CHAPTER 31 Disorders of the Oral Cavity, Pharynx, and Esophagus
MASSES, PROLIFERATIONS, AND INFLAMMATION OF THE OROPHARYNX
SIALOCELE
Etiology
Sialoceles are accumulations of saliva in subcutaneous tissues caused by salivary duct obstruction and/or rupture and subsequent leakage of secretions into subcutaneous tissues. Most cases are probably traumatic, but some are idiopathic.
Clinical Features
A large, usually painless swelling is found under the jaw or tongue or occasionally in the pharynx. Oral cavity sialoceles may cause dysphagia, whereas those located in the pharynx often produce gagging or dyspnea. If traumatized, sialoceles may bleed or cause anorexia due to discomfort.
Diagnosis
Aspiration with a large-bore needle reveals thick fluid with some neutrophils. The fluid usually resembles mucus, strongly suggesting its salivary gland origin. Contrast radiographic procedures (contrast sialograms) sometimes define which gland is involved.
SIALOADENITIS/SIALOADENOSIS/SALIVARY GLAND NECROSIS
Etiology
The etiology is unknown, but the condition apparently has occurred as an idiopathic event as well as secondary to vomiting/regurgitation.
Clinical Features
The condition may cause a painless enlargement of one or more salivary glands (usually the submandibular). If there is substantial inflammation, animals may be dysphagic. A syndrome has been reported in which noninflammatory swelling is associated with vomiting that is responsive to phenobarbital therapy. This syndrome has no established cause and effect.
Diagnosis
Biopsy and cytology or histopathology confirm that the mass is salivary tissue and determine whether inflammation or necrosis is present.
Treatment
If there is substantial inflammation and pain, surgical removal seems most efficacious. If the patient is vomiting, a search should be made for an underlying cause. If a cause is found, it should be treated and the size of the salivary glands monitored. If no other cause for vomiting can be found, phenobarbital may be administered at anticonvulsant doses (see Chapter 67).
NEOPLASMS OF THE ORAL CAVITY IN DOGS
Etiology
Most soft tissue masses of the oral cavity are neoplasms, and most of these are malignant (i.e., melanoma, squamous cell carcinoma, fibrosarcoma). However, acanthomatous ameloblastomas (previously called epulides), fibromatous epulides (classically in Boxers), oral papillomatosis, and eosinophilic granulomas (e.g., in Siberian Huskies and Cavalier King Charles Spaniels) also occur.
Clinical Features
The most common signs of tumors of the oral cavity are halitosis, dysphagia, bleeding, or a growth protruding from the mouth. Papillomatosis and fibromatous periodontal hyperplasia are benign growths that may cause discomfort when eating and occasionally cause bleeding, mild halitosis, or tissue protrusion from the mouth. The biologic behaviors of the different tumors are presented in Table 31-1.
Diagnosis
A thorough examination of the oral cavity (which may require that the animal be under anesthesia) usually reveals a mass involving the gingiva, although the tonsillar area, hard palate, and tongue can also be affected. Diagnosis requires cytologic or histopathologic analysis, although papillomatosis and melanomas may be strongly suspected on the basis of their gross appearance. The preferred diagnostic approach in a dog with a mass of the oral cavity is to perform an incisional biopsy and to obtain thoracic and skull radiographs or a computed tomography (CT) scan of the affected area. If malignancy is a diagnostic consideration, thoracic radiographs should be obtained to evaluate for metastases (seldom seen but a very poor prognostic sign if present), and maxillary and mandibular radiographs should be obtained to check for bony involvement. Fine-needle aspiration of regional lymph nodes, even if they appear normal, is indicated to detect metastases. Melanomas may be amelanotic and can cytologically resemble fibrosarcomas, carcinomas, or undifferentiated round cell tumors. Biopsy and subsequent histopathologic analysis may be required for a definitive diagnosis.
Treatment/Prognosis
The preferred therapeutic approach in dogs with confirmed malignant neoplasms of the oral cavity and lack of clinically detectable metastases is wide, aggressive surgical excision of the mass and surrounding tissues (e.g., mandibulectomy, maxillectomy). Enlarged regional lymph nodes should be excised and evaluated histopathologically, even if they are cytologically negative for neoplasia. Early complete excision of gingival or hard palate squamous cell carcinomas, fibrosarcomas, acanthomatous epulides, and (rarely) melanomas may be curative. Acanthomatous epulis and ameloblastomas may respond to radiation therapy alone (complete surgical excision is preferred), and squamous cell carcinomas or fibrosarcomas with residual postoperative disease may benefit from postoperative adjunctive radiation therapy. Lingual squamous cell carcinomas affecting the base of the tongue and tonsillar carcinomas have a very poor prognosis; complete excision or irradiation usually causes severe morbidity. Melanomas metastasize early and have a very guarded prognosis. Chemotherapy is usually not beneficial in dogs with squamous cell carcinoma, acanthomatous epulis, and melanoma, but an oncologist should be consulted about new protocols that may provide some benefit. Piroxicam can benefit some patients with squamous cell carcinoma. Combination chemotherapy may be beneficial in some dogs with fibrosarcoma (see Chapter 77). Radiotherapy plus hyperthermia has been successful in some dogs with oral fibrosarcoma.
Papillomatosis usually resolves spontaneously, although it may be necessary to resect some of the masses if they interfere with eating. Fibromatous epulides may be resected if they cause problems.
NEOPLASMS OF THE ORAL CAVITY IN CATS
Etiology
Tumors of the oral cavity are less common in cats than in dogs, but they are usually squamous cell carcinomas, which are diagnosed and treated as described for dogs. Cats are different from dogs in that they also have sublingual squamous cell carcinomas and eosinophilic granulomas (which mimic carcinoma but have a much better prognosis).
Clinical Features
Dysphagia, halitosis, anorexia, and/or bleeding are common features of these tumors.
Diagnosis
A large, deep biopsy specimen is needed because it is crucial to differentiate malignant tumors from eosinophilic granulomas. The superficial aspect of many masses of the oral cavity is ulcerated and necrotic as a result of the proliferation of normal oral bacterial flora, making it difficult to interpret this part of the mass.
Treatment
Surgical excision is desirable. Radiation therapy and/or chemotherapy may benefit cats with incompletely excised squamous cell carcinomas not involving the tongue or tonsil.
Prognosis
In general, the prognosis for cats with squamous cell carcinomas of the tongue or tonsil is guarded to poor (see Chapter 82).
FELINE EOSINOPHILIC GRANULOMA
Clinical Features
Feline eosinophilic granuloma complex includes indolent ulcer, eosinophilic plaque, and linear granuloma; however, it has not been established that these diseases are related. Indolent ulcers are classically found on the lip or oral mucosa of middle-aged cats. Eosinophilic plaque usually occurs on the skin of the medial thighs and abdomen. Linear granuloma is typically found on the posterior aspect of the rear legs of young cats but may also occur on the tongue, palate, and oral mucosa. Severe oral involvement of an eosinophilic ulcer or plaque typically produces dysphagia, halitosis, and/or anorexia. Cats with eosinophilic gran-ulomas of the mouth may have concurrent cutaneous lesions.
Diagnosis
An ulcerated mass may be found at the base of the tongue or on the hard palate, the glossopalatine arches, or anywhere else in the mouth. A deep biopsy specimen of the mass is necessary for accurate diagnosis. Peripheral eosinophilia is inconsistently present.
Treatment
High-dose corticosteroid therapy (oral prednisolone, 2.2 to 4.4 mg/kg/day) often controls these lesions. Sometimes cats are best treated with methylprednisolone acetate injections (20 mg every 2 to 3 weeks as needed) instead of oral prednisolone. Although effective, megestrol acetate may cause diabetes mellitus, mammary tumors, and uterine problems and probably should not be used except under extreme constraints. Chlorambucil might prove useful in resistant cases.
GINGIVITIS/PERIODONTITIS
Etiology
Bacterial proliferation and toxin production, usually associated with tartar buildup, destroy normal gingival structures and produce inflammation. Immunosuppression caused by feline leukemia virus (FeLV), feline immunodeficiency virus (FIV), and/or feline calicivirus may predispose some cats to this disease.
Clinical Features
Dogs and cats may be affected. Many are asymptomatic, but halitosis, oral discomfort, refusal to eat, dysphagia, drooling, and tooth loss may occur.
Diagnosis
Visual examination of the gums reveals hyperemia around the tooth margins. Gingival recession may reveal tooth roots. Accurate diagnosis can be made through probing and oral radiographs. The stage of periodontal disease is defined by radiographs.
Treatment
Supragingival and subgingival tartar should be removed, and the crowns should be polished. Antimicrobial drugs effective against anaerobic bacteria (e.g., amoxicillin, clindamycin, metronidazole; see Drugs Used in Gastrointestinal Disorders table, pp. 481–483) may be used before and after cleaning teeth. Regular brushing of the teeth and/or oral rinsing with a veterinary chlorhexidine solution formulated for that purpose helps to control the problem.
STOMATITIS
Etiology
There are many causes of canine and feline stomatitis (Box 31-1). The clinician should always consider the possibility of immunosuppression with secondary stomatitis (e.g., FeLV, FIV, diabetes mellitus, hyperadrenocorticism).
Clinical Features
Most dogs and cats with stomatitis have thick, ropey saliva; severe halitosis; and/or anorexia caused by pain. Some animals are febrile and lose weight.
Diagnosis
A thorough oral examination usually requires that the animal be under anesthesia. Stomatitis is diagnosed by gross observation of the lesions, but an underlying cause should be sought. Biopsy is routinely indicated, as are routine clinical pathology data and radiographs of the mandible and maxilla, including the tooth roots.
Treatment
Therapy is both symptomatic (to control signs) and specific (i.e., directed at the underlying cause). Thorough teeth cleaning and aggressive antibacterial therapy (i.e., systemic antibiotics effective against aerobes and anaerobes, cleansing oral rinses with antibacterial solutions such as chlorhexidine) often help. In some animals extracting teeth that are associated with the most severely affected areas may help. Bovine lactoferrin has been reported to ameliorate otherwise resistant lesions in cats.
FELINE LYMPHOCYTIC-PLASMACYTIC GINGIVITIS/PHARYNGITIS
Etiology
An idiopathic disorder, feline lymphocytic-plasmacytic gingivitis might be caused by feline calicivirus or any stimulus producing sustained gingival inflammation. Cats appear to have an excessive oral inflammatory response that can produce marked gingival proliferation.
Clinical Features
Anorexia and/or halitosis are the most common signs. Affected cats grossly have reddened gingiva around the teeth and/or posterior pillars of the pharynx. The gingiva may be obviously proliferative in severe cases and bleed easily. Dental neck lesions often accompany the gingivitis. Teeth chattering is also occasionally seen.
Diagnosis
Biopsy of affected (especially proliferative) gingiva is needed for diagnosis. Histologic evaluation reveals a lymphocytic-plasmacytic infiltration. Serum globulin concentrations may be increased.
Treatment
There is currently no reliable therapy for this disorder. Proper cleaning and polishing of teeth and antibiotic therapy effective against anaerobic bacteria may help. High-dose corticosteroid therapy (prednisolone, 2.2 mg/kg/day) is often useful. In some severe cases, multiple tooth extractions may alleviate the source of the inflammation. However, extraction of the canine teeth should be delayed. Immunosuppressive drugs such as chlorambucil also may be tried in obstinate cases.
DYSPHAGIAS
MASTICATORY MUSCLE MYOSITIS/ATROPHIC MYOSITIS
Etiology
Masticatory muscle myositis/atrophic myositis is an idiopathic, immune-mediated disorder that affects the muscles of mastication in dogs. The syndrome has not been reported in cats.
Clinical Features
In the acute stages the temporalis and masseter muscles may be swollen and painful. However, many dogs are not presented until the muscles are severely atrophied and the mouth cannot be opened.
Diagnosis
Atrophy of the temporalis and masseter muscles and the inability to open the dog’s mouth while it is anesthetized allow the clinician to establish a presumptive diagnosis. Muscle biopsy of the temporalis and masseter muscles provides confirmation. The presence of antibodies to type 2M fibers strongly supports this diagnosis.
Treatment
High-dose prednisolone therapy (2.2 mg/kg/day) with or without azathioprine (50 mg/m2 q24 h) is usually curative. Once control has been achieved, the prednisolone and azathioprine are administered every 48 hours and then the dose of prednisolone is tapered to avoid adverse effects. However, this tapering must be done slowly to prevent recurrence (see the section on immunosuppressive drugs in Chapter 103). If needed, a gastrostomy tube may be used until the animal can eat.
CRICOPHARYNGEAL ACHALASIA/DYSFUNCTION
Etiology
The cause of cricopharyngeal achalasia/dysfunction is unknown, but it is usually congenital. There is an incoordination between the cricopharyngeus muscle and the rest of the swallowing reflex, which produces obstruction at the cricopharyngeal sphincter during swallowing (i.e., the sphincter does not open at the proper time). The problem has a genetic basis in Golden Retrievers.
Clinical Features
Primarily seen in young dogs, cricopharyngeal achalasia rarely occurs as an acquired disorder. The major sign is regurgitation immediately after or concurrent with swallowing. Some animals become anorexic, and severe weight loss may occur. Clinically, this condition may be indistinguishable from pharyngeal dysfunction.
Diagnosis
Definitive diagnosis requires fluoroscopy or cinefluoroscopy while the animal is swallowing barium or another contrast media. A young animal that is regurgitating food immediately on swallowing is suggestive of the disorder, but pharyngeal dysphagia with normal cricopharyngeal sphincter function occasionally occurs as an apparently congenital defect and must be differentiated from cricopharyngeal disease.
PHARYNGEAL DYSPHAGIA
Etiology
Pharyngeal dysphagia is primarily an acquired disorder, and neuropathies, myopathies, and junctionopathies (e.g., localized myasthenia gravis) seem to be the main cause. The inability to form a normal bolus of food at the base of the tongue and/or to propel the bolus into the esophagus is often associated with lesions of cranial nerves IX or X. Simultaneous dysfunction of the cranial esophagus may cause food retention just caudal to the cricopharyngeal sphincter.
Clinical Features
Although pharyngeal dysphagia principally is found in older animals, young animals occasionally have transient signs. Pharyngeal dysphagia often clinically mimics cricopharyngeal achalasia; regurgitation is associated with swallowing. Pharyngeal dysphagia sometimes causes more difficulty with swallowing fluids than solids. Aspiration (especially associated with liquids) is common because the proximal esophagus is often flaccid and retains food, predisposing to later reflux into the pharynx.
Diagnosis
Fluoroscopy or cinefluoroscopy while the animal is swallowing barium is typically required for diagnosis. An experienced radiologist is needed to reliably distinguish pharyngeal dysphagia from cricopharyngeal dysphagia. With the former condition, the animal does not have adequate strength to properly push boluses of food into the esophagus, whereas in the latter the animal has adequate strength but the cricopharyngeal sphincter stays shut or opens at the wrong time during swallowing, thereby preventing normal movement of food from the pharynx to the proximal esophagus. It appears that some cases may be detected by electromyography of laryngeal, pharyngeal, and esophageal muscles.
Treatment
Although cricopharyngeal myotomy is often curative for animals with cricopharyngeal achalasia, it may be disastrous for animals with pharyngeal dysphagias because it allows food retained in the proximal esophagus to more easily reenter the pharynx and be aspirated. The clinician must either bypass the pharynx (e.g., gastrostomy tube) or resolve the underlying cause (e.g., treat or control myasthenia gravis).
ESOPHAGEAL WEAKNESS/MEGAESOPHAGUS
CONGENITAL ESOPHAGEAL WEAKNESS
Etiology
The cause of congenital esophageal weakness (i.e., congenital megaesophagus) is unknown. There is no evidence of demyelination or neuronal degeneration, and vagal efferent innervation appears to be normal.
Clinical Features
Affected animals (primarily dogs) are usually presented because of “vomiting” (actually regurgitation) with or without weight loss, coughing, or fever from pneumonia. Occasionally, coughing and other signs of aspiration tracheitis and/or pneumonia may be the only signs reported by the owner.
Diagnosis
The clinician usually first determines from the history that regurgitation appears likely (see p. 353). Then, after radiographic findings show generalized esophageal dilation that is not associated with obstruction (see Fig. 29-3, A), the clinician can presumptively diagnose esophageal weakness. Diverticula in the cranial thorax caused by esophageal weakness occur occasionally and are often confused with vascular ring obstruction (Fig. 31-1). Congenital, rather than acquired, disease is suspected if the regurgitation and/or aspiration began when the pet was young. If clinical features have been relatively mild or intermittent, the diagnosis might not be made until the animal is older, but consideration of the history should suggest that signs have been present since the animal was young. Endoscopy is not as useful as contrast radiographs for diagnosing this disorder. Collies may have dermatomyositis, which also causes esophageal weakness. Some breeds (e.g., Miniature Schnauzers, Great Danes, Dalmatians) appear to be at increased risk.
Treatment
Congenital esophageal weakness currently cannot be cured or resolved by medical therapy, although cisapride (0.25 mg/kg) seemingly ameliorates signs in rare cases (probably in patients with substantial gastroesophageal reflux). Conservative dietary management is used to try to prevent further dilation and aspiration. Classically, the animal is fed a gruel from an elevated platform that requires the pet to stand on its rear legs. In this manner, the cervical and thoracic esophagus is nearly vertical when food is ingested, which allows gravity to aid food passing through the esophagus and into the stomach. This position should be maintained for 5 to 10 minutes after the animal has finished eating and drinking. If the dog cannot stand, it may be backed into a corner, forced to sit on its haunches, and have its front legs lifted while the corner prevents the dog from falling over. Alternatively, it may be fed on stairs so that it is at least at a 45-degree angle when eating. Feeding several small meals a day also helps prevent esophageal retention.
Some animals do better if fed dry or canned dog food free choice throughout the day from such a platform. It is impossible to predict whether a given dog will respond better to gruel or dry dog food. Therefore trial and error are necessary to determine the diet that works best for a particular animal. In some dogs the dilated esophagus may partially return to normal size and function. Even if the esophagus remains dilated, some dogs may be managed by dietary change and have a good quality of life.
Gastrostomy tubes bypass the esophagus and can provide some relief from regurgitation and/or aspiration. However, animals may still regurgitate saliva and, if there is gastroesophageal reflux, may also regurgitate food. Some animals with gastrostomy tubes respond well for varying periods of time.
ACQUIRED ESOPHAGEAL WEAKNESS
Etiology
Acquired esophageal weakness in dogs is usually caused by a neuropathy, myopathy, or junctionopathy (e.g., myasthenia gravis; see Box 28-5). German Shepherds, Golden Retrievers, and Irish Setters might have increased risk. In cats esophagitis may be a cause of acquired esophageal weakness.
Clinical Features
Acquired esophageal weakness primarily occurs in dogs. The patients usually are presented because of “vomiting” (actually regurgitation), but some present with a cough and little or no obvious regurgitation (e.g., regurgitated material is sometimes reswallowed or re-eaten by the animal). Weight loss may occur if the dog regurgitates most of its food.
Diagnosis
The initial diagnostic step is to document that regurgitation, rather than vomiting, is occurring (see p. 353). Acquired esophageal weakness is usually diagnosed by finding generalized esophageal dilation without evidence of obstruction on plain and contrast radiographs (see Fig. 29-3, A). The severity of clinical signs does not always correlate with the magnitude of radiographic changes. Some symptomatic animals have segmental weakness primarily affecting the cervical esophagus, just behind the cricopharyngeus muscle. However, normal dogs often have minimal amounts of barium retained in this location, so it is important to distinguish insignificant from clinically important retention. It is important to rule out lower esophageal spasm and stricture, which, though very rare, radiographically mimic esophageal weakness but require surgical treatment. Ideally, fluoroscopy should be used to look for evidence of gastroesophageal reflux, which may benefit from prokinetic therapy (e.g., cisapride).
It is important to search for underlying causes of acquired esophageal weakness (see Box 28-5). The titer of antibodies to acetylcholine receptors (indicative of myasthenia gravis) should be measured in dogs. “Localized” myasthenia may affect only the esophagus and/or oropharyngeal muscles. An adrenocorticotropic hormone (ACTH)–stimulation test is indicated to look for otherwise occult hypoadrenocorticism (even if serum electrolyte concentrations are normal). Serum thyroxine, free thyroxine, and thyroid-stimulating hormone (TSH) concentrations may reveal hypothyroidism, which can very rarely be associated with esophageal dysfunction. Tests of thyroid gland function must be interpreted carefully because of potential confusion regarding the euthyroid sick syndrome (see Chapter 51). Electromyography may reveal generalized neuropathies or myopathies. Dysautonomia occurs occasionally and is suspected on the basis of clinical signs (i.e., dilated colon, dry nose, dilated pupils, keratocon junctivitis sicca, and/or bradycardia that responds poorly to atropine). Gastric outflow obstruction in cats can cause vomiting with secondary esophagitis. Other causes are rarely found (see Box 28-5). If an underlying cause cannot be found, the disease is termed idiopathic acquired esophageal weakness (i.e., idiopathic acquired megaesophagus).
Treatment
Dogs with acquired megaesophagus caused by localized myasthenia gravis or hypoadrenocorticism often respond to appropriate therapy (see Chapters 53 and 71). Localized myasthenia seems ultimately to respond best to immunosuppressive therapy (e.g., azathioprine), although pyridostigmine may help initially. Gastroesophageal reflux may respond to prokinetic and antacid therapy (cisapride at 0.25 mg/kg and omeprazole at 0.7 to 1.5 mg/kg are preferred). If the disease is idiopathic, conservative dietary therapy as described for congenital esophageal weakness is the only recourse. Although some dogs with congenital esophageal weakness regain variable degrees of esophageal function, this is rare in those with idiopathic acquired esophageal weakness. Severe esophagitis may cause secondary esophageal weakness, which resolves after appropriate therapy (discussed in more detail later in this chapter). Gastrostomy tubes diminish the potential for aspiration, ensure positive nitrogen balance, and help treat esophagitis if present. Some dogs benefit from the long-term use of a gastrostomy tube, but others continue to regurgitate and aspirate as a result of severe gastroesophageal reflux or simply the accumulation of large amounts of saliva in the esophagus.
Prognosis
All animals with acquired esophageal weakness are at risk for aspiration pneumonia and sudden death. If the underlying cause can be treated and the esophageal dilation and weakness can be resolved, the prognosis is good because the risk of aspiration is eliminated. The prognosis is guarded if the animal with idiopathic megaesophagus responds to dietary management (it is still at risk) and very poor if the animal does not respond to this protocol.
ESOPHAGITIS
Etiology
Esophagitis is principally caused by gastroesophageal reflux, persistent vomiting of gastric acid, esophageal foreign objects, and caustic agents. Pills (e.g., tetracycline) may be retained in the esophagus if they are not washed down with water or food and are thought to cause severe esophagitis in cats. An association between distal esophagitis (ostensibly caused by gastroesophageal reflux) and upper respiratory disease in brachycephalic dogs has been suggested.
Clinical Features
Regurgitation is expected, although anorexia and drooling may predominate if swallowing is painful. If a caustic agent (e.g., disinfectant) is ingested, the mouth and tongue are often hyperemic and/or ulcerated; anorexia is the primary sign.
Diagnosis
A history of vomiting followed by both vomiting and regurgitation suggests esophagitis secondary to excessive exposure to gastric acid. This sign may occur in parvoviral enteritis and in various other disorders. Likewise, regurgitation or anorexia begining shortly after an anesthetic procedure may indicate esophagitis caused by reflux. Plain and contrast radiographs may reveal hiatal hernias, gastroesophageal reflux, or esophageal foreign bodies. Contrast esophagrams do not reliably detect esophagitis; esophagoscopy with or without biopsy is needed to establish a definitive diagnosis.
Treatment
Decreasing gastric acidity, preventing reflux of gastric contents into the esophagus, and protecting the denuded esophagus are the hallmarks of treatment. H2 receptor antagonists (see Table 30-4) may be used, but proton pump inhibitors (e.g., omeprazole) are superior for decreasing gastric acidity, a critical factor in these animals. However, because it may take 2 to 5 days for omeprazole to achieve maximum efficacy, famotidine may be used concurrently during initial therapy. Metoclopramide stimulates gastric emptying, resulting in less gastric volume to reflux into the esophagus, but cisapride (0.25 to 0.5 mg/kg) tends to be more effective. Sucralfate (particularly suspensions) might protect denuded esophageal mucosa (see Table 30-5), but its usefulness is unknown. Antibiotics effective against anaerobes (e.g., amoxicillin, clindamycin; see Drugs Used in Gastrointestinal Disorders, pp. 481–483) have been used but are of unknown value. A gastrostomy feeding tube helps to protect the esophagus while the mucosa is healing and ensures a positive nitrogen balance. Corticosteroids (e.g., prednisolone, 1.1 mg/kg/day) may be administered in an attempt to prevent cicatrix, but their efficacy is dubious. Hiatal hernias may need to be surgically repaired.
HIATAL HERNIA
Etiology
Hiatal hernia is a diaphragmatic abnormality that allows part of the stomach (usually the cardia) to prolapse into the thoracic cavity. In severe cases it allows gastroesophageal reflux. The condition seems to be primarily congenital.
Clinical Features
Sharpei dogs seem to be predisposed to this disorder. Regurgitation is the primary sign in symptomatic individuals, but some animals are asymptomatic.
Diagnosis
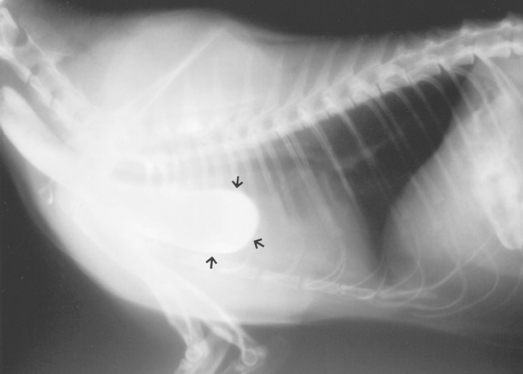
Plain radiographs or positive-contrast esophagrams may reveal gastric herniation into the thorax (Fig. 31-2); however, herniation may be intermittent and difficult to detect. It is sometimes necessary to put pressure on the abdomen during the radiographic procedure to cause displacement of the stomach during the study. Hiatal hernias are occasionally found endoscopically.
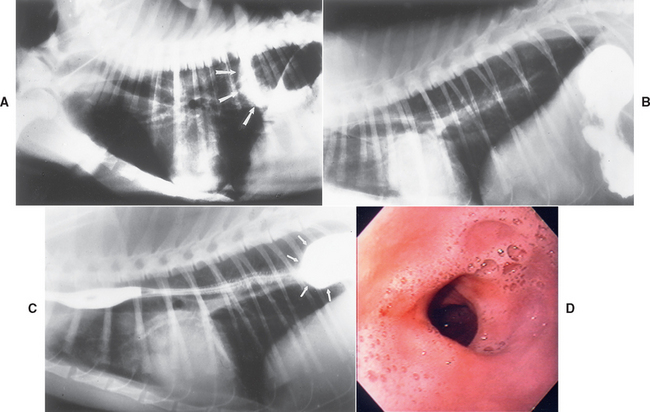
FIG 31-2 A, Lateral radiograph of a dog with a hiatal hernia showing the gastric shadow extending cranial to the diaphragm (arrows). B, Lateral view of contrast esophagram of a cat with hiatal hernia. There is no evidence of hernia on this radiograph because it has apparently slid back into the abdomen. C, Lateral view of contrast esophagram of the cat in B. The body of the stomach has now slid into the thoracic cavity (arrows), confirming that a hiatal hernia is present. D, An endoscopic image of the lower esophageal sphincter (LES) area of a dog with a hiatal hernia. Gastric rugal folds can be seen.
(A, Courtesy Dr. Russ Stickle, Michigan State University, East Lansing, Mich. B and C, Courtesy Dr. Royce Roberts, University of Georgia, Athens, Ga.)
Treatment
If the hiatal hernia is symptomatic at an early age, surgery is more likely to be required to correct it. If signs of hiatal hernia first appear later in life, aggressive medical management of gastroesophageal reflux (e.g., cisapride, omeprazole) is often sufficient. If medical management is not successful, surgery can be considered.
DYSAUTONOMIA
Etiology
Dysautonomia in dogs and cats is an idiopathic condition that causes loss of autonomic nervous system function. In at least some circumstances, it may be due to a clostridial toxin.
Clinical Features
Clinical signs vary substantially. Megaesophagus and subsequent regurgitation are common (not invariable); however, dysuria and a distended urinary bladder, mydriasis and lack of pupillary light response, dry mucous membranes, weight loss, constipation, vomiting, poor anal tone, and/or anorexia have all been reported. There appear to be geographic areas (e.g., Missouri and surrounding states) that currently have an increased incidence of the disease.
Diagnosis
Dysautonomia is usually first suspected clinically by finding dysuria, dry mucous membranes, and abnormal pupillary light responses. Radiographs revealing distention of multiple areas of the alimentary tract (e.g., esophagus, stomach, small intestine) also are suggestive. A presumptive, antemortem diagnosis is usually made by observing the effects of pilocarpine on pupil size after 1 to 2 drops of 0.05% pilocarpine are placed in one eye only. Finding that the treated eye rapidly constricts whereas the untreated eye does not is consistent with dysautonomia. Similarly, finding that a dysuric dog with a large urinary bladder can urinate after subcutaneous administration of 0.04 mg bethanechol/kg is also suggestive (although not all affected animals respond). Definitive diagnosis requires histopathology of autonomic ganglia, which can be obtained only at necropsy.
Treatment
Treatment is palliative. Bethanechol can be given (1.25 to 5 mg once daily) to aid in urinary evacuation. The urinary bladder should be expressed as needed. Gastric prokinetics (e.g., cisapride) may help lessen vomiting. Antibiotics may be administered for aspiration pneumonia secondary to megaesophagus.
ESOPHAGEAL OBSTRUCTION
VASCULAR RING ANOMALIES
Etiology
Vascular ring anomalies are congenital defects. An embryonic aortic arch persists, trapping the esophagus in a ring of tissue. Persistent right fourth aortic arch (PRAA) is the most commonly recognized vascular anomaly (see Chapter 5).
Clinical Features
Vascular ring anomalies occur in both dogs and cats. Regurgitation is the most common presenting complaint, although signs of aspiration may occur. Clinical features often begin shortly after the animal eats solid food for the first time. Some animals have relatively minor clinical signs and are not diagnosed until they are several years old.
Diagnosis
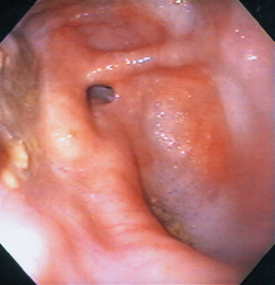
Definitive diagnosis is usually made by contrast esophagram (see Fig. 29-3, B). Typically, the esophagus cranial to the heart is dilated, whereas the esophagus caudal to the heart is normal. In rare cases the entire esophagus is dilated (the result of concurrent megaesophagus) except for a narrowing at the base of the heart. It has been suggested that if focal leftward deviation of the trachea is seen at the cranial border of the heart in the ventrodorsal or dorsoventral projections, this is sufficient to diagnose PRAA in young dogs that are regurgitating food. Endoscopically, the esophagus has an extramural narrowing (Fig. 31-3; i.e., not a mucosal proliferation or scar) near the base of the heart.
Treatment
Surgical resection of the anomalous vessel is necessary. Conservative dietary management (i.e., gruel diet) by itself is inappropriate because the dilation will persist and probably progress. In particular, the animal will be at risk for foreign body occlusion at the site of the PRAA. Dietary therapy may benefit some animals postoperatively.
Prognosis
Most patients improve dramatically after surgery. However, there are exceptions, and the more severe the preoperative dilation, the more likely regurgitation will continue postoperatively. Some dogs have concomitant esophageal weakness. A guarded prognosis is appropriate. If a postsurgical stricture occurs, esophageal ballooning or a second surgical procedure may be considered.
ESOPHAGEAL FOREIGN OBJECTS
Etiology
Almost anything may lodge in the esophagus, but objects with sharp points (e.g., bones, fishhooks) are probably most common. Most obstructions occur at the thoracic inlet, the base of the heart, or immediately in front of the diaphragm.
Clinical Features
Dogs are more commonly affected because of their less discriminating eating habits. Regurgitation or anorexia secondary to esophageal pain is common. Acute onset of regurgitation (as opposed to vomiting) is suggestive of esophageal foreign body. Clinical signs depend on where the obstruction occurs, whether it is complete or partial, and whether esophageal perforation has occurred. Complete obstructions cause regurgitation of solids and liquids, whereas partial obstructions may allow passage of liquids to the stomach. If an esophageal foreign object is impinging on airways, acute dyspnea may occur. Esophageal perforation usually causes fever and anorexia; dyspnea may occur as the result of pleural effusion or pneumothorax. Subcutaneous emphysema rarely occurs.
Diagnosis
Plain thoracic radiographs reveal most esophageal foreign bodies (see Fig. 29-2), although the clinician may have to search carefully to find poultry bones or other food items that are even less radiodense. It is also important to look for evidence of esophageal perforation (i.e., pneumothorax, pleural effusion, fluid in the mediastinum). Esophagrams are rarely necessary; esophagoscopy is diagnostic and typically therapeutic.
Treatment
Foreign objects are best removed endoscopically unless (1) they are too firmly lodged to pull free or (2) radiographs suggest perforation. Thoracotomy is indicated in these two situations, although in rare cases perforations may be treated medically. Objects that cannot be moved should not be pulled on vigorously because of the risk of creating or enlarging a perforation. An object should be pushed into the stomach only when the clinician is confident that there are no sharp edges on the other side of the foreign object. During the procedure the esophagus should be insufflated carefully to avoid rupturing weakened areas or causing tension pneumothorax. After an object has been removed, the esophageal mucosa should be reexamined endoscopically to evaluate the damage caused by the object. Thoracic radiographs should be repeated to look for pneumothorax, an indication of perforation. Treatment after foreign body removal may include antibiotics, H2 receptor antagonists or proton pump inhibitors, prokinetic agents, gastrostomy feeding tube, and/or corticosteroids (prednisolone, 1.1 mg/kg/day), depending on residual damage. Perforation usually requires thoracotomy to clean out septic debris and close the esophageal defect.
Prognosis
The prognosis for animals with esophageal foreign bodies without perforation is usually good, but the presence of perforation warrants a guarded prognosis depending on the severity of thoracic contamination. Cicatrix formation with obstruction is possible if substantial mucosal damage occurs.
ESOPHAGEAL CICATRIX
Etiology
Prior esophagitis from any cause may produce a stricture. Severe, deep inflammation of the esophagus (e.g., subsequent to foreign bodies or severe gastroesophageal reflux) is usually required for cicatrix to occur.
Clinical Features
Esophageal cicatrix occurs in both dogs and cats. The main sign is regurgitation (especially of solids). Some animals are clinically anorexic as a result of pain experienced when food becomes lodged at the stricture by forceful esophageal peristalsis.
Diagnosis
Partial obstructions may be difficult to diagnose. Positive-contrast esophagrams (often using barium mixed with food) are necessary (Fig. 31-4). Esophagoscopy is definitive, but a partial stricture may not be obvious in large dogs unless the endoscopist is experienced and the esophagus is carefully inspected.
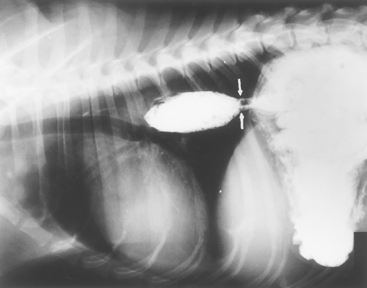
FIG 31-4 Lateral contrast esophagram using liquid barium mixed with moist food. Partial stricture (arrows) is preventing the bolus from readily entering the stomach. This stricture was not detected with barium paste, even when viewed fluoroscopically. However, when the barium-food mixture was used, the stricture was obvious and material was retained for minutes before passing. Endoscopically, there was a band of fibrous connective tissue at this spot.
Treatment
Treatment consists of correcting the suspected cause (e.g., esophagitis) and/or widening the stricture by ballooning or bougienage. Surgical resection is not recommended because iatrogenic strictures at the anastomotic site are common. Ballooning is less traumatic, has less chance of perforation, and may be accomplished during esophagoscopy. Angioplasty catheters or esophageal dilation balloons are more useful than Foley catheters because the former are less likely to slide to one side of the obstruction during inflation. Bougienage can more easily cause a rupture, but it is relatively safe and equally effective if done by a trained individual. After the stricture has been dilated, antibiotics and/or corticosteroids (prednisolone, 1.1 mg/kg/day) are often administered to help prevent infection and stricture reformation; however, their efficacy is unknown. Intralesional steroid injections performed endoscopically have been tried in severe cases, but their value is uncertain at this time. If esophagitis is present, it should be treated aggressively. Some animals are cured after one ballooning, whereas others require multiple procedures.
Early identification and appropriate treatment of high-risk animals (i.e., those with severe esophagitis or after foreign object removal) help decrease the likelihood of stricture formation. Resolving esophagitis decreases inflammation and lessens fibrous connective tissue formation. The efficacy of corticosteroids is uncertain, but they are worth trying in selected cases.
Prognosis
The shorter the length of esophagus involved and the sooner the corrective procedure is performed, hopefully the better the prognosis. Animals with extensive, mature strictures and/or continuing esophagitis often need repeated dilatory procedures and have a more guarded prognosis. Most animals with benign esophageal strictures can be helped. Long-term gastrostomy tubes may be necessary in some animals.
ESOPHAGEAL NEOPLASMS
Etiology
Primary esophageal sarcomas in dogs are often due to Spirocerca lupi. Primary esophageal carcinomas are of unknown etiology. Leiomyomas and leiomyosarcomas are found at the lower esophageal sphincter in older dogs. Thyroid carcinomas and pulmonary alveolar carcinomas may invade the esophagus in dogs. Squamous cell carcinomas are the most common esophageal neoplasm in cats.
Clinical Features
Dogs and cats with primary esophageal tumors may be asymptomatic until the tumor is far advanced, and these animals are diagnosed fortuitously when thoracic radiographs are obtained for other reasons. Regurgitation, anorexia, and/or fetid breath may occur if the tumor is large or causes esophageal dysfunction. If the esophagus is involved secondarily, clinical signs may result from esophageal dysfunction or tumor effects on other tissues.
Diagnosis
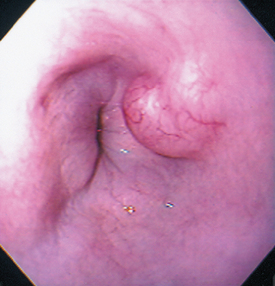
Plain thoracic radiographs may reveal a soft tissue density in the caudal lung fields. These tumors may be difficult to discern radiographically from pulmonary lesions and usually require contrast esophagrams to make this distinction (Fig. 31-5). Esophagoscopy easily locates intraluminal and intramural masses (Fig. 31-6) or strictures and is sensitive in finding extraluminal masses causing esophageal stricture (i.e., the endoscopist will not be able to normally distend the esophageal lumen). Retroflexing the tip of an endoscope while it is within the stomach is the best method of identifying lower esophageal sphincter leiomyomas and leiomyosarcomas.
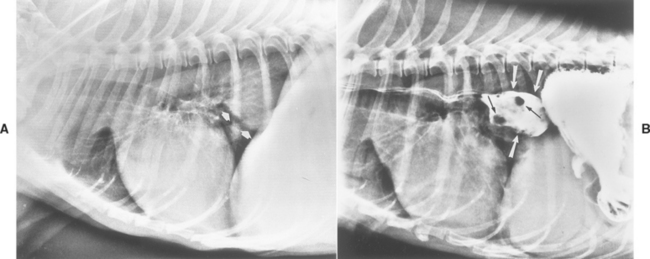
FIG 31-5 A, Lateral thoracic radiograph of a dog with a previously unsuspected mass (arrows) not obviously associated with the esophagus. B, Contrast esophagram in the same dog demonstrates that the esophagus is dilated (large arrows) and that there are intraesophageal filling defects (small arrows) in this dilated area. This dog had a primary esophageal carcinoma.
(A from Allen D, editor: Small animal medicine, Philadelphia, 1991, JB Lippincott.)
Treatment
Surgical resection is rarely curative (except for leiomyomas at the lower esophageal sphincter) because of the advanced nature of most esophageal neoplasms when they are diagnosed. Resection may be palliative. Photodynamic therapy may be beneficial in dogs and cats with small superficial esophageal neoplasms.
Bexfield NH, et al. Esophageal dysmotility in young dogs. J Vet Intern Med. 2006;20:1314.
Boydell P, et al. Sialadenosis in dogs. J Am Vet Med Assoc. 2000;216:872.
Buchanan JW. Tracheal signs and associated vascular anomalies in dogs with persistent right aortic arch. J Vet Intern Med. 2004;18:510.
Davidson AP, et al. Inheritance of cricopharygeal dysfunction in Golden Retrievers. Amer J Vet Res. 2004;65:344.
DeBowes LJ. Feline stomatitis and faucitis. In: Bonagura JD, editor. Current veterinary therapy XIII. Philadelphia: WB Saunders, 2000.
Gibbon KJ, et al. Phenobarbital-responsive ptyalism, dysphagia, and apparent esophageal spasm in a German Shepherd puppy. J Am Anim Hosp Assoc. 2004;40:230.
Graham JP, et al. Esophageal transit of capsules in clinically normal cats. Am J Vet Res. 2000;61:655.
Gualtieri M. Esophagoscopy. Vet Clinics N Am. 2001;31:605.
Gualtieri M, et al. Reflux esophagitis in three cats associated with metaplastic columnar esophageal epithelium. J Am Anim Hosp Assoc. 2006;42:65.
Han E, et al. Feline esophagitis secondary to gastroesophageal reflux disease: clinical signs and radiographic, endoscopic, and histopathologic findings. J Am Anim Hosp Assoc. 2003;39:161.
Harkin KR, et al. Dysautonomia in dogs: 65 cases (1993-2000). J Am Vet Med Assoc. 2002;220:633.
Jergans AE. Diseases of the esophagus. In Ettinger SJ, et al, editors: Textbook of veterinary internal medicine, ed 6, Philadelphia: WB Saunders, 2005.
Leib MS, et al. Endoscopic balloon dilation of benign esophageal strictures in dogs and cats. J Vet Intern Med. 2001;15:547.
Mears EA, et al. Canine megaesophagus. In: Bonagura JD, editor. Current veterinary therapy XIII. Philadelphia: WB Saunders, 2000.
Melendez LD, et al. Suspected doxycyline-induced esophagitis with esophageal stricture formation in three cats. Fel Pract. 2000;28:10.
Moore AH. Removal of oesophageal foreign bodies in dogs: use of the fluoroscopic method and outcome. J Small Anim Pract. 2001;42:227.
Moses L, et al. Esophageal motility dysfunction in cats: a study of 44 cases. J Am Anim Hosp Assoc. 2000;36:309.
Niles JD, et al. Resolution of dysphagia following cricopharyngeal myectomy in six young dogs. J Small Anim Pract. 2001;42:32.
Nunn R, et al. Association between Key-Gaskell syndrome and infection by Clostridium botulinum type C/D. Vet Rec. 2004;155:111.
O’Brien DP, et al. Diagnosis and management of dysautonomia in dogs. In: Bonagura JD, editor. Current veterinary therapy XIII. Philadelphia: WB Saunders, 2000.
Poncet CM, et al. Prevalence of gastrointestinal tract lesions in 73 brachycephalic dogs with upper respiratory syndrome. J Small Anim Pract. 2005;46:273.
Ranen E, et al. Spirocercosis-associated esophageal sarcomas in dogs a retrospective study of 17 cases (1997-2003). Vet Parasitol. 2004;119:209.
Ryckman LR, et al. Dysphagia as the primary clinical abnormality in two dogs with inflammatory myopathy. J Am Vet Med Assoc. 2005;226:1519-1523.
Sale C, et al. Results of transthoracic esophagotomy retrieval of esophageal foreign body obstructions in dogs: 14 cases (2000–2004). J Am Anim Hosp Assoc. 2006;42:450.
Schmidt BR, et al. Evaluation of piroxicam for the treatment of oral squamous cell carcinoma in dogs. J Am Vet Med Assoc. 2001;218:1783.
Sellon RK, et al. Esophagitis and esophageal strictures. Vet Clinics N Am. 2003;33:945.
Warnock JJ, et al. Surgical management of cricopharyngeal dysphagia in dogs: 14 cases (1989-2001). J Am Vet Med Assoc. 2003;223:1462-1468.
Wilson DV, et al. Postanesthetic esophageal dysfunction in 13 dogs. J Am Anim Hosp Assoc. 2004;40:455.
 TABLE 31-1
TABLE 31-1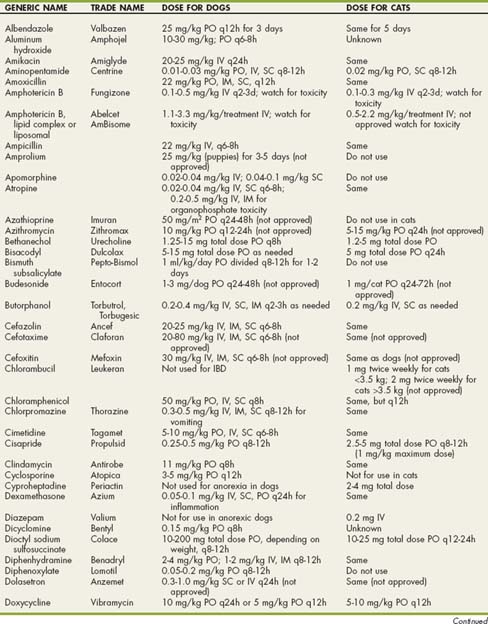
 BOX 31-1
BOX 31-1