Chapter 5 Neoplasia
See Targeted Therapy available online at studentconsult.com
Cancer is the second leading cause of death in the United States; only cardiovascular diseases exact a higher toll. Even more agonizing than the associated mortality is the emotional and physical suffering inflicted by neoplasms. Patients and the public often ask, “When will there be a cure for cancer?” The answer to this simple question is difficult, because cancer is not one disease but many disorders that share a profound growth dysregulation. Some cancers, such as Hodgkin lymphomas, are highly curable, whereas others, such as cancer of the pancreas, are virtually always fatal. The only hope for controlling cancer lies in learning more about its pathogenesis, and great strides have been made in understanding the molecular basis of cancer. This chapter deals with the basic biology of neoplasia—the nature of benign and malignant neoplasms and the molecular basis of neoplastic transformation. The host response to tumors and the clinical features of neoplasia also are discussed. Before we discuss the features of cancer cells and the mechanisms of carcinogenesis, it is useful to summarize the fundamental and shared characteristics of cancers:
• Cancer is a genetic disorder caused by DNA mutations that are (for the most part) acquired spontaneously or induced by environmental insults. In addition, cancers frequently show epigenetic changes, such as focal increases in DNA methylation and alterations in histone modifications, which may themselves stem from acquired mutations in genes that regulate such modifications. These genetic and epigenetic changes alter the expression or function of key genes that regulate fundamental cellular processes, such as growth, survival, and senescence.
• These genetic alterations are heritable, being passed to daughter cells upon cell division. As a result, cells harboring these alterations are subject to darwinian selection (survival of the fittest, arguably the most important scientific concept yet conceived), with cells bearing mutations that provide them with growth or survival advantages outcompeting their neighbors and thus coming to dominate the population. Darwinian selection also plays a role in the progression and recurrence of cancers, as discussed in more detail later. Because the selective advantages are conferred on a single cell that ultimately gives rise to the tumor, all tumors are clonal (i.e., the progeny of one cell).
• Accumulation of mutations gives rise to a set of properties that have been called hallmarks of cancer. These include (1) self-sufficiency in growth signals whereby the growth of cancers becomes autonomous and is unregulated by physiologic cues; (2) lack of response to growth inhibitory signals that control non-neoplastic cellular proliferations such as hyperplasias; (3) evasion of cell death, allowing cancer cells to survive under conditions that induce apoptosis in normal cells; (4) limitless replicative potential, thus making cancer cells immortal; (5) development of angiogenesis to sustain the growth of cancer cells; (6) ability to invade local tissues and spread to distant sites; (7) reprogramming of metabolic pathways—specifically, a switch to aerobic glycolysis even when there is abundant oxygen; and (8) ability to evade the immune system. The genetic alterations that give rise to these hallmarks of cancers are sustained and enabled by the development of genomic instability, adding fuel to the fire. The molecular underpinnings of these hallmarks are discussed in detail in a later section.
Understanding the cellular and molecular abnormalities in cancer cells is leading to a revolution in the treatment of cancer founded on basic research, and is one of the emerging triumphs of biomedical science.
Nomenclature
Neoplasia literally means “new growth.” Neoplastic cells are said to be transformed because they continue to replicate, apparently oblivious to the regulatory influences that control normal cell growth. Neoplasms therefore enjoy a certain degree of autonomy and tend to increase in size regardless of their local environment. Their autonomy is by no means complete, however. Some neoplasms require endocrine support, and such dependencies sometimes can be exploited therapeutically. All neoplasms depend on the host for their nutrition and blood supply.
In common medical usage, a neoplasm often is referred to as a tumor, and the study of tumors is called oncology (from oncos, “tumor,” and logos, “study of”). Among tumors, the division of neoplasms into benign and malignant categories is based on a judgment of a tumor’s potential clinical behavior.
• A tumor is said to be benign when its microscopic and gross characteristics are considered to be relatively innocent, implying that it will remain localized and is amenable to local surgical removal; the patient generally survives. Of note, however, benign tumors can produce more than localized lumps, and sometimes they are responsible for serious disease.
• Malignant tumors are collectively referred to as cancers, derived from the Latin word for “crab”—that is, they adhere to any part that they seize in an obstinate manner, similar to a crab’s behavior. Malignant, as applied to a neoplasm, implies that the lesion can invade and destroy adjacent structures and spread to distant sites (metastasize) to cause death. Not all cancers pursue so deadly a course. The most aggressive are also some of the most curable, but the designation malignant constitutes a red flag.
All tumors, benign and malignant, have two basic components: (1) the parenchyma, made up of transformed or neoplastic cells, and (2) the supporting, host-derived, non-neoplastic stroma, made up of connective tissue, blood vessels, and host-derived inflammatory cells. The parenchyma of the neoplasm largely determines its biologic behavior, and it is this component from which the tumor derives its name. The stroma is crucial to the growth of the neoplasm, since it carries the blood supply and provides support for the growth of parenchymal cells. Although the biologic behavior of tumors largely reflects the behavior of the parenchymal cells, there has been a growing realization that stromal cells and neoplastic cells carry on a two-way conversation that influences the growth of the tumor.
Benign Tumors
In general, benign tumors are designated by attaching the suffix -oma to the cell type from which the tumor arises. A benign tumor arising in fibrous tissue is a fibroma; a benign cartilaginous tumor is a chondroma. The nomenclature of benign epithelial tumors is more complex. They are classified sometimes on the basis of their microscopic pattern and sometimes on the basis of their macroscopic pattern. Others are classified by their cells of origin.
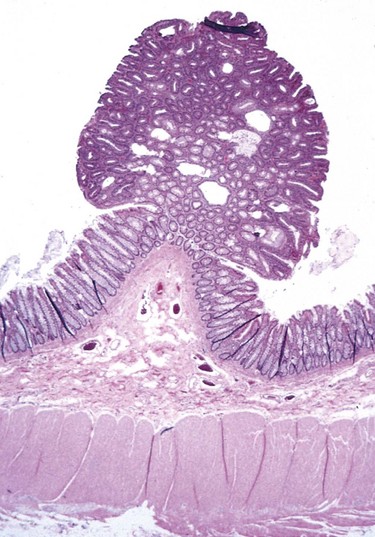
For instance, the term adenoma is generally applied to benign epithelial neoplasms producing gland patterns and to neoplasms derived from glands but not necessarily exhibiting glandular patterns. A benign epithelial neoplasm arising from renal tubule cells and growing in glandlike patterns is termed an adenoma, as is a mass of benign epithelial cells that produces no glandular patterns but has its origin in the adrenal cortex. Papillomas are benign epithelial neoplasms, growing on any surface, that produce microscopic or macroscopic finger-like fronds. A polyp is a mass that projects above a mucosal surface, as in the gut, to form a macroscopically visible structure (Fig. 5–1). Although this term commonly is used for benign tumors, some malignant tumors also may grow as polyps, whereas other polyps (such as nasal polyps) are not neoplastic but inflammatory in origin. Cystadenomas are hollow cystic masses that typically arise in the ovary.
Malignant Tumors
The nomenclature of malignant tumors essentially follows that of benign tumors, with certain additions and exceptions.
• Malignant neoplasms arising in “solid” mesenchymal tissues or its derivatives are called sarcomas, whereas those arising from the mesenchymal cells of the blood are called leukemias or lymphomas. Sarcomas are designated by the cell type of which they are composed, which is presumably their cell of origin. Thus, a cancer of fibrous tissue origin is a fibrosarcoma, and a malignant neoplasm composed of chondrocytes is a chondrosarcoma.
• While the epithelia of the body are derived from all three germ cell layers, malignant neoplasms of epithelial cells are called carcinomas regardless of the tissue of origin. Thus, a malignant neoplasm arising in the renal tubular epithelium (mesoderm) is a carcinoma, as are the cancers arising in the skin (ectoderm) and lining epithelium of the gut (endoderm). Furthermore, mesoderm may give rise to carcinomas (epithelial), sarcomas (mesenchymal), and hematolymphoid tumors (leukemias and lymphomas).
• Carcinomas are subdivided further. Carcinomas that grow in a glandular pattern are called adenocarcinomas, and those that produce squamous cells are called squamous cell carcinomas. Sometimes the tissue or organ of origin can be identified, as in the designation of renal cell adenocarcinoma. Sometimes the tumor shows little or no differentiation and must be called poorly differentiated or undifferentiated carcinoma.
The transformed cells in a neoplasm, whether benign or malignant, often resemble each other, as though all had been derived from a single progenitor, consistent with the monoclonal origin of tumors. In some unusual instances, however, the tumor cells undergo divergent differentiation, creating so-called mixed tumors. The best example is mixed tumor of salivary gland. These tumors have obvious epithelial components dispersed throughout a fibromyxoid stroma, sometimes harboring islands of cartilage or bone (Fig. 5–2). All of these diverse elements are thought to derive from epithelial cells or myoepithelial cells, or both, and the preferred designation for these neoplasms is pleomorphic adenoma. Fibroadenoma of the female breast is another common mixed tumor. This benign tumor contains a mixture of proliferating ductal elements (adenoma) embedded in a loose fibrous tissue (fibroma). Although only the fibrous component is neoplastic, the term fibroadenoma remains in common usage.
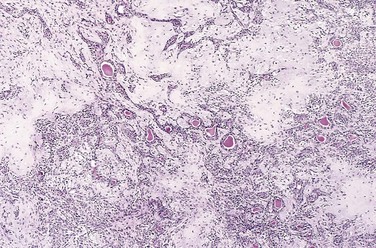
Figure 5–2 Mixed tumor of the parotid gland contains epithelial cells forming ducts and myxoid stroma that resembles cartilage.
(Courtesy of Dr. Trace Worrell, Department of Pathology, University of Texas Southwestern Medical School, Dallas, Texas.)
Teratoma is a special type of mixed tumor that contains recognizable mature or immature cells or tissues representative of more than one germ cell layer and sometimes all three. Teratomas originate from totipotential germ cells such as those normally present in the ovary and testis and sometimes abnormally present in sequestered midline embryonic rests. Germ cells have the capacity to differentiate into any of the cell types found in the adult body; not surprisingly, therefore, they may give rise to neoplasms that mimic, in helter-skelter fashion, bits of bone, epithelium, muscle, fat, nerve, and other tissues.
The specific names of the more common forms of neoplasms are presented in Table 5–1. Some glaring inconsistencies may be noted. For example, the terms lymphoma, mesothelioma, melanoma, and seminoma are used for malignant neoplasms. Unfortunately for students, these exceptions are firmly entrenched in medical terminology.
Table 5–1 Nomenclature of Tumors
| Tissue of Origin | Benign | Malignant |
|---|---|---|
| Composed of One Parenchymal Cell Type | ||
| Connective tissue and derivatives | Fibroma | Fibrosarcoma |
| Lipoma | Liposarcoma | |
| Chondroma | Chondrosarcoma | |
| Osteoma | Osteogenic sarcoma | |
| Endothelial and related tissues | ||
| Blood vessels | Hemangioma | Angiosarcoma |
| Lymph vessels | Lymphangioma | Lymphangiosarcoma |
| Mesothelium | Mesothelioma | |
| Brain coverings | Meningioma | Invasive meningioma |
| Blood cells and related cells | ||
| Hematopoietic cells | Leukemias | |
| Lymphoid tissue | Lymphomas | |
| Muscle | ||
| Smooth | Leiomyoma | Leiomyosarcoma |
| Striated | Rhabdomyoma | Rhabdomyosarcoma |
| Tumors of epithelial origin | ||
| Stratified squamous | Squamous cell papilloma | Squamous cell or epidermoid carcinoma |
| Basal cells of skin or adnexa | Basal cell carcinoma | |
| Epithelial lining of glands or ducts | Adenoma | Adenocarcinoma |
| Papilloma | Papillary carcinomas | |
| Cystadenoma | Cystadenocarcinoma | |
| Respiratory passages | Bronchial adenoma | Bronchogenic carcinoma |
| Renal epithelium | Renal tubular adenoma | Renal cell carcinoma |
| Liver cells | Liver cell adenoma | Hepatocellular carcinoma |
| Urinary tract epithelium (transitional) | Urothelial papilloma | Urothelial carcinoma |
| Placental epithelium | Hydatidiform mole | Choriocarcinoma |
| Testicular epithelium (germ cells) | Seminoma Embryonal carcinoma |
|
| Tumors of melanocytes | Nevus | Malignant melanoma |
| More Than One Neoplastic Cell Type—Mixed Tumors, Usually Derived from One Germ Cell Layer | ||
| Salivary glands | Pleomorphic adenoma (mixed tumor of salivary gland) | Malignant mixed tumor of salivary gland |
| Renal anlage | Wilms tumor | |
| More Than One Neoplastic Cell Type Derived from More Than One Germ Cell Layer—Teratogenous | ||
| Totipotential cells in gonads or in embryonic rests | Mature teratoma, dermoid cyst | Immature teratoma, teratocarcinoma |
There are other instances of confusing terminology:
• Hamartoma is a mass of disorganized tissue indigenous to the particular site. Histopathologic examination may show a mass of mature but disorganized hepatic cells, blood vessels, and possibly bile ducts within the liver, or a nodule in the lung containing islands of cartilage, bronchi, and blood vessels. Hamartomas have traditionally been considered developmental malformations, but some genetic studies have shown the presence of acquired translocations, suggesting a neoplastic origin.
• Choristoma is a congenital anomaly consisting of a heterotopic rest of cells. For example, a small nodule of well-developed and normally organized pancreatic tissue may be found in the submucosa of the stomach, duodenum, or small intestine. This heterotopic rest may be replete with islets of Langerhans and exocrine glands. The designation -oma, connoting a neoplasm, imparts to the heterotopic rest a gravity far beyond its usual trivial significance.
Although the terminology of neoplasms is regrettably not simple, a firm grasp of the nomenclature is important because it is the language by which the nature and significance of tumors are categorized.
Characteristics of Benign and Malignant Neoplasms
Nothing is more important to the patient with a tumor than being told: “It is benign.” In general, benign tumors appear to be genetically “simple,” harboring fewer mutations than cancers, and genetically stable, changing little in genotype over time. The latter feature probably explains why benign tumors such as lipomas and leiomyomas transform to malignancies rarely, if at all. In practice, the determination of benign versus malignant is made with remarkable accuracy using long-established clinical and anatomic criteria, but some neoplasms defy easy characterization. Certain features may indicate innocence, and others may indicate malignancy. Such problems are not the rule, however, and there are four fundamental features by which benign and malignant tumors can be distinguished: differentiation and anaplasia, rate of growth, local invasion, and metastasis.
Differentiation and Anaplasia
Differentiation and anaplasia are characteristics seen only in the parenchymal cells that constitute the transformed elements of neoplasms. The differentiation of parenchymal tumor cells refers to the extent to which they resemble their normal forebears morphologically and functionally.
• Benign neoplasms are composed of well-differentiated cells that closely resemble their normal counterparts. A lipoma is made up of mature fat cells laden with cytoplasmic lipid vacuoles, and a chondroma is made up of mature cartilage cells that synthesize their usual cartilaginous matrix—evidence of morphologic and functional differentiation. In well-differentiated benign tumors, mitoses are usually rare and are of normal configuration.
• Malignant neoplasms are characterized by a wide range of parenchymal cell differentiation, from surprisingly well differentiated (Fig. 5–3) to completely undifferentiated. For example, well-differentiated adenocarcinomas of the thyroid may contain normal-appearing follicles. Such tumors sometimes may be difficult to distinguish from benign proliferations. Between the two extremes lie tumors loosely referred to as moderately well differentiated. The stroma carrying the blood supply is crucial to the growth of tumors but does not aid in the separation of benign from malignant ones. The amount of stromal connective tissue does determine, however, the consistency of a neoplasm. Certain cancers induce a dense, abundant fibrous stroma (desmoplasia), making them hard, so-called scirrhous tumors.
• Malignant neoplasms that are composed of undifferentiated cells are said to be anaplastic. Lack of differentiation, or anaplasia, is considered a hallmark of malignancy. The term anaplasia literally means “backward formation”—implying dedifferentiation, or loss of the structural and functional differentiation of normal cells. It is now known, however, that at least some cancers arise from stem cells in tissues; in these tumors, failure of differentiation, rather than dedifferentiation of specialized cells, accounts for their undifferentiated appearance. Recent studies also indicate that in some cases, dedifferentiation of apparently mature cells does occur during carcinogenesis. Anaplastic cells display marked pleomorphism (i.e., variation in size and shape) (Fig. 5–4). Often the nuclei are extremely hyperchromatic (dark-staining) and large resulting in an increased nuclear-to-cytoplasmic ratio that may approach 1 : 1 instead of the normal 1 : 4 or 1 : 6. Giant cells that are considerably larger than their neighbors may be formed and possess either one enormous nucleus or several nuclei. Anaplastic nuclei are variable and bizarre in size and shape. The chromatin is coarse and clumped, and nucleoli may be of astounding size. More important, mitoses often are numerous and distinctly atypical; anarchic multiple spindles may produce tripolar or quadripolar mitotic figures (Fig. 5–5). Also, anaplastic cells usually fail to develop recognizable patterns of orientation to one another (i.e., they lose normal polarity). They may grow in sheets, with total loss of communal structures, such as glands or stratified squamous architecture.
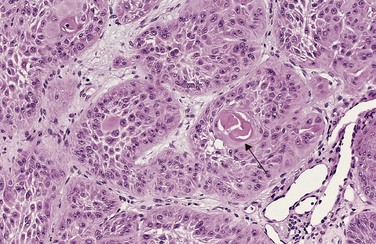
Figure 5–3 Well-differentiated squamous cell carcinoma of the skin. The tumor cells are strikingly similar to normal squamous epithelial cells, with intercellular bridges and nests of keratin (arrow).
(Courtesy of Dr. Trace Worrell, Department of Pathology, University of Texas Southwestern Medical School, Dallas, Texas.)
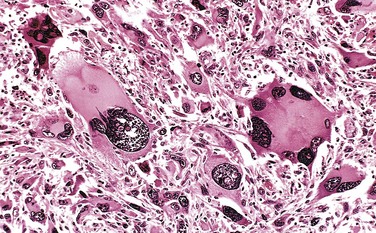
Figure 5–4 Anaplastic tumor of the skeletal muscle (rhabdomyosarcoma). Note the marked cellular and nuclear pleomorphism, hyperchromatic nuclei, and tumor giant cells.
(Courtesy of Dr. Trace Worrell, Department of Pathology, University of Texas Southwestern Medical School, Dallas, Texas.)
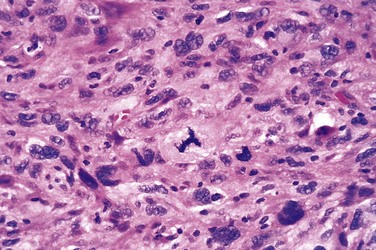
Figure 5–5 High-power detail view of anaplastic tumor cells shows cellular and nuclear variation in size and shape. The prominent cell in the center field has an abnormal tripolar spindle.
The more differentiated the tumor cell, the more completely it retains the functional capabilities of its normal counterparts. Benign neoplasms and even well-differentiated cancers of endocrine glands frequently elaborate the hormones characteristic of their origin. Well-differentiated squamous cell carcinomas produce keratin (Fig. 5–3), just as well-differentiated hepatocellular carcinomas secrete bile. In other instances, unanticipated functions emerge. Some cancers may elaborate fetal proteins not produced by comparable cells in the adult. Cancers of nonendocrine origin may produce so-called ectopic hormones. For example, certain lung carcinomas may produce adrenocorticotropic hormone (ACTH), parathyroid hormone–like hormone, insulin, glucagon, and others. More is said about these phenomena later. Despite exceptions, the more rapidly growing and the more anaplastic a tumor, the less likely it is to have specialized functional activity.
Of relevance in the discussion of differentiation and anaplasia is dysplasia, referring to disorderly but non-neoplastic proliferation. Dysplasia is encountered principally in epithelial lesions. It is a loss in the uniformity of individual cells and in their architectural orientation. Dysplastic cells exhibit considerable pleomorphism and often possess hyperchromatic nuclei that are abnormally large for the size of the cell. Mitotic figures are more abundant than usual and frequently appear in abnormal locations within the epithelium. In dysplastic stratified squamous epithelium, mitoses are not confined to the basal layers, where they normally occur, but may be seen at all levels and even in surface cells. There is considerable architectural anarchy. For example, the usual progressive maturation of tall cells in the basal layer to flattened squames on the surface may be lost and replaced by a disordered scrambling of dark basal-appearing cells (Fig. 5–6). When dysplastic changes are marked and involve the entire thickness of the epithelium, the lesion is referred to as carcinoma in situ, a preinvasive stage of cancer (Chapter 18). Although dysplastic changes often are found adjacent to foci of malignant transformation, and long-term studies of cigarette smokers show that epithelial dysplasia almost invariably antedates the appearance of cancer, the term dysplasia is not synonymous with cancer; mild to moderate dysplasias that do not involve the entire thickness of the epithelium sometimes regress completely, particularly if inciting causes are removed.
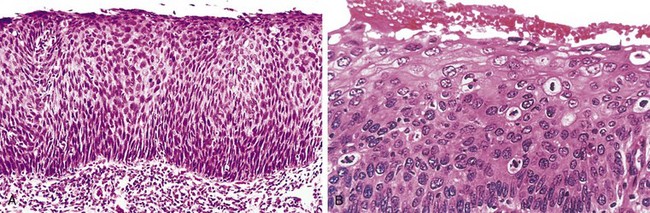
Figure 5–6 Carcinoma in situ. A, Low-power view shows that the entire thickness of the epithelium is replaced by atypical dysplastic cells. There is no orderly differentiation of squamous cells. The basement membrane is intact, and there is no tumor in the subepithelial stroma. B, High-power view of another region shows failure of normal differentiation, marked nuclear and cellular pleomorphism, and numerous mitotic figures extending toward the surface. The intact basement membrane (below) is not seen in this section.
Rate of Growth
Most benign tumors grow slowly, and most cancers grow much faster, eventually spreading locally and to distant sites (metastasizing) and causing death. There are many exceptions to this generalization, however, and some benign tumors grow more rapidly than some cancers. For example, the rate of growth of leiomyomas (benign smooth muscle tumors) of the uterus is influenced by the circulating levels of estrogens. They may increase rapidly in size during pregnancy and then cease growing, becoming largely fibrocalcific, after menopause. Other influences, such as adequacy of blood supply or pressure constraints, also may affect the growth rate of benign tumors. Adenomas of the pituitary gland locked into the sella turcica have been observed to shrink suddenly. Presumably, they undergo a wave of necrosis as progressive enlargement compresses their blood supply. Despite these caveats and the variation in growth rate from one neoplasm to another, it generally is true that most benign tumors increase in size slowly over the span of months to years.
The rate of growth of malignant tumors usually correlates inversely with their level of differentiation. In other words, poorly differentiated tumors tend to grow more rapidly than do well-differentiated tumors. However, there is wide variation in the rate of growth. Some grow slowly for years and then enter a phase of rapid growth, signifying the emergence of an aggressive subclone of transformed cells. Others grow relatively slowly and steadily; in exceptional instances, growth may come almost to a standstill. Even more exceptionally, some primary tumors (particularly choriocarcinomas) may become totally necrotic, leaving only secondary metastatic implants. Despite these rarities, most cancers progressively enlarge over time, some slowly, others rapidly, but the notion that they “emerge out of the blue” is not true. Many lines of experimental and clinical evidence document that most if not all cancers take years and sometimes decades to evolve into clinically overt lesions. This is true even of “acute” childhood leukemias, which often initiate during fetal development yet manifest as full-blown cancers years later. Rapidly growing malignant tumors often contain central areas of ischemic necrosis, because the tumor blood supply, derived from the host, fails to keep pace with the oxygen needs of the expanding mass of cells.
Cancer Stem Cells and Lineages
The continued growth and maintenance of many tissues that contain short-lived cells, such as the formed elements of the blood and the epithelial cells of the gastrointestinal tract and skin, require a resident population of tissue stem cells that are long-lived and capable of self-renewal. Tissue stem cells are rare and exist in a niche created by support cells, which produce paracrine factors that sustain the stem cells. As described in Chapter 2, tissue stem cells divide asymmetrically to produce two types of daughter cells—those with limited proliferative potential, which undergo terminal differentiation to form particular tissues, and those that retain stem cell potential. Cancers are immortal and have limitless proliferative capacity, indicating that like normal tissues, they also must contain cells with “stemlike” properties.
The cancer stem cell hypothesis posits that, in analogy with normal tissues, only a special subset of cells within tumors has the capacity for self-renewal. The concept of cancer stem cells has several important implications. Most notably, if cancer stem cells are essential for tumor persistence, it follows that these cells must be eliminated to cure the affected patient. It is hypothesized that, like normal stem cells, cancer stem cells are resistant to conventional therapies, because of their low rate of cell division and the expression of factors, such as multiple drug resistance-1 (MDR-1), that counteract the effects of chemotherapeutic drugs. Thus, the limited success of current therapies could be explained by their failure to kill the malignant stem cells that lie at the root of cancer. Cancer stem cells could arise from normal tissue stem cells or from more differentiated cells that, as part of the transformation process, acquire the property of self-renewal. Studies of certain leukemias (Chapter 11) suggest that both possibilities occur, in that chronic myelogenous leukemia originates from the malignant counterpart of a normal hematopoietic stem cell, whereas certain acute myeloid (myelogenous) leukemias are derived from more differentiated myeloid precursors that acquire an abnormal capacity for self-renewal. The identification of “leukemia stem cells” has spurred the search for cancer stem cells in solid tumors.
Local Invasion
A benign neoplasm remains localized at its site of origin. It does not have the capacity to infiltrate, invade, or metastasize to distant sites, as do malignant neoplasms. For example, as adenomas slowly expand, most develop an enclosing fibrous capsule that separates them from the host tissue. This capsule probably is derived from the stroma of the host tissue as the parenchymal cells atrophy under the pressure of the expanding tumor. The stroma of the tumor itself also may contribute to the capsule (Figs. 5-7 and 5-8). Of note, however, not all benign neoplasms are encapsulated. For example, the leiomyoma of the uterus is discretely demarcated from the surrounding smooth muscle by a zone of compressed and attenuated normal myometrium, but there is no well-developed capsule. Nonetheless, a well-defined cleavage plane exists around these lesions. A few benign tumors are neither encapsulated nor discretely defined; such lack of demarcation is particularly likely to be seen in some benign vascular neoplasms of the dermis. These exceptions are pointed out only to emphasize that although encapsulation is the rule in benign tumors, the lack of a capsule does not mean that a tumor is malignant.
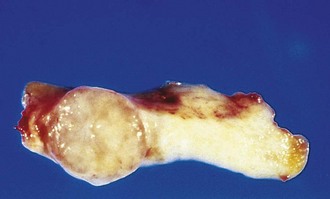
Figure 5–7 Fibroadenoma of the breast. The tan-colored, encapsulated small tumor is sharply demarcated from the whiter breast tissue.
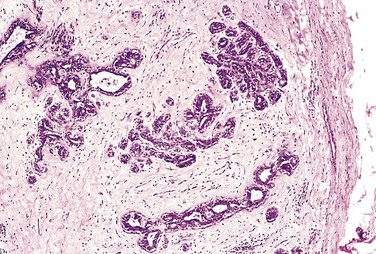
Figure 5–8 Microscopic view of fibroadenoma of the breast seen in Figure 5–7. The fibrous capsule (right) sharply delimits the tumor from the surrounding tissue.
(Courtesy of Dr. Trace Worrell, Department of Pathology, University of Texas Southwestern Medical School, Dallas, Texas.)
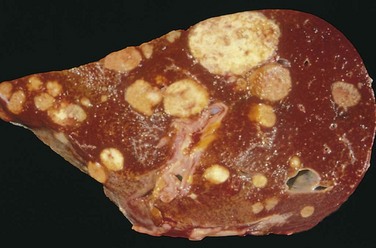
Cancers grow by progressive infiltration, invasion, destruction, and penetration of the surrounding tissue (Figs. 5-9 and 5-10). They do not develop well-defined capsules. There are, however, occasional instances in which a slowly growing malignant tumor deceptively appears to be encased by the stroma of the surrounding host tissue, but microscopic examination usually reveals tiny crablike feet penetrating the margin and infiltrating adjacent structures. The infiltrative mode of growth makes it necessary to remove a wide margin of surrounding normal tissue when surgical excision of a malignant tumor is attempted. Surgical pathologists carefully examine the margins of resected tumors to ensure that they are devoid of cancer cells (clean margins). Next to the development of metastases, local invasiveness is the most reliable feature that distinguishes malignant from benign tumors.
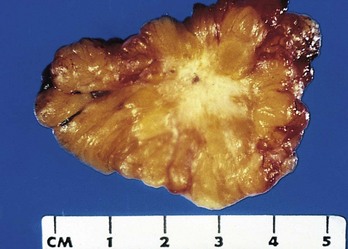
Figure 5–9 Cut section of invasive ductal carcinoma of the breast. The lesion is retracted, infiltrating the surrounding breast substance, and was stony-hard on palpation.
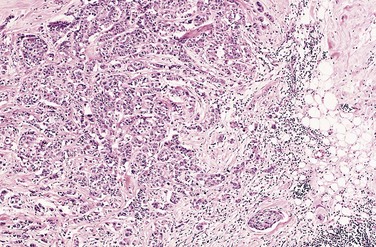
Figure 5–10 Microscopic view of breast carcinoma seen in Figure 5–9 illustrates the invasion of breast stroma and fat by nests and cords of tumor cells (compare with Fig. 5–8). Note the absence of a well-defined capsule.
(Courtesy of Dr. Trace Worrell, Department of Pathology, University of Texas Southwestern Medical School, Dallas, Texas.)
Metastasis
Metastases are secondary implants of a tumor that are discontinuous with the primary tumor and located in remote tissues (Fig. 5–11). More than any other attribute, the property of metastasis identifies a neoplasm as malignant. Not all cancers have equivalent ability to metastasize, however. At one extreme are basal cell carcinomas of the skin and most primary tumors of the central nervous system, which are highly invasive locally but rarely metastasize. At the other extreme are osteogenic (bone) sarcomas, which usually have metastasized to the lungs at the time of initial discovery.
Approximately 30% of patients with newly diagnosed solid tumors (excluding skin cancers other than melanomas) present with clinically evident metastases. An additional 20% have occult (hidden) metastases at the time of diagnosis.
In general, the more anaplastic and the larger the primary neoplasm, the more likely is metastatic spread, but as with most rules, there are exceptions. Extremely small cancers have been known to metastasize; conversely, some large and ominous-looking lesions may not. Dissemination strongly prejudices, and may preclude, the possibility of curing the disease, so obviously, short of prevention of cancer, no achievement would confer greater benefit on patients than the prevention of metastases.
Malignant neoplasms disseminate by one of three pathways: (1) seeding within body cavities, (2) lymphatic spread, or (3) hematogenous spread. Spread by seeding occurs when neoplasms invade a natural body cavity. This mode of dissemination is particularly characteristic of cancers of the ovary, which often cover the peritoneal surfaces widely. The implants literally may glaze all peritoneal surfaces and yet not invade the underlying tissues. Here is an instance of the ability to reimplant elsewhere that seems to be separable from the capacity to invade. Neoplasms of the central nervous system, such as a medulloblastoma or ependymoma, may penetrate the cerebral ventricles and be carried by the cerebrospinal fluid to reimplant on the meningeal surfaces, either within the brain or in the spinal cord.
Lymphatic spread is more typical of carcinomas, whereas hematogenous spread is favored by sarcomas. There are numerous interconnections, however, between the lymphatic and vascular systems, so all forms of cancer may disseminate through either or both systems. The pattern of lymph node involvement depends principally on the site of the primary neoplasm and the natural pathways of local lymphatic drainage. Lung carcinomas arising in the respiratory passages metastasize first to the regional bronchial lymph nodes and then to the tracheobronchial and hilar nodes. Carcinoma of the breast usually arises in the upper outer quadrant and first spreads to the axillary nodes. However, medial breast lesions may drain through the chest wall to the nodes along the internal mammary artery. Thereafter, in both instances, the supraclavicular and infraclavicular nodes may be seeded. In some cases, the cancer cells seem to traverse the lymphatic channels within the immediately proximate nodes to be trapped in subsequent lymph nodes, producing so-called skip metastases. The cells may traverse all of the lymph nodes ultimately to reach the vascular compartment by way of the thoracic duct.
A “sentinel lymph node” is the first regional lymph node that receives lymph flow from a primary tumor. It can be identified by injection of blue dyes or radiolabeled tracers near the primary tumor. Biopsy of sentinel lymph nodes allows determination of the extent of spread of tumor and can be used to plan treatment.
Of note, although enlargement of nodes near a primary neoplasm should arouse concern for metastatic spread, it does not always imply cancerous involvement. The necrotic products of the neoplasm and tumor antigens often evoke immunologic responses in the nodes, such as hyperplasia of the follicles (lymphadenitis) and proliferation of macrophages in the subcapsular sinuses (sinus histiocytosis). Thus, histopathologic verification of tumor within an enlarged lymph node is required.
Hematogenous spread is the favored pathway for sarcomas, but carcinomas use it as well. As might be expected, arteries are penetrated less readily than are veins. With venous invasion, the blood-borne cells follow the venous flow draining the site of the neoplasm, with tumor cells often stopping in the first capillary bed they encounter. Since all portal area drainage flows to the liver, and all caval blood flows to the lungs, the liver and lungs are the most frequently involved secondary sites in hematogenous dissemination. Cancers arising near the vertebral column often embolize through the paravertebral plexus; this pathway probably is involved in the frequent vertebral metastases of carcinomas of the thyroid and prostate.
Certain carcinomas have a propensity to grow within veins. Renal cell carcinoma often invades the renal vein to grow in a snakelike fashion up the inferior vena cava, sometimes reaching the right side of the heart. Hepatocellular carcinomas often penetrate portal and hepatic radicles to grow within them into the main venous channels. Remarkably, such intravenous growth may not be accompanied by widespread dissemination.
Many observations suggest that the anatomic localization of a neoplasm and its venous drainage cannot wholly explain the systemic distributions of metastases. For example, prostatic carcinoma preferentially spreads to bone, bronchogenic carcinomas tend to involve the adrenals and the brain, and neuroblastomas spread to the liver and bones. Conversely, skeletal muscles, although rich in capillaries, are rarely the site of secondary deposits. The molecular basis of such tissue-specific homing of tumor cells is discussed later on.
Thus, numerous features of tumors (Fig. 5–12), usually permit the differentiation of benign and malignant neoplasms.
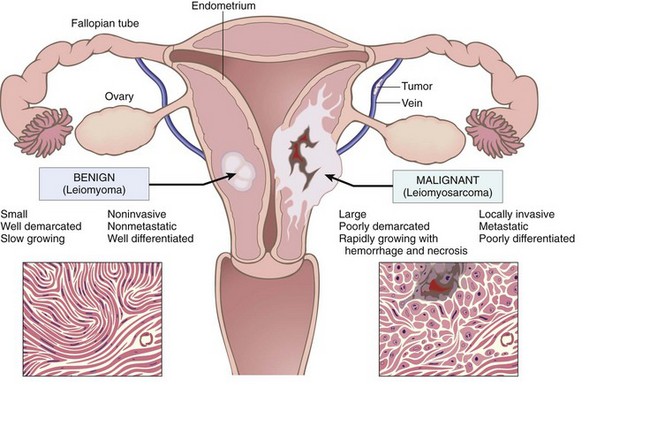
Figure 5–12 Comparison between a benign tumor of the myometrium (leiomyoma) and a malignant tumor of similar origin (leiomyosarcoma).
![]() Summary
Summary
Characteristics of Benign and Malignant Tumors
• Benign and malignant tumors can be distinguished from one another based on the degree of differentiation, rate of growth, local invasiveness, and distant spread.
• Benign tumors resemble the tissue of origin and are well differentiated; malignant tumors are poorly or completely undifferentiated (anaplastic).
• Benign tumors are slow-growing, whereas malignant tumors generally grow faster.
• Benign tumors are well circumscribed and have a capsule; malignant tumors are poorly circumscribed and invade the surrounding normal tissues.
• Benign tumors remain localized to the site of origin, whereas malignant tumors are locally invasive and metastasize to distant sites.
Epidemiology
Because cancer is a disorder of cell growth and behavior, its ultimate cause must be defined at the cellular and molecular levels. Cancer epidemiology can contribute substantially to knowledge about the origin of cancer. The now well-established concept that cigarette smoking is causally associated with lung cancer arose primarily from epidemiologic studies. A comparison of the incidence rates for colon cancer and dietary patterns in the Western world and in Africa led to the recognition that dietary fat and fiber content may figure importantly in the causation of this cancer. Major insights into the causes of cancer can be obtained by epidemiologic studies that relate particular environmental, racial (possibly hereditary), and cultural influences to the occurrence of specific neoplasms. Certain diseases associated with an increased risk of developing cancer (preneoplastic disorders) also provide clues to the pathogenesis of cancer.
The following discussion first summarizes the overall incidence of cancer to provide insight into the magnitude of the cancer problem and then reviews some issues relating to the patient and environment that influence the predisposition to cancer.
Cancer Incidence
Some perspective on the likelihood of developing a specific form of cancer can be gained from national incidence and mortality data. Overall, it is estimated that about 1.5 million new cancer cases occurred in 2011, and 569,000 people died of cancer in the United States that year. Incidence data for the most common forms of cancer, with the major killers identified, are presented in Figure 5–13.
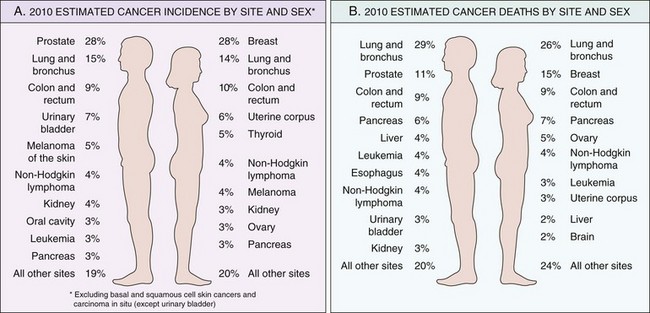
Figure 5–13 Cancer incidence and mortality by site and sex.
(Adapted from Jemal A, Siegel R, Xu J, Ward E: Cancer statistics, 2010. CA Cancer J Clin 60:277–300, 2010.)
Over several decades, the death rates for many forms of cancer have changed. Particularly notable is the significant increase in the overall cancer death rate among men that was attributable largely to lung cancer, but this has finally begun to drop. By contrast, the overall death rate among women has fallen slightly, mostly as a result of the decline in death rates for cancers of the uterine cervix, stomach, and large bowel. These welcome trends have more than counterbalanced the striking climb in the rate of lung cancer in women, which not long ago was a relatively uncommon form of neoplasia in this sex. The declining death rate from cervical cancer is directly related to widespread use of cytologic smear studies for early detection of this tumor and its precursor lesions. The development of the human papillomavirus (HPV) vaccine may eliminate this cancer altogether in the coming years. The causes of decline in death rates for cancers of the stomach are obscure; however, there have been speculations about decreasing exposure to dietary carcinogens.
Geographic and Environmental Variables
Although many impressive advances in understanding the molecular pathogenesis of cancer have been made by analyzing hereditary cancers, it is fair to state that environmental factors are the predominant cause of the most common sporadic cancers. This notion is supported by the geographic differences in death rates from specific forms of cancer. For example, death rates from breast cancer are about four to five times higher in the United States and Europe than in Japan. Conversely, the death rate for stomach carcinoma in men and women is about seven times higher in Japan than in the United States. Liver cell carcinoma is relatively infrequent in the United States but is the most lethal cancer among many African populations. Nearly all the evidence indicates that these geographic differences are environmental rather than genetic in origin. Nisei (second-generation Japanese living in the United States) have mortality rates for certain forms of cancer that are intermediate between those in natives of Japan and in Americans who have lived in the United States for many generations. The two rates come closer with each passing generation.
There is no paucity of environmental carcinogens. They lurk in the ambient environment, in the workplace, in food, and in personal practices. They can be as universal as sunlight, can be found particularly in urban settings (e.g., asbestos), or can be limited to a certain occupation (Table 5–2). Certain features of diet have been implicated as possible predisposing influences. Among the possible environmental influences, the most distressing in terms of prevention are those incurred in personal practices, notably cigarette smoking and chronic alcohol consumption. The risk of cervical cancer is linked to age at first intercourse and the number of sex partners (pointing to a causal role for venereal transmission of the oncogenic virus HPV). There is no escape: It seems that everything people do to earn a livelihood, to subsist, or to enjoy life turns out to be illegal, immoral, or fattening, or—most disturbing—possibly carcinogenic.
Table 5–2 Occupational Cancers
| Agent/Group of Agents | Human Cancer Site and Type for Which Reasonable Evidence Is Available | Typical Use/Occurrence |
|---|---|---|
| Arsenic and arsenic compounds | Lung, skin, hemangiosarcoma | Byproduct of metal smelting |
| Component of alloys, electrical and semiconductor devices, medications and herbicides, fungicides, and animal dips | ||
| Asbestos | Lung, mesothelioma; gastrointestinal tract (esophagus, stomach, large intestine) | Formerly used for many applications because of fire, heat, and friction resistance; still found in existing construction as well as fire-resistant textiles, friction materials (e.g., brake linings), underlayment and roofing papers, and floor tiles |
| Benzene | Leukemia | Principal component of light oil |
| Many applications exist in printing and lithography, paint, rubber, dry cleaning, adhesives and coatings, and detergents | ||
| Formerly widely used as solvent and fumigant | ||
| Beryllium and beryllium compounds | Lung | Missile fuel and space vehicles |
| Hardener for lightweight compounds metal alloys, particularly in aerospace applications and nuclear reactors | ||
| Cadmium and cadmium compounds | Prostate | Uses include yellow pigments and phosphors |
| Found in solders | ||
| Used in batteries and as alloy and in metal platings and coatings | ||
| Chromium compounds | Lung | Component of metal alloys, paints, pigments, and preservatives |
| Ethylene oxide | Leukemia | Ripening agent for fruits and nuts |
| Used in rocket propellant and chemical synthesis, in fumigants for foodstuffs and textiles, and in sterilants for hospital equipment | ||
| Nickel compounds | Nose, lung | Nickel plating |
| Component of ferrous alloys, ceramics, and batteries | ||
| Byproduct of stainless steel arc welding | ||
| Radon and its decay products | Lung | From decay of minerals containing uranium |
| Can be serious hazard in quarries and mines | ||
| Vinyl chloride | Angiosarcoma, liver | Refrigerant |
| Monomer for vinyl polymers | ||
| Adhesive for plastics | ||
| Formerly used as inert aerosol propellant in pressurized containers |
Modified from Stellman JM, Stellman SD: Cancer and workplace. CA Cancer J Clin 46:70–92, 1996, with permission from Lippincott Williams & Wilkins.
Age
In general, the frequency of cancer increases with age. Most cancer deaths occur between ages 55 and 75; the rate declines, along with the population base, after age 75. The rising incidence with age may be explained by the accumulation of somatic mutations associated with the emergence of malignant neoplasms (discussed later). The decline in immune competence that accompanies aging also may be a factor.
Cancer causes slightly more than 10% of all deaths among children younger than 15 years (Chapter 5). The major lethal cancers in children are leukemias, tumors of the central nervous system, lymphomas, and soft tissue and bone sarcomas. As discussed later, study of several childhood tumors, such as retinoblastoma, has provided fundamental insights into the pathogenesis of malignant transformation.
Heredity
The evidence now indicates that for many types of cancer, including the most common forms, there exist not only environmental influences but also hereditary predispositions. Hereditary forms of cancer can be divided into three categories based on their pattern of inheritance (Table 5–3).
Table 5–3 Inherited Predisposition to Cancer
| Autosomal Dominant Cancer Syndromes | |
|---|---|
| Gene(s) | Inherited Predisposition |
| RB | Retinoblastoma |
| TP53 | Li-Fraumeni syndrome (various tumors) |
| p16INK4A | Melanoma |
| APC | Familial adenomatous polyposis/colon cancer |
| NF1, NF2 | Neurofibromatosis 1 and 2 |
| BRCA1, BRCA2 | Breast and ovarian tumors |
| MEN1, RET | Multiple endocrine neoplasia 1 and 2 |
| MSH2, MLH1, MSH6 | Hereditary nonpolyposis colon cancer |
| PATCH | Nevoid basal cell carcinoma syndrome |
| Autosomal Recessive Syndromes of Defective DNA Repair | |
| Familial Cancers of Uncertain Inheritance | |
Autosomal Dominant Cancer Syndromes
Autosomal dominant cancer syndromes include several well-defined cancers in which inheritance of a single mutant gene greatly increases the risk of developing a tumor. The predisposition to these tumors shows an autosomal dominant pattern of inheritance. Childhood retinoblastoma is the most striking example of this category. Approximately 40% of retinoblastomas are familial. As is discussed later, inherited disabling mutations in a tumor suppressor gene are responsible for the development of this tumor in families. Carriers of this gene have a 10,000-fold increased risk of developing retinoblastoma. Unlike those with sporadic retinoblastoma, patients with familial retinoblastoma develop bilateral tumors, and they also have a greatly increased risk of developing a second cancer, particularly osteosarcoma.
Tumors within this group often are associated with a specific marker phenotype. There may be multiple benign tumors in the affected tissue, as occurs in familial polyposis of the colon and in multiple endocrine neoplasia (see Table 5–3). Sometimes, there are abnormalities in tissue that are not the target of transformation (e.g., Lisch nodules and café-au-lait spots in neurofibromatosis type 1) (Chapter 22).
Autosomal Recessive Syndromes of Defective DNA Repair
A group of rare autosomal recessive disorders is collectively characterized by chromosomal or DNA instability and high rates of certain cancers. One of the best-studied is xeroderma pigmentosum, in which DNA repair is defective. This and other familial disorders of DNA instability are described later.
Familial Cancers of Uncertain Inheritance
Virtually all the common types of cancers that occur sporadically have been reported to occur in familial forms where the pattern of inheritance is unclear. Examples are carcinomas of colon, breast, ovary, and brain. Features that characterize familial cancers include early age at onset, tumors arising in two or more close relatives of the index case, and sometimes multiple or bilateral tumors. Familial cancers are not associated with specific marker phenotypes. For example, in contrast with the familial adenomatous polyposis syndrome, familial colonic cancers do not arise in preexisting benign polyps. In general, siblings have a relative risk between 2 and 3. Segregation analysis of large families usually reveals that predisposition to the tumors is dominant, but incomplete penetrance or multifactorial inheritance cannot be easily ruled out.
In summary, no more than 5% to 10% of all human cancers fall into one of the three aforementioned categories. What can be said about the influence of heredity in the large preponderance of malignant tumors? There is emerging evidence that the influence of hereditary factors is subtle and sometimes indirect. The genotype may influence the likelihood of developing environmentally induced cancers. For example, polymorphisms in drug-metabolizing enzymes confer genetic predisposition to lung cancer in people who smoke cigarettes. More strikingly, genome-wide association studies (GWAS) in lung cancer, which sought to identify common genetic variants that increase risk for developing cancer, identified variants in a nicotinic acid receptor as being associated with development of lung cancer. Of interest, these variants were strongly associated with the number of cigarettes smoked, suggesting that they indirectly increase lung cancer risk by enhancing the addictiveness of cigarettes.
Acquired Preneoplastic Lesions
Just as some hereditary conditions increase the risk of getting certain cancers, so do certain acquired conditions. These are loosely referred to as preneoplastic lesions or simply “precancers.” These designations are unfortunate because they imply inevitability, but in fact, although such lesions increase the likelihood of malignancy, most do not progress to cancer. In many instances, precursor lesions arise in the setting of chronic tissue injury or inflammation, which may increase the likelihood of malignancy by stimulating continuing regenerative proliferation or by exposing cells to byproducts of inflammation, both of which can lead to somatic mutations (discussed later). Indeed, molecular analyses have shown that many precursor lesions possess some of the genetic lesions found in their associated cancers. Clinically, these precursor lesions are important to recognize, because their removal or reversal may prevent the development of a cancer. A brief listing of some of the chief precursor lesions follows:
• Squamous metaplasia and dysplasia of the bronchial mucosa, seen in habitual smokers—a risk factor for lung cancer
• Endometrial hyperplasia and dysplasia, seen in women with unopposed estrogenic stimulation—a risk factor for endometrial carcinoma
• Leukoplakia of the oral cavity, vulva, or penis, which may progress to squamous cell carcinoma
• Villous adenomas of the colon, associated with a high risk of transformation to colorectal carcinoma
In this context it may be asked, “What is the risk of malignant change in a benign neoplasm?”—or, stated differently, “Are benign tumors precancerous?” In general the answer is no, but inevitably there are exceptions, and perhaps it is better to say that each type of benign tumor is associated with a particular level of risk, ranging from high to virtually nonexistent. For example, adenomas of the colon as they enlarge can undergo malignant transformation in 50% of cases; by contrast, malignant change is extremely rare in leiomyomas of the uterus.
![]() Summary
Summary
Epidemiology of Cancer
• The incidence of cancer varies with age, race, geographic factors, and genetic backgrounds. Cancers are most common at the two extremes of age. The geographic variation results mostly from different environmental exposures.
• Most cancers are sporadic, but some are familial. Predisposition to hereditary cancers may be autosomal dominant or autosomal recessive. The former usually are linked to inheritance of a germ line mutation of cancer suppressor genes, whereas the latter typically are associated with inherited defects in DNA repair.
• Familial cancers tend to be bilateral and arise earlier in life than their sporadic counterparts.
• Some acquired diseases, known as preneoplastic disorders, are known to be associated with an increased risk for development of cancer.
Carcinogenesis: The Molecular Basis of Cancer
It could be argued that the proliferation of literature on the molecular basis of cancer has outpaced the growth of even the most malignant of tumors. Researchers and students alike can easily get lost in the growing forest of information. Accordingly, a review of some fundamental principles is presented as background for more detailed consideration of the genetic basis of cancer.
• As already discussed, nonlethal genetic damage lies at the heart of carcinogenesis. Such genetic damage (or mutation) may be acquired by the action of environmental agents, such as chemicals, radiation, or viruses, or it may be inherited in the germ line. The genetic hypothesis of cancer implies that a tumor mass results from the clonal expansion of a single progenitor cell that has incurred genetic damage (i.e., tumors are monoclonal). This expectation has been realized in all tumors that have been systematically analyzed by genomic sequencing.
• Four classes of normal regulatory genes—growth-promoting proto-oncogenes, growth-inhibiting tumor suppressor genes, genes that regulate programmed cell death (i.e., apoptosis), and genes involved in DNA repair—are the principal targets of genetic damage. Collectively, the genetic alterations in tumor cells confer growth and survival advantages over normal cells, as will be evident from the discussion that follows.
• Oncogenes are genes that induce a transformed phenotype when expressed in cells. A major discovery in cancer was the realization that most oncogenes are mutated or over expressed versions of normal cellular genes, which are called proto-oncogenes. Most known oncogenes encode transcription factors, growth regulating proteins, or proteins involved in cell survival and cell–cell and cell–matrix interactions. They are considered dominant because mutation of a single allele can lead to cellular transformation.
• Tumor suppressor genes are genes that normally prevent uncontrolled growth and, when mutated or lost from a cell, allow the transformed phenotype to develop. Usually both normal alleles of tumor suppressor genes must be damaged for transformation to occur. However, recent work has clearly shown that, in some cases, loss of a single allele of a tumor suppressor gene can promote transformation (haploinsufficiency).
• Tumor suppressor genes are usefully placed into two general groups, “governors” and “guardians.” “Governors” are classic tumor suppressor genes, such as RB, where mutation of the gene leads to transformation by removing an important brake on cellular proliferation. “Guardian” genes are responsible for sensing genomic damage. Some of these genes initiate and choreograph a complex “damage control response.” This response leads to the cessation of proliferation or, if the damage is too great to be repaired, the induction of apoptosis. TP53, the so-called “guardian of the genome,” is a prototypic tumor suppressor gene of this type. Other guardian genes are directly involved in recognizing and repairing specific kinds of DNA damage; these are the genes that are mutated in the autosomal recessive syndromes of DNA repair. Mutation of TP53 or other sensors of genomic damage does not directly transform cells, as loss of guardian function has no direct effect on cellular proliferation or apoptosis. Instead, loss of the guardian genes permits and accelerates the acquisition of mutations in oncogenes and tumor suppressor genes that can lead to the development of cancer. This increase in mutation rate is often referred to as a mutator phenotype.
• Genes that regulate apoptosis and DNA repair may act like proto-oncogenes (loss of one copy is sufficient) or tumor suppressor genes (loss of both copies).
Several types of alterations can affect cancer-causing genes and lead to cellular transformation, as detailed in a subsequent section. Presented next is a discussion of the varied genetic lesions that underlie mutation of genes in cancer.
Genetic Lesions in Cancer
The genetic changes that characterize cancer-associated mutations may be subtle (e.g., point mutations or insertions and deletions) or large enough to produce karyotypic changes. Point mutations can either activate or inactivate the resulting protein products. For example, point mutations in proto-oncogenes, such as RAS or EGFR, frequently result in overactivity of the protein, usually by altering an internal regulatory amino acid and producing a constitutively active protein. However, point mutations in tumor suppressors, such as those affecting RB or TP53 genes, reduce or disable the function of the encoded protein.
Karyotypic Changes in Tumors
The genetic lesion that activates oncogenes or inactivates tumor suppressor genes may be subtle (as described previously) or large enough to be detected in a karyotype. Some cancers have a virtually normal karyotype, while others are markedly aneuploid, with loss and gain of many entire chromosomes or chromosomal arms. In certain neoplasms, karyotypic abnormalities are nonrandom and common, or even characteristic of a particular tumor. Specific abnormalities have been identified in most leukemias and lymphomas and in an increasing number of nonhematopoietic tumors. The common types of nonrandom structural abnormalities in tumor cells are (1) balanced translocations, (2) deletions, and (3) cytogenetic manifestations of gene amplification.
Balanced Translocations
Balanced translocations are highly associated with certain malignancies, particularly specific kinds of hematopoietic and mesenchymal neoplasms. Translocations can activate proto-oncogenes in two ways:
• Some translocations result in overexpression of proto-oncogenes by removing them from their normal regulatory elements and placing them under control of an inappropriate, highly active promoter. Two different kinds of B cell lymphoma provide cardinal examples of this mechanism. In more than 90% of cases of Burkitt lymphoma the cells have a translocation, usually between chromosomes 8 and 14, which leads to overexpression of the MYC gene on chromosome 8 by juxtaposition with immunoglobulin heavy chain gene regulatory elements on chromosome 14. In follicular B cell lymphomas, a reciprocal translocation between chromosomes 14 and 18 leads to overexpression of the antiapoptotic gene, BCL2, on chromosome 18, also driven by immunoglobulin gene elements.
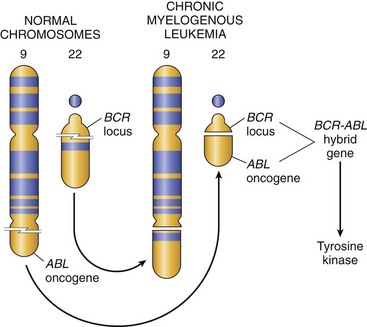
• Other oncogenic translocations create fusion genes encoding novel chimeric proteins. Most notable is the Philadelphia (Ph) chromosome in chronic myelogenous leukemia, consisting of a reciprocal and balanced translocation between chromosomes 22 and 9 (Fig. 5–14). As a consequence, the derivative chromosome 22 (the Philadelphia chromosome) appears abbreviated. This cytogenetic change, seen in more than 90% of cases of chronic myelogenous leukemia, is a reliable marker of this disease, and the few Ph chromosome–negative cases show molecular evidence of the BCR-ABL rearrangement, the crucial consequence of Ph translocation. As discussed later, such changes give rise to the BCR-ABL fusion gene with potent tyrosine kinase activity.
Lymphoid cells are most commonly the targets of gene rearrangements, which may take the form of translocations, inversions, or interstitial deletions, because these cells purposefully make DNA breaks during the processes of antibody or T cell receptor gene recombination. Two other types of mesenchymal tumors, myeloid neoplasms (acute myeloid leukemias and myeloproliferative disorders) and sarcomas, also frequently possess recurrent translocations, such as the t(11;22)(q24;12) translocation in Ewing sarcoma that results in fusion of the EWS transcription factor with Fli-1. The cause of the DNA breaks that lead to chromosomal translocations in myeloid neoplasms and sarcomas is unknown.
Identification of recurrent chromosomal rearrangements in carcinomas has lagged because of the complexity of the karyotypes of these tumors, but novel molecular techniques are beginning to unravel this tangled skein. As with hematologic malignancies and sarcomas, gene rearrangements in solid tumors can contribute to carcinogenesis either by increasing expression of an oncogene or by generation of a novel fusion gene. For example, various TMPRSS-ETS fusion genes found in prostate carcinomas place ETS family transcription factor genes under the control of the TMPRSS promoter, which is activated by androgens. The net effect of these rearrangements is the inappropriate, androgen-dependent expression of ETS family transcription factors. Rearrangements of the HMGA2 gene found in pleomorphic adenomas and other tumors lead to overexpression of the HMGA2 transcription factor through an unusual mechanism; they replace the 3′ untranslated region of HMGA2 with that of another gene, thus removing key negative regulatory microRNA binding sites. Although the mechanisms are not yet clear, overexpression of HGMA2 or ETS likely promotes carcinogenesis by altering expression of a number of genes that are the targets of these transcription factors. Another uncommon but clinically important type of rearrangement creates an EML4-ALK fusion gene, which is present in roughly 4% of lung carcinomas. The EML4-ALK kinase is constitutively active and upregulates signaling through a several pro-growth pathways. As discussed later, lung cancers expressing this fusion protein respond to inhibitors of the ALK kinase.
Deletions
Chromosomal deletions are the second most prevalent karyotypic abnormality in tumor cells. Compared with translocations, deletions large enough to be observed karyotypically are more common in nonhematopoietic solid tumors. At a molecular level, however, deletions are commonly found in hematopoietic tumors as well. Deletion of specific regions of chromosomes may result in the loss of particular tumor suppressor genes. Tumor suppressors generally require inactivation of both alleles in order for them to contribute to carcinogenesis. A common mechanism for this is an inactivating point mutation in one allele, followed by deletion of the other, nonmutated allele. Such deletions result in loss of heterozygosity (LOH), as formerly heterozygous genetic variants will now only have one allele, and all genetic variants within the deleted region will be detected as homozygous. As discussed later, deletions involving 13q14, the site of the RB gene, are associated with retinoblastoma, and deletion of 17p is associated with loss of p53.
Gene Amplifications
Proto-oncogenes may be converted to oncogenes by amplification, with consequent overexpression, of otherwise normal proteins. Such amplification may produce several hundred copies of the proto-oncogene in the tumor cell. The amplified genes can be readily detected by molecular hybridization with appropriate DNA probes. In some cases the amplified genes produce chromosomal changes that can be identified microscopically. Two mutually exclusive patterns are seen: multiple small, extrachromosomal structures called “double minutes” and homogeneously staining regions. The latter derive from the insertion of the amplified genes into new chromosomal locations, which may be distant from the normal location of the involved genes; because regions containing amplified genes lack a normal banding pattern, they appear homogeneous in a G-banded karyotype. The most interesting cases of amplification involve NMYC in neuroblastoma and ERBB2 in breast cancers. NMYC is amplified in 25% to 30% of neuroblastomas, and the amplification is associated with poor prognosis (Fig. 5–15). HER2/NEU (also known as ERBB2) amplification occurs in about 20% of breast cancers, and antibody therapy directed against this receptor has proved effective in this subset of tumors.
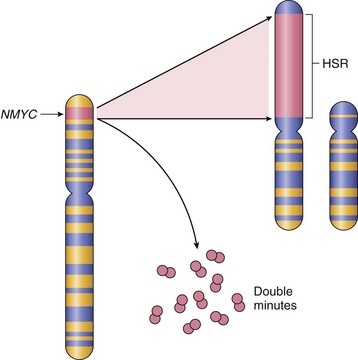
Figure 5–15 Amplification of the NMYC gene in human neuroblastoma. The NMYC gene, present normally on chromosome 2p, becomes amplified and is seen either as extrachromosomal double minutes or as a chromosomally integrated homogeneous-staining region (HSR). The integration involves other autosomes, such as 4, 9, or 13.
(Modified from Brodeur GM, Seeger RC, Sather H, et al: Clinical implications of oncogene activation in human neuroblastomas. Cancer 58:541, 1986. Reprinted by permission of Wiley-Liss, Inc, a subsidiary of John Wiley & Sons, Inc.)
Aneuploidy
Aneuploidy is defined as a number of chromosomes that is not a multiple of the haploid state; for humans that is a chromosome number that is not a multiple of 23. Aneuploidy is remarkably common in cancers, particularly carcinomas, and was proposed as a cause of carcinogenesis over 100 years ago. Aneuploidy frequently results from errors of the mitotic checkpoint, the major cell cycle control mechanism that acts to prevent chromosome mis-segregation. The mitotic checkpoint prevents aneuploidy by inhibiting the irreversible transition to anaphase until all of the replicated chromosomes have made productive attachments to spindle microtubules. Complete absence of the mitotic checkpoint leads to rapid cell-autonomous lethality as a consequence of massive chromosome missegregation. However, mechanistic data establishing aneuploidy as a cause of carcinogenesis, rather than a consequence, have been difficult to generate.
MicroRNAs and Cancer
As discussed in Chapter 6, microRNAs (miRNAs) are noncoding, single-stranded RNAs, approximately 22 nucleotides in length, that function as negative regulators of genes. They inhibit gene expression posttranscriptionally by repressing translation or, in some cases, by messenger RNA (mRNA) cleavage. In view of their important function to control cell growth, differentiation, and survival, it is not surprising that accumulating evidence supports a role for miRNAs in carcinogenesis.
As illustrated in Figure 5–16, miRNAs can participate in neoplastic transformation either by increasing the expression of oncogenes or reducing the expression of tumor suppressor genes. If an miRNA inhibits the translation of an oncogene, a reduction in the quantity or function of that miRNA will lead to overproduction of the oncogene product. Conversely, if the target of a miRNA is a tumor suppressor gene, then overactivity of the miRNA can reduce the tumor suppressor protein. Such relationships have already been established by miRNA profiling of several human tumors. For example, downregulation or deletion of certain miRNAs in some leukemias and lymphomas results in increased expression of BCL2, the antiapoptotic gene. Thus, by negatively regulating BCL2, such miRNAs behave as tumor suppressor genes. Similar miRNA-mediated upregulation of the RAS and MYC oncogenes also has been detected in lung tumors and in certain B cell leukemias, respectively.
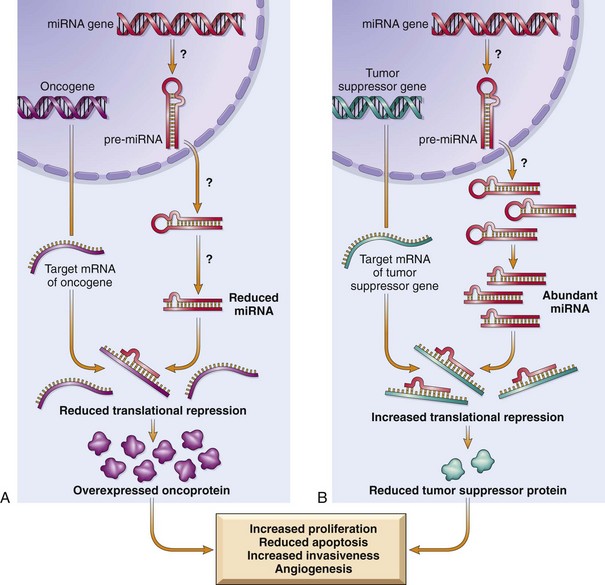
Figure 5–16 Role of microRNAs (miRNAs) in tumorigenesis. A, Reduced activity of an miRNA that inhibits translation of an oncogene gives rise to an excess of oncoproteins. B, Overactivity of an miRNA that targets a tumor suppression gene reduces the production of the tumor suppressor protein. Question marks in A and B are meant to indicate that the mechanisms by which changes in the level or activity of miRNA are not entirely known.
Epigenetic Modifications and Cancer
Epigenetics refers to reversible, heritable changes in gene expression that occur without mutation. Such changes involve posttranslational modifications of histones and DNA methylation, both of which affect gene expression. In normal, differentiated cells, the major portion of the genome is not expressed. These regions of the genome are silenced by DNA methylation and histone modifications. On the other hand, cancer cells are characterized by a global DNA hypomethylation and selective promoter-localized hypermethylation. Indeed, it has become evident during the past several years that tumor suppressor genes are sometimes silenced by hypermethylation of promoter sequences, rather than by mutation. As discussed later, CDKN2A is a complex locus that encodes two tumor suppressors, p14/ARF and p16/INK4a, produced from two different reading frames; p14/ARF is epigenetically silenced in colon and gastric cancers, while p16/INK4a is silenced in a wide variety of cancers. Since this locus produces two tumor suppressors that affect the p53 and Rb pathways, silencing this locus has the pleasing effect (from the cancer’s standpoint) of removing two checkpoints with a single alteration. Genome-wide hypomethylation has been shown to cause chromosomal instability and can induce tumors in mice. Thus, epigenetic changes may influence carcinogenesis in many ways. As an added wrinkle, deep sequencing of cancer genomes has identified mutations in genes that regulate epigenetic modifications in a number of cancers. Thus, certain genetic changes in cancers may be selected for because they lead to alterations of the “epigenome” that favor cancer growth and survival.
The epigenetic state of particular cell types—a feature described as the epigenetic context—also dictates their response to signals that control growth and differentiation. As mentioned earlier, epigenetic modifications regulate gene expression, allowing cells with the same genetic make-up (e.g., a neuron and a keratinocyte) to have completely different appearances and functions. In some instances, the epigenetic state of a cell dramatically affects its response to otherwise identical signals. For example, the gene NOTCH1 has an oncogenic role in T cell leukemia, yet acts as a tumor suppressor in squamous cell carcinomas. As it turns out, activated NOTCH1 turns on pro-growth genes in the epigenetic context of T cell progenitors (e.g., MYC) and tumor suppressor genes (e.g., p21) in the epigenetic context of keratinocytes.
![]() Summary
Summary
Genetic Lesions in Cancer
• Tumor cells may acquire mutations through several means, including point mutations, and nonrandom chromosomal abnormalities that contribute to malignancy; these include balanced translocations, deletions, and cytogenetic manifestations of gene amplification.
• Balanced translocations contribute to carcinogenesis by overexpression of oncogenes or generation of novel fusion proteins with altered signaling capacity. Deletions frequently affect tumor suppressor genes, whereas gene amplification increases the expression of oncogenes.
• Overexpression of miRNAs can contribute to carcinogenesis by reducing the expression of tumor suppressors, while deletion or loss of expression of miRNAs can lead to overexpression of proto-oncogenes.
• Tumor suppressor genes and DNA repair genes also may be silenced by epigenetic changes, which involve reversible, heritable changes in gene expression that occur not by mutation but by methylation of the promoter.
Carcinogenesis: A Multistep Process
Carcinogenesis is a multistep process resulting from the accumulation of multiple genetic alterations that collectively give rise to the transformed phenotype. Many cancers arise from non-neoplastic precursor lesions, which molecular analyses have shown already possess some of the mutations needed to establish a full-blown cancer. Presumably these mutations provide the cells of the precursor lesion with a selective advantage. Once initiated, cancers continue to undergo darwinian selection.
As discussed earlier, malignant neoplasms have several phenotypic attributes, such as excessive growth, local invasiveness, and the ability to form distant metastases. Furthermore, it is well established that over a period of time, many tumors become more aggressive and acquire greater malignant potential. This phenomenon is referred to as tumor progression and is not represented simply by an increase in tumor size. Careful clinical and experimental studies reveal that increasing malignancy often is acquired in an incremental fashion. At the molecular level, tumor progression and associated heterogeneity are most likely to result from multiple mutations that accumulate independently in different cells, generating subclones with different characteristics (Fig. 5–17) such as ability to invade, rate of growth, metastatic ability, karyotype, hormonal responsiveness, and susceptibility to antineoplastic drugs. Some of the mutations may be lethal; others may spur cell growth by affecting proto-oncogenes or cancer suppressor genes. Thus even though most malignant tumors are monoclonal in origin, by the time they become clinically evident their constituent cells may be extremely heterogeneous.
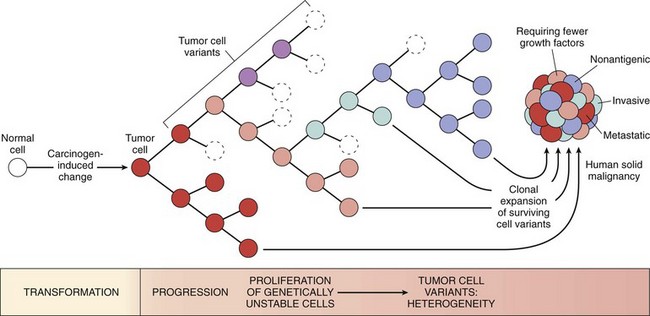
Figure 5–17 Tumor progression and generation of heterogeneity. New subclones arise from the descendants of the original transformed cell by multiple mutations. With progression, the tumor mass becomes enriched for variants that are more adept at evading host defenses and are likely to be more aggressive.
During progression, tumor cells are subjected to immune and nonimmune selection pressures. For example, cells that are highly antigenic are destroyed by host defenses, whereas those with reduced growth factor requirements are positively selected. A growing tumor, therefore, tends to be enriched for subclones that “beat the odds” and are adept at survival, growth, invasion, and metastasis. Finally, experience has shown that when tumors recur after chemotherapy, the recurrent tumor is almost always resistant to the drug regimen if it is given again. This acquired resistance, too, is a manifestation of selection, as subclones that by chance bear mutations (or perhaps epigenetic alterations) imparting drug resistance survive and are responsible for tumor regrowth. Thus, genetic evolution and selection can explain two of the most pernicious properties of cancers: the tendency for cancers to become (1) more aggressive and (2) less responsive to therapy over time.
Hallmarks of Cancer
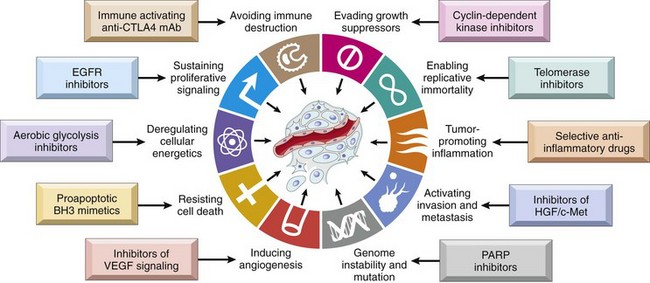
This overview serves as background for a more detailed consideration of the molecular pathogenesis of cancer and the carcinogenic agents that inflict genetic damage. In the past 30-some years, hundreds of cancer-associated genes have been discovered. Some, such as TP53, are commonly mutated; others, such as ABL, are affected only in certain leukemias. Each cancer gene has a specific function, the dysregulation of which contributes to the origin or progression of malignancy. It is best, therefore, to consider cancer-related genes in the context of several fundamental changes in cell physiology, the so-called hallmarks of cancer, which together dictate the malignant phenotype. Six of these are illustrated in Figure 5–18:
• Self-sufficiency in growth signals
• Insensitivity to growth inhibitory signals
• Limitless replicative potential
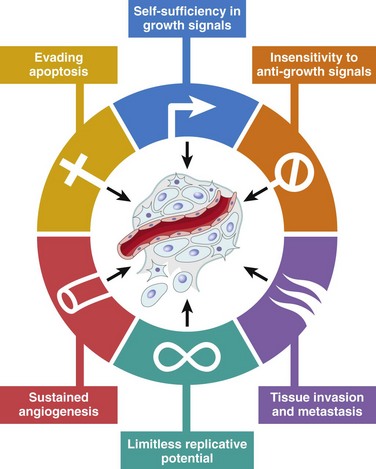
Figure 5–18 Six hallmarks of cancer. Most cancer cells acquire these properties during their development, typically by mutations in the relevant genes.
(From Hanahan D, Weinberg RA: The hallmarks of cancer. Cell 100:57, 2000.)
To this list may be added two “emerging” hallmarks of cancer, reprogramming of energy metabolism and evasion of the immune system, and two enabling characteristics, genomic instability and tumor-promoting inflammation.
Mutations in genes that regulate some or all of these cellular traits are seen in every cancer; accordingly, these traits form the basis of the following discussion of the molecular origins of cancer. Of note, by convention, gene symbols are italicized but their protein products are not (e.g., RB gene and Rb protein, TP53 and p53, MYC and MYC).
Self-Sufficiency in Growth Signals
Cancer cells use a number of strategies to drive their proliferation and become insensitive to normal growth regulators. To appreciate these phenomena, it is helpful to review briefly the sequence of events that characterize normal cell proliferation (introduced in Chapter 2). Under physiologic conditions, cell proliferation can be readily resolved into the following steps:
1 The binding of a growth factor to its specific receptor on the cell membrane
2 Transient and limited activation of the growth factor receptor, which in turn activates several signal-transducing proteins on the inner leaflet of the plasma membrane
3 Transmission of the transduced signal across the cytosol to the nucleus by second messengers or a cascade of signal transduction molecules
4 Induction and activation of nuclear regulatory factors that initiate and regulate DNA transcription
5 Entry and progression of the cell into the cell cycle, resulting ultimately in cell division
The mechanisms that endow cancer cells with the ability to proliferate can be grouped according to their role in the growth factor–induced signal transduction cascade and cell cycle regulation. Indeed, each one of the listed steps is susceptible to corruption in cancer cells.
Growth Factors
All normal cells require stimulation by growth factors to undergo proliferation. Most soluble growth factors are made by one cell type and act on a neighboring cell to stimulate proliferation (paracrine action). Normally, cells that produce the growth factor do not express the cognate receptor. This specificity prevents the formation of positive feedback loops within the same cell.
• Many cancer cells acquire growth self-sufficiency by acquiring the ability to synthesize the same growth factors to which they are responsive. For example, many glioblastomas secrete platelet-derived growth factor (PDGF) and express the PDGF receptor, and many sarcomas make both transforming growth factor-α (TGF-α) and its receptor. Similar autocrine loops are fairly common in many types of cancer.
• Another mechanism by which cancer cells acquire growth self-sufficiency is by interaction with stroma. In some cases, tumor cells send signals to activate normal cells in the supporting stroma, which in turn produce growth factors that promote tumor growth.
Growth Factor Receptors and Non-Receptor Tyrosine Kinases
The next group in the sequence of signal transduction is growth factor receptors, and several oncogenes that result from the overexpression or mutation of growth factor receptors have been identified. Mutant receptor proteins deliver continuous mitogenic signals to cells, even in the absence of the growth factor in the environment. More common than mutations is overexpression of growth factor receptors, which can render cancer cells hyperresponsive to levels of the growth factor that would not normally trigger proliferation. The best-documented examples of overexpression involve the epidermal growth factor (EGF) receptor family. ERBB1, the EGF receptor, is overexpressed in 80% of squamous cell carcinomas of the lung, 50% or more of glioblastomas, and 80% to 100% of epithelial tumors of the head and neck. The gene encoding a related receptor, HER2/NEU (ERBB2), is amplified in 25% to 30% of breast cancers and adenocarcinomas of the lung, ovary, and salivary glands. These tumors are exquisitely sensitive to the mitogenic effects of small amounts of growth factors, and a high level of HER2/NEU protein in breast cancer cells is a harbinger of poor prognosis. The significance of HER2/NEU in the pathogenesis of breast cancers is illustrated dramatically by the clinical benefit derived from blocking the extracellular domain of this receptor with anti-HER2/NEU antibodies. Treatment of breast cancer with anti-HER2/NEU antibody is an elegant example of “bench to bedside” medicine.
Downstream Signal-Transducing Proteins
A relatively common mechanism by which cancer cells acquire growth autonomy is mutations in genes that encode various components of the signaling pathways downstream of growth factor receptors. These signaling proteins couple growth factor receptors to their nuclear targets. They receive signals from activated growth factor receptors and transmit them to the nucleus, either through second messengers or through a cascade of phosphorylation and activation of signal transduction molecules. Two important members in this category are RAS and ABL. Each of these is discussed briefly next.
RAS Protein
RAS is the most commonly mutated proto-oncogene in human tumors. Indeed, approximately 30% of all human tumors contain mutated versions of the RAS gene, and the frequency is even higher in some specific cancers (e.g., colon and pancreatic adenocarcinomas).
• RAS is a member of a family of small G proteins that bind guanosine nucleotides (guanosine triphosphate [GTP] and guanosine diphosphate [GDP]), similar to the larger trimolecular G proteins.
• Normal RAS proteins flip back and forth between an excited signal-transmitting state and a quiescent state. RAS proteins are inactive when bound to GDP; stimulation of cells by growth factors such as EGF and PDGF leads to exchange of GDP for GTP and subsequent conformational changes that generate active RAS (Fig. 5–19). This excited signal-emitting state is short-lived, however, because the intrinsic guanosine triphosphatase (GTPase) activity of RAS hydrolyzes GTP to GDP, releasing a phosphate group and returning the protein to its quiescent GDP-bound state. The GTPase activity of activated RAS protein is magnified dramatically by a family of GTPase-activating proteins (GAPs), which act as molecular brakes that prevent uncontrolled RAS activation by favoring hydrolysis of GTP to GDP.
• The activated RAS stimulates downstream regulators of proliferation by two distinct pathways that converge on the nucleus and flood it with signals for cell proliferation. While details of the signaling cascades (some of which are illustrated in Fig. 5–19) downstream of RAS are not discussed here, an important point is that mutational activation of these “messengers” to the nucleus can mimic the growth promoting effects of activated RAS. For example, BRAF, which lies in the so-called RAF/ERK/MAP kinase pathway, is mutated in more than 60% of melanomas. Mutations of PI3 kinase in the PI3K/AKT pathway also occur with high frequency in some tumor types. Indeed, it appears that activating mutations of RAS as well as its downstream signaling molecules are very common in a wide variety of tumors.
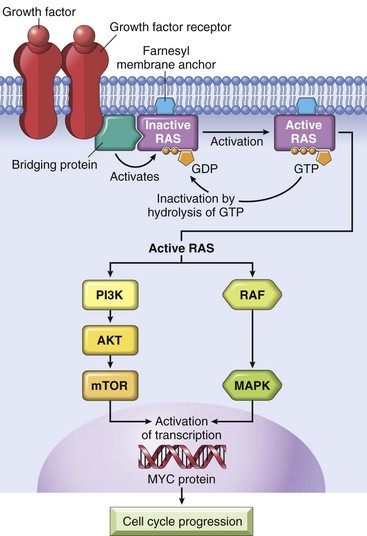
Figure 5–19 Model for action of RAS genes. When a normal cell is stimulated through a growth factor receptor, inactive (GDP-bound) RAS is activated to a GTP-bound state. Activated RAS transduces proliferative signals to the nucleus along two pathways: the so-called RAF/ERK/MAP kinase pathway and the PI3 kinase/AKT pathway. GDP, guanosine diphosphate; GTP, guanosine triphosphate; MAP, mitogen-activated protein; PI3, phosphatidylinositol-3.
The RAS protein most commonly is activated by point mutations in amino acid residues that are either within the GTP-binding pocket or in the enzymatic region essential for GTP hydrolysis. Both kinds of mutations interfere with GTP hydrolysis, which is essential to inactivate RAS. RAS is thus trapped in its activated, GTP-bound form, and the cell is forced into a continuously proliferating state. It follows from this scenario that the consequences of mutations in RAS protein would be mimicked by loss-of-function mutations in the GAPs with a failure to simulate GTP hydrolysis and thereby restrain normal RAS proteins. Indeed, disabling mutation of neurofibromin-1 (NF-1), a GAP, is associated with familial neurofibromatosis type 1 (Chapter 22).
ABL
In addition to RAS, several non–receptor-associated tyrosine kinases function as signal transduction molecules. In this group, ABL is the most well defined with respect to carcinogenesis.
• The ABL proto-oncogene has tyrosine kinase activity that is dampened by internal negative regulatory domains. In chronic myelogenous leukemia and certain acute leukemias, a part of the ABL gene is translocated from its normal abode on chromosome 9 to chromosome 22, where it fuses with part of the breakpoint cluster region (BCR) gene. The BCR-ABL hybrid protein maintains the tyrosine kinase domain; the BCR domain self-associates, a property that unleashes a constitutive tyrosine kinase activity. Of interest, there is cross-talk between BCR-ABL and RAS pathways, since BCR-ABL protein activates all of the signals that are downstream of RAS.
• The crucial role of BCR-ABL in transformation has been confirmed by the dramatic clinical response of patients with chronic myelogenous leukemia to BCR-ABL kinase inhibitors. The prototype of this kind of drug, imatinib mesylate (Gleevec), galvanized interest in design of drugs that target specific molecular lesions found in various cancers (so-called targeted therapy). BCR-ABL also is an example of the concept of oncogene addiction, wherein a tumor is profoundly dependent on a single signaling molecule. BCR-ABL fusion gene formation is an early, perhaps initiating, event that drives leukemogenesis. Development of leukemia probably requires other collaborating mutations, but the transformed cell continues to depend on BCR-ABL for signals that mediate growth and survival. BCR-ABL signaling can be seen as the central lodgepole around which the structure is built. If the lodgepole is removed by inhibition of the BCR-ABL kinase, the structure collapses. In view of this level of dependency, it is not surprising that acquired resistance of tumors to BCR-ABL inhibitors often is due to the outgrowth of a subclone with a mutation in BCR-ABL that prevents binding of the drug to the BCR-ABL protein.
Nuclear Transcription Factors
Ultimately, all signal transduction pathways enter the nucleus and have an impact on a large bank of responder genes that orchestrate the cell’s orderly advance through the mitotic cycle. Indeed, the ultimate consequence of signaling through oncoproteins such as RAS or ABL is inappropriate and continuous stimulation of nuclear transcription factors that drive the expression of growth-promoting genes. Growth autonomy may thus be a consequence of mutations affecting genes that regulate transcription of DNA. A host of oncoproteins, including products of the MYC, MYB, JUN, FOS, and REL oncogenes, function as transcription factors that regulate the expression of growth-promoting genes, such as cyclins. Of these, the MYC gene is involved most commonly in human tumors.
The MYC protein can either activate or repress the transcription of other genes. Those activated by MYC include several growth-promoting genes, including cyclin-dependent kinases (CDKs), whose products drive cells into the cell cycle (discussed next). Genes repressed by MYC include the CDK inhibitors (CDKIs). Thus, dysregulation of MYC promotes tumorigenesis by increasing expression of genes that promote progression through the cell cycle and repressing genes that slow or prevent progression through the cell cycle. MYC also is a key regulator of intermediate metabolism, upregulating genes that promote aerobic glycolysis (the so-called Warburg effect, described later) and the increased utilization of glutamine, two metabolic changes that are hallmarks of cancer cells. Dysregulation of the MYC gene resulting from a t(8;14) translocation occurs in Burkitt lymphoma, a B cell tumor. MYC also is amplified in breast, colon, lung, and many other cancers; the related NMYC and LMYC genes are amplified in neuroblastomas and small cell cancers of lung.
Cyclins and Cyclin-Dependent Kinases
The ultimate outcome of all growth-promoting stimuli is the entry of quiescent cells into the cell cycle. Cancers may become autonomous if the genes that drive the cell cycle become dysregulated by mutations or amplification. Before further consideration of this aspect of carcinogenesis, a brief review of the normal cell cycle is warranted (Fig. 5–20).
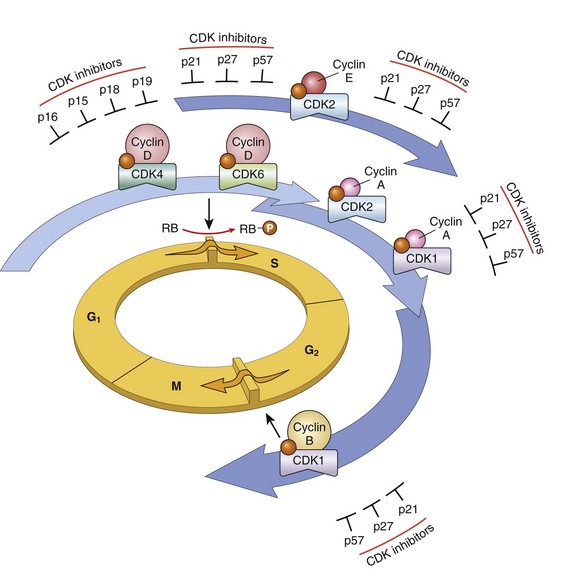
Figure 5–20 Role of cyclins, cyclin-dependent kinases (CDKs), and CDK inhibitors in regulating the cell cycle. The shaded arrows represent the phases of the cell cycle during which specific cyclin–CDK complexes are active. As illustrated, cyclin D–CDK4, cyclin D–CDK6, and cyclin E–CDK2 regulate the G1-to-S transition by phosphorylating the Rb protein (pRb). Cyclin A–CDK2 and cyclin A–CDK1 are active in the S phase. Cyclin B–CDK1 is essential for the G2-to-M transition. Two families of CDK inhibitors can block activity of CDKs and progression through the cell cycle. The so-called INK4 inhibitors, composed of p16, p15, p18, and p19, act on cyclin D–CDK4 and cyclin D–CDK6. The other family of three inhibitors, p21, p27, and p57, can inhibit all CDKs.
The Normal Cell Cycle
Cell proliferation is a tightly controlled process that involves a large number of molecules and interrelated pathways. The replication of cells is stimulated by growth factors or by signaling from ECM components through integrins. To achieve DNA replication and division, the cell goes through a tightly controlled sequence of events known as the cell cycle. The cell cycle consists of G1 (presynthetic), S (DNA synthesis), G2 (premitotic), and M (mitotic) phases. Quiescent cells that have not entered the cell cycle are in the G0 state. Each cell cycle phase is dependent on the proper activation and completion of the previous ones and the cycle stops at a place at which an essential gene function is deficient. Because of its central role in maintaining tissue homeostasis and regulating physiologic growth processes such as regeneration and repair, the cell cycle has multiple checkpoints, particularly during emergence from G0 into G1 and the transition from G1 to S phase.
Cells can enter G1 either from G0 (quiescent cells) or after completing mitosis (continuously replicating cells). Quiescent cells must first go through the transition from G0 to G1, the first decision step, which functions as a gateway to the cell cycle. Cells in G1 progress through the cell cycle and reach a critical stage at the G1-S transition, known as a restriction point, a rate-limiting step for replication. On passing this restriction point, normal cells become irreversibly committed to DNA replication. The cell cycle is tightly controlled by activators and inhibitors.
• Progression through the cell cycle, particularly at the G1-S transition, is regulated by proteins called cyclins, so called because of the cyclic nature of their production and degradation, and associated enzymes, the cyclin-dependent kinases (CDKs). CDKs acquire catalytic activity by binding to and forming complexes with the cyclins. The orderly progression of cells through the various phases of the cell cycle is orchestrated by CDKs, which are activated by binding to the cyclins.
• The CDK–cyclin complexes phosphorylate crucial target proteins that drive the cell through the cell cycle. On completion of this task, cyclin levels decline rapidly. More than 15 cyclins have been identified; cyclins D, E, A, and B appear sequentially during the cell cycle and bind to one or more CDKs. The cell cycle may thus be seen as a relay race in which each leg is regulated by a distinct set of cyclins: As one set of cyclins leaves the track, the next set takes over (Fig. 5–20). Activated CDKs in these complexes drive the cell cycle by phosphorylating proteins that regulate cell cycle transitions. One such protein is the retinoblastoma protein (Rb), discussed later.
• The activity of CDK–cyclin complexes is regulated by CDK inhibitors (CDKIs), which enforce cell cycle checkpoints. Embedded in the cell cycle are surveillance mechanisms that are geared to sensing damage to DNA and chromosomes. These quality control checks are called checkpoints; they ensure that cells with damaged DNA or chromosomes do not complete replication. The G1-S checkpoint monitors the integrity of DNA before DNA replication, whereas the G2-M checkpoint checks DNA after replication and monitors whether the cell can safely enter mitosis. When cells sense DNA damage, checkpoint activation delays the cell cycle and triggers DNA repair mechanisms. If DNA damage is too severe to be repaired, the cells are eliminated by apoptosis, or enter a nonreplicative state called senescence, primarily through p53-dependent mechanisms, discussed later on. Mutations in genes regulating these checkpoints allow cells with damaged DNA to divide, producing daughter cells carrying mutations.
• There are several families of CDKIs. One family, composed of three proteins called p21 (CDKN1A), p27 (CDKN1B), and p57 (CDKN1C), inhibits the CDKs broadly, whereas the other family of CDKIs has selective effects on cyclin CDK4 and cyclin CDK6. The four members of this family—p15 (CDKN2B), p16 (CDKN2A), p18 (CDKN2C), and p19 (CDKN2D)—are sometimes called INK4 (A to D) proteins.
Alterations in Cell Cycle Control Proteins in Cancer Cells
With this background it is easy to appreciate that mutations that dysregulate the activity of cyclins and CDKs would favor cell proliferation. Indeed, all cancers appear to have genetic lesions that disable the G1-S checkpoint, causing cells to continually reenter the S phase. For unclear reasons, particular lesions vary widely in frequency across tumor types.
• Mishaps increasing the expression of cyclin D or CDK4 seem to be a common event in neoplastic transformation. The cyclin D genes are overexpressed in many cancers, including those affecting the breast, esophagus, liver, and a subset of lymphomas and plasma cell tumors. Amplification of the CDK4 gene occurs in melanomas, sarcomas, and glioblastomas. Mutations affecting cyclins B and E and other CDKs also occur, but they are much less frequent than those affecting cyclin CDK4.
• The CDKIs frequently are disabled by mutation or gene silencing in many human malignancies. Germline mutations of CDKN2A are present in 25% of melanoma-prone kindreds. Somatically acquired deletion or inactivation of CDKN2A is seen in 75% of pancreatic carcinomas, 40% to 70% of glioblastomas, 50% of esophageal cancers, and 20% of non–small cell lung carcinomas, soft tissue sarcomas, and bladder cancers.
A final consideration of importance in a discussion of growth-promoting signals is that the increased production of oncoproteins does not by itself lead to sustained proliferation of cancer cells. There are two built-in mechanisms, cell senescence and apoptosis, that oppose oncogene-mediated cell growth. As discussed later, genes that regulate these two braking mechanisms must be disabled to allow unopposed action of oncogenes.
![]() Summary
Summary
Oncogenes That Promote Unregulated Proliferation (Self-Sufficiency in Growth Signals)
Proto-oncogenes: normal cellular genes whose products promote cell proliferation
Oncogenes: mutant or overexpressed versions of proto-oncogenes that function autonomously without a requirement for normal growth-promoting signals
Oncoproteins promote uncontrolled cell proliferation by several mechanisms:
• Stimulus-independent expression of growth factor and its receptor, setting up an autocrine loop of cell proliferation
• Mutations in genes encoding growth factor receptors or tyrosine kinases leading to constitutive signaling
 Fusion of ABL tyrosine kinase with BCR protein in certain leukemias generates a hybrid protein with constitutive kinase activity.
Fusion of ABL tyrosine kinase with BCR protein in certain leukemias generates a hybrid protein with constitutive kinase activity.• Mutations in genes encoding signaling molecules
 RAS commonly is mutated in human cancers and normally flips between resting GDP-bound state and active GTP-bound state; mutations block hydrolysis of GTP to GDP, leading to unchecked signaling.
RAS commonly is mutated in human cancers and normally flips between resting GDP-bound state and active GTP-bound state; mutations block hydrolysis of GTP to GDP, leading to unchecked signaling.• Overproduction or unregulated activity of transcription factors
 Translocation of MYC in some lymphomas leads to overexpression and unregulated expression of its target genes controlling cell cycling and survival.
Translocation of MYC in some lymphomas leads to overexpression and unregulated expression of its target genes controlling cell cycling and survival.• Mutations that activate cyclin genes or inactivate negative regulators of cyclins and cyclin-dependent kinases
 Complexes of cyclins with CDKs drive the cell cycle by phosphorylating various substrates. CDKs are controlled by inhibitors; mutations in genes encoding cyclins, CDKs, and CDK inhibitors result in uncontrolled cell cycle progression. Such mutations are found in a wide variety of cancers including melanomas, brain, lung, and pancreatic cancer.
Complexes of cyclins with CDKs drive the cell cycle by phosphorylating various substrates. CDKs are controlled by inhibitors; mutations in genes encoding cyclins, CDKs, and CDK inhibitors result in uncontrolled cell cycle progression. Such mutations are found in a wide variety of cancers including melanomas, brain, lung, and pancreatic cancer.Insensitivity to Growth Inhibitory Signals
Isaac Newton theorized that every action has an equal and opposite reaction. Although Newton was not a cancer biologist, his formulation holds true for cell growth. Whereas oncogenes encode proteins that promote cell growth, the products of tumor suppressor genes apply brakes to cell proliferation. Disruption of such genes renders cells refractory to growth inhibition and mimics the growth-promoting effects of oncogenes. The following discussion describes tumor suppressor genes, their products, and possible mechanisms by which loss of their function contributes to unregulated cell growth.
RB Gene: Governor of the Cell Cycle
It is useful to begin with the retinoblastoma gene (RB), the first tumor suppressor gene to be discovered and, as it happens, a prototypical representative. As with many advances in medicine, the discovery of tumor suppressor genes was accomplished by the study of a rare disease—in this case, retinoblastoma, an uncommon childhood tumor. Approximately 60% of retinoblastomas are sporadic, and the remaining ones are familial, the predisposition to develop the tumor being transmitted as an autosomal dominant trait. To account for the sporadic and familial occurrence of an identical tumor, Knudson, in 1974, proposed his now famous two-hit hypothesis, which in molecular terms can be stated as follows:
• Two mutations (hits) are required to produce retinoblastoma. These involve the RB gene, which has been mapped to chromosomal locus 13q14. Both of the normal alleles of the RB locus must be inactivated (hence the two hits) for the development of retinoblastoma (Fig. 5–21).
• In familial cases, children inherit one defective copy of the RB gene in the germ line; the other copy is normal. Retinoblastoma develops when the normal RB gene is lost in retinoblasts as a result of somatic mutation. Because in retinoblastoma families only a single somatic mutation is required for expression of the disease, the familial transmission follows an autosomal dominant inheritance pattern.
• In sporadic cases, both normal RB alleles are lost by somatic mutation in one of the retinoblasts. The end result is the same: a retinal cell that has lost both of the normal copies of the RB gene becomes cancerous.
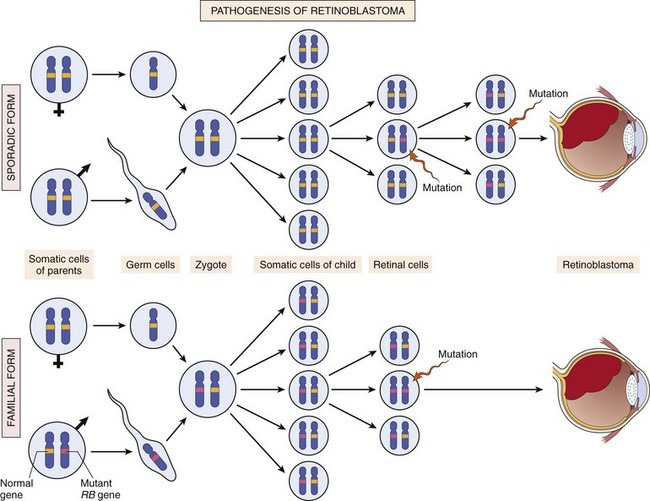
Figure 5–21 Pathogenesis of retinoblastoma. Two mutations of the RB chromosomal locus, on 13q14, lead to neoplastic proliferation of the retinal cells. In the familial form, all somatic cells inherit one mutant RB gene from a carrier parent. The second mutation affects the RB locus in one of the retinal cells after birth. In the sporadic form, both mutations at the RB locus are acquired by the retinal cells after birth.
Although the loss of normal RB genes initially was discovered in retinoblastomas, it is now evident that homozygous loss of this gene is a fairly common feature of several tumors, including breast cancer, small cell cancer of the lung, and bladder cancer. Patients with familial retinoblastoma also are at greatly increased risk for development of osteosarcomas and some soft tissue sarcomas.
At this point, some clarification of terminology is in order: A cell heterozygous at the RB locus is not neoplastic. Tumors develop when the cell loses its normal RB gene copy and thus becomes homozygous for the mutant allele.
In principle, antigrowth signals can prevent cell proliferation by several complementary mechanisms. The signal may cause dividing cells to enter G0 (quiescence), where they remain until external cues prod their reentry into the proliferative pool. Alternatively, the cells may enter a postmitotic, differentiated pool and lose replicative potential. Nonreplicative senescence, alluded to earlier, is another escape mechanism from sustained cell growth. And, as a last-ditch effort, the cells may be programmed for death by apoptosis. As we shall see, tumor suppressor genes have all these “tricks” in their toolbox designed to halt wayward cells from becoming malignant.
The subsequent discussion of growth inhibitory mechanisms and their evasion focuses initially on the prototypical tumor suppressor gene, the RB gene.
![]() Summary
Summary
Insensitivity to Growth Inhibitory Signals
• Tumor suppressor genes encode proteins that inhibit cellular proliferation by regulating the cell cycle. Unlike oncogenes, both copies of the gene must be dysfunctional for tumor development to occur.
• In cases with familial predisposition for development of tumors, affected persons inherit one defective (nonfunctional) copy of a tumor suppressor gene and lose the second one through somatic mutation. In sporadic cases, both copies are lost through somatic mutations.
The RB gene product is a DNA-binding protein that is expressed in every cell type examined, where it exists in an active hypophosphorylated state and an inactive hyperphosphorylated state. The importance of Rb lies in its regulation of the G1/S checkpoint, the portal through which cells must pass before DNA replication commences.
As background for an understanding of how tumor suppressors function, it is useful to briefly revisit the cell cycle: In embryos, cell divisions proceed at an amazing clip, with DNA replication beginning immediately after mitosis ends. As development proceeds, however, two gaps are incorporated into the cell cycle: gap 1 (G1) between mitosis (M) and DNA replication (S), and gap 2 (G2) between DNA replication (S) and mitosis (M) (Fig. 5–20). Although each phase of the cell cycle circuitry is monitored carefully, the transition from G1 to S is believed to be an extremely important checkpoint in the cell cycle “clock.” Once cells cross the G1 checkpoint they can pause the cell cycle for a time, but they are obligated to complete mitosis. In G1, however, cells can remove themselves entirely from the cell cycle, either temporarily (quiescence, or G0) or permanently (senescence). Indeed, during development, as cells become terminally differentiated, they exit the cell cycle and enter G0. Cells in G0 remain there until external cues, such as mitogenic signaling, push them back into the cell cycle. In G1, therefore, diverse signals are integrated to determine whether the cell should progress through the cell cycle, or exit the cell cycle and differentiate, and Rb is a key hub integrating external mitogenic and differentiation signals to make this decision.
To appreciate this crucial role of Rb in the cell cycle, it is helpful to review the mechanisms that enforce the G1/S transition.
• The initiation of DNA replication (S phase) requires the activity of cyclin E/CDK2 complexes, and expression of cyclin E is dependent on the E2F family of transcription factors. Early in G1, Rb is in its hypophosphorylated active form, and it binds to and inhibits the E2F family of transcription factors, preventing transcription of cyclin E. Hypophosphorylated Rb blocks E2F-mediated transcription in at least two ways (Fig. 5–22). First, it sequesters E2F, preventing it from interacting with other transcriptional activators. Second, Rb recruits chromatin remodeling proteins, such as histone deacetylases and histone methyltransferases, which bind to the promoters of E2F-responsive genes such as cyclin E. These enzymes modify chromatin at the promoters to make DNA insensitive to transcription factors.
• This situation is changed on mitogenic signaling. Growth factor signaling leads to cyclin D expression and activation of cyclin D–CDK4/6 complexes. These complexes phosphorylate Rb, inactivating the protein and releasing E2F to induce target genes such as cyclin E. Expression of cyclin E then stimulates DNA replication and progression through the cell cycle. When the cells enter S phase, they are committed to divide without additional growth factor stimulation. During the ensuing M phase, the phosphate groups are removed from Rb by cellular phosphatases, regenerating the hypophosphorylated form of Rb.
• E2F is not the sole target of Rb. The versatile Rb protein binds to a variety of other transcription factors that regulate cell differentiation. For example, Rb stimulates myocyte-, adipocyte-, melanocyte-, and macrophage-specific transcription factors. Thus, the Rb pathway couples control of cell cycle progression at G0-G1 with differentiation, which may explain how differentiation is associated with exit from the cell cycle.
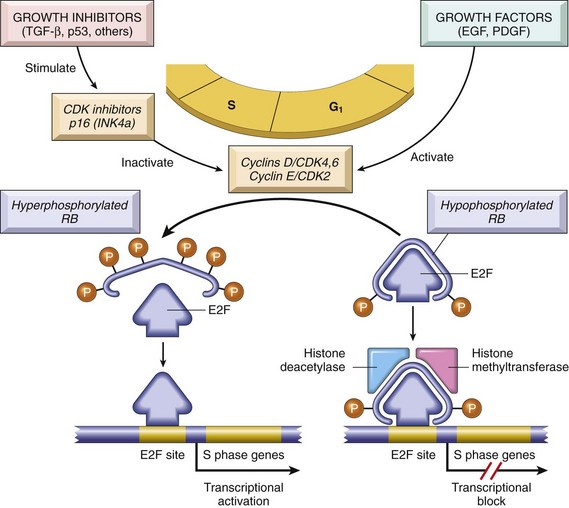
Figure 5–22 The role of Rb in regulating the G1–S checkpoint of the cell cycle.
Hypophosphorylated Rb in complex with the E2F transcription factors binds to DNA, recruits chromatin remodeling factors (histone deacetylases and histone methyltransferases), and inhibits transcription of genes whose products are required for the S phase of the cell cycle. When Rb is phosphorylated by the cyclin D–CDK4, cyclin D–CDK6, and cyclin E–CDK2 complexes, it releases E2F. The latter then activates transcription of S-phase genes. The phosphorylation of Rb is inhibited by CDKIs, because they inactivate cyclin-CDK complexes. Virtually all cancer cells show dysregulation of the G1–S checkpoint as a result of mutation in one of four genes that regulate the phosphorylation of Rb; these genes are RB, CDK4, cyclin D, and CDKN2A [p16]. EGF, epidermal growth factor; PDGF, platelet-derived growth factor.
In view of the centrality of Rb to the control of the cell cycle, an interesting question is why RB is not mutated in every cancer. In fact, mutations in other genes that control Rb phosphorylation can mimic the effect of RB loss; such genes are mutated in many cancers that seem to have normal RB genes. For example, mutational activation of CDK4 or overexpression of cyclin D favors cell proliferation by facilitating Rb phosphorylation and inactivation. Indeed, cyclin D is overexpressed in many tumors because of gene amplification or translocation. Mutational inactivation of CDKIs also would drive the cell cycle by unregulated activation of cyclins and CDKs. As mentioned earlier, the CDKN2A gene is an extremely common target of deletion or mutational inactivation in human tumors.
The emerging paradigm is that loss of normal cell cycle control is central to malignant transformation and that at least one of the four key regulators of the cell cycle (CDKN2A, cyclin D, CDK4, Rb) is mutated in most human cancers. Furthermore, the transforming proteins of several oncogenic human DNA viruses act, in part, by neutralizing the growth inhibitory activities of Rb. For example, the human papillomavirus (HPV) E7 protein binds to the hypophosphorylated form of Rb, preventing it from inhibiting the E2F transcription factors. Thus, Rb is functionally deleted, leading to uncontrolled growth.
![]() Summary
Summary
RB Gene: Governor of the Cell Cycle
• Rb exerts antiproliferative effects by controlling the G1-to-S transition of the cell cycle. In its active form, Rb is hypophosphorylated and binds to E2F transcription factor. This interaction prevents transcription of genes like cyclin E that are needed for DNA replication, and so the cells are arrested in G1.
• Growth factor signaling leads to cyclin D expression, activation of the cyclin D–CDK4/6 complexes, inactivation of Rb by phosphorylation, and thus release of E2F.
• Loss of cell cycle control is fundamental to malignant transformation. Almost all cancers have a disabled G1 checkpoint due to mutation of either RB or genes that affect Rb function, such as cyclin D, CDK4, and CDKIs.
• Many oncogenic DNA viruses, like HPV, encode proteins (e.g., E7) that bind to Rb and render it nonfunctional.
TP53 Gene: Guardian of the Genome
The p53-encoding tumor suppressor gene, TP53, is one of the most commonly mutated genes in human cancers. The p53 protein thwarts neoplastic transformation by three interlocking mechanisms: activation of temporary cell cycle arrest (termed quiescence), induction of permanent cell cycle arrest (termed senescence), or triggering of programmed cell death (termed apoptosis). If Rb “senses” external signals, p53 can be viewed as a central monitor of internal stress, directing the stressed cells toward one of these three pathways.
A variety of stresses trigger the p53 response pathways, including anoxia, inappropriate oncoprotein activity (e.g., MYC or RAS), and damage to the integrity of DNA. By managing the DNA damage response, p53 plays a central role in maintaining the integrity of the genome, as described next.
In nonstressed, healthy cells, p53 has a short half-life (20 minutes) because of its association with MDM2, a protein that targets p53 for destruction. When the cell is stressed, for example, by an assault on its DNA, “sensors” that include protein kinases such as ATM (ataxia telangiectasia mutated) are activated. These activated complexes catalyze post-translational modifications in p53 that release it from MDM2 and increase its half-life and enhance its ability to drive the transcription of target genes. Hundreds of genes whose transcription is triggered by p53 have been found. These genes suppress neoplastic transformation by three mechanisms:
• p53-mediated cell cycle arrest may be considered the primordial response to DNA damage (Fig. 5–23). It occurs late in the G1 phase and is caused mainly by p53-dependent transcription of the CDKI gene CDKN1A (p21). The p21 protein, as described earlier, inhibits cyclin–CDK complexes and prevents phosphorylation of Rb, thereby arresting cells in the G1 phase. Such a pause in cell cycling is welcome, because it gives the cells “breathing time” to repair DNA damage. The p53 protein also induces expression of DNA damage repair genes. If DNA damage is repaired successfully, p53 upregulates transcription of MDM2, leading to destruction of p53 and relief of the cell cycle block. If the damage cannot be repaired, the cell may enter p53-induced senescence or undergo p53-directed apoptosis.
• p53-induced senescence is a permanent cell cycle arrest characterized by specific changes in morphology and gene expression that differentiate it from quiescence or reversible cell cycle arrest. Senescence requires activation of p53 and/or Rb and expression of their mediators, such as the CDKIs. The mechanisms of senescence are unclear but seem to involve global chromatin changes, which drastically and permanently alter gene expression.
• p53-induced apoptosis of cells with irreversible DNA damage is the ultimate protective mechanism against neoplastic transformation. It is mediated by several pro-apoptotic genes such as BAX and PUMA (described later).
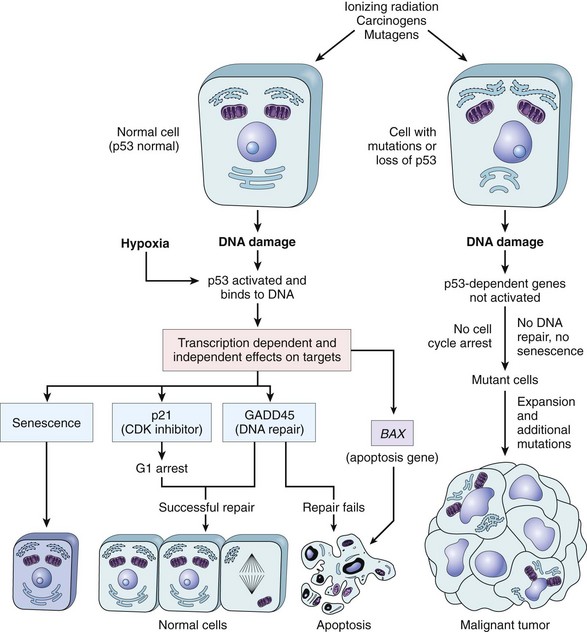
Figure 5–23 The role of p53 in maintaining the integrity of the genome. Activation of normal p53 by DNA-damaging agents or by hypoxia leads to cell cycle arrest in G1 and induction of DNA repair, by transcriptional upregulation of the cyclin-dependent kinase inhibitor CDKN1A (p21) and the GADD45 genes. Successful repair of DNA allows cells to proceed with the cell cycle; if DNA repair fails, p53 triggers either apoptosis or senescence. In cells with loss or mutations of TP53, DNA damage does not induce cell cycle arrest or DNA repair, and genetically damaged cells proliferate, giving rise eventually to malignant neoplasms.
Until recently it was thought that these functions of p53 were mediated exclusively by transcriptional activation of genes with antiproliferative, apoptotic, and senescence-inducing functions, as discussed earlier. But the waters were muddied when it was discovered that p53 represses a subset of pro-proliferative and anti-apoptotic genes as well. How could p53, a transcriptional activator, repress gene function? The answer came from the discovery that p53 can transcriptionally activate certain miRNAs (the “small guys with big clubs”). As discussed in Chapter 6, miRNAs can prevent translation of their target genes. The miRNAs activated by p53 can inhibit the translation of pro-proliferative genes such as cyclins and anti-apoptotic genes such as BCL2.
To summarize, p53 is activated by stresses such as DNA damage and assists in DNA repair by causing G1 arrest and inducing DNA repair genes. A cell with damaged DNA that cannot be repaired is directed by p53 to either enter senescence or undergo apoptosis (Fig. 5–25). In view of these activities, p53 has been rightfully called the “guardian of the genome.” With homozygous loss of the TP53 gene, DNA damage goes unrepaired, mutations become fixed in dividing cells, and the cell turns onto a one-way street leading to malignant transformation.
Confirming the importance of TP53 in controlling carcinogenesis, more than 70% of human cancers have a defect in this gene, and the remaining malignant neoplasms have defects in genes upstream or downstream of TP53. Biallelic loss of the TP53 gene is found in virtually every type of cancer, including carcinomas of the lung, colon, and breast—the three leading causes of cancer deaths. In most cases, inactivating mutations affecting both TP53 alleles are acquired in somatic cells. Less commonly, some patients inherit a mutant TP53 allele; the resulting disease is called the Li-Fraumeni syndrome. As with the RB gene, inheritance of one mutant allele predisposes affected persons to develop malignant tumors because only one additional hit is needed to inactivate the second, normal allele. Patients with the Li-Fraumeni syndrome have a 25-fold greater chance of developing a malignant tumor by age 50 compared with the general population. In contrast with tumors developing in patients who inherit a mutant RB allele, the spectrum of tumors that develop in patients with the Li-Fraumeni syndrome is varied; the most common types are sarcomas, breast cancer, leukemia, brain tumors, and carcinomas of the adrenal cortex. Compared with persons diagnosed with sporadic tumors, patients with Li-Fraumeni syndrome develop tumors at a younger age and may develop multiple primary tumors.
As with Rb protein, normal p53 also can be rendered nonfunctional by certain DNA viruses. Proteins encoded by oncogenic HPVs, hepatitis B virus (HBV), and possibly Epstein-Barr virus (EBV) can bind to normal p53 and nullify its protective function. Thus, DNA viruses can subvert two of the best-understood tumor suppressors, Rb and p53.
![]() Summary
Summary
TP53 Gene: Guardian of the Genome
• The p53 protein is the central monitor of stress in the cell and can be activated by anoxia, inappropriate oncogene signaling, or DNA damage. Activated p53 controls the expression and activity of genes involved in cell cycle arrest, DNA repair, cellular senescence, and apoptosis.
• DNA damage leads to activation of p53 by phosphorylation. Activated p53 drives transcription of CDKN1A (p21), which prevents Rb phosphorylation, thereby causing a G1-S block in the cell cycle. This pause allows the cells to repair DNA damage.
• If DNA damage cannot be repaired, p53 induces cellular senescence or apoptosis.
• Of human tumors, 70% demonstrate biallelic loss of TP53. Patients with the rare Li-Fraumeni syndrome inherit one defective copy in the germ line and lose the second one in somatic tissues; such persons develop a variety of tumors.
• As with Rb, p53 can be incapacitated by binding to proteins encoded by oncogenic DNA viruses such as HPV.
Transforming Growth Factor-β Pathway
Although much is known about the circuitry that applies brakes to the cell cycle, the molecules that transmit antiproliferative signals to cells are less well characterized. Best-known is TGF-β, a member of a family of dimeric growth factors that includes bone morphogenetic proteins and activins. In most normal epithelial, endothelial, and hematopoietic cells, TGF-β is a potent inhibitor of proliferation. It regulates cellular processes by binding to a complex composed of TGF-β receptors I and II. Dimerization of the receptor upon ligand binding leads to a cascade of events that result in the transcriptional activation of CDKIs with growth-suppressing activity, as well as repression of growth-promoting genes such as MYC, CDK2, CDK4, and those encoding cyclins A and E.
In many forms of cancer, the growth-inhibiting effects of the TGF-β pathways are impaired by mutations affecting TGF-β signaling. These mutations may alter the type II TGF-β receptor or SMAD molecules that serve to transduce antiproliferative signals from the receptor to the nucleus. Mutations affecting the type II receptor are seen in cancers of the colon, stomach, and endometrium. Mutational inactivation of SMAD4, 1 of the 10 proteins known to be involved in TGF-β signaling, is common in pancreatic cancers. In 100% of pancreatic cancers and 83% of colon cancers, at least one component of the TGF-β pathway is mutated. In many cancers, however, loss of TGF-β–mediated growth control occurs at a level downstream of the core signaling pathway, for example, loss of p21 and/or persistent expression of MYC. These tumor cells can then use other elements of the TGF-β–induced program, including immune system suppression–evasion or promotion of angiogenesis, to facilitate tumor progression. Thus, TGF-β can function to prevent or promote tumor growth, depending on the state of other genes in the cell. Indeed, in many late-stage tumors, TGF-β signaling activates epithelial-to-mesenchymal transition (EMT), a process that promotes migration, invasion, and metastasis, as described later.
Contact Inhibition, NF2, and APC
When nontransformed cells are grown in culture, they proliferate until confluent monolayers are generated; cell–cell contacts formed in these monolayers suppress further cell proliferation. Of importance, “contact inhibition” is abolished in cancer cells, allowing them to pile on top of one another. The mechanisms that govern contact inhibition are only now being discovered. Cell–cell contacts in many tissues are mediated by homodimeric interactions between transmembrane proteins called cadherins. E-cadherin (E for epithelial) mediates cell–cell contact in epithelial layers. How E-cadherin maintains normal contact inhibition is not fully understood. One mechanism that sustains contact inhibition is mediated by the tumor suppressor gene NF2. Its product, neurofibromin-2, more commonly called merlin, facilitates E-cadherin mediated contact inhibition. Homozygous loss of NF2 is known to cause a form of neural tumors associated with the condition called neurofibromatosis.
There are other mechanisms of E-cadherin regulation as well. One such mechanism is illustrated by the rare hereditary disease adenomatous polyposis coli (APC). This disorder is characterized by the development of numerous adenomatous polyps in the colon that have a very high incidence of transformation into colonic cancers. They consistently show loss of a tumor suppressor gene called APC (named for the disease). The APC gene exerts antiproliferative effects in an unusual manner. It encodes a cytoplasmic protein whose dominant function is to regulate the intracellular levels of β-catenin, a protein with many functions. On the one hand, β-catenin binds to the cytoplasmic portion of E-cadherin; on the other hand, it can translocate to the nucleus and activate cell proliferation. Here the focus is on the latter function of this protein. β-Catenin is an important component of the so-called WNT signaling pathway that regulates cell proliferation (illustrated in Fig. 5–24). WNT is a soluble factor that can induce cellular proliferation. It does so by binding to its receptor and transmitting signals that prevent the degradation of β-catenin, allowing it to translocate to the nucleus, where it acts as a transcriptional activator in conjunction with another molecule, called TcF (Fig. 5–24, B). In quiescent cells, which are not exposed to WNT, cytoplasmic β-catenin is degraded by a destruction complex, of which APC is an integral part (Fig. 5–24, A). With loss of APC (in malignant cells), β-catenin degradation is prevented, and the WNT signaling response is inappropriately activated in the absence of WNT (Fig. 5–24, C). This leads to transcription of growth-promoting genes, such as cyclin D1 and MYC, as well as transcriptional regulators, such as TWIST and SLUG, that repress E-cadherin expression and thus reduce contact inhibition.
Figure 5–24 A–C, The role of APC in regulating the stability and function of β-catenin. APC and β-catenin are components of the WNT signaling pathway. In resting cells (not exposed to WNT), β-catenin forms a macromolecular complex containing the APC protein. This complex leads to the destruction of β-catenin, and intracellular levels of β-catenin are low. When cells are stimulated by secreted WNT molecules, the destruction complex is deactivated, β-catenin degradation does not occur, and cytoplasmic levels increase. β-Catenin translocates to the nucleus, where it binds to TCF, a transcription factor that activates several genes involved in the cell cycle. When APC is mutated or absent, the destruction of β-catenin cannot occur. β-Catenin translocates to the nucleus and coactivates genes that promote the cell cycle, and cells behave as if they are under constant stimulation by the WNT pathway.
APC behaves as a typical tumor suppressor gene. Persons born with one mutant allele typically are found to have hundreds to thousands of adenomatous polyps in the colon by their teens or 20s; these polyps show loss of the other APC allele. Almost invariably, one or more polyps undergo malignant transformation, as discussed later. APC mutations are seen in 70% to 80% of sporadic colon cancers. Colonic cancers that have normal APC genes show activating mutations of β-catenin that render them refractory to the degrading action of APC.
![]() Summary
Summary
Transforming Growth Factor-β and APC–β-Catenin Pathways
• TGF-β inhibits proliferation of many cell types by activation of growth-inhibiting genes such as CDKIs and suppression of growth-promoting genes such as MYC and those encoding cyclins.
• TGF-β function is compromised in many tumors by mutations in its receptors (colon, stomach, endometrium) or by mutational inactivation of SMAD genes that transduce TGF-β signaling (pancreas).
• E-cadherin maintains contact inhibition, which is lost in malignant cells.
• APC gene exerts antiproliferative actions by regulating the destruction of the cytoplasmic protein β-catenin. With a loss of APC, β-catenin is not destroyed, and it translocates to the nucleus, where it acts as a growth-promoting transcription factor.
• In familial adenomatous polyposis syndrome, inheritance of a germ line mutation in the APC gene and sporadic loss of the sole normal allele causes the development of hundreds of colonic polyps at a young age. Inevitably, one or more of these polyps evolves into a colonic cancer. Somatic loss of both alleles of the APC gene is seen in approximately 70% of sporadic colon cancers.
Evasion of Cell Death
As discussed in Chapter 1, apoptosis, or programmed cell death, refers to an orderly dismantling of cells into component pieces that can then be consumed and disposed of by neighboring cells. It is now well established that accumulation of neoplastic cells may result not only from activation of growth-promoting oncogenes or inactivation of growth-suppressing tumor suppressor genes but also from mutations in the genes that regulate apoptosis.
The apoptotic pathway can be divided into upstream regulators and downstream effectors. The regulators are divided into two major pathways, one interpreting extracellular or extrinsic signals and the other interpreting intracellular signals. Stimulation of either pathway results in activation of a normally inactive protease (caspase-8 or caspase-9, respectively), which initiates a proteolytic cascade involving “executioner” caspases that disassemble the cell in orderly fashion. The cellular remains are then efficiently consumed by the cellular neighbors and professional phagocytes, without stimulating inflammation. Figure 5–25 shows, in simplified form, the sequence of events that lead to apoptosis by signaling through death receptors, which are members of the TNF receptor family (extrinsic pathway), and by DNA damage and other stresses (intrinsic pathway).
• The extrinsic (death receptor) pathway is initiated when a TNF receptor, such as CD95 (Fas), is bound to its ligand, CD95L, leading to trimerization of the receptor and its cytoplasmic death domains, which attract the intracellular adaptor protein FADD. This protein recruits procaspase-8 to form the death-inducing signaling complex. Procaspase-8 is activated by cleavage into smaller subunits, generating caspase-8. Caspase-8 then activates downstream caspases such as caspase-3, an executioner caspase that cleaves DNA and other substrates to cause cell death.
• The intrinsic (mitochondrial) pathway of apoptosis is triggered by a variety of stimuli, including withdrawal of survival factors, stress, and injury. Activation of this pathway leads to permeabilization of the mitochondrial outer membrane and release of molecules, such as cytochrome c, that initiate apoptosis.
Figure 5–25 Simplified schema of CD95 receptor–induced and DNA damage–triggered pathways of apoptosis and mechanisms used by tumor cells to evade cell death: 1, Reduced CD95 level. 2, Inactivation of death-induced signaling complex by FLICE protein. 3, Reduced egress of cytochrome c from mitochondrion as a result of upregulation of BCL2. 4, Reduced levels of pro-apoptotic BAX resulting from loss of p53. 5, Loss of APAF-1. 6, Upregulation of inhibitors of apoptosis.
The integrity of the mitochondrial outer membrane is regulated by pro-apoptotic and anti-apoptotic members of the BCL2 family of proteins. The pro-apoptotic proteins BAX and BAK are required for apoptosis and directly promote mitochondrial permeabilization. Their action is inhibited by the anti-apoptotic members of this family exemplified by BCL2 and BCL-XL. A third set of proteins, the so-called BH3-only proteins, which include BAD, BID, and PUMA, regulate the balance between the pro- and anti-apoptotic members of the BCL2 family. The BH3-only proteins promote apoptosis by neutralizing the actions of anti-apoptotic proteins like BCL2 and BCL-XL. When the sum total of all BH3 proteins expressed “overwhelms” the anti-apoptotic BCL2/BCLXL protein barrier, BAX and BAK are activated and form pores in the mitochondrial membrane. Cytochrome c leaks into the cytosol, where it binds to APAF-1 and activates caspase-9. Like caspase-8 of the extrinsic pathway, caspase-9 can cleave and activate the executioner caspases. Caspases can be inhibited by a family of proteins called inhibitor of apoptosis proteins (IAPs). Because of the pro-apoptotic effect of BH3 only proteins, efforts are underway to develop BH3 mimetic drugs to promote death of tumor cells.
Within this framework, it is possible to illustrate the multiple sites at which apoptosis is frustrated by cancer cells (Fig. 5–25). Of these candidates, perhaps best-established is the role of BCL2 in protecting tumor cells from apoptosis. Approximately 85% of B cell lymphomas of the follicular type (Chapter 11) carry a characteristic t(14;18) (q32;q21) translocation. As noted earlier, 14q32, the chromosomal locus for immunoglobulin heavy-chain genes, also is involved in the pathogenesis of Burkitt lymphoma. Juxtaposition of this transcriptionally active locus with BCL2 (located at 18q21) causes overexpression of the BCL2 protein. This overabundance in turn increases the BCL2/BCL-XL buffer, protecting lymphocytes from apoptosis and allowing them to survive for long periods; there is therefore a steady accumulation of B lymphocytes, resulting in lymphadenopathy and marrow infiltration. Because BCL2-overexpressing lymphomas arise in large part through reduced cell death rather than explosive cell proliferation, they tend to be indolent (slow-growing) compared to other lymphomas. In some instances, reduced levels of CD95 may render the tumor cells less susceptible to apoptosis by Fas ligand (FasL). Some tumors have high levels of FLIP, a protein that can bind death-inducing signaling complex and prevent activation of caspase 8.
As mentioned previously, TP53 is an important pro-apoptotic gene that induces apoptosis in cells that are unable to repair DNA damage. Similarly, unrestrained action of growth-promoting genes such as MYC also leads to apoptosis. Thus, both major oncogenic pathways—inability to repair DNA damage and inappropriate activation of oncogenes—converge on the apoptotic machinery, which, by causing cell death, acts as a major barrier to carcinogenesis.
Autophagy
As described in Chapter 1, autophagy is a key catabolic process that helps balance synthesis, degradation, and recycling of cellular products. During autophagy, cellular organelles, such as ribosomes and mitochondria, are sequestered from the rest of the cell by a membrane (autophagosome) and then fused to a lysosome, where they are degraded and utilized for cellular energy generation. The same process can signal cells to die if they cannot be rescued by the recycling of organelles. It is a tightly regulated process that plays an important role in normal cell function, and can help starving cells shift nutrients from unused cell processes to vital ones. Autophagy, like apoptosis, has regulatory and effector machinery. The effector components consist of proteins that lead to the formation of autophagosomes and direct their contents to lysosomes. Not surprisingly, the regulatory components of autophagy overlap with many of the signaling components that regulate apoptosis. For example, a protein, Beclin-1, required for autophagy, belongs to the BH3 domain containing proteins that regulate apoptosis. When cells sense internal stress (e.g., DNA damage), they may undergo apoptosis or Beclin-1–induced autophagy. Thus, autophagy, by analogy with apoptosis, appears to prevent the growth of tumor cells. Later in tumor growth, however, autophagy may be helpful to tumors. The metabolites generated by autophagy may supply crucial building blocks for growth and survival in the nutrient-poor environments that tumor cells inhabit. Indeed, autophagy may promote tumor survival in unfriendly climates or during therapy. Thus, autophagy may act as either a “friend” or a “foe,” depending on other internal and external factors.
![]() Summary
Summary
Evasion of Apoptosis
• Apoptosis can be initiated through extrinsic or intrinsic pathways.
• Both pathways result in the activation of a proteolytic cascade of caspases that destroys the cell.
• Mitochondrial outer membrane permeabilization is regulated by the balance between pro-apoptotic (e.g., BAX, BAK) and anti-apoptotic molecules (BCL2, BCL-XL). BH-3–only molecules activate apoptosis by tilting the balance in favor of the pro-apoptotic molecules.
• In 85% of follicular B cell lymphomas, the anti-apoptotic gene BCL2 is activated by the t(14;18) translocation.
• Stress may also induce cells to consume their components in a process called autophagy. Cancer cells may accumulate mutations to avoid autophagy, or may corrupt the process to provide parts for continued growth.
Limitless Replicative Potential
As discussed previously in the context of cellular aging (Chapter 1), most normal human cells have a capacity of 60 to 70 doublings. Thereafter, the cells lose the capacity to divide and enter senescence. This phenomenon has been ascribed to progressive shortening of telomeres at the ends of chromosomes. The consequences of such shortening, when pronounced, are drastic:
• Short telomeres seem to be recognized by the DNA repair machinery as double-stranded DNA breaks, leading to cell cycle arrest and senescence, mediated by TP53 and RB. In cells in which the checkpoints are disabled by TP53 or RB mutations, the nonhomologous end-joining pathway is activated in a last-ditch effort to save the cell, joining the shortened ends of two chromosomes.
• Such an inappropriately activated repair system results in dicentric chromosomes that are pulled apart at anaphase, resulting in new double-stranded DNA breaks. The resulting genomic instability from the repeated bridge–fusion–breakage cycles eventually produces mitotic catastrophe, characterized by massive apoptosis.
It follows that for tumors to grow indefinitely, as they often do, loss of growth restraints is not enough. Tumor cells also must develop ways to avoid both cellular senescence and mitotic catastrophe (Fig. 5–26). If during crisis a cell manages to reactivate telomerase, the bridge–fusion–breakage cycles cease, and the cell is able to avoid death. However, during this period of genomic instability that precedes telomerase activation, numerous mutations could accumulate, helping the cell march toward malignancy. Telomerase, active in normal stem cells, normally is absent from, or present at very low levels in, most somatic cells. By contrast, telomere maintenance is seen in virtually all types of cancers. In 85% to 95% of cancers, this is due to upregulation of the enzyme telomerase. A few tumors use other mechanisms, termed alternative lengthening of telomeres, which probably depend on DNA recombination.
Figure 5–26 Sequence of events in the development of limitless replicative potential.
Replication of somatic cells, which do not express telomerase, leads to shortened telomeres. In the presence of competent checkpoints, cells undergo arrest and enter nonreplicative senescence. In the absence of checkpoints, DNA repair pathways are inappropriately activated, leading to the formation of dicentric chromosomes. At mitosis, the dicentric chromosomes are pulled apart, generating random double-stranded breaks, which then activate DNA repair pathways, leading to the random association of double-stranded ends and the formation, again, of dicentric chromosomes. Cells undergo numerous rounds of this bridge–fusion–breakage cycle, which generates massive chromosomal instability and numerous mutations. If cells fail to reexpress telomerase, they eventually undergo mitotic catastrophe and death. Reexpression of telomerase allows the cells to escape the bridge–fusion–breakage cycle, thus promoting their survival and tumorigenesis.
Of interest, in a study of the progression from colonic adenoma to colonic adenocarcinoma, early lesions had a high degree of genomic instability with low telomerase expression, whereas malignant lesions had complex karyotypes with high levels of telomerase activity, consistent with a model of telomere-driven tumorigenesis in human cancer. Thus, it appears that in this model, unregulated proliferation in incipient tumors leads to telomere shortening, followed by chromosomal instability and mutation accumulation. If telomerase is then reactivated in these cells, telomeres are extended and these mutations become fixed, contributing to tumor growth. Several other mechanisms of genomic instability are discussed later.
![]() Summary
Summary
Limitless Replicative Potential
• In normal cells, which lack expression of telomerase, the shortened telomeres generated by cell division eventually activate cell cycle checkpoints, leading to senescence and placing a limit on the number of divisions a cell may undergo.
• In cells that have disabled checkpoints, DNA repair pathways are inappropriately activated by shortened telomeres, leading to massive chromosomal instability and mitotic crisis.
• Tumor cells reactivate telomerase, thus staving off mitotic catastrophe and achieving immortality.
Development of Sustained Angiogenesis
Even with all the growth advantages, as described previously, tumors cannot enlarge beyond 1 to 2 mm in diameter unless they are vascularized. Like normal tissues, tumors require delivery of oxygen and nutrients and removal of waste products; the 1- to 2-mm zone presumably represents the maximal distance across which oxygen, nutrients, and waste can diffuse from blood vessels. Cancer cells (and large benign tumors) can stimulate neoangiogenesis, during which new vessels sprout from previously existing capillaries, or, in some cases, vasculogenesis, in which endothelial cells are recruited from the bone marrow. Tumor vasculature is abnormal, however. The vessels are leaky and dilated, with a haphazard pattern of connection. Neovascularization has a dual effect on tumor growth: Perfusion supplies needed nutrients and oxygen, and newly formed endothelial cells stimulate the growth of adjacent tumor cells by secreting growth factors, such as insulin-like growth factors, PDGF, and granulocyte-macrophage colony-stimulating factor. Angiogenesis is required not only for continued tumor growth but also for access to the vasculature and hence for metastasis. Angiogenesis is thus a necessary biologic correlate of neoplasia, both benign and malignant.
How do growing tumors develop a blood supply? The emerging paradigm is that tumor angiogenesis is controlled by a balance between pro-angiogenic and inhibitory factors.
• The prototypical angiogenesis inducer and inhibitor are vascular endothelial growth factor (VEGF) and thrombospondin-1 (TSP-1), respectively. Early in their growth, most human tumors do not induce angiogenesis. They remain small or in situ for years until the angiogenic switch terminates this stage of vascular quiescence. Normal p53 induces synthesis of TSP-1.
• The molecular basis of the angiogenic switch involves increased production of angiogenic factors and/or loss of angiogenesis inhibitors. These factors may be produced directly by the tumor cells themselves or by inflammatory cells (e.g., macrophages) or other stromal cells associated with the tumors.
• Proteases, elaborated either by the tumor cells directly or from stromal cells in response to the tumor, also are involved in regulating the balance between angiogenic and anti-angiogenic factors. Many proteases can release the angiogenic basic FGF stored in the extracellular matrix (ECM); conversely, three potent angiogenesis inhibitors—angiostatin, endostatin, and vasculostatin—are produced by proteolytic cleavage of plasminogen, collagen, and transthyretin, respectively. TSP-1, on the other hand, is produced by stromal fibroblasts themselves in response to signals from the tumor cells.
• The angiogenic switch is controlled by several physiologic stimuli, such as hypoxia. Relative lack of oxygen stimulates production of a variety of pro-angiogenic cytokines, such as vascular endothelial growth factor (VEGF), through activation of hypoxia-inducible factor-1α (HIF-1α), an oxygen-sensitive transcription factor. HIF-1α is continuously produced, but in normoxic settings the von Hippel–Lindau protein (VHL) binds to HIF-1α, leading to ubiquitination and destruction of HIF-1α.
• In hypoxic conditions, such as in a tumor that has reached a critical size, the lack of oxygen prevents HIF-1α recognition by VHL, and it is not destroyed. HIF-1α translocates to the nucleus and activates transcription of its target genes, such as VEGF. Because of these activities, VHL acts as a tumor suppressor gene, and germline mutations of the VHL gene are associated with hereditary renal cell cancers, pheochromocytomas, hemangiomas of the central nervous system, retinal angiomas, and renal cysts (VHL syndrome).
• VEGF also increases the expression of ligands that activate the Notch signaling pathway, which regulates the branching and density of the new vessels. Because of the crucial role of angiogenesis in tumor growth, much interest is focused on anti-angiogenesis therapy. Indeed, anti-VEGF antibody is now approved for the treatment of several types of cancers.
![]() Summary
Summary
Development of Sustained Angiogenesis
• Vascularization of tumors is essential for their growth and is controlled by the balance between angiogenic and anti-angiogenic factors that are produced by tumor and stromal cells.
• Hypoxia triggers angiogenesis through the actions of HIF-1α on the transcription of the pro-angiogenic factor VEGF. Because of its ability to degrade HIF-1α and thereby prevent angiogenesis, VHL acts as a tumor suppressor. Inheritance of germ line mutations of VHL causes VHL syndrome, characterized by the development of a variety of tumors.
• Many other factors regulate angiogenesis; for example, p53 induces synthesis of the angiogenesis inhibitor TSP-1.
Ability to Invade and Metastasize
The spread of tumors is a complex process involving a series of sequential steps called the invasion–metastasis cascade (Fig. 5–27). These steps consist of local invasion, intravasation into blood and lymph vessels, transit through the vasculature, extravasation from the vessels, formation of micrometastases, and growth of micrometastases into macroscopic tumors. Predictably, this sequence of steps may be interrupted at any stage by either host-related or tumor-related factors. For the purpose of discussion, the metastatic cascade can be subdivided into two phases: (1) invasion of ECM and (2) vascular dissemination and homing of tumor cells.
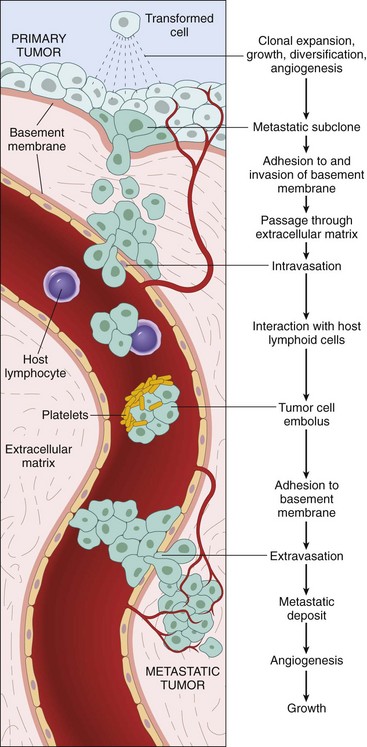
Figure 5–27 The metastatic cascade: The sequential steps involved in the hematogenous spread of a tumor.
Invasion of Extracellular Matrix (ECM)
As is well recognized, human tissues are organized into a series of compartments separated from each other by two types of ECM: basement membranes and interstitial connective tissue (Chapter 2). Although organized differently, each type of ECM is composed of collagens, glycoproteins, and proteoglycans. Tumor cells must interact with the ECM at several stages in the metastatic cascade (Fig. 5–27). A carcinoma first must breach the underlying basement membrane, then traverse the interstitial connective tissue, and ultimately gain access to the circulation by penetrating the vascular basement membrane. This cycle is repeated when tumor cell emboli extravasate at a distant site. Thus, to metastasize, a tumor cell must cross several different basement membranes, as well as negotiate its way through at least two interstitial matrices. Invasion of the ECM is an active process that requires four steps (Fig. 5–28):
• The first step in the metastatic cascade is a loosening of tumor cells. As mentioned earlier, E-cadherins act as intercellular glues, and their cytoplasmic portions bind to β-catenin (Fig. 5–24). Adjacent E-cadherin molecules keep the cells together; in addition, as discussed earlier, E-cadherin can transmit antigrowth signals by sequestering β-catenin. E-cadherin function is lost in almost all epithelial cancers, either by mutational inactivation of E-cadherin genes, by activation of β-catenin genes, or by inappropriate expression of the SNAIL and TWIST transcription factors, which suppress E-cadherin expression.
• The second step in invasion is local degradation of the basement membrane and interstitial connective tissue. Tumor cells may either secrete proteolytic enzymes themselves or induce stromal cells (e.g., fibroblasts and inflammatory cells) to elaborate proteases. Multiple different families of proteases, such as matrix metalloproteinases (MMPs), cathepsin D, and urokinase plasminogen activator, have been implicated in tumor cell invasion. MMPs regulate tumor invasion not only by remodeling insoluble components of the basement membrane and interstitial matrix but also by releasing ECM-sequestered growth factors. Indeed, cleavage products of collagen and proteoglycans also have chemotactic, angiogenic, and growth-promoting effects. For example, MMP-9 is a gelatinase that cleaves type IV collagen of the epithelial and vascular basement membrane and also stimulates release of VEGF from ECM-sequestered pools. Benign tumors of the breast, colon, and stomach show little type IV collagenase activity, whereas their malignant counterparts overexpress this enzyme. Concurrently, the levels of metalloproteinase inhibitors are reduced so that the balance is tilted greatly toward tissue degradation. Indeed, overexpression of MMPs and other proteases has been reported for many tumors.
• The third step in invasion involves changes in attachment of tumor cells to ECM proteins. Normal epithelial cells have receptors, such as integrins, for basement membrane laminin and collagens that are polarized at their basal surface; these receptors help to maintain the cells in a resting, differentiated state. Loss of adhesion in normal cells leads to induction of apoptosis, while, not surprisingly, tumor cells are resistant to this form of cell death. Additionally, the matrix itself is modified in ways that promote invasion and metastasis. For example, cleavage of the basement membrane proteins, collagen IV and laminin, by MMP-2 or MMP-9 generates novel sites that bind to receptors on tumor cells and stimulate migration.
• Locomotion is the final step of invasion, propelling tumor cells through the degraded basement membranes and zones of matrix proteolysis. Migration is a complex, multistep process that involves many families of receptors and signaling proteins that eventually impinge on the actin cytoskeleton. Such movement seems to be potentiated and directed by tumor cell–derived cytokines, such as autocrine motility factors. In addition, cleavage products of matrix components (e.g., collagen, laminin) and some growth factors (e.g., insulin-like growth factors I and II) have chemotactic activity for tumor cells. Stromal cells also produce paracrine effectors of cell motility, such as hepatocyte growth factor/scatter factor (HGF/SCF), which binds to receptors on tumor cells. Concentrations of HGF/SCF are elevated at the advancing edges of the highly invasive brain tumor glioblastoma multiforme, supporting their role in motility.
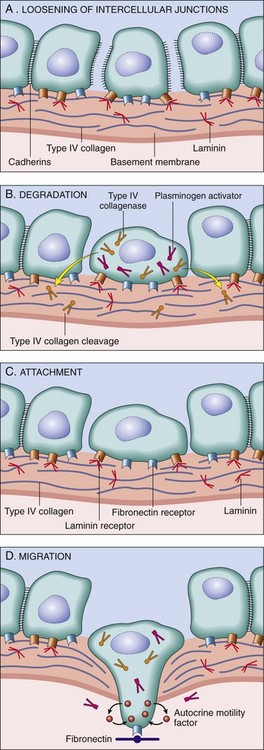
Figure 5–28 A–D, Sequence of events in the invasion of epithelial basement membranes by tumor cells. Tumor cells detach from each other because of reduced adhesiveness, then secrete proteolytic enzymes, degrading the basement membrane. Binding to proteolytically generated binding sites and tumor cell migration follow.
More recently, it has become clear that the stromal cells surrounding tumor cells do not merely present a static barrier for tumor cells to traverse but rather constitute a variable environment in which reciprocal signaling between tumor cells and stromal cells may promote or prevent tumorigenesis. Stromal cells that interact with tumors include innate and adaptive immune cells (discussed later), as well as fibroblasts. A variety of studies have demonstrated that tumor-associated fibroblasts exhibit altered expression of genes that encode ECM molecules, proteases, protease inhibitors, and various growth factors. Thus, tumor cells live in a complex and ever-changing milieu composed of ECM, growth factors, fibroblasts, and immune cells, with significant cross-talk among all the components. The most successful tumors may be those that can co-opt and adapt this environment to their own nefarious ends.
Vascular Dissemination and Homing of Tumor Cells
When in the circulation, tumor cells are vulnerable to destruction by host immune cells (discussed later). In the bloodstream, some tumor cells form emboli by aggregating and adhering to circulating leukocytes, particularly platelets; aggregated tumor cells are thus afforded some protection from the antitumor host effector cells. Most tumor cells, however, circulate as single cells. Extravasation of free tumor cells or tumor emboli involves adhesion to the vascular endothelium, followed by egress through the basement membrane into the organ parenchyma by mechanisms similar to those involved in invasion.
The site of extravasation and the organ distribution of metastases generally can be predicted by the location of the primary tumor and its vascular or lymphatic drainage. Many tumors metastasize to the organ that presents the first capillary bed they encounter after entering the circulation. In many cases, however, the natural pathways of drainage do not readily explain the distribution of metastases. As pointed out earlier, some tumors (e.g., lung cancers) tend to involve the adrenals quite often but almost never spread to skeletal muscle. Such organ tropism may be related to the following mechanisms:
• Expression of adhesion molecules by tumor cells whose ligands are expressed preferentially on the endothelium of target organs
• Expression of chemokines and their receptors. As discussed in Chapter 2, chemokines participate in directed movement (chemotaxis) of leukocytes, and it seems that cancer cells use similar tricks to home in on specific tissues. Human breast cancer cells express high levels of the chemokine receptors CXCR4 and CCR7. The ligands for these receptors (i.e., chemokines CXCL12 and CCL21) are highly expressed only in those organs to which breast cancer cells metastasize. On the basis of this observation, it is speculated that blockade of chemokine receptors may limit metastases.
• Once they reach a target, the tumor cells must be able to colonize the site. Factors that regulate colonization are not completely understood. However, it is known that after extravasation, tumor cells are dependent on a receptive stroma for growth. Thus, in some cases, the target tissue may be a nonpermissive environment—unfavorable soil, so to speak, for the growth of tumor seedlings. For example, although well vascularized, skeletal muscles are rarely the site of metastases.
Despite their “cleverness” in escaping their sites of origin, tumor cells are quite inefficient in colonizing of distant organs. Millions of tumor cells are shed daily from even small tumors. These cells can be detected in the bloodstream and in small foci in the bone marrow, even in patients in whom gross metastatic lesions never develop. Indeed, the concept of dormancy, referring to the prolonged survival of micrometastases without progression, is well described in melanoma and in breast and prostate cancer.
Although the molecular mechanisms of colonization are just beginning to be unraveled in mouse models, a consistent theme seems to be that tumor cells secrete cytokines, growth factors, and proteases that act on the resident stromal cells, which in turn make the metastatic site habitable for the cancer cell. With a better molecular understanding of the mechanisms of metastasis, the clinician’s ability to target them therapeutically will be greatly enhanced. Despite the foregoing considerations, the precise localization of metastases cannot be predicted with any form of cancer. Evidently, many tumors have not read the relevant chapters of the pathology textbooks!
Molecular Genetics of Metastasis
A long-held theory of tumor progression suggests that as tumors grow, individual cells randomly accumulate mutations, creating subclones with distinct combinations of mutations. According to this hypothesis, only a small subpopulation of the tumor cells contains all of the mutations necessary for metastasis. Recent experiments, however, in which gene profiling was performed for primary tumors and for metastatic deposits, have challenged this hypothesis. For example, a subset of breast cancers has a gene expression signature similar to that found in metastases, although no clinical evidence for metastasis is apparent. In these tumors, most if not all cells apparently acquire a predilection for metastatic spread early on, during primary carcinogenesis. Metastasis, according to this view, is not dependent on the stochastic generation of metastatic subclones during tumor progression, but is an intrinsic property of the tumor developed during carcinogenesis. Of note, however, gene expression analyses like those just described would not detect a small subset of metastatic subclones within a large tumor. Perhaps both mechanisms are operative, with aggressive tumors acquiring a metastasis-permissive gene expression pattern early in tumorigenesis that requires some additional random mutations to complete the metastatic phenotype.
An open question in cancer biology is whether there are genes whose principal or sole contribution to tumorigenesis is to control metastases. This question is of more than academic interest, because if altered forms of certain genes promote or suppress the metastatic phenotype, their detection in a primary tumor would have both prognostic and therapeutic implications. Among candidates for such metastasis oncogenes are those encoding SNAIL and TWIST, transcription factors whose primary function is to promote epithelial-to-mesenchymal transition (EMT). In EMT, carcinoma cells downregulate certain epithelial markers (e.g., E-cadherin) and upregulate certain mesenchymal markers (e.g., vimentin, smooth muscle actin). These molecular changes are accompanied by phenotypic alterations such as morphologic change from polygonal epithelioid cell shape to a spindly mesenchymal shape, along with increased production of proteolytic enzymes that promote migration and invasion. These changes are believed to favor the development of a promigratory phenotype that is essential for metastasis. Loss of E-cadherin expression seems to be a key event in EMT, and SNAIL and TWIST are transcriptional repressors that promote EMT by downregulating E-cadherin expression. How expression of these master regulator transcription factors is stimulated in tumors is not clear; however, experimental models suggest that interactions of tumor cells with stromal cells are a key stimulus for this change. Thus, acquisition of a metastatic phenotype may not require a set of mutations but may be an emergent property arising from the interactions of tumor cells and stroma.
![]() Summary
Summary
Invasion and Metastasis
• Ability to invade tissues, a hallmark of malignancy, occurs in four steps: loosening of cell–cell contacts, degradation of ECM, attachment to novel ECM components, and migration of tumor cells.
• Cell–cell contacts are lost by the inactivation of E-cadherin through a variety of pathways.
• Basement membrane and interstitial matrix degradation is mediated by proteolytic enzymes secreted by tumor cells and stromal cells, such as MMPs and cathepsins.
• Proteolytic enzymes also release growth factors sequestered in the ECM and generate chemotactic and angiogenic fragments from cleavage of ECM glycoproteins.
• The metastatic site of many tumors can be predicted by the location of the primary tumor. Many tumors arrest in the first capillary bed they encounter (lung and liver, most commonly).
• Some tumors show organ tropism, probably due to activation of adhesion or chemokine receptors whose ligands are expressed by endothelial cells at the metastatic site.
Reprogramming Energy Metabolism
Reprogramming of energy metabolism is so common to tumors that it is now considered a hallmark of cancer. Even in the presence of ample oxygen, cancer cells shift their glucose metabolism away from the oxygen-hungry but efficient mitochondria to glycolysis. This phenomenon, called the Warburg effect and also known as aerobic glycolysis, has been recognized for many years (indeed, Otto Warburg received the Nobel prize for discovery of the effect that bears his name in 1931) but was largely neglected until recently.
As is well known, aerobic glycolysis is less efficient than mitochondrial oxidative phosphorylation, producing 2 molecules of ATP per molecule of glucose, versus 36. Yet tumors that adopt aerobic glycolysis, such as Burkitt lymphoma, are the most rapidly growing of human cancers. Indeed, in clinical practice, the “glucose hunger” of such tumors is used to visualize tumors by positron emission tomography (PET) scanning, in which the patient is injected with 18F-fluorodeoxyglucose, a nonmetabolizable derivative of glucose. Most tumors are PET-positive, and rapidly growing ones are markedly so.
Importantly, it is now recognized that rapidly dividing normal cells, such as those in the embryo, also adopt Warburg metabolism, indicating that this mode of metabolism is favored when rapid growth is required. How can this be, given that aerobic glycolysis generates much less ATP per mole of glucose? In addition to doubling its DNA content before division, an actively dividing cell (whether normal or transformed) must also double all of its other components, including membranes, proteins, and organelles. This task requires increased uptake of nutrients, particularly glucose and amino acids. Studies of intermediate metabolism suggest that in rapidly growing cells glucose is the primary source of the carbons that are used for synthesis of lipids (needed for membrane assembly) as well as other metabolites needed for nucleic acid synthesis. This pattern of glucose carbon use is achieved by shunting pyruvate toward biosynthetic pathways at the expense of the oxidative phosphorylation pathway and ATP generation. Thus, the metabolism of cancer can also be viewed from a darwinian perspective; tumor cells that adapt this altered metabolism are able to divide more rapidly and outpace competing tumor cells that do not.
Since aerobic glycolysis continues in tumors in the face of adequate oxygen, it follows that the changes that promote the switch in metabolism must have become hard wired in the tumor cell. It is now becoming clear that oncogenes and tumor suppressors that favor cell growth, such as TP53, PTEN, and Akt (an intermediary in RAS signaling) stimulate glucose uptake by affecting glucose transporter proteins and favor aerobic glycolysis. Indeed the Warburg effect appears to be sufficiently central to the cancer phenotype that drugs that target this pathway are being developed for therapy.
Evasion of the Immune System
As mentioned at the outset, the ability of tumors to evade destruction by the immune system (like the reprogramming of the energy metabolism) is now considered a hallmark of cancer. Most tumors arise in immunocompetent hosts; accordingly, a likely strategy for success is to trick the immune system in such a way that the tumor fails to be recognized or eliminated despite the fact the affected person’s body has an army of cells that are quite capable of thwarting a microbial infection or rejecting an allogeneic organ transplant. Discussion of this hallmark is postponed to a later section, since it is best understood in the context of the nature of tumor antigens and how they might be recognized.
Genomic Instability as an Enabler of Malignancy
The preceding section identified eight defining features of malignancy and the genetic alterations that are responsible for the phenotypic attributes of cancer cells. How do these mutations arise? Although humans are awash in environmental agents that are mutagenic (e.g., chemicals, radiation, sunlight), cancers are relatively rare outcomes of these encounters. This state of affairs results from the ability of normal cells to repair DNA damage. The importance of DNA repair in maintaining the integrity of the genome is highlighted by several inherited disorders in which genes that encode proteins involved in DNA repair are defective. Persons born with such inherited defects in DNA repair proteins are at greatly increased risk for the development of cancer. Typically, genomic instability occurs when both copies of the gene are lost; however, recent work has suggested that at least a subset of these genes may promote cancer in a haploinsufficient manner. Defects in three types of DNA repair systems—mismatch repair, nucleotide excision repair, and recombination repair—are presented next. While these discussions focus on inherited syndromes, a point worthy of emphasis is that sporadic cancers often incur mutations in these genes as well, which in turn enable the accumulation of mutations in other genes whose dysfunction contributes to the hallmarks of cancer.
Hereditary Nonpolyposis Colon Cancer Syndrome
The role of DNA repair genes in predisposition to cancer is illustrated dramatically by hereditary nonpolyposis colon carcinoma (HNPCC) syndrome. This disorder, characterized by familial carcinomas of the colon affecting predominantly the cecum and proximal colon (Chapter 14), results from defects in genes involved in DNA mismatch repair. When a strand of DNA is being repaired, these genes act as “spell checkers.” For example, if there is an erroneous pairing of G with T, rather than the normal A with T, the mismatch repair genes correct the defect. Without these “proofreaders,” errors accumulate at an increased rate, a so-called mutator phenotype. Mutations in at least four mismatch repair genes have been found to underlie HNPCC (Chapter 14). Each affected person inherits one defective copy of one of several DNA mismatch repair genes and acquires the second hit in colonic epithelial cells. Thus, DNA repair genes affect cell growth only indirectly—by allowing mutations in other genes during the process of normal cell division. A characteristic finding in the genome of patients with mismatch repair defects is microsatellite instability (MSI). Microsatellites are tandem repeats of one to six nucleotides found throughout the genome. In normal people, the length of these microsatellites remains constant. By contrast, in patients with HNPCC, these satellites are unstable and increase or decrease in length. Although HNPCC accounts for only 2% to 4% of all colonic cancers, MSI can be detected in about 15% of sporadic cancers. The growth-regulating genes that are mutated in HNPCC include those encoding TGF-β receptor type II, BAX, and other oncogenes and tumor suppressor genes.
Xeroderma Pigmentosum
Patients with another inherited disorder, xeroderma pigmentosum, are at increased risk for the development of cancers of sun-exposed skin. The basis for this disorder is defective DNA repair. Ultraviolet (UV) rays in sunlight cause cross-linking of pyrimidine residues, preventing normal DNA replication. Such DNA damage is repaired by the nucleotide excision repair system. Several proteins are involved in nucleotide excision repair, and an inherited loss of any one of these can give rise to xeroderma pigmentosum.
Diseases with Defects in DNA Repair by Homologous Recombination
A group of autosomal recessive disorders comprising Bloom syndrome, ataxia-telangiectasia, and Fanconi anemia is characterized by hypersensitivity to other DNA-damaging agents, such as ionizing radiation (in Bloom syndrome and ataxia-telangiectasia), or to DNA cross-linking agents, such as nitrogen mustard (in Fanconi anemia). Their phenotype is complex and includes, in addition to predisposition to cancer, features such as neural symptoms (in ataxia-telangiectasia), anemia (in Fanconi anemia), and developmental defects (in Bloom syndrome). The gene mutated in ataxia-telangiectasia is ATM, which encodes a protein kinase that is important in recognizing DNA damage caused by ionizing radiation and initiating p53 activation.
Evidence for the role of DNA repair genes in the origin of cancer also comes from the study of hereditary breast cancer. Mutations in two genes, BRCA1 and BRCA2, account for 50% of cases of familial breast cancer. In addition to breast cancer, women with BRCA1 mutations have a substantially higher risk of epithelial ovarian cancers, and men have a slightly higher risk of prostate cancer. Likewise, mutations in the BRCA2 gene increase the risk of breast cancer in both men and women, as well as cancer of the ovary, prostate, pancreas, bile ducts, stomach, melanocytes, and B lymphocytes. Although the functions of these genes have not been elucidated fully, cells that lack these genes develop chromosomal breaks and severe aneuploidy. Indeed, both genes seem to function, at least in part, in the homologous recombination DNA repair pathway. For example, BRCA1 forms a complex with other proteins in the homologous recombination pathway and also is linked to the ATM kinase pathway. BRCA2 was identified as one of several genes mutated in Fanconi anemia, and the BRCA2 protein has been shown to bind to RAD51, a protein required for homologous recombination. Similar to other tumor suppressor genes, both copies of BRCA1 and BRCA2 must be inactivated for cancer to develop. Although linkage of BRCA1 and BRCA2 to familial breast cancers is established, these genes are rarely inactivated in sporadic cases of breast cancer. In this regard, BRCA1 and BRCA2 are different from other tumor suppressor genes, such as APC and TP53, which are inactivated in both familial and sporadic cancers.
Cancers Resulting From Mutations Induced by Regulated Genomic Instability: Lymphoid Neoplasms
A special type of DNA damage plays a central role in the pathogenesis of tumors of B and T lymphocytes. As described earlier, adaptive immunity relies on the ability of B and T cells to diversify their antigen receptor genes. Early B and T cells both express a pair of gene products, RAG1 and RAG2, that carry out V(D)J segment recombination, permitting the assembling of functional antigen receptor genes. In addition, after encountering antigen, mature B cells express a specialized enzyme called activation-induced cytosine deaminase (AID), which catalyzes both immunoglobulin gene class switch recombination and somatic hypermutation. Errors during antigen receptor gene assembly and diversification are responsible for many of the mutations that cause lymphoid neoplasms, described in detail in Chapter 11.
![]() Summary
Summary
Genomic Instability as Enabler of Malignancy
• Persons with inherited mutations of genes involved in DNA repair systems are at greatly increased risk for the development of cancer.
• Patients with HNPCC syndrome have defects in the mismatch repair system, leading to development of carcinomas of the colon. These patients’ genomes show MSI, characterized by changes in length of short tandem repeating sequences throughout the genome.
• Patients with xeroderma pigmentosum have a defect in the nucleotide excision repair pathway and are at increased risk for the development of cancers of the skin exposed to UV light, because of an inability to repair pyrimidine dimers.
• Syndromes involving defects in the homologous recombination DNA repair system constitute a group of disorders—Bloom syndrome, ataxia-telangiectasia, and Fanconi anemia—that are characterized by hypersensitivity to DNA-damaging agents, such as ionizing radiation. BRCA1 and BRCA2, which are mutated in familial breast cancers, are involved in DNA repair.
• Mutations incurred in lymphoid cells expressing gene products that induce genomic instability (RAG1, RAG2, AID) are important causes of lymphoid neoplasms.
Tumor-Promoting Inflammation as Enabler of Malignancy
Accumulating evidence suggests that inflammation, often thought of as a protective response against tumors, can paradoxically also enable malignancy. This occurs in two different settings:
• Persistent chronic inflammation in response to microbial infections or as part of an autoimmune reaction. This is exemplified by the increased risk of cancer in patients affected by a variety of chronic inflammatory diseases of the gastrointestinal tract. These include Barrett esophagus, ulcerative colitis, H. pylori gastritis, hepatitis B and C, and chronic pancreatitis. As with any cause of chronic tissue injury, there is a compensatory proliferation of cells in an attempt to repair the damage. This regenerative process is aided and abetted by a plethora of growth factors, cytokines, chemokines, and other bioactive substances produced by activated immune cells collected at the site. Persistent cell replication and reduced apoptosis under these conditions place the cells at risk of acquiring mutations in one or more of the genes involved in carcinogenesis. In addition, inflammatory cells such as neutrophils can contribute to carcinogenesis by secretion of reactive oxygen species, which in turn can inflict additional DNA damage in rapidly dividing cells.
• When inflammation occurs in response to tumors. Pathologists have known for quite some time that many tumors are infiltrated by leukocytes. The degree of inflammation varies, but virtually every tumor contains cells of the adaptive and innate components of the immune system. The conventional wisdom has been that the inflammatory reaction is protective since it represents an attempt by the host to destroy the tumor. Indeed, that may well be the purpose of the inflammatory reaction, but these cells can exert tumor-promoting activity by producing growth factors and inflicting additional DNA damage as described above.
Whatever the precise mechanism, the link between inflammation and cancer has practical implications. For instance, expression of the enzyme cyclooxygenase-2 (COX-2), which brings about the conversion of arachidonic acid into prostaglandins (Chapter 2), is induced by inflammatory stimuli and is increased in colon cancers and other tumors. The use of COX-2 inhibitors for cancer prevention and treatment is an active area of research.
Important clinical considerations emerge from the principles presented in the foregoing discussion of the hallmarks of cancer: These hallmarks provide a road map for the development of new therapeutic agents for the treatment of cancer (Fig. 5–29).
Multistep Carcinogenesis and Cancer Progression
As described earlier, the acquisition of several fundamental abnormalities is a prerequisite to development of malignancy. It follows, then, that each cancer must result from accumulation of multiple mutations. A dramatic example of incremental acquisition of the malignant phenotype is documented by the study of colon carcinoma. These lesions are believed to evolve through a series of morphologically identifiable stages: colon epithelial hyperplasia followed by formation of adenomas that progressively enlarge and ultimately undergo malignant transformation (Chapter 14). The proposed molecular correlates of this adenoma-carcinoma sequence are illustrated in Figure 5–30. According to this scheme, inactivation of the APC tumor suppressor gene occurs first, followed by activation of RAS and, ultimately, loss of a tumor suppressor gene on 18q and loss of TP53. The precise temporal sequence of mutations may be different in different tumors.