Chapter 19 Integumentary System
The integumentary system consists of the skin and its appendages: sweat glands, nails, hairs, sebaceous glands, arrector muscles of hairs (arrector pili muscles), mammary glands, and teeth.
Development of Skin and Appendages
The skin, the outer protective covering of the body, is a complex organ system and is the body’s largest organ. The skin consists of two layers (Fig. 19-1):
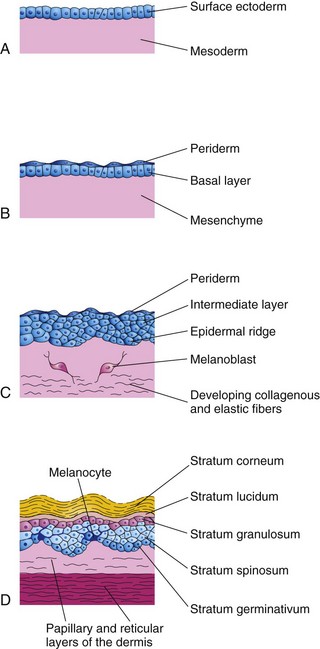
FIGURE 19–1 Illustrations of the successive stages of skin development. A, At 4 weeks. B, At 7 weeks. C, At 11 weeks. D, Neonate. Note the melanocytes in the basal layer of the epidermis and the way their processes extend between the epidermal cells to supply them with melanin.
Ectodermal (epidermal)/mesenchymal (dermal) interactions involve mutual inductive mechanisms that are mediated by a conserved set of signaling molecules of the Wnt, FGF, TGFβ, and Hedgehog pathways.
Skin structures vary from one part of the body to another. For example, the skin of the eyelids is thin and soft and has fine hairs, whereas the skin of the eyebrows is thick and has coarse hairs. The embryonic skin at 4 to 5 weeks consists of a single layer of surface ectoderm overlying the mesoderm (Fig. 19-1A).
Epidermis
Epidermal growth occurs in stages and results in an increase in epidermal thickness. The primordium of the epidermis is a single layer of surface ectodermal cells (Fig. 19-1A). These cells proliferate and form a layer of squamous epithelium, the periderm, and a basal layer (Fig. 19-1B and C). The cells of the periderm continually undergo keratinization (formation of a horny layer) and desquamation (shedding of the cuticle in scales), and are replaced by cells arising from the basal layer. The exfoliated peridermal cells form part of a white greasy substance—vernix caseosa—that covers the fetal skin. Later, the vernix contains sebum, a secretion from sebaceous glands (see Fig. 19-3). The vernix protects the developing skin from constant exposure to amniotic fluid, with its high content of urine, bile salts, and sloughed cells, during the fetal period. In addition, the greasy vernix facilitates birth of the fetus.
The basal layer of the epidermis becomes the stratum germinativum (Fig. 19-1D), which produces new cells that are displaced into the more superficial layers. By 11 weeks, cells from the stratum germinativum have formed an intermediate layer (Fig. 19-1C). Replacement of peridermal cells continues until approximately the 21st week; thereafter, the periderm disappears and the stratum corneum forms from the stratum lucidum (Fig. 19-1D).
Proliferation of cells in the stratum germinativum also forms epidermal ridges, which extend into the developing dermis (Fig. 19-2). These ridges begin to appear in embryos at 10 weeks and are permanently established by 19 weeks; those of the hand appear approximately one week earlier than in the feet. The epidermal ridges produce grooves on the surface of the palms and soles, including the digits (fingers and toes). The type of pattern that develops is determined genetically and constitutes the basis for examining fingerprints in criminal investigations and medical genetics. Abnormal chromosome complements affect the development of ridge patterns; for instance, infants with Down syndrome have distinctive patterns on their hands and feet that are of diagnostic value.
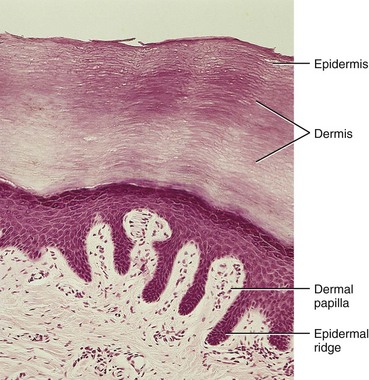
FIGURE 19–2 Light micrograph of thick skin (x132). Observe the epidermis and dermis as well as the dermal papilla interdigitating with the epidermal ridges.
(From Gartner LP, Hiatt JL: Color Textbook of Histology, 2nd ed. Philadelphia, WB Saunders, 2001.)
Late in the embryonic period, neural crest cells migrate into the mesenchyme of the developing dermis and differentiate into melanoblasts. Later these cells migrate to the dermoepidermal junction and differentiate into melanocytes (Fig. 19-1D). The differentiation of melanoblasts into melanocytes involves the formation of pigment granules. Wnt signaling regulates this process. Melanocytes appear in the developing skin at 40 to 50 days, immediately after the migration of neural crest cells. In Caucasians, the cell bodies of melanocytes are usually confined to basal layers of the epidermis; however, their dendritic processes extend between the epidermal cells (Fig. 19-1C).
Only a few melanin-containing cells are normally present in the dermis (Fig. 19-1D). The melanocytes begin producing melanin before birth and distribute it to the epidermal cells. Pigment formation can be observed prenatally in the epidermis of dark-skinned races; however, there is little evidence of such activity in light-skinned fetuses. The relative content of melanin inside the melanocytes accounts for the different colors of skin.
The transformation of the surface ectoderm into the multilayered definitive epidermis results from continuing inductive interactions with the dermis. Skin is classified as thick or thin based on the thickness of the epidermis.
Dermis
The dermis develops from mesenchyme, which is derived from the mesoderm underlying the surface ectoderm (Fig. 19-1A and B). Most of the mesenchyme that differentiates into the connective tissue of the dermis originates from the somatic layer of lateral mesoderm; however, some of it is derived from the dermatomes of the somites (see Chapter 14). By 11 weeks, the mesenchymal cells have begun to produce collagenous and elastic connective tissue fibers (Figs. 19-1D and 19-3).
As the epidermal ridges form, the dermis projects into the epidermis, forming dermal papillae, which interdigitate with the epidermal ridges (Fig. 19-2). Capillary loops of blood vessels develop in some of these papillae and provide nourishment for the epidermis (Fig. 19-3); sensory nerve endings form in other papillae. The developing afferent nerve fibers apparently play an important role in the spatial and temporal sequence of dermal ridge formation. The development of the dermatomal pattern of innervation of the skin is described in Chapter 16.
The blood vessels in the dermis begin as simple, endothelium-lined structures that differentiate from mesenchyme. As the skin grows, new capillaries grow out from the primordial vessels (angiogenesis). Such capillary-like vessels have been observed in the dermis at the end of the fifth week. Some capillaries acquire muscular coats through differentiation of myoblasts developing in the surrounding mesenchyme and become arterioles and arteries. Other capillaries, through which a return flow of blood is established, acquire muscular coats and become venules and veins. As new blood vessels form, some transitory ones disappear. By the end of the first trimester, the major vascular organization of the fetal dermis is established.
Development of Glands
The glands of the skin include eccrine and apocrine sweat glands, sebaceous glands, and mammary glands. They are derived from the epidermis and grow into the dermis.
Sebaceous Glands
Sebaceous glands are derived from the epidermis. Cellular buds develop from the sides of the developing epithelial root sheaths of hair follicles (Fig. 19-3). The buds invade the surrounding dermal connective tissue and branch to form the primordia of several alveoli (hollow sacs) and their associated ducts. The central cells of the alveoli break down, forming an oily substance—sebrum—that protects the skin against friction and dehydration. This secretion is released into the hair follicle and passes to the surface of the skin, where it mixes with desquamated peridermal cells to form vernix caseosa, a greasy, cheese-like substance, which protects the delicate fetal skin from abrasions and hardening that result from exposure to the amniotic fluid. Sebaceous glands, independent of hair follicles (e.g., in the glans of the penis and labia minora), develop as cellular buds from the epidermis that invade the dermis.
Sweat Glands
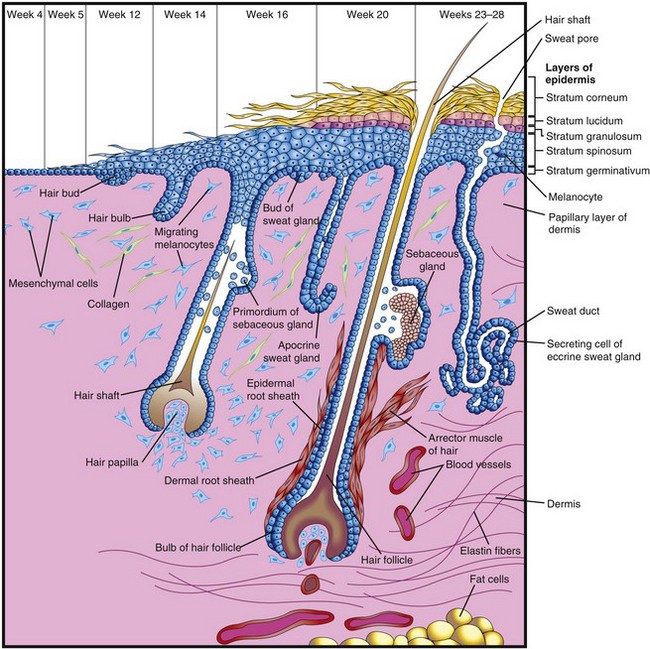
Coiled, tubular eccrine sweat glands are located in the skin throughout most of the body. They develop as cellular buds from the epidermis that grow into the underlying mesenchyme (Fig. 19-3). As the buds elongate, their ends coil to form the bodies of the secretory parts of the glands (Fig. 19-4A to D). The epithelial attachments of the developing glands to the epidermis form the primordia of the sweat ducts. The central cells of these ducts degenerate, forming lumina. The peripheral cells of the secretory parts of the glands differentiate into myoepithelial and secretory cells (Fig. 19-4D). The myoepithelial cells are thought to be specialized smooth muscle cells that assist in expelling sweat from the glands. Eccrine sweat glands begin to function shortly after birth.
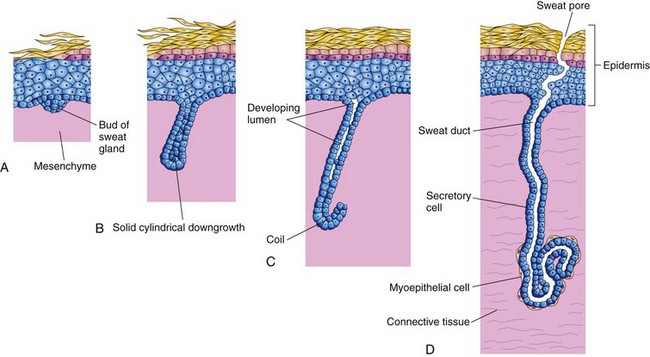
FIGURE 19–4 Illustrations of the successive stages of the development of a sweat gland. A and B, The cellular buds of the glands develop at approximately 20 weeks as a solid growth of epidermal cells into the mesenchyme. C, Its terminal part coils and forms the body of the gland. The central cells degenerate to form the lumen of the gland. D, The peripheral cells differentiate into secretory cells and contractile myoepithelial cells.
The distribution of the large sudoriferous (producing sweat) apocrine sweat glands is mostly confined to the axillary, pubic and perineal regions, and the areolae (circular pigmented areas) surrounding the nipples. The glands develop from downgrowths of the stratum germinativum of the epidermis (Fig. 19-3). As a result, the ducts of these glands open, not onto the skin surface, as do eccrine sweat glands, but into the canals of the hair follicles superficial to the entry of the sebaceous gland ducts. Secretion by apocrine sweat glands is influenced by hormones and does not begin until puberty.
Disorders of Keratinization
Ichthyosis is a general term that is applied to a group of skin disorders resulting from excessive keratinization—formation of horny layers of skin (Fig. 19-5B). The skin is characterized by dryness and scaling, which may involve the entire body surface.

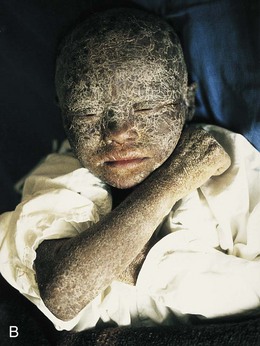
FIGURE 19–5 A, A child with congenital hypertrichosis and hyperpigmentation. Note the excessive hairiness on the shoulders and back. B, A child with severe keratinization of the skin (ichthyosis) from the time of birth.
(A, Courtesy of Dr. Mario Joao Branco Ferreira, Servico de Dermatologia, Hospital de Desterro, Lisbon, Portugal. B, Courtesy of Dr. Joao Carlos Fernandes Rodrigues, Servico de Dermatologia, Hospital de Desterro, Lisbon, Portugal.)
A harlequin fetus results from a rare keratinizing disorder that is inherited as an autosomal recessive trait with a mutation on the ABCA12 gene. The skin is markedly thickened, ridged, and cracked. Most affected neonates die during the first week of life.
A collodion infant is covered by a thick, taut membrane that resembles collodion (a protective film) or parchment. The membranous skin cracks with the first respiratory efforts and begins to fall off in large sheets. Deficiency of transglutaminase-1 is the most common cause. Complete shedding may take several weeks, occasionally leaving normal-appearing skin.
Lamellar ichthyosis is an autosomal recessive disorder. A neonate with this condition may at first appear to be a collodion baby; however, the scaling persists. Growth of hair may be curtailed and development of sweat glands is often impeded. Affected infants usually suffer severely in hot weather because of their inability to sweat.
Congenital Ectodermal Dysplasia
This skin condition represents a group of rare hereditary disorders involving tissues that are ectodermal in origin. The teeth are completely or partially absent and often the hairs and nails are affected. Ectrodactyly-ectodermal dysplasia-clefting syndrome is a congenital skin condition that is inherited as an autosomal dominant trait. It involves both ectodermal and mesodermal tissues, consisting of ectodermal dysplasia associated with hypopigmentation of skin and hair, scanty hair and eyebrows, absence of eyelashes, nail dystrophy, hypodontia and microdontia, ectrodactyly, and cleft lip and palate. This appears to be caused by a defect in the P63 gene coding for a transcription factor.
Angiomas of Skin
These vascular anomalies are developmental defects in which some transitory and/or surplus primitive blood or lymphatic vessels persist. Those composed of blood vessels may be mainly arterial, venous, or cavernous angiomas, but they are often of a mixed type. Angiomas composed of lymphatics are called cystic lymphangiomas or cystic hygromas (see Chapter 13). True angiomas are benign tumors of endothelial cells, usually composed of solid or hollow cords; the hollow cords contain blood.
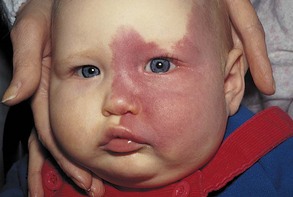
Nevus flammeus denotes a flat, pink or red, flame-like blotch that often appears on the posterior surface of the neck. A port-wine stain hemangioma is a larger and darker angioma than a nevus flammeus and is nearly always anterior or lateral on the face and/or neck (Fig. 19-6). It is sharply demarcated when it is near the median plane, whereas the common angioma (pinkish-red blotch) may cross the median plane. A port-wine stain in the area of distribution of the trigeminal nerve is sometimes associated with a similar type of angioma of the meninges of the brain and seizures at birth (Sturge-Weber syndrome). Hemangiomas are among the most common benign neoplasms found in infants and children.
Albinism
In generalized albinism, an autosomal recessive trait, the skin, hairs, and retina lack pigment; however, the iris usually shows some pigmentation. Albinism occurs when the melanocytes fail to produce melanin because of the lack of the enzyme tyrosinase or other pigment enzymes. In localized albinism—piebaldism—an autosomal dominant trait, there is a lack of melanin in patches of skin and/or hair.
Mammary Glands
Mammary glands are modified and highly specialized types of sweat glands. Development of these glands is similar in male and female embryos. The first evidence of mammary development appears in the fourth week when mammary crests (ridges) develop along each side of the ventral surface of the embryo. These crests extend from the axillary region to the inguinal region (Fig. 19-7A). The crests usually disappear except for the parts at the site of the future breasts (Fig. 19-7B). Involution of the remaining mammary crests in the fifth week produces the primary mammary buds (Fig. 19-7C). These buds are downgrowths of the epidermis into the underlying mesenchyme. The changes occur in response to an inductive influence from the mesenchyme. Each primary mammary bud soon gives rise to several secondary mammary buds, which develop into lactiferous ducts and their branches (Fig. 19-7D to E). Canalization (formation of canals) of these buds is induced by placental sex hormones entering the fetal circulation. This process continues until the late fetal period; by term, 15 to 19 lactiferous ducts are formed. The fibrous connective tissue and fat of the mammary glands develop from the surrounding mesenchyme.

FIGURE 19–7 Development of mammary glands. A, Ventral view of an embryo of approximately 28 days showing the mammary crests. B, Similar view at 6 weeks showing the remains of these crests. C, Transverse section of a mammary crest at the site of a developing mammary gland. D to F, Similar sections showing successive stages of breast development between the 12th week and birth.
During the late fetal period, the epidermis at the site of origin of the mammary gland becomes depressed, forming a shallow mammary pit (Fig. 19-7E). The nipples are poorly formed and depressed in neonates. Soon after birth, the nipples usually rise from the mammary pits because of proliferation of the surrounding connective tissue of the areola, the circular area of pigmented skin around the nipples. The smooth muscle fibers of the nipple and areola differentiate from surrounding mesenchymal cells.
The rudimentary mammary glands of newborn males and females are identical and are often enlarged. Some secretion, often called “witch’s milk” (galactorrhea), may be produced. These transitory changes are caused by maternal hormones passing through the placental membrane into the fetal circulation. The breasts of neonates contain lactiferous ducts but no alveoli (secretory parts of glands).
In females, the breasts enlarge rapidly during puberty (Fig. 19-8), mainly because of development of the mammary glands and the accumulation of the fibrous stroma and fat associated with them. Full development occurs at approximately 19 years (Fig. 19-8E). Normally, the lactiferous ducts of males remain rudimentary throughout life.
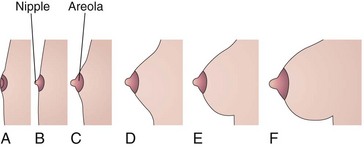
FIGURE 19–8 Sketches of progressive stages in the postnatal development of the female breasts. A, Neonate. B, Child. C, Early puberty. D, Late puberty. E, Young adult. F, Pregnant female. Note that the nipple is inverted at birth (A). At puberty (12–15 years), the breasts of females enlarge because of development of the mammary glands and the increased deposition of fat.
Several transcription factors, including the oncogene c-Myc, a basic-helix-loop-helix transcription factor, are essential for the formation of the lactiferous ducts and function of the female breast.
Gynecomastia
The rudimentary lactiferous ducts in males normally undergo no postnatal development. Gynecomastia refers to the development of the rudimentary lactiferous ducts in the male mammary tissue. During midpuberty, approximately two thirds of boys develop varying degrees of hyperplasia of the breasts. This subareolar hyperplasia may persist for a few months to 2 years. A decreased ratio of testosterone to estradiol is found in boys with gynecomastia. Approximately 80% of males with Klinefelter syndrome have gynecomastia (see Chapter 20), which is associated with an XXY chromosome complement.
Absence of Nipples (Athelia) or Breasts (Amastia)
These rare birth defects may occur bilaterally or unilaterally. They result from failure of development or disappearance of the mammary crests. These conditions may also result from failure of mammary buds to form. More common is hypoplasia of the breast, often found in association with gonadal agenesis and Turner syndrome (see Chapter 20). Poland syndrome is associated with hypoplasia or absence of the breast or nipple.
Aplasia of Breast
The breasts of postpubertal females often differ somewhat in size. Marked differences are regarded as anomalies because both glands are exposed to the same hormones at puberty. In these cases, there is often associated rudimentary development of muscles of the thoracic wall, usually the pectoralis major (see Chapter 15).
Supernumerary Breasts and Nipples
An extra breast (polymastia) or nipple (polythelia) occurs in approximately 1% of the female population (Fig. 19-9); it is an inheritable condition. An extra breast or nipple usually develops just inferior to the normal breast. Supernumerary nipples are also relatively common in males; often they are mistaken for moles (Fig. 19-10). Less commonly, supernumerary breasts or nipples appear in the axillary or abdominal regions of females. In these positions, the nipples or breasts develop from extra mammary buds that develop from remnants of the mammary crests. They usually become more obvious in women when they are pregnant. Approximately one third of affected persons have two extra nipples or breasts. Supernumerary mammary tissue very rarely occurs in a location other than along the course of the mammary crests. It probably develops from tissue that was displaced from these crests.
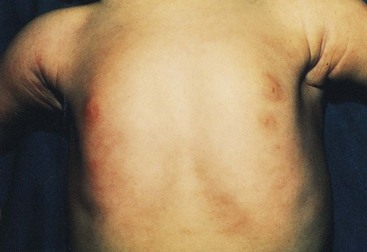
FIGURE 19–9 Female infant with an extra nipple (polythelia) on the left side.
(Courtesy of Dr. A.E. Chudley, Section of Genetics and Metabolism, Department of Pediatrics and Child Health, Children’s Hospital and University of Manitoba, Winnipeg, Manitoba, Canada.)
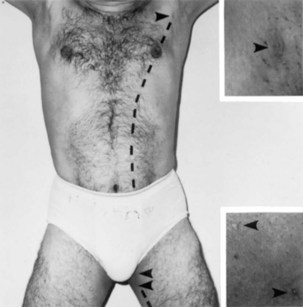
FIGURE 19–10 A man with polythelia (extra nipples) in the axillary and thigh regions. Insets are enlargements of the nipples (arrowheads). The broken line indicates the original position of the left mammary crests.
(Courtesy of Dr. Kunwar Bhatnagar, Professor of Anatomy, School of Medicine, University of Louisville, Louisville, Kentucky.)
Inverted Nipples
Sometimes the nipples fail to elevate above the skin surface after birth or during puberty, that is, they remain in their prenatal location (Figs. 19-7F and 19-8A). Inverted nipples may make breast-feeding of an infant difficult; however, a number of breast-feeding techniques can be used to reduce this difficulty.
Development of Hairs
Hairs begin to develop early in the fetal period (weeks 9–12), but they do not become easily recognizable until approximately the 20th week (Fig. 19-3). Hairs are first recognizable on the eyebrows, upper lip, and chin. The hair follicles begin as proliferations of the stratum germinativum of the epidermis, and extend into the underlying dermis. The hair buds soon become club shaped, forming hair bulbs (Fig. 19-3). The epithelial cells of the hair bulbs constitute the germinal matrix, which later produces the hair shafts.
The hair bulbs (primordia of hair roots) are soon invaginated by small mesenchymal hair papillae (Figs. 19-3 and 19-11). The peripheral cells of the developing hair follicles form the epithelial root sheaths, and the surrounding mesenchymal cells differentiate into the dermal root sheaths. As cells in the germinal matrix proliferate, they are pushed toward the surface, where they become keratinized to form hair shafts (Fig. 19-3). The hairs grow through the epidermis on the eyebrows and upper lip by the end of the 12th week.
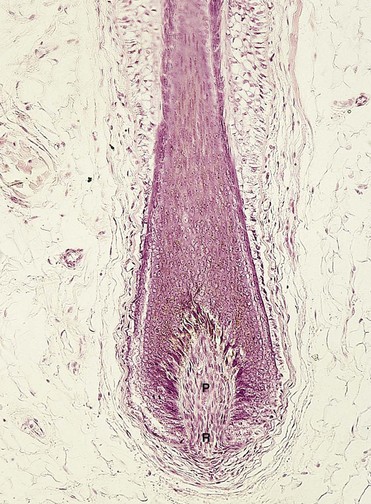
FIGURE 19–11 Light micrograph of a longitudinal section of a hair follicle with its hair root (R) and papilla (P) (x132).
(From Gartner LP, Hiatt JL: Color Textbook of Histology, 2nd ed. Philadelphia, WB Saunders, 2001.)
The first hairs to appear—lanugo (downy hair)—are fine, soft, and lightly pigmented. Lanugo begins to appear toward the end of week 12 and is plentiful by 17 to 20 weeks. These hairs help to hold the vernix caseosa on the skin. Lanugo is replaced by coarser hairs during the perinatal period. This hair persists over most of the body, except in the axillary and pubic regions, where it is replaced at puberty by even coarser terminal hairs. In males, similar coarse hairs also appear on the face and often on the chest (anterior wall of thorax).
Melanoblasts migrate into the hair bulbs and differentiate into melanocytes. The melanin produced by these cells is transferred to the hair-forming cells in the germinal matrix several weeks before birth. The relative content of melanin accounts for different hair colors.
Arrector muscles of hairs, small bundles of smooth muscle fibers, differentiate from the mesenchyme surrounding the hair follicles and attach to the dermal root sheaths of the hair follicles and the papillary layer of the dermis, which interdigitates with the epidermis (Figs. 19-1D and 19-3). Contractions of the arrector muscles depress the skin over their attachment and elevate the skin around the hair shafts, causing the hairs to stand up (“goose bumps”). The arrector muscles are poorly developed in the hairs of the axillary region and in certain parts of the face. The hairs forming the eyebrows and the cilia forming the eyelashes have no arrector muscles.
Alopecia
Absence or loss of scalp hairs may occur alone or with other defects of the skin and its derivatives. Congenital alopecia (hair loss) may be caused by failure of hair follicles to develop, or it may result from follicles producing poor-quality hairs.
Hypertrichosis
Excessive hairiness results from the development of supernumerary hair follicles, or from the persistence of lanugo hairs that normally disappear during the perinatal period. It may be localized (e.g., on the shoulders and back) or diffuse (Fig. 19-5A). Localized hypertrichosis is often associated with spina bifida occulta (see Chapter 17, Fig. 17-14).
Development of Nails
Toenails and fingernails begin to develop at the tips of the digits at approximately 10 weeks (Fig. 19-12). Development of fingernails precedes that of toenails by approximately 4 weeks (see Chapter 6). The primordia of nails appear as thickened areas or nail fields of the epidermis at the tip of each digit (Fig. 19-12A). Later these fields migrate onto the dorsal surfaces of the nails, carrying their innervation from the ventral surface. The nail fields are surrounded laterally and proximally by folds of epidermis, the nail folds (Fig. 19-12B). Cells from the proximal nail fold grow over the nail field and become keratinized to form the nail plate (Fig. 19-12C).
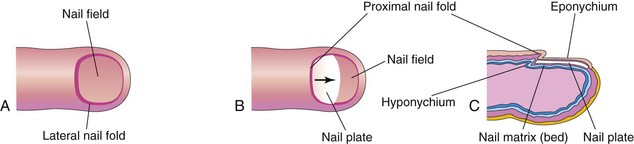
FIGURE 19–12 Successive stages in the development of a fingernail. A, The first indication of a nail is a thickening of the epidermis, the nail field, at the tip of the finger. B, As the nail plate develops, it slowly grows toward the tip of the finger. C, The fingernail reaches the end of the finger by 32 weeks.
At first, the developing nail is covered by a narrow band of epidermis, the eponychium (corneal layer of epidermis). This later degenerates, exposing the nail, except at its base, where it persists as the cuticle. The skin under the free margin of the nail is the hyponychium (Fig. 19-12C). The fingernails reach the fingertips by approximately 32 weeks; the toenails reach the toetips by approximately 36 weeks. Nails that have not reached the tips of the digits at birth indicate prematurity.
Aplastic Anonychia
Congenital absence of nails at birth is extremely rare. Anonychia results from failure of nail fields to form or from failure of the proximal nail folds to form nail plates. The abnormality is permanent. Aplastic anonychia (defective development or absence of nails) may be associated with extremely poor development of hairs and with defects of the teeth. Anonychia may be restricted to one or more nails of the digits of the hands and/or feet.
Development of Teeth
Two sets of teeth normally develop: the primary dentition or deciduous teeth and the secondary dentition or permanent teeth. Teeth develop from the oral ectoderm, mesenchyme, and neural crest cells. The enamel is derived from ectoderm of the oral cavity; all other tissues differentiate from the surrounding mesenchyme and neural crest cells. Neural crest cells are imprinted with morphogenetic information before, or shortly after, they migrate from the neural crest. The molecular mechanisms and signaling pathways involve the expression and effects of FGF, BMP, Shh, and Wnt. As the mandible and maxilla grow to accommodate the developing teeth, the shape of the face changes.
Odontogenesis (tooth development) is a property of the oral epithelium. The development is a continuous process involving reciprocal induction between neural crest mesenchyme and the overlying oral epithelium. It is usually divided into stages for descriptive purposes based on the appearance of the developing tooth. The first tooth buds appear in the anterior mandibular region (see Fig.19-14B); later, tooth development occurs in the anterior maxillary region and then progresses posteriorly in both jaws.
Tooth development continues for years after birth (Table 19-1). The first indication of tooth development occurs early in the sixth week of embryonic development as a thickening of the oral epithelium. These U-shaped bands—dental laminae—follow the curves of the primitive jaws (Figs. 19-13A and 19-14A).
Table 19–1 Order and Usual Time of Eruption of Teeth and Time of Shedding of Deciduous Teeth
| TOOTH | USUAL ERUPTION TIME | SHEDDING TIME |
|---|---|---|
| Deciduous | ||
| Medial incisor | 6–8 mo | 6–7 yr |
| Lateral incisor | 8–10 mo | 7–8 yr |
| Canine | 16–20 mo | 10–12 yr |
| First molar | 12–16 mo | 9–11 yr |
| Second molar | 20–24 mo | 10–12 yr |
| Permanent* | ||
| Medial incisor | 7–8 yr | |
| Lateral incisor | 8–9 yr | |
| Canine | 10–12 yr | |
| First premolar | 10–11 yr | |
| Second premolar | 11–12 yr | |
| First molar | 6–7 yr | |
| Second molar | 12 yr | |
| Third molar | 13–25 yr | |
* The permanent teeth are not shed.
Data from Moore KL, Dalley AF, Agur AMR: Clinically Oriented Anatomy, 6th ed. Baltimore, Williams & Wilkins, 2010.
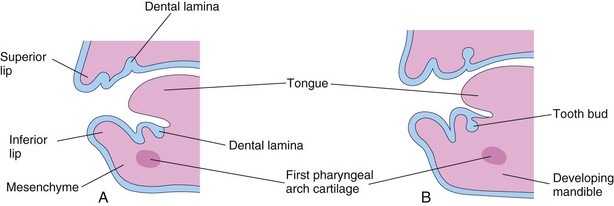
FIGURE 19–13 Sketches of sagittal sections through the developing jaws illustrating early development of the teeth. A, Early in the sixth week, showing the dental laminae. B, Later in the sixth week, showing tooth buds arising from the laminae.
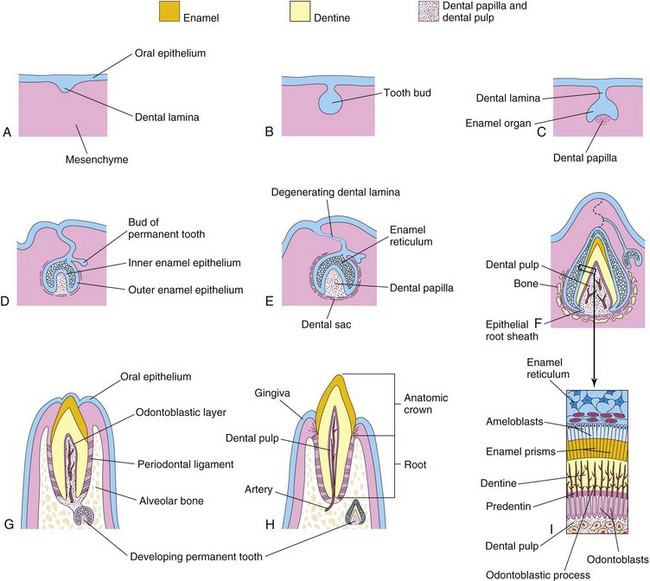
FIGURE 19–14 Schematic drawings of sagittal sections illustrating successive stages in the development and eruption of an incisor tooth. A, At 6 weeks, showing the dental lamina. B, At 7 weeks, showing the tooth bud developing from the dental lamina. C, At 8 weeks, showing the cap stage of tooth development. D, At 10 weeks, showing the early bell stage of a deciduous tooth and the bud stage of a permanent tooth. E, At 14 weeks, showing the advanced bell stage of tooth development. Note that the connection (dental lamina) of the tooth to the oral epithelium is degenerating. F, At 28 weeks, showing the enamel and dentine layers. G, At 6 months postnatally, showing early stage of tooth eruption. H, At 18 months postnatally, showing a fully erupted deciduous incisor tooth. The permanent incisor tooth now has a well-developed crown. I, Section through a developing tooth showing ameloblasts (enamel producers) and odontoblasts (dentine producers).
Bud Stage of Tooth Development
Each dental lamina develops 10 centers of proliferation from which swellings—tooth buds—grow into the underlying mesenchyme (Figs. 19-13B and 19-14B). These buds develop into the deciduous teeth (Table 19-1). The tooth buds for permanent teeth that have deciduous predecessors begin to appear at approximately 10 weeks from deep continuations of the dental lamina (Fig. 19-14D). They develop lingual (toward the tongue) to the deciduous tooth buds. The permanent molars have no deciduous predecessors and develop as buds from posterior extensions of the dental laminae (horizontal bands). The tooth buds for the permanent teeth appear at different times, mostly during the fetal period. The buds for the second and third permanent molars develop after birth. The deciduous teeth have well-developed crowns at birth (Fig. 19-14H), whereas the permanent teeth remain as tooth buds.
Cap Stage of Tooth Development
As each tooth bud is invaginated by mesenchyme—the primordium of the dental papilla and dental follicle—the buds become cap shaped (Fig. 19-15). The ectodermal part of the developing tooth, the enamel organ, eventually produces enamel. The internal part of each cap-shaped tooth, the dental papilla, is the primordium of dentin and dental pulp. Together, the dental papilla and enamel organ form the tooth bud. The outer cell layer of the enamel organ is the outer enamel epithelium, and the inner cell layer lining the papilla is the inner enamel epithelium (Fig. 19-14D).
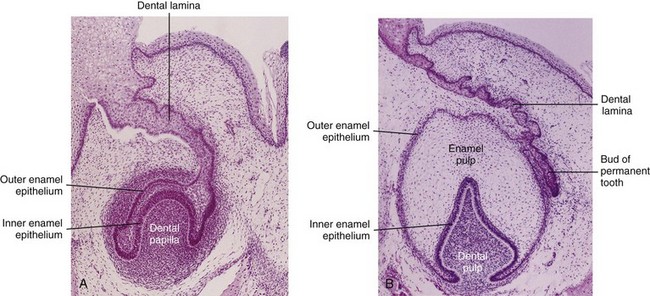
FIGURE 19–15 Photomicrograph of the primordium of a lower incisor tooth. A, A 12-week-old fetus (early bell stage). A cap-like enamel organ is formed and the dental papilla is developing beneath it. B, Primordium of a lower incisor tooth in a 15-week-old fetus (late bell stage). Observe the inner and outer enamel layers, the dental papilla, and bud of the permanent tooth.
(From Moore KL, Persaud TVN, Shiota K: Color Atlas of Clinical Embryology, 2nd ed. Philadelphia, WB Saunders, 2000.)
The central core of loosely arranged cells between the layers of enamel epithelium is the enamel reticulum (stellate reticulum). As the enamel organ and dental papilla develop, the mesenchyme surrounding the developing tooth condenses to form the dental sac (dental follicle), a vascularized capsular structure (Fig. 19-14E). The dental sac is the primordium of the cement and periodontal ligament. The cement is the bone-like, mineralized connective tissue covering the root of the tooth. The periodontal ligament, derived from neural crest cells, is a specialized vascular connective tissue that surrounds the root of the tooth, attaching it to the alveolar bone (Fig. 19-14G).
Bell Stage of Tooth Development
As the enamel organ differentiates, the developing tooth assumes the shape of a bell (Figs. 19-14D and E and 19-15). The mesenchymal cells in the dental papilla adjacent to the internal enamel epithelium differentiate into odontoblasts, which produce predentine and deposit it adjacent to the epithelium. Later, the predentine calcifies and becomes dentine, the second hardest tissue in the body.
As the dentine thickens, the odontoblasts regress toward the center of the dental papilla; however, their finger-like cytoplasmic processes—odontoblastic processes—remain embedded in the dentine (Fig. 19-14F and I).
Cells of the inner enamel epithelium differentiate into ameloblasts under the influence of the odontoblasts, which produce enamel in the form of prisms (rods) over the dentine. As the enamel increases, the ameloblasts migrate toward the outer enamel epithelium. Enamel is the hardest tissue in the body. It overlies and protects the dentine from being fractured (Fig. 19-16). The color of the translucent enamel is based on the thickness and color of the underlying dentine. Enamel and dentine formation begins at the cusp (tip) of the tooth and progresses toward the future root.
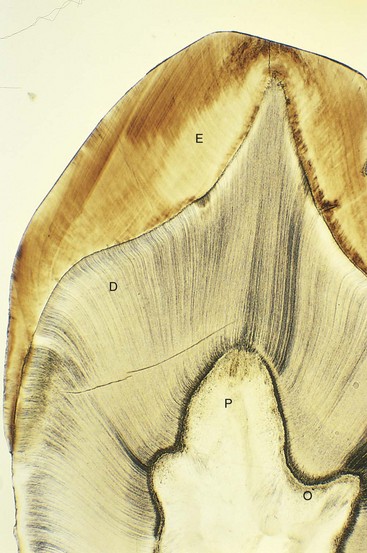
FIGURE 19–16 Photomicrograph of a section of the crown and neck of a tooth (x17). Observe the enamel (E), dentine (D), dental pulp (P), and odontoblasts (O).
(From Gartner LR, Hiatt JL: Color Textbook of Histology, 2nd ed. Philadelphia, WB Saunders, 2001.)
The root of the tooth begins to develop after dentine and enamel formation are well advanced (Figs. 19-14H and 19-17). The inner and outer enamel epithelia come together at the neck of the tooth (cementoenamel junction), where they form a fold, the epithelial root sheath (Fig. 19-14F). This sheath grows into the mesenchyme and initiates root formation.
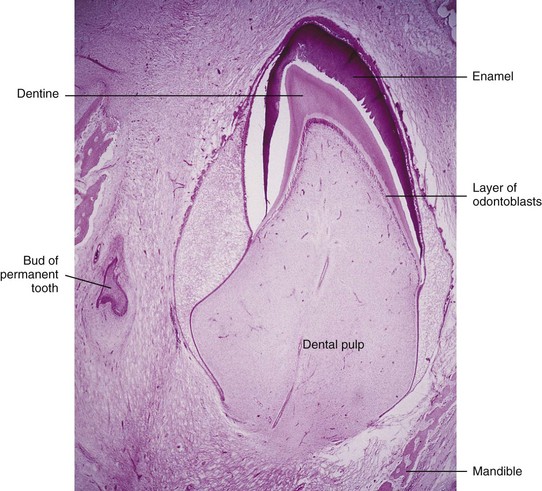
FIGURE 19–17 Photomicrograph of a section of a lower incisor tooth in a term fetus. The enamel and dentine layers and the pulp are clearly demarcated.
(From Moore KL, Persaud TVN, Shiota K: Color Atlas of Clinical Embryology, 2nd ed. Philadelphia, WB Saunders, 2000.)
The odontoblasts adjacent to the epithelial root sheath form dentine that is continuous with that of the crown. As the dentine increases, it reduces the pulp cavity to a narrow root canal through which the vessels and nerves pass (Fig. 19-14H). The inner cells of the dental sac differentiate into cementoblasts, which produce cement that is restricted to the root. Cement is deposited over the dentine of the root and meets the enamel at the neck of the tooth.
As the teeth develop and the jaws ossify, the outer cells of the dental sac also become active in bone formation. Each tooth soon becomes surrounded by bone, except over its crown. The tooth is held in its alveolus (bony socket) by the strong periodontal ligament, a derivative of the dental sac (Fig. 19-14G and H). Some fibers of this ligament are embedded in the cement of the root; other fibers are embedded in the bony wall of the alveolus.
Tooth Eruption
As the deciduous teeth develop, they begin a continuous slow movement toward the oral cavity (Fig. 19-14G). This process (eruption) results in the emergence of the tooth from the dental follicle in the jaw to its functional position in the mouth. The mandibular teeth usually erupt before the maxillary teeth, and girls’ teeth usually erupt sooner than boys’ teeth. The child’s dentition contains 20 deciduous teeth. As the root of the tooth grows, its crown gradually erupts through the oral epithelium. The part of the oral mucosa around the erupted crown becomes the gingiva (gum).
Usually eruption of the deciduous teeth occurs between the 6th and 24th months after birth (Table 19-1). The mandibular medial or central incisor teeth typically erupt 6 to 8 months after birth, but this process may not begin until 12 or 13 months in some children. Despite this, all 20 deciduous teeth are usually present by the end of the second year in healthy children. Delayed eruption of all teeth may indicate a systemic or nutritional disturbance such as hypopituitarism or hypothyroidism.
The complete permanent dentition consists of 32 teeth. The permanent teeth develop in a manner similar to that described for deciduous teeth. As a permanent tooth grows, the root of the corresponding deciduous tooth is gradually resorbed by osteoclasts (odontoclasts). Consequently, when the deciduous tooth is shed, it consists only of the crown and the uppermost part of the root. The permanent teeth usually begin to erupt during the sixth year and continue to appear until early adulthood (Fig. 19-18, Table 19-1).
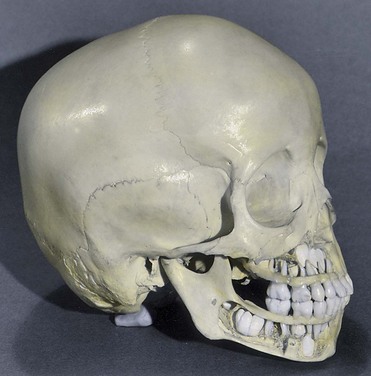
FIGURE 19–18 A 4-year-old child’s cranium. Bone has been removed from the mandible and maxilla to expose the relationship of the developing permanent teeth to the erupted deciduous teeth.
The shape of the face is affected by the development of the paranasal sinuses and the growth of the maxilla and mandible to accommodate the teeth (see Chapter 9). It is the lengthening of the alveolar processes (bony sockets supporting the teeth) that results in the increase in the depth of the face during childhood.
Natal Teeth
Natal teeth are erupted at birth. There are usually two in the position of the mandibular incisors. Natal teeth are observed in approximately one in 2000 newborn infants. Natal teeth may produce maternal discomfort during breast-feeding. In addition, the infant’s tongue may be lacerated or the teeth may detach and be aspirated; for these reasons, natal teeth are sometimes extracted. Because these are prematurely erupting decidual teeth, spacers may be required to prevent overcrowding of the other teeth.
Enamel Hypoplasia
Defective enamel formation causes pits and/or fissures in the enamel of teeth (Figs. 19-19 and 19-20A). These defects result from temporary disturbances of enamel formation. Various factors may injure ameloblasts, the enamel builders (e.g., nutritional deficiency, tetracycline therapy, and infectious diseases such as measles). Rickets occurring during the critical in utero period of tooth development (6–12 weeks) is a common cause of enamel hypoplasia. Rickets, a disease in children who are deficient in vitamin D, is characterized by disturbance of ossification of the epiphysial cartilages and disorientation of cells at the metaphysis (see Chapter 14).

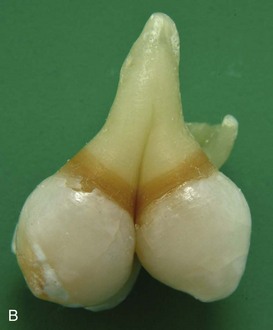


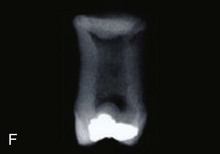
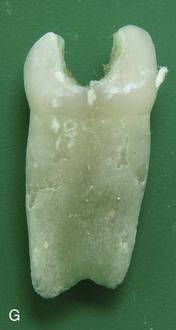
FIGURE 19–19 Some common birth defects of teeth. A, Enamel pearl (furcation of a permanent maxillary third molar). B, Gemination and tetracyline staining (maxillary third molar). C, Fusion (permanent mandibular central and lateral incisors). D, Abnormally short root (microdont permanent maxillary central incisor). E, Dens invaginatus (talon cusps on the lingual surface of the permanent maxillary central incisor). F, Taurodont tooth (radiograph of the mesial surface of the permanent maxillary second molar). G, Fusion (primary mandibular central and lateral incisors).
(Courtesy of Dr. Blaine Cleghorn, Faculty of Dentistry, Dalhousie University, Halifax, Nova Scotia, Canada.)
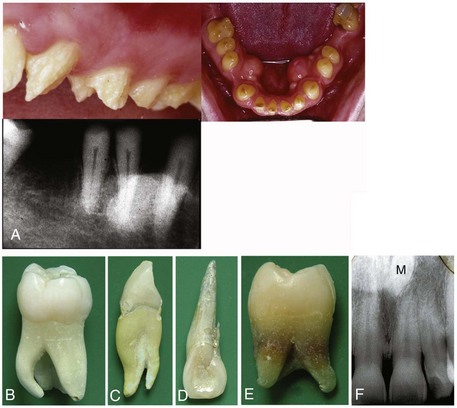
FIGURE 19–20 More common birth defects of teeth. A, Amelogenesis imperfecta. B, Extra root (mandibular molar). C, Extra root (mandibular canine). D, Accessory root (maxillary lateral incisor). Extra roots present challenges for root canal therapy and extraction. E, Tetracycline staining (root of maxillary third molar). F, A midline supernumerary tooth (M, mesiodens) located near the apex of the central incisor. The prevalence of supernumerary teeth is 1% to 3% in the general population
(A to E, Courtesy of Dr. Blaine Cleghorn, Faculty of Dentistry, Dalhousie University, Halifax, Nova Scotia, Canada. F, Courtesy of Dr. Steve Ahing, Faculty of Dentistry, University of Manitoba, Winnipeg, Manitoba, Canada.)
Variations of Tooth Shape
Abnormally shaped teeth are relatively common (Figs. 19-19A to G and 19-20A to E). Occasionally there is a spherical mass of enamel—enamel pearl—on the root of a tooth that is separate from the enamel of the crown (Fig. 19-19A). The pearl is formed by aberrant groups of ameloblasts. In other cases, the maxillary lateral incisor teeth may have a slender, tapering shape (peg-shaped incisors).
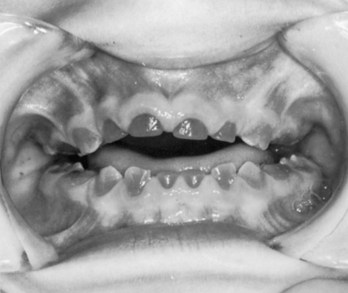
Congenital syphilis affects the differentiation of the permanent teeth, resulting in incisors with central notches in their incisive edges. The molars are also affected and are called mulberry molars because of their characteristic features.
Numerical Abnormalities
One or more supernumerary teeth (mesiodens) may develop, or the normal number of teeth may fail to form (Fig. 19-20F). Many studies report a higher prevalence in females. Supernumerary teeth usually develop in the area of the maxillary incisors and can disrupt the position and eruption of normal teeth. The extra teeth commonly erupt posterior to the normal ones (or remain unerupted) and are asymptomatic in most cases. In partial anodontia, one or more teeth are absent; this is often a familial trait. In total anodontia, no teeth develop; this is an extremely rare condition. It is usually associated with congenital ectodermal dysplasia.
Occasionally a tooth bud either partially or completely divides into two separate teeth. A partially divided tooth germ is called gemination. The result is a macrodontia (large teeth) with a common root canal system. Small teeth (microdontia) also occur. If the tooth germ completely divides into two separate teeth, the result is twinning with one additional tooth in the dentition. Fusion of two teeth results in one fewer tooth in the dentition. This condition can be differentiated radiographically from gemination by two separate root canal systems found with fusion.
Dentigerous Cyst
A cyst may develop in a mandible, maxilla, or maxillary sinus that contains an unerupted tooth. The dentigerous (tooth-bearing) cyst develops because of cystic degeneration of the enamel reticulum of the enamel organ of an unerupted tooth. Most cysts are deeply situated in the jaw and are associated with misplaced or malformed secondary teeth that have failed to erupt.
Amelogenesis Imperfecta
Amelogenesis imperfecta is a complex group of at least 14 different clinical entities that involve developmental aberrations in enamel formation in the absence of any systemic disorder. This is a inherited ectodermal birth defect that primarily affects the enamel only. The enamel may be hypoplastic, hypocalcified, or hypomature. Depending on the type of amelogenesis imperfecta, the enamel may be hard or soft, pitted or smooth, and thin or normal in thickness. The incidence of amelogenesis imperfecta ranges from 1 in 700 to 1 in 8000, depending on the population studied. Multiple modes of inheritance patterns are involved. Classification of this condition is based on clinical and radiographic findings, as well as mode of inheritance.
Dentinogenesis Imperfecta
The teeth are brown to gray-blue with an opalescent sheen because the odontoblasts fail to differentiate normally and poorly calcified dentine results. Both deciduous and permanent teeth are usually involved. The enamel tends to wear down rapidly, exposing the dentine. This anomaly is inherited as an autosomal dominant trait with the genetic defect in most cases localized on chromosome 4q and this condition is relatively common in Caucasian children (Fig. 19-21).
Discolored Teeth
Foreign substances incorporated into the developing enamel and dentine discolor the teeth. The hemolysis associated with erythroblastosis fetalis or hemolytic disease of the newborn (see Chapter 7) may produce blue to black discoloration of the teeth. All tetracyclines are extensively incorporated into the teeth. The critical period at risk is from approximately 14 weeks of fetal life to the 10th postnatal month for deciduous teeth, and from approximately 14 weeks of fetal life to the eighth postnatal year for permanent teeth.
Tetracycline staining affects both enamel and dentine because it binds to hydroxyapatite. The brownish-yellow discoloration (mottling) of the teeth, produced by tetracycline, is due to the conversion of tetracycline to a colored by-product under the action of light. The dentine is probably affected more than the enamel because it is more permeable than enamel after tooth mineralization is complete. The enamel is completely formed on all but the third molars by approximately 8 years of age. For this reason, tetracyclines should not be administered to pregnant women or children younger than 8 years of age.
Summary of Integumentary System
Clinically Oriented Problems
Case 19–2
The deciduous teeth of an infant had a brownish-yellow color and some hypoplasia of the enamel. The mother recalled that she had been given antibiotics during the second trimester of her pregnancy.
Case 19–3
An infant was born with a small, irregularly shaped, light-red blotch on the posterior surface of the neck. It was level with the surrounding skin and blanched when light pressure was applied.
References and Suggested Reading
Berkovitz BKB, Holland GR, Moxham BJ. Oral Anatomy, Histology, and Embryology, ed 4. Philadelphia: Mosby; 2009.
Buss PW, Hughes HE, Clarke A. Twenty-four cases of the EEC syndrome: Clinical presentation and management. J Med Genet. 1995;32:716.
Caton J, Tucker AS. Current knowledge of tooth development: Patterning and mineralization of the murine dentition. J Anat. 2009;214:407.
Coletta RD, McCoy EL, Burns V, et al. Characterization of the Six 1 homeobox gene in normal mammary gland morphogenesis. BMC Dev Biol. 2010;10:4.
Darmstadt GL, Sidbury R. The skin. In Behrman RE, Kliegman RM, Jenson HB, editors: Nelson Textbook of Pediatrics, ed 17, Philadelphia: Elsevier/Saunders, 2004.
Lee K, Gjorevski N, Boghaert E, et al. Snail1, Snail2, and E47 promote mammary epithelial branching morphogenesis. EMBO J. 2011;30:2662.
McDermottt KM, Liu BY, Tisty TD, et al. Primary cilia regulate branching morphogenesis during mammary gland development. Curr Biol. 2010;20:731.
Moore KL, Dalley AF, Agur AMR. Clinically Oriented Anatomy, ed 6. Baltimore: Williams & Wilkins; 2010.
Moore SJ, Munger BL. The early ontogeny of the afferent nerves and papillary ridges in human digital glabrous skin. Dev Brain Res. 1989;48:119.
Müller M, Jasmin JR, Monteil RA, et al. Embryology of the hair follicle. Early Hum Dev. 1999;26:59.
Nanci A. Ten Cate’s Oral Histology. Development, Structure, and Function, ed 7. St. Louis: CV Mosby; 2008.
Narendran V, Hoath SB. The skin. In Martin RJ, Fanaroff AA, Walsh MC, editors: Fanaroff and Martin’s Neonatal–Perinatal Medicine: Diseases of the Fetus and Infant, ed 8, Philadelphia: Mosby, 2006.
Osborne MP. Breast anatomy and development. In Harris JR, editor: Diseases of the Breast, ed 2, Philadelphia: Lippincott Williams & Wilkins, 2000.
Paller AS, Mancini AJ. Hurwitz Clinical Pediatric Dermatology: A Textbook of Skin Disorders of Childhood and Adolescence, ed 3. Philadelphia: WB Saunders; 2006.
Rudel RA, Fenton SE, Ackerman JM, et al. Environmental exposures and mammary gland development: state of the science, public health implications, and research recommendations. Environ Health Perspect. 2011;119:1053.
Smolinski KN. Hemangiomas of infancy: Clinical and biological characteristics. Clin Pediatr. 2005;44:747.
Tompkins K. Molecular mechanisms of cytodifferentiation in mammalian tooth development. Connect Tissue Res. 2006;47:111.
Watts A, Addy MA. Tooth discolouration and staining: A review of the literature. Br Dent J. 2001;190:309.
Winter GB. Anomalies of tooth formation and eruption. In Welbury RR, editor: Paediatric Dentistry, ed 2, Oxford, UK: Oxford University Press, 2001.