CHAPTER 32 Disorders of the Stomach
GASTRITIS
ACUTE GASTRITIS
Etiology
Ingestion of spoiled or contaminated foods, foreign objects, toxic plants, chemicals, and/or irritating drugs (e.g., nonsteroidal antiinflammatory drugs [NSAIDs]) are common causes of acute gastritis. Infectious, viral, and bacterial causes occur but are not well defined in dogs and cats.
Clinical Features
Dogs are more commonly affected than cats by acute gastritis, probably because of their less discriminating eating habits. Signs usually consist of acute onset of vomiting; food and bile are typically vomited, although small amounts of blood may be present. Affected animals are typically uninterested in food and may or may not feel sick. Fever and abdominal pain are uncommon.
Diagnosis
Unless the animal was seen eating some irritative substance, acute gastritis is usually a presumptive diagnosis of exclusion based on history and physical examination findings. Abdominal imaging and/or clinical pathologic data are indicated if the animal is severely ill or if other disease is suspected. After alimentary foreign body, obstruction, parvoviral enteritis, uremia, diabetic ketoacidosis, hypoadrenocorticism, hepatic disease, hypercalcemia, and pancreatitis are ruled out, acute gastritis is a reasonable tentative diagnosis. If the anorexia/vomiting resolves after 1 to 2 days of symptomatic and supportive therapy, the tentative diagnosis is generally assumed to be correct (pancreatitis is still possible; see Chapter 40). Gastroscopy in such animals might reveal bile or gastric erosions/hyperemia.
Because acute gastritis is a diagnosis of exclusion and its signs are suggestive of various other disorders (e.g., foreign bodies, intoxication), good history taking and physical examination are mandatory. The owner should monitor the pet, and if the animal’s condition worsens or does not improve within 1 to 3 days, imaging, a complete blood count (CBC), a serum biochemistry profile, and urinalysis are indicated.
Treatment
Parenteral fluid therapy and the withholding of food and water for 24 hours often suffice to control vomiting. If the vomiting persists or is excessive, or if the animal becomes depressed because of the vomiting, central-acting antiemetics (e.g., prochlorperazine, ondansetron, maropitant) may be administered parenterally (see p. 404). When feeding begins, small amounts of cool water are offered frequently. If the animal drinks without vomiting, small amounts of a bland diet (e.g., one part cottage cheese and two parts potato; one part boiled chicken and two parts potato) are offered. Antibiotics and corticosteroids are rarely indicated.
HEMORRHAGIC GASTROENTERITIS
Clinical Features
Hemorrhagic gastroenteritis occurs in dogs and is more severe than acute gastritis, typically causing profuse hematemesis and/or hematochezia. Classically occurring in smaller breeds that have not had access to garbage, this disorder has an acute course that can rapidly produce a critically ill animal. In severe cases the animal may be moribund by the time of presentation.
Diagnosis
These animals are typically hemoconcentrated (i.e., packed cell volume [PCV] ≥ 55%) with normal plasma total protein concentrations. The acute onset of typical clinical signs plus marked hemoconcentration allows a presumptive diagnosis. Thrombocytopenia and renal or prerenal azotemia may be seen in severely affected animals.
Treatment
Aggressive fluid therapy is initiated to treat or prevent shock, disseminated intravascular coagulation (DIC) secondary to hypoperfusion, and renal failure secondary to hypovolemia. Parenteral antibiotics (e.g., ampicillin, chloramphenicol; see pp. 481–483) are often used because of the fear that intestinal bacteria are proliferating, but their value has not been definitively established. If the patient becomes severely hypoalbuminemic during fluid therapy, synthetic colloids or plasma may be required.
CHRONIC GASTRITIS
Etiology
There are several types of chronic gastritis (e.g., lymphocytic/plasmacytic, eosinophilic, granulomatous, atrophic). Lymphocytic-plasmacytic gastritis might be an immune and/or inflammatory reaction to a variety of antigens. Helicobacter organisms might be responsible for such a reaction in some animals (especially cats). Physaloptera rara has seemingly been associated with a similar reaction in some dogs. Eosinophilic gastritis may represent an allergic reaction, probably to food antigens. Atrophic gastritis may be the result of chronic gastric inflammatory disease and/or immune mechanisms. Ollulanus tricuspis may cause granulomatous gastritis in cats.
Clinical Features
Chronic gastritis appears to be more common in cats than in dogs and may or may not be associated with chronic enteritis (e.g., inflammatory bowel disease). Anorexia and vomiting are the most common signs in affected dogs and cats. The frequency of vomiting varies from once weekly to many times per day. Some animals have only anorexia, ostensibly as a result of low-grade nausea.
Diagnosis
Clinical pathologic findings are not diagnostic, although eosinophilic gastritis inconsistently causes peripheral eosinophilia. Imaging sometimes documents mucosal thickening. Diagnosis requires gastric mucosal biopsy, and endoscopy is the most cost-effective method of obtaining these samples. Gastritis may be very localized, and endoscopy allows multiple biopsies over the entire mucosal surface, whereas surgical biopsy typically results in one sample that is taken blindly. Gastric biopsy should always be performed, regardless of the visual mucosal appearance. It must be remembered that enteritis is far more common than gastritis (which is why duodenal biopsies are usually more important than gastric biopsies). Gastric lymphoma can be surrounded by lymphocytic inflammation, and obtaining inappropriately superficial biopsy specimens may result in an incorrect diagnosis of inflammatory disease. Appropriate use of a scope with a 2.8-mm biopsy channel will usually prevent this misdiagnosis (unless the tumor is in the muscular layers of the stomach). Meaningful histopathologic interpretation of alimentary tissue can be difficult; the clinician should not hesitate to request a second histologic opinion if the diagnosis does not fit the patient or the response (or lack thereof) to therapy. If Ollulanus tricuspis is suspected, vomitus or gastric washings should be examined for the parasites, but they might also be found in gastric biopsy specimens. Physaloptera organisms are visible endoscopically.
Treatment
Lymphocytic-plasmacytic gastritis sometimes responds to dietary therapy (e.g., low-fat, low-fiber, elimination diets) alone (see p. 397). If such therapy is inadequate, corticosteroids (e.g., prednisolone, 2.2 mg/kg/day) can be used concurrently. Even if corticosteroids are required, dietary therapy may ultimately allow one to administer a substantially decreased dose, thus avoiding glucocorticoid adverse effects. If corticosteroid therapy is necessary, the dose should be gradually decreased to find the lowest effective dose. However, the dose should not be tapered too quickly after obtaining a clinical response or the clinical signs may return and be more difficult to control than they were initially. In rare cases, azathioprine or similar drugs will be necessary (see Chapter 30). Concurrent use of H2 receptor antagonists is sometimes beneficial. Ulceration should be treated as discussed on page 436.
Canine eosinophilic gastritis usually responds well to a strict elimination diet. If dietary therapy alone fails, corticosteroid therapy (e.g., prednisolone, 1.1 to 2.2 mg/kg/day) in conjunction with diet is usually effective. Feline hypereosinophilic syndrome responds poorly to most treatments.
Atrophic gastritis and granulomatous gastritis are more difficult to treat than lymphocytic-plasmacytic or canine eosinophilic gastritis. Diets low in fat and fiber (e.g., one part cottage cheese and two parts potato) may help control signs. Atrophic gastritis may respond to antiinflammatory, antacid, and/or prokinetic therapy; the latter is designed to keep the stomach empty, especially at night. Granulomatous gastritis is uncommon in dogs and cats and does not respond well to dietary or corticosteroid therapy.
Prognosis
The prognosis for canine and feline lymphocytic-plasmacytic gastritis is often good with appropriate therapy. Some researchers have suggested that lymphoma has been known to develop in cats with lymphocytic gastritis; however, it is possible that the original diagnosis of lymphocytic gastritis was incorrect or that lymphoma developed independently of the gastritis.
The prognosis for canine eosinophilic gastritis is typi-cally good. Feline eosinophilic gastritis can be a component of hypereosinophilic syndrome, which typically responds poorly to treatment. Hypereosinophilic syndrome has a guarded prognosis.
HELICOBACTER-ASSOCIATED DISEASE
Etiology
Helicobacter pylori is the principal spirochete found in human gastric mucosa, whereas Helicobacter felis, Helicobacter heilmannii, Helicobacter bizzozeronii, and Helicobacter salomonis may be the principal gastric spirochetes in dogs and cats. However, H. pylori has been found in cats.
Clinical Features
People with symptomatic H. pylori infections usually develop ulceration and gastritis with neutrophilic infiltrates. They can also develop a lymphocytic lesion that is indistinguishable from lymphoma but that can be cured with antibiotic therapy. Dogs and cats with gastric Helicobacter infections may have nausea, anorexia, and/or vomiting associated with lymphocytic and occasionally neutrophilic infiltrates; however, most dogs and cats with gastric Helicobacter infections are asymptomatic. Because so many infected animals are asymptomatic, the cause and effect have not been clearly established between Helicobacter organisms and canine or feline gastric disease. Cats colonized with H. pylori seem to have more severe histologic lesions than those with H. felis, which in turn may be associated with more severe lesions than those with H. heilmannii. Reasonable anecdotal evidence seems to suggest that some ill animals with gastric Helicobacter infections have their signs resolve when the organism is eliminated. Whether the “cure” is due to the elimination of Helicobacter organisms or something else remains in question, but it seems reasonable to assume that Helicobacter organisms cause disease in some animals.
Diagnosis
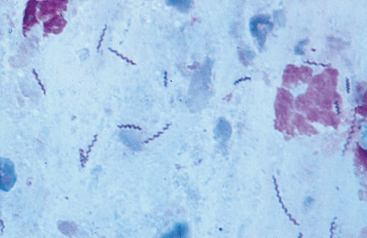
Gastric biopsy is currently required for a diagnosis of Helicobacter infection. The organisms are easy to identify if the pathologist is looking for them and uses special stains (e.g., Giemsa, Warthin-Starry). The bacteria are not uniformly distributed throughout the stomach, and it is best to obtain biopsy specimens from the body, fundus, and antrum. The clinician may also diagnose this infection by cytologic evaluation of the gastric mucosa (Fig. 32-1) or by looking for gastric mucosal urease activity (see Chapter 29). Because of the uncertain pathogenicity of Helicobacter spp., the clinician is advised to look first for other, better explanations for the animal’s clinical signs before deciding that a Helicobacter organism is causing disease.
Treatment
A combination of metronidazole, amoxicillin, and either famotidine or bismuth (either subsalicylate or subcitrate) seems to be effective in veterinary patients. Azithromycin and claritromycin have been substituted for bismuth in cats. Anecdotally, some animals seem to respond to just erythromycin or amoxicillin. Therapy should probably last for at least 10 days.
Prognosis
Animals with apparent Helicobacter-associated disease seem to respond well to treatment and have a good pro gnosis. However, because the cause and effect are uncertain, any animal that does not respond to therapy should be reexamined carefully to determine if other diseases are present. Recurrence of infection after treatment occurs, but it is not clear whether this represents a relapse of the original infection or reinfection from an outside sourse.
PHYSALOPTERA RARA
Etiology
Physaloptera rara is a nematode that has an indirect life cycle; beetles are the intermediate hosts.
Clinical Features
A single Physaloptera rara parasite can cause intractable vomiting. The parasite is primarily found in dogs. The vomiting usually does not resolve with antiemetics. Vomitus may or may not contain bile, and affected animals appear otherwise healthy.
Diagnosis
Ova are seldom found in feces. Furthermore, sodium dichromate or magnesium sulfate solutions are usually necessary to identify the eggs in feces. Most diagnoses are made when the parasites are found during gastroduodenoscopy (see Fig. 29-25). There may be only one worm causing clinical signs, and it can be difficult to find, especially if it is attached within the pylorus. Alternatively, empirical treatment (as described here) is reasonable.
OLLULANUS TRICUSPIS
Etiology
Ollulanus tricuspis is a nematode with a direct life cycle that is transmitted via vomited material.
Clinical Features
Cats are the most commonly affected species, although dogs and foxes are occasionally infected. Vomiting is the principal clinical sign, but clinically normal cats may harbor the parasite. Gross gastric mucosal lesions may or may not be seen in infested cats.
Diagnosis
Cattery situations promote infection because the parasite is passed directly from one cat to another. However, occasionally cats with no known contact with other cats are infected. Looking for parasites in gastric washings or vomited material with a dissecting microscope is the best means of diagnosis. The parasite can be seen occasionally in gastric mucosal biopsy specimens.
GASTRIC OUTFLOW OBSTRUCTION/GASTRIC STASIS
BENIGN MUSCULAR PYLORIC HYPERTROPHY (PYLORIC STENOSIS)
Etiology
The cause of benign muscular pyloric hypertrophy has not been definitively established, although some experimental research suggests that gastrin promotes the development of pyloric stenosis.
Clinical Features
Benign muscular pyloric stenosis typically causes persistent vomiting in young animals (especially brachycephalic dogs and Siamese cats) but can be found in any animal. These animals usually vomit food shortly after eating. The vomiting is sometimes described as projectile. The animals are otherwise clinically normal, although some pets may lose weight. Some cats with pyloric stenosis vomit so much that secondary esophagitis, megaesophagus, and regurgitation occur, confusing the clinical picture. Hypochloremic, hypokalemic, metabolic alkalosis sometimes occurs, but it is inconsistent and nonspecific for gastric outflow obstruction.
Diagnosis
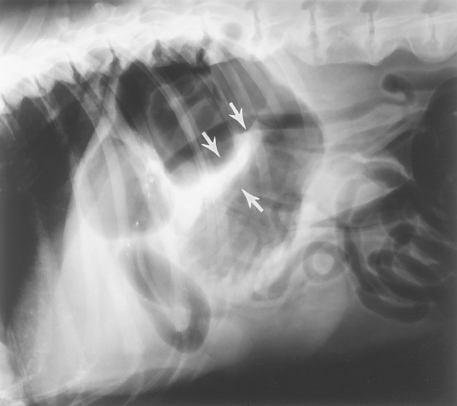
Diagnosing pyloric stenosis requires first finding gastric outflow obstruction during barium contrast-enhanced radiographs (Fig. 32-2), ultrasonography, gastroduodenoscopy, and/or exploratory surgery. Infiltrative disease of the pyloric mucosa then must be ruled out through biopsy. Endoscopically, the clinician may see prominent folds of normal-appearing mucosa at the pylorus. At surgery the serosa appears normal, but the pylorus is usually thickened when palpated. The surgeon can open the stomach and try to pass a finger through the pylorus to assess its patency. Extraalimentary tract diseases causing vomiting (see Box 28-6) should also be eliminated.
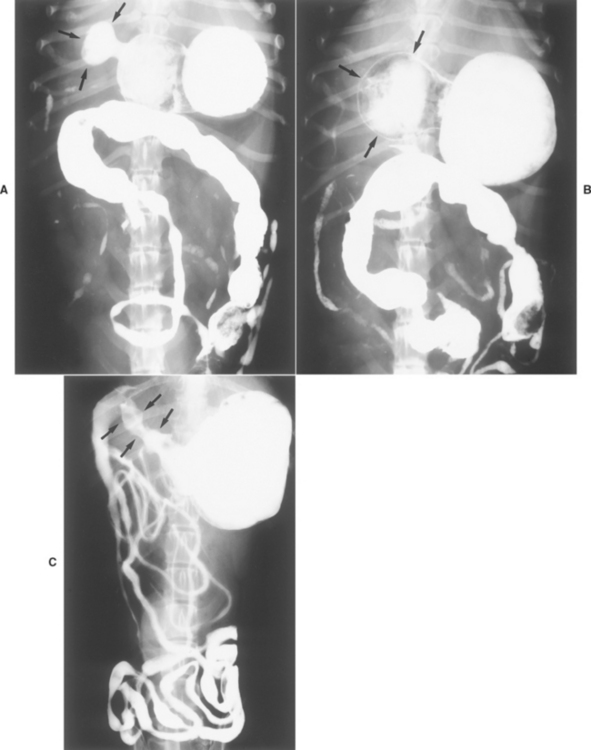
FIG 32-2 A and B, Ventrodorsal contrast radiographs of a dog with a gastric outflow obstruction. These radiographs were obtained approximately 3 hours after barium administration. There is inadequate gastric emptying despite obvious peristalsis. Note the smooth contour of barium in the antrum (arrows), which is in contrast to C. This is a case of pyloric stenosis. C, Dorsoventral contrast radiographs of a dog with gastric adenocarcinoma. The antrum has an irregular outline but is not distended (arrows). This failure to distend persisted on multiple radiographs and indicates an infiltrative lesion.
Treatment
Surgical correction is indicated. Pyloroplasty (e.g., a Y-U–plasty) is more consistently effective than pyloromyotomy. However, improperly performed pyloroplasty or pyloromyotomy can cause perforation or obstruction. Furthermore, the clinician should not routinely do a pyloric outflow procedure whenever an exploratory procedure fails to reveal another cause of vomiting.
GASTRIC ANTRAL MUCOSAL HYPERTROPHY
Etiology
Antral mucosal hypertrophy is idiopathic. Gastric outflow obstruction is caused by excessive, nonneoplastic mucosa that occludes the distal gastric antrum (Fig. 32-3). This disorder is different from benign muscular pyloric stenosis, in which the mucosa is thrown up into folds secondary to the submucosal thickening.
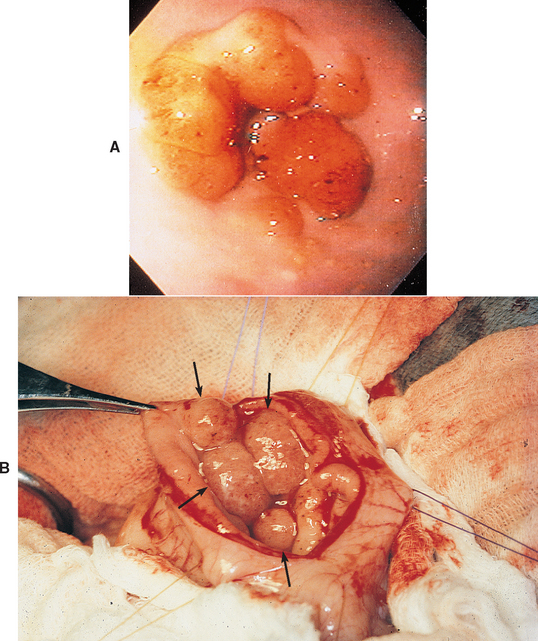
FIG 32-3 A, Endoscopic view of the pyloric region of a dog that has gastric antral mucosal hypertrophy. If biopsy is not performed, these folds may easily be mistaken for neoplasia. B, Intraoperative photograph of a dog’s opened pylorus. Note the numerous folds of mucosa that are protruding (arrows) as a result of gastric antral mucosal hypertrophy.
Clinical Features
Principally found in older, small-breed dogs, antral hypertrophy clinically resembles pyloric stenosis (i.e., animals usually vomit food, especially after meals).
Diagnosis
Gastric outlet obstruction is diagnosed radiographically, ultrasonographically, or endoscopically; however, definitive diagnosis of antral mucosal hypertrophy requires biopsy. Endoscopically, the antral mucosa is redundant and may resemble a submucosal neoplasm causing convoluted mucosal folds. In some cases the mucosa will be obviously reddened and inflamed. However, the mucosa in dogs with antral hypertrophy is usually not as firm or hard as expected in those with infiltrative carcinomas or leiomyomas. If antral mucosal hypertrophy is seen at surgery, there should be no evidence of submucosal infiltration or muscular thickening suggestive of neoplasia or benign pyloric stenosis, respectively. It is important to differentiate mucosal hypertrophy from these other diseases so that therapeutic recommendations are appropriate (e.g., gastric carcinomas typically have a worse prognosis, and surgery is not always indicated).
GASTRIC FOREIGN OBJECTS
Etiology
Objects that can pass through the esophagus may become a gastric or intestinal foreign object. Subsequently, vomiting may result from gastric outlet obstruction, gastric distention, or irritation. Linear foreign objects whose orad end lodges at the pylorus may cause intestinal perforation with subsequent peritonitis and must be dealt with expeditiously (see the section on intestinal obstruction on p. 464).
Clinical Features
Dogs are affected more commonly than cats because of their less discriminating eating habits. Vomiting (not regurgitation) is a common sign, but some animals demonstrate only anorexia, whereas others are asymptomatic.
Diagnosis
Acute onset of vomiting in an otherwise normal animal, especially a puppy, suggests foreign body ingestion. The clinician might palpate an object during physical examination or see it during plain radiographic imaging. Imaging and endoscopy are the most reliable means of diagnosis. However, diagnosis can be difficult if the stomach is filled with food. Some diseases closely mimic obstruction caused by foreign objects; canine parvovirus may initially cause intense vomiting, during which time viral particles might not be detected in the feces. Hypokalemic, hypochloremic, metabolic alkalosis is consistent with gastric outflow obstruction; however, these changes may be absent in animals with gastric obstruction and present in animals without obstruction. Therefore these electrolyte changes are neither sensitive nor specific for gastric outflow obstruction.
Treatment
Small foreign objects that are unlikely to cause trauma may pass through the gastrointestinal tract. If there is doubt, it is best to remove the object in question. Vomiting can be induced (e.g., apomorphine in the dog, 0.02 or 0.1 mg/kg administered intravenously or subcutaneously, respectively; hydrogen peroxide in the dog, 1 to 5 ml of 3% solution/kg administered orally; xylazine in the cat, 0.4 to 0.5 mg/kg administered intravenously) to eliminate gastric foreign objects if the clinician believes that the object will not cause problems during forcible ejection (i.e., it does not have sharp edges or points and is small enough to pass easily). If there is doubt as to the safety of this approach, the object should be removed endoscopically or surgically.
Before the animal is anesthetized for surgery or endoscopy, the electrolyte and acid-base status should be evaluated. Although electrolyte changes (e.g., hypokalemia) are common, they are impossible to predict with any accuracy. Hypokalemia predisposes to cardiac arrhythmias and should be corrected before anesthesia is induced.
Endoscopic removal of foreign objects requires a flexible endoscope and appropriate retrieval forceps. The animal should always be radiographed just before being anesthetized to ensure that the object is still in the stomach. Laceration of the esophagus and entrapment of the retrieval forceps in the object should be avoided. If endoscopic removal is unsuccessful, gastrostomy should be performed.
GASTRIC DILATION/VOLVULUS
Etiology
The cause of gastric dilation/volvulus (GDV) is unknown but may involve abnormal gastric motility. Thoracic confirmation seems correlated with risk; Irish Setters with a deeper thorax relative to width are more likely to experience GDV. Dogs with parents that had GDV may also be at increased risk. There are conflicting data regarding what predisposes dogs to GDV. Eating a large volume during a meal, eating once a day, eating rapidly, being underweight, eating from an elevated platform, being male, and advanced age seem to increase risk. Feeding dry food that is high in oil may also increase risk. GDV occurs when the stomach dilates excessively with gas (e.g., aerophagia, bacterial fermentation of carbohydrates, diffusion from the blood). The stomach may maintain its normal anatomic position (gastric dilation) or twist (GDV). In the latter situation the pylorus typically rotates ventrally from the right side of the abdomen below the body of the stomach to become positioned dorsal to the gastric cardia on the left side. If the stomach twists sufficiently, gastric outflow is obstructed and progressive distention with air results. Splenic torsion may occur concurrently with the spleen on the right side of the abdomen if the stomach twists sufficiently. Massive gastric distention obstructs the hepatic portal vein and posterior vena cava, causing mesenteric congestion, decreased cardiac output, severe shock, and DIC. The gastric blood supply may be impaired, causing gastric wall necrosis.
Clinical Features
GDV principally occurs in large- and giant-breed dogs with deep chests; it rarely occurs in small dogs or cats. Affected dogs typically retch nonproductively and may demonstrate abdominal pain. Marked anterior abdominal distention may be seen later. However, abdominal distention is not always obvious in large, heavily muscled dogs. Eventually, depression and a moribund state occur.
Diagnosis
Physical examination findings (i.e., a large dog with a large tympanic anterior abdomen that is retching unproductively) allow presumptive diagnosis of GDV but do not permit differentiation between dilation and GDV; plain abdominal radiographs, preferably with the animal in right lateral recumbency, are required. Volvulus is denoted by displacement of the pylorus and/or formation of a “shelf” of tissue in the gastric shadow (Fig. 32-4). It is impossible to distinguish between dilation and dilation/torsion on the basis of ability or inability to pass an orogastric tube.
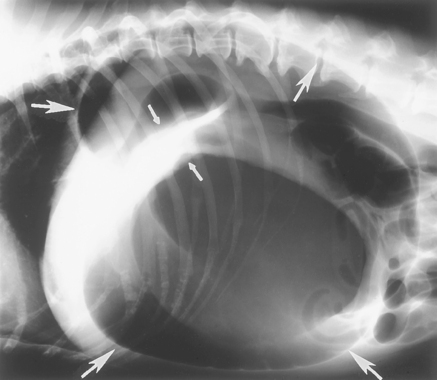
FIG 32-4 Lateral radiograph of a dog with gastric dilation/volvulus. The stomach is dilated (large arrows), and there is a “shelf” of tissue (small arrows), demonstrating that the stomach is malpositioned. Radiographs obtained from the right lateral position seem superior to those of other views in demonstrating this shelf. If the stomach were similarly distended but not malpositioned, the diagnosis would be gastric dilation.
Treatment
Treatment consists of initiating aggressive therapy for shock (hetastarch or hypertonic saline infusion [see p. 396] may make treatment for shock quicker and easier) and then decompressing the stomach unless the patient is asphyxiating, in which case the stomach is decompressed first. Gastric decompression is usually performed with an orogastric tube, after which the stomach is lavaged with warm water to remove its contents. The stomach of dogs with dilation and many with GDV can be decompressed in this manner. Mesenteric congestion caused by the enlarged stomach predisposes to infection and endotoxemia, making systemic antibiotic administration reasonable (e.g., cefazolin, 20 mg/kg administered intravenously). Serum electrolyte concentrations and acid-base status should be evaluated.
The orogastric tube should not be forced into the stomach against undue resistance because it could rupture the lower esophagus. If the tube cannot be passed into the stomach, the clinician may insert a large needle (e.g., a 3-inch, 12- to 14-gauge needle) into the stomach just behind the rib cage in the left flank to decompress the stomach (which usually causes some abdominal contamination) or perform a temporary gastrostomy in the left paralumbar area (i.e., the stomach wall is sutured to the skin, and then the stomach wall is incised to allow evacuation of accumulated gas and other contents). After the animal is stabilized, a second procedure is performed to close the temporary gastrostomy (if present), reposition the stomach, remove the spleen (if grossly infarcted), remove or invaginate the devitalized gastric wall, and perform a gastropexy. Gastropexy (e.g., circumcostal, belt loop, tube gastrostomy) is recommended to help prevent recurrence of torsion and may be correlated with prolongation of survival. Another option consists of immediately performing a laparotomy after decompressing the stomach but before stabilizing the animal. The decision as to whether to first stabilize the animal or immediately perform surgery is based on the condition of the dog at initial presentation and on whether the animal would be a considerably better anesthetic risk after stabilization.
If the dog has GDV (see Fig. 32-4), surgery is necessary to reposition the stomach; this is followed by gastropexy to prevent recurrence. This surgery should be performed as soon as the animal constitutes an acceptable anesthetic risk because torsion (even when the stomach is deflated) impairs gastric wall perfusion and may cause necrosis. Areas of gastric wall necrosis should be resected, or preferably invaginated, to prevent perforation and abdominal contamination. In dogs with gastric dilation without torsion, gastropexy is optional and may be performed after the dog is completely recovered from the current episode. Gastropexy almost always prevents torsions but does not prevent dilation.
Postoperatively, the animal should be monitored by electrocardiogram (ECG) for 48 to 72 hours. Lidocaine, procainamide, and/or soltolol therapy may be needed if cardiac arrhythmias diminish cardiac output (see Chapter 4). Hypokalemia is common and makes such arrhythmias refractory to medical control. Therefore hypokalemia should be resolved.
Prevention is difficult because the cause is unknown. Although preventing exercise after meals and feeding small meals of softened food would seem to be useful, there are no data to confirm this speculation.
Prognosis
The prognosis depends on how quickly the condition is recognized and treated. Mortality rates ranging from 20% to 45% have been reported. Early therapy improves the prognosis, whereas a delay lasting more than 5 hours between onset of signs and presentation to the veterinarian’s office, hypothermia at admission, preoperative cardiac arrhythmias, increased preoperative blood lactate concentrations, gastric wall necrosis, severe DIC, partial gastrectomy, splenectomy, and postoperative development of acute renal failure seem to worsen the prognosis. Although rare, gastric dilation may recur after gastropexy. Prophylactic gastropexy may be elected for animals believed to be at increased risk for GDV. Laparoscopy can be used to make prophylactic gastropexy a minimally invasive procedure.
PARTIAL OR INTERMITTENT GASTRIC VOLVULUS
Etiology
The causes for partial and intermittent gastric volvulus might be the same as for classic GDV.
Clinical Features
Dogs with partial or intermittent volvulus do not have the life-threatening, progressive syndrome characterizing classic GDV. Although occurring in the same breeds as GDV, partial gastric volvulus usually produces a chronic, intermittent, potentially difficult-to-diagnose problem. It may occur repeatedly and spontaneously resolve; dogs may appear normal between bouts. Some dogs have persistent, nondistended volvulus and are asymptomatic.
Diagnosis
Plain radiographs are usually diagnostic (Fig. 32-5). However, diagnosis may require repeated radiographs and/or contrast studies. Chronic volvulus will rarely be diagnosed endoscopically. It is possible, in rare cases, to cause a temporary gastric volvulus by manipulating the gastroscope in an air-distended stomach. Therefore the clinician must differentiate spontaneous from iatrogenic volvulus.
IDIOPATHIC GASTRIC HYPOMOTILITY
Etiology
Idiopathic gastric hypomotility refers to an anecdotal syndrome characterized by poor gastric emptying and motility despite the lack of anatomic obstruction, inflammatory lesions, or other causes.
Clinical Features
Idiopathic gastric hypomotility has primarily been diagnosed in dogs. Affected dogs usually vomit food several hours after eating but otherwise feel well. Weight loss may or may not occur.
Diagnosis
Fluoroscopic studies document decreased gastric motility, but diagnosis requires ruling out gastric outlet obstruction, infiltrative bowel disease, inflammatory abdominal disease, and extraalimentary tract diseases (e.g., renal, adrenal, or hepatic failure; severe hypokalemia or hypercalcemia).
Treatment
Metoclopramide (see Table 30-3) increases gastric peristalsis in some but not all affected dogs. Cisapride or erythromycin may be effective if metoclopramide fails. Diets low in fat and fiber promote gastric emptying and may be helpful.
BILIOUS VOMITING SYNDROME
Etiology
Bilious vomiting syndrome appears to be caused by gastroduodenal reflux that occurs when the dog’s stomach is empty for long periods of time (e.g., during an overnight fast).
Clinical Features
Bilious vomiting syndrome usually affects otherwise normal dogs that are fed once daily in the morning. Classically, the pet vomits bile-stained fluid once a day, usually late at night or in the morning just before eating.
Diagnosis
The clinician must rule out obstruction, gastrointestinal inflammation, and extraalimentary tract diseases. Elimination of these disorders, in addition to the history as described, strongly suggests bilious vomiting syndrome.
GASTROINTESTINAL ULCERATION/EROSION
Etiology
Gastrointestinal ulceration/erosion (GUE) is more common in dogs than in cats. There are several potential causes. Stress ulceration is associated with severe hypovolemic, septic, or neurogenic shock, such as occurs after trauma, surgery, and endotoxemia. These ulcers are typically in the gastric antrum, body, and/or duodenum. Extreme exertion (e.g., in sled dogs) causes gastric erosions/ulcers in the body and fundus, probably as a result of a combination of poor perfusion and high circulating levels of glucocorticoids.
NSAIDs (e.g., aspirin, ibuprofen, naproxen, piroxicam, flunixin) are a major cause of canine GUE because these drugs have longer half-lives in dogs than in people. Naproxen, ibuprofen, indomethacin, and flunixin are particularly dangerous to dogs. Concurrent use of more than one NSAID or use of an NSAID plus a corticosteroid (especially dexamethasone) increases the risk of GUE. The newer COX-2–selective NSAIDs (e.g., carprofen, dericoxib, meloxicam, etodolac) are less likely to cause GUE; however, GUE can still occur if these drugs are used inappropriately (e.g., excessive dose, failure to have an adequate washout period between use of different NSAIDs, concurrent use of corticosteroids). Use of NSAIDs in animals with poor visceral perfusion (e.g., those in cardiac failure, shock) may also increase the risk of GUE. Most steroids pose minimal risk unless the animal is otherwise at increased risk for GUE (e.g., anoxic gastric mucosa due to shock or anemia). Dexamethasone, however, is clearly ulcerogenic when used at high doses.
Mast cell tumors may release histamine (especially if radiation or chemotherapy is being used), which induces gastric acid secretion. Gastrinomas are apudomas principally found in the pancreas. Usually occurring in older dogs and rarely in cats, these tumors secrete gastrin, which produces severe gastric hyperacidity, duodenal ulceration, esophagitis, and diarrhea.
Renal failure seldom causes GUE, but hepatic failure seems to be an important cause in dogs. Foreign objects rarely cause GUE, but they prevent healing and increase blood loss from ulcers. Inflammatory bowel disease may be associated with GUE in dogs, although most animals with this condition do not have these lesions. Gastric neoplasms and other infiltrative diseases (e.g., pythiosis) may also cause GUE (see p. 438) Tumors are especially important as a cause in cats and older dogs.
Clinical Features
GUE is more common in dogs than in cats. Anorexia may be the principal sign. If vomiting occurs, blood (i.e., fresh or digested) may or may not be present. Anemia and/or hypoproteinemia occasionally occur and cause signs (i.e., edema, pale mucous membranes, weakness, dyspnea). Melena may occur if there is severe blood loss within a short period of time. Most affected dogs, even those with severe GUE, do not demonstrate pain during abdominal palpation. Perforation is associated with signs of septic peritonitis (see p. 476). Some ulcers perforate and seal over before generalized peritonitis occurs. In such cases a small abscess may develop at the site, causing abdominal pain, anorexia, and/or vomiting.
Diagnosis
A presumptive diagnosis of GUE is usually based on finding evidence of gastrointestinal blood loss (e.g., hematemesis, melena, iron-deficiency anemia) in an animal without a coagulopathy. The history and physical examination may identify an obvious cause (e.g., stress, NSAID administration, mast cell tumor). Perforation may cause peritonitis and signs of an acute abdomen and sepsis. Because mast cell tumors may resemble almost any cutaneous lesion, all cutaneous masses or nodules should be evaluated cytologically. Hepatic failure is usually diagnosed on the basis of the serum biochemistry profile. Contrast radiographs are diagnostic for foreign objects and sometimes for GUE (Fig. 32-6). Ultrasonography sometimes detects gastric thickening (such as would be seen in infiltrated lesions) and/or mucosal defects. Endoscopy is the most sensitive and specific tool for diagnosing GUE (see Figs. 29-1829-1929-20 to 29-21) and, in conjunction with biopsy, can be used to diagnose tumors (see Fig. 29-20), foreign bodies (see Fig. 29-24), and inflammation that may cause ulcers. Endoscopic findings may also suggest a gastrinoma if duodenal erosions are found. Serum gastrin concentrations should be measured if a gastrinoma is suspected or if there are no other likely causes.
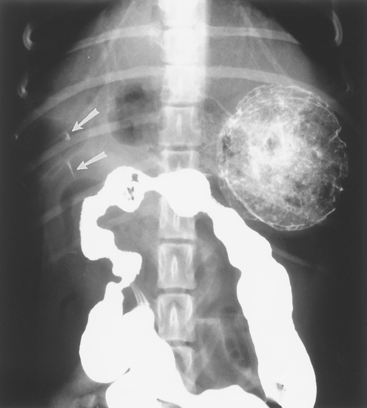
FIG 32-6 Contrast ventrodorsal radiograph of a dog with persistent vomiting. Note the small “sliver” representing retention of barium in the region of the pylorus (arrows). This area of contrast persisted on several radiographs. Endoscopy and surgery confirmed a large ulcer that had perforated and spontaneously sealed. This radiograph demonstrates how difficult radiographic diagnosis of gastrointestinal ulceration can be.
Treatment
Therapy depends on the severity of GUE and whether an underlying cause is detected. Animals with suspected GUE that is not obviously life threatening (i.e., there is no evidence of severe anemia, shock, sepsis, severe abdominal pain, or severe depression) may first be treated symptomatically if the clinician believes that he or she knows the cause.
Symptomatic therapy (e.g., H2 receptor antagonists, proton pump inhibitors, sucralfate, parenteral fluids, withholding food) is often successful. Eliminating the underlying etiology (e.g., NSAIDs, shock) is important, and any gastric foreign objects present should be removed. If appropriate medical therapy is unsuccessful after 5 or 6 days, or if the animal has life-threatening bleeding despite appropriate medical therapy, the ulcer(s) should usually be resected. The stomach should be examined endoscopically before surgery to determine the number and location of the ulcers; it is surprisingly easy to miss ulcers during laparotomy.
In animals with gastrinomas, H2-receptor antagonist therapy is often palliative for months. Animals with high serum gastrin concentrations may require more potent and/or higher doses of H2 receptor antagonists (e.g., famotidine) or the more potent proton pump inhibitors (see Table 30-4).
Prevention of GUE is preferable to treatment, and rational NSAID and steroid therapy are especially important. Sucralfate (Carafate; see Table 30-5) and H2 receptor antagonists (see Table 30-4) have been used in an attempt to prevent GUE in dogs receiving NSAIDs and steroids; however, there is no good evidence that these drugs are effective for this purpose in dogs and cats. Misoprostol (see Table 30-5) is designed to prevent NSAID-induced ulceration and is more effective than H2 receptor antagonists or sucralfate. However, it is not uniformly successful.
INFILTRATIVE GASTRIC DISEASES
NEOPLASMS
Etiology
Neoplastic infiltrations (e.g., adenocarcinoma, lymphoma, leiomyomas, and leiomyosarcomas in dogs; lymphoma in cats) may produce GUE through direct mucosal disruption. Gastric lymphoma is typically a diffuse lesion but can produce masses. The cause and significance of benign gastric polyps are unknown. They seem to occur more commonly in the antrum.
Clinical Features
Dogs and cats with gastric tumors are usually asymptomatic until the disease is advanced. Anorexia (not vomiting) is the most common initial sign. Vomiting caused by gastric neoplasia usually signifies advanced disease or gastric outflow obstruction. Adenocarcinomas are typically infiltrative and decrease emptying by impairing motility and/or obstructing the outflow tract. Weight loss is commonly caused by nutrient loss or cancer cachexia syndrome. Hematemesis occasionally occurs, but leiomyomas seem to be the tumor most likely to cause severe acute upper gastrointestinal bleeding. Other bleeding gastric tumors are more likely to cause iron deficiency anemia even if gastrointestinal blood loss is not obvious. Polyps rarely cause signs unless they obstruct the pylorus.
Diagnosis
Iron deficiency anemia in a dog or cat without obvious blood loss suggests gastrointestinal bleeding, often caused by a tumor. Plain and contrast imaging may reveal gastric wall thickening, decreased motility, and/or mucosal irregularities. The only sign of submucosal adenocarcinoma may be failure of one area to dilate (see Fig. 32-2, C). Ultrasound-guided aspiration of thickened areas in the gastric wall may produce cytologic preparations that are diagnostic for adenocarcinoma or lymphoma. Endoscopically, such areas may appear as multiple mucosal folds extending into the lumen without ulceration or erosion. Some tumors will be obvious endoscopically. When biopsy of such lesions is performed endoscopically, the sample must be deep enough to ensure that submucosal tissue is included. Furthermore, scirrhous adenocarcinomas may be so dense that the clinician cannot obtain diagnostic biopsy specimens with flexible endoscopic forceps. Mucosal lymphomas and nonscirrhous adenocarcinomas often produce GUE, and endoscopically obtained tissue samples are usually diagnostic. Polyps are usually obvious endoscopically, but a biopsy specimen should always be obtained and evaluated to ensure that adenocarcinoma is not present.
Treatment
Most adenocarcinomas are advanced before clinical signs are obvious, making complete surgical excision difficult or impossible. Leiomyomas and leiomyosarcomas are more likely to be resectable than adenocarcinomas. Gastroduodenostomy may palliate gastric outflow obstruction caused by an unresectable tumor. Chemotherapy is rarely helpful except for dogs and cats with lymphoma.
PYTHIOSIS
Etiology
Pythiosis is a fungal infection caused by Pythium insidiosum. This species is principally found in the Gulf coast area of the southeastern United States. Any area of the alimentary tract or skin may be affected. The fungus typically causes intense submucosal infiltration of fibrous connective tissue and a purulent, eosinophilic, granulomatous inflammation causing GUE. Such infiltration prevents peristalsis, causing stasis.
Clinical Features
Pythiosis principally affects dogs, typically causing vomiting, anorexia, diarrhea, and/or weight loss. Because gastric outflow obstruction occurs frequently, vomiting is common. Colonic involvement may cause tenesmus and hematochezia.
Diagnosis
Diagnosis requires serology or seeing the organism cytologically or histologically. Enzyme-linked immunosorbent assay (ELISA) and polymerase chain reaction (PCR) tests are available to look for antibodies or antigen, respectively. Biopsy samples should include the submucosa because the organism is more likely to be there than in the mucosa. Such diagnostic biopsy specimens can be procured by way of rigid endoscopy; however, because of the dense nature of the infiltrate, a sufficiently deep sample can rarely be obtained by flexible endoscopy. Cytologic analysis of a tissue sample obtained by scraping an excised piece of submucosa with a scalpel blade may be diagnostic; fungal hyphae that do not stain and appear as “ghosts” with typical Romanowsky-type stains are strongly supportive of a diagnosis. The organisms may be sparse and difficult to find histologically, even in large tissue samples.
Treatment
Complete surgical excision provides the best chance for cure. Itraconazole (5 mg/kg administered orally q12h) or liposomal amphotericin B (2.2 mg/kg/treatment) with or without terebinifin may benefit some animals for varying periods of time. Immunotherapy has recently become available, but critical evaluation of the efficacy of this therapy is not currently available
Beck JJ, et al. Risk factors associated with short-term outcome and development of perioperative complications in dogs undergoing surgery because of gastric dilatation-volvulus: 166 cases (1992-2003). J Am Vet Med Assoc. 2006;229:1934.
Bergh MS, et al. The coxib NSAIDs: potential clinical and pharmacologic importance in veterinary medicine. J Vet Intern Med. 2005;19:633.
Boston SE, et al. Endoscopic evaluation of the gastroduodenal mucosa to determine the safety of short-term concurrent adminstration of meloxicam and dexamethasone in healthy dogs. Am J Vet Res. 2003;64:1369.
Buber T, et al. Evaluation of lidocaine treatment and risk factors for death associated with gastric dilatation and volvulus in dogs: 112 cases (1997-2005). J Am Vet Med Assoc. 2007;230:1334.
Cohen M, et al. Gastrointestinal leiomyosarcoma in 14 dogs. J Vet Intern Med. 2003;17:107.
Davis MS, et al. Efficacy of omeprazole for the prevention of exercise-induced gastritis in racing alaskan sled dogs. J Vet Intern Med. 2003;17:163.
de Papp E, et al. Plasma lactate concentration as a predictor of gastric necrosis and survival among dogs with gastric dilatation-volvulus: 102 cases (1995–1998). J Am Vet Med Assoc. 1999;215:49.
Dowers K, et al. Effect of short-term sequential adminstration of nonsteroidal anti-inflammatory drugs on the stomach and proximal portion of the duodenum in healthy dogs. Am J Vet Res. 2006;67:1794.
Easton S. A retrospective study into the effects of operator experience on the accuracy of ultrasound in the diagnosis of gastric neoplasia in dogs. Vet Radiol Ultra. 2001;42:47.
Eggertsdottir AV, et al. Comparison of the recurrence rate of gastric dilatation with or without volvulus in dogs after circumcostal gastropexy versus gastrocolopexy. Vet Surg. 2001;30:546.
Glickman LT, et al. Incidence of and breed-related risk factors for gastric dilatation-volvulus in dogs. J Am Vet Med Assoc. 2000;216:40.
Glickman LT, et al. Non-dietary risk factors for gastric dilatation-volvulus in large and giant breed dogs. J Am Vet Med Assoc. 2000;217:1492.
Graham JP, et al. Ultrasonographic features of canine gastrointestinal pythiosis. Vet Radiol Ultra. 2000;41:273.
Grooters AM, et al. Development of a nested polymerase chain reaction assay for the detection and identification of Pythium insidiosum. J Vet Intern Med. 2002;16:147.
Grooters AM, et al. Development and evaluation of an enzyme-linked immunosorbent assay for the serodiagnosis of pythiosis in dogs. J Vet Intern Med. 2002;16:142.
Hensel P, et al. Immunotherapy for treatment of multicentric cutaneous pythiosis in a dog. J Am Vet Med Assoc. 2003;223:215.
Hilton LE, et al. Spontaneous gastroduodenal perforation in 16 dogs and seven cats (1982–1999). J Am Anim Hosp Assoc. 2002;38:176.
Lamb CR, et al. Ultrasonographic appearance of primary gastric neoplasia in 21 dogs. J Small Anim Pract. 1999;40:211.
Lascelles B, et al. Gastrointestinal tract perforation in dogs treated with a selective cyclooxygenase-2 inhibitor: 29 cases (2002–2003). J Am Vet Med Assoc. 2005;227:1112.
Liptak JM, et al. Gastroduodenal ulceration in cats: eight cases and a review of the literature. J Fel Med Surg. 2002;4:27.
Neiger R, et al. Gastric mucosal lesions in dogs with acute intervertebral disc disease: characterization and effects of omeprazole or misoprostol. J Vet Intern Med. 2000;14:33.
Neiger R, et al. Helicobacter infection in dogs and cats: facts and fiction. J Vet Intern Med. 2000;14:125.
Peters R, et al. Histopathologic features of canine uremic gastropathy: a retrospective study. J Vet Intern Med. 2005;19:315.
Raghavan M, et al. Diet-related risk factors for gastric dilatation-volvulus in dogs of high-risk breeds. J Am Anim Hosp Assoc. 2004;40:192-203.
Raghavan M, et al. The effect of ingredients in dry dog foods on the risk of gastric dilatation-volvulus in dogs. J Am Anim Hosp Assoc. 2006;42:28.
Rawlings CA, et al. Prospective evaluation of laparoscopic-assisted gastropexy in dogs susceptible to gastric dilatation. J Am Vet Med Assoc. 2002;221:1576.
Sennello K, et al. Effects of deracoxib or buffered aspirin on the gastric mucosa of healthy dogs. J Vet Intern Med. 2006;20:1291.
Simpson K, et al. The relationship of Helicobacter spp. infection to gastric disease in dogs and cats. J Vet Inter Med. 2000;14:223.
Steelman-Szymeczek SJ, et al. Clinical evaluation of a right-sided prophylactic gastropexy via a grid approach. J Am Anim Hosp Assoc. 2003;39:397.
Swan HM, et al. Canine gastric adenocarcinoma and leiomyosarcoma: a retrospective study of 21 cases (1986–1999) and literature reveiw. J Am Anim Hosp Assoc. 2002;38:157.
Tams TR. Endoscopic removal of gastrointestinal foreign bodies. In Tams TR, editor: Small animal endoscopy, ed 2, St Louis: Mosby, 1999.
Waldrop JE, et al. Packed red blood cell tranfusions in dogs with gastrointestinal hemorrhage: 55 cases (1999-2001). J Am Anim Hosp Assoc. 2003;39:523.
Ward DM, et al. The effect of dosing interval on the efficacy of misoprostol in the prevention of aspirin-induced gastric injury. J Vet Intern Med. 2003;17:282.
Webb C, et al. Canine gastritis. Vet Clin N Am. 2003;33:969.
Wiinberg B, et al. Quantitative analysis of inflammatory and immune responses in dogs with gastritis and their relationship to Helicobacter spp infection. J Vet Intern Med. 2005;19:4.