The High-Risk Newborn and Family
General Management of High-Risk Newborns,
Nursing Care of High-Risk Newborns
High-Risk Conditions Related to Dysmaturity
High Risk Related to Disturbed Respiratory Function
High Risk Related to Infectious Processes
High Risk Related to Cardiovascular and Hematologic Complications
http://evolve.elsevier.com/wong/ncic
Administration of Medication, Ch. 27
Assessment (Newborn), Ch. 8
Birth of a Child with a Physical Defect, Ch. 11
Birth Injuries, Ch. 9
Bodily Injury, Ch. 26
Clinical Assessment of Gestational Age, Ch. 8
Congenital Diaphragmatic Hernia, Ch. 11
Discharge Planning and Home Care, Ch. 26
Esophageal Atresia with Tracheoesophageal Fistula, Ch. 11
Family-Centered Home Care, Ch. 25
Gastroschisis, Ch. 11
Hyperbilirubinemia, Ch. 9
Hypocalcemia, Ch. 9
Hypoglycemia, Ch. 9
Immunizations, Ch. 12
Infant Mortality, Ch. 1
Latex Allergy, Ch. 11
Maintaining Healthy Skin, Ch. 27
Malformations of the Central Nervous System, Ch. 11
Nursing Care of the Surgical Neonate, Ch. 11
Omphalocele, Ch. 11
Pain Assessment; Pain Management, Ch. 7
Problems Caused by Perinatal Environmental Factors, Ch. 9
Promotion of Parent-Infant Bonding (Attachment), Ch. 8
General Management of High-Risk Newborns
Identification of High-Risk Newborns
![]() The high-risk neonate is defined as a newborn, regardless of gestational age or birth weight, who has a greater-than-average chance of morbidity or mortality, usually because of conditions or circumstances superimposed on the normal course of events associated with birth and the adjustment to extrauterine existence. The high-risk period begins at the time of viability (the gestational age at which survival outside the uterus is believed to be possible, or as early as 23 weeks of gestation) up to 28 days after birth and includes threats to life and health that occur during the prenatal, perinatal, and postnatal periods.
The high-risk neonate is defined as a newborn, regardless of gestational age or birth weight, who has a greater-than-average chance of morbidity or mortality, usually because of conditions or circumstances superimposed on the normal course of events associated with birth and the adjustment to extrauterine existence. The high-risk period begins at the time of viability (the gestational age at which survival outside the uterus is believed to be possible, or as early as 23 weeks of gestation) up to 28 days after birth and includes threats to life and health that occur during the prenatal, perinatal, and postnatal periods.
![]() Critical Thinking Exercise—The High-Risk Newborn
Critical Thinking Exercise—The High-Risk Newborn
There has been increased interest in late-preterm infants of 34 to 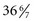 weeks of gestation who may receive the same treatment as term infants. Wang, Dorer, Fleming, and colleagues (2004) emphasize that late-preterm infants often experience similar morbidities to preterm infants: respiratory distress, hypoglycemia requiring treatment, temperature instability, poor feeding, jaundice, and discharge delays as a result of illness. Therefore assessment and prompt intervention in life-threatening perinatal emergencies often make the difference between a favorable outcome and a lifetime of disability. The nurse in the newborn nursery is familiar with the characteristics of neonates and recognizes the significance of serious deviations from expected observations. When providers can anticipate the need for specialized care and plan for it, the probability of successful outcome is increased.
weeks of gestation who may receive the same treatment as term infants. Wang, Dorer, Fleming, and colleagues (2004) emphasize that late-preterm infants often experience similar morbidities to preterm infants: respiratory distress, hypoglycemia requiring treatment, temperature instability, poor feeding, jaundice, and discharge delays as a result of illness. Therefore assessment and prompt intervention in life-threatening perinatal emergencies often make the difference between a favorable outcome and a lifetime of disability. The nurse in the newborn nursery is familiar with the characteristics of neonates and recognizes the significance of serious deviations from expected observations. When providers can anticipate the need for specialized care and plan for it, the probability of successful outcome is increased.
Late-Preterm Infant
Within the past two decades, several significant changes have occurred in neonatal care. Early postpartum discharge for term and preterm infants gained popularity as health care institutions attempted to cut health care costs. Another change occurred in newborn care, as infants who appeared to be “near” term began to be treated much like term infants, thus avoiding the costs of neonatal intensive care for infants who appeared to be healthy. Experts have recommended that infants born between 34 and 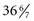 weeks of gestation be referred to as late-preterm infants rather than near-term infants (Engle, 2006; Engle, Tomashek, Wallman, et al, 2007). Late-preterm infants may be able to make an effective transition to extrauterine life; however, such infants, by nature of their limited gestation, remain at risk for problems related to feeding, neurodevelopment, thermoregulation, hypoglycemia, hyperbilirubinemia, sepsis, and respiratory function (Bakewell-Sachs, 2007; Darcy, 2009). In one study children born at 34 to 36 weeks were more than three times as likely as children born at term to be diagnosed with cerebral palsy (CP) (Petrini, Dias, McCormick, et al, 2009). It is now estimated that late-preterm infants represent 70% of the total preterm infant population and that the mortality rate for this group is significantly higher than that of term infants (7.9 versus 2.4 per 1000 live births, respectively) (Tomashek, Shapiro-Mendoza, Davidoff, et al, 2007). Because late-preterm infants’ birth weights often range from 2000 to 2500 g (4.4 to 5.5 lb) and they appear relatively mature in comparison to smaller preterm infants, they may be cared for in the same manner as healthy term infants, while risk factors for late-preterm infants are overlooked. Late-preterm infants are often discharged early from the birth institution and have a significantly higher rate of rehospitalization than term infants (Escobar, Clark, and Greene, 2006). Discussions regarding high-risk infants in this chapter also refer to late-preterm infants who are experiencing a delayed transition to extrauterine life.
weeks of gestation be referred to as late-preterm infants rather than near-term infants (Engle, 2006; Engle, Tomashek, Wallman, et al, 2007). Late-preterm infants may be able to make an effective transition to extrauterine life; however, such infants, by nature of their limited gestation, remain at risk for problems related to feeding, neurodevelopment, thermoregulation, hypoglycemia, hyperbilirubinemia, sepsis, and respiratory function (Bakewell-Sachs, 2007; Darcy, 2009). In one study children born at 34 to 36 weeks were more than three times as likely as children born at term to be diagnosed with cerebral palsy (CP) (Petrini, Dias, McCormick, et al, 2009). It is now estimated that late-preterm infants represent 70% of the total preterm infant population and that the mortality rate for this group is significantly higher than that of term infants (7.9 versus 2.4 per 1000 live births, respectively) (Tomashek, Shapiro-Mendoza, Davidoff, et al, 2007). Because late-preterm infants’ birth weights often range from 2000 to 2500 g (4.4 to 5.5 lb) and they appear relatively mature in comparison to smaller preterm infants, they may be cared for in the same manner as healthy term infants, while risk factors for late-preterm infants are overlooked. Late-preterm infants are often discharged early from the birth institution and have a significantly higher rate of rehospitalization than term infants (Escobar, Clark, and Greene, 2006). Discussions regarding high-risk infants in this chapter also refer to late-preterm infants who are experiencing a delayed transition to extrauterine life.
The Association of Women’s Health, Obstetric and Neonatal Nurses has published the Late Preterm Infant Assessment Guide (Askin, Bakewell-Sachs, Medoff-Cooper, et al, 2007) for the education of perinatal nurses regarding the late-preterm infant’s risk factors and appropriate care and follow-up care (Table 10-1).
TABLE 10-1
LATE-PRETERM INFANT ASSESSMENT AND INTERVENTIONS
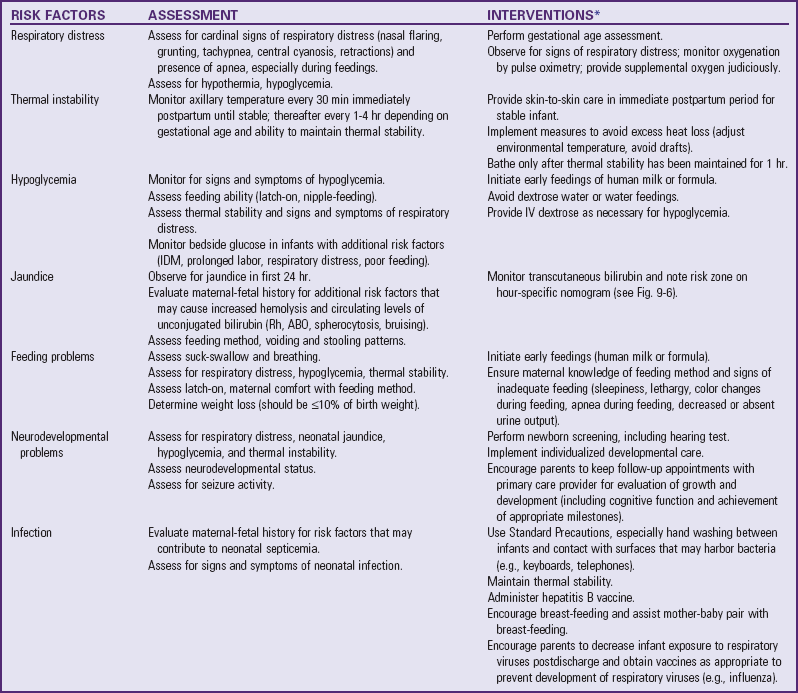
Portions adapted from Askin DF, Bakewell-Sachs S, Medoff-Cooper B, et al: Late preterm infant assessment guide, Washington, DC, 2007, Association of Women’s Health, Obstetric and Neonatal Nurses.
IDM, Infant of diabetic mother; IV, intravenous.
*This is not an exhaustive list of nursing interventions; additional interventions include those discussed under the care of the high-risk infant in this chapter.
Classification of High-Risk Newborns
High-risk infants are most often classified according to birth weight, gestational age, and predominant pathophysiologic problems. The more common problems related to physiologic status are closely associated with the infant’s state of maturity and usually involve chemical disturbances (e.g., hypoglycemia, hypocalcemia) and consequences of immature organs and systems (e.g., hyperbilirubinemia, respiratory distress, hypothermia). Box 10-1 outlines specific terminology describing the developmental status of the newborn.
Formerly, weight at birth reflected a reasonably accurate estimation of gestational age; that is, if an infant’s birth weight exceeded 2500 g (5.5 lb), the infant was considered to be mature. However, accumulated data have shown that intrauterine growth rates are not the same for all infants and that other factors (e.g., heredity, placental insufficiency, and maternal disease) influence intrauterine growth and the infant’s birth weight. From these data a more definitive and meaningful classification system that encompasses birth weight, gestational age, and neonatal outcome has been developed. It has also been determined that the lowest perinatal mortality occurs in the infant who weighs between 3000 and 4000 g (6.4 and 8.8 lb) and whose gestational age is more than 36 weeks and less than 42 weeks (Walsh and Fanaroff, 2006). (See Fig. 8-2 for size comparison of newborn infants.)
Many perinatal problems can be anticipated before delivery. Prenatal testing and labor monitoring have reduced the incidence of perinatal mortality, and specialized care of the distressed newborn is improving the survival rate. If the infant is likely to require special therapy at or soon after birth, plans can be made for the delivery to take place at a hospital with the facilities to provide such care. This eliminates delay in initiating needed care and averts some of the hazards associated with transporting the sick newborn. Prenatal evaluation of fetal well-being and advanced surgical and anesthetic techniques have made intrauterine treatment of certain pathologic conditions possible, thus enhancing the neonate’s chances for survival (Reed and Blumer, 2006).
Intensive Care Facilities
Rapid advances in our understanding of the pathophysiology of the neonate and increased capacity to apply this knowledge have emphasized the need for appropriate settings in which to care for the seriously ill infant. Advancements in electronics and biochemistry, new methods for monitoring cardiorespiratory function, microtechniques for biochemical determination from minute quantities of blood, noninvasive monitoring, and new methods for assisted ventilation and conservation of body heat have made it possible to effectively manage the newborn with serious illness.
Intensive care of the ill and immature newborn requires specialized knowledge and skill in a number of areas. Much of the equipment used in the care of the critically ill adult is unsuited to the singular needs of the very small infant; therefore equipment has been modified to meet these needs. Examples of modifications include ventilators that deliver small volumes of oxygen in the proper concentration and pressure, infusion pumps that accurately deliver very small amounts, and radiant heat warmers that provide a constant source of warmth and allow maximum access to the infant. Most important, advances in intensive care have created a need for highly skilled personnel trained in the art of neonatal intensive care.
The diversity of special care needs requires that the unit be arranged for graduated care of the infant population. There should be adequate facilities and skilled personnel to provide one-to-one nursing care for each seriously ill infant, as well as a means for graduation to one-to-three or one-to-four nursing care in a quieter area where infants require less intensive care until they are ready to be discharged to home. Family-centered care and a relatively quiet environment are often difficult to provide in a busy neonatal intensive care unit (NICU); therefore some units have developed step-down units and single-room units where high-risk infants may be observed by skilled staff. Such areas are designed for family-centered care along with appropriate neurodevelopmental care.
Organization of Services
The most efficient organization of services is a regionalized system of facilities within a designated geographic area. Neonatal intensive care facilities may provide three prescribed levels of care with special equipment, skilled personnel, and ancillary services concentrated in a centralized institution (American Academy of Pediatrics and American College of Obstetricians and Gynecologists, 2007):
Level I facility—Provides management of normal maternal and newborn care.
Level IIA facility—Provides a full range of maternity and newborn care and can provide care to infants born at more than 32 weeks of gestation and weighing more than 1500 g (3.3 lb) who are moderately ill with problems that are expected to resolve rapidly and who are not anticipated to need subspecialty care; or who are convalescing after intensive care.
Level IIB facility—In addition to the above, can provide mechanical ventilation for up to 24 hours and can provide continuous positive airway pressure (CPAP).
Level III facility—Neonatal intensive care
• Level IIIA units provide care for infants with birth weight of more than 1000 g (2.2 lb) and gestational age of more than 28 weeks. Life support is limited to conventional mechanical ventilation.
• Level IIIB units can provide care for extremely low–birth-weight (ELBW) infants with technology including high-frequency ventilation and inhaled nitric oxide, on-site access to pediatric medical subspecialists, and advanced diagnostic imaging and pediatric surgery available.
• Level IIIC units have the capabilities of a level IIIB NICU and, in addition, offer extracorporeal membrane oxygenation (ECMO) and surgical repair of serious congenital cardiac malformations.
Transporting High-Risk Newborns
When an at-risk infant is identified or anticipated, arrangements are made for care in the intensive care facility. The uterus is the ideal transport unit for the infant with anticipated difficulties; therefore, whenever possible, take the mother where special care is available for her delivery.
Some infants develop difficulties after a seemingly normal pregnancy and uncomplicated labor. Because it is impossible to always predict when infants will require intensive care, a coordinated system is needed to ensure them an optimum opportunity for survival. Each hospital that delivers infants should be able to provide for appropriate neonatal stabilization and arrange for transport to a tertiary care facility. The infant must be kept warm, be adequately oxygenated (including intubation if indicated), have vital signs and oxygen saturation monitored, and, when indicated, receive an intravenous (IV) infusion. The infant is transported in a specially designed incubator unit that contains a complete life-support system and other emergency equipment that can be carried by ambulance, van, plane, or helicopter.
The transport team may consist of one or more of the highly trained persons from the NICU: a neonatologist (or a fellow in neonatology), a neonatal nurse practitioner, a respiratory therapist, and one or more nurses. The professional assigned to accompany the infant must be constantly alert to every change in the infant’s condition and able to intervene appropriately. The neonate who must be moved from one place to another within the hospital (e.g., to surgery, or from delivery room to nursery) is transported in an incubator or radiant warmer and accompanied by the necessary personnel and equipment.
Nursing Care of High-Risk Newborns
Because the majority of infants admitted to intensive care facilities are born before the estimated date of delivery, this chapter focuses primarily on the preterm infant. (See p. 344 for a description of the characteristics of preterm infants.) The incidence of neonatal complications (e.g., respiratory distress and hypoglycemia) is highest in this group, and often other high-risk factors (e.g., sepsis and congenital malformations) are found in association with prematurity. This chapter discusses nursing problems encountered in the intensive care nursery, then considers common complications. Nursing care of high-risk infants with more serious disorders is examined in relation to specific high-risk conditions.
Assessment
At birth the newborn is given a cursory yet thorough assessment to determine any apparent problems and identify those that demand immediate attention. This examination is primarily concerned with the evaluation of cardiopulmonary and neurologic functions. The assessment includes the assignment of an Apgar score (see Chapter 8) and an evaluation for any obvious congenital anomalies or evidence of neonatal distress. The infant is stabilized and evaluated before being transported to the NICU for therapy and more extensive assessment. (See Clinical Assessment of Gestational Age, Chapter 8.)
A thorough, systematic physical assessment is an essential component in the care of the high-risk infant (see Nursing Care Guidelines box). Subtle changes in feeding behavior, activity, color, oxygen saturation (Spo2), or vital signs often indicate an underlying problem. The preterm infant, especially the ELBW infant, is not able to withstand prolonged physiologic stress and may die within minutes of exhibiting abnormal symptoms if the underlying pathologic process is not corrected. The alert nurse is aware of subtle changes and reacts promptly to implement interventions that promote optimum function in the high-risk neonate. The nurse notes changes in the infant’s status through ongoing observations of the infant’s adaptation to the extrauterine environment.
Observational assessments of the high-risk infant are made according to the infant’s acuity (seriousness of condition); the critically ill infant requires close observation and assessment of respiratory function, including continuous pulse oximetry, electrolytes, and blood gases. Accurate documentation of the infant’s status is an integral component of nursing care. With the aid of continuous, sophisticated cardiopulmonary monitoring, nursing assessments and daily care can be coordinated to allow for minimum handling of the infant (especially the very low–birth-weight [VLBW] or ELBW infant) to decrease the effects of environmental stress.
Monitoring Physiologic Data
Most neonates under intensive observation are placed in a controlled thermal environment and monitored for heart rate, respiratory activity, and temperature. The monitoring devices are equipped with an alarm system that indicates when the vital signs are above or below preset limits. However, a “hands on” assessment, including auscultation of heart tones and breath sounds, is essential.
The placement of electrodes may be challenging because of the lack of flat areas on the neonate’s chest, the limited space for alternating sites, the size of the electrodes, and irritation from the adhesive. Hydrogel electrodes are gentler on the skin and are easily removed by lifting an edge from the skin and moistening it with plain water to release the adhesive (Lund and Durand, 2006). If the same electrode is reapplied to the skin, rinse the hydrogel with plain water to remove accumulated sodium from perspiration, which can eventually irritate the skin. It is important to follow the manufacturer’s directions for care and handling of electrodes to avoid malfunction or burns to sensitive skin.
Monitor blood pressure routinely in the sick neonate by either internal or external means. Direct recording with arterial catheters is often used but carries the risks inherent in any procedure in which a catheter is introduced into an artery. An umbilical venous catheter may also be used to monitor the neonate’s central venous pressure. Oscillometry (Dinamap) or Doppler transcutaneous apparatus is a simple, effective means for detecting alterations in systemic blood pressure (hypotension or hypertension). Table 10-2 lists normal blood pressure ranges for healthy preterm infants. Infants who have birth asphyxia, have low Apgar scores, or are mechanically ventilated have lower systolic and diastolic pressures.
TABLE 10-2
BLOOD PRESSURE RANGES IN DIFFERENT WEIGHT GROUPS OF HEALTHY PRETERM INFANTS*
| BIRTH WEIGHT | SYSTOLIC PRESSURE (mm Hg) | DIASTOLIC PRESSURE (mm Hg) |
| 501-750 g (1.1-1.6 lb) | 50-62 | 26-36 |
| 751-1000 g (1.6-2.2 lb) | 48-59 | 23-36 |
| 1001-1250 g (2.2-2.7 lb) | 49-61 | 26-35 |
| 1251-1500 g (2.7-3.3 lb) | 46-56 | 23-33 |
| 1501-1750 g (3.3-3.8 lb) | 46-58 | 23-33 |
| 1751-2000 g (3.8-4.4 lb) | 48-61 | 24-35 |
*Defined as infants without a history of maternal hypertension, Apgar scores of <3 at 1 min and <6 at 5 min, pneumothorax, hematocrit 0.32, serum pH 7.1, use of dopamine, infusion of erythrocytes or colloid, mechanical ventilation, or cardiopulmonary resuscitation.
Modified from Hegyi T, Carbone MT, Anwar M, et al: Blood pressure ranges in premature infants, part I, The first hours of life, J Pediatr 124(4):630, 1994.
In the NICU frequent laboratory examinations and their interpretation are integral parts of the ongoing assessment of infants’ progress. The nurse keeps accurate intake and output records on all acutely ill infants. An accurate output can be obtained by collecting urine in a plastic urine collection bag specifically made for preterm infants (see Urine Specimens, Chapter 27) or by weighing the diapers, which is the simplest and least traumatic means of measuring urinary output. The preweighed wet diaper is weighed on a gram scale, and the gram weight of the urine is converted directly to milliliters (e.g., 25 g = 25 ml).
Urine obtained from cloth diapers and disposable diapers containing absorbent gel material may yield inaccurate results for urine specific gravity, pH, and protein. Urine samples obtained from 100%-cotton cottonballs strategically placed in the diaper proved to be the most accurate.
Blood examinations are a necessary part of the ongoing assessment and monitoring of the sick newborn’s progress. The tests most often performed are blood glucose, bilirubin, electrolytes, calcium, hematocrit, and blood gases. Samples may be obtained by heel stick; venipuncture; arterial puncture; or an indwelling catheter in an umbilical vein, umbilical artery, or peripheral artery. (See Atraumatic Care box, Heel Punctures, in Chapter 8.) In one study, the use of an automated incision device for heel blood sampling resulted in the need for fewer heel pokes, less bruising of both the foot and the leg, and less inflammation than manual lancets (Vertanen, Fellman, Brommels, et al, 2001). When skilled phlebotomists are available, venipuncture for blood collections may be preferred. A Cochrane review comparing heel punctures to venipuncture found that infants receiving a venipuncture for blood collection demonstrated less pain response than those receiving a heel lance and that use of venipuncture reduced the need for repeated heel punctures (Shah and Ohlsson, 2007a).
When numerous blood samples must be drawn, it is important to maintain an accurate record of the amount of blood being removed, especially in ELBW and VLBW infants, who cannot afford to lose blood during the acute phase of their illness.
When infants require close monitoring of oxygenation, pulse oximetry, a noninvasive measurement of the saturation or percent of oxygen in the hemoglobin, is typically used. Although used less frequently than pulse oximetry, some situations warrant the monitoring of transcutaneous oxygen (tcPo2) and carbon dioxide (tcPco2). The nurse notes changes in oxygenation (or other aspects being monitored) associated with handling and adjusts the infant’s care accordingly. The frequency of taking vital signs depends on the infant’s acuity level and response to handling.
Safety Measures
The increased sophistication of supportive technology, including delivery systems, monitors, ventilator devices, and warmers, is both boon and bane. Although built-in safety systems and better engineering have made these devices more reliable and easier to use, our increasing reliance on them carries with it the additional risks of electrical biohazards and inaccurate function. Additionally, untrained or inexperienced operators confer an extra element of risk. Parents need instruction regarding safety precautions and observations. They are usually uncomfortable around the equipment and atmosphere of an intensive care unit and therefore appreciate an explanation of the purposes and functions of the devices and pertinent safety aspects. Although most NICUs are closed units, parents must also learn about specific safety measures designed to prevent neonatal abduction. Most institutions have their own protocols for preventing such an occurrence. (See Protect from Infection and Injury, Chapter 8.)
Respiratory Support
The primary objective in the care of high-risk infants is to establish and maintain respiration. Many infants require supplemental oxygen and assisted ventilation. All infants require appropriate positioning to ensure an open airway and to maximize oxygenation and ventilation. Oxygen therapy is provided on the basis of the infant’s requirements and illness (see Respiratory Distress Syndrome, p. 347, and Oxygen Therapy, p. 352).
Thermoregulation
Concurrent with the establishment of respiration, the most crucial need of the low-birth-weight (LBW) infant is provision of external warmth. Prevention of heat loss in the distressed infant is absolutely essential for survival, and maintaining a neutral thermal environment is a challenging aspect of neonatal intensive nursing care. Heat production is a complicated process that involves the cardiovascular, neurologic, and metabolic systems, and the immature neonate has all the problems related to heat production that are faced by the full-term infant. (See Thermoregulation, Chapter 8.) However, LBW infants are placed at further disadvantage by a number of additional problems. They have an even smaller muscle mass and fewer deposits of brown fat for producing heat, lack insulating subcutaneous fat, and have poor reflex control of skin capillaries.
Pathophysiology: The immature neonate, unable to increase activity and lacking a shivering response, produces heat primarily through increased metabolic processes. Some heat continues to be generated by liver, heart, brain, and skeletal muscles, but the major source of increased heat production during cold stress is nonshivering thermogenesis. Norepinephrine, secreted by the sympathetic nerve endings in response to chilling, stimulates fat metabolism in the richly vascularized brown adipose tissue to produce internal heat, which is then conducted through the blood to surface tissues. A significant increase in metabolism requires increased oxygen consumption.
The consequences of cold stress that pose additional hazards to the neonate are (1) hypoxia, (2) metabolic acidosis, and (3) hypoglycemia. Increased metabolism in response to chilling creates a compensatory increase in oxygen and calorie consumption.
Norepinephrine, released in response to cold stress, causes pulmonary vasoconstriction, which further reduces the effectiveness of pulmonary ventilation. This decrease in oxygen intake diminishes the supply available for glucose metabolism. As a result, glucose is broken down by an alternate, hypoxic pathway (anaerobic glycolysis) that generates increased lactic acid. This, together with acid end-products of brown fat metabolism, contributes to the acidotic state. Anaerobic metabolism dissipates glycogen at a greatly increased rate over aerobic metabolism, thus precipitating hypoglycemia. This condition is especially marked when glycogen stores are diminished at birth and caloric intake is inadequate after birth.
Maintaining Thermoneutrality: To delay or prevent the effects of cold stress, at-risk newborns are placed in a heated environment immediately after birth, where they remain until they are able to independently maintain thermal stability—the capacity to balance heat production and conservation and heat dissipation. Because overheating produces an increase in oxygen and calorie consumption, the infant is also jeopardized in a hyperthermic environment. A neutral thermal environment is one that permits the infant to maintain a normal core temperature with minimum oxygen consumption and calorie expenditure. Studies indicate that optimum thermoneutrality cannot be predicted for every high-risk infant’s needs (Blackburn, 2007; Blake and Murray, 2006).
VLBW and ELBW infants, with thin skin and almost no subcutaneous fat, can control body heat loss or gain only within a limited range of environmental temperatures. In these infants heat loss from radiation, evaporation, and transepidermal water loss is three to five times greater than in larger infants, and a decrease in body temperature is associated with an increase in mortality.
The three primary methods for maintaining a neutral thermal environment are the use of an incubator, a radiant warming panel, and an open bassinet with cotton blankets. The healthy, full-term infant dressed and under blankets can maintain a stable temperature within a wider range of environmental temperatures; however, the infant requiring close observation or treatments such as phototherapy may need to be cared for in an incubator or under radiant heat (Fig. 10-1). The incubator should always be prewarmed before placing an infant in it. The use of double-walled incubators significantly improves the infant’s ability to maintain a desirable temperature and reduces energy expenditure related to heat regulation. The infant is clothed and warmly wrapped in blankets when removed from the warm environment of the incubator for feeding or cuddling. Inside or outside the incubator, head coverings are effective in preventing heat loss. A fabric-insulated cap is more effective than one fashioned from stockinette (Blackburn, 2007).

Fig. 10-1 Nurse caring for infant in radiant warmer. (Courtesy E. Jacobs, Texas Children’s Hospital, Houston.)
An effective means for maintaining the desired range of temperature in the infant is the use of an automatically controlled (servocontrolled) incubator. The mechanism, when set at the upper and lower limits of the desired circulating air temperature range, adjusts automatically in response to signals from a thermal sensor attached to the abdominal skin. If the infant’s temperature drops, the warming device is triggered to increase heat output. The servocontrol is usually set to a desired skin temperature between 36° and 36.5° C (96.8° and 97.7° F) (Blake and Murray, 2006).
Convective heat loss occurs when infants are exposed to increased air flow velocity and turbulence (e.g., drafts from doors, ventilation system, opening and closing incubator portholes and side panels). The infant being cared for in a radiant warmer also experiences convective heat losses in response to ventilation drafts and traffic flow around the bed; these losses may be partially countered with plastic wrap placed directly on the infant’s body or stretched over the side guards of the warmer unit (Fig. 10-2). Oxygen or any source of air, such as an oxygen mask or tube, should not blow directly on the infant’s face. Oxygen concentrated around the head, such as that supplied to a hood, must be warmed and humidified.
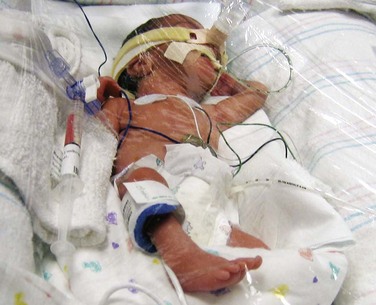
Fig. 10-2 Infant under plastic wrap, which produces a draft-free environment. (Courtesy E. Jacobs, Texas Children’s Hospital, Houston.)
Radiant heat loss is one of the greatest threats to temperature regulation in the incubator, since the temperature of circulating air within has no influence on heat loss to cooler surfaces without, such as windows, walls, or a lower nursery temperature. Such losses can be effectively reduced with the use of double-walled incubators; the infant radiates heat to the inner wall, which is surrounded by the warmed incubator air. The use of a cloth incubator cover further reduces radiant heat loss and provides some protection from exterior light sources.
A high-humidity atmosphere contributes to body temperature maintenance by reducing evaporative heat loss. Humidity is provided in some incubators by circulating air over a heated water reservoir, which has the additional advantage of decreasing heat loss by convection as the air flows over the infant. The water reservoir in older model incubators was often a source of water-borne bacteria, resulting in the need for frequent water changes. Newer technologies such as ultrasonic nebulizers may reduce the risk of such infections. Follow manufacturer’s recommendations in determining the frequency of water changes. The recommended humidity is 50% to 65%; higher humidity and a warmer environment are recommended for VLBW and ELBW infants.
A number of “microenvironments” may be used with the VLBW and ELBW infant to minimize evaporative and insensible water losses (IWLs). These include items such as bubble wrap blankets, humidified reservoirs for incubators, humidified tents, humidified Plexiglas boxes with plastic wrap coverings, polyethylene bags, and plastic wrap blankets. In cold-stressed infants, heat shields may be inappropriate because they may block heat from reaching the infant. The use of emollient cream to prevent transepidermal water loss has been used; however, this therapy has increased the risk of infection with coagulase-negative staphylococcus, and in preterm infants weighing 750 g or less, it should be used with caution (Association of Women’s Health, Obstetric and Neonatal Nurses, 2007).
The nurse can reduce conductive heat loss by warming all items that come in direct contact with the infant, such as scales, radiographic film, blankets, and the hands of caregivers. For example, the nurse can store blankets in a warming unit ready for use and place a freestanding warming unit or a heat lamp over a scale before weighing an infant.
Although the open radiant warmer unit allows easier access to the infant, there is an inherent increase in evaporative water loss (and evaporative heat loss) from the skin, especially in ELBW and VLBW infants. Transepidermal water losses, a form of IWL, may be increased by as much as 50% to 200%, thus predisposing the infant to dehydration; daily fluid requirements are generally increased to compensate for such losses. The use of plastic wrap over the ELBW or VLBW infant in a radiant warmer will help reduce IWL and convective losses.
The infant being cared for in a radiant warmer is kept warm using the servocontrol method. Air temperature manual control should not be used because of the danger of overheating the infant. A reflective aluminum temperature probe cover is used to allow proper function of the servocontrol heating unit. Traditionally, the temperature probe is placed over a nonbony, well-perfused tissue area such as the abdomen, flank, or back. In general, the probe site is changed when the infant’s position is changed to prevent the probe from coming in contact with the bed surface and potentially trapping heat at the probe site, causing an abnormal ambient temperature. Blackburn, De Paul, Loan, and colleagues (2001) found that abdominal and back skin temperatures varied considerably based on the infant’s position and the probe position; when infants were positioned prone and the probe was on the abdomen, the skin temperature rose. The researchers concluded that changing probe sites with repositioning may result in unstable body temperatures, that a consistent method of probe placement is needed, and that placement of the probe on the lateral abdomen may allow for frequent position changes (supine and prone) without the difficulties that occur when the infant lies on the probe.
The use of sterile cloth or disposable drapes also blocks radiant heat waves in a radiant warmer; during such procedures the use of a warmed blanket under the infant is appropriate. Clothing an infant on servocontrol in an incubator or radiant warmer is not recommended; head covering and foot covering (socks or booties) may be used with discretion.
Prolonged exposure to cold stress in the sick or preterm infant, particularly the ELBW or VLBW infant, may have disastrous results from which recovery may not be possible. Thermoregulation measures in the labor and delivery area and during transport to the NICU are essential. The use of a plastic bag or plastic wrap; careful drying; prewarming of equipment such as scales, stethoscopes, and incubators; and prompt placement of the VLBW or ELBW newborn in a proper heat source are essential for the prevention of further morbidity.
Hyperthermia may cause equally untoward effects because high-risk infants typically have a limited ability to perspire, thus decreasing heat dissipation. In high-risk neonates hyperthermia is usually a result of overheating rather than hypermetabolism. Therefore knowledge of proper care and use of external heating devices, such as radiant warmers or incubators, is as important as knowing the conditions for which they are being used.
Protection from Infection
Protection from infection is an integral part of all newborn care, but preterm and sick neonates are particularly susceptible. Thorough, meticulous, and frequent hand washing is the foundation of a preventive program. This includes all persons who come in contact with infants and their equipment. After handling another infant or equipment, no one should ever touch an infant without first washing hands.
Personnel with infectious disorders are either barred from the unit until they are no longer infectious or are required to wear suitable shields, such as masks or gloves, to reduce the likelihood of contamination. Standard Precautions as a method of infection control are instituted in all nursery areas to protect the infants and staff. (See Chapter 27.)
Readmission of infants from home or admission of infants delivered in unsterile conditions or infants suspected of having communicable illnesses is handled per institutional protocol. Such infants should at least be initially physically isolated from other highly susceptible high-risk infants. (See American Academy of Pediatrics and American College of Obstetricians and Gynecologists [2007] for further infection control recommendations, including nursery care of infants with specific communicable diseases.)
Hydration
High-risk infants often receive supplemental parenteral fluids to supply additional calories, electrolytes, and/or water. Adequate hydration is particularly important in preterm infants because their extracellular water content is higher (70% in full-term infants and up to 90% in preterm infants), their body surface area is larger in comparison to their weight, and the capacity for osmotic diuresis is limited in their underdeveloped kidneys. Therefore these infants are highly vulnerable to fluid depletion.
Parenteral fluids may be given to the high-risk neonate via several routes depending on the nature of the illness, the duration and type of fluid therapy, and unit preference. Common routes of fluid infusion include peripheral, peripherally inserted central venous (or percutaneous central venous), surgically inserted central venous or arterial, and, at times, umbilical venous or umbilical arterial catheterization. The preferred sites for peripheral IV infusions in neonates are the peripheral veins on the dorsal surfaces of the hands or feet. Alternative sites are scalp veins and antecubital veins. Special precautions and frequent observations (at least once every hour) must accompany the use of peripheral lines with hypertonic solutions (dextrose 10% to 12%) and parenteral hyperalimentation solutions. In many neonatal centers the percutaneous central venous catheter, also commonly called the peripherally inserted central venous catheter, is used for IV hydration therapy and medication administration because of less expense and decreased neonatal trauma, and because of the ease of insertion (Bradshaw, Turner, and Pierce, 2006).
In most facilities NICU nurses insert peripheral IV catheters and maintain the infusions. IV fluids must always be delivered by continuous infusion pumps that deliver minute volumes at a preset flow rate. Secure the catheter to the skin with transparent tape or a specialized IV dressing, taking care not to cause undue pressure from the needle hub and tubing. Because ELBW and VLBW infants are highly vulnerable to any fluid shifts, infusion rates are carefully regulated and checked hourly to prevent tissue damage from extravasation, fluid overload, or dehydration (Kerr, Starbuck, and Block, 2006). Pulmonary edema, congestive heart failure, patent ductus arteriosus (PDA), and intraventricular hemorrhage (IVH) may occur with fluid overload. Dehydration may cause electrolyte disturbances (particularly sodium), with potentially serious central nervous system (CNS) effects.
Small, fragile peripheral blood vessels are subject to rupture and subsequent infiltration. This situation is compounded by the use of infusion pumps that continue to infuse fluid into surrounding tissues. Observations are especially important when using hypertonic solutions (calcium, sodium bicarbonate, parenteral hyperalimentation) and IV drugs (antibiotics and vasoactive drugs such as dopamine and dobutamine), which can cause serious tissue damage. With flexible catheters and small IV catheter shields, arm boards and limb restraints are usually unnecessary. If used, restraints should be checked frequently to ensure that no harm to the patient’s extremity occurs and that peripheral circulation is adequate.
Infants who are ELBW, tachypneic, receiving phototherapy, or in a radiant warmer have increased IWL that require appropriate fluid adjustments. Nurses must monitor fluid status by taking daily (or more frequent) weights; accurately monitoring intake and output of all fluids, including medications and blood products; monitoring urine specific gravity as well as urine glucose and protein; and evaluating serum electrolyte levels. ELBW infants often require more frequent monitoring of these parameters because of their excessive transepidermal fluid loss, immature renal function, and propensity to dehydration or overhydration. Intolerance of even dextrose 5% is not uncommon in the ELBW infant, with subsequent glycosuria and osmotic diuresis. Alterations in behavior, alertness, or activity level in these infants receiving IV fluids may signal an electrolyte imbalance, hypoglycemia, or hyperglycemia. The nurse is also observant for tremors or seizures in the VLBW or ELBW infant, since these may be a sign of hyponatremia or hypernatremia.
A common problem observed in infants who have an umbilical arterial catheter in place is vasoconstriction of peripheral vessels, which can seriously impair circulation. The response is triggered by arterial vasospasm caused by the presence of the catheter, the infusion of fluids, or injection of medication. Blanching of the buttocks, genitalia, or legs or feet is an indication of vasospasm. The problem is recognized promptly and reported to the practitioner. The nurse must also observe for signs of thrombi in infants with umbilical venous or arterial lines. The precipitation of microthrombi in the vascular bed with the use of such catheters is commonly manifested by a sudden bluish discoloration seen in the toes, called “cath toes.” The problem is promptly reported to the practitioner because failure to alleviate the pathologic condition may result in permanent injury to the toes, foot, or leg.
Circulatory effects are observed first in the toes but may extend to include the legs and buttocks. The toes first flush and then turn a mulberry color; if the condition is not corrected, there may be serious complications involving the loss of a limb. The infant with an umbilical venous or arterial catheter should also be observed closely for catheter dislodgment and subsequent bleeding or hemorrhage; urinary output, renal function, and gastrointestinal function are also evaluated in these infants. Although the intent of such catheters is to effectively deliver IV fluids (and sometimes medications) and to obtain arterial blood gas samples, they are not without inherent complications.
Nutrition
Optimum nutrition is critical in the management of ELBW, VLBW, and LBW preterm infants, but difficulties arise in providing for their nutritional needs. The various mechanisms for ingestion and digestion of foods are not fully developed. The more immature the infant, the greater the problem.
Physiologic Characteristics: The preterm infant’s need for rapid growth and daily maintenance must be met in the presence of several anatomic and physiologic disabilities. Although infants demonstrate some sucking and swallowing activities before birth, coordination of these mechanisms does not occur until approximately 32 to 34 weeks of gestation, and they are not fully synchronized until 36 to 37 weeks. Initial sucking is not accompanied by swallowing, and esophageal contractions are uncoordinated. As infants mature, the suck-swallow pattern develops but is slow and ineffectual, and these reflexes may easily become exhausted.
As with most full-term infants, preterm infants have poor muscle tone in the area of the lower esophageal (cardiac) sphincter. This causes milk in the stomach to be easily regurgitated into the esophagus, where it can trigger the chemoreceptors and cause apnea (vagal stimulation) and bradycardia and increase the risk of aspiration. The stomach has a limited capacity in preterm infants and is easily overdistended, further compromising respiration.
Physiologically, preterm infants (LBW, not ELBW or VLBW) have approximately the same capacity to digest and absorb protein as full-term infants. However, carbohydrates and fats are less well tolerated. The secretion of lactase, a late-developing enzyme, is low in infants born before 34 weeks of gestation; therefore formulas containing lactose may not be well tolerated. Although amylase is deficient in preterm infants, an alternative enzyme (glucoamylase) is able to compensate in most neonates so that they can tolerate moderate amounts of starch. Preterm infants are inefficient in digesting and absorbing lipids, especially the saturated triglycerides of cow’s milk, because they have low levels of pancreatic lipase and low bile acid.
Nutritional Needs: The demand for nutrients in LBW infants is much higher than that in larger infants, and individual infants vary in activity level, ease of achieving basal energy expenditure, thermoneutrality, physical condition, and efficacy of nutrient absorption. The American Academy of Pediatrics, Committee on Nutrition (2009a), recommends an energy intake of 105 to 130 kcal/kg/day (taken enterally) for most preterm infants to achieve a satisfactory growth rate. It is estimated that for a daily weight gain of 15 g/kg, a caloric expenditure of 45 to 67 kcal/kg above the maintenance expenditure of 50 kcal/kg (Table 10-3) would be required (American Academy of Pediatrics, 2009a). Thus the amount of calories required for optimum growth in sick and VLBW infants is significantly higher than in their healthy full-term counterparts; the challenge of providing adequate calories for extrauterine growth in the preterm infant with limited capability to ingest and absorb nutrients is an important part of nursing care for this population.
TABLE 10-3
ESTIMATED ENERGY REQUIREMENT IN LOW-BIRTH-WEIGHT INFANTS
| ENERGY EXPENDITURE | AVERAGE ESTIMATION (kcal/kg/day) |
| Total energy used | 40-60 |
| Resting metabolic rate | 40-50* |
| Activity | 0-5* |
| Thermoregulation | 0-5* |
| Energy synthesis | 15† |
| Stored energy | 20-30† |
| Stool loss (energy) | 15 |
| Energy intake | 90-120† |
*Energy required for maintenance.
†Energy expenditure for growth.
Adapted from American Academy of Pediatrics, Committee on Nutrition: Pediatric nutrition handbook, ed 6, Evanston, Ill, 2009, The Academy; and Committee on Nutrition of the Preterm Infant, European Society of Paediatric Gastroenterology and Nutrition: Nutrition and feeding of preterm infants, Oxford, 1987, Blackwell Scientific Publications.
Table 10-3 shows the caloric requirements of healthy, growing preterm infants at 3 to 4 weeks of age. The energy requirements for sick and VLBW infants remains unknown; estimates are an intake of up to 105 to 115 kcal/kg/day, including a protein intake of 3 g/kg/day, for the ELBW infant (American Academy of Pediatrics, 2009a). Because most of the nutritional stores are accumulated in the final months of gestation, preterm infants also have low stores of calcium, iron, phosphorus, proteins, and vitamins A and C.
The infant’s size and condition determine the amount and method of feeding. Nutrition can be provided by either the parenteral or enteral route or by a combination of the two.
Total parenteral nutritional support of acutely ill infants may be accomplished with commercially available IV solutions specifically designed to meet the infant’s nutritional needs, including protein, amino acids, trace minerals, vitamins, carbohydrates (dextrose), and fat (lipid emulsion). Early protein intake (on day 1 of life) is also important in optimizing growth in LBW infants (Stephens, Walden, Gargus, et al, 2009). Daily monitoring of weight, electrolytes, renal function, calcium, and hydration status is carried out to ensure adequate therapy. As important as nutrition is the maintenance of adequate serum glucose homeostasis in sick preterm infants, who may depend on exogenous glucose sources for several days or weeks. Cornblath and Ichord (2000) recommend that in sick preterm infants an operational threshold blood glucose value of 45 to 50 mg/dl (2.6 to 2.8 mmol/L) be maintained.
Studies have revealed benefits to the early introduction of small amounts of enteral feedings in metabolically stable preterm infants (Hay, 2008). These minimum enteral feedings (MEFs; trophic feedings, gastrointestinal [GI] priming) have been shown to simulate the infant’s GI tract, preventing mucosal atrophy and subsequent enteral feeding difficulties. They have also been shown to reduce the risk of sepsis. MEFs with as little as 0.1 to 4 ml/kg formula or breast milk may be given by gavage as early as day one of life or as soon as the infant is medically stable. In the past early introduction of milk feedings was thought to increase the risk of a devastating intestinal complication, necrotizing enterocolitis (NEC). NEC occurs more frequently in preterm infants, but the etiology of the disease remains unclear. No increased incidence of NEC in those VLBW infants given MEF has been found (Terrin, Passariello, Canani, et al, 2009; Berseth, Bisquera, and Paje, 2003).
A Cochrane review showed that infants receiving trophic feedings versus no feedings had an overall reduction in the number of days to full feedings and a shorter length of stay (Tyson and Kennedy, 2005). However, the researchers suggested that there was insufficient evidence to conclude that trophic feedings would indeed prevent NEC.
Controversy still exists regarding the type of enteral feeding that best meets the nutritional needs of LBW infants. The predominant view supports the use of milk from an infant’s own mother. Alternatively, if breast milk is not available, commercial formulas designed specifically to meet the needs of small preterm infants that provide for adequate growth and metabolic stability can be used (Table 10-4). Prepared formulas have the advantage of allowing more concentrated feedings.
A number of studies regarding the effects of long-chain polyunsaturated fatty acids on cognitive development, visual acuity, and physical growth in full-term and preterm infants have prompted formula companies to add docosahexaenoic acid (DHA) and arachidonic acid (AA) to their infant formulas. AA and DHA are in human milk, and their presence has been assumed to lead to an increase in cognitive development in human milk–fed infants compared with infants fed a formula without these fatty acids (Gregory, 2004). One meta-analysis of four clinical trials demonstrated no clinically significant developmental benefits to supplementation of formula with AA and DHA in term and preterm infants at 18 months of age (Beyerlein, Hadders-Algra, Kennedy, et al, 2009).
Milk produced by mothers whose infants are born before term contains higher concentrations of protein, sodium, chloride, and immunoglobulin A (IgA). Thus mothers appear to be the preferred source of milk for their preterm infants. Growth factors, hormones, prolactin, calcitonin, thyroxine, steroids, and taurine (an essential amino acid) are also in human milk. The milk produced by mothers for their infants changes in content over the first 30 days postnatally, at which time it is similar to full-term human milk. Preterm infants who received human milk during their hospitalization demonstrated better intellectual performance scores at 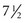 to 8 years of age compared with children who received formula (Schanler, 2001). Improved psychomotor development at 18 months has also been observed in preterm infants fed donor human milk compared with formula-fed preterm infants. Despite its benefits, LBW infants (<1500 g [3.3 lb]) who are exclusively fed unfortified human milk demonstrate decreased growth rates and nutritional deficiencies even beyond the hospitalization period. These infants often have inadequacies of calcium, phosphorus, protein, sodium, vitamins, and energy (Schanler, 2001). Specially designed supplements for human milk have been developed to address these deficits. Preterm infants fed fortified human milk (FHM) have shorter hospital stays and less infection and NEC than infants given preterm formulas. Fortifiers are commercially available, usually as a liquid or powder containing protein; carbohydrate; calcium; phosphorus; magnesium; sodium; and varied amounts of zinc, copper, and vitamins. Because fortifiers do not contain sufficient iron, an exogenous source must be administered after enteral feeding. Fortifiers should be added to milk as close as possible to feeding time, and FHM should be refrigerated until it is used.
to 8 years of age compared with children who received formula (Schanler, 2001). Improved psychomotor development at 18 months has also been observed in preterm infants fed donor human milk compared with formula-fed preterm infants. Despite its benefits, LBW infants (<1500 g [3.3 lb]) who are exclusively fed unfortified human milk demonstrate decreased growth rates and nutritional deficiencies even beyond the hospitalization period. These infants often have inadequacies of calcium, phosphorus, protein, sodium, vitamins, and energy (Schanler, 2001). Specially designed supplements for human milk have been developed to address these deficits. Preterm infants fed fortified human milk (FHM) have shorter hospital stays and less infection and NEC than infants given preterm formulas. Fortifiers are commercially available, usually as a liquid or powder containing protein; carbohydrate; calcium; phosphorus; magnesium; sodium; and varied amounts of zinc, copper, and vitamins. Because fortifiers do not contain sufficient iron, an exogenous source must be administered after enteral feeding. Fortifiers should be added to milk as close as possible to feeding time, and FHM should be refrigerated until it is used.
The antiinfectious attributes of human milk provide additional advantages for preterm infants. Secretory IgA concentration is higher in the milk from mothers of preterm infants than in the milk from mothers of full-term infants. IgA is important in the control of bacteria in the intestinal tract, where it inhibits adherence and proliferation of bacteria on epithelial surfaces. Additional protection from infection is provided by leukocytes, lactoferrin, and lysozyme, all of which are in human milk. Recent research suggests that administration of probiotics, live microbial supplements, decreases the incidence of NEC by normalizing intestinal flora, reducing intestinal permeability, and reducing gut inflammation (Alfaleh, Anabrees, and Bassler, 2009; Deshpande, Rao, and Patole, 2007).
NEC has been shown in several studies to be higher in formula-fed infants than in preterm infants fed human milk. Another report suggests that severity of NEC is lessened and the prevalence of intestinal perforation lowered when preterm infants are fed human milk (Schanler, 2001).
Preterm infants exclusively fed human milk have demonstrated significantly decreased NEC, fewer positive blood cultures, and decreased need for antibiotics. In one study infants fed human milk also received more skin-to-skin (STS) contact with their mothers and shorter hospital stays. Schanler (2001) suggests that STS contact might potentially stimulate the enteromammary immune system to produce specific antibodies against nosocomial pathogens in the nursery. Gastric emptying is improved with human milk feedings for preterm infants, primarily because of increased intestinal lactase and possibly decreased intestinal permeability. Finally, the psychologic advantages the mother gets from using her own milk cannot be overlooked.
For those infants who cannot be breast-fed but who also cannot survive except on human milk, banked donor milk is important. Because of the antiinfective and growth-promoting properties of human milk, as well as its superior nutrition, donor milk is used in many NICUs for preterm or sick infants when the mother’s own milk is not available (American Academy of Pediatrics, 2005). Unprocessed human milk from unscreened donors is not recommended because of the risk of transmission of infectious agents (American Academy of Pediatrics, 2005).
The Human Milk Banking Association of North America (HMBANA; www.hmbana.org) has established guidelines for the operation of donor human milk banks (Human Milk Banking Association, 2008). Donor milk banks collect, screen, process (pasteurize), and distribute milk donated by breast-feeding mothers who are feeding their own infants and pumping a few extra ounces each day for the milk bank. All donors are screened both by interview and serologically for communicable diseases. Donor milk is stored frozen until it is heat processed to kill potential pathogens (bacteria and viruses), and then it is refrozen for storage until it is dispensed for use. The heat processing adds a level of protection for the recipient that is not possible with any other donor tissue or organ. Milk is dispensed only by prescription. A per-ounce fee is charged by the bank for processing, but the HMBANA guidelines prohibit payment to donors.
Although the timing of the first feeding has been controversial, most authorities now believe that early feeding (provided that the infant is medically stable) reduces the incidence of complicating factors such as hypoglycemia and dehydration and reduces the degree of hyperbilirubinemia. The feeding regimen used varies in different units. One strategy for the prevention of NEC that has been supported by research is the use of standardized feeding protocols. A meta-analysis of six studies found a significant reduction in NEC in infants fed by a standard protocol that included cautious advancement in feeding volumes (Patole and de Klerk, 2005).
Feeding tolerance and feeding success are not entirely the same concept. Feeding tolerance is evaluated by the following: (1) soft abdomen; (2) absence of abdominal distention or visible bowel loops on the skin surface; (3) minimum or no aspirated gastric residual; (4) presence of bowel sounds; (5) usual frequency, color, and consistency of stools; (6) minimum to no spitting up or vomiting; (7) infant’s continued interest in feeding; and (8) consistent behavior pattern. Successful oral feeding should be safe, functional, and pleasurable. Feeding success can be measured by an infant’s ability to (1) participate in feeding with energy, (2) coordinate sucking and swallowing with adequate pauses for breathing, (3) maintain vital signs and oxygenation within normal limits, (4) maintain normal muscle tone in face and body, (5) complete feeding in about 20 to 25 minutes, (6) manage a liquid bolus with minimum or no loss of liquid from mouth, (7) sustain alertness for feeding, (8) maintain strength and endurance for entire feeding, and (9) measure appropriate-for-age on standard growth curve. A preterm infant’s success with feeding is first measured in terms of safety and functionality. Nurturing by holding close, but not socializing, during a feeding creates a warm and pleasurable experience. Later, after the infant is a competent feeder, socialization will enrich both parents’ and infant’s mealtime enjoyment.
Gavage Feeding: Gavage feeding is a safe means of meeting the nutritional requirements of infants who are not yet ready to feed orally. These infants are usually too weak to suck effectively, are unable to coordinate swallowing, or lack a gag reflex. A Cochrane review found that infants less than 1500 g (3.3 lb) fed by continuous tube-feeding took longer to reach full oral feeds than those fed intermittently; however, there was no difference in somatic growth or in the incidence of NEC (Premji and Chessell, 2003). Intermittent gavage feeding is used as an energy-conserving technique for infants learning to nipple-feed who become excessively tired, listless, or cyanotic.
A size 3.5, 5, 6, or 8 French feeding tube is usually used to instill the feeding, and the usual methods for determining correct placement are used. (See Chapter 27 for technique.) Although the more relaxed cardiac sphincter makes passage of the tube easier, the heart rate and blood pressure may change in response to vagal stimulation. The procedure is best accomplished when an infant is in a prone or a right side-lying position with the head slightly elevated. Small flexible nasogastric tubes (3.5 and 5 French) may be maintained as an indwelling feeding tube and used for prolonged periods without complications of intermittent removal and insertion.
The stomach is aspirated, the contents measured, and the aspirate returned as part of the feeding. However, this practice may vary, depending on circumstances and individual unit protocol.
The feeding is allowed to flow by gravity, and the length of time varies. This procedure is not used as a timesaving method for the nurse. Complications of indwelling tubes include the obstructed nares, mucous plugs, purulent rhinitis, epistaxis, infection, and possible stomach perforation.
The infant may be held during gavage feedings by the caregiver or parent. Also, nonnutritive sucking (NNS) on a pacifier helps infants associate the sucking with the feeling of satiety. A Cochrane review of NNS demonstrated a significant reduction in length of stay in preterm infants receiving an NNS intervention. Other positive outcomes of NNS included enhanced transition from tube- to bottle-feeding and better bottle-feeding performance (Pinelli and Symington, 2005).
Oral Feeding: Vigorous infants can be fed orally with little difficulty, whereas compromised preterm infants require alternative methods. The amount to be fed is determined largely by the infant’s weight gain and tolerance of previous feeding and is increased by small increments until a satisfactory caloric intake is ensured.
The rate of increase that is well tolerated varies from one infant to another, and determining this rate is often a nursing responsibility. Preterm infants require more time and patience to feed than full-term infants, and the oropharyngeal mechanism may be stressed by an attempt to feed too rapidly. It is important not to tire the infants or overtax their capacity to retain the feedings. When infants require a prolonged time (>30 minutes) to complete a feeding, gavage feeding may be considered for the next time.
The decision regarding when to start breast- or bottle-feeding is somewhat controversial. In many cases the decision is based on an evaluation of the infant’s developmental maturity, weight, activity level, respiratory status (absence of apnea and adequate oxygen saturation levels), and sucking capabilities. Infant behavioral organizational skills, such as the ability to maintain a quiet alert state and display engagement cues, also influence the preterm infant’s successful transition to oral feedings (Thoyre, Shaker, and Pridham, 2005). When infants are unable to tolerate breast- or bottle-feedings, intermittent feedings by gavage begin until they gain enough strength and coordination to use the nipple.
Although the nurse’s role in relation to feeding depends on the institution, the following are suggested nursing responsibilities: (1) recognize feeding readiness cues; (2) identify feeding behaviors typical of preterm infants; (3) understand the infant’s history and current medical condition; (4) consider environment, behavioral state, time of day, nipple type, and positioning; (5) understand rationale for different facilitation techniques and use appropriately; (6) evaluate feeding ability and tolerance; (7) identify infants with poor progress, structural defects, or abnormal feeding patterns who would benefit from specific therapy; and (8) play a supportive role for mothers who choose to breast-feed.
A developmental approach to feeding considers the individual infant’s readiness rather than initiating feedings based on weight and age. Feeding readiness is determined by each infant’s medical status, energy level, ability to sustain a brief quiet alert state, gag reflex (demonstrated with gavage tube insertion), spontaneous rooting and sucking behaviors, and functional sucking reflex (Hunter, 2001).
Oral feeding within a developmental framework involves three steps (Thoyre, Shaker, and Pridham, 2005):
1. Assessing individual physiologic, motor, and state behaviors during feeding
2. Individualizing the feeding plan based on specific infant cues
The goal of feeding must be well understood. A key concept is recognizing the difference between a successful feeding (volume and time) and a successful feeder (infant ability and enjoyment). This is the difference between task and developmental feeding techniques. Planning the progression and nature of feedings requires close monitoring and careful documentation. Baseline assessment data are collected before each feeding and observed during and after the feeding to make a comparative evaluation of feeding success. Assessment is ongoing throughout the feeding, and facilitation techniques are chosen based on the individual infant’s responses to improve the chance for feeding success and tolerance (Nye, 2008). Feeding stress and performance (Box 10-2) are evaluated and documented. Planning is done in collaboration with the health care team and family before the next feeding to determine appropriate strategies for the infant. Box 10-3 gives examples of ways to facilitate feeding.
Breast-Feeding: The American Academy of Pediatrics (2005) recommends human milk as the preferred food for all infants, including sick newborns and preterm infants (with rare exceptions). The academy recognizes that the choice of what to feed is the parents’ prerogative but advises that providers give parents complete and accurate information on the benefits and methods of breast-feeding so they can make an informed decision. Barriers to initiation and continuation of breast-feeding include physician indifference, misinformation, lack of prenatal education about breast-feeding, distracting hospital policies, lack of follow-up, working mother, unsupportive work environment, lack of support from family or society, hospital discharge packs with formula or coupons for formula, and media portrayal of bottle-feeding.
Studies indicate that even small preterm infants are able to breast-feed if they have adequate sucking and swallowing reflexes and no other contraindications, such as respiratory complications or concurrent illness (Dougherty and Luther, 2008; Morton, 2002). Mothers who wish to breast-feed their preterm infants should pump their breasts until their infants are sufficiently stable to tolerate breast-feeding. Appropriate guidelines for the storage of expressed mother’s milk should be followed to decrease the risk of milk contamination and destruction of its beneficial properties. (See Chapter 12.)
Preterm infants may be able to successfully breast-feed earlier than previously believed (28 to 36 weeks). In addition, preterm infants who are breast-fed rather than bottle-fed demonstrate fewer oxygen desaturation episodes; an absence of bradycardia; warmer skin temperature; and better coordination of breathing, sucking, and swallowing (Gardner, Snell, and Lawrence, 2006). The nurse should carefully evaluate the preterm infant for readiness to breast-feed, including assessment of behavioral state, ability to maintain body temperature outside an artificial heat source, respiratory status, and readiness to suckle at the mother’s breast. The latter may be accomplished with NNS at the breast during STS (or kangaroo) contact so the mother and newborn can become accustomed to each other (Gardner, Snell, and Lawrence, 2006). Nasal cannula oxygen may also be provided during breast-feeding if the infant requires it.
Time, patience, and dedication on the part of the mother and the nursing staff are necessary to help infants breast-feed. The process starts slowly, beginning with one oral feeding daily and gradually increasing the feedings as the infant tolerates them. Supplementary bottle-feeding is inefficient because the infant expends energy and calories to feed twice. Feeding more often and/or supplementing with gavage feeding is more energy and calorie efficient. Breast-feeding the preterm infant often requires additional guidance by a lactation consultant and continued support and encouragement by the nursing staff. In addition, postdischarge breast-feeding often requires further guidance, counseling, and support.
Social support for the mother is a major influence on the decision to breast-feed. To be effective advocates for mothers of all ethnicities, nurses must understand the cultural aspects that influence, whether positively or negatively, breast-feeding choices (McCarter-Spaulding, 2009; Gill, 2009). African-American women, for example, identify prenatal health care providers and friends as influential in decisions regarding breast-feeding. They tend to breast-feed less than women from other cultures and should be provided with appropriate information on breast-feeding by health care providers. Breast-feeding materials are available from organizations such as La Leche League International.*
Nipple-Feeding: The infant is positioned in the feeder’s arms or placed semiupright in the lap (Fig. 10-3) and is held with the back curved slightly to simulate the position assumed naturally by most full-term newborns. Stroking the infant’s lips, cheeks, and tongue before feeding helps promote oral sensitivity.
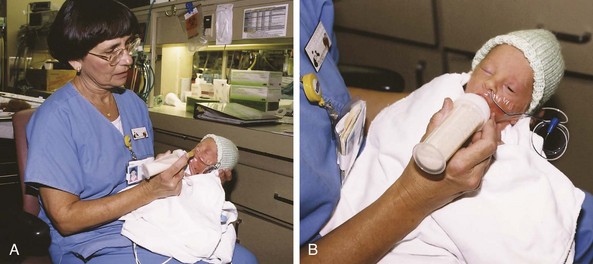
Fig. 10-3 Nipple-feeding the preterm infant. A, Infant is first brought to quiet alert state in preparation for feeding. B, After demonstrating readiness, infant is nipple-fed. (Courtesy Jeff Barnes, Education, and Eastern Oklahoma Perinatal Center, St. Francis Hospital, Tulsa.)
Hill, Kurkowski, and Garcia (2000) used cheek and jaw support for preterm infants between 32 and 34 weeks of gestation to facilitate feeding. Supported infants had fewer and shorter pauses during feeding and had higher postfeeding oxygen saturations than infants not receiving oral support. The groups did not differ in terms of oxygen saturation, heart rate, and respiratory rate during feeding, indicating the technique is as safe as traditional feeding techniques. This technique uses the thumb and index finger to provide gentle pressure (inward and forward) on the cheeks and the third finger to lift and stabilize the jaw under the mandible where the base of the tongue resides.
Bottle-feedings continue if infants are able to tolerate the feedings and take the required amount. The infant is best fed when fully alert. Drowsy infants feed more slowly, and liquid is more likely to fill the relaxed pharynx before the infant swallows, causing choking. It is believed that many digestive powers require signal stimulation to respond. Some preterm infants respond more slowly than full-term infants; therefore the feeding interval and amount are individualized. Preterm infants are often slow feeders and require patience, frequent rest periods, and burping (or bubbling).
A key ingredient for success is choosing an appropriate nipple. The nipple used should be relatively firm and stable. Although a high-flow, pliable nipple requires less energy to use, it may provide a flow rate that is too rapid for some preterm infants to manage without risk of aspiration. A firmer nipple facilitates a more “cupped” tongue configuration and allows for a more controlled, manageable flow rate.
Prodding techniques to encourage sucking can increase the risk of aspiration, especially if adequate breathing opportunities are not provided. The preterm infant has difficulty managing rapid or continuous milk flow with suck, swallow, and breathing coordination when the nipple is manipulated frequently by twisting or turning; the bottle is moved up and down or in and out of the mouth; or the infant’s jaw is moved up and down (not the same as cheek and jaw support). The infant will try to continue to suck or swallow at the risk of physiologic and behavioral consequences.
Research by Law-Morstatt, Judd, Snyder, and colleagues (2003) has demonstrated that a paced bottle-feeding protocol that was structured to limit the length of sucking bursts and lengthen the duration of swallowing and breathing resulted in earlier emergence of organized sucking patterns than traditional approaches to feeding. Similar findings emerged from work by Fucile, Gisel, and Lau (2005), who found that a systematic protocol of oral motor stimulation resulted in enhanced tongue and jaw muscle strength and coordination.
Feeding Resistance
Any feeding technique that bypasses the mouth precludes the opportunity for the affected child to practice sucking and swallowing, or the opportunity to experience normal hunger and satiation cycles. Infants may demonstrate aversion to oral feedings by such behaviors as averting the head to the presentation of the nipple, extruding the nipple by tongue thrust, gagging, or even vomiting.
Developmental delays have occurred in perceptual-motor performance among infants with feeding refusal as measured by standard tests, although intellectual function remains within normal limits. Other observations include disinterest in or active resistance to oral play, diminished spontaneity and motivation, and shallow interpersonal relationships, probably related to the absence of some early incorporative patterns of normal oral experiences. The longer the period of nonoral feeding, the more severe the feeding problems, especially if this period occurs during a time when the infant progresses from reflexive to learned and voluntary feeding actions. During infancy the mouth is the primary instrument for reception of stimulation and pleasure.
Infants identified as being at risk for feeding resistance should receive regular oral stimulation based on the child’s developmental level. Those who exhibit feeding aversion should begin a stimulation program to overcome resistance and acquire the ability to take nourishment by the oral route. Because management requires long-term commitment, successful implementation of a plan for oral stimulation depends on maximum parental involvement and promotion of primary nursing. Key components and interventions are in Box 10-4.
Skin Care
The skin of preterm infants is characteristically immature. Because of its increased sensitivity and fragility, no alkaline-based soap that might destroy the “acid mantle” of the skin is used. The increased permeability of the skin facilitates absorption of ingredients. All skin products (e.g., alcohol or povidone-iodine) are used with caution. The skin is rinsed with water afterward because these substances may cause severe irritation and chemical burns in LBW infants.
The skin is easily excoriated and denuded; therefore take care to avoid damage to the delicate structure. The total skin is thinner than that of full-term infants and lacks rete pegs, appendages that anchor the epidermis to the dermis. Therefore there is less cohesion between the thinner skin layers. Adhesives used after heel sticks or to secure monitoring equipment or IV infusions may excoriate the skin or adhere to the skin surface so well that the epidermis can be separated from the dermis and pulled away with the tape. The use of a zinc oxide–based tape such as Hy-Tape is encouraged to minimize epidermal stripping; the tape is flexible, waterproof, and, washable. The use of skin barriers protects healthy skin and helps excoriated skin heal.
Use scissors very carefully to remove dressings or tape from the extremities of very small and immature infants because it is easy to snip off tiny extremities or nick loosely attached skin. Avoid solvents to remove tape because they tend to dry and burn the delicate skin. Guidelines for skin care are given in the Nursing Care Guidelines box.
During skin assessment of preterm infants, nurses are alert to the subtle signs that indicate zinc deficiency, a common problem in these infants. Breakdown usually occurs in the areas around the mouth, buttocks, fingers, and toes. In VLBW infants it may also occur in the creases of the neck, wrists, and ankles and around wounds. Zinc deficiency is most likely to appear in infants with sepsis, those experiencing nasogastric losses, or those who have had surgery. Report suspicious lesions to the practitioner so that zinc supplements can be prescribed. In most preterm infants the skin barrier properties resemble those of the term infant by 2 to 3 weeks postnatal age, regardless of gestational age at birth.
Although no studies comparing the effectiveness of different commercially available neonatal bedding have been done, a number of products are useful in minimizing skin problems. The Nursing Care Guidelines box gives general information about bedding. Particularly vulnerable areas of the skin, such as bony prominences, can be protected with transparent dressings. Gel pads or mattresses can also be used to prevent pressure ulcers (Association of Women’s Health, Obstetric and Neonatal Nurses, 2007).
Skin injuries have been reported during use of phototherapy blankets. Caution is warranted in using these products with extremely preterm infants or infants with birth trauma, poorly perfused skin, or hypotension. Manufacturers of phototherapy blankets recommend the following during therapy: monitor skin color, observe for rashes or excoriation, keep skin clean with warm water, promptly clean perineum after stooling, reposition every 2 hours, carefully monitor cleanliness and skin integrity, and avoid direct contact of blanket with infant’s skin.
Administration of Medications
Administration of therapeutic agents, such as drugs, ointments, IV infusions, and oxygen, requires judicious handling and meticulous attention to detail. The computation, preparation, and administration of drugs in minute amounts often require collaboration between nurses, physicians, and pharmacists to reduce the chance for error. In addition, the immaturity of an infant’s detoxification mechanisms and inability to demonstrate symptoms of toxicity (e.g., signs of auditory nerve involvement from ototoxic drugs such as gentamycin) complicate drug therapy and require that nurses be particularly alert for signs of adverse reaction. (See Administration of Medication, Chapter 27.)
Nurses should be aware of the hazards of administering bacteriostatic and hyperosmolar solutions to infants. Benzyl alcohol, a common preservative in bacteriostatic water and saline, is toxic to newborns and should not be used to flush IV catheters or to dilute or reconstitute medications. It is recommended that medications with preservatives such as benzyl alcohol be avoided. Nurses must read labels carefully to detect the presence of preservatives in any medication administered to an infant.
Hyperosmolar solutions present a potential danger to preterm infants. Hyperosmolar solutions given orally to infants can produce clinical, physiologic, and morphologic alterations, the most serious of which is NEC. Oral or parenteral medications should be sufficiently diluted to prevent complications related to hyperosmolality.
Take caution to reduce adverse effects of medication administration in preterm infants. Strategies to heighten awareness and decrease unnecessary morbidity in such infants include having two registered nurses double check the dosages of potentially lethal medications (high-risk medications) and providing calculators in neonatal units to perform dosage calculations, double check unit dose medications, and check medications that are reconstituted by the nurse. Some other precautions are to have readily available quick-reference guides to weight-specific medication doses and appropriate dosages for preterm infants and to provide tables with medication cross-reactivity and incompatibility. One NICU developed a distinct neonatal formulary to reduce medication errors. Another strategy was to develop computerized guidelines for managing dose ranges based on the neonate’s most recent weight so medications ordered outside the appropriate dose range could be reevaluated by the pharmacist and practitioner This concept was also applied to computer-generated emergency medication sheets, which are updated weekly (Lucas, 2004).
Information technology (e.g., computerized practitioner order entry and clinical participation by a clinical pharmacist) is available to reduce medication errors, yet this technology does not provide the entire solution (Taylor, Loan, Kamara, et al, 2008). The human factor involved in many root-cause analyses for medication errors involves systems and the humans involved in those systems. Nurses, physicians, and pharmacists are at times affected by internal and external environmental factors that lead to a medication error: excessive workload; distractions such as a monitor alarm or questions asked during medication administration; boredom; work hours (nighttime or daytime); lack of updated, consistent drug information; ambiguous drug labeling; and dosage calculation errors (Lefrak, 2002). The variables involved are numerous and multifaceted yet can be decreased by simple cautionary measures, extensive education, and verification of medication orders written.
Developmental Outcome
Neonatal intensive care and rapid improvements in technology are associated with improved survival of critically ill newborn and preterm infants. Survival rates have increased to 93% for VLBW infants (1001 to 1500 g [2.2 to 3.3 lb]), 85% for ELBW infants (751 to 1000 g [1.6 to 2.2 lb]), and about 50% for infants weighing 501 to 750 g [1.1 to 1.6 lb] (Msall and Tremont, 2000). With decreasing mortality, morbidity rates have remained stable. At highest risk for unfavorable outcomes are preterm infants compromised during the neonatal period by respiratory distress syndrome (RDS), bronchopulmonary dysplasia (BPD, or chronic lung disease), NEC, sepsis, anemia, IVH, hydrocephalus, meningitis, or seizures (McGrath, Sullivan, and Lester, 2000; Vohr, Widen, Cone-Wesson, et al, 2000). These serious sequelae of prematurity correlate with the degree of immaturity, demonstrating the relationship of increasing morbidity with decreasing gestational age. A greater incidence of CP, attention deficit hyperactivity disorder, visual-motor deficits, mild to severe cognitive disabilities, hearing loss, speech and language impairment, and neuromotor problems has been reported in outcome studies of preterm infants (van Baar, van Wassenaer, Briet, et al 2005; Wilson-Costello, 2007; Hack and Costello, 2008). Reduced language and visual-motor skills have been reported in former LBW infants at 7 years of age (Pietz, Peter, Graf, et al, 2004).
An increased need for special school services, especially in reading and math, has been reported in former LBW infants at 12 years of age (Luu, Ment, Schneider, et al, 2009). Another longitudinal study of 532 VLBW infants from four countries found that, when evaluated between 8 and 11 years of age, more than half of all cohorts required special educational assistance and/or repeated a grade (Saigal, den Ouden, Wolke, et al, 2003). Neurodevelopmental impairment also occurs in preterm infants who have been spared the complications of IVH, sepsis, and hypoxemia, moving through the NICU with seemingly few problems. Conversely, some preterm infants do well and function at age level without evidence of neurobehavioral limitations. Improved developmental outcomes are more likely for these survivors of early gestation and LBW when emphasis is placed on providing the finest medical and nursing care within a developmentally supportive framework. This philosophy requires caregivers to evaluate their own knowledge, skills, and attitudes and expand their thinking beyond the traditional medical and nursing models of care.
Developmental Assessment: One approach to NICU care is based on Als’s (1982) synactive theory of infant development, which provides a framework for understanding the preterm infant’s development. The model proposes a systematic method for observing NICU infants to collect information concerning each infant’s competencies, vulnerabilities, and thresholds. This information forms the basis for planning individualized care appropriate for a particular infant (Table 10-5). The major assumption of this model is that infants, even ELBW infants, can communicate through physical and behavioral responses that provide us with the best information for planning their care. Communication by the infant is seen through three subsystems of function (autonomic, motor, and state) that can be readily observed in the clinical setting during rest, care, or procedures and during recovery from care or procedures. Responses by an infant’s autonomic (physiologic), motor, and state systems to the environment, physical care, or procedures help the nurse make necessary adjustments in technique to optimize the infant’s stability and function.
TABLE 10-5
SYNACTIVE THEORY OF DEVELOPMENT: NEUROBEHAVIORAL SUBSYSTEMS
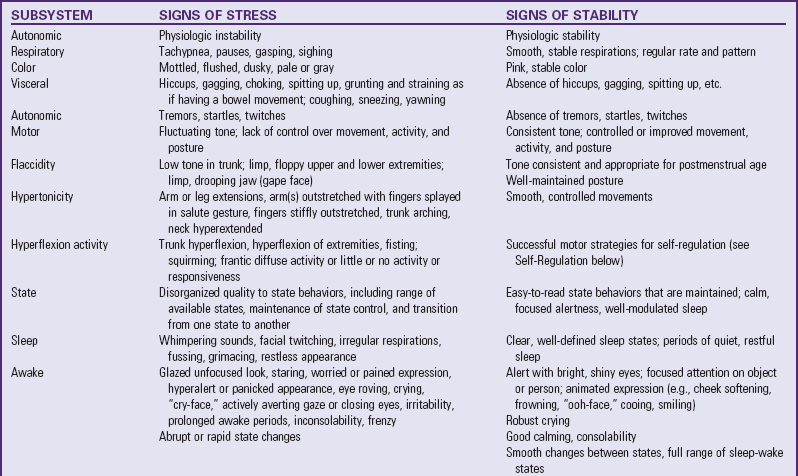
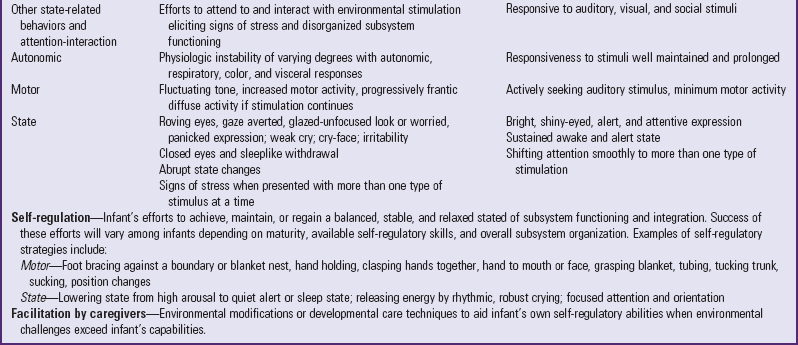
Modified from Als H: Toward a synactive theory of development: promise for the assessment and support of infant individuality, Inf Mental Health J 3(4):229-243, 1982; Als H: A synactive model of neonatal behavior organization: framework for the assessment of neurobehavioral development in the premature infant and for support of infants and parents in the neonatal intensive care environment, Phys Occup Therap Pediatr 6:3-55, 1986; and Hunter JG: The neonatal intensive care unit. In Case-Smith J, Allen AS, Pratt PN, editors: Occupational therapy for children, ed 4, St Louis, 2001, Mosby.
Individualized developmental care has had numerous positive effects on medical and neurobehavioral outcomes in high-risk newborn infants. Findings noted in a randomized controlled trial of developmental care for 92 preterm infants weighing less than 1250 g (2.7 lb) included shorter duration of parenteral feeding and time to full oral feeding; decreased time in intensive care and in hospital; lower incidence of NEC; improved weight, length, and head circumferences; enhanced autonomic, motor, state, attention, and self-regulatory function; and lowered family stress (Als, Gilkerson, Duffy, et al, 2003). In contrast, the Cochrane review done by Symington and Pinelli (2006) notes that, because of the inclusion of multiple interventions, it is difficult to ascribe specific benefits to the implementation of developmental care.
Because each infant is unique, supportive developmental care requires ongoing data collection of moment-by-moment responses and flexible care to address the infant’s cues. For example, an infant who demonstrates altered vital signs and even apnea after being weighed might benefit from swaddled weighing to support the infant’s competence and organization during a stressful procedure.
Knowledge of behavioral assessment and infant development assists the nurse in providing care that supports each infant’s ongoing function in a manner consistent with current evidence. Nurses have the greatest impact on the daily routine experienced by their tiny patients. The CNS is undergoing rapid and significant change during the preterm infant’s stay in the NICU. This vulnerable period of brain growth, differentiation, and organization is combined with the challenge of developing in environmental conditions that are not typical for the fetus and newborn (Blackburn, 2007). Brain organization peaks from about 20 weeks of gestation to several years after birth. The product of this complex process is establishment of an elaborate circuitry unique to the human brain.
Behavioral State Organization: Traditional nursing placed emphasis on interpreting physiologic data as the basis of caregiving. Developmentally supportive care uses both physiologic and behavioral information to better understand the needs of infants in the NICU setting. Behavioral states are highly individualized and formed by experience, maturation, circadian rhythms, and genetic inheritance. The emerging availability and regulation of arousal states mark a balancing of CNS inhibitory and excitatory processes that affect attention states and also mark executive functions (prefrontal cortex) that influence information processing, learning, and socialization. State organization has been described as a gating mechanism that protects the cortex from overstimulation and promotes coordination between attentional, executive, and sensory cortical systems.
Infant responsiveness to environmental stimuli depends on the quality, amount, and availability of particular states of arousal. States can be organized into five levels of arousal (Table 10-6). Transitional states such as drowsiness are not considered true states but are in-between levels of arousal in which the infant either moves toward wakefulness or back into sleep.
TABLE 10-6
| STATE | DESCRIPTION |
| Deep sleep | Regular breathing; eyes closed with no movement of eyes under lid; relaxed face; little or no movement or activity except for possible startle response |
| Active sleep | Sometimes called light sleep; may see rapid eye movements under closed lids, low activity level, breathing regular or irregular, occasional sighing or smiling |
| Drowsy | Eyes open or closed, unfocused expression; activity level varied |
| Quiet awake | Different qualities of alerting |
| Robust | Bright, shiny appearance to eyes; focused attention; minimum motor activity |
| Low level | Dull or unfocused eyes; little energy; appears to look through object or caregiver |
| Hyperalert | Wide eyes, panicked expression; may fixate on object or caregiver intensely and have trouble breaking away |
| Active awake | Active; eyes open or closed; fussy but not crying robustly |
| Crying | Highest level of arousal; agitated, rhythmic, and robust crying |
Modified from Als H: Manual for the naturalistic observation of newborn behavior, Newborn Individualized Developmental Care and Assessment Program (NIDCAP), Boston, 1995, Harvard Medical School.
Distinct sleep and awake states are observable in infants between 25 and 27 weeks (Holditch-Davis and Blackburn, 2007). Young preterm infants spend 70% or more of their time in active sleep. Developmental maturation for the young preterm infant is seen by a decrease in the amount of active sleep with an increase in quiet sleep, awake periods, and crying. Around 30 to 32 weeks, quiet alert states with some focused attention can occur. Before 28 weeks, attempts to attend to stimuli may have physiologic consequences for the immature infant (Blackburn, 1998). Responsiveness to sound and touch is greater during active or light (rapid eye movement [REM]) sleep, resulting in longer periods of vulnerability to sleep disturbance (Holditch-Davis, 1998). Maturation continues throughout the first year of life. By 6 months, the amount of quiet sleep is greater than that of active sleep. By 1 year, infants usually sleep 10 to 12 hours at night and take one or two naps during the day. Preterm infants generally sleep for shorter periods at night and awaken more frequently than full-term infants. Other maturational changes include organization of the standard sleep cycle and electroencephalogram sleep patterns comparable to those of adults. Neurologic insults, severity of illness, hyperbilirubinemia, and prenatal exposure to drugs can alter behavioral state patterns.
Physiologic parameters vary depending on level of arousal. Heart rate is higher during waking periods but more variable during active sleep. Blood pressure is higher during wakefulness. Cerebral blood flow is greater during active sleep (greater during quiet sleep in full-term infants). Respiratory rates fluctuate more and are higher in active sleep. Arterial oxygen and carbon dioxide levels are lower in active sleep than in quiet sleep or awake states. Hypoventilation and poorly coordinated chest wall and abdominal movements are reported during active sleep. Apneic pauses of less than 20 seconds are more frequent in active than quiet sleep in preterm infants.
Nursing care should be timed to the responsiveness of the infant as much as possible to optimize the development of sleep organization and enhance alerting as it emerges. Sensory stimulation can influence behavioral state as seen by either increased or decreased infant arousal when presented with a stimulus and its removal; the type of stimulus (e.g., loud bell or soft lullaby) also is a factor. The quality of each state, its duration, and the movement between states provide information concerning how well organized the state is and how much state control the infant has. Protection of sleep is an important goal for both the preterm and full-term infant. Environmental modifications and timing of care to provide longer episodes of undisturbed sleep should be planned into care.
Nurses can also support transitions between states. Gentle arousal to wakefulness by soft speech or gentle touch before caregiving is preferable to the traditional model in which care is begun without warning and with abrupt disruption of sleep. Slow movements and gentle handling support quiet alerting or return to sleep without periods of arousal after care is over. Nurses should facilitate return to sleep or interact with a quietly alert infant after care events.
An infant’s state of arousal allows for communication of responses that are valuable for individualized caregiving. Observing state patterns and individual responses of infants, nurses can better know their patients and support behavioral state organization. The nurse can also share this knowledge with parents to foster intimacy with the child.
Sensory System: Research with animal fetuses and infants has shown that atypical sensory experiences, whether overstimulating or depriving, can modify the developing brain (Glass, 2005; Lickliter, 2000a). In fact, much of the cerebral cortex is associated with the sensory system. Most sensory systems develop prenatally and are capable of functioning before birth. Onset of function of each sensory system proceeds in the same order for each individual (i.e., tactile, vestibular, gustatory-olfactory, auditory, and visual). The visual system becomes functional after birth. Sensory input provided before the stimulation would typically occur has been shown to interfere with perceptual and behavioral development (Lickliter, 2000b).
The normal experience for the preterm infant is within the womb and for the full-term infant is the home environment with a few primary caregivers. These environments are vastly different from the NICU. The NICU experience for the high-risk infant is made up of external conditions and interactions with caregivers. Often that experience is overstimulating to later-developing sensory systems (i.e., auditory and visual) and understimulating to earlier ones (i.e., tactile, vestibular, gustatory-olfactory). Alterations in the sensory environment may have developmental consequences. It is important for the nurse to consider the following while caring for high-risk infants: (1) timing of stimulation in relation to the infant’s current developmental stage, (2) amount of stimulation provided or denied, (3) type of stimulation, and (4) the infant’s response to the stimulation (Lickliter, 2000b).
By the age of viability, infants in the NICU have sophisticated perioral sensation and perceive pressure, pain, and temperature (Als, 1999). Touch in the NICU frequently involves routine, sometimes impersonal, caregiving and procedures that are either intrusive or painful. Even nonpainful care has been associated with adverse responses in preterm infants.
Preterm infants demonstrate cry expression, grimacing, and knee and leg flexion during major reposition changes. Hypoxemia, associated with nonpainful or routine caregiving activities such as suctioning, repositioning, taking vital signs, changing diapers, and removing electrodes, has been reported (Glass, 2005). Other physiologic changes involve blood pressure, heart rate, and respiratory rhythm and rate (Glass, 2005; Browne, 2000; Peters, 1999). Nursing activities that are painful or especially intrusive, such as needle puncture, suctioning, and chest physiotherapy, have resulted in acute decreases in Sao2 and behavioral state changes in preterm infants ranging from 23 to 37 weeks of gestation (Zahr and Balian, 1995). Increased motor activity, agitation, crying, and startle reflex have also been described as negative behavioral responses to touch (Browne, 2000).
Touch is the first sensory system to develop and forms the basis for early communication between infants and caregivers. In particular, touch is a powerful means of emotional exchange for parents and infants. Positioning and handling techniques promote comfort and minimize stress, while creating a balance between nurturing care and necessary interventions.
Therapeutic Handling: Using the developmental model of supportive care, the nurse closely monitors physiologic and behavioral signs to promote organization and well-being of high-risk infants during handling (Box 10-5). The type, timing, and amount of handling are carefully considered in terms of the infant’s current age, condition, vulnerabilities, thresholds for stress, and capabilities. Because touch can be disruptive to maturing sleep-wake states, avoid waking an infant for care or nurturing. Sleep deprivation may affect secretion of growth hormone and interfere with growth and development (Hunter, 2001).
Respectful approach before touching an infant allows more time for transition and adaptation from being alone to being handled. The nurse can use the infant’s own cues to determine optimum times for caregiving rather than following a rigid schedule. The best time for care is when an infant is awake. If the care or procedure cannot be postponed, softly calling an infant by name and then gently placing a hand on the body signals care is beginning and avoids the abrupt interruption that frequently precedes caregiving. Abrupt transitions can disrupt even organized functioning of an infant’s autonomic, motor, and state subsystems.
Infants who are unable to maintain a gently flexed position during repositioning or care procedures may benefit from containment. Gently holding the infant’s arms and legs in a tucked, flexed position close to the body can be accomplished with hands or blanket swaddling. Facilitated tucking before ET suctioning was shown to decrease physiologic and behavioral distress in preterm infants as young as 23 weeks of gestational age (Ward-Larson, Horn, and Gosnell, 2004). Blanket swaddling and nesting or containment decreased physiologic and behavioral stress during routine care procedures such as bathing, weighing, and heel lance (Byers, 2003).
Because repositioning has been associated with significant physiologic distress in immature infants, avoid sudden postural changes. Slow turning while containing the infant’s extremities in a gently tucked, midline position may reduce the impact of this procedure.
Stroking preterm infants who are not physiologically stable has been reported to result in behavioral signs of distress such as gasping, grunting, gaze aversion, and decreased tcPo2 levels. Some infants experience apnea and bradycardia during massage or tactile-kinesthetic stimulation (Glass, 2005). Other researchers report positive benefits of gentle human touch, including heart rate and oxygen saturation stability (Modrcin-Talbott, Harrison, Groer, et al, 2003). Individual infants show varied responses to tactile intervention, further supporting the need for close monitoring of behavioral and physiologic parameters.
Investigators have reported positive benefits of massage on stable, growing, preterm infants. Beachy (2003) found that, when infant massage therapy is properly applied to stable preterm infants, they respond with increased weight gain, enhanced developmental scores, and shortened hospital stays. Parents of the preterm infant also benefit because infant massage enhances bonding with their child and increases confidence in their parenting skills. Dieter, Field, Hernandez-Reif, and colleagues (2003) studied 16 preterm infants (mean gestational age of 30 weeks) and found that after 5 days of receiving massage therapy, infants in the treatment group averaged a 53% greater weight gain and spent less time sleeping than control infants.
Vickers, Ohlsson, Lacy, and colleagues (2004) evaluated literature relevant to infant massage, gentle touch, and gentle human touch. The researchers concluded that available studies, although demonstrating advantages to massage for preterm infants, lack sound methodologic bases on which firm recommendations can be made to advocate wide-scale use of this intervention.
Kangaroo care, or STS holding, has been advocated for fostering neurobehavioral development and supporting parent-infant intimacy and attachment. STS contact is maintained with the diaper-clad infant resting prone and semiupright on the bare chest of either parent, who encloses the infant in his or her own clothing to maintain temperature stability. Kangaroo care is reported to reduce incidence of severe illness and nosocomial infection, support breast-feeding duration until discharge, improve maternal satisfaction (Conde-Agudelo, Diaz-Rossello, and Belizan, 2000) and parental interaction (Feldman, Eidelman, Sirota, et al, 2002), and accelerate neurologic maturation (Feldman and Eidelman, 2003). Hurst, Valentine, Renfro, and colleagues (1997) reported a significant increase in milk volume in mothers providing kangaroo care to stable, ventilated, LBW infants (mean 27.7 weeks of gestation) compared with a control group. Others have reported advantages that include maintenance of skin temperature, reduction of apnea and bradycardia, stable tcPo2 level, increased frequency and duration of quiet sleep, less time crying, and lower activity levels during kangaroo care (Roberts, Paynter, and McEwan, 2000; Byers, 2003). Kangaroo care has been successfully initiated in stable preterm infants who weigh less than 1000 g (2.2 lb) (Neu, Browne, and Vojir, 2000).
However, in a study of preterm infants (mean gestational age of 29 weeks), kangaroo care was associated with a significant increase in bradycardia, less regular breathing, and hypoxemia. Temperatures increased (as measured rectally) every 2 hours (Bohnhorst, Heyne, Peter, et al, 2001). Although adverse effects are not usually associated with kangaroo care, monitoring to avoid potential harmful effects should include cardiorespiratory parameters, body temperature, and oxygenation.
Infants appear to be most vulnerable during the transfer from bed to parent and back to bed when kangaroo care is provided. Handling and repositioning necessary to prepare and move an infant into the STS position may result in similar disorganizing responses as previously described with routine nursing care.
Therapeutic Positioning: The American Academy of Pediatrics (2005) recommends the supine sleeping position for healthy infants in the first year of life as a preventive measure for sudden infant death syndrome (SIDS). Prone sleeping has decreased from more than 70% to about 13% in the United States since the guidelines were published in 1992. SIDS is the third highest cause of infant death after the neonatal period (28 days); the rate has decreased by more than 50% with the advent of supine sleeping. (See Sudden Infant Death Syndrome, Chapter 13.)
Parents of infants in the NICU should be educated on the safe sleeping position at home as part of discharge instructions. Supportive positioning in the NICU for acutely ill or recovering infants may look different from the academy’s recommendations, depending on each infant’s changing clinical condition, maturation, and readiness for the supine sleeping position and minimum bedding. It is important for staff to realize that routine care practices in the NICU may serve as a model for parents who, without proper instruction, may reproduce the environment and care techniques at home. Position and bedding choices in the unit, such as prone positioning, nests, and sheepskin, may be lethal for infants who have been discharged home.
Infants in the NICU are at increased risk for acquiring position-related deformities for a variety of reasons. Illness, weakness, low tone, immature motor control, the effects of gravity, and treatments such as ECMO or sedation are a few of the factors associated with prolonged immobility or decreased spontaneous movement (Hunter, 2001). Common position-related deformities include:
• Hyperabduction and flexion of the arms, causing upper extremity external rotation, resulting in a persistent W positioning of the arms; can interfere with later midline skills that form the foundation for feeding, crawling, reaching, and midline play with objects (Vaivre-Douret, Ennouri, Jrad, et al, 2004)
• Lower extremity external rotation deformities occurring when the trunk and pelvis are flat on the mattress, causing extreme hip abduction and outward rotation of the lower limbs, or the frog-leg appearance (Downs, Edwards, McCormick, et al, 1991)
• Neck extension and arching posture often observed in infants pulling away from ET tubes or nasal prongs during mechanical ventilation or nasal CPAP
• Motor asymmetries reported in preterm infants at 32 weeks of gestation or who are small for gestational age, occurring more often than in full-term infants even after 4 months corrected age (Samsom and de Groot, 2000, 2001)
Therapeutic positioning reduces the potential for acquired positional deformities that can affect motor development, play skills, attractiveness, and social attachment (Monterosso, Kristjanson, Cole, et al, 2003). Positioning can affect stability and comfort, and each infant must be observed for the effects of any position or repositioning. A position may also need to be adapted to accommodate necessary medical equipment or particular conditions, such as myelomeningocele, where the supine position is contraindicated before surgical repair of the defect. Deciding on which position and supportive aids to use requires the caregiver to consider the medical and developmental risks and benefits unique to a specific infant and situation (see Nursing Care Guidelines box).
The goal of therapeutic positioning for preterm and high-risk infants is to provide adequate support and containment as indicated to sustain flexed and midline postures, in an attempt to minimize positional deformities and assist infants in remaining calm and organized (Hunter, 2001).
The supine position requires support for the weak or immature infant. Because this position can create the most disorganization, make the position comfortable using positioning aids or blanket boundaries that support the head, trunk, and extremities according to the general positioning principles.
Although the prone position may appear to be the easiest to maintain, mistakes are often made with infants who are unable to sustain rounded shoulders, trunk, and pelvis without assistance. Use of a postural support roll has been shown to prevent scapular-humeral tightness and shoulder retraction commonly seen as a result of this position (Figs. 10-4 and 10-5) (Monterosso, Kristjanson, Cole, et al, 2003).
Fig. 10-4 Preterm infant slowly and gently transitioned to prone position on prone roll designed with stockinette-covered foam cut to individual specifications to prevent flattening of shoulders and pelvis against mattress and to support stable breathing base for the infant. (Courtesy Paul Vincent Kuntz, Halbouty Premature Nursery, Texas Children’s Hospital, Houston.)
Auditory Environment: The auditory system of the human fetus is mature enough for sound to produce physiologic effects as early as 23 weeks of gestation (Graven, 2000). Physical and behavioral responses to sudden, loud NICU noise have been observed in preterm and full-term infants (Philbin and Klaas, 2000; Bremmer, Byers, and Kiehl, 2003). Physiologic changes include apnea and bradycardia; fluctuations in heart and respiratory rates, blood pressure, and oxygen saturation; and changes in sleep-wake states (Philbin and Klaas, 2000, Bremmer, Byers, and Kiehl, 2003). These data demonstrate that infants in the NICU are capable of perceiving and responding to sounds around them.
The primary auditory environment in fetal life is made up of the maternal voice, respirations, heartbeat, and intestinal sounds. Soon after birth, newborn infants demonstrate preference for their own mother’s voice and the language heard in utero (Gerhardt and Abrams, 2000). The acoustic environment of most NICUs is vastly different from the uterine and home milieu. Currently no data are available on the effects of long-term exposure to NICU noise levels. Of serious concern is the increased risk of sensorineural hearing loss (Cristobal and Oghalai, 2008) and language delays in infants born prematurely (Pietz, Peter, Graf, et al, 2004). NICU noise may interfere with developing auditory pathways and mask socially relevant sounds of the human voice necessary for language development.
Maintaining recommended sound levels (<45 dB) in the NICU may provide some or all of the following benefits: (1) increased physiologic stability, (2) improved growth, (3) more natural and consistent neurosensory maturation, (4) enhanced parent-infant interaction and subsequent attachment, and (5) fewer speech and language difficulties (Graven, 2000).
Visual Environment: Vision is the least mature of the newborn’s senses. The preterm infant’s eyes undergo significant maturation and differentiation of the retina and its connections to the visual cortex that typically occur in utero during the last trimester of pregnancy (Glass, 2005; Hunter, 2001). A current concern is that early, intense visual stimulation for preterm infants could adversely affect visual pathways and alter the developmental course for other sensory systems.
Visual function in preterm infants is more limited than that in full-term infants who, although limited in ability to focus (accommodation to near and far distances) and discriminate (acuity), will actively explore the environment. Preterm infants are less responsive to visual stimulation and have less acuity and accommodation than full-term infants. The ability to visually attend emerges around 30 to 32 weeks, and the infant may become stressed if the visual stimulus is intense and prolonged. Strong visual stimulation such as high-contrast black and white patterns can evoke an obligatory staring response by the immature infant who is unable to break away from it. This behavior is neither appropriate nor desired.
A variety of lighting conditions exist for NICUs from continuous 24-hour illumination, continuous dim lighting, day-night cycled lighting, or unpredictable periods of light-dark depending on staff or situations. Ambient lighting in some NICUs is reported to range from 40 to 150 foot-candles (ft-c) during the day with levels over 1500 ft-c if sunlight is added (Glass, 2005; Blackburn, 1998). These levels drop dramatically at night if light is cycled to reported levels of 5 to 9 ft-c (Fielder and Merrick, 2000).
Staff needs to carefully consider the impact of visual stimuli on the NICU infant. For preterm infants whose visual system is undergoing maturation, it is probably more prudent to provide stimulation to the earlier-developing senses first and minimize the impact of the NICU visual milieu. As attention and alerting emerge, the most appropriate visual stimulus is likely to be the human face, especially that of the parent. Box 10-6 provides some suggested approaches to visual stimulation in the NICU (Glass, 2005; Hunter, 2001; Mirmiran and Ariagno, 2000).
Facilitating Parent-Infant Relationships
Because of their physiologic instability, preterm infants are immediately separated from their mothers and surrounded by a complex, impenetrable barrier of glass windows, mechanical equipment, and special caregivers. Increasing evidence indicates that the emotional separation that accompanies the physical separation of mothers and infants interferes with the normal maternal-infant attachment process, discussed in Chapter 8. Maternal attachment is a cumulative process that begins before conception, strengthens by significant events during pregnancy, and matures through maternal-infant contact during the neonatal period.
When an infant is sick, the necessary physical separation appears to be accompanied by an emotional estrangement in the parents, which may seriously damage the capacity for parenting their infant. This detachment is further hampered by the tenuous nature of the infant’s condition. When survival is in doubt, parents may be reluctant to establish a relationship with their infant. They prepare themselves for the infant’s death while continuing to hope for recovery. This anticipatory grief (see Chapter 23) and hesitancy to embark on a relationship are evidenced by behaviors such as delay in giving the infant a name, reluctance to visit the nursery (or focusing on equipment and treatments rather than on their infant when they do visit), and hesitancy to touch or handle the infant when given the opportunity.
Comprehensive management of high-risk newborns includes encouraging and facilitating parental involvement rather than isolating parents from their infant and associated care (Box 10-7). This is particularly important in relation to mothers; to reduce the effects of physical separation, mothers are united with their newborns at the earliest opportunity (Fig. 10-6). Preparing the parents to see their infant for the first time is a nursing responsibility.

Fig. 10-6 Encouraging interaction of mother and her preterm infant in intensive care unit facilitates mother-infant attachment process. (Courtesy E. Jacobs, Texas Children’s Hospital, Houston.)
Before the first visit, the nurse prepares parents for their infant’s appearance, the equipment attached to the child, and the general atmosphere of the unit. The initial encounter with the intensive care unit is stressful, and the frightening array of people, equipment, and activity is likely to be overwhelming. A book of photographs or pamphlets describing the NICU environment (infants in incubators or under radiant warmers, monitors, mechanical ventilators, and IV equipment) provides a useful and nonthreatening introduction to the NICU.
Encourage parents to visit their infant as soon as possible. Even if they saw the infant at the time of transport or shortly after birth, the infant may have changed considerably, especially if there are a number of medical and equipment requirements associated with the infant’s hospitalization. At the bedside the nurse should explain the function of each piece of equipment and the role it plays in facilitating recovery. Explanations may often need to be patiently repeated because parents’ anxiety over the infant’s condition and the surroundings may prevent them from really “hearing” what is said. When possible, some items related to therapy can be removed; for example, phototherapy can be temporarily discontinued and eye patches removed to permit eye-to-eye contact.
Parents appreciate the support of a nurse during the initial visit with their infant, but they may also want some time alone with the infant. It is important during the early visits to emphasize the positive aspects of their infant’s behavior and development so that parents can focus on their infant as an individual rather than on the equipment that surrounds the child. For example, the nurse may describe the infant’s spontaneous behaviors during care, such as grasp, sucking, and movement, or make comments about the infant’s biologic functions. Most institutions promote family-centered care and have open visiting policies so that parents and siblings can visit as often as they wish.
Parents vary greatly in the degree to which they are able to interact with their infant. Some may wish to touch or hold their infant during the first visit, whereas others may not feel comfortable enough to even enter the nursery. Parents may not be receptive to early and extended infant contact because they need time to adjust to the impact of an infant with birth problems and must be helped to grieve before they can accept their infant.
The parents’ inability to focus on their infant is a clue for the nurse to assist the parents in expressing feelings of guilt, anxiety, helplessness, inadequacy, anger, and ambivalence. Nurses can help parents deal with these distressing feelings and recognize that they are normal responses shared by other parents. It is important to point out and reinforce the positive aspects of parents’ behavior and interactions with their infant.
Ward’s (2001) research of parental needs in the NICU demonstrates the importance of nurses providing accurate information to the parent regarding treatment plan and procedures; answering parents’ questions honestly; actively listening to parents’ concerns, fears, and expectations; and helping parents understand infant responses to hospitalization.
Most parents feel shaky and insecure about initiating interaction with their infant. Nurses can sense parents’ level of readiness and offer encouragement in these initial efforts. Parents of preterm infants follow the same acquaintance process as do parents of full-term infants. They may quickly proceed through the process or may require several days, or even weeks, to complete it. Parents begin by touching their infant’s extremities with their fingertips and poking the infant tenderly, and then proceed to caresses and fondling (Fig. 10-7). Touching is the first act of communication between parents and child. Parents need to be prepared for their infant’s exaggerated and generalized startle responses to touch so that they will not interpret these as negative reactions to their overtures. It may be necessary to limit tactile stimuli when the infant is critically ill and labile, but the nurse can offer other options, such as speaking softly or sitting at the bedside.

Fig. 10-7 Mother and father interact with their preterm infant. (Courtesy E. Jacobs, Texas Children’s Hospital, Houston.)
Parents of acutely ill preterm infants may express feelings of helplessness and lack of control. Involving the parent in some type of caregiving activity, no matter how minor it may seem to the nurse, enables the parent to take on a more active role. Examples of such caregiving for the acutely ill infant who cannot be held and is seemingly not responding positively include moistening the infant’s lips with a small amount of sterile water on a cotton-tipped swab or slipping the diaper from under the infant when it is wet or soiled.
The nurse encourages parents to bring in clothes, a toy, a stuffed animal, or a family snapshot for their infant, and the nurse can help parents set goals for themselves and for the infant. Parents may become involved by reading a children’s storybook or nursery rhymes in a soft, soothing voice. The nurse encourages parents to visit at times when they can become involved in their infant’s care (Figs. 10-8 and 10-9).

Fig. 10-9 Mother consoling preterm infant. (Courtesy E. Jacobs, Texas Children’s Hospital, Houston.)
Throughout the parent-infant acquaintance process, the nurse listens carefully to what the parents say to assess their concerns and their progress toward incorporating their infant into their lives. The manner in which parents refer to their infant and the questions they ask reveal their worries and feelings and can serve as valuable clues to future relationships with the infant. The alert nurse is attuned to these subtle indications of parents’ needs, which provide guidelines for nursing intervention. Often all that the parents need is reassurance that they will have the nurse’s support during caregiving activities and that the behaviors about which they are concerned are normal reactions and will disappear as the infant matures (e.g., an exaggerated Moro reflex or inability to coordinate swallowing).
Parents need guidance in their relationships with their infant and assistance in their efforts to meet their infant’s physical and developmental needs. The nursing staff must help parents understand that their preterm infant offers few behavioral rewards and show them how to accept small rewards from their infant. They need reassurance that avoidance behaviors are not a reflection on their parenting skills. Teach parents to recognize their infant’s cues regarding stimulation, handling, and other interaction, especially aversive behaviors that indicate a need for rest. Nurses need to include parents in planning their infant’s care.
Above all, nurses must encourage and reinforce parents during their caregiving activities and interactions with their infant to promote healthy parent-child relationships. It is also helpful for the parents to have contact and communication with the infant’s primary nurse and associate primary nurse (according to unit model of nursing care). This decreases the amount of different information given to parents and often instills confidence that, although the parents cannot be at their infant’s bedside 24 hours a day, they can call competent and caring nurses to inquire about the infant’s status. Periodic parent conferences involving the primary practitioner, primary nurse, and associate primary nurse serve to clarify misunderstandings or problems related to the infant’s condition. Other members of the NICU team, such as the perinatal social worker, lactation consultant, discharge coordinator, or surgeon, may become involved as necessary.
Siblings: In the past, concerns about sibling visitation in the NICU focused on fears of infection and disruption of nursing routines. These fears have not been substantiated, and sibling visits should be a part of the normal operation of NICUs (Fig. 10-10).

Fig. 10-10 Siblings visiting in the neonatal intensive care unit. (Courtesy E. Jacobs, Texas Children’s Hospital, Houston.)
The birth of a preterm infant is a difficult time for siblings, who rely on the support of understanding parents. When the happy anticipation changes to sadness, worry, and altered routines, siblings are bewildered and deprived of their parents’ attention. They know something is wrong, but they do not completely understand what it is. Concern about the negative effects on visiting siblings of seeing the ill newborn has not been confirmed. Children have not hesitated to approach or touch the infant, and children less than 5 years of age have been less reluctant than older children. In addition, no measurable differences were found between previsit and postvisit behaviors.
The potential benefits of sibling visits must be weighed against the negatives of exposing the child to the NICU environment. Children must be prepared for the unfamiliar NICU atmosphere, but contact with the infant appears to have a positive effect on siblings by helping them deal with the reality rather than the bizarre fantasies that are characteristic of young children. Such visits also help bond the family as a unit.
Support Groups: Parents need to feel they are not alone. Parent support groups have been of immeasurable value to families of infants in the NICU. Some groups consist of parents who have infants in the hospital and share the same anxieties and concerns. Other groups include parents who have had infants in the NICU and who have dealt with the crisis effectively. The groups are usually under the leadership of a staff person and may involve physicians, nurses, and social workers, but it is the parents who can offer other parents something that no one else can provide.
Family Support America* (formerly Family Resource Coalition) is a North American network of family support programs designed to help families of preterm infants. An excellent resource for parents of preterm infants is the book by J. Zaichkin, Newborn Intensive Care: What Every Parent Needs to Know (2009). This resource has technical and anecdotal information regarding different problems facing preterm infants, common treatments and therapies, preparation for home discharge, and home care for the preterm infant.
Discharge Planning and Home Care
Parents become apprehensive and excited as the time for discharge approaches. They have many concerns and insecurities regarding the care of their infant. They fear the child may still be in danger, that they will be unable to recognize signs of distress or illness, and that the infant may not be ready for discharge. Nurses need to begin early to assist parents in acquiring or increasing their skills in the care of their infant. Appropriate instruction must be provided and sufficient time allowed for the family to assimilate the information and learn the continuing special care requirements. Where rooming-in or other live-in arrangements are available, parents can stay for a few days and nights and assume the care of their infant under the supervision and support of the nursery staff.
There should be appropriate medical and nursing follow-up care, including developmental follow-up, and referrals to services that can benefit the family. Parents of preterm infants should also receive information about immunizations, including respiratory syncytial virus (RSV) prophylaxis, as well as other discharge planning information. Home health agencies provide nursing supervision, counseling, and referrals for nursing visits. With early discharge, many hospital-based home health care agencies become involved in the follow-up monitoring and care of the NICU “graduate” in the home. For an infant being discharged with equipment such as an oxygen tank, apnea monitor, or even a ventilator, discharge planning requires multidisciplinary, collaborative practice to ensure that the family has not only the appropriate resources but also the available assistance for dealing with the infant’s needs. Many communities have organized support groups, including those discussed previously, those designed for parents of infants who require special care because of specific defects or disabilities, and those for parents of multiple births. (See Chapter 3.)
Car seat safety is an essential aspect of discharge planning. It is recommended that infants less than 37 weeks of gestation have a period of observation in an appropriate car seat to monitor for possible apnea, bradycardia, and decreased Sao2 (Bull, Engle, and American Academy of Pediatrics, 2009). One study found that mean oxygen saturation levels decreased from 97% to 94% for both term and preterm infants who were observed in their car seats; 12% of the preterm infants in the study had significant apneic or bradycardic events in their car seats (Merchant, Worwa, Porter, et al, 2001). Despite this evidence, a Cochrane review of predischarge car seat testing failed to find evidence that such testing prevents morbidity or mortality (Pilley and McGuire, 2006) (see Family-Centered Care box).
Several car seat models can be adapted for small infants with the placement of blanket rolls on each side of the infant, but never behind, to support the head and trunk. For adequate support without slumping, the seat back-to-crotch strap distance must be 14 cm (5.5 inches) or less. A small rolled blanket may be placed between the crotch strap and the infant to reduce slouching. If the child’s head drops forward because the position of the seat is upright, a roll cloth or blanket may be placed in the vehicle seat crease and under the safety base so the infant reclines at no more than a 45-degree angle. A car seat restraint without a shield is recommended; if the infant needs to be supine, a crash-tested car bed may be used (Bull, Engle, and American Academy of Pediatrics, 2009).
The rear-facing position provides support for the head, neck, and back, thereby reducing the stress to the neck and spinal cord in a vehicle crash. It is recommended that, before discharge from the hospital, the preterm infant have an evaluation in the designated car seat restraint by a staff member who is knowledgeable in car seat restraint positioning. Parents should learn how to properly restrain the child in the car seat for safe transportation (Bull, Engle, and American Academy of Pediatrics, 2009).
Additional guidelines are available from the American Academy of Pediatrics, including a videotape for the safe transportation of preterm and LBW infants. (See Chapter 12 for a discussion of infant car restraints and American Academy of Pediatrics website [www.aap.org] for a list of appropriate car seats for infants.)
An important part of discharge planning and care of the preterm infant is nutrition for continued growth; thus choice of feeding must be carefully addressed. Human milk should be fortified according to the infant’s corrected age and physiologic needs. An enriched postdischarge formula (usually 22 kcal/oz) has been recommended for preterm infants born at less than 36 weeks to meet appropriate growth standards (see Table 10-4) (Lucas, Fewtrell, Morley, et al, 2001; Carver, Wu, Hall, et al, 2001). However, a Cochrane review of studies examining growth in preterm infants fed an enriched postdischarge formula did not find strong evidence of enhanced growth and development compared with infants fed standard formula (Henderson, Fahey, and McGuire, 2007).
The term vulnerable child syndrome is applied to physically healthy children who are perceived by their parents to be at high risk for medical or developmental problems. The syndrome has been observed in parents of children who had an illness or injury from which they had not been expected to recover. The family continues to perceive the child as fragile, vulnerable, and “different” and as having needs that warrant special status in the family, which adversely affects the child’s and family’s behavior. The parents may lack confidence in their parenting ability that persists beyond the illness. The parents may also become overly indulgent and have difficulty setting limits, resulting in interference with normal development. Consequently, the child becomes dependent, demanding, and out of control. Overprotection and frequent visits to the health care provider are characteristic.
Problems that may arise in the high-risk newborn include overfeeding, underfeeding, feeding resistance, aversion to human touch or interaction, and difficulty separating the child from the parent. To help parents deal with the stress of home care for the infant, nurses can help families discuss their fears and anxieties, which are exaggerated in parents of preterm infants, and encourage them to create a normal routine in caring for the infant. Parents need to learn the normal developmental delays expected of formerly preterm infants and the importance of setting disciplinary limits and schedules. Continued explanations and clarification of the infant’s true health status and ongoing support of the parents’ efforts are important aspects of follow-up care.
Neonatal Loss
![]() The precarious nature of many high-risk infants makes death a real and ever-present possibility. Although infant mortality has been reduced sharply with improved technology, the mortality rate is still greatest during the neonatal period. Nurses in the NICU are the persons who must prepare the parents for an inevitable death and facilitate a family’s grieving process after an expected or unexpected death.
The precarious nature of many high-risk infants makes death a real and ever-present possibility. Although infant mortality has been reduced sharply with improved technology, the mortality rate is still greatest during the neonatal period. Nurses in the NICU are the persons who must prepare the parents for an inevitable death and facilitate a family’s grieving process after an expected or unexpected death.
![]() Critical Thinking Exercise—Neonatal Loss
Critical Thinking Exercise—Neonatal Loss
The loss of an infant has special meaning for the grieving parents. It represents a loss of a part of themselves (especially for mothers), a loss of the potential for immortality that offspring represent, and the loss of the dream child that has been fantasized throughout the pregnancy. The parents have a sense of emptiness and failure. In addition, when an infant has lived for such a short time, they may have few, if any, pleasant memories to serve as a basis for the identification and idealization that are part of the resolution of a loss.
To help parents understand that the death is a reality, it is important that they be given the opportunity to hold their infant before death and, if possible, be present at the time of death so their infant can die in their arms if they choose.
Parents should have the opportunity to actually “parent” the infant in any manner they wish or are able to before and after the death. This may include seeing, touching, holding, caressing, and talking to their infant privately. The parents may also wish to bathe and dress the infant. If parents are hesitant to see their dead infant, it is advisable to keep the body in the unit for a few hours, since many parents change their minds after the initial shock of the death.
Parents may need to see and hold the infant more than once—the first time to say “hello” and the last time to say “good-bye.” If parents wish to see the infant after the body has been taken to the morgue, the infant should be retrieved, wrapped in a blanket, rewarmed in a radiant warmer, and taken to the mother’s room or other private place. The nurse should plan to stay with the parents but also provide them an opportunity for private time alone with their dead infant if they wish. Individual grief responses of the mother and father should be recognized and handled appropriately. Gender differences and cultural and religious beliefs will affect the parents’ grief responses (Dyer, 2005).
Some units have implemented a hospice approach for families with infants for whom the decision has been made not to prolong life and who are receiving only palliative care. A special “family” room is set aside and contains all supportive equipment needed for the care of the infant. It also provides a homelike atmosphere for the family. All hospice services are available to the family, and the infant remains under the care and supervision of a primary nurse on the NICU staff. (See Chapter 23, End-of-Life Care, for further discussion of hospice care.)
A photograph of the infant taken before or after death is highly desirable. Parents may wish to have a special family portrait taken with the infant and other family members. This often helps personalize the experience and make it more tangible. The parents may not wish to see the photograph at the time of death, but the chance to refer to it later will help to make their infant seem more real, which is a part of the normal grieving process. A photograph of their infant being held by the hand or touched by an adult offers a more positive image than a morgue type of photograph. Many NICUs have a bereavement or memory packet made up for the grieving parents, which may include the infant’s handprints and footprints, a lock of hair, the bedside name card, and, if appropriate to the family’s religious beliefs, a certificate of baptism. The photographs and other personal effects of the deceased infant were perceived as critically important in the grieving process by one group of parents in a survey. Parents often indicated that the photographs were helpful in remembering the infant’s actual appearance during this stressful period (Anderson, 2001; Gold, Dalton, and Schwenk, 2007). Naming the deceased infant is an important step in the grieving process. Some parents may hesitate to give the newborn a name that had been chosen during the pregnancy for their special “baby.” However, it helps to have a tangible person for whom to grieve. In supporting parental responses to the loss of a child, care providers must understand and respect the cultural and religious practices.
A primary nurse who is familiar to the family should be present during the discussion about the dead or dying infant. A Resolve Through Sharing or bereavement counselor is often involved in helping the family through this difficult period. The nurse should talk with parents openly and honestly about funeral arrangements because few of them have had experience with this aspect of death. Many funeral homes now offer inexpensive arrangements for these special cases. Someone from the NICU should take the responsibility for acquiring this information. It is often helpful to parents for the NICU to have a list of local funeral homes, services offered, and prices. Families need to be informed of the options available, but a funeral is preferable because the ritual provides an opportunity for parents to feel the support of friends and relatives. A clergyman of the appropriate faith may be notified if the parents wish. Issues regarding an autopsy or organ donation (when appropriate) are approached in a multidisciplinary fashion (primary practitioner and primary nurse) with respect, tact, and consideration of the family’s wishes. (See also “Grief and Perinatal Loss” in Merenstein and Gardner, 2006.)
Before the parents leave the hospital, the nurse should provide them with the telephone number of the unit (if they do not have it) and invite them to call any time they have any further questions. Many intensive care units make it a point to contact the parents after a neonatal death to assess the parents’ coping mechanisms, evaluate the grieving process, and provide support as needed. Several organizations are available to offer support and understanding to families who have lost a newborn, including the Compassionate Friends* and Aiding Mothers and Fathers Experiencing Neonatal Death (AMEND).† (See Chapter 23 for further discussion of the family and the grieving process.)
Nurses who care for critically ill infants also experience grief. NICU nurses may feel helpless and sorrowful. It is important that such grief be allowed and that nurses attend the funeral or memorial service as a part of working through the grieving process. Nurses may fear that showing emotion is unprofessional and that the expression of grief demonstrates “loss of control”; these fears are unfounded. Studies have demonstrated that to continue to be effective managers and providers of care, nurses must be allowed to grieve and support each other through the process (Jansen, 2003).
Education regarding bereavement, end-of-life care, and culturally sensitive care of families and their dying infants may help nurses comfort families during this stressful period (Engler, Cusson, Brockett, et al, 2004).
Baptism: Many Christian parents wish to have their child baptized if death is anticipated or is a decided possibility. Whenever possible, it is most desirable that a representative of the parents’ faith (e.g., a Roman Catholic priest or a Protestant minister) perform such a ritual. When death is imminent, a nurse or a physician can perform the baptism by simply pouring water on the infant’s forehead (a medicine dropper is a convenient means) while repeating the words, “I baptize you in the name of the Father and of the Son and of the Holy Spirit.” This includes an infant of any gestational age, particularly when the parents are Roman Catholic.
When the faith of the parents is uncertain, a conditional baptism can be carried out by saying, “If you are capable of receiving baptism, I baptize you in the name of the Father and of the Son and of the Holy Spirit.” The fact of the baptism is recorded in the infant’s chart. Parents are informed at the first opportunity.
High-Risk Conditions Related to Dysmaturity
![]() Prematurity accounts for the largest number of admissions to an NICU. The immaturity not only places infants at risk for neonatal complications (e.g., hyperbilirubinemia and RDS, which has the highest incidence in preterm infants), but may also predispose the infant to problems that persist into adulthood (e.g., learning disabilities, growth deficiencies, asthma).
Prematurity accounts for the largest number of admissions to an NICU. The immaturity not only places infants at risk for neonatal complications (e.g., hyperbilirubinemia and RDS, which has the highest incidence in preterm infants), but may also predispose the infant to problems that persist into adulthood (e.g., learning disabilities, growth deficiencies, asthma).
![]() Case Study—Health Problems of the Newborn
Case Study—Health Problems of the Newborn
Etiology
A variety of maternal and pregnancy-related complications increase the risk of preterm delivery; however, the actual cause of prematurity is not known in most instances. The incidence of prematurity is lowest in the middle to high socioeconomic classes, in which pregnant women are generally in good health, are well nourished, and receive prompt and comprehensive prenatal care. The incidence is highest in the lower socioeconomic classes, in which a combination of deleterious circumstances is present. Other factors, such as multiple pregnancies, pregnancy-induced hypertension, and placental problems that interrupt the normal course of gestation before completion of fetal development, are responsible for a large number of preterm births.
The outlook for preterm infants is largely, but not entirely, related to the state of physiologic and anatomic immaturity of the various organs and systems at the time of birth. Infants at term have advanced to a state of maturity sufficient to allow a successful transition to the extrauterine environment. Infants born prematurely must make the same adjustments but with functional immaturity proportional to the stage of development reached at the time of birth. The degree to which infants are prepared for extrauterine life can be predicted to some extent by estimated gestational age. (See Clinical Assessment of Gestational Age, Chapter 8.) An understanding of prenatal development provides some concept of the status of the systems, at various stages of development, that must cope with functional changes that occur with birth.
Characteristics
Preterm infants have a number of distinct characteristics at various stages of development. Identification of these characteristics provides valuable clues to the gestational age and hence to the physiologic capabilities. The general, outward physical appearance changes as the fetus progresses to maturity. Characteristics of skin, general posture and tone, distribution of hair, and amount of subcutaneous fat provide clues to a newborn’s physical development. Observation of spontaneous, active movements and response to stimulation and passive movement contributes to the assessment of neurologic status. The appraisal is made as soon as possible after admission to the nursery because much of the observation and management of infants depend on this information.
On inspection, preterm infants are very small and appear scrawny because they lack or have only minimum subcutaneous fat deposits and have a proportionately large head in relation to the body, which reflects the cephalocaudal direction of growth. The skin is bright pink (often translucent, depending on the degree of immaturity), smooth, and shiny (may be edematous), with small blood vessels clearly visible underneath the thin epidermis. The fine lanugo is abundant over the body (depending on gestational age) but is sparse, fine, and fuzzy on the head. The ear cartilage is soft and pliable, and the soles and palms have minimum creases, resulting in a smooth appearance. The bones of the skull and the ribs feel soft, and before 26 weeks the eyes may be fused. Male infants have few scrotal rugae, and the testes are undescended; the labia minora and clitoris are prominent in females. Fig. 10-11 compares the features of full-term and preterm infants.
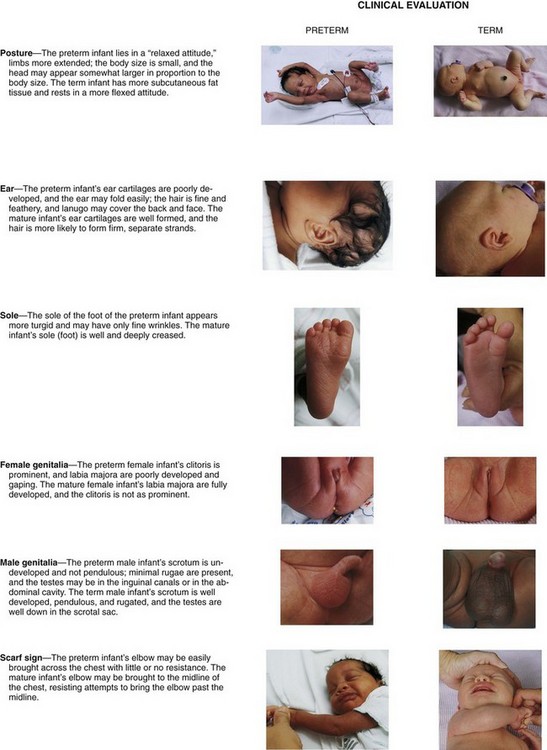
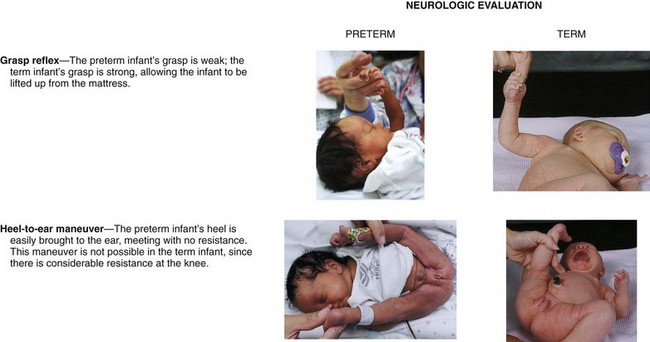
Fig. 10-11 Clinical and neurologic examinations comparing preterm and full-term infants. (Data from Pierog SH, Ferrara A: Medical care of the sick newborn, ed 2, St Louis, 1976, Mosby; photos courtesy Paul Vincent Kuntz, Texas Children’s Hospital, Houston.)
In contrast to full-term infants’ overall attitude of flexion and continuous activity, preterm infants are inactive and listless. The extremities maintain an attitude of extension and remain in any position in which they are placed. Physiologically immature, many preterm infants are unable to maintain body temperature, have limited ability to excrete solutes in the urine, and have increased susceptibility to infection. A pliable thorax, immature lung tissue, and an immature regulatory center lead to periodic breathing, hypoventilation, and frequent periods of apnea. These infants are more susceptible to biochemical alterations such as hyperbilirubinemia and hypoglycemia (see Chapter 9), and they have a higher extracellular water content that renders them more vulnerable to fluid and electrolyte imbalance. Preterm infants exchange fully half their extracellular fluid volume every 24 hours compared with one seventh of the volume turnover in adults.
The soft cranium is subject to characteristic unintentional deformation (dolichocephaly) caused by positioning from one side to the other on a mattress. The head looks disproportionately longer from front to back, is flattened on both sides, and lacks the usual convexity seen at the temporal and parietal areas. This positional molding is often a concern to parents and may influence their perception of the infant’s attractiveness and their responsiveness to the infant. Frequent repositioning of the infant and positioning on a gel mattress can reduce or minimize cranial molding.
Late-preterm infants may not have the immature appearance so commonly observed in preterm infants born at a lower gestational age (<34 weeks’ gestation). However, late-preterm infants are at risk for the development of some of the same physiologic adaptation problems as their preterm counterparts: respiratory distress syndrome, hyperbilirubinemia, thermoregulation difficulties, hypoglycemia, and feeding problems.
Therapeutic Management
When delivery of a preterm infant is anticipated, the intensive care nursery is alerted and a team approach implemented. Ideally, a neonatologist or a neonatal nurse practitioner, a staff nurse, and a respiratory therapist are present for the delivery. Infants who do not require resuscitation are immediately transferred in a heated incubator to the NICU, where they are weighed and where IV access, oxygen therapy, and other therapeutic interventions are initiated as needed. Resuscitation is conducted in the delivery area until infants can be safely transported to the NICU. Ongoing care is described elsewhere in the chapter.
Postterm Infants
Infants born of a gestation that extends beyond 42 weeks as calculated from the mother’s last menstrual period (or by gestational age assessment) are postterm, or postmature, regardless of birth weight. This constitutes 3.5% to 15% of all pregnancies. The cause of delayed birth is unknown. Some infants are appropriate for gestational age but show the characteristics of progressive placental dysfunction. These infants, often called postterm infants, display the characteristics of infants who are 1 to 3 weeks of age, such as absence of lanugo, little if any vernix caseosa, abundant scalp hair, and long fingernails. The skin is often cracked, parchmentlike, and desquamating. A common finding in postterm infants is a wasted physical appearance that reflects intrauterine nutritional deprivation. Depletion of subcutaneous fat gives them a thin, elongated appearance. The little vernix caseosa that remains in the skinfolds may be stained a deep yellow or green, which is usually an indication of meconium in the amniotic fluid.
Fetal and neonatal mortality increase significantly in postterm infants compared with those born at term. They are especially prone to fetal distress associated with the decreasing efficiency of the placenta, macrosomia, and meconium aspiration syndrome (MAS). The greatest risk occurs during the stresses of labor and delivery, particularly in infants of primigravidas, or women delivering their first child. Induction of labor is usually recommended when infants are significantly overdue.
High Risk Related to Disturbed Respiratory Function
Characteristically, preterm infants are periodic breathers. They have periods of rapid respiration separated by periods of very slow breathing, and often short periods with no visible or audible respirations. Apnea is primarily an extension of this periodic breathing and can be defined as a lapse of spontaneous breathing for 20 or more seconds, or shorter pauses accompanied by hypotonia, bradycardia, or color change (Stokowski, 2005).
Apnea of prematurity (AOP) is a common phenomenon in the preterm infant. Rarely observed in full-term infants, apneic spells increase in prevalence the younger the gestational age. Approximately one third of infants less than 33 weeks of gestation and more than half of apparently healthy infants less than 30 weeks of gestation have apneic spells (Stokowski, 2005). Apnea usually resolves as the infant approaches 37 weeks postmenstrual age.
AOP may be further classified according to origin. The three recognized types are (1) central apnea, an absence of diaphragmatic and other respiratory muscle function that causes a lack of respiratory effort and occurs when the CNS does not transmit signals to the respiratory muscles; (2) obstructive apnea, when air flow ceases because of upper airway obstruction, yet chest or abdominal wall movement is present; and (3) mixed apnea, a combination of central and obstructive apnea and the most common form of apnea seen in preterm infants (Poblano, Marquez, and Hernandez, 2006).
Pathophysiology
AOP reflects the immature and poorly refined neurologic and chemical respiratory control mechanisms in preterm infants. These infants are not as responsive to hypercarbia and hypoxemia, and their neurons have fewer dendritic associations than those of more mature infants. The respiratory reflexes of these infants are significantly less mature, which may be a contributing factor in the etiology. Overall weakness of the muscles of the thorax, diaphragm, and upper airway may also contribute to apneic episodes in the preterm infant. In addition, apnea is characteristically observed during periods of REM sleep. A variety of factors, including infection, intracranial hemorrhage (ICH), or PDA, can make apnea worse. Secondary causes of apnea should be investigated in infants with new-onset apnea or when there is a significant change in the frequency or severity of apneic episodes. Apnea in full-term infants should always be considered secondary and the cause investigated.
Clinical Manifestations
Factors that contribute to apnea in preterm neonates should be investigated and treated. Apnea can be anticipated in infants with a variety of conditions (Box 10-8); conversely, one of these disorders may be suspected in infants with persistent apneic spells. Although apnea is an expected event in preterm neonates, it should not be designated as being benign until all other causes have been ruled out. The observation of apnea is a reason to screen for any of the causes listed in Box 10-8.
Therapeutic Management
Administration of caffeine is often effective in reducing the frequency of primary apnea-bradycardia spells in newborns. Caffeine acts as a CNS stimulant to breathing. Neonates receiving caffeine must be closely observed for symptoms of toxicity. Caffeine has come to the forefront of pharmacologic therapy for AOP because it has fewer side effects than previously used aminophylline or theophylline, requires dosing once daily, has more predictable plasma concentrations, has slower elimination, and has a wider therapeutic range (trough, 5 to 20 mcg/ml). Caffeine citrate (Cafcit) has been approved for use in preterm infants with AOP. It is available in injectable and oral form. Weight and urinary output should be closely monitored because caffeine acts as a mild diuretic.
Nasal CPAP and, more recently, nasal intermittent positive pressure ventilation have been used as an adjunct treatment for AOP. CPAP acts to maintain airway patency; hence it is most effective for obstructive or mixed apnea.
Nursing Care Management
Management of apnea consists of monitoring respiration and heart rate routinely in all preterm infants and preventing contributing conditions. Cardiorespiratory monitors alert the staff to cessation of respiration according to a preset delay time—usually 15 to 20 seconds. Effective monitoring devices do not eliminate the need for alert nursing observation. Nursing observation combined with monitoring is the most effective means of identifying neonatal apnea.
If begun early, gentle tactile stimulation (e.g., rubbing the back or chest gently, turning the infant to a supine position) will stop most apneic spells. If tactile stimulation fails to reinstitute respiration, flow-by oxygen and suctioning of the nose and mouth may be required. If breathing does not begin, the chin is raised gently to open the airway, and sufficient pressure is applied with a resuscitation mask and bag to lift the rib cage. The infant is never shaken. After breathing is restored, the infant is assessed for possible precipitating factors, such as unstable temperature, abdominal distention (if not observed earlier), and supplemental oxygen (if any) being delivered before the episode. The use of pulse oximetry has helped detect the onset of an apneic episode.
It is important that nurses maintain a careful record of episodes of apnea, including the number of apneic spells, the infant’s appearance during and after the episode, and whether the infant self-recovers or whether tactile stimulation or other measures are needed to restore breathing. Subsequent investigation into the possible cause of the apneic episode is vital to the care of the preterm infant because it may signal an underlying condition such as sepsis or NEC.
Persistent and repeated periods of apnea may be treated by mechanical ventilation or CPAP. Various methods devised to provide an intermittent stimulus for breathing, such as oscillating beds and water beds, have achieved variable success in the treatment of AOP.
Respiratory Distress Syndrome
![]() RDS refers to a condition of surfactant deficiency and physiologic immaturity of the thorax. The terms respiratory distress syndrome and hyaline membrane disease are most often applied to this severe lung disorder. It is seen almost exclusively in preterm infants but may also be associated with multifetal pregnancies, infants of diabetic mothers, cesarean section delivery, delivery before 37 weeks of gestation, precipitous delivery, cold stress, asphyxia, and a history of previous RDS (Dudell and Stoll, 2007). The disorder is rarely observed in drug-exposed infants or infants who have been subjected to chronic intrauterine stress (e.g., maternal preeclampsia or hypertension). Respiratory distress of a nonpulmonary origin in neonates may also be caused by sepsis, cardiac defects (structural or functional), exposure to cold, airway obstruction (atresia), IVH, hypoglycemia, metabolic acidosis, acute blood loss, and drugs. Pneumonia in the neonatal period is respiratory distress caused by bacterial or viral agents and may occur alone or as a complication of RDS.
RDS refers to a condition of surfactant deficiency and physiologic immaturity of the thorax. The terms respiratory distress syndrome and hyaline membrane disease are most often applied to this severe lung disorder. It is seen almost exclusively in preterm infants but may also be associated with multifetal pregnancies, infants of diabetic mothers, cesarean section delivery, delivery before 37 weeks of gestation, precipitous delivery, cold stress, asphyxia, and a history of previous RDS (Dudell and Stoll, 2007). The disorder is rarely observed in drug-exposed infants or infants who have been subjected to chronic intrauterine stress (e.g., maternal preeclampsia or hypertension). Respiratory distress of a nonpulmonary origin in neonates may also be caused by sepsis, cardiac defects (structural or functional), exposure to cold, airway obstruction (atresia), IVH, hypoglycemia, metabolic acidosis, acute blood loss, and drugs. Pneumonia in the neonatal period is respiratory distress caused by bacterial or viral agents and may occur alone or as a complication of RDS.
![]() Nursing Care Plan—The High-Risk Infant with Respiratory Distress
Nursing Care Plan—The High-Risk Infant with Respiratory Distress
Pathophysiology
Preterm infants are born before the lungs are fully prepared to serve as efficient organs for gas exchange. This appears to be a critical factor in the development of RDS. RDS results from a combination of structural and functional immaturity of the lungs.
Because the final unfolding of the alveolar septa, which increases the surface area of the lungs, occurs during the last trimester of pregnancy, preterm infants are born with numerous underdeveloped and many uninflatable alveoli. In addition, the fetal chest wall is highly compliant because of the predominance of cartilage rather than bone; and the diaphragm, the dominant respiratory muscle, is prone to fatigue.
Functionally, the fetal lungs are deficient in surfactant, a surface-active phospholipid secreted by type II cells in the alveolar epithelium. Surfactant is first produced at about 24 weeks of gestational age, but the type II cells in the lung do not fully mature until about 36 weeks of gestation (Fig. 10-12). Acting much like a detergent, this substance reduces the surface tension of fluids that line the alveoli and respiratory passages, resulting in uniform expansion and maintenance of lung expansion at low intraalveolar pressure. Immature development of these functions produces consequences that seriously compromise respiratory efficiency. Deficient surfactant production causes unequal inflation of alveoli on inspiration and the collapse of alveoli on end expiration. Without surfactant, infants are unable to keep their lungs inflated and therefore exert a great deal of effort to reexpand the alveoli with each breath. It has been estimated that each breath requires as much negative pressure (60 to 75 cm H2O) as the initial lung expansion at birth. With increasing exhaustion they are able to open fewer and fewer alveoli. This inability to maintain lung expansion produces widespread atelectasis.
PATHOPHYSIOLOGY REVIEW
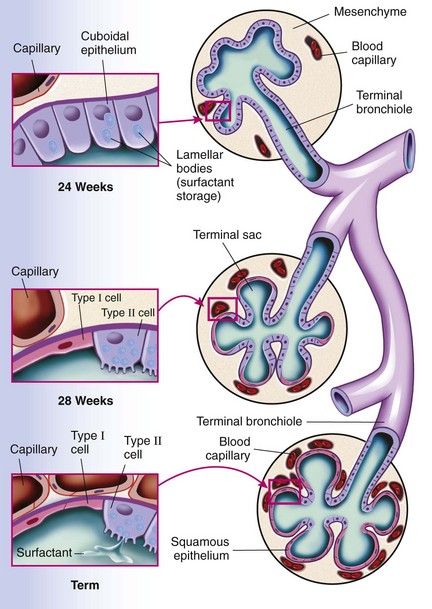
Fig. 10-12 Prenatal development of the alveolar unit. (From McCance K, Huether S: Pathophysiology: the biological basis for disease in adults and children, ed 6, St Louis, 2010, Mosby.)
In the absence of alveolar stability (normal functional residual capacity) and with progressive atelectasis, pulmonary vascular resistance (PVR) increases, whereas with normal lung expansion it would decrease. Consequently, there is hypoperfusion to the lung tissue, with a decrease in effective pulmonary blood flow. The increase in PVR causes partial reversion to the fetal circulation, with a right-to-left shunting of blood through the persisting fetal communications—the ductus arteriosus and foramen ovale.
Inadequate pulmonary perfusion and ventilation produce hypoxemia and hypercapnia. Pulmonary arterioles, with their thick muscular layer, are markedly reactive to diminished oxygen concentration. Thus a decrease in oxygen tension causes vasospasm in the pulmonary arterioles that is further enhanced by a decrease in blood pH. This vasoconstriction contributes to a marked increase in PVR. In normal ventilation with increased oxygen concentration, the ductus arteriosus constricts and the pulmonary vessels dilate to decrease PVR (Fig. 10-13).
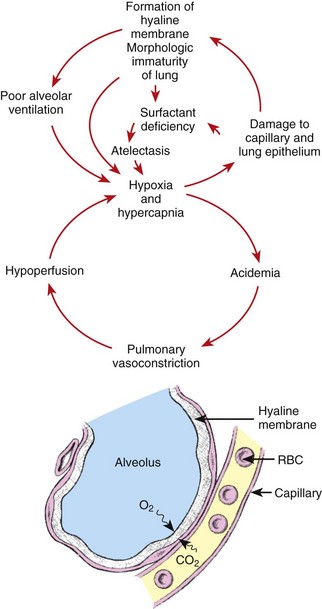
Fig. 10-13 Interdependent relationship of factors involved in pathology of respiratory distress syndrome. CO2, Carbon dioxide; O2, oxygen; RBC, red blood cell. (From Pierog SH, Ferrara A: Medical care of the sick newborn, ed 2, St Louis, 1976, Mosby.)
Prolonged hypoxemia activates anaerobic glycolysis, which produces increased amounts of lactic acid. An increase in lactic acid causes metabolic acidosis; inability of the atelectatic lungs to blow off excess carbon dioxide produces respiratory acidosis. Lowered pH causes further vasoconstriction. With deficient pulmonary circulation and alveolar perfusion, Pao2 continues to fall, pH falls, and the materials needed for surfactant production are not circulated to the alveoli.
Pulmonary edema observed in the early stages of RDS also contributes to impaired gas exchange. Factors believed to facilitate this fluid accumulation in the lungs include renal immaturity or insufficiency resulting from hypoxemia, high fluid intake and PDA, left ventricular dysfunction associated with papillary muscle necrosis, low serum protein concentration and low colloid osmotic pressure, increased alveolar surface tension that enhances the shift of interstitial fluid to alveolar spaces, oxygen toxicity, and high plasma vasopressin.
Pulmonary interstitial emphysema (PIE) may develop in preterm infants with RDS and immature lungs as a result of overdistention of distal airways. This condition further complicates adequate oxygenation in the immature airways (see Air Leak Syndromes, p. 358).
Deficiencies in other systems contribute to respiratory distress. For example, a high threshold of the respiratory center to afferent stimuli and weak or absent gag and cough reflexes reflect the immaturity of the nervous system. In addition, the persistence of fetal hemoglobin, so beneficial in prenatal existence, may place the infant at a disadvantage during respiratory distress. Although the binding power of fetal hemoglobin for oxygen is much greater than that of adult hemoglobin, this increased affinity also causes less oxygen to be released to the tissues at normal oxygen tension. In the newborn the arterial oxygen concentration must fall to a lower level for bound oxygen to be released from fetal hemoglobin.
A hyaline membrane is formed as hypoxemia and the increased pulmonary vascular pressure cause transudation of fluid into the alveoli. Necrotic cells from damaged alveoli plus the fibrin in the transudate form a membranous layer that lines the alveoli and inhibits gas exchange. The hyaline membrane contributes to respiratory difficulties by greatly diminishing lung distensibility, or compliance, the elastic quality of lung tissue that permits expansion in response to a given amount of applied pressure during inspiration. Affected lungs are stiffer and require far more pressure than do normal lungs to achieve an equal amount of expansion. Table 10-7 summarizes the major factors that produce RDS in immature infants.
TABLE 10-7
MAJOR FACTORS IN RESPIRATORY DISTRESS SYNDROME
| CAUSE | EFFECT |
| Increased pulmonary vascular resistance | Alveolar collapse; atelectasis; increased difficulty breathing |
| Impaired gas exchange | Hypoxemia and hypercapnia with respiratory acidosis |
| Increased transudation of fluid into lungs | Hypoperfusion of pulmonary circulation |
| Hypoperfusion (with hypoxemia) | Tissue hypoxia and metabolic acidosis |
| Hyaline membrane formation; impaired gas exchange | Increased surface tension of alveoli (surfactant deficiency) |
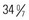 and
and  weeks of gestation, regardless of birth weight
weeks of gestation, regardless of birth weight