Inflammation and Repair
After studying this chapter, the student will be able to:
1 Define each of the words in the vocabulary list for this chapter.
2 List the five classic signs of inflammation that occur locally at the site of inflammation.
3 List and describe three major systemic signs of inflammation.
4 List and describe the microscopic events of the inflammatory process.
5 Describe the microscopic events associated with each of the local signs of inflammation.
6 List the two types of white blood cells that are involved in acute inflammation and describe how each is involved.
7 Describe the differences between acute and chronic inflammation.
8 Define and contrast hyperplasia, hypertrophy, and atrophy.
9 Describe the microscopic events that occur during the repair of a mucosal wound.
10 Describe and contrast healing by primary intention, healing by secondary intention, and healing by tertiary intention.
11 List local and systemic factors that can impair healing.
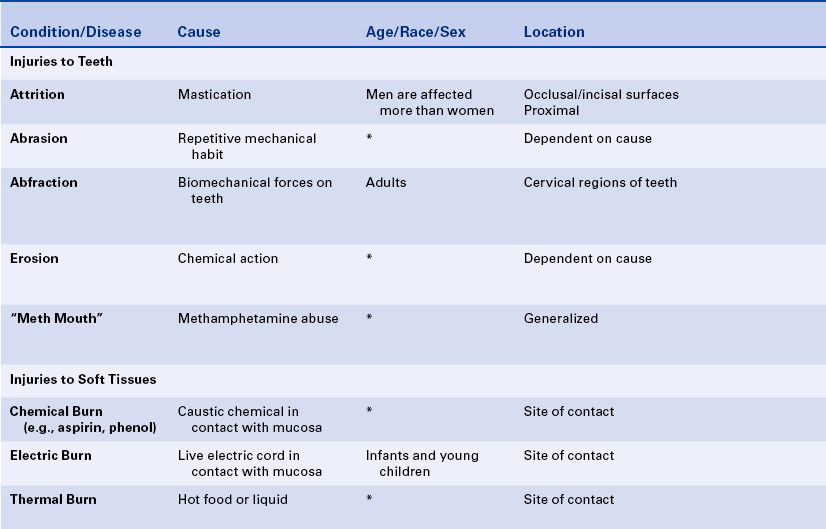
12 Describe and contrast attrition, abrasion, and erosion.
13 Describe the pattern of erosion seen in bulimia.
14 Describe the relationship between bruxism and abrasion.
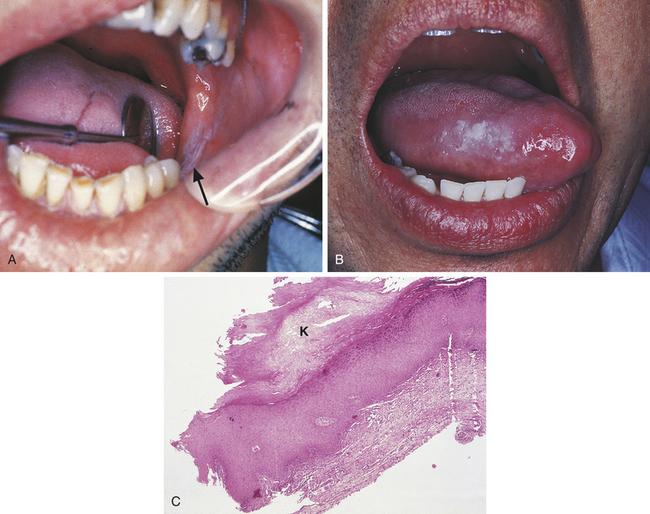
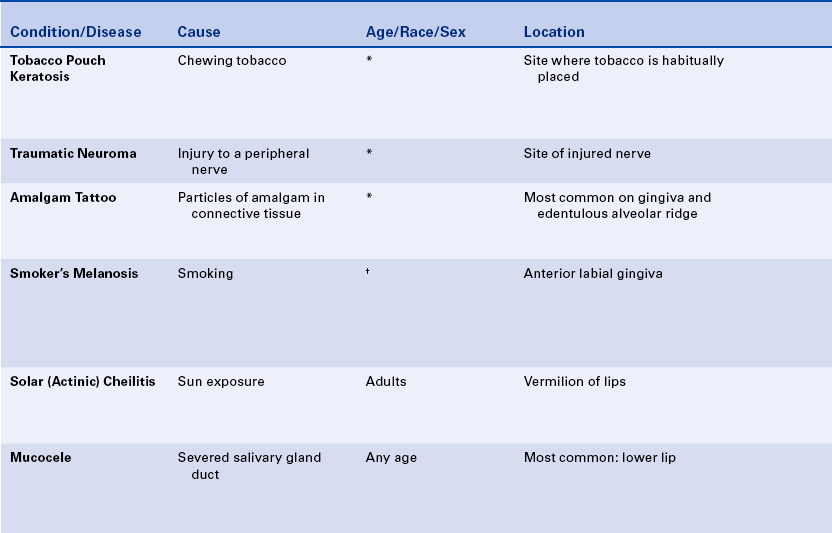
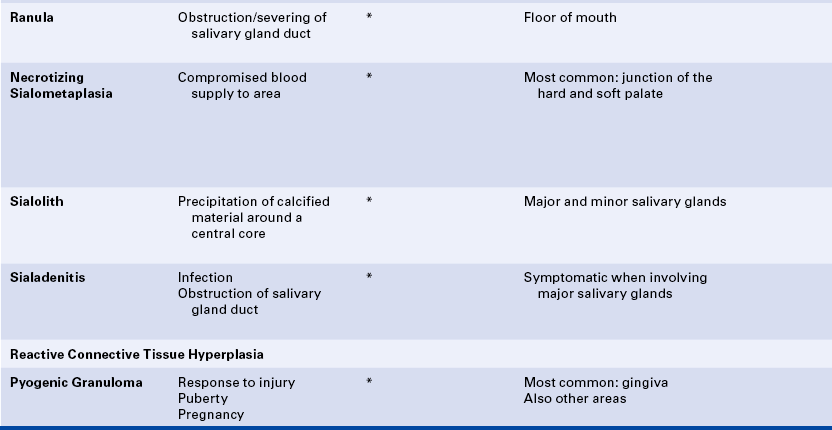
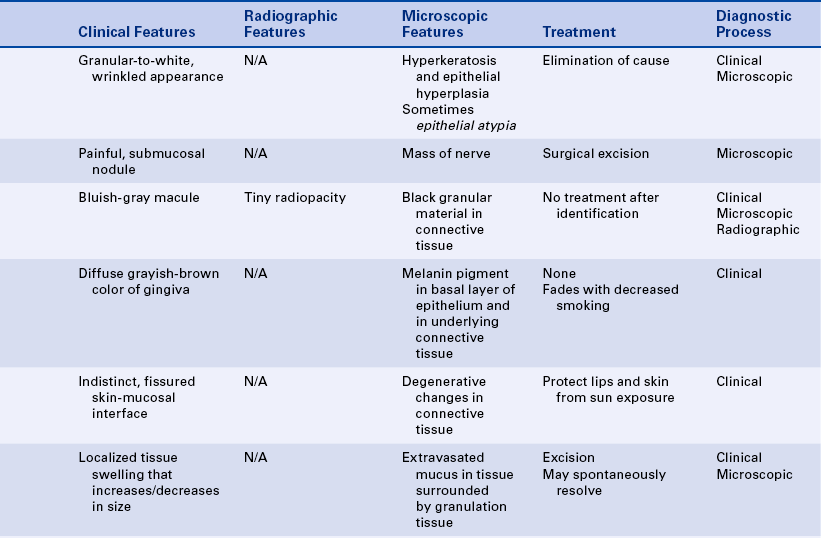
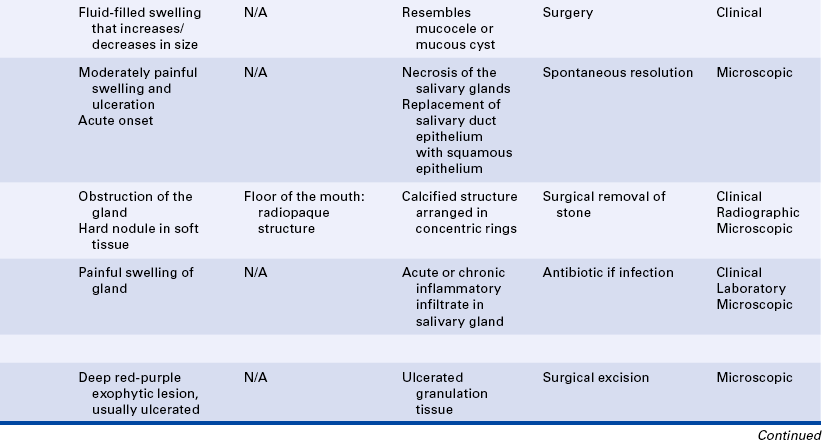
15 Describe the cause, clinical features, and treatment of each of the following: aspirin and phenol burns, electric burn, traumatic ulcer, frictional keratosis, linea alba, and nicotine stomatitis.
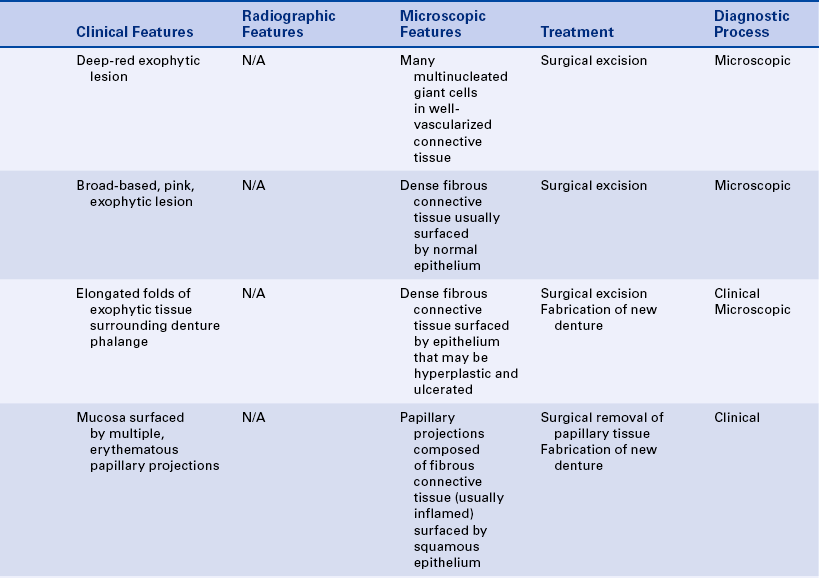
16 Describe the clinical features, cause (when known), treatment, and histologic appearance of each of the following: traumatic neuroma, post inflammatory melanosis, solar cheilitis, mucocele, ranula, necrotizing sialometaplasia, pyogenic granuloma, peripheral giant cell granuloma, chronic hyperplastic pulpitis, and irritation fibroma.
17 Describe the difference between a mucocele and a ranula.
19 Describe the difference between acute and chronic sialadenitis.
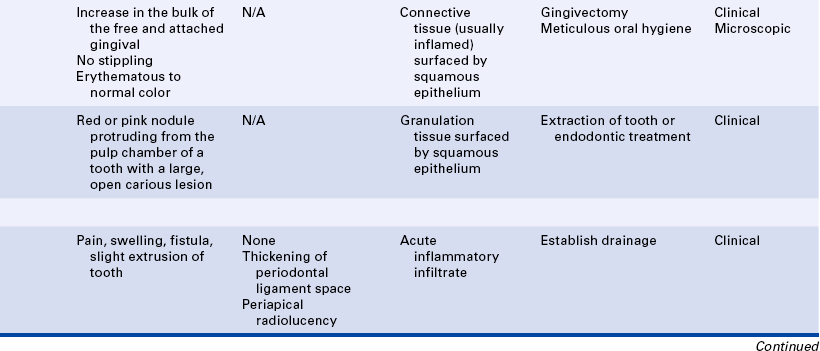
20 Describe the clinical features, radiographic appearance, and histologic appearance of a periapical abscess, a periapical granuloma, and a periapical (radicular) cyst.
21 Describe and contrast internal and external tooth resorption.
A collection of pus that has accumulated in a cavity formed by the tissue.
(ah-kūt) Of short duration or of short and relatively severe course.
(at′-ro-fe) The decrease in size and function of a cell, tissue, organ, or whole body.
(sen-′tral) Within bone.
(ke″mo-tak′sis) The directed movement of white blood cells to the area of injury by biochemical mediators.
(kron′ik) Persisting over a long time.
A protein produced in the liver that becomes elevated during episodes of acute inflammation or infection.
(e-dē′mă) Excess plasma or exudate in the interstitial space of the tissues that causes swelling.
(em″əgra′shun) The passage of white blood cells through the endothelium and wall of the microcirculation into the injured tissue.
(er″ə-the′mah)Redness of the skin or mucosa.
(eks′u-dat) Inflammatory fluid formed as a reaction to injury of tissues and blood vessels.
(fe′ver)An elevation of body temperature to greater than the normal of 37° C (98.6° F).
(hi″per-e′me-ah) An excess of blood in a part of the body.
(hi″per-pla′ze-ah) An enlargement of a tissue or organ resulting from an increase in the number of normal cells.
(hi-per′tro-fe) An enlargement of a tissue or organ resulting from an increase in size but not in number of cells.
(in′flă-m ′shŭn) A nonspecific response to injury that involves the microcirculation and its blood cells.
′shŭn) A nonspecific response to injury that involves the microcirculation and its blood cells.
(lo″kal) Confined to a limited part; not general or systemic.
(lim-fad″ĕ-nop′ah-thē) A condition associated with various disease processes that affect lymph nodes such that they become enlarged.
(mak′ro-f j) The second white blood cell to arrive at the site of injury; is involved in phagocytosis and also the immune response.
j) The second white blood cell to arrive at the site of injury; is involved in phagocytosis and also the immune response.
(mar′jĭ-n -′shun)A process during inflammation in which white blood cells tend to move to the periphery of the blood vessel wall.
-′shun)A process during inflammation in which white blood cells tend to move to the periphery of the blood vessel wall.
(mi″kro-sur-kūl-a′shin) Small blood vessels, including arterioles, capillaries, and venules, all of which can be affected by local changes as the result of inflammation.
(nĕ-kro′sis) The pathologic death of one or more cells or a portion of tissue or organ resulting from irreversible damage.
(nōō′tro-fil) The first white blood cell to arrive at the site of injury; the primary cell involved in acute inflammation; one of the white blood cells with a multilobed nucleus; also called a polymorphonuclear leukocyte.
(p v′ment-ing) Adherence of white blood cells to the walls of a blood vessel during inflammation.
v′ment-ing) Adherence of white blood cells to the walls of a blood vessel during inflammation.
(pĕ-rif′er-al) Located away from the center; indicates that the location of a lesion is in the soft tissue surrounding a bone.
(fag″o-si-tol′ĭ-sis) A process of ingestion and digestion by cells.
(loo″ko-si-to′sis) A temporary increase in the number of white blood cells circulating in blood.
(pu′roo-lent) Containing or forming pus.
(re-gen-er-a′-shun) The process by which injured tissue is replaced with tissue identical to that present before the injury.
(re-p r′) The restoration of damaged or diseased tissues.
r′) The restoration of damaged or diseased tissues.
(se′rus) Having a watery consistency relating to serum.
(sis-tem′ik) Pertaining to or affecting the body as a whole.
(hwēl) A localized swelling of tissue because of edema during inflammation, often accompanied by severe itching.
Inflammation, immunity, and repair are the body’s responses to injury. Inflammation allows the human body to eliminate injurious agents, contain injuries, and begin the process of healing. This chapter begins with a description of the inflammatory response, tissue regeneration, and repair and continues with a description of oral lesions that occur in response to injury. Many of these lesions are quite common and are likely to be encountered when the dental hygienist examines the hard and soft tissues of the oral cavity. Lesions that occur as a result of destruction through activity of the immune responses are included in Chapter 3. Lesions that occur as a result of infection are included in Chapter 4.
INJURY
Injury is an alteration in the environment that causes tissue damage. Injury to oral tissues can be caused by many different factors. Physical injury can affect teeth, soft tissue, and bone. Chemical injury can occur from the application of caustic substances to oral tissues. Microorganisms can cause injury by invading oral tissues. Nutritional deficiencies can render oral tissues more susceptible to injury from other sources.
NATURAL (INNATE) DEFENSES AGAINST INJURY
The body has a number of natural or innate defenses to protect against injury. Intact skin or mucosa acts as a physical barrier to injury. Cilia and mucus in the respiratory system serve as a mechanical defense system. Stomach acid kills most of the microorganisms that are taken into the body through the mouth. Components of saliva and tears have antimicrobial activity. The flushing action of tears, saliva, urine, and diarrhea also removes foreign substances. The process of inflammation and the white blood cells that are brought to the area of injury initially through the process of inflammation are innate or inborn responses to injury.
INFLAMMATION
Inflammation is a nonspecific response to injury and occurs in the same manner, regardless of the nature of the injury. The extent and duration of the injury determine the extent and duration of the inflammatory response. The inflammatory response may be local and limited to the area of injury, or it may become systemic if the injury is extensive. Inflammation of a specific tissue is denoted by the suffix -itis combined with the name of the tissue, such as in tonsillitis, pulpitis, and gingivitis.
The inflammatory response may be acute or chronic. If the injury is minimal and brief and its source is removed from the tissue, only acute inflammation occurs. The duration of acute inflammation is short, lasting only a few days. The tissue may return to its original state (regeneration), or repair of the tissue may begin immediately. If the inflammatory response is longer lasting, it is referred to as chronic inflammation. Chronic inflammation may last weeks, months, or even indefinitely. Chronic inflammation occurs if injury to the tissue continues. The inflammatory response is a dynamic process, changing continually.
Thus transitional stages exist during which the response is changing from one type of inflammation to the next and from an innate inflammatory response to an immune response (see Chapter 3). In addition, an acute inflammatory response may be superimposed over a chronic inflammatory response. Occasionally an overwhelming inflammatory response may lead to further injury. Repair of the tissue occurs only if the persistent source of injury is removed. Current research is demonstrating that chronic inflammation is a component of the pathogenesis of common disorders such as atherosclerosis, insulin resistance, colon cancer, and Alzheimer’s disease. Research focused on proinflammatory markers such as C-reactive protein is attempting to determine the link between chronic inflammation and a number of seemingly unrelated degenerative disorders.
MICROSCOPIC EVENTS AND CLINICAL SIGNS OF INFLAMMATION
Microscopic events occur within the injured tissues during both acute and chronic inflammation. These events cause changes that can be observed clinically. The local clinical changes at the site of injury are called the five classic (cardinal) signs of inflammation: redness, heat, swelling, pain, and loss of normal tissue function (Table 2-1). Systemic signs of inflammation may be present when the response is more extensive; these signs are discussed later in this chapter.
The microscopic events of inflammation involve the small blood vessels or microcirculation (arterioles, capillaries, and venules) in the area of injury, red blood cells, white blood cells, and chemicals in the body called biochemical mediators (Figure 2-1). Normally, blood and the cells it contains flow through the microcirculation. Exchange of oxygen and nutrients needed for the health of the surrounding tissue occurs as plasma passes between the endothelium lining the vessel walls of the arterioles and capillaries. Plasma is the fluid component of blood in which the blood cells are suspended; it is composed mainly of water and proteins. Normally most of the plasma that leaves the microcirculation reenters the circulation through the venules. The lymphatic vessels carry away any excess plasma that does not reenter the blood vessels.
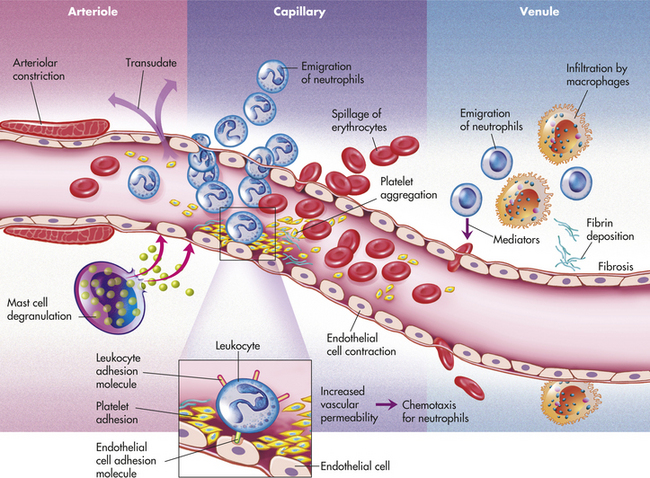
FIGURE 2-1 Microscopic events during inflammation. (From McCance K, Huether S: Pathophysiology, ed 5, St Louis, 2005, Mosby Elsevier).
In inflammation the underlying microscopic events proceed faster than the visible clinical changes. The sequence of microscopic events that occur during the inflammatory response is as follows:
2. Constriction of the microcirculation
3. Dilation of the microcirculation
4. Increase in permeability of the microcirculation
5. Exudate leaving the microcirculation
6. Increase in blood viscosity in the microcirculation
7. Decrease in blood flow through the microcirculation
8. Margination and pavementing of white blood cells
9. Emigration of white blood cells from the microcirculation
The first microscopic event of the inflammatory response is a brief, immediate reflex constriction of the microcirculation in the area of the injury. This is followed, within seconds, by a dilation of the same small blood vessels. Dilation is an increase in the diameter of the vessels and is caused by biochemical mediators that are released at the time of the injury. Dilation of the microcirculation results in increased blood flow through the vessels. The increased blood flow that fills the capillary beds in the injured tissue is called hyperemia. Hyperemia is responsible for two clinical signs of inflammation: redness (erythema) and heat. Erythema is easily visible in most inflamed orofacial tissues. Local temperature changes may be more difficult to recognize.
While hyperemia is occurring, the permeability of the vessels of the microcirculation also increases. As a result, plasma flows into the injured tissues as a fluid that is called an exudate. Two main types of exudate are (1) serous and (2) purulent. Serous exudate is composed mainly of plasma with just a few white blood cells. Purulent exudate (suppuration) contains tissue debris and many white blood cells in addition to the plasma. The presence of exudate in the injured tissue helps to dilute injurious agents that may be present and carry injurious agents through the lymphatic vessels to the lymph nodes.
As the exudate escapes into the tissue, excess plasma collects in the interstitial space. This excess plasma in the interstitial space is called edema and results in localized enlargement or swelling of the tissue, another clinical sign of inflammation (Figure 2-2). If the swollen tissue area is injured further, the exudate flows out of the tissue as either a thin, clear fluid (serous exudate) or a thick, white-to-yellow pus (purulent exudate). An abscess is a collection of pus that has accumulated in a cavity formed by the tissue.
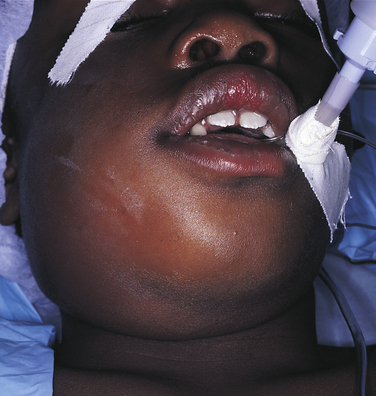
FIGURE 2-2 Swelling caused by increased local edema associated with a dental infection. The patient was hospitalized for treatment of the swelling. (Courtesy Dr. Sidney Eisig.)
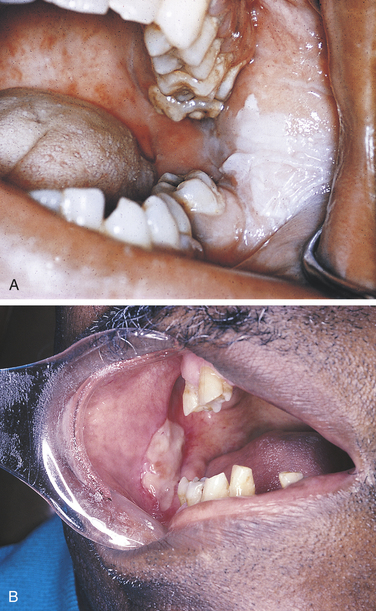
The formation of exudate may be so excessive that it interferes with repair of the tissue. The injured tissue may allow the excess exudate to drain by formation of a drainage passage that bores through the tissue, allowing drainage to the outside. This channel through the tissue is called a fistula (Figure 2-3); it is formed at the expense of healthy, functioning tissue in the area that is lost as the tissue becomes necrotic. Sometimes excessive exudate in damaged tissue has to be drained mechanically by making an incision in the surface of the swollen area and often placing a drainage tube in the site of the incision. This procedure is called incision and drainage and is usually accompanied by the administration of an antibiotic to treat infection and medication to reduce inflammation (Figure 2-4).
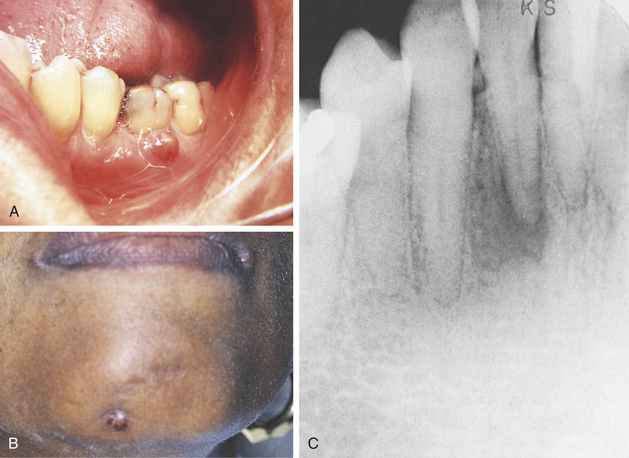
FIGURE 2-3 Fistulas formed from periapical abscesses. A, A fistula formed from an abscess associated with a mandibular first molar. B, The opening of a fistulous tract from a mandibular incisor is noted on the skin of the chin. C, A periapical radiolucency at the area of abscess causing the fistula to the skin in B.
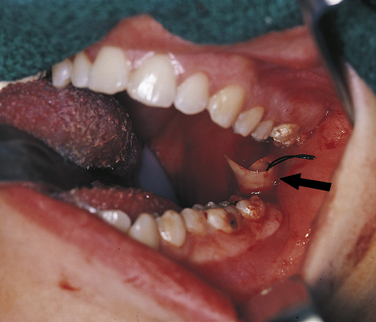
FIGURE 2-4 An intraoral abscess has been incised, and a drain (arrow) placed to allow the escape of purulent exudate from the tissue. (Courtesy Dr. Sidney Eisig.)
Exudate formation also results in another clinical sign of inflammation, pain, as the exudate presses on sensory nerves in the area. Some biochemical mediators present in inflamed tissue can also cause pain. The swelling and pain in tissue resulting from the inflammatory process may then cause a loss of normal tissue function, another clinical sign of inflammation.
In addition to exudate formation, blood vessel permeability leads to increased blood viscosity within the vessels because of the loss of plasma. The blood becomes thicker and cannot flow as easily. This eventually results in decreased flow through the microcirculation. As the blood flow slows down, the red blood cells begin to pile up in the center of the blood vessels, and the white blood cells are displaced to the periphery of the blood vessels. This movement of the white blood cells to the periphery is called margination. The white blood cells are now in position to adhere themselves to the inner walls of the injured blood vessels that have become “sticky” because of specific factors on the surfaces of the cells. This lining of the walls by white blood cells is called pavementing (Figure 2-5).
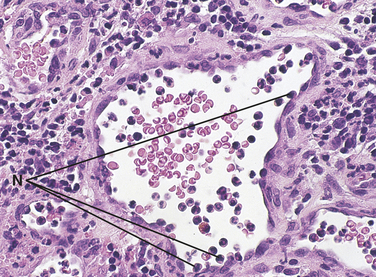
FIGURE 2-5 Microscopic view of a blood vessel showing margination and pavementing are present during inflammation. Neutrophils (N) are seen at the periphery of a small blood vessel.
After pavementing the vessel walls, the white blood cells begin to escape from the blood vessels along with the plasma and enter the injured tissue. This process by which the white blood cells escape from the blood vessels is called emigration. Emigration occurs as a result of opening of the cellular junctions of the endothelium lining the blood vessels as its cells retract in size because of biochemical mediators.
This directed movement of white blood cells toward the site of the injury is called chemotaxis; biochemical mediators that enhance this directed movement are called chemotactic factors. Emigration and chemotaxis of the white blood cells to the area of injury allow these cells to be mobilized in the body’s defense against the injury.
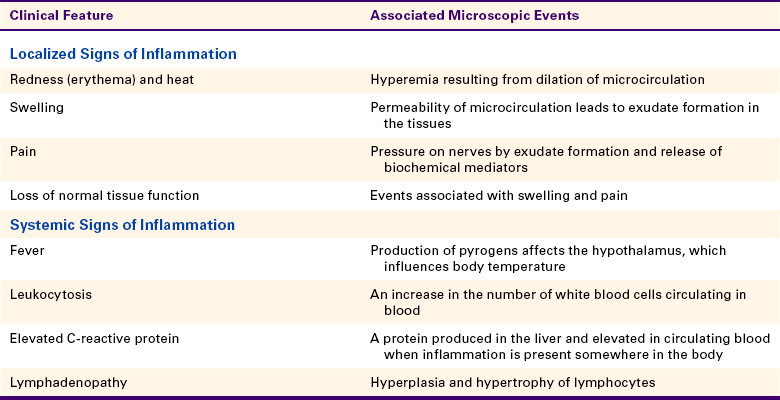
At first these cells try to wall off the site of the injury from the surrounding healthy tissue. Later in the injured tissue, the white blood cells also try to remove foreign substances from the site by ingesting and then digesting them. This is called phagocytosis (Figure 2-6). The foreign substances may include pathogenic microorganisms or tissue debris. The presence of these substances interferes with the repair process; therefore they must be removed for the inflammation to resolve and any necessary tissue repair to proceed.
WHITE BLOOD CELLS AND THEIR INVOLVEMENT IN THE INFLAMMATORY RESPONSE
Emigration of white blood cells (leukocytes) from the blood vessels into the site of injury and subsequent chemotaxis and phagocytosis are important components of the process of inflammation. Two types of white blood cells are involved initially in the inflammatory response: the neutrophil and the monocyte circulating in blood that becomes the macrophage in tissue. The neutrophil is sometimes called the polymorphonuclear leukocyte because it is the most prevalent of the white blood cells containing a multilobed nucleus. However, this term also includes other white blood cells; therefore neutrophil is a more specific name for this cell. Other cells within the blood and tissue such as the lymphocyte and plasma cell, eosinophil, and mast cell participate in both inflammatory and immune responses. The immune response and the involvement of these cells are included in Chapter 3.
As inflammation begins and continues over the 2-week time frame after an injury, changes take place in the types of white blood cells present in the tissue (Figure 2-7).
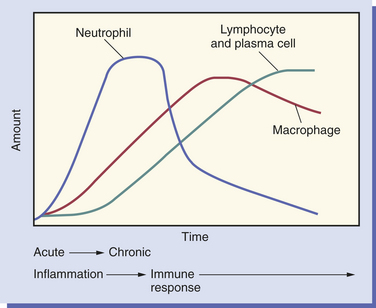
FIGURE 2-7 Changes in the white blood cell population of the injured tissue over time, starting with acute inflammation, continuing to chronic inflammation, and the beginning of the immune response.
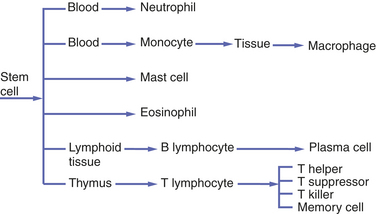
The neutrophil is the first cell to arrive at the site of injury and is the most common inflammatory cell present (Figure 2-8) during acute inflammation. The monocyte circulating in blood enters the tissue and becomes a macrophage. It is the second type of white blood cell to arrive at the site of injury. As inflammation continues, the number of neutrophils decreases. If the injury persists and chronic inflammation occurs, macrophages, lymphocytes, and plasma cells replace the neutrophils and become the predominant white blood cells in the tissue (Figure 2-9).
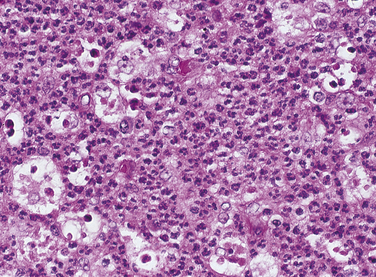
FIGURE 2-8 Microscopic view of acute inflammation showing an increase in the number of neutrophils. Macrophages are also present.
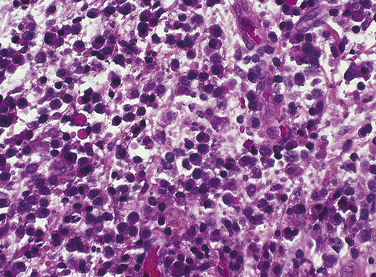
FIGURE 2-9 Microscopic view of chronic inflammation and the beginning of the immune response, showing lymphocytes, and plasma cells.
Neutrophils
Neutrophils are the first white blood cells recruited into the area of injury in response to chemotactic factors. The main function of the neutrophil is phagocytosis of substances such as pathogenic microorganisms and tissue debris. Lysosomal enzymes contained within vacuoles in the cytoplasm destroy substances after the cell has engulfed them. The removal of these substances from the site of injury is necessary to allow the process of healing to occur. The neutrophil dies shortly after phagocytosis; as a result, lysosomal enzymes and other damaging cellular substances that were meant only for intracellular destruction of foreign substances leak from the cells. This leakage can cause further tissue damage.
Neutrophils constitute 60% to 70% of the entire white blood cell population. Like all white blood cells, neutrophils are derived from stem cells in the bone marrow (Figure 2-10). Stem cells are undifferentiated cells in the spongy tissue that is found in the center shafts of certain long and flat bones of the body, such as the bones of the pelvis and sternum. When observed microscopically, neutrophils possess a multilobed nucleus, which is why they are sometimes called polymorphonuclear leukocytes, and a granular cytoplasm that contains enzymes called lysosomal enzymes (Figure 2-11). They are produced throughout life and are mobile cells.
Macrophages
The monocyte is the second white blood cell to emigrate from the blood vessels into the injured tissue, where it becomes a macrophage. Like neutrophils, monocytes are derived from stem cells in the bone marrow (see Figure 2-10). As a macrophage, it responds to chemotactic factors, is capable of phagocytosis, is mobile, and has lysosomal enzymes in its cytoplasm that assist in the destruction of foreign substances.
The macrophage is larger than its monocytic precursor. It has a single round nucleus and does not have granular cytoplasm (Figure 2-12). It constitutes 3% to 8% of the entire white blood cell population. The macrophage has a somewhat longer life span than the neutrophil. In addition to its role in phagocytosis, the macrophage is an important cell in the immune response (see Chapter 3).
BIOCHEMICAL MEDIATORS OF INFLAMMATION
Chemical agents in the body called biochemical or inflammatory mediators cause many of the events involved in the inflammatory response. Biochemical mediators are essential to the inflammatory response and can start or amplify the response. During the response, basic mediators of inflammation can recruit other mediators and immune mechanisms, thus escalating the overall process. Some biochemical mediators are circulating in blood, some come from endothelium, some from white blood cells, and some from platelets; others are produced by certain pathogenic microorganisms as they injure the tissue.
Three systems of plasma proteins circulating in the blood may be activated during inflammation: (1) the kinin system, (2) the clotting mechanism, and (3) the complement system. The activation of these plasma protein systems involves a sequential cascade of events. These systems are interrelated; interaction among the systems takes place during their activation, among their products, and within their various actions.
Kinin System
The kinin system mediates inflammation by causing increased dilation of the blood vessels at the site of injury and increasing the permeability of local blood vessels. This system is rapidly activated both by substances present in plasma and by those present in injured tissues. Its role is limited to the early phases of inflammation. Components of the kinin system also induce pain. The primary kinin is bradykinin.
Clotting Mechanism
The clotting mechanism functions primarily in the clotting of blood, which helps stop bleeding at the site of injury. It forms a fibrinous meshwork at the site of injury that protects adjacent tissues and keeps foreign substances corralled at the site. It also mediates inflammation because certain of its products that are activated when tissue is injured cause local vascular dilation and permeability by activating the kinin system. The clotting mechanism is also important in tissue repair since it forms a future framework for the repair process.
Complement System
The complement system is composed of a series of plasma proteins that are activated in a cascading fashion with one protein activating the next in the series. Components of the complement system function during both inflammation and immunity. Complement components can cause mast cells to release the granules in their cytoplasm that contain the biochemical mediator histamine and other mediators. Mast cells are a central cell in certain inflammatory reactions. They are located in large numbers in the loose connective tissue of skin and mucosa. When released, histamine causes an increase in vascular permeability and vasodilation. Other components of the complement system can cause cell death (cytolysis) by creating holes in the cell membrane. They also can form chemotactic factors for white blood cells and enhance phagocytosis. This enhancement of phagocytosis is called opsonization.
Other Biochemical Mediators of Inflammation
In addition to the biochemical mediators derived from circulating blood that have been described already, others are formed in the body during inflammation. Cell products from certain cells called cytokines effect the inflammatory response. These are described in Chapter 3 because they participate in the immune response. Prostaglandins are derived from cell membranes. They function in the inflammatory response by causing increased vascular dilation and permeability, tissue redness and pain, and changes in connective tissues. Lysosomal enzymes are released from the granules in white blood cells. They act as chemotactic factors and can cause damage to connective tissues and to the clot.
Endotoxin and lysosomal enzymes released by pathogenic microorganisms may also serve as biochemical mediators. Endotoxin, produced from the cell walls of gram-negative bacteria, can serve as a chemotactic factor, activate complement, and function as an antigen and damage bone tissue. During infection the lysosomal enzymes released from pathogenic microorganisms have a similar chemical composition and action to those released by white blood cells.
Antiinflammatory Drugs
Antiinflammatory drugs block or suppress the inflammatory response, preventing or reducing the clinical signs of inflammation and adverse reactions to the injury. Diseases and conditions such as asthma, arthritis, organ transplants, and surgical trauma are treated with nonsteroidal or steroidal antiinflammatory agents. Examples of nonsteroidal antiinflammatory agents (NSAIDs) include acetylsalicylic acid (aspirin) and ibuprofen. They exert their analgesic effects by inhibiting prostaglandin synthesis. Prednisone is a steroidal antiinflammatory drug. Another drug classification, antihistamines, reduces the effects of the mediator histamine.
Medications that are traditionally used to treat cancer such as methotrexate, sulfasalazine, leflunomide, cyclophosphamide, and mycophenolate are also being used to treat inflammatory diseases; the doses are significantly lower, and the risk of side effects tends to be considerably less than when prescribed in higher doses to treat cancer.
SYSTEMIC MANIFESTATIONS OF INFLAMMATION
In addition to the local features of inflammation, four major systemic signs may also occur: (1) fever, (2) an increase in the number of white blood cells (leukocytosis), (3) elevated C-reactive protein, and (4) enlargement of lymph nodes (lymphadenopathy) (see Table 2-1).
Fever
Body temperature is controlled by a regulatory center in the brain called the hypothalamic thermoregulatory center. Fever is a body temperature higher than 98.6° F (37° C) and is associated with a systemic inflammatory response. White blood cells and pathogenic microorganisms produce fever-producing substances known as pyrogens. Pyrogens exert their effects by action on the hypothalamus, the regulatory center in the brain that increases the temperature of the body by way of prostaglandins, producing fever.
The function of this increased body temperature is not clear. A moderately high fever may be helpful in combating some infections because increased temperature slows the growth of many pathogenic microorganisms. However, the body cannot tolerate excessively high fever for very long, and such fever could prove fatal. Drugs can be given to reduce high fever. Measuring body temperature with a thermometer is helpful in assessing whether a systemic inflammatory response is present.
Leukocytosis
Leukocytosis is an increase in the number of white blood cells circulating in blood. The normal value is 4,000 to 10,000/mm3. The complete blood count is an initial blood test that can be used to evaluate a patient for infection. During a systemic inflammatory response, particularly a response to infection, there is an increase in the number of circulating white blood cells (leukocytosis). The number increases to 10,000 to 30,000/mm3. This increase primarily involves neutrophils. The body increases the number of circulating white blood cells by increasing their formation and releasing immature forms from the bone marrow into the blood; this is called a “a shift to the left.” This increase is caused by biochemical mediators and is an attempt by the body to provide more cells for phagocytosis.
A complete blood count includes a “differential” white blood cell count. This measures the proportion of each white blood cell type and is useful in distinguishing a viral infection from a bacterial infection or an allergic reaction that involves eosinophils. These results provide a useful tool for evaluating patients but do not indicate the particular cause or site of inflammation in the body.
Elevated C-Reactive Protein
Another diagnostic laboratory test measures serum C-reactive protein. C-reactive protein is produced in the liver and plays the important role of interacting with the complement system. Normally low levels of C-reactive protein circulate in blood. Elevated C-reactive protein is present during episodes of acute inflammation or infection and may continue with chronic inflammation. Although a result above 10 mg/L is usually considered high for C-reactive protein, most infections and episodes of inflammation result in C-reactive protein levels at 100 mg/L. C-reactive protein levels drop as inflammation subsides.
C-reactive protein level can be used to help assess conditions such as rheumatoid arthritis and systemic lupus erythematosus and to determine if medication taken is effective. It may be used to monitor wound healing and as an early detection system for possible infections in patients who have had surgery, organ transplants, or severe burns. Chronically elevated C-reactive protein level is associated with an increased risk for cardiovascular disease. A high-sensitivity C-reactive protein (hs-CRP) assay is now available.
Lymphadenopathy
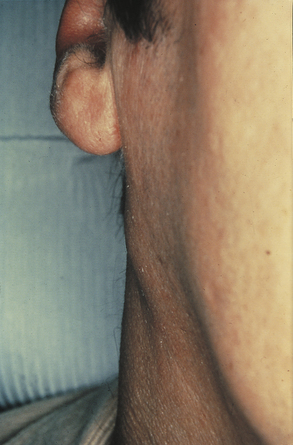
During the inflammatory process the lymph nodes enlarge or undergo lymphadenopathy (Figure 2-13). The enlarged lymph node or nodes, if located superficially, can be palpated as a mass or masses in the area of inflammation and possibly along the associated lymphatic drainage route (Figure 2-14). When palpated, the involved node feels firmer and larger than normal and may also be tender. Deeper lymph nodes may also be enlarged, but these cannot be palpated.
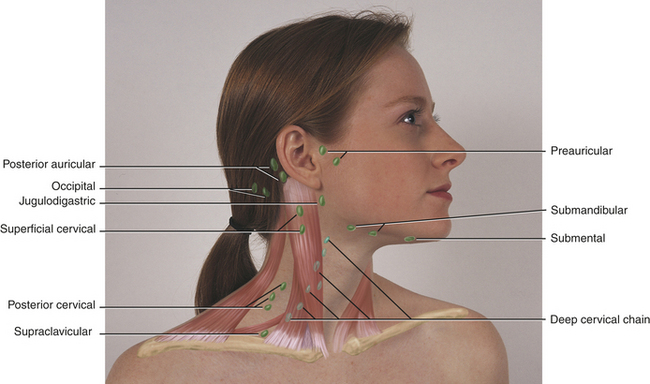
FIGURE 2-14 Location of lymph nodes in the neck. (From Jarvis C: Physical examination and health assessment, ed 5, St Louis, 2008, Saunders Elsevier.)
Lymphadenopathy results from changes in the lymphocytes that reside in the lymph node. Lymphocytes are white blood cells that mature in lymphoid tissue. They are the primary white blood cells of the immune response (see Chapter 3). They also travel from the lymph node to the tissues, where they are involved in the immune response. The changes in the lymphocytes cause the change in size of the lymph nodes. These changes include an increase in the number of cells (hyperplasia), resulting from increased cell division, and an enlargement of individual cells (hypertrophy), resulting from cellular maturation. The lymphoid tissue in Waldeyer’s ring, which includes the pharyngeal and lingual tonsillar tissue, may also undergo these changes. These changes in the lymphocyte population occur during prolonged or chronic inflammation.
CHRONIC INFLAMMATION
Chronic inflammation results from injuries that persist, often for weeks or months, possibly indefinitely. In addition to neutrophils and monocytes, other white blood cells are involved, as is the proliferation of fibroblasts. The cells involved in chronic inflammation include macrophages, lymphocytes, and plasma cells. Repair takes place at the same time that chronic inflammation proceeds, but it cannot be completed until the source of the injury is removed.
A distinctive form of chronic inflammation is called granulomatous inflammation. It is characterized by the formation of granulomas, which are microscopic groupings of macrophages surrounded by lymphocytes and occasional plasma cells. Granulomas usually contain large macrophages that have multiple nuclei called multinucleated giant cells. Foreign substances in tissue and certain infections such as tuberculosis tend to stimulate the formation of granulomas. The body is unable to destroy the offending substances and tries to enclose them in a mass of inflammatory cells.
HYPERPLASIA, HYPERTROPHY, AND ATROPHY
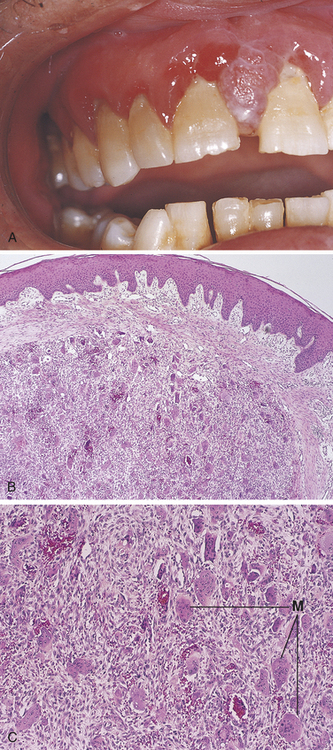
A cell or its associated tissue or organ may respond to injury by undergoing an adaptive response such as hyperplasia, hypertrophy, or atrophy. Hyperplasia is defined as an increase in the number of cells in a tissue or organ whereby the size of the tissue or organ is increased in response to conditions that cause cellular stress. Pathologic hyperplasia frequently occurs in oral tissues. In the oral cavity an increase in the number of epithelial cells and the increased thickness of the epithelium commonly occur in response to chronic irritation (Figure 2-15). As surface epithelial cells are lost, division of deeper basal epithelial cells increases to replace the lost cells. The production of new cells is in excess of the original number of cells; thus the epithelium becomes thickened, and the tissue appears paler or whiter.
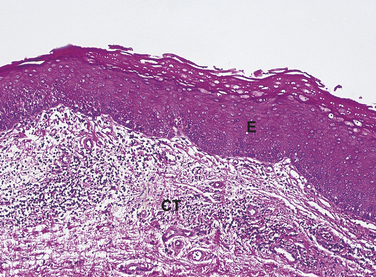
FIGURE 2-15 Microscopic appearance of epithelial hyperplasia (low magnification). The epithelium (E) is thickened because of an increase in the number of cells. CT, underlying connective tissue.
When the irritation subsides, the proliferation ceases; with time the epithelium usually returns to its normal size, and the color of the tissue returns to normal. However, sometimes the hyperplastic tissue persists even after the irritation is discontinued. Hyperplasia of fibrous connective tissue may also occur in response to chronic injury and is common in the oral cavity. Oral lesions caused by epithelial and fibrous hyperplasia are described later in this chapter.
In contrast, hypertrophy is a response to cellular stress that is defined as an increase in the size of a tissue or organ because of an increase in the size, not the number, of cells. For example, hypertrophy occurs in the smooth muscles of the uterus and the mammary glands in response to pregnancy, in cardiac muscle in response to long-standing high blood pressure, and in skeletal muscle in response to increased exercise. Hyperplasia and hypertrophy are often present together in one disease state.
Atrophy is the decrease in size and function of a cell, tissue, organ, or whole body in response to certain conditions of cellular stress. Atrophied cells are capable of increasing to their normal size after the stress is removed. Atrophy can be present in the muscular wasting that sometimes occurs in chronic disease states that do not allow mobility and thus function of the body (“use it or lose it”). It can also happen with changes in cellular growth, malnutrition, pressure, ischemia, or hormonal changes.
REGENERATION AND REPAIR
With resolution of the inflammatory response, the injured tissue undergoes wound healing as either regeneration or repair. When tissue damage has been slight, the inflamed area may return completely to its normal structure and function. This is called regeneration. Regeneration is the most favorable resolution of acute inflammation and involves complete removal of all cells, by-products, and inflammatory exudate that enter the tissue during inflammation and return of the microcirculation to its preinflammatory state.
In contrast, the process of repair takes place when complete return of the tissue to normal is not possible because the damage has been too great. Some tissues such as epithelium, fibrous connective tissue, and bone have the ability to undergo repair. Other tissues such as enamel do not.
Repair is the body’s final defense mechanism in its attempt to restore injured tissue to its original state. During the repair process destroyed cells and tissue are replaced with live cells and new tissue components, but the repair process cannot be completed until the source of injury is removed or the injurious agents are destroyed. Repair is not always a perfect process. Functioning cells and tissue components are often replaced by nonfunctioning scar tissue. Many studies are currently investigating ways to enhance the repair process.
MICROSCOPIC EVENTS DURING REPAIR
After an injury microscopic events occur in both the epithelium and connective tissue (Figure 2-16). These events are different for each of these tissues but occur almost simultaneously and are dependent on each other for optimal healing. If the source of the injury is removed, the repair process for both tissues is usually completed in 2 weeks. The repair process is slightly different in mucosa than in skin because mucosal tissues are wet and a scab does not form. There are three phases to repair that occur during these 2 weeks: (1) inflammation, (2) proliferation, and (3) maturation.
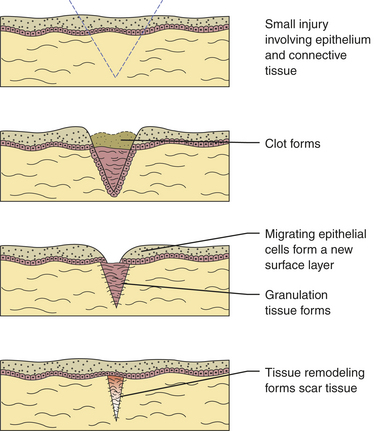
FIGURE 2-16 Underlying microscopic events of the repair process from the day of injury to 2 weeks later.
Day of Injury
A clot forms as the blood flows into the injured tissue. The clot is produced in the area of injury as a result of activation of the clotting mechanism. The clot consists of a meshwork structure comprised of locally produced fibrin, aggregated (clumped) red blood cells, and platelets. Platelets (thrombocytes) are cellular fragments found in blood and are extremely important in the formation of a clot. There are 250,000 to 400,000 platelets per cubic milliliter within the blood. The number of platelets is measured within the panel of the complete blood count. Hereditary factors, drugs, extensive injury, or certain diseases may affect red blood cells, platelets, and other factors involved in the formation of the clot and thus prevent or delay tissue repair.
One Day after Injury
Acute inflammation is taking place in the area of future repair. The neutrophils emigrate from the microcirculation into the injured tissue, and phagocytosis of foreign substances and necrotic tissue occurs as part of the inflammatory response.
Two Days after Injury
The monocytes emigrate from the microcirculation into the injured area as macrophages. Macrophages continue phagocytosis in a manner similar to that of the neutrophils. Neutrophils are reduced in number as the chronic inflammatory process proceeds. Fibroblasts proliferate within the injured connective tissue as a result of biochemical mediators from macrophages. Fibroblasts become the most important cells during wound healing as they begin to produce and secrete new collagen fibers, using the fibrinous meshwork as a scaffold.
The initial tissue formed in the connective tissue portion of the injury is called granulation tissue. It is an immature tissue, with many more capillaries and fibroblasts than the usual connective tissue that clinically appears a vivid pink or red. Sometimes the growth of this tissue is excessive (exuberant). It may interfere with the repair process until it is removed surgically.
If the surface epithelium has been destroyed by the injury, the epithelial cells create a new surface tissue at the same time that granulation tissue forms in the injured connective tissue. The epithelial cells from the borders of the healing injured area lose their cellular junctions and become mobile. They then divide and migrate across the injured tissue, using the fibrinous meshwork as a guide to form a new surface layer.
In addition to serving as a guide for migrating epithelial cells and as a scaffold for forming connective tissue, the fibrinous meshwork of the clot serves to protect the two newly formed deeper tissues from further injury. Thus it is important for the clot to remain in place during this time to allow optimal repair in both tissues. Dressings placed over the clot may prove beneficial to the healing process in some injuries.
At the end of 2 days, lymphocytes and plasma cells begin to immigrate from the surrounding blood vessels into the injured area as chronic inflammation and an immune response begin. The macrophages already present in the area now assist the lymphocytes in the immune response occurring at the site of injury.
Seven Days after Injury
The fibrinous meshwork of the clot is digested by tissue enzymes and sloughs off, and the initial repair of the tissues is completed. Clinically the surface of the repaired injury remains redder than normal because of the thinness of the new surface epithelium and the increased vascularity of the new underlying connective tissue. If the source of the injury has been removed completely, the inflammatory and immune responses in the tissue have completed their cycles. The immature type of collagen fibers found in granulation tissue is still present and remains fragile and at risk of reinjury.
Two Weeks after Injury
The initial granulation tissue and its fibers have been remodeled, giving the tissue its full strength. The new tissue has undergone maturation and is now called scar tissue; it appears whiter or paler at the surface of the repaired injury because of the increased number of collagen fibers and decreased vascularity. The amount of scar tissue remaining after an injury depends on many factors such as heredity, the strength and flexibility needed in the tissue, the type of repair that has occurred, and the tissue involved; the oral mucosa is less prone to scar formation than the skin.
TYPES OF REPAIR
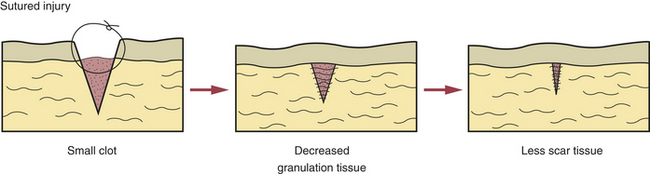
This refers to the healing of an injury in which little loss of tissue takes place, such as in a surgical incision. In this type of healing the clean edges of the incision are joined with sutures to form only a small clot, and very little granulation tissue forms (Figure 2-17). Thus less scar tissue forms, and the patient retains more normal tissue. The use of sutures is an attempt to try to join the edges of the injury surgically so that healing by primary intention occurs and scarring is minimized.
Healing by Secondary Intention
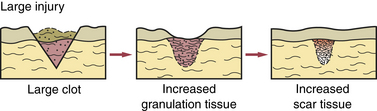
This involves injury in which tissue is lost; thus the edges of the injury cannot be joined during healing. A large clot slowly forms, resulting in increased formation of granulation tissue (e.g., an extraction site) (Figure 2-18). After healing, scar tissue increases, and normal tissue function is greatly reduced. This scar tissue formation can be so excessive that surgical correction is sometimes needed. Excessive scarring in skin that appears raised and extends beyond its original boundaries is called a keloid (Figure 2-19). A familial tendency to keloid formation has been noted.
Healing by Tertiary Intention
If infection occurs at the site of a surgical incision that is healing by primary intention, healing by tertiary intention may result. This transformation occurs because of an enlargement of the injured area and an increase in the magnitude and duration of the inflammatory and immune responses triggered by the presence of pathogenic microorganisms. In some cases an infected injury is left open, and the edges are not surgically joined until the infection is controlled. Waiting to perform surgical tissue repair until the infection is resolved is called healing by tertiary intention.
Factors That Impair Healing
Certain local factors impair healing; these include bacterial infection (primarily by Streptococcus species), tissue destruction and necrosis, hemorrhage into the tissue (hematoma), excessive movement of the injured tissue, and poor blood supply. Systemic factors such as those resulting from malnutrition (especially when protein, zinc, calcium, and vitamin C are severely reduced in the diet) can also impair healing. If the body is undergoing immunosuppression because of steroid use or chemotherapy, healing is also impaired. Certain genetic connective tissue disorders (osteogenesis imperfecta, Marfan syndrome) (see Chapter 6) and metabolic disorders resulting from age, renal failure, and diabetes mellitus (see Chapter 9) can reduce the effectiveness of the natural healing mechanism. Tobacco use has also been shown to impair healing.
BONE TISSUE REPAIR
Repair of a bone injury is similar to the process that takes place in fibrous connective tissue except that it involves the creation of bone tissue. Bone-forming cells called osteoblasts, which are found on the inner and outer surfaces of bone, produce this tissue. As with other tissues, nutrition, age, and tobacco use can influence the repair process of bone. At the site of the injury blood supply and growth factors can further modulate the process. Removal of osteoblast-producing tissues and excessive movement of the bone can interrupt healing. Inadequate movement of bone during the healing process can adversely affect repair. Injury, edema, or infection in the involved bone can delay repair.
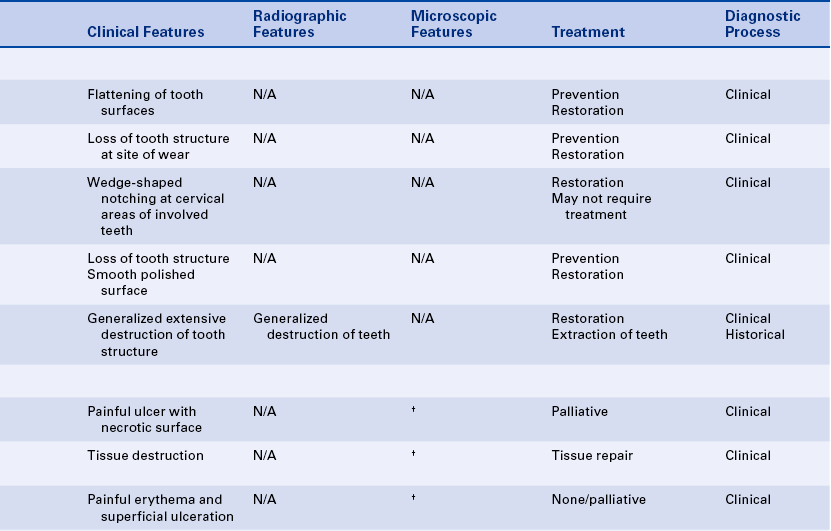
ATTRITION
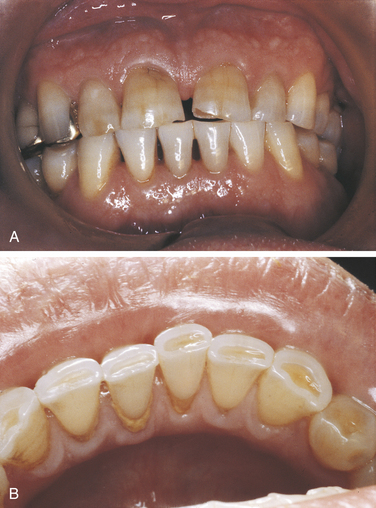
Attrition is the wearing away of tooth structure during mastication. It is a normal occurrence and happens as an individual ages. It involves the incisal, occlusal, and proximal surfaces of the teeth and is rarely seen on any other tooth surfaces unless teeth are abnormally placed in the arch (Figure 2-20). Attrition occurs in both deciduous and permanent dentitions; it is usually a slow process that starts as soon as the teeth are in contact and continues for the duration of the contact.
The first sign of attrition is the disappearance of the mamelons on incisal teeth and the flattening of the occlusal cusps (Figure 2-21). The rate of attrition is influenced by diet. A diet of more fibrous food causes greater attrition. Bruxism, the use of chewing tobacco, and certain occupations and environments in which abrasive dust particles enter the mouth accelerate attrition. Attrition increases as patients grow older, and the rate of attrition has been reported to be greater in men than in women.
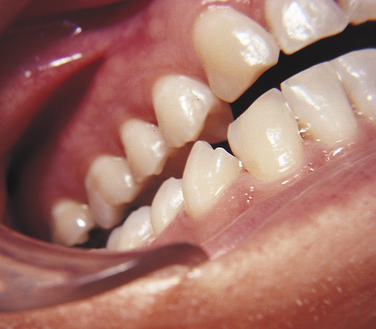
FIGURE 2-21 Attrition has caused the flattening of the cusps (wear facets) of both the maxillary and mandibular cuspid teeth.
Bruxism
Grinding and clenching the teeth together for nonfunctional purposes is called bruxism. The signs and symptoms resulting from bruxism and their extent are related to the intensity of the grinding and clenching. They are varied and include “wear facets” visible on enamel surfaces, an abnormal rate of attrition (Figure 2-22), hypertrophy of masticatory muscles (especially the masseter muscle), increased muscle tone, muscle tenderness, muscle fatigue, cheek biting, pain in the temporomandibular joint area (see Chapter 10), tooth mobility, and pulpal sensitivity to cold.
The incidence of bruxism varies greatly according to the population studied. In a university student population, 5% of individuals showed signs and symptoms of bruxism. In studies of patients with periodontal disease, 60% to 90% had evidence of bruxism. In children ages 2 to 5 years, the average prevalence reported was 20% to 30%, but the highest prevalence reported was 78%.
The cause of bruxism is unclear. Local factors such as occlusal interferences in combination with stress and tension are considered to be triggering factors. A relationship has been reported between bruxism and anxiety, hostility, and hyperactivity. Other studies have characterized the individual who engages in bruxism as emotionally fragile with meticulous character traits, more headaches and muscle pains, and more success in school. Certain conditions such as seizure disorders have been related to bruxism. The higher prevalence of bruxism reported in certain occupations is possibly related to the amount of stress associated with those occupations.
Management of the individual with bruxism includes eliminating the occlusal interferences through occlusal adjustments and protecting the teeth and supporting tissues from further destruction by fabricating an acrylic splint that can be worn as a protective device.
The dental hygienist may identify signs of bruxism while taking the patient’s history and during the oral examination. Active wear facets, muscle tenderness, and excessive attrition are clues to the presence of bruxism.
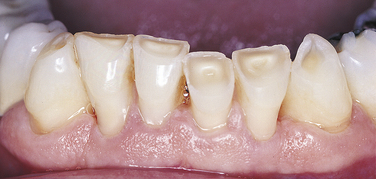
ABRASION
Abrasion is the pathologic wearing away of tooth structure that results from a repetitive mechanical habit. It is most commonly seen in exposed root surfaces because the cementum and dentin are not as hard as enamel; however, abrasion also occurs on enamel surfaces. The process of abrasion is usually slow, and the dentin responds by laying down a protective layer of secondary dentin. Therefore pulpal exposure does not usually result.
Abrasion most frequently presents as a notching of the root surface in areas of gingival recession and may occur from an improper toothbrushing technique, most commonly a back-and-forth scrubbing motion using excessive pressure. The use of an abrasive dentifrice or a hard toothbrush may also cause abrasion (Figure 2-23). Today most dentifrices manufactured in the United States have a very low abrasive index, and toothbrushes with soft bristles are recommended. Other causes of abrasion include opening bobby pins with the teeth or holding needles or pins in the teeth. These practices result in a notching of the maxillary incisors. Musicians who play wind instruments may also exhibit forms of abrasion of the teeth in the area of the mouth where the instrument is placed, and pipe smokers may show evidence of abrasion in the area of pipe placement.
The diagnosis of abrasion can often be made by correlating the clinical appearance of the lesions with information gained from questioning the patient about possible factors that may be causing the lesions. The patient should be informed of the cause of the abrasion, and corrective measures should be taken to prevent further destruction of tooth structure. Restorative dental treatment to repair the defect may be appropriate.
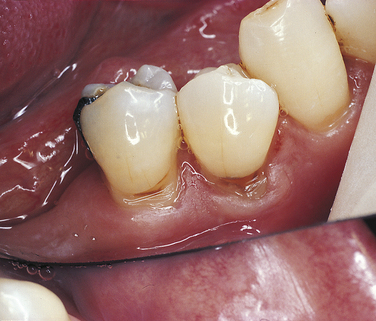
ABFRACTION
Abfraction typically appears as wedge-shaped lesions at the cervical areas of teeth. These lesions occur in adults; the cause may be related to fatigue, flexure, fracture, and deformation of tooth structure as the result of biomechanical forces on the teeth. The weakened tooth structure is more susceptible to abrasion, particularly toothbrush abrasion. The lesions may be treated using composite or glass ionomer materials, but the forces on the teeth may result in dislodging of the restorations.
EROSION
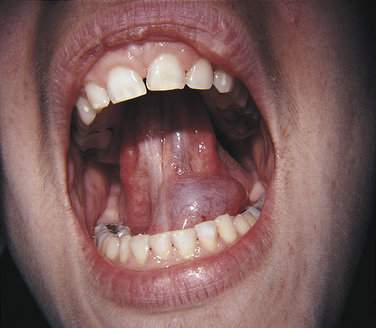
Erosion is the loss of tooth structure resulting from chemical action. The loss may occur on the smooth facial or lingual surfaces of the teeth and on the proximal and occlusal surfaces (Figure 2-24). The area of erosion appears smooth and polished and is usually extensive, involving several teeth. If erosion occurs in an area where restorations exist, the tooth structure is lost around the restoration, making it appear as if the restoration is standing on its own. This phenomenon is not seen in abrasion or attrition because the restoration would be worn down along with the tooth surface.
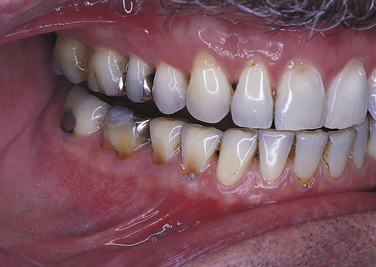
FIGURE 2-24 Erosion of buccal and labial surfaces of teeth that occurred as a result of accidental exposure to sulfuric acid.
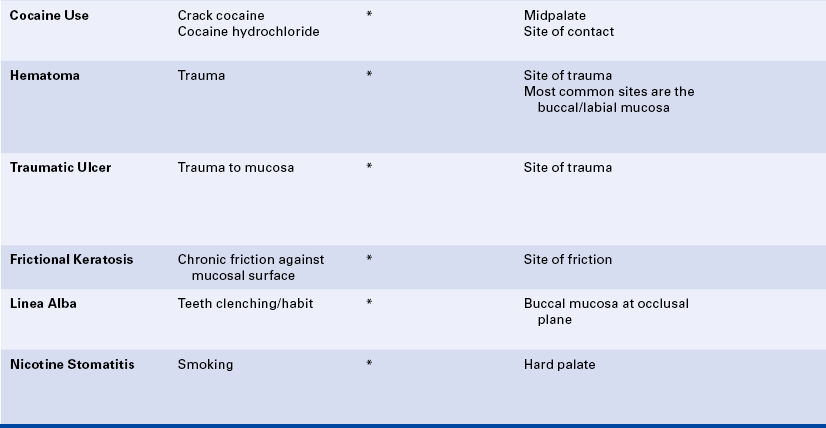
Erosion may be seen in individuals who work in industries in which acid is used, such as battery manufacturing, plating companies, and soft-drink manufacturing. The erosion occurs because the workers breathe the acid in the air. Erosion of teeth associated with intraorally applied cocaine hydrochloride has been reported. Erosion of the facial surfaces of the teeth may also occur as a result of frequently sucking on lemons; erosion of the lingual surfaces of the teeth may occur as a result of chronic vomiting.
The location of erosion and abrasion cannot reliably identify the cause. The patient’s history must be correlated with the location and cause.
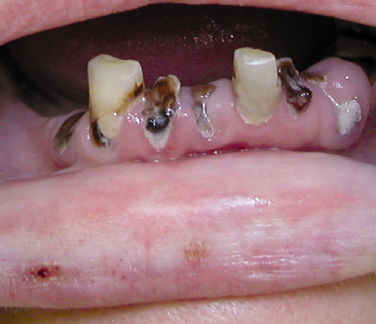
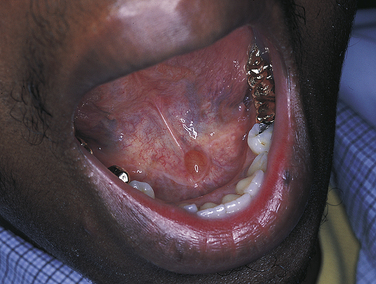
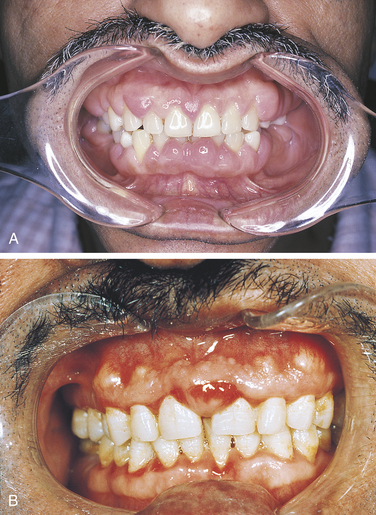
Bulimia is an eating disorder characterized by food binges, usually of very high caloric intake, followed by self-induced vomiting. The frequent vomiting in an individual with bulimia results in generalized erosion of the lingual surfaces of teeth (Figure 2-25). Because of the pattern of erosion of the lingual surfaces of the teeth caused by frequent vomiting, the dental hygienist may be the first health care professional to identify a patient with bulimia and may assist in encouraging the patient to seek treatment. Bulimia differs from anorexia nervosa, another eating disorder, which is characterized by a distorted perception of body image, in addition to depression, intense fear of gaining weight, and self-imposed starvation. The patient with bulimia maintains a normal body weight but is secretive about eating habits. Vomiting after eating is a component of bulimia and not of anorexia nervosa. Electrolyte imbalance and signs of malnutrition may be present. Irritation of the oral mucosa and lips may occur, and there may be lesions on the back of the fingers caused by their continual use to induce vomiting.
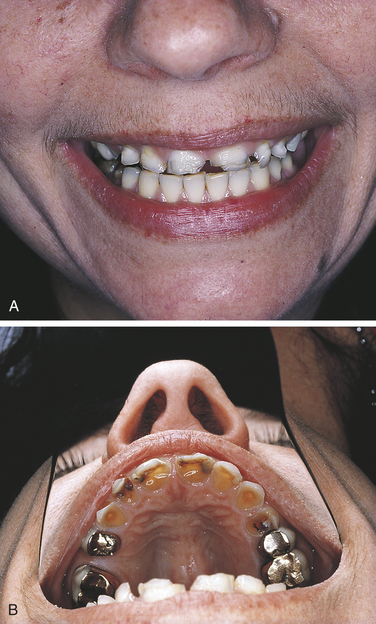
FIGURE 2-25 Erosion caused by bulimia. A, Decreased tooth size. B, Erosion of maxillary lingual surfaces.
Dental management of patients who vomit frequently includes an effort to minimize the effects of acid on tooth enamel by encouraging the daily use of fluoride rinse and toothpaste containing fluoride. Rinsing the mouth with water and thoroughly cleaning the teeth immediately after vomiting episodes also lessens the effects of acid.
METHAMPHETAMINE ABUSE
Recently the oral manifestations of methamphetamine abuse have been described. Acid content of methamphetamine, decreased salivary flow, and craving for high sugar–containing beverages combined with lack of oral hygiene care results in the extensive and rapid destruction of teeth that is called “meth mouth” (Figure 2-26).
ASPIRIN BURN
An aspirin burn generally occurs when a patient with a toothache places an aspirin tablet directly on the painful tooth instead of swallowing it. Aspirin (acetylsalicylic acid) is an analgesic (pain reliever) and antiinflammatory agent that must be ingested to be effective. Topical application is a common misuse of aspirin. As a result of placing the aspirin on the soft tissue, the tissue becomes necrotic and appears white. The lesion is painful, and the necrotic tissue may separate from the underlying connective tissue and slough off, resulting in a large ulcer (Figure 2-27). Questioning the patient should reveal the cause of the lesion, and the diagnosis is generally made without the need for biopsy of the tissue. An aspirin burn is painful and heals slowly because of the extent of destruction. However, the ulcer usually heals spontaneously in 7 to 21 days. The patient requires appropriate treatment of the painful tooth and medication for symptomatic relief of pain until the ulcer heals.
PHENOL BURN
Phenol is used in dentistry as a cavity-sterilizing and cauterizing agent. When phenol comes into contact with the soft tissues, a whitening of the exposed area occurs as a result of tissue destruction. The surface tissue may slough off, exposing the underlying connective tissue. The resulting ulcer is painful, and the duration of healing depends on the extent of the destruction. The phenol should be removed immediately to minimize the destruction. If phenol is ingested, the patient should drink large amounts of water and be referred for medical evaluation. Other agents used in dental treatment may also cause mucosal burns (Figure 2-28, A).
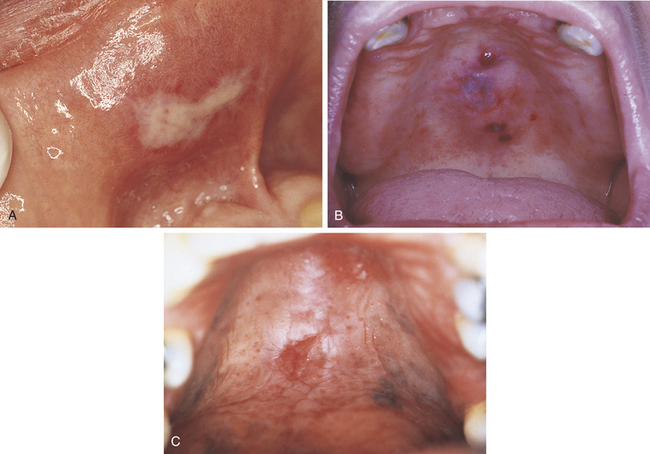
FIGURE 2-28 Mucosal burns. A, Chemical burn caused by contact with caustic material during endodontic treatment. B, Thermal burn of palate caused by contact with hot soup. C, Ulcer of midline of palate caused by heat generated during the use of crack cocaine. (C reprinted by permission of ADA Publishing Co., Inc. from Mitchell-Lewis DA et al: Identifying oral lesions associated with crack-cocaine use, J Am Dent Assoc 125:1104, 1994. Copyright 1994, American Dental Association.)
Phenol is also a component of some over-the-counter products that are advertised for relief of oral pain. Patients frequently misuse these preparations for oral ulcers, and the resulting destruction, in addition to being quite painful, may mask the clinical and microscopic diagnostic characteristics of the original ulcer.
ELECTRIC BURN
Electric burns in the oral area are usually seen in infants and young children who have bitten or chewed a live electric cord or have inserted something into an electric socket. The electric current can cause a great deal of destruction of the oral tissues. Any tissue in the area may be damaged, including the permanent tooth buds. Permanent disfigurement and scarring may result from this type of injury. Treatment may require a multidisciplinary approach that includes plastic surgery, oral surgery, and orthodontics.
OTHER BURNS
Mucosal burns from hot food are common. They occur most often on the palate and tongue (Figure 2-28, B). Over-the-counter products containing hydrogen peroxide or eugenol can also cause mucosal necrosis.
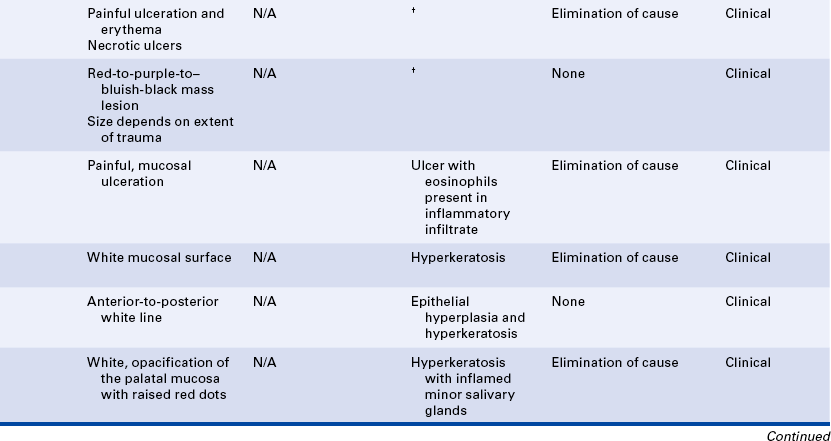
LESIONS ASSOCIATED WITH COCAINE USE
Lesions located at the midline of the hard palate that vary from ulcers to keratotic lesions to exophytic reactive lesions have been reported to result from the smoking of crack cocaine (Figure 2-28, C). When crack cocaine is smoked, the crack pipe directs extremely hot smoke to this part of the hard palate. Identification of these lesions is based on their location and the history of recent smoking of crack cocaine. Necrotic ulcers of the tongue and epiglottis related to smoking free-base cocaine have also been reported.
LESIONS FROM SELF-INDUCED INJURIES
Habits of which the patient may or may not be aware can cause injury. Chronic lip, cheek, or tongue biting (see Figure 2-31, B) and trauma to the gingiva by a fingernail (see Figure 2-30, A) are examples of habits that may cause oral lesions. These lesions range from ulceration to epithelial hyperplasia and hyperkeratosis. Ulcers caused by continual self-induced injuries may be of long duration and require biopsy and histologic examination to confirm the diagnosis. Treatment of self-induced lesions depends on the amount and type of destruction and may involve psychotherapy.
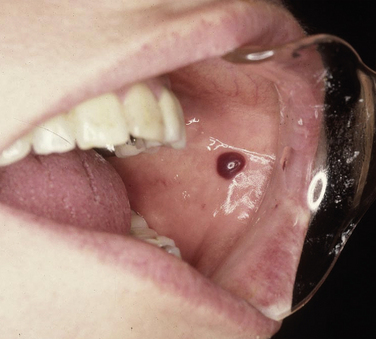
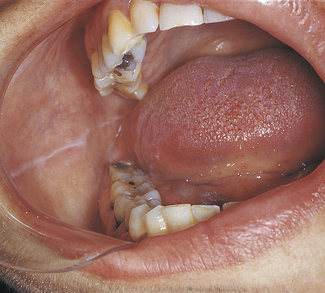
HEMATOMA
A hematoma is a lesion that results from the accumulation of blood within tissue as a result of trauma. In the oral cavity a hematoma appears as a red-to-purple-to–bluish-gray mass, most frequently seen on the labial or buccal mucosa (Figure 2-29). The size may vary from small to large, depending on the extent of the trauma. No treatment is required because the lesion will spontaneously resolve.
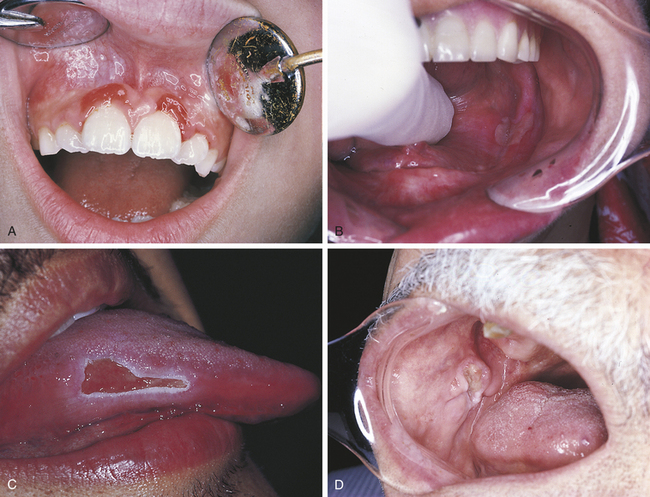
TRAUMATIC ULCER
A traumatic ulcer occurs as a result of some form of trauma (Figure 2-30). Sources of trauma vary. Biting the cheek, lip, or tongue may result in a traumatic ulcer, as can irritation from a complete or partial denture or mucosal injury from sharp edges of food. The removal of a dry cotton roll from the oral tissue after a dental procedure can cause a traumatic ulcer, and it is not uncommon to see a patient present for a dental hygiene appointment with a traumatic injury to the gingival tissues or vestibular mucosa that results from overzealous brushing before the appointment. Persistent trauma may result in a hard (indurated), raised lesion called a traumatic granuloma (see Figure 2-30, D).
Traumatic ulcers are usually diagnosed on the basis of the relationship of the history to the lesion. Healing is usually uneventful and occurs in 7 to 14 days unless the trauma persists. If trauma persists, ulcers may last for weeks to months. The patient is followed until healing is ensured. If an ulcer does not heal in 7 to 14 days, a biopsy is usually indicated. Microscopically the inflammatory infiltrate associated with a traumatic ulcer includes many eosinophils in addition to neutrophils, lymphocytes, and plasma cells. Persistent traumatic granulomas often heal rapidly following biopsy.
FRICTIONAL KERATOSIS
Chronic rubbing or friction against an oral mucosal surface may result in hyperkeratosis, a thickening of the keratin on the surface. This results in an opaque, white appearance of the tissue and represents a protective response. It is analogous to a callus on the skin. An example of frictional keratosis is an increase in surface keratin that results from chronic cheek and tongue chewing and chewing on edentulous alveolar ridges (Figure 2-31). Frictional keratosis is not associated with malignancy.
The diagnosis of frictional keratosis is made by identification of the trauma causing the lesion, elimination of the cause, and observing the resolution of the lesion. The keratosis may take a while to disappear on keratinized surfaces such as the gingiva.
Frictional keratosis must be distinguished from other white lesions. White lesions that are not caused by trauma and arise spontaneously are called leukoplakia. Leukoplakia may be a premalignant lesion (see Chapter 7). Biopsy is indicated for any questionable lesion.
LINEA ALBA
Linea alba is a white raised line that forms most commonly on the buccal mucosa at the occlusal plane and is usually considered a variant of normal (Figure 2-32). In some patients the line becomes prominent as a result of a teeth-clenching habit. The line follows the pattern of the teeth at the occlusal plane. Although it is most commonly seen on the buccal mucosa, linea alba may form on the labial mucosa as well. Histologically the white raised line is caused by epithelial hyperplasia and hyperkeratosis. No treatment is indicated. The prominence of linea alba may be helpful in evaluating the severity of the clenching habit.
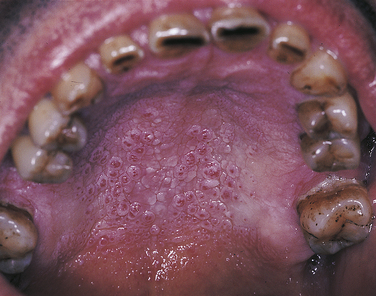
NICOTINE STOMATITIS
Nicotine stomatitis is a benign lesion on the hard palate most typically associated with pipe and cigar smoking; however, it also occurs with cigarette smoking. The development of the lesion indicates that the patient is smoking heavily. The intensity of smoking required to produce this lesion increases the patient’s risk for development of malignancy elsewhere in the oral cavity and respiratory tract.
The initial response of the palatal mucosa to the heat from these substances is an erythematous appearance. Over time keratinization occurs and results in increasing opacification. After the increase in keratinization, raised red dots are seen at the openings of the ducts of the minor salivary glands on the palatal surface (Figure 2-33). The minor salivary glands become inflamed as a result of obstruction by keratin at the mucosal opening of the ducts. The palate may develop a very similar clinical appearance as a result of the chronic intake of very hot liquids.
TOBACCO POUCH KERATOSIS
Individuals who chew tobacco may develop a white lesion in the area where the tobacco is habitually placed. The mucobuccal fold is the most common location. The epithelium usually has a granular or wrinkled appearance in early lesions. Long-standing lesions may be more opaquely white and have a corrugated surface (Figure 2-34). The lesion often disappears when the tobacco is no longer placed in the area.
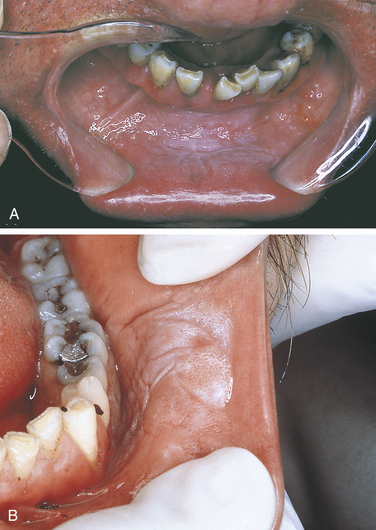
FIGURE 2-34 Tobacco pouch keratosis. Note the rough texture of the surface. A, Labial mucosa. B, Anterior buccal mucosa.
Long-term exposure to smokeless tobacco has been associated with an increased risk of squamous cell carcinoma. Lesions that do not resolve require biopsy and may show atypical dysplastic epithelium. In addition, the patient with this habit has an increased risk of caries, periodontal disease, attrition, and staining.
TRAUMATIC NEUROMA
A traumatic neuroma is a lesion caused by injury to a peripheral nerve. Nerve tissue is encased in a sheath composed of Schwann cells and their fibers. When this sheath is disrupted, the nerve loses its framework. When a nerve and its sheath are damaged, the proximal end of the damaged nerve proliferates into a mass of nerve and Schwann cells mixed with dense fibrous scar tissue. In the oral cavity injury to a nerve may occur from injection of local anesthesia, surgery, or other sources of trauma.
Traumatic neuromas are often painful. The pain may range from pain on palpation to severe and constant pain. Most traumatic neuromas occur in adults, and the mental foramen is the most common location. However, a traumatic neuroma may occur in other locations as well. Although the clinical features, particularly the pain that is characteristic, may suggest that a lesion is a traumatic neuroma, the diagnosis is made on the basis of a biopsy and microscopic examination. Traumatic neuromas are treated by surgical excision, and recurrence is rare.
AMALGAM TATTOO
An amalgam tattoo is a flat, bluish-gray lesion of the oral mucosa that results from the introduction of amalgam particles into the tissues (Figure 2-35, A). This may occur at the time of placement or removal of an amalgam restoration or at the time of tooth extraction if a piece of amalgam fractures off a restoration and remains in the tissue. The metallic particles disperse in the connective tissue and result in a permanent area of pigmentation. Over time the mercury-silver-tin amalgam changes, and it is mainly silver that remains in the tissue.
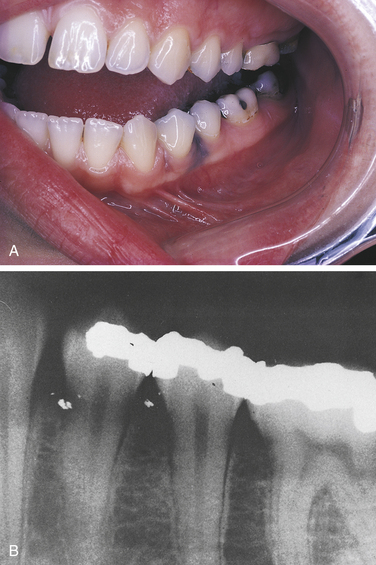
FIGURE 2-35 A, This bluish-gray pigmentation of the gingiva is an amalgam tattoo. B, Periapical radiograph showing amalgam particles in the gingival tissue.
Amalgam tattoos may be seen in any location in the oral cavity but are most commonly found on the gingiva or edentulous alveolar ridge. The posterior region of the mandible is the most common location.
An amalgam tattoo is usually diagnosed on the basis of clinical appearance of the pigmented area. Often the particles of amalgam can be seen on a periapical radiograph (Figure 2-35, B). As amalgam particles diffuse in the tissue over time, the size of the amalgam tattoo may increase. Biopsy may be necessary to distinguish an amalgam tattoo from a melanocytic lesion, particularly if it is located in an area other than the gingiva or alveolar ridge. Once the diagnosis of amalgam tattoo has been established, treatment is generally not indicated.
MELANOSIS
Normal physiologic pigmentation of the oral mucosa is common, particularly in dark-skinned individuals (see Figures 1-18 and 1-51). Melanin pigmentation may also occur after inflammation (Figure 2-36). The oral melanotic macule is a flat, well-circumscribed brown lesion of unknown cause. These are usually small (<1 cm in diameter) and may require biopsy and histologic examination for diagnosis. A similar lesion may occur on the vermilion of the lips. When occurring on the lips, the lesion may darken with exposure to sunlight.
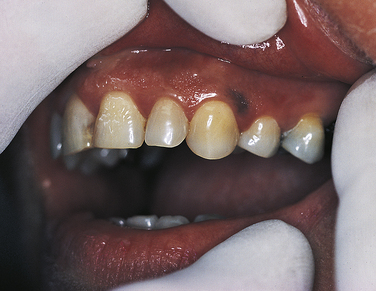
FIGURE 2-36 Posttraumatic melanin pigmentation. Area of melanin pigmentation on the gingiva after healing of a traumatic injury.
Another type of melanosis is called smoker’s melanosis or smoking-associated melanosis. In this type of melanosis the melanin pigmentation is associated with smoking, and the intensity is related to the amount and duration of smoking. The pigmentation fades when smoking is discontinued. However, this may take months to years. The anterior labial gingiva is the most commonly affected site. Women are affected more frequently than men. A relationship to female hormones and birth control pills has been suggested. Oral mucosal melanin pigmentation may also be associated with genetic, bone, and systemic diseases (see Chapters 6, 8, and 9).
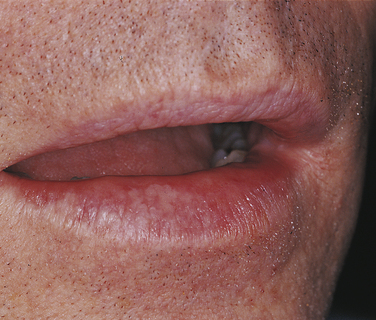
SOLAR CHEILITIS
Sun exposure, particularly in fair-skinned individuals, can result in degeneration of the tissue of the lips, called solar cheilitis (Figure 2-37). Solar cheilitis is also called actinic cheilitis. The development of this condition is related to the total cumulative exposure to sunlight and the amount of skin pigmentation; it may be seen in individuals of various ages. The lower lip is usually more severely involved than the upper lip. The epithelium is thinner than normal and often exhibits abnormal maturational changes (epithelial dysplasia). Degenerative changes are seen microscopically in the connective tissue.
The vermilion of the lips is affected. The color appears pale pinkish and mottled. The interface between the lips and the skin is indistinct, with fissures appearing at right angles to the skin and vermilion junction. The lips are dry and cracked.
No specific treatment is indicated. However, a strong relationship exists between these epithelial and connective tissue changes and the development of basal cell carcinoma and squamous cell carcinoma of the skin and squamous cell carcinoma of the vermilion of the lip. The risk is greater for the lower lip than the upper lip. Smoking may increase this risk. Biopsy is indicated for persistent scaling or ulceration. Identification of patients at high risk and with early indications of sun damage can be helpful in preventing future lesions. Patients at risk should be advised to avoid sun exposure and use sun-blocking agents for protection.
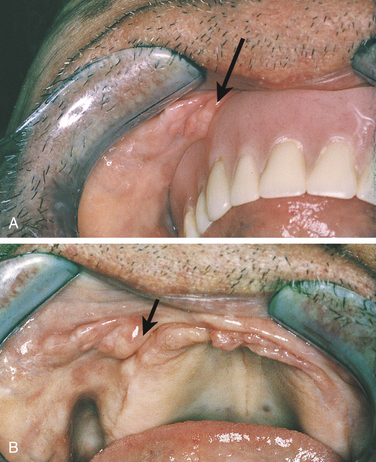
MUCOCELE
A mucocele is a lesion that forms when a salivary gland duct is severed and the mucous salivary gland secretion spills into the adjacent connective tissue. An inflammatory response occurs, granulation tissue forms, and the mucus is walled off to form a cystlike structure lined by compressed granulation tissue. This is not a true cyst because the cystic space is not lined by epithelium.
A mucocele presents as a swelling in the tissue that often increases and decreases in size over time. The lower lip is the most common site of occurrence (Figure 2-38). However, mucoceles may form in any area of the oral mucosa in which minor salivary glands are found. On the lower lip they are usually lateral to the midline. If a mucocele is near the surface, it may appear bluish. The color of the mucosa is normal if the mucocele is deeper in the tissue. A mucoepidermoid carcinoma may clinically resemble a mucocele and should be considered in the differential diagnosis. Most mucoceles occur in children and adolescents. However, they may occur in adults as well. If they are chronic or persistent, treatment is by surgical excision and removal of the adjacent minor salivary glands.
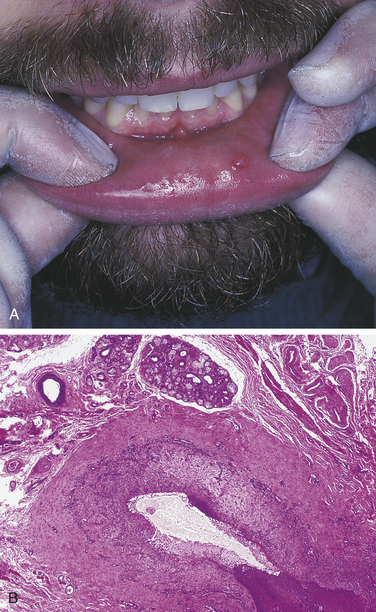
FIGURE 2-38 Mucocele of the lower lip. A, Fluid-filled lesion is seen on the lower lip. B, Microscopic appearance of a mucocele showing a cystlike space lined by granulation tissue (low magnification).
Occasionally an epithelium-lined cystic structure occurs in association with a salivary gland duct. This is also called a mucocele, mucous cyst, or mucous retention cyst (Figure 2-39) and occurs much less frequently than the type described previously. Most of these are not true cysts but rather dilated salivary gland ducts that are believed to develop as a result of salivary duct obstruction. A ballooning of the duct occurs, which appears microscopically as an epithelium-lined cyst. Mucous cysts usually occur in adults older than 50 years of age. They may occur anywhere in the oral cavity where minor salivary glands are found and are treated by removal of the affected minor salivary glands.
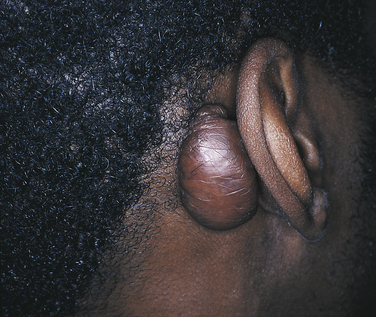
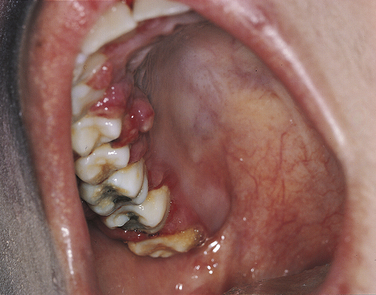
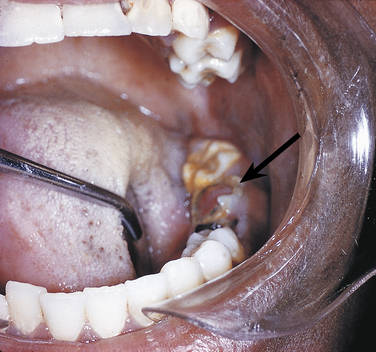
Ranula is a term used for a mucocele-like lesion that forms unilaterally on the floor of the mouth (Figure 2-40). It is associated with the ducts of the sublingual and submandibular glands. The name ranula is derived from rana, the Latin word for frog. The clinical appearance of the ranula resembles the outpouching that occurs under the jaw of the frog when croaking. Although obstruction of the duct is considered to be the most likely cause for its development, histologically a ranula may resemble either a mucocele or a mucous cyst. Ranulas are treated by surgery; and the cause of obstruction, often a salivary gland stone, must be removed.
NECROTIZING SIALOMETAPLASIA
Necrotizing sialometaplasia is a benign condition of the salivary glands characterized by moderately painful swelling and ulceration in the affected area (Figure 2-41). Histologically necrosis of the salivary glands is seen. The salivary gland duct epithelium is replaced by squamous epithelium (metaplasia) and appears microscopically as islands of squamous epithelium deep in the connective tissue. If the duration of the ulcer is prolonged, a biopsy is needed to establish the diagnosis. However, the ulcer heals spontaneously by secondary intention, usually within a few weeks. The junction of the hard and soft palates is most often affected. Necrotizing sialometaplasia is thought to result from blockage of the blood supply to the area of the lesion.
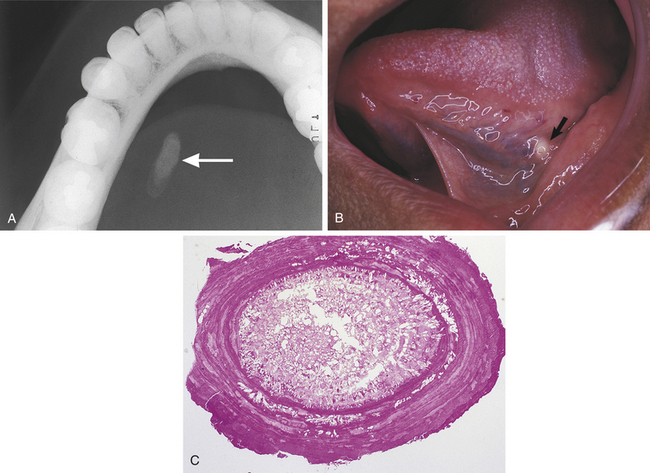
SIALOLITH
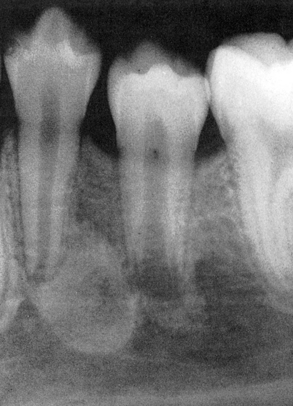
A sialolith is a salivary gland stone. Sialoliths occur in both major and minor salivary glands and form by precipitation of calcium salts around a central core. They may cause obstruction of the involved salivary gland; when they occur in the floor of the mouth, they can often be seen as a radiopaque structure on an occlusal radiograph (Figure 2-42).
ACUTE AND CHRONIC SIALADENITIS
Both acute and chronic sialadenitis may occur as a result of obstruction of a salivary gland duct. They may also occur as a result of infection. In some cases the cause cannot be identified. Sialadenitis presents as a painful swelling of the involved salivary gland, usually one of the major glands. Diagnosis may require injection of a radiopaque dye into the gland, followed by taking a radiograph of the gland (sialography). Antibiotics may be necessary in cases of infection.
REACTIVE CONNECTIVE TISSUE HYPERPLASIA
Reactive connective tissue hyperplasia consists of proliferating, exuberant granulation tissue and dense fibrous connective tissue. These lesions result from overzealous repair. They may occur as a response to a single event or as a chronic low-grade injury. The reason for the exuberant overgrowth of reparative tissue is not known.
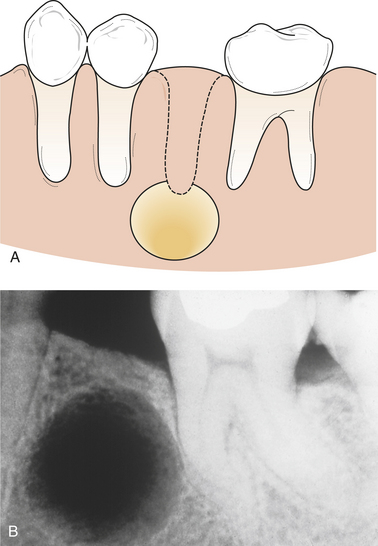
Periapical inflammation, radicular cyst, and internal and external tooth resorption are included in this classification because they are also examples of lesions associated with proliferating inflamed tissue.
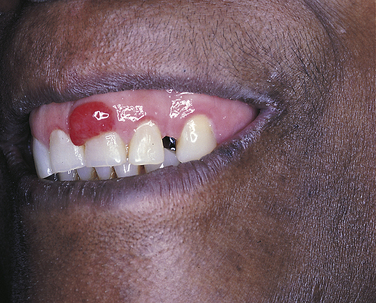
PYOGENIC GRANULOMA
A pyogenic granuloma is a commonly occurring intraoral lesion that is characterized by a proliferation of connective tissue containing numerous blood vessels and inflammatory cells. It occurs as a response to injury. The term pyogenic granuloma is a misnomer. The lesion does not produce pus (pyogenic) and is not a true granuloma.
The pyogenic granuloma (Figure 2-43) is usually ulcerated and soft to palpation and bleeds easily. It is deep red to purple because of the vascularity of the proliferating tissue. It is generally elevated and may be either sessile or pedunculated. When ulcerated, the fibrin membrane on the surface appears yellowish white. The gingiva is the most common location of occurrence, but the lesion also occurs in other areas such as the lips, tongue, and buccal mucosa. Pyogenic granulomas may vary considerably in size, from a few millimeters to several centimeters. They usually develop rapidly and then remain static. Although they are most common in teenagers and young adults, they may occur at any age. Some studies show predominance in females.
Pyogenic granulomas often occur in pregnant women and have been called pregnancy tumors (Figure 2-44). The lesions are identical to those seen in men and nonpregnant women and may be caused by changing hormonal levels and increased response to plaque. However, they often regress after delivery. Similar gingival lesions also occur during puberty.
The pyogenic granuloma is treated by surgical excision if it does not resolve spontaneously. Occasionally the lesion may recur if the injurious agent (e.g., calculus) remains.
PERIPHERAL GIANT CELL GRANULOMA
A giant cell granuloma is a lesion that contains many multinucleated giant cells and well-vascularized connective tissue. Red blood cells and chronic inflammatory cells are also seen in this lesion. The cause of the giant cell granuloma is not clear. It occurs only in the jaws, seems to originate from the periodontal ligament or the periosteum, and is thought to be a response to injury. The giant cell granuloma occurs both on the gingiva (peripheral giant cell granuloma) and within bone (central giant cell granuloma). The term peripheral pertains to lesions occurring outside the bone, generally on the gingiva or alveolar mucosa, and the term central refers to a lesion occurring within the maxilla and mandible. The description of the central giant cell granuloma is included in Chapter 8.
The peripheral giant cell granuloma always occurs on the gingiva or alveolar process, usually anterior to the molars. It is considered a reactive lesion, usually occurring as a result of local irritating factors. Peripheral giant cell granulomas associated with dental implants have been reported. The peripheral giant cell granuloma may resemble the pyogenic granuloma in clinical appearance (Figure 2-45). Peripheral giant cell granulomas may vary in size from 0.5 to 1.5 cm in diameter and are usually dark red because of the numerous blood vessels present. The lesion may occur at any age but has been reported to be more frequent in people between 40 and 60 years of age and more common in women than men. Peripheral giant cell granulomas may cause superficial destruction of the alveolar bone; they are treated by surgical excision of the lesion and generally do not recur.
IRRITATION FIBROMA
The irritation fibroma (also known as fibroma or traumatic fibroma) is a broad-based, persistent exophytic lesion that is composed of dense, scarlike connective tissue containing few blood vessels (Figure 2-46). It occurs as a result of chronic trauma or an episode of trauma. The irritation fibroma is usually a small lesion. Most are less than 1 cm in diameter; fibromas greater than 2 cm in diameter are rare. The fibroma occurs most frequently on the buccal mucosa. It also occurs on the gingival, tongue, lips, and palate. The color of the irritation fibroma is usually lighter than that of the surrounding mucosa. The surface is covered by stratified squamous epithelium and may appear opaque and white if it has a thick keratin surface, or it may be ulcerated because of local secondary trauma.
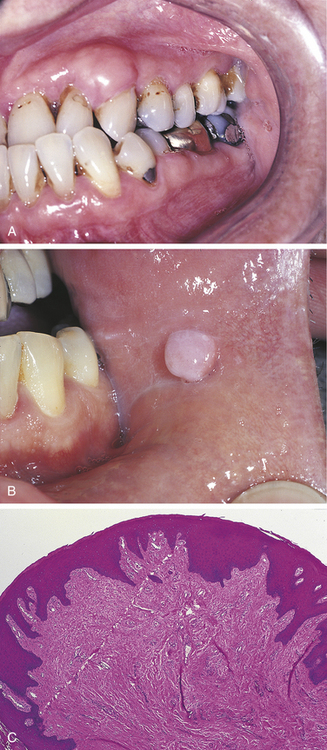
FIGURE 2-46 Irritation fibroma. A, Development of fibroma followed by the healing of a periodontal abscess. B, Irritation fibroma of the buccal mucosa. C, Microscopic appearance of a fibroma. (A courtesy Dr. Murray Schwartz.)
An irritation fibroma is removed surgically. Many benign soft tissue tumors resemble the irritation fibroma in clinical appearance. Excision and microscopic examination of the tissue are important for diagnosis if the clinician is unsure about the nature of the lesion. Irritation fibromas usually do not recur at the same site, but additional lesions may occur as a response to additional trauma.
The peripheral ossifying fibroma is a common gingival lesion. The cause is not known. It is thought to originate from the cells forming the periodontal ligament. However, it is not clear whether this lesion is a reactive or neoplastic lesion. The peripheral ossifying fibroma is included in Chapter 7.
DENTURE-INDUCED FIBROUS HYPERPLASIA
Denture-induced fibrous hyperplasia is commonly called epulis fissuratum or inflammatory hyperplasia. This lesion is caused by an ill-fitting denture and is located in the vestibule along the denture border. It is composed of dense, fibrous connective tissue surfaced by stratified squamous epithelium, the same type of tissue seen in the irritation fibroma. The lesion is usually somewhat larger than the irritation fibroma. It is arranged in elongated folds of tissue into which the denture flange fits (Figure 2-47). The surface of the lesion is often ulcerated. Because this lesion does not resolve even with prolonged removal of the denture, treatment involves surgical removal of the excess tissue and construction of a new denture.
PAPILLARY HYPERPLASIA OF THE PALATE
Papillary hyperplasia of the palate, or palatal papillomatosis, is a form of denture stomatitis. It is almost always associated with a removable full or partial denture or an orthodontic appliance. The palatal mucosa, most commonly the vault area, is covered by multiple erythematous papillary projections that give the area a granular or “cobblestone” appearance (Figure 2-48).
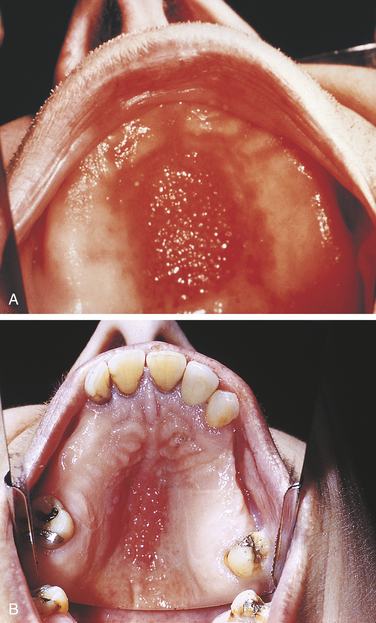
FIGURE 2-48 Papillary hyperplasia of the palate. A, Full denture. B, Partial denture. (Courtesy Dr. Edward V. Zegarelli.)
Each of the papillary projections consists of fibrous connective tissue, usually chronically inflamed and surfaced by stratified squamous epithelium. The precise cause of this type of hyperplasia is not known. It is usually related to an ill-fitting upper denture or other removable prosthetic device that is worn continuously, 24 hours a day. Surgical removal of the hyperplastic papillary tissue before construction of a new denture generally is necessary.
GINGIVAL ENLARGEMENT
Gingival enlargement is characterized by an increase in the bulk of the free and attached gingiva, especially that involving the interdental papillae (Figure 2-49). No stippling is seen, and the gingival margins are rounded. The tissue consistency may vary from soft to firm, and the appearance may vary from erythematous to a normal pink color, depending on the degree of inflammation. Gingival enlargement may be generalized or localized and may vary from mild focal enlargement of interdental papillae to severe generalized gingival enlargement that may cover the crowns of the teeth. The enlarged tissue may appear red, a normal mucosal color, or paler, depending on the amount of inflammation and degree of vascularity. Although generally called gingival hyperplasia, enlargement of the gingiva may also be the result of hypertrophy.
Most cases are the result of an unusual tissue response to chronic inflammation associated with local irritants such as plaque or calculus. Hormonal changes such as those occurring in pregnancy and puberty and certain drugs such as phenytoin, calcium channel blockers, and cyclosporine can increase the tissue response to local factors (see Chapter 9). Hereditary forms of gingival fibromatosis occur, beginning in early childhood (see Chapter 6). In some cases the cause of gingival hyperplasia cannot be identified.
If the tissue is inflamed and bleeds easily, biopsy and histologic examination may be necessary to rule out the gingival enlargement that may occur in individuals with leukemia (see Chapter 7).
Gingivoplasty (reshaping the gingiva) or gingivectomy (removing gingival tissue) may be necessary to recontour the gingival tissue. Meticulous oral hygiene is helpful in reducing additional hyperplasia.
CHRONIC HYPERPLASTIC PULPITIS
Chronic hyperplastic pulpitis, or pulp polyp, is an excessive proliferation of chronically inflamed dental pulp tissue. In children and young adults it occurs in teeth with large, open carious lesions; primary and permanent molars are often affected. Chronic hyperplastic pulpitis appears as a red or pink nodule of tissue that often fills the entire cavity in the tooth, with tissue protruding from the pulp chamber (Figure 2-50). It is usually asymptomatic; because the hyperplastic tissue contains so few nerves, it is usually insensitive to manipulation. The proliferation of pulp tissue rather than pulpal necrosis, which can also result from dental caries, may be related to the large root opening and blood supply of the tooth involved.
The hyperplastic tissue is granulation tissue. Inflammatory cells, primarily lymphocytes and plasma cells and sometimes neutrophils, are commonly present. The tissue is generally surfaced by stratified squamous epithelium. Because no epithelium exists in normal pulp tissue, this epithelium is thought to result from desquamation of the surface oral mucosa. Chronic hyperplastic pulpitis is treated by either extraction or endodontic treatment of the involved tooth.
INFLAMMATORY PERIAPICAL LESIONS
Dental caries or trauma to a tooth may result in a variety of responses: inflammation, infection, chronic hyperplastic pulpitis, and necrosis of the dental pulp. The inflammatory process begins in the dental pulp and then extends into the periapical area (the area surrounding the apical portion of the tooth root at the site of the apical foramen). This condition occurs because, once the inflammatory process has been established in the dental pulp, the only route it can follow is through the root canal into the periapical area. The presence of accessory canals may lead to areas of inflammation located on the lateral portion of the tooth root.
PERIAPICAL ABSCESS
An acute periapical abscess is composed of a purulent exudate, or pus, surrounded by connective tissue containing neutrophils and lymphocytes. The patient complains of severe pain, which is the result of inflammation. The inflammatory exudate puts pressure on nerves, and chemical mediators can also cause pain. The periapical abscess may develop directly from the inflammation in the pulp, but it more commonly develops in an area of previously existing chronic inflammation. The pus that forms seeks a path of least resistance and finds either a channel or a fistula out of the tissue, or it spreads to contiguous areas through oral and facial tissue spaces (see Figure 2-3). The tooth associated with the abscess is usually quite painful and may be slightly extruded from its socket. If the acute abscess develops directly from pulpal inflammation, there may be no radiographic changes except for a slight thickening of the apical periodontal ligament space. If the abscess develops in a preexisting area of periapical chronic inflammation, a distinct radiolucent area is seen at the apex.
The clinician treats a periapical abscess by establishing drainage, by either opening the pulp chamber or extracting the tooth. If the abscess has extended into adjacent tissue, an incision may be necessary to establish drainage. The patient may also be given antibiotic therapy.
DENTAL OR PERIAPICAL GRANULOMA
A periapical granuloma is a localized mass of chronically inflamed granulation tissue that forms at the opening of the pulp canal, generally at the apex of a nonvital tooth root (Figure 2-51). This is a chronic process from the outset. Most cases are completely asymptomatic. Sometimes the tooth is sensitive to pressure and percussion because of the inflammation in the apical area. The tooth may also appear slightly extruded from its socket. The radiographic change may vary from a slight thickening of the periodontal ligament space in the area of inflammation to a diffuse radiolucency to a distinct, well-circumscribed radiolucency surrounding the root apex.
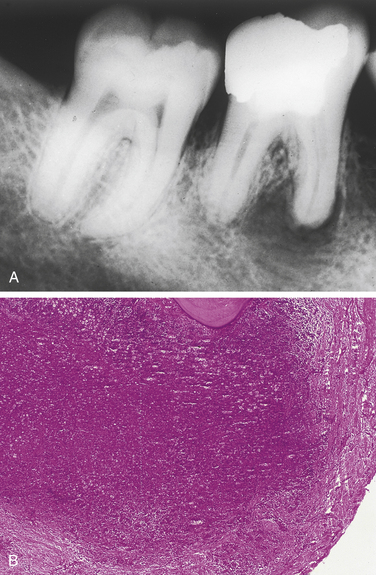
FIGURE 2-51 A, Radiograph of a periapical granuloma. B, Microscopic appearance of a periapical granuloma showing a collection of inflammatory cells at the apex of a tooth (low magnification). (A courtesy Dr. Herbert Frommer.)
The periapical granuloma is composed of granulation tissue containing lymphocytes, plasma cells, and macrophages. Neutrophils may also be present, and areas of dense fibrous connective tissue are often seen. The periapical granuloma is different from the granuloma or granulomatous inflammation, which is a distinctive type of inflammation characteristic of certain diseases (e.g., tuberculosis). Epithelial rests of Malassez, which are remnants of tooth-forming tissue, are often present in the periapical granuloma. The periapical granuloma is treated by root canal therapy or extraction of the tooth.
RADICULAR CYST (PERIAPICAL CYST)
A radicular cyst, or periapical cyst, is a true cyst consisting of a pathologic cavity lined by epithelium. It occurs in association with the root of a nonvital tooth (Figure 2-52). It is the most commonly occurring cyst in the oral region. The epithelial lining of the cyst develops in a periapical granuloma as a result of proliferation of the epithelial rests of Malassez.
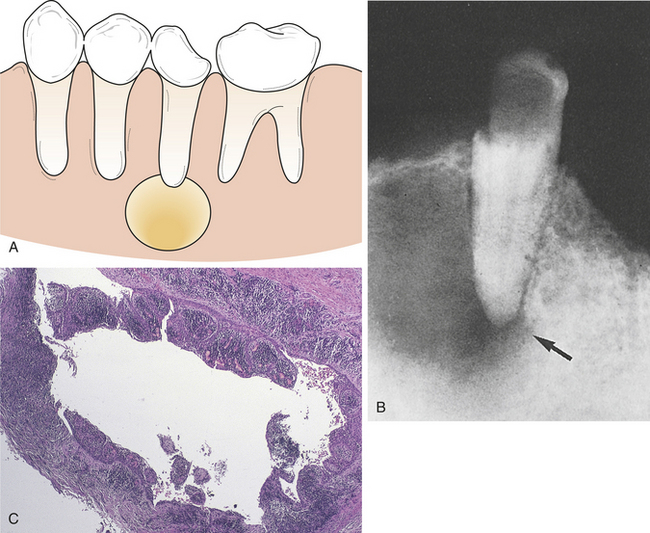
FIGURE 2-52 Radicular cyst. A, A radicular cyst located around the root of an erupted tooth. B, Radiograph showing a well-circumscribed radiolucency around the root of a tooth. C, Microscopic features of a radicular cyst.
A periapical cyst develops when the epithelium within the inflamed connective tissue of the periapical granuloma proliferates, forming an epithelial mass that increases in size through division of the peripheral cells. The peripheral cells are the equivalent of the basal cell layer of the epithelium. As the cells in the central portion of the mass become increasingly separated from the source of nutrition in the connective tissue, they degenerate centrally, forming a cavity (lumen) that is lined by epithelium and filled with fluid. Histologically the tissue surrounding this epithelial-lined cystic cavity is similar to that seen in the periapical granuloma.
Most periapical cysts are asymptomatic and discovered on radiographic examination. The radiographic appearance of the radicular cyst is the same as that of the periapical granuloma. It appears as a radiolucency, usually well circumscribed, that is attached to a tooth root. It is not possible to differentiate reliably a periapical granuloma from a radicular cyst on the basis of the radiographic appearance alone. A periapical cyst may form in association with any tooth. The cyst may occur lateral to the tooth root rather than at the apex if it is associated with a lateral pulp canal. Other types of cysts can resemble a periapical cyst radiographically; therefore removal of the cyst and microscopic examination of the tissue are necessary.
The radicular cyst is treated by root canal therapy, apicoectomy, or extraction and curettage of the periapical tissues. A residual cyst forms when the tooth is removed and all or part of a periapical cyst is left behind (Figure 2-53). Radiographically a residual cyst is a well-circumscribed radiolucency located at the site of tooth extraction. It is treated by surgical removal of the cyst.
RESORPTION OF TEETH
Tooth structure can be resorbed in the same manner as bone. This occurs normally in the process of exfoliation of deciduous teeth and may also occur in other situations. Resorption of the tooth structure beginning at the outside of the tooth is called external resorption. This usually involves the root of a tooth but can occasionally involve the crown of an impacted tooth.
Like bone resorption, tooth resorption can occur when inflammatory tissue is present. Resorption of the root of a tooth sometimes occurs when a periapical granuloma is present. Bone resorption as a response to pressure allows orthodontic tooth movement to occur. Pressure can also cause resorption of tooth structure. This can occur from excessive occlusal or orthodontic forces and with benign and malignant tumors. When a tooth that has been avulsed is reimplanted, the root is resorbed and replaced by bone. Occasionally resorption may involve the crown of an impacted tooth or the roots of teeth, and the cause cannot be identified. This is called idiopathic tooth resorption.
External root resorption first appears as a slight raggedness or blunting of the root apex and can proceed to severe loss of tooth substance (Figure 2-54). The condition is not reversible, but progression of the process can be avoided if the cause can be identified and removed. Resorption of impacted teeth can also occur. Generalized root resorption may also occur after orthodontic tooth movement.
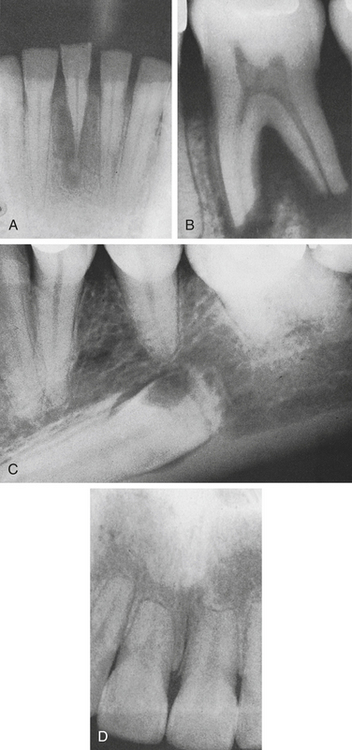
FIGURE 2-54 Tooth resorption. A, Root resorption of a mandibular anterior tooth associated with chronic inflammation. B, Resorption of tooth structure and bone because of chronic inflammation. C, Idiopathic resorption of an impacted tooth. D, Generalized root resorption associated with orthodontic tooth movement. (A courtesy Dr. Gerald P. Curatola.)
Internal tooth resorption can occur in any tooth. Usually only a single tooth is involved. In some cases no cause can be identified. However, it is usually associated with an inflammatory response in the pulp. If the process occurs in the coronal part of the tooth, it may be seen clinically as a pinkish area in the crown. The dental hard tissue has resorbed and is thinner than normal, and the pink color results from the vascular, inflamed connective tissue that can be seen through the remaining enamel and dentin. When the process involves the root, it can be seen only radiographically. A round-to-ovoid radiolucent area associated with the pulp is seen in the central portion of the tooth.
Root canal treatment can be performed successfully if internal resorption is discovered early. If not treated, the process can extend through the dental hard tissue and cause a perforation. After this perforation occurs, the tooth must be extracted.
FOCAL SCLEROSING OSTEOMYELITIS
Focal sclerosing osteomyelitis, also called condensing osteitis, is a change in bone near the apexes of teeth that is thought to be a reaction to low-grade infection. Radiographically a radiopaque area is seen extending below the roots of the involved tooth (Figure 2-55). The tooth most commonly associated with focal sclerosing osteomyelitis is the mandibular first molar. Occasionally the mandibular second molar and the mandibular premolars may also be involved.
Radiographically focal sclerosing osteomyelitis appears as a radiopaque area extending below the roots of the tooth. The borders may be diffuse or well defined. Occasionally the periphery of the area is radiolucent; in other cases a central radiolucency surrounded by radiopacity is seen. Microscopically it is dense bone with little marrow or connective tissue. Generally very little inflammation is present. Focal sclerosing osteomyelitis is often associated with a carious or restored tooth. It is generally asymptomatic. However, pain may be associated if pulpal inflammatory disease is present. Focal sclerosing osteomyelitis may be present at any age, but it is typically first seen in young adults.
Diagnosis of focal sclerosing osteomyelitis usually can be made on the basis of the characteristic radiographic appearance. Occasionally biopsy may be necessary to rule out other radiopaque lesions such as osteoma, complex odontoma, or ossifying fibroma. Treatment is not necessary. The sclerotic bone remains even after treatment of the involved tooth.
ALVEOLAR OSTEITIS (“DRY SOCKET”)
Alveolar osteitis or “dry socket” is a postoperative complication of tooth extraction. The most frequently affected area is the socket of an extracted mandibular third molar. After the extraction of the tooth, the blood clot breaks down and is lost before healing has taken place. Pain develops several days after the extraction. On examination the tooth socket appears empty, and the bone surfaces exposed. The patient complains of pain, bad odor, and bad taste. Because no infection exists, fever, swelling, and erythema are not present. Treatment of alveolar osteitis is directed at relief of pain and includes gentle irrigation and insertion of a medicated dressing.
Bath-Balogh, M., Fehrenbach, M.J. Illustrated dental embryology, histology, anatomy, ed 2. St Louis: Saunders, 2006.
Fehrenbach, M.J., Herring, S.W. Illustrated anatomy of the head and neck, ed 3. St. Louis: Saunders, 2007.
McCance, K., Huether, S. Pathophysiology, ed 5. St Louis: Mosby, 2005.
Kumar, V., Cotran, R.S., Robbins, S.L. Robbins basic pathology, ed 8. Philadelphia: Saunders, 2007.
Neville, B.W., et al. Oral and maxillofacial pathology, ed 3. St Louis: Saunders, 2009.
Regezi, J.A., Sciubba, J.J. Oral pathology: clinical-pathologic correlations, ed 5. St Louis: Saunders, 2008.
Broughton, G., II., Janis, J.E., Attinger, C.E. Wound healing: an overview. Plast Reconstr Surg. 2006;117(7 Suppl):S1e.
Eming, S.A., Krieg, T., Davidson, J.M. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127(3):514.
Kobayashi, S.D., et al. Neutrophils in the innate immune response. Arch Immunol Ther Exp (Warsz). 2005;53(6):505.
Krishnamoorthy, S., Honn, K.V. Inflammation and disease progression. Cancer Metastasis Rev. 2006;25(3):481.
Krishnaswamy, G., Ajitawi, O., Chi, D.S. The human mast cell: an overview. Methods Mol Biol. 2006;315:13.
Moore, K. An anatomy of an infection: overview of the infectious process. Crit Care Nurs Clin North Am. 2007;19(1):9.
Rankin, J.A. Biological mediators of acute inflammation. AACN Clin Issue. 2004;15(1):3.
Zamai, L., et al. NK cells and cancer. J Immunol. 2007;178(7):4011.
Lesions From Physical and Chemical Injuries to the Oral Tissues
Axell, T., Hedin, C. Epidemiologic study of excessive oral melanin pigmentation with special reference to the influence of tobacco habits. Scand J Dent Res. 1982;90:432.
Bouquot, J.E., Seime, R.J. Bulimia nervosa: dental perspectives. Pract Periodontics Aesthet Dent. 1997;9:655.
Buchner, A., Hansen, L.S. Amalgam pigmentation (amalgam tattoo) of the oral mucosa. Oral Surg Oral Med Oral Pathol. 1980;49:39.
Ferguson, M.M., et al. Enamel erosion related to wine making. Occup Med. 1996;46:159.
Gandara, B.K., Truelove, E.L. Diagnosis and management of dental erosion. J Contemp Dent Pract. 1999;1:16.
Geurtsen, W. Rapid general dental erosion by gas-chlorinated swimming pool water: review of the literature and case report. Am J Dent. 2000;13:291.
Gormley, M.B., et al. Thermal trauma: a review of 22 electrical burns of the lip. J Oral Surg. 1972;30:531.
Greer, R.O., Paulsion, T.C. Oral tissue alterations associated with the use of smokeless tobacco by teenagers. Oral Surg Oral Med Oral Pathol. 1983;56:275.
Hanemura, H., et al. Periodontal status and bruxism: a comparative study of patients with periodontal disease and occlusal parafunctions. J Periodontol. 1987;58:173.
Imbery, T.A., Edwards, P. Necrotizing sialometaplasia: literature review and case reports. J Am Dent Assoc. 1996;127:1087.
Järvinen, V., et al. Dental erosion and upper gastrointestinal disorders. Oral Surg Oral Med Oral Pathol. 1988;64:298.
Kapila, Y.L., Kashani, H. Cocaine-associated rapid gingival recession and dental erosion: a case report. J Periodontol. 1997;68:485.
Lineberry, T.W., et al. Methamphetamine abuse: a perfect storm of complications. Mayo Clin Proc. 2006;81:77.
Little, J.W. Eating disorders: dental implications. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:138.
Maron, F.S. Enamel erosion resulting from hydrochloric acid tablets. J Am Dent Assoc. 1996;127:781.
Mitchell-Lewis, D.A., et al. Identifying oral lesions associated with crack cocaine use. J Am Dent Assoc. 1994;125:1104.
Needleman, H.L., Berkowitz, R.J. Electric trauma to the oral tissues of children. J Dent Child. 1974;41:19.
Nemcovsky, C.E., Artizi, Z. Erosion-abrasion lesions revisited. Compend Contin Educ Dent. 1996;17:416.
Owens, B.M., Gallien, G.S. Noncarious dental “abfraction” lesions in an aging population. Compend Contin Educ Dent. 1995;16:552.
Owens, B.M., Johnson, W.W., Schuman, N.J. Oral amalgam pigmentation (tattoos): a retrospective study. Quintessence Int. 1992;23:805.
Owens, B.M., Schuman, N.J., Johnson, W.W. Oral amalgam tattoos: a diagnostic study. Compendium. 1993;14:210.
Piotrowski, B.T., Gillette, W.B., Handcock, E.B. Examining the prevalence and characteristics of abfraction-like cervical lesions in a population of US veterans. J Am Dent Assoc. 2001;132:1694.
Pollman, L., Berger, F., Pollman, S. Age and dental abrasion. Gerodontics. 1987;3:94.
Pullinger, A.G., Seligman, D.A. The degree to which attrition characterizes differentiated patient groups of temporomandibular disorders. J Orofac Pain. 1990;7:196.
Rawlinson, A. Case report: labial cervical abrasion caused by misuse of dental floss. Dent Health (London). 1987;26:3.
Richmond, N.L. Update on dental erosion: office and home treatment for hypersensitive teeth. J Indiana Dent Assoc. 1987;66:29.
Roberts, M.W., Li, S.H. Oral findings in anorexia nervosa and bulimia nervosa: a study of 47 cases. J Am Dent Assoc. 1987;115:497.
Robertson, P.B., Walsh, M.M., Greene, J.C. Oral effects of smokeless tobacco use by professional baseball players. Adv Dent Res. 1997;11:307.
Rossie, K.M., Guggenheimer, J. Thermally induced ‘nicotine’ stomatitis: a case report. Oral Surg Oral Med Oral Pathol. 1990;70:597.
Rugh, J.D., Harlan, J. Nocturnal bruxism and temporomandibular disorders. Adv Neurol. 1988;49:329.
Rytomaa, I., et al. Bulimia and tooth erosion. Acta Odontol Scand. 1998;56:36.
Simmons, M.S., Thompson, D.C. Dental erosion secondary to ethanol-induced emesis. Oral Surg Oral Med Oral Pathol. 1987;64:731.
Sist, T., Green, G. Traumatic neuroma of the oral cavity. Oral Surg Oral Med Oral Pathol. 1981;51:394.
Welsh, R.A., Donely, C. The association between occlusion and attrition. Aust Orthod J. 1992;12:138.
Wray, A., McGuirt, F. Smokeless tobacco usage associated with oral carcinoma. Arch Otolaryngol Head Neck Surg. 1993;119:929.
Zimmers, P.L., Gobetti, J.P. Head and neck lesions commonly found in musicians. J Am Dent Assoc. 1994;125:1487.
Reactive Connective Tissue Hyperplasia
Bodner, L., et al. Growth potential of peripheral giant cell granuloma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:548.
Bonetti, F., et al. Peripheral giant cell granuloma: evidence for osteoclastic differentiation. Oral Surg Oral Med Oral Pathol. 1990;70:471.
Brown, R.S., et al. Nitrendipine-induced gingival hyperplasia: first case report. Oral Surg Oral Med Oral Pathol. 1990;70:593.
Brunet, L., et al. Prevalence and risk of gingival enlargement in patients treated with anticonvulsant drugs. Eur J Clin Invest. 2001;31:781.
Cloutier, M., et al. An analysis of peripheral giant cell granuloma associated with dental implant treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:618.
Daley, T.D., Nartey, N.O., Wysocki, G.P. Pregnancy tumor: an analysis. Oral Surg Oral Med Oral Pathol. 1991;72:196.
Daley, T., Wysocki, G., Day, C. Clinical and pharmacologic correlations in cyclosporine-induced gingival hyperplasia. Oral Surg Oral Med Oral Pathol. 1986;62:417.
Fay, A.A., et al. Felodipine-influenced gingival enlargement in an uncontrolled type 2 diabetic patient. J Periodontol. 2005;76:1217.
Gould, A., Escobar, V. Symmetrical gingival fibromatosis. Oral Surg Oral Med Oral Pathol. 1981;51:62.
Lederman, D., et al. Gingival hyperplasia associated with nifedipine therapy: report of a case. Oral Surg Oral Med Oral Pathol. 1984;57:620.
Miranda, J., et al. Prevalence and risk of gingival enlargement in patients treated with nifedipine. J Periodontol. 2001;72:605.
Proia, N.K., et al. Smoking and smokeless tobacco-associated human buccal cell mutations and their association with oral cancer—a review. Cancer Epidemiol Biomarkers Prev. 2006;15:1061.
Roberson, J.B., Crocker, D.J., Schiller, T. The diagnosis and treatment of central giant cell granuloma. J Am Dent Assoc. 1997;128:81.
Silverstein, L.H., et al. The late development of oral pyogenic granuloma as a complication of pregnancy: a case report. Compendium. 1996;17:192.
Zain, R.B., Fei, Y.J. Fibrous lesions of the gingiva: a histopathologic analysis of 204 cases. Oral Surg Oral Med Oral Pathol. 1990;70:466.
Inflammatory Periapical Lesions
Gunraj, M.N. Dental root resorption. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:647.
Harris, E.F., Butler, M.I. Patterns of incisor root resorption before and after orthodontic correction in cases with anterior open bites. Am J Orthod Dentofacial Orthop. 1992;101:112.
Harris, E.F., Robinson, Q.C., Woods, M.A. An analysis of causes of apical root resorption in patients not treated orthodontically. Quintessence Int. 1993;24:417.
REVIEW QUESTIONS
1. Which of the following is the body’s initial response to injury?
2. What type of inflammation occurs if the injury is minimal and brief and the source is removed from the tissue?
3. In the inflammatory response, the first microscopic event is:
4. Which one of the following conditions is a chronic inflammatory lesion?
5. The directed movement of white blood cells to the area of injury is called:
6. Which cells are most common in chronic inflammation?
7. The macrophage has many functions. Which of the following is not a function of the macrophage?
8. Which of the following is the term used to describe blood plasma and proteins that leave the blood vessels and enter the surrounding tissues during inflammation?
9. The process of phagocytosis directly involves the:
A Ingestion of foreign substances by white blood cells.
B Escape of plasma fluids and proteins from the microcirculation into the surrounding tissues.
C Displacement of white blood cells to the blood vessel walls.
D Attachment of white blood cells to the blood vessel walls.
10. Which of the following statements is INCORRECT concerning the neutrophil? The neutrophil:
11. During the process of inflammation, the second type of white blood cell to emigrate from the blood vessel into the injured tissue is the:
12. Components of the complement system mediate the inflammatory process by:
13. Two days after injury, granulation tissue can be described as:
14. The enlargement of superficial lymph nodes that occurs as a systemic sign of inflammation is:
B Regulated by the hypothalamus.
C Caused by changes in their lymphocytes.
D A process that involves only the lymph nodes in the submental area.
15. Which statement concerning repair in the body is true?
A Repair can be completed with the injurious agents present.
B Functioning cells and tissue components are always replaced by functioning scar tissue.
C Repair always results in regeneration.
D The process of repair is initiated by the inflammatory response.
16. The clot that forms during repair:
A Consists of fibrous connective tissue.
B Serves as a guide for migrating epithelial cells.
17. Healing by secondary intention refers to healing of an injury when:
A The incision has clean edges joined by sutures.
C An infection forms in the injured area.
D The patient has increased formation of granulation tissue.
18. An increase in the size of an organ or tissue resulting from an increase in the number of its cells is termed:
19. Normal bone tissue repair can be delayed by:
A Maintenance of osteoblast-producing tissues.
B Inadequate movement of bone tissue.
20. Which one of the following would appear as a pigmented lesion?
21. Which of the following statements is false?
A Attrition is the wearing away of tooth structure during mastication.
B Bruxism is the same as mastication.
C Erosion is the loss of tooth structure resulting from chemical action.
22. Loss of tooth structure associated with bulimia is caused by:
24. A patient has a generalized white appearance of the palate. Tiny erythematous dots can be seen, surrounded by a thickened raised white-to-gray area. Overall the palate appears wrinkled. This condition is most likely:
25. The primary and most common cause of a mucocele is:
26. A ranula is located on the:
27. Which one of the following would not occur on the gingiva?
28. Generalized loss of tooth structure primarily on the lingual surfaces of maxillary anterior teeth is associated with:
29. External tooth resorption occurs as a result of:
30. Which one of the following is considered to be the most likely cause of necrotizing sialometaplasia?
31. The most common site for a mucocele to occur is the:
32. The peripheral giant cell granuloma only occurs on the:
A Chronic inflammation of a salivary gland.
B Acute inflammation of a salivary gland.
34. Which of the following statements is false?
A A periapical cyst develops from a periapical granuloma.
B A periapical abscess always causes radiographic periapical changes.
C A periapical granuloma is a circumscribed area of chronically inflamed tissue.
35. Epulis fissuratum results from irritation caused by:
36. Which of the following statements is true?
A A traumatic neuroma is never painful.
B Necrotizing sialometaplasia is considered a denture-related lesion.
C Chronic hyperplastic pulpitis is the same as gingival hyperplasia.
37. Loss of tooth structure caused by chemical action describes:
38. Which of the following cysts is characteristically associated with a tooth that is nonvital on pulp testing?
39. Which cyst results when a tooth is extracted without removing the periapical cystic sac?
40. The most common cause of the radicular cyst is:
41. The wearing away of tooth structure through an abnormal mechanical action defines:
42. Which one of the following is not associated with attrition?
43. Heavy plaque and calculus, mouth breathing, orthodontic appliances, and overhanging restorations best describe some of the causative factors for:
44. The central giant cell granuloma:
45. During examination of the dentition, the dental hygienist notes the presence of active wear facets. This indicates that the patient is:
46. A patient has a loss of tooth structure on the labial surfaces of the anterior teeth and reports a high intake of citrus fruit juices. The hygienist would most likely suspect:
47. The amalgam tattoo represents amalgam particles in the tissue and is most commonly observed in the oral cavity on the:
48. A pink protruding mass in the occlusal surface of a mandibular first or second molar is most likely:
49. Which of the following drugs does NOT cause gingival enlargement?