CHAPTER 29 Diagnostic Tests for the Alimentary Tract
PHYSICAL EXAMINATION
Routine physical examination is the first step in evaluating animals with alimentary tract disease, although oral examination is sometimes skipped in uncooperative animals. If oral, abdominal, or rectal disease is a major concern and the patient refuses to allow examination of the area, it is reasonable and appropriate to sedate or anesthetize the patient to examine and palpate this area. A common example of this is a vomiting cat with a possible linear foreign body lodged under the tongue; the clinician must thoroughly examine the mouth, even if it requires chemical restraint.
The clinician should methodically identify individual organs during abdominal palpation. In dogs the small intestine, large intestine, and urinary bladder can usually be found (unless there is an abdominal effusion, abdominal pain, or obesity). In cats both kidneys are usually palpable. In both species the clinician can usually detect substantial splenomegaly, hepatomegaly, intestinal or mesenteric masses, and intestinal foreign objects. Abdominal pain may be subtle; some animals will cry out during gentle palpation, whereas many just tense their abdomen (i.e., guarding) or try to move away. A rough palpation technique can cause a normal animal to tense up or vocalize during palpation, mimicking the reaction of an animal with abdominal pain. Light, careful palpation permits the definition of as much of the internal abdominal contents as possible. If sufficient abdominal fluid is present to prevent meaningful abdominal palpation, ballottement of the abdomen should produce a fluid wave.
During a rectal examination, the examiner should be able to identify and evaluate the colonic mucosa, anal sphincter, anal sacs, pelvic canal bones, muscular support for the rectum, urogenital tract, and luminal contents. However, it is particularly easy to misinterpret mucosal polyps as mucosal folds and to miss partial strictures that are large enough to allow a single digit to pass through easily.
ROUTINE LABORATORY EVALUATION
COMPLETE BLOOD COUNT
Complete blood counts (CBCs) are especially important in animals at risk for neutropenia (e.g., parvoviral enteritis, severe sepsis), infection (e.g., aspiration pneumonia), or anemia (e.g., pale mucous membranes, melena, hematemesis) and also in those that have fever, weight loss, or anorexia resulting from an occult cause. The clinician should always evaluate absolute numbers of the different types of white blood cells (WBCs), not the percentages, because an animal may have an abnormal percentage of a particular WBC and yet have a normal absolute number of cells (and vice versa). If the animal is anemic, the clinician should evaluate the CBC for evidence of iron deficiency (e.g., hypochromasia, microcytosis, thrombocytosis, increased red blood cell distribution width).
COAGULATION
A platelet count is important. Platelet numbers can be estimated on the basis of correctly made blood smears (i.e., a dog should have an average of 8 to 30 platelets per oil immersion field; finding 1 platelet per field suggests a platelet count of approximately 15,000 to 20,000/μl). Coagulation panels may detect unsuspected coagulopathies (e.g., disseminated intravascular coagulation). Activated clotting times are crude estimates of the intrinsic clotting pathway; partial thromboplastin times are more sensitive. Mucosal bleeding time is an excellent screening test for coagulopathies severe enough to cause clinical bleeding.
SERUM BIOCHEMISTRY PROFILE
Serum biochemistry profiles that include alanine transaminase and alkaline phosphatase activities, as well as the blood urea nitrogen, creatinine, total protein, albumin, sodium, potassium, chloride, total CO2, cholesterol, calcium, phosphorus, magnesium, bilirubin, and glucose concentrations, are important in animals with severe vomiting, diarrhea, ascites, unexplained weight loss, or anorexia. These values are crucial to correctly diagnosing the animal’s problem and appropriately treating it. The clinician cannot predict the changes that will occur or the magnitude of the changes in a particular animal, even when the cause of the disease is known. The total carbon dioxide concentration is not as definitive as blood gas analysis but helps define the acid-base status, which also cannot be accurately predicted.
The albumin concentration is more useful than the total protein concentration. Hyperglobulinemia, which has many causes (e.g., heartworms, chronic dermatitis, ehrlichiosis) in a hypoalbuminemic dog can cause the serum total protein concentration to be normal. Severe hypoalbuminemia (i.e., less than 2.0 g/dl) is important diagnostically; it is more commonly found in animals with infiltrative alimentary tract disease, parvoviral diarrhea, intestinal lymphangiectasia, gastrointestinal blood loss, or ascites. It is important to have the serum albumin measured by technology designed for canine and feline albumin; some techniques used for measuring human albumin result in falsely low measurements of canine albumin.
Ill animals (especially those receiving multiple drugs) are at risk for secondary renal or hepatic failure. Very young and very small animals easily become hypoglycemic if they cannot eat or absorb ingested nutrients. Finally, finding hypercalcemia or hypoalbuminemia may provide a clue to the underlying problem (i.e., make some disorders more likely) in animals with weight loss or anorexia.
FECAL PARASITIC EVALUATION
Fecal flotation is indicated in almost every animal with alimentary tract disease or weight loss, especially in puppies and kittens. Even if it is not the primary problem, parasitism may cause additional debilitation. Concentrated salt or sugar solutions are typically used for fecal flotation. The former are usually superior, although incorrectly made solutions may not force heavier ova (e.g., whipworms) to float. Moreover, concentrated salt solutions can distort Giardia cysts, making identification difficult. A zinc sulfate flotation solution is preferred for detecting nematode ova and Giardia cysts. Centrifugation is strongly recommended; it promotes separation of cysts from the fecal matter and results in a more sensitive fecal examination. Some parasites intermittently shed small numbers of ova or cysts, necessitating repeated fecal analyses for diagnosis. Whipworm and Giardia infections can be especially difficult to diagnose.
The ova of the most common tapeworm species are contained in segments and are not found by flotation techniques. Nanophyetus salmincola (the fluke that transmits salmon poisoning) is detected by many flotation solutions, although sedimentation examinations are required to detect most other fluke ova. Cryptosporidiosis can be detected by flotation techniques, but higher magnification (×1000) must be used. The clinician should send the feces to a laboratory that is familiar with this coccidium and is able to perform special procedures to detect it. ELISA methodology is more sensitive than fecal examination for finding cryptosporidia.
Direct fecal examination, although convenient, is not sensitive for nematodes and should not replace flotation techniques. However, occasionally amebiasis, strongyloidiasis, and whipworm infections missed by flotation procedures can be detected in this way. Motile Giardia and Tritrichomonas trophozoites may be found if the feces are very fresh and the smear is adequately diluted with saline solution. Direct examination seems about half as sensitive as zinc sulfate flotation techniques in detecting giardiasis.
Fecal sedimentation is time-consuming and offers no advantage in detecting common gastrointestinal tract parasites. However, it does detect fluke ova missed by other techniques, especially the ova of Eurytrema spp., Platynosomum spp., and Amphimerus spp. plus Heterobilharzia.
Feces may be preserved by mixing equal volumes of feces and 10% neutral buffered formalin or by using commercially available kits. Polyvinyl alcohol is used in the latter, and feces preserved in this manner can be examined weeks to months later. These techniques are especially useful if one cannot immediately examine feces for protozoal cysts.
FECAL DIGESTION TESTS
Examining feces for undigested food particles by staining thin fecal smears with the Sudan stain (for fat) or iodine (for starch and muscle fibers) is of dubious value. Although the finding of excessive amounts of undigested fecal fat is suggestive of exocrine pancreatic insufficiency (EPI), this test has many false-positive and false-negative results. If EPI is a differential diagnosis, serum trypsin-like immunoreactivity (TLI) is a better way to confirm the diagnosis (see the section on digestion and absorption tests).
Fecal analysis for proteolytic activity (i.e., the fecal trypsin content) also tests for EPI. Qualitative estimates (e.g., fecal film digestion, fecal gelatin digestion) are unreliable. Quantitative analysis is seldom needed because the TLI test is easier and more pleasant to perform. It is rarely necessary to quantitate fecal proteolytic activity to diagnose EPI caused by pancreatic duct obstruction, something TLI does not detect. In this test feces are collected for 3 consecutive days and stored frozen until sent to the laboratory. However, this is an exceedingly rare situation.
Quantitated fecal fat analysis is seldom indicated. Although sensitive for detecting fat malabsorption and maldigestion, it is expensive and unpleasant to perform and does not differentiate malabsorption from EPI.
Fecal occult blood analyses are seldom useful because most pets eat meat by-products that cause a positive reaction. False-positive reactions may also be produced by cimetidine, oral iron preparations, and some vegetables. Furthermore, the sensitivity of different techniques varies, making it difficult to accurately compare results. Finally, blood is often not distributed homogeneously throughout the feces, and a negative result could stem from a sampling error (especially in animals with lower intestinal tract problems).
If analysis for fecal occult blood is desired, the optimal approach is to feed the animal a meat-free diet for 3 to 4 days before performing the test. Tests using the reagents benzidine or orthotoluidine to detect hemoglobin tend to be very sensitive (and hence less specific), whereas those using guaiac are less sensitive (and thus more specific). A sensitive and specific fluorometric method has been validated in dogs. Repeated testing may be necessary to demonstrate intermittent bleeding.
MISCELLANEOUS FECAL ANALYSES
Enzyme-linked immunosorbent assays (ELISAs) can be used to detect various antibodies or antigens. The test for canine parvovirus seems to be very specific. However, virus may not be found in the feces for the first 24 to 48 hours, and it may be necessary to repeat the test in 2 to 3 days if initial results are negative in a dog strongly suspected of having parvoviral infection. In addition, although dogs with parvoviral diarrhea initially shed large amounts of virus, fecal shedding decreases substantially during the ensuing 7 to 14 days. A repeatedly negative test result therefore does not rule out parvoviral infection, but it does necessitate a consideration of other acute, febrile gastroenteritides (e.g., salmonellosis). This test is particularly valuable if there are epidemiologic considerations (e.g., breeding kennel).
ELISAs for detecting a Giardia-specific antigen in human (ProSpecT/Microplate ELISA assay for Giardia, Alexon, Inc.) and canine/feline feces (SNAP Giardia Test, Idexx Laboratories) are available. The SNAP Giardia test appears to be sensitive and specific but has not been carefully compared with multiple zinc sulfate flotation examinations in clinical patients. It has the advantage of being able to be performed in the practice. An IFA test (MERIFLUOR Cryptosporidium/Giardia direct immunofluorescent kit, Meridian Bioscience, Inc.) appears to be specific but has the disadvantage of requiring that feces be sent to a commercial laboratory.
ELISAs for detecting cryptosporidial antigens in feces (ProSpecT Cryptosporidium Microplate Assay, Meridian Diagnostics, Inc. and ProSpecT Cryptosporidium microplate assay, Remel Inc.) appear to be more sensitive than routine fecal examinations. Special staining of fecal smears with a modified Ziehl-Neelsen acid-fast technique is also sensitive, albeit more labor intensive. An IFA test (MERIFLUOR Cryptosporidium/Giardia direct immunofluorescent kit, Meridian Bioscience, Inc.) was not as sensitive as the ELISAs when looking for cryptosporidia.
Electron microscopy can be used to identify various viral particles (e.g., coronavirus, astrovirus) in feces. Because the ELISA is usually adequate for detecting parvovirus, electron microscopy is rarely necessary. However, it is reasonable to choose this technique if other test results are not diagnostic and there are epidemiologic considerations. Fecal samples for electron microscopy analysis should be obtained early in the disease because fecal viral concentrations may de-crease dramatically within 7 to 14 days after the onset of signs. Furthermore, some delicate viruses (e.g., coronavirus) degenerate quickly, and the feces from animals suspected of having such an infection must be handled appropriately if meaningful results are to be obtained. It is important that clinicians contact their laboratory for instructions on sample handling.
Assays for bacterial toxins in feces sometimes help implicate specific bacteria as causing diarrhea. There are numerous ELISA tests available for detecting Clostridum difficile toxin in human feces; however, the sensitivity of these tests for canine feces appears to be poor. The tissue culture assay for Clostridum difficile toxin in feces is sensitive but only performed in research laboratories. ELISA tests (Clostridium perfringens Enterotoxin Test, TechLab) and reverse passive latex agglutination tests (Oxoid PET-RPLA, Unipath Co.) tests for Clostridium perfringens enterotoxin are available. However, the results of these tests do not clearly correlate with presence or absence of disease. Their value in clinical cases is still being defined.
There are both culture techniques (InPouch TF, BioMed Dianonstics) and polymerase chan reaction (PCR) tests for Tritrichomonas fetus in feline feces. The culture technique can be done in the practice and appears to be sensitive and specific; it is more sensitive than direct fecal examination.
BACTERIAL FECAL CULTURE
Fecal culture is seldom indicated in small animals unless a contagious disease is strongly suspected or other test findings (e.g., endoscopy and biopsy) are nondiagnostic. Specific culture techniques for the detection of each pathogen are recommended. Therefore the clinician should contact the laboratory before submitting feces, informing them specifically what bacteria to attempt to grow and following their instructions regarding the handling of specimens. It is important to remember that fecal culture cannot be used to diagnose small intestinal antibiotic-responsive enteropathy (ARE).
The pathogens most likely to be cultured from feces from small animals are C. perfringens, C. difficile, Salmonella spp., Campylobacter jejuni, Yersinia enterocolitica, and verotoxin-producing strains of Escherichia coli. Confirmation of toxin production of isolates can be performed using PCR techniques or bioassay. Aeromonas spp. and Plesiomonas spp. may also cause diarrhea. Salmonella spp. are best cultured by inoculating at least 1 g of fresh feces into an enrichment medium and subsequently a selective medium specific for Salmonella spp. It is sometimes possible to culture Salmonella from the colonic mucosa. A PCR technique has been used recently in the evaluation of equine feces and may be useful for the evaluation of canine and feline feces. To culture C. jejuni, very fresh feces must be inoculated onto selective media and incubated at approximately 40° C instead of 37° C. If inoculation is to be delayed, special transport media should be used, not routine commercial transport devices (e.g., culturette swabs). PCR testing is available for Campylobacter spp. in canine and feline feces (GI Lab, Texas A&M University). The clinical value is still being defined.
It is important to note that the mere presence of any of these bacteria in an animal’s feces does not confirm that they are causing disease. Culture results must be correlated with clinical signs and the results of other laboratory tests.
Candida spp. are occasionally cultured from feces. The finding is often of uncertain significance, but the organisms may cause problems in some animals (e.g., those receiving chemotherapy).
CYTOLOGIC EVALUATION OF FECES
Fecal cytologic evaluations may identify etiologic agents or inflammatory cells. In this method a thin, air-dried smear is stained with Gram’s or a Romanowsky-type stain (e.g., Diff-Quik). The latter identifies cells better than Gram’s stain does.
Finding excessive numbers of spore-forming bacteria (e.g., more than 3 to 4 per 1000× field) was once thought to strongly suggest clostridial colitis (see Fig. 33-1). However, the presence of spores is neither specific nor sensitive for clostridial colitis. Finding that the bacterial population is relatively uniform morphologically is of uncertain value, other than to show that the normal bacterial flora is disrupted. However, no comments can be made relative to cause or effect.
Short, curved, gram-negative rods (i.e., “commas” or “seagull wings”) are suggestive of campylobacteriosis. The larger spirochetes, which are often plentiful in diarrheic feces, are not C. jejuni and are of uncertain pathogenicity. Although cytologic preparations are not critically analyzed in diarrheic small animals, fecal cytologic analysis for Campylobacter spp. is a specific, albeit insensitive, method in people. Fungal organisms (e.g., Histoplasma capsulatum, Cyniclomyces guttulatus Candida spp.) are rarely found by fecal examination; cytologic examination of mucosal scrapings or histologic examination of biopsy specimens is usually necessary to diagnose histoplasmosis.
The finding of leukocytes in feces indicates the presence of a transmural colonic inflammation instead of just a superficial mucosal inflammation. However, a definitive diagnosis of a particular cause is not possible.
RADIOGRAPHY OF THE ALIMENTARY TRACT
Imaging (i.e., radiography) allows structures to be evaluated that cannot be adequately assessed during physical examination (e.g., esophagus, stomach) and may detect abnormalities missed by abdominal palpation (e.g., gastric mass, foreign object, splenic parenchymal mass). Plain radiographs should always be obtained before contrast-enhanced radiographs because (1) the former may yield diagnostic findings, (2) contrast-enhanced radiographs may be contraindicated, and (3) plain radiographs are needed to ensure a correct radiographic technique during the contrast procedure. Contrast-enhanced radiographs may be able to detect abnormalities (e.g., a gastric outflow tract obstruction) that plain radiographs cannot.
Radiographs are generally useful in the diagnostic workup of animals with dysphagia, regurgitation, vomiting, abdominal mass or distention, abdominal pain, or acute abdomen. They are occasionally helpful in animals with constipation, weight loss, or anorexia of unknown cause, but other tests are usually indicated first in such animals and often render imaging unnecessary. Radiographic findings are rarely diag nostic in dogs or cats with diarrhea or copious abdominal effusion.
ULTRASONOGRAPHY OF THE ALIMENTARY TRACT
Ultrasonography may be done in combination with or instead of radiography; however, it is extremely operator dependent. It is often useful in animals with an acute abdomen, abdominal effusion, vomiting, diarrhea, weight loss, or anorexia of unknown cause and also in those with an abdominal mass, distention, or pain. Ultrasonography can be used to identify pancreatitis, infiltrations in various organs, and intussusceptions that radiography misses. Furthermore, effusions, which render radiographs useless, enhance ultrasonographic contrast. Ultrasonography can be more informative than radiography when determining whether an animal with an acute abdomen requires surgery. Finally, ultrasonography can be used to guide the percutaneous aspiration and biopsy of intraabdominal lesions that would otherwise necessitate surgery or laparoscopy.
Techniques
A 5 MHz probe is probably the most utilitarian. Hair is often clipped so that there is no trapped air that could compromise the quality of the image. Fluid can be infused into the abdomen or stomach to improve the evaluation, but this is infrequently needed.
Findings
The thickness, echodensity, and homogeneity of organs (e.g., liver, spleen, intestine, stomach, mesenteric lymph nodes, masses) may be assessed. Intraparenchymal infiltrates that cannot be detected radiographically may also be found. The particular ultrasonographic findings seen in specific disorders of the alimentary tract are discussed in subsequent chapters dealing with the disorders.
IMAGING OF THE ORAL CAVITY, PHARYNX, AND ESOPHAGUS
INDICATIONS
Animals with dysphagia, oral pain, halitosis of unknown cause, or a swelling or mass should generallly undergo imaging. If dysphagia of neuromuscular origin is suspected, dynamic studies (i.e., fluoroscopy) are recommended. Ultrasonography can be particularly informative in the evaluation of any infiltrates or masses.
Techniques
Anesthesia is necessary so that animals can be properly positioned for radiographs of the skull. Lateral, dorsoventral (DV), and oblique views are used to detect foreign objects or fractures. Open-mouth ventrodorsal (VD) views and end-on views of the nose may also be helpful. However, dynamic studies (i.e., fluoroscopy, cinefluoroscopy) are necessary if one is looking for dysphagia of neuromuscular origin. These studies are performed by feeding conscious animals various forms of barium (i.e., liquid, paste, and mixed with food).
Findings
Foreign objects, fractures, bone lysis, soft tissue masses or densities, and emphysema are commonly found. The bone surrounding the tooth roots should be examined for evidence of lysis and the temporomandibular joints for signs of arthritis. It is important to remember to consider the bilateral symmetry of the skull; one side should be compared with the other when evaluating the VD projection. When performing contrast-enhanced or dynamic studies, the clinician should watch for the aspiration of barium, the strength with which the bolus is propelled into the esophagus, and the synchronization of the opening of the cricopharyngeal muscle with the pharyngeal phase of swallowing.
INDICATIONS FOR IMAGING OF THE ESOPHAGUS
Indications for evaluating the esophagus include regurgitation (including pharyngeal dysphagia), pain when swallowing, unexplained recurrent pneumonia or cough, and thoracic “masses” (seen radiographically) of undetermined origin. A barium contrast–enhanced esophagram is necessary unless plain films reveal the presence of an esophageal foreign object, evidence of esophageal perforation (e.g., a pleural effusion or pneumothorax), or an obvious hiatal hernia. Finding obvious megaesophagus on plain radiographs is usually considered sufficient, but some dogs with megaesophagus on plain radiographs demonstrate normal function when barium is administered. Ultrasonography is seldom useful for dogs and cats with esophageal disease, unless there is a thoracic mass.
Techniques
Liquid barium is the best contrast agent for esophageal studies; it provides excellent detail and, if aspirated, is not as noxious as paste or food. The clinician must be careful not to administer drugs that affect esophageal motility (e.g., xylazine, ketamine, anesthesia). The animal should take several swallows of dilute barium from a syringe, after which right lateral and VD views are quickly obtained. If possible, the clinician should perform fluoroscopy as the animal swallows the barium to assess esophageal motility and look for partial esophageal obstruction, segmental esophageal weakness, gastroesophageal reflux, and esophageal-pharyngeal reflux (i.e., cricopharyngeal incompetence). Radiographs may be taken if a lesion is found fluoroscopically. If fluoroscopy is not available, multiple radiographs (usually lateral projections) are taken in rapid succession, beginning very shortly (i.e., 5 to 10 seconds) after swallowing.
Barium paste is acceptable if liquid is not available. Hypertonic, iodine-contrast agents do not achieve as good a contrast as barium and cause severe problems if aspirated; isotonic water-soluble iodine contrast agents are better. If radiographic studies performed with liquid or paste contrast agents do not detect an abnormality in an animal in which esophageal disease is strongly suspected, the study should be repeated using a mixture of barium and food (both canned food and dry kibble). Such studies may detect partial strictures or muscular weakness not found in previous studies.
If barium is retained in the esophagus but little or none enters the stomach, the animal should be held in a vertical position so that gravity facilitates the migration of barium into the stomach. If barium readily enters the stomach, this indicates that there is no lower esophageal sphincter obstruction. If a hiatal hernia is suspected but not seen, a lateral radiograph of the caudal thorax may be taken while the abdomen is manually compressed. This is done in an attempt to force the stomach to herniate into the thorax so that the hernia can be demonstrated.
If esophageal disease seems likely but is not found by static radiographs, fluoroscopic studies are required. It may be necessary to observe the esophagus for several minutes (or longer) before some abnormalities (e.g., gastroesophageal or esophageal-pharyngeal reflux) occur. In animals with marginal esophageal disease, fluoroscopy may be necessary to document that primary or secondary esophageal waves are present but are either weak or not readily stimulated.
If an esophageal perforation is suspected (e.g., septic pleuritis or mediastinitis, pneumomediastinum or pneumothorax), an isotonic, iodine contrast medium may be used. However, the only purpose of such a study is to localize the perforation. If the clinician already knows where the leakage is likely to be (e.g., there is a bone foreign body in the esophagus), radiographs are of dubious value; exploratory surgery is usually a better option.
Findings
Esophageal dilation, foreign objects, soft tissue densities, spondylosis suggestive of spirocercosis, and hiatal hernia may often be identified on plain films. An air-filled esophagus is not always diagnostic of pathologic esophageal weakness. Although it is tempting to use plain radiograph findings as the basis for the diagnosis of esophageal disease when there is an “obvious” abnormality, it is easy to misinterpret plain films or miss abnormalities that a barium contrastl-enhanced study reveals. Even the finding of a dilated, gas-filled esophagus on plain thoracic films does not definitively diagnose “megaesophagus.” Rarely, animals with a dilated, air-filled esophagus on plain films are found to have normal esophageal function when evaluated with barium contrast–enhanced radiographs (Fig. 29-1). Likewise, the appearance of an accumulation of foodlike material in the classic location for a vascular ring anomaly may be caused by a localized esophageal weakness or a thymic cyst.
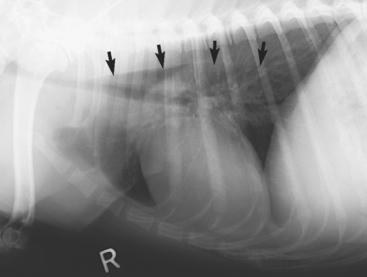
FIG 29-1 Lateral thoracic radiograph from a dog that was seen because of coughing. Note the dilated, air-filled esophagus (arrows). Contrast-enhanced esophagram (with fluoroscopy) obtained 2 days later documented normal esophageal size and function.
Many foreign objects in the esophagus (e.g., bones) can be seen on plain radiographs. However, excellent radiographic technique is necessary because some bones (especially poultry bones) as well as rawhide treats are relatively radiolucent (Fig. 29-2). An esophageal perforation sometimes causes pneumothorax, emphysematous mediastinitis, or a pleural or mediastinal effusion.
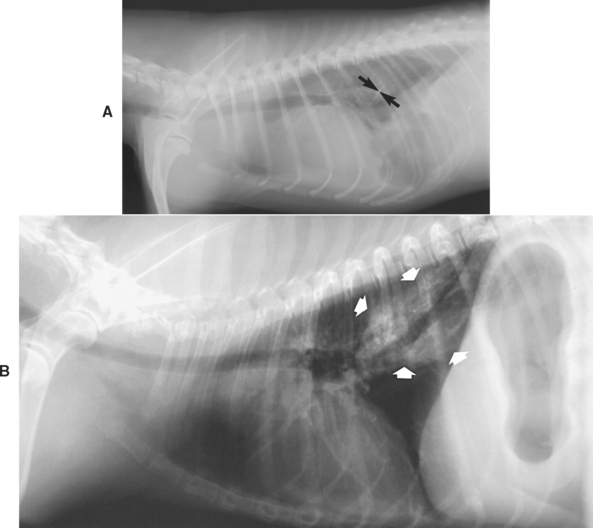
FIG 29-2 A, Lateral thoracic radiograph from a dog with a foreign object in the esophagus (arrows). Note the concomitant pleural effusion. A chicken bone had perforated the esophagus, and septic pleuritis was present. B, Lateral thoracic radiograph from a dog with a rawhide treat in the esophagus. The density representing the bone (arrows) is more diffuse than was seen in A and looks more like a pulmonary parenchymal density than a bone.
(A from Allen D, editor: Small animal medicine, Philadelphia, 1991, JB Lippincott.)
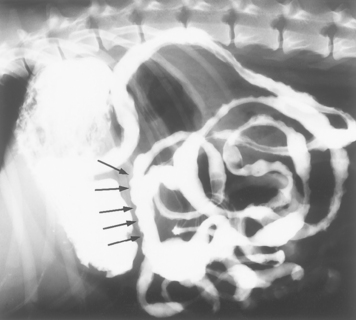
Contrast-enhanced esophagrams should be considered in animals with suspected esophageal disease and in those with unidentified thoracic masses because many esophageal tumors radiographically resemble pulmonary parenchymal masses (see Fig. 31-5). Contrast-enhanced esophagrams may also show that structures that seemingly involve the esophagus actually do not. An obstruction is suggested on contrast-enhanced esophagrams if the barium column terminates abruptly as it travels caudally; weakness usually causes contrast to be retained throughout the esophagus (Fig. 29-3) unless it is segmental. A partial obstruction is suggested by the retention of barium-impregnated food but not of liquid or paste (see Fig. 31-4).
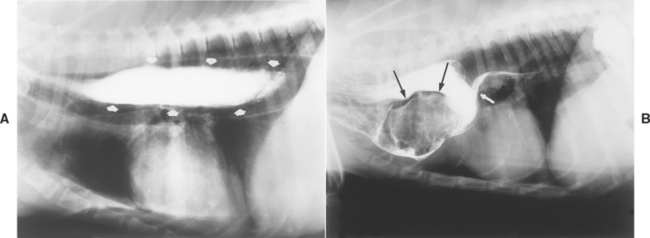
FIG 29-3 A, Lateral thoracic contrast-enhanced esophagram from a dog with generalized esophageal weakness. Note that barium is retained throughout the length of the esophagus (arrows). B, Lateral thoracic contrast-enhanced radiograph of a dog with an esophageal obstruction caused by a vascular ring anomaly. The column of barium stops abruptly (short arrow) in front of the heart, a finding characteristic of a persistent fourth aortic arch. A filling defect is also displacing barium in the dilated portion of the esophagus (long arrows).
(Courtesy Dr. Phillip F. Steyn, Colorado State University, Fort Collins, Colo.)
A barium contrast study may reveal malpositioning (e.g., hiatal hernia; see Fig. 31-2). However, the finding of a properly positioned structure on one study does not ensure that it will stay properly positioned (e.g., some hiatal hernias slide in and out of the diaphragm and may be normally positioned when the radiograph is taken). Gastroesophageal reflux and esophagitis also may be difficult to diagnose radiographically. Barium may adhere to a severely diseased mucosa, but less severe esophagitis may not be detected. In addition, normal dogs may have an episode of gastroesophageal reflux during a contrast study, whereas dogs with pathologic gastroesophageal reflux may not have reflux during a short examination.
If the animal is believed to be regurgitating but the barium contrast–enhanced radiographs are unrevealing, either the assessment of regurgitation is wrong or there is occult disease, in which case reexamination of the esophagus with fluoroscopy or endoscopy or both must be done.
IMAGING OF THE STOMACH AND SMALL INTESTINE
INDICATIONS FOR RADIOGRAPHIC IMAGING OF THE ABDOMEN WITHOUT CONTRAST MEDIA
Indications for plain abdominal radiography may include vomiting, acute abdomen, constipation, abdominal pain, enlargement, distention, or a mass. Plain radiographs are rarely beneficial in animals with a marked abdominal effusion (the fluid obliterates serosal detail) or with chronic diarrhea. Plain radiography is often not as cost-effective when the abdomen can be palpated thoroughly as when the area is difficult to examine (e.g., large or obese animals or animals in pain). In vomiting animals plain abdominal radiographs can be especially helpful in detecting radiodense foreign objects and alimentary tract dilation resulting from obstruction, foreign objects, or masses.
Techniques
The clinician always should obtain two radiographic views, usually right lateral and VD projections. Cleansing enemas may improve the diagnostic usefulness of radiographs in patients with a great deal of feces; however, a critically ill animal or one with an acute abdomen generally should not have an enema unless plain radiographs show it is necessary.
Findings
Plain abdominal radiographs may detect masses, foreign objects, a gas- or fluid-distended hollow viscus, misshapen or emphysematous parenchymal organs, pneumoperito neum, abdominal effusions, and displaced organs suggestive of a mass or adhesion.
Gastric outflow tract obstruction is easy to diagnose when there is marked gastric distention (Fig. 29-4). However, if the patient has recently vomited, the stomach may be empty and contracted. Gastric dilation, especially with volvulus, is easily recognized (see Fig. 32-4). Radiodense foreign objects are easily seen, but radiolucent foreign objects are seen only if they are outlined by swallowed air.
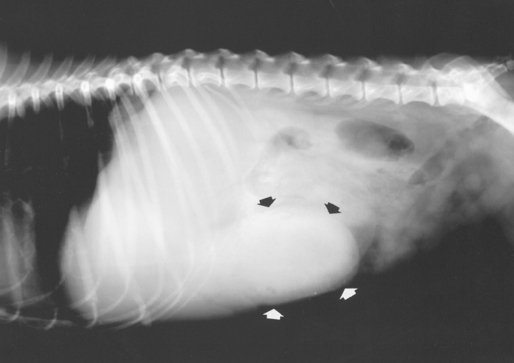
FIG 29-4 Plain lateral radiograph from a dog with gastric outflow obstruction. Note the dilated stomach protruding past the costal arch (arrows).
Intestinal obstructions are usually easier to diagnose on the basis of plain radiograph findings than are gastric obstructions; obstructed intestines distended with air, fluid, or ingesta are not readily emptied when the patient vomits (unless it is a high, duodenal obstruction). However, intestinal distention (i.e., ileus) may be caused by inflammation (i.e., adynamic or physiologic ileus) as well as obstruction (i.e., mechanical, occlusive, or anatomic ileus). Anatomic ileus (i.e., obstruction) typically produces a nonuniform intestinal distention with a greater degree of distention than is seen with physiologic ileus (Fig. 29-5). If “stacking” of the distended intestines or sharp bends and turns in the dilated intestines are seen, this also suggests anatomic ileus. Lateral radiographs obtained with the animal standing rarely aid in differentiating anatomic from physiologic ileus. Even experienced radiologists occasionally misdiagnose physiologic ileus as representing an obstruction. Thus diseases producing severe inflammation (e.g., parvoviral enteritis) may clinically and radiographically mimic an intestinal obstruction.
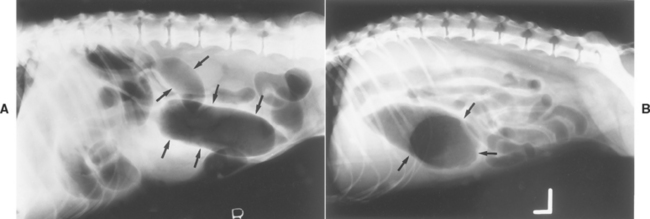
FIG 29-5 A, Plain lateral abdominal radiograph from a dog with an intestinal obstruction causing intestinal distention. Note the markedly increased diameter of the small intestinal lumen (arrows). B, Plain lateral abdominal radiograph from a dog with peritonitis causing physiologic ileus. Note the lesser degree of small intestinal distention compared with that in A. The large gas-filled structure is the gastric pylorus (arrows).
(Courtesy Dr. Kenita Rogers, Texas A&M University, College Station, Tex.)
Special types of intestinal obstruction are associated with unique radiographic findings. If the entire intestinal tract is uniformly distended with gas (Fig. 29-6) and the clinical signs fit, mesenteric volvulus may be diagnosed. If marked intestinal distention is found but is very localized and seems out of place (e.g., has herniated), a strangulated or incarcerated intestinal obstruction (see Fig. 33-9) should be considered.
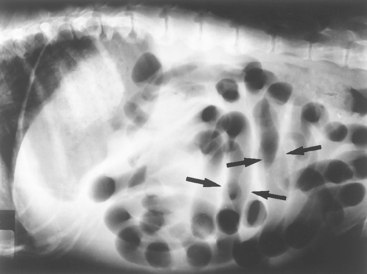
FIG 29-6 Lateral abdominal radiograph from a dog that had an acute onset of vomiting, abdominal pain, and shock. There is a uniform intestinal distention that is not as great as that in Fig. 29-5, A. However, distention is more than that seen in Fig. 29-5, B. Some intestinal loops have assumed a vertical orientation (arrows), which suggests the existence of an obstruction. This dog had a mesenteric volvulus.
(Courtesy Dr. Susan Yanoff, U.S. Military.)
Linear foreign bodies rarely produce gas-distended bowel loops. Instead, they tend to cause the intestines to bunch together, and sometimes small gas bubbles are present (see Fig. 33-10). This occurs because the intestines “gather” around the linear foreign object as they try to propel it aborad. This “gathering” or “bunching” plus the fact that linear foreign bodies tend primarily to affect the upper small intestines (i.e., duodenum) mean that it is rare that they cause gas-distended loops of bowel. Sometimes pleated (i.e., “accordian-like”) intestines can be seen on plain radiographs (see Fig. 33-10).
It is difficult to determine the thickness of intestines on plain radiographs. Animals with diarrhea and an increased amount of intestinal fluid are often misdiagnosed as having thickened intestinal walls.
Decreased serosal contrast is due to either lack of fat or excessive abdominal fluid (see Chapter 36). Displacement of an organ (Fig. 29-7) often means that there is a mass present. Pneumoperitoneum is diagnosed if both the thoracic and abdominal surfaces of the diaphragm or the serosal surfaces of the liver, stomach, or kidneys are easily seen (see Fig. 34-1, A). Pneumoperitoneum may also be documented by the finding of only a few gas bubbles in the peritoneal cavity (see Fig. 34-1, B).
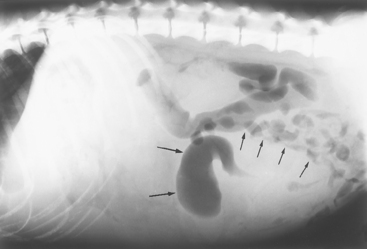
FIG 29-7 Lateral abdominal radiograph from a dog with a large granuloma caused by pythiosis. Small intestinal loops are displaced dorsally and caudally (small arrows). The border of the mass is not discernible except where it displaces small intestinal loops. The finding of a dilated intestinal loop (long arrows) is consistent with obstruction.
INDICATIONS FOR ULTRASONOGRAPHY OF THE STOMACH AND SMALL INTESTINES
Ultrasonography usually reveals almost any soft tissue change that plain radiographs detect in addition to infiltrations that radiographs cannot detect. Ultrasonography is particularly useful for detecting intussusceptions, pancreatitis, abdominal infiltrative disease, and small amounts of effusion not seen radiographically; for evaluating the hepatic parenchyma; and for identifying abdominal neoplasia in animals with a substantial effusion. Ultrasonography is much more revealing than radiography in animals with minimal body fat that have little or no radiographic contrast in the abdomen. However, very dehydrated animals may be difficult to image, and it is easy to miss small foreign objects (especially in the stomach if there is food and gas present). Ultrasonography will not detect bony changes and modest microhepatica that are easily detected by radiographs. The skill of the ultrasonographer determines the usefulness of the technique.
Technique
Before ultrasonography is performed, the abdominal hair usually should be clipped to improve the quality of the examination. This is not necessary in animals with minimal hair. Because air in the stomach or intestines limits the usefulness of ultrasonography, exercise, drugs (e.g., some narcotics) that cause hyperventilation, and enemas should be avoided before the examination.
Findings
Ultrasonography should detect almost any soft tissue change that plain radiographs detect, plus gastric and intestinal infiltrates (Fig. 29-8, A), intussusceptions (Fig. 29-8, B), enlarged lymph nodes (Fig. 29-8, C), masses (Fig. 29-8, D), some radiolucent foreign objects, and small amounts of free peritoneal fluid that radiographs cannot detect. If tissue infiltrates are found, they can sometimes be aspirated by the fine-needle technique.
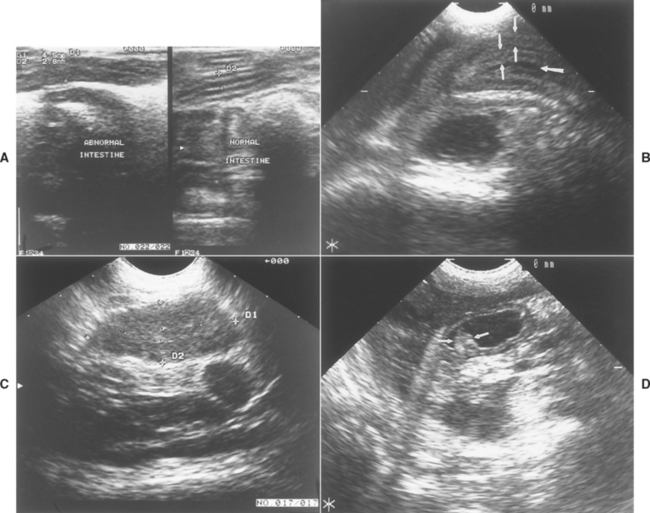
FIG 29-8 A, Ultrasonographic image of two sections of small intestine from a cat with an alimentary tract lymphoma. The normal intestine on the right is 2.8 mm thick (see the two “+’s” noted as D2), whereas the abnormal intestine on the left is 4.5 mm thick (D1) because of neoplastic infiltrates. B, Ultrasonographic image of an ileocolic intussusception that was not obvious on plain abdominal radiographs. There are two intestinal walls (small arrows) seen on each side of the lumen (large arrow). C, An enlarged mesenteric lymph node in a dog caused by lymphoma, seen by ultrasonography. The lymph node was not detected on radiographs or by abdominal palpation. D, Ultrasound image of the gastric antrum from a dog with benign gastric polyps. One polyp can be seen (arrows) protruding into the gastric lumen.
(Courtesy Dr. Linda Homco, Cornell University, Ithaca, N.Y.)
INDICATIONS FOR CONTRAST-ENHANCED GASTROGRAMS
Contrast-enhanced gastrography is principally performed in vomiting animals when ultrasound studies and plain abdominal radiographs are unrevealing. It is primarily used to detect a gastric outflow tract obstruction, gastric masses/foreign bodies, and gastric motility problems.
Technique
The animal should not be allowed to eat for at least 12 hours (preferably 24 hours) before the procedure, and feces should be removed with enemas. Plain radiographs should be obtained immediately before the contrast-enhanced films to verify that the abdomen has been properly prepared and the radiographic technique is correct and to determine whether the diagnosis cannot be made on the basis of the plain radiographic findings. Liquid barium sulfate is then administered orally (8 to 10 ml/kg in small dogs and cats and 5 to 8 ml/kg in large dogs). Iohexol can be administered orally (i.e., 700 to 875 mg I/kg, which is about 11/4 to 11/2 ml/kg). The agent should be administered via a stomach tube to ensure adequate gastric filling and optimal evaluation of the stomach. The animal should not receive motility-altering drugs (e.g., xylazine, parasympatholytics), which delay outflow.
Immediately after barium administration, radiographs are taken in the left and right lateral plus DV and VD projections. Radiographs in the lateral and DV projections should be obtained again at 15 and 30 minutes and perhaps also at 1 to 3 hours. The right lateral view causes barium to pool in the pylorus, the left lateral view causes it to pool in the gastric body, the DV view causes it to pool along the greater curvature, and the VD view allows better evaluation of the pylorus and antrum. Double-contrast gastrograms provide more detail than single-contrast gastrograms. They are performed by administering barium via a stomach tube, then removing most of the barium through the same tube and insufflating the stomach with gas until it is mildly distended.
If available, fluoroscopy is best performed immediately after administration of the barium. It can be used to evaluate gastric motility, gastric outflow, and the maximal opening size of the pylorus. If the animal is fed barium mixed with food (only recommended if gastric outflow tract obstruction is suspected despite normal liquid barium study findings), gastric emptying will be markedly delayed compared with that seen when the animal is fed liquid barium.
Findings
Gastric emptying is considered delayed if liquid barium does not enter the duodenum 15 to 30 minutes after administration or if the stomach fails to almost completely empty a liquid barium meal in 3 hours (see Fig. 32-2). Luminal filling defects (e.g., growths and radiolucent foreign objects), ulcers, pyloric lesions preventing gastric emptying, and infiltrative lesions may be seen using this method (see Fig. 32-2, C). However, normal peristalsis, ingesta, or gas bubbles may resemble an abnormality; therefore a change must be seen on at least two separate films before the clinician can diagnose disease.
Contrast-enhanced gastrograms are not as sensitive as endoscopy for detecting gastric ulceration, and they cannot detect erosions. Ulcers are documented radiographically if barium is seen to enter the gastric or duodenal wall or if a persistent spot of barium is identified in the stomach long after the organ has emptied itself of the contrast agent (see Fig. 32-6). The duodenum should be scrutinized in a search for constrictions and infiltrative lesions because many vomiting animals have disease there (e.g., inflammatory bowel disease, tumors) rather than in the stomach (see Chapter 33).
INDICATIONS FOR CONTRAST-ENHANCED STUDIES OF THE SMALL INTESTINE
Vomiting is the principal reason for performing contrast studies of the upper small intestine. Contrast-enhanced radiographs are particularly useful for distinguishing anatomic from physiologic ileus. Orad obstructions are easier to demonstrate than aborad ones if the contrast medium is administered orally. If a very aborad obstruction is suspected (e.g., ileocolic intussusception), a barium enema (or preferably ultrasonography) is often better than an upper gastrointestinal contrast series. Although linear foreign objects usually produce subtle findings on plain radiographs, they often cause a classic “pleating” or “bunching” of the intestines to be seen on contrast films (see Fig. 33-10, C).
Animals with diarrhea seldom benefit from contrast studies of the intestines because normal radiographic findings do not exclude the presence of severe intestinal disease, and even if radiographic findings indicate the presence of infiltrative disease, it is still necessary to obtain a biopsy specimen to determine the cause. Contrast series are sometimes useful if the clinician is trying to decide whether to perform endoscopy or surgery. However, it is usually more cost-effective to perform endoscopy or surgery and skip the contrast-enhanced radiographs.
Use of iodinated contrast agents (preferably iohexol) is reasonable if an alimentary tract perforation is suspected. However, if spontaneous septic peritonitis is strongly suspected, it can usually be definitively diagnosed by ultrasound-guided abdominocentesis and fluid analysis. If ultrasound is unavailable and blind abdominocentesis is unrevealing in such a patient, it is usually better to perform a thorough exploratory laparotomy than contrast-enhanced radiography.
Technique
Liquid barium sulfate is administered as described for contrast-enhanced gastrography. Lateral and VD radiographs should be obtained immediately and then 30, 60, and 120 minutes after barium administration. Additional films are obtained as necessary. The study is completed once contrast has reached the colon. If chemical restraint is absolutely necessary, acetylpromazine may be used. Fluoroscopy is rarely needed for these studies.
Hypertonic iodinated contrast agents are inferior to barium for small intestinal studies because they decrease the intestinal transit time and can cause considerable fluid shifts by osmotically drawing fluid into the gastrointestinal tract. Their potential advantages rarely outweigh the disadvantages. Iohexol is safer and produces better detail than the hypertonic iodinated compounds.
Findings
In a complete intestinal obstruction, the barium column cannot advance beyond a certain point, and the intestines orad to this point are typically dilated. A partial obstruction may be denoted by delayed passage past a certain point (there may or may not be dilation of the intestines orad to this point) or constriction of the lumen. Because it is easy to overinterpret contrast-enhanced radiographs of the intestines, changes must be seen on at least two different films taken at different times before a disease is diagnosed.
“Enteritis” is often incorrectly diagnosed if a fine “brush border” in the lumen is found. However, this finding actually results from the barium normally distributing itself among villi, not from enteritis. Infiltration is denoted by scalloped margins (sometimes called thumb-printing); such a pattern (Fig. 29-9) may be seen in the setting of neoplasia (e.g., lymphoma), inflammatory bowel disease, fungal infection (e.g., histoplasmosis), or parvoviral enteritis. However, its absence does not rule out the presence of infiltrative disease. Focal dilations not caused by obstruction (i.e., diverticula) are rare and usually represent a localized neoplastic infiltrate. In rare instances, unsuspected intestinal blind loops or short-bowel syndromes may be detected. Motility problems may cause slowed passage of the contrast through the alimentary tract.
INDICATIONS FOR BARIUM CONTRAST ENEMAS
If ultrasound and flexible colonoscopy are available, there is seldom any need for barium enemas. If only rigid colonoscopy is available, barium enemas are needed to evaluate the ascending and transverse colon, areas inaccessible to rigid scopes. If colonoscopy is unavailable, a barium enema may be useful for looking for infiltrative lesions (e.g., rectal-colonic neoplasia causing hematochezia), a partial or complete obstruction, or ileocolic or cecocolic intussusception. It can also evaluate the colon orad to a near-complete rectal obstruction to determine whether there are more infiltrative lesions or obstructions besides the one palpated near the rectum.
Technique
The patient should be fasted for at least 24 hours, and then the colon must be emptied and cleaned by enemas or ali mentary tract lavage solutions, or both. The animal should be anesthetized and a balloon-tipped catheter placed in the colon. The balloon is then inflated so that barium cannot leak out the rectum. Approximately 7 to 10 ml of liquid barium/kg at body temperature is infused into the colon until it is uniformly distended, and lateral and VD radiographs are obtained. The colon may then be emptied of barium and insufflated with air to achieve a double-contrast barium enema, which provides greater detail. If too much barium is administered, the ileum may fill with the contrast agent, obscuring colonic detail and making the study less useful.
Findings
Barium enemas unreliably detect mucosal disease (i.e., ulcers, inflammation). If the animal has been properly prepared, these enemas can reveal intraluminal filling defects representing ileocolic or cecocolic intussusception (see Fig. 33-11), proliferative colonic neoplasia (e.g., polyps, adenocarcinoma), extraluminal compression denoted by smooth-surfaced displacement of the barium from the colonic lumen, and infiltrative disease (i.e., a roughened, partial obstruction or an “apple core” lesion) (Fig. 29-10). However, it is imperative that a change be found on at least two films to ensure that it is not an artifact.
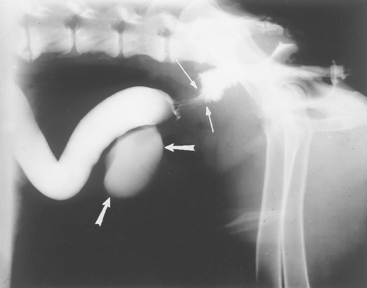
FIG 29-10 Lateral view of a dog that had a barium enema. There is circumferential narrowing with roughened borders (thin arrows) that is in distinction to the rest of the colon. This dog had infiltrative adenocarcinoma, which caused an obstruction. The urinary bladder is also seen as a result of the previous contrast procedure (thick arrows).
PERITONEAL FLUID ANALYSIS
Fluid analysis is discussed in detail in Chapter 36. The fluid is obtained by performing abdominocentesis with a syringe and needle. If this technique fails, a multifenestrated catheter (e.g., a dialysis catheter, a sterile teat cannula, or an 18-gauge cephalic catheter with additional holes cut with a scalpel) may be successful. It is sometimes best to allow fluid to drain out of the catheter without applying negative pressure.
If peritoneal inflammation is suspected but abdominal fluid cannot be retrieved, a diagnostic peritoneal lavage may be performed. In this method a sterile catheter (preferably with multiple fenestrations) is inserted into the abdomen and warm, sterile physiologic saline solution (20 ml/kg) is administered rapidly. The abdomen is massaged vigorously for 1 to 2 minutes, and then some of the fluid is aspirated. The aspirate is evaluated cytologically.
DIGESTION AND ABSORPTION TESTS
Exocrine pancreatic function may be tested by measuring fecal proteolytic activity (not recommended), fat absorption with and without pancreatic enzymes (not recommended), or serum TLI (recommended).
Fat absorption testing is simple but of questionable sensitivity and specificity. It is no longer recommended. The reader is referred to prior editions of this text for a description of the test and interpretation.
Serum TLI is the most sensitive and specific test for EPI and is convenient (i.e., submit 1 ml of refrigerated serum obtained after an overnight fast) and readily available. The TLI assay detects circulating proteins produced by a normally functioning exocrine pancreas and is even valid in animals receiving pancreatic enzyme supplements orally. Pancreatitis, renal failure, and severe malnutrition may increase the serum TLI concentrations, but this rarely causes results to be misinterpreted. However, if EPI is caused by obstruction of the pancreatic ducts (apparently rare) as opposed to acinar cell atrophy or destruction (common), the serum TLI test may not detect maldigestion. In such cases, a quantitative fecal proteolytic assay is required.
Normal dogs have serum TLI activities of 5.2 to 35 μg/L. Values of less than 2.5 μg/L confirm a diagnosis of EPI. Normal cats have higher values (28 to 115 μg/L). The serum TLI assay is primarily indicated in dogs with chronic small intestinal diarrhea or chronic weight loss of unknown origin. Because feline EPI is rare, the test is seldom necessary in cats. Although principally used to detect EPI, serum TLI values substantially greater than normal are suggestive of pancreatitis.
SERUM CONCENTRATIONS OF VITAMINS
Serum concentrations of cobalamin and folate are sometimes helpful in animals with chronic small intestinal diarrhea or chronic weight loss. These tests may provide evidence of severe small intestinal mucosal disease. Dietary cobalamin is absorbed in the intestine, principally the ileum. When ARE is present, bacteria sometimes bind cobalamin and prevent its absorption, decreasing the serum concentrations. Cobalamin concentrations are usually decreased in dogs with EPI, possibly because of the high incidence of ARE in such animals. Severe mucosal disease, especially in the region of the ileum, may also cause serum cobalamin concentrations to be decreased, ostensibly because of malabsorption of the vitamin. Perhaps the major indications for measuring serum cobalamin are to look for evidence of intestinal disease in patients with weight loss of uncertain cause and to better define cats with known small intestinal disease (cobalamin-deficient cats can experience metabolic complications). If the serum cobalamin is low in a patient with weight loss of unknown cause, it is likely that small intestinal disease is responsible. B-complex vitamin supplementation may cause an increased serum cobalamin concentration.
Dietary folate is absorbed in the small intestine. If there are many bacteria in the upper small intestine, these sometimes synthesize and release folate, causing the serum concentrations to be increased. Likewise, severe intestinal mucosal disease may decrease absorption, causing lower serum concentrations. B-complex vitamin supplementation may increase serum folate concentrations.
Because bright light degrades cobalamin, samples should be frozen and kept in the dark during storage and transport. The specificity of decreased serum cobalamin and increased folate concentrations for ARE is questionable.
OTHER SPECIAL TESTS FOR ALIMENTARY TRACT DISEASE
Antibodies to acetylcholine receptors should be measured if the clinician is looking for a cause of dysphagia or esophageal weakness that could be of neuromuscular origin (see p. 422). Serum is obtained and sent to a laboratory that can perform a validated assay for the species being evaluated. Increased titers to such antibodies are strongly suggestive of myasthenia gravis, even if there are no systemic signs. False-positive results are rare. Testing can be done by Dr. Diane Shelton (Comparative Neuromuscular Laboratory, Basic Science Building, University of California at San Diego, La Jolla, CA 92093-0612).
Measurement of antibodies to 2M muscle fibers can be helpful in dogs with suspected masticatory muscle myositis (see p. 420). These antibodies are typically not found in dogs with polymyositis, whereas most dogs with masticatory myositis have them. Serum is required for the test and can be sent to Dr. Diane Shelton for testing.
Serum gastrin concentrations are measured in animals with signs suggestive of gastrinoma (i.e., chronic vomiting, weight loss, and diarrhea in older animals, especially if there is concurrent esophagitis or duodenal ulceration). Gastrin stimulates gastric acid secretion and is trophic for the gastric mucosa. Serum for assay of gastrin is harvested from an animal after an overnight fast and rapidly frozen. The serum gastrin concentration may be increased in animals with gastrinoma, a gastric outflow tract obstruction, renal failure, short-bowel syndrome, or atrophic gastritis and in those receiving antacid therapy (e.g., H2-receptor antagonist and proton pump inhibitors). Resting serum gastrin concentrations may vary, with occasional values in the normal range in animals with gastrinoma. Provocative testing should be considered in dogs strongly suspected of having gastrinoma but with normal baseline serum gastrin concentrations (see Chapter 52).
Testing for urease activity in gastric mucosa is sometimes done if the clinician is looking for Helicobacter sp. in the stomach. This bacteria has strong urease activity. To perform this, one or preferably two fresh pieces of gastric mucosa are placed into urease agar and observed for up to 24 hours. If these urease-producing bacteria are present, their enzyme will split the urea in the agar into ammonia and the pH indicator in the agar will change from amber to pink (sometimes this occurs within 15 minutes). Tubes of urease agar may be obtained from microbiologic supply houses. There are also special kits designed to detect Helicobacter spp. In dogs and cats there is no good evidence that this test is more advantageous than special staining (e.g., Warthin-Starry) of multiple gastric biopsy specimens.
Intestinal permeability testing can be performed, and finding increased permeability seems to be a reliable marker of small intestinal disease. However, at this time it is impossible to diagnose a patient with increased small intestinal permeability as having a particular disease. Currently, the major value to such testing seems to be (1) determining that a patient with clinical signs of uncertain cause has small intestinal disease and (2) evaluating response to therapy in difficult-to-manage patients. This test is seldom done in clinical cases.
Fecal alpha-1 protease inhibitor can be measured in feces and is a marker for gastrointestinal protein loss. Clinically, this test is rarely indicated but could be helpful when trying to distinguish whether hypoalbuminemia is at least partly due to a protein-losing enteropathy in a patient with known renal protein loss or hepatic insufficiency.
Tests for Pythium insidiosum are available. ELISA tests for antibodies and PCR testing for antigen can be done at Louisana State University (Dr. Amy Grooters, College of Veterinary Medicine, Lousiana State University, Baton Rouge, LA 70803).
ENDOSCOPY
Endoscopy is often cost-effective if radiographic and ultrasonographic findings have been nondiagnostic in animals with chronic vomiting, diarrhea, or weight loss. It permits rapid exploration of selected sections of the alimentary tract and mucosal biopsy without the need for a thoracotomy or laparotomy. Although excellent for detecting morphologic changes (e.g., masses, ulcers, obstruction), it is insensitive for revealing abnormal function (e.g., esophageal weakness).
Rigid endoscopy is easier to perform and less expensive than flexible endoscopy, and it can provide excellent biopsy samples. Flexible endoscopes can be used to examine structures that cannot be inspected with a rigid endoscope. Flexible instruments are expensive, and it takes time to become proficient in their use. In addition, one is limited by how far the instrument can be advanced. Furthermore, tissue samples obtained through a flexible endoscope may have artifacts or may be too small to yield diagnostic findings unless the clinician’s technique is excellent.
Esophagoscopy is useful in looking for esophageal tumors (Fig. 29-11), foreign objects (Fig. 29-12), inflammation (Figs. 29-13 and 29-14), and obstructions caused by cicatrix (Fig. 29-15). Foreign objects and cicatrix are preferentially treated endoscopically. Esophagoscopy may also show partial obstructions not detected by contrast esophagrams. It is important in such procedures to enter the stomach and retroflex the scope’s tip to view the lower esophageal sphincter area to detect leiomyomas (Fig. 29-16) or other easily missed lesions. The esophageal lumen is covered with squamous epithelium, which cannot be pulled off with typical flexible endoscopic forceps. Therefore, if esophageal mucosal biopsy specimens are desired, flexible endoscopes are typically inadequate unless the distal feline esophagus is being biopsied or there is a tumor.
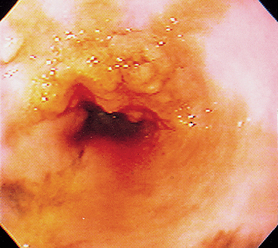
FIG 29-11 Endoscopic view of a polypoid mass in the esophagus of a Chow. This represents an adenocarcinoma.
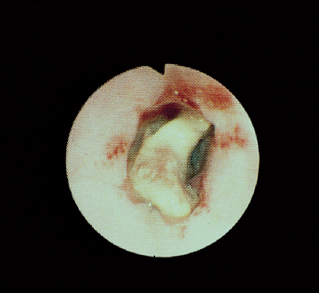
FIG 29-12 Endoscopic view of the esophagus of a dog with a chicken neck bone lodged in it. The bone was ultimately removed with a rigid scope and alligator forceps.
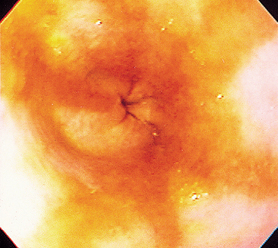
FIG 29-13 Endoscopic view of the lower esophageal sphincter of a dog with moderately severe reflux esophagitis secondary to vomiting. Note the hyperemic areas.
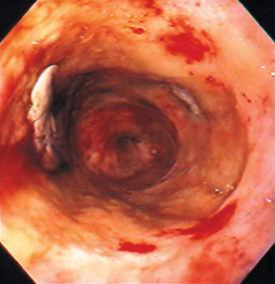
FIG 29-14 Endoscopic view of the distal esophagus of a dog with severe esophagitis secondary to a bone foreign body. Note the white plaque in the 9 o’clock position that is due to pressure necrosis from the foreign body.
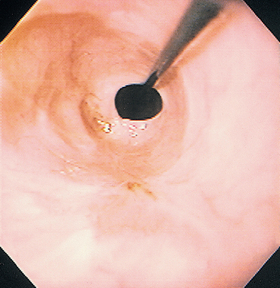
FIG 29-15 Endoscopic view of the same site as in Fig. 29-13 but 10 days later. A narrowing of the lumen is obvious; this is due to cicatrix formation. A guide wire has been passed through the cicatrix in preparation for balloon dilation.
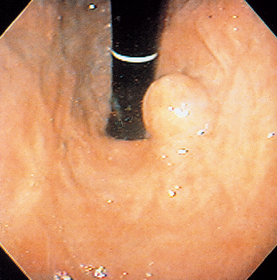
FIG 29-16 View of the lower esophageal sphincter (as seen from the stomach) of a dog with a leiomyoma. This lesion was causing vomiting and regurgitation and would easily have been missed if a careful, methodical examination had not been carried out.
Although esophagoscopy may occasionally detect esophageal weakness (Fig. 29-17), it is not sensitive for detecting this and other selected disorders (e.g., diverticula). Not all foreign objects can be safely removed endoscopically, and the clinician must guard against rupturing a diseased esophagus while trying to extract a foreign object. Finally, care must be taken to avoid creating a potentially fatal gastric distention in patients with esophageal strictures and a fataltension pneumothorax in animals with an esophageal perforation.
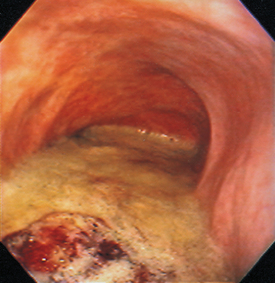
FIG 29-17 Endoscopic view of a dog with a megaesophagus. Note that the lumen is dilated and there is substantial food material accumulation.
Rigid endoscopy is often more useful than flexible endoscopy in removing esophageal foreign objects. The rigid endoscope can protect the esophagus during extraction of the object, and it allows the use of rigid forceps that can grasp the foreign object more tightly. Care must be taken to maintain the animal’s esophagus as straight as possible when using a rigid endoscope. If a flexible endoscope is used, it is often helpful to pass it through a rigid scope or tube that has been passed through the cricopharyngeal sphincter; this may facilitate passage of the foreign object through the sphincter.
Gastroduodenoscopy and biopsy are indicated in selected animals with vomiting, apparent upper gastrointestinal blood loss, apparent gastroduodenal reflux, or small intestinal disease. It is more sensitive and specific than radiography for detecting mucosal ulcers (Fig. 29-18), erosions (Fig. 29-19), tumors (Fig. 29-20), and inflammatory lesions (Figs. 29-21 to 29-23). Endoscopy is also quicker and less stressful to the animal than exploratory laparotomy. Many foreign objects in the upper gastrointestinal tract (Fig. 29-24) can be removed using endoscopy, and multiple biopsy specimens can be obtained. Occasionally, unexpected diagnoses (e.g., Physaloptera infection; Fig. 29-25) may be found. It may be necessary to use endoscopes with outer diameters of 9 mm or less in dogs and cats weighing less than 4 to 5 kg. Whenever possible, a scope with a 2.8-mm biopsy channel should be used to obtain larger specimens and allow the use of better foreign object retrieval devices.
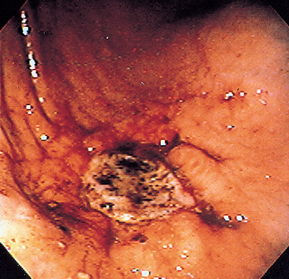
FIG 29-18 Endoscopic view of a gastric ulcer on the greater curvature in a Chow. Note that it is obvious that the mucosa is eroded to the level of the submucosa.
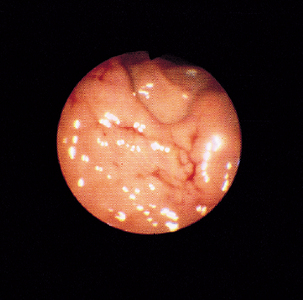
FIG 29-19 Endoscopic view of the gastric mucosa of a dog’s stomach that has obvious bleeding. This dog had received nonsteroidal drugs, and the bleeding represented erosions that could not be detected with radiographs or ultrasonography.
(From Fossum T, editor: Small animal surgery, St Louis, 1997, Mosby.)
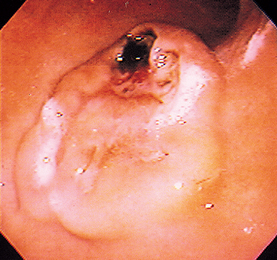
FIG 29-20 Endoscopic view of the stomach of a dog with an obvious mass in the greater curvature. This is an ulcerated leiomyosarcoma that was successfully removed.
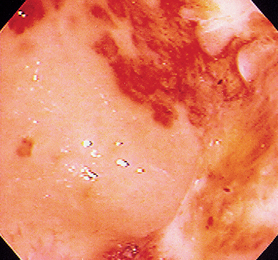
FIG 29-21 Endoscopic view of the stomach of a cat with diffuse inflammation, erosion, and ulceration of unknown cause.
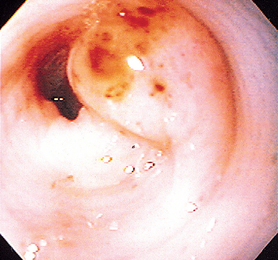
FIG 29-22 A focal gastritis near the pylorus of a dog. Note the reddened spots on the lesion, which were responsible for intermittent hematemesis.
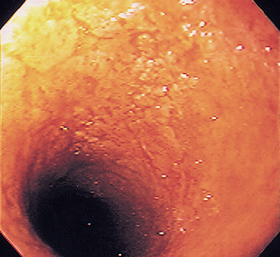
FIG 29-23 The duodenum of a dog with marked inflammatory bowel disease. Note the pseudomembrane-like appearance, which suggests severe disease.
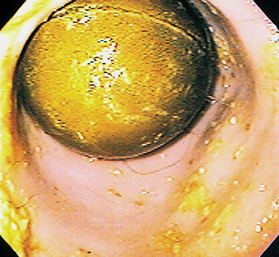
FIG 29-24 Endoscopic view of the antrum of a dog with a ball foreign object that has been present for months and was not detected on plain radiographs or by ultrasonography.
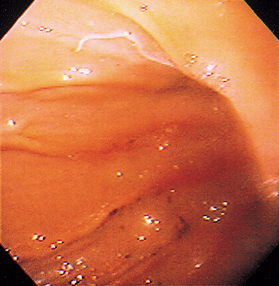
FIG 29-25 Endoscopic view of the greater curvature of the stomach of a dog with a Physaloptera attached.
The stomach must be as empty as possible when gastroduodenoscopy is performed, which usually necessitates at least a 24-hour fast; many animals undergoing gastroscopy may not empty their stomachs as rapidly as they normally would. During the procedure the stomach must be adequately inflated with air to allow thorough evaluation of its mucosa. Suction must be available to remove secretions or air. The endoscopist must inspect the mucosa methodically to keep from missing lesions. It is particularly easy to miss lesions (e.g., ulcers or Physaloptera) just inside the pylorus. Biopsy specimens of the gastric and duodenal mucosa should always be obtained because normal findings seen on visual examination do not rule out the presence of severe mucosal disease. Like esophagoscopy, gastroscopy is not sensitive in identifying functional problems (i.e., gastric hypomotility).
Proctoscopy or colonoscopy is indicated in dogs and cats with chronic large bowel disease unresponsive to appropriate dietary, antibacterial, or anthelmintic therapies as well as those that are losing weight or are hypoalbuminemic. Colonoscopy is more sensitive and definitive, yet comparable in cost to plain and contrast-enhanced radiography. Proctoscopy is used in animals with obvious rectal abnormalities (e.g., stricture felt on digital rectal examination). Rigid biopsy forceps obtain excellent tissue samples, which allows the identification of most lesions, including submucosal ones. Biopsy instruments used with flexible endoscopes do not obtain as deep a biopsy specimen but are adequate for obtaining specimens from mucosal lesions.
Proctoscopy and colonoscopy are easier to perform, require less animal restraint, and do not always require the more expensive flexible equipment demanded by other endoscopic procedures. The colon must be clean to allow proper inspection of the mucosa. All food should be withheld for at least 24 and preferably 36 hours before the procedure, a mild laxative (e.g., bisacodyl) should be administered the night before the procedure, and several copious warm water enemas should be given the night before and the morning of the procedure. Proctoscopy requires less cleaning than colonoscopy. Commercial intestinal lavage solutions (e.g., GoLytely, Colyte) clean the colon better than enemas and are particularly useful in larger dogs, those that will be undergoing ileoscopy (which necessitates a very clean ileocolic area), and animals in pain that resist enemas. The lavage solution is usually given to the animal twice the night before the procedure and perhaps once the morning of the procedure. In rare cases, it can cause gastric dilation or volvulus.
Sedation plus manual restraint can often be used instead of anesthesia; however, many animals undergoing colonoscopy have colonic or rectal irritation, and anesthesia is usually preferred. Suction should be available.
Normal colonic mucosa is smooth and glistening, and the submucosal blood vessels can be seen (Fig. 29-26); enema tubes may cause linear artifacts. The colon should distend to a uniform diameter, but it may have bends. If a flexible scope is used, the clinician should identify and inspect the ileocolic valve and the cecum (Figs. 29-27 and 29-28). The clinician should always biopsy the mucosa; normal gross findings do not rule out the presence of significant disease. Strictured areas with relatively normal-appearing mucosa are usually caused by a submucosal lesion, in which case biopsying must be aggressive enough to ensure that submucosal tissue is included in the specimen. Cytology can detect histoplasmosis, protothecosis, some neoplasms, and eosinophilic colitis.
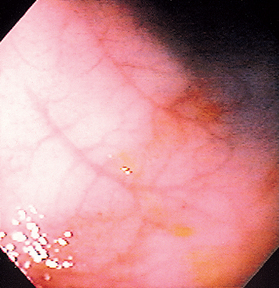
FIG 29-26 Endoscopic view of a normal colon in a dog, showing typical submucosal blood vessels. Inability to see such blood vessels may suggest inflammatory infiltrates.
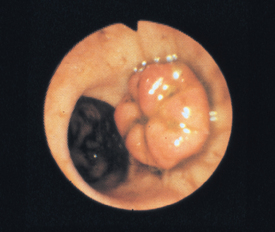
FIG 29-27 Normal ileocolic valve region in a dog. The ileocolic valve is the mushroomlike structure, and the opening below it is the cecocolic valve.
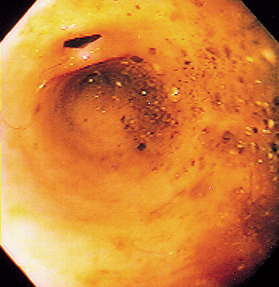
FIG 29-28 Endoscopic view of a normal ileocolic valve region from a cat. The blind pouch is the cecum, and the small opening above it is the ileocolic valve.
An adult or a pediatric human sigmoidoscope is usually adequate for rigid colonoscopy. The tip of the rigid biopsy forceps should have a shearing action (i.e., one part of the tip should fit into the other when it is closed, thus acting like a pair of scissors) instead of a clamshell (also called “double spoon”) action in which the edges of the top and bottom jaws simply meet.
Ileoscopy is principally indicated in dogs with diarrhea and in cats with vomiting or diarrhea. It is performed during flexible colonoscopy and requires thorough colonic cleansing so that the ileocolic valve can be visualized. It is difficult or impossible to enter the ileum of most cats (because of size), but one can often pass biopsy forceps through the ileocolic valve and blindly biopsy the ileal mucosa (Fig. 29-29). Ileoscopy can be particularly valuable in diagnosing lymphoma in cats when the duodenal biopsies are nondiagnostic.
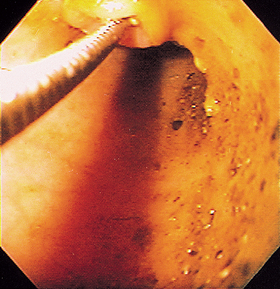
FIG 29-29 Same site as in Fig. 29-28. A biopsy instrument has been blindly passed into the ileum because the scope cannot be advanced through the narrow orifice.
BIOPSY TECHNIQUES AND SUBMISSION
FINE-NEEDLE ASPIRATION BIOPSY
Fine-needle aspiration or core biopsy of enlarged lymph nodes, abdominal masses, and infiltrated abdominal organs may be guided by abdominal palpation or ultrasonography. A 23- to 25-gauge needle is typically used so that any inadvertent intestinal or vascular perforation is insignificant (see Chapter 75).
ENDOSCOPIC BIOPSY
Rigid endoscopy usually provides excellent biopsy samples of the descending colon (i.e., large specimens that include the full thickness of the mucosa, including some muscularis mucosa), but the stomach and small intestine cannot be biopsied with this equipment. Flexible endoscopes can reach more of the alimentary tract, but the tissue samples obtained with these scopes may not always be deep enough to allow submucosal lesions to be diagnosed. Ideally, the tissue to be biopsied is visualized; however, the clinician may pass the biopsy forceps through the pylorus or ileocolic valve and biopsy the duodenum or ileum blindly if the tip of the endoscope cannot be advanced into these areas.
Not all laboratories are adept at processing and interpreting these samples. Endoscopes with 2.8-mm biopsy channel are generally preferred to those with a 2.0- or a 2.2-mm channel because the larger forceps allow retrieval of substantially larger and deeper tissue samples.
When intestinal or gastric mucosa is biopsied, the tissue sample must be handled carefully to minimize artifacts and distortion. The tissue should be carefully removed from the biopsy forceps with a 25-gauge needle. A squash preparation of one tissue specimen can be evaluated cytologically, and the remaining samples are fixed in formalin and evaluated histologically. The cytology slides should be evaluated by a pathologist familiar with gastrointestinal cytology. Cytologic preparations of the gastric mucosa may show adenocarcinoma, lymphoma, inflammatory cells, or large numbers of spirochetes (see Fig. 32-1). Cytologic studies of the intestinal mucosa may show eosinophilic enteritis, lymphoma, histoplasmosis, or protothecosis, and occasionally giardiasis, bacteria, or Heterobilharzia ova. The absence of cytologic findings suggestive of these disorders does not rule them out, but finding them cytologically is diagnostic.
The laboratory should be consulted regarding the proper way to submit endoscopic tissue sections. In the author’s lab, the samples are oriented on the surface of a plastic cassette sponge such that the submucosal side is on the sponge and the luminal side is away from the sponge. The sponge is placed in 10% neutral buffered formalin with the tissues down in the formalin. The clinician should place tissues from different locations in different vials of formalin; each vial should be properly labeled so that the pathologist can correctly identify the area evaluated. Small tissue samples should not be allowed to dry out or be damaged before placement in formalin.
Two common problems with endoscopically obtained tissue samples are that the sample is too small or there is excessive artifact. Lymphomas are sometimes relatively deep in the mucosa (or are submucosal), and a superficial biopsy specimen may then show only a tissue reaction above the tumor, resulting in a misdiagnosis of inflammatory bowel disease. Multiple biopsy specimens should be obtained until there are at least five to eight samples of excellent size and depth (i.e., the full thickness of mucosa). It is important to contact the pathologist and determine whether the quality of the tissue samples was adequate and if the severity of the histologic lesions found is consistent with the clinical signs.
FULL-THICKNESS BIOPSY
If endoscopy is not available, abdominal surgery may be needed to perform gastric and intestinal biopsies. Fullthickness biopsy specimens obtained surgically can have fewer artifacts than those obtained endoscopically; however, the clinician must consider the pros and cons of surgery in a potentially debilitated or ill animal. Endoscopy allows the clinician to direct the biopsy forceps to lesions that cannot be seen from the serosal surface. If surgery is performed, maximal benefit should be obtained from the procedure; the entire abdomen should be examined (i.e., literally from the beginning of the stomach to the end of the colon with all parenchymal organs). Biopsy specimens should be obtained from all obviously abnormal structures. Biopsy specimens of the stomach, duodenum, jejunum, ileum, mesenteric lymph nodes, and liver (and the pancreas in cats) should be obtained, regardless of how normal these organs appear, unless an obvious lesion is found (e.g., a large tumor). However, it is wise not to assume that a grossly impressive lesion is responsible for the clinical signs; rather, the clinician should perform a biopsy even when the diagnosis seems obvious. Dehiscence is a concern if the serum albumin concentration is less than 1.5 g/dl, but the use of nonabsorbable suture material and serosal patch grafting over intestinal suture lines minimizes the risk. The clinician should consider whether gastrostomy or enterostomy feeding tubes should be placed in emaciated animals before exiting the abdomen.
Baez JL, et al. Radiographic, ultrasonographic, and endoscopic findings in cats with inflammatory bowel disease of the stomach and small intestine: 33 cases (1990–1997). J Am Vet Med Assoc. 1999;215:349.
Bonfanti U, et al. Diagnostic value of cytologic examination of gastrointestinal tract tumors in dogs and cats: 83 cases (2001–2004). J Am Vet Med Assoc. 2006;229:1130.
Cave NJ, et al. Evaluation of a routine diagnostic fecal panel for dogs with diarrhea. J Am Vet Med Assoc. 2002;221:52.
Chouicha N, et al. Evaluation of five enzyme immunoassays compared with the cytotoxicity assay for diagnosis of Clostridium diffficile-associated diarrhea in dogs. J Vet Diagn Invest. 2006;18:182.
Dryden M, et al. Accurate diagnosis of Giardia spp. and proper fecal examination procedures. Vet Therap. 2006;7:4.
Goggin JM, et al. Ultrasonographic measurement of gastrointestinal wall thickness and the ultrasonographic appearance of the ileocolic region in healthy cats. J Am Anim Hosp Assoc. 2000;36:224.
Grooters AM, et al. Development of a nested polymerase chain reaction assay for the detection and identification of Pythyium insidiosum. J Vet Intern Med. 2002;16:147.
Grooters AM, et al. Development and evaluation of an enzyme-linked immunosorbent assay for the serodiagnosis of pythiosis in dogs. J Vet Intern Med. 2002;16:142-146.
Gualtieri M. Esophagoscopy. Vet Clin N Am. 2001;31:605.
Guilford WG. Upper gastrointestinal endoscopy. In: McCarthy TC, editor. Veterinary endoscopy. St Louis: Elsevier/Saunders, 2005.
Hall EJ, et al. Diseases of the small intestine. In Ettinger SJ, et al, editors: Textbook of veterinary internal medicine, ed 6, Philadelphia: WB Saunders, 2005.
Leib MS, et al. Complications associated with 355 flexible colonscopic procedures in dogs. J Vet Intern Med. 2004;18:642.
Marks SL, et al. Evaluation of methods to diagnose Clostridium perfringens–associated diarrhea in dogs. J Am Vet Med Assoc. 1999;214:357.
Marks SL, et al. Comparison of direct immunofluorescence, modified acid-fast staining, and enzyme immunoassay techniques for detection of Cryptosporidum spp. in naturally exposed kittens. J Am Vet Med Assoc. 2004;225:1549.
Marks SL, et al. Diarrhea in kittens. In August JR, editor: Consultations in feline internal medicine, ed 5, St Louis: Elsevier/Saunders, 2006.
Mansell J, et al. Biopsy of the gastrointestinal tract. Vet Clin N Am. 2003;33:1099.
Newell SM, et al. Sonography of the normal feline gastrointestinal tract. Vet Radiol Ultra. 1999;40:40.
Patsikas MN, et al. Ultrasonographic signs of intestinal intussusception associated with acute enteritis or gastroenteritis in 19 young dogs. J Am Anim Hosp Assoc. 2003;39:57.
Patsikas MN, et al. Normal and abnormal ultrasonographic findings that mimic small intestinal intussusception in the dog. J Am Anim Hosp Assoc. 2004;40:14.
Richter KP. Endoscopic evaluation of the colon. In: McCarthy TC, editor. Veterinary endoscopy. St Louis: Elsevier/Saunders, 2005.
Rudorf H, et al. Ultrasonographic evaluation of the thickness of the small intestinal wall in dogs with inflammatory bowel disease. J Small Anim Pract. 2005;46:322.
Vaden SL, et al. Evaluation of intestinal permeability and gluten sensitivity in Soft-Coated Wheaten Terriers with familial protein-losing enteropathy, protein-losing nephropathy, or both. Am J Vet Res. 2000;61:518.
Valentine BA. Endoscopic biopsy handling and histopathology. In: McCarthy TC, editor. Veterinary endoscopy. St Louis: Elsevier/Saunders, 2005.
Washabau RJ, et al. Diseases of the large intestine. In Ettinger SJ, et al, editors: Textbook of veterinary internal medicine, ed 6, Philadelphia: WB Saunders, 2005.
Weinstein WM. Mucosal biopsy techniques and interaction with the pathologist. Gastrointest Endosc Clin N Am. 2000;10(4):555.
Willard MD, et al. Quality of tissue specimens obtained endoscopically from the duodenum of dogs and cats. J Am Vet Med Assoc. 2001;219:474.
Willard MD, et al. Gastrointestinal, pancreatic, and hepatic disorders. In Willard MD, et al, editors: Small animal clinical diagnosis by laboratory methods, ed 4, Philadelphia: WB Saunders, 2004.
Willard MD, et al. Bacterial causes of enteritis and colitis. In August JR, editor: Consultations in feline internal medicine, ed 5, St Louis: Elsevier/Saunders, 2006.
Williams DA. Exocrine pancreatic disease and pancreatitis. In Ettinger SJ, et al, editors: Textbook of veterinary internal medicine, ed 6, Philadelphia: WB Saunders, 2005.
Zajac AM, et al. Evaluation of the importance of centrifugation as a component of zinc sulfate flotation examinations. J Am Anim Hosp Assoc. 2002;38:22.