Chapter 21 Drug Dependence and Social Pharmacology
Substance and drug misuse is one of the top public health issues in societies today. It is widespread despite legislation, enforcement and educational efforts to curb drug abuse. This chapter addresses the problem of drug misuse and abuse and the effects on individuals and society, the issues that affect drug abuse in professionals, problems in drug testing and the aetiological factors and pharmacological basis of dependence and tolerance. The drugs most commonly abused are identifi ed and discussed, especially opioids (heroin, morphine), CNS depressants (including alcohol, the benzodiazepines and inhalants), CNS stimulants (cocaine, amphetamines, ecstasy, caffeine and nicotine), psychotomimetics (cannabis) and hallucinogens (LSD, MDMA). Health-care professionals may need to recognise the signs of abuse of various drugs and know the appropriate interventions, pharmacological and non-pharmacological, used in clinical practice for treatment of acute overdose, detoxifi cation, substitution, withdrawal and maintenance.
Key abbreviations
cAMP cyclic adenosine monophosphate
ETS environmental tobacco smoke
5-HT 5-hydroxytryptamine (serotonin)
LSD lysergic acid diethylamide
MDMA 3,4-methylenedioxymethamphetamine
Drug dependence, misuse and abuse
Drug misuse or abuse
ALL drugs whether prescribed or self-administered have the potential to be misused or abused. The term drug misuse generally refers to inappropriate or indiscriminate use of drugs.
Drug abuse
Drug abuse refers to self-administration of a drug in chronically excessive quantities, in a manner that deviates from approved medical or social patterns in a given culture, resulting in physical or psychological harm. There are three important aspects to this definition:
Drug abuse has been known throughout history as one expression of an individual’s search for relief of physical, psychological, social or financial problems; it is not confined to any particular country, socioeconomic, cultural or ethnic group. Drug abuse may take a variety of forms:
Drugs commonly abused
The drugs that are most commonly abused in Western societies are caffeine, nicotine and ethanol (alcohol). There are double standards in terms of what is considered acceptable: for example, most governments condemn abuse of alcohol and tobacco products but do not ban the drugs, both for civil liberty reasons and because enormous amounts of revenue are received from taxes on the products. (In 2004/05, the Australian Government collected over A$6.7 billion from the importation and sale of tobacco products [AIHW 2008].) However, the revenue received falls far short of the amount required in health care for treatment of adverse reactions and chronic health and social problems arising from abuse of these two drugs. It is estimated that, for every 1000 Australian males who quit smoking, the savings over the next 10 years in health-care costs associated with heart attack, chronic obstructive pulmonary disease, lung cancer and stroke would be approximately $0.4 million (Hurley & Matthews 2008).
Apart from caffeine, nicotine and ethanol, drugs that are commonly abused are opioid analgesics (‘narcotics’, e.g. morphine and heroin), other CNS depressants (benzodiazepines, inhaled solvents), other CNS stimulants (cocaine, amphetamines) and psychotomimetics or hallucinogens (cannabis, lysergic acid diethylamide [LSD]). Aspects of these drug groups relevant to drug abuse or dependence will be considered in detail in this chapter; therapeutic uses are discussed in other chapters.
Drug dependence
Note that drug abuse does not always entail dependence on the drug: people may abuse simple analgesics or megadoses of vitamins or asthma puffers. Drug dependence is the condition in which administration of a drug is compulsively sought in the absence of a therapeutic indication and despite adverse psychological, social or physical effects; dependence may lead to disturbed behaviour to ensure further supplies of the drug. Dependence does not always cause problems; thus a person may be dependent on caffeine, which is safe and cheap, without breaking laws or suffering serious adverse effects or withdrawal reactions.
There are two main types of dependence:
Note that patients with chronic conditions may be in a state of medical dependence on a drug required for effective therapy; for example, patients with type 1 diabetes are said to be ‘insulin-dependent’.
Addiction (a term sometimes used synonymously with dependence) is a behavioural pattern of drug use characterised by an overwhelming involvement with the procurement and use of the drug and a high tendency to relapse back into drug dependence.1 The addictive pro cess involves impairment in three functional systems: motivation–reward, regulation of affect (mood) and behavioural inhibition.
Tolerance is a physical state in which repeated doses of the drug cause decreasing effects, or doses must be increased to maintain the same effects. Not all drugs of dependence induce tolerance; for example, tolerance develops rapidly to most of the effects of morphine (but not to the constipating or miotic effects), whereas there is little tolerance to marijuana.
Governments worldwide have regulated and restricted the use of most drugs of dependence (see Chapter 4); some drugs, however (alcohol, nicotine, caffeine), are considered differently and are readily available in most countries. In Australia drugs of dependence are generally listed in Schedule 8 (CONTROLLED DRUGS), and thus are subject to the strictest controls in terms of availability, storage, labelling and prescribing. (An exception is low-dose codeine, which is readily available in cough mixtures and compound analgesics.) Thus most drugs of dependence are illicit (illegal) outside of approved medical use on prescription.
Factors leading to drug abuse and dependence
Sociocultural factors
Societies use and accept certain drugs as legal, while they may restrict or ban the use of other drugs, depending on the particular society’s religious rules, typical ethos (aggressive or meditative), history, traditional medicine practices and experiences with the drugs. In many Middle Eastern societies cannabis is considered a legal drug, encouraging introspection and meditation and decreasing sex drive, whereas alcohol is usually a forbidden substance. By contrast, in most Western cultures use of alcohol is allowed despite its adverse effects on individuals, families and societies, while cannabis is illegal and may be considered an aphrodisiac (enhancing sex drive)! In highaltitude regions such as the South American Andes and Peru, coca leaves (the source of cocaine) are brewed as a tea or chewed to decrease hunger sensations, improve work performance and enhance a feeling of wellbeing.
As Rang et al (2007) memorably pointed out: ‘… drug-taking is clearly seen by society in a quite different light from other forms of addictive self-gratification, such as opera-going, football or sex’. In smaller units of society, whether or not a drug is ‘popular’ may depend on its availability, ease of sharing and peer-group pressures. Where particular drugs are illegal and in short supply, criminals may be motivated to obtain and sell the banned substances for profit.
Personality factors
The usual reason a person initially takes an illicit drug is belief that a desirable pharmacological effect will result. The drug generally is used as a (maladaptive) mechanism to provide relief from anxiety or from personal problems, to achieve pleasure or gratification or to alter the state of mind. Clinical Interest Box 21-1 summarises some theories on why people abuse drugs.
Clinical interest box 21-1 Why do some people abuse drugs?
Many theories have been proposed as to why some people abuse drugs. Theories that see drug abusers as deviants include:
Psychological theories to explain drug abuse include:
Note that there are different patterns of drug abuse in various sections of society, e.g. between men and women, indigenous and non-Indigenous people and adoles cents and adults. Theories (and management programs) need to be sensitive to these patterns of drug abuse.
Adapted from: Hamilton et al 1998.
Psychological studies on people dependent on or abusing drugs have shown the three most important predictors to be rebelliousness, tolerance of deviance and low school performance. Other implicated factors include curiosity; impulsiveness; a low threshold of frustration; boredom; peer pressure; alienation; hedonism (pleasure-seeking behaviour); affluence; feelings of fear, inadequacy, shame or failure; personal conflicts; a predisposition to depression, which may result in emotional and behavioural problems; aggressiveness in childhood; the need to escape; and the widely publicised attention to drug abuse in the mass media. In particular, the ‘alcoholic personality’ has a higher than average incidence of depression and antisocial tendencies plus a genetic predisposition to dependence on the drug. Organisations such as Alcoholics Anonymous have been developed to help individuals overcome their depend ence in a supportive, non-judgemental environment.
Pharmacological factors: CNS effects
All drugs likely to be abused have three characteristics: they act fast, make you feel good or stop you feeling bad. Obviously there would be little temptation to abuse a drug that took several hours to act—deferring gratification is not a common attribute among drug abusers.
Euphoria
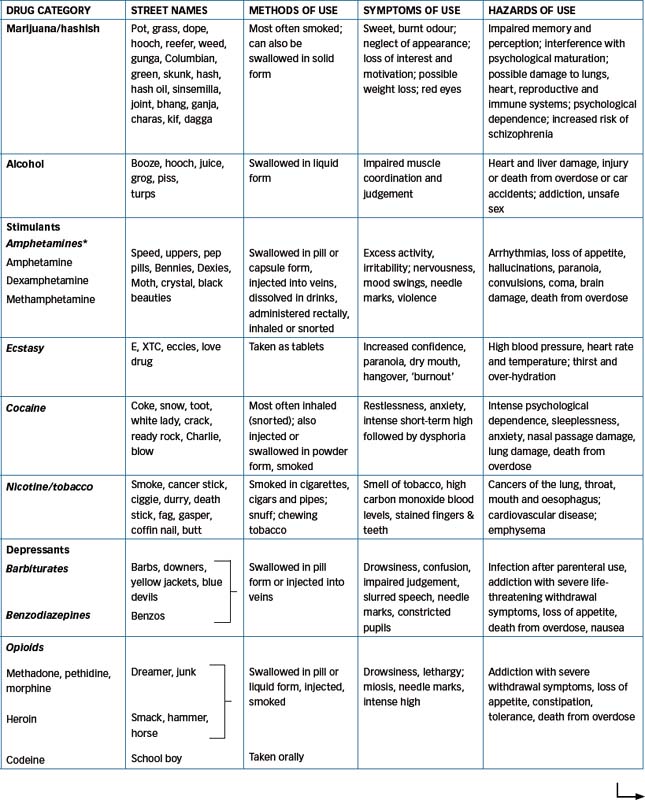
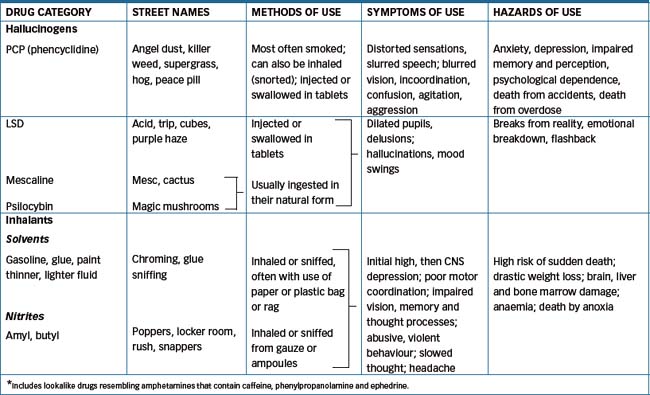
Desirable CNS effects include producing euphoria (‘feelgood’ effects), enhanced alertness, relief from anxiety or pain or hallucinations. Tolerance and/or physical dependence and a withdrawal syndrome may develop. Table 21-1 summarises several aspects of the main drugs of abuse. Other drugs that may induce altered states of perception, thought and feelings and drug-induced psychoses include the methylxanthines (caffeine and theophylline, found in coffee, tea, chocolate and colas), anticholinergics, corticosteroids, psychotropic agents and levodopa. The pharmacological effects of some of these mind-altering drugs are graphically illustrated in Figure 21-1, showing the webs woven by spiders sprayed with amphetamine, caffeine, chloral hydrate or marijuana (NASA 1995).

Figure 21-1 Effects of mind-altering drugs on spiders. In a technique developed to test the toxicity of chemicals, household spiders were sprayed with solutions of the chemicals and the shapes of the webs subsequently spun were analysed using techniques of statistical crystallography. The figure shows the effects on web-spinning prowess of marijuana (a drug causing relaxa tion and impairment of motor coordination and memory), amphetamine and caffeine (CNS stimulants) and chloral hydrate (a sedative drug). The technique was developed as an alternative to toxicity testing in higher animals, which is expensive, timeconsuming and subject to ethical concerns. The head of the research team, Dr David Noever, was quoted as saying that he did not expect complaints from animal rights groups: ‘We’re all concerned about tests on warm and fuzzy creatures, but in this case they are only fuzzy.’
(Quoted in The Sunday Age, 7 July 1995.) Reproduced from NASA Tech Briefs 1995; 19(4): 82, with permission.
Reinforcement and reward
Drugs of dependence have little in common in terms of their chemical structures. The one property they share is that of producing reinforcement or reward in animals or humans: subjects will carry out work to obtain further doses. Strong reinforcers include cocaine and morphine: animals trained to self-administer cocaine will press a bar many thousands of times to obtain a dose, to the point of toxicity. Weak reinforcers in animals include nicotine and caffeine; however, some drug addicts have found it easier to give up heroin than quit smoking. Non-reinforcers include cannabis: animals (non-human) will not bother to self-administer this drug.
Some drugs are in fact negative reinforcers, i.e. animals will learn to avoid them; phenothiazine antipsychotics such as chlorpromazine are examples, which helps explain the poor compliance of patients prescribed these drugs. The unpleasant withdrawal syndrome that occurs after stopping drugs that cause physical dependence can act as a negative reinforcement, encouraging addicts to seek another dose.
Dependence and tolerance
Much research has gone into the mechanisms of drug dependence. Currently the best accepted theory involves central dopaminergic pathways, in particular the meso limbic pathway from the substantia nigra through the nucleus accumbens to the frontal cortex. The role of dopamine (DA) appears to be in signalling incentives (reward, novel and unexpected stimuli), driving motivated behaviour and consolidating memory of important events. Evidence for the pivotal role of dopamine in addiction is as follows:
This explanation is oversimplified, however, and interactions with many other mediators and transmitters may also be involved, including 5-hydroxytryptamine (5-HT, serotonin), noradrenaline, endorphins, GABA and glutamate, endocannabinoids, neuropeptides, galanin, orexin, leptin, melanocortins and corticosteroids. (For a comprehensive review on this topic, see Goodman [2008]).
Tolerance, the tendency for successive doses to have lesser effects, may exist with either psychological or physical dependence. Receptor-site (pharmacodynamic) tolerance depends on both the concentration of the drug and the duration of the exposure: drug effects are reduced as the duration of exposure continues. Receptor synthesis may be downregulated, receptors may be lost or desensitised or there may be exhaustion of chemical mediators or transmitters. Neuroadaptations contributing to tolerance may exist also at the cellular, nerve network and body systems levels (see review by Christie [2008]).
The second type of tolerance is metabolic (pharmacokinetic): prolonged exposure to a drug increases drug clearance. With repeated ingestion of barbiturates, for example, steady-state blood concentrations fall progressively because of barbiturates’ inducing effect on hepatic microsomal enzymes, which increases barbiturate metabolism and inactivation.
Problems associated with drug abuse
The scale of trafficking in drugs
It is estimated that worldwide the trade in drugs amounts to 10% of all international trade. The global trade in illicit drugs—the big four groups being opioids, cocaine, cannabis and amphetamines—comprises a US$320 billion-a-year industry, making drugs one of the most ‘valuable’ commodities in the world. Law enforcement agencies cannot prevent the supply of illicit drugs, despite enormous operating budgets, which leads to the conclusion that prohibition is simply not working. However, the United Nations (2009) concluded that ‘illicit drugs continue to pose a health danger to humanity. That’s why drugs are, and should remain, controlled’.
Overall, the economic cost of drug misuse and abuse in Australia, including tangible and intangible costs, was estimated at more than A$56.1 billion in 2004/05. Of this, about 56% was due to tobacco, 27% due to alcohol and only 15% due to illicit drugs. Thus it is the licit drugs (alcohol and tobacco) that cause the most medical and economic harm in our community.
In the USA, alcohol is generally estimated to play a role in 40% of assaults reported, 50% of crimes, 50% of all traffic accidents, 35% of suicides and 50% of unintentional injury fatalities. Over 70% of all prison inmates are imprisoned for drug-related crimes.
Individual, family and society problems
Substance abuse is a major medical, social, economic and interpersonal problem, affecting people from all economic backgrounds and across the lifespan. The craving for further doses may come to dominate the individual’s life, leading to an unacceptable lifestyle or a life of crime to support the drug dependence. Clinical Interest Box 21-2 describes some of the myths related to drug abuse.
Clinical interest box 21-2 Myths related to drug abuse
Many myths circulate in the community related to drug misuse and abuse. Some examples of misinformation are:
Adapted from: Hamilton et al 1998; Walters 1996.
While deaths from overdose of heroin or other illicit drugs are tragic and newsworthy, the vast majority of non-prescription drug-related deaths in Australia are due to tobacco (72%) or alcohol (25%). In the state of Victoria, around 10% of hospital bed-days are used to treat conditions associated with the use of alcohol, tobacco or illicit drugs. Of clients attending specialist drug treatment services, about 50% of problems were related to alcohol, 25% to opioid abuse and 7% each to amphetamines or cannabis. Over 12% of clients reported multiple drug use and 38% admitted injecting drugs.
The harm done may be directly to the individual, from adverse drug reactions or interactions such as liver cirrhosis from chronic alcoholism, psychosis from amphetamines or lung cancer and cardiovascular disease from smoking. The signs and symptoms of acute drug intoxication are summarised in Table 21-2. There are indirect effects as well; for example, IV drug abuse may lead a person into the subculture of ‘shooting up’, with the risk of sharing of non-sterile needles and hence of blood-borne infections such as HIV–AIDS and hepatitis, or into a life of crime and possible imprisonment. As a consequence of all the above factors, the life-expectancy of people who are dependent on drugs is generally lower than normal.
Table 21-2 Signs and symptoms of acute drug intoxication
| DRUG(S) ABUSED | SIGNS AND SYMPTOMS |
| Cannabis drugs | Tachycardia and postural hypotension, conjunctival vascular congestion (red eyes), distortions of perception, dryness of mouth and throat, possible panic |
| Cocaine | Increased stimulation, euphoria, raised blood pressure and heart rate, anorexia, insomnia, agitation; in overdose: elevated body temperature, hallucinations, seizures, death |
| Opioids | Depressed blood pressure and respiration, fixed pinpoint pupils, coma, pulmonary oedema |
| Barbiturates and other general CNS depressants | Depressed blood pressure and respiration; ataxia, slurred speech, confusion, depressed tendon reflexes, coma, shock |
| Amphetamines | Elevated blood pressure, tachycardia, other cardiac arrhythmias, hyperactive tendon reflexes, pupils dilated and reactive to light, hyperpyrexia, perspiration, shallow respirations, circulatory collapse, possible hallucinations, paranoid feelings |
| Hallucinogenic agents | Elevated blood pressure, hyperactive tendon reflexes, piloerection, perspiration, pupils dilated and reactive to light, anxiety, distortion of body image and perception, delusions, hallucinations |
Families of drug-dependent persons may have to cope with aggressive behaviour, reduced earnings and resources, increased medical expenses, destructive relationships and increased dependence on state welfare for support. Physical injury and abuse as a result of drug-related incidents is suffered by 6.6% of Australian men and 3.5% of women (AIHW 2008).
At the society level there may be escalating crime in the community, with consequent requirements for increased policing, court procedures and prisons to deal with offences related to production, supply and possession of illicit drugs; and alcohol-related intoxication, violence and drink-driving (see Clinical Interest Box 21-3), with DRUGS AND ROAD SAFETY increased risk-taking and deaths. It is estimated that approximately 55% of prison inmates in Australia are there due to offences related to illicit drugs or alcohol. Abuse of injectable drugs (opioids, amphetamines and cocaine) leads to spread of infections such as viral hepatitis and HIV–AIDS. The most frequent ‘perpetration of drug-related harm’ reported in the 2007 Australian survey (AIHW 2008) was driving while under the influence of alcohol: 16.2% of men and 8% of women admitted to this. Alcohol is implicated in 40%–70% of all violent crimes.
Clinical interest box 21-3 Drugs and road safety
Some of the skills and attributes required for safe driving are: attentiveness, concentration, vigilance, hand-eye and footeye coordination, quick reaction time, multi-tasking and good visual fields, acuity and tracking ability. Many are adversely influenced by drugs, both prescribed and illicit. CNS depressants are particularly dangerous, and there are warnings on packets of such prescribed and OTC medicines. Significant risk of impaired driving skills especially occurs early in treatment.
Therapeutic drugs have been classified on the basis of their risk of impairing driving, as follows:
It is recommended that patients be warned of the increased risks, especially those driving at night, on shift work or using alcohol or other CNS depressants as well.
Source: Drummer 2008.
Drug abuse during pregnancy and breastfeeding
Drug abuse during pregnancy poses major problems for both mother and fetus and can lead to birth of drugdependent infants (see Clinical Interest Box 21-7; and Drugs at a Glance table at the end of this chapter). As usual, it is recommended that drug use be minimised during pregnancy to only what is essential to the health of the mother (and fetus). Most drugs of dependence, being lipidsoluble, are likely to cross the placental barrier and consequently adversely affect the fetus. Illicit drugs such as heroin, cannabis and ecstasy do not have pregnancy safety classifications, nor do non-scheduled substances such as caffeine, alcohol and tobacco. There are difficulties in gaining data on the safety of these drugs, particularly as drug-abusers often use many different drugs, and lifestyle factors may lead to poor antenatal care.
With respect to drugs of dependence generally, the following should be noted:
It is generally considered that the benefits of breastfeeding outweigh any potential risks to the baby from drugs that a mother may take, so moderate amounts of caffeine, alcohol, amphetamines and tobacco may be preferable to withdrawal syndromes or to weaning the infant. However, the ‘hard’ illicit drugs are of such risk to the infant that breastfeeding is not advised; cocaine in particular is contraindicated owing to the risk of toxicity to the infant.
Withdrawal syndromes
As well as acute adverse pharmacological effects and toxicity, there are longer-term problems of withdrawal after chronic administration. In many cases, the withdrawal syndrome is due to or manifests as a rebound in the systems affected by the drug. Thus withdrawal from chronic use of benzodiazepines (antianxiety agents and CNS depressants) is likely to lead to feelings of anxiety and agitation, while withdrawal from amphetamines (CNS stimulants) leads to depressed mood and drowsiness. Characteristics of individual withdrawal syndromes will be discussed in later sections under specific drug groups.
Problems among health professionals
Career pressures, long working hours and easy accessibility to drugs place health-care professionals, particularly doctors, pharmacists, nurses, anaesthetists and dentists, at greater risk of drug abuse. Studies among health professionals in the USA have shown that misuse of combined alcohol and other drugs was quite prevalent, especially with doctors and nurses. Health-care professionals who abused medications generally used more than four substances, including prescription drugs (opioid analgesics and benzodiazepines), alcohol, tobacco and nitrous oxide.
Policies related to drug abuse and its management
History of legislation against drugs of abuse
Opium, as a source of active alkaloids with analgesic, sedative (narcotic) and antidiarrhoeal activities, has been used in various cultures for thousands of years. An international Opium Convention was set up in 1912 to curb the trade; this was ratified after World War I. Other drugs of addiction were added to the charter, including cannabis in 1925 (see Chapter 4; and United Nations Office on Drugs and Crime [2009]).
The responsibility for worldwide control of ‘narcotics’ (by then defined to include cocaine and cannabis) was handed over to the United Nations after World War II (1946). The main international treaties are:
Narcotic Drugs and Psychotropic Substances.
Most countries now attempt to keep their official drug regulation legislation in line with that of the UN, but this can be problematic if a country wishes to trial an alternative policy (e.g. supervised injecting facilities).
Patterns of drug abuse worldwide
The United Nations’ latest report (2009) noted that, overall, growth in drug trafficking had flattened out: major markets for opium (Europe and Southeast Asia), cocaine (North America) and cannabis (North America, Oceania and Europe) were declining; however, consumption of synthetic stimulants was increasing, especially in East Asia and the Middle East. In 2008 there was a reduction in opium poppy cultivation in Afghanistan of 19% and a reduction in coca cultivation in Colombia of 18%; the other main producers of opium are Myanmar and Laos and of cocaine are Peru and Bolivia. It is more difficult to estimate production and trafficking in cannabis and amphetamines, as these can be grown or synthesised (respectively) virtually anywhere.
By UN estimates, between 172 and 250 million persons used illicit drugs at least once in 2007. However, the more important estimate is of ‘heavy or problematic users’ who consume the vast bulk of the supply, may be dependent on drugs, would benefit from treatment and whose level of use impacts on public health and public order—in 2007 there were probably between 18 and 38 million problem drug users worldwide.
Extent of drug abuse in Australia
To estimate the extent of drug abuse in Australia, large-scale population surveys on household drug use patterns, attitudes and behaviours have been carried out regularly every 3–4 years since 1985. The data collected from these surveys have formed the basis for the development of policies for Australia’s response to drug-related issues. Results from the 2007 survey are available from the Australian Institute of Health and Welfare (AIHW), Drug Statistics Series no. 22 (2008).
Some interesting facts and trends since the previous surveys are:
Tobacco and alcohol
As discussed in the previous section, the most commonly abused drugs are in fact legal: alcohol and tobacco. Overall, tobacco use has been dropping since the 1940s, when about 75% of Australian men smoked, to about 30% of men smoking in the mid-1990s and 18% in 2007. The proportion of young women smoking, however, has risen (16.6% in 2007); this group is particularly vulnerable to advertising and to the use of tobacco to decrease appetite. Australia is now ranked as one of the lowest among the Organisation for Economic Cooperation and Development (OECD) countries in terms of daily smoking.
With respect to alcohol, in 2007 Australians ranked about 22nd highest as per capita consumers (on average, 7.2 litres of pure alcohol per person per year). About 11% of men drink alcohol daily, and 5.5% of women. About 47% of teenagers aged 18–19 years drink at least weekly. Over the 200-odd years since alcohol was introduced into Australia, patterns of drinking have changed, from rum to beer to wines. Surveys attempting to estimate the extent of problem drinking (drinking above the accepted safe limits of four standard drinks per day for men or two for women) suggest that overall about 76% of men are responsible drinkers, 6.7% at-risk drinkers and 3.5% high-risk drinkers; the analogous figures for women are 70% responsible, 7.2% at-risk and 2.2% high-risk. There is a huge jump in use of drugs during the teenage years, especially between Year 7 at school (11–12-year-olds) and Year 11 (16–17-year-olds); alcohol and marijuana are most commonly used. Female teenagers are twice as likely as males to consume alcohol at risky levels.
Illicit drugs
The abuse of illicit drugs (especially cannabis, heroin and cocaine) is decreasing in Australia. Generally, men are more likely to use illicit drugs than women, and young people (<35 years) more so than older adults. Cannabis is the most widely used illicit drug, with about 34% of the Australian population (in large-scale surveys) admitting to having tried it and 10%–13% having used it in the previous 12 months; 55% of 20–29-year-olds have used it. In some states the laws against cannabis have been relaxed, such that the penalties for growing a plant for personal use do not lead to a criminal record.
With respect to use of other illicit drugs in Australia:
Therapeutic (licit) drugs
Therapeutic drugs (prescribed and OTC) can also be misused or abused for non-medical purposes; the extent of this is difficult to determine. The recent deaths of famous entertainers attributed to overdoses of prescription drugs have raised the level of awareness in the community. Drug regulations have become increasingly tight since the early 1900s, when narcotics were banned. Amphetamines were readily available in the 1950s and 1960s but are now indicated only for narcolepsy and ADHD. They are, however, fre quently abused by people wanting the CNSstimulant effects (e.g. long-distance drivers), and children prescribed stimulants for ADHD have been known to sell them on to schoolmates and adults. (Pseudoephedrine, another amphetamine-type stimulant, was previously readily available in cough and cold medicines; however, it has largely been removed, due to the ease with which it could be used to synthesise more dangerous amphetamines.) Codeine and other mild narcotic analgesics used in pain relievers and as cough suppressants are frequently abused.2 The benzodiazepine antianxiety drugs are renowned for causing dependence, which can be difficult to break. Doctors, other prescribers and pharmacists need to be aware that patients can become very persuasive in faking symptoms to get certain drugs prescribed, and often ‘shop around’ to augment their supply.
Policy approaches
Worldwide
Many possible policy approaches can be adopted by governments in response to problems of drug abuse in the community. Worldwide, prevention policies have been proven to be enormously expensive and ineffective and the extent of drug abuse has changed little over several decades. The United Nations now recognises that there is ‘a growing chorus among politicians, the press, and even public opinion saying: drug control is not working …’, and recom mends that, while drugs must remain controlled, there should be ‘different means to protect society against drugs, rather than … abandoning such protection’. Specifically, the UN Report recommends:
The conclusion was: ‘It is no longer sufficient to say: no to drugs. We have to state an equally vehement no to crime’. It was acknowledged that while international drug control has unfortunately led to organised crime involvement in trafficking, with a black-market for drugs, violence and corruption, it is still true that keeping dangerous drugs illegal does reduce the availability, and ‘protects millions from the adverse effects of drug abuse and addiction, particularly in the developing world’ (see United Nations Report [2009]).
Australian and New Zealand drug policies
Some aspects of New Zealand prevalence and policies related to drug abuse are given in Clinical Interest Box 21-4. The current policies in Australia are based on the American prohibition model of ‘zero tolerance’ for illicit drug abuse, as well as on demand reduction, supply reduction and harm minimisation (see www.national drugstrategy.gov.au). The National Drug Strategy 2004–2009 was the responsibility of the Ministerial Council on Drug Strategy, involving national, state and territory ministers for health, law enforcement and education to produce and implement a coordinated and integrated approach to issues of licit and illicit drugs.
Clinical interest box 21-4 Drug misuse in new zealand
Alcohol
Alcohol is the most commonly consumed drug in New Zealand. It is estimated that alcohol-related conditions account for 3.1% of male deaths and 1.41% of female deaths. In 2005/06, an estimated 14,000 New Zealanders died due to alcohol-related events.
Alcohol affects the road toll, street crime and petty dishonesty. It is related to:
Low–risk drinking (spirits and beer, not wine) has also recently been shown to increase the risk of a number of cancers, and it is suggested that this could be one of the reasons for the rise in breast cancer cases since the 1990s.
The Sale of Liquor Amendment Act 1999 introduced a number of major changes. The legal minimum purchase age was lowered from 20 to 18 years; minors under 18 years are not allowed to buy alcohol or consume it on licensed premises or in public places unless accompanied by a parent or guardian.
The upper legal limit of alcohol for licensed drivers aged over 20 years is 80 mg of alcohol per 100 mL of blood (0.08%) or 400 micrograms per litre of breath on a breathalyser. For all licensed drivers aged under 20 years, the upper legal limit is 30 mg of alcohol per 100 mL of blood or 150 micrograms per litre of breath.
To stay under the limit, male drivers should have no more than one 375-mL can of beer (4.5% alcohol) or two cans of low alcohol beer (2% alcohol). An average-size woman could go over the limit even after a double nip of spirits or a small glass of wine, or a can of beer. Under 20-year-olds are best advised not to drink and drive.
Smoking
Tobacco is the second most commonly used recreational drug after alcohol. It is estimated to kill approximately 4700 New Zealanders each year. Many people in New Zealand suffer from a wide range of chronic illnesses associated with smoking. On 3 December 2003, an amendment to the Smoke-free Environments Act 1990 was passed. Premises became smokefree and the display of tobacco products became restricted, as was the sale to under-18s. Herbal smoking products were included in the ban. The aim was to reduce the effects of second-hand smoke, which was reported to kill 350 New Zealanders annually.
The 2006/07 Health Survey showed that one in five adults and one in seven 15–17-year-olds were current smokers. Approximately 42.2% of Maori adults reported that they were current smokers, compared with 18.6% of European/Pakeha, 26.9% of Pacific Island and 11.2% of Asian adults. A smoking cessation scheme introduced in 1999 offering nicotinecontaining smoking cessation patches and gum from pharmacies and clinics in exchange for vouchers and a small fee has been taken up by New Zealanders in large numbers. In July 2003 the Maori Tobacco Control Strategy was launched. The culturally appropriate Aukati Kai Paipa programs have a quit rate of 29%; the 2006/07 Health Survey showed a significant decrease in current daily Maori smokers from 47.2% in 2002/03 to 37.6% in 2006/07.
Cannabis
Marijuana is the most popular illegal drug in New Zealand. Cannabis was the main illicit drug used in 1999, with only a small percentage reporting current or regular use and associated drug-related problems. It is used dis pro portionate ly by young males, Maori and some rural communities, particu larly in Northland and on the East Coast where cannabis is widely grown for economic purposes. It is these demographic areas which report the most cannabis-related harm.
New Zealand debated whether or not the possession or use of marijuana should be decriminalised; about 70% of the 18,720 prosecutions for offences involving cannabis resulted in a conviction in 1998. The National Organisation for the Reform of Marijuana Laws (NORML) works to end cannabis prohibition, but their campaign was opposed by the select committee of the Youth Parliament in 2000. In July 2005, the Labour Government reported that it would not introduce legislation to legalise marijuana.
Methamphetamine
Pseudoephedrine is used as a precursor substance in the manufacture of methamphetamine or ‘P’, an addictive drug misused for its stimulant potential. Methamphetamine sells on the street for between $180 and $1000 for 1 gram. Known also as ‘speed’, ‘pure’, ‘burn’ and ‘ice’, the name ‘P’ is used only in New Zealand.
Methamphetamine can be produced easily in a clandestine home laboratory using pseudoephedrine tablets (available without prescription in New Zealand), using simple extraction techniques, common household equipment and readily available chemicals to do the ‘bake’, following recipes available on the Internet. Police are observing the trend of ‘shoppers’ who move from pharmacy to pharmacy purchasing cold/flu products containing pseudoephedrine. There has been a marked increase in the number of clandestine laboratories for the manufacture of ‘P’, with a peak in 2003 of nearly 200 labs detected.
If it is contained in a cold/flu preparation containing less than 1.8 gram pseudoephedrine will be a partially exempt drug, as will controlled release formulations containing no more than 240 mg. This allows the sale of these medicines from pharmacies. In many pharmacies, identification is required for the purchase of pseudoephedrine-containing medicines. This is in accordance with the pharmacist’s Code of Ethics (Principle 3) of non-maleficence, which requires that the pharmacist plays an active role in preventing the sale of medicines for illegal purposes.
Pseudoephedrine and pseudoephedrine-containing products became controlled drugs from 15 October 2004. There was a steady increase in the size of parcels of pseudoephedrine products seized by Customs officials, with examples of parcels containing 1800, 2400, and 20,000 tablets in single imports. Such imports were often arranged through internet pharmacy sites. The National Government of New Zealand is currently exploring the removal of pseudoephedrine from non-prescription medicines.
Adapted from: www.moh.govt.nz, www.crime.co.nz , www.ndp.govt.nz, www.alac.org.nz/ [24 August 2009].
In recognition that some people will continue to abuse drugs and need some protection, the following practical advice to minimise harm has been prom ulgated:
Other possible drug policies
In the Netherlands and some other European countries, policies are based on normalisation and de-stigmatisation, whereby less harmful drugs (‘soft drugs’) are less tightly controlled and may be ignored by police (e.g. cannabis can be ordered in a coffee shop). Supervised injecting facilities have been demonstrated to operate well in some German cities, leading to decreased public nuisance, fewer heroin overdose deaths and decreased frequency of drug-related infections.
Treating drug dependence
General aspects of treatment
Many treatment modalities are possible in the area of drug abuse and the choice is determined by whether it is a case of acute toxicity, chronic abuse or long-term management. From an ethical point of view, the primary goal of treatments should be to reduce harm from illicit drugs, rather than to reduce supply or punish offenders. Drug dependence is a chronic relapsing–remitting disorder and, for any treatment to work, the person must first acknowledge that drug abuse has become a problem. Some general points relevant to treatment are:
Clinical interest box 21-5 Complementary and alternative therapies in management of drug dependence
Patients with problems related to drug abuse frequently turn to CAM methods for relief. Methods tried include prayer, removal to a sanatorium (with fresh air, controlled diet and healthy lifestyle), hypnosis, acupuncture and mutual support programs such as the famous 12-step program of Alcoholics Anonymous.
Few clinical trials have been done to test the efficacy of CAM methods and there is a high drop-out rate, with return to the abused drug (recidivism). Some evidence exists for the effectiveness of biofeedback in treatment of misuse of alcohol and opioids; transcendental meditation in opioid, nicotine, cocaine or alcohol abuse; rest and yoga in alcohol or nicotine abuse; acupuncture for cocaine abuse; and various herbs for detoxification and ‘liver cleansing’ in alcohol abuse.
Overall, combinations of Western and CAM therapies are common and often effective, e.g. specific replacement or antagonist drugs, along with behavioural psychotherapy, nutritional therapy and acupuncture.
Adapted from: Spencer & Jacobs 1999.
The guiding principle should be to consider a combination of therapeutic approaches, depending on the individual’s needs.
Treating acute overdose
The first aim of treatment is resuscitation of the patient, if necessary, then elimination of the drug taken, if possible, and treatment of toxic effects and complications. Specific antagonist drugs may be used to block the toxic effects of the drug of dependence, e.g. the opioid antagonists naloxone or nalorphine for opioid overdose or flumazenil for benzodiazepine overdose. Antidepressants such as fluoxetine or bupropion may be useful, particularly for withdrawal syndromes and quitting smoking. Drugs that may potentiate toxicity in the CNS or cardiovascular system should be avoided.
Treating chronic abuse
Initially, a comprehensive assessment of the person is required, for organic diseases, drug screening and a full history—medical, drug, social, family, psychological and psychiatric. The goals of treatment are to achieve total withdrawal from the drug, detoxification and treatment of any withdrawal reactions. Dopamine agonists may help reduce craving for the pleasure reinforcement. Antagonists (e.g. naltrexone on opioid receptors) will suppress the harmful effects of agonist drugs, but also suppress the euphoriant effects and provide no reinforcement, so compliance with them is poor. Naltrexone has moderate efficacy in treating more than one type of drug dependence (see Drug Monograph 21-1); the patient needs to be highly motivated and supported to stop dependence on opioids or alcohol. A multi-disciplinary approach to treatment is required and may be best carried out in a hospital or ‘detox’ facility. (An analogue, methylnaltrexone, is being used to treat opioid-induced constipation in palliative care patients by blocking opioid receptors in the GIT; as it does not pass the blood–brain barrier, it does not reduce the analgesic effects).
Drug monograph 21-1 Naltrexone
Naltrexone is a competitive opioid antagonist with no agonist actions; it reversibly blocks the effects of all opioids, including the physical dependence produced. In alcohol-dependent people, naltrexone reduces alcohol craving, alcohol consumption and relapse rates, presumably by antagonism of endogenous opioids involved in alcohol-dependence. Naltrexone can precipitate a withdrawal syndrome in opioid-dependent people.
Indications
This drug is indicated for adjuvant treatment in the detoxified opioid-dependent or alcohol-dependent person, in conjunction with a comprehensive psychological and social rehabilitation program.
Pharmacokinetics
Absorption is rapid but naltrexone undergoes an extensive first-pass metabolism in the liver to the active major metabolite 6-β-naltrexol, also an opioid antagonist. Oral bioavailability is only 5%–40%. The peak serum con centration is reached in 1 hour. Elimination half-life for naltrexone is 4 hours and, for its major meta bolite, about 13 hours. Its duration of action is dose-dependent and ranges from 24 to 72 hours. Excretion of the drug and its metabolites is via the kidneys; dose reduction is required in hepatic or renal impairment.
Adverse drug reactions
Serious adverse effects are uncommon, as naltrexone has no intrinsic agonist activity. Mild adverse effects are also the symptoms of opioid withdrawal and include anxiety, gastrointestinal and sleep disturbances and headache. Hepatotoxicity occurs rarely, and patients should be warned to watch for signs such as yellow eyes or dark urine; liver function should be monitored regularly.
Drug interactions
There have been few studies of drug interactions. In opioid-dependent people a serious withdrawal reaction is precipitated. The opioids present in other opioidcontaining medications (such as narcotic analgesics and cough suppressants) will be antagonised, leading to decreased effectiveness. In alcohol-dependent people the combination with disulfiram can lead to additive hepatotoxicity.
Warnings and contraindications
Naltrexone is contraindicated in patients receiving opioids, those dependent on them or those in acute withdrawal. Patients must be opioidfree for 7–10 days before starting naltrexone therapy. Naltrexone is contraindicated in severe liver disease.
There are dangers if a patient resumes opioid administration while on naltrexone or after stopping naltrexone therapy, as previous tolerance may have waned and there is risk of a potentially fatal overdose. If patients on naltrexone therapy suddenly require opioids for analgesia in an emergency, there are difficulties in overcoming the receptor blockade caused by naltrexone; other analgesics such as NSAIDs or anaesthetics should be used.
Naltrexone is classified B3 with respect to pregnancy warnings, as there has been limited use to establish safety. Similarly, safety in children or during breastfeeding has not been established.
Dosage and administration
Treatment with naltrexone is started cautiously, usually at a dose of 25 mg orally, with close monitoring for withdrawal signs and symptoms for about 1 hour. If no withdrawal effects occur, the balance of the daily dose is given. Maintenance is usually 50 mg orally daily. Compliance with therapy is improved if a trusted adult supervises administration of the drug.
Long-term maintenance
The preferred scenario is to achieve abstinence from any drug abuse; however, this is recognised as being very difficult and perhaps unrealistic, as withdrawal causes distress which commonly leads to resumption of drugtaking behaviours. A more reachable goal is harm minimisation, without reliance on pharmacological agents. A substitute drug may help maintain effects while reducing harms, e.g. oxazepam substituted for diazepam, or methadone for morphine or heroin.
A novel approach still undergoing research and trials is that of developing vaccines that stimulate the immune system to produce antibodies against a drug of dependence. Thus far vaccines have been developed against nicotine and cocaine, with some limited success.
Roles of health professionals
Health-care professionals have to remain alert for drugseeking behaviours. Many patients ‘shop around’ among prescribers to obtain prescriptions for drugs on which they are dependent, particularly seeking pethidine, codeine, oxycodone, amphetamines and benzodiazepines. A recent study in Australia revealed that over 850 people had seen more than 50 different doctors in one year, and over 20 000 people had seen 15 or more doctors.
While it is important that patients with genuine need of a drug are not denied it, drug seekers need to be identified and referred to an appropriate treatment facility. Signs of drug-seeking behaviours include:
Opioids
Heroin, morphine and other agonist opioids
Opiates are the narcotic drugs from natural sources and include the opium alkaloids morphine (see Figure 1-3A) and codeine. Related drugs are the semisynthetic compounds heroin and hydromorphone and the synthetic drugs pethidine, methadone and fentanyl. The term opioid is preferred because it refers to both natural and synthetic products that have morphine-like agonist effects on enkephalin (opioid) receptors. The pharmacology of these drugs when used clinically as analgesics is discussed in depth in Chapter 15.
Euphoria and tolerance
Because opioids can rapidly relieve pain, change or elevate mood, relieve tension, fear and anxiety, and produce feelings of peace, euphoria, and tranquillity, they are particularly likely to lead to physical and psychological dependence (see Clinical Interest Box 21-6). Tolerance develops to most of the effects, especially to analgesia, euphoria, sedation and respiratory depression, but not to the constipation or miosis. Heroin, morphine, pethidine, methadone and pholcodine are the most frequently abused; as described earlier, health-care professionals who have ready access to opioids are at particular risk.
Clinical interest box 21-6 Happy hoppies high on poppies
Farmers in northern Tasmania had occasionally noticed unusual circles in their fields of (legally-grown) opium poppies. The mystery was solved when a farmer noticed wallabies acting strangely in his fields: eating some poppies, hopping off, then returning for more and circling in the paddocks. The operations manager of Tasmanian Alkaloids also noticed a pattern in wallabies’ behaviour and suggested that, when other crops like grass are in short supply, wallabies graze on poppy capsules for the nutritious seeds inside.
A Tasmanian wild-life vet agreed that opium from the poppies could be affecting the animals’ behaviour, but suggested that if the wallabies became addicted, then other pharmacological effects like constipation and sedation would probably be significant too.
Emailed comments to the ABC’s website noted that it is a known risk for people to move on from ‘grass’ to harder drugs…
Source: ABC News, ‘The World Today’; 25 June 2009.
Opioid abuse
Heroin abuse
Diacetylmorphine (heroin, diamorphine) is a synthetic morphine derivative with no accepted medical use in Australia. It was initially introduced into medicine as a cough suppressant and to treat morphine addiction, but was banned in most countries because of its high potential for abuse and the increasing number of heroin addicts.
Heroin abuse and dependence is not an easy lifestyle: the drug has a short half-life, requiring frequent doses, and the practice is illegal and expensive, estimated at costing A$50–200 per day. Studies estimate that in 1997/98 there were about 75 000 dependent heroin users in Australia, i.e. about seven per thousand adults. The mortality rate is 1%–2% of users per annum; in 1998 (a year of high use) there were 737 reported deaths from heroin overdose. In 2007, approximately 1.6% of Australians over 13 years old admitted to have ever used heroin, but only 0.2% to have used it recently. In New Zealand a 1998 survey found that about 1% of the population reported using any opiate drug, with 0.6% as current users. These prevalence figures are similar to those in Britain and the European Union. The purity of heroin supplies varies widely—from 25% pure to 90% pure—and users can never be sure of the strength or purity of a sample (often adulterated with sugar, sedatives or amphetamines). It is therefore fatally easy for addicts to overdose. Impurities injected along with the opioid can cause collapsed veins, infections and organ damage.
Pharmacologically, heroin is a prodrug: after an oral dose it is rapidly converted in the liver to morphine. Heroin is highly lipid-soluble and quickly passes the blood–brain barrier, producing a rapid intense ‘rush’. It is highly addictive and tolerance develops rapidly to most effects. Controlled studies comparing heroin and morphine in terms of effects achieved when abused do not support the generally held belief that heroin is ‘better’.
Mode of administration
The opioids generally have low oral bioavailability and so are administered percutaneously (absorbed through the mucous membranes) by sniffing (snorting), by subcutaneous injection (skin popping) or by direct IV injection (mainlining, ‘shooting up’). The rate of absorption is increased by injection, with IV injection producing almost immediate drug effects.
Acute overdosage
Acute overdosage of opioids may result in severe pulmonary oedema and respiratory depression. These outcomes are dose-dependent, and what constitutes a lethal dose also depends on the individual’s tolerance for the drug. Symptoms occur rapidly in most patients: slow, shallow breathing, severe hypoxia, cold clammy skin, miosis, bradycardia, hypotension, muscle spasm and lethargy; urinary retention may also occur. The presence of thrombophlebitis, scarred veins and puckered scars from subcutaneous injections may help identify a patient with opioid dependence. The treatment of choice for acute overdosage is administration of an antagonist (e.g. naloxone) and respiratory and cardiovascular support.
Withdrawal syndrome
In a patient who is physically dependent on opioids, sudden withdrawal of the substance of abuse, or an abrupt reversal of opioid effects with an opioid antagonist, may precipitate an acute abstinence or withdrawal syndrome, with excitation and diarrhoea. While unpleasant, the withdrawal symptoms are not usually particularly dangerous. Milder symptoms (craving and sleep disturbances) may continue for many months, and psychological dependence may continue for the rest of the person’s life.
Babies born to women who are dependent on an opioid may suffer an immediate withdrawal reaction (see Clinical Interest Box 21-7).
Clinical interest box 21-7 An overdose in an opioid-dependent newborn baby
The coordinating centre of the Newborn Emergency Transport Service (NETS) in Victoria received a call from a small general hospital for advice about management of a very sick baby boy who had been born prematurely (at 35 weeks) to a woman who was on methadone maintenance treatment for opioid dependence and was also using heroin during the pregnancy. (Methadone requirements escalate during pregnancy due to placental metabolism, so dependent pregnant women are often tempted to resume heroin use due to ‘withdrawal’ cravings, despite huge doses of methadone up to 120 mg/day.)
The baby was withdrawing from the opioids to which he had been exposed in utero, and manifested many clinical features of the neonatal Narcotic Abstinence Syndrome (NAS), such as high-pitched crying, irritability, tremors, poor feeding, vomiting and diarrhoea, increased sweating, exaggerated reflexes and unstable temperature. Supportive treatment was given (swaddling, frequent small feeds), but he continued to have high ‘abstinence scores’ for 24 hours, indicating high risk of dangerous seizures. The baby was started on 4-hourly oral doses of morphine (0.5 mg/kg/day in 6 divided doses) to manage the withdrawal syndrome. Such infants are born with a high tolerance to morphine, and so are resistant to many of the adverse effects of opioids.
Unfortunately due to administration errors in the hospital (the dose was recorded in the chart as 0.5 mg/kg per dose rather than per day), the baby received 2 large 6-fold overdoses of morphine, which precipitated respiratory depression. Oxygen was administered, while advice was sought from the NETS paediatricians as to how best to manage the situation—normally, an opioid antagonist such as naloxone is contraindicated in such babies as they are already suffering opioid withdrawal, and an antagonist could precipitate a severe acute withdrawal reaction and seizures. However, in this case, the baby needed an antagonist to overcome the adverse effects of the morphine overdose.
Naloxone was administered both IV and IM, very carefully, to titrate the effects on opioid receptors of the antagonist (naloxone) against the agonists (morphine, plus any methadone or heroin remaining in the baby’s system). Happily, the baby survived the overdose, the abstinence syndrome was controlled, and then the detoxification process was implemented with the morphine maintenance dosage gradually reduced by 10% every 2–3 days, as permitted by ongoing NAS scores.
Acknowledgements to: Dr Philippa Shilson; NETS Victoria.
Treating opioid dependence
The main aim of treatment is to keep users alive and help them ‘mature out’ of their condition. Treatment programs concentrate on either withdrawal and continuing total abstinence, including ‘rapid detoxification’ programs then an opioid antagonist such as naltrexone (Drug Monograph 21-1); or (more realistically) withdrawal then substitution and ongoing maintenance with another less dangerous opioid such as methadone. Chilling statistics report that, on average after 10 years’ treatment, 30%–40% of former users remain abstinent, 40%–50% are active users or imprisoned, and 10%–20% have died.
Withdrawal and detoxification programs
Generally, opioid withdrawal is difficult and stressful, and repeated relapses occur. Abrupt and complete withdrawal (known as ‘going cold turkey’, due to the ‘goose-bumps’ induced) can be accomplished but is dangerous (especially in patients with a coexisting medical illness) and inhumane. Successively tapering the dosage over several days may be accomplished in a clinic with close medical supervision.
Therapeutic community programs (such as Odyssey House in several Australian cities) and halfway houses have been established; they offer group psychotherapy, support and self-help approaches.
Methadone substitution and withdrawal
One method of withdrawal is substitution of methadone for heroin or morphine, then withdrawal of methadone over a 6-week period. Methadone is a synthetic opioid agonist analgesic effective orally (see Drug Monograph 21-2); it has a slower onset of action than heroin and a much longer half-life. By virtue of cross-tolerance, methadone dependence can be substituted for heroin dependence. Methadone can forestall the euphoriant effects of heroin and the craving for the drug without producing heroin’s deleterious physical and mental effects, and without frequent parenteral administration and the attendant risks of infections. When properly administered, methadone allows the person to function adequately without intellectual or emotional impairment. Methadone withdrawal programs are not always successful, and relapse is common.
Drug monograph 21-2 Methadone oral syrup
Methadone is an orally active opioid agonist, safer than heroin, that helps reduce illicit drug use and the associated crime and social problems. Methadone is available in a syrup, the formulation usually used for treating opioid dependence, or as tablets or parenteral solution for pain relief as an alternative analgesic to morphine. Oral administration reduces IV drug habits, removes the opioid-taker from the ‘street drug’ scene and can be readily supervised.
Indications
Methadone is indicated either for short-term treatment and management of withdrawal symptoms during opioid detoxification programs or in long-term use for maintenance of opioid dependence in methadone maintenance programs; it is also used as an analgesic opioid in its own right.
Pharmacokinetics
Methadone is well absorbed orally and has good bioavailability but variable pharma co kinetics. Peak plasma levels are reached in 1–5 hours; it is widely distributed via the bloodstream; and protein binding ranges from 60% to 90%. Metabolism occurs in the liver, to at least two inactive metabolites; however, auto-induction of metabolising enzymes leads to a shorter half-life and tolerance. Methadone and its metabolites are excreted in urine and faeces. The half-life is variable (15–60 hours), so it takes several days to reach steadystate levels, and careful dose adjustment is required; some people may require more than one dose per day.
Adverse drug reactions
The adverse-reaction profile of methadone is similar to that of all opioids, i.e. euphoria, CNS and respiratory depression, GI and cardiovascular disturbances and spasm of biliary and renal-tract smooth muscle. Tolerance develops in a few weeks to most of the effects, so people on methadone maintenance can usually resume normal lifestyle and work patterns. In men, fertility may be impaired and gynaecomastia may develop.
Drug interactions
Any other CNS depressant, combined with methadone, will have additive depressant effects; this includes alcohol, antihistamines, sedatives and many psychotropic agents. Enzyme inducers can precipitate a withdrawal syndrome, thus requiring higher or more frequent methadone doses.
Warnings and contraindications
Methadone is contraindicated in respiratory depression, acute alcoholism or head injury and in severe hepatic or GI diseases. Prolonged use leads to dependence, but it is generally considered that it is easier to wean an addict off methadone than off heroin or morphine. Precautions are required in elderly patients (because of the prolonged half-life) and in patients with diabetes mellitus or other endocrine disorders. There are cautions against driving or operating machinery due to CNS depression. Methadone is in Category C with respect to pregnancy safety classification; higher doses may be required in pregnancy because of faster metabolism.
Dosage and administration
Methadone syrup is classified as Schedule 8 and there are strict regulations as to its prescribing, dispensing and admin istration. The strength of the formulation is 5 mg/mL. The initial dose is 10–20 mg, with the dosage increased gradually to the minimum required maintenance dosage, usually 30–50 mg/day, with a maximum of 80 mg/day. Many patients eventually choose to come off methadone by gradually reducing daily dosage.
During the opioid withdrawal phases, other pharmacological agents may be required to treat the withdrawal symptoms: antidiarrhoeal agents, anti spasmodics, nonsteroidal anti-inflammatory drugs and sedatives such as diazepam are used. Clonidine is specifically useful in treating the sympathetic nervous system symptoms and is helpful in lessening discomfort of the withdrawal syndrome. It is also under investigation for relieving the symptoms of acute withdrawal from other drugs, including nicotine and alcohol.
Naltrexone rapid detox programs
Naltrexone is a specific opioid antagonist used to prevent relapse in alcohol and opioid withdrawal and detoxification programs (and also to treat acute overdose with opioids; see Drug Monograph 21-1). Administration to an opioiddependent person precipitates an acute withdrawal syndrome within a few minutes, as the naltrexone binds to opioid receptors in the CNS and competitively inhibits their activation by endogenous enkephalins/endorphins or by administered opioids. Naltrexone is also being used in ‘rapid-detox’ procedures, in which the antagonist is administered under close medical supervision while the opioid-dependent person is under anaesthesia or sedation. In addition, naltrexone may be used in long-term abstinence programs, a daily dose being given to continuously block the effects of any opioids taken. Counselling and support are usually necessary to help the person remain com mitted to the opioid abstinence and on-going naltrexone treatment.
Methadone or buprenorphine maintenance
Methadone or buprenorphine maintenance is the long-term substitution of prescribed, supervised oral opioid for injected illicit opioids.
In Australia, methadone or buprenorphine programs must comply with the requirements of the state Department of Health or Human Services. The patient attends the pharmacy for a supervised oral dosing of methadone (daily) or buprenorphine (daily or alternate days). Occasional take-away doses are allowed to enable patients to go away for 1–2 days; take-away doses are dispensed in large volumes of cordial to obviate the risks of injection of the dose or inadvertent toxicity. Buprenorphine is a partial agonist at μ-opioid receptors (see Chapter 15) and as such is safer in overdose and can block effects of any heroin taken simultaneously. Due to its long half-life, buprenorphine is proving a useful alternative to methadone in maintenance therapy or detoxification programs. Sublingual (SL) tablets also contain a low dose of naloxone to deter IV usage: the naloxone has little clinical effect when taken SL, but if injected can precipitate an unpleasant withdrawal reaction.
A similar program operates in New Zealand (see Clinical Interest Box 21-8).
Clinical interest box 21-8 The new zealand methadone maintenance program
The objectives of the methadone maintenance program in New Zealand are in line with the national Drug Policy: the aim is to minimise the harms associated with the misuse of opioid drugs, a strategy referred to as ‘harm reduction’.
The Opioid Substitution Treatment New Zealand Practice Guidelines were published by the Ministry of Health in 2003. They replace the National Protocol for Methadone Treatment (1996) and emphasise the importance of the continuity of care, ranging from intensive intervention and stabilisation management to treatment through the GP primary-care network.
Each methadone client receives an individualised treatment plan that should be reviewed every 6 months. The first dose of methadone is usually in the range 10–40 mg and should not exceed 40 mg. The dose should be maintained for the first 3–4 days of treatment so as to reach steady state. Maximum daily doses are in the range 60–120 mg, and some individuals require ‘split doses’. The aim is to achieve effective management of withdrawal symptoms. Treatment should be started early in the week to allow monitoring during the working week; steady–state blood levels are often not achieved before 5 days’ treatment.
Methadone doses should be sufficient to provide clinical stability and minimisation of withdrawal symptoms. Clients should be able to continue their role in society, and remain in the program.
Adapted from: www.moh.govt.nz and www.mhc.govt.nz.
Analgesia for patients with opioid abuse disorders
Providing adequate analgesia to manage acute pain in patients with an opioid abuse or dependence disorder is difficult. Such patients are predisposed to some acutely painful conditions (pancreatitis, traumatic injuries), but may be tolerant to opioids, already using opioids, in remission or withdrawal or showing drug-seeking behaviours to obtain doses. Careful assessment is required, plus maximisation of non-opioid analgesics (paracetamol, NSAIDs), adjuvant therapies and non-pharmacological pain management techniques. Opioids should not be withheld if indicated for acute pain, but doses for patients in methadone programs may need to be higher than usual; opioids cannot be readily used for patients on naltrexone programs (see Drug Monograph 21-1).
Central nervous system depressants
Alcohols
The term ‘alcohol’, defined chemically, simply refers to a hydrocarbon derivative in which one or more of the hydrogen atoms (–H) has been replaced by a hydroxyl group (–OH). Phenols are aromatic alcohols, in which a hydrogen on a benzene ring has been replaced with a hydroxyl group (see Figure 1-3E). Although there are many different kinds of alcohol, the term alcohol in the medical or social context usually refers to ethanol (ethyl alcohol—see Clinical Interest Box 21-9). Methyl, propyl, butyl and amyl alcohols are examples of other alcohols; these are very toxic when taken orally.
Clinical interest box 21-9 Alcohols—what’s your poison?
The strengths of alcoholic solutions could scientifically (and logically) be expressed in SI units, e.g. in g/L, mg/mL or even molar terms; however, the unit % v/v is most commonly used (i.e. the number of millilitres of pure ethanol per 100 mL solution), and other archaic traditional units and terms are still in current use. In the British Pharmacopoeia, absolute alcohol, or dehydrated alcohol, refers to 100% pure ethanol, whereas Alcohol BP is a mixture of ethyl alcohol (approximately 96%) and water.
‘Proof spirit’ is an old term originally defined as ‘a solution of alcohol of such strength that it will ignite when mixed with gunpowder’ (an important concept in the early days of naval warfare) and more recently as ‘the alcoholic solution that weighs 12/13 of an equal measure of distilled water’. Proof spirit contains about 57% v/v ethanol in the UK. The strengths of alcoholic spirits are still sometimes stated in terms of proof spirit, thus ‘60 over proof’ refers to spirit of a strength such that 100 volumes contain as much alcohol as 160 volumes of proof spirit.
There are several forms of ‘methylated spirits’, all consisting largely of ethyl alcohol that has been purposely con tami nated with other solvents including methanol, acetone and pyridine to render it unfit for human consumption. It is used clinically for skin disinfection. Sadly, some ‘skid-row alcoholics’ in desperation resort to drinking ‘metho’ and suffer severe toxicity, mainly due to acute poisoning with methanol (see later section) which can cause severe abdominal pain, metabolic acidosis, blindness, coma and respiratory failure.
Alcoholic beverages contain varying amounts of ethanol, ranging from about 1%–5% v/v for beers, 9%–15% for wines, 16%–23% for fortified wines (sherry and port), to 40%–55% v/v for spirits such as brandy, rum, vodka and whisky. The standard measures of alcoholic drinks take these varying strengths into account, thus the large beer glass, medium-sized wine glass and small ‘shot-glass’ for spirits probably contain roughly the same ‘dose’ of alcohol, i.e. about 10–20 g.
Adapted from: Bowman & Rand 1980; Whelan 2002.
Ethanol (ethyl alcohol, ‘alcohol’)
Alcohols are naturally produced from the fermentation of cereals and fruits. Most wines are produced from fermentation of grapes from the plant species Vitis vinifera, while beer has been traditionally brewed from grains with hops added for flavouring. Spirits contain higher concentrations of alcohol as they are distilled to concentrate the alcohol: rum is distilled after fermentation of sugar cane, and other spirits from grains, fruits or vegetables (e.g. whisky from barley or rye).
Uses
Ethanol is the only alcohol used orally extensively in medicine and in alcoholic beverages. Therapeutically, ethanol has been used orally as an appetite stimulant and as a mild hypnotic. It was also used both clinically and traditionally to diminish uterine contractions in threatened spontaneous abortion. It is currently used as an antidote for acute methanol and ethylene glycol poisoning. Ethanol denatures proteins by precipitation and dehydration, which may be the basis for its germicidal, irritant and astringent effects. For local or in-vitro effects, ethanol has been used as a skin antiseptic and disinfectant, in topical pharmaceutical preparations, as a preservative in many formulations, in sclerotherapy (e.g. to cause hardening and closure of varicose veins) and to cause lesions to sensory nerves in neuralgias.
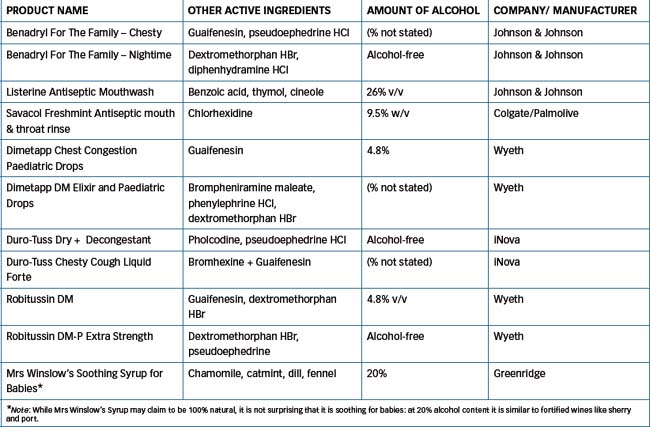
Alcohol is found in many oral pharmaceuticals, as a solvent or as a component of flavoured vehicles. (Table 21-3 lists the ethanol contents of various Australian OTC preparations.)
Ethanol is used in alcoholic drinks, and a low level of alcohol intake (e.g. two glasses of red wine daily for men, one glass for women) has been shown to be protective against some cardiac conditions.3 While ethanol is not usually taken for therapeutic purposes, it is a very commonly taken drug so it is discussed in the usual format (Drug Monograph 21-3).
Drug monograph 21-3 Alcohol (ethanol)
Taken orally, alcohol is a sedative and euphoriant; it is usually taken in the form of alcoholic drinks. Alcohol is the most commonly used and abused drug in Australia.
Pharmacokinetics
Being a very small molecule (molecular weight 46), ethanol does not require digestion before absorption; it readily diffuses through lipid membranes (as does water) and hence rapidly enters cells despite being very water-soluble. A small amount is absorbed from the stomach, while most is absorbed from the small intestine. Peak blood alcohol levels are reached about 30–60 minutes after administration. Alcohol is distributed in every tissue of the body as is water; the volume of distribution is about 35 L for a 70 kg adult. Analysis of the blood alcohol level gives a rough estimate of the quantity consumed and of the alcohol levels in the brain (see Table 21-4).
About 90% of alcohol absorbed is metabolised in the liver by alcohol dehydrogenase to acetaldehyde, then acetaldehyde is oxidised to acetic acid and eventually to carbon dioxide and water. The remainder of the ethanol is primarily excreted by way of the lungs, sweat and kidneys. The amount of alcohol excreted in expired air—as measured by ‘breathalyzers’—is very small: the amount in 2 L expired air is equivalent to that in 1 mL blood; however, this small amount may have considerable forensic importance if it indicates blood levels higher than the legal limit.
As plasma ethanol levels rise, the hepatic alcohol dehydrogenase pathway becomes saturated; the maximum rate of metabolism is about 120 mg/kg/h, and the clearance and half-life are dosedependent. Hence blood alcohol levels remain high if the person keeps drinking steadily. Plasma levels tend to be higher in women than in men after equivalent doses, both because women have lower levels of dehydrogenase enzymes and because they have a relatively smaller volume of distribution for water-soluble drugs. Heavy exercise may slightly increase the rate of elimination of alcohol. Chronic administration (i.e. in alcoholics) initially increases the rate of metabolism by the liver enzyme pathway, but as liver damage and cirrhosis develop, metabolism becomes impaired.
Adverse drug reactions
Alcohol affects many body systems (see Pharmacological effects in text). In particular, it causes euphoria and reduces inhibitions, causes sensorimotor impairment and increases gastric acidity. The ‘therapeutic index’ is estimated at about 4; i.e. while one or two drinks may make you the life of the party, 4–8 may make you raging drunk, comatose or incapable of driving safely. Alcohol has a diuretic effect both because of the increase in fluid intake (although this may be small with wines and spirits) and through inhibition of antidiuretic hormone (ADH) release. If an individual has preexisting renal disease, the kidney may be further damaged.
Chronic alcohol use may result in hyperlipidaemia, fatty deposits in the liver and, ultimately, alcoholic cirrhosis.
Drug interactions
Ethanol interacts with many prescription and OTC drugs, in particular with any other CNS depressant, resulting in frequent adverse drug interactions (see Drug Interactions 21–1).
Warnings and contraindications
Because of the increased risk of fetal alcohol syndrome (mental retardation, craniofacial dysgenesis and growth retardation; see Clinical Interest Box 9-1), pregnant women are advised by the World Health Organization to avoid alcohol throughout pregnancy; there is a 10% risk of fetal malformations if consumption exceeds 2 g ethanol/kg/day during the first trimester. Drinking alcohol is not recommended for breastfeeding women, as ethanol partitions into milk and causes depressant effects on the infant’s CNS and respiration.
Generally, alcohol is not recommended for people with liver disease or psychiatric problems, or patients taking any of the many drugs with which alcohol interacts.
Dosage and administration
The standard ‘measures’ of alcoholic drinks are such that an average drink contains in the range 5–20 grams of ethanol: the stronger the drink, the smaller is the typical container. Thus a beer stein is larger than a wine glass, and a sherry glass larger than a ‘shot’ glass for spirits. It is generally recommended that men drink no more than four standard drinks per day on a maximum of 3–4 days per week, and women no more than two standard drinks.
Pharmacological effects of ethanol
CNS-depressant actions
Contrary to popular belief, alcohol is not a stimulant but a CNS depressant, causing progressive depression in sequence of the cerebrum, cerebellum, medulla and spinal cord. What sometimes appears to be behavioural stimul ation results from depression of the higher faculties of the brain and represents the loss of inhibitions acquired by socialisation. Alcohol impairs transmission of nerve impulses at synaptic connections but the precise mechanism is not known. It inhibits calcium entry into nerve cells, possibly by enhancing γ-aminobutyric acid (GABA)-mediated inhibition and/or antagonising excitatory amino acid transmitters (e.g. glutamate). Other targets for alcohol actions are receptors for NMDA, glycine, 5-HT3 and acetylcholine (nicotinic), and some potassium channels.
The action of alcohol varies with the blood alcohol level, the individual’s tolerance, the presence or absence of extraneous stimuli, the rate of ingestion and gastric contents. Small or moderate quantities produce a feeling of wellbeing (euphoria) and increased confidence. Then finer powers of concentration, judgement and memory are lost, visual acuity is diminished and sensorimotor functions (including driving) are impaired. Many drivers will take chances when under the influence of alcohol that they would never take ordinarily, as accident statistics reveal (see Figure 21-2). Table 21-4 compares the blood alcohol level with clinical observations of behaviour and pharmacological effects.
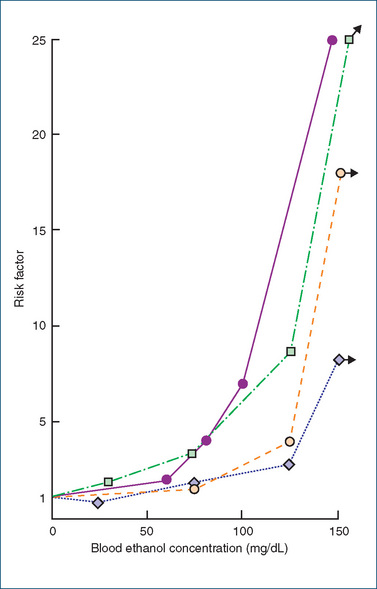
Figure 21-2 Results of four surveys on the relation between traffic accidents and blood ethanol concentration. The risk factor is the ratio of traffic accidents in subjects with a given blood ethanol concentration to all traffic accidents in the population from which the subjects are drawn. Note that 50 mg/dL equates to 0.05%.
From data reviewed in H Wallgren, M Berry, Actions of Alcohol, Amsterdam: Elsevier, 1970; as shown in Bowman & Rand 1980; used with permission.
Table 21-4 Dependence of pharmacological responses on blood alcohol level
| STAGE | BLOOD ALCOHOL (mg/dL)* | EFFECTS ON COGNITION & BEHAVIOUR |
| Subclinical | 30–100 | Possible slight deterioration in motor function, coordination, personality or mood and mental acuity |
| Emotional instability | 100–200 | Decreased inhibitions, emotional instability, some muscular incoordination, slowing of responses to stimuli, signs of intoxication |
| Confusion | 200–300 | Disturbance of sensation, decreased pain sense, staggering gait, slurred speech |
| Stupor | 300–400 | Marked decrease in response to stimuli, muscular incoordination approaching paralysis, impaired intelligence |
| Coma, death | 400 | Complete unconsciousness, depressed reflexes and respiration, subnormal temperature, anaesthesia, impairment of circulation, coma, possible death |
* Note: A blood alcohol level of 0.05% (the legally safe driving limit in most states of Australia), i.e. 0.05 g/100 mL, equates to 50 mg/dL.
Effects on other systems
The effects of alcohol on other body systems are as follows:
Methanol (methyl alcohol, wood alcohol)
If taken orally, methanol is a CNS toxin: the fatal dose is in the range 100–200 mL and as little as 10 mL has been known to cause permanent blindness. The reason for this severe toxicity compared with the very similar alcohol, ethanol, is that whereas ethanol is metabolised to acetaldehyde and then acetate, methanol is metabolised to formaldehyde (formalin) and formate, which are more toxic. Formic acid is slowly metabolised, with maximum concentrations in the blood 2–3 days after ingestion of methanol, and is the cause of severe metabolic acidosis. The specific ocular toxicity of formaldehyde is because it is a potent inhibitor of respiration and glycolysis in the retina.
Alcohol abuse and misuse
Prevalence of alcohol use and abuse
Alcohol is the most widely-used recreational drug in Australia, and the pattern of consumption has remained relatively stable over 20 years, with approximately 40% of the population having an alcoholic drink at least weekly. Overall trends show an increase in wine drinking since the 1970s and decrease in beer drinking. Compared with 39 other countries (in 2003), Australians ranked 22nd highest in total alcohol consumption (European countries were highest): 9th highest for beer (Czech Republic highest), 19th for wines (Luxembourg and France highest) and 30th for spirits (Russia and Latvia highest). Australian men in the age range 35–55 averaged about 15 drinks per week, and women about 7 per week.
Alcoholism
Alcohol abuse has been defined simply as any form of alcohol drinking that is more than customary, traditional or dietary use. Alcoholism is a physical or psychological dependence on alcohol, with a compulsion to consume despite adverse effects. It is a common, heritable, chronic relapsing disorder; the GABRA2 gene coding for a subunit on the GABA(A) receptor involved in the reward pathway is implicated in the disorder (see review by Enoch [2008]).
Problems from alcohol abuse
Problem drinking is considered to occur when people drink to escape problems or to the level of intoxication; work, drive, suffer injury or come into conflict with the law while intoxicated; or indulge in behaviour while intoxicated that they would not do when sober. Such problems include: social isolation ranging from family breakdowns to ‘skid-row’ lifestyle; increase in selfdestructive behaviours, suicide and motor vehicle accidents; neuropathies (peripheral and central) and myopathies (skeletal, cardiac); chronic hepatotoxicity and cirrhosis, with associated possible death from oesophageal varices; gastrointestinal (GI) or haematological toxicity; Korsakoff’s psychosis, alcohol dementia and cerebellar degeneration; and fetal abnormalities. It is estimated that 15%–25% of all male hospital admissions are due to alcohol-related causes (injuries, accidents, chronic disorders).
Tolerance develops to most effects of low doses of ethanol, particularly while blood alcohol levels are rising. There is both pharmacokinetic tolerance, due to induction of drug-metabolising enzymes in chronically alcoholic persons, and pharmacodynamic tolerance, due to some adaptation to the depressant effects. There is research evidence that ethanol increases opioid neurotransmission, which accounts for some of the euphoriant and reinforcing effects of alcohol and also for the tolerance that develops to some actions of alcohol. Humans with a family history of alcohol dependence show increased release of endorphins in response to an alcohol challenge, compared to those with no family history of alcohol abuse. Clinical trials of naltrexone in patients with alcohol dependence show a modest therapeutic effect of the opioid antagonist in reducing alcohol consumption.
Hangover and alcohol withdrawal syndrome
A ‘hangover’ is a mild withdrawal syndrome after acute intoxication. The symptoms (usually suffered on ‘the morning after the night before’) are headache, nausea, vertigo, pallor, sweating, tachycardia and nystagmus (rapid jerky eye movements). Various mechanisms have been suggested as contributing to hangover symptoms, including hypoglycaemia, dehydration (due to the diuretic effect of alcohol, possibly compounded by lack of other fluid intake, and vomiting), electrolyte imbalances and persistence of lactic acid and acetaldehyde in the bloodstream. A hangover is a withdrawal reaction, hence it can be ‘cured’ by another dose of the drug of dependence, i.e. alcohol, which is the basis for the old custom of treating hangover with another drink, known as taking ‘the hair of the dog that bit you’.
Treating alcohol abuse
Long-term treatment of ethanol withdrawal and abuse needs to be continued for many months or years; it includes the person’s admitting that he/she needs help, plus monitoring health status, symptom relief, preventing or treating complications, psychotherapy to improve coping skills and prevent relapses and developing long-term rehabilitation plans. Supportive care for the acute phase includes fluid, electrolyte and glucose replacement, adequate nutrition, thiamine to prevent development of Wernicke’s encephalopathy, antiemetics and psychotropic agents (if agitation is severe); hospitalisation may be necessary while the patient is on high doses of sedatives or anticonvulsants (oral diazepam). Naltrexone can be used to facilitate abstinence by reducing craving and relapses (Drug Monograph 21-1).
Disulfiram
There are also alcohol-sensitising drugs, in particular disulfiram, which when co-administered with alcohol make the person suffer such a severe drug interaction that this deters the person from drinking alcohol. Disulfiram inhibits the enzyme aldehyde dehydrogenase (which converts acetaldehyde to acetate); enzyme inhibition permits acetaldehyde to accumulate and cause the unpleasant toxic effects: vasodilation, hyper-ventilation and raised pulse rate, pounding headache and copious vomiting. Disulfiram is only effective if the patient is deterred by fear of unpleasant effects, and if compliance can be monitored.
An analogous drug used to treat acute poisoning by other alcohols (methanol, ethylene glycol) is fomepizole; it inhibits the enzyme alcohol dehydrogenase and thus reduces build-up of the toxic metabolite formic acid. It is not at present marketed in Australia, but may be made available via the Special Access Scheme.
Some drugs that interact with alcohol are listed in Drug Interactions 21-1.
Drug interactions 21-1 Alcohol
| Substances interacting with alcohol | Mechanism | Possible effects |
| Antihistamines, antidepressants, opioid analgesics, hypnotics, antianxiety agents, antipsychotic drugs | Additive | Enhanced CNS-depressant effects |
| Disulfiram, some cefalosporins, oral antidiabetic agents, griseofulvin, metronidazole, procarbazine, tinidazole | Inhibition of aldehyde dehydrogenase in metabolism of alcohol, leading to acetaldehyde accumulation (a ‘disulfiram-type reaction’) | Most severe effects seen with disulfiram and alcohol: flushing, stomach pain, head throbbing, raised heart rate, hypotension, sweating, nausea and vomiting; with antidiabetic agents, mild to severe hypoglycaemia |
| Phenytoin, warfarin | Increase or decrease in liver metabolism | In chronic alcohol abuse or anticoagulation: possible decrease in anticonvulsant or anticoagulant effect caused by increased metabolism (enzyme induction). In acute alcohol use: a possible decrease in metabolism, causing raised serum level of phenytoin or warfarin, and toxicity |
| Salicylates | Additive | Increased GI irritability and bleeding |
| Nitrates, glyceryl trinitrate | Additive | Vasodilation leading to hypotension, syncope |
| Anticholinergics, antispasmodics | Slowed GI functions | Slowed absorption of alcohol |
| Prokinetic drugs (metoclopramide) | Accelerated GI functions | Faster absorption of alcohol |
| Paracetamol | Additive | Enhanced hepatic toxicity of paracetamol |
Adapted from: Speight & Holford 1997; Australian Medicines Handbook 2010.
Acamprosate
The drug of choice in treating chronic alcohol abuse is now acamprosate (Drug Monograph 21-4), which is used in combination with counselling and lifestyle changes to maintain abstinence from alcohol. Acamprosate is chemically related to the neurotransmitters GABA, glutamate and taurine, and may restore inhibitory neurotransmission in these pathways. In rats it decreases alcoholseeking behaviour. Acamprosate reduces the symptoms of alcohol withdrawal (craving, anxiety, irritability and insomnia), but does not affect the metabolism of alcohol.
Drug monograph 21-4 Acamprosate
Acamprosate is a relatively new drug that reduces neuronal hyperexcitability and sometimes decreases alcohol craving and consumption; there is no abuse risk.
Indications
Acamprosate is indicated to help maintain abstinence from alcohol and reduce relapse rates in alcoholdependent people. It is used as an adjunct to psychological and social therapies.
Pharmacokinetics
Absorption after oral admin istration is moderate, but slow and variable; bioavailability is decreased by food in the GI tract. Acamprosate is not metabolised and is excreted unchanged in the urine; thus renal impairment reduces drug elimination. The half-life is 13–28 hours, with steady state reached in about 7 days.
Adverse drug reactions
The main adverse effects are in the GI tract (nausea and vomiting, diarrhoea, abdominal pain) and skin rashes.
Drug interactions
Few large-scale studies have been carried out. Acamprosate does not alter the CNS effects or metabolism of alcohol.
Other CNS depressants
Benzodiazepines and barbiturates
Misuse and abuse of other CNS depressants (benzodiazepines, barbiturates and chloral hydrate-type sedatives) have declined greatly in recent years, probably as a result of the availability of newer agents with greater safety and effectiveness profiles. (General information about these drugs is summarised in Tables 21-1 and 21-2; their pharmacological and clinical aspects are discussed in Chapters 16 and 17.) The general effects are similar to dependence on ethanol but the social setting is different: whereas men tend more to abuse alcohol, women are more likely to become dependent on benzodiazepines (‘mother’s little helpers’).
Benzodiazepines are commonly prescribed for anxiety, insomnia and convulsive disorders. They can cause de pendence and patients may ‘shop around’ among doctors and pharmacists to obtain supplies. Tolerance develops to the sedating and intoxicating effects but not to the respiratory depression. Managing benzodiazepine dependence should include gradual drug withdrawal. Flumazenil is a specific benzodiazepine-receptor antagonist that is administered intravenously to reverse sedation from benzodiazepines used during anaesthesia, and for treatment of benzodiazepine toxicity; it may precipitate seizures. As flumazenil has a short half-life (50 minutes), several doses may be required to treat the patient who has overdosed with a long-acting benzodiazepine.
Temazepam, a short-acting benzodiazepine used for insomnia, was formerly available in Australia in gel capsules containing temazepam in solution. Unfortunately these came to be abused, as injecting drug users injected the contents of the gel-caps, particularly at times of heroin shortage, in order to replace the depressant effects of the opioid or alcohol, to deal with anxiety and stress or to offset the effects of CNS-stimulant drugs. Common complications included abscesses, skin ulcers, deep venous thromboses, burst aneurysm and haemorrhage, ischaemia and gangrene requiring amputation. The gel-caps have now been withdrawn from the market.
Kava
Traditional uses
Kava (yaqona, ‘grog’) is an intoxicating drink made by fermenting the grated, pounded or chewed roots of Piper methysticum, a plant native to the south Pacific region, used at religious and welcoming ceremonies, to calm tensions and to improve socialising. Traditional medicinal uses include as a panacea for various complaints (cough and cold, convulsions, kidney and bladder problems), as a contraceptive, a poultice for wounds and to improve male sex drive. Proven pharmacological effects are similar to those of benzodiazepines or alcohol and include muscle relaxation, anticonvulsant effects, analgesia, reduced anxiety, mild stimulation, then sedation. Many active constituents have been identified chemically, in particular the kava lactone kawain and various dihydroderivatives.
Adverse effects
Chronic use has been associated with gastritis, hepatitis, red eyes, puffy face and skin lesions, especially noticeable on the legs. Kava has fewer detrimental effects than alcohol on cognitive functions and information processing; however, it can cause dependence, with compulsion to consume it, inability to control use, preoccupation with obtaining and preparing it and persistent use despite evidence of harm. Reports of acute liver failure (that led to the banning of kava in many countries) were later disproved, and bans on kava were relaxed. In many Pacific countries, the main problems caused by kava are social ones: kava is not consumed with food, but often in community drinking sessions that last for many hours; heavy consumers (usually men) then tend to sleep in late the next day and be unpunctual and inefficient at work; domestic violence is common, and families miss out on basic needs.
Gamma-hydroxybutyrate (GHB)
Actions and mechanism
GHB is a naturally occurring metabolite of the inhibitory neurotransmitter GABA; their structures are very similar:
GHB is a weak agonist at GABAB receptors (inhibitory), and stimulates the 5-HT systems and DA release; higher concentrations inhibit DA release. There are ‘GHB receptors’ in the CNS, activation of which increases levels of glutamate (excitatory) in the brain; thus GHB can have both CNS depressant and stimulatory effects. It has weak analgesic actions, but enhances the actions of narcotic analgesics and neuromuscular blocking agents. Other medical uses have included treatment of insomnia, depression, narcolepsy, alcohol withdrawal and fibromyalgia. It has been used by body-builders and athletes as it stimulates release of growth hormone. It is also structurally related to the ketone body beta-hydroxybutyrate, which accumulates in untreated diabetes mellitus.
Abuse of GHB
GHB (GBH, ‘grievous bodily harm’, ‘fantasy’, ‘liquid ecstasy’; chemically known as sodium oxybate) was originally used as an anaesthetic as it has CNS-depres sant effects: injected IV GHB causes a long-lasting unconsciousness (1.5–3 hours, and longer if taken orally mixed with alcohol) after a delay of a few minutes. It has sedative/hypnotic and amnesic actions that are additive with those of alcohol and is virtually tasteless, so is used as a ‘date-rape’ drug; in low doses it causes little depression of the cardiovascular or respiratory systems. High doses can cause unconsciousness, convulsions, vomiting, suppression of the gag reflex, respiratory depression and death.
GHB comes in a clear liquid form (although food colouring can be added) and is used in the rave dance music scene as an alternative to alcohol, or mixed with alcoholic drinks to cause reduced inhibitions, disorientation, impaired movement, unconsciousness and loss of memory. Particular risks with GHB are:
In October 1996, Australian Police in Victoria issued a warning after several people in a Gold Coast night club suffered respiratory depression and hallucinations; it is thought that their drinks were spiked with GHB. In New Zealand, the number of people admitted to accident and emergency departments after taking life-threatening overdoses of fantasy/grievous bodily harm has been increasing over the past few years; there have been some fatal overdoses.
Inhalants
Abuse of inhaled chemicals
Other CNS-depressant substances of abuse are the volatile hydrocarbons and solvents, grouped as solvents (including glue, liquid paper, petrol, lighter fluid, toluene, xylene, acetone and paint thinners); aerosols (hair spray, deodorant sprays and spray paint); anaesthetics (ether and nitrous oxide) and nitrates (amyl nitrate and butyl nitrite). Chemically, the substances are hydrocarbons (halogenated, aliphatic or aromatic), ketones, esters or ethers. They are general CNS depressants with pharmacological properties similar to those of the halogenated hydrocarbon general anaesthetics such as chloroform and halothane (see Chapter 14).
When abused, they are sniffed (inhaled); this procedure is referred to as ‘chroming’ or ‘glue sniffing’. This type of substance abuse is most common among children and teenagers (6–15 years of age). In economically depressed populations, inhalants are often the main drugs of abuse, particularly by teenage girls and men in their twenties.4
Adverse effects and toxicity
On inhalation these agents may produce a rapid general CNS depression with marked inebriation, dizziness, exhilaration, disinhibition and aggressiveness—similar to effects seen with alcohol intoxication. Inhalation may also result in bronchial and laryngeal irritation, cardiac arrhythmias, ventricular fibrillation and renal tubular acidosis, especially with glue sniffing. At high doses, confusion and coma occur, as well as blood dyscrasias. A serious risk is that the person may pass out while inhaling from a plastic bag and suffocate. Generally, treatment is with removal of the inhaled agent and bed-rest. Recovery from lower doses may be seen in 15 minutes to a few hours. Chronic inhalant abuse will lead to neurotoxicity, and hepatic and renal toxicity; deaths from cardiac arrhythmia, vagal inhibiton, anoxia and respiratory failure have been reported. Habituation and dependence can occur and tolerance also develops.
CNS stimulants
The primary CNS stimulants abused are the amphetamines and related ‘designer drugs’ (such as ecstasy) and nicotine, cocaine and caffeine. The pharmacological aspects and clinical uses of these drugs are discussed in Chapter 19 and also mentioned in Chapter 28 under ‘Respiratory stimulants’. The monoamine neurotransmitters are important in the mechanisms of actions of stimulants: the DA transporter (reuptake process) especially in the reinforcer and behavioural stimulant effects and noradrenaline and 5-HT in the release mechanism and in modulating the effects of psychostimulants. Glutamate systems help modulate DA functions, and inhibitory GABA systems modulate DA and glutamate release (see again Table 14-1 for CNS neurotransmitters).
Amphetamines
Amphetamines exemplify well the earlier statement that ‘drugs likely to be abused have three characteristics: they act fast, make you feel good, or stop you feeling bad’.
Actions and mechanisms
Chemically, amphetamines are similar to the natural catecholamines, adrenaline, noradrenaline and dopamine, but have fewer hydroxyl groups so are more lipid-soluble and CNS-active. They have weak agonist actions on adrenoreceptor sites and are classified as sympathomimetic agents. They increase the release of natural catecholamines and block their reuptake into neurons, which causes a ‘fight or flight’ response; they may also have mild monoamine oxidase inhibitory effects, which contributes to their sympathomimetic and CNS stimulant actions. The three main types of amphetamines (salts of racemic (±)-amphetamines, dextro-amphetamines and methamphetamines) vary in potency and peripheral effects. Dexamphetamine is said to have fewest peripheral effects, such as hypertension and tachycardia. Central effects include increases in mood, energy, alertness and mental and physical capacities and decreased appetite and sleep; euphoria and stereotyped behaviours also occur (see Drug Monograph 19-1).
Pharmacokinetics
Oral amphetamine is absorbed from the GI tract and concentrates in the brain, kidneys and lungs. It is metabolised in the liver and excreted via the kidneys. Amphetamine is a basic drug with a pKa (the pH at which half the drug amount is ionised and half un-ionised) of 9.9; therefore, alkaline urine with pH >7 reduces excretion of amphetamine and extends its half-life to about 20 hours. Acidic urine at pH 5, by contrast, increases excretion and reduces the half-life to 5–6 hours. People who abuse this drug are usually aware of the prolonged effect they can achieve by alkalinising their urine (with oral sodium or potassium citrate), whereas prescribers are aware that acidifying a person’s urine (with oral ammonium chloride) to a pH of 4.5–5.5 will enhance amphetamine excretion and help treatment of ampheta mine overdose.
Abuse of amphetamines
Amphetamines were widely used during World War II by servicemen to enhance alertness and reduce battle fatigue, and quickly became popular drugs of abuse, amphetamine as ‘benzedrine’ and methamphetamine or methedrine as ‘speed’. Results from the 2007 National Drug Survey in Australia showed that approximately 7.3% of persons aged 20–29 years had used amphetamines in the previous 12 months, and 11.2% had used the related drug ecstasy; the use of ecstasy increased steadily from 1991 to 2004. Most amphetamines are produced in illegal backyard laboratories, with no controls over the manufacturing practices or the purity or strength of the product, and sold illegally. Due to the unknown strength of street supplies, overdose is common and potentially fatal.
Amphetamines are generally taken orally, but can also be inhaled after vaporisation, inhaled as fine powders (‘snorted’) or injected. Intravenous amphetamine injection results in marked euphoria—a rush accompanied by a sense of great physical strength and clear thinking, and engagement in vigorous activity that may actually be inefficient and repetitious. Parenteral use carries the usual risks of IV drug abuse and associated infections.
The sympathomimetic stimulant properties of amphetamines can cause dramatic effects such as tachycardia, dyspnoea, chest pain and hypertension; infarcts, hyperpyrexia, hepatotoxicity, seizures and circulatory collapse have been reported. Severe anxiety, paranoia, schizophrenia-like symptoms (‘snow lights’), insomnia and weight loss also occur. Amphetamine users often use depressants or ‘downers’, such as large amounts of alcohol, marijuana, benzodiazepines, barbiturates or heroin to offset the overstimulation effects. Detoxification and use of usual treatments for medical complications, including anticonvulsants and antihypertensive agents, are necessary in treating acute toxicity.
There is a rapid fall-off in drug effects, which enhances the intense craving for the drug and rapidly leads to addiction. Drug withdrawal is followed by long periods of sleep and, on waking, the individual often feels hungry, extremely lethargic and profoundly depressed (anhedonia). This phenomenon is known as ‘crashing’ and is typical of the rebound swings after withdrawal of drugs of dependence. Suicide risk is quite possible during this period.
Amphetamine (especially methamphetamine) use is on the rise. Crystal methamphetamine (known as ‘ice’ or ‘crystal meth’) is gaining popularity because a ‘high’ occurs usually in less than a minute when these crystals are heated and the vapour is inhaled. In some instances, oral amphetamine users are also inhaling or smoking methamphetamine concurrently, which vastly increases the intensity and toxicity of the effect, and its duration (a ‘high’ can persist for 12 hours).
‘Designer drugs’
These are drugs designed and synthesised to be amphetamine look-alikes and mimic the CNS-stimulant effects of amphetamines and cocaine; the people designing the drugs are aiming at keeping a step ahead of the drug policy-makers and law-enforcement officers. The classic designer drug is 3,4-methylenedioxymethamphetamine (MDMA, better known as ‘ecstasy’, see Figure 21-4), originally synthesised in 1914 as an appetite suppressant but which has recently found popularity as a stimulant. Related compounds have varying chemical substituents (methoxy-, methyl-, halogen or sulfur) on the phenyl ring of the amphetamine or methamphetamine. They have similar mechanisms of action to amphetamine, interfering with uptake processes (transporters) in CNS neurons or enhancing release, to raise levels of monoamine neurotransmitters and cause CNS stimulation.
Abuse of designer drugs
‘The typical scene for abuse of ecstasy and other such drugs is at dance parties (the rave scene), where the drugs are taken to produce euphoria, feelings of closeness and confidence; hence the street names ‘ecstasy’ and ‘love drug’. It is estimated that about 7.5% of Australians aged over 14 have tried ecstasy (see Clinical Interest Box 21-10). Unwanted effects include jaw clenching and teeth grinding, anxiety, paranoia and confusion, mild hallucinations, impaired cognition, bizarre behaviour and possibly psychosis. Overdose can result in hypertension, tachycardia and hyperthermia; deaths have occurred from excess CNS and autonomic stimulation. Users of ecstasy in the dance scene are advised to take frequent rest breaks and sip water regularly to rehydrate.
Clinical interest box 21-10 The ecstasy con trick
Ecstasy (MDMA) is pushed as the risk-free feel-good drug of the world’s dance and club scene, and about 11% of young Australians (18–29 years old) think they have tried it. According to the Victorian Police Drug Squad, however, less than 10% of the drug in Australia is imported (likely to be true MDMA), and most of the white tablets made in Australian backyard laboratories and sold as ecstasy for $40–$60 per tablet are in fact imitations, or worse.
Chemical analyses of tablets seized in drug raids have shown that most contain not MDMA but methamphetamine, which is simpler and cheaper to make. More importantly, other contaminants found in the tablets included ketamine (a veterinary anaesthetic), codeine, paracetamol, ephedrine and pseudoephedrine, caffeine, benzodiazepines, antihistamines and even cocaine, heroin and LSD.
While the CNS and cardiovascular effects of some of the contaminant drugs may cancel out the actions of amphetamines, in other cases the effects could be additive, with potentially serious results. The risks to young people of inadvertently taking ‘hard’ addictive drugs such as heroin and cocaine, potentially becoming dependent and moving into the IV ‘shooting-up’ scene, are great. As one young rave party dancer stated, ‘If you buy things off complete strangers (at a party or nightclub), you’re pretty stupid’.
Adapted from: Christophersen 2000; Mundell 2001.
Nicotine and tobacco smoking
As discussed in Chapter 11, the major neurotransmitter at all autonomic ganglia is acetylcholine, where its effects were described as nicotinic because they were most closely mimicked by the known compound nicotine. Nicotine is the chief alkaloid in the tobacco plant Nicotiana tabacum; it is an oily liquid alkaloid, freely soluble in both organic solvents and water, which turns brown on exposure to air. Nicotine has no therapeutic use (other than in nicotine replacement therapy for smokers trying to quit), but is of great pharmacological and toxicological importance. Nicotine is readily absorbed from the GI tract, respiratory mucous membrane and skin. It is most commonly selfadministered by smoking cigarettes (which contain about 1 g nicotine each), cigars or pipes. Tobacco smoking was introduced into European societies from Central America in the 16th century.
Pharmacological effects
Peripheral effects
Many actions are dose-related, with small doses generally inducing activation or stimulation at receptors, and larger doses producing a decreased or depressed response. As stimulation of autonomic postganglionic fibres produces effects on smooth muscle, cardiac muscle and glands (see Figure 11-6), non-selective ganglionic stimulants that stimulate both parasympathetic and sympathetic pathways can result in a broad range of pharmacological effects. The actions and effects of nicotine on the cardiovascular system are complex. Heart rate is frequently slowed at first but later may be accelerated. The small blood vessels in peripheral parts of the body constrict but later may dilate, and the blood pressure rises then falls; this occurs in nicotine poisoning. Nicotine also has an antidiuretic action and decreases GI motility. Stimulation of all sympathetic and parasympathetic ganglia is followed by depression, which tends to last longer. Nicotinic-type acetylcholine receptors are also present at the end-plate in neuromuscular junctions, where effects are similar: stimulation then a depressant phase during which nicotine exerts a curare-like action on skeletal muscle.
CNS and neuromuscular effects
Acetylcholine is a neurotransmitter in the CNS and at the neuromuscular junction (at nicotinic receptors) as well, so nicotine stimulates the CNS, especially the medullary centres (respiratory, emetic and vasomotor) and skeletal muscles. Central effects commonly reported by humans are increased alertness and concentration and reduced boredom and anxiety; learning and performance have been shown to improve, and dependence occurs. Large doses may cause tremor and convulsions. Stimulation is followed by depression. Death may result from respiratory failure, caused mainly by the curare-like action of nicotine on nerve endings in the diaphragm.
Repeated administration of nicotine causes development of tolerance to some of its effects, particularly to the nausea, sweating, antidiuretic effects and feelings of unease, so that habitual smokers find smoking pleasurable and relaxing, whereas first-time smokers become anxious and nauseated. (It is an indication of the strong ‘rewarding’ effect and addictive potential of nicotine—and of the aggressive advertising practices and peer pressures encouraging smoking—that anyone goes on to smoke a second cigarette.)
Beneficial effects
Chronic smokers have a lower than average prevalence of both Alzheimer’s disease and Parkinson’s disease; the suggested rationale is that nicotine may enhance dopaminergic and cholinergic activity in central pathways. Nicotinic agonists have been tried in treatment of both conditions but, because of their slowly progressive course, clear-cut effects are difficult to prove and further long-term prospective studies are required.
Toxicity
Nicotine has both short- and long-term toxic effects that are extremely important in public health terms. Cases of nicotine toxicity have resulted from ingestion of tobacco products by small children and from percutaneous absorption after misuse of insecticides containing nicotine, which at times has led to the deaths of farm workers.
Drug interactions
There are potential interactions between nicotine and any other drug affecting acetylcholine functions, whether in the autonomic, motor or central nervous systems (see Drug Interactions 21-2); and indeed with any drug affecting neurotransmitter functions or balances generally. Tobacco smoking induces the drug-metabolising enzyme CYP1A2, hence there are potential drug interactions with other inducers (including carbamazepine and rifampicin), inhibitors (amiodarone, cimetidine, erythromycin) or substrates (amitriptyline, haloperidol, naproxen, paracetamol, warfarin…) of the same enzyme; reference texts should be consulted for individual potential interactions.
Drug interactions 21-2 Nicotine (or tobacco)
| Drug | Possible effects and management |
| Paracetamol, caffeine, oxazepam, propranolol and theophylline (and others) | Smoking increases metabolism of many drugs, which may result in lower blood concentrations, so higher or more frequent drug dosing may be required |
| Adrenergic agonists or blocking agents, catecholamines, corticosteroids | Smoking and nicotine raise catecholamine and cortisone levels; therapy with adrenoceptor agonists or antagonists or corticosteroids may require dosage adjustment based on the individual’s response |
| Insulin | Smoking cessation may result in an increased insulin effect; dosage reduction may be necessary; monitor closely for symptoms of hypoglycaemia |
| Autonomic drugs, including antihypertensives, bronchodilators and vasodilators | The effects of nicotine on autonomic ganglia are complicated and dosedependent; doses of other autonomic drugs may need adjusting |
| Vasoconstrictors | Nicotine decreases myocardial oxygen supply and increases demand; these effects are compounded by other vasoconstrictors |
| Acidic beverages (coffee, soft drinks) | May decrease buccal absorption of nicotine |
| Nicotine (in other forms, e.g. cigarettes, patches) | Additive effects, leading to chest pains and palpitations |
Tobacco smoking and nicotine
Tobacco smoke is an aerosol containing about 4 × 109 particles per mL, i.e. in the range 10–80 mg per cigarette; nicotine accounts for about 0.14–1.21 mg. Burning of tobacco also generates around 4000 compounds in the gaseous and particulate phases, including 60 known carcinogens such as tars, formaldehyde, hydrogen cyanide, benzene and nitrosamines, implicated as aetiological factors in cancers of the bladder, lung, buccal cavity, oesophagus and pancreas. Other smoking-related illnesses include pulmonary emphysema, chronic bronchitis, coronary heart disease, strokes, myocardial infarction and chronic dyspepsia. Male smokers have about one-third the sperm count of non-smokers. Cigarette smoking is thought to be responsible for more than 50% of all domestic fires. Smoking is also a considerable financial outlay: it has been estimated that someone who has smoked 10 cigarettes a day since the age of 20 and has the good luck to live to be 50 will be more than A$250,000 poorer than a non-smoker of the same age.
Smokers absorb sufficient nicotine to exert a variety of effects on the autonomic nervous system. In people with peripheral vascular disease such as thromboangiitis obliterans (Buerger’s disease), nicotine is generally believed to be a contributing factor by causing spasms of the peripheral blood vessels. Vasospasm in the retinal blood vessels of the eye, associated with smoking of tobacco, is thought to cause serious disturbance of vision. Most male smokers of 20 years duration are impotent by the age of 50, owing to microvascular damage. Mothers who smoke usually deliver infants with low birth weights and a higher incidence of congenital abnormalities; prematurity and stillbirth are more common.
Passive smoking
Passive smoking refers to the inhalation of cigarette smoke by non-smokers. Reports from studies in Australia, the USA and UK all indicate that:
Dependence on nicotine
Tobacco is Australia’s worst ‘killer’ drug, the most abused and the most harmful, killing ‘more people than alcohol, drugs, murder, suicide, road, rail and air crashes, poisoning, HIV, drowning, fires, falls, lightning, electrocution, snakes, spiders and sharks put together’ (Jamrozik & Le 2001). A 2007 national survey of drug use found that about 16.6% of Australians aged 14 years and over smoked regularly at least daily.
The addictive component of tobacco is nicotine. Monkeys trained to press a bar to receive an IV injection of nicotine will self-administer up to a point, at which the adverse effects presumably outweigh the rewarding effects. Nicotine is also postulated to have an antidepressant action, and ex-smokers who successfully quit and abstain often suffer clinical depression.
The dose of nicotine absorbed from one cigarette is estimated to amount to about 10–40 mcg/kg body weight, and smokers tend to maintain their plasma nicotine concentration at about 10–50 ng/mL. By comparison, nicotine is absorbed more slowly from cigars and pipes but the doses of nicotine achieved are in the same order, about 20–40 mcg/kg (see Figure 21-3).
Figure 21-3 Plasma concentration of nicotine after smoking. Habitual smokers used a cigarette, pipe or cigar; blood samples were taken and plasma levels of nicotine measured. The mean dose of nicotine over the period of smoking was estimated at, for a cigarette, 21 mcg/kg over 8–10 minutes; pipe, 45 mcg/kg over 20–30 minutes; cigar, 41 mcg/kg over 22 minutes.
Source: Bowman & Rand 1980, used with permission; data obtained by Dr MP Giles, Department of Pharmacology, University of Melbourne.
Treating nicotine dependence
Cessation of smoking
Smoking is notoriously hard to quit; the withdrawal syndrome, consisting of irritability, impatience, anxiety, restlessness and headaches, continues for several days; the craving persists for weeks, months or years. As with other drug-dependent states, treatment may be by replacement of the drug with a less harmful related drug (varenicline) or with nicotine delivered in a less harmful formulation such as a gum (Drug Monograph 21-5) or patch. Unfortunately there does not appear to be a nicotinic antagonist agent effective in treating dependence analogous to the use of naltrexone in opioid and alcohol dependence.
Drug monograph 21-5 Nicotine gum
Nicotine is an autonomic ganglion-stimulant agent plus has actions at the neuromuscular junction and in the CNS, hence has widespread effects in the body. When used medically in nicotine replacement therapy by smokers trying to quit, it decreases the severity of the tobacco withdrawal syndrome and increases the likelihood of smoking cessation. Nicotine is available in gum (resin), sublingual tablets, inhaler and transdermal systems (patches) for use in smoking cessation programs. The most effective treatment currently is a combination of patch (providing steady nicotine replacement) with gum or spray (to provide the ‘hit’ when a boost of euphoriant effect is required). Dependence on nicotine replacement therapy is considered easier to break than is the smoking habit.
Indications
The nicotine chewing gum is for a nicotinedependent patient undergoing acute cigarette withdrawal or trying to sustain abstinence from smoking. It is most effective in moderate to heavy smokers who are well motivated to quit and who have good counselling and social support. When the person has a strong urge to smoke, a stick of gum is chewed instead, which relieves withdrawal. The number of pieces of gum chewed is gradually reduced over a 2-to 3-month period. Nicotine replacement therapy is of little benefit to light smokers, for whom behavioural techniques to support quitting are recommended.
Pharmacokinetics
Pure nicotine is a colourless–pale yellow oily liquid. It is very lipid-soluble and rapidly absorbed across lipid membranes of the mouth, skin, airways and GI tract. When chewed as a gum (or from tobacco) it is absorbed through the buccal mucosa, more slowly than if inhaled while smoking. When saliva containing nicotine is swallowed, the drug is absorbed through the GI tract.
It is metabolised primarily in the liver, also in the kidney and lung. Most metabolites are inactive, although cotinine, the main oxidation product, is said to have antidepressant and psychomotor stimulant properties. The half-life is 1–2 hours. Elimination is primarily renal, with 10% excreted unchanged and the remainder as metabolites; the drug is excreted in breast milk. Adding menthol to tobacco in cigarettes slows the metabolism of nicotine and prolongs its half-life.
Adverse reactions
Adverse effects of nicotine include fast heart beat, mild headache, increased appetite, increased watering of mouth or dry mouth, sore mouth or throat, coughing, dizziness or light-headedness, hiccups, irritability, indigestion and difficulty in sleeping. Adverse reactions with nicotine gum include local injury to mouth, teeth or dental work. Transdermal patches may also cause pruritus and/or erythema under the patch. Some of the signs and symptoms may in fact be due to stopping smoking, rather than to nicotine gum.
Early signs of overdose are nausea and vomiting, severe increased salivation, severe abdominal pain, diarrhoea, cold sweat, severe headache and dizziness, disturbed hearing and vision, confusion and severe weakness; while signs of toxicity include fainting, hypotension, difficulty breathing and fast, weak or irregular pulse and convulsions.
Warnings and contraindications
Use with caution in people with cardiovascular disease, insulin-dependent diabetes mellitus, hyperthyroidism, peptic ulcer disease or phaeochromocytoma. Avoid use in patients with nicotine hypersensitivity, severe angina pectoris or life-threatening cardiac arrhythmias, and after myocardial infarction. Use is not recommended in pregnancy or breastfeeding; however, nicotine replacement therapy is less dangerous than smoking.
Dosage and administration
Nicotine gum comes in two strengths, either 2 mg or 4 mg nicotine per piece. The patient should be instructed to stop smoking before using nicotine replacements (gum, patches or inhaler) and not to use any other nicotine products.
The gum is chewed intermittently and very slowly when the individual has the urge to smoke. The user controls the dose by biting the gum to release nicotine. The oral dosage is 2 or 4 mg as a chewing gum, repeated as needed, with 10–12 pieces of gum per day, tapering off over 8–12 weeks.
Physician advice and follow-up can lead to maintained cessation rates of about 10%, and behavioural therapy has about a 20% success rate. Doctors in general practice are recommended to follow the ‘5As framework’ (see Litt [2005]) when encouraging patients to quit smoking:
This program, together with follow-up by QuitLine, advice offered by the nurse and pharmacist and combination pharmacotherapy, can significantly enhance quit rates.
Anti-smoking campaigns can effectively reduce the prevalence of smoking and smoking-related disease in the community: the Australian National Tobacco Campaign, an intensive mass-media campaign commenced in 1997 that has cost approximately A$9 million, is estimated to have reduced smoking prevalence by 1.4%. In terms of prevention of thousands of cases of lung cancer, heart attack, stroke and chronic obstructive pulmonary disease and hundreds of thousands of life-years gained, the campaign is credited with health-care cost savings of over A$740 million.
Nicotine by other routes
Nicotine replacement therapy (patches, gum, lozenges, sublingual tablets, spray or inhaler) doubles the cessation rate of advice or behavioural therapy alone. Patches are formulated in a range of doses providing from 21 mg/24 h to 7 mg/24 h; the dose is tapered off over several weeks. The patches and gum are available OTC (Schedule 2) but it has been suggested that, until nicotine replacement therapy is more available and cheaper than cigarettes, there are financial reasons why nicotine-dependent persons continue smoking.
Other aids to quitting
Varenicline is a new agent for treating nicotine dependence; studies have shown its efficacy to be greater than that of nicotine replacement or bupropion. It is a partial agonist at nicotinic receptors, hence substitutes for nicotine and helps reduce withdrawal symptoms and the pleasurable effects of smoking, with fewer adverse effects—this is analogous to the use of buprenorphine, a partial opioid agonist, in heroin addiction. Patients are advised to start taking varenicline 1–2 weeks before stopping smoking; other nicotine products should be avoided. Adverse effects (nausea, dizziness, GIT disturbances, insomnia) may also be related to nicotine withdrawal; the weight gain common after stopping smoking is not prevented. Interestingly, varenicline is also showing efficacy in treating alcohol dependence, which suggests a nicotinic pathway in other addictions.
Bupropion, which inhibits neuronal reuptake of dopamine and noradrenaline, was previously used as an antidepressant. When used as an aid to stop smoking, along with counselling, it enhances cessation rates to 10%–24%; it is thought that the antidepressant effects contribute to helping smokers give up smoking, as nicotine itself has antidepressant and antianxiety actions. When the cost of the drug was partly subsidised after its introduction into the Pharmaceutical Benefits Scheme (PBS), there was an unexpectedly high demand for the new therapy, with an estimated 200,000 Australians believed to have started using the drug within 4 months. Adverse effects (sleep disturbance, dizziness, headache, anxiety, suicidal ideation) may be related to stopping smoking and withdrawal from nicotine.
An alternative natural therapy to aid quitting is a product called Nicobrevin; oral capsules contain menthyl valerate, quinine, camphor and eucalyptus oil. However, search via the Cochrane database found no approved clinical trials showing evidence of long-term efficacy compared to placebo or other treatment.
Cocaine
Pharmacological properties
Cocaine is an alkaloid related to the belladonna alkaloids atropine and hyoscine; it is an ester-type local anaesthetic (see Clinical Interest Box 14-9) with vasoconstrictor effects. Topically and parenterally it has a limited local anaesthetic use in a few nasal and ophthalmic surgical procedures. Its central and autonomic effects are largely due to inhibition of reuptake of catecholamines back into nerve terminals, thus it potentiates the actions of noradrenaline, adrenaline and dopamine and has sympathomimetic effects. Cocaine has central stimulant properties, causing excitement, talkativeness, tremors, vomiting and increased respiration and blood pressure.
Abuse of cocaine
Cocaine is a powerful reinforcer, rapidly producing sensations of reward and exhilaration, and hence dependence. It is classified as a controlled substance (Schedule 8). While abuse of cocaine in Australia is nowhere near the problem it is in the USA, abuse is increasing: recent use in males aged 14 and older has doubled from 1.1% of the population in 1995 to 2.2% in 2007. Approximately 6% of the Australian population say they have ever used cocaine; the main age group using cocaine is young adults 20–29 years of age. There is no typical withdrawal syndrome, but rebound effects may be manifest as fatigue and depression.
Pharmacokinetics
Orally administered cocaine is readily absorbed but subject to an extensive first-pass effect; thus there is low oral bioavailability (as for all local anaesthetics) and short half-life (about 1 hour); cocaine abusers need to use the drug every half hour or less to maintain the high. When abused, cocaine is taken as a snuff (‘snorted’) or by injection; when coca leaves are chewed, cocaine is absorbed via the buccal mucosa. The free-base form (‘rock’ or ‘crack’) is smoked for a rapid, intense effect (see Clinical Interest Box 21-11).
Clinical interest box 21-11 Cocaine and coca-cola
Cocaine is the active ingredient of the leaves of the plant Erythroxylon coca and has been used for thousands of years in Central and South American countries, particularly in the Andes, where its stimulating properties suppressed hunger, alleviated misery and enhanced endurance at high altitudes.
It was introduced into Europe in the 16th century by returning Spanish conquistadors and rapidly achieved popularity. The potential medical usefulness of the new agent was recognised by European doctors, including Sigmund Freud, who experimented with the drug and described its CNS-stimulant effects, as well as the ‘fuzziness on the lips and palate’ (due to local anaesthesia). Freud’s colleague Carl Köller demonstrated the efficacy of cocaine as a local anaesthetic, particularly in eye surgery.
It was soon incorporated into wines, lozenges, toothache drops and tonic mixtures, most famously Coca-Cola, advertised as a ‘temperance drink’ that would aid digestion and stimulate the nervous system. Cocaine was removed from Coca-Cola only in 1908 because of fears about its addictive effects; after a lawsuit, the company was allowed to retain the word ‘coca’ in the name.
The acute toxicity of cocaine was quickly recognised, and studies of its chemical structure and pharmacological properties led to the synthesis of many chemical analogues as local anaesthetics with lower toxicity, including benzocaine, procaine and lignocaine (see Chapter 14 and Drug Monograph 14-4).
Caffeine
The xanthine alkaloids, caffeine, theophylline and theobromine, are found naturally in plants used for making the stimulating beverages coffee, tea and cocoa (see Clinical Interest Box 21-12).
Clinical interest box 21-12 Coffee, tea, cocoa or cola?
The plant from which coffee is extracted, Coffea arabica, is thought to have originated in Ethiopia; the technique for preparing the seeds to produce the stimulant beverage was developed in the 9th century in Yemen near the town Mocha. Coffee was introduced into Western Europe in the 16th century and rapidly became popular in coffee houses; coffee plantations were started in many colonies of the European powers, including in Africa, Brazil and New Guinea.
Tea is a beverage made by infusing dried leaves of the plant Camellia sinensis in boiling water; this has been done in China and Japan for over 1600 years. Tea also reached Europe in the 16th century and plantations were established in many colonies (India, Sri Lanka, Indonesia).
Cocoa and chocolate come from the seed of Theobroma cacao, discovered in the Aztec court in Mexico by Cortes in the early 16th century. The bitter beverage made from cacao beans, peppers and other herbs was called chocolatl, and was thought to have aphrodisiac properties. It was only when the beans were steeped in hot water, and vanilla and sugar added, that the drink became palatable and popular, as chocolate and cocoa.
Cola drinks have secret recipes; however, they are reported to contain sugar and/or artificial sweeteners, flavours such as citrus oils, cinnamon, vanilla, nutmeg and lavender, and phosphoric and citric acids; usually there is very little extract of the Cola nitida nuts (potentially containing caffeine and theobromine) that give the drinks their names. (The high acid content can damage tooth enamel and oral mucosa, and regular intake is associated with lowered bone mineral density and increased risk of bone fractures).
Caffeine is also present in the drink maté (popular in Spanish and Portuguese countries and colonies, an infusion of the leaves of the shrub Ilex paraguayensis) and guarana preparations (from fruit of the plant Paullinia cupana).
All of these beverages rely for their popularity and stimulant qualities on the content of the xanthine alkaloids caffeine, theophylline and theobromine. The caffeine content depends on how the drinks are brewed, with coffee containing about 40–180 mg caffeine per cup (decaffeinated 2–5 mg), tea 30–100 mg, yerba mate 25–150 mg, cocoa 4–70 mg, and chocolate bars 20–75 mg per 100 g. While caffeine can induce a mild dependence, those who claim to be ‘chocoholics’ are most likely addicted to the sugar and flavours in chocolate rather than the caffeine content. The average consumption from beverages by regular tea- or coffee-drinkers is about 200 mg caffeine/day.
Adapted from: Bowman & Rand 1980; Mann 1992, inter alia.
Xanthine alkaloids
Chemically, the xanthines are closely related to the purine bases adenine and guanine (building blocks for DNA and RNA, see Figure 42-1), and they are thought to act through antagonist effects on adenosine receptors. At higher concentrations, the xanthines also inhibit the enzyme phosphodiesterase (PDE), which leads to raised intracellular levels of cAMP and may contribute to some of caffeine’s actions (Figure 28-4). The xanthines have legitimate medical uses, as CNS stimulants (see Chapter 19) and as bronchodilators (Chapter 28); they also have mild diuretic and cardiac-stimulant effects and have been used to treat respiratory failure in premature infants. Caffeine (Drug Monograph 19-2) is the most powerful CNS stimulant of the xanthine alkaloids, and is the xanthine present in many OTC medications and in some prescribed medicines (see Table 21-6). Theophylline (Drug Monograph 28-3) is the most powerful smooth muscle relaxant, so it and its derivative aminophylline are used in asthma.
Social use of caffeine
Caffeine is the most widely used psychoactive substance worldwide; a large proportion of the world’s population have a cup of tea or coffee every day. About half the world’s annual coffee production is consumed in the USA, where the average daily caffeine dose is over 250 mg. By comparison, tea accounts for about 43% of all caffeine consumption; the British tend to drink more tea than coffee, on average over 300 mg caffeine daily. Two to three cups of strong coffee are sufficient to raise the caffeine levels in the plasma or brain to approximately 100 μM, a concentration at which adenosine-receptor blockade and some PDE inhibition occurs.
Pharmacological effects
Habitual moderate coffee intake does not represent a health hazard. Caffeine reduces fatigue, improves concentration (leading to decreased reaction times and increased speed of calculations in tests) and improves motor tasks. In contrast to the amphetamines, caffeine does not cause euphoria, stereotyped behaviours or psychoses. Some tolerance and dependence may develop to caffeine but there is little evidence of an acute withdrawal syndrome (possibly a mild irritability and headache). Animals cannot be trained to self-administer caffeine, which implies that it does not produce reward or dependence (or that they do not like the bitter taste); and caffeine does not act on the dopaminergic structures related to reward, motivation and addiction.
In large doses, caffeine can be mutagenic and teratogenic in animals but these effects have not been seen in humans. Large doses (300–600 mg) can cause insomnia, anxiety, palpitations, tremor, headache, increased gastric secretion and seizures. There have been suggestions that high regular intakes of caffeine are associated with increased incidence of cancers (breast, pancreatic, urogenital) but this association could be due to carcinogens from the roasted coffee beans (which contain more than 1000 different chemicals) rather than related to caffeine intake. Caffeine has also been implicated in female infertility and in low-birth-weight infants. Diterpenes (present in unfiltered coffee brews, e.g. from Turkish or Greek methods of making coffee) have been shown to raise cholesterol levels in the blood.
Tea, on the other hand, may be protective, as the tannins and flavonoids especially in green teas are antiatherosclerotic. Polyphenols in tea have been shown to have inhibitory effects on carcinogenesis in animals, delay cancer onset in humans and have protective antioxidant properties.
Overall, social use of caffeine is generally not problematic and any dependence is mild. Caffeine abuse is not considered sufficiently severe to warrant treatment and is usually self-limiting because of the negative adverse effects (diuresis, insomnia and dyspepsia) and lack of positive effects on reward pathways. However, dependence on the caffeine present in many soft drinks has been implicated in increasing obesity in people who drink large amounts of sweetened carbonated drinks (see Clinical Interest Box 21-13 and Table 21-5).
Clinical interest box 21-13 Caffeine in the diet
Gaining prominence in the soft-drink market are ‘formulated caffeinated beverages’. Marketed as ‘energy- or performanceenhancing drinks’, these products promise to ‘sustain energy levels’ and ‘improve mental acuity’. These drinks may also contain guarana, which consumers may not realise is a natural source of caffeine, as there is insufficient regulation of these products in regard to both caffeine levels and labelling.
Until recently in Australia, manufacture of these products was prohibited, although importation of products from New Zealand and other countries was allowed.
Safety concerns have been accentuated in the community by various media reports of caffeine intoxication. Examples have been the death of a 25-year-old woman from ventricular arrhythmias after ingesting energy drinks; her blood caffeine level was equivalent to that of ingesting 15–20 cups of coffee.
Also, the possibility of caffeine-induced psychosis or ‘caffeinism’ was discussed in a court case where an armed robbery occurred after the assailant consumed 11 cans of an energy drink. High caffeine intake can also induce insomnia, headache and migraine.
An expert working group under the auspices of Food Standards Australia New Zealand (FSANZ) has examined safety aspects of dietary caffeine. The effects on high-risk groups—children, pregnant or lactating women or those with hypersensitivity to caffeine—are under consideration, as is the potential for behavioural changes and caffeine dependence. Guidelines have been developed with respect to labelling of caffeine content of foods and drinks to which caffeine (or guarana) have been added; caffeinated cola drinks and formulated caffeine beverages will be permitted.
Table 21-5 Caffeine content of some medications and soft-drinks
| CAFFEINE CONTENT OF SELECTED MEDICATIONS | ||
| Medication (trade name) | Caffeine per tablet/capsule | Other active ingredients |
| Cafergot | 100 mg per tablet or suppository | Ergotamine tartrate |
| Endura Sports Energy gels | 8.5 mg | Sugars, electrolytes |
| No-Doz | 100 mg | |
| No-Doz Plus | 100 mg | Thiamine, nicotinic acid |
| Travacalm Original | 20 mg | Hyoscine HBr, dimenhydrinate |
| CAFFEINE CONTENT OF SOFT-DRINKS | ||
| Trade name | Approximate amount of caffeine | Other additives |
| Professor Head’s Smart Drink ‘Energy’ | 32 mg/100 mL | Ginseng, yerba maté,† taurine, guarana,‡ other (choline, leucine, valine, arginine, inositol) |
| Red Bull | 32 mg/100 mL | Taurine, inositol, vitamins |
| Diet Coke* | 12.8 mg/100 mL | Unspecified |
| Coca-cola*, Coca-cola Zero* | 9.7, 9.6 mg/100 mL | Unspecified |
| Solo Strong with Guarana | Not specified | Guarana extract‡ 0.1% |
| Pepsi-cola* | 10.6 mg/100 mL | Unspecified |
| Pepsi-cola Max*, Light* | 12 mg/100 mL | Unspecified |
| Energy Recharge | 4.4 mg/100 mL drink | Ginger, ginseng |
| V Guarana Energy Drink | 31 mg/100 mL | Guarana extract‡ 120 mg / 100 mL; taurine, inositol, vitamins |
| Zu Ballistic Energy Drink | 31 mg/100 mL (total caffeine?) | Taurine, guarana |
* Not specified on container, phone call to company is required to ascertain caffeine level.
† Yerba maté; leaves of Ilex paraguayensis (0.2%–2% caffeine).
‡ Guarana, from the ‘beans’ of the South American plant Paullina cupana (1%–5% caffeine).
Psychotomimetics
The term psychotomimetics literally means drugs that produce or mimic psychotic reactions, and could thus refer to cocaine, amphetamines, antidepressants, centrallyacting antimuscarinics and even some antimicrobial agents. It has, however, come to be used to refer to drugs with a history of religious, social and/or paramedical use in producing changes in perception or hallucinations. The main groups are the cannabinoids and hallucinogens such as lysergic acid diethylamide (LSD) and mescaline.
Cannabis drugs (marijuana, hashish)
Source and uses
The cannabis drugs are derived from hemp plants (Cannabis sativa), which were probably originally native to Central Asia. The plants have historically been used for the very strong fibres in the stems, which are fast-growing up to 5 m in length and are used for weaving into fabric (hence the term canvas), for twisting into ropes (hemp) and for making paper. Hempseed is used as birdseed and is a source of oil. There are also dermatological preparations (oils, soaps, lotions etc) and foods and fabrics based on cannabis.
The active drugs used for mental relaxation and euphoria (cannabinoids) are from the resin of the plant, exuded from the leaves, tops of the stems and the flowering tops of the plants. The potency of the main psychoactive ingredient (Δ9-tetrahydrocannabinol, THC), is greatest in the flowering tops and varies according to the climatic conditions under which the plant is grown, with the typical leaf containing 3%–10% THC. Marijuana grown under scientifically controlled conditions as a THC source may contain up to 15% THC. The other major ingredient, cannabidiol, does not impair cognition and may even have antipsychotic effects.
Preparations and active constituents
Marijuana (cannabis prepared for smoking) and hashish are the most common forms of cannabis in use. Hashish refers to the powdered form of the plant’s resin, which contains 7%–12% THC. Other forms of cannabis, used in such countries as Jamaica, Mexico, Africa, India and the Middle East, include bhang, ganja and charas. In Morocco kif is used and in South America a cannabis form called dagga.
Marijuana plants contain hundreds of different chemicals, generally termed cannabinoids; they have complex 3-ring structures. Of these, Δ9-THC and related compounds and cannabidiol have been most studied in humans to identify their pharmacological effects. Marijuana cigarettes contain about 0.5–2 g marijuana, of which only 0.5%–1% is THC.
Mode of administration and pharmacokinetics
Tetrahydrocannabinols are highly lipid-soluble, so are readily absorbed when administered by oral, subcutaneous or inhaled routes; they are most potent when inhaled. Either the pure resin or the dried leaves of the cannabis plant may be smoked in pipes or cigarettes. The smoke is inhaled deeply5 and retained in the lungs as long as possible to achieve maximal saturation of the absorbing surface; about 15%–50% of the THC present in preparations is actually absorbed after smoking.
The peak plasma level of THC after smoking one marijuana cigarette is reported to occur within minutes. THC is highly protein-bound, so only a small proportion enters the CNS; it persists in the body in adipose tissue depots (for over 4 weeks) and in the lungs and liver, with a long half-life. It is metabolised in the liver to various hydroxylation products, some of which are pharmacologically active. The metabolites are excreted in the urine, bile and faeces. Note that the long half-life of marijuana and the consequent prolonged period for detection of cannabinoids in the urine have made it difficult for accurate correlations to be made between blood cannabinoid concentrations and impaired driving performance, whereas with alcohol the correlation between alcohol in expired air (breath-testing) and impaired psychomotor performance is clear (Figure 21-2).
Pharmacological effects
The main effects are in the CNS, where the drug has intoxicating and mind-altering properties. The drug experience is highly subjective, with a high placebo reaction. Cannabinoids can affect most systems and organs of the body, as shown below:
Mechanism of action
The cannabinoids exert various distinct actions: they seem to act as CNS depressants, similar to ethanol, and also as mild hallucinogens like mescaline; THC has effects on lipid membranes similar to those of general anaesthetics. As dosage increases, the effects proceed from relief of anxiety, disinhibition and excitement, to anaesthesia. If dosage is high enough, respiratory and vasomotor depression may occur.
Much research work has gone into attempting to identify the mechanism of action of cannabinoids and the pos sible natural neurotransmitters or receptors involved. Cannabinoid receptors (CB1R) have been isolated and studied, and the search for a ‘natural’ endogenous cannabinoid (endocannabinoid) has shown up the compound anandamide, an arachidonic acid derivative related to the eicosanoids and prostaglandins. It is proposed that endocannabinoids (and cannabis) stimulate the G-protein-coupled CB1R in the frontal cortex, basal ganglia, hippocampus and cerebellum, and inhibit release of transmitters including glutamate and GABA.
Much active research work is focused on endocannabinoids and their functions, possible uses and exploitation in development of new classes of drugs. Metabolites, known as prostanoids and prostamides, may become effective drugs in inflammation and in glaucoma (see reviews by Woodward et al [2008]; Luzi et al [2008]).
Medical uses of cannabinoids
Synthetic cannabinoids (dronabinol, a pure preparation of Δ9-THC, and nabilone) have been prepared and tested for the treatment of nausea and vomiting induced by cancer chemotherapy and not responsive to standard therapies. Both products have a high potential for abuse and so are closely regulated; neither is available in Australia. Other potential uses of cannabinoids are as an adjunct in treating patients with wasting conditions such as HIV–AIDS and in chronic pain syndromes, insomnia, epilepsy, opioid withdrawal, glaucoma, asthma and neurological diseases with spasticity. Problems that can be envisaged related to medical use of cannabis are the issues of placebo reactions; consistency of dosing and bioavailability after adminis tration by smoking; and the long half-life and potential prolonged adverse effects on concentration and driving.
Marijuana abuse
Traditionally, marijuana was used in Eastern and African countries in religious ceremonies to enhance meditation and as a mild intoxicant (especially when alcohol was prohibited). Marijuana cigarettes (‘joints’) are illegal in Australia but in some states there is a more lenient attitude to marijuana use than to ‘harder’ illicit drugs such as opioids, amphetamines and cocaine, and a plant may be grown for personal use, despite prohibitions against sale and large-scale production. This has been shown to reduce the black market in marijuana products and the costs of enforcement and the criminal justice system, while increasing individuals’ use and civil liberties. In Australia, marijuana is by far the most commonly used illicit drug:6 in a 2007 survey, one-third of the adult population admitted to having used marijuana at some stage. It is now estimated that 40% of young Europeans have tried cannabis at least once in their life. ‘Recent use’ in Australia has been declining, from 18% in 1998 (then much higher than in the USA, UK, Canada or Spain) to 9.1% in 2007. The prohibitions on the use of cannabis are much debated, with arguments both for and against prohibition.
Tolerance, dependence and withdrawal
With regular doses at low levels, no tolerance develops to the effects of cannabis; on the contrary, there appears to be a type of ‘reverse tolerance’, in which users become more familiar with the administration techniques and effects and less anxious about the use of an illicit substance. There is no marked dependence, and withdrawal causes only mild ‘rebound’ effects such as anxiety, sleep disturbances and muscle weakness and tremor, which may persist for weeks. Craving for the drug can recur intermittently for months after the drug is stopped. If treatment for withdrawal is required, non-pharmacological interventions and an exercise program are preferred to substitution with another drug product.
Cannabis and psychosis
There is now strong evidence associating cannabis use with psychosis: acute cannabis intoxication causes brief psychotic symptoms including paranoia, disruption in normal thinking and speech patterns, depression, anxiety and visual, auditory and temporal illusions. In people with established psychosis, continued use of cannabis is associated with more frequent relapses. Large population studies in young people have shown that heavy use of marijuana at age 18 increased by sixfold the risk of schizophrenia later in life; overall, cannabis consumption caused a doubling of the schizophrenia risk. The risk is increased in those with a family history of psychosis; genetic vulnerability is associated with a polymorphism in the COMT gene (involved in metabolism of DA). Endocannabinoids function as crucial molecular signallers in the development of the fetal nervous system, and cohorts of children born to mothers who smoked marijuana heavily during pregnancy later show excessive impulsive, hyperactive and delinquent behaviours, and poorer performance in memory and reading skills.
The mechanism of increased risk of psychosis in heavy users of marijuana is still being elucidated: it appears to involve interaction between cannabis and dopamine pathways, with endocannabinoids, CB1 agonists and cannabis modulating both excitatory and inhibitor inputs to dopaminergic neurons, and increasing firing of mid-brain DA neurons and levels of DA in the striatum (see reviews by Luzi et al [2008]; Tucker [2009]).
Problems with chronic use
Heavy daily use over many years may be associated with the following adverse effects:
High-risk groups are adolescents, especially those who are poor performers at school, who are at risk of moving on to more dangerous illicit drugs; pregnant women and their offspring; people with pre-existing diseases, especially cardiovascular, respiratory or psychotic conditions; and drug-dependent people.
Overall, the health risks from regular use of cannabinoids are less dangerous than those from some legal drugs of dependence, particularly alcohol and tobacco. However, the now-proven association between heavy use in adolescence and increased risk of developing schizophrenia as a young adult is a sobering one.
Hallucinogens
A hallucinogen is a drug that produces auditory or visual hallucinations. The most common hallucinogenic agents include ecstasy, lysergic acid diethylamide (LSD) and its variants, mescaline, psilocybin and PCP; drugs based on amphetamine are also hallucinogenic (see earlier discussion). Various psychoactive hallucinogenic drugs have been used as adjuncts to religious ceremonies or were used experimentally by young people in the hippie scene in the 1960s and are now experiencing a resurgence in popularity. LSD (an ergot derivative; see Clinical Interest Box 38-4), dimethyltryptamine (DMT), PCP, mescaline, psilocybin and ecstasy (Clinical Interest Box 21-10) are examples of drugs that can produce distortions in perception or thinking at very low doses. Muscimol, a natural hallucinogenic ingredient of some mushrooms, is also an analogue of GHB with psychotomimetic effects.
Many of the hallucinogenic agents have chemical structures related to central neurotransmitters or are methylated derivatives of the transmitters (see Figure 21-4). This has led to the ‘methylated amine hypothesis’ of

Figure 21-4 Monoamine neurotransmitters and related hallucinogens. Chemical structures of the CNS neurotransmitters dopamine and 5-hydroxytryptamine (5-HT, serotonin) are shown, as well as various natural and synthetic hallucinogenic agents—all are methylated compounds in which a backbone structure similar to that of dopamine or serotonin can be traced.
Clinical interest box 21-14 other hallucinogens
MDMA (ecstasy, Adam, XTC) is a stimulant–hallucinogenic used largely by students and other young adults. High-dose or chronic use may lead to destruction of brain dopamine neurons, parkinsonian symptoms and eventually paralysis.
MDA, methylenedioxyamphetamine, is an amphetamine-type drug, similar in structure to MDMA, which destroys serotoninproducing neurons in the brain.
MPPP synthesis (a pethidine analogue used as a heroin substitute) usually produces a toxic by-product, MPTP, which has caused permanent, irreversible Parkinson’s disease in users by selectively destroying nigrostriatal dopaminergic neurons.
DOM, dimethoxymethamphetamine, is a hallucinogenic agent with about 50 times the potency of mescaline.
Bufotenine, a plant alkaloid also present in the skin of toads, and dimethyltryptamine (DMT) are weak hallucinogens and interfere with monoamine transmitters.
Hallucinogenic properties have been reported for many other natural compounds:
schizophrenia, as it raises the fascinating possibility that some neurological and psychiatric disturbances may be due to altered metabolic pathways producing endogenous methylated transmitters in CNS pathways, or to higher than normal levels of transmitters being shunted down unusual metabolic paths.
LSD (lysergic acid diethylamide; lysergide)
Lysergide is a very potent hallucinogenic drug that is usually available illicitly in doses of around 200 mcg. LSD is related to the ergot alkaloids (see Drug Monograph 38-4 and Clinical Interest Box 38-4) and thus can affect many body systems and neurotransmitters. It is an agonist at 5-HT2A receptors. After oral administration, it will cause a central sympathomimetic effect within 20 minutes: hypertension, dilated pupils, hyperthermia, tachycardia and enhanced alertness. Effects on mood are unpredictable, ranging from euphoria to severe depression and panic. The psychoactive effects occur in about 1–2 hours and have been described as heightened perceptions, distortions of the body and visual hallucinations.
Unpleasant experiences with LSD (a ‘bad trip’) are frequent. Clinically, evidence of impaired judgement in the toxic state is common, and altered states of consciousness may progress to psychosis. Feelings of acute panic and paranoia can result in homicidal or suicidal thoughts and actions. Long-term effects include flashback phenomena, in which unfavourable reactions induced by LSD, such as depression and long-term schizophrenic or psychotic reactions, recur weeks or even years after using the drug.
Mescaline
Mescaline is the chief alkaloid extracted from mescal buttons (flowering heads) of the peyote cactus. It produces subjective hallucinogenic effects similar to those produced by LSD but has only about 0.02% of the potency of LSD. The trimethoxy- and dimethoxy-derivatives of mescaline are also hallucinogenic. It is usually ingested in the form of a soluble crystalline powder that is either dissolved into teas or encapsulated. The usual dose of mescaline is 300–500 mg, which produces GI disturbances and sympathomimetic effects then vivid and colourful visual hallucinations. The half-life of mescaline is about 6 hours and it is excreted in the urine.
Psilocybin
Psilocybin and psilocyn are drugs derived from Mexican mushrooms. They produce subjective hallucinogenic effects similar to those produced by mescaline but of shorter duration. Within 0.5–1 hour after ingestion of 5–15 mg psilocybin, a hallucinogenic dysphoric state begins. A dose of 20–60 mg may produce effects lasting 5–6 hours. The mood is pleasant to some users, while others experience apprehension. The user has poor critical judgement capacities and impaired performance ability. Also seen are hyperkinetic compulsive movements, laughter, mydriasis, vertigo, ataxia, paraesthesia, muscle weakness, drowsiness and sleep.
Ketamine and phencyclidine (PCP)
Ketamine (‘K’, ‘special K’) is a dissociative anaesthetic with hallucinogenic properties; overdose can cause CNS stimulation and delirium. Dependence and flashbacks can occur.
PCP (phencyclidine, also known as ‘angel dust’) was originally introduced into medicine as an anaesthetic similar to ketamine, but its use was discontinued because of its hallucinogenic effects. PCP has a history of serious adverse outcomes, including many suicides, assaults and murders. Common peripheral signs include flushing, profuse sweating, nystagmus, diplopia, ptosis, analgesia and sedation. PCP produces a state similar to alcohol intoxication, with other perceptual distortions (visual or auditory) that can recur unpredictably, and symptoms that mimic schizophrenia. Toxic pressor effects may cause hypertensive crisis, intracerebral haemorrhage, convulsions, coma and death.
Other drugs of abuse
OTC and prescription drugs
Non-opioid analgesics (paracetamol, aspirin, ibuprofen)
Paracetamol, aspirin, ibuprofen and other non-steroidal anti-inflammatory drugs (NSAIDs—see Chapters 15 and 47) are OTC drugs that are readily available in many outlets such as pharmacies and supermarkets. These same ingredients may also be contained in combination formulations (with an opioid such as codeine) and sold with or without a prescription. Thus the potential for intentional and unintentional drug overdose exists with these drugs, although they do not cause dependence. They are particularly risky when combined with CNS depressants such as alcohol or benzodiazepines.
Overdoses from non-opioid analgesics are commonly seen in emergency departments; paracetamol (Drug Monograph 3-1) is especially dangerous when taken in large overdoses, owing to its toxic effects on the liver. In Australia, young teenage girls (aged 10–14) overdose on ‘headache tablets’ at 14 times the rate of boys of the same age. It is thought that the girls are beginning to selfmedicate at a turbulent time in their lives and that, whereas boys tend to use more violent means to harm themselves, girls are more likely to use drugs. About one in 100 females admitted to hospital for analgesic poisoning dies from the toxic effects.
Benzodiazepines
Benzodiazepines are prescribed for insomnia, anxiety or musculoskeletal problems; they are commonly used as tranquillisers or sleeping pills, but people can quickly become dependent on them—it can take as little as two weeks of regular use to become dependent. It is estimated that there are four times more Australians dependent on ‘benzos’ than on heroin (see earlier section under CNS depressants).
Drugs restricted in sport
Many drugs are restricted or prohibited in particular sports, such as anabolic steroids, opioid analgesics, ß-blockers, alcohol and even caffeine. This is a specific type of drug abuse, which is considered in Chapter 49.
Key points
 Drug dependence may be psychological and/or physical; related problems are addiction, tolerance and withdrawal syndromes. Trafficking in illicit drugs leads to problems in individuals, families and society; government policies attempt to reduce supply and demand and minimise harm arising from drug dependence.
Drug dependence may be psychological and/or physical; related problems are addiction, tolerance and withdrawal syndromes. Trafficking in illicit drugs leads to problems in individuals, families and society; government policies attempt to reduce supply and demand and minimise harm arising from drug dependence. Aetiological factors leading to drug abuse may be based on sociocultural aspects that dictate which drugs are prohibited, personality factors that predispose to drug dependence and pharmacological factors that cause some drugs to be reinforcing or rewarding. Drugs of dependence may be legal (alcohol, caffeine, nicotine) and prescribed (opioids, benzodiazepines, amphetamines), or illicit, such as heroin, marijuana and cocaine.
Aetiological factors leading to drug abuse may be based on sociocultural aspects that dictate which drugs are prohibited, personality factors that predispose to drug dependence and pharmacological factors that cause some drugs to be reinforcing or rewarding. Drugs of dependence may be legal (alcohol, caffeine, nicotine) and prescribed (opioids, benzodiazepines, amphetamines), or illicit, such as heroin, marijuana and cocaine. The drugs most commonly abused are opioids (heroin); CNS depressants, including alcohol, the benzodiazepines and inhalants; CNS stimulants (cocaine, amphetamines, caffeine and nicotine); psychotomimetics (cannabis) and hallucinogens (LSD, MDMA).
The drugs most commonly abused are opioids (heroin); CNS depressants, including alcohol, the benzodiazepines and inhalants; CNS stimulants (cocaine, amphetamines, caffeine and nicotine); psychotomimetics (cannabis) and hallucinogens (LSD, MDMA). Treating drug dependence involves managing the acute overdose situation or withdrawal reaction, attempts to detoxify, reduce dependence and maintain abstinence, or maintenance of dependence with the least harmful substitute drug.
Treating drug dependence involves managing the acute overdose situation or withdrawal reaction, attempts to detoxify, reduce dependence and maintain abstinence, or maintenance of dependence with the least harmful substitute drug. In the case of opioid dependence, naloxone is used as an opioid antagonist to treat acute toxicity, naltrexone is used for detoxification and methadone as an opioid substitute for long-term maintenance.
In the case of opioid dependence, naloxone is used as an opioid antagonist to treat acute toxicity, naltrexone is used for detoxification and methadone as an opioid substitute for long-term maintenance. Alcohol abuse is very common in Australian society and ranges from occasional problem drinking through to chronic alcoholism; treatment is with naltrexone or acamprosate. Other CNS depressants abused are the prescription sedatives, such as benzodiazepines, and solvents, which are inhaled.
Alcohol abuse is very common in Australian society and ranges from occasional problem drinking through to chronic alcoholism; treatment is with naltrexone or acamprosate. Other CNS depressants abused are the prescription sedatives, such as benzodiazepines, and solvents, which are inhaled. CNS stimulants including amphetamines, ecstasy and similar designer drugs, cocaine and caffeine are commonly abused for their euphoriant effects.
CNS stimulants including amphetamines, ecstasy and similar designer drugs, cocaine and caffeine are commonly abused for their euphoriant effects. The most problematic drug of dependence is nicotine, taken by smoking; smoking-related cardiovascular disease and cancers are a major public health problem. Nicotine dependence is treated by substituting nicotine by less dangerous routes, such as gum, patches or inhalers; or with bupropion, an antidepressant, or varenicline, a partial agonist at nicotinic receptors.
The most problematic drug of dependence is nicotine, taken by smoking; smoking-related cardiovascular disease and cancers are a major public health problem. Nicotine dependence is treated by substituting nicotine by less dangerous routes, such as gum, patches or inhalers; or with bupropion, an antidepressant, or varenicline, a partial agonist at nicotinic receptors.Review exercises
Analgesic Expert Group. Therapeutic Guidelines: Analgesic, version 5. Melbourne: Therapeutic Guidelines Limited; 2007.
Australian Institute of Health and Welfare (AIHW). 2007 National Drug Strategy Household Survey: First results. Cat. No. PHE 98 Canberra: Australian Institute of Health and Welfare, April 2008.
Australian Medicines Handbook 2010. Adelaide: AMH, 2010.
Bowman W.C., Rand M.J. Textbook of Pharmacology, 2nd edn. Oxford: Blackwell; 1980. [ch 42]
Cannon M.E., Cooke C.T., McCarthy J.S. Caffeine-induced cardiac arrhythmia: an unrecognised danger of healthfood products. Medical Journal of Australia. 2001;174:520-521.
Christie M.J. Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. British Journal of Pharmacology. 2008;154(2):384-396.
Christopherson A.S. Amphetamine designer drugs: an overview and epidemiology. Toxicology Letters. 2000;112–113:127-131.
Cooper J.R., Bloom F.E., Roth R.H. The Biochemical Basis of Neuropharmacology, 8th edn. New York: Oxford University Press; 2003.
Drummer O.H. The role of drugs in road safety. Australian Prescriber. 2008;31(2):33-35.
Enoch M.A. The role of GABA(A) receptors in the development of alcoholism. Pharmacology, Biochemistry & Behavior. 2008;90(1):95-104.
Goodman A. Neurobiology of addiction: an integrative review. Biochemical Pharmacology. 2008;75(1):266-322.
Guy G.W., Whittle B.A., Robson P.J. The Medicinal Uses of Cannabis and Cannabinoids. London: Pharmaceutical Press; 2004.
Hall W., Pacula R.L. Cannabis Use and Dependence: Public Health and Public Policy. Cambridge UK: Cambridge University Press; 2003.
Hamilton M., Kellehear A., Rumbold G. Drug Use in Australia: A Harm Minimisation Approach. Melbourne: Oxford University Press; 1998.
Howell L.L., Kimmel H.L. Monoamine transporters and psychostimulant addiction. Biochemical Pharmacology. 2008;75(1):196-217.
Hurley SF, Matthews JP. The Quit Benefits Model: a Markov model for assessing the health benefits and health care cost savings of quitting smoking. Cost effectiveness and resource allocation 2007; 5:2.(http://www.resource-allocation.com/content/5/1/2).
Hurley S.F., Matthews J.P. Cost-effectiveness of the Australian National Tobacco Campaign. Tobacco Control. 2008;17:379-384.
Intergovernmental Committee on Drugs the Australian National Council on Drugs. The National Drug Strategy: Australia’s Integrated Framework 2004-2009. Canberra: Commonwealth of Australia; 2004.
Jamrozik K., Le M. Tobacco’s uncounted victims. Medical Journal of Australia. 2001;174:490-491.
Litt J. What’s new in smoking cessation? Australian Prescriber. 2005;28(3):73-75.
Luzi S., Morrison P.D., Powell J., di Forti M., Murray R.M. What is the mechanism whereby cannabis use increases risk of psychosis? Neurotoxicity Research. 2008;14:105-112.
Mann J. Murder, Magic and Medicine. Oxford: Oxford University Press; 1992.
Moreira F.A., Lutz B. The endocannabinoid system: emotion, learning and addiction. Addiction Biology. 2008;13(2):196-212.
Mundell M. Ecstasy con: it’s the unreal thing. The Age 2001; Feb 3, News: 15.
NASA. Using spider-web patterns to determine toxicity. NASA Tech Briefs. 1995;19(4):82.
Nutt D., Lingford-Hughes A. Addiction: the clinical interface. British Journal of Pharmacology. 2008;154:397-405.
Paton A., Touquet R. ABC of Alcohol, 4th edn. Malden, MA: Blackwell, BMJ Books; 2005.
Penington D. Illicit Drugs, Community Perception and Public Policy Imperatives. Melbourne: University of Melbourne Centre for Public Policy; 1996.
Psychotropic Expert Group. Therapeutic Guidelines: Psychotropic, version 6. Melbourne: Therapeutic Guidelines Limited; 2008.
Rang H.P., Dale M.M., Ritter J.M., Moore P.K. Pharmacology, 6th edn. Edinburgh: Churchill Livingstone; 2007.
Roberts L.J. Managing acute pain in patients with an opioid abuse or dependence disorder. Australian Prescriber. 2008;31(5):133-135.
Scott T., Grice T. The Great Brain Robbery: What Everyone Should Know About Teenagers and Drugs. Sydney: Allen & Unwin; 2005.
Singh Y.N., editor. Kava: From Ethnology to Pharmacology. Boca Raton: CRC Press, 2004.
Speight T.M., Holford N.H.G., editors. Avery’s Drug Treatment, 4th edn, Auckland: Adis International, 1997.
Spencer J.W., Jacobs J.J. Complementary/Alternative Medicine: An Evidence-Based Approach. St. Louis: Mosby; 1999.
State Government of Victoria. Drugs, the Facts: A Practical Guide to Reducing the Harm From Drugs. Melbourne: State Government of Victoria, Department of Human Services, ‘Turning the Tide’ program; 1997.
Sudano I., Binggeli C., Spieker L., et al. Cardiovascular effects of coffee: is it a risk factor? Progress in Cardiovascular Nursing. 2005;20(2):65-69.
Tucker P. Substance misuse and early psychosis. Australasian Psychiatry. 2009;17(4):291-294.
United Nations Office on Drugs Crime. World Drug Report 2009. New York: United Nations; 2009.
Walters E. The Cruel Hoax: Street Drugs in Australia. Melbourne: Shield; 1996.
Whelan G. The management of the heavy drinker in primary care. Australian Prescriber. 2002;25(3):70-73.
Wills S. Drugs of Abuse, 2nd edn. London: Pharmaceutical Press; 2005.
Woodward D.F., Carling R.W., Cornell C.L., et al. The pharmacology and therapeutic relevance of endocannabinoid derived cyclo-oxygenase (COX)-2 products. Pharmacology and Therapeutics. 2008;120(1):71-80.
Australian Drug Foundation: www.adf.org.au/
Australian Government National Drugs Campaign: www.drugs.health.gov.au/internet/drugs/publishing.nsf/content/home-1
Australian Institute of Health and Welfare. 2007 National Drug Strategy Household Survey: Detailed Findings. AIHW cat. no. PHE 107 (Drug Statistics Series no. 22). Canberra: AIHW, 2008: www.aihw.gov.au/publications/index.cfm/title/10674
Food Standards Australia New Zealand: www.foodstandards.gov.au/foodmatters/caffeine/index.cfm
Inhalant misuse: www.healthinfonet.ecu.edu.au/health-risks/inhalants/reviews/background-information
Newborn Emergency Transport Service (Victoria): www.rch.org.au/nets/index.cfm?doc_id=338
Odyssey House Australia: www.odyssey.org.au/
Quit organisation: www.quit.org.au/
Turning Point Alcohol and Drug Centre: www.turningpoint.org.au/
Youth Mental Health Initiative: www.headspace.org.au/home/
More weblinks at: http://evolve.elsevier.com/AU/Bryant/pharmacology