Chapter 74Diseases of the Digital Flexor Tendon Sheath, Palmar Annular Ligament, and Digital Annular Ligaments
Synovial effusion of the digital flexor tendon sheath (DFTS) is common in all types of working horses. Frequently, effusion is idiopathic in origin and affects the DFTS of both hindlimbs without causing lameness. Occasionally synovial effusion is seen in a single limb along with lameness. Before the advent of ultrasonography and tenoscopy, injury to the soft tissue structures of the DFTS often remained unrecognized. The cause of chronic synovial effusion and lameness remained elusive, and a diagnosis of idiopathic tenosynovitis was made readily. Because modern diagnostic techniques have become commonplace in equine lameness practice, specific injuries of the structures of the DFTS have been identified.
Anatomical Considerations
The detailed anatomy of the DFTS and its contents was well described1 and is similar in forelimbs and hindlimbs. Reference is made to palmar and metacarpal region throughout, but the terms plantar region and metatarsal region strictly apply to the hindlimb. The DFTS is a thin-walled structure that encompasses the superficial digital flexor tendon (SDFT) and the deep digital flexor tendon (DDFT) from the level of the distal third of the metacarpal region to the T ligament, just proximal to the navicular bursa and the palmar pouch of the distal interphalangeal joint. The DFTS is mesenchymal in cell origin and is composed of two layers: an outer fibrous layer and an inner synovial layer. The fibrous layer provides strength and vascularity to the sheath. The synovial layer provides a smooth, frictionless surface and produces constituents of the synovial fluid. The palmar wall of the sheath incorporates three annular ligaments: the palmar annular ligament (PAL) and the proximal and the distal digital annular ligaments. These annular ligaments bind the digital flexor tendons to the palmar aspect of the digit, are effectively thickenings of the fibrous layer of the sheath wall with a transverse fiber orientation, and measure 2 mm or less in thickness.
The PAL or annular ligament of the fetlock joint inserts on the palmar border of the proximal sesamoid bones (PSBs), is continuous with the palmar (intersesamoidean) ligament of the fetlock joint, and thus converts the proximal scutum into an inelastic canal. The strong, transversely arranged fibers of the PAL bind down the digital flexor tendons into the proximal scutum. Distal to the PAL and immediately under the skin, the deep fascia forms a second, quadrilateral ligament: the proximal digital annular ligament. This ligament is a fibrous sheet that covers and adheres to the palmar surface of the SDFT and attaches laterally and medially by two bands to the proximal phalanx: one to the proximopalmar tuberosity and one that joins the insertion of the distal branch of the SDFT to the distal part of the proximal phalanx. This arrangement firmly binds the SDFT and DDFT, enveloped by the DFTS, in the palmar pastern region. The distal digital annular ligament adheres to the palmar surface of the distal part of the DDFT and binds down the terminal part of this tendon. The ligament is a crescent-shaped fibrous sheet attached by a strong band on either side of the middle of the proximal phalanx, covering the distal branches of the SDFT.
The dorsal wall of the DFTS is formed by the proximal scutum, middle scutum, and distal sesamoidean ligaments. The proximal scutum and the middle scutum are strong fibrocartilaginous pads that allow sliding of the digital flexor tendons along the palmar aspect of the fetlock and pastern regions, respectively. The proximal scutum is composed of the two PSBs and the thick intersesamoidean ligament. The latter is a thick sagittal structure made of transversely aligned collagen fibers. The intersesamoidean ligament covers and is attached strongly to the whole palmar and axial aspect of the PSBs and creates a solid union between these bones. The concave palmar face of the proximal scutum allows sliding of the digital flexor tendons in the palmar fetlock region. The proximal scutum extends proximally to the apex of each PSB between the two distal branches of the suspensory ligament (SL). Distally the proximal scutum gives origin to the distal sesamoidean ligaments, which represent the functional continuation of the SL and consist of the straight, oblique, cruciate, and short sesamoidean ligaments. The straight sesamoidean ligament inserts distally on the middle scutum, together with the distal branches of the SDFT and the palmar ligaments of the proximal interphalangeal joint. The middle scutum is a thick, fibrocartilaginous structure attached to the proximopalmar aspect of the middle phalanx. The middle scutum contacts the palmar aspect of the distal condyles of the proximal phalanx dorsally and the DDFT palmarly.
Within the DFTS, the SDFT and DDFT are intimately related. A fibrous ring (the manica flexoria) emanates from the lateral and medial borders of the SDFT and encircles the DDFT completely, from the proximal limit of the DFTS to the proximal aspect of the PSBs. The synovial lining of the DFTS adheres to the palmar surface of the SDFT in the sagittal midline proximal to the PAL, along the dorsal surface of the PAL and along the dorsal surface of the proximal digital annular ligament. The synovial lining of the DFTS also adheres to the palmar surface of the DDFT between the proximal digital annular ligament and the distal digital annular ligament, and along the dorsal surface of the distal digital annular ligament. The sagittal adhesion-like mesotendon between the SDFT and PAL is referred to as the vinculum of the SDFT. The DDFT also has a mesotendon that attaches to its palmar surface at the level of the proximal interphalangeal joint (see Figure 70-2, B). These mesotendon attachments contain vascular branches that contribute to the arterial supply of the intrasynovial part of the tendon.
The DFTS facilitates displacement of the digital flexor tendons during flexion and extension of the fetlock and interphalangeal joints. During metacarpophalangeal (metatarsophalangeal) joint movements, the two digital flexor tendons displace together, but during interphalangeal joint movements, displacement of the DDFT is greater than that of the SDFT.
Diagnostic Techniques
Diagnostic techniques that localize disease to the DFTS include synoviocentesis and synovial fluid analysis and intrathecal or perineural injection of local anesthetic solution. Synoviocentesis of the DFTS can be performed in one of the several recesses of the sheath. Access to the proximal pouch is possible when the sheath is distended with synovial fluid but difficult when it is not. Synoviocentesis can be achieved by introducing a 2.5-cm needle along the dorsal aspect of the DDFT, between the DDFT and the lateral branch of the SL, a few centimeters proximal to the lateral PSB. Easier access can be gained via the distal palmar pouch of the sheath, which extends between the two distal branches of the SDFT and between the two digital annular ligaments, along the palmar surface of the DDFT. One should remember that this pouch is divided sagittally by the mesotendon of the DDFT in its distal part. The needle can be introduced through the skin to one side of the midline and, to avoid iatrogenic damage to the DDFT, gently and slowly advanced at approximately 45 degrees to the skin surface until synovial fluid is seen at the needle hub. To increase distention of the distal palmar recess, it can be useful temporarily to compress the proximal pouch by firm application of an elasticated bandage (see Figure 124-1). Alternatively, the needle can be aimed to access this pouch between the lateral or medial border of the DDFT and the ipsilateral distal branch of the SDFT, which will prevent inadvertent needle penetration of the DDFT, although the palmar pouch of the proximal interphalangeal joint can be entered if the needle is introduced too deeply. The DFTS also can be accessed through its proximal or distal collateral recesses. The proximal collateral recess is situated in the triangular space palmaromedially or palmarolaterally, between the base of a PSB, the proximal insertion of the proximal digital annular ligament, and the dorsal border of the DDFT. The space can be entered 1 cm distal to the base of a PSB and 1 cm palmar or plantar to the neurovascular bundle. The distal collateral recess is located on the lateral (or medial) aspect of the pastern, between the digital flexor tendons and the distal sesamoidean ligaments and between the proximal and distal insertions of the proximal digital annular ligament. A cadaver study showed that synoviocentesis of the DFTS using these techniques was most consistently successful when performed at the level of the proximal lateral recess on a non–weight-bearing limb.2 In addition, a palmar-plantar axial sesamoidean approach was described where the needle was introduced axial to the PSB in the flexed limb. This technique was described as being optimal for synoviocentesis.3 Ten milliliters of local anesthetic solution is injected for adequate analgesia of the DFTS (see Chapter 10). It is important to recognize that intrathecal analgesia of the DFTS is not specific for elimination of pain from the contained structures and can influence pain from the oblique and straight sesamoidean ligaments.5 Moreover, in some horses with DFTS pathology, the response to perineural analgesia is better than to intrathecal analgesia.
Characteristics of synovial fluid of the DFTS do not vary from those of the distal limb synovial joints. Normal synovial fluid is clear yellow and has a nucleated cell count of 770 cells/mcL or less and a total protein concentration of 1.0 g/dL or less.4 More sophisticated analysis of the synovial fluid for molecular markers offers the prospect of better preoperative identification of the presence of intrathecal tendon pathology.6
Imaging of the Digital Flexor Tendon Sheath
Diagnostic ultrasonography is by far the most commonly used technique for evaluating the DFTS. The DFTS is first encountered at level 3A and continues distally to level P1C and beyond (see Chapter 16).8 The PAL can be demonstrated in normal horses as a thin (1 to 2 mm) echogenic band immediately adjacent to the palmar surface of the SDFT at level 3C. The proximal digital annular ligament and distal digital annular ligament usually cannot be recognized in the palmar midline, unless they are abnormally thickened. The vinculum of the SDFT at the level of the PSB is easily identifiable by ultrasonography, but the distal mesotendon of the DDFT in the phalangeal region is only occasionally visible, usually when distention of the DFTS provides negative contrast (see Figure 70-2, B). A normal synovial reflection or mesotendon joins the lateral and medial borders of the DDFT in the proximal recess of the DFTS, which should not be mistaken for an adhesion (see Figure 70-2, A). The thickness of the DFTS can be assessed at levels 3A and 3B, where the capsule is identifiable as an echogenic band dorsal to the DDFT and the manica flexoria.
Before the widespread use of ultrasonography for examining the soft tissues of the DFTS, negative contrast radiography was described for the assessment of tenosynovitis and annular desmitis.9 To perform this technique, a tourniquet is applied distal to the carpus in a standing or anesthetized horse; 50 to 100 mL of air is injected into the DFTS; and another 200 to 300 mL of air is injected subcutaneously. Sometimes extra air is injected between the SDFT and DDFT at the midmetacarpal level. One postinflation lateromedial radiograph obtained at half the milliampere second value of standard skeletal exposure for this region is normally sufficient to make an accurate diagnosis. Although this technique effectively demonstrates the thickness of the PAL, its use has become superseded by the widespread availability of diagnostic ultrasonography.
Survey radiography of the DFTS is performed to demonstrate evidence of intrathecal air or gas caused by a penetrating wound, the presence of metaplastic mineralization of injured soft tissue structures, or concurrent pathological conditions of the bone. Positive contrast radiography10 may provide the most conclusive evidence of wound communication with the synovial space and may also provide additional information in the diagnosis of synovioceles associated with the DFTS. Although fistulography and filling of the intrathecal space with sterile iodine-based contrast medium is diagnostic of communication between the wound and the DFTS, we prefer to access the DFTS by placing a needle at a site remote from the wound. This minimizes the risk of forcing bacteria or foreign material present in the deeper layers of the wound into the synovial space. Using a remote site further avoids the risk of inadvertently introducing bacteria while passing the needle through an area of cellulitis into the synovial cavity. Infiltration of 10 to 20 mL of sterile contrast medium followed by manipulation of the digit should result in flow of contrast medium from the wound and can be demonstrated radiologically (Figure 74-1).
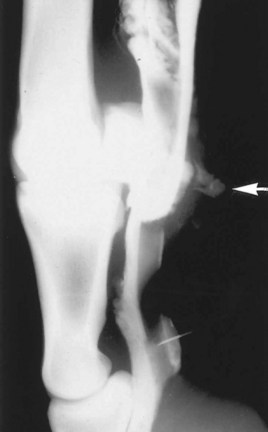
Fig. 74-1 Lateromedial radiographic image of the fetlock region. A positive-contrast tenogram of the digital flexor tendon sheath, followed by manipulation of the digit, resulted in flow of radiodense contrast medium from a wound (arrow).
Tenoscopy is the ultimate imaging modality for evaluating the internal structures of the DFTS.11 An endoscope is introduced routinely in the proximal collateral recess of the sheath, 1 cm distal to the base of the PSB and 1 cm palmar or plantar to the neurovascular bundle, but access to other synovial recesses is also possible (see Chapter 24). This approach allows for a complete examination of the DFTS and its contents, except for the palmar surface of the SDFT, unless substantial thickening of any of the annular ligaments or extensive subcutaneous fibrosis has occurred. Access in ponies and cob-types with very thick skin can also be difficult. The approach also facilitates therapeutic maneuvers within the DFTS, such as PAL desmotomy, adhesiotomy, synovial mass removal, and debridement of fibrillated or torn areas of the digital flexor tendons, manica flexoria, or intersesamoidean ligament.
Magnetic resonance imaging has superior soft tissue contrast and has been of use to identify occult lesions not seen radiologically or ultrasonographically such as isolated adhesions, lesions of the SDFT or DDFT, and injury of the distal digital annular ligament.
Diseases of the Digital Flexor Tendon Sheath
Noninfectious Tenosynovitis
Etiopathogenesis
Acute noninfectious tenosynovitis is a traumatic synovitis/capsulitis of the sheath lining. As for joints, a traumatic synovitis/capsulitis can be caused by accumulative low-grade trauma associated with normal exercise, acute trauma associated with direct impact force (e.g., overreach), or an abnormal force outside the normal range of movement of the fetlock region (e.g., hyperextension). Synovitis/capsulitis is more frequently secondary to damage to the internal or supporting structures of the DFTS, such as disruption of the visceral or parietal synovial layers, tearing of the vincula, tearing of the sheath wall with herniation or synoviocele formation, central or marginal damage to the flexor tendons, tearing of the manica flexoria, and spraining of the PAL or proximal digital annular ligament. Each of these complicating conditions is likely to result in continuous irritation of the sheath and cause chronic tenosynovitis. Chronic tenosynovitis may be associated with villonodular thickening of the sheath lining, especially in the proximal recess; adhesion formation between the visceral and parietal synovial lining; and fibrosis with reduced elasticity of the DFTS capsule. When these conditions are present, a self-perpetuating cycle occurs of improvement with rest, followed by repeated inflammation with exercise. This results in increased inflammation and lameness, further fibrosis, and eventually thickening of the PAL and stenosis of the fetlock canal (see the following discussion of PAL syndrome). Complex tenosynovitis has been defined as tenosynovitis with thickening of the PAL, synovial distention, and adhesions or synovial masses, or both (Figure 74-2).12 Windgalls, especially those occurring bilaterally in the hindlimbs, are another form of low-grade chronic tenosynovitis. Although lameness is not usually a feature of windgalls, the synovial effusion is still likely to reflect the presence of low-grade chronic synovitis of the DFTS, caused by the continuous stress of use-induced overloading.
Diagnosis
Acute tenosynovitis is characterized by a sudden onset of mild to severe lameness, accompanied by DFTS distention that can be palpated in the proximolateral and proximomedial pouches of the sheath and in the palmarodistal recess between both branches of the SDFT. The palmar or plantar region of the fetlock has increased skin temperature, and forced flexion of the fetlock is painful and exacerbates lameness. Chronic tenosynovitis produces similar signs, except for the signs of acute inflammation, although repeat injury may cause these signs to be superimposed on an established tenosynovitis. Disproportionate distention and pain in different regions of the DFTS can reflect the site of the primary pathology; pain on palpation of the proximal aspect of the DFTS is present when there is tearing of the digital flexor tendons in this location.
Ultrasonographic examination is essential for identifying any primary pathology responsible for the tenosynovitis, such as adhesions and complicating injuries of the digital flexor tendons (see Figure 69-8, A), the intersesamoidean ligament, or annular ligaments, and to document the staging of the condition.13 DFTS effusion may also accompany injury to the intrathecal part of the SDFT associated with a classic bowed tendon (see Chapter 69). However, intrathecal tendon injuries occur more commonly as focal core lesions in the DDFT (see Chapter 70) or as longitudinal tears of the SDFT, DDFT, or manica flexoria. DDFT tears usually involve the lateral or medial borders of the tendon in the forelimb; hence oblique ultrasonographic views can sometimes identify defects on these borders, although they are easily missed. Oblique images are also required to image the abaxial margins of the SDFT. Manica flexoria tears are more frequently seen in hindlimb tenosynovitis and are also easily missed using transverse ultrasonographic images because the tears are usually incomplete and located at the site of attachment of the manica flexoria to the SDFT. Recently, we have found that a midline longitudinal scan immediately proximal to the PSBs provides the best image for identifying instability and/or thickening of the manica flexoria that accompanies tears (Figure 74-3). However, negative ultrasonographic findings do not preclude a tear in the manica flexoria.
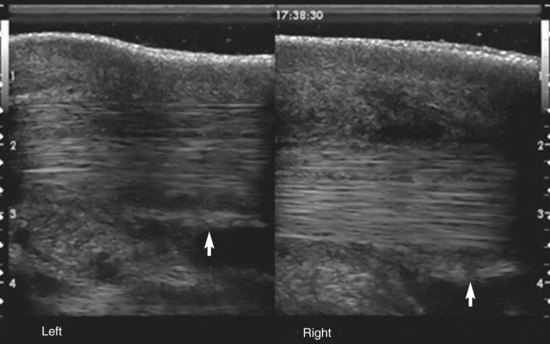
Fig. 74-3 Longitudinal ultrasonographic images from the midline immediately proximal to the metatarsophalangeal joint (distal to the left). The left limb has a normal manica flexoria (arrow). In the right hindlimb, the manica flexoria is displaced from the dorsal surface of the deep digital flexor tendon and is thickened (arrow).
Ultrasonographically, tenosynovitis has three progressive stages.14 Symmetrical distention of the DFTS without evidence of synovial proliferation represents stage 1, or the effusive stage of synovitis. More pronounced, often asymmetrical distention of the proximal pouch, which feels firm on palpation and is accompanied by synovial proliferation, is stage 2. In stage 3 synovitis, extensive synovial proliferation occurs with or without adhesion formation in the sheath. Adhesions are manifest as echogenic material between the tendon and wall of the DFTS and can be more easily seen when fluid from marked distention provides negative contrast. Care must be taken to avoid undue pressure on the ultrasound transducer, which will obliterate this fluid from the field of view. However, the presence of adhesions can be easily overestimated with ultrasonography.
Treatment
An important aspect of the decision making is the differentiation of primary versus secondary tenosynovitis. Treatment of horses with acute primary tenosynovitis consists of rest with bandage immobilization, cold therapy, and systemic antiinflammatory medication. The latter can be commenced with a single systemic dose of a short-acting corticosteroid (dexamethasone phosphate [0.06 mg/kg intravenously] or betamethasone phosphate [0.04 mg/kg intravenously]), followed by a 5-day systemic course of flunixin meglumine (1.1 mg/kg daily) or phenylbutazone (4.4 mg/kg daily). After the initial 7 to 14 days, in-handwalking can be resumed for 2 weeks, before returning the horse to riding. If the clinical signs have not resolved after 7 to 14 days, intrathecal injection of hyaluronan and a corticosteroid (40 to 80 mg of methylprednisolone acetate or 10 mg of triamcinolone acetonide) can be considered provided that no primary cause, such as tendon injury, of the tenosynovitis is suspected. A potential adverse effect of this treatment is that the intrathecal injection of a corticosteroid will interfere in the healing of an injured tendon and may enable a horse with a complicating digital flexor tendon injury to return to soundness temporarily, thereby potentially allowing it to exacerbate the underlying cause for continued irritation of the DFTS. Therefore the clinician should attempt to ensure, with the aid of ultrasonographic evaluation, that no injuries to the digital flexor tendons in the DFTS exist before intrathecal corticosteroids are administered. However, one should remember that many marginal tears of the SDFT or the DDFT within the DFTS may not be identifiable with ultrasonography, especially if they are located at the level of the ultrasonographic blind spot beneath the ergot. Evaluating specific images (see above), measuring the cross-sectional area of each digital flexor tendon (see Chapter 16), and comparing the same measurements in the contralateral limb may help veterinarians recognize subtle digital flexor tendon injuries, without obvious changes in echogenicity. One of the Editors (SJD) treats suspected primary DFTS tenosynovitis by intrathecal administration of corticosteroids immediately after recognition of lameness; persistence of lameness or recurrence of lameness within 2 to 3 weeks is a good indicator that there may be a more complicated injury.
However, in horses that are unresponsive to this treatment or in horses with suspected primary causes (such as those characterized by thickening and fibrosis of the PAL, adhesions, synovial masses, or tears to the digital flexor tendons), tenoscopic exploration of the DFTS is warranted. Tenoscopy provides both diagnostic and therapeutic capabilities. In forelimb injuries, the majority of the tendon lesions involve marginal tears of the lateral, most commonly, or medial margins of the DDFT proximal to the base of the PSBs (Figure 74-4).14,15 These tears are most appropriately managed by debridement of the prolapsed tendonous tissue. Open suture repair of the tears is invasive, does not offer any improvement over tenoscopic debridement, and is not recommended. In contrast, in hindlimbs, the more common abnormality (with the same clinical signs) is tearing of the lateral or medial attachments of the manica flexoria to the SDFT (Figure 74-5).15 These horses are managed by removing the manica flexoria in its entirety (see Figure 74-5). This is achieved using medial and lateral endoscopic portals and medial and lateral instrument portals to introduce a hook knife that is used to transect all remaining attachments of the manica flexoria to the medial and lateral borders of the SDFT. The manica is then pulled through one of the instrument portals (where enlargement of the skin portal is usually necessary) before the proximal synovial attachment is transected to complete removal.
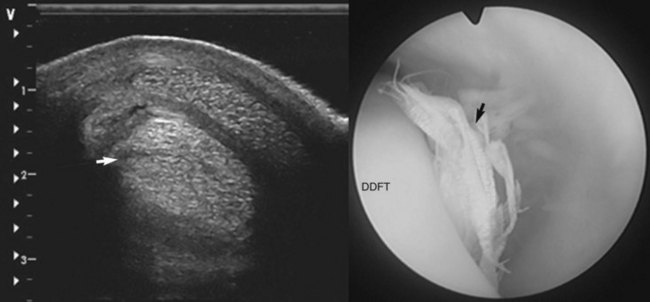
Fig. 74-4 Oblique transverse ultrasonographic image (left, lateral to the left) showing a lateral border tear of the deep digital flexor tendon (DDFT) and (right) the tenoscopic appearance with the torn fibers prolapsing from the border of the tendon (arrow).
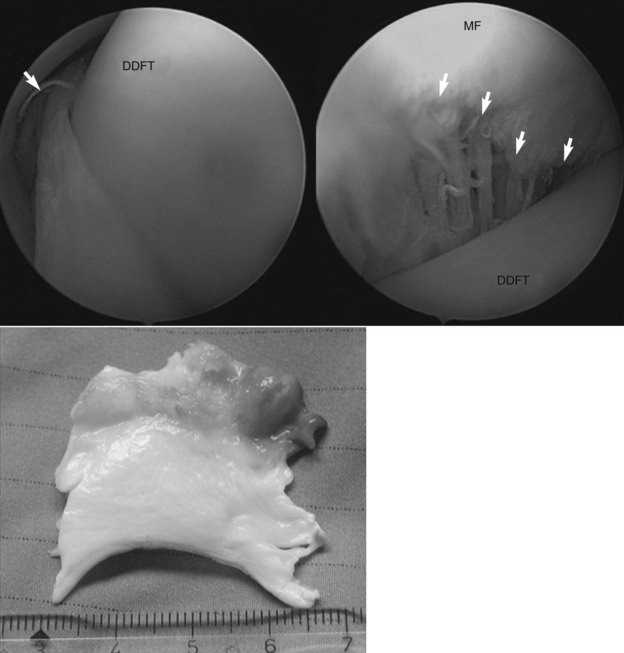
Fig. 74-5 Tenoscopic view of a manica flexoria tear (top images; arrows). The top left image shows a partial tear viewed from the abaxial part of the proximal aspect of the digital flexor tendon sheath. The top right image shows an almost complete tear taken with the endoscope positioned between the manica flexoria and the deep digital flexor tendon (DDFT). The bottom image shows the manica flexoria after resection. MF, Manica flexoria.
Prognosis
The prognosis for horses with acute, uncomplicated noninfectious tenosynovitis is favorable if treatment starts immediately. The prognosis for those with complex tenosynovitis with synovial masses and adhesions is more guarded but lameness can resolve after tenoscopic debridement and PAL desmotomy. In one report, 18 of 25 horses returned to athletic soundness.12 Of those horses with a primary tendon injury, the prognosis depends more on this primary pathology. Only 40% of horses with longitudinal tears of the DDFT within the DFTS returned to the same level of exercise after debridement, with the prognosis being even worse (18%) if the tears were long.15 This is similar to those horses with DDF tendonitis within the DFTS not associated with marginal defects, where seven of 24 horses (29%)16 made a full recovery (see Chapter 70). However, one of the Editors (SJD) has recently had much better success with management of horses with core lesions by intralesional injection of cultured mesenchymal stem cells. Seventy-six percent of horses with excision of manica flexoria tears returned to the previous level of work.15 Negative prognostic indicators in addition to the site of the tear were chronicity and marked preoperative distention of the DFTS.15 However, conservative management of horses with acute small marginal tears of the SDFT is often successful.
Infectious Tenosynovitis
Infectious tenosynovitis is a critical condition in the horse because of the severity of lameness, the difficulty in eliminating infection from the DFTS, and the high risk of long-term sequelae such as adhesions and fibrosis, which contribute to permanent lameness even if infection is eliminated.
Etiopathogenesis
The most common cause of DFTS infection is a penetrating wound. Occasionally, small puncture wounds that readily seal over may not be recognized as the cause of acute, severe lameness associated with DFTS infection. Penetrating injuries to the DDFT in the pastern region are frequently undiagnosed at the outset.15 Most penetrations in this region lead to infectious tenosynovitis and rarely infectious tendonitis of the DDFT. However, occasionally a traumatic penetration may enter the DDFT through its distal mesotendon and form a localized abscess that remains separate from the intrathecal space, thereby preventing infectious tenosynovitis. Infection may also occur after intrathecal injection or surgery of the PAL. Rarely, hematogenous spread of infection to the digital sheath may result from bacteremia.
Diagnosis
Heat and effusion of the DFTS and severe lameness are classic signs of DFTS infection. Typically horses are reluctant to put the heel of the affected limb to the ground. Exception to this may be seen in horses with an open wound to the DFTS that allows free drainage of infected synovial fluid. The reduction in intrathecal pressure and bacterial numbers provided by continuous flow of synovial fluid may reduce inflammation and lameness considerably. With a closed wound, however, the affected sheath is grossly distended. Generalized and painful lower limb edema caused by cellulitis associated with the entry wound may hinder specific palpation that would aid in identifying DFTS distention. Horses with penetrating injuries to the DDFT in the pastern region are characterized by moderate to severe lameness, localized swelling in the pastern, and a pronounced pain response to focal pressure on the palmar surface of the DDFT in the pastern region.
Radiography and ultrasonography are useful to recognize complicating factors such as osteitis or osteomyelitis, concurrent tendon injury, foreign bodies, and infectious tendonitis. Contrast radiography may help confirm a penetrating tract. In the absence of a penetrating tract, confirmation of the diagnosis relies on synovial fluid analysis. This should be performed as early as possible when DFTS infection is suspected. A total nucleated cell count greater than or equal to 30,000/µL, with more than 90% neutrophils, and a total protein concentration greater than or equal to 4.0 g/dL are considered pathognomonic for infection. Attempts should be made to identify bacteria by Gram stain and culture. Using broth culture bottles enhances the likelihood of obtaining a positive culture. Bacteria cultured from wounds or draining tracts are not representative of intrathecal bacterial populations. Positive cultures have been reported from 67% to 81% of horses with infectious tenosynovitis.18 Of the positive cultures in one study, 46% were mixed cultures. Streptococcus and Klebsiella species were the most common isolates from adult horses with infectious tenosynovitis.19 In another study, iatrogenic synovial infection was more likely to result in a pure culture of Staphylococcus aureus, whereas Enterobacteriaceae were most frequently cultured from synovial sepsis caused by a penetrating wound.20
Treatment
As for joint infections (see Chapter 65), the principles of antimicrobial therapy, synovial debridement, and drainage apply to infections of the DFTS. Treatment of infectious tenosynovitis must consist of aggressive intrasynovial and systemic broad-spectrum antimicrobial therapy with lavage of the sheath. Regional intravenous perfusion of antibiotics,21 slow-release antibiotic-depot systems in collagen or polymethylmethacrylate,22,23 and antibiotic infusion pumps (MILA Joint Infusion System; MILA International, Inc., Florence, Kentucky, United States)24 all have been used to maximize the intrathecal concentration of antibiotics over a protracted period.
The ideal surgical treatment for DFTS infection consists of tenoscopic debridement of fibrin, foreign material, adhesions, and synovial masses along with simultaneous lavage.25 Needle lavage is usually relatively less effective at achieving resolution of the infection because of the blind-ending pouches in the DFTS. Transection of the PAL can relieve pain that results from excessive fluid accumulation and fibrosis and thickening of the sheath wall in horses with chronic infectious tenosynovitis. This is best performed through a tenoscopic approach to minimize the risk of wound dehiscence that accompanies a transcutaneous approach. After tenoscopy, portals usually are closed but can be left open for continued drainage if desired with chronic infection. Indwelling fenestrated drains may be placed at surgery for continued through-and-through lavage. Fenestrated polyvinylchloride or silicone drains are preferable to Penrose drains because they allow for lavage and drainage. The drain is covered with a sterile dressing under a bulky pressure bandage, and lavage is continued twice daily for 3 to 5 days. Because adhesion formation and fibrosis are the most common causes for surviving horses’ failure to return to the intended use, specific recommendations have been made to reduce the incidence of these crippling complications. After removal of the drain and healing of the skin portals, hyaluronan is injected into the sheath at 14-day intervals to reduce adhesion formation. These injections are best started between 7 and 14 days after injury during the time of fibrosis and adhesion formation.
Provided adequate debridement and lavage have been achieved tenoscopically, primary closure of an open DFTS shortly after injury is usually indicated. However, closure may be contraindicated in some horses with extensive contamination and/or inadequate debridement because closure may trap bacteria in the sheath. In such horses, cleaning the wound thoroughly and maintaining it under sterile wraps to allow for healing by second intention to occur may be preferable, or consideration may be given to delayed closure when the wound is sufficiently clean and lameness has resolved.18
Early passive motion and handwalking exercise were advocated to prevent restriction of range of motion.18,19 Elevation of the heel often causes improved weight bearing and ambulation during this period. Cast immobilization of the distal limb is contraindicated during treatment for DFTS infection if the digital flexor tendons are intact. Although immobilization limits recurrent inflammation associated with continuous movement of the damaged tissues, it limits drainage and promotes the restrictive nature of any scar tissue and adhesions. However, immobilization effects more rapid soft tissue healing and therefore is indicated to decrease wound motion once infection has been resolved. A commercial or homemade splint is adequate for immobilization.
Prognosis
Horses with wounds of the DFTS have a good prognosis for return to soundness if the wound is diagnosed and treated before infectious tenosynovitis develops. If infection occurs, the prognosis is guarded. Although the prognosis for elimination of infection and survival was described as good in most studies (as high as 100% in one study20), another study reported a 45% failure of return to soundness and intended use for horses with infectious DFTS tenosynovitis. Even if infection can be resolved successfully, fibrosis, adhesions, and occasionally tendon rupture or osteomyelitis of the PSB may cause permanent lameness. Most authors believe that the chance of resolution is related directly to the duration of infection.18
Palmar Annular Ligament Syndrome
Etiopathogenesis
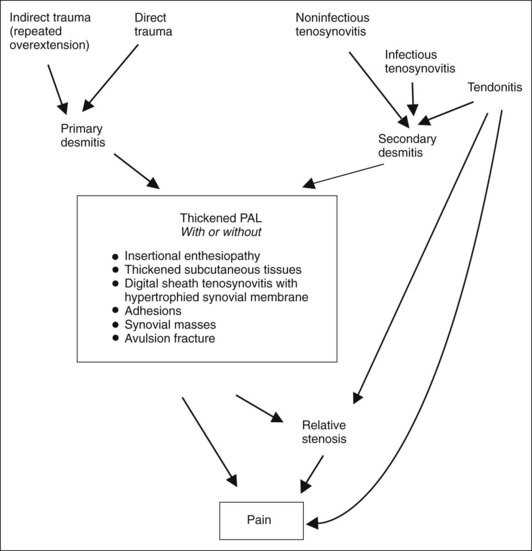
The PAL counteracts a tendency of the PSBs to move in a dorsal and abaxial direction during weight bearing. The medial and lateral branches and the extensor branches of the SL effect strong traction in a dorsal direction at the respective insertion sites on the abaxial surface of the PSBs during extension of the fetlock joint. This traction is balanced by the intersesamoidean ligament and the PAL.1 The role of disease of the PAL as a cause of lameness is a subject of debate. Thickening of the PAL has been associated with lameness in a number of different diseases of the DFTS and associated structures. All these diseases have characteristic clinical manifestation of distention of the DFTS and thickening of the PAL in common and therefore as a group have been referred to as palmar annular ligament syndrome. Other terms that have been used to describe this clinical presentation include stenosis of the fetlock canal, palmar annular ligament constriction, fetlock canal dysfunction, and stenosing palmar ligament desmitis. The role of desmopathy in this syndrome is supported by histopathological evidence of chronic inflammation and repair tissue in PAL biopsies from horses with clinical disease.24-28 Undoubtedly, however, the cause of PAL desmitis is multifactorial. Direct external trauma to the PAL may be caused by laceration or by direct impact (e.g., overreach). Overextension of the fetlock at high speed is likely to be associated with high tensile forces in the PAL and may lead to injury and failure of the PAL under tension. Excessive tendon swelling within the DFTS, although uncommon, may result in sustained pressure on and inflammation of the PAL (see Chapter 69, Figure 69-2). Finally, chronic inflammation of the DFTS (noninfectious or infectious tenosynovitis) leads to fibrosis and thickening of the fibrous part of the DFTS capsule, which includes the PAL. In some horses the subcutaneous connective tissue in the area of the PAL becomes greatly thickened and fibrotic. Once an inflammatory response is initiated, the PAL becomes thickened, whatever the cause. Thickening of the PAL reduces the space within the fetlock canal and results in a relative stenosis. The continuous pressure from the tendons associated with this relative stenosis is a source of trauma and perpetuates this sequence of events, leading to further inflammation, fibrosis, and thickening of the PAL and persistent pain (Figure 74-6).
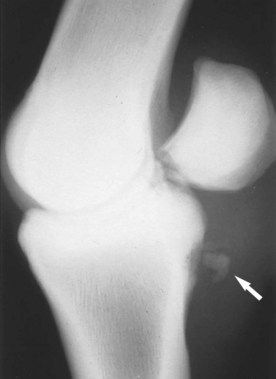
A positive relationship exists between incidence of PAL desmitis and increasing age of the horse, a predisposition in some breeds (Paso Fino and Warmbloods), and location (especially hindlimbs in the mentioned breeds).26 Rarely, small avulsion fractures may occur at the site of insertion of the PAL to the palmar border of a PSB after overextension injury or local trauma (see page 754 and Figure 72-17).29
Diagnosis
Occasionally, acute lameness may be caused by PAL injury without tenosynovitis. Clinical signs include localized heat, swelling, and pain on palpation of the palmar aspect of the fetlock. The clinical signs of the more common chronic PAL syndrome are characteristic. Horses have long-term, persistent, mild to moderate lameness that improves little with rest and worsens with return to exercise. A fetlock flexion test exacerbates lameness. DFTS distention with a notch in the palmar outline of the fetlock is virtually pathognomonic for PAL syndrome (Figure 74-7). This notch is caused by the inability of the DFTS to distend at the site of intimate attachment between the SDFT and the dorsal surface of the thickened, inelastic PAL. Sometimes enlargement of the PAL or the overlying subcutaneous tissues results in a localized bulge rather than a notch over the palmar aspect of the fetlock region. Extension of the affected fetlock often is decreased during weight bearing at walk or trot, and occasionally affected horses are reluctant to put the heel of the affected foot to the ground. This splinted fetlock posture is assumed by the affected horse to decrease pressure of the digital flexor tendons on the inflamed PAL.
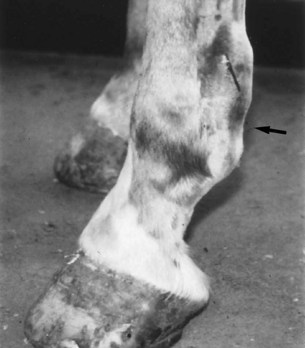
Fig. 74-7 Digital flexor tendon sheath distention with characteristic notch in the plantar outline of the fetlock region (arrow) in a horse with plantar annular ligament syndrome.
Lameness often is improved, but not always completely eliminated, by intrathecal analgesia. The response to a low four- or six-point nerve block is often better. Sometimes a palmar nerve block performed at the base of the PSBs improves lameness. Fibrosis of the PAL and DFTS may result in mechanical gait restriction, which cannot be abolished totally by regional analgesia.
Various imaging techniques can be used to support the clinical diagnosis of PAL desmitis. In horses with chronic lameness, entheseous new bone may be present at the PAL insertions on the palmar border of one or both PSBs. Air tenography or ultrasonography must be used to demonstrate thickening of the PAL or the overlying subcutis. The ultrasonographic appearance of a diseased PAL differs between affected horses and may be helpful in differentiating the etiopathogenic mechanisms behind each injury. An unaffected PAL can be difficult to identify because it is only 1 to 2 mm thick, and imaging the ligament in the sagittal midline is complicated by the vinculum between the PAL and the SDFT, which makes resolution of the ligament difficult. The PAL is identified most easily at its attachment to a PSB by tilting the transversely positioned transducer laterally or medially from the palmar midline. The ligament then can be followed toward the midline. Several measurements of the thickness of the PAL should be made on transverse and longitudinal images to obtain reliable data. Because of the difficulties in consistently identifying the PAL, some clinicians have proposed measuring the distance between the palmar surface of the SDFT and the skin surface.30 Any increase in thickness more than 5 mm is considered to be abnormal. However, three different structures contribute to this measurement, each of which can be thickened to varying degrees in different pathology and in different breeds: the subcutaneous tissues, the PAL, and the parietal and visceral layers of the DFTS.
Primary Palmar Annular Ligament Thickening
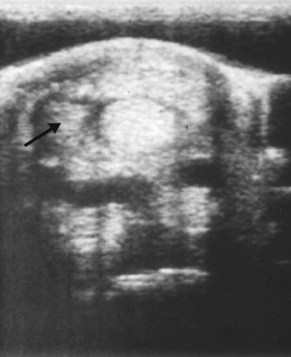
Primary PAL thickening is characterized by ultrasonographic evidence of thickening of the PAL, without evidence of pathological conditions of the structures within the DFTS (Figure 74-8). Although tenosynovitis is present, the synovial lining of the sheath is not substantially thickened. With acute desmitis, the ligament is enlarged and contains focal hypoechoic areas, or it is diffusely hypoechogenic. PAL thickening may be accompanied by extensive subcutaneous fibrosis. This condition occurs more commonly in hindlimbs than forelimbs, and ponies and cob-type horses seem particularly prone. Thickening may be present bilaterally in a unilaterally lame horse but is always greater in the lame limb. Some horses have developed sequential lameness in more than one limb.
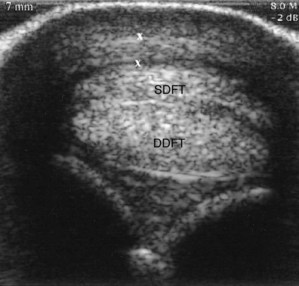
Fig. 74-8 Transverse ultrasonographic image of the distal metacarpal region at the level of the proximal sesamoid bones. There is primary enlargement of the palmar annular ligament. The palmar annular ligament (X to X) measured 7 mm in thickness. SDFT, Superficial digital flexor tendon; DDFT, deep digital flexor tendon.
Primary Tenosynovitis with Secondary Palmar Annular Ligament Thickening
Ultrasonographic differentiation between secondary PAL thickening and primary desmitis is sometimes difficult, but the synovial lining of the DFTS usually is considerably thickened, including the lining at the level of the PAL (Figure 74-9). Horses with chronic, complex tenosynovitis may have adhesions and synovial masses.
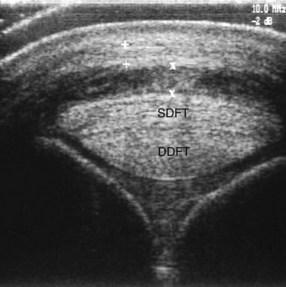
Fig. 74-9 Transverse ultrasonographic image of the distal metacarpal region at the level of the proximal sesamoid bones. There is thickening of the palmar annular ligament (+ to +) and the synovial membrane of the digital flexor tendon sheath (X to X). SDFT, Superficial digital flexor tendon; DDFT, deep digital flexor tendon.
Subcutaneous Fibrosis with a Normal or Minimally Enlarged Palmar Annular Ligament
Subcutaneous fibrosis in the region of the PAL can produce clinical signs similar to those of PAL thickening and often can be distinguished only by careful ultrasonographic imaging (Figure 74-10). These injuries frequently are caused by focal trauma to the PAL area and can involve portions of the PAL and the surface of the underlying SDFT.
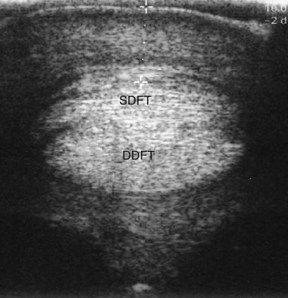
Fig. 74-10 Transverse ultrasonographic image of the distal metacarpal region. There is subcutaneous fibrosis, with minimal thickening of the palmar annular ligament (PAL), associated with clinical signs of PAL syndrome. Although the distance from the skin to the plantar surface of the superficial digital flexor tendon amounts to 9.19 mm (+ to +), the PAL is only 1.59 mm thick. SDFT, Superficial digital flexor tendon; DDFT, deep digital flexor tendon.
Tendon Injuries with Secondary Palmar Annular Ligament Thickening
Injuries of the SDFT, manica flexoria, or DDFT within the fetlock canal may result in inflammatory tenosynovitis, which also can lead to PAL thickening. Although tendonitis usually can be diagnosed by ultrasonography, many focal longitudinal tears of the SDFT and DDFT remain undetected, especially if they are located at the level of the ultrasonographic blind spot beneath the ergot.
Treatment and Prognosis
Treatment of horses with acute PAL desmitis with rest and topical antiinflammatory medication often results in rapid resolution of lameness and swelling. In many horses, however, PAL desmitis becomes a chronic condition, treatment of which requires surgical release (desmotomy) of the inflamed and thickened PAL. Desmotomy resolves pain from traction on the ligament during weight bearing and eliminates pressure from structures within the stenotic fetlock canal on the PAL. This can be performed via a traditional surgical approach, using an open or a minimally invasive technique. A minimally invasive approach through a small skin incision in the proximal recess of the DFTS is simple and quick, but subcutaneous transection of the PAL is performed blind (see Chapter 69). However, confirmation of a relative stenosis within the fetlock canal in these horses is difficult preoperatively unless movement of the tendons through the canal is visibly impeded. The best evaluation is achieved tenoscopically, albeit in a non–weight-bearing limb, by assessing the ease with which a 5-mm endoscopic cannula can be passed from distal to proximal through the fetlock canal. This has an additional benefit of enabling the surgeon to simultaneously evaluate the entire DFTS and treat any concurrent pathological conditions, such as longitudinal tendon tears, adhesions, and synovial masses, which may be the primary cause of the PAL thickening. Desmotomy of the PAL can then be achieved tenoscopically either with a slotted canula used for carpal tunnel release in people (Smith & Nephew Dyonics Inc., Andover, Massachusetts, United States) or by direct tenoscopic evaluation and use of a freehand, hook knife or Metzenbaum scissors.11 A minimally invasive approach (incisional or tenoscopic) decreases the risk of wound dehiscence and fistulation and allows for earlier, safe return to exercise. If a traditional open approach is used, particular attention should be paid to careful closure of the subcutaneous tissues in two layers, as well as of the skin, to minimize the risks of synovial fistulation and wound dehiscence. Contrary to previous reports, our opinion is that horses that have undergone an open surgical approach to the DFTS should not be returned to exercise before healing of the incision is complete. Instead the horse should be confined to a stable and the limb enclosed in a firm, padded bandage until the time of suture removal at 2 weeks. After this, a gradually increasing exercise regimen can be initiated (2 weeks walking in hand, 4 weeks lunging) before return to ridden work. Some authors report the need for 4 to 6 months of rest and controlled exercise before soundness can be expected.29
Specific recommendations for treating PAL syndrome can be made according to the specific ultrasonographic characteristics. Unresponsive primary desmitis requires PAL desmotomy. In horses with primary tenosynovitis with secondary PAL desmitis, therapeutic planning should be aimed at discovering and treating the primary cause of the synovitis (e.g., infection, traumatic tendon injuries, and hypertrophic synovitis), with or without the need for PAL desmotomy. Tenoscopy is indicated in these horses.
If the stenotic effect is caused by extensive subcutaneous fibrosis over the PAL, conservative management with rest and antiinflammatory medication is occasionally successful in resolving lameness. However, if this approach fails to bring resolution, surgical release of the PAL and subcutaneous ring of fibrosis may resolve lameness. Occasionally, complete resection of the PAL is performed in horses in which there is persistent focal and intense pain over the PAL. The use of a C-shaped skin incision to create a medially or laterally based flap and preservation of the subcutaneous tissues, including the fat of the ergot, followed by careful closure and short-term (2 to 4 weeks) cast immobilization can give remarkably good cosmetic and functional end results (Figure 74-11). However, resection should only be considered for horses with primary PAL pathology unresponsive to conservative management. Lameness associated with small avulsion fractures of the palmar border of the PSB may be relieved by PAL desmotomy near its insertion site. In one study, however, three horses regained soundness without surgery after 3 to 6 months of controlled exercise.27 Finally, the resolution of tendonitis of the SDFT or DDFT within the DFTS may be assisted by PAL desmotomy, although the benefit of surgical treatment has proved less obvious in these horses.13,30 Despite these reports, PAL desmotomy has proved useful in managing SDF tendonitis in Standardbreds (see Chapter 69).31 The prognosis after surgical desmotomy is generally favorable for soundness in horses with desmitis of the PAL without tendonitis and has ranged from 64% to 87%.12,29 Factors with a negative influence on outcome are the presence of tendonitis, infection, or adhesions. Although in one study adhesions reduced the return to soundness to 44% after PAL desmotomy,30 a different study showed that 72% of horses with complex tenosynovitis made a full recovery after tenoscopic debridement and PAL desmotomy.12 In a study of 71 horses with chronic PAL desmopathy with or without subcutaneous fibrosis, 22 of 44 (50%) returned to full athletic function, whereas only eight of 27 (33%) horses with concurrent tendon pathology returned to full function.27
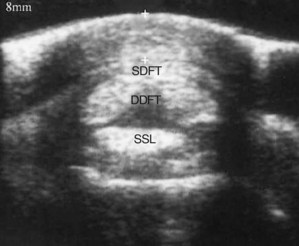
Fig. 74-11 Transverse ultrasonographic image of the proximal pastern region of a hindlimb. The distance from the skin surface to the plantar surface of the superficial digital flexor tendon (SDFT) (+ to +) is enlarged to 8 mm in this horse with chronic desmitis of the proximal digital annular ligament. DDFT, deep digital flexor tendon; SSL, straight sesamoidean ligament.
Diseases of the Intersesamoidean (Palmar) Ligament of the Fetlock
Etiopathogenesis
When the fetlock overextends, the proximal scutum slides distally to the metacarpal condyle and its palmar surface undergoes pressure from the flexor tendons. Moreover, in this position the distal medial and lateral extensor branches of the SL induce high tension on the abaxial surface of the PSBs, thus creating high tension on the intersesamoidean ligament.32 The effect of these forces could provide a biomechanical explanation for injuries to the intersesamoidean ligament. Post mortem examination of 305 pairs of PSBs revealed radiological changes in 25.8% and gross abnormalities of the intersesamoidean ligament in 25.9%,33 although premortem recognition of intersesamoidean ligament pathological conditions causing lameness has been considerably less common.30 Diseases of the intersesamoidean ligament have been classified by the ultrasonographic and radiological appearance as rupture of the intersesamoidean ligament, avulsion fracture of the intersesamoidean ligament from the PSBs, desmitis of the intersesamoidean ligament (noninfectious and infectious), and enthesopathy of the intersesamoidean ligament.32,35-37 Infectious desmitis and axial osteomyelitis of the PSBs usually are associated with infectious arthritis of the fetlock joint or infectious tenosynovitis of the DFTS. Which condition precedes the other is not always obvious. If infectious desmitis or osteomyelitis is the primary condition, a hematogenous route for the infection is likely.
Diagnosis
Injuries of the intersesamoidean ligament usually cause acute-onset, moderate to severe lameness. Horses with enthesopathy with thinning of the intersesamoidean ligament and erosion of the flexor surface of the corresponding PSB may have a more insidious course. Avulsion fractures of the axial border of the PSBs often are accompanied by fetlock effusion, usually involve the lateral PSB, and usually occur in conjunction with a displaced lateral condylar fracture of the third metacarpal bone31 Lameness accompanying these conditions is improved, but not always abolished, by intrasynovial analgesia of the fetlock joint or the DFTS and is eliminated by a low four-point block. Scintigraphy may be required to find the exact site of the pathological condition. In horses with suspected infectious desmitis or osteomyelitis, cytological examination of synovial fluid of the fetlock joint or DFTS often, but not always, indicates synovial infection.
Diagnosis of injury to the intersesamoidean ligament and associated structures mainly is based on abnormal imaging features. Evidence of osseous fragmentation or radiolucency along the axial border of the PSB, predominantly centered at the proximal half of the intersesamoidean space, indicates desmitis, enthesopathy with osteitis, avulsion fracture, or osteomyelitis. Evidence of osteolysis also may be observed along the palmar surface of the PSBs in association with enthesopathy of the intersesamoidean ligament. Ultrasonographic abnormalities include enlargement of the distance between both PSBs with intersesamoidean ligament rupture (>6 mm), enlargement of the intersesamoidean ligament, and alteration of the echogenicity within the intersesamoidean ligament in horses with desmitis, and asymmetrical reduction in the thickness of the intersesamoidean ligament and irregular bony outline to the palmar surface of a PSB in association with enthesopathy.
Treatment and Prognosis
No effective treatment exists for rupture of the intersesamoidean ligament with abaxial displacement of the PSB or for enthesopathy with thinning and degeneration of the intersesamoidean ligament within the DFTS, and the prognosis for soundness in either situation is grave. We have observed complete recovery in a general purpose riding and jumping horse with intersesamoidean desmitis and a small avulsion fracture of the axial border after a prolonged confinement (6 months). Some reports also have documented favorable outcome for horses with noninfectious desmitis with or without avulsion fracture after arthroscopic removal of fracture fragments and debridement of the osteochondral defect and the associated discolored area of the intersesamoidean ligament.35,36 Histological evaluation of the damaged intersesamoidean ligament in these horses revealed chronic inflammation and granulation tissue, with thrombosis of the microvasculature. All horses with noninfectious desmitis treated surgically in one study returned to previous level of performance, although the horses’ intended uses were not mentioned.35 Recovery time was 7 to 12 months. In the same study, similar treatment was generally unsuccessful for horses with infectious desmitis and osteomyelitis.35
Diseases of the Digital Annular Ligaments
Desmitis and avulsion fractures of the proximal insertion sites of the proximal digital annular ligament may occur.
Etiopathogenesis
Hyperextension of the fetlock causes differential movement between the SDFT and the proximal phalanx. The proximal digital annular ligament is adhered intimately to the palmar surface of the SDFT in this region, and the proximal digital annular ligament inserts to the proximal phalanx at two levels; thus this differential movement may result in injury of the proximal digital annular ligament or its insertions. Additionally, external trauma to the palmar aspect of the pastern may result in desmitis of the proximal digital annular ligament. Desmitis of the proximal digital annular ligament also has been described in association with injury of the SDFT or infectious tenosynovitis with thickening of the DFTS in horses with infectious desmitis and osteomyelitis.37 It has been suggested that thickening of the proximal digital annular ligament or fibrosis of the subcutaneous tissues on the palmar aspect of the pastern may result in functional constriction of the DFTS and the digital flexor tendons, causing lameness, as is the case with the PAL syndrome.36
Diagnosis
Characteristic clinical findings in horses with desmitis of the proximal digital annular ligament are soft tissue thickening in the palmar pastern region, prominent palmar protrusion of the DFTS distal to the proximal digital annular ligament, and improvement in lameness score by a palmar nerve block or intrathecal analgesia of the DFTS. Ultrasonography provides a definitive diagnosis. The normal proximal digital annular ligament is not visible on ultrasonographic examination, but the palmarodorsal thickness of the combined skin–proximal digital annular ligament layer should not exceed 2 mm. In horses with proximal digital annular desmitis, this distance increased to 4 to 5 mm (see Figure 74-11).36 It is important to rule out pathological conditions of the digital flexor tendons or distal sesamoidean ligaments as causes of soft tissue swelling in the palmar pastern region (see Chapter 82).
Avulsion fractures of the proximal insertion of the proximal digital annular ligament were seen as a cause of lameness in three horses.37 There were few localizing signs, and diagnosis relied on response to nerve blocks and radiography. Radiographs showed an avulsion fracture fragment at the insertion site of the proximal digital annular ligament on the palmarolateral or palmaromedial border of the proximal phalanx (Figure 74-12).
Treatment
Desmitis of the proximal digital annular ligament with constriction of the DFTS was treated successfully in two horses by desmotomy of the proximal digital annular ligament.36 Two horses with avulsion fractures of the proximal digital annular ligament were treated with 2 months of rest alone, and one horse was treated with 6 weeks of immobilization, followed by 6 weeks of rest. All three horses returned to the previous level of performance.36
Injury of the distal digital annular ligament has recently been recognized as a potential contributor to lameness, either as a primary injury39 or as one of a complex of injuries.40 Diagnosis required magnetic resonance imaging. Seven horses with pain localized to the digit had diffuse or focal thickening of the ligament associated with increased signal intensity in T1- and T2-weighted images, with or without concurrent lesions of the DDFT within the adjacent DFTS. Surgical transection of the ligament successfully resolved lameness in those horses in which the injury was considered primary.39 However, it is difficult to conceive how transection was achieved because of the intimate anatomical relationship between the DDFT and the distal digital annular ligament.
Synovial Ganglion or Hernia of the Digital Flexor Tendon Sheath
A synovial hernia or ganglion is a thin-walled cyst originating from a tendon sheath or joint capsule.41,42 Hernias of the DFTS result from a traumatic defect in the fibrous layer of the DFTS wall, allowing the synovial lining to herniate through it. Ganglia are thought to result from trauma involving all layers of the sheath wall and are not lined by synovium. Subsequent irritation to the hernial sac causes secretion of synovium and filling of the sac. The opening in some hernial sacs or ganglions can act as a one-way valve that prevents movement of synovial fluid back to the DFTS. Injection of a radiodense contrast medium into the DFTS has resulted in flow of the contrast medium from the parent synovial cavity into the ganglion (Figure 74-13), but not vice versa. We have encountered such a ganglion at the level of the proximolateral recess of the DFTS in five horses, associated with lameness, which was abolished by intrathecal analgesia of the DFTS.43 Diagnostic imaging and surgical exploration revealed a cystic structure, connected to a small slitlike opening in the DFTS wall. Resection and closure of the DFTS wall defect resulted in resolution of lameness. However, such structures can often be seen incidentally unassociated with lameness.
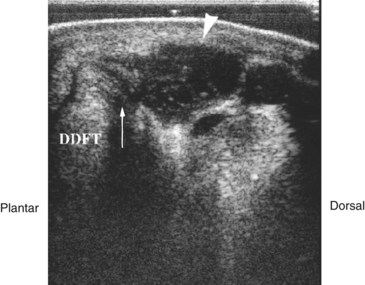
Fig. 74-13 Transverse ultrasonographic image over the lateral aspect of the distal metatarsal region of a horse with a synovial ganglion of the digital flexor tendon sheath (DFTS). There is subcutaneous fluid collection (arrowhead) outside the boundary of, but in communication with, the DFTS (arrow). DDFT, Deep digital flexor tendon.
Tendonitis of the Superficial and Deep Digital Flexor Tendons within the Digital Flexor Tendon Sheath
Tendonitis of the SDFT and DDFT within the digital sheath is discussed in Chapters 69 and 70.