Pharmacology and Pharmacy
When you have completed this chapter, you will be able to:
1 Define common terms related to pharmacology and pharmacy.
2 Describe the factors that affect the absorption and distribution of drugs and list mechanisms by which drugs may be biotransformed and eliminated.
3 List the dosage forms of medications, the routes by which medications may be administered, and factors that affect route selection.
4 Describe the classifications of drugs that affect the nervous, cardiovascular, and gastrointestinal (GI) systems and give examples of each.
5 List the classifications of agents used to treat common internal parasite infections of animals and name the parasite(s) that may be treated with each.
6 List the pharmacologic agents used in treatment and prevention of heartworm disease.
7 List the classes of compounds used to treat common external parasite infestations of animals.
8 List the classifications of antimicrobial agents used to treat animals and give examples of each.
9 List hormonal substances used in treatment of animals and describe indications for their use.
10 Describe legal issues and requirements related to purchasing, storing, dispensing, and administering pharmacologic agents.
11 Define compounding and explain legal issues related to compounding of medications.
12 Explain the purpose and uses of material safety data sheets.
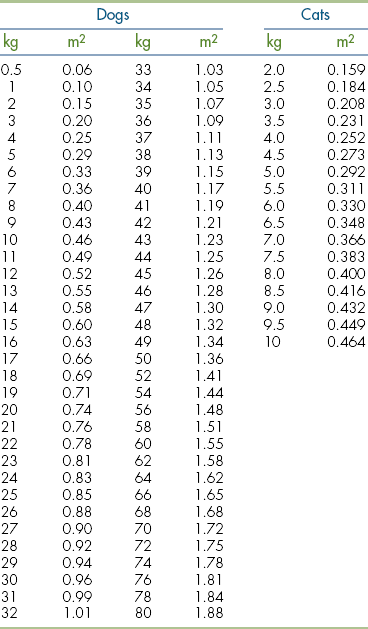
13 Calculate quantities of medications in a variety of dosage forms for dispensing or administering to patients.
14 Define inventory turnover rate and explain its importance in managing pharmacy inventory.
15 Describe procedures for procuring, organizing, and pricing pharmacy inventory.
PHARMACOLOGY
DEFINITIONS
A drug is defined as any chemical agent that affects living processes. These agents may be used to prevent, diagnose, or treat diseases. Pharmacology is a broad term defined as the study of drugs. Aspects of pharmacology include the history and source of drugs (pharmacognosy); physical and chemical properties of drugs and effects and actions of drugs on living organisms (pharmacodynamics); characteristic ability of living organisms to absorb, distribute, metabolize, and excrete drugs (pharmacokinetics); therapeutic uses of drugs (pharmacotherapeutics); and toxicology, the study of the symptoms, mechanisms, treatments, and detection of biologic poisoning. Toxicology has a set of related terms itself that need to be defined to better understand the study of pharmacology.
Therapeutic drug monitoring deals with the proper timing of blood samples drawn to determine the serum concentration of a drug. This value must be compared with accepted reported levels in consideration of the pharmacokinetic properties of the drug measured to ensure proper dosage and dose frequency.
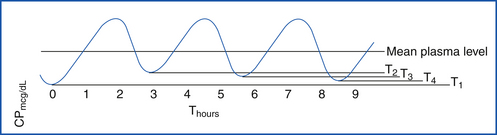
Half-life is the time required for the serum concentration of a drug to decrease by 50%. It shows the intradose fluctuation of a drug and is useful in estimating the time a drug concentration should approach zero. Half-life is most helpful in determining optimal dosing schedules of oral agents and time required to reach steady state.
Steady-state serum concentrations are values that recur with each dose and represent a state of equilibrium between the amount of drug administered and the amount eliminated in a given time interval. It takes five half-lives to reach steady state after dosing has begun.
Peak serum concentration is the point of maximum concentration of drug on the time-versus-serum concentration curve.
Trough serum concentration is the minimum drug serum concentration (Figure 25-1) during a given dosing interval.
Therapeutic window (range) is a range of a drug serum concentration associated with a high degree of efficacy and a low risk of undesired dose-related adverse reactions. Correct timing is important for sample collection. Steady-state concentrations should be achieved because low readings may cause premature and erroneous dose increases.
Toxic dose is a dose greater than the upper limit of the therapeutic range (Figure 25-2) that causes poisonous or toxic symptoms.
Therapeutic index is the ratio between the toxic dose and therapeutic dose of a drug used as a measure of the relative safety of the drug for a particular treatment. A drug that has a narrow therapeutic index may cause toxic results with small changes in doses. These drugs require constant monitoring so that the dose of drug can be adjusted as necessary to ensure uniform and safe results. These ranges should only be used as guides for dosing because there are differences among patients in the manner in which drugs are distributed and are available at the receptor site. Some patients may achieve adequate relief of symptoms before the drug level is within therapeutic range and may experience toxic symptoms when the drug level is within the target range. Examples of drugs with narrow therapeutic windows and indices are digoxin, theophylline, warfarin, phenobarbital, and levothyroxine.
LD50 is the dose of drug that kills 50% of the animals tested (LD = lethal dose). It is a standardized measure for expressing and comparing the toxicity of chemicals (see Figure 25-2).
ED50 is the minimum dose of drug required to cause the desired effect in 50% of the test subjects (ED = effective dose).
PRINCIPLES RELATING TO DRUG ACTIONS
The pharmacokinetic factors of a drug are absorption, distribution, metabolism, and excretion (ADME). These factors determine how the drug enters the body, reaches the site of action, and is removed from the body.
Drug Absorption: For drugs to exert an effect, they must reach their site of action (target tissue). For some drugs, a simple topical application accomplishes this. Most drugs, however, must cross several barriers of cell membranes to produce the desired action. Cell membranes also must be crossed for the subsequent deactivation and elimination of the drug from the body. Absorption is defined as the uptake of substances into or across tissues.
Drugs with systemic actions that are administered orally must cross the GI lining of the stomach or small intestine to be effective. Absorption of drugs from the GI tract will be influenced by several factors. To pass through the membrane lining of the GI tract, a drug must dissolve to some degree in oil (lipid soluble) because the membranes contain a high concentration of lipid (fat). Ionic (charged) forms of drugs do not easily pass through these membranes, whereas the nonionic forms of drugs pass more easily. Most drugs are weakly acidic or basic and have some lipid-soluble properties. The stomach is a highly acidic environment. The weakly basic drugs that are highly ionized (charged) in the acidic stomach will not be readily absorbed until they are farther down the digestive tract in the small intestine because it is basic in nature. In the small intestine, the weakly basic drugs exist in an unionized form, which permits easier transport across the lipid membrane. Drugs that are weak acids are unionized in the acidic stomach and diffuse more easily through the lipid membrane. They are rapidly absorbed from the stomach and therefore expected to exert their action more quickly than weakly basic drugs. Most drugs with poor lipid solubility cannot pass through cell membranes. Drugs, such as the antimicrobial aminoglycosides (e.g., gentamicin), have poor lipid solubility and therefore are inadequately absorbed and ineffective after oral administration.
Stomach contents may inactivate or trap certain drugs. The volume of stomach contents also may delay absorption, thus delaying action. In ruminants, one is confronted not only with slow absorption from dilution, but also with the effect of the action of the ruminal microorganisms on certain susceptible agents. Common drugs of plant origin, such as digoxin and atropine, are ineffective in the ruminant when administered orally because of digestive microorganisms.
Drugs that are administered by intradermal injection are deposited into the outer layer of the skin and are primarily used for diagnostic purposes, as in allergies and tuberculosis. The volume is less than 0.5 ml. The drug produces a local effect. Drugs that require injection subcutaneously or intramuscularly must be absorbed from the injection site to exert their action. The subcutaneous route is appropriate for small drug volumes (less than 1 ml) and drugs intended to be absorbed slowly. Because of limited blood flow, subcutaneous drug administration results in a more sporadic absorption compared with those drugs injected intramuscularly. Insulin and heparin are examples of drugs that are administered subcutaneously. In animals that are highly dehydrated, there is a restricted blood flow at body surfaces, so subcutaneous administration is not usually recommended.
Intramuscular injection is appropriate when a larger volume of drug must be administered. Absorption from the intramuscular site is faster than that from subcutaneous sites because muscles are better supplied with blood vessels than the skin.
Procaine penicillin is an example of a drug to be injected in the muscle.
Absorption from the subcutaneous or intramuscular site can be hastened by applying heat or massage to the site to accelerate blood flow. Applying ice packs at the injection site to decrease blood flow can slow absorption.
Drugs that are introduced into the vascular system (intravenous) will not go through an absorption phase. These drugs are placed directly into the plasma compartment and take effect immediately.
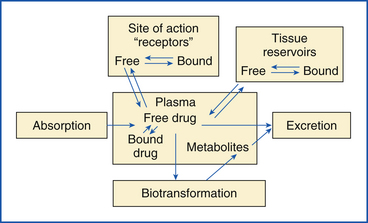
Drug Distribution: Drug distribution is the dispersion of the drug that is systemically available from the intravascular (within the vessels) space and extravascular (outside the vessels) fluid and tissues to the target receptor sites.
Figure 25-3 depicts the distribution of drugs after administration. Drug concentration is a dynamic process that continually varies at different sites until it is virtually all excreted. Generally, another dose of drug is administered before the complete removal of the previous dose, so the effective tissue levels (site of action) may be maintained. High lipid solubility and low protein binding are favorable characteristics indicative of the ability of a drug to diffuse through membranes. Drug transport into tissues involves passage through lipid-containing membranes. Diffusion is a difficult process for water-soluble compounds.
Most drugs in the bloodstream bind in varying degrees to plasma proteins, such as albumin. Only the unbound drug (free drug), which may be as little as 10%, is available to diffuse into tissues and produce biologic effects. As a rule, drugs bound to albumin or other proteins do not diffuse through capillary walls. Drug binding to albumin is a reversible process. Protein binding serves as a reservoir site because the drug becomes available as the plasma concentration of the free drug is reduced. Equilibrium is maintained at all times between protein-bound and free drug in the blood. A common form of drug interaction occurs when a second drug has a stronger affinity for the plasma protein. The first drug is replaced and becomes free to exert its effects in a greater concentration at its site of action.
Accumulation of drugs may occur in various body compartments, such as fat, muscle, and liver, prolonging the effects of the drug as it is released from these storage sites. The potential of a drug to accumulate at these different sites will vary greatly among drugs, depending on the physiochemical properties. For example, a highly lipid-soluble drug, such as thiopental, will accumulate in body fat. This accounts for the slow recovery of obese dogs from barbiturate anesthetics compared with leaner dogs, such as the greyhound.
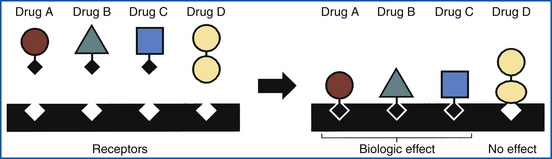
Although all the aforementioned distribution sites of a drug are important, the amount of a drug reaching its site of action is of primary concern. The place at which a drug interacts with cellular components to exert its effect is called a receptor. There are numerous sites throughout the body. Some sites are specific for certain drugs, whereas others are general and may respond or interact with several types of drugs.
The ability of a drug to bind to a specific receptor determines the biologic activity of the drug. The interaction of a drug with a specific receptor is similar to a lock-and-key fit (Figure 25-4). Only a certain critical portion of the drug is usually involved in binding with the receptor. Drugs that have similar critical portions but differ in other parts of the biologic molecule might be expected to have similar biologic activity.
A drug, in interacting with its receptor, may mimic the action of a natural body substance (transmitter). For example, acetylcholine is a natural transmitter that is secreted at terminal nerve endings, causing muscle contraction. A drug, such as bethanechol chloride, that is chemically similar to acetylcholine produces similar effects. Such drugs that directly produce the normal function of the receptor are termed agonists.
Drug Metabolism: For free drugs to be removed (cleared) from the blood, they must be excreted directly without change or metabolized (biotransformed). Biotransformation is the ability of a living organism to modify the chemical structure of drugs so that they are no longer active (inactive metabolites). The liver is the principal organ responsible for biotransformation, but some of the activity may occur in the kidneys, brain, lungs, small intestine, and other organs.
Simple changes in the drug molecule, such as the removal or addition of certain atoms, may completely inactivate the drug. Through the mammalian enzyme system, potentially toxic compounds are changed into water-soluble compounds, which are more easily eliminated from the body by the kidneys. One means of removing many of the lipid-soluble drugs is through conjugation. This process involves the attachment of various endogenous substances to the drug. An example is the attachment of glucuronic acid to aspirin. After conjugation, the aspirin complex is much more water soluble, making it more readily excreted by the kidney. Cats are deficient in the enzymes required to conjugate drugs with glucuronic acid. This accounts for the relatively longer action of certain drugs in cats compared with most other mammalian species that do not have this deficiency.
Other common biotransformations of drugs by the liver include hydroxylation and acetylation. Biotransformation often inactivates drugs, but it does not always produce inactive products. Drugs, such as codeine, diazepam, and amitriptyline, are changed by the liver into metabolites that also exert a pharmacologic effect. These are called active metabolites.
In older animals or animals with hepatic disease, the ability of the liver to biotransform drugs may be impaired. Newborns less than 30 to 60 days of age are generally not capable of metabolizing many drugs because the liver enzyme system is not yet fully developed. To prevent drug toxicity, it might be necessary to reduce the drug dosage, increase the interval between doses, or switch to a drug that is not metabolized by the liver.
A few drugs are administered in an inactive form and do not become active until they are biotransformed by the liver; these are called prodrugs (i.e., angiotensin-converting enzyme [ACE] inhibitor enalapril must be converted by the liver to enalaprilat before it will exert any biologic activity).
Bacteria may carry out some biotransformation within the colon. This process may limit absorption of the drug from the bowel after oral administration, or it may help to eliminate drugs from the blood after parenteral administration.
Excretion: The kidneys eliminate (excrete) most drugs or the metabolites, although some may be removed via the bowel or lungs or in some other minor way in limited amounts. The removal of drugs from the blood by the kidney is somewhat complex and will vary from drug to drug. One route of elimination involves the liver and kidney. Biotransformation of drugs by the liver tends to form more polar compounds, which can be more efficiently excreted by the kidneys. For example, chloramphenicol (CHPC) is metabolized by the liver to chloramphenicol glucuronide. In this form, the drug cannot be reabsorbed via the kidney tubules from the urine back into the blood and therefore is excreted in the urine.
The pH of the urine will also influence excretion of drugs. Urine pH is normally basic, so drugs that are weakly acidic will exist in the ionized state and be more readily excreted. The weakly basic drugs will be in an unionized state and more apt to be reabsorbed back from the urine. For example, the elimination of aspirin, a weak acid, is enhanced in more basic urine. The reverse is true of weak bases in acidic urine. Ammonium chloride can be used to produce more acidic urine, and sodium bicarbonate can be used to produce basic urine.
Some drugs are not extensively metabolized by any organ in the body and are excreted unchanged in the urine. Some are excreted through passive diffusion into the glomerular fluid and are not reabsorbed to any significant degree and therefore enter the urine. Other drugs are actively secreted by specific systems in the renal tubules, which lead to more rapid drug elimination.
Drugs that are excreted by the kidney will accumulate in the body when there is a loss of kidney function. Creatinine (a natural waste product) levels in the blood are sometimes measured to determine the extent of renal damage so that the dose of various drugs can be adjusted accordingly. Kidney function declines with age, even in the healthy animal. Elderly animals may show a reduced ability to excrete drugs in the urine. Certain drugs, such as the aminoglycosides, may directly damage the kidney (nephrotoxicity) and ultimately interfere with their own excretion.
Another route of drug excretion involves uptake by the liver, release into the bile, and elimination in the feces. Drugs in the bile enter the small intestine, in which they may be reabsorbed into the blood, returned to the liver, and secreted again into the bile. This process is called enterohepatic circulation. The drugs that are reabsorbed and resecreted will persist in the body much longer than the drugs that remain in the lumen of the intestine and pass out with the feces.
DOSAGE FORMS
To administer drugs through the various routes, manufacturers have produced products in different formulations to accomplish the desired effect. For oral administration, there are not only traditional tablets and capsules, but also chewable, flavored tablets to encourage animal acceptance and ease in owner administration. Care must be taken in dogs and cats with food allergies when considering the use of chewable flavor tablets. Many tablets are beef based and can cause adverse drug reactions in animals allergic to beef. Because of an undesirable flavor or high alcohol content, animals may not readily receive oral liquids developed for human use. Liquids specifically flavored and designed for dogs, cats, and exotic animals reduce stress for both client and patient during administration. Some cats are hard to orally administer drugs to. Compounding pharmacists can incorporate the drug into a gel that is placed on the outer or inner ear or a place with the least amount of hair. The advantages of using this dosage form are good absorption, high serum blood levels, and avoidance of the hepatic first-bypass effect. The two drugs that are currently available for this dosage form are methimazole and amitriptyline.
Equine owners often cannot administer many drugs to horses orally because of a disagreeable taste or odor and the amount to administer. Some crushed tablets and powders can be mixed with molasses or other suitable compounds then mixed with the animal's grain ration. Veterinary drug manufacturers have formulated granules and pellets for ease in oral administration. Oral paste forms, though somewhat more expensive, have gained popularity because of convenience to the owner and receptiveness of the animal.
Injectable drugs are frequently available in solutions or suspensions ready for use. Special buffers to maintain pH or absence of oxygen are required because of the instability of some components. Instability of some drugs may require a dry lyophilized powder mixed with a diluent (reconstituted), such as sterile water or saline, just before use.
Some vials of drugs in solution are designed for “single use only” because the preparation may not have a preservative or the drug is highly susceptible to oxygen in the air. Certain vaccines or intravenous products may advise on the labeling that unused portions be discarded.
A variety of other dosage forms exist for use in veterinary medicine, such as ophthalmic ointments, solutions, or suspensions; topical sprays, cream, ointments, and lotions; and otic drops. Most are designed for a local effect, although occasionally there may be sufficient absorption from the application site to produce some systemic side effect. Another dosage form that is gaining popularity is the transdermal system. A patch is designed for local application to produce systemic results. Duragesic (fentanyl) patches were introduced to veterinary medicine to control postsurgical pain. Compounding pharmacists are able to make a transdermal patch for any drug except antibiotics. The molecules of the antiotics are too large and will not pass through the lipid biolayer.
Intrauterine administration of some antibacterials is not uncommon in mares, cows, and other breeding stock. Antibiotics are also formulated for intramammary infusion for milk-producing animals. Some of these products are used to prevent (prophylactic) infections at the end of the milking period only. These agents are designated for use in dry cows and usually have a longer duration of action. Other mastitis preparations are for use in lactating cows to treat an infection during the milking period and for a time after the last treatment. The withdrawal time (usually 36 to 72 hours) will vary with the drug and formulation and is stated on the product label.
ROUTES OF ADMINISTRATION
Several methods are available for administering drugs to animals (Chapter 20). Each route of administration has advantages and disadvantages. The route selected will depend on a number of factors, including the patient's size, disease state, temperament, and unique species characteristics; the characteristics and commercial formulation of the drug; and the expertise and knowledge of the individual administering the drug. The cost of drugs should be a factor in the selection of a route of administration when all other clinical factors have been considered.
ORAL ADMINISTRATION
Oral administration is one of the most convenient methods used by clients and animal health personnel for giving drugs. Tablets and capsules are fairly economical and provide accurate and uniform doses. Oral liquids offer some convenience, but the amount of active ingredient administered may vary from dose to dose, depending on measurement or the animal's acceptance. Administration of oral liquids by force in cats usually results in an undesirable salivary gag reflex episode. Oral paste forms for horses and food-producing animals have gained popularity because of the ease of administration. The acceptance of oral granules and powders, although variable among animals, offers convenience for dosing larger species. Drugs formulated for mixing in the animal's drinking water are least desirable because water consumption is highly variable and unpredictable. However, when dealing with large numbers of sick animals in flocks or herds, the use of water mixes may be the only economical and feasible method of treatment. For small birds, medicated drinking water is sometimes used to prevent the stress that occurs with other methods.
Absorption of drugs administered orally depends on a number of factors. Even when accurate doses are given, the actual amount of drug absorbed may vary, altering the expected therapeutic response. Most medications that can be administered orally can also be administered via a feeding tube. It is preferred to give liquids by tube; however, some solid medications can be finely crushed and mixed with sufficient liquid to ensure complete passage of the drug into the stomach. Before administration of any drug via a tube, make sure that the tube is correctly placed and the drug can be crushed or mixed with aqueous solutions.
PARENTERAL ADMINISTRATION
Parenteral administration of drugs is usually accomplished by subcutaneous, intramuscular, intradermal (Figure 25-5), or intravenous injections. Parenteral administration of drugs requires sterile technique to reduce the possibility of introducing infection into the animal (see Chapter 20).
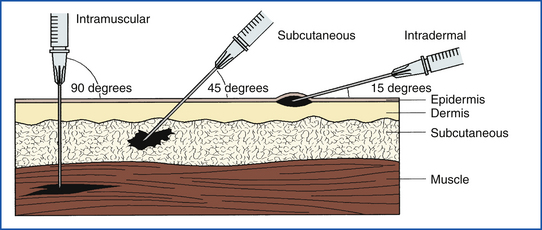
FIGURE 25-5 Comparison of angle of injection and location of medication deposit for IM, SQ, and ID injections.
An intradermal injection is made just below the outer layer of skin (epidermis). This route of administration is used for allergy testing and giving local anesthetics. The volume of drug injected is small, usually less than 0.5 ml.
Subcutaneous injections are common in veterinary medicine because they are less painful to the animal than intravenous or intramuscular injections and are easily administered. Some drugs cannot be given in this way because tissue irritation or sloughing may occur. Many vaccines are given subcutaneously, but some require intramuscular injection to produce the desired immune response.
Increased risks are inherent in the intramuscular administration of drugs. One must ensure that the drug will not be injected into a vein or an artery by accident. The potential also exists for injecting the drug in or near a major nerve fiber, which could cause paralysis. One must have knowledge of the location of major nerves to prevent accidental damage.
When giving drugs subcutaneously or intramuscularly, only a limited amount can be administered at the injection site. Multiple sites may be used for some preparations, but the absorption may be more erratic.
The absorption from an intramuscular or a subcutaneous injection site is primarily through simple diffusion. A number of factors will influence the rate of diffusion from the site. Of primary importance is capillary circulation in the area. Because circulation is limited at subcutaneous sites, compared with intramuscular sites, one would expect a lower absorption and longer action for drugs given subcutaneously.
Label directions should be followed regarding route of administration when administering drugs by injection. There may be a few exceptions for preparations with which sufficient experience exists for administration by routes other than those stated on the label. In most cases, however, there is a definite reason why the recommended route is stated. For example, antibiotics given by subcutaneous injection may not produce adequate blood levels to destroy microorganisms.
For intravenous administration, one must not only know the location of the larger veins that are used, but also possess some skill in placement of the needle or catheter within these blood vessels. An immediate effect can be obtained from drugs administered intravenously without the delay of absorption encountered with other administrative routes. This route may also be used when larger volumes are required. Even certain irritating compounds can be given intravenously if they are given slowly, allowing adequate blood dilution.
Although intravenous administration has advantages, it also has risks. One major disadvantage is the immediate effect seen with an intravenous administration. In situations involving overdose or inappropriate drug selection, the response in an attempt to prevent major problems may not be successful. Highly irritating drugs, such as phenylbutazone, sodium thiopental, and triple sulfa, can severely damage blood vessels and surrounding tissue if injected outside the vein (perivascularly). Injecting certain drugs too rapidly may lead to untoward effects, including circulatory collapse and death. Some drugs may irritate vein walls, stimulate vasoconstriction, and raise the pressure inside a blood vessel until it ruptures. Drugs that leave the vein, leak into the soft tissue surrounding the vein, and cause tissue damage are vesicants. The leakage of intravenous drugs from the vein into the surrounding tissue is called extravasation. Once extravasation has occurred, damage can continue for months and can involve nerves, tendons, and joints. It may cause full thickness of skin loss above the area of injury and may require skin grafting. Delayed treatment to the area may result in the need for surgical débridement, skin grafting, and even amputation. Injury from extravasation can occur with any medication that is highly acid or basic, cytotoxic, or has a high osmolarity. Drug items noted for extravasation are cytotoxic agents (cancer drugs), intravenous nutrition, and solutions of calcium, potassium, bicarbonate, and 10% dextrose.
To prevent extravasation, great care must be taken to ensure that the veins are intact with a good blood flow since drugs may leak from sites of previous or recent punctures or occluded veins. The insertion site should not be distal to a recent venipuncture or an extremity with compromised circulation.
The first line of treatment is to remove as much of the offending fluid as possible. One method reported has been to dilute the infiltrated fluid with saline. Small surgical incisions are made around the area and then suctioned with a liposuction device. Application of DMSO (dimethyl sulfoxide) has been used topically on the area to reduce inflammation. Hyaluronidase has been used with great success because it can work for a wide variety of fluids. It is injected into the area via a catheter or small injections. An enzyme degrades hyaluronic acid (involved with the inflammatory process), then enhances absorption of the extravasated fluid.
To treat tissue damage in most injuries, regular assessment of the site is all that is necessary. To facilitate healing of injuries leading to necrosis, follow wound care principles:
NEUROPHARMACOLOGY
Many different classes of drugs affect the nervous system, even though they are used for a variety of therapeutic uses. Some drugs will cause a direct effect, and others will alter functions of the nervous system as a side effect. The central nervous system (CNS) includes the brain and spinal cord. Its function is to monitor, convey, and process signals from receptors throughout the body.
Neurons (nerve cells) relay information from the CNS to the rest of the body. They use neurotransmitters (NTs) to contact neurons and other cells. An NT, a chemical substance released from the axon terminal of a presynaptic neuron or excitation (stimulation), diffuses across the synaptic cleft to either excite or inhibit the target cell (receptor). Most neurons make only one kind of NT. The receptor recognizes only one specific NT and initiates a cellular response to it. The binding of the NT to its receptor is reversible. The stimulation of the cell is terminated when the NT is degraded or removed away from the receptor.
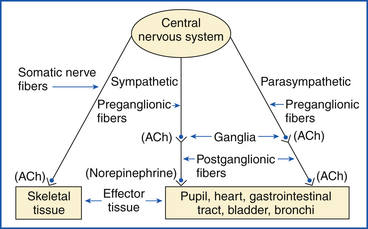
The nervous system is divided according to general function. The two primary divisions of the CNS are the autonomic nervous system, or involuntary system, and the somatic (motor) nervous system, or voluntary system. The somatic system initiates muscle contraction by both conscious and unconscious control. The autonomic system innervates involuntary activities of the body. Although both systems have efferent fibers leading from the CNS, the focus of this discussion is on those of the autonomic nervous system.
AUTONOMIC NERVOUS SYSTEM
The role of the autonomic nervous system is to monitor and control internal body functions, such as digestive processes, blood volume, cardiac output, and kidney function.
For impulse transmission to occur between nerves or between nerves and effector site (e.g., muscles, glands, organs), a small amount of NT must be released by the efferent nerve (Figure 25-6). Two major NTs exist in mammals: acetylcholine (ACh) and norepinephrine (NE). ACh is released into the synapse. ACh that diffuses into opposing membranes is degraded into acetate and choline by the membrane-bound enzyme acetylcholinesterase. ACh that diffuses into the blood is degraded by nonspecific cholinesterase in the blood and tissues. Enzymes deactivate NE, but reuptake of NE by the nerve that released it also occurs, and the NT is again stored in the granules.
The autonomic nervous system is subdivided into the sympathetic and parasympathetic nervous systems. Both divisions commonly act on a given organ, but they produce opposite responses. NE is the predominant NT in the sympathetic system, and ACh is the principal NT of the parasympathetic system. ACh is also the transmitter substance found at the ganglia and at the neuromuscular junction in the somatic nervous system.
Within the sympathetic nervous system, at least three different types of receptors exist (α, β1, and β2) with others postulated. All these receptors may be found within the same effector tissue, and the response to the transmitter will vary, depending in large part on the type of receptor that is predominant at the site and on the amount of transmitter substance present. The general response of various effector tissues to normal sympathetic and parasympathetic stimulation are listed in Table 25-1.
TABLE 25-1
Partial Listing of General Responses Seen at Effector Sites
| Effector Tissue | Sympathetic Stimulation (Dominant Receptor Type) | Parasympathetic Stimulation |
| Pupil | Dilated | Constricted |
| Glands | ||
| Salivary | Scanty viscous secretion | Copious secretion (watery) |
| GI tract | — | Increased |
| Bronchioles | Dilated | Constricted |
| Heart | ||
| Rate | Accelerated | Slowed |
| Contractile force | Increased | Decreased |
| Blood Vessels | ||
| Muscle (skeletal) | Dilated | — |
| Heart | Dilated | — |
| Skin | Constricted | Dilated |
| GI Tract | ||
| Muscle wall | ↓ Peristalsis and tone | ↑ Peristalsis and tone |
| Sphincter | ↑ Tone | ↓ Tone |
| Urinary bladder | ||
| Wall | Relaxed | Contracted |
| Sphincter | Contracted | Relaxed |
This antagonism allows full control of organ function according to body requirements. It should be noted that sympathetic response is a fight-or-flight response in that the animal's heart rate increases, bronchioles are dilated for better ventilation, and blood vessels to the heart and skeletal muscle dilate to increase blood supply. In the parasympathetic rest-and-digest response, the heart rate slows, bronchioles constrict to restrict airways, and blood vessels constrict in the heart and skeletal muscle.
Drugs affecting the autonomic nervous system may mimic or block all or selected effects of the NT, or they may alter the synthesis, storage, release or degradation, and uptake of the transmitter. The classification of these drugs is difficult, not only because there are so many different types of action possible, but also because most drugs possess more than one specific action. Drugs are generally classified based on the primary or predominant action.
Cholinomimetic (cholinergic or parasympathomimetic) agents are drugs that mimic the stimulatory effects of ACh. Cholinomimetic drugs can be further divided into muscarinic and nicotinic agents. Receptor sites that are found to be postganglionic in the effector tissue may be stimulated by a naturally occurring alkaloid, muscarine. Most other ACh receptor sites, including end plates of muscle, may be stimulated by nicotine. Anticholinergic (cholinergic blocking or parasympatholytic) agents are those that are capable of blocking ACh effects. They can also be subdivided according to the site or sites blocked.
Sympathomimetic (adrenergic) agents and sympatholytic (adrenergic blocking) agents are those drugs that mimic or block, respectively, the effects of NE. These agents also are further classified by the particular receptor that they stimulate or block.
AUTONOMIC DRUGS
ACh is not effective systemically as a drug because it is rapidly hydrolyzed by the enzyme acetylcholinesterase at the receptor site. Only an ACh ophthalmic formulation is available for the immediate constriction of the pupil during eye surgery.
Bethanechol is similar in structure to ACh and mimics much of its pharmacologic action. Bethanechol is sufficiently different from ACh in that it can resist hydrolysis by the cholinesterase enzymes; therefore it is a fairly long-acting drug. It is used as a smooth muscle stimulant. When given orally, indications for bethanechol use include gastric atony or stasis and urine retention when there is no obstruction.
Adverse reactions to bethanechol in small animals are mild and may include vomiting, diarrhea, salivation, and anorexia. Arrhythmias, hypotension, and asthma are most likely to occur in overdosage.
Several drugs are able to bind with the cholinesterase enzyme, preventing it from breaking down ACh. This not only allows ACh to act longer, but also creates increased concentration, resulting in exaggerated effects. These agents are toxic (some related compounds were used as nerve gases in World War II), and the therapeutic usefulness is limited to a few unique medical problems. In veterinary medicine, the use of cholinesterase inhibitors is primarily for treatment of parasites—both internal and external.
Cholinesterase inhibitors (anticholinesterases) are divided into three groups on the basis of reversibility: truly reversible (short acting, 5 minutes), edrophonium chloride; reversible (long acting, 30 minutes to 4 hours), physostigmine, pyridostigmine, and neostigmine; and irreversible, organophosphates and echothiophate iodide.
Edrophonium chloride is a drug used to diagnose myasthenia gravis, a disease of the nerves and muscles that is characterized by weakness and a marked fatigue of skeletal muscles. Edrophonium chloride induces an immediate improvement, although it is of short duration. The longer-acting agents, physostigmine, pyridostigmine, and neostigmine, are used to treat the disease in humans. Myasthenia gravis is a disease with a poor prognosis. It is a condition that is expensive to treat; therefore treatment is rare in veterinary medicine.
A common veterinary use of injectable neostigmine is in the treatment of ruminal atony or gut stasis. Neostigmine is relatively short acting (2 to 4 hours), but its stimulatory effects may be beneficial in returning the rumen and GI tract to normal peristaltic activity after surgery. This agent is sometimes employed to treat urine retention because of its stimulatory effects on smooth muscle in the urinary bladder.
Neostigmine and physostigmine can also be used to treat atropine intoxication and to reverse the effects of certain neuromuscular blocking agents (e.g., tubocurarine, gallamine, pancuronium) used during surgery.
Symptoms of overdose of the anticholinesterase agents include GI effects (nausea, vomiting, diarrhea), salivation, sweating, respiratory effects (increased bronchial secretions, bronchospasms, pulmonary edema), ophthalmic effects (miosis, blurred vision, lacrimation), cardiovascular effects (bradycardia or tachycardia, hypotension, cardiac arrest), muscle cramps, and weakness.
Other cholinesterase inhibitors are available only as ophthalmic preparations to treat glaucoma. Glaucoma is a disease complex that is characterized chiefly by an increase in intraocular pressure that may lead to blindness if left untreated. The anticholinesterase agents reduce the intraocular pressure by lowering the resistance to outflow of the aqueous humor.
Anticholinergics
As mentioned previously, nicotinic receptors are mainly at the end plates of skeletal muscle and autonomic ganglia. Muscarinic receptors are predominant in smooth muscle, heart, and glands. Some drugs have the capability of stimulating both types of receptors to varying degrees, whereas other drugs are capable of blocking both sites in varying degrees. Furthermore, some drugs may block the nicotinic effects at the skeletal muscle and not at the autonomic ganglia.
Drugs that inhibit the action of ACh at the muscarinic sites (antimuscarinic drugs) are used widely in veterinary medicine; the most popular drug in this class is atropine. Atropine is a belladonna alkaloid found in nature and commonly incriminated in plant poisoning. Other belladonna alkaloids, such as homatropine and scopolamine, are commercially available and have a slight difference in action.
Because anticholinergic drugs exhibit their usefulness in inhibiting the action of ACh by competing at a number of sites, their potential for correcting a disorder or altering a response is significant. One can rarely choose a single site for the therapeutic action without concomitant side effects occurring at other muscarinic sites. There have been numerous compounds synthesized in attempts to reduce certain unwanted actions and enhance desired effects. The success of such efforts has been limited, depending somewhat on the unique response of the individual patient.
The significant responses that are seen with therapeutic doses of atropine and related drugs are nearly the opposite of parasympathetic stimulation (see Table 25-1). The pharmacologic effects of atropine are dose related. Low doses will produce decreased salivation and bronchial secretions. Dilation of the pupil and increased intraocular pressure and heart rate are experienced with moderate systemic doses. High doses decrease motility and tone of the GI and urinary tracts.
The antimuscarinic drugs are frequently used before and during surgery in small animals to reduce or prevent secretions of the respiratory tract and to reduce bradycardia (decreased heart rate). Atropine and its analogs have been used in combination with other drugs to treat diarrhea (see later discussion of antidiarrheal agents).
Atropine is indicated in eye examinations and some ophthalmic surgery in which dilation of the pupil is desired. Atropine is long acting; therefore some of the shorter-acting mydriatics (dilating agents), such as tropicamide, are used. One of the most important uses of an antimuscarinic drug is to block spasms of the small ciliary eye muscles, thereby alleviating the associated pain.
Another significant use of atropine is as an antidote for organophosphates and other anticholinesterases found in many insecticides or parasiticides. Muscarine toxicity from poisonous mushrooms is also treated with atropine.
Atropine must be used with caution because of potential side effects, which are merely extensions of the pharmacologic effects. Some clinicians believe atropine is contraindicated in the horse except for life-threatening organophosphate toxicity because the decreased peristaltic activity in the lengthy gut of the horse leads to gas and toxin complications. Atropine can increase ocular pressure and is therefore contraindicated in the treatment of animals with certain types of glaucoma.
Neuromuscular Blockers
Neuromuscular blockers (NMBs) act at the junction of the nerve and skeletal muscle to paralyze skeletal muscle. These compounds are classified according to the onset and duration of action. Older agents, such as d-tubocurarine, succinylcholine, gallamine, and pancuronium, are still available commercially. Newer agents, such as vecuronium and atracurium, are widely used in veterinary medicine.
Some NMBs have been used in darts to capture animals, but this use is dangerous because respiratory paralysis occurs. The main clinical use of NMBs is as an adjuvant in surgical anesthesia to obtain relaxation of skeletal muscle, particularly of the abdominal wall, and in orthopedic surgery. These agents are selectively used in veterinary medicine. Guaifenesin, another type of muscle relaxant, is commonly used in equine and bovine surgery to selectively depress transmission of nerve impulses at the internuncial neurons of the spinal cord, brainstem, and subcortical regions of the brain. Symptoms of NMB overdose include increased risk for hypotension, histamine release, and prolonged muscle blockade.
Sympathomimetics
The sympathetic nervous system is extensively involved in regulating a number of body functions, including heart rate, blood pressure, bronchial airway tone, body temperature, carbohydrate and fatty acid metabolism, and appetite. Although NE is the primary transmitter substance, epinephrine is released from the adrenal gland when an animal is stressed through physical, psychologic, or other stimulatory means.
Because the NE molecule can be modified extensively and still possess some type of stimulatory properties, numerous agents are commercially available. Manufacturers seek a molecule that produces a desired response and eliminates or reduces all the other adrenergic effects. NE possesses only alpha effects and has limited therapeutic use in the treatment of certain hypotensive shock conditions.
Epinephrine has several therapeutic applications in veterinary medicine, although the actual frequency of use is limited. Clinical applications include the following:
• Allergic reactions (often lifesaving in the face of shock)
• Bronchospasm (provides rapid relief)
• Cardiac effects (sometimes used in specific heart disorders)
• Local hemostasis (may be used in dilute solution [1:100,000 to 1:20,000] to control surgical bleeding in highly vascular tissue)
• Prolongation of the effects of local anesthetics (even though there may be undesirable systemic effects from epinephrine if overused)
Isoproterenol, which has few alpha effects, but powerful beta effects, is useful as a bronchodilator in respiratory disorders and as a cardiac stimulant in certain heart conditions. Isoproterenol is available in many preparations for humans that are designed for inhalation use or as tablets for under the tongue (sublingual). Only the short-acting injectable form has application in veterinary medicine.
Epinephrine and NE are not available in oral forms because both are destroyed by stomach acid. In addition, both drugs are relatively short acting when given by injection. Epinephrine and phenylephrine hydrochloride are also commercially available as ophthalmic preparations. They cause the pupil to dilate, but unlike atropine, they directly stimulate those muscles of the eye controlled by sympathetic nerves. This mydriatic effect is useful in selected cases of glaucoma and in ophthalmic examinations.
Symptoms of toxicity include arrhythmias, pulmonary edema, dyspnea, vomiting, headache, and sharp rises in systolic, diastolic, and venous blood pressures.
Sympatholytics
Many chemicals interfere with the function of the sympathetic nervous system. Some agents act by interfering with the synthesis, storage, and release of the transmitter substance. Others interfere with the ability of receptors to interact effectively with NTs. Some blocking agents are specific in their action (e.g., prazosin hydrochloride is specific in blocking the α receptors). Other agents (e.g., the phenothiazine tranquilizers, such as acepromazine) are nonselective in activity, blocking α and β1 receptors (see Chapter 27 on information about preoperative drugs and drugs used in anesthetic emergencies).
α- and β-Adrenergic Blocking Agents
The α-adrenergic blocking agents, such as phenoxybenzamine, prazosin, and hydralazine, cause vasodilation and are used mainly in animals for lowering blood pressure or improving blood flow in certain vascular diseases.
Phentolamine, an expensive, injectable α-blocker, is used to diagnose adrenal gland tumors and during surgery to control abnormally high blood pressure. Adverse effects seen with use of α1-adrenergic blocking agents include first-dose syncope, transient lethargy and dizziness, nausea, vomiting, diarrhea, and constipation.
β-Adrenergic blocking agents, such as propranolol and atenolol, are therapeutically useful as antihypertensive agents and in the treatment of certain heart arrhythmias.
Betaxolol and timolol are two β-adrenergic blocking agents that are widely used in veterinary ophthalmology. After topical application to the eye, each reduces both elevated and normal intraocular pressure with or without glaucoma. Overuse of β-adrenergic blocking agents results in symptoms of hypotension, bradycardia, bronchospasms, depressed consciousness to seizures, hypoglycemia, respiratory depression, and atrioventricular block.
Tranquilizers
Tranquilizers are drugs that act on the CNS to produce a calmness of mind or detached serenity without loss of consciousness or marked depression. The use in veterinary medicine is to modify the behavior of the animal to make it more manageable or less responsive to external stimulation.
Phenothiazines
Phenothiazine was originally used in veterinary medicine as an anthelmintic. Derivatives of the drug (chlorpromazine and acepromazine) have been synthesized to enhance the sedative effects of phenothiazine. Some of the derivatives are used as antihypertensive agents because they exhibit peripheral α-adrenergic blocking activity and cause vasodilation. The exact mechanism of action for sedation is unknown, but phenothiazines block postsynaptic dopamine receptors. These drugs have found usefulness as antihistamines, antiemetics, and antimotion sickness agents.
The phenothiazine tranquilizers are used as preanesthetics by “taking the edge off” the animal and enhancing or prolonging the effects of certain anesthetics. Some side effects to be aware of when administering the phenothiazines include a drop in blood pressure, paralysis of the retractor penis muscle in horses, and lowering of the seizure threshold in dogs.
α2-Agonists
Although xylazine, detomidine, and medetomidine in the strictest sense may not be classified as tranquilizers, their sedative and analgesic properties are useful for chemical restraint, especially in the horse. Detomidine is approved for use only in the horse and has little application in other species. It appears to differ slightly from xylazine by producing greater analgesia and sedation. Although it is dose dependent, the duration of action of detomidine is longer than xylazine.
Both xylazine and detomidine are commonly used in combination with other sedatives, tranquilizers, and anesthetic agents. The effects of these drugs in combination are greatly potentiated and must be used with caution. Common side effects seen in the horse include muscle tremors, partial atrioventricular (AV) block, bradycardia, respiratory changes, sweating, penile prolapse, increased intracranial pressure, or decreased mucociliary clearance.
Xylazine has always been used widely in cattle. Only recently has the U.S. Food and Drug Administration (FDA) approved it for use in food-producing animals. The popularity in ruminants results from its excellent anesthetic properties. Ruminants are sensitive to xylazine, requiring approximately one tenth of the dose (based on body weight) used in horses. Adverse effects in cattle include ruminal atony, intestinal stasis, salivation, hypothermia, diarrhea, bloating, ataxia, and regurgitation with aspiration pneumonia.
Although xylazine is approved for the management of hyperexcitable behavior in the cat and dog, it is not widely used in these species. Vomiting is a common side effect seen in the dog and frequently in the cat soon after administration. A single episode usually occurs, but the use of antiemetics may delay this phenomenon. Xylazine is frequently used as an emetic when an emetic effect is desired (e.g., emptying stomach before surgery). Gaseous extension with use may occur in dogs, making radiographic interpretation difficult. Movement in response to sharp auditory stimuli may be observed. Increased urination may occur in cats following the use of xylazine.
Yohimbine is an α2-adrenergic receptor antagonist that competitively blocks and antagonizes CNS depression or sedation and the bradycardia and respiratory depression caused by xylazine.
Atipamezole hydrochloride, a synthetic α2-adrenergic antagonist, reverses the effects of medetomidine hydrochloride in dogs.
The use of propofol in veterinary medicine for induction of anesthesia in high-risk patients, such as those with compromised organ systems, is rapidly gaining popularity. It is used mainly for sedation and/or relaxation of 5 to 10 minutes in duration because it is rapidly metabolized. Because propofol can cause respiratory depression, its use should be restricted to situations where controlled intubation is available. Propofol may cause increased vasodilation and negative inotropy (weakening the force of muscular contraction) when used in conjunction with preanesthetic agents, such as acepromazine or opiates. Animals with preexisting cardiopulmonary disease, in shock, or suffering from trauma should be of particular concern. Propofol-induced bradycardia may be exacerbated in animals receiving opiate premedication, especially when anticholinergic agents are not given concurrently.
Drugs that inhibit the hepatic P-450 enzyme system and other basic lipophilic drugs may increase recovery times associated with propofol. Cats with liver disease as a preexisting condition may be susceptible to longer recovery time.
Anticonvulsants
Of the several different causes of seizures (convulsions) in dogs, only about two thirds can be controlled by the various anticonvulsant drugs. The benzodiazepine derivative diazepam may be the most popular injectable drug for use during seizures or in other emergency situations. This benzodiazepine agent depresses the subcortical levels of the CNS, thus exhibiting sedative, skeletal muscle relaxant, and anticonvulsant properties. Diazepam is relatively short acting (30 minutes to 2½ hours). Phenobarbital sodium, a barbiturate, is also available for injection when a longer effect (4 to 6 hours) is required.
Midazolam, an imidazobenzodiazepine, exhibits similar pharmacologic actions as other drugs in its class. The unique characteristic of lipid solubility at body pH gives it a rapid onset of action after injection. It is not used as an anticonvulsant, but finds use as a premedication before surgery, alone or in combination. When combined with potent analgesic and/or anesthetic drugs, such as ketamine or fentanyl, midazolam produces conscious sedation. Intracarotid artery injections must be avoided. Midazolam should be used cautiously in animals that are comatose, in shock, or have significant respiratory depression. Use in the first trimester of pregnancy should only occur when the benefits clearly outweigh the risks associated with the use. This drug should be used in an inpatient setting only or with direct professional supervision.
Adverse effects seen with benzodiazepine use include muscle fasciculations, weakness, and ataxia in the horse at sedative doses; irritability, possible development of hepatic failure, and aberrant demeanor in cats; and CNS excitement in the dog.
BARBITURATES
Phenobarbital is a barbiturate with CNS effects. The mechanism of action of this group of drugs is not quite understood, but they have been shown to inhibit the release of ACh, NE, and glutamate. Phenobarbital tends to depress motor activity without causing excessive sedation, which makes it a good anticonvulsant agent. One major side effect of this drug is dose-dependent respiratory depression.
An effective and inexpensive agent used to treat epilepsy (status epilepticus) and seizures caused by acute encephalitis or meningitis in dogs is oral phenobarbital. For some cases that are uncontrolled by phenobarbital, oral administration of potassium bromide has been effective. (Potassium bromide is not available in a commercial formulation. Authorization may be obtained from the FDA to compound preparations for treatment of refractory cases.) Compounding pharmacies may be a source for obtaining this product when its use is determined necessary.
Analgesics, Antipyretics, and Antiinflammatory Agents
Analgesics are agents that alleviate pain. Although local and general anesthetics inhibit the sensory perception of pain, analgesics are generally considered to increase the threshold of pain in the pain perception areas of the brain. Antiprostaglandins (e.g., aspirin, flunixin) inhibit the biosynthesis of these natural pain-producing substances and are also considered analgesics (see Chapter 26 for additional information on opioids).
Opioid Analgesics
The naturally occurring narcotics (e.g., morphine, codeine) and synthetic narcotics (e.g., hydrocodone, meperidine) are the most potent analgesics. These agents stimulate the μ-opioid receptor and are thought to have some activity at the δ-opioid receptor. Although these addictive agents are used for severe postsurgical or posttrauma pain in dogs and horses, their more common use is as an anesthetic or preanesthetic agent.
The pharmacologic effects differ somewhat among the various narcotics, but most will produce the following:
• CNS depression in the dog, monkey, and human
• CNS stimulation (excitement) in the cat and horse
• Cough sedation in the dog and human
• Respiratory depression (panting may initially be seen)
• Increased tone of intestinal smooth muscle, causing constipation
• The effects of these drugs are reversed by narcotic antagonists, such as naloxone
Unfortunately, narcotic analgesics are fairly short acting in the dog and the horse (2 to 4 hours). Gut stasis in the horse is a concern when considering opioid analgesics. The opioid analgesics have questionable efficacy in the ruminant.
The agonist activity of the synthetic opioid butorphanol is thought to be exerted at the к- and σ-receptors. Butorphanol, a morphine congener, has shown promise in dogs as a longer-acting (4 to 8 hours) analgesic. Adverse effects seen in dogs include sedation (occasionally), ataxia, and anorexia or diarrhea (rarely). Transient ataxia and sedation may occur in the horse at usual doses. Butorphanol is used in horses as an effective analgesic, although its stimulatory effects must be suppressed by the concurrent use of depressant drugs, such as xylazine. Butorphanol is approved by the FDA for use as an antitussive and analgesic in dogs.
Gaining popularity in veterinary medicine for pain relating to surgery is fentanyl. Fentanyl shares the actions of the opioid agonists; the same precautions should apply. One advantage of using fentanyl is that it is marketed in a transdermal patch system for chronic pain management that delivers continual analgesia for about 72 hours. The patch is not recommended for use in management in postsurgical pain.
Hydrocodone bitartrate is a phenanthrene-derivative opioid agonist that exhibits the characteristics of other opiate agonists. It is used in veterinary medicine mainly as an antitussive agent. The mechanism is thought to be a result of direct suppression of the cough reflex on the cough center in the medulla. Hydrocodone is more sedating than codeine, but not as constipating.
Opioid Antagonists
The opioid antagonists reverse the pharmacologic effects of narcotics and have no analgesic activity. Naloxone appears to be the only true antagonist because it possesses no other apparent pharmacologic effect at usual doses. (It reverses the majority of effects associated with high-dose opiate administration—respiratory and CNS depression.)
Although narcotic antagonists are used commonly in human addicts to reverse overdoses of self-administered narcotics, the principal use in veterinary medicine is to reverse the sedative and quieting effects of analgesics used for temporary restraint. Dogs receiving narcotic sedation for minor procedures (e.g., radiographs, suture removal) are easily “reversed” with naloxone; the animal is almost immediately alert. The duration of action of naloxone is shorter than that of most narcotics, and generally the effects of the unmetabolized analgesic are inadequate to cause the animal to return to its sedated state.
Corticosteroids
Corticosteroids are extremely active compounds that have numerous pharmacologic effects on all organ systems. They have been used in an attempt to treat practically every malady that afflicts animals. They are valuable in the treatment of certain conditions; however, there are significant risks when one considers the potential adverse effects.
Because corticosteroids are naturally occurring body substances (cortisol is derived from the adrenal gland), one indication for the use of steroids would be replacement therapy to correct a deficiency. Such a deficiency is relatively rare. Most steroids used in veterinary medicine are given for the antiinflammatory effect; the mechanism for the antiinflammatory response is complex. They suppress the tissue swelling and pain that normally follow injury. Because inflammation is common in a variety of diseases, there is extensive use, perhaps overuse, of these agents.
Steroids also possess antiimmunologic effects, altering the immune response of the body. Therefore they are used in certain allergic diseases because they reduce the hypersensitive and allergic reactions of the patient. Immunizations generally should not be given during corticosteroid therapy because of the potential for inadequate immune responses.
Common side effects seen with the long-term use of steroids include GI bleeding; increased susceptibility to infections or wounds that will not heal; potassium loss, causing irregular heartbeats, muscle cramps, and weakness; sodium and water retention (edema or ascites); muscle weakness resulting from protein breakdown; and behavioral changes. The primary adverse effects associated with long-term administration, especially if given at high doses or on an alternate-day regimen, are generally manifested as symptoms of hyperadrenocorticism (Cushing's disease).
Dexamethasone is one of the most popular steroids used in veterinary medicine; it is fairly long acting (more than 48 hours). Prednisolone and prednisone are used interchangeably and are available in tablet form. Triamcinolone and betamethasone are also used extensively in veterinary medicine.
Steroids are found in various dosage forms, including ophthalmic, otic, topical, injection, and oral. It should be noted that long-term use of these steroids as ophthalmic or topical agents may lead to some of the systemic toxic effects previously mentioned.
Nonsteroidal Antiinflammatory Drugs
To prevent side effects inherent to steroids, other agents possessing antiinflammatory action have been synthesized. These are called nonsteroidal antiinflammatory agents (NSAIDs). NSAIDs exhibit antipyretic, analgesic, and antiinflammatory activity. The major mechanism of therapeutic effect is believed to be the result of inhibition of prostaglandin (PG) synthesis. Many inhibit both COX-1 and COX-2 isoenzymes. Phenylbutazone, one of the original members of this group of compounds, remains one of the most widely used agents in equine medicine. Phenylbutazone is not frequently used in small animals, although there is a label claim for use in dogs. Dogs metabolize phenylbutazone rapidly, which makes it difficult to maintain therapeutic levels of the drug. Cats metabolize the drug slowly and thus become prone to its toxic effects. Blood dyscrasias have been reported in several species receiving phenylbutazone. The drug has the potential for reducing the effects of other drugs metabolized by the liver because it increases the hepatic microsomal enzymes necessary to deactivate these drugs.
Flunixin has gained popularity not only for its antiinflammatory effects, but also for its ability to reduce GI pain in horses and ruminants. Although not approved for food-producing animals, flunixin appears to be the best analgesic available for ruminants, providing relatively long, effective relief.
Ketoprofen and carprofen are propionic acid derivatives structurally related to ibuprofen and naproxen. Ketoprofen has been approved for use in the horse to alleviate inflammation and pain associated with skeletal disorders. Carprofen has been approved for dogs only to relieve pain and inflammation associated with osteoarthritis.
Oral administration of the NSAIDs is apparently irritating to the GI tract and may cause ulceration in the mouth, stomach, or intestines. Newer generation NSAIDs (etodolac, meloxicam, deracoxib, and tepoxalin) are gaining in popularity because of once-a-day dosing and decreased adverse effects on the GI tract. Firocoxib is a new agent that has been recently marketed for use in equine medicine.
DIURETIC AND CARDIOVASCULAR DRUGS
Fluid and electrolyte imbalances and the treatment are discussed in Chapter 21. The function of the kidney and its role in maintaining proper fluid volume and electrolyte concentration are also mentioned. Blood is initially filtered in the kidney, and most of the filtrate is reabsorbed from the kidney tubules back into the blood. Most diuretic drugs affect the reabsorption process, preventing the reabsorption of some sodium and water from the filtrate. As a result, urine output and sodium excretion are increased.
DIURETICS
Diuretic drugs are used primarily to relieve edema (the presence and abnormally large amounts of fluid in the intercellular tissue spaces of the body) associated with diseases of the kidney, heart, or liver. Although there are numerous diuretic agents, furosemide appears to be the most routinely used diuretic in veterinary medicine. Furosemide is a loop diuretic that primarily inhibits reabsorption of sodium (Na+) and chloride (Cl−) in the kidney. It is commercially available in convenient forms for oral and injectable administration in small and large animals. Besides being potent and effective in most cases, furosemide is rapid acting and usually produces diuresis within 5 minutes when given intravenously.
Furosemide can cause a “wasting” of potassium, so serum potassium levels should be monitored for animals that take furosemide. Potassium supplementation may be indicated during furosemide therapy.
Occasionally, when renal blood flow is inadequate because of trauma or shock, furosemide or similar diuretics are ineffective in altering tubular reabsorption. In such cases, an osmotic diuretic, such as mannitol, which is poorly absorbed from the glomerular filtrate, is used to produce diuresis. Animals that have been hit by cars may be likely candidates to receive mannitol. Crystallization may occur in solutions with concentrations greater than 15%. It is important to dissolve crystals before administering. Keeping the solution in a warm water bath prevents crystals from forming. The crystals can also be dissolved by running warm water on the bottle or rolling the bottle to and fro in the hands.
CARDIAC GLYCOSIDES
Cardiac (heart) drugs are probably the most potent and hazardous group of drugs used in medicine because of the effects on such a vital organ. Any carelessness in calculation, administration, or observation of the patient may lead to death. The dosage for these drugs should be individualized through frequent and careful monitoring to ensure the desired therapeutic response and prevent or minimize toxic effects.
The heart performs a relatively simple function (to circulate blood) and is essential to life. The heart consists primarily of myocardium (muscle), valves, and some specialized impulse-conducting nodes and fiber. Even though the heart has the ability to compensate for certain defects, disorders left untreated reduce the quality of life with severe disability, leading to premature death.
Significantly severe defects in the valves can only be treated surgically. Medical therapy is available for the treatment of a weakened myocardium and conductance disorders (arrhythmias).
The normal healthy heart can increase its output readily when demands, such as increased exercise, are placed on it. This increased cardiac output is a result of either an increased heart rate or an increase in the volume of blood pumped per beat (stroke volume), but usually, it is a combination of both. Heart muscle weakened with age does not contract as fully and therefore can lead to reduced output. Because the body cannot tolerate much decrease in cardiac output, the heart rate will increase slightly and the heart will become enlarged because the myocardium will thicken in an attempt to improve contractility. Congestive heart failure (CHF) is the condition of an enlarged heart with poor myocardium contractility.
Various glycosides found in the leaf of the digitalis plant have been found to be useful in the treatment of CHF. Digoxin is one of the glycosides that is commonly used in veterinary medicine. Digoxin is unique in that it not only improves the inotropic ability (contractility) of the myocardium, but also reduces the heart's demand for energy and oxygen. It also decreases the conduction of certain impulses within the heart and therefore decreases the heart rate. It is used for treatment of atrial fibrillation, an arrhythmic disorder of the heart.
Digoxin dosing is critical. Toxic effects of the cardiac glycosides are seen at doses close to the therapeutic dose (narrow therapeutic window) and therefore complicate its use. Owners should be aware of signs of toxicity, which include vomiting, diarrhea, loss of appetite, and depression. Associated with these symptoms are a decreased heart rate and drug-induced arrhythmias.
Further complications to digoxin therapy are animals with reduced liver or kidney function, as is common in the older animal. Good client compliance and close monitoring are essential in digoxin therapy because of the toxicity possibilities. The drug is available as an oral tablet, oral liquid, and injection.
Animals that are concurrently using a diuretic may have low serum potassium levels and are more susceptible to digoxin toxicity.
Although the cardiac glycosides are effective by injection in horses and cattle, and to some extent orally in horses, it is not feasible to use these drugs to treat CHF because of the long-term nature of the disease. These drugs are commonly used in dogs and cats. There is adequate absorption of digoxin from the GI tract in these species; however, it may differ somewhat among animals and can be influenced by feeding times.
ANTIARRHYTHMIA DRUGS
Arrhythmias of the heart fall into several categories and require skilled clinicians and electronic instrumentation for proper diagnosis and treatment. Some minor cardiac arrhythmias are likely to correct themselves and may be left untreated. The use of antiarrhythmic drugs in veterinary medicine is usually limited to treatment of those arrhythmias that are life threatening and require immediate attention.
Calcium channel blockers provide the veterinarian with an efficacious weapon in the treatment of certain cardiovascular disorders. This group of drugs has a low incidence of side effects. Of the numerous agents, diltiazem has surfaced as the most commonly used agent to treat supraventricular tachyarrhythmias in dogs and cats. It is used in the treatment of hypertrophic cardiomyopathy in cats. Diltiazem acts as an antihypertensive agent through arteriolar dilation, but the benefit of this action is not fully known.
Three older commonly used antiarrhythmic drugs are quinidine, procainamide, and lidocaine. The more ordinary uses are only mentioned because detailed discussion is beyond the scope of this chapter. Quinidine is used in horses and large dogs for the treatment of supraventricular and ventricular arrhythmias. Other uses include treatment of atrial fibrillation and atrial flutter. Procainamide is related chemically to procaine, and it is used in the treatment of ventricular extrasystoles and tachycardia, atrial arrhythmias, ectopic contraction and tachycardia, flutter, and fibrillation. Lidocaine, although used primarily as a local anesthetic, has therapeutic application in the treatment of ventricular tachyarrhythmias. Clinical monitoring and electronic evaluations should accompany the use of these drugs.
All antiarrhythmia drugs are toxic to the heart and may produce their own serious arrhythmias. In addition, in the horse, quinidine can produce urticarial wheals, GI disturbances (e.g., anorexia, colic, diarrhea), erythema, and edema of nasal mucosa with dyspnea and laminitis. Signs of quinidine toxicity in the dog include vomiting, depression, incoordination, and convulsions. Procainamide toxicities are exemplified in dogs by a loss of appetite, vomiting, and serious immunologic reactions with long-term use. A serious decrease in blood pressure may occur when procainamide is given intravenously. Lidocaine is not effective orally and has brief action when given intravenously. In large doses, lidocaine can produce a drop in blood pressure.
ANGIOTENSIN-CONVERTING ENZYME INHIBITORS
Vasodilatory drugs, or angiotensin-converting enzyme (ACE) inhibitors, prevent the conversion of angiotensin I to angiotensin II (a potent vasoconstrictor). The drugs compete with angiotensin I for the active site of ACE. In veterinary medicine, this group of drugs is primarily used to treat canine CHF. Captopril was the first agent in this class to be commercially available. Treatment presented risks, such as renal failure. Other ACE inhibitors have been synthesized; they are prodrugs because they require a functioning liver to convert them to the active metabolite. Enalapril is commercially available with label indication for use in veterinary medicine. Other ACE inhibitors that are in use are human-labeled benazepril hydrochloride and ramipril.
The side effect profile of the second generation of ACE inhibitors has improved, and the dosing schedule is one or two times per day, which should help with client compliance.
Hydralazine is a phthalazine-derivative antihypertensive, vasodilating agent. The main use of hydralazine is an afterload reducer for the adjunctive treatment in CHF in small animals, particularly if the primary cause is mitral valve insufficiency. It is usually administered in cases where enalapril is not effective in clinically improving dogs with mitral valve insufficiency. Hydralazine should be given with a diuretic because of the sodium and water retention associated with its use.
Pimobendan is a benzimidazole-pyridazinone inodilator (positive inotrope-vasodilator) and is the newest drug introduced to treat CHF, dilated cardiomyopathy, and mitral regurgitation in the dog. It is a nonsympathetic, nonglycoside, positive inotrope (through myocardial calcium sensitization), and vasodilator. Pimobendan should not be used as a monotherapy, but in conjunction with an ACE inhibitor, furosemide, or digoxin.
AGENTS USED TO TREAT PARASITISM
TREATMENT OF INTERNAL PARASITISM
Anthelmintics (dewormers) are an extremely important group of drugs in veterinary medicine. The presence of internal parasites in an animal can shorten its life span or reduce the quality of life. It can contribute to considerable economic loss in food-producing animals. Although several different parasites are capable of infecting each species, most parasite infections can be effectively prevented or treated with proper care and medication. Current anthelmintics are much improved because they are more effective in eradicating the parasite and less toxic to the host. In addition, dosage forms, such as pastes or chewable tablets, are now available. These formulations are much more easily administered, which reduces stress to the animal and client.
There are a vast number of anthelmintics currently available; however, this discussion is limited to a select, popular few. Parasite treatment summary charts and specific parasite information are given in Chapter 17.
Benzimidazoles
Benzimidazoles are a large class of anthelmintics. They inhibit the enzyme fumarate reductase and thereby interfere with parasitic carbohydrate metabolism. Thiabendazole, oxibendazole, mebendazole, albendazole, parbendazole, fenbendazole, cambendazole, and oxfendazole are safe and effective agents against several GI parasites. They are formulated primarily for large animals to eradicate strongyli, pinworms, and ascarids in the horse and roundworms and several other parasites in cattle, sheep, swine, and goats. Albendazole shows activity against liver flukes. Fenbendazole and mebendazole are available for use in small animals to eradicate roundworms, hookworms, whipworms, and some tapeworms, although neither is effective for the common Dipylidium tapeworm. Adverse effects are not usually seen at recommended doses of benzimidazoles. Thiabendazole, parbendazole, and cambendazole are not commercially available in the United States.
Organophosphates
Trichlorfon, coumaphos, and dichlorvos are a group of agents that bind irreversibly to cholinesterase in the parasite, leading to ACh “poisoning” of the parasite. These drugs would also be toxic to the host, but they are selectively formulated to be poorly absorbed from the GI tract of the animal. Precautions must be taken so animals dewormed with organophosphates are not exposed to other organophosphates, cholinesterase inhibitors, pesticides, or muscle relaxants, such as succinylcholine, until a few days after treatment. There is potential danger to humans in administration of these agents.
Common toxic signs of organophosphate poisoning (e.g., widespread parasympathetic stimulation) include miosis, salivation, breathing difficulties, vomiting, defecation, and muscle fasciculation. Atropine is used as a specific treatment to block the muscarinic effects. Pralidoxime (2-PAM) is an expensive product for humans and may be used in severe cases of organophosphate poisoning to reactivate the cholinesterase enzyme.
The organophosphates are fairly effective in treatment of a number of principal parasites in horses, cattle, swine, sheep, dogs, and cats. With the potential toxicity of the organophosphates, many are being replaced with safer agents. The use of organophosphates is no longer professionally accepted; however, they are still obtainable.
Tetrahydropyrimidines
Pyrantel and morantel are two drugs in the tetrahydropyrimidine class. These drugs act as a cholinergic agonist and depolarize neuromuscular junctions. They are effective against the adult nematodes, but not active against larvae form.
Morantel is an analog of pyrantel that is safer and more effective in sheep and cattle than pyrantel. It is available only as a feed additive.
Pyrantel is widely used in horses for ascarids, strongyli, and pinworms. In dogs, pyrantel is used in the prevention and treatment of hookworms and ascarids. Tetrahydropyrimidine products are safe and nontoxic to all species at the recommended therapeutic doses. There is no contraindication for use of these agents with other cholinergic drugs.
Imidazothiazoles
Two popular drug agents, febantel and levamisole, are in the broad category of imidazothiazoles. Febantel is a prodrug that is metabolized in vivo to fenbendazole. It is approved by the FDA for use in a number of species against parasites. It has only been recognized to treat the most common equine parasites except bots and is reported to be safe in pregnant mares. Febantel is only commercially available in the combination product (Drontal Plus, Bayer).
Levamisole has broad anthelmintic activity in a large number of hosts, including sheep, cattle, pigs, horses, chickens, dogs, and cats. Use and FDA approval are limited primarily to food-producing animals. Although levamisole is relatively safe, some signs of toxicity occur similar to those of organophosphate poisoning. The toxic doses are only one or two times the therapeutic dose. Muzzle foam may be seen in ruminants after oral administration, but it usually disappears within a few hours. Transitory excitement has been seen in horses after treatment.
Milbemycins
The milbemycins are macrocyclic lactones that act by interfering with the chloride-channel mediated neurotransmission in the parasite, thereby resulting in its paralysis and elimination.
Moxidectin is an oral dewormer and boticide for horses and ponies at least 4 months old. The label claims that one dose of the drug will suppress strongylus egg production through 84 days. Doramectin is an injectable drug marketed as a single dose for control of a wide range of roundworms and arthropod parasites in cattle and swine.
Ivermectins
The ivermectins enhance the release of gamma-aminobutyric acid (GABA), which paralyzes nematodes by blocking neurotransmission at excitatory motor neurons. Ivermectin has demonstrated effectiveness in a number of species against a wide variety of internal and external parasites. In cattle, swine, sheep, and goats, injectable ivermectin is used to treat infestations by numerous GI roundworms, lungworms, cattle grubs (cattle only), sucking lice, and mites. The paste and oral liquid forms of ivermectin have been approved for treatment of infestations by large and small strongyli, pinworms, and bots and for other equine parasite infestation. It is also approved for the treatment of ascaridiasis, although for some stages, it may be less effective than desirable.
Ivermectin has been approved for use in dogs and cats only for heartworm prevention; however, it has also been used at higher doses for treatment of other canine parasite infestations, including scabies. In certain dogs (most pure collie breeds) that are inherently sensitive to ivermectin, toxicities—sometimes fatal—have occurred with higher doses. Except for these unique toxicities, ivermectin has proved to be safe in other breeds and species when given at therapeutic doses. Overdose may manifest clinically as blindness, ataxia, and even death.
Agents Used In Heartworm Treatment and Prevention
There is considerable risk involved in the treatment of heartworms; therefore the American Veterinary Medicine Association (AVMA) Council on Veterinary Service established guidelines suggesting that first adult heartworms and then the microfilariae (the larvae forms of the filarial tissue) be eliminated. The adult worms usually stay in tissues, such as the heart, but the microfilariae migrate throughout the host. Heartworm disease is primarily seen in dogs; however, cats may also become infected. Dogs subject to infestation or reinfestation must be found free of microfilariae and adult heartworms before they are placed on a preventive heartworm regimen.
Melarsomine dihydrochloride is an arsenic agent used in treatment of heartworm disease caused by immature to adult infections. Dogs are at risk for posttreatment pulmonary thromboembolism; therefore they should be exercise restricted after treatment. The site of administration is critical for this drug, and it should be given only by deep intramuscular injection into the epaxial muscle. Adverse reactions observed with melarsomine dihydrochloride treatment include abdominal hemorrhage and pain, discolored urine, hematuria, tachypnea, disorientation, restlessness, and icterus. Melarsomine overdosage may show signs of arsenic toxicity. Dimercaprol (BAL) is an antidote for arsenic toxicity and may reduce signs of toxicity in overdoses. Co-administration of BAL may reduce the efficacy of melarsomine dihydrochloride.
Microfilariae ingested by mosquitoes from infected animals molt in the mosquitoes and are then introduced back into other animals when another blood meal is taken. It is these reintroduced microfilariae that molt again into larvae and become adult heartworms. Drugs used for heartworm prevention, such as diethylcarbamazine (DEC), ivermectin, milbemycin, and moxidectin, act by killing the tissue-migrating larvae. To review the heartworm life cycle, see Chapter 17.
DEC should be administered daily at the beginning of mosquito season and continued for 2 months after the season is over. If ivermectin or milbemycin is used, it should be given within 1 month of the initial exposure and then once monthly. The final dose is given within 30 days of the last exposure. If more than 45 days elapse between doses, animals should be retested for heartworms before restoration of preventative therapy. In mild climates where mosquitoes prevail year round, prophylactic treatment must be administered for the lifetime of the dog.
Although relatively nontoxic at the low dose used for heartworm prevention, DEC is somewhat irritating to the gastric mucosa. Oral administration is therefore recommended immediately after a meal to reduce nausea and vomiting. These adverse effects are usually seen only with the higher doses of DEC that are sometimes used to treat ascarids.
Milbemycin and ivermectin are available as one-per-month heartworm preventatives. Milbemycin (Interceptor, Novartis) and ivermectin/pyrantel (Heartgard Plus, Merial Ltd.) have the added protection against adult hookworms caused by Ancylostoma caninum. Ivermectin toxicities (although rarely observed at the low-dose heartworm preventative level) unique to collie breeds are not seen with milbemycin.
Moxidectin (ProHeart, Fort Dodge ) activity results in paralysis and death of the affected parasites at the tissue larvae stage. Not only is moxidectin indicated for heartworm prevention use in dogs 6 months and older, but also treatment of existing larvae and adult hookworm infections. Moxidectin is the first antimicrofilaria that is injectable and provides 6 months of continuous protection in one dose. It should be administered by a doctor of veterinary medicine (DVM). Professional administration avoids the need for the owner to remember when the dose is due.
Anticestodal Drugs
Anticestodal drugs kill and/or facilitate expulsion of tapeworms. The original drugs used were agents that temporarily paralyzed the tapeworms, causing them to lose their attachment to the GI tract. Even when these drugs contained purgative properties (causing the emptying, cleansing, or evacuation of the bowels) or were given with harsh laxatives, reattachment of a number of tapeworms was likely to occur. This treatment was stressful to the host because its ineffectiveness required repeated dosing. Newer drugs, although more expensive, kill the tapeworm and have replaced most other anticestodal drug on the market.
After oral administration, praziquantel is widely distributed throughout the body, which makes it unique in its effectiveness against various stages of tapeworm development, including the adult stage. In addition, it is nontoxic and has a wide margin of safety. It can also be given by injection.
Epsiprantel has proven to be safe and effective. Unlike praziquantel, only trace levels of it are absorbed after oral administrations, and it remains at the site of action within the GI tract. The exact mechanism of action has not been determined; however, this drug exerts its action directly on the tapeworm causing disruption of attachment to the host. The worm is vulnerable to digestion by the host animal.
Drugs Used To Treat Giardiasis
Giardia canis is a protozoan that may produce chronic diarrhea in dogs. Treatment with metronidazole is usually effective. In general, the toxicity is low; few adverse effects are reported during or after the 5-day treatment period.
Giardiasis is also found in cats, but clinically, it is usually not a problem (its diarrhea-producing role is not known). For treatment in the cat, metronidazole is given in a dosage regimen similar to that used in dogs. The margin of safety in cats is much narrower. Overdosing must be avoided because it may lead to death.
Investigations show that Giardia with presenting diarrhea may be successfully treated with an “alternating 7 days on-7 days off” regimen of fenbendazole. A label claim to this indication has not been made.
EXTERNAL PARASITE TREATMENT
Various chlorinated hydrocarbon compounds (e.g., lindane and methoxychlor) were once popular and marketed in several different formulations for a variety of uses in a number of species. The compounds were effective and possess rapid knockdown capability, with some having residual effects for several days. The long-lasting residual properties posed a threat as an environmental hazard, and as a result, many of these types of products have been banned. Although the degree of toxicity will vary among the various chlorinated hydrocarbons, they should all be treated with caution and used as advised on the container label. Some diluted aqueous suspensions and powders may be applied directly to livestock. Signs of toxicity include vomiting, weakness, and other CNS effects, such as tremors, incoordination, convulsions, coma, and respiratory failure. Young, debilitated, or lean animals are more susceptible to the toxic effects. There is no specific antidote for chlorinated hydrocarbon toxicity. The animal should be removed from further exposure and given supportive treatment, such as barbiturates, to control seizures, if necessary.
Organophosphates
Organophosphates (ronnel, coumaphos, trichlorfon, malathion) are formulated specifically for the treatment of external parasites. As with chlorinated hydrocarbons, a number of preparations exist, such as sprays, dips, foggers, pour-ons, and pest strips. These compounds have good insect-killing ability, but residual effects are related to the vehicle used to apply the agent. Topical application of these preparations permits significant absorption through the skin to produce signs of toxicity. Signs and treatment of toxicity are the same as those mentioned in the discussion on internal parasitism treatment. Persons applying these agents should avoid getting them in their eyes or on their skin. The use of disposable gloves and eye protection is recommended. Prolonged breathing of spray mists should also be avoided.
Pyrethrins
Pyrethrum flowers (chrysanthemums) have been used as insecticides for centuries. Formulations for animal use are reported to be nontoxic to mammals in addition to having little effect on the environment. Some toxicity has occurred in cats.
Pyrethrins are marketed in numerous formulations for convenient use. Most have chemicals, such as piperonyl butoxide, added to potentiate their killing power. Also, microencapsulation has significantly increased the residual activity of these compounds that were known initially for their quick “knockdown” effect.
Permethrin, a synthetic pyrethroid, is formulated and used similarly to the natural pyrethrins.
Miscellaneous Agents
Several manufacturers have marketed new once-per-month flea control products. Lufenuron is a benzoyl-phenyl-urea derivative classified as an insect development inhibitor. The product does not kill adult fleas, but instead safely and effectively controls flea populations by breaking the life cycle at the egg stage. Preexisting flea populations may continue to develop and emerge after flea treatment, so noticeable control may not be seen for several weeks after dosing. Lufenuron is available in tablet formulation for dogs and oral liquid and injectable formulations for cats older than 6 weeks of age.
Imidacloprid is a flea adulticide formulated for topical application. It is classified as a nitroguanidine and acts as an NT blocker in the insect. Imidacloprid will kill fleas within 1 day of treatment. The disadvantage with this product is that shampooing may shorten the duration of flea protection. The product is considered safe for dogs and cats older than 4 months of age.
Fipronil is classified as a phenylpyrazole and acts as a GABA inhibitor. It is a topical formulation for control of fleas by killing adult fleas. There is a product label claim for killing all stages of brown dog ticks, American dog ticks, Lone Star ticks, and deer ticks. After an application, the animal can be handled immediately and shampooed the following day. Fipronil is safe for dogs and cats 8 weeks of age and older.
Moxidectin is an endectocide of the milbemycin class used to treat infections caused by internal and external parasites in cattle.
Selamectin, a member of the avermectin class, is a once-per-month topical treatment for dogs and cats 6 weeks of age and older. Selamectin kills adult fleas and prevents flea eggs from hatching for 1 month. It is indicated for the prevention and control of flea infestations, prevention of heartworm disease, treatment and control of ear mite infestation, treatment and control of sarcoptic mange, control of tick infestation in dogs, and treatment of intestinal hookworm and roundworm infection in cats.
ANTIMICROBIAL AGENTS
Initially, antibiotics (antimicrobials) were defined as substances produced by microorganisms, which in low concentrations destroy or inhibit growth of other species of microorganisms. Many of these substances may be produced totally or in part through chemical synthesis. Because antibiotics have the potential to cure life-threatening infections, they are one of the most popular and useful groups of drugs in veterinary medicine.
It is important to know the characteristics and uses of the various antibiotics and have a proper understanding of the principles of antibiotic therapy (chemotherapy). It is beyond the scope of this chapter to present a thorough discussion of chemotherapy; however, some basic principles are discussed. Not all microorganisms are harmful or disease producing (pathogenic). Many bacteria normally found in the GI tract, mucous membranes, and skin are helpful to their host. They compete with invading harmful pathogens and keep them from proliferating, thereby preventing progression to a disease state.
Each antibiotic is effective against specific groups of microorganisms. Some antibiotics are bactericidal (destroy bacteria), and some are bacteriostatic (inhibit growth); some may be both, depending on the concentration of the antibiotic (Box 25-1). The various species of bacteria that are affected by the antibiotic are known as the spectrum. Broad-spectrum antibiotics are those that are effective against a wide range of microorganisms, both gram-positive and gram-negative.
One method of classification of bacteria is to determine the tendency to absorb dye (gentian violet) into the cell wall. Those absorbing stain are referred to as gram-positive (dark blue cell walls), and those that do not absorb the stain are known as gram-negative (light pink cell walls) (Box 25-2).
For an antibiotic to be effective, it must be able to reach the site of infection in a sufficient concentration to exert its effect on the microorganism. In addition, the antibiotic concentration must be maintained or reached frequently over a period of time to completely destroy all bacteria or inhibit bacterial growth and provide time for the natural defense mechanisms of the body to eradicate the pathogen.
The length of antibiotic therapy may vary, depending on factors, such as the site of infection, the microorganism, and the duration of infection. When antibiotics are prescribed, the treatment is usually for a minimum of 5 days. Although improvement may be seen with inadequate antibiotic therapy, it is an unwise practice to stop treatment until the total regimen has been given. Microorganisms exposed to subtherapeutic antibiotic levels may develop resistance to that particular antibiotic, which will then be ineffective even when given at high doses. Bacteria not only can develop resistance to several antibiotics, but can also pass resistance on to other species of bacteria. Multiple antibiotic-resistant bacteria are also a serious problem if resistance is developed in a hospital or practice. Nosocomial (originating in clinical settings) infections from resistant bacteria can be treated with only the most potent and expensive antibiotics. Nosocomial infections are discussed in Chapter 18.
The choice of antibiotic is obviously critical to successful therapy. The microorganism must be sensitive to the antibiotic chosen. A sample from the site of infection (blood, urine, or tissue) should be collected for culture and antibiotic sensitivity testing to determine the causative organism and the effective antibiotics (see Chapter 18). This is not always economically or clinically feasible, so potentially effective antibiotics are frequently just chosen (empiric treatment). Empiric treatment usually includes agents effective against gram-positive, gram-negative, fungal, and viral infections. In selecting an antibiotic, one tries to choose an agent that is most likely to be effective against the pathogen and least likely to disturb normal, nonpathogenic bacteria. Even the narrow-spectrum antibiotics are effective against a number of types of bacteria, both pathogenic and nonpathogenic. Destruction of the nonharmful bacterial flora may allow a second pathogen to manifest and proliferate.
Administered antibiotics that are not effective may actually worsen the disease by destroying nonpathogenic bacteria that are actively competing with the pathogen. Indiscriminate use of broad-spectrum antibiotics eventually leads to resistant strains, ineffective antibiotic use, and expensive, perplexing therapeutic problems.
PENICILLINS
The discovery of penicillin in 1920 has dramatically changed the outcome of many life-threatening infections. The basic penicillin molecule (Figure 25-7) has been continuously manipulated and changed to produce a number of improved penicillins with unique characteristics.
Penicillin G (benzylpenicillin), the first clinically used penicillin, is still used extensively in large animals in its procaine salt form. Procaine penicillin G is poorly soluble and is released slowly from its site of injection, providing adequate penicillin levels to allow once-daily dosing; however, twice-daily dosing is usually recommended. Penicillin G is effective when given orally, but high doses must be administered because only approximately one fourth of it is absorbed from the GI tract. Most of the antibiotic is destroyed by stomach acid, so it should not be given directly after feeding, when stomach acid is greatest.
Penicillin acts by blocking bacterial cell wall synthesis in the final stages of replication. Without a cell wall, the bacteria swell and cannot function properly, and some lysis (rupturing) may occur. New infections in a high-log growth phase are therefore most susceptible to penicillin. Penicillin has no direct effect on mammalian cells because they do not have cell walls.
Penicillin G is effective against most of the gram-positive microorganisms, including many of the streptococcal and staphylococcal species. Some staphylococcal species have the ability to produce penicillinase, an enzyme that hydrolyzes the lactam ring and thus renders the penicillin inactive. At high doses, penicillin G is effective against a few gram-negative species.
One alteration of the penicillin molecule was to make it more resistant to hydrolysis by stomach acid. For example, amoxicillin and potassium clavulanate, a specific β-lactamase inhibitor, is a combination product prepared to resist the action of penicillinase. Table 25-2 provides some comparison among various commercially available penicillins. From side-chain alteration of the molecule emerged penicillins that are effective against a wide variety of microorganisms. Some of the penicillins available for human use have a broad spectrum of activity and are the most important potent antibiotics for use against many gram-negative organisms that may be resistant to most other antibiotics.
TABLE 25-2
Comparison of Penicillin Products
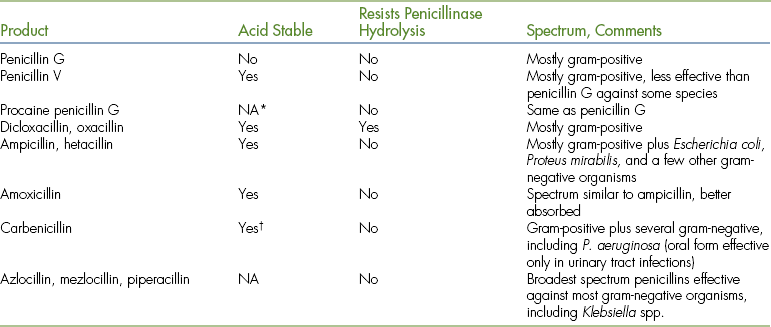
∗NA, Not applicable (no oral forms).
†Indanyl sodium salt for oral use.
There is documentation that some patients that are sensitive to the penicillins are also sensitive to another class of antibiotics: cephalosporins (cross-sensitivity). In general, the penicillins are safe. Allergic reactions, such as skin rashes, fever, urticaria, salivation, cutaneous edema, and other hypersensitivities, may occur and lead to justifiable concern.
AMINOGLYCOSIDES
Aminoglycosides (streptomycin, neomycin, kanamycin, amikacin, gentamicin) have a fairly broad spectrum, but are used primarily for the activity against gram-negative organisms. Aminoglycosides are not adequately absorbed when administered orally, but they may be used orally for intestinal tract infections or “sterilization” of the GI tract before surgery. Aminoglycosides exert their action by interfering with bacteria protein synthesis. Although toxicity may vary among agents, all are potentially ototoxic (affecting hearing balance) and nephrotoxic (renal toxicity). Neuromuscular blockage is also an adverse effect that is manifested by apnea and progressive paralysis of skeletal muscle. When aminoglycosides are administered to animals with preexisting renal damage, the patient must be closely monitored because potential for toxicity is much greater.
Resistance, toxicity, and expense are major considerations in the selection of these agents. Resistance demonstrated by organisms may be to a particular aminoglycoside or commonly to several aminoglycosides within this class (cross-resistance).
Neomycin is nephrotoxic and therefore finds its use primarily in topical or ophthalmic preparations. Kanamycin, gentamicin, and amikacin are commercially available as veterinary products. Although expensive for humans, other aminoglycosides are finding use in veterinary medicine for highly resistant organisms that are not susceptible to other antibiotics.
Aminoglycosides are frequently used simultaneously with some of the newer penicillins or cephalosporins to treat stubborn gram-negative infections. Because the combinations are more effective than the use of either agent alone, the activity of the combination is called synergism. The use of aminoglycosides and CHPC together is contraindicated because it is an antagonistic combination, resulting in decreased antibacterial action.
CEPHALOSPORINS
Cephalosporins (cephalexin, cefadroxil, cephradine, cephapirin, cefuroxime, cefotaxime, ceftazidime, and ceftiofur) are somewhat chemically similar (Figure 25-8) to the penicillins and share a similar mechanism of action and spectrum. Cephalosporins are not destroyed by penicillinase-producing bacteria, although some resistance to them exists.
The cephalosporins are subclassified primarily by spectrum into first, second, third, and fourth generations. Only minor differences exist in the spectrum of the first generation; all are effective against most gram-positive bacteria and several gram-negative species. The second generation has a somewhat broader spectrum, displaying activity against most clostridial species and adds more gram-negative coverage and less gram-positive coverage. Although Pseudomonas aeruginosa is not susceptible to the first- or second-generation cephalosporins, it may be treated with the third-generation cephalosporins. Severe infections, such as Pseudomonas infections, are usually treated with a combination of antibiotics to ensure eradication and limit the possibility of developing resistance. The fourth generation cephalosporin cefepime can be compared with the third generation, but is more resistant to some chromosomal β-lactams, such as those produced by Enterobacter.
The cost of the cephalosporins limits their use in veterinary medicine. Even with the availability of veterinary cephalosporin and generic products for humans, cost remains a major concern when considering second- and third-generation cephalosporins.
The cephalosporins have a low incidence of adverse effects. Long-term use of excessively large doses may lead to some complications similar to those of other antibiotics, including possible allergic reactions or overgrowth of nonsusceptible bacteria or fungi, leading to intestinal pain, bloating, and diarrhea.
QUINOLONES
Quinolones constitute a class of antibiotics finding extensive use in veterinary medicine for treatment of a wide variety of organisms, including P. aeruginosa. Enrofloxacin is approved primarily for urinary, skin, and respiratory infections in dogs and cats, but it is also being used to treat bone and other infections in several additional species. There is a subcutaneous injection approved for cattle not intended for food to treat bovine respiratory disease (BRD) associated with Pasteurella haemolytica, Pasteurella multocida, and Haemophilus somnus.
Enrofloxacin seems to be well tolerated, and few side effects have been noted in animals. It is contraindicated in puppies during the rapid growth phase because it can induce abnormal cartilage formation, leading to weakness or lameness. This potential adverse effect discourages the use of enrofloxacin in other young animals and in adult horses. Although bacterial resistance to enrofloxacin is not yet common, indiscriminate use to treat routine infections is likely to produce resistant strains, making this valuable drug worthless.
CHLORAMPHENICOL
Chloramphenicol use in humans is limited to a few specific infections because of a rare but potentially fatal occurrence of irreversible aplastic anemia. Personnel who handle and administer CHPC to animals should use care, avoiding direct contact with the drug. Although some blood dyscrasias have been seen in animals, particularly in neonates, the condition is usually reversible by withdrawal of the drug.
CHPC is an important antibiotic in veterinary equine medicine. Although CHPC is bacteriostatic, it has a fairly broad spectrum of activity. It is rapidly distributed to most body compartments and tissues in adequate therapeutic concentrations. A small amount of CHPC is excreted unchanged in the urine, but most undergo biotransformation in the liver to the inactive glucuronide conjugate.
Other adverse effects include anorexia, diarrhea, vomiting, and depression and other rare but severe effects. CHCP in combination with other antibiotics is usually contraindicated. It interacts with several specific drugs or groups of drugs, including anticonvulsants, penicillins, phenylbutazone, and lincomycin.
Florphenicol is a broad-spectrum, primarily bacteriostatic antibiotic with a range of activity similar to that of CHCP against many gram-negative and gram-positive organisms. It does not carry the risk of inducing blood dyscrasias.
TETRACYCLINES
Oxytetracycline and tetracycline are practically used interchangeably because of a similarity in spectrum and pharmacologic properties. One tetracycline, doxycycline, has gained acceptance for use in small animals. It requires less frequent dosing and penetrates the CNS better than other tetracyclines.
These bacteriostatic agents affect the vital protein synthesis of the microorganism. Although the tetracyclines possess a relatively broad spectrum of activity, the development of resistant organisms has been a factor that limits use. Through more judicious use of these agents, less-resistant strains are being encountered.
The absorption of tetracyclines from the GI tract is adequate, but is decreased in the presence of food, milk, or antacids. Some injectable preparations use propylene glycol as a solvent and are not recommended for intramuscular use because they are painful. When given intravenously, the tetracycline must be injected slowly because the solvent and drug may exert a blocking effect on the heart, causing the animal to temporarily collapse. Other injectable preparations contain povidone or similar agents, which reduce intramuscular irritation and eliminate the cardiac problem. The intramuscular product formulated for extended action must not be given intravenously.
Out-of-date or improperly stored tetracyclines should never be administered because they form nephrotoxic products.
Tetracyclines are relatively inexpensive and widely used, especially in food animals. The tetracyclines are also commonly used at low levels as a livestock feed additive to increase weight gain and decrease liver abscesses. This practice promotes the development of resistant strains of bacteria, rendering the tetracyclines useless for treatment even when given at therapeutic levels.
Although popular, the tetracyclines have toxicities. A common toxicity is the intestinal problems associated with disruption of the natural intestinal flora, including the possibility of superinfection by resistant organisms. Hypersensitivity reactions of rashes, fever, and liver damage may also occur with use of tetracyclines. The tetracyclines form complexes with calcium in developing bones and teeth; they should not be given to pregnant or young animals because tooth discoloration, increases in dental caries, and temporary suppression in bone growth may occur.
MISCELLANEOUS ANTIBIOTICS
Erythromycin and tilmicosin are classified as macrolide antibiotics because of their high molecular weight. The spectrum of activity is similar to that of penicillin; therefore they are commonly used instead of penicillin against penicillinase-producing microbes. Erythromycin does not alter intestinal flora extensively, but GI effects, such as vomiting and diarrhea, have been observed.
Tilmicosin is a macrolide used to treat bovine respiratory diseases, including those caused by Mycoplasma. A distinct advantage of tilmicosin is its long half-life, which allows a single-dose treatment. Tilmicosin must only be administered by subcutaneous or intramuscular injections because fatalities have been reported with intravenous dosage. Deaths have been reported after the use of tilmicosin in swine, horses, and nonhuman primates. The drug must be handled with extreme caution and administered in accordance with the detailed label instructions.
Azithromycin is a long-acting macrolide administered orally that is used to treat Chlamydia infections of the eye.
Lincomycin is in the class lincosamides. It has a spectrum of activity similar to erythromycin and is particularly effective against Staphylococcus and Streptococcus spp. It has been useful when resistant strains or hypersensitivities to other antibiotics exist. Favorable results have been reported in the treatment of bone infections and various skin disorders (pyoderma) with lincomycin. The drug is concentrated and excreted in the bile. Lincomycin causes severe intestinal flora disturbances in horses, hamsters, and rabbits, so it should be avoided in these species.
OTHER ANTIMICROBIAL AGENTS
In addition to the antibiotics discussed, other chemical agents exist that are effective against certain strains of microorganisms. The sulfa drugs were the first antimicrobial agents to be used systemically in the treatment of bacterial infections.
SULFONAMIDES
Numerous sulfonamides (sulfamethazine, sulfadiazine, sulfadimethoxine) have been formulated. Their value and use have declined with the discovery of newer antibiotics; however, a few sulfonamides remain useful for certain conditions. These agents are relatively inexpensive, which makes them attractive for use in large animals for herd or flock treatment. The sulfonamides are particularly useful in the treatment of various infections of the respiratory system and urinary tract, bacterial diarrhea, foot rot, and coccidial infections. Unfortunately, bacterial resistance to the sulfonamides limits their effectiveness. A toxicity seen with the original sulfonamides was crystalluria, a condition in which insoluble crystals formed in the urine, causing renal damage. Because the solubility of one sulfonamide is independent of other sulfonamides, the formulation of triple sulfa was developed to avert crystalluria. More soluble sulfonamides are also available, thereby further reducing concern. It is important that animals receiving sulfonamide have adequate water available.
The intravenous preparations of sulfonamides have a high (basic) pH and are therefore damaging to tissue when inadvertently given perivascularly (in spaces around blood vessels). In addition, the intravenous preparations should be given slowly to prevent acute toxicity demonstrated by CNS effects, such as salivation, vomiting, diarrhea, weakness, ataxia, and convulsions.
TRIMETHOPRIM-SULFONAMIDE COMBINATIONS
Effective antibacterials being used are combination products of one part trimethoprim and five parts sulfadiazine or sulfamethoxazole. Ormetoprim with sulfadimethoxine is a comparable combination with similar use and actions. These combinations block two essential sequential steps in the replication process of the bacteria, resulting in a synergistic antibacterial action. The combinations are effective against a wide range of organisms, but not Pseudomonas.
Undesirable side effects seen with these combinations are infrequent. Although vomiting may occur, diarrhea is seldom seen. Animals that are deficient in folic acid may be prone to develop blood disorders, as has been reported in humans.
NITROFURANS
The nitrofurans (nitrofurazone, nitrofurantoin, furazolidone) have been replaced to a great extent by newer, more effective, and safer antibacterials. These synthetic agents have a fairly broad spectrum of activity, but they are not effective against Pseudomonas.
Except for topical application, use in food-producing animals is strictly forbidden by the FDA because of carcinogenic properties.
Nitrofurantoin is sufficiently absorbed and has some use in small animals in the treatment of urinary tract infections. Nausea and vomiting, which are common adverse effects, can be reduced by administering nitrofurantoin with food or using the macrocrystal human preparations.
ANTIFUNGAL AGENTS
Numerous topical agents are available to treat fungal infections of the skin (dermatomycosis). Griseofulvin is administered orally; it has no antibacterial activity, but inhibits the growth of various skin fungi. It is an expensive product and is not usually the first-line choice unless the infection is widespread.
The treatment of systemic fungal infections (e.g., cryptococcosis, blastomycosis, histoplasmosis) is usually expensive, requiring lengthy treatment with limited success. Amphotericin B, an antibiotic used for various fungal infections, is toxic, causing kidney and liver damage, CNS abnormalities, and so on. A newer formulation of amphotericin B in a lipid complex suspension eliminates some toxic effects experienced with the original solution. Nystatin, another antibiotic, is relatively nontoxic, but has a narrow spectrum of activity. Nystatin has activity against a variety of fungal organisms, but is used systemically to treat oropharyngeal and GI Candida infections.
Ketoconazole, an expensive antifungal agent, has proven to be effective against a variety of fungal infections. Ketoconazole causes hepatotoxicity (liver damage), so liver enzymes should be monitored during therapy. Itraconazole is a newer agent that is efficacious against a variety of fungal infections and is less hepatotoxic than ketoconazole and more expensive.
HORMONES AND SYNTHETIC SUBSTITUTES
Hormones that act on or are released by various organs are also used in treatment of specific diseases and disorders.
THYROID HORMONES
The thyroid gland is controlled primarily by the amount of thyroid-stimulating hormone released from the pituitary gland. When stimulated, the thyroid gland releases thyroid hormones consisting primarily of thyroxin. Because the thyroid hormones affect the metabolism of carbohydrates, protein, and fats, thyroid-deficient (hypothyroid) animals show signs of lethargy, reduced alertness, increased body weight, poor hair coat, and other related signs. Insufficient amounts of iodine in the diet can result in inadequate production of thyroid hormones. Such hormone deficiencies can be treated with desiccated thyroid because it is effective orally. Sodium levothyroxine may be the most popular agent for the treatment of hypothyroidism. Sodium liothyronine, the other active component of desiccated thyroid, is also available commercially.
Feline hyperthyroidism is treated with methimazole. It interferes with iodine incorporation into tyrosyl residues of thyroglobulin, thereby inhibiting the synthesis of thyroid hormones.
INSULIN
Insulin is normally produced and released by islet cells of the pancreas. This hormone is necessary to facilitate the use of food by the body, especially sugar. Insulin enhances the absorption of glucose in most cells of the body. Animals with inadequate insulin will have abnormally high blood glucose levels (hyperglycemia) and other associated metabolic disorders. Dog and cat insulin are thought to resemble more closely porcine than beef insulin. Insulin injection (regular Iletin) is a solution of dissolved insulin crystals, which accounts for its immediate action and short duration. There are other insulin preparations that are intermediate acting (approximately 24 hours) to long acting (approximately 36 hours). Isophane insulin suspension (NPH), an intermediate-acting insulin, tends to be widely used in small animal medicine.
Protamine zinc insulin (PZI) may take 1 to 4 hours for onset of action to occur. The effect of PZI peaks between 5 and 20 hours after dosing and may persist up to 30 hours. Dogs are generally controlled with once-a-day dosing. In cats, PZI insulin would begin to decrease blood sugar in about 1 to 3 hours and has its peak effect in 4 to 10 hours. The duration of action may be 12 to 30 hours. Nearly all cats require twice-daily dosing for good control.
With the emergence of recombinant products, it may be difficult to obtain insulin of animal origin. The newest product on the market, glargine insulin (Lantus), is another recombinant product that is being introduced in veterinary medicine. Glargine is a once-a-day recombinant product that peaks in about 12 hours. It was designed to prevent the highs and lows that the diabetic patient experiences with use of other insulin products. Animals that were originally administered insulin from animal sources may need to have dose adjustments if switched to recombinant products. Any change in insulin should be made cautiously and under the medical supervision of the veterinarian.
Overdoses of insulin produce hypoglycemia, which, if severe, can lead to coma and death. Treatment of hypoglycemia consists of administration of intravenous dextrose or intramuscular injection of glucagon.
If an animal develops hypersensitivity (local or systemic reaction) or insulin resistance, a change in type or species of insulin should be tried.
OXYTOCIN
Oxytocin is a hormone released at the end of pregnancy to stimulate uterine contractions during parturition and induce milk letdown. The synthetically produced oxytocin is destroyed in the GI tract and must be administered parenterally. It is beneficial during delayed parturition, for aiding milk letdown, treatment of postpartum retained placenta, and metritis.
PROSTAGLANDINS
Prostaglandins are found in many mammalian tissues and have been shown to have a wide variety of effects on a number of body systems, including the CNS, cardiovascular, urinary, GI, and reproductive systems. Commercially available PGs, such as dinoprost and cloprostenol, are used because of the effects on the reproductive system.
In cattle, PGs can be used to regulate the heat cycle, so breeding and consequent calving times for a herd can be planned. PGs are approved by the FDA to abort feedlot heifers. For certain conditions in mares, PGs can effectively restore the normal heat cycle so that the animals can be bred.
Pregnant women should not handle these agents because they are abortifacients. Bronchospasm is another serious adverse effect in animals and humans that may occur as a result of contact with the product. Consequently, PGs should not be handled by asthmatics or used in animals with respiratory diseases.
GASTROINTESTINAL DRUGS
Certain species, such as horses, rabbits, and rodents, are unable to vomit, but protracted vomiting may become a problem in dogs, cats, and other species.
The vomiting reflex may be stimulated through at least four different pathways. For example, chemical substances in the blood (bacterial toxins or certain drugs) may mediate vomiting via the chemoreceptor trigger zone (CTZ) pathway (medulla of brain). Vomiting arising from movement of the head (motion sickness) is transmitted through another pathway (cortex of the brain). In selecting an antiemetic agent, it is desirable to know the underlying cause of vomiting and the pathway involved because some antiemetic drugs are specific in their site of action. Vomiting may be a symptom of a disease state, so initial attention should be directed to treatment of the primary disease.
Although independent of their antihistaminic activity, a few of the antihistamines (e.g., dimenhydrinate, cyclizine, clemastine, and meclizine) and scopolamine are effective in preventing vomiting induced by motion sickness. The principal side effect of the antihistamine is drowsiness, which may be desirable in pets that are traveling.
A number of phenothiazine tranquilizers (chlorpromazine, prochlorperazine, and triflupromazine) are classified as broad-spectrum antiemetics that control vomiting by blocking the CTZ at low doses and at the emetic center (in the medulla of the brain) at higher doses. Although these agents have the potential of producing a number of adverse effects, the risk of toxicity is low because of the low dose and short duration of therapy. Some potent broad-spectrum, human antiemetics (e.g., haloperidol and metoclopramide) are finding use in veterinary medicine.
Metoclopramide is a unique pharmacologic agent. Besides its potent antiemetic property, especially in drug-induced emesis (e.g., cancer chemotherapy), metoclopramide is also a peristaltic stimulant, increasing gut motility. It has been used for gastric stasis in a number of species, including horses and cattle. In addition, to facilitate radiologic examination of the stomach or small intestine, metoclopramide may be used to stimulate gastric emptying and intestinal transit of barium in cases where delayed emptying interferes. Reflux esophagitis in dogs and cats has also been treated with metoclopramide.
Cisapride is useful as an equine GI prokinetic agent (increases motility) in reflux conditions. Cisapride is no longer available commercially, but may be obtained from a compounding pharmacy.
Vomiting related to chemotherapy has been treated with ondansetron, granisetron, dolasetron, and butorphanol. Maropitant citrate (Cerenia, Pfizer) has been formulated specifically for targeting the mechanism that causes patients to vomit when undergoing chemotherapy.
EMETICS
Agents to induce vomiting are used clinically as a rapid means of eliminating certain poisons or to remove food from the stomach before induction of general anesthesia. A once common emetic used in veterinary medicine is apomorphine. Although still commercially available, it is extremely expensive and difficult to obtain. Apomorphine stimulates the CTZ and may be administered orally, intramuscularly, intravenously, or via the conjunctival sac of the eye. Because apomorphine depresses the emetic center, repeated dosage is not recommended when the initial dose is ineffective. Apomorphine should not be given to cats because it produces extreme excitement. Xylazine, a sedative analgesic, can be used as an emetic because of the routine vomiting it produces in the cat.
Ipecac, once used commonly in cats and occasionally in dogs, has the disadvantage of having to be administered via a stomach tube because of taste. In addition, its effects may be somewhat sporadic. Some toxic effects, including death, may be induced with ipecac in cats. However, ipecac syrup remains a popular, convenient emetic for children for the removal of accidentally ingested noncorrosive poison.
ANTIDIARRHEAL AGENTS
Diarrhea, like vomiting, may only be a symptom of an underlying problem. Ideally, it is best to identify the specific problem and correct it. Current trends are not to slow the gut, but to allow it to remain active to remove any present toxins or irritants. Most small animals with diarrhea recover regardless of therapy. Persistent diarrhea not only may be offensive to pet owners, but also may require supportive treatment, such as electrolyte and fluid replacement. Anticholinergics, such as the various belladonna alkaloids (atropine, homatropine, and scopolamine), have historically been used to treat diarrhea. Although peristalsis (propulsive intestinal contractions) is reduced, a minimal antidiarrheal effect results. The value of using anticholinergics is questionable because they have adverse effects, such as increased heart rate, dryness of mouth, and diarrhea from gut paralysis.
Opiates, including opium tincture, morphine, codeine, and similar derivatives, such as diphenoxylate, are unique in that they increase rhythmic segmentation contraction, which resists intestinal flow and decreases peristalsis. In addition, the opiates increase the tone of the various sphincters and valves in the GI tract, which further delays movement of the contents. The commercial product diphenoxylate hydrochloride with atropine sulfate is effective in treating diarrhea in dogs.
The use of antidiarrheal opiates in cats is controversial because this species may react with excitatory behavior. Opiate antidiarrheals should be used with caution in patients with head injuries or increased intracranial pressures and acute abdominal conditions, such as colic, because the opiates may obscure diagnosis or clinical course of the condition. Opiate antidiarrheals should be used with extreme caution in patients with hepatic disease and CNS symptoms of hepatic encephalopathy because hepatic coma may result.
The use of opiates in animals with acute diarrhea that may be bacterially induced may enhance bacterial proliferation, delay the disappearance of the microbe from the feces, and prolong the febrile state. Acute overdoses of the opiate antidiarrheals could result in central nervous, cardiovascular, or respiratory system toxicity.
Another over-the-counter (OTC) preparation that is extensively used in veterinary medicine is the human product loperamide. Loperamide is a synthetic piperidine derivative that slows intestinal motility through a direct effect on the nerve endings and/or intramural ganglia of the intestinal wall. In animals, loperamide does not have analgesic activity, even in extremely high doses. Loperamide is available in tablet, capsule, and oral liquid formulations.
Bismuth subsalicylate is thought to have weak antibacterial properties and is a protectant and antiendotoxic. Popular thought suggests that the compound is cleaved in the small intestine into bismuth carbonate and salicylate. The bismuth carbonate is responsible for the protective, antiendotoxic, and weak antibacterial properties. The salicylate component has antiprostaglandin activity, which may contribute to its effectiveness and reduce symptoms associated with secretory diarrhea. In humans, the preparation is used for other GI symptoms (e.g., indigestion, cramps, and gas pains) and in the treatment and prophylaxis of traveler's diarrhea.
CATHARTICS (LAXATIVES)
There are relatively few clinical reasons to use cathartics in veterinary medicine. Occasionally, an older animal may have constipation, but usually, alteration of the diet will correct the problem. Another indication might be for the treatment of hairballs in cats. After bowel or anal surgery, stool softeners may reduce stress at the surgery site until healing takes place. Cathartics and enemas may also be used before GI tract radiographic examinations, proctoscopy, or elective surgery. One of the most legitimate uses of cathartics is in treating food animals and horses suffering with overingestion of concentrated carbohydrates, such as grain. There are a few other unique circumstances in which the use of cathartics is appropriate; however, one is discouraged from overuse because it leads to dependence.
Cathartics increase the motility of the bowel by directly stimulating the smooth muscle or indirectly activating receptors through increased bulk. The irritant laxatives, which directly increase bowel motility, include (1) emodin, found in cascara sagrada, aloe, and senna; (2) sodium ricinoleate, a digestive end product of castor oil; and (3) danthron, a synthetic compound. Bulk-producing cathartics include (1) indigestible materials, such as psyllium seed, methyl cellulose, mineral oil, and white petrolatum, which not only increase bulk, but lubricate and soften fecal masses; (2) saline cathartics, such as magnesium sulfate, sodium sulfate, magnesium oxide, and phosphate salts, which draw water into the bowel; and (3) stool softeners, such as docusate sodium and dioctyl calcium sulfosuccinate. These are surface-active agents, such as soap, that increase bulk through water retention and lubricate and soften the fecal mass.
The cathartics as a group are relatively safe for short-term use, although some may be harsh and cause cramping and diarrhea. Chronic use of the petrolatum type of cathartics may lead to deficiencies in fat-soluble vitamins because of absorption interference.
ULCER MANAGEMENT DRUGS
Gastric ulceration and the subsequent blood loss appear to be related to acid damage commonly associated with high doses of corticosteroids or NSAIDs and to certain medical disorders. Several methods are currently available for treatment and prevention.
Antacids were initially used, but required around-the-clock administration every 2 to 3 hours to truly be effective. A major advancement in human medicine for ulcer management was the introduction of cimetidine, a histamine2-receptor antagonist. Although these agents are not approved for veterinary use, cimetidine, ranitidine, and others are used to block the acid-producing effects of histamine on the gastric parietal cells.
Sucralfate in an acid environment forms an ulcer-adherent complex providing a protective, Band-Aid-like barrier for the damaged mucosa. Sucralfate also inhibits pepsin activity.
Omeprazole is an agent that acts directly on the parietal cell, blocking acid secretion. Omeprazole is formulated as an oral gel that is indicated for treatment and prevention of recurrence of gastric ulcers in horses and foals 4 weeks of age and older. Misoprostol not only blocks gastric acid secretion, but also appears to enhance natural gastromucosal defense mechanisms.
PHARMACY
STATE LAWS
Most state pharmacy laws are primarily concerned with the distribution of drugs within the state. These laws specify who is authorized to prescribe and dispense legend drugs, the licensing of distributors, records required, and certain processing standards.
Because state laws are unique to each state, it is the responsibility of those practicing veterinary medicine to know the laws that apply to them. State laws work in conjunction with federal laws. Sometimes state laws are more restrictive than federal laws; in such cases, one should comply with the state law.
FEDERAL LAWS
Although the Food, Drug and Cosmetic Act of 1938 has been amended numerous times, it is still the basic federal law governing drugs in the United States. This law assures the public that drugs have been prepared through approved manufacturing standards and are safe and effective for the claims made. The Durham-Humphrey Amendment (1951) restricted the availability of certain drugs to prescription through licensed practitioners. This class of drugs, referred to as prescription drugs or legend drugs, is deemed unsafe for lay medication, even with clear and precise label directions.
Veterinary labeled prescription drugs bear the legend, “Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.” Human-labeled prescription drugs bear the legend, “Caution: Federal law prohibits dispensing without a prescription.” Commercial packaging may elect to use the “Rx” symbol on the label copy to denote drug product status as a legend drug instead of the written caution.
The FDA has the responsibility for determining the marketing status of a drug, whether or not it is possible to prepare adequate directions for use under which a layperson can use the drug safely and effectively. Nonprescription or “over-the-counter” drugs may be sold directly to clients, but must bear extensive labeling, which includes warnings and instructions for proper use.
The AVMA has approved the following guidelines regarding the use and distribution of veterinary drugs: (1) A prescription drug can be dispensed only by or upon the lawful written order of a licensed veterinarian within the course of his or her professional practice where a valid veterinarian-client-patient relationship (VCPR) exists; and (2) all veterinary prescription drugs must be properly labeled when dispensed.
VETERINARIAN-CLIENT-PATIENT RELATIONSHIP
A VCPR exists when all of the following conditions have been met:
1. The veterinarian has assumed the responsibility for making clinical judgments regarding the health of the animal(s) and the need for medical treatment, and the client has agreed to follow the veterinarian's instructions.
2. The veterinarian has sufficient knowledge of the animal(s) to initiate at least a general or preliminary diagnosis of the medical condition of the animal(s).
3. The veterinarian is readily available for a follow-up evaluation or has arranged for emergency coverage in the event of adverse reactions or failure of the treatment regimen.
LABEL REQUIREMENTS
Labeling requirements vary between states, but may include:
• Name, address, and telephone number of practice
• Quantity of medication dispensed
• Adequate directions for proper administration of medication
Auxiliary labels may also be required to caution or inform the client. Examples include “Shake well,” “Keep refrigerated,” “Do not use after (date),” “Poison,” “External use only,” and “For veterinary use only.” It is the responsibility of the veterinarian to inform clients to whom prescription drugs are delivered or dispensed about appropriate handling and storage.
The ultimate responsibility for any medication dispensed through a veterinary practice lies with the authorizing veterinarian. In some states, the technician may be allowed to assist the veterinarian by typing labels, counting or pouring, attaching labels, and pricing. The technician should not issue or refill medications without the veterinarian's approval. For most medications, this would be in violation of the federal law.
Readily retrievable dispensing records may be required by some states to safeguard the public's health. Accidental ingestion of prescription drugs by animals and small children is not uncommon. Proper records can provide attending physicians with the name and the amount of medication dispensed so that appropriate treatment can be provided.
The Federal Poison Prevention Packaging Act passed in 1970 requires pharmacists and physicians to dispense medications intended for oral human use in childproof containers. The AVMA recommends that prescription drugs to companion animal owners be placed in child-resistant containers. Certain states mandate the use of such. Veterinary practices failing to use such a safeguard would be highly vulnerable to legal action in a case of accidental poisoning.
CONTROLLED SUBSTANCES
The Controlled Substances Act of 1970 reduced drug abuse by defining certain legal and illegal acts regarding substances of high abuse potential. It established and authorized the Drug Enforcement Administration (DEA) to enforce this law. The law is designed to provide an approved means for proper manufacture, distribution, dispensing, and use of controlled substances through licensing of legitimate handlers of these drugs. This “closed” system has been effective in reducing widespread diversion of these drugs into the illicit market. Controlled substances are classified into five classes (schedules) according to the use or abuse potential (Table 25-3).
All veterinarians using these drugs in the course of their practice are required to have a DEA license number. Those who engage in administering or dispensing controlled substances in Schedules II, III, IV, and V are required to keep records of such transactions for 2 years. Receiving records for reports of controlled substances received must also be kept for 2 years.
In addition, practitioners who handle controlled substances are required to take an initial inventory at the opening of business of all controlled substances. Biannual inventories are required after the initial inventory. Records for receipts and dispensing of Schedule II substances must be kept separate from all other records. When records for Schedules III, IV, and V drugs are incorporated with other drugs, they should be identified with a red “C” in the lower right-hand corner of the record. All controlled substance records must be “readily retrievable.” Each commercial container of a controlled substance shall have printed on the label the symbol designating the Schedule in which such is listed, (CII). The word “schedule” need not be used.
Acquisition and distribution of controlled substances should be monitored by maintenance of a perpetual inventory (Figure 25-9) for each product stored in the practice. Drugs in Schedule II must be ordered on a DEA Form C-222 and completed when drugs are received.
A perpetual inventory is a “checkbook” balance system that provides an up-to-date balance of each drug. It is easier to reconcile inventory when this system is used.
It is best that those persons responsible for handling controlled substances be familiar not only with federal laws governing them but also with state laws, which may be more strict. Agencies, such as the state boards of pharmacy or the local DEA office, are quite helpful in answering questions concerning compliance.
The law states that, “A practitioner who has controlled substances stored in his office or practice must keep these drugs in a securely locked, substantially constructed cabinet or safe.” A secure area is usually interpreted as a double-locked container that cannot be picked up and moved. Examples would be a locked metal box stored inside a floor safe or an attached locked wall cabinet. The responsibility for access to controlled substances should be restricted to only one or two persons in the practice. Practitioners experiencing theft or a significant loss of controlled substances must report such a loss to the DEA regional office and the local police department when the loss is discovered.
A government publication entitled, Physician's Manual: An Information Outline on the Controlled Substances Act of 1970 is an excellent guide for proper handling of controlled substances. This government manual may be obtained free by request from the following:
COMPOUNDING
Compounding is the preparation of a drug product by mixing legally, obtainable ingredients and/or appropriate vehicles that have not been listed as an unapproved drug for animals by the regulatory action of the FDA, United States Department of Agriculture (USDA), or Environmental Protection Agency (EPA). When drug products are compounded, distributed, and used there is a possibility of harm to public health and animals if there is no adequate and well-controlled safety and effectiveness data and when there is no adherence to the good manufacturing practices (GMP). Death and adverse drug reactions may result from use of these compounded drugs.
Federal and state laws permit compounding because it sometimes provides value to patients. The practice of veterinary medicine continues to require medications to treat or prevent diseases and requires dosages for different animal species for which there are no commercially prepared, FDA-approved products currently available or efficacious. Concentrations, dosage forms, or combinations of medications that are unavailable can be compounded. Compounding can be used to prepare products that are hard to acquire or are temporarily unavailable from the manufacturer. Pharmacies require a written prescription specifying that the product can be compounded for a specific patient. Veterinarians may compound medicaments for their own patient use. Whether compounding is done at the local practice or in a pharmacy, a recipe is provided so that other pharmacists or veterinarians can provide refills of the same recipe.
Veterinarians, who compound themselves or make the decision to use a compounded product, must assume the responsibility for the safety of animals and wholesomeness of food of animal origin.
Both the pharmacist and veterinarian who compound must use professional judgment that is consistent with proper pharmaceutical and pharmacologic principles when compounding medications. The following points must be considered:
• The stability of the active ingredients
• The physical and chemical compatibility of the ingredients
• The pharmacodynamic compatibility of the ingredients
• The inactive ingredients and diluents must be of known compositions and not contaminated with harmful substances or agents or unapproved sources
• The prepared medication must be properly labeled before dispensing
• Compounded medications must not be advertised or displayed to the public
When compounded medications are used, appropriate records must be maintained. When compounded products are used in food-producing animals, appropriate residue tests, when available and practical, and other procedures for ensuring volatile residue avoidance should be instituted.
In the July 14, 2003, Federal Register, the FDA released a revised Compliance Policy Guide (CPG) section 608.400 entitled “Compounding of Drugs for Use in Animals” (61FR34840). The purpose of the guide is to ensure that the agency's enforcement policy regarding the compounding of drugs intended for use in animals is consistent, to the extent practical, with its policy regarding the compounding of drugs intended for use in humans. The FDA does not make a distinction between compounding and manufacturing or other processing of drugs for use in animals rather than in humans. It does acknowledge the use of compounding within certain areas of veterinary practice. Regulations specifically permit compounding of products from approved animal or human drugs under conditions set forth in 21CFR 530.13. The activity of compounding is not the subject of this guidance.
Veterinarians and pharmacies that are engaged in manufacturing and distributing unapproved new animal drugs in a manner that is clearly out of bounds of the traditional pharmacy or veterinary practice violate the act.
The three restrictions set for compounding by the FDA are:
• The drug product must not be identified by the FDA as a drug product that presents demonstrable difficulties for compounding in terms of safety or effectiveness.
• In states that have not entered into a “memorandum of understanding” with the FDA addressing the distribution of “inordinate amounts” of compounded drugs in interstate commerce, the pharmacy, pharmacist, or physician compounding the drug may not distribute compounded drugs out of state in quantities exceeding 5% of that entity's total prescription orders.
• The prescription must be “unsolicited,” [section 353a(a)], and the pharmacy, licensed pharmacist, or licensed physician compounding the drug may “not advertise or promote the compounding of any particular drug, class of drug, or type of drug.” The pharmacy, licensed pharmacist, or licensed physician may, however, “advertise and promote the compounding service” that they provide, [section 353a(c)]. Thompson v. Western States Medical Center.
Generally the FDA will defer to state authorities regarding the day-to-day regulation of compounding of animal and human drugs that are intended to be used in food-producing animals. When the scope and nature of activities raise concern associated with manufacturing resulting in significant violation of the new drug, adulteration, or misbranding provisions of the act, the FDA has determined it will seriously consider enforcement action. In determining whether or not to initiate such action, the agency will consider whether the veterinarian or pharmacist engages in any of the following acts:
• Compounding of drugs for use in a situation (a) where the health of the animal is not threatened and (b) where suffering or death of the animal is not likely to result from failure to treat.
• Compounding of drugs in anticipation of receiving prescriptions, except in limited quantities in relation to the amounts of drugs compounded after receiving prescriptions issued within the confines of a valid VCPR.
• Compounding of drugs that are prohibited for extralabel use in food-producing or non–food-producing animals under 21 CFR 530.41(a) and (b), respectively, because the drugs present a risk to the public health.
• Compounding finished drugs from human or animal drugs that are not the subject of an approved application or from bulk drug substances, other than those specifically addressed for regulatory discretion by the FDA, Center for Veterinary Medicine (e.g., antidotes). Inquiries about compounding from unapproved drugs or bulk drug substances should be directed to CVM, Division of Compliance, 240-276-9200.
• Compounding from approved human drugs for which the FDA has implemented a restricted distribution system.
• Using commercial scale manufacturing equipment for compounding drug products.
• Compounding drugs for third parties who resell to individual patients or offering compounded drug products at wholesale to other state licensed persons or commercial entities for resale.
• Failing to operate in conformance with applicable state law regulating the practice of pharmacy.
• Compounding of drugs for use in animals where an approved new animal drug or approved new human drug used as labeled or in conformity with 21 CFR Part 530 will, in the available dosage form and concentration, appropriately treat the condition diagnosed.
• Compounding from a human drug for use in food-producing animals if an approved animal drug can be used for the compounding.
• Instances where illegal residues occur in meat, milk, eggs, honey, aquaculture, or other food-producing animal products, and such residues were caused by the use of a compounded drug.
• Labeling a compounded drug with a withdrawal time established by the pharmacist instead of the prescribing veterinarian.
• Labeling of compounded drugs without sufficient information, such as withdrawal times for drugs for food-producing animals or other categories of information that are described in 21 DFR 530.12.
The foregoing list of factors is not intended to be all-inclusive. Other factors may be appropriate for consideration in a particular case.
Although many veterinarians say that in some cases the use of bulk drugs is medically necessary, the FDA regulations forbid the use of bulk drugs. The revised guide provides a list of bulk substances for compounding and subsequent use in animals that the FDA will not normally object to:
REGULATION OF NUTRACEUTICALS
Chapter 23 provides detailed information on nutraceuticals. The use of nutraceuticals has plummeted, even though most are unproven and uncontrolled. Most do not meet USP (United States Pharmacopeia) standards. None requires the scrutiny of the FDA because it considers nutraceuticals as dietary supplements, not drugs. Pet products are regulated under the auspices of specific animal regulatory agencies.
Preparations of nutraceuticals do vary widely between manufacturers. There is no mechanism to establish the effectiveness of levels of products consumed or support label claims. There are no guarantees that the product is stable enough to withstand extreme temperatures or prolonged storage. There is no supportive documentation of stability of raw ingredients. In addition, some nutraceuticals are poorly manufactured. Some may be contaminated with bacteria or heavy metal. A number of side effects have been identified, but not passed on to the consumer.
There is truly a need for some level of government to regulate the safe use of nutraceuticals as effective dietary supplements for animals and accurate function claims. Before purchasing pet supplements, owners need to make sure that the products are truly helpful, safe, and effective. They need to make sure that the products have undergone many of the same scientific and clinical evaluations that are now required of human products.
EXPIRATION DATES AND DISPOSAL OF DRUGS
The concept behind expiration dates is that the prescriber and consumer can be confident that the potency of the drug remains unaffected during the time of use. The expiration date guarantees if the drug is stored properly as instructed by the manufacturer, no toxic by-products will accumulate before completion of the drug regimen.
Some manufacturers first began putting expiration dates on drugs in the 1960s. Although it was not required, the FDA began to mandate this practice in 1979 to set uniform testing and reporting guidelines.
The expiration date is set by the manufacturer after stability studies have been submitted to the FDA. This date is required in all labeling and should be clearly expressed as month, day, and year (i.e., 1/5/04 = January 5, 2004), and not as a code.
If this date is stated only in terms of month and year, according to the USP, the product becomes expired the last day of the stated month (i.e., 3/04 = March 31, 2004).
If a drug has not been stored properly and the integrity of the product has been compromised, the product's safety and effectiveness is questioned and should not be used. If a bottle of pills is wet or has been kept in a room with extremely high humidity and temperatures, the medication may go bad before its expiration date. Freezing temperatures may also ruin a drug's effectiveness. If capsules are sticking together or the shiny coat of a tablet is rubbing off in your hands, the drug may be degrading because it is stored in a place that is too moist. If a solution changes color or consistency, the product is light sensitive and has been stored under direct light. It should not be used even if the expiration date has not passed.
Drugs regulated by the EPA (i.e., Advantage, Bayer Animal Health) have no labeled expiration dates. The required shelf life is a minimum of 5 years.
Homeopathic medicine uses medication that works with your whole body to restore your health and are made from all natural substances. Unlike synthetic or man-made drugs, they never lose their potency or efficacy because all of the components are natural and not combined with any unnatural substances that will expire. If exposed to strong scents, left out in extreme heat or cold for a period of time, or contaminated by returning pills back to the bottle after they have been handled, they may be ineffective. If this happens, it is better to throw the product away. Do not reuse the bottle or send it to a recycling center. Homeopathic medicines are exempt from labeling laws that require expiration dates.
Commercially prepared products that are reconstituted (mixed with a diluent) before administration or repackaged must be labeled with an appropriate period limiting the time of use. If products are compounded, the compounder must establish an expiration date and appropriate withdrawal times for food-producing animals.
DISPOSAL OF DRUGS
Regardless of how well managed inventory control is, every facility will have drugs expire or unwanted drugs that need disposal. The EPA states that a drug product only becomes outdated when the decision is made to discard it. Whereas flushing was once, and in many instances still is, the most commonly used method of disposing leftover and expired medications, there are environmental concerns resulting from hormones and antibiotics contaminating drinking water supplies.
The Department of Environmental Quality (DEQ), EPA, FDA, and local boards of pharmacy have established guidelines to regulate drug disposal.
All drugs for discard are to be separated from usable stock and clearly marked as “outdated.”
Medications that can be returned for credit are sent back to the manufacturer, distributor, or a reverse distribution company (RDC—a company that serves as a liaison between purchaser and vendor for credit). The purchaser will need to establish an account with the RDC of their choice before any drugs are sent to them.
When using an RDC, make sure it is understood which items were purchased or received using special programs so that problems will not be incurred in getting credit at the correct price. Understand that their fee for this service comes off the top, so your credit for returns will be less than the statement.
Items that are unacceptable for credit may also be discarded through the RDC on a “by the pound” basis. In most instances, this is determined after the medications reach the company. As an alternative, those items that are not accepted for credit may be destroyed through some waste disposal companies.
Open containers may be placed in the biologic waste collection containers for disposal. Prior arrangements must be made with the companies for destruction of medications.
The DEA regulates the disposal of controlled drugs. In no case should controlled drugs be forwarded to the DEA. The procedures established shall not be construed as altering, in any way, state laws or regulations for disposal of controlled substances.
The only approved method of disposal of controlled substances by the DEA is through the hire of an RDC. The registrant (practice) should complete a C-II request form (disposition and reporting form for expired Schedule II pharmaceuticals) from the RDC. This will list all C-II drugs that will need disposal, including partial containers. After completing the form and sending it back to the RDC, the registrant will receive the triplicate form C222 (U.S. Official Order Forms-Schedule I and II). The registrant now becomes the supplier and will keep the suppliers copy (now in brown color). The blue copy (purchaser's copy) and green copy (DEA copy) are included in the box of drugs to be sent to the RDC. The green copy is then forwarded to the DEA by the RDC.
An inventory should be taken of all controlled drugs in Schedules III to V. The registrant should submit a written or type written list of every item for disposal to the RDC. A copy of the list should be filed with the registrant.
After receipt and disposal of all drugs, the RDC will send the following records to the registrant as necessary:
1. C-II Manifest (DEA Form #41-Registrants Inventory of Drugs Surrendered)
All records pertaining to disposal of drugs should be kept for 2 years as required by the DEA.
Used drug containers should be disposed of properly to reduce the risk of environmental contamination with chemicals. Always use the manufacturer's label recommendation for disposal of empty or partial containers. Unused products should not be dumped down a drain, a toilet, or on the ground. Disinfectants should be added to unused portions of live virus or modified live virus vaccines to reduce accidental exposure to disease.
Disposal of medical wastes may be regulated by your state. Contact the agency in your state that oversees the disposal of medical waste. A list of agencies can be found at the following EPA website:
MATERIAL SAFETY DATA SHEETS
The U.S. Occupational Safety and Health Administration (OSHA) under the authorization of the U.S. Department of Labor sets standards for current practice relations and requirements. The OSHA Act of 1970 was enacted to ensure the safe and healthful working conditions for working men and women. The law was based on the simple concept that every employee has the basic “right to know” the potential hazard of any substance in the workplace. Employees also need to know what protective measures are available to prevent adverse effects from occurring. Along with the federal government, the U.S. chemical industry developed a chemical identification system. It requires a paper document to accompany every chemical shipped, used, or stored. This paper document is called a Material Safety Data Sheet (MSDS) and is sent to users, such as industries, hospitals, universities, practices, and others when the chemicals are sent. The company that produces the chemicals writes the MSDS. Every drug and pharmaceutical aid has an MSDS. A file of these fact sheets must by maintained in the veterinary practice and be accessible to every employee.
The essential parts to an MSDS are:
1. Name, address, and telephone number of the manufacturer or supplier.
2. Chemical name as it appears on the container's label: common name; scientific name: trade name or brand name that the manufacturer uses; synonyms for the mixture or chemicals.
3. For hazardous ingredients, the MSDS should list:
a. The permissible exposure level (PEL)—amount of an air contaminant a worker can be exposed to for 40 hr/wk over a working lifetime (30 years) without suffering adverse drug reaction.
b. The threshold value (TLV)—amount of a substance in the air nearly everyone can be exposed to daily without adverse drug reactions.
4. Physical properties: vapor pressure; specific gravity; appearance and odor; solubility; boiling point; melting point; freezing point; vapor density; evaporation rate.
5. Potential for fire and explosion data: flash point (when will a fire start and what should be done about it); flammability or explosive limit (lower and upper explosive limit) (LEL and UEL)—numbers used to describe the range in which a fire or explosion can occur; extinguishing media required to put out class A, B, C, and D fires.
a. Body entry by inhalation, ingestion, or transdermally.
b. The short-term (acute) and long-term (chronic) harmful effects.
c. Carcinogenicity (cancer-producing), corrosive, or sensitizer (an allergen or irritant that after an initial sensitizing exposure produced atopic or contact dermatitis), irritant, target organ effector.
7. Reactivity: conditions under which a chemical reaction will occur either by itself or with other materials; whether the chemical bonds are strong or weak and make the substance stable or unstable; incompatibility with other substances' storage compatibilities; if the substances will break down under conditions and release toxic or flammable vapor or gas; whether hazardous polymerization can occur (a chemical reaction that can cause a fire or explosion and possibly release hazardous gases).
8. Spill or leak procedures: Precautions for disposal of released substances should be taken during handling and storage.
9. Special precautions: Respiratory protection, ventilation, protective clothing and gear, and hygiene practices.
MSDSs are not intended for consumers. It reflects the hazard of working with a material in an occupational fashion. Employees must have ready access to MSDSs while in the workplace. MSDSs must be on hand for every hazardous chemical known to be present in the workplace in such a manner that employees may by exposed under normal conditions for use or in a foreseeable emergency.
CALCULATIONS
There is no need to fear calculations involved in dosing and compounding medications. Most are simple arithmetic. A methodical approach to each problem will simplify the concept and minimize the risk of error. Remember:
• If you are transferring data from a reference source, double-check what you have written down.
• Write down every step; expressing all quantities in the same system of units.
• Do not take short cuts; you are more likely to make a mistake.
• Try not to be totally dependent on your calculator. There is something to be said for “common sense.” Have an approximate idea of what the answer should be, and if you happen to hit the wrong button on the calculator, you are more likely to be aware that an error has been made.
• Finally, always double-check your calculations. There is frequently more than one way of doing a calculation, so if you get the same answer by two different methods, the chances are that your answer is correct. Alternatively, try working the problem in reverse to see if you get the starting numbers.
EXPRESSION OF CONCENTRATION
The metric system is the International System of Units (SI Units) for weight, volume, and length. The basic unit for weight is gram (g), and the basic unit for volume is the liter (L), and the basic unit of length is the meter (m). The prefix “milli” indicates one thousandth (10−3) and “micro” one millionth (10−6).
In some countries, the avoirdupois system (pounds and ounces) is still used in commerce and daily life. The apothecary system of volume (pints and gallons) is still a common system for commerce and household measurement. One should be aware of these systems to prevent serious errors in interpretation of prescriptions. It is important to be able to change between the systems (Box 25-3).
EXPRESSION OF STRENGTH
Ratio is the relative magnitude of two like quantities.
Ratio strength is the expression of a concentration by means of a ratio (e.g., 1:10).
Percentage strength is a ratio of parts per hundred (e.g., 10%).
Thus 1:10 = 1 part in 10 parts total volume of solution.
If 1 ml of glucose is in 10 ml of solution, the ratio is 1:10. Therefore 10 ml of glucose is in 100 ml of solution. This can be expressed as a percentage, so it is equivalent to a 10% volume/volume (vol/vol) solution. The same concept applies whether the expression is % volume/volume (vol/vol), % weight/volume (wt/vol), or % weight/weight (wt/wt).
1. Express 0.1% w/w as ratio strength.
Therefore 0.1 g/100 g = 1 part/Y parts.
2. Express 1:2500 as percentage strength.
You know: percentage is a ratio of parts/100 parts.
Therefore, 1 part/2500 parts = Y parts/100 parts.
3. Express 1 part per million (ppm) as percentage strength.
You know that ppm is another expression of ratio strength (ppm = 1 part per million = 1:1,000,000).
Let Y be the percentage strength.
Therefore 1 part/1,000,000 parts = Y part/100 parts. Y = 1 × 100/1,000,000 = 0.0001% = 1 × 10−4%.
Percentage weight-in-weight (wt/wt) is the number of grams of an active ingredient in 100 g (solid or liquid)
4. How many grams of a drug should be used to prepare 200 g of a 5% wt/wt solution?
Let Y = the weight of the drug needed.
Y = 5 × 200/100 = 10 g.Percentage weight-in-volume (wt/vol) is the number of grams of an active ingredient in 100 ml of liquid.
5. If 6 g of iodine are in 240 ml of iodine tincture, calculate the percentage of iodine in the tincture.
You know: % weight in volume is part (g)/100 ml.
Let Y = percentage of iodine in the tincture.
Therefore Y/100 ml = 6 g/240 ml.
Y = 6 × 100/240 = 2.5% (wt/vol).
Percentage volume-in-volume (vol/vol) indicates the number of milliliters (ml) of an active ingredient in 100 ml of liquid.
6. If 20 ml of Betadine are mixed with water to make 60 ml of solution, what is the percentage of Betadine in the solution? You know that % volume in volume is part (ml)/100 (ml). Let Y be the percentage of Betadine in the solution.
Therefore, Y/100 ml = 20 ml/60 ml.
Y = 20 × 100/60 = 33% (vol/vol).
7. Express 15 g of dextrose in 300 ml of solution as a percentage, indicating wt/wt, wt/vol, or vol/vol. You know that g (weight)/ml (volume) is expressed as % wt/vol.
Let Y grams be the weight of dextrose in 100 ml.
Therefore Y/100 ml = 15 g/300 ml
Y = 15 × 100/300 ml = 5% wt/vol.
8. What is the percentage of sodium chloride in the following syrup?
You know: Percentage is the number of grams (w) of sodium chloride in 100 ml (v) of syrup.
Calculating the Strength of a Drug Solution
The following basic equation is used to calculate the concentration of a liquid dosage form:
If you know any two of these quantities, the third can be found.
Example 1: What is the strength of a 1-L solution containing 50 g of drug?
Substitute all known quantities in the equation. Solve for the unknown:
Manipulation of this equation is frequently used for finding the quantity (mass) of a given volume of drug solution at a known concentration:
Mass (g) = volume (ml) × concentration (g/ml)
Example 2: How much drug is needed to prepare 4 oz of a 2% solution?
Substitute all known quantities in the equation. Solve for the unknown:
The original equation is also used to find out the total volume of drug solution that can be prepared at a desired concentration with a given quantity of drug:
Example 3: How much of a 10% solution can be prepared with 15 g of drug?
Substitute all known quantities in the equation. Solve for the unknown:
CALCULATING THE STRENGTH OF DILUTED SOLUTIONS
A basic equation can be used to solve problems for dilution stock (concentrated) solutions. (A more concentrated [stronger] solution can never be made from a diluted [weaker] solution without adding pure drug.)
Knowing any three of these quantities, one can solve for the unknown.
Example 1: Prepare 2 qt of a 1:1000 solution from a 20% solution.
1. Concentration of desired solution (1:1000 = 1 g/1000 ml)
2. Volume of desired solution (2 qt = approximately 2000 ml)
Substitute all known quantities in the equation. Solve for the unknown:
Example 2: How much of a 1% solution can be prepared from 6 ml of a 5% solution?
Substitute all known quantities in the equation. Solve for the unknown:
CALCULATING DRUG DOSAGES
A drug dosage is expressed as units or mass of drug per body weight (BW) of the patient. The usual dosage for human drugs is based on the ideal BW of 140 lb (70 kg). There is no ideal BW in veterinary medicine because of the variety of species and breeds of animals. The usual drug dose for animals is based on BW expressed in pounds or kilograms.
The following equation is used for calculating the quantity of drug to be administered based on BW:
Example 1: An 88-lb dog is to receive a drug dosage of 25 mg/kg of BW. How many milliliters of the supplied drug at 50 mg/ml are required?
Substitute all known quantities in the equation. Solve for the unknown:
Volume of drug = 40 kg × 25 mg/kg/50 ml/ml = 20 ml (dose)
The dosage of highly toxic drugs, such as antineoplastic (anticancer) agents, is calculated on the basis of body surface area (BSA). BSAs are difficult to calculate. Nomograms and charts (Table 25-4) have been constructed to help relate BW to BSA. BSA is expressed in square meters (m2).
To determine dosage based on BSA, modification of the previous equation will enable this:
Example 2: A 44-lb dog is to receive a dosage of 0.2 mg/m2. What volume of a drug should be given at a concentration of 1 mg/ml?
CALCULATING INFUSION RATES
Many drugs must be administered intravenously by slow infusion rather than as a rapid bolus injection. Large volumes of fluids are also given by intravenous infusion. Disposable intravenous sets and infusion pumps are used to deliver intravenous fluids at a steady rate over a period of time.
Calculations of infusion rates can be found by using the following equations:
Example 1: If 500 ml of a solution is to be infused over 6 hours, what is the correct infusion rate (drops/min) if the set delivers 10 drops/ml?
1. Drops/min calibration of intravenous set (10 drops/ml)
2. Volume of solution to be infused (500 ml)
Substitute all known quantities in the equation. Solve for the unknown.
Example 2: If a drug is to be infused at a dosage rate of 2 μg/kg/min into a 50-lb animal, what rate (ml/hr) should a pump be set on for a drug concentration of 400 mg in 250 ml of dextrose 5%?
2. Weight of patient = 50 lb (22.73 kg)
3. Concentration of drug solution 400 mg/250 ml = 1.60 mg/ml
Substitute all known quantities in the equations. Solve for the unknown:
INVENTORY CONTROL
The maintenance of an active working inventory requires both planning and continuous monitoring. Failure to keep abreast of use and needs results in shortage, inefficient use of time, increased costs, and added stress. The time invested to maintain appropriate levels of stock is therefore beneficial to the overall operation of the practice.
Veterinary technicians who demonstrate interest in an active inventory may find themselves acquiring an increasing role in inventory control and maintenance. This additional responsibility not only increases employee value in the practice, but also adds to job satisfaction.
Ideally the quantities of each item stocked should be as small as possible without running out between reasonable ordering periods. Because it is worse to have a shortage of certain items than to have extra, most practices lean toward a higher inventory than actually required. Inventory turnover (the number of times per year an item is bought and sold) should be at least four to six times per year. Some items, such as pet food, may turn over 12 to 14 times per year. With the assistance of a computer, monitoring of daily usage, and keeping helpful records, the average turnover rate can usually be increased. The higher the turnover, the lower the investment in the item. Drug ordering can become a full-time duty if care is not given to organization and planning.
Inventory Maintenance
The primary disadvantage of having a large inventory is the expense of having working capital tied up in drugs and supplies. A large inventory makes switching to equivalent products difficult, even at a cheaper price. There is great potential for product breakage, expiration, spoilage, and obsolescence when the inventory is large. Some states have an inventory tax that provides added incentive for keeping working stock to a minimum.
Occasionally, there is some justification for increasing the purchase of certain products. The “savings” claimed through many of the deals offered by vendors should be approached with caution. Unless one can accurately predict the use of certain products, quantity buying is difficult to justify. To participate in most marketing promotions, a significant financial commitment is usually required. Before entering into these agreements, one should truly determine whether the products offered are desirable and will be used within a reasonable period and whether the savings really merit the capital commitment.
Processing small orders is costly because the time commitment required to process the order is not much different from that of a larger order with several items. One is justified in increasing quantities on these small orders, especially if the items are inexpensive, to reduce ordering frequency and cost of acquisition. Some vendors charge handling fees if the total order is below a minimum required dollar amount or volume.
Availability of replacement goods is a factor that will affect the inventory turnover. With some items, one may be able to accurately predict monthly use and maintain a few weeks supply. Unfortunately the use of most items cannot be readily anticipated, which results in a larger inventory requirement, especially if delivery time cannot by predicted.
PROCUREMENT
One may purchase supplies through veterinary wholesale suppliers (distributors) or directly from manufacturers. Distributors may specialize in one class of items, such as surgical supplies or bulk pharmaceuticals. Some wholesale suppliers may offer a complete line of products, ranging from buckets to gas machines.
One advantage in dealing with wholesalers is the ability to reduce the number of small orders that would be required in purchasing from several individual vendors. A few manufacturers only sell their products directly to veterinarians rather than distributors. The Compendium of Veterinary Products (see Recommended Reading) offers a complete reference to veterinary pharmaceutical companies and their product lines.
Veterinary Practices
It is an excellent idea to establish and maintain a good working relationship with another practice in the area. In a crisis, you can borrow items from that practice to see you through the emergency. Borrowing seldom-used items in an emergency is encouraged rather than stocking them. However, your practice is expected to order the item and return it. Thus inventory of seldom-used items should be maintained elsewhere, and record keeping is not necessary. Purchasing some items from another practice may be helpful, especially for expensive, short-dated items.
Several large buying groups have been established by some practices to increase their purchasing power and decrease costs.
Pharmacies and Drug Wholesalers: Using the services of a retail pharmacy is nearly essential to the practice of quality veterinary medicine. Veterinarians have a need for various human products that are not obtainable through veterinary suppliers. Retail pharmacies may not stock many injectable products, but they can help with most ophthalmic and oral products and some topical preparations. In some locations, a human drug wholesaler may deal directly with the small, individual practitioner. Most, however, do not welcome these small accounts and will serve only as a distributor for hospitals and pharmacies. The veterinary practitioner must make arrangements with pharmacists to obtain human products for practice or client use. Most pharmacists welcome this opportunity to serve the veterinarian.
Human Hospitals and Hospital Suppliers: A local human hospital may be a valuable resource for the veterinary practice. Federal laws restrict hospitals with special buying privileges from selling to anyone outside their institution. As a result, it may be difficult for veterinarians and their clients to obtain some of the more potent, expensive, or rarely used medical supplies, except in an emergency. Practicing veterinarians should make human hospital contacts to determine the local availability of human drugs and supplies. The hospital's library and clinical laboratory may also provide some welcome assistance.
Local hospital suppliers will stock items, such as syringes, needles, cotton balls, tongue depressors, and other disposable supplies. Although veterinarians do not routinely purchase from the local hospital, do not overlook them as an immediate source in times of shortages.
Other Sources of Suppliers: In addition to bulk chemicals, major chemical suppliers will stock glassware, balances, disposable beakers, brushes, carboys, and other laboratory and clinic supplies and equipment that would be useful in a veterinary practice. Most of these suppliers are located in metropolitan areas and have addresses and telephone numbers listed in the telephone directory.
Numerous mail order suppliers exist that provide not only pharmaceuticals, but also a wide variety of veterinary products and equipment. The quality of product and service may vary greatly among these outlets. Of major concern is the return policy for handling inferior or unacceptable items.
Feed stores and lay veterinary drug outlets can be used for an occasional urgently needed item. One may at times also want to take advantage of certain specials offered through these suppliers.
ORGANIZING THE PHARMACY
A comprehensive list of all activities conducted in the pharmacy should be prepared, whether planning a major hospital complex or rearranging a small portion of a hospital. Activities related to the pharmacy include storage (refrigeration, security), ordering, receiving, cleanup, dispensing, withdrawal and administration of medication, compounding and manufacturing, product information, and so forth. In the design, the location of each activity must be determined, and each activity should be coordinated with other areas when required. Although most areas will be multifunctional, some activities may be unique and have their own special requirements.
Regulating agencies require that refrigerators that store vaccines and biologics should be kept at 40° C. Minor fluctuations in temperature may occur. A daily temperature log must be maintained (Figure 25-10). Personal foodstuff should not be stored in the refrigerator designated for pharmacy use.
A detailed list of functions pertaining specifically to the pharmacy inventory should include the following:
• Ordering requires a telephone, desk, file, and calculator.
• Receiving should be near an outside door and requires temporary counter or floor space.
• Returns require space for holding broken outdated, and damaged items.
• Storage areas must be adequate for working and backup stock. Refrigeration for perishable items and security for volatile hazardous bulk materials.
• Pricing involves the use of a computer or price book, markup schemes, record, and a collection of MSDSs for all products.
In addition, consideration should be given to the movement of items to areas of use for dispensing. Monitoring of inventory levels of all items is a much-needed function to ensure an adequate supply at demand without shortages.
Arrangement of Inventory
Working inventory should be placed on shelves in an organized fashion. One method is to arrange items by dosage form. Categories would include the following:
• Oral solids (tablets, capsules)
• Oral miscellaneous (boluses, powders, pastes)
• External miscellaneous (sprays, powders, ointments, creams)
Each section should be further arranged, perhaps by generic name, brand name, or the more common name used by individuals in the practice. One may wish to make exceptions for items that are popular, but they should be limited.
A different type of arrangement would be to group items by their most common therapeutic use. Classification would be similar to that in the discussion of drugs found in the first portion of this chapter:
Each drug class could then be further divided into more specific uses, such as GI drugs divided into antiemetics, emetics, and antidiarrheals. Some classes may have only two or three items. Disadvantages of using this system are the poor use of shelf space and the possibility of gallon jugs ending up next to ampules.
Another arrangement is to group items by company or vendor. This method may be acceptable for backup stock because it is helpful when preparing orders. In an active inventory, there may be poor use of shelf space. Perhaps the greatest disadvantage is trying to recall the last supplier for rarely used items. Another disadvantage is purchasing generic items from multiple vendors, which may lead to multiple locations of the same item and duplicate stock.
Pharmacy organization is desirable and has advantages, primarily by assisting each individual in locating items. The best method of organizing stock is probably a combination of the various preceding arrangements. Each practice should design its own method. In addition to the methods listed, placement of selected items in areas where they are frequently used should be considered.
INTERNET PHARMACY
The Internet is rapidly transforming the way we live and shop in all sectors of the economy. In the area of health care, it permits individuals to obtain medical information to help them understand health issues and treatment options for themselves and their animals. The Internet allows consumers to shop online for health care products and get prescriptions filled.
There are great benefits and challenges that the Internet presents when consumers use it to shop. One of the greatest benefits of shopping online to fill prescriptions is the ease with which consumers can comparison shop. Many pharmacies offer price comparisons between their charges and that of other legitimate pharmacies, which helps to stretch the health care dollar. Some Internet pharmacies sell drugs for less than traditional “brick-and-mortar” pharmacies, which is most important for people who love their animals, but have limited income.
Legitimate online pharmacies offer valuable health care information in a searchable format. Drug prices and drug information are accessed by the website. It may be requested by e-mail. Consumers do not have to wait on the telephone for an answer or travel to the pharmacy to get it.
There is convenience and flexibility to ordering and receiving medications without leaving home, which is a tremendous timesaver. For the pet owner who has limited time, online prescriptions allow for the convenience of shopping 24 hours per day. This is especially valuable to homebound pet owners for whom a trip to the pharmacy may be difficult.
Online pharmacies provide more privacy than traditional pharmacies. Sometimes consumers are too embarrassed to purchase certain items or health care products from the local pharmacy. They may find greater anonymity by ordering online where staff may not be able to put “face to name.”
CONCERNS ABOUT ONLINE SITES
As beneficial as computer technology is, the Internet also creates a new market place for illegal activity, such as the sale of unapproved new drugs and prescription drugs dispensed without a valid prescription. Consumers may encounter difficulties in identifying illegitimate sites.
One problem found with illegitimate online pharmacies is that they open and close on a daily basis. One company may have many URLs or web addresses, and they frequently sell customer links. Many customers are unable to contact the pharmacy because telephone lines are disconnected or there is no answer.
Often consumers experience nonreceipt of medications ordered, and they face credit card charges that these illegitimate pharmacies refuse to remove. Genuine risks exist when foreign drugs are dispensed.
Medications dispensed that are considered unsafe for laypersons to administer without monitoring by a licensed veterinarian are called legend drugs (they bear the word “caution,” which restricts the use of the drug). For the veterinarian to write a prescription, he should have established a valid VCPR with the animal and its owner. This cannot be done online. Because online pharmacies only provide a questionnaire to be filled out, the PE (physical examination)—a requirement within a VCPR—cannot be done. When a VCPR is established without the PE, inappropriate medications can worsen an underlying, undiagnosed, serious disease state. When pharmacies do not employ licensed professionals, the animal's life may be threatened because the pharmacy may not sell the right drug.
Illegitimate online pharmacies use patient questionnaires and fee-based cyberspace consultations. They will sell legend drugs and controlled drugs without a consult. It is no longer legal to sell controlled drugs over the Internet.
Many illegitimate sites will use drugs from foreign countries. The FDA generally prohibits the importation of foreign-made versions of prescription medications that are commercially available in the United States. Genuine risks exist when foreign drugs are dispensed. The safety and efficacy of these medications cannot be guaranteed. Online pharmacies may dispense expired, subpotent, contaminated, or counterfeit products; the wrong or contraindicated product; incorrect dose; or medications without adequate directions for use.
The prescription order should come directly from the prescriber to be valid—not the patient. Online sites that do not protect the integrity of the original prescription or do not verify the authenticity of the prescription may be in violation of the law.
REGULATIONS
The challenge for regulatory agencies concerning online prescriptions is to make sure that the protection for consumers is just as strong as that for consumers who purchase drugs at their corner pharmacy.
The FDA has actively engaged with a number of states in jointly pursuing illegal Internet sales. Regulation is primarily the jurisdiction of each state board of pharmacy with some federal oversight. Most states protect their citizens by licensing “out-of-state” pharmacies to ship medications in their jurisdiction. The National Association of Boards of Pharmacy (NABP) does not regulate online pharmacies.
The VIPPS is a voluntary certification to Verify Internet Pharmacy Practice Sites. The program offers an accompanying seal of approval that identifies to the public those online pharmacy sites that are appropriately licensed and are legitimately operating over the Internet. Those approved sites have successfully completed a rigorous review inspection.
The value of the VIPPS program is to provide members of the public a means to assure themselves that the Internet pharmacy they choose is a bona fide, fully licensed facility exercising competent Internet and interstate pharmacy practices. Regulations that apply to traditional “brick-and-mortar” mail-order pharmacies apply to online pharmacies.
VIPPS-certified pharmacies are required to offer customers free telephone consultations with a registered pharmacist and may offer free ask-a-pharmacist e-mail service.
VIPPS has a mechanism in place to report errors made by its certified pharmacies. They are to document, track, and analyze the types of errors to determine what went wrong and to make suggestions to prevent recurrences.
ADVICE FOR CONSUMERS
• Suspect the pharmacy if it will dispense medications without requiring a hard copy of the prescription to be mailed in.
• Suspect the pharmacy if it dispenses prescription medications and does not contact the prescriber to obtain a valid verbal prescription.
• Suspect the pharmacy if it will dispense medications solely based on a consumer questionnaire without a preexisting VCPR with a prescriber on-site.
• Suspect the pharmacy if it does not have a toll-free number and street address posted to the website.
• Suspect the site if the pharmacy merely has an e-mail feature as the sole communication between consumer and facility. Legitimate sites allow you to contact the pharmacist.
• Avoid the site if the site does not advertise the availability of a registered pharmacist for consultation.
• Avoid a pharmacy that does not have policies in place that address different issues.
Baumgartner, K., Hoffman, D. Controlled substances handbook. Washington, DC: Government Information Services; 1998.
Bonagura, J.D. Kirk's current veterinary therapy 14: small animal practice. St Louis: WB Saunders; 2009.
Compendium of veterinary products, ed 7, Port Huron, Mich, North American Compendium, 2003.
Hardarman, J.G., et al. The pharmacological basis of therapeutics, ed 9. New York: McGraw-Hill; 1995.
Physician's desk reference, ed 57, Montvale, NJ, Medical Economics, 2003.
Plumb, D.C. Veterinary drug handbook, ed 4. White Bear Lake, Minn: PharmaVet Publishing; 2002.
ed 23. USPDI: Drug information for the health care professional, Englewood, Colo, Micromedex, 2003;vol 1:.
Veterinary pharmaceutical and biologicals, ed 12, Lenexa, Kan, Veterinary Healthcare Communications, 2001.
 TECHNICIAN NOTE
TECHNICIAN NOTE