Molecular genetics and the skin
Recent and rapid advances in genetics have had an impact on our understanding of skin diseases. The Human Genome Project has now mapped all human genes, of which there are about 35 000. Genetics has been found to be more complicated than the original Mendelian concept, and common conditions such as atopy occur as a result of a complex interaction between multiple susceptibility genes and the environment. An average pregnancy carries a 1% risk of a single gene disease and a 0.5% risk of a chromosome disorder, but genetically influenced traits, e.g. atopy, are much more common.
The human chromosomes
The human genome comprises 23 pairs of chromosomes that are numbered by size (Fig. 1). Chromosomes are packets of genes with support proteins in a large complex. The karyotype is an individual’s number of chromosomes plus their sex chromosome constitution, i.e. 46XX for females and 46XY for males. The phenotype is the expression at a biological level of the genotype, e.g. blue eyes or atopy.
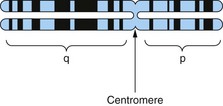
Divided by the centromere into the shorter (p) and the longer (q) arms, showing banding with the Giemsa stain.
Genes and DNA
Common variations in DNA sequence found in a population are called genetic polymorphisms and are inherited and not maintained by recurrent mutation. These polymorphisms may be functional (affect biological processes) or non-functional (‘silent’). A new change or inherited alteration in DNA sequence that causes pathology (disease) is a mutation. Investigation of genetic causation of disease can be undertaken from a population/phenotype level down (genome screen – statistical association of gene sequence alterations in disease versus control group) or gene level up (candidate gene analysis – sequencing genes of interest in families or populations with disease versus control populations).
Molecular methods
DNA sequence variations can be identified by the consequent change in polymerase chain reaction (PCR) amplification product size (Fig. 2), loss or gain of restriction endonuclease cutting, or sequence analysis. In recent years, DNA sequencing has become a high-throughput technology that has led to the concept of ‘whole genome sequencing’ studies of healthy versus controls. As this technique is so powerful, smaller numbers are required.
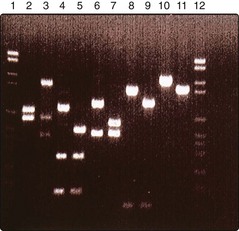
Fig. 2 Agarose gel electrophoresis, showing migration of DNA after cutting with enzymes, screening for mutations in a keratin gene.
To establish which molecular pathways may be important in disease pathogenesis, gene chip arrays and ‘next generation sequencing’ allow detailed and quantitative analysis of the transcribed genes in a diseased tissue (transcriptome). The transcribed genes are subject to further regulation by RNA degradation, silencing and inhibition. Thus, study of the protein repertoire in the diseased tissue (proteomics) may also be undertaken.
Molecular techniques can be used to:
 detect small amounts of DNA, e.g. of human papilloma virus within a skin cancer
detect small amounts of DNA, e.g. of human papilloma virus within a skin cancer
 sequence DNA from a ‘candidate’ section of an individual’s chromosome and compare the base sequences with family members similarly affected by a disorder, thus mapping a specific gene polymorphism characteristic for that disease (Table 1)
sequence DNA from a ‘candidate’ section of an individual’s chromosome and compare the base sequences with family members similarly affected by a disorder, thus mapping a specific gene polymorphism characteristic for that disease (Table 1)
 sequence the entire genome of an individual
sequence the entire genome of an individual
 identify the repertoire of genes that have been transcribed as a measure of the protein profile of the cell.
identify the repertoire of genes that have been transcribed as a measure of the protein profile of the cell.
Table 1 Skin conditions or characteristics with definite or probable gene localities on the chromosomes
| Chromosome site | Disease or characteristic |
|---|---|
| 1p34 | Porphyria cutanea tarda: enzyme (p. 46) |
| 2q31 | Ehlers–Danlos syndrome: collagen III (p. 93) |
| 3p21.3 | Dystrophic epidermolysis bullosa: collagen VII (p. 91) |
| 4, 4p | Red hair colour, psoriasis (Psors3 gene) |
| 6p21.3 | Psoriasis (Psors1 gene: 30% of susceptibility) |
| 9p21 | Familial malignant melanoma: kinase inhibitor (p. 102) |
| 9q22.3 | Xeroderma pigmentosum (p. 93) |
| 9q34 | Tuberous sclerosis: hamartin (p. 92) |
| 11q12 | Atopy: asthma and rhinitis: IgE response (p. 36) |
| 12q13 | Epidermolysis bullosa simplex: keratin 5 (p. 91) |
| 12q23 | Darier’s disease: adenosine triphosphatase (p. 90) |
| 14q11.2 | Ichthyosis: transglutaminase (p. 90) |
| 15q11.2 | Oculocutaneous albinism: homologue (p. 74) |
| 17q11.2, 17q25 | Neurofibromatosis NF1, psoriasis (Psors2) |
| 17q21.31 | Ehlers–Danlos syndrome: collagen I (p. 93) |
| 19 | Green/blue eye colour, brown hair colour |
| 21 trisomy | Down syndrome (p. 93) |
| Xq28 | Incontinentia pigmenti: nuclear factor (NF)-κB modulator (p. 93) |
| Xq22.32 | X-linked ichthyosis: steroid sulphatase (p. 90) |
Forms of inheritance
An individual with two different genes (alleles) at a particular locus is heterozygous, and one who has identical alleles is homozygous. Genes borne on chromosomes other than X and Y are autosomal, whereas those on X and Y are sex linked. Factors governing genetic penetrance are unclear.
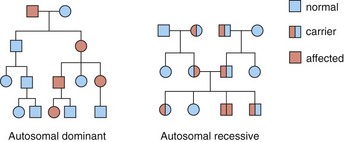
 Dominant. Affected individuals (both sexes) are heterozygous for the gene, will have an affected parent (except for new mutations) and have a 50% chance of passing it to their children (Fig. 3).
Dominant. Affected individuals (both sexes) are heterozygous for the gene, will have an affected parent (except for new mutations) and have a 50% chance of passing it to their children (Fig. 3).
 Recessive. An affected individual (of either sex) is homozygous for the gene, and both parents will be carriers and healthy. Consanguinity increases the risk. Recessive disorders are often severe. There is a 25% chance of heterozygotes passing the gene to the next generation.
Recessive. An affected individual (of either sex) is homozygous for the gene, and both parents will be carriers and healthy. Consanguinity increases the risk. Recessive disorders are often severe. There is a 25% chance of heterozygotes passing the gene to the next generation.
 X-linked recessive. Only affects males, as females are healthy carriers.
X-linked recessive. Only affects males, as females are healthy carriers.
 X-linked dominant. Affects males and females, although some disorders, e.g. incontinentia pigmenti, are lethal in males.
X-linked dominant. Affects males and females, although some disorders, e.g. incontinentia pigmenti, are lethal in males.
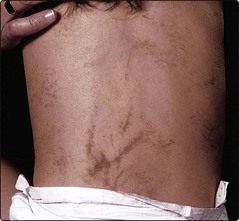
 Mosaicism. In mosaicism, an individual has two or more genetically different cell lines. The somatic (postconceptional) mutation of a single cell in an embryo results in a clone of subtly distinct cells. In the skin, this is revealed by the developmental growth pattern of Blaschko’s lines (Fig. 4). Certain dermatoses, e.g. naevi and incontinentia pigmenti (Fig. 5), follow these lines, resulting in streaky or whorled patterns where the abnormal clone meets normal cells. A dermatomal distribution (Fig. 6) suggests nerve involvement.
Mosaicism. In mosaicism, an individual has two or more genetically different cell lines. The somatic (postconceptional) mutation of a single cell in an embryo results in a clone of subtly distinct cells. In the skin, this is revealed by the developmental growth pattern of Blaschko’s lines (Fig. 4). Certain dermatoses, e.g. naevi and incontinentia pigmenti (Fig. 5), follow these lines, resulting in streaky or whorled patterns where the abnormal clone meets normal cells. A dermatomal distribution (Fig. 6) suggests nerve involvement.
 Imprinting. Imprinting involves the differential switching off of genes according to whether they have come from the father or the mother. It may be caused by methylation of DNA.
Imprinting. Imprinting involves the differential switching off of genes according to whether they have come from the father or the mother. It may be caused by methylation of DNA.
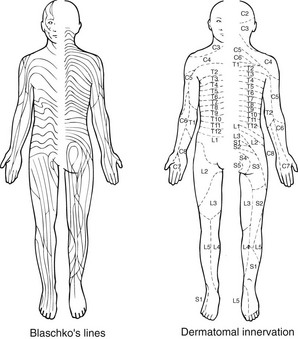
Fig. 4 Blaschko’s lines represent the growth trends of embryonic tissue, whereas the dermatomes map out areas of skin innervation.
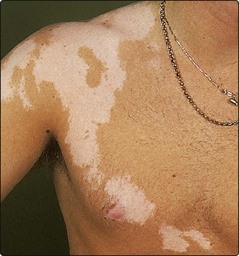
Rather than follow Blaschko’s lines, this occurs in a dermatomal distribution, suggesting a relationship with skin innervation.
Inheritance of specific skin disorders
In psoriasis (p. 24) and atopic eczema (p. 36), a family history is common, but the exact mode of inheritance is unclear. Psoriasis may be inherited polygenically or by an autosomal dominant gene with incomplete penetrance. Atopic eczema has recently been shown to be strongly associated with chromosome 1q21 mutations in the gene encoding the epidermal protein, filaggrin. Inheritance patterns in the rarer conditions are often clearer (Table 2). Epidermolysis bullosa simplex and dystrophica (p. 91), the porphyrias (p. 46), the Ehlers–Danlos syndromes (p. 93) and some other conditions may be dominantly or recessively inherited.
Table 2 The inheritance of selected skin disorders
| Inheritance | Disorder |
|---|---|
| Autosomal dominant | |
| Autosomal recessive | |
| X-linked recessive | X-linked ichthyosis (p. 90) |
| X-linked | Incontinentia pigmenti (p. 93) |
Some dermatoses are associated with polymorphisms in the human leucocyte antigen (HLA) complex on chromosome 6 (p. 11). These tend to show polygenic inheritance and an association with autoimmunity.
Gene therapy
DNA-based prenatal diagnosis is possible in several genodermatoses (p. 91). Although recessive disorders in which there is a single gene defect offer scope for genetic treatment and gene therapy, which has been shown to be possible in epidermolysis bullosa, this remains a highly complicated and expensive process with potentially severe complications including haematological malignancy.
Molecular genetics and the skin
 The human genome of 23 chromosomes (karyotype 46XY or 46XX) contains 35 000 genes, all of which have been mapped. Additionally, mitochondria encode 37 genes for oxidative enzymes.
The human genome of 23 chromosomes (karyotype 46XY or 46XX) contains 35 000 genes, all of which have been mapped. Additionally, mitochondria encode 37 genes for oxidative enzymes.
 DNA segments can be amplified by PCR and demonstrated by gel electrophoresis.
DNA segments can be amplified by PCR and demonstrated by gel electrophoresis.
 Whole genome sequencing and microarray technology have advanced the molecular approach to studying disease pathogenesis.
Whole genome sequencing and microarray technology have advanced the molecular approach to studying disease pathogenesis.
 Dominant, recessive and X-linked inheritances are seen, but heredity is still unclear in several disorders.
Dominant, recessive and X-linked inheritances are seen, but heredity is still unclear in several disorders.
 A dermatosis caused by mosaicism, due to a mutation producing more than one cell line, may appear in Blaschko’s lines.
A dermatosis caused by mosaicism, due to a mutation producing more than one cell line, may appear in Blaschko’s lines.
 Gene therapy should be possible for some recessive single gene disorders.
Gene therapy should be possible for some recessive single gene disorders.