Chapter 2 Functional anatomy of the respiratory tract
 In addition to conducting air to and from the lungs, the nose, mouth and pharynx have other important functions including speech, swallowing and airway protection.
In addition to conducting air to and from the lungs, the nose, mouth and pharynx have other important functions including speech, swallowing and airway protection.This chapter is not a comprehensive account of respiratory anatomy but concentrates on those aspects that are most relevant to an understanding of function. The respiratory muscles are considered in Chapter 6.
Mouth, Nose and Pharynx
Breathing is normally possible through either the nose or the mouth, the two alternative air passages converging in the oro-pharynx. Nasal breathing is the norm and has two major advantages over mouth breathing – filtration of particulate matter by the vibrissae hairs and humidification of inspired gas. Humidification by the nose is highly efficient because the nasal septum and turbinates greatly increase the surface area of mucosa available for evaporation and produce turbulent flow so increasing contact between the mucosa and air. However, the nose may offer more resistance to air flow than the mouth, particularly when obstructed by polyps, adenoids or congestion of the nasal mucosa. Nasal resistance may make oral breathing obligatory and many children and adults breathe only or partly through their mouths at rest. With increasing levels of exercise in normal adults, the respiratory minute volume increases and at a level of about 35 l.min−1 the oral airway comes into play. Deflection of gas into either the nasal or the oral route is under voluntary control and accomplished with the soft palate, tongue and lips. These functions are best considered in relation to a midline sagittal section (Figure 2.1).
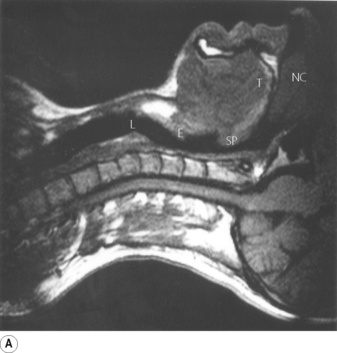
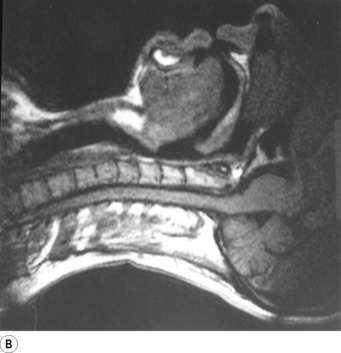
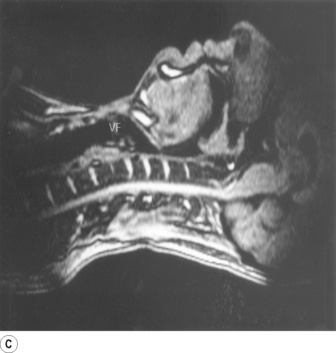
Fig. 2.1 Magnetic resonance imaging scans showing median sagittal sections of the pharynx in a normal subject. (A) Normal nasal breathing with the oral airway occluded by lips and tongue. (B) Deliberate oral breathing with the nasal airway occluded by elevation and backward movement of the soft palate. (C) A Valsalva manoeuvre in which the subject deliberately tries to exhale against a closed airway. Data acquisition for scans (A) and (B) took 45 seconds so anatomical differences between inspiration and expiration will not be visible. I am indebted to Prof M. Bellamy for being the subject. NC, nasal cavity; T, tongue; SP, soft palate; E, epiglottis; VF, vocal fold; L, Larynx.
Part (A) shows the normal position for nose breathing, with the mouth closed by occlusion of the lips, and the tongue lying against the hard palate. The soft palate is clear of the posterior pharyngeal wall.
Part (B) shows forced mouth breathing, as for instance when blowing through the mouth, without pinching the nose. The soft palate becomes rigid and is arched upwards and backwards by contraction of tensor and levator palati to lie against a band of the superior constrictor of the pharynx known as Passavant’s ridge which, together with the soft palate, forms the palatopharyngeal sphincter. Note also that the orifice of the pharyngotympanic (Eustachian) tube lies above the palatopharyngeal sphincter and the tubes can therefore be inflated by the subject only when the nose is pinched. As the mouth pressure is raised, this tends to force the soft palate against the posterior pharyngeal wall to act as a valve. The combined palatopharyngeal sphincter and valvular action of the soft palate is very strong and can easily withstand mouth pressures in excess of 10 kPa (100 cmH2O).
Part (C) shows the occlusion of the respiratory tract during a Valsalva manoeuvre. The airway is occluded at many sites: the lips are closed, the tongue is in contact with the hard palate anteriorly, the palatopharyngeal sphincter is tightly closed, the epiglottis is in contact with the posterior pharyngeal wall, and the vocal folds are closed so becoming visible in the midline in the figure.
During swallowing the nasopharynx is occluded by contraction of both tensor and levator palati. The larynx is elevated 2–3 cm by contraction of the infrahyoid muscles, stylopharyngeus and palatopharyngeus, coming to lie under the epiglottis. In addition, the aryepiglottic folds are approximated causing total occlusion of the entrance to the larynx. This extremely effective protection of the larynx is capable of withstanding pharyngeal pressures as high as 80 kPa (600 mmHg) which may be generated during swallowing.
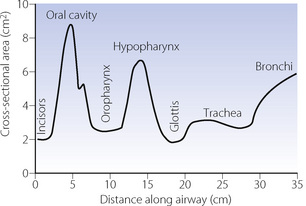
Upper airway cross-sectional areas can be estimated from conventional radiographs, magnetic resonance imaging (MRI) as in Figure 2.1, or acoustic pharyngometry. In the latter technique, a single sound pulse of 100 μs duration is generated within the apparatus and passes along the airway of the subject. Recording of the timing and frequency of sound waves reflected back from the airway allows calculation of cross-sectional area which is then presented as a function of the distance travelled along the airway1 (Figure 2.2). Acoustic pharyngometry measurements correlate well with MRI scans of the airway,2 and the technique is now sufficiently developed for use in clinical situations with real-time results. For example, acoustic pharyngometry has been used following the placement of a tracheal tube to differentiate between oesophageal and tracheal intubation,3 and to estimate airway size in patients with sleep disordered breathing (Chapter 16).4
The Larynx
The larynx evolved in the lungfish for the protection of the airway during such activities as feeding and perfusion of the gills with water. While protection of the airway remains important, the larynx now has many other functions, all involving varying degrees of laryngeal occlusion.
Speech.5 Phonation, the laryngeal component of speech, requires a combination of changes in position, tension and mass of the vocal folds (cords). Rotation of the arytenoid cartilages by the posterior cricoarytenoid muscles opens the vocal folds, while contraction of the lateral cricoarytenoid and oblique arytenoid muscles opposes this. With the vocal folds almost closed, the respiratory muscles generate a positive pressure of 5–35 cmH2O which may then be released by slight opening of the vocal folds to produce sound waves. The cricothyroid muscle tilts the cricoid and arytenoid cartilages backwards and also moves them posteriorly in relation to the thyroid cartilage. This produces up to 50% elongation and therefore tensioning of the vocal folds, an action opposed by the thyroarytenoid muscles, which draw the arytenoid cartilages forwards toward the thyroid and so shorten and relax the vocal folds. Tensioning of the cords results in both transverse and longitudinal resonance of the vocal fold allowing the formation of complex sound waves. The deeper fibres of the thyroarytenoids comprise the vocales muscles, which exert fine control over pitch of the voice by slight variations in both the tension and mass of the vocal folds. A more dramatic example of the effect of vocal fold mass on voice production occurs with inflammation of the laryngeal mucosa and the resulting hoarse voice or complete inability to phonate.
Effort closure. Tighter occlusion of the larynx, known as effort closure, is required for making expulsive efforts. It is also needed to lock the thoracic cage and so to secure the origin of the muscles of the upper arm arising from the rib cage, thus increasing the power which can be transmitted to the arm. In addition to simple apposition of the vocal folds described above, the aryepiglottic muscles and their continuation, the oblique and transverse arytenoids, act as a powerful sphincter capable of closing the inlet of the larynx, by bringing the aryepiglottic folds tightly together. The full process enables the larynx to withstand the highest pressures which can be generated in the thorax, usually at least 12 kPa (120 cmH2O) and often more. Sudden release of the obstruction is essential for effective coughing, when the linear velocity of air through the larynx is said to approach the speed of sound.
Laryngeal muscles are involved in controlling airways resistance, particularly during expiration, and this aspect of vocal fold function is described in Chapter 6.
The Tracheobronchial Tree
An accurate and complete model of the branching pattern of the human bronchial tree remains elusive, though several different models have been described.6 The most useful and widely accepted approach remains that of Weibel7 who numbered successive generations of air passages from the trachea (generation 0) down to alveolar sacs (generation 23). This ‘regular dichotomy’ model assumes that each bronchus regularly divides into two approximately equal size daughter bronchi. As a rough approximation it may therefore be assumed that the number of passages in each generation is double that in the previous generation, and the number of air passages in each generation is approximately indicated by the number 2 raised to the power of the generation number. This formula indicates one trachea, two main bronchi, four lobar bronchi, sixteen segmental bronchi, etc. However, this mathematical relationship is unlikely to be true in practice where bronchus length is variable, pairs of daughter bronchi are often unequal in size, and trifurcations may occur.
Work using computerised tomography to reconstruct, in three dimensions, the branching pattern of the airways has shown that a regular dichotomy system does occur for at least the first six generations.8 Beyond this point, the same study demonstrated trifurcation of some bronchi, and airways that terminated at generation 8. Table 2.1 traces the characteristics of progressive generations of airways in the respiratory tract.
Table 2.1 Structural characteristics of the air passages9
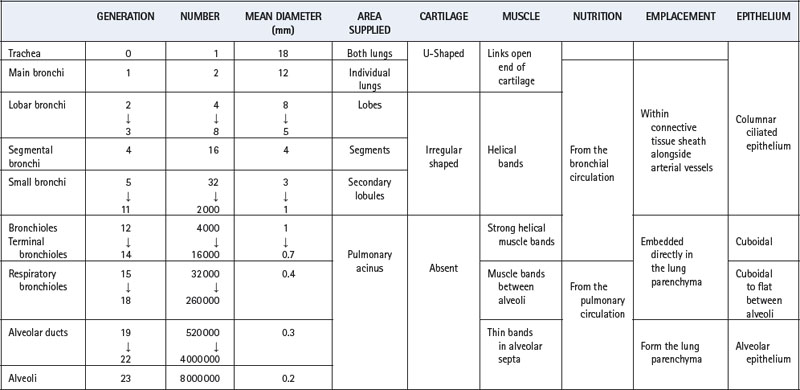
Trachea (Generation 0)
The adult trachea has a mean diameter of 1.8 cm and length of 11 cm. It is supported by U-shaped cartilages which are joined posteriorly by smooth muscle bands. The part of the trachea in the neck is not subjected to intrathoracic pressure changes, but it is very vulnerable to pressures arising in the neck due, for example, to tumours or haematoma formation. An external pressure of the order of 4 kPa (40 cmH2O) is sufficient to occlude the trachea. Within the chest, the trachea can be compressed by raised intrathoracic pressure during, for example, a cough, when the decreased diameter increases the linear velocity of gas flow and therefore the efficiency of removal of secretions.
Main, Lobar and Segmental Bronchi (Generations 1–4)
The trachea bifurcates asymmetrically, with the right bronchus being wider and making a smaller angle with the long axis of the trachea. Foreign bodies therefore tend to enter the right bronchus in preference to the left. Main, lobar and segmental bronchi have firm cartilaginous support in their walls, U-shaped in the main bronchi, but in the form of irregularly shaped and helical plates lower down with bronchial muscle between. Bronchi in this group (down to generation 4) are sufficiently regular to be individually named (Figure 2.3). Total cross-sectional area of the respiratory tract is minimal at the third generation (Figure 2.4).
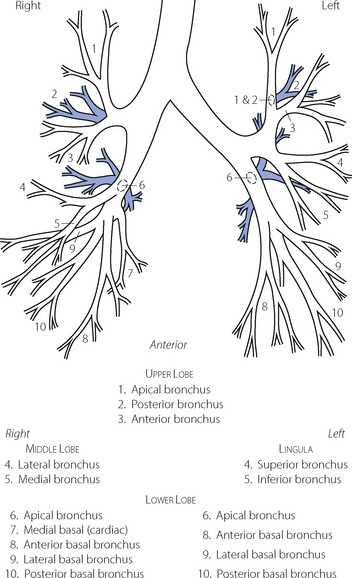
Fig. 2.3 Named branches of the tracheobronchial tree, viewed from the front.
(Reproduced by permission of the editors of Thorax.)
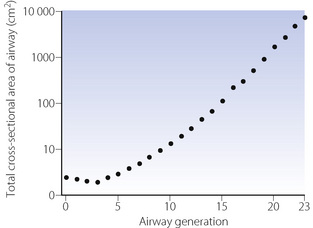
Fig. 2.4 The total cross-sectional area of the air passages at different generations of the airways. Note that the minimum cross-sectional area is at generation 3 (lobar to segmental bronchi). The total cross-sectional area becomes very large in the smaller air passages, approaching a square metre in the alveolar ducts.
These bronchi are subjected to the full effect of changes in intrathoracic pressure and will collapse when the intrathoracic pressure exceeds the intraluminar pressure by about 5 kPa (50 cmH2O). This occurs in the larger bronchi during a forced expiration so limiting peak expiratory flow rate (see Figure 4.7).
Small Bronchi (Generations 5–11)
The small bronchi extend through about seven generations with their diameter progressively falling from 3.5 to 1 mm. Down to the level of the smallest true bronchi, air passages lie in close proximity to branches of the pulmonary artery in a sheath containing pulmonary lymphatics, which can be distended with oedema fluid giving rise to the characteristic ‘cuffing’ that is responsible for the earliest radiographic changes in pulmonary oedema. Because these air passages are not directly attached to the lung parenchyma they are not subject to direct traction and rely for their patency on cartilage within their walls and on the transmural pressure gradient, which is normally positive from lumen to intrathoracic space. In the normal subject this pressure gradient is seldom reversed and, even during a forced expiration, the intraluminar pressure in the small bronchi rapidly rises to more than 80% of the alveolar pressure, which is more than the extramural (intrathoracic) pressure.
Bronchioles (Generations 12–14)
An important change occurs at about the eleventh generation where the internal diameter is approximately 1 mm. Cartilage disappears from the wall below this level and ceases to be a factor in maintaining patency. However, beyond this level the air passages are directly embedded in the lung parenchyma, the elastic recoil of which holds the air passages open like the guy ropes of a tent. Therefore the calibre of the airways beyond the eleventh generation is mainly influenced by lung volume, since the forces holding their lumina open are stronger at higher lung volumes. The converse of this factor causes airway closure at reduced lung volume (see Chapter 4). In succeeding generations, the number of bronchioles increases far more rapidly than the calibre diminishes (Table 2.1). Therefore the total cross-sectional area increases until, in the terminal bronchioles, it is about 100 times the area at the level of the large bronchi (Figure 2.4). Thus the flow resistance of these smaller air passages (less than 2 mm diameter) is negligible under normal conditions. However, the resistance of the bronchioles can increase to very high values when their strong helical muscular bands are contracted by the mechanisms described in Chapters 4 and 28. Down to the terminal bronchiole, the air passages derive their nutrition from the bronchial circulation and are thus influenced by systemic arterial blood gas levels. Beyond this point the smaller air passages rely upon the pulmonary circulation for their nutrition.
Respiratory Bronchioles (Generations 15–18)
Down to the smallest bronchioles, the functions of the air passages are solely conduction and humidification. Beyond this point there is a gradual transition from conduction to gas exchange. In the four generations of respiratory bronchioles there is a gradual increase in the number of alveoli in their walls. Like the bronchioles, the respiratory bronchioles are embedded in lung parenchyma, however, they have a well defined muscle layer with bands which loop over the opening of the alveolar ducts and the mouths of the mural alveoli. There is no significant change in calibre of advancing generations of respiratory bronchioles (approximately 0.4 mm diameter).
Alveolar Ducts (Generations 19–22)
Alveolar ducts arise from the terminal respiratory bronchiole, from which they differ by having no walls other than the mouths of mural alveoli (about 20 in number). The alveolar septa comprise a series of rings forming the walls of the alveolar ducts and containing smooth muscle. Some 35% of the alveolar gas resides in the alveolar ducts and the alveoli that arise directly from them.
Alveolar Sacs (Generation 23)
The last generation of the air passages differ from alveolar ducts solely in the fact that they are blind. It is estimated that about 17 alveoli arise from each alveolar sac and account for about half of the total number of alveoli.
Pulmonary Acinus
A pulmonary acinus is usually defined as the region of lung supplied by a first order respiratory bronchiole and includes the respiratory bronchioles, alveolar ducts and alveolar sacs distal to a single terminal bronchiole (Figure 2.5). This represents generations 15–23 above, but in practice the number of generations within a single acinus is quite variable being between 6 and 12 divisions beyond the terminal bronchiole. A human lung contains about 30 000 acini,9 each with a diameter of about 3.5 mm and containing in excess of 10 000 alveoli. A single pulmonary acinus is probably the equivalent of the alveolus when it is considered from a functional standpoint as gas movement within the acinus is by diffusion rather than tidal ventilation. Acinar morphometry therefore becomes crucial,10 in particular the path length between the start of the acinus and the most distal alveolus which in man is between 5 and 12 mm.9
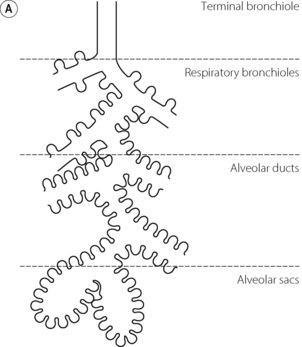
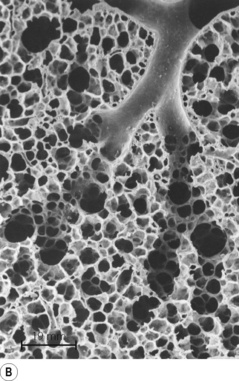
Fig. 2.5 (A) Schematic diagram of a single pulmonary acinus showing four generations between the terminal bronchiole and the alveolar sacs. The average number of generations in human lung is eight, but may be as many as 12. (B) Section of rabbit lung showing respiratory bronchioles leading to alveolar ducts and sacs. Human alveoli would be considerably larger. The scale bar is 0.5 mm.
(Photograph kindly supplied by Professor E. R. Weibel.)
Respiratory Epithelium11,12
Before inspired air reaches the alveoli it must be humidified, and airborne particles, pathogens and irritant chemicals removed. These tasks are undertaken by the respiratory epithelium and its overlying layer of airway lining fluid, and are described in Chapter 12. To facilitate these functions the respiratory epithelium contains numerous cell types.
Ciliated epithelial cells. These are the most abundant cell type in the respiratory epithelium. In the nose, pharynx and larger airways the epithelial cells are pseudo-stratified, gradually changing to a single layer of columnar cells in bronchi, cuboidal cells in bronchioles, and finally thinning further to merge with the Type I alveolar epithelial cells (see below). They are differentiated from either basal or secretory cells (see below) and are characterised by the presence of around 300 cilia per cell (page 218).
Goblet cells.13 These are present at a density of about 6000 per mm2 (in the trachea) and are responsible for producing the thick layer of mucous that lines all but the smallest conducting airways (page 218).
Submucosal secretory cells. Submucosal glands occur in the larger bronchi and in the trachea; in the latter there are about 10 submucosal openings per mm2. The glands comprise both serous cells and mucous cells, with serous cells occurring in the gland acinus, while mucous cells are found closer to the collecting duct. The serous cells have the highest levels of membrane-bound cystic fibrosis transmembrane conductance regulator (CFTR) in the lung (Chapter 28).
Basal cells. These cells lie underneath the columnar cells giving rise to the pseudo-stratified appearance, and are absent in the bronchioles and beyond. They are probably the stem cell responsible for producing new epithelial and goblet cells.
Mast cells. The lungs contain numerous mast cells which are located underneath the epithelial cells of the airways as well as in the alveolar septa. Some also lie free in the lumen of the airways and may be recovered by bronchial lavage. Their important role in bronchoconstriction is described in Chapter 28.
Non-ciliated bronchiolar epithelial (Clara) cells. These cells are found in the mucosa of the terminal bronchioles where they may be the precursor of epithelial cells in the absence of basal cells. They are metabolically active,14 secreting surfactant proteins A, B and D (page 29), antiprotease enzymes and a variety of other proteins whose functions are mostly unknown though some are involved in the metabolism of chemical toxins.
Neuro-epithelial cells. These cells are found throughout the bronchial tree, but occur in larger numbers in the intrapulmonary and terminal bronchioles. They may be found individually or in clusters as neuro-epithelial bodies, and are of uncertain function in the adult lung.15 Present in fetal lung tissue in a greater number they may be responsible for controlling lung development. Similar cells elsewhere in the body secrete a variety of amines and peptides with diverse effects such as calcitonin, gastrin releasing peptide, calcitonin gene related peptide and serotonin.
The Alveoli
The mean total number of alveoli has been estimated as 480 million, but ranges from about 270 million to 790 million, correlating with the height of the subject and total lung volume.16 The size of the alveoli is proportional to lung volume but due to gravity they are normally larger in the upper part of the lung except at maximal inflation when the vertical gradient in size disappears. At functional residual capacity the mean diameter is 0.2 mm.
The Alveolar Septa
The septa are under tension generated partly by collagen and elastin fibres, but more by surface tension at the air–fluid interface (page 28). They are therefore generally flat, making the alveoli polyhedral rather than spherical. The septa are perforated by small fenestrations known as the pores of Kohn (Figure 2.6), which provide collateral ventilation between alveoli. Collateral ventilation also occurs between small bronchioles and neighbouring alveoli, adjacent pulmonary acini and occasionally intersegmental communications,17 and is more pronounced in patients with emphysema (page 410).18
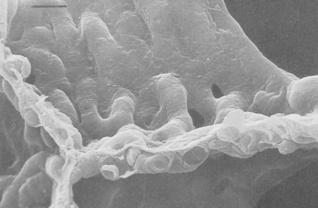
Fig. 2.6 Scanning electron micrograph of the junction of three alveolar septa which are shown in both surface view and section showing the polyhedral structure. Two pores of Kohn are seen to the right of centre. Red blood cells are seen in the cut ends of the capillaries. The scale bar is 10 μm.
(Reproduced from reference 19 by permission of the author and the publishers; © Harvard University Press.)
On one side of the alveolar wall the capillary endothelium and the alveolar epithelium are closely apposed, with almost no interstitial space, such that the total thickness from gas to blood is about 0.3 μm (Figures 2.7 and 2.8). This may be considered the ‘active’ side of the capillary and gas exchange must be more efficient on this side. The other side of the capillary, which may be considered the ‘service’ side, is usually more than 1–2 μm thick and contains a recognisable interstitial space containing elastin and collagen fibres, nerve endings and occasional migrant polymorphs and macrophages. The distinction between the two sides of the capillary has considerable pathophysiological significance as the active side tends to be spared in the accumulation of both oedema fluid and fibrous tissue (Chapter 29).
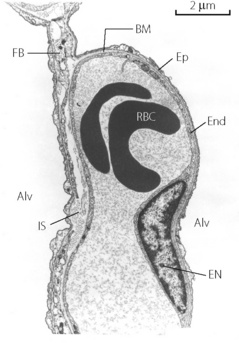
Fig. 2.7 Details of the interstitial space, the capillary endothelium and alveolar epithelium. Thickening of the interstitial space is confined to the left of the capillary (the ‘service’ side) while the total alveolar/capillary membrane remains thin on the right (the ‘active’ side) except where it is thickened by the endothelial nucleus. Alv, alveolus; BM, basement membrane; EN endothelial nucleus; End, endothelium; Ep, epithelium; IS, interstitial space; RBC, red blood cell; FB, fibroblast process.
(Electron micrograph kindly supplied by Professor E. R. Weibel.)
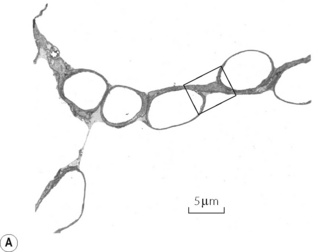
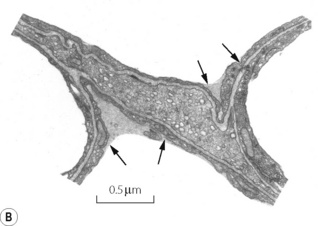
Fig. 2.8 (A) Transmission electron micrograph of alveolar septum with lung inflated to 40% of total lung capacity. The section in the box is enlarged in (B) to show alveolar lining fluid, which has pooled in two concavities of the alveolar epithelium and has also spanned the pore of Kohn in (A). There is a thin film of osmophilic material (arrows), probably surfactant, at the interface between air and the alveolar lining fluid.
(Reproduced from reference 20 by permission of the authors and the Editors of Journal of Applied Physiology.)
The fibre scaffold. The alveolar septum contains a network of fibres which forms a continuum between the peripheral fibres and the axial spiral fibres of the bronchioles. The septal fibre is in the form of a network, through which are threaded the pulmonary capillaries, which are themselves a network. Thus the capillaries pass repeatedly from one side of the fibre scaffold to the other (Figure 2.6), the fibre always residing on the thick (or ‘service’) side of the capillary allowing the other side to bulge into the lumen of the alveolus. The left side of the capillary in Figure 2.7 is the side with the fibres. Structural integrity of the fibre scaffold is believed to be maintained by the individual fibres being under tension, such that when any fibres are damaged, the alveolar septum disintegrates and adjacent alveoli change shape, ultimately leading to emphysema (page 410).21
At the cellular level, the scaffolding for the alveolar septa is provided by the basement membrane, which provides the blood–gas barrier with enough strength to withstand the enormous forces applied to lung tissue.22,23 At the centre of the basement membrane is a layer of type IV collagen, the lamina densa, approximately 50 nm thick and made up of many layers of a diamond-shaped matrix of collagen molecules. On each side of the lamina densa, the collagen layer is attached to the alveolar or endothelial cells by a series of proteins collectively known as laminins, of which seven sub-types are now known. The laminins are more than simple structural molecules, having complex interactions with membrane proteins and the intracellular cytoskeleton24 to help regulate cell shape and permeability etc. These aspects of the function of the basement membrane are important. It has been shown that increases in the capillary transmural pressure gradient above about 3 kPa (30 cmH2O) may cause disruption of endothelium and/or epithelium, while the basement membrane tends to remain intact, sometimes as the only remaining separation between blood and gas.25
Alveolar Cell Types
Capillary endothelial cells. These cells are continuous with the endothelium of the general circulation and, in the pulmonary capillary bed, have a thickness of only 0.1 μm except where expanded to contain nuclei (Figure 2.7). Electron microscopy shows the flat parts of the cytoplasm to be devoid of all organelles except for small vacuoles (caveolae or plasmalemmal vesicles) which may open onto the basement membrane or the lumen of the capillary or be entirely contained within the cytoplasm (see Figure 2.8). The endothelial cells abut against one another at fairly loose junctions which are of the order of 5 nm wide.26 These junctions permit the passage of quite large molecules, and the pulmonary lymph contains albumin at about half the concentration in plasma. Macrophages pass freely through these junctions under normal conditions, and polymorphs can also pass in response to chemotaxis (page 455).
Alveolar epithelial cells – type I. These cells line the alveoli and also exist as a thin sheet approximately 0.1 μm in thickness, except where expanded to contain nuclei. Like the endothelium, the flat part of the cytoplasm is devoid of organelles except for small vacuoles. Epithelial cells each cover several capillaries and are joined into a continuous sheet by tight junctions with a gap of only about 1 nm.26 These junctions may be seen as narrow lines snaking across the septa in Figure 2.6. The tightness of these junctions is crucial for prevention of the escape of large molecules, such as albumin, into the alveoli, thus preserving the oncotic pressure gradient essential for the avoidance of pulmonary oedema (see page 420). Nevertheless, these junctions permit the free passage of macrophages and polymorphs may also pass in response to a chemotactic stimulus. Figure 2.8 shows the type I cell covered with a film of alveolar lining fluid. Type I cells are end cells and do not divide in vivo. Their position in the lung means alveolar epithelial cells are exposed to the highest concentrations of oxygen of any mammalian cell, and they are sensitive to damage from high concentrations of oxygen (Chapter 26), but recent work suggests they may have active systems for preventing oxidative stress.27
Alveolar epithelial cells – type II. These are the stem cells from which type I cells arise.28 They do not function as gas exchange membranes, and are rounded in shape and situated at the junction of septa. They have large nuclei and microvilli (Figure 2.9). The cytoplasm contains characteristic striated osmiophilic organelles that contain stored surfactant (page 29). Type II cells are also involved in pulmonary defence mechanisms in that they may secrete cytokines and contribute to pulmonary inflammation. They are resistant to oxygen toxicity, tending to replace type I cells after prolonged exposure to high concentrations of oxygen (Chapter 26).
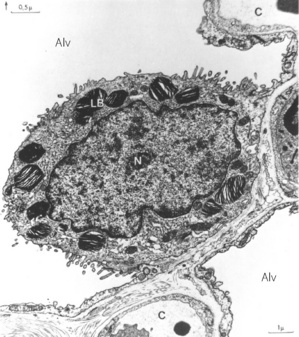
Fig. 2.9 Electron micrograph of a type II alveolar epithelial cell of a dog. Note the large nucleus, the microvilli and the osmiophilic lamellar bodies thought to release surfactant. Alv, alveolus; C, capillary; LB, lamellar bodies; N, nucleus.
(Reproduced from reference 29 by permission of Professor E. R. Weibel and the Editors of Physiological Reviews.)
Alveolar macrophages. The lung is richly endowed with these phagocytes which pass freely from the circulation, through the interstitial space and thence through the gaps between alveolar epithelial cells to lie on their surface within the alveolar lining fluid (Figure 2.10). They can re-enter the body but are remarkable for their ability to live and function outside the body. Macrophages form the major component of host defence within the alveoli, being active in combating infection and scavenging foreign bodies such as small dust particles. They contain a variety of destructive enzymes but are also capable of generating reactive oxygen species (Chapter 26). These are highly effective bactericidal agents but their presence in lung tissue may rebound to damage the host. Dead macrophages release the enzyme trypsin, which may cause tissue damage in patients who are deficient in the protein α1-antitrypsin.
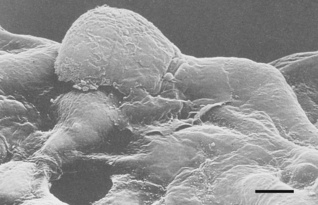
Fig. 2.10 Scanning electron micrograph of an alveolar macrophage advancing to the right over epithelial type I cells. The scale bar is 3 μm.
(Reproduced from reference 19 by permission of the author and the publishers; © Harvard University Press.)
The Pulmonary Vasculature
Pulmonary Arteries
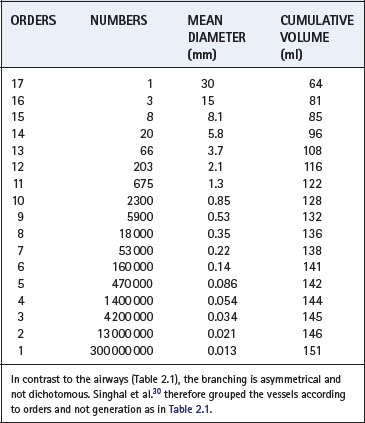
Although the pulmonary circulation carries roughly the same flow as the systemic circulation, the arterial pressure and the vascular resistance are normally only one-sixth as great. The media of the pulmonary arteries is about half as thick as in systemic arteries of corresponding size. In the larger vessels it consists mainly of elastic tissue but in the smaller vessels it is mainly muscular, the transition being in vessels of about 1 mm diameter. Pulmonary arteries lie close to the corresponding air passages in connective tissue sheaths. Table 2.2 shows a scheme for consideration of the branching of the pulmonary arterial tree.30 This may be compared with Weibel’s scheme for the airways (Table 2.1).
Pulmonary Arterioles
The transition to arterioles occurs at an internal diameter of 100 μm. These vessels differ radically from their counterparts in the systemic circulation, being virtually devoid of muscular tissue. There is a thin media of elastic tissue separated from the blood by endothelium. Structurally there is no real difference between pulmonary arterioles and venules.
Pulmonary Capillaries
Pulmonary capillaries tend to arise abruptly from much larger vessels, the pulmonary metarterioles. The capillaries form a dense network over the walls of one or more alveoli and the spaces between the capillaries are similar in size to the capillaries themselves (Figure 2.6). In the resting state, about 75% of the capillary bed is filled but the percentage is higher in the dependent parts of the lungs. Inflation of the alveoli reduces the cross-sectional area of the capillary bed and increases resistance to blood flow (Chapter 7). One capillary network is not confined to one alveolus but passes from one alveolus to another and blood traverses a number of alveolar septa before reaching a venule. This clearly has a bearing on the efficiency of gas exchange. From the functional standpoint it is often more convenient to consider the pulmonary microcirculation rather than just the capillaries. The microcirculation is defined as the vessels that are devoid of a muscular layer and it commences with arterioles of diameter 75 μm and continues through the capillary bed as far as venules of diameter 200 μm. Special roles of the microcirculation are considered in Chapters 12 and 29.
Pulmonary Venules and Veins
Pulmonary capillary blood is collected into venules that are structurally almost identical to the arterioles. In fact, Duke31 obtained satisfactory gas exchange when an isolated cat lung was perfused in reverse. The pulmonary veins do not run alongside the pulmonary arteries but lie some distance away, close to the septa which separate the segments of the lung.
Bronchial Circulation32
Down to the terminal bronchioles, the air passages and the accompanying blood vessels receive their nutrition from the bronchial vessels which arise from the systemic circulation. The bronchial circulation therefore provides the heat required for warming and humidification of inspired air, and cooling of the respiratory epithelium causes vasodilation and an increase in the bronchial artery blood flow. About one-third of the bronchial circulation returns to the systemic venous system, the remainder draining into the pulmonary veins, thereby constituting a physiological shunt (page 132).
The bronchial circulation also differs from the pulmonary circulation in its capacity for angiogenesis.33 Pulmonary vessels have very limited ability to remodel themselves in response to pathological changes while bronchial vessels, like other systemic arteries, can undergo prolific angiogenesis. As a result, the blood supply to most lung cancers (Chapter 30) is derived from the bronchial circulation.
Pulmonary Lymphatics
There are no lymphatics visible in the interalveolar septa, but small lymph vessels commence at the junction between alveolar and extra-alveolar spaces. There is a well developed lymphatic system around the bronchi and pulmonary vessels, capable of containing up to 500 ml of lymph, and draining towards the hilum. Down to airway generation 11 the lymphatics lie in a potential space around the air passages and vessels, separating them from the lung parenchyma. This space becomes distended with lymph in pulmonary oedema and accounts for the characteristic butterfly shadow of the chest radiograph. In the hilum of the lung, the lymphatic drainage passes through several groups of tracheobronchial lymph glands, where they receive tributaries from the superficial subpleural plexus. Most of the lymph from the left lung usually enters the thoracic duct whilst the right side drains into the right lymphatic duct. However, the pulmonary lymphatics often cross the midline and pass independently into the junction of the internal jugular and subclavian veins on the corresponding sides of the body.
References
1. Hoffstein V, Fredberg JJ. The acoustic reflection technique for non-invasive assessment of upper airway area. Eur Respir J. 1991;4:602-611.
2. Marshall I, Maran NJ, Martin S, et al. Acoustic reflectometry for airway measurements in man: implementation and validation. Physiol Meas. 1993;14:157-169.
3. Raphael DT. Acoustic reflectometry profiles of endotracheal and esophageal intubation. Anesthesiology. 2000;92:1293-1299.
4. Patel SR, Frame JM, Larkin EK, Redline S. Heritability of upper airway dimensions derived using acoustic pharyngometry. Eur Respir J. 2008;32:1304-1308.
5. Bannister LH. Anatomy of speech. In: Williams PL, editor. Gray’s Anatomy. London: Churchill-Livingstone; 1995:1651-1652.
6. Phillips CG, Kaye SR, Schroter RC. A diameter-based reconstruction of the branching pattern of the human bronchial tree. Part I. Description and application. Respir Physiol. 1994;98:193-217.
7. Weibel ER. Why measure lung structure? Am J Respir Crit Care Med. 2001;163:314-315.
8. Sauret V, Halson PM, Brown IW, Fleming JS, Bailey AG. Study of the three-dimensional geometry of the central conducting airways in man using computed tomographic (CT) images. J Anat. 2002;200:123-134.
9. Haefeli-Bleuer B, Weibel ER. Morphometry of the human pulmonary acinus. Anat Rec. 1988;220:401-414.
10. Sapoval B, Filoche M, Weibel ER. Smaller is better – but not too small: A physical scale for the design of the mammalian pulmonary acinus. Proc Natl Acad Sci USA. 2002;99:10411-10416.
11. Jeffery PK. Microscopic structure of normal lung. In: Brewis RAL, Corrin B, Geddes DM, Gibson GJ, editors. Respiratory Medicine. London: WB Saunders Company Ltd; 1995:54-72.
12. Knight DA, Holgate ST. The airway epithelium: structural and functional properties in health and disease. Respirology. 2003;8:432-436.
13. Rogers DF. Airway goblet cells: responsive and adaptable frontline defenders. Eur Respir J. 1994;7:1690-1706.
14. Singh G, Katyal SL. Clara cell proteins. Ann N Y Acad Sci. 2000;923:43-58.
15. Gosney J. Pulmonary neuroendocrine cell system in pediatric and adult lung disease. Micros Res Tech. 1997;37:107-113.
16. Ochs M, Nyengaard JR, Jung L, et al. The number of alveoli in the human lung. Am J Respir Crit Care Med. 2004;169:120-124.
17. Topol M. Collateral respiratory pathways of pulmonary acini in man. Folia Morphol. 1995;54:61-66.
18. Cetti EJ, Moore AJ, Geddes DM. Collateral ventilation. Thorax. 2006;61:371-373.
19. Weibel ER. The pathway for oxygen. Cambridge, Mass.: Harvard University Press; 1984.
20. Gil J, Bachofen H, Gehr P, Weibel ER. Alveolar volume-surface area relation in air and saline filled lungs fixed by vascular perfusion. J Appl Physiol. 1979;47:990-995.
*21. Weibel ER. How to make an alveolus. Eur Respir J. 2008;31:483-485.
22. Suki B, Ito S, Stamenovic D, Lutchen KR, Ingenito EP. Biomechanics of the lung parenchyma: critical roles of collagen and mechanical forces. J Appl Physiol. 2005;98:1892-1899.
23. Maina JN, West JB. Thin and strong! The bioengineering dilemma in the structural and functional design of the blood-gas barrier. Physiol Rev. 2005;85:811-844.
*24. Dudek SM, Garcia JGN. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91:1487-1500.
25. Tsukimoto K, Mathieu-Costello O, Prediletto R, Elliott AR, West JB. Ultrastructural appearances of pulmonary capillaries at high transmural pressures. J Appl Physiol. 1991;71:573-582.
26. DeFouw DO. Ultrastructural features of alveolar epithelial transport. Am Rev Respir Dis. 1983;127:S9-S11.
27. Berthiaume Y, Voisin G, Dagenais A. The alveolar type I cells: the new knight of the alveolus? J Physiol. 2006;572:609-610.
28. Uhal BD. Cell cycle kinetics in the alveolar epithelium. Am J Physiol. 1997;272:L1031-L1045.
29. Weibel ER. Morphological basis of alveolar-capillary gas exchange. Physiol Rev. 1973;53:419.
30. Singhal S, Henderson R, Horsfield K, Harding K, Cumming G. Morphometry of the human pulmonary arterial tree. Circ Res. 1973;33:190-197.
31. Duke HN. The site of action of anoxia on the pulmonary blood vessels of the cat. J Physiol. 1954;125:373.
32. Paredi P, Barnes PJ. The airway vasculature: recent advances and clinical implications. Thorax. 2009;64:444-450.
33. Mitzner W, Wagner EM. Vascular remodeling in the circulations of the lung. J Appl Physiol. 2004;97:1999-2004.