Noble Dental Alloys and Solders
Noble dental alloys have undergone a tremendous evolution because the U.S. government lifted its support on the price of gold in 1969. Before then, more than 95% of fixed dental prostheses in the United States were made of alloys containing a minimum of 75% gold and other noble metals by weight. However, when the price of gold increased from $35 per ounce to more than $800 per ounce in the early 1980s, the development of alternative alloys increased dramatically to reduce the cost of cast dental restorations. These alternatives included alloys with reduced gold content, but also alloys that contain no gold and alloys that contain no noble metal. Today, the majority of alloys used in the United States and a significant portion of those used in other countries are alternative alloys. In 1999, the tremendous increase in the price of palladium, from about $100 to more than $350 per ounce, again encouraged the development of new types of casting alloys.
This chapter will focus on dental casting alloys that have a noble metal content of at least 25% by weight. In addition, wrought noble alloys and solders will be discussed. The limit of 25 wt% is somewhat arbitrary, but represents the limit established by the ADA for alloys that can be classified as noble alloys. Base-metal alloys will be discussed in Chapter 16. The first section of this chapter will survey the metallic elements classified as noble dental alloys. In subsequent sections, the physical and chemical properties of noble dental alloys, both cast and wrought, will be presented. Finally, the compositions and properties of noble solders will be discussed. Techniques for casting and soldering will be discussed in Chapter 17.
METALLIC ELEMENTS USED IN DENTAL ALLOYS
For dental restorations, it is necessary to combine various elements to produce alloys with adequate properties for dental applications because none of the elements themselves have properties that are suitable. These alloys may be used for dental restorations as cast alloys, or may be manipulated into wire or other wrought forms. The metallic elements that make up dental alloys can be divided into two major groups, the noble metals and the base metals. A general discussion of the principles of alloys and metallurgy that are used in the current chapter can be found in Chapter 6.
NOBLE METALS
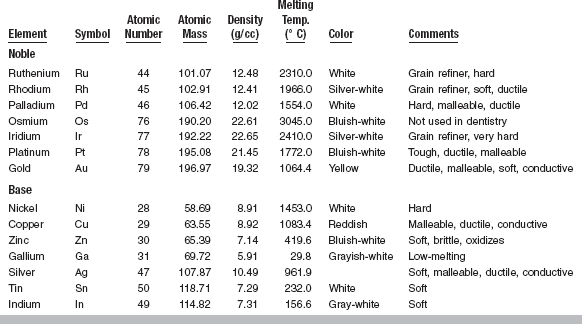
Noble metals are elements with a good metallic surface that retain their surface in dry air. They react easily with sulfur to form sulfides, but their resistance to oxidation, tarnish, and corrosion during heating, casting, soldering, and use in the mouth is very good. The noble metals are gold, platinum, palladium, iridium, rhodium, osmium, and ruthenium (Table 15-1, see Fig. 6-1). These metals can be subdivided into two groups. The metals of the first group, consisting of ruthenium, rhodium, and palladium, have atomic weights of approximately 100 and densities of 12 to 13 g/cm3. The metals of the second group, consisting of osmium, iridium, platinum, and gold, have atomic weights of about 190 and densities of 19 to 23 g/cm3. The melting points of members of each group decrease with increasing atomic weight. Thus, ruthenium melts at 2310° C, rhodium at 1966° C, and palladium at 1554° C. In the second group the melting points range from 3045° C for osmium to 1064° C for gold. The noble metals, together with silver, are sometimes called precious metals. The term precious stems from the trading of these metals on the commodities market. Some metallurgists consider silver a noble metal, but it is not considered a noble metal in dentistry because it corrodes considerably in the oral cavity. Thus, the terms noble and precious are not synonymous in dentistry.
Gold (Au)
Pure gold is a soft, malleable, ductile metal that has a rich yellow color with a strong metallic luster. Although pure gold is the most ductile and malleable of all metals, it ranks much lower in strength. The density of gold depends somewhat on the condition of the metal, whether it is cast, rolled, or drawn into wire. Small amounts of impurities have a pronounced effect on the mechanical properties of gold and its alloys. The presence of less than 0.2% lead causes gold to be extremely brittle. Mercury in small quantities also has a harmful effect. Therefore, scrap of other dental alloys, such as technique alloy or other base-metal alloys, including amalgam, should not be mixed with gold used for dental restorations. The addition of calcium to pure gold improves the mechanical properties of gold foil restorations (see subsequent discussion).
Air or water at any temperature does not affect or tarnish gold. Gold is not soluble in sulfuric, nitric, or hydrochloric acids. However, it readily dissolves in combinations of nitric and hydrochloric acids (aqua regia, 18 vol% nitric and 82 vol% hydrochloric acids) to form the trichloride of gold (AuCl3). It is also dissolved by a few other chemicals, such as potassium cyanide and solutions of bromine or chlorine.
Because gold is nearly as soft as lead, it must be alloyed with copper, silver, platinum, and other metals to develop the hardness, durability, and elasticity necessary in dental alloys, coins, and jewelry (Table 15-2). Through appropriate refining and purification, gold with an extremely high degree of purity may be produced. Such highly refined ingots of pure gold (99.99% by weight) serve as the starting material for gold foil.
TABLE 15-2
Physical and Mechanical Properties of Cast Pure Gold, Gold Alloys, and Condensed Gold Foil

*Values are for softened/hardened condition.
†Adapted from Rule RW: J Am Dent Assoc 24:583, 1937.
Gold foil is formed by a process known as gold beating. High-purity gold is first passed through a series of rollers and then annealed until the gold is in a ribbon about 0.0025 mm thick, which is comparable to the thickness of tissue paper. The ribbon is cut into small pieces, and each piece is placed between two sheets of paper, which are then placed one over the other to form a packet. The packet, which may contain 200 to 250 pieces of the small gold ribbons, is then beaten by a hammer until the desired thickness of gold is obtained, usually 0.00064 mm. The foil is then carefully weighed and annealed. Purity in the process of manufacturing gold foil is critical to maintaining its cohesive properties.
If uncontaminated, gold foil is cohesive; that is, it can be welded together at room temperature. This cohesive property has been exploited in the use of gold foil as a dental restorative material. If manipulated properly, small pieces of foil can be inserted and condensed into cavity preparations in teeth, providing a restoration with considerable longevity. As Table 15-2 shows, the tensile strength and hardness of condensed gold foil is more than twice that of pure (24k) cast gold. The reduced elongation of foil compared with cast gold is evidence of the considerable work hardening that the condensation process has accomplished (see Chapter 6, wrought alloys). It is this improvement of physical properties by work hardening that makes gold acceptable as a restoration in some areas of the mouth; without the improvement, cast gold would lack sufficient strength and hardness. The use of gold foil restorations has declined in recent years because of the time and skill required to properly place these materials and the development of more esthetic (tooth-colored) restorative materials for areas where foil restorations were placed.
Platinum (Pt)
Platinum is a bluish white metal; is tough, ductile, and malleable; and can be produced as foil or fine-drawn wire. Platinum has a hardness similar to that of copper. Pure platinum has numerous applications in dentistry because of its high fusing point and resistance to oral conditions and elevated temperatures. Platinum foil serves as a matrix for the construction of fused porcelain restorations because it does not oxidize at high temperatures. Platinum foil also has a higher melting point than porcelain and has a coefficient of expansion sufficiently close to that of porcelain to prevent buckling of the metal or fracture of the porcelain during changes in temperature (see Chapter 18). Platinum has been used for pins and posts in crown and bridge restorations, and alloys may be cast or soldered to the posts without damage.
Platinum adds greatly to the hardness and elastic qualities of gold, and some dental casting alloys and wires contain quantities of platinum up to 8% combined with other metals. Platinum is a major component of alloys used for precision attachments in complex crown and bridge restorations because these alloys have excellent wear characteristics and high melting ranges. The high melting range is necessary because other gold alloys must be cast to these attachments without causing distortion of the attachment. Platinum tends to lighten the color of yellow gold-based alloys.
Palladium (Pd)
Palladium is a white metal somewhat darker than platinum. Its density is a little more than half that of platinum and gold. Palladium has the quality of absorbing or occluding large quantities of hydrogen gas when heated. This can be an undesirable quality when alloys containing palladium are heated with an improperly adjusted gas-air torch.
Palladium is not used in the pure state in dentistry, but is used extensively in dental alloys. Palladium can be combined with gold, silver, copper, cobalt, tin, indium, or gallium for dental alloys. Alloys are readily formed between gold and palladium, and palladium quantities of as low as 5% by weight have a pronounced effect on whitening yellow gold-based alloys. Palladium-gold alloys with a palladium content of 10% or more by weight are white. Alloys of palladium and the other elements previously mentioned are available as substitutes for yellow-gold alloys, and the mechanical properties of the palladium-based alloys may be as good as or better than many traditional gold-based alloys. Although many of the palladium-based alloys are white, some, such as palladium-indium-silver alloys, are yellow.
Iridium (Ir), Ruthenium (Ru), and Rhodium (Rh)
Iridium and ruthenium are used in small amounts in dental alloys as grain refiners to keep the grain size small. A small grain size is desirable because it improves the mechanical properties and uniformity of properties within an alloy. As little as 0.005% (50 ppm) of iridium is effective in reducing the grain size. Ruthenium has a similar effect. The grainrefining properties of these elements are largely due to their extremely high melting points. Iridium melts at 2410° C and ruthenium at 2310° C. Thus these elements do not melt during the casting of the alloy and serve as nucleating centers for the melt as it cools, resulting in a fine-grained alloy.
Rhodium also has a high melting point (1966° C) and has been used in alloys with platinum to form wire for thermocouples. These thermocouples help measure the temperature in porcelain furnaces used to make dental restorations.
BASE METALS
Several base metals are combined with noble metals to develop alloys with properties suitable for dental restorations. Base metals used in dental alloys include silver, copper, zinc, indium, tin, gallium, and nickel (see Table 15-1, Fig. 6-1).
Silver (Ag)
Silver is a malleable, ductile white metal. It is the best-known conductor of heat and electricity, and is stronger and harder than gold but softer than copper. At 961.9° C, the melting point of silver is below the melting points of both copper and gold. It is unaltered in clean, dry air at any temperature, but combines with sulfur, chlorine, phosphorus, and vapors containing these elements or their compounds. Foods containing sulfur compounds cause severe tarnish on silver, and for this reason silver is not considered a noble metal in dentistry. Pure silver captures appreciable quantities of oxygen in the molten state, which makes it difficult to cast because the gas is evolved during solidification. As a result, small pits, porosity, and a rough casting surface develop. This tendency is reduced when 5% to 10% by weight of copper is added to the silver, for which reason castings are made of the alloy rather than the pure metal. This combination of elements is also used in silver-based dental solders to prevent pitting during soldering.
Pure silver is not used in dental restorations because of the black sulfide that forms on the metal in the mouth. Adding small amounts of palladium to silver-containing alloys prevents the rapid corrosion of such alloys in the oral environment. The electroforming of silver of high purity is readily accomplished and represents a popular method of forming metal dies.
Silver forms a series of solid solutions with palladium (see Fig. 15-1, D) and gold (see Fig. 15-1, C), and is therefore common in gold- and palladium-based dental alloys. In gold-based alloys, silver is effective in neutralizing the reddish color of alloys containing appreciable quantities of copper. Silver also hardens the gold-based alloys via a solidsolution hardening mechanism (see Chapter 6). More recent evidence indicates that a fine lamellar coherent precipitate of an Ag-rich phase at the grain boundaries may contribute to hardening as well. In palladium-based alloys, silver is important in developing the white color of the alloy. Although silver is soluble in palladium, the addition of other elements to these alloys, such as copper or indium, may cause the formation of multiple phases and increased corrosion.

FIGURE 15-1 Phase diagrams for binary combinations of A, copper and gold; B, copper and palladium; C, silver and gold; D, silver and palladium; E, palladium and gold; and F, gold and platinum. Atomic percentages are shown along the bottom of each graph; weight percentages are shown along the top. L, liquidus; S, solidus. (Adapted from Hansen M: Constitution of binary alloys, New York, 1958, McGraw Hill.) McGraw Hill
Copper (Cu)
Copper is a malleable and ductile metal with high thermal and electrical conductivity and a characteristic red color. Copper forms a series of solid solutions with both gold (Fig. 15-1, A) and palladium (Fig. 15-1, B), and is therefore an important component of noble dental alloys. When added to gold-based alloys, copper imparts a reddish color to the gold and hardens the alloy via a solid-solution or ordered-solution mechanism. The presence of copper in gold-based alloys in quantities between approximately 40% and 88% by weight causes allows the formation of an ordered phase. Copper is also commonly used in palladium-based alloys, where it can be used to reduce the melting point and strengthen the alloy through solid-solution hardening and formation of ordered phases when Cu is between 15 and 55 wt%. The ratio of silver and copper must be carefully balanced in gold- and palladium-based alloys, because silver and copper are not miscible. Copper is also a common component of most hard dental solders.
Zinc (Zn)
Zinc is a blue-white metal with a tendency to tarnish in moist air. In its pure form, it is a soft, brittle metal with low strength. When heated in air, zinc oxidizes readily to form a white oxide of relatively low density. This oxidizing property is exploited in dental alloys. Although zinc may be present in quantities of only 1% to 2% by weight, it acts as a scavenger of oxygen when the alloy is melted. Thus zinc is referred to as a deoxidizing agent. Because of its low density, the resulting zinc oxide lags behind the denser molten mass during casting, and is therefore excluded from the casting. If too much zinc is present, it will markedly increase the brittleness of the alloy.
Indium (In)
Indium is a soft, gray-white metal with a low melting point of 156.6° C. Indium is not tarnished by air or water. It is used in some gold-based alloys as a replacement for zinc, and is a common minor component of some noble ceramic dental alloys. Recently, indium has been used in greater amounts (up to 30% by weight) to impart a yellow color to palladium-silver alloys.
Tin (Sn)
Tin is a lustrous, soft, white metal that is not subject to tarnish in normal air. Some gold-based alloys contain limited quantities of tin, usually less than 5% by weight. Tin is also an ingredient in gold-based dental solders. It combines with platinum and palladium to produce a hardening effect, but also increases brittleness.
Gallium (Ga)
Gallium is a grayish metal that is stable in dry air but tarnishes in moist air. It has a very low melting point of 29.8° C and a density of only 5.91 g/cm3. Gallium is not used in its pure form in dentistry, but is used as a component of some gold- and palladium-based dental alloys, especially ceramic alloys. The oxides of gallium are important to the bonding of the ceramic to the metal.
Nickel (Ni)
Nickel has limited application in gold- and palladium-based dental alloys, but is a common component in non-noble dental alloys. Nickel has a melting point of 1453° C and a density of 8.91 g/cm3. When used in small quantities in gold-based alloys, nickel whitens the alloy and increases its strength and hardness. A more extensive discussion of these alloys can be found in Chapter 16.
BINARY COMBINATIONS OF METALS
Although most noble casting alloys have three or more elements, the properties of certain binary alloys are important because these binary combinations constitute the majority of the mass of many noble alloys. An understanding of the physical and manipulative properties of these binary alloys is therefore useful in understanding the behavior of the more complex alloys. Among the noble alloys, six binary combinations of elements are important: Au-Cu, Pd-Cu, Au-Ag, Pd-Ag, Au-Pd, and Au-Pt. Phase diagrams for these binary systems are shown in Fig. 15-1. Phase diagrams are powerful tools for understanding the physical and manipulative properties of binary alloys. A review of the theory of phase diagrams can be found in Chapter 6.
Alloy Composition and Temperature
In each phase diagram in Fig. 15-1, the horizontal axis represents the composition of the binary alloy. For example, in Fig. 15-1, A, the horizontal axis represents a series of binary alloys of gold and copper ranging in composition from 0% gold (or 100% copper) to 100% gold. The composition can be given in atomic percent (at%) or weight percent (wt%). Weight percent compositions give the relative mass of each element in the alloy, whereas atomic percentages give the relative numbers of atoms in the alloys. It is a simple calculation to convert weight percentages to atomic percentages, and vice versa. Note that for the binary alloys shown in Fig. 15-1, the atomic percent composition is shown along the bottom of the phase diagram, whereas the weight percent composition is shown along the top. The atomic and weight percent compositions of the binary alloys can differ considerably. For example, for the Au-Cu system shown in Fig. 15-1, A, an alloy that is 50% gold by weight is only 25% gold by atoms. For other systems, like the Au-Pt system in Fig. 15-1, F, there is little difference between atomic and weight percentages. The difference between atomic and weight percentages depends on the differences in the atomic masses of the elements involved. The bigger the difference in atomic mass, the bigger the difference between the atomic and weight percentages in the binary phase diagram. Because it more convenient to use masses in the manufacture of alloys, the most common method to report composition is by weight percentages. However, the physical and biological properties of alloys relate best to atomic percentages. It is therefore important to keep the difference between atomic and weight percent in mind when selecting and using noble dental casting alloys. Alloys that appear high in gold by weight percentage may in reality contain far fewer gold atoms than might be thought.
Other aspects of the phase diagrams that deserve attention are the liquidus and solidus lines. The y-axes in Fig. 15-1 show temperature. If the temperature is above the liquidus line (marked L), the alloy will be completely molten. If the temperature is below the solidus line (marked S), the alloy will be solid. If the temperature lies between the liquidus and solidus lines, the alloy will be partially molten. Note that the distance between the liquidus and solidus lines varies among systems in Fig. 15-1. For example, the temperature difference between these lines is small for the Ag-Au system (see Fig. 15-1, C), is much larger for the Au-Pt system (see Fig. 15-1, F), and varies considerably with composition for the Au-Cu system (see Fig. 15-1, A). From a manipulative standpoint, it is desirable to have a narrow liquidus-solidus range, because one would like to keep the alloy in the liquid state for as short a time as possible before casting. While in the liquid state, the alloy is susceptible to significant oxidation and contamination. If the liquidus-solidus range is broad, the alloy will remain at least partially molten for a longer period after it is cast. The temperature of the liquidus line is also important, and varies considerably among alloys and with composition. For example the liquidus line of the Au-Ag system ranges from 962° to 1064° C (see Fig. 15-1, C) but the liquidus line of the Au-Pd system ranges from 1064° to 1554° C (see Fig. 15-1, E). It is often desirable to have an alloy with a liquidus line at lower temperatures; the method of heating is easier, fewer side reactions occur, and shrinkage is generally less of a problem (see Chapter 17, Casting and Soldering Procedures).
Phase Structure of Noble Alloys
The area below the solidus lines in Fig. 15-1 is also important to the behavior of the alloy. If this area contains no boundaries, then the binary system is a series of solid solutions. This means that the two elements are completely soluble in one another at all temperatures and compositions. The Ag-Pd system (see Fig. 15-1, D) and Pd-Au system (see Fig. 15-1, E) are examples of solid-solution systems. If the area below the solidus line contains dashed lines, then an ordered solution is present within the dashed lines. An ordered solution occurs when the two elements in the alloy assume specific and regular positions in the crystal lattice of the alloy (for a discussion on metallic crystal lattices, see Chapter 6). This situation differs from a solid solution in which the positions of the elements in the crystal lattice are random. Examples of systems containing ordered solutions are the Au-Cu system (see Fig. 15-1, A) the Pd-Cu system (see Fig. 15-1, B), and the Au-Ag system (see Fig. 15-1, C). Note that the ordered solutions occur over a limited range of compositions because the ratios between the elements must be correct to support the regular positions in the crystal lattices. If the area below the solidus line contains a solid line, it indicates the existence of a second phase. A second phase is an area with a composition distinctly different from the first phase. In the Au-Pt system (see Fig. 15-1, F) a second phase forms between 20 and 90 at% platinum. If the temperature is below the phase boundary line within these compositions, two phases exist in the alloy. The presence of a second phase is important because it significantly changes the corrosion properties of an alloy. Fig. 15-2 shows electron micrographs of single- and multiple-phase alloys. The single-phase alloy has little visible microstructure because its composition is more or less homogeneous. In the multiple-phase alloy, areas that have distinct compositions are clearly visible. These areas correspond to the different phases under the solidus in a phase diagram. Because the different phases may interact electrochemically, the corrosion of multiple-phase alloys may be higher than for a single-phase alloy.
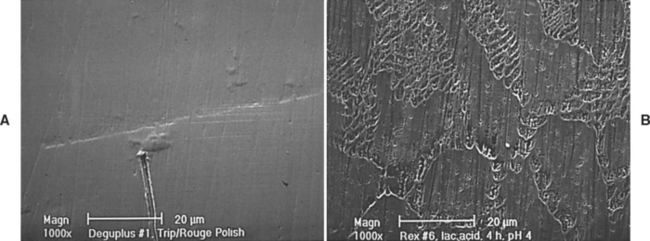
FIGURE 15-2 Electron micrographs of single-phase (A) and multiple-phase (B) alloys. A, Few distinguishing microstructure characteristics are seen because the alloy is nearly homogeneous. Only a few scratches from polishing and some debris on the surface are visible. B, A rich microstructure is evident, reflecting the several phases present. Each phase has a different composition.
Hardening of Noble Alloys
The use of pure cast gold is not practical for dental restorations because cast gold lacks sufficient strength and hardness. Solid-solution and ordered-solution hardening are two common ways of strengthening noble dental alloys sufficiently for use in the mouth. By mixing two elements in the crystal lattice randomly (forming a solid solution), the force needed to distort the lattice may be significantly increased. For example, adding just 10% by weight of copper to gold, the tensile strength increases from 105 to 395 MPa and the Brinell hardness increases from 28 to 85 (see Table 15-2). The 90:10 Au-Cu mixture is the composition used in U.S. gold coins. If the positions of the two elements become ordered (forming an ordered solution), the properties of the alloy are improved further (see Table 15-2). For a typical gold-based casting alloy, the formation of an ordered solution may increase yield strength by 50%, tensile strength by 25%, and hardness by at least 10%. It is important to note that the elongation of an alloy is reduced by the formation of the ordered solution. For the typical gold-based alloy, the percentage elongation will decrease from 30% to about 12%.
The formation of ordered solutions has been commonly used to strengthen cast dental restorations, particularly in gold-based alloys. As shown in Fig. 15-1, A, the Au-Cu system supports ordered solutions between about 20 and 70 at% gold. However, the manipulation of the alloy during casting will determine if the ordered solution will form. If Au-Cu containing about 50 at% gold is heated to the molten state and then cooled slowly, the mass will solidify at about 880° C as a solid solution. As the mass cools slowly to 424° C, the ordered solution will then form and will remain present at room temperature. However, if the mass is cooled rapidly to room temperature after the initial solidification, the ordered solution will not form because there is insufficient time for the mass to reorganize. Thus the alloy will be trapped in a nonequilibrium state of a solid solution and will be softer, weaker, and have greater elongation. The conversion between the ordered solution and solid solution is reversible in the solid state. By heating an alloy in either condition above 424° C (but below the solidus), the state of the alloy can be selected by picking the cooling rate. Rapid cooling will preserve the solid solution and the soft condition, whereas slow cooling will allow the formation of the ordered solution and the hardened condition. In alloys of gold and copper with other elements, Au-Cu ordered solutions are still possible as long as the ratio of copper to gold is greater than 30:70 (at%). As shown in Fig. 15-2, the formation of ordered solutions is possible in other noble alloy systems, such as Pd-Cu and Au-Pt. The ordered solution of the Ag-Au system exists but cannot be used in practice because the transition temperature is too low (almost body temperature).
The formation of a second phase has also been used to harden dental alloys, but this method is not commonly used for noble dental alloys. The dispersion of the second phase is very important to the effectiveness of the hardening. Recent evidence indicates that some Au-based alloys may contain Ag-rich coherent precipitates that, along with ordered solutions, contribute to alloy hardening. The advantages of the hardening must be balanced against the liabilities of the increased corrosion often seen with multiple-phase systems. It should be noted that, unlike the ordered solutions, the formation of second phases are not usually easily controlled by heat treatments and may not be reversible in the solid state. In fact, heat treatment commonly causes a deterioration of properties with these systems. Further discussion of cast and wrought base-metal alloys can be found in Chapter 16.
Formulation of Noble Alloys
The desired qualities of noble dental casting alloys determine the selection of elements that will be used to formulate the alloys. The ideal noble casting alloy should have (1) a low melting range and narrow solidus-liquidus temperature range, (2) adequate strength, hardness, and elongation, (3) a low tendency to corrode in the oral environment, and (4) low cost, among other properties. Traditionally, the noble elements gold and palladium have generally been the foundation to which other elements are added to formulate dental casting alloys. Gold and palladium are preferable to other noble elements because they have relatively low melting points, low corrosion, and form solid solutions with other alloy elements, such as copper or silver (see Fig. 15-1). Solidsolution systems are desirable for the formulation of alloys because they are generally easier to manufacture and manipulate, have a lower tendency to corrode than multiple phase systems, and provide increased strength through solid-solution or ordered-solution hardening. Furthermore, the systems shown in Fig. 15-1 generally have narrow liquidus-solidus ranges. Thus, it is not surprising that combinations of these elements have been extensively used in the formulation of noble dental casting alloys.
The Au-Pt alloys were initially developed out of fear that palladium posed a biological hazard. This fear persisted in spite of a lack of evidence that any hazard existed, other than a low frequency of allergic sensitivity. More recently, palladium-free alloys (Au-Pt and some other systems) have been popular because of the tremendous increase in the cost of palladium. Often, the palladium-free alloys have the disadvantages of high cost and limited compositional flexibility. As shown in Fig. 15-1, F, the addition of more than 20 at% platinum to gold forms a multiple-phase alloy. Thus these alloy systems generally have platinum concentrations below 20 at%. Because the Au-Pt systems may not be hard enough for oral applications, the addition of Zn as a dispersed phase-hardener has been used. Thus the corrosion of these alloys may be higher than the Au-Pd compositions they replaced. Furthermore, the cost of platinum is significantly higher than that of gold, and at least as expensive as palladium.
Carat and Fineness of Gold-Based Alloys
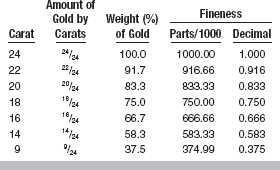
For many years the gold content of gold-containing alloys has been described on the basis of the carat, or in terms of fineness, rather than by weight percentage. The term carat refers only to the gold content of the alloy; a carat represents a 1/24 part of the whole. Thus, 24 carat indicates pure gold. The carat of an alloy is designated by a small letter k, for example, 18k or 22k gold.
The use of the term carat to designate the gold content of dental alloy is less common now. It is not unusual to find the weight percentage of gold listed or to have the alloy described in terms of fineness. Fineness also refers only to the gold content, and represents the number of parts of gold in each 1000 parts of alloy. Thus, 24k gold is the same as 100% gold or 1000 fineness (i.e., 1000 fine). The fineness represents a precise measure of the gold content of the alloy and is often the preferred measurement when an exact value is to be listed. An 18k gold would be designated as 750 fine, or, when the decimal system is used, it would be 0.750 fine; this indicates that 750/1000 of the total is gold. A comparison of the carat, fineness, and weight percentage of gold is given in Table 15-3. Both the whole number and the decimal system are in common use, especially for noble dental solders. The fineness system is somewhat less relevant today because of the introduction of alloys that are not gold-based. It is important to emphasize that the terms carat and fineness refer only to gold content, not noble-metal content.
CASTING ALLOYS
The number of dental casting alloys has increased dramatically in recent years. Formerly, the ADA Specification No. 5 classified these alloys as Types I through IV, with the type of alloy depending on its content of gold and platinum group metals. In the old system, the content of noble metals (Types I through IV) ranged from 83 to 75 wt%, respectively, and all alloys were gold based. This classification reflected the use of alloys in dentistry at that time. The current ADA specification also classifies alloys by composition, but in a much more inclusive manner, dividing alloys into three groups: (1) high-noble, with a noble metal content of at least 60 wt% and a gold content of at least 40%; (2) noble, with a noble metal content at least 25% (no stipulation for gold); and (3) predominately base metal, with a noble metal content less than 25%. The newer specification therefore includes non-noble alloys as well as those with no gold but high palladium. Under the current classification, all of the older alloy “types” are considered high-noble alloys. It is important to keep in mind that the percentages used as boundaries in the new specification are somewhat arbitrary.
The current ADA Specification No. 5 (ISO 1562) also uses a Type I through IV classification system in addition to the compositional classification previously described. However, in the current specification, the type of alloy (I through IV) is determined by its yield strength and elongation (Table 15-4). Thus, a high-noble alloy might be Type I or Type IV, depending on its mechanical properties. This situation is somewhat confusing, because in the old specification the alloy type was tied to its composition and virtually all alloys were gold-based. In the current system, each type of alloy is recommended for intraoral use based on the amount of stress the restoration is likely to receive. Type I alloys have high elongation and are therefore easily burnished, but can survive only in low-stress environments, such as inlays that experience no occlusal forces. Type IV alloys are to be used in clinical situations where very high stresses are involved, such as long-span, fixed, partial dentures.
Although the number of casting alloys is immense, it is possible to subdivide each ADA compositional group into several classes (Table 15-5). The predominately base-metal alloys are not shown, but will be discussed in Chapter 16. These classes are simply a convenient way of organizing the diverse strategies that have been used to formulate casting alloys. For each class of alloy shown in Table 15-5, there are many variations; the compositions shown are meant only to be representative. Note that both the wt% and at% compositions of the alloys are shown in Table 15-5 (see previous discussion in this chapter). For the sake of simplicity, the following discussion will be in terms of wt% composition. Most of the alloys contain some zinc as a deoxidizer and either Ir or Ru as grain refiners. Some of these compositions are used for both full metal castings and ceramic-metal restorations.
TABLE 15-5
Typical Compositions (wt%/at%) of Noble Dental Casting Alloys
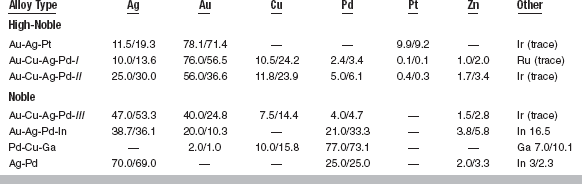
Note: Percentages may not add to exactly 100.0 because of rounding error in calculation of the atomic percentages (at%).
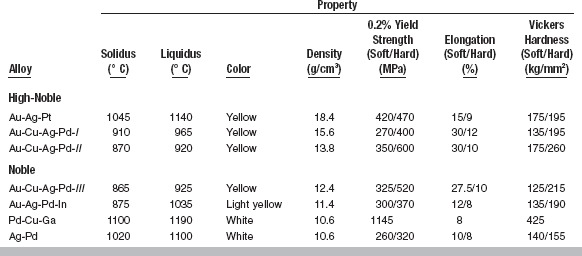
There are three classes of high-noble alloys: the Au-Ag-Pt alloys; the Au-Cu-Ag-Pd alloys with a gold content greater than 70 wt% (Au-Cu-Ag-Pd-I in Table 15-5); and the Au-Cu-Ag-Pd alloys with a gold content of about 50% to 65% (Au-Cu-Ag-Pd-II). The Au-Ag-Pt alloys typically consist of 78 wt% gold with roughly equal amounts of silver and platinum. These alloys have been used as casting alloys and porcelain-metal alloys. The Au-Cu-Ag-Pd-I alloys are typically 75 wt% gold with approximately 10 wt% each of silver and copper and 2 to 3 wt% palladium. These alloys are identical to the Type III alloys under the old ADA compositional classification. The Au-Cu-Ag-Pd-II alloys typically have less than 60 wt% gold, with the silver content increased to accommodate the reduced gold content. Occasionally, these alloys will have slightly higher palladium and lower silver percentages.
There are four classes of noble alloys: the Au-Cu-Ag-Pd alloys (Au-Cu-Ag-Pd-III in Table 15-5); Au-Ag-Pd-In alloys; Pd-Cu-Ga alloys; and Ag-Pd alloys. The Au-Cu-Ag-Pd-III alloys typically have a gold content of 40 wt%. The reduced gold is compensated primarily with silver, thus the copper and palladium contents are not changed much from the Au-Cu-Ag-Pd-II alloys. The Au-Ag-Pd-In alloys have a gold content of only 20 wt%, and have about 40 wt% silver, 20 wt% palladium, and 15 wt% indium. The Pd-Cu-Ga alloys have little or no gold, with about 75 wt% palladium and roughly equal amounts of copper and gallium. Finally, the Ag-Pd alloys have no gold, but have 70 wt% silver and 25 wt% palladium. By the ADA specification, these alloys are considered noble because of their palladium content.
As Table 15-5 shows, the wt% and at% of dental casting alloys can differ considerably. For example, by weight, the Au-Cu-Ag-Pd-I alloys have 76% gold. However, only 57% of the atoms in these alloys are gold. Other elements that have less mass than gold increase in atomic percentage. For these same alloys, the copper content by weight is 10%, but by atoms is 24%. For other alloys whose elements have similar mass, the differences between wt% and at% are less pronounced. For example, in the Ag-Pd alloys the weight and atomic percentages are similar. Weight percentages of the alloys are most commonly used by manufacturers in the production and sales of the alloys. However, the physical, chemical, and biological properties are best understood in terms of atomic percentages.
The compositions of casting alloys determine their color. In general, if the palladium content is over 10 wt%, the alloy will be white. Thus, the Pd-Cu-Ga and Ag-Pd alloys in Table 15-5 are white, whereas the other alloys are yellow. The Au-Ag-Pd-In alloys are an exception because they have a palladium content over 20% and retain a light yellow color. The color of this alloy results from interactions of the indium with the palladium in the alloy. Among the yellow alloys, the composition will modify the shade of yellow. Generally, copper adds a reddish color and silver lightens either the red or yellow color of the alloys.
GRAIN SIZE
Recent studies have described the influence of minute quantities of various elements on the grain size of dental casting alloys. In the past, many alloys had relatively coarse grain structures. Now, by the addition of small amounts (0.005% or 50 ppm) of elements such as iridium and ruthenium, fine-grained castings are produced (see Fig. 6-12). Adding one of these elements to the alloy is believed to develop centers for nucleating grains throughout the alloy. Most alloy manufacturers use grain refinement in present-day products. The mechanical properties of tensile strength and elongation are improved significantly (30%) by the fine grain structure in castings, which contributes to uniformity of properties from one casting to another. Other properties, however, such as hardness and yield strength, show less effect from the grain refinement.
PROPERTIES
Dental casting alloys do not have melting points, but rather melting ranges, because they are combinations of elements rather than pure elements. The magnitude of the solidus-liquidus melting range is important to the manipulation of the alloys (see Fig. 15-1, Table 15-6). The solidus-liquidus range should be narrow to avoid having the alloy in a molten state for extended times during casting. If the alloy spends a long time in the partially molten state during casting, there is increased opportunity for the formation of oxides and contamination. Most of the alloys in Table 15-6 have solidus-liquidus ranges of 70° C or less. The Au-Ag-Pt, Pd-Cu-Ga, and Ag-Pd alloys have wider ranges, which makes them more difficult to cast without problems.
The liquidus temperature of the alloys determines the burnout temperature, type of investment, and type of heat source that must be used during casting. In general, the burnout temperature must be about 500° C below the liquidus temperature (for details see Chapter 17 on casting). For the Au-Cu-Ag-Pd-I alloys, therefore, a burnout temperature of about 450° to 475° C should be used. If the burnout temperature approaches 700° C, a gypsum-bonded investment cannot be used because the calcium sulfate will decompose and embrittle the alloys. At temperatures near 700° C or greater, a phosphate-bonded investment is used. As shown in Table 15-6, a gypsum-bonded investment may be used with the Au-Cu-Ag-Pd-I, II, and III and the Au-Ag-Pd-In alloys, but a phosphate-bonded investment is advisable for the other alloys. The gas-air torch will adequately heat alloys with liquidus temperatures below 1100° C. Above this temperature, a gas-oxygen torch or electrical induction method must be used. Again from Table 15-6, a gas-air torch would be acceptable only for the Au-Cu-Ag-Pd-I, II, and III and the Au-Ag-Pd-In alloys.
The composition of the alloys determines the liquidus temperatures. If the alloy contains a significant amount of an element that has a high melting point, it is likely to have a high liquidus. Thus alloys that contain significant amounts of palladium or platinum, both of which have high melting points (see Table 15-1), will have high liquidus temperatures. In Table 15-6, these alloys include the Pd-Cu-Ga, Ag-Pd, and Au-Ag-Pt alloys.
The solidus temperature is important to soldering and formation of ordered phases, because during both of these operations, the shape of the alloys is to be retained. Therefore during soldering or hardening-softening, the alloy may be heated only to the solidus before melting occurs. In practice, it is desirable to limit heating to 50° C below the solidus to avoid local melting or distortion of the casting.
Density
Density is important during the acceleration of the molten alloy into the mold during casting. Alloys with high densities will generally accelerate faster and tend to form complete castings more easily. Among the alloys shown in Table 15-6, all have densities sufficient for convenient casting. Lower densities (7 to 8 g/cm3) seen in the predominately base-metal alloys sometimes present problems in this regard. Alloys in Table 15-6 with high densities generally contain higher amounts of denser elements such as gold or platinum. Thus the Au-Ag-Pt alloys and Au-Cu-Ag-Pd-I alloys are among the densest of the casting alloys.
Strength
Strength of alloys can be measured by either the yield strength or tensile strength. Although tensile strength represents the maximum strength of the alloy, the yield strength is more useful in dental applications because it is the stress at which permanent deformation of the alloys occurs (see Chapter 4). Because permanent deformation of dental castings is generally undesirable, the yield strength is a reasonable practical maximum strength for dental applications. The yield strengths for the different classes of alloys are shown in Table 15-6. Where applicable, the hard and soft conditions, resulting from the formation of ordered solutions, are shown. For several alloys, such as Au-Cu-Ag-Pd-I, II, and III, the formation of the ordered phase increases the yield strength significantly. For example, the yield strength of the Au-Cu-Ag-Pd-II alloys increases from 350 to 600 MPa with the formation of an ordered phase. For other alloys, such as the Au-Ag-Pt and Ag-Pd alloys, the increase in yield strength is more modest in the hardened condition. The Pd-Cu-Ga alloys do not support the formation of ordered phase because the ratio of palladium and copper are not in the correct range for ordered phase formation (see Table 15-5 and Fig. 15-1, B).
The yield strengths of these alloys range from 320 to 1145 MPa (hard condition). The strongest alloy is Pd-Cu-Ga, with a yield strength of 1145 MPa. The other alloys range in strength from 320 to 600 MPa. These latter yield strengths are adequate for dental applications and are generally in the same range as those for the base-metal alloys, which range from 495 to 600 MPa. The effect of solid-solution hardening by the addition of copper and silver to the gold or palladium base is significant for these alloys. Pure cast gold has a tensile strength of 105 MPa (see Table 15-2). With the addition of 10 wt% copper (coin gold), solid-solution hardening increases the tensile strength to 395 MPa. With the further addition of 10 wt% silver and 3 wt% palladium (Au-Cu-Ag-Pd-I), the tensile strength increases to about 450 MPa and 550 MPa in the hard condition.
Hardness
Hardness is a good indicator of the ability of an alloy to resist local permanent deformation under occlusal load. Although the relationships are complex, hardness is related to yield strength and gives some indication of the difficulty in polishing the alloy. Alloys with high hardness will usually have high yield strengths and are more difficult to polish. As Table 15-6 shows, the values for hardness generally parallel those for yield strength. In the hard condition, the hardness of these alloys ranges from 155 kg/mm2 for the Ag-Pd alloys to 425 kg/mm2 for the Pd-Cu-Ga alloys. More typically, the hardness of the noble casting alloys is around 200 kg/mm2. The Ag-Pd alloys are particularly soft because of the high concentration of silver, which is a soft metal. The Pd-Cu-Ga alloys are particularly hard because of the high concentration of Pd, which is a hard metal. The hardness of most noble casting alloys is less than that of enamel (343 kg/mm2), and typically less than that of base-metal alloys. If the hardness of an alloy is greater than enamel, it may wear the enamel of the teeth opposing the restoration.
Elongation
Elongation is a measure of the ductility of the alloy. For crown and bridge applications, a low value of elongation for an alloy is generally not a big concern, because permanent deformation of the alloys is generally not desirable. However, the elongation will indicate if the alloy can be burnished. Alloys with high elongation can be burnished without fracture. Elongation is sensitive to the presence or absence of an ordered phase, as shown in Table 15-6. In the hardened condition, the elongation will drop significantly. For example, for the Au-Cu-Ag-Pd-II alloys, the elongation is 30% in the soft condition versus only 10% in the hard condition. In the soft condition, the elongation of noble dental casting alloys ranges from 8% to 30%. These alloys are substantially more ductile than the base-metal alloys, which have elongation on the order of 1% to 2% (see Chapter 16).
Biocompatibility
The biocompatibility of noble dental alloys is equally important as other physical or chemical properties. A detailed discussion about the principles of biocompatibility can be found in Chapter 5, but a few general principles will be mentioned here. The biocompatibility of noble dental alloys is primarily related to elemental release from these alloys (i.e., their corrosion). Thus any toxic, allergic, or other adverse biological response is primarily influenced by elements released from these alloys into the oral cavity. The biological response is also influenced significantly by exactly which elements are released, their concentrations, and duration of exposure to oral tissues. For example, the short-term (more than 1 to 2 days) release of zinc may not be significant biologically, but longer-term (more than 2 to 3 years) release might have more significant effects. Similarly, equivalent amounts (in moles) of zinc, copper, or silver will have quite different biological effects, because each of the elements is unique in its interactions with tissues.
Unfortunately, there is currently no way of completely assessing the biocompatibility of noble alloys (or any other material), because the effects of elemental release on tissues are not completely understood. However, in general, several principles apply to alloy biocompatibility. The elemental release from noble alloys is not proportional to alloy composition, but rather is influenced by the numbers and types of phases in the alloy microstructure and the composition of the phases. In general, multiple-phase alloys release more mass than single-phase alloys. Some elements, such as copper, zinc, silver, cadmium, and nickel, are inherently more prone to be released from dental alloys than others, such as gold, palladium, platinum, and indium. Alloys with high noble metal content generally release less mass than alloys with little or no noble metal content. However, the only reliable way to assess elemental release is by direct measurement, because there are exceptions to each of the generalizations just mentioned. Similarly, it is difficult to predict, even knowing the elemental release from an alloy, what the biological response to the alloy will be. Thus the only reliable way is to measure the biological response directly, either in vitro, in animals, or in humans (see Chapter 5). It is important to also remember that combinations of alloys used in the mouth may alter their corrosion and biocompatibility.
The Identalloy program was developed in an effort to make dentists and patients more aware of the composition of dental alloys that are used. Under this program, each alloy has a certificate (Fig. 15-3) that lists the complete composition of the alloy, its manufacturer, name, and the ADA compositional classification (high-noble, noble, or predominately base metal). When the dental prosthesis is delivered by the laboratory to the dental office, a certificate is placed in the patient’s chart. In this manner, all parties know the exact composition of the material used. This information can be invaluable later if there are problems with the restoration; for example, if the patient develops an allergic reaction. This information is also useful when planning additional restorations that may contact the existing restoration, or if some modification (such as occlusal adjustment or contouring) becomes necessary.

FIGURE 15-3 An example of an Identalloy certificate showing the alloy name, manufacturer, composition, and ADA classification. This section of the certificate is for the dentist’s records. A duplicate retained by the laboratory is not shown here. Many dentists will give this information to the patient upon delivery of the crown.
GOLD-BASED ALLOYS FOR CERAMIC-METAL RESTORATIONS
Alloys used for ceramic-metal restorations are discussed in detail in Chapter 19. The application of porcelain imposes several additional requirements on alloys to be used for ceramic-metal restorations. However, the principles in the current chapter concerning composition, metallurgy, and physical properties all apply to ceramic-metal alloys. Several sintered (rather than cast) alloy systems have been introduced for ceramic-metal restorations. These systems use gold-based high-noble metals and follow the principles outlined in the current chapter.
WROUGHT ALLOYS
Alloys that are worked and adapted into prefabricated forms for use in dental restorations are described as wrought alloys. A wrought form is one that has been worked or shaped and fashioned into a serviceable form for an appliance (Fig. 15-4). The work done to the alloy is usually at a temperature far below the solidus, and is therefore referred to as cold work. Wrought forms may include precision attachments, backings for artificial teeth, and wire in various cross-sectional shapes. Wrought alloys are used in two ways in dental prostheses. First, they can be soldered to a previously cast restoration. An example is a wrought wire clasp on a removable partial denture framework. Second, they can be embedded into a cast framework by “casting to” the alloy, as a precision attachment is “cast to” the retainer of a crown, bridge, or partial denture. The physical properties required of the wrought alloy will depend on the technique used and the composition of the alloy in the existing appliance.
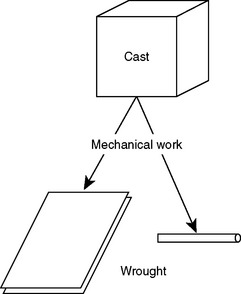
FIGURE 15-4 Diagram of the process of mechanical work that transforms cast structures into wrought structures. The microstructure and mechanical properties of cast and wrought forms are fundamentally different (see Chapter 6).
MICROSTRUCTURE
As indicated in Chapter 6, the microstructure of wrought alloys is fibrous. This fibrous structure results from the cold work applied during the operations that shape the alloy into its final form. Wires or other wrought forms normally have a measurable increase in tensile strength and hardness when compared with corresponding cast structures. The increase in these properties results from the entangled, fibrous internal structure created by the cold work.
Wrought forms will recrystallize during heating operations unless caution is exercised (see Figs. 6-18 and 6-19). During recrystallization, the fibrous microstructure is converted to a grained structure similar to the structure of a cast form. In general, the amount of recrystallization increases as both the heating time and temperature become excessive. For example, in most noble dental wires, a short heating cycle during the soldering operation is not sufficient to appreciably recrystallize the wire, even though the temperature approaches the fusion temperature. However, a prolonged heating period of 30 to 60 seconds or longer may cause recrystallization, depending on the time, temperature, alloy composition, and manner in which the wire was fabricated. Recrystallization results in a reduction in mechanical properties in proportion to the amount of recrystallization. Severe recrystallization can cause wrought forms to become brittle in the area of recrystallization. Therefore, heating operations must be minimized when working with wrought forms.
COMPOSITION
By the current ADA definitions, all alloys used for wrought forms are high-noble alloys except one, which is a noble alloy (Table 15-7). As with the casting alloys, several strategies have been used to formulate alloys with appropriate properties. The compositions in Table 15-7 are not inclusive of all available wrought alloys, but are intended to demonstrate typical alloys. These compositions are designed to provide a range of melting ranges and mechanical properties that are appropriate for wrought alloy applications. The Pt-Au-Pd alloys contain primarily platinum with equal amounts (27 wt%) of palladium and gold. These “PGP” alloys have been commonly used as clasping wires on removable partial dentures. The Au-Pt-Pd alloys are primarily gold with platinum and palladium. The Au-Pt-Cu-Ag, Au-Pt-Ag-Cu, and Au-Ag-Cu-Pd alloys contain approximately 60 wt% gold, but have adopted different strategies for the remaining 40% of the mass. The first two of these alloys contain about 15 wt% platinum with the balance in silver, copper, and palladium, whereas the third of these alloys contains no platinum and higher amounts of silver. The last alloy shown in Table 15-7 contains no appreciable gold or platinum, but consists of palladium and silver in approximately equal amounts with about 16 wt% copper. The Au-Ag-Cu-Pd wrought alloy (see Table 15-7) is similar to the Au-Cu-Ag-Pd-II casting alloy (see Table 15-5). These alloys differ only slightly in the gold/silver ratio. Other wrought alloys differ from the casting alloys primarily in their higher platinum contents and absence of iridium or ruthenium grain refiners. Platinum is added to increase the melting temperature of the alloys. The grain refinement is not necessary because these alloys are cold-worked into their final forms.
TABLE 15-7
Composition of Typical Wrought Dental Alloys (wt%)
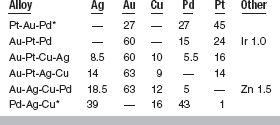
*Adapted from Lyman T: Metals Handbook, vol 1, Properties and selection of metals, ed 8, Metals Park, Ohio, 1961, American Society for Metals.
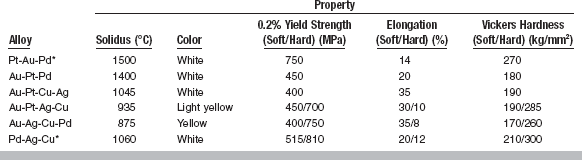
PROPERTIES
The properties of alloys used for wrought applications are shown in Table 15-8. The solidus of these alloys ranges from 875° C for Au-Ag-Cu-Pd to 1500° C for Pt-Au-Pd. If the wrought form is to be cast to or soldered to, the solidus must be sufficiently high so the form does not melt or lose its fibrous structure during burnout or casting operations. The solidus required will depend on the metals to be joined, the solder, and the burnout and casting temperatures to be used. In general, alloys with high solidus temperatures also have higher recrystallization temperatures. These alloys are mostly white because of the high platinum and palladium contents. Exceptions are the Au-Pt-Ag-Cu and Au-Ag-Cu-Pd alloys, which are light yellow and yellow, respectively. Yield strength, elongation, and hardness are properties relevant to wrought alloys (see Table 15-8). The wrought form must generally have a yield strength low enough to allow for adjustment (of a clasp or attachment), but be high enough that permanent distortion does not occur in service. Furthermore, the elongation must be sufficient to allow for adjustment without fracture. Three of the wrought alloys shown in Table 15-8 can be hardened by formation of ordered phases. The Au-Pt-Ag-Cu and Au-Ag-Cu-Pd alloys are hardened by an Au-Cu ordered phase, whereas the Pd-Ag-Cu alloys are hardened by a Pd-Cu ordered phase. As with the casting alloys, the ordered phase imparts significantly more strength and hardness to the alloy and lower elongation.
TABLE 15-8
Properties of Typical Wrought Dental Alloys
*Adapted from Lyman T: Metals Handbook, vol 1, Properties and selection of metals, ed 8, Metals Park, Ohio, 1961, American Society for Metals.
SOLDERS AND SOLDERING OPERATIONS
It is often necessary to construct a dental appliance in two or more parts and then join them together by either a soldering or welding process. The terms soldering, welding, and brazing have specific meanings in industry. The term welding is used if two pieces of metal are joined together directly (generally, but not always, without adding a third metal); that is, the metal pieces are heated to a high enough temperature so they attach to each other. The words soldering and brazing are used if two pieces of metal are joined by adding a third metal. If the temperature used in the process is below 425° C, the operation is soldering; however, if the temperature is above 425° C, the operation is brazing. In dentistry, the parts are joined at temperature above 425° C, so the operation should be called brazing. However, because it is most commonly called soldering, that is term used in this chapter.
TYPES OF SOLDERS
In general, solders may be divided into two major groups: soft and hard. The soft solders include the lead-tin alloys of eutectic type with a low melting point, sometimes known as “plumber’s” solder. The soft solders have several interesting properties, including a low fusion range of about 260° C or less, which permits them to be applied by simple means, such as by a hot soldering iron. Many also possess good working or mechanical properties, making them favorable for use in industry. However, the soft solders lack corrosion resistance, which makes them impractical for dental applications.
Hard solders have a much higher melting temperature than soft solders and possess greater hardness and strength. The high melting range of these solders precludes the use of soldering irons for melting. In industry, special melting methods are used, such as heating with a gas torch, in a furnace, or with other special heating devices.
Two types of hard solders are used in dentistry. Gold-based solders that have good tarnish and corrosion resistance are extensively used in crown and bridge applications. Silver-based solders are commonly used in orthodontic appliances. For dental applications, gold- or silver-based solders are normally melted with a specifically designed dental type of gas blowtorch. Occasionally a method involving an electric furnace or other heating equipment is used, but this is the exception rather than common practice.
Two techniques of dental soldering are used to assemble dental appliances. One is known as free-hand soldering, commonly used in assembling orthodontic and other appliances, and the other is investment soldering, customarily used in assembling bridges and similar restorations. In freehand soldering, the parts to be assembled are manually held in contact while the heat and solder are applied. As soon as the solder has flowed to position, the heating is discontinued and the appliance is cooled. In investment soldering, the parts to be assembled are mounted in a soldering investment (similar to a casting investment) and held in intimate contact by the hardened investment while the heat and solder are applied. These two techniques are described in detail in appropriate textbooks or manuals on orthodontics, crown and bridge prostheses, and operative dentistry.
Basis of Selecting Solders
Certain principles must be observed in selecting solders, regardless of the application. The ideal solder includes qualities such as (1) ease of flow at relatively low temperature, (2) sufficient fluidity to freely flow when melted, (3) strength compatible with that of the structure being soldered, (4) acceptable color to give an inconspicuous joint, (5) resistance to tarnish and corrosion, and (6) resistance to pitting during heating and application. No single, dental, gold-based solder has all of these qualities; therefore manufacturers provide solders that cover a range of fineness and a number of solders that have special qualities. Manufacturers will generally provide detailed information about which solder should be used with each of their alloys. The properties of solders are significantly influenced by the method used during the soldering operation. Thus, a recommended procedure must be faithfully followed to obtain the maximum quality from a product.
Composition
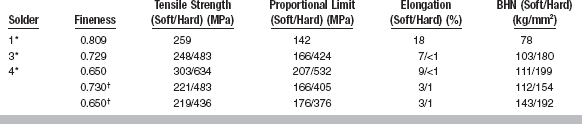
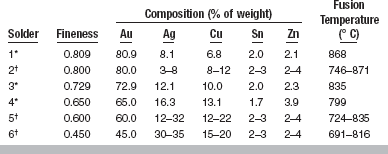
Gold-based solders for dental use are primarily alloys of gold, silver, and copper, with small quantities of tin, zinc, and sometimes phosphorus included to modify the fusion temperature and flow qualities. The typical composition and resulting fusion temperature values of a variety of gold solders are given in Table 15-9. The compositions of different solders vary considerably from one another. For example, the gold content may vary from 45 to 81 wt%, silver from 8 to 30 wt%, and copper from 7 to 20 wt%, with little variation in the tin or zinc content. Most solders have a copper/gold ratio to support the formation of a Au-Cu ordered phase. The fusion temperature is lower for alloys that have reduced gold content, but the reduction is not as great as sometimes believed. For example, the difference in fusion temperature between solders 1 and 4 is only 69° C when the gold content is reduced by approximately 16%.
TABLE 15-9
Typical Compositions and Fusion Temperatures of Dental Gold Solders
*Adapted from Coleman RL: Res Paper No 32, J Res Nat Bur Stand 1:894, 1928.
†Adapted from Lyman T: Metals handbook, vol 1, Properties and selection of metals, ed 8, Metals Park, Ohio, 1961, American Society for Metals.
In the past, solders were commonly referred to by a carat number. The number did not describe the actual carat of the solder, but rather the carat of the gold alloy on which the solder was to be used. This permitted a wide range of compositions, or actual carat values, to be used for solders described as a specific carat, because the solder was intended for use with an alloy of a definite carat. For example, solders that varied in gold content from 58.5 to 65 wt% might be described as 18k because they were to be used on an casting made of an 18k alloy. The gold content of an 18k alloy should be 75 wt% gold, but the solders contained only 58.5 to 65 wt% gold. This system led to much confusion. In recent years, the degree of fineness has been used to describe the various solders, such as those values designated in Tables 15-9 and 15-10. This designation is appropriate because it is specific, and when the fineness is changed, the actual carat change is indicated.
Easy-Flowing and Free-Flowing Qualities
Dental gold-based solders are frequently described by terms such as easy-flowing or free-flowing. Although these terms are sometimes interchanged, they refer to two different qualities in the solder alloys. An easy-flowing solder is one that has a relatively low fusion temperature; the lower the temperature of fusion, the easier to melt and form the joint. However, the difference in fusion temperatures of high- and low-fineness gold solder is approximately 56° C, so differences in flow caused by a lower fusion temperature are small among these solders (see Table 15-9).
The fusion temperature of the solder must be below that of the alloys being soldered, or the joined pieces will melt during the operation. Previous discussion of casting alloys indicated that the lowest solidus values for the casting alloys are for the Au-Cu-Ag-Pd-II and -III alloys and the Au-Ag-Pd-In alloys, ranging from 865° to 875° C (see Table 15-6). For wrought alloys, the lowest solidus values ranged from 875° to 935° C, although most of the wrought alloys have substantially higher solidus temperatures (see Table 15-8). As Table 15-9 indicates, most of the solders have fusion temperatures below the solidus temperatures for the casting or wrought alloys. Thus solders are available for even the lowest fusing alloys. In general, the fusion temperature of the solder should be at least 56° C below that of the parts being joined to prevent distortion.
The free-flowing quality refers specifically to the ability of the solder to spread and flow freely over the surfaces of the parts begin joined. This quality is closely related to the surface tension of the melted solder. The surface tension (and free-flowing qualities) control the capillary action, which causes the melted solder to penetrate into fine openings between the parts being assembled. In general, lower-fineness solders are more fluid in the molten state than higher-fineness solders because of their lower gold content and the presence of small quantities of alloying metals such as zinc and tin. For this reason, the lower-fineness solders are preferred when joining parts because the solder flows promptly and freely into the available spaces. Solders that spread slowly are often described as being sticky, and they resist spreading even when properly heated. As a result, they tend to penetrate or “burn through” the part being soldered if the solder is forced to spread by overheating. Obviously, the easy- and free-flowing qualities work together, because the lower-fineness gold solders have the lowest melting range and greatest freedom of flow.
Mechanical Properties
The values in Table 15-10 illustrate typical mechanical properties for dental solders. There are a wide range of strengths and hardness available. As stated previously, it is most desirable to use a solder with a strength similar to that of the parts being joined. All solders in Table 15-10 are subject to hardening by ordered-phase formation except solder 1. Solder 1 does not form an ordered phase because the gold/copper ratio is not appropriate (see Table 15-9). A considerable increase in strength and hardness can be obtained by slow-cooling the solder and allowing the ordered phase to form.
The percentages of elongation in noble solders are notably less than those of many noble casting alloys (see Tables 15-10 and 15-6). Even in the soft condition, the elongation ranges only from 3% to 9% for all of the solders except solder 1. In the hard condition the elongation is most often 1%, which is quite brittle. In general, it is more desirable in most appliances to obtain an improved hardness and proportional limit that accompany the ordered-phase formation than to sacrifice these properties for a small increase in elongation.
Color and Tarnish Resistance
The color of dental noble solders varies from deep yellow to light yellow and white in much the same manner as casting alloys. Although the ability to match the color of the appliances is an important quality of a solder, producing an inconspicuous solder joint is not difficult in practice. The location of the solder junction on the restoration and the total amount of solder applied are clearly factors that influence color matching.
Because of their gold content, the solders of high fineness are assumed to be more resistant to tarnish, discoloration, and corrosion in the mouth than the lower-fineness solders. However, there is little evidence or data to support such claims, because no good tests are available for tarnish and discoloration of such structures. High-fineness solders are often recommended to prevent tarnish in service. However, the qualities of increased fluidity and mechanical properties associated with the lower fineness (0.650 or less) may outweigh any increased tendency of those solders to tarnish in service. Actually, the lower-fineness solders are used extensively for the assembly of dental appliances without serious tendencies to discolor.
Biocompatibility
Little information exists about the biocompatibility of noble dental solders. However, the same principles govern the biocompatibility of solders as govern alloys (see previous discussion, this chapter). In vitro evidence indicates that the biological properties of solders may be completely different when the solder is tested by itself versus when it is tested in combination with a substrate alloy. Most solders appear to release less mass and have better in vitro biocompatibility when used on an appropriate substrate metal, but a few will be more cytotoxic on the substrate. The character of the substrate alloy also plays a role, as do the surface area of the solder, any pitting present, and other factors. Generally, these results indicate that the biological liabilities of any solder should be assessed with the solder-substrate combination versus the solder by itself.
Pitted Solder Joints
Hard solders have a tendency to be pitted after the soldering operation. In general, pitting results from improper heating of the solder, although some compositions of solder may be more susceptible to pitting than others. When solders of typical fineness are used, a pitted solder can often be associated with either excessive heating during the fusion of the solder or with improper fluxing during heating.
If the solder is heated to too high a temperature or for prolonged periods, the lower-melting point tin and zinc in the solder can boil or oxidize and form pits or porosities as the solder solidifies. These pits often become apparent only during finishing and polishing operations. If the solder is underheated and the flux is applied in excess or improperly melted, it may be trapped in the melted solder and form pits that are uncovered during polishing. To avoid pitting from these causes, the solder should be heated promptly to the fusion temperature, and heating should be stopped as soon as the solder has flowed into position.
MICROSTRUCTURE OF SOLDERED JOINTS
Microscopic examination of well-formed soldered joints has shown that the solder does not combine excessively with the parts being soldered. When the solder has fused properly and has not been overheated, a well-defined boundary forms between the solder and the soldered parts. When the joint is heated too high or for too long, diffusion of elements between the solder and the parts occurs in proportion to the time and temperature excess. Studies indicate that diffusion in the soldered joint reduces the strength and quality of the joint.
Fig. 15-5 shows a microscopic view of a soldered joint between a high-noble casting alloy and a section of a Pt-Au-Pd wire used as a clasp on a removable partial denture. The sample has been etched with acid to reveal its microstructure. The wire has a typical fibrous appearance of a wrought form, and the alloy has a typical granular appearance of a cast form. The solder also has a normal granular appearance, and a sharp boundary exists between the solder and wire and the solder and the cast alloy, indicating that time or temperature of the soldering procedure was not excessive.

FIGURE 15-5 Ideal soldered joint formed between gold-based casting alloy and a gold-based wire. Top, Granular microstructure of the cast alloy. Bottom, fibrous microstructure of the wire. Middle, Granular microstructure of the solder.
A less satisfactory soldered joint is shown in Fig. 15-6. The boundary between the wire and the solder is less sharp and is wider. This poorer adaptation may have resulted from either improper fluxing or improper heating when the solder was applied. The solder is also not ideally adapted to the cast alloy. However, there is no evidence of recrystallization of the wire and the grain size in the alloy is unchanged, indicating that the time and temperature of heating were not excessive. Overheating would also cause diffusion between the alloys. Recrystallization of the wire, grain growth of the casting alloy, and diffusion are all undesirable because they result in a loss of physical properties necessary for the successful function of the dental appliance.

FIGURE 15-6 Solder (middle) well attached to cast alloy (right) but with less satisfactory attachment to the wire (left).
The microstructure of an overheated wire is shown in Fig. 15-7. Some evidence of the original fibrous wire remains, but recrystallization has taken place throughout most of the wire. The section of wire shown in Fig. 15-7 was 2 mm from the soldered joint. More severe recrystallization occurred close to the solder, and the wire broke in service within the area adjacent to the solder. When cast structures are overheated in the presence of solder, the solder will diffuse into the alloy, resulting in a new alloy that lacks strength and ductility. Overheating can also cause warpage and distortion of the appliance from large dimensional changes.

FIGURE 15-7 Evidence of recrystallization resulting from excessive heating of gold wire. Right, Original fibrous microstructure of the wire; bottom and left, granular structure resulting from recrystallization. Heat was applied 2 mm from this point on the wire.
Silver Solder
Hard solders composed of silver-based alloys are used extensively in some industries, but their application in dentistry has been limited. These solders are also commonly known as silver solders. Silver-based solders are used when a low-fusing point solder is needed for soldering onto stainless steel or other base-metal alloys. Orthodontic appliances are commonly soldered using silver-based solders. In general, the resistance of silver solders to tarnish is not as good as gold-based solders, but the strengths of the two types of solders are comparable.
The silver-based solders are composed of silver (10 to 80 wt%), copper (15 to 30 wt%), and zinc (4 to 35 wt%), with some products containing small percentages of cadmium, tin, or phosphorus to further modify the fusion temperature. The formation of the silver-copper eutectic is responsible for the low melting range and higher corrosion rate found in the silver-based solders. The liquidus temperatures for these solders range from 620° to 700° C, which is slightly below those of gold-based solders. This difference is important in the soldering of stainless steel.
Anusavice, KJ. Phillips’ science of dental materials, ed 11. St Louis: Saunders, 2003.
Böning, K, Walter, M. Palladium alloys in prosthodontics: selected aspects. Int Dent J. 1990;40:289.
Cartwright, CB. Gold foil restorations. J Mich Dent Assoc. 1961;43:231.
Corso, PP, German, RM, Simmons, HD. Corrosion evaluation of gold-based dental alloys. J Dent Res. 1985;64:854.
Council on Dental Materials, Instruments, and Equipment. Classification system for cast alloys. J Am Dent Assoc. 1984;109:766.
Council on Dental Materials, Instruments, and Equipment. Revised ANSI/ADA Specification No. 5 for dental casting alloys. J Am Dent Assoc. 1989;118:379.
Craig, RG, Powers, JM, Wataha, JC. Dental materials: properties and manipulations, ed 8. St. Louis: Mosby, 2004.
Federation Dentaire Internationale. Alternative casting alloys for fixed prosthodontics. Int Dent J. 1990;40:54.
German, RM, Wright, DC, Gallant, RF. In vitro tarnish measurement on fixed prosthodontic alloys. J Prosthet Dent. 1982;47:399.
Gettleman, L. Noble alloys in dentistry. Curr Opin Dent. 1991;2:218.
Glantz, PO. Intraoral behaviour and biocompatibility of gold versus non precious alloys. J Biol Buccale. 1984;12:3.
Hodson, JT. Compaction properties of various gold restorative materials. J Am Acad Gold Foil Op. 1969;12:52.
Hollenback, GM, Collard, AW. An evaluation of the physical properties of cohesive gold. J Calif Dent Assoc. 1961;29:280.
Johansson, BI, Lemons, JE, Hao, SQ. Corrosion of dental copper, nickel, and gold alloys in artificial saliva and saline solutions. Dent Mater. 1989;5:324.
Keller, JC, Lautenschlager, EP. Metals and alloys. In: von Recum AF, ed. Handbook of biomaterials evaluation. New York: Macmillan, 1986.
Leinfelder, KF. An evaluation of casting alloys used for restorative procedures. J Am Dent Assoc. 1997;128:37.
Leinfelder, KF, Price, WG, Gurley, WH. Low-gold alloys: a laboratory and clinical evaluation. Quint Dent Technol. 1981;5:483.
Mahan, J, Charbeneau, GT. A study of certain mechanical properties and the density of condensed specimens made from various forms of pure gold. J Am Acad Gold Foil Op. 1965;8:6.
Malhotra, ML. Dental gold casting alloys: a review. Trends Tech Contemp Dent Lab. 1991;8:73.
Malhotra, ML. New generation of palladium-indium-silver dental cast alloys: a review. Trends Tech Contemp Dent Lab. 1992;9:65.
Mezger, PR, Stols, ALH, Vrijhoef, MMA, et al. Metallurgical aspects and corrosion behavior of yellow low-gold alloys. Dent Mater. 1989;5:350.
Moffa, JP. Alternative dental casting alloys. Dent Clin North Am. 1983;27:733.
Morris, HF, Manz, M, Stoffer, W, et al. Casting alloys: the materials and the “clinical effects,”. Adv Dent Res. 1992;6:28.
Nielsen, JP, Tuccillo, JJ. Grain size in cast gold alloys. J Dent Res. 1966;45:964.
O’Brien, WJ. Dental materials and their selection, ed 2. Carol Stream, IL: Quintessence, 1997.
Richter, WA, Cantwell, KR. A study of cohesive gold. J Prosthet Dent. 1965;15:772.
Richter, WA, Mahler, DB. Physical properties vs clinical performance of pure gold restorations. J Prosthet Dent. 1973;29:434.
Sarkar, NK, Fuys, RA, Jr., Stanford, JW. The chloride corrosion of low-gold casting alloys. J Dent Res. 1979;58:568.
Shell, JS, Hollenback, GM. Tensile strength and elongation of pure gold. J Calif Dent Assoc. 1966;34:219.
Stub, JR, Eyer, CS, Sarkar, NK. Heat treatment, microstructure and corrosion of a low-gold casting alloy. J Oral Rehabil. 1986;13:521.
Vermilyea, SG, Cai, Z, Brantley, WA, et al. Metallurgical structure and microhardness of four new palladium-based alloys. J Prosthodont. 1996;5:288.
Wataha, JC. Biocompatibility of dental casting alloys. J Prosthet Dent. 2000;83:223.
Wendt, SL. Nonprecious cast-metal alloys in dentistry. Cur Opin Dent. 1991;1:222.