The Child with Cardiovascular Dysfunction
CLINICAL CONSEQUENCES OF CONGENITAL HEART DISEASE
NURSING CARE OF THE FAMILY AND CHILD WITH CONGENITAL HEART DISEASE
Help Family Adjust to the Disorder
Educate Family About the Disorder
Help Families Manage the Illness at Home
On completion of this chapter the reader will be able to:
 Design a plan for assisting a child during a cardiac diagnostic procedure.
Design a plan for assisting a child during a cardiac diagnostic procedure.
 Demonstrate an understanding of the hemodynamics, distinctive manifestations, and therapeutic management of congenital heart disease.
Demonstrate an understanding of the hemodynamics, distinctive manifestations, and therapeutic management of congenital heart disease.
 Outline a care plan for an infant or child with congestive heart failure.
Outline a care plan for an infant or child with congestive heart failure.
 Describe the care for a child who has hypoxia.
Describe the care for a child who has hypoxia.
 Describe the care for an infant or a child with a congenital heart defect and its surgical repair.
Describe the care for an infant or a child with a congenital heart defect and its surgical repair.
 Discuss the nurse’s role in helping the child and family cope with congenital heart disease.
Discuss the nurse’s role in helping the child and family cope with congenital heart disease.
 Differentiate between rheumatic fever and rheumatic heart disease.
Differentiate between rheumatic fever and rheumatic heart disease.
 List the criteria for selected cholesterol screening of children.
List the criteria for selected cholesterol screening of children.
 Discuss the assessment and management of hypertension in children and adolescents.
Discuss the assessment and management of hypertension in children and adolescents.
 Outline a care plan for a child with Kawasaki disease.
Outline a care plan for a child with Kawasaki disease.
 Describe the emergency treatment for shock, including anaphylaxis.
Describe the emergency treatment for shock, including anaphylaxis.
CARDIOVASCULAR DYSFUNCTION
Cardiovascular disorders in children are divided into two major groups: congenital heart disease and acquired heart disorders. Congenital heart disease (CHD) includes primarily anatomic abnormalities present at birth that result in abnormal cardiac function. The clinical consequences of congenital heart defects fall into two broad categories: congestive heart failure (CHF) and hypoxemia. Acquired cardiac disorders are disease processes or abnormalities that occur after birth and can be seen in the normal heart or in the presence of congenital heart defects. They result from various factors, including infection, autoimmune responses, environmental factors, and familial tendencies.
History and Physical Examination
Taking an accurate health history is an important first step in assessing an infant or child for possible heart disease. Parents may have specific concerns, such as an infant with poor feeding or fast breathing, or a 7-year-old who can no longer keep up with friends on the soccer field. Others may not realize that their child has a medical problem; their baby has always been pale and fussy.
Asking details about the mother’s health history, pregnancy, and birth history are important in assessing infants. Mothers with chronic health conditions, such as diabetes or lupus, are more likely to have infants with heart disease. Some medications, such as phenytoin (Dilantin), are teratogenic to the fetus. Maternal alcohol use or illicit drug use increases the risk of congenital heart defects. Exposures to infections, such as rubella, early in pregnancy may result in congenital anomalies. Infants with low birth weight resulting from intrauterine growth restriction are more likely to have congenital anomalies. High-birth-weight infants have an increased incidence of heart disease.
A detailed family history is also important. There is an increased incidence of congenital cardiac defects if either parent or a sibling has a heart defect. Some diseases, such as Marfan syndrome, and some cardiomyopathies are hereditary. A family history of frequent fetal loss, sudden infant death, and sudden death in adults may indicate heart disease. Congenital heart defects are seen in many syndromes such as Down and Turner syndromes.
The physical assessment of suspected cardiac disease begins with observation of general appearance, then proceeds with more specific observations. The following are supplementary to the general assessment techniques described for physical examination of the chest and heart in Chapter 6.
Inspection: Nutritional state—Failure to thrive or poor weight gain is associated with heart disease.
Color—Cyanosis is a common feature of CHD, and pallor is associated with poor perfusion.
Chest deformities—An enlarged heart sometimes distorts the chest configuration.
Unusual pulsations—Visible pulsations of the neck veins are seen in some patients.
Respiratory excursion—This refers to the ease or difficulty of respiration (e.g., tachypnea, dyspnea, expiratory grunt).
Diagnostic Evaluation
A variety of invasive and noninvasive tests may be used in the diagnosis of heart disease (Table 25-1). Some of the more common diagnostic tools that require nursing assessment and intervention are described here.
TABLE 25-1
Procedures for Cardiac Diagnosis
| PROCEDURE | DESCRIPTION |
| Chest radiograph (x-ray) | Provides information on heart size and pulmonary blood flow patterns |
| Electrocardiography (ECG) | Graphic measure of electrical activity of heart |
| Holter monitor | 24-hour continuous ECG recording used to assess dysrhythmias |
| Echocardiography | Use of high-frequency sound waves obtained by a transducer to produce an image of cardiac structures |
| Transthoracic | Done with transducer on chest |
| M-mode | One-dimensional graphic view used to estimate ventricular size and function |
| Two-dimensional (2-D) | Real-time, cross-sectional views of heart used to identify cardiac structures and cardiac anatomy |
| Doppler | Identifies blood flow patterns and pressure gradients across structures |
| Fetal | Imaging fetal heart in utero |
| Transesophageal (TEE) | Transducer placed in esophagus behind heart to obtain images of posterior heart structures or in patients with poor images from chest approach |
| Cardiac catheterization | Imaging study using radiopaque catheters placed in a peripheral blood vessel and advanced into heart to measure pressures and oxygen levels in heart chambers and visualize heart structures and blood flow patterns |
| Hemodynamics | Measures pressures and oxygen saturations in heart chambers |
| Angiography | Use of contrast material to illuminate heart structures and blood flow patterns |
| Biopsy | Use of special catheter to remove tiny samples of heart muscle for microscopic evaluation; used in assessing infection, inflammation, or muscle dysfunction disorders; also to evaluate for rejection after heart transplant |
| Electrophysiology (EPS) | Special catheters with electrodes employed to record electrical activity from within heart; used to diagnose rhythm disturbances |
| Exercise stress test | Monitoring of heart rate, blood pressure, ECG, and oxygen consumption at rest and during progressive exercise on a treadmill or bicycle |
| Cardiac magnetic resonance imaging (MRI) | Noninvasive imaging technique; used in evaluation of vascular anatomy outside of heart (e.g., coarctation of the aorta, vascular rings), estimates of ventricular mass and volume; uses for MRI are expanding |
Electrocardiogram.: Bedside cardiac monitoring with the electrocardiogram (ECG) is commonly used in pediatrics, especially in the care of children with heart disease. The bedside monitor provides valuable information about heart rate and rhythm through a graphic display of the ECG tracing and a digital display. An alarm can be set with parameters for individual patient requirements and will sound if the heart rate is above or below the set parameters. Gelfoam electrodes are commonly used and placed on the right side of the chest (above the level of the heart) and on the left side of the chest, and a ground electrode is placed on the abdomen. Electrodes should be changed every 1 or 2 days because they irritate the skin. Bedside monitors are an adjunct to patient care and should never be substituted for direct assessment and auscultation of heart sounds. The nurse should assess the patient, not the monitor.
Echocardiography.: Echocardiography is one of the most frequently used tests for detecting cardiac dysfunction in children. Recent improvements in echocardiographic techniques have made it increasingly possible to confirm the diagnosis without resorting to cardiac catheterization. In more and more cases a prenatal diagnosis of CHD can be made by fetal echocardiography.
Echocardiography involves the use of ultra-high-frequency sound waves to produce an image of the heart’s structure. A transducer placed directly on the chest wall delivers repetitive pulses of ultrasound and processes the returned signals (echoes).
Although the test is noninvasive, painless, and associated with no known side effects, it can be stressful for children. The child must lie quietly in the standard echocardiographic positions; crying, nursing, or sitting up often leads to diagnostic errors or omissions. Therefore infants and young children may need a mild sedative; older children benefit from psychologic preparation for the test. The distraction of a video or movie is often helpful.
Cardiac Catheterization.: Cardiac catheterization is an invasive diagnostic procedure in which a radiopaque catheter is inserted through a peripheral blood vessel into the heart. The catheter is usually introduced through percutaneous technique, in which the catheter is threaded through a large-bore needle that is inserted into the vein. The catheter is guided through the heart with the aid of fluoroscopy. After the tip of the catheter is within a heart chamber, contrast material is injected, and films are taken of the dilution and circulation of the material (angiography). Types of cardiac catheterizations include:
Diagnostic catheterizations—These studies are used to diagnose congenital cardiac defects, particularly in symptomatic infants and before surgical repair. They are divided into right-sided catheterizations, in which the catheter is introduced through a vein (usually the femoral vein) and threaded to the right atrium (most common), and left-sided catheterizations, in which the catheter is threaded through an artery into the aorta and into the heart.
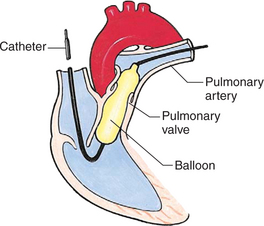
Interventional catheterizations (therapeutic catheterizations)—A balloon catheter or other device is used to alter the cardiac anatomy. Examples include dilating stenotic valves or vessels or closing abnormal connections (Table 25-2).
TABLE 25-2
Current Interventional Cardiac Catheterization Procedures in Children
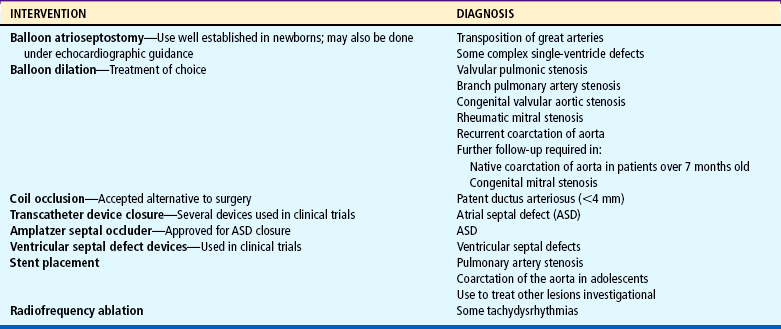
Data from Allen HD, Beekman RH 3rd, Garson A Jr, and others: Pediatric therapeutic cardiac catheterization: AHA scientific statement, Circulation 97:609-625, 1998; updated from Rome J, Kreutzer J: Pediatric interventional catheterization: reasonable expectations and outcomes, Pediatr Clin North Am 51:1589-1610, 2004.
Electrophysiology studies—Catheters with tiny electrodes that record the impulses of the heart directly from the conduction system are used to evaluate dysrhythmias and sometimes destroy accessory pathways that cause some tachydysrhythmias.
Nursing Care Management
Cardiac catheterization has become a routine diagnostic procedure and may be done on an outpatient basis. However, it is not without risks, especially in neonates and seriously ill infants and children. Possible complications include acute hemorrhage from the entry site (more likely with interventional procedures because larger catheters are used), low-grade fever, nausea, vomiting, loss of pulse in the catheterized extremity (usually transient, resulting from a clot, hematoma, or intimal tear), and transient dysrhythmias (generally catheter induced) (Uzark, 2001). Rare risks include stroke, seizures, tamponade, and death.
Preprocedural Care.: A complete nursing assessment is necessary to ensure a safe procedure with minimum complications. This assessment should include accurate height (essential for correct catheter selection) and weight. Obtaining a history of allergic reactions is important, since some of the contrast agents are iodine based. Specific attention to signs and symptoms of infection is crucial. Severe diaper rash may be a reason to cancel the procedure if femoral access is required. Because assessment of pedal pulses is important after catheterization, the nurse should assess and mark pulses (dorsalis pedis, posterior tibial) before the child goes to the catheterization room. The presence and quality of pulses in both feet are clearly documented. Baseline oxygen saturation using pulse oximetry in children with cyanosis is also recorded.
Preparing the child and family for the procedure is the joint responsibility of the patient care team. School-age children and adolescents benefit from a description of the catheterization laboratory and a chronologic explanation of the procedure, emphasizing what they will see, feel, and hear. Older children and adolescents may bring earphones and favorite music so they can listen during the catheterization procedure. Preparation materials such as picture books, videotapes, or tours of the catheterization laboratory may be helpful. Preparation should be geared to the child’s developmental level. The child’s caregivers often benefit from the same explanations. Additional information, such as the expected length of the catheterization, description of the child’s appearance after catheterization, and usual postprocedure care, should be outlined. (See also Prepare Child and Family for Invasive Procedures, p. 888.)
Methods of sedation vary among institutions and may include oral or intravenous (IV) medications (see Chapter 22). The child’s age, heart defect, clinical status, and type of catheterization procedure planned are considered when sedation is determined. General anesthesia may be needed for some interventional procedures. Children are allowed nothing by mouth (NPO) for 4 to 6 hours or more before the procedure according to institutional guidelines. Infants and patients with polycythemia may need IV fluids to prevent dehydration and hypoglycemia.
Postprocedural Care.: Patients may recover from the procedure in a recovery unit, their hospital room, or, occasionally, intensive care. Patients are placed on a cardiac monitor and a pulse oximeter for the first few hours of recovery. The most important nursing responsibility is observation of the following for signs of complications:
 Pulses, especially below the catheterization site, for equality and symmetry (Pulse distal to the site may be weaker for the first few hours after catheterization but should gradually increase in strength.)
Pulses, especially below the catheterization site, for equality and symmetry (Pulse distal to the site may be weaker for the first few hours after catheterization but should gradually increase in strength.)
 Temperature and color of the affected extremity, since coolness or blanching may indicate arterial obstruction
Temperature and color of the affected extremity, since coolness or blanching may indicate arterial obstruction
 Vital signs, which are taken as frequently as every 15 minutes, with special emphasis on heart rate, which is counted for 1 full minute for evidence of dysrhythmias or bradycardia
Vital signs, which are taken as frequently as every 15 minutes, with special emphasis on heart rate, which is counted for 1 full minute for evidence of dysrhythmias or bradycardia
 Blood pressure (BP), especially for hypotension, which may indicate hemorrhage from cardiac perforation or bleeding at the site of initial catheterization
Blood pressure (BP), especially for hypotension, which may indicate hemorrhage from cardiac perforation or bleeding at the site of initial catheterization
 Dressing, for evidence of bleeding or hematoma formation in the femoral or antecubital area
Dressing, for evidence of bleeding or hematoma formation in the femoral or antecubital area
 Fluid intake, both IV and oral, to ensure adequate hydration (Blood loss in the catheterization laboratory, the child’s NPO status, and diuretic actions of dyes used during the procedure put children at risk for hypovolemia and dehydration.)
Fluid intake, both IV and oral, to ensure adequate hydration (Blood loss in the catheterization laboratory, the child’s NPO status, and diuretic actions of dyes used during the procedure put children at risk for hypovolemia and dehydration.)
 Blood glucose levels for hypoglycemia, especially in infants, who should receive dextrose-containing IV fluids
Blood glucose levels for hypoglycemia, especially in infants, who should receive dextrose-containing IV fluids
Depending on hospital policy, the child may be kept in bed with the affected extremity maintained straight for 4 to 6 hours after venous catheterization and 6 to 8 hours after arterial catheterization to facilitate healing of the cannulated vessel. If younger children have difficulty complying, they can be held in the parent’s lap with the leg maintained in the correct position. The child’s usual diet can be resumed as soon as tolerated, beginning with sips of clear liquids and advancing as the condition allows. The child is encouraged to void to clear the contrast material from the blood. Generally, there is only slight discomfort at the percutaneous site. To prevent infection, the catheterization area is protected from possible contamination. If the child wears diapers, the dressing can be kept dry by covering it with a piece of plastic film and sealing the edges of the film to the skin with tape. However, the nurse must be careful to continue observing the site for any evidence of bleeding (see Family-Centered Care box and Critical Thinking Exercise).
CONGENITAL HEART DISEASE
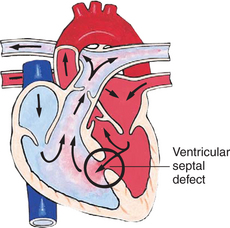
The incidence of CHD in children is approximately 5 to 8 per 1000 live births (Park, 2003). About 2 or 3 in 1000 infants will be symptomatic during the first year of life with significant heart disease that will require treatment (Hoffman and Kaplan, 2002). CHD is the major cause of death (other than prematurity) in the first year of life. Although there are more than 35 well-recognized cardiac defects, the most common heart anomaly is ventricular septal defect (VSD).
The exact etiology of most congenital cardiac defects is unknown. Most are thought to be a result of multifactorial inheritance: a complex interaction of genetic and environmental factors. The tremendous amount of information being discovered in molecular biology and the Human Genome Project will likely increase our understanding of the genetic causes of congenital heart defects.
Some risk factors are known to increase the incidence of congenital heart defects. Maternal factors include chronic illnesses such as diabetes or poorly controlled phenylketonuria, alcohol consumption, and exposure to environmental toxins and infections. Family history of a cardiac defect in a parent or sibling increases the likelihood of a cardiac anomaly. The risk of CHD increases if a first-degree relative (parent or sibling) is affected. The familial risk is higher with left-sided obstructive lesions.
Congenital heart anomalies are often associated with chromosomal abnormalities, syndromes, or congenital defects in other body systems. Down syndrome (trisomy 21) and trisomy 13 and 18 are highly correlated with congenital heart defects.
Recent research in gene mapping has identified deletion of part of chromosome 22 (22q11), which is present in the majority of patients with DiGeorge syndrome, velocardiofacial syndrome, and conotruncal anomaly face syndrome. The features of these syndromes include congenital cardiac defects, soft palate abnormalities, dysmorphic facial features, and speech and developmental delays. Mild immunologic abnormalities of T cells, absence or hypoplasia of the thymus, and parathyroid abnormalities resulting in hypocalcemia are seen with DiGeorge syndrome. Commonly associated cardiac defects are interrupted aortic arch, truncus arteriosus, tetralogy of Fallot, and posterior malaligned VSD (Goldmuntz, Clark, Mitchell, and others, 1998). This syndrome has variable clinical expression, with some patients minimally affected and others having all characteristics.
CIRCULATORY CHANGES AT BIRTH
During fetal life, blood carrying oxygen and nutritive materials from the placenta enters the fetal system through the umbilicus via the large umbilical vein. Oxygenated blood enters the heart by way of the inferior vena cava. Because of the higher pressure of blood entering the right atrium, it is directed posteriorly in a straight pathway across the right atrium and through the foramen ovale to the left atrium. In this way the better-oxygenated blood enters the left atrium and ventricle to be pumped through the aorta to the head and upper extremities. Blood from the head and upper extremities entering the right atrium from the superior vena cava is directed downward through the tricuspid valve into the right ventricle. From here it is pumped through the pulmonary artery, where the major portion is shunted to the descending aorta via the ductus arteriosus. Only a small amount flows to and from the nonfunctioning fetal lungs (Fig. 25-1, A).
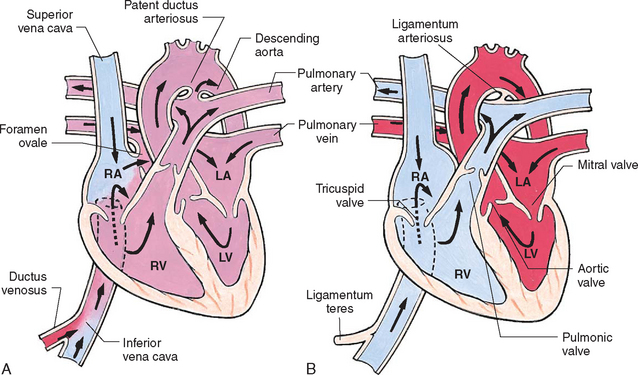
FIG. 25-1 Changes in circulation at birth. A, Prenatal circulation. B, Postnatal circulation. Arrows indicate direction of blood flow. Although four pulmonary veins enter the LA, for simplicity this diagram shows only two. RA, Right atrium; LA, left atrium; RV, right ventricle; LV, left ventricle.
Before birth the high pulmonary vascular resistance created by the collapsed fetal lung causes greater pressures in the right side of the heart and the pulmonary arteries. At the same time, the free-flowing placental circulation and the ductus arteriosus produce a low vascular resistance in the remainder of the fetal vascular system. With the cessation of placental blood flow from clamping of the umbilical cord and the expansion of the lungs at birth, the hemodynamics of the fetal vascular system undergo pronounced and abrupt changes (Fig. 25-1, B).
With the first breath, the lungs are expanded, and increased oxygen causes pulmonary vasodilation. Pulmonary pressures start to fall as systemic pressures, given the removal of the placenta, start to rise. Normally the foramen ovale closes as the pressure in the left atrium exceeds the pressure in the right atrium. The ductus arteriosus starts to close in the presence of increased oxygen concentration in the blood and other factors.
ALTERED HEMODYNAMICS
To appreciate the physiology of heart defects, it is necessary to understand the role of pressure gradients, flow, and resistance within the circulation. As with any fluid, blood flows from an area of high pressure to one of lower pressure and toward the path of least resistance in response to the pumping action of the heart. In general, the higher the pressure gradient, the greater the rate of flow; the higher the resistance, the lesser the rate of flow.
Normally the pressure on the right side of the heart is lower than that on the left side, and the resistance in the pulmonary circulation is less than that in the systemic circulation. Vessels entering or exiting these chambers have corresponding pressures. Therefore, if an abnormal connection exists between the heart chambers (such as a septal defect), blood will necessarily flow from an area of higher pressure (left side) to one of lower pressure (right side). Such a flow of blood is termed a left-to-right shunt. Anomalies resulting in cyanosis may result from a change in pressure so that the blood is shunted from the right to the left side of the heart (right-to-left shunt) because of either increased pulmonary vascular resistance or obstruction to blood flow through the pulmonic valve and artery. Cyanosis may also result from a defect that allows mixing of oxygenated and deoxygenated blood within the heart chambers or great arteries, such as occurs in truncus arteriosus.
CLASSIFICATION OF DEFECTS
Congenital heart defects have been divided into two categories. Traditionally, cyanosis, a physical characteristic, has been used as the distinguishing feature, dividing the anomalies into acyanotic defects and cyanotic defects. In clinical practice this system is problematic because children with acyanotic defects may develop cyanosis. Also, more often, those with cyanotic defects may appear pink and have more clinical signs of CHF.
A more useful classification system is based on hemodynamic characteristics (blood flow patterns within the heart). These blood flow patterns are (1) increased pulmonary blood flow; (2) decreased pulmonary blood flow; (3) obstruction to blood flow out of the heart; and (4) mixed blood flow, in which saturated and desaturated blood mix within the heart or great arteries. As a comparison, both classification systems are outlined in Fig. 25-2.
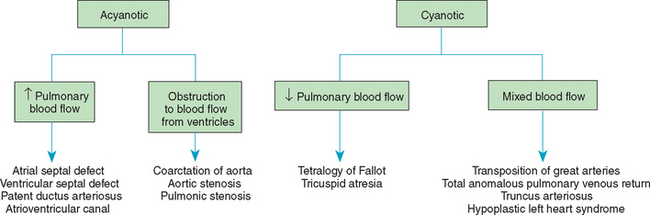
FIG. 25-2 Comparison of acyanotic-cyanotic and hemodynamic classification systems of congenital heart disease.
With the hemodynamic classification system, the clinical manifestations of each group are more uniform and predictable. Defects that allow blood flow from the higher pressure left side of the heart to the lower pressure right side (left-to-right shunt) result in increased pulmonary blood flow and cause CHF. Obstructive defects impede blood flow out of the ventricles; obstruction on the left side of the heart results in CHF, whereas severe obstruction on the right side causes cyanosis. Defects that cause decreased pulmonary blood flow result in cyanosis. Mixed lesions present a variable clinical picture based on the degree of mixing and amount of pulmonary blood flow; hypoxemia (with or without cyanosis) and CHF usually occur together. Using this classification system, the clinical presentation and management of the most common defects are outlined in the following sections and Box 25-1.
The outcomes of surgical treatment for patients with moderate to severe disease are variable. Patient risk factors for increased morbidity and mortality include prematurity or low birth weight, a genetic syndrome, multiple cardiac defects, a noncardiac congenital anomaly, and age at time of surgery (neonates are a higher-risk group). For example, aortic stenosis or coarctation manifesting in the first week of life is more severe and carries a higher mortality than if it becomes apparent at 1 year of age. Outcomes for surgical repair of similar congenital heart defects also vary among treatment centers. Most mortality rates are obtained from a large multicenter database maintained by the Society of Thoracic Surgeons (Jacobs, Mavroudis, Jacobs, and others, 2004) to reflect the more likely outcome of present day treatments. Individual center results may be better or worse than the Society of Thoracic Surgeons database. In general, the outcomes of surgical procedures have steadily improved in the past decade, with mortality rates for many severe defects below 10%, and the incidence of complications and length of hospital stay have declined.
Defects with Increased Pulmonary Blood Flow
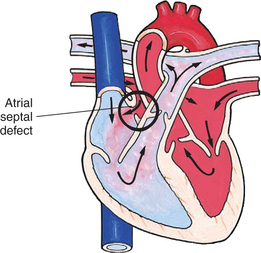
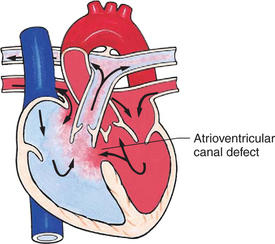
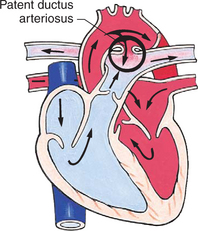
In this group of cardiac defects, intracardiac communications along the septum or an abnormal connection between the great arteries allows blood to flow from the higher pressure left side of the heart to the lower pressure right side of the heart (Fig. 25-3). Increased blood volume on the right side of the heart increases pulmonary blood flow at the expense of systemic blood flow. Clinically, patients demonstrate signs and symptoms of CHF. Atrial septal defect (ASD), VSD, and patent ductus arteriosus are typical anomalies in this group (Box 25-1).
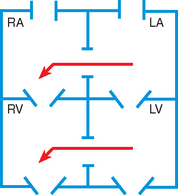
FIG. 25-3 Hemodynamics in defects with increased pulmonary blood flow. See Fig. 25-1 for abbreviations.
Obstructive Defects
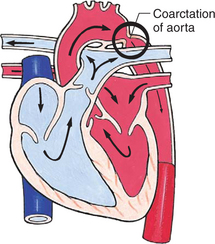
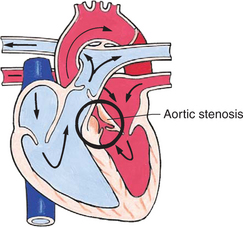
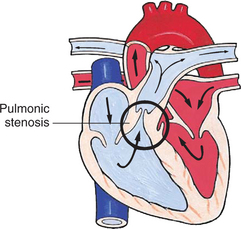
Obstructive defects are those in which blood exiting the heart meets an area of anatomic narrowing (stenosis), causing obstruction to blood flow. The pressure in the ventricle and in the great artery before the obstruction is increased, and the pressure in the area beyond the obstruction is decreased. The location of the narrowing is usually near the valve (Fig. 25-4), as follows:
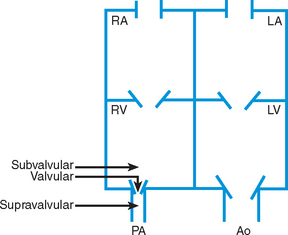
FIG. 25-4 Obstruction to ventricular ejection can occur at the valvular level (shown), below the valve (subvalvular), or above the valve (supravalvular). Pulmonary stenosis is shown here. Ao, Aorta; PA, pulmonary artery. See Fig. 25-1 for additional abbreviations.
Valvular—At the site of the valve itself
Subvalvular—Narrowing in the ventricle below the valve (also referred to as the ventricular outflow tract)
Coarctation of the aorta (narrowing of the aortic arch), aortic stenosis, and pulmonic stenosis are typical defects in this group (Box 25-2). Hemodynamically, there is a pressure load on the ventricle and decreased cardiac output. Clinically, infants and children exhibit signs of CHF. Children with mild obstruction may be asymptomatic. Rarely, as in severe pulmonic stenosis, hypoxemia may be seen.
Defects with Decreased Pulmonary Blood Flow
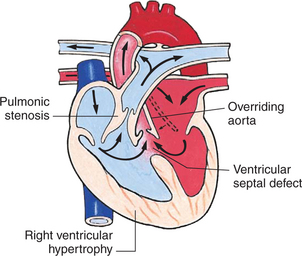
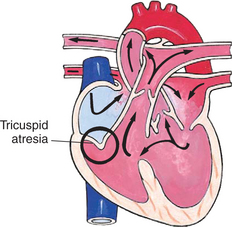
In this group of defects, there is obstruction of pulmonary blood flow and an anatomic defect (ASD or VSD) between the right and left sides of the heart (Fig. 25-5). Because blood has difficulty exiting the right side of the heart via the pulmonary artery, pressure on the right side increases, exceeding left-sided pressure. This allows desaturated blood to shunt right to left, causing desaturation in the left side of the heart and in the systemic circulation. Clinically, these patients are hypoxemic and usually appear cyanotic. Tetralogy of Fallot and tricuspid atresia are the most common defects in this group (Box 25-3).
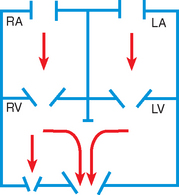
FIG. 25-5 Hemodynamic defects with decreased pulmonary blood flow. See Fig. 25-1 for abbreviations.
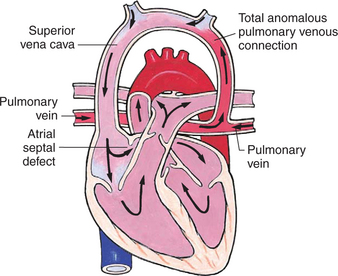
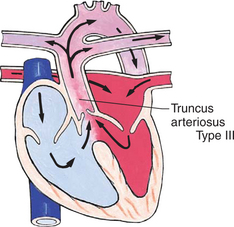
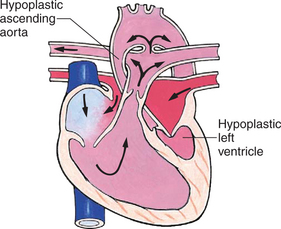
Mixed Defects
Many complex cardiac anomalies are classified together in the mixed category (Box 25-4) because survival in the postnatal period depends on mixing of blood from the pulmonary and systemic circulations within the heart chambers. Hemodynamically, fully saturated systemic blood flow mixes with the desaturated pulmonary blood flow, causing a relative desaturation of the systemic blood flow. Pulmonary congestion occurs because the differences in pulmonary artery pressure and aortic pressure favor pulmonary blood flow. Cardiac output decreases because of a volume load on the ventricle. Clinically, these patients have a variable picture that combines some degree of desaturation (although cyanosis is not always visible) and signs of CHF. Some defects, such as transposition of the great arteries, cause severe cyanosis in the first days of life and later cause CHF. Others, such as truncus arteriosus, cause severe CHF in the first weeks of life and mild desaturation.
CLINICAL CONSEQUENCES OF CONGENITAL HEART DISEASE
CHF is the inability of the heart to pump an adequate amount of blood to the systemic circulation at normal filling pressures to meet the body’s metabolic demands. In children, CHF most frequently occurs secondary to structural abnormalities (e.g., septal defects) that result in increased blood volume and pressure within the heart. It can also result from myocardial failure in which the contractility of the ventricle is impaired. This can occur with cardiomyopathy, dysrhythmias, or severe electrolyte disturbances. CHF can also occur because of excessive demands on a normal heart muscle, such as sepsis or severe anemia.
Pathophysiology
Heart failure is often separated into two categories: right-sided and left-sided failure. In right-sided failure the right ventricle is unable to pump blood effectively into the pulmonary artery, resulting in increased pressure in the right atrium and systemic venous circulation. Systemic venous hypertension causes hepatosplenomegaly and occasionally edema. In left-sided failure the left ventricle is unable to pump blood into the systemic circulation, resulting in increased pressure in the left atrium and pulmonary veins. The lungs become congested with blood, causing elevated pulmonary pressures and pulmonary edema.
Although each type of heart failure produces different signs and symptoms, clinically it is unusual to observe solely right- or left-sided failure in children. Because each side of the heart depends on adequate function of the other side, failure of one chamber causes a reciprocal change in the opposite chamber.
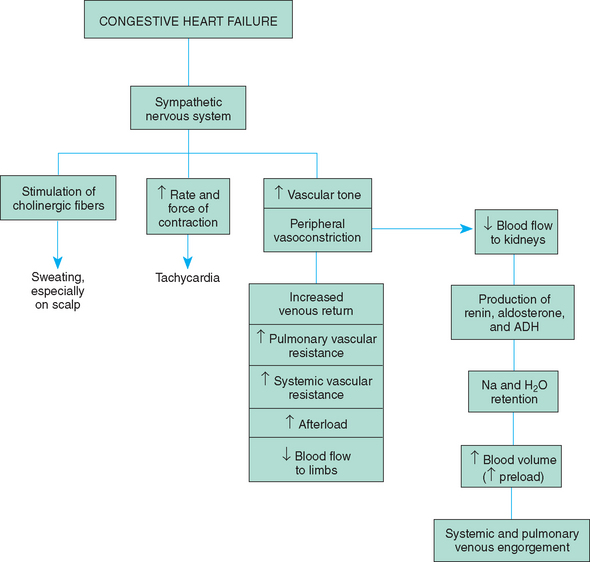
If the abnormalities precipitating CHF are not corrected, the heart muscle becomes damaged. Despite compensatory mechanisms, the heart is unable to maintain an adequate cardiac output. Decreased blood flow to the kidneys continues to stimulate sodium and water reabsorption, leading to fluid overload, increased workload on the heart, and congestion in the pulmonary and systemic circulations (Fig. 25-6).
The signs and symptoms of CHF can be divided into three groups: (1) impaired myocardial function, (2) pulmonary congestion, and (3) systemic venous congestion (Box 25-5). Because these hemodynamic changes occur from different causes and at differing times, the clinical presentation may vary among children.
Diagnostic Evaluation
Diagnosis is made on the basis of clinical symptoms such as tachypnea and tachycardia at rest, dyspnea, retractions, activity intolerance (especially during feeding in infants), weight gain caused by fluid retention, and hepatomegaly. A chest x-ray film demonstrates cardiomegaly and increased pulmonary blood flow. Ventricular hypertrophy appears on the ECG. An echocardiogram is done to determine the cause of CHF such as a congenital heart defect or poor ventricular function.
Therapeutic Management
The goals of treatment are to (1) improve cardiac function (increase contractility and decrease afterload), (2) remove accumulated fluid and sodium (decrease preload), (3) decrease cardiac demands, and (4) improve tissue oxygenation and decrease oxygen consumption. For most infants diagnosed with CHF, the cause is CHD. Infants are stabilized on medical therapy and then referred for surgical repair. For children newly diagnosed with CHF, the cause may be worsening ventricular function after a previous cardiac repair, cardiomyopathy, dysrhythmia, or other causes. In addition to management of CHF, the underlying cause is treated if possible.
Improve Cardiac Function.: Myocardial efficiency is improved through administration of digitalis glycosides. The beneficial effects are increased cardiac output, decreased heart size, decreased venous pressure, and relief of edema. In pediatrics, digoxin (Lanoxin) is used almost exclusively because of its more rapid onset. It is available as an elixir (0.05 mg/ml) for oral administration. For infants the dose is calculated in micrograms (1000 mcg = 1 mg).
Treatment consists of a digitalizing dosage, given orally or intravenously in divided doses over 24 hours to produce optimal cardiac effects, and a maintenance dosage, given orally twice a day to maintain blood levels. During digitalization the child is monitored by means of an ECG to observe for the desired effects (prolonged PR interval and reduced ventricular rate) and detect side effects, especially dysrhythmias.
A newer group of drugs used in the treatment of CHF are the angiotensin-converting enzyme (ACE) inhibitors. As their name implies, these drugs inhibit the normal function of the renin-angiotensin system in the kidney. The ACE inhibitors block the conversion of angiotensin I to angiotensin II so that, instead of vasoconstriction, vasodilation occurs. Vasodilation results in decreased pulmonary and systemic vascular resistance, decreased BP, and a reduction in afterload. Common medications used in pediatrics are captopril (Capoten), enalapril (Vasotec), and lisinopril. The principal side effects of ACE inhibitors are hypotension, cough, and renal dysfunction.
Carvedilol, a beta blocker, is the newest medication to be added to the treatment of some children with chronic CHF. It blocks the α- and β-adrenergic receptors, causing decreased heart rate, decreased BP, and vasodilation. It has been shown to decrease morbidity and mortality in some adults with heart failure and is being used selectively in children. Side effects included dizziness, headache, and hypotension.
A new and promising therapy for some patients with severe ventricular dysfunction may be biventricular pacing, also called resynchronization therapy. Both ventricles are paced to closely mimic normal ventricular conduction and thereby improve the mechanical function of the heart muscle (Rosenthal, Chrisant, Edens, and others, 2004).
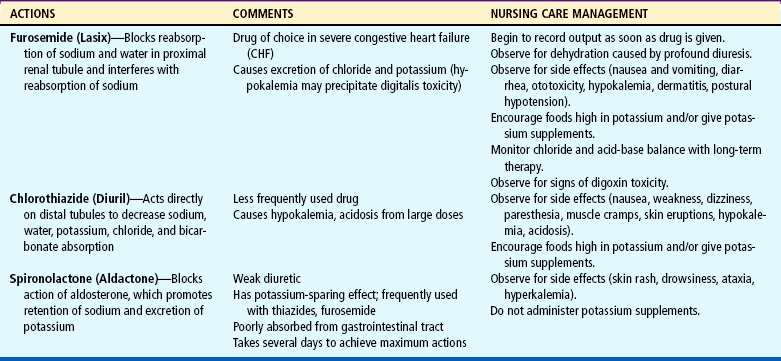
Remove Accumulated Fluid and Sodium.: Treatment consists of diuretics, possible fluid restriction, and possible sodium restriction. Diuretics are the mainstay of therapy to eliminate excess water and salt to prevent reaccumulation. The most frequently used agents are listed in Table 25-3. Because furosemide and the thiazides are potassium-losing diuretics, potassium supplements may be prescribed, and rich sources of the electrolyte are encouraged in the diet.
Fluid restriction may be required in the acute stages of CHF and must be carefully calculated to avoid dehydrating the child, especially if cyanotic CHD and significant polycythemia are present. Infants rarely need fluid restrictions because CHF makes feeding so difficult that they struggle to take maintenance fluids.
Sodium-restricted diets are used less often in children than in adults to control CHF because of their potential negative effects on appetite. If salt intake is restricted, additional table salt and highly salted foods are avoided.
Decrease Cardiac Demands.: The workload on the heart is reduced when metabolic needs are kept to a minimum. This is accomplished by limiting physical activity (bed rest), maintaining body temperature, treating any infections, reducing the effort of breathing (semi-Fowler position), and using medication to sedate an irritable child.
Improve Tissue Oxygenation.: All of the preceding measures serve to increase tissue oxygenation, either by improving myocardial function or by lessening tissue oxygen demands. In addition, supplemental cool humidified oxygen may be administered to increase the amount of available oxygen during inspiration. Oxygen administration is especially helpful in patients with pulmonary edema, intercurrent respiratory tract infections, and increased pulmonary vascular resistance (oxygen is a vasodilator that decreases pulmonary vascular resistance).
An oxygen hood, nasal cannula, or face tent is used to deliver oxygen. Nasal cannulas are ideal for long-term oxygen administration because the child can be ambulatory and can easily eat and drink. Cool humidification is necessary to counteract the drying effect of oxygen. The amount of cool humidity is carefully regulated to prevent chilling.
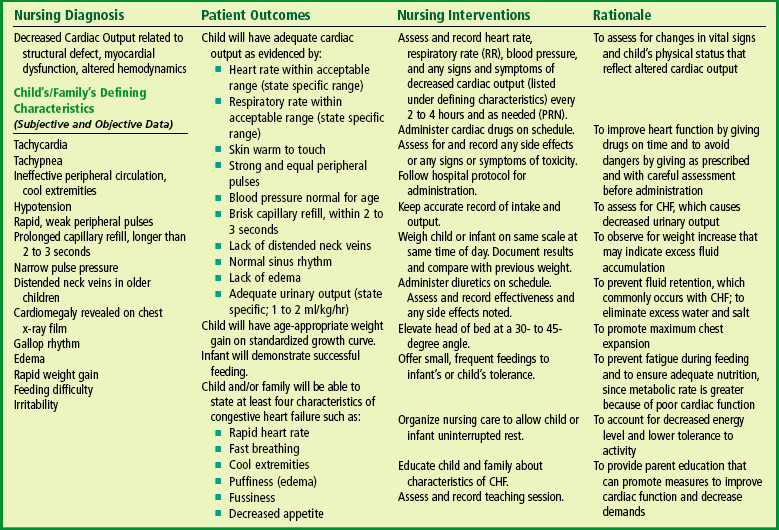
Nursing Care Management
The infant or child with CHF may be acutely ill, and some may require intensive care until the symptoms improve. Expert nursing care is essential to reduce the cardiac demands that strain the failing heart muscle. During this time the child and family require emotional support. Although the objectives of nursing care are the same, interventions differ depending on the child’s age (see Nursing Process box).
Assist in Measures to Improve Cardiac Function.: The nurse’s responsibility in administering digoxin includes calculating and administering the correct dosage, observing for signs of toxicity, and instituting parental teaching regarding drug administration at home. The child’s apical pulse is always checked before administering digoxin. As a general rule, the drug is not given if the pulse is below 90 to 110 beats/min in infants and young children or below 70 beats/min in older children (the cutoff point for adults is 60 beats/min). However, because the pulse rate varies in children in different age-groups, the written drug order should specify at what heart rate the drug is withheld. The nurse should also use judgment in evaluating the pulse rate. If it is significantly lower than the previous recording, the dose should be withheld until the practitioner is notified.
The apical rate is taken because a pulse deficit (radial pulse rate lower than apical) may be present with decreased cardiac output. It is auscultated for 1 full minute to evaluate alterations in rhythm. If the child is monitored by means of an ECG, a rhythm strip is obtained and attached to the chart for rate and rhythm analysis, such as abnormal lengthening of the PR interval (>50% increase over predigitalization interval) and dysrhythmias.
Digoxin is a potentially dangerous drug because of its narrow margin of safety of therapeutic, toxic, and lethal doses. Many toxic responses are extensions of its therapeutic effects. Therefore the nurse must maintain a high index of suspicion for signs of toxicity when administering digoxin (Box 25-6).
Because digoxin toxicity can occur from accidental overdose, great care must be taken in properly calculating and measuring the dosage. When converting milligrams to micrograms to milliliters, the nurse carefully checks the placement of the decimal point, since an error causes a significant change in dosage. For example, 0.1 mg is 10 times the dosage of 0.01 mg.
These same principles are taught to parents in preparation for discharge, although the correct dose in milliliters is usually specified on the container, thus reducing potential errors in calculation. The nurse watches the parent measure the elixir in the dropper and stresses the level mark as the meniscus of the fluid that is observed at eye level. Other instructions for administering digoxin are listed in the Family-Centered Care box and the Critical Thinking Exercise.
Parents are also advised of the signs of toxicity. According to the practitioner’s preference, they may be taught to take the pulse before giving the drug. A return demonstration of the procedure from the parents or another principal caregiver is included as part of the teaching plan. Their level of anxiety in counting the pulse is assessed, since overconcern about the heart rate may result in excessive withholding of the drug.
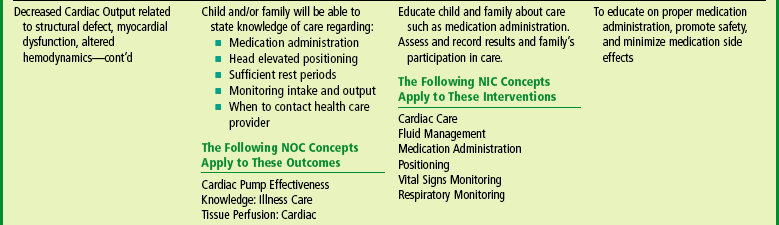
Monitor Afterload Reduction.: For patients receiving ACE inhibitors for afterload reduction, the nurse should carefully monitor BP before and after dose administration, observe for symptoms of hypotension, and notify the practitioner if BP is low. Numerous medications affecting the kidney can potentiate renal dysfunction, so children taking multiple diuretics and an ACE inhibitor require careful assessment of serum electrolytes and renal function.
Decrease Cardiac Demands.: The infant requires rest and conservation of energy for feeding. Every effort is made to organize nursing activities to allow for uninterrupted periods of sleep. Whenever possible, parents are encouraged to stay with their infant to provide the holding, rocking, and cuddling that help children sleep more soundly. To minimize disturbing the infant, changing bed linen and complete bathing are done only when necessary. Feeding is planned to accommodate the infant’s sleep and wake patterns. The child is fed at the first sign of hunger, such as when sucking on fists, rather than waiting until he or she cries for a bottle, since the stress of crying exhausts the limited energy supply. Because infants with CHF tire easily and may sleep through feedings, smaller feedings every 3 hours may be helpful. Gavage feedings may be instituted to provide adequate nutrition and allow the infant to rest.
Every effort is made to minimize unnecessary stress. Older children need an explanation of what is happening to them to decrease anxiety about their illness and necessary treatments such as cardiac monitoring, oxygen administration, and medications. Outlining a plan for the day, preparing the child for tests and procedures, providing quiet activities, and providing adequate rest periods are all helpful interventions with older children. Some infants and children require sedation during the acute phase of illness to allow them to rest.
Temperature is carefully monitored because hyperthermia or hypothermia increases the need for oxygen. Febrile states are reported to the physician, since infection must be promptly treated. Maintaining body temperature is of special importance in children who are receiving cool, humidified oxygen and in infants, who tend to be diaphoretic and lose heat by way of evaporation.
Skin breakdown from edema is prevented with a change of position every 2 hours (from side to side while in semi-Fowler position) and use of a pressure-relieving mattress or bed. The skin, especially over the sacrum, is checked for evidence of redness from pressure.
Reduce Respiratory Distress.: Careful assessment, positioning, and oxygen administration can reduce respiratory distress. Respirations are counted for 1 full minute during a resting state. Any evidence of increased respiratory distress is reported, since this may indicate worsening CHF.
Infants are positioned to encourage maximum chest expansion, with the head of the bed elevated; they should sit up in an infant seat or be held at a 45-degree angle. Children prefer to sleep on several pillows and remain in a semi-Fowler or high-Fowler position during waking hours. Safety restraints, such as those used with infant seats, are applied low on the abdomen and loosely enough to provide both safety and maximum expansion.
The infant or child is often given humidified supplemental oxygen via oxygen hood or tent, nasal cannula, or mask. The child’s response to oxygen therapy is carefully evaluated by noting respiratory rate, ease of respiration, color, and especially oxygen saturation as measured by oximetry.
Respiratory tract infections can exacerbate CHF and should be appropriately treated and prevented if possible. The child should be protected from persons with respiratory tract infections and have a noninfectious roommate. With an older child, it is advantageous to choose a roommate who is also confined to bed and relatively quiet to promote a restful environment. Good hand washing is practiced before and after caring for any hospitalized child. Antibiotics may be given to combat respiratory tract infection. The nurse ensures that the drug is given at equally divided times over a 24-hour schedule to maintain high blood levels of the antibiotic.
Maintain Nutritional Status.: Meeting the nutritional needs of infants with CHF or serious cardiac defects is a nursing challenge. The metabolic rate of these infants is greater because of poor cardiac function and increased heart and respiratory rates. Their caloric needs are greater than those of the average infant because of their increased metabolic rate, yet their ability to take in adequate calories is hampered by their fatigue. Feeding for a fragile infant with serious CHD is similar to exercising for an adult, and these infants often do not have the energy or cardiac reserve to do extra work. The nurse seeks measures to enable the infant to feed easily without excess fatigue and to increase the caloric density of the formula.
The infant should be well rested before feeding and fed soon after awakening so as not to expend energy on crying. A 3-hour feeding schedule works well for many infants. (Feeding every 2 hours does not provide enough rest between feedings, and a 4-hour schedule requires an increased volume of feeding, which many infants are unable to take.) The feeding schedule should be individualized to the infant’s needs. A feeding goal of 150 ml/kg/day and at least 120 kcal/kg/day is common for newborns with significant heart disease (Stetzler, Rudd, and Pick, 2005). A soft preemie nipple or a slit in a regular nipple to enlarge the opening decreases the infant’s energy expenditure while sucking. Infants should be well supported and fed in a semiupright position. The infant may need to rest frequently and may need to have the jaw and cheeks stroked to encourage sucking. Generally, giving an infant about a half hour to complete a feeding is reasonable. Prolonging the feeding time can exhaust the infant and decrease the rest period between feedings.
Infants with feeding difficulties are often gavage fed using a nasogastric tube to supplement their oral intake and ensure adequate calories. If they are very stressed and fatigued, in respiratory distress, or tachypneic to 80 to 100 breaths/min, oral feedings may be withheld and all nutrition given by gavage feedings. Gavage feedings are usually a temporary measure until the infant’s medical status improves and nutritional needs can be met through oral feedings. Some infants with severe CHF, neurologic deficits, or significant gastroesophageal reflux may need placement of a gastrostomy tube to allow adequate nutrition.
Caloric density of formulas is frequently increased by concentration and then adding Polycose, medium-chain triglyceride oil, or corn oil. Infant formulas provide 20 kcal/oz, and the use of additives can increase the calories to 30 kcal/oz or more. This allows the infant to obtain more calories despite a smaller volume intake of formula. The caloric density of the formula needs to be increased slowly (by 2 kcal/oz/day) to prevent diarrhea or formula intolerance. Breastfeeding mothers are encouraged to provide the infant with alternating feedings of breast milk and high-calorie formulas. Some lactating mothers prefer to feed the child expressed breast milk that has been fortified with Similac or Enfamil powder, Polycose, or corn oil to increase caloric intake. A supplemental nurser may also be helpful. A diet plan specific to the individual infant’s needs is calculated and prescribed by the nutritionist in collaboration with the other health personnel. The nurse needs to reinforce this information with the parents as necessary.
Assist in Measures to Promote Fluid Loss.: When diuretics are given, the nurse records fluid intake and output and monitors body weight at the same time each day to evaluate benefit from the drug. Because profound diuresis may cause dehydration and electrolyte imbalance (loss of sodium, potassium, chloride, bicarbonate), the nurse observes for signs indicating either complication, as well as signs and symptoms suggesting reactions to the drugs. Diuretics should be given early in the day to children who are toilet trained to avoid the need to urinate at night. If potassium-losing diuretics are given, the nurse encourages foods high in potassium, such as bananas, oranges, whole grains, legumes, and leafy vegetables, and administers prescribed supplements. Serum potassium levels are checked frequently.
Fluid restriction is rarely necessary in infants because of their difficulty in feeding. However, if fluids are restricted, the nurse plans fluid intake schedules for a 24-hour period, allowing for most fluids during waking hours. Toddlers and preschoolers should be given small amounts of liquid in small cups so that the containers appear full. Older children’s cooperation is gained by placing them in charge of recording fluid intake.
If salt is limited, the nurse discusses food sources of sodium with the family and discourages their bringing salt-containing treats to the child. At mealtime the child’s tray is checked to make sure the appropriate diet is given.
Support Child and Family.: CHF is a serious complication of heart disease. Parents and older children are usually acutely aware of the critical nature of the condition. Because stress places additional demands on cardiac function, the nurse should focus on reducing anxiety through anticipatory preparation, frequent communication with the parent regarding the child’s progress, and constant reassurance that everything possible is being done.
Home care involves many of the same interventions discussed under Plan for Discharge and Home Care (p. 891). The nurse teaches the family about the medications that need to be administered and alerts them to the signs of worsening CHF that require medical attention, such as increased sweating, decreased urinary output (noted in fewer wet diapers or infrequent use of the toilet), or poor feeding. Every effort is made to improve the family’s adherence to the medication schedule by adapting the schedule to their usual home routines, avoiding medications during the night, making it as simple as possible, and using charts or visual aids to remember when to give medications (see Chapter 22). Written instructions regarding correct administration of digoxin are essential (see Family-Centered Care box, p. 882), including an explanation regarding signs of toxicity.
If CHF is the end stage of a severe heart defect, the nurse cares for this child as for any child who is terminally ill, using the principles discussed in Chapter 18.
HYPOXEMIA
Hypoxemia refers to an arterial oxygen tension (or pressure, Pao2) that is less than normal and can be identified by a decreased arterial saturation or a decreased Pao2. Hypoxia is a reduction in tissue oxygenation that results from low oxygen saturations and Pao2 and results in impaired cellular processes. Cyanosis is a blue discoloration in the mucous membranes, skin, and nail beds of the child with reduced oxygen saturation. It results from the presence of deoxygenated hemoglobin (hemoglobin not bound to oxygen) in a concentration of 5 g/dl of blood. Cyanosis is usually apparent when arterial oxygen saturations are 80% to 85%. Determination of cyanosis is subjective. It can vary depending on skin pigment, quality of light, color of the room, or clothing worn by the child. The presence of cyanosis may not accurately reflect arterial hypoxemia, since both oxygen saturation and the amount of circulating hemoglobin are involved. Children with severe anemia may not be cyanotic despite severe hypoxemia, since the hemoglobin level may be too low to produce the characteristic blue color. Conversely, patients with polycythemia may appear cyanotic despite a near-normal Pao2. Heart defects that cause hypoxemia and cyanosis result from desaturated venous blood (blue blood) entering the systemic circulation without passing through the lungs.
Clinical Manifestations
Over time, two physiologic changes occur in the body in response to chronic hypoxemia: polycythemia and clubbing. Polycythemia, an increased number of red blood cells, increases the oxygen-carrying capacity of the blood. However, anemia may result if iron is not readily available for the formation of hemoglobin. Polycythemia increases the viscosity of the blood and crowds out clotting factors. Clubbing, a thickening and flattening of the tips of the fingers and toes, is thought to occur because of chronic tissue hypoxemia and polycythemia (Fig. 25-7). Infants with mild hypoxemia may be asymptomatic except for cyanosis and exhibit near-normal growth and development. Those with more severe hypoxemia may exhibit fatigue with feeding, poor weight gain, tachypnea, and dyspnea. Severe hypoxemia resulting in tissue hypoxia is manifested by clinical deterioration and signs of poor perfusion.
Hypercyanotic spells, also referred to as blue spells or tet spells because they are often seen in infants with tetralogy of Fallot, may occur in any child whose heart defect includes obstruction to pulmonary blood flow and communication between the ventricles. The infant becomes acutely cyanotic and hyperpneic because sudden infundibular spasm decreases pulmonary blood flow and increases right-to-left shunting (the proposed mechanism in tetralogy of Fallot). Spells, rarely seen before 2 months of age, occur most frequently in the first year of life. They occur more often in the morning and may be preceded by feeding, crying, defecation, or stressful procedures (see Critical Thinking Exercise). Because profound hypoxemia causes cerebral hypoxia, hypercyanotic spells require prompt assessment and treatment to prevent brain damage or possibly death.
Persistent cyanosis as a result of cyanotic heart defects places the child at risk for significant neurologic complications. Cerebrovascular accident (CVA, stroke), brain abscess, and developmental delays (especially in motor and cognitive development) may result from chronic hypoxia.
 IN TEXT
IN TEXT FAMILY-CENTERED CARE
FAMILY-CENTERED CARE CRITICAL THINKING EXERCISE
CRITICAL THINKING EXERCISE