CHAPTER 70 Disorders of the Spinal Cord
GENERAL CONSIDERATIONS
Spinal cord disorders can be caused by anomalies, degeneration, neoplasia, inflammatory conditions, external trauma, internal trauma from disk extrusion, hemorrhage, or infarction (Box 70-1). Clinical signs depend on lesion location and severity and frequently include focal or generalized pain, paresis, paralysis, and occasionally an inability to urinate. Examination of the signalment, history, onset, and progression of the disease can provide valuable information necessary for establishing a likely cause. Congenital malformations are present at birth, do not progress, and are often breed-associated. External trauma, type 1 intervertebral disk extrusion, and vascular disorders (hemorrhage or infarction) are usually associated with acute, nonprogressive signs. Infectious or noninfectious inflammatory disorders typically have a subacute and progressive course, whereas tumors and degenerative processes are most often slowly progressive.
 BOX 70-1 Common Causes of Spinal Cord Dysfunction
BOX 70-1 Common Causes of Spinal Cord Dysfunction
Subacute Progressive (Days to Weeks)
Noninfectious inflammatory disease
LOCALIZING SPINAL CORD LESIONS
Once a complete neurologic examination has been performed and postural reactions, proprioception, strength, muscle tone, and spinal reflexes have all been assessed, it is possible to identify the location of a spinal cord lesion. Functionally, the spinal cord can be divided into four regions: the cranial cervical spinal cord (C1-C5), the cervical intumescence (C6-T2), the thoracolumbar region (T3-L3), and the lumbar intumescence (L4-S3). Signs allowing localization of spinal cord lesion to each site and differential diagnoses considered for disease localizing to each site are listed in Table 70-1 and Box 70-2.
 TABLE 70-1 Neurologic Findings in Dogs and Cats with Spinal Cord Lesions
TABLE 70-1 Neurologic Findings in Dogs and Cats with Spinal Cord Lesions
| SITE OF LESION | THORACIC LIMBS | PELVIC LIMBS |
|---|---|---|
| C1-C5 | UMN | UMN |
| C6-T2 | LMN | UMN |
| T3-L3 | Normal | UMN |
| L4-S3 | Normal | LMN |
UMN, Upper motor neuron signs; LMN, lower motor neuron signs.
C1-C5 LESIONS
Lesions of the cranial cervical spinal cord cause upper motor neuron (UMN) paresis in all four limbs. Because the spinal cord pathways to the rear limbs are more superficial in the cord than those to the forelimbs, rear limb deficits are usually worse than forelimb deficits in patients with mild compressive C1-C5 spinal cord lesions. Central canal lesions (e.g., intramedullary neoplasia, infarcts, hydromyelia) in the C1-C5 region occasionally cause severe UMN deficits in the forelimbs with nearly normal rear limbs (central cord syndrome) as the superficially located white matter tracts to the rear limbs are spared. Most lesions of the C1-C5 spinal cord cause a long-strided, ataxic gait; postural reaction deficits, including decreased conscious proprioception (slow knuckling); increased extensor muscle tone; and normal to increased spinal reflexes in all four limbs. Unilateral lesions of the cervical cord cause hemiparesis and UMN signs only in the ipsilateral rear limbs and forelimbs. Cervical lesions are rarely severe enough to cause loss of deep pain sensation; such a severe injury would cause complete respiratory paralysis and rapid death.
C6-T2 LESIONS
Lesions of the spinal cord between C6 and T2 result in paresis of all four limbs and ataxia that is most pronounced in the rear limbs. The spinal cord segments containing the cell bodies of the nerves of the brachial plexus are affected in this region, so lower motor neuron (LMN) signs of weakness, a short-strided “choppy” gait, muscle atrophy, and hyporeflexia predominate in the forelimbs. Disruption of ascending and descending spinal cord tracts in this region causes UMN deficits in the rear limbs, including ataxia, a long stride, loss of conscious proprioception, delayed postural reactions, increased extensor muscle tone, and normal to increased reflexes. If the lesion affects only the central cord, sparing the superficially located long tracts to the rear limbs, the forelimb LMN signs may be much more pronounced than the rear limb UMN signs. When C6-T2 lesions are unilateral, ipsilateral forelimbs and rear limbs will be affected. Horner’s syndrome may be seen if the T1-T2 spinal cord segments or nerve roots are involved (see Chapter 66), and the ipsilateral cutaneous trunci reflex may be lost if the C8-T1 spinal cord segments or nerve roots are damaged. Because the phrenic nerve originates at C5 to C7, a severe lesion in this region could also cause diaphragmatic paralysis.
T3-L3 LESIONS
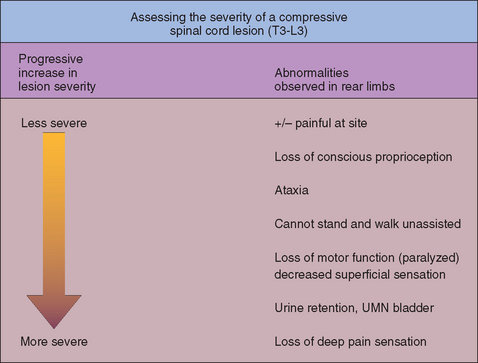
Lesions of the spinal cord between T3 and L3 cause UMN paresis and ataxia affecting the rear limbs (see Table 70-1), with normal forelimbs. Examination of the rear limbs reveals a long, incoordinated stride; loss of conscious proprioception; delayed postural reactions; increased extensor muscle tone; and normal to increased reflexes. As compressive lesions of the spinal cord in this region become more severe, there is a predictable worsening of the neurologic deficits (Fig. 70-1). With severe focal lesions in this region there may be a loss of the cutaneous trunci reflex caudal to the site of the lesion.
L4-S3 LESIONS
Lesions affecting the lumbar intumescence cause LMN signs in the rear limbs. Severe weakness, muscle atrophy, and loss of reflexes are apparent in the rear limbs, and forelimbs are normal. Animals that can still walk exhibit a short-strided rear limb gait. Bladder dysfunction and paresis or paralysis of the anal sphincter and tail are common with severe lesions. Lesions that compress the lumbar, sacral, and caudal nerve roots as they extend caudally from the end of the spinal cord within the vertebral canal (the cauda equina) usually cause pain at the site and, when severe, cause LMN dysfunction as well.
DIAGNOSTIC APPROACH
Lesions should be localized to a spinal cord region on the basis of the neurologic examination. It is important to recognize that spinal cord segments do not correlate directly with vertebral location in the dog and cat (Table 70-2; Fig. 70-2). The C6-T2 spinal cord segments of the cervical intumescence are located within vertebrae C4-T2. The L4-S3 spinal cord segments of the lumbar intumescence are located within vertebrae L3-L5 in dogs and L3-L6 in cats. The spinal cord is shorter than the vertebral canal, with the caudal segments ending at approximately the L6 vertebra in dogs and the L7 vertebra in cats. The nerve roots arising from the L7, sacral, and caudal spinal cord segments (the cauda equina) course caudally within the vertebral canal to their site of exit immediately caudal to the vertebra of the same number and are susceptible to compressive damage in the lumbosacral region (see the discussion of cauda equina syndrome.
 TABLE 70-2 Localization of Spinal Cord Segments Within Vertebral Bodies in the Dog
TABLE 70-2 Localization of Spinal Cord Segments Within Vertebral Bodies in the Dog
| SPINAL CORD SEGMENT | VERTEBRAL BODY |
|---|---|
| C1-C5 | C1-C4 |
| C6-T2 | C4-T2 |
| T3-L3 | T2-L3 |
| L4 | L3-L4 |
| L5, L6, L7 | L4-L5 |
| SI-S3 | L5 |
| Caudal | L6-L7 |
| Cauda equina spinal nerves | L5-sacrum |
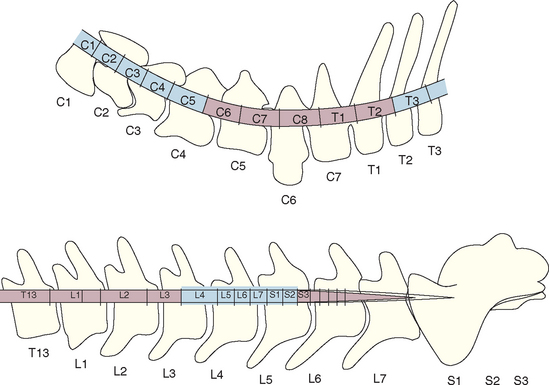
FIG 70-2 Position of the spinal cord segments within the cervical, cranial thoracic, and lumbar vertebrae. The cervical intumescence (C6-T2) and the lumbar intumescence (L4-S3) are highlighted.
Once spinal cord lesions are localized to the proper regional spinal cord segments and vertebrae, further diagnostic testing will usually be necessary to establish an etiology. Radiographs should be taken of the vertebral bodies that house the affected spinal cord segments. Vertebral radiographs may identify vertebral malformations, subluxation caused by trauma, diskospondylitis, vertebral fractures, intervertebral disk disease, and lytic vertebral neoplasms. A myelogram or other diagnostic imaging technique (e.g., computerized tomography [CT], magnetic resonance imaging [MRI]) may be performed to identify a compressive or expansive lesion in the spinal canal. Cerebrospinal fluid analysis can be performed to look for evidence of neoplasia or inflammation. When systemic infectious or neoplastic disorders are considered as differentials for a myelopathy, ancillary tests such as thoracic and abdominal radiographs, abdominal ultrasound, lymph node aspirates, complete ophthalmic examination, serology, and tissue biopsies may be helpful in determining the diagnosis. Rarely, surgical exploration of the spinal cord at the affected site will be required to achieve a diagnosis, gauge prognosis, and recommend treatment.
ACUTE SPINAL CORD DYSFUNCTION
TRAUMA
Traumatic injuries to the spinal canal are common, with fractures and luxations of the spine and traumatic disk extrusion being most frequent. Severe spinal cord bruising and edema can occur secondary to trauma, even without disruption of the bony spinal canal.
Clinical Features
The clinical signs associated with spinal trauma are acute and generally nonprogressive. Animals are usually in pain, and other evidence of trauma (e.g., shock, lacerations, abrasions, fractures) may be present. Neurologic findings depend on lesion location and severity. Neurologic examination should determine the location and extent of the spinal injury. Excessive manipulation or rotation of the animal should be avoided until the vertebral column is determined to be stable.
Diagnosis
The diagnosis of trauma is readily made on the basis of the history and physical examination findings. A thorough and rapid physical examination is important to determine whether the animal has life-threatening, nonneurologic injuries that should be addressed immediately. Concurrent problems may include shock, pneumothorax, pulmonary contusions, diaphragmatic rupture, ruptured biliary system, ruptured bladder, orthopedic injuries, and head trauma. Concern that the animal may have vertebral column instability warrants the use of a stretcher or board to restrain, examine, and transport the dog or cat in lateral recumbency.
The neurologic examination can be performed with the animal in lateral recumbency but will be limited to evaluation of mental status, cranial nerves, posture, muscle tone, voluntary movement, spinal reflexes, the cutaneous trunci reflex, and pain perception. Dogs with severe thoracic spinal cord lesions may exhibit the Schiff-Sherrington posture (see Fig. 63-8). The most important prognostic indicator after spinal trauma is the presence or absence of nociception or deep pain sensation. If deep pain is absent caudal to a traumatic spinal cord lesion, the prognosis for return of neurologic function is poor.
The neurologic examination allows determination of the neuroanatomic site of the lesion. Survey radiographs can then be used to more specifically localize the lesion, assess the degree of vertebral damage and displacement, and aid in prognosis. Manipulation or twisting of unstable areas of the spine must be avoided during radiography. If the animal is recumbent or restrained on a board, then lateral and cross-table ventrodorsal views allow assessment for the presence or absence of fractures or an unstable vertebral column. CT is a more accurate means to assess vertebral damage.
The entire spine should be assessed radiographically. Most spinal fractures and luxations occur at the junction of mobile and immobile regions of the spine, such as the lumbosacral junction or the thoracolumbar, cervicothoracic, atlantoaxial, or atlantooccipital regions. LMN lesions at an intumescence can mask a UMN lesion located more cra-nially in the spinal cord; therefore radiographic and clinical evaluation are important. Myelography, CT, or MRI should be used to look for radiographically inapparent lesions when radiographic lesions do not correspond with neuroanatomic localization.
Various classification schemes exist to determine the stability of vertebral injuries and the need for surgery. The vertebral body can be divided into three compartments and each assessed using radiographs or CT for damage (Fig. 70-3). When two of the three compartments are damaged or displaced, the fracture is considered unstable. Unstable fractures require surgical intervention or splinting, whereas stable fractures without significant ongoing spinal cord compression are managed conservatively. Splints are most effective when deep pain sensation is present, when ventral and middle compartments are intact, and when associated soft tissue injuries are minimal. Most dogs with cervical or lumbosacral injury are managed nonsurgically unless the patient deteriorates neurologically or remains in a great deal of pain 72 hours after injury, which suggests nerve root entrapment. Surgery is preferred for unstable thoracic and lumbar injuries.
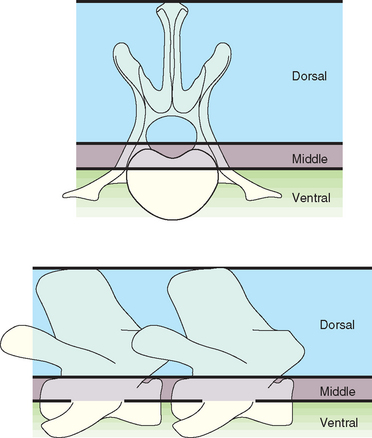
FIG 70-3 Illustration of the three-compartment model for radiographic evaluation of spinal fractures. The dorsal compartment includes the articular facets, laminae, pedicles, spinous processes, and supporting ligaments. The middle compartment contains the dorsal longitudinal ligament, the dorsal annulus, and the floor of the spinal canal. The ventral compartment consists of the remainder of the vertebral body and the annulus, the nucleus pulposus, and the ventral longitudinal ligament. When two or three of the compartments are damaged or displaced, surgical stabilization is indicated.
Treatment
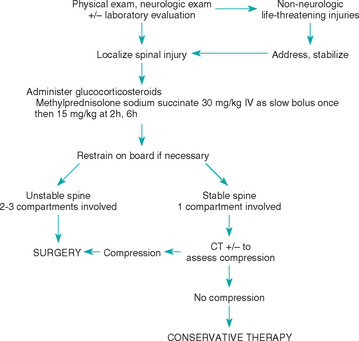
Primary treatment of animals with acute spinal injury involves evaluation for and treatment of other life-threatening injuries and maintenance of patient blood pressure, perfusion, and oxygenation. There is some evidence that the immediate IV administration of methylprednisolone sodium succinate (MPSS), a highly soluble corticosteroid with neuroprotective effects exerted primarily by its actions as a free radical scavenger, may be beneficial (Fig. 70-4). Unfortunately, dogs treated according to this protocol suffer from a high rate of gastrointestinal complications, and adverse effects should be monitored and may be decreased by concurrent administration of an H2-receptor blocker (ranitidine 2 mg/kg, given orally or intravenously q8h, or famotidine 0.5 mg/kg, given orally or intravenously q24h), a proton pump inhibitor (omeprazole 0.7 to 1.5 mg/kg/day) or a synthetic prostaglandin E1 analog (misoprostol 2 to 5 μg/kg, given orally q8h), and a mucosal protectant (sucralfate 0.25 to 1 g, given orally q8h; see Chapter 30).
Intensive nursing care is critically important in dogs and cats managed conservatively or surgically. Narcotic analgesics may be administered as needed (Table 70-3). Thickly padded, clean, dry cages and frequent turning of the patient will help prevent pressure sores. All impaired limbs should be moved repeatedly through a full range of motion many times each day. Maintenance of an indwelling urinary catheter ensures a dry animal but may increase the risk of urinary tract infection, particularly when kept in place for longer than 3 days. When long-term care is necessary, the bladder should be gently expressed or catheterized and emptied four to six times daily and urinary tract infections treated as they occur. In animals with UMN bladders (see Chapter 63) or those with urethral spasm, medical therapy (phenoxybenzamine 1 mg/kg q8h and diazepam 1.25 to 2.5 mg/kg q8h) may help relax the urethral sphincter, making bladder expression easier and less traumatic. When an animal starts to regain voluntary motion in the limbs, physical therapy is increased; hydrotherapy or swimming stimulates voluntary movement, improves circulation to the limbs, and cleans the skin.
 TABLE 70-3 Narcotic Analgesics Used to Treat Spinal Pain in Dogs
TABLE 70-3 Narcotic Analgesics Used to Treat Spinal Pain in Dogs
| DRUG | DOSAGE |
|---|---|
| oxymorphone | 0.05 mg/kg IM |
| morphine | 0.3-2.2 mg/kg SC or IM |
| butorphanol | 0.4-0.8 mg/kg SC |
| buprenorphine | 0.02-0.06 mg/kg IM or SC |
IM, Intramuscular; SC, subcutaneous.
Prognosis
Prognosis for recovery depends on the site and severity of injury. Unstable cervical vertebral fractures are associated with very high mortality at the time of trauma and also in the perioperative period. Prognosis for recovery is good if affected animals do not die acutely from respiratory dysfunction. Animals with thoracic and lumbar spinal cord injury and intact voluntary motion have a good prognosis for return of full function. Animals that are paralyzed but retain deep pain and normal bladder function have a fair prognosis for recovery, although they may have residual neurologic deficits. Animals presenting with no deep pain sensation rarely recover. Lesions of the white matter producing strictly UMN signs may have a better prognosis for full recovery than lesions affecting clinically important LMNs at the cervical or lumbar intumescence. In any animal with paralysis caused by a spinal cord injury, if no signs of improvement are evident by 21 days after injury, the prognosis for recovery is poor.
HEMORRHAGE/INFARCTION
Nontraumatic hemorrhage into the spinal canal causing acute neurologic deficits and sometimes pain (i.e., hyperesthesia) has been recognized in young dogs with hemophilia A, dogs of any age with von Willebrand’s disease, dogs and cats with acquired bleeding disorders (i.e., warfarin intoxication, thrombocytopenia), dogs with vascular anomalies (i.e., aneurysms, arteriovenous fistulas), and dogs and cats with primary or metastatic spinal neoplasia (i.e., lymphoma, hemangiosarcoma). Hemorrhage can be subdural or epidural. Signs occur acutely and are minimally progressive, with neurologic signs reflecting the site and severity of spinal cord damage. Antemortem diagnosis usually requires advanced diagnostic imaging (i.e., MRI), although identification of a systemic bleeding disorder or neoplasia can suggest the diagnosis. In addition to treatment to resolve the cause of bleeding, significant acute spinal cord compression caused by hemorrhage may require surgical decompression.
Spinal cord infarction by a blood clot is a rare cause of peracute neurologic dysfunction in dogs and cats. Signs are referable to the site and severity of the vascular compromise. Blood stasis, endothelial irregularity, hypercoagulability, and impaired fibrinolysis are all known predisposing factors for thromboembolism (see Chapter 12). Cardiomyopathy, hyperadrenocorticism, protein-losing nephropathy, immune-mediated hemolytic anemia, heartworm disease, vasculitis, and disseminated intravascular coagulation have all been associated with an increased risk of systemic thrombosis and can occasionally result in regional spinal cord infarction. Treatment consists of general supportive care and medications to decrease the risk of further infarction; however, antemortem definitive diagnosis is difficult.
ACUTE INTERVERTEBRAL DISK DISEASE
The intervertebral disks are composed of an outer fibrous layer (the annulus fibrosus) and a gelatinous center (the nucleus pulposus). With normal aging the nucleus is gradually replaced by fibrocartilage. In some dogs, particularly the chondrodystrophoid breeds, the nucleus matrix degenerates and mineralizes, making these dogs prone to acute disk rupture. Acute extrusion of mineralized nucleus pulposus into the spinal canal through the dorsal annulus causing bruising or compression of the spinal cord is classified as a Hansen’s type I disk (Fig. 70-5; for type II disk). This type of disk injury is most common in small breeds of dogs such as the Dachshund, Toy Poodle, Pekingese, Beagle, Welsh Corgi, Lhasa Apso, Shih Tzu, Chihuahua, and Cocker Spaniel, with a peak incidence between 3 and 6 years of age. Acute type I disk extrusions are also occasionally diagnosed in middle-aged large-breed dogs, particularly in Basset Hounds, Labrador Retrievers, Doberman Pinschers with caudal cervical vertebral instability, and German Shepherd Dogs. Intervertebral disk disease is a rare cause of clinically evident spinal cord compression in the cat, with predominantly acute type I disk prolapse occurring in older cats (mean age, 9.8 years) in the lower thoracic and lumbar regions (most commonly, L4/L5).
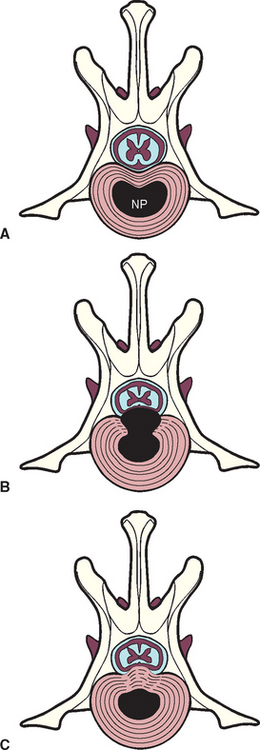
FIG 70-5 A, The normal relationship between the intervertebral disk and the spinal cord. NP, Nucleus pulposus. B, Hansen type I disk extrusion, wherein the NP herniated into the vertebral canal through a damaged AF, annulus fibrosus. C, Hansen type II disk protrusion, with bulging of the annulus into the vertebral canal.
Clinical Features
The predominant sign of cervical intervertebral disk disease (IVDD) is neck pain. The discomfort is often severe, and affected dogs may vocalize with the pain of movement. They may stand with their head and neck extended and may be reluctant to eat or drink from dishes placed on the floor. Some affected dogs lift one forelimb while standing to relieve the discomfort of nerve root irritation or cervical muscle spasm; this is called root signature (Fig. 70-6) and can be seen with cervical IVDD at any site. Compression of nerve roots and meninges causes neck pain. The vertebral canal in the cervical region has a very large diameter, such that even when large masses of disk material extrude into the spinal canal, significant spinal cord compression is unlikely. When significant spinal cord compression or concussion does occur, the result is usually UMN paresis or paralysis in all four legs, with rear limbs more severely affected than forelimbs. Caudal cervical disk extrusions (C6/7, C7/T1) can result in LMN forelimb weakness and scapular muscle atrophy together with UMN paresis in the rear limbs. Signs with spinal cord compression by type 1 disks are usually symmetric, although lateralized disk extrusions can result in asymmetry. The C2/3 intervertebral disk is most frequently involved, with the prevalence progressively decreasing from C3/4 to C7/T1. The C6/7 disk is more commonly affected in large-breed dogs as a component of cervical vertebral malformation malarticulation syndrome (also known as wobbler syndrome).
Diagnostic Approach
Cervical disk disease should be suspected on the basis of the signalment, history, physical examination, and neurologic findings. Most affected dogs show obvious signs of pain, but some stoic dogs do not exhibit discomfort during neck movement or manipulation. There should be no systemic signs of illness (e.g., fever, weight loss), and no specific neurologic abnormalities suggesting intracranial disease. Important differential diagnoses for dogs with neck pain include meningitis, diskospondylitis, vertebral neoplasia, polyarthritis, myositis, and trauma (see Box 69-1). Acute neurologic dysfunction caused by cervical disk disease must be distinguished through testing from cervical fracture/luxation, hemorrhage, or fibrocartilagenous embolism.
Spinal radiographs can be taken in an awake animal to look for evidence of disk disease and rule out other diseases (e.g., diskospondylitis, lytic vertebral tumor, fracture, atlantoaxial luxation). In animals with clinical features making surgery likely if disk extrusion is confirmed, radiographs are best obtained under general anesthesia to facilitate the optimal positioning and imaging necessary to detect subtle lesions.
Observation of calcified disk spaces confirms the presence of generalized intervertebral disk disease, but unless there is dorsal displacement of mineralized disk material into the spinal canal, this does not necessarily implicate the disk extrusion as the cause of neurologic dysfunction. Narrowing of the affected intervertebral space is commonly recognized (Fig. 70-7). Myelography or advanced diagnostic imaging (i.e., CT, MRI) are necessary to make a definitive diagnosis and determine which disk space is involved before surgical treatment. Myelography is the least expensive option, but it is also the most invasive and the least likely to provide lateralizing information. Analysis of cerebrospinal fluid (CSF) should always precede myelography, to rule out inflammatory central nervous system (CNS) disease (see the discussion of myelography, Chapter 64). CSF changes associated with disk extrusion are usually minimal but may include very slight increases in protein concentration and cell count. CT and MRI may be used to further delineate a compressive disk lesion identified myelographically, or they may be used as the sole technique for detecting and characterizing a disk lesion, particularly in regions where myelographic interpretation can be difficult and precise anatomic localization is important (e.g., caudal cervical; Fig. 70-8).
Treatment
Treatment decisions in dogs with cervical disk disease are based on the severity of disease noted at the time of presentation (Table 70-4). Dogs with a single episode of acute neck pain and no neurologic deficits are usually managed conservatively with strict cage confinement and analgesics. Animals should be kept in a small kennel crate or in the owner’s arms at all times except when walked outside with a harness to urinate and defecate. Nonsteroidal anti-inflammatory drugs or narcotic analgesics (see Table 70-3) can be administered for the first 3 to 5 days if strict confinement is likely to be enforced. Muscle relaxants (methocarbamol 15-20 mg/kg, administered orally q8h) will also decrease painful muscle spasms. After 3 to 4 weeks of strict crate confinement, 3 weeks of house confinement with no jumping or running and leash exercise should be recommended followed by a gradual increase in monitored exercise and (if necessary) a weight reduction program.
 TABLE 70-4 Classification of Dysfunction and Treatment Recommendations: Canine Cervical Disk Extrusion
TABLE 70-4 Classification of Dysfunction and Treatment Recommendations: Canine Cervical Disk Extrusion
| GRADE | CLINICAL FINDINGS | TREATMENT |
|---|---|---|
| 1 | Single episode of pain Normal neurologic exam | Cage rest +/- analgesics |
| 2 | Intractable pain or Recurrent pain | Surgical Decompression |
| 3 | Neurologic deficits +/- pain | Surgical Decompression |
Most dogs with neck pain and no neurologic deficits respond initially to this conservative medical management, but a few will have intractable pain. Approximately 40% of responding dogs will experience recurrent episodes of pain in the future. Dogs with cervical pain that does not resolve in 1 or 2 weeks, dogs with severe pain that cannot be controlled, dogs with recurrent episodes of neck pain, and dogs that develop paresis or paralysis indicating cervical spinal cord compression should be treated surgically. Even if cervical pain is the only clinical finding, most dogs with cervical intervertebral disk prolapse have a large amount of disk material within the spinal canal and these dogs will have a more complete and rapid recovery if surgery is performed. Myelography or MRI to locate the lesion and prompt surgical decompression using a ventral slot procedure are recommended. When the width of the ventral slot required to remove caudal cervical disk material is greater than 30% of the vertebral width, stabilization with a bone graft is recommended to prevent subluxation. Some surgeons recommend prophylactic fenestration of multiple cervical sites whenever a ventral slot surgery is performed to prevent further disk material prolapse and reduce the recurrence rate, but this is controversial. Most dogs are in a great deal less pain within 24 to 36 hours after decompressive surgery, and resolution of neurologic deficits occurs gradually over 2 to 4 weeks. Exercise is restricted for 2 weeks, followed by physiotherapy to enhance recovery. The prognosis for full recovery in dogs with neck pain alone or neck pain plus moderately severe tetraparesis is 80% to 90% at 4 weeks. Dogs with paralysis are more likely to have residual deficits, but approximately 80% of these dogs will become ambulatory. Rarely, vertebral subluxation occurs after ventral slot surgery, causing neck pain and worsening of neurologic deficits. Re-imaging (MRI preferred) followed by surgical distraction and stabilization is required in these dogs, which should result in a good prognosis for recovery.
Clinical Features
Most dogs with thoracolumbar disk disease are presented because of back pain and rear limb paresis or paralysis. The back pain in these dogs is usually less severe than that noted with cervical IVDD, but affected dogs may stand with an arched back and resent abdominal compression or palpation. The diameter of the vertebral canal is relatively small in the thoracolumbar region, so even small volumes of disk material extruded into the canal cause spinal cord compression and neurologic deficits. In addition to the compressive effect of the disk material, it is common to have impact injury to the spinal cord from explosive disk rupture. Most (>50 percent) of the disk extrusions in this region occur at the T12/13 or T13/L1 site, with 85% between T11/12 and L2/3. Disk extrusions at these sites cause UMN paresis or paralysis in the rear limbs. Only 10% to 15% of dogs will have a disk extrusion between the L3/4 and L6/7 disks, damaging the spinal cord at the lumbar intumescence and resulting in LMN signs.
The severity of the initial signs and the speed with which they progress are related not only to the volume of disk material extruded and the degree of resultant spinal cord compression but also to the force of the extrusion (see Fig. 70-1). In some dogs evidence of pain and subtle weakness resulting from partial disk rupture and mild spinal cord compression may be present for a few days or weeks before mild trauma or movement results in the extrusion of more disk material causing paralysis. The neurologic signs observed in dogs and cats with intervertebral disk disease are usually bilaterally symmetric. Affected animals usually exhibit pain on spinal palpation right over the affected disk because of meningeal and nerve root irritation at the site. When spinal cord damage is severe between T3 and L3, the cutaneous trunci reflex (see Fig. 63-17) can be used to further aid in lesion localization.
Diagnostic Approach
Trauma, fibrocartilaginous embolism (FCE), and vertebral neoplasia are the major differential diagnoses considered in animals with acute thoracolumbar disk extrusions. The lesion should be localized as precisely as possible on the basis of neurologic examination findings and detection of a specific area of spinal pain. Spinal survey radiographs can be taken in an awake animal to look for evidence of disk disease and rule out other diseases. Careful positioning of the suspected disk space in the center of the beam, with the dog anesthetized, is necessary for radiographic identification of subtle lesions, but this testing is usually reserved for potential surgical candidates, when preparations have been made for further diagnostic imaging and decompressive surgery during the same anesthetic episode.
Observation of calcified disk spaces confirms the presence of generalized intervertebral disk disease, but radiographs are only between 60% and 70% accurate in identifying the location of thoracolumbar disk extrusion. Radiographic changes consistent with herniation of an intervertebral disk in the thoracolumbar region include a narrowed or wedged disk space, a small or cloudy intervertebral foramen (i.e., “horse’s head”), narrowing of the facetal joints, and a calcified density within the spinal canal above the involved disk space (Figs. 70-9 and 70-10).
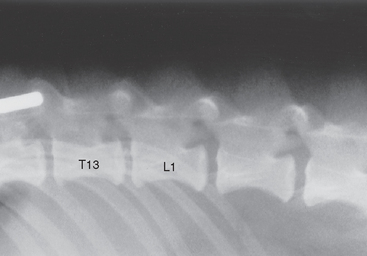
FIG 70-9 Lateral plain radiograph of vertebral column of a 4-year-old Pekingese with acute intervertebral disk prolapse. The intervertebral space between T13 and L1 is narrowed, the intervertebral foramen (“horse’s head”) is small, and a calcified density can be seen in the spinal canal above the T13-L1 disk space.
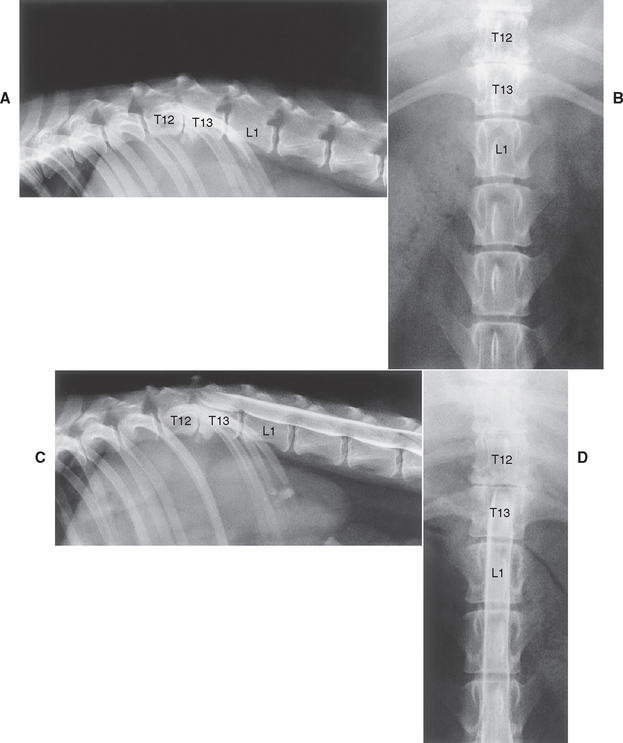
FIG 70-10 Lateral (A) and ventrodorsal (B) plain radiographs of the vertebral column of an 8-year-old Miniature Schnauzer with acute paralysis after a chronic history of intermittent back pain. Marked collapse of the intervertebral space at T12-T13, a small intervertebral foramen, and clouding of the foramen is evident. The T13-L1 space is also slightly narrowed. C and D, Myelography confirms the presence of a significant extradural mass at T12-T13, located ventrally and on the right, causing considerable cord compression and displacement. A minimal extradural mass effect exists as well at T13-L1 without significant compression. Surgery confirmed spinal cord compression by the disk material at T12-T13.
Myelography or advanced diagnostic imaging (i.e., CT, MRI) should be performed for definitive diagnosis before surgery. CSF is usually collected from the cerebello-medullary cistern before myelography. A cell count can be performed quickly to rule out meningitis/myelitis, and the sample can be saved for further diagnostic testing if the myelogram does not show a compressive lesion. A lumbar injection is preferred for myelography because the contrast medium must sometimes be injected under pressure to get past cord swelling in the area of the disk prolapse. CT is more accurate and faster than myelography; because it is much more reliable at determining what side the disk material is on, it is useful for surgical planning. MRI is superior to CT when extruded disk material is not mineralized and is best for spinal cord evaluation when the diagnosis of disk extrusion is uncertain (see Fig. 70-8). The increased sensitivity of CT and MRI can be problematic because clinically insignificant disk herniations not causing symptomatic spinal cord compression will also be identified.
Treatment
Treatment of acute thoracolumbar intervertebral disk extrusion may be nonsurgical or surgical (Table 70-5). Nonsurgical treatment is usually recommended when there are minimal or inapparent neurologic deficits and the dog is still able to rise and walk unassisted. Strict cage rest is the most important aspect of nonsurgical treatment and must be maintained for a minimum of 6 weeks to allow the annulus to repair. Analgesics (see Table 70-3) and antiinflammatory drugs are often administered as for cervical IVDD. Animals being treated nonsurgically must be evaluated frequently for deterioration in neurologic status because these dogs often deteriorate within 6 to 24 hours. If neurologic symptoms do not improve within 5 to 7 days or if even minor deterioration in neurologic status is seen, then surgical therapy is indicated. Persistent or recurrent pain is also an indication for decompressive surgery.
 TABLE 70-5 Classification of Dysfunction and Treatment Recommendations: Canine Thoracolumbar Disk Extrusion
TABLE 70-5 Classification of Dysfunction and Treatment Recommendations: Canine Thoracolumbar Disk Extrusion
| CLINICAL FINDINGS | TREATMENT |
|---|---|
| Single episode of pain | Cage rest |
| Normal neurologic exam | +/- analgesics |
| Intractable pain or | Surgical |
| Recurrent pain or | Decompression |
| Deterioration in neurologic status | |
| Ataxia, proprioceptive deficits | Cage rest |
| Paraparesis, able to stand and walk | +/- analgesics |
| Severe paraparesis unable to stand and walk | |
| Paralyzed |
Surgical treatment is recommended for all patients unable to walk at the time of presentation and for all dogs with signs suggesting less severe spinal cord compression (e.g., paresis, pain) if neurologic signs do not rapidly resolve with medical therapy. The rate of recovery is faster after decompression than after nonsurgical treatment, and the likelihood of residual neurologic deficits is decreased. Decompression is usually accomplished through a hemilaminectomy, and disk material is removed from the spinal canal. Preoperative imaging is essential to identify the affected interspace and to determine which side to decompress to gain access to disk material. Because clinical signs and myelography are not always reliable indicators of lateralized disk material, CT or MRI should be performed whenever possible. In addition to surgical decompression, many surgeons recommend concurrent fenestration at adjacent high-risk sites (T11 to L3) to help decrease the likelihood of subsequent herniations in dogs with generalized thoracolumbar disk disease.
Postsurgically, animals must be kept clean and confined. Pressure sores should be prevented in paralyzed patients through the use of padded bedding and frequent turning. Complete bladder emptying at least four times daily by manual expression, an indwelling catheter, or intermittent aseptic catheterization is necessary in dogs that have lost bladder function. In dogs with UMN bladders medical treatment with phenoxybenzamine and diazepam can lower sphincter pressure, facilitating manual expression and attempts by the animal to void. Massage of the limbs and passive physiotherapy, including limb abduction, may help prevent neurogenic atrophy and muscle fibrosis in the paraplegic animal. Towel walking of paraparetic dogs can improve attitude and promote early use of the affected limbs. Once the skin incision has healed, swimming may be instituted to encourage movement. In dogs with a prolonged anticipated recovery period, use of a paraplegic cart can provide a stimulus for recovery (Fig. 70-11). Improvement in neurologic function usually occurs within 1 week of surgery. No improvement after 21 days signals that the prognosis for recovery is poor.

FIG 70-11 The use of a paraplegic cart can provide a stimulus for recovery and improve mobility and attitude in paralyzed dogs recovering from thoracolumbar disk surgery.
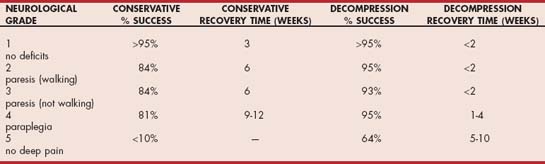
More than 90% of dogs with deep pain perception at the time of evaluation recover fully after effective decompression (Table 70-6). The best surgical results are obtained when decompression can be accomplished within 48 hours of the onset of neurologic signs. Dogs with very rapid progression to paralysis (grade 4 or grade 5) over less than 4 to 6 hours should be treated as a surgical emergency and decompressed without delay. There may be some benefit to preoperative treatment of this group of patients with methylprednisolone sodium succinate, as described for spinal trauma patients if they are presented within 8 hours of the onset of paralysis. This treatment is controversial insofar as the benefits are not well established and adverse effects are common. Dogs with loss of deep pain perception (grade 5) are very unlikely to recover without surgical intervention, but with rapid decompression (within 72 hours) 60% of small-breed dogs and 25% of large-breed dogs will make a functional recovery. If deep pain does not return within 4 weeks, the prognosis for recovery is very poor.
Acute, forceful, intervertebral disk extrusions sometimes cause considerable intramedullary hemorrhage and edema. In approximately 10% of dogs presenting for a rapid onset of complete paralysis and loss of deep pain perception, focal spinal cord damage and edema result in spinal cord ischemia and progressive myelomalacia of the cord cranial and caudal to the original lesion (i.e., ascending descending myelomalacia). This condition usually develops within 5 days of the original disk extrusion. This disorder should be suspected when the line demarcating the loss of the cutaneous trunci reflex moves cranially or the patellar and withdrawal reflexes are lost (LMN signs) in the rear limbs of a dog that previously had UMN paralysis in the rear limbs after disk extrusion. Most affected dogs are also very anxious and experience a great deal of pain. When ascending descending myelomalacia is recognized, euthanasia should be recommended because no chance for recovery exists and most affected dogs will die within a few days of respiratory paralysis.
FIBROCARTILAGINOUS EMBOLISM
Acute infarction and ischemic necrosis of the spinal cord parenchyma occur when fibrocartilage identical to that in the nucleus pulposus of the intervertebral disks is embolized into the very small arteries and veins supplying the spinal cord parenchyma and leptomeninges. This very acute, nonprogressive phenomenon can affect any region of the spinal cord and result in paresis or paralysis. The cause of this disorder is unknown. It is most common in medium-sized and large-breed dogs. It has also been described in small-breed dogs (especially the Miniature Schnauzer) and a few cats. Most affected dogs are middle aged, with the majority of cases between 3 and 7 years of age. A few dogs younger than 1 year of age have been recognized with FCE, especially Irish Wolfhounds. No gender predilection exists.
Clinical Features
The onset of neurologic signs is very sudden, and signs may worsen for 2 to 6 hours. In approximately half of all cases, FCE occurs immediately after minor trauma or during exertion. Neurologic examination reflects a focal spinal cord lesion, and the deficits observed depend on the region of spinal cord affected and the severity of cord involvement. The thoracolumbar cord (causing UMN signs in the rear limbs) and the lumbosacral intumescence (causing LMN signs in the rear limbs) are most often affected. The cervical cord is affected less frequently, but it is the site most often affected in small-breed dogs. Neurologic dysfunction may be mild or severe. Asymmetry is common, with the right and left sides affected to different degrees. Dogs commonly cry out as though in pain at the onset of signs, and dogs evaluated within 2 to 6 hours of onset sometimes exhibit focal spinal hyperpathia (i.e., painfulness); however, this resolves quickly, and most affected dogs do not exhibit pain by the time they are brought to a veterinarian, even on manipulation of their spine. The lack of pain and the asymmetry are very helpful in differentiating FCE from other disorders that cause acute nonprogressive neurologic dysfunction, such as acute intervertebral disk extrusion and fracture/luxation.
Diagnosis
FCE is suspected on the basis of the signalment, history, and recognition of acute, nonprogressive, nonpainful spinal cord dysfunction. Radiographs are normal in dogs and cats with FCE but assist in ruling out diskospondylitis, fractures, lytic vertebral neoplasia, and IVDD. CSF is usually normal, although an increase in protein (especially albumin) concentration may be observed in some (50%) cases. In the first 24 hours after the onset of clinical signs, a few dogs have a mild increase in neutrophil numbers within the CSF. Myelography is usually normal, although some animals exhibit focal intramedullary cord swelling. Myelography is most useful to rule out compressive lesions of the spinal cord for which surgery might be indicated, such as fractures, disk extrusion, and neoplasia.
CT is not useful in the diagnosis of FCE beyond excluding a compressive myelopathy. MRI may reveal focal cord density changes in severely affected dogs, but mild lesions will not be evident. The diagnosis of FCE be made only on the basis of clinical findings and exclusion of compressive and inflammatory acute spinal cord disorders (Fig. 70-12).

FIG 70-12 This adult Border Collie had an acute onset of lameness, decreased conscious proprioception, and hyporeflexia in the left rear limb while retrieving a Frisbee. The limb was not painful, and radiographs, cerebrospinal fluid (CSF) analysis, and myelogram were all normal. A presumptive diagnosis of fibrocartilaginous embolism (FCE) involving the lumbar and sacral spinal cord segments on the left side was made. This dog recovered uneventfully within a 3-week period.
Treatment
Treatment for FCE consists of nonspecific supportive measures and nursing care, as described for paralyzed dogs. Most affected dogs are large breeds, making this type of management difficult. In animals brought to the clinician during the first 6 hours of paralysis, it may be reasonable to treat aggressively with one dose of methylprednisolone sodium succinate, as recommended for the initial treatment of acute spinal cord trauma (see Figure 70-4). Most clinical improvement takes place within the first 7 to 10 days after the onset of neurologic signs, although it may take 6 to 8 weeks for a complete return to function. If no improvement is seen within 21 days, it is unlikely that the dog or cat will improve.
Prognosis
Recovery depends on the extent and location of spinal cord injury. The prognosis is best for recovery in dogs and cats with intact deep pain sensation and strictly UMN signs, including increased muscle tone and hyperactive reflexes. When the spinal cord is damaged at the brachial or lumbosacral intumescence (C6 to T2 or L4 to S3), causing LMN signs, a full recovery is less likely.
ATLANTOAXIAL INSTABILITY
Because many dogs with congenital atlantoaxial instability have slowly progressive waxing and waning tetraparesis, this condition will be discussed with chronic progressive spinal cord disease. Traumatic fracture of the dens leading to subluxation can occur in any dog or cat and will result in acute UMN dysfunction in all limbs.
NEOPLASIA
Neoplasms usually cause neurologic signs by compressing or infiltrating the spinal cord parenchyma. Neoplastic conditions will be discussed in this chapter with chronic progressive spinal cord diseases. Occasionally, neoplasia will cause acute nonprogressive neurologic signs as a result of tumor- or metastasis-associated intraparenchymal hemorrhage or lysis of vertebral bones, leading to fracture.
PROGRESSIVE SPINAL CORD DYSFUNCTION
Damage to the spinal cord that progresses over a few days to weeks (subacute) is most often caused by inflammatory (infectious or immune) processes or some type of neoplasia. Degenerative disorders and most cancers generally cause more slowly progressive spinal cord dysfunction. In all patients with progressive spinal cord dysfunction complete patient evaluation, including systemic evaluation for extraneural disease, should be recommended. The lesion should be localized and ancillary tests performed to reach a diagnosis and determine appropriate treatment.
SUBACUTE PROGRESSIVE DISORDERS
Infectious Inflammatory Disease
Most of the infectious inflammatory diseases discussed in Chapter 69 can result in myelitis (i.e., spinal cord inflammation), leading to progressive neurologic signs suggesting multifocal or focal spinal cord damage. CSF analysis is necessary to confirm that inflammatory disease is present. Additional diagnostic tests may be necessary to identify an etiology (see Chapter 69).
Noninfectious Inflammatory Disease
Noninfectious inflammatory diseases, specifically granulomatous meningoencephalitis (GME), steroid-responsive meningitis arteritis (SRMA), and feline polioencephalomyelitis, can affect the spinal cord. Cervical pain is a constant feature of SRMA, but neurologic deficits suggesting spinal cord parenchyma damage with this disorder are rare. Neurologic deficits are common, however, with focal or disseminated GME affecting the spinal cord. CSF analysis is necessary to confirm inflammatory myelitis, and additional tests are required to rule out infectious etiologies. See Chapter 69 for more information on these syndromes.
Diskospondylitis
Diskospondylitis is an infection of the intervertebral disks and adjacent vertebral endplates by bacterial or fungal organisms. Hematogenous spread of infection from infected foci in the body, extension from an infected local site, and migration of inhaled plant material (grass awns) have all been implicated. Numerous causative organisms have been isolated, with the most common being Staphylococcus intermedius, Streptococcus spp., and Escherichia coli. Brucella canis is less common but should be tested for because of human health implications. The fungal organisms Aspergillus terreus (in German Shepherd Dogs) and Paecilomyces varioti have been isolated from diskospondylitis lesions in a few dogs. Actinomyces spp. are commonly implicated in L2-L4 diskospondylitis caused by migration of inhaled grass awns.
Diskospondylitis occurs most often in young and middle-aged medium- to large-breed dogs. German Shepherd Dogs and Labrador Retrievers may have an increased prevalence of this disorder. Diskospondylitis is very rarely diagnosed in cats. Males are affected more often than females in both species.
Clinical Features
Spinal pain is the most common initial clinical sign of diskospondylitis. Palpation of the affected region of the spine usually allows lesion localization. Systemic signs such as fever, anorexia, depression, and weight loss occur in 30% of affected dogs, but hematologic inflammatory changes are rarely observed unless there is concurrent endocarditis or some other systemic infection. Secondary (i.e., reactive) polyarthritis may occur (see Chapter 74), resulting in a generally stiff, stilted gait in some dogs.
Neurologic deficits in dogs and cats with diskospondylitis are extremely uncommon. In chronic or untreated cases neurologic dysfunction can result from spinal cord compression by proliferating inflammatory tissue or from pathological fracture of lytic vertebrae. Occasionally, severe inflammation in the bone will cause functional abnormalities in the overlying spinal cord without any cord compression.
Diagnosis
The diagnosis of diskospondylitis is suspected after physical examination and confirmed by radiographic examination of the affected vertebrae. Radiographic changes of diskospondylitis characteristically include narrowing of the disk space, irregularity or lysis of one or both vertebral end plates (especially ventrally), sclerosis at the margins of bone loss, and osseous proliferation of adjacent vertebral bone (Fig. 70-13). The most commonly affected sites are the midthoracic, caudal cervical, thoracolumbar, and lumbosacral spine. It is common for diskospondylitis to affect more than one disk space (Fig. 70-14), so survey radiographs of the entire spine are recommended. Radiographic signs of diskospondylitis may not be apparent for several weeks after the onset of clinical signs. MRI or CT can identify subtle endplate erosion before radiographically apparent lesions are visible.
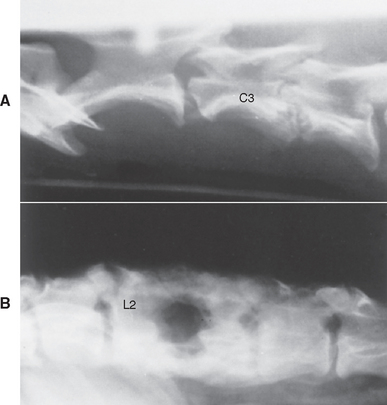
FIG 70-13 A, Lateral radiograph of cervical vertebral column of adult dog showing diskospondylitis between the third and fourth cervical vertebrae (C3 and C4). B, Lateral radiograph of lumbar vertebral column of an adult Pointer showing severe chronic diskospondylitis between the second and third lumbar vertebrae (L2 and L3).
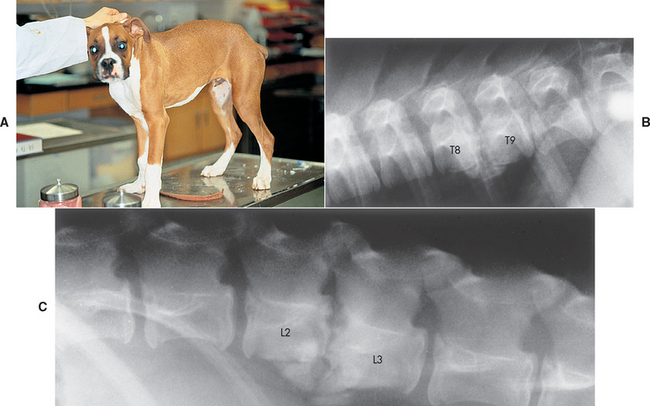
FIG 70-14 A, A 5-month-old Boxer puppy with back pain resulting from diskospondylitis. B and C, Lateral spinal radiographs reveal lesions at T8-T9 and L2-L3, with destruction of adjacent vertebral body end plates, collapse of the intervertebral disk spaces, shortening of the vertebral bodies, and new bone production around the ends of the affected vertebral bodies.
Blood culture is the most rewarding noninvasive method of isolating the organism responsible for the vertebral infection, yielding the organism in approximately half of the cases. Echocardiography and urine culture should be performed to evaluate the cardiac and urogenital systems as potential sources of infection. Percutaneous needle aspiration of the infected disk during general anesthesia using fluoroscopy has been effective in yielding positive cultures in some cases with negative blood and urine cultures, but this technique is usually reserved for cases in which other culture techniques have yielded negative results and the response to an empirically selected antibiotic is inadequate. A spinal needle is guided into the disk space using fluoroscopy or CT, and a small amount of sterile saline (0.3 to 0.5 ml) is injected and then aspirated for culture. Brucella serology or polymerase chain reaction (PCR) should be considered in all affected dogs because of the public health significance of brucellosis (see Chapter 58), despite its very low prevalence (<10%) in the United States and Canada.
Treatment
Initial treatment of diskospondylitis consists of antibiotics, cage rest, and analgesics. If an organism is isolated, susceptibility testing should guide antibiotic therapy. If an organism is not found, initial treatment attempts should be directed against Staphylococcus spp. Bactericidal antibiotics with a spectrum against gram-positive organisms and the ability to concentrate in bone are recommended. Firstgeneration cephalosporins (cefazolin 25 mg/kg, given intravenously q8h, cephalexin 22 mg/kg, given orally q8h) and amoxicillin with clavulanate (Clavamox 12.5 to 25 mg/kg, given orally q8h) have been effective. Quinolones can be added if gram-negative organisms are suspected. Ampicillin is the antibiotic of choice for Actinomyces infections associated with grass awn migration. Antibiotics are administered parenterally for the first 3 days whenever neurologic deficits are present, and then oral administration is continued for at least 8 weeks and up to 6 months, if necessary.
In addition to antibiotic therapy, the patient’s activity should be restricted to minimize discomfort and decrease the chance of pathologic fracture and luxation. Analgesics may be administered for 3 to 5 days, but their use will make it difficult to assess the efficacy of antibiotic therapy and may make it more difficult to enforce strict cage rest. Most dogs show very rapid clinical improvement within the first week of treatment. Dogs treated medically should be reevaluated clinically and radiographically every 3 weeks. With time, the lytic process should resolve and the affected vertebrae should fuse. Antibiotics should be administered for a minimum of 8 weeks. Antibiotics may then be discontinued if the spine is no longer painful over the affected sites and there is no radiographically visible lysis. Most treated animals do not relapse, unless the diskospondylitis is caused by a grass awn foreign body.
CHRONIC PROGRESSIVE DISORDERS
Neoplasia
Tumors that grow and compress or infiltrate spinal cord parenchyma frequently cause chronic, progressively worsening signs of spinal cord dysfunction. Spinal tumors can be primary or metastatic. The most common tumors affecting the spinal cord in the dog are extradural tumors arising from the vertebral body (e.g., osteosarcoma, chondrosarcoma, fibrosarcoma, myeloma) and extradural soft tissue tumors, including metastatic hemangiosarcoma, carcinoma, liposarcoma, and lymphoma. Intradural extramedullary tumors such as meningiomas, neuroepithelioma, and peripheral nerve sheath tumors are also common, comprising 35% of all spinal tumors. Intramedullary tumors (i.e., astrocytomas, ependymomas, metastatic tumors) are relatively rare in the dog, with the exception of metastatic hemangiosarcoma. Lymphoma can be extradural, intradural/extramedullary, or intramedullary in the dog and is usually a manifestation of multicentric disease. Extradural lymphoma is the only common spinal tumor in the cat.
Spinal tumors occur with equal frequency in males and females and can occur in any breed of dog or cat, although large-breed dogs are most often affected. Most spinal cord tumors are found in middle-aged and older dogs, with the mean age at the time of diagnosis being 5 to 6 years. Two noteworthy exceptions are lymphoma, which can affect dogs of any age, and neuroepithelioma, a primary intradural extramedullary tumor that has a predilection for T10 to L1 in young dogs, particularly German Shepherd Dogs and Golden Retrievers. In addition, vertebral osteomas may occur in young dogs and result in spinal cord compression, as can cartilaginous exostoses, benign proliferative lesions of the bone indistinguishable from neoplasia except by biopsy (Fig. 70-15; see also see Fig. 64-7). Spinal lymphoma is most common in young (mean age, 4 years) adult feline leukemia (FeLV)–positive cats. Certainly, spinal neoplasia cannot be eliminated as a differential diagnosis strictly on the basis of signalment.
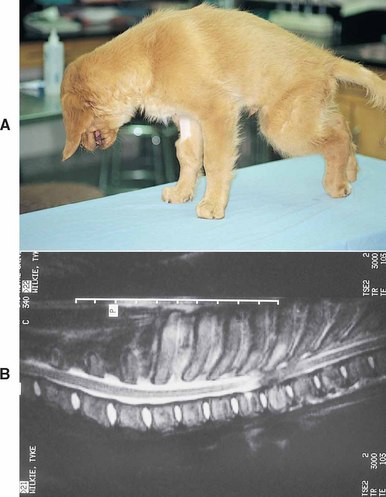
FIG 70-15 A, A 3-month old Golden Retriever puppy with spinal pain and progressive upper motor neuron (UMN) signs in both rear limbs resulting from a vertebral osteoma. B, Magnetic resonance imaging (MRI) showing severe compressive spinal cord damage from the caudal aspect of the T4 vertebral body extending caudally through the T6 vertebral body.
Clinical Features
Clinical signs are usually insidious and related to the location of the tumor. Early diagnosis is difficult because neurologic abnormalities are not clinically apparent until there has been significant compression or destruction of the spinal cord. Many animals have months of slowly progressive clinical signs before a diagnosis is made. Pain may be a prominent feature in dogs and cats with nerve root tumors encroaching on the spinal cord, tumors involving the meninges, and aggressive tumors involving vertebral bone. Progressively worsening lameness and pain on limb manipulation (i.e., radicular pain, root signature) without initial neurologic deficits are common in dogs with peripheral nerve sheath tumors involving nerve roots in the cervical or lumbar intumescence. An ipsilateral Horner’s syndrome and/or loss of the panniculus reflex may be seen if the thoracic nerve roots are involved. Pain is not a common feature of intramedullary spinal cord primary tumors or metastases. Although animals with compressive lesions of the T3-L3 spinal cord typically maintain urinary and fecal continence until after the limbs are paralyzed, some animals with intramedullary neoplasms will become incontinent while still able to walk.
Differential diagnoses must include other disorders that cause slowly progressive neurologic dysfunction, including type II disk protrusion and degenerative myelopathy (DM). Rapidly growing extradural tumors such as lymphoma and primary or metastatic intramedullary tumors sometimes cause rapidly progressive neurologic signs more typical of inflammatory myelitis. Acute paresis/paralysis may be seen in dogs or cats with tumor-associated hemorrhage or vertebral pathologic fractures.
Diagnosis
Whenever a neoplasm is considered as a differential diagnosis for spinal cord dysfunction, a thorough physical examination and clinicopathologic evaluation are necessary to look for sites of primary tumor and evidence of associated systemic disease. Fundic examination, palpation of lymph nodes, and rectal examination should be performed, as well as thoracic and abdominal radiographs, to identify a primary tumor site or metastasic lesions. Ultrasonographic examination of the spleen, liver, and heart should be performed in dogs whenever metastatic hemangiosarcoma is possible. Aspiration of the lymph nodes, spleen, and/or liver and examination of peripheral blood or bone marrow smears may yield the diagnosis in dogs with lymphoma. Patients with multiple myeloma often secrete paraproteins, causing a hyperproteinemia and a monoclonal gammopathy. Most cats with spinal lymphoma are FeLV-positive (>80%), and many have obvious systemic disease and hematologic evidence of bone marrow involvement.
Survey radiographs of the affected region of the spine are recommended. Bony changes will be seen primarily with vertebral tumors, in which obvious osteolysis or bone proliferation is common (Fig. 70-16). When a region of lysis is identified, fine needle aspiration of the lesion sometimes yields a diagnosis. The entire axial and appendicular skeleton should be surveyed for lytic lesions if clinical findings make multiple myeloma likely. Soft tissue tumors of the spinal cord are almost never visible using survey radiographs. Myelography is a fairly reliable method to identify, localize, and characterize spinal cord tumors, but it is relatively invasive and provides less useful diagnostic information than advanced imaging techniques such as CT and MRI. CSF analysis should always precede myelography. With tumors compressing the spinal cord, CSF analysis typically reveals nonspecific changes, including slight increases in protein concentration and a mild mononuclear pleocytosis. Neoplastic cells are rarely identified, except in cats and dogs with lymphoma (Fig. 70-17).
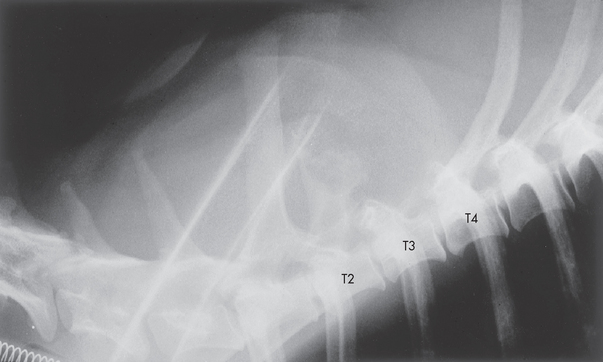
FIG 70-16 Lateral spinal radiograph from a 2-year-old Irish Setter with a 1-week history of progressive ataxia and a 12-hour history of upper motor neuron (UMN) paralysis of the rear limbs and Schiff-Sherrington syndrome. The entire spinous process of T3, the roof of T3, and most of the spinous process of T2 are destroyed, most consistent with a neoplastic process. An undifferentiated sarcoma at this site was identified on postmortem examination.
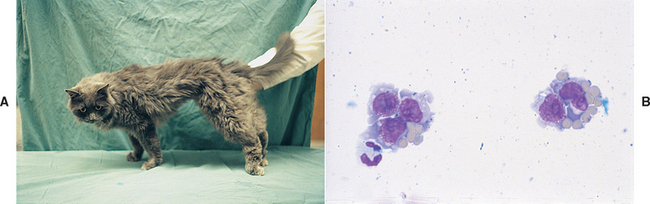
FIG 70-17 A, A 2-year-old cat with a 5-day course of progressive rear limb ataxia and upper motor neuron (UMN) paresis. B, Cerebrospinal fluid (CSF) analysis revealed an increased cell count consisting predominantly of neoplastic lymphoid cells.
Myelography allows most spinal cord tumors to be characterized as intramedullary, extramedullary-intradural, or extradural (see Fig. 64-6). When available, advanced imaging techniques (i.e., CT, MRI) add valuable information regarding precise tumor location and degree of spinal cord involvement, which may be important when considering surgical treatment and/or radiation therapy.
Treatment
Surgical decompression and attempts at complete tumor excision are usually limited to well-encapsulated intradural extramedullary tumors as a referral procedure. Feline menigiomas may have a good prognosis following surgical excision. Intramedullary tumors cannot usually be treated successfully with surgery because of their intimate involvement with neural tissue.
Chemotherapy and radiation therapy as primary or postoperative adjuvant therapies have met with limited success in the treatment of spinal tumors in dogs and cats. Radiation therapy may be of some benefit in dogs and cats with spinal lymphoma, plasma cell tumors, meningiomas, and some nerve sheath tumors. Corticosteroids, although they have little effect on most tumors, can decrease tumor-associated edema and inflammation and result in remarkable temporary improvement. Lymphoreticular tumors such as lymphoma and myeloma can also be treated with traditional chemotherapy protocols, although only a few of the drugs used cross the blood-brain barrier and the long-term prognosis is poor.
Intraspinal Articular Cysts
Cysts arising from the joint capsule of spinal facetal joints can, through enlargement, cause chronic progressive focal compression of the spinal cord or nerve roots. These cysts can result from an outpouching of the synovium (i.e., synovial cysts), or they may arise from mucinous degeneration of periarticular connective tissue (i.e., ganglion cysts). Synovial cysts and ganglion cysts are clinically indistinguishable, and both arise secondary to degenerative changes in the facetal joints. Degenerative changes occur because of congenital malformations, vertebral instability, or trauma. Signs are referable to the site and degree of resulting spinal cord or nerve root compression. Young giant breeds of dogs such as Mastiffs and Great Danes most commonly develop single or multiple cysts in the cervical region, which cause a UMN myelopathy and occasionally cervical pain. Older dogs, particularly German Shepherd Dogs, have been identified with thoracolumbar or lumbosacral articular cysts that cause spinal cord or cauda equina compression. Radiographs reveal degenerative changes of the articular facets. CSF analysis reveals normal cytology and slightly increased protein consistent with a noninflammatory chronic compressive myelopathy. Myelography reveals focal extradural dorsolateral compression of the spinal cord. MRI is necessary to identify the facetal joints as the origin of the cysts and to precisely localize the cysts before surgical therapy. Treatment consists of spinal cord decompression, cyst drainage, and arthrodesis of the facetal joint and usually produces excellent results. A similar syndrome with degeneration and bony proliferation of multiple thoracolumbar articular facets causing spinal cord compression has been reported as a hereditary condition in 4- to 10-month-old Shiloh Shepherds.
Arachnoid Cysts
Focal accumulations of CSF within cystlike structures within the subarachnoid space can lead to slowly progressive, nonpainful spinal cord compression in young dogs (Fig. 70-18). The cystlike structures containing CSF may represent a congenital diverticulum or a pocket caused by adhesions in the subarachnoid space secondary to trauma or disk extrusion. The cervical region and the caudal thoracic region are most often affected, and as CSF fills the arachnoid cyst, compression of the spinal cord occurs. Young large-breed dogs are most likely to be affected, with Rottweilers overrepresented. Cats are rarely affected. Myelography or MRI reveals the accumulation of CSF at the site. Exploration and marsupialization of the cyst is associated with a good prognosis for recovery if performed within 4 months of development of clinical signs and if neurologic deficits are not severe.
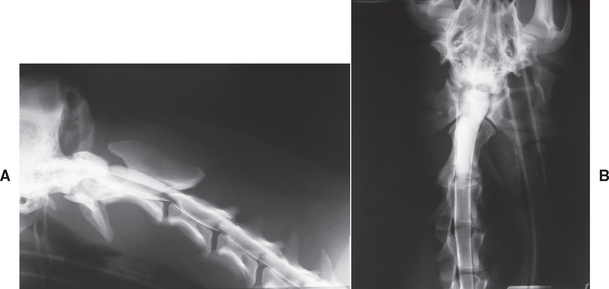
FIG 70-18 Lateral (A) and ventrodorsal (B) views of a myelogram from a 10-month-old Akita with progressive hypermetria of all four limbs and mild paraparesis. A well-defined, bulbous dilation of the dorsal subarachnoid space communicating with the rest of the subarachnoid space was present at C2-C3, suggesting an arachnoid cyst. Surgical exploration and marsupialization resulted in rapid and persistent (>6 years) return to normal gait.
Type II Intervertebral Disk Disease
Fibroid degeneration of the intervertebral disk occurs in some dogs as part of the aging process, and this can lead to prolapse of a small amount of disk nucleus into the annulus fibrosus. A fibrotic reaction ensues, resulting in a round, domelike dorsal bulging of the annulus so that it protrudes into the spinal canal and causes slowly progressive spinal cord compression (see Fig. 70-5). This type of disk protrusion (i.e., Hansen’s type II) is seen most commonly in aging large-breed nonchondrodystrophoid dogs, particularly German Shepherd Dogs, Labrador Retrievers, and Doberman Pinschers; however, it has also been recognized occasionally in small-breed dogs.
Clinical Features
Clinical signs result primarily from slowly progressive spinal cord compression, although spinal discomfort is apparent in a few dogs. Thoracolumbar type II disk protrusion results in UMN signs to the rear limbs, with normal forelimbs. Cervical type II disk disease may be seen in Doberman Pinschers, particularly in association with the cervical vertebral malformation-malarticulation syndrome (i.e., wobbler syndrome). In these dogs thoracic and pelvic limbs are affected, with UMN neurologic signs most prominent in the pelvic limbs.
Diagnosis
Slowly progressive signs of spinal cord dysfunction in an older dog should prompt consideration of type II disk protrusion, degenerative myelopathy (DM), and neoplasia. Neurologic examination localizes the lesion to a spinal cord region, but because the site is not usually painful, spinal palpation rarely results in more precise localization. Survey radiographs of the spine are normal in most affected dogs. Disk space narrowing, osteophyte production, and end-plate sclerosis may be seen at the site of type II disk protrusion in some dogs, but these abnormalities are common at multiple sites in older large-breed dogs; thus they are not very helpful in further localizing the lesion. Myelography or advanced imaging technique (i.e., CT, MRI) is necessary to determine the extent and location of the lesion and to distinguish type II disk protrusion from spinal neoplasia and DM.
Treatment
Medical therapy with antiinflammatory drugs (nonsteroidal antiinflammatory drugs or low-dose prednisone) and muscle relaxants will provide relief in dogs that are uncomfortable when the affected site is palpated or manipulated. Neurologic signs will progress, however, and surgery is recommended as the definitive treatment. Ventral decompression is performed if the cervical vertebrae are affected, whereas hemilaminectomy for decompression at the site is usually attempted for type II disks in the thoracolumbar spine. Effective surgical decompression is often difficult to achieve because of the chronic nature of the lesion and the difficulty encountered in removal of the dorsal annulus. The goal of therapy is to stabilize the animal’s neurologic status. The spinal cord has usually undergone considerable chronic compression before clinical signs appear; thus full recovery is rare. A few dogs experience temporary or permanent worsening of clinical signs postoperatively.
Degenerative Myelopathy
A degenerative disorder of the spinal cord white matter characterized by widespread myelin and axon loss occurs most often in aging German Shepherd Dogs and the Pembroke Welsh Corgi. DM has been recognized in dogs from 5 to 14 years of age and has rarely been seen in old dogs of other large breeds, in young German Shepherd Dogs, and in cats. A DM-like disorder has also been identified in the Pembroke Welsh Corgi. The thoracic and thoracolumbar spinal cord segments are most severely affected in all affected breeds; thus the neurologic findings suggest a lesion between T3 and L3.
Etiology
The cause of DM is uncertain. Some have speculated that deficiencies of nutrients or vitamins are responsible for the widespread demyelination and degeneration of axons observed histologically. An inherited cause has also been proposed in the German Shepherd Dog and the Pembroke Welsh Corgi. Whatever the initiating event, DM is generally considered to be an immune-mediated neurodegenerative disease similar to multiple sclerosis in humans. Depressed cell-mediated immunity and an increase in circulating immune complexes are consistent findings in dogs with DM, and spinal cord deposition of immunoglobulin and complement has been documented in association with histologic lesions of the disease.
Clinical Features
Clinically, DM results in a slowly progressive (e.g., 6 months to 2 years) UMN paraparesis and ataxia of the rear limbs. A loss of conscious proprioception results in knuckling of the toes, wearing of the dorsal nail surfaces of the digits of the rear limbs, and severe posterior ataxia.
Increased muscle tone and hyperactivity of the rear limb tendon reflexes result in clinical localization of the problem to the spinal cord between the T3 and L3 spinal cord segments. Thoracic limbs are normal, and urinary and fecal continence are maintained until very late in the course of the disease. Neurologic deficits may be asymmetric. In a very small number of cases (<10%) a decrease or loss of pelvic limb reflexes is observed late in the course of the disease as a result of involvement of the dorsal spinal nerve roots important for the afferent arm of the reflexes.
Diagnosis
A diagnosis of DM is suspected in any large-breed dog with slowly progressive UMN paresis in the rear limbs. Rear limb ataxia, a long-strided gait, toe scuffing, abnormal postural reactions (especially knuckling), and normal to increased rear limb reflexes are the most common findings. Affected dogs are systemically normal, with no site of localizable spinal pain. Neurologic findings distinguish DM from lumbosacral disease and from orthopedic disorders such as hip dysplasia and bilateral anterior cruciate ligament rupture. The primary differential diagnoses for chronic UMN paresis in the rear limbs include DM, spinal cord neoplasia, and type II disk disease.
The antemortem diagnosis of DM is one of exclusion. Radiographs of the spine are normal, as is CSF analysis, although a slight increase in CSF protein concentration is occasionally found. Myelography or MRI must be performed to rule out the presence of spinal cord compression or focal spinal cord neoplasia. Normal spinal radiographs, a cytologically normal CSF, and normal spinal cord imaging in an older dog with slowly progressive UMN signs to the pelvic limbs warrant a diagnosis of DM.
Treatment
No treatment has been proven effective in dogs with DM. Corticosteroids should not be administered because they cause muscle wasting and exacerbation of muscle weakness. Other immunosuppressive agents have not been shown to be beneficial. Some investigators have advocated vitamin (i.e., vitamin E, vitamin B complex, vitamin C) and omega-3 fatty acid supplementation, but conclusive evidence of their benefit is lacking. Exercise may be helpful in slowing the progression of the disease. Walking, running, and swimming for 30 minutes every other day is recommended. Some clinicians report success after long-term administration of aminocaproic acid (EACA; Amicar; Lederle Laboratories, American Cyanamide), 500 mg orally q8h. This drug blocks the final common pathway of tissue inflammation and may slow or halt the progression of DM in a few cases. Drawbacks of EACA therapy include gastrointestinal irritation, high cost, and a need to treat for 2 to 3 months before response to treatment is detectable. Administration of EACA in combination with the potent antioxidant acetylcysteine (25 mg/kg administered as a 5% solution orally q8h for 14 days, then every other day) has also been recommended. In the absence of other effective treatments for DM, these unproven treatments should be considered.
Cauda Equina Syndrome
In dogs the last three lumbar spinal cord segments (L5, L6, L7) are within the fourth lumbar vertebra, the sacral segments (S1, S2, S3) are within the body of the fifth lumbar vertebra, and the coccygeal segments are within the sixth lumbar vertebra. Nerve roots from these lumbar, sacral, and coccygeal segments of the spinal cord exit the spinal canal through the intervertebral foramina caudal to the vertebrae with the same number; thus they must course a considerable distance within the vertebral canal caudal to the point of termination of the spinal cord (Fig. 70-19). This collection of nerve roots descending in the vertebral canal is termed the cauda equina. The spinal nerves from the sacral and caudal segments overlie the lumbosacral junction, so compressive diseases of this region are likely to involve the L7, sacral, and caudal nerves.
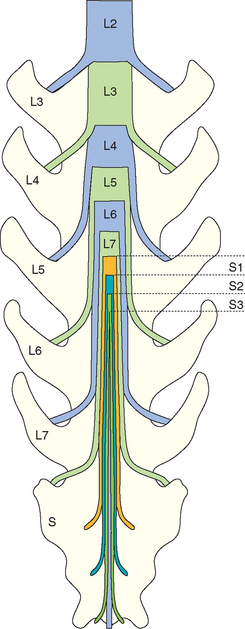
FIG 70-19 The anatomy of the cauda equina region in the dog. L5-L7 spinal cord segments sit within the L4 vertebra. S1-S3 spinal cord segments are within the L5 vertebra, and the coccygeal segments are within L6. Nerve roots from all of the lumbar, sacral, and coccygeal spinal cord segments leave the canal through the intervertebral foramen just caudal to the vertebra with the same number so that these nerve roots course a considerable distance within the vertebral canal.
Compression of the nerves of the cauda equina (cauda equina syndrome) is usually the result of acquired type II disk protrusion at the L7/S1 intervertebral space together with progressive proliferation of joint capsules and ligaments at that site, perhaps caused by excessive motion or instability. This disorder is most common in large-breed dogs, including German Shepherd Dogs, Labrador Retrievers, and Belgian Malinoises, and particularly affects male working dogs over the age of 5 years. Rarely, compression of the cauda equina may be caused by tumor, diskospondylitis, vertebral or sacral osteochondrosis, or congenital bony malformations.
Genetic predisposition, conformation, and physical activity are all factors proposed to cause increased mechanical stress on the intervertebral disk at the lumbosacral junction, promoting type II disk prolapse at this site. Loss of the structural strength of the disk worsens instability at the site, resulting in proliferative changes in the articular facets, joint capsules, and the interarcuate ligament (i.e., ligamentum flavum). Proliferative changes result in further narrowing of the vertebral canal, compression of the cauda equina, and compression of the nerve roots as they exit the foramina (degenerative lumbosacral stenosis).
Clinical Features
Compression of the cauda equina results in a very characteristic constellation of clinical signs. Affected dogs are slow to rise from a prone position and reluctant to run, sit up, jump, or climb stairs. Rear limb lameness worsens with exercise as the blood vessels accompanying the spinal nerve roots within the already crowded intervertebral foramen dilate and further compress the nerve roots (i.e., neurogenic claudication). Affected dogs may be reluctant to raise or wag their tails.
The most consistent physical examination finding is pain elicited by deep palpation of the dorsal sacrum or by dorsiflexion of the tail or hyperextension of the lumbosacral region (Fig. 70-20). Most dogs have no neurologic deficits at the time of initial evaluation, making it difficult to distinguish affected dogs from those with pain and lameness caused by diskospondylitis, prostatic disease, or degenerative joint disease. When lumbosacral spinal canal and foraminal narrowing progress to cause compression of the L7, sacral, and caudal spinal nerves, rear limb weakness, atrophy of the muscles of the caudal thigh and distal limb, and reduced or absent hock flexion during the withdrawal reflex will become apparent. The patellar reflex may appear increased in some dogs because there is a loss of tone in the opposing caudal thigh muscles (pseudohyperreflexia). In severely affected dogs decreased anal tone and fecal and urinary incontinence will occur. Hyperesthesia or paresthesia of the perineum may develop, with self-inflicted moist dermatitis of the perineum and tail base.
Diagnosis
Historical, physical, and neurologic examination findings are the primary basis for reaching a tentative diagnosis of cauda equina syndrome in affected dogs. Spinal survey radiographs are useful to rule out unusual causes of lumbosacral pain (e.g., diskospondylitis, lytic vertebral neoplasia, fracture/luxation). Radiographs of this region in dogs with cauda equina syndrome may be normal or may reveal end plate sclerosis and spondylosis of the L7 and S1 vertebral end plates and narrowing or collapse of the L7-S1 intervertebral disk space. These same abnormalities are common in clinically normal dogs.
Diagnosis is based on documentation of nerve compression using imaging. Myelography (cervical injection) can document cauda equina compression, but it will not be diagnostic in those dogs (20%) in which the dural sac ends cranial to the lumbosacral junction or in dogs in which the primary lesion is lateral compression of the spinal nerves at the intervertebral foramen. When available, MRI with the spine in extension provides the most sensitive, accurate, and noninvasive means of evaluating the lumbosacral region, allowing visualization of all components potentially involved in cauda equina compression (see Fig. 70-21). There is some concern that routine use of MRI for diagnosis may lead to overinterpretation of incidental minor compressive lesions of the cauda equina; therefore clinical findings must support the MRI diagnosis. When available, electrophysiological studies can be useful to confirm LMN disease and nerve root dysfunction of the rear limbs and tail.
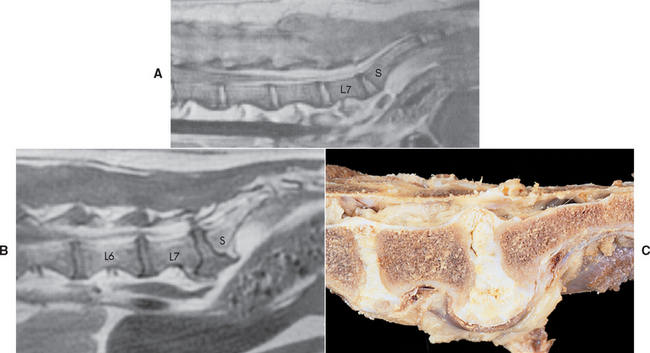
FIG 70-21 A, Normal midline sagittal T1 on a magnetic resonance image (MRI) scan of the lumbar spine of a dog. (The image reveals the high signal intensity [white] of the nucleus pulposus and the epidural fat, in contrast to the lesser signal density of the spinal cord and the nerve roots of the cauda equina [darker].) B, MRI from a dog with lumbosacral pain showing T1-weighted midline sagittal, displacement of epidural fat, and ventral and dorsal compression of the nerve roots at the L7-S1 disk space. Spondylosis deformans ventral to the L7-S1 intervertebral disk space and disk protrusion at the L6-L7 space can also be seen. C, Postmortem dissection of the lumbosacral region of a German Shepherd Dog with acquired degenerative lumbosacral stenosis and type II disk protrusion. The vertebral canal is compromised at the lumbosacral junction, resulting in compression of the nerves of the cauda equina.
(A and B, Courtesy Dr. Greg Daniel, University of Tennessee.)
Treatment
Restriction of exercise and the administration of analgesics or antiinflammatory drugs may result in temporary improvement in dogs with clinical signs limited to pain and lameness. Signs usually recur when normal activity is resumed. More definitive treatment involves lumbosacral dorsal laminectomy, excision of compressing tissues, and foraminal decompression by foraminotomy when necessary. Decompressive surgery together with lumbosacral distraction and stabilization have been recommended for dogs with marked neurologic deficits or severe pain. Descriptions of the surgical procedures are provided in Suggested Readings. Rapid relief from pain occurs in most dogs. Strict confinement is important for 4 to 8 weeks postoperatively, followed by a gradual return to exercise and work. The prognosis is excellent for resolution of lameness and mild neurologic deficits. Most dogs with mild to moderate deficits will return to working function. Dogs with severe LMN deficits or incontinence are likely to have permanent deficits.
Cervical Spondylomyelopathy (Wobbler Syndrome)
Cervical spondylomyelopathy (CSM), or canine wobbler syndrome, is a term used to describe caudal cervical spinal cord and nerve root compression in large-breed dogs that occurs secondary to developmental malformations, instability, or instability-associated changes in the spinal canal. Vertebral canal narrowing can be the result of malformed vertebral laminae, hypertrophy of the ligamentum flavum, articular facet enlargement, periarticular soft tissue hypertrophy, or a combination of these. In addition, changes in the vertebral body and end plates can result in instability that leads to intervertebral disk failure and the development of type II disk protrusions or occasionally type I disk herniation.
Typically, Great Danes and Doberman Pinschers are affected, but the condition has been reported in many large breeds of dogs. Males may be affected more often than females. Age at presentation varies from 7 weeks to 10 years. Stenosis of the cranial aspect of the cervical vertebrae (usually C4, C5, or C6) and articular facet deformities are the most common abnormalities in young Great Danes. Vertebral column instability with spinal cord compression by secondary soft tissue hypertrophy or disk, with or without congenital cervical vertebral malformation and canal stenosis (usually C5, C6, or C7), is more commonly recognized in middle-aged and older Doberman Pinschers. Genetic predisposition, overnutrition, and conformation have all been implicated in the development of this disorder.
Clinical Features
A slowly progressive course of paresis and an incoordinated or wobbling gait, particularly in the pelvic limbs, is characteristic of CSM. Affected dogs have a broad-based rear limb stance, ataxia, and abnormal postural reactions in the rear limbs (which are invariably more severely affected than the forelimbs). Neurologic findings in the forelimbs vary depending on whether spinal cord compression is centered in the cranial cervical region or in the caudal cervical region. Dogs with C1-C5 compression often have a floating or hypermetric front limb gait. Dogs with caudal cervical lesions may have a short-strided, weak front limb gait with a weak withdrawal reflex and pronounced atrophy of the supraspinatus and infraspinatus muscles over the scapula. Lameness and muscle atrophy in one thoracic limb or pain when traction is applied to a limb (i.e., root signature; see Fig. 70-6) suggests that nerve root compression is present. Slowly progressive deterioration in neurologic status is common, but occasionally a traumatic episode or an acute disk extrusion results in sudden tetraplegia. At the time of examination, neurologic deficits can be localized to the cervical spinal cord. Resistance to dorsal extension of the cervical spine is common, but overt cervical pain is rare unless secondary disk prolapse has occurred.
Diagnosis
The diagnosis is suspected on the basis of signalment, history, and clinical findings. Survey radiographs are useful to rule out other disorders associated with cervical spinal cord compression but are not definitive for CSM. Severe articular facet changes or vertebral body malformations should, however, raise the index of suspicion for CSM in a large-breed dog (Fig. 70-22A). Myelography is the standard means of confirming a diagnosis of CSM and has the advantage that the spinal cord compression can be observed with the spine in multiple positions, allowing differentiation between static, dynamic, and positional lesions. CSF should be evaluated before injection of contrast. Once contrast is injected, it should be concentrated in the cervical region through patient positioning and routine lateral, ventrodorsal, dorsoventral, and then lateral flexion and traction views should be taken. Compressive lesions that improve with traction (traction-responsive or dynamic lesions), include type II disks and ligamentous hypertrophy. Spinal cord compression by bone proliferation or by extruded nucleus pulposus (type 1 disk) will not resolve with traction (traction nonresponsive or static lesions; Fig. 70-22, B and C). Some dogs have spinal cord compression that is not evident in neutral or traction views but that becomes apparent with flexion or extension (positional lesions). Ideally, all conventional myelograms for CSM should be followed by a CT scan or MRI to improve surgical planning. Rational decisions regarding therapy and prognosis can be made after determining whether compression is at one site or many, is primarily dorsal or ventral, and is static or dynamic. Because a temporary worsening of neurologic status may occur after imaging dogs with CSM under general anesthesia, if surgery is planned, it should be scheduled 48 to 72 hours after imaging to allow recovery and neurologic stabilization. All affected animals should be evaluated for systemic disease before surgery, particularly Doberman Pinschers that may have concurrent hypothyroidism, von Willebrand’s disease, or cardiomyopathy.
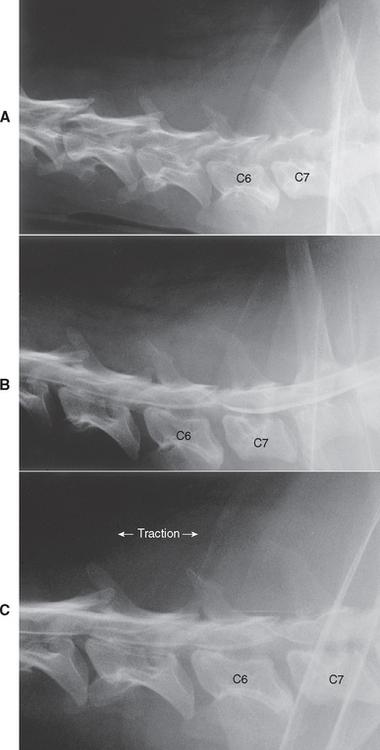
FIG 70-22 A, Radiographs of the cervical region in a 6-year-old Doberman Pinscher “wobbler” with a sudden onset of ataxia, paraparesis, proprioceptive deficits and hyperreflexia in the rear limbs, and mild cervical pain. A slight narrowing of the C6-C7 disk space can be seen; the vertebral canal is stenotic within the cranial aspect of C6 and C7. B, Myelography shows spinal cord compression by a ventral extradural mass at C6-C7 that is not altered significantly with traction, (C). Surgery revealed a large amount of disk material within the vertebral canal at this site.
Treatment
The clinical course of untreated wobbler syndrome is chronically progressive. Medical or surgical therapy can be used to attempt to relieve clinical signs. Severe exercise restriction, use of a harness, and administration of antiinflammatory doses of prednisone may result in temporary improvement in neurologic function. Sometimes, long-term management of dogs with minimal or mild signs of neurologic dysfunction is satisfactory with exercise restriction and corticosteroid therapy alone (prednisone 0.5 mg/kg orally q12h for 2 days; then 0.5 mg/kg once every day for 2 days; then 0.5 mg/kg once every other day for 14 days; then 0.25 mg/kg once every other day for 2 months).
Although initial improvement is common after medical therapy, the underlying compression and instability persist and generally progress without more definitive treatment. Surgical treatment is recommended in all dogs with persistent neurologic deficits. The main factor determining the specific surgical procedure to be recommended is the appearance of the cord myelographically, especially on the traction view. If the only lesion identified is static (traction nonresponsive) ventral spinal cord compression resulting from type 1 disk herniation, then ventral decompression is performed. If a single dynamic lesion is causing compression, such as a bulging annulus (ventrally) or a hypertrophied ligamentum flavum (dorsally), then a distraction/fusion technique is used to pull the vertebral bodies apart and maintain the separation, decreasing spinal cord compression and relieving pressure on the nerve roots (Fig. 70-23). The method used depends on the number of sites involved and surgeon preference. If the imaging studies indicate static dorsal compression of the spinal cord resulting from vertebral malformation or articular process osteophytes, then a dorsal decompressive technique must be attempted. Details of the surgical procedures and potential complications are discussed in Suggested Readings.
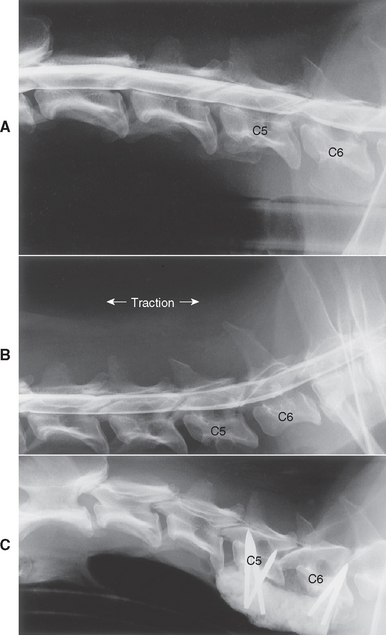
FIG 70-23 A, Cervical myelogram of an 11-year-old Doberman/Weimaraner cross with a chronic history of nonpainful ataxia and hypermetria of all four limbs. Narrowing of the C5-C6 disk space and thinning of the dorsal contrast column over this site (in association with dorsal deviation and thinning of the ventral contrast column) can be seen. B, The dramatic resolution of this spinal cord compression in the traction view suggests a dynamic compression by a bulging annulus fibrosus or ligamentum flavum. C, Surgery was performed to maintain traction on the spine at this site.
Prognosis
Dogs with wobbler syndrome have extremely variable prognoses, depending on their neurologic status, the temporal course of their disease, and the specific abnormalities that are present. Surgical results in ambulatory animals with a short history and only one lesion can be good, with up to 80% success reported. Multiple lesions, chronic disease, and an inability to walk are all associated with a poor prognosis.
PROGRESSIVE DISORDERS IN YOUNG ANIMALS
Breed-Associated Neuronal Abiotrophies and Degenerations
Neuronal abiotrophies and degenerative disorders have been recognized in many breeds of dogs. Progressive neurologic dysfunction usually begins early in life. In disorders affecting the entire spinal cord, clinical signs involving the rear limbs are often noted early in the course of disease with progression to tetraparesis. Disorders that primarily affect white matter and result in UMN signs are most often seen in Rottweilers, Afghan Hounds, Dalmatians, and Jack Russell Terriers. Disorders primarily affecting gray matter and causing LMN signs are seen in Alaskan Malamutes, Boxers, Brittany Spaniels, German Shepherd Dogs, English Pointers, and Maine Coon Cats. The disorders are diagnosed on the basis of the typical clinical course, the signalment, and the lack of any definable etiology on screening blood tests, spinal radiographs, CSF analysis, myelography, and other diagnostic testing. Diagnosis is confirmed by necropsy examination in most cases. No treatment is available.
Metabolic Storage Diseases
A large group of rare disorders, characterized pathologically by the accumulation of metabolic products in cells caused by a genetically based enzyme deficiency, may result in signs of spinal cord dysfunction. The enzyme deficiency itself or the accumulation of the metabolic intermediates within cells causes a gradual progression of neurologic signs. Spinal signs are usually UMN in nature, although peripheral nerve dysfunction may occur. Cortical signs (e.g., seizures) and cerebellar signs (e.g., hypermetria) are more common. Signs are gradually progressive and usually obvious within the first year or two of life. Metabolic storage diseases are diagnosed on the basis of the typical clinical course and signalment; the lack of any other identifiable etiology; and, in some cases, organomegaly, abnormal appearance, blindness, and other readily identifiable clinical abnormalities resulting from the accumulation of metabolic product in extraneural sites.
Atlantoaxial Instability and Luxation
Normally, the atlas (C1) and the axis (C2) are bound together by ligaments. The dens, a bony projection from the cranial aspect of the body of the axis, is held firmly against the floor of the atlas by the transverse ligament, maintaining alignment of these two vertebrae and integrity of the spinal canal. Malformation or absence of the dens leading to instability can be seen as a congenital defect in many small breeds of dogs, including the Yorkshire Terrier, Miniature or Toy Poodle, Chihuahua, Pomeranian, and Pekingese; it occurs rarely in large-breed dogs and in cats. The malformation and resultant atlantoaxial instability lead to repeated spinal cord trauma and slowly progressive signs of cranial cervical spinal cord compression. Alternatively, in young dogs with congenital atlantoaxial instability, mild trauma may cause C1/C2 luxation, precipitating a sudden onset of cervical pain, tetraparesis, or paralysis. Of course, in any normal dog severe trauma could result in traumatic luxation or fracture in this region with similar clinical findings.
Clinical Features
Dogs with congenital atlantoaxial instability typically develop UMN signs indicating cervical spinal cord compression before 2 years of age. Clinical signs include neck pain (30% to 60%), low head carriage, ataxia, tetraparesis and postural reaction and conscious proprioceptive deficits in all limbs. Manipulation of the spine should be avoided because it can exacerbate motor dysfunction. Paralysis is rare, but if it does occur, it may be accompanied by hypoventilation. Some dogs have a persistent head tilt or turn. Atlantoaxial luxation secondary to malformation should be suspected in any young (i.e., 6- to 18-month-old) toy-breed dog with a history of cervical pain, tetraparesis, or tetraplegia. Atlantoaxial luxation should also be considered as a possible differential diagnosis in any dog with evidence for high cervical spinal cord disease (UMN tetraplegia) after trauma.
Diagnosis
Radiographic examination should be performed initially without anesthesia when atlantoaxial luxation is suspected to prevent inadvertent overflexion or twisting of an unstable cervical spine. Lateral and oblique lateral views may aid in demonstrating absence or deformity of the dens. Accurate positioning with the region of interest located in the center of the film is important. Instability with significant luxation can be recognized on a lateral view as widening of the space between the dorsal arch of the atlas and the dorsal spinous process of the axis on the lateral view and dorsal displacement of the body of the axis (Fig. 70-24). In cases of congenital luxation the dens may be recognized as abnormal, and fracture of the dens may be apparent in traumatic luxations. If preliminary radiographs are not diagnostic, the animal should be anesthetized and the radiographs repeated with the head gently flexed. This may allow demonstration of the instability. Extreme care is critical when manipulating an animal suspected of having atlantoaxial instability under anesthesia because rotation or excessive flexion of the neck may result in further spinal cord compression, respiratory paralysis, and death. Splinting the animal’s head and neck in extension before anesthesia is recommended to prevent excessive flexion during induction of anesthesia and intubation.
FIG 70-24 Atlantoaxial subluxation in a 7-month-old Bichon Frise. The dens rises well above its normal position, consistent with rupture of its ligament and compression of the cervical spinal cord. The space between the arch of the atlas and the spinous process of the axis is increased. This dog had a chronic history of intermittent cervical pain and severe upper motor neuron (UMN) tetraparesis.
Treatment
Treatment for acute severe tetraparesis caused by atlantoaxial luxation should include medical treatment as for acute spinal cord trauma (see Fig. 70-4). Nonsurgical treatment should include cage rest, application of a neck brace, and administration of analgesics. The purpose of the splint is to immobilize the atlantoaxial junction, so the splint must extend from over the head cranial to the ears and go back to the chest. Nonsurgical treatment is recommended in small dogs that fracture a normal atlantoaxial articulation. It is also very effective short term in dogs with congenital lesions, but the long-term results are unknown. Surgical treatment is effective but may be associated with high perioperative morbidity and mortality. Dorsal and ventral techniques are described in Suggested Readings.
Prognosis
The prognosis for recovery is good in dogs with congenital lesions that survive the perioperative period. Outcome is most likely to be positive if the onset of signs occurs before the patient is 2 years of age, when signs have been present for less than 10 months, and if surgical reduction is good.
NONPROGRESSIVE DISORDERS IN YOUNG ANIMALS
Spina Bifida
Spina bifida results from embryonic failure of fusion of the two halves of the dorsal spinous processes of the vertebral arch. Although spina bifida may occur anywhere along the spinal canal, the caudal lumbar and lumbosacral region is most often affected. This malformation is most common in English Bulldogs and Manx cats. In the Manx cat the condition is an autosomal recessive trait and may be associated with caudal agenesis. Clinical signs are nonprogressive and present from birth, including rear limb LMN paresis, fecal and urinary incontinence, loss of perineal sensation, and decreased tone of the anal sphincter. No therapy is available.
Caudal Agenesis of Manx Cats
Congenital malformations of the sacrococcygeal spinal cord and vertebrae are common in tailless Manx cats. Clinical signs result from agenesis or dysgenesis of the caudal vertebrae and sacral spinal cord. Signs are typically present from birth and include hopping or crouched pelvic limb gait, fecal and urinary incontinence, and chronic constipation.
Spinal Dysraphism
Spinal dysraphism is an inherited congenital malformation of the spinal cord. It results from the abnormal development of the structures of the spinal cord along the central plane. The malformation includes a dilated or absent central canal, cavitation in the white matter, and the abnormal presence of ventral gray column cells across the median plane between the central canal and the ventral median fissure. Spinal dysraphism is recognized most commonly in Weimaraners, although other breeds are occasionally affected.
Clinical signs are present at birth. Affected dogs have a symmetric, bunny-hopping pelvic limb gait; a wide-based stance; and depressed proprioception. The patellar reflex is normal. The pelvic limb flexor reflex stimulated in one limb usually elicits simultaneous flexion of both pelvic limbs. Clinical signs caused by spinal dysraphism do not progress, and mildly affected dogs can live a normal life.
Syringomyelia/Hydromyelia
Cystic accumulations of fluid within the spinal cord causing compression of adjacent parenchyma are being recognized with increasing frequency as advanced diagnostic imaging techniques (i.e., CT, MRI) are used for neurologic diagnosis. Syringomyelia is the development of a CSF-filled cavity anywhere within the cord, and hydromyelia is the accumulation of excessive CSF within a dilated central canal. These disorders can develop as a result of altered CSF pressures within the spinal canal, a loss of spinal cord parenchyma, or secondarily to obstructed CSF flow caused by congenital malformations or inflammatory or neoplastic disorders.
Clinical signs reflect the site and degree of spinal cord parenchymal destruction. Ataxia and paresis are common. With cervical lesions UMN signs are more pronounced in the rear limbs if the dorsal and lateral portions of the cord are affected. When the spinal cord damage is more centrally located, ataxia and paresis will often be more significant in the forelimbs than in the rear limbs (i.e., central cord syndrome). Spinal pain may be seen because of stretching of nerve roots or meninges. Scoliosis occasionally develops as LMN cell body damage within the cord causes asymmetric denervation of the paraspinal muscles, resulting in vertebral deviation. Syringohydromyelia has been reported in numerous Cavalier King Charles Spaniels with occipital bone malformations leading to overcrowding at the foramen magnum. The onset of clinical signs in this breed is usually in young adult dogs, with most dogs presenting because of scratching at the shoulder region; intolerance to touching of the ear, limb, or neck of the affected side; and cervical pain. Muscle atrophy and LMN weakness of the associated thoracic limb and ataxia and UMN deficits of the rear limbs may also be seen. Diagnosis is most reliably made with MRI. Treatment with antiinflammatory doses of prednisone to decrease CSF production may result in clinical improvement. Decompression of the caudal fossa with an occipital craniectomy to reestablish normal CSF flow can be effective.
Bagley RS. Spinal fracture or luxation. Vet Clin N Am Sm Anim Pract. 2000;30(1):133.
Bagley RS, et al. Exogenous spinal trauma: surgical therapy and aftercare. Comp Cont Ed Sm An Pract Vet. 2000;22(3):218.
Bagley RS, et al. Syringomyelia and hydromyelia in dogs and cats. Comp Cont Educ Sm An Pract Vet. 2000;22(5):471.
Beaver DP, et al. Risk factors affecting the outcome of surgery for atlantoaxial subluxation in dogs: 46 cases (1978–1998). J Am Vet Med Assoc. 2000;216:1104.
Bush WW, et al. Functional outcome following hemilaminectomy without methylprednisolone sodium succinate for acute thoracolumbar disk disease in 51 non-ambulatory dogs. J Vet Emerg Crit Care. 2007;17:72.
Cauzinille L. Fibrocartilaginous embolism in dogs. Vet Clin N Am. 2000;30(1):155.
Coates JR. Paraparesis. In: Platt SR, Olby NJ, editors. BSAVA manual of canine and feline neurology. Gloucester: BSAVA, 2004.
de Risio L, Thomas WB, Sharp NJH. Degenerative lumbosacral stenosis. Vet Clin N Am. 2000;30(1):111.
de Risio L, et al. Dorsal laminectomy for caudal cervical spondylomyelopathy: postoperative recovery and long term followup in 20 dogs. Vet Surg. 2002;31:418.
Dickinson PJ, et al. Extradural spinal synovial cysts in nine dogs. J Small Anim Pract. 2001;42:502.
Havig ME, et al. Evaluation of nunsurgical treatment of atlantoaxial subluxation in dogs: 19 cases (1999-2001). J Am Vet Med Assoc. 2005;227:256.
Hawthorne JC, et al. Fibrocartilaginous embolic myelopathy in Miniature Schnauzers. J Am Anim Hosp Assoc. 2001;37:374.
Marioni-Henry K, et al. Prevalence of diseases of the spinal cord of cats. J Vet Intern Med. 2004;18:851.
Munana KR, et al. Intervertebral disk disease in cats. J Am Anim Hosp Assoc. 2001;37:384.
Olby NJ. Tetraparesis. In: Platt SR, Olby NJ, editors. BSAVA manual of canine and feline neurology. Gloucester: BSAVA, 2004.
Rusbridge C, et al. Syringohydromyelia in Cavalier King Charles Spaniels. J Am Anim Hosp Assoc. 2000;36:34.
Sharp JH, Wheeler SJ. Small animal spinal disorders. St Louis: Elsevier, 2005.