Chapter 39 Drugs Affecting the Male Reproductive System
Androgens, primarily testosterone and its ester derivatives, are male sex hormones with physiological actions in male sexual maturation and function and in development of male secondary sexual characteristics. They are used for replacement therapy in androgen deficiency and for treatment of advanced stages of breast cancer. (Androgenic anabolic steroids are abused in sport for their muscle-building effects.)
Antiandrogens such as flutamide are indicated for treatment of prostate cancer. Continuous administration of gonadotrophin-releasing hormone analogues also inhibits androgen synthesis and release, so this treatment is used for prostate cancer. Benign prostatic hyperplasia is a very common disorder that occurs in older men. The use of α1-adrenoceptor antagonists and 5α-reductase inhibitors for treating this condition is discussed.
Key abbreviations
BPH benign prostatic hyperplasia (or hypertrophy)
GnRH gonadotrophin-releasing hormone
Key background: the male reproductive system
Anatomy and physiology
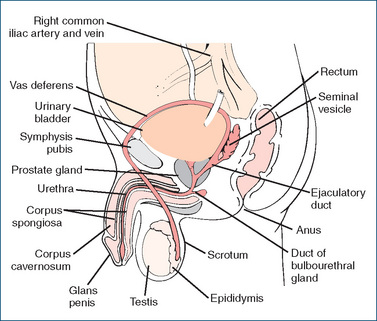
THE major organs of the male reproductive system consist of the testes, seminal vesicles, prostate gland, bulbourethral glands and penis (Figure 39-1).
The testes, the male gonads, are normally situated in the scrotum; they contain the seminiferous tubules in which the spermatozoa are produced. After maturation the sperm, in seminal fluid, travel through the epididymis (ducts that lie around the top of the testes), where motility is increased, to the vas deferens. The vas deferens extends over the bladder surface posteriorly to the ampulla to form the ejaculatory duct. Sperm may be stored in the vas deferens for several months without loss of viability, depending on sexual activity. The vas deferens passes within the spermatic cord, through the prostate gland to the urethra and through the penis, from which semen (sperm and seminal vesicle secretions) is ejaculated during sexual intercourse. (Thus a vasectomy, or severing of the vas deferens, will make a man sterile primarily because it interrupts the passage of sperm to the ejaculatory duct and urethra. Sperm are still produced, but they degenerate and are resorbed by phagocytes. Because the blood vessels are not cut, gonadotrophins continue to stimulate testosterone production, so libido and sexual performance are not impaired.)
Hypothalamic and pituitary control
The hypothalamic and pituitary controls of the reproductive organs (gonads) are described in Chapter 33, reviewed in chapter 38 with respect to the female system and shown diagrammatically in Figure 38-2. To summarise in the context of male reproductive functions:
GnRH and analogues
Gonadotrophin-releasing hormone (gonadorelin) is a 10-amino-acid peptide which stimulates release of FSH and ICSH from the anterior pituitary gland.
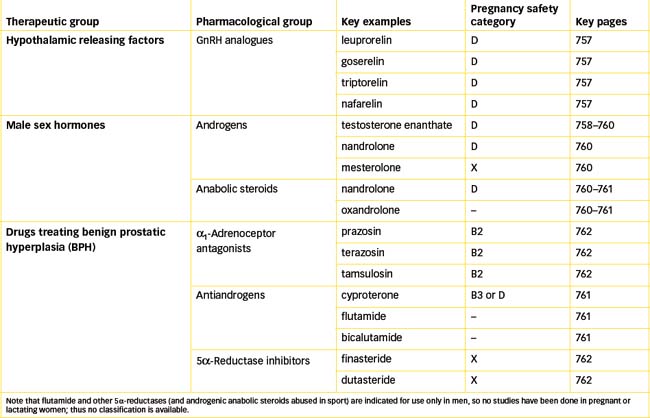
Gonadorelin analogues (i.e. GnRH agonists) have been developed to have longer half-lives and hence more useful activities when administered. Leuprorelin, goserelin and triptorelin (the latest analogue) are all indicated for continuous administration in palliative treatment of prostate cancer. The GnRH analogues cause initial stimulation of the gonads (and possibly a ‘flare-up’ of cancer symptoms), then suppression of testicular steroidogenesis and thus reduction in tumour growth and atrophy of the reproductive organs. (As their main indications for use are in treatment of cancers, these drugs are considered in more detail in Chapter 42.)
Nafarelin, another GnRH analogue, is used for analogous reasons in the female reproductive system: given regularly by nasal spray, it reduces oestrogen production and is indicated for treatment of endometriosis or to downregulate ovarian functions prior to controlled stimulation during in vitro fertilisation techniques.
Puberty
Puberty is the development stage during which reproductive capacity is attained. In girls it is easily marked by the onset of menstruation at the first menstrual period (menarche); in boys it is less easily defined. In both sexes the timing of puberty varies greatly, and the specific genes controlling onset of puberty are not known. There is increasing pulsatile secretion of hypothalamic GnRH stimulating release of pituitary gonadotrophins and in turn gonadal activity; other hormones including inhibin B and insulinfactor 3 are also involved.1 In boys, testicular growth is dependent on gonadotrophins, while androgens stimulate ‘virilisation’.
If puberty is long delayed in a boy, there is a risk of his failing to achieve target height and bone mass, plus delayed sexual and social integration in peer groups and associated adverse psychological and educational problems. In mild cases, no treatment may be required; however, short courses of low doses of testosterone have been shown to be effective.
‘Andropause’
In the later stages of adult life, male gonadal function decreases, with reduced androgen production. (However there is no distinct analogy to the clear-cut cessation of menstruation, at menopause, in women.) There may be loss of muscle mass, hypogonadism, decline in sexual functions, psychological changes and increased risk of bone fractures, atherosclerosis, ischaemic heart disease, metabolic syndrome and type 2 diabetes. Low circulating levels of androgens have also been associated with declining cognitive performance and increased levels of beta-amyloid protein, and as a risk factor for Alzheimer’s disease.
Testosterone replacement therapy is sometimes administered in cases of hypogonadism, analogous to oestrogen/progestogen HRT in post-menopausal women. It has been shown to have beneficial effects on visceral obesity, insulin sensitivity, glycaemic control and lipid profiles, as well as improving mood, sexual function and quality of life, in men with diagnosed hypogonadism (see review by Stanworth and Jones [2008]).
The male sexual response
Penile erection is a parasympathetic response that occurs during the phase of sexual excitement; typical parasympathetic effects of acetylcholine acting through muscarinic receptors are slow heart rate, dilation of blood vessels and stimulation of secretions. During sexual arousal the response consists of dilation of the arteries and arterioles in the penis, which compresses the veins in this area; thus, more blood enters the penis than leaves, the cavernous tissue of the penis becomes engorged with blood and erection occurs; at the same time, mucus is secreted by the associated glands. The stimulus that initiated erection will also help to move the sperm and secretions (semen) from the genital ducts to the prostatic urethra. Orgasm, the climax of the sexual act, moves the semen through the ejaculatory ducts. Ejaculation (emission and expulsion of the sperm in semen) is a reflex response, enabled by sympathetic stimulation and powerful contractions of muscles that enable the transfer of sperm from male to female during coitus (sexual intercourse). As in the female sexual response, there are increases in heart rate, blood pressure and respiratory rate and depth. (Aspects of reproductive functions related specifically to fertility and sexual functioning will be discussed in the following chapter.)
Male sex hormones
Androgens
Synthesis
Androgens, primarily testosterone, are the male sex hormones necessary for the normal development and maintenance of male sex functions and characteristics. Testosterone is produced in the Leydig (interstitial) cells of the testes, from precursors dehydroepiandrosterone (DHEA) and androstenedione synthesised in the adrenal cortex (see Figure 35-1). Some of the testosterone acts in the seminiferous tubules in production of sperm, and the rest is secreted into the bloodstream where it circulates, bound to steroid-binding proteins, to target tissues where its effects are exerted.
The actions of androgens are mediated in cells in androgen-sensitive tissues after conversion of testosterone (a steroid, see Figure 33-3E) by the 5α-reductase enzyme to the more active metabolite 5α-dihydrotestosterone (DHT). Androgens can also be converted to oestrogenic metabolites by the aromatase enzyme, which ‘aromatises’ the A ring of the steroid structure to an aromatic benzene ring as in oestradiol (Figure 33-3F).
Deficiencies
Levels of testosterone are low in conditions of deficiency of 5α-reductase, and also in men with obesity and associated metabolic syndrome, insulin resistance and diabetes. In obese males, aromatase enzymes in excess fat tissue convert the androgenic hormones to oestrogenic hormones, and thus cause low testosterone levels with high oestrogen levels, leading to male hypogonadism, possible erectile dysfunction and gynaecomastia. Excess aromatase activity can also cause gynaecomastia in men with liver disease, thyrotoxicosis or neoplasia of the testes, liver and adrenal glands, and sometimes in ageing and during puberty.
Androgen receptors
Testosterone and DHT act via binding to specific highaffinity androgen receptors (ARs), in the same superfamily of ligand-activated nuclear hormone receptors as oestro gen receptors (see previous chapter). The receptors act as ligand-inducible transcription factors: binding by ligand (androgen) induces a conformational change in the AR allowing translocation into the nucleus, phosphorylation and formation of dimers. In the nucleus the dimers activate target androgen-responsive genes by binding to response elements in their regulatory regions, hence regulating expression of the gene and increase or decrease of protein synthesis in tissues such as reproductive organs, bone and muscle. (ARs are being targeted in the search for new drugs in treatment of prostate cancer; see Chapter 42.)
Androgens can also exert rapid effects not mediated via genomic/transcriptional mechanisms. For example, via activation of a membrane receptor associated with sex hormone-binding globulin and a G-protein, second messenger activation of protein kinases or adenylate cyclase causes cellular effects such as smooth muscle relaxation, neurotransmission across the neuromuscular junction and neuronal plasticity (see review by Li and Al-Azzawi [2009]).
Androgen actions
Androgenic effects can be summarised into four main groups:
Pharmacokinetics and pharmaceutics
Testosterone has a high first-pass effect when given orally as a drug, being rapidly metabolised in the liver to androstenedione, then excreted via the urine, with a half-life of only 5–20 minutes. Consequently, it is formulated as ester compounds to prolong its duration of action. In the body tissues, the esters are rapidly hydrolysed to the active drug form. For example, testosterone undecanoate, formulated for oral administration in capsules, produces hormonal effects for 2–3 days. Testosterone enanthate as an oily solution for depot IM injection is much longer acting, usually administered once every 2–4 weeks (see Drug Monograph 39-1). Testosterone pellets are available for subcutaneous implantation. This form will also provide an extended duration of action; depending on the number of pellets used, effects may extend from 2 to 6 months before replacement pellets are necessary. Other synthetic androgens effective orally in tablet form include mesterolone and nandrolone. Transdermal testosterone systems are available for application to the skin as gel or patches. Because androgens are used for replacement therapy in conditions of androgen deficiency that are usually chronic, lifelong therapy may be required, hence the importance of long-acting derivatives and depot preparations.
Drug monograph 39-1 Testosterone enanthate depot injection
Testosterone enanthate has long-acting and intense androgenic actions.
INDICATIONS Testosterones are indicated for:
PHARMACOKINETICS The duration of action depends on the dose and the ester formulation administered. The longest duration of action for testosterone preparations is with the enanthate ester: after IM injection, the effects last for 2–4 weeks. Testosterone is metabolised in the liver, and metabolites are excreted by the kidneys.
DRUG INTERACTIONS Significant drug interactions have been reported when testosterone was given concurrently with oral anticoagulants (coumarin or indanedione), leading to enhanced anticoagulant effects, or with antidiabetic agents (enhanced hypoglycaemic effects).
ADVERSE REACTIONS The adverse reactions reported in males are urinary urgency, breast swelling or tenderness (gynaecomastia), frequent or continuous erections, testicular atrophy and impaired spermatogenesis. In children, there is impaired growth due to early closure of the epiphyses of the long bones. Other general adverse reactions include hair loss and balding, decreased gonadotrophin release and consequent impaired fertility, salt and fluid retention, hepatotoxicity and aggressive behaviour. Severe adverse effects occur particularly after inappropriate use in women, children and athletes. In females, the most frequent adverse reactions are an increase in oily skin or acne, deepening of the voice, increased hair growth and/or alopecia, enlarged clitoris and irregular menses. The deep voice or hoarseness may not be reversed, even when the medication is stopped.
DOSAGE AND ADMINISTRATION Testosterone enanthate is formulated for depot IM injection; other esters as capsules (PO), implants or transdermal gel or patches. The choice of dosage and length of therapy depend on the diagnosis, the patient’s age and sex and the intensity of the adverse reactions. For male hypogonadism, the usual IM dosage is initially 250 mg every 2–3 weeks, then, for maintenance, 250 mg every 3–6 weeks. The paediatric dosage for delayed puberty in males is 50–100 mg monthly for about 4–6 months.
Androgen replacement therapy
Testosterone, its derivatives and synthetic agents are commonly used as replacement therapy for males who lack the hormone (see Drug Monograph 39-1). In males with hypogonadism or eunuchoidism (a deficiency of male hormone) or delayed puberty, androgen therapy will produce marked changes in growth of the male sex organs, body contour, voice and other secondary sex characteristics.
Androgen deficiency is relatively common (1 in 200 men) and may be due to disorders of the hypothalamus, pituitary or testis, or to androgen-receptor defects (see Clinical Interest Box 39-1). Partial androgen deficiency may also occur in ageing men; this has been termed the male climacteric, or andropause (described above). While androgen replacement therapy may slow down deterioration in bone and muscle function, there is as yet no general recommendation for androgen replacement in ageing men.
Clinical interest Box 39-1 Akhenaton: a pharaoh with fröhlich’s syndrome?
The Egyptian pharaoh ruling circa 1369–1352 BC was Akhenaton, a powerful ruler and social reformer who overthrew the priestly caste and their gods. True-to-life statues at Karnak of the god-king in his later life show him with elongated and deformed skull, protruding lower jaw and stomach, narrow shoulders and arms, fat buttocks and thighs and prominent breasts (feminine physical characteristics), and no external male genitalia.
There has been much debate about Akhenaton, including suggestions that ‘he’ was a female masquerading as a male, that he had been castrated or that he suffered from rickets, pathological obesity or a pituitary tumour or infection.
The overgrowth of skull bones suggests an early excess of growth hormone from the pituitary gland, while the obesity and feminisation suggest a lack of male hormones. Overall, it is most likely that he suffered from a hypothalamic or pituitary disorder, maybe an infestation of tapeworm in the pituitary gland or a pituitary tumour. Fröhlich’s syndrome, or dystrophia adiposogenitalis, could account for the hypopituitarism and hypogonadism. However, Akhenaton was married to the fam ously beautiful Queen Nefertiti, who bore him six daughters, so hypogonadism and sterility are unlikely unless a tumour struck him after he had fathered several children. Until a mummy is found and identified with absolute certainty as Akhenaton’s, medical experts and archaeologists will con tinue to dispute the cause of his unusual representations in statues.
Based on: Leavesley 1984, chapter on ‘Akhenaton’.
Women need testosterone too
Women do naturally synthesise testosterone, with circulating levels of total testosterone approximately 1/15 of those in adult men (however, low levels are notoriously difficult to measure accurately and reproducibly). Androgen deficiency has been demonstrated in women with hypopituitarism, after adrenalectomy or oophorectomy, and in some women on oral oestrogen therapy. Symptoms include fatigue and low mood and libido (sexual interest). The symptoms are alleviated with low doses of testosterone or DHEA; adverse reactions in a minority of patients are typical androgenic effects: mild acne and hirsutism.
Androgens are also used in breast cancer in women and (in conjunction with an oestrogen) to treat severe osteoporosis in women.
Anabolic steroids
Anabolism refers to the metabolic processes in which small molecules are combined to form larger molecules and complex tissues, e.g. amino acids to proteins, or simple sugars to polysaccharides. This definition is extended to imply generalised increased building up of tissues and, in the context of pharmacology, to drugs that increase the bulk of the body, particularly muscle mass.
Androgens are potent anabolic agents; they stimulate the formation and maintenance of muscular and skeletal protein. Steroids used particularly for their anabolic effects include nandrolone, oxandrolone and stanozolol.2 They cause retention of nitrogen (essential to the formation of protein in the body) and enhance storage of inorganic phosphorus, sulfate, sodium and potassium. In the past, anabolic steroids have been used clinically to treat cachexia (generalised wasting), e.g. after long chronic illness, and for improving appetite, wellbeing and libido in people with ‘wasting diseases’, including osteoporosis, anaemia, adverse effects of corticosteroid therapy and terminal cancers. Given the lack of proof of efficacy and the availability of safer drugs with more specific actions, they are no longer recommended for such indications.
Adverse reactions are those of excessive androgenic actions. Weight gain may be caused by fluid retention, an adverse effect of androgen therapy. Other potential adverse reactions include testicular atrophy, sterility, gynaecomastia, increased risk of coronary heart disease, liver disease, mood swings and aggressiveness (‘steroid rage’) and induction of psychotic disorders. Women run the additional risks of virilisation, including irreversible deep voice changes.
Abuse in sport
Athletes have used anabolic androgenic steroids to increase weight, musculature and muscle strength, especially for endurance events requiring stamina (and to improve the ‘macho’ image). ‘Designer steroids’ are produced with the intention of avoiding detection by drug-control laboratories, and to maximise anabolic actions (in bone and muscle) and reduce androgenic effects (in testes, prostate and seminal vesicles). The potential risk of developing major serious adverse reactions from androgens far outweighs the advantages to be gained in athletic events. Several elite athletes (accused of using steroids) competing in international events, including long-distance cycling, have suffered death from cardiovascular collapse; some others became permanently sterile. Many major sporting events disqualify athletes whose use of such products is proved; many have been removed from Olympic Games competitions, or been later disqualified and stripped of their medals. (Additional information on the abuse of drugs can be found in Chapters 21 and 49; see also reviews by Kicman [2008] and Fragkaki et al [2009]).
Antiandrogens
Antiandrogen activity may be exhibited by many types of drugs, including those that block androgen receptors, decrease release of gonadotrophins, physiologically antagonise androgenic effects or inhibit enzymes for androgen synthesis. Cyproterone, for example, has weak antiandrogen activity and also has progestogen activity, hence its use in some oral contraceptives and HRT products.
Flutamide and analogues (nilutamide, bicalutamide) are more specific as orally active non-steroidal antiandrogens; they act by inhibiting androgen uptake or binding to receptors. They are used in advanced prostatic cancer, in combination with orchidectomy (removal of the testes) or to prevent the initial flare-up after starting treatment with a GnRH analogue. They can cause hepatic impairment, so liver function must be monitored.
Continuous administration of GnRH decreases gonadotrophin release (see GnRH analogues, above), and hence is used to treat prostate cancer, which is dependent on androgens for stimulation. Oestrogens and progesterone, by negative feedback loops, suppress gonadotrophin secretion and hence have both antiandrogen and anti-oestrogen/-progestogen actions. Danazol has weak androgenic activity but mainly suppresses the pituitary–ovarian axis and is used in female reproductive disorders such as endometriosis.
Selective androgen receptor modulators
Analogous to the rationale of selective oestrogen receptor modulators (SERMs) being useful in female endocrine disorders, there has been a search for drugs that would selectively act as agonists on some ARs, but antagonists on others—i.e. selective androgen receptor modulators (SARMs). SARMs could potentially be especially useful in treatment of prostate cancer.
Clinical uses
Antiandrogen agents have the following uses—low doses: acne, hirsutism in women; medium doses: hypersexuality in men; high doses: prostatic cancer. They have also been tested as male contraceptives. They are contraindicated in pregnant women, as they can cause feminisation of a male fetus. There is as yet no consensus on the issue of the optimal treatment for prostate cancer: surgery, radiation and ‘chemical castration’ with GnRH analogues or antiandrogens appear to be about equally effective (see Chapter 42). All carry the risks of impotence, osteo porosis, gynaecomastia, increased cardiovascular risk and decreased libido.
Benign prostatic hyperplasia
Pathology
 Testicular androgens are believed to have a permissive role in the development of benign adenomas of the prostate, i.e. benign prostatic hyperplasia (BPH), the excessive growth of the glandular and connective tissue in the portions of the prostate that surround the urethra (see Figure 39-1). This is considered a normal progressive age-related change that begins around age 40 in men. By age 70, about 75% of men will develop BPH symptoms severe enough to require professional intervention. BPH does not cause prostate cancer, but both types of hypertrophy are stimulated by androgens.
Testicular androgens are believed to have a permissive role in the development of benign adenomas of the prostate, i.e. benign prostatic hyperplasia (BPH), the excessive growth of the glandular and connective tissue in the portions of the prostate that surround the urethra (see Figure 39-1). This is considered a normal progressive age-related change that begins around age 40 in men. By age 70, about 75% of men will develop BPH symptoms severe enough to require professional intervention. BPH does not cause prostate cancer, but both types of hypertrophy are stimulated by androgens.
BPH obstructs the bladder neck and compresses the urethra, which results in urinary retention, increasing the risk of bacteriuria. If untreated, it may affect the ureters and kidneys and result in hydroureter, hydronephrosis and renal failure. The symptoms of BPH include hesitancy (difficulty starting the urinary stream); a decrease in the diameter and force of the stream; inability to terminate urination abruptly, resulting in post-void dribbling; and a sensation of incomplete bladder emptying, resulting in frequency and nocturia. Mild BPH does not require immediate treatment and benefits from watchful waiting. Surgical treatment (transurethral resection of the prostate, TURP) reduces the size of the prostate gland and is recommended for severe BPH, but many men are reluctant to submit to surgery in this area.
Pharmacological treatment
α-Blockers
Because the pathophysiology of BPH may also include increased smooth muscle tone in the bladder outlet and the prostate, mediated by α1-adrenergic receptors, pharmacological treatment of BPH with α1-adrenoceptor blockers has been tried. The antihypertensive drugs prazosin (Drug Monograph 12-3), terazosin, tamsulosin and alfuzosin are used for their smooth muscle relaxant actions in BPH and can improve urine flow rate; if effective, long-term therapy is required. Common adverse effects are those typical of vasodilators, i.e. headaches, dizziness and orthostatic hypotension, and also abnormal ejaculation during intercourse.
5α-Reductase inhibitors
Finasteride, a 5α-reductase inhibitor, is another drug available for the treatment of BPH (Drug Monograph 39-2). This drug acts by a very different mechanism: it is a specific inhibitor of the 5α-reductase enzyme type II, which in the prostate gland metabolises the conversion of testosterone to DHT, a more potent androgen responsible for prostate gland growth. Finasteride is highly effective at reducing the levels of DHT in the bloodstream and in the prostate, and thus markedly reduces the hypertrophy of the gland and reduces resistance to urinary outflow. It does not impair the synthesis of testosterone and so does not have antiandrogenic actions. It appears to be most effective in men with large prostates and may take 6 months of treatment for clinical improvement to be demonstrated.
Drug monograph 39-2 Finasteride
INDICATIONS Finasteride is indicated for mild to moderate symptoms of BPH with clinically demonstrated prostatomegaly, when surgical treatment is contraindicated or refused. It is also used to treat androgenic alopecia (hair loss) in men.
PHARMACOKINETICS Finasteride is well absorbed after oral administration, with maximum plasma concentrations being reached in about 2 hours; about 90% is bound to plasma proteins. Finasteride is inactivated in the liver, and metabolites are excreted in the urine, with an elimination half-life of about 6 hours. No dosage adjustments are required for elderly patients.
DRUG INTERACTIONS No significant drug interactions have been reported, although the drug is a known substrate for enzyme CYP3A4.
ADVERSE REACTIONS These include decreased libido, impotence, a decreased amount of ejaculate, gynaecomastia and allergic reactions.
WARNINGS AND CONTRAINDICATIONS Use should be avoided in patients with liver disease, obstructive uropathy or finasteride hypersensitivity. Finasteride is not indicated for use in women or children; women of child-bearing age should not handle broken or crushed tablets, as it is categorised X in terms of pregnancy safety.
Finasteride will reduce serum levels of prostate-specific antigen (PSA); hence it may interfere with diagnosis of prostate cancer.
DOSAGE AND ADMINISTRATION The drug is administered orally, 5 mg daily. Clinically useful effects may take 6–12 months to develop.
Dutasteride, another 5α-reductase inhibitor, is less specific for the prostate enzyme; 2 weeks treatment causes a reduction by 90% in DHT levels. It has a long half-life (up to 5 weeks) so takes some months to reach steady-state levels and full treatment effect. It causes a modest reduction in volume of the prostate gland and in urinary retention; it may adversely affect sexual function.
Other drugs
Studies of the genetics of susceptibility to prostate cancer and BPH have identified some enzymes of interest. Trials of drugs that inhibit the enzymes DNA methyltransferase and histone deacetylase are underway.
Extract of saw palmetto berries (Serenoa repens) has gained popularity as a proposed natural remedy for BPH; in some animal studies it has been shown to reduce the upregulation by testosterone of α1-adrenergic receptors. However, a recent Cochrane review of 30 randomised trials in over 5200 men showed that Serenoa repens was not more effective than placebo for treatment of urinary symptoms consistent with BPH. There is some evidence of efficacy from extracts of stinging nettle roots.
Other herbal remedies are extracted from bark of the African tree Pygeum africanum—the extract contains three active ingredients that reduce inflammation and enlargement of the prostate gland; also red clover extract and rye grass pollen extract. There are no large-scale randomised controlled trials of these products.
Key points
 Reproductive functions in the male are regulated by pituitary gonadotrophins and androgens from the testes; gonadotrophin-releasing hormone analogues are used by continuous administration in treatment of prostate cancer.
Reproductive functions in the male are regulated by pituitary gonadotrophins and androgens from the testes; gonadotrophin-releasing hormone analogues are used by continuous administration in treatment of prostate cancer. Sexual response in males is under hormonal, neuronal and psychological controls. The sexual response involves initially parasympathetic stimulation of erectile tissue, leading to engorgement of the penis, and sympathetic stimulation leading to ejaculation and muscular, respiratory and cardiovascular responses.
Sexual response in males is under hormonal, neuronal and psychological controls. The sexual response involves initially parasympathetic stimulation of erectile tissue, leading to engorgement of the penis, and sympathetic stimulation leading to ejaculation and muscular, respiratory and cardiovascular responses. Testosterone is the male sex hormone (androgen) necessary for the normal development and maintenance of male reproduction and sex characteristics.
Testosterone is the male sex hormone (androgen) necessary for the normal development and maintenance of male reproduction and sex characteristics. Testosterone is indicated for hormonal replacement therapy in males with androgen deficiency and for the treatment of advanced breast carcinoma. Because of its high first-pass effect, testosterone is usually administered as one of its ester derivatives in depot formulations or for transdermal administration.
Testosterone is indicated for hormonal replacement therapy in males with androgen deficiency and for the treatment of advanced breast carcinoma. Because of its high first-pass effect, testosterone is usually administered as one of its ester derivatives in depot formulations or for transdermal administration. The adverse reactions to androgens, such as cardiovascular disease, gynaecomastia in males and virilism in females, may be troublesome and even fatal.
The adverse reactions to androgens, such as cardiovascular disease, gynaecomastia in males and virilism in females, may be troublesome and even fatal.Review exercises
References and further reading
Ambler G.R. Androgen therapy for delayed male puberty. Current Opinion in Endocrinology, Diabetes and Obesity. 2009;16(3):232-239.
Australian Medicines Handbook 2010. Adelaide: AMH, 2010.
Braun L., Cohen M. Herbs and Natural Supplements: An Evidence-Based Guide, 2nd edn. Sydney: Elsevier Mosby; 2007.
Delemarre E.M., Felius B., Delemarre-van de Waal H.A. Inducing puberty. European Journal of Endocrinology. 2008;159(Suppl 1):S9-S15.
Drummond E.S., Harvey A.R., Martins R.N. Androgens and Alzheimer’s disease. Current Opinion in Endocrinology, Diabetes and Obesity. 2009;16(3):254-259.
Edwards J.L. Diagnosis and management of benign prostatic hyperplasia. American Family Physician. 2008;77(10):1403-1410.
Endocrinology Expert Group. Therapeutic Guidelines Endocrinology, version 4. Melbourne: Therapeutic Guidelines Limited; 2009.
Fargo K.N., Foecking E.M., Jones K.J., Sengelaub D.R. Neuroprotective actions of androgens on motoneurons. Frontiers in Neuroendocrinology. 2009;30(2):130-141.
Fragkaki A.G., Angelis Y.S., Koupparis M., Tsantili-Kakoulidou A., Kokotos G., Georgakopoulos C. Structural characteristics of anabolic androgenic steroids contributing to binding to the androgen receptor and to their anabolic and androgenic activities: applied modifications in the steroidal structure. Steroids. 2009;74:172-197.
Kicman A.T. Pharmacology of anabolic steroids. British Journal of Pharmacology. 2008;154:502-521.
Leavesley J.H. Medical By-Ways: Famous Diseases and Diseases of the Famous. Sydney: Australian Broadcasting Corporation/William Collins; 1984. [ch ‘Akhenaton’]
Li J., Al-Azzawi F. Mechanisms of androgen receptor action. Maturitas. 2009;63:142-148.
Miner M., Canty D.J., Shabsigh R. Testosterone replacement therapy in hypogonadal men: assessing benefits, risks and best practice. Postgraduate Medicine. 2008;120(3):130-153.
Stanworth R.D., Jones T.H. Testosterone for the aging male: current evidence and recommended practice. Clinical Interventions in Aging. 2008;3(1):25-44.
Tacklind J, MacDonald R, Rutks I, Wilt TJ. Serenoa repens for benign prostatic hyperplasia [update of Cochrane Database Syst Rev 2002; (3): CD001423; PMID: 12137626]. Cochrane Database of Systematic Reviews 2009; (2): CD001423.