Therapeutic Management
Newborns generally exhibit cyanosis within the first few days of life as the ductus arteriosus, which provided pulmonary blood flow, begins to close. Prostaglandin E1, which causes vasodilation and smooth muscle relaxation, thus increasing dilation and patency of the ductus arteriosus, is administered intravenously to reestablish pulmonary blood flow. The use of prostaglandins has been lifesaving for infants with ductus-dependent cardiac defects. The increase in oxygenation allows the infant to be stabilized and have a complete diagnostic evaluation performed before further treatment is needed.
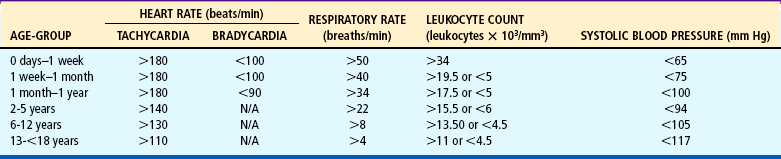
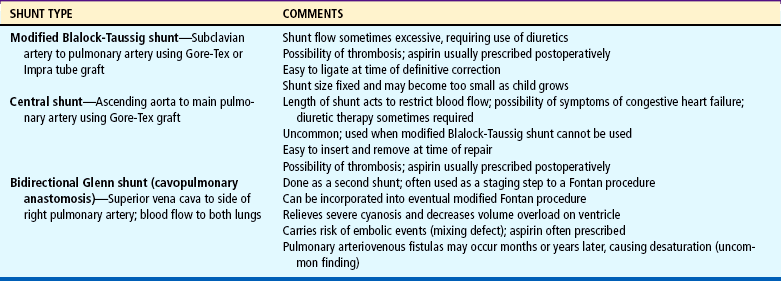
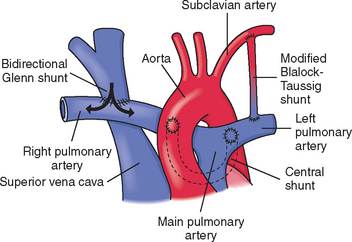
Hypercyanotic spells occur suddenly, and prompt recognition and treatment are essential. In the hospital setting, spells are often seen during blood drawing or IV insertion, when the child is highly agitated, or after cardiac catheterization. Treatment of a hypercyanotic spell is outlined in the Nursing Care Guidelines box. Morphine, administered subcutaneously or through an existing IV line, helps reduce infundibular spasm. A spell indicates the need for prompt surgical treatment if possible. In infants with defects not amenable to surgical repair, a shunt may be created surgically to increase blood flow to the lungs. Several commonly used shunt procedures are described in Table 25-4 and Fig. 25-9.
The cyanotic infant and child are well hydrated to keep the hematocrit and blood viscosity within acceptable limits to reduce the risk of CVAs. Fevers are carefully evaluated because bacteremia can result in bacterial endocarditis. The infant is monitored closely for anemia because of the risk of CVAs and the reduced arterial oxygen-carrying capacity that occurs. Iron supplementation and possibly blood transfusion are used as needed.
Respiratory tract infections or reduced pulmonary function from any cause can worsen hypoxemia in the cyanotic child. Aggressive pulmonary hygiene, chest physical therapy, administration of antibiotics, and use of oxygen to improve arterial saturations are important interventions.
Nursing Care Management
The general appearance of infants and children with significant cyanosis poses unique concerns. Blue lips and fingernails are obvious signs of their hidden cardiac defect. Clubbing and small, thin stature in older children further indicate severe heart disease. Adolescents are especially concerned about their body image; children with cyanosis are often teased about their appearance and singled out as different. Many children, when asked what surgery will do, reply, ‘Make me pink.’ Their joy and excitement after surgery are evident when they see their pink fingers. Parents are often fearful of their child’s bluish color, since cyanosis is usually associated with lack of oxygen and severe illness. They also must deal with comments from relatives, friends, and strangers about their child’s abnormal color. They need a simple explanation of hypoxemia and cyanosis and reassurance that cyanosis does not imply a lack of oxygen to the brain. Their questions and fears need to be addressed in a calm, supportive manner, and positive aspects of their child’s growth and development are emphasized. They are taught the treatment for hypercyanotic spells (see Nursing Care Guidelines box above).
Dehydration must be prevented in hypoxemic children because it potentiates the risk of CVAs. Fluid status is carefully monitored, with accurate intake and output and daily weight measurements. Maintenance fluid therapy is the minimum requirement, supplemental fluids should be readily available, and gavage feeding or IV hydration is given to children unable to take adequate oral fluids. Fever, vomiting, and diarrhea can cause dehydration and require prompt treatment. Parents are instructed in the importance of adequate fluid intake and measures to prevent dehydration. An oral electrolyte solution should be available at home in the event that the infant is unable to tolerate the usual formula. The practitioner should be notified of fever, vomiting, diarrhea, or other problems.
Preventive measures and accurate assessment of respiratory infection are important nursing considerations. Any compromise in pulmonary function will increase the infant’s hypoxemia. Good hand washing and protection from individuals with an obvious respiratory tract infection are important. Aggressive pulmonary hygiene, treatment with antibiotics or antiviral agents as indicated, and supplemental oxygen to decrease hypoxemia are necessary measures. Infants may need to be gavage fed or given parenteral hydration if respiratory distress prevents oral feeding.
 CRITICAL THINKING EXERCISE
CRITICAL THINKING EXERCISE

 FAMILY FOCUS
FAMILY FOCUS FAMILY-CENTERED CARE
FAMILY-CENTERED CARE