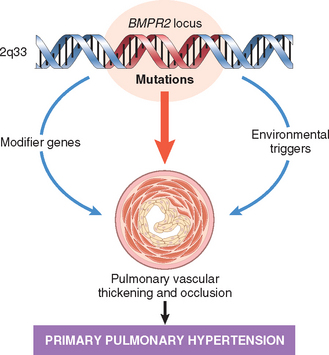
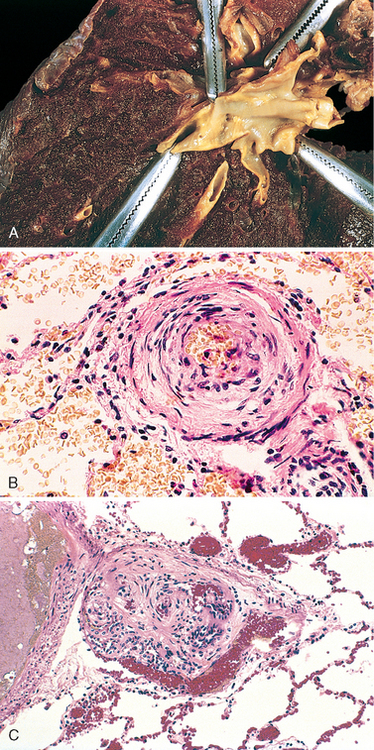
Chapter 15 The Lung
The lungs are ingeniously constructed to carry out their cardinal function: the exchange of gases between inspired air and blood. Developmentally, the respiratory system is an outgrowth from the ventral wall of the foregut. The midline trachea develops two lateral outpocketings, the lung buds. The right lung bud eventually divides into three branches—the lobar bronchi—and the left into two lobar bronchi, thus giving rise to three lobes on the right and two on the left. The right main stem bronchus is more vertical and more directly in line with the trachea. Consequently, aspirated foreign materials, such as vomitus, blood, and foreign bodies, tend to enter the right lung more than the left. The lobar right and left bronchi branch dichotomously, giving rise to progressively smaller airways. Accompanying the branching airways is the double arterial supply to the lungs, that is, the pulmonary and bronchial arteries.
Progressive branching of the bronchi forms bronchioles, which are distinguished from bronchi by the lack of cartilage and submucosal glands within their walls. Further branching of bronchioles leads to the terminal bronchioles, which are less than 2 mm in diameter. The part of the lung distal to the terminal bronchiole is called the acinus; it is roughly spherical, with a diameter of about 7 mm. An acinus is composed of respiratory bronchioles (which give off several alveoli from their sides), alveolar ducts, and alveolar sacs, the blind ends of the respiratory passages, whose walls are formed entirely of alveoli, which are the site of gas exchange. A cluster of three to five terminal bronchioles, each with its appended acinus, is referred to as the pulmonary lobule. This lobular architecture assumes importance in distinguishing the major forms of emphysema.
Except for the vocal cords, which are covered by stratified squamous epithelium, the entire respiratory tree, including the larynx, trachea, and bronchioles, is lined by pseudostratified, tall, columnar, ciliated epithelial cells. The bronchial mucosa also contains a population of neuroendocrine cells that have neurosecretory-type granules and can release a variety of factors, including serotonin, calcitonin, and gastrin-releasing peptide (bombesin). Numerous mucus-secreting goblet cells and submucosal glands are dispersed throughout the walls of the trachea and bronchi (but not the bronchioles).
The microscopic structure of the alveolar walls (or alveolar septa) consists, from blood to air, of the following (Fig. 15-1):
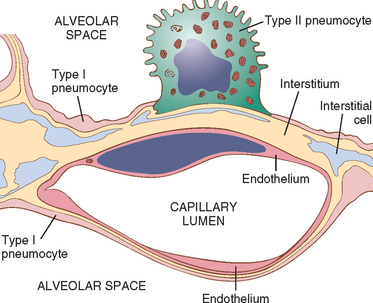
FIGURE 15-1 Microscopic structure of the alveolar wall. Note that the basement membrane (yellow) is thin on one side and widened where it is continuous with the interstitial space. Portions of interstitial cells are shown.
The alveolar walls are perforated by numerous pores of Kohn, which permit the passage of bacteria and exudate between adjacent alveoli (see Fig. 15-34B). Adjacent to the alveolar cell membrane is the pulmonary surfactant layer.

FIGURE 15-34 Stages of bacterial pneumonia. A, Acute pneumonia. The congested septal capillaries and extensive neutrophil exudation into alveoli corresponds to early red hepatization. Fibrin nets have not yet formed. B, Early organization of intra-alveolar exudate, seen in areas to be streaming through the pores of Kohn (arrow). C, Advanced organizing pneumonia featuring transformation of exudates to fibromyxoid masses richly infiltrated by macrophages and fibroblasts.
Primary respiratory infections, such as bronchitis and pneumonia, are commonplace in clinical and pathologic practice. With cigarette smoking, air pollution, and other environmental inhalants, chronic bronchitis and emphysema have become rampant. In men, malignancy of the lungs had been rising steadily but has now plateaued and is expected to decline in the future. Unfortunately, as more and more women are smoking, lung cancer has become the most common malignancy in women, surpassing even breast cancer and is now the most common lethal visceral malignancy in men and women. Although the lungs are secondarily involved in almost all forms of terminal disease, primary pulmonary diseases are emphasized in this chapter.
Congenital Anomalies
Developmental defects of the lung include the following1:
Only the more common anomalies are discussed here. Pulmonary hypoplasia is the defective development of both lungs (one may be more affected than the other) resulting in decreased weight, volume, and acini disproportional to the body weight and gestational age. It is caused by a variety of abnormalities that compress the lung(s) or impede normal lung expansion in utero such as congenital diaphragmatic hernia and oligohydramnions.
Foregut cysts arise from an abnormal detachment of primitive foregut and are most often located in the hilum or middle mediastinum. Depending on the wall structure, these cysts are classified as bronchogenic (most common), esophageal, or enteric. A bronchogenic cyst is rarely connected to the tracheobronchial tree. Microscopically, the cyst is lined by ciliated pseudostratified columnar epithelium with squamous metaplasia occurring in areas of inflammation. The wall contains bronchial glands, cartilage, and smooth muscle. Surgical resection is curative.
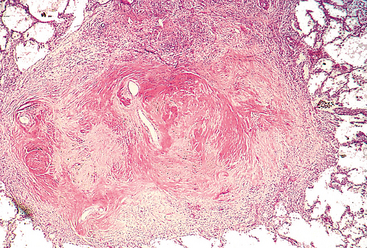
Pulmonary sequestration refers to the presence of a discrete mass of lung tissue without normal connection to the airway system. Blood supply to the sequestered area arises not from the pulmonary arteries but from the aorta or its branches. Extralobar sequestrations are external to the lung and may be located anywhere in the thorax or mediastinum. They most commonly come to attention in infants as abnormal mass lesions, and may be associated with other congenital anomalies. Intralobar sequestrations occur within the lung substance usually in older children and are often associated with recurrent localized infection or bronchiectasis.
Atelectasis (Collapse)
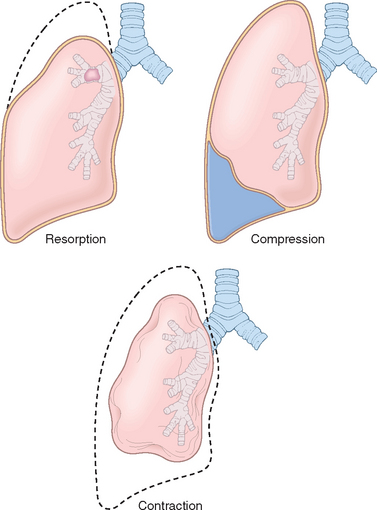
Atelectasis refers either to incomplete expansion of the lungs (neonatal atelectasis) or to the collapse of previously inflated lung, producing areas of relatively airless pulmonary parenchyma. Acquired atelectasis, encountered principally in adults, may be divided into resorption (or obstruction), compression, and contraction atelectasis (Fig. 15-2).
Resorption atelectasis is the consequence of complete obstruction of an airway, which in time leads to resorption of the oxygen trapped in the dependent alveoli, without impairment of blood flow through the affected alveolar walls. Since lung volume is diminished, the mediastinum shifts toward the atelectatic lung. Resorption atelectasis is caused principally by excessive secretions (e.g., mucus plugs) or exudates within smaller bronchi and is therefore most often found in bronchial asthma, chronic bronchitis, bronchiectasis, postoperative states, aspiration of foreign bodies and, rarely, bronchial neoplasms. Compression atelectasis results whenever the pleural cavity is partially or completely filled by fluid exudate, tumor, blood, or air (the last-mentioned constituting pneumothorax) or, with tension pneumothorax, when air pressure impinges on and threatens the function of the lung and mediastinum, especially the major vessels. With compressive atelectasis, the mediastinum shifts away from the affected lung. Contraction atelectasis occurs when local or generalized fibrotic changes in the lung or pleura prevent full expansion.
Significant atelectasis reduces oxygenation and predisposes to infection. Because the collapsed lung parenchyma can be re-expanded, atelectasis is a reversible disorder (except that caused by contraction).
Pulmonary Edema
A general consideration of edema is in Chapter 4, and pulmonary congestion and edema are described briefly in the context of congestive heart failure (Chapter 12). Pulmonary edema can result from hemodynamic disturbances (hemodynamic or cardiogenic pulmonary edema) or from direct increases in capillary permeability, as a result of microvascular injury (Table 15-1). Therapy and outcome depend on the underlying etiology.
TABLE 15-1 Classification and Causes of Pulmonary Edema
| HEMODYNAMIC EDEMA |
| EDEMA DUE TO MICROVASCULAR INJURY (ALVEOLAR INJURY) |
| Infections: pneumonia, septicemia |
| EDEMA OF UNDETERMINED ORIGIN |
Hemodynamic Pulmonary Edema
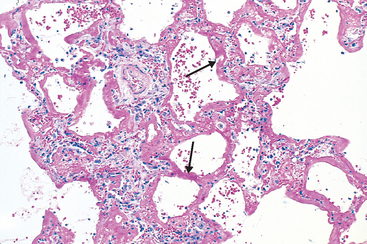
The most common hemodynamic cause of pulmonary edema is increased hydrostatic pressure, as occurs in left-sided congestive heart failure. Whatever the clinical setting, pulmonary congestion and edema are characterized by heavy, wet lungs. Fluid accumulates initially in the basal regions of the lower lobes because hydrostatic pressure is greater in these sites (dependent edema). Histologically, the alveolar capillaries are engorged, and an intra-alveolar granular pink precipitate is seen. Alveolar microhemorrhages and hemosiderin-laden macrophages (“heart failure” cells) may be present. In long-standing cases of pulmonary congestion, such as those seen in mitral stenosis, hemosiderin-laden macrophages are abundant, and fibrosis and thickening of the alveolar walls cause the soggy lungs to become firm and brown (brown induration). These changes not only impair normal respiratory function but also predispose to infection.
Edema Caused by Microvascular Injury
The second mechanism leading to pulmonary edema is injury to the capillaries of the alveolar septa. Here the pulmonary capillary hydrostatic pressure is usually not elevated, and hemodynamic factors play a secondary role. The edema results from primary injury to the vascular endothelium or damage to alveolar epithelial cells (with secondary microvascular injury). This results in leakage of fluids and proteins first into the interstitial space and, in more severe cases, into the alveoli. In most forms of pneumonia the edema remains localized and is overshadowed by the manifestations of infection. When diffuse, however, alveolar edema is an important contributor to a serious and often fatal condition, acute respiratory distress syndrome, discussed in the following section.
Acute Lung Injury and Acute Respiratory Distress Syndrome (Diffuse Alveolar Damage)
Acute lung injury (ALI) (also called noncardiogenic pulmonary edema) is characterized by the abrupt onset of significant hypoxemia and diffuse pulmonary infiltrates in the absence of cardiac failure.2 Acute respiratory distress syndrome (ARDS) refers to severe ALI. ARDS and ALI both have inflammation-associated increase in pulmonary vascular permeability, and epithelial and endothelial cell death. The histologic manifestation of these diseases is diffuse alveolar damage (DAD). Most cases of ALI are associated with an underlying etiology such as sepsis. In the absence of any etiologic association, such cases are called acute interstitial pneumonia (AIP).
ALI is a well-recognized complication of diverse conditions, including both direct injuries to the lungs and systemic disorders (Table 15-2). In many cases, a combination of predisposing conditions is responsible (e.g., shock, oxygen therapy, and sepsis). Nonpulmonary organ dysfunction may also be present in severe cases.
TABLE 15-2 Conditions Associated with Development of Acute Respiratory Distress Syndrome
| INFECTION |
| PHYSICAL/INJURY |
| INHALED IRRITANTS |
| CHEMICAL INJURY |
| HEMATOLOGIC CONDITIONS |
| PANCREATITIS |
| UREMIA |
| CARDIOPULMONARY BYPASS |
| HYPERSENSITIVITY REACTIONS |
* More than 50% of cases of acute respiratory distress syndrome are associated with these four conditions.
Morphology. In the acute stage, the lungs are heavy, firm, red, and boggy. They exhibit congestion, interstitial and intra-alveolar edema, inflammation, fibrin deposition, and diffuse alveolar damage. The alveolar walls become lined with waxy hyaline membranes (Fig. 15-3) that are morphologically similar to those seen in hyaline membrane disease of neonates (Chapter 10). Alveolar hyaline membranes consist of fibrin-rich edema fluid mixed with the cytoplasmic and lipid remnants of necrotic epithelial cells. In the organizing stage, type II pneumocytes undergo proliferation, and there is a granulation tissue response in the alveolar walls and in the alveolar spaces. In most cases the granulation tissue resolves, leaving minimal functional impairment. Sometimes, however, fibrotic thickening of the alveolar septa ensues, caused by proliferation of interstitial cells and deposition of collagen. Fatal cases often have superimposed bronchopneumonia.
Pathogenesis.
The alveolar capillary membrane is formed by two separate barriers: the microvascular endothelium and the alveolar epithelium. In ARDS the integrity of this barrier is compromised by either endothelial or epithelial injury or, more commonly, both.3 Markers of endothelial injury and activation such as endothelin and von Willebrand factor can be detected at high levels in the serum of patients with ARDS. Evidence of epithelial injury in the form of swelling, vacuolization, bleb formation, and frank necrosis is also noted early in the course of acute lung injury. The acute consequences of damage to the alveolar capillary membrane include increased vascular permeability and alveolar flooding, loss of diffusion capacity, and widespread surfactant abnormalities caused by damage to type II pneumocytes. Endothelial injury also triggers the formation of microthrombi that add the insult of ischemic injury (Fig. 15-4). Hyaline membranes so characteristic of ALI/ARDS result from inspissation of protein rich edema fluid that entraps debris of dead alveolar epithelial cells.
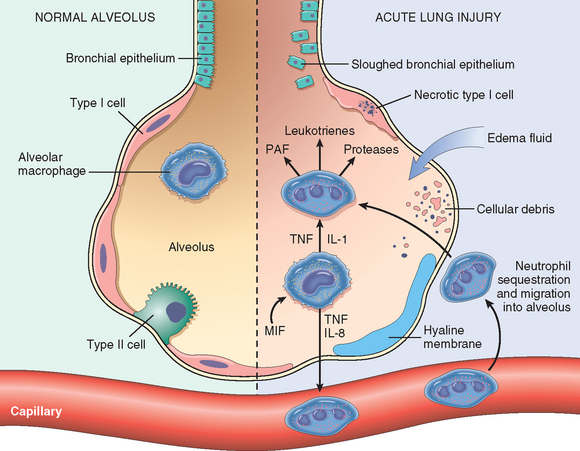
FIGURE 15-4 The normal alveolus (left side) compared with the injured alveolus in the early phase of acute lung injury and acute respiratory distress syndrome. Pro-inflammatory cytokines such as interleukin 8 (IL-8), interleukin 1 (IL-1), and tumor necrosis factor (TNF) (released by macrophages), cause neutrophils to adhere to pulmonary capillaries and extravasate into the alveolar space, where they undergo activation. Activated neutrophils release a variety of factors, such as leukotrienes, oxidants, proteases, and platelet-activating factor (PAF), which contribute to local tissue damage, accumulation of edema fluid in the airspaces, surfactant inactivation, and hyaline membrane formation. Macrophage migration inhibitory factor (MIF) released into the local milieu sustains the ongoing pro-inflammatory response. Subsequently, the release of macrophage-derived fibrogenic cytokines such as transforming growth factor β (TGF-β) and platelet-derived growth factor (PDGF) stimulate fibroblast growth and collagen deposition associated with the healing phase of injury.
(Modified with permission from Ware LB, Matthay MA: The acute respiratory distress syndrome. N Engl J Med 342:1334, 2000.)
Although the cellular and molecular basis of acute lung injury and ARDS remains an area of active investigation, it appears that in ARDS, lung injury is caused by an imbalance of pro-inflammatory and anti-inflammatory mediators.4 The most proximate signals leading to uncontrolled activation of the acute inflammatory response are not yet understood. However, nuclear factor κB (NF-κB), a transcription factor whose activation itself is tightly regulated under normal conditions, has emerged as a likely candidate shifting the balance in favor of a pro-inflammatory state. As early as 30 minutes after an acute insult, there is increased synthesis of interleukin-8 (IL-8), a potent neutrophil chemotactic and activating agent, by pulmonary macrophages. Release of this and similar compounds, such as IL-1 and tumor necrosis factor (TNF), leads to endothelial activation, and pulmonary microvascular sequestration and activation of neutrophils. Neutrophils are thought to have an important role in the pathogenesis of ARDS. Histologic examination of lungs early in the disease process shows increased numbers of neutrophils within the vascular space, the interstitium, and the alveoli. How neutrophils are sequestered in the lung is not entirely clear. There are two possible mechanisms. Firstly, neutrophils that are activated by cytokines like IL-8 and TNF upregulate the expression of adhesion molecules that allow them to bind to their ligands on activated endothelial cells. Secondly, activated neutrophils become “stiff” and less deformable and thus get trapped in the narrow capillary beds of the lung. Activated neutrophils release a variety of products (e.g., oxidants, proteases, platelet-activating factor, and leukotrienes) that cause damage to the alveolar epithelium and fuel the inflammatory cascade. Combined assault on the endothelium and epithelium perpetuate vascular leakiness and loss of surfactant that render the alveolar unit unable to expand. It should be noted that the destructive forces unleashed by neutrophils can be counteracted by an array of endogenous antiproteases, antioxidants, and anti-inflammatory cytokines (e.g., IL-10) that are upregulated by pro-inflammatory cytokines. Dysregulation of the coagulation system is also a feature of ARDS. Levels of tissue factor are increased and those of the anticoagulant, protein C, are decreased in the plasma and bronchoalveolar lavage fluid. The coagulation pathway itself is a powerful pro-inflammatory signal. Thrombin, for example, promotes adhesion of neutrophils to endothelium. In the end, it is the balance between the destructive and protective factors that determines the degree of tissue injury and clinical severity of ALI/ARDS.
Resolution of ARDS requires resorption of the exudate, removal of dead cells, and their replacement by new endothelium and alveolar epithelial cells. Removal of exudates and tissue debris is accomplished by macrophages as in any other form of tissue injury. Epithelial cells are recovered by an initial proliferation of surviving type II pneumocytes that line the denuded basement membrane. The recently discovered bronchoalveolar stem cells may also participate. Type II cells then give rise to type I cells that constitute the majority of alveolar epithelium. Endothelial restoration occurs both by migration from uninjured capillaries and marrow-derived endothelial progenitor cells (Chapter 3); the latter can be detected in the circulation during recovery from ARDS.
Clinical Course.
Individuals who develop ALI are usually hospitalized for one of the predisposing conditions listed earlier. Profound dyspnea and tachypnea herald ALI, followed by increasing cyanosis and hypoxemia, respiratory failure, and the appearance of diffuse bilateral infiltrates on radiographic examination. Hypoxemia can then become unresponsive to oxygen therapy, due to ventilation perfusion mismatching as described below, and respiratory acidosis can develop. Early in the course of the disease, lungs become stiff due to loss of functional surfactant. In a minority of patients, the exudate and diffuse tissue destruction that occur with ALI-ARDS do not resolve and result in scarring. The interstitial fibrosis in such cases produces stiff lungs and chronic pulmonary disease.
The functional abnormalities in ALI are not evenly distributed throughout the lungs. The lungs can be divided into areas that are infiltrated, consolidated, or collapsed (and thus poorly aerated and poorly compliant) and regions that have nearly normal levels of compliance and ventilation. Poorly aerated regions continue to be perfused, producing ventilationperfusion mismatch and hypoxemia. Due to improvements in therapy for sepsis, mechanical ventilation, and supportive care, the mortality rate among the 190,000 ALI cases seen yearly in the United States has decreased from 60% to about 40%.5 The majority of deaths are attributable to sepsis or multi-organ failure and, in some cases, direct lung injury.6
ACUTE INTERSTITIAL PNEUMONIA
Acute interstitial pneumonia is a clinicopathologic term that is used to describe widespread ALI associated with a rapidly progressive clinical course that is of unknownetiology (sometimes referred to as idiopathic ALI-DAD). It is an uncommon disease occurring at a mean age of 50 years with no sex predilection. Patients present with acute respiratory failure often following an illness of less than 3 weeks’ duration that resembles an upper respiratory tract infection. The radiographic and pathologic features are identical to those of the organizing stage of ALI. The mortality rate varies from 33% to 74%, with most deaths occurring within 1 to 2 months.7 In the surviving patients, recurrences and chronic interstitial disease may develop.8-10
Obstructive versus Restrictive Pulmonary Diseases
Based on pulmonary function tests, chronic noninfectious diffuse pulmonary diseases can be classified in one of two categories: (1) obstructive diseases (or airway diseases), characterized by an increase in resistance to airflow due to partial or complete obstruction at any level, from the trachea and larger bronchi to the terminal and respiratory bronchioles, and (2) restrictive diseases, characterized by reduced expansion of lung parenchyma and decreased total lung capacity. In individuals with diffuse obstructive disorders, pulmonary function tests show decreased maximal airflow rates during forced expiration, usually measured by forced expiratory volume at 1 second. Expiratory airflow obstruction may be caused by a variety of conditions listed in Table 15-3. They are distinguished by distinct anatomic lesions and hence different mechanisms for airflow obstruction. As discussed below, such neat distinctions are not always possible. In contrast, restrictive diseases are identified by a reduced total lung capacity, and an expiratory flow rate that is normal or reduced proportionately. Restrictive defects occur in two general conditions: (1) chest wall disorders (e.g., neuromuscular diseases such as poliomyelitis, severe obesity, pleural diseases, and kyphoscoliosis) and (2) chronic interstitial and infiltrative diseases, such as pneumoconioses and interstitial fibrosis of unknown etiology.
Obstructive Pulmonary Diseases
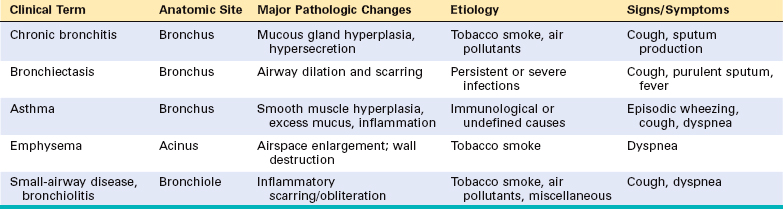
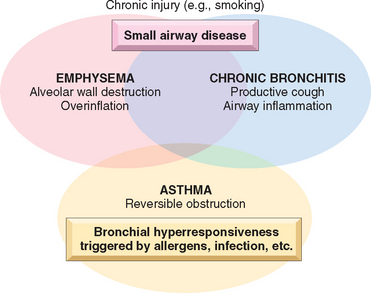
In their prototypical forms, these individual disorders—emphysema, chronic bronchitis, asthma, and bronchiectasis—have distinct anatomic and clinical characteristics (Table 15-3). Emphysema and chronic bronchitis are often clinically grouped together and referred to as chronic obstructive pulmonary disease (COPD), since many patients have overlapping features of damage at both the acinar level (emphysema) and bronchial level (bronchitis), almost certainly because one extrinsic trigger—cigarette smoking—is common to both. In addition, small-airway disease, a variant of chronic bronchiolitis, is now known to contribute to obstruction both in emphysema and chronic bronchitis.11 While asthma is distinguished from chronic bronchitis and emphysema by the presence of reversible bronchospasm, some patients with otherwise typical asthma also develop an irreversible component (Fig. 15-5). Conversely, some patients with otherwise typical COPD have a reversible component. It is clinically common to label such patients as having COPD/asthma. In a recent study the overlap between these three disorders was found to be substantial.12
In most patients, COPD is the result of long-term heavy cigarette smoking; about 10% of patients are nonsmokers.13,14 However, only a minority of smokers develop COPD, the reason for which is still unknown. Because of the increase in smoking (smoking is decreasing in the United States but is increasing worldwide), environmental pollutants, and other noxious exposures, the incidence of COPD has increased markedly in the last few decades and now ranks fourth in the United States as a cause of morbidity and mortality.
Recognizing the overlap between various forms of COPD, each of the components and the features that characterize them in pure forms are discussed next, because it is essential to understand the pathophysiologic basis of different causes of airflow obstruction. While currently they are treated on the basis of symptoms, an understanding of pathogenesis may lead to therapies that target the mechanisms.
EMPHYSEMA
Emphysema is a condition of the lung characterized by irreversible enlargement of the airspaces distal to the terminal bronchiole, accompanied by destruction of their walls without obvious fibrosis.15
Incidence.
COPD is a major public health problem. It is the fourth leading cause of morbidity and mortality in the United States16 and is projected to rank fifth by 2020 as a worldwide burden of disease.17 In one study there was a 50% combined incidence of panacinar and centriacinar emphysema at autopsy, and the pulmonary disease was considered to be responsible for death in 6.5% of these patients.18 There is a clear-cut association between heavy cigarette smoking and emphysema, and women and African Americans are more susceptible than other groups.19
Types of Emphysema.
Emphysema is classified according to its anatomic distribution within the lobule. Recall that the lobule is a cluster of acini, the terminal respiratory units. Although the term emphysema is sometimes loosely applied to diverse conditions, there are four major types: (1) centriacinar, (2) panacinar, (3) paraseptal, and (4) irregular. Of these, only the first two cause clinically significant airflow obstruction (Fig. 15-6). Centriacinar emphysema is far more common than the panacinar form, constituting more than 95% of cases.
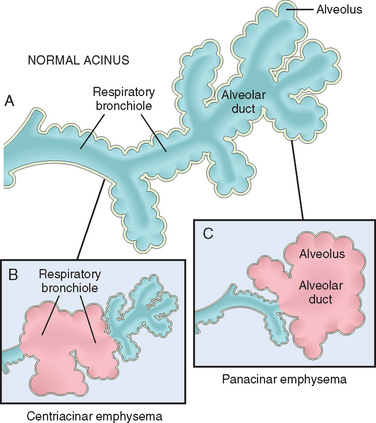
FIGURE 15-6 Major patterns of emphysema. A, Normal structure within the acinus. B, Centriacinar emphysema with dilation that initially affects the respiratory bronchioles. C, Panacinar emphysema with initial distention of the alveolus and alveolar duct.
Centriacinar (Centrilobular) Emphysema.
In this type of emphysema the central or proximal parts of the acini, formed by respiratory bronchioles, are affected, whereas distal alveoli are spared (Figs. 15-6B and 15-7A). Thus, both emphysematous and normal airspaces exist within the same acinus and lobule. The lesions are more common and usually more severe in the upper lobes, particularly in the apical segments. The walls of the emphysematous spaces often contain large amounts of black pigment. Inflammation around bronchi and bronchioles is common. In severe centriacinar emphysema, the distal acinus may also be involved, and differentiation from panacinar emphysema becomes difficult. Centriacinar emphysema occurs predominantly in heavy smokers, often in association with chronic bronchitis.
Panacinar (Panlobular) Emphysema.
In this type, the acini are uniformly enlarged from the level of the respiratory bronchiole to the terminal blind alveoli (Figs. 15-6C and 15-7B). The prefix “pan” refers to the entire acinus but not to the entire lung. In contrast to centriacinar emphysema, panacinar emphysema tends to occur more commonly in the lower zones and in the anterior margins of the lung, and it is usually most severe at the bases. This type of emphysemais associated with α1-antitrypsin (α1-AT) deficiency (Chapter 18).
Distal Acinar (Paraseptal) Emphysema.
In this type, the proximal portion of the acinus is normal, and the distal part is predominantly involved. The emphysema is more striking adjacent to the pleura, along the lobular connective tissue septa, and at the margins of the lobules. It occurs adjacent to areas of fibrosis, scarring, or atelectasis and is usually more severe in the upper half of the lungs. The characteristic findings are of multiple, continuous, enlarged airspaces from less than 0.5 cm to more than 2.0 cm in diameter, sometimes forming cystlike structures. This type of emphysema probably underlies many of the cases of spontaneous pneumothorax in young adults.
Airspace Enlargement with Fibrosis (Irregular Emphysema).
Irregular emphysema, so named because the acinus is irregularly involved, is almost invariably associated with scarring. Thus, it may be the most common form of emphysema, because careful search of most lungs at autopsy shows one or more scars from a healed inflammatory process. In most instances, these foci of irregular emphysema are asymptomatic and clinically insignificant.
Pathogenesis.
COPD is characterized by mild chronic inflammation throughout the airways, parenchyma, and pulmonary vasculature. Macrophages, CD8+ and CD4+ T lymphocytes, and neutrophils are increased in various parts of the lung. Activated inflammatory cells release a variety of mediators, including leukotriene B4, IL-8, TNF, and others, that are capable of damaging lung structures or sustaining neutrophilic inflammation.20 Although details of the genesis of the two common forms of emphysema—centriacinar and panacinar—remain unsettled, the most plausible hypothesis to account for the destruction of alveolar walls is the proteaseantiprotease mechanism, aided and abetted by imbalance of oxidants and antioxidants.
The protease-antiprotease imbalance hypothesis is based on the observation that patients with a genetic deficiency of the antiprotease α1-antitrypsin have a markedly enhanced tendency to develop pulmonary emphysema, which is compounded by smoking (Fig. 15-8). About 1% of all patients with emphysema have this defect. α1-antitrypsin, normally present in serum, tissue fluids, and macrophages, is a major inhibitor of proteases (particularly elastase) secreted by neutrophils during inflammation. α1-antitrypsin is encoded by codominantly expressed genes on the proteinase inhibitor (Pi) locus on chromosome 14. The Pi locus is extremely polymorphic, with many different alleles. Most common is the normal (M) allele and the corresponding phenotype. Approximately 0.012% of the US population is homozygous for the Z allele, associated with markedly decreased serum levels of α1antitrypsin. More than 80% of these individuals develop symptomatic panacinar emphysema, which occurs at an earlier age and with greater severity if the individual smokes. The following sequence is postulated:
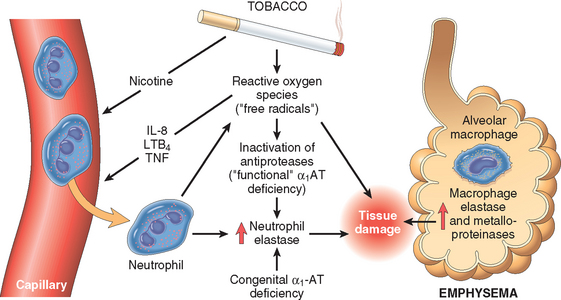
FIGURE 15-8 Pathogenesis of emphysema. Excessive protease activity and reactive oxygen species are additive in their effects and contribute to tissue damage. α1-antitrypsin (α1-AT) deficiency can be either congenital or “functional” as a result of oxidative inactivation. See text for details. IL-8, interleukin 8; LTB4, leukotriene B4; TNF, tumor necrosis factor.
Thus, emphysema is seen to result from the destructive effect of high protease activity in subjects with low antiprotease activity. The protease-antiprotease imbalance hypothesis also helps explain the effect of cigarette smoking in the development of emphysema, particularly the centriacinar form in subjects with normal amounts of α1-antitrypsin:
In addition, smoking has a seminal role in perpetuating the oxidant-antioxidant imbalance in the pathogenesis of emphysema. Normally, the lung contains a healthy complement of antioxidants (superoxide dismutase, glutathione) that keep oxidative damage to a minimum. Tobacco smoke contains abundant reactive oxygen species (free radicals), which deplete these antioxidant mechanisms, thereby inciting tissue damage (Chapter 1). Activated neutrophils also add to the pool of reactive oxygen species in the alveoli. A secondary consequence of oxidative injury is inactivation of native antiproteases, resulting in “functional” α1-antitrypsin deficiency even in patients without enzyme deficiency.
Since small airways are normally tethered by the elastic recoil of the lung parenchyma, the loss of elastic tissue in the walls of alveoli that surround respiratory bronchioles reduces radial traction and thus causes the respiratory bronchioles to collapse during expiration. This leads to functional airflow obstruction despite the absence of mechanical obstruction.
Until recently loss of elastic recoil was considered to be the sole mechanism of airflow obstruction in emphysema. However, careful studies in young smokers who died in accidents have revealed that inflammation of small airways, defined as bronchioles less than 2 mm in diameter, occurs early in the evolution of COPD. Several changes are seen:
Together these changes narrow the bronchiolar lumen and contribute to airway obstruction.21,25
One of the perplexing features of COPD is that smoldering inflammation and slow progressive destruction of the lung parenchyma often continue for decades after cessation of smoking.22 While there are no clear answers, there is emerging evidence that the initial insult in the form of tobacco smoke, or other irritants, triggers a maladaptive, self-perpetuating immune response in which both innate and adaptive components play a role. Fingers are pointing to pathogenic CD4+TH17 cells similar to those that are involved in other immunemediated inflammatory diseases such as Crohn disease, but much remains to be known.
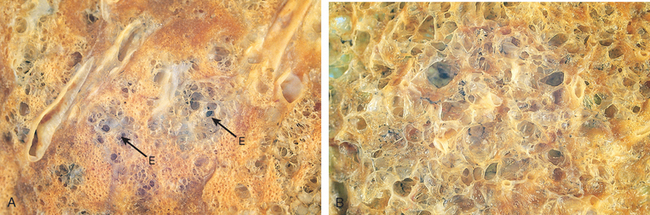
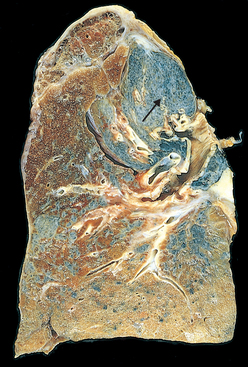
Morphology. Advanced emphysema produces voluminous lungs, often overlapping the heart and hiding it when the anterior chest wall is removed. Generally, the upper two thirds of the lungs are more severely affected. Large apical blebs or bullae are more characteristic of irregular emphysema secondary to scarring and of distal acinar emphysema. Large alveoli can easily be seen on the cut surface of formalin-inflated fixed lung (see Fig. 15-7).
Microscopically, there are abnormally large alveoli separated by thin septa with only focal centriacinar fibrosis. There is loss of attachments of the alveoli to the outer wall of small airways. The pores of Kohn are so large that septa appear to be floating or protrude blindly into alveolar spaces with a club-shaped end. As alveolar walls are destroyed, there is decrease in the capillary bed. With advanced disease, there are even larger abnormal airspaces and possibly blebs or bullae, which often deform and compress the respiratory bronchioles and vasculature of the lung. Inflammatory changes in small airways were described earlier.
Clinical Course.
The clinical manifestations of emphysema do not appear until at least one third of the functioning pulmonary parenchyma is damaged. Dyspnea is usually the first symptom; it begins insidiously but is steadily progressive. In some patients, cough or wheezing is the chief complaint, easily confused with asthma. Cough and expectoration are extremely variable and depend on the extent of the associated bronchitis. Weight loss is common and can be so severe as to suggest a hidden malignant tumor. Classically, the patient is barrel-chested and dyspneic, with obviously prolonged expiration, sits forward in a hunched-over position, and breathes through pursed lips. Expiratory airflow limitation, best measured through spirometry, is the key to diagnosis.
In individuals with severe emphysema, cough is often slight, overdistention is severe, diffusion capacity is low, and blood gas values are relatively normal at rest. Such patients may overventilate and remain well oxygenated, and therefore are somewhat ingloriously designated pink puffers (see Table 15-4). Development of cor pulmonale and eventually congestive heart failure, related to secondary pulmonary vascular hypertension, is associated with a poor prognosis. Death in most patients with emphysema is due to (1) respiratory acidosis and coma, (2) right-sided heart failure, and (3) massive collapse of the lungs secondary to pneumothorax. Treatment options include bronchodilators, steroids, bullectomy, and, in selected patients, lung volume reduction surgery and lung transplantation. Substitution therapy with α1-AT is being evaluated.23
TABLE 15-4 Emphysema and Chronic Bronchitis
| Predominant Bronchitis | Predominant Emphysema | |
|---|---|---|
| Age (yr) | 40–45 | 50–75 |
| Dyspnea | Mild; late | Severe; early |
| Cough | Early; copious sputum | Late; scanty sputum |
| Infections | Common | Occasional |
| Respiratory insufficiency | Repeated | Terminal |
| Cor pulmonale | Common | Rare; terminal |
| Airway resistance | Increased | Normal or slightly increased |
| Elastic recoil | Normal | Low |
| Chest radiograph | Prominent vessels; large heart | Hyperinflation; small heart |
| Appearance | Blue bloater | Pink puffer |
Other Forms of Emphysema.
Now we come to some conditions in which the term emphysema is applied less stringently and to some closely related conditions.
Compensatory Hyperinflation (Emphysema).
This term is sometimes used to designate dilation of alveoli but not destruction of septal walls in response to loss of lung substance elsewhere. It is best exemplified by the hyperexpansion of the residual lung parenchyma that follows surgical removal of a diseased lung or lobe.
Obstructive Overinflation.
In this condition the lung expands because air is trapped within it. A common cause is subtotal obstruction by a tumor or foreign object. Another example is congenital lobar overinflation in infants, probably resulting from hypoplasia of bronchial cartilage and sometimes associated with other congenital cardiac and lung abnormalities. Overinflation in obstructive lesions occurs either (1) because of a ball-valve action of the obstructive agent, so that air enters on inspiration but cannot leave on expiration, or (2) because the bronchus may be totally obstructed but ventilation through collaterals may bring in air from behind the obstruction. These collaterals are the pores of Kohn and other direct accessory bronchioloalveolar connections (the canals of Lambert). Obstructive overinflation can be a life-threatening emergency, because the affected portion distends sufficiently to compress the remaining normal lung.
Bullous Emphysema.
This is a descriptive term for large subpleural blebs or bullae (spaces more than 1 cm in diameter in the distended state) that can occur in any form of emphysema (Fig. 15-9). They represent localized accentuations of emphysema and occur near the apex, sometimes in relation to old tuberculous scarring. On occasion, rupture of the bullae may give rise to pneumothorax.
Interstitial Emphysema.
The entrance of air into the connective tissue stroma of the lung, mediastinum, or subcutaneous tissue is called interstitial emphysema. In most instances, alveolar tears in pulmonary emphysema provide the avenue of entrance of air into the stroma of the lung, but rarely, a wound of the chest that allows air to be sucked in or a fractured rib that punctures the lung substance may underlie this disorder. Alveolar tears usually occur when there is a combination of coughing plus some bronchiolar obstruction, producing sharply increased pressures within the alveolar sacs. Children with whooping cough and bronchitis, patients with obstruction to the airways (by blood clots, tissue, or foreign bodies) or who are being artificially ventilated, and individuals who suddenly inhale irritant gases are at risk.
CHRONIC BRONCHITIS
Chronic bronchitis is defined clinically as persistent cough with sputum production for at least 3 months in at least 2 consecutive years, in the absence of any other identifiable cause. Chronic bronchitis, so common among habitual smokers and inhabitants of smog-laden cities, is not nearly as trivial as was once thought. When persistent for years, it may (1) progress to COPD, (2) lead to cor pulmonale and heart failure, or (3) cause atypical metaplasia and dysplasia of the respiratory epithelium, providing a rich soil for cancerous transformation.
Pathogenesis.
The primary or initiating factor in the genesis of chronic bronchitis seems to be long-standing irritation by inhaled substances such as tobacco smoke (90% of patients are smokers), and dust from grain, cotton, and silica. The earliest feature of chronic bronchitis is hypersecretion of mucus in the large airways, associated with hypertrophy of the submucosal glands in the trachea and bronchi.24 Proteases released from neutrophils, such as neutrophil elastase and cathepsin, and matrix metalloproteinases, stimulate this mucus hypersecretion. As chronic bronchitis persists, there is also a marked increase in goblet cells of small airways—small bronchi and bronchioles—leading to excessive mucus production that contributes to airway obstruction. It is thought that both the submucosal gland hypertrophy and the increase in goblet cells are protective metaplastic reactions against tobacco smoke or other pollutants (e.g., sulfur dioxide and nitrogen dioxide).
Although mucus hypersecretion in large airways is the cause of sputum overproduction, it is now thought that accompanying alterations in the small airways of the lung (small bronchi and bronchioles, less than 2 to 3 mm in diameter) can result in physiologically important and early manifestations of chronic airway obstruction.25,26 This feature is similar to that described earlier in emphysema and seems to be a common denominator in COPD.
The role of infection seems to be secondary. It is not responsible for the initiation of chronic bronchitis but is probably significant in maintaining it and may be critical in producing acute exacerbations. Cigarette smoke predisposes to infection in more than one way. It interferes with ciliary action of the respiratory epithelium, it may cause direct damage to airway epithelium, and it inhibits the ability of bronchial and alveolar leukocytes to clear bacteria. Viral infections can also cause exacerbations of chronic bronchitis.
Morphology. Grossly, there is hyperemia, swelling, and edema of the mucous membranes, frequently accompanied by excessive mucinous or mucopurulent secretions. Sometimes, heavy casts of secretions and pus fill the bronchi and bronchioles. The characteristic histologic features are chronic inflammation of the airways (predominantly lymphocytes) and enlargement of the mucus-secreting glands of the trachea and bronchi. Although the numbers of goblet cells increase slightly, the major change is in the size of the mucous gland (hyperplasia). This increase can be assessed by the ratio of the thickness of the mucous gland layer to the thickness of the wall between the epithelium and the cartilage (Reid index). The Reid index (normally 0.4) is increased in chronic bronchitis, usually in proportion to the severity and duration of the disease. The bronchial epithelium may exhibit squamous metaplasia and dysplasia. There is marked narrowing of bronchioles caused by mucus plugging, inflammation, and fibrosis. In the most severe cases, there may be obliteration of lumen due to fibrosis (bronchiolitis obliterans).
Clinical Features.
The cardinal symptom of chronic bronchitis is a persistent cough productive of sputum. For many years no other respiratory functional impairment is present, but eventually dyspnea on exertion develops. With the passage of time, and usually with continued smoking, other elements of COPD may appear, including hypercapnia, hypoxemia, and mild cyanosis (“blue bloaters”). Differentiation of pure chronic bronchitis from that associated with emphysema can be made in the classic case (see Table 15-4), but, as has been mentioned, many patients with COPD have both conditions. Longstanding severe chronic bronchitis commonly leads to cor pulmonale with cardiac failure. Death may also result from further impairment of respiratory function due to superimposed acute infections.
ASTHMA
Asthma is a chronic inflammatory disorder of the airways that causes recurrent episodes of wheezing, breathlessness, chest tightness, and cough, particularly at night and/or in the early morning. These symptoms are usually associated with widespread but variable bronchoconstriction and airflow limitation that is at least partly reversible, either spontaneously or with treatment. The hallmarks of the disease are: increased airway responsiveness to a variety of stimuli, resulting in episodic bronchoconstriction; inflammation of the bronchial walls; and increased mucus secretion. Some of the stimuli that trigger attacks in patients would have little or no effect in subjects with normal airways. Many cells play a role in the inflammatory response, in particular lymphocytes, eosinophils, mast cells, macrophages, neutrophils, and epithelial cells.27
Individuals with asthma experience attacks of varying severity of dyspnea, coughing, and wheezing due to sudden episodes of bronchospasm. Rarely, a state of unremitting attacks, called status asthmaticus, proves fatal; usually, such patients have had a long history of asthma. Between the attacks, patients may be virtually asymptomatic. There has been a significant increase in the incidence of asthma in the Western world in the past four decades.
Asthma may be categorized into atopic (evidence of allergen sensitization, often in a patient with a history of allergic rhinitis, eczema) and non-atopic (without evidence of allergen sensitization). In either type, episodes of bronchospasm can be triggered by diverse mechanisms, such as respiratory infections (especially viral infections), environmental exposure to irritants (e.g., smoke, fumes), cold air, stress, and exercise. Recent studies have suggested that the recognition of subphenotypes of asthma based on the pattern of airway inflammation may also be useful. There is emerging evidence for differing patterns of airway inflammation: eosinophilic, neutrophilic, mixed inflammatory, and pauci-granulocytic asthma. These subgroups may differ in their etiology, immunopathology, and response to treatment.28 Asthma may also be classified according to the agents or events that trigger bronchoconstriction. These include seasonal, exercise-induced, drug-induced (e.g., aspirin), and occupational asthma, and asthmatic bronchitis in smokers.
Atopic Asthma.
This most common type of asthma is a classic example of type I IgE-mediated hypersensitivity reaction, discussed in detail in Chapter 6. The disease usually begins in childhood and is triggered by environmental allergens, such as dusts, pollens, roach or animal dander, and foods. A positive family history of asthma is common, and a skin test with the offending antigen in these patients results in an immediate wheal-and-flare reaction. Atopic asthma may also be diagnosed based on evidence of allergen sensitization by serum radioallergosorbent tests (called RAST), which identify the presence of IgE specific for a panel of allergens.
Non-Atopic Asthma.
The second group of individuals with asthma does not have evidence of allergen sensitization, and skin test results are usually negative. A positive family history of asthma is less common in these patients. Respiratory infections due to viruses (e.g., rhinovirus, parainfluenza virus) are common triggers in non-atopic asthma.29 In these patients hyperirritability of the bronchial tree probably underlies their asthma. It is thought that virus-induced inflammation of the respiratory mucosa lowers the threshold of the subepithelial vagal receptors to irritants. Inhaled air pollutants, such as sulfur dioxide, ozone, and nitrogen dioxide, may also contribute to the chronic airway inflammation and hyperreactivity that are present in some cases.
Drug-Induced Asthma.
Several pharmacologic agents provoke asthma. Aspirin-sensitive asthma is an uncommon yet fascinating type, occurring in individuals with recurrent rhinitis and nasal polyps. These individuals are exquisitely sensitive to small doses of aspirin as well as other nonsteroidal anti-inflammatory medications, and they experience not only asthmatic attacks but also urticaria. It is probable that aspirin triggers asthma in these patients by inhibiting the cyclooxygenase pathway of arachidonic acid metabolism without affecting the lipoxygenase route, thus tipping the balance toward elaboration of the bronchoconstrictor leukotrienes.
Occupational Asthma.
This form of asthma is stimulated by fumes (epoxy resins, plastics), organic and chemical dusts (wood, cotton, platinum), gases (toluene), and other chemicals (formaldehyde, penicillin products). Minute quantities of chemicals are required to induce the attack, which usually occurs after repeated exposure. The underlying mechanisms vary according to stimulus and include type I reactions, direct liberation of bronchoconstrictor substances, and hypersensitivity responses of unknown origin.
Pathogenesis.
The major etiologic factors in atopic asthma are a genetic predisposition to type I hypersensitivity (“atopy”) and exposure to environmental triggers that remain poorly defined.30 It is postulated that inheritance of susceptibility genes makes individuals prone to develop strong TH2 reactions against environmental antigens (allergens) that are ignored or elicit harmless responses in most individuals. In the airways the scene for the reaction is set by initial sensitization to inhaled allergens, which stimulate induction of TH2 cells (Fig. 15-10). TH2 cells secrete cytokines that promote allergic inflammation and stimulate B cells to produce IgE and other antibodies. These cytokines include IL-4, which stimulates the production of IgE; IL-5, which activates locally recruited eosinophils; and IL-13, which stimulates mucus secretion from bronchial submucosal glands and also promotes IgE production by B cells. As in other allergic reactions (Chapter 6), IgE coats submucosal mast cells, and repeat exposure to the allergen triggers the mast cells to release granule contents and produce cytokines and other mediators, which collectively induce the early-phase (immediate hypersensitivity) reaction and the late-phase reaction. The early reaction is dominated by bronchoconstriction, increased mucus production, and variable degrees of vasodilation with increased vascular permeability. Bronchoconstriction is triggered by direct stimulation of subepithelial vagal (parasympathetic) receptors through both central and local reflexes (including those mediated by unmyelinated sensory C fibers).
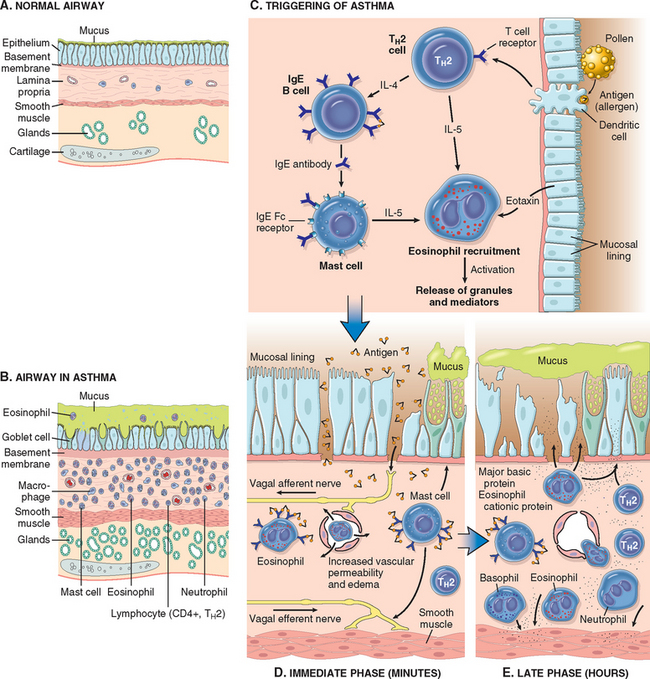
FIGURE 15-10 A and B, Comparison of a normal bronchus with that in a person with asthma. Note the accumulation of mucus in the bronchial lumen resulting from an increase in the number of mucus-secreting goblet cells in the mucosa and hypertrophy of submucosal glands. In addition, there is intense chronic inflammation due to recruitment of eosinophils, macrophages, and other inflammatory cells. Basement membrane underlying the mucosal epithelium is thickened, and there is hypertrophy and hyperplasia of smooth muscle cells. C, Inhaled allergens (antigen) elicit a TH2-dominated response favoring IgE production and eosinophil recruitment (priming or sensitization). D, On re-exposure to antigen (Ag), the immediate reaction is triggered by Ag-induced cross-linking of IgE bound to IgE receptors on mast cells. These cells release preformed mediators. Collectively, either directly or via neuronal reflexes, the mediators induce bronchospasm, increased vascular permeability, and mucus production, and recruit additional mediator-releasing cells from the blood. E, The arrival of recruited leukocytes (neutrophils, eosinophils, and basophils; lymphocytes and monocytes) signals the initiation of the late phase of asthma and a fresh round of mediator release from leukocytes, endothelium, and epithelial cells. Factors, particularly from eosinophils (e.g., major basic protein, eosinophil cationic protein), also cause damage to the epithelium. GM-CSF, granulocyte-macrophage colony-stimulating factor.
The late-phase reaction consists largely of inflammation with recruitment of leukocytes, notably eosinophils, neutrophils, and more T cells. Leukocyte recruitment is stimulated by chemokines produced by mast cells, epithelial cells and T cells, and by other cytokines (Chapter 2). Epithelial cells are known to produce a large variety of cytokines in response to infectious agents, drugs, and gases as well as to inflammatory mediators.31 This second wave of mediators stimulates the late reaction. For example, eotaxin, produced by airway epithelial cells, is a potent chemoattractant and activator of eosinophils.32 The major basic protein of eosinophils, in turn, causes epithelial damage31 and more airway constriction.33 Many mediators have been implicated in the asthmatic response, but the relative importance of each putative mediator in actual human asthma has been difficult to establish. The long list of “suspects” in acute asthma can be subclassified by the clinical efficacy of pharmacologic intervention with inhibitors or antagonists of the mediators.
It is thus clear that multiple mediators contribute to the acute asthmatic response. Moreover, the composition of this mediator soup might differ among different individuals or types of asthma. The appreciation of the importance of inflammatory cells and mediators in asthma has led to greater emphasis on anti-inflammatory drugs, such as corticosteroids, in the treatment of asthma.
Over time, repeated bouts of allergen exposure and immune reactions result in structural changes in the bronchial wall, referred to as “airway remodeling.” These changes, described later in greater detail, include hypertrophy and hyperplasia of bronchial smooth muscle, epithelial injury, increased airway vascularity, increased subepithelial mucus gland hypertrophy/hyperplasia, and deposition of subepithelial collagen. The complex interactions between the immune system, airway epithelium, and mesenchymal tissues in the airways are poorly understood. Infections with common respiratory pathogens, such as respiratory syncytial virus and influenza, can exacerbate the chronic changes and cause serious worsening of the clinical manifestations of the disease.
Although infections are often triggers for asthma, paradoxically, some infections may be protective. Epidemiologic studies first suggested that the incidence of asthma was greater in populations not exposed to microbes than in those living in an environment with abundant microbes, and this relationship may explain the increasing incidence of asthma in developed countries.35 These findings have led to the “hygiene hypothesis,” which states that eradication of infections may promote allergic and other harmful immune responses. Despite a fascination with this idea, there is no plausible explanation for the inverse relationship between infections and asthma.
Genetics of Asthma.
Asthma is a complex genetic trait in which multiple susceptibility genes interact with environmental factors to initiate the pathologic reaction. As in other complex traits (Chapter 5), there is considerable variability in the expression of these genes and in the combinations of polymorphisms present in individual patients, and even in the significance and reproducibility of reported polymorphisms. Of the more than 100 genes that have been reported to be associated with the disease, relatively few have been replicated in multiple patient populations. Many of these affect the immune response or tissue remodeling. Some genes may influence the development of asthma, while others modify asthma severity or the patient’s response to therapy.36 A few of these are discussed below:
The association between asthma and other forms of atopy with a polymorphism in the gene encoding the monocyte receptor for endotoxin, CD14, is worthy of additional comments since it is paradigmatic for studies of gene-environment interactions. In some studies, the TT genotype of CD14 has been associated with reduced levels of IgE and reduced risk for asthma and atopy. Other studies have revealed the opposite, i.e., an increased risk for atopy. Further analysis has revealed that the TT genotype is protective against asthma or allergic sensitization in individuals exposed to low (household) endotoxin levels, whereas the same genotype is associated with an increased risk for asthma or allergic sensitization in individuals exposed to high endotoxin levels (as may occur in those living on farms). These differences may relate to the influence of endotoxin levels on the regulation of TH1 vs. TH2 responses. In individuals with the TT genotype high endotoxin levels skew the response towards TH2 type, thus favoring more brisk IgE production and a predisposition to allergy. These studies indicate that the relationship between genotype and phenotype is context dependent, and help explain some of the discrepant results of association studies in different populations.37,38
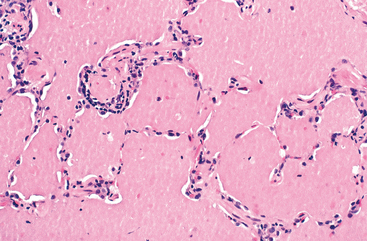
Morphology. In patients dying of status asthmaticus the lungs are overdistended because of overinflation, with small areas of atelectasis. The most striking macroscopic finding is occlusion of bronchi and bronchioles by thick, tenacious mucus plugs. Histologically, the mucus plugs contain whorls of shed epithelium, which give rise to the well-known spiral shaped mucus plugs called Curschmann spirals (these result either from mucus plugging in subepithelial mucous gland ducts which later become extruded or from plugs in bronchioles). Numerous eosinophils and Charcot-Leyden crystals are present; the latter are collections of crystalloid made up of an eosinophil lysophospholipase binding protein called galectin-10.42 The other characteristic histologic findings of asthma, collectively called “airway remodeling” (Fig. 15-10B), include:
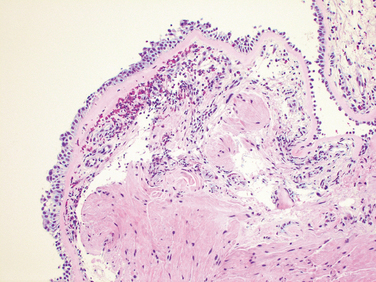
FIGURE 15-11 Bronchial biopsy specimen from an asthmatic patient showing sub-basement membrane fibrosis, eosinophilic inflammation, and muscle hyperplasia.
While acute airflow obstruction is primarily attributed to muscular bronchoconstriction, acute edema, and mucus plugging, airway remodeling may also contribute. Airway remodeling is commonly thought to contribute to chronic irreversible airway obstruction as well, although this is difficult to prove.
Clinical Course.
The classic acute asthmatic attack lasts up to several hours. In some patients these symptoms of chest tightness, dyspnea, wheezing, and cough with or without sputum production, persist at a low level constantly. In its most severe form, status asthmaticus, the severe acute paroxysm persists for days and even weeks, and under these circumstances airflow obstruction might be so extreme as to cause severe cyanosis and even death. The clinical diagnosis is aided by the demonstration of an increase in airflow obstruction (from baseline levels), difficulty with exhalation (prolonged expiration, wheeze), and elevated eosinophil count in the peripheral blood and the finding of eosinophils, Curschmann spirals, and Charcot-Leyden crystals in the sputum (particularly in patients with atopic asthma). In the usual case, with intervals of freedom from respiratory difficulty, the disease is more discouraging and disabling than lethal, being more of a problem in adult women than men. With appropriate therapy to relieve the attacks, most individuals with asthma are able to maintain a productive life. Up to 50% of childhood asthma remits in adolescence only to return in adulthood in a significant number of patients. In other cases there is a variable decline in baseline lung function.
BRONCHIECTASIS
Bronchiectasis is a disease characterized by permanent dilation of bronchi and bronchioles caused by destruction of the muscle and elastic tissue, resulting from or associated with chronic necrotizing infections. To be considered bronchiectasis the dilation must be permanent; reversible bronchial dilation often accompanies viral and bacterial pneumonia. Because of better control of lung infections, bronchiectasis is now an uncommon condition. Bronchiectasis develops in association with a variety of conditions, which include the following44,45:
Etiology and Pathogenesis.
Obstruction and infection are the major conditions associated with bronchiectasis, and it is likely that both are necessary for the development of full-fledged lesions, although either may come first. After bronchial obstruction, normal clearing mechanisms are impaired, there is pooling of secretions distal to the obstruction, and there is inflammation of the airway. Conversely, severe infections of the bronchi lead to inflammation, often with necrosis, fibrosis, and eventually dilation of airways.
These mechanisms, infection and obstruction, are most readily apparent in the severe form of bronchiectasis associated with cystic fibrosis (Chapter 10). In cystic fibrosis the primary defect in ion transport leads to defective mucociliary action, and accumulation of thick viscid secretions that obstruct the airways. This leads to a marked susceptibility to bacterial infections, which further damage the airways. With repeated infections there is widespread damage to airway walls, with destruction of supporting smooth muscle and elastic tissue, fibrosis, and further dilatation of bronchi. The smaller bronchioles become progressively obliterated as a result of fibrosis (bronchiolitis obliterans).47
In primary ciliary dyskinesia, an autosomal recessive syndrome with variable penetrance and a frequency of 1 in 15,000 to 40,000 births, poorly functioning cilia contribute to the retention of secretions and recurrent infections that in turn lead to bronchiectasis. There is an absence or shortening of the dynein arms that are responsible for the coordinated bending of the cilia. Approximately half of the patients with primary ciliary dyskinesia have Kartagener syndrome (bronchiectasis, sinusitis, and situs inversus or partial lateralizing abnormality).48 The lack of ciliary activity interferes with bacterial clearance, predisposes the sinuses and bronchi to infection, and affects cell motility during embryogenesis, resulting in the situs inversus. Males with this condition tend to be infertile, as a result of sperm dysmotility.
Allergic bronchopulmonary aspergillosis is a condition that results from a hypersensitivity reaction to the fungus Aspergillus fumigatus. It is also an important complication of asthma and cystic fibrosis.49 Characteristics are high serum IgE levels, serum antibodies to Aspergillus, intense airway inflammation with eosinophils, and the formation of mucus plugs, which play a primary role in its pathogenesis. There is evidence that neutrophil-mediated inflammation and a relative deficiency of anti-inflammatory cytokines such as IL-10 may also play a role.50 Clinically, there are periods of exacerbation and remission that may lead to proximal bronchiectasis and fibrotic lung disease.
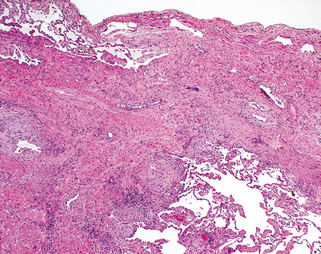
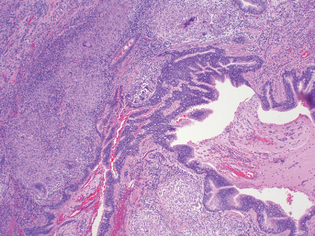
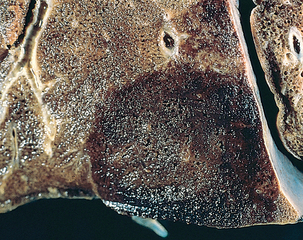
Morphology. Bronchiectasis usually affects the lower lobes bilaterally, particularly air passages that are vertical, and is most severe in the more distal bronchi and bronchioles. When tumors or aspiration of foreign bodies lead to bronchiectasis, the involvement may be sharply localized to a single segment of the lung. The airways are dilated, sometimes up to four times normal size. Characteristically, the bronchi and bronchioles are sufficiently dilated that they can be followed almost to the pleural surfaces. By contrast, in the normal lung, the bronchioles cannot be followed by ordinary gross dissection beyond a point 2 to 3 cm from the pleural surfaces. On the cut surface of the lung, the transected dilated bronchi appear as cysts filled with mucopurulent secretions (Fig. 15-12).
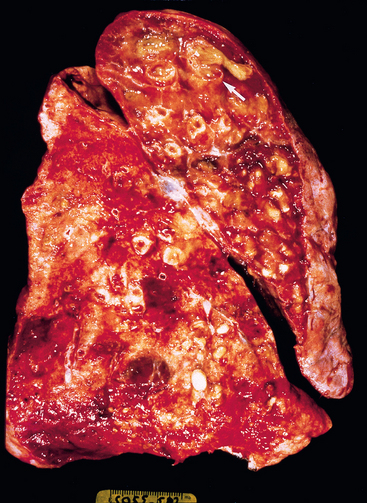
FIGURE 15-12 Bronchiectasis in a patient with cystic fibrosis, who underwent lung transplantation. Cut surface of lung shows markedly distended peripheral bronchi filled with mucopurulent secretions.
The histologic findings vary with the activity and chronicity of the disease. In the full-blown, active case there is an intense acute and chronic inflammatory exudation within the walls of the bronchi and bronchioles, associated with desquamation of the lining epithelium and extensive areas of necrotizing ulceration. There may be pseudostratification of the columnar cells or squamous metaplasia of the remaining epithelium. In some instances the necrosis completely destroys the bronchial or bronchiolar walls and forms a lung abscess. Fibrosis of the bronchial and bronchiolar walls and peribronchiolar fibrosis develop in the more chronic cases, leading to varying degrees of subtotal or total obliteration of bronchiolar lumens.
In the usual case of bronchiectasis, a mixed flora can be cultured from the involved bronchi, including staphylococci, streptococci, pneumococci, enteric organisms, anaerobic and microaerophilic bacteria, and (particularly in children) Haemophilus influenzae and Pseudomonas aeruginosa.51 In allergic bronchopulmonary aspergillosis a few fungal hyphae can be seen on special stains within the muco-inflammatory contents of the cylindrically dilated segmental bronchi. In late stages the fungus may infiltrate the bronchial wall.
Clinical Course.
Bronchiectasis causes severe, persistent cough; expectoration of foul-smelling, sometimes bloody sputum; dyspnea and orthopnea in severe cases; and occasional life-threatening hemoptysis. These symptoms are oftenepisodic and are precipitated by upper respiratory tract infections or the introduction of new pathogenic agents. Paroxysms of cough are particularly frequent when the patient rises in the morning, when changes in position lead to drainage of collections of pus and secretions into the bronchi. Obstructive respiratory insufficiency can lead to marked dyspnea and cyanosis. Cor pulmonale, brain abscesses, and amyloidosis are less frequent complications of bronchiectasis. However, due to current treatment with better antibiotics and physical therapy, outcome has improved considerably and life expectancy has almost doubled.
Chronic Diffuse Interstitial (Restrictive) Diseases
Chronic interstitial diseases are a heterogeneous group of disorders characterized predominantly by inflammation and fibrosis of the pulmonary connective tissue, principally the most peripheral and delicate interstitium in the alveolar walls. Many of the entities are of unknown cause and pathogenesis, some have an intra-alveolar as well as an interstitial component, and there is frequent overlap in histologic features among the different conditions. These disorders account for about 15% of noninfectious diseases seen by pulmonary physicians.
In general, the clinical and pulmonary functional changes are those of restrictive lung disease (see the earlier discussion of obstructive versus restrictive pulmonary diseases). Patients have dyspnea, tachypnea, end-inspiratory crackles, and eventual cyanosis, without wheezing or other evidence of airway obstruction. The classic physiologic features are reductions in carbon monoxide diffusing capacity, lung volume, and compliance. Chest radiographs show bilateral infiltrative lesions in the form of small nodules, irregular lines, or ground-glass shadows, hence the term infiltrative. Eventually, secondary pulmonary hypertension and right-sided heart failure with cor pulmonale may result. Although the entities can often be distinguished in the early stages, the advanced forms are hard to differentiate because they result in scarring and gross destruction of the lung, often referred to as end-stage lung or honeycomb lung. Diffuse restrictive diseases are categorized based on histology and clinical features (Table 15-5).
TABLE 15-5 Major Categories of Chronic Interstitial Lung Disease
| FIBROSING |
| GRANULOMATOUS |
| EOSINOPHILIC |
| SMOKING RELATED |
| OTHER |
| Pulmonary alveolar proteinosis |
FIBROSING DISEASES
Idiopathic Pulmonary Fibrosis
The term idiopathic pulmonary fibrosis (IPF) refers to a clinicopathologic syndrome with characteristic radiologic, pathologic, and clinical features. In Europe the term cryptogenic fibrosing alveolitis is more popular. The histologic pattern of fibrosis is referred to as usual interstitial pneumonia (UIP), which is required for the diagnosis of IPF but can also be seen in other diseases, notably connective tissue diseases, chronic hypersensitivity pneumonia, and asbestosis. The International Multidisciplinary Consensus Classification is an excellent reference for definitions and understanding of idiopathic interstitial pneumonias.52,53
Pathogenesis.
While the causative agent(s) of IPF remain unknown, our concepts of pathogenesis have evolved over the past several years.54 The earlier view was that IPF is initiated by an unidentified insult that gives rise to chronic inflammation resulting in fibrosis. The dismal failure of potent anti-inflammatory therapy in altering the course of the disease did not support this view. The current concept is that IPF is caused by “repeated cycles” of epithelial activation/injury by some unidentified agent. There is inflammation and induction of TH2 type T cell response characterized by the presence of eosinophils, mast cells, IL-4 and IL-13 in the lesions. But the significance of this inflammatory response is unknown. Abnormal epithelial repair at these sites gives rise to exuberant fibroblastic/myofibroblastic proliferation, leading to the “fibroblastic foci” that are so characteristic of IPF (Fig. 15-13). The circuits that drive such aberrant epithelial repair are not fully understood, but all evidence points to TGF-β1 as the driver of the process. TGF-β1 is known to be fibrogenic and is released from injured type I alveolar epithelial cells (Fig. 15-13). It favors the transformation of fibroblasts into myofibroblasts and deposition of collagen and other extracellular matrix molecules.55

FIGURE 15-13 Schematic representation of current understanding of the pathogenesis of idiopathic pulmonary fibrosis.
The concept that there is an intrinsic abnormality of tissue repair in IPF is supported by the finding that some patients with familial pulmonary fibrosis have mutations that shorten telomeres. Recall that telomeres control cell replications (see Chapters 1 and 7 and with shortening of telomeres alveolar epithelial cells undergo rapid senescence and apoptosis.56,57 Interestingly, TGF-β1 negatively regulates telomerase activity, thus facilitating epithelial cell apoptosis and the cycle of death and repair.58 Another molecule regulated by TGF-β1 is caveolin-1, the predominant structural protein of caveolae, flask-shaped invaginations of the plasma membrane present in many terminally differentiated cells. Caveolin-1 acts as an endogenous inhibitor of pulmonary fibrosis by limiting TGF-β1–induced production of extracellular matrix and restoring alveolar epithelial repair processes. Caveolin-1 is decreased in epithelial cells and fibroblasts of IPF patients, and overexpression of caveolin-1 in a mouse model limits fibrosis.59 Such down-regulation may be mediated by the ability of TGF-β1 to attenuate the expression of caveolin-1 in fibroblasts. Thus, it seems that TGF-β1 has its fingerprints on multiple pathways that regulate pulmonary fibrosis. Therapeutics directed toward neutralizing TGF-β1, enhancing telomerase activity or delaying telomere shortening, or augmenting caveolin-1 may lead to novel treatments for IPF in the future.60
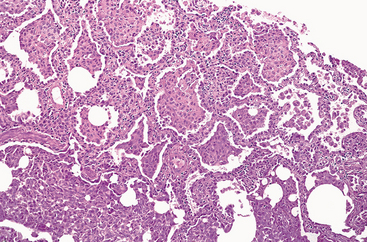
Morphology. Grossly, the pleural surfaces of the lung are cobblestoned as a result of the retraction of scars along the interlobular septa. The cut surface shows fibrosis (firm, rubbery white areas) of the lung parenchyma with lower-lobe predominance and a distinctive distribution in the subpleural regions and along the interlobular septa. Microscopically, the hallmark of UIP is patchy interstitial fibrosis, which varies in intensity (Fig. 15-14) and age. The earliest lesions contain exuberant fibroblastic proliferation (fibroblastic foci). With time these areas become more collagenous and less cellular. Quite typical is the coexistence of both early and late lesions (Fig. 15-15).The dense fibrosis causes the destruction of alveolar architecture and formation of cystic spaces lined by hyperplastic type II pneumocytes or bronchiolar epithelium (honeycomb fibrosis). With adequate sampling, these diagnostic histologic changes (i.e., areas of dense collagenous fibrosis with relatively normal lung and fibroblastic foci) can be identified even in advanced IPF. There is mild to moderate inflammation within the fibrotic areas, consisting of mostly lymphocytes, and a few plasma cells, neutrophils, eosinophils, and mast cells. Foci of squamous metaplasia and smooth muscle hyperplasia may be present. Pulmonary arterial hypertensive changes (intimal fibrosis and medial thickening) are often present. In acute exacerbations diffuse alveolar damage is superimposed on the UIP pattern.61
Clinical Course.
IPF begins insidiously, with gradually increasing dyspnea on exertion and dry cough. Most patients are 40 to 70 years old at the time of presentation. Hypoxemia, cyanosis, and clubbing occur late in the course. The progression in an individual patient is unpredictable. Most patients have a gradual deterioration of their pulmonary status, despite medical treatment (steroids, cyclophosphamide, or azathioprine). In some IPF patients, there are acute exacerbations of the underlying disease with a rapid downhill clinical course. The mean survival is 3 years or less. Lung transplantation is the only definitive therapy currently available.62
Nonspecific Interstitial Pneumonia
The concept of nonspecific interstitial pneumonia (NSIP) emerged when it was realized that there is a group of patients with diffuse interstitial lung disease of unknown etiology whose lung biopsies fail to show diagnostic features of any of the other well-characterized interstitial diseases. Despite its “nonspecific” name, NSIP has distinct radiologic and histologic features and is important to recognize, since these patients have a much better prognosis than do those with UIP.63
Morphology. On the basis of its histology, NSIP is divided into cellular and fibrosing patterns. The cellular pattern consists primarily of mild to moderate chronic interstitial inflammation, containing lymphocytes and a few plasma cells, in a uniform or patchy distribution. The fibrosing pattern consists of diffuse or patchy interstitial fibrosis without the temporal heterogeneity that is characteristic of UIP. Fibroblastic foci and honeycombing are absent. However, in some patients both NSIP and UIP patterns can be seen in different areas of the lung; the prognosis in these is the same as for UIP.64
Clinical Course.
Patients present with dyspnea and cough of several months’ duration. They are typically between 46 and 55 years of age. Those having the NSIP cellular pattern are somewhat younger than those with the fibrosing pattern or UIP. Patients with the cellular pattern have a better outcome than do those with fibrosing pattern and UIP.65
Cryptogenic Organizing Pneumonia
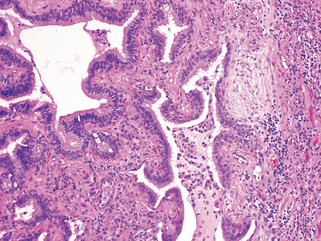
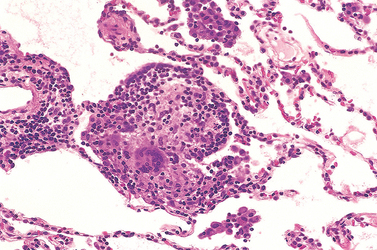
Cryptogenic organizing pneumonia is synonymous with the popular term bronchiolitis obliterans organizing pneumonia; however, the former is now preferred, since it conveys the essential features of a clinicopathologic syndrome of unknown etiology and avoids confusion with airway diseases such as bronchiolitis obliterans. Patients present with cough and dyspnea and have subpleural or peribronchial patchy areas of airspace consolidation radiographically. Histologically, cryptogenic organizing pneumonia is characterized by the presence of polypoid plugs of loose organizing connective tissue (Masson bodies) within alveolar ducts, alveoli (Fig. 15-16), and often bronchioles. The connective tissue is all of the same age, and the underlying lung architecture is normal. There is no interstitial fibrosis or honeycomb lung. Some patients recover spontaneously, but most need treatment with oral steroids for 6 months or longer for complete recovery.
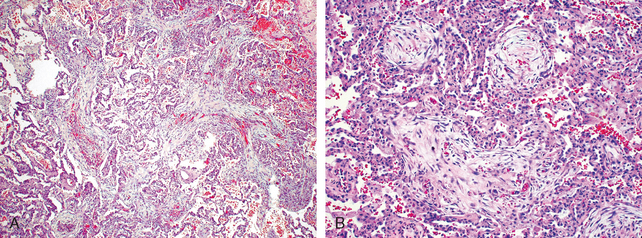
FIGURE 15-16 Cryptogenic organizing pneumonia. Some alveolar spaces are filled with balls of fibroblasts (Masson bodies), while the alveolar walls are relatively normal. A, Low power; B, high power.
It is important to recognize that organizing pneumonia with intra-alveolar fibrosis is also often seen as a response to infections or inflammatory injury of the lungs.66 These include viral and bacterial pneumonia, inhaled toxins, drugs, connective tissue disease, and graft-versus-host disease in bone marrow transplant recipients. The prognosis for these patients is the same as that for the underlying disorder.
Pulmonary Involvement in Connective Tissue Diseases
Many connective tissue diseases, notably systemic lupus erythematosus, rheumatoid arthritis, progressive systemic sclerosis (scleroderma), dermatomyositis-polymyositis, and mixed connective tissue disease, can involve the lung to a lesser or greater degree at some time in their course. Pulmonary involvement can occur in different patterns; NSIP, UIP (similar to that seen in IPF), vascular sclerosis, organizing pneumonia, and bronchiolitis are the most common.
Pulmonary involvement in these diseases is usually associated with a variable prognosis, partly dependent on the type of pulmonary disease, although it is still better than that of idiopathic UIP.67
Pneumoconioses
The term pneumoconiosis was originally coined to describe the non-neoplastic lung reaction to inhalation of mineral dusts encountered in the workplace. Now it also includes diseases induced by organic as well as inorganic particulates and chemical fumes and vapors. A simplified classification is presented in Table 15-6. Regulations limiting worker exposure have resulted in a marked decrease in dust-associated diseases.
TABLE 15-6 Lung Diseases Caused by Air Pollutants
| Agent | Disease | Exposure |
|---|---|---|
| MINERAL DUSTS | ||
| Coal dust | Anthracosis | Coal mining (particularly hard coal) |
| Macules | ||
| Progressive massive fibrosis | ||
| Caplan syndrome | ||
| Silica | Silicosis | Foundry work, sandblasting, hard rock mining, stone cutting, others |
| Caplan syndrome | ||
| Asbestos | Asbestosis | Mining, milling, fabrication, and installation and removal of insulation |
| Pleural plaques | ||
| Caplan syndrome | ||
| Mesothelioma | ||
| Carcinoma of the lung, larynx, stomach, colon | ||
| Beryllium | Acute berylliosis | Mining, fabrication |
| Beryllium granulomatosis | ||
| Lung carcinoma (?) | ||
| Iron oxide | Siderosis | Welding |
| Barium sulfate | Baritosis | Mining |
| Tin oxide | Stannosis | Mining |
| ORGANIC DUSTS THAT INDUCE HYPERSENSITIVITY PNEUMONITIS | ||
| Moldy hay | Farmer’s lung | Farming |
| Bagasse | Bagassosis | Manufacturing wallboard, paper |
| Bird droppings | Bird-breeder’s lung | Bird handling |
| ORGANIC DUSTS THAT INDUCE ASTHMA | ||
| Cotton, flax, hemp | Byssinosis | Textile manufacturing |
| Red cedar dust | Asthma | Lumbering, carpentry |
| CHEMICAL FUMES AND VAPORS | ||
| Nitrous oxide, sulfur dioxide, ammonia, benzene, insecticides | Bronchitis, asthma | Occupational and accidental exposure |
| Pulmonary edema | ||
| ARDS | ||
| Mucosal injury | ||
| Fulminant poisoning | ||
ARDS, acute respiratory distress syndrome.
Although the pneumoconioses result from well-defined occupational exposure to specific airborne agents, particulate air pollution also has deleterious effects on the general population, especially in urban areas. Studies have found increased morbidity (e.g., asthma incidence) and mortality rates in populations that are exposed to high ambient air particulate levels,68,69 leading to calls for greater efforts to reduce the levels of particulates in urban air.
Pathogenesis.
The development of a pneumoconiosis depends on (1) the amount of dust retained in the lung and airways; (2) the size, shape, and therefore buoyancy of the particles; (3) particle solubility and physiochemical reactivity; and (4) the possible additional effects of other irritants (e.g., concomitant tobacco smoking).
The amount of dust retained in the lungs is determined by the dust concentration in ambient air, the duration of exposure, and the effectiveness of clearance mechanisms. Any influence, such as cigarette smoking, that affects the integrity of the mucociliary apparatus significantly predisposes to the accumulation of dust. The most dangerous particles range from 1 to 5 μm in diameter because they may reach the terminal small airways and air sacs and settle in their linings. Under normal conditions there is a small pool of intra-alveolar macrophages, and this is expanded by recruitment of more macrophages when dust reaches the alveolar spaces. The protection provided by phagocytosis of particles, however, can be overwhelmed by a large dust burden by specific chemical interactions of the particles with cells.
The solubility and cytotoxicity of particles, which are influenced to a considerable extent by their size, modify the nature of the pulmonary response. In general, the smaller the particle, the more likely it is to appear in the pulmonary fluids and reach toxic levels rapidly, depending, of course, on the solubility of the agent. Therefore, smaller particles tend to cause acute lung injury. Larger particles resist dissolution and so may persist within the lung parenchyma for years. These tend to evoke fibrosing collagenous pneumoconioses, such as is characteristic of silicosis. Some of the particles may be taken up by epithelial cells or may cross the epithelial cell lining and interact directly with fibroblasts and interstitial macrophages. Some may reach the lymphatics by direct drainage or within migrating macrophages and thereby initiate an immune response to components of the particulates or to self-proteins modified by the particles or both. This response amplifies the intensity and the duration of the local reaction. Although tobacco smoking worsens the effects of all inhaled mineral dusts, the effects of asbestos are particularly magnified by smoking. The effects of inhaled particles are not confined to the lung alone, since solutes from particles can enter the blood and lung inflammation invokes systemic responses.70
In general, only a small percentage of exposed people develop occupational respiratory diseases, implying a genetic predisposition to their development.71 In one study, genetic variation of serum and erythrocytic proteins was shown to correlate with susceptibility to developing silicosis, chronic bronchitis, and occupational asthma.72 Many of the diseases listed in Table 15-6 are quite uncommon. Hence only a selected few that cause fibrosis of the lung are presented next.
Coal Workers’ Pneumoconiosis
Dust reduction measures in coal mines around the globe have drastically reduced the incidence of coal workers’ pneumoconiosis (CWP). The spectrum of lung findings in coal workers is wide, varying from (1) asymptomatic anthracosis to (2) simple CWP with little to no pulmonary dysfunction to (3) complicated CWP, or progressive massive fibrosis (PMF), in which lung function is compromised.73 The pathogenesis of complicated CWP, particularly what causes the lesions of simple CWP to progress to PMF, is incompletely understood. Contaminating silica in the coal dust can favor progressive disease. In most cases, carbon dust itself is the major culprit, and studies have shown that complicated lesions contain much more dust than simple lesions.
Morphology. Anthracosis is the most innocuous coal-induced pulmonary lesion in coal miners and is also seen to some degree in urban dwellers and tobacco smokers. Inhaled carbon pigment is engulfed by alveolar or interstitial macrophages, which then accumulate in the connective tissue along the lymphatics, including the pleural lymphatics, or in organized lymphoid tissue along the bronchi or in the lung hilus.
Simple CWP is characterized by coal macules (1 to 2 mm in diameter) and the somewhat larger coal nodules. The coal macule consists of carbon-laden macrophages; the nodule also contains small amounts of a delicate network of collagen fibers. Although these lesions are scattered throughout the lung, the upper lobes and upper zones of the lower lobes are more heavily involved. They are located primarily adjacent to respiratory bronchioles, the site of initial dust accumulation. In due course dilation of adjacent alveoli occurs, a condition sometimes referred to as centrilobular emphysema.
Complicated CWP (progressive massive fibrosis) occurs on a background of simple CWP and generally requires many years to develop. It is characterized by intensely blackened scars larger than 2 cm, sometimes up to 10 cm in greatest diameter. They are usually multiple (Fig. 15-17). Microscopically the lesions consist of dense collagen and pigment. The center of the lesion is often necrotic, most likely due to local ischemia.
FIGURE 15-17 Progressive massive fibrosis superimposed on coal workers’ pneumoconiosis. The large, blackened scars are located principally in the upper lobe. Note the extensions of scars into surrounding parenchyma and retraction of adjacent pleura.
(Courtesy of Drs. Werner Laquer and Jerome Kleinerman, the National Institute of Occupational Safety and Health, Morgantown, WV.)
Clinical Course.
CWP is usually a benign disease that causes little decrement in lung function. Even mild forms of complicated CWP fail to demonstrate abnormalities of lung function. In a minority of cases (fewer than 10%), PMF develops, leading to increasing pulmonary dysfunction, pulmonary hypertension, and cor pulmonale. Once PMF develops, it may become progressive even if further exposure to dust is prevented. Unlike silicosis (discussed later), there is no convincing evidence that coal dust increases susceptibility to tuberculosis. There is some evidence that exposure to coal dust increases the incidence of chronic bronchitis and emphysema, independent of smoking. Thus far, however, there is no compelling evidence that CWP in the absence of smoking predisposes to cancer.
Silicosis
Silicosis is a lung disease caused by inhalation of crystalline silicon dioxide (silica).74 Currently the most prevalent chronic occupational disease in the world, silicosis usually presents after decades of exposure as a slowly progressing, nodular, fibrosing pneumoconiosis. As shown in Table 15-6, workers in a large number of occupations are at risk, especially sandblasters and many mine workers. Less commonly, heavy exposure over months to a few years can result in acute silicosis, a disorder characterized by the accumulation of abundant lipoproteinaceous material within alveoli (identical morphologically to alveolar proteinosis, which is discussed later).
Pathogenesis.
Silica occurs in both crystalline and amorphous forms, but crystalline forms (including quartz, crystobalite, and tridymite) are much more fibrogenic. Of these, quartz is most commonly implicated in silicosis.After inhalation, the particles interact with epithelial cells and macrophages. Within the macrophages silica causes activation and release of mediators. Such mediators include IL-1, TNF, fibronectin, lipid mediators, oxygen-derived free radicals, and fibrogenic cytokines.75,76 Especially compelling is evidence incriminating TNF, since anti-TNF monoclonal antibodies can block lung collagen accumulation in mice given silica intratracheally. It has been noted that when mixed with other minerals, quartz has a reduced fibrogenic effect. This phenomenon is of practical importance because quartz in the workplace is rarely pure. Thus, miners of the iron-containing ore hematite may have more quartz in their lungs than some quartz-exposed workers and yet have relatively mild lung disease because the hematite somehow provides a protective effect. Although amorphous silicates are biologically less active than crystalline silica, heavy lung burdens of these minerals may also produce lesions.
Morphology. Silicosis is characterized grossly in its early stages by tiny, barely palpable, discrete pale to blackened (if coal dust is also present) nodules in the upper zones of the lungs. As the disease progresses, these nodules may coalesce into hard, collagenous scars (Fig. 15-18). Some nodules may undergo central softening and cavitation. This change may be due to superimposed tuberculosis or to ischemia. Fibrotic lesions may also occur in the hilar lymph nodes and pleura. Sometimes, thin sheets of calcification occur in the lymph nodes and are seen radiographically as eggshell calcification (i.e., calcium surrounding a zone lacking calcification). If the disease continues to progress, expansion and coalescence of lesions may produce progressive massive fibrosis. Histologic examination reveals that the nodular lesions consist of concentric layers of hyalinized collagen surrounded by a dense capsule of more condensed collagen (Fig. 15-19). Examination of the nodules by polarized microscopy reveals the birefringent silica particles.
Clinical Course.
Chest radiographs typically show a fine nodularity in the upper zones of the lung, but pulmonary functions are either normal or only moderately affected. Most patients do not develop shortness of breath until late in the course, after progressive massive fibrosis is present. The disease may be progressive even if the patient is no longer exposed. The disease is slow to kill, but impaired pulmonary function may severely limit activity. Silicosis is associated with an increased susceptibility to tuberculosis. It is postulated that silicosis results in a depression of cell-mediated immunity, and crystalline silica may inhibit the ability of pulmonary macrophages to kill phagocytosed mycobacteria. Nodules of silicotuberculosis often display a central zone of caseation. The relationship between silica and lung cancer is contentious. In 1997, the International Agency for Research on Cancer (IARC) concluded that crystalline silica from occupational sources is carcinogenic in humans. However, this subject continues to be controversial.
Asbestos-Related Diseases
Asbestos is a family of crystalline hydrated silicates that form fibers. Use of asbestos is seriously restricted in many developed countries; however, there is little, if any, control in less developed parts of the world.77 On the basis of epidemiologic studies, occupational exposure to asbestos is linked to78:
An increased incidence of asbestos-related cancer in family members of asbestos workers has alerted the general public to the potential hazards of asbestos in the environment. The proper public health policy toward low-level exposures that might be encountered in old buildings or schools is unsettled: some experts question the wisdom of expensive asbestos abatement programs for environments with airborne fiber counts that are as much as 100-fold lower than allowed by occupational standards.
Pathogenesis.
Concentration, size, shape, and solubility of the different forms of asbestos dictate whether it causes disease.79 There are two distinct geometric forms of asbestos: serpentine and amphibole. The serpentine chrysotile chemical form accounts for most of the asbestos used in industry. Amphiboles, even though less prevalent, are more pathogenic than chrysotiles particularly with respect to induction of malignant pleural tumors (mesotheliomas).
The greater pathogenicity of amphiboles is apparently related to their aerodynamic properties and solubility. Chrysotiles, with their more flexible, curled structure, are likely to become impacted in the upper respiratory passages and removed by the mucociliary elevator. Furthermore, once trapped in the lungs, chrysotiles are gradually leached from the tissues because they are more soluble than amphiboles. In contrast, the straight, stiff amphiboles may align themselves in the airstream and thus be delivered deeper into the lungs, where they can penetrate epithelial cells and reach the interstitium. Both amphiboles and serpentines are fibrogenic, and increasing doses are associated with a higher incidence of all asbestos-related diseases except mesothelioma, which is only associated with amphibole exposure.
In contrast to other inorganic dusts, asbestos can also act as a tumor initiator and promoter. Some of its oncogenic effects are mediated by reactive free radicals generated by asbestos fibers, which preferentially localize in the distal lung, close to the mesothelial layers. Potentially toxic chemicals adsorbed onto the asbestos fibers most likely contribute to the oncogenicity of the fibers. For example, the adsorption of carcinogens in tobacco smoke onto asbestos fibers may well contribute to the remarkable synergy between tobacco smoking and the development of lung carcinoma in asbestos workers. One study of asbestos workers found a fivefold increase of lung carcinoma with asbestos exposure alone, while asbestos exposure and smoking together led to a 55-fold increase in the risk of lung cancer.80
The occurrence of asbestosis, like the other pneumoconioses, depends on the interaction of inhaled fibers with lung macrophages and other parenchymal cells. The initial injury occurs at bifurcations of small airways and ducts, where the asbestos fibers land and penetrate. Macrophages, both alveolar and interstitial, attempt to ingest and clear the fibers and are activated to release chemotactic factors and fibrogenic mediators that amplify the response. Chronic deposition of fibers and persistent release of mediators eventually lead to generalized interstitial pulmonary inflammation and interstitial fibrosis.
Morphology. Asbestosis is marked by diffuse pulmonary interstitial fibrosis, which is indistinguishable from diffuse interstitial fibrosis resulting from other causes, except for the presence of multiple asbestos bodies. Asbestos bodies appear as golden brown, fusiform or beaded rods with a translucent center and consist of asbestos fibers coated with an iron-containing proteinaceous material (Fig. 15-20). They arise when macrophages attempt to phagocytose asbestos fibers; the iron is presumably derived from phagocyte ferritin. Other inorganic particulates may become coated with similar iron-protein complexes and are called ferruginous bodies. Rare single asbestos bodies can be found in the lungs of normal people.
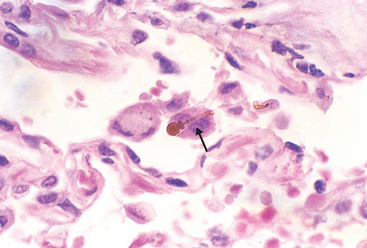
FIGURE 15-20 High-power detail of an asbestos body, revealing the typical beading and knobbed ends (arrow).
Asbestosis begins as fibrosis around respiratory bronchioles and alveolar ducts and extends to involve adjacent alveolar sacs and alveoli. The fibrous tissue distorts the architecture, creating enlarged airspaces enclosed within thick fibrous walls; eventually the affected regions become honeycombed. The pattern of fibrosis is similar to that seen in UIP, with fibroblastic foci and varying degrees of fibrosis, the onlydifference being the presence of numerous asbestos bodies. In contrast to CWP and silicosis, asbestosis begins in the lower lobes and subpleurally. The middle and upper lobes of the lungs become affected as fibrosis progresses. The scarring may trap and narrow pulmonary arteries and arterioles, causing pulmonary hypertension and cor pulmonale.
Pleural plaques, the most common manifestation of asbestos exposure, are well-circumscribed plaques of dense collagen (Fig. 15-21), often containing calcium. They develop most frequently on the anterior and posterolateral aspects of the parietal pleura and over the domes of the diaphragm. The size and number of pleural plaques do not correlate with the level of exposure to asbestos or the time since exposure.81 They do not contain asbestos bodies; however, only rarely do they occur in individuals who have no history or evidence of asbestos exposure. Uncommonly, asbestos exposure induces pleural effusions, which are usually serous but may be bloody. Rarely, diffuse visceral pleural fibrosis may occur and, in advanced cases, bind the lung to the thoracic cavity wall.

FIGURE 15-21 Asbestos-related pleural plaques. Large, discrete fibrocalcific plaques are seen on the pleural surface of the diaphragm.
(Courtesy of Dr. John Godleski, Brigham and Women’s Hospital, Boston, MA.)
Both lung carcinomas and mesotheliomas (pleural and peritoneal) develop in workers exposed to asbestos. The risk of lung carcinoma is increased about fivefold for asbestos workers; the relative risk of mesotheliomas, normally a rare tumor (2 to 17 cases per 1 million persons), is more than 1000-fold greater. Concomitant cigarette smoking greatly increases the risk of lung carcinoma but not that of mesothelioma.
Clinical Course.
The clinical findings in asbestosis are very similar to those caused by other diffuse interstitial lung diseases (discussed earlier). Dyspnea is usually the first manifestation; at first, it is provoked by exertion, but later it is present even at rest. The dyspnea is usually accompanied by a cough associated with production of sputum. These manifestations rarely appear fewer than 10 years after first exposure and are more common after 20 years or more. Chest x-rays reveal irregular linear densities, particularly in both lower lobes. With advancement of the pneumoconiosis, a honeycomb pattern develops. The disease may remain static or progress to respiratory failure, cor pulmonale, and death. Pleural plaques are usually asymptomatic and are detected on radiographs as circumscribed densities. Asbestosis complicated by lung or pleural cancer is associated with a particularly grim prognosis.
Complications of Therapies
Drug-Induced Lung Diseases.
Drugs can cause a variety of both acute and chronic alterations in respiratory structure and function, interstitial fibrosis, bronchiolitis obliterans, and eosinophilic pneumonia (Table 15-7).82 For example, cytotoxic drugs used in cancer therapy (e.g., bleomycin) cause pulmonary damage and fibrosis as a result of direct toxicity of the drug and by stimulating the influx of inflammatory cells into the alveoli. Amiodarone, a drug used to treat cardiac arrhythmias, is preferentially concentrated in the lung and causes significant pneumonitis in 5% to 15% of patients receiving it.
TABLE 15-7 Examples of Drug-Induced Pulmonary Disease
| Drug | Pulmonary Disease |
|---|---|
| Bleomycin | Pneumonitis and fibrosis |
| Methotrexate | Hypersensitivity pneumonitis |
| Amiodarone | Pneumonitis and fibrosis |
| Nitrofurantoin | Hypersensitivity pneumonitis |
| Aspirin | Bronchospasm |
| β-Antagonists | Bronchospasm |
Radiation-Induced Lung Diseases.
Radiation pneumonitis is a well-known complication of therapeutic radiation of thoracic tumors (lung, esophageal, breast, mediastinal).83 It most often involves the lung within the radiation port but occasionally may extend to other areas of the same lung or even the contralateral lung. It occurs in acute and chronic forms. One to six months after fractionated irradiation, acute radiation pneumonitis (lymphocytic alveolitis or hypersensitivity pneumonitis) occurs in 10% to 20% of patients. It is manifest by fever, dyspnea out of proportion to the volume of lung irradiated, pleural effusion, and radiologic infiltrates that usually correspond to an area of previous irradiation. With steroid therapy, these symptoms may resolve completely in some patients without long-term effects,84 while in others there is progression to chronic radiation pneumonitis (pulmonary fibrosis). The latter is a consequence of the repair of injured endothelial and epithelial cells within the radiation portal. Morphologic changes are those of diffuse alveolar damage, including severe atypia of hyperplastic type II cells and fibroblasts. Epithelial cell atypia and foam cells within vessel walls are also characteristic of radiation damage.
GRANULOMATOUS DISEASES
Sarcoidosis
Sarcoidosis is a systemic disease of unknown cause characterized by noncaseating granulomas in many tissues and organs. Sarcoidosis presents many clinical patterns, but bilateral hilar lymphadenopathy or lung involvement is visible on chest radiographs in 90% of cases. Eye and skin lesions occur next in frequency. Since other diseases, including mycobacterial and fungal infections and berylliosis, can also produce noncaseating (hard) granulomas, the histologic diagnosis of sarcoidosis is made by exclusion.85
The prevalence of sarcoidosis is higher in women than in men but varies widely in different countries and populations. In the United States the rates are highest in the Southeast; they are 10 times higher in American blacks than in whites. In contrast, the disease is rare among Chinese and Southeast Asians.
Etiology and Pathogenesis.
Although the etiology of sarcoidosis remains unknown, several lines of evidence suggest that it is a disease of disordered immune regulation in genetically predisposed individuals exposed to certain environmental agents.86 The role of each of these three contributory factors is summarized below.
Immunological Factors.
There are several immunological abnormalities in the local milieu of sarcoid granulomas that suggest the development of a cell-mediated response to an unidentified antigen.87 The process is driven by CD4+ helper T cells. These abnormalities include88:
Additionally, there are systemic immunological abnormalities in individuals with sarcoidosis:
Genetic Factors.
Evidence of genetic influences are the familial and racial clustering of cases and the association with certain HLA genotypes (e.g., HLA-A1 and HLA-B8).
Environmental Factors.
These are possibly the most tenuous of all the associations in the pathogenesis of sarcoidosis. As with many other diseases of unknown etiology, suspicion falls on microbes. Indeed several putative microbes have been proposed as the inciting agent for sarcoidosis (e.g., mycobacteria, Propionibacterium acnes, and Rickettsia species).89 Alas there is no unequivocal evidence that sarcoidosis is caused by an infectious agent.
Morphology. Histologically, all involved tissues show the classic well-formed noncaseating granulomas (Fig. 15-22), each composed of an aggregate of tightly clustered epithelioid cells, often with Langhans or foreign body–type giant cells. Central necrosis is unusual. With chronicity the granulomas may become enclosed within fibrous rims or may eventually be replaced by hyaline fibrous scars. Laminated concretions composed of calcium and proteins known as Schaumann bodies and stellate inclusions known as asteroid bodies enclosed within giant cells are found in approximately 60% of the granulomas. Though characteristic, these microscopic features are notpathognomonic of sarcoidosis, because asteroid and Schaumann bodies may be encountered in other granulomatous diseases (e.g., tuberculosis). Pathologic involvement of virtually every organ in the body has been cited at one time or another.
The lungs are common sites of involvement.90 Macroscopically there is usually no demonstrable alteration, although in advanced cases the coalescence of granulomas produces small nodules that are palpable or visible as 1 to 2 cm, noncaseating, noncavitated consolidations. Histologically, the lesions are distributed primarily along the lymphatics, around bronchi and blood vessels, although alveolar lesions are also seen. The relative frequency of granulomas in the bronchial submucosa accounts for the high diagnostic yield of bronchoscopic biopsies. There seems to be a strong tendency for lesions to heal in the lungs, so varying stages of fibrosis and hyalinization are often found. The pleural surfaces are sometimes involved.
Lymph nodes are involved in almost all cases, particularly the hilar and mediastinal nodes, but any other node in the body may be involved. Nodes are characteristically enlarged, discrete, and sometimes calcified. The tonsils are affected in about one quarter to one third of cases.
The spleen is affected microscopically in about three quarters of cases, but it is enlarged in only one fifth. On occasion, granulomas may coalesce to form small nodules that are barely visible macroscopically. The capsule is not involved. The liver is affected slightly less often than the spleen. It may also be moderately enlarged and contains scattered granulomas, more in portal triads than in the lobular parenchyma. Needle biopsy can be diagnostic.
The bone marrow is involved in about one fifth of cases of systemic sarcoidosis. The radiologically visible bone lesions have a particular tendency to involve phalangeal bones of the hands and feet, creating small circumscribed areas of bone resorption within the marrow cavity and a diffuse reticulated pattern throughout the cavity, with widening of the bony shafts or new bone formation on the outer surfaces.
Skin lesions are encountered in one third to one half of cases. Sarcoidosis of the skin assumes a variety of macroscopic appearances (e.g., discrete subcutaneous nodules; focal, slightly elevated, erythematous plaques; or flat lesions that are slightly reddened and scaling, and resemble those of lupus erythematosus). Lesions may also appear on the mucous membranes of the oral cavity, larynx, and upper respiratory tract. The eye, its associated glands, and the salivary glands are involved in about one fifth to one half of cases. The ocular involvement takes the form of iritis or iridocyclitis, either bilaterally or unilaterally. Consequently, corneal opacities, glaucoma, and total loss of vision may occur. These ocular lesions are frequently accompanied by inflammation of the lacrimal glands, with suppression of lacrimation. Bilateral sarcoidosis of the parotid, submaxillary, and sublingual glands constitutes the combined uveoparotid involvement designated as Mikulicz syndrome (Chapter 16). Muscle involvement is often underdiagnosed, since it may be asymptomatic. Muscle weakness, aches, tenderness, and fatigue should prompt consideration of occult sarcoid myositis.91 Muscle biopsy can be useful for diagnosis when clinical features point to sarcoidosis. Sarcoid granulomas occasionally occur in the heart, kidneys, central nervous system, and endocrine glands, particularly in the pituitary, as well as in other body tissues.
Clinical Course.
Because of its varying severity and the inconstant distribution of the lesions, sarcoidosis is a protean clinical disease. It may be discovered unexpectedly on routine chest films as bilateral hilar adenopathy or may present with peripheral lymphadenopathy, cutaneous lesions, eye involvement, splenomegaly, or hepatomegaly. In the great majority of cases, however, individuals seek medical attention because of the insidious onset of respiratory abnormalities (shortness of breath, cough, chest pain, hemoptysis) or of constitutional signs and symptoms (fever, fatigue, weight loss, anorexia, night sweats).
Sarcoidosis follows an unpredictable course characterized by either progressive chronicity or periods of activity interspersed with remissions, sometimes permanent, that may be spontaneous or induced by steroid therapy. Overall, 65% to 70% of affected patients recover with minimal or no residual manifestations. Twenty percent have permanent loss of some lung function or some permanent visual impairment. Of the remaining 10% to 15%, some die of cardiac or central nervous system damage, but most succumb to progressive pulmonary fibrosis and cor pulmonale.
Hypersensitivity Pneumonitis
The term hypersensitivity pneumonitis describes a spectrum of immunologically mediated, predominantly interstitial, lung disorders caused by intense, often prolonged exposure to inhaled organic antigens.92 Affected individuals have an abnormal sensitivity or heightened reactivity to the antigen, which, in contrast to that occurring in asthma, involves primarily the alveoli (thus the synonym “allergic alveolitis”).93 It is important to recognize these diseases early in their course because progression to serious chronic fibrotic lung disease can be prevented by removal of the environmental agent.
Most commonly, hypersensitivity results from the inhalation of organic dust containing antigens made up of spores of thermophilic bacteria, true fungi, animal proteins, or bacterial products. Numerous specifically named syndromes are described, depending on the occupation or exposure of the individual. Farmer’s lung results from exposure to dusts generated from harvested humid, warm hay that permits the rapid proliferation of the spores of thermophilic actinomycetes. Pigeon breeder’s lung (bird fancier’s disease) is provoked by proteins from serum, excreta, or feathers of birds. Humidifier or air-conditioner lung is caused by thermophilic bacteria in heated water reservoirs.
Several lines of evidence suggest that hypersensitivity pneumonitis is an immunologically mediated disease:
Finally, the presence of noncaseating granulomas in two thirds of the patients suggests the development of a T cell–mediated (type IV) delayed-type hypersensitivity against the implicated antigen(s).
Morphology. Histologic changes in subacute and chronic forms are characteristically centered on bronchioles.94 They include (1) interstitial pneumonitis consisting primarily of lymphocytes, plasma cells, and macrophages; (2) noncaseating granulomas in two thirds of patients (Fig. 15-23); and (3) interstitial fibrosis, honeycombing, and obliterative bronchiolitis (in late stages). In more than half the patients there is also evidence of an intra-alveolar infiltrate.
Clinical Features.
The clinical manifestations are varied. Acute attacks, which follow inhalation of antigenic dust in sensitized patients, consist of recurring episodes of fever, dyspnea, cough, and leukocytosis. Diffuse and nodular infiltrates appear in the chest radiograph, and pulmonary function tests show an acute restrictive disorder. Symptoms usually appear 4 to 6 hours after exposure. If exposure is continuous and protracted, a chronic form of the disease supervenes with progressive respiratory failure, dyspnea, and cyanosis and a decrease in total lung capacity and compliance—a picture similar to other forms of chronic interstitial disease.
PULMONARY EOSINOPHILIA
Several clinical and pathologic pulmonary entities are characterized by an infiltration of eosinophils, recruited in part by elevated alveolar levels of eosinophil attractants such as IL-5.95
Pulmonary eosinophilia is divided into the following categories96:
Acute eosinophilic pneumonia with respiratory failure is an acute illness of unknown cause. It has a rapid onset with fever, dyspnea, and hypoxemic respiratory failure. The chest radiograph shows diffuse infiltrates, and bronchoalveolar lavage fluid contains more than 25% eosinophils. There is a prompt response to corticosteroids.
Simple pulmonary eosinophilia is characterized by transient pulmonary lesions, eosinophilia in the blood, and a benign clinical course. CT scans are often quite striking, with shadows of varying size and shape in any of the lobes, suggesting irregular intrapulmonary densities. The alveolar septa are thickened by an infiltrate composed of eosinophils and occasional interspersed giant cells, but there is no vasculitis, fibrosis, or necrosis.
Chronic eosinophilic pneumonia is characterized by focal areas of cellular consolidation of the lung substance distributed chiefly in the periphery of the lung fields. Prominent in these lesions are heavy aggregates of lymphocytes and eosinophils within both the septal walls and the alveolar spaces. These patients have high fever, night sweats, and dyspnea, all of which respond to corticosteroid therapy. Chronic eosinophilic pneumonia is diagnosed when other causes of chronic pulmonary eosinophilia are excluded.
SMOKING-RELATED INTERSTITIAL DISEASES
Smoking-related diseases can be grouped into obstructive diseases (emphysema and chronic bronchitis, already discussed) and restrictive or interstitial diseases. A majority of individuals with idiopathic pulmonary fibrosis are smokers; however, the role of cigarette smoking in its pathogenesis has not been clarified yet. Desquamative interstitial pneumonia (DIP) and respiratory bronchiolitis-associated interstitial lung disease are thought to represent two ends of a spectrum of smoking-associated interstitial lung diseases.97
Desquamative Interstitial Pneumonia
The large collections of airspace macrophages that char-acterize DIP were originally thought to be desquamated pneumocytes, thus the misnomer “desquamative interstitial pneumonia.”
Morphology. The most striking histologic finding is the accumulation of a large number of macrophages with abundant cytoplasm containing dusty brown pigment (smokers’ macrophages) in the airspaces. Finely granular iron may be seen in the macrophage cytoplasm. Some of the macrophages contain lamellar bodies (composed of surfactant) within phagocytic vacuoles, presumably derived from necrotic type II pneumocytes. The alveolar septa are thickened by a sparse inflammatory infiltrate of lymphocytes, plasma cells, and occasional eosinophils (Fig. 15-24). The septa are lined by plump, cuboidal pneumocytes. Interstitial fibrosis, when present, is mild. Emphysema is often present.
DIP usually presents in the fourth or fifth decade of life, and is more common in men than in women by a ratio of 2 : 1. Virtually all patients are cigarette smokers. Presenting symptoms include an insidious onset of dyspnea and dry cough over weeks or months, often associated with clubbing of digits. Pulmonary function tests usually show a mild restrictive abnormality with a moderate reduction of the diffusing capacity of carbon dioxide. Patients with DIP typically have a good prognosis with close to 100% response to steroid therapy and cessation of smoking.65,98
Respiratory Bronchiolitis-Associated Interstitial Lung Disease
Respiratory bronchiolitis is a common histologic lesion found in cigarette smokers. It is characterized by the presence of pigmented intraluminal macrophages within first- and second-order respiratory bronchioles. In its mildest form, it is seen most often as an incidental histologic finding in the lungs of smokers or ex-smokers.99 The term respiratory bronchiolitis-associated interstitial lung disease is used for patients who develop significant pulmonary symptoms, abnormal pulmonary function, and imaging abnormalities.
Morphology. The changes are patchy at low magnification and have a bronchiolocentric distribution. Respiratory bronchioles, alveolar ducts, and peribronchiolar spaces contain aggregates of dusty brown macrophages (smokers’ macrophages) similar to those seen in DIP. There is a patchy submucosal and peribronchiolar infiltrate of lymphocytes and histiocytes. Mild peribronchiolar fibrosis is also seen, which expands contiguous alveolar septa. Centrilobular emphysema is common but not severe. Histologic overlap with DIP is often found in different parts of the same lung.
Symptoms are usually mild, consisting of gradual onset of dyspnea and cough in patients who are typically current smokers in the fourth or fifth decade of life with average exposures of over 30 pack-years of cigarette smoking. There is a 2 : 1 male predominance. Cessation of smoking usually results in improvement.
PULMONARY ALVEOLAR PROTEINOSIS
Pulmonary alveolar proteinosis (PAP) is a rare disease that is characterized radiologically by bilateral patchy asymmetric pulmonary opacifications and histologically by accumulation of acellular surfactant in the intra-alveolar and bronchiolar spaces. There are three distinct classes of this disease—acquired, congenital, and secondary PAP—each with a different pathogenesis but with a similar spectrum of histologic changes.
Acquired PAP represents 90% of all cases of PAP and lacks any familial predisposition. Unexpectedly, researchers working with knockout mice lacking the gene for the hematopoietic growth factor granulocyte-macrophage colony-stimulating factor (GM-CSF) found that these mice had impaired surfactant clearance by alveolar macrophages, leading to a condition that resembled human PAP. Subsequently, a GM-CSF–neutralizing autoantibody was found in the serum and bronchial fluid of individuals with acquired PAP that was not present in those with congenital or secondary PAP. Currently, it is thought that the anti–GM-CSF antibody is responsible for the development of the disease.100 These antibodies inhibit the activity of endogenous GM-CSF, leading to a state of functional GM-CSF deficiency. The systemic production of the antibody also provides an explanation for the recurrence of PAP following bilateral-lung transplantation. Thus, acquired PAP is an autoimmune disorder.
Congenital PAP is a rare cause of immediate-onset neonatal respiratory distress. Thus far, mutations have been identified in multiple genes including those encoding ATP-binding cassette protein member A3 (ABCA3) (which may be the most frequent), surfactant protein B (SP-B), surfactant protein C (SP-C), GM-CSF, and GM receptor (GM-CSF/IL-3/IL-5) β chain. ABCA3 is localized to the lamellar body membrane andis probably involved in transport of surfactant components.101 SP-B deficiency is transmitted in an autosomal recessive manner and is most often caused by a frameshift mutation in the SP-B gene. This leads to an unstable SP-B messenger RNA, reduced or absent SP-B, secondary disturbances of SP-C, and intra-alveolar accumulation of SP-A and SP-C.
Secondary PAP is uncommon. The underlying causes include hematopoietic disorders, malignancies, immunodeficiency disorders, lysinuric protein intolerance, and acute silicosis and other inhalational syndromes.
Morphology. The disease is characterized by a peculiar homogeneous, granular precipitate within the alveoli, causing focal-to-confluent consolidation of large areas of the lungs with minimal inflammatory reaction (Fig. 15-25). On section, turbid fluid exudes from these areas. As a consequence there is a marked increase in the size and weight of the lung. The alveolar precipitate is periodic acid–Schiff positive and also contains cholesterol clefts. Immunohistochemical stains show the presence of surfactant proteins A and C in congenital SP-B deficiency and all three proteins in the acquired form. Ultrastructurally, abnormalities in lamellar bodies in type II pneumocytes can be seen in mutations of SP-B, SP-C, and ABCA3.102
Adult patients, for the most part, present with nonspecific respiratory difficulty of insidious onset, cough, and abundant sputum that often contains chunks of gelatinous material. Some have symptoms lasting for years, often with febrile illnesses. These patients are at risk for developing secondary infections with a variety of organisms. Progressive dyspnea, cyanosis, and respiratory insufficiency may occur, but some patients tend to have a benign course, with eventual resolution of the lesions. Whole-lung lavage remains the current standard of care, while GM-CSF therapy is effective in 50% of patients.103
Congenital PAP is a fatal respiratory disorder that is usually immediately apparent in the newborn. Typically, the infant is full term and rapidly develops progressive respiratory distress shortly after birth. Without lung transplantation, death ensues between 3 and 6 months of age.
Diseases of Vascular Origin
PULMONARY EMBOLISM, HEMORRHAGE, AND INFARCTION
Blood clots that occlude the large pulmonary arteries are almost always embolic in origin. Large-vessel in situ thromboses are rare and develop only in the presence of pulmonary hypertension, pulmonary atherosclerosis, and heart failure. The usual source of pulmonary emboli—thrombi in the deep veins of the leg in more than 95% of cases—and the magnitude of the clinical problem were discussed in Chapter 4, in which the disturbing frequency of pulmonary embolism and infarction was emphasized. Pulmonary embolism causes more than 50,000 deaths in the United States each year. Its incidence at autopsy has varied from 1% in the general population of hospital patients to 30% in patients dying after severe burns, trauma, or fractures to 65% of hospitalized patients in one study in which special techniques were applied to discover emboli at autopsy. It is the sole or a major contributing cause of death in about 10% of adults who die acutely in hospitals.
Pulmonary embolism is a complication principally in patients who are already suffering from some underlying disorder, such as cardiac disease or cancer, or who are immobilized for several days or weeks, those with hip fracture being at high risk. Hypercoagulable states, either primary (e.g., factor V Leiden, prothrombin mutations, and antiphospholipid syndrome) or secondary (e.g., obesity, recent surgery, cancer, oral contraceptive use, pregnancy), are frequent risk factors. Indwelling central venous lines can be a nidus for right atrial thrombus, which can be a source of pulmonary embolism.
The pathophysiologic response and clinical significance of pulmonary embolism depend on the extent to which the pulmonary artery blood flow is obstructed, the size of the occluded vessel(s), the number of emboli, the overall status of the cardiovascular system, and the release of vasoactive factors such as thromboxane A2 from platelets that accumulate at the site of the thrombus. Emboli result in two main pathophysiologic consequences: respiratory compromise due to the nonperfused, though ventilated, segment and hemodynamic compromise due to increased resistance to pulmonary blood flow engendered by the embolic obstruction.
Morphology. Large emboli lodge in the main pulmonary artery or its major branches or at the bifurcation as a saddle embolus (Fig. 15-26). Sudden death often ensues, largely as a result of the blockage of blood flow through the lungs. Death may also be caused by acute failure of the right side of the heart (acute cor pulmonale). Smaller emboli travel out into the more peripheral vessels, where they may cause hemorrhage or infarction. In patients with adequate cardiovascular function, the bronchial arterial supply can sustain the lung parenchyma. Hemorrhages may occur, but there is no infarction. The underlyingpulmonary architecture is preserved, and resorption of the blood permits reconstitution of the preexisting architecture.
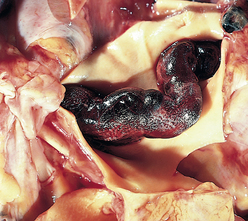
FIGURE 15-26 Large saddle embolus from the femoral vein lying astride the main left and right pulmonary arteries.
(From the teaching collection of the Department of Pathology, University of Texas Southwestern Medical School, Dallas, TX.)
Only about 10% of emboli actually cause infarction, which occurs when the circulation is already inadequate, as in patients with heart or lung disease. Thus, pulmonary infarcts tend to be uncommon in the young. About three fourths of all infarcts affect the lower lobes, and in more than half, multiple lesions occur. They vary in size from lesions that are barely visible to the naked eye to massive involvement of large parts of an entire lobe. Typically, they extend to the periphery of the lung substance as a wedge with the apex pointing toward the hilus of the lung. In many cases, an occluded vessel can be identified near the apex of the infarct. Pulmonary embolus can be distinguished from a post-mortem clot by the presence of the lines of Zahn in the thrombus (Chapter 4).
The pulmonary infarct is classically hemorrhagic and appears as a raised, red-blue area in the early stages (Fig. 15-27). Often, the apposed pleural surface is covered by a fibrinous exudate. The red cells begin to lyse within 48 hours, and the infarct becomes paler and eventually red-brown as hemosiderin is produced. With the passage of time, fibrous replacement begins at the margins as a gray-white peripheral zone and eventually converts the infarct into a contracted scar. Histologically, the diagnostic feature of acute pulmonary infarction is the ischemic necrosis of the lung substance within the area of hemorrhage, affecting the alveolar walls, bronchioles, and vessels. If the infarct is caused by an infected embolus, it is modified by a more intense neutrophilic inflammatory reaction. Such lesions are referred to as septic infarcts, and some convert to abscesses.
Clinical Course.
A large pulmonary embolus is one of the few causes of virtually instantaneous death. During cardiopulmonary resuscitation in such instances, the patient frequently is said to have electromechanical dissociation, in which the electrocardiogram has a rhythm but no pulses are palpated because no blood is entering the pulmonary arterial circulation. If the patient survives after a sizable pulmonary embolus, however, the clinical syndrome may mimic myocardial infarction, with severe chest pain, dyspnea, shock, fever, and increased levels of serum lactate dehydrogenase. Pulmonary hemorrhages due to small emboli induce only transient chest pain and cough. Infarcts manifest as dyspnea, tachypnea, fever, chest pain, cough, and hemoptysis. An overlying fibrinous pleuritis may produce a pleural friction rub.
Findings on chest radiograph are variable and can be normal or disclose a pulmonary infarct, usually 12 to 36 hours after it has occurred, as a wedge-shaped infiltrate. The diagnosis of pulmonary embolism is usually made with spiral computed tomographic angiography. Rarely, other diagnostic methods, such as ventilation perfusion scanning or pulmonary angiography are required. Alternatively, deep vein thrombosis can be diagnosed with duplex ultrasonography. After the initial acute insult, emboli often resolve via contraction and fibrinolysis, particularly in the relatively young. Unresolved, multiple small emboli over the course of time may lead to pulmonary hypertension, pulmonary vascular sclerosis, and chronic cor pulmonale. Perhaps most important is that a small embolus may presage a larger one. In the presence of an underlying predisposing condition, patients with a pulmonary embolus have a 30% chance of developing a second embolus. Repeated emboli can result in pulmonary arterial hypertension.
Prevention of pulmonary embolism constitutes a major clinical problem for which there is no easy solution. Prophylactic therapy includes early ambulation in postoperative and postpartum patients, elastic stockings and graduated compression stockings for bedridden patients, and anticoagulation in high-risk individuals. It is sometimes necessary to resort to insertion of a filter (“umbrella”) into the inferior vena cava or to ligation of this vein, which are not minor procedures in an already seriously ill patient. Treatment of existing pulmonary embolism often includes anticoagulation, preceded by thrombolysis in some cases.
PULMONARY HYPERTENSION
The pulmonary circulation is normally one of low resistance, and pulmonary blood pressure is only about one eighth of systemic blood pressure. Pulmonary hypertension (PH) occurs when mean pulmonary pressure reaches one fourth of systemic levels. The clinical classification of PH groups entities that share similarities in pathophysiologic mechanisms, clinical presentation, and therapeutic options. The groups are (1) pulmonary arterial hypertension, (2) PH with left heart disease, (3) PH associated with lung diseases and/or hypoxemia, (4) PH due to chronic thrombotic and/or embolic disease, and (5) miscellaneous PH.104
PH is most frequently associated with structural cardiopulmonary conditions that increase pulmonary blood flow or pressure (or both), pulmonary vascular resistance, or left heart resistance to blood flow. These include the following:
Uncommonly, PH is encountered sporadically in patients in whom all known causes of increased pulmonary pressure are excluded; this is referred to as idiopathic pulmonary arterial hypertension. Even less common is the familial form of pulmonary arterial hypertension with autosomal dominant mode of inheritance. Within these families, there is incomplete penetrance, and only 10% to 20% of the family members actually develop overt disease.
Pathogenesis.
As is often the case, much has been learned about the pathogenesis of PH by investigating the molecular basis of the uncommon familial form of the disease. These studies have revealed that familial PH is caused by mutations in the bone morphogenetic protein receptor type 2 (BMPR2) signaling pathway.105
To understand how such a mutation causes PH, it is essential to review the vascular pathology of the disease and to understand the physiologic functions of BMPR2 signaling. PH is associated with obstruction to the vasculature caused by proliferation of endothelial, smooth muscle, and intimal cells accompanied by concentric laminar intimal fibrosis. How does BMPR2 cause these changes?
BMPR2 is a cell surface protein belonging to the TGF-β receptor superfamily, which binds a variety of cytokines, including TGF-β, bone morphogenetic protein (BMP), activin, and inhibin. Although originally described in the context of bone growth, BMP-BMPR2 signaling is now known to be important for embryogenesis, apoptosis, and cell proliferation and differentiation. The specific effects depend on the tissue and its microenvironment. In vascular smooth muscle cells BMPR2 signaling causes inhibition of proliferation and favors apoptosis. Thus, in the absence of such signaling, increased smooth muscle survival and proliferation may be expected. In keeping with this, inactivating germline mutations in the BMPR2 gene are found in 50% of the familial cases of pulmonary arterial hypertension and 25% of sporadic cases. In many families, even without mutations in the coding regions of the BMPR2 gene, linkage to the BMPR2 locus on chromosome 2q33 can be established, thus indicating that other possible lesions such as gene rearrangements, large deletions, or insertions could be involved.
Despite these discoveries, several questions remain unanswered. First, how does loss of a single allele of the BMPR2 gene lead to complete loss of signaling? Two possibilities exist: the mutation might act as a dominant negative (Chapter 5), or a secondary loss of the normal allele might occur in the vascular wall, thus leading to a homozygous loss of BMPR2. This is reminiscent of how germline mutations in tumor suppressor genes give rise to neoplasia. Interestingly, in some studies microsatellite instability has been reported in the proliferating endothelial cells within the vascular lesions. This could be a mechanism by which the normal allele is lost in the vasculature. Note that a similar mechanism can inactivate TGF-β receptors in hereditary nonpolyposis colon cancer (Chapters 7 and 17. The second unanswered question is why the phenotypic disease occurs only in 10% to 20% of individuals with BMPR2 mutations. This strongly points toward the existence of modifier genes and/or environmental triggers. Among the modifier genes are those that control vascular tone, including endothelin, prostacyclin synthetase, and angiotensinconverting enzymes. The nature of the environmental factors remains unknown, but presumably they cause dysfunction of vasoregulatory mechanisms. Thus, as with tumor suppressor genes, a two-hit model has been proposed whereby a genetically susceptible individual with a BMPR2 mutation requires additional genetic or environmental insults to develop the disease (Fig. 15-28).
In secondary forms of PH, endothelial cell dysfunction is produced by the process that initiates the disorder, such as the increased shear and mechanical injury associated with leftto-right shunts or the biochemical injury produced by fibrin in thromboembolism. Decreased elaboration of prostacyclin, decreased production of nitric oxide, and increased release of endothelin all promote pulmonary vasoconstriction. Also, decreased elaboration of prostacyclin and nitric oxide promotes platelet adhesion and activation. Moreover, endothelial activation, as detailed in Chapter 11, makes endothelial cells thrombogenic and promotes the persistence of fibrin. Finally, production and release of growth factors and cytokines induce the migration and replication of vascular smooth muscle cells and elaboration of extracellular matrix.
Some individuals with PH have a vasospastic component; in such patients, pulmonary vascular resistance can be rapidly decreased with vasodilators. Pulmonary arterial hypertension has also been reported after ingestion of certain plants ormedicines, including the leguminous plant Crotalaria spectabilis, which is indigenous to the tropics and used medicinally in bush tea; the appetite depressant aminorex; adulterated olive oil; and the anti-obesity drugs fenfluramine and phentermine.106 It has been suggested that such substances might act through effects on serotonin transporter expression or activity.
Morphology. All forms of PH have some common pathologic features regardless of their etiology, that is, medial hypertrophy of muscular and elastic arteries, atheromas of pulmonary artery and its major branches, and right ventricular hypertrophy.107 The presence of many organizing or recanalized thrombi favors recurrent pulmonary emboli as the cause, and the coexistence of diffuse pulmonary fibrosis, or severe emphysema and chronic bronchitis, points to chronic hypoxia as the initiating event. The vessel changes can involve the entire arterial tree, from the main pulmonary arteries down to the arterioles (Fig. 15-29). In the most severe cases, atheromatous deposits form in the pulmonary artery and its major branches, resembling (but lesser in degree than) systemic atherosclerosis. The arterioles and small arteries (40 to 300 μm in diameter) are most prominently affected, with striking increases in the muscular thickness of the media (medial hypertrophy) and intimal fibrosis, sometimes narrowing the lumens to pinpoint channels. One extreme in the spectrum of pathologic changes, present most prominently in idiopathic and familial pulmonary arterial hypertension, unrepaired congenital heart disease with left-to-right shunts, and PH associated with drugs and HIV, is the plexiform lesion, so called because a tuft of capillary formations is present, producing a network, or web, that spans the lumens of dilated thin-walled, small arteries and may extend outside the vessel. Dilated vessels and arteritis may also be present. Rarely, extensive occlusion of the veins by fibrous tissue can be the cause of PH.108
Clinical Course.
Idiopathic PH is most common in women who are 20 to 40 years of age and is also seen occasionally in young children. Clinical signs and symptoms of all forms of hypertension become evident only with advanced disease. In cases of idiopathic disease, the presenting features are usually dyspnea and fatigue, but some patients have chest pain of the anginal type. Over time, severe respiratory distress, cyanosis, and right ventricular hypertrophy occur, and death from decompensated cor pulmonale, often with superimposed thromboembolism and pneumonia, usually ensues within 2 to 5 years in 80% of patients.109
Conventional therapies (oxygen supplementation, calcium channel blockers, anticoagulation, digoxin, and diuretics) are helpful in the short course.110 However, recently developed specific therapies such as prostacyclin analogues, endothelial receptor antagonists, inhaled nitric oxide, and phosphodiesterase-5 inhibitors111-113 have improved the outcome in many patients. Lung transplantation provides definitive treatment for selected patients. Gene therapy has been successful in animals and may be possible for humans in the future.114
DIFFUSE PULMONARY HEMORRHAGE SYNDROMES
Hemorrhage from the lung is a dramatic complication of some interstitial lung disorders.115,116 Among these so-called pulmonary hemorrhage syndromes (Fig. 15-30) are (1) Goodpasture syndrome, (2) idiopathic pulmonary hemosiderosis, and (3) vasculitis-associated hemorrhage, which is found in conditions such as hypersensitivity angiitis, Wegener granulomatosis, and lupus erythematosus (Chapter 11).
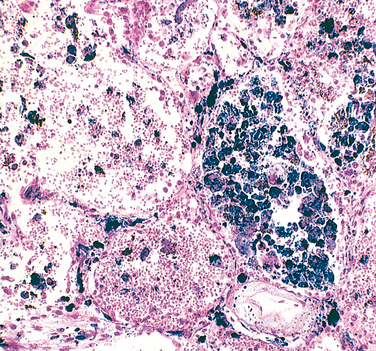
FIGURE 15-30 Diffuse pulmonary hemorrhage syndrome. Acute intra-alveolar hemorrhage and hemosiderin-laden macrophages, reflecting previous hemorrhage, are common features (Prussian blue stain for iron).
Goodpasture Syndrome
Goodpasture syndrome is an uncommon autoimmune disease in which kidney and lung injury are caused by circulating autoantibodies against the noncollagenous domain of the α3 chain of collagen IV. The antibodies initiate inflammatory destruction of the basement membrane in renal glomeruli and pulmonary alveoli,117 giving rise to proliferative, usually rapidly progressive glomerulonephritis and a necrotizing hemorrhagic interstitial pneumonitis. Most cases occur in the teens or 20s, and in contrast to many other autoimmune diseases, there is a male preponderance. In one study 89% of patients were active smokers.118
Pathogenesis.
The immunopathogenesis of the syndrome and the nature of the Goodpasture antigens are described in Chapter 20. The trigger that initiates the anti–basement membrane antibodies is still unknown. Since the epitopes that evoke anti-collagen antibodies are normally hidden within the molecule, it is presumed that some environmental insult such as viral infection, exposure to hydrocarbon solvents (used in the dry cleaning industry), or smoking is required to unmask the cryptic epitopes. As in other autoimmune disorders, a genetic predisposition is indicated by association with certain HLA subtypes (e.g., HLA-DRB1*1501 and *1502).115
Morphology. In the classic case, the lungs are heavy, with areas of red-brown consolidation. Histologically, there is focal necrosis of alveolar walls associated with intra-alveolar hemorrhages. Often the alveoli contain hemosiderin-laden macrophages (see Fig. 15-30). In later stages there may be fibrous thickening of the septae, hypertrophy of type II pneumocytes, and organization of blood in alveolar spaces. In many cases immunofluorescence studies reveal linear deposits of immunoglobulins along the basement membranes of the septal walls. The kidneys have the characteristic findings of focal proliferative glomerulonephritis in early cases or crescentic glomerulonephritis in patients with rapidly progressive glomerulonephritis. Diagnostic linear deposits of immunoglobulins and complement are seen by immunofluorescence studies along the glomerular basement membranes even in the few patients without renal disease.
Clinical Features.
Most cases begin clinically with respiratory symptoms, principally hemoptysis, and radiographic evidence of focal pulmonary consolidations. Soon, manifestations of glomerulonephritis appear, leading to rapidly progressive renal failure. The most common cause of death is uremia. The once dismal prognosis for this disease has been markedly improved by intensive plasmapheresis. This procedure is thought to be beneficial by removing circulating anti–basement membrane antibodies as well as chemical mediators of immunological injury. Simultaneous immunosuppressive therapy inhibits further antibody production, ameliorating both lung hemorrhage and glomerulonephritis.
Idiopathic Pulmonary Hemosiderosis
Idiopathic pulmonary hemosiderosis is a rare disorder characterized by intermittent, diffuse alveolar hemorrhage. Most cases occur in young children, although the disease has been reported in adults as well.119 It usually presents with an insidious onset of productive cough, hemoptysis, anemia, and weight loss associated with diffuse pulmonary infiltrations similar to Goodpasture syndrome.
The cause and pathogenesis are unknown, and no anti–basement membrane antibodies are detectable in serum or tissues. However, favorable response to long-term immunosuppression with prednisone and/or azathioprine indicates that an immunological mechanism could be involved in the pulmonary capillary damage underlying alveolar bleeding. In addition, long-term follow-up of patients shows that some of them develop other immune disorders.120
Wegener Granulomatosis
This autoimmune disease most often involves the upper respiratory tract and/or the lungs, with hemoptysis being the common presenting symptom. Its features are discussed in Chapter 11. Here, it is enough to emphasize that a transbronchial lung biopsy might provide the only tissue available for diagnosis. Since the amount of tissue is small, necrosis and granulomatous vasculitis might not be present. Rather, the diagnostically important features are capillaritis and scattered, poorly formed granulomas (unlike those of sarcoidosis, which are rounded and well-defined).