CHAPTER 14
George B. Richerson
When we are awake, we are constantly aware of sensory input from our external environment, and we consciously plan how to react to it. When we are asleep, the nervous system has a variety of mechanisms to dissociate cortical function from sensory input and somatic motor output. Among these mechanisms are closing the eyes, blocking the transmission of sensory impulses to the cortex as they pass through the thalamus, and effecting a nearly complete paralysis of skeletal muscles during rapid eye movement (REM) sleep to keep us from physically acting out our dreams.
The conscious and discontinuous nature of cortical brain function stands in sharp contrast with those parts of the nervous system that are responsible for control of our internal environment. These “autonomic” processes never stop attending to the wide range of metabolic, cardiopulmonary, and other visceral requirements of our body. Autonomic control continues whether we are awake and attentive, preoccupied with other activities, or asleep. While we are awake, we are unaware of most visceral sensory input, and we avoid any conscious effort to act on it unless it induces distress. In most cases, we have no awareness of motor commands to the viscera, and most individuals can exert voluntary control over motor output to the viscera only in minor ways. Consciousness and memory are frequently considered the most important functions of the human nervous system, but it is the visceral control system—including the autonomic nervous system (ANS)—that makes life and higher cortical function possible.
We have a greater understanding of the physiology of the ANS than of many other parts of the nervous system, largely because it is reasonably easy to isolate peripheral neurons and to study them. As a result of its accessibility, the ANS has served as a key model system for the elucidation of many principles of neuronal and synaptic function.
Output from the central nervous system (CNS) travels along two anatomically and functionally distinct pathways: the somatic motor neurons, which innervate striated skeletal muscle; and the autonomic motor neurons, which innervate smooth muscle, cardiac muscle, secretory epithelia, and glands. All viscera are richly supplied by efferent axons from the ANS that constantly adjust organ function.
The autonomic (from the Greek for “self-governing,” functioning independently of the will) nervous system was first defined by Langley in 1898 as including the local nervous system of the gut and the efferent neurons innervating glands and involuntary muscle. Thus, this definition of the ANS includes only efferent neurons and enteric neurons. Since that time, it has become clear that the efferent ANS cannot easily be dissociated from visceral afferents as well as from those parts of the CNS that control the viscera and other autonomic functions. This larger visceral control system monitors afferents from the viscera and the rest of the body, compares this input with current and anticipated needs, and controls output to the body’s organ systems.
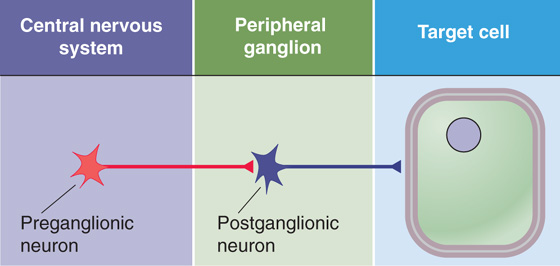
The ANS has three divisions: sympathetic, parasympathetic, and enteric. The sympathetic and parasympathetic divisions of the ANS are the two major efferent pathways controlling targets other than skeletal muscle (Fig. 14-1). Each arises in the CNS, and each innervates target tissue by a two-synapse pathway. The cell bodies of the first neurons lie within the CNS. These preganglionic neurons are found in columns of cells in the brainstem and spinal cord and send axons out of the CNS to make synapses with postganglionic neurons in peripheral ganglia interposed between the CNS and their target cells. Axons from these postganglionic neurons then project to their targets. The sympathetic and parasympathetic divisions can act independently of each other. However, in general, they work synergistically to control visceral activity and often act in opposite ways, like an accelerator and brake to regulate visceral function. An increase in output of the sympathetic division occurs under conditions of stress, anxiety, physical activity, fear, or excitement; parasympathetic output increases during sedentary activity, eating, or other “vegetative” behavior.

Figure 14-1 Organization of the sympathetic and parasympathetic divisions of the ANS.
The enteric division of the ANS is a system of afferent neurons, interneurons, and motor neurons that form networks of neurons called plexuses (from the Latin “to braid”) that surround the gastrointestinal tract. It can function as a separate and independent nervous system, but it is normally controlled by the CNS through sympathetic and parasympathetic fibers.
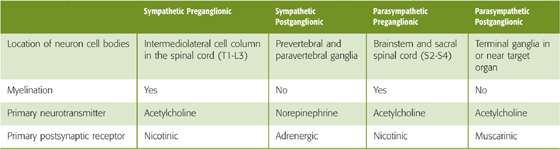
Preganglionic Neurons The cell bodies of preganglionic sympathetic motor neurons are located in the thoracic and upper lumbar spinal cord between levels T1 and L3. At these spinal levels, autonomic neurons lie in the intermediolateral cell column, or lateral horn, between the dorsal and ventral horns (Fig. 14-2). Axons from preganglionic sympathetic neurons exit the spinal cord through the ventral roots along with axons from somatic motor neurons. After entering the spinal nerves, sympathetic efferents diverge from somatic motor axons to enter the white rami communicantes. These rami, or branches, are white because most preganglionic sympathetic axons are myelinated.
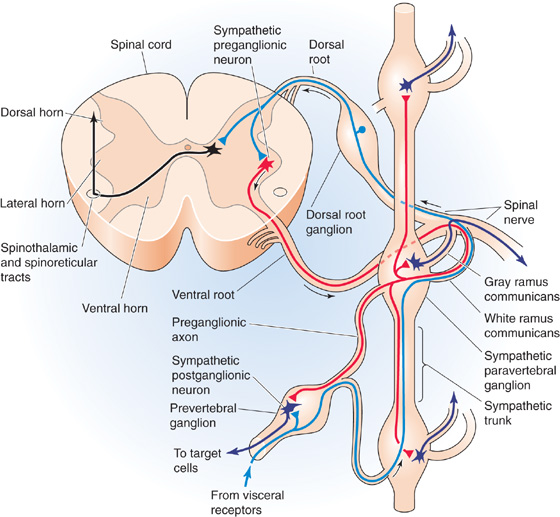
Figure 14-2 Anatomy of the sympathetic division of the ANS. The figure shows a cross section of the thoracic spinal cord and the nearby paravertebral ganglia as well as a prevertebral ganglion. Sympathetic preganglionic neurons are shown in red and postganglionic neurons in dark blue-violet. Afferent (sensory) pathways are in blue. Interneurons are shown in black.
Paravertebral Ganglia Axons from preganglionic neurons enter the nearest sympathetic paravertebral ganglion through a white ramus. These ganglia lie adjacent to the vertebral column. Although preganglionic sympathetic fibers emerge only from levels T1 to L3, the chain of sympathetic ganglia extends all the way from the upper part of the neck to the coccyx, where the left and right sympathetic chains merge in the midline and form the coccygeal ganglion. In general, one ganglion is positioned at the level of each spinal root, but adjacent ganglia are fused in some cases. The most rostral ganglion, the superior cervical ganglion, arises from fusion of C1 to C4 and supplies the head and neck. The next two ganglia are the middle cervical ganglion, which arises from fusion of C5 and C6, and the inferior cervical ganglion (C7 and C8), which is usually fused with the first thoracic ganglion to form the stellate ganglion. Together, the middle cervical and stellate ganglia, along with the upper thoracic ganglia, innervate the heart, lungs, and bronchi. The remaining paravertebral ganglia supply organs and portions of the body wall in a segmental fashion.
After entering a paravertebral ganglion, a preganglionic sympathetic axon has one or more of three fates. It may synapse within that segmental paravertebral ganglion, travel up or down the sympathetic chain to synapse within a neighboring paravertebral ganglion, or enter the greater or lesser splanchnic nerve to synapse within one of the ganglia of the prevertebral plexus.
Prevertebral Ganglia The prevertebral plexus lies in front of the aorta and along its major arterial branches and includes the prevertebral ganglia and interconnected fibers (Fig. 14-3). The major prevertebral ganglia are named according to the arteries that they are adjacent to and include the celiac, superior mesenteric, aorticorenal, and inferior mesenteric ganglia. Portions of the prevertebral plexus extend down the major arteries and contain other named and unnamed ganglia and plexuses of nerve fibers, altogether making up a dense and extensive network of sympathetic neuron cell bodies and nerve fibers.
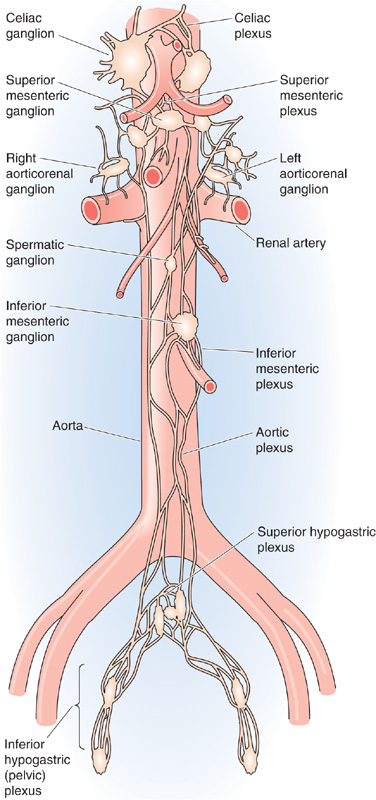
Figure 14-3 Anatomy of the sympathetic prevertebral plexus. The ganglia and each associated plexus are named after the artery with which they are associated.
Each preganglionic sympathetic fiber synapses on many postganglionic sympathetic neurons that are located within one or several nearby paravertebral or prevertebral ganglia. It has been estimated that each preganglionic sympathetic neuron branches and synapses on as many as 200 postganglionic neurons, thus enabling the sympathetic output to have more widespread effects. However, any impulse arriving at its target end organ has only crossed a single synapse between the preganglionic and postganglionic sympathetic neurons.
Postganglionic Neurons The cell bodies of postganglionic sympathetic neurons that are located within paravertebral ganglia send out their axons through the nearest gray rami communicantes, which rejoin the spinal nerves (Fig. 14-2). These rami are gray because most postganglionic axons are unmyelinated. Because preganglionic sympathetic neurons are located only in the thoracic and upper lumbar spinal segments (T1 to L3), white rami are found only at these levels (Fig. 14-4, left). However, because each sympathetic ganglion sends out postganglionic axons, gray rami are present at all spinal levels from C2 or C3 to the coccyx. Postganglionic sympathetic axons from paravertebral and prevertebral ganglia travel to their target organs within other nerves or by traveling along blood vessels to reach their target organ. Because the paravertebral and prevertebral sympathetic ganglia lie near the spinal cord and thus relatively far from their target organs, the postganglionic axons of the sympathetic division tend to be long. On their way to reach their targets, some postganglionic sympathetic axons travel through parasympathetic terminal ganglia or cranial nerve ganglia without synapsing. (See Note: Tracing of Nerve Tracts Using Pseudorabies Virus)
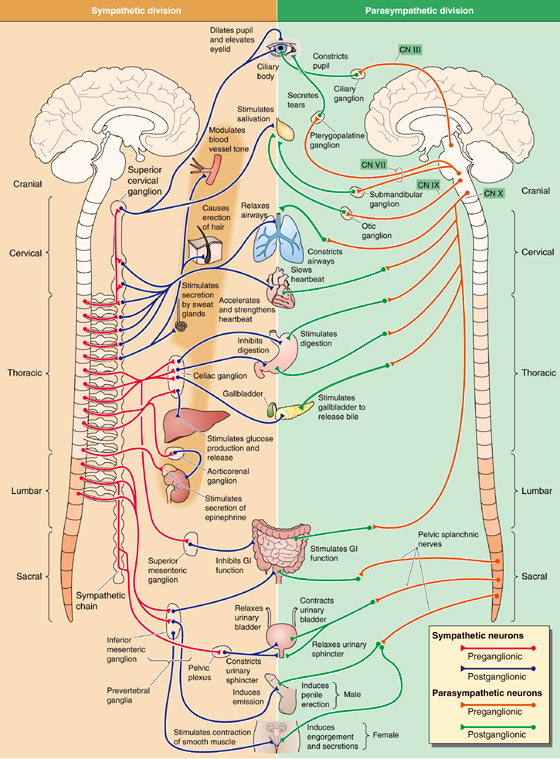
Figure 14-4 Organization of the sympathetic and parasympathetic divisions of the ANS. The left panel shows the sympathetic division. The cell bodies of sympathetic preganglionic neurons (red) are in the intermediolateral column of the thoracic and lumbar spinal cord (T1-L3). Their axons project to paravertebral ganglia (the sympathetic chain) and prevertebral ganglia. Postganglionic neurons (blue) therefore have long projections to their targets. The right panel shows the parasympathetic division. The cell bodies of parasympathetic preganglionic neurons (orange) are either in the brain (midbrain, pons medulla) or in the sacral spinal cord (S2-S4). Their axons project to ganglia very near (or even inside) the end organs. Postganglionic neurons (green) therefore have short projections to their targets.
The cell bodies of preganglionic parasympathetic neurons are located in the medulla, pons, and midbrain and in the S2 through S4 level of the spinal cord (Fig. 14-4, right). Thus, unlike the sympathetic—or thoracolumbar—division, whose preganglionic cell bodies are in the thoracic and lumbar spinal cord, the parasympathetic—or craniosacral—division’s preganglionic cell bodies are cranial and sacral. The preganglionic parasympathetic fibers originating in the brain distribute with four cranial nerves: the oculomotor nerve (CN III), the facial nerve (CN VII), the glossopharyngeal nerve (CN IX), and the vagus nerve (CN X). The preganglionic parasympathetic fibers originating in S2 through S4 distribute with the pelvic splanchnic nerves.
Postganglionic parasympathetic neurons are located in terminal ganglia that are more peripherally located and more widely distributed than are the sympathetic ganglia. Terminal ganglia often lie within the walls of their target organs. Thus, in contrast to the sympathetic division, postganglionic fibers of the parasympathetic division are short. In some cases, individual postganglionic parasympathetic neurons are found in isolation or in scattered cell groups rather than in encapsulated ganglia.
Cranial Nerves III, VII, and IX The preganglionic parasympathetic neurons that are distributed with CN III, VII, and IX originate in three groups of nuclei.
1. The Edinger-Westphal nucleus is a subnucleus of the oculomotor complex in the midbrain (Fig. 14-5). Parasympathetic neurons in this nucleus travel in the oculomotor nerve (CN III) and synapse onto postganglionic neurons in the ciliary ganglion (Fig. 14-4, right). The postganglionic fibers project to two smooth muscles of the eye: the constrictor muscle of the pupil and the ciliary muscle, which controls the shape of the lens (see Fig. 15-6A).
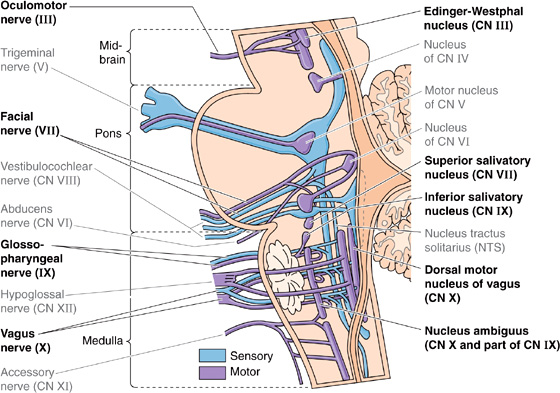
Figure 14-5 Supraspinal nuclei containing neurons that are part of the ANS. These nuclei contain the cell bodies of the preganglionic parasympathetic neurons (i.e., efferent). The Edinger-Westphal nucleus contains cell bodies of preganglionic fibers that travel with CN III to the ciliary ganglion. The superior salivatory nucleus contains cell bodies of preganglionic fibers that travel with CN VII to the pterygopalatine and submandibular ganglia. The inferior salivatory nucleus contains cell bodies of preganglionic fibers that travel with CN IX to the otic ganglion. The rostral portion of the nucleus ambiguus contains preganglionic cell bodies that distribute with CN IX; the rest of the nucleus ambiguus and the dorsal motor nucleus of the vagus contain cell bodies of preganglionic fibers that travel with CN X to a host of terminal ganglia in the viscera of the thorax and abdomen. NTS, which is not part of the ANS, receives visceral afferents and is part of the larger visceral control system. The figure also illustrates other cranial nerves that are not involved in controlling the ANS (gray labels).
2. The superior salivatory nucleus is in the rostral medulla (Fig. 14-5) and contains parasympathetic neurons that project, through a branch of the facial nerve (CN VII), to the pterygopalatine ganglion (Fig. 14-4, right). The postganglionic fibers supply the lacrimal glands, which produce tears. Another branch of the facial nerve carries preganglionic fibers to the submandibular ganglion. The postganglionic fibers supply two salivary glands, the submandibular and sublingual glands.
3. The inferior salivatory nucleus and the rostral part of the nucleus ambiguus in the rostral medulla (Fig. 14-5) contain parasympathetic neurons that project through the glossopharyngeal nerve (CN IX) to the otic ganglion (Fig. 14-4, right). The postganglionic fibers supply a third salivary gland, the parotid gland.
Cranial Nerve X Most parasympathetic output occurs through the vagus nerve (CN X). Cell bodies of vagal preganglionic parasympathetic neurons are found in the medulla within the nucleus ambiguus and the dorsal motor nucleus of the vagus (Fig. 14-5). This nerve supplies parasympathetic innervation to all the viscera of the thorax and abdomen, including the gastrointestinal tract between the pharynx and distal end of the colon (Fig. 14-4, right). Among other effects, electrical stimulation of the nucleus ambiguus results in activation of striated muscle in the pharynx, larynx, and esophagus and slows the heart. Stimulation of the dorsal motor nucleus of the vagus initiates secretion of gastric acid, insulin, and glucagon. Preganglionic parasympathetic fibers of the vagus nerve join the esophageal, pulmonary, and cardiac plexuses and travel to terminal ganglia that are located within their target organs.
Sacral Nerves The cell bodies of preganglionic parasympathetic neurons in the sacral spinal cord (S2 to S4) are located in a position similar to that of the preganglionic sympathetic neurons—although they do not form a distinct intermediolateral column. Their axons leave through ventral roots and travel with the pelvic splanchnic nerves to their terminal ganglia in the descending colon and rectum (see Chapter 41) as well as to the bladder (see Chapter 33) and reproductive organs (see Chapters 54 and 55).
All internal organs are densely innervated by visceral afferents. Some of these receptors monitor nociceptive (painful) input. Others are sensitive to a variety of mechanical and chemical (physiological) stimuli, including stretch of the heart, blood vessels, and hollow viscera, as well as PCO2, PO2, pH, blood glucose, and temperature of the skin and internal organs. Many visceral nociceptive fibers travel in sympathetic nerves (blue projections in Fig. 14-2). Most axons from physiological receptors travel with parasympathetic fibers. As is the case with somatic afferents (see Chapter 10), the cell bodies of visceral afferent fibers are located within the dorsal root ganglia or cranial nerve ganglia (e.g., nodose and petrosal ganglia). Ninety percent of these visceral afferents are unmyelinated.
The largest concentration of visceral afferent axons can be found in the vagus nerve, which carries non-nociceptive afferent input to the CNS from all viscera of the thorax and abdomen. Most fibers in the vagus nerve are afferents, even though all parasympathetic preganglionic output (i.e., efferents) to the abdominal and thoracic viscera also travels in the vagus nerve. Vagal afferents, whose cell bodies are located in the nodose ganglion, carry information about the distention of hollow organs (e.g., blood vessels, cardiac chambers, stomach, bronchioles), blood gases (e.g., PO2, PCO2, pH), and body chemistry (e.g., glucose concentration) to the medulla.
Internal organs also have nociceptive receptors that are sensitive to excessive stretch, noxious chemical irritants, and very large decreases in pH. In the CNS, this visceral pain input is mapped (see Chapter 16) viscerotopically at the level of the spinal cord because most visceral nociceptive fibers travel with the sympathetic fibers and enter the spinal cord at a specific segmental level along with a spinal nerve (Fig. 14-2). This viscerotopic mapping is also present in the brainstem but not at the level of the cerebral cortex. Thus, awareness of visceral pain is not usually localized to a specific organ but is instead “referred” to the dermatome (see Chapter 10) that is innervated by the same spinal nerve. This referred pain results from lack of precision in the central organization of visceral pain pathways. Thus, you know that the pain is associated with a particular spinal nerve, but you do not know where the pain is coming from (i.e., from the skin or a visceral organ). For example, nociceptive input from the left ventricle of the heart is referred to the left T1 to T5 dermatomes and leads to discomfort in the left arm and left side of the chest, whereas nociceptive input from the diaphragm is referred to the C3 to C5 dermatomes and is interpreted as pain in the shoulder. This visceral pain is often felt as a vague burning or pressure sensation.
The enteric nervous system (ENS) is a collection of nerve plexuses that surround the gastrointestinal tract, including the pancreas and biliary system. Although it is entirely peripheral, the ENS receives input from the sympathetic and parasympathetic divisions of the ANS. The ENS is estimated to contain more than 100 million neurons, including afferent neurons, interneurons, and efferent postganglionic parasympathetic neurons. Enteric neurons contain many different neurotransmitters and neuromodulators. Thus, not only does the total number of neurons in the enteric division exceed that of the spinal cord, but the neurochemical complexity of the ENS also approaches that of the CNS. The anatomy of the ENS as well as its role in controlling gastrointestinal function is discussed in Chapter 41.
The plexuses of the ENS are a system of ganglia sandwiched between the layers of the gut and connected by a dense meshwork of nerve fibers. The myenteric or Auerbach’s plexus (Fig. 14-6) lies between the external longitudinal and the deeper circular smooth muscle layers; the submucosal or Meissner’s plexus lies between the circular muscle and the most internal layer of smooth muscle, the muscularis mucosae (see Fig. 41-3A). In the intestinal wall, the myenteric plexus is involved primarily in the control of motility, whereas the submucosal plexus is involved in the control of ion and fluid transport. Both the myenteric and the submucosal plexuses receive pre ganglionic parasympathetic innervation from the vagus nerve (or sacral nerves in the case of the distal portion of colon and rectum). Thus, in one sense, the enteric division is homologous to a large and complex parasympathetic terminal ganglion. The other major input to the ENS is from postganglionic sympathetic neurons. Thus, the ENS can be thought of as “postganglionic” with respect to the parasympathetic division and “post-postganglionic” with respect to the sympathetic division. Input from both the sympathetic and parasympathetic divisions modulates the activity of the ENS, but the ENS can by and large function normally without extrinsic input. The isolated ENS can respond appropriately to local stimuli and control most aspects of gut function, including initiating peristaltic activity in response to gastric distention, controlling secretory and absorptive functions, and triggering biliary contractions.

Figure 14-6 The myenteric (Auerbach’s) plexus. The photograph is a scanning electron micrograph of the myenteric plexus of the mouse large intestine. The external longitudinal muscle of the intestine was removed so that the view is of the plexus (the highly interconnected meshwork of neuron cell bodies, axons, and dendrites on the surface) spreading over the deeper circular layer of muscle. (From Burnstock G: Autonomic neuromuscular junctions: Current developments and future directions. J Anat 1986; 146:1-30.)
PHOX2B: Master Gene of the Visceral Control System
During development, a complex genetic program expressed in each progenitor cell determines cell fate—ensuring that the cell migrates to the correct location and differentiates into the correct mature cell type. The genes that encode some transcription factors (see Chapter 4) turn on at a specific time during development and trigger normal migration or differentiation of specific cell types. Phox2b is a transcription factor required for development of nearly all neurons within the visceral control system and almost no other class of neuron.
Phox2b is expressed early in development in all neurons of the mammalian visceral control system—including preganglionic and postganglionic parasympathetic neurons, postganglionic (but not preganglionic) sympathetic neurons, enteric neurons, all visceral afferents, and neurons of the nucleus tractus solitarii (NTS), on which they synapse. No other cells express Phox2b, except for neurons of the locus coeruleus in the pons (which plays an important role in cardiovascular control) and certain cranial nerve nuclei (most of which are important for respiratory output and for feeding). Knockout of the mouse Phox2b gene leads to loss of development of all these neurons and is fatal. In humans, heterozygous mutations in PHOX2B cause congenital central hypoventilation syndrome (CCHS), a congenital form of Ondine’s curse (see Chapter 32). Infants with this condition have problems with breathing while they sleep, probably because of a deficiency in detection of O2 or CO2 in their blood by peripheral chemoreceptors or defective integration of this information by the NTS. A subset of patients with PHOX2B mutations also have Hirschprung disease (see Chapter 41), in which the ENS does not develop normally in a portion of the colon. This combination of CCHS and Hirschprung disease is called Haddad syndrome. Some CCHS patients also develop tumors of derivatives of the sympathetic nervous system called neuroblastomas. (See Note: Phox2b)
As a rule, the neural circuits that carry out individual tasks or behaviors (e.g., locomotion, sleep, vision) are formed by several classes of neurons that follow unrelated developmental pathways before they assemble into a circuit. So far, the visceral control system is unique in that most of its constituent neuronal types differentiate under the control of the same, highly specific transcription factor, Phox2b. PHOX2B can thus be considered the “master gene” of the visceral control system.
All innervation of skeletal muscle in humans is excitatory. In contrast, many visceral targets receive both inhibitory and excitatory synapses. These antagonistic synapses arise from the two opposing divisions of the ANS, the sympathetic and the parasympathetic.
In organs that are stimulated during physical activity, the sympathetic division is excitatory and the parasympathetic division is inhibitory. For example, sympathetic input increases the heart rate, whereas parasympathetic input decreases it. In organs whose activity increases while the body is at rest, the opposite is true. For example, the parasympathetic division stimulates peristalsis of the gut, whereas the sympathetic division inhibits it.
Although antagonistic effects of the sympathetic and parasympathetic divisions of the ANS are the general rule for most end organs, exceptions exist. For example, the salivary glands are stimulated by both divisions, although stimulation by the sympathetic division has characteristics different from parasympathetic stimulation (see Chapter 43). In addition, some organs receive innervation from only one of these two divisions of the ANS. For example, sweat glands, piloerector muscles, and most peripheral blood vessels receive input from only the sympathetic division.
Synapses of the ANS are specialized for their function. Rather than possessing synaptic terminals that are typical of somatic motor axons, many postganglionic autonomic neurons have bulbous expansions, or varicosities, that are distributed along their axons within their target organ (Fig. 14-7). It was once believed that these varicosities indicated that neurotransmitter release sites of the ANS did not form close contact with end organs and that neurotransmitters needed to diffuse long distances across the extracellular space to reach their targets. However, we now recognize that many varicosities form synapses with their targets, with a synaptic cleft extending ~50 nm across. At each varicosity, autonomic axons form an “en passant” synapse with their end-organ target. This arrangement results in an increase in the number of targets that a single axonal branch can influence, with wider distribution of autonomic output.

Figure 14-7 Synapses of autonomic neurons with their target organs. Many axons of postganglionic neurons make multiple points of contact (varicosities) with their targets. In this scanning electron micrograph of the axon of a postganglionic sympathetic neuron from a guinea pig grown in tissue culture, the arrows indicate varicosities. (From Burnstock G: Autonomic neuromuscular junctions: Current developments and future directions. J Anat 1986; 146:1-30.)
At synapses between postganglionic neurons and target cells, the two major divisions of the ANS use different neurotransmitters and receptors (Table 14-1). However, in both the sympathetic and parasympathetic divisions, synaptic transmission between preganglionic and postganglionic neurons (termed ganglionic transmission because the synapse is located in a ganglion) is mediated by acetylcholine (ACh) acting on nicotinic receptors (Fig. 14-8). Nicotinic receptors are ligand-gated channels (i.e., ionotropic receptors) with a pentameric structure (see Chapter 8). Table 14-2 summarizes some of the properties of nicotinic receptors. The nicotinic receptors on postganglionic autonomic neurons are a molecular subtype (N2) different from that found at the neuromuscular junction (N1). Both are ligand-gated ion channels activated by ACh or nicotine. However, whereas the N1 receptors at the neuromuscular junction (see Chapter 8) are stimulated by decamethonium and preferentially blocked by d-tubocurarine, the autonomic N2 receptors are stimulated by tetramethylammonium but resistant to d-tubocurarine. When activated, N1 and N2 receptors are both permeable to Na+ and K+. Thus, nicotinic transmission triggered by stimulation of preganglionic neurons leads to rapid depolarization of postganglionic neurons.
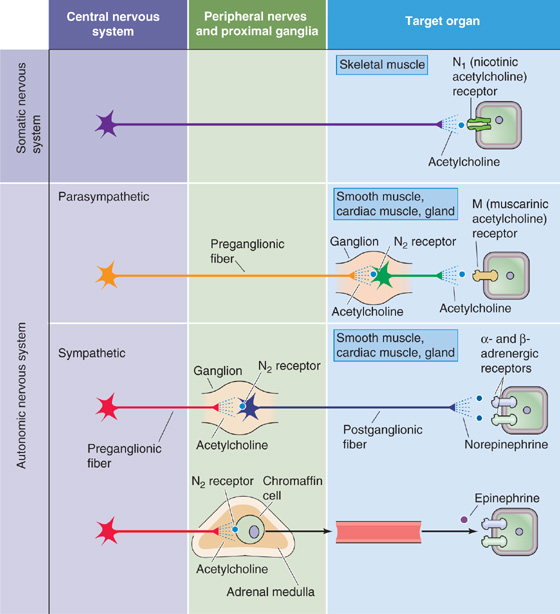
Figure 14-8 Major neurotransmitters of the ANS. In the case of the somatic neuron, the pathway between the CNS and effector cell is monosynaptic. The neuron releases ACh, which binds to N1-type nicotinic receptors on the postsynaptic membrane (i.e., skeletal muscle cell). In the case of both the parasympathetic and sympathetic divisions, the preganglionic neuron releases ACh, which acts at N2-type nicotinic receptors on the postsynaptic membrane of the postganglionic neuron. In the case of the postganglionic parasympathetic neuron, the neurotransmitter is ACh, but the postsynaptic receptor is a muscarinic receptor (i.e., GPCR) of one of five subtypes (M1 to M5). In the case of most postganglionic sympathetic neurons, the neurotransmitter is norepinephrine. The postsynaptic receptor is an adrenergic receptor (i.e., GPCR) of one of two major subtypes (α and β).
Table 14-1 Properties of the Sympathetic and Parasympathetic Divisions
All postganglionic para sympathetic neurons act through muscarinic ACh receptors on the postsynaptic membrane of the target cell (Fig. 14-8). Activation of this receptor can either stimulate or inhibit function of the target cell. Cellular responses induced by muscarinic receptor stimulation are more varied than are those induced by nicotinic receptors. Muscarinic receptors are G protein–coupled receptors (GPCRs; see p. 52)—also known as metabotropic receptors—that (1) stimulate the hydrolysis of phosphoinositide and thus increase [Ca2+]i and activate protein kinase C, (2) inhibit adenylyl cyclase and thus decrease cyclic adenosine monophosphate (cAMP) levels, or (3) directly modulate K+ channels through the G protein βγ complex. Because they are mediated by second messengers, muscarinic responses, unlike the rapid responses evoked by nicotine receptors, are slow and prolonged.
Muscarinic receptors exist in five different pharmacological subtypes (M1 to M5) that are encoded by five different genes. All five subtypes are highly homologous to each other but very different from the nicotinic receptors, which are ligand-gated ion channels. Subtypes M1 to M5 are each stimulated by ACh and muscarine and are blocked by atropine. These muscarinic subtypes have a heterogeneous distribution among tissues, and in many cases a given cell may express more than one subtype. Although a wide variety of antagonists inhibit the muscarinic receptors, none is completely selective for a specific subtype. However, it is possible to classify a receptor on the basis of its affinity profile for a battery of antagonists. Selective agonists for the different isoforms have not been available. (See Note: Muscarinic Receptors)
A molecular characteristic of the muscarinic receptors is that the third cytoplasmic loop (i.e., between the fifth and sixth membrane-spanning segments) is different in M1, M3, and M5 on the one hand and M2 and M4 on the other. This loop appears to play a role in coupling of the receptor to the G protein downstream in the signal transduction cascade. In general M1, M3, and M5 preferentially couple to Gαq and then to phospholipase C, with release of IP3 and diacylglycerol. On the other hand, M2 and M4 preferentially couple to Gαi or Gαo to inhibit adenylyl cyclase and thus decrease [cAMP]i (see Chapter 3).
Table 14-2 Signaling Pathways for Nicotinic, Muscarinic, Adrenergic, and Dopaminergic Receptors
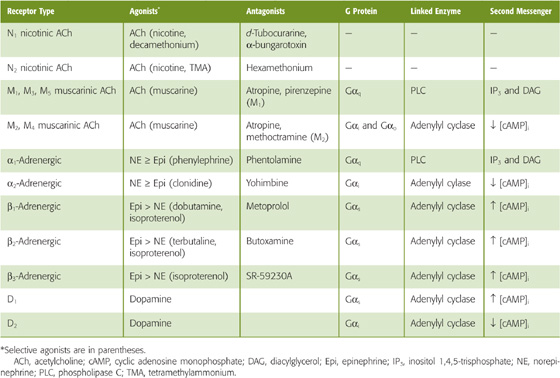
Most postganglionic sympathetic neurons release norepinephrine (Fig. 14-8), which acts on target cells through adrenergic receptors. The sympathetic innervation of sweat glands is an exception to this rule. Sweat glands are innervated by sympathetic neurons that release ACh and act through muscarinic receptors. The adrenergic receptors are all GPCRs and are highly homologous to the muscarinic receptors (see Chapter 24). Two major types of adrenergic receptor are recognized, α and β, each of which exists in multiple subtypes (e.g., α1, α2, β1, β2, and β3). In addition, there are heterogeneous α1 and α2 receptors, with three cloned subtypes of each. Table 14-2 lists the signaling pathways that are generally linked to these receptors. For example, β1 receptors in the heart activate the Gs heterotrimeric G protein and stimulate adenylyl cyclase, which antagonizes the effects of muscarinic receptors. (See Note: Cholinergic Sympathetic Neurons)
Adrenergic receptor subtypes have a tissue-specific distribution. α1 Receptors predominate on blood vessels, α2 on presynaptic terminals, β1 in the heart, β2 in high concentration in the bronchial muscle of the lungs, and β3 in fat cells. This distribution has permitted the development of many clinically useful agents that are selective for different subtypes and tissues. For example, α1 agonists are effective as nasal decongestants, and α2 antagonists have been used to treat impotence. β1 Agonists increase cardiac output in congestive heart failure, whereas β1 antagonists are useful antihypertensive agents. β2 Agonists are used as bronchodilators in patients with asthma and chronic lung disease.
The adrenal medulla (see Chapter 50) is a special adaptation of the sympathetic division, homologous to a sympathetic ganglion (Fig. 14-8). It is innervated by preganglionic sympathetic neurons, and the postsynaptic target cells, which are called chromaffin cells, are analogous to postganglionic sympathetic neurons. Thus, chromaffin cells have nicotinic ACh receptors. However, rather than possessing axons that release norepinephrine onto a specific target organ, the chromaffin cells reside near blood vessels and release epinephrine into the bloodstream. This neuroendocrine component of sympathetic output enhances the ability of the sympathetic division to broadcast its output throughout the body. Norepinephrine and epinephrine both activate all five subtypes of adrenergic receptor, but with different affinities (Table 14-2). In general, the α receptors have a greater affinity for norepinephrine, whereas the β receptors have a greater affinity for epinephrine.
The simplified scheme described in the preceding discussion is very useful for understanding the function of the ANS. However, two additional layers of complexity are superimposed on this scheme. First, some postganglionic neurons, both sympathetic and parasympathetic, have muscarinic in addition to nicotinic receptors. Second, at all levels of the ANS, certain neurotransmitters and postsynaptic receptors are neither cholinergic nor adrenergic. We discuss the first exception in this section and the second in the following section.
If we stimulate the release of ACh from preganglionic neurons or apply ACh to an autonomic ganglion, many postganglionic neurons exhibit both nicotinic and muscarinic responses. Because nicotinic receptors (N2) are ligand-gated ion channels, nicotinic neurotransmission causes a fast, monophasic excitatory postsynaptic potential (EPSP). In contrast, because muscarinic receptors are GPCRs, neurotransmission by this route leads to a slower electrical response that can be either inhibitory or excitatory. Thus, depending on the ganglion, the result is a multiphasic postsynaptic response that can be a combination of a fast EPSP through a nicotinic receptor plus either a slow EPSP or a slow inhibitory postsynaptic potential (IPSP) through a muscarinic receptor. Figure 14-9A shows a fast EPSP followed by a slow EPSP.
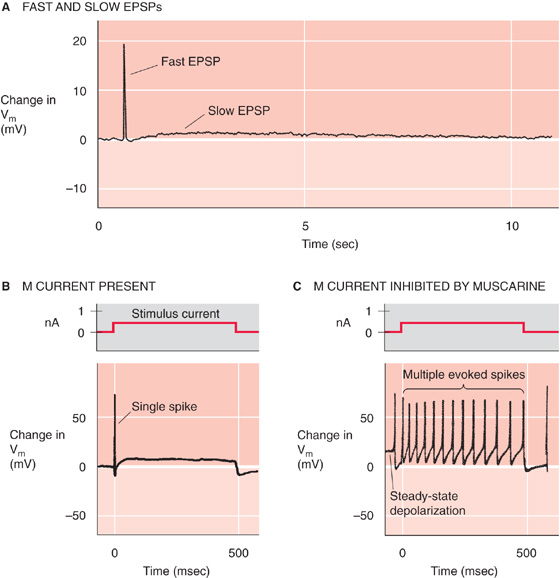
Figure 14-9 Dual nicotinic and muscarinic neurotransmission between sympathetic preganglionic and postganglionic neurons. A, Stimulation of a frog preganglionic sympathetic neuron releases ACh, which triggers a fast EPSP (due to activation of nicotinic receptors on the postganglionic sympathetic neuron), followed by a slow EPSP (due to activation of muscarinic receptors on the postganglionic neuron). B, The M current (mediated by a K+ channel) normally hyperpolarizes this rat sympathetic postganglionic neuron, thereby inhibiting trains of action potentials. Thus, injection of current elicits only a single action potential. C, In the same experiment as that in B, the addition of muscarine stimulates a muscarinic receptor (i.e., GPCR) and triggers a signal transduction cascade that blocks the M current. One result is a steady-state depolarization of the cell. In addition, injection of current now elicits a train of action potentials. (A, Data from Adams PR, Brown DA: Synaptic inhibition of the M-current: Slow excitatory post-synaptic potential mechanism in bullfrog sympathetic neurones. J Physiol 1982; 332:263-272. B and C, Data from Brown DA, Constanti A: Intracellular observations on the effects of muscarinic agonists on rat sympathetic neurones. Br J Pharmacol 1980; 70:593-608.)
A well-characterized effect of muscarinic neurotransmission in autonomic ganglia is inhibition of a specific K+ current called the M current. The M current is widely distributed in visceral end organs, autonomic ganglia, and the CNS. In the baseline state, the K+ channel that underlies the M current is active, thereby producing slight hyperpolarization. In the example shown in Figure 14-9B, with the stabilizing M current present, electrical stimulation of the neuron causes only a single spike. If we now add muscarine to the neuron, activation of the muscarinic receptor turns off the hyperpolarizing M current and thus leads to a small depolarization. If we repeat the electrical stimulation in the continued presence of muscarine (Fig. 14-9C), repetitive spikes appear because loss of the stabilizing influence of the M current increases the excitability of the neuron. The slow, modulatory effects of muscarinic responses greatly enhance the ability of the ANS to control visceral activity beyond what could be accomplished with only fast nicotinic EPSPs.
In the 1930s, Sir Henry Dale first proposed that sympathetic nerves release a transmitter similar to epinephrine (now known to be norepinephrine) and parasympathetic nerves release ACh. For many years, attention was focused on these two neurotransmitters, primarily because they mediate large and fast postsynaptic responses that can be easily studied. In addition, a variety of antagonists are available to block cholinergic and adrenergic receptors and thereby permit clear characterization of the roles of these receptors in the control of visceral function. More recently, it has become evident that some neurotransmission in the ANS involves neither adrenergic nor cholinergic pathways. Moreover, many neuronal synapses use more than a single neurotransmitter. Such cotransmission is now known to be common in the ANS. As many as eight different neurotransmitters may be found within some neurons, a phenomenon known as co-localization (see Table 13-1). Thus, ACh and norepinephrine play important but not exclusive roles in autonomic control. (See Note: Sir Henry H. Dale)
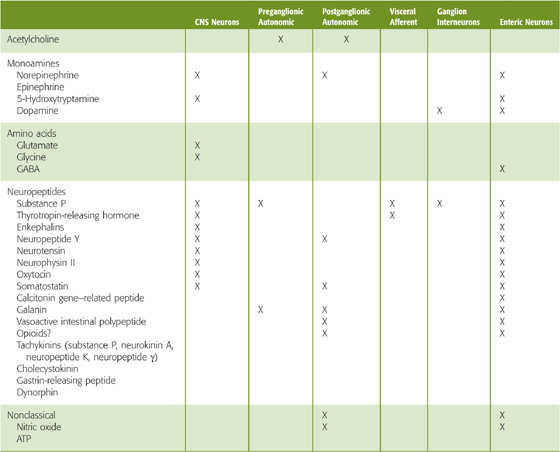
The distribution and function of nonadrenergic, noncholinergic transmitters are only partially understood. However, these transmitters are found at every level of autonomic control (Table 14-3), where they can cause a wide range of postsynaptic responses. These nonclassic transmitters may cause slow synaptic potentials or may modulate the response to other inputs (as in the case of the M current) without having obvious direct effects. In other cases, nonclassic transmitters have no known effects and may be acting in ways that have not been determined.
Table 14-3 Neurotransmitters Present Within the Autonomic Nervous System
Although co-localization of neurotransmitters is recognized as a common property of neurons, it is not clear what controls the release of each of the many neurotransmitters. In some cases, the proportion of neurotransmitters released depends on the level of neuronal activity (see Chapter 13). For example, medullary raphe neurons project to the intermediolateral cell column in the spinal cord, where they co-release serotonin, thyrotropin-releasing hormone, and substance P onto sympathetic preganglionic neurons. The proportions of released neurotransmitters are controlled by neuronal firing frequency: at low firing rates, 5-hydroxytryptamine is released alone; at intermediate firing rates, thyrotropin-releasing hormone is also released; and at high firing rates, all three neurotransmitters are released. This frequency-dependent modulation of synaptic transmission provides a mechanism for enhancing the versatility of the ANS.
It was not until the 1970s that a nonadrenergic, noncholinergic class of sympathetic or parasympathetic neurons was first proposed by Geoffrey Burnstock and colleagues, who suggested that adenosine triphosphate (ATP) might act as the neurotransmitter. This idea, that a molecule used as an intracellular energy substrate could also be a synaptic transmitter, was initially difficult to prove. However, it is now clear that neurons use a variety of classes of molecules for intercellular communication (see Chapter 13). Two of the most surprising examples of nonclassic transmitters, nitric oxide (NO) and ATP, were first identified and studied as neurotransmitters in the ANS, but they are now known to be more widely used throughout the nervous system.
Adenosine Triphosphate ATP is co-localized with norepinephrine in postganglionic sympathetic vasoconstrictor neurons. It is contained in synaptic vesicles, is released on electrical stimulation, and induces vascular constriction when it is applied directly to vascular smooth muscle. The effect of ATP results from activation of P2 purinoceptors on smooth muscle, which include ligand-gated ion channels (P2X) and GPCRs (P2Y and P2U). P2X receptors are present on autonomic neurons and smooth muscle cells of blood vessels, the urinary bladder, and other visceral targets. P2X receptor channels have a relatively high Ca2+ permeability (see Chapter 13). In smooth muscle, ATP-induced depolarization can also activate voltage-gated Ca2+ channels (see Chapter 7) and thus lead to an elevation in [Ca2+]i and a rapid phase of contraction (Fig. 14-10). Norepinephrine, by binding to α1-adrenergic receptors, acts through a heterotrimeric G protein (see Chapter 3) to facilitate the release of Ca2+ from intracellular stores and thereby produce a slower phase of contraction. Finally, the release of neuropeptide Y may, after prolonged and intense stimulation, elicit a third component of contraction.

Figure 14-10 Cotransmission with ATP, norepinephrine, and neuropeptide Y in the ANS. In this example, stimulation of a postganglionic sympathetic neuron causes three phases of contraction of a vascular smooth muscle cell. Each phase corresponds to the release of a different neurotransmitter or group of transmitters. In phase 1, ATP binds to a P2X purinoceptor (a ligand-gated cation channel) on the smooth muscle cell, leading to depolarization, activation of voltage-gated Ca2+ channels, increased [Ca2+]i, and the rapid phase of contraction. In phase 2, norepinephrine binds to an α1-adrenergic receptor, which—through a Gq/PLC/IP3 cascade, leads to Ca2+ release from internal stores and the second phase of contraction. In phase 3, when it is present, neuropeptide Y binds to a Y1 receptor and somehow causes an increase in [Ca2+]i and thus produces the slowest phase of contraction. ER, endoplasmic reticulum; IP3, inositol 1, 4, 5-trisphosphate; PLC, phospolipase C.
Nitric Oxide In the 1970s, it was also discovered that the vascular endothelium produces a substance that induces relaxation of vascular smooth muscle. First called endothelium-derived relaxation factor, it was identified as the free radical NO in 1987. NO is an unusual molecule for intercellular communication because it is a short-lived gas. It is produced locally from L-arginine by the enzyme nitric oxide synthase (NOS; see Chapter 3). The NO then diffuses a short distance to a neighboring cell, where its effects are primarily mediated by the activation of guanylyl cyclase.
NOS is found in the preganglionic and postganglionic neurons of both the sympathetic and parasympathetic divisions as well as in vascular endothelial cells. It is not specific for any type of neuron inasmuch as it is found in both norepinephrine-and ACh-containing cells as well as in neurons containing a variety of neuropeptides. Figure 14-11 shows how a parasympathetic neuron may simultaneously release NO, ACh, and vasoactive intestinal polypeptide, each acting in concert to lower [Ca2+]i and to relax vascular smooth muscle. Why NO is so ubiquitous and when its release is important are not known. However, evidence now indicates that abnormalities of the NO system are involved in the pathophysiological processes of adult respiratory distress syndrome, high-altitude pulmonary edema, stroke, and other diseases. Understanding of its physiological and pathophysiological roles has led to the introduction of clinical treatments that modulate the NO system. Examples include the use of gaseous NO for treatment of pulmonary edema, NO generators such as nitroglycerin for treatment of angina, and cGMP phosphodiesterase inhibitors such as sildenafil (Viagra) for treatment of erectile dysfunction.
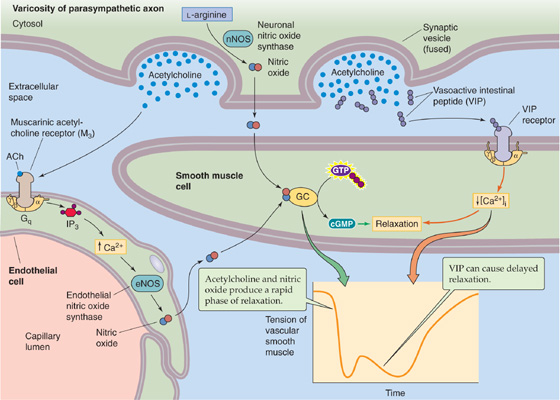
Figure 14-11 Action of NO in the ANS. Stimulation of a postganglionic parasympathetic neuron can cause more than one phase of relaxation of a vascular smooth muscle cell, corresponding to the release of a different neurotransmitter or group of transmitters. The first phase in this example is mediated by both NO and ACh. The neuron releases NO, which diffuses to the smooth muscle cell. In addition, ACh binds to M3 muscarinic receptors (i.e., GPCR) on endothelial cells, leading to production of NO, which also diffuses to the smooth muscle cell. Both sources of NO activate guanylyl cyclase and raise [cGMP]i in the smooth muscle cell and contribute to the first phase of relaxation. In the second phase, which tends to occur more with prolonged or intense stimulation, the neuropeptide VIP (or a related peptide) binds to receptors on the smooth muscle cell and causes a delayed relaxation through an increase in [cAMP]i or a decrease in [Ca2+]i. cGMP, cyclic guanosine monophosphate; GC, guanylyl cyclase; VIP, vasoactive intestinal polypeptide.
In 1915, Walter Cannon proposed that the entire sympathetic division is activated together and has a uniform effect on all target organs. In response to fear, exercise, and other types of stress, the sympathetic division produces a massive and coordinated output to all end organs simultaneously, and parasympathetic output ceases. This type of sympathetic output is used to ready the body for life-threatening situations—the so-called fight-or-flight response. Thus, when a person is presented a fearful or menacing stimulus, the sympathetic division coordinates all body functions to respond appropriately to the stressful situation. This response includes increases in heart rate, cardiac contractility, blood pressure, and ventilation of the lungs; bronchial dilatation; sweating; piloerection; liberation of glucose into the blood; inhibition of insulin secretion; reduction in blood clotting time; mobilization of blood cells by contraction of the spleen; and decreased gastrointestinal activity. This mass response is a primitive mechanism for survival. In some people, such a response can be triggered spontaneously or with minimal provocation; each individual episode is then called a panic attack. (See Note: Walter B. Cannon)
The fight-or-flight response is an important mechanism for survival, but under normal nonstressful conditions, output of the sympathetic division can also be more discrete and organ specific. In contrast to Cannon’s original proposal, the sympathetic division does not actually produce uniform effects on all visceral targets. Different postganglionic sympathetic neurons have different electrophysiological properties and, in addition to norepinephrine, release other neurotransmitters. This specific distribution of neuroactive chemicals among neurons is called chemical coding. For example, depolarization of guinea pig postganglionic sympathetic neurons in the lumbar sympathetic chain ganglia causes a brief burst of action potentials in 95% of the neurons and release of norepinephrine together with ATP and neuropeptide Y. These neurons are thought to innervate arteries and to induce vasoconstriction (Fig. 14-10). In contrast, depolarization of postganglionic sympathetic neurons in the inferior mesenteric ganglion causes sustained firing in 80% of the neurons and release of norepinephrine together with somatostatin. These neurons appear to control gut motility and secretion. Thus, sympathetic neurons have cellular properties that are substantially variable. This variability permits the sympathetic division to produce different effects on targets with different functions.
As opposed to the sympathetic division, neurons in the parasympathetic division function only in a discrete, organ-specific, and reflexive manner. Together with specific visceral afferents and a small number of interneurons, parasympathetic neurons mediate simple reflexes involving target organs. For example, the output for the baroreceptor reflex (see Chapter 23) is mediated by preganglionic parasympathetic neurons in the dorsal motor nucleus of the vagus. Other examples include urination in response to bladder distention (see Chapter 33); salivation in response to the sight or smell of food (see Chapter 43); vagovagal reflexes (see Chapter 41) in the gastrointestinal tract, such as contraction of the colon in response to food in the stomach; and bronchoconstriction in response to activation of receptors in the lungs (see Chapter 32). The pupillary light reflex is an example of an involuntary parasympathetic reflex that can be tested at the bedside (see Chapter 15).
In addition to nuclei that contain parasympathetic preganglionic neurons (Fig. 14-5), a variety of other brainstem structures are also involved in visceral control. These structures include the nucleus tractus solitarii, area postrema, ventrolateral medulla, medullary raphe, reticular formation, locus coeruleus, and parabrachial nucleus. These nuclei within the lower part of the brainstem mediate autonomic reflexes, control specific autonomic functions, or modulate the general level of autonomic tone. In some cases, these nuclei play a well-defined role in one specific autonomic function. For example, stimulation of a group of neurons in the rostral portion of the ventrolateral medulla increases sympathetic output to the cardiovascular system—without affecting respiration or sympathetic output to other targets. In other cases, these nuclei are linked to more than one autonomic function. For example, the medullary raphe contains serotonergic neurons that project to cardiovascular and respiratory neurons, gastrointestinal neurons, the reticular activating system, and pain pathways. Therefore, these neurons can affect the background level of autonomic tone. The specific functions of some nuclei are not known, and their involvement in autonomic control is inferred from their anatomical connections, a correlation between neuron activity and activity in autonomic nerves, or the effect of lesions.
One of the most important lower brainstem structures is the nucleus tractus solitarii (NTS) in the medulla. The NTS contains second-order sensory neurons that receive all peripheral chemoreceptor (see Chapter 32) and baroreceptor input (see Chapter 23) as well as non-nociceptive afferent input from every organ of the thorax and abdomen. Visceral afferents from the vagus nerve make their first synapse within the NTS, where they combine with other afferent impulses derived from the glossopharyngeal (CN IX), facial (CN VII), and trigeminal (CN V) nerves. These visceral afferents form a large bundle of nerve fibers—the tractus solitarius—that the NTS surrounds. Afferent input is distributed to the NTS in a viscerotopic manner, with major subnuclei devoted to respiratory, cardiovascular, gustatory, and gastrointestinal input. The NTS also receives input and sends output to many other CNS regions (Table 14-4), including the brainstem nuclei described earlier as well as the hypothalamus and the forebrain. These widespread interconnections allow the NTS to influence and to be influenced by a wide variety of CNS functions. Thus, the NTS is the major lower brainstem command center for visceral control. It integrates multiple input from visceral afferents and exerts control over autonomic output, thereby participating in autonomic reflexes that maintain the homeostasis of many basic visceral functions.
Table 14-4 Connections to and from the Nucleus Tractus Solitarii
Receives input from |
Vagus nerve (peripheral chemoreceptor/aortic bodies and aortic baroreceptor, as well as non-nociceptive afferent input from every organ of the thorax and abdomen) |
Glossopharyngeal nerve (taste and peripheral chemoreceptors/carotid bodies, carotid baroreceptor) |
Facial nerve (taste) |
Trigeminal nerve (teeth, sinuses) |
Ventrolateral medulla |
Medullary raphe |
Area postrema |
Periaqueductal gray |
Parabrachial nucleus |
Hypothalamus |
Cerebral cortex |
Sends output to |
Intermediolateral cell column (preganglionic sympathetic neurons) and sacral parasympathetic neurons |
Phrenic motor nucleus and other respiratory output pathways |
Dorsal motor nucleus of the vagus (preganglionic parasympathetic neurons from the vagus nerve) |
Nucleus ambiguus (preganglionic parasympathetic neurons from the vagus nerve) |
Ventrolateral medulla |
Medullary raphe |
Area postrema |
Parabrachial nuclei |
Reticular formation |
Forebrain nuclei |
Hypothalamus |
Only a subset of the nervous system is necessary to maintain autonomic body homeostasis under most conditions. The necessary structures include (1) the brainstem nuclei discussed in the preceding section, (2) the brainstem nuclei that contain the parasympathetic preganglionic neurons, (3) the spinal cord, and (4) the peripheral ANS. These components are capable of acting autonomously, even without input from higher (i.e., rostral) forebrain regions. However, forebrain regions do play a role in coordinating and modulating activity in the lower centers. Many rostral CNS centers influence autonomic output; these centers include the hypothalamus, amygdala, prefrontal cortex, entorhinal cortex, insula, and other forebrain nuclei.
The hypothalamus, especially the paraventricular nucleus, is the most important brain region for coordination of autonomic output. The hypothalamus projects to the parabrachial nucleus, medullary raphe, NTS, central gray matter, locus coeruleus, dorsal motor nucleus of the vagus, nucleus ambiguus, and intermediolateral cell column of the spinal cord. Thus, the hypothalamus can initiate and coordinate an integrated response to the body’s needs, including modulation of autonomic output as well as control of neuroendocrine function by the pituitary gland (see Chapter 47). The hypothalamus coordinates autonomic function with feeding, thermoregulation, circadian rhythms, water balance, emotions, sexual drive, reproduction, motivation, and other brain functions and thus plays a dominant role in the integration of higher cortical and limbic systems with autonomic control. The hypothalamus can also initiate the fight-or-flight response. (See Note: Fight or Flight Response)
The hypothalamus often mediates interactions between the forebrain and the brainstem. However, a number of forebrain regions also have direct connections to brainstem nuclei involved in autonomic control. Most of these forebrain regions are part of the limbic system rather than the neocortex. The paucity of direct neocortical connections probably explains why individuals trained to control autonomic output by biofeedback can generally produce only relatively minor effects on overall autonomic activity rather than regulate output to specific organs. Most individuals are incapable of even limited cortical control over the ANS. However, even though we may have only minimal conscious control of autonomic output, cortical processes can strongly modulate the ANS. Emotions, mood, anxiety, stress, and fear can all alter autonomic output (Table 14-5). The pathways for these effects are unknown, but they could be mediated by direct connections or through the hypothalamus.
Table 14-5 Interactions Between Cortical and Autonomic Function
Examples of descending cortical control of autonomic output |
Fear: initiates fight-or-flight response |
Panic attacks: initiate spontaneous fight-or-flight response |
Emotional stress (e.g., first day in gross anatomy laboratory) or painful stimuli: lead to massive vasodilation and hypotension, i.e., vasovagal syncope (fainting) |
Seizures: can induce sudden cardiac death from massive sympathetic output and arrhythmias |
Chronic stress: can lead to peptic ulcers from increased gastric acid secretion |
Sleep deprivation: in rats leads to death from loss of thermoregulation and cardiovascular control |
Cognitive activity: can initiate sexual arousal |
Nervousness (e.g., before an examination) can lead to diarrhea |
Examples in which visceral afferents overwhelm cortical function (i.e., nothing else seems to matter) |
Hunger |
Nausea |
Dyspnea |
Visceral pain |
Bladder and bowel distention |
Hypothermia, hyperthermia |
Not only does forebrain function influence the ANS, visceral activity also influences forebrain function. Visceral afferents reach the neocortex. However, because these afferents are not represented viscerotopically, they cannot be well localized. Nevertheless, visceral afferents can have profound effects on cortical function. Visceral input can modulate the excitability of cortical neurons (see the box titled Vagus Nerve Stimulation in the Treatment of Epilepsy) and, in some cases, can result in such overpowering sensory stimuli that it is not possible to focus cortical activity on any other purpose (Table 14-5).
The ANS maintains physiological parameters within an optimal range by means of feedback loops made up of sensors, afferent fibers, central autonomic control centers (discussed in the preceding section), and effector systems. These feedback loops achieve homeostasis by monitoring input from visceral receptors and adjusting the output of both the sympathetic and parasympathetic divisions to specific organs so that they maintain activity at a set-point determined by involuntary CNS control centers. As we have already noted, the sympathetic and parasympathetic divisions usually act in opposite ways to make these adjustments. Blood pressure control is an example of a visceral feedback loop in which the CNS monitors current blood pressure through afferents from baroreceptors, compares it with an internally determined set-point, and appropriately adjusts output to the heart, blood vessels, adrenal gland, and other targets. An increase in blood pressure (see Chapter 23) causes a reflex decrease in sympathetic output to the heart and an increase in parasympathetic output.
Instead of merely responding through feedback loops, the ANS also anticipates the future needs of the individual. For example, when a person begins to exercise, sympathetic output increases before the increase in metabolic need to prevent an exercise debt from occurring (see Chapter 60). Because of this anticipatory response, alveolar ventilation rises to such an extent that blood levels of CO2 (a byproduct of exercise) actually drop at the onset of exercise. This response is the opposite of what would be expected if the ANS worked purely through feedback loops, in which case an obligatory increase in CO2 levels would have preceded the increase in respiratory output (see Chapter 32). Similarly, a trained athlete’s heart rate begins to increase several seconds before the starting gun fires to signal the beginning of a 100-meter dash. This anticipation of future activity, or feedforward stimulation during exercise, is a key component of the regulation of homeostasis during stress because it prevents large changes in physiological parameters that could be detrimental to optimal function. This type of response probably resulted in an evolutionary advantage that permitted the body to respond rapidly and more efficiently to a threat of danger. A system relying solely on feedback could produce a response that is delayed or out of phase with respect to the stimulus. The central neuronal pathways responsible for this anticipatory or feedforward response are not known.
Vagus Nerve Stimulation in the Treatment of Epilepsy
It is often not appreciated just how much effect the ANS can have on cortical function. Table 14-5 lists several examples in which strong input from visceral afferents can overwhelm cortical function, to the point that concentrating on anything else is nearly impossible. As we have already noted, not only does the vagus nerve contain parasympathetic preganglionic motor fibers, t also contains a wide variety of sensory fibers from viscera in the thorax and abdomen. Discovery of the influence of vagal afferent input on seizures has led to development of the vagus nerve stimulator, which is used clinically. The surgically implanted device electrically stimulates the vagus nerve for 30 seconds every 5 minutes, 24 hours per day. In addition, when patients feel a seizure coming on (an aura), they can activate the device with a hand-held magnet to deliver extra pulses. Clinical studies have shown that this treatment reduces the number of seizures by about half in about one in four patients. It remains to be determined whether a subgroup of patients may be particularly responsive to this treatment. Side effects include hoarseness, coughing, and breathlessness. That this approach works at all indicates how important visceral input is to cortical function. Vagal input can influence many rostral brain structures, but it is not yet clear whether stimulation of peripheral chemoreceptor afferents, pulmonary afferents, or other visceral afferent pathways is important for the anticonvulsant effect. If the specific pathways could be identified, it might be possible to selectively stimulate these pathways or to activate them pharmacologically to produce an anticonvulsant effect with fewer side effects.
The human nervous system is built in a hierarchy that mirrors phylogenetic evolution (see Chapter 10). Each of the successively more primitive components is capable of independent, organized, and adaptive behavior. In turn, the activity of each of the more primitive levels is modulated by rostral, more phylogenetically advanced components. (See Note: Hierarchical Reflex Loops in the Autonomic Nervous System)
The enteric nervous system of humans is homologous to the most primitive nervous system, the neural net of jellyfish. In both cases, the component neurons control motility and nutrient absorption and respond appropriately to external stimuli.
Crosstalk Between Autonomic Functions Can Be Pathological
Visceral control of each of the body’s organs occurs relatively independently of the others. However, some overlap in control systems can be noted for different components of the ANS. For example, stimulation of the baroreceptors causes inhibition of respiration. Conversely, the decrease in thoracic pressure that occurs during inspiration normally triggers a reflex decrease in heart rate. Many neurons in the brainstem and spinal cord have a firing pattern that is modulated in time with both the heartbeat and respiratory activity. This spillover may be responsible for the frequent observation of sinus arrhythmia on electrocardiograms of normal patients, whereby the heart rate is irregular because of an exaggeration of the normal influence of respiration on heart rate. These phenomena have no clear evolutionary advantage. Instead, they may simply be due to an error in separating closely related physiological control mechanisms.
In some cases, overlap between physiological control mechanisms can have serious consequences. For example, control of micturition overlaps with cardiorespiratory control. An increase in bladder pressure can lead to apnea and hypertension. Conversely, each breath is accompanied by an increase in neural outflow to the bladder. In patients with obstruction of urinary outflow, as can be seen in men with enlarged prostates, the bladder can become severely distended. If this obstruction is relieved suddenly by insertion of a catheter and the bladder is drained too rapidly, blood pressure can drop precipitously. In extreme cases, the hypotension causes syncope (fainting) or a stroke. A similar phenomenon can occur in some people with less provocation. During emptying of a relatively full bladder, blood pressure can drop precipitously and lead to postmicturition syncope, with the patient suddenly falling unconscious on the bathroom floor.
The autonomic ganglia are homologous to ganglionic nervous systems, such as those of annelid worms. Autonomic ganglia were previously considered a simple relay station for signals from the CNS to the periphery, but it is now clear that they integrate afferent input from the viscera and have substantial independent control mechanisms. The largest of the sympathetic ganglia, the superior cervical ganglion, contains about 1 million neurons. In addition to postganglionic cell bodies, autonomic ganglia also contain interneurons. Axons from interneurons, sensory receptors located in the end organs, and preganglionic neurons converge with postganglionic neuron dendrites to form a dense network of nerve fibers, or a neuropil, within the ganglion. This neuropil confers considerable computational capability on the ganglia. As opposed to feedback from skeletal muscle, which occurs only in the CNS, the peripheral synapses of visceral afferents result in substantial integration of autonomic activity at peripheral sites. This integration is enhanced by the variety of neurotransmitters released, for example, by interneurons in autonomic ganglia (Table 14-3). Thus, although fast neurotransmission from preganglionic neurons to postganglionic neurons is an important role of the autonomic ganglia, the ganglia are not simply relays.
The spinal cord, which coordinates activity among different root levels, first appeared with the evolution of chordates. The CNS of amphioxus, a primitive chordate, is essentially just a spinal cord. In humans who suffer transection of the low cervical spinal cord—and in whom the outflow of the respiratory system is spared (see Chapter 32)—the caudal spinal cord and lower autonomic ganglia can still continue to maintain homeostasis. However, these individuals are incapable of more complex responses that require reflexes mediated by the cranial nerve afferents and cranial parasympathetic outflow. In many patients, this situation can lead to maladaptive reflexes such as autonomic hyperreflexia, whereby a full bladder results in hypertension and sweating (see the box on pathological crosstalk).
Horner Syndrome
One of the keys to neurological diagnosis has always been neuroanatomical localization (Fig. 14-12). A classic condition in which it is important to define neuroanatomy is Horner syndrome: the combination of unilateral ptosis (drooping eyelid), miosis (small pupil), and anhidrosis (lack of sweating). Sympathetic neurons innervate the smooth muscle that elevates the eyelid, the pupillary dilator muscle, and the sweat glands of the face. Horner syndrome results from loss of the normal sympathetic innervation on one side of the face. The differential diagnosis of this syndrome is large, but it can be narrowed if the site of involvement of the sympathetic pathways can be identified. Involvement of first-order sympathetic neurons can occur at their cell bodies in the hypothalamus or along their axons traveling down to the ipsilateral intermediolateral column of the spinal cord. Thus, a first-order Horner syndrome can be due to ischemia of the lateral medulla (e.g., occlusion of the posterior inferior cerebellar artery, the so-called Wallenberg syndrome). In this case, other brainstem abnormalities will also be present. The second-order sympathetic neurons, or preganglionic neurons, can be affected at their origin in the intermediolateral column or along their axons. Those that supply the eye synapse in the superior cervical ganglion. A second-order Horner syndrome can be the first sign that a Pancoast tumor exists in the apex of the lung and is encroaching on the sympathetic nerves as they travel to the superior cervical ganglion. Finally, third-order sympathetic neurons, or postganglionic neurons, can be involved at the ganglion or along their course to the eye. Because they travel within the wall of the carotid artery, these sympathetic nerves can be damaged during a carotid artery “dissection.” Dissection is damage to the wall of the artery, often caused by a neck injury. In time, the damage to the vessel can lead to a blood clot that will obstruct blood flow. Thus, a Horner syndrome can be a warning that without treatment, a stroke may be imminent. The key to proper diagnosis is to determine what nearby structures may be involved (the company that it keeps). Two pharmacological tests can also be administered. A dilute 2% to 10% cocaine solution blocks norepinephrine re-uptake into synaptic terminals so that the buildup of norepinephrine near the pupil dilator muscle will dilate the pupil in a healthy person. Cocaine treatment will have less effect on the pupil of a patient with Horner syndrome regardless of where the lesion is because less norepinephrine is in the synaptic cleft. To determine if the Horner syndrome is postganglionic, a solution containing hydroxyamphetamine (Paredrine) can then be given. This drug will cause release of norepinephrine from synaptic terminals if they are present, so it will not cause pupillary dilation in a patient with a third-order Horner syndrome. A combination of a careful neurological examination with these tests will usually allow one to determine where in the sympathetic pathways damage has occurred, thus narrowing the differential diagnosis.
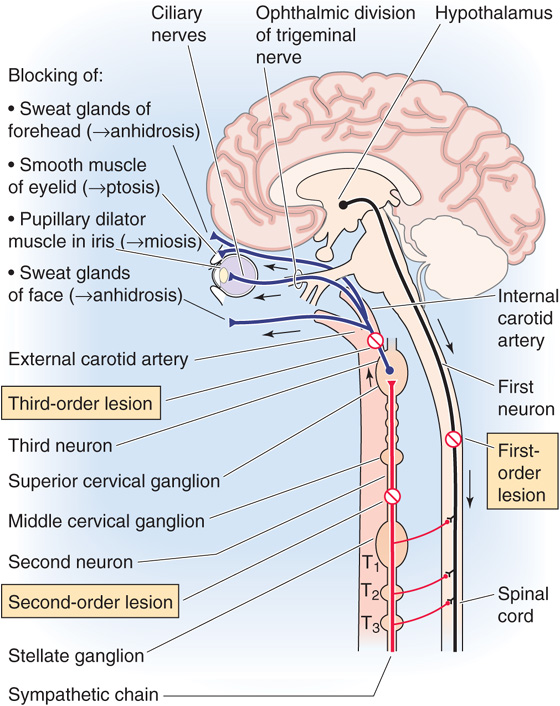
Figure 14-12 Anatomy of the sympathetic innervation to the eyelid, pupil, and facial sweat glands—Horner syndrome. Diagram showing pathways from (1) the hypothalamus to the intermediolateral column in the spinal cord (first-order neuron). (2) A preganglionic sympathetic neuron with the cell body in the intermediolateral column gets a synapse from (1) and sends an axon to the superior cervical ganglion. (3) A postganglionic sympathetic neuron with the cell body in the superior cervical ganglion sends axons to pupillary dilator (smooth) muscles.
All vertebrates have a brain that is segmented into three parts (see Chapter 10), the prosencephalon, mesencephalon, and rhombencephalon. With evolution, the more rostral parts took on a more dominant role. The brain of the ammocoete larva of the lamprey is dominated by the medulla, which is also the most vital part of the human brain; in contrast to more rostral structures, destruction of the medulla leads to instant death in the absence of life support. The medulla coordinates all visceral control and optimizes it for survival. In humans, normal body homeostasis can continue indefinitely with only a medulla, spinal cord, and peripheral ANS.
In fish, the midbrain became the dominant CNS structure in response to the increasing importance of vision. The brain of primitive reptiles is only a brainstem and paleocortex, without a neocortex; the corpus striatum is the dominant structure. Thus, the brainstem is sometimes referred to as the reptilian brain. Finally, the neocortex appeared in mammals and became dominant. The phylogenetically advanced portions of the CNS rostral to the medulla—including the hypothalamus, limbic system, and cortex—coordinate activity of the ANS with complex behaviors, motivations, and desires, but they are not required for normal homeostasis.
As a result of this hierarchy, impulses from most visceral afferents never reach the cortex, and we are not usually conscious of them. Instead, they make synapses within the enteric plexuses, autonomic ganglia, spinal cord, and brainstem, and they close reflex loops that regulate visceral output at each of these levels.
Books and Reviews
Andresen MC, Kunze DL: Nucleus tractus solitarius—gateway to neural circulatory control. Annu Rev Physiol 1994; 56:93-116.
Bennett MR: Transmission at sympathetic varicosities. News Physiol Sci 1998; 13:79-84.
Caulfield MP, Birdsall NJ: International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev 1998; 50:279-290.
Janig W, McLachlan E: Characteristics of function-specific pathways in the sympathetic nervous system. Trends Neurosci 1992; 15:475-481.
Lundberg JM: Pharmacology of cotransmission in the autonomic nervous system: Integrative aspects on amines, neuropeptides, adenosine triphosphate, amino acids and nitric oxide. Pharmacol Rev 1996; 48:113-178.
Journal Articles
Evans RJ, Derkach V, Surprenant A: ATP mediates fast synaptic transmission in mammalian neurons. Nature 1992; 357:503-505.
Furchgott RF, Zawadzki JV: The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980; 288:373-376.
Haddad GG, Mazza NM, Defendini R, et al: Congenital failure of automatic control of ventilation, gastrointestinal motility and heart rate. Medicine (Baltimore) 1978; 57:517-526.
Jansen ASP, van Nguyen X, Karpitskiy V, et al: Central command neurons of the sympathetic nervous system: Basis of the fight-or-flight response. Science 1995; 270:644-646.
Palmer RMJ, Ferrige AG, Moncada S: Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987; 327:524-526.
Pattyn A, Morin X, Cremer H, et al: The homeobox gene Phox2bx is essential for the development of autonomic neural crest derivatives. Nature 1999; 399:366-370.