Spread of Infection
1 Define and pronounce the key terms and anatomic terms in this chapter.
2 Discuss the spread of odontogenic infection to the sinuses and by the vascular system, lymphatic system, and spaces in the head and neck region.
3 Trace the routes of odontogenic infection in the head and neck region on a diagram, skull, and patient.
4 Discuss the complications that can occur with the spread of odontogenic infection in the head and neck region.
5 Discuss the prevention of the spread of odontogenic infection during patient dental care.
6 Correctly complete the review questions and activities for this chapter.
7 Integrate an understanding of the anatomic considerations for the spread of odontogenic infection into clinical dental practice.
(ab-ses) Infection with suppuration resulting from the entrapment of pathogens in a contained space.
(em-bol-us, em-bol-eye) Foreign material or thrombus (or thrombi) traveling in the blood that can block the vessel.
(fis-chool-ah, fis-chool-ay) Passageway(s) in the skin, mucosa, or bone allowing drainage of an abscess at the surface.
(lood-vig an-ji-nah) Serious infection of the submandibular space, with a risk of spread to the neck and chest.
(lim-fad-in-op-ah-thee) Process in which there is an increase in the size and a change in the consistency of lymphoid tissue.
(pus-tule) Small, elevated, circumscribed suppuration-containing lesion of either the skin or the oral mucosa.
Infection Process Overview
The healthy body usually lives in balance with a number normal flora in residence. However, certain nonresident microorganisms called pathogens can invade and initiate an infection. Pathogens contain certain factors that help further the infection process such as capsules, spores, and toxins. A dental professional should understand the infection process that allows a microorganism to create disease.
Odontogenic Infection
Dental infection or odontogenic infection (also known as dentoalveolar infections by the medical community) involving the teeth or associated tissue is caused by oral pathogens that are mainly anaerobic and usually of more than one species. These polymicrobial organisms inhabit the surfaces of the teeth and oral mucous membranes and are also found in the gingival sulci and saliva. These infections can be of dental origin or can arise from a nonodontogenic or secondary source. Infections of dental origin usually originate from progressive dental caries or extensive periodontal disease, or even with implant placement (periimplantitis).
Thus most odontogenic infections result initially from the increased formation of dental biofilm (plaque) from the surrounding area. Pathogens can also be introduced deeper into the oral tissue by the trauma caused by dental procedures such as the contamination of dental surgery sites (e.g., tooth extraction) and needle tracks made during local anesthetic administration. Treatment consists of removal of the source of infection, systemic antibiotics, and area drainage.
Some odontogenic infections are secondary infections incited by an infection from the tissue surrounding the oral cavity such as the skin, tonsils, ears, or sinuses. These nonodontogenic sources of infections must be diagnosed and treated early if noted during dental care; prompt medical referral will prevent further spread and potential complications. A dental professional may want to refer to an oral or systemic pathology text for more information on this topic (see Appendix A).
Odontogenic Infection Lesions
Odontogenic infections can result in various types of pathologic lesions, depending on the location of the infection and thus the type of tissue involved. Pathologic lesions in the head and neck from odontogenic infections can include abscess, cellulitis, or osteomyelitis.
Abscess
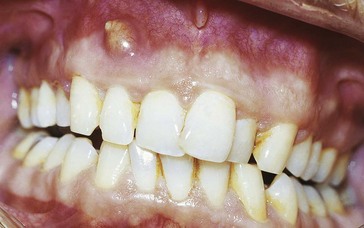
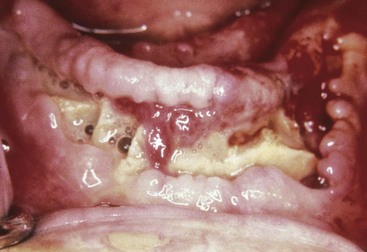
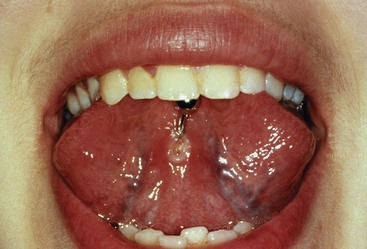
An abscess in the oral cavity occurs when there is localized entrapment of pathogens from a chronic odontogenic infection in a well circumscribed but closed tissue space such as that created by the oral mucosa (Figures 12-1 to 12-5). The abscess becomes filled with suppuration and feels fluctuant with palpation. Suppuration or pus contains pathogenic bacteria, white blood cells, tissue fluid, and debris. Periapical abscess formation can occur with progressive caries, when pathogens invade the usually sterile pulp and the infection spreads apically. Pathogens can also become entrapped in deepened gingival sulci (or periodontal pockets) in cases of severe periodontal disease and cause a periodontal abscess. With the right circumstances, an erupting mandibular third molar can cause a pericoronal abscess or pericoronitis.
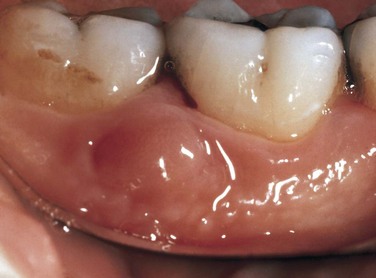
FIGURE 12-1 Intact periodontal abscess formation noted between the apices of the mandibular first and second molars.
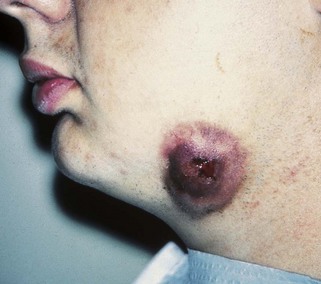
FIGURE 12-2 Extraoral abscess on the cheek skin surface resulting from the formation of a fistula and stoma caused by periapical involvement of the mandibular second molar. (Courtesy Dr. Mark Gabrielson.)
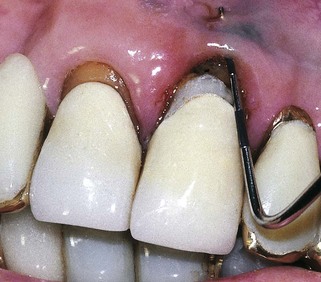
FIGURE 12-3 Periodontal abscess of the maxillary central incisor with fistula and stoma formation (probe inserted) in the maxillary vestibule.
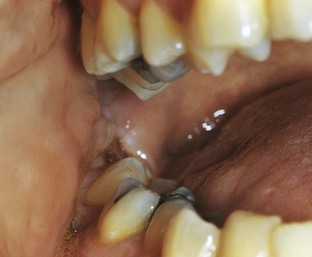
FIGURE 12-5 Pericoronal abscess of the mandibular third molar with abscess formation (pericoronitis).
An abscess can be either acute or chronic. Abscess formation may not be detectable on radiographs during the early acute stages. However, in the later stages of infection, chronic abscess formation can lead to the formation of a tract(s) or fistula (plural, fistulae) in the skin, oral mucosa, or bone. These passageway(s) allows drainage of the infection with suppuration noted on a surface (see Figures 12-2 and 12-3). The infection process causes the overlying tissue to undergo necrosis, which allows this tract to form in the tissue. The opening of the fistula from the tract is called a stoma. If the alveolar bone surrounds the odontogenic infection, it will break down the bone in its thinnest part (either the facial or lingual cortical plate), following the path of least resistance, and thus will then be noted as a radiolucency on radiographs.
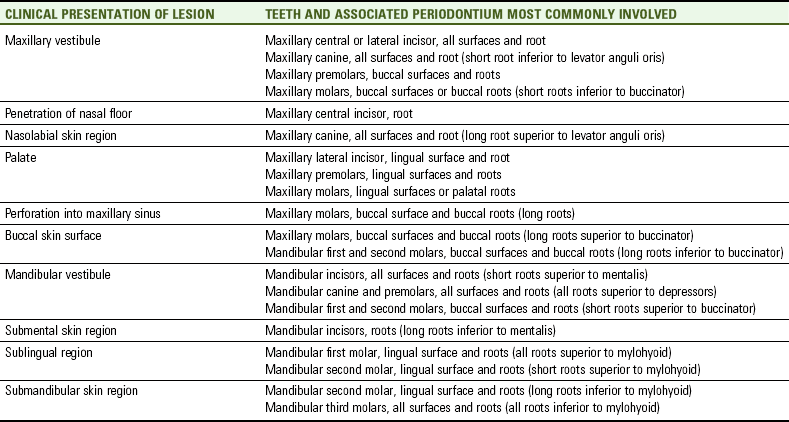
The soft tissue over a fistula in the alveolar bone may also have a associated extraoral or intraoral pustule. A pustule is a small, elevated, circumscribed lesion of either the skin or oral mucosa that contains suppuration (see Figure 12-4). The position of the pustule is determined largely by the relationship between the fistula and the overlying muscle attachments. Again, the infection will follow the path of least resistance (Table 12-1). Importantly, muscle attachments to the bones, unlike the other facial soft tissue, may serve as barriers to the spread of infection.
Cellulitis
Cellulitis of the face and neck can also occur with odontogenic infections, resulting in an acute level of diffuse inflammation of soft tissue spaces unlike a more localized abscess (Figure 12-6; see Chapter 11). The clinical signs and symptoms are pain, tenderness, redness, and diffuse edema of the involved soft tissue space, causing a massive and firm swelling that feels doughy to indurated with palpation (Table 12-2). Difficulty swallowing (dysphagia) or restricted eye opening (ptosis) may also happen if the cellulitis occurs within the pharynx or orbital regions, respectively. Usually the infection forms an additional facial abscess trying to contain the infection; if not initially treated, however, it may discharge on the facial surface.
TABLE 12-2
Clinical Presentations of Cellulitis*
| CLINICAL PRESENTATION OF LESION | SPACE INVOLVED | TEETH AND ASSOCIATED PERIODONTIUM MOST COMMONLY INVOLVED IN INFECTION |
| Infraorbital, zygomatic, and buccal regions | Buccal space | Maxillary premolars and maxillary and mandibular molars |
| Posterior border of mandible | Parotid space | Not generally of odontogenic origin |
| Submental region | Submental space | Mandibular anterior teeth |
| Unilateral submandibular region | Submandibular space | Mandibular posterior teeth |
| Bilateral submandibular region | Submental, sublingual, and submandibular spaces with Ludwig angina | Spread of mandibular infection |
| Lateral cervical region | Parapharyngeal space | Spread of mandibular infection |
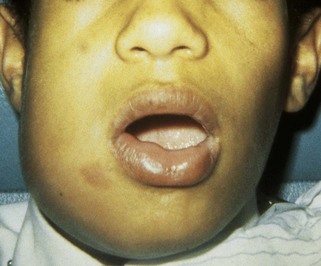
FIGURE 12-6 Cellulitis involving the buccal space resulting from an abscess of the mandibular first molar with swelling. (Courtesy Dr. Mark Gabrielson.)
Without treatment, cellulitis can spread due to perforation of the surrounding bone, becoming more generalized and causing serious complications such as Ludwig angina (discussed later). Cellulitis is treated by administration of antibiotics and removal of the cause of the infection.
Osteomyelitis
Another type of lesion that can be related to odontogenic infection is osteomyelitis, an inflammation of the bone marrow. Osteomyelitis can locally involve any bone in the body or can be generalized. This inflammation develops from the invasion of the tissue of a long bone by pathogens, usually from a skin or pharyngeal infection. In osteomyelitis involving the jawbones, the pathogens are most likely to derive from a periapical abscess, from an extension of cellulitis, or from contamination of a surgery site (Figure 12-7).
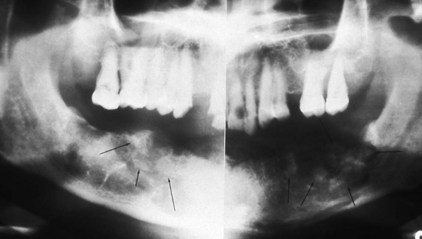
FIGURE 12-7 Panoramic radiograph of osteomyelitis, with bone resorption and formation of sequestra (arrows).
Osteomyelitis most commonly occurs in the mandible; it occurs only rarely in the maxillae because of the mandible’s thicker cortical plates and reduced vascularization. Continuation of osteomyelitis leads to bone resorption and formation of sequestra, which are pieces of dead bone separated from the sound bone within the area. These bone changes can be detected by radiographic evaluation (see Figure 12-7).
Paresthesia, evidenced by burning or prickling (“pins and needles”), may develop in the mandible if the infection involves the mandibular canal carrying the inferior alveolar nerve. Localized paresthesia of the lower lip may occur if the infection is distal to the mental foramen where the mental nerve exits. Treatment consists of drainage, removal of any sequestra by surgery, and antibiotic administration; in some patients the additional use of hyperbaric oxygen may be necessary. Today, osteomyelitis of the jawbone is uncommon because dental care and antibiotics are readily available.
Infection Resistance Factors
More than half of the gram-negative anaerobic bacteria are capable of producing the beta-lactamase enzyme, which is responsible for the initial tissue damage caused by head and neck infections, as well as many treatment failures in odontogenic infections. This enzyme may not only survive penicillin therapy but also may shield penicillin-susceptible co-pathogens from the activity of penicillin by releasing the free enzyme into their environment. Careful use of antimicrobials in the future may reduce and control the emergence of penicillin-resistant organisms.
Medically Compromised Patients
The normal flora found in the oral cavity usually do not create an infection. If, however, the body’s natural defenses are compromised, they can create opportunistic infections. Medically compromised individuals include those with HIV infection, diabetes, or cancer and those undergoing cancer or transplant therapy (Figure 12-8). Some patients also have a higher risk of complications resulting from odontogenic infections because of their medical histories. Patients in this category include those at risk for infective endocarditis or infection of their implanted prosthetic joints.
Spread of Odontogenic Infection
Many odontogenic infections that start in the teeth and associated oral tissue can have significant consequences if they spread to vital structures, tissue, or organs. Usually a localized abscess establishes a fistula in the skin, oral mucosa, or associated bone, allowing natural drainage of the infection and diminishing the risk of its spread. However, fistula formation and drainage do not always occur.
Occasionally, odontogenic infection can spread to the paranasal sinuses or can be spread by the vascular system, lymphatic system, or spaces in the head and neck. Reviewing the communication patterns in tissue, structures, or organs is important to the understanding of the possible routes of the spread of infection.
Spread to Paranasal Sinuses
The paranasal sinuses of the skull can become infected as a result of the direct spread of infection from the teeth and associated dental tissue, resulting in secondary sinusitis (see Chapter 3). A perforation, an abnormal hole in the wall of the sinus, can also be caused by an infection, which may then further the spread of infection.
Maxillary Sinusitis
Secondary sinusitis of dental origin occurs mainly in the maxillary sinuses because the maxillary posterior teeth and associated tissue are close to these sinuses (see Figure 3-46). Thus maxillary sinusitis can result from the spread of infection from a periapical abscess initiated by a maxillary posterior tooth that perforates the sinus floor to involve the sinus mucosa. In addition, a contaminated tooth or root fragment can be displaced into the maxillary sinus during an extraction, possibly creating an infection.
However, most infections of the maxillary sinuses are not of dental origin but are caused by an upper respiratory infection, an infection in the nasal region that spreads to the sinuses. An infection in one sinus can travel through the nasal cavity to other sinuses and lead to serious complications for the patient such as infection of the cranial cavity and brain. Thus it is important that any sinusitis be treated aggressively by medical referral to eliminate the initial infection.
The symptoms of sinusitis are headache, usually near the involved sinus, and foul-smelling nasal or pharyngeal discharge, possibly with fever and weakness. The skin over the involved sinus can be tender, hot, and red due to the inflammation in the area when palpated and examined (see Figure 3-58). Difficulty in breathing (dyspnea) occurs, as well as pain, when the nasal passages become blocked by tissue inflammation. Early radiographic evidence of sinusitis is the thickening of the sinus walls. Subsequent radiographic evaluation shows increased radiopacity (or cloudiness) and possibly perforation, usually using bilateral comparisons of the paired sinuses; magnetic resonance imaging (MRI) may also be indicated (see Figure 3-56).
Acute sinusitis usually responds to antibiotic therapy, while drainage is aided by the use of decongestants. Surgery may be necessary in cases of prolonged chronic maxillary sinusitis to enlarge the ostia of the lateral walls in the nasal cavity so that adequate drainage can diminish the effects of the infection. The approach to the maxillary sinus during surgery is through the thin bone of the canine fossa. Recent studies show that removal of the toxic mucus with its inflammatory products is also of prime importance in chronic sinusitis (see Chapter 3).
Spread by Vascular System
The vascular system of the head and neck can allow the spread of infection from the teeth and associated oral tissue because pathogens can travel in the veins and drain the infected oral site into other tissue, structures, or organs (see Chapter 6). The spread of odontogenic infection by way of the vascular system can occur because of bacteremia or an infected thrombus.
Bacteremia
Bacteria traveling in the vascular system can cause bacteremia, which can occur during dental treatment. In an individual with a high risk for infective endocarditis, these bacteria may lodge in the compromised tissue and set up serious infection deep in the heart, which can result in massive and fatal heart damage. These patients may need antibiotic premedication to prevent bacteremia from occurring during invasive dental treatment. Bacteremia may also be implicated in patients at risk for deep tissue infection surrounding a newly placed prosthesis or in medically compromised patients. These patients may also need antibiotic premedication to prevent bacteremia from occurring during invasive dental treatment.
Cavernous Sinus Thrombosis
An infected intravascular clot or thrombus (plural, thrombi) can dislodge from the inner blood vessel wall and travel as an infected embolus (plural, emboli) (see Figures 6-16 and 6-17 ). An infected embolus can travel in the veins, draining the oral cavity into areas such as the dural venous sinuses within the cranial cavity. These dural venous sinuses are channels by which blood is conveyed from the cerebral veins into the veins of the head and neck, particularly the internal jugular vein. However, because these veins lack functional valves, venous blood can flow both into and out of the cranial cavity, possibly spreading infection.
The cavernous sinus is most likely to be involved in the possible fatal spread of odontogenic infection (see Figure 6-12). The cavernous sinus is located on the lateral surface of the body of the sphenoid bone. Each cavernous sinus communicates by anastomoses across the midline with the contralateral sinus and also with the pterygoid plexus of veins and the superior ophthalmic vein, which anastomoses with the facial vein. These major veins drain the teeth through the posterior superior and inferior alveolar veins and the lips through the superior and inferior labial veins.
None of these major veins that communicate with the cavernous sinus have valves to prevent the retrograde flow of blood back into the cavernous sinus. Therefore odontogenic infections that drain into these major veins may initiate an inflammatory response resulting in an increase in blood stasis, thrombus formation, and increasing extravascular fluid pressure. Increased pressure can reverse the direction of venous blood flow, enabling the transport of the infected thrombus (or thrombi) as embolus (or emboli) into this venous sinus thus causing cavernous sinus thrombosis.
Needle track contamination can also result in the spread of infection to the pterygoid plexus of veins if a posterior superior alveolar block is incorrectly administered (see Figure 9-7). In addition, nonodontogenic infections of the area that includes the orbital region, nasal region, and paranasal sinuses, may result in the spread of infection to the cavernous sinus. That is why this area is considered to be the “dangerous triangle” of the face.
The signs and symptoms of cavernous sinus thrombosis include fever, drowsiness, and rapid pulse. In addition, there is loss of function of the sixth cranial nerve or abducens because it runs through the cavernous sinus, resulting in abducens nerve paralysis. Because the muscle supplied by the nerve moves the eyeball laterally, inability to perform this movement suggests nerve damage. Additionally, the patient usually has double vision (diplopia) due to restricted movement of one eye, as well as edema of the eyelids and conjunctivae, tearing (lacrimation), and extruded eyeballs (exophthalmus), depending on the course of the infection (Figure 12-9).
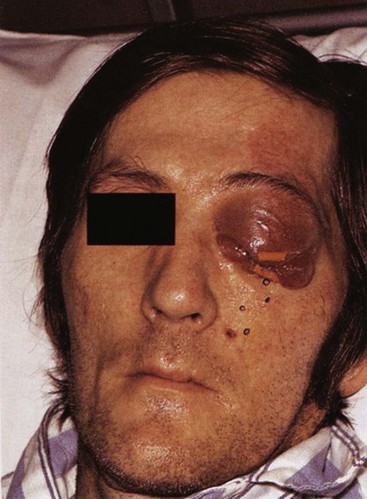
FIGURE 12-9 Cavernous sinus thrombosis, an infection of the cavernous sinus, with its edema of the eyelids and conjunctivae, tearing, and extruded eyeballs. (From Reynolds PA, Abrahams PH: McMinn’s interactive clinical anatomy: head and neck, ed 2, London, 2001, Mosby Ltd.)
With cavernous sinus thrombosis there may also be damage to the other cranial nerves such as the oculomotor nerve (third) and trochlear nerve (fourth), as well as to the ophthalmic and maxillary divisions of the trigeminal nerve (fifth) and changes in the tissue they innervate because all these nerves travel in the cavernous sinus wall. Finally, this infection can be fatal because it may lead to meningitis, inflammation of the meninges in the brain or spinal cord, which requires immediate hospitalization with intravenous antibiotics and anticoagulants (see Chapter 8).
Spread by Lymphatic System
The lymphatic system of the head and neck can allow the spread of infection from the teeth and associated oral tissue (see Chapter 10). This occurs because the pathogens can travel in the lymph through the lymphatic vessels that connect the series of nodes from the oral cavity to other tissue or organs. Thus these pathogens can move from a primary node near the infected site to a secondary node at a distant site.
The route of odontogenic infection traveling through the nodes varies according to the teeth involved. The submandibular nodes are the primary nodes for all the teeth and associated tissue, except the maxillary third molars (superior deep cervical nodes) and mandibular incisors (submental nodes) (Figure 12-10 and see Table 10-1). The submandibular nodes then empty into the superior deep cervical nodes.
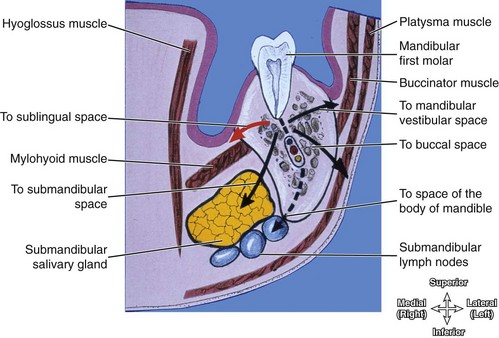
FIGURE 12-10 Coronal section showing the possible spread of an odontogenic infection from a mandibular first molar. It would spread first into the sublingual space and then possibly to other spaces as noted. Note that the submandibular nodes are primary nodes for all teeth and associated tissue, except for the maxillary third molars and mandibular incisors. (From Reynolds PA, Abrahams PH: McMinn’s interactive clinical anatomy: head and neck, ed 2, St Louis, 2001, Mosby.)
The superior deep cervical nodes empty into either the inferior deep cervical nodes or directly into the jugular trunk and then into the vascular system (see Figure 10-1). Once the infection is in the vascular system, it can be spread to other tissue, structures, and organs, as previously discussed.
Lymphadenopathy
A lymph node involved in infection undergoes hypertrophy, which results in lymphadenopathy, which results in a size increase and a change in the consistency of the lymph node so that it becomes palpable (see Figure 10-22). This change in the lymph node allows it to better fight the infection process. Evaluation of the involved nodes can determine the degree of regional involvement in the infection process, which is instrumental in the diagnosis and management of the infection.
Spread by Spaces
The spaces of the head and neck can allow the spread of infection from the teeth and associated oral tissue because the pathogens can travel within the fascial spaces, from one space near the infected site to another, more distant space by means of the spread of the related inflammatory exudate (see Chapter 11). When involved in infections, the space can undergo cellulitis (see Table 12-2), which can cause a change in the normal proportions of the face (see Chapter 2).
If the maxillary teeth and associated tissue are infected, the infection can spread into the vestibular space of the maxilla, buccal space, or canine space. If the mandibular teeth and associated tissue are infected, the infection can spread into the vestibular space of the mandible, buccal space, submental space, sublingual space, submandibular space, masticator spaces, or the space of the body of the mandible, depending on the location of the tooth and extent of infection (Figures 12-10 and 12-11).
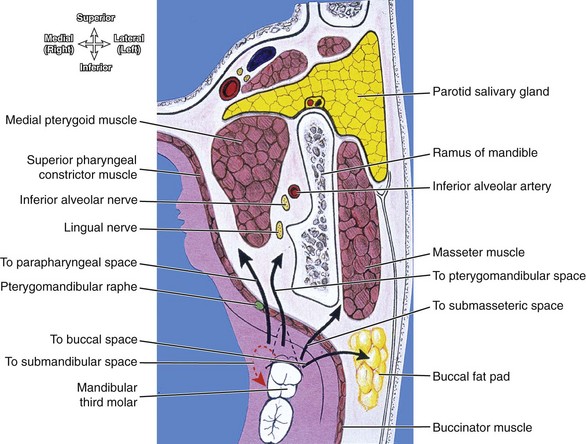
FIGURE 12-11 Horizontal section showing the spread of an odontogenic infection from a mandibular third molar directly into submandibular space and then possibly to other spaces as noted. (From Reynolds PA, Abrahams PH: McMinn’s interactive clinical anatomy: head and neck, ed 2, London, 2001, Mosby Ltd.)
The insertion of the mylohyoid muscle along the mandible dictates which mandibular subspace is initially affected by an odontogenic infection. The apex of the mandibular first molar is superior to the mylohyoid muscle, so involvement of this tooth or teeth anterior to it will first involve the sublingual space (see Figure 11-12). In contrast, the apices of the mandibular second and third molars are inferior to the mylohyoid muscle, and infection here will directly spread to the submandibular space. However, these spaces freely communicate around the posterior border of the mylohyoid muscle, and so both subspaces may become involved if the infection continues.
From these spaces, the infection can spread into other spaces of the jaws and neck such as the parapharyngeal and retropharyngeal spaces, causing serious complications (discussed next).
Ludwig Angina
One of the most serious lesions of the jaw region is Ludwig angina, which is cellulitis of the submandibular space (Figure 12-12 and see Figure 11-12). It involves the spread of infection from an abscess from any of the mandibular teeth or associated tissue to one space initially—the submental, the sublingual, or even the submandibular space itself.

FIGURE 12-12 Ludwig angina with its massive bilateral swelling showing involvement of both the submandibular and submental spaces resulting from an abscess of the mandibular third molar. (Courtesy Dr. Mark Gabrielson.)
The infection progresses to involve the submandibular space bilaterally, with a risk of spreading to the parapharyngeal space and then onto the retropharyngeal space of the neck (see Figures 11-13 and 11-14 ). With this lesion, there is initially massive bilateral submandibular regional swelling, which can extends down the anterior cervical triangles to the clavicles. Swallowing, speaking, and breathing may be difficult, with high fever and drooling evident. Respiratory obstruction develops rapidly as continued swelling elevates the tongue, displacing it backward, blocking the pharyngeal airway. Contrast-enhanced computed tomography scan has become the imaging modality of choice in the evaluation of the patient with a deep tissue cervical infection.
As the retropharyngeal space, which is considered the “danger space” of the neck by dental professionals, becomes involved with edema of the larynx there can be complete respiratory obstruction, asphyxiation, and death. Thus Ludwig angina is an acute medical emergency requiring immediate hospitalization, and may necessitate an emergency cricothyrotomy to quickly create a patent airway due it being compromised.
With the advent of earlier care of abscessed teeth and more routine antibiotic treatment, Ludwig angina has become an uncommon dental emergency in healthy patients. However, symptoms may be masked in partially treated cases, and risk is exponentially increased in medically compromised patients. Also, with the rising popularity of oral piercings, there has been an increase in the serious infection of oral sites to include Ludwig angina (Figure 12-13).
Prevention of Spread of Infection
Early diagnosis and treatment of infections must occur in all patients. Particular care must be taken not to contaminate sites of surgery such as those resulting from extraction, implant placement, or periodontal treatment. There must also be strict adherence to standard infection control measures during other types of dental treatment so as to prevent the spread of infection during, for example, the removal of heavy dental biofilm (dental plaque) accumulations before restorative and periodontal treatment.
Using an antiseptic oral rinse before the treatment protocol, a rubber dam, or an antimicrobial-laced external water supply when ultrasonic instrumentation or irrigation are used may help prevent the spread of infection. Also important is not administering a local anesthetic through an area of odontogenic infection so as to avoid moving the pathogens deeper into the tissue by needle-track contamination. After treatment, an antiseptic oral rinse or antibiotic coverage might be prescribed if the risk of infection exists.
A thorough medical history with periodic updates will allow the dental professional to perform safe treatment of medically compromised patients and avoid serious complications due to active dental disease. These patients may require antibiotic premedication before dental treatment or other changes in the dental treatment plan so as to prevent serious sequelae. A medical consultation is indicated when there is uncertainty as to the risk of opportunistic infection.
Oral infections can have significant medical ramifications including death. As the healthcare professional most familiar with a patient’s oral health, the dental professional must be knowledgeable about the appearances, causes, and symptoms of the lesions of all types of oral infections, as well as expert in the means of their prevention. In addition, the dental professional must keep up with recent guidelines for standard precautions for infection control such as those reported by the Centers for Disease Control.
Finally, scientific evidence has linked severe oral infections with increased susceptibility to certain important systemic diseases and conditions such as cardiovascular disease, diabetes mellitus, adverse pregnancy outcomes, pulmonary infections, and possibly rheumatoid arthritis. This is because the gram-negative bacteria involved in oral infections, such as with periodontal and endodontic disease, trigger production of lipopolysaccharides, heat-shock proteins, and proinflammatory cytokines that may be related to those serious systemic disease states. Due to these associations, it is imperative that oral infections be prevented when possible or promptly recognized and adequately treated.
1. Which of the following complications is MOST likely with untreated Ludwig angina?
2. Which of the following cranial nerves is MOST likely involved with cavernous sinus thrombosis?
A Oculomotor and trochlear nerves
B Vagus and glossopharyngeal nerves
3. Which of the following statements is CORRECT concerning an oral abscess?
4. Which of the following statements concerning cavernous sinus thrombosis is CORRECT?
5. If an infection involves the lingual surface of the mandibular third molar, where is a swelling MOST likely to be observed?
6. Which of the following types of infection processes presents as a diffuse inflammation of soft tissue spaces?
7. Which of the following is an infection-resistance factor noted with gram-negative anaerobic bacteria?
A Ability to be killed by penicillin
B Production of protective cell envelopes
8. Which of the following anatomic structures do NOT have functional valves, thus aiding in the spread of dental infections by blood backflow?
9. Which of the following lymph nodes are primary nodes for all the teeth (except the mandibular incisors and maxillary third molars) and thus can be involved in the spread of dental infections?
10. Which of the following can ONLY present as pathogens traveling in the vascular system?
11. Which of the following processes can occur to lymph nodes when involved in an infection?
12. When considering the signs and symptoms for abducens nerve paralysis, which of the following is NOT included?
13. What part of the face is considered part of the “dangerous triangle” by dental professionals?
14. When a dental patient is first diagnosed with an inflammation of the meninges, what form of care is needed?
A Wait and see if the infection progresses to the thorax
B Drainage of the localized infection and daily oral antibiotics
C Immediate hospitalization with intravenous antibiotics and anticoagulants
D NO treatment is possible due to the poor level of prognosis for the patient
15. What type of treatment may be necessary in cases of prolonged chronic maxillary sinusitis?