28 The Abdomen of the Ruminant
CONFORMATION AND SURFACE ANATOMY
The form of the abdomen varies with age, obesity, and physiological condition. In adult animals it is both deep and wide, and the floor, which dips behind the sternum, ascends very steeply in its caudal part to join the pubic brim. This marked contraction is not obvious on first inspection because the caudal part of the abdomen is covered by the thighs and the skin folds that pass between the flanks and stifle joints and is overlain ventrally by the udder or the prepuce. The considerable extent of the abdomen under cover of the ribs follows from the curvature of the diaphragm (Figure 27–3). The abdomen is usually bilaterally symmetrical, although advanced pregnancy or excessive distention of the rumen may cause one side to bulge more markedly. The upper part of the flank is dished, forming the paralumbar fossa beside the loins (see Figure 26–1, B, E), while the lower convex part merges with the floor.
In the younger calf the abdomen is shallower and laterally compressed, and the floor slopes more gradually to the pelvis; the spreading of the caudal ribs, the deepening of the trunk, and the depressions beside the vertebral column develop with growth of the rumen.
The lateral and ventral abdominal walls are bounded by the last rib and costal arch, the extremities of the lumbar transverse processes, the coxal tuber, and the terminal line of the pelvic inlet (see Figure 26–1, A). Not all of these are palpable, although identification of the margin of the thoracic cage, the coxal tuber, and most transverse processes normally presents no problem. Palpation should be performed with care because correct identification of the bones is important in certain anesthetic techniques. There are six lumbar vertebrae in cattle. Recognition of the second to fifth vertebrae is easy and may even be possible without palpation in lean cattle; the first process cannot always be located because it is short, tucked into the angle between the last rib and the spine, and generally overlain by a pad of fat; the last one always eludes the fingers because it lies medial to the coxal tuber below a thick covering of muscle (see Figure 26–5). There are occasionally seven lumbar vertebrae in sheep and goats.
THE VENTROLATERAL WALL OF THE ABDOMEN
STRUCTURE
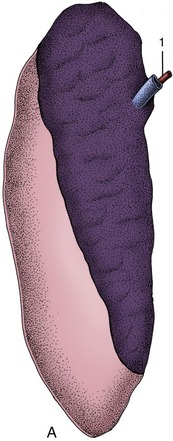
The ventrolateral wall of the abdomen is composed of as many as 9 or 10 layers, although not all cover the entire extent. The skin is freely movable except over the coxal tuber. The cutaneous muscle is thick over the lower parts of the flank but thins dorsally and does not extend over the paralumbar fossa; it also leaves the abdominal floor bare except for detached fascicles that supply the male animal with cranial and caudal muscles of the prepuce. The cutaneous muscle extends through the flank fold to end in an aponeurosis over the lateral surface of the thigh (Figure 28–1, A).
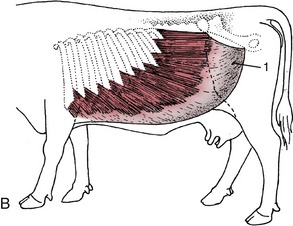
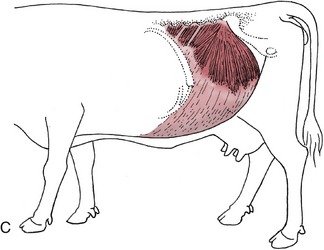
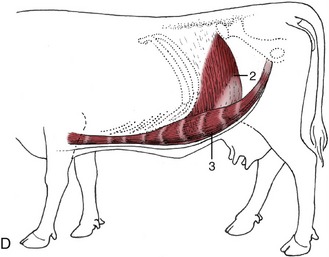
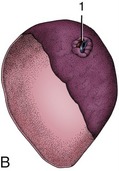
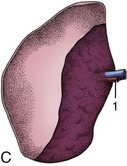
Figure 28–1 Cutaneous trunci and abdominal muscles. A, Cutaneous trunci, especially well-developed ventrally. B, External abdominal oblique with superficial inguinal ring (1) in its aponeurosis. C, Internal abdominal oblique. D, Transversus abdominis (2) and rectus abdominis (3). Note the reduction in the thickness of the wall along the caudal part of the rectus margin.
The loose superficial fascia provides pathways for the cutaneous nerves and encloses certain lymph nodes. The elongated subiliac node lies vertically within the skin fold, pressed against the cranial margin of the thigh some distance above the patella; it can always be found on palpation. It drains the more superficial layers of the body wall as far forward as the caudal part of the thorax and also receives lymph coming from the skin and superficial muscles of the thigh and croup (see Figure 29–46). A number of smaller nodes within the paralumbar fossa drain the surrounding parts; they normally escape notice but appear as circumscribed swellings when enlarged. The subcutaneous abdominal (“milk”) vein runs forward over the abdominal floor from the udder (see Figures 29–34 and 29–44).
The deep fascia is transformed into an elastic tunica flava, attached to the underlying muscle and sharing in supporting the viscera. Ventrally it gives origin to the external spermatic fascia or the medial lamina of the suspensory apparatus of the udder.
The muscle layer is broadly arranged as in other species. In the flank it consists of a triple layer of flat muscles that take origin from the ribs, lumbar transverse processes, and ilium (see Figure 28–1). These are continued over the abdominal floor by aponeurotic tendons that enclose the rectus muscles to each side of the linea alba where the aponeuroses attach (see Figure 1–37). The linea alba runs from the xiphoid process of the sternum to the center of the prepubic tendon, where it blends with the end tendons of the recti.
The most superficial muscle of the flank, the external oblique, arises by fleshy serrations from the outer surfaces of the last eight ribs. Its most dorsal fibers run more or less horizontally toward the coxal tuber, but the greater number slope caudoventrally to find attachment to the linea alba (Figure 28–1, B). The gap that intervenes between the dorsal border and transverse processes is closed by a sheet of fascia. The fleshy part is succeeded by an aponeurotic tendon, and the transformation occurs along a line that first drops vertically, from a point roughly level with the coxal tuber, before sweeping cranially. A split within the aponeurosis provides the superficial opening (ring) of the inguinal canal.
The second muscle, the internal oblique, has a tendinous origin from the coxal tuber and the pelvic tendon of the external oblique and several independent fleshy origins from the tips of the lumbar transverse processes. It radiates to insert on the last rib and into the linea alba. Most fibers run cranioventrally, but the thicker, most caudal fascicles pass slightly behind the plane of the tuber. The muscle–tendon junction slopes caudoventrally, and only the most caudal strip is fleshy where the muscle crosses the margin of the rectus (Figure 28–1, C). The aponeuroses of the two oblique muscles become increasingly interwoven where they pass ventral to the rectus and together furnish the external layer of the rectus sheath. The flesh of the internal oblique forms the inner wall of the inguinal canal.
The third, the transversus abdominis, arises from the last ribs and the extremities of the lumbar transverse processes. Its craniodorsal triangle is tendinous, but most of the part covering the flank is fleshy; before reaching the edge of the rectus, the flesh gives way to an aponeurosis that crosses the dorsal face of the rectus to gain the linea alba, thus forming the inner layer of the rectus sheath. Most fibers run transversely, and none pass behind the plane of the coxal tuber; the dorsal surface of the rectus is thus left uncovered in its most caudal part (Figure 28–1, D).
The rectus abdominis muscle is interrupted in the usual way by several tendinous intersections (Figure 28–1/3). It arises from the outer surfaces of the lower ends of the last 10 ribs and continues as a wide band separated from its neighbor by the flattened linea alba; it narrows suddenly as it approaches the pubic brim, and the tendon that succeeds the flesh twists to form with its fellow and the linea alba a V-shaped trough that continues as the central part of the prepubic tendon. Before reaching the pubic brim, which it approaches almost vertically from below, the prepubic tendon is strengthened by joining the decussation formed by the contralateral parts of the pectineus muscles (each of which arises from both pubic bones) and by additional contributions from the aponeuroses of the abdominal oblique muscles. Ultimately, and after partial decussation, the rectus tendons end in common on the symphysial crest of the pelvis and on the medial symphysial tendon that arises here. A rounded median depression of the internal surface of the prepubic tendon is ascribed to the drag of the udder (see Figure 29–40).
A thin fascia covers the abdominal muscles internally and supports the parietal peritoneum. The largest deposits of fat in the subperitoneal tissues are encountered toward the pelvic inlet. The wholly tendinous nature of a region of the abdominal wall, along the border of the rectus in front of the stifle, merits emphasis.
The inguinal canal resembles that of the horse (p. 549) so closely that a separate description is unnecessary. Inguinal hernias are infrequent in cattle but common in male sheep, although there are no obvious differences in the adult anatomy. It is probable that the frequent incidence in rams is connected with inherited anomalies in gubernacular development.
INNERVATION AND VASCULARIZATION
The most important nerves of the abdominal wall are the last thoracic (T13) and the first and second lumbar nerves, although the floor ventral to the costal arch is served by continuations of the caudal intercostal nerves. A knowledge of the topography and distribution of the nerves to the flank is of practical importance in obtaining local anesthesia.
The skin of the abdomen is supplied by branches from both dorsal and ventral primary rami, but the muscles and other deep structures are supplied by ventral rami alone (see Figure 1–37). The skin is divided into bands (dermatomes) that encircle the trunk, and each is the territory of a particular spinal nerve. The peritoneal regions supplied by spinal nerves correspond very closely to the dermatomes.
The dorsal rami (Figure 1–37/4) of the thoracic and lumbar nerves supply the epaxial muscles and the strip of skin extending from the dorsal midline roughly to the level of the patella. Below this line the skin is supplied by two tiers of branches from the ventral rami (Figure 1–37/5).
The ventral rami are much widened where they enter the flank between the internal oblique and transverse muscles. Each possesses a rather constant relationship to the skeleton that is a useful guide when the nerves are blocked with anesthetics. These nerves run obliquely, deviating in an increasingly caudal direction (Figure 28–2). The last thoracic ventral branch usually passes below the tip of the first lumbar transverse process, the first lumbar branch (iliohypogastric nerve) passes below the tip of the second, and the second lumbar branch (ilioinguinal nerve) passes below the tip of the fourth (Figure 28–3). Most variations affect the last of these three nerves, which sometimes passes below the transverse process of the third lumbar vertebra.
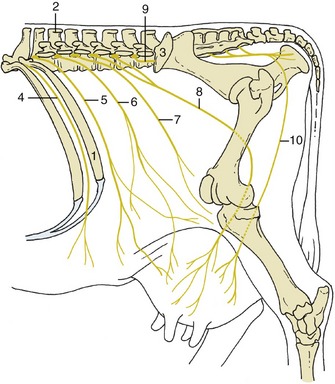
Figure 28–2 Topography of the nerves to the flank and udder, simplified. The dorsal branches of the spinal nerves to the upper part of the flank are not shown. 1, Last rib; 2, spinous process of L2; 3, coxal tuber; 4, twelfth intercostal n. (T12); 5, T13 (costoabdominal n.); 6, L1 (iliohypogastric n.); 7, L2 (ilioinguinal n.); 8, L3, L4 (genitofemoral n.); 9, L5 (nerve); 10, ventral perineal n.
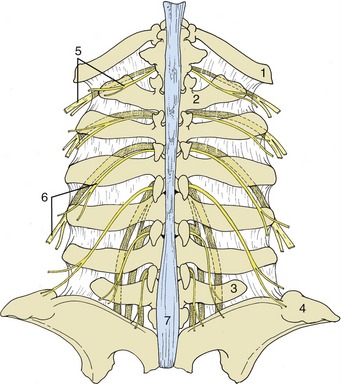
Figure 28–3 Relationship of the lumbar spinal nerves to the transverse processes of the bovine lumbar vertebrae. 1, Last rib; 2, first lumbar vertebra; 3, sixth lumbar vertebra; 4, coxal tuber; 5, dorsal and ventral branches of thirteenth thoracic nerve (the ventral branch is partly stippled); 6, dorsal and ventral branches of second lumbar nerve; 7, supraspinous ligament.
An exception to the general pattern of innervation of the abdominal wall is provided by the nerve to the cutaneous muscle; this is supplied by a branch from the brachial plexus.
It can now be appreciated that incisions of the upper flank require blockage of both dorsal and ventral branches. Anesthesia is most conveniently obtained by paravertebral injection of the relevant nerves close to their foramina of emergence from the vertebral canal. Anesthesia of the lower flank and abdominal floor requires blockage of the ventral branches only, and these are most conveniently reached where they pass close to the tips of the lumbar transverse processes (paralumbar block). Variation in topography makes the procedure less reliable than might be wished, unless the anesthetic agent is diffused rather widely. Lumbar epidural injection provides an alternative procedure. The specific innervation of the cutaneous muscle must be kept in mind regardless of the method chosen.
The abdominal wall receives blood vessels from several sources. The ventral part obtains its supply through the cranial and caudal epigastric arteries, which are branches of the internal thoracic and external pudendal arteries, respectively. The flanks are supplied from parietal branches of the aorta, of which the most important surgically is the deep circumflex iliac artery, which comes from the external iliac to pierce the flank a little cranial to the coxal tuber. The veins are initially satellite, but in the parous cow the arrangement is modified with the formation of the “milk vein” (p. 723).
THE SPLEEN
A general impression of the visceral topography should be obtained from Figure 28–4 before the individual organs are considered.
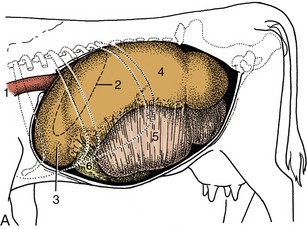
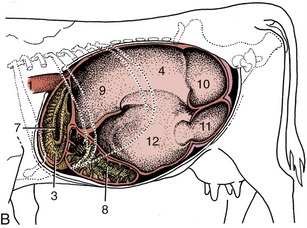

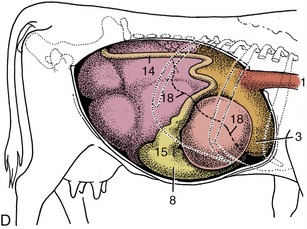
Figure 28–4 Topography of the abdominal viscera. A, Relationship of abdominal viscera to the left abdominal wall. B, The interior of the stomach seen from the left. C, Relationship of abdominal viscera to the right abdominal wall; the liver has been removed. D, Position of the parts of the stomach seen from the right. 1, Esophagus; 2, outline of spleen; 3, reticulum; 4, dorsal sac of rumen; 5, ventral sac of rumen, covered by superficial wall of greater omentum; 6, fundus of abomasum, covered by superficial wall of greater omentum; 7, reticular groove; 8, body of abomasum; 9, atrium ruminis; 10, caudodorsal blind sac; 11, caudoventral blind sac; 12, ventral sac of rumen (opened); 13, omasum, covered by lesser omentum; 14, descending duodenum; 15, pyloric part of abomasum; 16, greater omentum covering the intestinal mass; 17, lesser omentum cut away from the liver; 18, position of caudoventral border of liver.
The flat oblong spleen is situated over the craniodorsal part of the rumen, against the left half of the diaphragm, and is attached to both these organs. Its upper end lies under the dorsal ends of the last few ribs, and its axis extends ventrally, with a slight cranial inclination, across the line of the ribs to end in the region of the seventh costochondral joint (Figure 28–4, A/2 and Figure 28–5/6). In most animals the lower end passes onto the reticulum, which brings risk of involvement in the common abscesses and perforations of that organ. The upper part of the spleen is retroperitoneal: the line of serosal reflection runs cranioventrally over both parietal and visceral surfaces. The hilus is confined to the dorsocranial angle of the medial side, and to reach this site, the splenic vessels must first pass over the roof of the rumen.
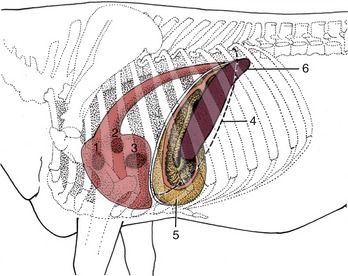
Figure 28–5 Left lateral projection of certain organs on the bovine thoracic wall. 1, Pulmonary valve; 2, aortic valve; 3, left atrioventricular valve; 4, position of basal border of the lung; 5, reticulum, opened (note position of reticular groove); 6, spleen.
The capsule contains little muscle, and physiological variation in spleen size is therefore rather restricted. Occasionally an enlarged spleen may extend behind the last rib in the angle between this and the lumbar spine, but for practical purposes the spleen may be regarded as out of reach for palpation or percussion. Access for a biopsy is normally made through the upper end of the eleventh intercostal space and involves little risk of injury to the lung, particularly if the needle is introduced during expiration.
The spleen has a relatively soft consistency. Its color varies considerably, tending to be steel blue in cows and more reddish in males and younger animals. The division of the pulp into red and white areas is very obvious; the white corpuscles are somewhat larger than pinheads.
The spleen is relatively small in sheep and goats, in which its form, position, and attachments resemble those of the dorsal extremity of the bovine organ. It is roughly triangular in sheep and quadrilateral in goats (Figure 28–6, B-C).
THE STOMACH
GENERAL CONSIDERATIONS
The stomach is composed of four chambers—rumen, reticulum, omasum, and abomasum—through which the food passes successively (Figure 28–7). The first three, collectively known as the forestomach (proventriculus), are developed to cope with the complex carbohydrates that form so large a part of the normal diet of ruminants, and only the last chamber is comparable in structure and function to the simple stomach of most other species. All are derived, however, from the gastric spindle of the embryo (Figure 28–8).

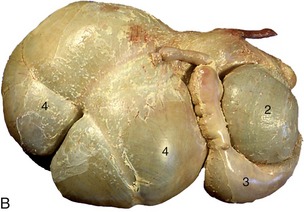
Figure 28–7 A, Bovine stomach, left side. B, Bovine stomach, right side. 1, Reticulum; 2, omasum; 3, abomasum; 4, rumen.

Figure 28–8 The attachments of the greater and lesser omenta on the developing ruminant stomach. The simple stomach to the right shows the correspondence of its parts to the compartments of the ruminant stomach. 1, Esophagus; 1′, cardia; 2, atrium ruminis; 3, dorsal sac of rumen; 4, ventral sac of rumen; 5, reticulum; 6, omasum; 7, abomasum; 7′, pylorus; 8, greater omentum; 9, lesser omentum; 10, part of greater curvature corresponding to the right longitudinal groove of the rumen; 11, part of greater curvature corresponding to the left longitudinal groove of the rumen.
The topography of the ruminant abdomen is dominated by the enormous development of the stomach, which in adult cattle almost fills the left half of the cavity and occupies a substantial portion of the right (Figure 27–1 and Figures 28–9, 28–10, 28–11, and 28–12). Its capacity measures about 60 L. This figure, which is much more modest than many estimates, may be apportioned between the various chambers as follows: rumen, 80%; reticulum, 5%; omasum, 8%; and abomasum, 7%. The proportions in small ruminants are somewhat different, being perhaps 75% rumen, 8% reticulum, 4% omasum, and 13% abomasum. The relative volumes are fairly constant in the short term because the enormous storage capacity of the first chambers and the more or less continuous passage of ingesta into the distal parts minimize the effects of intermittent feeding.
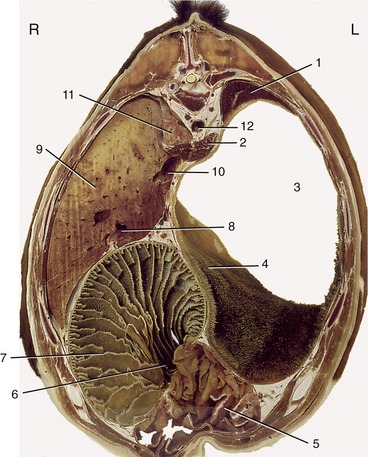
Figure 28–9 Transverse section of the bovine trunk at the level of the tenth thoracic vertebra. 1, Spleen; 2, crura of diaphragm; 3, atrium ruminis; 4, cranial pillar; 5, abomasum; 6, omasoabomasal opening; 7, omasum; 8, portal vein; 9, liver; 10, caudal vena cava; 11, right lung; 12, aorta.
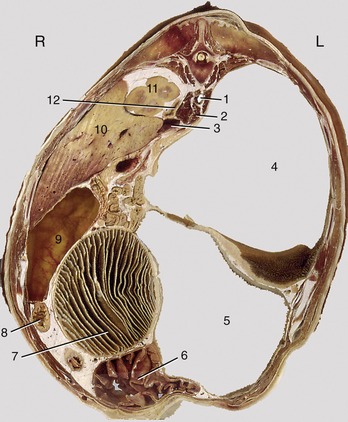
Figure 28–10 Transverse section of the bovine trunk at the level of the thirteenth thoracic vertebra. 1, Aorta; 2, right crus of diaphragm; 3, caudal vena cava; 4, dorsal sac of rumen; 5, ventral sac of rumen; 6, abomasum; 7, omasum; 8, duodenum; 9, gallbladder; 10, liver; 11, cranial pole of right kidney; 12, right adrenal gland.

Figure 28–11 Transverse section of the bovine trunk at the level of the third lumber vertebra. 1, Aorta: 2, caudodorsal blind sac; 3, dorsal coronary pillar; 4, caudal pillar; 5, left longitudinal pillar; 6, ventral coronary pillar; 7, caudoventral blind sac; 8, descending duodenum; 9, left kidney; 10, caudal vena cava; 11, milk vein; 12, intestinal mass.
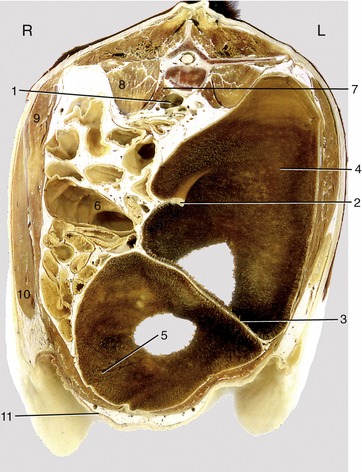
Figure 28–12 Transverse section of the bovine trunk at the level of the fifth lumbar vertebra. 1, Bifurcation of aorta and formation of caudal vena cava; 2, right dorsal coronary pillar; 3, caudal pillar; 4, caudodorsal blind sac; 5, caudoventral blind sac; 6, colon; 7, psoas minor; 8, psoas major; 9, internal abdominal oblique; 10, external abdominal oblique; 11, milk vein.
The different chambers are identifiable as expansions of the foregut spindle in the early embryo. They increase at unequal rates throughout the embryonic and fetal periods, as first one takes the lead and then another. At one stage the fetal stomach has an almost adult configuration, but during the last months of intrauterine life the abomasum outstrips the others; at birth it accounts for more than half the weight and capacity of the entire organ—which is appropriate because it is the only part that has an immediate function to perform. The postnatal changes through which the adult proportions and topography are acquired are described later (p. 692).
THE RUMEN AND RETICULUM
The rumen and reticulum together form the vessel in which the unpromising food material, invulnerable to attack by mammalian digestive enzymes, is reduced by processes of microbial fermentation. Some of the simpler products are assimilated directly, while others are susceptible to conventional digestion lower in the digestive tract.
The rumen is laterally compressed and extends from the cardia—which lies a little way above the middle of the seventh intercostal space or eighth rib—to the pelvic inlet, from the abdominal roof to the floor, and from the left body wall across the midline, especially caudally and ventrally, where it may reach the lower right flank (see Figure 28–12). The much smaller reticulum lies cranial to the rumen under cover of the sixth to eighth ribs and mainly to the left of the median plane. It reaches from the cardia to the most forward part of the diaphragm and occupies the full height of this shallower part of the abdomen; it also passes across the midline, especially ventrally, where it lies above the xiphoid process of the sternum (see Figures 27–7/8 and 28–4/3). This position allows the application of external pressure in the expectation of eliciting pain when the reticulum is diseased.
The rumen and reticulum are so intimately related in structure and function that many now prefer to describe a combined ruminoreticular compartment. There is much in favor of this convention. The division of the rumen from the reticulum, though more complete, is achieved in exactly the same way as the subdivision of the rumen, namely by the inflection of the walls to form a series of pillars (pilae) that project internally. The whole thickness of the stomach wall, except the peritoneum, participates in these formations. The divisions and the pillars that bound them are illustrated in Figure 28–4, B. The rumen and reticulum communicate over the U-shaped ruminoreticular fold. The principal ruminal pillars encircle the organ, dividing dorsal and ventral major sacs, while lesser coronary pillars mark off the caudal blind sacs. The cranial pillar has an oblique direction that partially divides the cranial extremity from the remainder of the dorsal sac, emphasizing the association of the former part (atrium ruminis) with the reticulum. External grooves correspond to the positions of all these folds. The relative proportions of the compartments vary among the domestic ruminants. The smaller size of the dorsal sac and the extensive caudal projection of the ventral blind sac give the rumen of sheep and goats an unbalanced appearance when compared with the more symmetrical bovine rumen. There are also differences in the development of the grooves visible externally, but these are altogether without significance.
The serosa covers the entire surface of the rumen and reticulum, except dorsally where the ruminal wall is directly adherent to the abdominal roof from the esophageal hiatus of the diaphragm to the level of the fourth lumbar vertebra (Figure 28–13/12), and over certain grooves where it is reflected to continue into the greater omentum. The limited attachment allows the ruminoreticulum the freedom necessary for the incessant and reciprocal contractions and enlargements of its various parts.
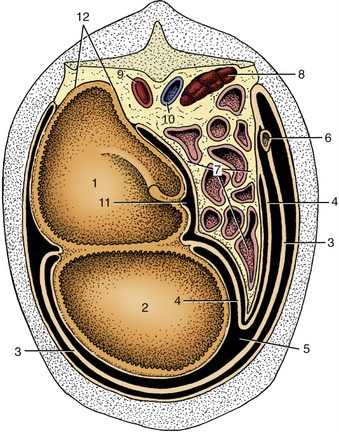
Figure 28–13 Schematic transverse section of the abdominal cavity to show the disposition of the greater omentum. 1, Dorsal sac of rumen; 2, ventral sac of rumen; 3, superficial wall of greater omentum; 4, deep wall of greater omentum; 5, omental bursa; 6, descending duodenum; 7, intestinal mass; 8, right kidney; 9, aorta; 10, caudal vena cava; 11, supraomental recess; 12, retroperitoneal attachment of rumen.
The relationships are most easily studied by reference to the illustrations (see Figures 28–4, A-B; 28–7; and 28–10). The most important points are contact between the reticulum and the diaphragm and liver cranially; insinuation of the abomasum between the two chambers (ventral sac of rumen and reticulum) ventrally; relation of the right surface of the rumen to the intestinal mass, omasum, abomasum, pancreas, and kidneys; and the intrusion of the superficial wall of the greater omentum between the ventral sac of the rumen and the abdominal wall. The rumen also has a variable relationship to the uterus and other organs at the entrance to the pelvis, where the dorsal sac may be palpated per rectum. The direct contact of the dorsal sac with the upper part of the left flank makes auscultation and palpation simple. It also facilitates trocarization for the relief of tympany.
The interior of the ruminoreticulum communicates with the esophagus and omasum through openings placed at the extremities of the reticular groove, a prominent gutter that descends from the cardia over the right face of the reticulum toward the fundus (Figure 28–14/4,5). The groove is bounded by spiral fleshy lips; the upper end of the left (cranial) lip is expanded to overhang the slitlike cardiac opening, while a similar thickening of the lower end of the right (caudal) lip partly conceals the round exit into the omasum. The cardia is placed at the junction of the rumen and reticulum and discharges into both chambers. In the unweaned animal the reticular groove may be converted into a closed tube, forming a channel that conveys milk directly from the esophagus to the omasal canal, whence it drops into the abomasum. The muscular contractions that draw the lips together are reflexly stimulated by sucking from the dam or by the presentation of suitable bucket feeds. As the animal matures, alterations in diet and feeding regimen result in decreasing use of this route, although even in the adult a portion of the soluble nutrients released into the saliva during mastication succeeds in bypassing the ruminoreticulum. The groove reflex is stimulated by antidiuretic hormone (ADH), which indicates that the reflex may have some function in adult life. ADH is produced in response to dehydration or an increase in plasma osmolality. ADH is associated with thirst, and its effect on the reticular groove may cause a portion of the water drunk by dehydrated animals to bypass the ruminoreticulum. Closure of the groove can be stimulated by certain chemicals (e.g., copper sulfate). This provides a useful strategy when it is desirable to introduce drugs to the abomasum without prior dilution in the forechambers.
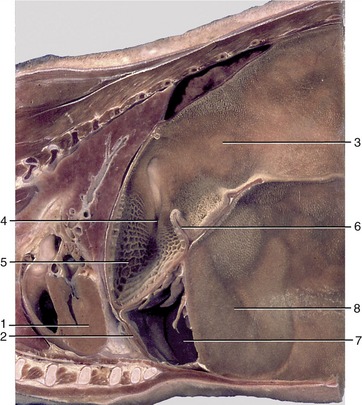
Figure 28–14 Paramedian section of part of the trunk of a goat. 1, Heart; 2, diaphragm; 3, atrium ruminis; 4, reticular groove; 5, reticulum; 6, ruminoreticular fold; 7, abomasum; 8, ventral sac of rumen.
The ruminoreticular mucosa is lined by a harsh stratified cutaneous epithelium (Figure 28–15, A-B) that is stained a greenish brown; the floor of the reticular groove, however, is smooth and pale. The reticular mucosa has a distinctive pattern formed by ridges about 1 cm high that outline four-, five-, and six-sided “cells” (see Figures 27–7/8 and 28–16, B). These ridges and the cell floors between them carry low papillae. The reticulate pattern becomes less regular toward the junction with the rumen and gradually modifies to merge with the papillated surface of this chamber. The upper keratinized layer of the epithelium protects against abrasion by the rough, fibrous diet, whereas the deeper layers metabolize volatile short-chain fatty acids. Histologically, the epithelium shows many similarities with the epidermis. The lamina propria–submucosa, formed by a network of collagen and elastic fibers, includes bands of smooth muscle within the distal parts of the reticular ridges (Figure 28–15, A). The ruminal papillae vary in prominence according to age, diet, and location (Figure 28–16, A, and Figure 28–14). Normally they are largest and most densely strewn within the blind sacs, fewer and less prominent in the ventral sac, and least developed over the center of the roof and toward the free margins of the pillars. Individual papillae vary from low rounded elevations through conical and tonguelike forms to flattened leaves about 1 cm long. The ruminal epithelium resembles that of the reticulum. A thick lamina propria beneath the epithelium forms the core of the papilla; apart from collagen, elastic, and reticular fibers, it includes a dense capillary network. There is no muscularis mucosae. The looser submucosa is located directly against the lamina propria and also contains a vascular network (Figure 28–15, B).
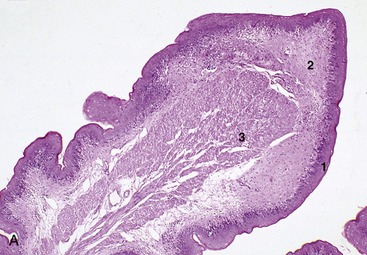
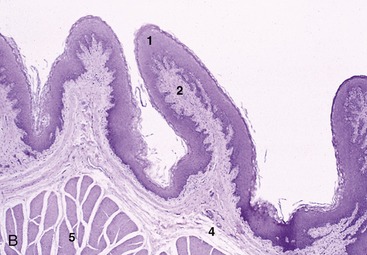
Figure 28–15 A, Reticulum (goat) (28×). B, Rumen (goat) (28×). 1, Stratified squamous epithelium; 2, lamina propria; 3, lamina muscularis mucosae; 4, submucosa; 5, muscularis interna.
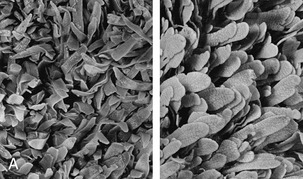

Figure 28–16 A, Rumen papillated mucosa taken from a Waterbuck (left) and a lesser Kudu. B, Reticulum: mucosal ridges outlining “cells” characteristic of the reticular mucosa (cow).
The rugose nature of the ruminoreticular lining was formerly interpreted as an adaptation for the mechanical disruption of the macerating ingesta. Since it became known that the volatile fatty acids produced by microbial fermentation are absorbed in the rumen and reticulum, it has been regarded as primarily a device to increase the epithelial surface. Papillary development is stimulated by these acids (especially butyric), and their absorption is facilitated by the very rich subepithelial capillary plexus. In some wild ruminants, striking changes in papillary prominence and size, and thus in the ruminal surface area (Figure 28–16, A), accompany seasonal changes in forage quality.* Changes in papillary development tend to be more restrained in domestic species, whose diet is subject to human influence to a greater or lesser degree.
The reticulum of the small ruminants is relatively larger than that of cattle. Although it extends farther caudally, its contact with the abdominal floor is subject to much functional variation (Figure 28–14/5). There are conspicuous species differences in its lining. The ridges that bound the reticular “cells” are relatively much lower and have more prominently serrated margins. The papillated “ruminal” mucosa also extends over a larger part of the reticular wall.
The smooth muscle of the ruminoreticular wall is arranged in two coats that continue the striated muscle of the esophagus. The thin outer coat runs craniocaudally over the rumen but has an oblique course on the reticulum. Most bundles of the much thicker inner layer run more or less at right angles to the superficial coat and thus encircle the long axis of the rumen. They extend into the pillars and form the bases of these structures. The thicker parts of the ruminoreticular muscle are sold for consumption as tripe.
The regular sequence of ruminoreticular contractions mixes and redistributes the stomach contents. The cycle consists of a biphasic reticular contraction (relaxation between contraction phases is more consistent in cattle than in sheep), which throws the reticular contents into the atrium ruminis, followed by contraction of first the dorsal and later the ventral rumen sacs. The wave of contraction passes over each in a craniocaudal direction. The process is centrally regulated, and the tempo and vigor are adjusted according to information supplied by intramural receptors that are stimulated by stretching of the wall and by contact with floating fragments. Both the sensory and the motor pathways travel within the vagus nerves.
Regurgitation of food for remastication requires the coordination of the stomach movements with those of the thoracic wall and throat. It is preceded by an additional reticular contraction that floods the cardiac region; the ingesta are drawn into the esophagus on expansion of the thorax with a closed upper airway and are then carried orally by an antiperistaltic wave. The heavy remasticated cud, now further sodden and divided, tends to drop from the cardia into the reticulum.
In eructation (the discharge of gas through the esophagus), ruminal contractions in which the reticulum does not participate are substituted for the normal pattern of activity. These contractions originate in the ventral sac and generally spread to the dorsal sac, where they begin caudally and extend cranially; they force the ruminal gas forward to the cardiac area whence it is aspirated into the esophagus, through which it is hurried orally by an antiperistaltic wave. It then passes through the relaxed pharyngoesophageal sphincter into the pharynx. Some escapes from the mouth, but part is directed to the lungs.
The content of the rumen shows some stratification: food of recent ingestion is piled above the heavier, more sodden remasticated material. It is therefore the lighter material that is most liable to be regurgitated for further mastication and insalivation (Figure 28–17).
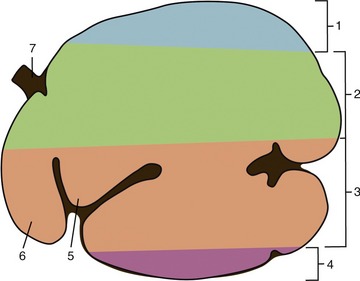
Figure 28–17 Stratification of ingesta in the ruminoreticulum, left lateral view. 1, Gas bubble; 2, coarse forage (“floating mat”); 3, more finely ground material with higher specific gravity than 2; 4, liquid zone; 5, atrium ruminis; 6, reticulum; 7, esophagus.
Cattle are notoriously careless feeders and often ingest foreign bodies, especially pieces of wire, with their forage. These bodies tend to collect within the reticulum and, when sharp, may be driven through the reticular wall by the contractions of this organ (traumatic reticulitis—”hardware disease”). Common sequelae include abscessation of the liver and possibly other abdominal organs and, more critically, a purulent pericarditis when the object penetrates the diaphragm. Some of these bodies corrode, while others may be immobilized by introducing a magnet through the mouth (Figure 28–18/2 and inset).
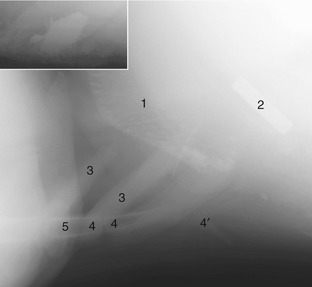
Figure 28–18 Lateral radiograph of the vicinity of the reticulum of a young cow (cranial is to the left). The inset shows a close-up of a magnet with adhering metal objects. 1, Cranial wall of reticulum with sediment in its “cells”; 2, magnet; 3, costal cartilages; 4, sternebrae; 4′, xiphoid cartilage; 5, proximal epiphysis of ulna (olecranon).
THE OMASUM
The omasum lies within the intrathoracic part of the abdomen to the right of the midline, between the rumen and reticulum to the left and the liver and body wall to the right (Figures 28–9/7 and 28–7/2). It is bilaterally flattened and displays a long convex border that faces dextrocaudally and a much shorter lesser curvature that looks in the opposite direction. The long axis is more or less vertical in the cadaver, but the position and orientation of the living organ alter constantly. Most of the omasum lies under cover of the eighth to eleventh ribs, but in cattle the lower pole generally projects onto the abdominal floor below the costal arch (Figure 28–19/5). Although its position places most of the omasum beyond direct manual reach, the organ may be examined by auscultation and percussion. The lower pole of the omasum has an extensive attachment to the fundic region of the abomasum around the omasoabomasal orifice. Much of its right surface is covered by and partly connected to the lesser omentum (Figure 28–4, C/13).
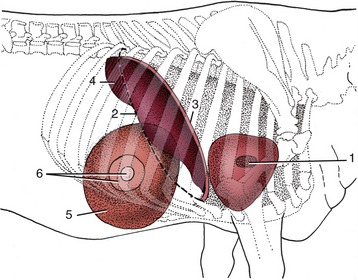
Figure 28–19 Right lateral projection of certain organs on the bovine thoracic wall. 1, Right atrioventricular valve; 2, position of basal border of lung; 3, cranial extent of diaphragm and liver; 4, field of liver percussion; 5, omasum; 6, field for percussion and auscultation of omasum.
The omasum is relatively smaller in sheep and goats, in which it is bean-shaped. It maintains an almost vertical position when the stomach is at rest. It projects on the eighth and ninth ribs but, because of the intervention of the liver, makes no direct contact with the body wall.
The interior is occupied by about a hundred crescentic laminae that arise from the sides and greater curvature and project toward the lesser curvature, where there is a more open passage, the omasal canal (see Figure 28–9). The laminae are of several lengths, and those of different sizes alternate so as to divide the lumen into a series of narrow and fairly uniform recesses (Figure 28–10/7). The reticulo-omasal orifice is situated at the upper end of the short canal; the large, oval omasoabomasal opening (Figure 28–10/6) at the other extremity is partly occluded by the prolapse of abomasal folds. The floor of the canal (known as the omasal groove) is smooth except for a few low ridges that run along its length and a scattering of clawlike projections that guard the upper opening.
The keratinized stratified squamous epithelium over the laminae is raised to cover numerous papillae. Most are small and lenticular, but there are a few larger, conical projections that point distally and perhaps promote the onward movement of the ingesta. The mucosa is further characterized by a lamina propria that includes a dense subepithelial capillary network and encloses a thick muscularis mucosae consisting of a thin outer longitudinal layer and a thicker inner circular layer. The inner layer is continuous with the muscle of the omasal wall. The contents of the omasal recesses are finely divided and rather dry; they impart a firmness to the organ that allows it to be readily recognized on palpation at laparotomy, directly, or from within the rumen after opening that chamber.
Omasal contractions are biphasic. The first phase squeezes ingesta from the omasal canal into the recesses between the laminae; the second phase is a mass contraction. The principal effect is to squeeze fluid from the material within the recesses, which is a process essential to the continuing movement of ingesta to the abomasum. These contractions occur at a much slower and more deliberate tempo than those of the ruminoreticulum. Although the rough surfaces and muscular cores of the laminae suggest that these folds triturate the food by rubbing against each other, there is no evidence of such activity. Absorption is continued in the omasum.
THE ABOMASUM
The abomasum lies flexed on the abdominal floor, embracing the lower pole of the omasum from behind (Figure 28–7/3). The larger of the two limbs forms a piriform sac that reaches forward to the left to make contact with the body wall between the reticulum and the atrium and ventral sac of the rumen (Figure 28–4, A/6). This limb is divided by analogy with the simple stomach into fundus and body, but the boundary between these parts is imprecise. In fact, the location of the omasoabomasal opening in the living animal is not known with certainty; it is possible that it is terminal, and in that case no blind diverticulum and therefore no true fundus exists. The cranial part of the fundus is extensively connected to the reticulum, atrium, and ventral sac by muscle bundles.
The narrower and more uniform distal limb constitutes the pyloric part of the organ. It passes transversely, or with a slightly cranial inclination, toward the right body wall and ascends to terminate at the pylorus, caudal to the lower part of the omasum (Figure 28–4, D/15). The abomasum does not usually come into contact with the liver in adult cattle.
The abomasum of the sheep and the goat is relatively large. In contrast to the situation in adult cattle, it is usually allowed direct contact with the liver by the smaller size of the omasum.
The position and relations of the abomasum depend on the fullness of the different parts of the stomach, intrinsic abomasal activity, and, most importantly, the contractions of the rumen and reticulum to which the abomasum is attached. Age and pregnancy are also an influence (Figure 28–20). Although it is difficult to specify abomasal relations exactly, it is vital to appreciate that there are limits beyond which deviations produce digestive disturbance and may endanger life. Abomasal displacement, which may be to the right or left, is a well-recognized disorder, particularly in dairy cows (see further on).
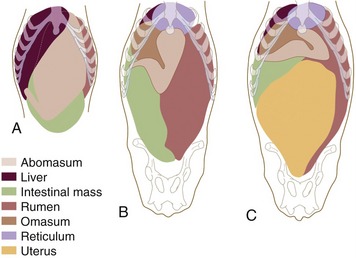
Figure 28–20 Ventral views of the abdominal viscera of a newborn calf (A), a 5-year-old cow (B), and a 6-year-old heavily pregnant cow (C) based on reconstructions of transverse sections of animals frozen in the standing position.
The abomasum is lined by a pink, slime-covered, glandular mucosa that is in striking contrast to the harsh lining of the forestomach. At the omasoabomasal junction the epithelium changes abruptly to a simple columnar epithelium with occasional goblet cells. The lamina propria is less dense than that of the omasum, and frequently, solitary lymph nodules are observed at the junction with the epithelium. The mucosa of the abomasum has all the characteristics of that of the simple stomach (Figure 28–21, A-C). The area is increased about sixfold by the presence of almost a dozen large folds that arise around the entrance and course over the walls of the fundus and body before subsiding as the flexure is approached (Figure 28–22/2). Approximation of the proximal ends of these folds forms a mucosal valve or “plug” that discourages the reflux of ingesta into the omasum. The mucosa of the pyloric part is most remarkable for the large swelling or torus that projects from the lesser curvature to narrow the pyloric passage (Figure 28–22/6). The vascular arrangements within the torus suggest that it is capable of a form of erection, but the possible functional significance of this (and of the entire structure, for that matter) is unknown. The dark mucosa of the body and fundus contains true peptic glands; the glands of the lighter pyloric part secrete mucus alone.

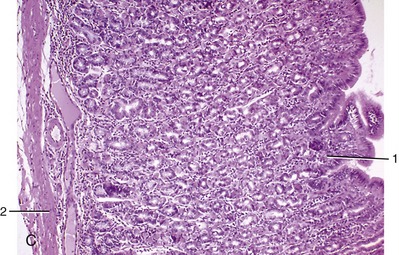
Figure 28–21 A, Internal surface of omasum (cow). 1, Omasal laminae. B, Internal surface of abomasum (cow). 1, Abomasal folds. C, Abomasum (goat) (70×). 1, Gastric pit; 2, lamina muscularis mucosae.
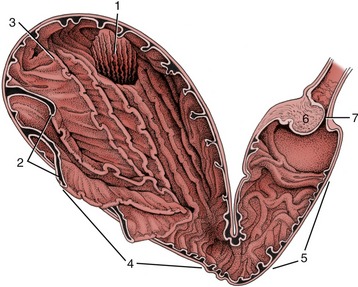
Figure 28–22 Opened abomasum as seen from behind, above, and slightly from the left. 1, Omasoabomasal opening through which the omasal laminae can be seen; 2, abomasal folds; 3, fundus; 4, body; 5, pyloric part; 6, torus pyloricus; 7, pylorus.
The abomasal wall is relatively thin. The serous covering is deficient only at the attachment to the other stomach chambers and along the origins of the omenta. The muscle coat consists of longitudinal and circular strata. The longitudinal muscle is confined to the curvatures of the fundus and body but forms a thicker and wider covering for the pyloric part. The circular fibers provide a more complete layer that is better developed over the pyloric part, especially distally.
The movements of the adult abomasum are rather sluggish. They consist of general contractions of the proximal limb and more forceful peristalsis confined to the pyloric part. The latter activity often appears to be prompted by the tipping of the ingesta toward the pylorus when the fundic region is elevated by reticular contraction. It is possible that these normal alterations in position facilitate morbid displacements. Atony, with the accumulation of gas in the fundus, is a constant finding in these cases, and it may be that a slight initial displacement is worsened because this gas is denied its usual escape through the omasoabomasal opening when this comes to lie below the gas bubble.
Displacements are commonly related to the high proportion of concentrates to roughage in the ration of stabled cows, which leads to atony of the abomasum and accumulation of liquid ingesta and gas. Pregnancy may be a predisposing factor (Figure 28–20, C). Because the abomasum is well fixed proximally to the heavy omasum and distally by the lesser omentum, it is its middle part that travels farthest from its usual position on the abdominal floor. Contractions of the ruminoreticulum may allow the abomasum, buoyed by the gas within, to work its way under the atrium of the rumen and up on the left side. The loop formed by the middle part of the abomasum eventually comes to lie between the rumen and the left abdominal wall, deep to the last three or four ribs, where it can be identified by simultaneous percussion and auscultation (left displacement of the abomasum; LDA). In right displacement (RDA) the loop formed by the middle part of the abomasum slides to the right and lies between the right abdominal wall and the intestines and liver. Displacements to the right are often complicated by twisting of the loop. Treatment of uncomplicated displacements consists of returning the abomasum to its normal position by placing the cow on her back, by deflating the organ through a paramedian incision of the abdominal wall, and by including its muscular coat in the closing of the incision (abomasopexy).
THE OMENTA
The attachment of the greater omentum begins dorsal to the esophagus. The two serosal sheets of which it is composed pass directly onto the rumen but are so widely separated that the immediately postcardiac part of the rumen roof is enabled to attach directly to the abdominal roof (Figure 28–13/12). This retroperitoneal space is closed caudally where the two serosal sheets come together halfway along the right longitudinal groove to form a conventional duplicature attaching to the stomach. The attachment of this fold may be traced along the right longitudinal groove, through the caudal groove between the caudal blind sacs, and then forward along the left longitudinal groove. It now crosses the atrium ruminis and widens to make a broad attachment to the reticulum before bending sharply to the right, ventral to the ruminoreticulum, to reach the greater curvature of the abomasum (Figures 28–4, A,C and 28–8/8). It follows this to the pylorus and continues onto the caudal aspect of the first (vertical) part of the duodenum from which it extends onto the descending duodenum and later the mesoduodenum. The omental attachment is reflected where the duodenum turns cranially, and it retraces its attachment along the descending duodenum until carried back to the cranial duodenal flexure at the porta of the liver. It then returns to the right face of the rumen via the pancreas.
The lesser omentum arises from the visceral surface of the liver, between the porta and the esophageal impression (Figure 28–23), and passes to the region of the reticular groove, the right face of the omasum, and thence along the lesser curvature of the abomasum to the first part of the duodenum, which returns it to the liver (Figure 28–4, C).
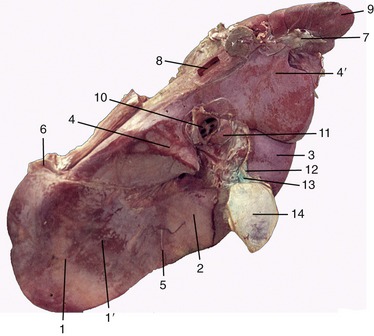
Figure 28–23 Visceral surface of the bovine liver. 1, Left lobe; 1′, omasal impression; 2, quadrate lobe; 3, right lobe; 4, 4′, papillary and caudate processes of caudate lobe; 5, round ligament; 6, left triangular ligament; 7, right triangular ligament; 8, caudal vena cava; 9, right kidney; 10, portal vein; 11, hepatic lymph node; 12, bile duct; 13, cystic duct; 14, gallbladder.
The omental sheets enclose a space, the omental bursa, which is completely divided from the greater peritoneal cavity except at the epiploic foramen near the porta of the liver. The bursa is a mere capillary cleft in life, but it is simpler for descriptive purposes to envisage it as distended. A first impression of its topography may be obtained from the schema, in which it can be seen that the ventral sac of the rumen projects into it (Figure 28–24, B/6,2′). Of the omental sheets that run transversely across the abdomen, one lies against the abdominal wall and the other lies against the viscera (chiefly, the intestines) (Figure 28–13/3,4). The superficial and deep sheets pass into each other caudally and, in this way, close the bursa behind (Figure 28–24, A). The omasum, abomasum, and lesser omentum provide most of the cranial bursal wall. The entrance to the bursal cavity, the epiploic foramen, is situated dorsocranially between the liver and the duodenum or, more precisely, between the caudal vena cava dorsally and the portal vein ventrally.
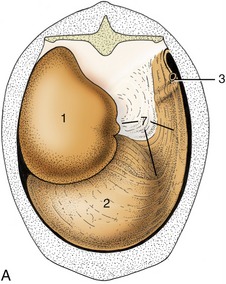
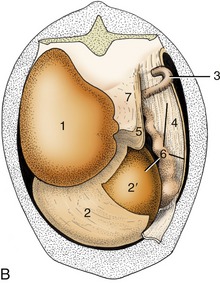
Figure 28–24 Attachment of the greater omentum to the stomach and the dorsal body wall. A, Caudal view of intact greater omentum. B, Caudal view of greater omentum fenestrated to permit a view into omental bursa. 1, Dorsal sac of rumen; 2, ventral sac of rumen, covered by superficial wall of greater omentum; 2′, ventral sac of rumen projecting into omental bursa; 3, caudal flexure of duodenum; 4, superficial wall of greater omentum; 5, deep wall of greater omentum; 6, omental bursa; 7, supraomental recess.
The greater omentum is an important store of fat that is first deposited along the small vessels that ramify between the peritoneal layers; usually the fat is present in such large amounts that the whole omentum becomes thickened and opaque. (In many cows one such thickening forms a short offshoot near the pylorus known as “pig’s ear”; it can be palpated during surgery and marks the position of the pylorus.) The superficial sheet screens the ventral sac of the rumen from view when the lower left flank is opened, and both superficial and deep sheets intervene between the organs that lie ventral to the duodenum and the right flank (Figure 28–4, A,C). The intestines are closeted in the space above the bursa and to the right of the rumen, which is known as the supraomental recess (Figures 28–24/7 and 28–13/11); it is freely open behind and is often entered by the pregnant uterus.
INNERVATION AND VASCULARIZATION
The principal gastric nerves, parasympathetic efferent and afferent, run in the trunks formed along the esophagus by the regrouping of vagal fibers (see Figure 27–3/19,20). The sympathetic nerves that reach the stomach through periarterial plexuses have a subordinate role.
Section of both vagal trunks abolishes all motor activity of the forechambers. Section of the dorsal trunk alone results in almost complete but not necessarily permanent paralysis of the rumen, while the effect on the reticulum is generally less marked. The effects of division of the ventral trunk are unpredictable and range from little or no discernible change to almost complete paralysis of the forechambers. It is presumed that these inconstant results can be explained by differences in the regrouping of fibers where the vagus nerves combine to form the dorsal and ventral trunks and by the later assumption of part of these functions by association neurons in the stomach wall.
Abomasal contractions are greatly reduced after bilateral vagal section but are not wholly interrupted, possibly because some intrinsic control is vested in a submucosal nerve plexus present in the wall of this chamber alone. Division of the splanchnic nerves brings only slight alteration to the gastric movements. Clinically, disturbances of stomach function may follow involvement of the vagus nerves at any point along their courses from the brainstem; the most common causes are mediastinal infections and traumatic reticulitis.
The stomach is supplied with blood through several branches of the celiac artery. The large right ruminal artery runs caudally in the right longitudinal groove and continues into the left groove by passing between the dorsal and ventral blind sacs. It supplies most of the rumen wall and ends in anastomosis with the left ruminal artery, which follows the cranial groove (between atrium and ventral sac) to supply adjoining parts of the rumen and reticulum. The omasum and abomasum are supplied by the left gastric and left gastroepiploic arteries that follow their curvatures.
The veins are mainly satellite to the arteries. The left ruminal vein joins veins draining other chambers of the stomach; the right one, the veins leading from the spleen; their union produces a major radicle (splenic vein) of the portal vein.
Many small lymph nodes are scattered over the stomach, particularly in the ruminal grooves and over the omasal and abomasal curvatures. Lymph from the forechambers leads, after serial passage through these peripheral nodes, to a number of large atrial nodes situated between the cardia and omasum and thence to the visceral root of the cisterna chyli. The nodes placed along the abomasal curvatures direct their efferent vessels to the hepatic lymph nodes.
POSTNATAL DEVELOPMENT
At birth the ruminant stomach is prepared for the digestion of milk. The abomasum predominates and is remarkable not only for its size, which surpasses the combined capacity of the other chambers, but also for the degree of structural maturity that it has attained. Its full extent is apparent directly after the consumption of a generous feed, when it extends from the liver and diaphragm to the pelvic entrance, from one flank to the other, and from the floor well into the upper half of the abdomen (Figures 28–20, A, and 28–25/4). Its capacity may already exceed 60% of the adult measure. So large an organ inevitably impinges on almost all other abdominal contents, but only the extensive contact with the liver, which in the neonate reaches far across the median plane, need be mentioned. The abomasal mucosa is at first not quite mature, and a few days elapse before the fundic glands become fully active; presumably this is a provision to allow the absorption of unaltered antibodies from the colostrum.
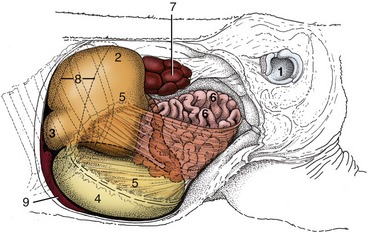
Figure 28–25 Topography of the abdominal organs in a newborn calf, left lateral view. The left abdominal wall and the left hindlimb have been removed. 1, Left acetabulum; 2, rumen; 3, reticulum; 4, abomasum; 5, greater omentum; 6, small intestine; 7, left kidney; 8, position of spleen; 9, liver.
In contrast to the abomasum, the rumen and reticulum of the newborn calf are very small. They are confined to the left dorsal and cranial corner of the abdomen and are generally found crumpled and collapsed (Figure 28–25/2,3); they are bypassed by milk feeds and normally contain only a small amount of fluid—secretions of the respiratory tract (swallowed in utero) in the youngest animals and saliva in those a little older. The omasum is also retarded in development and forms a relatively inconspicuous bridge between the reticulum and the abomasal fundus. The walls of the forechambers are thin and deficient in muscle, and while their mucosae possess the characteristic adult features, these are present in subdued form.
No striking changes in proportions and structure are to be observed before the young calf shows serious interest in solid food, generally from the time it is 2 or 3 weeks old. Thereafter the abomasum continues to increase at a slow but steady rate while the rumen and reticulum enter a period of spectacular growth. They have generally overtaken the abomasum by 8 weeks, and at 12 weeks they are more than twice as large. This unequal growth continues—but more slowly—until the time when the definitive topography and proportions are established. It is difficult to specify this age, for many variable factors are involved; some authors assert that the conformation is virtually adult after 3 months, but others believe that it does not become so until near the end of the first year.
Normal development depends on the availability of a normal diet of solid forage, but there remain some uncertainties concerning the precise stimuli that are involved. At one time it was thought that roughage not only stretched the stomach wall and stimulated its muscular growth but also promoted the differentiation of the mucosa. Later it was shown that many gross and microscopic features of the mucosa develop only with exposure to certain end products of microbial fermentation, notably butyric acid. Exposure to these stimuli must be continued for some time if development is to follow its normal course, and the return of a young, partly weaned calf to a wholly milk diet may result in the arrest and sometimes even reversal of the maturation processes.
The abomasum is initially the most vigorous chamber, but its activity diminishes as the raminoreticulum, first inert and then only spasmodically active, establishes a regular cycle of contraction by the second month. The feeding habits, the structural changes, and the motor and chemical activities of the stomach, when taken in conjunction, define three phases of development. A neonatal period, in which milk forms the sole diet, may last for 2 or at most 3 weeks and may be followed by a transitional period when the stomach is adapting to solid food. From the eighth week onward the anatomy and the processes of digestion may be essentially those of the adult. The chronology will clearly be different in dairy and suckler calves.
Changes in abdominal topography are not confined to the stomach. In the newborn the liver is relatively large and lies across the midline, extensively related to the abomasum. As the rumen and reticulum increase in size the liver is pressed toward the right and dorsally, and it rotates so that its left lobe comes to lie cranioventral to the right one and out of the reach of the abomasum. The intestines are simultaneously pushed away from the left flank and become confined to the right side; the expansion of the dorsal ruminal sac also displaces the left kidney, thrusting it across the midline until it comes to rest below and caudal to its fellow (Figures 28–11/9 and 29–9/10).
THE INTESTINES
The intestines lie almost entirely to the right of the midline, packed mainly into the dorsal part of the abdomen and in part lying under cover of the ribs. Although said to measure as much as 50 m in adult cattle, their capacity is relatively slight, which is a feature correlated with the efficiency of gastric digestion. Adhesion of the mesenteries of the small intestine and ascending colon during the fetal period results in these parts of the intestine sharing a common support in which they are flexed and coiled in a complex arrangement (see Figure 28–26) difficult to unravel in situ.
The duodenum takes origin below the ribs. Its first part rises almost vertically toward the visceral surface of the liver; it then runs toward the pelvis as the descending duodenum but turns when almost level with the coxal tuber. The ascending part then returns toward the liver, passing to the left of the cranial mesenteric artery, to enter the fringe of the mesentery. It is continued by the jejunum. The first part of the duodenum is joined to the liver by the lesser omentum. The other border of the first and descending parts gives attachment, directly or at slight remove, to both walls of the greater omentum (Figures 28–4, C, and 28–24). Only the descending duodenum is immediately visible on opening the right flank.
The jejunum forms many short coils within the free margin of the mesentery. Their general course takes them ventrally, then caudally, and finally dorsally toward the large bowel. The position of these coils depends on the fullness of the rumen and the size of the uterus; usually most lie within the supraomental recess, but some may spill from this to insinuate themselves behind the rumen and so appear against the left flank. The extent of the short ileum is defined by the ileocecal fold (Figure 28–26/4,6).
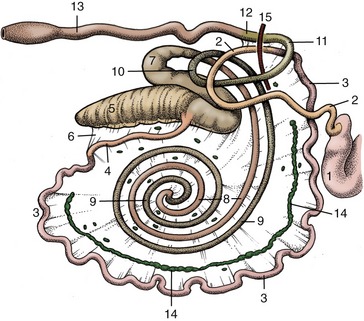
Figure 28–26 Right lateral view of the bovine intestinal tract, schematic. 1, Pyloric part of abomasum; 2, duodenum; 3, jejunum; 4, ileum; 5, cecum; 6, ileocecal fold; 7-10, ascending colon; 7, proximal loop of ascending colon; 8, centripetal turns of spiral colon; 9, centrifugal turns of spiral colon; 10, distal loop of ascending colon; 11, transverse colon; 12, descending colon; 13, rectum; 14, jejunal lymph nodes; 15, cranial mesenteric artery.
The cecum continues into the colon without obvious change in diameter; the junction is marked only by the entrance of the ileum. Its rounded blind tip projects caudally from the supraomental recess and floats high when gas-filled. When greatly distended with gas for protracted periods, it must be deflated surgically. Rotation of the cecum together with the proximal loop of the colon (Figure 28–26/7) is common, compromises its function and blood supply, and requires surgical correction.
The colon is divided into the usual ascending, transverse, and descending parts (see Figure 3–45/Ru). The first of these is wound in a very elaborate manner. On leaving the cecum it forms a flattened sigmoid flexure (see Figure 3–45/11) before narrowing and turning ventrally to trace a double spiral attached to the left side of the mesentery. Two centripetal turns are succeeded by two centrifugal turns that restore the colon toward the periphery of the mesentery, where it continues into a distal loop that carries it first toward and then away from the pelvis (see Figure 3–45/11′). Beyond this it joins the short transverse colon that crosses the midline in front of the mesenteric artery and leads directly into the descending colon. This part runs toward the pelvic entrance within a mesentery that is thickened by fat and fused with neighboring parts of the gut. The mesentery of the descending colon is at first short but lengthens in front of the sacrum, where the colon forms a sigmoid flexure before continuing as the rectum. This looseness gives the hand of the veterinarian considerable range in rectal exploration (p. 720). The rectum is described with the pelvic viscera.
The ascending colon of small ruminants performs three or four turns in each direction. A more significant difference lies in the “pearl necklace” appearance of the centrifugal turns, in which the contents are already segmented into the pellets so characteristic of the feces. The string of these pellets in the ascending colon is replaced by their massing in a thicker column in the wider descending colon and rectum.
Few features of the interior of the intestines call for comment. In cattle the accessory pancreatic duct opens far down the descending duodenal limb, the bile duct opens more proximally, where the duodenum lies against the liver. In the small ruminants the greater pancreatic duct is usually present. The ileum projects into the cecum, and a low rampart is thus present around the ileal orifice. Lymphoid tissue is generously spread through the mucosa, especially in the small intestine, where both solitary and aggregated nodules occur. The aggregated nodules may reach lengths of 25 cm and are distinguished by their irregular cribriform surfaces. Usually one of these patches extends through the ileal orifice into the large gut.
The bulk of the intestines is supplied by the cranial mesenteric artery; however, the first part of the duodenum is supplied from the celiac artery and the descending colon is supplied from the caudal mesenteric artery. The intestinal veins combine to form the cranial mesenteric radicle of the portal vein. Many jejunal lymph nodes are found within the mesentery, where they form a more or less continuous chain of giant nodes placed between the peripheral festoons of small intestine and the more central coils of the spiral colon (Figure 28–26/14). The largest may be as much as a meter in length. In the small ruminants this chain of nodes lies central to the last centrifugal turn of the spiral colon. Other small nodes are scattered beside the cecum, colon, and rectum. The efferent stream from the mesenteric nodes joins the cisterna chyli. The nerves that reach the gut along the cranial mesenteric artery consist of both sympathetic and vagal fibers.* The parasympathetic nerves to the last part of the colon are derived from the sacral outflow.
THE LIVER
The liver of the adult animal lies almost entirely within the right half of the abdomen, related to the caudal face of the diaphragm and under cover of the ribs (Figure 28–9/9). Its projection extends between the ventral third of the sixth intercostal space to the upper part of the last (Figure 28–19/4). The visceral surface is related to the reticulum, atrium ruminis, omasum, duodenum, gallbladder, and pancreas, most of which impress their form on the living organ; the indentations are retained by the specimen hardened in situ (see Figure 28–23). The thick dorsal border extends farthest caudally and is partly fashioned by the blunt caudate process; this is separated from the main mass by a recess into which fits the cranial pole of the right kidney. The medial (originally dorsal) border follows the midline rather closely; toward its lower end it is marked by an impression that gives passage to the esophagus, and below this a small part spreads across into the left half of the abdomen. The caudal vena cava (Figure 28–23/8) tunnels through this edge of the liver and in its course receives its hepatic tributaries (Figure 28–9/10).
The thin lateral border is marked by the fissure that divided the right and left “halves” of the fetal organ, and in most adult cattle this provides entrance for the round ligament, the remains of the umbilical vein (Figure 28–23/5). The blind vertex of the piriform gallbladder (Figure 28–23/14) projects beyond the lateral margin of the right lobe; it lies against the diaphragm opposite the ventral part of the tenth or eleventh rib.
The liver is retained in position by certain ligaments attaching it to the diaphragm and, more importantly, by visceral pressure. Its position may be verified by dullness on percussion over an area centered on the dorsal part of the eleventh rib and eleventh intercostal space. The percussion area is small in relation to the size of the organ and corresponds to the area of direct contact with the body wall (Figure 28–10/10). A detectable increase in its extent generally signifies a disproportionate enlargement of the organ.
The relationship of the liver to the right pleural sac should be noted so that biopsy specimens may be obtained with the least risk (Figure 28–19/2,4). The preferred site for puncture is through the eleventh intercostal space in the plane of the lower part of the coxal tuber. The trocar is directed to meet the diaphragm and thus the liver at right angles so that a clean puncture is ensured; this route avoids the larger vessels. The relatively larger size of the liver of the young calf may allow the organ to be palpated behind the last rib.
The structure of the liver shows no species-specific features of importance. The organ is enclosed within a tough fibrous capsule, but the extensions into the parenchyma do not outline obvious lobules as in the liver of the pig. The hepatic ducts join together in the portal region to form a single channel from which the cystic duct branches to the gallbladder. The continuation beyond this junction constitutes the bile duct, which enters the duodenum. The most superficial hepatic ducts may be visible through the covering liver tissue, especially when thickened by disease; in many countries, most ostensibly normal animals show this evidence of fluke infestation (distomiasis).
The liver receives blood from the hepatic artery and portal vein, which enter at the porta. Blood from both sources returns to the general circulation through the hepatic veins, which enter the embedded portion of the caudal vena cava. The openings of the major hepatic veins are arranged in two widely separated clusters; intrahepatic anastomoses between the two sets provide a potential collateral pathway that becomes important when the intervening stretch of the caudal vena cava is obstructed.
The efferent lymphatic vessels pass mainly to the hepatic group of nodes scattered about the porta; the lymph thence drains into the visceral radicle of the cisterna chyli. Some lymph is routed via accessory hepatic (on the caudal vena cava) and caudal mediastinal nodes.
Although the livers of the sheep and the goat generally resemble that of cattle, size alone prohibits confusion of the adult organs. They are distinguished from the liver of the calf by the much deeper umbilical fissure, narrower and less bluntly shaped caudate process, more elongated gallbladder, and absence of the sizable vestige of the umbilical vein that is evident on the liver of the young calf. An extensive contact with the abomasum is retained throughout life.
THE PANCREAS
The pancreas is of irregular form and of pinkish-yellow color. The pancreas of the calf is consumed as a delicacy, together with the thymus, under the title of sweetbread. For descriptive purposes it may be regarded as consisting of two lobes that join in a body located cranial to the portal vein, where the gland is adherent to the liver. The left lobe extends across the abdomen, insinuated between the liver, diaphragm, and great vessels dorsally and the intestinal mass and dorsal ruminal sac ventrally; it thus enters the retroperitoneal area above the rumen. The right lobe has a more complete peritoneal covering and follows the mesentery of the descending part of the duodenum, ventral to the right kidney and against the flank.
Although developed from dorsal and ventral primordia, the excretory system is usually reduced in cattle to a single (accessory) duct when the ventral outgrowth loses its direct connection to the gut. The surviving duct enters the descending duodenum about 20 to 25 cm past the entry of the bile duct. Its orifice is raised on a slight papilla.
The pancreas of small ruminants is very similar in form and topography to that of cattle. A single ventral duct is present, and it opens into the duodenum with the bile duct, usually by means of a common trunk.
THE KIDNEYS AND ADRENAL GLANDS
The kidneys of adult cattle retain much of their fetal lobation and are divided by surface fissures into about a dozen lobes (see Figure 5–21). The right kidney has a flattened ellipsoidal form and lies in a conventional position with a dorsal retroperitoneal attachment to the sublumbar musculature. It is received cranially into the renal impression of the liver. The left kidney is less regular, being flattened at its cranial pole and thickened caudally. Its position below and behind its fellow is unusual and is the consequence of the postnatal growth of the rumen (see Figure 29–9/10). Although surrounded by considerable accumulations of fat (capsula adiposa), both kidneys vary in position with the phase of respiration and according to the pressure exerted by other viscera. In the cadaver the right kidney is commonly found below the last rib and first two or three lumbar transverse processes, while the left one lies at a more ventral level under the second to fourth lumbar vertebrae. The left kidney is thus within easy reach on rectal exploration, but contact with the right one is not usually attainable. The left kidney may return to the left side when the pressure on it is relieved by fasting in life or after evisceration in the course of an autopsy.
The numerous relations of the right kidney need not be described at length. They include the liver, pancreas, duodenum, colon, and, in most animals, the adrenal gland. The hilus is widely open and lies ventromedially; the ureter runs from it, crossing the medial margin to follow a winding retroperitoneal course below the abdominal roof that carries it into the pelvis.
The left kidney is swung through about 90° around the axis of the aorta in moving from its fetal (see Figure 28–26) to its adult location against the right face of the dorsal sac; it hangs in a relatively long fold, rests on the intestinal mass, and is flattened by contact with the rumen. The left ureter crosses the dorsal aspect of the kidney to regain the left half of the abdomen. Its later course is similar to that of the right duct.
In structure the bovine kidneys are of the multipyramidal type (Figure 28–27). The separate medullary pyramids are capped by a continuous cortex, although on casual inspection this also appears fragmented by fissures extending inward from its surface (Figure 28–28). The cortex (Figure 28–27/4) is clothed in a tough capsule that is easily stripped from the healthy organ, except toward the hilus, where it blends with the wall of the ureter. The cortical and medullary regions are distinguishable in gross sections by the much lighter color of the former and by the cut vessels that mark their mutual boundary. The glomerular vascular tufts scattered through the cortex may be visible to the naked eye. The apex (papilla; Figure 28–27/3) of each medullary pyramid fits into a calyx or cup formed by one of the terminal branches of the ureter; these branches eventually unite to form two major channels that converge from the cranial and caudal poles to yield a single ureter (see Figure 5–23). There is thus no large central expansion corresponding to a renal pelvis.

Figure 28–27 Bovine kidney dissected to show its interior, semischematic. 1, Principal branches of ureter; 2, calyx; 3, renal papillae; 4, renal cortex; 5, interlobular artery.
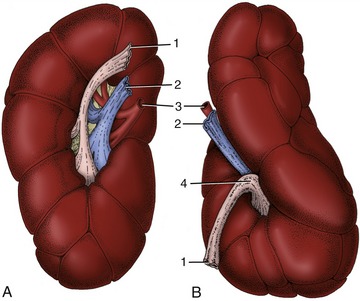
Figure 28–28 Ventral views of the right (A) and left (B) bovine kidneys. 1, Ureter; 2, renal vein; 3, renal artery; 4, renal sinus.
The renal arteries are derived from the aorta; the renal veins join the caudal vena cava. Lymphatic vessels lead to the renal nodes, enlarged members of the lumbar aortic series, and these in turn drain into the lumbar lymph trunk.
The kidneys of the sheep and goat are quite unlike those of cattle but conform closely in external appearance and internal structure to those of the dog (see Figure 5–23). They are more regular in shape than the dog’s, being protected from distorting pressures by enclosure in thick masses of fat. The fat cushion makes the left kidney less subject to displacement by the rumen.
The adrenal glands are located close to the kidneys. The right gland is heart shaped and usually lies against the medial margin of the cranial extremity of the corresponding kidney (Figure 28–10/12). The left one is less regular in form and less constant in position; generally it is found within the perirenal fat some centimeters cranial to the left kidney. The division into cortex and medulla is very evident in gross sections.
THE LYMPH NODES OF THE ABDOMINAL ROOF
A number of important lymph nodes are scattered about the bifurcation of the aorta and between its terminal branches. Most belong to the medial iliac group, which collects lymph from the hindlimbs, pelvic walls, and pelvic viscera (see Figure 29–4). The large deep inguinal (iliofemoral) node, in the angle between the external and deep circumflex iliac arteries, receives the flow from the udder; when enlarged it can be palpated per rectum near the cranial border of the ilium. The efferent stream forms the lumbar trunk, which runs forward over the aorta to enter the cisterna chyli.
A few much smaller (lumbar aortic) nodes that are spread along the psoas musculature are concerned with the lymphatic drainage of the vertebrae and neighboring muscles. The renal nodes belong to this series.