Chapter 38 Urinary elimination
Mastery of content will enable you to:
• Define the key terms listed.
• Describe the physiological process of micturition.
• Identify factors that commonly influence urinary elimination.
• Compare and contrast common alterations in urinary elimination.
• Obtain a nursing history and perform a physical examination for a patient with urinary elimination problems.
• Describe characteristics of normal and abnormal urine.
• Describe the nursing implications of common diagnostic tests of the urinary system.
• Identify nursing diagnoses appropriate for patients with alterations in urinary elimination.
• Discuss nursing interventions to promote normal voiding.
• Discuss nursing interventions to promote continence.
• Discuss nursing interventions to reduce urinary tract infection.
• Apply a urinary sheath/condom drainage device.
• Describe routine care and assessment of a person with an indwelling urinary catheter.
Normal elimination of waste products via the urine is a basic function most people take for granted. When the renal system fails to function properly, virtually all organ systems will eventually be affected. Similarly, problems with function of the lower urinary tract can significantly affect a person’s health and quality of life.
Scientific knowledge base
Urinary system
Urinary elimination depends on the function of the kidneys, ureters, bladder and urethra. Kidneys remove wastes from the blood to form urine. Ureters transport urine from the kidneys to the bladder. The bladder holds urine until the urge to urinate develops. Urine leaves the body through the urethra. The kidneys and ureters form the upper urinary tract; the bladder and urethra form the lower urinary tract. All organs of the urinary system must be intact and functional for successful removal of urinary wastes (Figure 38-1).

FIGURE 38-1 Organs of the urinary system.
From Potter PA, Perry AG 2013 Fundamentals of nursing, ed 8. St Louis, Mosby.
Kidneys
The kidneys are reddish-brown, bean-shaped organs, one on either side of the vertebral column, posterior to the peritoneum and lying against the deep muscles of the back. The kidneys extend to the twelfth thoracic and third lumbar vertebrae. Normally, the left kidney is 1.5–2 cm higher than the right because of the anatomical position of the liver on the right side of the abdomen. Each kidney typically measures approximately 12 × 7 cm and weighs 120–150 g. Each kidney is covered by a tough capsule and surrounded by a cushion of fat.
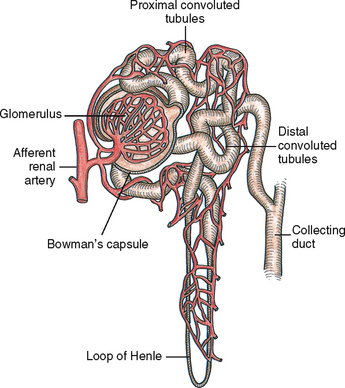
The kidneys filter the waste products of metabolism that collect in the blood. Blood reaches each kidney by a renal artery that branches from the abdominal aorta. The renal artery enters the kidney at the hilum. Approximately 20–25% of the cardiac output circulates each minute through the kidneys. The nephron, the functional unit of the kidney, forms the urine. The nephron is composed of the glomerulus, Bowman’s capsule, proximal convoluted tubule, loop of Henle, distal tubule and collecting duct (Figure 38-2).
Blood reaches nephrons through the afferent arterioles. A cluster of these blood vessels forms the capillary network of the glomerulus, which is the initial site of filtration of the blood and the beginning of urine formation. The glomerular capillaries are porous and permit filtration of water and substances such as glucose, amino acids, urea, creatinine and major electrolytes into the Bowman’s capsule. Large proteins and blood cells do not normally filter through the glomerulus. The presence of large proteins in the urine (proteinuria) is one sign of glomerular injury. The glomerulus filters approximately 125 mL of filtrate per minute. Initially the filtrate closely approximates the composition of blood plasma minus the large proteins.
Only a small amount of the glomerular filtrate is excreted as urine. About 99% of the filtrate is reabsorbed into the plasma, with the remaining 1% excreted as urine. Thus the kidneys play a key role in fluid and electrolyte balance (see Chapter 39). Although urine output does depend on intake, the normal adult 24-hour output of urine is about 1500–1600 mL. An output of less than 0.5 mL/kg per hour may indicate alterations in renal function.
The kidneys also produce several hormones vital to production of red blood cells (RBCs), blood pressure regulation and bone mineralisation. The kidneys are responsible for maintaining a normal RBC volume by producing erythropoietin. As a hormone, erythropoietin functions within the bone marrow to stimulate RBC production and maturation (McCance and Huether, 2010). Erythropoietin also prolongs the life of mature RBCs. People with chronic alterations in kidney function cannot produce sufficient quantities of this hormone and are, therefore, prone to anaemia.
Renin is another hormone produced by the kidneys. Its major role is the regulation of the systemic circulation in times of renal ischaemia (decreased blood supply to the kidneys). Renin is released from juxtaglomerular cells (Figure 38-3).
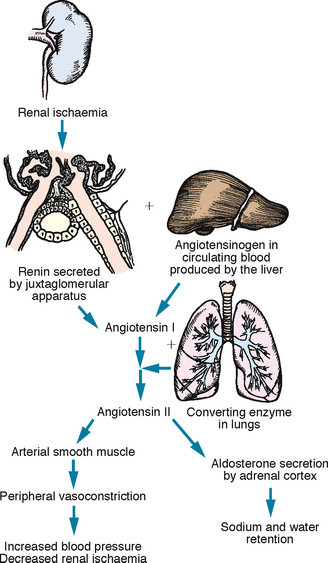
FIGURE 38-3 Physiological effects of the renin–angiotensin mechanism.
From Potter PA, Perry AG 2013 Fundamentals of nursing, ed 8. St Louis, Mosby.
Renin functions as an enzyme to convert angiotensinogen (a plasma substance synthesised by the liver) into angiotensin I. As angiotensin I circulates through the lungs, it is converted to angiotensin II and angiotensin III. Angiotensin II exerts its effect on vascular smooth muscle to cause vasoconstriction and stimulates aldosterone release from the adrenal cortex. Aldosterone causes retention of water, which increases blood volume. Angiotensin III exerts similar effects, but to a lesser degree. The net effect of both these mechanisms is an increase in arterial blood pressure and renal blood flow (McCance and Huether, 2010).
The kidneys also play a role in calcium and phosphate regulation. They are responsible for producing a substance that converts vitamin D into its active form. People with chronic alterations in kidney function do not make sufficient amounts of the active vitamin D metabolite. They are therefore prone to developing renal bone disease resulting from the demineralisation of bone secondary to impaired intestinal calcium absorption unless the active form of vitamin D is supplied.
Ureters
Urine enters the renal pelvis from the collecting ducts. A ureter joins each renal pelvis to the urinary bladder. Ureters are tubular structures measuring 25–30 cm in length and 0.5 cm in diameter in the adult (Getliffe and Dolman, 2007). They extend retroperitoneally to enter the urinary bladder in the pelvic cavity at the ureterovesical junction. Urine draining from the ureters to the bladder is usually sterile.
Three layers of tissue form the wall of the ureter. The inner layer is a mucous membrane continuous with the lining of the renal pelvis and urinary bladder. The middle layer consists of smooth muscle fibres that transport urine through the ureters by peristaltic waves stimulated by distension with urine. An outer layer of fibrous connective tissue supports the ureters.
Peristaltic waves cause the urine to enter the bladder in spurts, rather than steadily. The ureters enter obliquely through the posterior bladder wall. This arrangement normally prevents the reflux of urine from the bladder into the ureters during the act of micturition by the compression of the ureter at the ureterovesical junction (the juncture of the ureters with the bladder). An obstruction within a ureter, such as a kidney stone (renal calculus), results in strong peristaltic waves that attempt to move the obstruction into the bladder. These strong peristaltic waves result in pain often referred to as renal colic.
Bladder
The urinary bladder is a hollow, distensible, muscular organ that is both a reservoir for urine and the organ of excretion. The size and shape of the bladder will vary depending on its state of fullness. When empty, the bladder lies in the pelvic cavity behind the symphysis pubis. In men the bladder lies against the anterior wall of the rectum and in women it rests against the anterior wall of the uterus and vagina.
The bladder expands as it becomes filled with urine. Pressure within the bladder is usually low, even when partly full, a factor that protects against reflux of urine back up the ureters into the kidneys. In a healthy adult, bladder capacity is approximately 500 mL and when the bladder is emptied there is no residual urine volume. Both men and women will void at 3- to 5-hour intervals during the day and usually do not need to void at night (Getliffe and Dolman, 2007). Keep in mind, however, that the habits of individuals will vary. When the bladder is full, it expands and extends above the symphysis pubis. A greatly distended bladder may reach the umbilicus. In a pregnant woman the developing fetus pushes against the bladder, causing a feeling of fullness and reducing the bladder’s capacity. This effect is more likely to occur in the first and third trimesters.
The trigone (a smooth, triangular area on the inner surface of the bladder) is at the base of the bladder. An opening exists at each of the trigone’s three angles. Two are for the ureters, and one is for the urethra. This area does not change shape very much during bladder filling and is very sensitive to stretch due to the large number of sensory nerve endings it contains (Getliffe and Dolman, 2007).
The wall of the bladder has four layers: an inner mucous coat, a submucous layer of connective tissue, a muscular layer and an outer serous coat. The muscular layer has bundles of muscle fibres that form the detrusor muscle. Parasympathetic nerve fibres stimulate the detrusor muscle during urination. In the male, the bladder neck provides a powerful sphincter mechanism. It also prevents retrograde ejaculation into the bladder during orgasm. The male internal urethral sphincter is made of a ring-like band of muscle which has a rich nerve supply. In the female the bladder neck is a far weaker structure than in the male (Getliffe and Dolman, 2007): the smooth muscle fibres extend longitudinally along the urethra; there are no circular muscles.
Urethra
Urine travels from the bladder through the urethra and passes outside the body through the urethral meatus. Normally, the turbulent flow of urine through the urethra washes it free of bacteria. Mucous membrane lines the urethra, and urethral glands secrete mucus into the urethral canal. The mucus is believed to be bacteriostatic and forms a mucous plug to prevent entrance of bacteria. Thick layers of smooth muscle surround the urethra. In addition, the urethra descends through a layer of skeletal muscle called the pelvic floor muscles. When these muscles are contracted, it is possible to prevent urine flow through the urethra (McCance and Huether, 2010).
In women the urethra is approximately 4–6.5 cm long. The external urethral sphincter, located about halfway down the urethra, permits voluntary flow of urine. The short length of the urethra predisposes women to ascending infection. Bacteria can easily enter the urethra from the perineal area. In men the urethra, which is both a urinary canal and a passageway for semen, is 18–22 cm long. The male urethra has three sections: the prostatic urethra, the membranous urethra and the cavernous or penile urethra. The prostate gland encircles the male urethra at the base of the bladder. In a female the urinary meatus (opening) is located between the labia minora, above the vagina and below the clitoris. In a male the meatus is located at the distal end of the penis.
Pelvic floor muscles
The bony pelvis forms the solid structure for the muscles and ligaments of the anterior, posterior and lateral pelvic walls and pelvic floor, which play an important role in supporting the position of the pelvic organs. The pelvic floor muscles include the coccygeus muscle and the levator ani and are referred to as the pelvic diaphragm. The anal canal, urethra and vagina pass through the pelvic diaphragm. The levator ani muscles support the pelvic organs and function as a sphincter for the anal canal and urethra (Jenkins and others, 2010).
The perineum is located inferior to the pelvic diaphragm. The perineum extends from the symphysis pubis anteriorly to the coccyx posteriorly and the ischial tuberosities laterally. The muscles of the perineum are in two layers: superficial and deep. The deep muscle layer of the perineum includes the external urethral sphincter and external anal sphincter which assist in maintaining urinary and faecal continence (Jenkins and others, 2010).
Micturition
Several brain structures influence bladder function, including the cerebral cortex, thalamus, hypothalamus and brainstem. Together they suppress contraction of the bladder’s detrusor muscle until a person decides to urinate (void). Once voiding (micturition) occurs, the response is a contraction of the bladder and coordinated relaxation of the pelvic floor muscles.
The desire to urinate can be sensed when the bladder contains approximately 150–200 mL of urine in an adult and 50–200 mL of urine in a child. As the volume increases, the bladder walls stretch, sending sensory impulses to the micturition centre in the sacral spinal cord. Parasympathetic impulses from the micturition centre stimulate the detrusor muscle to contract rhythmically. The internal urethral sphincter also relaxes so that urine may enter the urethra, although voiding does not yet occur. As the bladder contracts, nerve impulses travel up the spinal cord to the pons and cerebral cortex. A person is thus conscious of the need to urinate. Older children and adults can respond to or ignore this urge, thus making urination under voluntary control. If the person chooses not to void, the external urinary sphincter remains contracted, and the micturition reflex is inhibited. However, when a person is ready to void, the external sphincter relaxes, the micturition reflex stimulates the detrusor muscle to contract, and efficient emptying of the bladder occurs.
Nursing knowledge base
Factors affecting urinary elimination
Many factors influence the volume and quality of urine and a person’s ability to urinate. Some pathophysiological conditions may be acute and reversible (e.g. acute urinary tract infection), while others may be chronic and irreversible (e.g. slow, progressive development of renal dysfunction). There are changes that occur to the urinary system from normal growth and development; for example, development of continence in a child. There are also a number of changes that occur with normal ageing that predispose older people to problems related to their kidneys and bladder.
Growth and development
Infants and young children cannot effectively concentrate urine. Their urine thus appears light yellow or clear. In relation to their small body size, infants and children excrete large volumes of urine. For example, a 6-month-old infant who weighs 6–8 kg excretes 400–500 mL of urine daily.
A child cannot control micturition voluntarily until 18–24 months old. A child must be able to recognise the feeling of bladder fullness, to hold urine for 1–2 hours and to communicate the sense of needing to void to an adult. The young child needs parents’ understanding, patience and consistency. A child may not gain full control of micturition until age 4 or 5 years. Daytime control of micturition is easier to accomplish than night-time control and occurs earlier in the child’s development, usually by 2–3 years of age (see Working with diversity).
The adult normally voids 1500–1600 mL of urine daily. The kidney concentrates urine, normally producing amber-coloured urine. A person does not normally wake to void during sleep because of reduction of renal blood flow during rest and the kidneys’ ability to concentrate urine.
Changes in kidney and bladder function also occur with ageing. The glomerular filtration rate declines, but the kidneys’ ability to concentrate urine also declines. Thus the older adult often experiences nocturia (urination at night). The bladder loses its muscle tone and capacity to hold urine, resulting in increased urinary frequency. Because the bladder cannot contract as effectively, an older person often retains urine in the bladder after voiding (residual urine). Older men may also suffer from benign prostatic hypertrophy, which makes them prone to urinary retention and incontinence. These changes increase the risk for bacterial growth and development of urinary tract infections (UTIs). Another factor related to urinary elimination difficulties is constipation. Constipation and resulting bowel fullness may put external pressure on the bladder, reducing the effective capacity and causing frequency or even incontinence (see Chapter 37).
WORKING WITH DIVERSITY FOCUS ON INFANTS AND CHILDREN
Bedwetting (nocturnal enuresis) is common in children and can occur until the teen years. According to the Continence Foundation of Australia, 1 in 5 Australian children wet their bed. There are three main causes of bedwetting:
• the inability to waken to the sensation of a full bladder at night
• overactivity of the bladder at night, causing loss of urine
• an abnormal amount of urine production occurring at night (nocturnal polyuria) when the child is asleep and unable to sense the fullness of the bladder.
Most children will gain night-time continence with time. The Continence Foundation of Australia suggests parents should seek assistance from a healthcare professional if a child who has been dry at night suddenly starts wetting at night; the episodes are frequent after 5 years of age; the wetting bothers the child or makes them angry or frustrated; the child is socially affected; or if the child states they want to become dry.
There are several approaches—bladder-training programs to increase the child’s functional bladder capacity; night alarms which wake the child when there is leakage, so they learn to wake when their bladder is full; and the use of some medications.
At home, parents can praise success and ignore failure; encourage the child to drink plenty of water; encourage the child to take responsibility for the problem (assist when changing sheets, etc); provide a high-fibre diet, as constipation can aggravate the problem; and avoid ‘toileting’ the child during the night—this does not improve bladder control.
Parents can be advised to seek help from:
• Continence Foundation of Australia, www.continence.org.au/index.php or helpline 1800 33 00 66
• New Zealand Continence Association, www.continence.org.nz or helpline 0800 650 659.
In the female, childbearing and/or the hormonal changes of menopause may cause changes that lead to urinary difficulties. During a pregnancy, urinary frequency is common and susceptibility to UTIs is increased. Temporary or permanent changes that result from repeated vaginal deliveries or hormonal changes may result in decreased pelvic floor muscle tone, leading to urgency and stress incontinence (see Chapter 22). The changes in the urethral mucosa associated with loss of oestrogen during and after menopause also contribute to increased susceptibility to infection (McCance and Huether, 2010).
Fluid balance
The kidneys maintain a sensitive balance between retention and excretion of fluids (see Chapter 39). If fluids and the concentration of electrolytes and solutes are in equilibrium, an increase in fluid intake causes an increase in urine production. Ingested fluids increase the body’s circulating plasma and thus increase the volume of glomerular filtrate and urine excreted.
This amount varies with food and fluid intake. The volume of urine formed at night is about half that formed during the day, because both intake and metabolism decline. This results in a reduction in the volume of renal blood flow. In a healthy person, the intake of water in food and fluids balances the output of water in urine, faeces and insensible losses in perspiration and respiration. An excessive output of urine is known as polyuria.
Ingestion of certain fluids directly affects urine production and excretion. Coffee, tea, cocoa and cola drinks that contain caffeine promote increased urine formation (diuresis). Alcohol inhibits the release of antidiuretic hormone (ADH), resulting in increased water loss in urine. Foods that contain a high fluid content, such as fruits and vegetables, may also increase urine production.
Febrile conditions affect urine production. The patient who becomes diaphoretic loses a large amount of fluids through insensible water loss, which decreases urine production. However, the increased body metabolism associated with fever increases accumulation of body wastes. Although urine volume may be reduced, it is highly concentrated.
Changes to renal function
Disease processes that primarily affect renal function (changes in urine volume or quality) are generally categorised as prerenal, renal or postrenal in origin (Box 38-1). Prerenal alterations in urinary elimination decrease circulating blood flow to and through the kidneys, with subsequent decreased perfusion to renal tissue. In other words, the alterations are outside the urinary system. The decrease in renal perfusion leads to oliguria (diminished capacity to form urine) or, less commonly, anuria (inability to produce urine). Renal alterations result from factors that cause injury directly to the glomerulus or renal tubule, interfering with their normal filtering, reabsorptive and secretory functions. Postrenal alterations result from obstruction to the urinary collecting system anywhere from the calyces (drainage structures within the kidney) to the urethral meatus (i.e. outside the kidney but within the urinary system). Urine is formed by the urinary system but cannot be eliminated by normal means.
Changes to bladder function
Several diseases can affect the ability to void normally. Any lesion of peripheral nerves leading to the bladder causes loss of bladder tone, reduced sensation of bladder fullness and difficulty in controlling urination. For example, diabetes mellitus and multiple sclerosis cause neuropathic conditions that alter bladder function.
Illness and frailty
Reduced mobility or disability sometimes makes it difficult for a person to reach a toilet in time or to manage clothing or hygiene requirements. Rheumatoid arthritis, degenerative joint disease and Parkinson’s disease are examples of conditions that make it difficult to reach and use toilet facilities. A person with rheumatoid arthritis often cannot sit on or rise from a toilet without an elevated seat or handrails.
Any alteration in cognitive function can impair a person’s perception of the need to void or impair their ability to get themselves to the toilet and complete toileting activities. This is particularly relevant when a person who has dementia is admitted to an acute care hospital or moves from home to residential care. The change in environment can increase the person’s confusion and make episodes of urinary incontinence more likely. A major goal when caring for a person in this situation is to reduce the impact of the change in environment by close observation of the person’s responses, so that the nurse can anticipate the cues that the person needs to go to the toilet, reinforce the location of the toilet and provide frequent reassurance.
Psychological and environmental factors
Anxiety and emotional stress may cause a sense of urgency and increased frequency of urination. An anxious person may have the urge to void even after voiding only a few minutes earlier. Anxiety may also prevent a person from being able to urinate completely. Emotional tension makes it difficult to relax abdominal and pelvic floor muscles. If the external urethral sphincter is not completely relaxed, voiding may be incomplete and urine is retained in the bladder.
When people are hospitalised they are often reluctant to void in a shared ward environment or to use a bedpan or urinal. Where possible the person should be assisted to the toilet to void. Men may need assistance to stand to void. Staying close by and leaving the person with a call bell may encourage a more normal environment in which they feel comfortable for voiding to occur.
Surgical procedures
The stress of surgery initially triggers the general adaptation syndrome (see Chapter 42). The posterior pituitary gland releases an increased amount of ADH, which increases water reabsorption and reduces urine output. The surgical patient is often in an altered state of fluid balance before surgery due to the disease process or preoperative fasting, which aggravates the reduction in urine output. The stress response also elevates the level of aldosterone, resulting in reduction of urine output in an effort to maintain circulatory fluid volume.
Anaesthetic and narcotic analgesics may slow the glomerular filtration rate, reducing urine output. These pharmacological agents also impair sensory and motor impulses between the bladder, spinal cord and brain. Patients recovering from anaesthesia and deep analgesia are often unable to sense bladder fullness and are unable to initiate or inhibit micturition. Spinal anaesthetics, in particular, create the risk of urinary retention because of an inability to sense the need to void and a possible inability of the bladder muscles and sphincters to respond (Brown and Edwards, 2011).
Surgery of lower abdominal and pelvic structures can impair voiding because of local trauma to surrounding tissues. Pain can interfere with relaxation of pelvic floor and sphincter muscles and make voiding difficult or painful. After returning from surgery involving the ureters, bladder and urethra, patients routinely have an indwelling urinary catheter.
Medications
Diuretics prevent reabsorption of water and certain electrolytes to increase urine output. Urinary retention may be caused by use of anticholinergics (e.g. atropine), antihistamines (e.g. pseudoephedrine), antihypertensives (e.g. methyldopa) or beta-adrenergic blockers (e.g. propranolol). Some medications change the colour of urine. Amitriptyline causes a green or blue discolouration, while levodopa may discolour the urine to brown or black (Bryant and Knights, 2010). Cancer chemotherapy drugs may also colour the urine and be toxic to the kidneys or the bladder. Patients with alterations in kidney function require dosage adjustments of medications excreted by the kidneys.
Common urinary elimination problems
People with urinary problems most commonly have disturbances in voiding that involve a failure to store urine or a failure to empty urine. These disturbances result from impaired bladder function, obstruction to urine outflow or inability to voluntarily control micturition. Chronic kidney disease is a significant health problem in the Australian and New Zealand community, causing a significant and ongoing health burden on the person, their family and the healthcare system.
Lower urinary tract infections
Urinary tract infections are very common throughout the life span but are more prevalent in older people. It is estimated that 1 in 3 women and 1 in 20 men will develop a UTI during their lifetime. Approximately 1 in 3 women will have a UTI requiring treatment before the age of 24 years (Masson and others, 2009). When the infection is confined to the bladder and urethra it is termed a lower urinary tract infection. Bacteria in the urine (bacteriuria) may lead to the spread of organisms into the kidneys (upper urinary tract infection).
The most common organisms that cause UTIs in otherwise healthy people are Escherichia coli (85%), Staphylococcus saprophyticus (10%), Enterococcus faecalis and other enterobacteriacae (Gray and Robinson, 2010). Microorganisms most commonly enter the urinary tract through the urethral route. Bacteria inhabit the distal urethra, external genitalia and vagina in women. Organisms enter the urethral meatus easily and travel up the inner mucosal lining to the bladder. Risk factors for developing a UTI include being a postmenopausal woman, sexual activity in women, pregnancy, and underlying anatomical abnormality (vesico-ureteric reflux, history of recurrent UTI, urinary obstruction, presence of renal calculi). Older adults and people with progressive underlying disease or decreased immunity are also at increased risk (Gray and Moore, 2009).
Most children and adults with lower UTIs have pain or burning during urination (dysuria) as urine flows past inflamed tissues. An irritated bladder causes a frequent and urgent sensation of the need to void. Irritation to bladder and urethral mucosa can result in blood-tinged urine (haematuria). The urine can appear concentrated and cloudy because of the presence of white blood cells (WBCs) or bacteria. The signs and symptoms of UTI in older adults can be less obvious. Often the first symptoms may be confusion and dehydration. An infection in the upper urinary tract (kidneys—pyelonephritis) can cause more-severe symptoms such as (commonly) flank pain, tenderness, nausea, vomiting, fever and chills.
The most common cause of infection in hospitalised patients is the introduction of instruments into the urinary tract. For example, the introduction of a catheter through the urethra provides a direct route for microorganisms. Catheter-associated urinary tract infections (CAUTIs) are common. The incidence of bacteriuria increases with the duration that the catheter is in situ. According to Gray and Moore (2009:105), nearly all people with an indwelling urinary catheter will have bacteriuria within 30 days. Hence, a goal of care is to remove the urinary catheter as soon as possible. Bacteria ascend along the outside of the catheter on the urethral wall or travel up the catheter’s lumen. The catheter interferes with the normal voiding mechanism that acts as a defence against organisms entering the urethra. Local irritation to the urethra or bladder further predisposes tissues to bacterial invasion. The majority of CAUTIs do not cause generalised symptoms and are generally not treated with antibiotics. Use of antibiotics in this situation can result in bacterial resistance.
Urinary incontinence
Urinary incontinence is defined as the complaint of any involuntary loss of urine (Abrams and others, 2009). It may be temporary or permanent. Urinary incontinence can be further defined according to the person’s symptoms: stress incontinence, urge incontinence, mixed incontinence (a mix or stress and urge symptoms), nocturnal enuresis, post-micturition dribble, continuous urinary leakage, overactive bladder and functional incontinence (Abrams and others, 2009; Newman and Wein, 2009a) (see Table 38-1 for definitions, risk factors and defining characteristics).
TABLE 38-1 TYPES OF URINARY INCONTINENCE
| DESCRIPTION | RISK FACTORS | SYMPTOMS |
|---|---|---|
| URGE URINARY INCONTINENCE | ||
| Involuntary leakage of urine after a strong sense of urgency to void | Decreased bladder capacity; irritation of bladder stretch receptors; alcohol or caffeine ingestion; increased fluid intake; infection | Significant urinary urgency—inability to defer voiding once the urge has occurred, often with frequency (more often than every 2 hours) |
| STRESS URINARY INCONTINENCE | ||
| Involuntary leakage of urine on effort or exertion or on sneezing or coughing | Coughing, laughing, sneezing or lifting with a full bladder; obesity; full uterus in third trimester; incompetent bladder outlet; vaginal or uterine prolapse; weak pelvic floor muscles | Loss of urine with increased intra-abdominal pressure. Usually small amounts of urine lost |
| MIXED URINARY INCONTINENCE | ||
| Involuntary leakage of urine associated with urgency and also with effort or exertion or on sneezing or coughing | Coughing, laughing, sneezing or lifting with a full bladder; obesity; full uterus in third trimester; incompetent bladder outlet; vaginal or uterine prolapse; weak pelvic floor muscles | Loss of urine with increased intra-abdominal pressure, urinary urgency and frequency. Can be a small or large amount of urine lost |
| FUNCTIONAL URINARY INCONTINENCE | ||
| Involuntary urine loss that occurs because of impaired functional status—impaired cognitive status, impaired mobility and dexterity and/or environmental barriers that block toilet access | Change in environment: sensory, cognitive or mobility deficits | Urge to void that causes loss of urine before reaching appropriate receptacle. The patient with cognitive changes may have forgotten what to do or not recognise the place to void |
| NOCTURNAL ENURESIS | ||
| Involuntary loss of urine occurring during sleep | Abnormal night-time secretion of antidiuretic hormone, developmental delay in development of control of the micturition reflex, family history of enuresis. Exacerbated by urinary tract infection | Urinary loss while asleep |
| OVERACTIVE BLADDER | ||
| Symptoms of urgency with or without urge incontinence, usually with frequency and nocturia | Neurological disorders such as stroke, dementia, Parkinson’s disease; stress urinary incontinence, inflammation, idiopathic | Diagnosed with urodynamic testing. Bothersome urgency usually associated with frequency and nocturia |
| POST-MICTURITION DRIBBLE | ||
| Feeling of involuntary urine loss immediately after finishing voiding | Urinary retention, urethral abnormalities such as a diverticulum in women and post prostatectomy in men | Men will complain of urine loss immediately after voiding; women will complain of urine loss on rising from the toilet |
| NEUROGENIC DETRUSOR OVERACTIVITY (‘REFLEX’ INCONTINENCE) AND DETRUSOR SPHINCTER DYSSYNERGIA | ||
| Urinary incontinence, urinary retention. Urine leakage occurs without warning | Disruption of the central nervous system regulation of the micturition reflex as seen in neurological disorders such as spinal cord injury, multiple sclerosis, etc | Urine leakage occurs without warning |
Adapted from: Abrams P, Cardozo P, Khoury S and others, editors, 2009 Incontinence, ed 4. Paris, Health Publications Ltd, Paris; Gray M, Moore KN 2009 Urologic disorders: adult and paediatric care. St Louis, Mosby; Newman DK, Wein A 2009 Managing and treating urinary incontinence. Baltimore: Health Professionals Press.
Urinary incontinence affects up to 13% of Australian men and up to 37% of Australian women (Australian Institute of Health and Welfare, 2006). In New Zealand, 17% of adult women have bothersome urinary incontinence. The prevalence is even higher in adult Māori women, at 47% (New Zealand Continence Association 2011).
Urinary incontinence significantly affects a person’s quality of life, including the person’s daily activities, sexuality, body image and self-esteem. Efforts to cope with the problem may lead to the person reducing their fluid intake, increasing the frequency of voiding and avoiding social contact (Newman and Wein, 2009a; St John and others, 2010). There is also evidence in the literature that continence problems have significant impact on family caregivers, including depression (Gotoh and others, 2009; Hayder and Schnepp, 2008).
Prostatic hypertrophy and prostate cancer
Benign enlargement of the prostate gland that can occur with ageing can obstruct the urethra and make voiding difficult. Benign prostatic hypertrophy (BPH) is a common condition affecting men from the age of approximately 50 years. According to Gray and Moore (2009:50), 50% of 60-year-old men and 80% of 80-year-old men will experience some bothersome lower urinary tract symptoms (LUTS). The typical symptoms are decreased urinary stream, hesitancy, intermittent stream, nocturia, frequency and urgency (see Table 38-2 for a description of common types of lower urinary symptoms). Some men may develop an acute or chronic urinary retention (see below). Treatment for men who have symptomatic BPH include medications which aim to relax the smooth muscles at the bladder neck (alpha-blockers) or to reduce the size of the prostate gland (5-alpha-reductase inhibitors), or surgery—for example trans-urethral resection of the prostate (Bright and Abrams, 2010).
TABLE 38-2 COMMON LOWER URINARY TRACT SYMPTOMS
| SYMPTOMS | DESCRIPTION | CAUSES OR ASSOCIATED FACTORS |
|---|---|---|
| STORAGE/FILLING SYMPTOMS | ||
| Frequency | Voiding more than 8 times in a 24-hour period | Increased fluid intake, bladder inflammation, increased pressure on bladder (pregnancy, psychological stress), overactive bladder. |
| Nocturia | Urination, particularly excessive or frequent, at night | Excessive fluid intake before bed (especially coffee or alcohol), renal disease, ageing process, prostate enlargement, heart failure, incomplete bladder emptying, use of sedatives and hypnotics |
| Urgency | Strong and immediate need to void immediately that is not easily deferred | Bladder irritation or inflammation from infection, incompetent urethral sphincter, psychological stress |
| EMPTYING/VOIDING SYMPTOMS | ||
| Dysuria | Painful or difficult urination—often described as a ‘burning’ sensation | Bladder inflammation, trauma or inflammation of urethral sphincter |
| Hesitancy | Difficulty initiating urination and delay in onset of voiding | Prostate enlargement, anxiety, urethral oedema |
| Incomplete bladder emptying | The sensation that urine remains in the bladder after micturition | Abnormal bladder sensations, bladder outlet obstruction, neurological diseases, pelvic organ prolapse |
Adapted from Newman DK, Wein A 2009 Managing and treating urinary incontinence. Baltimore: Health Professionals Press.
Prostate cancer is the most common cancer that occurs in men. The risk of developing prostate cancer to age 75 years is 1 in 8 for Australian men (Australian Institute of Health and Welfare, 2008). There are similar rates of prostate cancer in New Zealand men (Ministry of Health, 2011). Prostate cancer is essentially a disease of older men, with 85% diagnosed after 65 years of age (Cancer Council Australia 2011). Prostate cancer is rare before the age of 40 years and its incidence rises rapidly after age 60 years. The cause of prostate cancer is unknown. Apart from advancing age and being male, the strongest established risk factor is a family history of the disease (Bright and Abrams, 2010). Part of the concern for men and for healthcare workers is the non-specific nature of the typical presenting signs and symptoms associated with prostate cancer. In the early stages they are likely to be asymptomatic. Differential diagnosis involves a health assessment and will include a prostatic-specific antigen (PSA) test, digital rectal examination (DRE) and transrectal ultrasound and biopsy. Treatment options for prostate cancer include surgery (radical prostatectomy), androgen deprivation therapy and radiation therapy.
Urinary retention
Urinary retention is defined as the inability to fully empty the bladder after voiding or a complete inability to urinate (Gray and Moore, 2009). Urinary retention can be acute or chronic. In acute retention, key signs are bladder distension and absence of urine output over several hours. The bladder may hold as much as 2000–3000 mL of urine. The person may experience significant discomfort, tenderness over the symphysis pubis, restlessness and diaphoresis (sweating). Acute retention is most common in men due to obstruction of the urethra as a result of benign enlargement of the prostate gland. It is a medical emergency that requires immediate intervention.
Chronic retention results from incomplete bladder emptying over a period of time. It results from either a partial obstruction of the urethra (e.g. urethral stricture, enlarged prostate, chronic constipation) and/or from reduced ability of the detrusor (bladder) muscle to contract during voiding (e.g. spinal cord injury, nerve damage from diabetes mellitus) (Gray and Moore, 2009). As retention progresses, retention with overflow may develop. Pressure in the bladder builds to a point where the external urethral sphincter is unable to hold back urine. The person may have an urge to void but is only able to void small amounts. Some people may experience intermittent episodes of incontinence, and others constant dribbling of urine. Depending on the cause of the chronic retention, the person may or may not be aware of the incomplete bladder emptying. Chronic retention caused by partial obstruction can result in increased pressures within the urinary tract causing damage to the kidneys (postrenal obstruction). Incomplete bladder emptying can predispose the person to UTIs.
Chronic kidney disease
Diseases that cause irreversible damage to the glomerulus or tubules result in permanent alterations in renal function. Chronic kidney disease (CKD—formally known as chronic renal failure) or end-stage kidney disease (ESKD) are the terms used to describe the resulting decline in kidney function from these processes. CKD is damage to the kidneys or reduced renal function lasting more than 3 months (Australian Institute of Health and Welfare, 2009). It is estimated that 13% of Australians are living with CKD (Australian Institute of Health and Welfare, 2009). Aboriginal and Torres Strait Islander people, especially those living in remote communities, are at high risk of developing CKD and the incidence is increasing (Australian Institute of Health and Welfare, 2011). There is a similar incidence of CKD in New Zealand, with 16% of New Zealanders having some form of kidney damage, and a higher incidence in Māori and Pacific Islanders (Joshy and others, 2010). A major risk factor for developing CKD is diabetes. Other risk factors include hypertension, cigarette smoking, obesity, family history of CKD and being over 50 years of age (Kidney Health Australia, 2011).
The person with ESKD manifests numerous metabolic disturbances that require treatment for survival. The associated symptoms experienced by the patient occur as a result of the uraemic syndrome. This syndrome is characterised by an increase in nitrogenous wastes in the blood, altered regulatory functions (causing marked fluid and electrolyte abnormalities), nausea, vomiting, headache, coma and convulsions. Treatment options include methods to correct these biochemical derangements. The problem may be managed conservatively with medications and a regimen of dietary and fluid restrictions. However, as worsening of the uraemic symptoms becomes evident, more-aggressive treatment is indicated. These treatments are known as renal replacement therapies. Dialysis (see Box 38-2) and organ transplant are the two methods of renal replacement; the two methods of dialysis are haemodialysis and peritoneal dialysis. Both dialysis methods can be applied for a short or a long time, and they require specialised equipment and appropriately qualified nurses.
BOX 38-2 INDICATIONS FOR DIALYSIS
• Renal failure that can no longer be controlled by conservative management (i.e. dietary modifications and administration of medications to correct electrolyte abnormalities)
• Worsening of uraemic syndrome associated with end-stage kidney disease (i.e. nausea, vomiting, neurological changes, pericarditis)
• Severe electrolyte and/or fluid abnormalities that cannot be controlled by simpler measures (e.g. hyperkalaemia, pulmonary oedema)
Haemodialysis involves using a machine equipped with a semipermeable filtering membrane (artificial kidney) that removes accumulated waste products from the blood. In the dialysis machine, dialysate fluid is pumped through one side of the filter membrane (artificial kidney) while the patient’s blood passes through the other side. The processes of diffusion, osmosis and ultrafiltration clean the patient’s blood, and it is returned through a specially placed vascular access device (LaRocco, 2011).
An organ transplant is the replacement of the patient’s diseased kidney with a healthy one from a living or cadaveric donor of compatible blood and tissue type. After the patient (recipient) is deemed medically and psychosocially suitable, the organ is surgically implanted. Immunosuppressive medications are administered for life to prevent the body rejecting the transplanted organ. Unlike the other treatments, a successful organ transplant offers the patient the potential for restoration of normal kidney function for varying periods of time.
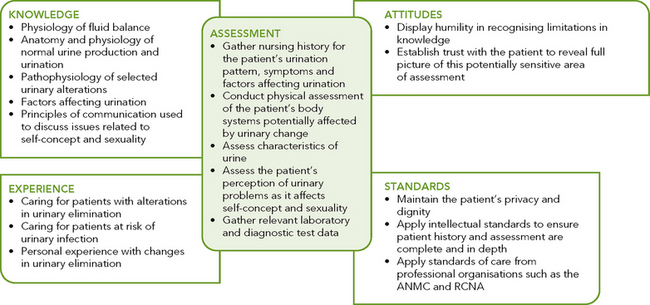
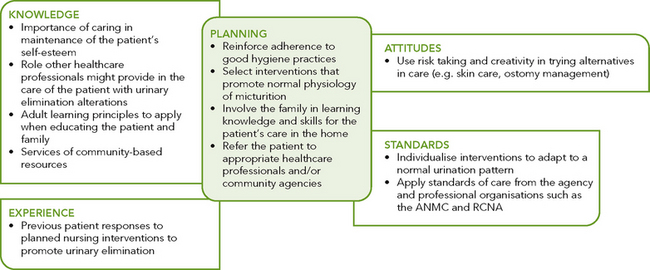
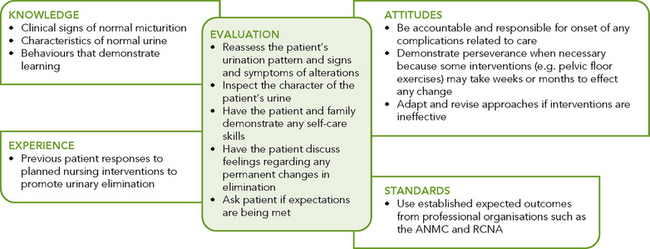
Critical thinking synthesis
Successful critical thinking requires a synthesis of knowledge, experience, information gathered from patients, critical-thinking attitudes, and intellectual and professional standards. Assessment of a person for risk factors or actual urinary elimination problems requires specific knowledge and sensitivity in approaching health issues that the person may find difficult to discuss (see Figure 38-4).
NURSING PROCESS AND URINARY ELIMINATION
ASSESSMENT
Assessing urinary elimination patterns and determining the aetiology of symptoms requires a focused nursing history, physical assessment including abdominal examination, assessment of skin, mobility and where necessary cognition, fluid balance, voiding patterns and urinalysis. In most cases you would also need to assess bowel function (see Chapter 37).
Nursing history
The focused nursing history provides a review of the person’s usual urinary elimination pattern and habits. The purpose of this nursing history is to identify normal and abnormal patterns, habits and the person’s perception of normal and abnormal urinary function. This information enables identification of possible problems and gives direction to the physical examination. The nursing history includes a review of the patient’s elimination pattern, LUTS and an assessment of other factors that may be affecting the ability to urinate normally.
• Determine the person presenting’s concern. It is important to acknowledge the person’s perception of their urinary elimination problem and how this affects their quality of life. How does this problem affect their work, school, activities, relationships, etc?
• Determine the usual voiding pattern. Ask the person about the frequency and pattern of urination, including frequency and times of day, normal volume at each voiding and any recent changes. Frequency varies among individuals and varies with intake and other types of fluid losses. The common times for urination are on waking, after meals and before bedtime. Most people void an average of five or more times a day. Information about the pattern of urination establishes a baseline for comparison. Having the person or caregiver complete a ‘voiding or bladder diary’ can enhance accurate assessment of the person’s urinary pattern. The information collected in a typical voiding diary includes: time of voiding and the measured amount voided, fluid intake and type, presence of symptoms (urge, incontinence, etc). To improve accuracy, the person completing the diary must understand what information must be recorded. Normally a voiding diary would be kept for three 24-hour periods so that the person’s normal urinary elimination patterns can be determined. Keep in mind that normal changes with ageing predispose older adults to certain elimination problems (see Research highlight).
• Characteristics of the urine. Assess colour, odour, clarity, etc.
• Fluid intake/fluid balance. In a hospitalised patient, assessment of fluid balance is often undertaken. The data is recorded on a fluid balance chart, and intake and output are compared over a 24-hour period (see below). The information in the voiding diary will help you determine the amount and types of fluid that the person usually consumes. If the person has not completed a voiding diary, ask them about their usual fluid intake (type and amount) and pattern of intake.
• Pain. There are several sites for pain associated with urinary tract dysfunction. These include flank pain (costovertebral angle), usually unilateral, which is associated with a problem in the kidney or renal pelvis; and suprapubic pain which is associated with UTI. Ask about the frequency and character of the pain. Is the pain associated with voiding?
• Lower urinary tract symptoms. LUTS may occur in more than one type of disorder. During assessment ask the patient about the LUTS listed in Table 38-2. Also investigate the factors that precipitate or aggravate symptoms. It is important to specifically ask people about the presence of urinary incontinence. People are embarrassed about this problem and are often reluctant to talk about it (Keyock and Newman, 2011).
• Other symptoms. Generalised symptoms can also result from urinary elimination problems, especially CKD and upper UTI. Another important association is heart failure; people who have heart failure tend to retain fluid over the day. In addition to a sense of breathlessness on exertion, the person may complain of nocturia. This happens because of the diuresis that may occur in these people when they lie down and their peripheral oedema is relieved. Ask the person about the presence of fever, nausea and vomiting, weight gain or loss, peripheral oedema, fatigue and lethargy, headache, itching skin, etc.
Research focus
Lower urinary tract symptoms (LUTS) are common in the community and can cause decreased quality of life, loss of dignity, social isolation and depression and can interfere with activities of daily living. People living with LUTS use a variety of self-management strategies to deal with the symptoms, including voiding frequently, pelvic floor muscle exercises, use of continence products and fluid manipulation. Little is known about how people experiencing LUTS use fluid manipulation to manage their symptoms.
Research abstract
The purpose of this project was to determine how individuals use fluid manipulation to self-manage the urinary symptoms of daytime frequency, urgency and urine leakage and the underlying rationale for this behaviour. A mixed-methods design included statistical analysis of data from a population-based survey of urological symptoms and qualitative analysis of in-depth interviews. Quantitative data were collected from 5503 participants participating in a community health survey of urological symptoms in Boston (USA). Qualitative data were derived from in-depth interviews from a random subsample of men and women with LUTS.
The findings revealed that fluid intake was greater in men and women reporting frequency (p < 0.001). Women with frequency drank significantly more water (p < 0.001), while women with urgency drank significantly less water (p = 0.047). Qualitative data analysis revealed that some respondents restricted fluid intake while others increased it, in both cases with the expectation of improved symptoms. This study found divergent expectations of the role of fluids in alleviating symptoms, leading some individuals to restrict and others to increase fluid intake.
Evidence-based practice
Individuals with LUTS may need guidance in fluid management. Nurses should be aware that patients may self-manage LUTS by restricting fluid intake, putting them at risk for dehydration, constipation and urinary tract infection, but also that they may be increasing their fluid intake, which could worsen symptoms.
• Past health history. Personal or family history of health issues affecting the urinary tract; for example, infection, urinary calculi, family history of renal or bladder cancer, neurological disorders, surgery including gynaecological, spinal or bowel surgery, diabetes, urinary catheterisation or any issues with constipation. For men, ask about history of prostate cancer. For women, ask about obstetric history (number of births, birthweights, type of delivery, etc) and, if relevant, menopausal symptoms.
• History of smoking. Smoking is a known risk factor for bladder and renal cancer and can contribute to voiding problems.
• Self-care practices. Ask about activity and exercise patterns, toileting and hygiene, use of continence aids and appliances. The patient’s mobility and dexterity need to be evaluated to determine whether the patient needs assistive devices or personnel. You need to make a judgment about the need for questioning in this area based on your observation of the person. For example, questions regarding assistance needed to get to the toilet, undressing/redressing, hygiene, and use of toileting aids (commode chairs, raised toilet seats, etc). Where patients live may affect their toileting habits. If the patient is sharing living quarters, how many bathrooms are there? Do patients have their own bathroom, or do they need to share and thus adjust the time they use the bathroom to accommodate others?
• Medications (prescribed and over the counter) currently being used. Many medications can affect bladder function.
Physical examination
The physical examination is focused on body systems and functions related to urinary elimination problems (see Chapter 27). You will need to make a judgment about the specific areas to include in your examination, which may include assessment of fluid balance (including skin and mucous membranes), abdomen and perineal area/skin. You will also need to complete a urinalysis and may be involved in specimen collection for microscopic examination and microbiological and biochemical analysis.
SKIN AND MUCOSAL MEMBRANES
Problems with urinary elimination are often associated with fluid and electrolyte disturbances. By assessing skin turgor and the oral mucosa, you are assessing the person’s hydration status. A more general skin assessment may be required if the person has urinary incontinence. Specifically assess the health of skin on the upper thighs, groin and perineal area.
ABDOMINAL ASSESSMENT
As mentioned previously, often an assessment of urinary elimination is done at the same time as an assessment of bowel elimination. Specific aspects of an abdominal assessment relevant to urinary elimination involve inspection, palpation and percussion.
Inspect all four abdominal quadrants for contour, shape, symmetry and skin colour. Inspection also includes noting masses, scars, stomas and lesions. In adults the bladder rests below the symphysis pubis and cannot be examined abdominally. When distended, the bladder rises above the symphysis pubis at the midline of the abdomen and may extend to just below the umbilicus. A distended bladder may appear as a protuberance on the central lower abdomen.
Palpation of the abdomen is performed to determine distension (a drum-like tightness), areas of tenderness or the presence of masses (see Chapter 27). It is important for the person to relax. Tensing abdominal muscles interferes with palpating underlying organs or masses. If the examination reveals significant tenderness in any area of the abdomen, note the location of the tenderness and do not progress with further palpation or percussion. Refer the person to a medical practitioner for further assessment, as the pain/tenderness may indicate serious underlying pathology such as acute appendicitis or inflammation of the bowel.
The partially filled bladder normally feels smooth and rounded. As the nurse applies light pressure to the bladder, the patient may feel tenderness or even pain. Even when the bladder is not visible, palpation may cause the urge to urinate. A person with urinary retention may have a significantly distended bladder that you may be able to palpate centrally on the lower abdomen. The suprapubic area may also be tender if the person has a UTI.
Percussion detects lesions, fluid or gas within the abdomen. On percussion a distended bladder will elicit a dull percussion tone. If the kidneys become infected or inflamed, flank pain typically develops. Percussion at the costovertebral angle (the angle formed by the spine and the twelfth rib) may indicate inflammation of the kidney. If you find that the person has kidney pain, they should be referred to a medical practitioner for further assessment.
In most acute care hospitals you will have access to a bladder scanner. This is an ultrasound device which detects and measures the volume of urine in the bladder. It is commonly used to determine the volume (if any) of residual urine left in the bladder after voiding. A conductive gel is applied to the lower abdomen and the transducer is placed on the skin and moved about until a reading is obtained. Once the bladder volume is determined, the person is asked to void normally. The scan is repeated to determine the presence of a residual urine volume. The accuracy of the scan is determined by correct use of the equipment and patient selection. You will need to follow the manufacturer’s instructions on correct use of the bladder scanner.
URETHRAL MEATUS
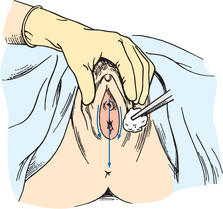
Assess the urinary meatus to note the presence of discharge, inflammation and lesions. This assessment screens for infections and other abnormalities. To examine the female, a dorsal recumbent position provides full exposure of the genitalia. While wearing gloves, retract the labial folds to see the urethral meatus. Normally the meatus is pink and appears as a small slit-like opening below the clitoris and above the vaginal orifice. There is normally no discharge from the meatus. If present, specimens of urethral discharge should be obtained before the patient voids.
Women with vaginal infections are susceptible to UTIs because the vaginal discharge may travel easily to the urethral meatus. Older women commonly have vaginitis as a result of hormonal deficiencies. Inspect the vaginal orifice and note any discharge and the appearance of the labial and vaginal mucosa. Normally the mucosa is pink and moist.
The male urethral meatus is normally a small opening at the tip of the penis. Inspect the meatus for discharge, inflammation and lesions. It may be necessary to retract the foreskin in uncircumcised men to see the meatus.
ASSESSMENT OF INTAKE AND OUTPUT
As mentioned previously, assessment of fluid balance is often undertaken for a hospitalised patient. The data are recorded on a fluid balance chart, and intake and output are compared over a 24-hour period. All sources are included, including oral intake, intravenous fluid infusions, tube feedings and fluid instilled into nasogastric or gastric tubes.
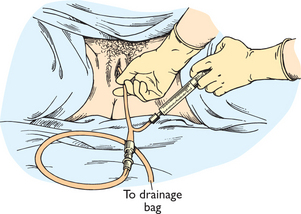
The volume of urinary output is measured (with plastic receptacles, bed pans or urinals) with each voiding. If the person has a urinary catheter, precise measurement of the volume of urine is done by using a measuring device which is part of the urinary drainage bag. The urine drains passively into the urimeter, which holds 100–200 mL of urine. After measuring the urine in the urimeter, typically at hourly or 2-hourly intervals, the urine can be drained directly into the large drainage bag without opening the closed drainage system. If there is no need to measure hourly or 2-hourly urine output, urine volume can be measured by draining the urine from the drainage bag into a plastic graduated measuring receptacle (Figure 38-5). Standard infection-control precaution measures should be used when measuring urine.

FIGURE 38-5 Urine drainage bag.
From Potter PA, Perry AG 2013 Fundamentals of nursing, ed 8. St Louis, Mosby.
Report any extreme increase or decrease in urine volume. An hourly output of less than 0.5 mL/kg for more than 2 hours is cause for concern. Similarly, consistently high volumes of urine (polyuria), more than 2000–2500 mL daily, should be reported to the person’s medical practitioner.
ASSESSMENT OF URINE
The characteristics of the urine should also be recorded. Inspect the urine for colour, clarity and odour. Normal urine ranges from a pale straw colour to amber, depending on its concentration. Urine is usually more concentrated in the morning or with fluid volume deficits. As the person drinks more fluids, urine becomes less concentrated. Bleeding from the kidneys or ureters causes urine to become dark red or tea-coloured; bleeding from the bladder or urethra causes bright-red urine. Various medications also change urine colour. Eating beetroot, rhubarb or blackberries may cause red urine. Special dyes used in intravenous diagnostic studies eventually discolour urine. Dark-amber urine may be the result of high concentrations of bilirubin caused by liver dysfunction. Document and report any abnormal colour or sediment, especially if the cause is unknown.
Normal urine is transparent at voiding; urine that stands several minutes in a container becomes cloudy. Freshly voided urine in patients with renal disease may be cloudy or foamy because of high protein concentrations. Urine can be thick and cloudy as a result of bacteria.
Urine has a characteristic odour. The more concentrated the urine, the stronger the odour. Stagnant urine has an ammonia odour, which is common in patients who are repeatedly incontinent. A sweet or fruity odour occurs from acetone or acetoacetic acid (by-products of incomplete fat metabolism) seen with diabetes mellitus or starvation. Keep in mind that some foods, for example asparagus, can change the odour of urine. If you detect an unusual odour, especially when there are no other abnormalities in the urine, ask the person about what they have been eating in the last 12–24 hours.
Urine testing
Urine specimens are often collected for laboratory testing. The type of test determines the method of collection. Specimens that need to be sent to the laboratory for analysis should be labelled with the patient’s name and date and time of collection. They should be transported to the laboratory in a timely fashion to ensure accuracy of test results. Standard infection-control precaution measures should be used when collecting urine specimens (see Chapter 29). In all cases you need a fresh urine sample.
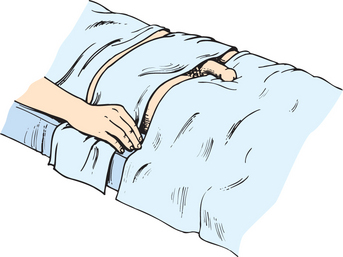
COLLECTING A CLEAN VOIDED URINE SPECIMEN
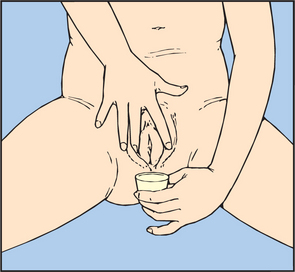
A clean, freshly voided specimen is collected so that urinalysis and inspection of the urine can be performed. The person is asked to void into a clean urine cup, urinal or bed pan. Most people are able to do this independently; however, if the person has poor mobility they may require assistance. The person should void before defecating so that faeces does not contaminate the specimen. Female patients are also instructed not to place toilet tissue in the bed pan. Only 50–100 mL of urine is needed for accurate testing. After the specimen is collected, the urine is inspected and then tested using the appropriate testing strips (dipsticks).
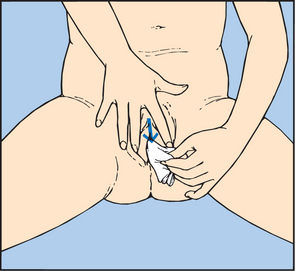
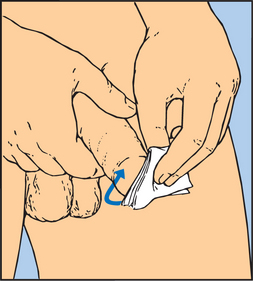
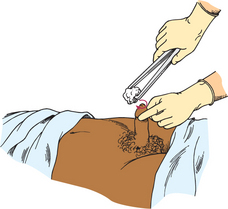
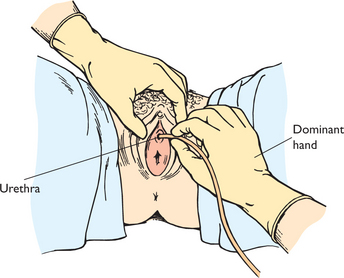
COLLECTING A MIDSTREAM URINE SPECIMEN
To obtain a specimen relatively free of the microorganisms growing in the lower urethra, the patient will need to be instructed about the method for obtaining a midstream urine specimen (Skill 38-1). This type of specimen is needed to culture urine to determine the presence of bacteriuria and to check for antibiotic sensitivity (micro-culture and sensitivity or MC&S test). After appropriate cleaning of the external genitalia, the person begins voiding, allowing a small amount of urine to escape; then during the middle portion of voiding, the person collects the specimen in a sterile container. The initial stream of urine cleans or flushes the urethral orifice and meatus of resident bacteria. It is easiest for a patient to obtain midstream urine specimens while using toilet facilities.
SKILL 38-1 Collecting a midstream (clean-voided) urine specimen
DELEGATION CONSIDERATIONS
If appropriate, an alert patient who is physically able may be instructed to collect the specimen.
EQUIPMENT
• Soap or cleaning solution, washcloth and towel or disposable cleansing towel
• Sterile specimen collection jar with lid
• If the person cannot collect the specimen themselves, a bed pan, bedside commode or specimen hat
| STEPS | RATIONALE | ||
|---|---|---|---|
| May indicate bladder fullness. | |||
| Reveals patient’s ability to cooperate during procedure. | |||
| Determines level of assistance. | |||
| Information allows you to clarify misunderstandings and promotes patient cooperation. | |||
| Helps patient understand the procedure. | |||
| Faeces change characteristics of urine and may cause abnormal values. | |||
| Improves likelihood of patient being able to void. | |||
| Privacy allows patient to relax and produce specimen more quickly. | |||
| Prevents transmission of microorganisms to nurse, provides easy access to perineal area to collect specimen. | |||
|
7. Open the sterile specimen container, placing cap with sterile inside surface up; do not touch inside of container or cap (see Chapter 29). |
Aseptic technique is essential to maintain sterility of equipment and specimen. Contaminated specimen is most frequent reason for inaccurate reporting of urine cultures and sensitivities. | ||
| Provides access to urethral meatus. | |||
| Clean from area of least contamination to area of greatest contamination, to decrease bacterial levels. | |||
| Initial stream flushes out microorganisms that accumulate at urethral meatus and prevents transfer into specimen. | |||
| Clean from area of least contamination to area of greatest contamination, to decrease bacterial levels. | |||
| Initial stream flushes out microorganisms that accumulate at urethral meatus and prevents transfer into specimen. | |||
| Prevents contamination of specimen with skin flora. | |||
| If foreskin not replaced, swelling and constriction may occur, causing pain and possible obstruction to urine flow. | |||
| Retains sterility of inside of container and prevents spillage of urine. | |||
| Prevents transfer of microorganisms to others. | |||
| Promotes relaxing environment. | |||
| Prevents inaccurate identification that could lead to errors in diagnosis or treatment. | |||
| Critical decision point: If patient is menstruating, indicate information on laboratory requisition. | |||
| Reduces transmission of infection. | |||
| Bacteria grow quickly in urine, and specimen should be analysed immediately to obtain correct results. | |||
| RECORDING AND REPORTING | HOME CARE CONSIDERATIONS |
|---|---|
COLLECTING A URINE SPECIMEN FROM A PATIENT WITH AN INDWELLING URINARY CATHETER
You may need to perform a urinalysis using dipsticks or collect a specimen for laboratory analysis from a person who has an indwelling urinary catheter. A urine specimen is not collected for culture from a urine drainage bag unless it is the first urine drained into a new, sterile bag. Bacteria grow rapidly in the drainage bags and could cause an inaccurate result. The aim is to obtain a fresh urine sample while maintaining the closed system between the catheter and the urine drainage bag. The sample needs to be drawn from the needleless sampling port in the drainage bag tubing. You should not disconnect the catheter from the drainage bag tubing, nor insert a needle into the catheter or the drainage bag tubing. The following steps should be undertaken:
• Inform the patient of the purpose of collecting the urine.
• Place the urine drainage bag tubing so that a loop near the drainage port is lying flat on the patient’s bed or chair (so that urine will collect in the tubing). In some cases you may need to clamp off the tubing; however, make sure that you return to take the sample within 15 minutes so that urine does not build up in the bladder.
• After performing hand hygiene, don non-sterile gloves.
• Assemble a sterile 10 mL syringe.
• If the specimen is for laboratory analysis, have a sterile pathology specimen container close by—do not touch the inside of the container or the lid. Make sure that the container is labelled with the patient’s details and date and time of the specimen collection. Also make a note on the label that the urine was obtained from a patient with an indwelling catheter.
• Wipe the needleless port with an antimicrobial swab. When this has dried, carefully insert the hub of the syringe through the needleless port into the catheter lumen. Aspirate 5–10 mL of urine and remove the syringe from the sampling port. Transfer the urine into the sterile container using an aseptic technique (see Chapter 29).
• Return the drainage bag tubing to the usual position so that urine will flow freely into the drainage bag.
• Remove the gloves and properly dispose of equipment. Perform hand hygiene.
The specimen is transferred to the laboratory in a sealed plastic bag with the request slip for testing. It generally takes 24 hours to culture the bacteria and 48 hours to identify sensitivity to antimicrobial agents.
COLLECTING URINE OVER A SPECIFIC TIME PERIOD
Some tests of renal function and urine composition, such as measuring levels of adrenocortical steroids or hormones, creatinine clearance or protein quantitation tests, require collection of urine over 2-, 12- or 24-hour intervals. The urine is collected each time the person voids and placed in a specially labelled container over the prescribed period of time. The timed collection period begins after the person urinates. This sample is discarded and the collection begins at this time. You will need to inform the patient that all urine must now be collected, and note the starting time on the collection container and on the laboratory requisition. The person then collects all urine voided in the timed period. Each voiding is collected in a clean container and immediately emptied into the larger container. Any missed specimens will make test results inaccurate. The person should be reminded to void before defecating so that faeces or toilet paper do not contaminate urine. The person should void the last specimen at the end of the timed period. For example, for a 24-hour collection the person first voids at 0800. This sample is discarded and the 24-hour time period begins. All urine from this time onwards is saved until 0800 the next day. The person voids as close to 0800 as possible. This urine is added to the collection container and the time period is completed.
COLLECTING URINE FROM CHILDREN
Specimen collection from infants and children is often difficult. Adolescents and school-age children are usually able to cooperate, although they may be embarrassed. Preschool children and toddlers have difficulty voiding on request. Offering a young child fluid 30 minutes before requesting a specimen may help. Gain cooperation from the parents in obtaining the specimen.
A young child may be reluctant to void in unfamiliar receptacles. A potty-chair or specimen hat placed under the toilet seat is usually effective. For infants or toddlers who are not toilet-trained, clear plastic, single-use bags with self-adhering material (paediatric urine collector) can be attached over the child’s urethral meatus. Specimens should not be obtained by squeezing urine from the nappy material. When the specimen is obtained, it is placed in a sterile container and labelled with the child’s details and date and time of the specimen collection. Also, make a note on the label that the urine was obtained using a paediatric urine-collecting device.
COMMON URINE TESTS
Urine tests include urinalysis, specific gravity and urine culture.
Urinalysis is a test commonly performed by nurses in all healthcare settings. The urine specimen should be fresh. The purpose of the urinalysis is to determine the constituents of the urine and determine the presence of abnormalities. Using standard infection-control guidelines, dip the reagent strips (often called dipsticks) into the urine, making sure that all of the reagent pads are submerged in the urine. Taking care to drain the urine off the dipstick, observe for a colour change in the time interval designated on the packaging (Figure 38-6). Various brands of urine dipsticks test for varying abnormalities. Typically the strips test for glucose, bilirubin, ketones, specific gravity, microscopic blood, pH, protein, urobilinogen, nitrites and leucocytes. The exact detail of the results of the urinalysis is recorded in the patient’s clinical progress notes. Urine may also be sent to the laboratory where urinalysis is performed using specialised equipment. Table 38-3 lists normal values for a urinalysis.
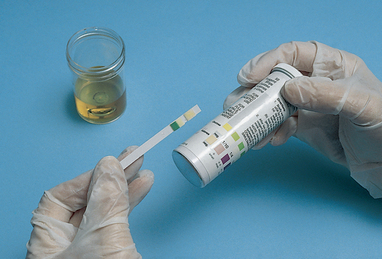
FIGURE 38-6 Checking results of a chemical reagent strip dipped in urine.
From Potter PA, Perry AG 2013 Fundamentals of nursing, ed 8. St Louis, Mosby.
TABLE 38-3 ROUTINE URINALYSIS—ADULTS
Adapted from: Royal Society of Pathologists of Australia Manual 2009. Online. Available at http://rcpamanual.edu.au; Gormella LG, editor, 2009 The 5-minute urology consult. Philadelphia, Wolters Kluwer/Lippincott Williams & Wilkins.
Diagnostic examinations
The urinary system is one of the few organ systems amenable to accurate diagnostic study by several radiographical techniques. The two approaches for viewing urinary structures, direct and indirect techniques, can be quite simple or very complex, requiring extensive nursing intervention. These procedures are further subdivided into invasive or non-invasive categories.
NON-INVASIVE PROCEDURES
ABDOMINAL X-RAY
Also referred to as plain abdominal film or KUB (kidney, ureter, bladder), X-ray of the abdomen is commonly used to assess the gross structures of the urinary tract for abnormalities. It can determine size, symmetry, shape and location of the kidneys, ureters and bladder structures. It is also useful in showing calculi (if calcified) or tumours in these organs. In addition, the ribs or other surrounding support structures can be assessed for fractures or abnormalities. Lack of positive findings on the X-ray does not rule out the possibility of abnormalities in the urinary tract; additional diagnostic studies may be needed. No special preparation is needed prior to this procedure.
INTRAVENOUS PYELOGRAM (IVP)
This technique enables visualisation of the collecting ducts and renal pelvis and outlines the ureters, bladder and urethra. Although this procedure is non-invasive, it requires the patient to receive an intravenous injection of a radio-opaque dye. Because the kidneys and ureters lie behind the intestines, it is necessary that the patient receives a bowel preparation to empty the intestines before the procedure.
During the IVP, X-ray studies are taken at specific intervals over 30–60 minutes as the dye concentrates. The patient may also be asked to void during the procedure, to measure bladder emptying. Diseases or disorders of the urinary tract that should be investigated by this means include renal artery occlusion, tumours, cysts or calculi, vesico-urethral reflux and traumatic injuries.
RADIONUCLIDE TESTS
Radionuclide tests such as renal scans allow indirect viewing of urinary tract structures after an intravenous injection of radioactive isotopes. The emissions from the radionuclides can be photographed by special cameras. The isotope can be detected without the need for bowel preparation. A very low dose of radioisotope is used. No precautions against radioactive exposure are needed except for the use of disposable gloves if the patient uses a bed pan or urinal to void. Rinse bed pan or urinal and double-flush urine down the toilet to dilute any possible remaining radiation hazard.
After a radionuclide is injected, it circulates through the kidneys and is excreted. The renal scan measures radioactive concentrations. Except for the venepuncture, it is painless. The scanning procedure is completed in about 1 hour. Information pertaining to renal blood flow, anatomical structures and their excretory function can be obtained from this procedure.
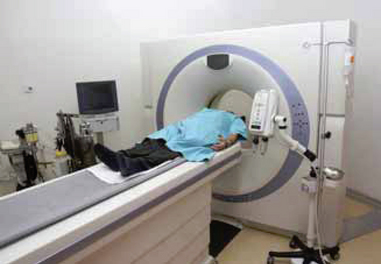
COMPUTED TOMOGRAPHY (CT)
This is a computerised X-ray procedure used to obtain detailed images of structures within a selected plane of the body. The tomographic scanner is a large machine that contains specialised computers and X-ray detector systems that function simultaneously to photograph internal structures in thin, transverse cross-sections (Figure 38-7). The computer, through a series of complex manipulations, is able to ‘reconstruct’ the cross-sectional image as a recognisable photograph on the television monitor. With this procedure it is possible to see abnormal pathological conditions such as tumours, obstructions, retroperitoneal masses and lymph node enlargement. Although this procedure is non-invasive, in some examinations oral or intravenous contrast material is used to enhance the areas under study. If intravenous contrast is used, it may be necessary to administer a bowel-cleaning solution orally (such as GoLYTELY), especially if additional organs in the abdominal cavity will be examined.
ULTRASONOGRAPHY
A valuable non-invasive diagnostic tool in the assessment of urinary disorders, ultrasonography or ultrasound makes use of high-frequency inaudible sound waves that reflect off tissue structures. Some of the sound waves are reflected back to the transducer as echoes; the velocity of the sound waves varies with tissue density. The patient is usually prone during the procedure but can be positioned in a sitting position. Ultrasound is often used to identify gross renal structures and structural abnormalities of the kidneys or lower urinary tract, and to assist with percutaneous biopsy. Abnormalities such as tumours or cysts in the kidney are easily identified. If a Doppler is used with the transducer, examination of blood flow through the kidney can also be performed. This procedure is painless.
INVASIVE PROCEDURES
ENDOSCOPY
Endoscopy is the visual inspection of a hollow body organ with the aid of a fibre-optic instrument. A cystoscopy enables visualisation of the interior of the bladder and urethra. The cystoscope is inserted through the patient’s urethra into the bladder. The instrument has an outer plastic or rubber sheath, an obturator that keeps the scope rigid during insertion, a telescope for viewing the bladder and urethra, and a channel for inserting catheters or special surgical instruments.
A flexible cystoscopy is performed using local anaesthesia (xylocaine gel) which is inserted into the urethra prior to inserting the endoscope. During the test, urine and tissue specimens may be collected. This procedure can be performed in an operating room or in a procedure room in a clinic. In some hospitals, registered nurses who have undertaken specific education perform this procedure, especially in situations where patients are having frequent procedures to monitor bladder tumours. This test is commonly performed as an outpatient. The person may eat and drink as normal prior to the test and will be able to leave the hospital shortly after the procedure. They should be encouraged to observe their urine for signs of bleeding or infection and be advised to increase fluid intake.
URODYNAMIC TESTING
A variety of studies may be done to measure the transport, storage and elimination of urine in the lower urinary tract. A cystometrogram (CMG) is one such test that determines the level of function of the detrusor muscle. This test is used to determine the type and degree of urinary incontinence. A catheter is inserted, residual volume is measured and discarded and the bladder is filled with sterile saline in predetermined increments. Pressure readings are taken at those increments. During the filling time, the patient’s perceptions related to bladder fullness, urge to void and the ability to inhibit voiding are documented.
Patient expectations
Patients depend on their caregivers to recognise and meet their needs. The patient with needs related to urinary function expects that the nurse will be respectful of privacy needs and sensitive to the impact of urinary impairments on their quality of life and self-concept. The patient should be included in the plan of care and in the development of goals that are mutually acceptable. Cultural practices and personal preferences should also be considered.
NURSING DIAGNOSIS
A thorough assessment of the patient’s urinary elimination function reveals patterns of data that allow the nurse to make relevant and accurate nursing diagnoses. The diagnosis may be an actual problem or a problem that the patient is at risk of developing (Box 38-3).
The diagnosis may focus on a specific urinary elimination alteration or on associated problems such as impaired skin integrity related to urinary incontinence. Identification of defining characteristics leads the nurse to select an appropriate diagnosis (Box 38-3). Specifying related factors for each diagnosis allows selection of individualised nursing interventions.
PLANNING
Critical thinking ensures that the patient’s plan of care integrates knowledge about the individual as well as key critical thinking elements (Figure 38-8). Professional standards are especially important to consider when developing a plan of care. These standards often establish scientifically proven guidelines for selecting effective nursing interventions.
The plan incorporates health promotion activities and therapeutic interventions for patients with urinary elimination problems. Preventive interventions may be required for patients at risk of urinary problems. It is important in the nursing process to consider the patient’s home environment and normal elimination routines when planning therapies. In planning care for some patients, consultation with other healthcare professionals may be needed. For example, the physiotherapist can design an exercise plan to increase strength and endurance so the patient will be able to walk to the bathroom.
Goals and outcomes
Planning care also involves an understanding of the patient’s need to control body function. Alterations in urinary elimination can be embarrassing, uncomfortable and often frustrating. The nurse and patient work together to establish ways of maintaining patient involvement in nursing care and to maintain normal urinary elimination. General goals for the patient may include:
Setting priorities
Associated problems such as anxiety may require interventions that often have no direct effect on urinary elimination. Unless the nurse intervenes, however, associated problems are likely to continue. Problems involved with urinary elimination alterations are often interrelated and complex. You also need to anticipate problems that may develop as a result of therapy. For example, the diagnosis of risk of infection is appropriate when a patient has an indwelling catheter. Determining the most imperative diagnosis will depend on each individual patient’s condition.
Continuity of care
For hospitalised patients, planning should include preparations for discharge; for example, assessment of the need for any aids that will be required and the patient’s educational needs. Health education is important in enabling people to care for themselves. For example, a patient being discharged with an indwelling catheter will need to perform catheter care, understand ways to empty the drainage bag safely, measure urine accurately and know signs and symptoms of urinary infection. The need for home health services should be explored, and appropriate referrals should be made. Good discharge planning will result in the patient’s smooth transition from healthcare agency to home.
FUNCTIONAL INCONTINENCE
ASSESSMENT*
Jenny Smith (a registered nurse) is assisting Mrs Christine Bryant to have a shower. Mrs Bryant, 81 years of age, was admitted to the orthopaedic ward 5 days previously via the emergency department after sustaining a fractured pubic ramus from a fall in the backyard of her house. She has a medical history of osteoporosis and has been taking daily strontium (Protos) for the past 2 years. She is otherwise well and does not take any regular medication. She is active in the local bowls club and is involved in local community groups. She lives with her husband (84 years old) who has chronic obstructive pulmonary disease and macular degeneration that limits his activities to staying around the house. Since admission she has been on bed rest and has been getting up to go to the toilet and shower with assistance and a gutter frame walker. She is taking oxycodone (OxyNorm) for pain which has good effect when she is resting; however, she finds walking very painful. While in the shower she tells Jenny that she has been losing urine on the way to the toilet, especially if there is a delay in the nurse coming to assist her after she has rung the call bell. Jenny tells her she will assist her to get dried and dressed and they can talk about this further.
The assessment reveals the following data:
• Normally has no urinary incontinence, never needs to use a pad.
• No other LUTS—no frequency, urgency or dysuria. She feels she can hold on a long time but because she needs assistance it sometimes takes a long time for a nurse to give her assistance to get to the toilet. She says that she is reluctant to call for help because ‘the nurses are so busy’.
• Only small amounts of urine lost—only on walking to the toilet when she has a very full bladder. Gets up once at night to go to the toilet.
• Normally no loss of urine on sneezing or coughing except when she has a very full bladder.
• History of three vaginal births (all over 3.5 kg birthweight); 1st delivery with forceps.
• No history of constipation. Bowels open yesterday—Bristol type 3.
• Eating and drinking normally—drinking 5 cups of tea per day and water (approx. 1 litre).
• Beginning to mobilise well with assistance. Likelihood of a two-week hospital stay.
• Abdominal examination—soft abdomen, bowel sounds in all four quadrants.
• Skin on upper thighs and perineum intact and healthy.
• Urine analysis—urine clear, pH 6.5, no abnormalities detected.
NURSING DIAGNOSIS: Functional incontinence related to impaired mobility. Mild stress incontinence (when bladder very full) related to weakened pelvic floor muscles (age and childbirth).
PLANNING
| GOALS | EXPECTED OUTCOMES |
|---|---|
| Patient will have reduced episodes of incontinence within 2 days. | Patient will report less-frequent episodes of incontinence. |
| INTERVENTION | RATIONALE |
|---|---|
| Reduces the likelihood that she will have a very full bladder. Gives her more time to get to the toilet within current mobility constraints. | |
| –the need to call for assistance when she first feels the need to void | Encourages her to feel empowered to take control and not feel a burden on nursing staff |
| –that she is not causing a problem for the nursing staff | |
| –the importance of strong pelvic floor muscles | |
| –the importance of not becoming constipated. | Motivates her to participate in PFM training. |
| –the need to keep drinking fluids, especially water. | |
| Makes it easy for her to be independent without risk of falling. | |
| Pelvic floor exercises strengthen the muscles that control continence; 4-6 weeks of exercises will result in decreased leakage. |
*Defining characteristics are shown in bold type.
IMPLEMENTATION
Promotion of normal voiding patterns
Many nursing measures have been designed to promote normal voiding in patients at risk of urination difficulties and in patients with established urination problems. The nurse can initiate many of these interventions independently.
PROVIDE PRIVACY AND TIME TO VOID
Encourage patients to not rush voiding, so that their bladder empties fully. Privacy is essential. If the patient cannot reach the bathroom, make sure that a curtain encloses the bedside area. In the home the debilitated patient may prefer using a bedside commode enclosed behind a partition or room divider. Some patients are embarrassed by the sound of voiding. Running water or flushing the toilet masks the sound. Young children are often unable to void in the presence of persons other than their parents.
It is very important to respond to patients’ need to urinate in a timely manner. Delay in helping patients to the bathroom may interfere with normal voiding and contribute to incontinence. Attempts by frail older people or those with a disability or a mobility problem to get to the toilet alone can contribute to falls (DuBeau and others, 2009).
STIMULATING MICTURITION REFLEX
It is often difficult for hospitalised patients to void due to the effects of surgery, an anaesthetic, pain or medications. The patient’s ability to void depends on feeling the urge to urinate, being able to control the urethral sphincter and being able to relax during voiding. You can help a patient learn to relax and stimulate the reflex to void by assuming the normal position for voiding. A woman is better able to void in a sitting position, leaning forward with feet flat on the floor. This position promotes contraction of the pelvic and intra-abdominal muscles that assist in sphincter control and bladder contraction. If the patient is unable to use toilet facilities, assist the person to position themselves in a squatting position on a bed pan (see Chapter 37) or bedside commode. A man voids more easily in the standing position. If the man cannot reach the toilet, he may stand at the bedside and void into a urinal (a metal or plastic receptacle for urine) (Figure 38-9). At times it may be necessary for one or more nurses to assist a man to stand.
FIGURE 38-9 Types of male urinals.
From Potter PA, Perry AG 2013 Fundamentals of nursing, ed 8. St Louis, Mosby.
Other measures that promote relaxation and the ability to void include sensory stimuli. The sound of running water may help the patient void through the power of suggestion. If the patient is confined to bed the nurse can also pour warm water over their perineum and create the sensation to urinate. If urine output is to be measured, the nurse must first measure the volume of water to be poured over the perineal area. Offering fluids the patient will drink may also promote voiding.
MAINTAINING ADEQUATE FLUID INTAKE
A simple method of promoting normal micturition is by maintaining good fluid intake. A patient with normal renal function who is not on fluid restriction should drink 1500–2000 mL of fluid daily. However, an average daily intake of 1200–1500 mL of fluids is usually adequate (in hot weather, increase fluid intake to compensate for increased loss through perspiration).
When fluid intake is increased, the excreted urine flushes out solutes or particles that may collect in the urinary system. Because a patient may be unwilling to drink 2000 mL of water daily, encourage fluids that the patient prefers. Many vegetables and fruits also have a high fluid content. At home it may help to set a schedule for drinking fluids (e.g. with meals or medications).
MAINTAINING NORMAL BOWEL FUNCTION
Constipation can cause obstruction of the urethra and contribute to a sense of urinary urgency. Promotion of normal bowel function needs to be part of the care plan for any patient who has a urinary elimination problem. See Chapter 37 for details on promoting normal bowel function.
PATIENT EDUCATION
The success of therapies aimed at eliminating or minimising urinary elimination problems depends in part on the person understanding their urinary elimination problem. For example, people who experience recurrent UTIs may benefit from learning about normal sterility of the urinary tract and ways to prevent infection. Health teaching can be incorporated when giving nursing care, for example discussing the benefits of increasing fluid intake while giving fluids with medications or meals.
CATHETERISATION
Catheterisation of the bladder involves introducing a tube, usually made of silicone- or hydrogel-coated latex, through the urethra and into the bladder. The catheter provides a continuous flow of urine in patients unable to control voiding or those with urethral obstructions. It also provides a means of assessing hourly urine outputs in haemodynamically unstable patients. Because bladder catheterisation carries the risk of UTI and trauma to the urethra, it is preferable to rely on other measures for either specimen collection or management of incontinence.
WORKING WITH DIVERSITY FOCUS ON CULTURAL CARE
The Victorian Continence Resource Centre conducted a project titled ‘Awareness of incontinence in ethnic communities’. The aim of the project was to explore the awareness of people from different ethnic communities in relation to the prevention, treatment and management of incontinence.
Twenty focus groups were undertaken in selected ethnic communities in Victoria. Participants (n = 218) included older men and women, and middle-aged women (167 female and 52 male). The communities represented included Arabic-speaking, Chinese, Greek, Italian, Polish, Vietnamese, Macedonian, Russian, Spanish-speaking and Turkish. Interpreters were used when needed.
• Knowledge of incontinence was low across all ethnic groups.
• Not all languages have a term to describe ‘incontinence’ or the translated term often has a negative or detrimental meaning.
• Limitation to social participation was a common impact of incontinence.
• Incontinence could lead to religious restriction for Muslim men and women.
• Cultural and religious influences were expressed in relation to preferences for information in a specific language and for healthcare professionals who spoke their language or were a specific gender.
• Communication and language barriers were perceived to limit access to information and support services.
Continence Foundation Australia (Victoria Branch). Awareness of incontinence in ethnic communities. Kew: CFA (Vic Branch). Online. Available at: www.continencevictoria.org.au/node/138, 2011. 27 Feb 2012.
Incontinence information for supporting ethnic communities available at: www.continencevictoria.org.au/node/138.
METHODS OF ACHIEVING DRAINAGE OF THE URINARY TRACT
There are several methods for achieving drainage of the urinary tract:
• An indwelling or Foley catheter has a small inflatable balloon that encircles the catheter just below the tip. When inflated, the balloon rests against the bladder outlet to anchor the catheter in place. The indwelling retention catheter also has two or three lumens within the body of the catheter (Figure 38-10B). One lumen drains urine through the catheter to a collecting tube. A second lumen carries sterile water to and from the balloon when it is inflated or deflated. A third (optional) lumen may be used to instil fluids or medications into the bladder. It is easy to determine the number of lumens by the number of drainage and injection ports at the catheter’s end. An indwelling catheter is used when constant drainage is required, for example when there has been surgery to the abdomen or pelvic area, or when accurate measurement of urine is required. Indwelling catheters can be left in for long periods of time. It is necessary to change indwelling catheters periodically—usually every 6–8 weeks.
• Intermittent catheterisation. With the intermittent technique, a straight single-use catheter (Figure 38-10A) is introduced long enough to drain the bladder (5–10 minutes). When the bladder is empty, the catheter is withdrawn. The straight single-use catheter has a single lumen with a small opening about 1.3 cm from the tip. Urine drains from the tip, through the lumen, to a receptacle. Intermittent catheterisation can be repeated as necessary, usually several times per day. Some people require intermittent catheterisation for many months or years and are taught to catheterise themselves; this is termed intermittent self-catheterisation (ISC).
• Suprapubic catheterisation. A suprapubic catheter is surgically inserted into the bladder via a puncture wound on the lower abdomen. These are used in patients who have chronic retention of urine, neurological disease (such as spinal cord injury or multiple sclerosis), prior pelvic or gynaecological surgery, urethral trauma or those who require long-term drainage of urine (Harrison and others, 2010). They can be more comfortable for people who require long-term urinary drainage. Suprapubic catheters are self-retaining—they have a balloon at the catheter tip. Suprapubic catheters need to be changed the same way that indwelling urethral catheters are changed. Registered nurses who have had appropriate training are able to change these catheters.
• Other methods to achieve drainage from the urinary tract. There are several other devices that can be used to drain urine from the upper urinary tract. Nephrostomy tubes are inserted in the flank region straight into the pelvis of the kidney. These are used when there is obstruction in the upper ureter from urinary calculi or tumours or resulting from oedema when there has been surgery to the upper tract. A sterile drainage bag is applied to the person’s skin and urine can drain freely. Stents can also be inserted into the ureter so that drainage is maintained. These fine tubes are inserted via a cystoscope in an operating room.
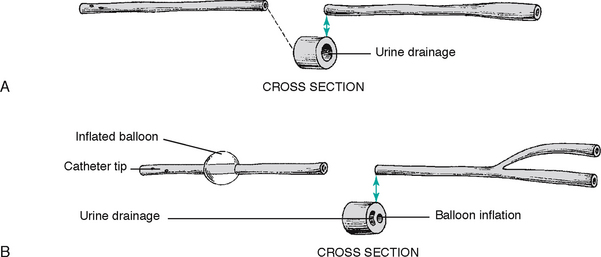
FIGURE 38-10 Types of urinary catheters. A, Non-retaining or straight catheter. B, Self-retaining indwelling (Foley) catheter.
From Potter PA, Perry AG 2013 Fundamentals of nursing, ed 8. St Louis, Mosby.
Catheters come in many diameters to fit the size of a patient’s urethral canal. Suggestions on catheter selection are provided in Box 38-4.
BOX 38-4 GUIDELINES FOR APPROPRIATE CATHETER SELECTION
• The catheter size should be determined by the size of the person’s urethral canal. When the French system (Charriére or French Gauge) is used, the larger the gauge number, the larger the catheter size. One Charriére is ⅓ of a millimetre. Generally, children require an 8–10 FG, women require a 12–14 FG and men require a 16–18 FG. To prevent trauma, the smallest effective catheter size is preferred.
• The expected time required for the catheterisation will determine the catheter material selection.
• Balloon size is also important in selecting an indwelling catheter. Use the smallest balloon size possible, as a large balloon can cause irritation at the bladder neck.
• Only sterile water should be used to inflate the balloon as saline may crystallise, resulting in incomplete deflation of the balloon at the time of removal.
Adapted from Australian and New Zealand Urological Nurses Society 2009 Catheterisation guidelines. Online. Available at www.anzuns.org/?page_id=105 22 May 2012; Turner B, Dickens N 2011 Long-term urethral catheterisation. Nurs Stand 25(24):49–56.
INDICATIONS FOR CATHETERISATION
Catheterisation may be indicated for many reasons. When catheterisation time will be short and minimising infection is a priority, the intermittent method is best. Intermittent catheterisation is also preferred for people who have chronic retention of urine (without urethral obstruction), such as a person with diabetes who has developed an atonic bladder. By intermittently draining the bladder on a routine basis, these patients have fewer infections (Newman and Wilson, 2011). Indwelling catheterisation is used when long-term bladder emptying is necessary. Box 38-5 outlines specific indications for catheterisation.
BOX 38-5 INDICATIONS FOR CATHETERISATION
• Relief of acute or chronic urinary retention.
• Measurement of urinary output in acutely ill patients:
• Following surgery on the bladder, urethra and surrounding structures:
Adapted from Australian and New Zealand Urological Nurses Society 2009 Catheterisation guidelines. Online. Available at www.anzuns.org/?page_id=105 22 May 2012; Turner B, Dickens N 2011 Long-term urethral catheterisation. Nurs Stand 25(24):49–56.
CATHETER INSERTION
Urethral catheterisation may require a medical prescription. Aseptic technique is used to minimise the risk of introducing microorganisms into the bladder (see Chapter 29). The steps for inserting indwelling and single-use straight catheters are basically the same. The difference lies in the procedure taken to inflate the indwelling catheter balloon and secure the catheter. Skill 38-2 lists steps for performing female and male urethral catheterisation.
SKILL 38-2 Inserting a straight or indwelling catheter
DELEGATION CONSIDERATIONS
Catheterisation requires the problem-solving and knowledge application skills of registered nurses. Insertion of a urinary catheter normally requires a medical request.
EQUIPMENT
| STEPS | RATIONALE | ||
|---|---|---|---|
| Bladder fullness may be detected with deep palpation above the symphysis pubis. | |||
| Reveals the patient’s ability to cooperate and level of explanation needed. | |||
| Affect way the nurse positions patient. | |||
| Determines catheter size: see Box 38-4. | |||
| Causes pain. Can indicate need to insert catheter if patient is unable to void independently. | |||
| Determines condition of the perineum. | |||
| Obstruction prevents passage of catheter through urethra into the bladder. | |||
| Determines allergy to antiseptic, tape, latex and lubricant. Betadine allergies are common; if the patient is unaware of allergy, ask if allergic to shellfish. | |||
| Determines purpose of inserting catheter. Assess for previous catheterisation, including catheter size, response of patient and time of last catheterisation. | |||
| Reveals need for patient instruction. | |||
| Promotes cooperation. | |||
| Patient may be unable to assume positioning for procedure. | |||
| Catheterised patients are at risk of urinary complications. | |||
| Reduces transmission of microorganisms. | |||
| Offers privacy, reduces embarrassment and aids in relaxation during procedure. | |||
| Promotes use of proper body mechanics. | |||
| Successful catheter insertion requires nurse to assume comfortable position with all equipment easily accessible. The materials in the kit are arranged in sequence of use. | |||
| Promotes patient safety. | |||
| Prevents soiling of bedclothes. | |||
| Provides good view of perineal structures. | |||
| Legs may be supported with pillows to reduce muscle tension and promote comfort. | |||
| This position is used if patient cannot abduct leg at hip joint (e.g. if patient has arthritic joints). Also, this position may be more comfortable for patient. | |||
| Comfortable position for patient that gives good view. | |||
| Avoids unnecessary exposure of body parts and maintains patient’s comfort. | |||
| Permits accurate identification and good view of urethral meatus. | |||
| Reduces microorganisms near urethral meatus and allows further opportunity to see perineum and landmarks. | |||
| Wait several minutes before inserting the catheter so that the anaesthetic agent has time to take effect. | |||
| Prevents transmission of microorganisms from table or work area to sterile supplies. | |||
|
21. Put on sterile gloves (see Chapter 29). |
Allows nurse to handle sterile supplies without contamination. | ||
| Maintains principles of surgical asepsis and organises work area. | |||
| Outer surface of drape covering hands remains sterile. Sterile drape against sterile gloves is sterile. | |||
| Maintains sterility of work surface. | |||
|
(1)Two methods are used for draping depending on preference. First method: apply drape over thighs and under penis without completely opening fenestrated drape. Second method: apply drape over thighs just below penis. Pick up fenestrated sterile drape, allow it to unfold and drape it over penis with fenestrated slit resting over penis. |
Maintains sterility of work surface. | ||
| Provides easy access to supplies during catheter insertion. Maintains aseptic technique during procedure. | |||
| Full view of urethral meatus is provided. Full retraction prevents contamination of urethral meatus during cleaning. | |||
|
(2)Using forceps in sterile dominant hand, pick up gauze square saturated with antiseptic solution/sterile water and clean perineal area, wiping from front to back from clitoris towards anus. Using a new gauze square for each area, wipe along the far labial fold, near labial fold and directly over centre of urethral meatus (see illustration). |
|||
| Critical decision point: Closure of labia during cleaning requires that the cleaning procedure be repeated because the area has become contaminated. | |||
| Accidental release of foreskin or dropping of penis during cleaning requires process to be repeated because area has become contaminated. | |||
| Reduces number of microorganisms at urethral meatus and moves from area of least to most contamination. Dominant hand remains sterile. | |||
| Female urethra is short. Appearance of urine indicates that catheter tip is in bladder or lower urethra. Advancement of catheter ensures bladder placement. | |||
| Critical decision point: If no urine appears, check that catheter is in vagina. If misplaced, leave catheter in vagina as landmark indicating where not to insert, and insert another (you will need to start the procedure again unless you have another nurse attending who can open another sterile catheter onto the sterile field). | |||
| Bladder or sphincter contraction may cause accidental expulsion of catheter. | |||
| Over-inflation of the balloon may cause it to burst or block off the drainage holes of the catheter. Under-inflation can result in the catheter falling out. | |||
| Straightens urethral canal to ease catheter insertion. | |||
| The adult male urethra is long. It is normal to meet resistance at the prostatic sphincter. When resistance is met, nurse should hold catheter firmly against sphincter without forcing catheter. After a few seconds, the sphincter relaxes, and the catheter is advanced. Appearance of urine indicates catheter tip is in bladder or urethra. Further advancement of catheter ensures proper placement. | |||
| Catheter may be accidentally expelled by bladder or urethral contraction. Collection of urine prevents soiling and provides output measurement. | |||
| Over-inflation of the balloon may cause it to burst or block off the drainage holes of the catheter. Under inflation can result in the catheter falling out. | |||
| Paraphimosis (retraction and constriction of the foreskin behind the glans penis) secondary to catheterisation may occur if foreskin is not reduced. | |||
| For indwelling catheterisation—attach end of catheter to tube of drainage bag system (see illustration). Make sure drainage port at the base of the drainage bag is closed. Drainage bag must be below level of bladder; attach bag to bed frame, do not place bag on side rails of bed. | Establishes a closed system for urine drainage. | ||
| Reduces transmission of microorganisms. | |||
| Anchoring catheter to inner thigh reduces pressure on urethra, thus reducing possibility of tissue injury and reduces risk of urinary tract infection. | |||
| Anchoring catheter to lower abdomen reduces pressure on urethra at junction of penis and scrotum, thus reducing possibility of tissue injury. | |||
| Maintains comfort and security. | |||
| Reduces transmission of microorganisms. | |||
| Determines whether distension is relieved. | |||
| Determines whether patient’s sensation of discomfort or fullness has been relieved. | |||
| Determines whether urine is flowing adequately. | |||
| Prevents injury to patient’s skin. | |||
CLOSED DRAINAGE SYSTEMS
After inserting an indwelling catheter, a closed urinary drainage system is maintained to minimise the risk of infection. Urinary drainage bags are plastic and can hold about 1000–1500 mL of urine. The bag should hang on the bed frame or wheelchair without touching the floor. When the patient moves about, the nurse or patient carries the bag below the patient’s waist. The drainage bag should never be raised above the level of the patient’s bladder, so that urine does not flow back from the tubing into the bladder.
Most drainage bags contain an anti-reflux valve to prevent urine in the bag from re-entering the drainage tubing and contaminating the patient’s bladder. A valve at the base of the bag provides a means for emptying the bag. The valve should always be closed, except during emptying, and tucked into the protective pouch on the side of the bag. To keep the drainage system patent, frequently check for kinks or bends in the tubing, avoid positioning the patient on the drainage tubing and observe for clots or sediment that may occlude the collecting tubing.
For the person who will go home from hospital with an indwelling urinary catheter, a smaller leg bag is connected to the catheter. Leg bags are designed to be strapped to the person’s leg so that they can carry on their usual daily activities without having to carry a large urine bag. Because the leg bag only holds approximately 500 mL of urine, the leg bag will need to be emptied frequently throughout the day. This can easily be done into a toilet or plastic jug. A larger overnight bag is connected (at the base of the leg bag) before the person goes to sleep so that their sleep is not interrupted by the need to empty the smaller leg bag.
ROUTINE CATHETER CARE
Patients with indwelling catheters have a number of special care needs. Nursing measures are directed at preventing infection and maintaining an unobstructed flow of urine through the catheter drainage system.
Build-up of secretions or encrustation at the catheter insertion site is a source of irritation and potential infection. The person can shower normally with a catheter, and they should be encouraged to use mild soap and water to wash their genital area at least daily (Turner and Dickens, 2011). If the patient is unable to shower on their own they will need assistance with their personal hygiene either in the shower or during a bed bath.
All patients with catheters should have a daily intake of 2000–2500 mL of fluid if not contraindicated. This can be achieved through oral intake or intravenous infusion. A high fluid intake produces a large volume of urine that flushes the bladder and keeps the catheter tubing free of sediment.
As mentioned previously, constipation can affect urinary elimination. Promotion of normal bowel elimination is also important in catheterised patients. Assessment of bowel function should be conducted daily.
A patient who has a urinary catheter needs focused assessment at least three times per day (once per shift). The assessment should include:
• daily inspection of the urethral meatus for signs of inflammation and leakage of urine
• checking of the closed drainage system for patency
• inspection of the urine for colour and clarity
• abdominal palpation for bladder distension
• checking that the catheter is secured to the upper thigh or lower abdomen with an appropriate device such as a catheter strap.
PREVENTING INFECTION
Infection can develop in a catheterised patient in many ways. A CAUTI is defined as a symptomatic UTI in a person with a urinary catheter (Gray and Moore, 2009). The longer the catheter is left in situ, the higher the likelihood of developing a CAUTI. According to Hameed and others (2010), there is a 5% risk of developing a CAUTI each day that the catheter is in situ and this rate increases over time. After 30 days of catheterisation nearly all patients will have bacteriuria, although not all will have symptomatic infection (Gray and Moore, 2009). Bacteria can enter the bladder at several points. Sites at risk are the site of catheter insertion, the drainage bag, the spigot, the tube junction and the junction of the tube and the bag. Maintaining a closed urinary drainage system is important in infection control, as a break in the system can lead to introduction of microorganisms.
Pooling of urine in the drainage bag tubing should also be avoided. Urine in the drainage bag is an excellent medium for microorganism growth. Bacteria can travel up drainage tubing to grow in pools of urine in the tubing. If this urine flows back into the patient’s bladder, an infection is likely to develop. Suggestions for ways to prevent infections in catheterised patients are provided in Box 38-6.
BOX 38-6 TIPS FOR PREVENTING INFECTION IN CATHETERISED PATIENTS
• Follow hand hygiene guidelines (see Chapter 29).
• Maintain a closed system—catheter, drainage tubing and drainage bag.
• Do not open the drainage system at connecting points to collect specimens.
• If the drainage tube becomes disconnected, do not touch the ends of the catheter or tubing. Wipe the end of the tubing and catheter with an antimicrobial solution before reconnecting.
• Prevent pooling of urine in the tubing and reflux of urine into the bladder:
• Do not allow the drainage port on the drainage bag to touch the floor.
• Empty the drainage bag at least every 8 hours. If large outputs are noted, empty more frequently.
• Remove the catheter as soon as clinically warranted.
• Secure the catheter appropriately for the patient (see Skill 38-2).
REMOVAL OF INDWELLING URETHRAL CATHETER
The aim when removing an indwelling catheter is to prevent trauma to the urethra. Following catheter removal, time to first void and volume of first void are assessed as well as return to normal voiding patterns.
The equipment required to remove a catheter is a disposable towel or plastic bed protection pad; a rubbish receptacle; and a sterile syringe the same size as the volume of the catheter balloon. The distal end of each catheter contains a label that denotes the volume of solution (5–30 mL) within the balloon. Standard infection-control precautions are also recommended.
• Explain the procedure to the patient. The removal of the catheter is likely to cause minor discomfort but is unlikely to be painful.
• Position the patient in the same position as during catheterisation.
• After removing catheter-securing devices such as a catheter strap, place the towel/bed protection pad between a female patient’s thighs or over a male patient’s thighs.
• Insert the syringe into the balloon port. Slowly withdraw all of the solution to deflate the balloon totally. If a portion of the solution remains, the partially inflated balloon will traumatise the urethral canal as the catheter is removed.
• When all of the solution from the balloon has been aspirated, pull the catheter out smoothly and slowly. If the balloon does not deflate or you have trouble removing the catheter, do not cut the tubing. Refer the problem to a specialist clinical nurse (for example a urology or continence nurse) or a medical practitioner.
It is normal for the patient to experience some dysuria, especially if the catheter has been in place for several days or weeks. The catheter causes inflammation of the urethral canal. Until the bladder regains full tone, the patient may also experience frequency of urination or urinary retention.
Assesses the patient’s urinary function by noting the time of first voiding after catheter removal, and document the time and amount of voiding for the next 24 hours. Encourage the patient to drink water. If the voided amounts are small (less than 100 mL), frequent assessment for retention of urine is necessary. Most hospitals use ultrasonic bladder scanners to assess post-void residual urine volumes. Guidelines vary on the amount of residual urine that is considered acceptable. The post-void residual volume is compared with the actual amount voided. If over 8 hours elapse without voiding in a patient who is drinking good amounts, it may become necessary to reinsert a catheter.
Interventions to promote continence
All of the interventions mentioned previously that promote normal voiding patterns are also used to promote continence. There are a number of interventions that are used to treat urinary incontinence. The treatment plan can only be developed after careful assessment of the problem. Implementing toileting programs without a detailed assessment and identification of the specific continence issue is not likely to result in an improvement in the person’s continence.
STRENGTHENING PELVIC FLOOR MUSCLES
Continence, especially in women, relies on strong pelvic floor muscles. Pelvic floor muscle exercises (PFEs), also known as Kegel exercises, are designed to improve the strength of pelvic floor muscles. They consist of repetitive contractions of specific muscle groups. PFEs can be used to promote continence as well as for rehabilitation when a continence problem exists. As PFEs involve the identification and conscious recruitment of the pelvic floor muscles, the person must be able to follow instructions and repeat a series of exercises without supervision over a long period of time. Therefore, people who have short-term memory problems, inattention or impaired conscious state may not be suitable candidates for a PFE program. The elements of a PFE program are described in Box 38-7. Both the Continence Foundation of Australia and the New Zealand Continence Association have excellent brochures, posters and teaching materials related to PFEs.
BOX 38-7 HEALTH EDUCATION: PELVIC FLOOR MUSCLE EXERCISES
IMPORTANT POINTS
• Have the person attempt to tighten PFMs during voiding by attempting to stop the flow of urine or get them to visualise squeezing the muscles between vagina and anus (or scrotum and anus in men). Pull muscles up into the body. Get the sense of the squeezing and lifting sensation. (Don’t tighten the buttocks, abdominal muscles or thighs.)
• Have the person squeeze and lift these muscles and hold for 8 seconds before relaxing. Breathe in and out during the contraction. Repeat the squeeze and lift 8–12 times.
• When they have perfected the technique, advise them to repeat these exercises three times a day (8–12 squeeze-and-lifts with a rest in between) each day while lying down, sitting or standing.
OTHER POINTS
• Provide written instructions with diagrams of anatomy (you can get brochures from the Continence Foundation of Australia or the New Zealand Continence Association).
• Talk with the person about good bladder and bowel habits (drink well, eat a healthy diet, healthy weight, stop smoking, good toilet habits, avoiding constipation).
• If the person has difficulty in identifying their PFMs refer to a continence nurse or continence physiotherapist.
• Refer to a continence nurse or the person’s general practitioner if they have any other lower urinary tract symptoms.
RESOURCES
• Brochures and teaching materials available from the Continence Foundation of Australia (www.continence.org.au/index.php) or New Zealand Continence Association (www.continence.org.nz).
• For further assistance to locate a continence nurse or continence physiotherapist or for advice call the relevant continence helpline: Australia—1800 33 00 66, New Zealand—0800 650 659.
WORKING WITH DIVERSITY FOCUS ON OLDER ADULTS
• When frail older adults can no longer maintain their independence in personal care, nurses must assume responsibility for their urinary care.
• Dilute urine discourages bacterial growth, so older adults should be encouraged to drink at least 6–8 glasses of fluids a day, unless medically contraindicated.
• Since acidic urine prevents or inhibits bacterial growth in urine, fluids that acidify the urine (e.g. cranberry juice) should be offered daily.
• Urinary catheterisation increases the incidence of nosocomial infection. Indwelling catheters should not be used in a routine manner.
• Incontinence is not a normal part of ageing, and efforts should be made to assess incontinence and provide interventions to promote return to continence.
• Hospitalised older adults who have no pre-existing urinary incontinence should not be given continence pads ‘just in case’ they are incontinent or instead of being assisted to go to the toilet.
CONTINENCE AIDS AND APPLIANCES
Aids and appliances form the key to achieving social continence. Social continence refers to the use of continence aids and appliances to keep urinary leakage and odour contained, enabling the person to engage in normal daily activities. Continence aids and appliances are an important part of active treatment plans for many people because they give the person the confidence they need to engage in normal daily activities and social interactions. For some people, social continence may be the goal of treatment. There are various continence aids and appliances available. These include continence pads and pants, absorbent bed sheets and chair covers, and other products that assist with voiding such as bed pans, urinals, commode chairs, raised toilet seats and chairs; most hospitals and healthcare agencies will have a selection in stock. Some of these products can be misused. A study by Zisberg (2011) found that continent adult women, especially those with impaired mobility, were being given continence pads in the acute care setting. Zisberg makes the point that using continence pads for these people is likely to result in reduced self-esteem and quality of life, increased risk of skin problems and urinary tract infections and may increase the risk of urinary incontinence in frail older people. It is not acceptable practice to give a patient a continence pad when they do not have any urinary incontinence.
A person with an ongoing continence problem should be referred to a continence nurse specialist for assessment and management of their continence problem. Continence nurse specialists are registered nurses who have postgraduate qualifications and experience in promoting continence and managing incontinence. A continence nurse specialist can provide expert advice on appropriate continence aids and appliances and can assist the person to access government funding schemes. Individual needs must be taken into account when selecting continence products. These include the amount of urine that the pad needs to contain, rate of loss of urine, gender, size of the person, cognitive function, manual dexterity and strength, the person’s lifestyle, access to laundry and/or disposal facilities, and the personal preference of the person (Cottenden and others, 2009).
URINARY SHEATH
The urinary sheath (or the ‘condom’ in condom drainage) is a non-invasive soft, pliable, silicone or latex sheath that slips over the penis (Skill 38-3). It is suitable for men who have urinary incontinence but still have complete and spontaneous bladder emptying. It may be worn continuously or at night only, depending on the person’s needs. The success of the urinary sheath relies on correct choice of size of the device and correct application of the device to the penis. Urinary sheaths come in varying lengths and diameters. Each brand of sheath will come with a sizing guide; care must be taken to ensure that whatever type or size is used, blood supply to the penis is not impaired. Each sheath has adhesive on the inside so that the sheath is comfortably fitted and stays in place without leaking. The penis needs to be clean and dry for the adhesive to stick properly. Some men may need to reduce the amount of pubic hair (using a personal trimmer) so that the adhesive sticks properly.
SKILL 38-3 Applying a sheath/condom drainage device
DELEGATION CONSIDERATIONS
Applying a sheath/condom can be delegated to enrolled nurses, the person if he is able or a family carer in the home context.
• Ensure that person/caregiver knows standard precautions guidelines relating to body fluids.
• Clarify that skin of penile shaft is intact and free of swelling, redness or open lesions before sheath/condom drainage device is applied.
• Clarify the person’s/caregiver’s understanding of how to apply the adhesive strip that secures the sheath/condom drainage device.
EQUIPMENT
• Sheath/condom drainage device kit
• Urinary collection bag with tubing or leg bag with straps
• Basin with warm water and soap
| STEPS | RATIONALE |
|---|---|
| Patients who are incontinent are at high risk of skin breakdown. | |
| Maintains patient’s self-esteem. | |
| Some male patients may be incontinent only at night. Teaching can be implemented to instruct patient on self-application. | |
| Provides a baseline to compare changes in condition of skin after condom application. | |
| Reveals need for patient instruction. | |
| Reduces anxiety and promotes cooperation. | |
| Reduces transmission of microorganisms. | |
| Promotes patient safety and proper use of body mechanics by nurse. | |
| Promotes patient comfort and prevents unnecessary exposure of body parts. | |
| Provides easy access to drainage equipment after sheath/condom is applied. | |
| Sheath/condoms come in a variety of sizes (circumference and length). It is very important to use the correct size for comfort and so that the sheath/condom remains in place for the required period of time. | |
|
12. Wash area with soap and water or ask the patient to do this for himself if he is able (see Skill 38-2), and dry thoroughly. |
Removes irritating secretions. Rubber/latex sheath rolls onto dry skin more easily. The adhesive will adhere to dry skin. Be aware of possible latex allergy. |
| Hair adheres to sheath/condom and is pulled during sheath/condom removal or may get caught as sheath/condom is applied. | |
|
14. Roll sheath/condom onto penis—try to keep the glans centrally within the sheath. You may need to put gentle tension on the penis to keep it straight within the sheath. As the adhesive is on the inside of the sheath/condom, you need to gently press the sheath onto the shaft of the penis as you unroll it (see illustrations). |
|
| Critical decision point: Allow 2-5 cm of space between tip of glans penis and end of sheath/condom (see illustration, Step 14). | |
| Allows urine to be collected and measured. Keeps patient dry. Twisted sheath/condom obstructs urine flow. | |
| Promotes free drainage of urine. | |
| Promotes safety and comfort. | |
| Reduces spread of microorganisms. | |
| Determines whether normal voiding is occurring. | |
| Determines whether sheath/condom has been applied correctly. | |
| Indicates whether sheath/condom or urine is causing irritation. Frequent assessment of good blood circulation to glans penis is important to determine whether sheath/condom has been applied too tightly. | |
The distal end of the sheath fits into plastic drainage tubing. A drainage bag can be attached to the side of the bed, or a leg bag can be strapped to the patient’s leg. The sheath itself poses little risk of infection. Infections with urinary sheaths usually result from a build-up of secretions around the urethra, trauma to the urethral meatus or a build-up of pressure in the outflow tubing.
The man (or their carer) should be advised to gently remove and discard the sheath daily. This provides a good opportunity to check the skin of the penis for irritation and inflammation. Normal personal hygiene can be attended to, such as showering, and a new sheath fitted.
For a man with a retracted penis, maintaining a conventional urinary sheath may prove difficult. Special devices are available to help alleviate this problem (Figure 38-11). Manufacturers’ guidelines for product application should be consulted.
FIGURE 38-11 Retracted penis pouch external urinary device.
From Potter PA, Perry AG 2013 Fundamentals of nursing, ed 8. St Louis, Mosby.
• CRITICAL THINKING
Mr Miller is a 75-year-old widower who has had prostate surgery for benign prostatic hypertrophy. He thought his problems would be over, but now he is experiencing continual dribbling of urine. He has been trying to deal with the problem by using an absorbent pad in his underwear, but he feels as though everyone knows his problem. The embarrassment of having an odour often keeps him at home. He has given up attending his senior citizens centre.
Maintenance of skin integrity
The normal acidity of urine is irritating to skin. Urine allowed to be in contact with the skin becomes alkaline, causing encrustations or precipitates to collect on the skin, fostering breakdown. Continuous exposure of the perineal area leads to gradual maceration and excoriation (see Chapter 30). Washing with mild soap and warm water is the best way to remove urine from skin. A non-perfumed body lotion keeps skin moisturised, and skin barriers (for example zinc and castor oil cream) provide a barrier to the urine.
If the skin becomes inflamed, the doctor may prescribe a cream containing a corticosteroid such as hydrocortisone. If a fungal infection develops, a topical antifungal containing an agent such as nystatin or clotrimazole is effective.
Voiding programs
Voiding programs should not be commenced until a detailed assessment has been undertaken and the specific continence problem(s) identified. Bladder training programs aim to increase bladder capacity by gradually increasing the interval between voids, for example in a person with urge incontinence who may be voiding frequently. These programs consist of an educational program about how the bladder works and how continence is maintained; a voiding program based on the person’s current voiding pattern (determined by a voiding diary); and an ongoing support program as the program is likely to take several months to achieve the desired increase in voiding frequency (Newman and Wein, 2009b).
Habit training, timed voiding and prompted voiding are methods of bladder re-education aimed at re-establishing a normal voiding pattern. These programs are used for people with stress, urge and mixed incontinence and are often used with frail or cognitively impaired people to reduce incontinence episodes (Newman and Wein, 2009b). Habit training involves identification of the person’s usual voiding pattern and pre-empting incontinence episodes by decreasing voiding intervals, while keeping these intervals as long as possible. Timed voiding is a program of voiding according to a schedule that is determined from a voiding diary (Ostaszkiewicz and others, 2010a). Prompted voiding is a program in which the nurse (or carer) checks the person at regular intervals to see if they are wet or dry, provides feedback to the person about their own perception of being wet or dry and offers assistance with toileting. Positive responses are praised and incontinent episodes are managed without comment. This type of voiding program is most suited to people with mild to moderate cognitive impairment or with functional incontinence due to immobility (Eustice and others, 2009). While these programs are extensively used in practice, there is very little published evidence on their efficacy and patient outcomes (Eustice and others, 2009; Ostaszkiewicz and others, 2010a, 2010b).
EVALUATION
The patient is the best source of evaluation of outcomes and responses to nursing care (see Figure 38-12). Although most patients will be able to assess for themselves whether their goals have been met, the nurse will also evaluate the effectiveness of nursing interventions through comparisons with baseline data. The nurse evaluates for change in the patient’s voiding pattern, presence of urinary tract alteration, and physical condition. Outcomes are compared with expected outcomes to determine the patient’s health status. Continuous evaluation allows the nurse to determine whether new or revised therapies are required or if any new nursing diagnoses have developed.
Patient expectations
If the nurse has developed a relationship of trust with a patient, indications of the patient’s degree of satisfaction with the care will be evident. However, the nurse needs to confirm whether the patient’s expectations have been met to full satisfaction. The nurse may need to ask specifically about the patient’s degree of urinary control and comfort. Keep in mind that it may take several months before any measurable improvement in urinary incontinence can be determined. These people need ongoing support so that their treatment goals can be realised. The nurse can also assist the patient in redefining unrealistic patient goals when impairment in function is not likely to be altered as completely as the patient might like.
• CRITICAL THINKING
Mrs Joseph is a 78-year-old woman with mild cognitive changes associated with Alzheimer’s disease. Her daughter, with whom she lives, has brought her to the general practitioner’s clinic for assessment and advice. You are the practice nurse in the practice. As you assess Mrs Joseph, you ask her daughter how she is coping with caring for her mother. The daughter replies that her mother does not seem to remember how to go to the toilet. Mrs Joseph will go into the bathroom but forget to pull down her underwear before going to the toilet. After such an incident she becomes upset and blames her daughter for her wetness. The daughter asks you for suggestions on how to manage, as she has noticed that her mother’s perineal skin is reddened and sore.
What assessments would you need to complete before planning interventions for Mrs Joseph’s care?
KEY CONCEPTS
• The act of micturition or voiding is influenced by voluntary control from higher brain centres and involuntary control from the spinal cord.
• Symptoms common to urinary disturbances include urgency, frequency, dysuria, incontinence, nocturia, polyuria, oliguria and difficulty in starting the urinary stream.
• Methods of promoting the micturition reflex help patients sense the urge to urinate and control urethral sphincter relaxation.
• An increased fluid intake results in increased urine formation that flushes particles and solutes from the urinary system.
• An indwelling urinary catheter remains in the bladder for an extended period, making the risk of infection greater than with intermittent catheterisation.
• One of the main functions of the elimination process is fluid and electrolyte balance.
• A catheter drainage system should remain a closed system and the drainage bag should be positioned to allow free drainage of urine by gravity.
• Incontinence is a significant health issue. An accurate assessment must be undertaken to determine the type of continence problem before a treatment program can be developed.
• Alterations in the urinary system can cause alterations in other organ systems.