Chapter 22 The Female Genital Tract*
 OVARIES
OVARIES
Development
The development of the female genital tract is relevant to both anomalies in this region and the histogenesis of various tumors. The primordial germ cells arise in the wall of the yolk sac by the fourth week of gestation; by the fifth or sixth week they migrate into the urogenital ridge. The mesodermal epithelium of the urogenital ridge then proliferates, to eventually produce the epithelium and stroma of the gonad. The dividing germ cells, which are of endodermal origin, are incorporated into the proliferating mesodermal epithelium to form the ovary.1
A second component of female genital development is the müllerian duct. At about the sixth week, invagination and subsequent fusion of the coelomic lining epithelium forms the lateral müllerian (or paramesonephric) ducts. Müllerian ducts progressively grow caudally to enter the pelvis, where they swing medially to fuse with the urogenital sinus at the müllerian tubercle (Fig. 22-1A). Further caudal growth brings these fused ducts into contact with the urogenital sinus, formed when the cloaca is subdivided by the urorectal septum. The urogenital sinus eventually becomes the vestibule of the external genitalia (Fig. 22-1B). Normally the unfused portions mature into the fallopian tubes, the fused caudal portion develop into the uterus and upper vagina, and the urogenital sinus forms the lower vagina and vestibule. Consequently, the entire lining of the uterus and tubes as well as the ovarian surface is ultimately derived from coelomic epithelium (mesothelium). This close embryologic relationship between the mesothelium and müllerian system may be reflected in adult life in the form of benign (endometriosis) and malignant (endometrioid and serous neoplasia) lesions, which may arise in both the surface of the ovaries and the peritoneal surfaces. In addition, it explains the morphologic overlap of tumors arising in the various parts of the female genital tract (e.g., serous, endometrioid, clear cell).
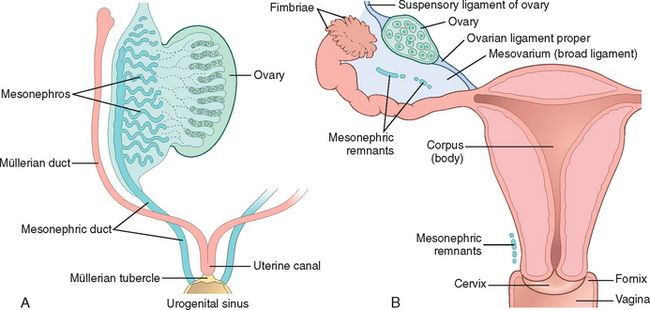
FIGURE 22-1 Embryology and anatomy of the female genital tract. A, Early in development the mesonephric (blue) and müllerian (red) ducts merge at the urogenital sinus to form the müllerian tubercle. B, By birth the müllerian ducts have fused to form the fallopian tubes, uterus, and endocervix (red), merging with the vaginal squamous mucosa. The mesonephric ducts regress but may be found as a remnant in the ovary, adnexa, and cervix (Gartner duct).
(Adapted from Langman J: Medical Embryology. Baltimore, Williams and Wilkins, 1981.)
The epithelium of the vagina, cervix, and urinary tract is formed by induction of basal cells from the underlying stroma, which undergo squamous and urothelial differentiation.2 A portion of these cells remains uncommitted, forming the reserve cells of the cervix. The latter are capable of both squamous and columnar cell differentiation.3
In males, müllerian inhibitory substance4 from the developing testis causes regression of the müllerian ducts, and the paired wolffian (or mesonephric) ducts form the epididymis and the vas deferens. Normally the mesonephric duct regresses in the female, but remnants may persist into adult life as epithelial inclusions adjacent to the ovaries, tubes, and uterus. In the cervix and vagina these rests may be cystic and are termed Gartner duct cysts. Many of the events in the formation of the internal and external genitalia and their epithelial coverings result from reciprocal epithelial-stromal signaling, leading to mesenchymal remodeling and changes in epithelial cell fate.2,5
Anatomy
During active reproductive life, the ovaries measure about 4 × 2.5 × 1.5 cm in dimension. The ovary is divided into a cortex and a medulla. The cortex consists of a layer of closely packed stromal cells and a thin covering of relatively acellular collagenous connective tissue. Follicles in varying stages of maturation are found within the outer cortex. With each menstrual cycle, one follicle develops into a graafian follicle, which is transformed into a corpus luteum following ovulation. Corpora lutea ranging from recent to senescent (corpora albicans) may be found in the cortex of the adult ovary.
The medulla of the ovary consists of loosely arranged mesenchymal tissue and contains remnants of the mesonephric duct (rete ovarii) and small clusters of round to polygonal, epithelioid cells (hilus cells) around vessels and nerves. These hilus cells are vestigial remains of the gonad from its primitive “ambisexual” phase, are steroid producing, and resemble the interstitial cells of the testis. Rarely, these cells give rise to masculinizing tumors (hilar cell tumors).
The fallopian tube mucosa is composed of numerous delicate papillary folds (plica) consisting of three cell types: ciliated columnar cells; nonciliated, columnar secretory cells; and so-called intercalated cells, which may simply represent inactive secretory cells.
The uterus varies in size depending on the age and parity of the individual. It weighs about 50 gm and measures about 8.0 × 6.0 × 3.0 cm in nulliparous reproductive age women. Following pregnancies, uteri are slightly larger (up to 70 gm in weight), then diminish to half their weight and dimension following menopause.
The uterus has three distinctive anatomic and functional regions: the cervix, the lower uterine segment, and the corpus. The cervix is further divided into the vaginal portio (ectocervix) and the endocervix. The portio is visible to the naked eye on vaginal examination and is covered by a stratified nonkeratinizing squamous epithelium continuous with the vaginal vault. The squamous epithelium converges centrally at a small opening termed the external os. In the nulliparous woman, this os is virtually closed. Just cephalad from the os is the endocervix, which is lined by columnar, mucus-secreting epithelium that dips down into the underlying stroma to produce endocervical glands. The point at which the squamous and endocervical mucinous columnar epithelium meet is termed the squamocolumnar junction (Fig. 22-2). The position of the junction is variable because of both the cervical anatomy and age-related hormonal influences. The differentiation of basal/reserve cells at the squamocolumnar junction into either squamous or glandular cell type governs the microanatomy of this region and results in a progressive upward migration of the squamocolumnar junction with age. The area of the cervix where the columnar epithelium is ultimately replaced by squamous epithelium is termed the transformation zon (see Fig. 22-2). Metaplasia of glandular epithelium to squamous epithelium at the squamocolumnar junction produces multilayered, initially immature, squamous epithelium known as “squamous metaplasia.” These immature squamous cells are susceptible to human papillomavirus (HPV) infection and, as discussed below, it is at the squamocolumnar junction where precancerous lesions and cervical carcinomas develop.6
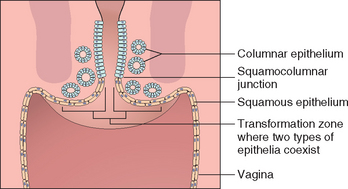
FIGURE 22-2 Schematic of the cervical transformation zone where squamous and endocervical columnar epithelia coexist undergoing metaplasia (“transformation”) from glandular to squamous differentiation.
The corpus consists of the endometrium surrounded by the myometrium. Changes in the endometrium that occur during the menstrual cycle (discussed below) are keyed to the rise and fall in the levels of ovarian hormones, and the reader should be familiar with the complex but fascinating interactions among hypothalamic, pituitary, and ovarian factors underlying maturation of ovarian follicles, ovulation, and the menstrual cycle.
Diseases of the female genital tract are extremely common and include complications of pregnancy, infections, tumors, and hormonally induced effects. The following discussion presents the pathology of the major diseases that result in clinical problems. Details can be found in current textbooks of gynecologic pathology and clinical obstetrics and gynecology.7,8 The pathologic conditions peculiar to each segment of the female genital tract are discussed separately, but first we briefly review infections and pelvic inflammatory disease because they can affect many of the various anatomic structures concomitantly.
Infections of the Female Genital Tract
A large variety of organisms can infect the female genital tract. Infections with some microorganisms, such as Candida, Trichomonas, and Gardnerella, are extremely common and may cause significant discomfort with no serious sequelae. Others, such as Neisseria gonorrhoeae and Chlamydia infections, are major causes of female infertility, and others still, such as Ureaplasma urealyticum and Mycoplasma hominis infections, are implicated in preterm deliveries. Viruses, especially herpes simplex viruses (HSVs) and human papillomaviruses (HPVs), also account for considerable morbidity; HSVs cause painful genital ulcerations, whereas HPVs are involved in the pathogenesis of cervical, vaginal, and vulvar cancers.
Many of these infections are sexually transmitted, including trichomoniasis, gonorrhea, chancroid, granuloma inguinale, lymphogranuloma venereum, syphilis, mycoplasma, chlamydia, HSV, and HPV.9 Most of these conditions have been considered in Chapter 8. Here we touch only on selected aspects relevant to the female genital tract, including pathogens confined to the lower genital tract (vulva, vagina, and cervix) and those that involve the entire genital tract and are implicated in pelvic inflamatory disease. Papillomaviruses are also discussed in Chapter 7.
Infections of the Lower Genital Tract
Genital herpes simplex virus infection is common and involves, in the order of frequency, the cervix, vagina, and vulva. HSVs are DNA viruses that include two serotypes, HSV-1 and HSV-2. HSV-1 typically results in oropharyngeal infection, whereas HSV-2 usually involves genital mucosa and skin; however, depending on the sexual practices HSV-1 may be detected in the genital region and HSV-2 may cause oral infections as well (see also Chapter 8). The frequency of genital herpes has increased dramatically in the past decades, particularly in teenagers and young women. By the age of 40, 20% of women are seropositive for antibodies against HSV-2.10
Clinical symptoms are seen in about one third of infected individuals. The initial lesions typically develop 3 to 7 days after sexual transmission and consist of red papules that progress to vesicles and then to painful coalescent ulcers. Such lesions are clinically apparent on vulvar skin and mucosa, while cervical or vaginal lesions present with severe purulent discharge and pelvic pain. Lesions around the urethra may cause painful urination and urine retention. The initial infection typically produces systemic symptoms such as fever, malaise, and tender inguinal lymph nodes. The vesicles and ulcers contain numerous viral particles, accounting for the high transmission rate during active infection. The mucosal and skin lesions heal spontaneously in 1 to 3 weeks, but as with herpetic infections elsewhere, the virus migrates to the regional lumbosacral nerve ganglia establishing a latent infection. Because of viral latency, HSV infections persist indefinitely and any decrease in immune system surveillance, as well as stress, trauma, ultraviolet radiation, and hormonal changes, can trigger reactivation of the virus and recurrence of the skin and mucosal lesions.9 As expected, recurrences are much more common in immunosuppressed individuals. In addition, HSV-2 infections are more likely to recur than HSV-1 infections.
Transmission of HSV may occur during both the active and latent phases (subclinical virus shedding), although it is much less likely in asymptomatic carriers. Condoms offer limited protection against HSV infection, since a large genital area may be affected by the virus. As with other sexually transmitted diseases, women are more susceptible to transmission than men. Previous infection with HSV-1 seems to reduce susceptibility to HSV-2 infection. The gravest consequence of HSV infection is transmission to the neonate during birth. This risk is highest if the infection is active during delivery and particularly if it is a primary (initial) infection in the mother. Cesarian section is warranted in such cases.
The diagnosis is based on typical clinical findings and HSV detection. For diagnosis the purulent exudate is aspirated from the lesions and inoculated into a tissue culture. After 48 to 72 hours the viral cytopathic effect can be seen, and the virus may then be isolated and serotyped. In addition, some laboratories offer more sensitive polymerase chain reaction, enzyme-linked immunosorbent assays, and direct immunofluorescent antibody tests for detection of HSV in the lesional secretions. Individuals with primary, acute-phase HSV infection do not have serum anti-HSV antibodies. Detection of anti-HSV antibodies in the serum is indicative of recurrent/latent infection.
There is no effective treatment for latent HSV; however, antiviral agents like acyclovir or famciclovir may shorten the length of the initial and recurrent symptomatic phase. Several prophylactic and therapeutic vaccine strategies have been developed using animal models, and several clinical trials are currently underway.11
Molluscum contagiosum is a poxvirus infection of the skin and the mucous membranes. There are four types of molluscum contagiosum viruses (MCVs), MCV-1 to -4, with MCV-1 being the most prevalent and MCV-2 being most often sexually transmitted. The infections are common in young children between 2 and 12 years of age and are transmitted through direct contact or shared articles (e.g., towels). Molluscum may affect any area of the skin but is most common on the trunk, arms, and legs. In adults, molluscum infections are typically sexually transmitted and affect the genitals, lower abdomen, buttocks, and inner thighs. The average incubation period is 6 weeks. Diagnosis is based on the characteristic clinical appearance of pearly, dome-shaped papules with a dimpled center. The papules measure 1 to 5 mm in diameter, and their central waxy core contains cells with intracytoplasmic viral inclusions (Fig. 22-3).
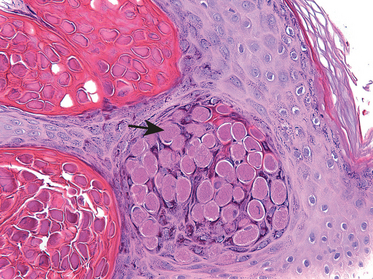
FIGURE 22-3 Lesion caused by molluscum contagiosum infection. Arrow points to intracytoplasmic viral inclusions.
Fungal infections, especially those caused by yeasts (Candida), are extremely common; in fact, yeasts are part of many women’s normal vaginal microflora and the development of symptomatic candidiasis is typically a result of a disturbance in the patient’s vaginal microbial ecosystem. Diabetes mellitus, antibiotics, pregnancy, and conditions resulting in compromised cell-mediated immunity are permissive to symptomatic infection, which manifests itself by marked vulvovaginal pruritus, erythema, swelling, and curdlike vaginal discharge. Severe infection may result in mucosal ulcerations. The diagnosis is made by finding the pseudospores or filamentous fungal hyphae in wet KOH mounts of the discharge or on Pap smear. Even though sexual transmission of yeast infection has been documented, candidiasis is not considered a sexually transmitted disease.
Trichomonas vaginalis is a large, flagellated ovoid protozoan that can be readily identified in wet mounts of vaginal discharge or Pap smear of infected patients. The infection is usually transmitted by sexual contact and develops within 4 days to 4 weeks. The patients may be asymptomatic or may complain of yellow, frothy vaginal discharge, vulvovaginal discomfort, dysuria (painful urination), and dyspareunia (painful intercourse). The vaginal and cervical mucosa typically has a fiery-red appearance, with marked dilatation of cervical mucosal vessels resulting in characteristic colposcopic appearance of “strawberry cervix.”
Gardnerella vaginalis is a gram-negative bacillus that is implicated as the main cause of bacterial vaginosis (vaginitis). Patients typically present with thin, green-gray, malodorous (fishy) vaginal discharge. Pap smears reveal superficial and intermediate squamous cells covered by a shaggy coat of multiple coccobacilli. Bacterial cultures in such cases reveal G. vaginalis and other bacteria including anaerobic peptostreptococci and aerobic α-hemolytic streptococci. In pregnant patients, bacterial vaginosis has been implicated in premature labor.
Ureaplasma urealyticum and Mycoplasma hominis species account for some cases of vaginitis and cervicitis, and have been implicated in chorioamnionitis and premature delivery in pregnant patients.12
Most Chlamydia trachomatis infections take the form of cervicitis. However, in some patients it ascends to the uterus and fallopian tubes, resulting in endometritis and salpingitis, and thus is one of the causes of pelvic inflammatory disease, as discussed below.
For description of genital lesions caused by Treponema pallidium, see Chapter 8. Description of HPV infections is presented in this chapter under “Cervix”, and gonorrhea infections are described below.
Infections Involving The Lower and Upper Genital Tract
Pelvic Inflammatory Disease (PID)
PID is an ascending infection that begins in the vulva or vagina and spreads upward to involve most of the structures in the female genital system, resulting in pelvic pain, adnexal tenderness, fever, and vaginal discharge. Gonococcus continues to be a common cause of PID, the most serious complication of gonorrhea in women. Chlamydia infection is another well-recognized cause of PID. Besides these two organisms, infections after spontaneous or induced abortions and normal or abnormal deliveries (called puerperal infections) are important causes of PID. In these situations the infections are typically polymicrobial and may be caused by staphylococci, streptococci, coliform bacteria, and Clostridium perfringens.
With gonococcus, inflammatory changes start to appear approximately 2 to 7 days after inoculation. Endocervical mucosa is the most common site of initial involvement. Gonococcal inflammation may also begin in the Bartholin gland and other vestibular, or periurethral, glands. From any of these sites, the organisms may spread upward to involve the fallopian tubes and tubo-ovarian region. The non-gonococcal bacterial infections that follow induced abortion, dilation and curettage of the uterus, and other surgical procedures of the female genital tract are thought to spread from the uterus upward through the lymphatics or venous channels rather than on the mucosal surfaces. Therefore, these infections tend to produce less mucosal involvement but more reaction within the deeper layers of the organs.
Morphology. Wherever it occurs, gonococcal disease is characterized by marked acute inflammation largely confined to the superficial mucosa. Smears of the inflammatory exudate disclose the intracellular gram-negative diplococcus; however, definitive diagnosis requires culture, or detection of gonoccocal RNA or DNA. If spread occurs, the endometrium is usually spared for unclear reasons. Once the infection reaches the tubes, an acute suppurative salpingitis ensues. The tubal mucosa becomes congested and diffusely infiltrated by neutrophils, plasma cells, and lymphocytes. Gonococcal lipopolysaccharide and inflammatory mediators such as TNF cause epithelial injury and sloughing of the plicae. The tubal lumen fills with purulent exudate that may leak out of the fimbriated end. The infection may further spill over to the ovary to create a salpingo-oophoritis. Collections of pus within the ovary and tube (tubo-ovarian abscesses) or tubal lumen (pyosalpinx) may occur (Fig. 22-4). In the course of time the infecting organisms may disappear, leaving the sequelae of chronic follicular salpingitis and hydrosalpinx (dilated, fluid-filled fallopian tube). The tubal plicae, denuded of epithelium, adhere to one another and slowly fuse in a reparative, scarring process that forms glandlike spaces and blind pouches, referred to as chronic follicular salpingitis. The lumen of such tubes may be impenetrable for the oocyte, resulting in infertility or ectopic pregnancy. Hydrosalpinx develops as a consequence of the fusion of the fimbriae and the subsequent accumulation of the tubal secretions and tubal distention. Hydrosalpinx is another cause of post-PID infertility, since lack of flexible tubal fimbriae prevents uptake of the oocyte after ovulation.
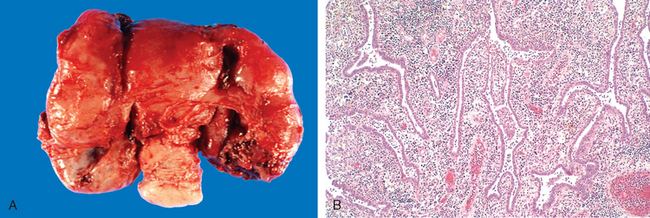
FIGURE 22-4 A, Acute salpingo-oophoritis, with tubo-ovarian abscess. The fallopian tubes and ovaries have coalesced into an inflammatory mass adherent to the uterus. B, Salpingitis with edematous tubal plicae expanded by inflammatory cell infiltrates.
PID caused by staphylococci, streptococci, and the other puerperal invaders tends to have less exudation within the lumen of the tube and less involvement of the mucosa, but a greater inflammatory response within the deeper tissue layers. These infections often spread throughout the wall to involve the serosa and the broad ligaments, pelvic structures, and peritoneum. Bacteremia is a more frequent complication of streptococcal or staphylococcal PID than of gonococcal infections.
The acute complications of PID include peritonitis and bacteremia, which in turn may result in endocarditis, meningitis, and suppurative arthritis. The remote sequelae of PID include infertility and tubal obstruction, increased risk of ectopic pregnancy, pelvic pain, as well as intestinal obstruction due to adhesions between the bowel and pelvic organs.
In the early stages, gonococcal infections are readily controlled with antibiotics, although penicillin-resistant strains have regrettably emerged. When the infection becomes walled off in tubo-ovarian abscesses, it is difficult to achieve sufficient levels of antibiotics within such infectious foci and it sometimes becomes necessary to remove the organs surgically. Postabortion and postpartum PIDs are also amenable to treatment with antibiotics but are far more difficult to control than the gonococcal infections because of the broad spectrum of pathogens that may be involved.
VULVA
Diseases of the vulva in the aggregate constitute only a small fraction of gynecologic practice. Many inflammatory dermatologic diseases that affect skin elsewhere on the body may also occur on the vulva, such as psoriasis, eczema, and allergic dermatitis. The vulva is more prone to skin infections, because it is constantly exposed to secretions and moisture. Nonspecific vulvitis is particularly likely to occur in the setting of immunosuppression. Most skin cysts (epidermal inclusion cysts) and skin tumors can also occur in the vulva. Here we discuss disorders particular to the vulva, including Bartholin cyst, non-neoplastic epithelial disorders, benign exophytic lesions, and tumors of the vulva.
Bartholin Cyst
Infection of the Bartholin gland produces an acute inflammation within the gland (adenitis) and may result in an abscess. Bartholin duct cysts are relatively common, occur at all ages, and result from obstruction of the duct by an inflammatory process. The resulting cysts are lined by the ductal squamous metaplastic and/or epithelium. They may become large, up to 3 to 5 cm in diameter, and produce pain and local discomfort. Bartholin duct cysts are either excised or opened permanently (marsupialization).
Non-Neoplastic Epithelial Disorders
A heterogeneous group of lesions of the vulva presents as opaque, white, plaquelike mucosal thickening that may produce itching (pruritus) and scaling. Because of their appearance, these disorders have traditionally been termed leukoplakia by clinicians. This is a non-specific descriptive term, as white plaques may represent a variety of benign, premalignant, or malignant lesions including (1) inflammatory dermatoses (e.g., psoriasis, chronic dermatitis); (2) vulvar intraepithelial neoplasia, Paget disease, or even invasive carcinoma; and (3) epithelial disorders of unknown etiology. Excluding neoplasms and specific disease entities, nonneoplastic epithelial disorders of unknown etiology are classified into two categories: (1) lichen sclerosus and (2) squamous cell hyperplasia (also known as lichen simplex chronicus). The two disorders may coexist and the lesions are often multiple, making their clinical management particularly difficult.
LICHEN SCLEROSUS
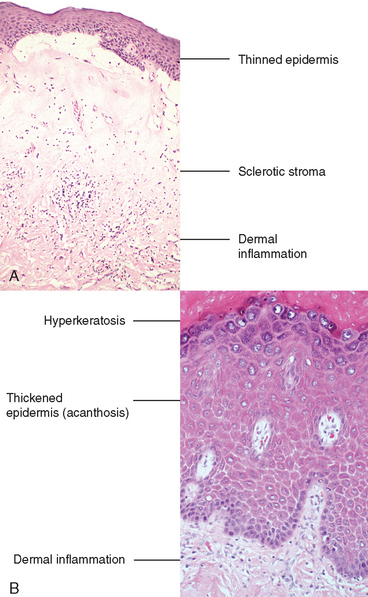
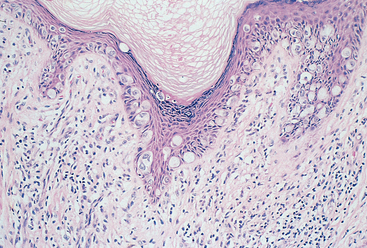
This lesion is characterized by thinning of the epidermis and disappearance of rete pegs, hydropic degeneration of the basal cells, superficial hyperkeratosis, and dermal fibrosis with a scant perivascular, mononuclear inflammatory cell infiltrate (Fig. 22-5). The lesions appear clinically as smooth, white plaques or papules that in time may extend and coalesce. The surface is smoothed out and sometimes resembles parchment. When the entire vulva is affected, the labia become somewhat atrophic and stiffened, and the vaginal orifice is constricted. It occurs in all age groups but is most common in postmenopausal women. It may also be encountered elsewhere on the skin. The pathogenesis is uncertain, but the presence of activated T cells in the subepithelial inflammatory infiltrate and the increased frequency of autoimmune disorders in these women suggests an autoimmune reaction may be involved. Although the lesion in lichen sclerosus is not pre-malignant by itself, women with symptomatic lichen sclerosus have a somewhat increased chance of developing squamous cell carcinoma in their lifetime.13
SQUAMOUS CELL HYPERPLASIA
Previously called hyperplastic dystrophy, or lichen simplex chronicus, squamous cell hyperplasia is a nonspecific condition resulting from rubbing or scratching of the skin to relieve pruritus. It is marked by epithelial thickening, expansion of the stratum granulosum, and significant surface hyperkeratosis. It appears clinically as an area of leukoplakia. The epithelium may show increased mitotic activity in both the stratum basalis and spinosum. Leukocytic infiltration of the dermis is sometimes pronounced. The hyperplastic epithelial changes show no atypia (see Fig. 22-5B). There is generally no increased predisposition to cancer, but suspiciously, lichen simplex chronicus is often present at the margins of established cancer of the vulva.
Benign Exophytic Lesions
Benign raised (exophytic) or wartlike conditions of the vulva may be caused by an infection or are of unknown etiology. Condyloma acuminatum, a papillomavirus-induced lesion, also called a genital wart, and syphilitic condyloma latum (described in Chapter 8) are consequences of sexually transmitted infections. Vulvar fibroepithelial polyps, or skin tags, are similar to skin tags occurring elsewhere on the skin. Vulvar squamous papillomas are benign exophytic proliferations covered by nonkeratinized squamous epithelium, which develop on vulvar mucosal surfaces and may be single or numerous (vulvar papillomatosis). The etiology of fibroepithelial polyps and squamous papillomas is unknown; however, these lesions are not related to any known infectious agent.
CONDYLOMA ACUMINATUM
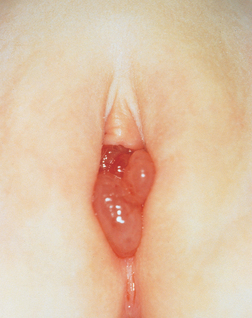
Condylomata acuminata are sexually transmitted, benign lesions that have a distinct verrucous gross appearance (Fig. 22-6A). Although they may be solitary, they are more frequently multifocal: they may involve vulvar, perineal, and perianal regions as well as the vagina and, less commonly, the cervix. The lesions are identical to those found on the penis and around the anus in males (Chapter 21). On histologic examination, they consist of branching, treelike cores of stroma covered by squamous epithelium with characteristic viral cytopathic changes referred to as koilocytic atypia (Fig. 22-6B). Condylomata acuminata are caused by low oncogenic risk HPVs, principally types 6 and 11, and represent productive viral infection in which HPV replicates in the squamous cells. The virus life cycle is completed in the mature superficial cells, resulting in distinct cytologic changes—koilocytotic atypia—characterized by nuclear enlargement and atypia as well as a cytoplasmic perinuclear halo (see also “Cervix”). Condylomata acuminata are not considered precancerous lesions.
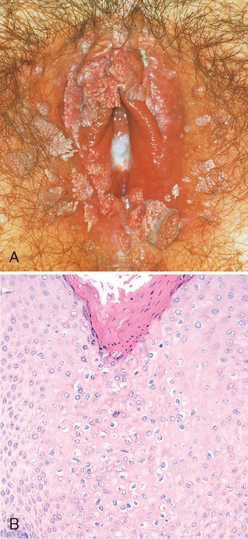
FIGURE 22-6 A, Numerous condylomas of the vulva encircling the introitus. B, Histopathology of condyloma acuminatum showing acanthosis, hyperkeratosis, and koilocytic atypia with enlarged, atypical nuclei and cytoplasmic vacuolation (center of microphotograph).
(A, Courtesy of Dr. Alex Ferenczy, McGill University, Montreal, PQ, Canada.)
Squamous Neoplastic Lesions
VULVAR INTRAEPITHELIAL NEOPLASIA AND VULVAR CARCINOMA
Carcinoma of the vulva is an uncommon malignant neoplasm (approximately one eighth as frequent as cervical cancer) representing about 3% of all genital cancers in the female; approximately two thirds occur in women older than 60 years. Squamous cell carcinoma is the most common histologic type of vulvar cancer. In terms of etiology, pathogenesis, and histologic features, vulvar squamous cell carcinomas are divided into two groups: basaloid and warty carcinomas related to infection with high oncogenic risk HPVs (30% of cases) and keratinizing squamous cell carcinomas, not related to HPV infection (70% of cases).14
Invasive basaloid and warty carcinomas develop from a precancerous in situ lesion called classic vulvar intraepithelial neoplasia (classic VIN). This form of VIN includes lesions designated formerly as carcinoma in situ or Bowen disease. Classic VIN is characterized by nuclear atypia of the squamous cells, increased mitoses, and lack of cellular maturation (Fig. 22-7A). It is analogous to cervical squamous intraepithelial lesions (SILs, see under “Cervix”). It most commonly occurs in reproductive-age women, and the risk factors are the same as those associated with cervical squamous intraepithelial lesions (e.g., young age at first intercourse, multiple sexual partners, male partner with multiple sexual partners), since both cervical squamous intraepithelial lesions and classic VIN are related to HPV infection. VIN is frequently multicentric in the vulva, and 10% to 30% of patients with VIN also have vaginal or cervical HPV-related lesions. The majority of cases of classic VIN are positive for HPV 16, and less frequently for other high-risk HPV types, like HPV 18 or 31. Spontaneous regression of VIN lesions has been reported, usually in younger women; the risk of progression to invasive carcinoma is higher in women older than 45 years of age or in women with immunosuppression.
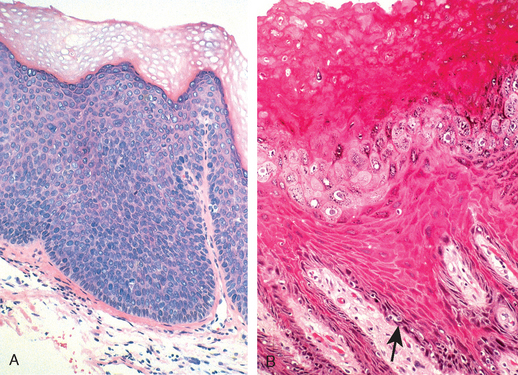
FIGURE 22-7 A, Histopathology of classic vulvar intraepithelial neoplasia (HPV positive) with diffuse cellular atypia, immaturity, nuclear crowding, and increased mitotic activity. B, Differentiated VIN (HPV negative), showing maturation of the superficial layers, hyperkeratosis, and basal cell atypia (arrow).
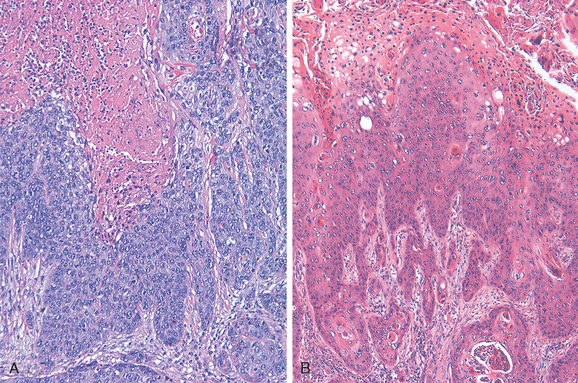
Morphology. HPV-associated vulvar squamous cell carcinomas begin as classic VIN lesions, which present as discrete white (hyperkeratotic), flesh-colored or pigmented, slightly raised lesions. Coexisting carcinomas may be exophytic or indurated, frequently with ulceration. On histologic examination, basaloid carcinoma (Fig. 22-8A) shows an infiltrating tumor characterized by nests and cords of small, tightly packed malignant squamous cells lacking maturation that resemble immature cells from the basal layer of the normal epithelium. The tumor may have foci of central necrosis.
Warty carcinoma is characterized by exophytic, papillary architecture and prominent koilocytic atypia (Fig. 22-8B).
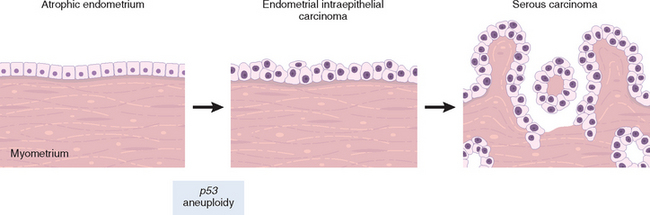
Non-HPV-related keratinizing squamous cell carcinomas frequently arise in individuals with long-standing lichen sclerosus or squamous cell hyperplasia. The mean age of the patients is 76 years. The immediate premalignant lesion is referred to as differentiated vulvar intraepithelial neoplasia (differentiated VIN) or VIN simplex (see Fig. 22-7B).14 Differentiated VIN is characterized by marked atypia of the basal layer of the squamous epithelium with apparently normal epithelial maturation and differentiation in the superfical layers, thus the designation “differentiated VIN.” The etiology of differentiated VIN is unknown, but it is postulated that chronic epithelial irritation in lichen sclerosus or squamous cell hyperplasia may contribute to a gradual evolution of the malignant phenotype. The putative molecular events leading to malignant transformation in lichen sclerosus, squamous cell hyperplasia, and differentiated VIN are under investigation. A report of allelic imbalance in lichen sclerosus and squamous cell hyperplasia supports the hypothesis that both conditions pose a risk for neoplasia despite the lack of morphologic evidence of atypia. Rare cases of lichen sclerosus, differentiated VIN, and adjacent carcinoma with identical p53 gene mutations have been reported. Overall, however, p53 gene mutation is an infrequent and rather late event in vulvar carcinogenesis.15
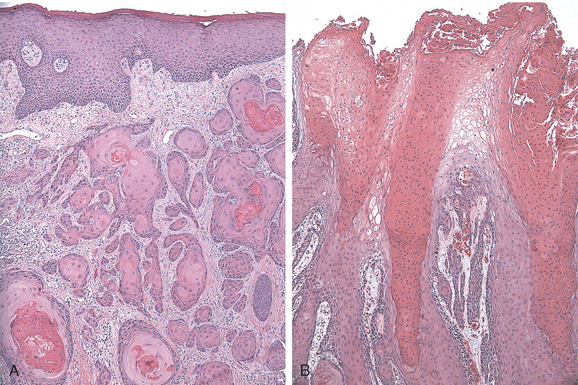
Morphology. Carcinomas associated with lichen sclerosus, squamous cell hyperplasia, and differentiated VIN may develop as nodules in a background of vulvar inflammation. The often-subtle emergence of cancer may be misinterpreted as dermatitis, eczema, or leukoplakia for long periods. The clinical manifestations are nonspecific, including local discomfort, itching, and exudation because of superficial secondary infection, and underscore the importance of repeated examination in women with vulvar inflammatory disorders. Histologic examination reveals infiltrating tumor characterized by nests and tongues of malignant squamous epithelium with prominent central keratin pearls (Fig. 22-9A).
Risk of cancer development in VIN is principally a function of age, extent, and immune status.16 Once invasive cancer develops, metastatic spread is linked to the size of tumor, depth of invasion, and involvement of lymphatic vessels. The initial spread is to inguinal, pelvic, iliac, and periaortic lymph nodes. Ultimately, lymphohematogenous dissemination to the lungs, liver, and other internal organs may occur. Patients with lesions less than 2 cm in diameter have a 60% to 80% 5-year survival after treatment with vulvectomy and lymphadenectomy; however, larger lesions with lymph node involvement have a 5-year survival rate of less than 10%.
Rare variants of squamous cell carcinoma include verrucous carcinomas (Fig. 22-9B), which are fungating tumors resembling condyloma acuminatum, and basal cell carcinomas, which are identical to their counterparts in the skin. Neither tumor is associated with papillomaviruses. Both tumors rarely metastasize and are successfully cured by wide excision.
Glandular Neoplastic Lesions
PAPILLARY HIDRADENOMA
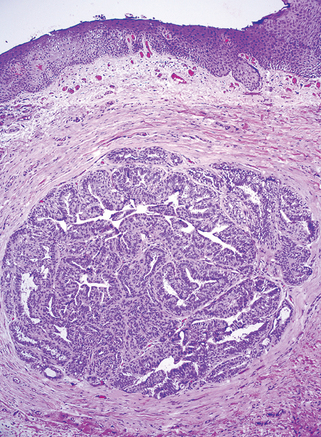
Like the breast, the vulva contains modified apocrine sweat glands. In fact, the vulva may contain tissue closely resembling breast (“ectopic breast”) and develop two tumors with counterparts in the breast, namely papillary hidradenoma and extramammary Paget disease. Papillary hidradenoma presents as a sharply circumscribed nodule, most commonly on the labia majora or interlabial folds, and may be confused clinically with carcinoma because of its tendency to ulcerate.
Morphology. On histologic examination hidradenoma is identical in appearance to intraductal papillomas of the breast and consists of papillary projections covered with two layers of cells: the top columnar, secretory cells and an underlying layer of flattened “myoepithelial cells.” These myoepithelial elements are characteristic of sweat glands and sweat gland tumors (Fig. 22-10).
EXTRAMAMMARY PAGET DISEASE
This curious and rare lesion of the vulva, and sometimes the perianal region, is similar in its manifestations to Paget disease of the breast (Chapter 23). As a vulvar neoplasm, it presents as a pruritic, red, crusted, sharply demarcated, maplike area, occurring usually on the labia majora. It may be accompanied by a palpable submucosal thickening or nodule.
Morphology. Paget disease is a distinctive intraepithelial proliferation of malignant cells. The diagnostic microscopic feature is the presence of large tumor cells lying singly or in small clusters within the epidermis and its appendages. These cells are distinguished by a clear separation (“halo”) from the surrounding epithelial cells (Fig. 22-11) and a finely granular cytoplasm containing mucopolysaccharide that stains with periodic acid–Schiff (PAS), Alcian blue, or mucicarmine stains. Ultrastructurally, Paget cells display apocrine, eccrine, and keratinocyte differentiation and presumably arise from primitive germinal cells of the mammary-like gland ducts of the vulvar skin.17,18
In contrast to Paget disease of the nipple, in which 100% of patients show an underlying ductal breast carcinoma, vulvar lesions are most frequently confined to the epidermis of the skin and adjacent hair follicles and sweat glands. Paget disease is treated with wide local excision and shows a high recurrence rate. Typically, Paget cells spread beyond the confines of the grossly visible lesion, and therefore are frequently present beyond the margins of surgical excision. Intraepidermal Paget disease may persist for many years, even decades, without invasion or metastases. Invasion develops rarely, and in such patients the prognosis is poor.
Malignant Melanoma
Melanomas of the vulva are rare, representing less than 5% of all vulvar cancers and 2% of all melanomas in women. Their peak incidence is in the sixth or seventh decade; they tend to have the same biologic and histologic characteristics as melanomas occurring elsewhere in the skin and are capable of widespread metastatic dissemination. The 5-year survival rate is less than 32%, presumably because of delays in detection and because the majority of these tumors rapidly enter a vertical growth phase following inception (Chapter 25). Prognosis is linked principally to depth of invasion, with greater than 60% mortality for lesions invading deeper than 1 mm.
Because it is initially confined to the epithelium, melanoma may resemble Paget disease, both grossly and histologically. It can usually be differentiated by its uniform reactivity with antibodies to S100 protein, absence of reactivity with antibodies to cytokeratin, and lack of mucopolysaccharides, both of which are present in Paget disease.
VAGINA
The vagina is a portion of the female genital tract that is remarkably free from primary disease. In the adult, inflammations often affect the vulva and perivulvar structures and spread to the cervix without significant involvement of the vagina. Primary lesions of the vagina are rare; the most serious of which is primary vaginal carcinoma. Thus, they are discussed only briefly.
Developmental Anomalies
Septate, or double, vagina is an uncommon anomaly that arises from failure of total fusion of the müllerian ducts and accompanies a double uterus (uterus didelphys). These and other anomalies of the external genitalia may be the manifestations of genetic syndromes, in utero exposure to diethylstilbestrol (DES) used to prevent threatened abortions in the 1940s through 1960s, or other disturbances associated with abnormalities in reciprocal epithelial-stromal signaling during fetal development.19
Vaginal adenosis is a remnant of columnar, endocervical-type epithelium that during embryonal development extends from the endocervix and covers the ectocervix as well as the upper vagina and is subsequently replaced by the squamous epithelium advancing upwardly from the urogenital sinus. Small patches of unreplaced glandular epithelium may persist focally into adult life. Adenosis presents clinically as red, granular areas contrasting with the normal pale-pink vaginal mucosa. On microscopic examination, adenosis consists of columnar mucinous epithelium indistinguishable from endocervical epithelium. Adenosis, while normally present in a small percentage of adult women, has been reported in 35% to 90% of women exposed to DES in utero. Rare cases of clear cell carcinoma (Fig. 22-12) arising in DES-related adenosis were recorded in teens and young women in the 1970s and 1980s, resulting in discontinuation of DES treatment.
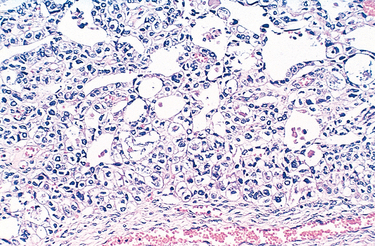
FIGURE 22-12 Clear cell adenocarcinoma of the vagina showing vacuolated tumor cells in clusters and glandlike structures.
Gartner duct cysts are relatively common lesions found along the lateral walls of the vagina and derived from wolffian (mesonephric) duct rests. They are 1- to 2-cm fluid-filled cysts that occur in the submucosal location. Other cysts, including mucus cysts, which occur in the proximal vagina, are derived from müllerian epithelium. Another müllerian-derived lesion, endometriosis (described later), may occur in the vagina and simulate a neoplasm.
Premalignant and Malignant Neoplasms
Most of the benign tumors of the vagina occur in reproductive-age women and include stromal tumors (stromal polyps), leiomyomas, and hemangiomas. The most common malignant tumor of the vagina is carcinoma metastatic from the cervix, followed by a primary squamous cell carcinoma of the vagina. Infants may develop a unique, rare malignancy—embryonal rhabdomyosarcoma (sarcoma botryoides).
VAGINAL INTRAEPITHELIAL NEOPLASIA AND SQUAMOUS CELL CARCINOMA
Primary carcinoma of the vagina is an extremely uncommon cancer (about 0.6 per 100,000 women yearly) accounting for about 1% of malignant neoplasms in the female genital tract. Almost all of these tumors are squamous cell carcinomas associated with high oncogenic risk HPVs. The greatest risk factor is a previous carcinoma of the cervix or vulva; 1% to 2% of women with an invasive cervical carcinoma eventually develop a vaginal squamous cell carcinoma. Squamous cell carcinoma of the vagina arises from a premalignant lesion, vaginal intraepithelial neoplasia, analogous to cervical squamous intraepithelial lesions (SILs, see under “Cervix”). Most often the invasive tumor affects the upper posterior vagina, particularly along the posterior wall at the junction with the ectocervix. The lesions in the lower two thirds of the vagina metastasize to the inguinal nodes, whereas upper lesions tend to involve the regional iliac nodes.
EMBRYONAL RHABDOMYOSARCOMA
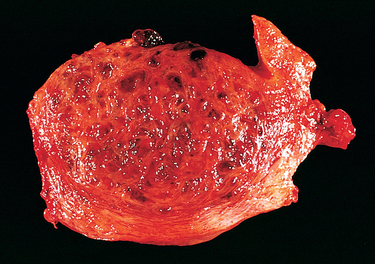
Also called sarcoma botryoides, this uncommon vaginal tumor is most frequently found in infants and in children younger than 5 years of age and consists predominantly of malignant embryonal rhabdomyoblasts.20 These tumors tend to grow as polypoid, rounded, bulky masses that sometimes fill and project out of the vagina; they have the appearance and consistency of grapelike clusters (hence the designation botryoides = grapelike) (Fig. 22-13). On histologic examination, the tumor cells are small and have oval nuclei, with small protrusions of cytoplasm from one end, resembling a tennis racket. Rarely, striations can be seen within the cytoplasm. Beneath the vaginal epithelium, the tumor cells are crowded in a so-called cambium layer, but in the deep regions they lie within a loose fibromyxomatous stroma that is edematous and may contain many inflammatory cells. For this reason the lesions can be mistaken for benign inflammatory polyps, leading to unfortunate delays in diagnosis and treatment. These tumors tend to invade locally and cause death by penetration into the peritoneal cavity or by obstruction of the urinary tract. Conservative surgery, coupled with chemotherapy, seems to offer the best results in cases diagnosed sufficiently early.
CERVIX
The cervix is both a sentinel for potentially serious upper genital tract infections and a target for viruses and other carcinogens, which may lead to invasive carcinoma. Worldwide, cervical carcinoma is the second most common cancer in women, with an estimated 493,000 new cases each year, over half of which are fatal. In the United States, 11,150 women were diagnosed with cervical cancer and 3670 women died from this disease in 2007. The potential threat of cancer is central to Papanicolaou (Pap) smear screening programs and histologic interpretation of biopsy specimens by the pathologist.
Inflammations
ACUTE AND CHRONIC CERVICITIS
At the onset of menarche, the production of estrogens by the ovary stimulates maturation of the cervical and vaginal squamous mucosa and formation of intracellular glycogen vacuoles in the squamous cells. As these cells are shed, the glycogen provides a substrate for endogenous vaginal aerobes and anaerobes, including streptococci, enterococci, Escherichia coli, and staphylococci; however, the normal vaginal and cervical flora is largely dominated by lactobacilli. Lactobacilli produce lactic acid that maintains the vaginal pH below 4.5, suppressing the growth of other saprophytic and pathogenic organisms. In addition, at low pH, lactobacilli produce bacteriotoxic hydrogen peroxide (H2O2).21 At higher, more alkaline pH caused by bleeding, sexual intercourse, vaginal douching as well as during antibiotic treatment, lactobacilli decrease H2O2 production, permitting the overgrowth of other microorganisms, which may result in clinically apparent cervicitis or vaginitis. Some degree of cervical inflammation may be found in virtually all women, and it is usually of little clinical consequence. However, infections by gonococci, chlamydiae, mycoplasmas, and herpes simplex virus may produce significant acute or chronic cervicitis and are important to identify due to their association with upper genital tract disease, complications during pregnancy, and sexual transmission. Marked cervical inflammation produces reparative and reactive changes of the epithelium and shedding of atypical-appearing squamous cells, and therefore may cause a nonspecific, abnormal Pap test result.
Endocervical Polyps
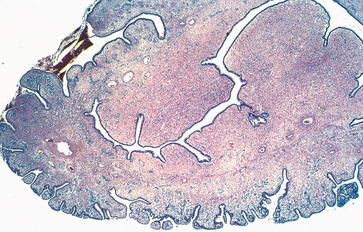
Endocervical polyps are benign exophytic growths that occur in 2% to 5% of adult women. Perhaps the major significance of polyps lies in their production of irregular vaginal “spotting” or bleeding that arouses suspicion of some more ominous lesion. Most polyps arise within the endocervical canal and vary from small and sessile to large, 5-cm masses that may protrude through the cervical os. All are soft, almost mucoid, lesions composed of a loose fibromyxomatous stroma harboring dilated, mucus-secreting endocervical glands, often accompanied by inflammation (Fig. 22-14). Simple curettage or surgical excision effects a cure.
Premalignant and Malignant Neoplasms
No form of cancer better documents the remarkable effects of screening, early diagnosis, and curative therapy on the mortality rate than does cancer of the cervix. Fifty years ago, carcinoma of the cervix was the leading cause of cancer deaths in women in the United States, but the death rate has declined by two thirds to its present rank as the eighth leading cause of cancer mortality. In sharp contrast to this reduced mortality, the detection frequency of early cancers and precancerous lesions is high. Much credit for these dramatic gains belongs to the effectiveness of the Pap test in detecting cervical precancers and to the accessibility of the cervix to colposcopy (visual examination of the cervix with a magnifying glass) and biopsy. While there are an estimated 11,000 new cases of invasive cervical cancer in the United States annually, there are nearly 1 million precancerous lesions of varying grade that are discovered yearly by cytologic examinations. Thus, it is evident that Pap smear screening not only has increased the detection of potentially curable, low-stage cancers but has also allowed the detection and eradication of preinvasive lesions, some of which would have progressed to cancer if not discovered and treated.
Pathogenesis.
The pathogenesis of cervical carcinoma has been delineated by a series of epidemiologic, clinicopathologic, and molecular genetic studies. Epidemiologic data have long implicated a sexually transmitted agent, which is now established to be HPV. For his discovery of HPV as a cause of cervical cancer, Harald zur Hausen was awarded the Nobel Prize in 2008. HPVs are DNA viruses that are typed based on their DNA sequence and subgrouped into high and low oncogenic risk. High oncogenic risk HPVs are currently considered to be the single most important factor in cervical oncogenesis. High oncogenic risk HPVs have also been detected in vaginal squamous cell carcinomas and in a subset of vulvar, penile, anal, tonsillar, and other oropharyngeal carcinomas, as detailed in Chapter 7. As noted earlier, low oncogenic risk HPVs are the cause of the sexually transmitted vulvar, perineal, and perianal condyloma acuminatum. There are 15 high oncogenic risk HPVs that are currently identified. From the point of view of cervical pathology, HPV 16 and HPV 18 are the most important. HPV 16 alone accounts for almost 60% of cervical cancer cases, and HPV 18 accounts for another 10% of cases; other HPV types contribute to less than 5% of cases, individually.22 The risk factors for cervical cancer are related to both host and viral characteristics such as HPV exposure, viral oncogenicity, inefficiency of immune response, and presence of co-carcinogens.23 These include:
Genital HPV infections are extremely common; most of them are asymptomatic, do not cause any tissue changes, and therefore are not detected on Pap test. Figure 22-15 shows age-dependent prevalence of HPVs in cervical smears in women with normal Pap test results. The high peak of HPV prevalence in 20-year-olds is related to sexual début, while the subsequent decrease in prevalence reflects acquisition of immunity and monogamous relationships. Most HPV infections are transient and are eliminated by the immune response in the course of months. On average, 50% of HPV infections are cleared within 8 months, and 90% of infections are cleared within 2 years. The duration of the infection is related to HPV type; on average, infections with high oncogenic risk HPVs last longer than infections with low oncogenic risk HPVs, 13 months versus 8 months, respectively.24 Persistent infection increases the risk of the development of cervical precancer and subsequent carcinoma.
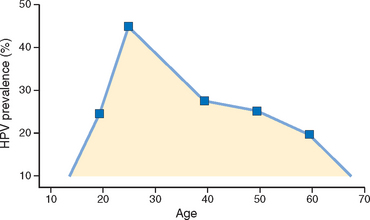
FIGURE 22-15 Age-dependent prevalence of HPVs in cervical smears in women with normal Pap test results in the US population.
(Adapted from Dunne EF et al.: Prevalence of HPV infection among females in the United States. JAMA 297:813, 2007.)
HPVs infect immature basal cells of the squamous epithelium in areas of epithelial breaks, or immature metaplastic squamous cells present at the squamocolumnar junction (Fig. 22-16). HPVs cannot infect the mature superficial squamous cells that cover the ectocervix, vagina, or vulva. Establishing HPV infection in these sites requires damage to the surface epithelium, which gives the virus access to the immature cells in the basal layer of the epithelium. The cervix, with its relatively large areas of immature squamous metaplastic epithelium, is particularly vulnerable to HPV infection as compared, for example, with vulvar skin and mucosa that are covered by mature squamous cells. This difference in epithelial susceptibility to HPV infection accounts for the marked difference in incidence of HPV-related cancers arising in different sites, and explains the high frequency of cervical cancer in women or anal cancer in homosexual men and a relatively low frequency of vulvar and penile cancer.
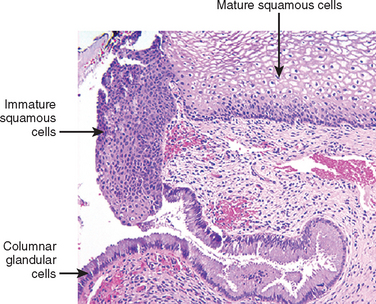
FIGURE 22-16 Cervical squamocolumnar junction showing mature, glycogenized (pale) squamous epithelium, immature (dark pink) squamous metaplastic cells, and columnar endocervical glandular epithelium.
Although the virus can infect only the immature squamous cells, replication of HPV occurs in the maturing squamous cells and results in a cytopathic effect, “koilocytic atypia,” consisting of nuclear atypia and a cytoplasmic perinuclear halo. To replicate, HPV has to induce DNA synthesis in the host cells. Since HPV replicates in maturing, nonproliferating squamous cells, it must reactivate the mitotic cycle in such cells. Experimental studies have shown that HPV activates the cell cycle by interfering with the function of Rb and p53, two important tumor suppressor genes (Chapter 7).
Viral E6 and E7 proteins are critical for the oncogenic effects of HPV. They can promote cell cycle by binding to RB and up-regulation of cyclin E (E7); interrupt cell death pathways by binding to p53 (E6); induce centrosome duplication and genomic instability (E6, E7); and prevent replicative senescence by up-regulation of telomerase (E6) (Chapter 7). HPV E6 induces rapid degradation of p53 via ubiquitin-dependent proteolysis, reducing p53 levels by two- to three-fold. E7 complexes with the hypophosphorylated (active) form of RB, promoting its proteolysis via the proteosome pathway. Because hypophosphorylated RB normally inhibits S-phase entry via binding to the E2F transcription factor, the two viral oncogenes cooperate to promote DNA synthesis while interrupting p53-mediated growth arrest and apoptosis of genetically altered cells. Thus, the viral oncogenes are critical in extending the life span of epithelial cells—a necessary component of tumor development.
The physical state of the virus differs in different lesions, being integrated into the host DNA in cancers, and present as free (episomal) viral DNA in condylomata and most precancerous lesions. Certain chromosome abnormalities, including deletions at 3p and amplifications of 3q, have been associated with cancers containing specific (HPV-16) papillomaviruses.
Even though HPV has been firmly established as a causative factor for cancer of the cervix, the evidence does not implicate HPV as the only factor. A high percentage of young women are infected with one or more HPV types during their reproductive years, and only a few develop cancer. Other cocarcinogens, the immune status of the individual, and hormonal and other factors influence whether the HPV infection will regress or persist and eventually progress to cancer.23
In addition to infecting squamous cells, HPVs may also infect glandular cells or neuroendocrine cells present in the cervical mucosa and cause malignant transformation, resulting in adenocarcinomas, and adenosquamous and neuroendocrine carcinomas; these tumor subtypes, however, are less common since glandular and neuroendocrine cells do not support effective HPV replication.
CERVICAL INTRAEPITHELIAL NEOPLASIA
The classification of cervical precancerous lesions has evolved over time and the terms from the different classification systems are currently used interchangeably. Hence a brief review of the terminology is warranted. The oldest classification system classified lesions as having mild dysplasia on one end and severe dysplasia/carcinoma in situ on the other. This was followed by cervical intraepithelial neoplasia (CIN) classification, with mild dysplasia termed CIN I, moderate dysplasia CIN II, and severe dysplasia termed CIN III. Because the decision with regard to patient management is two-tiered (observation versus surgical treatment), the three-tier classification system has been recently simplified to a two-tiered system, with CIN I renamed low-grade squamous intraepithelial lesion (LSIL) and CIN II and CIN III combined into one category referred to as high-grade squamous intraepithelial lesion (HSIL) (Table 22-1).
TABLE 22-1 Classification Systems for Premalignant Squamous Cervical Lesions
| Dysplasia/Carcinoma in Situ | Cervical Intraepithelial Neoplasia (CIN) | Squamous Intraepithelial Lesion (SIL), Current Classification |
|---|---|---|
| Mild dysplasia | CIN I | Low-grade SIL (LSIL) |
| Moderate dysplasia | CIN II | High-grade SIL (HSIL) |
| Severe dysplasia | CIN III | High-grade SIL (HSIL) |
| Carcinoma in situ | CIN III | High-grade SIL (HSIL) |
LSILs are associated with productive HPV infection, but show no significant disruption or alteration of the host cell cycle. Most LSILs regress spontaneously, with only a small percentage progressing to HSIL. LSIL does not progress directly to invasive carcinoma. For these reasons LSIL is not treated like a premalignant lesion. In HSIL, there is a progressive deregulation of the cell cycle by HPV, which results in increased cellular proliferation, decreased or arrested epithelial maturation, and a lower rate of viral replication, as compared with LSIL. HSILs are one tenth as common as LSILs.
Morphology. Figure 22-17 illustrates a spectrum of morphologic alterations that range from normal to high grade dysplasia. The diagnosis of SIL is based on identification of nuclear atypia characterized by nuclear enlargement, hyperchromasia (dark staining), presence of coarse chromatin granules, and variation of nuclear sizes and shapes. The nuclear changes may be accompanied by cytoplasmic halos indicating disruption of the cytoskeleton before release of the virus into the environment. Nuclear alterations and perinuclear halo are termed koilocytic atypia. The grading of SIL into low or high grade is based on expansion of the immature cell layer from its normal, basal location. If the atypical, immature squamous cells are confined to the lower one third of the epithelium, the lesion is graded as LSIL; if they expand to two thirds of the epithelial thickness, it is graded as HSIL.
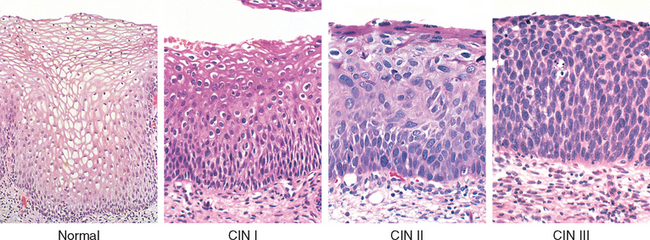
FIGURE 22-17 Spectrum of cervical intraepithelial neoplasia: normal squamous epithelium for comparison; LSIL (CIN I) with koilocytic atypia; HSIL (CIN II) with progressive atypia and expansion of the immature basal cells above the lower third of the epithelial thickness; HSIL (CIN III) with diffuse atypia, loss of maturation, and expansion of the immature basal cells to the epithelial surface.
Figure 22-18A illustrates the histologic features of LSIL. The adjacent panel, Figure 22-18B, shows detection of HPV DNA using an in situ hybridization test. The staining is most intense in the superficial layers of the epithelium, which contain the highest viral load. Figures 22-18C and D show immunostaining for Ki-67 and p16. Ki-67 is a marker of cellular proliferation, and in normal squamous mucosa is confined to the basal layer of the epithelium. In contrast, in SILs, Ki-67 positivity is seen throughout the entire thickness of epithelium, indicating abnormal expansion of the epithelial proliferative zone (see Fig. 22-18C). p16, a cyclin kinase inhibitor, is a cell cycle–regulatory protein, which inhibits the cell cycle by preventing the phosphorylation of RB. It has been shown that in cells infected with oncogenic HPVs, there is overexpression of p16 (see Fig. 22-18D). Despite high levels of p16, however, the HPV-infected cells continue to proliferate because RB, the target of p16 inhibitory activity, is inactivated by the E7 HPV oncoprotein. Both Ki-67 and p16 staining are highly correlated with HPV infection and are useful for confirmation of the diagnosis in equivocal cases of SIL.
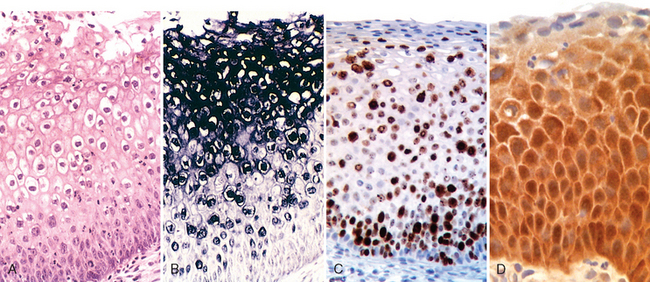
FIGURE 22-18 A, LSIL—routine H&E staining. B, In situ hybridization test for HPV DNA. The dark granular staining denotes HPV DNA, which is typically most abundant in the koilocytes. C, Diffuse immunostaining for the proliferation marker Ki-67, illustrating abnormal expansion of the proliferating cells from the normal basal location to the superficial layers of the epithelium. D, Up-regulation of p16INK4 (seen as intense brown immunostaining) characterizes high oncogenic risk HPV infections.
More than 80% of LSILs and 100% of HSILs are associated with high oncogenic risk HPVs. HPV 16 is the single most common HPV type detected in both categories of lesions. Table 22-2 shows rates of regression and progression of SILs within 2-year follow-up.25 Although the majority of HSILs develop from LSILs, approximately 20% of cases of HSIL develop de novo, without the preexisting LSIL.26 The rates of progression are by no means uniform, and although HPV type—especially HPV 16—is associated with increased risk, it is difficult to predict the outcome in an individual patient. These findings underscore that the risk of developing precancer and cancer is conferred only in part by HPV type, and depends also on immune status and environmental factors. Progression to invasive carcinoma, when it occurs, may take place in a few months to more than a decade.
CERVICAL CARCINOMA
Squamous cell carcinoma is the most common histologic subtype of cervical cancer, accounting for approximately 80% of cases. As outlined above, HSIL is an immediate precursor of cervical squamous cell carcinoma. The second most common tumor type is cervical adenocarcinoma, which constitutes about 15% of cervical cancer cases and develops from a precursor lesion called adenocarcinoma in situ. Adenosquamous and neuroendocrine carcinomas are rare cervical tumors that account for the remaining 5% of cases. All of the above tumor types are caused by high oncogenic risk HPVs. The clinical characteristics and risk factors are the same for each tumor type, with the exception that adenocarcinomas and adenosquamous and neuroendocrine carcinomas typically present with advanced-stage disease. This unfortunate outcome occurs because Pap screening is less effective in detecting these cancers. Patients with adenosquamous and neuroendocrine carcinomas, therefore, have a less favorable prognosis than patients with squamous cell carcinomas or adenocarcinomas. The peak incidence of invasive cervical carcinoma is 45 years. With the advent of widespread screening, many cervical carcinomas are detected at a subclinical stage, during evaluation of an abnormal Pap smear.
Morphology. Invasive cervical carcinoma may manifest as either fungating (exophytic) or infiltrative cancers.
On histologic examination, squamous cell carcinomas are composed of nests and tongues of malignant squamous epithelium, either keratinizing or nonkeratinizing, invading the underlying cervical stroma (Fig. 22-19). Adenocarcinomas are characterized by proliferation of glandular epithelium composed of malignant endocervical cells with large, hyperchromatic nuclei and relatively mucin-depleted cytoplasm, resulting in dark appearance of the glands, as compared with the normal endocervical epithelium (Fig. 22-20A). Adenosquamous carcinomas are tumors composed of intermixed malignant glandular and malignant squamous epithelium. Neuroendocrine cervical carcinomas typically have an appearance similar to small-cell carcinoma of the lung (see Chapter 15); however, in contrast to the lung tumor, which is not related to HPV infection, cervical small-cell carcinomas are positive for high oncogenic risk HPVs.
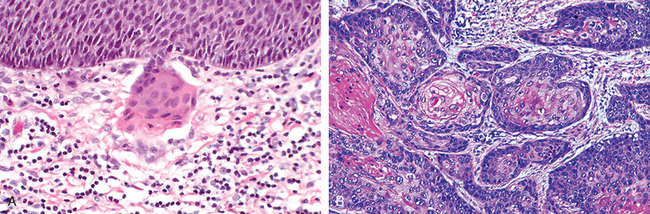
FIGURE 22-19 Squamous cell carcinoma of the cervix. A, Microinvasive squamous cell carcinoma with invasive nest breaking through the basement membrane of HSIL. B, Invasive squamous cell carcinoma.
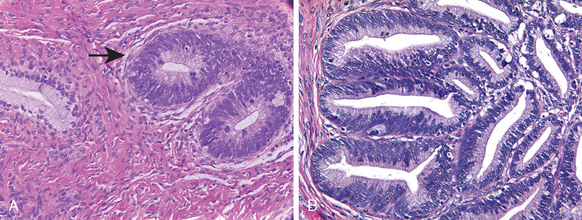
FIGURE 22-20 Adenocarcinoma of the cervix. A, Adenocarcinoma in situ (arrow) showing dark glands adjacent to normal, pale endocervcial glands. B, Invasive adenocarcinoma.
Advanced cervical carcinoma extends by direct spread to involve contiguous tissues, including the paracervical tissues, urinary bladder, ureters, rectum, and vagina. Local and distant lymph nodes are also involved. Distant metastases may be found in the liver, lungs, bone marrow, and other structures.
Cervical cancer is staged as follows:
| Stage 0. | Carcinoma in situ (CIN III, HSIL) |
| Stage I. | Carcinoma confined to the cervix |
|
Ia1. Stromal invasion no deeper than 3 mm and no wider than 7 mm (so-called microinvasive carcinoma) (see Fig. 22-19A)
|
|
| Stage II. | Carcinoma extends beyond the cervix but not to the pelvic wall. Carcinoma involves the vagina but not the lower third. |
| Stage III. | Carcinoma has extended to the pelvic wall. On rectal examination there is no cancer-free space between the tumor and the pelvic wall. The tumor involves the lower third of the vagina. |
| Stage IV. | Carcinoma has extended beyond the true pelvis or has involved the mucosa of the bladder or rectum. This stage also includes cancers with metastatic dissemination. |
Clinical Features.
More than half of invasive cervical cancers are detected in women who did not participate in regular screening. While early invasive cancers of the cervix (microinvasive carcinomas) may be treated by cone biopsy alone, most invasive cancers are managed by hysterectomy with lymph node dissection and, for advanced lesions, irradiation. The prognosis and survival for invasive carcinomas depend largely on the stage at which cancer is first discovered and to some degree on the cell type, with small-cell neuroendocrine tumors having a very poor prognosis. With current methods of treatment there is a 5-year survival rate of at least 95% for stage Ia (including microinvasive) carcinomas, about 80% to 90% with stage Ib, 75% with stage II, and less than 50% for stage III and higher. Most patients with stage IV cancer die as a consequence of local extension of the tumor (e.g., into and about the urinary bladder and ureters, leading to ureteral obstruction, pyelonephritis, and uremia) rather than distant metastases. However, as mentioned above, early detection has reduced the number of patients with stage IV cancer by more than two thirds in the past 50 years.
Cervical Cancer Screening and Prevention
Cervical cancer prevention and control can be divided into several components. One includes cytologic screening and management of Pap smear abnormalities. Another is the histologic diagnosis and removal of precancerous lesions. Still another component is surgical removal of invasive cancers, with adjunctive radiation therapy and chemotherapy. A new aspect is an HPV vaccination program, approved by the US Food and Drug Administration (FDA) for preventing HPV infection. HPV vaccines are also being evaluated for effectiveness as a therapeutic tool in cervical precancers.
The reason that cytologic screening is so effective in preventing cervical cancer is that the majority of cancers are preceded by a long-standing precancerous lesion. This lesion may exist in the noninvasive stage for years and shed abnormal cells that can be detected on cytologic examination. Pap tests are cytologic preparations of exfoliated cells from the cervical transformation zone that are stained with the Papanicolaou method. Using a spatula or brush, the transformation zone of the cervix is circumferentially scraped and the cells are smeared or spun down onto a slide. Following fixation and staining, the cytotechnologist, a person specifically trained to identify cytologic abnormalities, screens the smears. The cellular changes on Pap test illustrating the spectrum from normal, through LSIL to HSIL, are shown in Figure 22-21.
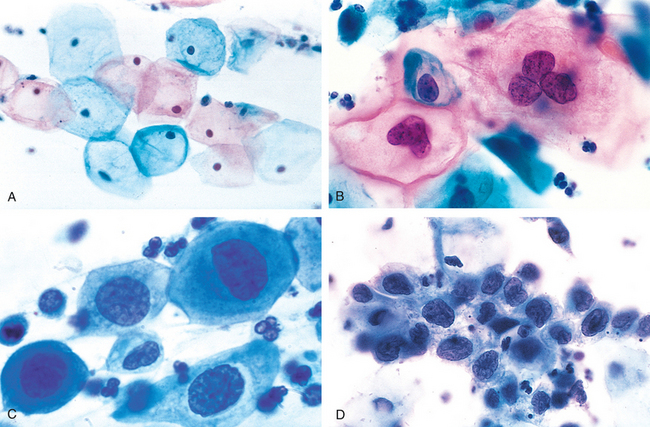
FIGURE 22-21 The cytology of cervical intraepithelial neoplasia as seen on the Papanicolaou smear. Normal cytoplasmic staining in superficial cells (A and B) may be either red or blue. A, Normal exfoliated superficial squamous cells. B, LSIL—koilocytes. C, HSIL (CIN II). D, HSIL (CIN III). Note the reduction in cytoplasm and the increase in the nucleus-to-cytoplasm ratio, which occurs as the grade of the lesion increases. This reflects the progressive loss of cellular differentiation on the surface of the lesions from which these cells are exfoliated.
(Courtesy of Dr. Edmund S. Cibas, Brigham and Women’s Hospital, Boston, MA.)
The false-negative error rate of the Pap test is around 10% to 20%. Most of these false-negative tests stem from sampling errors. Recommendations for the frequency of Pap screening vary, but in general the first smear should be at age 21 years or within 3 years of onset of sexual activity, and thereafter on an annual basis. After age 30, women who have had three consecutive normal cytology results may be screened every 2 to 3 years.27
As an adjunct to cytology, HPV DNA testing may be added to cervical cytology for screening in women aged 30 years or older. Women with normal cytology result and negative HPV DNA testing may be rescreened every 3 years. Women with a normal cytology result, but who are high-risk HPV DNA–positive, should have cervical cytology repeated at 6 to 12 months.28 HPV testing of women younger than 30 is not recommended because of the high prevalence of HPV infection in this age group and the low specificity of the positive result (see Fig. 22-15).
When the Pap test is abnormal, a colposcopic examination of the cervix and vagina is performed to delineate the extent of the lesion and to target the areas to be biopsied. Application of acetic acid to the cervix highlights abnormal areas. After confirmation by tissue biopsy, women with LSIL can be followed in a conservative fashion with repeat smears and close follow-up. Some gynecologists use local ablative measures based upon their experience with the disease and reliability of patient follow-up. HSILs are treated with cervical conization (excision).29 Follow-up smears and clinical examinations should continue for life, since vaginal, vulvar, or cervical precancers and cancers may later develop.
In 2006 the FDA licensed a quadrivalent, prophylactic HPV vaccine for HPV types 6, 11, 16, and 18. This vaccine is designed to reduce the incidence of cervical cancer caused by HPV 16 and HPV 18 (together accounting for approximately 70% of cervical cancer cases22) and vulvar condylomas (HPV 6 and 11). In phase III trials the vaccine prevented 100% of HPV 16/18–associated HSILs. The vaccine is prepared from noninfectious, DNA-free virus-like particles produced by recombinant technology. It induces high levels of serum antibodies in all vaccinated individuals. In women who have no evidence of past or current infection with the HPV genotypes included in the vaccine, there is protection from HPV infection for up to 5 years after vaccination; longer follow-up studies are still pending. Since the HPV vaccine does not eliminate the risk of cervical cancer due to other oncogenic HPV types, cervical cancer screening should still continue according to past guidelines to minimize cancer incidence.30
BODY OF UTERUS AND ENDOMETRIUM
The uterus has two major components: the myometrium and the endometrium. The myometrium is composed of tightly interwoven bundles of smooth muscle that form the wall of the uterus. The internal cavity of the uterus is lined by the endometrium composed of glands embedded in a cellular stroma. The uterus is subject to a variety of disorders, the most common of which result from endocrine imbalances, complications of pregnancy, and neoplastic proliferation. Together with the lesions that affect the cervix (causing abnormal Pap smears), the lesions of the corpus of the uterus and the endometrium (causing abnormal vaginal bleeding) account for most patient visits to gynecologic practices.
Endometrial Histology in the Menstrual Cycle
The endometrium is a dynamic tissue that undergoes physiologic and characteristic morphologic changes during the menstrual cycle as a result of the effect of sex steroid hormones coordinately produced in the ovary. The ovary, in turn, is influenced by hormones produced by the pituitary. Together the hypothalamic, pituitary, and ovarian factors and their interactions regulate maturation of ovarian follicles, ovulation, and menstruation.
“Dating” the endometrium by its histologic appearance is often used clinically to assess hormonal status, document ovulation, and determine causes of endometrial bleeding and infertility (Fig. 22-22). The cycle begins with the shedding of the upper half to two thirds of the endometrium, referred to as the functionalis (the hormonally responsive upper zone), during menses. Under the influence of estrogen, produced by the granulosa cells of the developing follicle in the ovary, the remaining third (basalis) of the endometrium undergoes extremely rapid growth of both glands and stroma (proliferative phase). During the proliferative phase the glands are straight, tubular structures lined by regular, tall, pseudostratified columnar cells. Mitotic figures are numerous, and there is no evidence of mucus secretion or vacuolation. The endometrial stroma is composed of thickly compacted spindle cells that have scant cytoplasm but abundant mitotic activity (Fig. 22-22A).
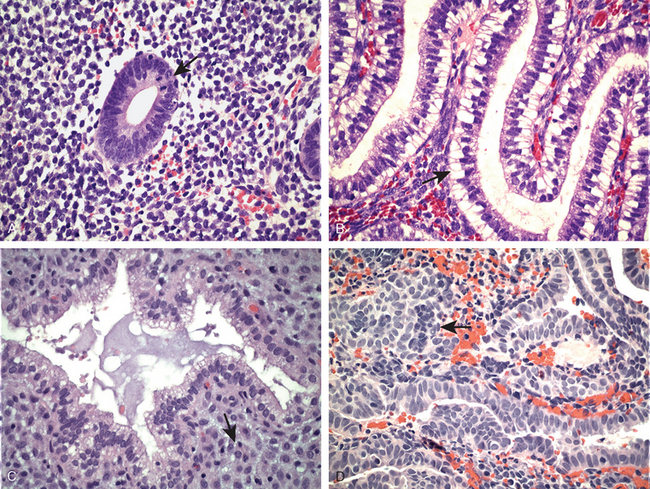
FIGURE 22-22 Histology of the menstrual cycle. A, Proliferative phase with mitoses (arrow). B, Early secretory phase with subnuclear vacuoles (arrow). C, Late secretory exhaustion and predecidual changes (arrow). D, Menstrual endometrium with stromal breakdown (arrow) (see text).
At the time of ovulation the endometrium slows in its growth, and it ceases mitotic activity within days after ovulation, at which time the corpus luteum is producing progesterone in addition to estrogen. The postovulatory endometrium is initially marked by secretory vacuoles beneath the nuclei in the glandular epithelium (Fig. 22-22B). This secretory activity is most prominent during the third week of the menstrual cycle, when the basal vacuoles progressively push past the nuclei. By the fourth week, the secretions are discharged into the gland lumens. When secretion is maximal, between 18 and 24 days, the glands are dilated. By the fourth week the glands are tortuous, producing a serrated appearance when they are cut in their long axis. This serrated or “saw-toothed” appearance is accentuated by secretory exhaustion and shrinking of the glands.
The stromal changes in late secretory phase, due predominantly to progesterone, are important for dating the endometrium and consist of the development of prominent spiral arterioles by days 21 to 22. A considerable increase in ground substance and edema between the stromal cells occurs and is followed in days 23 to 24 by stromal cell hypertrophy with accumulation of cytoplasmic eosinophilia (predecidual change) and resurgence of stromal mitoses (Fig. 22-22C). Predecidual changes spread throughout the functionalis during days 24 to 28 and are accompanied by scattered neutrophils and occasional lymphocytes, which in this context do not imply inflammation. With the dissolution of the corpus luteum and the subsequent lack of progesterone, the disintegration of the functionalis begins with the escape of blood into the stroma, marking the beginning of menstrual shedding (Fig. 22-22D).
Although the molecular mechanism(s) by which estrogen and progesterone cause these profound changes in the endometrium are not well understood, it is known that these hormones induce local production of molecules that act in an autocrine and paracrine fashion.31 Much of the hormonal action occurs through their cognate nuclear receptors (estrogen receptor α, progesterone receptor A, and progesterone receptor B). However, they may also act through alternate receptors or perhaps even by receptor-independent pathways.32 In addition, there is considerable cross-talk between the glands and stroma. For example, much of the effect of estrogen on glandular proliferation occurs via stromal cells, which in response to estrogen produce growth factors (e.g., insulin-like growth factor 1 and epidermal growth factor) that bind receptors expressed on the epithelial cells. In the secretory phase, progesterone initially inhibits proliferation in both the glands and the stroma. It also promotes differentiation of the glands and causes profound alterations of the stroma. Interestingly, progesterone secretion leads to a decrease in estrogen receptor expression in both the glands and stroma, making the endometrium relatively unresponsive to estrogen still being produced by the ovary. To further elucidate the mechanisms responsible for the hormonal effects, global gene expression studies are being used.33 It is thought that such information will aid in the treatment of women with disorders of the endometrium that range from infertility to cancer, as discussed below.
Functional Endometrial Disorders (Dysfunctional Uterine Bleeding)
During active reproductive life, the endometrium is in a dynamic state of proliferation, differentiation, and shedding, in preparation for implantation of an embryo. As discussed above, this cycle is exquisitely controlled by the rise and fall of pituitary and ovarian hormones, which is executed by proper timing of hormone release in both absolute and relative amounts. Abnormalities in this system result in abnormal uterine bleeding.
Although abnormal uterine bleeding can be caused by well-defined organic pathologic conditions, such as chronic endometritis, endometrial polyp (see Fig. 22-23C), submucosal leiomyomas (see Fig. 22-23D), or endometrial neoplasms, the largest single group encompasses functional disturbances, referred to as dysfunctional uterine bleeding (DUB; Table 22-3). DUB is a clinical term for uterine bleeding not caused by any underlying organic (structural) abnormality. The most common causes of DUB are discussed.
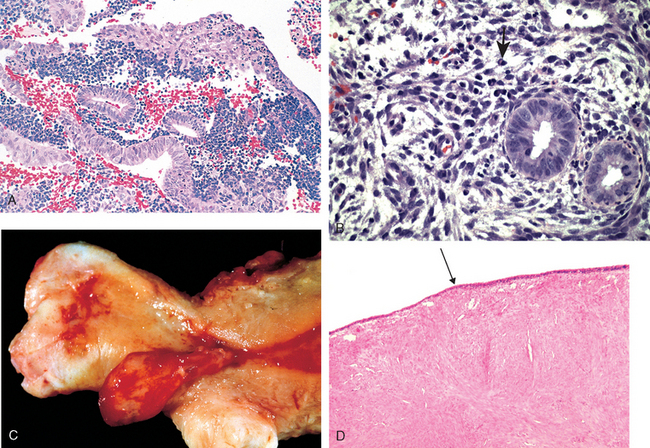
FIGURE 22-23 Common causes of abnormal uterine bleeding. A, The most common is dysfunctional uterine bleeding, seen here as anovulatory endometrium with stromal breakdown. Note breakdown associated with proliferative glands. B, Chronic endometritis with numerous plasma cells (arrow). C, Endometrial polyp. D, Submucosal leiomyoma with attenuation of the endometrial lining (arrow).
TABLE 22-3 Causes of Abnormal Uterine Bleeding by Age Group
| Age Group | Causes |
|---|---|
| Prepuberty | Precocious puberty (hypothalamic, pituitary, or ovarian origin) |
| Adolescence | Anovulatory cycle, coagulation disorders |
| Reproductive age | |
| Perimenopausal | |
| Postmenopausal |
ANOVULATORY CYCLE
In most instances, dysfunctional bleeding is due to the occurrence of an anovulatory cycle. Anovulation results in excessive and prolonged estrogenic stimulation without the counteractive effect of the progestational phase that regularly follows ovulation. In most women anovulatory cycles have no obvious cause, occurring most likely due to subtle hormonal imbalances. Anovulatory cycles are most common at menarche and in the perimenopausal period. Less commonly, lack of ovulation is the result of (1) an endocrine disorder, such as thyroid disease, adrenal disease, or pituitary tumors; (2) a primary lesion of the ovary, such as a functioning ovarian tumor (granulosa-theca cell tumors) or polycystic ovaries (see “Ovaries”); or (3) a generalized metabolic disturbance, such as marked obesity, severe malnutrition, or any chronic systemic disease.
Failure of ovulation results in prolonged, excessive endometrial stimulation by estrogens. Under these circumstances the endometrial glands undergo mild architectural changes, including cystic dilation, that are usually selflimited by the occurrence of the next ovulatory cycle. Unscheduled breakdown of the stroma may also occur (“anovulatory menstruation”), with no evidence of endometrial secretory activity (see Fig. 22-23A). More severe consequences of repeated anovulation are discussed under “Endometrial Hyperplasia.”
INADEQUATE LUTEAL PHASE
This term refers to a condition that is thought to stem from inadequate corpus luteum function resulting in low progesterone output, with subsequent early menses. The condition often manifests clinically as infertility, with either increased bleeding or amenorrhea. Endometrial biopsy performed at an estimated postovulatory date shows secretory endometrium, which, however, lags in its secretory characteristics expected at that date.
ENDOMETRIAL CHANGES INDUCED BY ORAL CONTRACEPTIVES
As might be suspected, oral contraceptives containing synthetic or derivative ovarian steroids induce a wide variety of endometrial changes, depending on the steroids used, the method of administration (combined or sequential regimen), and the dose. A common response pattern is a discordant appearance between glands and stroma, usually with inactive glands amid a stroma showing large cells with abundant cytoplasm reminiscent of the decidua of pregnancy. When such therapy is discontinued, the endometrium reverts to normal. All these changes have been minimized with newer low-dose contraceptives.
MENOPAUSAL AND POSTMENOPAUSAL CHANGES
Because the menopause is characterized by anovulatory cycles, architectural alterations in the endometrial glands may be present transiently, followed by ovarian failure and atrophy of the endometrium. As discussed later in this chapter, anovulatory cycles and uninterrupted estrogen production can induce mild hyperplasias with cystic dilation of glands. If this is followed by complete ovarian atrophy and loss of stimulus, the cystic dilation may remain, while the ovarian stroma and gland epithelium undergo atrophy. In this case, so-called cystic atrophy results. Such cystic changes should not be confused with simple hyperplasia, which shows evidence of glandular and stromal proliferation.
Inflammation
The endometrium and myometrium are relatively resistant to infections, primarily because the endocervix normally forms a barrier to ascending infection. Thus, although chronic inflammation in the cervix is an expected and frequently insignificant finding, it is of concern in the endometrium, excluding the menstrual phase.
ACUTE ENDOMETRITIS
Acute endometritis is uncommon and limited to bacterial infections that arise after delivery or miscarriage. Retained products of conception are the usual predisposing influence; the causative agents include group A hemolytic streptococci, staphylococci, and other bacteria. The inflammatory response is chiefly limited to the interstitium and is entirely nonspecific. Removal of the retained gestational fragments by curettage, accompanied by antibiotic therapy, is promptly followed by remission of the infection.
CHRONIC ENDOMETRITIS
Chronic inflammation of the endometrium occurs in the following settings: (1) in patients suffering from chronic PID; (2) in postpartum or post-abortion patients with retained gestational tissue; (3) in women with intrauterine contraceptive devices; and (4) in women with tuberculosis, either from miliary spread or, more commonly, from drainage of tuberculous salpingitis. The last is distinctly rare in Western countries. The chronic endometritis in all these cases is secondary to another underlying cause.
In about 15% of cases no cause is obvious, yet plasma cells (which are not present in normal endometrium) are seen together with macrophages and lymphocytes (see Fig. 22-23B). Some women with this so-called nonspecific chronic endometritis have gynecologic complaints such as abnormal bleeding, pain, discharge, and infertility. Chlamydia may be involved and is commonly associated with both acute (e.g., polymorphonuclear leukocytes) and chronic (e.g., lymphocytes, plasma cells) inflammatory cell infiltrates. The organisms may or may not be successfully cultured.34 Importantly, antibiotic therapy is indicated because it may prevent other sequelae (e.g., salpingitis).
Endometriosis and Adenomyosis
Endometriosis is the presence of endometrial tissue outside of the uterus. It most commonly consists of both endometrial glands and stroma, but rarely consists only of endometrial stroma. It occurs in the following sites, in descending order of frequency: (1) ovaries; (2) uterine ligaments; (3) rectovaginal septum; (4) cul de sac; (5) pelvic peritoneum; (6) large and small bowel and appendix; (7) mucosa of the cervix, vagina, and fallopian tubes; and (8) laparotomy scars.
Endometriosis is an important clinical condition; it often causes infertility, dysmenorrhea (painful menstruation), pelvic pain, and other problems. The disorder is principally a disease of women in active reproductive life, most often in the third and fourth decades, affecting approximately 10% of women. Uncommonly, endometriosis can show features (metastasis and invasion) similar to malignant tumors. When these features are present they often contribute to significant complications. For example, invasion of the muscular wall of the bowel can result in intestinal symptoms (Fig. 22-24).
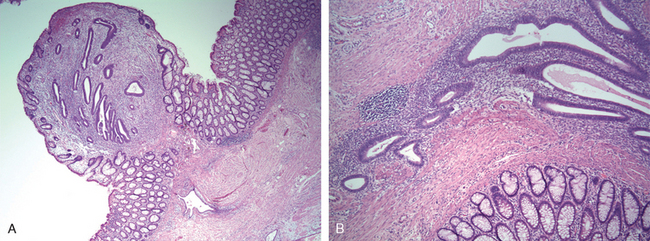
FIGURE 22-24 Endometriosis. A, Endometriosis is present in the mucosa of the colon. B, Higher magnification reveals the presence of both endometrial glands and stroma.
Two major theories for the development of endometriosis have been proposed.7
The metastatic theory is most widely accepted and provides a plausible explanation for the majority of cases of endometriosis. However, it fails to explain some situations in which endometriosis arises. For example, the presence of endometriosis in women who are amenorrheic because of a variety of underlying etiologies (e.g., gonadal dysgenesis) cannot be due to displaced menstrual endometrium. In addition, the relatively low incidence of endometriosis despite the common occurrence of retrograde menstruation (76% to 90% of women) suggests that specific individual factors must predispose women. Other factors that have been postulated include genetic, hormonal, and immune factors.35 Molecular analysis, including gene expression profiling, have provided novel insights into pathogenesis of endometriosis. Some of the specific abnormalities that distinguish normal endometrium from endometriotic tissue are highlighted below35:
These abnormalities seem related to epigenetic changes in key genes that encode two nuclear receptors: steroidogenic factor-1, and estrogen receptor-β. Substantially decreased methylation of the promoters of these genes cause their pathologic overexpression leading to activation of a downstream molecular cascade favoring overproduction of estrogen and prostaglandin and resistance to progesterone action. These defects are present not only in ectopic endometriotic tissue, but, to a lesser degree, also in the endometrium lining the uterus in patients with endometriosis suggesting that they are not secondary to abnormal location.
Some studies have suggested that endometriosis is clonal, yet other studies have demonstrated polyclonality.38,39 Moreover, recent studies have detected mutations in endometriotic cysts that are similar to those found in ovarian endometrioid adenocarcinoma,40 and clinicopathologic studies have long reported an association between the two. Collectively, these findings suggest that endometriosis can give rise to carcinoma.
Morphology. The foci of endometriosis respond to both extrinsic cyclic (ovarian) and intrinsic hormonal stimulation with periodic bleeding. This produces nodules with a red-blue to yellow-brown appearance on or just beneath the mucosal and/or serosal surfaces in the site of involvement. When the disease is extensive, organizing hemorrhage causes extensive fibrous adhesions between tubes, ovaries, and other structures and obliterates the pouch of Douglas. The ovaries may become markedly distorted by large cystic masses (3 to 5 cm in diameter) filled with brown fluid resulting from previous hemorrhage; these are often referred to clinically as chocolate cysts or endometriomas. Aggressive forms of endometriosis can infiltrate tissues and cause fibrosis and subsequent adhesions.
The histologic diagnosis of endometriosis is usually straightforward but may be difficult in long-standing cases in which the endometrial tissue is obscured by the secondary fibrosis. A histologic diagnosis of endometriosis is readily made when both endometrial glands and stroma are present (Fig. 22-24B), with or without the presence of hemosiderin. In rare cases only stroma is identified; however, if only glands are present it must be distinguished from other entities, such as endosalpingiosis, that have different clinical ramifications.
Clinical Features.
Clinical signs and symptoms usually consist of severe dysmenorrhea, dyspareunia (pain with intercourse), and pelvic pain due to the intrapelvic bleeding and periuterine adhesions. Pain on defecation indicates rectal wall involvement, and dysuria results from involvement of the serosa of the bladder. Intestinal disturbances may appear when the small intestine is affected. Menstrual irregularities are common, and infertility is the presenting complaint in 30% to 40% of women. In addition, though uncommon, malignancies can develop in this setting, suggesting that endometriosis contains “at-risk” epithelium.
A closely related disorder, adenomyosis, is defined as the presence of endometrial tissue within the uterine wall (myometrium). Adenomyosis remains in continuity with the endometrium, presumably signifying downgrowth of endometrial tissue into and between the smooth muscle fascicles of the myometrium. Adenomyosis occurs in up to 20% of uteri (Fig. 22-25). On microscopic examination, irregular nests of endometrial stroma, with or without glands, are arranged within the myometrium, separated from the basalis by at least 2 to 3 mm. Like endometriosis, the clinical symptoms of adenomyosis include menometrorrhagia (irregular and heavy menses), colicky dysmenorrhea, dyspareunia, and pelvic pain, particularly during the premenstrual period.
Endometrial Polyps
Endometrial polyps are exophytic masses of variable size that project into the endometrial cavity. They may be single or multiple and are usually sessile, measuring from 0.5 to 3 cm in diameter, but are occasionally large and pedunculated. Polyps may be asymptomatic or may cause abnormal bleeding (intramenstrual, menometrorrhagia, or postmenopausal) if they ulcerate or undergo necrosis. Most commonly the glands within polyps are hyperplastic or atrophic, but they can occasionally demonstrate secretory changes (functional polyps). Hyperplastic polyps may develop in association with generalized endometrial hyperplasia and are responsive to the growth effect of estrogen but show little or no progesterone response (see Fig. 22-23C). Atrophic polyps, which largely occur in postmenopausal women, most likely represent atrophy of a hyperplastic polyp. Rarely, adenocarcinomas arise within endometrial polyps. Endometrial polyps have been observed in association with the administration of tamoxifen. This drug is often used in the therapy of breast cancer due to its anti-estrogenic activity on the breast.41 However, tamoxifen has weak estrogenic effects in the endometrium. Cytogenetic studies indicate that the stromal cells in endometrial polyps contain chromosome (6p21) rearrangements involving the HMGIY gene, which is also rearranged in a variety of other benign mesenchymal tumors.42
Endometrial Hyperplasia
Endometrial hyperplasia, an important cause of abnormal bleeding, is defined as an increased proliferation of the endometrial glands relative to the stroma, resulting in an increased gland-to-stroma ratio when compared with normal proliferative endometrium. Endometrial hyperplasia deserves special attention because of its relationship with endometrial carcinoma. Clinicopathologic and epidemiologic studies have supported the malignant potential of endometrial hyperplasia and the concept of a continuum of proliferative glandular lesions culminating, in some cases, in carcinoma.43 Molecular studies have confirmed this relationship, since endometrial hyperplasia and carcinoma share specific molecular genetic alterations.
Endometrial hyperplasia is associated with prolonged estrogen stimulation of the endometrium, which can be due to anovulation, increased estrogen production from endogenous sources, or exogenous estrogen. Thus, conditions associated with hyperplasia include obesity, menopause, polycystic ovarian disease (including Stein-Leventhal syndrome), functioning granulosa cell tumors of the ovary, excessive cortical function (cortical stromal hyperplasia), and prolonged administration of estrogenic substances (estrogen replacement therapy). These are the same influences postulated to be of pathogenetic significance in some endometrial carcinomas, discussed later.
A common genetic alteration found in a significant number of hyperplasias and related endometrial carcinomas is inactivation of the PTEN tumor suppressor gene.44 PTEN is located on chromosome 10q23.3 and encodes a dual-specificity phosphatase capable of dephosphorylating both lipid and protein molecules. Its main function in tumorigenesis, as presently understood, is dephosphorylation of the lipid molecule phosphatidylinositol (3,4,5)-trisphosphate (PIP3), which blocks the phosphorylation of AKT, a central factor in the phosphatidylinositol 3-kinase (PI3K) growth-regulatory pathway. When PTEN is inactive, AKT phosphorylation is enhanced, and it stimulates protein synthesis and cell proliferation and inhibits apoptosis. Mutations in PTEN have been found in more than 20% of hyperplasias, both with and without atypia, and in 30% to 80% of endometrial carcinomas, suggesting that alterations in PTEN occur at a relatively early stage in endometrial tumorigenesis.45,46 You will also recall that patients with Cowden syndrome, which is caused by germline mutations in PTEN, have a high incidence of endometrial carcinoma. Although it is clear that PTEN plays a central role in the development of hyperplasia and carcinoma, the mechanism by which its loss contributes to endometrial tumorigenesis is not yet well understood. It has been shown that loss of PTEN, resulting in the activation of AKT, can lead to phosphorylation of the estrogen receptor in a ligand (estrogen)-independent manner.47 Thus, loss of PTEN function may activate pathways normally activated by estrogen.
Morphology. Based on architectural and cytologic features, endometrial hyperplasia is divided into four major categories:
Simple hyperplasia without atypia, also known as cystic or mild hyperplasia, is characterized by glands of various sizes and irregular shapes with cystic dilatation. There is a mild increase in the gland-to-stroma ratio. The epithelial growth pattern and cytology are similar to those of proliferative endometrium, although mitoses are not as prominent (Fig. 22-26A). These lesions uncommonly progress to adenocarcinoma (approximately 1%) and largely reflect a response to persistent estrogen stimulation. Simple hyperplasia may evolve into cystic atrophy when the estrogen stimulation is withdrawn.
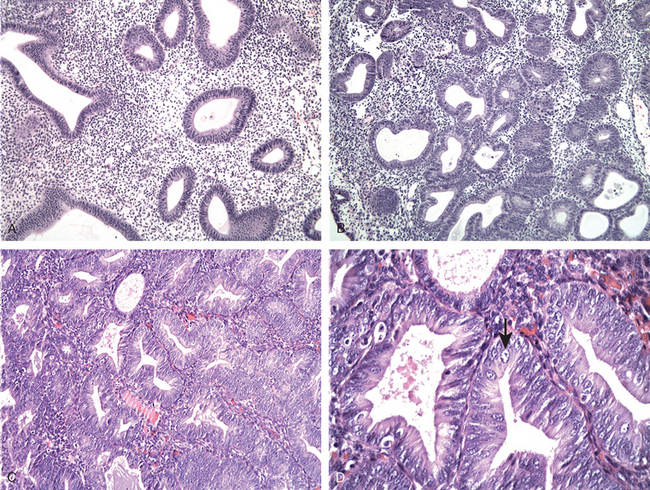
FIGURE 22-26 A, Simple hyperplasia without atypia with architectural abnormalities including mild glandular crowding and cystic glandular dilatation. B, Complex hyperplasia without atypia demonstrates increased glandular crowding with areas of back-to-back glands with cytologic features similar to proliferative endometrium. C, Complex hyperplasia with atypia has architectural features similar to complex hyperplasia without atypia, but the cytologic features have changed. D, High magnification of complex hyperplasia with atypia showing rounded, vesicular nuclei with prominent nucleoli (arrow).
Simple hyperplasia with atypia is uncommon. Architecturally it has the appearance of simple hyperplasia, but there is cytologic atypia within the glandular epithelial cells, as defined by loss of polarity, vesicular nuclei, and prominent nucleoli. Morphologically the cells become rounded and lose the normal perpendicular orientation to the basement membrane. In addition, the nuclei have an open chromatin pattern and conspicuous nucleoli. Approximately 8% of such lesions progress to carcinoma.
Complex hyperplasia without atypia shows an increase in the number and size of endometrial glands, marked gland crowding, and branching of glands. As a result, the glands may be crowded back-to-back with little intervening stroma and abundant mitotic figures (Fig. 22-26B). However, the glands remain distinct and nonconfluent, and the epithelial cells remain cytologically normal. This class of lesions has about a 3% progression to carcinoma, lower than that of simple hyperplasia with atypia.
Complex hyperplasia with atypia has considerable morphologic overlap with well-differentiated endometrioid adenocarcinoma (as discussed below), and an accurate distinction between complex hyperplasia with atypia and cancer may not be possible without hysterectomy (Fig. 22-26C and D).48 It has been found that approximately 23% to 48% of women with a diagnosis of complex hyperplasia with atypia have carcinoma when a hysterectomy is performed shortly after the endometrial biopsy or curettage.49 In one study, in which women with complex hyperplasia with atypia were treated with progestin therapy alone, 50% had persistent disease, 25% recurred, and 25% progressed to carcinoma.50 Currently, complex hyperplasia with atypia is managed by hysterectomy or, in young women, a trial of progestin therapy and close follow-up. The low rate of regression usually requires the removal of the uterus.
A proportion of endometrial hyperplasias are less easily classified, including complex lesions without cellular atypia (uncommon) and those with altered cellular differentiation (metaplasia), such as squamous, ciliated cell, and mucinous metaplasia. The latter may result from alterations in epithelial-stromal interactions inducing the basal endometrial cells to follow different differentiation pathways.51 Because of these nuances of cell growth and differentiation, interpretation of endometrial hyperplasia may be highly subjective, and thus precise classification of every change is not possible. Any assessment of a suspected hyperplasia should include the degree of atypia in a manner clearly understandable by the clinician because the impact on therapy is great. For the patient it may mean the difference between cyclic progestin therapy on one hand and continuous high-dose progestin therapy or hysterectomy (or both) on the other.
Malignant Tumors of the Endometrium
CARCINOMA OF THE ENDOMETRIUM
Endometrial carcinoma is the most common invasive cancer of the female genital tract and accounts for 7% of all invasive cancer in women, excluding skin cancer. At one time, it was far less common than cancer of the cervix, but earlier detection and eradication of squamous intraepithelial lesions and an increase in endometrial carcinomas in younger age groups have reversed this ratio. There are now 39,000 new endometrial cancers per year, compared with 11,000 new invasive cervical cancers. Although they occur at a high frequency, endometrial cancers arise mainly in postmenopausal women. Because they cause abnormal (postmenopausal) bleeding, early detection and cures are possible.
Molecular Pathogenesis.
Carcinoma of the endometrium is uncommon in women younger than 40 years of age. The peak incidence is in 55- to 65-year-old women. Clinicopathologic studies and molecular analyses support the classification of endometrial carcinoma into two broad categories, referred to as type I and type II, as summarized in Table 22-4.52 Because of their distinct pathogenesis they will be discussed separately.
TABLE 22-4 Characteristics of Type I and Type II Endometrial Carcinoma
| Characteristics | Type I | Type II |
|---|---|---|
| Age | 55–65 yr | 65–75 yr |
| Clinical setting | ||
| Morphology | Endometrioid | |
| Precursor | Hyperplasia | Endometrial intraepithelial carcinoma |
| Molecular genetics | ||
| Behavior |
* MSI, microsatellite instability.
Type I carcinomas.
These are the most common type, accounting for greater than 80% of all cases. The majority are well differentiated and mimic proliferative endometrial glands and, as such, are referred to as endometrioid carcinoma. As discussed above, they typically arise in the setting of endometrial hyperplasia and like endometrial hyperplasia they are associated with (1) obesity, (2) diabetes (abnormal glucose tolerance is found in more than 60%), (3) hypertension, (4) infertility (women who develop cancer of the endometrium tend to be nulliparous and have a history of functional menstrual irregularities consistent with anovulatory cycles), and (5) unopposed estrogen stimulation. Recent molecular studies have provided further evidence that endometrial hyperplasia is a precursor to endometrioid carcinoma (Fig. 22-27).53
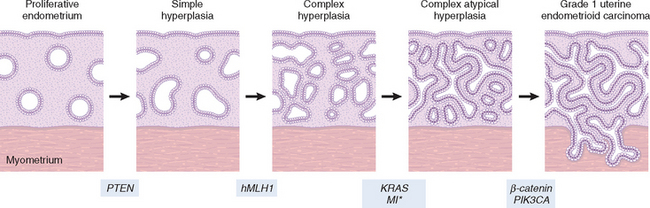
FIGURE 22-27 Schematic diagram depicting the development of type I endometrial carcinoma arising in the setting of hyperplasia. The most common molecular genetic alterations are shown at the time they are most likely to occur during the progression of the disease. *MI, microsatellite instability.
As mentioned earlier, mutations in the PTEN tumor suppressor gene have been identified in 30% to 80% of endometrioid carcinomas and in approximately 20% of endometrial hyperplasias, both with and without atypia. In hysterectomy specimens containing complex hyperplasia with atypia and carcinoma, identical PTEN mutations have been identified in each component.54 These findings support complex hyperplasia with atypia as a precursor to carcinoma and demonstrate that PTEN mutations occur before the development of invasion. Of interest, PIK3CA mutations have recently been reported in approximately 39% of endometrioid carcinomas, and they have been found in tumors with and without PTEN mutations.55 PIK3CA is the catalytic subunit of PI3K, a lipid kinase that phosphorylates PIP2 to PIP3, directly antagonizing the action of PTEN. However, in contrast to PTEN mutations, PIK3CA mutations rarely occur in complex hyperplasia with atypia, suggesting that mutations in PIK3CA play a role in invasion.56 Additional molecular changes that are common in type I carcinomas include microsatellite instability and mutations in the KRAS and β-catenin oncogenes. Microsatellite instability occurs in about 20% of sporadic tumors but is also found in tumors in women from families with hereditary non-polyposis colorectal carcinoma (HNPCC), as discussed in Chapter 17. While microsatellite instability in HNPCC-related carcinomas is caused by germline mutations, in sporadic endometrioid carcinomas it is most commonly due to epigenetic silencing (via promoter hypermethylation) of one of the DNA mismatch repair genes. Mutations in KRAS are found in approximately 25% of cases and have also, but less commonly, been found in complex atypical hyperplasia. These molecular genetic alterations in endometrioid carcinoma, are rarely, if ever, found in type II carcinomas. One of the genes altered in both tumor types is p53. In the more poorly differentiated endometrioid carcinomas, mutations in p53 can be found in up to 50% of cases. They are not identified in well-differentiated tumors or in complex atypical hyperplasias. Thus, it is thought that p53 mutations are a late-occurring event in endometrioid carcinoma, in contrast to what is seen in serous endometrial carcinoma, as discussed below.
Morphology. On gross inspection, endometrial carcinoma can be either a localized polypoid tumor or a diffuse tumor involving the endometrial surface (Fig. 22-28A). Spread generally occurs by direct myometrial invasion with eventual extension to the periuterine structures by direct continuity. Spread into the broad ligaments may create a palpable mass. Dissemination to the regional lymph nodes eventually occurs, and in the late stages, the tumor may metastasize to the lungs, liver, bones, and other organs.
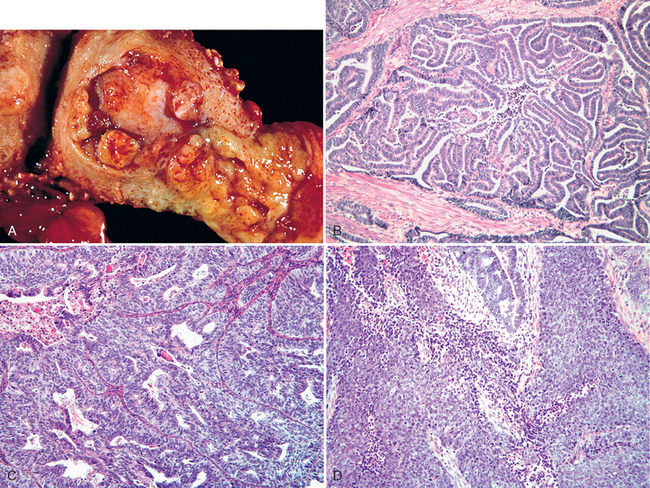
FIGURE 22-28 Type I carcinoma. A, Endometrial adenocarcinoma presenting as a fungating mass in the fundus of the uterus. B, Well-differentiated (grade 1) endometrioid adenocarcinoma with preserved glandular architecture but lack of intervening stroma, distinguishing it from hyperplasia. C, Moderately differentiated (grade 2) endometrioid adenocarcinoma with glandular architecture admixed with solid areas. D, Poorly differentiated (grade 3) endometrioid adenocarcinoma with predominantly solid growth.
On histologic examination, most endometrial carcinomas (about 85%) are endometrioid adenocarcinomas characterized by gland patterns resembling normal endometrial epithelium. A three-step grading system is applied to endometrioid tumors and includes well differentiated (grade 1) (Fig. 22-28B), with easily recognizable glandular patterns; moderately differentiated (grade 2) (Fig. 22-28C), showing well-formed glands mixed with solid sheets of malignant cells; or poorly differentiated (grade 3) (Fig. 22-28D), characterized by solid sheets of cells with barely recognizable glands and a greater degree of nuclear atypia and mitotic activity (see below).
G1. Well-differentiated adenocarcinoma, less than 5% solid growth
G2. Moderately differentiated adenocarcinoma with partly (less than 50%) solid growth
G3. Poorly differentiated adenocarcinoma with predominantly solid growth (greater than 50%)
Up to 20% of endometrioid carcinomas contain foci of squamous differentiation. Squamous elements may be histologically benign-appearing when they are associated with well-differentiated adenocarcinomas. Less commonly, moderately or poorly differentiated endometrioid carcinomas contain squamous elements that appear frankly malignant. Current classification systems grade the carcinomas based on glandular differentiation alone and do not include areas of solid squamous differentiation when considering grading.
Type II carcinomas.
These generally occur in women a decade later than type I carcinoma, and in contrast to type I carcinoma they usually arise in the setting of endometrial atrophy (Fig. 22-29). Type II tumors are by definition poorly differentiated (grade 3) tumors and account for approximately 15% of cases of endometrial carcinoma. The most common subtype is serous carcinoma, referred to as such because of morphologic and biologic overlap with ovarian serous carcinoma. There are less common histologic subtypes (clear cell carcinoma and malignant mixed müllerian tumor) within this category, but very little is known about their pathogenesis. The most frequent alteration described thus far in serous endometrial carcinoma is mutation of the p53 tumor suppressor gene. Alterations in other genes have been described, but at much lower frequency. Mutations in p53 are present in at least 90% of serous endometrial carcinoma.57 The majority of mutations are missense mutations that result in an accumulation of the altered protein that can be detected with immunohistochemistry as strong, diffuse staining of the tumor cell nuclei (Fig. 22-30B and D).
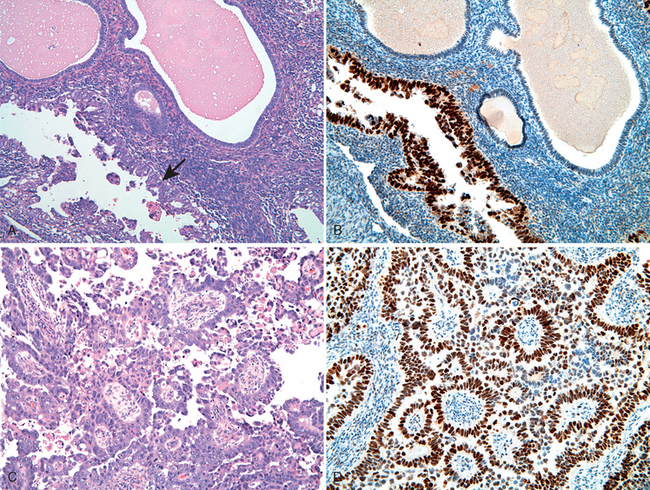
FIGURE 22-30 Type II carcinoma. A, Endometrial intraepithelial carcinoma, the precursor to serous carcinoma showing malignant cells (arrow) with morphologic features identical to serous carcinoma lining the surfaces of the endometrial glands without obvious stromal invasion. B, Strong, diffuse expression of p53 as detected by immunohistochemistry in endometrial intraepithelial carcinoma. C, Serous carcinoma of the endometrium with papillary growth pattern consisting of malignant cells with marked cytologic atypia including high nuclear-to-cytoplasmic ratio, atypical mitotic figures, and hyperchromasia. D, As with the previous lesion, there is an accumulation of p53 protein in the nucleus.
The precursor of serous carcinoma, endometrial intraepithelial carcinoma (EIC), consists of cells identical to those of serous carcinoma but lacks identifiable stromal invasion. Mutations in p53 are found in approximately 75% of these lesions, suggesting that mutation of p53 is an early event in serous endometrial carcinoma. Thus, serous carcinoma presumably begins as a surface epithelial neoplasm that extends into adjacent gland structures and later invades endometrial stroma. Their generally poorer prognosis is thought to be a consequence of a propensity to exfoliate, undergo transtubal spread, and implant on peritoneal surfaces like their ovarian counterparts. They have often spread outside of the uterus at the time of diagnosis.
Morphology. Generally, serous carcinomas arise in small atrophic uteri and are often large bulky tumors or deeply invasive into the myometrium. The precursor lesion, endometrial intraepithelial carcinoma, consists of malignant cells identical to those of serous carcinoma but they remain contained to the gland surface without identifiable stromal invasion (see Fig. 22-30A and B). The invasive lesions may have a papillary growth pattern composed of cells with marked cytologic atypia including high nuclear-to-cytoplasmic ratio, atypical mitotic figures, heterochromasia, and prominent nucleoli (see Fig. 22-30C and D). However, they can also have a predominantly glandular growth pattern that can be distinguished from endometroid carcinoma by the marked cytologic atypia. All of the non-endometrioid carcinomas are classified as grade 3 irrespective of histologic pattern. Serous carcinoma, despite relatively superficial endometrial involvement, may be associated with extensive peritoneal disease, suggesting spread by routes (i.e., tubal or lymphatic transmission) other than direct invasion.
Clinical Course.
There is no currently available screening test for carcinoma of the endometrium. Although it may be asymptomatic for a period of time, it usually produces irregular or postmenopausal vaginal bleeding with excessive leukorrhea. Uterine enlargement may be absent in the early stages. The diagnosis of endometrial cancer must ultimately be established by biopsy or curettage and histologic examination of the tissue.
As would be anticipated, the prognosis depends heavily on the clinical stage of the disease when it is discovered, and its histologic grade and type. In the United States, most women (about 80%) present in stage I and have well-differentiated or moderately differentiated endometrioid carcinomas. Surgery, alone or in combination with irradiation, gives about 90% 5-year survival in stage I (grade 1 or 2) disease. This rate drops to approximately 75% for grade 3/stage I and to 50% or less for stage II and III endometrial carcinomas.
As mentioned, serous carcinoma has a propensity for extrauterine (lymphatic or transtubal) spread, even when apparently confined to the endometrium or its surface epithelium. Overall, fewer than 50% of patients with these tumors are alive 3 years after diagnosis and 35% after 5 years. If peritoneal cytology and adnexal histologic exam are negative, the 5-year survival of those with stage I disease is approximately 80% to 85%.58 The additional advantage of prophylactic radiation or chemotherapy in early-stage disease is unclear.59,60
MALIGNANT MIXED MÜLLERIAN TUMORS
MMMTs (previously referred to as carcinosarcomas) consist of endometrial adenocarcinomas with malignant changes in the stroma.61 The stroma tends to differentiate into a variety of malignant mesodermal components, including muscle, cartilage, and even osteoid. The epithelial and stromal components are presumably derived from the same cell, a concept supported by immunohistochemical and molecular studies.62 Both clinicopathologic and molecular studies suggest that the vast majority of these tumors are carcinomas with sarcomatous differentiation. The mechanisms underlying the sarcomatous transformation are unknown. MMMTs occur in postmenopausal women and present with postmenopausal bleeding.
Morphology. In gross appearance, MMMTs are fleshier than adenocarcinomas, may be bulky and polypoid, and sometimes protrude through the cervical os. On histology, the tumors consist of adenocarcinoma (endometrioid, serous, or clear cell) mixed with the malignant mesenchymal (sarcoma) elements (Fig. 22-31A); alternatively, the tumor may contain two distinct and separate epithelial and mesenchymal components. Sarcomatous components may also mimic extrauterine tissues (e.g., striated muscle, cartilage, adipose tissue, and bone). Metastases usually contain only epithelial components (Fig. 22-31B).
Outcome of MMMTs is determined primarily by depth of invasion and stage. As with endometrial carcinomas, the prognosis is influenced by the grade and type of the adenocarcinoma, being poorest with serous differentiation. These tumors are highly malignant, with 5-year survival rate of 25% to 30%.61
Staging of types I and II of endometrial adenocarcinoma and MMMTs is as follows:
| Stage I. | Carcinoma is confined to the corpus uteri itself. |
| Stage II. | Carcinoma involves the corpus and the cervix. |
| Stage III. | Carcinoma extends outside the uterus but not outside the true pelvis. |
| Stage IV. | Carcinoma extends outside the true pelvis or involves the mucosa of the bladder or the rectum. |
Tumors of the Endometrium with Stromal Differentiation
These are relatively uncommon tumors and comprise less than 5% of endometrial cancers. One group is composed of stromal neoplasias in association with benign glands (adenosarcomas). The other group consists of pure stromal neoplasms, ranging from benign (stromal nodule) to malignant (stromal sarcoma).
ADENOSARCOMAS
Adenosarcomas present most commonly as large broad-based endometrial polypoid growths that may prolapse through the cervical os. The diagnosis is based on malignant-appearing stroma, which coexists with benign but abnormally shaped endometrial glands. These tumors predominate in women between the fourth and fifth decades and are generally considered to be a low-grade malignancy; recurrences develop in one fourth and are nearly always confined to the pelvis.63 The principal diagnostic dilemma is distinguishing these tumors from large benign polyps. The distinction is important, because oophorectomy is typically performed in cases of adenosarcoma, since they are estrogen-sensitive.
STROMAL TUMORS
The endometrial stroma occasionally gives rise to neoplasms that may resemble normal stromal cells. Similar to most neoplasms, they may be well or poorly differentiated. Stromal neoplasms are divided into two categories: (1) benign stromal nodules and (2) endometrial stromal sarcomas.
Morphology. Stromal nodule is a well-circumscribed aggregate of endometrial stromal cells in the myometrium that does not penetrate the myometrium and is of little consequence. Stromal sarcoma consists of neoplastic endometrial stroma lying between muscle bundles of the myometrium and is distinguished from stromal nodules by either diffuse infiltration of myometrial tissue or the invasion of lymphatic channels (previously termed endolymphatic stromal myosis).
About half of stromal sarcomas recur, with relapse rates of 36% to over 80% for stage I and stage III/IV tumor, respectively; relapse cannot be predicted by either mitotic index or degree of cytologic atypia.64 Distant metastases may occur decades after initial diagnosis, and death from metastatic tumor occurs in about 15% of cases. Five-year survival rates average 50%. A recurrent chromosomal translocation, t(7;17)(p15;q21), occurs in endometrial stromal sarcoma. This translocation leads to the fusion of two polycomb group genes, JAZF1 and JJAZ1, with production of a fusion transcript with anti-apoptotic properties.65 Interestingly, even normal endometrial stromal cells express the fusion gene, derived not by translocation, but by the “stitching” together of m-RNAs. Thus, it appears that a pro-survival gene in the normal endometrium is somehow subverted to become pro-neoplastic.