CHAPTER 28 Clinical Manifestations of Gastrointestinal Disorders
DYSPHAGIA, HALITOSIS, AND DROOLING
Dysphagia, halitosis, and drooling may co-exist in many animals with oral disease. Dysphagia (i.e., difficulty in eating) usually results from oral pain, masses, foreign objects, trauma, neuromuscular dysfunction, or a combination of these (Box 28-1). Halitosis typically signifies an abnormal bacterial proliferation secondary to tissue necrosis, tartar, periodontitis, or the oral or esophageal retention of food (Box 28-2). Drooling occurs because animals are unable to or are in too much pain to swallow (i.e., pseudoptyalism). Excessive salivation is usually due to nausea; animals that are not nauseated rarely produce excessive saliva (Box 28-3). Although any disease causing dysphagia may have an acute onset, the clinician usually should first consider foreign objects or trauma as the cause in such an animal. The environment and vaccination history should also be assessed to determine whether rabies is a possibility.
Oral Pain
Periodontitis or caries (especially cats)
Mandibular or maxillary osteomyelitis
Stomatitis, glossitis, pharyngitis, gingivitis, tonsillitis, or sialoadenitis
Pain associated with swallowing: esophageal stricture or esophagitis
The next step is a thorough oral, laryngeal, and cranial examination. This examination is often the most important diagnostic step because most problems producing oral pain can be partially or completely defined on the basis of physical examination findings. Ideally, this is done without chemical restraint to allow pain to be detected. However, the animal often must be anesthetized for the oral examination to be performed adequately. A search for anatomic abnormalities, inflammatory lesions, pain, and discomfort should always be made. If pain is found, the clinician should determine whether it occurs when the mouth is opened (e.g., retrobulbar inflammation), is associated with extraoral structures (e.g., muscles of mastication), or originates from the oral cavity. The clinician should also search for fractures, lacerations, crepitus, masses, enlarged lymph nodes, inflamed or ulcerated areas, draining tracts, loose teeth, excessive temporal muscle atrophy, inability to open the mouth while the animal is under anesthesia, and ocular problems (e.g., proptosis of the eye, inflammation, or strabismus suggestive of retrobulbar disease). If oral pain is apparent but cannot be localized, retrobulbar lesions, temporomandibular joint disease, and posterior pharyngeal lesions should be considered. A concurrent clinicopathologic evaluation may be useful, especially if oral examination findings indicate the presence of systemic disease (e.g., lingual necrosis resulting from uremia, chronic infection secondary to hyperadrenocorticism).
Biopsies should be done of mucosal lesions (e.g., masses, inflamed or ulcerated areas) and painful muscles of mastication. Masses that do not disrupt the mucosa, especially those on the midline and dorsal to the larynx, can be difficult to discern and are sometimes found only by careful digital palpation. Fine-needle aspiration and cytologic evaluation are reasonable first steps for the diagnosis of masses. Remember that fine-needle aspirates can only find disease; they cannot exclude disease. Subtle masses or those dorsal to the larynx may sometimes be aspirated more accurately with ultrasonographic guidance. Multiple aspirations are usually done before a wedge or punch biopsy is performed.
Incisional biopsy specimens must include generous amounts of submucosal tissues. Many oral tumors cannot be diagnosed on the basis of findings from superficial biopsy specimens because of superficial necrosis and inflammation caused by normal oral flora. Biopsies of these lesions are often not done aggressively because they bleed profusely and are hard to suture. The clinician should avoid major vessels (e.g., the palatine artery) and use silver nitrate to stop hemorrhage. It is better to have difficulty stopping hemorrhage after obtaining an adequate biopsy specimen than less difficulty stopping hemorrhage after obtaining a nondiagnostic specimen. If diffuse oral mucosal lesions are noted, search carefully for vesicles (e.g., pemphigus), and if these are found, remove them intact for histopathologic and immunofluorescent studies. If vesicles are not found, then at least two or three tissue samples representing a spectrum of new and old lesions should be submitted for analysis.
If oral examination findings are not helpful, plain oral and laryngeal radiographs are usually the best next steps. Oral cultures are rarely cost-effective because the normal oral flora makes interpretation of the results difficult. Even animals with severe halitosis or stomatitis secondary to bacterial infection rarely benefit from bacterial culture, unless there is a draining tract or abscess.
Halitosis often accompanies dysphagia, in which case it is usually more productive to determine the cause of the dysphagia. If halitosis occurs without dysphagia, the clinician should first be sure that the odor is abnormal and then check for the ingestion of odoriferous substances (e.g., feces). A thorough oral examination is still the most important test. Halitosis not attributable to an oropharyngeal lesion may be originating from the esophagus. Contrast-enhanced radiographs or esophagoscopy may reveal the presence of tumors or retained food secondary to stricture or weakness. If the history and oral examination are unrevealing except for the finding of mild-to-moderate tartar accumulation, the teeth should be cleaned to try to alleviate the problem.
Drooling is usually caused by nausea, oral pain, or dysphagia. The approach to the diagnosis of oral pain and dysphagia is described under the appropriate headings. Nausea is considered in the section on vomiting.
Dysphagic animals without demonstrable lesions or pain may have neuromuscular disease. Dysphagia of muscular origin usually results from atrophic myositis (see Chapter 31). The finding of swollen, painful temporal muscles suggests acute myositis. The combination of severe temporal-masseter muscle atrophy and difficulty opening the mouth (even when the animal is anesthetized) is suggestive of chronic temporal-masseter myositis. Biopsy of affected muscles is indicated, but the clinician must ensure that muscle tissue is retrieved; it is easy to obtain only fibrous scar tissue. It may help to have serum analyzed for antibodies to type 2M muscle fibers, a finding consistent with masticatory muscle myositis but not polymyopathy.
Neurogenic dysphagia is caused by disorders in the oral (i.e., also called prehensile), pharyngeal, or cricopharyngeal phases of swallowing (disorders of the latter two phases are discussed in the section on regurgitation). Rabies should always be considered, despite its relative rarity. After rabies is presumptively ruled out, cranial nerve deficits (especially deficits of cranial nerves V, VII, IX, XII) should be considered. Because the clinical signs vary depending on the nerve (or nerves) affected, a careful neurologic examination must be done.
Inability to pick up food or having food drop from the mouth while eating usually indicates a prehensile disorder. Dysphagia may be noticeable in dogs and cats with pharyngeal and cricopharyngeal dysfunction, but regurgitation is often more prominent. Dynamic contrast-enhanced radiographic studies (e.g., cinefluoroscopy or fluoroscopy) are best for detecting and defining neuromuscular dysphagia. If neuromuscular problems are seemingly ruled out by these radiographic studies, then anatomic lesions and occult causes of pain (e.g., soft tissue inflammation or infection) must be reconsidered.
DISTINGUISHING REGURGITATION FROM VOMITING FROM EXPECTORATION
Regurgitation is the expulsion of material (i.e., food, water, saliva) from the mouth, pharynx, or esophagus. It must be differentiated from vomiting (the expulsion of material from the stomach and/or intestines) and expectoration (the expulsion of material from the respiratory tract). Historical and physical examination findings sometimes allow differentiation of these three (Table 28-1). Expectoration is generally associated with coughing at the time of the event. However, because dogs that cough and gag excessively may stimulate themselves to vomit as well, careful history taking is important. Animals that regurgitate and occasionally those that vomit may cough as a result of aspiration, but oral expulsion is not consistently correlated with coughing in these patients.
 TABLE 28-1 Aids to Differentiate Regurgitation from Vomiting*
TABLE 28-1 Aids to Differentiate Regurgitation from Vomiting*
| SIGN | REGURGITATION | VOMITING |
|---|---|---|
| Prodromal nausea† | No | Usually |
| Retching‡ | No | Usually |
| Material produced | ||
| Food | ± | ± |
| Bile | No | ± |
| Blood | ± (undigested) | ± (digested or undigested) |
| Amount of material | Any amount | Any amount |
| Time relative to eating | Anytime | Anytime |
| Distention of cervical esophagus | ± | No |
| Dipstick analysis of material | ||
| pH | ≥7 | ≤5 or ≥8 |
| Bile | No | ± |
* These are guidelines that often help distinguish vomiting from regurgitation. However, occasional animals will require plain and/or contrast-enhanced radiographs to distinguish between the two.
† May include salivation, licking lips, pacing, and an anxious expression. The owner may simply state that the animal is aware that it will soon “vomit.”
‡ These are usually forceful, vigorous abdominal contractions or dry heaves. This is not to be confused with gagging.
The criteria in Table 28-1 are only guidelines. Some animals that appear to be regurgitating are vomiting and vice versa. If the clinician cannot distinguish between the two on the basis of the history and physical examination findings, he or she may use a urine dipstick to determine the pH and whether there is bilirubin in freshly “vomited” material. If the pH is 5 or less, the material has originated from the stomach and probably resulted from vomiting. If the pH is more than 7 and there is no evidence of bilirubin, this is most consistent with regurgitation. The presence of bilirubin indicates that the material has originated from the duodenum (i.e., vomiting). A positive finding of blood in the urine dipstick test is not useful.
If vomiting and regurgitation still cannot be distinguished, plain and/or contrast-enhanced radiographs will usually detect esophageal dysfunction. However, some esophageal disorders (e.g., hiatal hernia, partial stricture, partial or segmental motility defect) are easily missed unless a careful radiographic technique and/or fluoroscopy are used. Endoscopy is rarely required to detect esophageal lesions missed by imaging (e.g., esophagitis).
REGURGITATION
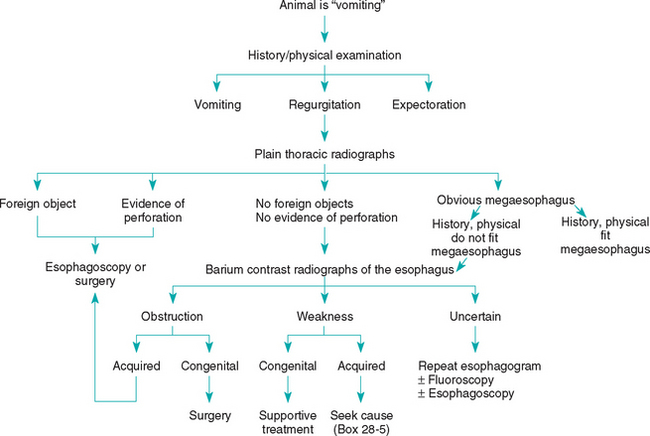
Once regurgitation is confirmed, the disease should be localized to the oral cavity/pharynx or esophagus (Fig. 28-1). The history, in combination with observation of the pet eating, should allow the clinician to detect evidence of dysphagia (e.g., undue stretching or flexing of the neck during swallowing, repeated efforts at swallowing, food falling from the mouth during swallowing) if it is present. Some animals with dysphagia associated with neuromuscular disorders have more difficulty swallowing liquids than solid foods, probably because it is easier to aspirate liquids. Attempts to swallow water may produce coughing in these animals.
If a regurgitating animal is dysphagic, oral, pharyngeal, and cricopharyngeal dysfunctions should be considered; the latter two mimic each other. Fluoroscopic evaluation of swallowing during a barium meal is necessary to differentiate pharyngeal from cricopharyngeal dysfunction. If they are not accurately differentiated, inappropriate therapy may cause morbidity or mortality.
If the regurgitating animal is not dysphagic, esophageal dysfunction is most likely. The two main reasons for esophageal regurgitation are obstruction and muscular weakness. Plain thoracic radiographs, with or without barium contrast-enhancement, are the best tools for initially defining these problems. Fluoroscopy is often necessary in animals with a partial loss of peristalsis, segmental aperistalsis, gastroesophageal reflux, or sliding hiatal hernias. If the animal seems to be regurgitating but the contrast-enhanced radiographs fail to reveal esophageal dysfunction, either the assessment of regurgitation is wrong or there is occult disease (e.g., partial stricture of the esophagus, esophagitis, gastroesophageal reflux). Procedures involving the use of liquid barium sulfate may miss some lesions (e.g., partial strictures). Repeating contrast-enhanced esophagography using barium plus food or performing esophagoscopy (or both) is appropriate in such patients.
Esophageal obstruction is principally caused by foreign objects and vascular anomalies, although cicatrix, tumors, and achalasia of the lower esophageal sphincter may also be responsible (Box 28-4). Obstruction should be characterized as congenital or acquired and as intraluminal, intramural, or extraesophageal. Congenital obstructions are usually extraesophageal vascular ring anomalies. Acquired intraluminal obstructions are usually caused by foreign objects or cicatrix secondary to esophagitis. The clinician should always determine whether animals with esophageal foreign objects also have a partial esophageal stricture that has predisposed them to the obstruction. Endoscopy may be both diagnostic and therapeutic in these animals; thoracotomy is seldom needed for the management of cicatrix or intraluminal foreign objects.
Esophageal weakness may be congenital or acquired. Congenital weakness is of uncertain cause, and further diagnostics are typically unfruitful. Acquired esophageal weakness usually results from an underlying neuromuscular problem. Although an underlying cause is infrequently diagnosed, finding one may lead to a permanent cure as opposed to supportive therapy, which only treats symptoms. A complete blood count (CBC), serum biochemistry profile, determination of serum antibody titers to acetylcholine receptors, an adrenocorticotropic hormone (ACTH)–stimulation test (see Chapter 53), and/or fecal examination for Spirocerca lupi ova are performed to look for causes of acquired esophageal weakness (Box 28-5). One may also consider searching for lead intoxication (nucleated red blood cells and basophilic stippling in the CBC, serum and urine lead concentrations), canine distemper (retinal lesions), and neuropathy-myopathy (electromyography, nerve biopsy, muscle biopsy). Chagas’ disease causes esophageal disease in people, but it is unknown whether it causes esophageal weakness in dogs.
Esophagoscopy may detect esophagitis or small lesions (e.g., partial strictures) that contrast-enhanced esophagrams do not reveal. If esophagitis is found, the clinician should look carefully for a cause (e.g., hiatal hernia, gastric outflow obstruction). After entering the stomach, the clinician retroflexes the tip of the endoscope and examines the lower esophageal sphincter for leiomyomas. Gastroduodenoscopy is performed concurrently to look for gastric and duodenal reasons for gastroesophageal reflux or vomiting. If fluoroscopy is available, the lower esophageal sphincter should be observed for several minutes to detect the frequency and severity of gastroesophageal reflux (normal animals may show occasional reflux).
VOMITING
Vomiting is usually caused by (1) motion sickness, (2) ingestion of emetogenic substances (e.g., drugs), (3) gastrointestinal (GI) tract obstruction, (4) abdominal (especially alimentary tract) inflammation or irritation, and (5) extragastrointestinal tract diseases that may stimulate the medullary vomiting center or the chemoreceptor trigger zone (Box 28-6). Occasionally, central nervous system (CNS) disease, behavior, and learned reactions to specific stimuli may cause vomiting. If the cause of the vomiting is not apparent on the basis of the history and physical examination findings, the next step depends on whether the vomiting is acute or chronic and whether there is hematemesis (Figs. 28-2 and 28-3). Remember that blood in vomitus may be fresh (i.e., red) or partially digested (i.e., “coffee grounds” or “dregs”).
Emetogenic Substances (Acute)
Drugs: almost any drug can cause vomiting (especially drugs administered orally [PO]), but the following drugs seem especially likely to cause vomiting:
* It is necessary to determine whether this is the cause of vomiting or an effect of vomiting.
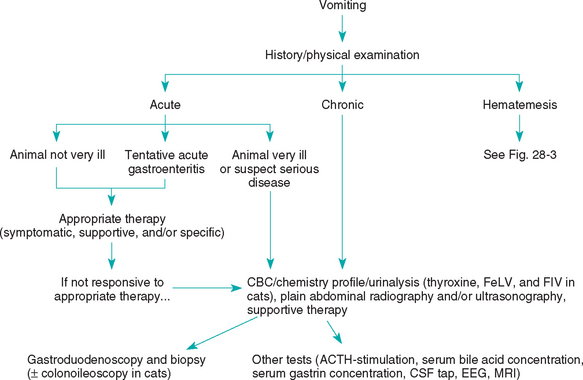
FIG 28-2 General diagnostic approach to vomiting in the dog and cat. CBC, Complete blood count; FeLV, feline leukemia virus; FIV, feline immunodeficiency virus; CSF, cerebrospinal fluid; EEG, electroencephalogram; MRI, magnetic resonance imaging.
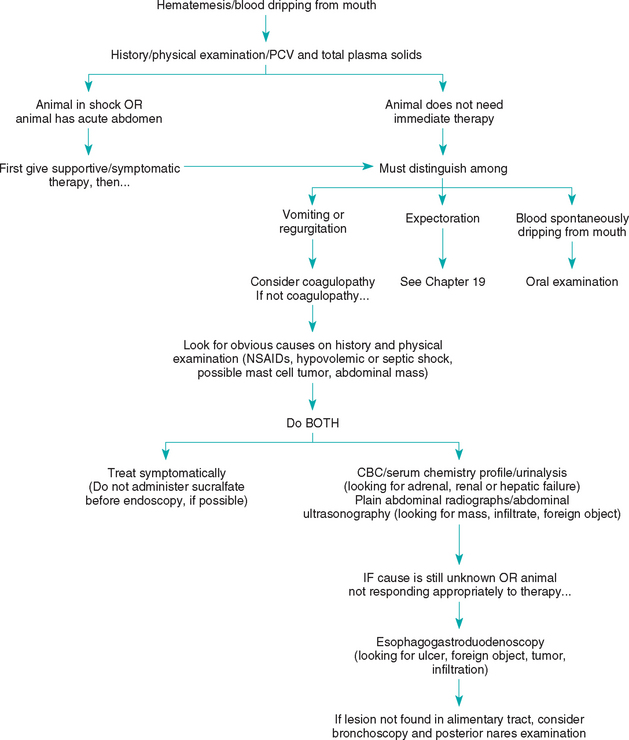
FIG 28-3 General diagnostic approach to hematemesis in the dog and cat. PCV, Packed cell volume; CBC, complete blood count.
In animals with acute vomiting without hematemesis, the clinician should first search for obvious causes (e.g., ingestion of a foreign body, intoxication, organ failure, parvovirus) as well as for secondary fluid, electrolyte, or acid-base abnormalities or sepsis that require prompt, specific therapy. If the animal’s condition seems stable and there is no obvious cause, symptomatic treatment is often used for 1 to 3 days. If the animal is too sick for the clinician to take a chance on guessing wrong, if the vomiting persists for 2 to 4 days after the start of symptomatic therapy, or if the condition worsens during this initial time, then more aggressive diagnostic testing is usually indicated.
The clinician should search for historical evidence of the ingestion of foreign objects, toxins, inappropriate food, or drugs. Physical examination is used to look for abdominal abnormalities (e.g., masses), linear foreign objects caught under the tongue, and evidence of extraabdominal disease (e.g., uremia, hyperthyroidism). The clinician should always consider the possibility of linear foreign bodies in vomiting cats and carefully examine the base of the tongue. Chemical restraint (e.g., ketamine HCl, 2.2 mg/kg of body weight given intravenously) may be necessary to examine this area properly. The abdomen is palpated to search for masses or pain, but even careful palpation may miss short ileocolic intussusceptions in the craniodorsal area of the abdomen. It is reasonable to perform fecal examination for parasites because they can be the cause of vomiting. If a cause cannot be found and the animal is not unduly ill, the clinician may prescribe a therapeutic trial (e.g., pyrantel and a dietary trial; see Table 30-7 and Chapter 30). Therapeutic trials should be designed so that the failure of a treatment allows the clinician to exclude at least one disease and then look for others.
If acute vomiting does not respond to symptomatic therapy or if the animal is so sick that the clinician cannot take a chance on symptomatic therapy being ineffective, aggressive diagnostic testing is indicated. Animals with acute or chronic vomiting without hematemesis should undergo abdominal imaging (i.e., radiography, ultrasonography) to look for problems such as an intestinal obstruction, foreign objects, masses, pancreatitis, peritonitis, poor serosal contrast in the region of the pancreas, free abdominal fluid, or free abdominal gas. Abdominal ultrasonography can be more revealing than plain radiographs; however, radiographs may be more sensitive in revealing some foreign bodies. A CBC, serum biochemistry profile, and urinalysis are also indicated. Cats should be tested for feline leukemia virus, feline immunodeficiency virus, and hyperthyroidism. It may be necessary to measure serum bile acid concentrations (or blood ammonia concentrations) or perform an ACTH–stimulation test (or at least resting serum cortisol concentrations) to identify hepatic or adrenal insufficiency that is not indicated by results of routine serum biochemistry profiles.
If results of the CBC, chemistry profile, urinalysis, and routine abdominal imaging are not diagnostic, the next step is usually either contrast-enhanced abdominal radiography or endoscopy plus biopsy. Endoscopy is usually more cost-effective than contrast-enhanced radiography in vomiting patients. During endoscopy the clinician should biopsy the stomach and duodenum, regardless of the gross mucosal appearance. In cats endoscopic biopsy of the ileum and ascending colon may be required to reveal the cause of vomiting. If laparotomy is chosen over endoscopy, the entire abdomen should be examined and biopsy of the stomach, duodenum, jejunum, ileum, mesenteric lymph node, liver, and, in cats, the pancreas should be performed.
If the cause of vomiting is undiagnosed after biopsy, the basis for previously excluding the different diseases should be reviewed. Diseases may be inappropriately ruled out (or diagnosed) because the clinician does not understand the limitations of certain tests. For example, dogs with hypoadrenocorticism may have normal electrolyte concentrations; inflammatory gastric and bowel disease may be localized to one area of the stomach or intestine and rarely causes significant changes in the white blood cell count; hyperthyroid cats may have normal serum thyroxine concentrations; dogs and cats with hepatic failure may have normal serum alanine aminotransferase and alkaline phosphatase activities; dogs and cats with pancreatitis may have normal serum amylase and lipase activities and normal abdominal ultrasound examinations; and Physaloptera infections are almost never diagnosed on the basis of fecal examination results. Finally, the clinician may have to consider less common diseases that are more difficult to diagnose (e.g., idiopathic gastric hypomotility, occult CNS disease, “limbic epilepsy”).
HEMATEMESIS
The clinician must often use history and physical examination to help identify hematemesis as well as distinguish it from other problems. Hematemesis may involve expulsion of digested blood (i.e., “coffee grounds”) or fresh blood. Animals with oral lesions that have blood dripping from their lips do not have hematemesis. Likewise, hemoptysis (i.e., coughing up blood) is not hematemesis.
The clinician should further distinguish vomiting that produces specks of blood from vomiting in which there is substantial blood present. The former may be caused by gastric mucosal trauma secondary to vigorous vomiting from any cause, and animals with such “hematemesis” should generally be treated as described in the previous section on vomiting. Patients that produce more substantial amounts of blood generally should be approached differently. Although hematemesis is usually caused by gastroduodenal ulceration and erosion (GUE), the clinician should not automatically start treating affected patients with antacids, cytoprotective agents, or sucralfate. Shock (e.g., hypovolemic, septic) and acute abdominal conditions should be eliminated first. The clinician should check the hematocrit and plasma total protein concentration to determine whether a blood transfusion is necessary (see Fig. 28-3). The clinician should next try to identify the cause, whether it is a coagulopathy (uncommon), the ingestion of blood from another site (e.g., the respiratory tract), or GUE (Box 28-7). Historical and physical examination findings may help in ruling out a coagulopathy or respiratory tract disease as the cause. However, platelet counts and the clotting capability (e.g., one-stage prothrombin time, partial thromboplastin time, buccal mucosal bleeding time) are preferred. The clinician should then look for obvious causes of GUE (e.g., acute gastritis, hemorrhagic gastroenteritis [HGE], ulcerogenic drugs [e.g., nonsteroidal antiinflammatory drugs, dexamethasone], recent severe hypovolemic shock, systemic inflammatory response syndrome, abdominal masses that may involve the gastric mucosa, cutaneous mast cell tumors). It is important to remember that a mast cell tumor can grossly mimic almost any other benign or malignant neoplasm, especially lipomas.
 BOX 28-7 Causes of Hematemesis
BOX 28-7 Causes of Hematemesis
Alimentary Tract Lesion
Gastrointestinal tract ulceration/erosion (important)
Gastric mucosal trauma from vigorous vomiting*
* Hematemesis caused by vigorous vomiting usually consists of specks of blood as opposed to larger quantities
If acute gastritis, HGE, nonsteroidal antiinflammatory drug–induced GUE, or GUE resulting from shock is strongly suspected, the clinician may elect a limited diagnostic workup (e.g., CBC, serum biochemistry panel) to define the degree of blood loss and look for evidence of renal or hepatic or adrenal failure. Then the animal can be treated symptomatically for 3 to 5 days (see pp. 407–409) to see what effect this has in controlling clinical signs. Endoscopy is not neces sarily helpful in many of these cases because it cannot reliably distinguish between ulcers that will heal with medical therapy and those that will require surgical resection. However, if the cause is unknown and especially if the vomiting or blood loss is severe or chronic, more aggressive diagnostic tests (e.g., abdominal imaging, gastroduodenoscopy) should be done (see Fig. 28-3). The stomach and duodenum should be imaged, preferably by abdominal ultrasonography with or without plain radiographs to look for alimentary tract infiltrations, foreign objects, and masses. Endoscopy is the most sensitive and specific means of finding and evaluating gastroduodenal ulcers and erosions. The principal indications for endoscopy in animals with upper GI blood loss include (a) distinguishing potentially resectable ulcers from widespread, unresectable erosions in patients with life-threatening GI bleeding; (b) localizing ulcers when considering surgical resection; and (c) determining the cause of GUE in patients with upper GI blood loss of unknown cause. During endoscopy the clinician should generally biopsy mucosa in an effort to rule out neoplasia or inflammatory bowel disease. Abdominal exploratory surgery may be performed instead of endoscopy, but it is easy to miss bleeding mucosal lesions when examining the serosal surface; intraoperative endoscopy (i.e., endoscopic examination of the mucosal surface of the stomach and duodenum while the abdomen is opened) may be useful in finding lesions that the surgeon cannot discern from the serosal surface.
If the source of bleeding cannot be found using gastroduodenoscopy, the clinician should consider possible bleeding sites beyond the reach of the endoscope; blood being swallowed from a lesion in the mouth, posterior nares, trachea, or lungs; hemorrhage from the gallbladder; or an intermittently bleeding gastric or duodenal lesion. Endoscopy of the trachea and choana can be diagnostic in some cases.
DIARRHEA
Diarrhea is excessive fecal water. Fecal mucus is principally caused by large bowel disorders and is discussed in the section on chronic large bowel diarrhea. The best approach to the assessment of animals with diarrhea is to first distinguish acute from chronic problems.
Acute diarrhea is usually caused by diet, parasites, or infectious diseases (Box 28-8). Dietary problems are often detected by history; parasites by fecal examination; and infectious diseases by history (i.e., evidence of contagion or exposure), CBC, fecal enzyme–linked immunosorbent assay for canine parvoviral antigen, and the exclusion of other causes. If acute diarrhea becomes unduly severe or persistent, additional diagnostic tests are recommended. The diagnostic approach for such a patient is similar to that adopted for the assessment of animals with chronic diarrhea.
Animals with chronic diarrhea should first be examined for evidence of parasites; multiple fecal examinations looking for nematodes, Giardia, and Tritrichomonas are indicated. Next, the clinician should determine whether the diarrhea originates from the small or large intestine. History is the best tool (Table 28-2). Failure to lose weight or body condition despite chronic diarrhea almost always indicates large bowel disease. Weight loss usually indicates the presence of small bowel disease, although severe large bowel diseases (e.g., pythiosis, histoplasmosis, malignancy) may cause weight loss. Animals with weight loss resulting from severe large bowel disease usually have obvious signs of colonic involvement (i.e., fecal mucus, marked tenesmus, hematochezia). If there is tenesmus, the clinician must ascertain whether it was present when the disease began; if tenesmus did not begin until late in the course, it may be due simply to perineal scalding or anal soreness resulting from chronic irritation.
 TABLE 28-2 Differentiation of Chronic Small Intestinal from Large Intestinal Diarrheas
TABLE 28-2 Differentiation of Chronic Small Intestinal from Large Intestinal Diarrheas
| SIGN | SMALL INTESTINAL DIARRHEA | LARGE INTESTINAL DIARRHEA |
|---|---|---|
| Weight loss* | Expected | Rare* |
| Polyphagia | Sometimes | Rare to absent |
| Frequency of bowel movements | Often near normal | Sometimes very increased |
| Volume of feces | Often increased | Sometimes decreased (because of the increased frequency) |
| Blood in feces | Melena (rare) | Hematochezia (sometimes†) |
| Mucus in feces | Uncommon | Sometimes |
| Tenesmus | Uncommon (but may occur later in chronic cases) | Sometimes |
| Vomiting | May be seen | May be seen |
* Failure to lose weight or condition is the most reliable indication that an animal has large bowel disease. However, animals with colonic histoplasmosis, pythiosis, lymphoma, or similar infiltrative diseases may have weight loss despite large bowel involvement.
† Hematochezia becomes much more important as a differentiating feature in animals that are losing weight. Its presence in such animals confirms the presence of large bowel involvement (either by itself or in combination with small bowel disease) despite weight loss.
Chronic small intestinal diarrhea can be categorized as maldigestion, nonprotein-losing malabsorptive disease, and protein-losing malabsorptive disease. Maldigestion is principally caused by exocrine pancreatic insufficiency (EPI) and rarely causes significant hypoalbuminemia (i.e., serum albumin concentration of 2.0 g/dl or less if the normal range is 2.5 to 4.4 g/dl). Film digestion tests for fecal trypsin activity, Sudan staining of feces for undigested fats, and fat absorption tests yield many false-negative and false-positive results. The most sensitive and specific test for EPI is measuring the serum trypsin-like immunoreactivity (TLI; see p. 388), which is indicated in dogs with chronic small intestinal diarrhea. The cPLI test may have use in diagnosing EPI, but this is not yet certain. EPI is rare in cats, but if suspected, an fTLI (feline TLI) is recommended.
Diagnosing EPI by treating the animal and evaluating its response to therapy is not recommended. If the animal has apparently responded to pancreatic enzyme supplementation, the enzymes should be repeatedly withheld and then readministered to ensure that the enzymes are responsible for resolution of the diarrhea. A false-positive diagnosis of EPI results in the unnecessary supplementation of expensive enzymes. Second, up to 15% of dogs with EPI do not respond when enzymes are added to their diet. If EPI is incorrectly ruled out in such a case, then unnecessary endoscopies or operations often result. Antibiotic-responsive enteropathy (ARE) may be responsible for causing such a failure to respond to proper enzyme supplements and dietary changes. Therefore the clinician should definitively diagnose or rule out EPI before proceeding with other diagnostic tests or treatments.
Malabsorptive intestinal disease may be protein-losing (PLE) or nonprotein-losing (Fig. 28-4). The serum albumin concentration will usually be markedly decreased (i.e., 2.0 g/dl or less; normal, 2.5 to 4.4 g/dl) in the former but not in the latter; hypoglobulinemia may develop in patients with PLE. Diarrhea occurs only if the absorptive capacity of the colon is exceeded. Therefore a dog or cat can be losing weight because of small intestinal malabsorption and not have diarrhea (see the section on weight loss). If an animal has marked hypoproteinemia not resulting from protein-losing nephropathy, hepatic insufficiency, or skin lesions, then PLE must be the main consideration.
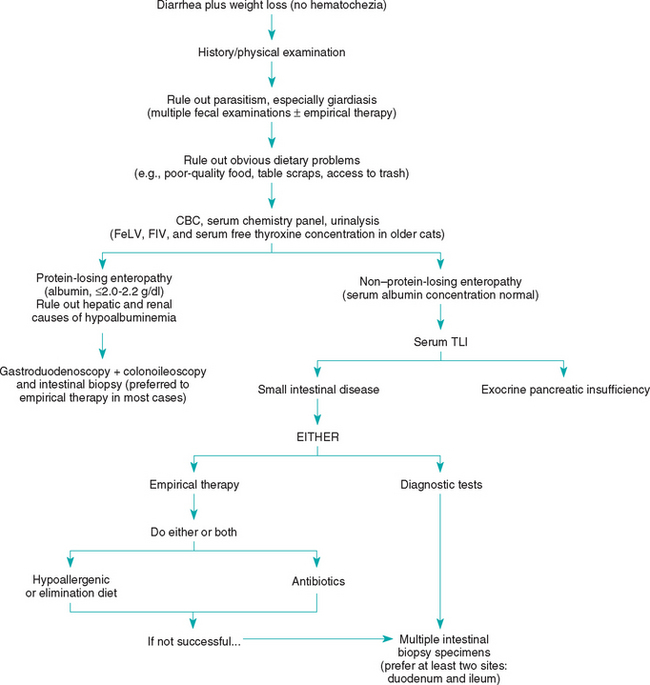
FIG 28-4 General diagnostic approach to small intestinal diarrhea in the dog and cat. CBC, Complete blood count; FeLV, feline leukemia virus; FIV, feline immunodeficiency virus, TLI, trypsin-like immunoreactivity.
In patients with nonprotein-losing malabsorptive disease, the clinician may perform additional diagnostic tests (e.g., intestinal biopsy) or design therapeutic trials depending on how ill the patient is. Therapeutic trials are the best way to diagnose antibiotic responsive enteropathy (ARE) or dietary responsive disease. ARE cannot reliably be diagnosed on the basis of quantitated duodenal culture, and decreased serum cobalamin plus increased serum folate concentrations are of dubious sensitivity. However, if a therapeutic trial is performed, the clinician must be sure that it is done properly (e.g., long enough, correct dose) so that it will almost certainly succeed if the animal has the suspected disease. If the patient seems particularly ill (e.g., substantial weight loss) or if PLE is suspected, ultrasonography and intestinal biopsy are often the preferred next steps because spending 2 to 3 weeks waiting to see if a therapeutic trial will work can be disasterous if the therapy is incorrect and the disease pro gresses. If diagnostic tests are chosen, abdominal imaging (especially ultrasonography) followed by gastroduodenoscopy or colonoscopy are typical next steps because the findings can help determine the cause of PLE and nonprotein-losing enteropathies in patients that do not have ARE or dietary responsive disease (Boxes 28-9 and 28-10). Absorptive tests and barium contrast–enhanced radiographs are rarely helpful. Abdominal ultrasonography may be diagnostic if it shows lymphadenopathy or intestinal infiltrates that can be aspirated percutaneously. Laparotomy or endoscopy can be performed to obtain biopsy specimens. If ultrasonography reveals a localized lesion that cannot be reached with an endoscope, then laparotomy is necessary as opposed to endoscopy. Otherwise, endoscopy is quicker and safer than laparotomy and may allow the clinician to biopsy lesions not seen from the serosal surface. Endoscopic biopsy specimens can be nondiagnostic if the endoscopist has not been carefully trained in taking biopsy specimens. If laparotomy is performed in hypoalbuminemic animals, it may be prudent to use nonabsorbable suture material and/or perform intestinal serosal patch grafting. The presence of distended intestinal lymphatics or lipogranulomas is suggestive of lymphangiectasia. If a cause is not shown by intestinal biopsy specimens, the main possible reasons for this are that the specimens were inadequate (e.g., not deep enough, from the wrong place, too much artifact), the animal has occult giardiasis, the animal has ARE, the animal has a dietary intolerance, or there is localized lymphangiectasia or inflammation at a site other than the one biopsied.
 BOX 28-10 Major Causes of Protein-Losing Enteropathy*
BOX 28-10 Major Causes of Protein-Losing Enteropathy*
Dog
Intestinal lymphangiectasia (common and important)
Alimentary tract lymphoma (common and important)
Severe inflammatory bowel disease
Alimentary tract fungal infections
Chronic intussusception (especially young dogs)
Alimentary tract hemorrhage (e.g., ulceration or erosion, neoplasia, parasites)
Unusual enteropathies (e.g., chronic purulent enteropathy, severe ectasia of mucosal crypts)
Massive hookworm or whipworm infestation (regionally important)
Cat
Severe inflammatory bowel disease
Alimentary hemorrhage (e.g., neoplasia, duodenal polyps, idiopathic ulceration)
* Any gastrointestinal disease can cause protein-losing enteropathy, but these are the most common causes. Except for lymphangiectasia, these diseases do not consistently produce protein-losing enteropathy.
Dogs with chronic large intestinal diarrhea (Box 28-11) should first undergo a digital rectal examination to search for mucosal thickening or proliferation. The rectum is the most common site of canine colonic neoplasia, and finding obvious mucosal lesions indicates the need for biopsy. If the rectal mucosa seems normal and the animal has not lost weight or become hypoalbuminemic (i.e., albumin <2.0 g/dl), it is often most appropriate to first try therapeutic trials. However, multiple fecal examinations to detect whipworms, Giardia (a small bowel problem that can mimic large bowel disease), and Tritrichomonas are appropriate. Therapeutic trials usually consist of high-fiber diets, hypoallergenic diets, antibiotics to control “clostridial” colitis, or treatment for whipworms.
 BOX 28-11 Major Causes of Chronic Large Intestinal Diarrhea
BOX 28-11 Major Causes of Chronic Large Intestinal Diarrhea
Dog
Dietary responsive (intolerance or allergy; important and common)
Fiber-responsive (important and common)
Additional diagnostic tests that may be done instead of therapeutic trials principally include obtaining biopsy specimens of the colonic mucosa by colonoscopy, fecal cultures, assays for clostridial toxin, and antigen tests for specific organisms (e.g., Campylobacter). Fecal cultures for specific pathogens (e.g., Salmonella spp.) should be done if the history indicates the possiblity of a contagious disorder or if the animal is not responding to seemingly appropriate therapy. Fecal cultures should be done before the animal receives enemas or intestinal lavagae solutions. Unless there is some epidemiologic reason to suspect an infectious bacteria, fecal cultures tend to be low-yield procedures that are difficult to interpret.
If the results of these tests are not diagnostic, the clinician must consider three main possibilities. First, the biopsy specimens may not be representative of the entire colonic mucosa. For example, if the disease is localized to the region of the ileocolic valve, it will be necessary to use a flexible endoscope to reach the area. Second, the pathologist may not have recognized the lesions. This occasionally happens, especially if animals have colonic histoplasmosis or neoplasia. Third, there may be no mucosal lesions. This typically occurs in animals with a dietary intolerance or allergy, “clostridial” colitis, chronic giardiasis, or irritable bowel syndrome (i.e., fiber-responsive diarrhea), all common problems in dogs.
HEMATOCHEZIA
If the patient has hematochezia (fresh blood in the feces) and diarrhea, the problem should usually be approached in the same way as that for animals with large bowel diarrhea (see p. 362). The patient with normal stools plus hematochezia is approached slightly differently. Streaks of blood on the outside of otherwise normal feces usually indicates the presence of a distal colonic or rectal lesion, whereas blood that is mixed into the feces suggests that bleeding is occurring higher in the colon. Coagulopathies are rarely a cause of rectal bleeding only. Focal bleeding lesions in the distal colon, rectum, or perineal region (Box 28-12) are especially important. Acute hematochezia may also result from trauma.
 BOX 28-12 Major Causes of Hematochezia*
BOX 28-12 Major Causes of Hematochezia*
Anal-Rectal Disease
Anal sacculitis (important and common)
Anal-rectal trauma (e.g., foreign body, thermometer, enema tube, fecal loop, pelvic fractures)
Colonic/Intestinal Disease
Dietary responsive (intolerance or allergy; common)
“Clostridial” colitis (common)
Hemorrhagic gastroenteritis (important)
Parvoviral enteritis (important and common)
* These diseases do not consistently produce hematochezia; however, when hematochezia is present, these are the most common causes.
A thorough digital rectal examination is the best initial step (even if anesthesia is necessary). The clinician should express each anal sac repeatedly and examine the contents. If the problem is chronic and results of these tests are uninformly negative, then colonoscopy and biopsy are usually indicated. An excellent barium enema is usually inferior to a good endoscopic examination. Biopsy specimens should include the submucosa, or some neoplastic lesions will be missed. Hematochezia is rarely severe enough to cause anemia; however, a CBC can be performed to look for and evaluate the cause of anemias.
MELENA
Melena is caused by digested blood and is seen as coal tar black (not dark) feces. The clinician must be extremely careful to distinguish melena from stools that are intensely dark green. Melena is strongly suggestive of upper alimentary tract bleeding or the ingestion of blood (Box 28-13). However, a lot of blood must enter the GI tract in a short time to produce melena, which is why most animals with upper GI hemorrhage do not have melena. A CBC is indicated to look for iron deficiency anemia (i.e., microcytosis, hypochromasia, thrombocytosis). Measuring the total serum iron concentration and the total iron-binding capacity plus staining the bone marrow for iron are more definitive tests for iron deficiency anemia. Ultrasonongraphy is very useful when looking for infiltrated, bleeding lesions (e.g., an intestinal tumor). Gastroduodenoscopy is the most sensitive test for GUE (which is often missed by ultrasonography). If gastroduodenoscopy is nonrevealing, then contrast-enhanced radiography may detect small intestinal lesions beyond the reach of the endoscope. If imaging reveals a lesion beyond the reach of the endoscope, exploratory laparotomy is required. The clinician may elect to perform exploratory surgery immediately, but it is easy to miss bleeding mucosal lesions when examining the serosa or palpating the bowel. Intraoperative endoscopy may be helpful if surgery is performed but no lesion is detected.
 BOX 28-13 Major Causes of Melena*
BOX 28-13 Major Causes of Melena*
* These diseases do not consistently produce melena; however, if melena is present, these are the most common causes.
TENESMUS
Tenesmus (i.e., ineffectual or painful straining at urination or defecation) and dyschezia (i.e., painful or difficult elimination of feces from the rectum) are principally caused by obstructive or inflammatory distal colonic or urinary bladder or urethral lesions (Box 28-14). Colitis, constipation, perineal hernias, perianal fistulas, prostatic disease, and cystic/urethral disease are the most common causes of tenesmus. Most rectal masses and strictures cause hematochezia; however, some do not disrupt the colonic mucosa and cause only tenesmus.
The first goal (especially in cats) is to distinguish lower urinary tract from alimentary tract disease. In cats tenesmus secondary to a urethral obstruction is often misinterpreted as constipation. By observing the animal, the clinician may be able to determine whether the animal is attempting to urinate or defecate. The clinician palpates the bladder (a distended urinary bladder indicates an obstruction; a small, painful bladder indicates inflammation); performs a urinalysis; and, if necessary, catheterizes the urethra to determine whether it is patent.
If the clinician suspects tenesmus resulting from alimentary tract disease, he or she should palpate the abdomen and rectum and visualize the anus and perineal areas. The clinician should not assume that constipation, if present, is causing the tenesmus. Severe pain (e.g., that resulting from proctitis) may make the animal refuse to defecate and cause secondary constipation. Most strictures, perineal hernias, masses, enlarged prostates, pelvic fractures, and rectal tumors can be detected during a digital rectal examination. The clinician may need to use two fingers to detect partial strictures when examining large dogs. Perianal fistulae are usually visible but may be detected only as perirectal thickenings. Next, the clinician expresses the anal sacs and examines their contents. Finally, the clinician evaluates the feces to determine whether they are excessively hard or have abnormal contents (e.g., hair, trash).
A biopsy should be done of any mass, stricture, or infiltrative lesion found by rectal examination. A rectal scraping is sometimes sufficient (e.g., histoplasmosis), but biopsy specimens that include the submucosa are usually preferred. Fine-needle aspiration should be performed on extracolonic masses because abscesses occasionally occur in extracolonic locations.
If the clinician is confused by the findings from a physical examination, observing the animal defecate may help define the underlying process. Animals with inflammation often continue to strain after defecating, whereas a constipated animal strains before feces are produced. Tenesmus that occurs when an animal is in a squatting position often results from colitis, whereas tenesmus that occurs when an animal is in a semiwalking or partial squatting position usually results from constipation.
CONSTIPATION
Constipation (the infrequent and difficult evacuation of feces) and obstipation (intractable constipation) have several causes (Box 28-15). The initial use of symptomatic therapy is often successful, but it is also important to look for causes because some problems may become harder to treat if symptomatic therapy masks the signs while the underlying disease progresses.
 BOX 28-15 Causes of Constipation
BOX 28-15 Causes of Constipation
Colonic Obstruction
Deviation of rectal canal: perineal hernia
A search of the history for iatrogenic, dietary, environmental, or behavioral causes should be done. Feces should be examined to determine whether they contain plastic, bones, hair, popcorn, or other such material. Physical and rectal examinations are done to search for rectal obstruction or infiltration. Plain pelvic radiographs can help show whether the animal has anatomic abnormalities or a previously undetected colonic obstruction (e.g., prostatomegaly, enlarged sublumbar lymph node). Ultrasonography is the preferred technique when looking for infiltrates. A serum biochemistry panel may reveal causes of colonic inertia (e.g., hypercalcemia, hypokalemia, hypothyroidism).
Colonoscopy is indicated if the clinician suspects an obstruction too orad to be detected by digital examination. Ultrasound-guided fine-needle aspiration of infiltrative colonic lesions sometimes yields diagnostic findings, but colonoscopy (especially rigid) allows a more reliable biopsy specimen to be obtained. If a thorough diagnostic workup fails to identify a cause in a patient with a grossly dilated colon, idiopathic megacolon may be present.
FECAL INCONTINENCE
Fecal incontinence is caused by neuromuscular disease (e.g., cauda equine syndrome, lumbosacral stenosis) or a partial rectal obstruction. Severe irritative proctitis may cause urge incontinence. Animals with rectal obstructions continually try to defecate because the anal canal is filled with feces. Proctitis is suspected on the basis of rectal examination findings and confirmed by proctoscopy and biopsy findings. Neuromuscular disease is suspected if an abnormal anal reflex is found, usually in conjunction with other neurologic defects in the anal, perineal, hindlimb, or coccygeal region. Defects in the coccygeal region are discussed in Chapter 70.
WEIGHT LOSS
Weight loss is usually caused by one of several categories of problems (Box 28-16). If other problems with more defined lists of differentials (e.g., ascites, vomiting, diarrhea, polyuria/polydipsia) are also present, they should usually be investigated first because it may be easier to find the cause. If there are no such concurrent problems that allow relatively prompt localization of the disease, the clinician should then determine what the animal’s appetite was when the weight loss began (Fig. 28-5). Almost any disease can cause anorexia. Weight loss despite a good appetite usually indicates maldigestion, malabsorption, or excessive utilization (e.g., hyperthyroidism, lactation) or inappropriate loss (e.g., diabetes mellitus) of calories.
 BOX 28-16 Causes of Weight Loss
BOX 28-16 Causes of Weight Loss
Food
Not enough (especially if there are multiple animals)
Poor quality or low caloric density
Anorexia (see Box 28-17)
Dysphagia (see Box 28-1)
Regurgitation/Vomiting (i.e., losing enough calories to account for weight loss; see Boxes 28-4 to 28-6)
Maldigestive Disease
Exocrine pancreatic insufficiency (usually but not always associated with diarrhea)
Malabsorptive Disease (see Box 28-9)
Small intestinal disease (may be associated with normal stools)
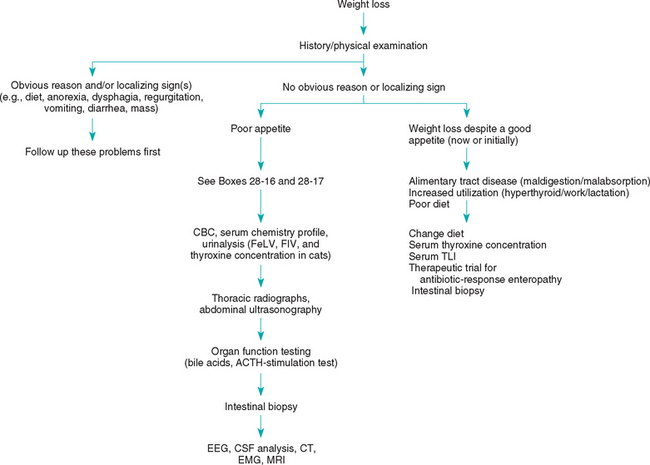
FIG 28-5 General diagnostic approach to weight loss in the dog and cat. CBC, Complete blood count; FeLV, feline leukemia virus; FIV, feline immunodeficiency virus; ACTH, adrenocorticotropic hormone; EEG, electroencephalography; EMG, electromyography; CT, computed tomography; CSF, cerebrospinal fluid; MRI, magnetic resonance imaging.
The animal’s history should be reviewed for evidence of dietary problems, dysphagia, regurgitation, vomiting, or increased use of calories (e.g., lactation, work, extremely cold temperature). Signalments suggestive of particular diseases (e.g., hyperthyroidism in older cats, hepatic failure in younger dogs with signs of portosystemic shunts) should be recognized. It is important to remember that diarrhea may be absent in animals with severe small intestinal disease.
Physical examination is performed to identify abnormalities that might help localize the problem to a particular body system (e.g., nasal disease preventing normal olfaction, dysphagia, arrhythmia suggestive of cardiac failure, weakness suggestive of neuromuscular disease, abnormally sized or shaped organs, abnormal fluid accumulations). Retinal examination may identify inflammatory or infiltrative diseases, especially in cats.
A CBC, serum biochemistry profile, and urinalysis should be done next to search for evidence of inflammation, organ failure, or a paraneoplastic syndrome. Cats should be tested for circulating feline leukemia virus antigen and antibodies to feline immunodeficiency virus. Serum T4 (and sometimes fT4) concentrations should be determined in middle-aged to older cats. If clinical pathology data are not helpful, imaging is usually the next step. Thoracic radiographs (ventrodorsal and both lateral views) are important because significant thoracic disease cannot be ruled out on the basis of physical examination findings alone. Most cats and some dogs can be palpated well enough that abdominal radiographs are not cost-effective early in the workup. However, abdominal ultrasonography may reveal focal or infiltrative lesions that cannot be palpated (plain radiographs reveal such lesions less frequently).
If the cause of weight loss remains unknown after these steps have been taken, additional tests are necessary. Daily physical examinations can be an important means of localizing the problem. Fever of unknown origin may be noted (see Chapter 90). Organ function testing (e.g., serum bile acid concentrations, ACTH–stimulation testing, serum TLI, serum cobalamin) is reasonable. Likewise, if serum T4 concentrations are normal in a cat with suspected hyperthyroidism, the serum fT4 concentration should be determined or other tests (e.g., nuclear scintigraphy) performed (see Chapter 51).
If the cause of weight loss still remains undiagnosed, the clinician should consider performing gastric and intestinal biopsy (preferably endoscopically). If a laparotomy is performed instead, the entire abdomen should be examined, multiple biopsy samples of the alimentary tract obtained, and biopsy of the liver and mesenteric lymph nodes done. Biopsy of the pancreas should also be done in cats.
Other possible diagnostic tools include tests to evaluate the CNS (i.e., cerebrospinal fluid analysis, electroencephalography, computed tomography, magnetic resonance imaging; animals that are anorectic as a result of severe CNS disease do not always have obvious cranial nerve deficits or seizures) and peripheral nerves and muscles (i.e., electromyography, muscle or nerve biopsies; sometimes the weakness associated with neuropathies and myopathies is mistaken for lethargy). (See Chapter 64.) If the cause of the weight loss still remains undiagnosed and the history and physical examination findings are still noncontributory, occult cancer becomes a major differential diagnosis. In such cases, the clinician may have to wait and retest later with the hope that the disease will progress enough to be detected.
Causes of weight loss that can be particularly difficult to diagnose include gastric disease that does not cause vomiting, intestinal disease that does not cause vomiting or diarrhea, hepatic disease with normal serum alanine aminotransferase or alkaline phosphatase activities, occult inflammatory disease, hypoadrenocorticism with normal serum electrolyte concentrations, occult cancer, “dry” feline infectious peritonitis, and CNS disease without cranial nerve deficits or seizures.
ANOREXIA
The approach to the diagnostic evaluation of animals with anorexia of uncertain cause is similar to that for animals with weight loss (see Fig. 28-5), and the differential diagnoses are also similar (Box 28-17). Inflammatory disease is often detected by the CBC or the finding of fever (see Chapter 90). GI disease may produce anorexia without vomiting or diarrhea. Cancer cachexia (with anorexia as the predominant sign) may stem from relatively small tumors that are not grossly detectable, although this is rare. Finally, CNS disease must be considered whenever there is altered mentation. However, altered mentation may resemble the depression and lethargy commonly seen in animals with other diseases.
 BOX 28-17 Major Causes of Anorexia
BOX 28-17 Major Causes of Anorexia
Alimentary Tract Disease
Dysphagia (especially resulting from pain)
Nausea (stimulation of the medullary vomiting center for any reason, even if it is not sufficient to cause vomiting, especially gastric or intestinal disease; see Box 28-6)
ABDOMINAL EFFUSION
Abdominal effusion is usually caused by hypoalbuminemia, portal hypertension, or peritoneal inflammation. Effusions resulting from alimentary tract disorders are primarily caused by PLE or alimentary tract rupture (i.e., septic peritonitis). Some animals with PLE have normal stools, with ascites being the presenting complaint. Malignant tumors may obstruct lymphatic flow or increase vascular permeability, causing modified transudates to form or nonseptic peritonitis to develop. Modified transudates usually result from hepatic or cardiac disease or from malignant conditions. For further information on abdominal effusions, see Chapters 35 and 36.
ACUTE ABDOMEN
Acute abdomen refers to various abdominal disorders producing shock (hypovolemic or septic), sepsis, or severe pain (Box 28-18). Causes may include alimentary tract obstruction or leakage, vascular compromise (e.g., congestion, torsion, volvulus, ischemia), inflammation, neoplasia, or sepsis. The diagnostic evaluation of this problem is determined by the severity of the clinical signs (Fig. 28-6).
 BOX 28-18 Major Causes of Acute Abdomen
BOX 28-18 Major Causes of Acute Abdomen
Septic Inflammation
Organ Distention or Obstruction
Gastric dilation or volvulus (important and common)
Intestinal obstruction resulting from many causes (important and common)
Ischemia
Torsion of spleen, liver lobe, testicle, or other organ
Thromboembolism of abdominal organ(s)
Other Causes of Abdominal Pain (see Box 28-19)
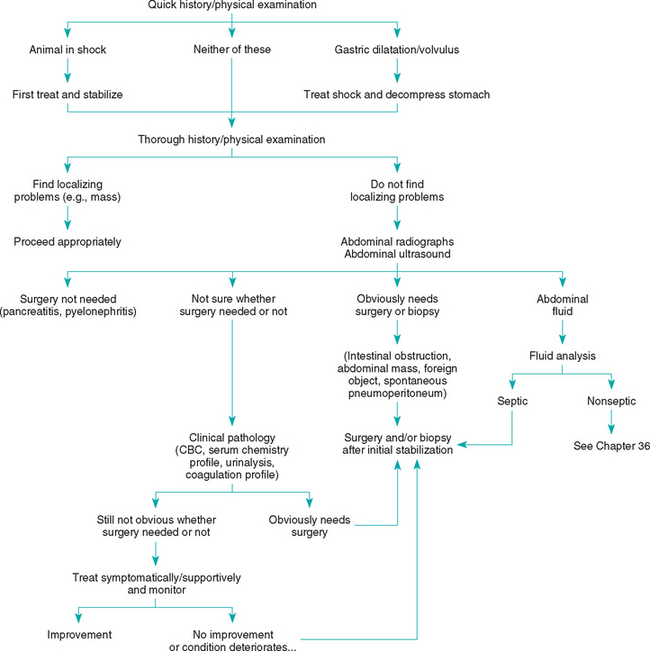
FIG 28-6 General diagnostic approach to acute abdomen in the dog and cat. CBC, Complete blood count.
Shock and gastric dilation or volvulus (GDV) must be identified and treated immediately. Once these conditions are eliminated, the next major decision is whether to perform exploratory surgery or initiate medical therapy. Animals with abdominal masses, foreign objects, bunched-up loops of painful small intestine (e.g., linear foreign body), or spontaneous septic peritonitis should typically undergo surgery as soon as supportive therapy has made the risk of anesthesia acceptable. If the cause of the acute abdomen is uncertain, it can be difficult to determine whether surgery is indicated. Surgery is not necessarily beneficial and may even be detrimental to animals with conditions such as pancreatitis, parvoviral enteritis, pyelonephritis, or prostatitis. Typically, abdominal imaging (i.e., plain abdominal radiography or ultrasonongraphy) and clinical pathologic studies (i.e., CBC, chemistry panel) should be performed before a laparotomy is performed. Ultrasound can reveal changes (e.g., infiltration) that radiographs cannot detect, sometimes allowing diagnosis via aspiration (and potentially eliminating the need for surgery). However, radiographs occasionally detect lesions (e.g., small foreign bodies) that were missed ultrasonographically. Imaging may reveal spontaneous pneumoperitoneum, abdominal masses, foreign objects, alimentary tract obstruction, gastric or mesenteric torsion (these require surgical treatment), or free peritoneal fluid (this requires abdominocentesis and fluid analysis for management). A barium series is seldom needed and may complicate later therapy/surgery.
If optimal medical therapy is being given and the animal’s condition is clearly deteriorating or does not improve after 2 to 5 days of therapy, or if the animal continues to have excruciating pain, it is often appropriate to recommend exploratory surgery. Inform the client that you may discover the animal has a disorder not surgically correctable (especially pancreatitis) or that nothing abnormal may be found. In the latter case, the clinician should biopsy various abdominal organs and then treat the animal’s symptoms while awaiting biopsy results.
ABDOMINAL PAIN
“Abdominal” pain must first be determined to be abdominal and not extraabdominal in origin (e.g., thoracolumbar pain is often erroneously assessed as being abdominal in origin). An animal with true abdominal pain may show obvious discomfort (e.g., it paces or repeatedly assumes different positions, repeatedly looks at or licks its abdomen) and may whine, growl, or snap if the abdomen is touched. Some dogs stretch out and assume a “praying” position (i.e., the “position of relief”). Other animals have inconspicuous signs (e.g., the animal grunts or tries to walk away when palpated, the abdomen is tensed) that are easily missed. On the other hand, a poor or rough abdominal palpation technique in normal animals may elicit a guarding response that can mimic abdominal pain. The main causes of abdominal pain are listed in Box 28-19.
 BOX 28-19 Causes of Abdominal Pain
BOX 28-19 Causes of Abdominal Pain
Gastrointestinal Tract
Foreign object (especially linear)
See also Box 28-18, under Organ Distention or Obstruction
If the patient has abdominal pain, the goal is to determine the source. If the pain is originating from within the abdominal cavity, the diagnostic approach depends on its severity, the progression of disease, and whether there are any obvious causes. The steps taken in diagnosing the cause of abdominal pain are similar to those taken in an animal with acute abdomen. Some causes of abdominal pain can be difficult to diagnose (e.g., acute pancreatitis, localized peritonitis).
ABDOMINAL DISTENTION OR ENLARGEMENT
Abdominal distention or enlargement may be associated with an acute abdomen, but these conditions are typically separate problems. It is best to believe clients who claim there is abdominal enlargement until good cause is found to do otherwise. There are six main causes of abdominal distention (Box 28-20).
 BOX 28-20 Causes of Abdominal Enlargement
BOX 28-20 Causes of Abdominal Enlargement
Tissue
Hepatomegaly (infiltrative or inflammatory disease, lipidosis, neoplasia)
Splenomegaly (infiltrative or inflammatory disease, neoplasia, hematoma)
Renomegaly (neoplasia, infiltrative disease, compensatory hypertrophy)
The first concern is whether an acute abdomen is present (e.g., GDV, septic peritonitis, hemoabdomen plus shock). After an acute abdomen is ruled out, it should be possible to classify the enlargement on the basis of the physical examination and abdominal imaging (i.e., radiography or ultrasonography) findings, according to the criteria in Box 28-20. Obesity and pregnancy should be obvious. Specimens of free abdominal fluid should be obtained and analyzed as described in Chapter 36. Biopsy should be performed on abdominal masses and enlarged organs, unless there is a reason not to (e.g., hepatomegaly caused by severe right-sided heart failure). Fine-needle aspiration is usually safe, although the leakage of septic contents or implantation of neoplastic cells may occur. Ultrasonography helps determine the potential for hemorrhage or leakage (e.g., cyst, mass with ultrasonographic characteristics of hemangiosarcoma). The finding of a spontaneous pneumoperitoneum suggests alimentary tract rupture or septic peritonitis and is an indication for immediate surgical exploration. A hollow viscus dilated with gas may indicate obstruction (i.e., gastric dilation, intestinal obstruction) or physiologic ileus (see pp. 384 and 436; Figs. 29-5 and 32-4). Surgery is indicated if an obstruction seems likely. If abdominal musculature weakness is suspected, the clinician should test for hyperadrenocorticism. Results of a CBC, serum biochemistry panel, and urinalysis are used to look for specific organ involvement (e.g., hyperadrenocorticism). Contrast-enhanced alimentary or urinary tract radiographs may be useful in selected cases, although ultrasonography often makes such techniques unnecessary.
Harkin KR. Constipation, tenesmus, dyschezia, and fecal incontinence. In Ettinger SJ, et al, editors: Textbook of veterinary internal medicine, ed 6, Philadelphia: WB Saunders, 2005.
Hoover JP, et al. Anorexia. In Ettinger SJ, et al, editors: Textbook of veterinary internal medicine, ed 6, Philadelphia: WB Saunders, 2005.
Kelly KM. Melena and hematochezia. In Ettinger SJ, et al, editors: Textbook of veterinary internal medicine, ed 6, Philadelphia: WB Saunders, 2005.
Marretta SM. Ptyalism. In Ettinger SJ, et al, editors: Textbook of veterinary internal medicine, ed 6, Philadelphia: WB Saunders, 2005.
Steiner JM. Diarrhea. In Ettinger SJ, et al, editors: Textbook of veterinary internal medicine, ed 6, Philadelphia: WB Saunders, 2005.
Twedt DC. Vomiting. In Ettinger SJ, et al, editors: Textbook of veterinary internal medicine, ed 6, Philadelphia: WB Saunders, 2005.
Willard MD, et al. Gastrointestinal, pancreatic, and hepatic disorders. In Willard MD, et al, editors: Small animal clinical diagnosis by laboratory methods, ed 4, Philadelphia: WB Saunders, 2004.