Traumatic Brain Injury
Coma and levels of consciousness
Decorticate, decerebrate, and motor rigidity
Abnormal muscle tone and spasticity
Decreased functional endurance
Evaluation of the lower-level individual
Intervention for the lower-level individual
Evaluation of the intermediate- to higher-level individual
Intervention for the intermediate- to higher-level individual
After studying this chapter, the student or practitioner will be able to do the following:
1 Describe the pathology underlying traumatic brain injury (TBI).
2 State current medical, surgical, and pharmaceutical interventions for acute TBI.
3 Identify levels of consciousness in individuals with TBI by using standard scales.
4 Describe the clinical picture of individuals with TBI, including common physical, cognitive, and psychosocial sequelae.
5 Identify occupational therapy evaluation methods for lower-, intermediate-, and higher-level individuals with TBI.
6 Identify several standard occupational therapy assessments for physical, cognitive, and psychosocial impairment after TBI.
7 Describe occupational therapy intervention methods for lower-, intermediate-, and higher-level individuals with TBI.
8 Describe the continuum of care services available for an individual with TBI in the acute, subacute, and postacute stages of rehabilitation.
Introduction and Epidemiology
Traumatic brain injury (TBI) is defined as damage to brain tissue caused by an external mechanical force with resultant loss of consciousness, post-traumatic amnesia, skull fracture, or objective neurologic findings that can be attributed to the traumatic event on the basis of radiologic findings or physical or mental status examination.25,68,73 It is the most common cause of death and disability in young people.25 More than 50,000 Americans die; 235,000 are hospitalized and 1.1 million are treated and released from an emergency department. The number of people with TBI who are not seen in an emergency department or who receive no care is unknown. The direct and indirect costs of TBI in the United States have been estimated to be in excess of $60 billion annually.10 Survivor costs account for more than $31 billion, and fatal brain injuries cost another $16.6 billion.74,82 The Centers for Disease Control and Prevention estimates that at least 5.3 million Americans currently have a long-term or lifelong need for help to perform activities of daily living (ADLs) as a result of TBI.9
The etiology of TBI is closely associated with age and gender. Children younger than 5 years tend to be injured in falls, in motor vehicle crashes, and by adults inflicting violence. Those between the ages of 5 and 15 are also injured on bicycles, skateboards, and horses; as pedestrians; and during sports activities. Between the ages of 15 and 40, high-speed motor vehicle and motorcycle crashes are the most common causes of TBI. After the age of 40, the incidence of violence-related injury approaches that of motor vehicles, particularly in metropolitan areas. Young and middle-aged adult men are 1.5 times more likely to be injured than their female counterparts. The two age groups at highest risk for TBI are 0- to 4-year-olds and 15- to 19-year-olds.9 Elderly individuals are injured just as often by a fall or during a pedestrian mishap as they are in motor vehicles accidents.21,46 Blasts are a leading cause of TBI in active duty military personnel in war zones.9
Nontraumatic brain injuries include toxicity from drug overdose, chronic substance abuse, carbon monoxide poisoning, or environmental exposure; anoxia from cardiopulmonary arrest or near-drowning; brain abscess, meningitis, and encephalitis from bacteria, viruses, acquired immunodeficiency syndrome, fungi, or parasites; nutritional deficiencies; genetic and congenital disorders; chronic epilepsy; and degenerative diseases such as dementia.49
Although the aforementioned conditions often have characteristics similar to those resulting from TBI (particularly with regard to rehabilitation approaches), this chapter’s primary focus is assessment of and intervention for individuals with traumatic injury.
Substance abuse is strongly linked to TBI and many nontraumatic causes. Use of alcohol close to the time of injury is prevalent in more than 50% of adults with TBI.41 An even greater number of individuals have a previous history of alcohol or other substance abuse in the year preceding the injury. As defined by the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, fourth edition, substance abuse is defined as follows: (1) failure to fulfill major role obligations at work, school, or home; (2) use in situations in which it is physically hazardous; (3) substance-related legal problems; or (4) continued use despite having persistent or recurrent social or interpersonal problems related to use.3,17,41,47 Therefore, knowledge of the acute and chronic sequelae of substance abuse disorders is crucial in assessing and treating individuals with brain injuries.
Recovery from any type of brain injury depends on the patient’s age and preinjury capabilities, the severity of the injuries incurred, and the quality of intervention and support available during the patient’s recovery. Recurrent brain injury is unfortunately all too common and occurs in those who have previously sustained trauma, have developmental disabilities, or have acquired disabilities associated with other causes.
Prevention of secondary complications is critical throughout all stages of the recovery process: at the time that the person is resuscitated (i.e., at the site of injury), in acute medical care settings, during acute and postacute rehabilitation programs, and when the individual is trying to reintegrate into his or her family and community. Many of the available medical and therapeutic interventions target these secondary complications. A well-coordinated team of knowledgeable professionals, family members, and supportive community caregivers can optimize the outcome for a given individual.12
Pathophysiology
Neuropathologists and neurosurgeons currently categorize the early stages of TBI as primary (occurring at the moment of impact) and secondary (occurring in the days to several weeks after the injury). Prevention of primary injury includes the use of safety belts, protective helmets, air bags, and roadside barriers, all of which can minimize the impact of the initial injury-causing agent. Prevention of secondary injury typically begins at the point of contact with those providing first aid at the scene of the trauma. It continues with emergency medical services, resuscitation and transport, and acute medical and surgical management and carries over to rehabilitation settings. It is particularly the secondary interventions that will be discussed later in the section on medical interventions. An individual with TBI will typically have some combination of primary focal and diffuse brain injury, depending on the cause and mechanism of the initial injury; in best-case scenarios, there is a minimal amount of secondary brain damage and functional disability that occurs as a result of brain swelling, hypotension, hypoxia, and systemic injury. Prompt recognition of these complications improves survival and functional outcome when an appropriate intervention is implemented.25
Focal Brain Injury
Focal brain injury is caused by a direct blow to the head after collision with an external object or fall, a penetrating injury resulting from a weapon, and collision of the brain with the inner tables of the skull. The bones of the face or skull may or may not be fractured. Common findings in individuals with focal injury resulting from falls include intracerebral and brain surface contusions, particularly in the inferior and dorsal-lateral frontal lobes, anterior and medial temporal lobes, and less commonly, the inferior cerebellum. Assaults and missile wounds can occur anywhere in the brain depending on the direction of force. Other surface areas of the brain, including those not directly below the blow to the head, can also suffer contusions as a result of collision of the brain with the inner tables of the skull. The directly injured area is referred as the coup, and the site of the indirect injury is known as the contrecoup.29,30
If there are injuries to the coverings of the brain, especially the dura, pia, and arachnoid, other focal hemorrhages occur. Epidural hematomas (EDHs) are associated with skull fractures in adults with disruption of the integrity of the meningeal arteries; children may have arterial disruption with or without a skull fracture. Individuals with an EDH may initially be alert after the blow to the skull; as the hematoma develops between the skull and the dura, it can cause pressure on underlying brain tissue (secondary injury) with rapid deterioration in mental and physical status. Prompt recognition and neurosurgical treatment can save lives and limit morbidity.36
Subdural hematomas (SDHs) occur between the dura and the brain surface through tearing of bridging veins. The rate of hemorrhage is often slower than that of an EDH because venous bleeding is more gradual than arterial. An SDH may occur just as frequently on the side of the head opposite the direct blow; therefore, an EDH can occur on one side of the brain adjacent to the trauma and an SDH on the other. SDHs tend to spread around the entire surface of one hemisphere or less commonly in the posterior fossa. Acute SDH is diagnosed within 48 hours of injury, subacute SDH within 2 to 14 days after injury, and chronic SDH after 2 weeks. The fall or blow to the head in individuals with subacute or chronic SDH may have occurred days before the person arrives at the hospital, with symptoms being typical of changes in mental status. The urgency of treating SDH depends on the clinical condition of the individual and the extent of adjacent brain tissue affected as observed on radiology.67
Multifocal and Diffuse Brain Injury
With multifocal and diffuse brain injuries, there may be sudden deceleration of the body and head with variable forces transmitted to the surface and deeper portions of the brain. Motor vehicle, bicycle, and skateboard crashes are typical etiologic factors, but falls from a high surface or off a horse or bull can also result in multifocal and diffuse injuries.
Intracerebral hemorrhage (ICH) is nearly always present as a focal injury with missile wounds and is common after falls and assaults. Within the first week after TBI, particularly in clients with blood-clotting abnormalities, ICH may appear on follow-up computed tomography (CT) scans. With high-speed deceleration injury, multiple small, deep ICHs occur throughout the neuraxis; on high-resolution CT or magnetic resonance imaging (MRI), they are typically visible at the junction between gray and white matter and in the basal ganglia, corpus callosum, midbrain, and/or cerebellum.
Subarachnoid hemorrhage (SAH) and intraventricular hemorrhage (IVH) occur when the pia or arachnoid is torn. SAH caused by trauma is less frequently associated with vasospasms than is SAH caused by rupture of an aneurysm. A large IVH can block the flow of cerebrospinal fluid (CSF) and result in acute hydrocephalus. Thus, clinical evaluation of the possibility of a ruptured aneurysm causing brain dysfunction, which may result from a fall or motor vehicle crash, is important with either of these entities.
Diffuse axonal injuries (DAIs) are prototypic lesions caused by rapid deceleration. The degree of injury may vary from primary axonotomy, with complete disruption of the nerve, to axonal dysfunction, wherein the structural integrity of the nerve remains intact but there is loss of ability to transmit normally along neuronal pathways. The clinical severity of DAI is measured by the depth and length of coma (i.e., the time from onset of the injury until the individual performs purposeful activity) and associated signs such as pupillary abnormalities.47
Prevention of Secondary Brain Injuries
The brain, like any body tissue, reacts to injury with swelling or edema, neurochemical injury cascades, changes in blood flow, and inflammation. Unlike other tissues, the brain is confined in a closed container, the skull, which protects it from outside injury but also confines the amount of swelling or blood accumulation that can occur. The brain is also the organ least able to tolerate loss of blood flow or oxygen. Secondary injury occurs as a result of the effects of brain swelling in a closed space, loss of perfusion, and decreased delivery of oxygen to healthy and damaged tissue. Recovery is related to the extent of the initial pathology and secondary injury.25
Guidelines for the management of severe TBI to minimize the impact of secondary injury have been developed over the last 10 years by the American Association of Neurological Surgeons (AANS) and the Brain Trauma Foundation. Some of these areas include resuscitation of blood pressure and oxygenation, management of elevated intracranial pressure (ICP) or hypertension, nutrition after acute trauma, and seizure prophylaxis. These care recommendations are based on a critical review of the available literature on the management of individuals with TBI and are categorized into the following groups: standards, which represent a high degree of clinical certainty; guidelines, which represent a moderate degree of clinical certainty; and options, which represent a low degree of clinical certainty.11 Each of these terms—standards, guidelines, and options—are used as labels for forms of intervention in individuals who have sustained a TBI.
Areas of intervention that are considered standards are relatively few and relate to interventions that may be more harmful than helpful. They include the following:
• In the absence of increased ICP, chronic prolonged hyperventilation should be avoided.
• Steroids are not recommended to reduce ICP.
• Prophylactic anticonvulsants are not recommended for preventing late (i.e., after the first week) post-traumatic seizures (PTSs).
Guidelines, which have a moderate degree of certainty regarding efficacy, include the following for individuals with severe TBI: (1) all regions should have an organized trauma care system; (2) hypotension (systolic blood pressure <90 mm Hg) or hypoxia (apnea, cyanosis, oxygen saturation <90% in the field, or a Pao2 of 60 mm Hg) must be monitored and corrected immediately; (3) ICP monitoring is appropriate for injuries in individuals younger than 40 years with a systolic blood pressure lower than 90 mm Hg, with Glasgow Coma Scale (GCS) scores between 3 and 8, or when CT scans show hematomas, contusions, edema, or compressed basal cisterns; (4) intervention should be initiated to lower ICP if it exceeds 20 to 25 mm Hg; (5) effective ICP treatments include mannitol, high-dose barbiturate therapy, ventriculostomy for drainage of CSF, and craniectomy (i.e., removal of portions of the skull to allow external brain swelling [bone flap]); and (6) enteral or parenteral nutritional support at 140% of the basal rate in nonparalyzed and 100% in paralyzed individuals, with 15% of calories provided as protein within 7 days of TBI.
PTSs are classified as immediate when they occur during the first 24 hours after injury, early when they occur during the first 7 days, and late after the first 7 days. Prophylactic treatment with phenytoin or carbamazepine during the first week after TBI is an intervention option recognized by both the AANS and the American Academy of Physical Medicine and Rehabilitation. Both organizations recognize that the efficacy of prophylactic treatment diminishes greatly after the first week and thus recommend discontinuation of anticonvulsant medication as standard treatment after the first week.10,11 If late PTSs develop, treatment is warranted because the reoccurrence rate is greater than 80%.33 All patients and caregivers should learn recognition of and first aid for seizures, as well as risk modification. Avoidance of alcohol, street drugs, and prescribed medications that lower the seizure threshold is important for clients recovering from TBI. The groups at highest risk are those with penetration of metal and bone into the brain substance, biparietal contusions, multiple intracranial operations, and any injury that causes more than a 5-mm lateral shift on CT.20
Implementation of the aforementioned standards and guidelines more typically takes place in designated trauma centers, where physicians, nurses, and allied health providers are accustomed to treating large numbers of individuals with TBI. Studies in both academic and community hospital settings have shown that morbidity and mortality can be reduced appreciably by following the AANS guidelines.59
After survival from the initial injury, ongoing optimal medical and health management can facilitate an individual’s recovery and ability to participate in his or her own rehabilitation. Early detection and prompt management of sleep and mood disorders, pain, hydrocephalus, heterotopic ossification, and endocrinopathies, which are all common sequelae of TBI, must be addressed. Medical therapeutic interventions should be based on behavioral, cognitive, and functional performance factors that are observable and measurable by members of a rehabilitation team.
Coma and Levels of Consciousness
A TBI typically results in an altered level of consciousness. The continuum of consciousness includes coma at one end and conscious awareness at the opposite end. After a brain injury, an individual’s progression along this continuum of consciousness depends on age; previous health status; severity of the injury; and the methods of medical, therapeutic, and environmental management.
Consciousness is a state of environmental awareness and self-awareness. Coma involves the absence of awareness of self and the environment despite maximal external stimuli. No periods of wakefulness occur in the coma state.60 When sedating and hypnotic medications are discontinued, the coma rarely lasts more than 4 weeks. When the coma resolves, the person becomes either partially aware of self and the environment (“minimally conscious”) or, if no awareness is present, “vegetative.”
The GCS has been the traditional method used by health care professionals to assess levels of consciousness after TBI (Table 34-1). The GCS has been used to quantify the severity of brain injury and predict outcome. The three behavioral areas assessed in the GCS are motor responses, verbal responses, and eye opening. The most reliable is the motor score; when it reaches 5, which signifies a purposeful response to pain, such as pushing away noxious stimuli, or 6, which represents an ability to follow simple commands, the injured individual is no longer in a coma or vegetative state. This is an important landmark in recovery from TBI.74
TABLE 34-1
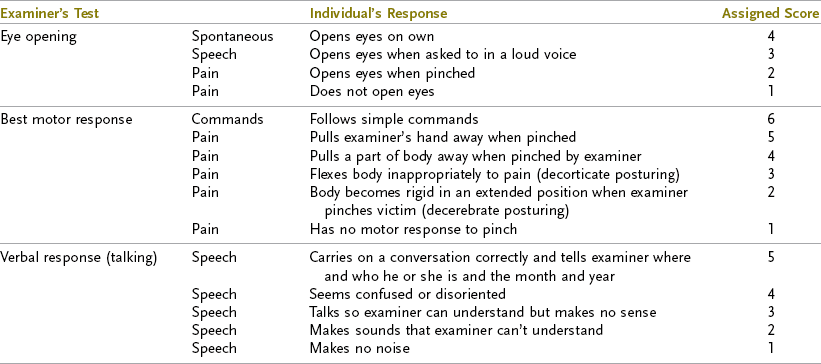
From Rosenthal M: Rehabilitation of the head-injured adult, Philadelphia, Pa, 1984, FA Davis.
The vegetative state is most succinctly described as wakefulness without awareness. A person in a vegetative state has the following characteristics: (1) no awareness of self or the environment and an inability to interact with others; (2) no sustained, reproducible, or voluntary behavioral responses to sensory stimuli; (3) no language comprehension or expression; (4) sleep-wake cycles of variable length; (5) ability to regulate temperature, breathing, and circulation to permit survival with routine medical and nursing care; (6) incontinence of bowel and bladder; and (7) variably preserved cranial nerve and spinal reflexes. A persistent vegetative state refers to a condition of past and continuing disability with an uncertain future; the typical onset is within 1 month of a traumatic or nontraumatic brain injury or after a month-long metabolic or degenerative condition. The condition may improve and the client may achieve a minimally conscious state (MCS) over time. If the client does not improve, the term permanent vegetative state is appropriate and signifies that the chance of regaining consciousness before death is exceedingly small.71 Recovery of consciousness is rare for individuals in a persistent vegetative state 12 months after a TBI or 3 months after a nontraumatic brain injury.72
Practice parameters regarding the care of individuals in a persistent vegetative state indicate that appropriate diagnosis of the condition is crucial; a physician with experience in this area should participate in the determination. Once the diagnosis is established, the prognosis should be explained in detail to the family, surrogates, and caregivers. Appropriate care respects the individual’s comfort, hygiene, and dignity. Careful observation of any signs of emergence to MCS is important in determining the intensity of therapeutic interventions. Positioning and other interventions to manage tone and prevent contractures should be included. The amount of extraordinary care will be guided by the advanced directives or presumed directives supplied by the patient’s surrogate.62
Many individuals emerge from a persistent vegetative state to MCS in which there is definite behavioral evidence of awareness of self, environment, or both. Clearly discernible, reproducible behavior in one or more of the following areas must be demonstrated: (1) ability to follow commands, (2) gestural or verbal yes/no responses (regardless of accuracy), (3) intelligible verbalizations, and (4) purposeful movements or affective responses that are appropriate reactions to environmental stimuli. Examples include reaching for objects; touching or holding objects and accommodating for their size and shape; engaging in eye pursuit movements or sustained fixation in direct response to stimuli; and smiling, crying, vocalizing, or gesturing in response to relevant stimuli. Convenient ways to assess for MCS are to test an individual with situational orientation questions (e.g., “Are you standing? Are you in a chair?”) and giving the individual an object of common use, such as a washcloth or comb, and seeing whether the patient tries to use it. Testing for MCS should occur in a quiet environment when the patient is alert (i.e., not under sedating medication or in a physical position that encourages inattention). Requested commands should not exceed the patient’s physical capabilities and not involve reflexive movements.26 Serial assessment tools are available that can be useful for measuring the cognitive progress of individuals in MCS, such as the JFK Coma Recovery Scale (JFK), Wessex Head Injury Matrix (WHIM), Coma–Near Coma Scale, Sensory Stimulation Assessment Measure, and the Western Neuro Sensory Stimulation Profile.14,57
Another important landmark in recovery is post-traumatic amnesia (PTA), which is probably the single best measurable predictor of functional outcome in the research literature (Table 34-2). PTA is the length of time from the injury to the moment when the individual regains ongoing memory of daily events. Some evidence suggests that the duration of PTA is highly correlated with individual outcomes. Longer PTA is associated with poorer long-term cognitive and motor abilities and a decreased ability to return to work and school. PTA lasting 4 weeks or longer is correlated with significant long-term disability.53 Measurement of PTA is performed with the Galveston Orientation and Amnesia Test or the Orientation Log.48 The latter test is easier to administer to individuals with moderate to severe TBI in which the examiner may not know the details of circumstances that took place immediately before the injury and in which the injured individual has begun to remember events.39,48
TABLE 34-2
Duration of Post-traumatic Amnesia and Severity of Injury
| PTA Duration | Severity |
| Less than 5 min | Very mild |
| 5 to 60 min | Mild |
| 1 to 24 hr | Moderate |
| 1 to 7 days | Severe |
| 1 to 4 weeks | Very severe |
| More than 4 weeks | Extremely severe |
From Rosenthal M: Rehabilitation of the head-injured adult, Philadelphia, Pa, 1984, FA Davis.
The Rancho Los Amigos Scale of Cognitive Functioning is a descriptive measurement of levels of awareness and cognitive function.63 Progression through the various levels occurs most typically with traumatic injuries. However, the recovery curve of some individuals who are very severely injured may actually skip a level, typically Rancho IV, an agitated confused state. Others may never be as low as Rancho level I or II but may be agitated and confused for several weeks (Rancho IV). These individuals may experience periods during which they also function at Rancho V or VI levels. Thus, this scale can be helpful to treating staff and family members in designing specific behavioral interventions for a given patient. Box 34-1 contains a complete description of the scale.
Although many studies have analyzed factors such as age, severity, cause of the injury, substance abuse, and psychosocial status in predicting outcomes after TBI, they have definite limitations regarding the recovery of an individual patient.8,12,17,22,35,78 Individuals with TBI improve over a period of months to years, especially once the individual becomes aware of his or her altered capabilities.61 Monitoring an individual’s personal rate of recovery is probably more predictive of future recovery than any other factor.
Clinical Picture
An individual with TBI may exhibit a variety of symptoms, depending on the type, severity, and location of the injury. Individuals may have limitations in most of the areas listed in the following sections, or they may have subtle deficits evident only during more complex activities. Table 34-3 shows some of the most common clinically diagnosed physical signs and symptoms of a client who has suffered a TBI.
TABLE 34-3
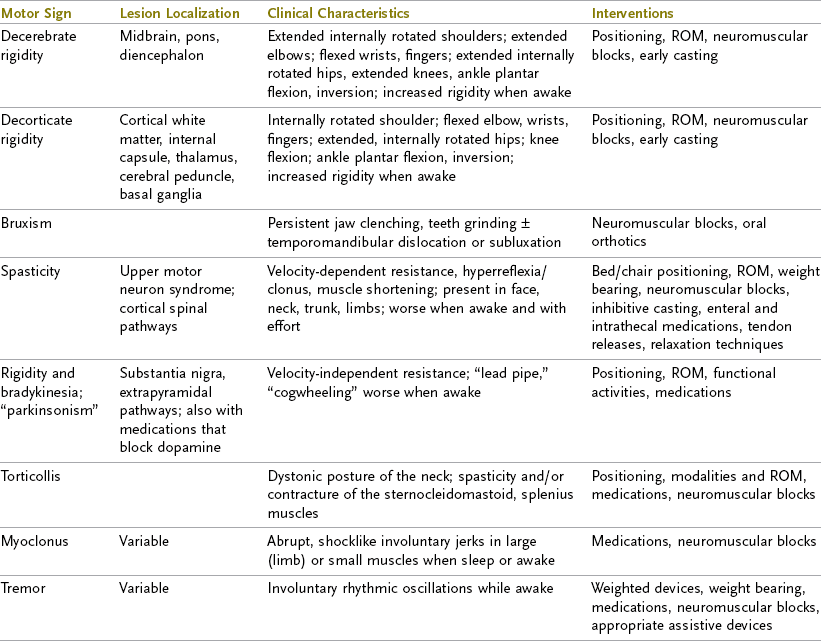
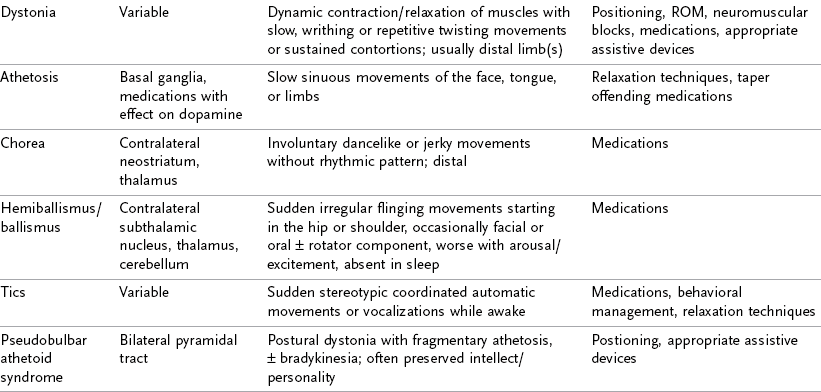
Data from Mayer NH, Keenan MAE, Esquenzi A: Limbs with restricted or excessive motion after traumatic brain injury. In Rosenthal M, Griffith ER, Kreutzer JS, Pentland B, editors: Rehabilitation of the adult and child with traumatic brain injury, ed 3, Philadelphia, Pa 1999, FA Davis; Mayer NH: Choosing upper limb muscles for focal intervention after traumatic brain injury, J Head Trauma Rehabil 19(2):119, 2004; Zafonte R, Elovic E, Lombard L: Acute care management of post-TBI spasticity, J Head Trauma Rehabil 19(2):89, 2004.
Decorticate, Decerebrate, and Motor Rigidity
Rigidity is the presence of increased resistance to passive movement throughout most of the range that is independent of stretch velocity.51 Comatose individuals often display one of two common positions: decorticate rigidity and decerebrate rigidity. In decorticate rigidity, the upper extremities (UEs) are in a spastic flexed position with internal rotation and adduction. The lower extremities (LEs) are in a spastic extended position but also internally rotated and adducted. Decorticate rigidity results from damage to the cerebral hemispheres (particularly the internal capsules) that causes an interruption in the corticospinal tracts—those that emerge from the cortex and send voluntary motor messages to all extremities.
In decerebrate rigidity, both the UEs and LEs are in a position of spastic extension, adduction, and internal rotation. The wrist and fingers flex, the plantar surfaces of the feet flex and invert, the trunk extends, and the head retracts. Decerebrate rigidity occurs as a result of damage to the brainstem and extrapyramidal tracts—the tracts that send involuntary motor messages from the brainstem to the extremities. Individuals with decerebrate rigidity have a poorer prognosis than do those exhibiting decorticate rigidity.15,29
Cogwheel or lead pipe rigidity resembling Parkinson’s disease can occur, typically in individuals with severe TBI. It may respond to dopamine agonists such as levodopa/carbidopa or amantadine. Dystonia of the neck (torticollis), jaw, or distal ends of the limbs can also occur and may require treatment with motor point blocks.51
Abnormal Muscle Tone and Spasticity
Although decorticate rigidity and decerebrate rigidity are associated with the most severe types of abnormal muscle tone and tend to occur in comatose individuals, hypertonicity may range from minimal to severe in any muscle group. Individuals who are functioning at a higher cognitive level than coma generally display a combination of both hypotonicity (i.e., decreased tone, or flaccidity) and hypertonicity (i.e., increased tone, or spasticity). Flaccidity, or hypotonicity, is a decrease in normal muscle tone. It is usually attributed to peripheral nerve injury resulting in soft muscle feel, in which the muscles offer no resistance to passive movement. Spasticity, also known as hypertonia, is an involuntary increase in muscle resistance that is dependent on velocity.47 Because individuals with spasticity cannot voluntarily relax their limbs, voluntary movement of an affected limb may be impossible. Spasticity can be seen as early as a few days after brain injury, or it may take between 3 and 6 months to develop. In as little as 2 weeks, spasticity may cause muscles to shorten permanently, which in turn can cause joints to lose motion. The condition of permanent shortening is called a muscle contracture.
Primitive Reflexes
If damage to the midbrain has occurred, impaired righting reactions are commonly observed. Similarly, damage to the basal ganglia can result in the absence of equilibrium reactions and protective extension. The absence of righting reactions, equilibrium reactions, and protective extension places the individual at a significant risk for further injury from falls during such activities as transfers, getting out of bed, toileting, bathing, and dressing.
Muscle Weakness
A decrease in muscle strength without the presence of spasticity can occur as a result of peripheral nerve or plexus injuries and lack of physical activity caused by secondary factors associated with TBI (e.g., compromised respiration, fractures, and infection). A functional muscle and sensory test may be indicated when an individual exhibits decreased strength in the limbs. Additionally, impaired gross and fine motor coordination will be evident and should be assessed.
Decreased Functional Endurance
Decreased endurance and vital capacity usually accompany reduced muscle strength as a result of medical complications such as infections, poor nutrition, or prolonged bedrest. Increasing the individual’s muscle strength and endurance is a primary goal in the acute stage and in the initial stages of rehabilitation.
Ataxia
Ataxia results from impairment of the cerebellum itself or the motor pathways leading to and from the cerebellum; it can also occur with impaired proprioception. Ataxia is a movement abnormality characterized by incoordination, impaired sitting and standing balance, or both.7 Ataxia can occur in the entire body, in the trunk, or in the UEs and LEs. An individual with ataxia has lost the ability to perform the small adjustments in the distal and proximal ends of extremities that are necessary for smooth, coordinated movement. The degree of ataxia ranges from mild to severe. An individual with ataxia in the trunk displays impaired postural stability when sitting and standing. He or she has difficulty maintaining the trunk in a stable position to free the UEs or LEs for activities. The individual may compensate for this deficit by grasping a stable surface such as a tabletop. Ataxia in the UEs causes dysfunction in activities in which the individual attempts to perform a combination of gross and fine motor movements, such as bringing a glass of water to the mouth. The UE oscillates back and forth, thus causing the water to spill. Ataxia in the LEs results in an impaired ability to ambulate while maintaining balance; falls can easily occur with this condition.
Postural Deficits
Postural deficits develop as a result of an imbalance in muscle tone throughout the body. An individual may inadvertently accentuate the postural deficits by using ineffective strategies to compensate for impaired motor control, delayed or absent righting reactions, or impaired vision, cognition, or perception. Therapists must possess thorough knowledge of the postural deficits of their clients to position them properly in a wheelchair with the appropriate seating system, which is necessary to obtain an upright posture, maintain good postural alignment, and prevent further postural deformities. Frequently exhibited abnormal postures include the following:
1. Pelvis. Posterior pelvic tilt is often due to prolonged bedrest in the supine position, which causes loss of range of motion (ROM) in the lower part of the back. Posterior pelvic tilt results in sacral sitting and facilitates kyphosis. Pelvic obliquity is observed when one side of the pelvis sits lower than the other side as a result of hypertonicity of the quadratus lumborum on the involved side.
2. Trunk. Kyphosis, scoliosis, and lordosis may all be present secondary to weak or spastic trunk muscles (e.g., pectoralis, abdominal, spinal, and paraspinal). It is also common to observe lateral flexion toward the involved side (trunk shortening) with elongation of muscle on the opposite side.
3. Head and neck. Forward flexion or hyperextension of the neck and lateral flexion of the head often accompany lateral flexion of the trunk.
4. Scapula. The scapula may be depressed, protracted or retracted, downwardly rotated, or all of these at once. This results from an imbalance in scapular muscle tone; some muscles are hypertonic, whereas others are hypotonic.
5. Upper extremities. UEs may be bilaterally or unilaterally involved. In unilateral involvement, it is common to see variations in ROM, tone, and strength in each muscle group and joint of the arm, forearm, wrist, and hand.
6. Lower extremities. Severe extension patterns are often observed in both LEs, which can pose a problem with wheelchair positioning; this is evident when the individual thrusts forward and slides out of the seating system. Hip adduction, internal rotation, knee flexion, plantar flexion, and inversion of the feet can all be observed.
Limitations of Joint Motion
Loss of ROM in the joints is a common problem. It is often difficult to distinguish between several possible causes of decreased ROM, such as increased muscle tone, volitional resistance, contractures, heterotopic ossification, fractures or dislocations, and pain. Because the intervention addressing decreased ROM depends on the cause, the therapist should consult a physician to determine the cause of the decreased ROM before initiating intervention. Distal limb fractures are often overlooked in acute trauma settings when patients are unable to communicate because of cognitive deficits. Therapists are typically the first to detect the hard end feeling of joints with limited ROM typical of heterotopic ossification, the formation of lamellar bone in soft tissue.34
Sensation
Clients with TBI may exhibit signs of absent or diminished sensation, including problems with light touch, differentiation between sharp and dull sensations, proprioception, temperature, pain, and kinesthesia. Additionally, impaired senses of taste and smell, caused by cranial nerve injury, may be observed.7 Lost or diminished stereognosis, two-point discrimination, and graphesthesia (i.e., the ability to interpret letters written on the hand without visual input) may be present. Hypersensitivity, which can often interfere with postural alignment, may also occur.
Integration of Total Body Movements
Total body movements involve the integration of head, neck, and trunk control with dynamic sitting and standing balance while reaching, bending, stooping, and ambulating. To perform total body movements, the individual must coordinate and modulate gross and fine motor movements of the trunk, head, neck, and limbs while performing ADLs. An individual with severe physical involvement often displays poor sitting and standing balance and is unable to maintain an upright position to free the UEs for activities. Individuals functioning at a more advanced level may exhibit subtle deficits in total body movements that make it difficult to bend down, reach overhead to retrieve items in a cabinet, or stoop to retrieve an item that has fallen to the floor. Integrated total body movements are necessary for the performance of all ADLs.
Dysphagia
Dysphagia, or difficulty completing the four stages of chewing and swallowing, is caused by cranial nerve damage (see Chapter 27). There is a higher incidence of oral preparatory–, oral-, and pharyngeal-stage dysphagia than esophageal-stage dysphagia. Typically, more than one stage of chewing and swallowing is impaired.5,6
An individual may display oral muscular hypotonicity or hypertonicity, instability of the jaw, and abnormal oral reflexes such as rooting, biting, sucking, gagging, and coughing, which prevent or impair the activity of speaking or eating. As a result of cognitive deficits, the individual may experience difficulty in sequencing chewing, swallowing, and breathing.
Self-Feeding
Clients with TBI may be unable to sustain attention long enough to feed themselves. If impulsivity is apparent, these clients will have difficulty monitoring the amount and rate of food brought to the mouth, thus causing coughing and possibly aspiration. Oral apraxia, an inability to perform an intended action or execute an act on command with the mouth or lips, may occur. If clients have ideational apraxia, they will have difficulty understanding the demands required of the self-feeding activity and will be unable to recognize utensils as tools for eating. Because they may also have lost the motor plan for self-feeding (ideomotor apraxia), they may be unable to gain access to the neurologic motor pattern for bringing food to the mouth. Hemianopia (visual field cut) or visual neglect may prevent them from seeing half of the plate of food.
Cognitive Status
Cognitive deficits are always evident to varying degrees and can affect many aspects of the individual’s quality of life, as mentioned in previous sections. The most common include decreased attention and concentration, impaired memory, impaired initiation and termination of activities, decreased safety awareness and poor judgment, impulsivity, and difficulty with executive functions and abstract thinking (e.g., problem solving, planning, integration of new learning, and generalization).
Attention and Concentration
Reduced attention and concentration impair the ability to maintain focus on an activity without becoming distracted and the ability to resume an activity when interrupted. Clients with TBI often lose the ability to concentrate for a length of time and the ability to filter out distractions from the surrounding environment. The inability to attend to and concentrate on activities severely impedes the ability to function at work and school and complete ADLs. Although deficits in attention and concentration can diminish as neurologic recovery progresses, such deficits can remain to varying degrees throughout an individual’s life. Even patients who experience mild TBI can demonstrate subtle deficits in attention and concentration that often linger for years after the injury and can affect their everyday functioning.
Memory
Impaired memory, the most frequently observed cognitive deficit in patients with TBI, can remain a lifelong problem. Memory impairment ranges from forgetting several words just heard (immediate memory), to forgetting which family members visited the night before (short-term memory), to forgetting events that occurred years before the injury (long-term memory). Despite neurologic recovery, most of these patients continue to demonstrate difficulty in learning new information. Safety concerns include getting lost, leaving doors unlocked, and leaving a stove on; patients with impaired memory typically require supervision if compensatory methods cannot be used. This loss of independence can be emotionally devastating because patients with TBI often have insight into who they were before the injury, as well as their accomplishments, goals, and plans for the future—many of which are severely disrupted and perhaps lost as a result of TBI.
Memory losses are also labeled in relation to the time of the injury or brain damage. Loss of memories for events before the time of the specific injury is referred to as retrograde amnesia. The client may forget events that occurred just before the accident or forget events that happened several days, weeks, and even months before the accident. Following TBI the client is often unable to form new memories, and this is referred to as anterograde amnesia. This period when the client cannot form new memories can last for days, weeks, or even months after the injury.
Initiation and Termination of Activities
Impaired initiation and termination of activities affect the ability to start and end activities. An inability to initiate activities without assistance affects the individual’s ability to live independently. In general, patients who exhibit deficits in initiation will demonstrate the greatest progress in a rehabilitation setting that provides assistance and structure. After returning home, patients may regress and have difficulty completing basic daily activities if the necessary structure has not been set up. Similarly, patients may exhibit difficulty terminating an activity once it is started, which is a type of perseveration. For example, patients may initiate brushing their teeth and be unable to end the task because they feel compelled to continue. Perseveration sometimes involves a thought process. Clients may be unable to concentrate on one activity because they are perseverating on the idea that another activity (e.g., the laundry) must be completed.
Safety Awareness and Judgment
Frontal lobe damage often results in an impairment in insight regarding a person’s limitations, as well as in impulsivity, or the inability to consider consequences before acting. Such individuals demonstrate poor safety awareness and judgment. For example, the client may attempt to rise out of a wheelchair without locking the brakes or moving the footrests. A more mobile client who has been reintegrated into the community may attempt to cross streets without observing traffic signals or remove pots from the stove or oven without using protective oven mitts or pot holders. It is important for the occupational therapist to structure the client’s environment to reduce accidents and increase the client’s awareness of his or her limitations through repeated opportunities to practice and relearn safe and appropriate behavior.
Processing of Information
Most people with TBI experience some degree of difficulty processing external information from the environment. A delay in response time is often noted and can range from a few seconds to several minutes. It is important for the therapist to recognize the presence of delayed processing and distinguish the delay from the absence of function. For example, during sensory evaluation a client may exhibit a delay in response to a dull stimulus. The therapist may mistakenly interpret the individual’s delayed processing time as an absence seizure or impairment of sensory awareness. A delay in the processing of external information from the environment can involve visual, auditory, sensory, and perceptual processing.
Executive Functions and Abstract Thought
Executive function skills include the ability to plan, organize, set goals, understand the consequences of one’s actions, and modify behavior in accordance with environmental responses. Abstract thinking is the ability to hold and manipulate a concept in one’s mind by using critical reasoning and analytic skills. Many clients with TBI exhibit concrete thinking, in which they are able to interpret information only at the most literal level. For example, individuals with impaired executive and abstract functions may be able to complete a meal preparation activity accurately and safely only if step-by-step directions are provided. If the directions do not specifically direct the reader to modify the cooking temperature, these individuals may burn the food because they are unable to foresee the consequences of maintaining the stove on a high setting.
Generalization
Generalization of new learning is the ability to learn a specific task and transfer the skills needed for that task to a similar activity. Deficits in executive function skills, abstract thinking, and short-term memory significantly impair the generalization of new learning. For example, an individual who has learned in a day treatment setting the skills for completing a laundry task may be unable to transfer the skills at home or at a different laundromat. Such deficits often occur as a result of concrete thinking and the inability to form abstractions. Although the cognitive pattern for completing laundry tasks with the laundry machine in the clinical setting is established, the individual cannot transfer that cognitive pattern to a similar but unfamiliar laundry machine in a different environment. Impaired generalization of new learning is one of the most significant problems impeding the individual’s ability to resume independent functioning in a community setting.
Visual Status
Visual skills involve the ability to accurately see stimuli from the external environment (see Chapter 24). Visual skills do not involve the identification of objects, which is a function of perception. Among the many deficits in visual skills that may result from TBI are accommodative dysfunction (causing blurred vision), convergence insufficiency (the inability to maintain a single vision while fixating on an object), lateral or medial strabismus, nystagmus, hemianopia, and impairment of scanning and pursuits. Saccades (fast, jerky movements of the eyes as they change from one position of gaze to another, as needed to read a book) may also be compromised by TBI. Reduced blink rate, ptosis (drooping of the eyelid), and lagophthalmos (incomplete eyelid closure) are also common visual deficits resulting from damage to the oculomotor nerve.7
Dysfunction in any of these visual elements can profoundly affect daily life function. Individuals rely on vision indirectly in social and interpersonal interactions. Vision is used as a cueing and feedback system for motor skills such as ambulation and for eye-hand coordination activities. Deficits in vision can affect all daily life activities, including the areas of hygiene and grooming, meal preparation and eating, wheelchair mobility, reading and writing, and driving.
Perceptual Skills
Perception is the ability to interpret stimuli from the external environment (see Chapter 25). Perception is a function of the secondary cortical areas of the right hemisphere, including the secondary visual area, the secondary somatosensory area, the secondary auditory area, and the multimodal parietal-occipital-temporal area. Perceptual deficits are more often a result of right hemisphere damage but may also occur with lesions in the left hemisphere. Perception can be grouped into the following categories: visual perception, body schema perception, motor perception, and speech and language perception. An individual with impairments in visual perceptual may exhibit difficulty in right-left discrimination, figure-ground discrimination, form constancy, position in space, and topographic orientation. Visual perceptual deficits also include visual agnosia, in which the individual displays difficulty recognizing familiar objects and people. For example, prosopagnosia is the inability to connect faces with names. Prosopagnosia results from damage to the multimodal association area.7
Body schema perception is awareness of the spatial characteristics of a person’s own body. This awareness is derived from the neural synthesis of tactile, proprioceptive, and pressure sensory associations about the body and its individual parts. A common problem in persons with TBI is anosognosia, a failure to recognize deficits or limitations. This may lead to the body schema perceptual dysfunction of unilateral neglect syndrome, in which the individual has lost the ability to integrate perceptions from one side of the body or environment (usually the left). Unilateral neglect is commonly caused by a lesion in the right parietal lobe but can also occur as a result of frontal and occipital lobe damage. Patients with left unilateral neglect may disown their left extremities and treat them as though they belong to someone else. For example, these individuals may shave only the right side of their face or dress only the right side of their body.7
Aphasia is a disturbance in comprehension or formulation of language (or both) caused by dysfunction in specific brain regions, typically the left hemisphere.19 A few left-handed individuals have language dominance in the right hemisphere. Establishing reliable communication is crucial in treating anyone with aphasia. If auditory comprehension is compromised, gestural demonstration of instructions or activities will be more reliable. Common types of aphasia involving disorders of comprehension include the Wernicke and transcortical sensory types. Affected individuals will have longer periods of PTA because they do not understand orientation questions, and although their spoken language is fluent, it includes verbal paraphasias, or word substitutions. Clients with aphasia may also misinterpret speech and become suspicious and agitated. Insight into their communication deficit may be limited.
The nonfluent aphasias (Broca’s aphasia and transcortical motor aphasia) are characterized by relatively preserved comprehension but effortful or explosive speech with phonemic paraphasias (e.g., “bork” for “fork”). Conduction aphasia (i.e., intact comprehension, fluent speech, impaired repetition) and anomic aphasia are characterized by circumlocution and frequently paraphasias. Individuals with these types of aphasia are typically aware of their problems and are often frustrated by their limitations. They should be encouraged to use gestures to express their immediate desires and needs.
Dyslexia (disturbance in reading), agraphia (disorder of writing), and dyscalculia (disturbance in calculation) frequently accompany the aphasias. However, with traumatic aphasias, these capabilities may be better preserved than with stroke; treating therapists should always attempt alternative modes of communication.
Dysprosody or aprosody is impaired production or comprehension of the tonal inflections or emotional tone of speech. Executive dysprosody is an inability to inflect one’s voice to convey emotion. It can occur with cerebellar, basal ganglia, or right frontal lobe injury. Receptive dysprosody is an inability to perceive the emotional content of other people’s spoken language. It occurs with right temporal or parietal injury and less often with left hemisphere injury. Individuals with this disorder may miss the point of a joke or story because they cannot comprehend the subtle innuendoes and implicit meanings conveyed through tonal qualities and inflections.75 Even more disabling is the inability to interpret anger, humor, or sarcasm during communication with others.18 Perceptual-motor dysfunction is impairment in motor planning, or an apraxia. It is a disorder of learned movement that cannot be explained by weakness, lack of sensation, inattention, or comprehension of the requested task.18 The apraxias are usually a result of impairment of the premotor cortex, corpus callosum, or connections between the temporal/parietal lobes and frontal motor cortex.37 It is in these cortical areas that established motor patterns for specific activities are stored and accessed for the execution of common movement patterns. Ideational apraxia is an inability to understand the demands of a task or use of the wrong motor plan for a specific task. For example, individuals suffering from ideational apraxia may not understand that a shirt is an item of clothing to be placed on the torso and UEs. Not understanding the demands of the task, they may be unable to activate the motor plan for UE dressing or may activate the wrong plan and attempt to place their legs through the sleeve holes. This deficit is sometimes referred to as a dressing apraxia. Ideomotor apraxia is loss of the kinetic memory of a movement pattern for a specific activity. Individuals with this disorder may understand that a shirt is an item of clothing to be placed on the torso and UEs but be unable to execute the appropriate movement plan because it is no longer accessible. Constructional apraxia is an inability to accurately assemble pieces of an object to form a three-dimensional whole. For example, a former carpenter who suffers from constructional apraxia may be unable to put together the wooden pieces of a basic birdhouse kit.
Psychosocial Factors
Researchers have found that the greatest concerns of clients 1 or more years after TBI are the psychosocial deficits that prevent them from rebuilding a satisfactory quality of life. As the time after injury increases, clients and family members view such psychosocial factors as being more detrimental than both the physical and cognitive sequelae of TBI.
For example, in Marisol’s case, it was initially difficult to assess her psychosocial status because she was nonvocal. The team was able to assess her mood on the basis of her level of participation and affect during therapy. Marisol would laugh appropriately and become visibly more interactive, with brighter affect, when her boyfriend arrived to visit.
Marisol continued to engage in positive interactions throughout her 4-month inpatient and day treatment stay. Her discharge plan involved moving to Georgia with her mother. When the move was discussed with Marisol, it was evident that she was quite saddened by the knowledge that she would no longer be able to see her boyfriend. The team observed her closely to assess whether her sadness would eventually culminate in depressive symptoms.
Self-Concept
One of the most difficult psychosocial sequelae of TBI is alteration of the individual’s self-concept. Self-concept is the internal image that a person holds regarding personal human identity, sexual and gender identity, body image, personal strengths and limitations, and position in the family, peer group, and community systems. An individual’s self-concept changes drastically after TBI. One of the most difficult characteristics of TBI is that although short-term memory is often impaired, long-term memory commonly remains intact. Persons with TBI often have a clear memory of who they were before their injury and must now resolve the emotional conflict of having to replace their preinjury self-concept with a postinjury self-concept that is both meaningful and satisfying. Affected individuals sometimes describe this process as an unwanted death and rebirth. They say that the person who lived before the injury is now gone, replaced by someone who is very different from the person that they remember themselves to be.58
Social Roles
Self-concept is derived largely from the social roles that the person attains in the family, peer group, and larger community systems. Frequently, an individual with TBI loses most preinjury roles and the activities that supported those roles. Family and peer group roles change. Family members and friends are often readily visible during the acute and subacute stages of TBI rehabilitation. However, as the time after injury increases, family and friends become progressively less involved with the individual, which frequently leads to feelings of isolation and abandonment. Many individuals with TBI report that the feeling of isolation and the inability to form and maintain social relationships are their most troubling postinjury concerns. Loss of the role of dating partner or spouse commonly leaves clients with TBI with a deep sense of loss and failure if they cannot rebuild a postinjury life that includes intimacy with another human being, partnership in a committed relationship, and parenting of children. Loss of the work role and inability to support oneself are intimately tied to feelings of dependence and lack of personal control.44
Independent Living Status
As a result of the physical, cognitive, and psychosocial sequelae of TBI, many affected individuals find that they require supportive living arrangements or must live with their parents. Loss of the ability to live independently in the community further reinforces feelings of dependence and decreased personal control. As a result of these role losses, adults who sustain TBI commonly experience role strain and feel that they cannot re-enter their communities. The TBI, particularly if it occurred between the ages of 18 and 30, disrupts the developmental transition from adolescence to adulthood and leaves individuals feeling inadequate and unable to attain a postinjury adult status. Depression, withdrawal, and apathy are common psychosocial sequelae of the alterations in self-concept discussed earlier and of the loss of desired social roles.52
Dealing with Loss
Persons with TBI and their family members often go through a process that resembles the stages of death and dying experienced by the terminally ill.45 These stages begin with denial, in which affected individuals deny that they are experiencing physical, cognitive, or psychosocial deficits. Denial can impede therapy because these clients may refuse to participate in the belief that it is unnecessary. Denial gradually subsides as they continually confront their limitations in ADLs. Anger follows denial. Clients grow increasingly aware of their deficits and become frustrated and angry because recovery is slower than desired. Bargaining is the next stage. Clients strike a deal with the Creator or the fates and offer to work as diligently as possible in therapy if only their preinjury lifestyle could be restored. The bargaining stage is often marked by increased motivation and optimism. Depression tends to emerge next. Eventually, clients begin to realize the severity of the injury and its effect on the rest of their lives. Acceptance of the injury and resultant limitations, the next stage in the process, is necessary for clients to become sufficiently motivated to attempt to build a postinjury life that although drastically different from their preinjury goals and expectations, is nevertheless meaningful and personally valuable. These stages may require years of transition. Frequently, denial, anger, and bargaining occur in the first few months to a year after the injury. Depression sets in as the individual is able to let go of some of the denial and becomes aware of the effect that the injury will have on the future. It may take years before he or she can truly accept the injury and alterations in personality, skill, and lifestyle and move on to rebuild a new life.
The process of denial, anger, depression, and acceptance does not generally proceed in a linear fashion. Clients with TBI commonly experience repeated periods of denial, anger, and depression throughout their years of rehabilitation. Renewed denial, anger, and depression may occur in response to a new environmental demand, such as a change in life condition (e.g., a need to move from the parental home to a community group home) or the development of further physical, cognitive, or psychosocial deterioration over time (e.g., the need for increased ambulatory assistance because of deterioration in visual skills).
Affective Changes
Depression, increased emotional lability, and decreased affect can result from the neurologic damage itself. Individuals with left hemisphere damage tend to exhibit increased depression and emotional lability. Lesions of the left orbitofrontal lobe often cause severe depression and heightened affect (including excitement, agitation, and tearfulness). Lesions of the left dorsolateral frontal lobe commonly result in a decreased or flat affect. Individuals with these lesions may appear depressed even though they feel fine. Neurologic damage to the right hemisphere frequently causes a strange sense of euphoria or lack of emotional response to the severity of injury.58
Behavioral Status
Behavioral impairments are a natural part of the recovery process. The Rancho Los Amigos cognitive level IV is typically described by the rehabilitation team as the “agitated, confused” level.63 During this stage of recovery, affected individuals can be described as restless and combative. They may be responding to internal body experiences, or some external environmental stimuli may be provoking the agitation. Commonly observed behavior includes yelling, swearing, grabbing, and biting. Behavioral problems can be disturbing to both the individual’s family and the intervention team; therefore, behavioral management is an essential component in TBI rehabilitation.
A comprehensive behavior management program should be established for anyone who exhibits behavior that interferes with active participation in therapy and achievement of goals. The goals and objectives of a comprehensive program include maintaining a safe environment for individuals and staff at all times, developing and consistently implementing behavior management techniques, minimizing the use of all restrictive modalities, and providing an environment that facilitates participation and appropriate behavior in the hospital setting and after discharge.65
Interventions used in an effective behavior management program include one-on-one coaching, intervention with psychotropic medications, and individually designed behavior management guidelines and interventions. One-on-one coaching, usually performed by a trained nursing assistant or rehabilitation technician, is especially necessary for clients who are at risk of harming themselves or others. In many cases, implementation of a behavior management program is necessary 24 hours a day, 7 days a week. A coach will help reinforce the client’s behavior plan and redirect inappropriate or maladaptive behavior. Medications are required to regulate sleep and minimize agitation and combative behavior until clients can control the behavior on their own. Because pain may often provoke agitation, assessing patients’ level of pain and providing appropriate medication may resolve the restlessness, agitation, and/or inability to sleep. Medications must be chosen carefully to prevent side effects such as clouding of awareness and psychomotor slowing. Clients should have specific behavioral end points, such as establishing adequate sleep at night, facilitating attention during functional activities, and decreasing the frequency of verbal or physical outbursts.
Environmental modifications are a proactive approach to prevent and minimize undesirable behavior. Such modifications may include use of a cubicle or net bed, an alarm system, a helmet, and walkie-talkies. A drug and alcohol policy is frequently necessary; it is well documented that many individuals with TBI have pre-existing alcohol or drug problems (or both).66
The first step in becoming more comfortable when working with clients who have behavior problems is to understand why they occur and how they manifest themselves.
Clients who are exhibiting agitation, combativeness, disinhibition, and refusal to cooperate and participate in activities typically have difficulty filtering distractions and can become agitated in noisy environments. Providing a quiet room during interventions and use of a cubicle bed may help minimize noise and reduce outbursts.
A disinhibited individual may lack awareness of the external environment and may make indiscriminate sexual remarks or gestures to others. Ignoring comments, redirecting inappropriate behavior, and modeling acceptable behavior are typical therapeutic interventions.
Clients who refuse to cooperate and participate in intervention can be the most challenging because this attitude may affect their ability to remain in an acute rehabilitation program. The lack of participation is typically organically based and due to cognitive deficits such as impaired initiation and lack of insight into their disability. It is important to document such behavior throughout the course of care; however, when clients refuse to participate, documenting progress can be challenging.
Interventions include providing consistent structure through daily schedules and goal sheets that provide visual cues for expectations, as well as visual and physical guidance through activities until clients are capable of completing tasks without assistance.
Evaluation of the Lower-Level Individual
Clients emerging from coma and at the beginning stages of the injury (Rancho Los Amigos level I to III) may exhibit minimal arousal and limited purposeful movements. It may be necessary to evaluate such individuals in short sessions and at different times of the day. A quiet environment with minimal distractions will enhance the client’s ability to attend to and follow commands. Evaluation includes assessment of the following:
1. Level of arousal and cognition—Can the client visually attend to the speaker and follow commands such as “open your mouth” and “squeeze your eyes closed”? Can he or she communicate through verbalizations, gestures, or eye movements? Does he or she demonstrate purposeful movements such as pulling at vital tubes? How easy/difficult is it to wake the client, and how long can the client stay awake?
2. Vision—Is the client able to visually scan or attend to a person, object, or activity? Can the client maintain eye contact?
3. Sensation—Does the client respond to external stimulation such as pain, temperature, and movement of the joints?
4. Joint ROM—Has the client lost ROM in certain joints as a result of decorticate or decerebrate posturing, increased tone or spasticity, contractures, or heterotopic ossification?
5. Motor control—Does the client exhibit decorticate or decerebrate posturing? Is there an increase in tone and spasticity? Is there decreased tone and hypotonicity? Are deep tendon responses present, diminished, or absent? Does the client exhibit the presence of primitive reflexes? Does the client engage in spontaneous motor movements, such as scratching the face?
6. Dysphagia—Does the client handle his or her own secretions, drool, or swallow spontaneously? Does the client demonstrate poor oral-motor control? Answers to these questions provide valuable information on whether a swallowing evaluation is indicated.
7. Emotional and behavioral factors—Is the client’s affect flat or expressive? Are responses such as crying or laughing observed in response to interactions with the rehabilitation team or family members?
Evaluation of lower-level individuals with TBI is generally accomplished with tools such as a goniometer, clinical muscle and tone testing, traditional neurologic screening, and clinical observations. Many acute TBI rehabilitation facilities have developed their own initial evaluation forms. A variety of scales can be used to establish a baseline and predict recovery. The GCS and Rancho Los Amigos Scale are common; however, newer cognitive scales such as the JFK and WHIM are also being used.62 Some clients tend to emerge quickly and move expeditiously through the Rancho levels, whereas others (e.g., those with anoxia) may demonstrate limited or slow recovery. A subacute program or a rehabilitation center that specializes in slow-to-recover clients may be necessary. In either case, a rehabilitation program with active therapeutic intervention is necessary to prevent contractures, encourage activity, and facilitate the client’s progress through the rehabilitative process.
In the case of Marisol, the team decided to address her spasticity and joint contractures first by providing appropriate medical interventions, including blockade of her musculocutaneous nerve to decrease her left UE spasticity. This was followed by casting to reduce her elbow contracture. The team also gave Marisol a comprehensive activity schedule in a multistimulus environment. This schedule involved transferring her out of bed and into a customized wheelchair, in which she would remain between 6 and 8 hours a day, getting up into a standing frame daily, and requiring active participation in all therapies 4 hours per day. Her cognitive level was assessed weekly with the JFK scale, as well as assessment of her ability to follow-through with basic mobility and self-care tasks.
Intervention for the Lower-Level Individual
The general aim of intervention for those at Rancho levels I through III is to increase the individual’s level of response and overall awareness of self and environment. All stimulation should be well structured and broken down into simple steps and commands. Allotting sufficient time for an individual’s response is necessary because cognitive processing is often significantly delayed during this phase of recovery. Intervention at this stage can be grouped into six areas: sensory stimulation, bed positioning, casting or splinting, wheelchair positioning, management of dysphasia, and emotional and behavioral management, as well as family and caregiver education. Interventions may occur simultaneously to optimize progress. Each intervention affects and enhances the next. Because clients often respond more to the familiar and routine, it is important to incorporate close family members and friends into sessions.
Sensory Stimulation
Intervention for clients emerging from coma should start as soon as they are medically stable. Intervention generally begins in the intensive care unit. At this stage, clients frequently lack responsiveness to pain, touch, sound, or sight. They may exhibit a generalized response to pain that appears reflexive (e.g., attempting to pull away from painful stimuli). The goal of intervention is to increase the client’s level of awareness by trying to increase arousal with controlled sensory input. Sensory regulation increases neurologic signals to the reticular activation system, the structure of the brainstem that alerts the brain to important sensory input from the external environment.
Sensory stimulation can be introduced in a variety of ways and methods. Introducing isolated visual, auditory, tactile, olfactory, and gustatory stimulants to the individual may heighten arousal. For example, a flashlight may be used to elicit eye opening and visual tracking. Playing familiar music may facilitate autonomic responses, such as a change in the respiratory rate or changes in blood pressure. Introducing olfactory stimulation through a variety of scents may elicit eye opening or head turning. Gustatory stimulation involves the controlled presentation of taste to the client’s lips and tongue through use of a cotton swab. Such stimulants may include salty, sweet, bitter, and sour tastes. Any response from the client is noted.
Kinesthetic input is incorporated early in the intervention. One of the most effective ways to facilitate volitional movement is by actively guiding movements in a normalized fashion while performing functional activities. The therapist actively helps the client perform simple movements, such as rolling from side to side, and perform simple functional activities such as wiping the mouth with a washcloth, combing hair, and applying lotion to the skin. The theoretic aim of functional sensory stimulation is to reactivate highly processed neural pathways that had been established before the injury. Other activities related to functional sensory stimulation include sitting the individual up at the edge of the bed and having the client stand by using a tilt table or a hydraulic standing frame. During all these activities, the therapist observes the client for any changes such as visual tracking, turning of the head, physical responses, vocalizations, and ability to follow verbal commands.
Wheelchair Positioning
Seating and positioning are important components of treatment in lower-level patients. Being properly positioned in a wheelchair allows these patients to interact with their immediate environment in an upright, midline posture. Proper positioning aims to facilitate head and trunk control so that clients can see and interact with people and objects in the environment. A proper wheelchair seating position helps prevent skin breakdown and joint contractures, facilitate normal muscle tone, inhibit primitive reflexes, increase sitting tolerance, enhance respiration and swallowing function, and promote function (Figure 34-1).
Effective seating and positioning require a stable base of support at the pelvis, maintenance of the trunk in the midline, and facilitation of the head in an upright, midline position. This position frees the UEs for use and allows the client to visually scan the environment. Once the client has a seating system that encourages and promotes function, therapy sessions can be more effective and beneficial. For example, clients generally find it easier to handle their secretions in this position, so swallowing trials may be safer and more effective.
Marisol required a wheelchair and specific positioning devices when she entered the acute rehabilitation program. Given her motor abilities and deficits, what type of wheelchair set-up would you prescribe?
Pelvis
Wheelchair positioning should begin at the pelvis. Poor hip placement adversely alters trunk and head alignment and influences tone in the extremities. Because sling-seat wheelchairs contribute to internal rotation and adduction of the hips, it is important to insert a firm, solid seat (padded with foam and covered by vinyl) to facilitate a neutral to slightly anterior pelvic tilt. A lumbar support may also help maintain the natural curve in the lumbar spine. A wedged seat insert (with the downward slope pointing toward the back of the chair) can be used to facilitate hip flexion and inhibit extensor tone in the hips and LEs. The individual’s buttocks should bear weight evenly, with both ischial tuberosities firmly resting on the wheelchair seat. A seatbelt angled across the pelvis helps maintain this desired position. Since patients have spent a significant amount of time in bed, loss of anterior pelvic tilt is present. Before postioning clients in a wheelchair, significant pelvis and trunk stretching is often necessary to achieve neutral pelvic alignment and upper trunk extension. These stretches often facilitate upright, symmetric trunk alignment, which may have occurred secondarily to prolonged bedrest and abnormal tone, reflexes, and patterns.
Trunk
The trunk should be positioned after the pelvis because it is the next most proximal body structure. A solid back insert or firm contoured back should be placed behind the client’s back to maintain the spine in an erect posture. A back insert that is contoured to the curves in the spine will maintain the lumbar and thoracic curves. Lateral trunk supports can be used to reduce scoliosis and lateral trunk flexion caused by imbalanced tone of the intrinsic muscles of the back. A chest strap (with easily opened Velcro fasteners) can be used to decrease kyphosis, facilitate shoulder retraction and abduction, and expand the upper part of the chest for proper diaphragmatic breathing and UE use.
Lower Extremities
An abductor wedge placed between the LEs just proximal to the knees may be used to decrease hip adduction and internal rotation. If hip abduction is present, a padded abductor wedge can be placed along the lateral aspect of the thigh to reduce LE abduction. Ideally, the knees should be positioned at 90 degrees, with the heels slightly behind the knees while sitting. It is desirable to maintain both feet securely on the foot plates to provide proprioceptive input and facilitate weight bearing in both heels to normalize tone.
Upper Extremities
The UEs should be positioned with the scapulae in a neutral position (neither elevated nor depressed), the shoulders slightly externally rotated and abducted, the elbows in a neutral position of slight flexion with forearm pronation, and the wrists and digits in a functional position. This position is often difficult to achieve because of severe spasticity and soft tissue contractures of the UEs. A splint or cast may be applied to decrease spasticity and facilitate a functional position of the UEs. Frequently, a lap tray is used to provide support for the UEs and encourage bilateral UE weight bearing and use.
Head
Clients with TBI at a lower functioning level often have little or no active head control. Attaining a neutral-midline head position, which allows optimal visual contact with the environment, is difficult. A dynamic head-positioning device (Figure 34-2) can be used to maintain neutral head alignment and facilitate head control. A contoured headrest that cradles the head posteriorly and laterally may be used to support the head in a midline position. A forehead strap (fabricated from soft, padded material) may be used to prevent the head from falling forward. Slightly reclining the wheelchair also prevents the client’s head from falling forward and facilitates visual interaction with the environment. The client should be reclined between 10 and 15 degrees; reclining the client beyond this point reduces weight bearing through the trunk and pelvis and tends to encourage extensor tone, a posterior pelvic tilt, and sacral sitting. If the patient has had a portion of the skull removed, a helmet is necessary to protect the brain during all mobility and when getting the patient out of bed into a wheelchair.

FIGURE 34-2 A dynamic head-positioning device maintains neutral head alignment and facilitates head control.
As the client progresses in rehabilitation, wheelchair seating and positioning should be continually re-evaluated to better meet his or her specific needs. Devices should be modified gradually or removed as the client begins to control his or her body actively and manipulate more items in the environment. A schedule is necessary to indicate the length of time that the client can tolerate being seated in the wheelchair. Keeping the client in a wheelchair longer than can be tolerated may result in fatigue, which can subsequently interfere with active participation in therapy.
Bed Positioning
Proper bed positioning is critical in the early stages of TBI. Because the client tends to spend a lot of time in bed, proper bed positioning is crucial to prevent pressure sores, facilitate normal muscle tone, and prevent loss of pelvis and trunk ROM and mobility. It is often difficult to maintain optimal positioning because of spasticity and abnormal posturing. Other complications that may interfere with proper positioning are casts or splints, intravenous tubes, nasogastric tubes, fractures, or any other medical precautions that must be maintained while in bed.
If the client exhibits abnormal tone or posturing, a side-lying or semiprone position is preferable. This position assists in normalizing tone and providing sensory input. A supine position may elicit a tonic labyrinthine reflex and extensor tone. A supine position with the head in a lateral position could elicit an asymmetric tonic neck reflex. Clients with TBI generally have bilateral involvement requiring a program for side-lying on both sides. The traditional bed-positioning techniques used for patients who have suffered a cerebrovascular accident may require modification depending on the extent of bilateral involvement. Pillows, foam wedges, and splints may be incorporated into the bed-positioning program to facilitate normal positions and prevent abnormal postures such as extreme elbow flexion, head and neck extension, and footdrop deformity.
Splinting and Casting
Splinting or casting may be indicated when (1) spasticity interferes with functional movement and independence in ADLs, (2) joint ROM limitations are present, and (3) soft tissue contractures are possible. Splints have been thought to provide elongation and inhibition by positioning the joint in a static position with the muscles and soft tissues on stretch. Splinting of the elbows, wrists, and hands is often implemented to maintain a functional position at rest and to reduce tone. Serial casting is a more aggressive intervention to increase ROM in the joints when contractures have formed or spasticity is present (or both). Splinting and casting not only reduce contractures and increase ROM but also prevent skin breakdown. Because clients with TBI often have limited active ROM, the UE joints often assume a position of flexion (particularly when severe finger flexor spasticity has caused the fingers and nails to embed in the palmar surface of the hand), and this may cause moisture, redness, and breakdown of the skin.
A resting or functional position splint (Figure 34-3) is one that is worn when the client is not involved in active movements or functional tasks. Once the splint has been fitted, a wearing schedule must be established for the rehabilitation team and caregivers to follow. A typical splint schedule during the day requires the client to wear the splint for repeated, alternating 2-hour periods (2 hours on, followed by 2 hours off). The client must be monitored frequently for any skin breakdown or tonal changes that may change the initial fit of the splint. The team and caregivers should be trained in proper application and removal of each splint.
Other common splints worn at this stage of recovery are cone splints, used in the palm of the hand to keep the fingers from digging into the palmar surface. Frequently, rolled cloths are put into the clenched hand; however, because this may facilitate increased spasticity, a hard cone splint is more appropriate. An antispasticity splint (Figure 34-4) not only positions the hand and wrist in a functional position but also abducts the fingers to further decrease spasticity. Splints are modified as needed and may eventually be discontinued if the individual’s motor control and tone improve.
A serial casting program is indicated when moderate to severe spasticity cannot be managed by splints. The goal of casting is to increase ROM and decrease tone gradually by using a progressive succession of separately fabricated casts, each worn continuously for a period of weeks. Casts are often left on for 5 to 7 days, which places the muscle and tendons on prolonged stretch and reduces tone. Successive casts are designed to increase ROM further until a functional joint range is achieved and maintained. A common difficulty that prevents serial casting from being successful is skin breakdown. If skin breakdown occurs because of a cast that is worn for several days, the cast must be removed until the skin has healed. While wound healing is occurring, spasticity again increases and any gain in joint ROM is often lost.
The most common UE casts are the elbow cast, which is used for loss of passive range of motion (PROM) in the elbow flexors, and the wrist-hand cast, which is used for loss of PROM in the wrist and finger flexors. Other variations of these casts include elbow dropout, wrist, thumb, hand, and individual finger casts. However, casting of more than one joint at a time often leads to skin breakdown as a result of multiple pressure points. It is thus recommended that casting be applied to one joint at a time.28
Casting is frequently used in conjunction with motor point, nerve blocks or botulinum toxin injections. Blocks involve the injection of a chemical substance (e.g., lidocaine, bupivacaine, phenol) into the nerve or motor point to inhibit the innervation of spastic muscles temporarily. Botulinum toxin is injected directly into the target muscle and works by causing presynaptic blockade of acetylcholine release.
Indications for termination of a casting program include achieving functional ROM or plateauing (e.g., the individual has not gained significant improvement in ROM after two consecutive casts). When improvement in ROM has been made and the goal has been achieved, the final cast is cut in half lengthwise, the edges are finished, and the cast is used as a bivalve splint to maintain the functional position. Velcro straps or elastic wrap bandages are used to secure them in place (Figure 34-5). A wearing schedule is then established.
Casting is an advanced intervention technique that carries some risk. Competent use of the technique requires knowledge and advanced clinical training. Marisol had increased spasticity and reduced function in her UEs. What splint or cast (or both) would best suit her needs initially?
Dysphagia
Patients emerging from coma are fed through a nasogastric or gastrointestinal tube. Once the patient is alert and more oriented, the physician decides when evaluation for dysphagia is indicated. Dysphagia programs usually begin in the intermediate- to advanced-level stages of rehabilitation (see Chapter 27).
Behavioral and Cognition
As clients emerge from coma and become more alert and aware of their surroundings, it is important to track their improvement and attempt to establish a form of communication. In acute rehabilitation, tracking the level of arousal and awareness is important because it demonstrates progress. Several scales and assessments are available, including the WHIM, JFK, and Orientation Log. These measurement tools will document improvements in visual attention, visual tracking, and ability to follow commands.
Establishing a way for the client to communicate wants and needs is of the utmost importance because it helps guide intervention. It also allows the team to more accurately assess the client’s cognitive level. A reliable yes/no system should typically be implemented. Examples include eye blinks, eye gaze, head nods, and motor movements such as thumb up and down. Once a system is established, communication is possible. In the case of Marisol, she gained some active movement of her right UE and with guiding was able to use large colored buttons to answer yes and no to questions. The yes/no buttons were positioned on the lap tray of her wheelchair and provided a buzz sound when touched. She was effectively able to answer simple questions such as do you need to use the bathroom or are you in pain when asked.
Family and Caregiver Education
Education of family members and caregivers starts immediately because they are an integral part of the intervention team. Family members often play an essential role in eliciting the client’s responses and implementing the sensory regulation program, positioning the client in bed, and contributing to the ROM program. At the earliest stage after the injury, therapy may be limited; therefore, setting up a simple intervention plan for the family to implement is important in fostering the client’s recovery and maintaining passive joint motion. Family members often feel helpless, and allowing them to be actively involved helps alleviate their feelings of helplessness and focus their array of emotions. Later, when the individual is more alert and mobile, family members can be involved in transfers, wheelchair positioning, feeding programs, and ADL retraining. Providing several education materials is helpful for family members. Brain injury patient education booklets and Internet Websites are effective tools in educating patients and family members.
Evaluation of the Intermediate- to Higher-Level Individual
During the intermediate to advanced level of recovery (Rancho Los Amigos Scale level of IV to VIII), the client is alert but often displays confused, agitated, and inappropriate responses. The client may be able to follow simple two- to three-step verbal commands but is easily distracted. Minimal or moderate cues are often necessary to assist intermediate- to higher-level clients in the performance of ADLs. In general, they can complete most components of the occupational therapy evaluation; however, they may require several breaks during the evaluation process because of distractibility or agitation. The evaluation is similar to that for beginning-level clients in that physical status, dysphagia, psychosocial and behavioral factors, vision, sensation, and perception are assessed. Additionally, these patients require more extensive evaluation of ADLs (including driving), work readiness, and ability to reintegrate into the community.
Physical Status
The physical status evaluation includes an assessment of joint ROM, muscular strength, sensation, proprioception, kinesthesia, fine and gross motor control, and total body control (i.e., dynamic sitting or standing balance). Limitations in physical status are usually the result of abnormal tone, spasticity, and muscle weakness without abnormal tone, heterotopic ossification, fractures, soft tissue contractures, and peripheral nerve compression. Tools to assess physical status include goniometers, dynamometers, manual muscle testing, and clinical observation. Standard assessments may include the Jebsen Hand Function Test,40 the Minnesota Rate of Manipulation Tests,54 the Minnesota Manual Dexterity test,54 and the Purdue Pegboard.70
Dysphagia
Dysphagia assessment may include both clinical (bedside) evaluation and videofluoroscopy. The bedside examination provides the therapist with a variety of information. For example, aspiration can be caused by impulsivity because the client may gulp large portions of food quickly. Pocketing food and drooling may be apparent and are a result of impaired oral motor control. The dysphagia examination can also provide the therapist with information regarding cognitive status. Does the client appear to understand what to do with the utensils and food items? Is neglect present and causing the client to leave one side of the plate untouched? Does the client know the names of the utensils and food items, or is aphasia suspected?
Performed by a speech pathologist or a trained occupational therapist, videofluoroscopy provides information regarding the anatomy and physiology of the oral, pharyngeal, and esophageal stages of swallowing. Videofluoroscopy is the only dysphagia assessment tool that can provide information on the individual’s ability to manage liquids and solid foods. This information will be used to design a feeding program that may require a diet of thick liquids and puréed foods. Swallowing status should be re-evaluated as the individual improves in rehabilitation and can progress to thin liquids and solid foods. (See Chapter 27 for more information on dysphagia.)
Improper positioning, behavioral disorders, and cognitive-perceptual impairment have all been implicated as factors contributing to swallowing disorders. Dysphagia intervention must address seating and positioning and cognitive-perceptual distortions. Formal assessments to evaluate dysphagia include the Dysphagia Evaluation Protocol 4 and the Evaluation of Oral Function in Feeding.69
Cognition
Cognitive skills are assessed within functional tasks (e.g., ADLs, meal preparation, money management, and community skills). Tasks that involve paper and pencil can also provide valuable information, although they are only part of the equation. Assessment of a client’s cognition during preparation of a cold meal may include the following skills: (1) following two- to three-step written or spoken directions, (2) correctly sequencing the order of steps, (3) attending to the task with minimal distraction, and (4) displaying good safety and judgment. The therapist may evaluate the client’s cognitive status by measuring any of the following: (1) counting the number of errors and correct responses, (2) assessing the amount of assistance or cueing required (minimal, moderate, or maximal), and (3) determining the percentage of the task that was completed correctly. Assessment of the complexity of the activity (simple versus multistep or basic to complex) and the conditions of the environment (isolated versus multistimulus or quiet to distracting) is also important.
When assessing an individual’s cognitive skills, the therapist must consider and document other factors that may affect performance. Such factors include language barriers (e.g., the presence of aphasia, a primary language other than English), visual-perceptual deficits, the effects of medication on cognitive level, educational and cultural background, and previous experience with the task. Formal cognitive assessments that may be used with a TBI population include the Allen Cognitive Level Test,2 the Loewenstein Occupational Therapy Cognitive Assessment,50 the Rivermead Behavioral Memory Test,77 Kohlman Evaluation of Living Skills,43 and the Cognitive Assessment of Minnesota.64
Vision
Clients with TBI should undergo vision screening. The vision screening should be completed as early as possible in the rehabilitation process because early detection of visual deficits will allow the intervention team to obtain more reliable information regarding the client’s overall health status. For example, diplopia (double vision) or accommodative dysfunction (inability to adjust focus for changes in distance) will probably influence the results of the neuropsychology or speech-language pathology assessments.
Vision screening is a tool that allows therapists to identify potential deficits in vision. Although therapists cannot diagnose conditions of vision dysfunction, they can determine whether an individual passes or fails a visual screening based on standard criteria. The screening is a means of determining which clients require a referral to an optometrist or ophthalmologist for a complete evaluation and intervention. A comprehensive vision intervention program is designed by an optometrist and implemented by an occupational therapist or vision therapist. A visual history questionnaire should be completed as well. The questionnaire should contain an ophthalmologic history, questions regarding the use of glasses and contact lenses, and questions about the presence of blurred vision, dizziness, headaches, eyestrain, diplopia, and visual field loss.
Common areas evaluated in a vision screening include visual attention, near and distant acuity, ocular movement (e.g., pursuits and saccades), convergence, accommodation, ocular alignment, depth perception (stereopsis), and visual field function. Visual dysfunction can also be identified during clinical observation of the individual’s performance in functional activities. Tilting the head as a result of a field deficit, closing or covering one eye to decrease blurred vision, and bumping into walls or objects in the environment because of a field deficit or unilateral neglect are all easily observed behavior indicative of visual dysfunction.
Perceptual Function
Perceptual evaluation should be performed when the therapist has obtained a clear understanding of the individual’s cognitive, sensory, motor, and language status because deficits in these areas may skew the client’s performance on a perceptual evaluation. Evaluation of visual perception should include right-left discrimination, form constancy, position in space, topographic orientation, and naming of objects. Evaluation of perceptual-speech and language function should assess for aphasia, and anomia. Evaluation of perceptual motor function should include the functions of ideational praxis, ideomotor praxis, three-dimensional constructional praxis, and body schema perception (including identification of unilateral neglect). Formal perceptual assessments that can be used for a population of adults with TBI include the Hooper Visual Organization Test,38 Motor-Free Visual Perception Test—Revised,16 Rivermead Perceptual Assessment Battery,76 and Loewenstein Occupational Therapy Cognitive Assessment.50
Activities of Daily Living
Intermediate-level clients should be assessed in all basic ADLs (e.g., grooming, oral hygiene, bathing, toileting, dressing, functional mobility, and emergency response). Advanced-level clients should also be assessed with regard to instrumental activities of daily living (IADLs), such as hot and cold meal preparation, money management, community shopping (Figure 34-6), household maintenance, cleaning and clothing care, safety procedures, medication routine, and work readiness. The therapist will have ample opportunity during assessment to observe cognitive skills, perceptual skills, and behavioral appropriateness.31 Formal assessments that can be used for a population with TBI to assess ADL skills include the Arnadottir OT-ADL Neurobehavioral Evaluation,4 Assessment of Motor and Process Skills,23 Functional Independence Measure,35 and the Klein-Bell Activities of Daily Living Scale.42
Clients with a history of alcohol abuse require assessment of leisure patterns. An interest history and interest checklist may reveal healthful leisure interests that can replace alcohol use. The combination of leisure skills development and substance abuse rehabilitation will help clients manage time more effectively and thereby refrain from using alcohol after discharge.
Driving
Many states require physicians to report anyone to the Department of Motor Vehicles who has lapses of consciousness, seizure disorders, and cognitive, visual, and perceptual dysfunction caused by TBI. Regulations regarding such disorders often mandate that the driver’s license be revoked until further assessment confirms that the person can drive without posing a safety risk to self or others.
Advanced-level clients with TBI who do not have seizure disorders or severe cognitive deficits must undergo a comprehensive driving evaluation to assess their ability to resume driving. Two types of driving evaluation can be completed: a clinical assessment (evaluation of the individual’s visual, cognitive, perceptual, and physical status as it relates to driving) and an on-road assessment. Both types of evaluation are necessary because the client may fail the clinical assessment but pass the on-road assessment by using compensatory strategies. Conversely, the client may perform successfully on the clinical assessment but fail the on-road assessment (see Chapter 11).
Clients with TBI frequently exhibit deficits (e.g., visual processing disorders, figure-ground discrimination dysfunction, and impulsivity) that significantly affect their ability to drive safely. When visual processing is delayed, affected persons hesitate during driving maneuvers and stop in an unsafe manner (e.g., in the middle of the road or at a corner) to allow themselves adequate time to process visual information. Those with figure-ground impairments may be unable to identify stop signs and traffic signals at intersections or locate the gearshift near the dashboard. Impulsive individuals may respond aggressively rather than defensively when driving, thereby increasing the risk for accidents. They may use poor judgment when making driving decisions and be unable to inhibit inappropriate responses. The Elemental Driving Simulator27 and Driving Assessment System27 are off-the-road clinical driving assessments that can be used with an on-road assessment to determine a client’s ability to resume driving after brain injury. Occasionally, individuals have a strong desire to drive and poor insight into whether they possess safe skills to resume driving. Since most rehabilitation centers and outpatient settings do not have an adaptive driving program and expensive driving simulators, occupational therapists need to use low-tech options to determine driving readiness and educate patients on whether they are safe to drive. There are many commercially available computerized driving assessments and training tools that are easy to download and inexpensive. One option is the Roadwise Review distributed by the American Automotive Association (AAA).1 This computer-based tool measures the functional abilities scientifically linked to risk for crashing in older drivers. Since it assesses useful field of view, visual-perceptual skills, and reaction times, it is an excellent tool that can be used in the TBI population.
Vocational Rehabilitation
Advanced-level clients may be evaluated to determine whether they are ready to return to work. It has been well documented that return to work after moderate to severe TBI is generally unsuccessful. High unemployment rates have been attributed to the adverse emotional, behavioral, and neuropsychologic changes arising from TBI. Substance abuse in the TBI population is also a major factor inhibiting the ability to return to and maintain employment.26
Vocational assessment for advanced-level clients must involve assessment in the actual work setting because psychometric tests and job simulations in themselves do not accurately determine work potential. The client is often able to compensate in a familiar work setting for deficits that may appear to be significant impairments on a psychometric test. The therapist’s vocational evaluation should summarize the individual’s interests, strengths, and areas of deficit. The report should conclude with recommendations stating the modifications required, realistic job goals, and a plan for achieving these goals with professional assistance as needed.
Psychosocial Skills
Advanced-level clients who will be discharged to the home setting or a community-supported living residence should also undergo a psychosocial skill evaluation. Such an evaluation should assess role loss, social conduct, interpersonal skills, self-expression, time management, and self-control. The therapist should also assess the client’s social support system, ability to form and maintain friendships, and resources to decrease feelings of isolation (such as TBI support groups). The ability to form and maintain intimate and sexual relationships after TBI will be of paramount concern to single clients who sustained their TBI between the ages of 18 and 30. Child rearing and care of family members will be of concern for clients who are responsible for children and other family members.
Assessment of psychosocial skills in clients with TBI is critical. For 1 or more years after injury, clients with TBI report that their psychosocial deficits significantly diminish life satisfaction and are a greater problem than the physical and cognitive deficits combined. Psychosocial impairment is often neglected in the rehabilitation setting, which prioritizes intervention for acute physical, cognitive, and perceptual deficits. Psychosocial difficulties are more apparent after discharge, when the individual has left the structured and safe setting of the rehabilitation hospital to re-enter the community. It is important to address psychosocial difficulties before the individual is discharged. Psychosocial assessment tools that can be used for this population include the Assessment of Communication and Interaction Skills,65 the Occupational Role History,24 and the Role Checklist.56
Marisol received skilled occupational therapy intervention throughout a 3-month inpatient rehabilitation stay, followed by 6 weeks in a day treatment program. Marisol participated in a multifaceted spasticity reduction program and neuromuscular re-education, which improved ROM and functional use of her right UE. As her ROM and selective movement improved, Marisol learned how to feed, dress, and bathe herself with minimal assistance. As Marisol’s head and trunk control improved, she was able to participate in all aspects of bed mobility and transfers. Daily guided self-care tasks were a critical part of her morning schedule. Performing meaningful, routine tasks allowed Marisol to work on her basic cognitive abilities. Spontaneous neurologic recovery, cognitive re-education, and memory strategies improved Marisol’s ability to plan, organize, and sequence her ADLs and recall her daily therapy schedule with occasional verbal cues. Marisol was referred to an outpatient occupational therapy program after discharge from the day treatment program.
Intervention for the Intermediate- to Higher-Level Individual
Intervention for intermediate- to higher-level individuals involves two primary approaches: the rehabilitative model and the compensatory model. The rehabilitative model is supported by the theory of neuroplasticity, which holds that the brain can repair itself or reorganize its neural pathways to allow relearning of functions that had been lost as a result of neural damage sustained in the accident. The compensatory model holds that repair of damaged brain tissue either has occurred to its fullest extent or cannot occur, with the individual being left unable to perform lost functions without external assistance. Tools used in the compensatory model are adaptive equipment, environmental modification, and compensatory strategies that allow the client to perform ADLs. It is valuable to approach intervention via both the rehabilitative and compensatory approaches through addressing neuromuscular impairment, cognitive deficits, perceptual deficits, vision dysfunction, and behavioral disorders. In general, a rehabilitative approach is used in the acute stage of TBI recovery until the client has plateaued or progress has slowed, at which time a compensatory approach is attempted.
Neuromuscular Impairments
As in beginning-level individuals with TBI, numerous types of neuromuscular impairment can be present in intermediate- to advanced-level individuals. Spasticity, rigidity, soft tissue contractures, the presence of primitive reflexes, diminished or lost postural reactions, muscular weakness, and impaired sensation affect the ability to perform activities independently and with normal control (see Table 34-3). The prerequisites for normal movement include normal postural tone, balanced integration of flexor control (reciprocal innervation), normal proximal stability, and the ability to implement selective movement patterns.
The common principles of intervention for neuromuscular impairment are to facilitate control of muscle groups, progressing proximally to distally; encourage symmetric posture; facilitate integration of both sides of the body into activities; encourage bilateral weight bearing; and introduce a normal sensory experience. Effective rehabilitation techniques for such individuals include neurodevelopmental treatment (NDT), proprioceptive neuromuscular facilitation (PNF), myofascial release, Rood techniques, and some physical agent modalities (see Chapters 29 and 31). These clinical interventions require education beyond the entry level and must be either incorporated into or followed by a meaningful functional activity that requires the same movement. The following brief overview of principles is merely an introduction and cannot substitute for training in the specific techniques.
Intervention for impaired neuromuscular control should begin at the pelvis because positioning of the pelvis affects the motor control of all other body parts. A variety of approaches may be used to normalize pelvic positioning. For example, clients with TBI commonly have a posterior pelvic tilt. To move the client to a more functional erect pelvic position, a therapist trained in NDT might use anterior pelvic tilt mobilization. A therapist with a different approach might use a bedsheet behind the pelvis to lift and rotate the pelvis forward over the heads of the femurs. In either case, individuals would be directed to raise their head and sit up tall.
The trunk is positioned after the pelvis. Proper positioning of the trunk frees the UEs for functional activities. Major principles include the following: (1) facilitating trunk alignment, (2) stimulating reciprocal trunk muscle activity, (3) encouraging the individual to shift weight out of a stable posture into all directions (bending forward, bending backward, reaching to each side while laterally flexing the trunk), and (4) helping the individual move the lower part of the trunk on a stable upper trunk or move the upper trunk on a stable lower trunk. Once trunk control improves, intervention should progress to the UEs.
Competent practitioners may apply rehabilitative techniques in a variety of ways. A client with soft tissue contractures or spasticity in a particular muscle group may benefit from NDT mobilization and inhibitory techniques for the agonistic muscle group. A client with low tone or weak muscles (without the presence of spasticity) may benefit from NDT, PNF, Rood, and physical agent modalities. Kinesio-taping can assist in providing stability to weak muscle groups. Neuromuscular electrical stimulation can effectively stimulate UE muscle groups, including the triceps, pronators, supinators, and wrist and finger extensors, to enhance muscle strength, increase sensory awareness, and assist in motor learning and coordination.13
Many advanced-level clients have fairly intact motor control and are able to ambulate independently and use both UEs in functional activities. However, close observation reveals subtle trunk and extremity deficits related to coordination and speed of movement. The intervention for trunk control focuses on developing full isolated movements of the trunk and extremities, good dynamic standing balance for all activities (including reaching and bending to high and low surfaces), and the ability to shift weight naturally from one LE to the other during activities. UE intervention programs are designed to increase scapular stability and improve fine motor control. A goal of intervention is to improve the client’s speed while maintaining good coordination and minimizing compensatory strategies (Figure 34-7).
Ataxia
Ataxia is a common motor dysfunction that occurs primarily as a result of damage to the cerebellum or to the neural pathways leading to and from the cerebellum. Ataxia develops early in the acute stage of recovery and may remain permanently. It is a clinical problem for which rehabilitation methods are generally ineffective. More often, therapists train the client in use of compensatory strategies to control the effects of ataxia. For example, weighting of body parts and the use of resistive activities often improve control during the performance of tasks but show inconsistent carryover of muscular control when the resistance is removed. When applying weights to clients, the therapist must identify at which joint (or joints) the tremor originates. Applying weights to clients’ wrists when the tremor emerges from the trunk and shoulders is ineffective. Weighted eating utensils and cups are also used as compensatory aids to reduce the effects of ataxia on the UEs; however, these assistive devices are limited in their effectiveness.
Cognition
Intervention designed to enhance cognitive skills should be implemented through functional ADLs and IADLs. A common impairment in cognition is concrete thinking, in which the individual is likely to have difficulty with abstract concepts. Activities that require generalization of skills from one task to another will be difficult for clients with TBI. It is best to engage these clients in activities that they need to participate in everyday life. For example, if the client will return to a community environment in which it is necessary to use public transportation, interpreting bus schedules is a meaningful and relevant activity that addresses many critical cognitive skills, including problem solving, planning, organization, concentration, tolerance of frustration, sequencing, money management, and categorization. Another way to address the aforementioned cognitive skills is by planning a trip to the hardware store to purchase supplies necessary to install a hand-held showerhead.
Clients at an advanced level of recovery who demonstrate high-level cognitive skills often display subtle cognitive deficits in the areas of organization, planning, sequencing, and short-term memory.
Generally, neuropsychologists and cognitive educators have implemented the use of computers in cognitive retraining. However, use of computer programs has not been shown to generalize to the cognitive skills needed to improve performance in IADLs.55 Computers can be used in therapy if they are meaningful in the client’s daily life; the therapist should address the client’s specific computer needs. For example, by simplifying tool bars and menus and programming step-by-step written directions that appear on screen, therapists may reprogram a client’s home computer to make it less complicated to use. Software programs that do not represent functional activities should be avoided. Since 36% of Americans with a disability use a computer at home (U.S. Census, 2008) and 60% of working-age adults are likely to benefit from the use of accessible technology because of disabilities, it is important to introduce computers into treatment as a therapeutic modality. The occupational therapist can not only set up a computer by using the built-in accessibility options and utilities but also provide specialty assistive technology products such as voice recognition, alternative keyboards, and trackballs to allow patients successful access and use.32 Computer access not only facilitates cognitive retraining opportunities but can also be a source of communication and address visual, perceptual, and motor deficits.
Vision
Intervention alternatives for clients with TBI and visual dysfunction include the use of corrective lenses, occlusion (e.g., patching one eye), prism lenses, vision exercises, environmental adaptations, and corrective surgery. An optometrist or ophthalmologist can evaluate the client’s vision and prescribe glasses to address any accommodative dysfunction caused by brain injury. However, the glasses should not be prescribed until the client has passed the subacute phase of rehabilitation because an accommodative dysfunction that appears in the acute stage of brain injury may improve during the recovery process.
A common technique to eliminate double vision (diplopia) is patching, or occlusion. The client wears a patch over one eye to block the image seen by that eye and eliminate diplopia. Patching is a temporary compensatory strategy. An optometrist may prescribe prism glasses or binasal occluders for clients with consistent diplopia resulting from permanent oculomotor nerve damage. The prisms assist the eyes in fusing images. Prism glasses are not effective for those with significant lateral strabismus or exotropia (outward eye turn). Binasal occluders encourage the malaligned eye to fixate centrally. Prism glasses and binasal occluders are used conjointly with vision exercises. The goal of this intervention is to decrease the diplopia and eventually eliminate the need for prisms or occluders.
Vision exercises consist of a series of activities that (1) maximize residual vision, (2) enhance impaired vision skills (the rehabilitative approach), (3) increase the client’s awareness of his or her visual deficits, and (4) help the client learn compensatory strategies. Intervention progresses from monocular to binocular vision and follows a developmental progression (supine to sitting to standing). Exercises initially address basic skills such as visual attention, pursuits, and saccades and may progress to more difficult skills such as fusion and stereopsis. These vision exercises are based on the rehabilitative model, which holds that impaired visual skills can improve with training.
Environmental adaptations for visual deficits are based on the compensatory model. Compensatory strategies for visual deficits include using a colored border along one side of a page to facilitate reading. A colored strip of tape along one side of a plate or meal tray to promote self-feeding is one option. Use of large objects, such as a clock with bold numbers or a telephone with enlarged buttons, is another compensatory technique. Contrasting colors to highlight controls and knobs (e.g., marking TV/VCR remote control buttons with fluorescent paint) may be helpful. Increasing lighting in an environment and using textures as cues (e.g., placing textured tape on a banister by the bottom step to alert the individual that the bottom step is coming and thereby reduce falls) may be used for clients with low vision. The latter compensatory strategy is also valuable for clients with vertical gaze paralysis who can look neither up nor down. Those who have lost pupil constriction should wear sunglasses whenever they are in bright light.
Corrective surgery performed by an ophthalmologist may be indicated to align the eyes and eliminate double vision; however, the individual must wait at least a year after the injury to allow any improvement that may occur naturally in the course of recovery.
Perception
Treatment of perceptual deficits involves both rehabilitative and compensatory intervention. For example, impairment of figure-ground perception might be treated via a rehabilitative approach through the repeated practice of locating objects against a similar background (e.g., finding a white shirt on a bed with white sheets or finding a spoon in a drawer of similar stainless steel utensils). Using a compensatory approach, the therapist would help the client arrange the kitchen drawers so that utensils are categorized (perhaps color-coded) and distinctly divided to facilitate identification.
Aphasia (a perceptual-speech disorder) can also be treated by both rehabilitative and compensatory approaches. Expressive aphasia may be treated rehabilitatively through repeated conversation exercises in which clients are given feedback regarding their incorrect spoken words and challenged to express the words that they meant to verbalize. If the client has not made significant gains in expressive speech through use of the rehabilitative approach, the compensatory approach should be used to help the client articulate his or her needs to caregivers. For example, a chart with letters, words, or pictures (or a combination of the three) of important items in the client’s environment can be used to help the client identify needs such as eating, toileting, and medications. Such a chart may be used concomitantly with rehabilitative approaches.
Through a rehabilitative approach, apraxia can be treated by helping the client perform specific tasks (e.g., hair combing) hand over hand (i.e., the therapist’s hands guide the client’s hands during hair combing). The rehabilitative approach holds that through repeated hand-over-hand exercise, the client’s brain can repair the neural pathways that mediate specific motor patterns, such as those needed for hair combing, or can reorganize pathways so that different, undamaged areas of the brain can establish new pathways for specific motor patterns. Using a compensatory approach, the client may comb his or her hair by following steps through the visual interpretation of pictures sequentially depicted (pictures) or listed (words) on a poster or note card.
Neglect syndrome (a disorder of body schema) can also be addressed by using rehabilitation and compensatory strategies. Severe neglect syndromes tend to decrease as a natural part of the recovery process. However, some neglect syndromes may continue into the postacute rehabilitation stage. With a rehabilitative approach, the client is encouraged to use the neglected extremity for all ADLs. The client’s room may be rearranged to encourage interaction with the neglected part of the environment (e.g., placing the television or standing bed tray in the left side of the room if the client has left-sided neglect). A compensatory model is used when the client has not demonstrated significant improvement in attending to the neglected side of the body or environment. The meal tray may be placed within the client’s field of vision to maximize success. A colored border may be placed on the left side of book pages to cue the individual to scan the entire line while reading.
Behavioral Management
The types of intervention strategies used to decrease and eliminate problem behavior may be divided into two categories: environmental and interactive. Environmental interventions alter objects or other environmental features to facilitate appropriate behavior, inhibit unwanted behavior, and maintain individual safety. Agitated clients should be placed in a quiet, isolated room without a roommate. All extraneous stimuli (e.g., radios and televisions) should be removed. Similarly, therapy is provided in a private, quiet room away from other people and extraneous stimuli.
An agitated client who demonstrates severe behavioral problems may require one-to-one care. The client is assigned a rehabilitation aide who remains with the client throughout the day (including during therapy) to monitor and regulate his or her behavior. The rehabilitation aide may wear an alarm bracelet that signals staff when the client attempts to wander away from the appropriate floor or out of the building. Walkie-talkies and pagers may be used with those who are at risk of eloping. One walkie-talkie or pager remains in the nursing station; the other is held by the therapist or staff member who is providing one-to-one care to the client. If the client begins to act aggressively or attempts to elope, the rehabilitation aide can alert the staff that assistance is needed.
Interactive interventions are the approaches that staff and caregivers use to interact with the client. The entire team should implement these interventions in a consistent way. Consistent implementation includes speaking in a calm and concise manner and deliberately refraining from detailed explanations that will only increase the client’s confusion and frustration. For safety’s sake, therapists should also keep the door open when working with the client at the bedside and should always maintain awareness of the individual in relation to self.
A client who is in the postacute stages of rehabilitation and who continues to exhibit behavioral problems should be placed in a behavioral management program. Such a program should allow the client to experience the natural consequences of inappropriate behavior (e.g., losing community recreational privileges) in an effort to encourage more appropriate responses. Drug therapy may be used for those who do not make significant improvements in their behavior and who present a safety risk to themselves and others.
Dysphagia and Self-Feeding
Intervention strategies for dysphagia follow the same guidelines as for other neurologic impairments; intervention, however, may be more complex in this population as a result of bilateral neurologic involvement, cognitive and behavioral issues, and severe neuromuscular impairments.5,6 A self-feeding program may begin in a quiet area, such as the client’s room. Eating is then advanced to more social situations, such as the hospital dining room. Common pieces of adaptive equipment, such as a rocker knife, plate guard, and nonspill mug, may be used if the client demonstrates diminished strength, coordination, or perceptual deficits. If the client displays decreased attention, introducing one piece of adaptive equipment at a time may help. Clients with heightened impulsivity may benefit from the strategy of placing the fork down after each bite to ensure that they completely chew and swallow before initiating the next bite. Depending on the client’s level of dysphagia (i.e., pre-oral, oral, pharyngeal, and esophageal), a diet of thick liquids or puréed foods may be indicated until the client makes progress toward recovery.
Functional Mobility
Mobility training can be subdivided into bed mobility, transfer training, wheelchair mobility, functional ambulation during performance of ADLs, and community mobility. The NDT principles of bilateral extremity use, equal weight bearing, and tone normalization are used in intervention strategies that address functional mobility. The rehabilitation model, based on the principles of NDT and PNF, should be used for intermediate-level clients with TBI in the acute and subacute stages of rehabilitation. Allowing a client with loss of function to use compensatory strategies, such as grabbing a bed rail with one hand and rolling or standing on one leg to transfer, may appear to enable the client to function more independently earlier. However, use of such strategies diminishes the client’s ability to perform activities with a bilateral UE pattern at a later point. In time, unilateral performance of activities results in hemiplegic postures, contractures, and abnormal gait deviations. Compensatory strategies should be used only in the later stages of recovery and when the client has not been able to demonstrate significant improvement in functional mobility skills and thus must learn compensatory strategies to enhance the ability to live independently in the community.
Bed Mobility
An intermediate-level client with TBI may require training in bed mobility skills, including (1) scooting up and down in bed, (2) rolling, (3) bridging, and (4) moving from a supine position to and from sitting and standing positions.
Wheelchair Management
Wheelchair management includes the ability to manage wheelchair parts (e.g., removing footrests and locking brakes) and propelling the wheelchair both indoors and outdoors on a variety of surfaces (e.g., low-pile carpeting, sidewalks, and ramps). Customized wheelchairs may be ordered for a client who is in the postacute stage of rehabilitation and continues to exhibit neuromuscular impairment that requires the use of a wheelchair for long-term mobility needs. A custom wheelchair provides a seating and positioning system that contours the client’s body for comfort and skin protection, includes adaptive supports for proper pelvic and trunk alignment, and offers a seating position that enhances the client’s ability to interact with the environment. Clients who cannot propel or control a manual wheelchair may require an electric wheelchair for independent home or community mobility.
Functional Ambulation
Functional ambulation refers to the ability to walk during functional activities. Whereas physical therapists address gait training, occupational therapists facilitate the carryover of ambulation skills into ADLs. Ambulation during performance of ADLs often requires the integrated use of UEs and LEs to carry and manipulate objects (e.g., carrying a plate to a table, holding a book bag or purse, sweeping with a broom or vacuum cleaner, carrying an infant). Functional ambulation also requires the ability to negotiate an ambulatory device (e.g., straight or quad cane and walker) with one or both UEs during performance of ADLs. This is a high-level activity that requires eye-hand coordination and the integration of total body movements. Compensatory aids to improve the client’s ability to negotiate an ambulatory device while performing ADLs include walker bags and baskets, wheeled carts (to provide balance and support while transporting items such as plates to a table), canes with built-in reachers, pouch belts (to hold keys, wallet, and memory books), and an apron during meal preparation (see also Chapter 10).
Community Travel
For clients who will be discharged to home or a community supportive living arrangement, the ability to negotiate their environment must be considered. Negotiating uneven sidewalks and curb cutouts and correctly interpreting traffic light signals, as well as the direction and speed of oncoming traffic, are important skills to practice for safe and independent community mobility. Functional ambulation in the community requires the client to respond and initiate actions quickly—for example, to cross the street after the light turns green and before it turns red. Clients must perceive depth and spatial relationships (to correctly judge the distance and speed of oncoming and turning traffic) and visually identify and avoid environmental hazards that could cause falls (e.g., potholes and cracks in the sidewalk). Power mobile scooters or power wheelchairs are often recommended for clients who must perform long-distance mobility in the community but who fatigue easily or are unable to walk independently. Use of a power mobile scooter or power wheelchair requires good static sitting balance and the ability to quickly integrate UE hand control and cognitive decisions regarding the environment. Practicing with clients during the wheelchair evaluation is crucial to determine whether they are able to safely propel a power system within the community.
Transfers
Because individuals with TBI commonly have memory deficits and limited carryover of information, transfer training should be consistent (in technique and sequence) among all staff members treating the client. It is preferable that transfers for intermediate- and advanced-level clients be practiced while moving to both the right and the left sides. Without such practice, clients who become proficient in a transfer toward the uninvolved side (in the hospital) may be dismayed to find that the home setting or public restroom requires transfers toward the opposite side. Additionally, teaching them to transfer to both sides provides weight bearing on both LEs, the use of bilateral trunk muscles, and bilateral sensory input.
Family members and caregivers should be trained in proper transferring techniques (including proper body mechanics) and cleared by a therapist before transferring the individual alone. The decision about when to begin caregiver training depends on the client’s functional level and ability to cooperate, the discharge date, and the caregiver’s physical and cognitive abilities.
Home Management
As the client’s skills and independence in self-care, dressing, self-feeding, and functional mobility increase, intervention is expanded to include home management skills in preparation for discharge to the community. Home management skills include meal preparation, laundry, cleaning, money management (e.g., balancing a checkbook, paying bills, and budgeting), home repairs (e.g., changing a washer in a leaking faucet), and community shopping (which includes making a shopping list, locating the correct items in the store, and paying the correct amount of money at the cash register). Examples of high-level activities include planning a monthly budget, organizing a file cabinet, ordering from a catalog or the Internet, and filing income taxes. These are skills that adults need to live independently in the community and are thus relevant for most clients with TBI.
The degree to which clients participate in home management activities varies. For example, some prepare only simple meals using a microwave oven. For those who must prepare meals to live independently in the community but who are not interested in cooking, the goal is to help them safely prepare simple hot and cold meals at home. Some clients perform no household cleaning activities other than making their bed and doing the laundry. Common sense dictates that therapeutic interventions first address the activities that the client performed before the injury.
As in all other areas of intervention, home management skills are graded to accommodate the client’s functional level. Beginning meal preparation tasks may involve making a cold sandwich, whereas beginning money management skills may involve learning to perform basic cash transactions. As clients progress in home management skills, the meal preparation task may be graded to preparing a two-item hot meal using a stovetop, oven, or microwave oven. Money management skills may be graded to writing checks and balancing a checkbook. As the client continues to gain skills, activities requiring higher-level demands are performed until the client reaches the desired goals.
Child care, if appropriate, must not be overlooked as an area of intervention. Family involvement is critical if a mother or father is to return effectively to his or her role as a spouse and parent. Sensory overload and its resultant agitation in a parent with TBI are a commonly reported problem for families. Occupational therapy sessions should gradually reintroduce parents to their role of caring for their children. Some hospitals have a family suite in which family members can practice ADLs and interpersonal skills with the client on weekends in preparation for discharge. This allows family members to gain a greater awareness of their loved one’s impairments and need for assistance. It also makes the transition from hospital to home less stressful for both clients and family members.
The occupational therapist can also assist parents with TBI in the adaptation of strollers, cribs, and child care equipment to make handling of such items easier. Safely bathing or carrying a baby, preparing a meal while simultaneously caring for children, and one-handed diapering and dressing techniques are all examples of areas that could be addressed by occupational therapy services.
Community Reintegration
Clients who will be discharged from the acute rehabilitation hospital to home or to a postacute, residential supportive living arrangement should receive training to facilitate the transition from the hospital to the community. Clients who achieve a maximal level of independence in the protected and structured environment of the rehabilitation hospital may find that community reintegration holds even greater challenges. Community trips—in which an advanced-level client with TBI is accompanied in the community by the occupational therapist (and perhaps a family member) to practice IADLs in the natural environment—should be implemented to provide the client with the opportunity to rebuild daily life skills. Depositing or withdrawing money from the bank or ATM, using the public transportation system, planning a shopping list, and purchasing items at the grocery or hardware store are activities that will facilitate initiation of the client’s re-entry into the community. Having the client perform ADLs within the community setting will also allow the therapist to observe the client’s ability to interact successfully or otherwise with the environment. The client will be provided a chance to receive valuable feedback from others in the community regarding his or her behavior.
Some clients are discharged from the acute rehabilitation center to a transitional living center. Transitional living centers are designed to develop daily life skills by providing the client an opportunity to temporarily live in a community group setting with 24-hour staff supervision and assistance. The goal of transitional living centers is to facilitate progression from supervised living to greater independence in community living. The client is usually discharged from the transitional living center to a relative’s home or to a residential supportive living facility that provides various levels of living arrangements (e.g., community apartments and shared community group homes). Because long-term residential community facilities for people with TBI are expensive and not covered by most insurance companies, many are discharged home, where they receive continued intervention in outpatient rehabilitation or in day treatment programs that provide community, work, and school re-entry training.
Psychosocial Skills
Individuals with TBI commonly report 1 or more years after the injury that psychosocial impairment is the greatest obstacle to rebuilding a meaningful lifestyle. Many report feeling a deep sense of isolation and loneliness. Loss of roles such as partner or spouse, worker or student, independent home maintainer, friend, and community member often leaves individuals feeling as though they have lost their identity. The goal of the occupational therapist, particularly in postacute TBI centers (e.g., day treatment programs, outpatient rehabilitation, transitional living sites, and long-term community supportive living arrangements), is to help rebuild desired occupational and social roles. This involves a three-step process: (1) identifying the desired roles that were lost secondary to TBI, (2) identifying activities that would support the desired roles, and (3) identifying rites of passage that were either lost or never transitioned through as a result of TBI. Rites of passage are socially recognized events that mark the transition from one life stage to another. Common rites of passage in Western society include obtaining a driver’s license, graduating from secondary school or obtaining a higher education degree, securing full-time employment, living independently in the community, dating, marrying, and parenting.
Once the desired occupational and social roles, activities, and rites of passage have been identified, the therapist facilitates the client’s use of adaptation, compensatory strategies, and integration of new learning. The therapist will also help the client enhance or regain interpersonal skills, self-expression, social appropriateness, time management, and self-control. Such psychosocial skills will be critical if the client is to re-enter the community—to live in a neighborhood setting, hold a job, perform volunteer work in the community, and participate in desired recreational opportunities along with other adult community members.
Group intervention is beneficial because it enables the client to meet others experiencing the same life concerns (thereby decreasing feelings of isolation). Groups can offer exposure to peer reactions, which is particularly helpful if the client exhibits socially inappropriate behavior. Groups may also facilitate problem solving by providing the opportunity to speak with others who have successfully dealt with the same or similar problems. Participants who have been in the group longer can become peer mentors to new group members. The opportunity to help others—to share one’s experience of having a brain injury with others who can benefit from that knowledge—has been shown to enhance an individual’s life satisfaction, feelings of competence, and sense of usefulness. Many states have support groups for individuals with TBI that are run by state associations for brain injury.
Substance Use
If the client’s preinjury history includes substance use, the client should receive drug and rehabilitation services specifically designed for individuals with TBI. Clients with a history of substance use may not display any signs of a desire to return to substance use while in the structured and protective environment of the subacute rehabilitation facility. Substance use may become a problem only after the client is discharged to home, a community-supported living arrangement, or any residential situation in which long periods may be spent alone and unsupervised. Drug rehabilitation services are critical for clients with a substance abuse history because return to substance use after brain injury has been closely implicated in the occurrence of a second TBI.
Discharge Planning
Planning for discharge from occupational therapy services begins at the initial evaluation and continues until the last day of intervention. Components of discharge planning include a home safety evaluation (if the client will be discharged home), equipment evaluation and ordering, family and caregiver education, recommendations for a driver’s training program (if indicated), and recommendations for successful return to school or vocational retraining and work skills.
Home Safety
If the client is to be discharged home, the therapist should visit the home (or transitional living setting) to recommend modifications for increased safety. For example, clients with balance difficulties should have grab bars in the shower stall. Increased lighting should be provided as necessary for clients with visual deficits because low lighting has been linked to falls. Recommendations should also be made regarding the client’s ability to handle sharp items (e.g., knives or glass items that could shatter easily), use the stove, and remember to turn off the faucet and other appliances. The temperature setting on the hot water system should be set at or below 120° F to prevent scalding. Anything that could be tripped over (e.g., throw rugs, appliance cords, furniture legs, objects placed on steps) should be removed. If feasible, nonslip flooring should be added to slippery surfaces (e.g., bathroom and kitchen tiles). If a wheelchair is indicated, the therapist should recommend modifications to doorways and bathroom spaces and should suggest replacement of high-pile carpeting with tile, wood, or other surfaces that can easily be traversed by a wheelchair. Additionally, family members and caregivers should be educated in the appropriate steps to follow during a seizure, should understand how to evacuate the individual in case of emergency, and should practice methods by which to transfer the individual safely. Caregivers should be able to identify unsafe activities in which their loved one should not participate and should know the length of time that he or she can be left alone safely (if possible at all).
Equipment Evaluation and Ordering
Clients who will be discharged from the acute rehabilitation facility will require an evaluation of the equipment needed in the next setting. This may necessitate re-evaluation of the client’s equipment needs because many of the adaptive devices that were valuable in the beginning and intermediate stages of rehabilitation may be discarded as the client improves. For example, a tub bench or shower chair may have been needed initially because of dynamic standing balance difficulties. The client may have progressed sufficiently during the course of rehabilitation to stand in the shower while using only a grab bar.
Family and Caregiver Education
Family members and caregivers should be involved in the client’s rehabilitation from the beginning of treatment and should be considered members of the intervention team. Education of caregivers in such activities as transfers, wheelchair mobility, ADLs, bed positioning, splint schedules, use of equipment, ROM exercises, and self-feeding techniques will facilitate follow-through with the skills that have been learned in the rehabilitation hospital. Individual safety is of primary importance for caregiver education. The caregiver should be trained in implementation of the home program (in either written or videotape form). Home programs may include the areas listed previously, as well as specific activities for the improvement of cognition, vision, perception, and motor control.
Recommendations for Driver’s Training
If the client passes the clinical driver’s evaluation, the occupational therapist may recommend a specific number of hours of driver’s training. An occupational therapist or a driving instructor who has experience working with individuals with TBI (see Chapter 11) should implement the driver’s training.
Recommendations for Vocational Training and Work Skills
If indicated, an occupational therapist may make recommendations for vocational training if the client is discharged to a day treatment program, outpatient rehabilitation center, or a transitional living site. Vocational training is an extended process that requires the involvement of an occupational therapist and possibly a vocational counselor. The client’s eventual return to work may require the assistance of a job coach. The success of a client’s return to work depends highly on the environment to which he or she is returning and the supportiveness of the environment. These are all aspects that must be considered when evaluating a client’s potential.
Summary
Treatment of adults with TBI is challenging and requires flexibility, stamina, and creativity. Behavioral and psychosocial deficits greatly influence recovery. Substance abuse, a possible contributing factor, must be assessed and addressed. Most clients have a multitude of problems requiring intervention. Coordination of evaluation and goal setting with the interdisciplinary team (including the client and family) is assumed. Intervention should be individualized and oriented toward functional outcomes that are meaningful to the client. An effective transition from acute care to intermediate-level care and then to the community requires the therapist to plan thoughtfully and communicate clearly. For persons with TBI, recovery and adjustment may be a lifelong challenge. Since these individuals face challenges throughout their lives and their needs continue to evolve, providing resources throughout the continuum is essential for an ongoing productive outcome.
1. What are two important measurable landmarks of recovery from TBI?
2. Name five types of neuromuscular impairment that may be present in a client with TBI.
3. Describe the types of care settings available for clients with TBI in the acute, subacute, and postacute stages of rehabilitation.
4. Describe the psychosocial deficits that may be present in a client with TBI.
5. List two components of a behavioral management program.
6. Name three standard assessments for a population with TBI and describe the performance components and areas that they assess.
7. List four visual skills that are evaluated in a vision screening.
8. Why is it important for a client with TBI to complete an on-road driving assessment?
9. What are the goals of a proper wheelchair-positioning program?
10. What are the indications for splinting? Casting?
11. Describe three areas that should be addressed during discharge planning.
12. Why is it important to address substance use in populations with TBI?
References
1. AAA Foundation for Traffic Safety. Available at http://www.aaafoundation.org-Roadwise Review.
2. Allen, CK. Occupational therapy for psychiatric diseases: measurement and management of cognition disabilities. Boston, Mass: Little, Brown; 1985.
3. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, ed 4. Washington, DC: American Psychiatric Association; 1994.
4. Arnadottir, G. The brain and behavior. Philadelphia, Pa: Mosby; 1990.
5. Avery-Smith, W, Dellarosa, DM. Approaches to treating dysphagia in individuals with brain injury. Am J Occup Ther. 1994;48(3):235.
6. Avery-Smith, W, Dellarosa, DM, Brod Rosen, A. Dysphagia evaluation protocol. San Antonio, Tex: Therapy Skill Builders; 1996.
7. Bennett, SE, Karnes, JL. Neurological disabilities. Philadelphia, Pa: JB Lippincott; 1998.
8. Bombardier, CH, Temkin, NR, Machamer, J, Dikmen, SS. The natural history of drinking and alcohol-related problems after traumatic brain injury. Arch Phys Med Rehabil. 2003;84(2):185.
9. Brain Injury Association. Fact sheets. Washington, DC: Brain Injury Association; 2006.
10. Brain Injury Special Interest Group of the American Academy of Physical Medicine and Rehabilitation. Practice parameter: antiepileptic drug treatment of posttraumatic seizures. Arch Phys Med Rehabil. 1998;79(5):594.
11. Brain Trauma Foundation and American Association of Neurological Surgeons, Management and prognosis of severe traumatic brain injury, Brain Trauma Foundation, 2000. http://www.braintrauma.org.
12. Brain Trauma Foundation and American Association of Neurological Surgeons, Management and prognosis of severe traumatic brain injury. Part II: early indicators of prognosis in severe brain injury, Brain Trauma Foundation, 2000. http://www.braintrauma.org.
13. Carmick, J. Clinical use of neuromuscular electric stimulation for children with cerebral palsy. Part 2: upper extremity. Phys Ther. 1993;73(8):514.
14. Center for Outcome Measures in Brain Injury, Santa Clara Valley Medical Center. Available at www.tbi-sci.org.
15. Chestnut, RM. The management of severe traumatic brain injury. Emerg Med Clin North Am. 1997;15(3):581.
16. Colarusso, RP, Hammill, DD, Mercier, L. Motor-Free Visual Perception Test—Revised. Novato, Calif: Academic Therapy Publications; 1995.
17. Corrigan, JD, Bogner, JA, Mysiw, WJ, et al. Systemic bias in outcome studies of persons with traumatic brain injury. Arch Phys Med Rehabil. 1997;78(2):132.
18. Cummings, JL, Mega, MS. Neuropsychiatry and behavioral neuroscience. Oxford, England: Oxford University Press; 2003.
19. Damasio, AR. Aphasia. N Engl J Med. 1992;326(8):531.
20. Englander, J, Bushnik, T, Duong, TT, et al. Analyzing risk factors for late posttraumatic seizures: a prospective, multicenter investigation. Arch Phys Rehabil. 2003;84(3):365.
21. Englander, J, Cifu, DX. The older adult with traumatic brain injury. In Rosenthal M, Griffith ER, Kreutzer JS, Pentland B, eds.: Rehabilitation of the adult and child with traumatic brain injury, ed 3, Philadelphia, Pa: FA Davis, 1999.
22. Englander, J, Cifu, DX, Wright, JM, Black, K. The association of early computed tomography scan findings and ambulation, self-care and supervision needs at rehabilitation discharge and at 1 year after traumatic brain injury. Arch Phys Med Rehabil. 2003;84(2):214.
23. Fisher, AG. Assessment of motor and process skills. Fort Collins, Colo: Colorado State University; 1994.
24. Florey, LL, Michelman, SM. Occupational role history: a screening tool for psychiatric occupational therapy. Am J Occup Ther. 1982;36(5):301.
25. Ghajar, J. Traumatic brain injury. Lancet. 2000;356:923.
26. Giacino, JT, Ashwal, S, Childs, N, et al. The minimally conscious state: definition and diagnostic criteria. Neurology. 2002;58(3):349.
27. Giatnutsos, R. Elemental driving simulator and driving assessment system. Bayport, NY: Life Sciences Associates; 1994.
28. Goga-Eppenstein, P, et al. Casting protocols for the upper and lower extremities. Gaithersburg, Md: Aspen; 1999.
29. Graham, DI. Pathophysiological aspects of injury and mechanisms of recovery. In Rosenthal M, Griffith ER, Kreutzer JS, Pentland B, eds.: Rehabilitation of the adult and child with traumatic brain injury, ed 3, Philadelphia, Pa: FA Davis, 1999.
30. Graham, DI, Adams, JH, Nicoll, JA, et al. The nature, distribution and causes of traumatic brain injury. Brain Pathol. 1995;5(4):397.
31. Granger, CV, Cotter, AC, Hamilton, BB, Fiedler, RC. Functional assessment scales. Arch Phys Med Rehabil. 1993;74(2):133.
32. Green, JL. Technology for communication and cognitive treatment: the clinician’s guide. Potomac, Md.: Innovative Speech Therapy; 2007.
33. Haltiner, AM, Temkin, NR, Dikmen, SS. Risk of seizure recurrence after the first late posttraumatic seizure. Arch Phys Med Rehabil. 1997;78(8):835.
34. Hammond, FM, McDeavitt, JT. Medical and orthopedic complications. In Rosenthal M, Griffith ER, Kreutzer JS, Pentland B, eds.: Rehabilitation of the adult and child with traumatic brain injury, ed 3, Philadelphia, Pa: FA Davis, 1999.
35. Hart, T, Millis, S, Novack, T, et al. The relationship between neuropsychological function and level of caregiver supervision at 1 year after traumatic brain injury. Arch Phys Med Rehabil. 2003;84(2):221.
36. Haselberger, K, Pucher, R, Auer, LM. Prognosis after acute subdural or epidural hemorrhage. Acta Neurochir (Wien). 1988;90(3-4):111.
37. Heilman, KM, Gonzales Rothi, LJ. Apraxia. In Heilman KM, Valenstein E, eds.: Clinical neuropsychology, ed 4, New York, NY: Oxford University Press, 2003.
38. Hooper, HE. Hooper Visual Organization Test. Los Angeles, Calif: Western Psychological Services; 1983.
39. Jackson, WT, Novack, TA, Dowler, RN. Effective serial management of cognitive orientation in rehabilitation: the Orientation Log. Arch Phys Med Rehabil. 1998;79(6):718.
40. Jebsen, RH, Taylor, N, Trieschmann, RB, et al. An objective and standardized test of hand function. Arch Phys Med Rehabil. 1969;50(6):311.
41. Kraus, JF, Morgenstern, H, Fife, D, et al. Blood alcohol tests, prevalence of involvement, and outcomes following brain injury. J Public Health. 1989;79(3):294.
42. Klein, RM, Bell, BJ. Klein-Bell Activities of Daily Living Scale. Seattle, Wash: Health Science Center for Educational Resources; 1982.
43. Kohlman Thomson, L. The Kohlman Evaluation of Living Skills, ed 3. Bethesda, Md: American Occupational Therapy Association; 1992.
44. Kreuter, M, Sullivan, M, Dahllöf, AG, Siösteen, A. Partner relationships, functioning, mood and global quality of life in persons with spinal cord injury and traumatic brain injury. Spinal Cord. 1998;36(4):252.
45. Kubler-Ross, E. On death and dying. New York, NY: Macmillan; 1969.
46. Langlois, JA, Kegler, SR, Butler, JA, et al. Traumatic brain injury–related hospital discharges: results from a 14-state surveillance system, 1997. MMWR Surveill Summ. 2003;52(4):1.
47. Levi, L, Guilburd, JN, Lemberger, A, et al. Diffuse axonal injury: analysis of 100 individuals with radiological signs. Neurosurgery. 1990;27(3):429.
48. Levin, HS, O’Donnell, VM, Grossman, RG. The Galveston Orientation and Amnesia Test: a practical scale to assess cognition after head injury. J Nerv Ment Dis. 1979;167(11):675.
49. Levy, DE, Bates, D, Caronna, JJ, et al. Prognosis in nontraumatic coma. Ann Intern Med. 1981;94(3):293.
50. Loewenstein Rehabilitation Hospital, Israel. Loewenstein Occupational Therapy Cognitive Assessment. Pequannock, NJ: Maddak; 1990.
51. Mayer, NH, Keenan, ME, Esquenazi, A. Limbs with restricted or excessive motion after traumatic brain injury. In Rosenthal M, Griffith ER, Kreutzer JS, Pentland B, et al, eds.: Rehabilitation of the adult and child with traumatic brain injury, ed 3, Philadelphia, Pa: FA Davis, 1999.
52. Mazaux, JM, Masson, F, Levin, HS, et al. Long-term neuropsychological outcome and loss of social autonomy after traumatic brain injury. Arch Phys Med Rehabil. 1997;78(12):1316.
53. McKinlay, WM, Watkiss, AJ. Cognitive and behavioral effects of brain injury. In Rosenthal M, Griffith ER, Kreutzer JS, Pentland B, eds.: Rehabilitation of the adult and child with traumatic brain injury, ed 3, Philadelphia, Pa: FA Davis, 1999.
54. Minnesota rate of manipulation tests. Circle Pines, Minn: American Guidance Service, 1969.
55. Novack, TA, Caldwell, SG, Duke, LW, et al. Focused versus unstructured intervention for attentional deficits after traumatic brain injury. J Head Trauma Rehabil. 1996;11(3):52.
56. Oakley, FM. The role checklist. Occup Ther J Res. 1986;6(3):157.
57. O’Dell, MW, Jasin, P, Lyons, N, et al. Standardized assessment instruments for minimally-responsive, brain-injured patients. NeuroRehabilitation. 1996;6(1):45.
58. Ownsworth, TL, Oei, TP. Depression after traumatic brain injury: conceptualization and treatment considerations. Brain Inj. 1998;12(9):735.
59. Palmer, S, Bader, MK, Qureshi, A, et al. The impact on outcomes in a community hospital setting of using the AANS traumatic brain injury guidelines. J Trauma. 2001;50(4):657.
60. Plum, F, Posner, JB. The diagnosis of stupor and coma, ed 3. Philadelphia, Pa: FA Davis; 1980.
61. Prigitano, GP, Schacter, DL. Awareness of deficit after brain injury: clinical and theoretical issues. New York, NY: Oxford University Press; 1991.
62. Quality Standards Subcommittee of the American Academy of Neurology. Practice parameters: Assessment and management of patients in the persistent vegetative state (summary statement). Neurology. 1995;45:1015–1018.
63. Ranchos Los Amigos Medical Center. Levels of cognitive functioning. Downey, Calif: Rancho Los Amigos Medical Center; 1980.
64. Rustad, RA, et al. Cognitive assessment of Minnesota. San Antonio, Tex: Therapy Skill Builders; 1999.
65. Salamy, M, et al. Assessment of communication and interaction skills. Chicago, Ill: Department of Occupational Therapy, University of Illinois at Chicago; 1993.
66. Santa Clara Valley Medical Center. Behavior management program policy and procedure manual. San Jose, Calif: Santa Clara Valley Medical Center; 2006.
67. Seelig, JM, Becker, DP, Miller, JD, et al. Traumatic acute subdural hematoma: major mortality reduction in comatose patients treated within four hours. N Engl J Med. 1981;304(25):1511.
68. Sosin, DM, Sniezek, JE, Thurman, DJ. Incidence of mild and moderate brain injury in the United States, 1991. Brain Inj. 1996;10(1):47.
69. Stratton, M. Behavioral assessment scale of oral functions in feeding. Am J Occup Ther. 1981;35(11):719.
70. Tiffan, J. Purdue pegboard. Lafayette, Ind: Lafayette Instruments; 1960.
71. The Multi-Society Task Force Report on PVS. Medical aspects of the persistent vegetative state (first of two parts). N Engl J Med. 1994;330(21):1499.
72. The Multi-Society Task Force Report on PVS. Medical aspects of the persistent vegetative state (second of two parts). N Engl J Med. 1994;330(22):1572.
73. Thurman, DJ, Jeppson, L, Burnett, CL, et al. Guidelines for surveillance of central nervous system injury. Atlanta, Ga: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 1995.
74. Traumatic Brain Injury Model Systems National Data Base Syllabus, 2008.
75. Tucker, FM, Hanlon, RE. Effects of mild traumatic brain injury on narrative discourse production. Brain Inj. 1998;12(9):783.
76. Whiting, S, et al. Rivermead Perceptual Assessment Battery. Los Angeles, Calif: NFER-Nelson; 1985.
77. Wilson, B, Cockburn, J, Baddeley, A. The Rivermead Behavioural Memory Test. Gaylord, Minn: National Rehabilitation Services; 1991.
78. Zasler, ND. Prognostic indicators in medical rehabilitation of traumatic brain injury: a commentary and review. Arch Phys Med Rehabil. 1997;78(8 Suppl 4):12.