Large Animal Medical Nursing
When you have completed this chapter, you will be able to:
1 List the common diseases and disorders of horses and describe the causes, symptoms, treatment, and control.
2 List the physiologic parameters used to monitor hospitalized equine patients.
3 Describe unique requirements for care of hospitalized recumbent and infectious equine patients.
4 Describe concerns related to the placement and care of intravenous (IV) catheters in horses.
5 List common medications used on equine patients and describe their indications.
6 Describe routine laboratory studies performed on equine patients.
7 List the common diseases and disorders of food animals and describe the causes, symptoms, treatment, and control.
8 List the common diseases and disorders of small ruminants and describe the causes, symptoms, treatment, and control.
9 List the common diseases and disorders of swine and describe the causes, symptoms, treatment, and control.
10 List the common diseases and disorders of camelids and describe the causes, symptoms, treatment, and control.
THE IMPORTANCE OF PHYSICAL EXAMINATION
A thorough physical examination is an integral part of the diagnostic assessment and monitoring of large animals. Because veterinary technicians play an important role in the day-to-day monitoring of hospitalized patients, learning to perform a thorough physical examination is vital. Refer to Chapter 8 for a detailed description of how to complete a physical examination on large animal species. Review the normal parameters for a horse in Box 22-1. It is crucial to record all observations and findings of the physical examination in the medical record. The medical record provides the only record of the patient’s progress or deterioration and is a legal document. Refer to examples of medical records designed for the large animal patients in Chapter 5.
EQUINE MEDICINE
COMMON DISEASES AND CONDITIONS IN HORSES
RESPIRATORY DISEASES
Strangles: Strangles is a common, highly contagious respiratory disease of horses caused by the bacterial pathogen Streptococcus equi equi. Strangles typically produces swelling and abscesses of the submandibular and retropharyngeal lymph nodes. Affected horses have fever, depression, poor appetite, and painful swellings under the mandible. The abscesses under the mandible enlarge, rupture, and drain purulent exudate. Horses may develop abscesses within the guttural pouch, thorax, abdomen, and central nervous system (CNS). The development of an abscess in abnormal locations is termed bastard strangles. These cases are particularly difficult to treat successfully. Horses with complicated cases of strangles should be treated with antibiotics, and S. equi equi is typically sensitive to penicillin. Horses with strangles should be maintained under a strict isolation protocol. Recovered horses remain contagious and represent a threat to susceptible horses for approximately 6 weeks after recovering from clinical disease.
Immunization against S. equi can be helpful. The recently developed modified live virus intranasal vaccine induces mucosal immunity, providing better protection and fewer side effects, but it should not be given to pregnant mares and young foals. This attenuated vaccine will not completely prevent infection in horses, but minimizes clinical signs.
Guttural Pouch Empyema and Mycosis: The guttural pouches are two large symmetric dilations of the eustachian tube that are present in all Equidae. They are located just above the pharynx and larynx and can be accessed during an endoscopic examination through small openings in the dorsal lateral nasopharynx. The internal and external carotid arteries and several cranial nerves travel superficially under the surface of the guttural pouch lining and are vulnerable to damage from pathologic conditions. The purpose of the guttural pouches may be to lower the temperature of the blood to the brain (internal and external carotid arteries) during exercise. A bacterial infection of the guttural pouch is termed guttural pouch empyema and is often associated with strangles. A fungal infection of the guttural pouch is termed guttural pouch mycosis, and the causative agent is often Aspergillus spp.
A bacterial infection of the guttural pouch (empyema) usually is a sequela to strangles or retropharyngeal lymph node abscesses. Clinical signs include swelling in the throat-latch region and bilateral mucopurulent nasal discharge. Horses with guttural pouch empyema can be treated conservatively with antimicrobials and guttural pouch lavage; this may be effective in many horses that are treated early in the course of the disease. However, in more chronic cases, the mucopurulent material becomes inspissated and forms gelatinous concretions (chondroids) that lie in the floor of the guttural pouches. A resolution of empyema requires removal of the chondroids. Long-term effective drainage can usually only be achieved with surgical drainage.
During guttural pouch mycosis, fungal plaque usually forms over the internal carotid artery, adjacent to nerves that control swallowing. Horses may have a life-threatening blood loss from rupture of the internal carotid artery or dysphagia from damage to the nerves.
The accumulation of air in guttural pouches (guttural pouch tympany) occurs in foals and weanlings and is usually associated with an abnormality of the opening to the pouches. It can occur unilaterally or bilaterally and is characterized by a fluctuant, nonpainful swelling in the throat-latch region.
Influenza: Influenza is a highly contagious viral respiratory disease in horses characterized by an increased body temperature, e.g., 40.8° C (104.8° F), cough, and depression. The incubation period is short (2 to 3 days), and horses remain ill for 3 to 4 days. The equine influenza virus is transmitted through a herd via aerosolization of virus during coughing. The virus damages the clearance mechanisms in the lung and predisposes horses to bacterial pneumonia. Horses should be rested for a minimum of 3 weeks after recovery from viral respiratory disease.
Immunization against influenza is recommended. The intramuscular influenza vaccines do not provide consistent protection from an influenza virus challenge, but vaccination programs do reduce the incidence of disease within the herd and the severity and duration of disease in individual horses. On the other hand, intranasal influenza vaccine closely resembles the protective immunity achieved with natural infection. Sedentary adult horses not exposed to other horses are at a low risk for contracting influenza and may be vaccinated only once or twice per year. Young horses and horses engaged in performance activities (racing, showing, training) are at high risk for contracting influenza because of the exposure to other horses and should be vaccinated every 3 to 4 months or 3 to 4 weeks before exposure to other horses. Broodmares should be vaccinated with the injectable vaccine against influenza in the tenth month of pregnancy to ensure adequate colostral transfer of antibodies against influenza for the foal. Some horses may suffer a transient systemic reaction characterized by fever, inappetence, and depression several days after influenza vaccination.
Herpes: Equine herpesvirus (the causative agent of rhinopneumonitis) is a contagious virus that produces respiratory disease, abortion, and neonatal and neurologic disease (ascending paralysis) in horses. It is a reportable disease in some states. The clinical signs of respiratory disease caused by equine herpesvirus are milder, but hardly distinguishable from equine influenza. The incubation period is longer (2 to 10 days), and horses may remain ill for 4 to 5 days. Equine herpesvirus is transmitted through the herd by aerosol transmission, respiratory secretions, and fomite transmission. Protection against respiratory disease following equine herpesvirus vaccination is inconsistent and relatively short lived. Abortion secondary to equine herpesvirus occurs in the seventh to eleventh month of gestation and the mare does not appear sick at the time of abortion. Vaccination is recommended for performance horses and broodmares. Neurologic disease caused by equine herpesvirus is not common; however, there have been a few large outbreaks in the United States over the last few years with high morbidity and mortality. Affected horses demonstrate signs of incoordination, inability to urinate, and poor tail tone. The recovery from neurologic diseases is prolonged (2 to 3 months), and horses may not return to completely normal neurologic function. Standard quarantine protocols (e.g., quarantine all new horses for 30 days) and using individual equipment with proper disinfecting techniques are important to minimize disease transmission.
Protection against respiratory disease following equine herpesvirus vaccination is inconsistent and relatively short lived. None of the currently available vaccines claims to provide protection against the neurologic form of herpesvirus in horses. Sedentary adult horses not exposed to other horses may not be vaccinated or vaccinated only once or twice per year, whereas young horses and horses engaged in performance activities should be vaccinated every 3 to 4 months. Inactivated univalent vaccines should be administered to broodmares during the third, fifth, seventh, and ninth months of pregnancy to prevent abortion. Although 100% protection against abortion is not achieved, the incidence of abortion caused by equine herpesvirus is significantly decreased by adherence to a proper vaccination program.
Viral Arteritis: Equine viral arteritis is a contagious viral disease that produces limb swelling, conjunctivitis, abortion, and respiratory disease in horses. Limb swelling is painful and results from vasculitis (inflammation of blood vessels). Stallions infected after puberty develop a persistent infection in the accessory sex glands (ampullae) and transmit the viral infection to mares during breeding. Abortion can occur at any point during gestation and results from viral damage to the blood vessels of the placenta. The vaccine for equine viral arteritis is approved for use in stallions and nonpregnant mares under the supervision of the United States Department of Agriculture (USDA). Pregnant mares should not be vaccinated against equine viral arteritis. Vaccination induces seropositivity and may interfere with testing requirements for export. Therefore a negative status should be confirmed before vaccination.
Heaves: Heaves or recurrent airway obstruction (RAO) is an allergic airway disease caused by airway inflammation, narrowing of small airways (bronchoconstriction), and excessive mucus production. The clinical signs of heaves are cough, nasal discharge, flared nostrils, increased respiratory rate, increased expiratory effort, and wheezing. The severity of clinical signs may range from exercise intolerance to severe respiratory distress (dyspnea) at rest. Most affected horses are allergic to dust and molds present in hay and straw. Management, such as changing the environment to remove offending allergens, is the most important intervention to treat these cases. Ideally, horses should be maintained at pasture and not fed hay to minimize dust. Horses cannot be “cured” of heaves, but can often be controlled with appropriate management practices. Medical therapy of horses with heaves may be intermittently necessary in moderate to severely affected horses. Corticosteroids to reduce inflammation and bronchodilator therapy to relax small airways are used. They may be administered systemically (IV, IM, PO) or by inhalation using a special mask (e.g., Aeromask®).
CARDIOVASCULAR DISEASE
Equine Infectious Anemia: Equine infectious anemia (EIA) is a persistent viral disease of horses causing anemia, fever, and weight loss; however, some horses may appear healthy, but are inapparent carriers. The virus is transmitted from infected horses by large biting flies (tabanids). Once infected, horses become permanently infected and become carriers of the virus for the rest of their lives. Infected horses produce antibodies to the virus, so they will have a positive test result in the agar gel immunodiffusion test (Coggin’s test) or the enzyme-linked immunosorbent assay (ELISA) for EIA virus. Horses must have a negative (Coggin’s) test result for EIA within 6 months for the issuance of health certificates for interstate travel, international travel and show, and sale. A USDA-accredited veterinarian must draw blood for testing and provide a detailed description of the horse on specified forms. The health certificate for interstate travel cannot be issued until the negative test result is returned from a state or federally recognized laboratory. Horses not traveling or sold should still be tested on a yearly basis. If a positive test result is obtained, the entire herd is quarantined until all horses on the premises are tested (usually 60 days). Only the state veterinarian can release the quarantine. Because horses that have a positive test result for EIA are persistent carriers, they are a reservoir of the virus. Therefore infected horses must be quarantined for life (within a distance greater than 200 yards from other horses) or euthanized.
GASTROINTESTINAL DISEASE
Colic: See Colic section of Chapter 33, Emergency Nursing and Abdominal Surgery section of Chapter 31.
Gastric and Colonic Ulceration: Young horses are particularly prone to the development of gastric ulceration. Stress, a high-grain diet, musculoskeletal pain, and the administration of nonsteroidal antiinflammatory drugs (NSAIDs) are common predisposing factors. Clinical signs of gastric ulceration are bruxism (grinding teeth), hypersalivation, and abdominal pain after eating. Foals with gastric ulceration will often lie still in dorsal recumbency with their forelimbs over their head or extended out straight. Human antiulcer medications, such as histamine H2 blockers, intestinal protectants, and hydrogen-ion pump blockers, are used to treat gastric ulceration in horses.
Phenylbutazone (NSAID) toxicosis in horses can produce renal insufficiency and oral, gastric, and colonic ulceration in horses. The colonic ulcers occur in the right dorsal colon and are difficult to treat. Colonic ulcers secondary to phenylbutazone toxicity can produce abdominal pain, marked protein loss, melena (blood in manure), peritonitis, colonic stricture, or colonic rupture. Dehydration and excessive dosages are the most important predisposing factors for development of phenylbutazone toxicosis.
Colitis: Colitis in horses can result in a rapid, life-threatening fluid loss (hypovolemia), shock, toxemia, electrolyte loss, and acid-base imbalance as a result of diarrhea. Some horses may develop hypovolemic shock and electrolyte imbalance before the appearance of diarrhea. In addition to diarrhea, clinical signs of colitis include depression, inappetence, abdominal pain, tachycardia (increased heart rate), injected (brick red) mucous membranes, and prolonged capillary refill time. Etiologic agents that produce life-threatening diarrhea in horses include Salmonella spp., Clostridium spp., and Ehrlichia risticii. Horses with diarrhea should be considered contagious and maintained under an isolation protocol. IV fluid therapy is crucial to support the cardiovascular system, replace fluid losses, and correct electrolyte and acid-base imbalance. Complications of colitis include laminitis (founder), cardiovascular collapse, cardiac arrhythmias, and thrombophlebitis.
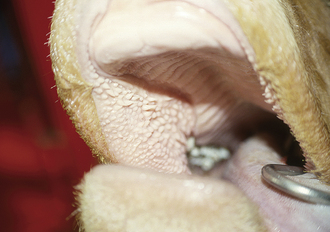
Choke: Choke indicates obstruction of the esophagus. Chronic dental disease and retained deciduous caps are common predisposing conditions for the development of choke. Horses in overcrowded environments may eat feed too quickly and choke. Removing the competition usually alleviates this behavior. The esophagus is usually obstructed by grain or hay. Many horses will continue to attempt to eat despite their inability to swallow. Clinical signs include anxiety, gagging, excessive salivation, and feed and saliva coming from the nostrils. The obstruction can be visualized via an endoscopic examination (Figure 22-1) and in most instances can be relieved by sedation and time. Occasionally, manipulation and hydropulsion using a nasogastric tube is required. Horses must be heavily sedated to lower their head during manipulation of the nasogastric tube to prevent water and feed from entering the trachea. Aspiration pneumonia is a significant complication, therefore a preventative treatment is warranted. An esophageal stricture or rupture is a less common complication and occurs in horses with circumferential damage to the esophageal mucosa.
Potomac Horse Fever: Potomac horse fever is caused by E. risticii and produces diarrhea, fever, abortion, and laminitis. The mode of transmission is not completely elucidated, but it is suspected to involve an arthropod vector. Geographically, clinical disease is observed preponderantly in states east of the Mississippi. Two inactivated bacterins are commercially available. Although the vaccine is not effective, horses living in affected areas may be vaccinated. Vaccination should precede the months of peak disease incidence (June through October).
NEUROLOGIC DISEASE
Brain and Brainstem Disorders: The four most common disorders of the brain and brainstem in horses are rabies, equine viral encephalitis (Alphaviruses: eastern, western, Venezuelan; Flavivirus: West Nile), leukoencephalomalacia (moldy corn toxicity), and head trauma. Damage to the cerebrum may produce altered mentation, altered states of consciousness, head pressing, and seizures (Figure 22-2). Damage to the brainstem may potentially damage the cranial nerves, which control the muscles of facial expression, facial sensation, mastication, swallowing, balance, vision, taste, and ocular position (Figure 22-3). Brainstem lesions also lead to incoordination of the limbs and altered breathing patterns. Diagnostic aids for the evaluation of horses with cerebral or brainstem dysfunction include cerebrospinal fluid (CSF) analysis and skull radiographs.

FIGURE 22-3 Horse with facial nerve paralysis (flaccid facial musculature, droopy ear and eyelid on the affected side) as signs of brainstem dysfunction.
Rabies: Rabies is a zoonotic infection and is universally fatal. Horses usually acquire the infection by a bite wound from a wild animal. Skunks, foxes, raccoons, and bats are the most common reservoirs in North America. Clinical signs are highly variable, but often begin as fever, hind limb ataxia, and hyperesthesia (hyperresponsiveness to touch). Neurologic signs rapidly progress to involve the brain and brainstem. The duration of neurologic signs before death is relatively short, varying from 3 to 10 days.
Horses should be vaccinated against rabies on an annual basis. Vaccinated horses that have been exposed to a rabid animal should be revaccinated promptly and observed for 90 days. Unvaccinated horses with a known rabies exposure should be observed for 6 months and should not be vaccinated.
There is no accurate antemortem test for rabies; it is important to be cautious when handling horses with suspected rabies. The diagnosis of rabies is confirmed by fluorescent antibody stain of brain tissue. People handling potentially rabid horses should avoid contact with saliva, wear gloves, protective eyewear, disposable outerwear, wash hands thoroughly, and avoid contact with CSF. A list of individuals that had contact with the potentially rabid horse must be kept, and these individuals must be informed of the result of the test (generally 24 to 48 hours). A postexposure rabies vaccination should be administered to humans in contact with rabid animals. Individuals with occupational exposure to livestock and wildlife should undergo a prophylactic rabies vaccination series.
Viral Equine Encephalitis: There are four main types of viral equine encephalitis: Eastern, Western, Venezuelan, and West Nile. The viral equine encephalitides produce rapidly progressive, highly fatal neurologic disease in horses. Mosquitoes transmit the infection to horses; therefore disease incidence is seasonal in most geographic regions. Clinical signs of Eastern, Western, and Venezuelan encephalitis are practically indistinguishable and include profound depression, fever, ataxia, head pressing, dementia, and multiple cranial nerve abnormalities. The clinical signs of West Nile encephalitis include weakness, ataxia, muscle fasciculations, and cranial nerve deficits (such as droopy lip). Hyperesthesia (extreme sensitivity to touch) around the head and neck is a common clinical sign. The mortality rate is extremely high with Eastern Equine encephalitis (75% to 100%), moderate with Venezuelan (40% to 80%), and lower with western (30% to 50%) and West Nile (36% to 44%). The treatment consists of supportive care to provide hydration; nutrition; and a clean, dry environment. The prognosis is poor with eastern equine encephalitis and guarded with Western, Venezuelan, and West Nile encephalitides. The diagnosis is confirmed by serologic test (a high titer or a fourfold increase in antibodies to Eastern, Western, and Venezuelan viruses identified by complement-fixation, neutralization, or hemagglutination-inhibition assays or a positive IgM-capture ELISA for West Nile and Venezuelan). Fluorescent antibody or virus isolation in brain tissue is used to make a diagnosis from postmortem samples. The viral encephalitides can be prevented by vaccination 1 month before mosquito season. In southern regions of the United States, vaccinations should be administered two or three times per year.
Vaccines for Eastern and Western equine encephalitis are highly efficacious, and clinical disease in vaccinated horses is rare. A vaccine for West Nile has been available since 2001, and it appears to be efficacious. Horses in the United States should be vaccinated against eastern and western equine and West Nile encephalomyelitis viruses before the mosquito season in the spring. Horses living in southern states with a year-round mosquito season should be vaccinated in the fall in addition to the spring vaccination. Broodmares should receive a booster of their vaccination in the tenth month of gestation (use only killed-virus vaccines in pregnant animals) to ensure adequate colostral antibody protection for the foal. Vaccination against Venezuelan equine encephalomyelitis is not routinely recommended because the disease has not been reported recently in the United States and does not currently pose a threat to the U.S. horse population except those near the Mexican border.
Leukoencephalomalacia: Equine leukoencephalomalacia (moldy corn toxicity) is caused by the ingestion of a fungal toxin produced by Fusarium moniliforme. This mold has a predilection for corn, and affected kernels are usually pink to brown. The fungal toxin produces liquefactive necrosis of the cerebral cortex. Clinical signs include profound depression, head pressing, altered states of consciousness, incoordination, and aimless wandering. The treatment consists of supportive care, and the prognosis for recovery is poor. Horses often die within 24 hours of manifesting neurologic signs.
Head Trauma: Horses acquire two types of skull fractures depending on the nature of the traumatic injury. Horses that suffer a frontal impact with a solid object develop depression fractures of the frontal and parietal bones. The common neurologic signs observed in horses with this type of fracture are due to cerebral damage and include depression, seizure, stupor, and aimless wandering. Horses that flip over backward develop fractures of the petrous temporal bone and the junction of the basisphenoid and basioccipital bone. Neurologic signs associated with these fractures include abnormalities of balance, incoordination of limbs, nystagmus (rhythmic eye movement), abnormal respiratory patterns, and coma. The diagnosis is confirmed by a radiographic examination of the skull. The treatment consists of supportive care and antiinflammatory therapy (corticosteroids, dimethyl sulfoxide [DMSO]). Surgical decompression of frontal and parietal fractures may improve the neurologic status of some horses.
Spinal Cord Disorders: The five most common disorders of the spinal cord are cervical vertebral malformation caused by stenotic or dynamic compression of the spinal cord (wobbler syndome), equine protozoal myelitis, equine herpesvirus myeloencephalopathy (rhinopneumonitis), equine degenerative myeloencephalopathy, and vertebral fracture. Damage to the spinal cord causes spinal ataxia (incoordination of the limbs without abnormalities of the brain and brainstem), which may progress to dog sitting and recumbency (Figure 22-4). Muscle atrophy from a lower motor neuron disorder can also indicate a spinal cord disorder (Figure 22-5). Diagnostic aids to differentiate these diseases include a neurologic examination, cervical radiographic examination, myelographic examination, and CSF analysis. CSF can be obtained at the lumbosacral space in standing, sedated horses and at the atlantooccipital space in anesthetized horses. The CSF travels from the cranial area in a caudal direction. Typically, a CSF collection is performed at the lumbosacral space in a standing horse. A CSF collection can also be performed at the atlantooccipital space in horses while using a general anesthetic. Because some neurologic infectious diseases have zoonotic potential, most notably rabies, barrier precautions (e.g., face mask, double gloves) must be taken when collecting and handling CSF samples from horses with neurologic signs to minimize exposure to the infectious agent.
FIGURE 22-5 Asymmetrical gluteal muscle atrophy in a horse with spinal cord dysfunction involving a lower motor neuron.
Wobbler Syndrome: Cervical vertebral malformation is a manifestation of developmental orthopedic disease characterized by compression of the cervical spinal cord by malformed or unstable cervical vertebrae. Males are affected four times more frequently than females, and Thoroughbreds appear to be predisposed. Clinical signs of symmetric incoordination usually begin between 6 months and 3 years of age. The hind limbs are usually more severely affected than the forelimbs. The likelihood of disease is determined by the evaluation of plain film cervical radiographs, and the diagnosis is confirmed by a myelographic examination. Surgical stabilization improves the neurologic status of some patients.
Equine Protozoal Myelitis: Equine protozoal myelitis (EPM) causes ataxia in horses. Horses are dead-end, aberrant hosts of the protozoan parasites. Sarcocystis neurona is the most common protozoan parasite that causes spinal cord disease in horses; opossums are the primary hosts of this parasite, and horses are likely infected via fecal-oral transmission. Birds are the secondary hosts and do not appear to be infectious for horses. The clinical signs of EPM are directly referable to the location of the organism in the CNS. Therefore EPM should be considered in a horse demonstrating neurologic signs. Most horses with EPM (85%) demonstrate signs such as ataxia, weakness, and muscle atrophy as a result of spinal cord damage. Clinical signs are often asymmetrical. Other signs such as cranial nerve deficits may occur. The diagnosis is confirmed by the identification of antibodies to the organism in CSF. The treatment of EPM consists of the administration of antiprotozoal drugs; the most common treatment for EPM is ponazuril. Another treatment is a combination of two antibiotics that inhibit folic acid metabolism: sulfadiazine and pyrimethamine, treating for an average of approximately 90 to 120 days.
Herpes: Equine herpesvirus can produce respiratory disease, abortion, and neonatal and neurologic disease in horses. The neurologic form is characterized by ascending paralysis with hind limbs more severely affected than forelimbs. Horses often demonstrate urinary incontinence, poor tail tone, and penile prolapse. The diagnosis is confirmed by a cytologic analysis of CSF. The administration of corticosteroids may improve recovery if administered early in the disease process. The prognosis for return to normal neurologic function is approximately 80%. Recently, there have been a number of outbreaks in the United States with a higher mortality than in the past. Cases are now considered reportable to the state veterinarian.
Equine Degenerative Myelopathy: Equine degenerative myelopathy results in symmetric spinal ataxia with both the forelimbs and hind limbs equally affected. Clinical signs appear between 6 months and 2 years of age. The disease appears to be familial in some breeds. There is no definitive antemortem diagnostic test, and the diagnosis is usually made on the basis of the neurologic examination, CSF analysis, cervical radiographs, and myelographic examination. Dietary supplementation with vitamin E may prevent the progression of disease and may result in improvement in clinical signs in some instances. The prognosis for return to normal neurologic function is poor.
Vertebral Fracture: The cervical vertebrae, caudal thoracic vertebrae, and thoracolumbar junction are the most common sites of vertebral fracture. A cervical vertebral fracture results in tetraparesis (weakness of all four limbs), whereas fracture of the thoracic and lumbar vertebrae produces paraparesis (weakness of hind limbs) or paraplegia (paralysis of hind limbs). The diagnosis is confirmed by radiography. If the fracture is nondisplaced, nuclear scintigraphy may aid in the identification of the fracture site. Surgical correction may be attempted for fractures of the cervical vertebrae, but the repair of thoracic or lumbar vertebrae is not attempted. The most consistent clinical sign associated with vertebral fracture is pain.
Tetanus: Tetanus is a highly fatal neurologic disease in horses characterized by a stiff, stilted gait; hyperexcitability; seizure; and coma. The causative organism is commonly present in the environment. The most common portals of entry for disease in horses include a subsolar abscess, penetrating wound, or infected intramuscular injection site. Tetanus toxoid (inactivated) is a safe and efficacious vaccine for preventing clinical disease. Healthy horses without risk factors should be vaccinated for tetanus annually. Unvaccinated horses at high risk for the development of tetanus (wounds, subsolar abscess, surgery) should receive tetanus antitoxin in addition to tetanus toxoid to provide immediate protection against disease. Tetanus antitoxin is associated with fatal serum hepatitis, and its administration should be limited to cases at a high risk for disease.
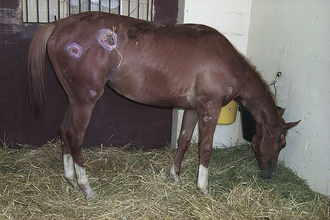
Botulism: Botulism is a rapidly progressive, often fatal neurologic disease in horses characterized by profound weakness, muscle fasciculations, and dysphagia (inability to swallow). The causal organism produces a neurotoxin that may gain entry to the body by colonizing the intestinal tract (foals), infected wounds, or contaminating feedstuff (Figure 22-6). Colonization of the intestinal tract in foals occurs in particular geographic regions of the United States, especially Pennsylvania, Ohio, and Kentucky (Figure 22-7). This is a preventable disease, and it is imperative to vaccinate for botulism in affected areas. The vaccine is effective, and unvaccinated horses can die quickly after an infection. The initial series is administered monthly for 3 months, then once a year.
DERMATOLOGIC DISEASE
Ringworm: Equine dermatophytosis (ringworm) is a fungal infection of the superficial layer of skin. The fungi commonly involved are Trichophyton and Microsporum spp. The transmission of the fungal infection is by direct contact between affected animals. Younger animals (less than 4 years old) are more likely to be affected. Infected areas of skin have a bull’s-eye appearance with circular patches of hair loss with a circle of inflammation at the periphery of the lesion. The diagnosis is confirmed by a fungal culture on commercially available dermatophyte culture medium. Although the infection is usually self-limiting, the application of topical antifungal drugs will speed recovery.
Dermatophilosis (rain scald, rain rot) is a common bacterial infection caused by Dermatophilus congolensis that produces crusting lesions. The crusts can be pulled out with a tuft of hair, and the remaining lesion is a glistening yellow crater. The organisms readily colonize wet, macerated skin; therefore the disease is common in the winter and spring. An impression smear of the tuft should be stained with Wright stain. Organisms are identified as a double chain of cocci with a “railroad track” appearance. The organisms are usually easily cultured and form an applesauce-like colony on specialized growth medium. Affected horses should be bathed with an iodine-based or chlorhexidine shampoo and placed in a dry environment. The administration of penicillin will speed recovery in severely affected horses.
Culicoides Hypersensitivity: Culicoides hypersensitivity is a syndrome characterized by mane and tail rubbing whereby affected horses develop an allergic pruritic skin condition secondary to the bite of Culicoides flies. The classic body regions affected include the face, ears, mane, withers, rump, base of the tail, and ventral abdomen. The dermatitis usually begins as a seasonal condition, but its severity and duration increase as the horse ages. Pruritus usually is noted during the fly season, but will vary in length depending on geographic location. The condition is diagnosed by correlating the time of year with physical evidence of self-mutilation, especially in the mane and tail areas. Intradermal skin testing can be useful in confirming the diagnosis. The treatment involves reducing insect exposure and concomitant use of antiinflammatory medication. Because Culicoides breeds in stagnant waters, affected horses should be moved away from ponds, lakes, or irrigation canals. Water troughs and barrels should be cleaned frequently and the water kept fresh to prevent use as breeding sites by the flies. Because Culicoides feeds primarily at dusk, night, and dawn, horses should be kept stabled during these times. Stabling is most effective if the doors and windows can be closed and if the stall is lined with a fine-mesh screen. Frequent application of insecticide to the screen may also be useful. Fans are helpful to reduce exposure because Culicoides cannot fly well in brisk breezes. The application of insecticides and repellents is a necessary part of disease control. The most effective products are those containing pyrethrins with synergists and repellents. Frequent bathing not only decreases scale and crust, but also seems to decrease pruritus. Corticosteroid therapy is often necessary in these cases to control the pruritus.
Sarcoid: Equine sarcoid is a benign, locally invasive tumor of skin and is the most common tumor in horses. These tumors produce either raised, hairless lesions with a corrugated surface that often bleed when traumatized, known as fibroblastic sarcoids, or a flattened form known as verrucous sarcoids. The cause of sarcoid is unknown, but a viral agent is suspected. Surgical resection, cryotherapy (freezing), laser therapy, immunotherapy (intralesional mycobacterial cell wall extract), radiotherapy (iridium 191), and chemotherapy (intralesional cisplatin) are accepted treatment modalities with variable success. It is difficult to predict the response to a given treatment modality, and combination therapy is often necessary.
Melanomas: Melanomas are relatively common skin tumors, particularly in gray horses. They occur most commonly in the perineal region, but can occur on other areas of the body. Melanomas appear as darkly pigmented nodules in the skin. They are usually benign, but tend to enlarge, causing mechanical problems, such as interfering with defecation. Most clinicians believe it is better not to attempt surgical removal unless they are located in an area that interferes with tack or they are so large that they interfere with normal body functions. The administration of cimetidine has been reported to be effective in many horses to reduce the size of melanomas. Once cimetidine is discontinued, the tumors usually enlarge. Autologous vaccines (making a vaccine from the horse’s tumor) have also been used with some success.
OPHTHALMOLOGIC DISEASE
Equine Recurrent Uveitis: Equine recurrent uveitis (moon blindness) is the most common cause of blindness in horses. It is an immune-mediated condition, and many factors have been implicated (heredity, parasites, leptospirosis); however, the inciting cause is often unknown. Affected horses experience episodes of intraocular inflammation characterized by swelling of the eyelids, corneal edema, and hypopyon (inflammatory cellular exudate in the anterior chamber). Over time, the episodes become more frequent, severe, and produce permanent ocular damage, including retinal degeneration, cataracts, and synechiae (adhesions of the iris to either the lens or the anterior chamber). One or both eyes may be affected. Recurrent uveitis cannot be cured, but can often be controlled with long-term antiinflammatory therapy, including atropine and aspirin. Acute episodes are treated with ophthalmic preparations containing atropine and corticosteroids if there is no corneal ulceration. Systemic antiinflammatory therapy is beneficial (e.g., flunixin meglumine). Horses with end-stage uveitis are blind and have small, collapsed, ocular globes (phthisis bulbi). If a corneal ulcer is present, antimicrobials and NSAIDs are used. Once the ulcer completely resolves, a topical steroid may be used. These cases require diligent observation and chronic treatment.
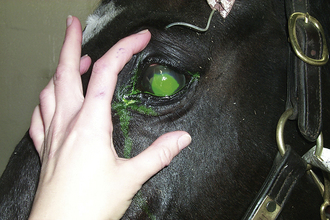
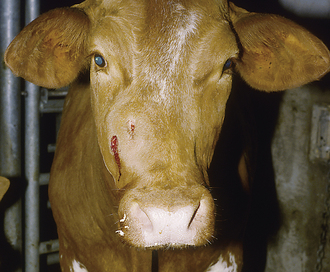
Corneal Ulceration: Corneal ulceration commonly results from ocular trauma. Fluorescein stain is used to detect corneal abrasions because it adheres to abnormal cornea (Figure 22-8). Defects in the corneal surface will stain an apple-green color. Corneal ulceration in most horses responds readily without complications to the administration of ophthalmic antibacterial ointment (bacitracin, neomycin, polymyxin B). In some instances, the ulcer will be colonized by fungus (e.g., Pseudomonas or Aspergillus spp.). These organisms produce collagenase, which destroys the cornea and creates a “melting” corneal ulcer. These ulcers are rapidly progressive, and the eye is prone to rupturing. Frequent antimicrobial dosage regimens may require the placement of a subpalpebral lavage system (Figure 22-9) to allow frequent medication administration for a painful eye. Aggressive topical antimicrobial therapy may be successful, but suturing a conjunctival pedicle flap to provide blood supply to the affected area may be necessary to save the globe in some instances. Deep, melting corneal ulcers often heal with a fibrous scar that may impair vision in the future.
MUSCULOSKELETAL DISEASE
Exertional rhabdomyolysis (myositis, tying up, azoturia, Monday morning sickness) is an acute inflammatory disease of muscle. It can be caused by exertion or a change in diet or exercise. It is characterized by a stiff, stilted gait with firm or hard muscles. The most commonly affected muscles are the hind limbs and back. Severely affected horses may be reluctant to move. Some may become recumbent and unable to rise. Affected horses are often anxious, sweat excessively, and have increased heart and respiratory rates and body temperature. Horses often have dark, discolored urine secondary to myoglobinuria from muscle damage. The confirmation of this disease is often based on increased serum muscle enzyme (creatine phosphokinase, aspartate aminotransferase) concentrations. The treatment involves exercise restriction, diet modification, IV fluid therapy, NSAIDs (phenylbutazone, flunixin meglumine), muscle relaxants, and tranquilization.
CARE OF THE HOSPITALIZED EQUINE PATIENT
In the equine hospital setting, veterinary technicians are responsible for primary patient monitoring, administration of medications, general daily care of horses, and supervision of lay technical support. This section provides an overview of the daily management of equine patients in the hospital setting.
PATIENT MONITORING
The level of patient monitoring required for a hospitalized horse depends on the severity and nature of the disease. Horses with infectious disease require frequent patient monitoring (e.g., every 6 hours). Any critically ill patients need constant IV fluid administration and intensive care monitoring. Most will be monitored frequently for signs of discomfort, heart rate, respiratory rate, hydration, capillary refill time, abdominal pain, respiratory distress, shock, laminitis, and gastrointestinal motility. An increased heart rate (tachycardia) is indicative of pain. 60 beats per minute (bpm) or greater indicates serious pain in an adult horse.
Patient monitoring forms are designed to identify trends in physical signs. Patient treatment forms coordinate treatment periods when several individuals may be responsible for administering medications. Treatment sheets and monitoring forms may be combined for low-maintenance, elective patients. However, for intensive care patients, monitoring should be more detailed, and many hospitals use a flow sheet. It is important to recognize that monitoring and treatment forms are a permanent part of the medical record, which represents a legal document to record all events during hospitalization.
Horses with contagious diseases should be hospitalized in isolation facilities. The most common diseases that require an isolation protocol are colitis (Salmonellosis) and strangles (S. equi equi). Personnel wear disposable gloves, boots, and body suits while attending to isolation cases. A disinfectant foot dip should be used when entering and exiting each stall. Protective boots, gloves, and suits should be discarded when exiting the isolation area. Horses in isolation should not be walked in areas where other horses are grazing. Waste from the stall should be disposed of in an inaccessible area. If possible, personnel attending to isolation cases should not attend to foals or immunocompromised patients.
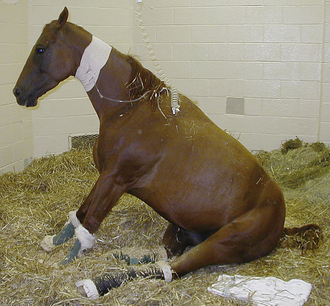
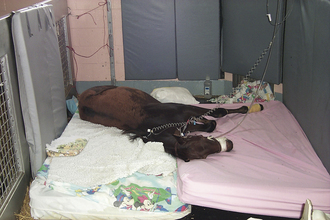
Recumbent horses are a particular challenge to manage effectively in a hospital setting. Neurologic and musculoskeletal diseases are the most common problems resulting in recumbency in horses. Recumbent horses and foals will quickly develop pressure sores (decubital ulcers) over the pelvis (tuber coxae), elbows, and head if not properly managed (see Figure 22-7). Manure- and urine-soaked bedding must be removed frequently because the horse will develop irritated skin and sores. Pressure sores rapidly become deep and may infect underlying bony structures. In addition, recumbent horses may have decreased intestinal motility and fail to void urine. Therefore soft feed, such as fresh grass, should be offered to recumbent horses to facilitate fecal evacuation and prevent impaction. Horses unable to defecate should have feces manually removed twice daily. The placement of an indwelling urinary catheter or periodic catheterization of the urinary bladder is often necessary when managing recumbent patients. Recumbent horses should be deeply bedded on straw, placed on a padded mat, or placed on a mattress to prevent the development of pressure sores (see Figure 22-6). The horse’s position should be changed every 6 hours; multiple attendants are required to move an adult recumbent horse. A sling can only be used in horses that can support their own weight but are not able to stand on their own (Figure 22-10, A, B, C, and D). Horses cannot be supported solely by a sling because of the constriction of breathing and development of sling-induced pressure sores. Recumbent adult horses can rarely be managed for more than 1 or 2 weeks without the development of life-threatening complications (pneumonia, urinary tract infection, colic, pressure sores).
FEEDING
Whenever possible, hospitalized patients should be offered feed similar to what they are fed at home. Sudden changes in diet predispose horses to colic or diarrhea. When a horse is admitted to the veterinary hospital, it is imperative to ask the owner or trainer for details on the horse’s typical diet. In some instances, feeding must be specialized to accommodate the patient’s disease. After the medical resolution of colic, horses should be offered soft feed, such as bran mash, fresh grass, and small amounts of good-quality hay. Feed should be offered frequently in small quantities to horses with gastrointestinal tract disease rather than offering two large daily meals. Horses with heaves (recurrent airway obstruction) should be offered water-soaked hay and a dust-free complete pelleted diet. Inappetent horses should be offered highly palatable, calorie-dense feed to increase energy intake.
THERAPEUTICS
An IV catheter can be placed for repeated administration of medications or continuous fluid infusion. IV catheters are usually placed in the jugular vein. Alternate sites include cephalic and lateral thoracic veins (Figures 22-11 and 22-12). Catheters should be placed aseptically and sutured appropriately to prevent dislodgment. Teflon catheters are relatively irritating and should be replaced every 3 days. Silastic catheters can remain in the vein as long these are patent and show no signs of infection. Central venous catheterization using a wire-guided polyurethane catheter is often used in critically ill patients. Polyurethane catheters are less traumatic, less thrombogenic, and ideal in peripheral veins, such as lateral thoracic and cephalic veins. IV catheters should be flushed with heparinized saline flush (2 to 10 U/ml) every 6 hours and monitored twice daily for heat, swelling, and pain. Infection at the catheter site may occur in the subcutaneous tissue or in the vein (septic thrombophlebitis). Septic thrombophlebitis can be life threatening in horses.
The ideal antimicrobial is effective against a wide range of bacterial organisms (broad spectrum), easy to administer, and nontoxic. Penicillin has good efficacy against common gram-positive pathogens in the horse (Streptococcus zooepidemicus, S. equi equi) and is relatively safe. It is frequently administered intramuscularly (procaine penicillin) and IV (potassium penicillin). Procaine penicillin should never be administered IV. Life-threatening anaphylactic reactions are reported with procaine penicillin administration and should be treated with epinephrine. Aminoglycoside antimicrobials (gentamicin, amikacin sulfate) are efficacious against gram-negative pathogens and can be administered intramuscularly or IV. These antimicrobials are nephrotoxic, so renal function should be monitored during therapy. Trimethoprim-sulfa antimicrobials have a moderate gram-positive and gram-negative spectrum and are administered orally or IV. Ceftiofur sodium has a good gram-positive and gram-negative spectrum and may be administered intramuscularly or IV. Metronidazole is administered orally or per rectum to treat anaerobic bacterial infections. There are specific indications for the administration of other antimicrobials, but some are not widely used because of the risk of antimicrobial-induced colitis. Chloramphenicol is used sparingly in horses because of the human health risk for idiosyncratic, fatal aplastic anemia from exposure during patient administration.
There are many analgesic medications available for horses. Phenylbutazone, ketoprofen, and flunixin meglumine are NSAIDs that provide mild to moderate pain relief. NSAIDs also reduce fever (antipyretic) and inflammation. Phenylbutazone is most effective for the treatment of musculoskeletal pain. Ketoprofen, meclofenamic acid, and naproxen are less commonly used drugs that provide mild to moderate analgesia for musculoskeletal pain. Flunixin meglumine is more effective for soft tissue and visceral (abdominal) pain. In addition, flunixin meglumine may combat the effects of toxemia in horses with gastrointestinal tract disease. Sedatives that also provide analgesia include xylazine and detomidine (alpha2 agonists). Xylazine provides approximately 20 minutes of sedation and analgesia. Detomidine provides up to 1 hour of sedation and analgesia. Butorphanol is a narcotic agonist that provides up to 1 hour of sedation and analgesia for moderate to severe pain. Acepromazine has no analgesic properties and only provides moderate tranquilization. Acepromazine also causes hypotension and can cause persistent paraphimosis in stallions.
Corticosteroids have potent antiinflammatory properties and are administered for allergic airway disease, allergic skin conditions, immune-mediated disease, and joint inflammation. Corticosteroids are administered topically, orally, parenterally (IV or intramuscularly), and intraarticularly. Adverse effects of corticosteroid administration include immunosuppression, polyuria or polydipsia, poor hair coat, muscle wasting, poor wound healing, laminitis, and progression of degenerative joint disease. Therefore corticosteroids are administered with caution and only when specifically indicated.
DMSO is an antiinflammatory drug occasionally used in horses to relieve swelling and edema associated with CNS trauma, traumatic musculoskeletal injuries, laminitis, and myositis. DMSO may be administered topically, orally, or IV (diluted in crystalloid fluids as a 10% to 20% solution). Nitrile gloves are used while handling the product, because it can be absorbed wearing latex gloves. Rapid IV administration may result in hemolysis, hematuria, and sweating in horses.
LABORATORY STUDIES
Clinicopathologic testing provides important information for the veterinarian to identify an impairment of an organ system, confirm a clinical diagnosis, assess the response to therapy, and formulate a prognosis. The normal values of many clinicopathologic tests vary among species. In addition, there are species-specific characteristics associated with diseases and the significance of abnormal findings. This section concentrates solely on equine-specific alterations in clinicopathologic values in health and disease.
Hematology
A complete blood count (CBC) provides information pertaining to the red blood cell (RBC) count, RBC morphology, total white blood cell (WBC) count, WBC differential (including neutrophils, lymphocytes, eosinophils, monocytes), WBC morphology, and fibrinogen concentration. Samples for a CBC should be submitted in a tube with ethylenediaminetetraacetic acid (EDTA) anticoagulant (purple-top tube). The RBCs are most easily estimated using the packed-cell volume (PCV); low PCV (less than 30%) is indicative of anemia. Horses have a large muscular spleen that normally contains up to one third of the circulating RBC volume. With excitement and exercise, the PCV in horses can increase by as much as 50% secondary to splenic contraction. Therefore the resting PCV is highly variable and must be serially evaluated in excitable patients. In addition, the response of the spleen to massive hemorrhage precludes the use of the PCV to estimate the magnitude of blood loss for at least 24 hours. The normal range of the PCV depends on the breed, but generally is between 32% and 45%. Hot-blooded breeds (Thoroughbreds, Arabians, Quarter horses) have higher resting RBC counts compared with ponies and draft horses.
An evaluation of the total and differential WBC count is important to identify the presence of infection. In most instances, a bacterial infection will manifest as an increase in WBC count (leukocytosis) characterized by an increase in the number of mature neutrophils (mature neutrophilia). Fibrinogen is a coagulation factor and an acute-phase protein in horses, produced by the liver in response to inflammation. Fibrinogen concentrations remain increased until the infection is resolved.
Horses are particularly sensitive to circulating endotoxins released from the cell wall of gram-negative bacteria. Endotoxins cause margination and sequestration of WBCs. Therefore a profoundly low WBC count (leukopenia) characterized by low neutrophil count (neutropenia) and immature band neutrophils (left shift) is indicative of gram-negative septicemia or gastrointestinal disease with inflammation allowing mucosal absorption of gram-negative bacteria. High eosinophil counts (eosinophilia) are indicative of a massive parasite infestation or possibly allergic diseases. Low lymphocyte counts (lymphopenia) may be observed in horses with early viral infections.
Serum Chemistry
A serum chemistry panel provides specific information pertaining to the liver, kidney, muscle, and serum electrolyte concentrations. The serum sample should be drawn into a tube without anticoagulant (red-top tube) and submitted to the laboratory. Some laboratories can perform chemistry profiles in heparinized blood samples (green top). If there will be more than a 1-hour delay in submission, the tube should be centrifuged, serum or plasma removed, and stored in the refrigerator. Delayed sample submission without centrifuging produces artificially low serum glucose and high serum potassium concentrations. Horses normally have a yellow tint to the serum as a result of increased serum bilirubin levels because horses do not have gallbladders. Serum bilirubin concentrations will increase dramatically if feed is withheld for more than 24 hours as a result of a normal physiologic response and does not indicate liver disease. Most species develop low serum albumin levels with chronic liver disease because of decreased production; however, horses maintain production of albumin even with a marked impairment of liver function. Reliable indicators of liver dysfunction in horses are high serum γ-glutamyltransferase (GGT) activity, high serum sorbitol dehydrogenase (SDH) activity, high serum bile acid concentrations, low blood urea nitrogen (BUN) concentrations, and increased ammonia levels.
In most species, renal failure produces low serum calcium and high serum phosphorus concentrations. Horses are obligate calcium excreters, and chronic renal failure often produces a marked increase in the serum calcium concentration. Reliable indicators of renal failure in horses include high serum creatinine and BUN and electrolyte abnormalities, including low sodium and chloride and high potassium and calcium levels. The large colon of horses exchanges a vast amount of electrolytes and fluids on a daily basis. Horses with colonic inflammation may develop marked electrolyte abnormalities before the development of diarrhea. Low serum sodium, chloride, and potassium levels in horses with abdominal pain or depression often indicate a loss of electrolytes into the lumen of the colon and impending diarrhea.
Serum creatine phosphokinase (CK) is an indicator of muscle damage in all species. Horses have large muscle masses in comparison with ruminants and small animals. Moderate increases in serum CK levels (two to four times normal) readily occur in horses following prolonged transport, prolonged recumbency, exercise in an unconditioned horse, or rolling from abdominal pain. Moderate increases do not usually indicate primary muscle disease. Horses with primary muscle disease, such as exertional rhabdomyolysis (tying up, azoturia, Monday morning sickness) have increases in serum CK activity of up to 200 times normal values.
Urinalysis
A urinalysis is essential for evaluation of primary renal disease. Urine can be collected as a voided sample or after catheterization of the bladder. Urinary catheterization is performed in the standing horse. Females should have the perineum washed with 1% iodine and water. A sterile gloved hand is inserted into the vagina approximately 10 cm. The sterile catheter is gently inserted into the urethral orifice on the floor of the vagina to obtain urine. Males need to be sedated, and the penis is then grasped, gently extruded, and washed thoroughly with 1% iodine and water. While wearing sterile gloves, a sterile flexible stallion catheter is inserted into the urethral orifice and advanced until urine flows from the catheter. If no urine is spontaneously voided, slight negative pressure from a syringe attached to the catheter may produce a sample.
Normal horse urine is usually alkaline (pH 7 to 9) and contains many calcium carbonate crystals. Alkaline urine usually produces a false-positive reaction for protein on urine dipsticks. Horses have a large number of mucous glands located within the renal pelvis; therefore normal horse urine may appear thick and mucoid. Red urine is abnormal and results from the presence of frank blood (primary urinary tract disease), hemoglobin (hemolytic anemia), or myoglobin (myositis). The differentiation of these sources of red urine requires special testing of urine and serum samples. Urine specific gravity and urinary electrolyte excretion ratios should be obtained to investigate primary renal function. Urine specific gravity indicates the ability of the kidney to concentrate urine, and normal values in resting horses should be greater than 1.030. Urinary electrolyte excretion ratios indicate the ability of the kidney to conserve electrolytes. The identification of WBCs and numerous bacteria indicate a urinary tract infection. Protein in the urine (proteinuria), glucose in the urine (glucosuria), and casts indicate renal disease.
Evaluation of Body Fluids
The evaluation of cerebrospinal, synovial (joint), and abdominal cavity fluid provides important information pertaining to inflammation, an infection, or neoplasia within that particular body cavity. These body fluids are analyzed for total protein, total cell count, differential cell count, and bacterial culture.
Some neurologic diseases in horses require a CSF analysis for diagnosis. Because some neurologic diseases, such as rabies, have zoonotic potential, CSF must be collected and handled with caution (e.g., protective eyewear or face shields, lab coats, and gloves) to prevent exposure to the infectious agent. CSF is collected in standing, sedated horses with spinal cord disease from the lumbosacral space using a 6-inch 18-gauge spinal needle. In horses with brain and brainstem disease, CSF is collected in anesthetized horses from the atlantooccipital space using a 3-inch, 18-gauge spinal needle. Normal nucleated cell counts are less than five cells per microliter (predominately lymphocytes). The normal total protein concentration is variable depending on the laboratory, but is usually less than 80 mg/dl (higher than in other species). Abnormalities in protein and cell counts can identify an inflammatory, infectious, or neoplastic process, but CSF analyses are often nonspecific. Antibodies to the agents of several equine neurologic diseases (rabies, protozoal myelitis, herpes myeloencephalopathy, equine encephalomyelitis) can be detected in CSF, which provides specific information regarding the cause of neurologic signs. Complications associated with a CSF tap include iatrogenic (operator-induced) spinal cord trauma and the introduction of bacteria into the CNS.
Abdominal pain, an abnormal rectal examination, abdominal distention, and fever of unknown origin are indications for abdominocentesis in horses. Abdominal fluid is obtained by placing an 18-gauge, 1.5-inch needle into the peritoneal space of the ventral abdomen. The needle should be placed one hand’s breadth behind the sternum, off the midline to the right of the horse (to avoid the spleen). If a 1.5-inch needle is insufficient to reach the peritoneal cavity, a teat cannula may be used. The use of a teat cannula is more invasive and increases the risk of traumatic bowel rupture. The normal abdominal fluid total protein is less than 2.5 mg/dl, and the normal total nucleated cell count is less than 5000/ml (50% neutrophils). The analysis of abdominal fluid can identify devitalized bowel in horses with acute abdominal pain (colic), an abdominal abscess, a tumor in horses with a mass in the abdomen identified via rectal palpation, and a ruptured bladder in foals with abdominal distention. Complications of abdominocentesis include traumatic bowel rupture, intraabdominal hemorrhage from trauma to the spleen, and iatrogenic septic peritonitis.
Bacterial Culture and Susceptibility Testing
The veterinary technician often plays an important role in the bacteriologic testing of specimens collected from patients with infectious diseases. Specimens (blood, joint fluid, abdominal fluid, urine, wound exudate, infected bone, etc.) are frequently collected from horses with infectious diseases for culture. Following proper procedures during the collection and transport of these specimens to the laboratory for culture and susceptibility testing improves the chances of growing the causative organism. There are specific guidelines followed for the collection and transport of different types of specimens. For example, blood is usually placed in a special enhancement medium immediately after collection for transport to the laboratory. There are also special methods for the collection and transport of samples submitted for aerobic and anaerobic culture. Identifying the causative agent in an infectious process and determining its in vitro susceptibility pattern to antibiotics are often critical in choosing the appropriate antibiotic regimen. Fecal samples are often submitted for Salmonella spp. or Clostridium spp. cultures from horses with diarrhea. Fecal samples for Salmonella spp. culture should be submitted daily for 5 consecutive days. If culture results are negative for these five samples, the horses are not shedding Salmonella organisms. Fecal samples may be tested for Clostridium toxins and Clostridium spp. culture; samples should be submitted daily for 3 consecutive days. Fecal samples may be submitted to other diagnostic tests, such as ELISA for rotavirus.
Delivering exceptional medical care to the equine patient requires a team approach, and the veterinary technician plays a vital role in the diagnosis and treatment of these patients. Carefully monitoring hospitalized cases for potential complications is another important role for the veterinary technician. These horses often respond well to the treatment, and it is rewarding to see the horse recover with proper care.
FOOD ANIMAL MEDICINE
With fewer veterinarians choosing food animal practice, practice owners have been finding it increasingly difficult to hire an associate. For these individuals, optimizing the use of veterinary technicians is of great importance. Capitalization of technician skills can help meet client needs and improve practice productivity and efficiency. As a part of the professional team, the veterinary technician can assist in the restraint and handling of animals for an examination, sample collection, diagnosis, and treatment. Technicians can also prepare equipment, supplies, and animals for surgery and assist during the surgical procedure.
Other tasks performed by veterinary technicians in food animal practice include performing laboratory procedures and diagnostic tests, patient care, diagnostic imaging, anesthesia, preparation of pharmacologic and biologic agents, administration of injections and other treatments, ration balancing, body condition scoring in cattle and small ruminants, metabolic profiling of herds or flocks, monitoring records of postparturient cows, and planning herd consultation visits. Technicians are also valuable in educating clients. For example, instructing producers about the proper technique for the placement of growth implants in feedlot cattle and explanation of proper injection techniques based on meat quality assurance guidelines.
Veterinary technicians can also perform necropsies and collect tissue specimens. They record their findings and take digital pictures of the necropsy for subsequent review by the veterinarian. Technicians may also possess special talents or training, such as expertise in artificial insemination or corrective foot work, that could be offered as a service to clients. A cognizant, well-trained, and knowledgeable technician can anticipate the needs of the veterinarian and producer thereby enhancing the productivity of the professional team.
Finally, with current concerns of bioterrorism and foreign animal diseases in the United States (currently bovine spongiform encephalopathy and foot and mouth disease), the food animal veterinary technician might play a key role in the dissemination of information when handling public questions and concerns.
COMMON DISEASES AND CONDITIONS OF CATTLE
CARE OF THE NEONATE AND NEONATAL DISEASES
Food animal veterinarians are often asked to assist cows and heifers having difficulty calving. Often calves that are born via forced fetal extraction or cesarean section (C-section) are compromised. While the veterinarian attends to the mother, especially in the case of a C-section, the veterinary technician can provide intensive care to the neonate, if necessary. The most important step is to make sure the calf is breathing. All mucus should be cleared from the nose, mouth, and upper airway. There are several ways to achieve this. The calf may be hung upside down with the head off the ground to allow drainage of these fluids. A bulb syringe also works well to remove mucus from the nose and mouth, and it also provides nasal stimulus to breathe. A piece of straw or hay can also be used to tickle the nose and stimulate respiration. If all else fails, a technique known as gin chung, placing a small-gauge needle in the nasal septum, is often a successful respiratory stimulus. If these techniques fail, artificial respiration can be provided by mouth-to-nose resuscitation or by raising and lowering the uppermost forelimb while simultaneously pressing and releasing the rib cage. For difficult cases, a small amount of doxapram hydrochloride, a respiratory stimulant, may be injected under the tongue to induce respiration. Once the calf is breathing, it should be vigorously rubbed dry with a towel and the umbilical cord dipped in strong (7%) iodine.
Colostrum ingestion soon after birth is an important part of neonatal survival and prevention of infectious disease. Cattle and other ruminants (including sheep and goats) have a thick syndesmochorial placentation that prevents in utero transfer of high-molecular-weight immunoglobulins. These species are essentially agammaglobulinemic at birth and rely on the ingestion and absorption of colostrum-rich antibodies and nonantibody immune factors.
The transfer of immunity can be compromised by colostrum deficiencies, ingestion failure, or absorption failure. Immunoglobulins are absorbed by pinocytosis by specialized epithelial cells in the jejunum and ileum. These cells are replaced soon after birth by normal intestinal epithelium, and absorption terminates. Therefore it is important that the neonate receives colostrum within the first 6 to 8 hours of life. The calf should receive colostrum at the rate of 10% of its body weight within the first 12 hours (4.5 L/45 kg of body weight in 12 hours).
Several tests are available for the detection of failure of passive transfer (FPT), including single radial immunodiffusion (SRID), sodium sulfite precipitation, zinc sulfate turbidity, and serum protein analysis. The serum concentration of IgG using SRID should be greater than 1600 mg/dl. Serum protein can be measured using a refractometer. A serum protein concentration greater than 5 mg/dl in the absence of dehydration is indicative of successful passive transfer; values less than 4.5 mg/dl are consistent with FPT. For valuable calves, the treatment may be achieved by plasma transfusion at the rate of 20 to 40 ml/kg body weight.
Partial or total FPT can make a calf susceptible to a variety of disease conditions. Compromised calves are more likely to develop umbilical infections (omphalophlebitis), which may lead to any combination of the following problems: septicemia, septic arthritis, anterior uveitis, meningitis, vegetative endocarditis, pneumonia, and diarrhea. Calves experiencing these problems should immediately be given broad-spectrum antimicrobial agents, supportive therapy, such as fluids with dextrose and electrolytes, and other treatments specific to the problems encountered. If omphalophlebitis is present and does not respond to antimicrobial therapy, surgical removal of infected umbilical remnants should be considered if the patient is a good surgical candidate. Anterior uveitis and hypopyon often respond to the application of ophthalmic preparations of antibiotics and atropine. Septic arthritis may be treated with IV regional antibiotic perfusion or implantation of antibiotic-impregnated polymethylmethacrylate (PMMA) beads near affected joints.
Diarrhea, a common problem in young dairy and beef calves, has multiple infectious and noninfectious causes. Viral causes include rotavirus, coronavirus, and bovine diarrhea virus (BVD). Bacterial enteritis may result if the calf is infected with Escherichia coli, Salmonella spp., or clostridial and protozoal diseases, such as cryptosporidiosis and coccidiosis, may also cause calf diarrhea. In addition, a variety of management conditions, including stress, poor nutrition, and improper sanitation, may cause or contribute to the development of diarrhea. Regardless of cause, affected calves often develop watery diarrhea, rapidly leading to severe dehydration, metabolic acidosis, hypoglycemia, shock, and hypothermia. The treatment is aimed at quick replacement of lost fluids and correction of acidosis and electrolyte abnormalities. Sick calves should be started on warm IV fluids, such as Normosol (Abbott Laboratories) supplemented with dextrose and bicarbonate as indicated. Milk and milk products should be withheld during the treatment of diarrhea, but for no longer than 48 hours total. In herds in which calf diarrhea is a persistent problem, it may be necessary to vaccinate cows and heifers before calving. Many polyvalent vaccines are available, and the product(s) used can be tailored to the needs of the individual herd.
Calves should be fed whole milk or quality calf milk replacer. Although milk replacers are never as complete as whole milk, the product chosen should be of good quality and meet the following standards: protein level 20% to 22%, and all protein must be milk derived (dried skimmed milk, dried whey, whey protein, casein, etc.), crude fat 18% to 20%, and fiber less than 10%. In general, calves are fed 10% of their body weight divided twice daily. This amount may need to be adjusted based on individual needs. It is important to carefully follow label directions when mixing milk replacers since a solution that is too concentrated or too dilute can cause problems, such as nutritional diarrhea. Well-informed veterinary technicians can question clients concerning the quality of their milk replacers, the method of mixing the milk replacer, and the technique of calf feeding and can then make appropriate recommendations to owners.
CONDITIONS OF THE DIGESTIVE SYSTEM
Actinomycosis and Actinobacillosis
Two common conditions of the head in cattle include actinobacillosis (woody tongue) and actinomycosis (lumpy jaw). Woody tongue is caused by a gram-negative bacterium, Actinobacillus lignieresii, which is a normal inhabitant of the mouth of cattle. The organism may gain entry into the soft tissues of the mouth through wounds caused by weed or plant awns resulting in a granulomatous abscess, usually of the tongue. The tongue becomes hard with a diffuse nodular swelling. Clinical signs include excessive salivation and the inability of the animal to prehend food normally causing anorexia and weight loss. The tongue may become so swollen that it protrudes from the mouth (Figure 22-13). The diagnosis is usually based on clinical signs and an examination of the tongue; however, a biopsy and culture of the organism confirms the diagnosis. The successful treatment of this disease involves using sodium iodide and antibiotics. Sodium iodide is administered IV, and care must be taken during administration because perivascular injection may result in tissue sloughing. Woody tongue typically responds to treatment within a few days, but occasionally, repeated doses of sodium iodide may be necessary until signs of iodism occur (lacrimation, anorexia, dandruff, coughing). Prevention includes reduced exposure of the cattle to scabrous feed and plant awns (foxtails, horse nettles, pigweed) by keeping pastures as clean as possible.
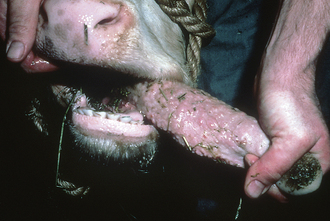
FIGURE 22-13 Bull with actinobacillosis (woody tongue). The tongue is enlarged and nodular in appearance.
Lumpy jaw is the common name for the disease caused by Actinomyces bovis. This organism is gram positive and also a normal inhabitant of the mouth of ruminants. This bacteria gains entry through wounds in the mouth and the infection results in osteomyelitis of the mandible and less commonly the maxilla. Affected cattle have a hard, immovable, nonpainful bony mass of the mandible or maxilla (Figure 22-14). If the mass becomes large enough or involves tooth roots, it may result in pain, an inability to masticate, and subsequent anorexia with weight loss. The treatment of lumpy jaw is the same as described for woody tongue; however, this disease is much less responsive to treatment. Repeated doses of sodium iodide are often necessary just to arrest the growth of the lesion. A more radical treatment involves surgical débridement and curettage of fibrous tissue and infected bone. The surgery is not without risk because these patients often lose significant amounts of blood and may require blood transfusions during the surgical procedure. However, surgical treatment followed by medical treatment offers the best long-term prognosis for valuable animals.
Pharyngeal Trauma and Abscessation
Pharyngeal trauma occurs relatively frequently in cattle and may result in cellulitis, abscess, or hematoma formation. It is almost always caused by trauma associated with the improper use of a balling gun, long dose syringe, speculum, paste dewormer gun, or a rigid stomach tube. Less commonly, a foreign body (sharp stick or wire) may penetrate the pharynx.
Clinical signs include anorexia, salivation, malodorous breath, extension of the head and neck, feed coming from the nares, and mild bloat. In more severe cases, fever, obvious pharyngeal swelling, dysphagia, coughing, and aspiration pneumonia may occur. Careful digital palpation of the pharynx per os is often diagnostic. Always wear gloves during palpation of the mouth or pharynx in cattle because of the concern of rabies. Endoscopy and radiography may be of great benefit in diagnosing the site of the lesion, extent of the cellulitis, and the presence of a foreign body.
The treatment requires the aggressive use of antimicrobial drugs for 10 to 14 days. NSAIDs may help reduce the inflammation in the early stages of the disease. Supportive therapy is also important, especially if the animal cannot eat or drink. Feed and water may be forced with the use of a soft stomach tube or via a temporary rumenostomy. If a retropharyngeal abscess develops, it is best to drain the abscess into the pharynx by manually enlarging the original laceration.
The best way to prevent this condition is to exercise caution when using balling guns, dose syringes, and stomach tubes and make sure restraint is adequate to prevent excessive movement of the animal and subsequent injury. The veterinary technician can play an important role in educating the client-producer concerning the proper use of this equipment.
Grain Overload (Carbohydrate Engorgement, Lactic Acidosis)
Grain overload in ruminants results from the consumption of excessive amounts of highly fermentable carbohydrate feed with the production of large quantities of lactic acid in the rumen. Cattle with grain overload rapidly develop clinical signs of depression, anorexia, bloat, diarrhea with large amounts of grain, dehydration, incoordination, and recumbency leading to death.
Excess carbohydrate ingestion leads to an increased production of volatile fatty acids in the rumen, which lowers rumen pH and decreases rumen motility. S. bovis organisms then multiply producing lactic acid, which further lowers rumen pH (4.0 to 5.0). The acid-resistant Lactobacillus spp. then proliferate, producing more lactic acid. With the increased osmolarity of the rumen fluid, body water is drawn into the rumen creating a “splashy rumen” and leading to a loss of body water with severe dehydration and metabolic acidosis. Thus affected animals if not treated early may develop severe metabolic acidosis leading to shock and acute death. Animals that do not die from the acute acidosis may subsequently develop secondary problems, such as rumenitis with liver abscesses, laminitis (founder), and/or polioencephalomalacia.
The diagnosis is based on a history of sudden exposure to large amounts of grain, typical clinical signs, and a rumen pH of less than 5.0. Ruminal fluid analysis can be a valuable tool in the diagnosis of this and other rumen abnormalities.
Medical treatment involves removal of the rumen contents with a large-bore stomach tube (Kingman tube), which is accomplished by repeated flushing of water into the rumen followed by the outflow of rumen contents (lavage) (Figure 22-15). In addition, animals are given oral antacids, antibiotics (usually penicillin), NSAIDs when rehydrated, and thiamine, which all help prevent liver abscesses, polioencephalomalacia, and laminitis. In more severe cases, IV fluids with sodium bicarbonate should be administered to correct dehydration and acidosis. It may be necessary in some cases to perform a rumenotomy to remove all rumen contents (see Chapter 31). Whether an animal is treated medically or surgically, rumen transfaunation is often helpful to reestablish normal rumen microflora and improve appetite during the convalescent period. Prevention of grain overload involves making dietary changes gradually. Rumen adaptation to dietary changes may take as long as 6 weeks. The veterinary technician can be instrumental in helping owners understand nutrition and the importance of making slow dietary adjustments in ruminants.
The analysis of rumen fluid is useful to establish the cause of indigestion from abnormal fermentation, such as occurs with the previously described grain overload. These are diagnostic tests that can be performed relatively quickly by a veterinary technician, providing the veterinarian with diagnostic information as to the cause of the indigestion and a treatment appropriate to the condition. Samples may be collected by using a 2- to 3-m–long × 1-cm-inner–diameter (ID) stomach tube. Nasogastric passage of the tube prevents continuous struggling during sample collection and reduces the amount of saliva (no mouth device) that can contaminate the sample and falsely elevate its pH. Samples may also be obtained via centesis of the left paralumbar area using an 18-gauge, 12- to 15-cm needle.
The evaluation of the fluid sample includes the assessment of color, consistency, odor, pH, sedimentation velocity, microscopic examination, rumen chloride, and redox potential. The normal color of rumen fluid is olive or brownish green. Grain overload results in fluid that is milky gray, whereas prolonged stasis or decomposition in the rumen changes the color to dark green or black. The consistency of normal rumen fluid is slightly viscous, and salivary contamination increases viscosity. The odor of normal rumen fluid is aromatic and strong, but it develops an acid smell with grain overload, a putrid odor with stasis and decomposition, and an ammonia scent with urea toxicity.
The pH of rumen fluid is normally 6.5 to 6.8 (5.5 to 6.5 with high grain diets). In general, anorexia will usually result in an elevation of rumen pH. Values below 5.5 and above 7.0 can cause anorexia because of an alteration in the normal microbial population. A pH less than 5.5 is suggestive of grain overload, whereas a pH greater than 7.5 may indicate the overzealous use of antacids or urea toxicity.
An evaluation of sedimentation velocity should be performed soon after sampling and serves as a crude evaluation of microbial activity. The sample is placed in a tube and observed for the time it takes for sedimentation to occur. Fine particles sink, and coarse particles float, buoyed by gas bubbles of fermentation. Normal sedimentation takes 4 to 8 minutes. Grossly inactive fluid shows rapid sedimentation, and frothy ingesta may show no sedimentation.
A microscopic examination is performed to assess the type of bacteria present and protozoal activity. The observation of protozoa requires no stain, and they can be easily seen at 40× to 100× magnification. Normally, ciliate and flagellate forms of various sizes and shapes are present and active. The importance of protozoa is their sensitivity to abnormalities in rumen fluid; the larger species are more sensitive to change, and all protozoa die at a pH less than 5. In the normal animal, gram-negative bacteria are predominate in the rumen. Grain overload results in mostly gram-positive bacteria (Streptococcus spp. and Lactobacillus spp.).
The rumen chloride concentration is normally less than 25 mEq/L and may be elevated in cases of vagus indigestion (failure of pyloric outflow and reflux of chloride back into the rumen). The redox potential test uses a new methylene blue (NMB) test to evaluate anaerobic fermentation. To perform the test, 1 ml of 0.03% NMB is mixed with 20 ml rumen fluid (control sample is untreated rumen fluid). The microflora, if active, reduce NMB, and it loses its color, and this usually takes about 3 minutes (1 to 3 minutes if on a grain diet, 3 to 6 minutes if on a hay diet). Animals with prolonged anorexia, after grain overload, or those receiving indigestible roughage may have a redox potential greater than15 minutes.
Rumen Tympany (Bloat)
Gas production is a normal occurrence during rumen fermentation, and healthy animals are capable of eructating far more gas than the rumen produces. However, in some cases, abnormal distention of the rumen with gas may occur resulting in bloat. Bloat is classified as either primary where eructation is normal but the gas cannot be expelled or secondary that is due to a failure of eructation. Primary bloat occurs when large amounts of legumes or grain are ingested resulting in development of froth in the rumen. A failure of eructation or secondary bloat may be associated with esophageal foreign bodies (choke); motor function abnormalities of the rumen, such as vagus indigestion; body position (lateral recumbency); hypocalcemia; or pharyngitis. Bloat is also described as free gas bloat or frothy bloat depending on the cause.
Clinical signs include distention of the left paralumbar fossa, discomfort (grunting, colic), dyspnea with open-mouth breathing, anorexia, salivation, anxiety, depression terminally, and sudden death.
The treatment of free-gas bloat involves passing a stomach tube either via the nasogastric or orogastric route. If bloat is due to the animal’s position, the animal should be helped into sternal recumbency or a standing position. If hypocalcemia is the underlying problem, administration of calcium is therapeutic. Forced exercise also stimulates rumen motility and eructation. In addition, rumen stimulants improve motility and normal belching. If an animal is critically bloated and passing a stomach tube is too stressful, an emergency procedure called rumen trocarization should be performed using a large-bore trocar to enter the rumen through the left paralumbar fossa. The surgical treatment of chronic bloat is covered in Chapter 31.
Frothy bloat requires a different treatment in that the froth must first be consolidated into larger pockets of gas before it can be expelled. To reduce surface tension, several products may be used, including poloxalene, household detergent (Tide, 2 to 3 oz), mineral oil, or dioctyl sodium sulfosuccinate (DSS). Once the frothy bloat becomes a free gas bloat, it can be eructated or relieved via a tube. Nutritional management, such as preventing excessive exposure to grain or legumes, is important to prevent bloat in ruminants.
Traumatic Reticuloperitonitis
Traumatic reticuloperitonitis (TRP), or hardware disease, which results from the penetration of the reticulum by a foreign body, is one of the most common gastrointestinal problems affecting the forestomach compartments of mature dairy cattle. The indiscriminate eating habits of cattle, in contrast to small ruminants, can lead to the accidental ingestion of foreign materials that settle in the reticulum (Figure 22-16). The foreign bodies ingested by cattle are most often wires and nails, but also include steel objects. Most foreign bodies are ferromagnetic. Subsequent to ingestion of a foreign body, four outcomes are possible:
FIGURE 22-16 Metallic foreign body (wire) found in the reticulum of a cow with traumatic reticuloperitonitis (hardware disease).
1. Attachment of the object to a magnet without further disease problems
2. Penetration of the reticular wall with acute inflammation and mild clinical disease if there is not penetration into the peritoneal cavity
3. Perforation of the reticular wall into the peritoneal cavity with acute localized TRP
4. Migration of the foreign body with penetration into the peritoneal or thoracic cavity and resulting abscessation (thoracic, reticular, hepatic), vagal indigestion, pericarditis, myocarditis, or other secondary problems
Acute cases of TRP result in anorexia, a sharp decrease in milk production, reluctance to rise or move, cranial abdominal pain, and kyphosis. Uncomplicated cases may improve in 3 to 5 days, but progression of the severity of signs may indicate either a failure to contain a localized peritonitis or extension of the infection to other organs. A heart rate greater than 90 bpm or a fever greater than 40° C generally indicates more severe disease, such as diffuse peritonitis or pericarditis. A heart rate less than 64 bpm is suggestive of vagus indigestion syndrome.
A differential white blood cell count (WBC) is a more reliable indicator of inflammation than is a total WBC. A neutrophilia (greater than 4000 cells/μl) with a left shift can be expected in acute cases, but in chronic cases, these changes are less consistent. A high plasma fibrinogen concentration (greater than 1000 mg/dl) usually occurs in both acute and chronic cases of TRP.
A peritoneal fluid analysis may be helpful in the diagnosis of TRP, especially in chronic cases when WBC changes are infrequently observed. It may be necessary to sample multiple sites because of the size of the rumen and that cattle are capable of localizing an infection by the formation of a large amount of fibrin. Failure to obtain a fluid sample does not rule out TRP. A relatively accurate diagnosis of peritonitis can be made if the nucleated cell count is greater than 6000 cells/μl and the total protein is greater than 3 g/dl (a more accurate diagnosis can be made if a differential is performed, and neutrophils account for greater than 40% of the cells and eosinophils for less than 10%).
Reticular or cranioventral abdominal radiography or ultrasonography can offer valuable assistance in the diagnosis and treatment of TRP.
Medical treatment of TRP is often successful. Even if a foreign body has perforated the reticular wall, in approximately half of the cases, it will return to the lumen. Medical treatment is geared toward treating reticulitis and/or peritonitis and preventing further perforation of the reticulum by the use of broad-spectrum antimicrobials and the oral administration of a magnet.
Surgical intervention may be necessary if TRP fails to respond to conservative treatment (see Chapter 31 for surgical details). Most abscesses that form secondary to TRP are located on the medial wall of the reticulum and are usually tightly adhered. These abscesses are the most common causes of vagal indigestion associated with TRP. These tightly adhered abscesses can be lanced and drained into the reticulum or omasum and then explored for the presence of a foreign body.
Indications for performing paracentesis or abdominocentesis include an evaluation of accumulated abdominal fluid and diagnosis of abdominal diseases, such as TRP, peritonitis, abomasal ulcers (perforating), abomasal rupture, ruptured bladder or ureters, abdominal neoplasia (lymphosarcoma, mesothelioma), or intestinal obstruction or rupture. In monogastric animals, the most ventral aspect of the abdomen on the midline is the usual site for abdominocentesis; however, in cattle, centesis on the midline usually results in puncture of the rumen. Because bovine abdominal disorders are often localized (or effectively walled off), centesis of a single site might not reflect disease elsewhere in the abdomen. Centesis of four sites on the ventrolateral abdomen may be most productive. These areas represent four abdominal quadrants: left cranial, left caudal, right cranial, and right caudal.
Sampling should begin where the problem is suspected, such as start with the left cranial quadrant if hardware disease is likely. The animal should be standing and properly restrained. The area of centesis is surgically clipped and prepared. To approach the left cranial quadrant, palpate the foramen of the subcutaneous abdominal vein. The site to tap is one hand’s breadth cranial and one hand’s breadth (6 cm) medial to the foramen, or halfway between the xyphoid and umbilicus and half way between the midline and milk vein. The right cranial quadrant has the same landmarks as the left cranial quadrant only on the right side. This site would be most useful if abomasal disease is suspected. The left caudal quadrant can be located directly anterior to the attachment of the udder to the body (comparable location in the male) in the paramedian area. The right caudal quadrant has the same landmarks as the left caudal quadrant only on the right side. These sites are sampled using an 18-gauge, 3.8-cm needle and collecting any fluid in EDTA and serum tubes. Each quadrant sample should be individually analyzed if several sites are tapped unless it is necessary to combine samples because of small volumes. A sterile teat cannula may be used instead of a needle, but this requires some local analgesia and a small skin incision.
The sample is evaluated and classified as a transudate, which is a serous fluid accumulation as a result of an alteration in pressure, or an exudate, which may be a result of an abdominal infection. Transudates are clear, colorless, odorless, have a protein less than 2.5 g/dl and cell counts less than 5000/μl, and may be due to hypoproteinemia or hypoalbuminemia. Exudates are usually turbid and have a protein greater than 3 g/dl and cell counts greater than 10,000 cells/μl. The evaluation of peritoneal fluid in cattle includes the assessment of volume, color, turbidity, total protein, chemical analysis (urea nitrogen, creatinine), cell count, and cytology. In clinically normal animals, the ratio of neutrophils to mononuclear cells (eosinophils) is 1:1 with the exception of normal cows less than 2 weeks postpartum that may have total nucleated cell counts greater than 10,000 cells/μl, and neutrophils or eosinophils are predominant.
To evaluate cytology, a direct smear may be prepared or the sample may need to be centrifuged if cellularity is low. Hematologic stains, such as Wrights stain, NMB, or Diff-Quik, (Baxter S/P) can be used to examine neutrophil morphology (degenerative changes, bacteria). The classification of inflammatory fluids (exudates) is as follows:
1. Acute inflammation with 80% to 85% neutrophils (the rest of the cells are lymphocytes, eosinophils, macrophages), which may be nondegenerate as with a noninfectious irritant or degenerate as may occur with sepsis
2. Chronic active (subacute) inflammation with 50% to 70% nondegenerate neutrophils and 20% to 50% monocytes or macrophages
3. Chronic inflammation with greater than 50% monocytes and macrophages
Diarrhea in the Adult
Common causes of acute diarrhea in adults include coccidiosis, dietary gastroenteritis, acute salmonellosis, acute bovine virus diarrhea (BVD), and winter dysentery. Chronic diarrhea in the adult may be due to gastrointestinal (GI) parasites, Johne’s disease, chronic BVD, chronic salmonellosis, bovine leukemia virus (BLV), chronic renal disease, or chronic liver disease.
Winter dysentery, believed to be caused by a coronavirus, is an acute, contagious diarrheal disease of adult cattle. It is seen more commonly in dairy herds in the winter, and it is considered an epizootic disease since it spreads rapidly through infected herds. Morbidity is high, but mortality rare. The disease has a rapid onset of explosive diarrhea, mild depression, partial anorexia, and a decreased milk production in affected animals. The disease can spread through an entire herd in 2 weeks, and those individuals affected first usually recover within 1 week. The greatest economic loss to the producer is a loss of milk production as a result of body water loss secondary to severe diarrhea. No treatment is available, and most animals recover spontaneously; however, sick cows can be treated symptomatically with intestinal astringents, protectants, and absorbents. The provision of fresh drinking water and free-choice salt is also beneficial.
The most frequent cause of chronic diarrhea in cattle is parasitic infection, especially by Ostertagia ostertagi and Nematodirus. In adult cattle, the most common clinical parasitic disease is type II or pre-type II ostertagiasis.
In pre-type II disease, the fourth-stage Ostertagia larvae burrow into the abomasal wall and encyst instead of maturing into adults. This occurs in the fall in the northern United States and in the spring in the south. During encystment, the acid-secreting parietal cells of the abomasum are destroyed causing an increase in the pH of the abomasum. With the pH above 5, pepsinogen is no longer converted to pepsin, resulting in impaired protein digestion.
Type II ostertagiasis occurs in the spring in the northern United States and in the late summer, early fall in the south when large numbers of encysted larvae emerge into the lumen of the abomasum and mature. Much more damage occurs to the abomasal glands during emergence than during dormancy. Because of the extensive damage, clinical signs often persist long after the parasites are removed by effective anthelmintics.
Clinical signs include unthriftiness, weight loss, pale mucous membranes, diarrhea, and dependent edema in severe cases. Severe parasitism and associated clinical cases are more likely to occur when conditions are crowded, nutrition is marginal, and deworming programs are inadequate.
Parasite eggs may be absent in some cases of type II ostertagiasis. An increased serum pepsinogen concentration may be helpful in the diagnosis in cases of type II ostertagiasis (excessive amounts of pepsinogen are in the GI tract as a result of mucosal damage or decreased conversion to pepsin; the pepsinogen leaks into the bloodstream). For the treatment of these parasitic conditions, please refer to Chapter 17.
Johne’s disease, characterized by chronic diarrhea and weight loss, is caused by Mycobacterium paratuberculosis, a slow-growing, acid-fast organism. The bacterium is transmitted from infected cows to their calves via the fecal-oral route, with calves less than 6 months the most susceptible to infection. Once the organisms have been ingested by the calf, these are taken up from the intestinal lumen by cells covering the Peyer’s patches, particularly in the distal ileum, and are phagocytized by macrophages. The organisms reside and grow within macrophages eventually causing a severe granulomatous reaction with thickening of the intestinal wall. This leads to protein malabsorption and a protein-losing enteropathy so that even though these animals have good appetites, they continue to experience diarrhea and weight loss (Figure 22-17). Although the infection occurs early in life, clinical signs do not usually develop until the animal is at least 2 years of age.

FIGURE 22-17 Severe emaciation, intermandibular edema, and chronic diarrhea in a cow with Johne’s disease.
Johne’s disease is a terminal disease with no effective treatment. It can be difficult to diagnose because of a lack of reliable tests. There are several serologic tests available that may help diagnose this disease; however, most tests are clouded by false-positive and false-negative reactions. A deoxyribonucleic acid (DNA) test has recently become available, but it is relatively expensive and not 100% reliable. Once clinical signs are advanced, a rectal mucosal biopsy may be beneficial to achieve a quick diagnosis. Often the diagnosis is made based on history, clinical signs, and a lack of response to conventional treatments, such as antimicrobial and anthelmintic therapy (for both nematodes and trematodes) (Case Presentation 22-1).
Control programs have been outlined for producers who wish to eliminate the disease from their herd. These programs involve the use of repeated serologic testing, fecal cultures, culling of positive animals and clinically ill animals, and maintenance of separate disease-free and infected herds. These programs are costly and labor intensive, so most producers choose to live with the disease, culling clinically affected cows and the exposed offspring. A vaccine, the use of which is state controlled, is available and is used to control the development of clinical disease in herds with a high incidence of Johne’s disease.
Salmonellosis typically manifests as an acute diarrhea, but in rare cases, individuals become chronically infected and have recurring bouts of diarrhea with or without fever, anorexia, and dehydration. Chronically infected cattle usually lose weight and become unthrifty. Salmonellosis is often associated with leukopenia, hyponatremia, hypokalemia, and hypoproteinemia. The submission of multiple fecal samples of adequate volume (up to 60 ml) for culture is necessary to establish a diagnosis. A culture of a rectal biopsy may enhance recovery of the organism.
Salmonellosis should be suspected when chronic diarrhea is preceded by an outbreak of acute diarrhea in a herd, especially if the outbreak was associated with the introduction of new livestock, a feed change, a water change, or a flood.
Other clinical diseases caused by Salmonella include septicemia, especially in the neonate, and abortion in adults. Severe cases may progress to endotoxemia, shock, and subsequent death. Animals that survive may continue to carry and shed the organism, posing a threat to other animals. The treatment includes antimicrobial drugs, NSAIDs, and fluid therapy, particularly in young calves with septicemia and/or endotoxemia.
BVD is a common virus-induced gastroenteritis affecting all ages of cattle. BVD manifests as sudden onset of fever, depression, anorexia, oral and GI ulcers and erosions, and diarrhea (sometimes with blood and mucus). The disease may progress rapidly through a group of animals. BVD also plays an important role in respiratory tract diseases in cattle by causing immunosuppression and susceptibility to secondary bacterial pathogens causing pneumonia. The virus is also responsible for abortions, in utero infections, and birth defects. The exposure of pregnant cattle to the noncytopathic strain of BVD between days 80 and 125 of gestation may result in an infected fetus, which becomes immunotolerant to the virus. Later in life, the calf may be exposed to cytopathic strains of BVD, which results in acute mucosal disease (MD) or chronic diarrhea (chronic BVD or BVD-MD), which has a nearly 100% mortality rate; affected calves rarely live to 1 year of age. Clinical signs of BVD-MD include intermittent or persistent diarrhea, weight loss, anorexia, unthriftiness, crusty eyes or muzzle, blunting of oral papillae (Figure 22-18), and chronic coronary band lesions. These cattle are also leukopenic and anemic and have no titer to BVD when serology is performed.
The diagnosis of BVD is made on a physical examination, characteristic necropsy findings, including linear erosions in the esophagus, necrosis of the Peyer’s patches, blunted papillae in the buccal mucosa, and epithelial erosions on the tongue and ruminal pillars, virus isolation from tissues or
the buffy coat of whole blood samples, and serology (paired samples 2 to 4 weeks apart are most helpful).
There is no treatment for BVD, but antimicrobials are often used to prevent secondary bacterial infections. Vaccination of dairy and beef animals is important to help prevent the disease. Both killed virus and modified live virus vaccines are available. The use of the modified live virus vaccines should be avoided in pregnant cows, calves nursing pregnant cows, and immunosuppressed animals. Vaccination of calves experiencing chronic BVD using the modified live virus vaccine may result in the death of the animal.
DISEASES OF THE RESPIRATORY SYSTEM
Bovine Respiratory Disease Syndrome
Bovine respiratory disease syndrome (BRDS) affects all ages of cattle, but particularly beef calves during the first 45 days in the feedlot and dairy calves younger than 6 months of age. The syndrome is caused by a complex interaction of respiratory viruses, bacteria, and stress. Transportation, cold weather, close confinement, stress with immunosuppression, and exposure to viral and bacterial pathogens predispose to the development of respiratory disease.
A number of viruses including infectious bovine rhinotracheitis (IBR), BVD, parainfluenza virus (PI3), bovine respiratory syncytial virus (BRSV), and respiratory coronavirus in addition to bacteria, such as Mannheimia haemolytica and Histophilus somnus, produce respiratory disease in susceptible, immunocompromised animals. Generally, infection with one or more of the respiratory viruses occurs first, followed by bacterial infection of the lower respiratory tract or bronchopneumonia. BRDS in feedlot cattle is often referred to as shipping fever.
Cattle with BRDS experience depression, standing with their heads lowered, anorexia, fever 40° C to 41.5° C (104° F to 107° F), mucopurulent ocular and nasal discharge, cough, and dyspnea (Figure 22-19). Morbidity and mortality within a group may be quite high.
The diagnosis of BRDS is based on a history of calves undergoing stress, typical clinical signs of pneumonia, and the presence of bronchopneumonia on necropsy. Samples from a transtracheal aspirate can be submitted for cytology, bacterial isolation, and antimicrobial sensitivity and virus isolation in cases of respiratory disease in cattle. A successful treatment of the disease hinges on early diagnosis and the institution of appropriate antimicrobial therapy. Individual sick animals should be isolated from the rest of the group and treated with broad-spectrum antimicrobial therapy for at least 5 days. Antimicrobial agents typically used for the treatment of shipping fever include ceftiofur sodium, ceftiofur hydrochloride, or ceftiofur crystalline free acid (Naxcel, ExcenelRTU, or Excede all produced by Pharmacia & Upjohn, Division of Pfizer Inc., New York), florfenicol (Nuflor, Schering-Plough, Union, N.J.), tilmicosin (Micotil, Elanco, Indianapolis, Ind.), tulathromycin (Draxxin, Pfizer Animal Health, New York), tetracycline (LA200, Liquamycin, Pfizer Animal Health, New York), and fluoroquinolone (Baytril, Bayer, Shawnee Mission, Kan.). When large numbers of animals are ill, antimicrobial agents may be added to the water to simplify the treatment. In addition, fresh water, hay, and adequate shelter should be provided. A procedure known as metaphylaxis is now commonly employed by feedlots and involves the treatment of cattle with long-acting antimicrobial agents, such as Micotil, upon arrival at the feedlot. Preconditioning (castration, dehorning, implanting, acclimation to feed and water, deworming, and vaccination) of calves before weaning and vaccination before transport from stocker to feeder operations decreases stress on the cattle during these transitions and may prevent the development or lessen the severity of disease. Vaccines typically used to prevent respiratory disease outbreaks include IBR, PI3, BVD, BRSV, M. haemolytica, and H. somnus.
DISEASES OF THE MUSCULOSKELETAL SYSTEM
Lameness is commonly encountered in cattle and is most often (88%) caused by lesions or problems in the foot. Upper leg problems, such as anterior cruciate ligament rupture, coxofemoral (hip) luxation, fractures, and arthritis, account for the remaining 12% of lameness. When foot problems occur, they are most often seen in the claws that bear the most weight, front medial and hind lateral claws.
Common foot conditions, especially in dairy cattle, include foot rot, laminitis, white-line abscess (“gravel”), sole ulcer, underrun heel and sole, and hairy heel warts. It is important to examine all foot problems early since many conditions can quickly lead to osteomyelitis and/or septic arthritis if not properly treated. Regardless of the cause of lameness, it can lead to a loss of production as a result of decreased milk production, weight loss, delayed breeding or anestrus, and culling. Intensive housing and feeding of large groups of animals has lead to an increased incidence of lameness.
Interdigital Necrobacillosis
Interdigital necrobacillosis, or foot rot, in cattle is an infection of the interdigital skin and underlying tissues caused by the synergistic effects of Fusobacterium necrophorum and other anaerobic bacteria. The infection results in an ulcerated, foul-smelling area between the claws with resultant lameness (Figure 22-20). There may be swelling apparent above the coronary band, and in severe cases, cellulitis up to the carpus or hock may occur. The disease is particularly prevalent when cattle are kept in wet, muddy conditions. If left untreated, foot rot can invade the deeper tissues of the foot causing septic arthritis, osteomyelitis, and chronic lameness.
The treatment of foot rot in cattle can be successfully accomplished with aggressive topical treatment. In some cases, it may be necessary to débride necrotic tissue and even bandage the foot with antimicrobial agents to promote healing. In cases where cellulitis has occurred, it may be necessary to use parenteral antibiotics or the regional antibiotic perfusion technique. Foot rot usually has a low morbidity in any given herd, but in cases where there is a high incidence of foot rot, it may be necessary to initiate the use of a footbath containing zinc sulfate for the prevention or treatment of foot rot.
Papillomatous Digital Dermatitis
Papillomatous digital dermatitis (PDD), or hairy heel wart, has become an important cause of lameness in dairy cattle worldwide. Initially, PDD appears as a superficial inflammation of the skin of the bovine digit, but progresses to the typical mature lesions that are circumscribed, erosive, or proliferative and are usually located on the hind feet, adjacent to the interdigital ridge and heel bulbs (Figure 22-21). Granulation tissue develops with outgrowths of dermal tissue that grossly resemble hair, thus the common name, hairy heel wart. The disease seems to be contagious with a fairly high morbidity within any given herd.
FIGURE 22-21 Papillomatous digital dermatitis or hairy heel warts of the hind feet cause severe lameness in affected dairy cattle.
At this time, the cause of PDD is still controversial; it is believed to be a multifactorial disease in which infectious agents are primarily involved. The economic impact includes a decreased milk production, impaired reproductive performance, an increased number of cows culled, and the cost of treatment and control. Antibiotics, such as oxytetracycline, lincomycin, or lincomycin-spectinomycin, have been used in addition to nonantibiotic products, such as triplex (solubilized copper, a peroxy compound and a cationic agent). The treatment is beneficial, but does not appear to be curative because disease outbreaks are common once a herd is infected.
Laminitis
Laminitis, or founder, is a diffuse, aseptic inflammation of the corium (sensitive lamina) of the feet. Acute laminitis occurs sporadically and may be due to sudden excess grain ingestion (see Grain Overload), or it may occur secondary to other diseases that occur during the postparturient period, such as retained placenta, metritis, mastitis, ketosis, or abomasal displacement. Chronic laminitis is more often associated with constant feeding of high-grain diets as occurs commonly in high-producing dairy cows, feedlot cattle, or show cattle.
Clinical signs include stiffness, pain, reluctance to walk, and difficulty in rising. Affected animals spend a lot of time lying down, and when they do stand, they may stand with their backs arched, front legs crossed, or kneeling on the front legs in an attempt to redistribute body weight because of the pain. Acute cases of laminitis are treated by correcting any existing underlying problem and the administration of NSAIDs. Laminitis often leads to serious sequelae, including sole ulcers, white-line disease, abnormal hoof growth with horizontal or vertical hoof-wall cracks (normal hoof growth is about 0.5 cm per month), underrun heel and sole, and even osteomyelitis or septic arthritis. Frequent foot trimming helps prevent the development of these problems.
Sole (Rusterholz) Ulcers
A sole ulcer is a circumscribed loss of sole that exposes the corium (sensitive lamina or “quick”). The typical location of the lesion is near the axial (inside) aspect of the hind lateral claws near the heel-sole junction. Lesions are usually bilateral involving both hind lateral claws. In some cases, sole loss is not evident, but there is a circular area of hemorrhage beneath the sole or the sole is yellow and soft; the ulcer becomes apparent when the undermined sole is trimmed away. Lameness usually is not severe until granulation tissue develops from the exposed corium and protrudes from the defect in the sole. This granulation tissue retards the development of new sole. These ulcers may become infected with extension of the infection into the deeper tissues (navicular bursa, coffin joint, and flexor tendons).
Sole ulcers are probably the direct result of laminitis. The higher incidence of ulcers in the lateral hind claws versus medial hind claws suggests an anatomic or mechanical difference, but it is not certain. The pressure on impact is greater at the heel-sole junction of the lateral claw. Localized ischemia from laminitis can lead to erosion of the sole.
Lameness can be severe and is worse when the granulation tissue protrudes or if deeper tissues are involved. The animal may abduct its leg slightly (an attempt to put more weight on the medial claw) or stand with the heel extending beyond the gutter. Since the condition is often bilateral, both hind feet should be checked, even though lameness may be apparent in only one leg (usually the lesion is more advanced in one foot than the other). The treatment is aimed at controlling granulation tissue and preventing extension of the infection into the deeper tissues. The treatment involves the excision of excessive granulation tissue, placement of a wooden or acrylic block on the medial claw to take weight bearing off the lateral claw, and use of a caustic substance, such as copper sulfate or phenol-formalin, with a bandage to control the granulation tissue. Prognosis is good if there is no deep infection; however, the treatment requires time and money.
White-Line Disease
The white line is the area of fibrous connective tissue that joins the rigid hoof wall to the more resilient sole. Since the white line is soft, it is more vulnerable to penetration by foreign material. Dirt-filled cracks and fissures in the white line are not uncommon in normal feet, but when abscesses develop under the sole, lameness follows. Factors predisposing to white-line disease are continuously wet feet and animals with previous bouts of laminitis, which result in poor horn quality and decreased white-line strength.
A serious sequela to white-line disease is an infection of the navicular bursa. Although the bursa is protected from direct penetration or infection by the flexor tendon, it is still vulnerable at the edges of the tendon. Occasionally, the infection extends up along the wall, forms an abscess in the navicular bursa, and drains above the abaxial coronet (called gravel).
White-line abscesses cause lameness. The animal may walk or stand with the heel slightly raised or the limb abducted (an attempt to shift weight to the medial claw). Hoof testers help localize the lesions that are not obvious. Black lines, cracks, and fissures should be followed and pared out. The opening of an abscess will often result in release of watery black exudate and gas. If the navicular bursa is involved, the heel will be swollen, hot, and painful. The sole should be pared to allow adequate opening and drainage of the abscess, and the lateral wall should be trimmed to prevent packing of dirt and manure back into the abscess hole.
Underrun Heel and Sole
This condition is also referred to as stable foot rot and occurs primarily in the hind feet. The erosive process begins at the heel bulb initially as pits or pockmarks, and then parallel horizontal grooves develop and fill with black necrotic material. The anaerobic bacteria F. necrophorum and Bacteroides nodosus have both been isolated from these cases. The sole can separate to form a flap that may extend to the toe with a new sole developing underneath (Figure 22-22). Debris can become packed between the sole layers causing lameness. Underrun heels may not cause lameness unless there is infection of deeper structures or a lot of debris gets packed between the sole layers.
FIGURE 22-22 Debris packed between layers of sole causing lameness in a bull with underrun heel and sole.
This condition may result from chronic laminitis, which causes hoof overgrowth (especially at the toe) and shifting of the animal’s weight to the area of the heel. The abnormal and underrun sole should be removed to treat this condition. If sensitive lamina is exposed, the foot may need to be topically treated with antimicrobials and bandaged until the area becomes tough enough to bear weight. A wooden block on the opposite claw may be helpful during the healing process. Prevention includes regular hoof trimming to keep the animal from walking on its heels and the use of footbaths or regular topical treatment to control anaerobic bacterial infection in the area.
 TECHNICIAN NOTE
TECHNICIAN NOTE