7 PAIN
INTRODUCTION
Of all the sensations that arise from the human body, pain is one of the most clinically important. It is the sensation of pain that often motivates people to seek medical help as a result of a traumatic injury or a progressive disease,1 and it is often a consequence of surgical interventions used to treat injuries or disease. Pain is also clinically important as it encourages patients to adopt behaviours that enhance healing (such as limb immobilisation following a fracture).
Pain also has a very important physiological role, as it acts as the conditioning stimulus that teaches us to avoid environmental stimuli that cause harm. As children, we learn not to touch sharp objects because it hurts, and even as adults this sensation helps us to adapt to new or unusual environments. For example, as a postgraduate student in England I remember staring in disbelief as a visiting scientist from Australia (wearing only shorts) walked into a bush of head-high stinging nettles to retrieve a Frisbee. Never having been exposed to the leaves of this plant, he had no idea of the effect that they have on exposed skin — until he emerged screaming in agony from the bushes that many of us had learned as children cause intense pain.
In this chapter we consider the physiological basis of pain and then explore some of the clinical consequences of this very important sensation. However, the focus of this chapter is not to explore different types of pain that patients may experience — such as headaches, angina or postsurgical pain — but to detail how pain comes about, how it is detected and the neural pathways involved in the transmission of pain signals. We start with an overview of the biopsychosocial phenomenon of pain.
THE DEFINITION OF PAIN
Pain is a complex and highly subjective sensation that is affected by a large number of variables and consequently can be difficult to describe. In 1979 the International Association for the Society of Pain (IASP) defined pain is ‘an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage’.2 Although this definition is a little cryptic it does highlight some of the key concepts related to pain. So to help you understand some of the most important elements of this complex sensation, let us have a closer look at this definition.
The first thing that the IASP definition reminds us of is that pain is unpleasant. Whether the pain is caused by touching something hot or is the result of a surgical procedure or the growth of a tumour, the experience is likely to be a negative one. Although the intensity of the experience may vary from individual to individual and has been shown to be influenced by sex, race, age, culture, beliefs, previous experience, fear and anxiety,3–5 4 6 pain is invariably unpleasant and therefore is something we avoid or try to minimise.
The second concept that emerges from the definition is that pain is a sensory experience. In some respects pain is no different from other senses such as hearing, taste or vision. Just as we can look up into the sky at night and define the position of a star, describe how bright it is and even attribute it with a colour, so we can identify the site and intensity of a painful stimulus applied to our bodies. Humans are, in general, very good at identifying the site of an injury that causes pain and can readily distinguish between stimuli of different intensities and indeed modalities. A pin prick applied to the anterior surface of the arm is thus easily distinguishable from a drop of acid applied to the wrist, not just by location but by the sharp quality of the former and the burning sensation produced by the latter. Indeed, psychophysical experiments using carefully controlled thermal stimuli have shown that humans can distinguish between two high-intensity thermal stimuli that differ by as little as 0.1°C.6 This ability to locate a painful stimulus and describe both its intensity and its quality is referred to as the sensory-discriminative aspect of pain. These sensations are controlled through afferent nerves fibres, the spinal cord, brainstem and higher brain centres (see Chapter 6), which results in prompt withdrawal from the painful stimulus.
The definition highlights that pain is much more than a sensation — it is also an emotional experience. Because it is unpleasant it elicits an emotional response that produces changes in both mental (affective) state and behaviour (motivation). Individuals exposed to a painful stimulus become anxious, tense, distressed or scared and, if the pain is ongoing, they may become depressed, develop a feeling of hopelessness and, ultimately, perhaps consider suicide. In addition, individuals are motivated to remove themselves from the source of the pain, avoid similar environments in the future and/or adopt behaviours that enhance healing. The degree to which these responses manifest themselves depends on a number of factors, including the intensity of the pain, its duration, the individual’s past experience and the availability of treatment. However, it is the emotional aspect of pain that predominates in severe and/or ongoing pain. The changes in mental state and behaviour that are part of the emotional component of pain are collectively referred to as the affective-motivational aspect of pain. These behaviours are controlled through the interaction of the reticular activating system, the limbic system and the brainstem.
The next part of the definition states that pain is caused by ‘actual or potential tissue damage’. This serves to remind us of two very important components about pain. First, pain is normally produced by stimuli that are sufficiently intense to cause peripheral tissue damage — that is, damage to tissues such as the skin, muscles or internal organs. That is why cutting the skin, reducing blood flow to the heart or damaging the wall of the digestive tract all cause pain, because they result in ‘actual’ tissue damage. In other words, the stimulus is sufficient to damage tissue, which we are aware of because it causes pain. Second, pain may result from stimuli that have the ‘potential’ to cause tissue damage. Therefore, touching something hot or stepping on a sharp object may cause pain, but little or no tissue damage, because the rapid withdrawal reflex removes your limb from the pain source (see Chapter 6). This is possible because the threshold for pain in some tissues is lower than the threshold for tissue damage, so behavioural modifications allow us to remove ourselves from the source of the stimulus or adopt behaviours that minimise tissue damage. Therefore, one important function of pain is to advise us of the presence of stimuli that could cause us harm and thereby provide us with the opportunity to prevent it happening (or reduce its impact).
The last part of the definition is probably the most cryptic as it is not immediately obvious what is meant by the phrase ‘or described in terms of such damage’. We know that pain is usually caused by stimuli that cause tissue damage (or have the potential to), but this part of the definition implies that you can experience pain that feels like it is caused by tissue damage without there being any peripheral tissue damage. Although this sounds counterintuitive, this is exactly the point. People can experience pain in parts of their body that do not exhibit any sign of disease or trauma. For example, a patient may complain of a severe burning pain in their left foot without the limb having been exposed to a heat source or showing any signs of peripheral tissue damage. The pain feels like it was caused by a burn injury, but there is no evidence of any peripheral tissue damage. In the absence of any physical evidence, in the past individuals experiencing this type of pain were sometimes considered hysterical or delusional and the pain was attributed to a psychological disorder. We now know that most such types of pain are the result of damage to the parts of the peripheral and central nervous systems responsible for sensations that arise from the affected tissue. Thus, although there is no peripheral tissue damage, the pain feels like it is caused by such damage.
One of the factors that can contribute to the variable nature of the pain experienced is that the pain can be modified. In other words, the intensity of the sensation can be affected by behaviour, cognitive factors and clinical intervention. For example, in response to minor burns or cuts many people immediately rub the site of the injury. We do this because we have learned that this reduces the intensity of the pain (i.e. produces analgesia). Furthermore, soldiers in combat situations may suffer quite severe injuries but experience comparatively little pain because of the stress associated with the life-threatening situation. This type of pain modification is not limited to the battlefield, as anyone who has watched any Australian or New Zealand football code can attest. Analgesia can occur in trance-like states associated with certain religious and cultural rituals, and in the clinical environment analgesia can be produced by pharmacological agents, such as opioids, but also through other avenues, such as transcutaneous electrical nerve stimulation, acupuncture and cognitive behaviour modification.
Collectively, pain can be considered a negative multidimensional experience that is typically associated with peripheral tissue damage but in some pathological circumstances may exist in the absence of tissue damage. As a subjective experience, pain is highly variable and may be modified by cultural, situational and psychological as well as clinical interventions.
Just how important pain is to the wellbeing of humans is perhaps best illustrated by considering the rather serious consequences of being unable to feel pain. Individuals born with a congenital insensitivity to pain suffer horrendous injuries because they are unaware of the damage they are doing to their bodies when, for example, they sit too close to a fire or bite into their tongue while eating (see Figure 7-1).7,8 They do not make the regular adjustments to their posture necessary to avoid damaging joints and can be completely oblivious to internal damage caused by disease. Not surprisingly, these types of injuries often become infected, are slow to heal and can be life-threatening.
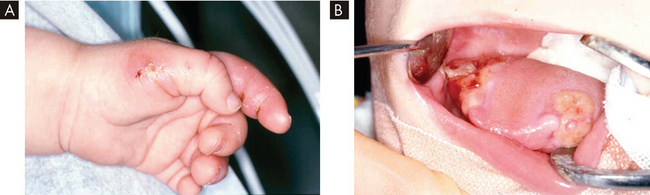
FIGURE 7-1 Congenital insensitivity to pain.
Photographs illustrating tissue damage in a 9-month-old boy with congenital insensitivity to pain. A Damage to the left thumb and index finger caused by biting. B Severe mutilation of the tongue.
Source: Butler J, Fleming P, Webb D. Congenital insensitivity to pain: review and report of a case with dental implications. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006; 101(1):58–62.
TYPES OF PAIN
It can greatly aid clinical practice if we classify different types of pain. This allows the clinician to distinguish the origin of the pain and, importantly, ascertain effective treatment options. Most common types of pain fall into one of three categories that vary in terms of their aetiology (or cause), duration and ease of treatment.
Nociceptive pain
Nociceptive pain is the most common type of pain and its defining characteristic is that it is produced by nociceptive stimuli, which cause or have the potential to cause peripheral tissue damage. Nociceptive pain can be subdivided into two subtypes:
 External damage. Pain due to external damage is the most common form of nociceptive pain and you are likely to have experienced it. As the name implies, this type of pain usually involves trauma to the skin but may extend to the underlying tissues. It is relatively mild and has a comparatively short time course, lasting a few seconds to a few days. Treatment of this type of pain usually involves simple interventions that assist the healing process of the affected tissues. Moreover, pain relief is relatively easy to achieve through the use of milder forms of analgesics, such as non-steroidal anti-inflammatory drugs (NSAIDs).
External damage. Pain due to external damage is the most common form of nociceptive pain and you are likely to have experienced it. As the name implies, this type of pain usually involves trauma to the skin but may extend to the underlying tissues. It is relatively mild and has a comparatively short time course, lasting a few seconds to a few days. Treatment of this type of pain usually involves simple interventions that assist the healing process of the affected tissues. Moreover, pain relief is relatively easy to achieve through the use of milder forms of analgesics, such as non-steroidal anti-inflammatory drugs (NSAIDs). Internal damage. Pain due to internal damage is less common and usually more severe than that associated with external damage. It has numerous causes. One of the principal causes is severe trauma, for instance due to bone fractures, surgery or childbirth. This type of pain is also associated with disease. In fact, pain is a symptom of virtually every disease (e.g. cancer, arthritis) at some point during the disease progression. The duration is usually quite a bit longer than that following external injury and typically is in the order of a few days to weeks. Treatment involves removing the cause of the tissue damage, but interim management of pain can be achieved with more powerful narcotic analgesics, such as opioids.
Internal damage. Pain due to internal damage is less common and usually more severe than that associated with external damage. It has numerous causes. One of the principal causes is severe trauma, for instance due to bone fractures, surgery or childbirth. This type of pain is also associated with disease. In fact, pain is a symptom of virtually every disease (e.g. cancer, arthritis) at some point during the disease progression. The duration is usually quite a bit longer than that following external injury and typically is in the order of a few days to weeks. Treatment involves removing the cause of the tissue damage, but interim management of pain can be achieved with more powerful narcotic analgesics, such as opioids.Neuropathic pain
As the term implies, neuropathic pain is caused by injury or disease of the nervous system rather than a peripheral tissue.9 Fortunately, this type of pain is less common than nociceptive pain. Neuropathic pain is usually more severe and has a time course that can last from a few months to many years — and sometimes the rest of a person’s life. Because of the complex aetiology and the lack of knowledge of the underlying mechanisms, treatment is challenging. In some cases pharmacological interventions, particularly when used in combination, produce positive outcomes for patients. However, for others, pain relief is less satisfactory and specialised ongoing therapies are required in an effort to alleviate the effects of neuropathic pain. Forms of neuropathic pain are dealt with in more detail later in this chapter.
Psychogenic pain
It is well recognised that some people report pain that may be severe and persistent but for which there appears to be no underlying pathology. The pain experienced by these patients (typically headaches, abdominal pain, back pain) is indistinguishable from that experienced by people with identifiable injuries or disease. Such pain can be debilitating and consequently interferes with their ability to function normally. In the absence of any physical explanation for the symptoms, despite exhaustive clinical examination and testing, the assumption is made that the pain is the result of a psychological disorder, so it is termed psychogenic pain. It is very likely that some pain of neuropathic origin was at one time incorrectly diagnosed as psychogenic pain due to similarities in symptoms and the absence of any visible cause. However, due to improvements in diagnosis and more sophisticated medical imaging, diagnosis of psychogenic pain is less common.10
PAIN TERMINOLOGY
Whereas some types of pain have a relatively short time course, others last a very long time indeed. In order to discriminate between these types of pain, the terms acute pain and chronic pain are used. Acute pain refers to any pain that lasts less than 3 months. In contrast, chronic pain is any pain that lasts longer than 3 months. The timelines outlined in the previous section suggest that most neuropathic pain is chronic pain, and although this is often the case, the two terms should not be used interchangeably. The terms acute and chronic refer exclusively to the time course of the pain, irrespective of aetiology. For instance, neuropathic pain that resolves within 3 months is considered acute, while cancer-related nociceptive pain that persists for more than 3 months is chronic. Approximately one-fifth of Australians suffer from chronic pain11 and are about 5 times more likely to use health-care services than those without chronic pain.12 Therefore, patients with chronic pain are a concern in the health-care industry and you are likely to encounter them in all areas of the health sector.
Another important aspect of pain is the location of the pain. Typically, neuropathic pain feels like it is coming from the part of the body that the affected nerve innervates. For example, a compression injury of branches of the sciatic nerve (shown in Figure 6-15) caused by damage to a vertebral disc can result in pain in the leg or foot. Interestingly, this disconnection between injury site and pain location is not restricted to neuropathic pain, but also occurs in some forms of nociceptive pain. For example, the first symptom of appendicitis is usually tenderness in the midline abdominal region, despite the inflamed appendix being located in the right lower quadrant (see Chapter 27). Similarly, one of the classical signs of myocardial infarction (see Chapter 23) is a shooting pain referred to the left shoulder or arm, when the tissue damage is actually occurring in the heart (see Figure 7-2). We refer to this type of pain as referred pain.
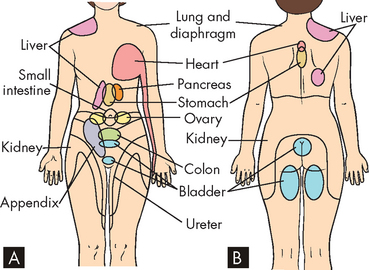
FIGURE 7-2 Visceral referred pain.
Examples of some of the typical sites of referred pain from visceral structures on A the anterior and B the posterior surface of the body.
Acute pain Pain that lasts less than 3 months.
Allodynia Pain due to a stimulus that does not normally provoke pain.
Analgesia Absence of pain in response to a stimulus that would normally be painful.
Chronic pain Pain that lasts longer than 3 months.
Hyperalgesia An increased response to a stimulus that is normally painful.
Referred pain Pain perceived as occurring in a region of the body topographically distinct from the region in which the actual source of pain is located.
Source: Merskey H, Bogduk N (eds). Classification of chronic pain. 2nd edn. Seattle: IASP Press; 1994.
Following injury the affected tissue becomes very sensitive to subsequent stimuli. Thus, we tend to protect a bruised heel or burnt hand because any additional stimulation greatly enhances the pain. The increased sensitivity that follows peripheral tissue damage can be attributed to two factors: there can be an amplified neural response to any subsequent painful stimulus and/or a decrease in the threshold such that previously non-painful stimuli now cause pain. To accommodate these two different characteristics we use the term hyperalgesia to describe the situation where the original tissue damage augments the pain produced by subsequent high-intensity (damaging) stimuli. The term allodynia refers to a reduction in the threshold such that pain is now produced by low-intensity (non-damaging) stimuli. These changes in sensitivity occur in nociceptive pain and many forms of neuropathic pain. For example, individuals with trigeminal neuralgia (an alteration to cranial nerve V causing episodes of excruciating pain to the face) rarely wear high collars or scarves because of mechanical allodynia, where even the gentle touch of such clothing can elicit excruciating pain referred to the face.13
THE PHYSIOLOGY OF PAIN
Since pain is the conscious perception of a stimulus that causes tissue damage, this means that the presence of the stimulus has to be detected in the tissue and then information about the tissue damage relayed to the cerebral cortex, where it is consciously perceived as pain. In order to enable this, small patches of each peripheral tissue (skin, muscle and viscera) are connected to regions of the cerebral cortex by a three-neuronal pain pathway (see Figure 7-3). The first-order neuron in this pathway carries the information from the periphery to the spinal cord, where it synapses with the second-order neuron. This second-order neuron relays the information to a number of sites in the brain. Some of the brain sites are involved in the autonomic nervous system reflex responses to injury (such as increases in cardiac output or ventilation rate). However, conscious perception of tissue damage is transmitted from the thalamus via the third-order neuron, which relays the information to the cerebral cortex. It is here that the individual becomes aware of the pain. Large numbers of these three-neuronal relays are arranged in parallel to ensure that most tissues in the body are connected to the cerebral cortex. The structure and function of each of these three classes of neuron are considered in more detail in the following section.
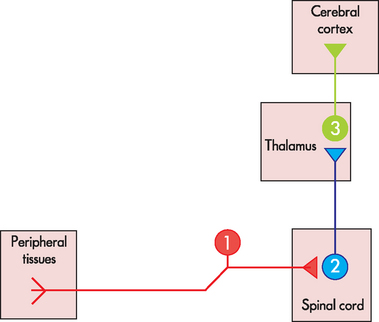
FIGURE 7-3 Basic arrangement of the pain pathway.
Schematic diagram showing that the pain pathway consists of three neurons, which connect peripheral tissues (skin, muscle and viscera) with the cerebral cortex. Large numbers of these three neuronal pathways are arranged in parallel to ensure that most tissues in the body are connected to the cortex.
Nociceptors
Nociceptors are the first-order neurons in the pain pathway. They are a subpopulation of the primary sensory neurons that collectively are responsible for the detection of all sensations, such as touch, temperature, muscle length and joint position (see Chapter 6). Like other primary sensory neurons, they have a cell body located in the posterior root ganglia, just outside the spinal cord, and an axon that runs from the peripheral tissue through a peripheral nerve and terminates within the superficial layers of the posterior horn of the spinal cord (see Figure 7-4).
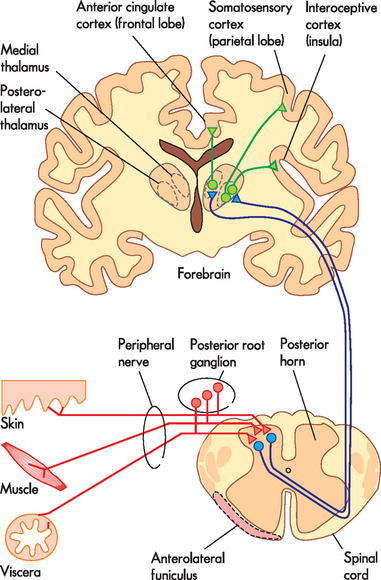
FIGURE 7-4 Detailed arrangement of the pain pathway.
The relationship between nociceptors (red), spinothalamic tract neurons (blue) and thalamocortical neurons (green) as they course through the peripheral and central nervous systems.
The important thing about nociceptors is that they are selectively activated by high-intensity stimuli that have the potential to cause tissue damage. Thus, they are not activated by low-intensity (innocuous) stimuli, and only respond when the stimulus is of sufficient intensity to threaten the integrity of the tissue they innervate.
In addition, tissue damage leads to the release of substances that produce pain. These substances are intricately involved in the inflammation process (see Chapter 13 for more detail) and often cause a cascade of inflammatory events, such as vasodilation, local swelling and stimulation of nociceptors. The most recognised substances are bradykinin, histamine and prostaglandin. Some substances are released by cells that migrate to the injured site, such as mast cells and white blood cells, while other substances are released by the tissues themselves. These substances flood the injured site during the inflammatory process and have been referred to as a ‘soup’ (see Figure 7-5). Collectively, they stimulate nociceptors, which transmit the pain signals to the brain and then the individual becomes aware of the injured tissue.
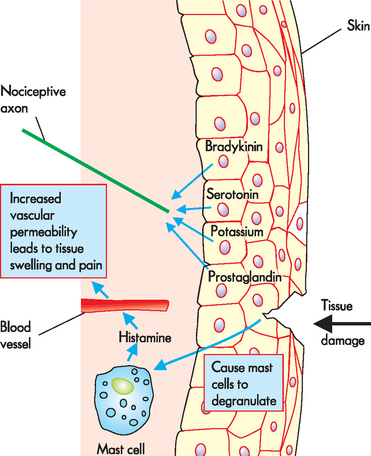
FIGURE 7-5 Tissue damage leading to inflammation and stimulation of nociceptors.
In this example, tissue damage has occurred to the skin causing the release of a ‘soup’ of substances, namely bradykinin, serotonin, potassium and prostaglandin, which stimulate nociceptors. Mast cells degranulate (release the contents of) histamine when triggered by tissue damage, which stimulates the nerve ending but also causes the blood vessels to allow fluid to leak into the extracellular space, causing swelling and more pain stimulation.
Source: Based on Boron WF, Boulpaep EL. Medical physiology: a cellular and molecular approach. 2nd edn. Philadelphia: Saunders; 2009.
Not surprisingly, nociceptors have been identified in virtually every peripheral tissue from which we experience pain. However, a lot more is known about the properties of the nociceptors responsible for sensations that arise from the skin than from other structures. Therefore, we begin by examining the properties of these cutaneous nociceptors before considering what is known about the neurons responsible for detecting tissue damage in deeper tissues such as muscle and viscera.
Cutaneous nociceptors
It is well-established that there are two distinct pain sensations — sharp pain and burning pain — that differ in their timing and quality. These can be clearly demonstrated by a mechanical injury such as that produced by a paper cut or pin-prick. Very shortly after a mechanical injury we experience a pain that has a ‘sharp’ quality. A few seconds later we experience a second pain sensation that is usually described as ‘burning’. The burning pain can also be elicited by exposure to high temperatures and by the application of pain-producing chemicals such as acid. Underlying these two sensations are two classes of nociceptor in the skin that are quite distinct in structural and functional properties: high-threshold mechanoreceptors and polymodal nociceptors (see Table 7.1).
Table 7-1 COMPARISON OF THE STRUCTURAL AND FUNCTIONAL PROPERTIES OF CUTANEOUS NOCICEPTORS
| FEATURE | HIGH-THRESHOLD MECHANORECEPTORS | POLYMODAL NOCICEPTORS |
|---|---|---|
| Axon diameter | Small | Small |
| Myelin sheath | Yes | No |
| Conduction velocity | 5–15 m/sec | 0.5–2.5 m/sec |
| Activated by low-threshold stimulus | No | No |
| Activated by mechanical injury | Yes | Yes |
| Activated by thermal injury | No | Yes |
| Activated by damaging chemicals | No | Yes |
High-threshold mechanoreceptors
Structurally, high-threshold mechanoreceptors have small-diameter myelinated axons in which action potentials travel along their length at around 5–15 m/sec. The axons terminate within the dermis of the skin as simple free nerve endings that resemble small knob-like swellings at their ends. These endings are the most common type of nerve terminal and are referred to as unencapsulated, which means that the ending is not enclosed in a connective tissue capsule.
As the name suggests, high-threshold mechanoreceptors are activated by high-intensity mechanical stimuli such as a pin-prick, cutting or pinching the skin. They are completely unresponsive to low-threshold stimuli (touch, vibration, warmth) or to painful thermal or chemical stimuli.
Clearly this class of cutaneous nociceptor is very well-suited to detecting mechanical injury of the skin as they are activated only by mechanical stimuli at intensities high enough to cause damage. In addition, because these neurons have myelinated axons they are able to relay information about a mechanical injury to the spinal cord rapidly. For these reasons, high-threshold mechanoreceptors are responsible for detecting the fast, sharp pain that follows a mechanical injury of the skin.
Polymodal nociceptors
Like high-threshold mechanoreceptors, cutaneous polymodal nociceptors have free nerve endings in the dermis, with some projecting into the epidermis. Therefore, they are well-positioned to detect stimuli affecting the skin. They have small-diameter axons but no myelin sheath and consequently action potentials travel along these unmyelinated axons relatively slowly (0.5–2.5 m/sec) compared to high-threshold mechanoreceptors.
As their name suggests, this class of nociceptors respond to multiple modalities of high-intensity stimuli. They respond to the same high-intensity mechanical stimuli that activate high-threshold mechanoreceptors but in addition are activated by high temperatures (above 45°C) and a range of chemicals (such as acid). This is important, because these intense thermal and chemical stimuli will cause pain when exposed to the skin. Thus, this class of nociceptor is truly polymodal in that they respond to mechanical, thermal and chemical stimuli of sufficient intensity to cause damage to the skin. As polymodal nociceptors are activated by high-intensity mechanical stimuli, but have unmyelinated axons and hence a slow conduction velocity, they are responsible for detecting the slow-burning pain that follows mechanical injury of the skin. In addition, the burning sensation that is characteristic of heat or chemical injury to the skin is relayed by these receptors.
A mechanical injury such as a cut will, of course, activate both high-threshold mechanoreceptors and polymodal nociceptors in the skin at exactly the same time. However, because action potentials travel along myelinated axons much more quickly than along unmyelinated axons, the information carried by the high-threshold mechanoreceptors reaches the spinal cord before that carried by the polymodal nociceptors. This time difference is maintained all the way through to the cerebral cortex, where the sensation of pain is first perceived. Therefore, high-threshold mechanoreceptors transmit the fast, sharp pain and this is followed a couple of seconds later by a burning sensation because of the activity of polymodal nociceptors (slow-burning pain).
Musculoskeletal and visceral nociceptors
Studies of nociceptors in deeper tissues (muscles, joints and visceral organs) suggest that the vast majority have unmyelinated axons and therefore relatively slow conduction velocities.14–16 4 6 Some of these are activated by high-intensity mechanical stimuli as well as a variety of pain-producing chemicals. Although the temperature sensitivity of these neurons can be difficult to test, many of them respond to high temperatures, suggesting that they are like polymodal nociceptors. As these neurons are not activated by low-intensity stimuli they are very well-suited at detecting the twisting of joints, the distension of visceral organs and ischaemia, which often causes pain in deeper tissues.
A new class of nociceptor has been identified in joints, muscle and visceral organs that is completely insensitive to stimulation in normal healthy tissue. These nociceptors cannot be activated by either low-intensity or high-intensity stimulation and are called silent (or ‘sleeping’) nociceptors.16 However, if the peripheral tissue these neurons innervate becomes inflamed, they are activated and may even begin to respond to relatively low-threshold stimuli — that is, stimuli that would not cause pain in healthy tissue. Interestingly, these neurons do not appear to have any physiological role but instead are responsible for the sometimes debilitating pain that is often associated with tissues that become inflamed because of damage or disease.
Spinothalamic tract neurons
The nociceptors that relay action potentials intersect with the spinal cord. The ascending pathway connects the spinal cord with the thalamus and consequently is referred to as the spinothalamic tract (refer to Figure 6-19). Spinothalamic tract neurons are second-order neurons in the pain pathway and they receive information about peripheral tissue damage from nociceptors and relay it to the thalamus.
Spinothalamic tract neurons have their cell bodies located in the superficial layers of the grey matter of the posterior horn of the spinal cord (see Figure 7-4). Therefore, they are well-positioned to receive the inputs from the nociceptors that terminate there. The axons of spinothalamic tract neurons cross the midline of the spinal cord underneath the central canal and then ascend along the length of the spinal cord in the white matter on the opposite side. The axons of spinothalamic tract neurons from different parts of the body appear to run together in a white matter tract (the funiculus, which translates as ‘slender cord’), which is located between the anterior funiculus and lateral funiculus and is known as the anterolateral funiculus (see Figure 7-4).
The axons of spinothalamic tract neurons project out of the spinal cord, through the brainstem and terminate in the thalamus. The thalamus is a large, oval-shaped structure located deep within the forebrain underneath the cerebral hemispheres and lateral to the third ventricle (see Figure 7-4). It is a major component of the diencephalon, and receives information from all the senses (except smell) and passes this on to the cerebral cortex. The thalamus is made up of a large number of nuclei, named according to their anatomical position. Spinothalamic tract neurons terminate in two structurally distinct regions of the thalamus. Some neurons terminate in a group of nuclei in the posterolateral (back and to the side location) part of the thalamus, while another population synapse in the medial (towards the midline) region (see Figure 7-4). These two different termination sites form the basis for projections from the thalamus to the cerebral cortex.
Thalamocortical neurons
The third-order neurons of the pain pathway collect the information relayed to the thalamus by spinothalamic tract neurons and relay this to the cerebral cortex, and so are referred to as thalamocortical neurons. As there are two thalamic sites that receive information about peripheral tissue damage from the spinothalamic tract, there are two distinct populations of thalamocortical neurons:
 Thalamocortical neurons with their cell bodies in the posterolateral parts of the thalamus are activated by high-intensity stimulation of small, well-defined regions in the periphery and relay this information in a highly ordered fashion to the somatosensory cortex of the parietal lobe and the adjacent insular cortex. The insula is the lobe of the cerebral cortex hidden behind the parietal and temporal lobes, and the region where these lateral thalamocortical neurons terminate is referred to as the interoceptive cortex (see Figure 7-4) as it receives sensory information about the physiological condition of the whole body.
Thalamocortical neurons with their cell bodies in the posterolateral parts of the thalamus are activated by high-intensity stimulation of small, well-defined regions in the periphery and relay this information in a highly ordered fashion to the somatosensory cortex of the parietal lobe and the adjacent insular cortex. The insula is the lobe of the cerebral cortex hidden behind the parietal and temporal lobes, and the region where these lateral thalamocortical neurons terminate is referred to as the interoceptive cortex (see Figure 7-4) as it receives sensory information about the physiological condition of the whole body. Thalamocortical neurons that are located in the medial thalamus tend to be activated by painful stimulation of large areas of the body — for instance, a whole limb — and relay this information to the anterior cingulate cortex of the frontal lobe (see Figure 7-4).
Thalamocortical neurons that are located in the medial thalamus tend to be activated by painful stimulation of large areas of the body — for instance, a whole limb — and relay this information to the anterior cingulate cortex of the frontal lobe (see Figure 7-4).Cortical representation of pain
There are three known cortical regions that are responsible for the perception of pain: the lateral pain pathway that terminates in the parietal and insular lobes, and a medial pain pathway that terminates in the frontal lobe. Functional brain imaging and analyses of patients with damage to these different cortical areas suggest that they are responsible for different aspects of the pain experience.
Brain imaging analyses of both somatosensory and interoreceptive cortices have revealed that they are activated by painful peripheral stimuli.17 In addition, electrical stimulation of the interoreceptive cortex in conscious humans has shown that the pain is located in well-defined peripheral tissues.18 This is supported by the observation that damage to this part of the cortex interferes with pain perception.19 These observations have led to the suggestion that the lateral pain pathway is responsible for the sensory-discriminative aspect of pain (i.e. the position, intensity and modality of the tissue damage).
Analysis of the anterior cingulate cortex with functional imaging has revealed that it is activated by peripheral tissue damage and that these responses can be alleviated by hypnosis.20,21 Furthermore, patients who have damage in this region can still feel pain but are less troubled by it.22 Therefore, it is believed that the medial pain pathway is involved in the affective-motivational aspect of pain.
Neuromodulation of pain
As well as the neural pathways, several substances are capable of altering the pain pathways. Collectively, these are referred to as neuromodulators. They are found in the pathways that control information about painful stimuli throughout the nervous system. Neuromodulators are substances, other than neurotransmitters, which are released by neurons and transmit signals to other neurons that change the activities of these neurons. It is thought that neuromodulators are released in response to tissue injury (such as prostaglandins, bradykinin) and chronic inflammatory changes (cytokines; see Chapter 12). Excitatory neuromodulators (ones that propagate the action potential that excites the neuron) include substances such as glutamate, substance P, somatostatin and vasoactive intestinal polypeptide. Inhibitory neuromodulators (ones that delay or stop nerve transmission) include gamma-aminobutyric acid (GABA), 5-hydroxytryptamine (serotonin), noradrenaline and endorphins.
BOX 7-2 MECHANISMS OF REFERRED PAIN
Although the origin of cutaneous and musculoskeletal pain is usually fairly well-defined (i.e. we can determine the origin of the pain fairly well), visceral pain can be a little more difficult to localise. For example, pain from the heart is often referred to the left shoulder and arm. It is thought that when many nociceptors from different peripheral tissues merge onto the same spinothalamic tract neurons, the pain will be referred. So, a population of spinothalamic tract neurons may receive input from nociceptors in the left arm as well as the heart. Typically, they are activated by injury of the skin or muscles of the left arm, and the cerebral cortex associates activity in these neurons with arm pain. However, the cerebral cortex cannot distinguish between this and activation of the same neurons by nociceptors in the heart, so the angina pain is referred to the left arm. The exact reason for this is not known.
Endorphins (morphines produced by the body) inhibit transmission of pain impulses in the spinal cord and brain by binding to opioid receptors. These are receptors in the central nervous system that block pain transmission. You may be familiar with the term endorphins as they are released during exercise and produce the ‘runner’s high’ that gives the exercising individual a sense of euphoria, despite being physically drained and experiencing pain. It is thought that endorphins attach to the limbic and prefrontal areas of the brain (involved in emotion), which alters the individual’s mood. Beta-endorphin is a potent substance, released from the hypothalamus and pituitary gland, which results in analgesic effects. It may also be responsible for general sensations of wellbeing. Enkephalin, found in the neurons of the brain and spinal cord, is a weaker analgesic than other endorphins but is more potent and longer lasting than morphine.
All endorphins attach to opiate receptors on the cell membrane of the afferent neuron (see Figure 7-6) and inhibit the release of excitatory neurotransmitters. Opiate drugs relieve pain by attaching to the opiate receptors and enhancing the natural endorphin response. Stress, excessive physical exertion, acupuncture, sexual intercourse and other factors increase the levels of circulating endorphins, serotonin, noradrenaline and other neurotransmitters, thereby raising the pain threshold.
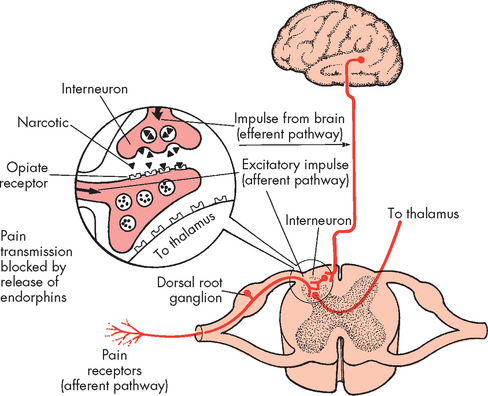
FIGURE 7-6 Descending pathway and endorphin release.
Endorphin receptors are located close to the known pain receptors in the peripheral tissues and pain pathways in the spinal cord.
CLINICAL MANIFESTATIONS OF PAIN
Pain is complex and highly subjective, meaning that no two people are likely to experience the same level of pain for a given painful stimulus. Furthermore, pain has cognitive, affective, behavioural, social and cultural implications beyond sensory recognition. For instance, pain tolerance differs between individuals. Pain tolerance is the amount of time or the intensity of pain that an individual will endure before initiating overt pain responses. Pain tolerance varies greatly among individuals, as well as in the same individual over time, because of the body’s ability to respond differently to noxious stimuli. It is influenced by cultural perceptions, expectations, role behaviours, gender and physical and mental health.23 It generally decreases with repeated exposure to pain, fatigue, anger, boredom, apprehension and sleep deprivation; and may increase with increased alcohol consumption, medication, hypnosis, warmth, distracting activities and strong beliefs or faith.
The pain threshold is the lowest intensity at which a stimulus is perceived as pain and may be influenced by genetics.24 Intense pain at one location may increase the threshold in another location. For example, a person with severe pain in one knee is less likely to experience chronic back pain that is less intense. Therefore, an individual with many painful sites may report only the most painful one. Then, when the dominant pain is diminished, the individual identifies other painful areas.
While pain is a subjective symptom, it may result in changes in the level of autonomic nervous system activation. Patients often experience an increase in heart rate, blood pressure, ventilation, nausea and vomiting, as well as sweating, when experiencing pain, especially acute surgical pain. However, these clinical signs are not universal in all individuals who are experiencing pain. When there is damage to the internal organs, this can also result in activation of the sympathetic nervous system, causing an elevation in cardiovascular and respiratory responses.
EVALUATION AND TREATMENT
The evaluation of pain is often difficult. Nurses are usually the primary clinicians responsible for assessing a patient’s pain. Foremost in the evaluation of pain is to listen to, and believe, what the patient says about their level of pain. There is no single test that assesses an individual’s level of pain and, despite numerous measures to provide an objective measure of pain, the most useful is asking the patient to relate the location, type and duration of the pain, as well as any therapies previously used to effectively alleviate pain.
As well as receiving information from the patient about their pain, there are numerous pain measurement tools that the clinician can use to obtain more information about the patient’s pain (see Figure 7-7). These can be used to assess changes in the patient’s level of pain and response to therapies. In addition, facial pain scales can be used for individuals who may not be able to vocalise their feelings of pain or who cannot speak (for example, children).
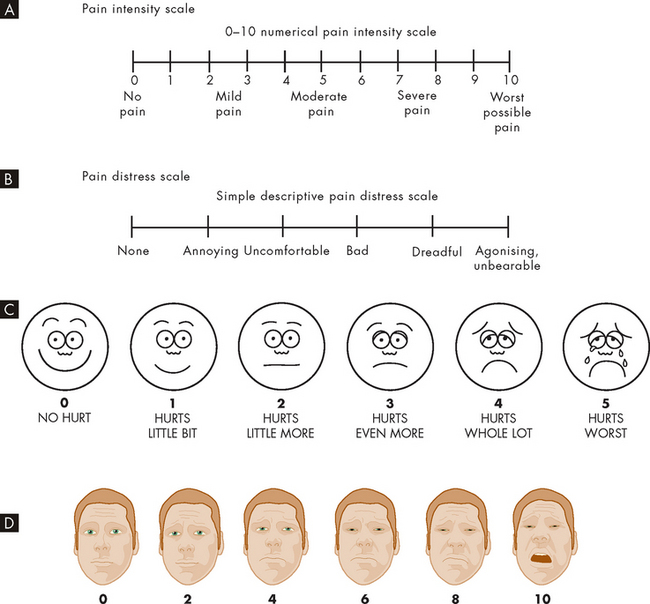
FIGURE 7-7 Scales for rating the intensity of pain.
The numerical and facial scales can be used by patients to self-rate their pain. A Pain intensity scale. B Pain distress scale. C Facial pain scale for children. D Facial pain scale for adults, used in multiple languages.
Source: A & B Bryant B, Knights K. Pharmacology for health professionals. 2nd edn. Sydney: Elsevier; 2007; adapted from Salerno E. Pharmacology for health professionals. St Louis: Mosby; 1999; Carr DB et al. Acute pain management: operative or medical procedures and trauma. Clinical practice guideline. AHCPR pub no 92-0032. Rockville MD: Agency for Health Care Policy and Research, Public Health Service, US Department of Health and Human Services, 1992. C Hockenberry M, Wilson D. Wong’s essentials of pediatric nursing. 8th edn. St Louis: Mosby; 2009. D Bieri D et al. The faces pain scale for the self-assessment of the severity of pain experienced by children: development, initial validation, and preliminary investigation for ratio scale properties. Pain 1990; 41:139–150.
The management of pain can be divided into pharmacological and non-pharmacological therapies, or a combination of both. The principal management aim should be to treat the cause of pain, if possible. Second, choose appropriate therapies that target the type of pain. For instance, opioids and NSAIDs target nociceptive pain. If using pharmacological agents, provide doses regularly and prevent pain from arising, such as postsurgical pain. The administration route also becomes important when deciding the severity of the pain. While originally developed for the management of cancer pain, the World Health Organization (WHO) analgesic ladder provides a simple scale for the determination of pharmacological agents in pain management (see Figure 7-8). The effect of different types of analgesic agents on pain pathways is illustrated in Figure 7-9.
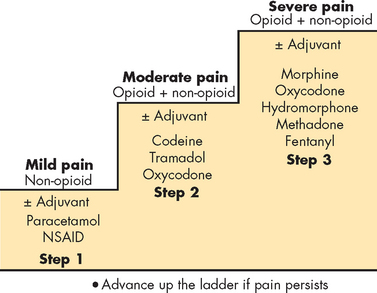
FIGURE 7-8 Strategy for pharmacological management of pain using the World Health Organization analgesic ladder.
Multiagent therapy is usually required for optimal pain management. Patients with mild pain should be started on a non-opioid analgesic, and those with moderate pain on a step 2 opioid. Many patients can benefit from the addition of a non-opioid to the opioid (e.g. for bone pain) or an adjuvant agent to the opioid (e.g. for neuropathic pain). If this combination does not produce adequate relief or the patient presents with severe pain, step 3 opioids should be begun initially.
Source: Hoffman R et al. Hematology: basic principles and practice. 5th edn. Philadelphia: Churchill Livingstone; 2009.
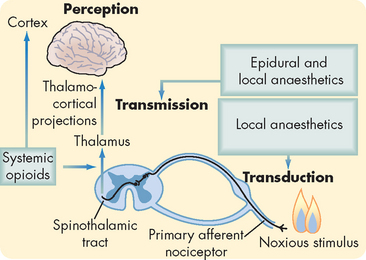
FIGURE 7-9 Schematic diagram outlining the nociceptive pathway for transmission of painful stimuli.
Interventions that prevent nociceptive transmission are shown at the points in the pathway that are thought to be their sites of action. Agents that block the transduction of pain actually prevent the generation of pain action potentials. In contrast, agents that block transmission actually stop the relay of action potentials to the cerebral cortex.
Source: Based on Ferrante FM, VadeBoncouer TR. Postoperative pain management. New York: Churchill Livingstone; 1993.
BOX 7-3 SURGICAL TREATMENT OF CHRONIC PAIN
Due to their well-characterised anatomical location, the axons of spinothalamic tract neurons are sometimes targeted in surgical approaches for the treatment of severe chronic pain in patients whose pain is inadequately controlled by analgesics.25,26 Because of the importance of these neurons in the onward transmission of the pain signal, cutting the axons should result in abolition of the pain.
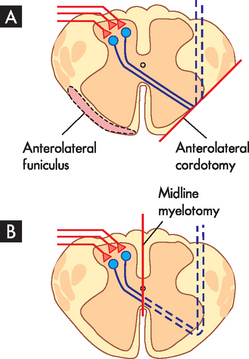
In an anterolateral cordotomy (-otomy refers to surgical cutting) the objective is to cut the axons of the spinothalamic tract as they course through the anterolateral funiculus. At one time this involved exposing the lateral surface of the spinal cord with an operation called a laminectomy, so that the cordotomy could be performed under direct visual control. Today, it is performed percutaneously (through the skin) and involves the insertion of an electrode into the anterolateral funiculus of the upper cervical segments of the spinal cord, which is visualised using computed tomography (CT) scanning. The cut is made by heating the tip of the electrode. Anterolateral cordotomy is particularly useful when the pain is unilateral, as the lesion only affects the axons of the spinothalamic tract on one side of the body (see Figure 7-10A).
If the pain is bilateral, then more effective relief can be produced by a midline myelotomy. The objective of this procedure is to cut the axons of the spinothalamic tract neurons as they cross the midline underneath the central canal (see Figure 7-10B). A midline myelotomy is a complicated procedure as it usually requires a laminectomy to access the posterior surface of the spinal cord and special care to ensure that the lesion is made at the correct level in order to target the axons of the appropriate spinothalamic tract neurons. However, the advantage of the procedure is that it cuts the axons of the spinothalamic tract neurons crossing the midline from both sides of the spinal cord and so produces bilateral analgesia, which is particularly beneficial when the pain is of visceral origin.
PAIN AND THE LIFE SPAN
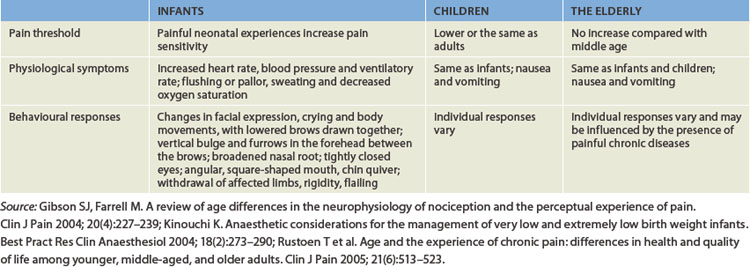
Although infants and children have the same anatomical and physiological pain pathways as adults, it was previously thought that their nervous systems were not fully developed and so their ability to differentiate pain was inhibited. We now know that this is incorrect, and several key signs and symptoms can alert the clinician to the pain of infants. For instance, crying is one of the most common symptoms of pain in the paediatric population. This may be accompanied by changes in facial expression and increases in heart rate, blood pressure and ventilatory rate (see Table 7-2).
In contrast, studies of the pain threshold in the elderly have revealed conflicting results. There are theories that state that the pain threshold is elevated in the elderly and this may be associated with changes in the skin and peripheral neuropathies. However, this is not conclusive. In addition, pain can be influenced by chronic illness, renal and liver metabolism, and the effect of pharmacological agents in the elderly. Often, pain with chronic illness in the elderly leads to increased rates of depression and lack of independence.
PATHOPHYSIOLOGY OF PAIN
So far we have considered pain from a physiological perspective and have identified it as both having a protective function (warning us about environments that can cause us harm) and acting as a stimulus to adopt behaviours that enhance healing. However, when the nervous system components that are responsible for detecting pain are themselves affected by injury or disease, the consequences can be severe and persistent neuropathic pain. It has been estimated that chronic pain affects around 3.2 million Australians and that the total cost of chronic pain on the Australian economy is about $34.3 billion per year — that is, more than $10,000 per person with chronic pain.27 Healthcare costs are estimated to comprise 20% of these costs (about $7 billion per year), mainly due to inpatient, outpatient and out-of-hospital medical costs.27 The different types of peripheral pathophysiological pain are summarised in Table 7-3.
Table 7-3 CLINICAL FEATURES AND LIKELY PATHOPHYSIOLOGY OF PERIPHERAL NEUROPATHIES
| PAIN SYNDROME | PATHOPHYSIOLOGY | CLINICAL FEATURES |
|---|---|---|
| Complex regional pain syndrome (type II) | Traumatic injury of peripheral nerves causing spontaneous action potentials to be generated in both damaged and intact nociceptors | Ongoing pain, allodynia, hyperalgesia, oedema, cutaneous blood flow and sweating abnormalities |
| Painful diabetic neuropathy | Hyperglycaemia leading to degeneration of unmyelinated sensory neurons | Loss of sensation and burning pain in feet and hands |
| Postherpetic neuralgia | Infection of peripheral nerves with varicella zoster virus leading to sensory neuron loss | Loss of sensation, pain and allodynia in dermatome of infected nerve |
| Lumbosacral radicular pain | Herniated intervertebral disc, resulting in compression injury of spinal nerve causing spontaneous activity in nociceptors | Lancinating (stabbing, piercing sensation) pain in the thigh or lower leg |
| Phantom limb pain | Spontaneous activation of nociceptors in neuroma and sensitisation of central neurons as a result of nerve transection during amputation | Ongoing cramping or aching pain referred to amputated limb |
| Trigeminal neuralgia (Tic douloureux) | Compression of the trigeminal nerve (sometimes by an atypical blood vessel) as it enters the brain | Episodes of severe, sharp, piercing pain referred to the facial region sometimes triggered by light touch |
| Chemotherapy-induced peripheral neuropathy | Chemotherapy-induced neurotoxicity of myelinated primary sensory neurons | Loss of sensation and spontaneous burning pain |
Peripheral neuropathic pain
Peripheral neuropathic pain results from damage to the peripheral nervous system including the cranial nerves, spinal nerves and any of the peripheral nerves that branch from these. Typically the pain feels like it is coming from the parts of the body that the affected nerves innervate — that is, referred pain — and the pain often has a characteristic quality (e.g. burning or shooting). The term mononeuropathy is used where only a single nerve is involved and polyneuropathy is used where multiple nerves are affected. The nerve damage that causes the pain can be the result of a range of factors, including physical injury, disease, infection or poisoning (e.g. chemotherapy).
One of the most common causes of painful peripheral neuropathies is where a peripheral nerve is damaged by a traumatic injury. The damage can be caused by a variety of mechanical insults such as nerve compression (following a crush injury) or transection (as the result of a penetrating injury or surgical procedure). The resultant pain is severe, often has a burning quality and is characterised by both hyperalgesia and allodynia. These types of pain used to be referred to as causalgias but are now known as complex regional pain syndromes (CRPSs).28
Painful diabetic neuropathy is an example of a neuropathic pain caused by either type 1 or type 2 diabetes mellitus. Unfortunately, it occurs in more than 50% of individuals with long-standing diabetes. Painful diabetic neuropathy appears to be due to hyperglycaemia, as the pain can be reduced in severity and delayed in onset by careful control of blood glucose levels.29 The longer the length of the axons, the more susceptible the neurons are to hyperglycaemia, which is why painful diabetic neuropathy is usually characterised by numbness and burning pain in the distal extremities (a ‘stocking and glove’ type distribution) (see Figure 7-11) that gradually spreads proximally and becomes more severe.30 The lack of sensation in the affected areas may result in the formation of ulcers, which may require limb amputation if they become gangrenous. The major cause of the painful neuropathy appears to be degeneration of unmyelinated axons caused directly by hyperglycaemia, as well as ischaemia caused by hyperglycaemia-induced damage to the blood vessels supplying the peripheral nerves. Further descriptions of diabetes are in Chapter 35.
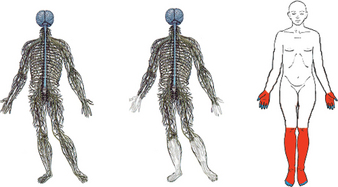
FIGURE 7-11 Painful diabetic neuropathy.
In poorly controlled diabetes, hyperglycaemia affects the longest sensory neurons first. A Normal intact nervous system. B Diabetes-affected nervous system with degeneration of axons in the distal extremities, resulting in C diabetic neuropathy with the characteristic ‘stocking and glove’ distribution.
Source: Edwards L et al. Diabetic neuropathy: mechanisms to management. Pharmacol Therapeut 2008; 120:1–34.
Infections of the peripheral nerves can also result in neuropathic pain. Around 20% of Australians will experience shingles (herpes zoster) during their lifetime. In shingles, the virus responsible for chicken pox (varicella) can lie dormant in the sensory nerves for years and then spontaneously erupt to cause a rash and painful blisters in the skin innervated by the infected nerves. Although the rash and blisters usually heal, in some sufferers the pain persists and is referred to as postherpetic neuralgia.
Given the diversity of causes of these peripheral neuropathies, it is perhaps not surprising that the mechanisms that underlie them differ and indeed in many cases remain poorly understood. Probably one of the better understood pathologies is that following a traumatic injury of a peripheral nerve responsible for CRPS. Where the nerve is completely transected (cut) or crushed, the portions of the axons distal to the injury typically degenerate because they are separated from their supporting cell bodies in the posterior root ganglia. This results in sensory loss due to the denervation of the peripheral tissue innervated by the affected nerve, as well as the formation of a neuroma at the site of the nerve damage. A neuroma consists of a disorganised array of scar tissue, neuroglial cells and inflammatory cells, as well as the axons of neurons attempting to regenerate along the damaged nerve and reinnervate the peripheral tissues. The regenerating axons within the neuroma itself appear to be highly sensitive and this is partly responsible for some of the symptoms associated with this type of injury. Action potentials appear to arise spontaneously from the neuroma, which may partly explain the pain in the absence of any peripheral stimuli. The structure itself is also very sensitive to mechanical stimuli, with gentle tapping of the skin overlying the neuroma or movement of the affected limb enough to elicit action potentials and produce pain.31
In some patients, the sympathetic nervous system also appears to play a role in CRPSs, because local anaesthetic blockade of sympathetic ganglia (or pharmacological block of adrenergic receptors) alleviates the pain. This pain appears to be a consequence of intact nociceptors in tissues that have been denervated by the nerve injury, that then become sensitive to noradrenaline released from sympathetic postganglionic neurons nearby.32 These intact nociceptors (which have axons in nerves not affected by the peripheral nerve injury) become sensitised and exhibit spontaneous activity and augmented responses to peripheral stimuli.
In addition to these changes in the properties of primary sensory neurons brought about by damage to the nerve, there is evidence to suggest that changes in the properties of neurons within the central nervous system may also contribute to neuropathic pain. Second-order neurons in the parts of the spinal cord receiving input from damaged nerves exhibit higher rates of spontaneous activity, show greatly enhanced responses to nociceptive stimuli applied to the periphery and become sensitive to low-intensity stimuli. The enhanced responsiveness of these neurons in the central nervous system is referred to as central sensitisation and appears to be a consequence of changes in the excitability of the second-order neurons themselves, as well as a reduction in the ongoing inhibitory influence exerted on these neurons by endogenous pain control circuits (disinhibition). Together with the peripheral mechanisms detailed above, these central changes are thought to be responsible for the spontaneous pain, hyperalgesia and allodynia associated with some forms of neuropathic pain.
Central pain syndromes
Forms of pain caused by damage to the central nervous system are known as central pain syndromes. Such forms of pain are typically very intense, aching, shooting pains that feel like they are due to damage to peripheral tissues but where clinical examination fails to identify any peripheral tissue damage or peripheral nervous system pathology. The causes of central pain syndromes were first identified by postmortem examination of the brains of individuals with CRPSs, which revealed damage in parts of the brain and/or spinal cord known to be involved in the processing of pain.33 The damage may be the result of a number of factors, including traumatic injury, tumour, stroke or even the side effects of neurosurgery (see Table 7-4). Because the cause of the pain is not visible, such pain can be particularly frustrating for patients and diagnosis can sometimes be a long and challenging process.
Table 7-4 CAUSES OF CENTRAL PAIN SYNDROMES
| Vascular lesions (infarction, haemorrhage) |
| Traumatic brain injury |
| Neurosurgery |
| Brain tumours |
| Multiple sclerosis |
| Spinal cord injury |
| Epilepsy |
The mechanisms responsible for central pain syndromes are not well understood, but it is likely that damage to spinothalamic tract neurons and thalamocortical neurons by the original insult is responsible for triggering ongoing activity in neurons at subsequent levels of the pain pathway. It has been postulated that this activity may be a response to the loss of normal input to these neurons (caused by the damage earlier in the pathway) or the loss of normal inhibitory influences that normally suppress their activity.
The definition of pain
 Pain is clinically important as it encourages patients to seek medical help and adopt behaviours that enhance healing.
Pain is clinically important as it encourages patients to seek medical help and adopt behaviours that enhance healing. Pain has an important physiological role as it teaches us to avoid environmental stimuli that cause harm.
Pain has an important physiological role as it teaches us to avoid environmental stimuli that cause harm. Individuals born with a congenital insensitivity to pain suffer horrendous injuries because they are unaware of the damage they are experiencing.
Individuals born with a congenital insensitivity to pain suffer horrendous injuries because they are unaware of the damage they are experiencing. Pain is a subjective sensation that is affected by a large number of variables and consequently can be difficult to define.
Pain is a subjective sensation that is affected by a large number of variables and consequently can be difficult to define. The International Association for the Society of Pain’s definition of pain highlights most of the key elements of pain.
The International Association for the Society of Pain’s definition of pain highlights most of the key elements of pain. The ability to locate a painful stimulus and determine its intensity and quality is referred to as the sensory-discriminative aspect of pain.
The ability to locate a painful stimulus and determine its intensity and quality is referred to as the sensory-discriminative aspect of pain. Pain produces changes in both mental state (affect) and behaviour (motivation), which are referred to as the affective-motivational aspect of pain.
Pain produces changes in both mental state (affect) and behaviour (motivation), which are referred to as the affective-motivational aspect of pain.Types of pain
 Nociceptive pain due to external damage is relatively mild and has a comparatively short time frame.
Nociceptive pain due to external damage is relatively mild and has a comparatively short time frame. Nociceptive pain due to internal damage is less common, is usually more severe and has a longer time frame.
Nociceptive pain due to internal damage is less common, is usually more severe and has a longer time frame.Pain terminology
The physiology of pain
 There are two classes of cutaneous nociceptor: high-threshold mechanoreceptors and polymodal nociceptors.
There are two classes of cutaneous nociceptor: high-threshold mechanoreceptors and polymodal nociceptors. Joints, muscle and viscera appear to be innervated by neurons with properties similar to cutaneous polymodal nociceptors.
Joints, muscle and viscera appear to be innervated by neurons with properties similar to cutaneous polymodal nociceptors. Joints, muscle and viscera also appear to be innervated by silent (or ‘sleeping’) nociceptors that only become active when these tissues become inflamed as the result of damage or disease.
Joints, muscle and viscera also appear to be innervated by silent (or ‘sleeping’) nociceptors that only become active when these tissues become inflamed as the result of damage or disease. Although there are a number of neural pathways relaying information about tissue damage between the spinal cord and brain, the most important in terms of the conscious perception of pain is the spinothalamic tract.
Although there are a number of neural pathways relaying information about tissue damage between the spinal cord and brain, the most important in terms of the conscious perception of pain is the spinothalamic tract. Spinothalamic tract neurons are the second-order neurons in the pain pathway and they collect the information delivered to the spinal cord by nociceptors and relay this to the thalamus.
Spinothalamic tract neurons are the second-order neurons in the pain pathway and they collect the information delivered to the spinal cord by nociceptors and relay this to the thalamus. The axons of spinothalamic tract neurons are sometimes targeted in surgical approaches to the treatment of severe chronic pain through a midline myelotomy or anterolateral cordotomy.
The axons of spinothalamic tract neurons are sometimes targeted in surgical approaches to the treatment of severe chronic pain through a midline myelotomy or anterolateral cordotomy. Thalamocortical neurons with their cell bodies in the posterolateral parts of the thalamus relay information about tissue damage to the somatosensory cortex and interoreceptive cortex.
Thalamocortical neurons with their cell bodies in the posterolateral parts of the thalamus relay information about tissue damage to the somatosensory cortex and interoreceptive cortex. Thalamocortical neurons located in the medial thalamus relay information about peripheral tissue damage to the anterior cingulate cortex.
Thalamocortical neurons located in the medial thalamus relay information about peripheral tissue damage to the anterior cingulate cortex. Through the information that they receive from the posterolateral parts of the thalamus, the somatosensory and interoreceptive cortices are thought to be responsible for the sensory-discriminative aspect of pain.
Through the information that they receive from the posterolateral parts of the thalamus, the somatosensory and interoreceptive cortices are thought to be responsible for the sensory-discriminative aspect of pain. The medial thalamic projection to the anterior cingulate cortex is thought to be responsible for the affective-motivational aspect of pain.
The medial thalamic projection to the anterior cingulate cortex is thought to be responsible for the affective-motivational aspect of pain. Neuropathic pain affects up to 7% of the population and contributes significantly to the cost of healthcare in developed countries.
Neuropathic pain affects up to 7% of the population and contributes significantly to the cost of healthcare in developed countries. Modulators of pain include substances that stimulate pain receptors (i.e. prostaglandins, bradykinins, substance P, glutamate) and substances that suppress pain (i.e. endorphins, gamma-aminobutyric acid (GABA), serotonin).
Modulators of pain include substances that stimulate pain receptors (i.e. prostaglandins, bradykinins, substance P, glutamate) and substances that suppress pain (i.e. endorphins, gamma-aminobutyric acid (GABA), serotonin).Clinical manifestations of pain
Evaluation and treatment
Pathophysiology of pain
 Mononeuropathy is where only a single nerve is involved and polyneuropathy is where multiple nerves are affected.
Mononeuropathy is where only a single nerve is involved and polyneuropathy is where multiple nerves are affected. The neural mechanisms responsible for painful peripheral neuropathies are complex and in many cases remain poorly understood.
The neural mechanisms responsible for painful peripheral neuropathies are complex and in many cases remain poorly understood. Following a traumatic injury of a peripheral nerve the portions of the axons distal to the injury sometimes degenerate and a neuroma may form at the site of the nerve damage.
Following a traumatic injury of a peripheral nerve the portions of the axons distal to the injury sometimes degenerate and a neuroma may form at the site of the nerve damage. Action potentials can occur spontaneously in the neuroma and it may be very sensitive to mechanical stimuli, resulting in pain if it is touched or disturbed by movement.
Action potentials can occur spontaneously in the neuroma and it may be very sensitive to mechanical stimuli, resulting in pain if it is touched or disturbed by movement. In some cases noradrenaline released from sympathetic postganglionic neurons appears to exacerbate the pain produced by nerve injury.
In some cases noradrenaline released from sympathetic postganglionic neurons appears to exacerbate the pain produced by nerve injury.Peter was a 59-year-old shearer whose best friend was a blue heeler called Red. After noticing blood in his stools Peter visited his local general practitioner and was subsequently diagnosed with carcinoma of the colon. A colon resection was performed and he underwent a complete course of radiotherapy and chemotherapy. Six months later, Peter started to experience abdominal pain. On investigation it was revealed that the malignancy had spread into his urinary bladder and urethra. Peter was readmitted to hospital and in subsequent weeks his pain increased progressively until eventually it could only be controlled with high doses of intravenous morphine. Twelve months after the onset of the pain, Peter had a midline myelotomy in the mid-thoracic region of the spinal cord. Ten days following this operation, he was able to leave hospital and lived pain-free until his death 5 years later.