
Barry W. Connors
Sensation is a cognitive process that requires the full powers of the central nervous system (CNS). Sensation begins with the sensory receptors that actually interface with the world, and these receptors use energy from the environment to trigger electrochemical signals that can be transmitted to the brain—a process called sensory transduction. Understanding of transduction processes is crucial for several reasons. Without these processes, sensation fails. Moreover, a variety of diseases that specifically affect sensory receptors can impair or abolish sensation without damaging the brain. Transduction also sets the basic limits of perception. It determines the sensitivity, range, speed, versatility, and vigor of a sensory system.
We have a variety of senses, each tuned to particular types of environmental energy. These sensory modalities include the familiar ones of seeing, hearing, touching, smelling, and tasting as well as our senses of pain, balance, body position, and movement. In addition, other intricate sensory systems of which we are not conscious monitor the internal milieu and report on the body’s chemical and metabolic state. Early in the 19th century, the physiologist Johannes Müller recognized that neurons that are specialized to evaluate a particular type of stimulus energy will produce the appropriate sensation regardless of how they are activated. For example, banging your eye can produce perceptions of light even in the dark, and seizure activity in a region of the cortex devoted to olfaction can evoke repulsive smells even in a rose garden. This property has been called univariance; in other words, the sensory receptor and its subsequent neural circuits do not know what stimulated them—they give the same type of response regardless. Specificity for each modality is ensured by the structure and position of the sensory receptor.
Evolution is a conservative enterprise. Good ideas are retained, and with slight modification they are adapted to new purposes. Sensory transduction is a prime example of this principle. The sensory processes that are now understood at the molecular level use systems that are closely related to the ubiquitous signaling molecules in eukaryotic cells. Some modalities (vision, olfaction, some types of taste, and other chemoreception) begin with integral membrane proteins that belong to the superfamily of G protein–coupled receptors (GPCRs; see Chapter 5). The second-messenger pathways use the same substances that are used for so many nonsensory tasks in cells, such as cyclic nucleotides, inositol phosphates, and kinases. Other sensory systems (mechanoreceptors, including the hair cells of audition and the vestibular organs, as well as some taste cells) use modified membrane ion channels in the primary transduction process. Although the structures of most of these channels have not yet been determined, their biophysical properties are generally unremarkable, and they are likely to be related to other, nonsensory ion channels. Indeed, the gating of many ion channels (see Chapter 6) from “nonsensory” cells is sensitive to the physical distortion of the membrane that they lie in, which implies that mechanosensitivity is a widespread (although perhaps epiphenomenal) feature of integral membrane proteins.
To achieve a specificity for certain stimulus energies, many sensory receptors must use specialized cellular structures. These, too, are usually adapted from familiar components. Various receptors are slightly modified epithelial cells. Some situate their transduction sites on modified cilia, whereas others use muscle cells or collagen fibers to channel the appropriate forces to the sensory axon. Many are neurons alone, often just bare axons with no specialization visible by microscopy. Most sensory transduction cells (e.g., oxygen and taste sensors, but not olfactory receptors) lack their own axon to communicate with the CNS. For these cells, the communication system of choice is a relatively standard, Ca2+-dependent system of synaptic transmission onto a primary sensory neuron.
Functionally, sensory transducers follow certain general steps. Obviously, they must detect stimulus energy, but they must do so with enough selectivity and speed that stimuli of different types, from different locations, or with different timing are not confused. In most cases, transduction also involves one or more steps of signal amplification so that the sensory cell can reliably communicate small stimuli (e.g., a few stray photons or a smattering of drifting molecules) to a large brain in an environment with much sensory noise. The sensory cell must then convert the amplified signal into an electrical change by altering the gating of some ion channel. This channel gating leads to alterations of the membrane potential (Vm) in the receptor cell—otherwise known as a receptor potential. The receptor potential is not an action potential but a graded electrotonic event (see Chapter 7) that can either modulate the activity of other channels (e.g., voltage-gated Na+ or Ca2+ channels) or trigger action potentials in a different portion of the same cell. Very often, the receptor potential regulates the flux of Ca2+ into the cell and thus controls the release of some synaptic transmitter molecule onto the sensory afferent neuron.
Ultimately, receptor potentials determine the rate and pattern with which action potentials fire in a sensory neuron. This firing pattern is the signal that is actually communicated to the CNS. Useful information may be encoded in many features of the firing, including its rate, its temporal patterns, its periodicity, its consistency, and its patterns compared with other sensory neurons of the same or even different modalities.
Every cell is bathed in chemicals. Molecules can be food or poison, or they may serve as signals of communication between cells, organs, or individuals. The ability to recognize and to respond to environmental chemicals can allow cells to find nutrients, to avoid harm, to attract a mate, to navigate, or to regulate a physiological process. Chemoreception has basic and universal advantages. It is the oldest form of sensory transduction, and it exists in many forms. Chemoreception does not even require a nervous system. Single-celled organisms such as bacteria can recognize and respond to substances in their environment. In the broadest sense, every cell in the human body is chemosensitive, and chemical signaling between cells is the basis for internal communication through endocrine systems and neurotransmission. In this chapter, we restrict ourselves to chemoreception as a sensory system, the interface between the nervous system and the external and internal chemical milieu.
Chemicals reach the human body by oral or nasal ingestion, contact with the skin, or inhalation, and once there, they diffuse or are carried to the surface membranes of receptor cells through the various aqueous fluids of the body (e.g., mucus, saliva, tears, cerebrospinal fluid, blood plasma). The nervous system constantly monitors these chemical comings and goings with a diverse array of chemosensory receptors. The most familiar of these receptors are the sensory organs of taste (gustation) and smell (olfaction). However, chemoreception is widespread throughout the body. Chemoreceptors in the skin, mucous membranes, and gut warn against irritating substances, and chemoreceptors in the carotid bodies (see Chapter 32) measure blood levels of O2, CO2, and [H+].
The tasks of gustatory and olfactory receptors appear similar at first glance. Both recognize the concentration and identity of dissolved molecules, and they communicate this information to the CNS. In fact, the two systems operate in parallel during eating, and the flavors of most foods are strongly dependent on both taste and smell. However, the receptor cells of the two systems are quite different. Olfactory receptors are neurons. Each olfactory cell has small dendrites at one end that are specialized to identify chemical stimuli, and at the other end an axon projects directly into the brain. Taste receptor cells are not neurons but rather modified epithelial cells that synapse onto the axons of sensory neurons that communicate with the CNS.
Taste Receptor Cells Taste receptors are located mainly on the dorsal surface of the tongue (Fig. 15-1A), concentrated within small but visible projections called papillae (Fig. 15-1B). Papillae are shaped like ridges, pimples, or mushrooms, and each is a few millimeters in diameter. Each papilla in turn has numerous taste buds (Fig. 15-1C). One taste bud contains 50 to 150 taste receptor cells, numerous basal and supporting cells that surround the taste cells, plus a set of sensory afferent axons. Most people have 2000 to 5000 taste buds, although exceptional cases range from 500 to 20,000.

Figure 15-1 Taste receptors.
The chemically sensitive part of a taste receptor cell is a small apical membrane region near the surface of the tongue. The apical ends have thin extensions called microvilli that project into the taste pore, a small opening on the surface of the tongue where the taste cells are exposed to the contents of the mouth. Taste cells form synapses with the primary sensory axons near the bottom of the taste bud. However, processing may be more complicated than a simple receptor-to-axon relay. Receptor cells also make both electrical and chemical synapses onto some of the basal cells, some basal cells synapse onto the sensory axons, and some type of information-processing circuit may be present within each taste bud itself.
Cells of the taste bud undergo a constant cycle of growth, death, and regeneration. This process depends on the influence of the sensory nerve because if the nerve is cut, taste buds degenerate.
Olfactory Receptor Cells We smell with receptor cells in the thin olfactory epithelium, which is placed high in the nasal cavity (Fig. 15-2A). The olfactory epithelium has three main cell types: olfactory receptor cells are the site of transduction; support cells are similar to glia and, among other things, help produce mucus; and basal cells are the source of new receptor cells (Fig. 15-2B). Olfactory receptors (similar to taste receptors) continually die, regenerate, and grow in a cycle that lasts ~4 to 8 weeks. Olfactory receptor cells are one of the very few types of neurons in the mammalian nervous system that are regularly replaced throughout life.
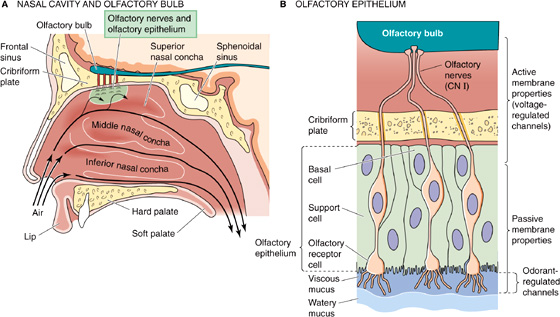
Figure 15-2 Olfactory reception.
As we breathe or sniff, chemical odorants waft through the many folds of the nasal passages. However, to contact the receptor cells, odorants must first dissolve in and diffuse through a thin mucous layer, which has both a viscous and a watery portion. The normal olfactory epithelium exudes a mucous layer 20 to 50 μm thick. Mucus flows constantly and is normally replaced about every 10 minutes. Mucus is a complex, water-based substance containing dissolved glycosaminoglycans (see Chapter 2); a variety of proteins, including antibodies, odorant-binding proteins, and enzymes; and various salts. The antibodies are critical because olfactory cells offer a direct route for viruses (e.g., rabies) or bacteria to enter the brain. Odorant-binding proteins in the mucus probably facilitate the diffusion of odorants toward and away from the receptors. Enzymes may help clear the mucus of odorants and thus speed recovery of the receptors from transient odors.
Both the absolute size and the receptor density of the olfactory epithelium vary greatly among species, and they help determine olfactory acuity. The surface area of the human olfactory epithelium is only ~10 cm2, but this limited area is enough to detect some odorants at concentrations as low as a few parts per trillion. The olfactory epithelia of some dogs may be over 170 cm2, and dogs have more than 100 times as many receptors in each square centimeter as humans do. The olfactory acuity of some breeds of dog is legendary and far surpasses that of humans. Dogs can often detect the scent of someone who walked by hours before.
Studies of taste discrimination in humans imply that we can distinguish among 4000 to 10,000 different chemicals with our taste buds. However, behavioral evidence suggests that these discriminations represent only five primary taste qualities: bitter, salt, sweet, and sour plus a primary quality called umami (“delicious” in Japanese). Umami is epitomized by the taste of the amino acid glutamate (monosodium glutamate [MSG] is the familiar culinary form). Unlike an olfactory receptor cell, which apparently expresses only one receptor type (see later), a taste receptor cell may express several.
In many cases, there is an obvious correlation between the chemistry of tastants (i.e., chemicals being tasted) and the quality of their taste. Most acids taste sour and most salts taste salty. However, for many other tastants, the linkage between taste and chemical structure is not clear. The familiar sugars (e.g., sucrose and fructose) are satisfyingly sweet, but certain proteins (e.g., monellin) and artificial sweeteners (e.g., saccharin and aspartame, which is made from two amino acids: L-aspartyl-L-phenylalanine methyl ester) are 10,000 to 100,000 times sweeter by weight than these sugars. Bitter substances are also chemically diverse. They include simple ions such as K+ (KCl actually simultaneously evokes both bitter and salty tastes), larger metal ions such as Mg2+, and complex organic molecules such as quinine.
If the tongue has only four or five primary taste qualities available to it, how does it discriminate among the myriad complex flavors that embellish our lives? First, the tongue’s response to each tastant reflects distinct proportions of each of the primary taste qualities. In this sense, the taste cells are similar to the photoreceptors of our eyes; with only three different types of color-selective photoreceptive cone cells, we can distinguish a huge palette of colors (see later). Second, the flavor of a tastant is determined not only by its taste but also by its smell. Taste and smell operate in parallel, with information converging in the CNS to aid the important discrimination of foods and poisons. For example, without the aid of olfaction, an onion tastes much like an apple—and both are quite bland. Third, the mouth is filled with other types of sensory receptors that are sensitive to texture, temperature, and pain, and these modalities enhance both the identification and enjoyment of foods. A striking example is the experience of spicy food, which is enjoyable to some but painful to others. The spiciness of hot peppers is generated by the chemical capsaicin, not because of its activation of taste receptor cells but because of its stimulation of heat-sensitive pain receptors in the mouth (see later).
The chemicals that we taste have diverse structures, and taste receptors have evolved a variety of mechanisms for transduction. The taste system has adapted many types of membrane-signaling systems to its purposes. Tastants may pass directly through ion channels (salt), bind to ion channels (sour), or bind to membrane receptors that activate second-messenger systems, which in turn open or close ion channels (sweet, bitter, and umami). Taste cells have simply used specialized variations of these processes to initiate meaningful signals to the brain.
The receptor potentials of taste cells are usually depolarizing. At least some taste receptor cells can fire action potentials, similar to those of neurons; but if the membrane is sufficiently depolarized by whatever means, voltage-gated Ca2+ channels open, and Ca2+ enters the cytoplasm and triggers the release of transmitter molecules. The identity of the taste receptor’s transmitter or transmitters is unknown.
Some believe that each taste-receptor cell responds to only one of the five basic taste modalities. It is generally accepted that a receptor cell responds to only one out of the group of sweet, bitter, and umami—all of which share a common signal transduction mechanism. Finally, some evidence suggests that each taste-receptor cell is hard-wired to the CNS to convey a particular taste quality. For example, if we express a bitter receptor in sweet taste-receptor cells, a mouse—naturally attracted to sweet tastants—will now be attracted to bitter tastants that now taste sweet.
The complex diversities of taste transduction are not yet fully understood. Many of the details have come from research on the taste cells of catfish, mudpuppies, mice, and rats. Each animal has certain experimental advantages (e.g., very large taste cells), but the differences among species suggest that we may be surprised when it becomes possible to study human mechanisms directly. The following is a summary of the best-understood transduction processes for the five primary taste qualities (Fig. 15-3).
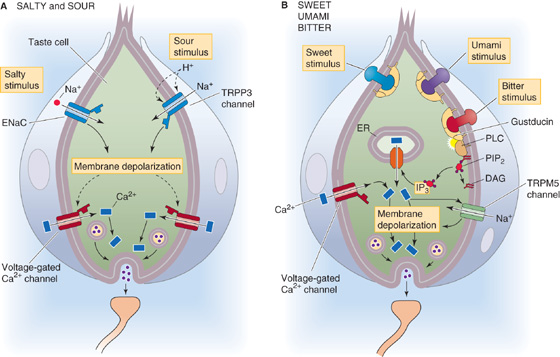
Figure 15-3 Cellular basis of taste transduction. A, Salty taste is mediated by an epithelial Na+ channel (ENaC) that is sensitive to amiloride. Sour is mediated as extracellular H+ activates TRPP3 channels. B, Sugars and umami compounds bind to GPCRs consisting of T1R heterodimers. Bitter substances bind to GPCRs consisting of dimers made up of members of the T2R family. DAG, diacylglycerol; ER, endoplasmic reticulum; IP3, inositol 1, 4, 5-triphosphate; PDE, phosphodiesterase; PIP2, phosphatidyl inositol 4, 5-biphosphate; PLC, phospholipase C.
Salt The most common salty tasting chemical is NaCl, or table salt. The taste of salt is mainly the taste of the cation Na+, and transduction of [Na+] in taste cells is relatively simple. Salt-sensitive taste cells have an Na+-selective channel called ENaC (Fig. 15-3A), common to many epithelial cells, that is blocked by the drug amiloride (see Chapter 35). Unlike the Na+ channel that generates action potentials in excitable cells, the taste channels are relatively insensitive to voltage and stay open at rest. However, transduction of the [Na+] in a mouthful of food is somewhat analogous to the behavior of a neuron during the upstroke of an action potential. When [Na+] rises outside the receptor cell, the gradient for Na+ across the membrane becomes steeper, Na+ diffuses down its electrochemical gradient (i.e., it flows into the cell), and the resultant inward current causes the membrane to depolarize to a new voltage. Neurons depolarize during their action potential by increasing Na+ conductance at a fixed Na+ gradient (see Fig. 7-4). In contrast, Na+-sensitive taste cells depolarize by increasing the Na+ gradient at a fixed Na+ permeability. The resultant graded depolarization of the taste cell is defined as its receptor potential.
Anions may affect the taste of salts by modulating the saltiness of the cation or by adding a taste of their own. NaCl tastes saltier than Na acetate, perhaps because the larger an anion is, the more it inhibits the salty taste of the cation. Na saccharin is sweet because the anion saccharin activates sweetness receptors; it is not salty because Na+ is present at a very low concentration.
Sour Sourness is evoked by protons (H+ ions). The key player is the non-selective cation channel TRPP3 (Fig. 15-3A), a member of the transient receptor potential (TRP) family of ion channels (Table 6-2 on p. 167). Decreases in pH—presumably extracellular pH—activate TRPP3, thereby depolarizing the sour-receptor cell. TRPP3 is also known as PKD2L1 because it is a close relative of polycystin 2 (PKD2), a mutation in which can cause autosomal dominant polycystic kidney disease. The taste of carbonation (i.e., CO2 in drinks) arises as GPI-linked extracellular carbonic-anhydrase IV (p. 654) converts CO2 to HCO−3 plus H+, the latter activating TRPP3.
Sweet Sweetness is sensed when molecules bind to specific receptor sites on the taste cell membrane and activate a cascade of second messengers (Fig. 15-3B). Two families of taste receptor genes—the T1R family and T2R family—seem to account for sweet, bitter, and umami transduction. These taste receptors are GPCRs, and all use the same basic second-messenger pathway. In the case of sweet transduction, the tastant (e.g., a sugar molecule) binds to a taste receptor that consists of a dimer of T1R2 and T1R3 proteins. The activated receptor then activates a G protein that stimulates phospholipase C, which in turn increases its production of the messenger inositol trisphosphate (IP3; see Chapter 3). IP3 triggers the release of Ca2+ from internal stores, and the rise in [Ca2+]i then activates the TRPM5 channel that is specific for taste cells. TRPM5 is a relatively nonselective cation channel that depolarizes the taste cell, triggering the release of neurotransmitter onto the primary gustatory axon (Fig. 15-3B). The sweet receptor complex—the T1R2/T1R3 dimer—is broadly sensitive to sweet-tasting substances. Despite the appearance in Figure 15-3B, sweet-sensing taste cells do not express receptors for either bitter or umami.
Bitter Bitterness usually warns of poison. Perhaps because poisons are so chemically diverse, we have about 30 different types of bitter receptors to sense them. These are GPCRs in the T2R family. Animals are not very good at distinguishing between different bitter substances because each bitter taste cell expresses the majority of the 30 T2Rs. It may be more important to recognize that something is bitter, and potentially poisonous, than it is to recognize precisely what type of poison it may be. Stimulation of the T2Rs activates a second-messenger pathway that is apparently identical to the one that sweet receptors activate: G proteins, PLC, IP3, [Ca2+]i increase, and TRPM5 channel opening. We do not confuse the tastes of sweet and bitter substances because even though they trigger similar signaling systems, each transduction cascade occurs within a specific sweet or bitter taste cell. Moreover, each taste cell makes synaptic contact with a different primary gustatory axon that leads into the CNS.
Amino Acids Amino acids are critical nutrients that are vital as an energy source and for constructing proteins. Probably as a consequence, many amino acids taste good, although some taste bitter. The umami taste, which we know well from Chinese restaurants, is triggered by a mechanism very similar to that for sweet tasting. The umami receptor is a dimer comprising two members of the T1R family, T1R1 and T1R3. Note that the umami and sweet receptors share T1R3. The taste for amino acids seems to depend on T1R1 because mice that lack it are unable to discriminate glutamate and other amino acids, although they retain their ability to detect sweet substances. The umami receptor activates the same signaling mechanisms that sweet and bitter receptors do: G proteins, PLC, IP3, [Ca2+]i increase, and TRPM5 channel opening. Again, by isolating the umami receptors in taste cells that do not also express sweet and bitter receptors, the CNS can distinguish the various tastes from one another by somehow knowing which taste cell connects to a particular gustatory axon.
Our ability to smell chemicals is better developed than our ability to taste them. By one estimate, we can smell more than 400,000 different substances. Interestingly, ~80% of them smell unpleasant. As with taste, it seems likely that smell evolved to serve important protective functions, such as warning us away from harmful substances. With the ability to discriminate so many different smells, you might also expect many different types of transduction mechanisms, as in the taste system. In fact, olfactory receptors probably use only one second-messenger mechanism. Figure 15-4 summarizes the chain of events that leads to an action potential in the olfactory nerve (i.e., CN I):
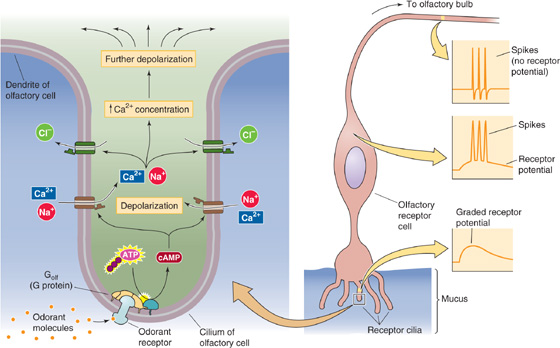
Figure 15-4 Cellular mechanism of odor sensation. ATP, adenosine triphosphate.
Step 1: The odorant binds to a specific olfactory receptor protein in the cell membrane of a cilium of an olfactory receptor cell.
Step 2: Receptor activation stimulates a heterotrimeric G protein called Golf (see Chapter 3).
Step 3: The α subunit of Golf in turn activates adenylyl cyclase, which produces cAMP.
Step 4: The cAMP binds to a cAMP-gated cation channel.
Step 5: Opening of this channel increases permeability to Na+, K+, and Ca2+.
Step 6: The net inward current leads to membrane depolarization and increased [Ca2+]i.
Step 7: The increased [Ca2+]i opens Ca2+-activated Cl− channels. Opening of these channels produces more depolarization because of the relatively high [Cl−]i of olfactory receptor neurons.
Step 8: If the receptor potential exceeds the threshold, it triggers action potentials in the soma that travel down the axon and into the brain.
All this molecular machinery, with the exception of the action potential mechanism, is squeezed into the thin cilia of olfactory receptor cells. Moreover, additional modulatory schemes also branch from this basic pathway.
Olfactory receptor cells express a huge family of receptor proteins; in fact, they are the largest family of mammalian genes known! Their discovery in the early 1990s earned Linda Buck and Richard Axel the 2004 Nobel Prize. Rodents have more than 1000 different olfactory receptor genes. Humans have ~350 genes that encode functional receptor proteins. This family of olfactory receptor proteins belongs to the superfamily of GPCRs (see Chapter 3) that also includes the phototransduction protein rhodopsin and the taste receptors for sweet, bitter, and umami described before as well as the receptors for a wide variety of neurotransmitters. (See Note: Richard Axel and Linda Buck)
The extracellular surfaces of olfactory receptor proteins have odorant binding sites, each slightly different from the others. Presumably, each receptor protein can bind only certain types of odorants; therefore, some degree of selectivity is conferred to different olfactory receptor cells. Remarkably, each receptor cell seems to express only a single gene of the 1000 different odorant receptor genes in rodents. Thus, 1000 different types of olfactory receptor cells are present, each identified by the one receptor gene that it expresses. Because each odorant may activate a large proportion of the different receptor types, the central olfactory system’s task is to decode the patterns of receptor cell activity that signals the identity of each smell.
The structure of the olfactory cAMP-gated channel is closely related to the light-activated channel in photoreceptors of the retina, which is normally gated by an increase in intracellular cyclic guanosine monophosphate ([cGMP]i). The olfactory channel and the photoreceptor channel almost certainly evolved from one ancestral cyclic nucleotide–gated channel, just as the olfactory receptor and photoreceptor proteins probably evolved from an ancestral receptor with seven membrane-spanning segments.
Termination of the olfactory response occurs when odorants diffuse away, scavenger enzymes in the mucous layer break them down, or cAMP in the receptor cell activates other signaling pathways that end the transduction process.
The environment of most species is enveloped by light (Fig. 15-5). Animals have evolved a variety of mechanisms to transduce and to detect light. Their brains analyze visual information to help them locate food, to avoid becoming food, to find a mate, to navigate, and generally to recognize distant objects. Light is an exceptionally useful source of information about the world because it is nearly ubiquitous and can travel far and fast and in straight lines with relatively little dispersion of its energy. The vertebrate eye, which we describe here, has two major components: an optical part to gather and focus light and to form an image and a neural part (the retina) to convert the optical image into a neural code.
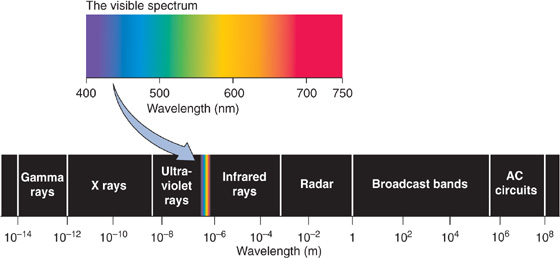
Figure 15-5 The electromagnetic spectrum. AC, alternating current.
The optical structures of the eye are among the most sophisticated of the specialized non-neural sensory endings, and they are often compared with a camera. As cameras have become more technologically sophisticated, the analogy has improved because the eye has systems to focus automatically, to adjust its sensitivity for widely different light levels, to move to track and to stabilize a target, and even to keep its surface washed and clear (obviously, cameras still have room for improvement). The similarity to a camera breaks down when we consider the retina, which is decidedly not like standard photographic film or electronic light detectors.
Figure 15-6A shows a cross section through the human eye. A ray of light entering the eye passes through several relatively transparent elements to reach the retina; these elements include a thin film of tears and then the cornea, the aqueous humor, the lens, and finally the vitreous humor. Tears are a surprisingly complex liquid, based on a plasma ultrafiltrate. They bathe the cornea in a layer that is less than 10 μm thick, keep it wet, and allow O2 to diffuse from the air to the corneal cells. Tears also contain lysozymes and antibodies to counter infection, a superficial oily layer that greatly slows evaporation and prevents spillage at the lid margins, and a thin mucoid layer to wet the surface of the cornea and to allow the tears to spread smoothly. Tears also help flush away foreign substances. The cornea is a thin, transporting epithelium that is devoid of blood vessels and has a cell structure specialized to maintain its high transparency. The ciliary epithelium, a part of the ciliary body, constantly secretes aqueous humor, a protein-free ultrafiltrate of blood plasma, into the posterior chamber of the eye. The aqueous humor then flows between the iris and the anterior surface of the lens and reaches the anterior chamber through the pupil. This aqueous humor keeps the anterior portion of the eye slightly pressurized (~20 mm Hg), which helps maintain the eye’s shape. The canals of Schlemm drain the aqueous humor. Excess pressure in the anterior chamber produces a disease called glaucoma. In the most common form of glaucoma, blockage of the canals of Schlemm leads to increased intraocular pressure. Pressure damages and kills ganglion cell axons at the optic disc, where they leave the eye and enter the optic nerve. The lens is an onion-like structure with closely packed columnar cells that are arranged in concentric shells and encased by a thin, tough, transparent capsule that is composed of epithelial cells. The cells of the lens have a high concentration of proteins called α-crystallins, which help increase the density of the lens and enhance its focusing power. The posterior chamber, which is filled with a gelatinous substance called vitreous humor, is also kept pressurized by the production of aqueous humor.
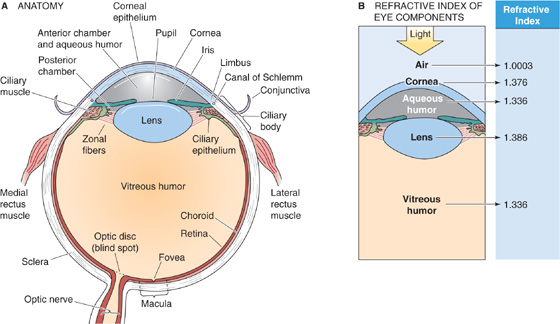
Figure 15-6 The eye. A, Cross section of the right human eye, viewed from the top. B, Bending of light by a structure depends not only on the radius of curvature but also on the difference in the indices of refraction of the two adjoining media.
The light must be focused to generate a clear optical image on the retina. This is accomplished by the cornea and, to a lesser extent, the lens. Focusing requires the path of the light to be bent, or refracted. Refraction can occur when light passes from a medium in which it travels relatively fast into a medium in which it travels relatively slowly, or vice versa. The index of refraction for a substance is essentially a measure of the speed of light within it; for example, light travels faster through air (index of refraction, 1.0003) than through the denser substance of the cornea (index of refraction, 1.376). Two things determine how much a light ray is refracted: the difference in the refractive indices of the two media and the angle between the incident light and the interface between the two media. Simple convex lenses use curved surfaces to control the refraction of light rays so that they converge (or focus) on a distant surface. The focal power (D) of one surface of a spherical lens is
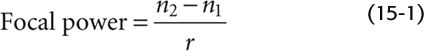
Here, n1 and n2 are the refractive indices of the first and second medium and r is the radius of curvature of the lens in meters. The unit of focal power is a diopter (1 D = 1 m−1). Focal power is the reciprocal of focal length. Thus, parallel light rays entering a 1-D lens are focused at 1 m, and those entering a 2-D lens are focused at 0.5 m.
In the case of the eye, most of the focusing takes place at the interface between the air and the tear-covered anterior surface of the cornea because this region is where light encounters the greatest disparity in refractive index on its path to the retina (Fig. 15-6B). With a change of 0.376 in refractive index and a radius of outer curvature of 7.8 mm in a typical human cornea, the focal power is 48.2 D. The curvature on the inner surface of the cornea is reversed, so some focal power is lost as light passes into the aqueous humor. However, the change in refractive index at this surface is only 0.040, so the change is only −5.9 D. The lens of the eye, with convex curvature on both sides, has a potentially greater focal power than the cornea. However, because of the small difference in refractive index between the substance of the lens and the aqueous and vitreous humors surrounding it, the effective focal power of the lens is lower. The summed focal power of the optics of the relaxed eye is ~60 D, which allows it to focus light from distant objects onto the retina, the center of which is ~24 mm behind the surface of the cornea (Fig. 15-7A). The position of the retinal image is, of course, upside down relative to the object that produced it.
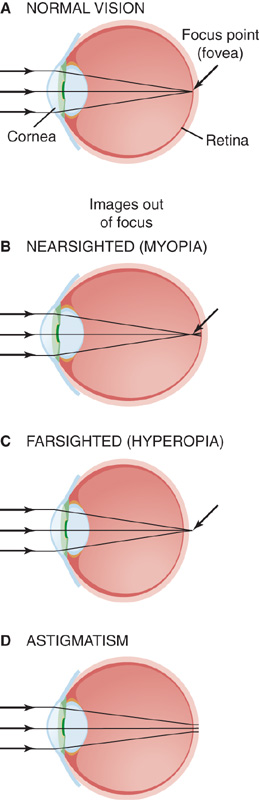
Figure 15-7 Light paths from a distant object to the eye.
A normal resting eye is focused on distant objects, beyond ~7 m. If it were fixed in this position, it would be impossible to see objects that are close up. To focus objects that are closer than 7 m away, the eye needs to increase its focal power, a process called accommodation. The eye achieves this goal by changing the shape of the lens. At rest, the lens is suspended around its edge by elastic zonal fibers that keep its capsule stretched and relatively flattened. To accommodate, the ciliary muscle fibers contract and release some of the tension in the zonal fibers. Relieved of the radial pull of its fibers, the lens becomes rounder. This increased curvature means increased focal power and a shift of the focal point closer to the eye. There are limits to accommodation, of course, and they are strongly age dependent. Young children have the most pliable lenses and can increase their focal power up to 12 to 14 D. Their near point, the closest distance that they are able to focus, is about at the end of their nose.
With age, the lens becomes stiffer and less able to round up and accommodate. By age 30, the near point is ~10 cm, and by the mid-40s, it stretches beyond arm’s length. The loss of accommodation with age is called presbyopia (from the Greek presbus for “old” and ops for “eye”); it is the reason that glasses for reading are unavoidable for almost everyone past middle age. Additional refractive flaws may be caused by an eye that is too long or short for its focusing power or by aberrations in the refracting surfaces of the eye. Myopia, or nearsightedness, occurs when the eye is too long; distant objects focus in front of the retina and appear blurred (Fig. 15-7B). Hyperopia (or hypermetropia), or farsightedness, is a feature of eyes that are too short; even with the lens fully accommodated, a near object focuses behind the retina and appears blurry (Fig. 15-7C). People with myopia can wear concave lenses that move the focal plane of all images back toward the retina. Those with hyperopia can wear convex lenses that move the focal plane forward. Astigmatism is caused by uneven curvature of the refractive surfaces of the eye. As a result, a point source of light cannot be brought to a precise focus on the retina (Fig. 15-7D). The resultant diffuse focusing leads to blurring of the image. Most people with astigmatic vision can also wear lenses that compensate for aberrant focusing properties of their eyes.
The iris is the colored structure that is visible through the window of the cornea. The iris’s hue comes from pigments in its cells, but its function is to create and to adjust the round opening that it encircles—the pupil. The pupil is like the aperture of the camera, and the iris is the diaphragm that regulates the amount of light allowed to enter the eye. The iris has sphincter muscles, innervated by postganglionic parasympathetic fibers from the ciliary ganglion (Fig. 15-8; see also Fig. 14-4), that allow it to constrict (miosis). The iris also has radially oriented muscles, innervated by postganglionic sympathetic fibers from the superior cervical ganglion (see Figs. 14-4 and 14-12), that allow it to dilate (mydriasis). Pupil size depends on the balance of the two autonomic inputs. The regulation of pupillary size by ambient light levels is called the pupillary light reflex (Fig. 15-8). Light striking the retina stimulates fibers in the optic nerve (neuron 1) that synapse in the brainstem in the pretectal nucleus. Neuron 2 projects to the Edinger-Westphal nuclei on both sides of the brain (see Fig. 14-5), stimulating preganglionic parasympathetic neurons (neuron 3) that project to the ciliary ganglia. These neurons activate postganglionic parasympathetic neurons (neuron 4) that constrict both pupils. Thus, control of the pupils in the two eyes is “yoked”: an increase in light to only one eye causes its pupil to constrict (the direct light response), but it also causes an identical constriction in the other eye, even if that eye saw only constant light levels (the consensual light response). Pupillary responses serve two functions: (1) they regulate the total amount of light that enters the eye (over a range of ~16-fold), and (2) they affect the quality of the retinal image in the same way that the aperture affects the depth of focus of a camera (a smaller pupil diameter gives a greater depth of focus). (See Note: Importance of Pupil Size for Depth of Focus)
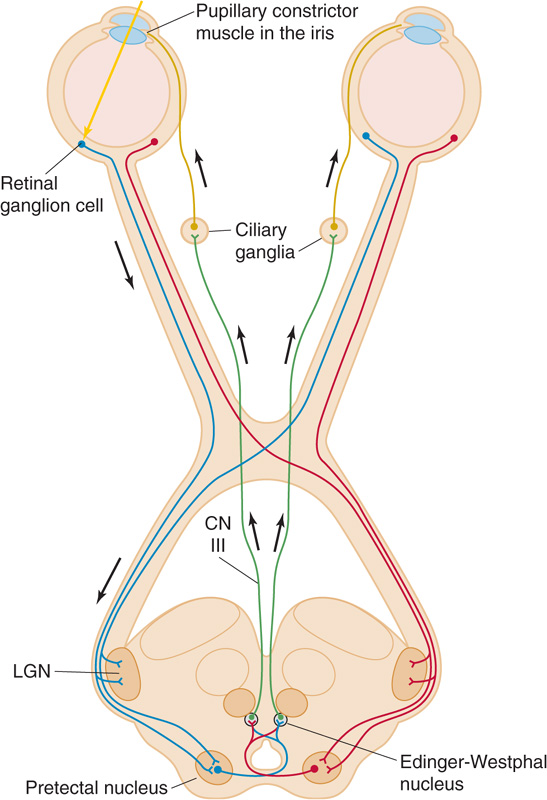
Figure 15-8 The pupillary light reflex. The figure shows the parasympathetic pathways that lead to constriction of the pupils. Pupil diameter depends on the balance between these parasympathetic pathways as well as the sympathetic pathways shown in Figure 14-12. CN III, oculomotor nerve; LGN, lateral geniculate nucleus.
Other peripheral structures are also essential to proper visual function. The most important are the extraocular muscles that control eye movements and thus the direction of gaze, the tracking of objects, and the coordination of the two eyes to keep their retinal images aligned as the eye, head, and visual world move about. Nuclei in the brainstem also control these tracking functions.
The retina is a very thin (~200 μm thick in humans) sheet of tissue that lines the back of the eye and contains the light-sensitive cells, the photoreceptors. Photoreceptors capture photons, convert their light energy into chemical free energy, and ultimately generate a synaptic signal for relay to other visual neurons in the retina.
The retina is, histologically and embryologically, a part of the CNS. Not only does it transduce light into neural signals, but it also does some remarkably complex processing of visual information before passing it on to other regions of the brain. In addition to the photoreceptor cells, the retina has four additional types of neurons that form an orderly but intricate neural circuit (Fig. 15-9). One type, the ganglion cell, generates the sole output of the retina by sending its axons to the thalamus through the optic nerve (CN II).
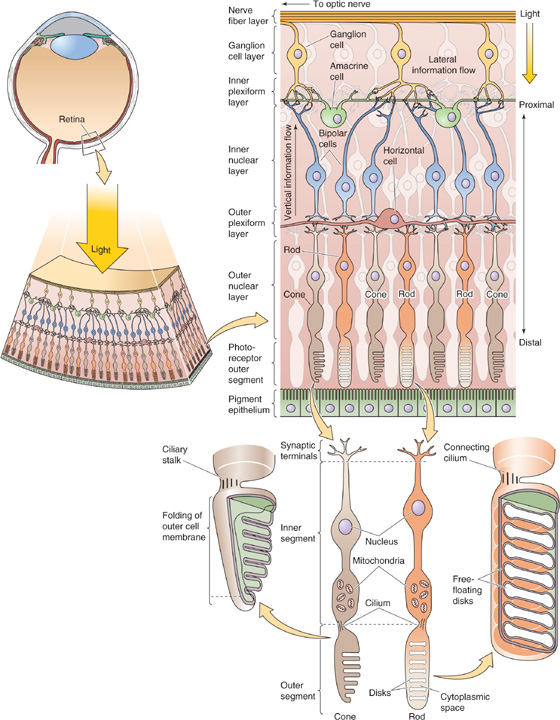
Figure 15-9 The retina—the neural circuits in the retina of a primate. Notice that the incoming light reaches the photoreceptor cells (rods and cones) only after passing through several thin, transparent layers of other neurons. The pigment epithelium absorbs the light that is not absorbed by the photoreceptor cells and thus minimizes reflections of stray light. The ganglion cells communicate to the thalamus by sending action potentials down their axons. However, the photoreceptor cells and other neurons communicate by graded synaptic potentials that are conducted electrotonically.
The retina is a highly laminated structure. Through a quirk of evolution, the photoreceptors of the vertebrate eye are on the outer surface of the retina, that is, the side facing away from the vitreous humor and incoming light. Thus, to reach the transducing cells, light has to first pass through all the retinal neurons. This path causes only minor distortion of image quality because of the thinness and transparency of the neural layers. This seemingly inverted arrangement may actually be an advantage for housekeeping of the eye. Photoreceptors undergo a continuous process of renewal, sloughing off membrane from their outer segments and rebuilding them. They also demand a relatively high energy supply. Because they face the back of the eye, photoreceptors are close to the pigment epithelium, which aids the renewal process, and to the blood vessels that supply the retina. These poorly transparent structures (i.e., pigment epithelium and blood vessels) are thus isolated from the light path. In fact, the pigment epithelium also absorbs photons that are not first captured by photoreceptors, before they can be reflected and degrade the visual image.
Each human eye has more than 100 × 106 photoreceptors but only 1 × 106 ganglion cells, which implies a high degree of convergence of information as it flows from the transducing cells to the output cells. Some of this convergence is mediated by a set of interneurons (i.e., cells that make synaptic connections only within the retina) called bipolar cells, which directly connect photoreceptors and ganglion cells in a mainly radial direction (Fig. 15-9). The two remaining types of retinal neurons, horizontal cells and amacrine cells, are interneurons that mainly spread horizontally. Horizontal cells synapse within the outer layer of the retina and interconnect photoreceptors and bipolar cells to themselves and to each other. Horizontal cells often mediate interactions over a wide area of retina. Amacrine cells synapse within the inner layer of the retina and interconnect both bipolar cells and ganglion cells. The circuitry of the retina is much more complex than this picture implies. One hint of this complexity is that its four primary types of neurons are in turn divided into at least 10 to 20 distinct subtypes, each with different physiological and morphological features.
The thinness of the mammalian retina has an interesting biophysical consequence. Because signaling distances are so short, synaptic potentials can spread effectively within its neurons without the help of conventional action potentials. Electrotonic spread of potentials along the dendrites is generally enough. The main exceptions are the ganglion cells, which use action potentials to speed visual information along their axons to the thalamus.
The two main types of photoreceptors, rods and cones, are named for their characteristic shapes (Fig. 15-9). The human retina has only one type of rod, which is responsible for our monochromatic dark-adapted vision, and three subtypes of cones, which are responsible for the color-sensitive vision that we experience in brighter environments. Rods outnumber cones by at least 16:1, and each is spread in a distinct pattern across the retina.
In the central area of the primate retina is a small pit 300 to 700 μm in diameter (which accounts for 1 to 2.3 degrees of visual angle) called the fovea, which collects light from the center of our gaze (Fig. 15-6). Several adaptations of the fovea allow it to mediate the highest visual acuity in the retina. Neurons of the inner layer of retina are actually displaced laterally to the side of the fovea to minimize light scattering on the way to the receptors. In addition, within the fovea, the ratio of photoreceptors to ganglion cells falls dramatically. Most foveal receptors synapse on only one bipolar cell, which synapses on only one ganglion cell (Fig. 15-10A). Because each ganglion cell is devoted to a very small portion of the visual field, central vision has high resolution. In other words, the receptive field of a foveal ganglion cell (i.e., the region of stimulus space that can activate it) is small. At the periphery, the ratio of receptors to ganglion cells is high (Fig. 15-10B); thus, each ganglion cell has a large receptive field. The large receptive field reduces the spatial resolution of the peripheral portion of the retina but increases its sensitivity because more photoreceptors collect light for a ganglion cell. Foveal vision is purely cone mediated, and the sheet of foveal photoreceptors consists of only the smallest cones packed at the highest density (~0.3 μm from the center of one cone to another). Cone density falls to very low levels outside the fovea, and rod density rises. Peripheral vision (i.e., nonfoveal vision, or vision at visual angles more than 10 degrees away from the center of the fovea and thus the center of gaze) is mediated by both rods and cones.
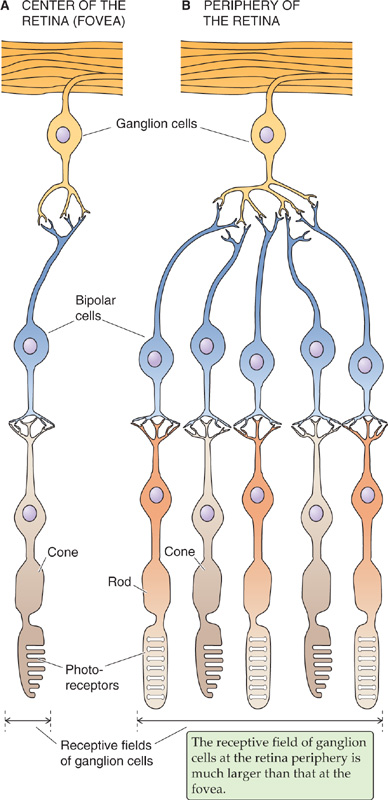
Figure 15-10 Comparison of receptive fields in the fovea and periphery of the retina.
Photoreceptors are elongated cells with synaptic terminals, an inner segment, and an outer segment (Fig. 15-9). The synaptic terminals connect to the inner segment by a short axon. The inner segment contains the nucleus and metabolic machinery; it synthesizes the photopigments and has a high density of mitochondria. The inner segment also serves an optical function—its high density funnels photons into the outer segment. A thin ciliary stalk connects the inner segment to the outer segment. The outer segment is the transduction site, although it is the last part of the cell to see the light. Structurally, the outer segment is a highly modified cilium. Each rod outer segment has ~1000 tightly packed stacks of disk membranes, which are flattened, membrane-bound intracellular organelles that have pinched off from the outer membrane. Cone outer segments have similarly stacked membranes, except that they are infolded and remain continuous with the outer membrane. The disk membranes contain the photopigments—rhodopsin in rods and molecules related to rhodopsin in cones. Rhodopsin moves from its synthesis site in the inner segment through the stalk and into the outer segment through small vesicles whose membranes are packed with rhodopsin to be incorporated into the disks.
The remarkable psychophysical experiments of Hecht and colleagues in 1942 demonstrated that five to seven photons, each acting on only a single rod, are sufficient to evoke a sensation of light in humans. Thus, the rod is performing at the edge of its physical limits because there is no light level smaller than one photon. To detect a single photon requires a prodigious feat of signal amplification. As Denis Baylor has pointed out, “the sensitivity of rod vision is so great that the energy needed to lift a sugar cube one centimeter, if converted to a blue-green light, would suffice to give an intense sensation of a flash to every human who ever existed.”
Phototransduction involves a cascade of chemical and electrical events to detect, to amplify, and to signal a response to light. As in many other sensory receptors, photoreceptors use electrical events (receptor potentials) to carry the visual signal from the outer segment to their synapses. Chemical messengers diffusing over such a distance would simply be too slow. The surprising fact about the receptor potential of rods and cones is that it is hyperpolarizing. Light causes the cell’s Vm to become more negative than the resting potential that it maintains in the dark (Fig. 15-11A). At low light intensities, the size of the receptor potential rises linearly with light intensity; but at higher intensities, the response saturates.
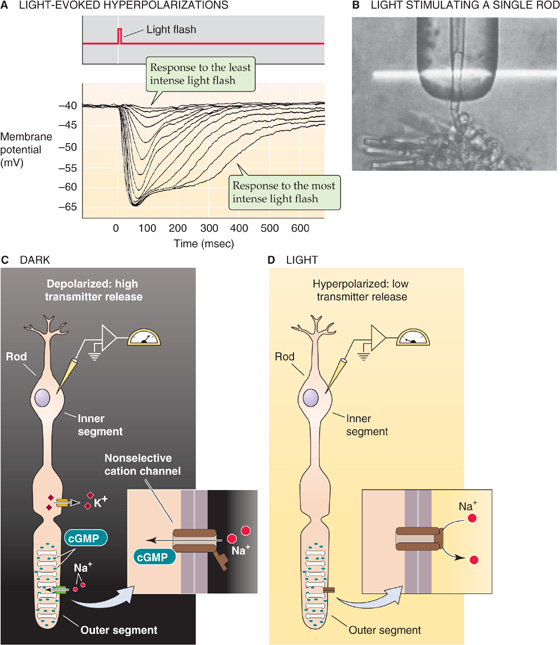
Figure 15-11 Phototransduction. A, The experiment summarized here was performed on a red-sensitive cone from a turtle. A brief flash of light causes a hyperpolarization of the photoreceptor cell. The size of the peak and the duration of the receptor potential increase with the increasing intensity of the flash. At low light intensities, the magnitude of the peak increases linearly with light intensity. At high intensities, the peak response saturates, but the plateau becomes longer. (Data from Baylor DA, Hodgkin AL, Lamb TD: The electrical response of turtle cones to flashes and steps of light. J Physiol 1974; 242:685-727.) B, A single rod has been sucked into a pipette, allowing the investigators to monitor the current. The horizontal white band is the light used to stimulate the rod. (Reproduced from Baylor DA, Lamb TD, Yau K-W: Responses of retinal rods to single photons. J Physiol [Lond] 1979; 288:613-634.) C, In the absence of light, Na+ enters the outer segment of the rod through cGMP-gated channels and depolarizes the cell. The electrical circuit for this dark current is completed by K+ leaving the inner segment. The dark current, which depolarizes the cell, leads to constant transmitter release. D, In the presence of light, Na+ can no longer enter the cell because cGMP levels are low, and the cGMP-gated channel closes. The photoreceptor cell thus hyperpolarizes, and transmitter release decreases.
Hyperpolarization is an essential step in relaying the visual signal because it directly modulates the rate of transmitter release from the photoreceptor onto its postsynaptic neurons. This synapse is conventional in that it releases more transmitter—in this case glutamate—when its presynaptic terminal is depolarized and less when it is hyperpolarized. Thus, a flash of light causes a decrease in transmitter secretion. The upshot is that the vertebrate photoreceptor is most active in the dark.
How is the light-induced hyperpolarization generated? Figure 15-11B shows a method to measure the current flowing across the membrane of the outer segment of a single rod. In the dark, each photoreceptor produces an ionic current that flows steadily into the outer segment and out of the inner segment. This dark current is carried mainly by inwardly directed Na+ ions in the outer segment and by outwardly directed K+ ions from the inner segment (Fig. 15-11C). Na+ flows through a nonselective cation channel of the outer segment, which light indirectly regulates, and K+ flows through a K+ channel in the inner segment, which light does not regulate. Na+ carries ~90% of the dark current in the outer segment, and Ca2+, ~10%. In the dark, Vm is about −40 mV. Na-K pumps, primarily located within the inner segments, remove the Na+ and import K+. A Na-Ca exchanger removes Ca2+ from the outer segment.
Absorption of photons leads to closure of the nonselective cation channels in the outer segment. The total conductance of the cell membrane decreases. Because the K+ channels of the inner segment remain open, K+ continues to flow out of the cell, and this outward current causes the cell to hyperpolarize (Fig. 15-11D). The number of cation channels that close depends on the number of photons that are absorbed. The range of one rod’s sensitivity is 1 to ~1000 photons. Cones are less sensitive, but they are faster than rods; moreover, cone responses do not saturate even at the brightest levels of natural light.
Baylor and colleagues measured the minimum amount of light required to produce a change in receptor current (Fig. 15-11B). They found that absorption of one photon suppresses a surprisingly large current, equivalent to the entry of more than 106 Na+ ions, and thus represents an enormous amplification of energy. At the peak of the response, this decrease in Na+ influx represents ~3% of the cell’s entire dark current. The single-photon response is also much larger than the background electrical noise in the rod, as it must be to produce the rod’s high sensitivity to dim light. Cones respond similarly to single photons, but they are inherently noisier and their response is only ~1/50 the size of that in the rod.
How can a single photon stop the flow of 1 million Na+ ions across the membrane of a rod cell? The process begins when the photon is absorbed by rhodopsin, the light receptor molecule. Rhodopsin is one of the most tightly packed proteins in the body, with a density of ~30,000 molecules per square micrometer in the disk membranes. Thus, the packing ratio is 1 protein molecule for every 60 lipid molecules! One rod contains ~109 rhodopsin molecules. This staggering density ensures an optimized capture rate for photons passing through a photoreceptor. Even so, only ~10% of the light entering the eye is used by the receptors. The rest is either absorbed by the optical components of the eye or passes between or through the receptors. Rhodopsin has two key components: retinal and the protein opsin. Retinal is the aldehyde of vitamin A, or retinol (~500 Da). Opsin is a single polypeptide (~41 kDa) with seven membrane-spanning segments (Fig. 15-12A). It is a member of the superfamily of GPCRs (see Chapter 3) that includes many neurotransmitter receptors as well as the odor receptor molecules.
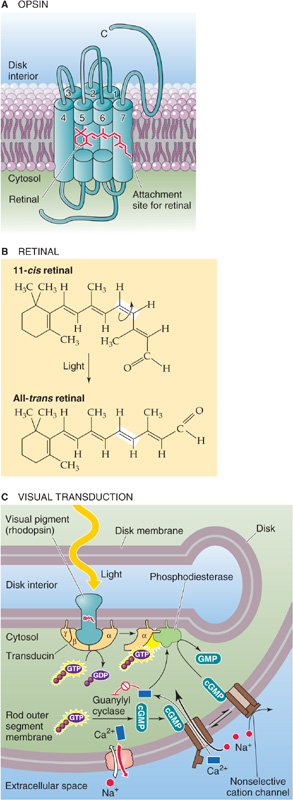
Figure 15-12 Rhodopsin, transducin, and signal transduction at the molecular level. A, The opsin molecule is a classic seven-transmembrane receptor that couples to transducin, a G protein. The attachment site of the retinal is amino acid residue 296 in the seventh (i.e., most C-terminal) membrane-spanning segment. B, The absorption of a photon by 11-cis retinal causes the molecule to isomerize to all-trans retinal. C, After rhodopsin absorbs a photon of light, it activates many transducins. The activated α subunit of transducin (Gαt) in turn activates phosphodiesterase, which hydrolyzes cGMP. The resultant decrease in [cGMP]i closes cGMP-gated channels and produces a hyperpolarization (receptor potential). GMP, 5′-guanylate monophosphate.
To be transduced, photons are actually absorbed by retinal, which is responsible for rhodopsin’s color. The tail of retinal can twist into a variety of geometric configurations, one of which is a kinked and unstable version called 11-cis retinal (Fig. 15-12B). The cis form sits within a pocket (comparable to the ligand binding site of other GPCRs) of the opsin and is covalently bound to it. However, because of its instability, the cis form can exist only in the dark. If 11-cis retinal absorbs a photon, it isomerizes within 1 picosecond to a straighter and more stable version called all-trans retinal. This isomerization in turn triggers a series of conformational changes in the opsin that lead to a form called metarhodopsin II, which can activate an attached molecule called transducin. Transducin carries the signal forward in the cascade and causes a reduction in Na+ conductance. Soon after isomerization, all-trans retinal and opsin separate in a process called bleaching; this separation causes the color to change from the rosy red (rhodon is Greek for the color “rose”) of rhodopsin to the pale yellow of opsin. The photoreceptor cell converts all-trans retinal to retinol (vitamin A), which then translocates to the pigment epithelium and becomes 11-cis retinal. This compound makes its way back to the outer segment, where it recombines with opsin. This cycle of rhodopsin regeneration takes a few minutes.
Transducin is so named because it transduces the light-activated signal from rhodopsin into the photoreceptor membrane’s response (Fig. 15-12C). Transducin was the first of the large family of guanosine triphosphate (GTP)–binding proteins (G proteins) to be identified, and its amino acid sequence is very similar to that of other GPCRs (see Chapter 3). When it is activated by metarhodopsin, the α subunit of transducin exchanges a bound guanosine diphosphate (GDP) for a GTP and then diffuses within the plane of the membrane to stimulate a phosphodiesterase that hydrolyzes cGMP to 5′-guanylate monophosphate.
cGMP is the diffusible second messenger that links the light-activated events of the disk membranes to the electrical events of the outer membrane. A key discovery by Fesenko and colleagues in 1985 showed that the “light-sensitive” cation channel of rods is actually a cGMP-gated cation channel (see Chapter 6). This cyclic nucleotide–gated channel was the first of its kind to be discovered (we have already discussed a similar channel in olfactory receptors). In the dark, a constitutively active guanylyl cyclase that synthesizes cGMP from GTP keeps cGMP levels high within the photoreceptor cytoplasm. This high [cGMP]i causes the cGMP-gated cation channels to spend much of their time open and accounts for the dark current (Fig. 15-11C). Because light stimulates the phosphodiesterase and thus decreases [cGMP]i, light reduces the number of open cGMP-gated cation channels and thus reduces the dark current. The photoreceptor then hyperpolarizes, transmitter release falls, and a visual signal is passed to retinal neurons.
Strong amplification occurs along the phototransduction pathway. The absorption of 1 photon activates 1 metarhodopsin molecule, which can activate ~700 transducin molecules within ~100 ms. These transducin molecules activate phosphodiesterase, which increases the rate of cGMP hydrolysis by ~100-fold. One photon leads to the hydrolysis of ~1400 cGMP molecules by the peak of the response, thus reducing [cGMP] by ~8% in the cytoplasm around the activated disk. This decrease in [cGMP]i closes ~230 of the 11,000 cGMP-gated channels that are open in the dark. As a result, the dark current falls by ~2%.
The cGMP-gated channel has additional interesting properties. It responds within milliseconds when [cGMP]i rises, and it does not desensitize in response to cGMP. The concentration-response curve is very steep at low [cGMP]i because opening requires the simultaneous binding of three cGMP molecules. Thus, the channel has switch-like behavior at physiological levels of cGMP. Ion conductance through the channel also has steep voltage dependence because Ca2+ and Mg2+ strongly block the channel (as well as permeate it) within its physiological voltage range. This open-channel block (see Fig. 7-20D) makes the normal single-channel conductance very small, among the smallest of any ion channel; the open channel normally carries a current of only 3 × 10−15 amperes (3 fA)! The current of ion channels is inherently “noisy” as they flicker open and closed. However, the 11,000 channels—each with currents of 3 fA—summate to a rather noise-free dark current of 11,000 channels × 3 fA per channel = 33 pA. In contrast, if 11 channels—each with currents of 3 pA—carried the dark current of 33 pA, the 2% change in this signal (0.66 pA) would be smaller than the noise produced by the opening and closing of a single channel (3 pA). Thus, the small channels give the photoreceptor a high signal-to-noise ratio.
The [cGMP]i in the photoreceptor cell represents a dynamic balance between the synthesis of cGMP by guanylyl cyclase and the breakdown of cGMP by phosphodiesterase. Ca2+, which enters through the relatively nonselective cGMP-gated channel, synergistically inhibits the guanylyl cyclase and stimulates the phosphodiesterase. These Ca2+ sensitivities set up a negative feedback system. In the dark, the incoming Ca2+ prevents runaway increases in [cGMP]i. In the light, the ensuing decrease in [Ca2+]i relieves the inhibition on guanylyl cyclase, inhibits the phosphodiesterase, increases [cGMP]i, and thus poises the system for channel reopening.
The process of termination of the light-activated state of the photoreceptor cell has not been as well defined as the activation process. One mechanism appears to involve the channels themselves. As described in the preceding paragraph, closure of the cGMP-gated channels in the light leads to a fall in [Ca2+]i, which helps replenish cGMP and facilitates channel reopening. Two additional mechanisms involve the proteins rhodopsin kinase and arrestin. Rhodopsin kinase phosphorylates light-activated rhodopsin and allows it to be recognized by arrestin. Arrestin, an abundant cytosolic protein, binds to the phosphorylated light-activated rhodopsin and helps terminate the activated state of the receptor.
The human eye can operate effectively over a 1010-fold range of light intensities, which is the equivalent of going from almost total darkness to bright sunlight on snow. However, moving from a bright to a dark environment, or vice versa, requires time for adaptation before the eye can respond optimally. Adaptation is mediated by several mechanisms. One mechanism mentioned earlier is regulation of the size of the pupil by the iris, which can change light sensitivity by ~16-fold. That still leaves the vast majority of the range to account for. During dark adaptation, two additional mechanisms with very different time courses are evident, as we can see from a test of the detection threshold for the human eye (Fig. 15-13). The first phase of adaptation is finished within ~10 minutes and is a property of the cones; the second takes at least 30 minutes and is attributed to the rods. A fully dark-adapted retina, relying on rods, can have a light threshold that is as much as 500 times lower than a retina relying on fully dark-adapted cones. In essence, then, the human eye has two retinas in one, a rod retina for low light levels and a cone retina for high light levels. These two systems can operate at the same time; when dark adapted, the rods can respond to the lowest light levels, but cones are available to respond when brighter stimuli appear.
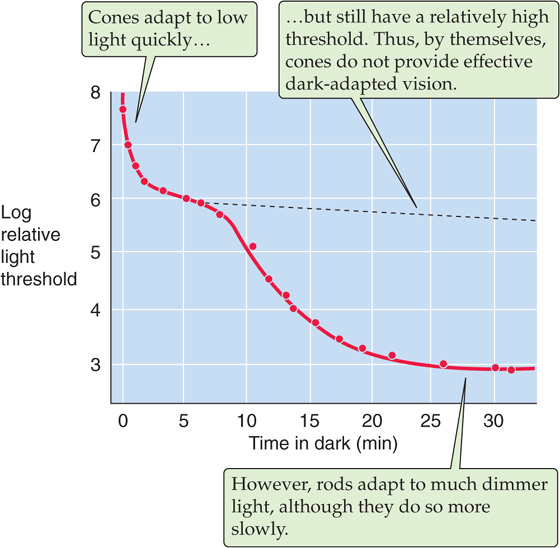
Figure 15-13 The effect of dark adaptation on the visual threshold. The subject was exposed to light at a level of 1600 millilumens and then switched to the dark. The graph is a plot of the time course of the subject’s relative threshold (on a log scale) for detecting a light stimulus. (Data from Hecht S, Shlaer S, Smith EL, et al: The visual functions of the complete color blind. J Gen Physiol 1948; 31:459-472.)
The rapid and slow phases of adaptation that are discussed in the preceding paragraph have both neural and photoreceptor mechanisms. The neural mechanisms are relatively fast, operate at relatively low ambient light levels, and involve multiple mechanisms within the neuronal network of the retina. The photoreceptor mechanisms involve some of the processes that are described in the previous section. Thus, in bright sunlight, rods become ineffective because most of their rhodopsin remains inactivated, or bleached. After returning to darkness, the rods slowly regenerate rhodopsin and become sensitive once again. However, a component of the cGMP system also regulates photoreceptor sensitivity. In the dark, when baseline [cGMP]i is relatively high, substantial amounts of Ca2+ enter through cGMP-gated channels. The resultant high [Ca2+]i inhibits guanylyl cyclase and stimulates phosphodiesterase, thereby preventing [cGMP]i from rising too high. Conversely, when background light levels are high, this same feedback system causes baseline [cGMP]i to remain high so that [cGMP]i can fall in response to further increases in light levels. Otherwise, the signal transduction system would become saturated. In other words, the photoreceptor adapts to the increased background light intensity and remains responsive to small changes. Additional adaptation mechanisms regulate the sensitivity of rhodopsin, guanylyl cyclase, and the cGMP-gated channel. Clearly, adaptation involves an intricate network of molecular interaction.
The human eye responds only to a small region of the electromagnetic spectrum (Fig. 15-5); but within it, we are exquisitely sensitive to the light’s wavelength. We see assorted colors in a daytime panorama because objects absorb some wavelengths while reflecting, refracting, or transmitting others. Different sources of light may also affect the colors of a scene; the light from tungsten bulbs is reddish, whereas that of fluorescent bulbs is bluish.
Research on color vision has a long history. In 1801, Thomas Young first outlined the trichromatic theory of color vision, which was championed later in the 19th century by Hermann von Helmholtz. These investigators found that they could reproduce a particular sample hue by mixing the correct intensities of three lights with the primary hues blue, green, and red. They proposed that color vision, with its wide range of distinct, perceived hues, is based on only three different pigments in the eye, each absorbing a different range of wavelengths. Microspectrophotometry of single cones in 1964 amply confirmed this scheme. Thus, although analysis of color by the human brain is sophisticated and complex, it all derives from the responses of only three types of photopigments in cones.
Our sensitivity to the wavelength of light depends on the retina’s state of adaptation. When it is dark adapted (also called scotopic conditions), the spectral sensitivity curve for human vision is shifted toward shorter wavelengths compared with the curve obtained after light adaptation (photopic conditions; Fig. 15-14A). The absolute sensitivity to light can also be several orders of magnitude higher under scotopic conditions (Fig. 15-13). The primary reason for the difference in these curves is that rods are doing the transduction of dim light under dark-adapted conditions, whereas cones transduce in the light-adapted eye. As we would predict, the spectral sensitivity curve for scotopic vision is quite similar to the absorption spectrum of the rods’ rhodopsin, with a peak at 500 nm.
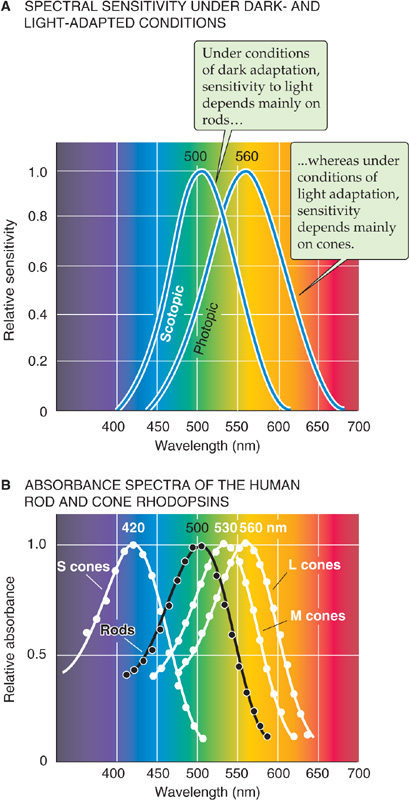
Figure 15-14 Sensitivity of vision and photoreceptors at different wavelengths of light. A, The figure shows the results of a psychophysical experiment. Under dark-adapted (scotopic) conditions, the eye is maximally sensitive at ~500 nm. Under light-adapted (photopic) conditions, the human eye is maximally sensitive at ~560 nm. (Data from Knowles A: The biochemical aspects of vision. In Barlow HB, Mollon JD [eds]: The Senses, pp 82-101. Cambridge: Cambridge University Press, 1982.) B, The spectral sensitivity of rods (obtained with a spectrophotometer) peaks at ~500 nm; that of the three types of cones peaks at ~420 nm for the S (blue), ~530 nm for the M (green), and ~560 nm for the L (red). The absorbance spectrum for each type of cone has been normalized to its peak sensitivity. (Data from Dartnell HJ, Bowmaker JK, Mollon JD: Microspectrophotometry of human photoreceptors. In Mollon JD, Sharpe LT [eds]: Colour Vision, pp 69-80. London: Academic Press, 1983.)
The spectral sensitivity of the light-adapted eye depends on the photopigments in the cones. Humans have three different kinds of cones, and each expresses a photopigment with a different absorbance spectrum. The peaks of their absorbance curves fall at ~420, 530, and 560 nm, which correspond to the violet, yellow-green, and yellow-red regions of the spectrum (Fig. 15-14B). The three cones and their pigments were historically called blue, green, and red, respectively. They are now more commonly called S, M, and L (for short, medium, and long wavelengths); we use this terminology. Because the absolute sensitivity of the short-wavelength cone is only one tenth that of the other two, the spectral sensitivity of photopic human vision is dominated by the two longer wavelength cones (compare the spectral sensitivity functions in Fig. 15-14A with the absorbance spectra of the cones in Fig. 15-14B).
Single cones do not encode the wavelength of a light stimulus. If a cone responds to a photon, it generates the same response regardless of the wavelength of that photon. A glance at Figure 15-14B shows that each type of cone pigment can absorb a wide range of wavelengths. The pigment in a cone is more likely to absorb photons when their wavelength is at its peak absorbance, but light hitting the cone on the fringe of its absorbance range can still generate a large response if the light’s intensity is sufficiently high. This property of response univariance is the reason that vision in an eye with only one functioning pigment (e.g., scotopic vision using only rods) can only be monochromatic. With a single pigment system, the distinction between different colors and between differences in intensity is confounded. Two different cones (as in most New World monkeys), each with a different but overlapping range of wavelength sensitivity, remove much of the ambiguity in encoding the wavelength of light stimuli. With three overlapping pigments (as in Old World monkeys and humans), light of a single wavelength stimulates each of the three cones to different degrees, and light of any other wavelength stimulates these cones with a distinctly different pattern. Because the nervous system can compare the relative stimulation of the three cone types to decode the wavelength, it can also distinguish changes in the intensity (luminance) of the light from changes in its wavelength.
Color capabilities are not constant across the retina. The use of multiple cones is not compatible with fine spatial discrimination because of wavelength-dependent differences in the eye’s ability to focus light (chromatic aberration) and because very small objects may stimulate only single cones. The fovea has only M and L cones, which limits its color discrimination in comparison to the peripheral portions of the retina but leaves it best adapted to discriminate fine spatial detail.
The four different human visual pigments have a similar structure. The presence of retinal and the mechanisms of its photoisomerization are essentially identical in each. The main difference is the primary structure of the attached protein, the opsin. M and L opsins share 96% of their amino acids. Pairwise comparisons among the other opsins show only 44% or lower sequence similarity, however. Apparently, the different amino acid structures of the opsins affect their charge distributions in the region of the 11-cis retinal and shift its absorption spectrum to give the different pigments their specific spectral sensitivities.
Inherited Defects in Color Vision
Inherited defects in color vision are relatively common, and many are caused by mutations in visual pigment genes. For example, 8% of white males and 1% of white females have some defect in their L or M pigments caused by X-linked recessive mutations. A single abnormal pigment can lead to either dichromacy (the absence of one functional pigment) or anomalous trichromacy (the absorption spectrum of one pigment shifted relative to normal), often with a consequent inability to distinguish certain colors. Jeremy Nathans and colleagues found that men have only one copy of the L pigment gene; but located right next to it on the X chromosome, they may have one to three copies of the M pigment gene. He proposed that homologous recombination could account for the gene duplication, loss of a gene, or production of the hybrid L-M genes that occur in red-green color blindness. Hybrid L-M pigments have spectral properties intermediate between those of the two normal pigments, probably because their opsins consist of a combination of the traits of the two normal pigments.
Lack of two of the three functional cone pigments leads to monochromacy. The number of people who have such true color blindness is very small, less than 0.001% of the population. For example, S-cone monochromacy is a rare X-linked disorder in which both L and M photopigments are missing because of mutations on the X chromosome. The S pigment is on chromosome 7.
Balancing on one foot and listening to music both involve sensory systems that have similar transduction mechanisms. Sensation in both the vestibular and auditory systems begins with the inner ear, and both use a highly specialized kind of receptor called the hair cell. Common structure and function often suggest a common origin, and indeed, the organs of mammalian hearing and balance both evolved from the lateral line organs present in all aquatic vertebrates. The lateral line consists of a series of pits or tubes along the flanks of an animal. Within each indentation are clusters of sensory cells that are similar to hair cells. These cells have microvilli-like structures that project into a gelatinous material that in turn is in contact with the water in which the animal swims. The lateral line is exquisitely sensitive to vibrations or pressure changes in the water in many animals, although it is also sensitive to temperature or electrical fields in some species. Reptiles abandoned the lateral line during their evolution, but they retained the hair-cell–centered sensory structures of the inner ear that evolved from the lateral line.
The vestibular system generates our sense of balance, and the auditory system provides our senses of hearing. Vestibular sensation operates constantly while we are awake and communicates to the brain the head’s orientation and changes in the head’s motion. Such information is essential for generation of muscle contractions that will put our body where we want it to be, to reorient the body when something pushes us aside (vestibular-spinal reflexes), and to move our eyes continually so that the visual world stays fixed on our retinas even though our head may be nodding about (vestibular-ocular reflexes). Vestibular dysfunction can make it impossible to stabilize an image on our moving retinas, and it causes the disconcerting feeling that the world is uncontrollably moving around—vertigo. Walking and standing can be difficult or impossible. With time, compensatory adjustments are made as the brain learns to substitute more visual and proprioceptive cues to help guide smooth and accurate movements. (See Note: Vestibulo-Ocular Reflexes)
Auditory sensation is often at the forefront of our conscious experience, unlike vestibular information, which we rarely notice unless something goes wrong. Hearing is an exceptionally versatile process that allows us to detect things in our environment, to precisely identify their nature, to localize them well at a distance, and, through language, to communicate with speed, complexity, nuance, and emotion.
Hair cells are mechanoreceptors that are specialized to detect minuscule movement along one particular axis. The hair cell is an epithelial cell; the hair bundles project from the apical end, whereas synaptic contacts occur at the basal end. Hair cells are somewhat different in the vestibular and auditory systems. In this section, we illustrate concepts mainly with the vestibular hair cell (Fig. 15-15A), which comes in two subtypes. Vestibular type I cells have a bulbous basal area, surrounded by a calyx-shaped afferent nerve terminal (Fig. 15-15B, left). Vestibular type II hair cells are more cylindrical and have several simple, bouton-shaped afferent nerve terminals (Fig. 15-15B, right). As we will see, auditory hair cells also come in two varieties, inner hair cells and outer hair cells. However, all hair cells sense movement in basically the same way.
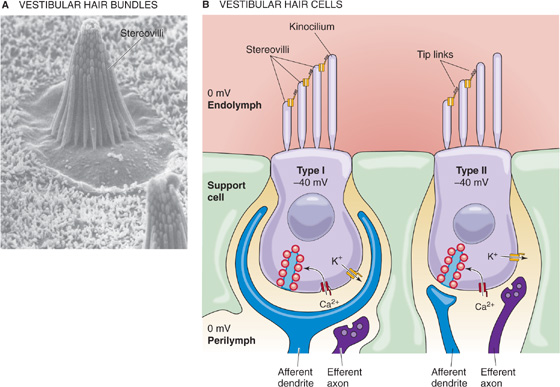
Figure 15-15 Vestibular hair cells. A, Scanning electron micrograph of a bullfrog hair cell from the sensory epithelium of the saccule. (From Corey DP, Assad JA: In Corey DP, Roper SD [eds]: Sensory Transduction. New York: Rockefeller University Press, 1992.) B, Type I and type II cells. (Data from Philine Wangemann, Kansas State University.)
As part of their hair bundles, vestibular hair cells (Fig. 15-15B) have one large kinocilium, which is a true cilium with the characteristic 9 + 2 pattern of microtubules (see Fig. 2-11A). The role of the kinocilium is unknown. In mammals, auditory hair cells lose their kinocilium with maturity.
Both vestibular and auditory hair cells have 50 to 150 stereovilli, which are filled with actin and are more akin to microvilli. The stereovilli—often called stereocilia, although they lack the typical 9 + 2 pattern of true cilia—are 0.2 to 0.8 μm in diameter and are generally 4 to 10 μm in height. These “hairs” are arranged in a neat array. In the vestibular system, the kinocilium stands tallest along one side of the bundle and the stereovilli fall away in height to the opposite side (Fig. 15-15B). Stereovilli are narrower at their base and insert into the apical membrane of the hair cell, where they make a sort of hinge before connecting to a cuticular plate. Within the bundle, stereovilli are connected one to the next, but they can slide with respect to each other as the bundle is deflected side to side. The ends of the stereovilli are interconnected with very fine strands called tip links, which are visible by electron microscopy.
The epithelium of which the hair cells are a part separates perilymph from endolymph. The perilymph bathes the basolateral side of the hair cells. In composition (i.e., relatively low [K+], high [Na+]), perilymph is similar to cerebrospinal fluid. Its voltage is zero—close to that of most other extracellular fluids in the body. The basolateral resting potential of vestibular hair cells and auditory inner hair cells is about −40 mV (Fig. 15-15B). The endolymph bathing the stereovilli is singular in composition. It has a very high [K+] (150 mM) and a very low [Na+] (1 mM), more like cytoplasm than extracellular fluid. It also has a relatively high [HCO−3] (30 mM). The voltage of the vestibular endolymph is ~0 mV relative to perilymph. Across the apical membrane of vestibular hair cells, the chemical gradient for K+ is small. However, the electrical gradient is fairly large, ~40 mV. Thus, a substantial force tends to drive K+ into the vestibular hair cell across the apical membrane. Later, we will see that the driving force for K+ influx is even higher in the auditory system.
The appropriate stimulus for a hair cell is the bending of its hairs, but not just any deflection will do. Bending of the hair bundle toward the longer stereovilli (Fig. 15-16A) excites the cell and causes a depolarizing receptor potential. Bending of the hair bundle away from the longer stereovilli (Fig. 15-16B) hyperpolarizes the cell. Only tiny movements are needed. In auditory hair cells, as little as 0.5 nm (which is the diameter of a large atom) gives a detectable response, and the response is saturated at ~150 nm, about the diameter of one stereovillus! In fact, the sensitivity of hair cells is limited only by noise from the brownian motion of surrounding molecules. The cell is also exquisitely selective to direction. If the hairs are bent along the axis 90 degrees to their preferred direction, they are less than one tenth as responsive.
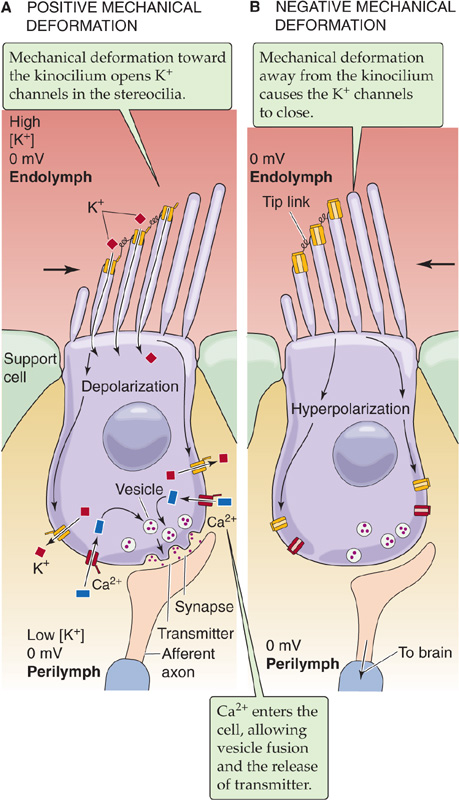
Figure 15-16 Mechanotransduction in the hair cell. A, At rest, a small amount of K+ leaks into the cells, driven by the negative membrane potential and high apical [K+]. Mechanical deformation of the hair bundle toward the longer stereovilli increases the opening of nonselective cation channels at the tips of the stereovilli, allowing K+ influx, depolarizing the cell. In all hair cells except the auditory outer hair cells, the depolarization activates voltage-sensitive Ca2+ channels on the basal membrane, causing release of synaptic vesicles and stimulating the postsynaptic membrane of the accompanying sensory neuron. B, Mechanical deformation of the hair bundle away from the longer stereovilli causes the nonselective cation channels to close, leading to hyperpolarization.
Mechanotransduction in hair cells seems to be accomplished by directly linking the movement of the stereovilli to the gating of apical mechanosensitive cation channels. Electrical measurements, as well as the imaging of intracellular Ca2+, imply that the transduction channels are located near the tips of the stereovilli. How is channel gating connected to movement of the hairs? The latency of channel opening is extremely short, less than 40 μs. If one deflects the hairs more rapidly, the channels are activated more quickly. This observation suggests a direct, physical coupling inasmuch as diffusion of a second messenger would take much longer. Corey and Hudspeth have suggested a spring-like molecular linkage between the movement of stereovilli and channel gating. Indirect evidence suggests that the tip links may be the tethers between stereovilli and the channels. Brief exposure to low-Ca2+ solutions abolishes transduction, and it also destroys the tip links without otherwise causing obvious harm to the cells.
The mechanosensitive channels at the tips of the stereovilli are relatively large (~100 pS each) and unselective in that they allow monovalent cations and some divalent cations, including Ca2+, to pass easily. Each hair cell has fewer than 100 channels. Under physiological conditions, K+ carries most of the current. When the cell is at rest—hairs straight up—a small but steady leak of depolarizing K+ current flows through the cell. This leak allows the hair cell to respond to both positive and negative deflections of its stereovilli. A positive deflection—toward the tallest stereovilli—further opens the apical channels, leading to influx of K+ and thus depolarization. K+ leaves the cell through mechano in sensitive K+ channels on the basolateral side (Fig. 15-16A), along a favorable electrochemical gradient. A negative deflection closes the apical channels and thus leads to hyperpolarization (Fig. 15-16B).
The mechanosensitive channel in hair cells seems to be a member of the transient receptor potential (TRP) superfamily of ion channels, specifically the TRPA1 channel expressed at the tips of stereovilli. Knocking down TRPA1 abolishes hair cell transduction. The TRPA1 protein has a long chain of ankyrin repeats leading up to the channel domain. The ankyrin repeats may be part of a “gating spring” that is observed in biophysical studies of channel gating.
A hair cell is not a neuron. Hair cells do not project an axon of their own, and most do not generate action potentials. Instead—in the case of vestibular hair cells and auditory inner hair cells—the membrane near the presynaptic (i.e., basolateral) face of the cell has voltage-gated Ca2+ channels that are somewhat active at rest but more active during mechanically induced depolarization (i.e., the receptor potential) of the hair cell. The Ca2+ that enters the hair cell through these channels triggers the release of glutamate as well as aspartate in the case of vestibular hair cells. These excitatory transmitters stimulate the postsynaptic terminal of sensory neurons that transmit information to the brain. The greater the transmitter release, the greater the rate of action potential firing in the postsynaptic axon.
In mammals, all hair cells—whether part of the vestibular or auditory system—are contained within bilateral sets of interconnected tubes and chambers called, appropriately enough, the membranous labyrinth (Fig. 15-17A, B). The vestibular portion has five sensory structures: two otolithic organs, which detect gravity (i.e., head position) and linear head movements, and three semicircular canals, which detect head rotation. Also contributing to our sense of spatial orientation and motion are proprioceptors and the visual system (see later). The auditory portion of the labyrinth is the spiraling cochlea, which detects rapid vibrations (sound) transmitted to it from the surrounding air. (See Note: Spatial Orientation)
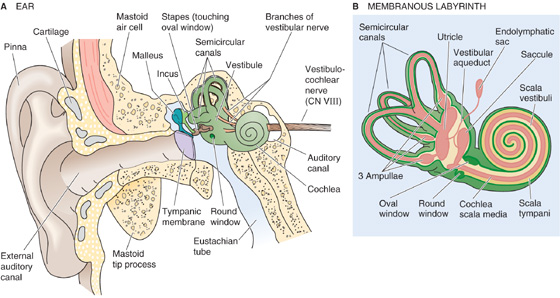
Figure 15-17 The ear, cochlea, and semicircular canals. A, This section through the right ear of a human shows the outer, the middle, and the inner ear. B, The labyrinth consists of an auditory and a vestibular portion. The auditory portion is the cochlea. The vestibular portion includes the two otolithic organs (the utricle and saccule) and the three semicircular canals.
The ultimate function of each of these sensory structures is to transmit mechanical energy to their hair cells. In each case, transduction occurs in the manner described earlier. The specificity of the transduction process depends much less on the hair cells than on the structure of the labyrinth organs around them.
The otolithic organs are a pair of relatively large chambers—the saccule and the utricle—near the center of the labyrinth (Fig. 15-17B). These otolithic organs as well as the semicircular canals are (1) lined by epithelial cells, (2) filled with endolymph, (3) surrounded by perilymph, and (4) encased in the temporal bone. Within the epithelium, specialized vestibular dark cells secrete K+ and are responsible for the high [K+] of the endolymph. The mechanism of K+ secretion is similar to that by the stria vascularis in the auditory system (see later).
The saccule and utricle each has a sensory epithelium called the macula, which contains the hair cells that lie among a bed of supporting cells. The stereovilli project into the gelatinous otolithic membrane, a mass of mucopolysaccharides that is studded with otoliths or otoconia (Fig. 15-18A, B). These crystals of calcium carbonate, 1 to 5 μm in diameter, give the otolithic membrane a higher density than the surrounding endolymph. With either a change in the angle of the head or a linear acceleration, the inertia of the otoconia causes the otolithic membrane to move slightly, deflecting the stereovilli.
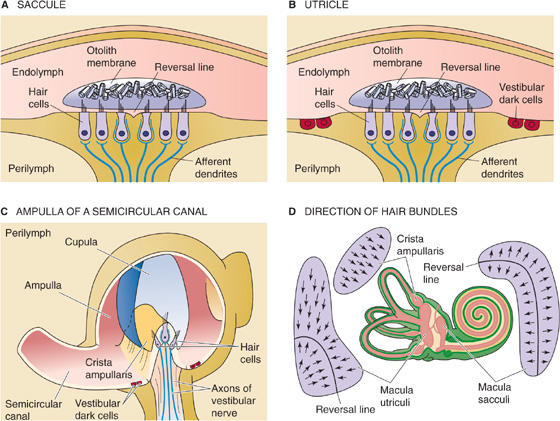
Figure 15-18 Vestibular sensory organs. In A, the longer stereovilli point toward the reversal line. In B, the longer stereovilli point away from the reversal line. In C, all stereovilli point in the same direction. In D, the arrows point toward the longer stereovilli (and kinocilia) and thereby indicate the directions of greatest sensitivity for opening of the transduction channels. (Data from Philine Wangemann, Kansas State University.)
The macula is vertically oriented (in the sagittal plane) within the saccule and horizontally oriented within the utricle when the head is tilted down by ~25 degrees, as during walking. In the saccule, the kinocilia point away from a curving reversal line that divides the macula into two regions (Fig. 15-18A and D). In the utricle, the kinocilia point toward the reversal line (Fig. 15-18B and D). The saccule and utricle respond well to changes in head angle and to acceleration of the sort that you experience as a car or an elevator starts or stops. Of course, the head can tilt or experience acceleration in many directions. Indeed, the orientation of hair cells of the saccule and utricle covers a full range of directions. Any tilt or linear acceleration of the head will enhance the stimulation of some hair cells, reduce the stimulation of others, and have no effect on the rest.
Each hair cell synapses on the ending of a primary sensory axon that is part of the vestibular nerve, which in turn is a branch of the vestibulocochlear nerve (CN VIII). The cell bodies of these sensory neurons are located in Scarpa’s ganglion within the temporal bone. The dendrites project to multiple hair cells, increasing the signal-to-noise ratio. The axons project to the ipsilateral vestibular nucleus in the brainstem. Because the saccule and utricle are paired structures (one on each side of the head), the CNS can simultaneously use information encoded by the full population of otolithic hair cells and unambiguously interpret any angle of tilt or linear acceleration. The push-pull arrangement of increased/decreased activity within each macula (for hair cells of opposite orientation) and between maculae on either side of the head enhances the fidelity of the signal. (See Note: Vestibular Innervation)
Semicircular canals (Fig. 15-17B) also sense acceleration, but not the linear acceleration that the otolithic organs prefer. Angular acceleration generated by sudden head rotations is the primary stimulus for the semicircular canals. Shake your head side to side or nod it up and down. Each rotation of your head will excite some of your canals and inhibit others.
The semicircular canals stimulate their hair cells differently from the otolithic organs. In each canal, the hair cells are clustered within a sensory epithelium (the crista ampullaris) that is located in a bulge along the canal called the ampulla (Fig. 15-18C). The hair bundles—all of which have the same orientation—project into a gelatinous, dome-shaped structure called the cupula, which spans the lumen of the ampulla. The cupula contains no otoconia, and its mucopolysaccharides have the same density as the surrounding endolymph. Thus, the cupula is not sensitive to linear acceleration. However, with a sudden rotation of the canal, the endolymph tends to stay behind because of its inertia. The relatively stagnant endolymph exerts a force on the movable cupula, much like wind on a sail. This force bows the cupula, which bends the hairs and (depending on the direction of rotation) either excites or suppresses the release of transmitter from the hair cells onto the sensory axons of the vestibular nerve. This arrangement makes the semicircular canals very sensitive to angular acceleration of the head. If head rotation is maintained at a constant velocity, the friction of endolymph with the canal walls eventually makes the two move together, so that the bending of the cupula gradually extinguishes within seconds. When rotation is then stopped, the inertia of the endolymph causes bending of the cupula in the other direction and thus gives a temporary sensation of counterrotation. (See Note: Head Rotation and the Vestibular-Ocular Reflex Vestibulo-Ocular Reflexes)
Each side of the head has three semicircular canals that lie in approximately orthogonal planes. The anterior canal is tilted ~41 degrees anterolaterally from the sagittal plane, the posterior canal is tilted ~56 degrees posterolaterally from the sagittal plane, and the lateral canal is tipped ~25 degrees back from the horizontal plane. Because each canal best senses rotation about a particular axis, the three together give a good representation of all possible angles of head rotation. This complete representation is further ensured because each canal is paired with another on the opposite side of the head. Each member of a pair sits within the same plane and responds to rotation about the same axis. However, whereas rotation excites the hair cells of one canal, it inhibits the canals of its contralateral axis mate. This push-pull arrangement presumably increases the sensitivity of detection.
Sound is a perceptual phenomenon that is produced by periodic longitudinal waves of low pressure (rarefactions) and high pressure (compressions) that propagate through air at a speed of 330 to 340 m/s. Absolute sound intensity is the amplitude of the longitudinal wave, measured in pascal (Pa). Intensities of audible sounds are commonly expressed in decibel sound pressure level (dB SPL), which relates the absolute sound intensity (PT) to a reference pressure (Pref) of 20 μPa, close to the average human threshold at 2000 Hz.
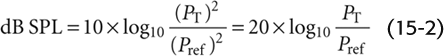
The logarithmic scale compresses the wide extent of sound pressures into a convenient range. An increase of 6 dB SPL corresponds to a doubling of the absolute sound pressure level; an increase of 20 dB SPL corresponds to a 10-fold increase.
Sound can be a pure tone of a single frequency, measured in hertz (Hz). Sounds produced by musical instruments or the human voice consist of a perceived fundamental frequency (pitch) and overtones. Sound that is noise contains no recognizable periodic elements. Pure tones are used clinically for the determination of hearing thresholds (pure-tone audiogram). Humans do not perceive as equally loud sounds of the same sound pressure level but different frequency. The psychoacoustic phon scale accounts for these differences in perception. (See Note: Sound Waveforms; Phon Scale)
Sound waves vary in frequency, amplitude, and direction; our auditory systems are specialized to discriminate all three. We can also interpret the rapid and intricate temporal patterns of sound frequency and amplitude that constitute words and music. Encoding of sound frequency and amplitude begins with mechanisms in the cochlea, followed by further analysis in the CNS. To distinguish the direction of a sound along the horizontal plane, the brain compares signals from the two ears.
All mammalian ears are strikingly similar in structure. The ear is traditionally divided into outer, middle, and inner components (Fig. 15-17A). We discuss the outer and middle ear here. The inner ear consists of the membranous labyrinth, with both its vestibular and auditory components.
Outer Ear Proceeding from outside to inside, the part most visible is the pinna, a skin-covered flap of cartilage, and its small extension, the tragus. Together, they funnel sound waves into the external auditory canal. These structures, which compose the outer ear, focus sound waves on the tympanic membrane. Many animals (e.g., cats) can turn each pinna independently to facilitate hearing without changing head position. The shape of the pinna and tragus tends to emphasize certain sound frequencies over others, depending on their angle of incidence. The external ear parts in humans are essential for localization of sounds in the vertical plane. Sound enters the auditory canal both directly and after being reflected; the sound that we hear is a combination of the two. Depending on a sound’s angle of elevation, it is reflected differently off the pinna and tragus. Thus, we hear a sound coming from above our head slightly differently than a sound coming from straight in front of us.
The external auditory canal is lined with skin and penetrates ~2.5 cm into the temporal bone, where it ends blindly at the eardrum (or tympanic membrane). Sound causes the tympanic membrane to vibrate, much like the head of a drum.
Middle Ear The air-filled chamber between the tympanic membrane on one side and the oval window on the other is the middle ear (Fig. 15-17A). The eustachian tube connects the middle ear to the nasopharynx and makes it possible to equalize the air pressure on opposite sides of the tympanic membrane. The eustachian tube can also provide a path for throat infections and epithelial inflammation to invade the middle ear, leading to otitis media. The primary function of the middle ear is to transfer vibrations of the tympanic membrane to the oval window (Fig. 15-19). The key to accomplishing this task is a chain of three delicate bones called ossicles: the malleus (or hammer), incus (anvil), and stapes (stirrup). The ossicles are the smallest bones in the body.
Figure 15-19 The middle ear. Displacement of the stapes and the oval window moves fluid in the scala vestibuli, causing opposite fluid movement in the scala tympani and thus an opposite displacement of the round window. (Data from Philine Wangemann, Kansas State University.)
Vibration transfer is not as simple as it might seem because sound starts as a set of pressure waves in the air (within the ear canal) and ends up as pressure waves in a watery cochlear fluid within the inner ear. Air and water have a very different acoustic impedance, which is the tendency of each medium to oppose movement brought about by a pressure wave. This impedance mismatch means that sound traveling directly from air to water has insufficient pressure to move the dense water molecules. Instead, without some system of compensation, more than 97% of a sound’s energy would be reflected when it met a surface of water. The middle ear serves as an impedance-matching device that saves most of the aforementioned energy by two primary methods. First, the tympanic membrane has an area that is ~20-fold larger than that of the oval window, so a given pressure at the air side (the tympanic membrane) is amplified as it is transferred to the water side (the footplate of the stapes). Second, the malleus and incus act as a lever system, again amplifying the pressure of the wave. Rather than being reflected, most of the energy is successfully transferred to the liquids of the inner ear. (See Note: Acoustic Impedance)
Two tiny muscles of the middle ear, the tensor tympani and the stapedius, insert onto the malleus and the stapes, respectively. These muscles exert some control over the stiffness of the ossicular chain, and their contraction serves to dampen the transfer of sound to the inner ear. They are reflexively activated when ambient sound levels become high. These reflexes are probably protective and may be particularly important for suppression of self-produced sounds, such as the roar you produce in your head when you speak or chew.
The auditory portion of the inner ear is mainly the cochlea, a tubular structure that is ~35 mm long and coiled 2.5 times into a snail shape about the size of a large pea (Fig. 15-20). Counting its stereovilli, the cochlea has a million moving parts, making it the most complex mechanical apparatus in the body.
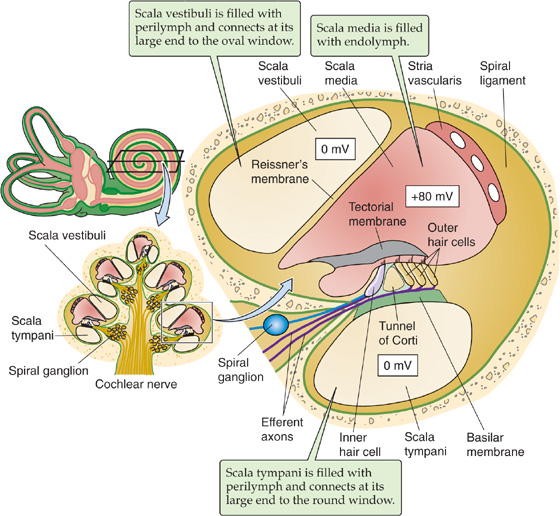
Figure 15-20 The cochlea. Reissner’s membrane and the basilar membrane divide the cochlea into three spiraling fluid-filled compartments: the scala vestibuli, the scala media, and the scala tympani. (Data from Philine Wangemann, Kansas State University.)
The cut through the cochlea in the lower left panel of Figure 15-20 reveals five cross sections of the spiral. We see that in each cross section, two membranes divide the cochlea into three fluid-filled compartments. On one side is the compartment called the scala vestibuli (Fig. 15-20, right panel), which begins at its large end near the oval window—where vibrations enter the inner ear. Reissner’s membrane separates the scala vestibuli from the middle compartment, the scala media. The other boundary of the scala media is the basilar membrane, on which rides the organ of Corti and its hair cells. Below the basilar membrane is the scala tympani, which terminates at its basal or large end at the round window. Both the oval and round windows look into the middle ear.
Both the scala vestibuli and scala tympani are lined by a network of fibrocytes and filled with perilymph. Like its counterpart in the vestibular system, this perilymph is akin to cerebrospinal fluid (i.e., low [K+], high [Na+]). Along the lengths of scala vestibuli and scala tympani, the two perilymphs communicate through the leaky interstitial fluid spaces between the fibrocytes. At the apex of the cochlea, the two perilymphs communicate through a small opening called the helicotrema. Cochlear perilymph communicates with vestibular perilymph through a wide passage at the base of the scala vestibuli (Fig. 15-17B), and it communicates with the cerebrospinal fluid through the cochlear aqueduct.
The scala media is filled with endolymph. Like its vestibular counterpart—with which it communicates through the ductus reuniens (Fig. 15-17B)—auditory endolymph is extremely rich in K+. Unlike vestibular endolymph, which has the same voltage as the perilymph, auditory endolymph has a voltage of +80 mV relative to the perilymph (Fig. 15-20, right panel). This endocochlear potential, which is the highest transepithelial voltage in the body, is the main driving force for sensory transduction in both inner and outer hair cells. Moreover, loss of the endocochlear potential is a frequent cause of hearing loss. A highly vascularized tissue called the stria vascularis secretes the K+ into the scala media, and the K+ gradient between endolymph and perilymph generate the endocochlear potential.
The stria vascularis is functionally a two-layered epithelium (Fig. 15-21). Marginal cells separate endolymph from a very small intrastrial compartment inside the stria vascularis, and basal cells separate the intrastrial compartment from the interstitial fluid of the spiral ligament, which is contiguous with perilymph. Gap junctions connect one side of the basal cells to intermediate cells and the other side of the basal cells to fibrocytes of the spiral ligament. This architecture is essential for generation of the endocochlear potential.
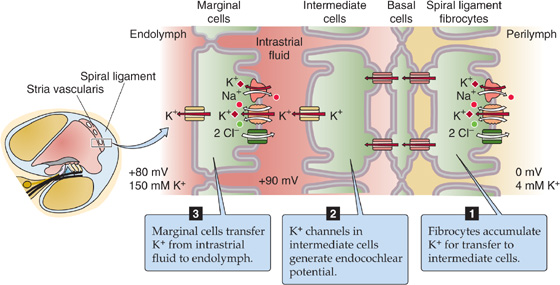
Figure 15-21 K+ secretion into the endolymph by the stria vascularis. (Data from Philine Wangemann, Kansas State University.)
The fibrocytes are endowed with K+ uptake mechanisms that maintain a high [K+]i in the intermediate cells. The KCNJ10 K+ channel of the intermediate cells generates the endocochlear potential. The K+ equilibrium potential of these cells is extremely negative because of the combination of their very high [K+]i and the very low [K+] of the intrastrial fluid. Finally, the marginal cells support the endocochlear potential by mopping up the K+ from the intrastrial fluid—keeping the intrastrial [K+] very low—and depositing the K+ in the endolymph through a KCNQ1 K+ channel.
The business end of the cochlea is the organ of Corti, the portion of the basilar membrane that contains the hair cells. The organ of Corti stretches the length of the basilar membrane and has four rows of hair cells: one row of ~3500 inner hair cells and three rows with a total of ~16,000 outer hair cells (Fig. 15-22). In the auditory system, the arrangement of stereovilli is also quite orderly. The hair cells lie within a matrix of supporting cells, with their apical ends facing the endolymph of the scala media (Fig. 15-23A). The stereovilli of inner hair cells (Fig. 15-23B) are unique in that they float freely in the endolymph. The stereovilli of the outer hair cells (Fig. 15-23C) project into the gelatinous, collagen-containing tectorial membrane. The tectorial membrane is firmly attached only along one edge, with a sort of hinge, so that it is free to tilt up and down. (See Note: Alfonso Giacomo Gaspare Corti)
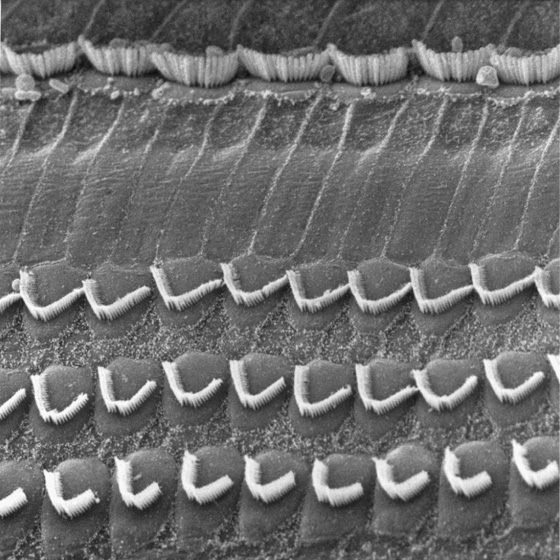
Figure 15-22 Hair cells of the cochlea. A scanning electron microscopic image of outer and inner hair cells of the organ of Corti of a chinchilla after removal of the tectorial membrane. The three rows of outer hair cells are on the bottom, and the single row of inner hair cells is along the top. (Courtesy of I. Hunter-Duvar, MD, and R. Harrison, PhD, DSc, The Hospital for Sick Children, Toronto, Canada.)
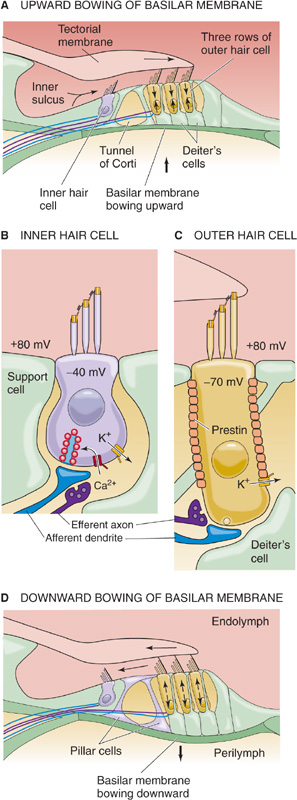
Figure 15-23 Organ of Corti. A, Upward movement of basilar membrane tilts hair bundles toward longer stereovilli, opening transduction channels. B, In inner hair cells, depolarization causes enhanced transmitter release. C, In outer hair cells, depolarization causes prestin to contract. D, Downward movement of basilar membrane tilts hair bundles away from longer stereovilli, closing transduction channels. (Data from Philine Wangemann, Kansas State University.)
How do air pressure waves actually stimulate the auditory hair cells? Movements of the stapes against the oval window create traveling pressure waves within the cochlear fluids. Consider, for example, what happens as sound pressure falls in the outer ear.
Step 1: Stapes moves outward. As a result, the oval window moves outward, causing pressure in the scala vestibuli to decrease. Because the perilymph that fills the scala vestibuli and scala tympani is incompressible and the cochlea is encased in rigid bone, the round window moves inward (Fig. 15-19B).
Step 2: Scala vestibuli pressure falls below scala tympani pressure.
Step 3: Basilar membrane bows upward. Because Reissner’s membrane is very thin and flexible, the low scala vestibuli pressure pulls up the incompressible scala media, which in turn causes the basilar membrane (and the organ of Corti) to bow upward.
Step 4: Organ of Corti shears toward hinge of tectorial membrane. The upward bowing of the basilar membrane creates a shear force between the hair bundle of the outer hair cells and the attached tectorial membrane.
Step 5: Hair bundles of outer hair cells tilt toward their longer stereovilli.
Step 6: Transduction channels open in outer hair cells. Because K+ is the major ion, the result is depolarization of the outer hair cells (Fig. 15-16A)—mechanical to electrical transduction. The transduction-induced changes in membrane potential are called receptor potentials. The molecular mechanisms of these Vm changes are basically the same as in vestibular hair cells.
Step 7: Depolarization contracts the motor protein prestin. Outer hair cells express very high levels of prestin (named for the musical notation presto, or fast). The contraction of myriad prestin molecules—each attached to its neighbors—causes the outer hair cell to contract, electrical to mechanical transduction or electromotility. Conversely, hyperpolarization (during downward movements of the basilar membrane) causes outer hair cells to elongate. Indeed, imposing changes in Vm causes cell length to change by as much as ~5%. The change in shape is fast, beginning within 100 μs. The mechanical response of the outer hair cell does not depend on adenosine triphosphate (ATP), microtubule or actin systems, extracellular Ca2+, or changes in cell volume. Prestin is a member of the SLC26 family of anion transporters (see Chapter 5), although it is not clear whether prestin also functions as an anion transporter.
Step 8: Contraction of outer hair cells accentuates upward movement of basilar membrane. Conversely, outer hair cell elongation (during downward movements of the basilar membrane) accentuates the downward movement of the basilar membrane. Thus, outer hair cells act as a cochlear amplifier—sensing and then rapidly accentuating movements of the basilar membrane. The electromotility of outer hair cells is a prerequisite for sensitive hearing and, as we will see later, the ability to discriminate frequencies sharply. In the absence of prestin, the cochlear amplifier ceases to function and animals become deaf.
Step 9: Endolymph sloshes beneath the tectorial membrane, out of the inner sulcus. The upward movement of the basilar membrane—accentuated by the cochlear amplifier—forces endolymph to flow out of the inner sulcus, beneath the tectorial membrane, toward its tip.
Step 10: Inner hair cell hair bundles bend toward longer stereovilli. The flow of endolymph now causes the free-floating hair bundles of the inner hair cells to bend.
Step 11: Transduction channels open in inner hair cells. As in the outer hair cells, the result is a depolarization.
Step 12: Depolarization opens voltage-gated Ca2+ channels. [Ca2+]i rises in the inner hair cells.
Step 13: Synaptic vesicles fuse, releasing glutamate. The neurotransmitter triggers action potentials in afferent neurons, relaying auditory signals to the brainstem. Note that the response to depolarization is very different in the two types of hair cells. The outer hair cell contracts and thereby amplifies the movement of the basilar membrane. The inner hair cell releases neurotransmitter.
When the stapes reverses direction and moves inward, all of these processes reverse as well. The basilar membrane bows downward. In the outer hair cells, transduction channels close, causing hyperpolarization and cell elongation. The accentuated downward movement of the basilar membrane causes endolymph to slosh into the inner sulcus. In inner hair cells, transduction channels close, causing hyperpolarization and reduced neurotransmitter release.
A fascinating clue to the existence of the cochlear amplifier was the early observation that not only does the ear receive sounds, it also generates them! Short click sounds trigger an “echo,” a brief vibration of the tympanic membrane that far outlasts the click. A microphone in the auditory canal can detect the echo. On occasion, damaged ears may produce spontaneous “otoacoustic emissions” that can even be loud enough to be heard by a nearby listener. (See Note: Otoacoustic Emissions)
The cochlea receives sensory and motor innervation from the auditory or cochlear nerve, a branch of CN VIII. We discuss the motor innervation later. The cell bodies of the sensory or afferent neurons of the cochlear nerve lie within the spiral ganglion, which corkscrews up around the axis of the cochlea (Fig. 15-20, lower left inset). The dendrites of these neurons contact nearby hair cells, whereas the axons project to the cochlear nucleus in the brainstem (see Fig. 16-15). Not surprisingly, ~95% of the roughly 30,000 sensory neurons (i.e., type I cells) of each cochlear nerve innervate the relatively few inner hair cells—the true auditory sensory cells. The remaining 5% of spiral ganglion neurons (i.e., type II cells) innervate the abundant outer hair cells, which are so poorly innervated that they must contribute very little direct information about sound to the brain.
The subjective experience of tonal discrimination is called pitch. Young humans can hear sounds with frequencies from ~20 to 20,000 Hz. This range is modest by the standards of most mammals because many hear up to 50,000 Hz, and some, notably whales and bats, can hear sounds with frequencies greater than 100,000 Hz. (See Note: Auditory Frequency Range)
A continuous, pure tone produces a wave that travels along the basilar membrane and has different amplitudes at different points along the base-apex axis (Fig. 15-24A). Increases in sound amplitude cause an increase in the rate of action potentials in these sensory neurons—rate coding. The frequency of the sound determines where along the cochlea the cochlear membranes vibrate most—high frequencies at one end and low at the other—and thus which hair cells are stimulated. This selectivity is the basis for place coding in the auditory system, that is, the frequency selectivity of a hair cell depends mainly on its longitudinal position along the cochlear membranes. The cochlea is essentially a spectral analyzer that evaluates a complex sound according to its pure tonal components, with each pure tone stimulating a specific region of the cochlea. (See Note: Rate Coding)
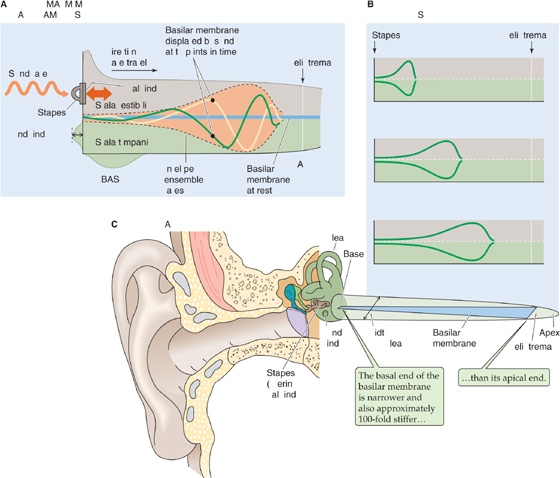
Figure 15-24 Waves along the basilar membrane of the cochlea. A, As a wave generated by a sound of a single frequency travels along the basilar membrane, its amplitude changes. The green and yellow curves represent a sample wave at two different times. The upper and lower broken lines (i.e., the envelope) encompass all maximum amplitudes of all waves, at all points in time. Thus, a wave can never escape the envelope. The figure exaggerates the amplitudes of the traveling waves ~1 million-fold. B, For a pure tone of 10,000 Hz, the envelope is confined to a short region of the basilar membrane near the stapes. For pure tones of 4000 Hz and 200 Hz, the widest part of the envelope moves closer to the helicotrema. C, The cochlea narrows in diameter from base to apex, whereas the basilar membrane tapers in the opposite direction.
Using optical methods to study cadaver ears, Georg von Békésy found that sounds of a particular frequency generate relatively localized waves in the basilar membrane and that the envelope of these waves changes position according to the frequency of the sound (Fig. 15-24B). Low frequencies generate their maximal amplitudes near the apex. As sound frequency increases, the envelope shifts progressively toward the basal end (i.e., near the oval and round windows). For his work, von Békésy received the 1961 Nobel Prize in Physiology or Medicine. (See Note: Georg von Békésy)
Two properties of the basilar membrane underlie the low-apical to high-basal gradient of resonance: taper and stiffness (Fig. 15-24C). If we could unwind the cochlea and stretch it straight, we would see that it tapers from base to apex. The basilar membrane tapers in the opposite direction—wider at the apex, narrower at the base. More important, the narrow basal end is ~100-fold stiffer than its wide and floppy apical end. Thus, the basilar membrane resembles a harp. At one end—the base, near the oval and round windows—it has short, taut strings that vibrate at high frequencies. At the other end—the apex—it has longer, looser strings that vibrate at low frequencies.
Although von Békésy’s experiments were illuminating, they were also paradoxical. A variety of experimental data suggested that the tuning of living hair cells is considerably sharper than the broad envelopes of von Békésy’s traveling waves on the basilar membrane could possibly produce. Recordings from primary auditory nerve cells are also very sharp, implying that this tuning must occur within the cochlea, not in the CNS. Some enhancement of tuning comes from the structure of the inner hair cells themselves. Those near the base have shorter, stiffer stereovilli, which makes them resonate to higher frequencies than possible with the longer, floppier stereovilli on cells near the apex. (See Note: Sharpening of Cochlear Tuning)
The blue curve in Figure 15-25 approximates von Békésy’s envelope of traveling waves for a passive basilar membrane from cadavers. It is important to note that von Békésy used unnaturally loud sounds. With reasonable sound levels, the maximum passive displacement of the basilar membrane would be slightly more than 0.1 nm. This distance is less than the pore diameter of an ion channel and also less than the threshold (0.3 to 0.4 nm) for an electrical response from a hair cell. However, measurements from the basilar membrane in living animals (the orange curve in Fig. 15-25) by very sensitive methods show that movements of the basilar membrane are much more localized and much larger than predicted by von Békésy. The maximal physiological displacement is ~20-fold greater than threshold and ~40-fold greater than that predicted by the passive von Békésy model. Moreover, the physiological displacement decays sharply on either side of the peak, more than 100-fold within ~0.5 mm (recall that the human basilar membrane has a total length of more than 30 mm).
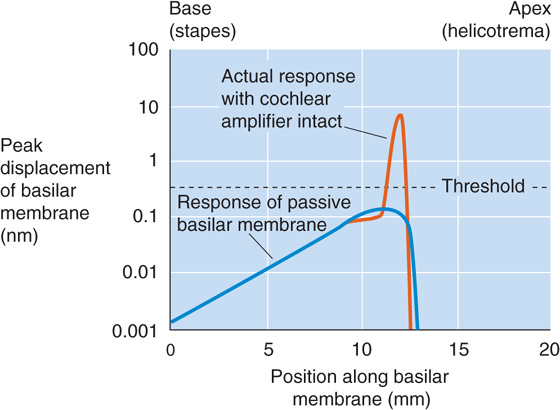
Figure 15-25 Peak movement of basilar membrane. The graph illustrates the displacement of the basilar membrane—in response to a pure tone—as a function of distance along the base-to-apex axis. The dashed line indicates the displacement threshold for triggering of an electrical response. (Data from Ashmore JF: Mammalian hearing and the cellular mechanisms of the cochlear amplifier. In Corey DP, Roper SD [eds]: Sensory Transduction, pp 396-412. New York: Rockefeller University Press, 1992.)
Both the extremely large physiological excursions of the basilar membrane and the exquisitely sharp tuning of the cochlea depend on the cochlear amplifier that we introduced earlier. Indeed, selectively damaging outer hair cells—with large doses of certain antibiotics, for example—considerably dulls the sharpness of cochlear tuning and dramatically reduces the amplification.
Tuning of hair cells is also under motor control of neurons that arise in the superior olivary complex in the brainstem and synapse mainly on the outer hair cells and, sparsely, on the afferent axons that innervate the inner hair cells. Stimulation of these olivocochlear efferent fibers suppresses the responsiveness of the cochlea to sound and is thought to provide auditory focus by suppressing responsiveness to unwanted sounds—allowing us to hear even in noisy environments. The main efferent neurotransmitter is acetylcholine, which activates ionotropic ACh receptors (see Chapter 6)—nonselective cation channels—and triggers an entry of Ca2+. The influx of Ca2+ activates Ca2+-activated K+ channels, causing a hyperpolarization—effectively an inhibitory postsynaptic potential—that suppresses the electromotility of outer hair cells and action potentials in afferent dendrites. Thus, the efferent axons allow the brain to control the gain of the inner ear. (See Note: Central Processing of Auditory Patterns)
Somatic sensation is the most widespread and diverse of the body’s sensory systems (soma means “body” in Greek). Its receptors are distributed throughout the body instead of being condensed into small and specialized sensory surfaces, as most other sensory systems are arranged. Somatosensory receptors cover the skin, subcutaneous tissue, skeletal muscles, bones and joints, major internal organs, epithelia, and cardiovascular system. These receptors also vary widely in their specificity. The body has mechanoreceptors to transduce pressure, stretch, vibration, and tissue damage; thermoreceptors to gauge temperature; and chemoreceptors to sense a variety of substances. Somatic sensation (or somesthesia) is usually considered to be a combination of at least four sensory modalities: the senses of touch, temperature, body position (proprioception), and pain (nociception).
Cochlear Implants
The most common cause of human deafness is damage to the hair cells of the cochlea. This damage can be caused by genetic factors, a variety of drugs (e.g., some antibiotics, including quinine), chronic exposure to excessively loud sounds, and other types of disease. Even when all hair cells have been destroyed, if the auditory nerve is intact, it is often possible to restore substantial hearing with a cochlear implant. (See Note: Conductive Hearing Loss)
A cochlear implant is essentially an electronic cochlea. Most of the system resides outside the body. The user wears a headpiece with a microphone, which is connected to a small, battery-powered digital speech processor. This processor sends signals to a miniature radio transmitter next to the scalp, which transmits digitally encoded signals—no wires penetrate the skin—to a receiver/decoder that is surgically implanted in the mastoid bone behind the ear. A very thin and flexible set of wires carries the signals through a tiny hole into the basal end of the cochlea, where an array of 8 to 22 electrodes lies adjacent to the auditory nerve endings (where healthy hair cells would normally be) along the cochlea. Each electrode activates a small portion of the auditory nerve axons. (See Note: Cochlear Implants)
The cochlear implant exploits the tonotopic arrangement of auditory nerve fibers. By stimulating near the base of the cochlea, it is possible to trigger a perception of high-frequency sounds; stimulation toward the apex evokes low-frequency sounds. The efficacy of the implant can be extraordinary. Users require training of a few months or more, and in many cases, they achieve very good comprehension of spoken speech, even as it comes across on a telephone.
As the technology and safety of cochlear implants have improved, so has their popularity. By 2004, more than 60,000 people were using cochlear implants, ~20,000 of them children. The best candidates for cochlear implants are young children and older children or adults whose deafness was acquired after they learned some speech. Adults whose deafness preceded any experience with speech generally do not fare as well with cochlear implants. Sensory systems, including the auditory system, need to experience normal inputs at a young age to develop properly. When the auditory system is deprived of sounds early in life, it can never develop completely normal function even if sensory inputs are restored during adulthood.
To meet a wide array of sensory demands, many kinds of specialized receptors are required. Somatic sensory receptors range from simple, bare nerve endings to complex combinations of nerve, muscle, connective tissue, and supporting cells. As we have seen, the other major sensory systems have only one type of sensory receptor or a set of very similar subtypes.
Mechanoreceptors, which are sensitive to physical distortion such as bending or stretching, account for many of the somatic sensory receptors. They exist throughout our bodies and monitor the following: physical contact with the skin, blood pressure in the heart and vessels, stretching of the gut and bladder, and pressure on the teeth. The transduction site of these mechanoreceptors is one or more unmyelinated axon branches. Our progress in understanding the molecular nature of mechanosensory transduction has been relatively slow. Similar to the transduction process in hair cells, cutaneous mechanoreceptive nerve endings probably involve the gating of ion channels. As in hair cells, some of these channels belong to the TRP superfamily. To date, at least seven types of TRP channels, from nearly all of the subfamilies, have been implicated in mechanosensation (in various species and tissues).
The mechanisms by which mechanical force is transferred from cells and their membranes to mechanosensitive channels is unclear. The ion channels may be physically coupled to either extracellular structures (e.g., collagen fibers, like the tip link proteins in hair cells) or cytoskeletal components (e.g., microtubules) that transfer energy from deformation of the cell to the gating mechanism of the channel. It is also possible that some channels may be sensitive to stresses in the lipid bilayer itself and require no other types of anchoring proteins. Mechanically sensitive axons are often surrounded by specialized structures that give them much of their specific sensitivity to different stimuli.
Thermoreceptors respond best to changes in temperature, whereas chemoreceptors are sensitive to various kinds of chemical alterations. In the next three sections, we discuss mechanoreceptors, thermoreceptors, and chemoreceptors that are located in the skin.
Skin protects us from our environment by preventing evaporation of body fluids, invasion by microbes, abrasion, and damage from sunlight. However, skin also provides our most direct contact with the world. The two major types of mammalian skin are hairy and glabrous. Glabrous skin (or hairless) is found on the palms of our hands and fingertips and on the soles of our feet and pads of our toes (Fig. 15-26A). Hairy skin makes up most of the rest and differs widely in its hairiness. Both types of skin have an outer layer, the epidermis, and an inner layer, the dermis, and sensory receptors innervate both. The receptors in the skin are sensitive to many types of stimuli and respond when the skin is vibrated, pressed, pricked, and stroked or when its hairs are bent or pulled. These are quite different kinds of mechanical energy, yet we can feel them all and easily tell them apart. Skin also has exquisite sensitivity; for example, we can reliably feel a dot only 0.006 mm high and 0.04 mm across when it is stroked across a fingertip. The standard Braille dot is 167 times higher!
Figure 15-26 Sensors in the skin.
The sensory endings in the skin take many shapes, and most of them are named after the 19th century European histologists who observed them and made them popular. The largest and best studied mechanoreceptor is Pacini’s corpuscle, which is up to 2 mm long and almost 1 mm in diameter (Fig. 15-26B). Pacini’s corpuscle is located in the subcutaneous tissue of both glabrous and hairy skin. It has an ovoid capsule with 20 to 70 onion-like, concentric layers of connective tissue and a nerve terminal in the middle. The capsule is responsible for the rapidly adapting nature of the Pacini corpuscle’s responses. When the capsule is compressed, energy is transferred to the nerve terminal, its membrane is deformed, and mechanosensitive channels open. Current flowing through the channels generates a depolarizing receptor potential that, if large enough, causes the axon to fire an action potential (Fig. 15-26B, left panel). However, the capsule layers are slick, with viscous fluid between them. If the stimulus pressure is maintained, the layers slip past one another and transfer the stimulus energy away so that the underlying axon terminal is no longer deformed and the receptor potential dissipates (Fig. 15-26B, right panel). When pressure is released, the events reverse themselves and the terminal is depolarized again. In this way, the non-neural covering of Pacini’s corpuscle specializes the corpuscle for sensing of vibrations and makes it almost unresponsive to steady pressure. Pacini’s corpuscle is most sensitive to vibrations of 200 to 300 Hz, and its threshold increases dramatically below 50 Hz and above ~500 Hz. The sensation evoked by stimulating Pacini’s corpuscle is a poorly localized humming feeling.
Werner Lowenstein and colleagues in the 1960s showed the importance of the Pacini corpuscle’s capsule to its frequency sensitivity. With fine microdissection, they were able to strip away the capsule from single corpuscles. They found that the resultant naked nerve terminal is much less sensitive to vibrating stimuli and much more sensitive to steady pressure. Clearly, the capsule modifies the sensitivity of the bare mechanoreceptive axon. The encapsulated Pacini’s corpuscle is an example of a rapidly adapting sensor, whereas the decapsulated nerve ending is an example of a slowly adapting sensor.
Several other types of encapsulated mechanoreceptors are located in the dermis, but none has been studied as well as Pacini’s corpuscle. Meissner’s corpuscles (Fig. 15-26A) are located in the ridges of glabrous skin and are about one tenth the size of Pacini’s corpuscle. They are rapidly adapting, although less so than Pacini’s corpuscles. Ruffini’s corpuscles resemble diminutive Pacini’s corpuscles and, like Pacini’s corpuscles, occur in the subcutaneous tissue of both hairy and glabrous skin. Their preferred stimuli might be called “fluttering” vibrations. As relatively slowly adapting receptors, they respond best to low frequencies. Merkel’s disks are also slowly adapting receptors made from a flattened, nonneural epithelial cell that synapses on a nerve terminal. They lie at the border of the dermis and epidermis of glabrous skin. It is not clear whether it is the nerve terminal or epithelial cell that is mechanosensitive. The nerve terminals of Krause’s end bulbs appear knotted. They innervate the border areas of dry skin and mucous membranes (e.g., around the lips and external genitalia) and are probably rapidly adapting mechanoreceptors.
The receptive fields of different types of skin receptors vary greatly in size. Pacini’s corpuscles have extremely broad receptive fields (Fig. 15-27A), whereas those of Meissner’s corpuscles (Fig. 15-27B) and Merkel’s disks are very small. The last two seem to be responsible for the ability of the fingertips to make very fine tactile discriminations. Small receptive fields are an important factor in achieving high spatial resolution. Resolution varies widely, a fact easily demonstrated by measuring the skin’s two-point discrimination. Bend a paper clip into a U shape. Vary the distance between the tips and test how easily you can distinguish the touch of one tip versus two on your palm, your fingertips, your lips, your back, and your foot. To avoid bias, a colleague—rather than you—should apply the stimulus. Compare the results with standardized data (Fig. 15-27C).
Figure 15-27 Receptive fields and spatial discrimination of skin mechanoreceptors. A, Each of the two black dots indicates an area of maximal sensitivity of a single corpuscle. Each green area is the receptive field of a corpuscle (i.e., the corpuscle responds when stimulus strength increases sufficiently anywhere within the area). B, Each dot represents the entire receptive field of a single Meissner’s corpuscle. Note that the fields are much smaller than in A. C, The horizontal bars represent the minimum distance at which two points can be perceived as distinct at various locations over the body. Spatial discrimination depends on both receptor density and receptive field size.
Two things determine the sensitivity of spatial discrimination in an area of skin. The first is the size of the receptors’ receptive fields—if they are small, the two tips of your paper clip are more likely to stimulate different sets of receptors. The second parameter that determines spatial discrimination is the density of the receptors in the skin. Indeed, two-point discrimination of the fingertips is better than that of the palm, even though their receptive fields are the same size. The key to finer discrimination in the fingertips is their higher density of receptors. Crowding more receptors into each square millimeter of fingertip has a second advantage: because the CNS receives more information per stimulus, it has a better chance of transducing very small stimuli.
Although we rarely think about it, hair is a sensitive part of our somatic sensory system. For some animals, hairs are a major sensory system. Rodents whisk long facial vibrissae (hairs) and feel the texture, distance, and shape of their local environment. Hairs grow from follicles embedded in the skin, and each follicle is richly innervated by free, mechanoreceptive nerve endings that either wrap around it or run parallel to it. Bending of the hair causes deformation of the follicle and surrounding tissue, which stretches, bends, or flattens the nerve endings and increases or decreases their firing frequency. Various mechanoreceptors innervate hair follicles, and they may be either slowly or rapidly adapting.
Neurons are sensitive to changes in temperature, as are all of life’s chemical reactions. This temperature sensitivity has two consequences: first, neurons can measure temperature; but second, to work properly, most neural circuits need to be kept at a relatively stable temperature. Neurons of the mammalian CNS are especially vulnerable to temperature changes. Whereas skin tissue temperatures can range from 20°C to 40°C without harm or discomfort, brain temperature must be near 37°C to avoid serious dysfunction. The body has complex systems to control brain (i.e., body core) temperature tightly. Even though all neurons are sensitive to temperature, not all neurons are thermoreceptors. Because of specific membrane mechanisms, some neurons are extremely sensitive to temperature and seem to be adapted to the job of sensing it. Although many temperature-sensitive neurons are present in the skin, they are also clustered in the hypothalamus and the spinal cord. The hypothalamic temperature sensors, like their cutaneous counterparts, are important for regulation of the physiological responses that maintain stable body temperature (see Chapter 59).
Perceptions of temperature apparently reflect warmth and cold receptors located in the skin. Thermoreceptors, like mechanoreceptors, are not spread uniformly across the skin. When you map the skin’s sensitivity to temperature with a small cold or warm probe, you find spots ~1 mm across that are especially sensitive to either hot or cold, but not to both. In addition, some areas of skin in between are relatively insensitive. The spatial dissociation of the hot and cold maps shows that they are separate submodalities, with separate receptors to encode each. Recordings from single sensory fibers have confirmed this conclusion. The responses of thermoreceptors adapt during long stimuli, as many sensory receptors commonly do. Most cutaneous thermoreceptors are probably free nerve endings, without obvious specialization. Their axons are small, either unmyelinated C fibers or the smallest-diameter myelinated Aδ fibers (see Table 12-1).
We can perceive changes in our average skin temperature of as little as 0.01°C. Within the skin are separate types of thermoreceptors that are sensitive to a range of relatively hot or cold temperatures. Figure 15-28A shows how the steady discharge rate of both types of receptors varies with temperature. Warmth receptors begin firing above ~30°C and increase their firing rate until 44°C to 46°C, beyond which the rate falls off steeply and a sensation of pain begins, presumably mediated by nociceptive endings (see the next section). Cold receptors have a much broader temperature response. They are relatively quiet at skin temperatures of ~40°C, but their steady discharge rate increases as the temperature falls to 24°C to 28°C. Further decreases in temperature cause the steady discharge rate of the cold receptors to decrease until the temperature falls to ~10°C. Below that temperature, firing ceases and cold becomes an effective local anesthetic.
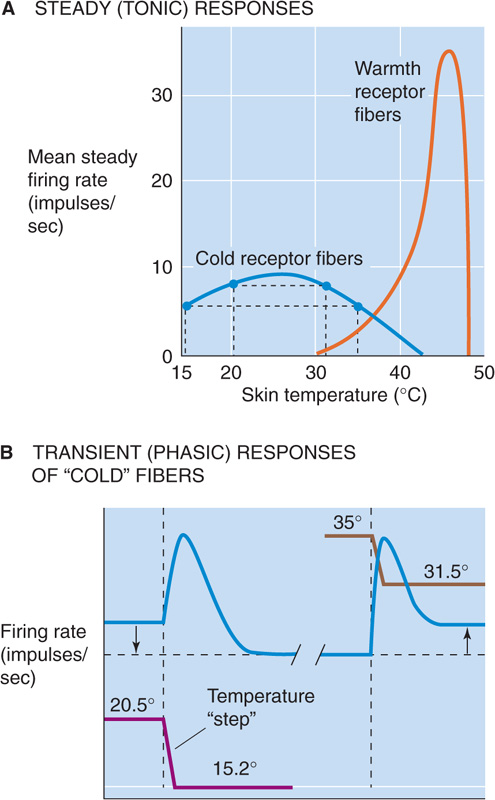
Figure 15-28 Temperature sensitivity of cutaneous thermoreceptors. A, The curves represent the mean, steady firing rates of neurons from warmth receptors and cold receptors. B, These two experiments on cold receptors show effects of cooling steps of nearly identical magnitude but starting from different temperatures (20.5°C and 35°C). In both instances, the transient (phasic) responses are the same: an increase in the firing rate. When the starting temperature is 20.5°C (to the left of the peak of the blue curve in B), the final firing rate is less than the initial one. However, when the initial temperature is 35°C (to the right of the peak of the blue curve in B), the final rate is greater than the initial one. (Data from Somjen GG: Sensory Coding in the Mammalian Nervous System. New York: Appleton-Century-Crofts, 1972.)
In addition to the tonic response just described (i.e., the steady discharge rate), cold receptors also have a phasic response that enables them to report changes in temperature. As shown in Figure 15-28B, when the temperature suddenly shifts from 20.5°C to 15.2°C (both points are to the left of the peak in Fig. 15-28A), the firing rate transiently increases (i.e., the phasic response). However, the new steady-state level is lower, as suggested by the left pair of points in Figure 15-28A. When the temperature suddenly shifts from 35°C to 31.5°C (both points are to the right of the peak in Fig. 15-28A), the firing rate in Figure 15-28B transiently increases, and the new steady-state level is higher, as suggested by the right pair of points in Figure 15-28A.
The transduction of relatively warm temperatures is carried out by several types of TRPV channels (specifically TRPV1 to 4). TRPV1 is a vanilloid receptor—it is activated by the vanilloid class of compounds that includes capsaicin, the pungent ingredient that gives spicy foods their burning quality. Aptly enough, chili peppers taste “hot” because they activate some of the same ion channels that heat itself activates! TRPV1 channels have a high temperature threshold (about 43°C) and thus help mediate the painful aspects of thermoreception (see later). Other TRPV channels are activated at more moderate temperatures and presumably provide our sensations of warmth.
Yet another TRP channel, TRPM8, mediates sensations of moderate cold. TRPM8 channels begin to open at temperatures below ~27°C and are maximally activated at 8°C. In a remarkable analogy to the hot-sensitive TRPV1 channel (the capsaicin receptor), the cool-sensitive TRPM8 channel is a menthol receptor. Menthol evokes sensations of cold because it activates the same ion channel that is opened by cold temperatures. In yet another strange twist, the TRPA1 channel that seems to be the hair cell transduction channel (see earlier) is also a painfully cold-sensitive channel in somatic sensory neurons.
Physical energy that is informative at low and moderate levels can be destructive at higher intensity. Sensations of pain motivate us to avoid such situations. Nociceptors are the receptors mediating painful feelings to warn us that body tissue is being damaged or is at risk of being damaged (as their Latin roots imply: nocere, to hurt, and recipere, to receive). The pain-sensing system is entirely separate from the other modalities we have discussed; it has its own peripheral receptors and a complex, dispersed, chemically unique set of central circuits.
Nociceptors vary in their selectivity. Mechanical nociceptors respond to strong pressure, in particular, pressure from sharp objects. Thermal nociceptors signal either burning heat (above ~45°C, when tissues begin to be destroyed) or unhealthy cold; the heat-sensitive nociceptive neurons express the TRPV1 and TRPV2 channels, whereas the cold-sensitive nociceptors express TRPA1 and TRPM8 channels. Chemically sensitive, mechanically insensitive nociceptors respond to a variety of agents, including K+, extremes of pH, neuroactive substances such as histamine and bradykinin from the body itself, and various irritants from the environment. Finally, polymodal nociceptors are single nerve endings that are sensitive to combinations of mechanical, thermal, and chemical stimuli. Nociceptive axons include both fast Aδ fibers and slow, unmyelinated C fibers. Aδ axons mediate sensations of sharp, intense pain; C fibers elicit more persistent feelings of dull, burning pain.
Nociceptors are free nerve endings that are widely distributed throughout the body. They innervate the skin, bone, muscle, most internal organs, blood vessels, and heart. Ironically, they are generally absent from the brain substance itself, although they are in the meninges.
Sensations of pain can be modulated in a variety of ways. Skin, joints, or muscles that have been damaged or inflamed are unusually sensitive to further stimuli. This phenomenon is called hyperalgesia, and it can be manifested as a reduced threshold for pain, an increased intensity of painful stimuli, or spontaneous pain. Primary hyperalgesia occurs within the area of damaged tissue, but within ~20 minutes after an injury, tissues surrounding a damaged area may become supersensitive by a process called secondary hyperalgesia. Hyperalgesia seems to involve processes near peripheral receptors (Fig. 15-29) as well as mechanisms in the CNS. Damaged skin releases a variety of chemical substances from itself, blood cells, and nerve endings. These substances include bradykinin, prostaglandins, serotonin, substance P, K+, H+, and others; they trigger the set of local responses that we know as inflammation. As a result, blood vessels become more leaky and cause tissue swelling (or edema) and redness (see Chapter 20 for the box on interstitial edema). Nearby mast cells release the chemical histamine, which directly excites nociceptors. By a mechanism called the axon reflex, action potentials can propagate along nociceptive axons from the site of an injury into side branches of the same axon that innervate neighboring regions of skin. The spreading axon branches of the nociceptors themselves may release substances that sensitize nociceptive terminals and make them responsive to previously nonpainful stimuli. Such “silent” nociceptors among our small Aδ and C fibers are normally unresponsive to stimuli—even destructive ones. Only after sensitization do they become responsive to mechanical or chemical stimuli and contribute greatly to hyperalgesia.
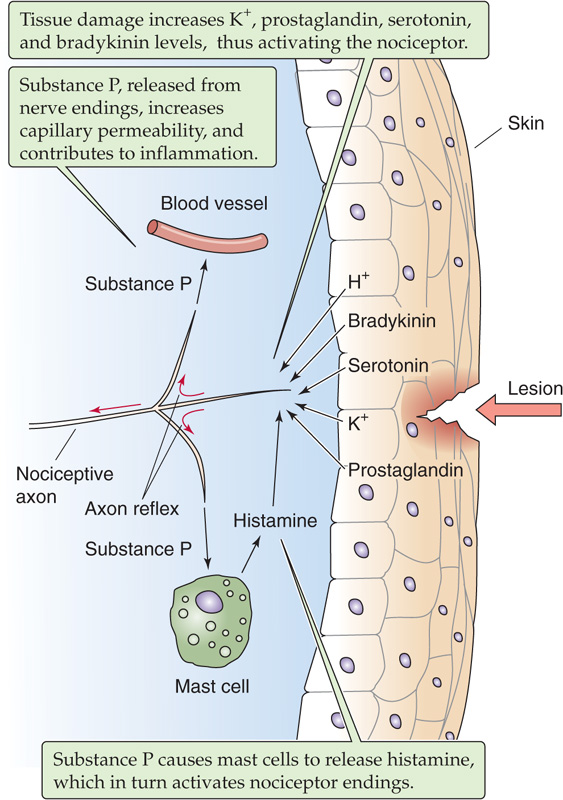
Figure 15-29 Hyperalgesia of inflammation.
The cognitive sensations of pain are under remarkably potent control by the brain, more so than other sensory systems. In some cases, nociceptors may fire wildly, although perceptions of pain are absent; on the other hand, pain may be crippling, although nociceptors are silent. Pain can be modified both by nonpainful sensory input and by neural activity from various nuclei within the brain. For example, pain evoked by activity in nociceptors (Aδ and C fibers) can be reduced by simultaneous activity in low-threshold mechanoreceptors (Aα and Aβ fibers). This phenomenon is a familiar experience—some of the discomfort of a burn, cut, or bruise can be relieved by gentle massage or rubbing (stimulating mechanoreceptors) around the injured area. In 1965, Melzack and Wall proposed that this phenomenon involves a circuit in the spinal cord that can “gate” the transmission of nociceptive information to the brain; control of the gate could be provided by other sensory information (e.g., tactile stimulation) or by descending control from the brain itself.
A second mechanism for modifying the sensation of pain involves the relatively small peptides called endorphins. In the 1970s, it was discovered that a class of drugs called opioids (including morphine, heroin, and codeine) act by binding tightly and specifically to opioid receptors in the brain and, furthermore, that the brain itself manufactures “endogenous morphine-like substances,” collectively called endorphins (see Chapter 13).
The somatic sensory receptors described thus far provide information about the external environment. However, the body also needs detailed information about itself to know where each of its parts is in space, whether it is moving, and if so, in which direction and how fast. Proprioception provides this sense of self and serves two main purposes. First, knowledge of the positions of our limbs as they move helps us judge the identity of external objects. It is much easier to recognize an object if you can actively palpate it than if it is placed passively into your hand so that your skin is stimulated but you are not allowed to personally guide your fingers around it. Second, proprioceptive information is essential for accurately guiding many movements, especially while they are being learned.
Skeletal muscles, which mediate voluntary movement, have two mechanosensitive proprioceptors: the muscle spindles (or stretch receptors) and Golgi tendon organs (Fig. 15-30). Muscle spindles measure the length and rate of stretch of the muscles, whereas the Golgi tendon organs gauge the force generated by a muscle by measuring the tension in its tendon. Together, they provide a full description of the dynamic state of each muscle. The different sensitivities of the spindle and the tendon organ are due partly to their structures but also to their placement: spindles are located in modified muscle fibers called intrafusal muscle fibers, which are aligned in parallel with the “ordinary” force-generating or extrafusal skeletal muscle fibers. On the other hand, Golgi tendon organs are aligned in series with the extrafusal fibers.
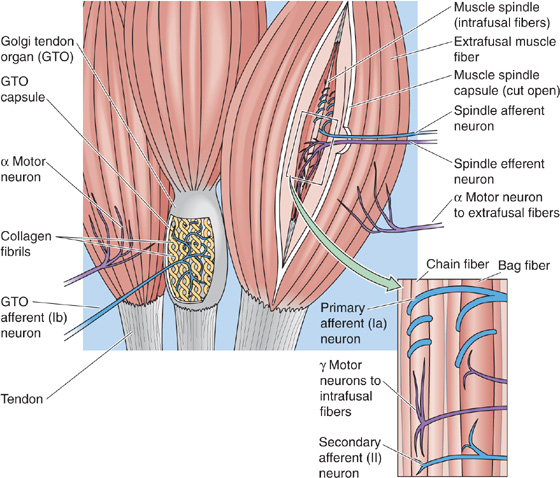
Figure 15-30 Golgi tendon organ and muscle spindle fibers. A muscle contains two kinds of muscle fibers, extrafusal fibers (ordinary muscle fibers that cause contraction) and intrafusal fibers (in parallel with the extrafusal fibers). Some of the extrafusal fibers have Golgi tendon organs located in series between the end of the muscle fiber and the macroscopic tendon. The intrafusal fibers contain muscle spindles, which receive both afferent (sensory) and efferent (motor) innervation. The spindle (inset) contains both bag fibers, with nuclei bunched together, and chain fibers, with nuclei in a row.
The Golgi tendon organ consists of bare nerve endings of group Ib axons (see Table 12-1). These endings intimately invest an encapsulated collagen matrix and usually sit at the junction between skeletal muscle fibers and the tendon. When tension develops in the muscle as a result of either passive stretch or active contraction, the collagen fibers tend to squeeze and distort the mechanosensitive nerve endings, triggering them to fire action potentials.
The mammalian muscle spindle is a complex of modified skeletal muscle fibers (intrafusal fibers) combined with both afferent and efferent innervation. The spindle does not contribute significant force generation to the muscle but serves a purely sensory function. A simplified summary of the muscle spindle is that it contains two kinds of intrafusal muscle fibers (bag and chain), with two kinds of sensory endings entwined about them (the primary and secondary endings). The different viscoelastic properties of the muscle fibers make them differentially sensitive to the consequences of muscle stretch. Because the primary sensory endings of group Ia axons coil around and strongly innervate individual bag muscle fibers (in addition to chain fibers), they are very sensitive to the dynamics of muscle length (i.e., changes in its length). The secondary sensory endings of group II axons mainly innervate the chain fibers and most accurately transduce the static length of the muscle; in other words, they are slowly adapting receptors. The discharge rate of afferent neurons increases when the whole muscle—and therefore the spindle—is stretched.
What is the function of the motor innervation of the muscle spindle? Consider what happens when the α motor neurons stimulate the force-generating extrafusal fibers and the muscle contracts. The spindle, connected in parallel to the extrafusal fibers, quickly tends to go slack, which makes it insensitive to further changes in length. To avoid this situation and to continue to maintain control over the sensitivity of the spindle, γ motor neurons cause the intrafusal muscle fibers to contract in parallel with the extrafusal fibers. This ability of the spindle’s intrafusal fibers to change their length as necessary greatly increases the range of lengths over which the spindle can work. It also means that the sensory responses of the spindle depend not only on the length of the whole muscle in which the spindle sits but also on the contractile state of its own intrafusal muscle fibers. Presumably, the ambiguity in this code is sorted out centrally by circuits that simultaneously keep track of the spindle’s sensory output and the activity of its motor nerve supply.
In addition to the muscle receptors, various mechanoreceptors are found in the connective tissues of joints, especially within the capsules and ligaments. Many resemble Ruffini, Golgi, and Pacini end organs; others are free nerve endings. They respond to changes in the angle, direction, and velocity of movement in a joint. Most are rapidly adapting, which means that sensory information about a moving joint is rich. Nerves encoding the resting position of a joint are few. We are nevertheless quite good at judging the position of a joint, even with our eyes closed. It seems that information from joint receptors is combined with that from muscle spindles and Golgi tendon organs, and probably from cutaneous receptors as well, to estimate joint angle. Removal of one source of information can be compensated by use of the other sources. When an arthritic hip is replaced with a steel and plastic one, patients are still able to tell the angle between their thigh and their pelvis, even though all hip joint mechanoreceptors are long gone.
Books and Reviews
Copeland BJ, Pillsbury HC: 3rd: Cochlear implantation for the treatment of deafness. Annu Rev Med 2004; 55:157-167.
Jordt SE, McKemy DD, Julius D: Lessons from peppers and peppermint: The molecular logic of thermosensation. Curr Opin Neurobiol. 2003; 13:487-492.
Kung C: A possible unifying principle for mechanosensation. Nature 2005; 436:647-654.
Lin SY, Corey DP: TRP channels in mechanosensation. Curr Opin Neurobiol 2005; 15:350-357.
Mombaerts P: Genes and ligands for odorant, vomeronasal and taste receptors. Nat Rev Neurosci 2004; 5:263-278.
Santos-Sacchi J: New tunes from Corti’s organ: The outer hair cell boogie rules. Curr Opin Neurobiol 2006; 13:459-468.
Journal Articles
Buck L, Axel R: A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell 1991; 65:175-187.
Corey DP, Garcia-Anoveros J, Holt JR, et al: TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature 2004; 432:723-730.
Liberman MC, Gao J, He DZ, et al: Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature 2002; 419:300-304.
Nelson G, Hoon MA, Chandrashekar J, et al: Mammalian sweet taste receptors. Cell. 2001; 106:381-390.
Zhao GQ, Zhang Y, Hoon MA, et al: The receptors for mammalian sweet and umami taste. Cell 2003; 115:255-266.