Normal flora
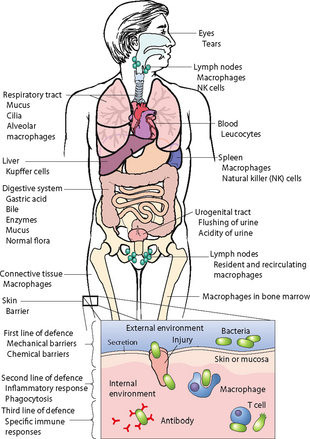
Microorganisms normally reside on the surface and in the deep layers of the skin, in the saliva and oral mucosa, in the respiratory tract and in the gastrointestinal and genitourinary tracts. Normal flora do not generally cause disease when residing in their usual location within the body. Instead, they help maintain health by creating an environment which inhibits colonisation by other organisms—by altering the pH, thereby inhibiting the growth of other bacteria; by excreting antibacterial chemicals; and by producing vitamins which benefit the host.
The skin contains a large population of resident flora. These organisms are permanent residents of the skin, where they survive and multiply. The skin’s normal flora exert a protective action by inhibiting multiplication of other microorganisms. Large numbers of normal skin flora live in warm, moist areas of the body, for example nasal passages, axillae, groin, hair follicles and sweat glands, with fewer normal flora found on exposed skin surfaces. Resident organisms are not easily removed by washing with plain soaps and detergents unless considerable friction is used.
Resident microorganisms in the mouth and pharynx impair growth of invading microbes. Upper-respiratory normal flora include streptococci, staphylococci, diphtheroids and Gram-negative cocci.
A person normally excretes trillions of microbes daily through the intestines. A large number of normal flora exist in the large intestine without causing disease. These serve a protective function by competing with disease-producing microorganisms for food, thereby limiting colonisation and disease. Normal flora also secrete antibacterial substances within the intestinal wall.
In the genitourinary tract, the lower portion of the urethra contains microorganisms similar to those present on the skin. Although usually flushed out by acidic urine, microorganisms can increase in alkaline urine, causing UTIs. The action of lactobacilli can lower the pH of the vaginal fluid to 5 (acidic), which inhibits the growth of other organisms. A decrease in lactobacillus through long-term antibiotic use allows the colonisation of other organisms, for example C. albicans.
The mass of normal flora within the body maintains a sensitive balance with other microorganisms to prevent infection. Any factor disrupting this balance places a person at increased risk of acquiring an infectious disease. For example, the use of broad-spectrum antibiotics for the treatment of infection can lead to suprainfection—normal bacterial flora are eliminated, allowing disease-producing microorganisms to multiply.
Body system defences
A number of the body’s organ systems have unique defences against infection (Table 29-3). The skin, respiratory tract and gastrointestinal tract are easily accessible to microorganisms. Pathogenic organisms can adhere to the skin’s surface, be inhaled into the lungs or be ingested with food. Each organ system has defence mechanisms physiologically suited to their structure and function. Although the lungs cannot completely control the entrance of microorganisms, the airways are lined with hair-like projections, termed cilia, which rhythmically beat to move a blanket of mucus and adherent organisms up the respiratory tract to the pharynx, to be exhaled or expectorated.
TABLE 29-3 NORMAL DEFENCE MECHANISMS AGAINST INFECTION
| DEFENCE MECHANISM |
ACTION |
FACTORS THAT MAY ALTER DEFENCE |
| SKIN |
| Intact multilayered surface (body’s first line of defence against infection) |
Provides barrier to microorganisms |
Cuts, abrasions, puncture wounds, areas of maceration |
| Shedding of outer layer of skin cells |
Removes organisms that adhere to skin’s outer layers |
Failure to bathe regularly |
| Sebum |
Contains fatty acid that kills some bacteria |
Excessive bathing |
| MOUTH |
| Intact multilayered mucosa |
Provides mechanical barrier to microorganisms |
Lacerations, trauma, extracted teeth |
| Saliva |
Washes away particles containing microorganisms
Contains microbial inhibitors (e.g.
lysozyme)
|
Poor oral hygiene, dehydration |
| RESPIRATORY TRACT |
| Cilia lining upper airway, coated by mucus |
Trap inhaled microbes and sweep them outwards in mucus to be expectorated or swallowed |
Smoking, high concentration of oxygen and carbon dioxide, decreased humidity, cold air |
| Macrophages |
Engulf and destroy microorganisms that reach lung’s alveoli |
Smoking |
| URINARY TRACT |
| Flushing action of urine flow |
Washes away microorganisms on lining of bladder and urethra |
Obstruction to normal flow by urinary catheter placement, obstruction from growth or tumour, delayed micturition |
| Intact multilayered epithelium |
Provides barrier to microorganisms |
Introduction of urinary catheter, continual movement of catheter in urethra |
| GASTROINTESTINAL TRACT |
| Acidity of gastric secretions |
Chemically destroys microorganisms incapable of surviving low pH |
Administration of antacids |
| Rapid peristalsis in small intestine |
Prevents retention of bacterial contents |
Delayed motility resulting from impaction of faecal contents in large bowel or mechanical obstruction by masses |
| VAGINA |
| At puberty, normal flora causing vaginal secretions to achieve low pH |
Inhibit growth of many microorganisms |
Antibiotics and oral contraceptives disrupting normal flora |
Inflammatory response
The body’s cellular response to injury or infection is inflammation. The inflammatory response, a protective vascular and cellular reaction, neutralises pathogens and repairs body cells. The inflammatory response may be triggered by physical agents such as mechanical trauma, temperature extremes and radiation, chemical agents, external and internal irritants such as harsh poisons, gastric acid or by microorganisms. When tissues are injured, a well-coordinated inflammatory response occurs.
VASCULAR AND CELLULAR RESPONSES
Acute inflammation is an immediate response to cellular injury. This protective vascular reaction delivers increased blood products and nutrients to interstitial tissues in an area of injury. The process neutralises and eliminates pathogens or dead (necrotic) tissues and establishes a means of repairing body cells and tissues. Arterioles supplying the injured area dilate, allowing more blood into the local circulation. The increase in local blood flow causes the characteristic redness of inflammation. The symptoms of localised warmth and swelling result from a greater volume of blood accumulating at the inflamed site. Local vasodilation delivers blood and white blood cells (WBCs) to injured tissues.
Injury causes tissue necrosis and, as a result, the body releases histamine, bradykinin, prostaglandin and serotonin. These chemical mediators or transmitters increase the permeability of small blood vessels. Fluid, protein and cells then enter interstitial spaces. Accumulated interstitial fluid results in localised swelling, termed oedema.
The cellular response of inflammation involves WBCs arriving at the site. WBCs pass through blood vessels and into the tissues. Through the process of phagocytosis, specialised WBCs, called neutrophils and monocytes, ingest and destroy microorganisms or other small particles. As inflammation becomes systemic, other signs and symptoms develop. Leucocytosis—an increase in the number of circulating WBCs—is evident as the body responds to WBCs leaving blood vessels. A serum WBC count is normally in the range of 4–11 × 109/L; this may rise to 14–22 × 109/L or higher during inflammation. Fever is caused by the phagocytic release of pyrogens from bacterial cells, causing a rise in the hypothalamic set point, which regulates temperature (see Chapter 28).
INFLAMMATORY EXUDATES
Exudates include a serous, protein-rich, pale pink liquid in which blood cells are suspended, known as haemoserous exudate, containing both serous liquid and some blood cells or frank blood. Inflammatory exudate, consisting of an accumulation of fluid, dead microorganisms, tissue cells and WBCs, forms at the site of inflammation. Platelets and plasma proteins such as fibrinogen produce a mesh-like matrix at the site of inflammation to prevent its spread. Eventually the exudate is cleared away through lymphatic drainage. Purulent exudate, termed pus, consists of dead cells and microorganisms and is indicative of infection.
TISSUE REPAIR
Injury to tissue cells initiates a healing process involving defensive, reconstructive and maturative stages (see Chapter 30).
Immune response
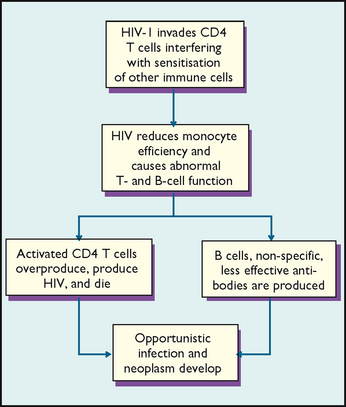
When an invading microorganism enters the body, it is initially attacked by monocytes. Foreign material—antigens—are remnants of the microorganism that triggered the immune response. Antigens are usually composed of proteins not normally found in a person’s body and which often exist as part of the bacterium or virus structure. The series of immune responses changes the body’s biological makeup. Reactions to subsequent exposure to this particular antigen, therefore, are different from the initial reaction. These altered immune responses ensure a repeated antigen is neutralised, destroyed or eliminated. After an antigen enters the body, it travels in the blood or lymph and initiates cell-mediated immunity or humoral immunity.
CELL-MEDIATED IMMUNITY
There are two classes of lymphocytes: T lymphocytes (CD4 T-cells) and B lymphocytes (B-cells). T-cells play a major role in cell-mediated immunity. There are antigen receptors on the surface membranes of CD4 T-cells. When an antigen identifies a cell with surface receptors that fit the antigen, binding occurs. This binding activates the CD4 T-cell to divide rapidly to form sensitised cells. Sensitised CD4 T-cells travel to the area of inflammation or injury, bind with antigens and release chemical compounds called lymphokines. The lymphokines attract macrophages and stimulate them to attack antigens. Eventually the antigens are killed. The cell-mediated response is altered by HIV infections, which cause AIDS (Figure 29-3).
HUMORAL IMMUNITY
Stimulation of B-cells triggers the humoral immune response, causing synthesis of immunoglobulins or antibodies that destroy antigens. After a B-cell binds with an antigen, it causes formation of plasma and memory B-cells. Plasma cells synthesise and secrete large amounts of antibodies. Memory B-cells prepare the body against future antigen invasion. When an antigen enters the body a second time, antibodies form more rapidly than during the first exposure, and immunoglobulin levels remain high to attack the antigen.
ANTIBODIES
Antibodies are large protein molecules. The five classes of antibody immunoglobulins are identified by the letters M, G, A, E and D. Immunoglobulin M (IgM) is the predominant early antibody formed after initial contact with an antigen. This initial contact is the primary immune response, and the presence of IgM denotes current inflammation. The most abundant circulating antibody is IgG, which is formed after subsequent contact with antigens or during the secondary immune response, and its presence denotes past contact with a particular antigen.
The basis of immunisation against disease involves the formation of antibodies. This is either a natural or an artificially induced event. Natural immunity results after having a certain disease, such as measles, and usually lasts a lifetime. Artificial immunity follows the receipt of a vaccine, such as tetanus or polio vaccine. Depending on the duration of immunity, a booster vaccine may be required. Passive immunity is usually of short duration and can occur transplacentally, from mother to child.
COMPLEMENT
A complement is an inactive protein compound found in blood serum. It is activated when an antigen and an antibody bind together. After a complement is activated, a rapid sequence of catalytic activity changes the shape of antigenic cells; for example, the foreign bacteria assume the shape of a doughnut. The complement actually makes a hole through the antigen’s cell membrane. Ions and water enter the cell, causing it to burst. This process is called cytolysis.
INTERFERON
When viruses invade certain cells, they synthesise the protein interferon. Interferon interferes with the ability of viruses to multiply and protects body cells from simultaneous infection with other viruses. Interferon also directly inhibits the growth and division of tumour cells.
• CRITICAL THINKING
Mrs Tendulka accompanies her 12-year-old daughter to have her viral meningitis strain C immunisation. She asks you to explain how a virus causes disease. How would you explain this to her?
THE NURSING PROCESS IN INFECTION CONTROL
ASSESSMENT
The nurse assesses the patient’s defence mechanisms, susceptibility and knowledge of infections. By recognising the early signs and symptoms of infection, a nurse can alert others in the healthcare team about the need for therapy and can implement appropriate nursing measures.
Status of defence mechanisms
A review of physical assessment findings and the patient’s medical condition can reveal the status of normal defence mechanisms against infection. For example, any break in the skin or mucosa is a potential site for infection. Similarly, a patient who is a chronic smoker is at greater risk of acquiring a respiratory tract infection following general anaesthesia, because the cilia of the lung are generally less active or able to propel retained mucus from the lungs. Any reduction in the body’s primary or secondary defences against infection places a patient at risk (Box 29-4).
BOX 29-4RISK FACTORS FOR INFECTION
INADEQUATE PRIMARY DEFENCES
• Broken skin or mucosa
• Traumatised or burnt tissue
• Decreased respiratory ciliary action
• Obstructed urine outflow
• Altered gastrointestinal peristalsis
• Change in pH of secretions
• Decreased mobility
INADEQUATE SECONDARY DEFENCES
• Reduced haemoglobin level
• Suppression of white blood cells (WBCs) (drug- or disease-related)
• Suppressed inflammatory response (drug- or disease-related)
• Low WBC count (leucopenia)
Patient susceptibility
Many factors influence susceptibility to infection. The nurse gathers information about each factor by assessing the patient, including their family history. A review of disease history with the patient and family may reveal an exposure to a communicable disease. Analysis of laboratory findings may provide information about a patient’s defence against infection. Knowledge of the factors that increase patient susceptibility or risk of infection enables the nurse to effectively plan infection prevention and control techniques.
AGE
Throughout the life span, susceptibility to infection changes. An infant has immature defences against infection. Born with only the antibodies provided by their mothers, the immune systems of infants are incapable of producing the necessary immunoglobulins and WBCs to adequately fight some infections. Breastfed infants have greater immunity than bottle-fed infants, because they receive the mother’s antibodies through breast milk. As the child grows, their immune system matures, but the child is still susceptible to organisms that cause the common cold, intestinal infections and, if not vaccinated, infectious diseases such as mumps and measles.
The young or middle-aged adult has refined defences against infection. Normal flora, body system defences, inflammation and the immune response provide protection against invading microorganisms. Viruses are the most common cause of infectious illness in young or middle-aged adults.
Defences against infection may change with ageing. The immune response, particularly cell-mediated immunity, declines, leaving elderly patients with a lower resistance to infection (Weiskopf and others, 2009). Older adults also undergo alterations in the structure and function of the skin, urinary tract and lungs. Loss of skin turgor and thinning of the epithelium increase the risk of abrasions and tears and subsequent invasion by pathogens (Table 29-4).
TABLE 29-4 ASSESSING THE RISK OF INFECTION IN OLDER ADULTS
| COMPONENT |
POSSIBLE CHANGES WITH AGE |
OUTCOME |
| Skin |
Thinner dermal and epidermal layers, decreased collagen strength, decreased skin elasticity, decreased ability to perspire (sweat) |
Pressure ulcers |
| Peripheral nerves |
Reduced sensitivity, particularly in patients with history of alcohol abuse, vitamin B12 deficiency and diabetes mellitus |
Pressure ulcers, ignored trauma leading to infection |
| Circulation |
Heart disease: congestive heart failure, calcified mitral and aortic valves |
Pneumonia, bacterial endocarditis |
| Peripheral circulation |
More-elastic veins, less-effective venous valves, blood pooling in lower extremities, dependent oedema in lower limbs |
Venous stasis ulcers |
| Mouth |
Dehydration, loss of saliva production, functional inability to maintain oral hygiene |
Parotid gland infection, periodontal disease, localised abscess, bacteraemia |
| Gastrointestinal tract |
Loss of ability to secrete stomach acid in 30% of persons over 70 years of age |
Salmonella diarrhoea |
| Pulmonary system |
Increased colonisation of oropharynx, impaired mucociliary clearance, decreased macrophage function, decreased cough reflex |
Viral and bacterial pneumonia |
| Urinary tract |
Prostatic hyperplasia, urethral strictures, age- related hormonal changes in vaginal wall, pelvic floor relaxation, ureterocele or cystocele, degeneration of nerves leading to neurogenic bladder, use of tricyclic antidepressants, dehydration |
Asymptomatic bacteriuria, cystitis, pyelonephritis |
| Nutrition |
Malnutrition, vitamin deficiency (vitamin A, pyridoxine and riboflavin), protein and caloric malnutrition |
Impaired immune response to infection |
| Drug therapy |
Corticosteroid and cytotoxic drugs |
Impaired immune response to infection |
| Nursing home residency |
Exposure to nosocomial infections, including influenza, Proteus and Providencia organisms with an indwelling catheter, tuberculosis and wound infections |
Frequent serious infection, increased risk of pneumonia |
Modified from Tideiksaar R 1987 Infections in the elderly: I. Diagnosis and treatment. Physician Assist 11(2):17.
Not only does immunity to infection decrease with advancing age, but alterations in the immune system may even trigger events associated with the ageing process (Gomez and others, 2008). Cells of the immune system, such as lymphocytes, become more diversified with age, and the body undergoes a progressive loss of cellular regulation. When viruses or other antigens and corresponding antibodies lodge in sites such as the kidney and arteries, factors injurious to the tissues are released and deterioration begins. With ageing and autoimmune diseases, cellular changes such as depletion of lymphoid tissues occur. The basic mechanism for the ageing process is not well understood.
NUTRITIONAL STATUS
When protein intake is inadequate due to poor diet or a debilitating disease, the rate of protein breakdown exceeds that of tissue synthesis. A reduction in the intake of protein and other nutrients such as carbohydrates and fats reduces the body’s defences against infection and impairs wound healing (see Chapter 30).
Patients with illnesses or problems that increase protein requirements are at further risk. Such problems include traumatic injury, extensive burns and conditions causing fever. Patients who have had surgery also require increased protein.
The nurse must assess each patient’s dietary intake and their ability to tolerate solid foods. Patients with swallowing difficulties or alterations in digestion, or those who are too confused or weak to feed themselves, are at increased risk of nutritional deficits. A dietitian may be consulted to calculate the energy value of foods ingested. In preparation for discharge, the nurse should evaluate the patient’s and carers’ understanding of nutrition in relation to health.
STRESS
The body responds to emotional or physical stress (surgery, trauma or hospitalisation) via the general adaptation syndrome (see Chapter 42). Stress increases the body’s basal metabolic rate and use of energy stores. Adrenocorticotrophic hormone (ACTH) and the release of cortisone increase serum glucose levels and decrease unnecessary anti-inflammatory responses. If stress continues, elevated cortisone levels decrease resistance to infection. Continued stress leads to exhaustion, depletion of energy stores and decreased resistance to invading organisms.
HEREDITY
Certain hereditary conditions impair a person’s response to infection. Pre-existing medical problems may reveal known hereditary disorders. For example, agammaglobulinaemia is a rare inherited or acquired disorder characterised by the absence of serum antibodies. A patient with this disorder has virtually no ability to initiate defences.
DISEASE PROCESS
Patients with diseases of the immune system are at particular risk of infection. Leukaemia, AIDS, lymphoma and aplastic anaemia are conditions that compromise a host by weakening their defences against infectious organisms. Patients with leukaemia, for example, are unable to produce enough WBCs to ward off infection.
Patients with chronic diseases such as diabetes mellitus and multiple sclerosis are also more susceptible to infection because of the disease process and impaired health status. Diseases such as pulmonary emphysema and bronchitis cause impaired ciliary action and thickened mucus. Cancer alters the immune response; peripheral vascular disease reduces blood flow to injured tissues; and burns or damage to skin surfaces impairs body system defences and increases a person’s susceptibility to infection.
MEDICAL THERAPY
A review of medical and medication therapies will reveal potential for compromised immunity to infection. Glucocorticoids, for example prednisolone, can cause protein breakdown and impair the inflammatory response against bacteria and other pathogens. Cytotoxic or antineoplastic drugs attack cancer cells but cause side effects, including bone-marrow depression and normal cell toxicity. Bone-marrow depression renders the body unable to produce sufficient lymphocytes and WBCs. When antineoplastic agents alter normal cells, cellular defences against infection fail. Cyclosporin and other immunosuppressant drugs can decrease the body’s immune response, and are commonly prescribed for recipients of organ transplants. Immunosuppressants prevent organ and tissue rejection but increase a person’s susceptibility to infection. The massive doses of radiation received by patients with cancer destroy cancerous cells but increase their risk of infection by depressing bone marrow and destroying normal cells.
Clinical examination: signs and symptoms of infection
Signs of local infection may include swelling, redness, localised heat, pain or tenderness, accumulation of body fluids (serous, haemoserous or purulent) and loss of function in the affected body part. Localised infections most commonly occur in areas of skin or mucous membrane breakdown, such as surgical and traumatic wounds, pressure ulcers and mouth lesions. Infections can also develop locally in cavities beneath the skin, for example an abscess. Assessment for localised infection requires inspection for redness and swelling caused by inflammation and drainage from open lesions or wounds. Infected drainage may be yellow, green or brown, depending on the infecting pathogen. The nurse should ask the patient to describe the pain, tenderness or discharge experienced around the site of infection.
Swollen, inflamed tissues increase pressure on nerve endings and this causes pain. Chemical substances such as histamine are released and stimulate nerve endings. The physiological changes associated with infection can cause a temporary loss of function to the involved body part. For example, a localised infection of the hand causes the fingers to become swollen, painful and discoloured. Joints may become stiff as a result of swelling, but function returns to the fingers once the inflammation subsides. If the nurse suspects a patient’s hand may swell, they are advised to ensure the patient removes all rings, watches and bracelets from the hand, to prevent constricting damage to tissues.
When inflammation becomes systemic, other signs and symptoms can develop. These include fever, increased pulse and respiratory rate, leucocytosis, malaise, anorexia, lymph-node enlargement (lymphadenopathy) and an increase in antibodies (e.g. IgM is elevated for a current infection and IgG is elevated for a past infection). Lymph nodes that drain the area of infection often become enlarged, swollen and tender to palpation. For example, an abscess in the peritoneal cavity may cause enlargement of lymph nodes in the groin. An infection of the upper respiratory tract may cause cervical lymph-node enlargement. If an infection is serious and widespread, all major lymph nodes may enlarge.
Systemic infections develop after treatment for localised infection has failed and may initially result in changes in the patient’s level of activity and responsiveness. As a systemic infection develops, the patient may become lethargic and complain of decreased energy, an elevation in temperature, increased pulse and increased respiratory rates and hypoxia. Involvement of major body systems may produce specific signs. For example, a pulmonary infection may result in a productive cough with purulent sputum. A UTI may result in cloudy, foul-smelling urine.
Older adults may not present with typical signs and symptoms of an infection because of reduced inflammatory and immune responses. Their temperature may not elevate initially if they regularly use aspirin or non-steroidal anti-inflammatory drugs (NSAIDs). Atypical symptoms such as confusion, incontinence or agitation may be the only symptoms of an infectious illness. Some older adults with the influenza complication of pneumonia do not present with typical signs and symptoms such as fever, rigors, chills and productive sputum. Their only symptoms may be tachycardia, aching joints or generalised fatigue.
Laboratory data
A review of laboratory test results may reveal infection (Table 29-5). Laboratory values alone are not enough to detect infection and should be combined with an interpretation of other clinical signs and symptoms. Factors other than infection may alter test values. For example, trauma and physical stress can cause an elevation in the number of neutrophils. A culture result may show growth of an organism in the absence of clinical signs and symptoms of infection.
TABLE 29-5 LABORATORY TESTS TO SCREEN FOR INFECTION*
| LABORATORY TEST |
NORMAL (ADULT) VALUES |
INDICATION OF INFECTION |
| C-reactive protein (CRP) |
Negative or < 5 mg/L |
Increased in acute and chronic inflammatory conditions |
| Cultures of urine and blood |
Normally sterile, without microorganism growth |
Presence of infectious microorganism growth |
| Cultures of wound, sputum and throat |
Possible normal flora |
Presence of infectious microorganism growth |
| Erythrocyte sedimentation rate |
Up to 15 mm/h for men and 20 mm/h for women |
Elevated in presence of inflammatory process |
| Iron level |
13–36 micromol/L (males)
12–31 micromol/L (females)
|
Decreased in chronic infection |
| WBC count |
4–11 × 109/L |
Increased in acute infection, decreased in certain viral or overwhelming infections |
| DIFFERENTIAL COUNT (PERCENTAGE OF EACH TYPE OF WBC) |
| Basophils |
0.06–0.1 × 109/L |
Normal during infection |
| Eosinophils |
0.04–0.4 × 109/L |
Increased in parasitic infection |
| Lymphocytes |
1.5–4.0 × 109/L |
Increased in chronic bacterial and viral infection, decreased in sepsis |
| Monocytes |
0.2–0.8 × 109/L |
Increased in protozoal, rickettsial and tuberculosis infections |
| Neutrophils |
2.0–7.5 × 109/L |
Increased in acute suppurative infection, decreased in overwhelming bacterial infection (older adult) |
Patients with infection
A patient with infection may have a variety of health problems. Infection may affect the patient and family’s physical, psychological, social or economical needs. For example, a patient with a chronic disease such as AIDS may experience serious psychological problems as a result of self-imposed isolation or rejection by their family and friends. Assessment of the patient and family’s ability to adjust to the disease and initial and ongoing resources is required.
NURSING DIAGNOSIS
During assessment, the nurse gathers objective findings (e.g. an open incision or a reduced kilojoule intake) and subjective data (such as a patient’s complaint of tenderness over a surgical wound site). The nurse then interprets the data carefully, looking for defining characteristics or risk factors that suggest a specific nursing diagnosis (Box 29-5). Data should then be validated (e.g. by inspecting the integrity of a wound) and additional data such as laboratory findings assessed. Selection of appropriate nursing diagnoses depends on correct analysis of data (Box 29-6).
BOX 29-5 NURSING DIAGNOSES
INFECTION
Body image disturbance
Infection, risk of
Injury, risk of
Nutrition, altered: less than body requirements
Oral mucous membrane, altered
Skin integrity, impaired, risk of
Social isolation
Tissue integrity, impaired
BOX 29-6 SAMPLE NURSING DIAGNOSTIC PROCESS
| INFECTION |
| ASSESSMENT ACTIVITIES |
DEFINING CHARACTERISTICS |
NURSING DIAGNOSIS |
| Check results of laboratory tests. |
White cell count < 4 × 109/L. |
Risk of infection related to lowered immunity. |
| Review current medications. |
Patient receiving azathioprine (Imuran), an immunosuppressant. |
|
| Identify potential sites of infection. |
IV. catheter in right forearm, in place for 3 days.
Foley catheter draining amber-coloured urine.
|
|
| Inspect condition of dependent pressure points. |
Area 2 cm in diameter, superficial broken skin over sacrum. |
Impaired skin integrity related to pressure and exposure to faecal irritants. |
| Observe for skin contamination. |
Patient incontinent (semi-liquid stool). |
|
The diagnosis must take into account aetiological factors in the establishment of an appropriate comprehensive plan. For example, minimising the risk of infection related to impaired skin integrity requires modified hygiene measures and wound care. Minimising the risk of infection related to malnutrition requires nutritional support and fluid balance.
Nursing diagnoses may include a risk of infection or the impact of an infection on the patient’s health status. The nurse’s success in planning appropriate nursing interventions depends on the accuracy of the diagnosis and the ability to implement effective care to meet the patient’s needs.
PLANNING
The patient’s care plan is based on each nursing diagnosis and related factors. The plan should set attainable outcomes and purposeful, appropriate interventions. Caring for a patient with a nursing diagnosis of risk of infection related to impaired skin integrity includes skin care measures to promote healing. The expected outcomes of ‘reduction in wound size by 1 cm’ and ‘absence of drainage’ enables improvements to be measured. Once these outcomes are met, the goal of ‘skin intact and without drainage’ can be set. Interventions are selected in collaboration with the patient, the family and other healthcare providers. Common goals of care may include the following:
• prevention of exposure to infectious agents
• controlling or reducing the spread of infection
• maintaining resistance to infection
• educating the patient and family about infection prevention and control techniques
• educating the patient and family about nutritional requirements for healing.
The nurse establishes priorities for the goals of care. If a patient has an open wound, suffers a debilitating disease such as cancer and has been unable to tolerate solid foods, administering therapies to promote wound healing would take priority over educating the patient to assume self-care therapies at home. When the patient’s condition improves, the priorities will change and patient education becomes an essential intervention prior to discharge.
The nurse may initiate appropriate referrals, for example to a dietitian, infection-control professional or home health nurse, to collaborate with the patient’s care. The nurse plans for the home environment to be consistent with informed infection-control practice. Educating patients and families about the importance of infection control is also an important preventive measure.
Infection-control guidelines
The development of a care plan includes infection prevention and control practices. You should refer to the current infection control guidelines published by the NHMRC (2010) in Australia and the standards for infection control published by Standards New Zealand (2008). These guidelines provide recommendations that outline the critical aspects of infection prevention and control (see Box 29-7). It is recognised that the level of risk differs in different healthcare settings and therefore some recommendations should be justified by risk assessment.
BOX 29-7 SUMMARY OF RECOMMENDATIONS OF THE AUSTRALIAN GUIDELINES FOR THE PREVENTION AND CONTROL OF INFECTION IN HEALTHCARE
STANDARD PRECAUTIONS
HAND HYGIENE
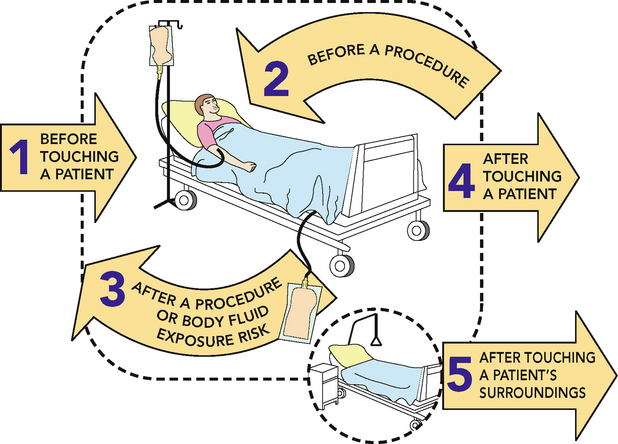
1. Routine hand hygiene
Hand hygiene must be performed before and after every episode of patient contact. This includes:
•
before touching a patient
•
after a procedure or body substance exposure risk
•
after touching a patient
• after touching a patient’s surroundings.
Hand hygiene must also be performed after the removal of gloves.
2. Choice of product for routine hand hygiene practices
For all routine hand hygiene practices in healthcare settings, use alcohol-based hand rubs that:
•
contain between 60% and 80% v/v ethanol or equivalent
•
meet the requirements of EN1500.
3. Choice of hand hygiene product when hands are visibly soiled
If hands are visibly soiled, hand hygiene should be performed using soap and water.
4. Hand hygiene for Clostridium difficile and non-enveloped viruses
Hand hygiene should be performed using soap and water when Clostridium difficile or non-enveloped viruses such as norovirus are known or suspected to be present and gloves have not been worn. After washing, hands should be dried thoroughly with single-use towels.
PERSONAL PROTECTIVE EQUIPMENT
5. Wearing of aprons/gowns
Aprons or gowns should be appropriate to the task being undertaken. They should be worn for a single procedure or episode of patient care and removed in the area where the episode of care takes place.
6. Use of face and protective eyewear for procedures
A surgical mask and protective eyewear must be worn during procedures that generate splashes or sprays of blood, body substances, secretions or excretions into the face and eyes.
7. Wearing of gloves
Gloves must be worn as a single-use item for:
•
each invasive procedure
•
contact with sterile sites and non-intact skin or mucous membranes
• any activity that has been assessed as carrying a risk of exposure to blood, body substances, secretions and excretions.
Gloves must be changed between patients and after every episode of individual patient care.
8. Sterile gloves
Sterile gloves must be used for aseptic procedures and contact with sterile sites.
HANDLING AND DISPOSAL OF SHARPS
9. Safe handling of sharps
Sharps must not be passed directly from hand to hand and handling should be kept to a minimum. Needles must not be recapped, bent or broken after use.
10. Disposal of single-use sharps
The person who has used the single-use sharp must be responsible for its immediate safe disposal. Used disposable sharps must be discarded into an approved sharps container at the point-of-use. These must not be filled above the mark that indicates the bin is three-quarters full.
ROUTINE ENVIRONMENTAL CLEANING
11. Routine cleaning of surfaces
Clean frequently touched surfaces with detergent solution at least daily, and when visibly soiled and after every known contamination.
Clean general surfaces and fittings when visibly soiled and immediately after spillage.
12. Cleaning of shared clinical equipment
Clean touched surfaces of shared clinical equipment between patient uses with detergent solution. Exceptions to this should be justified by risk assessment.
13. Surface barriers
Use surface barriers to protect clinical surfaces (including equipment) that are:
•
touched frequently with gloved hands during the delivery of patient care
•
likely to become contaminated with blood or body substances
14. Site decontamination after spills of blood or other potentially infectious materials
Spills of blood or other potentially infectious materials should be promptly cleaned as follows:
•
wear utility gloves and other PPE appropriate to the task
•
confine and contain spill, clean visible matter with disposable absorbent material and discard the used cleaning materials in the appropriate waste container
• clean the spill area with a cloth or paper towels using detergent solution.
Use of chemical disinfectants such as sodium hypochlorite should be based on assessment of risk of transmission of infectious agents from that spill.
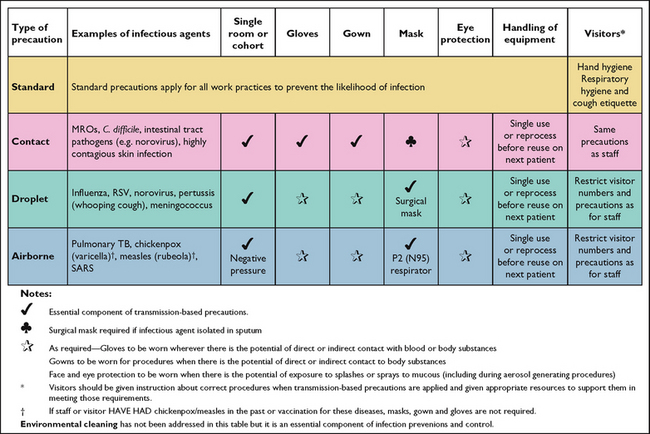
TRANSMISSION-BASED PRECAUTIONS
CONTACT PRECAUTIONS
15. Implementation of contact precautions
In addition to standard precautions, implement contact precautions in the presence of known or suspected infectious agents that are spread by direct or indirect contact with the patient or the patient’s environment.
16. Hand hygiene and personal protective equipment to prevent contact transmission
When working with patients who require contact precautions:
•
put on gloves and gown upon entry to the patient-care area
•
ensure that clothing and skin do not contact potentially contaminated environmental surfaces
•
remove gown and gloves and perform hand hygiene before leaving the patient-care area.
17. Patient-care equipment for patients on contact precautions
Use patient-dedicated equipment or single-use non-critical patient-care equipment.
If common use of equipment for multiple patients is unavoidable, clean the equipment and allow it to dry before use on another patient.
DROPLET PRECAUTIONS
18. Implementation of droplet precautions
In addition to standard precautions, implement droplet precautions for patients known or suspected to be infected with agents transmitted by respiratory droplets that are generated by a patient when coughing, sneezing or talking.
19. Personal protective equipment to prevent droplet transmission
When entering the patient-care environment, put on a surgical mask.
20. Placement of patients requiring droplet precautions
Place patients who require droplet precautions in a single-patient room.
AIRBORNE PRECAUTIONS
21. Implementation of airborne precautions
In addition to standard precautions, implement airborne precautions for patients known or suspected to be infected with infectious agents transmitted person-to-person by the airborne route.
22. Personal protective equipment to prevent airborne transmission
Wear a correctly fitted P2 respirator when entering the patient-care area when an airborne-transmissible infectious agent is known or suspected to be present.
23. Placement of patients requiring airborne precautions
Patients on airborne precautions should be placed in a negative pressure room or in a room from which the air does not circulate to other areas.
Exceptions to this should be justified by risk assessment.
24. Implementation of core strategies in the control of MROs (MRSA, MRGN, VRE)
Implement transmission-based precautions for all patients colonised or infected with a multi-resistant organism, including:
•
performing hand hygiene and putting on gloves and gowns before entering the patient-care area
•
using patient-dedicated or single-use non-critical patient-care equipment
•
using a single-patient room or, if unavailable, cohorting patients with the same strain of multi-resistant organism in designated patient-care areas
•
ensuring consistent cleaning and disinfection of surfaces in close proximity to the patient and those likely to be touched by the patient and healthcare workers.
MRO = multi-resistant organisms; MRGN = multi-resistant Gram-negative bacilli; MRSA = methicillin-resistant Staphyloccus aureus; VRE = vancomycin-resistant enterococci.; From National Health and Medical Research Council (NHMRC) 2010 Australian guidelines for the prevention and control of infection in healthcare. Canberra, NHRMC. Online. Available at www.nhmrc.gov.au/_files_nhmrc/publications/attachments/cd33_exec_summary.pdf 29 Sep 2011.
IMPLEMENTATION
By recognising and assessing each patient’s risk factors and implementing measures that break the chain of infection, the nurse can help minimise infection.
Standard and transmission-based precautions
The risk of transmitting HAIs or infectious diseases among patients is high. When a patient has a suspected or known infection, health workers follow infection-control practices. However, health workers may not be aware that some patients have infections, as the majority of organisms causing HAIs are found in the colonised body substances of patients, regardless of whether a culture has confirmed infection and a diagnosis has been made. Both New Zealand and Australia have endorsed the use of standard precautions to minimise risk of transmission of infectious organisms. Standard precautions include the use of barrier measures in all situations to prevent cross-contamination (NHMRC, 2010; Standards New Zealand, 2008). The NHMRC recommends standard precautions as the minimum requirement in work practices to promote infection control (see Box 29-8).
BOX 29-8 STANDARD PRECAUTIONS
Standard precautions include:
• hand hygiene, before and after every episode of patient contact (i.e. five moments for hand hygiene)
• the use of personal protective equipment
• the safe use and disposal of sharps
• routine environmental cleaning
• reprocessing of reusable medical equipment and instruments
• respiratory hygiene and cough etiquette
• aseptic non-touch technique
• waste management
• appropriate handling of linen.
Source: National Health and Medical Research Council (NHMRC) 2010 Australian guidelines for the prevention and control of infection in healthcare. Canberra, NHMRC. Online. Available at www.nhmrc.gov.au/guidelines/publications/cd33 16 June 2012.
Standard precautions are recommended for the treatment and care of all patients, regardless of their perceived infectious status. Standard precautions should be used in the handling of: blood, including dried blood; all other body substances, secretions and excretions (excluding sweat), regardless of whether they contain visible blood; non-intact skin; and mucous membranes (NHMRC, 2010).
• CRITICAL THINKING
You are a graduate registered nurse working in a post-anaesthesia care unit (PACU) in an adult public hospital and notice it is normal practice for nurses to highlight patients on the theatre lists with known infectious diseases such as hepatitis C or HIV with red pen and highlighter. You find that medical and nursing staff emphasise this information during hand-over (e.g. ‘This patient is an IV drug user.’) and take ‘extra precautions’ such as wearing additional PPE during care and clinical procedures with these patients.
1. Are these actions justifiable?
2. What are the possible implications of this practice for patients and staff?
Transmission-based precautions are recommended in addition to standard precautions for patients with a known or suspected infection or colonisation with highly transmissible pathogens that can cause infection (see Box 29-9 for an example). This second tier of additional precautions focuses on three transmission categories: airborne, droplet and direct or indirect contact with skin in accordance with a patient’s diagnosis and the suspected organism (see Box 29-10). Airborne precautions are implemented for infections spread in small particles in the air, such as chickenpox (varicella). Droplet precautions are implemented for infections spread in large droplets by coughing, talking or sneezing, such as rubella, pharyngitis or influenza. Contact precautions are implemented for infections spread by skin or mucous membrane contact, such as herpes simplex virus.
BOX 29-9 AIRBORNE PRECAUTIONS FOR A PATIENT WITH ACTIVE TUBERCULOSIS (TB)
The Australian infection-control guidelines recommend:
• Single patient room maintained under negative pressure
• Doors kept closed except when entering or leaving the room
• Negative pressure monitored daily using a differential pressure sensing device
• Minimum of 12 air exchanges per hour
• HEPA filtration of air to 99% clean, as it exits the isolation room
• Use of appropriate personal respiratory protective devices capable of efficient filtration of the infecting organism, applied prior to entering the room (Figure 29-7)
• Use of a mask by the patient when out of the room (with the patient leaving the room only if necessary)
• Screening of health workers for TB before commencement of employment, regular surveillance for TB, offering BCG vaccination for staff with positive Mantoux tests and follow up chest X-rays.
Regardless of the type of isolation system, the nurse must follow these basic principles:
• Wash hands thoroughly before entering and leaving the room of a patient in isolation
• Dispose of contaminated supplies and equipment in a manner that prevents spread of microorganisms to other persons as indicated by the mode of transmission of the organism
• Apply knowledge of disease processes and the mode of infection transmission when using protective barriers
• Protect all people who might be exposed during transport of patients outside their private room.
BCG = bacille Calmette-Guérin; HEPA = high-efficiency particulate air; TB = tuberculosis.; Adapted from National Health and Medical Research Council (NHMRC) 2010 Australian guidelines for the prevention and control of infection in healthcare. Canberra, NHMRC. Online. Available at www.nhmrc.gov.au/guidelines/publications/cd33 12 Mar 2012.
BOX 29-10 TRANSMISSION-BASED PRECAUTIONS
Transmission-based precautions may include one or any combination of the following:
• continued implementation of standard precautions
• appropriate use of personal protective equipment (including gloves, apron or gowns, surgical masks or P2 respirators and protective eyewear)
• patient-dedicated equipment
• allocation of single rooms or cohorting of patients
• appropriate air-handling requirements
• enhanced cleaning and disinfecting of the patient environment
• restricted transfer of patients within and between facilities.
From National Health and Medical Research Council (NHMRC) 2010 Australian guidelines for the prevention and control of infection in healthcare. Canberra, NHMRC. Online. Available at www.nhmrc.gov.au/guidelines/publications/cd33 16 Jun 2012.
Transmission-based precautions should be used in combination with standard precautions. This two-tiered approach to infection control ensures a high level of protection for patients, family members and staff involved in the care of infectious patients (see Figure 29-4).
Hand hygiene
Hand hygiene is the single most important intervention for reducing HAIs and preventing the spread of antimicrobial resistance. While hand hygiene may be the cornerstone of infection prevention and control today, it is interesting to note that the original proponents of clinical handwashing during the 19th century were rejected by the medical community (Stewardson and Pittet, 2011). Despite its central importance, compliance with hand hygiene among healthcare workers is poor (Gould and others, 2010).
The purpose of handwashing is to remove debris and transient microorganisms from the hands and to reduce total microbial counts over time. Handwashing must last for at least 15 seconds and the hands should be patted dry rather than rubbed, to preserve skin integrity. A more thorough, lengthy surgical hand and arm scrub is performed preoperatively by surgical personnel to remove transient bacteria and reduce resident hand microorganisms. Subsequent surgical hand- and arm-washing with an antimicrobial solution provides an accumulative, residual effect by lowering microbe populations.
Contaminated hands commonly cause cross-infection. For example, a nurse caring for a patient with excessive pulmonary secretions may help the patient expectorate sputum and dispose of tissues in a bedside container. The patient’s room-mate may ask the nurse to open a container of food on their meal tray; the nurse then leaves the room to prepare a dose of medication due in 5 minutes. If the nurse fails to wash hands before opening the container of food or preparing medication, organisms from the first patient’s sputum can easily be transmitted to the room-mate’s food or to the medication container.
Fingernails of health workers should be kept short, clean and free from infection. Research has linked long fingernails, artificial nails, nail additives and nail polish to the harbouring and transmission of pathogens, including bacteria and fungi (Boyce and Pittet, 2002; Grayson and others, 2010).
National programs established in both Australia (Hand Hygiene Australia, 2012) and New Zealand (Hand Hygiene New Zealand, 2009) have been developed to target hand hygiene behaviour. Consistent with the World Health Organization guidelines (WHO, 2009), both countries advocate, and have incorporated into their respective national hand hygiene programs, the ‘five moments for hand hygiene’ (Figure 29-5) as critical to the prevention and control of infections.
Effective handwashing should last at least 15 seconds unless the hands are visibly soiled, in which case additional time may be required. Routine hand hygiene (Skill 29-1) may be performed with antimicrobial soap, an alcohol-based hand rub or antimicrobial solution. Following washing, the hands should be thoroughly dried with a disposable towel. The towel or elbow should be used to turn off the tap. Using hot water should be avoided, as repeated exposure may increase the risk of dermatitis (Boyce and Pittet, 2002). Fingernails should be cleaned with a disposable nail pick at the commencement of the shift. The use of scrub brushes is not recommended unless hands are grossly soiled; brushes can cause microscopic abrasions to the outer layer of the skin, breaching the protective barrier and providing a portal of entry for normal flora to penetrate, thus increasing the risk of allergic reactions to antimicrobial solutions.
SKILL 29-1 Handwashing
DELEGATION CONSIDERATIONS
All staff caring for patients must wash their hands using correct handwashing techniques. All staff and carers should:
• Gain instruction and demonstrate competency in correct handwashing techniques.
• Report any skin irritation from soaps or antimicrobial solutions immediately.
• Gain instruction regarding the importance of caring for and maintaining the integrity of their skin.