Migraine and other headaches
Headache has many causes and most headaches are not produced by any structural abnormality or metabolic disturbance (primary headache; see Box 26.1). Tension is by far the most common primary cause, accounting for about two-thirds of cases, while migraine is the second most frequent cause. When headache is present for a prolonged time, or is recurrent, secondary causes may need to be excluded by a full history and examination for associated neurological symptoms and signs.
Migraine is an episodic headache, typically lasting 4–72 h. The diagnostic features of migraine are listed in Box 26.2. These are the only symptoms in the majority of migraineurs. However, in up to one-third the headache is preceded or accompanied by focal neurological symptoms (migraine with aura), usually visual disturbances but occasionally more severe focal neurological episodes such as hemiparesis.
Pathogenesis of migraine
The pathogenesis of migraine is imperfectly understood but involves both neuronal and vascular dysfunction (Fig. 26.1). Headache in people with migraine is associated with sensitisation of the pain pathways in both the meninges (peripheral sensitisation) and the central processing of sensory stimuli in the brainstem (central sensitisation). Genetic predisposition is a factor in migraine, and abnormal function of voltage-dependent ion channels in cortical and brainstem structures may provide the basis for increased excitability in the neuronal circuits that are responsible for the headache.
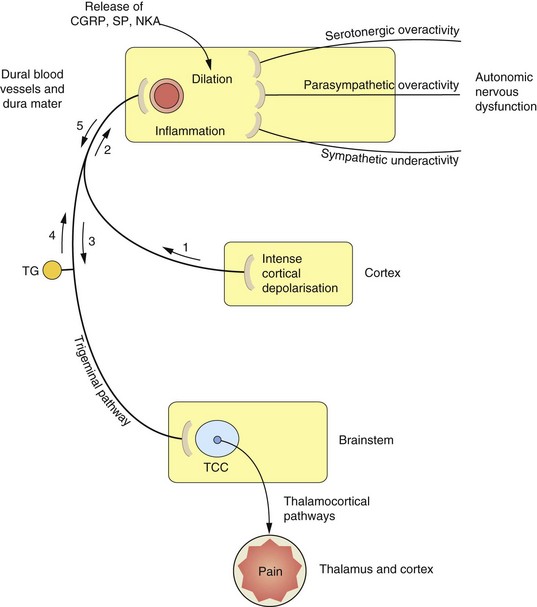
Fig. 26.1 Proposed mechanisms associated with the processes of migraine.
The sequence and nature of neurogenic and vascular involvement in migraine are vigorously debated and may vary in different kinds of migraine-type headache. One scenario is that the trigger is a wave of cortical depression (1) which sensitizes and stimulates the trigeminal pathway in either direction (2, 3). This results in stimulation of the trigeminocervical complex (TCC) (3) in the brainstem, and onward stimulation of the thalamus and other areas can cause pain and nausea. Other reflex pathways from the brainstem via the superior salivatory nucleus to the dural blood vessels, which result in vasodilation, can also be activated at this stage. The stimulation of trigeminal nerves innervating the dural blood vessels (2, 4) results in the release of mediators such as calcitonin gene-related peptide (CGRP), substance P (SP) and neurokinin A (NKA), which cause vasodilation and participate in inflammation. CGRP and possibly other mediators are able to stimulate nociceptors in the trigeminal nerve endings, resulting in further activation of the pathways to the TCC and thalamus (5) and consequently further pain. The control of vascular tone in the dural blood vessels is complex, with sympathetic, parasympathetic and serotonergic systems contributing to the migraine process. Vasoconstrictor innervation of these vessels is by sympathetic nerves and vasodilation occurs by parasympathetic innervation. Serotonergic pathways produce vasoconstriction by stimulation of 5-HT1B receptors and vasodilation via 5-HT2 receptors. TG, trigeminal ganglion.
An aura precedes a migraine attack in 15% of cases with transient visual symptoms (such as flashing or jagged lights) or sensory symptoms. Visual symptoms are probably caused by an initial intense but brief neuronal excitation producing cortical hyperaemia. This is followed by a slowly propagated wave of cortical depolarisation that transiently depresses spontaneous and evoked neuronal activity (cortical spreading depression). The depressant wave is associated with vasoconstriction, which may produce focal neurological symptoms and signs. The majority of migraineurs who do not experience an aura appear to have ‘silent’ cortical spreading depression and this suppression of electrical activity may therefore be the trigger for the migraine headache.
The hallmark of the migraine process is activation of the trigeminal nerve pathway (Fig. 26.1). Increased dopaminergic and glutamatergic neurotransmission, with reduced serotonergic neurotransmission, are all thought to contribute to this activation.
The migraine process involves vasodilation and inflammation of the dural blood vessels and dura mater (neurogenic inflammation). This results from activation of afferents in the trigeminal pathway from the trigeminocervical complex in the brainstem to the dural blood vessels. The triggers that activate the pathway are unknown, but are probably chemical. Activation of the pathway releases vasoactive peptides such as calcitonin gene-related peptide (CGRP) and substance P from the perivascular axons in the dura mater. As a result, the local concentrations of cytokines, serotonin, histamine and nitric oxide increase, producing vasodilation and activating endothelial cells, mast cells and platelets. Mediators released from these cells further contribute to neurogenic inflammation. Sensory nociceptors on the trigeminal nerves in the dura are stimulated, and the nociceptive impulses are relayed from the dura through the trigeminocervical complex in the brainstem, from where they are transmitted to the thalamus. Thalamic stimulation produces pain, nausea and vomiting. Monoaminergic pathways in the trigeminocervical complex modulate the nociceptive pain (Ch. 19) via afferent nerves to intra- and extracranial blood vessels. There are also reflex parasympathetic connections back to the dural vessels that potentiate vasodilation (Fig. 26.1). The trigeminocervical complex is rich in serotonin 5-HT1B/1D receptors, and their stimulation (particularly 5-HT1B receptors) inhibits neurotransmission to the thalamus.
Drugs for migraine
Specific drugs for the acute migraine attack
Triptans
Mechanisms of action and effects: The triptans are serotonin 5-HT1B/1D receptor agonists, which may alter the pathophysiology of migraine (Fig. 26.2) by:
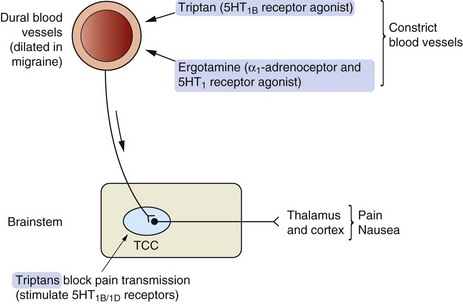
Fig. 26.2 Possible mechanisms of action of 5-HT receptor agonists in migraine.
The trigeminocervical complex (TCC) is rich in 5-HT1B/1D receptors and stimulation of these receptors by so-called second-generation triptans inhibits neurotransmission to the thalamus and cortex. Triptans also cause vasoconstriction acting at 5-HT1B receptors on blood vessels.
 intracranial vasoconstriction (5-HT1B),
intracranial vasoconstriction (5-HT1B),
 inhibition of neurotransmission in the trigeminocervical complex and inhibition of release of pro-inflammatory and vasoactive mediators from perivascular trigeminal neurons (5-HT1B/1D).
inhibition of neurotransmission in the trigeminocervical complex and inhibition of release of pro-inflammatory and vasoactive mediators from perivascular trigeminal neurons (5-HT1B/1D).
Sumatriptan does not easily cross the blood–brain barrier since it is water-soluble, but this barrier may be impaired during a migraine attack. Naratriptan, zolmitriptan and other ‘second-generation’ triptans penetrate the blood–brain barrier more readily. These drugs directly inhibit excitability of the trigeminocervical complex in the brainstem and relieve both the pain and the nausea associated with migraine.
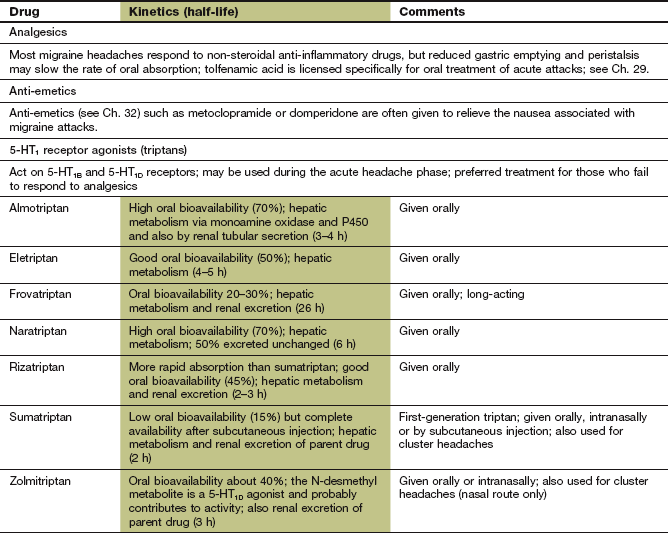
Pharmacokinetics: Absorption of sumatriptan from the gut is rapid but erratic, whereas second-generation triptans such as naratriptan and zolmitriptan have better absorption. Effective plasma concentrations of these drugs are usually reached within 30 min of oral administration. Sumatriptan and zolmitriptan undergo first-pass metabolism by monoamine oxidase A (MAO-A) and have a low oral bioavailability. Subcutaneous injection or administration by nasal spray avoid first-pass metabolism with these drugs and relieves symptoms within 15 min. These routes can be more effective if the headache is accompanied by nausea, because gastric stasis often delays oral drug absorption during a migraine attack. Naratriptan is not a substrate for MAO-A, which gives it high oral bioavailability. Elimination of most triptans is partially by hepatic metabolism and partially by renal excretion. Most triptans have short half-lives of between 2 and 6 h, but the half-life of frovatriptan is longer, at 26 h.
Unwanted effects: The frequency and intensity of unwanted effects is highest after subcutaneous use of sumatriptan:
Ergotamine
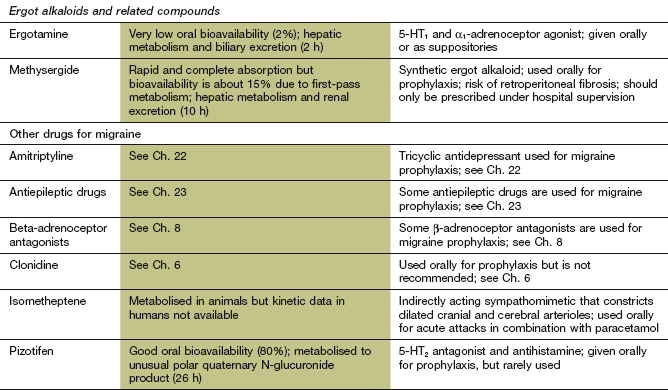
Mechanism of action: Ergotamine probably has an antimigraine action similar to the triptans by stimulating 5-HT1 receptors. Unwanted effects arise from agonist activity at several other receptors, including α1-adrenoceptors and dopamine D2 receptors.
Pharmacokinetics: Oral administration is often accompanied by nausea, and absorption is poor, erratic and delayed. Ergotamine undergoes extensive metabolism in the liver and has a short half-life (2 h). However, tight receptor binding produces a longer duration of action.
 Nausea and vomiting are caused by dopamine receptor stimulation at the chemoreceptor trigger zone (Ch. 32).
Nausea and vomiting are caused by dopamine receptor stimulation at the chemoreceptor trigger zone (Ch. 32).
 Severe vasoconstriction as a result of α1-adrenoceptor stimulation can lead to peripheral gangrene (acute ergotism). Ergotamine should be avoided in people with known atheromatous vascular disease (including ischaemic heart disease).
Severe vasoconstriction as a result of α1-adrenoceptor stimulation can lead to peripheral gangrene (acute ergotism). Ergotamine should be avoided in people with known atheromatous vascular disease (including ischaemic heart disease).
 Chronic intoxication with dependence can occur after prolonged use. Withdrawal then produces nausea and headache similar to an acute migraine attack. For this reason, ergotamine treatment should not be repeated at intervals of less than 4 days.
Chronic intoxication with dependence can occur after prolonged use. Withdrawal then produces nausea and headache similar to an acute migraine attack. For this reason, ergotamine treatment should not be repeated at intervals of less than 4 days.
Prophylactic drugs
In migraine, β-adrenoceptor antagonists without intrinsic sympathomimetic activity, such as propranolol, timolol and metoprolol, have the greatest evidence for efficacy, while drugs with partial agonist activity that cause vasodilation (e.g. pindolol) are ineffective. The antagonist action at β1-adrenoceptors is complemented by 5-HT receptor antagonist activity with some β-adrenoceptor antagonists. Effects on central nervous system function include reduced neuronal firing in noradrenergic neurons in the locus coeruleus and inhibition of cortical potentials evoked by auditory and visual stimuli, suggesting a modulation of neuronal excitability. Full details of β-adrenoceptor antagonists can be found in Chapter 5.
Antiepileptic drugs
The mechanism of action of sodium valproate, gabapentin and topiramate in the prophylaxis of migraine is not well understood. They almost certainly work by multiple mechanisms, including γ-aminobutyric acid (GABA)-mediated suppression of neurotransmission through the trigeminocervical complex in the brainstem, and modulating neuronal excitability through effects on voltage-gated Na+ channels. Blockade of P/Q-type Ca2+ channels may also contribute by reducing glutamate release. Antiepileptic drugs with a predominant single mechanism of action seem to be ineffective in treatment of migraine. Full details of these drugs can be found in Chapter 23.
Amitriptyline
The mechanism of action of amitryptiline in migraine is unknown, but probably relates to multiple neurotransmitter–receptor interactions that modulate the processing of nociceptive impulses. In addition to reducing neuronal reuptake of noradrenaline and serotonin, amitriptyline is also a 5-HT2 receptor antagonist, and may enhance the pain threshold by α2-adrenoceptor activation. Amitriptyline works in migraine at lower doses than those used to treat depression. Several other antidepressants have been studied but, with the possible exception of fluoxetine, they are ineffective. Full details of amitriptyline can be found in Chapter 22.
Management of migraine and other headaches
Withdrawal of possible triggers such as cheese, chocolate, citrus fruits or alcoholic drinks may reduce the frequency of attacks by up to 50%. The combined oral hormonal contraceptive is a potential exacerbating factor, although it can be helpful for prevention of menstrual-related migraine.
Simple analgesia with a non-steroidal anti-inflammatory drug (NSAID) such as aspirin or ibuprofen (Ch. 29) may be sufficient for the relief of a mild acute attack of migraine. Nausea frequently accompanies a migraine attack and delays gastric emptying. If this occurs, absorption of the analgesic will be more rapid if an anti-emetic such as metoclopramide or domperidone (Ch. 32) is given concurrently. If the person is vomiting, rectal or intramuscular analgesia, for example with an NSAID such as diclofenac or naproxen, can be used combined with rectal domperidone. Analgesics are usually more effective when given early after the onset of pain. Opioid analgesics are not recommended as they are short-acting, produce dependence and frequent use can also promote medication-overuse headaches (see below).
If attacks are poorly controlled by simple analgesics or are moderate to severe in intensity, a triptan is usually highly effective. It can relieve pain even if taken more than 4 h after the onset of an attack, but is less effective in those migraineurs who have developed cutaneous allodynia (a form of neuropathic pain; Ch. 19) in the trigeminal nerve distribution in association with the headache. Subcutaneous sumatriptan or intranasal sumatriptan or naratriptan are useful if a rapid response is required or if nausea precludes oral therapy. The response of individuals varies to the different triptans and it is worth changing to an alternative after two unsuccessful attempts at treatment with one drug. Headache recurs in about 40% of those who initially gain relief from a triptan, although the risk of recurrence may be less with frovatriptan, which has a long half-life. A second dose of a triptan can be used for recurrent headache if there was a good response to the first dose. If migraine headaches are prolonged or recur frequently despite treatment with a triptan then the combination of a triptan with an NSAID can be tried.
Ergotamine can be used to treat acute attacks, but is not recommended concurrently with a triptan. The risk of vasospasm and habituation means that ergotamine should be avoided in older people (who may have cardiovascular disease) and in those with frequent attacks. For these reasons, and because of the frequency of other unwanted effects, ergotamine is now infrequently used.
Prophylaxis of migraine
Prophylaxis is usually recommended for people experiencing at least two attacks of migraine each month that significantly interfere with the person's daily routine. Beta-adrenoceptor antagonists are widely held to be the best choice, providing there are no contraindications. The antiepileptic drugs sodium valproate and topiramate are effective second-line alternatives. There is less consistent evidence for the use of gabapentin, which is used as a third-line option. The major disadvantage of these agents is the risk of teratogenicity in women of childbearing age. Amitriptyline may be particularly effective when tension headache coexists with migraine. Methysergide and pizotifen (see the Compendium at the end of this chapter) are less commonly used.
Botulinum toxin type A (Ch. 24), by injection into sites such as the forehead, temporalis, cervical and trapezius muscles may reduce symptoms for up to 3 months. The mechanism in unclear, but inhibition of peripheral sensory neurons may indirectly reduce central neuronal sensitisation. Some evidence suggests that people who describe their headache as ‘being crushed from the outside’ or have ‘eye-popping’ headache (imploding or ocular headaches) respond better than those who describe a build-up of pressure inside the head (exploding headache). This suggests that botulinum toxin works best if there is an extracranial trigger for the pain.
The efficacy of all current prophylactic treatments is limited. Although the response to an individual drug class is unpredictable, only about half of all migraineurs can expect to have a 50% reduction in the frequency of attacks.
Medication-overuse headache
Regular use of opioids or compound analgesic drugs, especially those containing caffeine, to treat any type of headache, or triptans for migraine, can lead to a chronic daily headache known as medication-overuse headache (also called analgesic or rebound headache). It is the third most common cause of headache. The mechanisms underlying this are not well understood, but probably involve downregulation of receptor or enzyme targets of the drugs with consequent sensitisation of the pain pathways. Medication-overuse headache is most common in women aged between 30 and 60 years, and is diagnosed when headache is present for at least 15 days each month for at least 3 months in someone who is taking regular medication to treat headache. The person experiencing the headache is often reluctant to consider that the drugs are the cause, but abrupt withdrawal of the causative treatment is advised to achieve resolution. NSAIDs can be used for withdrawal headaches, and prophylactic treatment for the underlying tension or migrainous headache given prior to withdrawal.
Management of other headaches
Most other primary headaches are managed as for any other form of acute pain, usually using step 1 of the World Health Organization (WHO) analgesic ladder (Ch. 19). Care must be taken to avoid medication-overuse headache. Tension-type headache is usually bilateral and not disabling. Frequent tension headaches may respond to prophylactic treatment with amitriptyline at low dosage. Cluster headache is characterised by severe attacks of unilateral pain in the distribution of the trigeminal nerve that are associated with autonomic features on the side of the headache (such as ptosis, miosis, conjunctival injection, lacrimation and rhinorrhoea). Cluster headache usually responds to a triptan, but if prophylaxis is needed for recurrent episodes, then the calcium channel blocker verapamil (Ch. 5) is the drug of choice.
Suspected secondary headache requires treatment of the precipitating cause. Red flag features include thunderclap headache with peak severity achieved in seconds or minutes (usually primary headache but possible subarachnoid haemorrhage), focal neurological signs or new cognitive disturbance (possible intracranial pathology) and headache worse on standing (possible low-pressure headache due to cerebrospinal fluid leak). Other symptoms suggesting raised intracranial pressure are headache that wakes the person from sleep or worsening of headache with coughing, laughing or straining. Meningitis and temporal arteritis can also present with headache. Headache due to focal intracerebral pathology that is causing raised intracranial pressure is often alleviated by dexamethasone (Ch. 44), which reduces cerebral oedema.
True/false questions
1. Diet plays little part in the precipitation of migraine attacks.
2. Serotonin is released in a migraine attack.
3. Agonists at 5-HT1B/1D receptors are used for the treatment of migraine.
4. Ergotamine is used prophylactically for migraine.
5. Sumatriptan is not useful for acute attacks of migraine as it is slow-acting.
6. Sumatriptan causes chest discomfort in 40% of people as a result of coronary vasoconstriction.
7. Regular use of triptans can cause rebound headache.
8. Pizotifen is used prophylactically and blocks 5-HT2 receptors.
9. Ergotamine is safe to use in ischaemic heart disease.
10. Tricyclic antidepressants and β-adrenoceptor antagonists may be useful in prophylaxis of migraine.
11. Where migraine is associated with vomiting, metoclopramide and paracetamol given together is a useful combination.
12. Pindolol is effective in prophylaxis of migraine.
13. The combined oral contraceptive pill reduces the frequency of migraine attacks in women.
14. Prophylactic treatment for migraine is highly effective.
15. Injections of botulinum toxin may reduce migraine symptoms.
1. False. In some people, stress and dietary items like chocolate, cheese and alcohol may provoke migraine attacks.
2. True. Serotonin, histamine, nitric oxide and other vasoactive mediators contribute to neurogenic inflammation in migraine.
3. True. The triptans are 5-HT1B/1D agonists used in acute migraine.
4. False. Because of unwanted effects, ergotamine should not be used prophylactically, or more than twice a month for acute attacks.
5. False. Oral sumatriptan acts within 30 min, and more quickly when given by subcutaneous or nasal routes of administration.
6. False. Although sumatriptan causes chest discomfort and is contraindicated in people with ischaemic heart disease, chest discomfort in people without ischaemic heart disease is probably caused by oesophageal spasm, not myocardial ischaemia.
7. True. Medication-overuse headache may occur with triptans, opioids and compound analgesics when used regularly for headache.
8. True. Pizotifen is not commonly used but reduces perivascular inflammation, vasodilation and pain by blocking 5-HT2 receptors.
9. False. Ergotamine causes vasoconstriction and should be avoided in people with vascular diseases.
10. True. Beta-adrenoceptor antagonists like propranolol are first-line drugs for migraine prophylaxis.
11. True. Metoclopramide is an anti-emetic and increases gastric emptying, so improving the rate of absorption of paracetamol.
12. False. Beta-adrenoceptor antagonists with partial agonist activity like pindolol are not effective as they may exacerbate vasodilation.
13. False. The combined oral hormonal contraceptive may exacerbate migraine, although it can be helpful in women with menstrual-related migraine.
14. False. The prophylaxis of migraine is effective in only about half of those who are treated.
15. True. Pericranial injections of botulinum toxin type A can reduce the frequency of migraine attacks.
Drug and Therapeutics Bulletin. Management of medication overuse headache. BMJ. 2010;340:968–972.
Fenstermacher, N, Levin, M, Ward, T. Pharmacological prevention of migraine. BMJ. 2011;342:540–543.
Galletti, F, Cupini, LM, Corbelli, P, et al. Pathophysiological basis of migraine prophylaxis. Prog Neurobiol. 2009;89:176–192.
Goadsby, PJ. Recent advances in the diagnosis and management of migraine. BMJ. 2006;332:25–29.
Loder, E. Triptan therapy in migraine. N Engl J Med. 2010;363:63–70.
Nesbitt, AD, Goadsby, PJ. Cluster headache. BMJ. 2012;344:e2407.