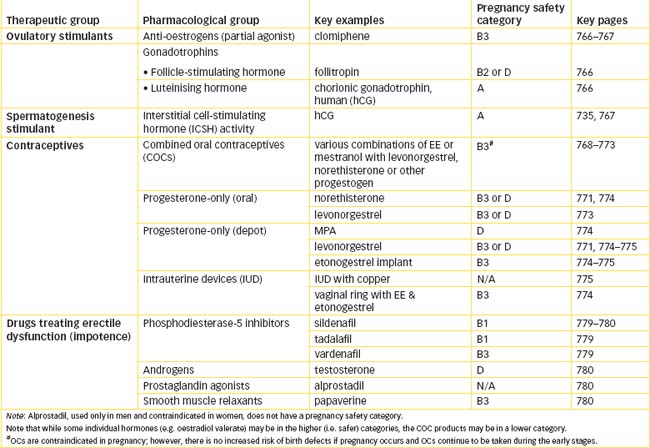
Chapter 40 Drugs Affecting Fertility or exual Functioning
In this chapter, two important aspects of reproductive pharmacology are considered more specifically: fertility and sexual functioning. Infertility is a serious problem for many couples; the common causes of infertility in men and women are described, and the drugs used to treat infertility discussed.
For many people, the main issue in sexual functioning is contraception: the various methods of contraception for women and men are described, with drugs, devices and ‘natural’ methods; their relative advantages, disadvantages, rates of use and failure are compared.
Many drugs can influence both sexuality and sexual functions; drugs that may enhance or reduce sexual desire or functioning are discussed.
COC combined oral contraceptive
FSH follicle-stimulating hormone
GnRH gonadotrophin-releasing hormone
hCG human chorionic gonadotrophin
ICSH interstitial cell-stimulating hormone
LSD lysergic acid diethylamide
Drugs affecting fertility
FERTILITY in humans requires effective, coordinated and appropriately timed functioning of several reproductive processes and many hormones (all described in the previous two chapters): production of viable gametes in both man and woman, deposition and motility of sufficient spermatozoa in the female reproductive tract, fertilisation of a mature oocyte, then its implantation and development in the primed uterine mucosa and maintenance of pregnancy. Defects in any step can lead to infertility.
Infertility
Infertility is defined as the absence of conception after more than one year of regular sexual intercourse between a couple without contraception. It affects 10%–15% of all cohabiting couples, and can cause great emotional distress to a man and woman hoping to conceive a baby. Effective treatment requires careful assessment of possible causes in both partners and, if possible, identification of the specific cause.
Aetiologies
The causes are estimated to be attributed to male factors in about 40% of cases, female factors in about 40% of cases and couple factors in 20% of cases. In men, common problems are abnormalities of sperm production, duct obstruction, hypothalamic or pituitary dysfunction, disorders of ejaculation or exposure to radiation. In women, cycles may be anovulatory due to hyperprolactinaemia, hypothalamic or pituitary dysfunction or ovarian dysfunction; or, if ovulation is normal, conception may be impossible due to tubal damage, endometriosis or uterine or vaginal abnormalities. Diagnosis of the cause of infertility in a particular couple and its treatment are highly specialised areas of medicine, usually provided in clinics attached to teaching hospitals.
Treatment of female infertility
Some conditions causing female infertility are treatable with hormone replacement or stimulation, and have been discussed previously. Pulsatile administration of gonadotrophin-releasing hormone (GnRH), for example, can mimic the physiological release of GnRH at intervals of approximately 1–2 hours during the follicular phase of the menstrual cycle. A typical regimen is initially downregulation of the hypothalamic–pituitary axis with continuous administration of a GnRH agonist, then GnRH 10–20 mcg by SC pump every 90 or 120 minutes, to stimulate pituitary production of gonadotrophins. This usually results in follicular development, ovulation and normal luteal function. The gonadotrophins themselves (follicle-stimulating hormone [FSH] and luteinising hormone [LH]) can be administered as well to stimulate normal functioning of the ovaries.
In some cases, the treatment of choice may be microsurgery or assisted conception techniques, such as in-vitro fertilisation (IVF), intrauterine insemination (IUI, in which the semen or isolated motile spermatozoa are directly injected into the uterus) or gamete intrafollicular transfer (GIFT). All such techniques require prior controlled ovarian hyperstimulation to stimulate development of a single or multiple follicles. The technical aspects of such procedures are beyond the scope of this text (see review by Hrometz and Gates [2009]).
Ovulatory stimulants
Anovulation, the absence of ovulation, is physiological in women who are pregnant, breastfeeding or postmenopausal. It becomes a suspected pathological condition in women of reproductive age with abnormal bleeding or infertility. The incidence of anovulation is unknown and cannot readily be ascertained, but diagnostic tests may determine its presence.
Clomiphene (Drug Monograph 40-1) and urofollitrophin are ovulatory stimulants used to treat anovulatory infertility in women. These treatments carry the risks of excessive stimulation leading to multiple pregnancies or potentially fatal ovarian hyperstimulation syndrome, and therefore are carried out only in specialist centres of reproductive medicine. Pregnancy must first be excluded.
Gonadotrophins
If clomiphene treatment is unsuccessful, then the more complicated gonadotrophin therapy for controlled ovarian stimulation is carried out, to harvest multiple eggs for IVF procedures. This involves sequential use of firstly a GnRHantagonist (cetrorelix or ganirelix) to prevent premature ovulation in preparation for controlled ovarian stimulation, then the gonadotrophins FSH (e.g. follitropin) to recruit and mature follicles, then LH (as human chorionic gonadotrophin [hCG], see Drug Monograph 38-1; or lutropin alfa) to induce ovulation and luteinisation. A GnRH analogue (goserelin, leuprorelin and nafarelin, see Table 38-1) may be administered beforehand to downregulate the pituitary gland before ‘controlled ovarian stimulation’.
Other drugs
Other drugs sometimes used as adjunct therapies in female infertility include supplementary progesterone (for its usual actions to aid implantation of the embryo and maintain pregnancy) and oral contraceptives to regularise cycles. In women with anovulation due to hyperprolactinaemia, the dopamine agonist bromocriptine is used to suppress prolactin release from the pituitary gland (see Clinical Interest Box 33-5).
Metformin
In women with polycystic ovary syndrome (PCOS, discussed in Chapter 38), common symptoms are anovulation and hyperandrogenism, with infertility and high prevalence of spontaneous abortion, gestationaldiabetes and pre-eclampsia. Insulin reduction strategies are helpful in treating the associated infertility, especially simple weight reduction. In women with a high body mass index, an insulin-sensitising agent such as metformin may be used to assist ovulation and weight reduction. Metformin (500 mg orally, 3 times daily with meals, continued until the first positive pregnancy test) has been shown to be as effective as clomiphene citrate as first-line therapy of PCOS infertility. The mechanisms are not clear; however, metformin reduces hyperinsulinaemia and improves insulin sensitivity, improves cardiovascular risk factors such as high blood pressure and dyslipidaemia, reduces free fatty acids, reduces high androgen levels, promotes weight loss and overall improves the chance of ovulation and outcome of pregnancy (see review by Diamanti-Kandarakis et al [2010]).
Treatment of male infertility
Many cases of male infertility are due to anatomical abnormalities, inadequate numbers or quality of sperm or problems with sexual functioning or technique. Endocrine causes such as hyper- or hyposecretion of thyroid or adrenal glands usually respond to appropriate hormone therapy. Combinations of androgens, antioestrogens and antioxidants have been tried. Erectile dysfunction (ED) may also require treatment, and drugs that impair sexual functioning need to be avoided (see discussions later in this chapter). An IVF technique, involving aspiration of sperm and direct injection in vitro into the cytoplasm of the oocyte (intracytoplasmic sperm injection, ICSI), can be an effective technique for extreme oligospermia (very low sperm count).
In gonadotrophin-deficient men, gonadotrophin therapy can induce spermatogenesis and hence increase fertility. Human chorionic gonadotrophin (which has mainly interstitial cell-stimulating hormone [ICSH] activity; see Drug Monograph 38-1) is given 2–3 times weekly for several months, then, if necessary, FSH is added for several months. Semen analysis is used to assess sperm numbers, and pregnancy is often achieved despite oligospermia (see review by Check [2007]).
Contraception
For many sexually active men and women, an ongoing problem is not inability to conceive, but contraception, i.e. prevention of pregnancy after sexual intercourse.1 The average rate of pregnancy following a year of regular unprotected intercourse between a couple is about 85%. It was estimated in 2000 that worldwide each day there were about 100 million acts of sexual intercourse, leading to about 910,000 conceptions (of which 25% were unwanted) and about 150,000 abortions. These days, 50% of couplesuse some contraceptive technique, yet the rates of abortion and unplanned pregnancies remain high. In teenagers the most common method is condom use, followed by use of condoms (to prevent sexually transmitted infections [STIs]) plus oral contraceptives.
An ideal contraceptive technique should be safe, 100% effective, immediately functional, not interfere with sex life, easy to use and rapidly reversible. Although no technique totally fulfils these criteria, many methods have remarkably low failure rates when used correctly by a highly-motivated couple. Table 40-3 lists the failure rates of several methods, showing the range of pregnancy rates. It is important to be aware that most contraceptive techniques, while protecting against pregnancy, do not protect against transmission of STIs (only condoms do the latter).
Data on contraceptive method usage from different countries are not readily comparable. However, Table 40-4 shows estimates of usage of various methods for world wide data and for Australian couples (Vollenhoven 2005; Australian Medicines Handbook 2010). Readiness of availability, requirement for prescription or fitting by a doctor and cost may be significant factors in choice of method; in Australia, preparations subsidised on the PBS are usually significantly cheaper than others.
Contraception in females
Contraceptive methods in women include oral tablets (usually provided in monthly calendar packs), intrauterine devices, intravaginal rings and diaphragms, intramuscular implants and female condoms. Some of these products are shown in Figure 40-1.
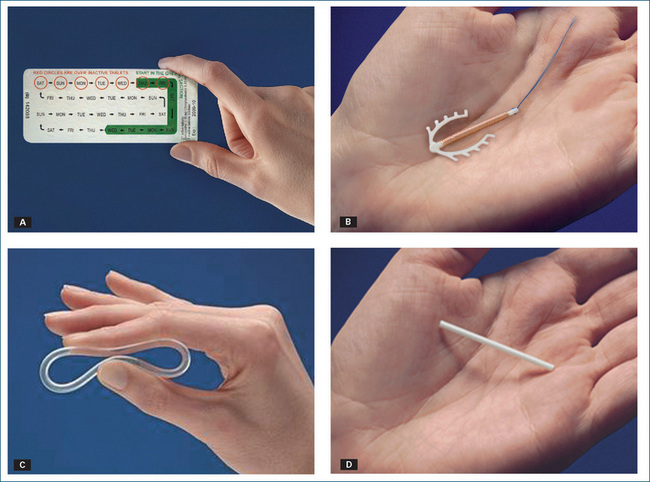
Figure 40-1 Different types of female contraceptive products: A 28-day pack of COC; B copper IUD; C intra-vaginal ring; D intramuscular implant.
Source: Schering-Plough/Organon, North Ryde, NSW; reproduced with kind permission.
Oral contraceptives
A very effective form of reversible birth control is combined oral hormonal contraception, i.e. oral administration of an oestrogen–progestogen combination in various doses in a 28-day cyclical regimen to mimic the normal physiological pattern of the menstrual cycle. Since the 1960s, millions of women have used combined oral contraceptives (COCs) for decades during their reproductive lives (see Clinical Interest Box 40-1). Through this experience, an enormous amount of information has been collected about the specific drugs, their effectiveness and the relationship of risk factors and contraindications to adverse reactions, drug interactions and morbidity and mortality.
Clinical interest Box 40-1 History of the oral contraceptive pill
The rationale for thinking that oestrogens and progestogens might act as reversible contraceptive agents was the fact that ovulation does not occur again during pregnancy, when levels of oestrogens and progestogens are high, i.e. multiple pregnancies of different gestation stages do not occur.
It was realised at the time that availability of an oral contraceptive pill heralded not only a pharmacological revolution but also sexual and social revolutions: for the first time in history, women now had a reliable and safe method of controlling their own fertility, which provided them with opportunities to be sexually active without fear of pregnancy, to separate career choices from family planning and to compete more equally with men in the workforce.
Hormone components
The aim of most COC formulations is to mimic closely the sequence and relative levels of oestrogen and progestogen in the menstrual cycle (see Figure 38-3): oestrogen levels are low early in the cycle, high in midcycle and medium late in the cycle; progestogen levels are very low or absent until after the midcycle surge of gonadotrophins, then rise in the latter half of the cycle. Although the use of exogenous oestrogenic substances alone will inhibit ovulation, undesirable ‘break-through’ bleeding frequently occurs during the latter phase of the cycle. If oestrogen levels are increased to prevent this, severe nausea, breast tenderness and thromboembolic adverse reactions may occur. Hence progestogens are combined with oestrogens in COCs.
In the typical 21/7 COC regimen, withdrawal of the active hormones after 21 days (when 7 placebo tablets or no tablets are substituted for active tablets) precipitates the withdrawal bleed, i.e. the next menstrual cycle. Phasic dosing, i.e. varying levels of each hormone) was introduced in the belief that it might interfere less with a woman’s normal metabolism but this has not been substantiated clinically.
Note that menstruation can be effectively suppressed by continuous taking of the active tablets (see Clinical Interest Box 38-1). In some countries an ‘extended’ formulation is available with 84 hormone tablets followed by a 7-day break, which effectively causes amenorrhoea within one year in most women using this formulation. This 91-day pack reflects the fact that many women skip the inactive tablets for a couple of months at times when menstruation would be inconvenient.
Mechanisms of actions
Much research has gone into identifying the actions of the hormones that together bring about contraceptive effects. Current views are that the oestrogen component causes decreased FSH release and thus impaired selection of a dominant ovarian follicle and impaired follicle development, with consequent decreased likelihood of ovulation and decreased chance of implantation. The actions of the progestogen component are to modify the secretory activity of the uterine cervix, decrease LH release, impair ovulation and impair tubal motility, decreasing likelihood of fertilisation.
Taken together over a few cycles, oestrogen–progestogen combinations effectively inhibit secretion of hypothalamicGnRH, pituitary gonadotrophins FSH and LH and endogenous ovarian steroids. This causes production of thickened cervical mucus that impairs sperm transport; decreases endometrial proliferation, secretions and menstrual flow; and reduces likelihood of ovulation and implantation. Overall, pregnancy does not occur.
The mechanisms of action of female contraceptive methods are compared diagrammatically in Figure 40-2.
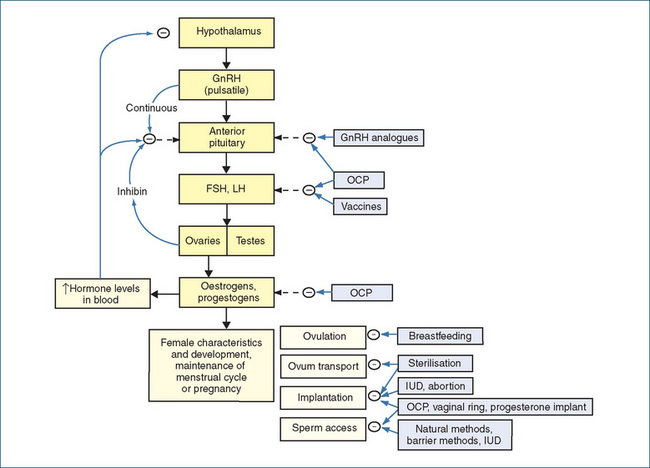
Figure 40-2 Sites of action of various contraceptive methods in the female reproductive tract (compare Figure 38-2). [ – ] = effect decreased or inhibited; GnRH = gonadotrophin-releasing hormone; IUD = intrauterine device; OCP = oral contraceptive pill.
Clinical use of oral contraceptives
Oral contraceptives are indicated for use in menstrual disorders and premenstrual syndrome as well as for contraception. In addition, they can be used for emergency contraception (described later). For detailed information on the pharmacokinetics, adverse effects, drug interactions and contraindications of the component drugs, see the drug monographs for typical oestrogens (oestradiol valerate, Drug Monograph 38-2) and progestogens (medroxyprogesterone acetate, Drug Monograph 38-3) in Chapter 38.
Before prescribing OC therapy, a prescriber should perform a thorough history and physical examination, including breast examination and a complete drug history. Teaching and monitoring of patients are also necessary; in particular, a woman needs to know:
Combined oral contraceptive formulations
Many different types of COC formulations are available, with different combinations and sequences of semisynthetic hormones selected to optimise activity and minimise adverse effects. Table 40-1 lists the compositions, doses and brand names of some typical OCs used in these methods; a typical calendar pack is shown in Figure 40-1A. However, formulations change frequently, so current reference lists should be consulted. Other concurrent medical conditions may determine the preferred drug or contraceptive method (see Table 40-2).
Table 40-1 Examples of hormonal contraceptives
| Brand name | Oestrogen (mcg) | Progestogen (mg) |
| Monophasic COC | ||
| Microgynon 20, Loette, Microlevlen | EE 20 | Levonorgestrel 0.1 |
| Yaz or Yasmin | EE 20 or 30 | Drospirenone 3.0 |
| Marvelon | EE 30 | Desogestrel 0.15 |
| Valette | EE 30 | Dienogest 2.0 |
| Levlen, Microgynon 30, Monofeme, Nordette | EE 30 | Levonorgestrel 0.15 |
| Femoden, Minulet | EE 30 | Gestodene 0.075 |
| Brenda-35, Diane-35, Estelle-35, Juliet-35, Laila-35 | EE 35 | Cyproterone acetate 2.0 |
| Brevinor, Brevinor-1; Norimin, Norimin-1 | EE 35 | Norethisterone 0.5 or 1.0 |
| Norinyl-1 | Mestranol 50 | Norethisterone 1.0 |
| Microgynon 50 | EE 50 | Levonorgestrel 0.125 |
| Triphasic COC | ||
| Triphasil, Logynon, Trifeme, Triquilar (6/5/10/7 tabs) | EE 30/40/40/0 | Levonorgestrel 0.05/0.075/0.125/0 |
| Improvil, Synphasic (6/9/6/7 tabs) | EE 35/35/35/0 | Norethisterone 0.5 /1.0/0.5/0 |
| Combined, depot | ||
| NuvaRing (vaginal ring, for 3 weeks) | EE 2.7 mg | Etonogestrel 11.7 mg |
| Progestogen-only (oral) | ||
| Locilan 28, Micronor, Noriday 28 | None | Norethisterone 0.35 |
| Microlut | None | Levonorgestrel 0.03 |
| Levonelle-1, Postinor-1; Norlevo | None | Levonorgestrel 1.5 or 0.75 (for emergency contraception) |
| Progestogen-only (depot/IUD) | ||
| Depro-Provera | None | Medroxyprogesterone acetate 150 mg IM every 3 months |
| Implanon implant | None | Etonogestrel 68 mg subdermal every 3 years |
| Mirena intrauterine device | None | Levonorgestrel 52 mg; replaced every 5 years |
Source: AMH 2010; MIMS OnLine.
Table 40-2 Recommendations for selecting a contraceptive method
| Conditions | Contraceptive management |
| Sexually active women (teenagers to age 35) | Low oestrogen (30–35 mcg)/low progestogen. Discourage smoking |
| Adolescents | COC pill most popular method; progesterone-only implant or depot obviates daily tablet-taking; IUDs more difficult; access to emergency contraception is important |
| Heavy smokers, † smokers >35 y and non-smokers >40 y | Increased risk of cardiovascular adverse reactions. Use alternative methods of contraception |
| Postpartum women | Condoms can be used; 3 weeks postpartum minipill possible; IUD or diaphragm from 6 weeks postpartum |
| Breastfeeding women (lactation improves contraception) | Progestogen-only minipill or implant, male condoms, copper IUDs all effective |
| Women whose family is complete | Sterilisation of male or female partner; COC, copper IUD; progestogen-only implant or IUD provides increased safety in this age group |
| Peri-menopause | Contraception if needed should be continued for 12 months after last menstrual period; preferred methods are those with minimal or no oestrogen component |
| Concurrent medical conditions | |
| Cancer (breast, uterus, cervix, liver) | Oral contraceptives contraindicated |
| Cerebrovascular disease, coronary artery disease or thromboembolic disorders | Oral contraceptives contraindicated |
| Liver impairment; smokers aged >35; history of CVA, uncontrolled hypertension and migraine | Progestogen-only minipill |
| Epilepsy; or taking other enzyme-inducing drugs | Higher doses of OC may be required: depot medroxyprogesterone recommended (See Drug Interactions 38-1 for interactions with oestrogens) |
| Diabetes, hypertension, depression, jaundice, hyperlipidaemias, pre- or post-surgery | Existing condition may be exacerbated—monitor closely; possible additional risk of thrombosis |
| Management of adverse reactions | |
| Acne, oily skin, hirsutism, sebaceous cysts, weight gain | Trial OC with low progestogen dose, or use cyproterone (with antiandrogen activity) as the progestogen component |
| Breakthrough bleeding | May reduce over time; oestrogen and/or progestogen dosage may be increased; other possible causes of bleeding should be checked |
| Withdrawal bleeding absent | First, rule out pregnancy; if no pregnancy, then an OC with a low progestogen dose or higher oestrogen may be prescribed |
† > 15 cigarettes/day. CVA = cerebrovascular accident (stroke).
Source: AMH 2010.
Most formulations in Australia contain ethinyloestradiol (EE); the only other oestrogen used is mestranol. (New oestrogens being trialled include estetrol [not yet available in Australia or New Zealand in 2010], a steroid produced in the human liver.) Because naturally occurring pro gesterone is inactivated or extremely weak in its effect when taken orally, and must be given by injection to be effective, synthetic progestogens with stronger actions were developed. Several are used in OC preparations: norethisterone, levonorgestrel; desogestrel, gestodene or drospirenone (the latter three are less likely to have androgenic effects), or cyproterone (with antiandrogen activity). New progestogens with antiandrogenic activity undergoing trials in other countries include dienogest (currently available in Australia and New Zealand)and nomegestrol (not available in Australia or New Zealand).
Monophasic OCs
Monophasic OCs contain a fixed ratio of oestrogen and progestogen and are taken for 21 (or 24) days of the normal menstrual cycle, then placebo tablets may be taken for the next 7 (or 4) days. These are generally found to be less confusing than the phasic types, provide good protection against mood swings and can be used to extend the cycle, e.g. to put off the next menstrual period until after sporting competitions, holidays or examinations. The formulation with only four inactive tablets may result in more effective ovarian suppression, lighter withdrawal bleeds and fewer instances of oestrogen-withdrawal headache.
Triphasic OCs
Triphasic OCs most closely simulate the normal oestrogen and progesterone levels during the menstrual cycle. The dose of oestrogen is kept at a low level during the 21-day dosing period, or may increase (‘mid-cycle surge’) in the middle of the cycle, while the progestogen is progressively phased up (increased twice) to mimic the natural release of hormones; placebo tablets are taken for the last 7 days. Because the lowest dosages of hormones possible are used in this type of formulation, the incidence and severity of adverse reactions reported are lower than with the monophasic formulations; however, controlled trials show little difference in cycle control compared with monophasic types.
Progestogen-only oral contraception
Minipill
Low-dosage progestogen-only OCs (the minipill) do not contain an oestrogen; this type was developed for women unable to take oestrogens, e.g. during lactation, or for women with a history of thromboembolic disorders or those who smoke. They are generally prescribed for 28 days of the menstrual cycle, are usually slightly less effective than the combination products and have a higher incidence of breakthrough bleeding. Advantages are that they generally do not cause the more serious adverse reactions associated with oestrogen therapy, and that they can be taken by women who are breastfeeding, as there is no oestrogen component that might inhibit lactation. Because the dose is low, the timing of taking the tablet each day is critical; it is recommended that, if the tablet is delayed by more than 3 hours, an additional method of contraception should be used for the next 48 hours.
Emergency contraception
Emergency contraception (the ‘morning-after pill’) is a high-dose progestogen indicated for use within 72 hours of unprotected intercourse or in possible failure of contraceptive method in women not wanting to conceive. It is administered to reduce the frequency of unwanted pregnancies and abortions. One 1.5 mg levonorgestrel tablet is taken; the alternative method is 2 × 750 mcg tablets 12 hours apart. The mechanism of action is to inhibit the mid-cycle surge of LH from the pituitary gland and thus delay or prevent ovulation and fertilisation. Levonorgestrel does not prevent implantation or development of the embryo if the woman is already pregnant.
This preparation is available in Australia without prescription, as the benefits far outweigh the risks; the method prevents up to 85% of otherwise-expected pregnancies. Levonorgestrel emergency contraception has been shown in societies in which it is readily available to reduce the rate of abortion. The main contraindication to these regimens is known pregnancy. An alternative non-hormonal method of emergency contraception is insertion of a copper-impregnated intrauterine device (IUD), effective up to 5 days after unprotected intercourse.
Interestingly, the COX-2 inhibitors meloxicam and rofecoxib have been shown to prevent rupture of the dominant follicle after the LH surge, so might improve the efficacy of levonorgestrel emergency contraception.
(The use of antiprogestogens such as mife pristone, RU486, at a single dose of 10 mg to terminate pregnancy has been described in Chapter 38; this method is usually classified as use of an abortifacient rather than as emergency contraception, as it is applied some time after conception
SPRMs as contraceptives
A new method undergoing trials is the use of a selective progesterone receptor modulator (SPRM) such as mifepristone, which is available in New Zealand but not Australia, (or a new drug ulipristal that is not yet marketed in Australia or New Zealand) to block receptors in the ovary and inhibit the LH surge. Daily doses of 5 mg mifepristone, or weekly doses of 25 mg, effectively inhibit pregnancies and lead to fewer bleeding days and more amenorrhoea than the progesterone-only pill.
Risk–benefit analysis
Because OC preparations are taken by millions of women for most of their reproductive lives, it is crucial that long-term safety, risks and benefits be identified. The risks need to be compared with risks associated with pregnancy and childbirth, particularly in countries where obstetric care is inadequate, and with the risks of abortion in the case of unwanted pregnancy. Major long-term studies over millions of woman–years have demonstrated that the newer low-dose OCs have many advantages over earlier preparations, and fewer risks than pregnancy (see Clinical Interest Box 40-2).
Clinical interest Box 40-2 ‘The pill’
More than 40 years of worldwide clinical experience have shown the OC pill to be safe and effective. Provided that risk factors (smoking, hypertension, thromboembolic disease or obesity) are minimised, COCs are safe for most women for most of their reproductive lives.
On balance, the life-threatening risks associated with using modern low-dose OC formulations are statistically lower than those in having a baby or driving a car.
Absolute contraindications for the use of OC are: thromboembolic disease, coronary artery disease, stroke, active liver disease, oestrogen-dependent cancers, focal migraine, porphyrias and pregnancy. OC is relatively contraindicated in hypertension, diabetes, previous cholestasis, undiagnosed vaginal bleeding, elective surgery within 4 weeks, sickle cell disease and severe depression, and in women over 35 years with risks for coronary artery disease.
Common adverse reactions and drug interactions are described under ‘Oestrogens’ and ‘Progestogens’ (Chapter 38).
Non-oral hormonal contraception
The non-oral hormonal methods (depot injections, transdermal patches, vaginal rings and depot IM rods) have many advantages over oral formulations:
The main reasons given by women for discontinuationof these methods are the irregularity of bleeding andunpredictable return of ovulation.
New pharmacological methods currently under investigation include contraceptive vaccines, selective progesterone receptor modulators and suppression of oocyte maturation.
Combined vaginal ring
A new technique that provides controlled release of a combination of hormones is the vaginal ring that contains both a progestogen and an oestrogen (see Figure 40-1C). It is a flexible polymer ring that slowly releases EE (15 mcg/24 hours) and etonogestrel (120 mcg/24 hours). It is inserted by the woman into her vagina and left for 3 weeks, then removed; her next menstrual period usually starts within 2–3 days. A new ring is inserted 1 week after the previous ring was removed. Absorption of the steroid through the vaginal epithelium is rapid, and first-pass metabolism is avoided. Rings have now been made that continuously release hormones for up to one year. Progestogen-only rings can be used by lactating women.
The ring has the same possible adverse effects, precautions and contraindications as combined oestrogen–progestogen products taken orally in the COC pill. There are additional warnings and advice as to changing from COCs or progestogen-only products, and what the woman should do if the ring is accidentally removed, left in too long or not replaced on time.
Combined depot injections
Depot injections containing both a progestogen (either MPA or norethisterone) with an oestrogen (oestriol valerate or cypionate) have been trialled and gone into clinical use in some countries. The combined depot preparations disturb bleeding patterns less than the progestogen-only injections, and allow earlier return to ovulation after stopping use.
Progestogen-only depot preparations
Depot injections of progestogen (e.g. MPA 150 mg given IM every 3 months) have the lowest failure rate of all reversible contraceptive methods. Progestogen depots inhibit gonadotrophin secretion, thus preventing follicular maturation and ovulation, resulting in contraception. This method is suitable for women who do not want to take a daily tablet or who cannot take oestrogens. There may be a period of infertility for some months after completing the course. Adverse reactions include vaginal bleeding, irregular periods and weight gain. New formulations containing lower doses of micronised medroxyprogesterone acetate (MPA) for depot IM injection are undergoing clinical trials; the MPA is absorbed more slowly and reaches lower peak levels, with fewer adverse effects but good efficacy.
A subdermal depot implant containing a progestogen is also available, consisting of a polymer rod (4 cm long, 2 mm diameter) impregnated with 68 mg etonogestrel (see Figure 40-1 D). It is implanted using aseptic procedures under the skin of the inner aspect of the upper arm,2 and is left in place for 3 years, during which there is a gradually reducing rate of release of active drug (from 60–70 to 25–30 mcg/day). The contraceptive effect is due mainly to inhibition of ovulation. It is then removed and a new rod is inserted if continuing contraception is desired. Fertility will return after removal of the implants, with rapid return to normal menstrual periods. New versions of the implant containing nestorone or nomegestrol, and biodegradable implants, are being trialled.
A plastic IUD impregnated with 52 mg levonorgestrel, a slow-release progestogen, is also available. It is useful for long-term contraception (5 years), to treat menorrhagia and to provide the progestogen content of continuous combined HRT.
Other pharmacological contraceptive methods
Diaphragm plus spermicidal agent
An occlusive vaginal diaphragm may be used in conjunction with a spermicidal gel (containing surfactant chemicals) as a non-hormonal method of contraception. The gel is applied before placement to the surface of the diaphragm to be in contact with the cervix and around the rim. The diaphragm should remain in situ for 6–8 hours after sexual intercourse. A fresh application of spermicidal gel should be made (using an intravaginal applicator) for subsequent sexual activity during this time frame. Adverse effects may include mild irritation or dermatitis; neither diaphragms nor spermicides protect against sexually transmissible diseases. Some spermicides have been shown to damage the vaginal epithelium and potentially increase the risk of sexually-transmitted infections.
Intrauterine devices
Intrauterine devices (IUDs) impregnated with copper (see Drug Monograph 40-2 and Figure 40-1B) progestogen (levonorgestrel) are used for long-term (5–8 years) reversible contraception. They alter the intrauterine environment to decrease sperm motility and viability, inhibit nidation (attachment of the fertilised ovum in the endometrium) and inhibit or decrease ovulation and follicular development. They can cause dysmenorrhoea, pelvic inflammatory disease or uterine perforation, and the IUD can be expelled from the uterus; they do not protect against STDs.
Other methods
Other methods tested or under trial in women include continuous administration of GnRH drugs that inhibit luteinising hormone-releasing hormone; luteolytic agents, including prostaglandin F2α; and vaccines against hCG, GnRH or sperm antigens. An anti-hCG vaccine has gone through phase 2 clinical trials, but provided effective contraception in only 60%–80% of women using it.
Contraceptive methods in males
Currently the main options for male contraception are surgical methods such as vasectomy (which is usually irreversible) and barrier methods such as condoms. In traditional Chinese medicine, gossypol, a natural antifertility agent in cottonseed oil, has been found to be effective. This is a naphtholphenol derivative (see Figure 1-3F for structure) that decreases the number and motility of sperm and impairs spermatogenesis after 2 months of daily oral administra tion. Adverse effects include fatigue and decreased libido, and the effects are not always reversible.
The ‘male pill’
Male contraceptive methods involving drugs include androgens, antiandrogens, FSH inhibitors, progestogens or GnRH analogues to inhibit gonadotrophin secretion, the so-called ‘male pill’ (see Clinical Interest Box 40-3); and chlorinated sugar compounds. Immunological methods include vaccines against various hormones and against components of the reproductive tract or of spermatozoa (see review Naz and Rowan [2009]).
Clinical interest Box 40-3 The ‘male pill’
Testosterone has a negative-feedback effect on gonadotrophin production and hence decreases spermatogenesis and causes oligospermia. However, high doses of weekly testosterone enanthate IM in men (or a subdermal implant) are required to reduce sperm count so low as to prevent pregnancy, and the resulting adverse effects include decreased libido. Combinations of IM testosterone with a low daily oral dose of a progestogen or cyproterone (an antiandrogen) are effective contraceptive regimens in most men and have been called ‘the male pill’. Prelimi nary trials in some ethnic groups have shown a high success rate for (reversible) con traception. Another approach has been to search for selective androgen-receptor modulators that maintain desired anabolic effects (and male sex characteristics), while reducing specific androgenic actions such as high gonadotrophin and testosterone levels and spermatogenesis.
The methods are unlikely to gain universal appeal, partly because many men prefer not to take drugs that they fear may interfere with their virility or prefer to leave contraception to the female partner. In addition, studies have shown that many women do not trust men to take contraceptive medica tion regularly and prefer to rely on their own contraceptive efficiency to prevent pregnancy.
The male pill may gain acceptance as a niche method, e.g. for couples in a stable relationship who are highly motivated to space pregnancies, and when the female partner cannot or prefers not to use the female methods of contraception. In 2010, a formulation consisting of a 2-monthly combined injection of testosterone plus a progestin is proving effective in trials carried out at Melbourne’s Prince Henry’s Institute, collaborating with seven other research institutes worldwide; however, a commercial backer for the product has not yet come forward (see http://www.princehenrys.org/news/164/contraception-men).
Non-drug contraception
Non-drug methods (except for condoms) generally have much lower success rates in preventing pregnancies (see Table 40-3 for failure rates for various contraceptive methods and Table 40-4 for estimates as to world-wide rates of usage of different methods).
Table 40-3 Failure rates for contraceptive methods
| Contraceptive method | Contraceptive failure rate* (= % women with pregnancy during 1 year of use) |
| No method | 85 |
| OC pill: combination | 0.3–8 |
| OC pill: progestogen-only | 0.3–8 |
| IUD + levonorgestrel | 0.2 |
| IUD + copper | 0.6–0.8 |
| Combined hormone vaginal ring | 0.3–8 |
| Depot IM MPA | 0.3–3 |
| Implant etonogestrel | 0.01–0.06 |
| Sterilisation (male) | 0.1–0.15 |
| Sterilisation (female) | 0.2–0.4 |
| Spermicide | 18–29 |
| Diaphragm + spermicide | 6–18 |
| Cap or diaphragm | 6–20 |
| Periodic abstinence (‘rhythm method’) | 9–20 |
| Withdrawal (‘coitus interruptus’) | 4–18 |
| Male condom | 2–15 |
| Female condom | 5–21 |
IUD = intrauterine device; OC = oral contraceptive.
* Ranges are lowest expected (perfect use) rate to typical rate in normal use.
Sources: Speroff & Darney 1992; AMH 2010.
Table 40-4 Who uses which contraceptive method?
| Contraceptive method | Worldwide usage (2000)* | Australian usage (2001)† |
| Combined OC | 9% | 26.8% |
| Barrier method | 5.3% | 23.4% |
| Sterilisation (male/female) | 20% | 20% |
| Withdrawal/natural | 6.9% | 9.9% |
| Depot MPA | ? | 1.9% |
| IUD | 10.6% | 1.2% |
| Other or none | 47% | 32.8% |
IUD = intrauterine device; MPA = medroxyprogesterone acetate; OC = oral contraceptive.
* Worldwide data for couples of reproductive age.
† Australian data for women aged 18–49 years.
Data from: Associate Professor Beverley Vollenhoven, Department of Obstetrics & Gynaecology, Monash University, Melbourne 2006; adapted with kind permission.
Barrier methods
Barrier methods of contraception are those that impose a physical barrier between sperm ejaculated by the man during sexual intercourse and an oocyte in the woman’s uterine tube. Some barrier methods or occlusive devices have the advantage of offering some protection against STIs and cervical cancer; condoms are especially effective for this purpose.
Condoms—thin rubber sheaths stretched over the erect penis before intercourse—prevent sperm from entering the vagina and effectively protect against both pregnancy and STIs. They are safe and inexpensive and have been widely used throughout history. When used properly and regularly, they are 97% effective as contraceptives, especially when used with spermicides. Condoms made from materials other than latex rubber are being trialled; they can have longer shelf-lives and can be used with oil-based lubricants.
The female condom consists of a thin rubber pouch that is inserted in the vaginal canal. It also protects against STIs.
‘Diaphragms’ or ‘caps’ inserted in the vagina prevent access of sperm to the cervical canal. They are most effective when used with a spermicidal cream, inserted before intercourse and left in place for at least 6 hours afterwards. Complications include irritation or pain and allergic reactions. They do not prevent STIs.
‘Natural’ methods
The ‘natural’ methods of family planning or contraception include total abstinence from sexual activity, periodic abstinence planned to avoid the most fertile days of a woman’s cycle (‘rhythm methods’), withdrawal of the penis before ejaculation (‘coitus interruptus’) and reliance on the antiovulation effects of prolactin during breastfeeding (see Clinical Interest Box 40-4).
Clinical interest Box 40-4 ‘Rhythm methods’ for natural family planning
Natural family planning methods are commonly used by people who for various reasons prefer not to use devices or drugs to prevent conception. They rely on attempts to predict the day of ovulation and avoidance of intercourse for several days before and after ovulation (as the ovum is viable for about 2 days and sperm for up to 7 days after intercourse).
The method works only if the woman has regular periods and can predict the time of ovulation (usually 14 days before the next period).
The methods require abstinence from sex for at least half the cycle (days 8–20 in a 28-day cycle); e.g. safe days in an average 28-day cycle might be from day 21 of one cycle through the menstrual period to day 7 of the next cycle.
Accuracy in predicting the day of ovulation can be im proved by measuring the small rise in basal body temperature that occurs in response to the midcycle rise in progesterone or by identifying changes in mucus secretions, abdominal pain, breast tenderness or cervix or mood changes before, during and after ovulation.
The unreliability of such methods has contributed to the high birth rates and/or high abortion rates in societies in which these methods are relied on.
Sterilisation
Sterilisation, while not (usually) reversible, is virtually 100% effective as a contraceptive method and is the most widely used form of contraception in the world. The most common techniques in women involve ligation or clipping of the fallopian tubes (‘tubal ligation’) and in men, vasectomy, i.e. surgical interruption of the vas deferens to prevent transport of sperm. Severe complications are rare. A new technique in women involves insertion of a ‘microcoil’ into the openings of the fallopian tubes to occlude them; the procedure can be carried out in conscious women as an outpatient procedure.
Other physical methods used in men rely on the fact that sperm are inactivated by higher temperatures than those in the scrotum. The thermal effects of hot water, microwaves, ultrasound and infrared heat have been trialled to produce azoospermia (absence of viable sperm).
Drugs that affect sexual functioning
Sexuality and sexual behaviour have physiological, psychological and social ramifications that are beyond the scope of this discussion of drugs affecting sexuality. Many contributing factors are involved, such as general health, partner availability, appropriate environment, self-esteem, religious beliefs, society’s standards and lifestyle factors.
The anatomical and hormonal aspects of female and male reproductive physiology and physiological aspects of human sexual responses are described in the previous two chapters, and summarised briefly below. Various drugs and conditions can produce adverse effects on sexual function, such as:
CNS control
Many physiological functions significant to sexual pleasure are controlled by higher centres in the central nervous system (CNS) and by the autonomic nervous system (see also Unit 4). The male and female sexual organs are composed of homologous tissues—although the shapes of the organs differ, they correspond, part for part, in structure, position and embryological origin. The embryo is characteristically female initially and does not differentiate until fetal androgens begin to masculinise tissues (7th to 12th weeks of pregnancy). Thus it is not surprising that the mature organs function in analogous ways and can be influenced by hormones of both sexes. Female hormones generally increase sexual functions in women and decrease them in men, and male hormones do the opposite. Central nervous system involvement in sexual functioning includes both behavioural and endocrine effects, with stimuli integrated via nuclei in the thalamus and hypothalamus. Many drugs that affect dopaminergic transmission have effects on hypothalamic–pituitary pathways, and any drugs with CNS-depressant effects may depress sexual interest or functions.
ANS control
Male
In the male, parasympathetic stimulation controls penile erection. This response results from vasodilation and congestion of the vascular sinuses in the penile corpora, caused by parasympathetic nerve action mediated by release of nitric oxide, which relaxes smooth muscle and hence dilates arterioles. (Priapism, a state of persistent painful erection without sexual excitation, is due to failure of drainage of blood from the penis; it requires urgent treatment, as blood clotting can cause permanent damage.) Drugs that interfere with parasympathetic neurotransmission, such as atropinic anticholinergic drugs, can cause erectile dysfunction. Because many drugs have atropinic effects, this is a common problem in men.
Sympathetic (adrenergic) impulses in the male produce emission and ejaculation by causing contraction of the vas deferens and seminal vesicles and of prostatic smooth muscle, along with effects on the bulbocavernosus and ischiocavernosus muscles. Ejaculation is a spinal reflex, with sympathetic stimulation causing rapid muscular contractions and expulsion of semen and simultaneous cardiovascular stimulation that increases heart rate and blood pressure and constricts arterioles, which leads to waning of the erection. Impotence is the inability of a man to achieve or maintain a penile erection to allow sexual intercourse. It may be caused by drugs that block adrenergic impulses and thus impair ejaculatory function. In addition, ganglionic blocking agents, which may block both sympathetic and parasympathetic nerve transmission, can cause complete impotence and impaired sexual functioning. Androgens are required for normal seminal fluid content and volume and play important roles in libido and erections and responsiveness to erotic stimuli.
Female
In the female, parasympathetic (cholinergic) impulses cause arterial dilation and venoconstriction, which produce clitoral erection and vasocongestion of the vulva, transudation (oozing of a fluid through pores) of lubricating secretions from the vaginal walls and swelling of the introitus (vaginal opening). Continued sexual stimulation may then produce orgasm, with sympathetic responses including sudden skeletal muscle contractions, increases in heart rate and blood pressure and intense pleasurable sensations. Oestrogens and progestogens are involved in sexual behaviour and responsiveness, and androgens are involved in enhancing libido in women as well as in men. Anticholinergic actions of drugs may depress the parasympathetic responses and hence decrease sexual functioning.
Drugs that may enhance sexual functioning
Treatment of erectile dysfunction (impotence)
Erectile dysfunction in men (ED), or impotence, is the condition in which a man is unable to attain or maintain an erection long enough for sexual intercourse, or is unable to ejaculate. It is estimated that 25% of men over 55 years of age are impotent. Very few, however, present for treatment because of embarrassment or denial. There are many possible causes of impotence:
Attention to psychological factors and treatment of any underlying disorder are important in all cases.
Phosphodiesterase type 5 inhibitors
The final common pathway leading to impotence is a lack of the vasodilator nitric oxide (NO), which normally relaxes the smooth muscle of the penile arteries and allows congestion. Sildenafil (which rapidly became renowned worldwide by its trade name Viagra) specifically improves erectile function and treats impotence (see Drug Monograph 40-3).sildenafil was the first oral medication approved for efficacy in treating impotence; later drugs in this group are tadalafil and vardenafil. Tadalafil has a significantly longer half-life (17.5 hours compared to 2–5 hours) and duration of action (12–36 hours compared to 4–5 hours).
Mechanism of action
The mechanism of action is secondary to sexual stimulation, which increases the release of NO. This activates the enzyme guanylate cyclase, thus increasing the levels of cyclic guanosine monophosphate (cGMP), a smooth muscle relaxant. Sildenafil enhances the NO effects by inhibiting phosphodiesterase type 5 (PDE5), an enzyme (found primarily in the penis) that degrades cGMP. The increased levels of cGMP in the corpus cavernosum enhance the smooth muscle relaxation and inflow of blood and maintain erection via a localised vasodilator action. Sildenafil has no effect in the absence of sexual stimulation.
Precautions
Since the release and enthusiastic acceptance of sildenafil (Clinical Interest Box 40-5), post-marketing surveillance has disclosed various adverse reactions, and several reports of fatality have been associated with its use; however, other current medications or disease states may have been involved in these deaths. There is now a warning that sildenafil should not be taken with concomitant administration by any route of an organic nitrate (also vasodilator). This contraindication is based on the combination causing severe hypotension and possibly a decrease in coronary perfusion, which may result in myocardial ischaemia and infarction. Health-care professionals should be aware that a man without a history of angina who takes sildenafil for sexual impotence and develops his first angina attack should not receive any nitrate products in the emergency room. This includes glyceryl trinitrate tablets, patches or ointments, and other nitrates or nitrites. Men need advice as to treatment of priapism if it occurs: if the erection lasts more than 2 hours, pseudoephedrine (2 × 60 mg tablets) is useful as a vasoconstrictor; more than 4 hours becomes a medical emergency.
Clinical interest Box 40-5 Sex, serendipity, sildenafil and share prices
Sildenafil is said to have been discovered by accident: it was being tested as a vasodilator treatment for angina, but was not significantly effective. However, many men taking the active drug in the clinical trial reported an unexpected effect, experiencing better erections during sexual intercourse. This effect was followed up and the mechanism was elucidated: inhibition of PDE5 in the penis, leading to improved erectile function.
Other interesting facts to emerge from the clinical trials of sildenafil were that many of the men in the group taking the active drug were reluctant to return leftover tablets after the trial, and that 25% of the men in the placebo group also reported improved sexual function (compared with 62% on low-dose sildenafil).
Sildenafil had the fastest track ever through drug-regulating agencies in the United States, reflecting the fact that demand for the drug was expected to be high (and that many staff in the drug-regulating authorities are older men?).
Sildenafil had one of the rosiest futures in pharmacology, with sales reaching billions of US dollars in the first few years. Share prices for the drug company Pfizer increased dramatically when rumours about the drug spread on Wall Street.
It may be no coincidence that, as the post-World War II babyboomers reach late middle age, other drugs recently fasttracked have included fluoxetine (Prozac) for depression and minoxidil (Rogaine Topical) for baldness. Other drug groups that are also big sellers are combination hormone replacement therapy products (HRT) for menopausal symptoms and drugs that reduce cholesterol concentrations (statins).
Drug monograph 40-1 Clomiphene citrate
Clomiphene is a non-steroidal anti-oestrogen with some partial oestrogenic effects. Although its exact mechanism of action is unknown, it has been postulated that it competes with oestrogen for receptor sites in the hypothalamus, inhibits the actions of the stronger oestrogens and thus interferes with the normal negative-feedback effect, allowing increased release of the pituitary gonadotrophins FSH and LH (see Figure 38-2). The result is ovarian stimulation, maturation of the ovarian follicle, ovulation and development of the corpus luteum. A single course of therapy (5 days) usually results in a single ovulation. If the first course is unsuccessful, i.e. sexual intercourse or intrauterine insemination does not result in conception, further courses are tried or the dose is doubled to increase the likelihood of ovulation. Assuming there is no other reason for infertility in the couple, about 70% of women will ovulate and 30% conceive after clomiphene therapy.
INDICATIONS Clomiphene is indicated to treat female anovulatory infertility.
PHARMACOKINETICS It is well absorbed orally and recirculated in the enterohepatic system, which may account for its prolonged duration of action in the body. It has a plasma half-life of 5–7 days, with ovulation usually occurring 6–12 days after a course of treatment. Clomiphene is metabolised in the liver and metabolites excreted in the faeces and bile.
DRUG INTERACTIONS It has no known significant drug interactions.
ADVERSE REACTIONS These include hot flushes, abdominal pain or gas, visual disturbances, ovarian enlargement or cyst formation, nausea and vomiting, abnormal uterine bleeding and ovarian hyperstimulation syndrome. There is an increased incidence of multiple pregnancies.
CONTRAINDICATIONS Avoid use in women with clomiphene hypersensitivity, severe liver function impairment, endometrial carcinoma, ovarian cyst or enlargement that is not associated with polycystic ovary syndrome, abnormal vaginal bleeding (undiagnosed) or fibroid tumours in the uterus; not indicated for use during pregnancy.
DOSAGE AND ADMINISTRATION The dose for female infertility is 50–100 mg orally daily for 5 days, starting on the second day of the menstrual period if bleeding occurs, or at any time in women who have no recent uterine bleeding. This cycle is repeated until conception occurs, for a maximum of six cycles.
Drug monograph 40-2 Intrauterine device with copper
The contraceptive intrauterine device (IUD) with copper consists of a Y-shaped flanged polyethylene device with copper wire wound around the stem; there are threads attached to facilitate checking of position (see Figure 40-1 B). The device is supplied in an insertion tube, all sterile. The copper is radioopaque, which allows its detection by imaging techniques.
Studies of the mechanism of action of the device have shown that it induces an inflammatory reaction to the foreign body in the uterus, with increases in leucocyte counts. The copper also hinders transport of sperm and ova. Overall, the viability and union of the sperm and ova are impaired, thus reducing the likelihood of conception and implantation after intercourse.
INDICATIONS The IUD with copper is indicated for contraception, in both nulliparous and multiparous women (i.e. those who have never been pregnant and those who have). It may be used during breastfeeding. It is 99% effective for emergency contraception if inserted within 5 days after unprotected intercourse.
PHARMACOKINETICS After the device is inserted into the uterus, it slowly releases copper over the period (up to 5 years) that it is left in place. Any absorption into the systemic circulation is too low to raise the levels of copper in the plasma, as the average daily release is estimated to be less than 1% of the average daily copper intake via the diet.
ADVERSE REACTIONS Adverse effects occurring during insertion or removal include syncope (fainting) and slowed heart rate. In the first few weeks after insertion, bleeding and cramps can occur. In the long term, there may be urticarial skin reactions to copper, embedding of part of the IUD in the uterus wall or cervix, penetration of the abdominal cavity, expulsion of the device, heavier menstrual bleeding and hence anaemia, and higher risk of pelvic inflammatory disease and thus reduction in future fertility.
WARNINGS AND CONTRAINDICATIONS Use with caution in women with valvular heart disease. If pregnancy occurs despite the use of IUDs, there is higher risk of abortion, sepsis, or ectopic pregnancy and its associated dangers.
Avoid use in women with copper hypersensitivity, during pregnancy and in women with current or previous disorders of the uterus or of menstruation, pelvic or genital inflammation or infection or STIs.
DRUG INTERACTIONS There are no clinically significant drug interactions.
DOSAGE AND ADMINISTRATION The copper wire in the device is 29 cm long (when unwound), and 0.4 mm in diameter; it provides a copper surface area of 375 mm2.
The IUD must be inserted using strict aseptic procedures and following manufacturer’s instructions closely. Its correct position should be checked soon after insertion, thence every 6 months. If there are no problems, it can be left in place for up to 5–8 years, then removed and another IUD inserted if contraception is still desired.
Drug monograph 40-3 Sildenafil
Sildenafil (Viagra) is a selective inhibitor of cGMP-specific PDE5 (phosphodiesterase type 5). After oral administration, it is active particularly in the penis, where it potentiates the vasodilator actions of nitrates released during sexual excitement.
INDICATIONS Sildenafil is indicated for erectile dysfunction in men, except for those taking nitrates or for whom sexual intercourse is inadvisable.
PHARMACOKINETICS Sildenafil is rapidly absorbed after PO administration and peak blood concentrations are reached after about 60 minutes; bioavailability is around 40%. Absorption is delayed by a high-fat meal. The drug and its major metabolite (also active as a PDE5 inhibitor) are highly protein-bound and widely distributed in tissues. Metabolites are mainly excreted in faeces, with a terminal half-life of 3–5 hours. Clearance is reduced in patients with severe liver or kidney disease.
DRUG INTERACTIONS Sildenafil should not be used with other vasodilators, especially nitrate preparations and selective α-blockers, as the hypotensive vasodilator effects are synergistic. The PDE5 inhibitors are metabolised mainly by CYP3A4, so there may be interactions with all other drugs that inhibit or induce these enzymes, including many anticonvulsants, corticosteroids, hypoglycaemic agents, antibiotics, antivirals, antifungals, warfarin and grapefruit juice; reference lists should be consulted for specific interactions and doses varied accordingly. Food may delay the onset of action.
ADVERSE REACTIONS These include typical vaso dilator effects such as headache, facial flushing, nasal congestion, dizziness and cardiovascular events (e.g. angina pectoris, tachycardia and hypotension). Gastric distress, diarrhoea, allergic reaction and priapism can occur, and at higher doses the drug may cause some visual changes, including a blue–green colour tinge in the field of vision, light sensitivity and blurred vision, and hearing loss.
WARNINGS AND CONTRAINDICATIONS Use with caution in patients with cardiovascular diseases, bleeding disorders, retinal disorders, Peyronie’s disease (an anatomical abnormality of the penis) or conditions that predispose to priapism, such as multiple myeloma, leukaemia and sickle cell anaemia. Avoid use in men with sildenafil hypersensitivity or concurrent use of organic nitrates.
DOSAGE AND ADMINISTRATION The usual adult dose is 50–100 mg, taken about 1 hour before sexual activity to a maximum of 100 mg in any day.
Other drugs used to treat erectile dysfunction
Second-line drugs used to treat ED are testosterone derivatives, prostaglandins and papaverine. These must all be given by injection, hence the major advantage of oral sildenafil. Alprostadil, a synthetic form of prostaglandin E1 (PGE1), is given by penile injection (into the corpora cavernosa) or transurethral application; it dilates the cavernosal arteries and thus assists erectile function. Testosterone implants, IM depot injections and patches or transdermal gels are tried if the ED is due to androgen deficiency (see Drug Monograph 39-1); however, the role of testosterone in the human erectile response is not well defined. Papaverine, a smooth muscle relaxant, is given by intracavernosal injection; it relaxes all vascular components of the penile erectile system.
Bromocriptine is effective in patients in whom the cause of ED is hyperprolactinaemia. The local anaesthetic lignocaine is available in a spray formulation as a local surface penile desensitiser to be used to prevent premature ejaculation. A variety of combination therapies have also been tried, along with surgical procedures and prosthetic devices (see review by Ellsworth and Kirshenbaum [2008]).
Drugs to increase libido
Substances that will increase libido (sexual potency or drive) have been sought throughout history. Inscriptions in the ruins of ancient cultures have described the preparation of ‘erotic potions’, and an endless number of ‘aphrodisiacs’ have been hopefully described since then. In contemporary society, many drugs and chemicals that modify mood and behaviour are claimed to have aphrodisiac properties.
In reality, very few drugs specifically enhance libido or sexual performance, and chemicals taken for this purpose without medical advice, and especially in combination with other drugs, pose the danger of adverse reactions, drug interactions or overdose. Many pharmacologically active agents, however, temporarily modify both physiological responsiveness and subjective perception to enhance the enjoyment, if not the fulfilment, of the sex act. Some of these agents are considered in this section; most are of psycho- or ethno-pharmacological interest only and are not available clinically.
Vasodilators
As well as the PDE5 inhibitors, other drugs with vasodilator activity may enhance the sexual response.
One of the few compounds with proven activity is yohimbine, an alkaloid derived from the West African tree Coryanthe yohimbe and related chemically to the ergot alkaloids. Yohimbine produces a competitive α-adrenergic block, leading to vasodilation, enhanced erection and increased ejaculatory reflexes. There is no convincing evidence that it acts as a sexual stimulant, and it has no medical use as it has low efficacy.
Vasodilators such as the organic nitrates (glyceryl trinitrate [see Drug Monograph 23-1]; isosorbide mononitrate) might be expected to be useful in dilating blood vessels in the penis and improving erections; however, such drugs have non-specific vasodilator actions and thus powerful adverse effects, causing headaches, hypotension and fainting, which would be counter-productive. Amyl nitrite, a drug used in the past to treat angina pectoris, is alleged to enhance sexual activity in humans and has been reported to intensify the orgasmic experience for men if inhaled at the moment of orgasm. No effect of amyl nitrite on libido has been reported, but loss of erection or delayed ejaculation may result. Women generally experience negative effects on orgasm when taking this drug.
Social drugs and psychoactive agents
The use of drugs such as morphine, heroin, cocaine, alcohol, marijuana, lysergic acid diethylamide (LSD), amphetamines and ‘designer drugs’ in the hope of aphrodisiac actions has become widespread in contemporary society (see Chapter 21). These agents can, in certain circumstances, enhance the enjoyment of the sexual experience for some people. More commonly, however, sexual function decreases. Responsiveness varies because these agents have no particular properties that specifically increase sexual potency—rather they tend to affect the user according to expectations. Thus the user’s state of mind, the amount of drug(s) consumed and the surrounding company and environment contribute considerably to the effect perceived. Like alcohol, these drugs act on the CNS to weaken inhibitions, which are often the cause of problems involving sexual behaviour. Taken in excess or too often, however, these drugs have the opposite effect and inhibit sexual drive and function. Because of these variations, researchers are sceptical of their clinical value.
Opioids such as morphine, pethidine and heroin are general CNS depressants and cause disorientation and mental confusion. They are said to have an ‘orgasmic effect’, but habitual users have low libido and impaired potency.
Marijuana (cannabis), an extract of the Cannabis sativa plant, is considered by many to be a sexual stimulant. Its effects, however, like those of alcohol, result indirectly from relaxation and release of inhibitions surrounding sexual activity. The active ingredient in marijuana is tetrahydrocannabinol. The pharmacological effects resulting from smoking marijuana depend on the expectations and personality of the user, the dose and the prevailing circumstances. Usually, the effects of marijuana are to distort time and enhance suggestibility, producing the illusion that sexual climax is prolonged. Thus the expectation that marijuana is an aphrodisiac may enhance enjoyment of the sex act. Studies of marijuana for a specific effect on sexual behaviour, however, have shown that it has no such enhancing effect. On the contrary, there is evidence that marijuana smokers have a higher incidence of decreased libido, potency and fertility than non-users do, possibly because of its oestrogenic-type effects and a decrease in release of the gonadotrophins FSH and LH. In addition, chronic intensive use of marijuana depresses plasma testosterone levels and produces gynaecomastia in some male users and decreases prolactin levels and ovulation in women. Chromosomal breaks have also been reported, with the risk of congenital abnormalities.
LSD is another drug that, although considered an aphrodisiac by some, has potentially adverse effects on sexual function and behaviour. As with marijuana, any alteration of sexual performance produced by LSD is principally subjective. This drug acts as an agonist at central 5-hydroxytryptamine (5-HT, serotonin) receptors (see Figure 21-4), causing facilitated sensory input, altered sensations and improved mood. The repeated use of LSD may produce serious psychological problems, which could overall adversely affect sexual interest or activity. Teratogenic effects have also been reported.
Amphetamines (‘speed’) have also been used to stimulate sexual function. These drugs release catecholamines from neuronal stores and inhibit their reuptake. They also have a powerful central stimulant action at dopamine and 5-HT receptors and peripheral sympathomimetic effects. The main effects are wakefulness and alertness, mood elevation, increased motor and speech activity, often elation and euphoria and decreased fatigue. The effects of ampheta mines on sexual performance, however, are inconsistent and may be attributed to overcoming feelings of shyness or inadequacy or to delaying sleep.
Nicotine is a CNS stimulant and has complex peripheral effects due to its actions at acetylcholine nicotinic receptors in autonomic ganglia and skeletal muscle. Overall, it tends to increase heart rate and vasoconstriction, inhibit spinal reflexes and increase release of ADH and oxytocin. No clear-cut acute effect of smoking on sexual functions is evident. However long-term effects are well known: statistically, 50% of men who smoke regularly from their 20s are impotent by their 50s, due to impaired cardiovascular functions.
Other drugs that may stimulate sexual behaviour
Hormones involved in the reproductive systems have effects in sexual functions; thus oestrogens and androgens may increase sexual activity in people of the appropriate sex. Oxytocin appears to have roles in mating and parenting behaviours in humans as well as in animals studied.
Premature ejaculation, the occurrence of a male orgasm too early in sexual intercourse, is a condition that impairs sexual functioning in many relationships. A topical spray or cream formulation of the local anaesthetic lignocaine is available; it is used to desensitise the surface of the penis and prolong time to ejaculation.
Levodopa, useful in treating parkinsonism by enhancing dopamine transmission in the CNS, is reported to increase libido and has caused priapism. Elderly men on levodopa have been observed to have a sexual rejuvenation, and studies with younger men complaining of decreased erectile ability have shown that levodopa increases libido and incidence of penile erections. Overall, however, these effects are short-lived and do not reflect continued satisfactory sexual function and potency. Thus levodopa is not a true aphrodisiac, but the increased sexual activity experienced by parkinsonian patients treated with levodopa may reflect improved wellbeing and partial recovery of normal sexual and motor functions impaired by Parkinson’s disease.
Clinical interest Box 40-6 Beware the blistering beetle!
Cantharidin, a legendary and notorious reputed sexual stimulant, is a powerful irritant, vesicant (blistering agent) and potent corrosive systemic poison. It is a powder made from dried beetles (Lytta vesicatoria, formerly called Cantharidin vesicatoria, the blistering beetle, or Spanish fly) found in southern Europe. Cantharidin can produce severe illness characterised by vomiting, diarrhoea, abdominal pain, corrosion of mucous membranes and shock. When taken internally, it causes irritation and inflammation of the genitourinary tract and dilation of the blood vessels of the penis and clitoris, sometimes producing prolonged erections (priapism) or engorgement, usually without increased sexual desire.
Deaths have been reported from the abuse of cantharidin as an aphrodisiac. The most infamous user of cantharidin was the French nobleman the Marquis de Sade (after whom the sexual perversion sadism was named). He was convicted in 1772 of several counts of poisoning prostitutes and guests at his banquets with sweets dipped in cantharadin, administered as an aphrodisiac to inflame their sexual desires and encourage their participation in orgies of sexual perversions. Many of his victims died of corrosive poisoning and shock. It is currently recognised that cantharidin is not an effective sexual stimulant.
Vitamin E compounds (the tocopherols) act as antioxidants and have metabolic roles and have been postulated to be involved in sexual functions because deficiencies of the vitamin impair reproductive ability. Much has been said about the positive effects of vitamin E on sexual performance and ability in human beings, but beneficial effects are not proven. Because sexual performance is often influenced by mental attitude, a person who believes vitamin E improves sexual prowess may actually find improvement.
Drugs that decrease sexual functioning
As described earlier, effective sexual activity depends on adequate CNS, ANS and endocrine system functions, as well as behavioural, social and lifestyle aspects. Not surprisingly, many drugs can impair sexual functioning, libido or sexual gratification. In particular, drugs that depress the CNS and ANS, and reproductive hormone antagonists, are likely to have adverse effects on sexual functions; these are summarised below. (The pharmacology of the drugs is described fully in the relevant chapters.)
Antihypertensives
Early antihypertensive drugs, especially ganglion blockers and adrenergic neuron-blocking agents, had such severe deleterious effects on sexual functions and potency in men (by blocking both parasympathetic and sympathetic nervous systems) that compliance with the therapy was often very poor. Modern, more specific drugs cause fewer problems in this area.
Ganglion-blocking agents
These drugs occupy nicotinic acetylcholine receptor sites at all autonomic ganglia and block the actions of released acetylcholine. They thus block all sympathetic and parasympathetic responses and effectively ‘wipe out’ the entire ANS. Because of their widespread actions and severe adverse effects, including hypotension, inhibition of gut functions, urinary retention and impairment of both erectile capability and ejaculatory function, they are no longer used. Examples were hexamethonium and mecamylamine.
Adrenergic neuron-blocking agents
Adrenergic neuron-blocking agents act by decreasing release of adrenergic transmitter (noradrenaline) and depleting transmitter stores. They thus impair sympathetic nervous system functions, causing vasodilation and hypotension as well as ejaculatory disturbances and impotence. Reserpine, a drug affecting central transmitter stores of catecholamines and 5-HT, was formerly used as an antihypertensive drug and in psychiatry, but is no longer used because of its many adverse effects.
Other antihypertensive agents
Centrally acting α2-agonists used as antihypertensives, such as methyldopa and clonidine, have been associated with frequent reports of impotence, sexual dysfunction, dec reased libido and gynaecomastia. The α-blocker phenoxy benzamine, an effective hypotensive drug, decreases ejaculation. (This drug has been referred to as the male contraceptive: interestingly, it has been used successfully in men with premature ejaculation problems.) Beta-blockers such as propranolol and metoprolol have also been reported to cause impotence and sexual dysfunction, as have calcium channel blockers (nifedipine, verapamil) and angiotensinconverting enzyme inhibitors (like captopril)
The thiazide diuretic hydrochlorothiazide may induce sexual dysfunction through its hypotensive and vasodilator actions. Spironolactone, an aldosterone antagonist, has both diuretic and endocrine effects and has been associated with impotence, gynaecomastia and a decrease in libido.
CNS-active drugs
A wide variety of centrally acting agents affect sexual interest and capability both directly and indirectly. The phenothiazines and other neuroleptics, antidepressants, benzodiazepines and barbiturates are often associated with sexual dysfunction.
Antipsychotic agents
The phenothiazine tranquillisers, such as chlorpromazine, prochlorperazine, thioridazine and fluphenazine, are commonly prescribed antischizophrenic agents that are thought to act by inhibiting dopaminergic transmission in the CNS. They very frequently have endocrine-type adverse effects mediated through their stimulation of prolactin release, causing gynaecomastia (in men) or galactorrhoea (in women), and through their decreased release of pituitary gonadotrophins and thus of sex hormones, causing priapism and ejaculatory disorders or impaired menstruation and ovulation. The phenothiazines are very ‘dirty’ drugs, affecting many transmitter systems (see Tables 18-1 and 18-2), and can block α-receptors (causing hypotension and ejaculatory disorders) and cholinergic receptors (causing ED). They have general CNS-depressant effects, including sedation, which may partly account for decreased sexual interest in people undergoing phenothiazine therapy. There have been some reports of enhanced libido, particularly in women. Because of the many serious adverse reactions, patient compliance with phenothiazine therapy is often low.
Other non-phenothiazine antipsychotics (neuroleptics, antischizophrenics) may cause similar adverse effects because all appear to act by inhibiting dopamine transmission. Such drugs include the thioxanthenes (flupenthixol and analogues), haloperidol and droperidol, and pimozide. The ‘atypical’ neuroleptics, clozapine and olanzapine, appear to be less problematic than all other neuroleptics in these respects.
Antidepressants
Antidepressant drugs generally elevate mood and thus may increase sexuality, as depression is often associated with diminished sexual interest, drive and activity (see Chapter 18). Unfortunately, however, antidepressants can influence sexual behaviour adversely by causing impotence, menstrual disorders, ejaculatory disturbances or gynaecomastia. All groups of antidepressants have been implicated: tricyclic antidepressants such as imipramine and amitriptyline (possibly related to their peripheral anticholinergic effects); monoamine oxidase (MAO) inhibitors such as phenelzine and moclobemide; and even the more specific selective serotonin reuptake inhibitors (SSRIs) such as fluoxetine (Prozac).
Hypnotics/sedatives
Benzodiazepine compounds (diazepam, alprazolam etc) are commonly prescribed antianxiety medications that are also useful as skeletal muscle relaxants. The sedative and relaxing effects of these drugs may account for the reported decreased interest in sexual activity, anorgasmia in men and women and ejaculation failure. Alternatively, the judicious use of benzodiazepines has been considered of value in the treatment of sexual impotence and other problems involving sexual performance where excessive anxiety was a factor in decreased sexual performance. Buspirone, a non-benzodiazepine antianxiety agent, has also been associated with reports of increased or decreased libido and (rarely) with impotence or delayed ejaculation.
Barbiturates such as phenobarbitone and thiopentone are sedative–hypnotic drugs that have general depressant effects on all nervous tissues. They are used variously as antiepileptics, sedatives or induction anaesthetics. These drugs, in prescribed dosage, produce relaxation, hypnosis and sleep, with depression of various body functions, including sexual performance and ability.
The classical antihistamines act as competitive inhibitors of histamine at H1-receptor sites; these drugs include diphenhydramine, promethazine and chlorpheniramine. They are commonly taken as antiemetics and mild sedatives and for the control of allergy symptoms and travel sickness. Most antihistamines cause anticholinergic effects such as dry mouth, urinary retention and constipation. Continuous use of these drugs may interfere with sexual activity. The histamine H2-receptor antagonists such as cimetidine and ranitidine, used to treat peptic ulcers, have been reported to cause gynaecomastia and impotence in men in high doses; ranitidine is probably the safest drug in this respect.
Ethyl alcohol
Ethanol is, for its effects on human sexual function and behaviour, a drug of individual and unique notoriety (see Chapter 21 and Drug Monograph 21-3). Revered for centuries as a sexual stimulant and cure of all ills, alcohol is in fact a CNS depressant, but in moderate amounts may enhance sexual activity by relieving anxieties and reducing the inhibitions that often impair sexual behaviour. In the oftenquoted words of Shakespeare (the porter at the gate, in Macbeth, Act II, Scene III, in response to Macduff’s question ‘What three things does drink especially provoke?’): ‘Lechery, sir, it provokes and it unprovokes: it provokes the desire, but it takes away the performance: therefore, much drink may be said to be an equivocator with lechery: it makes him, and it mars him…’ Or, as William Osler put it (quoted by Bowman and Rand 1980): ‘Alcohol does not make people do things better, it makes them less ashamed of doing them badly’.
Beyond a certain limit, however, neither desire nor potency will overcome the depressed physical capability that alcohol causes. The CNS is more affected by alcohol than is any other system of the body. Electrophysiological studies show that alcohol first depresses the part of the brain responsible for integrating the various activities of the nervous system, causing impaired sensorimotor performance. The first mental processes affected are related to sobriety and self-restraint, producing a less inhibited and less restrained approach to sexual behaviour and other activities normally inhibited by previous training or experience. With continued consumption of alcohol, cerebral functions become depressed, reflexes become slowed, blood vessels are dilated and the capacity for sexual function is diminished. In addition, alcohol produces a potent diuretic effect, which can also interfere with sexual function.
Typically, the male alcoholic after years of chronic alcoholism experiences delayed ejaculation and impotence after drinking. Vascular changes, peripheral neuropathy and lower testosterone levels because of liver damage are thought to cause the impotence; body image changes such as testicular atrophy, ‘beer gut’ and gynaecomastia compound the problems.
Hormones and derivatives
Sex hormones act on the endocrine system, CNS and other body organs to influence reproductive functions and sexual and aggressive behaviours, as well as mood and emotional outlook. Thus variations in female hormones may produce the anxiety, irritability and depression associated with premenstrual syndrome, whereas male hormones are traditionally associated with aggression and increased sexual interest (libido).
The anabolic steroids are derived from or related to the male sex hormone testosterone (Drug Monograph 39-1). They have been misused by athletes and other people to promote muscle growth and endurance. As adverse effects, these drugs in women cause virilisation, hirsutism, libido changes and clitoral enlargement; in men, testicular atrophy, impotence, chronic priapism and oligospermia. Antiandrogens (e.g. cyproterone) are also used to reduce sexual drive in overaggressive men.
Other drugs
Ketoconazole, an antifungal agent, may cause transient decreases in testosterone levels, with oligospermia and decreased libido in men. Various other medications have been reported rarely to cause sexual dysfunction; these effects would be listed in reference texts of adverse drug reactions or interactions.
Many of the drugs described earlier as reputedly having aphrodisiac or sexual stimulating actions (including opioids, marijuana, LSD, nitrates and cantharidin) have been shown to be more likely to cause sexual dysfunction, and so should probably be included in this rather than the previous section.

 Key points
Key points
 Infertility is a common problem in the community and has many possible causes. There are many types of treatments, including pharmacological and IVF methods.
Infertility is a common problem in the community and has many possible causes. There are many types of treatments, including pharmacological and IVF methods. Anovulatory infertility in women may be treated with ovulatory stimulants, such as clomiphene, or with gonadotrophins.
Anovulatory infertility in women may be treated with ovulatory stimulants, such as clomiphene, or with gonadotrophins. Contraception is practised by about 50% of couples to limit their fertility or plan their family. Drug and non-drug methods are available for the female or male partner, with varying usage rates, failure rates and degrees of reversibility. ‘Barrier’ methods are most effective at preventing STIs.
Contraception is practised by about 50% of couples to limit their fertility or plan their family. Drug and non-drug methods are available for the female or male partner, with varying usage rates, failure rates and degrees of reversibility. ‘Barrier’ methods are most effective at preventing STIs. Oral contraception with combinations of an oestrogen and a progestogen is a very effective reversible form of birth control.
Oral contraception with combinations of an oestrogen and a progestogen is a very effective reversible form of birth control. Types of contraceptive formulations include combined continuous and phased preparations, progestogen-only tablets and implants and emergency contraception regimens.
Types of contraceptive formulations include combined continuous and phased preparations, progestogen-only tablets and implants and emergency contraception regimens. Risk–benefit analysis shows that, for most women, use of oral contraception is safe, effective, reversible and inexpensive and protects against many gynaecological problems. Adverse reactions are generally mild with low- or no-oestrogen formulations.
Risk–benefit analysis shows that, for most women, use of oral contraception is safe, effective, reversible and inexpensive and protects against many gynaecological problems. Adverse reactions are generally mild with low- or no-oestrogen formulations. Because oral contraceptives are primarily for selfadministration, patient education is important for accurate and safe administration and for early recognition of adverse reactions, particularly thromboembolism.
Because oral contraceptives are primarily for selfadministration, patient education is important for accurate and safe administration and for early recognition of adverse reactions, particularly thromboembolism. Although many drugs have been postulated or tried as sexual stimulants (aphrodisiacs), very few are actually active and none prescribed for this purpose. Psychoactive agents, including social drugs and hallucinogens, tend merely to suppress inhibitions or alter sensations.
Although many drugs have been postulated or tried as sexual stimulants (aphrodisiacs), very few are actually active and none prescribed for this purpose. Psychoactive agents, including social drugs and hallucinogens, tend merely to suppress inhibitions or alter sensations.Review exercises
Allen K. Catching up on contraception. Australian Family Physician. 2009;38(6):380-382.
Australian Medicines Handbook 2010. Adelaide: AMH, 2010.
Bayram N., van Wely M., van der Veen F. Pulsatile gonadotrophin releasing hormone for ovulation induction in subfertility associated with polycystic ovary syndrome. Cochrane Database of Systematic Reviews. 2004. (1): CD000412
Beck J.I., Boothroyd C., Proctor M., Farquhar C., Hughes E. Oral anti-oestrogens and medical adjuncts for subfertility associated with anovulation. Cochrane Database of Systematic Reviews. 2005. (1): CD002249
Bowman W.C., Rand M.J. Textbook of Pharmacology, 2nd edn. Oxford: Blackwell; 1980. [chs 20, 42]
Check J.H. Treatment of male infertility. Clinical and Experimental Obstetrics and Gynecology. 2007;34(4):201-206.
Cibula D. Women’s contraceptive practices and sexual behaviour in Europe. European Journal of Contraception and Reproductive Health Care. 2008;13(4):362-375.
Diamanti-Kandarakis E., Christakou C.D., Kandaraki E., et al. Metformin: an old medication of new fashion: evolving new molecular mechanisms and clinical implications in polycystic ovary syndrome. European Journal of Endocrinology. 2010;162:193-212.
Ellertson C., Trussell J., Stewart F.H., Winikoff B. Should emergency contraceptive pills be available without prescription? Journal of the American Medical Women’s Association. 1998;53(5 Suppl 2):226-232.
Ellsworth P., Kirshenbaum E.M. Current concepts in the evaluation and management of erectile dysfunction. Urologic Nursing. 2008;28(5):357-369.
Endocrinology Expert Group. Therapeutic Guidelines Endocrinology, version 4. Melbourne: Therapeutic Guidelines Limited; 2009.
Foran T.M. New contraceptive choices across reproductive life. Medical Journal of Australia. 2003;178:616-620.
Fraser I.S. Forty years of combined oral contraception: the evolution of a revolution. Medical Journal of Australia. 2000;173:541-544.
Frost J.J., Darroch J.E., Remez L. Improving contraceptive use in the United States. Issues Brief (Alan Guttmacher Inst). 2008;1:1-8.
Ghanem H., Shamloul R. An evidence-based perspective to the medical treatment of male infertility: a short review. Urologia Internationalis. 2009;82(2):125-129.
Guillebaud J. Contraception: Your Questions Answered, 4th edn. Edinburgh: Churchill Livingstone; 2004.
Hrometz S.L., Gates V.A. Review of available infertility treatments. Drugs of Today. 2009;45(4):275-291.
Naz R.K., Rowan S. Update on male contraception. Current Opinion in Obstetrics and Gynecology. 2009;21(3):265-269.
Richardson D., Green J., Ritcheson A., Goldmeier D., Harris J.R. A review of controlled trials in the pharmacological treatment of premature ejaculation. International Journal of STD and AIDS. 2005;16(10):651-658.
Rowlands S. New technologies in contraception. BCOG: an International Journal of Obstetrics & Gynaecology. 2008;116(2):230-239.
Shufelt C., Merz N.B. Contraceptive hormone use and cardiovascular disease. Journal of the American College of Cardiology. 2009;53(3):221-231.
Speroff L., Darney P. A Clinical Guide for Contraception. Baltimore: Williams & Wilkins; 1992.
Turok D.K., Simonsen S.E., Marshall N. Trends in levonorgestrel emergency contraception use, births and abortions: the Utah experience. Medscape Journal of Medicine. 2009;11(1):30. Epub 29 January 2009
Vollenhoven B. Contraception. [Unpublished review.] Melbourne: Monash University, 2005.
Weisberg E. Contraception, hormone therapy and thrombosis. Australian Prescriber. 2002;25(3):57-59.
New Zealand Medicines and Medical Devices Safety Authority: www.medsafe.govt.nz
More weblinks at: http://evolve.elsevier.com/AU/Bryant/pharmacology