Chapter 37 Bowel elimination
Mastery of content will enable you to:
• Define the key terms listed.
• Discuss the role of gastrointestinal organs in digestion and elimination.
• Describe four functions of the large intestine.
• Explain the physiological aspects of normal defecation.
• Discuss psychological and physiological factors that influence the elimination process.
• Describe common physiological alterations in elimination.
• Assess a patient’s elimination pattern.
• List nursing diagnoses related to common alterations in elimination.
• Describe nursing implications for common diagnostic examinations of the gastrointestinal tract.
• Administer a pre-packaged enema.
• Describe nursing measures that promote normal bowel function.
• Discuss the relationship between the structure and function of bowel diversions and the nursing care required.
• Use critical thinking in the provision of care to patients with alterations in bowel elimination.
Regular elimination of bowel waste products is essential for normal body functioning. Alterations in elimination are often early signs or symptoms of problems within the gastrointestinal or other body systems. Because bowel function depends on the balance of several factors, elimination patterns and habits vary among individuals.
People who are hospitalised have additional risk factors for bowel problems, such as immobility, bed rest, altered diet, surgery and narcotic-type pain medications. To promote normal function and manage patients’ elimination problems, an understanding of normal elimination and factors that promote, impede or cause alterations in elimination is required.
Scientific knowledge base
The gastrointestinal (GI) tract is a series of hollow, mucous-membrane-lined muscular organs. The purposes of these organs are to absorb fluid and nutrients, prepare food for absorption and use by the body’s cells, and provide for temporary storage of faeces (Figure 37-1). The volume of fluids absorbed by the GI tract is high, making fluid balance a key function of the GI system. In addition to ingested fluids and foods, the GI tract receives secretions from organs such as the gallbladder and the pancreas. Any condition that seriously impairs normal absorption or secretion of GI fluids could cause fluid imbalance.
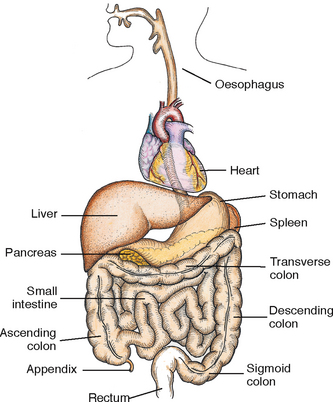
FIGURE 37-1 Organs of the gastrointestinal tract (with the heart as a reference point).
From Potter PA, Perry AG 2013 Fundamentals of Nursing, ed 8. St Louis, Mosby.
Mouth
Digestion begins in the mouth, where mechanical and chemical breakdown of nutrients occurs. The teeth masticate (chew) food, breaking it down to a suitable size for swallowing. Salivary secretions contain enzymes, such as ptyalin, that initiate digestion of certain food elements. Saliva dilutes and softens the bolus of food in the mouth for easier swallowing.
Oesophagus
As food enters the upper oesophagus, it passes through the upper oesophageal sphincter, which is a circular muscle that prevents air from entering the oesophagus and food from refluxing (moving backwards) into the throat. The bolus of food travels approximately 25 cm down the oesophagus. Food is pushed along by slow peristalsis produced by alternating involuntary contractions and relaxations of smooth muscle. As a portion of the oesophagus contracts above the food bolus, the circular muscle below (or in front) of the bolus relaxes. This alternate contraction–relaxation of smooth muscle propels food towards the next wave (Figure 37-2).
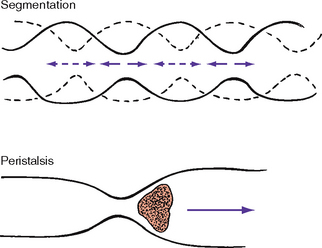
FIGURE 37-2 Segmented and peristaltic waves.
From Potter PA, Perry AG 2013 Fundamentals of Nursing, ed 8. St Louis, Mosby.
In 15 seconds the bolus of food moves down the oesophagus and reaches the lower oesophageal sphincter. The lower oesophageal (or cardiac) sphincter lies between the oesophagus and stomach and prevents backward movement of fluids from the stomach to the oesophagus. Factors influencing cardiac sphincter efficacy include antacids, which minimise reflux, and fatty foods and nicotine, which increase reflux.
Stomach
In the stomach, food is temporarily stored and mechanically and chemically broken down for digestion and absorption (see Chapter 36). The stomach secretes hydrochloric acid (HCl), mucus, the enzyme pepsin, and intrinsic factor. The concentration of hydrochloric acid influences stomach acidity and the body’s acid–base balance (see Chapter 39), and helps mix and break down food. Prostaglandins assist in the formation of mucus, which protects the stomach mucosa from acidity and enzyme activity. Pepsin digests proteins, although not much digestion occurs in the stomach. Intrinsic factor is the essential component needed for vitamin B12 absorption in the intestine and subsequent normal red blood cell formation. Lack of this intrinsic factor results in a condition called pernicious anaemia.
Before food leaves the stomach, it is changed into a semifluid material called chyme. Chyme is more easily digested and absorbed than solid food. Patients who have portions of their stomachs removed, have had a gastroplasty or have rapid stomach emptying (as with gastritis) may have serious digestive problems because food is not broken down into chyme.
Small intestine
During normal digestion, chyme leaves the stomach and enters the small intestine. The small intestine is a tube about 2.5 cm in diameter and 6 m long. It contains three divisions: duodenum, jejunum and ileum. Chyme mixes with digestive enzymes (e.g. bile and amylase) while travelling through the small intestine. Segmentation (alternating contraction and relaxation of smooth muscle) churns the chyme, further breaking down food for digestion (see Figure 37-2). These alternating contractions occur about 12 times a minute. As chyme mixes, forward peristaltic movement temporarily ceases, permitting absorption. Chyme travels slowly through the small intestine to allow absorption of nutrients and electrolytes.
Enzymes from the pancreas (e.g. amylase) and bile from the gallbladder are released into the duodenum. The enzymes in the small intestine break down fats, proteins and carbohydrates into basic elements (see Chapter 36). Nutrients are almost entirely absorbed by the duodenum and jejunum, although the ileum absorbs certain vitamins, iron and bile salts. If the function of the small intestine is impaired, the digestive process is greatly altered. For example, inflammation, surgical resection or obstruction can disrupt peristalsis, reduce the area of absorption or block passage of chyme.
Large intestine
The lower GI tract is called the large intestine because it is larger in diameter than the small intestine. However, its length of 1.5–1.8 m is much shorter. The large intestine is divided into the caecum, colon and rectum (Figure 37-3). It is responsible for the absorption of water and is the primary organ of bowel elimination.
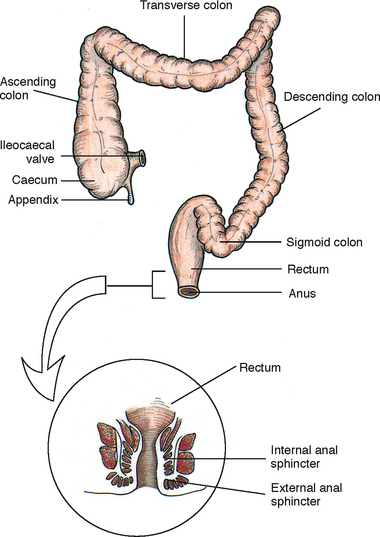
FIGURE 37-3 Divisions of the large intestine.
From Potter PA, Perry AG 2013 Fundamentals of Nursing, 8 ed. St Louis, Mosby.
Caecum
Unabsorbed chyme enters the large intestine at the caecum through the ileocaecal valve. This valve is a circular muscle layer that prevents colon contents from regurgitating and returning to the small intestine. Located at the end of the caecum is the vermiform appendix. Inflammation of this area can result in blockage of the appendix, leading to appendicitis.
Colon
Although watery chyme enters the colon, the volume of water lessens as chyme moves along it. The colon is divided into the ascending, transverse, descending and sigmoid colon. The colon is made of muscular tissue, which allows it to accommodate and thus eliminate large quantities of waste.
The colon has four interrelated functions: absorption, protection, secretion and elimination. A large volume of water and significant amounts of sodium and chloride are absorbed by the colon daily. As food passes through the colon, haustral contractions occur. These are similar to segmental contractions of the small intestine but last longer, up to 5 minutes. The contractions produce large sacs in the colon’s wall, providing a large surface area for absorption.
As much as 2.5 L of water can be absorbed by the colon in 24 hours. On average, 55 mmol of sodium and 23 mmol of chloride are absorbed daily. The amount of water absorbed from chyme depends on the speed at which colonic contents move. Chyme is normally a soft, formed mass. If the speed of peristaltic contractions is abnormally fast, there is less time for water to be absorbed and the stool will be more liquid. If peristaltic contractions slow down, water continues to be absorbed and a hard mass of stool forms, resulting in constipation.
The colon protects itself by secreting a supply of mucus. Mucus is normally clear to opaque, with a stringy consistency. Mucus lubricates the colon, preventing trauma to its inner walls. Lubrication is especially important near the distal end of the colon, where contents become drier and harder. The secretory function of the colon aids in electrolyte balance. Bicarbonate is secreted in exchange for chloride. About 4–9 mmol of potassium is released each day by the large intestine. Serious alterations in colon function, such as diarrhoea, can cause electrolyte imbalance due to loss of potassium and chloride.
Finally, the colon eliminates waste products and gas (flatus). Flatus results from air swallowing, diffusion of gas from the bloodstream into the intestine, and bacterial action on non-absorbable carbohydrates. Fermentation of carbohydrates (such as in cabbage and onions) produces intestinal gas, which can stimulate peristalsis. An adult normally forms 400–700 mL of flatus daily.
Slow peristaltic contractions move contents through the colon. Intestinal content is the main stimulus for contraction. Waste products and gas exert pressure against the walls of the colon. The muscle layer stretches, stimulating the reflex that initiates contraction. Mass peristaltic movements push undigested food towards the rectum. These movements occur only three or four times daily, unlike the frequent peristaltic waves in the small intestine (usually heard during auscultation). When these mass peristaltic movements occur, large segments of the colon contract as a result of gastrocolic reflex and duodenocolic reflex responses. These occur when the stomach or duodenum is filled with food. Filling initiates nerve impulses that stimulate the colon’s muscular walls. Mass peristalsis is strongest during the hour after a meal.
Rectum
Waste products that reach the sigmoid portion of the colon are called faeces. The sigmoid stores faeces until just before defecation. The rectum is the final division of the GI tract. Its length varies according to age:
| Infant | 2.5–4 cm |
| Toddler | 5 cm |
| Preschooler | 7.5 cm |
| School-age child | 10 cm |
| Adult | 15–20 cm |
Normally the rectum is empty of faeces until defecation. It contains vertical and transverse folds of tissue. Each vertical fold contains an artery and veins. If the veins become distended from pressure during the straining, haemorrhoids form. Haemorrhoids can make defecation painful.
When the faecal mass or gas moves into the rectum to distend its walls, defecation begins. The process involves involuntary and voluntary control. The internal sphincter is a smooth muscle innervated by the autonomic nervous system. As the rectum distends, sensory nerves are stimulated and carry impulses that cause the internal sphincter to relax, allowing more faeces to enter the rectum. At the same time, impulses travel to the brain to create awareness of the need to defecate.
As the internal sphincter relaxes, so does the external sphincter. Adults and toilet-trained children can voluntarily control the external sphincter. If the time for defecation is not right, constriction of the levator ani muscles closes the anus and defecation is delayed. At the time of defecation, the external sphincter relaxes. Pressure can be exerted to expel faeces through an increase in intra-abdominal pressure, or a Valsalva manoeuvre. A Valsalva manoeuvre is voluntary contraction of abdominal muscles during forced expiration with a closed glottis (holding one’s breath while straining).
Nursing knowledge base
Factors affecting bowel elimination
Many factors influence the process of bowel elimination. Knowledge of these factors enables identification of people at risk and the appropriate selection of intervention(s) to prevent or alleviate the symptoms of altered bowel elimination.
Age
Developmental changes that affect elimination occur throughout life. An infant has a small stomach capacity and less secretion of digestive enzymes. Some foods, such as complex starches, are tolerated poorly. Food passes quickly through an infant’s intestinal tract because of rapid peristalsis. The infant is unable to control defecation because of a lack of neuromuscular development (see Working with diversity). This development usually does not take place until 2–3 years of age.
During adolescence, there is rapid growth of the large intestine. The secretion of HCl increases, particularly in boys. Adolescents typically eat more.
WORKING WITH DIVERSITY FOCUS ON INFANTS AND CHILDREN
• Normally, a healthy newborn infant has a bowel motion within 24–36 hours of birth. This first stool is called meconium and is a thick greenish-black material.
• The frequency of bowel movements in babies can be affected by their feeding regimen. Breastfed babies tend to have more-frequent bowel actions than those fed with formula. They may have an average of three bowel actions per day.
• The stools of babies are a yellow colour, gradually assuming more adult characteristics as solid food is introduced into the diet after 6 months of age. By the age of 3 years, a child will normally have one soft bowel movement each day.
Adapted from Greenwald B 2010 Clinical practice guidelines for paediatric constipation. J Acad Nurse Pract 22:332–8; Hockenberry M, Wilson D 2011 Wong’s nursing care of infants and children, ed 9. St Louis, Mosby.
Older adults often experience changes in the GI system that impair digestion and elimination (Ebersole and Hess, 2012). These changes in the GI tract that occur with ageing are given in Table 37-1. In addition, peristaltic action declines with age, and oesophageal emptying slows. Sluggish emptying of the oesophagus can cause discomfort in the epigastric section of the abdomen. Absorptive properties of the intestinal mucosa change, causing protein, vitamin and mineral deficiencies. Older adults may also have decreased tone in the pelvic floor muscles and anal sphincter. Although the integrity of the external sphincter may remain intact, frail older adults may have difficulty controlling bowel evacuation. Because of slowing of nerve impulses, some are less aware of the need to defecate and are likely to become constipated.
TABLE 37-1 NORMAL CHANGES IN THE GASTROINTESTINAL TRACT FROM AGEING
| PORTION OF GI TRACT | CHANGES | CAUSES |
|---|---|---|
| Mouth | Degeneration of cells, use of some medications | |
| Oesophagus | Reduced peristalsis, especially in lower third in late older age (85+ years) | Degeneration of neural cells |
| Stomach | Decrease in acid secretions | |
| Decrease in motor activity | Delayed gastric emptying and fewer hunger contractions | |
| Decrease in mucosal thickness | Loss of parietal cells also leads to loss of intrinsic factor, which is needed for vitamin B12 absorption | |
| Small intestine | Absorption of most nutrients and minerals not significantly affected | |
| Large intestine | ||
| Liver | Reduced storage capacity and ability to synthesise protein and medications |
Source: Feldstein RC, Tepper RE, Katz S 2010 Geriatric gastroenterology—an overview. In Fillit HM, Rockwood K, Woodhouse A, editors, Brocklehurst’s Textbook of geriatric medicine and gerontology, ed 7. Philadelphia, Saunders, pp. 106–10. DOI: 10.1016/B978-1-4160-6231-8.10017-0.
Diet
The food that a person eats influences elimination. Regular daily food intake helps maintain a regular pattern of peristalsis in the colon. Fibre, the indigestible residue in the diet, provides the bulk in faecal material. Bulk-forming foods absorb fluids, thereby increasing stool mass. The bowel walls are stretched, creating peristalsis and initiating the defecation reflex. An infant’s immature bowel cannot usually tolerate fibre-containing foods until several months of age. The following foods contain a high amount of fibre (see Chapter 36):
• raw fruits (apples, bananas, oranges)
• cooked fruits (prunes, apricots)
• raw vegetables (celery, green beans, zucchini)
• whole grains (cereal, bran flakes, breads) (National Health and Medical Research Council, 2008).
Ingestion of a high-fibre diet improves the likelihood of a normal elimination pattern if other factors are normal. Gas-producing foods such as onions, cauliflower and beans also stimulate peristalsis. The gas formed distends intestinal walls, increasing colon motility. Some spicy foods can increase peristalsis but can also cause indigestion and watery stools.
Some foods, such as milk and milk products, are difficult or impossible for some people to digest. These people are known as lactose intolerant; this condition bears a genetic link. Lactose-intolerant people are unable to digest lactose (milk sugar) due to an inadequate production of the enzyme lactase. Intolerance to lactose-containing foods results in diarrhoea, abdominal cramping and gaseous distension. Some children with lactose intolerance can tolerate cheese and yoghurt (McCance and Huether, 2010).
Fluid intake
An inadequate intake of fluids affects the character of faeces. Fluid liquefies the intestinal contents, easing its passage through the colon. Inadequate fluid intake may slow passage of food through the intestine and can result in hardening of stool contents.
Physical activity
Physical activity promotes peristalsis. Therefore, it is important to encourage people to move around as quickly as possible following surgery or serious illness. Additionally, weakened abdominal and pelvic floor muscles impair the ability to increase intra-abdominal pressure and to control the external sphincter.
Personal habits
Personal elimination habits influence bowel function. Most people benefit from being able to use their own toilet facilities at a time that is most effective and convenient for them. A busy work schedule may prevent the individual from responding appropriately to the urge to defecate, disrupting regular habits and causing possible alterations such as constipation.
Hospitalised patients can rarely maintain privacy during defecation. Bathroom facilities are often shared with another person whose hygiene habits might be quite different. The patient’s illness often limits physical activity and requires the use of a bed pan or bedside commode. The sights, sounds and odours associated with sharing toilet facilities or using bed pans are often embarrassing. Embarrassment prompts patients to ignore the urge to defecate, which can begin a vicious circle of constipation and discomfort.
Position during defecation
Squatting is the normal position during defecation. Modern toilets are designed to facilitate this posture, allowing the person to lean forward, exert intra-abdominal pressure and contract the thigh muscles. For the patient immobilised in bed, defecation is often difficult. In a supine position it is impossible to contract the muscles used during defecation. If allowable within the patient’s condition, raise the head of the bed; this helps the patient to a more normal sitting position on a bed pan, enhancing the ability to defecate.
Psychological factors
The function of almost all body systems can be impaired by prolonged emotional stress (see Chapter 42). If an individual becomes anxious, afraid or angry, the stress response is initiated, allowing the body to restore defences. The digestive process is accelerated, and peristalsis is increased to provide nutrients needed for defence. Side effects of increased peristalsis are diarrhoea and gaseous distension.
Pain
Normally the act of defecation is painless. However, a number of conditions, including haemorrhoids, rectal surgery, rectal fistulas and abdominal surgery, can result in discomfort. In these instances the patient often suppresses the urge to defecate to avoid pain. Constipation is a common consequence for people who experience pain during defecation.
Pregnancy
As pregnancy advances and the size of the fetus increases, pressure is exerted on the rectum. A temporary obstruction created by the fetus impairs passage of faeces. Slowing of peristalsis during the third trimester often leads to constipation.
Surgery and anaesthesia
Some general anaesthetic agents used during surgery cause temporary cessation of peristalsis (see Chapter 44). The patient who receives local or regional anaesthesia is less at risk of elimination alterations because bowel activity is affected minimally or not at all.
Surgery that involves direct manipulation of the bowel can temporarily stop peristalsis. This condition, called paralytic ileus, usually lasts about 24–48 hours. If the patient remains inactive or is unable to eat after surgery, return of normal bowel function may be further delayed.
Infection
It is known that Helicobacter pylori is an infection that contributes to the development of gastric ulcers and gastritis, and can lead to gastric cancers (Bornschein and others, 2010). Chronic gastritis can occur in the older adult, leading to degeneration of the stomach wall and altered digestion and elimination (McCance and Huether, 2010).
Medications
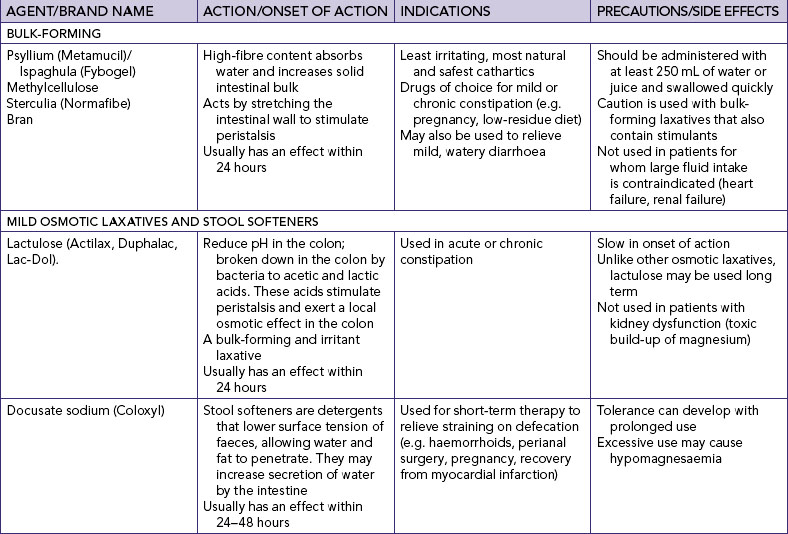
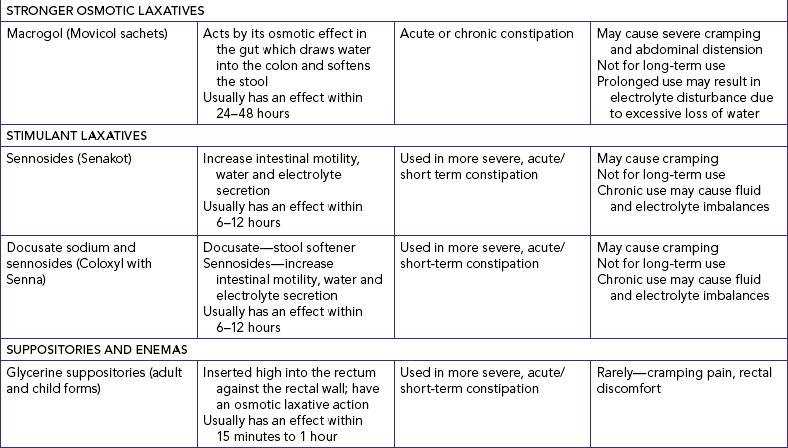
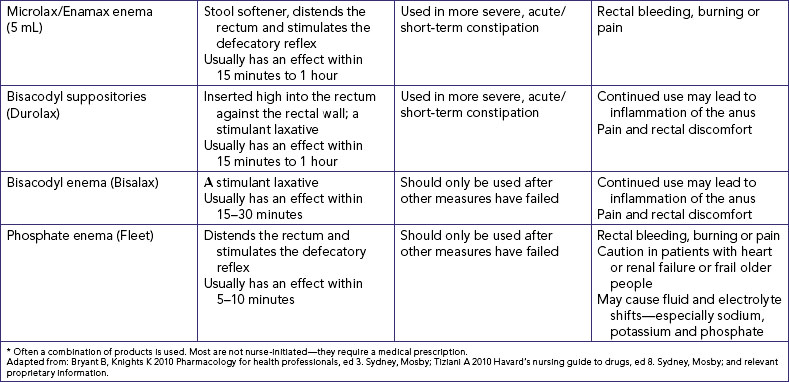
Many common medications can affect bowel elimination (Table 37-2). These effects include slowing peristalsis, increasing peristalsis and changing the bowel flora. Laxatives, cathartics and stool softeners soften the stool and promote peristalsis. Although similar to cathartics, laxatives are milder in action. When used correctly, laxatives and cathartics safely promote and maintain normal elimination patterns. However, long-term use of cathartics causes the large intestine to lose muscle tone and become less responsive to stimulation by laxatives. Laxative overuse can also cause serious diarrhoea that can lead to dehydration and electrolyte depletion.
TABLE 37-2 MEDICATIONS AND THE GASTROINTESTINAL SYSTEM
| MEDICATIONS | ACTION |
|---|---|
| Antibiotics | May produce diarrhoea by disrupting the normal bacterial flora in the GI tract, especially if administered orally. If the diarrhoea and associated abdominal cramping become severe, the patient might need to change medications |
| Anticholinergic drugs, such as atropine | Inhibit gastric acid secretion and depress gastrointestinal (GI) motility (Bryant and Knights, 2010). Although useful in treating hyperactive bowel disorders, anticholinergics can cause constipation |
| Anticholinergic/antispasmodic drugs, such as dicyclomine HCl (Merbentyl) | Suppresses peristalsis and can decrease gastric emptying |
| Aspirin | A prostaglandin inhibitor, it can interfere with the formation and production of protective mucus and can predispose patients to gastritis |
| Histamine-2 (H2) antagonists | Suppress the secretion of hydrochloric acid and may interfere with the digestion of some foods |
| Iron | Can cause discolouration of the stool (black) and lead to constipation |
| Narcotic analgesics | Slow peristalsis and segmental contractions, often resulting in constipation |
| Non-steroidal anti-inflammatory drugs | Promote gastrointestinal irritation that can range from dyspepsia to life-threatening haemorrhage (Bryant and Knights, 2010) |
Bryant B, Knights K 2010 Pharmacology for health professionals, ed 3. Sydney, Mosby; Tiziani A 2010 Havard’s nursing guide to drugs, ed 8. Sydney, Mosby.
Diagnostic tests
Diagnostic examinations that involve viewing of GI structures often require portions of the bowel to be empty of contents. In the case of evaluation through the use of an enema or endoscopy, the patient usually has a low-residue diet for 3 days and receives cathartics until the bowel contents that are expelled are clear. Such emptying of the bowel can interfere with elimination until normal eating is resumed. The patient is not allowed to eat or drink after midnight of the day preceding examinations such as endoscopy of the lower GI tract (colonoscopy and sigmoidoscopy).
Common bowel elimination problems
Nurses commonly care for people who have, or are at risk of, elimination problems because of physiological changes in the GI tract, surgical alteration of intestinal structures, other prescribed therapy or disorders impairing defecation.
Constipation
Constipation is noted by a decrease in frequency of normal bowel movements, normally accompanied by straining and prolonged or difficult passage of hard, dry stools (Brown and Edwards, 2011). Constipation is a common health issue in the community-dwelling population. According to an epidemiological study by Peppas and others (2008), the incidence of constipation in the Australasian population is between 6% and 30%. The incidence in hospitalised patients is likely to be much higher.
Constipation is a symptom that should not be ignored, as it may indicate a serious underlying disease or motility disorder of the GI tract (Apau, 2010). When intestinal motility slows, the faecal mass becomes exposed for an increased time to the intestinal walls and most of the faecal water content is absorbed. Little water is left to soften and lubricate stools. Passage of a dry, hard stool may cause rectal pain. An important consideration when assessing a person’s bowel elimination pattern is that defecation patterns vary between individuals. Not every adult has a daily bowel movement. A bowel movement only every 3 or more days may be considered normal, if it is not associated with pain, passage of hard faeces or bloating.
The causes of constipation are multifactorial. Primary causes include insufficient dietary fibre, lack of exercise and deferring defecation. Secondary causes include anal and colonic disorders such as anal stricture and colon cancer; neurological disorders such as spinal cord injury or damage to the sacral nerves; metabolic disorders such as hypothyroidism; and laxative abuse (Apau, 2010). Common medications are also a significant cause of constipation. These include antacids, iron supplements, anticholinergics, antidepressants, calcium channel blockers, beta-blockers, non-steroidal anti-inflammatory drugs (NSAIDs), diuretics and narcotic analgesics (Toglia, 2009) (Table 37-2). Prolonged constipation can have significant adverse health effects, including the development of haemorrhoids, faecal impaction, urinary incontinence (due to direct pressure of a faecal mass on the bladder), urinary retention and urinary tract infection (due to incomplete bladder emptying) (Kyle and Prynn, 2007).
Impaction
Faecal impaction results from unrelieved constipation. It is a collection of hardened faeces, wedged in the rectum, which cannot be expelled. In cases of severe impaction, the mass can extend up into the sigmoid colon. Frail older adults, who are debilitated or confused (e.g. people with dementia), or people who are unconscious or have an intellectual disability are most at risk for impaction. They are too frail, unaware of the need to defecate or may be dehydrated so that the stool becomes too hard and dry to pass.
BOX 37-1 COMMON CAUSES OF CONSTIPATION
• Irregular bowel habits and ignoring the urge to defecate
• Slowed peristalsis, loss of abdominal muscle elasticity and reduced sense of rectal fullness
• Reduced physical or cognitive functioning (including dementia)
An obvious sign of impaction is the inability to pass a stool for several days, despite a repeated urge to defecate. When a continuous oozing of diarrhoeal stool develops, impaction should be suspected. The liquid portion of faeces located higher in the colon seeps around the impacted mass. Loss of appetite (anorexia), abdominal distension and cramping, and rectal pain may accompany the condition.
WORKING WITH DIVERSITY FOCUS ON OLDER ADULTS
Constipation is a common health issue in the community. Older adults are prone to developing constipation because of the interaction of a number of factors:
• Physiological—lack of dietary fibre intake, poor fluid intake, inappropriate dietary habits, poor dentition
• Functional—decrease in mobility due to chronic joint disease, increase in sedentary lifestyle, ignoring the urge to defecate, poor lifelong bowel habits
• Mechanical—any condition that slows peristalsis or obstructs the colon and rectum; lack of sufficient abdominal muscle tone
• Psychological—depression, confusion, stress, avoidance of defecation (e.g. in a 4-bed ward), recent environmental change
• Systemic—conditions that alter the physiology of the gastrointestinal tract
• Pharmacological—numerous medications that slow peristalsis, alter absorption or change the physiology of the gastrointestinal tract, e.g. antacids, opioid analgesics, antidepressants, sedatives, overuse of laxatives
Adapted from Ebersole P, Hess P 2012 Toward healthy aging, ed 8. St Louis, Mosby.
Diarrhoea
Diarrhoea is an increase in the number of stools and the passage of liquid, unformed faeces. It is a symptom of disorders affecting digestion, absorption and secretion in the GI tract. Intestinal contents pass through the small and large intestines too quickly to allow the usual absorption of fluid and nutrients. Irritation within the colon can result in an increased mucus secretion. As a result, faeces become watery and the person may be unable to control the urge to defecate.
It is often difficult to assess diarrhoea in infants. An infant who is bottle-fed may have one firm stool every second day, whereas a breastfed baby may pass 5–8 small soft stools daily. Any sudden increase in the number of stools, any reduction in faecal consistency with an increase in fluid content and a tendency for faeces to be greenish may indicate the need for further assessment.
Excess loss of colonic fluid can result in serious fluid and electrolyte or acid–base imbalances. Infants and older adults are particularly susceptible to associated complications (see Chapter 39). Because repeated passage of diarrhoeal stools also exposes the skin of the perineum and buttocks to irritating intestinal contents, meticulous skin care is needed to prevent skin breakdown (see Chapter 34), and containment of faecal drainage is needed.
Diarrhoea can be acute or chronic and can be caused by many conditions (Table 37-3). The aims of treatment are to remove precipitating conditions and to slow peristalsis. Box 37-2 lists nursing interventions that can be used in the treatment of acute diarrhoea in a hospitalised patient.
TABLE 37-3 CONDITIONS AND INTERVENTIONS THAT CAUSE DIARRHOEA
| CONDITION/INTERVENTION | PHYSIOLOGICAL EFFECTS |
|---|---|
| Emotional stress (anxiety) | Increased intestinal motility |
| Colon disease (colitis, Crohn’s disease) | Inflammation and ulceration of intestinal walls, reduced absorption of fluids, increased intestinal motility |
| Food allergies | Reduced digestion of food elements |
| Food intolerance (greasy foods, coffee, alcohol, spicy foods) | Increased intestinal motility, increased mucus secretion in colon |
| Intestinal infection (streptococcal or staphylococcal enteritis) | Inflammation of intestinal mucosa, increased mucus secretion in colon |
| Laxatives (short term) | Increased intestinal motility |
| Medications | |
| Iron | Irritation of intestinal mucosa |
| Antibiotics | Superinfection allowing overgrowth of normal flora, inflammation and irritation of mucosa |
| Surgical alterations | |
| Gastrectomy | Loss of reservoir function of stomach, improper absorption because food is moved into duodenum too quickly |
| Colon resection | Reduced size of colon, reduced amount of absorptive surface |
| Tube feedings | Hyperosmolarity of some enteral solutions results in diarrhoea, because hyperosmolar fluids draw fluids into the gastrointestinal tract |
BOX 37-2 SUMMARY OF NURSING INTERVENTIONS FOR THE MANAGEMENT OF ACUTE DIARRHOEA IN A HOSPITALISED PATIENT
• Reduce risk of spread of infective organism—use standard and transmission-based infection control precautions.
• Fluid replacement—provide measures to maintain, or restore, fluid status and electrolyte balance.
• Assessment—observe vital signs for systemic manifestations such as fever, leucocytosis, fluid volume deficits, electrolyte imbalances.
• Nutrition—consult dietitians and encourage foods that are nourishing and tempting, little and often.
• Skin care—maintain perianal skin integrity by cleansing area and drying well.
Adapted from Australian Commission on Quality and Safety in Healthcare 2010 Australian guidelines for the prevention and control of infection in healthcare. Canberra, Australian Government; Hall V 2010 Acute uncomplicated diarrhoea management. Pract Nurs 21(3):118–22.
Incontinence
Faecal incontinence is the inability to control passage of faeces and flatus (gas) from the anus that is sufficient enough to become a social or hygienic problem (Abrams and others, 2009). It is a debilitating condition that is estimated to affect up to 10% of community-dwelling adults, with the incidence rising to 40% in the nursing-home population (Bliss and others, 2006; Smith, 2010). Physical conditions that impair anal sphincter function or control can cause incontinence; for example, damage to the anal sphincter during childbirth, pelvic floor dysfunction and neurological disorders such as multiple sclerosis, Parkinson’s disease and spinal cord injury (Toglia, 2009). Conditions that create frequent, loose, large-volume, watery stools also predispose to incontinence; for example, inflammatory bowel disease.
Consider for a moment how you would feel if you were not confident you could get to the toilet on time. Incontinence significantly affects a person’s quality of life and can lead to depression, anxiety and a decrease in general wellbeing (Chien and Bradway, 2010). The embarrassment of soiling clothes can lead to social isolation.
Flatulence
As gas accumulates in the lumen of the intestines, the bowel wall stretches and distends (flatulence). This is a common cause of abdominal fullness, pain and cramping. Normally, intestinal gas escapes through the mouth (burping) or the anus (passing of flatus). However, if there is a reduction in intestinal motility resulting from opiates, general anaesthetics, abdominal surgery or immobilisation, flatulence can become severe enough to cause abdominal distension and severe sharp pain.
Haemorrhoids
Haemorrhoids are dilated engorged veins in the lining of the rectum. They are either external or internal. External haemorrhoids are clearly visible on the outside of the anus as protrusions of skin. If the underlying vein is hardened, there can be a purplish discolouration (thrombosis). This causes increased pain and the haemorrhoid may need to be excised. Internal haemorrhoids have an outer mucous membrane. Increased venous pressure from straining at defecation, pregnancy, obesity, heart failure and chronic liver disease can cause haemorrhoids.
Bowel diversions
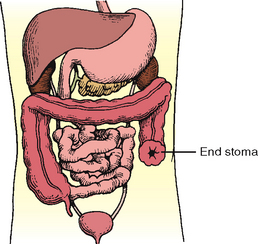
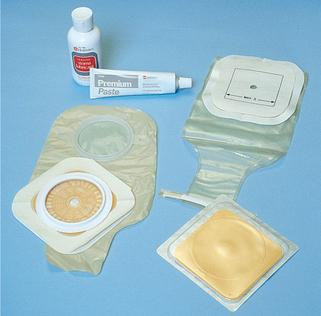
Certain diseases cause conditions that prevent normal passage of faeces through the rectum. This creates the need for a temporary or permanent artificial opening (stoma) in the abdominal wall. Surgical openings (ostomies) are most commonly formed in the ileum (ileostomy) or colon (colostomy) (Figure 37-4). Ends of the intestines are brought through a surgical opening in the abdominal wall to create the stoma (Burch, 2011). A stoma can be permanent or temporary. Depending on the type of surgical procedure done, the patient either will have no control over when the faecal material exits the stoma (incontinent ostomy) or will have control (continent ostomy). For incontinent ostomies, the stoma is covered with a pouch (appliance), what patients refer to as ‘a bag’, to collect faecal material.
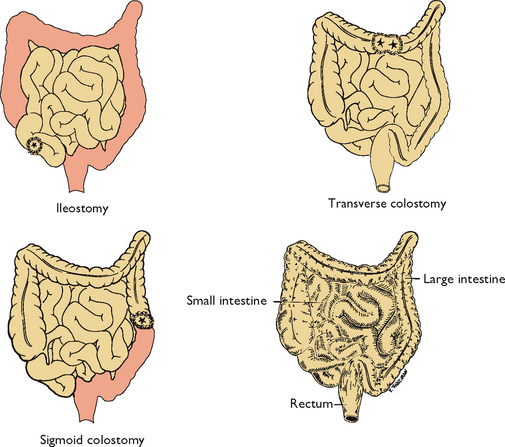
FIGURE 37-4 Normal intestines (bottom right) and three types of ostomy. Shaded areas indicate excised tissue.
INCONTINENT OSTOMIES
The location of the ostomy determines the consistency of stool. An ileostomy bypasses the entire large intestine. As a result, stools are frequent and liquid. The same is true for a colostomy of the ascending colon. A colostomy of the transverse colon generally results in a more solid, formed stool. The sigmoid colostomy emits near-normal stools. The location of a colostomy is determined by the patient’s medical problem and general condition. There are three types of colostomy construction: loop colostomy, end colostomy and double-barrel colostomy.
LOOP COLOSTOMY
A loop colostomy is usually performed in a medical emergency when closure of the colostomy is anticipated. These are usually temporary large stomas constructed in the transverse colon (Figure 37-5). The surgeon pulls a loop of bowel onto the abdomen. An external supporting device such as a plastic rod, bridge or rubber catheter is temporarily placed under the bowel loop to keep it from slipping back. The surgeon then opens the bowel and sutures it to the skin of the abdomen. A communicating wall remains between the proximal and distal bowel. The loop ostomy has two openings through the stoma. The proximal end drains stools, whereas the distal portion drains mucus. Within 7–10 days the external supporting device is removed.
END COLOSTOMY
The end colostomy consists of one stoma formed from the proximal end of the bowel with the distal portion of the GI tract either removed or sewn closed (called a Hartmann’s pouch) and left in the abdominal cavity. For many patients, end colostomies are a result of surgical treatment of colorectal cancer. In such cases the rectum might also be removed. Patients with diverticulitis who are treated surgically often have a temporary end colostomy with a Hartmann’s pouch (Figure 37-6).
DOUBLE-BARREL COLOSTOMY
Unlike the loop colostomy, the bowel is surgically severed in a double-barrel colostomy, and the two ends are brought out onto the abdomen (Figure 37-7). The double-barrel colostomy consists of two distinct stomas: the proximal functioning stoma and the distal non-functioning stoma.
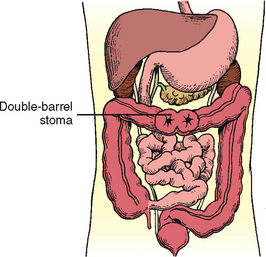
FIGURE 37-7 Double-barrel colostomy. Both ends of transected colon are brought out to skin.
Modified from Phillips N 2007 Berry & Kohn’s Operating room technique. St Louis, Mosby.
Ostomies that emit frequent liquid stools (e.g. ileostomy) create a management challenge. A pouch must always be worn. Control of defecation cannot be achieved because of a continuous oozing of liquid stool. The pouch must be emptied, washed and, if a two-piece ostomy system is being used, even replaced throughout the day. Skin care is vital to prevent exposure to faecal irritants.
A colostomy in the transverse or sigmoid colon needs less-frequent emptying of the pouch. Although some patients might choose to not wear a pouch at all times, most patients with sigmoid colostomies wear a pouch at all times even though bowel movements may occur only once or twice daily. Selected foods can be eaten at prescribed intervals so that bowel movements occur at a convenient time.
CONTINENT OSTOMIES
Certain types of surgery may provide continence for select colectomy patients. These continent ostomies are also called continent diversions or continent reservoirs. In a procedure called an ileoanal pull-through, the colon is removed and the ileum is anastomosed or connected to an intact anal sphincter. Not every colectomy patient is a candidate for this procedure. Selection criteria require close coordination between the patient and surgeon.
ILEOANAL RESERVOIR
Another surgical procedure based on the ileoanal pull-through is the ileoanal reservoir (IAR). The ileoanal reservoir is also called a restorative proctocolectomy, ileal pouch–anal anastomosis, or pelvic pouch. In this procedure, the patient has no permanent external stoma and therefore does not need to wear an ostomy pouch. Patients have an internal pouch created from their ileum. These ileum pouches can be constructed in various configurations such as in a lateral, S, J or W shape. The end of the pouch is then sewn or anastomosed to the anus (Figure 37-8). The surgery is done in stages, 8–12 weeks apart, and the patient may have a temporary ostomy until the surgically created ileum pouch has healed. When healing has occurred and the patient has successfully learned Kegel exercises to strengthen the pelvic floor and sphincter muscles, the temporary ostomy is removed. The patient then has bowel movements, less frequently, from only the anal area. Nursing care for patients with an ileoanal reservoir should focus on emotional support, perianal skin care of washing carefully, drying and the use of a perineal pad if necessary, sphincter re-education and prompt recognition of complications. Information regarding self-care management should be available in verbal and written format (Brown and Edwards, 2011).

FIGURE 37-8 Ileoanal reservoirs (IARs). A, S-shaped configuration for IAR. Three 10 cm limbs of ileum are used, the antimesenteric surface of each limb opened and adjacent bowel walls anastomosed. B, J-shaped configuration for IAR. Distal ileum is aligned in J shape; the antimesenteric surface of J shape opened, and adjacent bowel walls anastomosed. Side-to-end anastomosis of bowel to dentate line is evident. C, Lateral or side-by-side ileoanal pouch configuration.
From Hampton BG, Bryant RA 1992 Ostomies and continent diversions: nursing management. St Louis, Mosby.
KOCK CONTINENT ILEOSTOMY
The Kock continent ileostomy is another type of continent ostomy. In this procedure an internal reservoir or pouch is created from a piece of the patient’s small intestine (Figure 37-9A). Part of the pouch is brought out onto the patient’s abdomen as an external stoma (Figure 37-9B). Unlike other ostomy stomas, the external stoma from a Kock continent ileostomy is usually very low on the patient’s abdomen; usually below the line of the patient’s underpants. At the end of the internal part of the pouch is a one-way nipple valve, which is how continence is accomplished (see Figure 37-9B). This valve allows faecal contents to drain from the pouch only when an external catheter is intermittently placed into the stoma. As faecal contents are eliminated from the Kock pouch only when intubated with the catheter, the patient, unlike other people with an ostomy, does not have to wear an ostomy pouch. Nursing care of patients with a Kock reservoir focuses on emotional support, teaching self-intubation technique, determining an intubation schedule, diet teaching and recognising complications.
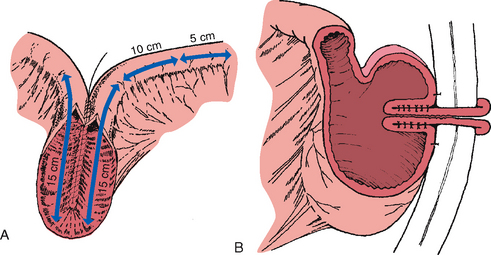
FIGURE 37-9 Construction of Kock continent ileostomy—Kock pouch. A, Two 15 cm limbs are used to create pouch, and one 15 cm limb is used to fashion a nipple valve and stoma. B, Distal limb is intussuscepted into reservoir to create one-way valve and accomplish continence. Sutures or staples, or both, are placed to stabilise and maintain intussuscepted nipple. Anterior surface of reservoir is anchored to anterior peritoneal wall.
From Hampton BG, Bryant RA 1992 Ostomies and continent diversions: nursing management. St Louis, Mosby.
PSYCHOLOGICAL CONSIDERATIONS
A stoma can cause serious body image changes, particularly if it is permanent. Changes to the person’s ability to complete daily tasks (including their usual work), changes to sexuality and sexual function (including erectile dysfunction in men), social isolation, changes to general wellbeing and perception of quality of life are all possible consequences of living with a bowel stoma (Boyles, 2010). An important factor in the person’s reaction to living with a stoma is the character of faecal secretions and the ability to control them. Foul odours, the fear of spillage or leakage of liquid stools and inability to regulate bowel movement can severely affect the person’s quality of life.
Critical thinking synthesis
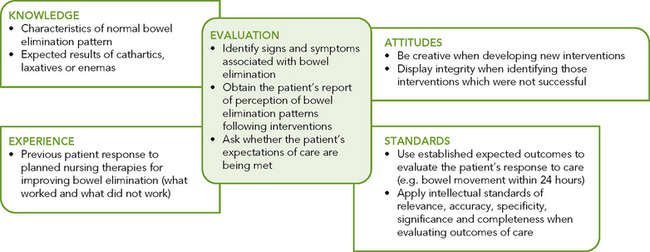
Successful clinical decision making requires a synthesis of knowledge, experience, information gathered from patients, critical thinking attitudes and intellectual and professional standards. Assessment of a person for risk factors or actual bowel elimination problems requires specific knowledge and sensitivity in approaching health issues that the person may find difficult to discuss (see Figure 37-10).
NURSING PROCESS AND BOWEL ELIMINATION
ASSESSMENT
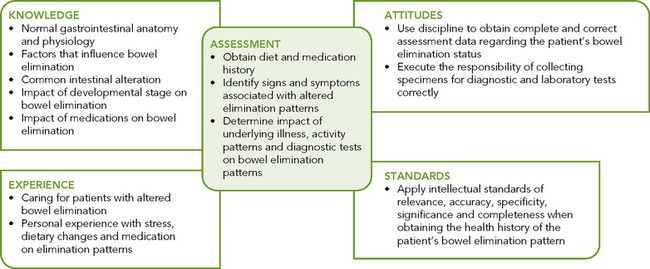
To assess bowel elimination patterns and determine the aetiology of symptoms requires a focused nursing history, physical assessment including abdominal examination, specimen inspection and skin assessment.
Nursing history
The focused nursing history provides a review of the person’s usual bowel pattern and habits. Identifying normal and abnormal patterns, habits and the person’s perception of normal and abnormal bowel function enables identification of possible problems and gives direction to the physical examination. Much of the nursing history can be organised around the factors that affect elimination:
• Determination of the person presenting’s concern. It is important to acknowledge the person’s perception of their bowel elimination problem and how this affects their quality of life.
• Determination of the usual bowel elimination pattern. Frequency and time of day are included. Accurate assessment of the current bowel elimination pattern can be enhanced by having the person or caregiver complete a ‘bowel diary’. To improve accuracy, the person completing the diary must understand what information must be recorded. Normally a bowel diary would be kept for three 24-hour periods so that the person’s normal bowel elimination patterns can be determined.
• Description of any recent change in elimination pattern. This information is perhaps the most significant, because elimination patterns are variable and the patient can best detect change. For example, a change in character or frequency of bowel elimination, history of recent overseas travel, eating take-away food, etc. Ask the person to describe any change in detail.
• Bowel symptoms and description of usual characteristics of stool. Ask the person to describe any abnormal symptoms they are experiencing. For example, presence of blood or mucus in the stool, presence of pain before, during or after defecation, sense of incomplete emptying, or anal symptoms such as itchiness or pain. Ask the person to describe their usual stool using the Bristol Stool Chart (Lewis and Heaton, 1997). This chart is a validated tool for describing stool appearance. It has a 7-point scale (see Figure 37-11). Stool types 3 and 4 are considered to be the most usual or normal consistency (Kyle and Prynn, 2007).
• Routines followed to promote normal elimination. Examples are drinking hot liquids, using laxatives, eating specific foods or taking time to defecate during a certain part of the day.
• Usual diet. The most accurate way of collecting this information is for the person to complete a food diary. If this is not possible, ask the person to recall their food intake over the last 24-hour period. Take note of types of food consumed (fresh or processed), the amount of dietary fibre in the diet and the variety of foods eaten. The information may indicate the need for referral to a dietitian for specific dietary advice.
• Description of daily fluid intake. This includes the type and amount of fluid. The person might have to estimate the amount using common household measurements.
• Exercise. Describe the type and amount of daily exercise, as lack of exercise is linked to constipation.
• Medication history. Question the person regarding their usual medications (such as laxatives, antacids, iron supplements and analgesics) that might alter defecation or faecal characteristics.
• Past health history. Personal or family history of health issues affecting the GI tract; for example abdominal surgery, neurological disease, family history of bowel cancer. If the patient has an ostomy, ask them about the frequency of faecal drainage, character of faeces, appearance and condition of the stoma (colour, swelling and irritation), type of appliance used and methods used to maintain the ostomy’s function.
• Mobility and dexterity—ability to care for self. The patient’s mobility and dexterity need to be evaluated to determine whether the patient needs assistive devices or personnel. You need to make a judgment about the need for questioning in this area based on your observation of the person; for example, on assistance needed to get to the toilet, undressing/redressing, hygiene, and use of toileting aids (commode chairs, raised toilet seats, etc). Where patients live may affect their toileting habits. If the patient is sharing living quarters, how many bathrooms are there? Do patients have their own bathroom, or do they need to share and thus adjust the time they use the bathroom to accommodate others?
• Other self-care practices. Health screening related to faecal occult blood testing (FOBT), rectal examination and colonoscopy (where there is a risk of bowel cancer). Use of continence pads or pants if the person has a bowel control problem.
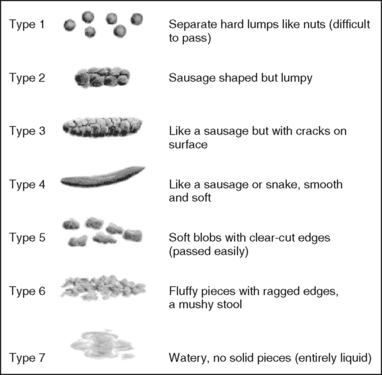
FIGURE 37-11 Bristol Stool Chart
developed by KW Heaton and SJ Lewis at the University of Bristol, England.
Research abstract
Constipation is a common symptom for people of all ages. Constipation is a major health concern for many hospitalised patients, especially older, immobile patients; those with neurological impairment; and patients with multiple healthcare needs. The causes of constipation are multifactorial and influenced by physical, psychological, physiological, emotional and environmental factors. This paper defines constipation and describes the symptoms and types and risk factors for constipation. It also describes assessment of constipation using the Norgine Risk Assessment for Constipation.
Findings
In this paper Kyle defines constipation, and raises the point that patients tend to define constipation on the basis of their symptoms while healthcare professionals define constipation in terms of frequency and stool consistency. This difference in emphasis, she argues, is likely to contribute to the problem not being attended to until it has become a significant problem.
Constipation presents as two main syndromes: slow colonic transit (slow transit of stool through the bowel to the rectum); and evacuation difficulties (stool not able to be evacuated from the rectum). Often symptoms are from a combination of both types.
The assessment of constipation must take into account all possible causes and aims to establish a detailed symptom and risk profile. In addition to history, there are a number of tools that can be used in assessment including the Bristol Stool Chart, food and fluid diaries and bowel diaries.
Kyle also emphasises the importance of the physical assessment, including functional assessment, posture, gait and mobility; abdominal examination; digital rectal examination including the assessment of anal sphincter tone; and general skin condition.
Kyle advocates the use of the Norgine risk assessment tool (developed by Kyle and others, 2007). The validity and reliability of this tool has been tested and it has been found to have good inter-rater reliability (95% confidence interval). The tool requires a series of questions to be answered when assessing the patient, which are scored. The higher the score, the greater the risk the patient has of developing constipation. Kyle makes the point that the tool is still in development and encourages use, further development and evaluation.
Evidence-based practice
• Accurate assessment of the patient requires a comprehensive knowledge of the different types of constipation and risk factors.
• Clear identification of risk factors will assist in preventing constipation in high-risk patients.
• The Norgine risk assessment tool provides a valid tool for use in all clinical settings in determining patient risk.
• CRITICAL THINKING
A 17-year-old male with a history of good health and regular exercise is seen by the school nurse. He complains of increasing diarrhoea and abdominal cramping. He states that on rare occasions he has noticed blood on the toilet paper he has used. What additional pieces of assessment data do you need?
Physical assessment
Focused physical examination (see Chapter 27) of body systems and functions related to elimination problems should be carried out. You will need to make a judgment about the specific areas to include in your examination, which may include assessment of the mouth, abdomen, perianal area and rectum.
Mouth
An assessment includes inspection of the patient’s teeth, tongue and gums. Poor dentition or poorly fitting dentures influence the ability to chew (see Chapter 34). Sores and oral Candida (thrush) in the mouth can make eating not only difficult but painful.
Abdomen
Inspect all four abdominal quadrants for contour, shape, symmetry and skin colour. Inspection also includes noting masses, scars, stomas and lesions. Abdominal distension appears as an overall outward protuberance of the abdomen. Intestinal gas, large tumours, or fluid in the peritoneal cavity may cause distension. The skin of a distended abdomen appears taut, as if stretched.
Auscultation of the abdomen with the stethoscope is performed to assess bowel sounds in each quadrant (see Chapter 27). Auscultation of the bowel sounds is not done in the usual order of physical assessment, but is done prior to palpation. Normal bowel sounds occur every 5–15 seconds and last 1 second to several seconds. While auscultating, note the character and frequency of bowel sounds.
• An increase in pitch or a tinkling sound may be heard with abdominal distension.
• Absent or hypoactive sounds (< 5 sounds per minute) occur with paralytic ileus, such as after abdominal surgery.
• High-pitched and hyperactive bowel sounds (35 or more sounds per minute) occur with small-intestine obstruction and inflammatory disorders.
Palpation of the abdomen is performed to determine distension (a drum-like tightness), areas of tenderness or the presence of masses (see Chapter 27). It is important for the person to relax. Tensing abdominal muscles interferes with palpating underlying organs or masses. If the examination reveals significant tenderness in any area of the abdomen, note the location of the tenderness and do not progress with further palpation or percussion. Refer the person to a medical practitioner for further assessment, as the pain/tenderness may indicate serious underlying pathology such as acute appendicitis or inflammation of the bowel. A person with severe constipation or faecal impaction may have faecal matter in the sigmoid or descending colon which you may be able to palpate on the left lower abdominal region.
Percussion detects lesions, fluid or gas within the abdomen. Familiarity with the five percussion notes (see Chapter 27) also permits identification of underlying abdominal structures, for example a distended bladder. Gas or flatulence creates a tympanic note. Masses, tumours and fluid are dull to percussion.
Perianal area
Inspect the area around the anus for lesions, discolouration, inflammation and haemorrhoids. Abnormalities should be carefully recorded (see Chapter 27).
Laboratory tests
Laboratory and diagnostic examinations yield useful information concerning elimination problems. Laboratory analysis of faecal contents can detect pathological conditions such as tumours, GI haemorrhage, infection and parasites.
FAECAL SPECIMENS
Ensure that specimens are accurately obtained, properly labelled in appropriate containers and transported to the laboratory on time. Institutions provide special containers for faecal specimens. Some tests require specimens to be placed in chemical preservatives.
Aseptic technique should be used during collection of stool specimens (see Chapter 29). Because about 25% of the solid portion of a stool is bacteria from the colon, you must wear disposable gloves when handling specimens.
Hand hygiene is necessary for anyone who might come in contact with the specimen. Often the patient can obtain the specimen if properly instructed. Explain that faeces cannot be mixed with urine or water. For this reason the patient must defecate into a clean, dry bed pan or special container placed under the toilet seat.
Tests performed by the laboratory for occult (microscopic) blood in the stool and stool cultures require only a small sample; collect about 2 cm of formed stool or 15–30 mL of liquid diarrhoeal stool. Tests for measuring the output of faecal fat require a 3- to 5-day collection of stool. All faecal material must be saved throughout the test period.
After obtaining a specimen, label and tightly seal the container and complete the laboratory requisition forms. Then record the specimen collection in the patient’s medical record. It is important to avoid delays in sending specimens to the laboratory. Some tests, such as measurement for ova and parasites, require the stool to be warm; this is called a ‘hot’ specimen. When stool specimens are allowed to stand at room temperature, bacteriological changes that alter test results can occur.
FAECAL OCCULT BLOOD TEST
The FOBT is a common laboratory test which measures microscopic amounts of blood in faeces. There are two main types of tests for FOB: guaiac and immunochemical. Immunochemical techniques are considered to be more reliable, as their accuracy is not influenced by diet and the use of certain medications. The FOBT is useful as a diagnostic screening test for colon cancer. The Australian Federal Government funds a National Bowel Screening Program aimed at detecting bowel cancer early so that it can be effectively treated. The New Zealand Ministry of Health has a similar bowel screening program for New Zealanders. See Box 37-3.
BOX 37-3 SCREENING FOR COLORECTAL CANCER
SCREENING TESTS
• Faecal occult blood test (FOBT)—the Australian National Bowel Screening Program screens at ages 50, 55 and 65 years. The Cancer Council Australia recommends screening every two years for people over 50 years of age. New Zealand is currently (2011–2013) carrying out a pilot to test whether bowel screening should be introduced nationally.
• Colonoscopy is undertaken every 1–3 years for people at high risk or who have had a FOBT.
Cancer Council Australia 2008 Clinical practice guidelines for the prevention, early detection and management of colorectal cancer. Sydney, Cancer Council Australia. Online. Available at www.cancer.org.au/File/HealthProfessionals/ClinicalpracticeguidelinesJuly2008.pdf; National Bowel Cancer Screening Program 2012 About the program. Canberra, Department of Health and Ageing. Online, Available at www.cancerscreening.gov.au/internet/screening/publishing.nsf/Content/bowel-about.
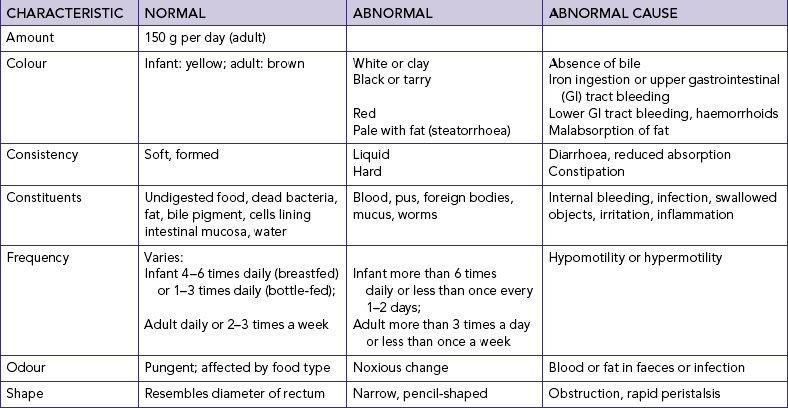
Faecal characteristics
Inspection of faecal characteristics (Table 37-4) reveals information about the nature of elimination alterations. Several factors can influence each characteristic. A key to assessment is determining whether there have been any recent changes in bowel elimination patterns and the characteristics of the faeces.
Diagnostic examinations
A patient may have a diagnostic test as an outpatient or inpatient. Viewing of GI structures may be by direct or indirect approach. Many facilities use conscious sedation during these procedures. Midazolam, a sedative-antianxiety agent, may be used, possibly augmented with a narcotic analgesic along with a local anaesthetic spray to help overcome gagging (Bryant and Knights, 2010). It is essential to understand the safety precautions involved concerning the use of this form of anaesthesia. Most institutions require nurses to have specialised skills in anaesthetic care nursing to care for such patients. Resuscitation equipment must be present at the bedside, and the patient must be monitored continuously with pulse oximetry.
DIRECT VIEWING
Instruments introduced through the mouth (upper GI viewing, or UGI) or the rectum (lower GI viewing) allow the medical practitioner to inspect the integrity of mucosa, blood vessels and organ parts. A colonoscopy is usually the test of choice; this uses a fibre-optic endoscope with a lens viewer, a long flexible tube and a light source at the end. It allows viewing of structures at the tip of the tube and insertion of special instruments for biopsy. Proctoscopes and sigmoidoscopes are rigid, tube-shaped instruments with attached light sources; the proctoscope looks like a speculum with a light. These instruments are less flexible than fibre-optic scopes and more capable of causing discomfort.
UGI endoscopy or gastroscopy allows viewing of the oesophagus, stomach and duodenum. The doctor inspects for tumours, vascular changes, mucosal inflammation, ulcers, hernias and obstructions. A gastroscope enables the doctor to remove tissue specimens (biopsy), remove abnormal tissue growth (e.g. polyps) and coagulate sources of bleeding. Lower GI examinations require bowel preparation for several days prior to the procedure. The person has a restricted, low-fibre diet for several days and then clear fluids on the day prior to the procedure. They are also required to take a strong stimulant laxative (e.g. ColonLYTELY) the day prior to the procedure. This is taken orally, usually 1 litre of fluid per hour for 2 hours. It has a strong osmotic effect in the bowel and may cause abdominal distension and cramping. The person should be advised to stay close to the toilet as it will result in high volumes of watery diarrhoea within 30 minutes to 3 hours following ingestion (Tiziani, 2010).
Nursing implications before diagnostic procedures concerning the GI system include the following:
• Record baseline observations including oxygen saturation (SpO2).
• Check that the patient has signed an informed consent form after the steps of the procedure have been explained to them.
• Check that the patient has performed the necessary bowel preparations.
• Check that the patient has not had anything to eat or drink for the required period of time prior to the procedure (as instructed by the medical practitioner).
• If conscious sedation is used, explain to the patient that they will have an intravenous cannula inserted prior to the procedure. The sedation will make them very sleepy, but they may hear voices of the staff involved in the procedure. They will recover quickly following the procedure.
For procedures involving the upper GI tract:
• If conscious sedation is not used, explain that the patient may feel fullness in the throat and a sense of gagging during the test.
• Explain that the patient will be unable to speak as the endoscope enters the oesophagus.
For procedures involving the lower GI tract:
• During the test, air is used to distend the bowel for better viewing; explain that the patient may have ‘wind pains’ after the procedure.
• For upper GI procedures—instruct the patient to avoid eating or drinking until the gag reflex returns (2–4 hours). To check for the gag reflex, the nurse places a tongue blade at the back of the patient’s tongue. Hoarseness and a sore throat are normal for several days; cool fluids and Normal saline gargling relieve soreness. The person should not drive a car or operate machinery for 12 hours after the procedure when conscious sedation is used.
• For lower GI procedures—the nurse observes for bleeding, fever, abdominal pain and blood in the stool. The person should not drive a car or operate machinery for 12 hours after the procedure when conscious sedation is used.
INDIRECT VIEWING
When direct viewing is impossible (as with deeper GI structures), the doctor relies on indirect X-ray examination. The patient ingests a contrast medium or has the medium given as an enema. One of the most common media is barium, a white, chalky, radio-opaque substance that the patient drinks like a milkshake. It is used in upper GI studies and barium enemas. Contrast media usually contain a flavouring agent to improve the taste.
The upper GI study is an X-ray study of an ingested contrast medium that allows the doctor to view the lower oesophagus, stomach and duodenum. The doctor notes ulcerations, inflammation, tumours and anatomical malposition of organs. The patency of organs and the pyloric valve are also observed. Nursing implications before the test include the following:
• Signed an informed consent form—explain the steps of the procedure to the patient, including that the test might take several hours and requires frequent position changes; explain that discomfort is minimal except for lying on a hard examination table.
• Performed the necessary bowel preparations (if applicable).
• Not had anything to eat or drink for the required period of time prior to the procedure (as instructed by the medical practitioner).
• Been informed that the radio-opaque medium meglumine diatrizoate (Gastrografin) may have a chalky taste and that the patient can resume eating after the test.
Small-bowel follow-through (continuation of upper GI viewing) enables close examination of the small intestine. The flow of meglumine through the intestine may suggest motility problems. A barium enema enables indirect viewing of the lower colon to reveal location of tumours, polyps and diverticula as well as structural abnormalities such as rectovaginal prolapse.
Patient expectations
Patients expect the nurse to be able to answer all their questions regarding diagnostic tests and the preparation for those tests. Patients will be concerned about discomfort and exposure of their more personal areas. Fear of loss of control over bowel elimination is especially worrisome. Patients will need reassurance that their needs will be met and that the nurse will be supportive.
NURSING DIAGNOSIS
A nursing assessment of the patient’s bowel function may reveal data that indicates an actual or potential elimination problem or a problem resulting from elimination alterations (Box 37-4). Associated problems, such as body-image changes or skin breakdown, require interventions unrelated to bowel-function impairment. However, in some instances the nurse must direct as much attention to the elimination problem as to the associated problem.
The ability to identify the correct diagnosis depends not only on the thoroughness of the assessment but also on recognition of the defining characteristics and factors that can impair elimination (Box 37-5). The nurse determines the patient’s risk and institutes measures to ensure maintenance of normal bowel function.
BOX 37-5 SAMPLE NURSING DIAGNOSTIC PROCESS
| ACUTE DIARRHOEA | |
| Possible nursing diagnosis | Diarrhoea related to infection, changes in diet or alteration in gastrointestinal functioning |
| ASSESSMENT | ACTIVITIES DEFINING CHARACTERISTICS |
|---|---|
| Have patients describe pain, cramping, or any associated factors. | Pain is colicky in nature and spasmodic. |
| Have patient describe onset of symptoms, relationship to diet, recent travel, etc. | |
| Auscultate bowel sounds. | Bowel sounds will be hyperactive and may be audible without a stethoscope. |
| Assess frequency of stools. | Frequency is an early indication of increased risk of fluid and electrolyte imbalance, which is further indicated by muscle cramps. |
| Assess hydration status. | |
| Evaluate perianal area for redness and irritation. | Frequent stools lead to breakdown of perianal tissues. |
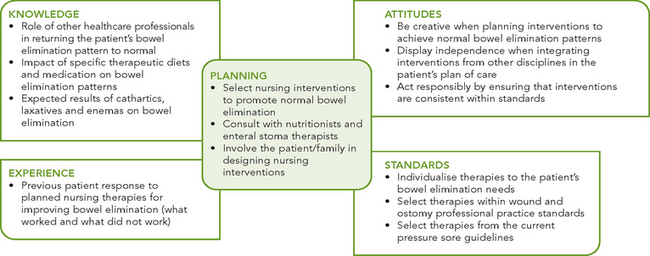
PLANNING
During the planning of care, the nurse synthesises information from multiple resources (Figure 37-12). Critical thinking ensures that the plan of care integrates knowledge about the patient and the clinical problem. The guidelines on urinary incontinence (see Chapter 38) should be used in care planning to protect the patient’s skin, promote continence and reduce the embarrassment associated with faecal incontinence. In addition, the use of current guidelines for the reduction of pressure injury helps in the development of care for patients with bowel incontinence (see Chapter 30). When the person requires surgical intervention, a care pathway may be used to coordinate the activities of the multidisciplinary healthcare team.
Goals and outcomes
The care plan establishes goals and outcomes by incorporating the patient’s elimination patterns or routines as much as possible. The person may need to be assisted to develop knowledge and new elimination patterns. Defecation patterns vary among individuals. For this reason, the nurse and patient must work together closely to plan effective interventions (see Sample nursing care plan).
The goals of care for patients with elimination problems include the following:
• understanding normal elimination
• attaining regular defecation patterns
• understanding and maintaining healthy fluid and food intake
Outcomes for a goal of attaining regular defecation patterns would be that the patient maintains soft, formed bowel motions every 1–3 days, identifies measures to prevent constipation, demonstrates regular bowel habits, increases fibre in the diet and increases fluid intake.
Setting priorities depends on the patient’s condition and perceived need. Some patients perceive that they are constipated if they do not open their bowels every day; others do not worry until they become uncomfortable.
Continuity of care
Patients who are discharged with unresolved bowel problems may require ongoing community care. Appropriate nursing services (e.g. Royal District Nursing Services) will be involved in teaching and monitoring the patient and meeting any ongoing needs. As well, the patient with alterations in bowel elimination may require intervention from many members of the healthcare team—the general practitioner, pharmacist and physiotherapist can provide information, support, expertise and assistance. When patients have a disability or are debilitated by illness, it is necessary to include the family in the plan of care.
Certain tasks, such as assisting patients onto the bed pan or bedside commode, are appropriate to delegate to nursing assistants (for example a personal care worker or health assistant). It is, however, important to remind the assistant to report any abnormal findings or difficulties with elimination.
Implementation
Success of the nursing interventions depends on improving patients’ and family members’ understanding of bowel elimination.
Health promotion
One of the most important concepts a nurse can teach regarding bowel elimination is to take time for defecation. To establish regular bowel elimination patterns, a person must know when the urge to defecate normally occurs. Advise the person to begin establishing a routine during a time when defecation is most likely to occur, usually an hour after a meal. If the person is restricted to bed or requires assistance in moving, the person should be offered a bed pan or assistance to reach the toilet.
Many people have established rituals for defecation. In a hospital or extended-care facilities, make certain that treatment routines do not interfere with these schedules. It is also important to provide privacy. When patients are forced to use a bed pan or share rooms with other people, pull the curtain around the area so that patients can relax, knowing that interruptions will not occur. The call light should always be placed within a patient’s reach. Bathroom doors should be closed, although you may need to stand close in case the patient needs assistance.
BOWEL ELIMINATION ALTERATIONS
ASSESSMENT*
Chris White (a registered nurse working in a rehabilitation centre) is assessing Mr John Moore on admission to the centre from an acute care hospital following a right total hip replacement 4 days ago. Mr Moore is 75 years old and has a history of osteoarthritis for approximately 15 years. This has caused pain and reduced movement in both hips and knees. He had his left hip replaced 18 months ago. He has no other health problems and his only regular medication prior to surgery was ‘Panadol osteo’. He was taking 2 tablets three times a day. He has progressed well following surgery and is keen to be involved in a rehabilitation program. The wound is healing well and has no signs of redness or swelling. Mr Moore is progressing well with increasing his mobility. His main health concern relates to bowel elimination. On this issue, the assessment revealed the following data:
• Normally has one soft bowel movement per day. Experienced constipation following previous hip replacement surgery.
• Has a poor appetite since the surgery, eating only small amounts. Drinking approximately 1500 mL of water and cups of tea per day.
• Feels bloated, feels like he needs to have his bowels opened but can’t. Last bowel movement 4 days ago.
• Had patient-controlled analgesia of morphine for 24 hours postoperatively and then Oxynorm for pain.
• Abdominal examination: slightly convex shape to abdomen, bowel sounds decreased in all four quadrants. Tender on palpation, especially in left lower quadrant.
NURSING DIAGNOSIS: Constipation related to opiate-containing pain medication, change of diet and mobility.
PLANNING
| GOALS | EXPECTED OUTCOMES |
|---|---|
*Defining characteristics are shown in bold type.
PROMOTION OF NORMAL DEFECATION
To help patients evacuate bowel contents normally and without discomfort, a number of interventions can stimulate the defecation reflex, affect the character of faeces or increase peristalsis.
TOILETING POSITION
You might need to give assistance to patients who have difficulty getting onto a toilet because of muscular weakness and mobility problems. Regular toilets are often too low for people who are unable to lower themselves to a squatting position because of joint- or muscle-wasting diseases. Patients can purchase elevated toilet seats for the home. With such a seat, less effort is needed to sit or stand.
POSITIONING ON BED PAN
Patients restricted to bed must use bed pans for defecation. Women use bed pans to pass both urine and faeces, whereas men use bed pans only for defecation. Sitting on a bed pan can be extremely uncomfortable. You may need to assist the person to get positioned comfortably. Two types of bed pans are available (Figure 37-13). The regular bed pan, made of metal or hard plastic, has a curved smooth upper end and a sharp-edged lower end and is about 5 cm deep. A fracture or slipper pan, designed for patients with body or leg casts, has a shallow upper end about 1.3 cm deep. The upper end of the pan fits under the buttocks toward the sacrum, with the lower end just under the upper thighs. The pan should be high enough so that faeces enter the pan.

FIGURE 37-13 Types of bed pans. From left, regular bed pan and fracture or slipper pan.
From Potter PA, Perry AG 2013 Fundamentals of Nursing, 8 ed. St Louis, Mosby.
When positioning a patient, it is important to prevent muscle strain and discomfort. A patient should never be placed on a bed pan and then left with the bed flat unless activity restrictions demand this. If the bed is flat, the hips remain hyperextended. It may be necessary to have the bed flat when placing the patient on the bed pan. After the patient is on the pan, the nurse raises the head of the bed 30 degrees. Patients who have had abdominal surgery will be hesitant to exert strain on suture lines.
Figure 37-14 shows correct and incorrect positions on bed pans. The best method is to be sure the patient is positioned high in bed. Raise the patient’s head about 30 degrees, to prevent hyperextension of the back and to provide support to the upper torso, as the patient raises the hips by bending the knees and lifting the hips upwards. The use of an overbed handrail to assist the patient to pull themselves off the mattress may also be used. When handling a bed pan, always wear gloves.
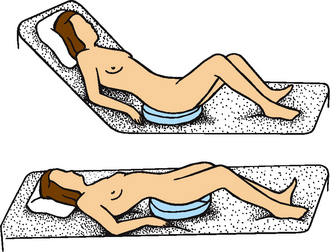
FIGURE 37-14 Positions on a bed pan. Top, Proper position reduces patient’s back strain. Bottom, Improper positioning of patient.
From Potter PA, Perry AG 2013 Fundamentals of Nursing, 8 ed. St Louis, Mosby.
If the patient is immobile or it is unsafe to allow the patient to exert such effort, the patient can roll onto the bed pan by using the following steps:
1. Lower the head of the bed flat and help the patient roll onto one side, backside towards you.
2. You may need to apply talcum powder lightly to the back and buttocks to prevent skin from sticking to the pan.
3. Place the bed pan firmly against the buttocks, down into the mattress with the open rim towards the patient’s feet (Figure 37-15).
4. Keeping one hand against the bed pan, place the other around the patient’s upper hip. Ask the patient to roll back onto the pan, flat in bed. Do not push the pan under the patient.
5. With the patient positioned comfortably, raise the head of the bed 30 degrees.
6. Place a rolled towel or small pillow under the lumbar curve of the patient’s back for added comfort.
7. Ask the patient to bend the knees to assume a squatting position.
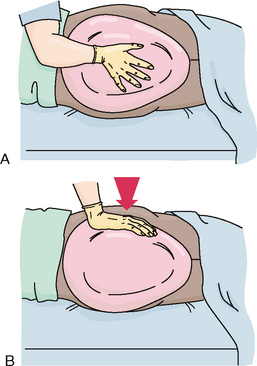
FIGURE 37-15 Positioning an immobilised patient on a bedpan. A, Place bed pan firmly against buttocks. B, Push buttocks down into mattress with open rim towards feet. Once bed pan is in this position, place one hand against pan and the other on the patient’s upper hip and ask the patient to roll onto the pan.
The privacy of a patient using a bed pan needs to be maintained. The call light and a supply of toilet paper should be within easy reach. Ensure the bed rails are raised to provide a secure environment for the patient. When the patient finishes, respond to the call signal immediately and remove the pan. To remove the pan, ask the patient to roll off to the side or raise the hips. Hold the pan steady to avoid spilling—the bed pan should be covered with a paper pan cover before taking it to the utility room for cleaning. Avoid pulling or shoving the pan from under the patient’s hips because this can pull the patient’s skin and cause tissue injury such as a pressure ulcer (see Chapter 30). After the pan is removed, assist the patient to clean the anal and perineal areas.
After assessing the stool, immediately empty the bed pan’s contents into the pan flusher in the utility room. The bed pan is sterilised before being used by another person. After removing the gloves and performing hand hygiene, chart the characteristics of the faeces. After using a bed pan offer the patient a warm face washer or bowl of water and a towel so that they can wash their hands.
People who are unable to get to the toilet should be offered a bed pan often. Patients may accidentally soil bedclothes if forced to wait. Many patients try to avoid using a bed pan because it is embarrassing and uncomfortable. They may try to get to the toilet even though their conditions prohibit ambulation. The nurse must warn patients about the risk of falls or accidents.
FACILITATION OF THE GASTROCOLIC REFLEX
As described previously, when food is chewed and swallowed, and moved into the stomach, a gastrocolic reflex is triggered causing an increase in peristalsis throughout the rest of the GI tract. The contents of the bowel are moved into the sigmoid colon and rectum, giving the person a sensation of wanting to defecate. The urge to defecate usually occurs approximately 30 minutes after eating, especially after a period of fasting, such as after breakfast. The aim in people with bowel problems is to harness this natural reflex. It is a useful strategy, even for people who are confused and may not be able to communicate the need to defecate (such as a person with dementia) or who have impaired sensation such as paraplegia.
The reflex can be harnessed by establishing a routine time for breakfast (which is high in fibre and includes fluids), encouraging light exercise where possible and going to the toilet approximately 30 minutes after eating. Some people may need to wait for a while on the toilet for the sensation to become evident. However, it is recommended that the person should not sit on the toilet for more than 15 minutes as rectal prolapse can occur due to prolonged relaxation of the pelvic floor (Kyle and Prynn, 2007). It is important to inform people not to ignore the urge to defecate, because once the urge is overridden it may not return for 24 hours.
MAINTENANCE OF FLUID AND FOOD INTAKE
In choosing a diet for promoting normal elimination, consider the frequency of defecation, characteristics of faeces and types of foods that impair or promote defecation. The patient with frequent constipation or impaction requires an increased intake of high-fibre foods and more fluids. However, the patient should realise that diet therapy provides only long-term relief of elimination problems and may not give immediate relief from problems such as constipation. When diet alone does not provide enough fibre, a fibre supplement such as psyllium husk powder (e.g. Metamucil) can be added. Psyllium husk powder can be sprinkled on cereals or taken with a glass of water.
When acute diarrhoea is a problem, consider recommending foods with low fibre content and discouraging consumption of foods that typically cause gastric upset or abdominal cramping, for example spicy foods. If the patient cannot tolerate foods or liquids orally, intravenous therapy (with potassium supplements) is often necessary. The patient returns to a normal diet slowly, often beginning with fluids. Excessively hot or cold fluids stimulate peristalsis, causing abdominal cramps and further diarrhoea. As the tolerance to liquids improves, solid foods are introduced. For a person with chronic diarrhoea, diet is also important; however, determining the exact cause for the diarrhoea is necessary before dietary modifications are made. People with chronic diarrhoea are often referred to a continence nurse advisor for ongoing assessment and evaluation of their treatment plan.
During the first weeks after bowel surgery requiring formation of an ostomy, many surgeons recommend low-fibre diets, particularly for ileostomy patients, because the small bowel requires time to adapt to the diversion. Low-fibre foods include bread, noodles, rice, cream cheese, eggs (not fried), strained fruit juices, lean meats, fish and poultry. As ostomies heal and the patient’s condition improves, they can eat almost any food. High-fibre foods such as fresh fruits and vegetables help ensure a more solid stool, needed to achieve a more regular elimination pattern for the person with a sigmoid colostomy. Ileostomy patients should eat slowly and chew food completely to reduce the risk of intestinal blockage caused by undigested foods. Drinking 10–12 glasses of water daily also prevents blockage. Ostomy patients may benefit from avoiding foods that cause gas and odour, for example broccoli, cabbage, cauliflower, dried beans and Brussels sprouts.
PROMOTION OF REGULAR EXERCISE
A daily exercise program helps prevent elimination problems. For community-dwelling people, walking, riding a stationary bicycle or swimming stimulates peristalsis. People who are sedentary at work are most in need of regular exercise. Hospitalised patients who are temporarily immobilised should be encouraged to walk as soon as possible. If the condition permits, assist the postoperative patient to walk to a chair on the evening of the day of surgery. The patient should be encouraged to walk further each day.
PROMOTION OF COMFORT
Many patients have discomfort from alterations in elimination. Pain results when haemorrhoidal tissues are directly irritated. Flatus production can also create discomfort, particularly if distension develops.
The main goal for the patient with haemorrhoids is to have soft-formed, painless stools. A high fibre diet, fluids and regular exercise improve the likelihood of stools being soft. If the patient becomes constipated, passage of hard stools may cause bleeding and irritation. Local heat may provide temporary relief to swollen haemorrhoids. A sitz bath is the most effective means of heat application (see Chapter 30).
To relieve the discomfort of gaseous distension, use interventions that aim to reduce flatus or promote its escape. Swallowing of air increases flatus. The patient can reduce the amount of air swallowed by not drinking carbonated beverages, not using straws for drinking and not chewing gum or hard lollies. Some patients drink herbal teas (peppermint) to reduce the discomfort of flatus.
When flatus results in abdominal cramping, encourage the patient to walk as this promotes passage of flatus. Having the patient walk down the hall may be enough to stimulate peristalsis and relieve gas.
MAINTENANCE OF SKIN INTEGRITY
The patient with diarrhoea or faecal incontinence is at risk of skin breakdown when faecal contents remain on the skin. The same problem exists for the patient with an ostomy that drains liquid stool. Liquid stool is usually acidic and contains digestive enzymes. Irritation from repeated wiping with toilet paper aggravates skin breakdown. Bathing the skin after soiling helps, but may result in more breakdown unless the skin is thoroughly dried.
When caring for a debilitated, incontinent person who is unable to ask for assistance, assess often for episodes of incontinence. The anal areas can be protected with a protective barrier cream (for example a zinc oxide and olive oil cream) that protects and holds moisture in the skin, preventing drying and cracking. Yeast infections (for example Candida albicans—‘thrush’ infection) of the skin can develop easily. Several powdered antifungal agents are effective against this type of infection. Baby powder should not be used, because it has no medical properties and frequently cakes on the skin and becomes difficult to remove.
PROMOTION OF SELF-CONCEPT
Bowel elimination problems can have significant psychological impact. Frequent incontinence, foully odorous stools and an ostomy appliance are just a few factors that may cause a patient to perceive a change in body image. The result could be a patient who avoids socialising with others or who is unwilling to assume responsibility for self-care. The nurse can play an important role in restoring a patient’s self-concept through the following interventions:
• Give the patient an opportunity to discuss concerns or fears about elimination problems.
• Provide the patient and family with information to understand and manage the elimination problem.
• Give positive feedback when the patient attempts self-care measures.
• Help the patient manage the condition and allow time for adjustment.
• Provide privacy during care.
• Show acceptance and understanding. Remember that the patient will be watching the nurse during ostomy care and pouch changes for facial expressions and other non-verbal clues that demonstrate acceptance of the ostomy.
CATHARTICS AND LAXATIVES
If dietary and other interventions as described above have not resulted in a normal bowel action, further interventions may be required to alleviate constipation. Cathartics and laxatives have the short-term action of emptying the bowel. They are also used in bowel evacuation for patients undergoing GI tests and abdominal surgery. Although the terms ‘cathartic’ and ‘laxative’ are often used interchangeably, cathartics have a stronger effect on the intestines. Five types of laxatives and cathartics are available (Table 37-5). Cathartics and laxatives are available in oral tablet and suppository dosage forms (see Chapter 31). You should always aim to start with the milder type of laxative first. For example, start with a mild osmotic laxative such as lactulose syrup before moving onto a stronger laxative (e.g. Movicol or Coloxyl with senna). However, this will depend on your assessment of the patient. If the patient has significant symptoms or is taking narcotic analgesia, you may need to start with a stronger laxative and use a combination of laxatives.
RECTAL SUPPOSITORIES AND ENEMAS
Suppositories and enemas are used when the patient has significant symptoms associated with their constipation to provide them with immediate relief. They work more quickly than oral preparations because of their local stimulant effect on the rectal mucosa.
RECTAL SUPPOSITORIES
A suppository is a preparation that is solid until it is inserted into the rectum, where it melts and releases the active substance. The most commonly used preparation for the treatment of constipation is glycerine suppositories. They usually have an effect within 15 minutes to 1 hour. Stimulant suppositories such as bisacodyl (e.g. Durolax) can act within 30 minutes. Frail older adults often get a strong sudden urge to defecate with bisacodyl. Ensure the patient can reach the call bell, or has easy and quick access to the toilet area.
ENEMAS
An enema is the instillation of a solution into the rectum and sigmoid colon. The main reason for an enema is to promote defecation by stimulating peristalsis and softening the faecal mass. The most common use for an enema is temporary relief of acute constipation. Other indications include removing impacted faeces and to empty the bowel before diagnostic tests or surgery.
The most commonly administered enemas are commercial preparations administered with a 5 mL plastic squeeze tube and a long insertion nozzle, for example a Microlax microenema (see Table 37-5). Sterile technique is unnecessary when administering an enema as the colon normally contains bacteria. However, gloves are worn to prevent the transmission of faecal microorganisms onto the hands. You should explain the procedure, including the position to assume, precautions to take to avoid discomfort, and the length of time necessary to retain the solution before defecation. If the patient is to receive the enema at home, explain the procedure to a family member or carer. Skill 37-1 outlines the steps for an enema administration.
SKILL 37-1 Administering a prepared enema
DELEGATION AND OTHER CONSIDERATIONS
This task requires the problem-solving and knowledge-application skills of registered nurses. For this reason, delegation of this task to nurse assistants or personal care workers is inappropriate.
EQUIPMENT
• Waterproof, absorbent pad to cover bed sheets
• Blanket or large towel to cover patient
• Bed pan, bedside commode or access to toilet
• Washbasin, washcloths, towel and soap (if person cannot get to the bathroom)
| STEPS | RATIONALE | |
|---|---|---|
| Determines factors indicating need for enema and influencing the type of enema used. An enema should only be used when less-invasive methods have been used and have not resulted in relief of constipation. | ||
| Conditions contraindicate use of enemas. | ||
| Allows nurse to plan for appropriate teaching measures. | ||
| Order by doctor is required. Determines number and type of enema to be given. | ||
| Information promotes patient cooperation and reduces anxiety. | ||
| Reduces embarrassment for patient. | ||
| Promotes good body mechanics and patient safety. Allows enema solution to flow downwards by gravity along natural curve of sigmoid colon and rectum, thus improving retention of solution. | ||
| Reduces transmission of microorganisms. | ||
| Critical decision point: If patient is suspected of having poor sphincter control, position on bed pan. Patient will have difficulty retaining enema solution. | ||
| Prevents soiling of bedclothes. | ||
| Provides warmth, reduces exposure of body parts and allows patient to feel more relaxed and comfortable. | ||
| Used in case patient is unable to retain enema solution. | ||
| Lubrication provides for smooth insertion of rectal tube without causing rectal irritation or trauma. | ||
| Breathing out promotes relaxation of external rectal sphincter. | ||
| Gentle insertion prevents trauma to rectal mucosa. Insertion beyond proper limit can cause bowel perforation. | ||
| Only small volumes, to stimulate defecation. | ||
| Length of retention varies with type of enema and patient’s ability to contract rectal sphincter. Longer retention promotes more-effective stimulation of peristalsis and defecation. | ||
| Reduces transmission and growth of microorganisms. | ||
| Normal squatting position promotes defecation. | ||
| Note abnormalities such as presence of blood or mucus. | ||
| Faecal contents can irritate skin. Hygiene promotes patient’s comfort. | ||
| Determines whether distension is relieved. | ||
| RECORDING AND REPORTING | HOME CARE CONSIDERATIONS |
|---|---|
ANTIDIARRHOEAL AGENTS
For patients with diarrhoea, frequent passage of liquid stools becomes a problem. The most effective antidiarrhoeal agents are opiates such as codeine phosphate and diphenoxylate (e.g. Lomotil). Antidiarrhoeal opiate agents decrease intestinal muscle tone to slow the passage of faeces. Opiates inhibit peristaltic waves that move faeces forward, but they also increase segmental contractions that mix intestinal contents. As a result, the intestinal walls absorb more water. Opiate antidiarrhoeal agents should be used with caution because opiates are habit-forming and there is the risk of retaining harmful bacteria in the lower bowel.
Care of ostomies
Patients who have temporary or permanent bowel diversions face unique healthcare problems. Their patterns of bowel elimination differ from those of patients with intact colons. People with incontinent ostomies must wear pouches or appliances to collect stools emitted from the stomas. Patients with ostomies must also follow good health practices such as maintaining proper dietary habits and exercising regularly to maintain normal elimination patterns. Patients with an ostomy have many education needs (Box 37-6).
BOX 37-6 CLIENT TEACHING FOR STOMAL CARE (INCONTINENT OSTOMY)
TEACHING STRATEGIES
• Ascertain the person’s current knowledge about stomal care and their readiness to learn.
• Instruct patient to avoid using alcohol in cleaning around the stoma. Alcohol dilates capillaries and can cause bleeding of the stomal margin.
• Demonstrate how to wash around the stoma with water and a mild soap. Pat the skin dry, but do not rub.
• Instruct patient not to use skin creams on skin because it prevents the pouch or skin barrier from adhering to the skin.
• Explain to the patient that peroxide is an irritant and should not be used.
• Instruct patient that if a yeast infection occurs, thoroughly clean the area, pat dry and apply an antifungal agent such as clotrimazole or nystatin.
• Show patient how to inspect the stoma daily and observe a stoma that is moist, shiny and dark pink to red.
• Teach patient to observe for and report excessive bleeding, oedema, or abnormal discharge or colour to the nurse or doctor.
• Teach patient how to select and apply correctly sized skin barrier and ostomy pouch.
POUCHING OSTOMIES
An incontinent ostomy requires a pouch to collect faecal material. An effective pouching system protects the skin, contains faecal material, remains odour-free and is comfortable and inconspicuous. A person wearing a pouch should feel secure in participating in any activity.
Many pouching systems are available. To ensure that a pouch fits well and meets the patient’s needs, the nurse considers the location of the ostomy, type and size of the stoma, type and amount of ostomy drainage, size and contour of the abdomen, condition of the skin around the stoma, physical activities of the patient, the patient’s personal preference, age and dexterity, and cost of equipment. A stomal therapist is a registered nurse educated to specialise in the care of patients with an ostomy. The patient is usually referred to the stomal therapist prior to surgery who will direct the preoperative and postoperative care. The stomal therapist will work with the patient to decide which pouching system is the most suitable.
A pouching system consists of a pouch and a skin barrier. Some pouching systems, such as Squibb-Convatec, Hollister, Coloplast and Smith & Nephew, are attached to the patient’s skin along the product’s adhesive surface, whereas other pouching systems are non-adhesive systems. Pouches come in one- and two-piece systems that are disposable or reusable. Some pouches have the opening precut by the manufacturer; others require the stoma opening to be custom-cut to the patient’s specific stoma size.
Skin barriers include wafers, pastes, powders and liquid film that are applied to the skin around the stoma. Some wafer skin barriers are permanently attached to the ostomy pouch. These are called one-piece pouch systems. In a two-piece system, the pouch can be detached from the skin barrier for emptying or changing. This allows the skin barrier to remain around the patient’s stoma for several days, thus minimising the chance of skin damage from too-frequent removal of the skin barrier from the peristomal skin. When using a two-piece pouching system, it is important to remember that the skin barrier and pouch must correspond in size and be from the same manufacturer. The pouch from one manufacturer will not fit correctly on the skin barrier from another manufacturer. The nurse must be sure to use an ostomy pouch made for collecting faecal matter (colostomy or ileostomy) and not one for collecting urine.
It is important to measure the stoma size carefully when selecting and cutting out the opening on the wafer skin barrier. A good skin barrier protects the skin, prevents irritation from repeated removal of the pouch and is comfortable for the patient to wear. Skill 37-2 describes general steps for applying one- and two-piece pouching systems.
DELEGATION CONSIDERATIONS
Pouching an ostomy, especially a newly established ostomy, requires the problem-solving and knowledge-application skills of a registered nurse (RN). Delegation is inappropriate. Pouching of an established ostomy can be delegated to enrolled nurses.
EQUIPMENT (SEE ILLUSTRATION)
• Pouch, clear drainable colostomy/ileostomy in correct size for two-piece system or custom cut-to-fit one-piece type with attached skin barrier
• Pouch closure device, such as clamp
Inserting and maintaining a nasogastric tube
A patient’s condition or situation may warrant special interventions to decompress the GI tract. Such conditions include abdominal surgery (see Chapter 44), infections of the GI tract, trauma to the GI tract and conditions in which peristalsis is absent (such as bowel obstruction). When a nasogastric (NG) tube is used for decompression of the GI tract, the aim is to remove any gastric secretions (or food and fluids) from the stomach to prevent their build-up in the stomach and small intestine.
An NG tube is a pliable tube that is inserted through the patient’s nasopharynx into the stomach. The tube has a hollow lumen that allows removal of gastric secretions and introduction of solutions into the stomach. Nasogastric insertion has several purposes (Table 37-6); however, the following discussion only refers to use of an NG tube for decompression.
TABLE 37-6 PURPOSES OF NASOGASTRIC INTUBATION
| PURPOSES | DESCRIPTION | TYPE OF TUBE |
|---|---|---|
| Compression | Internal application of pressure by means of inflated balloon to prevent internal oesophageal or gastrointestinal (GI) haemorrhage | Sengstaken-Blakemore |
| Decompression | Removal of secretions and gaseous substances from GI tract; prevention or relief of abdominal distension | Large bore: Salem sump, Levin |
| Feeding (gavage) (see Chapter 36) | Instillation of liquid nutritional supplements or feedings into stomach for patients unable to swallow fluid | Fine bore: radio-opaque enteral feeding tube |
| Lavage | Irrigation of stomach in cases of active bleeding, poisoning or gastric dilation | Levin, Salem sump |
The Levin tube is most commonly used for stomach decompression. It is a single-lumen tube with holes near the tip. It may be connected to a drainage bag or to an intermittent suction device to drain stomach secretions.
Nasogastric tube insertion (Skill 37-3) does not require sterile technique. The patient may experience a burning sensation as the tube passes through the sensitive nasal mucosa and when the tube reaches the back of the pharynx, and may begin to gag. You will need to encourage the patient to relax to make tube insertion easier. Some institutions allow lignocaine jelly (e.g. topical Xylocaine) to be used when inserting the tube as it increases patient comfort during the procedure.
SKILL 37-3 Inserting and maintaining a nasogastric tube (for decompression)
DELEGATION CONSIDERATIONS
Inserting and maintaining a nasogastric (NG) tube requires the problem-solving and knowledge-application skills of registered nurses. Enrolled nurses may measure and record the drainage from the NG tube and provide oral and nasal hygiene and comfort measures.
EQUIPMENT
• No. 14 or no. 16 Fr NG tube (smaller lumen catheters are not used for decompression in adults because they must be able to remove thick secretions)
• Water-soluble lubricating jelly
• pH test strips (measure gastric aspirate acidity)
• 2.5 cm wide hypoallergenic tape
• Safety pin and rubber band/tape
• Clamp, drainage bag or suction machine or pressure gauge if wall suction is to be used
| STEPS | RATIONALE | ||
|---|---|---|---|
| Provides privacy. | |||
| Baseline condition of nasal and oral cavity determines need for special nursing measures for oral hygiene after tube placement. | |||
| Nurse should insert tube into uninvolved nasal passage. Procedure may be contraindicated if surgery is recent. | |||
| Baseline determination of level of abdominal distension later serves as comparison once tube is inserted. | |||
| Determines patient’s ability to assist in procedure. | |||
| Critical decision point: If patient is confused, disoriented or unable to follow commands, get help from another staff member to insert the tube. | |||
| Procedure requires a written medical order. Adequate decompression depends on NG suction. | |||
| Identification prevents error of placing tube in wrong patient. Explanation gains patient’s cooperation and lessens possibility that patient will remove tube. | |||
| Reduces transmission of microorganisms. | |||
| Promotes patient’s ability to swallow during procedure. Good body mechanics prevent injury to nurse or patient. | |||
| Allows easiest manipulation of tubing. | |||
| Prevents soiling of patient’s gown. Tube insertion through nasal passages may cause eye-watering and coughing with increased salivation. | |||
| Tube passes more easily through naris that is more patent. | |||
| Tube should extend from nares to stomach; distance varies with each patient. | |||
| Marks amount of tube to be inserted from nares to stomach. | |||
| Tape will be used after tube insertion to anchor the tube securely. | |||
| Curving tube tip aids insertion and decreases stiffness of tube. | |||
| Minimises friction against nasal mucosa and aids insertion of tube. | |||
| Decreases patient anxiety and increases patient cooperation. | |||
| Facilitates initial passage of tube through naris and maintains clear airway for open naris. | |||
| Minimises discomfort of tube rubbing against upper nasal turbinates. Resistance is caused by posterior nasopharynx. Downward pressure helps tube curl around corner of nasopharynx. | |||
| Forcing against resistance can cause trauma to mucosa. Resting helps relieve patient’s anxiety. | |||
| Critical decision point: If unable to insert tube in either naris, stop procedure and notify doctor. | |||
| Relieves patient’s anxiety; eye-watering is natural response to mucosal irritation, and excessive salivation may occur because of oral stimulation. | |||
| Sipping of water aids passage of NG tube into oesophagus. | |||
| Flexed position closes off upper airway to trachea and opens oesophagus. Swallowing closes epiglottis over trachea and helps move the tube into the oesophagus. Swallowing water reduces gagging or choking. Water can be removed later from stomach by suction. | |||
| Tubing may accidentally enter larynx and initiate cough reflex. Gagging is eased by swallowing water. Risk of aspiration increases if vomiting occurs. | |||
| Critical decision point: If vomiting occurs, help patient clear airway; oral suctioning may be needed. Do not proceed until airway is cleared. | |||
| Tube may enter larynx and obstruct airway. | |||
| Tube may coil around itself in back of throat and stimulate gag reflex. | |||
| Tip of tube should be within stomach to decompress properly. | |||
| Tube should be partially anchored before placement is checked. | |||
| Patient is unable to talk if NG tube has passed through vocal cords. | |||
| Tube is pliable and can coil up in back of pharynx instead of advancing into oesophagus. | |||
| Gastric contents are usually cloudy and green, but may be off white, tan, bloody or brown. Aspiration of contents provides means to measure fluid pH and thus determine tube tip placement in gastrointestinal tract. | |||
| Other common aspirate colours include the following: duodenal placement (yellow or bile-stained), oesophagus (may or may not have saliva-appearing aspirate). | |||
| Gastric aspirates have highly acidic pH values, preferably 4 or less in adults and 5 or less in children, compared with intestinal aspirates, which are usually greater than 4, or respiratory secretions, which are usually greater than 5.5 (Gilbertson and others, 2011; Phang and others, 2004). | |||
| Tube must be in stomach to provide decompression. | |||
| Drainage bag is used for gravity drainage. Intermittent suction is most effective for decompression. Patient going to the operating room often has tube clamped. | |||
| Prevents tissue necrosis. Tape anchors tube securely. | |||
| Benzoin prevents loosening of tape if patient perspires. | |||
| Reduces pressure on the nares if tube moves. | |||
| Helps prevent oesophageal reflux and minimises irritation of tube against posterior pharynx. | |||
| Adaptation to continued sensory stimulus. | |||
| Reduces transmission of microorganisms. | |||
| The mark or tube length is to be used as a guide to indicate whether displacement may have occurred. | |||
| Reduces transmission of microorganisms. | |||
| Reduces transmission of microorganisms. | |||
| Determines whether tube is decompressing stomach of contents. | |||
| Determines success of abdominal decompression and the return of peristalsis. | |||
| Evaluates onset of skin and tissue irritation. | |||
| Determines whether tension is being applied to nasal structures. | |||
| Evaluates level of patient’s discomfort. | |||
| Written medical order required for procedure. | |||
| Minimises anxiety and increases cooperation. Tube passes out smoothly. | |||
| Reduces transmission of microorganisms. | |||
| Have tube free of connections before removal. | |||
| Allows easiest manipulation of tube. | |||
| Patient may wish to blow nose after tube is removed. Towel may keep gown from getting soiled. Airway will be temporarily obstructed during tube removal. | |||
| Clamping prevents tube contents from draining into oropharynx. Reduces trauma to mucosa and minimises patient’s discomfort. Towel covers tube, which can be an unpleasant sight. Holding breath helps to prevent aspiration. | |||
| Provides accurate measure of fluid output. Proper disposal of equipment and soiled gloves prevents spread of microorganisms and ensures proper exchange procedures. | |||
| Promotes comfort. | |||
| Depends on doctor’s order. Sometimes patients are allowed nothing by mouth (NBM) for up to 24 hours. When fluids are allowed, the order usually begins with a small amount of ice chips each hour and increases as patient is able to tolerate more. | |||
One of the greatest challenges in caring for a patient with an NG tube is maintaining comfort. The tube is a constant irritation to nasal mucosa. Frequent assessment of the condition of the nares and mucosa with regard to inflammation and excoriation should be undertaken. The tape used to anchor the tube often becomes soiled, and should be changed every day to lessen irritation. Frequent lubrication of the nares also minimises excoriation. With one naris occluded, the patient may breathe through the mouth. Frequent mouth care (at least every 2 hours) helps minimise drying of oral mucosa. A glass of cool water for rinsing is useful, but the patient who is allowed nothing by mouth (NBM) should not swallow the water. The patient will frequently complain of a sore throat. An ice bag applied externally to the throat sometimes helps. Gargling with topical lignocaine jelly and/or lozenges may be used if ordered by the medical practitioner.
After the tube is inserted, its patency must be maintained. If the tip of the tubing rests against the stomach wall or if the tube becomes blocked with thick secretions, irrigation may be necessary. Flushing the tube with water by way of an irrigation syringe clears blockage within the tube (see Skill 37-3). If an NG tube continues to drain improperly after irrigation, it must be repositioned by advancing or withdrawing it slightly. Any change in tube position requires reassessment of the placement of the tube in the patient’s GI tract.
The NG tube can cause distension. The presence of the tube causes many patients to swallow large volumes of air. Channels of gastric secretions also form along the walls of the stomach and bypass the suction holes. Repositioning the patient regularly helps to collapse the channels and promote emptying of stomach contents.
EVALUATION
Patient care
The effectiveness of care depends on success in meeting the goals and expected outcomes of care. At best, the patient will be able to defecate regularly, producing soft-formed, painless stools. The patient is the only one who is able to determine whether the bowel elimination problems have been relieved and which therapies were the most effective (Figure 37-16). The patient will also be able to demonstrate information gained regarding establishment of a normal elimination pattern. The patient will be able to demonstrate any skills learned, such as ostomy protocols and skin protection. The patient will be able to accomplish normal defecation by manipulating natural components of daily living such as diet, fluid intake and exercise. The patient will have minimal reliance on artificial means of defecation such as enemas and laxative use.
Patient expectations
If you have been successful in establishing a therapeutic relationship with the patient, the patient will feel comfortable in discussing the intimate details often associated with bowel elimination. The patient will not be embarrassed and will have a feeling of comfort and freedom from pain as elimination needs are met within the limits of their health condition and treatment.
KEY CONCEPTS
• Mechanical breakdown of food elements, gastrointestinal (GI) motility and selective absorption and secretion of substances by the large intestine influence the character of faeces.
• Food high in fibre content and an increased fluid intake keep faeces soft.
• Vagal stimulation, which slows the heart rate, may occur during straining while defecating, taking rectal temperatures and during enemas.
• The greatest danger from acute diarrhoea is development of fluid and electrolyte imbalance.
• The location of an ostomy influences consistency of stools.
• Assessment of elimination patterns should focus on bowel habits, factors that normally influence defecation, recent changes in elimination and a physical examination.
• Indirect and direct viewing of the lower GI tract usually requires cleansing the bowel before the procedure.
• The nurse should consider frequency of defecation, faecal characteristics and effect of foods on GI function when advising on a diet promoting normal elimination.
• Proper positioning on a bedpan enables the patient to assume a position similar to squatting without experiencing muscle strain.
• Normal bowel elimination patterns can be promoted by attention to toileting posture, facilitating the gastrocolic reflex, appropriate diet and fluid intake and an increase in exercise.
• Proper selection and use of an ostomy pouching system is necessary to prevent damage to the skin around the stoma.
• Skin breakdown can occur after repeated exposure to liquid stools.