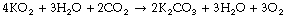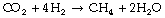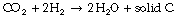Chapter 19 Respiration in closed environments and space
 Environments in which a closed atmosphere suitable for breathing is maintained include closed-circuit anaesthesia, submarines and space vehicles.
Environments in which a closed atmosphere suitable for breathing is maintained include closed-circuit anaesthesia, submarines and space vehicles. Problems of maintaining acceptably low carbon dioxide concentrations and low levels of inhaled contaminants are common to all these environments.
Problems of maintaining acceptably low carbon dioxide concentrations and low levels of inhaled contaminants are common to all these environments.The fascination of the human race with exploration has taken humans well beyond the high altitude and underwater environments described in Chapters 17 and 18. Our ability to maintain life in space, the most hostile of environments yet explored, was developed as a result of techniques used to sustain breathing in other seemingly unrelated environments on Earth. All these environments share the problems common to maintaining respiration while separated from the Earth’s atmosphere.
Closed-System Anaesthesia
This may not represent the most dramatic example of closed-environment breathing but it is by far the most common. Careful control of the composition of respired gas is the hallmark of inhalational anaesthesia. The anaesthetist must maintain safe concentrations of oxygen and carbon dioxide in the patient’s lungs, while controlling with great precision the dose of inhaled anaesthetic. It was recognised over 100 years ago that anaesthesia could be prolonged by allowing the patient to rebreathe some of their expired gas, including the anaesthetic vapour.1 Provided oxygen is added and carbon dioxide removed, other gases can be circulated round a breathing system many times, providing beneficial effects such as warm and humid inspired gas. More recently, rebreathing systems have become popular as a method of reducing both the amount of anaesthetic used and pollution of the operating theatre environment.
A totally closed system during anaesthesia means that all expired gases are recirculated to the patient, with oxygen added only to replace that consumed and anaesthetic agent added to replace that absorbed by the patient. In practice, low-flow anaesthesia, in which over half of the patient’s expired gases are recirculated, is much more commonly used.1 In each case, carbon dioxide is absorbed by chemical reaction with combinations of calcium, sodium, potassium or barium hydroxides, resulting in the formation of the respective carbonate and water. The reaction cannot be reversed, and the absorbent must be discarded after use.
Widespread use of closed-circuit anaesthesia is limited by perceived difficulties with maintaining adequate circuit concentrations of gases that the patient is consuming, such as oxygen and anaesthetic agent. However, gas-monitoring systems are now almost universally used with low-flow and closed circuit anaesthesia, allowing accurate control of circuit gas composition.
Accumulation of other Gases in Closed Circuits
Closed-circuit systems with a constant inflow and consumption of oxygen will allow retention of other gases entering the circuit either with the fresh gas or from the patient. This affects the patient in two quite distinct ways. First, essentially inert gases such as nitrogen and argon may accumulate to such an extent that they dilute the oxygen in the system. Secondly, small concentrations of more toxic gases may arise within the breathing system.
Nitrogen enters the circuit from the patient at the start of anaesthesia. Body stores of dissolved nitrogen are small, but air present in the lungs may contain 2–3 litres of nitrogen, which will be transferred to the circuit in the first few minutes. If nitrogen is not intended to be part of the closed-circuit gas mixture, the patient must ‘denitrogenate’ by breathing high concentrations of oxygen before being anaesthetised, or higher fresh gas flow rates must be used initially to flush the nitrogen from the closed circuit.
Argon is normally present in air at a concentration of 0.93%. Oxygen concentrators effectively remove nitrogen from air, and so concentrate argon in similar proportions to oxygen, resulting in argon concentrations of around 5%. In a study of closed-circuit breathing in volunteers using oxygen from an oxygen concentrator, argon levels in the circuit reached 40% after only 80 minutes.2 Cylinders of medical grade oxygen and hospital supplies from liquid oxygen evaporators contain negligible argon, so the risk of significant accumulation is low.
Methane is produced in the distal colon by anaerobic bacterial fermentation and is mostly excreted directly from the alimentary tract. Some methane is, however, absorbed into the blood, where it has low solubility so is rapidly excreted by the lung, following which it will accumulate in the closed circuit. There is a large variation between subjects in methane production and, therefore, the concentrations seen during closed-circuit anaesthesia. Mean levels in the circle system in healthy patients reached over 900 ppm, well below levels regarded as unacceptable in other closed environments, but sufficient to cause interference with some anaesthetic gas analysers.3
Acetone, ethanol and carbon monoxide all have high blood solubility, so concentrations in the closed circuit remain low, but rebreathing causes accumulation in the blood. Levels achieved are generally low,3 but acetone accumulation may be associated with postoperative nausea.4 Closed-circuit anaesthesia is not recommended in patients with increased excretion of acetone or alcohol, such as uncontrolled diabetes mellitus, recent alcohol ingestion or during prolonged starvation.1
Submarines
Submersible ships have been used for almost 100 years, almost exclusively for military purposes until the last few decades when they have become more widespread for undersea exploration and industrial use. Atmospheric pressure in the submarine remains approximately the same as at surface level during a dive, the duration of which is limited by the maintenance of adequate oxygen and carbon dioxide levels for the crew in the ship.
Diesel powered. Submarines were used extensively during both world wars, and were powered by diesel engines like surface-based warships. Clearly, the oxygen requirement of the engines precluded them from use during dives and battery-powered engines were used, thus limiting the duration of dives to just a few hours. A more significant limitation to dive duration was atmospheric regulation. No attempt was made to control the internal atmosphere, and, after ventilation at the surface, the submarine dived with only the air contained within. After approximately 12 hours the atmosphere contained 15% oxygen, 5% carbon dioxide and a multitude of odours and contaminants. The need to return to the surface was apparent when the submariners became short of breath and were unable to light their cigarettes due to low levels of oxygen.5
Nuclear powered. Short dive duration severely limited the use of diesel-powered submarines. The development of nuclear power allowed submarines to generate an ample supply of heat and electricity completely independent of oxygen supply, and so allowed prolonged activity underwater. Atmospheric regeneration was therefore needed. Current nuclear-powered submarines have a crew of up to 180, and routinely remain submerged for weeks.
Atmosphere regeneration.5,6 The plentiful supply of sea water and electricity make hydrolysis of water the obvious method for oxygen generation. Sea water must first have all electrolytes removed by a combination of evaporation and de-ionisation. Theoretically, one litre of water can yield 620 litres of oxygen, so, even with less than 100% efficient electrolysis, large volumes of oxygen are easily produced. Submarine atmosphere oxygen concentration is maintained at 21 ± 2%.
Atmospheric CO2 in submarines is absorbed by passage through monoethanolamine, which chemically combines with CO2 to produce carbonates. When fully saturated, the absorber can either be replaced or be regenerated by heating with steam, when the CO2 is released and can be vented into the sea. This method maintains the CO2 concentration in submarines at 0.5–1.5%, and though further reduction is possible, the energy cost of doing so is prohibitive.
Atmospheric contamination during prolonged submarine patrols is well recognised, many hundreds of substances entering the atmosphere, originating from both machinery and crew. These substances include volatile hydrocarbons such as benzene, oil droplets, carbon monoxide, cadmium, and microbial organisms, with varying concentrations in different parts of the submarine. Continuous monitoring of many compounds is now routine, and maximum allowable levels during prolonged patrols are defined.7 Submarine air-conditioning units include catalytic burners that oxidise carbon monoxide, hydrogen and other hydrocarbons to CO2 and water, and charcoal absorbers to absorb any remaining contaminants. The health risks from submarine occupation are therefore believed to be extremely small.5,7,8
Physiological Effects of Prolonged Hypercapnia
Definition of a ‘safe’ level of atmospheric CO2 over long periods has concerned submarine designers for some years. The respiratory response to inhalation of low concentrations of CO2 (< 3%) is similar to that at higher levels (page 69), but compensatory acid–base changes seem to be quite different.
Respiratory changes.9,10 Atmospheric CO2 levels of 1% cause an elevation of inspired Pco2 of 1 kPa (7.5 mmHg), which results in an average increase in minute ventilation of 2–3 l.min−1. However, the degree of hyperventilation is highly variable between subjects, and presumably relates to their central chemoreceptor sensitivity to CO2 (page 69). Measurements of arterial blood gases in submariners show that the elevated minute volume limits the increase in arterial Pco2 to an average of only 0.14 kPa (1 mmHg). After a few days, the increase in ventilation declines, and minute volume returns towards normal, allowing arterial Pco2 to increase further to reflect the inspired Pco2. The time course of the decline in ventilation is too short to result from blood acid–base compensation (see below), and is believed to reflect a small attenuation of the central chemoreceptor response. On return to the surface, ventilation may be temporarily reduced following withdrawal of the CO2 stimulus.
Calcium metabolism.9,11 Elevation of arterial Pco2 causes a respiratory acidosis, which is normally, over the course of one or two days, compensated for by the retention of bicarbonate by the kidney (page 359). The changes in pH seen when breathing less than 3% CO2 appear to be too small to stimulate measurable renal compensation, and pH remains slightly lowered for some time. During this period, CO2 is deposited in bone as calcium carbonate, and urinary and faecal calcium excretion is drastically reduced to facilitate this. Serum calcium levels also decrease, suggesting a shift of extracellular calcium to the intracellular space.11 After about three weeks, when bone stores of CO2 are saturated, renal excretion of calcium and hydrogen ions begins to increase and pH tends to return to normal. Abnormalities of calcium metabolism have been demonstrated with inspired CO2 concentrations as low as 0.5%.
Some other effects of low levels of atmospheric CO2 during space travel are described below (page 307).
Space6,12-14
Space represents the most hostile environment into which humans have sojourned. At 80 km (50 miles) above Earth there is insufficient air to allow aerodynamic control of a vehicle, and at 200 km (125 miles) there is an almost total vacuum. True space begins above 700 km (435 miles), where particles become so scarce that the likelihood of a collision between two atoms becomes negligible. Even under these conditions, there are estimated to be 108 particles (mainly hydrogen) per cubic metre compared with 1025 on the Earth’s surface. Maintenance of a respirable atmosphere in these circumstances is challenging, and both American and Soviet space pioneers lost their lives during the development of suitable technology. Current experience is based on expeditions in close proximity to Earth, involving Earth orbit or travel to the moon. This means that the raw materials for atmosphere regeneration can be repeatedly supplied from Earth.
Atmosphere Composition
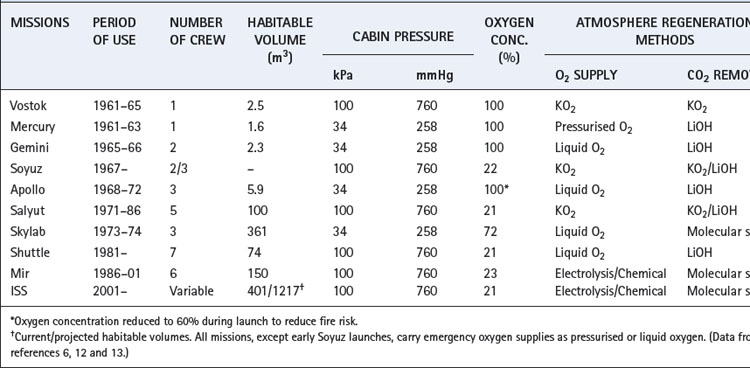
A summary of manned space missions and the atmospheres used is shown in Table 19.1. Spacecraft have an almost totally sealed, closed-circuit system of atmospheric control, and early Soviet space vehicles aimed to be completely sealed environments. Their designers had such confidence in the structure that emergency stores of oxygen were considered unnecessary until Soyuz 11 depressurised on re-entry in 1971, tragically killing all three cosmonauts. American Apollo missions leaked approximately 1 kg of gas per day in space, even with a lower atmospheric pressure (Table 19.1).
The use of a total pressure of 34.5 kPa (259 mmHg) in early US space vehicles required a high atmospheric oxygen concentration to provide an adequate inspired Po2 (Table 19.1). Because of the fatal fire on the launch pad in 1967, the composition of the atmosphere during launch was changed from 100% oxygen to 64% oxygen in 36% nitrogen at the same pressure, which still gave an inspired Po2 in excess of the normal sea level value. Previous Soviet designs were all based on maintaining normal atmospheric pressure, and space vehicles in current use continue to do so with inspired oxygen concentrations of near 21%. Extravehicular activity in space presents a particular problem. In order to maintain a functionally acceptable flexibility of the space suit in the vacuum of space, the internal pressure is only 28 kPa (212 mmHg). This entails the use of 100% oxygen after careful decompression and denitrogenation of the astronaut.
Oxygen Supply
Storage of oxygen and other gases in space presents significant problems. The weight of the containers used is critical during launch, and storage of significant quantities of oxygen requires high pressures and therefore strong, heavy tanks. Liquid oxygen presents a greatly improved storage density, but the behaviour of stored liquids in weightless conditions is complex.
Chemical generation of oxygen has been used mainly by Soviet space missions. Potassium superoxide releases oxygen on exposure to moisture, a reaction that generates potassium hydroxide as an intermediate and so also absorbs carbon dioxide:
One kilogram of KO2 can release over 200 litres of oxygen, but the reaction is irreversible and the used canisters must be discarded. Sodium chlorate candles release oxygen when simply ignited, and were used for emergency oxygen generation in Soviet space missions and are still used for atmospheric regeneration in disabled submarines.15
Electrolysis of water is an efficient way to produce oxygen in space where solar panels provide the electricity supply. In contrast to submarines, water is scarce in space vehicles, again because of weight considerations at launch. In the International Space Station oxygen is generated by electrolysis using waste water from the occupants, though this alone does not produce sufficient oxygen for a reasonably active astronaut.
Carbon Dioxide Removal
Chemical absorption by lithium hydroxide has been the mainstay of the US space program, whilst the Soviet program used KO2 as described above. Reversible chemical reactions such as those used in submarines have been adapted for space use, and can be regenerated by exposure to the vacuum of space.
Molecular sieves allow CO2 to be adsorbed into a chemical matrix without undergoing any chemical reaction. When saturated with CO2, exposure to the space vacuum causes release of the adsorbed gas. Use of two- or four-bed molecular sieves allows continuous CO2 removal by half the processors whilst the others are regenerated.
Maintenance of low levels of CO2 on prolonged future space missions is likely to have unacceptable costs in terms of energy and consumables.16 This fact led to three space agencies worldwide undertaking a joint research programme to study the effects of 1.2% and 0.7% atmospheric CO2 on a wide range of physiological systems. The study involved normal volunteers spending 22 days in a closed mock ‘space station’ on the ground.16 Some of the results have already been described above (page 305).10,11 Effects at 0.7% atmospheric CO2 were generally concluded to be minimal.17 At 1.2% however, changes in respiration and calcium metabolism were significant and, more important, mental performance was impaired with a loss of alertness and visuomotor performance.18
Atmospheric Contamination
Chemical contamination within space vehicles is mainly from within the habitable area of the vehicle, with external contamination from propellants etc. being very rare.12 The greatest contribution to atmospheric contamination is the astronauts themselves, but the compounds released such as carbon monoxide, ammonia, methane and indole are easily dealt with by standard methods. More complex chemicals may be released into the atmosphere by a process called off-gassing. Almost any non-metallic substance, but particularly plastics, release small quantities of volatile chemicals for many months and years after manufacture. This is more likely to occur at low atmospheric pressure as on the earlier space missions. Within a closed environment, these chemicals may accumulate to toxic levels and air-conditioning units similar to those described for submarines (page 305) are required.
Long-Term Space Travel
Manned space travel to planets more distant than the moon requires expeditions of years’ duration with no access to supplies from Earth. For example, the journey time to Mars is around 6 months, so the minimum realistic mission duration would be 2 years. The estimated mass of provisions required to sustain 6 crew members for this duration would be over 45 tons, which far exceeds the capacity of current space vehicles.19 Regenerative life support systems have, therefore, been studied extensively in recent years and aim to reverse the effects of animal metabolism on a closed atmosphere. Biological solutions are believed by many to be the only feasible option, and biospheres are discussed below. Physico-chemical methods are, however, now realistic options, and are likely to act as valuable backup systems.
CO2 reduction reactions convert carbon dioxide back into oxygen, and two main methods are described.20 The Sabatier reaction requires hydrogen to produce methane and water:
Methane can then be converted to solid carbon and hydrogen gas, which re-enters the Sabatier reactor. The Bosch reaction produces solid carbon in one stage:
Electrolysis of water generates oxygen and hydrogen gas, and the latter enters the Bosch or Sabatier reactions and the water produced is recycled. Both reactions ultimately generate solid carbon, which must be removed from the reactors periodically: current hardware can convert CO2 into oxygen for 60 person-days before the carbon deposits must be emptied.20
In-situ resource utilisation on Mars.21 The atmosphere of Mars is composed of 95.3% CO2, 2.7% N2, 1.6% Ar, 0.13% O2 and 0.07% CO. For any mission to Mars, these gases could be used for atmospheric regeneration as shown in Figure 19.1. Separation of the gases in the atmosphere will produce a small amount of oxygen, and larger volumes of nitrogen and argon, which may be used as buffer gas in the atmosphere. On prolonged missions, loss of buffer gas from the vehicle by leakage and from activation of airlocks is a substantial problem. The abundant CO2 on Mars can enter a Sabatier reactor and the methane produced may then be used as a propellant for the mission, the water used either by the crew or to provide oxygen for the life support systems and the hydrogen can re-enter the Sabatier reactor.
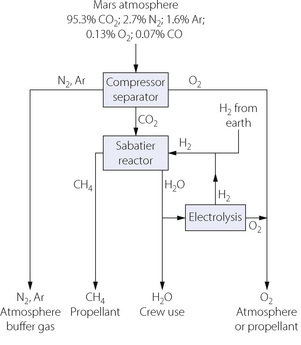
Fig. 19.1 In-situ resource utilisation on Mars. Using a series of simple physicochemical processes, the atmosphere of Mars, supplemented by hydrogen transported from Earth, may be used to provide buffer gas and oxygen for the space vehicle atmosphere, methane as propellant for the vehicle and water for use by the crew or for generation of oxygen.
(After reference 21.)
Microgravity22,23
All bodies with mass exert gravitational forces on each other, so zero gravity is theoretically impossible. Once in space, away from the large mass of Earth or other planets, gravitational forces become negligible and are referred to as microgravity. Space vehicles in orbit around Earth are still subject to its considerable gravitational forces, but these are matched almost exactly by the centrifugal force from the high tangential velocity of the space vehicle.13 Occupants of orbiting space vehicles are normally subject to a gravitational force of approximately 10−6 times that on Earth’s surface.
Chapter 8 contains numerous references to the effect of gravity on the topography of the lung and the distribution of perfusion and ventilation. Microgravity may therefore be predicted to have significant effects on respiratory function.
The first studies of short-term microgravity used a Lear jet flying in a series of Keplerian arcs, which gave 20–25 seconds of weightlessness. Unfortunately, between each period of microgravity the subject is exposed to a similar duration of increased gravitational forces (2 G) as the jet pulls out of the free-fall portion of the flight,24 and this may influence the results of physiological studies. Sustained microgravity has been studied in space. In 1991 an extended series of investigations on seven subjects was undertaken in Spacelab SLS-1, which was carried into orbit by the space shuttle for a nine day mission, and studies of prolonged microgravity on the International Space Station are now being published.25
Lung volumes. Chest radiography in the sitting position during short-term microgravity showed no striking changes other than a tendency for the diaphragm to be slightly higher in some of the subjects at functional residual capacity (FRC).26 This accords with a 413 ml reduction in FRC also measured in Keplerian arc studies on seated subjects.24 Abdominal contribution to tidal excursion was increased at microgravity in the seated position, probably because of loss of postural tone in the abdominal muscles,24 an observation now confirmed in space studies.27
During sustained microgravity subdivisions of lung volume were again found to be intermediate between the sitting and supine volumes at 1 G, except for residual volume which was reduced below that seen in any position at 1 G (Figure 19.2).28 The FRC was reduced by 750 ml compared with preflight standing values. These changes in lung volume are ascribed to altered respiratory mechanics and increased thoracic blood volume.
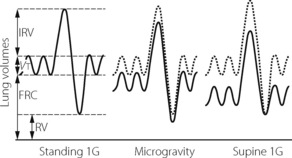
Fig. 19.2 Static lung volumes during sustained microgravity after nine days in Earth orbit. Dotted line shows the normal standing values on Earth for comparison. Volumes at microgravity are generally intermediate between standing and supine values at 1 G, except residual volume, which is further reduced. FRC, functional residual capacity; IRV, inspiratory reserve volume; RV, residual volume; Vt, tidal volume.
Topographical inequality of ventilation and perfusion.22 Early results in the Lear jet, using single-breath nitrogen washout (page 123), indicated a substantial reduction in topographical inequality of ventilation and perfusion during weightlessness, as expected.29 However, the more detailed studies in Spacelab showed that a surprising degree of residual inequality of blood flow30 and ventilation31 persisted despite the major improvement at zero gravity. Ventilatory inequality with microgravity is believed to result from continued airway closure at low lung volume,31 airway closure possibly occurring in a patchy fashion.32 The most likely explanation for the continued perfusion inequality is the central to peripheral ‘radial’ gradient within each horizontal slice of lung (page 124), which is mostly overshadowed at 1G by the large vertical perfusion gradient.
Breathing during sleep in space.33 Snoring and airway obstruction during sleep are common, and many factors are involved in their initiation (page 271). Reduced activity of pharyngeal dilator muscles and increased compliance of pharyngeal structures both encourage the normal gravitational force on Earth to initiate obstruction. This important contribution of gravity to sleep-disordered breathing has been confirmed by studies of astronauts sleeping in the orbiting space shuttle.34 Compared with sleeping at 1G before the mission, in microgravity there were dramatic reductions in their apnoea-hypopnoea index (page 271) and snoring was virtually eliminated.
Biospheres13
A biosphere is defined as ‘a closed space of two or more connected ecosystems in equilibrium with their environment’.35 Only energy enters and leaves a biosphere. Earth is the largest and most successful known biosphere, though the equilibrium between its ecosystems is almost certainly changing (Chapter 1). Attempts to create smaller biospheres have mostly been driven by the prospect of long-term space travel.36 Physico-chemical methods of sustaining life, as described above, have many limitations whereas a biological system has numerous advantages. Plants perform the complex CO2-reduction chemistry using chlorophyll, and at the same time, rather than generating carbon, they produce varying amounts of food.37 Plants also act as efficient water-purification systems via transpiration.
Small Scale Biological Atmospheric Regeneration
The first report of prolonged biological atmosphere regeneration was described in 1961 when a single mouse was maintained in a closed chamber for 66 days.38 Air from the chamber was circulated through a second chamber containing four litres of Chlorella alga solution illuminated with artificial light. Over the course of the experiment, oxygen concentration in the chamber increased from 21% to 53%, and carbon dioxide concentrations remained below 0.2%. Subsequent experiments by both American and Soviet researchers demonstrated the feasibility of human life support by Chlorella, culminating in a 30-day closure of a single researcher in a 4.5 m3 room, maintained by just 30 litres of alga solution. Algae alone are unsuitable for long-term life support.13 Their excellent atmospheric regeneration properties result from a very fast rate of growth but Chlorella is generally regarded as inedible, and so presents a significant disposal problem in a totally closed system. In addition, if the algal solution becomes acidic for any reason, such as bacterial contamination, algae produce carbon monoxide in unacceptable quantities.
Unknown to the scientific community at large, from 1963 the Soviet Union ran a ‘Bios’ research centre at the Institute of Biophysics in Krasnoyarsk, Siberia.13,39 A whole series of progressively more complex biospheres were constructed. In 1983, two researchers successfully spent five months in a biosphere (Bios 3), which provided all their atmospheric regeneration needs and over three-quarters of their food.39 In these studies, plants were grown hydroponically – that is, without soil with their roots bathed in carefully controlled nutrient solution. Light was provided with continuous xenon lighting to maximise growth, to such an extent that, under these conditions, wheat can be harvested six times per year. An estimated 13 m2 of planted area will then produce enough oxygen for one human, though over 30 m2 is probably required to produce almost enough food as well. Beds of Chlorella algae were also used to maximise oxygen production and, along with larger planted areas, resulted in excess oxygen. This was reduced by incineration of the non-edible portion of the plants, and so enabled the researchers to exercise some control over the balance between oxygen and carbon dioxide concentration within the biosphere.
American research of controlled ecological life support systems (CELSS) began in 1977 and has focused on basic plant physiology.13 Plant species, light, humidity, nutrients and atmospheric gas concentrations all have profound effects on the design of a CELSS. Atmospheric regeneration is usually the easiest problem to overcome, whilst the plant species used has important implications for the dietary intake and psychological well-being of the CELSS inhabitants.37,39 In contrast to this American project, the European Space Agency is developing a life-support system for long-term space travel centred around micro-organisms, which are more versatile biochemically.40 For example, bacteria may be used to compost crew waste and inedible plant components into nitrogenous compounds to enhance the plant growth.
Biosphere 213
Small-scale biosphere experiments never attained a totally closed system, particularly with respect to food supplies and waste disposal, and always struggled with the accumulation of toxic atmospheric compounds. With these problems in mind, an ambitious series of biosphere experiments were established in Arizona, USA, culminating in the biosphere 2 project in 1991.
A totally sealed complex, covering 3.15 acres (1.3 hectares) was purpose built with a stainless steel underground lining and principally glass cover. Two flexible walls, or ‘lungs’, were included to minimise pressure changes within the complex with expansion and contraction of the atmosphere. A two-year closure was planned, with the complex containing a wide range of flora and fauna, including eight humans. Soil was chosen as the growing medium for all plants in preference to hydroponic techniques used previously. This was to facilitate air purification by soil bed reactors, in which atmospheric air is pumped through the soil where bacterial action provides an adaptable and efficient purification system.13 A CO2‘scrubber’ system was included in biosphere 2 to control atmospheric CO2 levels, particularly during winter when shorter days reduce photosynthetic activity. Also, the amount of O2-consuming biomass relative to atmosphere volume was known to be high, and therefore small increases in CO2 levels were anticipated.
Biosphere 2 aimed, wherever possible, to use ecological engineering. By the inclusion of large numbers of species (3800 in total), it was hoped that there would be sufficient flexibility between systems to respond to changes in the environment. In particular, microbial diversity is believed to be extremely important in maintaining biosphere 1 (Earth), and multiple habitats were established to facilitate this type of diversity in biosphere 2.
Outcome from the two-year closure. Concentrations of oxygen and carbon dioxide in biosphere 2 were very unstable (Figure 19.3), and after 16 months, oxygen concentration had fallen to only 14%. Extensive symptoms were reported by the human inhabitants, including significantly reduced work capacity, and external oxygen had to be added to the atmosphere. Carbon dioxide levels did increase slightly during winter months (Figure 19.3) when the CO2 was removed by the scrubber system.
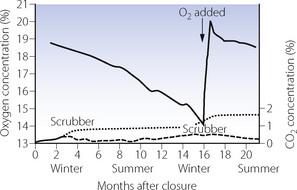
Fig. 19.3 Changes in atmospheric concentrations of oxygen (solid line) and carbon dioxide (dashed line) during the two-year closure of biosphere 2. Less daylight during winter months reduces photosynthesis causing increased levels of CO2. Carbon dioxide was therefore removed using a CO2 ‘scrubber’ system, during the periods shown. Even when CO2 absorption by the scrubbers is taken into account (dotted line), it can clearly be seen that the reduction in O2 concentration exceeds the increase in CO2 concentration; after 16 months, O2 had to be added to the biosphere. See text for details.
(Data from references 13 and 38.)
It was never expected that all species introduced into biosphere 2 would survive, and extinction of some species was seen as a natural response to stabilisation of the ecosystem. However, after 21 months, extinct species were numerous, including 19 of 25 vertebrates and most insects, including all pollinators.41 In contrast, ants and cockroaches thrived.
The success of biosphere 2 as a closed ecosystem was therefore limited, and, in contrast to the smaller biospheres previously used, basic atmospheric regeneration was a significant problem. Any increase in CO2 concentration should be matched by an equivalent decrease in O2 concentration, as biological reactions between CO2 and O2 are generally equimolar. Even when the CO2 removed by the recycling system is taken into account, it can clearly be seen from Figure 19.3 that oxygen losses were much greater. The explanation for this is believed to be two-fold.42 First, oxygen depletion occurred due to respiration in the biosphere proceeding faster than photosynthesis, most likely as a result of excessive microbial activity in the soil. Secondly, much of the CO2 produced by this respiration was lost from the atmosphere by chemical reaction with the concrete from which the biosphere complex was built.
We remain some way away from being able to establish a long-term habitable atmosphere away from Earth.
References
1. Baum JA, Aitkenhead AR. Low-flow anaesthesia. Anaesthesia. 1995;50(supp):37-44.
2. Parker CJR, Snowdon SL. Predicted and measured oxygen concentrations in the circle system using low fresh gas flows with oxygen supplied by an oxygen concentrator. Br J Anaesth.. 1988;61:397-402.
3. Versichelen L, Rolly G, Vermeulen H. Accumulation of foreign gases during closed-system anaesthesia. Br J Anaesth.. 1996;76:668-672.
4. Strauß JM, Hausdörfer J. Accumulation of acetone in blood during long-term anaesthesia with closed systems. Br J Anaesth.. 1993;70:363-364.
5. Knight DR, Tappan DV, Bowman JS, O’Neill HJ, Gordon SM. Submarine atmospheres. Toxicol Lett.. 1989;49:243-251.
6. Wieland PO. Designing for human presence in space: an introduction to environmental control and life support systems, NASA Reference Publication 1324. National Aeronautics and Space Administration. 1994.
7. Dean MR. Benzene exposure in Royal Naval submarines. J R Soc Med.. 1996;89:286P-288P.
8. Lambert RJW. Environmental problems in nuclear submarines. Proc R Soc Med.. 1972;65:795-796.
9. BJW Pingree. Acid-base and respiratory changes after prolonged exposure to 1% carbon dioxide. Clin Sci Mol Med.. 1977;52:67-74.
10. Elliott AR, Prisk GK, Schöllmann C, Hoffmann U. Hypercapnic ventilatory response in humans before, during, and after 23 days of low level CO2 exposure. Aviat Space Environ Med.. 1998;69:391-396.
11. Drummer C, Friedel V, Börger A, et al. Effects of elevated carbon dioxide environment on calcium metabolism in humans. Aviat Space Environ Med. 1998;69:291-298.
12. Nicogossian AE, Huntoon CL, Pool SL. Space physiology and medicine, 3rd ed. Philadelphia: Lea & Febiger; 1994.
13. Churchill SE. Fundamentals of space life sciences. Malabar, Florida: Krieger Publishing; 1997.
14. West JB. Historical aspects of the early Soviet/Russian manned space program. J Appl Physiol.. 2001;91:1501-1511.
15. Risberg J, Österberg C, Svensson T, et al. Atmospheric changes and physiological responses during a 6-day “disabled submarine” exercise. Aviat Space Environ Med.. 2004;75:138-149.
16. Wenzel J, Luks N, Plath G, Wilke D, Gerzer R. The influence of CO2 in a space-like environment: study design. Aviat Space Environ Med.. 1998;69:285-290.
17. Frey MAB, Sulzman FM, Oser H, Ruyters G. The effects of moderately elevated ambient carbon dioxide levels on human physiology and performance: A joint NASA-ESA-DARA study – overview. Aviat Space Environ Med.. 1998;69:282-284.
18. Manzey D, Lorenz B. Effects of chronically elevated CO2 on mental performance during 26 days of confinement. Aviat Space Environ Med.. 1998;69:506-514.
19. Grigoriev AI, Kozlovskaya IB, Potapov AN. Goals of biomedical support of a mission to Mars and possible approaches to achieving them. Aviat Space Environ Med.. 2002;73:379-384.
20. Noyes GP. Carbon dioxide reduction processes for spacecraft ECLSS: A comprehensive review. Proceedings of the 18th intersociety conference on environmental systems, paper 881042. San Francisco: July 1988.
21. Sridhar KR, Finn JE, Kliss MH. In-situ resource utilisation technologies for Mars life support systems. Adv Space Res.. 2000;25:249-255.
*22. Prisk GK. Microgravity and the lung. J Appl Physiol.. 2000;89:385-396.
*23. Prisk GK. The lung in space. Clin Chest Med.. 2005;26:415-438.
24. Paiva M, Estenne M, Engel LA. Lung volumes, chest wall configuration, and pattern of breathing in microgravity. J Appl Physiol.. 1989;67:1542-1550.
25. Prisk GK, Fine JM, Cooper TK, West JB. Vital capacity, respiratory muscle strength, and pulmonary gas exchange during long-duration exposure to microgravity. J Appl Physiol.. 2006;101:439-447.
26. Michels DB, Friedman PJ, West JB. Radiographic comparison of human lung shape during normal gravity and weightlessness. J Appl Physiol.. 1979;47:851-857.
27. Wantier M, Estenne M, Verbanck S, Prisk GK, Paiva M. Chest wall mechanics in sustained microgravity. J Appl Physiol.. 1998;84:2060-2065.
28. Elliott AR, Prisk GK, Guy HJB, West JB. Lung volumes during sustained microgravity on Spacelab SLS-1. J Appl Physiol.. 1994;77:2005-2014.
29. Michels DB, West JB. Distribution of pulmonary ventilation and perfusion during short periods of weightlessness. J Appl Physiol.. 1978;45:987-998.
30. Prisk GK, Guy HJB, Elliott AR, West JB. Inhomogeneity of pulmonary perfusion during sustained microgravity on SLS-1. J Appl Physiol.. 1994;76:1730-1738.
31. Guy HJB, Prisk GK, Elliott AR, Deutschman RA, West JB. Inhomogeneity of pulmonary ventilation during sustained microgravity as determined by single breath washouts. J Appl Physiol.. 1994;76:1719-1729.
32. Dutrieue B, Verbanck S, Darquenne C, Prisk GK. Airway closure in microgravity. Respir Physiol Neurobiol.. 2005;148:97-111.
33. Dinges DF. Sleep in space flight. Breath easy – sleep less. Am J Respir Crit Care Med.. 2001;164:337-340.
34. Elliott AR, Shea SA, Dijk D-J, et al. Microgravity reduces sleep-disordered breathing in humans. Am J Respir Crit Care Med.. 2001;164:478-485.
35. Walford RL, Bechtel R, MacCallum T, Paglia DE, Weber LJ. ‘Biospheric medicine’ as viewed from the two-year first closure of biosphere 2. Aviat Space Environ Med.. 1996;67:609-617.
36. Schwartzkopf SH. Human life support for advanced space exploration. Adv Space Biol Med.. 1997;6:231-253.
37. Mitchell CA. Bioregenerative life-support systems. Am J Clin Nutr.. 1994;60:820S-824S.
38. Bowman RO, Thomae FW. Long-term nontoxic support of animal life with algae. Science. 1961;134:55-56.
39. Ivanov B, Zubareva O. To mars and back again on board Bios. Soviet Life. 1985:22-25. April
40. Hendrickx L, De Wever H, Hermans V, et al. Microbial ecology of the closed artificial ecosystem MELiSSA (Micro-Ecological Life Support System Alternative): Reinventing and compartmentalising the Earth’s food and oxygen regeneration system for long-haul space exploration missions. Res Microbiol.. 2006;157:77-86.
41. Cohen JE, Tilman D. Biosphere 2 and biodiversity: the lessons so far. Science. 1996;274:1150-1151.
42. Severinghaus JP, Broecker WS, Dempster WF, MacCallum T, Wahlen M. Oxygen loss in biosphere 2. EOS. 1994;75:33-40.