nurses (Gamulka, Mendoza, and Connolly, 2005). The most common insertion site is the antecubital area using the median, cephalic, or basilic vein. The catheter is threaded either with or without a guide wire into the superior vena cava. The modified Seldinger technique is often used with ultrasonography to access very small veins in infants and children (Mickler, 2008). PICCs are sometimes inserted a shorter length; this is often referred to as a “midline” catheter. The insertion length of the midline catheter is usually somewhere between the insertion site and the axilla; in some cases the midline catheter may be placed in a scalp vein and threaded into the external jugular vein (Petit, 2006). The midline has an average dwell time of 6 to 10 days in neonates (Petit, 2006). Centrally placed catheter tips are associated with fewer complications (phlebitis, occlusion, leaking) than noncentrally placed catheters (Racadio, Doellman, Johnson, et al, 2001).
If the catheter is threaded midline, total parenteral nutrition (TPN) should not be administered, because the high concentration of glucose irritates the vessel; it should be infused through a central catheter.
The decision to insert a PICC needs to be made before several attempts at IV insertions are done. In one center PICC lines were inserted in children undergoing operative procedures. The PICCs were inserted in the operating room in pediatric patients under general anesthesia whose hospital stay was expected to be at least 4 days; when compared with similar patients undergoing surgery and with only a PIV, children in the PICC group experienced fewer needlesticks for blood draws and failed PIV complications postoperatively. The cost of PICC insertion was higher than PIV, but satisfaction was greater and complications were lower (Schwengel, McGready, Berenholtz, et al, 2004). Once the antecubital veins have been punctured repeatedly, they are not considered candidates for this type of catheter. Because this catheter is the least costly VAD and has a lower risk of complications than other central VADs, it is an excellent choice for many pediatric patients. This catheter is also usually inserted in the unit’s treatment room.
PICC lines may be used for blood draws, although there is often a greater number of occlusions with blood draws (Knue, Doellman, Rabin, et al, 2005).
PICCs can create problems with removal. Causes for resistance in removal include infectious processes, fibrin formation, and endothelial thrombosis. Methods to free the catheter include gentle traction to the catheter, taping the catheter to create tension on the line, and warm soaks to the site. Aggressive pulling of the catheter is contraindicated.
Long-Term Central Venous Access Devices
![]() Long-term central VADs include tunneled catheters (Broviac, Hickman, or Groshong) and implanted infusion ports (see Table 28-8). They may have single, double, or triple lumens. Several lumens (multilumen catheters) allow more than one therapy to be administered at the same time. Reasons to use multilumen catheters include repeated blood sampling, TPN infusion, administration of blood products or infusion of large quantities or concentrations of fluids, administration of incompatible drugs or fluids at the same time (through different lumens), and central venous pressure monitoring.
Long-term central VADs include tunneled catheters (Broviac, Hickman, or Groshong) and implanted infusion ports (see Table 28-8). They may have single, double, or triple lumens. Several lumens (multilumen catheters) allow more than one therapy to be administered at the same time. Reasons to use multilumen catheters include repeated blood sampling, TPN infusion, administration of blood products or infusion of large quantities or concentrations of fluids, administration of incompatible drugs or fluids at the same time (through different lumens), and central venous pressure monitoring.
![]() Critical Thinking Exercises—Central Venous Access Device
Critical Thinking Exercises—Central Venous Access Device
With the patient under local or general anesthesia, the long-term catheter of choice is placed with aseptic technique. A vein, such as the jugular or subclavian, is entered through a small cutdown site, and the catheter is threaded to the junction of the superior vena cava and right atrium, confirmed by radiography with fluoroscopic dye injection, and then sutured in place. To stabilize the catheter and reduce the risk of infection, the remainder is tunneled beneath the skin to exit through a small incision at a convenient location on the anterior aspect of the chest or upper abdomen (Fig. 28-10, A and B). One or two Dacron cuffs or VitaCuffs on the catheter remain in the subcutaneous tunnel; as tissue adheres to the cuff, the cuff provides a barrier to infection. The cutdown site is surgically closed, the catheter is sutured to the skin at the exit site, and a sterile dressing is applied. A radiograph is taken to ascertain the location of the catheter before instillation of fluids. With any of the central venous catheters, medications are easily instilled through the injection cap. Maintenance of the catheter includes dressing changes, flushing to maintain patency, and prevention of occlusion or dislodgment.
Fig. 28-10 Venous access devices. A, External central venous catheter insertion and exit site. B, Child with external central venous catheter (dressing removed for photo). C, Child with implanted port with Huber needle in place. (note: Dressing usually covers needle insertion site; removed for photo only.) D, Side view of implanted port.
Children may benefit from an implanted port, which consist of a small, circular “port of entry” that is placed under the skin (while the patient is under local or general anesthesia) over a bony prominence to provide a stable surface, usually under the distal third of the clavicle (Fig. 28-10, C and D). A tunnel is created from the port to the point where the catheter enters a central vein leading to the entrance to the right atrium. Medication or other solution is injected with a special Huber needle (a 90-degree angled needle) through the skin into the port. The device can remain situated indefinitely and may be placed in small infants as well as older children and adolescents.
With the implanted device, palpate the port for placement and stabilize it with the thumb and index finger. Cleanse the overlying skin, and use only special noncoring Huber needles to pierce the port’s diaphragm on the top or side, depending on the style. A special infusion set with a Huber needle and extension tubing with Luer connection can be used to access the port when blood work or infusion therapy (chemotherapy or blood product administration) is required. A small gauze pad is placed under the horizontal portion of the protruding needle and a transparent dressing is placed over the protruding needle and gauze for stabilization and infection control. In small, curious children the tubing may be tunneled under a cotton shirt or one-piece suit and out the leg or back to prevent the child from pulling on the port tubing.
With the port accessed, the injection procedure is the same as for the venous catheters. To prevent infection, use meticulous aseptic technique anytime the devices are accessed, including instillation of heparin or saline to prevent clotting. Protocols are established in most centers for periodic flushing with normal saline and heparin solution (amount and frequency). Maintenance includes intermittent flushing to prevent clotting and a dressing change if the Huber needle is left in place for long periods (>1 week). There should be a protocol stating that the Huber needle needs to be changed at established intervals, usually 5 to 7 days. Advantages to the port include cosmetic appearance, which may be important for older school-aged children and adolescents, and relatively easy access for blood work and fluid and medication administration. When the port must be accessed with a Huber needle, apply a topical anesthetic such as EMLA or LMX4 before the procedure to reduce the pain of penetrating the skin with the Huber needle (see Atraumatic Care box, p. 1073).
Chapter 10 discusses umbilical artery and venous catheters used in neonates.
Complications
Central venous line bacteremia can be of major concern in a child. The mean incidence of catheter-related bloodstream infection in children varies in the literature, but it averages around 3% to 7% with an estimated cost of $9000 to $46,000 per episode (Kline, 2005). Centrally placed catheters are associated with fewer complications than are peripherally placed catheters (Racadio, Doellman, Johnson, et al, 2001).
Catheter-related bloodstream infections have a significant impact on the duration of the catheter and cost of care. Studies have shown that the following practices result in a decrease in catheter-related bloodstream infections (Krein, Hofer, Kowalski, et al, 2007; Morgan and Thomas, 2007; Smith, 2008):
• Using maximum barrier techniques during insertion
• Practicing good hand washing
• Performing skin antisepsis with 2% chlorhexidine
Guidelines for prevention, diagnosis, and management of intravascular catheter–related infections are available from the Infectious Diseases Society of America (Mermel, Allon, Bouza, et al, 2009).
Line connections should have a Luer-Lok connection device or be wrapped with tape to prevent accidental disconnection. The nurse should minimize the number of line accesses for blood withdrawal or medication administration. Central line dressing protocols should be developed, and nurses experienced in dressing change techniques should be responsible for line care. Family teaching must include how to care for the line, what to do if the line becomes disconnected or broken, and how to flush the line using aseptic technique.
Catheter-related central venous thrombosis and catheter occlusion can also be serious problems in children (McCloskey, 2002). Approximately 60% of all catheter-related occlusions occur as a result of thrombus formation (Fisher, Deffenbaugh, Poole, et al, 2004). Small thrombi at the tip of the catheter can usually be prevented with regular heparin flushes (Table 28-9) and, if present, lysed with a thrombolytic solution. The most common drug used to treat catheter-related thrombi is alteplase, a tissue plasminogen activator, which initiates fibrinolysis and clot dissolution. Alteplase has been demonstrated to be safe and effective for treating catheter-related occlusions in children (Blaney, Shen, Kerner, et al, 2006; Fisher, Deffenbaugh, Poole, et al, 2004; Kerner, Garcia-Careaga, Fisher, et al, 2006; Shen, Li, Murdock, et al, 2003). Written protocols should be available for the administration of alteplase for catheter occlusion in children in health care institutions and home care.
TABLE 28-9
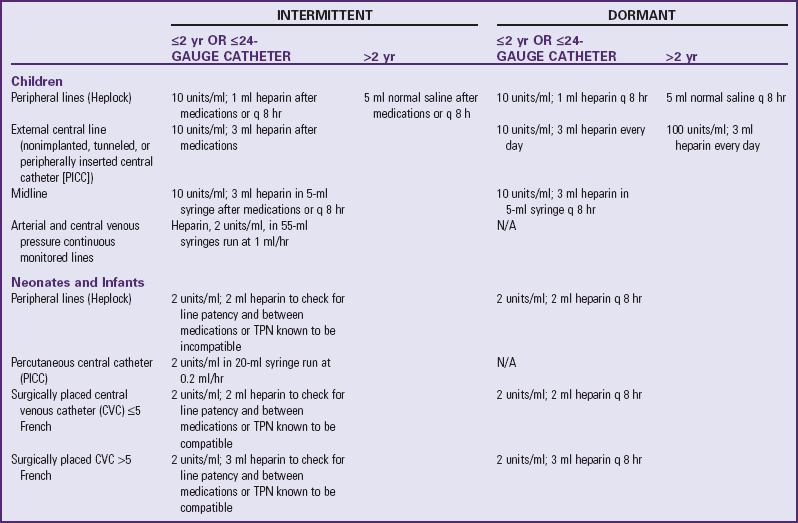
N/A, Not applicable; TPN, total parenteral nutrition.
Adapted from Texas Children’s Hospital: Heparin flush guidelines, Houston, 2002, The Hospital.
Catheter occlusion may be partial, in which case fluid can be flushed into the device, or complete, in which case fluid or blood can be neither withdrawn nor flushed through the catheter (McCloskey, 2002). A fibrin sheath may form over the inner tip of the catheter within the vessel; the catheter may flush easily, but when attempts to withdraw fluid or blood are made, the sheath falls over the tip of the catheter, thus precluding withdrawal of blood or fluid (McCloskey, 2002). Larger thrombi outside of the catheter may require removal and anticoagulant therapy. A central venous thrombosis may also develop as the catheter brushes against the vessel wall, prompting thrombus formation, which may lead to superior vena cava syndrome (McCloskey, 2002). Symptoms of large thrombi include signs of superior vena cava occlusion, such as facial swelling and cyanosis, distended neck veins, blurred vision, vertigo, and swelling of the upper arm.
Complications associated with indwelling ports include port migration and difficulty accessing the port; the latter problem may occur if the Huber needle is not long enough to penetrate the subcutaneous skin.
Parent and Child Teaching
Regardless of which catheter is used, teach the child and family the care and management of the device with practice under supervision. It can be frightening to both child and parents to know that the catheter tip is situated near the heart. They need reassurance that with reasonable care they will do no harm to the apparatus. It is often useful to introduce the family to other children and families who are using central venous catheters successfully and with whom they can share concerns and helpful tips regarding care and management. This sharing is especially valuable for teenage patients. Because teenagers usually have a positive attitude toward use of the catheter, it is beneficial for them to share their experiences with adolescents who face the prospects of catheter placement.
Parents of children who engage in outside activities, go to school, or are otherwise under the supervision of another adult should inform the teacher, school nurse, coach, and baby-sitter about the presence of the central venous catheter. Vigorous contact sports, such as football, soccer, and hockey, are generally not allowed. Provide a written information sheet concerning the VAD, including its purpose, pertinent facts about any restrictions for the child, and directions related to management of the device, for their reference. The nurse or the parents should teach grandparents and other family members who care for the child the care and management of the catheter.
Procedures and published standards for catheter care vary widely among organizations, and there is no evidence that one method is superior. For example, some advocate covering the healed catheter site with a dressing; others do not. All companies that manufacture central catheters have patient and professional teaching kits. The user should become thoroughly familiar with the specific device selected for use.
The catheter is not a deterrent to most routine daily activities, including showers or tub bathing. However, the practitioner is consulted before activities such as swimming or physical contact sports are attempted. Swimming is usually prohibited but may be allowed in certain situations. If the exit site is healed and the cuff adheres to the tissue, place a transparent dressing over the catheter and exit site. Swimming may be permitted for a limited time, such as 1 hour or less, in a chlorinated pool. Most contact sports are prohibited because of the possibility of the catheter being hit or pulled. A protective vest can prevent active children from accidentally dislodging the catheter.
Family members need to know the signs of infection and an occluded catheter. Signs of a localized infection are redness, swelling, and pain at the vein entry site. Bacteremia is a serious complication that produces fever, chills, general malaise, and an ill appearance. Prevent uncapping by taping the cap securely to the catheter and the clamped line to the dressing. Prevent leaks by using a smooth-edged or padded clamp. Caution parents to keep scissors away from the child to prevent accidental cutting of the catheter. If the catheter leaks, they are instructed to tape it above the leak (between the leak and the entry site on the skin) and then clamp the catheter at the taped site. The child should be taken to the practitioner as soon as possible to prevent infection, blood loss, or clotting following a catheter leak.
Total Parenteral Nutrition and Total Nutrient Admixture
TPN, also known as IV alimentation, provides for the nutritional needs of infants or children who cannot consume an adequate amount of nutrients to support physical growth, positive nitrogen balance, and water and electrolyte homeostasis. Parenteral nutrition (PN) is defined by the American Society for Parenteral and Enteral Nutrition as any PN delivered into a large diameter vessel such as the subclavian vein (Teitelbaum, Guenter, Howell, et al, 2005). Total nutrient admixture (TNA) refers to the PN formula with carbohydrates, lipids, amino acids, vitamins, minerals, water, trace elements, and other additives in a single container (Teitelbaum, Guenter, Howell, et al, 2005). TNA may also be referred to as a three-in-one admixture, trimix, or all-in-one parenteral admixture. In many instances TPN may refer to a dextrose and amino acid solution with lipids piggybacked into the administration setup.
The following discussion on TPN encompasses TNA as well. Although the terms are not always synonymous in a clinical context, the complications and assessments are often the same for both TPN and TNA.
Common conditions for which TPN is used therapeutically include chronic intestinal obstruction from peritoneal sepsis or adhesions, extensive bowel resections with necrotizing enterocolitis, and conditions such as gastroschisis or large omphalocele that prevent optimum bowel functioning soon after surgery. TPN is also used for bowel fistulas; inadequate intestinal length with subsequent malabsorption; chronic, nonremitting, severe diarrhea; extensive body burns; and abdominal tumors treated by surgery, irradiation, and chemotherapy. TPN may also be initiated prophylactically when prolonged starvation is expected. Since the advent of PN in the 1960s, it has been recognized that prolonged periods of gut starvation (NPO) cause alterations in the gastrointestinal mucosa that affect the ability to absorb nutrients effectively once the gut has healed from surgical resection or manipulation. Therefore providers make attempts to resume some type of trophic feedings to minimize intestinal mucosal atrophy.
PN therapy involves IV infusion of solutions of protein, glucose, electrolytes, microminerals, and other nutrients on a short-term basis. Lipid emulsion is often initiated after a few days (although the time is variable depending on patient status) of PN to prevent essential fatty acid deficiency; lipids may be initially added as a piggyback infusion to the PN to ascertain tolerance and to avoid changing out the entire PN admixture if further changes in the solution are required. The alimentation solution is infused through conventional tubing with a special filter attached to remove particulate matter or microorganisms that may have contaminated the solution. The solution of glucose, lipids, amino acids, at times insulin, and other nutrients can be mixed together in a bag and delivered through an infusion pump. The solutions require infusion into a vessel with sufficient volume to allow for rapid dilution to minimize phlebitis and irritation. The wide-diameter vessels selected are the superior vena cava and innominate or intrathoracic subclavian veins approached by way of the external or internal jugular veins. In some situations the inferior vena cava from a femoral vein serves as an alternative route.
Central VADs are ideal for long-term and home parenteral nutrition (HPN). The TNA solution is prepared under sterile conditions, usually under a laminar-flow hood in a pharmacy equipped to appropriately mix the required ingredients on a daily basis. The IV tubing and administration set is likewise assembled under a laminar hood as a single unit to prevent contamination and minimize bacterial growth. Because concentrated glucose and protein solutions are optimum breeding sources for bacterial growth, take special care to connect the administration set and tubing to the patient’s VAD using strict aseptic technique and to avoid adding other solutions or medications to the bag once it leaves the pharmacy.
Assessment of tolerance to TPN includes those tests performed for patients receiving parenteral fluids and who have a VAD. These include serum and urine chemistry and electrolyte profile, liver function tests, triglycerides, albumin, renal function, and body weight and height measurements. Hyperglycemia and hypoglycemia are common complications of TPN or TNA therapy until the child adjusts to the glucose load when high glucose solutions are used. The nurse is alert for signs and symptoms of hypoglycemia or hyperglycemia, and bedside glucose monitoring is instituted as necessary. Insulin is often added to the TNA to promote glucose utilization at the cellular level and growth; therefore monitoring for serum glucose levels is important. Hyperglycemia may cause an osmotic diuresis and increased loss of body fluid with subsequent fluid, electrolyte, and glucose imbalances for which the nurse should be vigilant. Once the child’s condition stabilizes, the TNA is adjusted according to metabolic needs to promote growth and maintain a positive nitrogen balance, in addition to fluid and electrolyte homeostasis.
Children on full TPN programs may receive TPN either on a 24-hour basis or according to a plan established so the child receives the infusion at night (over 8 to 12 hours) while asleep to minimize disruption of daily activities such as school. Cycling TPN also allows the liver to rest for a period and is believed to prevent TPN-induced liver damage (Hwang, Lue, and Chen, 2000). However, evidence demonstrating significantly improved liver function with cyclic TPN in children is inconclusive (Jensen, Goldin, Koopmeiners, et al, 2009; Shulman and Phillips, 2003). In some cases the child may take regular fluids throughout the day for maintenance purposes and therefore is not dependent on the infusion for maintenance of electrolyte balance (depending on the nature of the illness). A plan may be established wherein the child may take small amounts of oral fluids or even small amounts of specific kinds of solid foods as tolerated during the day to provide a normal mealtime atmosphere. TPN may be combined with an enteral feeding program, as gastrointestinal tolerance allows, so the child is not totally dependent on TPN.
If prolonged nonoral feedings are required, the infant is allowed nonnutritive sucking to meet those needs. A consultation with a feeding specialist may be helpful so the child does not experience an aversion to foods fed orally when these are eventually implemented.
Because many children are treated with TPN regimens for long periods, it is especially important to be attuned to the child’s growth and development needs. An individualized developmental care program is initiated as early as feasible to prevent developmental delays. (See Developmental Outcome, Chapter 10.) Delays in the areas of gross motor and language skills observed in infants receiving long-term PN (>3 months) may be caused by reduced mobility and social interaction.
Complications
Complications from TPN and TNA are numerous, and a major nursing responsibility is to prevent these when possible and to be alert to signs of their development. Complications are either (1) related to the infusate (metabolic complications); or (2) mechanical complications related to the indwelling catheter, the administration set, or the infusion pump.
Metabolic complications are associated with the infant’s or child’s capacity for the various components of the TNA or TPN solution. Excessive intake of any of the components will create an imbalance, such as hyperglycemia, azotemia, acid-base disorders, anemia, bone demineralization, vitamin and mineral deficiencies, hyperosmotic dehydration and coma, fluid overload, and a variety of electrolyte imbalances.
Liver disease is the most important gastrointestinal complication in pediatric populations. The cause is obscure, but liver disease appears to be more prevalent in preterm infants who were begun on TPN at an early age. In general, the lower the birth weight and gestational age, the higher the incidence of TPN-associated cholestasis; approximately 50% of infants weighing less than 1000 g develop TPN-associated cholestasis (Xanthakos and Balistreri, 2007). Affected infants develop cholestasis, hepatocellular necrosis, and, in advanced disease, cirrhosis or hepatic failure. Manifestations are often insidious and include hepatomegaly; jaundice; and elevated serum aminotransferase, bilirubin, and alkaline phosphatase levels, which become evident approximately 2 weeks after initiation of TPN (Xanthakos and Balistreri, 2007). In the low-birth-weight infant on TPN, onset of jaundice may overlap the phase of neonatal jaundice; therefore fractionated serum bilirubin measurements are recommended (Xanthakos and Balistreri, 2007). TPN-associated cholestasis is less common in older children. Cholelithiasis is an uncommon but possible occurrence in pediatric patients. Therefore children receiving TNA should be assessed periodically for signs and symptoms of cholelithiasis or cholecystitis.
Mechanical complications include catheter-related complications such as those involving catheter placement: pneumothorax, hemothorax, perforation, catheter dislodgment, and thrombus formation. However, the major complication associated with the catheter is infection: infection at the catheter entrance site, catheter “seeding” sepsis, venous thrombosis with infection and embolization, and endocarditis.
Pediatric TPN solution generally has a higher concentration of calcium and phosphorus, which makes some solutions more susceptible to precipitation. In the event of observed precipitation, stop the infusion and disconnect the container and tubing from the child; notify the practitioner so that a replacement infusion of dextrose can be started to prevent hypoglycemia. Other additives, including electrolytes, may need to be added depending on the child’s status and the contents of the original solution. The nurse should take the original solution to the originating pharmacy for analysis.
Home Parenteral Nutrition
Some children require TPN over an extended period, often weeks, months, or even years. For many children, HPN is an alternative to long-term hospitalization. The child must be one who is unable to maintain adequate enteral alimentation, has no medical problems requiring hospitalization, has a parent who is able to manage the home care (or is an older child who can participate in his or her own care), and has the potential to benefit from the treatment.
Before a home care program can be implemented, a thorough assessment is made of the family and the home situation. The parents must be capable of performing the technical aspects of the procedure and be able to adapt to the changes inherent in the home program. Psychosocial readiness of the family; family support systems; and practical considerations are investigated, including availability of a pharmacy to prepare the parenteral alimentation solution, a practitioner to handle day-to-day emergency needs, and a cooperating insurance company or agency (because of the exorbitant cost of maintaining long-term parenteral feeding). In many areas home health care agencies are able to assume the major management of HPN for families; however, a shortage of skilled nursing staff and decreased third-party reimbursement often require a family member to assume major responsibility for the child’s home care. (See also Family-Centered Home Care, Chapter 25.)
The major nursing responsibilities for the child and family with HPN include assurance that the proper solution is infusing, proper maintenance of the VAD, prevention of sepsis and other mechanical complications, monitoring of infusion rate, and assessment of the patient’s tolerance to the solution. In most cases a family member may assume responsibility for starting (hanging) and monitoring the infusion. The TPN or TNA solution is prepared under sterile conditions, usually in a hospital pharmacy or home health agency pharmacy, and then is distributed to the primary caretaker by a home health aide or family member. Teach family members how to maintain the child’s VAD, including dressing changes and routine flushing as required, and how to evaluate the progress of the infusion. In most cases the infusion is delivered by an infusion pump to prevent too rapid an infusion, which may cause fluid and electrolyte problems. HPN complications include those listed above for long-term TPN: infection (catheter-related sepsis), exit site infection, catheter occlusion, catheter tears, thrombosis, fluid and electrolyte imbalance, and liver dysfunction (Howard and Ashley, 2003).
In a study of adult and pediatric patients on HPN, Howard and Ashley (2003) found that the single most important factor influencing the outcome of home TPN was the patient’s primary diagnosis. Mortality and morbidity for patients with cancer was much greater than for those with bowel disorders such as obstruction and Crohn disease.
Colomb, Dabbas-Tyan, Taupin, and colleagues (2007) also noted that most children on HPN with primary digestive disease survived if early referral occurred; survival probabilities in the study at 2, 5, 10, and 15 years were 97%, 89%, 81%, and 72%, respectively. In this study the primary diagnosis strongly influenced the outcome and survival rate of the child on HPN. Johnson and Sexton (2006) noted that HPN was associated with fewer catheter infections than hospital PN; however, HPN was associated with more physical and psychologic stress for families. Social isolation, depression, and loneliness were identified in children and families with HPN.
An important goal with HPN is the normalization of the child to optimize ability to perform activities of daily living and promote a healthy lifestyle in anticipation of the day when TPN or TNA is no longer required. (See also Chapter 25.) One study found that the quality of life for children and their families on HPN was not significantly different from that of a reference group of healthy children without HPN (Gottrand, Staszewski, Colomb, et al, 2005). The researchers concluded that children on HPN developed appropriate coping skills; parents of children on HPN, especially parents of infants, had lower quality-of-life scores than controls, and adolescents on HPN had quality-of-life scores similar to those of controls, except for those with ileostomy, who scored lower on quality-of-life issues.
Before beginning HPN, the parents are prepared for taking over the child’s total care. Teaching may occur in the hospital or at home, depending on the policies of insurance companies and the home health care agency monitoring the family. The parents assume full responsibility for the child’s care, with help being readily available when needed.
The emotional and economic benefits of this approach are readily apparent. The familiar environment and the atmosphere of normality are enormously therapeutic, and the stress of separation is avoided. With multidisciplinary support from health professionals, a home care program can be the ideal alternative to hospitalization for a capable, motivated family of a child who requires TNA.
Encourage the family to make the home life as normal as possible for the child within the limits imposed by the therapy. For example, having the infant or child at the table during mealtimes and including the child in family activities contribute to a normal family atmosphere. Activity restrictions often depend on the child’s illness, the type of VAD, and period of HPN (continuous versus intermittent), but the provider should promote normalization of all allowed activities (Fig. 28-11). Allow infants and toddlers to crawl and pull up to a standing position to promote optimum development. The length of tubing can be adjusted to accommodate such activities. Running the tubing under a one-piece clothing outfit and out the back often encourages ambulation. At times the nurse must give the parents permission to allow the child to play because they may be frightened by the tubing and VAD setup. Once the parents or caregivers becomes familiar with the usual complications of mobility, they are resourceful at adapting the clothing and home environment to allow the child to play with minimum restrictions. It is also important to make certain the child’s dental care is not neglected.

Fig. 28-11 Home total parenteral nutrition requires modifications in lifestyle and adjustments in activities for child and family.
The family is referred to community agencies that provide support and practical assistance. The Oley Foundation,* a nonprofit research and education organization, maintains a national registry of persons receiving HPN and publishes a bimonthly newsletter for consumers, families, clinicians, and home care services.
Key Points
• Water distribution and maintenance are determined by solutes, physical forces, internal control mechanisms, and boundary organs through which external exchanges occur.
• Infants are subject to fluid depletion because of their relatively greater surface area, their high rate of metabolism, and their immature kidney function.
• Management of fluid volume disturbances focuses on volume of body fluids, osmolality, hydrogen ion status, electrolyte deficits, and disturbances in mineral skeleton and body fluid equilibrium.
• Fluid disturbances experienced by children are dehydration, water intoxication, and edema.
• Dehydration may be classified as isotonic, hypotonic, or hypertonic.
• Parenteral fluid therapy is initiated to meet ongoing daily physiologic losses, restore previous deficits, and replace ongoing abnormal losses.
• Fluid gains or losses from the interstitial spaces depend on venous hydrostatic pressure, colloidal osmotic pressure, semipermeable capillary wall, tissue tension, and lymphatic flow.
• Edema formation is caused by increased venous pressure, capillary permeability, diminished plasma proteins, lymphatic obstruction, or decreased tissue tension.
• Disturbances in acid-base balance are respiratory acidosis, respiratory alkalosis, metabolic acidosis, and metabolic alkalosis.
• Respiratory acidosis may result from factors that depress the respiratory center, affect the lungs, or interfere with the bellows action of the chest wall.
• Respiratory alkalosis results primarily from CNS stimulation.
• Metabolic acidosis is a lowered plasma pH caused by any process that reduces base bicarbonate concentration or increases metabolic acid formation.
• Metabolic alkalosis is an elevated plasma pH that occurs when there is a reduction of hydrogen ion concentration or an excess of base bicarbonate.
• Nursing assessment of fluid and electrolyte disturbances entails observation of general appearance, vital signs, daily weights, I&O measurement, and review of relevant laboratory results.
• Long-term venous access is accomplished by intermittent IV devices; central venous catheters, including short-term (subclavian, femoral, and jugular), short-term to moderate-term (PICCs), and long-term (tunneled) catheters and ports; or implanted ports.
• PN provides for total nutritional needs when feeding via the gastrointestinal tract is impossible, inadequate, or hazardous.
• Before initiating HPN, the nurse assesses the parents’ ability to perform the procedure, existence of family support systems, availability of nearby pharmacies, and insurance coverage.
References
American Academy of Pediatrics. Pediatric nutrition handbook, ed 6. Elk Grove Village, Ill: The Academy; 2009.
Aschner, JL, Poland, RL. Sodium bicarbonate: basically useless therapy. Pediatrics. 2008;122(4):831–835.
Blaney, M, Shen, V, Kerner, JA, et al. Alteplase for the treatment of central venous catheter occlusion in children: results of a prospective, open-label, single-arm study (the Cathflo Activase Pediatric Study). J Vasc Interv Radiol. 2006;17(11 Pt 1):1745–1751.
Carson, SM. Chlorhexidine versus povidone-iodine for central venous catheter site care in children. J Pediatr Nurs. 2004;19(1):74–80.
Colomb, V, Dabbas-Tyan, M, Taupin, P, et al. Long-term outcome of children receiving home parenteral nutrition: a 20-year single-center experience in 302 patients. J Pediatr Gastroenterol Nutr. 2007;44(3):347–353.
Crosby, CT, Mares, A. Skin antisepsis: past, present, and future. J Vasc Access Devices. 2001;6(1):26–31.
Curley, MAQ, Moloney-Harmon, PA. Critical care nursing of infants and children, ed 2. Philadelphia: Saunders; 2001.
de Caen, AR, Reis, A, Bhutta, A. Vascular access and drug therapy in pediatric resuscitation. Pediatr Clin North Am. 2008;55(4):909–927.
DeCamp, LR, Byerly, JS, Doshi, N, et al. Use of antiemetic agents in acute gastroenteritis: a systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2008;162(9):858–865.
Doellman, D. Pharmacologic versus nonpharmacologic techniques in reducing venipuncture psychological trauma in pediatric patients. J Infusion Nurs. 2003;26(2):103–109.
Ellis, JA, Sharp, D, Newhook, K, et al. Selling comfort: a survey of interventions for needle procedures in a pediatric hospital. Pain Manage Nurs. 2004;5(4):144–152.
Emond, S. Dehydration in infants and young children. Ann Emerg Med. 2009;53(3):395–397.
Fann, B. Fluid and electrolyte balance in the pediatric patient. J Intraven Nurs. 1998;21(3):153–159.
Farion, KJ, Splinter, KL, Newhook, K, et al. The effect of vapocoolant spray on pain due to intravenous cannulation in children: a randomized controlled trial. CMAJ. 2008;179(1):31–36.
Fiorito, BA, Mirza, F, Doran, TM, et al. Intraosseous access in the setting of pediatric critical care transport. Pediatr Crit Care Med. 2005;6(1):50–53.
Fisher, AA, Deffenbaugh, C, Poole, R, et al. The use of alteplase for restoring patency to occluded central venous access devices in infants and children. J Infusion Nurs. 2004;27(3):171–174.
Ford, DM. Fluid, electrolyte, and acid-base disorders. In Hay WW, Levin MJ, Sondheimer JM, et al, eds.: Current diagnosis and treatment, ed 19, Philadelphia: McGraw Hill, 2009.
Freedman, SB, Adler, M, Seshadri, R, et al. Oral ondansetron for gastroenteritis in a pediatric emergency department. N Engl J Med. 2006;354(16):1698–1705.
Friedman, A. Fluid and electrolyte therapy: a primer. Pediatr Nephrol. 2009. [E-pub ahead of print May 15].
Gamulka, B, Mendoza, C, Connolly, B. Evaluation of a unique, nurse-inserted, peripherally inserted central catheter program. Pediatrics. 2005;115(6):1602–1606.
Gillies D, O’Riordan L, Wallen M, et al: Optimal timing for intravenous administration set replacement, Cochrane Database Syst Rev 19(4): CD003588, 2005.
Gillies, D, O’Riordan, L, Wallen, M, et al. Timing of intravenous administration set changes: a systematic review. Infect Control Hosp Epidemiol. 2004;25(3):240–250.
Gottrand, F, Staszewski, P, Colomb, V, et al. Satisfaction in different life domains in children receiving home parenteral nutrition and their families. J Pediatr. 2005;146(6):793–797.
Greenbaum, LA. Pathophysiology of body fluids and fluid therapy. In Behrman RE, Kliegman RM, Jenson HB, eds.: Nelson textbook of pediatrics, ed 18, Philadelphia: Saunders, 2007.
Hazinsky, MF, Zaritsky, AL, Nadkarni, VM, et al. PALS provider manual. Dallas: American Heart Association; 2002.
Howard, L, Ashley, C. Management of complications in patients receiving home parenteral nutrition. Gastroenterology. 2003;124(6):1651–1661.
Huether, SE. The cellular environment: fluids and electrolytes, acids and bases. In McCance KL, Huether SE, Brashers VL, et al, eds.: Pathophysiology: the biologic basis for disease in adults and children, ed 6, St Louis: Mosby, 2010.
Hwang, TL, Lue, MC, Chen, LL. Early use of cyclic TPN prevents further deteriorations of liver functions for the TPN patients with impaired liver function. Hepatogastoenterology. 2000;47(35):1347–1350.
Infusion Nurses Society. Policies and procedures for infusion nursing, ed 3. Norwood, Mass: The Society; 2006.
Jensen, AR, Goldin, AB, Koopmeiners, JS, et al. The association of cyclic parenteral nutrition and decreased incidence of cholestatic liver disease in patients with gastroschisis. J Pediatr Surg. 2009;44(1):183–189.
Jimenez, N, Bradford, H, Seidel, KD, et al. A comparison of a needle-free injection system for local anesthesia versus EMLA for intravenous catheter insertion in the pediatric patient. Anesth Analag. 2006;102(2):411–414.
Johnson, T, Sexton, E. Managing children and adolescents on parenteral nutrition: challenges for the nutritional support team. Proc Nutr Soc. 2006;65(3):217–221.
Kee, J, Paulanka, B. Handbook of fluid, electrolyte and acid-base imbalances. Albany, NY: Delmar; 2000.
Kerner, JA, Garcia-Careaga, MG, Fisher, AA, et al. Treatment of catheter occlusion in pediatric patients. J Parenter Enteral Nutr. 2006;30(Suppl 1):S73–S81.
Kline, AM. Pediatric catheter-related bloodstream infections: latest strategies to decrease risk. AACN Clin Issues. 2005;16(2):185–198.
Knue, M, Doellman, D, Rabin, K, et al. The efficacy and safety of blood sampling through peripherally inserted central catheters devices in children. J Infus Nurs. 2005;28(1):30–35.
Krein, SL, Hofer, TP, Kowalski, CP, et al. Use of central venous catheter–related bloodstream infection prevention practices by US hospitals. Mayo Clin Proc. 2007;82(6):672–678.
Lamagna, P, MacPhee, M. Phlebitis and infiltration: troubleshooting pediatric peripheral IVs. Nurse Week (Heartland ed). 2004;5(4):20. [26, 28].
Lee, OK, Johnston, L. A systematic review for effective management of central venous catheters and catheter sites in acute care paediatric patients. Worldviews Evid Based Nurs. 2005;2(1):4–13.
Linder, N, Prince, S, Barzilai, A, et al. Disinfection with 10% povidone-iodine versus 0.5% chlorhexidine gluconate in 70% isopropanol in the neonatal intensive care unit. Acta Paediatr. 2004;93(2):205–210.
Luhmann, J, Hurt, S, Shootman, M, et al. A comparison of buffered lidocaine versus Ela-Max before peripheral intravenous catheter insertions in children. Pediatrics. 2004;113(3 Pt 1):e217–e220.
Marini, MA, Giangregorio, M, Kraskinski, JC. Complying with the Occupational Safety and Health Administration’s bloodborne pathogen standard: implementing needleless systems and intravenous safety devices. Pediatr Emerg Care. 2004;20(3):209–214.
McCloskey, DJ. Catheter-related thrombosis in pediatrics. Pediatr Nurs. 2002;28(2):97–105.
Mermel, LA, Allon, M, Bouza, E, et al, Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009;49:1–45. available at www.cdc.gov/publiccomments/comments/guidelines-for-the-prevention-of-intravascular-catheter-related-infections/1862.ashx [(accessed March 8, 2010)].
Metheny, N. Fluid and electrolyte balance, ed 4. Philadelphia: Lippincott; 2000.
Mickler, PA. Neonatal and pediatric perspectives in PICC placement. J Infusion Nurs. 2008;31(5):282–285.
Milstone, AM, Passaretti, CL, Perl, TM. Chlorhexidine: expanding the armamentarium for infection control and prevention. Clin Infect Dis. 2008;46(2):274–281.
Morgan, LM, Thomas, DJ. Implementing evidence-based nursing practice in the pediatric intensive care unit. J Infus Nurs. 2007;30(2):105–112.
O’Grady, NP, Alexander, M, Dellinger, EP, et al. Guidelines for the prevention of intravascular catheter-related infections. MMWR. 2002;51(RR10):1–26.
Petit, J. Fostering a new era of vascular access device selections in neonates. Newborn Infant Nurs Rev. 2006;6(4):186–192.
Racadio, JM, Doellman, DA, Johnson, ND, et al. Pediatric peripherally inserted central catheters: complication rates related to catheter tip location. Pediatrics. 2001;107(2):e28.
Roslund, G, Hepps, TS, McQuillen, KK. The role of oral ondansetron in children with vomiting as a result of acute gastritis/gastroenteritis who have failed oral rehydration therapy: a randomized controlled trial. Ann Emerg Med. 2008;52(1):22–29.
Schwengel, DA, McGready, J, Berenholtz, SM, et al. Peripherally inserted central catheters: a randomized, controlled, prospective trial in pediatric surgical patients. Anesthesiol Analg. 2004;99(4):1038–1043.
Shen, V, Li, X, Murdock, M, et al. Recombinant tissue plasminogen activator (alteplase) for restoration of function to occluded central venous catheters in pediatric patients. J Pediatr Hematol Oncol. 2003;25(1):38–45.
Shulman, RJ, Phillips, S. Parenteral nutrition in infants and children. J Pediatr Gastroent Nutr. 2003;36(5):587–607.
Smith, MJ. Catheter-related bloodstream infections in children. Am J Infect Control. 36(10), 2008. [S173e1–e3].
Spandorfer, PR, Alessandrini, EA, Joffe, MD, et al. Oral versus intravenous rehydration of moderately dehydrated children: a randomized, controlled trial. Pediatrics. 2005;115(2):295–301.
Spanos, S, Booth, R, Koenig, H, et al. Jet injection of 1% buffered lidocaine versus topical ELA-Max for anesthesia before peripheral intravenous catheterization in children: a randomized controlled trial. Pediatr Emerg Care. 2008;24(8):511–515.
Steiner, MJ, DeWalt, D, Byerly, JS. Is this child dehydrated? JAMA. 2004;291(22):2746–2754.
Steiner, MJ, Nager, AL, Wang, VJ. Urine specific gravity and other urinary indices: inaccurate tests for dehydration. Pediatr Emerg Care. 2007;23(5):298–303.
Stevens, B, Taddio, A, Ohlsson, A, et al. The efficacy of sucrose for relieving procedural pain in neonates: a systematic review and meta-analysis. Acta Paediatr. 1997;86(8):837–842.
Taddio, A, Shah, V, Hancock, R, et al. Effectiveness of sucrose analgesia in newborns undergoing painful medical procedures. CMAJ. 2008;179(1):37–43.
Teitelbaum, D, Guenter, P, Howell, WH, et al. Definition of terms, style, and conventions used in ASPEN guidelines and standards. Nutr Clin Pract. 2005;20(2):281–285.
Thompson, DG. Utilizing an oral sucrose solution to minimize neonatal pain. JSPN. 2005;10(1):3–10.
Wade, G. Fluid problems in infants and children. In Kee JL, Paulanka BJ, Polek C, eds.: Handbook of fluid, electrolytes, and acid-base imbalance, ed 3, Clifton Park, NY: Delmar, Cengage Learning, 2010.
Weise, KL, Nahata, MC. EMLA for painful procedures in infants. J Pediatr Healthcare. 2005;19(1):42–47.
Whitby, M, McLaws, ML, Slater, K. Needlestick injuries in a major teaching hospital: the worthwhile effect of hospital-wide replacement of conventional hollow-bore needles. Am J Infect Control. 2008;36(3):180–186.
Wong, DL. Topical anesthetics: two products for pain relief during minor procedures. AJN. 2003;103(6):42–45.
Xanthakos, SA, Balistreri, WF. Liver disease associated with systemic disorders. In Kliegman RM, Behrman RE, Jenson HB, et al, eds.: Nelson textbook of pediatrics, ed 18, Philadelphia: Saunders, 2007.
Zempsky, WT. Pharmacologic approaches for reducing venous access pain in children. Pediatrics. 2008;122(Suppl 3):S140–S153.
*315 Norwood Park South, Norwood, MA 02062; 781-440-9408; www.ins1.org.
*214 Hun Memorial, MC-28, Albany Medical Center, Albany, NY 12208-3478; 800-776-6539 (in United States and Canada), 518-262-5079 (in other countries); www.oley.org.
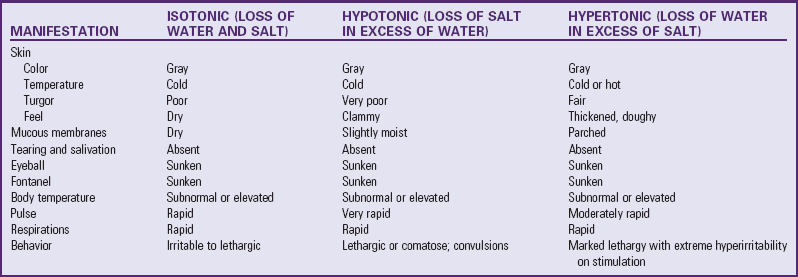
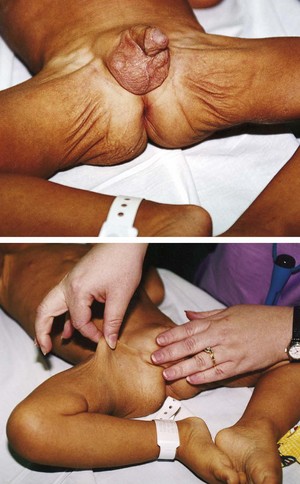
 tsp) table salt
tsp) table salt tsp) potassium chloride or potassium salt
tsp) potassium chloride or potassium salt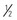 tsp) baking soda
tsp) baking soda
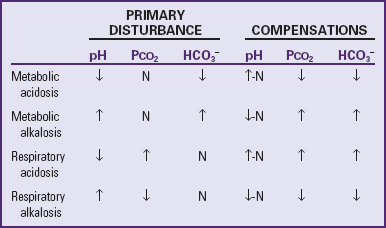
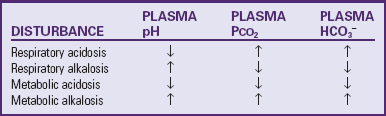
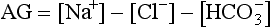
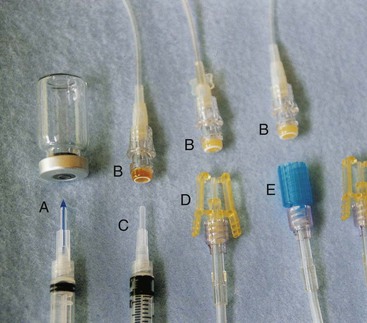
 to
to  inch) to ensure that both the metal stylet and catheter are inside the vein. Look closely at the intravenous (IV) catheter before inserting it and notice that the stylet tip is slightly longer than the catheter. It is necessary to have both pieces inside the vein before advancing the catheter. Holding the stylet steady, push the catheter off the stylet and into the vein until the catheter hub is situated against the skin at the insertion site. Activate safety mechanism if necessary (some safety catheters are passive and activate automatically), remove the stylet, and discard into sharps container. Apply pressure to catheter within the vein to prevent backflow of blood before attaching extension tubing.
inch) to ensure that both the metal stylet and catheter are inside the vein. Look closely at the intravenous (IV) catheter before inserting it and notice that the stylet tip is slightly longer than the catheter. It is necessary to have both pieces inside the vein before advancing the catheter. Holding the stylet steady, push the catheter off the stylet and into the vein until the catheter hub is situated against the skin at the insertion site. Activate safety mechanism if necessary (some safety catheters are passive and activate automatically), remove the stylet, and discard into sharps container. Apply pressure to catheter within the vein to prevent backflow of blood before attaching extension tubing. - to
- to  -inch strip of clear tape across the width of the transparent dressing and the catheter hub but avoid the insertion site. This will serve as an anchor tape strip, and all other tape will be affixed to this strip (tape-on-tape method). This strip will not compromise the transparent dressing properties or interfere with visual inspection of the catheter-skin insertion site.
-inch strip of clear tape across the width of the transparent dressing and the catheter hub but avoid the insertion site. This will serve as an anchor tape strip, and all other tape will be affixed to this strip (tape-on-tape method). This strip will not compromise the transparent dressing properties or interfere with visual inspection of the catheter-skin insertion site. inches of clear tape that is
inches of clear tape that is  to
to  inch wide, adhesive side up, to the underneath side of the catheter hub and junction securement device at their connection. Wrap the ends of the tape around the connections and meet on top to form a
inch wide, adhesive side up, to the underneath side of the catheter hub and junction securement device at their connection. Wrap the ends of the tape around the connections and meet on top to form a