Rheumatoid arthritis, other inflammatory arthritides and osteoarthritis
Other types of inflammatory arthritis: the spondyloarthritides
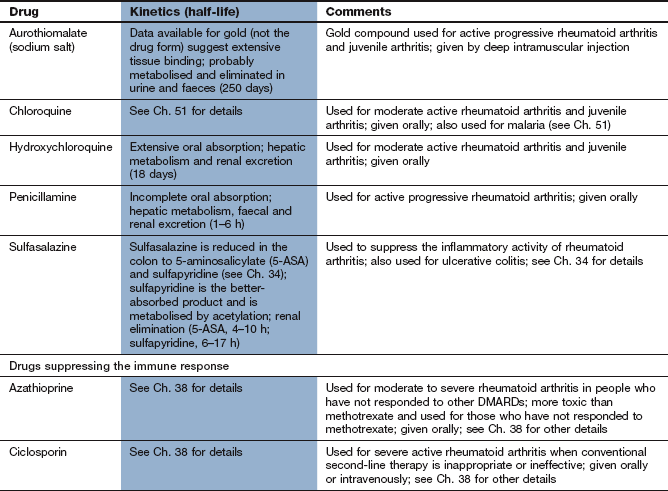
Conventional disease-modifying antirheumatic drugs for rheumatoid arthritis
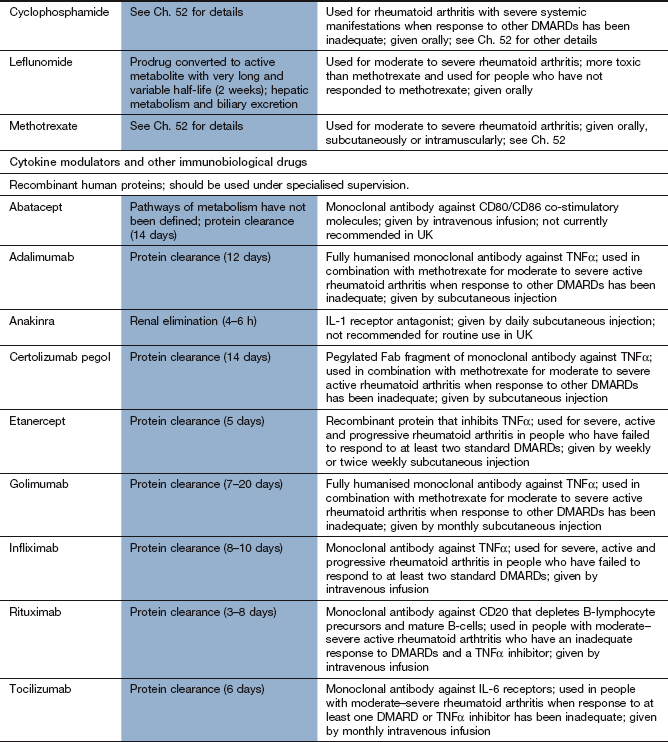
Biological drugs for rheumatoid arthritis
Antibodies against tumour necrosis factor α
Interleukin-1 receptor antagonist
Interleukin-6 receptor antagonist
Management of rheumatoid arthritis
Rheumatoid arthritis
Rheumatoid arthritis is a chronic systemic inflammatory autoimmune condition to which some people are genetically predisposed. The symptoms of rheumatoid arthritis usually appear gradually and most often involve the proximal interphalangeal joints of the fingers, metacarpophalangeal joints and wrists. Inflammation of other joints such as the ankles and hips may be the presenting complaint, or they may become involved later. The affected joints are warm, swollen and painful. Stiffness is troublesome, particularly in the morning, as a result of an increase in extracellular fluid in and around the joint. Systemic disturbance is common, including general fatigue, malaise and weight loss, while extra-articular manifestations such as vasculitis and neuropathy can occur.
Autoimmune processes contribute to the maintenance of the rheumatoid arthritis, but it is uncertain whether it is initiated by an autoimmune reaction or by an exogenous antigen. Two autoantibodies, rheumatoid factor (IgM autoantibodies reactive with IgG) and anti-citrullinated protein antibodies, may be detected, but are absent in about one-third of cases. The initiating antigen is believed to bind to Toll-like receptors (TLRs) – pattern-recognition molecules that bind to both foreign and self structures – on dendritic cells and macrophages, which then initiate a response by the innate immune system (Ch. 38). The primary response is lymphoid cell infiltration of the synovium around the joint, formation of new blood vessels and a proliferation of the synovial membrane. The synovium becomes locally invasive (pannus) and osteoclasts destroy joint cartilage and bone. Apart from psoriatic arthritis, other forms of inflammatory arthritis do not produce erosive changes in periarticular bone or marked joint destruction to the same degree.
The chronic inflammatory process is initiated by T-helper 1 (Th1) lymphocytes that migrate into the joint. Failure of suppression of Th1 cells by regulatory T-cells may be important in the pathogenesis of the disease. Activated T-cells produce a gamut of pro-inflammatory cytokines, including tumour necrosis factor α (TNFα) and interleukin-1 (IL-1). These cytokines stimulate B-lymphocytes, macrophages, fibroblasts, chondrocytes and osteoclasts (Fig. 30.1). B-cells play an important role in the pathology of rheumatoid arthritis. They act as antigen-presenting cells that co-stimulate T-cells, generate inflammatory cytokines such as TNFα and produce rheumatoid factor antibody. Antigen-presenting cells express CD20, CD80 and CD86 surface markers and are involved in the activation of T-cells (Fig. 30.1). TNFα has a prominent role in orchestrating the production of other inflammatory mediators and the recruitment of further immune and inflammatory cells into the joint. This pattern of inflammation differs from most other immune-mediated diseases. The ubiquitous gene transcription factor nuclear factor κB (NF-κB) is also thought to be involved at many steps in cell activation and cytokine production in the destructive cycle of events.
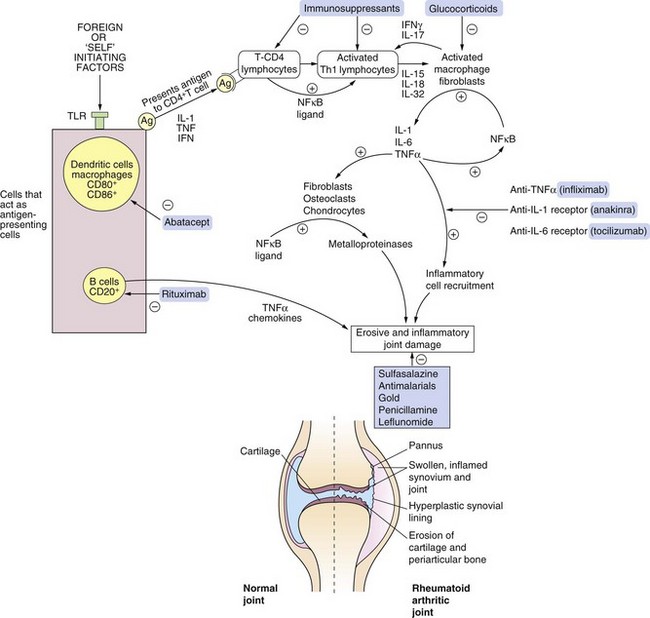
Fig. 30.1 The biology of rheumatoid arthritis and sites of drug action.
The affected synovial joint is characterised by inflamed and swollen synovium with angiogenesis and increased presence of fibroblasts, osteoclasts, plasma cells, mast cells and B-lymphocytes. The synovial fluid contains increased numbers of neutrophil leucocytes and there is erosion of cartilage and adjacent bone. The cascade of self-perpetuating inflammatory events involves many factors, including upregulation of the ubiquitous nuclear transcription factor NF-κB and generation of cytokines including IL-1, IL-6 and TNFα. Some of the drugs shown act at multiple sites. APCs, antigen-presenting cells; IFN, interferon; IL, interleukin; NF-κB, nuclear factor κB; TLR, Toll-like receptor; TNFα, tumour necrosis factor α.
TNFα and IL-1 aid the recruitment of inflammatory cells such as leucocytes by increasing the expression of adhesion molecules (integrins) on vascular endothelial cells. These cytokines also stimulate synovial fibroblasts, osteoclasts and chondrocytes to release tissue-destroying matrix metalloproteinases (MMPs) and to express chemokine receptors. Activated macrophages, lymphocytes and fibroblasts stimulate angiogenesis in the synovium.
Antibodies are produced to the collagen exposed in the damaged cartilage. Rheumatoid factor forms complexes with collagen in damaged cartilage, which then activate the complement pathway. The relevance of this to joint damage is not known. The activated osteoclasts increase bone resorption. The result of this complex inflammatory process is irreversible destruction of cartilage and erosion of periarticular bone.
The plethora of cells that enter the synovium, and the bewildering array of cytokines that are involved, provide a large number of potential targets for disease-modifying antirheumatic drugs affecting the immune system (Fig. 30.1).
Other types of inflammatory arthritis: the spondyloarthritides
There are many types of inflammatory arthritis that have a pattern of joint involvement different from that of rheumatoid arthritis. They involve the sacroiliac joints and can affect small or large joints peripherally. Collectively they are called the spondyloarthritides, and include ankylosing spondylitis, psoriatic arthritis, arthritis associated with inflammatory bowel disease and some juvenile idiopathic arthritis. There is a genetic predisposition to this type of arthritis, varying from a single genetic risk factor such as HLA-B27 in ankylosing spondylitis to polygenic influences in other disorders.
Spondyloarthritides are considered to be autoinflammatory syndromes and probably arise from activation of innate immune processes in response to bacterial or mechanical stress. This distinguishes them from autoimmune conditions that are triggered by activation of the adaptive immune system (Ch. 38). A variety of cytokines appear to be involved in the inflammatory response, including TNFα, and various interleukins such as IL-1, IL-6, IL-17 and IL-23. The inflammation is characteristically associated with enthesopathy (inflammation at the bone insertion of tendons and ligaments) and formation of new endochondral bone.
The presence of different pathophysiological mechanisms in rheumatoid arthritis and the spondyloarthritides explains the different responses to treatments designed to modify disease progression.
Conventional disease-modifying antirheumatic drugs for rheumatoid arthritis
Non-steroidal anti-inflammatory drugs (NSAIDs; Ch. 29) provide symptomatic relief but do not alter the long-term progression of joint destruction in rheumatoid arthritis. A diverse group of compounds can reduce the rate of progression of joint erosion and destruction, leading to improvement both in symptoms and in the clinical and serological markers of rheumatoid arthritis activity. These drugs produce long-term depression of the inflammatory response even though they have little direct anti-inflammatory effect. They all have a slow onset of action, with many producing little improvement until about 3 months after starting treatment. Such drugs are grouped together and known as disease-modifying antirheumatic drugs (DMARDs).
Sulfasalazine
The action of sulfasalazine in arthritis is poorly understood. It is cleaved in the colon by bacterial enzymes to 5-aminosalicylic acid (which is not believed to contribute to the antirheumatic action) and sulfapyridine. Sulfapyridine in the colon may reduce the absorption of antigens that promote joint inflammation. However, sulfasalazine and sulfapyridine are both absorbed and are found at similar concentrations in synovial fluid. Sulfasalazine can suppress several signal transduction pathways involved in the synthesis of pro-inflammatory cytokines, which may contribute to its efficacy.
High doses of sulfasalazine are required for the treatment of rheumatoid arthritis and these often produce gastrointestinal upset. This can be minimised by increasing the dose slowly and by using an enteric-coated formulation. Other problems include reversible oligospermia (therefore sulfasalazine should be avoided in males who wish to have a family) and blood dyscrasias. Sulfasalazine is discussed more fully in Chapter 34.
Antimalarials
Hydroxychloroquine is weakly basic, which permits its uptake and concentration in a non-ionised form within cells. Having entered the lysosomes inside the cell, the acidic environment traps and concentrates the drug in its ionised state. Macrophages depend on acid proteases in their lysosomes for digestion of phagocytosed protein. Hydroxychloroquine slightly increases the pH inside the macrophage lysosomes, which alters the processing of peptide antigens and reduces their subsequent presentation on the cell surface. Thus, the interaction between T-helper cells and antigen-presenting macrophages responsible for joint inflammation is reduced, with a reduction in the inflammatory response. Hydroxychloroquine also reduces the activation of plasmacytoid dendritic cells by blocking Toll-like receptors on their cell membrane.
Retinal toxicity is a potential problem due to selective binding to photoreceptor cells in the macula and subsequent disruption of lysosomal function. It is rare with recommended doses of hydroxychloroquine, but specialist assessment of the eyes is recommended before treatment and again during treatment if there is a change in visual acuity or blurring of vision or if treatment continues for more than five years. The pharmacokinetics and other unwanted effects of hydroxychloroquine can be found in Chapter 51.
Leflunomide
Leflunomide is an isoxazole derivative that inhibits dihydroorotate dehydrogenase, a key mitochondrial enzyme in the de novo synthesis of the pyrimidine ribonucleotide uridine monophosphate (UMP). Activated lymphocytes require an eightfold increase in their pyrimidine pool to proliferate. Inadequate provision of UMP increases the expression of the tumour-suppressor molecule p53 which translocates to the cell nucleus and arrests the cell cycle in the G1 phase. This cytostatic action reduces the expansion of the activated autoimmune T- and B-lymphocyte pool, thereby suppressing immunoglobulin production and cellular immune processes. Other dividing cells can obtain adequate pyrimidines from a separate salvage pathway that reuses existing ribonucleotides and is not affected by leflunomide.
There are other potential mechanisms of immunomodulation by leflunomide, such as inhibition of tyrosine kinases and suppression of transcription factors that stimulate osteoclast formation, but they are probably of lesser importance.
Pharmacokinetics
Leflunomide is a prodrug. It is well absorbed from the gut and is converted non-enzymatically, mainly in the intestinal mucosa and plasma, to its active metabolite. The metabolite is excreted via the bile, and enterohepatic circulation contributes to its very long plasma half-life (15 days).
Unwanted effects
 Gastrointestinal upset, especially diarrhoea.
Gastrointestinal upset, especially diarrhoea.
 Headache, dizziness, lethargy.
Headache, dizziness, lethargy.
 Leucopenia, anaemia or thrombocytopenia.
Leucopenia, anaemia or thrombocytopenia.
 Hepatotoxicity, especially in the first 6 months.
Hepatotoxicity, especially in the first 6 months.
 Teratogenicity: it is advised that conception should be avoided for two years after stopping treatment in women and for three months in men.
Teratogenicity: it is advised that conception should be avoided for two years after stopping treatment in women and for three months in men.
Prevention and management of unwanted effects
Monitoring of full blood count and liver function should be carried out regularly during treatment. If serious unwanted effects occur, elimination of the drug can be increased by the use of colestyramine (Ch. 48) or activated charcoal to bind the active metabolite present in the gut after biliary excretion, thereby interrupting its enterohepatic circulation (Ch. 2).
Immunosuppressant drugs
Several drugs with immunosuppressant actions have been shown to be effective in rheumatoid arthritis. These include:
Methotrexate is one of the most effective antirheumatic drugs. Although its primary mechanism of action is by folate antagonism, co-administration of folic acid supplements prevents much of the mucosal and gastrointestinal toxicity of the drug but does not reduce its immunomodulatory effect. A possible additional mechanism of action to explain the effect of methotrexate in arthritis is inhibition of the deamination of adenosine, causing its accumulation. Adenosine is an intermediate in purine biosynthesis and a potent anti-inflammatory mediator. It suppresses neutrophil adhesion and cytokine production, reduces macrophage function and impairs the expression of endothelial adhesion molecules. Methotrexate is usually given orally once a week for the treatment of inflammatory arthritis. It can be given intramuscularly if oral use produces intractable gastrointestinal symptoms or if absorption by the oral route is inadequate.
Gold
Mechanism of action
The precise mechanism by which gold compounds act is unknown. A popular concept is that the compound is taken up by mononuclear cells and inhibits their phagocytic function. This will reduce the release of inflammatory mediators and inhibit inflammatory cell proliferation. Production of inflammatory cytokines such as IL-1, IL-6 and TNFα is inhibited, and superoxide generation by neutrophils is reduced. There is also evidence for inhibition of other cell signalling pathways involved in inflammation, including NF-κB.
The advent of more effective and less toxic drugs has reduced the use of gold salts in current clinical practice.
Pharmacokinetics
Sodium aurothiomalate is given by deep intramuscular injection. An initial test dose is given to screen for acute toxicity (see below), followed by injections at weekly intervals to gradually achieve a therapeutic concentration in the tissues. Subsequently, a smaller dose is used to maintain remission. Gold binds readily to albumin and several tissue proteins and accumulates in many tissues such as the liver, kidney, bone marrow, lymph nodes and spleen. Accumulation also occurs in the synovium of inflamed joints. Elimination is largely renal. Gold has a half-life of several weeks, probably as a result of its extensive tissue binding.
Unwanted effects
The unwanted effects can be serious and all but the most minor effects should lead to immediate cessation of treatment:
Prevention and management of unwanted effects
Urine should be checked for protein and a full blood count obtained before each injection of gold, and regularly during oral therapy. Major complications may require treatment with dimercaprol or penicillamine to chelate the gold (Ch. 53) and increase its elimination. Corticosteroids can be helpful to treat blood dyscrasias. Gold should not be used if there is a history of renal or hepatic disease, blood dyscrasias or severe rashes. Gold should be stopped if stomatitis, a pruritic rash, neutropenia, thrombocytopenia or significant proteinuria (>1 g in 24 h) develops.
Penicillamine
The mechanisms of action of penicillamine are uncertain. Modulation of the immune system is believed to be important, including a reduction in the number of activated lymphocytes, reduced synthesis of immunoglobulins and stabilisation of lysosomal membranes in inflammatory cells. Penicillamine has not been shown to slow the progression of joint erosions and is no longer widely used for rheumatoid arthritis.
Penicillamine is a thiol compound that can chelate many metals. This is probably of little relevance to its use in arthritis but has given the drug a role in the management of poisoning (Ch. 53) and in Wilson's disease, a genetically determined illness that is associated with copper overload.
Pharmacokinetics
Penicillamine is well absorbed from the gut, although oral iron supplements substantially reduce its absorption.
Unwanted effects
Unwanted effects occur frequently and are responsible for cessation of treatment in about 30% of people. They can be reduced by slow increases in dose. Many unwanted effects resemble those of gold:
 nausea, vomiting, abdominal discomfort and rashes (often with fever), especially early in treatment,
nausea, vomiting, abdominal discomfort and rashes (often with fever), especially early in treatment,
 loss of taste is common, but may resolve despite continued treatment,
loss of taste is common, but may resolve despite continued treatment,
 proteinuria, which is caused by immune-complex glomerulonephritis and is dose-related; nephrotic syndrome can occur,
proteinuria, which is caused by immune-complex glomerulonephritis and is dose-related; nephrotic syndrome can occur,
 blood disorders, especially thrombocytopenia, but also neutropenia and, rarely, aplastic anaemia.
blood disorders, especially thrombocytopenia, but also neutropenia and, rarely, aplastic anaemia.
Regular monitoring of urine, protein and blood counts should be carried out during treatment.
Biological drugs for rheumatoid arthritis
Antibodies against tumour necrosis factor α
Mechanism of action
TNFα stimulates several inflammatory processes (see above and Fig. 30.1). It acts by binding to one of two cell surface receptors, type 1 (p55) and type 2 (p75), which are found in several tissues. There are several antibody derivatives available that block the action of TNFα.
 Adalimumab and golimumab are fully humanised monoclonal antibodies specific for TNFα.
Adalimumab and golimumab are fully humanised monoclonal antibodies specific for TNFα.
 Certolizumab pegol is a pegylated Fab fragment of a humanized monoclonal antibody for TNFα.
Certolizumab pegol is a pegylated Fab fragment of a humanized monoclonal antibody for TNFα.
 Etanercept is a fusion protein consisting of two recombinant soluble extracellular portions of the human Type 2 (p75) TNF receptor, fused to the constant (Fc) domain of human immunoglobulin (IgG1). It binds to TNFα and the cytokine lymphotoxin α (also known as TNFβ).
Etanercept is a fusion protein consisting of two recombinant soluble extracellular portions of the human Type 2 (p75) TNF receptor, fused to the constant (Fc) domain of human immunoglobulin (IgG1). It binds to TNFα and the cytokine lymphotoxin α (also known as TNFβ).
 Infliximab is a chimaeric monoclonal antibody comprising the variable region of a murine antibody, which neutralises TNFα, spliced to the constant region of a human antibody.
Infliximab is a chimaeric monoclonal antibody comprising the variable region of a murine antibody, which neutralises TNFα, spliced to the constant region of a human antibody.
Pharmacokinetics
Adalimumab, certolizumab pegol, etanercept and golimumab are given by subcutaneous injection and infliximab by intravenous infusion. The mechanism of elimination of these recombinant compounds is poorly defined but likely to be by proteolysis. They have very long half-lives between 5 and 20 days, enabling dosing at frequencies ranging from twice weekly to monthly.
Unwanted effects
 All these biological drugs can produce gastrointestinal upset, decompensation of heart failure, hypersensitivity reactions, injection-site reactions, fever, headache and depression. Blood disorders, including anaemia, leucopenia and thrombocytopenia, also occur.
All these biological drugs can produce gastrointestinal upset, decompensation of heart failure, hypersensitivity reactions, injection-site reactions, fever, headache and depression. Blood disorders, including anaemia, leucopenia and thrombocytopenia, also occur.
 Increased risk of pulmonary tuberculosis: screening for evidence of tuberculosis is recommended before initiation of therapy. Septicaemia and reactivation of hepatitis B virus occur more frequently.
Increased risk of pulmonary tuberculosis: screening for evidence of tuberculosis is recommended before initiation of therapy. Septicaemia and reactivation of hepatitis B virus occur more frequently.
Interleukin-1 receptor antagonist
Mechanism of action
Anakinra is a recombinant human IL-1 receptor antagonist (Fig. 30.1). IL-1 is actually a family of three cytokines, comprising two IL-1 receptor antagonists (IL-1α and IL-1β) and an IL-1 receptor antagonist. The theoretical basis for the use of anakinra is that joint destruction arises from an imbalance between the agonists and the antagonist. IL-1 agonists are pro-inflammatory cytokines released by macrophages and fibroblasts in inflamed synovium, and by neutrophils in synovial fluid. The IL-1 peptides compete for occupancy of the IL-1 receptor on the membrane of synovial cells, and as little as 2–3% occupancy by the agonists produces maximal pro-inflammatory cell activation. Anakinra blocks the receptors and suppresses the inflammatory response. In the UK anakinra is not currently recommended for routine use in rheumatoid arthritis.
Interleukin-6 receptor antagonist
Mechanism of action
Tocilizumab is a recombinant humanized monoclonal antibody that acts as a competitive antagonist at the IL-6 receptor. IL-6 is a pro-inflammatory cytokine produced by a variety of cell types including T- and B-lymphocytes, monocytes and fibroblasts. IL-6 is involved in T-cell activation, immunoglobulin secretion and stimulation of hematopoietic precursor cell proliferation and differentiation. IL-6 is also produced by synovial and endothelial cells leading to local production of IL-6 in joints affected by inflammatory processes such as rheumatoid arthritis.
T-cell co-stimulation modulator
Mechanism of action and uses
T-lymphocyte activation requires recognition of a specific antigen carried by an antigen-presenting cell, and a second co-stimulatory signal. A major co-stimulatory signal involves binding of CD80 and CD86 molecules on the surface of antigen-presenting cells to the CD28 receptor on T-cells. Abatacept is a monoclonal antibody that selectively binds to CD80 and CD86 and blocks the co-stimulatory signal. It therefore reduces the subsequent production of inflammatory mediators and pro-inflammatory cytokines. Abatacept is used for people who have failed to respond to, or are intolerant of, a TNFα inhibitor, but is not currently recommended in the UK. Another co-stimulation inhibitor, belatacept, is used as an immunosuppressant in renal transplantation (Ch. 38).
Anti-CD20 B-cell depleter
Mechanism of action and uses
Rituximab specifically depletes CD20+ B-lymphocytes by binding to the CD20 antigen expressed on the cell surface (Fig. 30.1). The depletion of mature and differentiating B-cells will reduce antigen presentation, stimulation of T-lymphocytes, cytokine production and production of autoantibodies. Responses usually last for up to six months, and relapse corresponds with B-cell repopulation.
Pharmacokinetics
Rituximab is given by intravenous infusion. Its metabolism is unknown, and it has a very long half-life of about 3–8 days.
Unwanted effects
 Cytokine release syndrome with fever, chills, nausea, vomiting and allergic reactions occurs in about one-third of people with the first infusion. Premedication with an anti-histamine and sometimes a corticosteroid will reduce these reactions.
Cytokine release syndrome with fever, chills, nausea, vomiting and allergic reactions occurs in about one-third of people with the first infusion. Premedication with an anti-histamine and sometimes a corticosteroid will reduce these reactions.
 Exacerbation of angina, arrhythmia or heart failure can occur in people with cardiovascular disease.
Exacerbation of angina, arrhythmia or heart failure can occur in people with cardiovascular disease.
Management of rheumatoid arthritis
Progressive joint damage is common in rheumatoid arthritis. There is now a substantial body of evidence that early use of DMARDs (within three months of the onset of symptoms) leads to a better long-term outcome.
DMARDs do not have significant anti-inflammatory action and require 2–3 months before an effect is established. Therefore, they are almost always used initially in combination with a corticosteroid or an NSAID. Methotrexate, given with folic acid supplementation, is the first-choice DMARD. It is usually taken orally, but parenteral use can be considered if gastrointestinal tolerability is a problem. A combination of two DMARDs is now recommended as standard first-line therapy and is more effective than a single drug. Sulfasalazine, leflunomide or hydroxychloroquine are most frequently used in combination with methotrexate. Immunosuppressant drugs such as ciclosporin or azathioprine are generally reserved as third-line agents. Gold and penicillamine are less widely used due to toxicity and, in the case of penicillamine, limited efficacy. If disease activity cannot be suppressed by two DMARDs then a combination of three DMARDs (such as methotrexate, sulfasalazine and hydroxychloroquine) can be tried. Cyclophosphamide is useful for the management of extra-articular manifestations of rheumatoid disease, such as vasculitis, pericarditis or pleurisy.
Intra-articular injections of corticosteroid are used for individual inflamed joints (especially knee and shoulder). Pulsed intramuscular corticosteroid therapy can be given for disease flares, or to ameliorate symptoms in the first few weeks after initiating DMARD therapy (because of the slow response). In early rheumatoid disease a low dose of corticosteroid, given for six months in combination with DMARDs, retards bone destruction and slows disease progression. Intramuscular methylprednisone is often used and is preferred to oral prednisolone, which can be difficult to withdraw (Ch. 44).
Anti-TNFα drugs such as etanercept (the most frequently used biological agents for rheumatoid arthritis) are usually reserved for those who fail to respond to combination therapy with DMARDs, or who are intolerant of several DMARDs. They are typically used in combination with methotrexate, or leflunomide if methotrexate is not tolerated, which produces a better response than the biological agent given alone. The combination produces remission and halts disease progression in up to 60% of people who are treated. If one anti-TNFα drug is ineffective or poorly tolerated, then a different drug from the same class should be tried. Abatacept, tocilizumab and rituximab have been shown to be effective for people with rheumatoid arthritis who fail to respond to, or who cannot tolerate, TNFα blockers. Anakinra is less effective than anti-TNFα drugs and is currently not recommended in the UK.
NSAIDs (Ch. 29) are useful for symptomatic treatment of all types of inflammatory arthritis since they reduce both pain and stiffness. However, they do not affect the long-term course of the disease, and DMARDs have superseded them as the mainstay of treatment of rheumatoid arthritis. There is considerable variation in responses to different NSAIDs, and there is no way of predicting effectiveness in an individual. Propionic acid derivatives, such as ibuprofen, are often used first. They have a weaker anti-inflammatory activity than other classes of NSAID, but generally have fewer unwanted effects. More powerful drugs such as naproxen can be used when ibuprofen fails to control symptoms, although the increased risk of gastrointestinal irritation and cardiovascular events limit their use, especially in the elderly. Morning stiffness is often disabling in inflammatory arthritis. This is helped by giving a late-evening dose of an NSAID with a long half-life, a modified-release formulation of a compound with a short half-life or an NSAID suppository. Topical NSAIDs applied over the affected joint(s) are not usually recommended. Selective cyclo-oxygenase (COX)-2 inhibitors are usually reserved for people who are intolerant of NSAIDs, or who have a higher risk of serious gastrointestinal complications with an NSAID (Ch. 29).
Physical aids such as splinting and bed rest can help acute flares of joint inflammation. There is an increased risk of cardiovascular disease in people with rheumatoid arthritis, and attention to the conventional risk factors for prevention of atherosclerosis is important (Ch. 5).
Management of spondyloarthritides
NSAIDs remain the first-line treatment for this group of disorders, and will relieve pain and improve function. This should be combined with a tailored exercise regimen. Although most evidence for the use of DMARDs has been obtained in the treatment of rheumatoid arthritis, there is limited evidence for their efficacy in the seronegative spondyloarthritides. Sulfasalazine and methotrexate give some benefit for peripheral joint disease in these forms of inflammatory arthritis, but do not improve axial joint inflammation or enthesitis. Anti-TNFα agents can produce remission of disease in ankylosing spondylitis and psoriatic arthritis in about one-third of those treated, with considerable symptomatic benefit in others. Unlike DMARDs, these agents can help most aspects of the disease, with the exception of new bone formation. If treatment with an anti-TNFα agent is stopped after remission is achieved then relapse usually occurs within 6–12 months. The efficacy of biological agents that block other cytokines is unproven, and T- or B-lymphocyte targeted therapies are ineffective.
Osteoarthritis
Osteoarthritis is the clinical manifestation of joint degeneration that results from loss of articular cartilage and becomes more common with increasing age. Most osteoarthritis is idiopathic (when it can be localised or generalised) but a small proportion is secondary to other conditions such as joint injury or chondrocalcinosis. The cardinal symptom of osteoarthritis is pain in the affected joint during physical activity, which is relieved by rest. Pain also occurs at rest with advanced disease. Stiffness may be troublesome for a few minutes after rest. Various small joints can be involved, particularly the distal interphalangeal joints of the fingers and the carpometacarpal joint of the thumb. Large joints such as the knee, hip, elbow and shoulder are often asymmetrically affected.
The integrity of articular cartilage depends on the balance of synthetic and catabolic activity of the chondrocytes embedded in the cartilage matrix. Mechanical compression of cartilage produces many physical and biochemical stimuli that influence chondrocyte metabolism. Mechanical overload, the principal cause of secondary osteoarthritis, produces changes that promote matrix destruction and apoptotic chondrocyte death. Osteoarthritis is caused by an imbalance of cartilage degradation compared to synthesis of cartilage, with loss of the normal cartilage matrix. Synovial inflammation results from release of cartilage debris into the joint accompanied by catabolic mediators and results in joint swelling.
Synthesis of cartilage is promoted by the expression of growth factors by chondrocytes, particularly insulin-like growth factor 1 and transforming growth factor β (TGFβ). Degradation of cartilage proteins is carried out by matrix metalloproteinases (MMPs), particularly stromelysin 1 (MMP-3) in early osteoarthritis and gelatinase A (MMP-2) and MMP-13 in late disease. MMPs are synthesised by chondrocytes in response to stimulation by the pro-inflammatory cytokines IL-1β and TNFα released by inflammatory cells. Synovial inflammation often occurs adjacent to the damaged cartilage. Chondrocytes produce a chemokine, RANTES (regulated upon activation, normal T-cell expressed and secreted), and synovial fluid cells express chemokine receptors including CXC chemokine receptor type 4 (CXCR4), which may have a role in recruiting inflammatory cells to the joint.
Loss of matrix leads to disruption of the cartilage, with swelling and fissuring of the surface. Subchondral bone becomes increasingly vascular and new bone is laid down. It is uncertain whether the initiating factors for osteoarthritis originate in the articular cartilage or subchondral bone. However, recent evidence suggests that stiffening of subchondral bone, with less effective shock absorption, may be the trigger for cartilage loss.
Management of osteoarthritis
Treatment of osteoarthritis currently remains symptomatic. Non-pharmacological therapy such as weight loss, exercise, physical therapy and orthotics is often useful. Glucosamine sulphate or chondroitin sulphate supplements (over-the-counter preparations in the UK) are often used by people with osteoarthritis, but there is conflicting evidence of benefit. If pain is troublesome, simple analgesics such as paracetamol should usually be considered as first-line treatment. NSAIDs may be helpful for inflammatory episodes or if paracetamol is ineffective. In vitro studies suggest that some NSAIDs may accelerate the loss of articular cartilage in osteoarthritis. Clinical studies are inconclusive, but avoidance of powerful NSAIDs is probably desirable. The risks of gastrointestinal and cardiac toxicity may also limit the value of NSAIDs.
Intra-articular or periarticular injection of a corticosteroid (Ch. 44) can provide short-term symptomatic relief in osteoarthritis, even if there is little clinical evidence of joint inflammation. Corticosteroids inhibit pro-inflammatory mediators in synovial tissue, such as IL-1 and TNFα. Joint injection with hyaluronic acid remains a controversial treatment, with conflicting evidence of efficacy from clinical trials.
The recognition that osteoarthritis involves inflammatory flares and that cartilage repair can occur has opened up potential new approaches to management. However, there is no proven disease-modifying therapy currently available for the treatment of osteoarthritis. Long-term management of osteoarthritis may eventually require surgical joint replacement.
True/false questions
1. The action of most disease-modifying antirheumatic drugs (DMARDs) is slow in onset.
2. Non-steroidal anti-inflammatory drugs (NSAIDs) reduce the symptoms of rheumatoid disease and retard its progress.
3. If penicillamine does not lead to clinical benefit within six months, it should be stopped.
4. Intramuscular gold can cause proteinuria.
5. Methotrexate has a rapid onset of action (4–6 weeks).
6. During methotrexate therapy, folic acid is contraindicated.
7. Methotrexate has relatively few unwanted effects compared with other DMARDs.
8. The active component of sulfasalazine in rheumatoid disease is 5-aminosalicylic acid.
9. Combination therapy with DMARDs should not be used in rheumatoid arthritis.
10. Leflunomide selectively inhibits pyrimidine synthesis in lymphocytes.
11. Intra-articular injections of corticosteroids slow the progression of erosions.
12. Prolonged treatment with high doses of corticosteroids can cause adrenal atrophy.
13. The antimalarial hydroxychloroquine is of little benefit in rheumatoid arthritis.
14. Rituximab depletes B-cells.
15. Adalimumab and golimumab are fully humanised anti-tumour necrosis factor α (TNFα) monoclonal antibodies.
16. Abatacept blocks antigen presentation to T-lymphocytes
17. Anakinra and tocilizumab activate cytokine receptors.
18. DMARDs are effective treatments for ankylosing spondylitis.
Case-based questions
A 30-year-old woman had developed painful wrists gradually over four weeks; she had not experienced similar episodes of pain before. On examination, both wrists and the metacarpophalangeal joints of both hands were tender but not deformed.
A What course of treatment would you suggest?
There was some initial symptomatic improvement, but subsequently the pain, stiffness and swelling of the hands persisted and eight weeks later both knees became similarly affected. She saw a rheumatologist, who confirmed that she was suffering from rheumatoid arthritis and altered her treatment.
B What treatment option would now be appropriate?
She was commenced on treatment that required folic acid supplements to counteract folate depletion.
1. True. Most DMARDs take several weeks to show a clinical improvement.
2. False. NSAIDs do not slow disease progression; indeed, some evidence suggests they may hasten progress of the disease.
3. True. The second-line drugs take a long time to act (4–6 months) but they should be discontinued if there is no sign of improvement by that time.
4. True. Proteinuria occurs associated with immune-complex nephritis. Only 15% of people continue with gold treatment after 5–6 years because of unwanted effects.
5. True. Methotrexate is an immunosuppressant often chosen as initial disease-modifying therapy for rheumatoid arthritis because of its rapid onset of action.
6. False. Methotrexate inhibits reduction of folic acid to dihydrofolate and tetrahydrofolate, which are essential for nucleotide synthesis. Folic acid can be given daily to prevent gastrointestinal and haematological complications of methotrexate.
7. True. More than 50% of people who take methotrexate for rheumatoid arthritis continue taking the drug for five years or more, but a similar proportion have to cease treatment with most other DMARDs within two years.
8. True. Sulfasalazine is converted in the colon to 5-aminosalicylic acid, which is the active moiety in the treatment of inflammatory bowel disease, and to sulfapyridine, which is probably the main active moiety in rheumatoid arthritis.
9. False. The combination of methotrexate with ciclosporin, sulfasalazine or hydroxychloroquine has shown significant benefit in people with severe rheumatoid disease.
10. True. The active metabolite of leflunomide inhibits synthesis of uridine monophosphate and this slows the proliferation of T- and B-lymphocytes.
11. False. Corticosteroids can give dramatic relief of symptoms in rheumatoid arthritis, but there is no evidence they slow progression of the disease.
12. True. Adrenal atrophy caused by negative feedback on the hypothalamo–pituitary–adrenal axis can last for many months following prolonged treatment.
13. False. Hydroxychloroquine can cause remission of rheumatoid arthritis but does not slow the progression of joint damage.
14. True. Rituximab depletes B-lymphocytes by binding to their CD20 surface antigen.
15. True. Adalimumab, golimumab and other TNFα inhibitors are the most commonly used biological agents for moderate–severe rheumatoid arthritis, usually in combination with methotrexate or other DMARDs.
16. True. Abatacept blocks the CD80 and CD86 co-stimulatory molecules on antigen-presenting cells, preventing them interacting with CD28 on T-lymphocytes.
17. False. They are cytokine receptor antagonists; anakinra is an interleukin (IL)-1 receptor antagonist and tocilizumab blocks IL-6 receptors.
18. False. There is limited evidence for efficacy of DMARDs in the spondyloarthritides; TNFα inhibitors may be effective in inducing remission.
Case-based answers
A The brief duration of the symptoms and their mild nature warrant the initial administration of an NSAID, such as ibuprofen, and follow-up.
B The persistence of the symptoms and their spread to the knees suggest that a DMARD should be started. Guidelines now advise that DMARDs should be considered for persistent inflammatory joint disease of more than 8 weeks' duration.
C Methotrexate is a DMARD that requires folate supplements (see answer to question 6, above). Methotrexate takes 4–6 weeks for its onset of action. Methotrexate and an NSAID (or a corticosteroid) should be given together to cover this interim period.
Rheumatoid arthritis and other inflammatory arthritides
Braun, J, Sieper, J. Ankylosing spondylitis. N Engl J Med. 2007;369:1379–1390.
Brockbank, J, Gladman, D. Diagnosis and management of psoriatic arthritis. Drugs. 2002;62:2447–2457.
Donahue, KE, Gartlehner, G, Jonas, DE, et al. Systematic review: comparative effectiveness and harms of disease-modifying medications for rheumatoid arthritis. Ann Intern Med. 2008;148:162–163.
Dougados, M, Baeten, D. Spondyloarthritis. Lancet. 2011;377:2127–2137.
Emery, P. Treatment of rheumatoid arthritis. BMJ. 2006;332:152–155.
Goldblatt, F, Isenberg, DA. Anti-CD20 monoclonal antibody in rheumatoid arthritis and systemic lupus erythematosus. Handb Exp Pharmacol. 2008;2008:163–181.
Katz, WA. Use of nonopioid analgesics and adjunctive agents in the management of pain in rheumatic diseases. Curr Opin Rheumatol. 2002;14:63–71.
Klarenbeek, NB, Kerstens, PJSM, Huizinga, TWJ, et al. Recent advances in the management of rheumatoid arthritis. BMJ. 2010;341:c6942.
Klareskog, L, Catrina, AI, Paget, S. Rheumatoid arthritis. Lancet. 2009;373:659–672.
Olsen, NJ, Stein, CM. New drugs for rheumatoid arthritis. N Engl J Med. 2004;350:2167–2179.
Scott, DL, Kingsley, GH. Tumour necrosis factor inhibitors for rheumatoid arthritis. N Engl J Med. 2006;355:704–712.
Scott, DL, Wolfe, F, Huizinga, TW. Rheumatoid arthritis. Lancet. 2010;376:1094–1108.
Smolen, JS, Aletaha, D, Weisman, MH, et al. New therapies for the treatment of rheumatoid arthritis. Lancet. 2007;370:1861–1874.
Bijlsma, JW, Berenbaum, F, Lafeber, FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377:2115–2126.
Dieppe, P, Brandt, KD. What is important in treating osteoarthritis? Whom should we treat and how should we treat them? Rheum Dis Clin North Am. 2003;29:687–716.
Felson, DT. Osteoarthritis of the knee. N Engl J Med. 2006;354:841–848.
Hunter, DJ, Felson, DT. Osteoarthritis. BMJ. 2006;332:639–642.
Sharma, S. Nonpharmacological management of osteoarthritis. Curr Opin Rheumatol. 2002;14:603–607.