Chapter 15 Exercise
 The extra tissue oxygen requirement is provided by increases in cardiac output and blood oxygen extraction.
The extra tissue oxygen requirement is provided by increases in cardiac output and blood oxygen extraction.The respiratory response to exercise depends on the level of exercise performed, which can be conveniently divided into three grades:
Oxygen Consumption during Exercise
There is a close relationship between the external power that is produced and the oxygen consumption of the subject (Figure 15.1). The oxygen consumption at rest (the basal metabolic rate) is of the order of 200–250 ml.min−1. As work is done, the oxygen consumption increases by approximately 12 ml.min−1 per watt. Exercise intensity is commonly described in terms of metabolic equivalents (METs), which refer to the number of multiples of the normal resting oxygen consumption. For example, walking briskly on the level requires an oxygen consumption of about 1 l.min−1 or 4 METs, whilst running at 12 km per hour (7.5 miles per hour) requires about 3 l.min−1 of oxygen and is rated as 12 METs of activity. Further examples are shown in Figure 15.1.
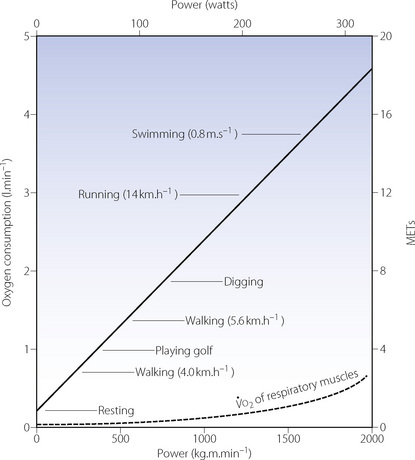
Fig. 15.1 Steady state oxygen consumption with varying degrees of exercise. The continuous straight line denotes whole body oxygen consumption as a function of the level of power developed. The broken curve is an estimate of the oxygen cost of breathing for the increasing hyperventilation of exercise.1 MET, metabolic equivalent, which is the number of multiples of basal oxygen consumption required for different activities.
Time Course of the Increase in Oxygen Consumption1
Oxygen consumption rises rapidly at the onset of a period of exercise, with an accompanying increase in carbon dioxide production and a small increase in blood lactate. With moderate exercise (Figure 15.2A) a plateau is quickly reached and the lactate level remains well below the normal maximum resting level (<3.5 mmol.l−1). With heavy exercise 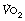 ,
, 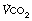 and lactate all increase more quickly, again reaching constant levels within a few minutes, the magnitude of which relates to the power generated and the fitness of the subject (Figure 15.2B). If the level of exercise exceeds approximately 60% of the subject’s maximal exercise ability (see below), there is usually a secondary ‘slow component’ to the increase in oxygen consumption, associated with a continuing increase in blood lactate level, which ultimately prevents the exercise from continuing (Figure 15.2C). There have been many explanations proposed for this slow component of
and lactate all increase more quickly, again reaching constant levels within a few minutes, the magnitude of which relates to the power generated and the fitness of the subject (Figure 15.2B). If the level of exercise exceeds approximately 60% of the subject’s maximal exercise ability (see below), there is usually a secondary ‘slow component’ to the increase in oxygen consumption, associated with a continuing increase in blood lactate level, which ultimately prevents the exercise from continuing (Figure 15.2C). There have been many explanations proposed for this slow component of 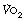 , including increased temperature, the oxygen cost of breathing, lactic acidosis1 and changes in muscle metabolism secondary to the use of differing fibre types with prolonged exercise.2 The physiological mechanism underlying the linkage between oxygen requirement and its delivery during exercise, and the time course of this response, remain incompletely explained.3
, including increased temperature, the oxygen cost of breathing, lactic acidosis1 and changes in muscle metabolism secondary to the use of differing fibre types with prolonged exercise.2 The physiological mechanism underlying the linkage between oxygen requirement and its delivery during exercise, and the time course of this response, remain incompletely explained.3
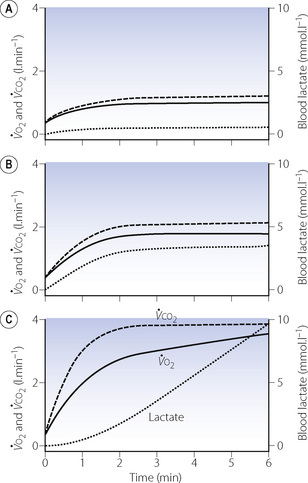
Fig. 15.2 Changes in oxygen consumption (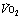 , solid line), CO2 production (
, solid line), CO2 production (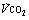 , dashed line) and blood lactate (dotted line) with the onset of varying levels of exercise. (A) Light to moderate exercise with little or no increase in lactate; (B) heavy exercise with an increase in lactate to an increased, but steady, level; (C) severe exercise, above the anaerobic threshold when levels continue to rise as exercise proceeds. Note that with severe exercise (C), the increase in oxygen consumption is biphasic with a second ‘slow’ component.
, dashed line) and blood lactate (dotted line) with the onset of varying levels of exercise. (A) Light to moderate exercise with little or no increase in lactate; (B) heavy exercise with an increase in lactate to an increased, but steady, level; (C) severe exercise, above the anaerobic threshold when levels continue to rise as exercise proceeds. Note that with severe exercise (C), the increase in oxygen consumption is biphasic with a second ‘slow’ component.
Maximal Oxygen Uptake
Maximal oxygen uptake (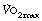 ) refers to the oxygen consumption of a subject when exercising as hard as possible for that subject. A fit and healthy young adult of 70 kg should be able to maintain a
) refers to the oxygen consumption of a subject when exercising as hard as possible for that subject. A fit and healthy young adult of 70 kg should be able to maintain a 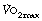 of about 3 l.min−1, but this decreases with age to about 2 l.min−1 at the age of 70. A sedentary existence without exercise can reduce
of about 3 l.min−1, but this decreases with age to about 2 l.min−1 at the age of 70. A sedentary existence without exercise can reduce 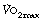 to 50% of the expected value. Conversely,
to 50% of the expected value. Conversely, 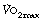 can be increased by regular exercise, and athletes commonly achieve values of 5 l.min−1. The highest levels (over 6 l.min−1) are attained in rowers, who utilise a greater muscle mass than other athletes. For example elite oarsmen may attain, for a brief period, a mean oxygen consumption of 6.6 l.min−1 on the treadmill.4 This requires a minute volume of 200 l.min−1 (tidal volume 3.29 litres at a frequency of 62 breaths per minute).
can be increased by regular exercise, and athletes commonly achieve values of 5 l.min−1. The highest levels (over 6 l.min−1) are attained in rowers, who utilise a greater muscle mass than other athletes. For example elite oarsmen may attain, for a brief period, a mean oxygen consumption of 6.6 l.min−1 on the treadmill.4 This requires a minute volume of 200 l.min−1 (tidal volume 3.29 litres at a frequency of 62 breaths per minute).
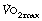 is commonly used in exercise physiology as a measure of cardiorespiratory fitness. Subjects undertake a period of graduated exercise while
is commonly used in exercise physiology as a measure of cardiorespiratory fitness. Subjects undertake a period of graduated exercise while 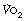 is measured continuously by a spirometric method (page 211). In all but severe exercise, within a few minutes
is measured continuously by a spirometric method (page 211). In all but severe exercise, within a few minutes 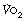 reaches a plateau (Figure 15.2), which is the subject’s
reaches a plateau (Figure 15.2), which is the subject’s 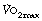 . At higher levels of exercise, as seen in athletes, defining when maximal oxygen uptake is reached may be difficult because of the slow component of oxygen consumption. Many varying definitions of
. At higher levels of exercise, as seen in athletes, defining when maximal oxygen uptake is reached may be difficult because of the slow component of oxygen consumption. Many varying definitions of 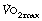 have therefore been used over the years,5 none of which are universally accepted. Elite athletes rarely reach a satisfactory plateau in
have therefore been used over the years,5 none of which are universally accepted. Elite athletes rarely reach a satisfactory plateau in 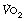 , and secondary criteria such as high plasma lactate levels or a raised respiratory exchange ratio need to be used to define
, and secondary criteria such as high plasma lactate levels or a raised respiratory exchange ratio need to be used to define 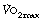 .5
.5
At 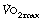 in trained athletes, approximately 80% of the oxygen consumed is used by locomotor muscles. With the high minute volumes seen during exercise, the oxygen consumption of respiratory muscles also becomes significant, being around 5% of total
in trained athletes, approximately 80% of the oxygen consumed is used by locomotor muscles. With the high minute volumes seen during exercise, the oxygen consumption of respiratory muscles also becomes significant, being around 5% of total 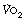 with moderate exercise and 10% at
with moderate exercise and 10% at 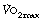 (Figure 15.1).6,7
(Figure 15.1).6,7
Response of the Oxygen Delivery System
A 10- or 20-fold increase in oxygen consumption requires a complex adaptation of both circulatory and respiratory systems.
Oxygen delivery. This is the product of cardiac output and arterial oxygen content (page 202). The latter cannot be significantly increased, so an increase in cardiac output is essential. However, the cardiac output does not, and indeed could not, increase in proportion to the oxygen consumption. For example, an oxygen consumption of 4 l.min−1 is a 16-fold increase compared with the resting state. A typical cardiac output at this level of exercise would be only 25 l.min−1 (Figure 15.3), which is only five times the resting value. Therefore, there must also be increased extraction of oxygen from the blood. Figure 15.3 shows that the largest relative increase in cardiac output occurs at mild levels of exercise. At an oxygen consumption of 1 l.min−1 cardiac output is already close to 50% of its maximal value.
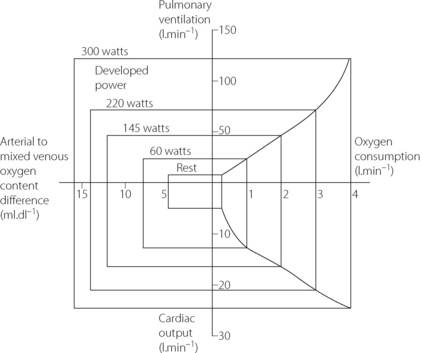
Fig. 15.3 Changes in ventilation, oxygen consumption, cardiac output and oxygen extraction at different levels of power developed.
Oxygen extraction. In the resting state, blood returns to the right heart with haemoglobin 70% saturated. This provides a substantial reserve of available oxygen and the arterial/mixed venous oxygen content difference increases progressively as oxygen consumption is increased, particularly in heavy exercise when the mixed venous saturation may be as low as 20% (Figure 15.3). This decrease in mixed venous saturation covers the steep part of the oxygen dissociation curve (Figure 11.9) and therefore the decrease in Po2 is relatively less (5 to 2 kPa, or 37.5 to 15 mmHg). High levels of blood lactate seen during heavy exercise may contribute to the increase in oxygen extraction by shifting the dissociation curve to the right at a capillary level.1
The additionally desaturated blood returning to the lungs and the greater volume of blood require that the respiratory system transport a larger quantity of oxygen to the alveoli. If there were no increased oxygen transport to the alveoli, the reserve oxygen in the mixed venous blood would be exhausted in one or two circulation times. Fortunately the respiratory system normally responds rapidly to this requirement.
Anaerobic Metabolism
During heavy exercise, the total work exceeds the capacity for aerobic work, which is limited by oxygen transport (see below). The difference is made up by anaerobic metabolism, of which the principal product is lactic acid (see Figure 11.13), which is almost entirely ionised to lactate and hydrogen ions. The anaerobic threshold may be defined as the highest intensity of exercise at which measured oxygen uptake can account for the entire energy requirement.8 Exercise intensity at the anaerobic threshold depends not only on the power produced but also on many other factors including environmental temperature, the degree of training undertaken by the subject and altitude. An additional factor is the muscle groups that are used to accomplish the work, as different skeletal muscle fibres, and therefore muscle groups, have different metabolic products.1
During severe exercise the lactate level continues to rise (Figure 15.2C) and begins to cause distress at levels above about 11 mmol.l−1, 10 times the normal resting level. Lactate accumulation seems to be the limiting factor for sustained heavy work, and the progressive increase in blood lactate results in the level of work being inversely related to the time for which it can be maintained. Thus there is a reciprocal relationship between the record time for various distances and the speed at which they are run.
Oxygen Debt
The difference between the total work and the aerobic work is achieved by anaerobic metabolism of carbohydrates to lactate, which is ultimately converted to citrate, enters the citric acid cycle and is then fully oxidised (page 199). Like glucose, lactate has a respiratory quotient of 1.0. Although this process continues during heavy exercise, lactate accumulates and the excess is oxidised in the early stages of recovery. Oxygen consumption remains above the resting level during recovery for this purpose. This constitutes the ‘repayment of the oxygen debt’ and is related to the lactate level attained by the end of exercise.
Repayment of the oxygen debt is especially well developed in the diving mammals such as seals and whales. During a dive, their circulation is largely diverted to heart and brain, and the metabolism of the skeletal muscles is almost entirely anaerobic (page 298). On regaining the surface, very large quantities of lactate are suddenly released into the circulation and are rapidly metabolised while the animal is on the surface between dives.
Excess post-exercise oxygen consumption. Sustained heavy exercise results in an increased 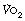 even when the subject’s blood lactate remains only mildly elevated. Excess oxygen consumption may occur for several hours, and is related to both the intensity and duration of exercise undertaken. Previous hypotheses put forward to explain the excess
even when the subject’s blood lactate remains only mildly elevated. Excess oxygen consumption may occur for several hours, and is related to both the intensity and duration of exercise undertaken. Previous hypotheses put forward to explain the excess 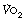 included an increase in body temperature and increased fat metabolism, though proof of these is lacking. Exercise at around 75% of
included an increase in body temperature and increased fat metabolism, though proof of these is lacking. Exercise at around 75% of 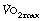 raises levels of catabolic hormones such as cortisol and catecholamines, which may explain the excess
raises levels of catabolic hormones such as cortisol and catecholamines, which may explain the excess 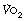 .9
.9
The Ventilatory Response to Exercise
Time course.10 In the previous section it was seen that exercise without a rapid ventilatory response would be dangerous if not fatal. In fact, the respiratory system does respond with great rapidity (Figure 15.4). There is an instant increase in ventilation at, if not slightly before, the start of exercise (phase I). During moderate exercise, there is then a further increase (phase II) to reach an equilibrium level of ventilation (phase III) within about 3 minutes. With heavy exercise there is a secondary increase in ventilation that may reach a plateau, but ventilation continues to rise in severe work. At the end of exercise, the minute volume falls to resting levels within a few minutes. After heavy and severe exercise, return to the resting level of ventilation takes longer, as the oxygen debt is repaid and lactate levels return to normal.
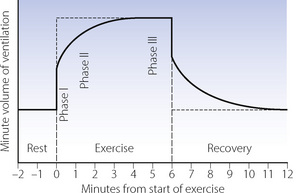
Fig. 15.4 The time course of changes in ventilation in relation to a short period of moderate exercise. Note the instant increase in ventilation at the start of exercise before the metabolic consequences of exercise have had time to develop.
The ventilation equivalent for oxygen. The respiratory minute volume is normally very well matched to the increased oxygen consumption, and the relationship between minute volume and oxygen consumption is approximately linear up to an oxygen consumption of about 2 l.min−1 in the untrained subject and more following training (Figure 15.5). The slope of the linear part is the ventilation equivalent for oxygen and is within the range 20–30 l.min−1 ventilation per l.min−1 of oxygen consumption. The slope does not appear to change with training.
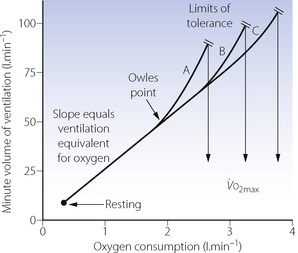
Fig. 15.5 Changes in minute volume of ventilation in response to the increased oxygen consumption of exercise. The break from linearity (Owles point) occurs at higher levels of oxygen consumption in trained athletes, who can also tolerate higher minute volumes. A to C shows progressive levels of training. Both mechanisms combine to enable the trained athlete to increase his maximum oxygen consumption.
In heavy exercise, above a critical level of oxygen consumption (Owles point), the ventilation increases above the level predicted by an extrapolation of the linear part of the ventilation/oxygen consumption relationship (Figure 15.5). This is surplus to the requirement for gas exchange and is accompanied by hypocapnia with arterial Pco2 decreasing by levels of the order of 1 kPa (7.5 mmHg). The excess ventilation is probably driven by lactic acidosis, though other possible explanations exist such as a humoral agent from muscle metabolism affecting the carotid body, possibly potassium.11 In the trained athlete, the break from linearity occurs at higher levels of oxygen consumption. This together with improved tolerance of high minute volumes allows the trained athlete to increase his 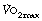 as shown in Figure 15.5.
as shown in Figure 15.5.
Minute volume and dyspnoea. It is generally believed that the ventilatory system does not limit exercise in normal subjects, although the evidence for this view is elusive.12 One study13 found that 50–60% of maximal breathing capacity (MBC) was required for work at 80% of aerobic capacity. However, the breaking point of exercise is usually determined by breathlessness, which occurs when the exercise ventilation utilises a high proportion of the MBC. There is a close correlation between MBC and 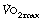 .
.
Minute volumes as great as 200 l.min−1 have been recorded during exercise although the normal subject cannot maintain a minute volume approaching MBC for more than a very short period. Tidal volume during maximal exercise is about half vital capacity, and 70–80% of MBC can normally be maintained, with difficulty, for 15 minutes by fit young subjects.13 Ventilation approximates to 60% of MBC at maximal oxygen consumption. The usable fraction of the MBC can, however, be increased by training.
Diffusing capacity. Diffusion across the alveolar/capillary membrane does not normally limit the increased oxygen consumption at sea level but this is a limiting factor at altitude (see Chapters 9 and 17). Exercise-induced hypoxia, which is seen fairly commonly in elite endurance athletes, is believed to be caused in part by diffusion limitation along with maldistribution of pulmonary ventilation/perfusion ratios and airflow limitation.14
Control of Ventilation
Elucidation of the mechanisms that underlie the remarkably efficient adaptation of ventilation to the demands of exercise has remained a challenge to generations of physiologists, and a complete explanation remains elusive.10,15
Neural factors. It has long been evident that neural factors play an important role, particularly as ventilation normally increases at or even before the start of exercise (phase I), when no other physiological variable has changed except cardiac output (Figure 15.4). There is evidence in humans that the phase I ventilatory response may be in part a ‘learned’ response to the onset of exercise.16 Simply imagining exercising in an otherwise relaxed subject causes an increase in ventilation. Under these conditions, positron emission tomography shows activation of several areas of the cerebral cortex, again indicating that the early increase in ventilation with exercise is a behavioural response.15
Arterial blood gas tensions and the chemoreceptors. There is a large body of evidence that, during exercise at sea level with oxygen consumption up to about 3 l.min−1, there is no significant change in either Pco2 or Po2 of arterial blood. In one study, even at the point of exhaustion (oxygen consumption 3.5 l.min−1), the arterial Po2 was the same as the resting value and Pco2 was reduced. In healthy subjects, blood gas tensions do not therefore seem at first sight to be the main factor governing the increased minute volume. There is a caveat to this conclusion.
ThePo2/ventilation response curve is known to be steeper during exercise (see Figure 5.7), so ventilation will respond to small fluctuations in normal arterial Po2 under these circumstances. Carotid body resection17 or administration of dopamine to inhibit carotid body activity18 reduces the ventilatory response to exercise, particularly phase II (Figure 15.4). Thus it seems likely that the peripheral chemoreceptors are responsible for developing exercise-induced hyperpnoea, particularly during the non-steady state.10,19 This response may not result from changes in Po2, but from oscillations in arterial Pco2.20 Unlike in the resting state when gas flow within the alveolus is by diffusion (page 19) during the deep breathing that accompanies exercise, air flow into the alveoli becomes more tidal in nature, and the arterial Pco2 rises and falls with each breath. The magnitude of these oscillations is believed to affect respiratory drive via the carotid bodies irrespective of the mean Pco2 (to which the central chemoreceptors respond), an effect which is exaggerated under hypoxic conditions.
Humoral mechanisms. Humoral factors play a comparatively minor role in moderate exercise but are more important in heavy and severe exercise when metabolic acidosis is an important factor. Lactic acidosis contributes to excess ventilation during heavy and severe exercise (Figure 15.5), causing a slight reduction in arterial Pco2. Slight additional respiratory drive may result from hyperthermia.
Fitness and Training
The definitions of moderate, heavy and severe exercise at the beginning of this chapter are not transferable between individuals. A given amount of energy expenditure that constitutes severe exercise to a sedentary unfit subject is likely to represent less than moderate exercise to a trained athlete. The linear relationship between power generated and 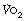 (Figure 15.1) is remarkably consistent irrespective of fitness and training, but the distance a subject may progress along this line, that is their
(Figure 15.1) is remarkably consistent irrespective of fitness and training, but the distance a subject may progress along this line, that is their 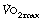 , is extremely variable.
, is extremely variable.
In healthy untrained subjects, rapidly increasing lactate levels normally limit exercise tolerance. Intracellular lactic acidosis in muscles gives rise to weakness and cramp, the respiratory stimulation rapidly takes the subject towards an intolerable minute ventilation, and exhaustion occurs. Training changes many aspects of exercise physiology. For example, improved cardiovascular fitness results in improved oxygen delivery, such that the 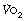 at which lactate rises is greatly increased. Muscle in trained athletes releases less lactate than in untrained subjects (see below), and animal studies indicate that training may improve the ability of the liver to remove circulating lactate.1 Finally, trained athletes can tolerate much higher blood lactate levels, up to 20 mmol.l−1, or twice that of untrained subjects. There are two respiratory aspects of training that merit further consideration.
at which lactate rises is greatly increased. Muscle in trained athletes releases less lactate than in untrained subjects (see below), and animal studies indicate that training may improve the ability of the liver to remove circulating lactate.1 Finally, trained athletes can tolerate much higher blood lactate levels, up to 20 mmol.l−1, or twice that of untrained subjects. There are two respiratory aspects of training that merit further consideration.
Minute volume of ventilation. Maximal expiratory flow rate is limited by flow-dependent airway closure (page 49), and is relatively unaffected by training.13 However, within the limits of MBC, it is possible to increase the strength and endurance of the respiratory muscles. It is therefore possible to improve the fraction of the MBC that can be sustained during exercise. Highly trained athletes may be able to maintain ventilations as much as 90% of their MBC.
Ventilation equivalent for oxygen. There is no evidence that training can alter the slope of the plot of ventilation against oxygen consumption (Figure 15.5). However, the upward inflection of the curve (Owles point) is further to the right in the trained subject. This permits the attainment of a higher oxygen consumption for the same minute volume. Prolongation of the straight part of the curve is achieved by improving metabolic processes in skeletal muscle to minimise the stimulant effect of lactic acid. There is ample evidence that training can improve the aerobic performance of muscles by many adaptations, including, for example, the increased density of the capillary network in the muscles. The consequent reduction in lactic acidosis and therefore the excess ventilation, together with an increase in the tolerable minute volume, combine to increase the 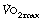 as shown in Figure 15.5. It would seem that the major factor in increasing the
as shown in Figure 15.5. It would seem that the major factor in increasing the 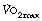 is improved performance of skeletal muscle and the cardiovascular system, rather than any specific change in respiratory function.
is improved performance of skeletal muscle and the cardiovascular system, rather than any specific change in respiratory function.
Cardiorespiratory Disease1,2,21
Patients with cardiovascular or respiratory disease have poor exercise tolerance for three main reasons. First, the ventilatory response to exercise is more rapid so a greater minute volume is required to achieve a given 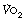 . Secondly, the proportion of MBC that a patient can tolerate is reduced, and when combined with the previous observation this results in an extreme limitation of exercise tolerance before shortness of breath intervenes. Hypoxia or hypercapnia occur more commonly during exercise in patients with respiratory disease. Thirdly, a limited increase in cardiac output in response to exercise means that mixed venous oxygen levels will fall to low levels more quickly, and also causes inadequate muscle blood flow, impairing the function of respiratory and other muscles. Anaerobic metabolism therefore occurs much more quickly, leading to extra ventilatory requirements and exhaustion.
. Secondly, the proportion of MBC that a patient can tolerate is reduced, and when combined with the previous observation this results in an extreme limitation of exercise tolerance before shortness of breath intervenes. Hypoxia or hypercapnia occur more commonly during exercise in patients with respiratory disease. Thirdly, a limited increase in cardiac output in response to exercise means that mixed venous oxygen levels will fall to low levels more quickly, and also causes inadequate muscle blood flow, impairing the function of respiratory and other muscles. Anaerobic metabolism therefore occurs much more quickly, leading to extra ventilatory requirements and exhaustion.
Cardiopulmonary exercise (CPX) testing. The limited respiratory response to exercise seen in patients with cardiorespiratory disease has led to the use of exercise testing as a means of quantifying the extent of their disease. During a progressively increasing workload a variety of measures may be made, including peak 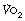 , anaerobic threshold (
, anaerobic threshold (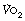 at Owles point), and ventilation equivalent for oxygen (
at Owles point), and ventilation equivalent for oxygen (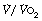 ) and carbon dioxide (
) and carbon dioxide (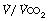 ). These measures are invariably reduced in patients with cardiac and/or respiratory disease, and have been shown to be predictive of poor outcomes in patients with heart failure22 or those having major, high-risk surgery.23
). These measures are invariably reduced in patients with cardiac and/or respiratory disease, and have been shown to be predictive of poor outcomes in patients with heart failure22 or those having major, high-risk surgery.23
References
1. Wasserman K. Coupling of external to cellular respiration during exercise: the wisdom of the body revisited. Am J Physiol.. 1994;266:E519-E539.
2. Poole DC, Barstow TJ, Gaesser GA, Willis WT, Whipp BJ.  slow component: physiological and functional significance. Med Sci Sports Exer.. 1994;26:1354-1358.
slow component: physiological and functional significance. Med Sci Sports Exer.. 1994;26:1354-1358.
3. Burnley M. Found in translation: the dependence of oxygen uptake kinetics on O2 delivery and O2 utilization. J Appl Physiol.. 2008;105:1387-1388.
4. Clark JM, Hagerman FC, Gelfand R. Breathing patterns during submaximal and maximal exercise in elite oarsmen. J Appl Physiol.. 1983;55:440-446.
5. Howley ET, Bassett DR, Welch HG. Criteria for maximal oxygen uptake: review and commentary. Med Sci Sports Exerc.. 1995;27:1292-1301.
6. Aaron EA, Seow KC, Johnson BD, Dempsey JA. Oxygen cost of exercise hyperpnea: implications for performance. J Appl Physiol.. 1992;72:1818-1825.
7. Dempsey JA, Harms CA, Ainsworth DM. Respiratory muscle perfusion and energetics during exercise. Med Sci Sports Exerc.. 1996;28:1123-1129.
8. Svedahl K, MacIntosh BR. Anaerobic threshold: The concepts and methods of measurement. Can J Appl Physiol.. 2003;28:299-323.
9. Quinn TJ, Vroman NB, Kertzer R. Postexercise oxygen consumption in trained females: effect of exercise duration. Med Sci Sports Exerc.. 1994;26:908-913.
10. Whipp BJ. Peripheral chemoreceptor control of exercise hyperpnea in humans. Med Sci Sports Exerc.. 1994;26:337-347.
11. Cotes JE, Chinn DJ, Miller MR. Lung Function. Physiology, Measurement and Application in Medicine. Oxford: Blackwell Publishing; 2006.
12. Bye PTP, Farkas GA, Roussos C. Respiratory factors limiting exercise. Annu Rev Physiol.. 1983;45:439-451.
13. Shephard RJ. The maximum sustained voluntary ventilation in exercise. Clin Sci.. 1967;32:167-176.
14. Dempsey JA. Is the healthy respiratory system (always) built for exercise? J Physiol.. 2006;576:339-340.
15. Thornton JM, Guz A, Murphy K, et al. Identification of higher brain centres that may encode the cardiorespiratory response to exercise in humans. J Physiol.. 2001;533:823-836.
*16. Helbling D, Boutellier U, Spengler CM. Modulation of the ventilatory increase at the onset of exercise in humans. Respir Physiol.. 1997;109:219-229.
17. Wasserman K, Whipp BJ, Koyal SN, Cleary MG. Effect of carotid body resection on ventilatory and acid-base control during exercise. J Appl Physiol.. 1975;39:354-358.
18. Boetger CL, Ward DS. Effect of dopamine on transient ventilatory response to exercise. J Appl Physiol.. 1986;61:2102-2107.
19. Forster HV, Pan LG. The role of carotid chemoreceptors in the control of breathing during exercise. Med Sci Sports Exerc.. 1994;26:328-336.
*20. Collier DJ, Nickol AH, Milledge JS, et al. Alveolar Pco2 oscillations and ventilation at sea level and at high altitude. J Appl Physiol.. 2008;104:404-415.
*21. Epstein FH. Exercise limitation in health and disease. N Engl J Med.. 2000;343:633-641.
22. Gitt AK, Wasserman K, Kilkowski C, et al. Exercise anaerobic threshold and ventilatory efficiency identify heart failure patients for high risk of early death. Circulation. 2002;106:3079-3084.
23. Carlisle J, Swart M. Mid-term survival after abdominal aortic aneurysm surgery predicted by cardiopulmonary exercise testing. Br J Surg.. 2007;94:966-969.