Chapter 18 High pressure and diving
 When diving in water the increased density of inhaled gases and immersion in water cause an increase in the work of breathing, which can impair gas exchange during exercise.
When diving in water the increased density of inhaled gases and immersion in water cause an increase in the work of breathing, which can impair gas exchange during exercise.Humans have sojourned temporarily in high-pressure environments since the introduction of the diving bell. The origin of this development is lost in antiquity but Alexander the Great was said to have been lowered to the seabed in a diving bell.
The environment of the diver is often, but not invariably, aqueous. Saturation divers spend most of their time in a gaseous environment in chambers that are held at a pressure close to that of the depth of water at which they will be working. Tunnel and caisson workers may also be at high pressure in a gaseous environment. Those in an aqueous environment also have the additional effect of different gravitational forces applied to their trunks, which influence the mechanics of breathing and other systems of the body. Workers in both environments share the physiological problems associated with increased ambient pressures and partial pressures of respired gases.
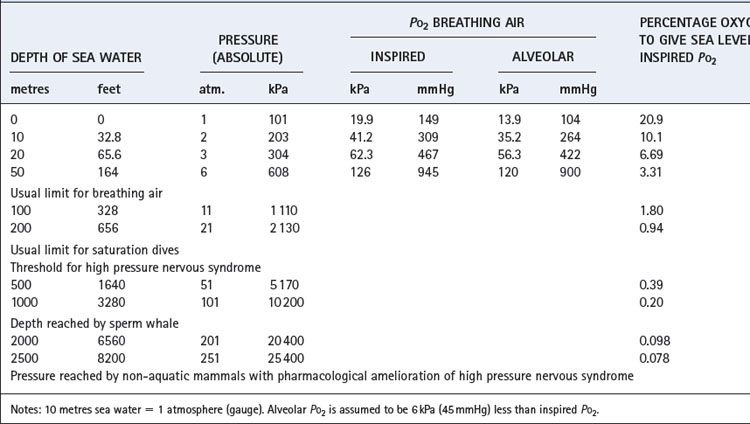
In this field, as in others, we cannot escape from the multiplicity of units, and some of these are set out in Table 18.1. Note particularly that ‘atmosphere gauge’ is relative to ambient pressure. Thus 2 atmospheres absolute (ATA) equals 1 atmosphere gauge relative to sea level. Throughout this chapter atmospheres of pressure refer to absolute and not gauge.
Exchange of Oxygen and Carbon Dioxide1
Effect of Pressure on Alveolar Pco2 and Po2
Pressure has complicated and very important effects on Pco2 and Po2. The alveolar concentration of CO2 equals its rate of production divided by the alveolar ventilation (page 167). However, both gas volumes must be measured under the same conditions of temperature and pressure. Alveolar CO2 concentration at 10 ATA will be about one tenth of sea level values, i.e. 0.56% compared with 5.3% at sea level. When these concentrations are multiplied by pressure to give Pco2, values are similar at sea level and 10 atmospheres. Thus, as a rough approximation, alveolar CO2 concentration decreases inversely to the environmental pressure, but the Pco2 remains near its sea level value.
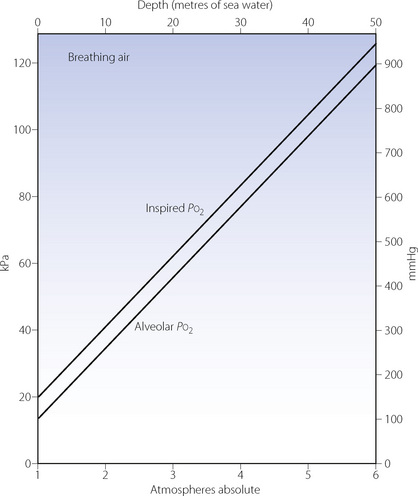
Effects on the Po2 are slightly more complicated but no less important. The difference between the inspired and alveolar oxygen concentrations equals the ratio of oxygen uptake to inspired alveolar ventilation. This fraction, like the alveolar CO2 concentration, decreases inversely with the increased pressure. However, the corresponding partial pressure will remain close to the sea level value, as does the alveolar Pco2. Therefore the difference between the inspired and alveolar Po2 will remain roughly constant, and the alveolar Po2, to a first approximation, increases by the same amount as the inspired Po2 (Figure 18.1). However, these considerations only take into account the direct effect of pressure on gas partial pressures. There are other, more subtle, effects on respiratory mechanics and gas exchange which must now be considered.
Effect on Mechanics of Breathing2,3,4
Two main factors must be considered. First, there is the increased density of gases at pressure, although this can be reduced by changing the composition of the inspired gas. The second factor is the pressure of water on the body, which alters the gravitational effects to which the respiratory system is normally exposed.
Gas density is increased in direct proportion to pressure. Thus air at 10 atmospheres has 10 times the density of air at sea level, which increases the resistance to turbulent gas flow (page 45) and limits the maximal breathing capacity (MBC) that can be achieved. In fact, it is usual to breathe a helium/oxygen mixture at pressures in excess of about 6 atmospheres because of nitrogen narcosis (see below). Helium has only one-seventh the density of air and so is easier to breathe. Furthermore, lower inspired oxygen concentrations are both permissible and indeed desirable as the pressure increases (Table 18.1). Therefore, at 15 atmospheres it would be reasonable to breathe a mixture of 98% helium and 2% oxygen. This would more than double the MBC that the diver could attain while breathing air at that pressure. Hydrogen has even lower density than helium, and has been used in gas mixtures for dives to more than 500 metres deep.5
The effect of immersion is additional to any change in the density of the respired gases. In open-tube snorkel breathing, the alveolar gas is close to normal atmospheric pressure but the trunk is exposed to a pressure depending on the depth of the subject, which is limited by the length of the snorkel tube. This is equivalent to a standing subatmospheric pressure applied to the mouth and it is difficult to inhale against a ‘negative’ pressure loading of more than about 5 kPa (50 cmH2O). This corresponds to a mean depth of immersion of only 50 cm, and it is therefore virtually impossible to use a snorkel tube at a depth of 1 metre. However, the normal length of a snorkel tube assures that the swimmer is barely more than awash, and so these problems should not arise.
‘Negative’ pressure loading is prevented by supplying gas to the diver’s airway at a pressure that is close to the hydrostatic pressure surrounding the diver. This may be achieved by providing an excess flow of gas with a pressure-relief valve controlled by the surrounding water pressure. Such an arrangement was used for the traditional helmeted diver supplied by an air pump on the surface. Free-swimming divers carrying their own compressed gas supply rely on inspiratory demand valves, which are also balanced by the surrounding water pressure.
These arrangements supply gas that is close to the hydrostatic pressure surrounding the trunk. However, the precise ‘static lung loading’ depends on the location of the pressure-controlling device in relation to the geometry of the chest. Minor differences result from the various postures that the diver may assume. Thus, if he is ‘head-up’ when using a valve at mouthpiece level, the pressure surrounding the trunk is higher than the airway pressure by a mean value of about 3 kPa (30 cmH2O). If he is ‘head-down’, airway pressure is greater than the pressure to which the trunk is exposed. The head-down position thus corresponds to positive pressure breathing and the head-up position to negative pressure breathing. The latter causes a reduction of functional residual capacity (FRC) of about 20–30% but breathing is considered to be easier head-up than head-down.1
Apart from these considerations, immersion has relatively little effect on respiratory function, and the additional respiratory work of moving extracorporeal water does not seem to add appreciably to the work of breathing.
Effect on Efficiency of Gas Exchange4
Dead space/tidal volume ratio in divers increases with greater depth.2,6 Changes are seen at relatively low pressures, for example, in one study dead space/tidal volume ratio increased from 37% at sea level to 42% at 2.8 ATA.2 During exercise at this pressure, values decreased to around 20%.
The best measure of the efficiency of oxygenation of the arterial blood is the alveolar/arterial Po2 gradient. Measurement of arterial blood gas tensions presents formidable technical difficulties at high pressures. However, studies at 2.8, 47 and 66 ATA have reported only small increases in alveolar/arterial Po2 gradient.2,6 Since it is customary to supply deep divers with an inspired oxygen tension of at least 0.5 ATA, arterial hypoxaemia is unlikely to occur either from hypoventilation or from maldistribution of pulmonary ventilation and perfusion in healthy subjects.6
The position as regards arterial Pco2 is less clear. Hypercapnia is a well-recognised complication of diving, and divers are known to have a blunted Pco2/ventilation response, though the cause of this is unknown.1 Hypercapnia in divers at rest is uncommon, but during exercise elevated end-tidal and arterial Pco2 levels are described. Arterial Pco2 during exercise at 2.8 ATA were around 5 kPa (37 mmHg),2 but at pressures of 47 and 66 ATA were in the range 6.2–8.3 kPa (47–62 mmHg).6 This is potentially hazardous because 9 kPa is approaching the level at which there may be some clouding of consciousness, and that is potentially dangerous at depth. High gas density at depth causing increased work of breathing is believed to be responsible for the inadequate ventilation during exercise.
Oxygen Consumption
The relationship between power output and oxygen consumption at pressures up to 66 ATA, whether under water or dry, is not significantly different from the relationship at normal pressure6 shown in Figure 15.1. Oxygen consumption is expressed under standard conditions of temperature and pressure, dry (STPD, see Appendix C) and therefore represents an absolute quantity of oxygen. However, this volume, when expressed at the diver’s environmental pressure, is inversely related to the pressure. Thus, an oxygen consumption of 1 l.min−1 (STPD) at a pressure of 10 atmospheres would be only 100 ml.min−1 when expressed at the pressure to which the diver is exposed. Similar considerations apply to carbon dioxide output.
The ventilatory requirement for a given oxygen consumption at increased pressure is also not greatly different from the normal relationship shown in Figure 15.5, provided that the oxygen consumption is expressed at STPD, and minute volume is expressed at body temperature, saturated with water vapour, and at the pressure to which the diver is exposed (BTPS, see Appendix C). Considerable confusion is possible as a result of the different methods of expressing gas volumes, and though the differences are trivial at sea level they become very important at high pressures.
Exercise.5 Oxygen consumption may reach very high values during free swimming (see Figure 15.1) and are of the order of 2–3 l.min−1 (STPD) for a swimming speed of only 2 km h−1. Maximal oxygen consumption (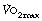 ) during exercise is improved slightly at modest high pressures (<20 ATA), an observation that results from hyperoxia (0.3 ATA oxygen) normally used at this depth. With deeper dives, there is a progressive reduction in exercise capacity, irrespective of the oxygen pressure, as a result of respiratory limitation secondary to higher gas density.
) during exercise is improved slightly at modest high pressures (<20 ATA), an observation that results from hyperoxia (0.3 ATA oxygen) normally used at this depth. With deeper dives, there is a progressive reduction in exercise capacity, irrespective of the oxygen pressure, as a result of respiratory limitation secondary to higher gas density.
Effects Attributable to the Composition of the Inspired Gas
Air
Oxygen. When breathing air at a pressure of 6 ATA, the inspired Po2 will be about 126 kPa (945 mmHg) and the alveolar Po2 about 120 kPa (900 mmHg). This is below the threshold for oxygen convulsions of about 2 ATA, but above the threshold for pulmonary oxygen toxicity if exposure is continued for more than a few hours (see Chapter 26).1,3
Nitrogen. It is actually nitrogen that limits the depth to which air may be breathed. It has three separate undesirable effects.
First, nitrogen is an anaesthetic and, in accord with its lipid solubility, can cause full surgical anaesthesia at a partial pressure of about 30 ATA. The narcotic effect of nitrogen is first detectable when breathing air at about 4 ATA and there is usually serious impairment of performance at 10 atmospheres. This effect is known as nitrogen narcosis or ‘the rapture of the deep’. It is a general rule that nitrogen narcosis precludes the use of air at depths greater than 100 metres of sea water (11 ATA pressure) and, in fact, air is not used today at pressures greater than 6 ATA. Helium is the preferred substitute at higher pressures and has no detectable narcotic properties up to at least 100 ATA.
The second problem attributable to nitrogen at high pressures is its density, which causes greatly increased hindrance to breathing at high pressure (see above). Helium has only one-seventh of the density of nitrogen and this is the second reason for its choice.
The third problem with nitrogen is its solubility in body tissues, with the resultant formation of bubbles on decompression. This is discussed in more detail below. Other inert gases, particularly helium, are less soluble in body tissues and this is the third reason for the use of helium at high pressures.
Helium/Oxygen Mixtures (Heliox)
For the three reasons outlined in the previous section, helium is the preferred diluent inert gas at pressures above 6 ATA. The concentration of oxygen required to give the same inspired gas Po2 as at sea level is shown in Table 18.1. In fact, it is usual practice to provide an inspired Po2 of about 0.5 ATA (50 kPa or 375 mmHg) to give a safety margin in the event of error in gas mixing and to provide protection against hypoventilation or defective gas exchange. This level of Po2 appears to be below the threshold for pulmonary oxygen toxicity, even during prolonged saturation dives.
A special problem of helium is its very high thermal conductivity, which tends to cause hypothermia unless the diver’s environment is heated. Heat loss from radiation and evaporation remain generally unchanged, but convective heat loss from the respiratory tract and skin is greatly increased.5 It is usual for chambers to be maintained at temperatures as high as 30–32°C during saturation dives on helium/oxygen mixtures.
Helium/Oxygen/Nitrogen Mixtures (Trimix)
The pressure that can be attained while breathing helium/oxygen mixtures is currently limited by the high pressure nervous syndrome (HPNS).5,7 This is a hyperexcitable state of the central nervous system which appears to be due to hydrostatic pressure per se and not to any changes in gas tensions. It becomes a serious problem for divers at pressures in excess of about 50 ATA, but is first apparent at about 20 ATA.
Various treatments can mitigate this effect and so increase the depth at which a diver can operate safely. The most practicable is the addition of 5–10% percent nitrogen to the helium oxygen mixture. This in effect reverses HPNS with partial nitrogen narcosis, whilst the HPNS reverses the narcosis that would be caused by the nitrogen. Trimix containing 5% nitrogen allows divers to function normally at depths of over 600 metres.5
Types of Diving Activity and their Respiratory Effects
Snorkelling is the simplest form of human diving but, as described above, respiratory effects limit the diver to the top 50 cm of water. Many other forms of diving have therefore evolved.
Breath-Hold Diving8,9,10
The simplest method of diving is by breath holding, and this is still used for collecting pearls, sponges and food from the sea bed. After breathing air, breath holding time is normally limited to 60–75 seconds, and the changes in alveolar gas tensions are shown in Figure 5.9. Astonishingly, the depth record is currently 214 metres.11 Many remarkable mechanisms interact to make this possible.
Lung volume. As pressure increases lung volume decreases by Boyle’s law (page 515). Thus at 10 ATA, an initial lung volume of 6 litres would be reduced to about 600 ml, well below residual volume (RV), and with the loss of 5.4 kg of buoyancy. During descent a point is reached when the body attains neutral buoyancy and the body will sink below that depth. To increase lung volume before diving, breath-hold divers have developed a technique called glossopharyngeal insufflation, in which air is taken into the oropharynx and compressed before being forced into the already fully inflated lung.12 Pulmonary pressure is increased above atmospheric and lung volumes increased above normal total lung capacity by 2 litres or more. A similar procedure, glossopharyngeal exsufflation, allows the divers to practice reducing their lung volumes below residual volume. For dives to 200 m depth the lungs must be almost totally collapsed, and must be reinflated on the return ascent, and the ability of a human to perform this manoeuvre contradicts much of what we currently understand about lung mechanics.11
Alveolar Po2. increases with greater depth as the alveolar gas is compressed, providing a doubling of Po2 at about 8 metres deep. More of the alveolar oxygen is therefore available at depth. Conversely, during ascent, alveolar Po2 decreases due partly to oxygen consumption, but mainly to decreasing pressure. There is thus danger of hypoxia just before reaching the surface. However, when the alveolar Po2 falls below the mixed venous Po2, there is a paradoxical transfer of oxygen from mixed venous blood to alveolar gas, and the arterial Po2 is maintained above the very low partial pressure that would otherwise occur in the alveoli. This may be an important factor in preventing loss of consciousness in the final stages of ascent.
Alveolar Pco2. By a similar mechanism, alveolar Pco2 is greater during a breath-hold dive than during a simple breath hold at sea level. At an environmental pressure of only 12 kPa (90 mmHg) gauge, the alveolar Pco2 will be increased above the mixed venous Pco2, and there will be a paradoxical transfer of carbon dioxide from alveolus to arterial blood. Fortunately there is a limited quantity of carbon dioxide in the alveolar gas, and the process is reversed during late descent and ascent.
Adaptations in the diving mammals.13 Diving mammals rely on breath holding for dives and have adaptations that permit remarkably long times under water and the attainment of great depths. For example, Sperm whales can attain depths of 1000 metres and Weddell seals can reach 500 metres and remain submerged for 70 minutes. Such feats depend on a variety of biochemical, cardiovascular and respiratory adaptations. It seems likely that the lungs of the Weddell seal collapse completely at depths between 25 and 50 metres, thus preventing the partial pressure of nitrogen increasing above the level of 320 kPa (2400 mmHg) which has been recorded at depths between 40 and 80 metres.
Many diving mammals use the spleen as a reservoir for oxygenated blood during dives. In some diving species, the spleen represents over 10% of body mass, and contains a much more muscular capsule than terrestrial animals. Splenic contraction is the probable cause of an increase of haemoglobin concentration from 15 to 25 g.dl−1 during long dives.14 Furthermore, these animals have twice the blood volume per kilogram body weight relative to humans, so oxygen stored in blood for a dive is proportionately about three times that of humans.
Limited Duration Dives
Most dives are of relatively brief duration and involve a rapid descent to operating depth, a period spent at depth, followed by an ascent, the rate of which is governed by the requirement to avoid release of inert gas dissolved in the tissues. The profile and the duration of the ascent are governed by the depth attained, the time spent at depth and the nature of the diluent inert gas.
The diving bell. The simplest and oldest technique was the diving bell. Air was trapped on the surface but the internal water level rose as the air was compressed at depth. Useful time at depth was generally no more than 20–30 minutes. More recently, additional air was introduced into the bell under pressure from the surface.
The helmeted diver. From about 1820 until recent times, the standard method of diving down to 100 metres has been by a helmeted diver supplied with air pumped from the surface into the helmet and escaping from a relief valve controlled by the water pressure. This gave much greater mobility than the old diving bell and permitted the execution of complex tasks.
SCUBA diving.15 There was for some years a desire to move towards free-swimming divers carrying their own gas supply (SCUBA – self-contained underwater breathing apparatus), first achieved in 1943. The system is based on a demand valve that is controlled by both the ambient pressure and the inspiration of the diver. Air-breathing SCUBA dives are usually restricted to depths of 30 metres. Greater depths are possible but special precautions must then be taken to avoid decompression sickness. SCUBA divers are far more mobile than helmeted divers and can work in almost any body position.
Caisson and tunnel working. Since 1839, tunnel and bridge foundations have been constructed by pressurising the work environment to exclude water. The work environment is maintained at pressure, normally of less than 4 ATA, with staff entering and leaving by air locks. Entry is rapid but exit requires adherence to the appropriate decompression schedule if the working pressure is in excess of 2 ATA.
Free submarine escape. It is possible to escape from a submarine by free ascent from depths down to about 100 metres. The submariner first enters an escape chamber which is then pressurised to equal the external water pressure. He then opens a hatch communicating with the exterior and leaves the chamber. During the ascent, the gas in his lungs expands according to Boyle’s law. It is therefore imperative that he keeps his glottis and mouth open, allowing gas to escape in a continuous stream. If gas is not allowed to escape, barotrauma is almost certain to occur (see below). In an uneventful escape, the time spent at pressure is too short for there to be any danger of decompression sickness. Thorough training is necessary and all submariners are trained in a vertical tank of 100 feet depth.
Saturation Dives
When prolonged and repeated work is required at great depths, it is more convenient to hold the divers in a dry chamber, kept on board a ship or oil rig, and held at a pressure close to the pressure of their intended working depth. Divers transfer to a smaller chamber at the same pressure, which is lowered to depth as and when required. The divers then leave the chamber for work, without any major change in pressure, but remaining linked to the chamber by an umbilical. On return to the chamber, they can be raised to the surface where they wait, still at pressure, until they are next required. A normal tour of duty is about 3 weeks, the whole of which is spent at operating pressure, currently up to about 20 atmospheres breathing helium/oxygen mixtures. During the long period at pressure, tissues are fully saturated with inert gas at the chamber pressure and prolonged decompression is then required which may last for several days.
Respiratory Aspects of Decompression Illness3
Returning to the surface following a dive is a hazardous procedure, and can give rise to a variety of complications variously known as ‘bends’, ‘chokes’ or caisson disease. In its mildest form, subjects have short-lived joint pain, but more serious presentations include pulmonary barotrauma or neurological deficit that can result in permanent disability. In the late 19th century, before decompression illness was understood, the effects on caisson workers were severe. For example, of the 600 men involved in building the underwater foundations of the St Louis Bridge in the USA, 119 of them had serious decompression illness and 14 died.16 Nowadays some form of decompression illness is thought to affect 1 in 3500–10 000 recreational dives,17 and one in 500–1000 commercial dives.16 Nomenclature of the many syndromes associated with decompression is confusing, but there are two main ways in which illness arises.
Barotrauma
Barotrauma as a result of change in pressure will affect any closed body space containing gas, and tends to occur during ascent when the gas expands. The ears, sinuses and teeth are the most commonly affected areas, but pulmonary barotrauma, although rare, is much more dangerous. Pulmonary barotrauma may occur during rapid ascent in untrained subjects, for example during submarine escape training (see above) when the subject forgets to exhale during ascent.18 Barotrauma results in disruption of the airway or alveolar wall, and air may enter either the pulmonary vessels or interstitial tissue, from where it spreads to the pleura, mediastinum or subcutaneous tissues. Mediastinal or pleural air pockets continue to expand during ascent, until chest pain or breathing difficulties occur within a few minutes of surfacing.
Some divers develop barotrauma during relatively shallow dives, and efforts have been made to identify which divers are at risk.19 In this case, barotrauma is believed to result from expansion of air trapped in the periphery of the lung by small airway closure. Subjects with reduced expiratory flow rates at low lung volume, including some asthmatics, are therefore at a theoretically greater risk.19 There is currently only weak evidence that this is a practical problem in asthmatic patients taking part in recreational diving.20
Decompression Sickness
Tissue bubble formation1,16,21 occurs when tissues become ‘supersaturated’ with an inert gas, usually nitrogen. As decompression occurs, tissue Pn2 becomes greater than the ambient pressure, and bubbles form, exactly as occurs when opening a carbonated drink. The increase in tissue Pn2 during descent and the decrease in Pn2 on ascent are both exponential curves. Tissues poorly perfused with blood have the slowest half-time for both uptake and elimination, hence on decompression tissue Pn2 decreases most slowly in poorly perfused tissues such as cartilage, giving rise to the ‘bends’.
Arterial gas embolism. Venous bubbles occur commonly during decompression, and the filtration provided by the lung is extremely effective. Overload of the filtration system may result in arterial gas embolism, but this is only believed to be the case in severe decompression sickness. There is an increasing body of evidence that arterial gas embolism follows shunting of blood containing air bubbles from the right to left sides of the heart through an otherwise asymptomatic atrial septal defect (page 217).17 Whatever the origins, arterial gas embolism is believed to be the major factor causing the neurological deficits of decompression sickness, and may be contributing to long-term neurological damage in professional divers.22
Treatment of decompression sickness is best achieved by avoidance. Detailed and elaborate tables have been prepared to indicate the safe rate of decompression depending on the pressure and time of exposure. Administration of oxygen will reduce the blood Pn2 and so accelerate the resorption of bubbles in both blood and tissue. In severe cases, including all divers with neurological deficits, urgent recompression in a chamber is required, followed by slow decompression with oxygen and other therapeutic interventions.23
Altitude Decompression Sickness24,25
Flying at high altitude by military aircraft exposes the pilots to significant degrees of decompression, a cabin altitude of 9000 m (30 000 ft) being equivalent to approximately 0.3 ATA (see Chapter 16). During actual flights, symptoms of decompression sickness tend to be under-reported because these elite pilots may fear restrictions on their flying activities. However, during their careers, three-quarters of pilots experience problems, and almost 40% of trainee pilots develop symptoms during hypobaric chamber testing to normal cabin altitudes.25 Joint pain is predictably the most common symptom, whilst the ‘chokes’ (sub-sternal pain, cough and dyspnoea) occurs in 1–3% of cases. Breathing oxygen prior to altitude exposure is likely to significantly ameliorate the symptoms seen, and is required by the US Air Force prior to altitude exposure.
Flying in the partially pressurised cabin (page 287) of commercial aircraft shortly after underwater diving increases the risk of decompression sickness. The likelihood of developing symptoms is increased by both greater depth of the last dive and shorter duration of time between the dive and flying. Dives to less than 18.5 m deep, and leaving over 24 hours between diving and flying, are generally accepted as resulting in a minimal, but not zero, risk of decompression sickness.26
References
1. Lundgren CEG, Harabin A, Bennett PB, Van Liew HD, Thalmann ED. Gas physiology in diving. In: Fregly MJ, Blatteis CM, editors. Handbook of Physiology, Section 4: Environmental physiology. New York & Oxford: Oxford University Press; 1996:999-1019.
2. Mummery HJ, Stolp BW, Dear GdeL, et al. Effects of age and exercise on physiological dead space during simulated dives at 2.8 ATA. J Appl Physiol.. 2003;94:507-517.
3. Tetzlaff K, Thorsen E. Breathing at depth: physiologic and clinical aspects of diving while breathing compressed gas. Clin Chest Med.. 2005;26:355-380.
4. Moon RE, Cherry AD, Stolp BW, Camporesi EM. Pulmonary gas exchange in diving. J Appl Physiol.. 2009;106:668-677.
5. Hong SK, Bennett PB, Shiraki K, Lin Y-C, Claybaugh JR. Mixed gas saturation diving. In: Fregly MJ, Blatteis CM, editors. Handbook of Physiology, Section 4: Environmental Physiology. New York & Oxford: Oxford University Press; 1996:1023-1045.
6. Salzano JV, Camporesi EM, Stolp BW, Moon RE. Physiological responses to exercise at 47 and 66 ATA. J Appl Physiol.. 1984;57:1055-1068.
7. Halsey MJ. The effects of high pressure on the central nervous system. Physiol Rev.. 1982;62:1341-1377.
8. Ferretti G. Extreme human breath-hold diving. Eur J Appl Physiol.. 2001;84:254-271.
9. Muth C-M, Ehrmann U, Radermacher P. Physiological and clinical aspects of apnea diving. Clin Chest Med.. 2005;26:381-394.
*10. Lindholm P, Lundgren CE. The physiology and pathophysiology of human breath-hold diving. J Appl Physiol.. 2009;106:284-292.
11. Fahlman A. The pressure to understand the mechanism of lung compression and its effect on lung function. J Appl Physiol.. 2008;104:907-908.
12. Whittaker LA, Irvin CG. Going to extremes of lung volume. J Appl Physiol.. 2007;102:831-833.
*13. Butler PJ, Jones DR. Physiology of diving of birds and mammals. Physiol Rev.. 1997;77:837-895.
14. Qvist J, Hill RD, Schneider RC, et al. Hemoglobin concentrations and blood gas tensions of free-diving Weddell seals. J Appl Physiol.. 1986;61:1560-1569.
15. Lynch PR. Historical and basic perspectives of SCUBA diving. Med Sci Sports Exerc.. 1996;28:570-572.
16. Moon RE, Vann RD, Bennett PB. The physiology of decompression illness. Sci Am Aug.. 1995;273:54-61.
*17. Foster PD, Boriek AM, Butler BD, Gernhardt ML, Bove AA. Patent foramen ovale and paradoxical systemic embolism: a bibliographic review. Aviat Space Environ Med.. 2003;74:B1-B40.
18. Broome CR, Jarvis LJ, Clark RJ. Pulmonary barotrauma in submarine escape training. Thorax. 1994;49:186-187.
19. Bove AA. Pulmonary barotrauma in divers: can prospective pulmonary function testing identify those at risk? Chest. 1997;112:576-578.
20. Koehle M, Lloyd-Smith R, McKenzie D, Taunton J. Asthma and recreational SCUBA diving. Sports Med.. 2003;33:109-116.
21. Barak M, Katz Y. Microbubbles: pathophysiology and clinical implications. Chest. 2005;128:2918-2932.
22. Wilmshurst P. Brain damage in divers. BMJ. 1997;314:689-690.
23. Moon RE, Sheffield PJ. Guidelines for treatment of decompression illness. Aviat Space Environ Med.. 1997;68:234-243.
24. Bendrick GA, Ainscough MJ, Pilmanis AA, Bisson RU. Prevalence of decompression sickness among U-2 pilots. Aviat Space Environ Med.. 1996;67:199-206.
25. Balldin UI, Pilmanis AA, Webb JT. Pulmonary decompression sickness at altitude: early symptoms and circulating gas emboli. Aviat Space Environ Med.. 2002;73:996-999.
26. Freiberger JJ, Denoble PJ, Pieper CF, Uguccioni DM, Pollock NW, Vann RD. The relative risk of decompression sickness during and after air travel following diving. Aviat Space Environ Med.. 2002;73:980-984.