Health Promotion of the Newborn and Family
Adjustment to Extrauterine Life
Nursing Care of the Newborn and Family
Initial Assessment: Apgar Scoring
Transitional Assessment: Periods of Reactivity
Assessment of Attachment Behaviors
Maintain a Stable Body Temperature
Protect from Infection and Injury
Promote Parent-Infant Bonding (Attachment)
http://evolve.elsevier.com/wong/ncic
Administration of Medication, Ch. 27
Ambiguous Genitalia, Ch. 11
Birth Injuries, Ch. 9
Blood Specimens, Ch. 27
Cardiac Development and Function: Embryologic Development, Ch. 34
Changes in Fluid Volume Related to Growth, Ch. 28
Congenital Heart Disease, Ch. 34
Dermatologic Problems in the Newborn, Ch. 9
Diaper Dermatitis, Ch. 13
Fluoride, Ch. 14
The High-Risk Newborn and Family, Ch. 10
Motor Vehicle Injuries, Chs. 12 and 14
Multiple Births, Ch. 3
Neonatal Pain, Ch. 7
Nutrition, Ch. 12
Physical Examination, Ch. 6
Sibling Rivalry, Ch. 14
The Skin, Ch. 18
Adjustment to Extrauterine Life
![]() The most profound physiologic change required of the newborn is transition from fetal or placental circulation to independent respiration. The loss of the placental connection means the loss of complete metabolic support, particularly the supply of oxygen and the removal of carbon dioxide. The normal stresses of labor and delivery cause alterations in placental gas exchange patterns, acid-base balance in the blood, and cardiovascular activity in the neonate. Factors that interfere with normal transition or increase fetal hypoxia, hypercapnia, and acidosis will affect the fetus’s adjustment to extrauterine life.
The most profound physiologic change required of the newborn is transition from fetal or placental circulation to independent respiration. The loss of the placental connection means the loss of complete metabolic support, particularly the supply of oxygen and the removal of carbon dioxide. The normal stresses of labor and delivery cause alterations in placental gas exchange patterns, acid-base balance in the blood, and cardiovascular activity in the neonate. Factors that interfere with normal transition or increase fetal hypoxia, hypercapnia, and acidosis will affect the fetus’s adjustment to extrauterine life.
Immediate Adjustments
The most critical and immediate physiologic change required of the newborn is the onset of breathing. The stimuli that help initiate respiration are primarily chemical and thermal. Chemical factors in the blood (low oxygen, high carbon dioxide, and low pH) initiate impulses that excite the respiratory center in the medulla. The primary thermal stimulus is the sudden chilling of the infant, who leaves a warm environment and enters a relatively cooler atmosphere. This abrupt change in temperature excites sensory impulses in the skin that are transmitted to the respiratory center. Tactile stimulation may assist in initiating respiration. Descent through the birth canal and normal handling during delivery, such as drying the skin, help stimulate respiration in uncompromised infants. Acceptable methods of tactile stimulation include slapping or flicking the soles of the feet or gently rubbing the newborn’s back, trunk, or extremities (American Academy of Pediatrics, 2006a). Slapping the newborn’s buttocks or back is a harmful technique and should not be done. Prolonged tactile stimulation, beyond one or two slaps or flicks to the soles of the feet or rubbing the back once or twice, can waste precious time in the event of respiratory difficulty and can cause additional damage in infants who have become hypoxemic before or during the birth process.
The initial entry of air into the lungs is opposed by the surface tension of the fluid that filled the fetal lungs and alveoli. Some fetal lung fluid is removed during the normal forces of labor and delivery. As the chest emerges from the birth canal, fluid is squeezed from the lungs through the nose and mouth. After complete emergence of the neonate’s chest, a brisk recoil of the thorax occurs. Air enters the upper airway to replace the lost fluid. In cesarean birth the chest is not compressed, and the newborn may need additional respiratory support or monitoring until remaining fetal lung fluid is absorbed by the pulmonary capillaries and lymphatic vessels.
In the alveoli the fluid’s surface tension is reduced by surfactant, a substance produced by the alveolar epithelium that coats the alveolar surface. Chapter 10 discusses the effect of surfactant in facilitating breathing in relation to respiratory distress syndrome.
Circulatory System
As important as the initiation of respiration are the circulatory changes that allow blood to flow through the lungs. These changes occur more gradually and are the result of pressure changes in the lungs, heart, and major vessels. The transition from fetal circulation to postnatal circulation involves the functional closure of the fetal shunts: the foramen ovale, the ductus arteriosus, and eventually the ductus venosus. (For a brief review of fetal circulation, see Chapter 34.)
Once the lungs are expanded, the inspired oxygen dilates the pulmonary vessels, which decreases pulmonary vascular resistance and consequently increases pulmonary blood flow. As the lungs receive blood, the pressure in the right atrium, right ventricle, and pulmonary arteries decreases. At the same time, there is a progressive rise in systemic vascular resistance and an increased volume of blood as a result of cord clamping. This increases the pressure in the left side of the heart. Because blood flows from an area of high pressure to one of low pressure, the circulation of blood through the fetal shunts is reversed (see Fig. 34-2).
The most important factor controlling ductal closure is the increased oxygen concentration of the blood. Secondary factors are the fall in endogenous prostaglandins and acidosis. The foramen ovale closes functionally at or soon after birth from compression of the two portions of the atrial septum. The ductus arteriosus is closed functionally by the fourth day in well neonates, but closure may be delayed in ill or preterm infants. Anatomic closure from deposition of fibrin and cell products takes considerably longer. Because of the reversible flow of blood through the ductus arteriosus during the early neonatal period, a functional murmur is occasionally heard.
Physiologic Status of Other Systems
Next to establishing respiration, heat regulation is most critical to the newborn’s survival. Although the newborn’s capacity for heat production is adequate, several factors predispose the newborn to excessive heat loss. First, the newborn’s large surface area relative to his or her weight facilitates heat loss to the environment. The newborn’s large body surface is partially compensated for by a usual position of flexion, which decreases the amount of surface area exposed to the environment.
The second factor that contributes to loss of body heat is the newborn’s thin layer of subcutaneous fat. Since core body temperature is approximately 1° F (~0.5° C) higher than surface body temperature, this temperature gradient (difference) causes a heat transfer from a higher to lower temperature.
A third factor is the newborn’s mechanism for producing heat. Unlike the child or adult, who can increase heat production through shivering, the chilled neonate cannot shiver but produces heat through nonshivering thermogenesis (NST). NST (or chemical thermogenesis) is produced by stimulating cellular respiration, resulting in increased need for oxygen and glucose (see Thermoregulation, Chapter 10). A thermogenic source unique to the full-term newborn is brown adipose tissue (BAT), or brown fat, which owes its name to its larger content of mitochondrial cytochromes. BAT has a greater capacity for heat production through intensified metabolic activity than does ordinary adipose tissue. Heat generated in the brown fat is distributed to other parts of the body by the blood, which is warmed as it flows through the layers of this tissue. Superficial deposits of brown fat are located between the scapulae, around the neck, in the axillae, and behind the sternum. Deeper layers surround the kidneys, trachea, esophagus, some major arteries, and adrenals. The location of the brown fat may explain why the nape of the neck often feels warmer than the rest of the body. Brown fat can affect the accuracy of axillary temperature measurement (see p. 242).
Because of these factors predisposing infants to loss of body heat, it is essential that newly born infants are quickly dried and either provided with warm, dry blankets or placed skin-to-skin with their mothers after delivery.
Although concern is usually for newborns’ ability to conserve heat, they also can have difficulty dissipating heat in an overheated environment, increasing the risk of hyperthermia.
Hematopoietic System
The blood volume of the newborn depends largely on the amount of blood transferred via the placenta before clamping of the cord. The blood volume of the full-term infant is about 80 to 85 ml/kg of body weight. Immediately after birth the total blood volume averages 300 ml, but, depending on how long the infant is attached to the placenta, as much as 100 ml can be added to the blood volume. Common laboratory test blood values for the newborn are listed in Appendix C.
Fluid and Electrolyte Balance
Changes occur in the total body water volume, extracellular fluid volume, and intracellular fluid volume during the transition from fetal to postnatal life. The fetus is composed almost entirely of water early in gestation and at term is 73% fluid, compared with 58% in the adult. The fetus has more extracellular fluid than intracellular fluid, but this shifts progressively throughout postnatal life, probably because of the growth of cells at the expense of extracellular fluid. The infant has a proportionately higher ratio of extracellular fluid than the adult and consequently has a higher level of total body sodium and chloride and a lower level of potassium, magnesium, and phosphate (see Chapter 28).
An important aspect of fluid balance is its relationship to other systems. The rate of fluid exchange is seven times greater in the infant than in the adult, and the infant’s rate of metabolism is twice as great in relation to body weight. As a result, twice as much acid is formed, leading to more rapid development of acidemia. In addition, the immature kidneys cannot sufficiently concentrate urine to conserve body water. These three factors make the infant more prone to dehydration, acidosis, and overhydration.
Gastrointestinal System
The newborn’s ability to digest, absorb, and metabolize food is adequate but limited in certain functions. Enzymes are available to catalyze proteins and simple carbohydrates (monosaccharides and disaccharides), but deficient production of pancreatic amylase impairs utilization of complex carbohydrates (polysaccharides). A deficiency of pancreatic lipase limits the absorption of fats, especially with ingestion of foods that have high saturated fatty acid content, such as cow’s milk. Human milk, despite its high fat content, is easy to digest and absorb because it contains enzymes such as lipase, which assist in digestion.
The liver is the most immature of the gastrointestinal organs. The activity of the enzyme glucuronyl transferase is reduced, affecting the conjugation of bilirubin with glucuronic acid, which contributes to physiologic jaundice of the newborn. The liver is also deficient in forming plasma proteins, which likely plays a role in the edema usually seen at birth. Prothrombin and other coagulation factors are also low. The liver stores less glycogen at birth than later in life. Consequently, the newborn is prone to hypoglycemia, which may be prevented by early and effective feeding, especially breast-feeding.
Some salivary glands are functioning at birth, but the majority do not begin to secrete saliva until about age 2 to 3 months, when drooling is common. The stomach capacity is limited to about 90 ml in an average-sized full-term infant (3.4 kg [7.5 lb]); thus the infant requires frequent small feedings. Newborns who breast-feed usually have more frequent feedings and more frequent stools than infants who receive formula.
The infant’s intestine is longer in relation to body size than that in the adult. Therefore it has a proportionately larger number of secretory glands and a larger surface area for absorption compared with the adult’s intestine. Rapid peristaltic waves and simultaneous nonperistaltic waves occur along the entire intestine. These waves, called the migrating motor complex (MMC), propel nutrients forward. The relative immaturity of the MMC, combined with decreased lower esophageal sphincter (LES) pressure, inappropriate relaxation of the LES, and delayed gastric emptying, makes regurgitation a common occurrence. Progressive changes in the stooling pattern indicate a properly functioning gastrointestinal tract (Box 8-1).
Renal System
All structural components are present in the renal system, but the kidney has a functional deficiency in its ability to concentrate urine and to cope with fluid and electrolyte fluctuations, such as dehydration or a concentrated solute load.
Total volume of urinary output per 24 hours is about 200 to 300 ml by the end of the first week. The bladder involuntarily empties when stretched by a volume of 15 ml, resulting in as many as 20 voidings per day. The first voiding should occur within 24 hours. The urine is colorless and odorless and has a specific gravity of approximately 1.020.
Integumentary System
At birth all the structures within the skin are present, but many of the functions of the integument are immature. The two layers of the skin, the epidermis and dermis, are loosely bound to each other and are very thin. Rete pegs, which later in life anchor the epidermis to the dermis, are not developed. Slight friction across the epidermis, such as from rapid removal of tape, can cause separation of these layers and blister formation or loss of the epidermis. In full-term infants the transitional zone between the cornified and living layers of the epidermis is effective in preventing fluid from reaching the skin surface.
The sebaceous glands are active late in fetal life and in early infancy because of high levels of maternal androgens. They are most densely located on the scalp, face, and genitalia and produce the grayish white, greasy vernix caseosa that covers the infant at birth. Plugging of the sebaceous glands causes milia.
The eccrine glands, which produce sweat in response to heat or emotional stimuli, are functional at birth, and by 3 weeks of age palmar sweating on crying reaches levels equivalent to those of anxious adults. Observing palmar sweating is helpful in assessing pain. The eccrine glands produce sweat in response to higher temperatures than those required in adults, and the retention of sweat may result in milia. The apocrine glands, sweat glands that develop as attachments to hair follicles, remain small and nonfunctional until puberty.
The growth phases of hair follicles usually occur simultaneously at birth. During the first few months the synchrony between hair loss and regrowth is disrupted, and there may be overgrowth of hair or temporary alopecia. Boys’ hair grows faster than girls’ hair, and in both sexes scalp hair growth is slower at the crown.
Because the amount of melanin is low at birth, newborns are lighter skinned than they will be as children. Consequently, infants are more susceptible to the harmful effects of ultraviolet light such as the sun.
Musculoskeletal System
At birth the skeletal system contains larger amounts of cartilage than ossified bone, although the process of ossification is fairly rapid during the first year. The nose, for example, is predominantly cartilage at birth and is frequently flattened by the force of delivery. The six skull bones are relatively soft and not yet joined. The sinuses are incompletely formed as well.
Unlike the skeletal system, the muscular system is almost completely formed at birth. Hypertrophy, rather than hyperplasia, of cells causes the growth in the size of muscular tissue.
Defenses Against Infection
The infant is born with several defenses against infection. The first line of defense is the skin and mucous membranes, which protect the body from invading organisms. The second line of defense is the cellular elements of the immunologic system, which produces several types of cells capable of attacking a pathogen. The neutrophils and monocytes are phagocytes, cells that engulf, ingest, and destroy foreign agents. Eosinophils also probably have a phagocytic property because in the presence of foreign protein they increase in number. The lymphocytes (T and B cells) are capable of being converted to other cell types, such as monocytes and antibodies. Although the phagocytic properties of the blood are present in the infant, the tissues’ inflammatory response to localize an infection is immature.
The third line of defense is the formation of specific antibodies to an antigen. This process requires exposure to various foreign agents for antibody production to occur. Infants are generally not capable of producing their own immunoglobulins until the beginning of the second month of life, but they receive considerable passive immunity in the form of immunoglobulin G (IgG) from the maternal circulation and from human milk (see p. 260). They are protected against most major childhood diseases, including diphtheria, measles, poliomyelitis, and rubella, for about 3 months, provided that the mother has developed antibodies to these illnesses.
Endocrine System
Ordinarily, the newborn’s endocrine system is adequately developed, but its functions are immature. For example, the posterior lobe of the pituitary gland produces limited quantities of antidiuretic hormone, or vasopressin, which inhibits diuresis. This renders the newborn highly susceptible to dehydration.
The effect of maternal sex hormones is particularly evident in the newborn. The labia are hypertrophied, and the breasts in both sexes may be engorged and secrete milk (witch’s milk) during the first few days of life to as long as 2 months of age. Female newborns may have pseudomenstruation (more often seen as a milky secretion rather than actual blood) from a sudden drop in progesterone and estrogen levels.
Neurologic System
At birth the nervous system is incompletely integrated but sufficiently developed to sustain extrauterine life. Most neurologic functions are primitive reflexes. The autonomic nervous system is crucial during transition because it stimulates initial respirations, helps maintain acid-base balance, and partially regulates temperature control.
Myelination of the nervous system follows the cephalocaudal-proximodistal (head-to-toe–center-to-periphery) laws of development and is closely related to the observed mastery of fine and gross motor skills. Myelin is necessary for rapid and efficient transmission of some, but not all, nerve impulses along the neural pathway. Tracts that develop myelin earliest are the sensory, cerebellar, and extrapyramidal. This accounts for the acute senses of taste, smell, and hearing and the perception of pain in the newborn. All cranial nerves are myelinated except the optic and olfactory nerves.
Sensory Functions
The newborn’s sensory functions are remarkably well developed and have a significant effect on growth and development, including the attachment process.
Vision: At birth the eye is structurally incomplete. The fovea centralis is not yet completely differentiated from the macula. The ciliary muscles are also immature, limiting the eyes’ ability to accommodate and fixate on an object for any length of time. The pupils react to light, the blink reflex is responsive to minimum stimulus, and the corneal reflex is activated by a light touch. Tear glands usually do not begin to function until 2 to 4 weeks of age.
The newborn has the ability to momentarily fixate on a bright or moving object that is within 20 cm (8 inches) and in the midline of the visual field. In fact, the infant’s ability to fixate or coordinate movement is greater during the first hour of life than during the succeeding several days. Visual acuity is reported to be between 20/100 and 20/400, depending on the vision measurement techniques (see Table 6-9).
The infant also demonstrates visual preferences: medium colors (yellow, green, pink) over dim or bright colors (red, orange, blue); black-and-white contrasting patterns, especially geometric shapes and checkerboards; large objects with medium complexity rather than small, complex objects; and reflecting objects over dull ones.
Hearing: Once the amniotic fluid has drained from the ears, the infant probably has auditory acuity similar to that of an adult. The newborn is able to detect a loud sound of about 90 dB and reacts with a startle (Moro) reflex. The newborn’s response to sounds of low frequency and high frequency differs; the former, such as a heartbeat, metronome, or lullaby, tends to decrease an infant’s motor activity and crying, whereas the latter elicits an alerting reaction.
Infants have an early sensitivity to the sound of human voices and to specific speech sounds. For example, infants younger than 3 days of age can distinguish their mother’s voice from that of other females. As early as 5 days, newborns can differentiate between stories read by their mother’s voice (in utero) versus stories read by another woman’s voice after birth.
The internal and middle ear structures are large at birth, but the external canal is small. The mastoid process and the bony part of the external canal have not yet developed. Consequently, the tympanic membrane and facial nerve are close to the surface and can be easily damaged.
Smell: Newborns react to strong odors such as alcohol or vinegar by turning their heads away. Breast-fed infants are able to smell breast milk and will cry for their mothers when the breasts are leaking. Infants are also able to differentiate their mother’s breast milk from that of other women by scent alone. Many believe maternal odors influence the attachment process and successful breast-feeding. Unnecessary routine washing of the breasts may interfere with establishment of early breast-feeding.
Taste: The newborn can distinguish between tastes, and various types of solutions elicit differing facial reflexes. A tasteless solution elicits no facial expression; a sweet solution elicits an eager suck and a look of satisfaction; a sour solution causes the usual puckering of the lips; and a bitter liquid produces an angry, upset expression.
Touch: The newborn perceives tactile sensation in any part of the body, although the face (especially the mouth), hands, and soles of the feet seem to be most sensitive. Sufficient evidence now shows that touch and motion are essential components in the attachment process and in normal growth and development. Gentle patting of the back or rubbing of the abdomen usually elicits a calming response from the infant. However, painful stimuli, such as a pinprick, elicit an upset response.
Nursing Care of the Newborn and Family
![]() The newborn requires thorough, skilled observation to ensure a satisfactory adjustment to extrauterine life. Physical assessment after delivery is divided into four phases: (1) the initial assessment, which includes the Apgar scoring system; (2) transitional assessment during periods of reactivity; (3) assessment of gestational age; and (4) a comprehensive, systematic physical examination. In addition, the nurse must be aware of those behaviors that signal successful attachment between the infant and parents. Awareness of the expected normal findings during each assessment process helps the nurse recognize any deviation that may prevent the infant from progressing uneventfully through the early postnatal period. With shorter hospital stays, accomplishing a thorough newborn assessment and comprehensive parent teaching may be a challenge (see p. 271).
The newborn requires thorough, skilled observation to ensure a satisfactory adjustment to extrauterine life. Physical assessment after delivery is divided into four phases: (1) the initial assessment, which includes the Apgar scoring system; (2) transitional assessment during periods of reactivity; (3) assessment of gestational age; and (4) a comprehensive, systematic physical examination. In addition, the nurse must be aware of those behaviors that signal successful attachment between the infant and parents. Awareness of the expected normal findings during each assessment process helps the nurse recognize any deviation that may prevent the infant from progressing uneventfully through the early postnatal period. With shorter hospital stays, accomplishing a thorough newborn assessment and comprehensive parent teaching may be a challenge (see p. 271).
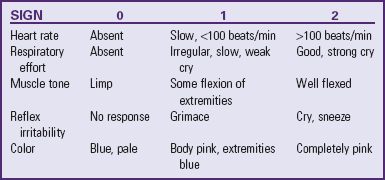
Initial Assessment: Apgar Scoring
The most frequently used method to assess the newborn’s immediate adjustment to extrauterine life is the Apgar scoring system (Stoll, 2007). The score is based on observation of heart rate, respiratory effort, muscle tone, reflex irritability, and color (Table 8-1). Each item is given a score of 0, 1, or 2. Evaluations of all five categories are made 1 and 5 minutes after birth and are repeated every 5 minutes until the infant’s condition stabilizes. Total scores of 0 to 3 represent severe distress, scores of 4 to 6 signify moderate difficulty, and scores of 7 to 10 indicate absence of difficulty in adjusting to extrauterine life. Many healthy newborns do not achieve a score of 10 because the body is not completely pink. The degree of physiologic immaturity affects the Apgar score. For example, a healthy preterm infant may receive a low score due to low tone and reduced reflex irritability. Infection, congenital anomalies, maternal sedation or analgesia, hypovolemia, and neuromuscular disorders also affect the Apgar score.
The Apgar score reflects the infant’s general condition at 1 and 5 minutes based on the five parameters described above. The Apgar score is not a tool, however, that stands on its own to either interpret past events or predict future events linked to the infant’s neurologic or physical status. The Apgar score is not used to determine the newborn’s need for resuscitation at birth; when necessary, resuscitative efforts should begin before the 1-minute Apgar score is obtained (American Academy of Pediatrics, 2006b).
Transitional Assessment: Periods of Reactivity
The newborn exhibits behavioral and physiologic characteristics that can at first appear to be signs of stress. During a newborn’s initial 24 hours, changes in heart rate, respiration, motor activity, color, mucus production, and bowel activity occur in an orderly, predictable sequence, which is normal and indicates lack of stress. Distressed infants also progress through these stages but at a slower rate.
For 6 to 8 hours after birth the newborn is in the first period of reactivity. During the first 30 minutes the infant is alert, cries vigorously, may suck his or her fingers or fist, and appears interested in the environment. At this time the neonate’s eyes are usually open; thus this is an excellent opportunity for mother, father, and child to see each other. Because the healthy, full-term newborn has a vigorous suck reflex, this is an opportune time to begin breast-feeding. The newborn usually grasps the nipple quickly, satisfying both mother and child. This is particularly important to remember, because it is likely that after this initial highly active state the infant may be sleepy and uninterested in sucking. Physiologically the respiratory rate can be as high as 80 breaths/min, crackles may be heard, heart rate may reach 180 beats/min, bowel sounds are active, mucus secretions are increased, and temperature may decrease slightly.
After this initial stage of alertness and activity, the infant enters the second stage of the first reactive period, which generally lasts 2 to 4 hours. Heart and respiratory rates decrease, temperature continues to fall, mucus production decreases, and urine or stool is usually not passed. The infant is in a state of sleep and relative calm. Any attempt at stimulation usually elicits a minimal response. Because of the decrease in body temperature, avoid undressing or bathing the infant during this time.
The second period of reactivity begins when the infant wakes from this deep sleep; it lasts about 2 to 5 hours and provides another excellent opportunity for child and parents to interact. The infant is again alert and responsive, heart and respiratory rates increase, the gag reflex is active, gastric and respiratory secretions are increased, and passage of meconium commonly occurs. This period is usually over when the amount of respiratory mucus has decreased. After this stage is a period of stabilization of physiologic systems and a vacillating pattern of sleep and activity.
Behavioral Assessment
An important area of assessment is observation of behavior. Infants’ behavior helps shape their environment, and their ability to react to various stimuli affects how others relate to them. The principal areas of behavior for newborns are sleep, wakefulness, and activity such as crying.
One method of systematically assessing the infant’s behavior is use of the Brazelton Neonatal Behavioral Assessment Scale (BNBAS) (Brazelton and Nugent, 1996). The BNBAS is an interactive examination that assesses the infant’s response to 28 items organized in clusters (Box 8-2). It is generally used as a research or diagnostic tool and requires special training.
In addition to its use as an initial and ongoing tool to assess neurologic and behavioral responses, the scale can be used to assess initial parent-child relationships; to help identify caregivers who may benefit from a role model; and to guide parents, helping them focus on their infant’s individuality and develop a deeper attachment. Studies have demonstrated that, by showing parents the unique characteristics of their infant, health care providers can enable them to develop a more positive perception of the infant, with increased interaction between infant and parents.
Patterns of Sleep and Activity: Newborns begin life with a systematic schedule of sleep and activity that is initially evident during the periods of reactivity. For the next 2 or 3 days most infants sleep almost constantly to recover from the exhausting birth process.
Infants have six distinct sleep-wake states, which represent a particular form of neural control (Table 8-2). As gestational and postconceptional maturity increases, each state becomes more precisely defined according to the behaviors observed. State is defined as a “group of characteristics that regularly occur together” (Blackburn, 2007); these include body activity, eye and facial movements, respiratory pattern, and response to internal and external stimuli. The six sleep-wake states are quiet (deep) sleep, active (light) sleep, drowsy, awake (quiet), active alert, and crying. Infants respond to internal and external environmental factors by controlling sensory input and regulating the sleep-wake states; the ability to make smooth transitions between states is called state modulation. The ability to regulate sleep-wake states is essential in the infant’s neurobehavioral development. The more immature the infant, the less he or she is able to cope with factors, external or internal, that affect the sleep-wake patterns.
TABLE 8-2
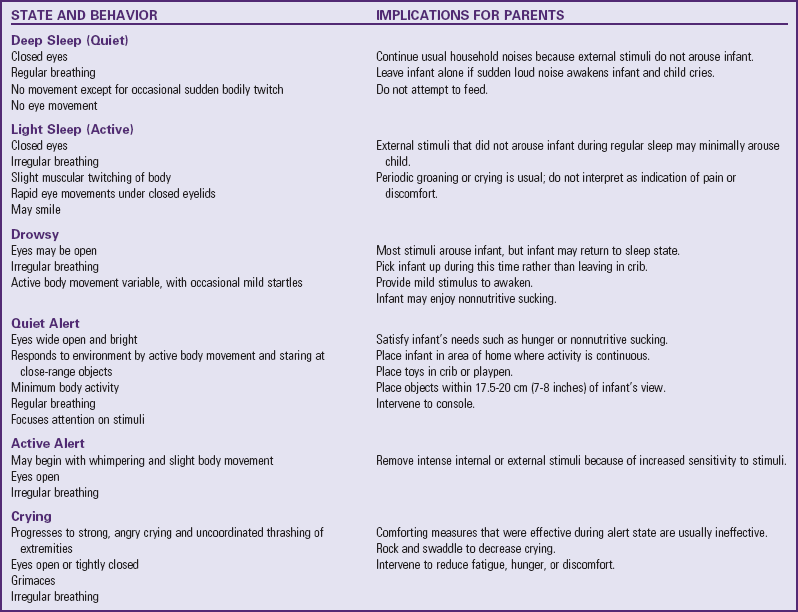
Portions adapted from Blackburn S, Loper DL: Maternal, fetal, and neonatal physiology: a clinical perspective, Philadelphia, 1992, Saunders.
Recognition and knowledge of sleep-wake states are important in planning nursing care. It is also important for nurses to help parents and caregivers understand the significance of the infant’s behavioral responses to daily caregiving and how these states can be altered. A classic example is the newborn who feeds vigorously in the active alert state but not in the deep sleep state. The neurologic assessment of a newborn will differ significantly in these two states. Newborns typically spend as much as 16 to 18 hours a day sleeping and do not necessarily follow a pattern of light-dark diurnal rhythm. With increasing age, sleep-wake states change, with increasing amounts of time spent in awake alert states and decreasing amounts in sleep time. Approximately 50% of total sleep time is spent in irregular or rapid eye movement sleep.
Cry: The newborn should begin extrauterine life with a strong, lusty cry. The sounds produced by crying can be described as hunger, anger, pain, and “bid for attention” cries. Discomfort (pain) sounds initially consist of gasps and cries in which the consonant H is clearly distinguishable. The duration of crying in each infant varies as much as the duration of sleep. Some newborns may cry for as little as 5 minutes or as much as 2 hours or more per day.
Variations in the initial cry can indicate underlying abnormalities. A weak, groaning cry or grunting during expiration usually indicates a respiratory disturbance. Absent, weak, or constant crying may suggest a pathologic state such as tension pneumothorax or perinatal asphyxia. Crying status alone, however, is not a diagnostic tool.
Assessment of Attachment Behaviors
One of the most important areas of assessment is careful observation of those behaviors thought to indicate the formation of emotional bonds between the newborn and family, especially the mother. Although bonding and attachment are sometimes referred to as separate phenomena, with bonding representing the development of emotional ties from parent to infant and attachment representing the emotional ties from infant to parent, in this discussion and the one on p. 268, the terms are used interchangeably to denote both processes.
Unlike physical assessment of the neonate, which follows concrete guidelines, assessment of parent-child attachment requires much more skill in terms of observation and interviewing. The assessment process is even more challenging with short hospital stays for mothers and their newborn infants. Rooming-in of mother and infant and visits by father, siblings, and grandparents facilitate recognition of behaviors that demonstrate positive or negative attachment. Guidelines for assessment of bonding behaviors are in the Nursing Care Guidelines box.
Talking to the parents uncovers many variables that can affect the development of attachment and parenting. (See also Child Maltreatment, Chapter 16.) What expectations do they have for this child? In other words, how similar are their predictions of the fantasy child and their realizations about the real child? Encourage them to talk about their relationship with their own parents, since the type of parenting that parents received as children influences their childrearing practices. Is this a planned birth? How do they see the addition of a dependent family member affecting their lifestyle? What arrangements have they made for such changes in lifestyle? What support system or significant others are available for assistance? What are their views regarding childrearing?
The labor process significantly affects the immediate attachment of mothers to their newborn children. Factors such as a long labor, feeling tired or “drugged” after delivery, and problems with breast-feeding may delay the development of initial positive feelings toward the newborn.
During pregnancy, and often even before conception, parents develop an image of the “ideal or fantasy infant.” The unborn child has an imagined appearance, pattern of behavior, expected accomplishments, and predetermined effect on the family’s lifestyle. At birth the fantasy infant becomes the real infant. How closely the dream child resembles the real child influences the bonding process. Assessing such expectations during pregnancy and at the time of the infant’s birth allows identification of discrepancies in the parents’ fantasy versus the real child.
The Neonatal Perception Inventories (NPI) (Broussard, 1979) assess the mother’s perception of her real infant compared with her image of an “average” infant. It has been hypothesized that, for optimum mothering to occur, the mother needs to see her infant as better than an “average” baby. Mothers who do not rate their infants as better than average may be at risk for developing parenting abilities that fail to meet the infant’s needs. The NPI II (completed 4 weeks after delivery) accurately predicted later childhood adjustment problems, whereas the NPI I (completed 1 or 2 days after the infant’s birth) did not (Broussard, 1976). In follow-up studies over a 19-year period, infants who were negatively perceived had the greatest risk for developing behavioral and emotional disorders (Broussard, 1984).
Because attachment involves a mutually reciprocal interchange, observing the interaction between parent and infant is important. An excellent opportunity exists during feeding. A useful instrument for systematically describing the parent’s and infant’s behaviors is the Feeding Scale developed by the Nursing Child Assessment Satellite Training (NCAST) program (Barnard, 1994). It consists of 76 behavioral items; 50 items describe the parent’s behavior regarding (1) sensitivity to cues, (2) response to child’s distress, (3) social-emotional growth fostering, and (4) cognitive growth fostering. Twenty-six items focus on the child’s behavior in terms of (1) clarity of cues and (2) responsiveness to parent. The results can be shared with parents to encourage discussion of feelings about the infant and to highlight behaviors of the couple that foster successful interaction. The Feeding Scale is appropriate for use with infants during the first year. Training to become a certified tester is available through the NCAST program.*
Clinical Assessment of Gestational Age
Assessment of gestational age is important because perinatal morbidity and mortality are related to gestational age and birth weight. A frequently used method is the Simplified Assessment of Gestational Age by Ballard, Novack, and Driver (1979) (Fig. 8-1, A). The Ballard scale, an abbreviated version of the Dubowitz scale (Dubowitz and Dubowitz, 1977), measures gestational ages of infants between 35 and 42 weeks. It assesses six external physical and six neuromuscular signs. Each sign has a number score, and the cumulative score correlates with a maturity rating of 26 to 44 weeks of gestation.
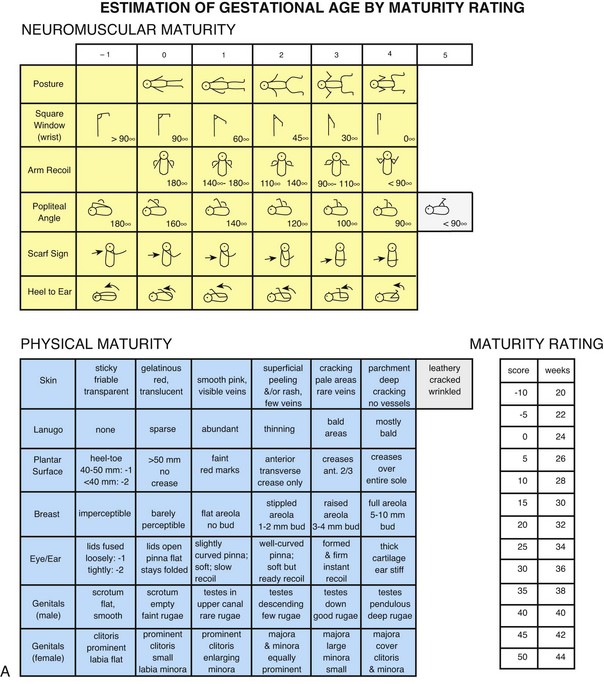
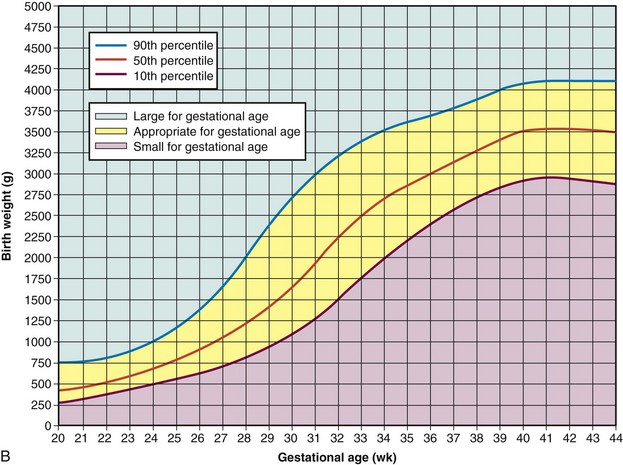
Fig. 8-1 A, Ballard scale for newborn maturity rating. Expanded scale includes extremely premature infants and has been refined to improve accuracy in more mature infants. (A, From Ballard JL, Khoury JC, Wedig K, et al: New Ballard score expanded to include extremely premature infants, J Pediatr 119:417, 1991.) B, Intrauterine growth: birth weight percentiles based on live single births at gestational ages 20 to 44 weeks. (B, Data from Alexander GR, Himes JH, Kaufman RB, et al: A United States national reference for fetal growth, Obstet Gynecol 87(2):163-168, 1996.)
The New Ballard Scale, a revision of the original scale, can be used with newborns as young as 20 weeks of gestation (Ballard, Khoury, Wedig, et al, 1991). The tool has the same physical and neuromuscular sections but includes scores that reflect signs of extremely premature infants, such as fused eyelids; imperceptible breast tissue; sticky, friable, transparent skin; no lanugo; and square-window (flexion of wrist) angle of greater than 90 degrees (see Fig. 8-1, A, and a description of the tests in Box 8-3). The examination of infants with a gestational age of 26 weeks or less should be performed at a postnatal age of less than 12 hours. For infants with a gestational age of at least 26 weeks, the examination can be performed up to 96 hours after birth. To ensure accuracy, it is recommended that the initial examination be performed within the first 48 hours of life. In a study of preterm infants ranging from 29 to 35 weeks at birth, Ballard scores completed 7 days after birth were found to either overestimate or underestimate gestational age by up to 2 weeks (Sasidharan, Dutta, and Narang, 2009).
The New Ballard Scale overestimates gestational age by 2 to 4 days in infants younger than 37 weeks of gestation, especially at gestational ages of 32 to 37 weeks (Ballard, Khoury, Wedig, et al, 1991). In one study, the Ballard scale was shown to overestimate gestational age of infants less than 28 weeks by as much as 1.3 to 3.3 weeks (Donovan, Tyson, Ehrenkranz, et al, 1999), so other indices of gestational age must also be used in this age-group.
Trotter (2009) indicates that the optimal timing for gestational age assessment in extremely preterm neonates has not been determined primarily because of neuromuscular adjustments following birth; in addition, conditions such as asphyxia or maternal medications administered during delivery may alter neuromuscular function. One suggested alternative to an inconclusive neuromuscular evaluation is to assess the physical criteria, multiply by a factor of 2, and assign a gestational age based on the score obtained with this method (Trotter, 2009).
Weight Related to Gestational Age: The infant’s weight at birth also correlates with the incidence of perinatal morbidity and mortality. Birth weight alone, however, is a poor indicator of gestational age and fetal maturity. Maturity implies functional capacity—the degree to which the neonate’s organ systems are able to adapt to the requirements of extrauterine life. Therefore gestational age is more closely related to fetal maturity than is birth weight. Because heredity influences size at birth, it is important to note the sizes of other family members as part of the assessment process.
Intrauterine growth curves developed by Battaglia and Lubchenco (1967) have been used to classify infants according to birth weight and gestational age. Since that time, other intrauterine growth charts have emerged to reflect a more heterogeneous sample population than previously described (Cunningham, Gant, Leveno, et al, 2001). The primary intrauterine growth charts that provide national reference data include the work of Alexander, Himes, Kaufman, and colleagues (1996), which represents more than 3.1 million live births in the United States (see Fig. 8-1, B); Thomas, Peabody, Turnier, and colleagues (2000); and Arbuckle, Wilkins, and Sherman (1993) and Kramer, Platt, Wen, and colleagues (2001), which represent intrauterine growth among the Canadian population. Thomas, Peabody, Turnier, and colleagues (2000) concluded that intrauterine growth measured by head circumference, birth weight, and length varies according to race and gender. These researchers also found that altitude did not seem to significantly affect birth weight as other authors have suggested. It is recommended that the reader access and use the most current intrauterine growth chart specific to the population being evaluated. Classification of infants at birth by both weight and gestational age provides a satisfactory method for predicting mortality risks and providing guidelines for management of the neonate.
The infant’s birth weight, length, and head circumference are plotted on standardized graphs that identify normal values for gestational age. The infant whose weight is appropriate for gestational age (AGA) (between the 10th and 90th percentiles) can be presumed to have grown at a normal rate regardless of the length of gestation—preterm, term, or postterm. The infant who is large for gestational age (LGA) (>90th percentile) can be presumed to have grown at an accelerated rate during fetal life; the small-for-gestational-age (SGA) infant (<10th percentile) can be presumed to have grown at a restricted rate during intrauterine life. When gestational age is determined according to the Ballard scale, the newborn will fall into one of the following nine possible categories for birth weight and gestational age: AGA—term, preterm, postterm; SGA—term, preterm, postterm; or LGA—term, preterm, postterm. Fig. 8-2 illustrates the disparity between birth weights of three preterm infants of the same gestational age. Birth weight influences mortality: the lower the birth weight, the higher the mortality. The same is true for gestational age: the lower the gestational age, the higher the mortality.
Physical Assessment
The discussion of physical examination focuses on normal findings, variations from the norm that require little or no intervention, and specific potential danger signs that require more careful observation. General guidelines for conducting a physical examination are in the Nursing Care Guidelines box. Table 8-3 summarizes the physical examination of the newborn. (See Chapter 6 for further discussion of examination techniques.)
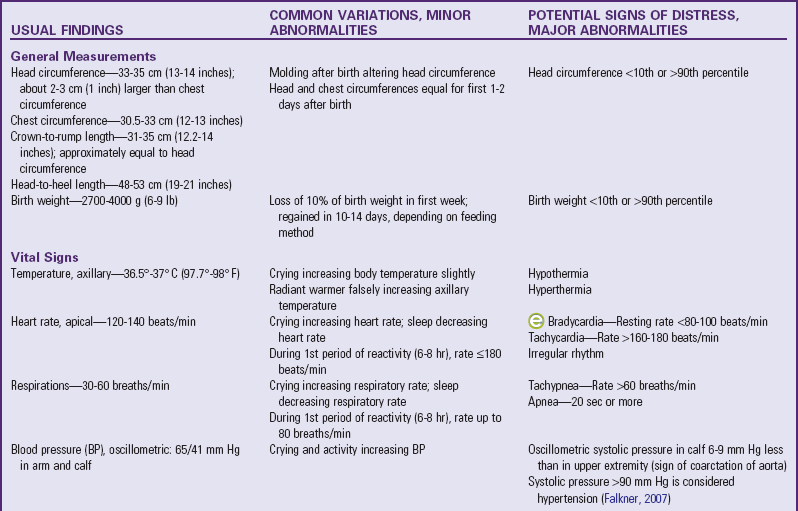
General Measurements: Several important measurements of the newborn are significant when compared with each other, as well as when recorded over time on a graph. For the full-term infant, average head circumference is between 33 and 35 cm (13 and 14 inches). Head circumference may be somewhat less than that immediately after birth because of the molding process that occurs during vaginal delivery. Usually by the second or third day the normal size and contour of the skull have replaced the molded one.
Chest circumference is 30.5 to 33 cm (12 to 13 inches). Head circumference is usually about 2 to 3 cm (about 1 inch) greater than chest circumference. Because of the molding of the head during delivery, these measurements may initially appear equal. However, if the head is significantly smaller than the chest, microcephaly or premature closure of the sutures (craniosynostosis) is a possibility. If the head is more than 4 cm (1.6 inches) larger than the chest in circumference and this relationship remains constant or increases over several days, then hydrocephalus must be considered. Other causes of increased head circumference are caput succedaneum, cephalhematoma, subgaleal hemorrhage, and subdural hematoma. Prematurity and intrauterine malnutrition also can cause the head measurement to be significantly larger than the chest circumference, but this is because of decreased chest size, not increased head circumference.
Head circumference may also be compared with crown-to-rump length, or sitting height. Crown-to-rump measurements are usually 31 to 35 cm (12.5 to 14 inches) and are approximately equal to head circumference. The relationship of the head and crown-to-rump measurements is more reliable than that of the head and chest. Severn (1994) noted that neonatal head circumference and crown-to-rump length provide a more accurate means for identifying infants at risk; head circumference was shown to be equal to or up to 1 cm (0.4 inch) more than crown-to-rump length in 62% of the infants examined and determined to be normocephalic.
Head-to-heel length is also measured. It is important to extend the legs completely when measuring total body length (Fig. 8-3). The average length of the newborn is 48 to 53 cm (19 to 21 inches).
Abdominal circumference need not be routinely measured in the newborn, but should be done in the event of abdominal distention to determine changes in girth over time. Abdominal circumference is measured just above the level of the umbilicus. Because the umbilical cord is still attached, making measurements across the umbilicus (see Fig. 6-9) is too variable in newborns. Measuring the abdominal circumference below the umbilical region is also unsuitable because bladder status may affect the reading.
Measure body weight soon after birth because weight loss occurs fairly rapidly. Normally the newborn loses up to 10% of the birth weight by 3 to 4 days of age because of loss of excessive extracellular fluid and meconium, as well as limited food intake, especially in breast-fed infants. The birth weight is usually regained by the tenth to fourteenth day of life, depending on method of feeding. Most newborns weigh 2700 to 4000 g (6 to 9 lb), the average weight being about 3400 g (7.5 lb). Accurate birth weights and lengths are important because they provide a baseline for assessment of risk status and future growth.
Vital Signs: Another category of measurements is vital signs. Axillary temperatures are taken because insertion of a thermometer into the rectum can cause perforation of the mucosa. (See Table 6-3 and Fig. 8-4.) Core (internal) body temperature varies according to the period of reactivity but is normally 36.5° to 37.6° C (97.7° to 99.7° F). Skin temperature is slightly lower than core body temperature. Therefore axillary temperature is generally less than rectal temperature, measuring about 0.2° C lower (Hussink Muller, van Berkel, and de Beaufort, 2008).
Fig. 8-4 Mother taking axillary temperature with digital thermometer. (Courtesy E. Jacob, Texas Children’s Hospital, Houston.)
Because BAT is located in the axillary pocket, axillary readings may be elevated whenever NST occurs (see p. 228). However, axillary readings are normal in cold-stressed infants when NST is not triggered or is overwhelmed. The single best method for determining the newborn infant’s temperature in any given situation remains elusive when considering the available studies. Controversy exists regarding the accuracy of tympanic membrane sensors. Bliss-Holtz (1993) compared axillary and tympanic membrane temperatures in neonates and found that tympanic membrane temperature measurements were helpful in determining the infant’s thermal state. However, other studies have found tympanic membrane temperatures to have high variability according to neonatal environment (bassinet or open crib, radiant warmer, and incubator) (Hicks, 1996; Leick-Rude and Bloom, 1998) and limited usefulness in critically ill neonates (Weiss, Poeltler, and Gocka, 1993; Wilshaw, Beckstrand, Waid, et al, 1999). One study concluded that tympanic membrane temperatures were unacceptable for detecting fever in children under 6 years of age (Lanham, Walker, Klocke, et al, 1999). A meta-analysis of 101 studies comparing tympanic membrane temperatures with rectal temperatures in children concluded that the tympanic method demonstrated a wide range of variability, limiting its application in a pediatric setting (Craig, Lancaster, Taylor, et al, 2002).
The Canadian Paediatric Society (2009) outlines concerns regarding safety and accuracy of tympanic temperature measurement in newborns because of the size of a newborn’s external ear canal relative to the size of the thermometer probe. To ensure accuracy, the probe, which may be up to 8 mm (0.3 inch) in diameter, must be deeply inserted into the ear canal to allow orientation of the sensor near or against the tympanic membrane. At birth, the average diameter of the canal is just 4 mm (0.16 inch); at 2 years of age it is just 5 mm (0.2 inch). The Canadian Paediatric Society concurs with earlier writers Bliss-Holtz (1995) and Yetman, Coody, West, and colleagues (1993) in their conclusion that infrared tympanic membrane technology has yet to meet clinical needs for use in newborn infants. Infrared axillary and digital thermometers are used in many neonatal units because they give rapid readings and are easy to clean. Studies demonstrate their usefulness in well, full-term newborns (Sganga, Wallace, Kiehl, et al, 2000), but accuracy with critically ill neonates is less predictable (Seguin and Terry, 1999; Wilshaw, Beckstrand, Waid, et al, 1999). Bailey and Rose (2001) concluded that tympanic membrane temperatures (versus axillary) in healthy preterm infants were reliable, were cost-effective, and decreased the amount of handling required for monitoring. Skin temperature readings have also been found to vary with probe site placement; bed type; environmental temperature; and the use of blankets, clothing, and nesting devices (Leick-Rude and Bloom, 1998). Jones, Kleber, Eckert, and colleagues (2003) compared rectal temperatures of infants under 2 months of age with calibrated digital thermometers and mercury glass thermometers. The study of 120 infants found that the digital thermometers measured a higher temperature (mean average of 0.7° F; range 0° to 1.6° F) than the mercury glass thermometers. The researchers concluded that the error in measurement was attributable to the digital thermometer used.
Advantages of digital thermometers in neonatal care include relatively easy readability by parents and caretakers in the home, improvement of discharge planning effectiveness, and decreased risk of breakage and associated complications compared with glass thermometers. Both the American Academy of Pediatrics (2003b) and the Canadian Paediatric Society (2009) have advised that mercury thermometers no longer be used in hospitals, clinics, and homes to decrease mercury exposure hazard.
Temporal artery thermometers (TATs) are available for use in the general pediatric population, and parents often report ease of use and less discomfort with such methods. Greenes and Fleisher (2001) concluded that the TAT had limited sensitivity in infants for detecting rectal fever, yet the TAT was more accurate than the tympanic thermometer. Siberry, Diener-West, Schappell, and colleagues (2002) compared the infrared TAT (using an infrared device [Exergen, Watertown, Massachusetts]) with the rectal temperature measurement (digital thermometer) and found poor predictability for fever in children 0 to 3 months old. The authors concluded that the TAT could be used as a rapid assessment screening tool to identify rectal fever in children 3 to 24 months old, but TAT was unreliable as a screening tool for infants under 3 months. Schuh, Komar, Stephens, and colleagues (2004) compared rectal temperatures with TAT measurements in an emergency department population of children under 24 months and concluded that the TAT was not predictable for fever in children under 3 months but could be used as a screening tool for detecting fever of at least 38.3° C (100.9° F) in children 3 to 24 months. Holzhauer, Reith, Sawin, and colleagues (2009) determined that TAT did not accurately detect rectal fever in infants and children aged 3 to 36 months; the researchers did find that TAT was less traumatic for small children than rectal temperature measurement.
In most studies regarding newborn temperature, the glass mercury thermometer is the gold standard against which other methods are compared. Nurses must be cognizant of the many variables involved and be able to make clear clinical decisions based on accurate and objective data. Some examples of variables include:
Site—Axillary, rectal, tympanic, skin
Environment—Radiant warmer, open crib, incubator, clothing, or nesting
Purpose—Fever, possible sepsis (in which case the temperature may be lower than normal in newborns), and thermoregulation in the transition phase
Nurses must also consider cost-effectiveness (nursing care time and instrument operation cost) and potential cross-contamination risks when evaluating neonatal temperature measurement (Sganga, Wallace, Kiehl, et al, 2000). Ease of use and infant comfort are important factors to consider when teaching parents about taking the newborn’s temperature at home. Further research is needed to perfect thermometers that accurately reflect the infant’s core temperature to effectively plan nursing care and maintain a stable temperature.
Pulse and respirations vary according to the periods of reactivity and to the infant’s behavior but are usually 120 to 140 beats/min and 30 to 60 breaths/min. Both are counted for a full 60 seconds to detect irregularities in rate, rhythm, and quality. The heart rate is taken apically with a stethoscope, and the brachial and femoral arteries are palpated for equality of strength or fullness.
Measurement of blood pressure (BP) provides baseline data and may indicate cardiovascular problems. BP is affected by gestational age and birth weight. During the first postnatal week BP increases by 1 to 2 mm Hg/day; during the first 6 weeks of life it increases by approximately 1 mm Hg/wk (Tulassy, Ramanathan, Evans, et al, 2005). Falkner (2007) suggests that the upper 95% confidence limit for a 40-week term infant is 90 mm Hg (systolic) and that a term infant with a systolic blood pressure exceeding this limit is considered hypertensive. For infants with murmurs, suspicion of congenital heart disease, or any concerns regarding tissue perfusion or fluid volume, BP is most easily and accurately assessed using oscillometry (Dinamap), although the device is less reliable when the mean arterial BP is below 35 mm Hg (Fig. 8-5). Oscillometric BP is more accurate when the newborn is in quiet or sleep state and an appropriate cuff width–to-arm or calf ratio of 0.45 to 0.70 (approximately 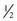 to
to 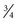 ) is used (Nuntnarumit, Yang, and Bada-Ellzey, 1999). It is also recommended that two or three BP measurements be taken in sick infants and that the mean BP be used because there is less error than when using systolic and diastolic readings. For healthy term infants, the average oscillometric systolic/diastolic BP is 65/45 mm Hg on day 1 of life, changing to 69.5/44.5 by day 3 (Kent, Kecskes, Shadbolt, et al, 2007). Compare BP in the upper and lower extremities, which should be equal.
) is used (Nuntnarumit, Yang, and Bada-Ellzey, 1999). It is also recommended that two or three BP measurements be taken in sick infants and that the mean BP be used because there is less error than when using systolic and diastolic readings. For healthy term infants, the average oscillometric systolic/diastolic BP is 65/45 mm Hg on day 1 of life, changing to 69.5/44.5 by day 3 (Kent, Kecskes, Shadbolt, et al, 2007). Compare BP in the upper and lower extremities, which should be equal.

Fig. 8-5 Measurement of blood pressure using oscillometry. (Courtesy E. Jacob, Texas Children’s Hospital, Houston.)
Calf BP measurements are comparable to brachial pressures in newborns and infants less than 1 year of age and are often more accessible. For consistency, some recommend that baseline calf and brachial pressures be taken initially and the site documented (Axton, Smith, Bertrand, et al, 1995). Although recent normative BP values are for healthy term infants (Kent, Kecskes, Shadbolt, et al, 2007), there remains a distinct need for further studies to clarify the norms for preterm newborns (Tulassy, Ramanathan, Evans, et al, 2005; Nuntnarumit, Yang, and Bada-Ellzey, 1999).
A suggested schedule for monitoring heart and respiratory rates and temperature is within the first hour of life, then once every 8 hours until discharge. This schedule may vary according to institutional policy. Any change in the infant’s color, breathing, muscle tone, or behavior necessitates more frequent monitoring.
Although uncommon, neonatal hypertension may be a sign of a significant underlying problem such as renal, cardiac, or thromboembolic pathologic condition, or it may be associated with a medication treatment regimen. The nurse should bring neonatal hypertension to the primary practitioner’s attention for further evaluation.
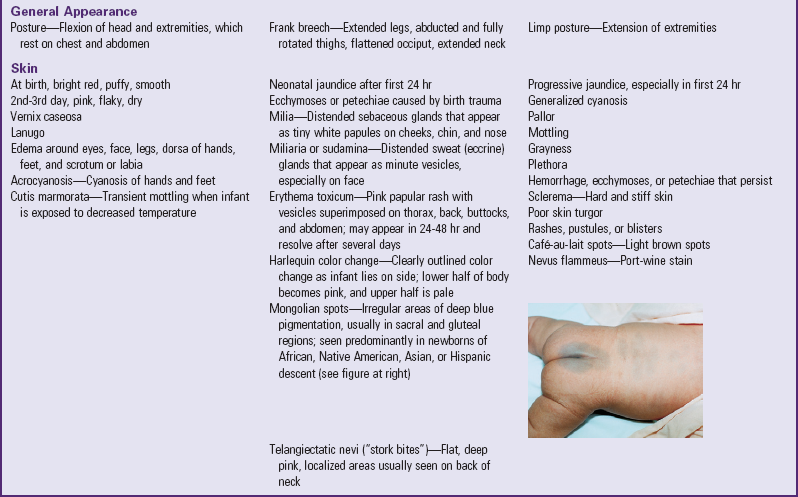
General Appearance: In the full-term newborn the posture is one of flexion, a result of in utero position (Fig. 8-6). Most infants are born in a vertex (head first) presentation and keep the head flexed, with the chin resting on the upper chest. The arms are flexed at the elbows and rest, folded, on the chest with hands clenched or fisted. The legs are flexed at the knees, the hips are flexed with thighs resting on the abdomen, and the feet are dorsiflexed against the anterior aspect of the legs. The vertebral column is also flexed.
Note any deviation from this characteristic fetal position. For example, preterm and hypoxic infants do not assume an attitude of total flexion but rather one of limp or hypotonic extension. Nonvertex presentations also result in variations in posture. In breech presentations the posture depends on the presenting part; a frank breech presentation results in extended legs, abducted and fully rotated thighs, a flattened head on top, and a neck that appears elongated.
Observe the infant’s behavior, especially the degree of alertness, drowsiness, and irritability, which are common signs of neurologic problems. Ask the following questions when assessing behavior:
Skin: The texture of the newborn’s skin is velvety smooth and puffy, especially about the eyes, the legs, the dorsal aspect of the hands and feet, and the scrotum or labia.
Skin color depends on racial and familial background and varies greatly among newborns. In general, the Caucasian infant is usually pink to red; the African-American newborn may appear a pinkish or yellowish brown; infants of Hispanic descent may have an olive tint or a slight yellow cast to the skin; infants of Asian descent may be a rosy or yellowish tan; and the color of Native American newborns depends on the tribe and can vary from light pink to dark reddish brown. By the second or third day the skin turns to its more natural tone and is drier and flakier.
Observe the color of the skin in relation to activity, position, and temperature changes. In general, the infant becomes redder when crying and may demonstrate transient periods of cyanosis during the first few hours of life (not associated with apnea or bradycardia). Table 8-3 describes several other color changes and minor skin blemishes.
At birth the skin may be covered with a grayish white, cheeselike substance called vernix caseosa, a mixture of sebum and desquamating cells. If it is not removed during the bath, it will be absorbed by about 24 to 48 hours. A fine, downy hair called lanugo may be present on the skin, especially on the forehead, cheeks, shoulders, and back.
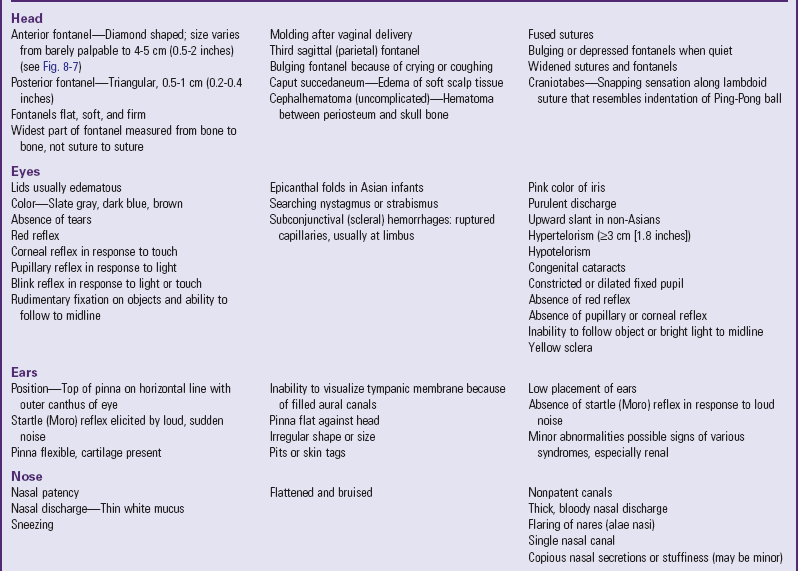
Head: General observation of the head’s contour is important because molding occurs in almost all vaginal deliveries. In a vertex delivery the head is usually flattened at the forehead, with the apex rising and forming a point at the end of the parietal bones, and the posterior skull or occiput dropping abruptly. The usual, more oval contour of the head is apparent by 1 or 2 days after birth. The change in shape occurs because the bones of the cranium are not fused, allowing for overlapping of the edges of these bones to accommodate to the size of the birth canal during delivery. Such molding does not occur in infants born by cesarean section unless there has been prolonged labor or the head has been engaged in the pelvis.
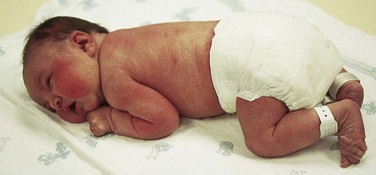
Six bones—the frontal, occipital, two parietals, and two temporals—constitute the cranium. Between the junctions of these bones are bands of connective tissue called sutures. At the junction of the suture are wider spaces of unossified membranous tissue called fontanels. ![]() The two most prominent fontanels are the anterior fontanel, formed by the junction of the sagittal, coronal, and frontal sutures, and the posterior fontanel, formed by the junction of the sagittal and lambdoid sutures (Fig. 8-7, A).
The two most prominent fontanels are the anterior fontanel, formed by the junction of the sagittal, coronal, and frontal sutures, and the posterior fontanel, formed by the junction of the sagittal and lambdoid sutures (Fig. 8-7, A).
![]() Anatomy Review—Location of Sutures and Fontanels
Anatomy Review—Location of Sutures and Fontanels
Two other fontanels—the sphenoidal and mastoid—are normally present but are not usually palpable. An additional fontanel located between the anterior and posterior fontanels along the sagittal suture is found in some neonates but is also found in some infants with Down syndrome. Note and record the presence of this sagittal or parietal fontanel.
Palpate the skull for all sutures and fontanels by using the tip of the index finger and running it along the ends of the bones (Fig. 8-7, B). Sutures feel like cracks between the skull bones; fontanels feel like wider “soft spots” at the junction of sutures. Note their size, shape, molding, and any abnormal closure.
The anterior fontanel is diamond shaped and measures 4 to 5 cm (about 2 inches) at its widest point (from bone to bone, rather than from suture to suture). The posterior fontanel is triangular, measuring between 0.5 and 1 cm (<0.5 inch) at its widest part. It is easily located by following the sagittal suture toward the occiput. The posterior fontanel may not be palpable after birth because of edema (caput) or other cranial molding.
The fontanels should feel flat, firm, and well demarcated against the bony edges of the skull. Frequently pulsations are visible at the anterior fontanel. Coughing, crying, or lying down may temporarily cause the fontanels to bulge and become more taut.
Palpate the skull for any unusual masses or prominences, particularly those resulting from birth trauma, such as caput succedaneum or cephalhematoma. (See Chapter 9.) Because of the pliability of the skull, exerting pressure at the margin of the parietal and occipital bones along the lambdoid suture may produce a snapping sensation similar to the indentation of a Ping-Pong ball. This phenomenon, known as physiologic craniotabes, may be found normally, especially in newborns of breech birth, but also may be associated with hydrocephalus, congenital syphilis, or rickets.
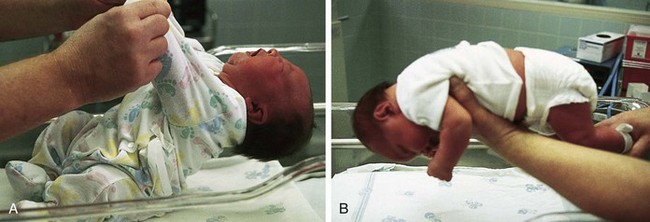
Assess the degree of head control. Although head lag is normal, the full-term newborn has some ability to control the head in certain positions. If the supine infant is pulled by the arms into a semi-Fowler position, head lag and hyperextension occur (Fig. 8-8, A). However, as infants are brought forward into a sitting position, they attempt to control their heads in an upright position. As the head falls forward onto the chest, many infants attempt to right it into the erect position. If they are held in ventral suspension (i.e., held prone above and parallel to the examining surface), the head is held in a straight line with the spinal column (Fig. 8-8, B). When lying on the abdomen, newborns have the ability to lift the head slightly, turning it from side to side. Marked head lag is seen in neonates with Down syndrome, prematurity, hypoxia, and metabolic and neurologic disorders.
Eyes: Because newborns tend to keep their eyes tightly closed, begin the examination of the eyes by observing the lids for edema, which is normally present for 2 days after delivery. Observe the eyes for symmetry and for hypertelorism (wide spacing between the eyes), but do not measure the distance between the inner canthi unless there is cause for further investigation. Tears may be present at birth, but purulent discharge from the eyes shortly after birth is abnormal.
To visualize the surface structures of the eye, hold the infant supine and gently lower the head. The eyes will usually open, similar to the mechanism of a doll’s eyes. The sclera should be white and clear.
Examine the cornea for any opacities or haziness. The corneal reflex is present at birth but is generally not elicited unless cerebral or eye damage is suspected. The pupil usually responds to light by constricting. Absence of the pupillary reflex, particularly by 3 weeks of age, suggests blindness. A fixed, dilated, or constricted pupil may indicate central nervous system damage. A searching nystagmus is common after birth. Strabismus is a normal finding because of the lack of binocularity.
Note the color of the iris. Most light-skinned newborns have slate gray or dark blue eyes, whereas dark-skinned infants have brown eyes. Absence of color is characteristic of albinism.
Although it is difficult to perform a complete funduscopic examination of the retina, the nurse should elicit a red reflex (see p. 155).
Ears: Examine the ears for position, structure, and auditory function. The pinna is often flattened against the side of the head from pressure in utero. An otoscopic examination may not always be performed because the canals are filled with vernix caseosa and amniotic fluid, making visualization of the tympanic membrane difficult. Periauricular skin tags, sinuses, and misshapen or low-set ears may be familial or associated with other congenital defects such as trisomy 18 and renal defects.
One way to assess auditory ability is by making a sharp, loud noise close to the infant’s head and noting the presence of the startle reflex (Table 8-4) or twitching of the eyelids. However, the absence of a response to a loud noise in the newborn is not diagnostic for hearing deficit. Full-term newborns have the ability to habituate to noxious stimuli such as noise and may not react every time. Also, be aware of newborns considered at risk for hearing loss so that early testing can be performed (see Universal Newborn Hearing Screening, p. 254). (See Auditory Testing, Chapter 6, and Hearing Impairment, Chapter 24.)
Nose: Assess patency of the nasal canals by holding your hand over the infant’s mouth and one canal and noting the passage of air through the unobstructed opening. If nasal patency is questionable, report it, since most newborns are obligatory nose breathers and are unable to breathe orally in response to nasal occlusion.
The nose is usually flattened after birth, and bruises are common, especially if forceps were used. Thin, white mucus is common in the newborn, but a thick, bloody nasal discharge should be evaluated. Sneezing is common.
Mouth and Throat: Inspect the mouth’s existing structures. The palate is normally high arched and somewhat narrow. Inspect the hard and soft palates for any clefts, which warrant further investigation. A common finding is Epstein pearls—small, white, epithelial cysts along both sides of the midline of the hard palate. They are insignificant and disappear in several weeks.
The frenulum of the upper lip is a band of thick, pink tissue that lies under the inner surface of the upper lip and extends to the maxillary alveolar ridge. It usually disappears as the maxilla grows. It is particularly evident when the infant yawns or smiles.
The lingual frenulum attaches the underside of the tongue midway between the ventral surface of the tongue and the tip to the lower palate. It is estimated that 4% to 5% of newborns have a tight lingual frenulum, sometimes referred to as tongue-tie, which may restrict adequate sucking (Ricke, Baker, Madlon-Kay, et al, 2005; Messner, Lalakea, Aby, et al, 2000; Griffiths, 2004). Further evaluation may be required to ascertain sucking ability, particularly in breast-fed infants (Masaitis and Kaempf, 1996; Wiessinger and Miller, 1995). The treatment for a tight lingual frenulum advocated by the American Academy of Pediatrics is frenotomy, a safe and effective surgical procedure that improves comfort, effectiveness, and ease of breast-feeding for mother and infant (Ballard, Auer, and Khoury, 2002; Coryllos, Genna, and Salloum, 2004; Dollberg, Botzer, Grunis, et al, 2006).
Elicit the sucking reflex by placing a nipple or gloved finger in the infant’s mouth. The infant should exhibit a strong, vigorous suck. To stimulate the rooting reflex, stroke the cheek and note the infant’s response of turning toward the stimulated side and sucking. Assess the gag reflex when using a tongue blade to visualize the oropharynx.
Inspect the uvula while the infant is crying and the chin is depressed. However, the uvula may be retracted upward and backward during crying. Tonsillar tissue is generally not seen in the newborn. Natal teeth (teeth present at birth as opposed to neonatal teeth—teeth that erupt during the first month of life) are seen infrequently and erupt chiefly at the position of the lower central incisors. They are reported because they are sometimes found in infants with developmental abnormalities and syndromes, including cleft lip and palate. Most natal teeth are loosely attached. However, current research suggests preserving them until they exfoliate naturally (Leung and Robson, 2006; McDonald, Avery, and Dean, 2004), unless breast-feeding is impaired by the neonate biting the breast or the teeth are very loose (Wright, 2000).
Neck: Because the newborn’s neck is short and covered with folds of tissue, for adequate assessment allow the head to fall gently backward in slight hyperextension while supporting the back in a slightly raised position. Observe for range of motion, shape, and any abnormal masses, and palpate each clavicle for possible fractures. (See Fractures, Chapter 9.)
Chest: The shape of the newborn’s chest is almost circular, with equal anteroposterior and lateral diameters. The ribs are flexible, and slight intercostal retractions are normal on inspiration. The xiphoid process is commonly visible as a small protrusion at the end of the sternum. The sternum is raised and slightly curved.
Inspect the breasts for size; shape; and nipple formation, location, and number. Maternal hormones cause breast enlargement that appears in many newborns of either gender by the second or third day. Occasionally the infant’s breasts will secrete a milky substance (sometimes called witch’s milk). Infrequently, more than two nipples are present; if these are found, evaluate the kidneys because of the association of extra nipples with renal anomalies.
Lungs: The newborn’s normal respirations are irregular and abdominal, and the rate is between 30 and 60 breaths/min. Periods of apnea lasting more than 20 seconds are abnormal and may be accompanied by bradycardia. After the first forceful breaths required to initiate respiration, subsequent breaths should be easy and fairly regular in rhythm. Occasional irregularities occur in relation to crying, sleeping, and feeding. Periodic breathing is common in full-term newborns and consists of rapid nonlabored respirations followed by pauses of less than 20 seconds; periodic breathing may be more prominent during sleep and is not accompanied by status changes such as cyanosis or bradycardia.
Perform auscultation when the infant is quiet. Bronchial breath sounds should be equal bilaterally. Report any differences in auscultatory findings between symmetric sites. Crackles soon after birth may indicate areas of atelectasis or the presence of fluid, which represents the normal transition of the lungs to extrauterine life. However, wheezes, persistence of crackles, and stridor should be reported.
Heart: Heart rate may range from 100 to 180 beats/min shortly after birth and, when the infant’s condition has stabilized, from 120 to 140 beats/min. Palpate to find the point of maximum intensity (PMI), which is usually in the fourth to fifth intercostal space, medial to the left midclavicular line. The PMI gives some indication of the location of the heart, which may be displaced in conditions such as congenital diaphragmatic hernia or pneumothorax. Dextrocardia, an anomaly wherein the heart is on the right side of the body, should be reported (the abdominal organs may also be reversed), along with associated circulatory abnormalities.
Auscultation of the specific components of the heart sounds is difficult because of the rapid rate and effective transmission of respiratory sounds. However, the first (S1) and second (S2) sounds should be clear and well defined; the second sound is somewhat higher in pitch and sharper than the first. Murmurs are frequently heard in the newborn, especially over the base of the heart or at the left sternal border in the third or fourth interspace. Ordinarily they are not associated with specific cardiac defects but frequently represent the incomplete functional closure of fetal shunts (Fuloria and Kreiter, 2002). (Chapter 6 discusses grading of heart murmurs.) However, always record and report any murmur or other unusual sounds.
Abdomen: The normal contour of the abdomen is cylindric and prominent with visible veins. Bowel sounds are audible within the first 15 to 20 minutes after birth. Visible peristaltic waves may be observed in thin newborns but should not be seen in well-nourished infants.
Inspect the umbilical cord to determine the presence of two arteries, which look like papular structures, and one vein, which has a larger lumen than the arteries and a thinner vessel wall. At birth the cord appears bluish white and moist. After clamping, it begins to dry and appears a dull, yellowish brown. It progressively shrivels and turns greenish black. If the umbilical cord appears unusually large in diameter at the base, inspect for the presence of a hematoma or small omphalocele. The cord must not be clamped over an omphalocele, since part of the intestine will be clamped, causing tissue necrosis. One practical rule of thumb is to cut the cord distally 10 to 12 cm (4 to 5 inches) from a questionable enlargement until further examination is carried out by a practitioner. The extra length can be cut once normal anatomy has been identified.
A cord that is draining and erythematous at the base should be investigated by the primary practitioner. The cord undergoes a process of dry gangrene decay, which has an odor; therefore odor alone may not be a reliable index of suspicion for omphalitis.
Palpate after inspecting the abdomen. The liver is normally palpable 1 to 3 cm (about 0.5 to 1 inch) below the right costal margin. The tip of the spleen can sometimes be felt, but a palpable spleen more than 1 cm below the left costal margin suggests enlargement and warrants further investigation. Although the nurse should palpate both kidneys, this maneuver requires considerable practice. When felt, the lower half of the right kidney and the tip of the left kidney are 1 to 2 cm (about 0.5 to 1 inch) above the umbilicus. During examination of the lower abdomen, palpate for femoral pulses, which should be strong and equal bilaterally.
Female Genitalia: Normally the labia majora and minora (the minora may be more prominent) and clitoris are edematous, especially after a breech delivery. However, carefully inspect the labia and clitoris to identify any evidence of ambiguous genitalia or other abnormalities. Normally in a girl the urethral opening is located behind the clitoris. Any deviation from this may mistakenly suggest that the clitoris is a small penis, which can occur in conditions such as congenital adrenal hyperplasia.
A hymenal tag is occasionally visible from the posterior opening of the vagina. It is composed of tissue from the hymen and the labia minora and usually disappears in several weeks. Generally, the vaginal vault is not inspected.
Vaginal discharge may be noted during the first week of life. This pseudomenstruation is a manifestation of the abrupt decrease in maternal hormones and usually disappears by 2 to 4 weeks of age. Fecal discharge from the vaginal opening indicates a rectovaginal fistula and must be reported. Vernix caseosa may be present in large amounts between the labia. Vigorous attempts to remove all the vernix through bathing are avoided to prevent tissue damage. With routine bathing and care, vernix will disappear after several days.
Male Genitalia: Inspect the penis for the urethral opening, which is located at the tip. However, the opening may be totally covered by the prepuce, or foreskin, which covers the glans penis. A tight prepuce is a common finding in newborns and does not indicate phimosis. It should not be forcefully retracted; locating the urinary meatus is usually possible without retracting the foreskin (Cavaliere, 2009). Smegma, a white cheesy substance, is commonly found around the glans penis, under the foreskin. An erection is common in the newborn. Small, white, firm lesions called epithelial pearls may be seen at the tip of the prepuce.
The scrotum may be large, edematous, and pendulous in the full-term neonate, especially in the infant born in breech position. It is more deeply pigmented in dark-skinned infants. A noncommunicating hydrocele commonly occurs unilaterally and disappears within a few months. Palpate the scrotum for the presence of testes. (See Chapter 6.)
In small newborns, particularly premature infants, the undescended testes may be palpable within the inguinal canal. Absence of the testes may also be a sign of ambiguous genitalia, especially when accompanied by a small scrotum and penis. Inguinal hernias may or may not be manifested immediately after birth. A hernia is easier to detect when the infant is crying. Palpable lymph nodes are most commonly found in the inguinal area. Report a discolored or dusky scrotum, scrotal edema, or palpation of a small mass to the practitioner because these may be a sign of testicular torsion (Juretschke, 2000).
Back and Anus: Inspect the spine with the infant prone. The shape of the spine is gently rounded, with none of the characteristic S-shaped curves seen later in life. Any abnormal openings or sinuses, masses, dimples, or soft areas are noted. A protruding sac anywhere along the spine, but most commonly in the sacral area, indicates some type of spina bifida. A small sinus, which may or may not be communicating with the spine, is a pilonidal sinus. It is frequently covered with a tuft of hair. Although it may have no pathologic significance, a pilonidal cyst may indicate the existence of spina bifida occulta or be a portal of entry into the spinal column. With the infant still prone, note symmetry of the gluteal folds. Report any evidence of asymmetry. Skilled examiners test for developmental dysplasia of the hip. (See Chapter 11.)
The presence of an anal orifice and passage of meconium through the orifice during the first 24 to 48 hours of life indicates anal patency. If the nurse suspects an imperforate anus, report this to the practitioner for further evaluation. The presence of meconium or stool on the perineum is not an indication of rectal patency; a fistula may exist wherein stool is evacuated via the vagina, scrotum, or raphe. Therefore it is imperative to check anal patency with a small rubber catheter should any doubt regarding patency exist.
Extremities: Examine the extremities for symmetry, range of motion, and signs of malformation or trauma. Count the fingers and toes, and note supernumerary digits (polydactyly) or fusion of digits (syndactyly). A partial syndactyly between the second and third toes is a common variation seen in otherwise normal infants. More extensive fusion is abnormal, and the nurse should report this to the practitioner.
Observe range of motion of the extremities throughout the entire examination. The newborn will demonstrate full range of motion in the elbow, hip, shoulder, and knee joints. Movements should be symmetric, smooth, and unrestricted. The absence of arm movement signals a potential birth injury paralysis such as Klumpke or Erb-Duchenne paralysis. (See Birth Injuries, Chapter 9.) An asymmetric or partial Moro reflex should alert the practitioner to further evaluate upper extremity mobility. Examine the lower extremities for limb length, symmetry, and hip abduction and flexion.
Examine the nails; the nail beds should be pink, although slight blueness is evident in acrocyanosis. Persistent cyanosis of the nail beds may indicate hypoxia or vasoconstriction. Yellowing of the nail beds may indicate intrauterine distress, postterm birth, or hemolytic disease. Short or absent nails are seen in preterm infants, whereas long nails, extending over the ends of the fingers, are characteristic of postterm newborns.
The palms of the hands should have the usual creases (see Fig. 6-14). A transverse palmar crease (simian crease) suggests Down syndrome but may also be a normal finding. The full-term newborn usually has creases covering the entire sole of the foot. In postterm infants the sole is covered with deep creases, and in preterm infants the creases may partially cover the sole or may be absent. The soles are flat with prominent fat pads. Note any foot abnormalities.
Two reflexes are elicited. The first is the grasp reflex. Touching the palms or soles near the base of the digits causes flexion or grasping (Fig. 8-10, A). The other is Babinski reflex. Stroking the outer sole of the foot upward from the heel across the ball of the foot causes the big toe to dorsiflex and the other toes to hyperextend (Fig. 8-10, B, and Table 8-4).
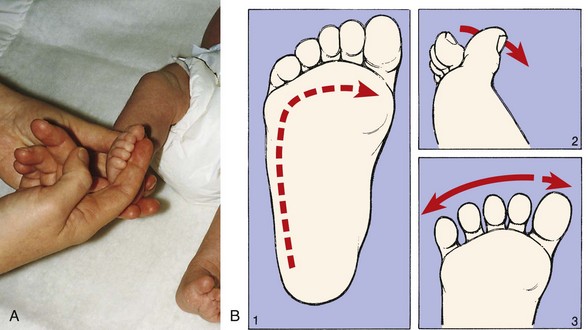
Fig. 8-10 A, Plantar or grasp reflex. B, Babinski reflex. 1, Direction of stroke. 2, Dorsiflexion of big toe. 3, Fanning of toes. (A from Zitelli BJ, Davis HW: Atlas of pediatric physical diagnosis, ed 4, St Louis, 2002, Mosby.)
Inspect the extremities for evidence of fractures from birth trauma. The clavicle, humerus, and femur are most commonly involved. Limitation of movement, crepitus, visible deformity, asymmetry of reflexes, and malposition of the site suggest a fracture.
It is important to assess neonatal muscle tone. By attempting to extend a flexed extremity, determine whether tone is equal bilaterally. Extension of any extremity is usually met with resistance, and when released, the extremity returns to its previous flexed position. Hypotonia suggests some degree of hypoxia, neurologic or muscular disorder, or Down syndrome. Asymmetry of muscle tone may indicate a degree of paralysis from damage to the central nervous system. Failure to move the lower limbs suggests a spinal cord lesion or injury. Sustained rhythmic tremors, twitches, and myoclonic jerks characterize neonatal seizures or may indicate neonatal abstinence syndrome. (See Neonatal Seizures and Drug-Exposed Infants, Chapter 10.) Sudden asynchronous jerking movements, quivering, or momentary tremors are usually normal.
Neurologic System: Assessing neurologic status is a critical part of the physical examination of the newborn. Much of the neurologic testing takes place during evaluation of body systems, such as eliciting localized reflexes and observing posture, muscle tone, head control, and movement. However, several important mass (total body) reflexes also need to be elicited. Test these at the end of the examination, since they may disturb the infant and interfere with auscultation. Table 8-4 describes these reflexes and several local reflexes. Record and report the absence, asymmetry, persistence, or weakness of a reflex.
The nursing process in the care of the healthy newborn is outlined in Box 8-4.
Maintain a Patent Airway
Establishing a patent airway is the primary objective in the delivery room. When the newborn is supine, a neutral neck position (avoiding neck flexion or hyperextension) is critical to achieving and maintaining a patent airway. After feeding, position the infant to facilitate drainage of secretions.
The American Academy of Pediatrics (2005a) recommends the supine position during sleep for all newborns. This recommendation is based on the association between sleeping prone and sudden infant death syndrome. (See Chapter 13.) Since the initial recommendation in 1992 that all infants be placed in the supine position to sleep, there has been no evidence of an increased number of complications, such as choking or vomiting, when infants are placed in a supine sleep position (Malloy, 2002). There has, however, been an increase in the number of infants with cranial asymmetry, particularly unilateral flattening of the occiput (American Academy of Pediatrics, 2003a). Health care professionals must educate parents on prevention of deformational plagiocephaly by encouraging alternate positions when baby is awake. (See also Positional Plagiocephaly, Chapter 11.)
Suctioning, if needed, may be done with a bulb syringe. Used bulb syringes should be replaced every 24 hours in the hospital and boiled for 10 minutes after use in the home to prevent bacterial contamination. If more forceful removal of secretions is required, mechanical suction is used. Use of the proper-sized catheter and correct suctioning technique are essential to prevent mucosal damage and edema. Gentle suctioning is necessary to prevent laryngospasm, reflex bradycardia, and other cardiac arrhythmias from vagal stimulation. Oropharyngeal suctioning is performed for up to 5 seconds with sufficient time between each attempt to allow the infant to reoxygenate. If nasal suctioning is necessary, it must be done after oral suctioning to minimize the possibility of aspiration of oropharyngeal contents (American Academy of Pediatrics, 2006a).
The stomach may be emptied (aspirated) to remove the contents; passing a catheter to the stomach may also rule out esophageal atresia. Closely monitor vital signs, and report immediately any indication of respiratory distress.
To avoid aspiration of amniotic fluid or mucus, clear the mouth and pharynx first, then the nasal passages (remember m before n), to prevent a gasp and inspiration of large amounts of oropharyngeal secretions. Compress the bulb syringe before insertion to avoid forcing secretions into the bronchi.
Maintain a Stable Body Temperature
Conserving the newborn’s body heat is an essential nursing goal. At birth a major cause of heat loss is evaporation, the loss of heat through moisture. The amniotic fluid that bathes the infant’s skin favors evaporation, especially when combined with the cool atmosphere of the delivery room. Rapidly drying the skin and hair with a warmed towel and placing the infant in a heated environment will minimize heat loss through evaporation.
Another source of heat loss is radiation, the loss of heat to cooler solid objects in the environment that are not in direct contact with the infant. Loss of heat through radiation increases as these solid objects become colder and closer to the infant. The temperature of ambient (surrounding) air in the incubator essentially has no effect on loss of heat through radiation. This is a critical point to remember when attempting to maintain a constant temperature for the infant, because even when the temperature of the ambient air is optimum, the infant can become hypothermic. The use of a radiant heating device, such as a heat lamp, with an incubator may cause the infant to overheat because the neonate cannot effectively dissipate radiant heat through the Plexiglas wall of the incubator. For this same reason, do not expose an incubator to direct sunlight.
An example of radiant heat loss is the placement of the incubator close to a cold window or air conditioning unit. The cold from either source will cool the incubator walls and subsequently the neonate’s body. To prevent this, the incubator is placed as far away as possible from walls, windows, or ventilating units.
Heat loss can also occur through conduction and convection. Conduction involves loss of body heat from direct skin contact with a cooler solid object. The nurse can minimize this by placing the infant on a padded, covered surface rather than directly on a cool hard table and by providing insulation with clothes and blankets. Placing the newborn nestled close to the mother, such as in her arms or on her abdomen immediately after delivery in skin-to-skin (kangaroo care) contact, is beneficial in terms of conserving newborn heat and fostering maternal attachment and breast-feeding.
Convection is similar to conduction, except that heat loss is aided by surrounding air currents. For example, placing the infant in the direct flow of air from a fan or air conditioner vent causes rapid heat loss through convection. Transporting the neonate in a crib with solid sides reduces airflow around the infant.
Protect from Infection and Injury
The most important practice for preventing cross-infection is thorough hand washing by all individuals involved in the infant’s care. Other procedures to prevent infection include eye care, umbilical care, bathing, and care of the circumcision. Artificial and long fingernails are discouraged for those working in neonatal care because the former have been implicated in the transmission of Pseudomonas organisms (Moolenaar, Crutcher, San Joaquin, et al, 2000; Foca, Jacob, Whittier, et al, 2000); contaminated hand soaps have been identified in one neonatal Serratia marcescens outbreak (Rabier, Bataillon, Jolivet-Gougeon, et al, 2008). Vitamin K is administered to protect against hemorrhage.
Identification
Proper identification of the newborn is essential. The nurse must verify that identifying bands are securely fastened on the newborn and verify the information (name, sex, mother’s admission number, date, and time of birth) against the birth records and the child’s gender. Some institutions use methods of infant identification such as a color photograph kept in the medical record, storage of blood for DNA genotyping, and electronic surveillance systems for infant security. Footprinting or fingerprinting alone is not currently recommended for newborn identification (American Academy of Pediatrics and American College of Obstetricians and Gynecologists, 2007); however, many institutions continue to practice footprinting. Electronic tags that give off a radio frequency are also being used to prevent newborn abductions (Shogan, 2002). Another measure to decrease infant abduction is to discontinue the publication of birth announcements in the local newspaper.
A proactive hospital emergency plan should be implemented to prevent infant abduction and to respond promptly and effectively if one occurs (Geller, 2000). A mock newborn abduction drill is an effective method to evaluate staff competence and response to the incident (Shogan, 2002). All hospital personnel should be educated regarding newborn abduction, preventive strategies, and methods to identify the potential risk of such an occurrence.
The nurse needs to discuss safety issues with the mother before and after delivery of the newborn to ensure adequate understanding of safety measures to prevent newborn abduction. Thirty-two percent of infant abductions occur from health care facilities (Burgess, Carr, Nahirny, et al, 2008). A written copy of safety instructions should also be given to the parent. Instruct parents to look at identification badges of nurses and any hospital personnel who come in contact with the infant and not to relinquish their infants to anyone without proper identification. Some hospital employees in newborn and maternity areas wear color-coded badges or symbols that are changed frequently to decrease the likelihood of infant abduction. Mothers are also advised not to leave the infant alone in the crib while they shower or use the bathroom; rather, they should ask to have the infant returned to the nursery if a family member is not present in the room. Encourage parents and staff to use a password system when the newborn is taken from the room as a routine security measure. The nurse should document in the chart that these instructions were given and that appropriate identification band checks are routinely made throughout each shift.
Nursing staff are also educated regarding the “typical” abductor profile and to be constantly aware of visitors with unusual behavior. The typical abductor is a female between the ages of 15 and 44 who often has a large build and low self-esteem. She may be emotionally disturbed because of the loss of her own child or inability to conceive and may have a strained relationship with her husband or partner. The typical abductor may also be seen visiting the newborn nursery or neonatal intensive care unit (NICU) before the abduction and may ask questions about the care of or the health of a specific newborn. The abductor may familiarize herself with the hospital routine and may also impersonate a health care worker.
The nurse should advise parents that infant safety measures must be implemented after discharge from hospital as well, since home abductions have nearly doubled in recent years, and make up 49% of all infant abductions; those from public places have tripled to 9% of abductions (Burgess, Carr, Nahirny, et al, 2008). Instruct parents to be cautious of anyone they do not know well, particularly persons they met during the pregnancy who show excessive interest or who show up at their home unannounced (Burgess, Carr, Nahirny, et al, 2008).
Eye Care
Prophylactic eye treatment against ophthalmia neonatorum, infectious conjunctivitis of the newborn, includes the use of (1) silver nitrate (1%) solution, (2) erythromycin (0.5%) ophthalmic ointment or drops, or (3) tetracycline (1%) ophthalmic ointment or drops (preferably in single-dose ampules or tubes) (see Nursing Care Guidelines box). Chlamydia trachomatis is the major cause of ophthalmia neonatorum in the United States. Silver nitrate is effective against gonococcal conjunctivitis. Topical antibiotics (tetracycline and erythromycin) and silver nitrate have not proved to be effective in the prevention and treatment of chlamydial conjunctivitis. A 14-day course of oral erythromycin or ethylsuccinate may be given for chlamydial conjunctivitis (American Academy of Pediatrics, 2009b). The administration of oral erythromycin to infants less than 6 weeks old has been associated with the development of infantile hypertrophic pyloric stenosis; therefore parents should be informed of the potential risks and signs of the illness (American Academy of Pediatrics, 2009b).
Although eye prophylaxis is mandatory in the United States, health care facilities are free to choose specific drugs. Effective prophylaxis may be better directed at treating maternal chlamydial infection in areas where that organism is prevalent.
Studies on maternal attachment suggest that in the first hour of life a newborn has a greater ability to focus on coordinated movement than at any other time during the next several days. Because eye contact is important in the development of maternal-infant bonding, routine administration of silver nitrate or antibiotics can be postponed for up to 1 hour after birth. However, practitioners must ensure that the drug is given by 1 hour of age.