Chapter 21 The Lower Urinary Tract and Male Genital System
THE LOWER URINARY TRACT
Despite differing embryonic origins, the various components of the lower urinary tract have many morphologic similarities. The renal pelves, ureters, bladder, and urethra (save for its terminal portion) are lined by a special form of transitional epithelium (urothelium). The surface layer consists of large, flattened “umbrella cells” with abundant cytoplasm that horizontally cover several underlying cells. The umbrella cells have a trilaminar asymmetric unit membrane and possess apical plaques composed of specific proteins called uroplakins. The underlying urothelium is composed of several layers of cells with oval smaller nuclei often with linear nuclear grooves and less cytoplasm. This epithelium rests on a well-developed basement membrane, beneath which is a lamina propria. The lamina propria in the urinary bladder contains wisps of smooth muscle that form a discontinuous muscularis mucosae. It is important to differentiate the muscularis mucosae from the deeper well-defined larger muscle bundles of the detrusor muscle (muscularis propria), since bladder cancers are staged on the basis of invasion of the latter. The bladder musculature is capable, with obstruction to the flow of urine, of great thickening.
The ureters lie throughout their course in a retroperitoneal position. Retroperitoneal tumors or fibrosis may trap the ureters in neoplastic or dense, fibrous tissue, sometimes obstructing them. As ureters enter the pelvis, they pass anterior to either the common iliac or the external iliac artery. In the female pelvis they lie close to the uterine arteries and are therefore vulnerable to injury in operations on the female genital tract. There are three points of slight narrowing—at the ureteropelvic junction, where they enter the bladder, and where they cross the iliac vessels—all providing loci where renal calculi may become impacted when they pass from the kidney to the bladder. As the ureters enter the bladder they pursue an oblique course, terminating in a slitlike orifice. The obliquity of this intramural segment of the ureteral orifice permits the enclosing bladder musculature to act like a sphincteric valve, blocking the upward reflux of urine even in the presence of marked distention of the urinary bladder. As discussed in Chapter 20, a defect in the intravesical portion of the ureter leads to vesicoureteral reflux.
The close relationship of the female genital tract to the bladder makes possible the spread of disease from one tract to the other. In middle-aged and elderly women, relaxation of pelvic support leads to prolapse (descent) of the uterus, pulling with it the floor of the bladder. In this fashion the bladder is protruded into the vagina, creating a pouch (cystocele) that fails to empty readily with micturition. In males the seminal vesicles and prostate have similar close relationships, being situated just posterior and inferior to the neck of the bladder. Thus, enlargement of the prostate, so common in middle to later life, constitutes an important cause of urinary tract obstruction. In the subsequent sections we discuss the major pathologic lesions in the ureters, urinary bladder, and urethra separately.
Ureters
CONGENITAL ANOMALIES
Congenital anomalies of the ureters are found in about 2% or 3% of all autopsies. Although most have little clinical significance, certain anomalies may contribute to obstruction to the flow of urine and thus cause clinical disease. Anomalies of the ureterovesical junction that potentiate reflux are discussed with pyelonephritis in Chapter 20.
Double and bifid ureters. Double ureters are almost invariably associated either with totally distinct double renal pelves or with the anomalous development of a large kidney having a partially bifid pelvis terminating in separate ureters. Double ureters may pursue separate courses to the bladder but commonly are joined within the bladder wall and drain through a single ureteral orifice. The majority of double ureters are unilateral and of no clinical significance.
Ureteropelvic junction (UPJ) obstruction, a congenital disorder, results in hydronephrosis. It usually presents in infants or children, much more commonly in boys. However, it is bilateral in 20% of cases and may be associated with other congenital anomalies. It is the most common cause of hydronephrosis in infants and children. In adults, UPJ obstruction is more common in women and is most often unilateral. The condition has been ascribed to abnormal organization of smooth muscle bundles at the UPJ, to excess stromal deposition of collagen between smooth muscle bundles, or rarely to congenitally extrinsic compression by polar renal vessels. There is agenesis of the kidney on the opposite side in a significant number of cases, probably resulting from obstructive lesions in utero.
Diverticula, saccular outpouchings of the ureteral wall, are uncommon lesions that are usually asymptomatic and are found on imaging studies. They appear as congenital or acquired defects and are of importance as pockets of stasis and secondary infections. Dilation (hydroureter), elongation, and tortuosity of the ureters may occur as congenital anomalies or as acquired defects.
INFLAMMATION
Ureteritis, though associated with inflammation, is typically not associated with infection and is of little clinical consequence.
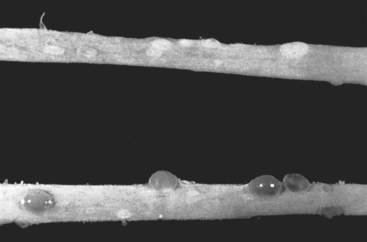
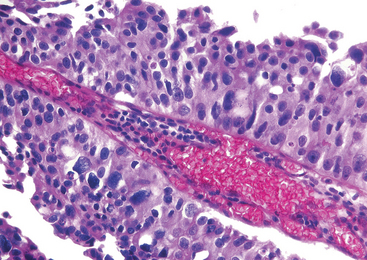
Morphology. The accumulation or aggregation of lymphocytes forming germinal centers in the subepithelial region may cause slight elevations of the mucosa and produce a fine granular mucosal surface (ureteritis follicularis). At other times the mucosa may become sprinkled with fine cysts varying in diameter from 1 to 5 mm lined by flattened urothelium (ureteritis cystica) (Fig. 21-1).
TUMORS AND TUMOR-LIKE LESIONS
Primary tumors of the ureter are rare. Small benign tumors of the ureter are generally of mesenchymal origin. Fibroepithelial polyp is a tumor-like lesion that grossly presents as a small mass projecting into the lumen, often in children. The lesion occurs more commonly in the ureters but may also appear in the bladder, renal pelves, and urethra. The polyp is composed of a loose, vascularized connective tissue mass lying beneath the mucosa.
Primary malignant tumors of the ureter resemble those arising in the renal pelvis, calyces, and bladder. The majority are urothelial carcinomas (Fig. 21-2). They are found most frequently during the sixth and seventh decades of life and cause obstruction of the ureteral lumen. They are sometimes multiple and commonly occur concurrently with similar neoplasms in the bladder or renal pelvis.
OBSTRUCTIVE LESIONS
A great variety of pathologic lesions may obstruct the ureters and give rise to hydroureter, hydronephrosis, and sometimes pyelonephritis (Chapter 20). It is not the ureteral dilation that is of significance in these cases, but the consequent involvement of the kidneys. The more important causes, divided into those of intrinsic or extrinsic origin, are listed in Table 21-1. Unilateral obstruction typically results from proximal causes, whereas bilateral obstruction arises from distal causes, such as nodular hyperplasia of the prostate. Only sclerosing retroperitoneal fibrosis is discussed further.
TABLE 21-1 Major Causes of Ureteral Obstruction
| Type of Obstruction | Cause |
|---|---|
| INTRINSIC | |
| Calculi | Of renal origin, rarely more than 5 mm in diameter |
| Larger renal stones cannot enter ureters | |
| Impact at loci of ureteral narrowing—ureteropelvic junction, where ureters cross iliac vessels, and where they enter bladder—and cause excruciating “renal colic” | |
| Strictures | Congenital or acquired (inflammations) |
| Tumors | Transitional cell carcinomas arising in ureters |
| Rarely, benign tumors or fibroepithelial polyps | |
| Blood clots | Massive hematuria from renal calculi, tumors, or papillary necrosis |
| Neurogenic | Interruption of the neural pathways to the bladder |
| EXTRINSIC | |
| Pregnancy | Physiologic relaxation of smooth muscle or pressure on ureters at pelvic brim from enlarging fundus |
| Periureteral inflammation | Salpingitis, diverticulitis, peritonitis, sclerosing retroperitoneal fibrosis |
| Endometriosis | With pelvic lesions, followed by scarring |
| Tumors | Cancers of the rectum, bladder, prostate, ovaries, uterus, cervix; lymphomas, sarcomas |
Sclerosing Retroperitoneal Fibrosis.
This refers to an uncommon cause of ureteral narrowing or obstruction characterized by a fibrous proliferative inflammatory process encasing the retroperitoneal structures and causing hydronephrosis.1 The disorder occurs in middle to late age. In some cases specific causes can be identified, such as drugs (ergot derivatives, β-adrenergic blockers), adjacent inflammatory conditions (vasculitis, diverticulitis, Crohn disease), or malignant disease (lymphomas, urinary tract carcinomas). However, 70% of cases have no obvious cause and are considered primary or idiopathic (Ormond disease). Several cases have been reported with similar fibrotic changes in other sites (such as mediastinal fibrosis, sclerosing cholangitis, and Riedel fibrosing thyroiditis), suggesting that the disorder is systemic in distribution but preferentially involves the retroperitoneum. Thus, an autoimmune reaction, sometimes triggered by drugs, has been proposed as the immediate cause of the systemic disease.
On microscopic examination the inflammatory fibrosis is marked by a prominent infiltrate of lymphocytes, often with germinal centers, plasma cells, and eosinophils. Treatment involves surgical extrication of the ureters from the surrounding fibrous tissue (ureterolysis).
Urinary Bladder
Diseases of the bladder, particularly inflammation (cystitis), constitute an important source of clinical signs and symptoms. Usually, however, these disorders are more disabling than lethal. Cystitis is particularly common in young women of reproductive age. Tumors of the bladder are an important source of both morbidity and mortality.
CONGENITAL ANOMALIES
Diverticula.
A bladder or vesical diverticulum consists of a pouchlike evagination of the bladder wall. Diverticula may arise as congenital defects but more commonly are acquired lesions caused by persistent urethral obstruction.
The congenital form may be due to a focal failure of development of the normal musculature or to some urinary tract obstruction during fetal development. Acquired diverticula are most often seen with prostatic enlargement (hyperplasia or neoplasia), producing obstruction to urine outflow and marked muscle thickening of the bladder wall. The increased intravesical pressure causes outpouching of the bladder wall and the formation of diverticula. They are frequently multiple and have narrow necks located between the interweaving hypertrophied muscle bundles. In both the congenital and the acquired forms, the diverticulum usually consists of a round to ovoid, saclike pouch that varies from less than 1 cm to 5 to 10 cm in diameter.
Although most diverticula are small and asymptomatic, they may be clinically significant, since they constitute sites of urinary stasis and predispose to infection and the formation of bladder calculi. They may also predispose to vesicoureteral reflux as a result of impingement on the ureter. Rarely, carcinomas may arise in bladder diverticuli. When invasive cancers arise in diverticula, they tend to be more advanced in stage as a result of the thin or absent muscle wall of a diverticulum.
Exstrophy.
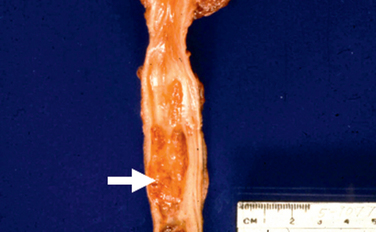
Exstrophy of the bladder is a developmental failure in the anterior wall of the abdomen and the bladder, so that the bladder either communicates directly through a large defect with the surface of the body or lies as an opened sac (Fig. 21-3). The exposed bladder mucosa may undergo colonic glandular metaplasia and is subject to infections that often spread to upper levels of the urinary system. Patients have an increased risk of adenocarcinoma arising in the bladder remnant.2 These lesions are amenable to surgical correction, and long-term survival is possible.
Miscellaneous Anomalies.
Vesicoureteral reflux is the most common and serious anomaly. As a major contributor to renal infection and scarring, it was discussed earlier, in Chapter 20, in the consideration of pyelonephritis. Abnormal connections between the bladder and the vagina, rectum, or uterus may create congenital vesicouterine fistulas.
Rarely, the urachus (the canal that connects the fetal bladder with the allantois) may remain patent in part or in whole. When totally patent, a fistulous urinary tract is created that connects the bladder with the umbilicus. At times, only the central region of the urachus persists, giving rise to urachal cysts, lined by either urothelium or metaplastic glandular epithelium. Carcinomas, mostly glandular tumors, may arise from such cysts (see “Neoplasms”). These account for only a minority of all bladder cancers (0.1% to 0.3%) but 20% to 40% of bladder adenocarcinomas.
INFLAMMATION
Acute and Chronic Cystitis
The pathogenesis of cystitis and the common bacterial etiologic agents are discussed in Chapter 20 in the consideration of urinary tract infections. As emphasized earlier, bacterial pyelonephritis is frequently preceded by infection of the urinary bladder, with retrograde spread of microorganisms into the kidneys and their collecting systems. The common etiologic agents of cystitis are the coliforms: Escherichia coli, followed by Proteus, Klebsiella, and Enterobacter. Women are more likely to develop cystitis as a result of their shorter urethras. Tuberculous cystitis is almost always a sequel to renal tuberculosis. Candida albicans and, much less often, cryptococcal agents cause cystitis, particularly in immunosuppressed patients or those receiving long-term antibiotics. Schistosomiasis (Schistosoma haematobium) is rare in the United States but is common in certain Middle Eastern countries, notably Egypt. Viruses (e.g., adenovirus), Chlamydia, and Mycoplasma may also cause cystitis. Predisposing factors include bladder calculi, urinary obstruction, diabetes mellitus, instrumentation, and immune deficiency. Finally, irradiation of the bladder region gives rise to radiation cystitis.
Morphology. Most cases of cystitis take the form of nonspecific acute or chronic inflammation of the bladder. In gross appearance there is hyperemia of the mucosa, sometimes associated with exudate. Patients receiving cytotoxic antitumor drugs, such as cyclophosphamide, may develop hemorrhagic cystitis.3 Adenovirus infection also causes a hemorrhagic cystitis.
Persistence of the infection leads to chronic cystitis, which differs from the acute form only in the character of the inflammatory infiltrate. Follicular cystitis, characterized by the aggregation of lymphocytes into lymphoid follicles within the bladder mucosa and underlying wall, is not necessarily associated with infection. Eosinophilic cystitis, manifested by infiltration with submucosal eosinophils, typically also represents nonspecific subacute inflammation, although rarely it is a manifestation of a systemic allergic disorder. The ubiquitous presence of mild chronic inflammation in the bladder unaccompanied by clinical symptoms should not be given the diagnosis of chronic cystitis.
All forms of cystitis are characterized by a triad of symptoms: (1) frequency, which in acute cases may necessitate urination every 15 to 20 minutes; (2) lower abdominal pain localized over the bladder region or in the suprapubic region; and (3) dysuria—pain or burning on urination.
The local symptoms of cystitis may be disturbing, but these infections are also important as antecedents to pyelonephritis. Cystitis is sometimes a secondary complication of some underlying disorder such as prostatic enlargement, cystocele of the bladder, calculi, or tumors. These primary diseases must be corrected before the cystitis can be relieved.
Special Forms of Cystitis
Several variants of cystitis are distinctive by their morphologic appearance or causation.
Interstitial Cystitis (Chronic Pelvic Pain Syndrome).
This is a persistent, painful form of chronic cystitis occurring most frequently in women.4 It is characterized clinically by intermittent, often severe suprapubic pain, urinary frequency, urgency, hematuria and dysuria without evidence of bacterial infection, and cystoscopic findings of fissures and punctate hemorrhages (glomerulations) in the bladder mucosa after luminal distention. Some but not all patients show morphologic features of chronic mucosal ulcers (Hunner ulcers); this is termed the late (classic, ulcerative) phase. Although mast cells are characteristic of this disease, there is no uniformity in the literature about their specificity and diagnostic utility. Late in the disease, transmural fibrosis may ensue, leading to a contracted bladder. The major role of biopsy is not to specifically diagnose the disease as much as it is to rule out carcinoma in situ, which may mimic interstitial cystitis clinically. Its etiology is unknown, its evaluation and diagnosis remain controversial, and its treatment is largely empiric.5
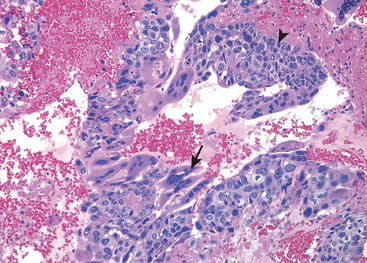
Malacoplakia.
This designation refers to a peculiar pattern of vesical inflammatory reaction characterized macroscopically by soft, yellow, slightly raised mucosal plaques 3 to 4 cm in diameter (Fig. 21-4), and histologically by infiltration with large, foamy macrophages mixed with occasional multinucleate giant cells and interspersed lymphocytes.6 The macrophages have an abundant granular cytoplasm due to phagosomes stuffed with particulate and membranous debris of bacterial origin. In addition, laminated mineralized concretions resulting from deposition of calcium in enlarged lysosomes, known as Michaelis-Gutmann bodies, are typically present within the macrophages (Fig. 21-5). Similar lesions have been described in the colon, lungs, bones, kidneys, prostate, and epididymis.
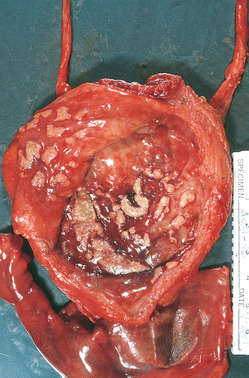
FIGURE 21-4 Cystitis with malacoplakia of bladder showing inflammatory exudate and broad, flat plaques.
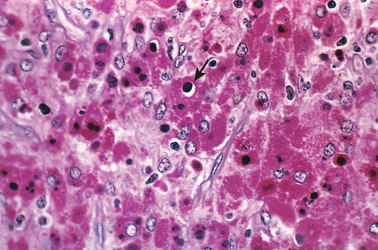
FIGURE 21-5 Malacoplakia, periodic acid–Schiff (PAS) stain. Note the large macrophages with granular PAS-positive cytoplasm and several dense, round Michaelis-Gutmann bodies surrounded by artifactual cleared holes in the upper middle field (arrow).
Malacoplakia is clearly related to chronic bacterial infection, mostly by E. coli or occasionally Proteus species. It occurs with increased frequency in immunosuppressed transplant recipients. The unusual-appearing macrophages and giant phagosomes point to defects in phagocytic or degradative function of macrophages, such that phagosomes become overloaded with undigested bacterial products.
Polypoid Cystitis.
Polypoid cystitis is an inflammatory condition resulting from irritation to the bladder mucosa.7,8 Although indwelling catheters are the most commonly cited culprits, any injurious agent may give rise to this lesion. The urothelium is thrown into broad bulbous polypoid projections as a result of marked submucosal edema. Polypoid cystitis may be confused with papillary urothelial carcinoma both clinically and histologically.
METAPLASIC LESIONS
Cystitis Glandularis and Cystitis Cystica.
These terms refer to common lesions of the urinary bladder in which nests of urothelium (Brunn nests) grow downward into the lamina propria and undergo transformation of their central epithelial cells into cuboidal or columnar epithelium lining (cystitis glandularis) or cystic spaces filled with clear fluid lined by flattened urothelium (cystitis cystica). Because the two processes often coexist, the condition is typically referred to as cystitis cystica et glandularis. In a variant of cystitis glandularis goblet cells are present, and the epithelium resembles intestinal mucosa (intestinal or colonic metaplasia). Both variants are common microscopic incidental findings in relatively normal bladders, although they can also arise from inflammation and metaplasia. In contrast to earlier reports, lesions showing extensive intestinal metaplasia are not associated with an increased risk for the development of adenocarcinoma (except when associated with exstrophy).9
Squamous Metaplasia.
As a response to injury, the urothelium is often replaced by squamous epithelium, which is a more durable lining. This should be distinguished from glycogenated squamous epithelium that is normally found in women at the trigone.
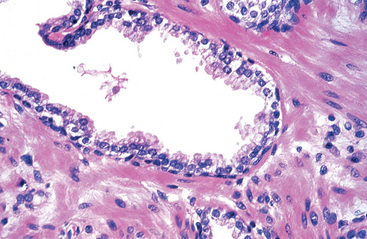
Nephrogenic Adenoma.
Nephrogenic adenoma is an unusual lesion that in the past was believed to represent metaplasia of the urothelium in response to injury.10,11 It has now been demonstrated to result from shed renal tubular cells that implant in sites of injured urothelium.12 The term nephrogenic adenoma was originally given because the lesion resembles renal tubules histologically, but the term also reflects the pathogenesis of the lesion. The overlying urothelium may be focally replaced by cuboidal epithelium, which can assume a papillary growth pattern. In addition, a tubular proliferation in the underlying lamina propria and superficial detrusor muscle can mimic a malignant process.13 Although typically less than a centimeter, lesions may be sizable, and may resemble cancer clinically.
NEOPLASMS
Bladder cancer accounts for approximately 7% of cancers and 3% of cancer mortality in the United States.14 About 95% of bladder tumors are of epithelial origin, the remainder being mesenchymal tumors (Table 21-2). Most epithelial tumors are composed of urothelial (transitional cell) type and are thus interchangeably called urothelial or transitional tumors, but squamous and glandular carcinomas also occur. Here we focus on urothelial tumors and touch briefly on the others.
TABLE 21-2 Tumors of the Urinary Bladder
| Mixed carcinoma |
| Adenocarcinoma |
| Small-cell carcinoma |
| Sarcomas |
Urothelial Tumors
Urothelial tumors represent about 90% of all bladder tumors and run the gamut from small benign lesions that may never recur to aggressive cancers associated with a high risk of death. Many of these tumors are multifocal at presentation. Though most commonly seen in the bladder, any of the urothelial lesions described below may be seen at any site where there is urothelium, from the renal pelvis to the distal urethra.
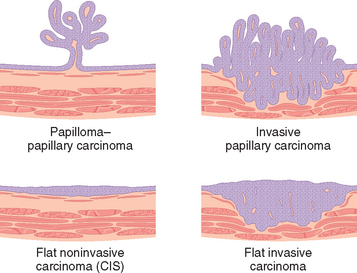
There are two distinct precursor lesions to invasive urothelial carcinoma: non-invasive papillary tumors, and flat non-invasive urothelial carcinoma. The most common precursor lesions are the non-invasive papillary tumors, which originate from papillary urothelial hyperplasia.15 These tumors have a range of atypical changes, and are graded according to their biological behavior. The other precursor lesion to invasive carcinoma, flat non-invasive urothelial carcinoma is referred to as carcinoma in situ or CIS. As discussed in Chapter 7, CIS is a histologic term used to describe epithelial lesions that have cytologic changes of malignancy, but are confined to the epithelium, without basement membrane invasion.16 Such lesions are considered to be high grade. In about one half of individuals with invasive bladder cancer, the tumor has already invaded the bladder wall, at the time of presentation, and no precursor lesions may be detected. It is presumed that the precursor lesion has been destroyed by the high-grade invasive component, which typically appears as a large frequently ulcerated mass. Although invasion into the lamina propria worsens the prognosis, the major decrease in survival is associated with invasion of the muscularis propria (detrusor muscle). Once muscularis propria invasion occurs, there is a 30% 5-year mortality rate.
In Table 21-3, we have listed two of the most common grading systems of these tumors. The World Health Organization (WHO) 1973 classification grades tumors into a rare totally benign papilloma and three grades of transitional cell carcinoma (grades I, II, and III). A more recent classification, based on a consensus reached at a conference by the International Society of Urological Pathology (ISUP) in 1998 and adopted by the WHO in 2004, recognizes a rare benign papilloma, a group of papillary urothelial neoplasms of low malignant potential, and two grades of carcinoma (low and high grade).16,17
TABLE 21-3 Grading of Urothelial (Transitional Cell) Tumors
| WHO/ISUP Grades |
| WHO Grades |
WHO, World Health Organization; ISUP, International Society of Urological Pathology.
Morphology. The gross patterns of urothelial tumors vary from purely papillary to nodular or flat (Fig. 21-6). Papillary lesions appear as red, elevated excrescences varying in size from less than 1 cm in diameter to large masses up to 5 cm in diameter (Fig. 21-7). Multicentric origins may produce separate tumors. As noted, the histologic changes encompass a spectrum from benign papilloma to highly aggressive anaplastic cancers. Overall, the majority of papillary tumors are low grade. Most arise from the lateral or posterior walls at the bladder base.
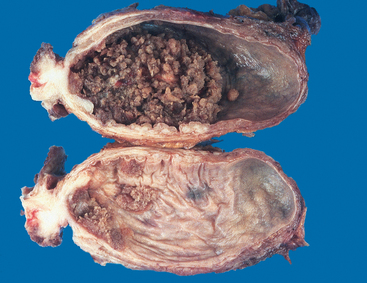
FIGURE 21-7 Cross-section of bladder with upper section showing a large papillary tumor. The lower section demonstrates multifocal smaller papillary neoplasms.
(Courtesy of Dr. Fred Gilkey, Sinai Hospital, Baltimore, MD.)
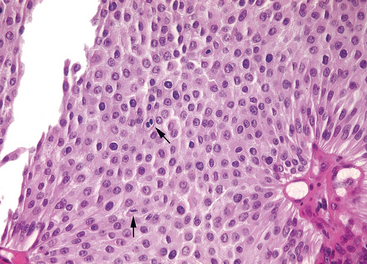
FIGURE 21-9 Low-grade papillary urothelial carcinoma with an overall orderly appearance, with a thicker lining than papilloma and scattered hyperchromatic nuclei and mitotic figures (arrows).
In most analyses, less than 10% of low-grade cancers invade, but as many as 80% of high-grade urothelial carcinomas are invasive.21,22 Aggressive tumors may extend not only into the bladder wall, but, in more advanced stages, invade the adjacent prostate, seminal vesicles, ureters, and retroperitoneum. Some tumors produce fistulous communications to the vagina or rectum. About 40% of these deeply invasive tumors metastasize to regional lymph nodes. Hematogenous dissemination, principally to the liver, lungs, and bone marrow, may result.
Carcinoma in situ (CIS or flat urothelial carcinoma) is defined by the presence of cytologically malignant cells within a flat urothelium.16,23-26 CIS may range from full-thickness cytologic atypia to scattered malignant cells in an otherwise normal urothelium, the latter termed pagetoid spread (Fig. 21-11). A common feature similar to high-grade papillary urothelial carcinoma is the lack of cohesiveness, which leads to the shedding of malignant cells into the urine. When shedding is widespread, it may result in a denuded urothelium with only a few CIS cells clinging to the basement membrane. CIS usually appears grossly as an area of mucosal reddening, granularity, or thickening without producing an evident intraluminal mass. It is commonly multifocal and may involve most of the bladder surface and extend into the ureters and urethra. If untreated, 50% to 75% of CIS cases progress to muscle-invasive cancer.
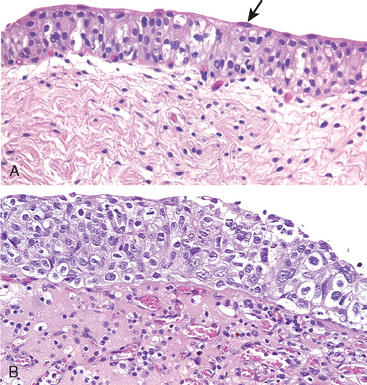
FIGURE 21-11 A, Normal urothelium with uniform nuclei and well-developed umbrella cell layer (arrow). B, Flat carcinoma in situ with numerous cells having enlarged and pleomorphic nuclei.
Invasive urothelial cancer (Fig. 21-12) may be associated with papillary urothelial cancer, usually high grade, or CIS. The extent of the invasion into the muscularis mucosae is of prognostic significance, and understaging on biopsy is a significant problem. The extent of spread (staging) at the time of initial diagnosis is the most important factor in determining the outlook for a patient (Table 21-4). Almost all infiltrating urothelial carcinomas are high grade, such that grading of the infiltrating component is not critical, as opposed to the importance of grading noninvasive papillary urothelial carcinoma.
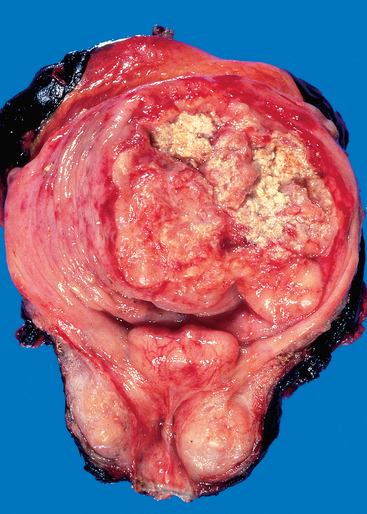
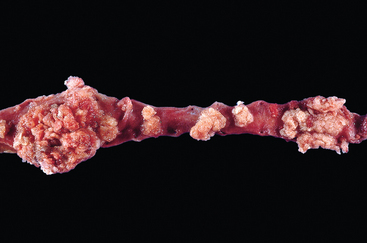
FIGURE 21-12 Opened bladder showing a high-grade invasive transitional cell carcinoma at an advanced stage. The aggressive multinodular neoplasm has fungated into the bladder lumen and spread over a wide area. The yellow areas represent areas of ulceration and necrosis.
TABLE 21-4 Pathologic T (Primary Tumor) Staging of Bladder Carcinoma
| Depth of Invasion | AJCC/UICC |
|---|---|
| Ta | Noninvasive, papillary |
| Tis | Carcinoma in situ (noninvasive, flat) |
| T1 | Lamina propria invasion |
| T2 | Muscularis propria invasion |
| T3a | Microscopic extra-vesicle invasion |
| T3b | Grossly apparent extra-vesicle invasion |
| T4 | Invades adjacent structures |
AJCC/UICC, American Joint Commission on Cancer/Union Internationale Contre le Cancer.
Variants of Urothelial Carcinoma. Unusual variants of urothelial cancer include the nested variant with deceptively bland cytology, lymphoepithelioma-like carcinoma, and micropapillary carcinoma.27-32
Other Epithelial Tumors.
Squamous cell carcinomas represent about 3% to 7% of bladder cancers in the United States, but in countries where urinary schistosomiasis is endemic, they occur much more frequently.33,34 Pure squamous cell carcinomas are nearly always associated with chronic bladder irritation and infection. Mixed urothelial carcinomas with areas of squamous carcinoma are more frequent than pure squamous cell carcinomas. Most are invasive, fungating tumors or are infiltrative and ulcerative. The level of cytologic differentiation varies widely, from highly differentiated lesions producing abundant keratin to more anaplastic tumors with only focal evidence of squamous differentiation.
Adenocarcinomas of the bladder are rare and histologically identical to adenocarcinomas seen in the gastrointestinal tract.35,36 Some arise from urachal remnants or in association with extensive intestinal metaplasia (discussed earlier).
Small-cell carcinomas, indistinguishable from small-cell carcinomas of the lung, arise in the bladder often in association with urothelial, squamous, or adenocarcinoma.37
Epidemiology and Pathogenesis.
The incidence of carcinoma of the bladder is higher in men than in women, in developed than in developing nations, and in urban than in rural dwellers. The male-to-female ratio for urothelial tumors is approximately 3 : 1. About 80% of patients are between the ages of 50 and 80 years. Bladder cancer, with rare exceptions, is not familial.
Several factors have been implicated in the causation of urothelial carcinoma. Some of the more important contributors include the following:
Several genetic alterations have been observed in urothelial carcinoma.38-42 Particularly common (occurring in 30% to 60% of tumors) are chromosome 9 monosomy or deletions of 9p and 9q as well as deletions of 17p, 13q, 11p, and 14q. The chromosome 9 deletions are the only genetic changes present frequently in superficial papillary tumors and occasionally in noninvasive flat tumors. The 9p deletions (9p21) involve the tumor suppressor gene p16 (INK4a), which encodes an inhibitor of a cyclin-dependent kinase (Chapter 7), and also the related tumor suppressor gene p15. The identity of the putative second tumor suppressor locus on chromosome 9q is not yet known. On the other hand, many invasive urothelial carcinomas show deletions of 17p, including the region of the p53 gene, as well as mutations in p53, suggesting that alterations in p53 contribute to the progression of urothelial carcinoma. Mutations in p53 are also found in CIS.
On the basis of these findings, a model for bladder carcinogenesis has been proposed. In this two-pathway model the first pathway is initiated by deletions of tumor suppressor genes on 9p and 9q, leading to superficial papillary tumors, a few of which may then acquire p53 mutations and progress to invasion; a second pathway, possibly initiated by p53 mutations, leads to CIS and, with loss of chromosome 9, progression to invasion (Chapter 7).
Clinical Course of Bladder Cancer.
Bladder tumors classically produce painless hematuria. This is their dominant and sometimes only clinical manifestation. Frequency, urgency, and dysuria occasionally accompany the hematuria. When the ureteral orifice is involved, pyelonephritis or hydronephrosis may follow. About 60% of neoplasms, when first discovered, are single, and 70% are localized to the bladder.
Individuals with urothelial tumors, whatever their grade, have a tendency to develop new tumors after excision, and recurrences may show a higher grade. The risk of recurrence and progression is related to several variables, including tumor size, stage, grade, multifocality, prior recurrence rate, and associated dysplasia and/or CIS in the surrounding mucosa.43-48 Although the term recurrence is used, most of the subsequent tumors arise at different sites from the original lesion. Recurrent tumors reflect in some cases new tumors, and in other instances they share the same clonal abnormalities as the initial tumor and represent a true recurrence of the initial lesion caused by shedding and implantation of the original tumor cells.
The prognosis depends on the histologic grade of the papillary tumor and the stage at diagnosis. Papillomas, papillary urothelial neoplasms of low malignant potential, and low-grade papillary urothelial cancer yield a 98% 10-year survival rate regardless of the number of recurrences; only a few patients (<10%) have progression of their disease to higher grade lesions. High-grade papillary urothelial carcinomas invade and lead to death in about 25% of cases. Patients with primary (de novo) CIS, as opposed to CIS associated with infiltrating urothelial carcinoma, are less likely to progress to muscle-invasive cancer (28% versus 59%) or die of disease (7% versus 45%).49 Invasive urothelial carcinoma is associated with a 30% mortality rate once tumor invades into the lamina propria. Overall, squamous cell carcinoma and adenocarcinoma are associated with a worse prognosis than urothelial carcinoma, yet stage for stage they are all similar.
The clinical challenge with these neoplasms is early detection and adequate follow-up. A significant issue is that 50% of invasive bladder cancers present with muscle-invasive disease and a relatively poor prognosis despite therapy. For tumors detected at an earlier stage, cystoscopy and biopsy are the mainstays of diagnosis. Of value in these circumstances are cytologic examinations and newer urine tests that detect the presence of various markers such as human complement factor H–related protein, telomerase, fibrin-fibrinogen degradation products, mucins, carcinoembryonic antigen, hyaluronic acid, hyaluronidase, nuclear matrix proteins, and chromosomal abnormalities detected by fluorescent in situ hybridization in cells in the urine.50,51 The major limitation of cytologic examination is the under-recognition of low-grade papillary neoplasms, whereas tests measuring urine markers have relatively low specificity, due to positive results caused by other conditions associated with injured urothelium.
The treatment for bladder cancer depends on the grade, stage, and whether the lesion is flat or papillary.52 For small, localized papillary tumors that are not high grade, the initial diagnostic transurethral resection is the only surgical procedure done. Patients are closely followed with periodic cystoscopies and urine cytologies for the rest of their lives to detect recurrence. Research is ongoing to determine whether less invasive urine marker studies can be used as follow-up tests to increase the interval between cystoscopic procedures. After the biopsy site has healed, patients at high risk of recurrence and/or progression (CIS; papillary tumors that are high grade, multifocal, have a history of rapid recurrence, or are associated with lamina propria invasion) receive topical immunotherapy consisting of intravesicle instillation of an attenuated strain of tuberculous bacillus called bacillus Calmette-Guérin (BCG). The bacteria elicit a local inflammatory reaction that destroys the tumor. Radical cystectomy is typically performed for (1) tumor invading the muscularis propria, (2) CIS or high-grade papillary cancer refractory to BCG, and (3) CIS extending into the prostatic urethra and extending down the prostatic ducts, where BCG will not come into contact with the neoplastic cells. Advanced bladder cancer is treated by chemotherapy.
Mesenchymal Tumors
Benign Tumors.
A great variety of benign mesenchymal tumors may arise in the bladder, having the histologic features of their counterparts elsewhere. Collectively, they are rare. The most common is leiomyoma.53 They all tend to grow as isolated, intramural, encapsulated, oval-to-spherical masses, varying in diameter up to several centimeters.
Sarcomas.
True sarcomas are distinctly uncommon in the bladder. Inflammatory myofibroblastic tumors and various carcinomas may assume sarcomatoid growth patterns and be mistaken histologically for sarcomas.54,55 As a group, sarcomas tend to produce large masses (varying up to 10 to 15 cm in diameter) that protrude into the vesicle lumen. Their soft, fleshy, gray-white gross appearance suggests their sarcomatous nature. The most common sarcoma in infancy or childhood is embryonal rhabdomyosarcoma.56 In some of these cases they manifest as a polypoid grapelike mass (sarcoma botryoides). The most common sarcoma in the bladder in adults is leiomyosarcoma53 (Chapter 26).
Secondary Tumors
Secondary malignant involvement of the bladder is most often by direct extension from primary lesions in nearby organs, cervix, uterus, prostate, and rectum. Lymphomas may involve the bladder as a component of systemic disease, but also, rarely, as primary bladder lymphoma.57
OBSTRUCTION
Obstruction to the bladder neck is of major clinical importance, mainly because of its eventual effect on the kidney. In males the most important lesion is enlargement of the prostate gland due to nodular hyperplasia (Fig. 21-13). Bladder obstruction is somewhat less common in females and is most often caused by cystocele of the bladder. Infrequent causes are (1) congenital urethral strictures, (2) inflammatory urethral strictures, (3) inflammatory fibrosis and contraction of the bladder, (4) bladder tumors, either benign or malignant, (5) invasion of the bladder neck by tumors arising in contiguous organs, (6) mechanical obstructions caused by foreign bodies and calculi, and (7) injury to the innervation of the bladder causing neurogenic bladder.
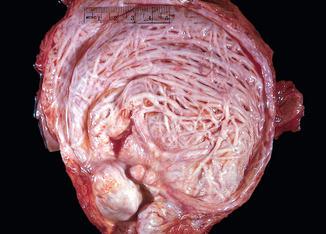
FIGURE 21-13 Hypertrophy and trabeculation of bladder wall secondary to polypoid hyperplasia of the prostate.
Morphology. In the early stages there is only some thickening of the bladder wall due to smooth muscle hypertrophy. With progressive hypertrophy the individual muscle bundles greatly enlarge and produce trabeculation of the bladder wall. In the course of time, crypts form and may then become converted into diverticula.
In some cases of acute obstruction or in terminal disease when the patient’s normal reflex mechanisms are depressed, the bladder may become extremely dilated. The enlarged bladder may reach the brim of the pelvis or even the level of the umbilicus. In these cases the bladder wall is markedly thinned and without trabeculations.
Urethra
INFLAMMATION
Urethritis is classically divided into gonococcal and nongonococcal. Gonococcal urethritis is one of the earliest manifestations of this venereal infection. Nongonococcal urethritis is common and can be caused by a variety of bacteria, among which E. coli and other enteric organisms predominate. Urethritis is often accompanied by cystitis in women and by prostatitis in men. In many instances bacteria cannot be isolated. Various strains of Chlamydia (e.g., C. trachomatis) are the cause of 25% to 60% of nongonococcal urethritis in men and about 20% in women. Mycoplasma (Ureaplasma urealyticum) also accounts for the symptoms of urethritis in many cases. Urethritis is also one component of Reiter syndrome, which comprises the clinical triad of arthritis, conjunctivitis, and urethritis (Chapter 26).
The morphologic changes are entirely typical of inflammation in other sites within the urinary tract. The urethral involvement is not itself a serious clinical problem but may cause considerable local pain, itching, and frequency, and may represent a forerunner of more serious disease at higher levels of the urogenital tract.
TUMORS AND TUMOR-LIKE LESIONS
Urethral caruncle is an inflammatory lesion presenting as a small, red, painful mass about the external urethral meatus, typically in older females. It may be covered by an intact mucosa but is extremely friable, and the slightest trauma may cause ulceration of the surface and bleeding. On histologic examination, it is composed of an inflamed granulation tissue polyp. Surgical excision affords prompt relief and cure.
Benign epithelial tumors of the urethra include squamous and urothelial papillomas, inverted urothelial papillomas, and condylomas.
Peyronie disease results in fibrous bands involving the corpus cavernosum of the penis. Although some classify it as a variant of fibromatosis, its etiology remains an enigma. Clinically, the lesion results in penile curvature and pain during intercourse.
Primary carcinoma of the urethra is an uncommon lesion (Fig. 21-14). Tumors arising within the proximal urethra tend to show urothelial differentiation and are analogous to those occurring within the bladder. Those lesions found within the distal urethra are more typically squamous carcinomas. Glandular carcinomas occur less frequently in the urethra generally in women. A rare variant is clear cell adenocarcinoma. Some neoplastic lesions of the urethra are similar to those described in the bladder, arising through metaplasia or, less commonly, from periurethral glands. Cancers arising within the prostatic urethra are dealt with in the section on the prostate.
Penis
The penis can be affected by congenital anomalies, inflammations, and tumors, inflammations and tumors being the most important. The venereal infections (e.g., syphilis and gonorrhea) usually begin with penile lesions. Carcinoma of the penis is an uncommon neoplasm in North America.
CONGENITAL ANOMALIES
The penis is the site of many forms of congenital anomalies, only some of which have clinical significance.
Hypospadias and Epispadias
Malformation of the urethral groove and urethral canal may create abnormal openings either on the ventral surface of the penis (hypospadias) or on the dorsal surface (epispadias).58 Though more frequent with epispadias, either of these two anomalies may be associated with failure of normal descent of the testes and with malformations of the urinary tract. Hypospadias, the more common of the two, occurs in approximately 1 in 300 live male births.59 Even when isolated, these urethral defects may have clinical significance, because the abnormal opening is often constricted, resulting in urinary tract obstruction and an increased risk of ascending urinary tract infections. When the orifices are situated near the base of the penis, normal ejaculation and insemination are hampered or totally blocked. These lesions therefore are possible causes of sterility in men.
Phimosis
When the orifice of the prepuce is too small to permit its normal retraction, the condition is designated phimosis. Such an abnormally small orifice may result from anomalous development but is more frequently the result of repeated attacks of infection that cause scarring of the preputial ring.60 Phimosis is important because it interferes with cleanliness and permits the accumulation of secretions and detritus under the prepuce, favoring the development of secondary infections and possibly carcinoma.
INFLAMMATION
Inflammations of the penis almost invariably involve the glans and prepuce and include a wide variety of specific and nonspecific infections. The specific infections—syphilis, gonorrhea, chancroid, granuloma inguinale, lymphopathia venerea, genital herpes—are sexually transmitted and are discussed in Chapter 8. Only the nonspecific infections causing so-called balanoposthitis require description here.
Balanoposthitis refers to infection of the glans and prepuce caused by a wide variety of organisms. Among the more common agents are Candida albicans, anaerobic bacteria, Gardnerella, and pyogenic bacteria.61 Most cases occur as a consequence of poor local hygiene in uncircumcised males, with accumulation of desquamated epithelial cells, sweat, and debris, termed smegma, acting as local irritant. Persistence of such infections leads to inflammatory scarring and, as mentioned earlier, is a common cause of phimosis.
TUMORS
Tumors of the penis are, on the whole, uncommon. The most frequent neoplasms are carcinomas and a benign epithelial tumor, condyloma acuminatum.
Benign Tumors
Condyloma Acuminatum
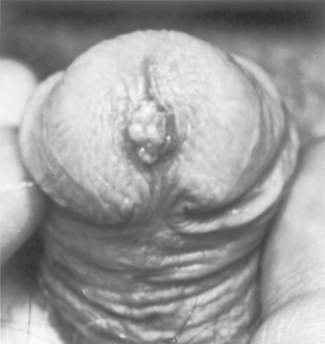
Condyloma acuminatum is a benign sexually transmitted tumor caused by human papillomavirus (HPV). It is related to the common wart and may occur on any moist mucocutaneous surface of the external genitals in either sex. HPV type 6, and less frequently type 11, are the most frequent agents that cause condylomata acuminata.
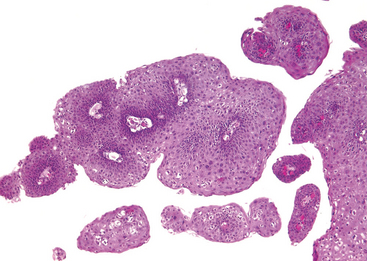
Morphology. Condylomata acuminata may occur on the external genitalia or perineal areas. On the penis these lesions occur most often about the coronal sulcus and inner surface of the prepuce. They consist of single or multiple sessile or pedunculated, red papillary excrescences that vary from 1 mm to several millimeters in diameter (Fig. 21-15). Histologically a branching, villous, papillary connective tissue stroma is covered by epithelium that may have considerable superficial hyperkeratosis and thickening of the underlying epidermis (acanthosis) (Fig. 21-16). The normal orderly maturation of the epithelial cells is preserved. Cytoplasmic vacuolization of the squamous cells (koilocytosis), characteristic of HPV infection, is noted in these lesions (Fig. 21-17). Cells may have degenerative (viral) atypia but true dysplasia is rare. Condylomata acuminata tend to recur but only rarely progress into in situ or invasive cancers.
Malignant Tumors
Carcinoma in Situ (CIS)
In the external male genitalia, two distinct lesions display histologic features of CIS: Bowen disease and bowenoid papulosis. These lesions have a strong association with infection by HPV, most commonly type 16.62
Bowen disease occurs in the genital region of both men and women, usually in those over the age of 35 years. In men it tends to involve the skin of the shaft of the penis and the scrotum. Grossly it appears as a solitary, thickened, gray-white, opaque plaque. It can also manifest on the glans and prepuce as single or multiple shiny red, sometimes velvety plaques. Histologically the epidermis shows proliferation with numerous mitoses, some atypical. The cells are markedly dysplastic with large hyperchromatic nuclei and lack of orderly maturation (Fig. 21-18). Nevertheless, the dermal-epidermal border is sharply delineated by an intact basement membrane. Over the span of years, Bowen disease may transform into infiltrating squamous cell carcinoma in approximately 10% of patients. Bowen disease may also be associated with visceral cancer, such as that of the colon or breast, but not as frequently as initially reported.
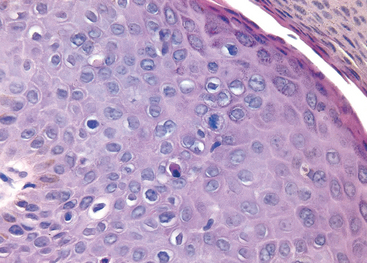
FIGURE 21-18 Bowen disease (carcinoma in situ) of the penis. Note the hyperchromatic, dysplastic dyskeratotic epithelial cells with scattered mitoses above the basal layer. The intact basement membrane is not readily seen in this picture.
Bowenoid papulosis occurs in sexually active adults. Clinically, it differs from Bowen disease by the younger age of patients and the presence of multiple (rather than solitary) reddish brown papular lesions. Histologically, bowenoid papulosis is indistinguishable from Bowen disease and is also related to HPV type 16. However, in contrast to Bowen disease, bowenoid papulosis virtually never develops into an invasive carcinoma and in many cases spontaneously regresses.
Invasive Carcinoma
Squamous cell carcinoma of the penis is an uncommon malignancy in the United States, accounting for fewer than 1% of cancers in males. By contrast, in some parts of Asia, Africa, and South America the incidence of squamous cell carcinoma of the penis ranges from 10% to 20% of male malignancies. Circumcision confers protection, and hence this cancer is extremely rare among Jews and Moslems and is correspondingly more common in populations in which circumcision is not routinely practiced. It is postulated that circumcision is associated with better genital hygiene, which, in turn, reduces exposure to carcinogens that may be concentrated in smegma and decreases the likelihood of infection with potentially oncogenic types of HPV. HPV DNA can be detected in penile squamous cancer in approximately 50% of patients.62 HPV type 16 is the most frequent culprit, but HPV 18 is also implicated. Cigarette smoking elevates the risk of developing cancer of the penis.63 Carcinomas are usually found in patients between the ages of 40 and 70.
Morphology. Squamous cell carcinoma of the penis usually begins on the glans or inner surface of the prepuce near the coronal sulcus. Two macroscopic patterns are seen—papillary and flat. The papillary lesions simulate condylomata acuminata and may produce a cauliflower-like fungating mass. Flat lesions appear as areas of epithelial thickening accompanied by graying and fissuring of the mucosal surface. With progression, an ulcerated papule develops (Fig. 21-19). Histologically, both the papillary and the flat lesions are squamous cell carcinomas with varying degrees of differentiation. Verrucous carcinoma is an exophytic well-differentiated variant of squamous cell carcinoma that has low malignant potential. These tumors are locally invasive, but they rarely metastasize. Other, less common, subtypes of penile squamous carcinoma include basaloid, warty, and papillary variants.64,65
Clinical Features.
Invasive squamous cell carcinoma of the penis is a slowly growing, locally invasive lesion that often has been present for a year or more before it is brought to medical attention.66 The lesions are nonpainful until they undergo secondary ulceration and infection. Metastases to inguinal lymph nodes characterize the early stage, but widespread dissemination is extremely uncommon until the lesion is far advanced. Clinical assessment of regional lymph node involvement is notoriously inaccurate; 50% of men with penile squamous cell carcinoma and clinically enlarged inguinal nodes have only reactive lymphoid hyperplasia when examined histologically. The prognosis is related to the stage of the tumor. In persons with limited lesions without invasion of the inguinal lymph nodes, there is a 66% 5-year survival rate, whereas metastasis to the lymph nodes carries a grim 27% 5-year survival.
Testis and Epididymis
Distinct pathological conditions affect the testis and epididymis. In the epididymis, the most important and frequent conditions are inflammatory diseases, whereas in the testis the major lesions are tumors.
CONGENITAL ANOMALIES
With the exception of undescended testes (cryptorchidism), congenital anomalies are extremely rare and include absence of one or both testes and fusion of the testes (so-called synorchism).
Cryptorchidism
Cryptorchidism is found in approximately 1% of 1-year-old boys.67 This anomaly represents a complete or incomplete failure of the intra-abdominal testes to descend into the scrotal sac. It usually occurs as an isolated anomaly but may be accompanied by other malformations of the genitourinary tract, such as hypospadias.
Testicular descent occurs in two morphologically and hormonally distinct phases.68 During the first, the transabdominal, phase, the testis comes to lie within the lower abdomen or brim of the pelvis. This phase is believed to be controlled by a hormone called müllerian-inhibiting substance. In the second, or the inguinoscrotal, phase, the testes descend through the inguinal canal into the scrotal sac. This phase is androgen dependent and is possibly mediated by androgen-induced release of calcitonin gene–related peptide, from the genitofemoral nerve. Although testes may be arrested anywhere along their pathway of descent, defects in transabdominal descent are uncommon, accounting for approximately 5% to 10% of cases. In most patients the undescended testis is palpable in the inguinal canal. Even though testicular descent is controlled by hormonal factors, cryptorchidism is only rarely associated with a well-defined hormonal disorder. The condition is completely asymptomatic, and it is found by the patient or the examining physician only when the scrotal sac is discovered not to contain the testis.
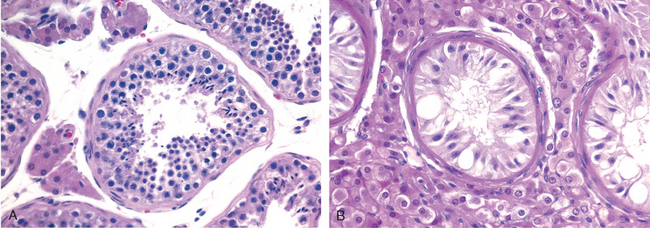
Morphology. Cryptorchidism is unilateral in most cases, but it may be bilateral in 25% of patients. Histologic changes in the malpositioned testis begin as early as 2 years of age. They are characterized by an arrest in the development of germ cells associated with marked hyalinization and thickening of the basement membrane of the spermatic tubules (Fig. 21-20). Eventually the tubules appear as dense cords of hyaline connective tissue outlined by prominent basement membranes. There is concomitant increase in interstitial stroma. Because Leydig cells are spared, they appear to be prominent. As might be expected with progressive tubular atrophy, the cryptorchid testis is small in size and is firm in consistency as a result of fibrotic changes. Histologic deterioration, associated with a paucity of germ cells, has also been noted in the contralateral (descended) testis in males with unilateral cryptorchidism, supporting an intrinsic defect in testicular development.
In addition to sterility, cryptorchidism can be associated with other morbidity. When the testis lies in the inguinal canal, it is particularly exposed to trauma and crushing against the ligaments and bones. A concomitant inguinal hernia accompanies the undescended testis in about 10% to 20% of cases. In addition, the undescended testis is at a greater risk of developing testicular cancer than is the descended testis.69 During the first year of life the majority of inguinal cryptorchid testes descend spontaneously into the scrotum. Those that remain undescended require surgical correction, preferably before histologic deterioration sets in at around 2 years of age.70 Orchiopexy (placement in the scrotal sac) does not guarantee fertility; deficient spermatogenesis has been reported in 10% to 60% of patients in whom surgical repositioning was performed.67,70 To what extent the risk of cancer is reduced after orchiopexy is also unclear. According to some studies, orchiopexy of unilateral cryptorchidism before 10 years of age protects against cancer development.71 This is not universally accepted, however.72 Malignant change may occur in the contralateral, normally descended testis. These observations suggest that cryptorchidism is associated with a defect in testicular development and cellular differentiation that is unrelated to anatomic position.
REGRESSIVE CHANGES
Atrophy and Decreased Fertility
Atrophy is a regressive change that affects the scrotal testis and can have any of several causes, including (1) progressive atherosclerotic narrowing of the blood supply in old age, (2) the end stage of an inflammatory orchitis, (3) cryptorchidism, (4) hypopituitarism, (5) generalized malnutrition or cachexia, (6) irradiation, (7) prolonged administration of antiandrogens (treatment for advanced carcinoma of the prostate), and (8) exhaustion atrophy, which may follow the persistent stimulation produced by high levels of follicle-stimulating pituitary hormone. The gross and microscopic alterations follow the pattern already described for cryptorchidism. Atrophy occasionally occurs as a primary failure of genetic origin, such as in Klinefelter syndrome (discussed in Chapter 5).
Atrophy is an end-stage pattern of testicular injury. Before this terminal histologic appearance is reached, several other patterns are associated with decreased fertility.73 These include hypospermatogenesis, maturation arrest, and findings associated with vas deferens obstruction. In some instances a specific cause for the testicular injury can be found, and if it can be removed before the development of atrophy, testicular function can be restored.
INFLAMMATION
Inflammations are distinctly more common in the epididymis than in the testis. Of the three major specific inflammatory states that affect the testis and epididymis, gonorrhea and tuberculosis almost invariably arise in the epididymis, whereas syphilis affects first the testis.
Nonspecific Epididymitis and Orchitis
Epididymitis and possible subsequent orchitis are commonly related to infections in the urinary tract (cystitis, urethritis, prostatitis), which reach the epididymis and the testis through either the vas deferens or the lymphatics of the spermatic cord.
The cause of epididymitis varies with the age of the patient. Though uncommon in children, epididymitis in childhood is usually associated with a congenital genitourinary abnormality and infection with gram-negative rods. In sexually active men younger than age 35 years, the sexually transmitted pathogens C. trachomatis and Neisseria gonorrhoeae are the most frequent culprits. In men older than age 35 the common urinary tract pathogens, such as E. coli and Pseudomonas, are responsible for most infections.
Morphology. The bacterial invasion induces nonspecific acute inflammation characterized by congestion, edema, and infiltration by neutrophils, macrophages, and lymphocytes. Although the infection, in the early stage, is more or less limited to the interstitial connective tissue, it rapidly extends to involve the tubules and may progress to frank abscess formation or complete suppurative necrosis of the entire epididymis (Fig. 21-21). Usually, having involved the epididymis, the infection extends into the testis to evoke a similar inflammatory reaction. Such inflammatory involvement of the epididymis and testis is often followed by fibrous scarring, which in many cases leads to sterility. Usually the interstitial cells of Leydig are not totally destroyed, so sexual activity is not disturbed.
Granulomatous (Autoimmune) Orchitis
Idiopathic granulomatous orchitis presents in middle age as a moderately tender testicular mass of sudden onset sometimes associated with fever. It may appear insidiously, however, as a painless testicular mass mimicking a testicular tumor, hence its importance. Histologically the orchitis is distinguished by granulomas restricted to spermatic tubules. The lesions closely resemble tubercles but differ in that the granulomatous reaction is present diffusely throughout the testis and is confined to the seminiferous tubules. Although an autoimmune basis is suspected, the cause of these lesions remains unknown.
Specific Inflammations
Gonorrhea
Extension of infection from the posterior urethra to the prostate, seminal vesicles, and then to the epididymis is the usual course of a neglected gonococcal infection. Inflammatory changes similar to those described for nonspecific infections occur, with the development of frank abscesses in the epididymis, which may lead to extensive destruction of this organ. In neglected cases, the infection may spread to the testis and produce suppurative orchitis.
Mumps
Mumps is a systemic viral disease that most commonly affects school-aged children. Testicular involvement is extremely uncommon in this age group. In postpubertal males, however, orchitis may develop and has been reported in 20% to 30% of male patients. Most often, acute interstitial orchitis develops about 1 week after the onset of swelling of the parotid glands.
Tuberculosis
Tuberculosis almost invariably begins in the epididymis and may spread to the testis. The infection invokes the classic morphologic reactions of caseating granulomatous inflammation characteristic of tuberculosis elsewhere.
Syphilis
The testis and epididymis are affected in both acquired and congenital syphilis, but almost invariably the testis is involved first by the infection. In many cases, the orchitis is not accompanied by epididymitis. The morphologic pattern of the reaction takes two forms: the production of gummas or a diffuse interstitial inflammation characterized by edema and lymphocytic and plasma cell infiltration with the characteristic hallmark of all syphilitic infections (i.e., obliterative endarteritis with perivascular cuffing of lymphocytes and plasma cells).
VASCULAR DISORDERS
Torsion
Twisting of the spermatic cord typically cuts off the venous drainage of the testis. The thick-walled arteries remain patent, so that the intense vascular engorgement may be followed by hemorrhagic infarction. There are two types of testicular torsion. Neonatal torsion occurs either in utero or shortly after birth. It lacks any associated anatomic defect to account for its occurrence. Adult torsion is typically seen in adolescence presenting as sudden onset of testicular pain. It often occurs without any inciting injury; sudden pain heralding the torsion may even occur during sleep. Torsion is one of the few urologic emergencies. If the testis is explored surgically and manually untwisted within approximately 6 hours after the onset of torsion, there is a good chance that the testis will remain viable. In contrast to neonatal torsion, adult torsion results from a bilateral anatomic defect where the testis has increased mobility, giving rise to what is termed the bell-clapper abnormality. To prevent the catastrophic occurrence of subsequent torsion in the contralateral testis, the testis unaffected by torsion is surgically fixed to the scrotum (orchiopexy).
Morphology. Depending on the duration of the process, the morphologic changes range from intense congestion to widespread extravasation of blood into the interstitial tissue to hemorrhagic testicular infarction (Fig. 21-22). In these late stages the testis is markedly enlarged and is converted virtually into a sac of soft, necrotic, hemorrhagic tissue.
SPERMATIC CORD AND PARATESTICULAR TUMORS
Lipomas are common lesions involving the proximal spermatic cord, identified at the time of inguinal hernia repair. Although diagnosed as “lipomas,” many of these lesions probably represent retroperitoneal adipose tissue that has been pulled into the inguinal canal along with the hernia sac, rather than a true neoplasm.
The most common benign paratesticular tumor is adenomatoid tumor. Although these lesions are mesothelial in nature, they are not referred to as mesotheliomas to distinguish them from other mesothelial lesions that may occur at this site. Adenomatoid tumors are usually small nodules, typically occurring near the upper pole of the epididymis. Although grossly well circumscribed, microscopically they may be minimally invasive into the adjacent testis. The importance of this lesion is that it is one of the few benign tumors that occur near the testis. If the pathologist can identify the nature of this lesion in intraoperative frozen sections, local excision of the adenomatoid tumor can spare the patient orchiectomy.
The most common malignant paratesticular tumors located at the distal end of the spermatic cord are rhabdomyosarcomas in children and liposarcomas in adults.
TESTICULAR TUMORS
Testicular neoplasms span an amazing gamut of anatomic types.17,74 They are divided into two major categories: germ cell tumors and sex cord–stromal tumors (Table 21-5). Approximately 95% of testicular tumors arise from germ cells. Germ cell tumors are subdivided into seminomas and non-seminomas. Most germ cell tumors are aggressive cancers capable of rapid, wide dissemination, although with current therapy most can be cured.75 Sex cord–stromal tumors, in contrast, are generally benign.
TABLE 21-5 Pathologic Classification of Common Testicular Tumors
Germ Cell Tumors
The incidence of testicular tumors in the United States is approximately 6 per 100,000, resulting in approximately 300 deaths per year. For unexplained reasons there is a worldwide increase in the incidence of these tumors. In the 15- to 34-year age group, they constitute the most common tumor of men and cause approximately 10% of all cancer deaths. In the United States these tumors are much more common in whites than in blacks (ratio 5 : 1).
Environmental Factors and Genetic Predisposition.
Environmental factors play a role in the incidence of testicular germ cell tumors, as demonstrated by population migration studies. The incidence of testicular germ cell tumors in Finland is about two times lower than in Sweden; second generation Finnish immigrants to Sweden, have a tumor incidence that approaches that of the Swedish population. Testicular germ cell tumors are associated with a spectrum of disorders known as testicular dysgenesis syndrome (TDS). This syndrome includes cryptorchidism, hypospadias, and poor sperm quality, and it has been proposed that some of these conditions might be influenced by in utero exposures to pesticides and nonsteroidal estrogens. Cryptorchidism, which is associated with approximately 10% of testicular germ cell tumors, is the most important risk factor. Klinefelter syndrome (a TDS condition) is associated with an increased risk (50 times greater than normal) for the development of mediastinal germ cell tumors, but these patients do not develop testicular tumors.
There is a strong family predisposition associated with the development of testicular germ cell tumors. The relative risk of development of these tumors in fathers and sons of patients with testicular germ cell tumors is four times higher than normal, and is 8 to 10 times higher between brothers. It is possible that genetic polymorphisms at the Xq27 locus may be responsible for this susceptibility, but further studies are needed to validate this hypothesis.
Classification and Pathogenesis.
A simple classification of the most common types of testicular tumors is presented in Table 21-5. Two broad groups are recognized. Seminomatous tumors are composed of cells that ressemble primordial germ cells or early gonocytes. The non-seminomatous tumors may be composed of undifferentiated cells that resemble embryonic stem cells, as in the case of embryonal carcinoma, but the malignant cells can differentiate into various lineages generating yolk sac tumors, choriocarcinomas and teratomas. Germ cell tumors may have a single tissue component, but in approximately 60% of cases, the tumors contain mixtures of seminomatous and non-seminomatous components and multiple tissues. In teratomas, tissues of the three germ layers are represented as a result of the differentiation of embryonal carcinoma cells. Seminomas constitute approximately 50% of all testicular germ cell neoplasms and are the most common testicular tumor.
Most testicular germ cell tumors originate from lesions called intratubular germ cell neoplasia (ITGCN), which is also referred to as intratubular germ cell neoplasia unclassified (ITGCNU).76,77 However, ITGCN has not been implicated as a precursor lesion of pediatric yolk sac tumors and teratomas, or of adult spermatocytic seminoma. ITGCN is believed to occur in utero and stay dormant until puberty, when it may progress into seminomas or non-seminomatous tumors. The lesion consists of atypical primordial germ cells with large nuclei and clear cytoplasm, which are about twice the size of normal germ cells. These cells retain the expression of the transcription factors OCT3/4 and NANOG, which are associated with pluripontentiality (Chapter 3), and are expressed in normal embryonic stem cells. ITGCN share some of the genetic alterations found in germ cell tumors such as the gain of additional copies of the short arm of chromosome 12 (12p) in the form of an isochromosome of its short arm, i(12p). This change is invariably found in invasive tumors regardless of histological type. Activating mutations of c-KIT, which may be present in seminomas, are also present in ITGCN. About 50% of individuals with ITGCN develop invasive germ cell tumors within five years after diagnosis, and it has been proposed that practically all patients with ITGCN eventually develop invasive tumors. ITGCN is essentially a type of carcinoma in situ (CIS), although the term CIS is not frequently used to refer to this lesion.
Seminoma
Seminomas are the most common type of germ cell tumor, making up about 50% of these tumors. The peak incidence is the third decade and they almost never occur in infants. An identical tumor arises in the ovary, where it is called dysgerminoma (Chapter 22). Seminomas contain an isochromosome 12p, and express OCT3/4 and NANOG. Approximately 25% of these tumors have c-KIT activating mutations. c-KIT amplification has also been repeated, but increased c-KIT expression may occur without genetic defects.
Morphology. If not otherwise specified, “seminoma” refers to “classical” or “typical” seminoma that consists of a uniform population of cells. Spermatocytic seminoma, despite its nosologic similarity, is a distinct tumor discussed later. Seminomas produce bulky masses, sometimes ten times the size of the normal testis. The typical seminoma has a homogeneous, gray-white, lobulated cut surface, usually devoid of hemorrhage or necrosis (Fig. 21-23). Generally the tunica albuginea is not penetrated, but occasionally extension to the epididymis, spermatic cord, or scrotal sac occurs.
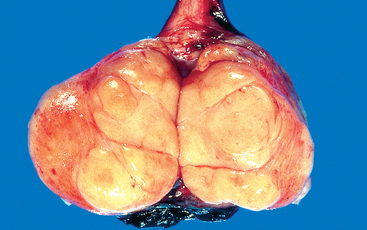
FIGURE 21-23 Seminoma of the testis appears as a fairly well-circumscribed, pale, fleshy, homogeneous mass.
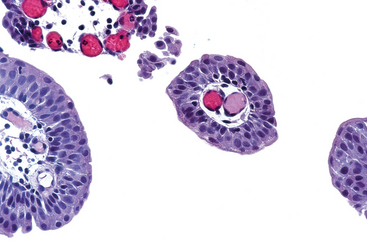
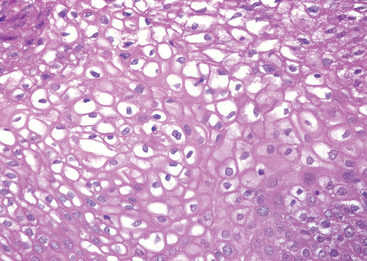
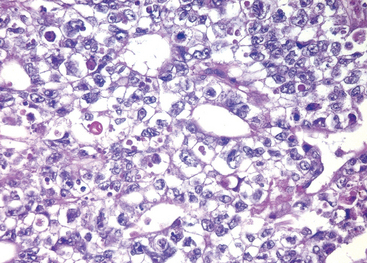
Microscopically the typical seminoma is composed of sheets of uniform cells divided into poorly demarcated lobules by delicate septa of fibrous tissue containing a moderate amount of lymphocytes (Fig. 21-24A). The classic seminoma cell is large and round to polyhedral and has a distinct cell membrane; a clear or watery-appearing cytoplasm; and a large, central nucleus with one or two prominent nucleoli (Fig. 21-24B). Mitoses vary in frequency. The cytoplasm contains varying amounts of glycogen. Seminoma cells are diffusely positive for c-KIT, (regardless of c-KIT mutational status) OCT4, and placental alkaline phosphatase (PLAP), with sometimes scattered keratin-positive cells.
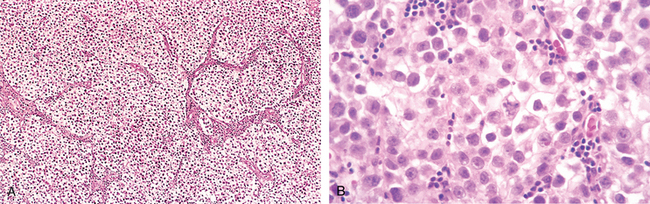
FIGURE 21-24 Seminoma. A, Low magnification shows clear seminoma cells divided into poorly demarcated lobules by delicate septa. B, Microscopic examination reveals large cells with distinct cell borders, pale nuclei, prominent nucleoli, and a sparse lymphocytic infiltrate.
Approximately 15% of seminomas contain syncytiotrophoblasts. In this subset of patients, serum human chorionic gonadotropin (HCG) levels are elevated, though not to the extent seen in patients with choriocarcinoma. Seminomas may also be accompanied by an ill-defined granulomatous reaction, in contrast to the well-formed discrete granulomas seen with tuberculosis.
The term anaplastic seminoma is used by some to indicate greater cellular and nuclear irregularity with more frequent tumor giant cells and many mitoses. However, since “anaplastic seminoma” is not associated with a worse prognosis when matched stage for stage with classic seminoma and is not treated differently, most authorities do not recognize anaplastic seminoma as a distinct entity.
Spermatocytic Seminoma
Though related by name to seminoma, spermatocytic seminoma is a distinctive tumor both clinically and histologically.78 Spermatocytic seminoma is an uncommon tumor, representing 1% to 2% of all testicular germ cell neoplasms. The age of involvement is much later than for most testicular tumors: Affected individuals are generally over the age of 65 years. In contrast to classic seminoma, it is a slow-growing tumor that does not produce metastases, and hence the prognosis is excellent. In contrast to typical seminomas, spermatocytic seminomas lack lymphocytes, granulomas, syncytiotrophoblasts, extra-testicular sites of origin, admixture with other germ cell tumors, and association with ITGCN (see “Clinical Features of Testicular Tumors” discussed later).
Morphology. Grossly, spermatocytic seminoma tends to have a soft, pale gray, cut surface that sometimes reveal mucoid cysts. Spermatocytic seminomas contain three cell populations, all intermixed: (1) medium-sized cells, the most numerous, containing a round nucleus and eosinophilic cytoplasm; (2) smaller cells with a narrow rim of eosinophilic cytoplasm resembling secondary spermatocytes; and (3) scattered giant cells, either uninucleate or multinucleate. The chromatin in some intermediate-sized cells is similar to that seen in the meiotic phase of non-neoplastic spermatocytes (spireme chromatin).
Embryonal Carcinoma
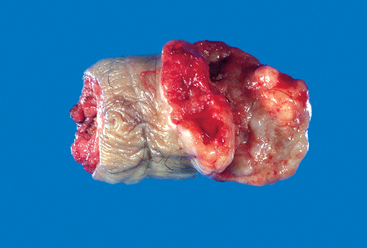
Embryonal carcinomas occur mostly in the 20- to 30-year age group. These tumors are more aggressive than seminomas.
Morphology. Grossly, the tumor is smaller than seminoma and usually does not replace the entire testis. On cut surfaces the mass is often variegated, poorly demarcated at the margins, and punctuated by foci of hemorrhage or necrosis (Fig. 21-25). Extension through the tunica albuginea into the epididymis or cord frequently occurs. Histologically the cells grow in alveolar or tubular patterns, sometimes with papillary convolutions (Fig. 21-26). Embryonal carcinomas lack the well-formed glands with basally situated nuclei and apical cytoplasm seen in teratomas. More undifferentiated lesions may display sheets of cells. The neoplastic cells have an epithelial appearance, are large and anaplastic, and have hyperchromatic nuclei with prominent nucleoli. In contrast to seminoma, the cell borders are usually indistinct, and there is considerable variation in cell and nuclear size and shape. Mitotic figures and tumor giant cells are frequently seen. Embryonal carcinomas share some markers with seminomas such as OCT 3/4 and PLAP, but differ by being positive for cytokeratin and CD30, and negative for c-KIT.79
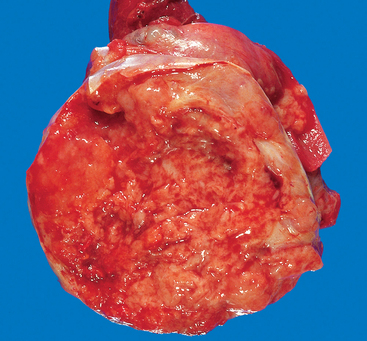
FIGURE 21-25 Embryonal carcinoma. In contrast to the seminoma illustrated in Figure 21-23, the embryonal carcinoma is a hemorrhagic mass.
Yolk Sac Tumor
Also known as endodermal sinus tumor, yolk sac tumor is of interest because it is the most common testicular tumor in infants and children up to 3 years of age. In this age group it has a very good prognosis. In adults the pure form of this tumor is rare; instead, yolk sac elements frequently occur in combination with embryonal carcinoma.
Morphology. Grossly, the tumor is nonencapsulated, and on cross-section it presents a homogeneous, yellow-white, mucinous appearance. Characteristic on microscopic examination is a lacelike (reticular) network of medium-sized cuboidal or flattened cells. In addition, papillary structures, solid cords of cells, and a multitude of other less common patterns may be found. In approximately 50% of tumors, structures resembling endodermal sinuses (Schiller-Duval bodies) may be seen; these consist of a mesodermal core with a central capillary and a visceral and parietal layer of cells resembling primitive glomeruli. Present within and outside the cytoplasm are eosinophilic, hyaline-like globules in which α-fetoprotein (AFP) and α1-antitrypsin can be demonstrated by immunocytochemical staining. The presence of AFP in the tumor cells is highly characteristic, and it underscores their differentiation into yolk sac cells.
Choriocarcinoma
Choriocarcinoma is a highly malignant form of testicular tumor. In its “pure” form choriocarcinoma is rare, constituting less than 1% of all germ cell tumors.
Morphology. Often they cause no testicular enlargement and are detected only as a small palpable nodule. Typically, these tumors are small, rarely larger than 5 cm in diameter. Hemorrhage and necrosis are extremely common. Histologically the tumors contain two cell types (Fig. 21-27). The syncytiotrophoblastic cells are large and have many irregular or lobular hyperchromatic nuclei and an abundant eosinophilic vacuolated cytoplasm. HCG can be readily demonstrated in the cytoplasm. The cytotrophoblastic cells are more regular and tend to be polygonal, with distinct borders and clear cytoplasm; they grow in cords or masses and have a single, fairly uniform nucleus. More anatomic details are available in the discussion of these neoplasms in the female genital tract (Chapter 22).
Teratoma
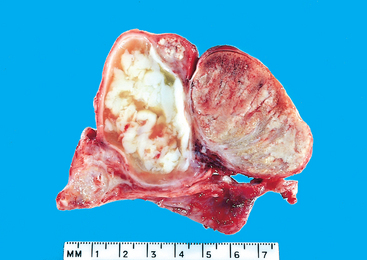
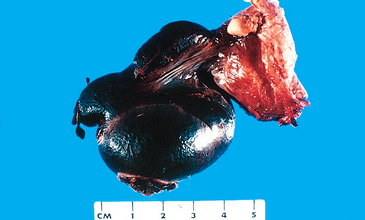
The designation teratoma refers to a group of complex testicular tumors having various cellular or organoid components reminiscent of normal derivatives from more than one germ layer. They may occur at any age from infancy to adult life. Pure forms of teratoma are fairly common in infants and children, second in frequency only to yolk sac tumors. In adults, pure teratomas are rare, constituting 2% to 3% of germ cell tumors. However, the frequency of teratomas mixed with other germ cell tumors is approximately 45%.
Morphology. Grossly, teratomas are usually large, ranging from 5 to 10 cm in diameter. Because they are composed of various tissues, the gross appearance is heterogeneous with solid, sometimes cartilaginous, and cystic areas (Fig. 21-28). Hemorrhage and necrosis usually indicate admixture with embryonal carcinoma, choriocarcinoma, or both.
FIGURE 21-28 Teratoma of testis. The variegated cut surface with cysts reflects the multiplicity of tissue found histologically.
Teratomas are composed of a heterogeneous, helter-skelter collection of differentiated cells or organoid structures, such as neural tissue, muscle bundles, islands of cartilage, clusters of squamous epithelium, structures reminiscent of thyroid gland, bronchial or bronchiolar epithelium, and bits of intestinal wall or brain substance, all embedded in a fibrous or myxoid stroma (Fig. 21-29). Elements may be mature (resembling various adult tissues) or immature (sharing histologic features with fetal or embryonal tissue). Dermoid cysts and epidermoid cysts, are a form of teratoma that are common in the ovary (Chapter 22), but rare in the testis. Unlike testicular teratomas, they have a uniformly benign behavior.
FIGURE 21-29 Teratoma of the testis consisting of a disorganized collection of glands, cartilage, smooth muscle, and immature stroma.
Rarely, a malignant non–germ cell tumors may arise in teratoma.80 This phenomenon is referred to as “teratoma with malignant transformation,” where there is malignancy in derivatives of one or more germ cell layers. Thus, there may be a focus of squamous cell carcinoma, mucin-secreting adenocarcinoma, or sarcoma. The importance of recognizing a non–germ cell malignancy arising in a teratoma is that the non–germ cell component does not respond to chemotherapy when it spreads outside of the testis. In this case, the only hope for cure resides in the resectability of the tumor. These non–germ cell malignancies have an isochromosome 12p, similar to the germ cell tumors from which they arose.
In the child, differentiated mature teratomas usually follow a benign course. In the postpubertal male all teratomas are regarded as malignant, capable of metastatic behavior whether the elements are mature or immature. Consequently, it is not critical to detect immaturity in a testicular teratoma of a postpubertal male.
Mixed Tumors
About 60% of testicular tumors are composed of more than one of the “pure” patterns. Common mixtures include: teratoma, embryonal carcinoma, and yolk sac tumor; seminoma with embryonal carcinoma; and embryonal carcinoma with teratoma (teratocarcinoma). In most instances the prognosis is worsened by the inclusion of the more aggressive element.
Clinical Features of Germ Cell Testicular Tumors.
Although painless enlargement of the testis is a characteristic feature of germ cell neoplasms, any solid testicular mass should be considered neoplastic unless proved otherwise. Biopsy of a testicular neoplasm is associated with a risk of tumor spillage, which would necessitate excision of the scrotal skin in addition to orchiectomy. Consequently, the standard management of a solid testicular mass is radical orchiectomy based on the presumption of malignancy.
Testicular tumors have a characteristic mode of spread. Lymphatic spread is common to all forms of testicular tumors. In general retroperitoneal para-aortic nodes are the first to be involved. Subsequent spread may occur to mediastinal and supraclavicular nodes. Hematogenous spread is primarily to the lungs, but liver, brain, and bones may also be involved. The histology of metastases may sometimes be different from that of the testicular lesion. For example, an embryonal carcinoma may present a teratomatous picture in the secondary deposits. As discussed earlier, because all these tumors are derived from pluripotent germ cells, the apparent “forward” and “backward” differentiation seen in different locations is not entirely surprising. Another explanation for the differing morphologic patterns in the primary and metastatic site is that minor components in the primary tumor that were unresponsive to chemotherapy survive, resulting in the dominant metastatic pattern.
From a clinical standpoint, tumors of the testis are segregated into two broad categories: seminoma and nonseminomatous germ cell tumors (NSGCTs). Seminomas tend to remain localized to the testis for a long time, and hence approximately 70% present in clinical stage I (see later). In contrast, approximately 60% of males with NSGCTs present with advanced clinical disease (stages II and III). Metastases from seminomas typically involve lymph nodes. Hematogenous spread occurs later in the course of dissemination. NSGCTs not only metastasize earlier but also use the hematogenous route more frequently. The rare pure choriocarcinoma is the most aggressive NSGCT. It may not cause any testicular enlargement but instead spreads predominantly and rapidly by the bloodstream. Therefore, lungs and liver are involved early in virtually every case. From a therapeutic viewpoint, seminomas are extremely radiosensitive, whereas NSGCTs are relatively radioresistant. To summarize, as compared with seminomas, NSGCTs are biologically more aggressive and in general have a poorer prognosis.
In the United States, three clinical stages of testicular tumors are defined:
Germ cell tumors of the testis often secrete polypeptide hormones and certain enzymes that can be detected in blood by sensitive assays.81 Such biologic markers include HCG, AFP, and lactate dehydrogenase, which are valuable in the diagnosis and management of testicular cancer. The elevation of lactate dehydrogenase correlates with the mass of tumor cells, and provides a tool to assess tumor burden. Marked elevation of serum AFP or HCG levels are produced by yolk sac tumor and choriocarcinoma elements, respectively. Both of these markers are elevated in more than 80% of individuals with NSGCT at the time of diagnosis. As stated earlier, approximately 15% of seminomas have syncytiotrophoblastic giant cells and minimal elevation of HCG levels, which does not affect prognosis. In the context of testicular tumors, the value of serum markers is fourfold:
The therapy and prognosis of testicular tumors depend largely on clinical stage and on the histologic type. Seminoma, which is extremely radiosensitive and tends to remain localized for long periods, has the best prognosis. More than 95% of patients with stage I and II disease can be cured. Among NSGCTs, the histologic subtype does not influence the prognosis significantly, and hence these are treated as a group. Approximately 90% of patients with NSGCTs can achieve complete remission with aggressive chemotherapy, and most can be cured. Pure choriocarcinoma has a poor prognosis. However, when it is a minor component of a mixed germ cell tumor, the prognosis is less adversely affected. With all testicular tumors, distant metastases, if present, usually occur within the first 2 years after treatment.
Tumors of Sex Cord–Gonadal Stroma
As indicated in Table 21-5, sex cord–gonadal stroma tumors are subclassified based on their presumed histogenesis and differentiation. The two most important members of this group—Leydig cell tumors and Sertoli cell tumors—are described here. Details of other tumors in this group can be found in a review.82
Leydig Cell Tumors
Tumors of Leydig cells are particularly interesting, because they may elaborate androgens and in some cases both androgens and estrogens, and even corticosteroids.83,84 They may arise at any age, although most cases occur between 20 and 60 years of age. As with other testicular tumors, the most common presenting feature is testicular swelling, but in some patients gynecomastia may be the first symptom. In children, hormonal effects, manifested primarily as sexual precocity, are the dominating features.
Morphology. These neoplasms form circumscribed nodules, usually less than 5 cm in diameter. They have a distinctive golden brown, homogeneous cut surface. Histologically, neoplastic Leydig cells usually are remarkably similar to their normal counterparts in that they are large and round or polygonal, and they have an abundant granular eosinophilic cytoplasm with a round central nucleus. The cytoplasm frequently contains lipid granules, vacuoles, or lipofuscin pigment, and, most characteristically, rod-shaped crystalloids of Reinke occur in about 25% of the tumors. Approximately 10% of the tumors in adults are invasive and produce metastases; most are benign.
Sertoli Cell Tumors
Most Sertoli cell tumors are hormonally silent and present as a testicular mass.85
Morphology. These neoplasms appear as firm, small nodules with a homogeneous gray-white to yellow cut surface. Histologically the tumor cells are arranged in distinctive trabeculae that tend to form cordlike structures and tubules. Most Sertoli cell tumors are benign, but occasional tumors (∼10%) pursue a malignant course.
Gonadoblastoma
Gonadoblastomas are rare neoplasms containing a mixture of germ cells and gonadal stromal elements, that almost always arise in gonads with some form of testrcular dysgenesis (discussed earlier). In some cases the germ cell component becomes malignant, giving rise to seminoma.
Testicular Lymphoma
Although an uncommon tumor of the testis, testicular lymphoma is included here because affected patients present with only a testicular mass, mimicking other, more common, testicular tumors. Aggressive non-Hodgkin lymphomas account for 5% of testicular neoplasms, and are the most common form of testicular neoplasms in men over the age of 60. In most cases, the disease is already disseminated at the time of detection. The most common testicular lyphomas, in decreasing order of frequency, are diffuse large B cell lymphoma, Burkitt Lymphoma, and EBV-positive extranodal NK/T cell lymphoma (Chapter 13). Patients with testicular lymphomas have a higher incidence of central nervous system involvement than those similar tumors located elsewhere.
MISCELLANEOUS LESIONS OF TUNICA VAGINALIS
Brief mention should be made of the tunica vaginalis, which is a mesothelial-lined surface exterior to the testis that may accumulate serous fluid (hydrocele) causing considerable enlargement of the scrotal sac. By transillumination it is usually possible to define the clear, translucent character of the contained fluid. Hydrocele sacs are frequently lined by mesothelial cells. Rarely, malignant mesotheliomas also can be seen arising from the tunica vaginalis.
Hematocele indicates the presence of blood in the tunica vaginalis. It is an uncommon condition usually encountered only when there has been either direct trauma to the testis or torsion of the testis with hemorrhagic suffusion into the surrounding tunica vaginalis or in hemorrhagic diseases associated with widespread bleeding diatheses. Chylocele refers to the accumulation of lymph in the tunica and is almost always found in patients with elephantiasis who have widespread, severe lymphatic obstruction caused, for example, by filariasis (Chapter 8). Spermatocele refers to a small cystic accumulation of semen in dilated efferent ducts or ducts of the rete testis. Varicocele is a dilated vein in the spermatic cord. Varicoceles may be asymptomatic but have also been implicated in some men as a contributing factor to infertility. They can be corrected by surgical repair.
Prostate
In the normal adult the prostate weighs approximately 20 gm. The prostate is a retroperitoneal organ encircling the neck of the bladder and urethra, and is devoid of a distinct capsule. In the adult, prostatic parenchyma can be divided into four biologically and anatomically distinct zones or regions: the peripheral, central, and transitional zones, and the region of the anterior fibromuscular stroma (Fig. 21-30).87 The types of proliferative lesions are different in each region. For example, most hyperplasias arise in the transitional zone, whereas most carcinomas originate in the peripheral zone.
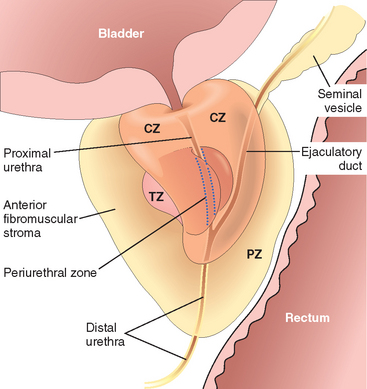
FIGURE 21-30 Adult prostate. The normal prostate contains several distinct regions, including a central zone (CZ), a peripheral zone (PZ), a transitional zone (TZ), and a periurethral zone. Most carcinomas arise from the peripheral glands of the organ and may be palpable during digital examination of the rectum. Nodular hyperplasia, in contrast, arises from more centrally situated glands and is more likely to produce urinary obstruction early than is carcinoma.
Histologically the prostate is composed of glands lined by two layers of cells: a basal layer of low cuboidal epithelium covered by a layer of columnar secretory cells (Fig. 21-31). In many areas there are small papillary infoldings of the epithelium. These glands are separated by abundant fibromuscular stroma. Testicular androgens control the growth and survival of prostatic cells. Castration leads to atrophy of the prostate caused by widespread apoptosis.
Only three pathologic processes affect the prostate gland with sufficient frequency to merit discussion: inflammation, benign nodular enlargement, and tumors. Of these three, the benign nodular enlargements are by far the most common and occur so often in advanced age that they can almost be construed as a “normal” aging process. Prostatic carcinoma is also an extremely common lesion in men and therefore merits careful consideration. We begin our discussion with consideration of the inflammatory processes.
INFLAMMATION
Prostatitis may be divided into several categories: acute and chronic bacterial prostatitis, chronic abacterial prostatitis, and granulomatous prostatitis.
Acute bacterial prostatitis typically results from bacteria similar to those that cause urinary tract infections. Thus, most cases are caused by various strains of E. coli, other gramnegative rods, enterococci, and staphylococci. The organisms become implanted in the prostate usually by intraprostatic reflux of urine from the posterior urethra or from the urinary bladder, but occasionally they seed the prostate by lymphohematogenous routes from distant foci of infection. Prostatitis sometimes follows surgical manipulation of the urethra or prostate gland itself, such as catheterization, cystoscopy, urethral dilation, or resection procedures on the prostate. Clinically, acute bacterial prostatitis is associated with fever, chills, and dysuria. On rectal examination the prostate is exquisitely tender and boggy. The diagnosis can be established by urine culture and clinical features.
Chronic bacterial prostatitis is difficult to diagnose and treat. It may present with low back pain, dysuria, and perineal and suprapubic discomfort. Alternatively, it may be virtually asymptomatic. Patients often have a history of recurrent urinary tract infections (cystitis, urethritis) caused by the same organism. Because most antibiotics penetrate the prostate poorly, bacteria find safe haven in the parenchyma and constantly seed the urinary tract. Diagnosis of chronic bacterial prostatitis depends on the demonstration of leukocytosis in the expressed prostatic secretions, along with positive bacterial cultures. In most cases, there is no antecedent acute attack, and the disease appears insidiously and without obvious provocation. The implicated organisms are the same as those cited as causes of acute prostatitis.
Chronic abacterial prostatitis is the most common form of prostatitis seen today. Clinically, it is indistinguishable from chronic bacterial prostatitis. There is no history, however, of recurrent urinary tract infection. Expressed prostatic secretions contain more than 10 leukocytes per high-power field, but bacterial cultures are uniformly negative.
Granulomatous prostatitis may be specific, where an etiologic infectious agent may be identified or non-specific.88 In the United States the most common cause is related to instillation of BCG within the bladder for treatment of superficial bladder cancer, discussed earlier in this chapter.89,90 BCG is an attenuated mycobacterial strain that gives rise to a histologic picture indistinguishable from that seen with systemic tuberculosis. However, in this setting the finding of granulomas in the prostate is of no clinical significance, and requires no treatment. Fungal granulomatous prostatitis is typically seen only in immunocompromised hosts. Nonspecific granulomatous prostatitis is relatively common and represents a reaction to secretions from ruptured prostatic ducts and acini.91 Although some of these men have a recent history of urinary tract infection, bacteria are not seen within the tissue in nonspecific granulomatous prostatitis.
Morphology. Acute prostatitis may appear as minute, disseminated abscesses; as large, coalescent focal areas of necrosis; or as diffuse edema, congestion, and boggy suppuration of the entire gland.
In men with symptoms of acute or chronic prostatitis, biopsy or surgical specimens are uncommonly examined microscopically, because the disease is diagnosed on clinical and laboratory findings. In fact biopsy of a man with acute prostatitis is contraindicated, as it may lead to sepsis. It is common in prostate specimens removed surgically to find histologic evidence of acute or chronic inflammation in men with no clinical symptoms of acute or chronic prostatitis. In these instances etiologic infectious agents have yet to be identified.92 So as not to be confused with the clinical syndromes of acute and chronic prostatitis, these prostate specimens are instead diagnosed in descriptive terms as showing “acute inflammation” or “chronic inflammation” and not as “prostatitis.”
BENIGN ENLARGEMENT
Benign Prostatic Hyperplasia (BPH) or Nodular Hyperplasia
BPH is an extremely common disorder in men over age 50.94 It is characterized by hyperplasia of prostatic stromal and epithelial cells, resulting in the formation of large, fairly discrete nodules in the periurethral region of the prostate. When sufficiently large, the nodules compress and narrow the urethral canal to cause partial, or sometimes virtually complete, obstruction of the urethra.
Incidence.
Histologic evidence of BPH can be seen in approximately 20% of men 40 years of age, a figure that increases to 70% by age 60 and to 90% by age 80. There is no direct correlation, however, between histologic changes and clinical symptoms. Only 50% of those who have microscopic evidence of BPH have clinically detectable enlargement of the prostate, and of these individuals, only 50% develop clinical symptoms. BPH is a problem of enormous magnitude, with approximately 30% of white American males over 50 years of age having moderate to severe symptoms.
Etiology and Pathogenesis.
Despite the fact that there is an increased number of epithelial cells and stromal components in the periurethral area of the prostate, there is no clear evidence of increased epithelial cell proliferation in human BPH. Instead, it is believed that the main component of the “hyperplastic” process is impaired cell death. It has been proposed that there is an overall reduction of the rate of cell death, resulting in the accumulation of senescent cells in the prostate.94 In keeping with this androgens (discussed below), which are required for the development of BPH, can not only increase cellular proliferation, but also inhibit cell death.
The main androgen in the prostate, constituting 90% of total prostatic androgens, is dihydrotestosterone (DHT). It is formed in the prostate from the conversion of testosterone by the enzyme type 2 5α-reductase.93-96 This enzyme is located almost entirely in stromal cells; epithelial cells of the prostate do not contain type 2 5α reductase, with the exception of a few basal cells. Thus stromal cells are responsible for androgen-dependent prostatic growth. Type 1 5α-reductase is not detected in the prostate, or is present at very low levels. However this enzyme may produce DHT from testosterone in liver and skin, and circulating DHT may act in the prostate by an endocrine mechanism.
DHT binds to the nuclear androgen receptor (AR) present in both stromal and epithelial prostate cells. DHT is more potent than testosterone because it has a higher affinity for AR and forms a more stable complex with the receptor. Binding of DHT to AR activates the transcription of androgen-dependent genes. DHT is not a direct mitogen for prostate cells, instead DHT-mediated transcription of genes results in the increased production of several growth factors and their receptors. Most important among these are members of the fibroblast growth factor (FGF) family, and particularly FGF-7 (keratinocyte growth factor; Chapter 3). FGF-7, produced by stromal cells, is probably the most important factor mediating the paracrine regulation of androgen-stimulated prostatic growth. Other growth factors produced in BPH are FGFs 1 and 2, and TGFβ, which promote fibroblast proliferation. Although the ultimate cause of BPH is unknown, it is believed that DHT-induced growth factors act by increasing the proliferation of stromal cells and decreasing the death of epithelial cells.
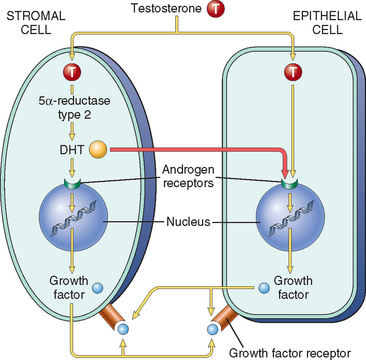
FIGURE 21-32 Simplified scheme of the pathogenesis of prostatic hyperplasia. The central role of the stromal cells in generating dihydrotestosterone (DHT) should be noted. DHT may also be produced in skin and liver by both type 1 and 2 5α-reductase.
Morphology. In the usual case of prostatic enlargement, the prostate weighs between 60 and 100 gm. Nodular hyperplasia of the prostate originates almost exclusively in the inner aspect of the prostate gland (transition zone). The early nodules are composed almost entirely of stromal cells, and later predominantly epithelial nodules arise. From their origin in this strategic location the nodular enlargements may encroach on the lateral walls of the urethra to compress it to a slitlike orifice (Fig. 21-33). In some cases, nodular enlargement may project up into the floor of the urethra as a hemispheric mass directly beneath the mucosa of the urethra, which is termed median lobe hypertrophy by clinicians.
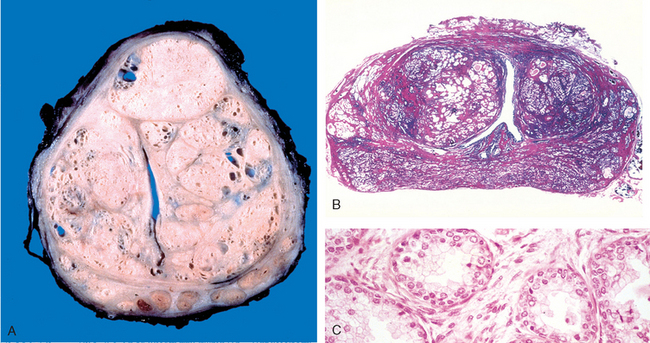
FIGURE 21-33 Nodular prostatic hyperplasia. A, Well-defined nodules of BPH compress the urethra into a slitlike lumen. B, A microscopic view of a whole mount of the prostate shows nodules of hyperplastic glands on both sides of the urethra. C, Under high power the characteristic dual cell population: the inner columnar and outer flattened basal cell can be seen.
On cross-section, the nodules vary in color and consistency. In nodules that contain mostly glands, the tissue is yellow-pink with a soft consistency, and a milky-white prostatic fluid oozes out of these areas. In nodules composed primarily of fibromuscular stroma, each nodule is pale gray, is tough, does not exude fluid, and is less clearly demarcated from the surrounding uninvolved prostatic tissue. Although the nodules do not have true capsules, the compressed surrounding prostatic tissue creates a plane of cleavage about them.
Microscopically, the hallmark of BPH is nodularity (Fig. 21-33B). The composition of the nodules ranges from purely stromal fibromuscular nodules to fibroepithelial nodules with a glandular predominance. Glandular proliferation takes the form of aggregations of small to large to cystically dilated glands, lined by two layers, an inner columnar and an outer cuboidal or flattened epithelium (Fig. 21-33C). The diagnosis of BPH cannot usually be made on needle biopsy, since the histology of glandular or mixed glandular-stromal nodules of BPH cannot be appreciated in limited samples. Also, needle biopsies do not typically sample the transition zone where BPH occurs. Occasionally foci of reactive squamous metaplasia histologically mimicking urothelial carcinoma can be seen adjacent to prostatic infarcts in prostates with prominent BPH.
Clinical Features.
The pathophysiology of BPH is complex, as it involves several factors. The increased size of the gland, and the smooth muscle-mediated contraction of the prostate cause uretheral obstruction. The increased resistance to urinary outflow leads to bladder hypertrophy and distension, accompanied by urine retention. The inability to empty the bladder completely creates a reservoir of residual urine that is a common source of infection. Patients experience increased urinary frequency, nocturia, difficulty in starting and stopping the stream of urine, overflow dribbling, dysuria (painful micturition), and have an increased risk of developing bacterial infections of the bladder and kidney. In many cases, sudden, acute urinary retention appears for unknown reasons that requires emergency catheterization.
Mild cases of BPH may be treated without medical or surgical therapy, such as by decreasing fluid intake, especially before bedtime; moderating the intake of alcohol and caffeinecontaining products; and following timed voiding schedules. The most commonly used and effective medical therapy for symptoms relating to BPH are α-blockers, which decrease prostate smooth muscle tone via inhibition of α1-adrenergic receptors.97,98 Another common pharmacologic therapy aims to decrease symptoms by physically shrinking the prostate with an agent that inhibits the synthesis of DHT. Inhibitors of 5-α-reductase fall into this category. For moderate to severe cases recalcitrant to medical therapy, a wide range of more invasive procedures exist. Transurethral resection of the prostate (TURP) has been the gold standard in terms of reducing symptoms, improving flow rates, and decreasing post-voiding residual urine. It is indicated as a first line of therapy in certain circumstances, such as recurrent urinary retention. As a result of its morbidity and cost, alternative procedures have been developed. These include high-intensity focused ultrasound, laser therapy, hyperthermia, transurethral electrovaporization, and transurethral needle ablation using radiofrequency. Nodular hyperplasia is not considered to be a premalignant lesion.
TUMORS
Adenocarcinoma
Adenocarcinoma of the prostate is the most common form of cancer in men, accounting for 29% of cancer in the United States in 2007.99 However, prostate cancer is tied with colorectal cancer in terms of cancer mortality, causing 9% of cancer deaths in the United States in 2007. There is a one in six lifetime probability of being diagnosed with prostate cancer. Over the last 20 years there has been a significant drop in prostate cancer mortality. It is one of the most remarkable tumors, exhibiting a wide range of clinical behaviors from very aggressive lethal cancers to incidentally discovered clinically insignificant cancers.
Incidence.
Cancer of the prostate is typically a disease of men over age 50. However, in men who are at increased risk (see “Etiology”), screening for prostate cancer is recommended to begin at age 40. Consideration has also been given to screen all men at age 40 and again at age 45 to detect uncommon cases of young men with prostate cancer before the disease becomes incurable. The incidence of prostatic cancer at autopsy is quite high. It increases from 20% in men in their 50s to approximately 70% in men between the ages of 70 and 80 years. There are some remarkable and puzzling national and racial differences in the incidence of the disease.100 Prostatic cancer is uncommon in Asians and occurs most frequently among blacks. In addition to hereditary factors, environment plays a role, as evidenced by the rise in the incidence of the disease in Japanese immigrants to the United States, though not nearly to the level of that of native-born Americans. Also, as the diet in Asia becomes more westernized, the incidence of clinically significant prostate cancer in this region of the world seems to be increasing. Whether this is due to dietary factors or other lifestyle changes is not clear.
Etiology and Pathogenesis.
Our knowledge of the causes of prostate cancer is far from complete. Several factors, including age, race, family history, hormone levels, and environmental influences are suspected to play a role. The increased incidence of this disease upon migration from a low-incidence region to one with a high incidence is consistent with a role for environmental influences. There are many candidate environmental factors, but none has been proven to be causative. For example, increased consumption of fats has been implicated. Other dietary products suspected of preventing or delaying prostate cancer development include lycopenes (found in tomatoes), selenium, soy products, and vitamin D.101
Androgens play an important role in prostate cancer. Like their normal counterparts, the growth and survival of prostate cancer cells depends on androgens, which bind to the androgen receptor (AR) and induce the expression of pro-growth and pro-survival genes. Of interest with respect to differences in prostate cancer risk among races, the X-linked AR gene contains a polymorphic sequence composed of repeats of the codon CAG (which codes for glutamine). Very large expansions of this stretch of CAGs cause a rare neurodegenerative disorder, Kennedy disease, characterized by muscle cramping and weakness. However, even in normal individuals, there is sufficient variation in the length of the CAG repeats to affect AR function. ARs with the shortest stretches of polyglutamine have the highest sensitivity to androgens. The shortest polyglutamine repeats on average are found in African Americans, while Caucasians have an intermediate length and Asians have the longest, paralleling the incidence and mortality of prostate cancer in these groups. More directly, the length of the repeats is inversely related to rate at which prostate cancer develops in mouse models.102
The importance of androgens in maintaining the growth and survival of prostate cancer cells can be seen in the therapeutic effect of castration or treatment with anti-androgens, which usually induce disease regression. Unfortunately, most tumors eventually become resistant to androgen blockade. Tumors escape through a variety of mechanisms, including acquisition of hypersensitivity to low levels of androgen (e.g., through AR gene amplification); mutations in AR that allow it to be activated by non-androgen ligands; and other mutations or epigenetic changes that activate alternative signaling pathways, which may bypass the need for AR altogether.103 Among the latter are changes that lead to increased activation of the P1-3 kinase/AKT signaling pathway, which is observed most often in tumors that have become resistant to anti-androgen therapy.
There is much interest in the role of other inherited polymorphisms in the development of prostate cancer.104-108 Compared with men with no family history, men with one first-degree relative with prostate cancer have twice the risk and those with two first-degree relatives have five times the risk of developing prostate cancer. Men with a strong family history of prostate cancer also tend to develop the disease at an earlier age. Men with germline mutations of the tumor suppressor BRCA2 have a 20-fold increased risk of prostate cancer, but the vast majority of familial prostate cancers are due to variation in other loci that confer a small increase in cancer risk. Family and genome-wide association studies have identified a number of risk-associated loci, including one at 8q24 that appears to selectively increase the risk among African American men.108 Of possible interest, a number of the candidate genes in these regions are involved in innate immunity, leading to speculation that inflammation may set the stage for the development of prostate carcinoma, as has been shown with respect to other human cancers (Chapter 7).
Other work is focused on the role of tumor-specific acquired somatic mutations and epigenetic changes. One very common type of somatic mutation in prostate cancer gives rise to chromosomal rearrangements that juxtapose the coding sequence of an ETS family transcription factor gene (most commonly ERG or ETV1) next to the androgen-regulated TMPRSS2 promoter.110 These rearrangements place the involved ETS gene under the control of the TMPRSS2 promoter and lead to their over-expression in an androgen-dependent fashion. Over-expression of ETS transcription factors makes normal prostate epithelial cells more invasive, possibly through the upregulation of matrix metalloproteases. In addition, tumors with rearranged ETS genes have certain distinctive morphologic features111 and a different gene expression signature than those lacking ETS gene rearrangements,112 suggesting that ETS gene rearrangements define a specific molecular sub-class of prostate cancer. ETS rearrangements may also have implications for prostate cancer screening and early diagnosis, as it is possible to detect ETS fusion genes in the urine using sensitive PCR assays.
The most common epigenetic alteration in prostate cancer is hypermethylation of glutathione S-transferase (GSTP1) gene which down-regualtes GSTP1 expression. The GSTP1 gene is located on chromosome 11q13 and is an important part of the pathway that prevents damage from a wide range of carcinogens.113 Other genes silenced by epigenetic modifications in a subset of prostate cancers include a number of tumor suppressor genes, including PTEN, RB, p16/INK4a, MLH1, MSH2, and APC.
In addition to prostate specific antigen (PSA, discussed below), other genes and proteins that may serve as biomarkers in prostate cancer have emerged, and some of these appear to play a direct role in the biology of the disease. Three worthy of brief mention are EZH-2 (enhancer of zeste-2), alpha-methylacyl-CoA racemase (AMACR), and PCA3. Prostate cancers show a relatively frequent loss of E-cadherin,114 an adhesion protein that is also down-regulated in invasive signet ring carcinoma of the stomach and lobular carcinoma of the breast. Loss of E-cadherin from prostate cancer cells is associated with expression of high levels of EZH-2, a transcriptional repressor that may contribute to prostate cancer progression.115 AMACR, an enzyme involved in the beta-oxidation of branched chain amino acids, is selectively upregulated in prostate cancer and its possible precursor lesions as compared to normal prostate (described below)116,117, as is PCA3, a gene on chromosome 9q that appears to encode a regulatory RNA.118,119
As can be surmised from the multiplicity of abnormalities, prostate carcinoma (like other cancers) is the product of some critical combination of acquired somatic mutations and epigenetic changes. A putative precursor lesion, prostatic intraepithelial neoplasia (PIN), has been described. Prostates containing cancer have a higher frequency and a greater extent of PIN, which is also often seen in proximity to cancer. Studies have revealed that many of the molecular changes seen in invasive cancers are present in PIN (for example, rearrangements involving ETS genes are found in a subset120,121), strongly supporting the argument that PIN is a precursor of invasive cancer. What remains unclear is whether PIN inevitably progresses to cancer, or instead sometimes remains latent or even regresses.122
Morphology. When the terms “prostate cancer” or “prostate adenocarcinoma” are used without qualifications it refers to the common or acinar variant of prostate cancer. In approximately 70% of cases, carcinoma of the prostate arises in the peripheral zone of the gland, classically in a posterior location, where it may be palpable on rectal examination (Fig. 21-34). Characteristically, on cross-section of the prostate the neoplastic tissue is gritty and firm, but when embedded within the prostatic substance it may be extremely difficult to visualize and be more readily apparent on palpation. Local extension most commonly involves periprostatic tissue, seminal vesicles, and the base of the urinary bladder, which in advanced disease may result in ureteral obstruction. Metastases first spread via lymphatics initially to the obturator nodes and eventually to the para-aortic nodes. Hematogenous spread occurs chiefly to the bones, particularly the axial skeleton, but some lesions spread widely to viscera. Massive visceral dissemination is an exception rather than the rule. The bony metastases are typically osteoblastic and in men point strongly to prostatic cancer (Fig. 21-35). The bones commonly involved, in descending order of frequency, are lumbar spine, proximal femur, pelvis, thoracic spine, and ribs.
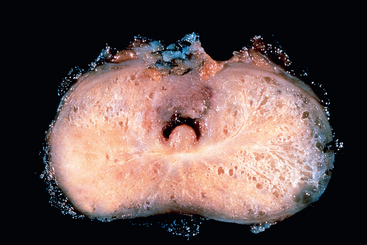
FIGURE 21-34 Adenocarcinoma of the prostate. Carcinomatous tissue is seen on the posterior aspect (lower left). Note solid whiter tissue of cancer in contrast to spongy appearance of benign peripheral zone in the contralateral side.
Histologically, most lesions are adenocarcinomas that produce well-defined, readily demonstrable gland patterns.123,124 The glands are typically smaller than benign glands and are lined by a single uniform layer of cuboidal or low columnar epithelium. In contrast to benign glands, prostate cancer glands are more crowded, and characteristically lack branching and papillary infolding. The outer basal cell layer typical of benign glands is absent. The cytoplasm of the tumor cells ranges from pale-clear as seen in benign glands to a distinctive amphophilic appearance. Nuclei are large and often contain one or more large nucleoli. There is some variation in nuclear size and shape, but in general pleomorphism is not marked. Mitotic figures are uncommon.
The histologic diagnosis of prostate cancer on biopsy specimens is one of the more difficult challenges for pathologists.125 In part, difficulty stems not only from the scant amount of tissue available for histologic examination removed by the needle biopsy, but also that biopsy often only samples a few malignant glands among many benign glands (Fig. 21-36). Morphologically, prostate cancer is difficult to diagnose in that the clues to malignancy may be subtle, increasing the likelihood of underdiagnosis. There are also many benign mimickers of cancer that can lead the unwary pathologist to a misdiagnosis of cancer. Although there are a few histologic findings on biopsy that are specific for prostate cancer, such as perineural invasion, in general the diagnosis is made based on a constellation of architectural, cytologic, and ancillary findings (Fig. 21-37). As discussed earlier, one distinguishing feature between benign and malignant prostate glands is that benign glands contain basal cells whereas they are absent in cancer (compare benign and malignant glands in Fig. 21-36A, and benign glands in Fig. 21-33C with cancerous glands in Fig. 21-36B).126 Pathologists have exploited this finding, by using various immunohistologic markers to label basal cells. α-methylacyl-coenzyme A-racemase (AMACR) is up-regulated in prostate cancer and can be detected by immunohistochemistry. The majority of prostate cancers are positive for AMACR, the sensitivity varying among studies from 82% to 100%. The use of all of these markers, while improving the accuracy of the diagnosis of prostate cancer, have their limitations with false positive and false negatives and must be used in conjunction with the routine H&E-stained sections.
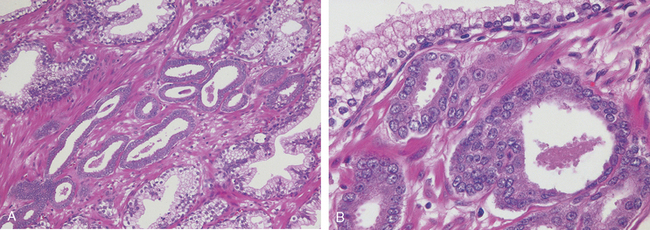
FIGURE 21-36 A, Photomicrograph of small focus of adenocarcinoma of the prostate demonstrating small glands crowded in between larger benign glands. B, Higher magnification shows several small malignant glands with enlarged nuclei, prominent nucleoli, and dark cytoplasm, as compared with larger benign gland (top).

FIGURE 21-37 Carcinoma of prostate showing perineural invasion by malignant glands. Compare to benign gland (left).
In approximately 80% of cases, prostatic tissue removed for carcinoma also harbors presumptive precursor lesions, referred to as high-grade prostatic intraepithelial neoplasia (PIN).127-128 PIN consists of architecturally benign prostatic acini lined by cytologically atypical cells with prominent nucleoli. Cytologically PIN and carcinoma may be identical, yet architecturally PIN involves larger branching glands with papillary infolding, in contrast to invasive cancer that is typically characterized by small crowded glands with straight luminal borders. PIN glands are surrounded by a patchy layer of basal cells and an intact basement membrane. There are several lines of evidence relating PIN to invasive cancer. First, both PIN and cancer typically predominate in the peripheral zone and are relatively uncommon in other zones. If one compares prostates without cancer to those with cancer, prostates containing cancer have a higher frequency and a greater extent of PIN. PIN is also often seen in proximity to cancer, in some cases the cancer appearing to bud off of the PIN. Many of the molecular changes seen in invasive cancers are also present in PIN, supporting the view that PIN is an intermediate lesion between normal and invasive cancer. Despite all this evidence, we do not know the natural history of PIN, and in particular how often it progresses to cancer. Thus, unlike in cancer of the cervix, the term “Carcinoma in situ” is not used for PIN. There are many other secrets about prostate cancer that have yet to be revealed.
Grading and Staging.
The grading schema used for prostate cancer is the Gleason system.129,130 According to this system, prostate cancers are stratified into five grades on the basis of glandular patterns of differentiation. Grade 1 represents the most well-differentiated tumors, in which the neoplastic glands are uniform and round in appearance and are packed into well-circumscribed nodules (Fig. 21-38A). By contrast, grade 5 tumors show no glandular differentiation, and the tumor cells infiltrate the stroma in the form of cords, sheets, and nests (Fig. 21-38C). The other grades fall in between. Most tumors contain more than one pattern, where one assigns a primary grade to the dominant pattern and a secondary grade to the second most frequent pattern. The two numeric grades are then added to obtain a combined Gleason grade or score. Thus, for example, a tumor with a dominant grade 3 and a secondary grade 4 would achieve a Gleason score of 7. Tumors with only one pattern are treated as if their primary and secondary grades are the same, and hence, the number is doubled. An exception to the rule is if three patterns are present on biopsy, the most common and highest grades are added together to arrive at the Gleason score. Thus, under this schema the most well-differentiated tumors have a Gleason score of 2 (1 + 1), and the least-differentiated tumors merit a score of 10 (5 + 5). Gleason scores are often combined into groups with similar biologic behavior, with grades 2 through 4 representing well-differentiated cancer, 5 and 6 intermediate-grade tumor, 7 moderate to poorly differentiated cancer, and 8 through 10 high-grade tumor. Gleason scores of 2 through 4 are typically found in small tumors within the transition zone. In surgical specimens, such low-grade cancer is typically an incidental finding on TURP performed for symptoms of BPH. The majority of potentially treatable cancers detected on needle biopsy as a result of screening have Gleason scores of 5 through 7. Tumors with Gleason scores 8 through 10 tend to be advanced cancers that are unlikely to be cured. Although there is some evidence that prostate cancers can become more aggressive with time, most commonly, the Gleason score remains stable over a period of several years. Grading is of particular importance in prostatic cancer, because grade and stage are the best prognostic predictors.
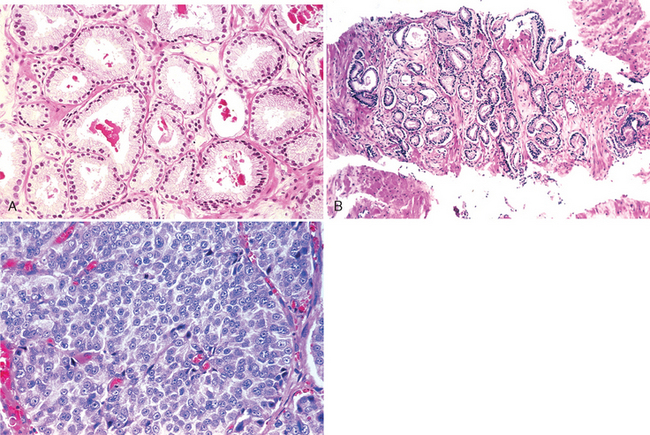
FIGURE 21-38 A, Low-grade (Gleason score 1 + 1 = 2) prostate cancer consisting of back-to-back uniform-sized malignant glands. Glands contain eosinophilic intraluminal prostatic crystalloids, a feature more commonly seen in cancer than benign glands and more frequently seen in lower grade than higher grade prostate cancer. B, Needle biopsy of the prostate with variably sized, more widely dispersed glands of moderately differentiated Gleason score 3 + 3 = 6) adenocarcinoma. C, Poorly differentiated (Gleason score 5 + 5 = 10) adenocarcinoma composed of sheets of malignant cells.
Staging of prostatic cancer is also important in the selection of the appropriate form of therapy (Table 21-6). Stage T1 refers to incidentally found cancer, either on TURP done for BPH symptoms (T1a and T1b depending on the extent and grade) or on needle biopsy typically performed for elevated serum prostate-specific antigen (PSA) levels (stage T1c).131-133 Stage T2 is organ-confined cancer. Stage T3a and T3b tumors show extra-prostatic extension, with and without seminal vesicle invasion, respectively. Stage T4 reflects direct invasion of contiguous organs. Any spread of tumor to the lymph nodes regardless of extent is eventually associated with a fatal outcome, such that the staging system merely records the presence or absence of this finding (N0/N1).
TABLE 21-6 Staging of Prostatic Adenocarcinoma Using the TNM System
| TNM Designation | Anatomic Findings |
|---|---|
| Extent of Primary Tumor (T) | |
| T1 | CLINICALLY INAPPARENT LESION (BY PALPATION/IMAGING STUDIES) |
| T1a | Involvement of ≤5% of resected tissue |
| T1b | Involvement of >5% of resected tissue |
| T1c | Carcinoma present on needle biopsy (following elevated PSA) |
| T2 | PALPABLE OR VISIBLE CANCER CONFINED TO PROSTATE |
| T2a | Involvement of ≤5% of one lobe |
| T2b | Involvement of >5% of one lobe, but unilateral |
| T2c | Involvement of both lobes |
| T3 | LOCAL EXTRAPROSTATIC EXTENSION |
| T3a | Extracapsular extension |
| T3b | Seminal vesical invasion |
| T4 | INVASION OF CONTIGUOUS ORGANS AND/OR SUPPORTING STRUCTURES INCLUDING BLADDER NECK, RECTUM, EXTERNAL SPHINCTER, LEVATOR MUSCLES, OR PELVIC FLOOR |
| Status of Regional Lymph Nodes (N) | |
| N0 | NO REGIONAL NODAL METASTASES |
| N1 | METASTASIS IN REGIONAL LYMPH NODES |
| Distant Metastases (M) | |
| M0 | NO DISTANT METASTASES |
| M1 | DISTANT METASTASES PRESENT |
| M1a | Metastases to distant lymph nodes |
| M1b | Bone metastases |
| M1c | Other distant sites |
PSA, prostate-specific antigen.
Clinical Course.
It is generally accepted that most men with incidentally discovered focal (stage T1a) cancer found on TURP do not show evidence of progression when followed for 10 or more years. Older patients with stage T1a disease are typically followed, but younger men with a longer life expectancy may undergo needle biopsy to look for additional cancer in the peripheral zone of the prostate. Stage T1b lesions are more ominous and are treated the same as tumors that are found on needle biopsy, since they have a mortality of 20% if left untreated.
Localized prostate cancer is asymptomatic, and is usually discovered by the detection of a suspicious nodule on rectal examination or elevated serum PSA level (discussed later). Most prostatic cancers arise peripherally away from the urethra, and therefore urinary symptoms occur late. Patients with clinically advanced prostatic cancer may present with urinary symptoms, such as difficulty in starting or stopping the stream, dysuria, frequency, or hematuria. Today it is uncommon for patients to come to attention because of back pain caused by vertebral metastases. The finding of osteoblastic metastases by skeletal surveys or the much more sensitive radionuclide bone scanning is virtually diagnostic of this form of cancer in men. These patients have a universally fatal outcome.
Digital rectal examination may detect some early prostatic carcinomas because of their posterior location, although the test suffers from both low sensitivity and specificity. Although there are characteristic findings of prostate cancer on transrectal ultrasonography and other imaging modalities, the poor sensitivity and specificity of these tests also limit their diagnostic utility. Typically a transrectal needle biopsy is required to confirm the diagnosis.
PSA is the most important test used in the diagnosis and management of prostate cancer.134 PSA is a product of prostatic epithelium and is normally secreted in the semen. It is a serine protease whose function is to cleave and liquefy the seminal coagulum formed after ejaculation. In normal men, only minute amounts of PSA circulate in the serum. Elevated blood levels of PSA occur in association with localized as well as advanced cancer. In most laboratories a serum level of 4 ng/mL is used as a cutoff point between normal and abnormal. However, as discussed below, this simplified approach to serum PSA tests is not appropriate, and has led to the delay in diagnosis of many prostate cancers.
PSA is organ-specific, yet not cancer-specific. Although serum levels of PSA are elevated to a lesser extent in BPH, there is considerable overlap. Other factors such as prostatitis, infarct, instrumentation of the prostate, and ejaculation also increase serum PSA levels. Furthermore, 20% to 40% of patients with organ-confined prostate cancer have a PSA value of 4.0 ng/mL or less.
Whereas most readers of this text will not directly practice pathology, almost all will be confronted with evaluation of a serum PSA test, either as a treating primary care physician, in addressing the results of a family member’s or friend’s test, or for the male readers reviewing one’s own test results. The widespread use of this test, along with its complexity and the corresponding increased risk of it being interpreted incorrectly, warrant a detailed discussion of this topic. This test differs from most other laboratory tests that a physician may order in that it is a cancer detection test. Consequently, physicians should ensure that tests come back from the laboratory, abnormal values are recorded, and patients are contacted for follow-up of elevated levels. Numerous medical malpractice cases result from the mishandling of serum PSA test results and the subsequent delay in diagnosis.
Several refinements in the estimation and interpretation of PSA values have been proposed. These include the ratio between the serum PSA value and volume of prostate gland (PSA density), the rate of change in PSA value with time (PSA velocity), the use of age-specific reference ranges, and the ratio of free and bound PSA in the serum. Men with enlarged hyperplastic prostate glands have higher total serum PSA levels than men with small glands. The measurement of serum PSA density factors out the contribution of benign prostatic tissue to serum PSA levels. It is calculated by dividing the total serum PSA level by the estimated gland volume (usually determined by transrectal ultrasound measurements) to estimate the PSA produced per gram of prostate tissue. As men age, their prostates tend to enlarge with BPH. One would then anticipate that overall older men would have higher serum PSA levels than younger men. The upper age-specific PSA reference ranges are 2.5 ng/mL for men 40 to 49 years of age, 3.5 ng/mL for men 50 to 59 years, 4.5 ng/mL for men 60 to 69 years, and 6.5 ng/mL for men 70 to 79 years. Consequently, a serum PSA value of 3.5, while it will appear as a normal value on a laboratory test, is a worrisome finding in a man in his 40s, warranting additional evaluation. Another means of interpreting serum PSA tests is by assessing PSA velocity or the rate of change of PSA. Men with prostate cancer demonstrate an increased rate of rise in PSA as compared with men who do not have prostate cancer. The rate of change in PSA that best distinguishes between men with and without prostate cancer is 0.75 ng/mL per year. If this test is to be valid, there must be at least three PSA measurements available over a period of 1.5 to 2 years, as there is substantial short-term variability (up to 20%) between repeat PSA measurements. A man who has a significant rise in serum PSA levels even though the latest serum PSA test may be below the normal cutoff (<4 ng/mL), should undergo additional work-up. Studies have revealed that immunoreactive PSA (the form detected by the widely used antibody test) exists in two forms: a major fraction bound to α1-antichymotrypsin and a minor free fraction. The percentage of free PSA (free PSA/total PSA × 100) is lower in men with prostate cancer than in men with benign prostatic diseases. Free PSA higher than 25% indicates a lower risk of cancer, as compared with free PSA values of less than 10%, which are of concern for cancer.
Because many small cancers localized to the prostate may never progress to clinically significant invasive cancers, there is considerable uncertainty regarding the management of small lesions that are detected because of an elevated PSA level. This has created some controversy about the role of widespread screening for prostate cancer. Much effort is therefore focused in devising criteria by which those localized lesions most likely to advance can be distinguished from those that may remain innocuous.
Serial measurements of PSA are of great value in assessing the response to therapy. For example, a rising PSA level after radical prostatectomy or radiotherapy for localized disease is indicative of recurrent or disseminated disease. Immunohistochemical localization of PSA on tissue sections can also help the pathologist to determine whether a metastatic tumor originated in the prostate.135
Cancer of the prostate is treated by surgery, radiation therapy, and hormonal manipulations. More than 90% of patients who receive such therapy can expect to live for 15 years. Currently, the most common treatment for clinically localized prostate cancer is radical prostatectomy. The prognosis following radical prostatectomy is based on the pathologic stage, margin status, and Gleason grade. Alternative treatments for localized prostate cancer are either external-beam radiation therapy or interstitial radiation therapy, the latter consisting of placing radioactive seeds throughout the prostate (brachytherapy). External-beam radiation therapy is also used to treat prostate cancer that is too locally advanced to be cured by surgery. Since some prostate cancers have a relatively indolent course, wherein it may take 10 years to see benefit from surgery or radiation therapy, active surveillance is appropriate for many older men or those with significant co-morbidity or even some younger men with low serum PSA values and limited lower grade cancer on biopsy. Advanced, metastatic carcinoma is treated by androgen deprivation either by orchiectomy or by administration of synthetic agonists of luteinizing hormone–releasing hormone (LHRH). Long-term administration of LHRH agonists suppresses normal LHRH, achieving in effect a pharmacologic orchiectomy. Although anti-androgen therapy induces remissions, eventually tumors develop testosterone-resistance, followed by a rapid progression of disease and death.
Miscellaneous Tumors and Tumor-like Conditions
Prostate adenocarcinomas may also arise from prostatic ducts. Ductal adenocarcinomas arising in peripheral ducts may present in a similar fashion to ordinary prostate cancer, whereas those arising in the larger periurethral ducts may show signs and symptoms similar to urothelial cancer, causing hematuria and urinary obstructive symptoms.136,137 Ductal adenocarcinomas are associated with a relatively poor prognosis. Prostate cancer may show squamous differentiation, either following hormone therapy or de novo, resulting in either adenosquamous or pure squamous cancer. Prostate cancer that reveal abundant mucinous secretions are termed colloid carcinoma of the prostate.138 The most aggressive variant of prostate cancer is small-cell cancer.139 Almost all cases are rapidly fatal, only a few surviving with aggressive combination chemotherapy.
The most common tumor to secondarily involve the prostate is urothelial cancer.139 Two distinct patterns of involvement exist. Large invasive urothelial cancers can directly invade from the bladder into the prostate. Alternatively, CIS of the bladder can extend into the prostatic urethra and down into the prostatic ducts and acini.
The same mesenchymal tumors described earlier that involve the bladder may also manifest in the prostate.140-142 In addition, there exist unique mesenchymal tumors of the prostate derived from the prostatic stroma.143 Although lymphomas may appear to first arise in the prostate, most patients shortly thereafter demonstrate systemic disease.144
1 Kottra JJ, Dunnick NR. Retroperitoneal fibrosis. Radiol Clin North Am. 1996;34:1259.
2 Smeulders N, Woodhouse CR. Neoplasia in adult exstrophy patients. BJU Int. 2001;87:623.
3 deVries CR, Freiha FS. Hemorrhagic cystitis: a review. J Urol. 1990;143:1.
4 Nickel JC. Interstitial cystitis. Etiology, diagnosis, and treatment. Can Fam Physician. 2000;46:2430.
5 Wyndaele JJ. Evaluation of patients with painful bladder syndrome/interstitial cystitis. Sci World J. 2005;5:942.
6 Long JPJr, Althausen AF. Malacoplakia: a 25-year experience with a review of the literature. J Urol. 1989;141:1328.
7 Young RH. Papillary and polypoid cystitis. A report of eight cases. Am J Surg Pathol. 1988;12:542.
8 Lane Z, Epstein JI. Polypoid/papillary cystitis: a series of 41 cases misdiagnosed as papillary urothelial neoplasia. Am J Surg Pathol. 2008;32:758.
9 Corica FA, et al. Intestinal metaplasia is not a strong risk factor for bladder cancer: study of 53 cases with long-term follow-up. Urology. 1997;50:427.
10 Young RH, Scully RE. Nephrogenic adenoma. A report of 15 cases, review of the literature, and comparison with clear cell adenocarcinoma of the urinary tract. Am J Surg Pathol. 1986;10:268.
11 Allan CH, Epstein JI. Nephrogenic adenoma of the prostatic urethra: a mimicker of prostate adenocarcinoma. Am J Surg Pathol. 2001;25:802.
12 Mazal PR, et al. Derivation of nephrogenic adenomas from renal tubular cells in kidney-transplant recipients. N Engl J Med. 2002;347:653.
13 Allan CH, Epstein JI. Nephrogenic adenoma of the prostatic urethra: a mimicker of prostate adenocarcinoma. Am J Surg Pathol. 2001;25:802.
14 Jemal A, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43.
15 Taylor DC, et al. Papillary urothelial hyperplasia. A precursor to papillary neoplasms. Am J Surg Pathol. 1996;20:1481.
16 Epstein JI, et al. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol. 1998;22:1435.
17 Eble JN, et al. The World Health Organization Classification of Tumours of the Urinary System and Male Genital System. Lyon: IARC Press, 2004.
18 Magi-Galluzzi C, Epstein JI. Urothelial papilloma of the bladder: a review of 34 de novo cases. Am J Surg Pathol. 2004;28:1615.
19 Cheville JC, et al. Inverted urothelial papilloma: is ploidy, MIB-1 proliferative activity, or p53 protein accumulation predictive of urothelial carcinoma? Cancer. 2000;88:632.
20 Witjes JA, et al. The prognostic value of a primary inverted papilloma of the urinary tract. J Urol. 1997;158:1500.
21 Gilbert HA, et al. The natural history of papillary transitional cell carcinoma of the bladder and its treatment in an unselected population on the basis of histologic grading. J Urol. 1978;119:488.
22 Heney NM, et al. Superficial bladder cancer: progression and recurrence. J Urol. 1983;130:1083.
23 Melamed MR, et al. Natural history and clinical behavior of in situ carcinoma of the human urinary bladder. 1964. CA Cancer J Clin. 1993;43:348.
24 Elliott GB, et al. “Denuding cystitis” and in situ urothelial carcinoma. Arch Pathol. 1973;96:91.
25 Farrow GM, et al. Clinical observations on sixty-nine cases of in situ carcinoma of the urinary bladder. Cancer Res. 1977;37:2794.
26 Melicow MM, Hollowell JW. Intra-urothelial cancer: carcinoma in situ, Bowen’s disease of the urinary system: discussion of thirty cases. J Urol. 1952;68:763.
27 Drew PA, et al. The nested variant of transitional cell carcinoma: an aggressive neoplasm with innocuous histology. Mod Pathol. 1996;9:989.
28 Volmar KE, et al. Florid von Brunn nests mimicking urothelial carcinoma: a morphologic and immunohistochemical comparison to the nested variant of urothelial carcinoma. Am J Surg Pathol. 2003;27:1243.
29 Amin MB, et al. Lymphoepithelioma-like carcinoma of the urinary bladder. Am J Surg Pathol. 1994;18:466.
30 Talbert ML, Young RH. Carcinomas of the urinary bladder with deceptively benign-appearing foci. A report of three cases. Am J Surg Pathol. 1989;13:374.
31 Tamas EF, et al. Lymphoepithelioma-like carcinoma of the urinary tract: a clinicopathological study of 30 pure and mixed cases. Mod Pathol. 2007;20:828.
32 Kamat AM, et al. Micropapillary bladder cancer: a review of the University of Texas M. D. Anderson Cancer Center experience with 100 consecutive patients. Cancer. 2007;110:62.
33 Sakamoto N, et al. Urinary bladder carcinoma with a neoplastic squamous component: a mapping study of 31 cases. Histopathology. 1992;21:135.
34 El-Bolkainy MN, et al. The impact of schistosomiasis on the pathology of bladder carcinoma. Cancer. 1981;48:2643.
35 Grignon DJ, et al. Primary adenocarcinoma of the urinary bladder. A clinicopathologic analysis of 72 cases. Cancer. 1991;67:2165.
36 Xiaoxu L, et al. Bladder adenocarcinoma: 31 reported cases. Can J Urol. 2001;8:1380.
37 Trias I, et al. Small cell carcinoma of the urinary bladder. Presentation of 23 cases and review of 134 published cases. Eur Urol. 2000;39:85.
38 Brandau S, Bohle A. Bladder cancer. I. Molecular and genetic basis of carcinogenesis. Eur Urol. 2000;39:491.
39 Jung I, Messing E. Molecular mechanisms and pathways in bladder cancer development and progression. Cancer Control. 2000;7:325.
40 Gibas Z, Gibas L. Cytogenetics of bladder cancer. Cancer Genet Cytogenet. 1997;95:108.
41 Spruck CH, et al. Two molecular pathways to transitional cell carcinoma of the bladder. Cancer Res. 1994;54:784.
42 Luis NM, et al. Molecular biology of bladder cancer. Clin Transl Oncol. 2007;9:5.
43 Holmang S, et al. Stage progression in Ta papillary urothelial tumors: relationship to grade, immunohistochemical expression of tumor markers, mitotic frequency and DNA ploidy. J Urol. 2001;165:1124.
44 Malmstrom PU, et al. Recurrence, progression and survival in bladder cancer. A retrospective analysis of 232 patients with greater than or equal to 5-year follow-up. Scand J Urol Nephrol. 1987;21:185.
45 Koss LG. Mapping of the urinary bladder: its impact on the concepts of bladder cancer. Hum Pathol. 1979;10:533.
46 Melicow MM. Histological study of vesical urothelium intervening between gross neoplasms in total cystectomy. J Urol. 1952;68:261.
47 Smith G, et al. Prognostic significance of biopsy results of normal-looking mucosa in cases of superficial bladder cancer. Br J Urol. 1983;55:665.
48 Murphy WM, Soloway MS. Developing carcinoma (dysplasia) of the urinary bladder. Pathol Annu. 1982;17(Pt 1):197.
49 Orozco RE, et al. Carcinoma in situ of the urinary bladder. Clues to host involvement in human carcinogenesis. Cancer. 1994;74:115.
50 Murphy WM, et al. Urinary cytology and bladder cancer. The cellular features of transitional cell neoplasms. Cancer. 1984;53:1555.
51 Nielsen ME, et al. Urinary markers in the detection of bladder cancer: what’s new? Curr Opin Urol. 2006;16:350.
52 Herr HW, et al. Bacillus Calmette-Guérin therapy for superficial bladder cancer: a 10-year followup. J Urol. 1992;147:1020.
53 Martin SA, et al. Smooth muscle neoplasms of the urinary bladder: a clinicopathologic comparison of leiomyoma and leiomyosarcoma. Am J Surg Pathol. 2002;26:292.
54 Montgomery EA, et al. Inflammatory myofibroblastic tumors of the urinary tract: a clinicopathologic study of 46 cases, including a malignant example inflammatory fibrosarcoma and a subset associated with high-grade urothelial carcinoma. Am J Surg Pathol. 2006;30:1502.
55 Lopez-Beltran A, et al. Carcinosarcoma and sarcomatoid carcinoma of the bladder: clinicopathological study of 41 cases. J Urol. 1998;159:1497.
56 Scholtmeijer RJ, et al. Embryonal rhabdomyosarcoma of the urogenital tract in childhood. Eur Urol. 1983;9:69.
57 Kempton CL, et al. Malignant lymphoma of the bladder: evidence from 36 cases that low-grade lymphoma of the MALT-type is the most common primary bladder lymphoma. Am J Surg Pathol. 1997;21:1324.
58 Diamond DA, Ransley PG. Male epispadias. J Urol. 1995;154:2150.
59 Belman AB. Hypospadias update. Urology. 1997;49:166.
60 Davenport M. ABC of general surgery in children. Problems with the penis and prepuce. BMJ. 1996;312:299.
61 Edwards S. Balanitis and balanoposthitis: a review. Genitourin Med. 1996;72:155.
62 Cupp MR, et al. The detection of human papillomavirus deoxyribonucleic acid in intraepithelial, in situ, verrucous and invasive carcinoma of the penis. J Urol. 1995;154:1024.
63 Dillner J, et al. Etiology of squamous cell carcinoma of the penis. Scand J Urol Nephrol Suppl. 2000;205:189.
64 Cubilla AL, et al. Morphological features of epithelial abnormalities and precancerous lesions of the penis. Scand J Urol Nephrol Suppl. 2000;205:215.
65 Cubilla AL, et al. Histologic classification of penile carcinoma and its relation to outcome in 61 patients with primary resection. Int J Surg Pathol. 2001;9:111.
66 Burgers JK, et al. Penile cancer. Clinical presentation, diagnosis, and staging. Urol Clin North Am. 1992;19:247.
67 Rozanski TA, Bloom DA. The undescended testis. Theory and management. Urol Clin North Am. 1995;22:107.
68 Hutson JM, et al. Normal testicular descent and the aetiology of cryptorchidism. Adv Anat Embryol Cell Biol. 1996;132:1.
69 Swerdlow AJ, et al. Risk of testicular cancer in cohort of boys with cryptorchidism. BMJ. 1997;314:1507.
70 Davenport M. ABC of general paediatric surgery. Inguinal hernia, hydrocele, and the undescended testis. BMJ. 1996;312:564.
71 United Kingdom Testicular Cancer Study Group. Aetiology of testicular cancer: association with congenital abnormalities, age at puberty, infertility, and exercise. BMJ. 1994;308:1393.
72 Buetow SA. Epidemiology of testicular cancer. Epidemiol Rev. 1995;17:433.
73 Nistal M, Paniagua R. Testicular biopsy. Contemporary interpretation. Urol Clin North Am. 1999;26:555.
74 Ulbright TM. Germ cell neoplasms of the testis. Am J Surg Pathol. 1993;17:1075.
75 Bosl GJ, Motzer RJ. Testicular germ-cell cancer. N Engl J Med. 1997;337:242.
76 McIntyre A, et al. Genes, chromosomes and the development of testicular germ cell tumors of adolescents and adults. Genes, Chromosomes, Cancer. 2008;47:547.
77 Looijenga LHS, et al. Chromosomes and expression in human testicular germ-cell tumors. Insight into their origin and pathogenesis. Ann NY Acad Sci. 2007;1120:187.
78 Eble JN. Spermatocytic seminoma. Hum Pathol. 1994;25:1035.
79 Emerson RE, Ulbright TM. The use of immunohistochemistry in the differential diagnosis of tumors of the testis and paratestis. Semin Diagn Pathol. 2005;22:33.
80 Motzer RJ, et al. Teratoma with malignant transformation: diverse malignant histologies arising in men with germ cell tumors. J Urol. 1998;159:133.
81 Doherty AP, et al. The role of tumour markers in the diagnosis and treatment of testicular germ cell cancers. Br J Urol. 1997;79:247.
82 Dilworth JP, et al. Non–germ cell tumors of testis. Urology. 1991;37:399.
83 Kim I, et al. Leydig cell tumors of the testis. A clinicopathological analysis of 40 cases and review of the literature. Am J Surg Pathol. 1985;9:177.
84 Cheville JC, et al. Leydig cell tumor of the testis: a clinicopathologic, DNA content, and MIB-1 comparison of nonmetastasizing and metastasizing tumors. Am J Surg Pathol. 1998;22:1361.
85 Young RH, et al. Sertoli cell tumors of the testis, not otherwise speci-fied: a clinicopathologic analysis of 60 cases. Am J Surg Pathol. 1998;22:709.
86 Ferry JA, et al. Malignant lymphoma of the testis, epididymis, and spermatic cord. A clinicopathologic study of 69 cases with immunophenotypic analysis. Am J Surg Pathol. 1994;18:376.
87 McNeal JE. Normal and pathologic anatomy of prostate. Urology. 1981;17:11.
88 Wise GJ, Silver DA. Fungal infections of the genitourinary system. J Urol. 1993;149:1377.
89 Oates RD, et al. Granulomatous prostatitis following bacillus Calmette-Guerin immunotherapy of bladder cancer. J Urol. 1988;140:751.
90 Mukamel E, et al. Clinical and pathological findings in prostates following intravesical bacillus Calmette-Guérin instillations. J Urol. 1990;144:1399.
91 Epstein JI, Hutchins GM. Granulomatous prostatitis: distinction among allergic, nonspecific, and post-transurethral resection lesions. Hum Pathol. 1984;15:818.
92 Kohnen PW, Drach GW. Patterns of inflammation in prostatic hyperplasia: a histologic and bacteriologic study. J Urol. 1979;121:755.
93 Roehrborn CG, McConnell. Benign prostatic hyperplasia: etiology, pathophysiology, epidemiology and natural history. Wein AJ, editor. Campbell-Walsh Urology. Philadelphia: WB Saunders; 2007;vol XVI:2727.
94 Umtergasser G, et al. Benign prostatic hyperplasia: age related tissue-remodeling. Exp Gerontol. 2005;40:121.
95 Marks LS, et al. Prostate tissue androgens: history and current clinical relevance. Urology. 2008;72:247.
96 Heracek J, et al. Tissue and serum levels of principal androgens in benign prostatic hyperplasia and prostate cancer. Steroids. 2007;72:375.
97 Roehrborn GC. Current medical therapies for men with lower urinary tract symptoms and benign prostatic hyperplasia: achievements and limitations. Rev Urol. 2008;10:14.
98 Barkin J. management of benign prostatic hyperplasia by primary care physicians in the 21st century: the new paradigm. Can J Urol Suppl. 2008;1:21.
99 Jemal A, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43.
100 Ekman P. Genetic and environmental factors in prostate cancer genesis: identifying high-risk cohorts. Eur Urol. 1999;35:362.
101 Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266.
102 Albertelli MA, et al. Replacing the mouse androgen receptor with human alleles demonstrates glutamine tract length-dependent effects on physiology and tumorigenesis in mice. Mol Endocrin. 2006;20:1248.
103 Nieto M, et al. Prostate cancer: Refocusing on androgen receptor signaling. Int J Biochem Cell Biol. 2007;39:1562.
104 DeMarzo AM, et al. Pathological and molecular aspects of prostate cancer. Lancet. 2003;361:955.
105 Nelson WG, et al. Prostate cancer. N Engl J Med. 2003;349:366.
106 Prowatke I, et al. Expression analysis of imbalanced genes in prostate carcinoma using tissue microarrays. Br J Cancer. 2007;96:82.
107 Tomlins SA, Rubin MA, Chinnaiyan AM. Integrative biology of prostate cancer progression. Annu Rev Pathol. 2006;1:243.
108 Wiklund F, Gillanders EM, Albertus JA, Bergh A, Damber JE, Emanuelsson M, et al. Genome-wide scan of Swedish families with hereditary prostate cancer: suggestive evidence of linkage at 5q11.2 and 19p13.3. Prostate. 2003;57:290.
109 Freedman ML, et al. Admixture mapping identifies 8q24 as a prostate cancer risk locus in African-American men. Proc Natl Acad Sci USA. 2006;103:14068.
110 Kumar-Sinha C, Tomlins SA, Chinnaiyan AM. Recurrent gene fusions in prostate cancer. Nature Rev Cancer. 2008;8:497.
111 Mosquera JM, et al. Morphologic features of TMPRSS2-ERG gene fusion prostate cancer. J Pathol. 2007;212:91.
112 Iljin K, et al. TMRPSS2 fusions with oncogenic ETS factors in prostate cancer involve unbalanced genomic rearrangements and are associated with HDAC1 and epigenetic reprogramming. Cancer Res. 2006;66:10658.
113 Carmen J, et al. Quantitation of GSTP1 methylation in non-neoplastic prostatic tissue and organ-confined prostate adenocarcinoma. J Natl Cancer Inst. 2001;93:1671.
114 Schalken JA, et al. Molecular prostate cancer pathology: current issues and achievements. Scand J Urol Nephrol Suppl. 2005;216:82.
115 Varambally S, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624.
116 Jiang Z, et al. Discovery and clinical application of a novel prostate cancer marker: alpha-methylacyl CoA racemase (P504S). Am J Clin Pathol. 2004;122:275.
117 Luo J, et al. Alpha-methylacyl-CoA racemase: a new molecular marker for prostate cancer. Cancer Res. 2002;62:2220.
118 Groskopf J, et al. APTIMA PCA3 molecular urine test: development of a method to aid in the diagnosis of prostate cancer. Clin Chem. 2006;52:1089.
119 Marks LS et al.: PCA3 molecular urine assay for prostate cancer in men.
120 Cerveira N, et al. TMPRSS2-ERG gene fusion causing ERG overexpression precedes chromosome copy number changes in prostate carcinomas and paired HGPIN lesions. Neoplasia. 2006;8:826.
121 Perner S, et al. TMPRSS2: ERG fusion prostate cancer: an early molecular event associated with invasion. Am J Surg Pathol. 2007;31:882.
122 Epstein JI, Herawi M. Prostate needle biopsies containing prostatic intraepithelial neoplasia or atypical foci suspicious for carcinoma: implications for patient care. J Urol. 2006;175:820.
123 Epstein JI, Netto GJ. Biopsy Interpretation of the Prostate. Philadelphia: JB Lippincott Williams & Wilkins, 2008.
124 Eble JN, et al. Pathology and Genetics: Tumors of the urinary system and male genital organs. WHO classification of tumors. Geneva: World Health Organization, 2004.
125 Epstein JI. Diagnostic criteria of limited adenocarcinoma of the prostate on needle biopsy. Hum Pathol. 1995;26:223.
126 Wojno KJ, Epstein JI. The utility of basal cell-specific anti-cytokeratin antibody (34 beta E12) in the diagnosis of prostate cancer. A review of 228 cases. Am J Surg Pathol. 1995;19:251.
127 McNeal JE. Significance of duct-acinar dysplasia in prostatic carcinogenesis. Urology. 1989;34:9.
128 McNeal JE, Bostwick DG. Intraductal dysplasia: a premalignant lesion of the prostate. Hum Pathol. 1986;17:64.
129 Epstein JI, et al. Update on the Gleason grading system for prostate cancer: results of an international consensus conference of urologic pathologists. Adv Anat Pathol. 2006;13:57.
130 Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol. 1974;111:58.
131 Epstein JI, et al. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271:368.
132 Matzkin H, et al. Stage T1A carcinoma of prostate. Urology. 1994;43:11.
133 Eble JN, Epstein JI. Stage A carcinoma of the prostate. In: Roth LM, editor. Pathology of the Prostate, Seminal Vesicles, and Male Urethra. New York: Churchill Livingstone; 1990:61-82.
134 Gretzer MB, Partin AW. PSA markers in prostate cancer detection. Urol Clin North Am. 2003;30:677.
135 Epstein JI. PSAP and PSA as immunohistochemical markers. Urol Clin North Am. 1993;20:757.
136 Brinker DA, et al. Ductal adenocarcinoma of the prostate diagnosed on needle biopsy: correlation with clinical and radical prostatectomy findings and progression. Am J Surg Pathol. 1999;23:1471.
137 Ro JY, et al. Mucinous adenocarcinoma of the prostate: histo-chemical and immunohistochemical studies. Hum Pathol. 1990;21:593.
138 Wang W, Epstein JI. Small cell carcinoma of the prostate: a morphological and immunohistochemical study of 95 cases. Am J Surg Pathol. 2008;32:65.
139 Oliai BR, et al. A clinicopathologic analysis of urothelial carcino-mas diagnosed on prostate needle biopsy. Am J Surg Pathol. 2001;25:794.
140 Sexton WJ, et al. Adult prostate sarcoma: the M.D. Anderson Cancer Center Experience. J Urol. 2001;166:521.
141 Raney RB, et al. Rhabdomyosarcoma and undifferentiated sarcoma in the first two decades of life: a selective review of intergroup rhabdomyosarcoma study group experience and rationale for Intergroup Rhabdomyosarcoma Study V. J Pediatr Hematol Oncol. 2001;23:215.
142 Hansel DE, Epstein JI. Sarcomatoid carcinoma of the prostate: a study of 42 cases. Am J Surg Pathol. 2006;30:1316.
143 Herawi M, Epstein JI. Specialized stromal tumors of the prostate: a clinicopathologic study of 50 cases. Am J Surg Pathol. 2006;30:694.
144 Bostwick DG, Mann RB. Malignant lymphomas involving the prostate. A study of 13 cases. Cancer. 1985;56:2932.