15 The Pelvis and Reproductive Organs of the Dog and Cat
GENERAL ANATOMY OF THE PELVIS AND PERINEUM
(See also pp. 55–56.)
The bony pelvis is formed by the pelvic girdle, sacrum, and first few caudal vertebrae; of course the caudal limit of the roof is, as always, difficult to define precisely. The bones were described in Chapter 2, and the surface landmarks they create are mentioned in Chapter 17. It will therefore be sufficient at this point to recapitulate a few general features of the anatomy of the pelvis.
The pelvic cavity is smaller than might be supposed from examination of the intact animal or the isolated girdle. The discrepancy between expectation and reality is due to the shallowness of the caudal part of the abdomen and to the acute angle (about 20°) formed between the ilia and the vertebral column (Figure 15–1, A-B). The pronounced obliquity of the inlet places the pubic brim level with, or even behind, the caudal limit of the sacrum. The iliac shafts are not quite parallel, and the inlet is widest in its middle part and narrowest dorsally. The pelvic outlet is less confined than the inlet and possesses a considerable capacity for further enlargement through elevation of the tail behind the very short sacrum. Only a small part of the lateral wall is bony, as neither the ischial spine nor the ischial tuber rises to any great height. In the dog the sacrotuberous ligament is reduced to a narrow cord (under cover of the superficial gluteal muscle) extending between the ischial tuber and the caudolateral corner of the sacrum (Figure 15–1, A).
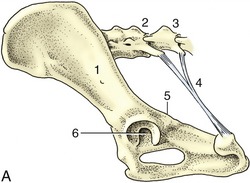
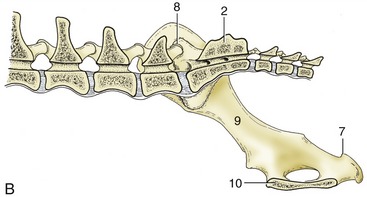
Figure 15–1 A, Canine sacrotuberous ligament, left lateral view. B, The right half of the canine bony pelvis, medial view. 1, Ilium; 2, sacrum; 3, caudal vertebra(e); 4, sacrotuberous ligament; 5, ischial spine; 6, acetabulum; 7, ischial tuber; 8, sacroiliac joint; 9, shaft of ilium; 10, symphysis.
The pelvic girdle of the cat shows some differences. Cranially, the ilia diverge slightly, producing a somewhat funnel-shaped entrance to the pelvis from the abdominal cavity. The wings of these bones are relatively smaller and shallower, which also eases the transition. The ischial tubers stand closer together than in the dog, which gives the pelvis a more rectangular appearance in the ventrodorsal view and a more confined exit (Figure 15–2). In consequence of the last feature the perineum is narrow. There are no sacrotuberous ligaments in this species.
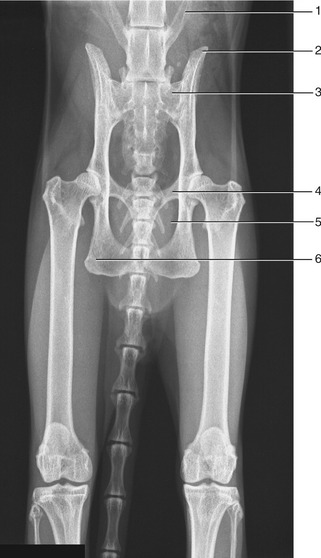
Figure 15–2 Radiograph of the feline pelvis. 1, Transverse process of last lumbar vertebra (L7); 2, iliac crest; 3, sacrum; 4, pecten of the pubis; 5, obturator foramen; 6, ischial tuber.
The axis of the short pelvic canal is almost straight, and in general the conformation appears well adapted for easy parturition. Sexual dimorphism is not pronounced, and pelvic measurements have not been given much attention in small animal obstetrics. An ill match of the proportions of the fetus and the dam is most common in cases in which the litter is small (and the individual fetus relatively large) in toy dogs, and in those breeds in which a measure of achondroplasia is a feature of the conformation. On rectal examination the pelvic canal of young dogs is shaped like an hourglass, which may mistakenly suggest a pelvic fracture.
The perineum slopes somewhat ventrocaudally and is largely concealed when the tail is carried low. When the tail is raised, it exhibits a shield of naked integument about the anal orifice and, at some distance ventral to this, the vulva or root of the penis; these features are considered in more detail later. The ischiorectal fossa between the anus and the ischial tuber naturally varies in prominence with the character of the coat and the degree of obesity. The fossa is bounded by the sacrotuberous ligament and the deep face of the superficial gluteal muscle laterally and by the superficial face of the coccygeus medially. It is traversed by the large caudal gluteal vessels that run against the lateral wall and by the main trunks and certain branches of the internal pudendal vessels and pudendal nerve placed more medially, toward the floor (Figure 15–17/2,3).
The pelvic diaphragm has the usual composition. The lateral muscle, the coccygeus, has a tendinous origin from the ischial spine and inserts on the lateral aspect of the tail between the second and fifth vertebrae (Figure 3–48 and Figure 15–17). The deeper and thinner levator ani (Figure 3–48/2) has a wider origin, which extends from the iliac shaft onto the pelvic floor along which it runs, directly to the side of the symphysis (Figure 15–3/7). The part arising from the pelvic floor closely embraces the pelvic viscera in its passage to its insertion on the tail, reaching as far caudally as the seventh vertebra. The levator fibers run more obliquely than those of the coccygeus, and part of the levator emerges superficially behind that muscle. The levator has only a passing fascial connection with the external sphincter of the anus, and like the coccygeus, it is primarily a depressor of the tail. However, its fascial attachment enables it to help fix the position of the anus during defecation. The tone of both muscles is important in retaining the pelvic viscera in place, and perineal hernia—in which pelvic organs are displaced to form a swelling to the side of the anus—may be a sequel to their paralysis or atrophy. Surgical repair of this condition involves suture of the external sphincter to the coccygeus, internal obturator, and sacrotuberous ligament about the margins of the space.
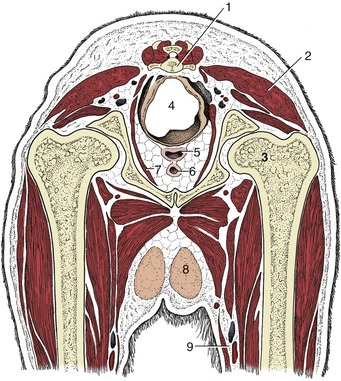
Figure 15–3 Transverse section of the canine pelvis at the level of the hip joint. 1, Caudal vertebra; 2, superficial gluteal muscle; 3, head of femur in acetabulum; 4, rectum suspended by a short mesorectum; 5, vagina; 6, urethra; 7, levator ani; 8, inguinal mammary gland; 9, femoral artery and vein.
The pelvic blood vessels and nerves were sufficiently described in the general accounts (pp. 251 and 325). Because there are only three sacral spinal nerves, the origins of the pudendal, caudal rectal, and pelvic nerves are rather compressed; variations in the branching patterns of the first two are common. The pudendal and caudal rectal nerves supply afferent and efferent fibers to the perineum, and their integrity is necessary for the execution of the perineal reflex that provides a means of gauging the depth of narcosis. The modified skin about the anus is especially sensitive, and even a gentle touch evokes a brisk contraction of the anal sphincter of the conscious or lightly anesthetized animal.
THE RECTUM AND ANUS
(See also pp. 133 and 134.)
The rectum joins the anal canal ventral to the second or third caudal vertebra. Its cranial part is intraperitoneal and joined to the pelvic roof by a short mesorectum (Figure 15–3/4); the caudal part becomes entirely retroperitoneal once the serous covering has been reflected onto the pelvic walls and the dorsal surface of the reproductive tract (bitch) or prostate (dog). The dorsal relations of the rectum include the ventral muscles of the tail and certain smooth muscle bundles (rectococcygeus) that run caudally from the rectal wall to the undersurface of the tail; these bundles probably help draw the anus caudally when a column of feces descends from the colon. The ventral relations of the rectum of the bitch are the cervix and, possibly, the body of the uterus in addition to the vagina; in the male dog they are the prostate and urethra. Laterally, the rectum is bounded by the levator muscle and crossed by the internal pudendal vessels (Figure 15–17) and the sciatic, pelvic, pudendal, and caudal rectal nerves; the rectum has some freedom to deviate from its usual median course because of its mesorectum and its cushioning by fat.
The mucosa of the short (ca. 7-mm) initial columnar portion of the anal canal is fashioned by underlying vessels into a series of longitudinal ridges whose interdigitation helps maintain continence (Figure 15–4). These ridges end on an anocutaneous line that represents the junction between the columnar intestinal epithelium and the stratified cutaneous epithelium. The outer cutaneous zone is of variable extent; the modified skin that lines this last part of the passage may be everted to appear as a purplish patch on the perineal surface, especially when defecation impends. At this time the anal orifice takes on a triangular form in place of the transverse slit generally displayed (Figure 10–29, A).
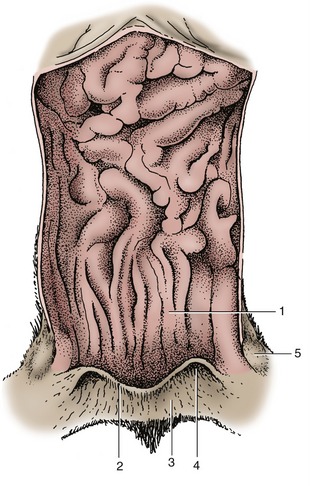
Figure 15–4 Feline anal canal opened dorsally. 1, Columnar zone; 2, anocutaneous line; 3, cutaneous zone; 4, opening of the right anal sac; 5, right anal sac.
Developmental errors lead to an imperforate anus, which results from the persistence of an unusually thick anal membrane, or the absence of a longer portion of patent bowel, which results from the failure of the rectum to make proper connection with the anal pit.
All fissiped carnivores (other than bears) possess paired anal sacs (sinus paranales) enclosed between the external and internal anal sphincters. In the dog, each is about 1 cm in diameter and discharges through a short duct that opens ventrolateral to the anal orifice at the level of the anocutaneous line, concealed or exposed on the perineal surface according to the physiological condition (Figure 3–47/1). In cats, the ducts of the anal sacs open on small projections some distance lateral to the anus and not at the mucocutaneous junction as in dogs. Modified sweat glands are located beneath the epithelium and discharge into the lumen of the sac. In the dog, only apocrine glands are found, but in cats both sebaceous and apocrine sweat glands are present. Because occlusion of the duct of the anal sac is frequently encountered in dogs but is rare in cats, it is thought that the lipid component of these sebaceous secretions is responsible for this difference. The evil-smelling content of the anal sacs is normally expressed in the later stages of defecation and serves as a marker that identifies the animal to other members of its species.
Apart from the clinical importance imparted by the frequent blockage of the ducts, the anal sacs of dogs obtain an additional significance from the malignant tumors that sometimes develop in the apocrine glands, until now only reported in bitches. A common feature of these tumors is the production of a parathormone-like hormone that raises the blood calcium levels.
The lymphatics of the anal sac drain to the sacral, hypogastric, and medial iliac lymph nodes.
There are, in addition, small anal glands within the columnar zone and much larger and more numerous circumanal or perianal glands within the cutaneous zone. In dogs, the circumanal glands are lobulated, modified sebaceous glands located in a ring about the anus, extending outward for a distance of perhaps 3 cm from the anocutaneous junction. These glands can be identified shortly after birth and increase in size throughout adult life in response to androgens. In older male dogs, slow-growing, generally benign tumors of these glands commonly develop near the anus.
THE KIDNEYS
The positions and relations of the kidneys were described in the previous chapter.
The right kidney usually lies below the first three lumbar vertebrae, and the left one lies below the second to fourth, although both may be found a full vertebral length more caudally. In the bitch the caudal poles of both kidneys reach close to, or make contact with, the fat-filled mesovaria. Although described as unipyramidal (p. 177), the canine kidney retains clear evidence of the former existence of a number of separate pyramids. The renal arteries, direct branches from the aorta, usually divide before entering the kidneys; they may be assisted by small arteries of aberrant origin. The renal veins pass directly to the caudal vena cava (Figure 14–21). There are no features of major specific interest in the sympathetic and parasympathetic nerve supply.
The kidneys of the cat are relatively larger, shorter, and thicker than those of the dog and obtain a distinctive appearance from the capsular veins that converge toward the hilus, where they enter the renal vein (Figure 15–5). The cut surface of the kidney is red to yellowish red because of a large amount of intracellular fat stored in the proximal convoluted tubules; the fat content is greatest in castrated males and pregnant females. There are fewer vestiges of the multipyramidal stage of development. The kidneys are more mobile than in dogs, especially the left one, which can be displaced rather far cranially or caudally from its usual position (Figure 14–13); it has been mistaken for a pathological swelling. In cats, both kidneys are readily palpable.

Figure 15–5 Ventral view of feline abdominal roof. 1, Liver; 2, kidneys (with stellate v.v.); 3, caudal vena cava (injected); 4, aorta; 4′, ovarian a. (injected); 5, uterine horn; 6, ovary.
In the dog (if not the cat) it is generally thought more prudent to expose a kidney by laparotomy when a biopsy specimen is required rather than to attempt a blind puncture.
The muscle of the renal pelvis is strongest at the transition to the ureter, presumably to impel urine into the narrower tube. The abdominal part of the ureter runs retroperitoneally close to the aorta or vena cava (Figures 14–21, 14–22/3, and 15–5), passing over the dorsal (lateral) surface of the gonadal vessels before crossing the ventral face of the deep circumflex iliac vessels and the terminal branches of the aorta (and corresponding veins). It is carried into the pelvis in the base of the broad ligament or genital fold, which brings it to the dorsal surface of the bladder; in the male it crosses above the deferent duct toward the end of its course. It penetrates the bladder wall very obliquely. The inclusion of the ureter within the genital fold places it at some risk in the common spay operation.
Survey radiographs of the abdomen will adequately reveal the external anatomy of the kidneys when, as is usually the case, they are enclosed in fat. (Deficiency of fat occurs in very young pups and in emaciated older subjects.) Visualization of internal features requires the intravenous injection of an appropriate contrast agent that is then excreted in the urine; suitably staged radiographs will show general opacification of the cortex and medulla (Figure 14–22), renal pelvic morphology (Figure 5–29), and, later, the status of the ureters and bladder. Because the passage of urine is assisted by peristaltic contraction, a single radiograph does not usually depict a healthy ureter along its entire length.
THE BLADDER AND FEMALE URETHRA
(See also pp. 181–184.)
Although the neck of the canine bladder extends a little way into the pelvic cavity, the bulk of the organ is visible as soon as the floor of the abdomen is removed, as it is not covered by the greater omentum (Figure 15–6). Its size varies greatly and when excessively distended, as in house-trained animals denied opportunity for relief, it may reach to or even beyond the umbilicus (see Figure 15–27). In dogs allowed freedom, the bladder is rarely very large because the frequent discharge of urine performs a social (scent-marking) as well as eliminative function.* The bladder may be identified on abdominal palpation when moderately (or more greatly) distended. Unless handled with care, a grossly distended bladder may rupture when compressed through the abdominal wall to induce micturition. The oblique passage of the ureters through the bladder wall normally affords protection against reflux of urine to the kidneys, but even cautious compression, if too long maintained, may overcome this protection and may cause the introduction of contamination from an infected bladder. A moderate increase in bladder size is not accompanied by increased tension, and radiographs obtained with the (contrasted) bladder in this state show its contours molded to those of adjacent organs (Figure 5–30). The organ is globular when the thick detrusor muscle is fully contracted.
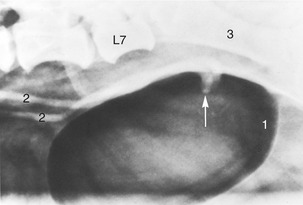
Figure 15–6 The canine bladder made visible by the introduction of air. The arrow indicates the terminations of the ureters in the dorsal wall of the bladder, superimposed here on the air-filled lumen. 1, Caudal end of bladder; 2, ureters; 3, shaft of ilium.
The peritoneal covering, which extends onto the cranial part of the urethra, is reflected into the usual lateral and ventral folds.
The bladder receives its blood supply through the cranial vesical artery, a branch of the umbilical artery, and the caudal vesical artery, an indirect branch of the internal iliac artery. The hypogastric nerve supplies the sympathetic innervation, the pelvic nerve (S1–S3) supplies the parasympathetic innervation, and the pudendal nerve (S1–S3), the somatic innervation.
The female urethra is relatively long. It originates within the cranial part of the pelvis and follows the symphysis to open on the floor of the vestibule, immediately caudal to the vestibulovaginal junction. In the bitch, the orifice is raised on a tubercle that continues some way over the vestibular floor, flanked by well-marked depressions. Although blind catheterization is difficult in small subjects, the procedure is less troublesome in larger bitches, in which a finger may be introduced to locate the tubercle and guide the instrument.
The bladder of the cat is more cranially placed than that of the dog and lies wholly within the abdomen at all times. As a result, the urethra is unusually long and some authors have been tempted to interpret the intraabdominal part as a curiously drawn-out bladder neck (Figure 15–7). The urethra of the queen is more or less uniformly wide (unlike its counterpart in the tom) and makes a more discrete entry into the vestibule than that of the bitch.
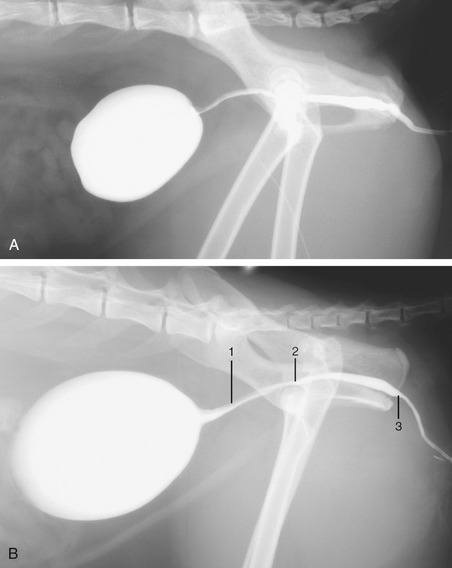
Figure 15–7 Radiographs of the feline bladder when moderately (A) and markedly (B) full. 1, Preprostatic urethra: the upper gray part is the urethral crest, the lower white part is the lumen filled with contrast medium; 2, slight dorsal dip marks the seminal colliculus; 3, isthmus, narrowing of lumen.
The male urethra of both species is considered with the reproductive organs.
The urachus, which connects the bladder with the allantoic sac of the fetus, normally closes at birth, but sometimes there is leakage at the umbilicus for a time. A more important anomaly is the persistence of part of the urachus as a diverticulum of the bladder, as this seems to predispose to recurrent bladder infections.
Congenital urinary incontinence in dogs and cats is most often caused by ectopic ureters, those which terminate at a site other than the normal one at the trigone of the bladder. Sometimes they take an unusual course through the bladder wall, and sometimes they bypass the organ to enter a more distal part of the urogenital tract.
Acquired urinary incontinence occurs most often after the spaying of bitches and is caused by urethral sphincter incompetence, for which a number of explanations, some likelier than others, have been suggested: low urethral pressure, a short urethra, estrogen deficiency, or an intrapelvic position of the bladder. This type of incontinence is most often associated with relaxation or recumbency, particularly at night. Several surgical techniques have been developed to relocate the bladder neck to an intraabdominal position with the use of the prepubic tendon as an anchor.
THE FEMALE REPRODUCTIVE ORGANS
THE OVARIES AND UTERINE TUBES
(See also pp. 197–199.)
The distal mesovarium and the mesosalpinx fuse to create a bursa into which the ovary projects and within which it is entrapped (Figure 5–60). In bitches these folds contain much fat that largely conceals the ovary (Figure 15–8), which is a firm, flattened, ellipsoidal structure measuring about 15 × 10 × 6 mm. Its contours are obviously less regular in phases of the estrous cycle in which large follicles or corpora lutea are present (Figure 15–9). The wall of the ovarian bursa of cats commonly contains conspicuously less fat than that of the bitch and covers only the lateral surface of the ovary, which is consequently more immediately visible.
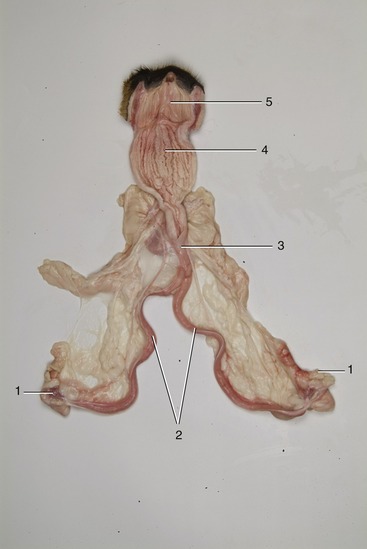
Figure 15–8 Overview of canine female reproductive tract. Vagina has been opened. 1, Ovaries; 2, uterine horns; 3, uterus body; 4, vagina; 5, vestibulum.
The ovaries (within the bursae) lie close to, or even in contact with, the caudal poles of the kidneys; in conformity with the asymmetrical position of the kidneys the left ovary is placed a little caudal to its fellow. Although most spays (the removal of ovaries and [parts of] the uterine horns; ovariectomy/ovariohysterectomy) are now performed by midline incision, an alternative lateral approach is quite often used in cats. The flank incision is made midway between the iliac crest and the last rib in the confident expectation that the ovary will be within easy reach. The right ovary is usually found dorsal or dorsolateral to the ascending colon, and the left one is found between the dorsal extremity of the spleen and the descending colon. Lengthening of the attachments in parous animals allows the ovaries a greater mobility.
The ovary is fixed additionally by suspensory and proper ligaments. The former is a peritoneal fold, thickened along its free margin, that attaches to the transverse fascia close to the last rib in the dog (Figure 15–10/6); it is prolonged caudally as the proper ligament, which extends beyond the ovary to merge with the tip of the uterine horn. The anchorage provided by the suspensory ligament makes surgical exteriorization of the ovary difficult. The suspensory ligament in the cat reaches the diaphragm and allows the ovary greater mobility.

Figure 15–10 Canine (A) and feline (B) ovaries and uterus in situ, ventral view. 1, Psoas muscles; 2, aorta; 3, caudal vena cava; 4, 4′, left kidney and ureter; 5, ovary; 5′, ovarian vessels; 6, suspensory ligament of ovary; 7, uterine horn; 8, body of uterus; 9, rectum; 10, bladder, reflected caudally.
The entrance to the canine bursa is reduced to a slit in the medial wall, usually made obvious by the protrusion of a few reddish infundibular fimbriae. The infundibulum is continued by the narrower part of the uterine tube, which is not obviously divided between ampulla and isthmus. These parts follow a tortuous course within the walls of the bursa; disregarding minor kinks and bends, the tube runs in a broad sweep that first passes forward in the distal mesovarium before crossing cranial to the ovary to continue caudally in the mesosalpinx (Figure 5–60). It ends in an abrupt junction with the horn of the uterus. Although in most subjects much of the tube is concealed by fat deposits, the terminal part is usually visible. The infundibulum may transmit bacteria into the bursa (or abdominal cavity) in the case of pyometra.
Parovarian cysts originate from remnants of either mesonephric or paramesonephric ducts. They are more frequently encountered during ovariohysterectomy in dogs than in cats and are located between the ovary and uterine horn.
THE UTERUS
(See also pp. 199–201.)
The uterus, which lies mainly dorsal to the small intestine, consists of a very short (ca. 2- to 3-cm) body from which two long and slender (ca. 12- × 1-cm) horns diverge (Figure 15–10/7,8 and Figure 15–11). The body is near the pubic brim but may be abdominal or pelvic in position. It is, in fact, even shorter than external inspection suggests because a short internal septum continues caudally from the junction of the horns. The cervix is also very short—the canal is barely 1 cm long—but the tissue thickening extends beyond the external ostium as a fold on the roof of the vagina (Figure 15–11/3,3′). Transverse grooves frequently divide this fold into cranial, middle, and caudal tubercles; these become much swollen at certain stages of the cycle. The ostium of the cervix generally faces caudoventrally, and this orientation, combined with the asymmetry of the fornix and the fissuration of the cervical prolongation, may make its identification rather difficult, even with the aid of an endoscope.
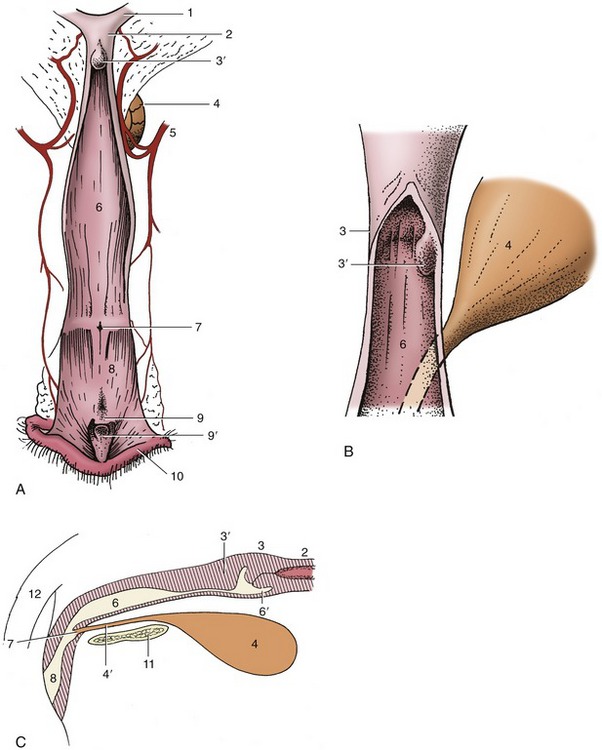
Figure 15–11 A, Canine vagina, vestibule, and vulva, opened dorsally. B, Enlarged view of the cervix. C, Schematic median section of the organs shown in A. 1, Right uterine horn; 2, body of uterus; 3, cervix; 3′, dorsal fold, may extend a considerable distance into the vagina; 4, bladder; 4′, urethra; 5, vaginal artery; 6, vagina; 6′, fornix; 7, external urethral orifice; 8, vestibule; 9, clitoris; 9′, clitoral fossa; 10, right labium of vulva; 11, pelvic symphysis; 12, tail.
The feline cervix feels like a hard oval knot at the uterovaginal junction and, although small, is readily distinguished from the adjoining parts by the thickness of its wall. As in the bitch, the cervical mucosa is smooth, without conspicuous folds.
The broad ligaments also commonly contain much fat. They are wider in their middle parts than toward their extremities and allow the horns of the uterus considerable mobility. An unusual feature is the detachment from the lateral surface of a peritoneal fold that extends toward, and in the bitch through, the inguinal canal to end variously between the groin and the vulva. The fold is thickened at its free margin (the round ligament), and this slightly dilates the canal, which predisposes to inguinal hernia, an almost male prerogative in other species. Because the uterine horn is the most likely organ to be herniated, the bizarre situation sometimes arises in which a portion of the pregnant uterus is trapped subcutaneously; a fetus developing in this situation must be delivered by separate section if the herniated part is not restored to the abdomen in good time.
The vascularization of the uterus depends on the uterine branch of the ovarian artery and the uterine artery, a branch of the vaginal artery (Figure 15–12/1,5). The two vessels anastomose within the broad ligament and must be ligated when ovariohysterectomy is performed. These vessels lie close to the extremities of the uterus but swing away in the intermediate part of the broad ligament. The proximity of the uterine artery to the cervix allows an arterial ligature to be securely anchored to the uterine stump to prevent slippage when the bulk of the uterus is removed surgically. Almost the entire uterus is drained by a large uterine tributary of the ovarian vein, which empties into the renal vein on the left but generally proceeds directly to the caudal vena cava. The ovarian artery and vein do not closely accompany each other within the mesovarium.
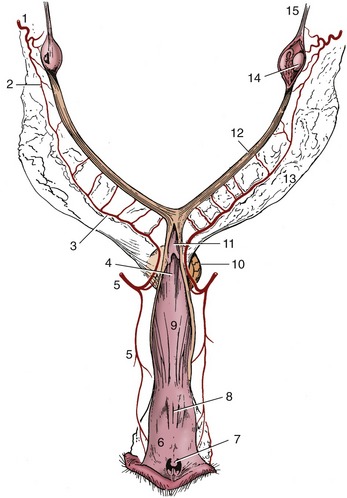
Figure 15–12 Blood supply of the reproductive organs of the bitch, dorsal view. The right ovarian bursa and the caudal parts of the tract have been opened. 1, Ovarian artery; 2, uterine branch of ovarian artery; 3, uterine artery; 4, dorsomedian fold continuing the cervix; 5, vaginal artery; 6, vestibule; 7, clitoris; 8, external urethral orifice; 9, vagina; 10, bladder; 11, cervix; 12, right uterine horn; 13, broad ligament; 14, right ovary; 15, suspensory ligament of ovary.
The lymphatic drainage of the ovary and uterus passes to the medial iliac and aortic lumbar nodes.
THE VAGINA, VESTIBULE, AND VULVA
(See also pp. 201–203.)
The vagina of the bitch is very long (ca. 12 cm) and extends horizontally through the pelvis before dipping beyond the ischial arch to join the vestibule (Figure 5–35/5,9). Apart from the prominent dorsomedian fold that continues the cervix for a short distance, the interior of the undistended organ is obstructed by the irregular folds into which the wall naturally falls. These end at the junction with the vestibule (Figure 15–11 and Figure 15–12). The vestibule continues the downward slope of the vagina, which must be kept in mind when introducing a vaginal speculum or other instrument (Figure 5–2). This must be passed in a craniodorsal direction to clear the ischial arch before it can be advanced horizontally. During such examinations the dorsal fold combines with the lateral and ventral vaginal walls to simulate a cervix (pseudocervix).
The cranial part of the vestibular floor (of the bitch) displays the tubercle and flanking depressions associated with the opening of the urethra, while the caudal part presents the fossa into which the glans of the clitoris projects (Figure 15–11/9,9′) The functional significance of the urethral tubercle is not known. Darker patches of the lateral walls betray the positions of the vestibular bulbs, which are well-developed in the bitch but slighter and more diffuse (even insignificant) in the female cat. Vestibular glands are present only in the cat.
The thick labia of the vulva meet in a rounded dorsal and a pointed ventral commissure. More lateral folds that are sometimes apparent are believed to be homologous with the labia majora of human anatomy. The crura and body of the clitoris possess a little erectile tissue; the glans is largely of fatty fibrous tissue but sometimes contains a small bone, the os clitoridis. The queen has only a corpus cavernosum clitoridis and not a glans clitoridis.
FUNCTIONAL CHANGES
It is often stated that bitches come in heat twice a year, in spring and autumn. In fact, three heats are not uncommon, although even when this is so, the greater part of the year is occupied by periods of anestrus. Cats are even less dependable in these matters, and even four cycles are possible in place of the usual two. The first heat occurs at the age of 6 to 9 months or thereabouts in bitches and at 6 to 12 months in young queens, depending on the season of their birth.
The reproductive organs, quiescent during anestrus, develop rapidly in proestrus when, over a period of a week, a batch of follicles enlarges. The uterus now increases in length and in thickness; its endometrium proliferates, and the entire reproductive tract becomes hyperemic. A thickened, edematous vulva discharges the serous uterine secretion, which is tinged with blood, the result of diapedesis from the widened endometrial vessels. Estrus also lasts about a week and can be distinguished from proestrus by the female’s readiness to accept a male. The endometrial hypertrophy and hyperemia continue, but the discharge gradually becomes less blood-stained. Ovulation, which occurs about the second day of estrus, is succeeded by very rapid formation of corpora lutea, which may be mature by the end of estrus.* The separation of diestrus and metestrus is difficult to determine because there is often a period (2 to 8 weeks) of pseudopregnancy during which the bitch exhibits the usual physical and behavioral signs of pregnancy, even though fertilization has not occurred; pseudopregnancy can perhaps be likened to a greatly extended period of diestrus. The cervix is tightly closed during diestrus and metestrus, and secretions that would have been utilized for embryo nutrition then accumulate in amounts that may distend the uterus; infection often supervenes, producing a condition (pyometra) that may necessitate hysterectomy.
The responses of the vaginal epithelium to changes in hormonal levels are more pronounced than in other domestic species, and smears taken from the vagina provide evidence of the stage within the cycle. Both cornified epithelial cells and erythrocytes are present in large numbers during proestrus, but while the former persist through estrus, the latter gradually become fewer as leukocytes become present. The stages of the cycle are also reflected in the gross appearance of the vaginal lining, including that covering the dorsomedian fold. In proestrus the lining becomes edematous and forms prominent soft folds. As estrogen levels drop rapidly during estrus, the vaginal wall becomes less oedematous and the lining wrinkles until about 4 days after ovulation, when the surface is said to resemble crepe paper. A few days later the mucosa becomes flat and patchy; with the desquamation of the cornified superficial layer of epithelium the blood vessels are able to shine through once more.
Ova enter the uterus about the sixth day after ovulation. If fertilized, they implant after a further 10 days, and this delay also allows appropriate spacing. An omphalovitelline (yolk sac) attachment is first established, but though effective in early pregnancy, it is later replaced by the definitive chorioallantoic placenta (Figure 15–13/6). This develops through the invasion of the endometrium by villi growing from a broad band of the chorion encircling the trunk of the fetus, as a continuation of the erosion that started in the nonvascular (chorioamniotic) regions and about the yolk sac attachment. The erosion leads to the interdigitation of thin plates of fetal tissue, and endometrial lamellae are reduced to little more than the maternal capillary endothelium (Figure 5–70, E-H). The tissue barrier of this basically chorioendothelial placenta is further reduced at the margins of the zonary band, where blood extravasated from maternal vessels directly bathes the fetal tissue. Hemoglobin breakdown in these marginal hematomas is responsible for the brilliant green pigmentation that contrasts with the deep red of the major part of the placenta (Figure 5–67, A). In short, this type of placenta consists of three zones: a transfer zone (around the embryo for nutrient transfer), a pigmented zone at either end of the transfer zone (maternal hematomas, probably important for iron transport from dam to fetus), and a relatively nonvascular zone, the allantochorion that is thought to be responsible for resorption from the uterine lumen. Only a certain proportion of the antibodies the pup receives from the dam penetrates the placenta; the greater share (about 75%) of the passive immunization of the newborn is dependent on the colostrum.

Figure 15–13 The feline fetal membranes in transverse and longitudinal section, schematic. 1, Amnion; 2, amniotic cavity; 3, yolk sac; 4, chorioallantois; 5, allantoic cavity; 6, zonary placenta.
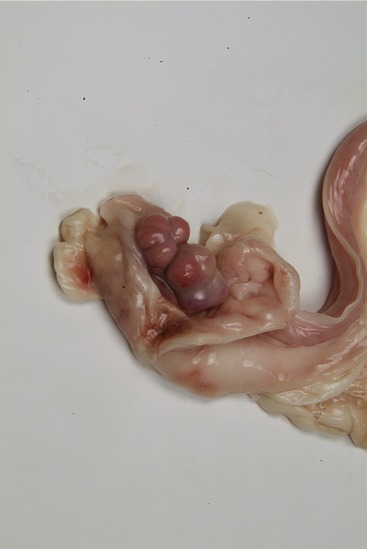
Initially the uterus enlarges locally, and each conceptus is confined within a globular swelling that is bounded by regions of constriction. The separate ampullae persist until about the 40th day (in a gestation that averages 63 days, measured from the date of ovulation*), when there begins to be a gradual relaxation of the constrictions, eventually creating an almost uniformly expanded uterus. The positions of the individual fetuses are still obvious on inspection of the exposed organ as the whole thickness of the uterine wall is very vascular at the placental sites. The uterine horns are relatively fixed at their extremities, and when they lengthen, they are forced into loops that first bend cranially from the ovarian attachment before sweeping ventrally, then caudally, to join the body (Figure 15–15). The pattern of coiling is even more complicated when the litter is large, and radiographs obtained in late pregnancy (when there is mineralization of the fetal skeletons) sometimes show the puppies arranged in a confusing jumble (Figure 15–14, B).
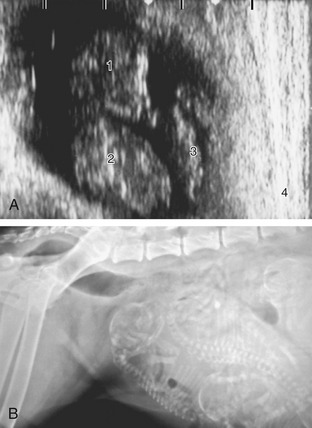
Figure 15–14 A, Ultrasonographic (transabdominal) view of a 33-day (after a single mating) Beagle fetus in its ampulla; the scale on top is in centimeters. 1, Head of fetus; 2, thorax of fetus; 3, yolk sac; 4, uterine wall. B, Pregnant bitch with several almost full-term fetuses. Note the gas in the rectum.
Pregnancy diagnosis by abdominal palpation is possible from 18 to 21 days of gestation onward, first by the presence of round swellings of approximately 1 cm in diameter and later, between 24 and 32 days, by palpation of swellings of about 2.5 to 4 cm in diameter. From 35 to 45 days of gestation, the swellings enlarge, elongate, become flaccid, and are found ventrally in the abdomen. For a few days, starting from about the 50th day, it is no longer possible to palpate individual swellings, but from the 55th day of gestation individual fetuses are easily palpable.
In the later stages of pregnancy abdominal radiographs not only serve to determine the number of pups in the litter but also provide a means of assessing fetal age, thus predicting the date of parturition. Mineralization commences in the axial skeleton by about the 45th day and is soon followed by the progressive mineralization of the appendicular skeleton in proximodistal sequence (Figure 5–74; Table 15–1). Mineralization of the skeleton of kittens follows the same pattern, but each element makes its appearance a few days earlier than in pups.
Table 15–1 Guide to the Mineralization of Dog Fetuses
| Days | Skeletal Elements |
|---|---|
| 45 | Skull, vertebrae, and ribs |
| 48 | Proximal long bones of limbs |
| 52 | Distal long bones of limbs |
| 54 | Pelvis |
| 60 | Minor bones of limbs |
Modified from Concannon P, Rendano V: Radiographic diagnosis of canine pregnancy: onset of fetal skeletal radiopacity in relation to times of breeding, preovulatory leuteinizing hormone release, and parturition, Am J Vet Res 44:1506–1512, 1983; and Yaeger AE, Mohammed HO, Meyers-Wallen V et al: Ultrasonographic appearance of the uterus, placenta, fetus and fetal membranes throughout accurately timed pregnancy in beagles, Am J Vet Res 53:324–329, 1992.
For some time now, ultrasonography has provided an alternative or additional means of diagnosing pregnancy and predicting term. Its advantages and disadvantages for these purposes, when compared with radiography, are dependent to a large extent on the stage of pregnancy when the examination is made. It has been claimed to be successful in recognizing uterine enlargement at a very early stage, but confident diagnosis requires a longer wait (perhaps 28 days). Even then, exact litter size cannot be determined. In cats, a gestational sac is visible about days 11 to 14, and fetal cardiac activity is present at day 14.
Parturition is facilitated by pelvic rotation at the sacroiliac joints and by elevation of the tail, which are both maneuvers that significantly increase the dimensions of the pelvis. In both dogs and cats some 60% to 80% of fetuses present the head toward the cervix at term, which is a bias that has yet to receive a satisfactory explanation of how it is achieved. Fetuses tend to be delivered from each horn in alternation, and when each is delivered, the emptied segment of the uterus contracts and brings those littermates left behind closer to the exit. When expelled, each fetus is still attached to its placenta, from which it is freed by the dam’s biting through the umbilical cord. The “afterbirth,” with which considerable maternal tissue is shed, is normally consumed.
Although less often useful to the clinician, some information on the development of certain external features of fetuses will be found in Tables 15–2 and 15–3.
Table 15–2 Guide to the Aging of Dog Fetuses
| Weeks | Crown–Rump Length (cm) | External Features |
|---|---|---|
| 3 | ≈1 | Embryo C-shaped; limb buds forming |
| 4 | ≈2 | Hand plate present; shallow grooves between digits |
| 5 | ≈3 | Eyelids partly cover eye; pinna covers acoustic meatus; external genitalia differentiated; digits separated distally |
| 6 | ≈7 | Eyelids fused; hair follicles present on body; digits widely spread; claws formed |
| 7 | ≈11 | Hair almost completely covering body; color markings present; full term: on average 63–64 days |
From Evans HE, Sack WO: Prenatal development of domestic and laboratory animals. Growth curves, external features and selected references, Anat Histol Embryol 2:11–45, 1973.
Table 15–3 Guide to the Aging of Cat Fetuses
| Weeks | Crown–Rump Length (cm) | External Features |
|---|---|---|
| 3 | ≈1 | Acoustic meatus forming; eye well formed and pigmented; forelimb hand plate notched |
| 4 | ≈3 | All digits widely spread; pinna almost covers acoustic meatus; claws forming; eyelids partly cover eyes |
| 5 | ≈5 | Eyelids fused; tactile hairs present on face |
| 6 | ≈7 | Fine hairs appearing on body; claws begin to harden |
| 7 | ≈10.5 | Fine hairs cover body; claws white and hard; color markings present; full term: on average 65 days (counted from first mating) |
From Evans HE, Sack WO: Prenatal development of domestic and laboratory animals. Growth curves, external features and selected references. Anat Histol Embryol 2:11–45, 1973.
The cat is sexually mature at 6 to 9 months of age. The proestrus stage, the nonacceptance of a male, lasts 12 to 48 hours. In cats, pea-sized swellings can be palpated at 21 days of gestation. By 28 days, the swellings are firm and are about 2.0 to 2.5 cm in diameter. The uterus is evenly distended during days 35 and 50 and may be difficult to differentiate from pyometra.
Potentially embarrassing mistakes in the determination of the sex of newborn kittens are relatively easily made. The difficulty arises from the orientation of the penis. This brings the anal and genital openings relatively close together in the tom, and the spacing is inconveniently similar to that in the female (Figure 15–16).
THE MALE REPRODUCTIVE ORGANS
THE SCROTUM AND TESTES
(See also pp. 184–191.)
The rather pendulous scrotum of the dog is globular and placed in a position intermediate between the perineum and the groin (Figure 15–17/11). It is most easily inspected from behind, and because it is sparsely haired, its close molding on the testes is obvious. A deep groove defines the boundary between the internal compartments occupied by the generally asymmetrical testes. The thin scrotal skin and underlying fasciae do not impede palpation, which normally allows recognition of the body and tail of the epididymis, the deferent duct, and the spermatic cord, in addition to the testis itself. The scrotal skin of dogs is richly supplied with sweat glands. The scrotum of the cat is perineal, sessile, and commonly concealed by a dense covering of hair.
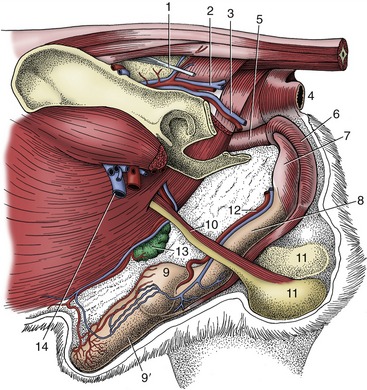
Figure 15–17 Deep dissection of the external reproductive organs of the dog. 1, Sacrotuberous ligament; 2, caudal gluteal vessels; 3, internal pudendal vessels; 4, anus; 5, pelvic urethra; 6, bulb of penis enclosed by bulbospongiosus; 7, ischiocavernosus over left crus; 8, body of penis; 9, 9′, bulbus and pars longa glandis; 10, spermatic cord; 11, testes in scrotum; 12, dorsal artery and vein of the penis; 13, superficial inguinal lymph nodes and caudal superficial epigastric vessels; 14, femoral vessels.
The testes are relatively small in both species. They are carried horizontally in dogs but with their caudal extremities tipped toward the anus in cats. Each testis is roughly oval in outline, laterally compressed, and related to the epididymis along its dorsal (in cats, craniodorsal) margin. The head and tail of the epididymis adhere to the testis, but the body is partly free, which creates a testicular bursa. The constituents of the compact spermatic cord disperse at the internal inguinal ring. Because of the very caudal position of the scrotum, the spermatic cord in the tom is unusually long. Perhaps it is because of this that the cremaster muscle of the cat is very weak. The striated cremaster muscle originates from the iliac fascia on the ventral aspect of the psoas muscles just craniomedial to the caudal border of the internal oblique muscle, inserts on the internal spermatic fascia, and is innervated by the genitofemoral nerve.
THE URETHRA AND ACCESSORY REPRODUCTIVE GLANDS
(See also pp. 192–193.)
The very short first part of the male dog’s urethra is completely surrounded by the prostate (Figure 5–1/9). It presents a lumen indented by a dorsal ridge, locally raised to form a seminal colliculus that is perforated to each side by the narrow opening of the deferent duct and the numerous pores that drain the prostate. The remaining part of the pelvic urethra is provided with a thin sleeve of spongy tissue within the striated urethralis muscle. The urethral lumen widens caudal to the prostate but narrows again as it leaves the pelvis at the ischial arch. In the tom, the prostate is located 3 to 4 cm caudal to the bladder neck, and the preprostatic part of the urethra has sometimes been described as an elongated bladder neck. The striking radiographic appearance of the feline urethra is shown in Figure 15–7/1,2,3.
The ampullary glands and prostate provide the entire complement of accessory sex glands in the dog. In dogs, sometimes remnants of the paramesonephric duct (vagina masculine) are present in the genital fold, covered dorsally by the prostate.
The cat, which lacks ampullary glands, has small bulbourethral glands located on the urethra, level with the ischial arch. These glands are important landmarks in perineal urethrostomy (removal of the penis in chronic urethral obstruction). The pudendal nerve courses over the ventral part of the bulbourethral glands.
In both species the prostate contributes the bulk of the seminal fluid. In the dog, it comprises a large compact mass about the urethra and neck of the bladder and a small disseminate part spread within the urethral mucosa. The compact part varies greatly in size, and this obviously affects its position and relations. It may be within the pelvic cavity when small, but more usually, and especially in mature and older dogs, it is mainly if not entirely intraabdominal (Figure 15–18/2). A dorsal groove and internal septum divide it into right and left lobes, which are subdivided into lobules by finer septa that radiate outward to the capsule. The right and ventral lobes do not join ventral to the urethra in cats.

Figure 15–18 Lateral radiographic view of the canine caudal abdomen to show the position of the prostate. 1, 1′, Descending colon containing gas and feces; 2, prostate; 3, bladder; 4, abdominal floor.
The prostate is extremely sensitive to hormonal influences, and it is difficult to suggest normal dimensions because hyperplasia of the parenchymatous part commonly develops in early middle age and fibrosis and shrinkage are common senile changes. The hyperplasia sometimes affects the different lobes unequally. An enlarged prostate may press on the large intestine, producing constipation and difficulties in defecation; however, in contrast to the human experience, interference with micturition is unusual unless the condition is very gross. The state of the prostate—its size, firmness, and regularity of form—may be assessed by digital examination per rectum, a procedure facilitated by pushing the bladder toward the pelvis by pressure through the abdominal wall. The proportions of parenchyma and supporting tissue may be estimated from gross sections of autopsy specimens: connective tissue normally predominates in the prostate of the very young, glandular tissue predominates in those from animals in their prime, and the relationship is inconstant in the glands of aged dogs. It has been reported that the prostate is proportionately much larger (by a factor of four) in the Scottish terrier than in other breeds.
Enlargement of the prostate is sometimes treated by castration. Alternatively, or if castration fails, surgical removal may be performed. It is then relevant to note that generally only the craniodorsal aspect of the gland has a peritoneal covering. The trunk of the prostatic artery continues over the lateral aspect of the gland as the supply to the bladder after detaching prostaticovesical and prostaticourethral branches. The other structure at risk is the plexus formed by the pelvic and hypogastric autonomic nerves.
Beyond the prostate, the urethra widens before narrowing on leaving the pelvis and becoming incorporated in the penis. It is narrowest just before opening to the exterior at the tip of the glans, where urinary calculi, a frequent affliction of male cats, are often held up. Little is known of age changes to the prostate of this species, in which enlargement is a much less frequently encountered problem (see Figure 15–22/8).
THE PENIS AND PREPUCE
(See also pp. 193–195.)
The penis of carnivores presents several unusual features, and additional differences between the organs of the dog and cat make separate description necessary.
The penis of the dog is slung between the thighs, where it may be palpated along its whole length. The root is formed of two slender crura that arch forward from their ischial attachments to combine in a common body that is little stouter than either contributor (Figure 15–19/4′). The urethra is incorporated at the same level and runs forward on the ventral surface of the body (Figure 15–19/3). At the level of the ischial arch the corpus spongiosum (that surrounds the urethra) expands to form the paired bulbus penis (covered by the bulbospongiosus muscle, a continuation of the urethralis muscle); further distally, the corpus spongiosum expands to form the glans penis, which is unusually extensive and clearly divided, both externally and internally, into a proximal expanded part (bulbus glandis; Figure 15–19/7) and a distal cylindrical part (pars longa glandis; Figure 15–19/7′), which provides the apex. About half the bulbus and the whole pars longa project into the preputial cavity, where they may be palpated. The cavernous parts of both crura combine within the proximal part of the body to form a single corpus cavernosum (Figure 15–19/4) with a tough outer fibrous covering and a substantial median septum; these are connected by radial trabeculae that divide and enclose relatively meager cavernous spaces. The corpus cavernosum comes to a premature end because its distal part is converted into a bone, the os penis, within the core of the organ (Figure 15–19/5). This bone is grooved ventrally for the reception and protection of the urethra within its spongy covering; the bone tapers toward its distal extremity, which is prolonged by a short, ventrally deflected rod of fibrocartilage that reaches almost to the very apex of the penis. The fibrocartilage remains unossified even in aged animals. The partial enclosure of the urethra within the groove of the os penis impedes the passage of urethral calculi, which therefore tend to lodge at the caudal end of the bone.
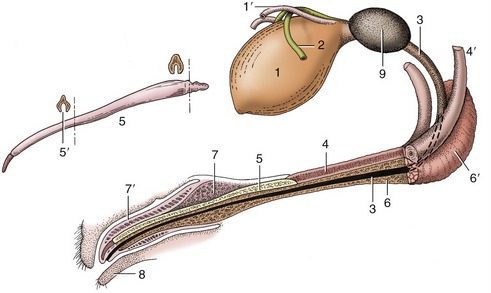
Figure 15–19 Canine bladder, urethra, and penis (in section). 1, Bladder; 1′, left ureter; 2, left deferent duct; 3, urethra; 4, corpus cavernosum; 4′, left crus; 5, os penis; 5′, urethral groove; 6, corpus spongiosum; 6′, bulb of penis; 7, bulbus glandis; 7′, pars longa glandis; 8, prepuce; 9, prostate.
The caudal (or proximal) part of the glans penis, the bulbus glandis, is considerably expanded, even in the quiescent state. It is firmly anchored to the bone and considerably overlapped by the elongated distal division, which presents the urethral orifice toward its tip. The pars longa is more loosely attached to the bone. Both contain large blood spaces enclosed by relatively weak trabeculae.
The structure and connections of the various erectile bodies and their relationships to the supplying and draining vessels require close attention if the mechanism of erection is to be understood (Figure 15–20, A-B and Figure 15–21, A-F). The penis is supplied by the continuation (beyond the origin of its perineal branch) of the internal pudendal artery, which now becomes the artery of the penis (Figure 15–20/1′). The artery of the penis divides into three. One division, the artery of the bulb (Figure 15–20/2), supplies the bulb (of the penis) and then runs distally within the organ to supply the corpus spongiosum about the urethra and later, on approaching the apex of the penis, the elongated portion of the glans. The second, the deep artery of the penis (Figure 15–20/3), supplies several branches to both the tissues and the blood spaces of the corpus cavernosum. The third, the dorsal artery of the penis (Figure 15–20/4), may be regarded as the direct continuation of the main trunk. It first runs on the dorsal aspect of the penis before sinking to the side and dividing close to the caudal limit of the bulbus. A superficial branch runs almost to the tip of the organ below the skin over the ventral aspect of the glans; a deep branch penetrates the bulbus to run apically on the os penis to enter the pars longa; and a preputial branch forks into a division that runs over the dorsal aspect of the bulbus to supply the dorsal aspect of the pars longa and the prepuce.
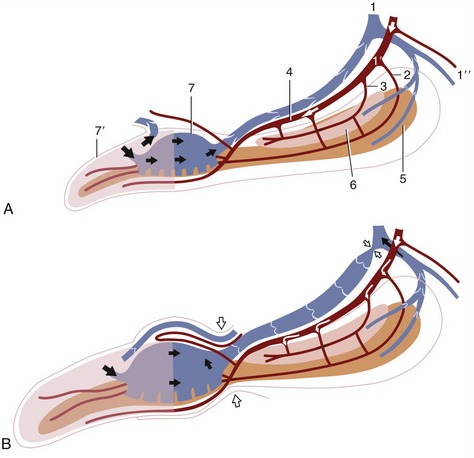
Figure 15–20 Schematic representation of the blood supply and the blood spaces of the quiescent (A) and erect (B) canine penis. 1, Internal pudendal vessels; 1′, artery of the penis; 1″, perineal branches; 2, artery of the bulb; 3, deep artery of the penis; 4, dorsal artery of the penis; 5, corpus spongiosum; 6, corpus cavernosum; 7, bulbus glandis; 7′, pars longa glandis.
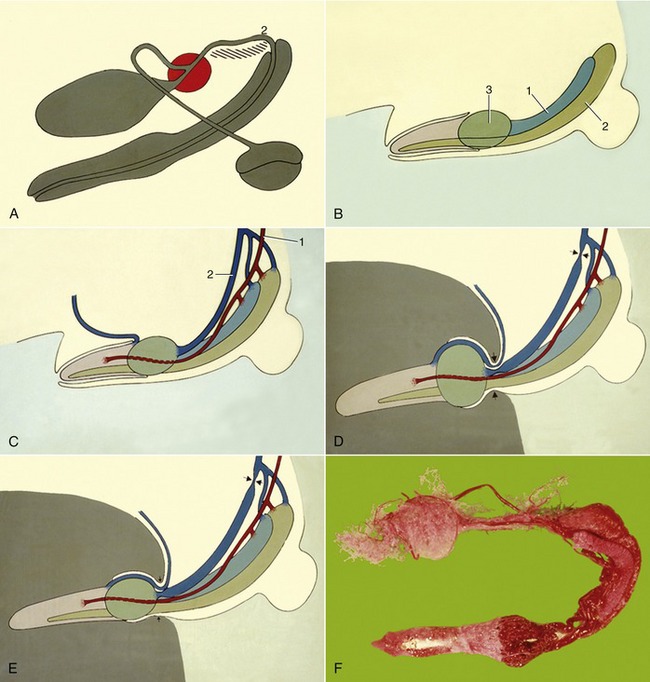
Figure 15–21 A, Schematic representation of canine male reproductive organs. B, Major vascular parts of canine penis. 1, Corpus cavernosum; 2, corpus spongiosum; 3, bulbus glandis. C, D, and E, Stages in the erection process. 1, Penile a.; 2, dorsal penile v. F, Corrosion cast of the arterial supply to prostate and penis.
The veins are broadly satellite to the arteries. The dorsal vein leaves the lateral aspect of the bulbus and runs caudally, gradually shifting toward the dorsal aspect of the penis, where it is joined by a common trunk formed of the veins corresponding to the deep artery and that of the bulb. The augmented dorsal vein then bends around the ischial arch to enter the pelvis, where it provides the main radicle of the internal pudendal vein. Other veins assist in the drainage of the glans. A superficial vein leaves the pars longa to wind around the fornix of the prepuce before joining the external pudendal vein. A deep vein within the glans drains blood from the pars longa to the bulbus; it is valved so that reflux of blood is impossible and is so arranged that it may either provide a through passage to the dorsal vein or open into the blood spaces of the bulbus, from where the blood then enters the dorsal vein.
The usual muscles are present. The retractor, largely composed of smooth muscle, loops to the side of the anal canal before converging on its fellow to form a band that runs along the urethral aspect of the penis to a termination by the preputial fornix. A few small fascicles are detached to the scrotum. Short but powerful ischiocavernosus muscles cover the crura. The bulbospongiosus forms a transverse covering over the urethra from the bulb to its incorporation in the penis. A small ischiourethralis passes from the ischial tuber to a fibrous ring that encloses the dorsal veins at their entry to the pelvis. The two large muscles at the root of the penis can be identified on palpation (Figure 15–17/6,7).
The prepuce of the dog is rather pendulous toward its cranial extremity, where it is suspended below the abdomen by a fold of skin. It has a simple arrangement, and the parietal part of its lining is studded with lymph nodules, which give it a rather irregular appearance. There are also small scattered preputial glands. Paired preputial muscles, detachments from the cutaneous muscle of the trunk, run over the abdominal floor to meet and partially decussate in the skin of the prepuce caudal to the T-shaped orifice. Congenital or acquired narrowing of the preputial orifice may prevent protrusion of the penis (phimosis). Those acquired cases that are due to scar formation after an earlier inflammation may be treated surgically. The wisdom of surgical intervention may be questioned when the defect is congenital and possibly hereditary. Paraphimosis, in which the erect penis is unable to subside and cannot be withdrawn into the prepuce, requires more urgent attention because the interruption of the circulation may cause tissue death within hours.
At birth, the epithelial surface of the prepuce and penis adhere through a frenulum. Separation of the prepuce from the penis is under androgenic influence and usually occurs at puberty.
The penis of the cat is unique (among domestic species) in retaining the embryonic position: the apex is directed caudoventrally, and the urethral surface is uppermost (Figure 15–22/6 and Figure 15–23). It is relatively much shorter than the penis of the dog but has a similar construction, including the transformation of the distal part of the corpus cavernosum into bone. Kittens lack the os penis until 3 months of age. The existence of an apical ligament extending between the os penis and the proximal part of the corpus cavernosum appears to be responsible for the ventral deflection of the penis that occurs with erection. The dorsal artery only supplies the prepuce and not the penis. The glans is small, and its free surface is generously ornamented with small, keratinized spines in the tom; these develop during the first few months of postnatal life and regress to a very insignificant state in castrated animals (Figure 15–23 and Figure 15–24). Approximately 120 in number, they lie flat against the surface of the glans in the nonerect state but rise, as a result of the congestion of the blood spaces at their bases, on erection. The stimulus they provide to the queen is believed to be important in inducing ovulation.

Figure 15–22 The reproductive organs of the tomcat in situ, left lateral view. 1, Shaft of ilium; 2, sciatic nerve; 3, pudendal nerve; 4, anus; 5, left testis in scrotum; 5′, spermatic cord; 6, penis; 6′, prepuce; 7, bulbourethral gland; 8, prostate; 9, deferent duct; 9′, testicular vessels; 10, bladder; 11, left ureter.
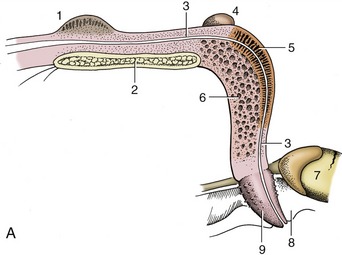
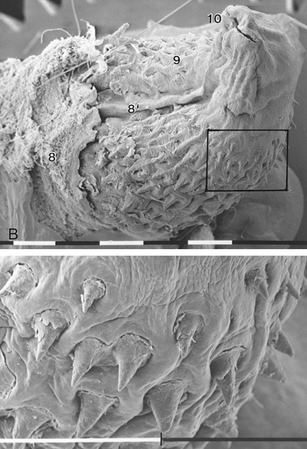
Figure 15–23 A, Median section of the feline penis, left lateral view. B, Scanning electron micrograph of a feline glans and enlargement of the marked area (bar = 1 mm). 1, Prostate; 2, pelvic symphysis; 3, urethra; 4, right bulbourethral gland; 5, corpus spongiosum; 6, corpus cavernosum; 7, right testis; 8, prepuce; 8′, preputial frenulum; 9, glans (with spines); 10, external urethral orifice.
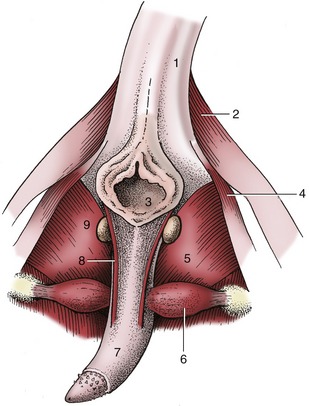
Figure 15–24 Feline penis in situ, caudal view. 1, Tail (raised); 2, gluteofemoralis; 3, anus; 4, coccygeus; 5, internal obturator; 6, ischiocavernosus; 7, penis; 8, left retractor penis; 9, left bulbourethral gland.
The cat’s prepuce is thick but short and often much obscured by hair; its orifice faces caudally, and urine is ejected in this direction. The spraying of urine by the tom is a social gesture marking territory (Figure 15–25). The sites are not always discretely chosen and are often inconvenient to the owner, which is one reason for the common practice of castration.*
AGE AND FUNCTIONAL CHANGES
Although there has been little detailed study of the postnatal development, it is known that the testes most often remain within the abdomen until about the third day after birth. Their descent through the inguinal canal then commences, and although it is completed within a couple of days, another 4 or 5 weeks are required before the testes occupy their definitive positions within the scrotum. The seminiferous tissue increases markedly in volume during this time, but spermatogenesis does not begin until about the 6th month. Because the testes attain their definitive locations so precociously, some have advocated castration of male kittens at much younger ages—6 to 14 weeks—rather than the 5 or 6 months conventionally adopted. It is claimed that the operation is well tolerated by these very young animals.* If descent fails—the cryptorchid condition—the testis may be located anywhere between the caudal pole of the kidney and the inguinal canal. It is most easily located by following the deferent duct, readily picked up at the lateral ligament of the bladder. Although the germinal epithelium fails to develop normally at the core temperature of the body, Leydig cells produce androgens and the full range of secondary sex characteristics may develop in bilaterally cryptorchid animals.
The mating behavior of dogs is most unusual. The dog mounts the bitch in the usual way, but shortly after intromission he drops to her side and reverses so that the pair stand rear to rear during the remainder of the “tie,” which may last for a further 45 minutes or even longer. There has been surprisingly little consideration of the anatomy of this process.
Although all erectile tissues of the penis become engorged when erection is complete, they attain very different degrees of expansion and turgidity (Figure 15–20). The corpus cavernosum swells least, and its construction allows it to remain flexible about a vertical axis, though not about a horizontal one, even in this state. The bulbus glandis is most capable of expansion and swells to twice its resting thickness, becoming very tense in the process. The pars longa stiffens least but elongates considerably, which causes it to slide apically on the os penis to which it is only loosely attached; it then extends well beyond the fibrocartilaginous extension of the bone and presents an indentation about the urethral orifice in consequence of the tighter anchorage of this part.
Intromission necessarily occurs before the penis is markedly enlarged (Figure 15–21, D-E). The labia thrust the prepuce caudally when the dog mounts and introduces the glans into the vagina. The slope of the female passage requires a dorsocranial penetration and the relatively soft tip of the glans is diverted ventrally by its impingement (through the soft tissues) on the pelvic roof. This deflection allows the penis to be advanced toward the fornix and perhaps explains the necessity for the softer nature of the pars longa and the early termination of its bony support. When the stud dog dismounts and turns through 180°, the body of the penis is bent laterally and then caudally; withdrawal of the penis is prevented by the swollen bulbus glandis and the grip exerted on it by the engorged vestibular bulbs and muscles associated with the female tract. The reversal of position twists the prepuce, tightening the preputial muscles into a cord that presses on the veins draining the glans. The dorsal veins of the penis, buckled by the flexion of the penis, are further obstructed by being pressed against the ischial arch by the contraction of the ischiourethralis. Detumescence is probably eventually achieved by relaxation of the bulbospongiosus, which allows the spaces within the corpus spongiosum to provide alternative channels for the escape of blood from the engorged penis.
The initial, sperm-rich fraction of the ejaculate is discharged during the first stage of coitus when the dog is mounted in the fashion conventional for quadrupeds. The second stage is occupied in pumping out the much larger fraction—perhaps 30 mL—provided by the prostate; the tide sweeps the sperm-rich part through the cervix into the body of the uterus. It is known that short matings—in which only first-stage coitus occurs—may be fertile. The purpose of the second-stage coitus may encourage uterine rather than vaginal insemination. Turning around discourages detumescence of the penis and therefore maintains high intravaginal pressure.
The penis of the cat increases considerably in length on erection and then curves downward and forward. This change in orientation, allied to a ventral flexion of the pelvic region, enables coitus to be performed in a fashion not greatly different from that usual in quadrupeds (Figure 15–26).
THE ANATOMY OF ABDOMINAL AND RECTAL PALPATION
In the previous chapter the process of abdominal palpation was described, together with the examination of most abdominal organs. The remaining organs are now considered, together with the information that may be obtained from digital examination via the rectum.
Although the right kidney cannot be found in most dogs, the caudal pole of the left one is generally identifiable. Indeed, in some dogs, generally of the larger breeds, the left kidney “floats” as both kidneys normally do in cats.
The dog’s bladder can be found extending forward from the pubic brim; when grossly distended, it lies over a large part of the abdominal floor. Micturition may be induced by gentle compression through the abdominal wall, which is a procedure not free from risk if performed incautiously. The bladder of the cat is located more cranially than that of the dog, well forward of the pubic brim. The prostate, notoriously variable in size and position (p. 468), may sometimes be palpated between the pubic brim and the bladder (Figure 15–27).
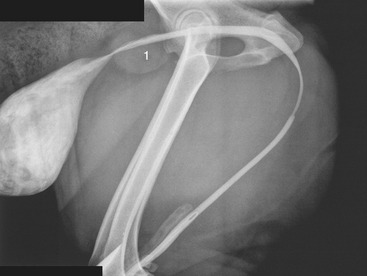
Figure 15–27 Contrast medium in the male canine bladder and urethra. The prostatic urethra appears to be less distensible. 1, Prostate.
The empty uterus cannot normally be palpated. The gravid uterus is readily identified at certain stages of pregnancy by its beaded form or general enlargement or by the recognition of individual fetuses. The separate loculi (p. 465) within which the embryos initially develop are largest about the beginning of the 6th week (bitch), but this stage is soon followed by that in which the horns are uniformly swollen. A little later, individual fetuses may be palpated, although it is not always possible to make an exact count when the litter is large. The gravid uterus may affect the position of other abdominal organs markedly. It always occupies the most ventral position in the abdomen because it contains no gas; therefore it is heavier than most freely movable abdominal organs. In advanced pregnancy it may almost fill the ventral half of the abdominal cavity.
Digital examination per rectum, a procedure possible only in subjects of a certain size, may provide additional information. In addition to revealing the tone of the anal sphincter and the condition of the rectum and its mucosa, digital examination may be used to explore the pelvic skeleton for evidence of fracture or deformity. The anal sacs may be palpated and their content expressed with the aid of a finger within the rectum. The only other visceral organs that may usually be examined are the urethra and the prostate in the male and the vagina in the female. Evaluation of the prostate requires consideration of its size, consistency, and symmetry. In large dogs the gland may be out of reach, but the prostate and the neck of the bladder may be made more accessible by coordinating the rectal examination with manipulation of the abdomen to press the caudal abdominal contents toward the pelvic entrance.
Palpation of the abdominal wall of the laterally recumbent animal will reveal the position of the superficial inguinal ring, from which the spermatic cord may then be traced toward the scrotum in the male. The location of the ring is determined by recognition of its tense medial crus, which may be traced over the abdominal wall from the origin of the pectineus muscle (which forms the conspicuous swelling on the medial surface of the thigh). The superficial inguinal lymph nodes lie a little cranial to the ring. They are contained within the fold of skin that supports the prepuce in the male but are more difficult to find in the bitch, especially the parous bitch, because they lie deep to the inguinal mammary gland. In perineal hernia the pelvic diaphragm fails to support the rectal wall, which stretches and deviates. In this condition it is possible to deviate the inserted finger to the side of the hernia.
MAIN VESSELS IN THE PELVIS
The internal iliac artery supplies blood to the pelvic wall and the pelvic organs. The sacral median artery courses over the ventral surface of the sacrum and continues as the median caudal artery in the tail. The internal iliac artery divides into the caudal gluteal artery (wide) and internal pudendal artery (smaller), after detaching the umbilical artery. In the mature dog and cat this vessel gives off branches to the bladder, after which it becomes the ligament in the cranial edge of the lateral bladder ligament.
The internal pudendal artery courses at the inside of the pelvic wall and branches off the prostatic artery or the vaginal artery, which continues cranially as the uterine artery. This vessel also detaches branches to the rectum, bladder, and urethra. Near the anus the internal pudendal artery detaches the ventral perineal artery before continuing as the artery of the penis or clitoris.