27 ALTERATIONS OF DIGESTIVE FUNCTION ACROSS THE LIFE SPAN
INTRODUCTION
The primary function of the digestive system is to digest and absorb nutrients into the blood, to ultimately provide valuable substances for the functioning of all body cells. Impairment of the digestive system can lead to systemic abnormalities and may become life-threatening. Structural and neural abnormalities can slow, obstruct or accelerate the movement of chyme at any level of the gastrointestinal tract. Fast movement through the digestive system causes diarrhoea and malabsorption of foods, while slowed or obstructed movement causes constipation.
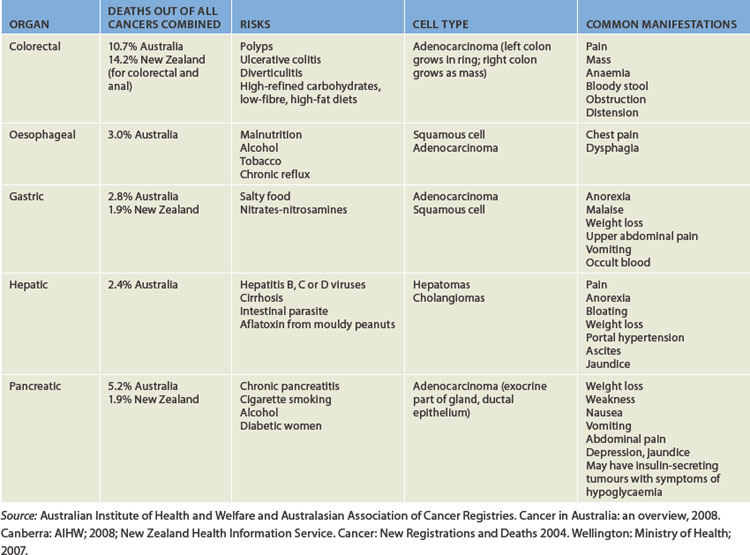
Cancers are a main affliction for this system, with the chief cancer of the gastrointestinal tract being colorectal cancer — a leading cause of death in Australia and New Zealand. It is likely that the modern lifestyle, particularly the choice of foods, adopted by many people in the community increases the risk of developing this cancer. Changes in the cells lining the colon and rectum, often seen in the form of polyps, can be precancerous for a long period, so regular screening may allow for these changes to be detected and treated prior to them progressing to cancer. Cancers of the accessory organs are also seen, with pancreatic cancer being particularly devastating, having a 100% mortality rate.
Inflammatory and ulcerative conditions of the gastrointestinal wall disrupt secretion, motility and absorption. Inflammation or obstruction of the liver, pancreas or gallbladder can alter metabolism and result in local and systemic symptoms. Many clinical manifestations of gastrointestinal tract disorders are nonspecific and can be caused by a variety of impairments.
Nutritional disorders such as lactose intolerance and coeliac disease are receiving increasing awareness within the community. Those with lactose intolerance have options such as lactose-free dairy foods, and although coeliacs can avoid gluten to manage their condition, many coeliacs unfortunately remain undiagnosed.
The liver is a vital organ that has a wide variety of essential functions not only for digestive system function, but also for homeostasis of other body systems. Impairment of the liver by conditions such as alcoholic liver disease or hepatitis can lead to serious complications including insufficient production of blood-clotting factors (which can result in bleeding), inadequate metabolism of toxic substances such as alcohol and other drugs and encephalopathy (altered brain structure). Therefore, the liver should be considered as far more than an accessory organ of digestion: it assists in homeostasis of other systems, such that liver disease can be fatal.
DISORDERS OF THE GASTROINTESTINAL TRACT
Cancers of the gastrointestinal tract
Cancers of the gastrointestinal tract are often associated with nutritional factors, as these factors can directly impact on the epithelial cells lining the tract. For example, colorectal cancer, the most prevalent cancer of the digestive system, is associated with a diet high in fats and red meat and low in vegetables. Cancers of the upper parts of the digestive system are associated with alcohol intake (as well as smoking). Stomach cancer is also associated with bacterial infection. Other cancers of the gastrointestinal tract are quite rare: the incidence of cancers of the lip, tongue, mouth, pharynx, larynx, small intestine and anus each are less than 1% of the total incidence of cancers in the Australian population.1 Gastrointestinal cancers with the highest mortality in Australia are shown in Table 27-1. General principles of cancers are discussed in Chapter 36; here we consider the impact on the digestive system.
Colorectal cancer
The most serious disorder of the digestive system in Australia and New Zealand is colorectal cancer: it is the most prevalent cancer of this system and has a high mortality rate when detected at an advanced stage. Colorectal cancer (or bowel cancer) is the term used to encompass cancers of the caecum, colon and through to the rectum. Of the 13,000 Australians diagnosed with colorectal cancer each year, approximately two-thirds have cancers in the colon and one-third have cancers in the rectum.1
Colorectal cancer has the second-highest incidence rate out of all reportable cancers for both males and females, being second only to prostate cancer for males and breast cancer for females. (Skin cancers have a very high rate but these are not all reportable, therefore not all skin cancers are included in cancer incidence statistics.) In fact, 13% of all cancers in Australia and 14% in New Zealand are colorectal cancers1,2 — these rates are among the highest in the world.3,4 The rate is actually lower in Indigenous populations than in the non-Indigenous populations in both countries.5,6 Approximately 80 Australians die from colorectal cancer each week,7 and it is the sixth highest cause of death for all Australians;8 24 New Zealanders also die of this disease each week.9
Cancer of the colon tends to occur mainly in individuals older than 50 years of age, so early screening is aimed at those aged 50 and over.3 Clearly age cannot be modified; however, a substantial number of modifiable risk factors that can lower the risk of developing colorectal cancer have been identified (see Table 27-2). For example, increasing physical activity is preventative in a dose-dependent manner, such that more exercise will further reduce the risk;10 avoiding obesity decreases the risk;11 and a diet rich in vegetables, grains, fruit, folate and calcium and low in fat can also decrease the risk.12 These modifiable dietary and lifestyle factors account for up to 70% of the risk factors for developing colorectal cancers.13 Regular consumption of aspirin is also preventative against colorectal cancer,14,15 and aspirin is now recommended for patients with previous colorectal cancer to prevent further disease.3
Table 27-2 MODIFIABLE RISK FACTORS FOR PREVENTION OF COLORECTAL CANCER
|
• Maintain a healthy body mass index — less than 25 (calculations for body mass index are in Chapter 35)
• Limit alcohol intake — no more than 2 standard drinks for men, and 1 standard drink for women, per day
• Limit energy intake — men: less than 10,480 kilojoules (2500 calories) per day; women: less than 8360 kilojoules (2000 calories) per day
|
Source: Australian Cancer Network Colorectal Cancer Guidelines Revision Committee. Guidelines for the Prevention, Early Detection and Management of Colorectal Cancer. Sydney: The Cancer Council Australia and Australian Cancer Network; 2005.
Lifestyle factors help prevent colorectal cancer in the following ways:16,17
Clearly, lifestyle factors have a substantial influence on a range of processes involved in the early stages of cancer. It is important as healthcare professionals that we and, in particular, the individuals we encounter are educated about these lifestyle risk factors.
The development of colorectal cancer appears to be a result of both these environmental factors and genetic or inherited susceptibility (which is typical of most cancers; see Chapter 37).3 Familial adenomatous polyposis (FAP) and hereditary nonpolyposis colorectal cancer (HNPCC) are genetic conditions (autosomal-dominant inheritance traits) that are implicated in up to 25% of colorectal cancers.19 Both genetic syndromes appear to have a substantial link to cancers that occur earlier in life (approximately age 35–40). Clustering in families is common, with genes for both inherited syndromes having been identified.20,21
PATHOPHYSIOLOGY
Colorectal polyps are closely associated with the development of cancer. A polyp is a finger-like projection arising from the mucosal epithelium. Most polyps are benign; however, because it can be difficult to distinguish between benign and malignant types, there is currently a strong preference to remove all polyps.3
Neoplastic polyps are benign, premalignant lesions and are classified as tubular (the most prevalent), villous or tubulovillous adenomas (see Figure 27-1). Those with the villous shape are more likely to become cancerous, although flat adenomas may be more aggressive.3 The larger the polyp, the greater the risk of colorectal cancer — those larger than 1.5 cm are more likely to be malignant than those smaller than 1 cm. Most colorectal cancers arise from these adenomatous polyps. Small adenomas may grow slowly, even appearing unchanged for years.3 These tumours have a long pre-invasive phase and, even when they invade, their growth is still often slow. Adenomas can be detected early, before the submucosa has been penetrated.
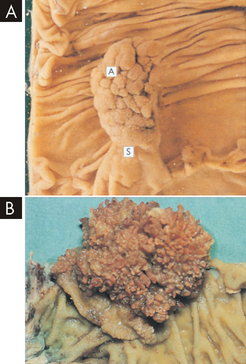
FIGURE 27-1 Neoplastic polyps.
A Tubular adenomas (A) are rounded lesions 0.5–2 cm in size that are generally red and sit on a stalk (S) of normal mucosa that has been dragged up by traction of the polyp in the bowel lumen. B Villous adenomas are velvety lesions about 0.6 cm thick that occupy a broad area of mucosa generally 1–5 cm in diameter.
Source: Stevens A, Lowe J. Pathology. 2nd edn. Edinburgh: Mosby; 2000.
Colorectal carcinoma starts in the glands of the mucosal lining, forming an adenocarcinoma. Many cancers are most serious when they undergo metastasis, which is the spread of the original carcinoma to other body tissues. Because the lymphatic channels are located under the muscularis mucosae (the thin layer of muscle within the mucosal layer), colorectal cancer must traverse this layer before metastasis can occur. Once the malignant cells cross the muscularis mucosae, the tumour becomes invasive and highly malignant. The liver and lungs are the most common sites of metastasis (see Figure 27-2).
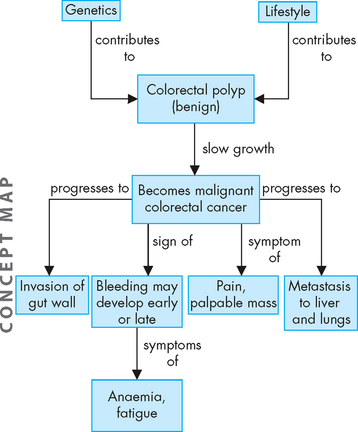
FIGURE 27-2 The development of colorectal cancer.
A combination of genetic and lifestyle factors may contribute to changes that can become malignant and lead to effects throughout the body.
Cancer of the colon is also associated with genetic events. Deletion of genes is linked to the transformation of normal colon epithelial cells to benign and malignant adenocarcinomas. Mutations of oncogenes, tumour-suppressor genes and repair genes are all associated with colon cancer.22 Dietary factors, including a diet high in fat, low in fibre and low in calcium, may promote genetic mutations.
CLINICAL MANIFESTATIONS
Symptoms of colorectal cancer depend on the location, size and shape of the lesion (see Figure 27-3). Tumours of the right (ascending) colon and left (descending) colon form into two distinct tumour types:23
 Right side lesions are polyp-shaped and extend along one wall of the caecum and ascending colon (see Figure 27-4). These tumours may be silent, evolving to pain, palpable mass in the lower right quadrant, anaemia, fatigue and dark-red or mahogany-coloured blood mixed with the stools. These tumours can become large and bulky with necrosis (cell damage) and ulceration, contributing to persistent blood loss and anaemia. Obstruction is unusual because the growth does not readily encircle the colon.
Right side lesions are polyp-shaped and extend along one wall of the caecum and ascending colon (see Figure 27-4). These tumours may be silent, evolving to pain, palpable mass in the lower right quadrant, anaemia, fatigue and dark-red or mahogany-coloured blood mixed with the stools. These tumours can become large and bulky with necrosis (cell damage) and ulceration, contributing to persistent blood loss and anaemia. Obstruction is unusual because the growth does not readily encircle the colon. Left side tumours start as small, elevated, button-like masses. They grow circumferentially, encircling the entire bowel wall and eventually ulcerating in the middle as the tumour penetrates the blood supply — this creates a typical ‘apple-core’ lesion seen on imaging. Obstruction is common but occurs slowly, and stools become narrow and pencil-shaped. Manifestations include progressive abdominal distention, pain, vomiting, constipation, need for laxatives, cramps and bright red blood on the surface of the stool.
Left side tumours start as small, elevated, button-like masses. They grow circumferentially, encircling the entire bowel wall and eventually ulcerating in the middle as the tumour penetrates the blood supply — this creates a typical ‘apple-core’ lesion seen on imaging. Obstruction is common but occurs slowly, and stools become narrow and pencil-shaped. Manifestations include progressive abdominal distention, pain, vomiting, constipation, need for laxatives, cramps and bright red blood on the surface of the stool.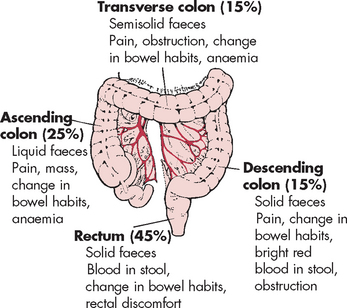
FIGURE 27-3 Signs and symptoms of colorectal cancer by location of primary lesion.
Clinical manifestations are listed in order of frequency for each region (lymphatics of colon also shown).
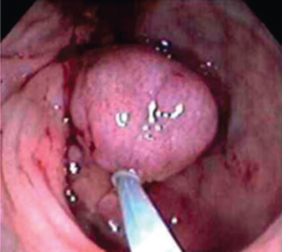
FIGURE 27-4 Colorectal cancer.
A polyp within the large intestine, viewed using colonoscopy.
Source: Klatt EC. Robbins and Cotran atlas of pathology. 2nd edn. Philadelphia: Saunders; 2010.
Rectal carcinomas (occurring up to 15 cm from the anal opening) can spread through the rectal wall to nearby structures: the prostate in men and the vagina in women. Penetration occurs readily in the lower third of the rectum because it has no serosal covering (serous membrane lining of the gastrointestinal tract). Systemic metastasis occurs through the blood supply known as the haemorrhoidal plexus, which drains into the vena cava. Metastasis commonly occurs in the liver and lungs.
Some other conditions commonly confused with colorectal cancer are listed in Table 27-3.
Table 27-3 CONDITIONS COMMONLY CONFUSED WITH COLORECTAL CANCER
| CONDITION | SIGNIFICANT CHARACTERISTICS |
|---|---|
| Diverticulitis | Left-sided pain similar to that of appendicitis; tender lower left quadrant; associated findings: nausea, vomiting, fever, obstruction, anorexia and leucocytosis; mucosa is intact, and perforation, peritonitis and abscesses occur more often than in cancer; sigmoidoscopy (examination of the lower colon using a scope) or barium enema used to distinguish from cancer |
| Ulcerative colitis | Younger people with chronic attacks of bloody diarrhoea, crampy abdominal pain, fever, malnutrition and dehydration; usually involves the left colon and rectum; endoscopy, barium enema and biopsy performed for definitive diagnosis |
| Crohn’s disease | Generally involves the right colon; chronic diarrhoea with abdominal cramps, fever, weight loss and often a palpable abdominal mass; endoscopic examination and barium enema used to distinguish from cancer |
| Appendicitis | Vague abdominal symptoms, often with a tender or non-tender mass in the lower right quadrant; associated symptoms: mild fever and leucocytosis; barium enema used to distinguish from cancer |
| Thrombosed haemorrhoids | Examination shows a tender, swollen, bluish painful mass in the anus; patient will have a history of haemorrhoids |
EVALUATION AND TREATMENT
Screening for colorectal cancer can be performed by several different methods:
Early detection screening recommendations for Australians are summarised in Table 27-4. Regular screening using the faecal occult blood test can decrease the mortality rate from colorectal cancer by approximately 33%.26 The Australian government is currently undertaking a large trial leading towards the introduction of a national bowel cancer screening program using the faecal occult blood test. The first phase of the program commenced in 2006 and the second phase is currently underway.1,7 The immunochemical faecal occult blood test is being used, which does not require restrictions on diet or medications.26 As of early 2010, a national screening program was in the planning stages for New Zealand.6
Table 27-4 SCREENING FOR COLORECTAL CANCER
| From the age of 50 years, men and women of the Australian population should be screened using: |
| From the age of 50 years, people who have a moderately increased risk due to their family history of colorectal cancer should be screened using: |
| From the age of 25 years, people who have a potentially high risk due to a more extensive family history of colorectal cancer should be screened using: |
| In a symptomatic patient over 40 years, it is recommended that a full colonoscopy be performed |
Source: Australian Cancer Network Colorectal Cancer Guidelines Revision Committee. Guidelines for the Prevention, Early Detection and Management of Colorectal Cancer. Sydney: The Cancer Council Australia and Australian Cancer Network; 2005.
In addition to screening, physical examination of the abdomen detects liver enlargement and ascites; and appropriate lymph nodes are palpated. Evaluation of the liver and lungs for metastasis includes using X-ray, CT scans, MRI and ultrasound.
Treatment for cancer of the colon is almost always surgical, with or without chemotherapy or radiotherapy.3 Resection (cancer removal) and anastomosis (intestine rejoining) can be performed for cancer of the ascending, transverse, descending or sigmoid colon and upper rectum. Surgery is performed through abdominal incisions and natural defecation is preserved. Growths in the lower portion of the rectum require removal of the entire rectum with the formation of a permanent colostomy (faeces exit the body via an alternative opening made in the abdominal wall). Prognosis after surgery depends on the stage and location of the tumour. Resection of liver metastases can prolong survival.27 As the diagnosis and treatment of colorectal cancer has improved in recent decades, we have seen an overall increase in the 5-year survival rate from 49% in 1982–1986 to 62% in 1998–2004.1
Oesophageal cancer
Carcinoma of the oesophagus is a rare type of cancer, affecting only 1165 Australians in 2005 and accounting for approximately 1.2% of all cancers.1 However, although new diagnoses are relatively rare, oesophageal cancer was the tenth highest cause of death from cancer in 20051 as it is able to undergo metastasis, thereby rapidly increasing the mortality rate (see Table 27-1). In New Zealand in 2005, almost as many people died of the disease as were newly diagnosed.9
PATHOPHYSIOLOGY
Carcinoma of the oesophagus is usually squamous cell carcinoma or, less commonly, adenocarcinoma. Adenocarcinomas are often secondary to infiltration by a gastric carcinoma or to the presence of Barrett’s oesophagus. In this case, the epithelium is altered such that columnar rather than squamous epithelium in the lower oesophagus is present, which is associated with chronic gastro-oesophageal reflux.28 Carcinomas can occur at any level of the oesophageal tract but are most common at the gastro-oesophageal junction.
RISK FACTORS FOR OESOPHAGEAL CANCER
Source: The Cancer Council Victoria. Stomach and oesophageal cancer: information for people with cancer, their family and friends. Melbourne: The Cancer Council Victoria; 2008.
The pathogenesis of oesophageal carcinoma is facilitated by: (1) alterations of oesophageal structure and function that permit food and drink to remain in the oesophagus for prolonged periods; (2) ulceration and metaplasia caused by oesophageal reflux; (3) chronic exposure to irritants, such as alcohol and tobacco, which cause neoplastic transformation; and (4) obesity (see Chapter 35).29 Chronic inadequate nutrition can impair oesophageal structure and function (see the box ‘Risk factors: oesophageal cancer’).
CLINICAL MANIFESTATIONS
The two frequent symptoms of oesophageal carcinoma are chest pain and dysphagia (difficulty swallowing). The most common type of pain is heartburn. It is initiated by eating spicy or highly seasoned foods and lying down. Dysphagia is usually pressure-like and may radiate posteriorly between the scapulae. Dysphagia usually progresses rapidly and is mostly painless during the early stages of oesophageal carcinoma.
EVALUATION AND TREATMENT
Individuals with dysphagia undergo endoscopy with biopsy, and the tissue is examined for neoplastic change. Endoscopic ultrasound is also used for staging. Prevention of gastro-oesophageal reflux is essential to the management of Barrett’s oesophagus. Oesophageal cancer metastasises rapidly and therefore has a poor prognosis — this explains why, despite moderately low incidence, it ranks high on the cancer causes of death. If the malignancy has not spread beyond the local lymph nodes, surgical removal of the tumour usually has excellent prognosis. If metastasis has occurred, incomplete resection is of little benefit for survival. Treatment is combined radiation and chemotherapy.30
Gastric cancer
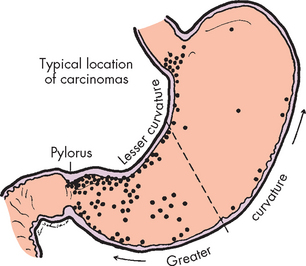
Gastric (stomach) cancer was diagnosed in 1900 Australians and 138 New Zealanders in 2005, accounting for approximately 1.9% of all cancers in both countries.1,9 The incidence rate is greater in males than in females. Gastric cancer traditionally has been prevalent in countries where the diet is high in salted and preserved foods (see Table 27-1), such as in some Asian countries. A combination of international dietary influences and immigration may be contributing to the rate of gastric cancer in Australia.
The most important environmental causative factors of gastric cancer are: (1) infection with Helicobacter pylori; (2) intake of heavily salted and preserved foods (namely nitrates in pickled or salted foods such as bacon); (3) low intake of fruits and vegetables; and (4) use of tobacco and alcohol. Dietary salt enhances the conversion of nitrates to carcinogenic nitrosamines (cancer-causing chemical compounds) in the stomach. Salt is also caustic to the stomach and can cause chronic atrophic gastritis. Nitrates are thought to cause stomach cancer once atrophic gastritis has occurred. Helicobacter pylori–associated gastritis increases the risk for gastric adenocarcinoma and gastric mucosa–associated lymphoid tissue lymphoma.31 Finally, the presence of salt delays gastric emptying — this increases the time during which carcinogenic nitrosamines can exert their effects on the stomach mucosa.
Other non-environmental risk factors are a family history of gastric adenocarcinoma and pernicious anaemia (see Chapter 17), which results from atrophy of the gastric mucosa in the same locations where gastric tumours arise. Loss of tumour suppressor genes and other genetic alterations may be important in gastric cancer.32
PATHOPHYSIOLOGY
Gastric adenocarcinoma usually begins in the glands of the distal stomach mucosa. Approximately 50% of cases develop in the pre-pyloric antrum (see Figure 27-5). Inflammation and atrophic gastritis associated with Helicobacter pylori infection and intestinal metaplasia are strongly linked to the development of gastric cancer. Insufficient acid secretion by the atrophic mucosa creates a relatively alkaline environment that permits bacteria to multiply, whereby they can metabolise the nitrates to nitrosamines. The resulting increase in nitrosamines further damages the DNA of mucosal cells, promoting metaplasia and neoplasia (replacement of cells with different cell types or tumorous cells, respectively). Duodenal reflux may also contribute to intestinal metaplasia. The reflux contains caustic bile salts that destroy the mucosal barrier, which normally protects the stomach.
CLINICAL MANIFESTATIONS
The early stages of gastric cancer are generally asymptomatic or produce vague symptoms such as loss of appetite (especially for meat), malaise (tiredness) and indigestion. Later manifestations include unexplained weight loss, upper abdominal pain, vomiting, change in bowel habits and anaemia caused by persistent occult (unseen) bleeding. The prognosis is poor because symptoms do not occur until the tumour has penetrated the muscle layers of the stomach, spread to surrounding tissues and entered the draining lymph nodes and veins, causing distant metastases, particularly to the liver and peritoneal structures. Generally the first manifestations of carcinoma are caused by distant metastases and the disease is already in an advanced stage.
EVALUATION AND TREATMENT
Most symptoms suggest a problem in the upper gastrointestinal tract and a barium X-ray film shows the lesion. Direct endoscopic visualisation using a scope down the oesophagus and biopsy usually establish the diagnosis. Another definitive technique is microscopic examination of exfoliated cells obtained by lavage during endoscopy. Surgery is the usual treatment for gastric cancer. Radiation therapy is generally unsuccessful and immunotherapy is still experimental. Chemotherapy combined with radiation reduces the tumour.33 Individuals who respond well to chemotherapy generally live longer than those who do not.
Inflammatory processes of the gastrointestinal tract
Ulcerative colitis and Crohn’s disease together are known as the inflammatory bowel diseases. Although the diseases have some unique differences, factors such as genetics, immune dysregulation, epithelial barrier dysfunction and microbial flora have some role for both in disease pathogenesis. In some patients, distinguishing between ulcerative colitis and Crohn’s disease may be difficult (see Table 27-5).
Table 27-5 FEATURES OF ULCERATIVE COLITIS AND CROHN’S DISEASE
| FEATURE | ULCERATIVE COLITIS | CROHN’S DISEASE |
|---|---|---|
| Incidence | ||
| Age at onset | 20–40 years most common | 20–40 years most common |
| Family history | Less common | More common |
| Cancer risk | Increased | Increased |
| Pathophysiology | ||
| Location of lesions | Large intestine Left side more common | Large and small intestine, ‘skip’ lesions common Right side more common |
| Inflammation and ulceration | Mucosal layer involved | Entire intestinal wall involved |
| Granulomas | Rare | Common |
| Friable mucosa | Common | Less common |
| Anal and perianal fistulae | Rare | Common; abscesses |
| Narrowed lumen and possible obstruction | Rare | Common; obstruction |
| Clinical manifestations | ||
| Abdominal pain | Mild to severe | Moderate to severe |
| Diarrhoea | Common; 4 times/day | Common; 4 times/day |
| Bloody stools | Common | Less common |
| Abdominal mass | Rare | Common |
| Small intestinal malabsorption | Rare | Common |
| Steatorrhoea | Rare | Common |
| Clinical course | Remissions and exacerbations | Remissions and exacerbations |
Ulcerative colitis
Ulcerative colitis is a chronic inflammatory disease that causes ulceration of the colonic mucosa, usually in the rectum and sigmoid colon. The lesions appear in susceptible individuals between 20 and 40 years old.34 Risk factors include a family history of disease. It is estimated that it affects 33,000 people in Australia and 670 in New Zealand.34,35
Although the cause of ulcerative colitis is unknown, infectious, genetic and immunological factors have all been suggested.36 Immune system causes include humoral immunological factors and activated macrophages. Anticolon antibodies have been identified in the sera (lining of the bowel) of individuals with ulcerative colitis. T lymphocytes in individuals with ulcerative colitis may have cytotoxic effects on the epithelial cells of the colon. Furthermore, autoimmune disorders such as systemic lupus erythematosus may accompany ulcerative colitis.
PATHOPHYSIOLOGY
The primary lesion of ulcerative colitis begins with inflammation at the base of the crypt of Lieberkühn in the large intestine. The disease is most severe in the rectum and sigmoid colon (see Figure 27-6). The mucosa is hyperaemic (contains excess blood) and may appear dark red and velvety. Small erosions form and develop into ulcers. Abscess formation causes areas of inflammation and pus, while necrosis indicates death of cells. Ragged ulceration of the mucosa ensues. Oedema and thickening of the muscularis mucosae may narrow the lumen of the involved colon. Mucosal destruction causes bleeding, cramping pain and an urge to defecate. Frequent diarrhoea, with passage of small amounts of blood and purulent mucus (containing pus), is common. Loss of the absorptive mucosal surface and rapid colonic transit time cause large volumes of watery diarrhoea.
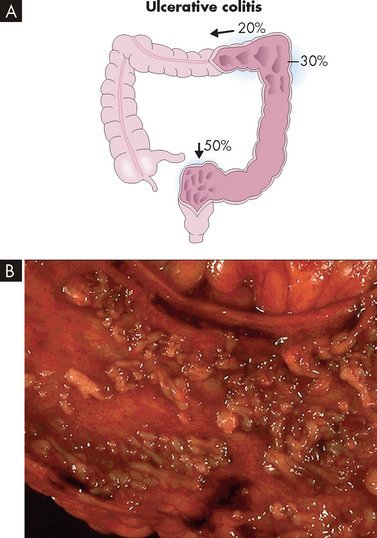
FIGURE 27-6 Ulcerative colitis.
A Approximately 50% of cases of ulcerative colitis occur in the rectosigmoid area with 30% of cases extending to hepatic flexure. In 20% of cases ulcerative colitis is diffuse. B The mucosa has been ulcerated away in a severe case of ulcerative colitis.
Source: A Damjanov I. Pathology. Philadelphia: Saunders; 2009. B Klatt EC. Robbins and Cotran atlas of pathology. 2nd edn. Philadelphia: Saunders; 2010.
CLINICAL MANIFESTATIONS
The course of ulcerative colitis consists of intermittent periods of remission and exacerbation. Mild ulcerative colitis involves less mucosa, so that frequency of bowel movements, bleeding and pain are minimal. Severe forms may involve the entire colon and are characterised by fever, elevated pulse rate, frequent diarrhoea (10–20 stools per day), urgency, obviously bloody stools and continuous crampy pain. Dehydration, weight loss, anaemia and fever result from fluid loss, bleeding and inflammation. Complications include anal fissures, haemorrhoids (dilated anal veins, often referred to as piles) and perirectal abscess. Severe haemorrhage is rare. Oedema, strictures (narrowing) or fibrosis (increased fibrous tissue) can obstruct the colon. Perforation is an unusual but possible complication. The risk of colon cancer increases significantly after 10 years of ulcerative colitis and therefore regular colonoscopies are recommended.37 The systemic manifestations include polyarthritis (arthritis at two or more joints), episcleritis (inflammation of the sclera of the eyes), disorders of the liver and alterations in coagulation.
EVALUATION AND TREATMENT
Diagnosis of ulcerative colitis is based on the medical history and clinical manifestations. Sigmoidoscopy, barium enema and X-ray films are used in addition to laboratory data. Infectious causes are ruled out by stool culture.
Treatment is individualised and depends on the severity of symptoms and the extent of mucosal involvement. The disease is often treated with sulfasalazine (a combination of a sulfa drug and aspirin) or mesalazine, which is of particular benefit in that it helps to protect against colorectal cancer.37 Steroids suppress the inflammatory response and help to alleviate the cramping pain. Broad-spectrum antibiotics may be prescribed if bacterial infection is suggested. Severe, unremitting disease can require hospitalisation and administration of intravenous fluids and support nutrition, such as parenteral nutrition. Surgical resection of the colon may be performed if other forms of therapy are unsuccessful or if there are acute serious complications (sepsis, haemorrhage, perforation or obstruction). Proctocolectomy and ileal pouch anal anastomosis have become the standard surgery for mucosal ulcerative colitis.38
Crohn’s disease
Crohn’s disease (also known as granulomatous colitis or regional enteritis) is an inflammatory disorder that affects both the large and the small intestines. The rectum is seldom involved. Risk factors and theories of causation are the same as those for ulcerative colitis, including genetic predisposition and an altered immune response to normal bowel flora.39 In some cases, Crohn’s disease is difficult to differentiate from ulcerative colitis (see Table 27-5),
The prevalence of Crohn’s disease may be lower than ulcerative colitis in Australia (affecting 28,000 people),35 but higher in New Zealand (affecting more than 700 people).34 Like ulcerative colitis, Crohn’s disease is usually diagnosed between the ages of 20 and 40.34 Of affected individuals, 10–20% have a positive family history. Increased suppressor T cell activity, alterations in immunoglobulin A (IgA) production, macrophage activation, bacterial flora, antigens and susceptibility genes are factors associated with Crohn’s disease.40 Psychological stresses also appear to be causative of both Crohn’s disease and ulcerative colitis. Smoking increases the risk of developing severe disease.41
PATHOPHYSIOLOGY
The inflammation process of Crohn’s disease begins in the intestinal submucosa and spreads inwards and outwards to involve the mucosa and serosa. Activated neutrophils and macrophages promote inflammation and cause tissue injury. The ascending colon and the transverse colon are the most common sites of the disease, but the ileum of the small intestine may also be involved. The inflammation can affect some segments of the intestine but not others, creating ‘skip lesions’ (see Figure 27-7). Also one side of the intestinal wall may be affected and not the other. The ulcerations of Crohn’s disease produce fissures (tears) that extend inflammation into lymphoid tissue. The typical lesion is a granuloma (small nodule of inflammation) with a cobblestone appearance from projections of inflamed tissue surrounded by ulceration. Fistulae (connections) may form in the perianal area between loops of intestine or extend into the bladder, such that one part of the intestine is able to connect directly into another area. Strictures may develop, promoting obstruction.
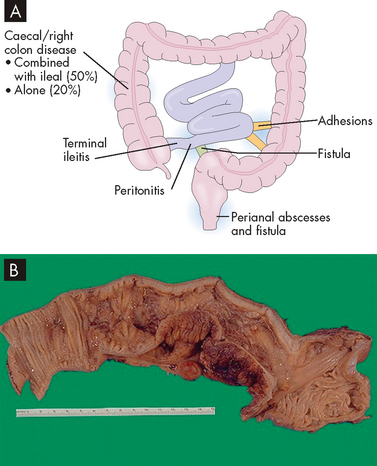
A The disease is localised to the terminal ileum and ascending colon and the transverse colon in most cases. B A segment of colon that has a thickened wall and in which the mucosa has lost the regular folds, indicative of Crohn’s disease.
Source: A Damjanov I. Pathology. Philadelphia: Saunders; 2009. B Klatt EC. Robbins and Cotran atlas of pathology. 2nd edn. Philadelphia: Saunders; 2010.
CLINICAL MANIFESTATIONS
Individuals with Crohn’s disease may have no specific symptoms other than an ‘irritable bowel’ for several years. Diarrhoea is the most common sign and, occasionally, colonic bleeding. Weight loss and lower abdominal pain accompany Crohn’s disease. If the ileum is involved, the individual may be anaemic as a result of malabsorption of vitamin B12. There may also be deficiencies in folic acid and vitamin D absorption. In addition, proteins may be lost, leading to hypoalbuminaemia (low blood albumin). Complications in other organs are similar to those occurring in ulcerative colitis. There is increased risk for colon cancer and cancer of the small intestine with long-standing disease.42
EVALUATION AND TREATMENT
The diagnosis and treatment of Crohn’s disease are similar to the diagnosis and treatment of ulcerative colitis. Surgery is generally performed to manage complications such as fistula, abscess or obstruction. Routine endoscopy for cancer screening should be performed for long-standing disease.
The new treatment adalimumab (anti-TNF-α) has improved the disease for 85% of patients in Australia, with 60% of patients in remission.35 It has also improved quality of life for patients, as a decrease in symptoms is accompanied by an alleviation of depression.43 Mesalazine is often ineffective; alternatively, there is a focus on anti-inflammatory medications, including steroids.37 Intravenous administration of iron may be required, as the inflammatory tissue may prevent absorption of oral iron.37
Irritable bowel syndrome
Irritable bowel syndrome is a functional gastrointestinal disorder with no specific structural or alterations as a cause of disease. It is broadly characterised by abdominal pain and discomfort associated with altered bowel habits. About 20% of the world’s population is estimated to have the disorder and it is more common in women, with a higher prevalence during youth and middle age. Individuals with this syndrome are also more likely to have anxiety and depression. Symptoms of irritable bowel syndrome can negatively affect quality of life and activity and present a significant economic burden.44
PATHOPHYSIOLOGY
The pathophysiology of irritable bowel syndrome is difficult to define, as structural changes do not occur. Some alternative mechanisms for explaining the symptoms are listed here.
CLINICAL MANIFESTATIONS
Irritable bowel syndrome is characterised by lower abdominal pain or discomfort; predominant diarrhoea, predominant constipation or alternating diarrhoea and constipation; gas and bloating; and nausea. Symptoms are usually relieved with defecation and do not interfere with sleep.
EVALUATION AND TREATMENT
The diagnosis of irritable bowel syndrome is based on signs and symptoms, as there are no specific tests to confirm this syndrome. The diagnostic criteria include abdominal pain being relieved by defecation, and changes in the frequency and appearance of the faeces.51 Diagnostic procedures to rule out other causes of symptoms may include endoscopic evaluations, scans or abdominal ultrasound, blood tests, and tests for lactose intolerance or coeliac disease (discussed in ‘Nutritional disorders’ below). The patient may be evaluated for food allergies, parasites or bacterial growth.
There is no cure for irritable bowel syndrome and treatment is individualised to symptoms. Options may include laxatives and fibre, antidiarrhoeals, antispasmodics, low-dose antidepressants and visceral analgesics. Drugs that interact at the serotonin (or 5-hydroxytryptomine) receptors are used for more severe symptoms; for example, 5-hydroxytryptomine 4 receptor agonists may be used for severe constipation, and 5-hydroxytryptomine 3 receptor antagonists may be used for severe diarrhoea. Alternative therapies include probiotics. Research continues to advance the management of this complex syndrome.52
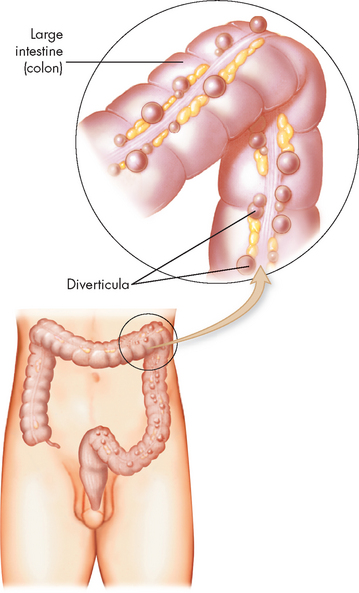
Diverticular disease
Diverticula are herniations or saclike outpouchings of mucosa through the muscle layers, usually in the wall of the sigmoid colon. Diverticulosis is asymptomatic diverticular disease. Diverticulitis represents inflammation. Diverticular disease is most common in the elderly, but the incidence is increasing in younger individuals, particularly when much of the diet consists of refined foods.
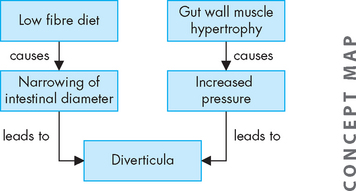
PATHOPHYSIOLOGY
Although diverticula can occur anywhere in the gastrointestinal tract, the most common site is the sigmoid colon. They arise from increases in intraluminal pressure, particularly at weak points in the colon wall, usually where arteries penetrate the tunica muscularis to nourish the mucosal layer (see Figure 27-8). A common associated finding is thickening of the circular and longitudinal muscles surrounding the diverticula.53 Hypertrophy and contraction of these muscles increase intraluminal pressure and degree of herniation. Regular consumption of a low-fibre diet reduces faecal bulk, thus reducing the diameter of the colon. The resulting pressure within the narrow lumen can increase enough to rupture the diverticula (see Figure 27-9).
CLINICAL MANIFESTATIONS
Symptoms of diverticular disease may be vague or absent. Cramping pain of the lower abdomen can accompany constriction of the hypertrophied colonic muscles. Diarrhoea, constipation, distension or flatulence may occur. If the diverticula become inflamed or abscesses form, the individual develops fever, leucocytosis (increased white blood cell count) and tenderness of the lower left quadrant. Severe complications, such as haemorrhage, peritonitis, bowel obstruction and fistula formation, are rare.
EVALUATION AND TREATMENT
Diverticula are often discovered during diagnostic procedures performed for other problems. Sigmoidoscopy permits direct observation of the lesions. Ultrasound and barium enema reveal the muscle hypertrophy, but barium may become trapped in the diverticula and form hard masses. Abdominal CT scanning is used for complicated cases.
An increase of dietary fibre intake often relieves symptoms. Importantly, fluid intake must also be increased for fibre to be effective. Surgical resection may be required for diverticulitis or if there are severe complications.54
Appendicitis
Appendicitis is an inflammation of the appendix and is the most common surgical emergency of the abdomen as it affects 7–12% of the population. It generally occurs between 20 and 30 years of age, although it may develop at any age. Almost 20,000 Australians required hospitalisation for this condition in 2007–2008.55
PATHOPHYSIOLOGY
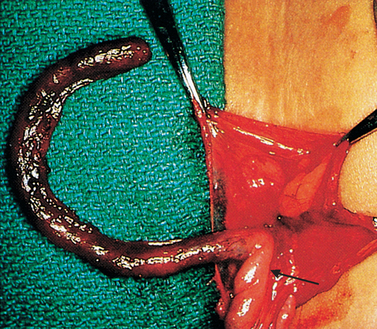
The exact mechanism of the cause of appendicitis is controversial. Obstruction of the lumen with stool, tumours or foreign bodies with consequent bacterial infection is the most common theory. The obstructed lumen does not allow drainage of the appendix and, as mucosal secretion continues, intraluminal pressure increases. The increased pressure decreases mucosal blood flow and the appendix becomes hypoxic. The mucosa ulcerates, promoting bacterial or other microbial invasion with further inflammation and oedema. Inflammation may involve the distal or entire appendix. Gangrene develops (see Figure 27-10) from thrombosis of the luminal blood vessels, followed by perforation of the appendix.56
CLINICAL MANIFESTATIONS
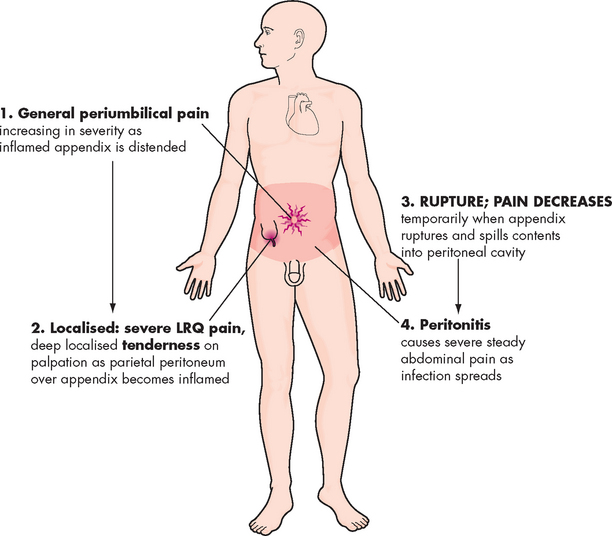
Gastric or peri-umbilical (around the umbilical region) pain is the typical symptom of an inflamed appendix (see Figure 27-11). The pain may be vague at first, increasing in intensity over 3–4 hours. It may subside and then recur in the right lower quadrant, indicating extension of the inflammation to the surrounding tissues. Nausea, vomiting and anorexia follow the onset of pain and a low-grade fever is common. Diarrhoea occurs in some individuals, particularly children; others have a sensation of constipation. Perforation, peritonitis and abscess formation are the most serious complications of appendicitis.
EVALUATION AND TREATMENT
In addition to clinical manifestations, the clinician can usually locate the painful site with one finger. Rebound tenderness (pain evident upon sudden release of pressure from the abdomen, usually indicative of inflammation in the peritoneum) is usually referred to in the right lower quadrant. There may be an increased white blood cell count, indicative of inflammation (see Chapter 13). Ultrasonography and CT scans can assist in differentiating appendicitis from perforated ulcer or cholecystitis. Laparoscopy (insertion of a scope into the abdominal cavity) may be necessary.
Appendectomy is the treatment for simple or perforated appendicitis. This surgery provides quick recovery for simple appendicitis.
Gastritis
Gastritis is an inflammatory disorder of the gastric mucosa. It can be acute or chronic and affect the fundus or antrum, or both.
Acute gastritis erodes the surface epithelium and usually results only in superficial damage. The stomach’s protective mucosal barrier is damaged by some drugs, with aspirin well-known for being harsh on the stomach lining. Aspirin and some other anti-inflammatory drugs may cause gastritis by inhibiting prostaglandins that normally stimulate the secretion of mucus. Hence, there is less protective mucus in the stomach. Digoxin, a drug used to increase the force of contraction of the heart, can also contribute to the development of gastritis. Acute gastritis occurs most commonly due to alcohol consumption. The clinical manifestations of acute gastritis can include vague abdominal discomfort, epigastric tenderness (in the upper middle portion of the abdomen) and bleeding. Healing usually occurs spontaneously within a few days. Discontinuing injurious drugs, using antacids or decreasing acid secretion facilitates healing.
Chronic gastritis tends to occur in the elderly and causes thinning and degeneration of the stomach wall. Chronic fundal gastritis, also called atrophic gastritis, is the most severe type. The gastric mucosa degenerates extensively in the body and fundus of the stomach, leading to gastric atrophy. Loss of chief cells and parietal cells diminishes acid secretion. Pernicious anaemia develops because less intrinsic factor is secreted to facilitate vitamin B12 absorption in the ileum. The pathogenesis may result from an autoimmune disorder, as indicated by the presence of antibodies to the parietal cells, intrinsic factor and gastric cells. Helicobacter pylori infection can also promote mucosal atrophy and tissue injury.57 Chronic fundal gastritis is a risk factor for gastric carcinoma, particularly in individuals who develop pernicious anaemia.
Chronic antral gastritis generally involves the antrum only and occurs approximately four times more often than fundal gastritis. It is not associated with decreased hydrochloric acid secretion, pernicious anaemia or the presence of parietal cell antibodies. Helicobacter pylori is also a major causative factor57 and mucosal atrophy is rare. In approximately 10% of cases, antibodies to gastrin-secreting cells are found in the serum. Chronic reflux of bile and pancreatic enzymes may contribute to the gastritis by persistently disrupting the mucosal barrier.
Gastroscopy (direct visualisation of the stomach with a camera called an endoscope) and biopsy may show a long-standing inflammatory process and gastric atrophy in an individual with no history of abdominal distress. Gastric secretions can be evaluated for hydrochloric acid and intrinsic factor secretion. Individuals may report vague symptoms, including anorexia, fullness, nausea, vomiting and epigastric pain. Gastric bleeding may be the only clinical manifestation of gastritis.
Symptoms can usually be managed with smaller meals; a soft, bland diet; and avoidance of alcohol and aspirin. Helicobacter pylori infection is treated with antibiotics, and vitamin B12 is administered to correct pernicious anaemia.
Peptic ulcer disease
A peptic ulcer is a break, or an ulceration, in the protective mucosal lining of the stomach or duodenum. Such breaks expose submucosal areas to gastric secretions and autodigestion (digestion of gut mucosa by the body’s secretions). Peptic ulcers can be acute or chronic, superficial or deep. Superficial ulcerations are called erosions because they erode the mucosa but do not penetrate the muscularis mucosae. True ulcers extend through the muscularis mucosae and damage blood vessels, causing haemorrhage, or perforate the gastrointestinal wall (see Figure 27-12). Risk factors for peptic ulcer disease are summarised in the box ‘Risk factors: peptic ulcer’.
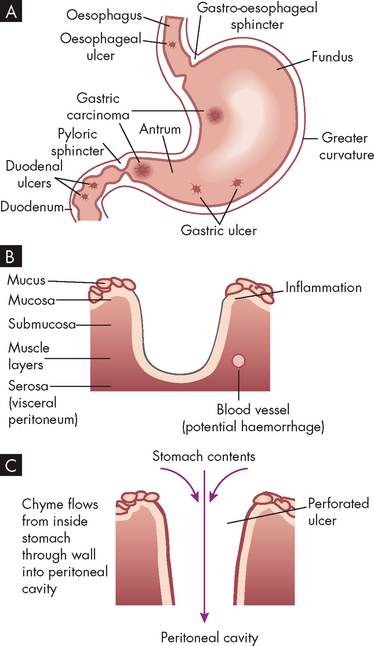
A Common locations. B Peptic ulcer. C Perforated ulcer.
Source: Gould BE. Pathophysiology for the health professions. 3rd edn. Philadelphia: Saunders; 2006.
Source: Hawkey CJ et al. Relative contribution of mucosal injury and Helicobacter pylori in the development of gastroduodenal lesions in patients taking non-steroidal anti-inflammatory drugs. Gut 2002; 51(3):336–343; Yuan Y, Padol IT, Hunt RH. Peptic ulcer disease today. Nat Clin Pract Gastroentrol Hepatol 2006; 3(2):80–89.
Gastric ulcers
Gastric ulcers are ulcers of the stomach and occur about equally in males and females, usually between the ages of 55 and 65 years. They are about one-quarter as common as duodenal ulcers (see Table 27-6).
Table 27-6 CHARACTERISTICS OF GASTRIC AND DUODENAL ULCERS
| CHARACTERISTICS | GASTRIC ULCER | DUODENAL ULCER |
|---|---|---|
| Incidence | ||
| Age at onset | 50–70 years | 20–50 years |
| Family history | Usually negative | Positive |
| Sex (prevalence) | Equal in women and men | Greater in men |
| Stress factors | Increased | Average |
| Ulcer producing drugs | Normal use | Increased use |
| Cancer risk | Increased | Not increased |
| Pathophysiology | ||
| Abnormal mucus | May be present | May be present |
| Parietal cell mass | Normal or decreased | Increased |
| Acid production | Normal or decreased | Increased |
| Serum gastrin | Increased | Normal |
| Associated gastritis | More common | Usually not present |
| Helicobacter pylori | Usually present (95–100%) Stimulates acid hypersecretion | |
| Clinical manifestations | ||
| Pain | ||
| Clinical course | ||
PATHOPHYSIOLOGY
Use of non-steroidal anti-inflammatory drugs (NSAIDs) and Helicobacter pylori infection are major causes of gastric ulcer. Generally, gastric ulcers develop in the antral region, adjacent to the acid-secreting mucosa of the body of the stomach. The mucosal barrier becomes altered, such that acid (hydrogen ions) can penetrate to deeper tissue layers more easily. Gastric secretion may be normal or less than normal.
Reflux of bile from the duodenum into the stomach disrupts the gastric mucosa and can permit acid (hydrogen ions) to diffuse into the mucosa, where they damage cellular structure (see Figure 27-13). A vicious cycle can be established as the damaged mucosa liberates histamine, which further stimulates the increase of acid production (see Figure 27-14). Destruction of small vessels causes bleeding.
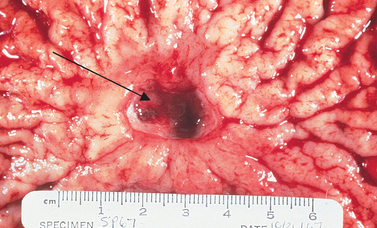
Folds in the stomach mucosa surround the ulcer (arrow), seen as a break in the mucosa and filled with blood.
Source: Patton KT, Thibodeau GA. Anatomy & physiology. 7th edn. St Louis: Mosby; 2010.
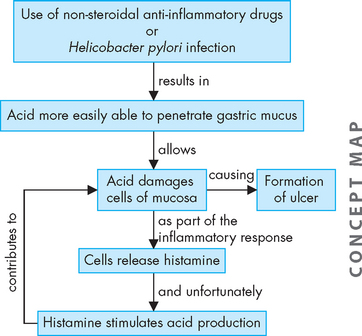
FIGURE 27-14 A combination of acid penetrating the mucosa and increased acid production contribute to the formation of ulcers.
Chronic gastritis is often associated with development of gastric ulcers and may precipitate ulcer formation by limiting the mucosa’s ability to secrete a protective layer of mucus.
CLINICAL MANIFESTATIONS
The main clinical manifestation of gastric ulcers is intermittent pain in the epigastric region. Pain is relieved by ingestion of food, which creates a typical pain–food–relief pattern. However, the pain of gastric ulcers also may occur immediately after eating. Gastric ulcers tend to be chronic rather than alternating between periods of remission and exacerbation. Gastric ulcers cause anorexia, vomiting and weight loss. Complications include perforation of ulcer, resulting in acute bleeding, a common cause of mortality.
Duodenal ulcers
Duodenal ulcers occur with greater frequency than other types of peptic ulcers and tend to develop in younger persons.58
PATHOPHYSIOLOGY
Infection with Helicobacter pylori and use of NSAIDs are the major cause of duodenal ulcer.59 Hypersecretion of acid and pepsin is the primary cause and inadequate secretion of bicarbonate by the duodenal mucosa may also be involved.60 Factors that contribute to ulcer formation include the following:
All these factors, singly or in combination, cause acid and pepsin concentrations in the duodenum to penetrate the mucosal barrier and cause ulceration.
CLINICAL MANIFESTATIONS
Clinical manifestations of duodenal ulcers are similar to gastric ulcers, with the main symptom of chronic intermittent pain in the epigastric area. Some individuals may have no symptoms; the first manifestation may be haemorrhage or perforation, particularly with a history of aspirin or anticoagulant use.
Complications include bleeding, perforation and obstruction of the duodenum or outlet of the stomach. Bleeding is the most common cause of mortality, particularly among the elderly. Perforation occurs with destruction of all layers of the duodenal wall and causes sudden severe epigastric pain. Obstruction may be the result of oedema from inflammation or scarring from chronic injury.
Duodenal ulcers often heal spontaneously but recur within months without treatment. Relief of pain accompanies healing. Bleeding from duodenal ulcers causes haematemesis (vomiting blood) or melaena (digested blood appearing in the faeces).
Stress ulcers
A stress ulcer is an acute form of peptic ulcer that tends to accompany severe illness, systemic trauma or neural injury.61 Emotional stress may cause peptic ulcer.62 Usually multiple sites of ulceration are distributed within the stomach or duodenum. Decreased mucosal blood flow, mucosal ischaemia and reperfusion injury are important contributing events in stress ulcer formation.63 Stress ulcers may be classified as follows:
The primary clinical manifestation of stress ulcers is bleeding. Acid suppression with antacids and proton pump inhibitors may provide the best prophylactic treatment.61 Stress ulcers seldom become chronic.
Treatment of ulcers
The evaluation and treatment of gastric and duodenal ulcers are similar. X-ray examinations using barium may show an anatomical deformity created by the ulcer crater. Flexible endoscopic evaluations may also be performed. Testing gastrin levels can identify ulcers associated with gastric carcinomas (see Table 27-6). Helicobacter pylori can be detected by endoscopic evaluation and biopsy, serology or non-invasively with a urea breath test or stool antigen.64
Management of duodenal and gastric ulcers is aimed at: (1) relieving the causes and effects of hyperacidity; (2) administering antacids and drugs that reduce acid secretion (such as proton pump inhibitors) and antibiotics, if Helicobacter pylori is present.65 Lifestyle modifications can assist in the treatment of ulcers. For example, smoking cessation has been shown to assist ulcer healing. The risk of ulcer may be reduced with a diet high in vitamin A and fibre.66 Endoscopic heater probes (which diathermy the ulcer) are effective to stop bleeding. Complications are treated with either endoscopic or surgical approaches.
Necrotising enterocolitis
Necrotising enterocolitis is an ischaemic, inflammatory condition of the bowel that causes necrosis (cell damage), perforation and death if untreated. Necrotising enterocolitis occurs primarily in premature infants; affected infants have a mean gestational age of 31 weeks and weigh less than 1500 g (this is less than half the average full-term birth weight). As premature birth is associated with increased maternal age, the incidence of this disorder may increase as women in Australia and New Zealand have their babies at older ages. The risk of necrotising enterocolitis decreases as the gastrointestinal tract matures. The cause is unknown.67
PATHOPHYSIOLOGY
The exact pathophysiological mechanisms responsible for necrotising enterocolitis have yet to be identified. It is likely that the condition is multifactorial with several mechanisms proposed. Factors contributing to the development of necrotising enterocolitis include maternal age older than 35 years, infections, immunological injury, perinatal stress and the effects of medications and feeding practices. Accumulation of gas in the mucosa and submucosa leads to reduced mucosal blood flow, ischaemia and necrosis of intestinal segments. The injury leads to release of inflammatory mediators, bacterial invasion or perforation of the bowel wall, or both.68
CLINICAL MANIFESTATIONS
Manifestations of necrotising enterocolitis usually appear within 2 weeks of birth. They range from mild abdominal distension to bowel perforation, sepsis and death. Abdominal pain, unstable temperature, bradycardia (low heart rate) and apnoea (not spontaneously breathing) are nonspecific signs. Affected infants have occult or grossly bloody stools, gastric retention, abdominal distention and septicaemia (infection within the blood), with elevated white blood cell and falling platelet counts. Premature infants often have more severe disease and other disorders such as respiratory distress syndrome (see Chapter 25).
EVALUATION AND TREATMENT
Diagnosis is based on clinical manifestations, laboratory results and plain films of the abdomen that show gas accumulation in the intestine. Prevention of necrotising enterocolitis includes prenatal glucocorticoids and standardised feeding schedules.69,70 Treatments include cessation of feeding, gastric suction to decompress the intestines, fluid and electrolyte maintenance, and administration of antibiotics to control sepsis. Surgical resection and peritoneal drainage are the treatment of choice for perforation;71,72 however, for very ill infants weighing less than 1000 g, peritoneal drainage without laparotomy may improve survival.73 Overall mortality is 20–40%; however, the incidence is decreasing.74
Nutritional disorders
Lactose intolerance
Lactose intolerance occurs in people who are deficient in the enzyme necessary for digesting lactose, a sugar (carbohydrate) found in milk. As you may recall from Chapter 26, the names of most digestive enzymes end in the letters ‘-ase’; this is also true for the enzyme lactase, responsible for the breakdown of lactose (milk sugar). Normally, lactase breaks down lactose into the smaller components, glucose and galactose; however, when the lactose cannot be digested, it is not absorbed across the intestinal wall.
PATHOPHYSIOLOGY
The deficiency of the enzyme lactase allows undigested lactose to remain in the intestines, where bacterial fermentation causes large quantities of gases to form. Undigested lactose also increases the osmotic gradient in the intestines, causing fluids to remain in the intestines and leading to irritation and osmotic diarrhoea.
CLINICAL MANIFESTATIONS
Clinical manifestations of lactase deficiency are bloating, crampy abdominal pain, diarrhoea and flatulence. Because of the fluid volume that remains in the intestines (rather than being absorbed), dehydration may also occur. Lactase deficiency usually does not develop until adulthood. Secondary (acquired) lactase deficiency can be caused by several diseases of the intestine, including coeliac disease, gastroenteritis and bacterial overgrowth.
EVALUATION AND TREATMENT
The disorder is diagnosed by a lactose-tolerance test, whereby blood glucose levels are monitored following ingestion of lactose; in the lactose-intolerant person, the blood glucose level will not rise normally, as the lactose is not digested to glucose and absorbed into the blood. Avoiding milk and adhering to a lactose-free diet relieves symptoms.
Hydrogen lactose breath testing, a biopsy of the jejunum and a laboratory test for enzyme activity may also assist with diagnosis.16 Treatment consists of reducing milk consumption; other dairy products such as cheese may need to be increased in the diet to ensure adequate calcium intake.16 Alternatively, oral lactase supplementation may be used to replace the deficient enzyme. Some people can tolerate lactose in fermented forms, such as cheese and yoghurt, or by adding soy food.75
Coeliac disease
Coeliac disease (or gluten-sensitive enteropathy) is the loss of mature intestinal villous epithelium caused by hypersensitivity to gluten, the protein component of cereal grains. The gluten in wheat, rye, oats and barley causes a T cell–mediated (see Chapter 12) autoimmune injury to the intestinal epithelial cells of genetically susceptible individuals.76 There is currently discrepancy in the literature regarding whether oats are detrimental or not, as some individuals with coeliac disease are able to tolerate oats much better than others.77,78 Pathogenesis appears to be complex, involving dietary, genetic and immunological factors. Coeliac disease has been associated with other immune disorders, including diabetes mellitus and thyroid disease.79
The number of patients diagnosed with coeliac disease is on the rise, with approximately 1% of the population affected.80 Coeliac disease was previously considered a disorder of early childhood; however, it is now apparent that only 20% of cases are diagnosed before the age of 20, with many people being diagnosed between the ages of 30 and 40 and some people being diagnosed in their 60s.16,81 There is also evidence that many people are undiagnosed and may be asymptomatic.82
PATHOPHYSIOLOGY
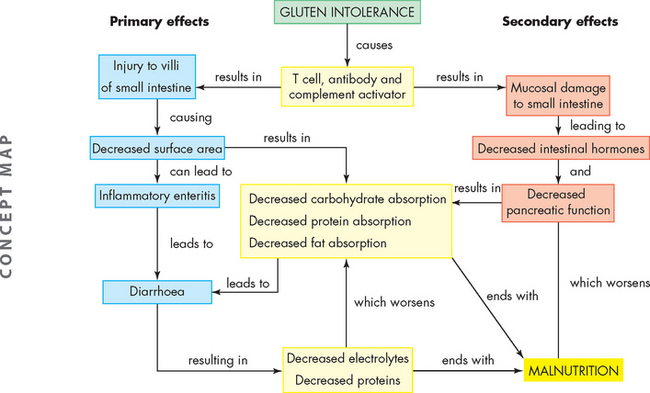
The major pathophysiological characteristics of coeliac disease are atrophy and flattening of villi in the duodenum and jejunum of the small intestine, which results in malabsorption of most nutrients (see Figure 27-15). Gluten leads to destruction of mucosal cells, which causes inflammation, and water and electrolytes are secreted, leading to watery diarrhoea. Potassium loss leads to muscle weakness. Magnesium and calcium malabsorption can cause seizures or tetany (involuntary contraction of skeletal muscles, often causing pain). Unabsorbed fatty acids combine with calcium within the intestinal lumen, and phosphorus excretion leads to bone reabsorption. The secretion of intestinal hormones, such as secretin and cholecystokinin, may be diminished, so secretion of pancreatic enzymes and expulsion of bile from the gallbladder decrease, contributing to malabsorption.
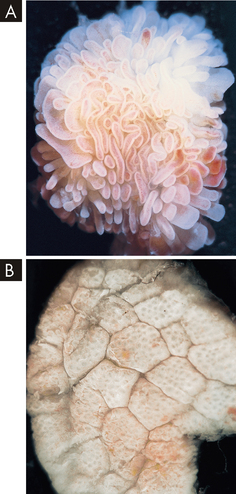
FIGURE 27-15 A Normal small intestinal mucosa with villi appearing as fingers and leaves. B Coeliac disease — flat intestinal mucosa with total villus atrophy.
Source: Gould BE. Pathophysiology for the health professions. 3rd edn. Philadelphia: Saunders; 2006.
Fat malabsorption in the jejunum is the major cause of steatorrhoea (fatty stools). Deficiencies of fat-soluble vitamins are common in those with coeliac disease. Vitamin K malabsorption leads to hypoprothrombinaemia (deficiency of prothrombin, integral for blood clotting; see Chapter 16). In one-third of cases, iron and folic acid malabsorption is manifested as cheilosis (chapped lips and mouth), anaemia and a smooth red tongue. Vitamin B12 absorption is impaired in those with extensive ileal disease, and folate and iron deficiencies are common (see Figure 27-16). As autoimmune antibodies circulate in the blood with this disease, other systemic symptoms include skin, liver and joint conditions.83
CLINICAL MANIFESTATIONS
The onset of clinical manifestations of coeliac disease in children depends on the infant’s age when gluten-containing cereals are added to the diet. In 50% of affected children, onset occurs by 18 months of age, with latent intervals varying from months to years. The classic symptoms in children diagnosed under the age of 2 years are diarrhoea, irritability and weight loss,84 with diarrhoea being a usual early sign. The stools are pale, bulky, greasy and foul smelling. As early as 3 or 4 months of age, growth failure,85 anorexia and constipation can begin. In older children, constipation is occasionally seen despite steatorrhoea (fatty stools). Abdominal pain is more prominent in older children.84
In children, manifestations of malabsorption, such as rickets, tetany, frank bleeding or anaemia, may be obvious. The tongue is smooth and red and the child may bruise and bleed easily. Hypomagnesaemia and hypocalcaemia (low blood levels of magnesium and calcium) cause irritability, tremor, convulsions, tetany, bone pain, osteomalacia and dental abnormalities. If vitamin D deficiency is prolonged, rickets (see Chapter 21) and clubbing of the terminal phalanges are likely. Eighty-six per cent of older children have fingerprint changes (ridge atrophy). In older children, delayed puberty and infertility may be manifestations of otherwise subtle coeliac disease.86
In adults, symptoms are similar, with diarrhoea being the main symptom, as well as bloating and abdominal pain. Other symptoms such as tiredness and anaemia result from malabsorption of nutrients.16 While previously there was a tendency for the development of osteoporosis (as the damaged small intestine could not absorb sufficient calcium), it is now common practice to screen all diagnosed coeliacs using bone scans (dual-energy X-ray absorptiometry) to allow early identification of those at particular risk.87
EVALUATION AND TREATMENT
Blood tests for autoantibodies (IgA, IgG, antigliaden from gluten and anti-tissue transglutaminase) are useful and intestinal biopsy is mandatory to detect the classic mucosal changes caused by coeliac disease, particularly the loss of villi. The initial biopsy is generally followed by a second intestinal biopsy to demonstrate regeneration of intestinal villi after treatment with a gluten-free diet. A wide variety of screening tests for malabsorption may also be useful.
Treatment consists of the immediate and permanent institution of a diet free of cereal grains (wheat, rye, barley and oats). As products and flours from these cereals can be found in a wide variety of foods, patient education is critical for adherence to a gluten-free diet. Lactose intolerance may accompany coeliac disease, due to the destruction of villi in the small intestine where the enzyme lactase is located. Infants and adults may need vitamin D, iron and folic acid supplements to treat deficiencies. There is an increased incidence of malignant disease, particularly lymphoma, in individuals who fail to respond to gluten-free diets.88
Malnutrition
Malnutrition is lack of nourishment from inadequate amounts of kilojoules (calories), protein, vitamins or minerals, and is caused by improper diet, alterations in digestion or absorption, or a combination of these factors. It is perhaps surprising to learn that malnutrition is a relatively common complaint for groups of our population — approximately 20% of hospitalised patients are malnourished,89 while approximately 30% of patients in public acute hospitals and 50% of residents of aged-care facilities have malnutrition in Australia.90 Furthermore, almost two-thirds of patients undergoing rehabilitation for conditions such as stroke either have malnutrition or are at risk of it developing.91 The importance of this issue cannot be underestimated, as malnutrition limits the body’s ability to recover from illness, as well as decreasing the overall health status of the elderly population.
Starvation
Starvation is a state of extreme malnutrition and hunger from lack of nutrients. Short-term starvation (1–14 days of fasting) and long-term starvation (14–60 days of fasting) have different effects.92,93 Therapeutic short-term starvation is part of some weight-reduction programs because it causes an initial rapid weight loss that reinforces the individual’s motivation to diet. Therapeutic long-term starvation is used in medically controlled environments to facilitate rapid weight loss in morbidly obese individuals. Pathological long-term starvation can be caused by poverty, chronic diseases of the cardiovascular, pulmonary, hepatic, renal and digestive systems; malabsorption syndromes; and cancer. Cachexia is physical wasting with loss of weight and muscle atrophy, fatigue and weakness. Inflammatory mediators (i.e. TNF-α, interferon gamma or interleukin 6) associated with advanced cancer, AIDS, tuberculosis and other major chronic progressive diseases contribute to cachexia. Anorexia (loss of appetite) and cachexia (severe tissue wasting) often occur together. Cachexia is not the same as starvation. A healthy person’s body can adjust to starvation by slowing metabolism, but in cachexia the body does not make this adjustment.
Re-feeding syndrome
Re-feeding syndrome occurs in severely malnourished individuals when parenteral or enteral nutritional therapy is initiated. During starvation, loss of body minerals causes the movement of phosphate, magnesium and potassium out of the cells and into the plasma. When re-feeding starts, an increase in insulin levels stimulates the intracellular movement of glucose and these ions; subsequently, the plasma concentrations can decrease to dangerously low levels causing hypophosphataemia, hypomagnesaemia and hypokalaemia (low phosphate, magnesium and potassium in the blood). Rapid expansion of the extracellular fluid volume can also occur with carbohydrate re-feeding and may cause fluid overload. Hypophosphataemia contributes to alterations in red blood cell shape and function, contributing to tissue hypoxia and increased respiratory drive. The consequences of these alterations include life-threatening arrhythmias, congestive heart failure, muscle weakness (including the respiratory muscles) and death. Individuals at greatest risk are those with starvation from any cause including anorexia nervosa, chronic alcoholism, morbid obesity with massive weight loss and prolonged fasting. Re-feeding syndrome is prevented by slowly reinstituting feeding and monitoring plasma phosphate, potassium, magnesium and calcium.
Source: Marinella MA. Refeeding syndrome and hypophosphatemia. J Intensive Care Med 2005; 20(3):155–159; Azumagawa K et al. Anorexia nervosa and refeeding syndrome: a case report. Scientific World J 2007; 7:400–403; Ladage E. Refeeding syndrome. ORL Head Neck Nurs 2003; 21(3):18–20.
Short-term starvation, or extended fasting, consists of several days of total dietary abstinence or deprivation. Once all available energy has been absorbed from the intestine, glycogen (stores of glucose) in the liver is released to the blood as glucose (through glycogenolysis) after 4–8 hours. Following this, other molecules (such as lactate and amino acids) are used by the liver to produce glucose (by gluconeogenesis). Both of these processes deplete stored nutrients and thus cannot meet the body’s energy needs indefinitely. Fatigue decreases physical activity and energy expenditure. Interestingly, some hospitalised patients may have short-term starvation. Patients who are kept nil by mouth for days, due to a range of reasons, may experience a decrease in dietary energy intake, because the intravenous fluids provide very little nutritional value.
Long-term starvation begins after several days of dietary abstinence and eventually causes death. The major characteristic of long-term starvation is an increased use of ketone bodies (products of lipid and pyruvate metabolism) as a cellular energy source. Depressed insulin and glucagon levels result in lipid stores being released as fatty acids (through lipolysis), which supply energy to cardiac and skeletal muscle cells. Although this meets most energy needs of the cells, glucose is still an essential fuel for neurons. Once the supply of adipose tissue is depleted, proteolysis begins (breakdown of proteins). The breakdown of muscle protein is the last process to supply energy for life. Organ failure of the renal and respiratory systems follows and death results from cardiac failure due to severe alterations in electrolyte balance.
Adequate ingestion of appropriate nutrients is the obvious treatment for starvation — see the box ‘Health alert: refeeding syndrome’. In medically induced starvation, the body is maintained in a ketotic state until the desired amount of adipose tissue has been lysed. Starvation imposed by chronic disease, long-term illness or malabsorption is treated with enteral or parenteral nutrition.
Failure to thrive
Failure to thrive is the inadequate physical development of an infant or a child. It is manifested as a deceleration in weight gain, a low weight/height ratio or a low weight/height/head circumference ratio. It is a nutritional disorder that may be due to underlying pathophysiology or psychosocial causes.
PATHOPHYSIOLOGY
Pathophysiological causes of failure to thrive include gastro-oesophageal reflux, pyloric stenosis (narrowing of the pylorus), gastroenteritis, infection by intestinal parasites or congenital anomalies, or chronic diseases of major body systems. All these disorders reduce the availability of nutrients for maintenance and growth. This can also create developmental problems, psychosocial problems and emotional problems for the child.
Failure to thrive may also result from environmental causes and can be complicated by families having inadequate economic resources and parental lack of knowledge. Parental stressors may include:
CLINICAL MANIFESTATIONS
Clinical manifestations are retarded growth accompanied by manifestations of the underlying disease. For the psychosocial causes, failure to thrive involves retarded growth plus reduced energy level, reduced responsiveness and interaction with the environment, social isolation, spasticity and rigidity when held or touched, inability to make eye contact or smile and rejection of foods. Weight loss and decelerated growth are accompanied by overall retardation of development.
EVALUATION AND TREATMENT
Failure to thrive is suggested if a child exhibits particularly slow growth (falls below the third percentile on the growth curve or is falling off a previously established growth curve). If no genetic, endocrine or other systemic disorders are identified and if the physical and laboratory examinations show no abnormalities other than delayed growth, an environmental cause is indicated.
Hospital admission is recommended if the diagnosis is unclear or the child is in nutritional or emotional jeopardy. Eating patterns, food preferences, kilojoule intake and family interactions can be assessed during the hospital stay. If the cause is environmental, the hospitalised child usually begins to gain weight. If a pathophysiological problem has been identified, management of failure to thrive consists of treating the cause. Management involves the immediate total care of the child and measures to address both the psychosocial and the emotional problems of the caregivers and the parent–child interactions.94
Obesity
Excessive intake of food or energy can lead to obesity — this is an epidemic in countries such as Australia and New Zealand and is discussed in detail in Chapter 35.
Disorders of motility
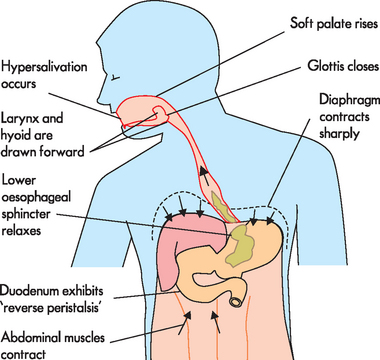
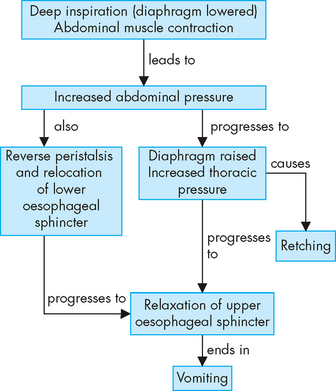
Gastro-oesophageal reflux
Gastro-oesophageal reflux is the reflux of chyme (partially digested food mixed with gastric secretions) from the stomach to the oesophagus. The lower oesophageal sphincter may relax inappropriately and transiently 1–2 hours after eating, permitting gastric contents to regurgitate into the oesophagus. The acid is usually cleared from the oesophagus by peristaltic action within 1–3 minutes and the sphincter then closes again. In some individuals, however, a combination of factors causes an inflammatory response to reflux called reflux oesophagitis.95 This is a relatively common condition, with more than 50,000 Australians requiring hospitalisations for gastro-oesophageal reflux disease in 2007–2008.55
PATHOPHYSIOLOGY
Most of the time, the lower oesophageal sphincter remains closed, thereby preventing gastro-oesophageal reflux (see Figure 27-17). In individuals who develop reflux oesophagitis, this muscle tone tends to be lower than normal, such that the sphincter is contracted less. Vomiting, coughing, lifting or bending that increases abdominal pressure can contribute to the development of reflux oesophagitis. The severity of the oesophagitis depends on the composition of the gastric contents and the length of time they are in contact with the oesophageal mucosa. As the lining of the oesophagus is not protected from acid (in the same way as the stomach mucosa), the oesophagus is quite susceptible to damage from the acidic chyme (see Figure 27-18). If the chyme is highly acidic or contains bile salts and pancreatic enzymes, reflux oesophagitis can be severe. In individuals with weak oesophageal peristalsis, refluxed chyme remains in the oesophagus longer than usual. Delayed gastric emptying contributes to reflux oesophagitis by both lengthening the period during which reflux is possible and increasing the acid content of chyme. Disorders that delay emptying include gastric or duodenal ulcers, which can cause pyloric oedema (swelling due to fluid), and hiatal hernia, which can weaken the lower oesophageal sphincter.96
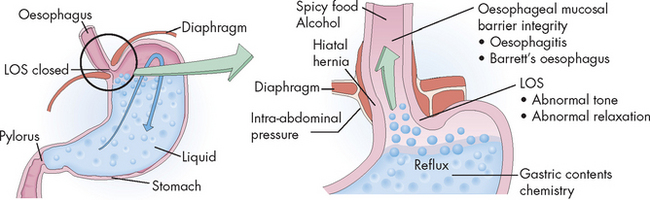
FIGURE 27-17 The causes of gastro-oesophageal reflux.
LOS = lower oesophageal sphincter.
Source: Damjanov I. Pathology. 3rd edn. Philadelphia: Saunders; 2009.
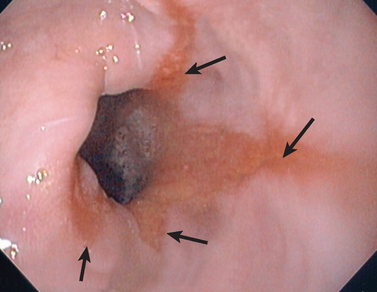
FIGURE 27-18 Gastro-oesophageal reflux disease.
Endoscopic view of oesophageal inflammation (oesophagitis) caused by ‘splashing back’ of acids from the stomach in a patient with gastro-oesophageal reflux disease. The arrows indicate the extent of the acid damage.
Source: Based on Patton KT, Thibodeau GA. Anatomy & physiology. 7th edn. St Louis: Mosby; 2010.
CLINICAL MANIFESTATIONS
The clinical manifestations of reflux oesophagitis are heartburn, acid regurgitation, dysphagia (difficulty swallowing), chronic cough, asthma and upper abdominal pain within 1 hour of eating. The symptoms worsen if the individual lies down or if intra-abdominal pressure increases (for example, as a result of coughing, vomiting or straining at defecation). Oedema, strictures, oesophageal spasm or decreased oesophageal motility may result in dysphagia with weight loss. Alcohol or acid-containing foods, such as citrus fruits, can cause discomfort during swallowing.
EVALUATION AND TREATMENT
Diagnosis of reflux oesophagitis is based on clinical manifestations, oesophageal endoscopy that shows oedema and erosion, and ambulatory pH monitoring of the oesophagus. Endoscopy also allows evaluation for metaplastic changes (termed Barrett’s oesophagus) and the development of oesophageal carcinoma.97 A barium swallow is used to identify associated conditions, such as hiatal hernia, gastric ulcers and abnormal contours of the oesophageal lumen.
Antacids relieve symptoms by neutralising gastric contents. Weight reduction and cessation of smoking also help to alleviate symptoms. Proton pump inhibitors (such as omeprazole) are the agents of choice for controlling symptoms and healing oesophagitis.98 Sucralfate will coat ulcerated tissue; smooth muscle stimulants (such as cisapride) can increase lower oesophageal sphincter motility and rate of gastric emptying. If other treatments fail or if reflux oesophagitis does not heal, the lumen of the lower oesophageal sphincter may be narrowed with laparoscopic surgery.
Gastro-oesophageal reflux disease
In newborns, gastro-oesophageal reflux is normal because neuromuscular control of the lower oesophageal (gastro-oesophageal) sphincter is not fully developed, thereby allowing return of stomach contents into the oesophagus. The frequency of reflux is highest in premature infants and decreases during the first 6–12 months of postnatal life. Normal infants and children have been shown to have some reflux but may be asymptomatic. Gastro-oesophageal reflux can progress to gastro-oesophageal reflux disease when complications such as bleeding or failure to thrive develop.99
PATHOPHYSIOLOGY
Delayed maturation of the lower oesophageal sphincter or impaired hormonal response mechanisms are possible causes. Factors that maintain integrity of this sphincter in children include anatomical issues such as the angle at which the oesophagus enters the stomach. Irritation of the mucosa by acidic gastric contents results in inflammation of the oesophageal epithelium and stimulation of the vomiting reflex.
CLINICAL MANIFESTATIONS
Of affected infants, 85% vomit excessively during the first week of life and usually have other symptoms by 6 weeks. Aspiration pneumonia develops in one-third of infants with gastro-oesophageal reflux. In cases that persist into childhood, chronic cough, wheezing and recurrent pneumonia are common. Inadequate retention of nutrients can adversely impact growth and weight gain. Oesophagitis resulting from exposure of the oesophageal mucosa to acidic gastric contents is manifested by pain, bleeding and eventually stricture formation and abnormal motility. Approximately 25% have iron deficiency anaemia caused by frank or occult blood loss.100
EVALUATION AND TREATMENT
The clinical manifestations are often adequate to confirm a diagnosis of gastro-oesophageal reflux. A barium swallow and oesophageal pH monitoring with a probe are useful diagnostic procedures in complex cases.
Mild gastro-oesophageal reflux resolves without treatment. Small, frequent feedings and frequent burping are also accepted strategies for managing reflux. Medications to increase motility, to increase lower oesophageal sphincter pressure or to decrease gastric acid production have been used to treat this condition. If no improvement is seen with medical management or the child has life-threatening events with reflux, an anti-reflux surgical procedure, including gastropexy (whereby the stomach is stitched to the abdominal wall) and fundoplication (whereby the fundus of the stomach is wrapped around the oesophageal sphincter and stitched to strengthen the valve such that reflux does not occur), is performed.101
Pyloric stenosis
Pyloric stenosis is an obstruction of the pyloric sphincter caused by hypertrophy of the sphincter muscle; the cause is unknown. One of the most common disorders of early infancy, it affects infants between the ages of 2 weeks and 4 months. The incidence of pyloric stenosis is approximately 1 to 5 in 1000.102 Full-term infants are affected more often than premature infants.
PATHOPHYSIOLOGY
Individual muscle fibres thicken, so the whole pyloric sphincter becomes enlarged and inelastic. The mucosal lining of the pyloric opening is folded and narrowed by the encroaching muscle. Because of the extra peristaltic effort necessary to force the gastric contents through the narrow pylorus, the muscle layers of the stomach may become hypertrophied (enlargement of the tissue) as well.
CLINICAL MANIFESTATIONS
Between 2 and 3 weeks after birth, an infant who has fed well and gained weight begins to vomit without apparent reason. The vomiting gradually becomes more forceful (projectile). Food is often regurgitated through the nose. The vomiting usually occurs immediately after eating and the vomitus (material that has been vomited) consists of the bulk of the feeding plus some food retained from previous feedings, but is almost always free of bile. In severe, untreated cases, increased gastric peristalsis and vomiting lead to severe fluid and electrolyte imbalances, malnutrition and weight loss that can be fatal within 4–6 weeks. Infants with pyloric stenosis are irritable because of hunger and they may have oesophageal discomfort caused by repeated vomiting and oesophagitis. The vomitus may be blood-streaked because of rupture of gastric and oesophageal vessels.
EVALUATION AND TREATMENT
Diagnosis is based on the history, clinical manifestations and findings on ultrasound.103 Occasionally, gastric peristalsis is observable over the abdomen. A firm, small, movable mass, approximately the size of an olive, is felt in the right upper quadrant in 70–90% of infants with pyloric stenosis. Ultrasound clearly shows the hypertrophied pyloric muscles and narrowed pyloric channel.
The standard treatment for hypertrophic pyloric stenosis is a pyloromyotomy, in which the muscles of the pylorus are surgically split and separated. Preoperative and postoperative medical management to correct fluid and electrolyte imbalance has been the key to the high success rate and low complication rates with this surgery.104
Some infants may respond to medical and nutritional management, which is based on the theory that the pylorus will open spontaneously by 6–8 months of age if nutrition can be maintained. Antispasmodic drugs are given to relax the pyloric spasm and the infant is re-fed after vomiting. Rehydration of infants following vomiting is particularly important, as infants are susceptible to dehydration. Endoscopic balloon dilation has also shown some success.
Faecal incontinence
Faecal incontinence may be due to an inability to have a voluntary bowel movement (linked to constipation) or an inability to control bowel movements (linked to diarrhoea). Although it affects those who were born with an anorectal malformation, it is being seen increasingly in the elderly, those with previous bowel surgery and older women who have had previous obstetric complications.105–107 4 6 Those with irritable bowel syndrome are at greater risk of faecal incontinence, due to the effects of diarrhoea.105 Faecal incontinence may also result from diarrhoea induced by laxatives or excessive consumption of some artificial sweeteners.
Although approximately 2% of the population are thought to suffer from faecal incontinence, in a rural Australian setting 20% of patients attending gynaecology and colorectal clinics were found to have faecal incontinence,106 indicating that there may be more sufferers than suspected. Many individuals may not report their symptoms to medical staff due to embarrassment, so underreporting may be common.
Treatment may include medications to assist in faecal bulk formation (Metamucil) and antidiarrhoeal drugs (loperamide). An individualised bowel management program, where the type of enema, medication and diet are adjusted based on the patient’s symptoms, and a contrast enema may be particularly useful.107
Structural abnormalities of the gastrointestinal tract
PATHOPHYSIOLOGY
Hiatal hernia is the protrusion (herniation) of the upper part of the stomach through the diaphragm and into the thorax (see Figure 27-19).109 The two most common types of hiatal hernia are as follows:
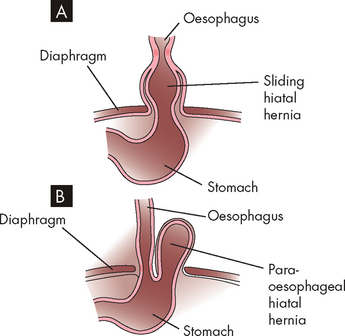
FIGURE 27-19 Types of hiatal hernia.
A Sliding hiatal hernia. Part of the stomach protrudes into the thoracic cavity by sliding superiorly. B Para-oesophageal hiatal hernia. Part of the stomach protrudes by sliding alongside the oesophagus.
Hiatal hernias of both types tend to occur in conjunction with several other diseases, including reflux oesophagitis, peptic ulcer, cholecystitis (gallbladder inflammation), cholelithiasis (gallstones), chronic pancreatitis and diverticulosis.
CLINICAL MANIFESTATIONS
Generally, a wide variety of symptoms develop later in life, although hiatal hernias are often asymptomatic. Manifestations include gastro-oesophageal reflux, dysphagia, heartburn and epigastric pain. Regurgitation and substernal discomfort after eating are common.
EVALUATION AND TREATMENT
Diagnostic procedures include examinations using barium as a contrast medium, endoscopy and chest X-ray. Treatment for sliding hiatal hernia is usually conservative. The individual can diminish reflux by eating small, frequent meals and avoiding the recumbent (lying down) position after eating. Abdominal supports and tight clothing should be avoided and weight control is recommended for obese individuals. Antacids alleviate reflux oesophagitis. Individuals who are uncomfortable at night benefit from sleeping in a semi-upright position. Surgery may be performed if medical management fails to control symptoms.
Intestinal obstruction
Intestinal obstruction can be caused by any condition that prevents the normal flow of chyme through the intestinal lumen (see Table 27-7). Obstructions can occur in either the small or the large intestine. Intestinal obstruction is classified by cause as simple or functional. Simple obstruction is mechanical blockage of the lumen; functional obstruction is a failure of motility (paralytic ileus), often occurring after abdominal surgery. Simple obstruction of the small intestine is the most common type of intestinal obstruction. Acute obstructions usually have mechanical causes, such as adhesions or hernias (see Figure 27-20A, D). Chronic or partial obstructions are more often associated with tumours or inflammatory disorders, particularly of the large intestine.
Table 27-7 COMMON CAUSES OF INTESTINAL OBSTRUCTION
| CAUSE | PATHOPHYSIOLOGY |
|---|---|
| Hernia | Protrusion of the intestine through a weakness in the abdominal muscles or through the inguinal ring |
| Intussusception | Telescoping of one part of the intestine into another; this usually causes strangulation of the blood supply; more common in infants 10–15 months of age than in adults |
| Volvulus | Twisting of the intestine on its mesenteric pedicle, with occlusion of the blood supply; often associated with fibrous adhesions; occurs most often in middle-aged and elderly men |
| Diverticulosis | Inflamed saccular herniations (diverticuli) of the mucosa and submucosa through the tunica muscularis of the colon; diverticuli are interspersed between thick, circular, fibrous bands; most common in obese individuals older than 60 years |
| Fibrous adhesions | Peritoneal irritation from surgery or trauma leads to formation of fibrin and adhesions that attach to intestine, omentum or peritoneum and can cause obstruction; most common in the small intestine |
| Paralytic ileus | Loss of peristaltic motor activity in the intestine; associated with abdominal surgery, peritonitis, hypokalaemia, ischaemic bowel, spinal trauma or pneumonia |
| Tumour | Tumour growth into the intestinal lumen; adenocarcinoma of the colon and rectum is the most common tumoral obstruction; most common in individuals older than 60 years |
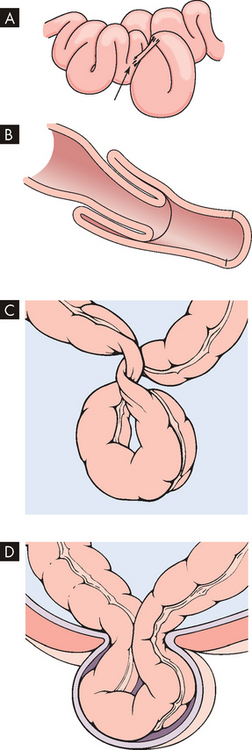
FIGURE 27-20 Intestinal obstructions.
A Constriction adhesions. B Intussusception. C Volvulus. D Hernia.
Source: A–C Damjanov I. Pathology for the health professions. 3rd edn. Philadelphia: Saunders; 2006; D Monahan FD et al. Phipps’ medical–surgical nursing: concepts and clinical practice. 8th edn. St Louis: Mosby; 2007.
Intussusception is the telescoping or invagination of one portion of the intestine into another (see Figure 27-20B). Usually, the ileum invaginates the caecum and part of the ascending colon by collapsing through the ileocaecal valve (ileocolic intussusception). In adults, it can occur after previous abdominal surgery and it accounts for 80–90% of intestinal obstructions in infants. As the proximal portion of the intestine collapses into the distal portion, it drags its mesentery into the enveloping lumen. This results in constriction of the mesentery and obstructs venous return. Oedema and compression obstruct the flow of chyme through the intestine. Unless the intussusception is treated, gangrene ensues.
Volvulus occurs when a section of the intestine twists on itself, resulting in an emergency as the intestinal blood supply is restricted (see Figure 27-20C). In children, it can occur due to anatomical abnormalities. A loop of the intestine forms a ‘U’ shape and the adjacent regions at the top of the U then rotate around each other, forming the twist of volvulus. Dilation of the stomach occurs, which results in vomiting, leading to dehydration and electrolyte imbalances. This condition may be asymptomatic and discovered during unrelated abdominal surgery or it may cause minor abdominal complaints, such as nausea after meals, vomiting or abdominal pain. Surgical treatment is necessary to avoid gangrene.
An abdominal hernia occurs when the intestine protrudes through an opening of the abdominal wall muscles, often due to muscle weakening (see Figure 27-20D). The most common type of abdominal hernia is the inguinal hernia, where the intestine protrudes down into the genital area (in males into the scrotal sac). In some mild cases, the patient may actually be experienced in physically manipulating the gut back into place. However, the hernia can become strangulated, when it progresses to obstruction, limiting progression of intestinal contents.
Clinical manifestations of gastrointestinal tract alterations
In the previous sections, you have learned about the alterations to the digestive system that have the greatest impact in Australia and New Zealand — disorders that are the most prevalent in our community. We now expand on this knowledge with a detailed discussion of the signs and symptoms that are associated with these conditions. For healthcare professionals, a focus on the clinical manifestations is particularly important.
Vomiting
Vomiting (emesis) is the forceful emptying of stomach and intestinal contents (chyme) through the mouth. Stimuli initiating the vomiting reflex include severe pain; distension (stretching) of the stomach or duodenum; and activation of the chemoreceptor trigger zone in the medulla. A wide variety of medical conditions, disorders and medications can induce vomiting, so it is likely that you will encounter patients with vomiting in many areas of clinical practice.
Hirschsprung’s disease
Hirschsprung’s disease, also known as congenital aganglionic megacolon, is a functional obstruction of the colon caused by inadequate motility. The term aganglionic means without ganglia (a collection of neuronal cell bodies), because the enteric nervous system of the digestive system is incomplete. It is the most common cause of colon obstruction, accounting for about one-third of all gastrointestinal obstructions in infants. The incidence rate is 1 in 5000, with a preponderance in males. There is an increased incidence in children with Down syndrome.110
PATHOPHYSIOLOGY
Hirschsprung’s disease is caused by a malformation of the parasympathetic nervous system characterised in the enteric nerve plexuses. In most cases, the aganglionic segment is limited to the rectal end of the sigmoid colon. The abnormally innervated colon obstructs faecal movements, causing the proximal colon to become distended — hence the term megacolon (see Figure 27-21).
CLINICAL MANIFESTATIONS
Mild to severe constipation is the usual manifestation. Diarrhoea may be the first sign because only water can travel around the impacted faeces. The most serious complication in the neonatal period is enterocolitis (inflammation of the intestines) related to faecal impaction (blockage). Bowel dilation stretches and partly occludes the encircling blood and lymphatic vessels, causing oedema, ischaemia, infarction of the mucosa and significant outflow of fluid into the bowel lumen. Copious liquid stools result. Infarction and destruction of the mucosa enable enteric microorganisms to penetrate the bowel wall. Frequently, gram-negative sepsis occurs, accompanied by fever and vomiting. Severe and rapid electrolyte changes may take place, causing collapse and death.
EVALUATION AND TREATMENT
The definitive diagnosis is made by rectal biopsy showing an absence of ganglion cells in the submucosa of the colon. X-ray films show dilated loops of colon and contrast films show aganglionic areas.111
The affected segment is resected within the first few months of life. Alternatively, enemas are given until the lumen is clear and then stool softeners are prescribed for life. In general, the prognosis of Hirschsprung’s disease is satisfactory for children who undergo surgical treatment. Bowel training may be prolonged, but most children achieve bowel continence before puberty.
PATHOPHYSIOLOGY
Vomiting is a protective response to a number of factors that may potentially be harmful to the body — so important is this response, that there is a vomiting control centre in the medulla of the brainstem, located near the other control centres essential for life (namely, the cardiac and respiratory control centres). The vomiting control centre is responsible for coordinating sensory stimuli and then directing motor output to the muscles involved. Examples of sensory stimuli that may produce this reflex include extreme emotions such as fear, unpleasant sights or odours, severe pain, increased intracranial pressure and motion or balance signals from the equilibrium apparatus of the inner ear. Another important area that sends signals to the vomiting control centre is the chemoreceptor trigger zone: neurons of this zone located in the brainstem (beside the fourth ventricle) receive inputs more specifically associated with the gastrointestinal system, such as contaminated food, toxic substances in the stomach and anti-cancer drugs (chemotherapy).
CLINICAL MANIFESTATIONS
Vomiting begins with deep inspiration: the glottis closes, intrathoracic pressure falls and the abdominal muscles contract, creating a pressure gradient from abdomen to thorax. The lower oesophageal sphincter relaxes and waves of peristalsis move in reverse, forcing chyme from the stomach and duodenum up into the oesophagus. The contractions of the abdominal muscles are extremely strong, forcing the diaphragm high into the thoracic cavity. The high intrathoracic pressure forces the upper oesophageal sphincter to open and chyme is expelled from the mouth — this material is now referred to as the vomitus (see Figures 27-22 and 27-23). The upper part of the oesophagus contracts, forcing the remaining chyme back into the stomach, and the lower oesophageal sphincter then closes. The cycle may be repeated.
TREATMENT
Disturbances in hydration, electrolytes and acid–base balance can become severe consequences of vomiting. This is of particular concern for babies and young children, who are more susceptible to changes in fluid balance and thus need careful monitoring. Therefore, the treatment of this condition requires restoring the balance of fluids and electrolytes. Oral rehydration solutions usually contain electrolytes (sodium and potassium), as well as glucose and/or sucrose. During vomiting, intravenous restorations may be necessary, as orally administered substances are likely to be vomited before being absorbed into the bloodstream.
Some of the widely used treatments are metoclopramide and prochlorperazine, which are usually given parenterally (although oral doses may be taken as prophylaxis). Chemotherapy medications can cause severe nausea and vomiting, and anti-emetic treatments are targeted specifically for this use (such as ondansetron). Commonly used drugs are blockers (or antagonists) of particular receptors associated with the vomiting response (metoclopramide and prochlorperazine block dopamine receptors associated with the chemoreceptor trigger zone; ondansetron blocks the 5-hydroxytryptamine or serotonin receptors associated with the gastrointestinal tract that send signals to the vomiting centre).112
Nausea
Nausea and retching usually precede vomiting. Nausea is a subjective experience of feeling likely to vomit; this may or may not progress to retching and vomiting. Retching is similar to vomiting, except that no food or chyme reaches the mouth, as the upper oesophageal sphincter remains closed. As the abdominal muscles relax, the contents of the oesophagus drop back into the stomach. This process may be repeated several times before vomiting occurs or it may not progress to vomiting at all.
A response by the ‘fight or flight’ branch of the nervous system (sympathetic nervous system) causes tachycardia (increased heart rate), tachypnoea (increased respiratory rate) and sweating that accompany retching and vomiting. Also, the ‘rest and digest’ branch (parasympathetic nervous system) mediates copious salivation, increased gastric motility and relaxation of the upper and lower oesophageal sphincters. This is an example of a body process that has both the sympathetic and the parasympathetic branches working together for the same function.
Spontaneous vomiting not preceded by nausea or retching is called projectile vomiting. It is caused by direct stimulation of the vomiting centre by neurological lesions (such as tumours or aneurysms) involving the brainstem, or it can be a symptom of gastrointestinal obstruction (pyloric stenosis in children). The metabolic consequences of vomiting are fluid, electrolyte and acid–base disturbances (see Chapter 29).
Constipation
Constipation is difficult or infrequent defecation. It is a common complaint caused by personal habits and various disorders and drugs. It usually means a decrease in the number of bowel movements per week, hard stools and difficult evacuation, but the definition must be individually determined. Normal bowel habits range from 1 to 3 evacuations per day to 1 per week.
PATHOPHYSIOLOGY
Constipation can be caused by neurogenic disorders of the large intestine in which neural pathways or neurotransmitters are altered and delay transit time.113 A low-fibre diet (the habitual consumption of highly refined foods) decreases the volume and number of stools and causes constipation. Other contributing factors include a sedentary lifestyle, lack of regular exercise and consistent suppression of the urge to empty the bowel. Excessive use of antacids containing calcium carbonate or aluminium hydroxide often results in constipation. Opioid analgesics, particularly codeine, tend to inhibit bowel motility and therefore patients receiving these medications require adequate education about dietary changes to prevent constipation. Ageing may also result in changes in neuromuscular function causing constipation.114
CLINICAL MANIFESTATIONS
Constipation can be a serious medical problem, as the increased abdominal pressure created during straining (in an attempt to pass stools) can affect the heart. During straining (also known as the Valsalva manoeuvre), temporary bradycardia (slowing of the heart rate) occurs as venous return is slowed; however, when straining ceases, the sudden increase in venous return can cause cardiac overload and acute myocardial infarction (see Chapter 23). Changes in bowel evacuation patterns, such as less frequent defecation, smaller stool volume, difficulty in evacuating the rectum or a feeling of bowel fullness and discomfort, require investigation. When faeces are passed, they are often hard and dry.
EVALUATION AND TREATMENT
The history and physical examination and stool diaries provide precise information regarding the nature of constipation. Functional constipation — that is, constipation resulting from lifestyle or bowel habits — usually has a long history. Dysfunctional constipation is more likely to be sudden; constipation can accompany the development of physical changes and requires careful evaluation.
The individual’s description of frequency, stool consistency, associated pain and presence of blood is significant. In assessing frequency, it is important to discover whether bowel evacuation was stimulated by enemas or laxatives. Palpation of the abdomen discloses colonic distension, masses and tenderness. Digital examination of the rectum is performed to assess muscle tone of the anal sphincters and detect anal lesions. Stool transit time is evaluated. Sigmoidoscopy is used to visualise the lumen directly. A barium enema may be required if no lesions are directly visualised and symptoms continue after simple treatment.
The treatment for dysfunctional constipation is to manage the underlying disorder. Management of functional constipation usually consists of bowel retraining, in which the individual establishes a satisfactory bowel evacuation routine without becoming preoccupied with bowel movements. The individual may also need to engage in moderate exercise, drink more fluids and increase fibre intake. Bulk supplements (such as Metamucil), stool softeners (such as coloxyl) and laxative agents (for example, glycoprep) are useful for some individuals. Enemas are fluids inserted into the anus and can be used to establish bowel routine, but they should not be used habitually.
Diarrhoea
Diarrhoea is an increase in the frequency of defecation and the fluidity and volume of faeces. More than 3 stools per day is considered abnormal. Many factors determine stool volume and consistency, including the water content of the colon and the presence of unabsorbed food and intestinal secretions. Stool volume in the normal adult averages less than 200 grams per day; in children it depends on age and size, and an infant may pass up to 100 grams per day.
PATHOPHYSIOLOGY
Diarrhoea in which the volume of faeces is increased is called large-volume diarrhoea. It is generally caused by excessive amounts of water or secretions, or both, in the intestines. Small-volume diarrhoea, in which the volume of faeces is not increased, usually results from excessive intestinal motility.
The major mechanisms of diarrhoea are osmotic and secretory (see Figure 27-24):
FIGURE 27-24 The pathophysiology of diarrhoea.
In all cases, the chyme contains more fluid than usual. A In small-volume diarrhoea, increased intestinal motility causes the passage of chyme to pass too quickly for water to be absorbed. B In osmotic diarrhoea, a type of large-volume diarrhoea, the undigested sugars within the intestines draw water out of the mucosal cells into the lumen. C In secretory diarrhoea, a type of large-volume diarrhoea, an excessive amount of mucus is secreted by the gut wall, which means that more mucus passes with the chyme than usual.
CLINICAL MANIFESTATIONS
Apart from the mechanism, diarrhoea can be classified as acute or chronic, depending on its cause. Symptoms of acute diarrhoea include fatigue and drowsiness, thirst, loss of appetite, nausea, headache and faintness, as well as decreased urinary volume.16 Systemic effects of prolonged diarrhoea are dehydration and electrolyte imbalance. Normally, faeces do not contain much sodium, but are high in potassium; however, diarrhoea can cause an extensive loss of both potassium and sodium, which can become serious if untreated.16 Manifestations of acute bacterial or viral infection include fever, with or without cramping pain. Fever, cramping pain and bloody stools accompany diarrhoea caused by inflammatory bowel disease. Steatorrhoea (fat in the stools) and diarrhoea are common signs of malabsorption syndromes.
EVALUATION AND TREATMENT
A thorough history is taken to document the onset and frequency of diarrhoea. Exposure to contaminated food or water is indicated if the individual has travelled in foreign countries or areas where drinking water might be contaminated. Physical examination helps identify underlying systemic disease. Stool culture, examination of stool specimens for blood, abdominal X-ray films and intestinal biopsies provide more specific data.
Treatment for diarrhoea includes restoration of fluid and electrolyte balance, management of distressing symptoms and treatment of causal factors. Nutritional deficiencies need to be corrected in cases of chronic diarrhoea or malabsorption. The use of probiotic food products (foods that contain live bacteria, such as yoghurt) may be useful in the prevention of diarrhoea resulting from Clostridium difficile, as may be other infection control techniques (such as adequate hand-washing) and avoiding excessive use of broad-spectrum antibiotics.117
Diarrhoea in children
Diarrhoea is a common gastrointestinal problem during infancy and early childhood and is the leading cause of death in children younger than 5 years of age.118 Infants have low fluid reserves and relatively rapid gut peristalsis and metabolism. Therefore, the danger of dehydration is great with prolonged diarrhoea. Disturbances can occur by processes that increase fluid secretion into the gastrointestinal lumen (secretory diarrhoea), draw fluid into the lumen by osmosis (osmotic diarrhoea) or prevent fluid absorption in the intestine. Most episodes are self-limiting in that once the original cause in the gastrointestinal tract has been passed (along with the diarrhoea), recovery begins and usually resolves within 72 hours.
Acute diarrhoea
Acute diarrhoea in children is almost synonymous with acute viral or bacterial gastroenteritis. Rotavirus is the single most significant cause of gastroenteritis in infants and young children. Severe dehydration results from vomiting and diarrhoea.119 Vaccines for rotavirus are now available (see the box ‘Health alert: rotavirus vaccine’). Viral gastroenteritis tends to be self-limiting. Bacterial gastroenteritis is treated with antibiotics if the causal pathogen can be identified. Other causes of acute diarrhoea in the older child include antibiotic therapy, appendicitis, chemotherapy, inflammatory bowel disease, parasitic infestation, parenteral infections and ingestion of toxic substances.
Chronic diarrhoea
Children with acute gastroenteritis often remain mildly symptomatic for up to 4 weeks; therefore, diarrhoea that persists for longer than 4 weeks is considered to be chronic. Children with chronic diarrhoea can be divided into two groups: (1) otherwise well children whose growth is normal; and (2) ill children whose growth is retarded. Causes of chronic diarrhoea in the first group include abnormal colonic motility, lactose intolerance, encopresis (involuntary defecation), parasitic infestation and antibiotic use. Chronic diarrhoea in the second group is usually caused by a disease that impairs absorption.
PATHOPHYSIOLOGY
Common causes of acute diarrhoea in infants include Hirschsprung’s disease, infections and milk protein allergies. Infectious diarrhoea in newborns is usually associated with day-care epidemics involving pathogens such as Escherichia coli, Klebsiella, staphylococci, Salmonella and Shigella. Diarrhoea caused by these agents has a rapid onset and acidosis and shock can occur quickly. Clostridium difficile, often associated with previous antibiotic therapy, can cause acute, profuse, watery diarrhoea and symptoms of colitis.
Rotavirus vaccine
An early vaccine, Rotashield®, was associated with incidence of intussusception, and therefore was quickly withdrawn from the overseas market in 1999. It was never released in Australia. The currently used rotavirus vaccination became available for babies from July 2007 and is on the National Immunisation Program schedule for Australia (see Chapter 14). In Australia, the vaccines are Rotarix® and Rotaq®, with both given as multiple doses between the ages of 2 and 6 months old. Children who receive the vaccination are less likely to require hospitalisation for gastroenteritis.
Source: National Centre for Immunisation Research and Surveillance of Vaccine Preventable Diseases. Fact sheet: rotavirus vaccines for Australian children — information for GPs and immunisation providers. Available at www.ncirs.usyd.edu.au/facts/rotavirus_vaccine_for_children_june_2007.pdf Accessed 25 June 2009.
Dysphagia
Dysphagia is difficulty swallowing. Functional dysphagia is caused by neural or muscular disorders that interfere with voluntary swallowing or peristalsis. Disorders that affect the striated muscles of the upper oesophagus interfere with the oropharyngeal (voluntary) phase of swallowing. This is common in patients who have suffered from cerebrovascular accidents (stroke) and therefore is reasonably common in elderly patients. Other neurological impairments such as Parkinson’s disease may also cause dysphagia (see Chapter 9).
Dysphagia can also result from mechanical obstruction of the oesophagus or a disorder that impairs oesophageal motility. Intrinsic obstructions originate in the wall of the oesophageal lumen and include tumours, strictures and diverticular herniations (outpouchings). Extrinsic mechanical obstructions originate outside the oesophageal lumen and narrow the oesophagus by pressing inwards on the oesophageal wall. The most common cause of extrinsic mechanical obstruction is tumour.
CLINICAL MANIFESTATIONS
Distension and spasm of the oesophageal muscles during eating or drinking may cause a mild or severe stabbing pain at the level of obstruction. Discomfort occurring 2–4 seconds after swallowing is associated with upper oesophageal obstruction. Discomfort occurring 10–15 seconds after swallowing is more common in obstructions of the lower oesophagus. If obstruction results from a growing tumour, dysphagia begins with difficulty swallowing solids and advances to difficulty swallowing semisolids and liquids. If motor function is impaired, both solids and liquids are difficult to swallow. Regurgitation of undigested food, unpleasant taste, vomiting, aspiration and weight loss are common manifestations of dysphagia. Aspiration of oesophageal contents can lead to pneumonia.
EVALUATION AND TREATMENT
Knowledge of the patient’s history and clinical manifestations contributes significantly to a diagnosis of dysphagia. A barium swallow is used to visualise the contours of the oesophagus and identify structural defects. Manometry uses an instrument to determine the duration and amplitude of abnormal pressure changes associated with obstruction or loss of neural regulation. Oesophageal endoscopy is performed to examine the oesophageal mucosa and obtain biopsy specimens.
The individual is taught to manage symptoms by eating slowly, eating small meals, taking fluid with meals and sleeping with the head elevated to prevent regurgitation and aspiration. Anticholinergic drugs block the effects of acetylcholine at the neuromuscular junction and result in muscle relaxation — a well-known example, botox (botulism toxin), may relieve symptoms of dysphagia.120 (See Box 9-1 for a description of botox.)
Anorexia
Anorexia is a clinical manifestation that needs clarification. It is simply a lack of desire to eat despite physiological stimuli that would normally produce hunger. This nonspecific symptom may be associated with a large number of disorders and diseases including nausea, abdominal pain, diarrhoea, cancer, heart disease, renal disease and psychosocial distress. It may be relatively short-lasting and resolve if the underlying cause improves. Anorexia nervosa is a more specific condition of decreased food intake of neurological origin and is discussed in Chapter 38.
Gastrointestinal bleeding
Upper gastrointestinal bleeding, which is defined as bleeding in the oesophagus, stomach or duodenum, is characterised by frank (easily observed), bright-red bleeding or ‘coffee-ground’ material that has been affected by stomach acids. Haematemesis refers to vomiting blood and should be distinguished from haemoptysis, which is coughing up blood-stained sputum from the lungs. Upper gastrointestinal bleeding is commonly caused by bleeding varices (varicose veins) in the oesophagus, peptic ulcers or a Mallory-Weiss tear at the oesophageal–gastric junction from severe retching (see Figure 27-25A). Lower gastrointestinal bleeding, or bleeding from the jejunum, ileum, colon or rectum, can be caused by polyps, inflammatory disease, cancer or haemorrhoids (see Figure 27-25B). Acute, severe gastrointestinal bleeding is life-threatening, depending on the volume and rate of blood loss, associated disease, patient’s age and effectiveness of treatment.
FIGURE 27-25 Gastrointestinal bleeding.
A Causes of upper gastrointestinal bleeding numbered in order of frequency. B Causes of lower gastrointestinal bleeding numbered in order of frequency. IBD = inflammatory bowel disease.
Source: Damjanov I. Pathology. Philadelphia: Saunders; 2009.
The physiological response to gastrointestinal bleeding depends on the amount and rate of the loss. Changes in blood pressure and heart rate are the best indicators of massive blood loss in the gastrointestinal tract. During the early stages of blood volume depletion, the peripheral vascular compartment constricts to shunt blood to vital organs, particularly the brain, heart and lungs. A sign that this is happening is postural hypotension (a drop in blood pressure that occurs with postural change from lying to sitting or standing), light-headedness and loss of vision. If blood loss continues, hypovolaemic (low blood volume) shock progresses (see Figure 27-26; and also Figure 23-62 for further details on hypovolaemic shock). Diminished blood flow to the kidneys causes decreased urine output and may lead to oliguria (low urine output), tubular necrosis and renal failure. Ultimately, insufficient cerebral and coronary blood flow causes irreversible anoxia and death.
The accumulation of blood in the gastrointestinal tract is irritating and increases peristalsis, causing diarrhoea. If bleeding is from the lower gastrointestinal tract, the diarrhoea contains large amounts of frank blood. Bleeding from the upper gastrointestinal tract is usually manifest as melaena — black or tarry stools that are sticky and have a characteristic foul odour. It results in partial digestion of the blood components. Small amounts of blood in the faeces may go unnoticed — this is occult, or unseen, bleeding. The presence of bright-red blood in the faeces is usually due to bleeding in the rectal or anal area, particularly with a tear or haemorrhoids.