Chapter 1 Drugs and Medicines
Optimal selection and clinical use of drugs require a thorough knowledge of pharmacological principles. This chapter focuses on: the origin, development and scope of pharmacology; physical and chemical characteristics of drugs; drug nomenclature, classification and sources; dosage measurements and calculations; and an overview of drug information sources. An understanding of these basic areas of pharmacology is important in the application of pharmacology in health-care professions.
Key abbreviations
AMH Australian Medicines Handbook
APF Australian Pharmaceutical Formulary
CMI consumer medicine information
PBS Pharmaceutical Benefits Scheme
S4 Schedule 4 [Prescription-only Medicine]
Introduction and definitions
Pharmacology and drugs
PHARMACOLOGY is the study of drugs, including their actions and effects in living systems. The word ‘drug’ is defined by the World Health Organization as ‘any substance or product that is used or intended to be used to modify or explore physiological systems or pathological states for the benefit of the recipient’.1 The prefix pharmaco- is derived from the Greek word pharmakon, meaning drug or medicine. Hence we have related terms such as pharmacy, pharmacodynamics, pharmacokinetics, pharmaceutics and pharmacopoeia (Table 1-1). The terms medication and medicine in this context usually refer to drugs mixed in a formulation with other ingredients to improve the stability, taste or physical form, in order to allow appropriate administration of the active drug.
Table 1-1 Some common pharmacological terms*
| Adverse drug reaction | An unintended and undesirable response to a drug |
| Clinical pharmacology | Pharmacology applied to the treatment of patients; the study of drugs ‘at the bedside’ |
| Dose | The quantity of a drug to be administered at one time; determined by experience as likely to be safe and effective in most people |
| Dose form/formulation | The form in which the drug is administered, e.g. as a tablet, injection, eye-drop or ointment |
| Drug | A substance used to modify or explore the physiological system or pathological state for the benefit of the recipient |
| Indication | An illness or disorder for which a drug has a documented specific usefulness |
| Medicine | Drug(s) given for therapeutic purposes; possibly a mixture of drug(s) plus other substances to provide stability in the formulation; also, the branch of science devoted to the study, prevention and treatment of disease |
| Pharmaceutics | The science of the preparation and dispensing of drugs |
| Pharmacokinetics | How the body affects a specific drug after administration; i.e. how a drug is altered as it travels through the body (by absorption, distribution, metabolism and excretion) |
| Pharmacodynamics | What drugs do to the body and how they do it; refers to the interaction of drug molecules with their target receptors or cells, and their biochemical, physiological and possibly adverse effects |
| Pharmacology | The study of drugs, including their actions and effects in living systems |
| Pharmacopoeia | A reference book listing standards for drugs approved in a particular country; may also include details of standard formulations and prescribing guidelines (a formulary) |
| Pharmacy | The branch of science dealing with preparing and dispensing drugs; also the place where a pharmacist carries out these roles |
| Pregnancy safety | A method of classifying drugs according to documented risks in pregnancy |
| Receptor | A structure on or within a cell or membrane that is capable of binding to a specific substance (such as a transmitter, hormone or drug) and as a result causing a response in the cell |
| Route | The pathway by which a drug is administered to the body; e.g. oral route: drug is taken by mouth and swallowed |
| Side effect | A drug effect that is not necessarily the primary purpose for giving the drug in the particular condition; side effects may be desirable or undesirable. This term has been virtually superseded by the term adverse drug reaction, which is used throughout this book |
| Toxicology | The study of the nature, properties, identification, effects and treatment of poisons, including the study of adverse drug reactions |
* See the Glossary (Appendix 4) for a more complete list of pharmacological terms.
Pharmacology deals with all drugs used in society today—legal and illegal, prescription and ‘over-thecounter’ medications, natural and synthetic products, with beneficial or potentially toxic effects. This includes endogenous substances (those produced within the body) such as enzymes, hormones, antibodies, neurotransmitters and ions, and indeed many such chemicals are used therapeutically. Pharmacologists may study the origins, isolation, purification, chemical structure and synthesis, assay (measurement), uses, economics, genetic aspects and toxicity of drugs, as well as their fate in the body, medical uses and effects. The pharmacological agents available today have controlled, prevented, cured, diagnosed and in some instances eradicated diseases, and have improved the quality of life of billions of people.
Medications also have the potential to cause harm, as indicated by the fact that the Greek word for drug was also the word for poison. Health-care professionals should be well informed about each medication before administering or advising it to a patient, or when considering the possible effects of drugs their patients are taking, whether prescribed or self-administered, and whether for medical or social reasons. To administer a drug safely, one must know the usual dose, frequency and route of administration, indications and contraindications, significant adverse reactions, major drug interactions, dietary implications (if applicable) and appropriate monitoring techniques and interventions, and apply this knowledge to the particular patient and situation.
Characteristics of drugs
Potency, selectivity and specificity
By our broad definition of a drug as a chemical having useful action on living tissue, many substances could be classed as drugs: even oxygen, sugar, salt and water affect the body but can be toxic in overdose. To make the definition more descriptive, we can say that useful drugs usually have other important attributes: potency, selectivity and specificity (Clinical Interest Box [CIB] 1-1).
Clinical interest Box 1-1 Is alcohol a useful drug?
Two commonly taken sedative substances, lorazepam and alcohol, may be compared in terms of their potencies, selectivities and specificities: lorazepam (Ativan) is used in treatment of insomnia and anxiety and in premedication before surgery (similarly to diazepam, DM 16-1), and alcohol (ethanol, DM 21-3) is used as a solvent, disinfectant and social drug.
| LORAZEPAM | ETHANOL | |
| Potency: effective at concentrations of: | 10−8–10−5 M (20-30 ng/mL) | 10−2–10−1 M* (0.5-5 mg/mL) |
| Dose: | 1–4 mg | 5–20 g |
| Biological selectivity: | Facilitates GABA binding to GABAA receptors; has antianxiety, muscle relaxant, antiepileptic and sedative/ hypnotic actions | Increases disorder in lipid membranes, depresses neuronal activity in most excitable cells and tissues |
| Chemical specificity: | High (closely related to all benzodiazepines) | Low (depressant actions related to GABA and NMDA receptors and calcium channels) |
| Specific antagonists? | Yes — antagonised by flumazenil at the GABAA receptor | No — nonspecific antagonism by central nervous system stimulants such as caffeine and amphetamines |
DM = Drug Monograph; GABA = γ-aminobutyric acid; M = molar strength solution; NMDA = N-methyl-D-aspartate.
*Note that 0.05% blood alcohol level (“point 05”) is approximately equivalent to 1.1 × 10−2 M.
As chemicals need to have potency, selectivity and specificity in order to be useful as drugs, by our definition, alcohol is not a useful drug: it requires high doses and has only general effects on most cells of the body.
Potency refers to the amount of chemical required to produce an effect; it is an inverse relationship—the more potent the drug, the lower the dose required for a given effect (see Chapter 5 and Figure 5-4). One of the most potent chemicals known is the natural bacterial product botulinum toxin, for which the minimum lethal dose in a mouse is as low as 10−12 g (one-millionth of one-millionth of a gram); it has found uses in medicine in treating spasm of eye muscles and spasticity, and in cosmetic surgery (see Drug Monograph [DM] 31-3).
Selectivity refers to the narrowness of a drug’s range of actions on particular receptors, cellular processes or tissues. The antidepressant drugs known as selective serotonin reuptake inhibitors (SSRIs), such as fluoxetine (Prozac, see CIB 18-10), have fewer adverse effects than older antidepressants because they are more selective in inhibiting the transport of the neurotransmitter serotonin into cells.
The term specificity may be used loosely like ‘selectivity’ to refer to the narrowness of the range of actions of a drug, e.g. cardiospecific or cardioselective β-blocking agents, which are less likely to cause asthma as an adverse effect than are non-specific β-blockers because they are more selective for β1-receptors, which are found mainly in cardiac tissue. Specificity may also refer to the relation between the chemical structure of a drug and its pharmacological actions; for example, the effects of pseudoephedrine (DM 28-6) and related compounds are due to their chemical similarity to the neurotransmitter noradrenaline.
The ideal drug
In designing a new drug, a research pharmacologist might aim for it to be: easily administered (preferably orally) and fully absorbed from the gastrointestinal tract, not highly protein-bound in the blood plasma, potent, highly specific, selective, with rapid onset and useful duration of action, of high therapeutic index (no adverse drug reactions, no interference with body functions), unlikely to interact with any other drugs or foodstuffs, spontaneously eliminated, stable chemically and microbiologically, readily formulated into an easily taken form and inexpensive.
Sadly, not even pharmacologists live in an ideal world, and so we must admit that there is no ideal drug, whether natural product or synthetic. It has been well said that any substance powerful enough to be useful is also powerful enough to do some harm. In all cases, the decision to prescribe, administer or take a drug requires a risk–benefit analysis based on the best information available: do the likely therapeutic benefits outweigh the possible harmful effects? This balance is indeed the main theme of this textbook.
Physical aspects of drugs
In terms of their physical state, drugs may be solids, liquids or gases. Most are solids at room temperature, but some are liquids in the pure state, such as nicotine, halothane (a general anaesthetic) and ethanol, and some are gases, especially general anaesthetics such as nitrous oxide and cyclopropane. The solids may be formulated in solid dose forms, such as tablets, capsules, creams, powders or patches or, when dissolved, may be formulated in liquid preparations such as cough mixtures, injectable solutions, aerosol sprays, eye-drops or paints. These aspects of the formulation of drugs will be covered in the section on pharmaceutics in Chapter 2.
Chemical aspects of drugs
All drugs, whether found naturally in plants, animals, minerals or microorganisms, or synthesised in a laboratory, are chemicals of one sort or another, as described in Clinical Interest Box 1-2. They may be inorganic molecules, such as calcium salts used to prevent and treat osteoporosis, minerals such as iodine to prevent deficiencies or fluorides used to prevent dental decay. The vast majority of drugs, however, are organic molecules, i.e. they contain carbon in their structures. All the major classes of organic compounds, including hydrocarbons, proteins, lipids, carbohydrates, nucleic acids and steroids, are represented in pharmacopoeias (see Figure 1-3 later). Many drug molecules are acids or bases, which is important not only for their taste and irritant effects but also for how the drugs move across membranes (see Figure 6-3) or are affected by the normal body processes of metabolism and excretion (pharmacokinetics).
Clinical interest Box 1-2 There’s no such thing as a chemical-free lunch!
The term ‘chemical’ simply refers to any substance made up of elements (i.e. hydrogen, carbon, oxygen etc), and thus refers to all matter. Unfortunately, ‘chemical’ has come to have derogatory connotations, largely due to misleading advertising by the ‘natural products’ industry, implying that natural products do not have chemicals in them.
The Royal Society of Chemistry (RSC) in Britain has become so irate at this misuse of the term that in 2008 they offered a reward of £1 million to the first member of the public who could place in their hands any material shown to be chemicalfree. As explained in their press release: ‘The truth, as any right-minded person will say, is that everything we eat, drink, drive, play with and live in is made of chemicals—both natural and synthetic chemicals are essential for life as we know it.’ Indeed, water and oxygen—the basic essentials for life—are of course chemicals, and anyone suggesting they are not, or that any product is chemical-free, is simply showing their scientific ignorance (see http://www.rsc.org/AboutUs/News/PressReleases/2008/ChemicalFree.asp).
PS: Despite the tantalising reward it offered, the RSC has not yet had to pay out…
The size of drug molecules can also vary enormously, ranging from tiny lithium, the third-lightest element with an atomic weight of about 7, used as a specific antimanic agent (DM 18-4), through to proteins such as insulin (DM 36-2), erythropoietin (DM 49-1) and influenza vaccine (DM 28-7). Most drugs are in a more intermediate size range, with molecular weights (relative molecular masses) between 100 and 1000. For example, gabapentin, an anticonvulsant, has a molecular weight of 171, aspirin 180, caffeine 194, testosterone (a steroid hormone) 288, penicillin 373, digoxin (a cardiac glycoside) 781 and cyclosporin (an immunosuppressant with a cyclic polypeptide structure) 1203. By comparison, insulin, a relatively small protein, has a molecular weight of about 5700; erythropoietin, a large glycoprotein, about 30,400; and heparin, a glycosaminoglycan polymer, between 4000 and 20,000. Again, the size and nature of the molecule has important implications for the pharmacokinetic handling of the drug: proteins taken orally would be digested in the gut, so they must be administered by injection; large molecules will not readily pass through cell membranes and may need to be administered directly into the bloodstream or to their site of action.
A brief history of pharmacology
Medicines in antiquity
For many thousands of years, people have searched for substances to prevent, treat and cure disease, so the history of pharmacology (see CIB 1-3) goes back a long way. Archaeological diggings show that Stone Age people used opium poppies (see CIB 15-4) and Inca civilisations used
Clinical interest box 1-3 timeline of major drug discoveries and inventions
| TIME PERIOD | COMMENTS |
| 1500 BC | Ebers Papyrus, with details of Egyptian pharmacy and surgical practices; disease considered due to wrath of the gods |
| 400 BC | Hippocrates, Greek physician: emphasis on humours and doctrine of opposites |
| 1st century AD | Dioscorides’ De Materia Medica: information on use of >600 medicinal plants; translated into Latin, Arabic and Persian. Celsus’ medical textbook |
| 2nd century | Galen, Greek physician/surgeon/druggist: pharmacy based on ‘simples’ and complex mixtures now called galenicals |
| 5th–11th centuries | Dark Ages in Europe: herbal medicine, magic and cosmology interwoven in monasteries. Meanwhile in Arabia, China and India, medicine and herbal pharmacy developed, with teaching hospitals and medical libraries |
| 11th century | Persian physician, Avicenna, ‘the father of clinical pharmacology’ |
| 12th–14th centuries | In Europe, medical schools developed in Salerno, Bologna and Montpellier; apoth e caries documented use of herbs and spices |
| 16th–17th centuries | More scientific: Vesalius (anatomist), Gerard and Culpepper (herbalists) and Paracelsus (alchemist, botanist); opium tincture, coca (cocaine), ipecac and antiscorbutic agents (antiscurvy) |
| 18th century | Digitalis: source of cardiac glycosides (digoxin, digitoxin); smallpox vaccine developed |
| 19th century | Important alkaloids isolated: morphine, quinine, atropine and codeine; ether and chloroform, first general anaesthetics available (rare or obsolete now) |
| 1860s | Important advances in chemistry, especially coal-tar (organic) chemistry |
| 20th century | Application of organic and synthetic chemistry to drug discovery |
| 1922 | Insulin isolated, the most important discovery for treatment of diabetes mellitus |
| 1930s–1940s | First safe oral antimicrobials: sulfonamides and penicillins devel oped. Use of muscle relaxants with general anaesthetics making major surgery safer |
| 1949 | Cortisone, an important hormone from the adrenal cortex, identified and synthetically prepared |
| 1940s–1950s | Autonomic pharmacology studies, structure–activity relationships on α- and β-receptors; tuberculosis cured with combination antimicrobial therapy |
| 1952 | Chlorpromazine, the first effective antipsychotic drug, revolutionised treatment of schizophrenia (see CIB 18-2) |
| 1950s | Oral contraceptives developed—similar to natural oestrogen and progesterone hormones; revolutionised family planning |
| 1955, 1961 | Poliovirus vaccines eliminating deaths and paralysis from polio epidemics |
| 1960s | Levodopa used to treat Parkinson’s disease; immunosuppressants made organ transplantation feasible; effective treatment of hypertension with thiazide diuretics and β-blockers helped prevent strokes; cytotoxic agents (alkylating agents, antime tabolites and antibiotics) developed to treat cancers. The thalidomide disaster, when thousands of infants were born with severe malformations, led to tightening of regulations for drug testing |
| 1970s | Antivirals developed for prophylaxis and treatment of viral diseases. Childhood leukaemia treated successfully with cytotoxics and steroids. Ovulatory stimulants used in in-vitro fertilisation |
| 1980s–1990s | New drugs for thrombolysis, reduction of cholesterol levels, inhibition of synthesis of angiotensin or prostaglandins, combination therapy of AIDS and treatment of impotence; new antineoplastic agents for chemotherapy of cancers, inhaled corticosteroids for asthma, atypical antipsychotics for schizophrenia; refinement of treatment protocols |
| 2000–present | Recent innovations include chiral versions of optically active drugs (levobupivacaine, escitalopram), genetically engineered molecules (insulin glargine), prostaglandin analogues for glaucoma (latanoprost, travoprost), thiazolidinediones and incretin enhancers for type II diabetes and tyrosine kinase inhibitors (imatinib) and monoclonal antibodies (trastuzumab) in cancer chemotherapy |
cocaine (CIB 21-11). The oldest prescriptions found were on a clay tablet written by a Sumerian physician around 3000 BC, i.e. 5000 years ago; these included vegetable and mineral drugs dissolved in milk, beer and wine, showing the longstanding use of alcohol in medicine. Presumably, knowledge of pharma cology developed by trial and error, with many fatalities and adverse reactions along the way. Supernatural healing rituals and magical practices involving drugs were—and sometimes still are—carried out by healers and shamans in primitive cultures.
Throughout the ancient Egyptian period (3000–30 BC) people believed that disease was caused by evil spirits living in the body. Imhotep, the god of medicine, and Isis and Horus, gods of pharmacy, were worshipped. The Ebers Papyrus, dating from about 1500 BC and translated into English in 1875 AD, described formulations of over 700 drugs from plant, mineral and animal sources.
Chinese medicine dates back beyond 2000 BC. Methods included the use of herbs, poisons and antidotes, acupuncture, diets and moxibustion (burning of herbs for incense and heating the skin). The common practice of using boiling water to make tea probably prevented many intestinal infections, and there is documentation of the use of ephedra (ephedrine) for asthma and seaweeds (iodine) for goitre. Ancient Indian (Ayurvedic) medicine, recorded in sacred writings (the Vedas), described many surgical practices and over 1000 natural drugs, including wine (alcohol) and hemp (marijuana), used for pain relief.
Medicine in the Greek and Roman civilisations
In the Ancient Greek civilisation (1100–146 BC), the god Asclepius was considered to be the principal god of healing. He combined religion and healing in a temple setting, and his large family represented health or medical ideology. His wife Epione, for example, soothed pain; his daughter Hygeia, the goddess of health, represented the prevention of disease; and Panacea, another daughter, represented treatment. His large temple settings were used to treat both the rich and the poor to cure their illnesses.
Hippocrates (5th century BC) advanced the idea that disease results from natural causes and can be understood only through a study of natural laws and from careful diagnosis. He believed that health was due to a balance of four ‘humours’ ebbing and flowing in the body (blood, phlegm, black bile and yellow bile); hence we have the related terms sanguine, phlegmatic, bilious, choleric and melancholic. He realised that the body has healing powers, and saw the health-care provider’s role as assisting the recuperative process. His ‘doctrine of opposites’, i.e. the concept that opposites cure (cold treats fever, bleeding treats excess humours), was the basis of medicine for many hundreds of years and eventually held up advances in more accurate medical knowledge. Known today as the father of medicine, Hippocrates influenced the principles that control the practice of medicine today, including versions of the Hippocratic Oath that are still read at some medical graduation ceremonies (see Figure 1-1A and CIB 4-6).

Figure 1-1 Famous people from medical history: A Hippocrates; B Galen; C Hildegarde von Bingen; D Avicenna.
Medicine during the Roman Empire (about 100 BC to AD 400) was largely based on Greek traditions of herbal remedies and healing gods. The Romans introduced excellent public health measures, including water supplies and sanitation. Folk remedies included wound dressings of wine, vinegar, eggs, honey, worms and pig dung. Ephedra (ephedrine, a sympathomimetic agent) was used, with good pharmacological rationale, for asthma, cough and haemorrhage. Famous medical men during this era included Dioscorides, a military physician who published a text entitled De Materia Medica (On the Materials of Medicine) on the sources, preparation and uses of hundreds of medically useful natural remedies, including analgesics, antiseptics, emetics and laxatives. Celsus described the four cardinal signs of inflammation, and stressed the importance of moderation, exercise, knowledge of anatomy, and prevention of infection and haemorrhage. Galen of Pergamon (Figure 1-1B) wrote voluminously on medical, scientific, philosophical, ethical and religious issues and considered that bleeding (removal of large volumes of blood) was appropriate treatment for virtually all disorders, as they were all due to an excess of a humour in the body. Galen was famous for his knowledge of drugs, both ‘simples’, i.e. simple herbal or mineral remedies, and complex mixtures that might include exotic herbs, amulets, excrement and antidotes, which came to be known as ‘galenicals’.
The Dark Ages and mediaeval times
The fall of the Roman Empire marked the beginning of the mediaeval period (400–1500 AD). Constantinople (now Istanbul) became the eastern capital of the Byzantine Empire, while the West sank into the Dark Ages as barbarians overran Western Europe. The practice of medicine reverted to folklore and tradition similar to that of the Greeks before Hippocrates. During this time, Christian religious orders built monasteries that became sites for learning, including pharmacy and medicine. They aided the sick and needy with food, rest and herbal medicines from their monastery gardens. Learning was carried out in Latin, and libraries held versions of Greek, Roman and Arabic medical texts. Medicine was a combination of both spiritual methods (prayer, exorcism, trust in relics of the saints) and physical methods (diet, drugs, bleeding and surgery).
One of the most famous women of the Middle Ages, Hildegard of Bingen, was a remarkable writer, composer, prophet, healer and abbess (Figure 1-1C). Her books described the causes of many mental and physical diseases, and medical and toxic properties of herbal, animal and mineral preparations. It is thought that her visions were probably due to the migraines from which she suffered. In some countries, women at this period were allowed to practise medicine and midwifery.
Hospitals have been called the greatest medical innovation of the Middle Ages. They were generally hospices attached to monasteries and had multiple purposes, providing religious, nursing and charitable care and also acting as leper houses. Particular saints were attributed the power to heal specific diseases, e.g. St Anthony and ergotism (see CIB 38-4), so a pilgrimage to the appropriate shrine was believed to help cure the condition. Battle wounds always provided a need for surgical and medical care, as victims usually succumbed to infection, haemorrhage or shock. The soporific (sleepinducing) and analgesic effects of the herbs poppy, henbane and mandrake were known and valued; a ‘soporific sponge’ containing a mixture of these herbs was prepared for chewing or inhalation by the patient.
In 1240 AD, the head of the Holy Roman Empire, Frederick II, declared pharmacy to be separate from medicine. Pharmacy was not, however, truly established separately until the 16th century, when Valerius Cordus compiled the first pharmacopoeia (reference text with standard formulae and recipes) as an authoritative standard.
Byzantine and Persian Empires
During this period (324 AD–15th century), in the Byzantine (Eastern Roman) Empire centred around Constantinople (Istanbul) and that of Persia (Iran) occurred the Golden Age of Islamic medicine. The Arabians’ interest in medicine, pharmacy and chemistry was reflected in the hospitals and schools they built, the many new drugs they contributed and their formulation of the first set of drug standards. Folk medicines included camphor, henna, syrup, aloes, amber and musk. The classic Greek medical works were translated into Arabic and an extensive library was collected in Baghdad. The great contribution of Islamic medicine was the establishment of teaching hospitals such as those in Baghdad, Cairo and Damascus; medical education has depended ever since on this style of training for doctors.
The most famous ancient Persian physician is Avicenna (Abu-Ali Ibn Sina Balkhi), who lived in central Asia and Persia around 980–1037 AD (Figure 1-1D). He was a ‘man for all seasons’—physician, philosopher, astronomer, chemist, mathematician, poet, teacher… —and his most famous works, The Book of Healing and The Canon of Medicine, were the standard medical textbooks for hundreds of years, even in French medical schools. Ibn Sina is considered the father of clinical pharmacology, as he introduced systematic experimentation, quantification, randomised clinical trials and efficacy tests into the study of physiology and infectious diseases.
Medicine in the Renaissance and scientific eras
In the Renaissance (14th to 16th centuries AD), there was a rebirth of interest in and knowledge of the arts, sciences, politics and economics in Europe. In the medical field, Paracelsus (1493–1541), a professor of physics and surgery at Basel in Switzerland and an alchemist and pharmacologist, denounced ‘humoral pathology’ and substituted the ‘like cures like’ theory—that diseases are actual entities to be combated with specific remedies, especially minerals. He recognised the relationship between cretinism and goitre (CIB 34-1) and that between gout and the deposition of crystals in tissues, and improved pharmacy and therapeutics for succeeding centuries, introducing new remedies and reducing the overdosing that was prevalent in that period.
Many important pharmacological discoveries were made in the 16th and 17th centuries, including:
Meanwhile, great progress was being made in pharmacy and chemistry. The first London pharmacopoeia appeared in 1618 and many preparations introduced at that time are still in use today, including opium tincture (CIB 15-4), cocaine and ipecac. Other important national pharmacopoeias were the French Codex (1818), followed by the United States Pharmacopoeia in 1820, the British Pharmacopoeia in 1864 and Germany’s in 1872.
In the 18th and 19th centuries, deliberate clinical testing of drugs for their actions was carried out. The gas nitrous oxide and the volatile liquids ether and chloroform were used in surgery, dentistry and obstetrics (see CIB 14-5) and provided the first safe painless surgery. A local anaesthetic, cocaine, had been in use for millennia in extracts of coca bark (CIB 14-10). This was studied, purified and used in eye surgery in the 1870s, and safer synthetic analogues were soon developed. Hypnotics and sedatives such as bromides and chloral hydrate helped relieve insomnia. Antiseptics such as carbolic acid were synthesised and found to be effective in vitro (in test tubes or Petri dishes) in reducing infection from wounds, but were too toxic in vivo (in the living organism) to be given to patients. The study of dose–response relationships led to the safer use of drugs. Rational medicine was replacing empiricism.
Into the 20th and 21st centuries
Early in the 20th century, drugs commonly used in medicine were aspirin and codeine as analgesics, sodium bicarbonate and glycerine for gastrointestinal problems, sodium bromide as a sedative, sodium salicylate as an antiinflammatory and antipyretic analgesic, strychnine as a ‘tonic’ and ammonium chloride as an expectorant and urinary acidifier. As knowledge of chemistry, physiology and medicine developed, it was applied to the problem of finding drugs to treat specific conditions. Advances in synthetic organic chemistry led to the establishment of large-scale chemical manufacturing plants to produce drugs, among other chemicals. Structure–activity studies identified series of molecules with agonist or antagonist actions on many types of receptor. The importance of using a control group when testing drugs or other treatments was recognised and the randomised controlled clinical trial became the expected standard (see CIB 4-3).
This was the era of the ‘magic bullet’3 with major developments being the production of safe, orally active antimicrobials, both synthetic (sulfonamides) and natural (penicillins). In the 1930s and 1940s penicillin was discovered, isolated and purified (by Fleming, Florey and Chain), which revolutionised the treatment of microbial infections and became the precursor of many other antibiotics, e.g. streptomycin for tuberculosis. These successes led to the expectation that a drug would soon be found to treat every previously life-threatening disease.
During the 20th century, medicine made enormous advances, leading to therapeutic revolutions in all areas of medicine (CIB 1-3). Of 36 major events identified as the most significant in modern medicine from 1935 to 1999 (Le Fanu 1999), at least half have been directly due to the development of effective drugs that treated diseases that were previously life-threatening or permitted safe surgery or diagnosis.
It is interesting to note at the beginning of the 21st century that, as major acute conditions are generally now treatable with drugs, most of the ‘top 10 drugs’ (Table 1-4) now prescribed are for lifestyle diseases, including statins for high cholesterol levels, calcium channel blockers and angiotensin-converting enzyme inhibitors for cardiovascular diseases, proton-pump inhibitors for peptic ulcers and metformin for type II diabetes.
The scientific revolution brought about by molecular biology techniques has enabled the identification and cloning of genes that code for therapeutically useful proteins, including monoclonal antibodies. In addition, many receptors have been purified, identified and cloned, and the biochemical pathways important in control of cell division are being elucidated, leading to new anticancer agents (see CIBs 42-1 and 42-2). The recognition that many treatments used in medicine have never been subjected to scientific scrutiny has encouraged the development of meta-analysis techniques to analyse the results of all clinical trials and medical research and to evaluate scient ific data to encourage implementation of evidence-based medicine.
Sources of drugs
Where drugs come from
Drugs and biological products have been identified or derived from several main sources:

Figure 1-2 Natural sources of important drugs: APenicillium notatum, source of penicillin; BCoffea arabica, source of caffeine (and coffee); CDigitalis purpurea, source of digoxin; DPapaver somniferum, source of morphine and codeine.
The processes and stages of drug discovery and development are discussed in more detail in Chapter 4.
It has been pointed out that, as biodiversity is lost worldwide, we are losing many potentially life-saving natural products from endangered species, rainforests and coral reefs. For example, the recent extinction of Australia’s gastric-brooding frogs means we will now never know how the frog’s eggs avoided digestion in the mother frog’s stomach or being passed on into her small intestine—actions potentially very useful in treating peptic ulcers and other gastrointestinal tract disorders. Research into threatened bear species could elucidate their mechanisms for surviving months of hibernation without losing bone mass or dying of uraemia (see Chivian and Bernstein [2008]).
Safety of natural products
There is a widely held belief that ‘natural’ products are safer than synthetic, man-made drugs (see again CIB 1-1); this belief is encouraged by many in the health-food industry and by alternative therapy practitioners. However, a quick scan through a list of naturally occurring substances such as arsenic, botulinum toxin, cantharidin, cocaine, cyanide, deadly nightshade, ipecacuanha, mercury, methanol, physostigmine, strychnine, thallium, tobacco and uranium will prove the belief false. It would be foolish to expect all substances extracted from microorganisms, plants, animals or minerals to be automatically safer than those synthesised in laboratories—or vice versa. In all cases, whether natural or synthetic, a drug’s safety and efficacy must be tested and proved before it is approved for clinical use (see Smith [2002]).
Active constituents of plant drugs
The leaves, roots, seeds and other parts of some plants may be dried, crushed, boiled and extracted or otherwise processed for use as a medicine and, as such, are known as crude drugs or herbal remedies; these are discussed in Chapter 3. Although they may appear more ‘natural’ than tablets, ointments or injections, their therapeutic effects are produced by the chemical substances they contain (see Table 1-2). Indeed, the natural antidepressant St John’s wort had been shown to have a similar mechanism of action—and hence similar adverse effects—as the synthetic selective serotonin reuptake inhibitors.
Table 1-2 Pharmacological properties of some plant drugs
| DRUG | SOURCE | MAIN PHARMACOLOGICAL ACTIONS |
| Aromatic oils | E.g. from eucalyptus, pine, mint | Decongestant, Rx common cold, mild antiseptics |
| Atropine | Atropa belladonna (belladonna lily) | Antimuscarinic, premedication, Rx asthma |
| Benzoin | Resin from Styrax spp. | Inhalant, decongestant, antiseptic, astringent |
| Bran | Indigestible vegetable fibre | Laxative, Rx constipation |
| Caffeine | Coffea arabica (coffee) | CNS stimulant, diuretic |
| Cocaine | Erythroxylum coca | CNS stimulant, local anaesthetic |
| Colchicine | Colchicum autumnale (crocus) | Anti-inflammatory, Rx gout |
| Coumarins | Sweet clover | Anticoagulants, prevent thrombosis |
| Digoxin | Digitalis lanata (foxglove) | Cardiac glycoside, Rx heart failure |
| Emetine | Ipecacuanha (Cephaelis) root | Antiamoebic, Rx dysentery |
| Ephedrine | Ephedra sinica | Sympathomimetic, Rx asthma |
| Ergot alkaloids, e.g. ergometrine | Mould on Claviceps spp. | Oxytocic, Rx postpartum bleeding |
| Ipecacuanha | Cephaelis root | Expectorant, emetic, Rx poisoning |
| Morphine | Papaver somniferum (opium poppy) | Analgesic, sedative, constipating, cough suppressant |
| Nicotine | Nicotiana tabacum (tobacco) | Vasoconstrictor, CNS stimulant, addictive |
| Paclitaxel | Yew tree bark | Antineoplastic, Rx cancer |
| Phyto-oestrogens | Clover, soybeans | Oestrogenic, Rx menopausal symptoms |
| Pilocarpine | Pilocarpus microphyllus | Muscarinic agonist, Rx glaucoma |
| Quinine, quinidine | Cinchona bark | Antimalarial, Rx cardiac arrhythmias |
| Salicylates | Salix spp. (willow) | Anti-inflammatory, analgesic, antipyretic |
| Strychnine | Strychnos nux vomica | CNS stimulant, convulsant |
| Vincristine | Vinca rosea (periwinkle plant) | Antineoplastic, Rx cancer |
CNS = central nervous system; Rx = treatment of.
Source: Evans (2002), Trease & Evans’ Pharmacognosy, 15th edn [ch 6].
When the pharmacologically active constituents are separated from the crude preparation and purified and quantified, the resulting substances usually have similar pharmacological actions to the crude drugs but are more potent, usually produce effects more reliably and are less likely to be affected by other constituents in the crude preparations. Some of the types of pharmacologically active compounds found in plants, grouped according to their physical and chemical properties, are alkaloids, glycosides, steroids, hydrocarbons, alcohols, proteins, gums and oils. Note that the groups are not mutually exclusive—there can be glycoproteins and phenolic glycosides etc. Figure 1-3 shows the chemical formulae of some drugs that are extracted from plant sources.
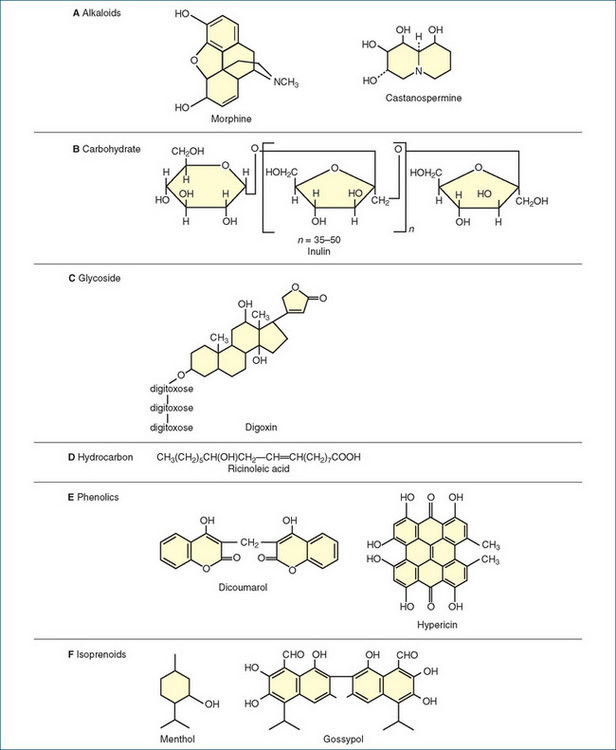
Figure 1-3 Chemical structures of some active drugs derived from plant sources. A Alkaloids: morphine and castanospermine. B A carbohydrate: inulin. C A glycoside: digoxin. D A hydrocarbon: ricinoleic acid. E Phenolics: dicoumarol and hypericin. F Isoprenoids: menthol and gossypol.
The pharmacological actions of drugs from plants are determined by their active chemical ingredients. Useful plant drugs with actions affecting virtually every body system have been found, as shown in Table 1-2.
Alkaloids
Alkaloids are organic nitrogen-containing compounds that are alkaline and usually bitter-tasting; the nitrogen atom is usually in a heterocyclic ring of carbon atoms (Figure 1-3A); as many alkaloid drugs are amines, their names often end in the suffix ‘-ine’. They are combined as salts to make them more soluble, e.g. morphine sulfate. It is thought that plants may have evolved the ability to synthesise bitter alkaloids as a defence mechanism against herbivorous animals. Examples of some pharmacologically useful plant alkaloids are listed below, with cross-references to interesting relevant Drug Monographs (DM) and Clinical Interest Boxes (CIBs):
Formerly, the drug company Drug Houses of Australia (earlier known as Felton, Grimwade and Duerdins Pty Ltd) manufactured hyoscine and atropine from Australian Duboisia species; this was important during World War II, when supplies of the antinauseant drug hyoscine from European sources ran out.4 In Tasmania, the opium poppy Papaver somniferum is grown and harvested for production of opium alkaloids. Castanospermum australe is a source of the effective antiretroviral alkaloid castanospermine.
Carbohydrates
Carbohydrates are organic compounds of carbon, hydrogen and oxygen. Carbohydrates used in medicine include sugars such as glucose, starches and fibres such as cellulose and inulin (a fructose–furanose polysaccharide used in kidney function tests [Figure 1-3B]), gelling agents such as agar, and gums such as tragacanth and Aloe vera products (CIB 48-2).
Gums and mucilages are plant exudates. When water is added, some of them will swell and form gelatinous masses. When taken orally, they tend to remain unchanged in the gastrointestinal tract, where they act as hydrophilic (waterattracting) colloids, forming watery bulk and exerting a laxative effect. Agar and psyllium seeds are examples of natural laxative gums, whereas methylcellulose and sodium carboxymethylcellulose are synthetic colloids. Gums are also used to soothe irritated skin and mucous membranes, and may be a rich source of starch.
Glycosides
Glycosides are a particular type of carbohydrate, which, on hydrolysis, yields a sugar plus one or more additional active substances. The sugar part is believed to increase the solubility, absorption, permeability and cellular distribution of the glycoside. An important plant glycoside used in medicine is digoxin (Figure 1-3C), found in Digitalis (foxglove) plants and known as a cardiac glycoside because of its stimulant actions on the heart. Glycosides present in other Australian plants, including the oleanders Cerbera and Carissa, are responsible for the poisonous nature of these plants. Cane toads also contain cardioactive glycosides (see ‘Myth busting—cane toads in Australia’, Capon et al, www.raci.org.au/chemaust/docs/pdf/2009/CiA_May09p3.pdf).
Glycosides are also produced during the processes of drug metabolism in the human body, particularly in the liver; in phase II metabolic processes many drug molecules and their metabolites are conjugated with glucuronic acid. Such large compound metabolites are known as glucuronides and are more soluble and hence more excretable than the parent drug molecules; they are also less pharmacologically active, as the large drug–glucuronide combination cannot activate receptors in the same way that the parent drug does (see Table 6-4 and Figure 6-5).
Hydrocarbons
Plants contain many hydrocarbon components, including fats and waxes; oils such as castor, olive and coconut oil; and fatty acids, prostaglandins and balsams. Derivatives such as organic alcohols and esters contribute the fragrances to many plants and perfumes. Castor oil is mainly composed of ricinoleic acid (Figure 1-3D). Hydrocarbons are commonly used by drug companies and hospital pharmacies when preparing formulations of drugs, especially creams and ointments.
Phenols
Many pharmacologically active plant constituents are phenolic, i.e. they contain a benzene ring with a hydroxyl substituent. Examples are the salicylates, including aspirinlike compounds and flavouring agents (e.g. vanillin); isoflavones, including phyto-oestrogens (CIB 38-3); coumarins, including the anticoagulant dicoumarol (Figure 1-3E); cannabinols from marijuana; hypericin (from St John’s wort, used in depression, CIB 18-9 and Figure 1-1E); and poisonous aflatoxins from mouldy peanuts (ethanol from fermented plants and grains is not phenolic but is the prototype alcohol).
Tannins are astringent plant phenolics that have the ability to tan hides (animal skins) by precipitating proteins. Tannins are common plant constituents, especially in bark, and account for some of the brown colour in swamps and rivers and also in cups of tea. In Australian native medicine, kino, the gum exuded from eucalyptus trees, was an important source of tannins, which were used to treat diarrhoea, haemorrhages and throat infections.
Terpenes and steroids
Many plant chemicals, including steroids, are synthesised naturally from terpenes, 10-carbon molecules built up from small 5-carbon building blocks called isoprenes. Plant steroids, with their characteristic 4-ring structures (Figure 33-3), are used as the starting material for the production of many hormones. For example, the production of oestrogenic hormones for use as contraceptives was very difficult and expensive until methods were devised to use the plant sterol diosgenin, from Dioscorea species, in the synthesis of oestrogenic compounds. Other isoprenoid compounds are gossypol, a Chinese male contraceptive agent (Figure 1-3F); the active ingredients of the herbs gentian, valerian, feverfew and ginkgo; carotenoids such as β-carotene (CIB 48-4, DM 48-2); and the poison picrotoxin.
Salicylates, a group of phenolic terpenoid compounds, are important analgesic drugs based on saligenin from willow tree bark; the chemical name for aspirin is acetylsalicylic acid (CIB 15-7, DM 15-2). Pyrethrins, terpene-type compounds with effective insecticidal actions, have been used for centuries: it is reported that Napoleon ordered that the dried flowers of the chrysanthemum plant be used to delouse the French army! Australian research in the pyrethrin industry has discovered semisynthetic derivatives that have longer half-lives than the natural compounds and are therefore more useful in the plant production industries and as insecticides for animals.
Oils
Oils are highly viscous liquids that are high in hydrocarbon content, often flammable and immiscible with water and aqueous solvents. They may be terpene-type compounds, and contain many types of functional groups including ketones, phenols, alcohols, esters and aldehydes. Oils are classified as being of two kinds, volatile or fixed: a fixed oil dropped onto filter paper will leave a greasy stain, whereas a volatile oil will not, as it evaporates. Volatile oils may impart aromas to a plant.
Oils are frequently used as flavouring agents, in perfumery, in chemical industries and for therapeutic actions as antiseptics, carminatives (soothing to the stomach) and antispasm agents. Eucalyptus, peppermint and clove oils are examples of volatile oils used in medicine. Castor oil is an example of a fixed oil used in medicine, while olive oil is a fixed oil used in cooking. Camphor, menthol (Figure 1-3F) and thymol are related aromatic agents used in respiratory medicine. The Australian species myrtaceae and melaleucas contain many fragrant and useful oils, including eucalyptus and tea-tree oils (CIB 28-12).
Drug names and classifications
Drug names
As a drug passes through the investigational stages before it is approved and marketed, it collects three different types of name: the chemical name; the approved (or generic or non-proprietary) name; and the proprietary (or brand or trade) name or names (CIB 1-4). For example, the chemical name of amoxycillin, a commonly prescribed antibacterial antibiotic, is D(–)-α-amino-p-hydroxybenzylpenicillin. Its approved (generic) name, amoxycillin, is derived from parts of its chemical name, and it is marketed under dozens of proprietary names, including Alphamox, Amoxil, Bgramin, Cilamox, Fisamox, Moxacin and Ranmoxy, in various formulations such as injections, capsules, tablets, syrups, suspensions and paediatric drops, and in combinations with other antibacterials and proton-pump inhibitors. Note that approved/generic names start with a lower-case letter, whereas trade names start with a capital (upper-case) letter.
It would be helpful if all drugs had names related to other similar drugs; however, this tends to be true only of the more recent drug groups. Names can be deceiving: names of most β-blockers end in ‘-olol’; however, stanozolol is not a β-blocker but an anabolic steroid and nystatin is an antifungal agent, not a ‘statin’, so it is not safe to assume that drugs whose names sound similar always have similar effects and uses. Similarly, while drugs ending in ‘-mycin’ all come from bacteria (e.g. the Actinomycetes aka Actinobacteria) or fungi (Eumycetes) or are related to fungal metabolites, a ‘-mycin’ drug could be an antibacterial antibiotic or an anticancer antibiotic. And Table 1-2 cannot be read backwards, i.e. while the suffix ‘-vir’ implies the drug is probably an antiviral, not all antiviral drugs end in -vir (think zidovudine and ribavirin).
Chemical names
The chemical name is a precise description of the drug’s chemical composition and molecular structure. It is particularly meaningful to medicinal chemists—who should be able to draw the chemical structure if given the chemical name—but may be virtually unintelligible to others. As chemical names are too complicated to remember easily, or fit on a prescription pad or pharmacy bottle label, drugs likely to reach the market and be used medically are allocated or given a name that is simpler, more euphonious and easier to spell.
Clinical interest box 1-4 what’s in a (drug) name?
Pharmacists, pharmacologists and doctors may have noticed that, in recent years, increasing numbers of drug names (both generic and trade) have odd letters like q, x and z in them. In a delightful ‘Christmas Offering’ in the Medical Journal of Australia (2000; 173: 662–663), Dr Gordon Parker, Professor of Psychiatry at the University of New South Wales, analysed and discussed this trend, surveying a total of 33 psychotropic drug names and comparing old and new. The new names, he decided, ‘resemble the loser’s board in a last round of Scrabble’, while the older names were rated by the psychiatry registrars as more attractive and thus more prescribable. Old names were evocative, such as the hypnotic Halcion, the antipsychotic Serenace and the alcohol deterrent Antabuse, whereas new names (such as reboxetine, venlafaxine, quetiapine and zuclopenthixol) tended to be unpronounceable and of questionable appeal; there is now even a group known as the ‘Z-drugs’. Professor Parker feared that we would soon see a hypnotic named Zizzzz, and a wonder-drug with the winning name X-Plozox. He suggested that more euphonious names that drug companies could consider are Cloud9 for an antidepressant, ChillOut for an antipsychotic, and Care-Less for an antianxiety agent. (For his suggestion for a better trade name for sildenafil—a drug used to treat male impotence—than Viagra, the reader is advised to consult the original article.)
Approved (generic) names
The approved name is usually suggested by the manufacturer and approved by the local drug regulating authority; it becomes the official drug name, e.g. the Australian Approved Name (AAN) or European Approved Name (EAN). It is a shorter name, usually derived from the chemical name, and is the name listed in official compendia such as the Australian Medicines Handbook or the British Pharmacopoeia. The approved name needs to be distinct in sound and spelling so that it is not easily confused with other drugs (but see CIB 1-5), and preferably related to the names of pharmacologically similar drugs (see Table 1-3).5
| PREFIX OR SUFFIX | DRUG GROUP | EXAMPLE GENERIC NAME |
| cefa/o- | Cefalosporins | cefotaxime |
| gli- | Sulfonylureas | glibenclamide |
| -a/oquine | Quinine antimalarials | chloroquine |
| -artan | Angiotensin-II-receptor antagonists | candesartan |
| -a/ovir | Antivirals | aciclovir |
| -azepam | Benzodiazepines | diazepam |
| -azole | Azole antifungal agents | ketoconazole |
| -caine | Local anaesthetics | lignocaine, bupivacaine |
| -cillin | Penicillins | ampicillin |
| -coxib | Cyclo-oxygenase-2 inhibitors | celecoxib |
| -cycline | Tetracycline antibiotics | doxycycline |
| -dipine | Calcium channel blockers | nifedipine |
| -dronate | Bisphosphonates | alendronate |
| -eplase | Fibrinolytic agents | alteplase |
| -floxacin | Quinolone antibiotics | ciprofloxacin |
| -glitazone | Thiazolidinediones (glitazones) | rosiglitazone |
| -i/ythromycin | Macrolide antibiotics | erythromycin |
| -lutamide | Antiandrogens | flutamide |
| -mab | Monoclonal antibodies | trastuzumab |
| -olol (most) | β-Blockers | propranolol |
| -onidine | α2-Adrenoceptor agonist | clonidine |
| -oprost | Prostaglandin analogues | latanoprost |
| -oxacin | Quinolone antibiotics | norfloxacin |
| -prazole | Proton-pump inhibitors | omeprazole |
| -pril | ACE inhibitors | captopril |
| -setron | 5-HT3 antagonists | ondansetron |
| -statin (some) | HMG-CoA reductase inhibitors | simvastatin |
| -stim | Colony-stimulating factors | filgrastim |
| -tidine | Histamine H2-receptor antagonists | cimetidine |
| -tinib | Tyrosine kinase inhibitors | imatinib |
| -triptan | 5-HT1 agonists | sumatriptan |
| -zolamide | Carbonic anhydrase inhibitor | acetazolamide |
ACE = angiotensin-converting enzyme (converts angiotensin I to angiotensin II, which acts to decrease the diameter of arteries and arterioles [vasoconstriction] and hence to raise blood pressure); HMG-CoA = 3-hydroxy-3-methylglutaryl coenzyme A (a coenzyme involved in the early stages of cholesterol synthesis).
Strictly speaking, the term generic name refers to a group name, e.g. the penicillins, the salicylates, the β-blockers; however, it has come to be used interchangeably with the approved name. For example, we speak of ‘generic prescribing’, meaning prescribing using the approved name of a drug (amoxycillin) rather than one proprietary
Clinical interest box 1-5 an eye-drop by another name
A 50-year-old woman who had been treated surgically for raised intraocular pressure (glaucoma) also required eyedrops and was prescribed Azopt brand (brinzolamide 1%). She noticed that the second bottle of drops looked different from the first, but as the pharmacist’s label obscured the original bottle label she assumed it was a different brand of the same drug, so used the drops at night. Next morning she had bilateral dilated pupils, severe glare intolerance and blurred vision—very different from her response to the first bottle of drops.
After the product and prescription were checked, it was found that the pharmacist dispensing the prescription had mistakenly selected a bottle of Atropt drops (atropine, a powerful and long-lasting mydriatic drug used in ocular examinations) instead of Azopt. Atropine raises intraocular pressure and could potentially have caused blindness in this susceptible patient; luckily she suffered only glare and blurring for 5 days.
The case emphasises the risks inherent in drug names that look and sound very similar, and the importance of careful writing of prescriptions and labelling of drugs. Prescribing and labelling with the generic name rather than trade name might have avoided the potentially dangerous situation.
Source: Dunlop 2009.
or brand name (e.g. Amoxil). In this text we will always use generic (approved) names for drugs but may sometimes add a trade name6 if it is well known enough (e.g. Valium, Prozac or Viagra) to help students identify a particular drug. Note that approved names use lower-case letters, whereas a trade name always begins with an upper-case letter.
Generic prescribing and bioequivalence
As numerous brand names may exist for the same drug, such as those shown above for amoxycillin, prescribers are encouraged to use the generic name. The use of generic names is also widely advocated to avoid confusion between drugs with similar trade names, and to reduce costs. With some exceptions, most generic drug products sold (assuming same dose and type of formulation) are considered therapeutically equivalent (bioequivalent), and some ‘generic’ products are much less expensive than a particular brand name drug.
For this reason, and because pharmacists cannot possibly carry and store every brand of every marketed drug, in some defined situations pharmacists are allowed to substitute between brand names if the named products are considered to be identical in terms of bioequivalence (dose, availability to sites of action, pharmacokinetic parameters etc—see Chapter 6). Thus the Australian Pharmaceutical Benefits Scheme (PBS; see Chapters 2 and 4) allows brand substitution between several brands of amoxycillin, for tablets of the same strength (dose), unless the prescriber checks a box on the prescription form to indicate ‘Brand substitution not permitted’ (see Figure 2-3B). The substitution can be confusing to patients if, for example, the colour, shape, name, taste or packaging of the tablet changes but the pharmacist insists that the medicine is the same; this situation requires sensitive counselling (see McLachlan et al [2007]).
Proprietary (trade or brand) names
When a drug company markets a particular drug product, it selects and copyrights a proprietary or trade name for its drug (CIB 1-4). This copyright restricts the use of the name to that individual drug company and refers only to that formulation of the drug. To encourage doctors to prescribe particular versions of the drug and to promote sales of trade name drugs, extensive advertising is usually necessary; this expense is eventually borne by the consumer.
International Nonproprietary Names (INN) and European Approved Names
Since the United Kingdom’s entry into the European Community (EC) in 1973, and the recent adoption of European Approved Names (EAN) for drugs, the British medical and pharmaceutical establishments have had to accept the use of INN in the EC as the EAN for drugs. Examples of INN are norepinephrine (formerly noradrenaline), sulfonamides (sulphonamides), furosemide (frusemide), diethylstilbestrol (stilboestrol) and ciclosporin (cyclosporin) (see Longmore et al [2008]). These changes have not yet been adopted in Australia or New Zealand. We suspect and hope that the old terminology will continue in Australia and New Zealand; for example, although the INN for the sympathomimetic neurotransmitter is ‘norepinephrine’ in the EC, USA and Canada, the type of neurotransmission is still called ‘noradrenergic’ and the receptors ‘adrenoceptors’.
American names
It would be ideal for safety and convenience if the approved name for a drug molecule could be the same worldwide; indeed, the World Health Organization (WHO) is encouraging the use of International Non-proprietary Names (INN). Approved names in Australia generally follow the British names, as Australian pharmacy has long been legally dependent on the British Pharmacopoeia as the standard for drugs. Sometimes, however, other approved names are used in the USA (USAN, the US Approved Name), Canada and countries that follow their lead, so Australian and New Zealand students can become confused if they do not realise, for example, that adrenaline (UK, Australia, New Zealand) = epinephrine (USA). The commonest drug with very different names is paracetamol (known as acetaminophen in the USA/Canada).
Major reference texts such as Martindale: The Complete Drug Reference (Sweetman 2009) usually list alternative approved and many trade names, which helps clarify the issue.
Drug classifications
Classification systems
Drug classification can be approached from many perspectives. Using the example of amoxycillin again, this could be classified by:
Not surprisingly, students are often confused by drug classification, particularly as sometimes the same drug may be classified into various groups depending on the clinical use, e.g. aspirin-like drugs may be classified as analgesics, antipyretics, anti-inflammatory agents or antithrombotics. Probably the most useful methods involve classification by clinical indication, by body system or by mechanism of action. This book uses these approaches where appropriate; examples include the titles of Chapter 24, ‘Lipid-lowering drugs’, and Chapter 45, ‘Antifungal and antiviral drugs’. An example of drugs classified by body system can be found in Unit IV, ‘Drugs affecting the central nervous system’, whereas in Chapter 18, ‘Psychotropic agents’, antidepressants are grouped together under ‘Tricyclic antidepressants’ (a chemical class), ‘Monoamine oxidase inhibitors’ or ‘Selective serotonin reuptake inhibitors’ (mechanisms of action). Such drug classifications can help the health-care professional understand and learn about the individual agents available for drug therapy.
Prototype drugs
Pharmacology is easier to understand and learn when key, or prototype, drugs are studied. A prototype drug is usually the most important drug in a particular drug class, to which other drugs in the class can be compared. In this text, many prototype drugs are described in detail in a consistent format called a Drug Monograph (DM); thus, diazepam can be viewed as the prototype benzodiazepine antianxiety agent (DM 16-1). When a new similar drug becomes available, the practitioner can associate it with its drug group and prototype, and make inferences about many of its basic qualities before focusing on specific properties (usually pharmacokinetic) to differentiate it from the prototype and other drugs in the same group.
Prescription-only or OTC drugs
A drug may be classified as a prescription-only drug, which means that it requires a legal prescription to obtain it, or it may be a non-prescription or over-the-counter (OTC) drug, which means that it may be purchased without a prescription, possibly in a pharmacy, supermarket or general store. Some prescription drugs may be purchased OTC, usually in lower drug dosages that are considered to be relatively safe for sale, for conditions that may not warrant a person visiting a doctor or for which important drugs need to be readily available. An example is the nonsteroidal anti-inflammatory drug naproxen, which is available as an OTC drug (S2, Pharmacy-Only) in a 220-mg to 275-mg strength (packs of of × 12, 24 or 30 tablets) for treatment of headache/toothache or dysmenorrhoea, but requires a prescription (S4) for the 250-( × 50 or × 100), 500-or 550-mg ( × 50) or for the 750- or 1000-mg ( × 28) tablets for arthritis and bone pain.7 Drug schedules are considered in more detail in Chapters 2 and 4 and Appendix 5, and OTC medicines in Chapter 3.
WHO Essential Medicines List
It is recognised that with the enormous range of drugs available, few countries or health services can subsidise or provide the whole range of drugs, and no retail or hospital pharmacies could stock them all. To assist in decision making with respect to which drugs are the most important, the World Health Organization (WHO) through its Expert Committee on the Selection and Use of Essential Medicines has derived a Model List of about 280 core drugs in some 27 main categories (see Appendix 6), which are considered essential to provide ‘minimum medicine needs for a basic health-care system, listing the most efficacious, safe and cost-effective medicines for priority conditions’. This is useful for all countries attempting to curtail rapidly increasing expenditure on drugs, and is particularly useful for developing countries, allowing them to concentrate on providing the most important drugs. A statement by WHO defined essential drugs as ‘those that satisfy the healthcare needs of the majority of the population… they should therefore be available at all times in adequate amounts and in the appropriate dosage forms, and at a price that individuals and community can afford’.
The selection of drugs is determined by a committee of scientists and clinicians and is updated at regular intervals. These drugs first require market approval on the basis of efficacy, safety and quality as well as value for money. Listing ‘essential’ drugs inevitably raises concerns, particularly from the manufacturers of drugs not on the list, which may be seen as ‘non-essential’. A table adapted from the WHO Model List of drugs is included as Appendix 6, showing categories of essential drugs and, where possible, an example of therapeutic groups.
There is a separate Model List of medicines for children, and a ‘complementary list’ of approximately 67 medicines for which specialised doctors or facilities are required.
Australian top 10 drugs
The Australian Commonwealth Department of Health regularly audits the usage of prescription drugs in Australia and publishes lists of the top 10 drugs, scored by numbers of daily doses, by prescription counts and by cost to the government (i.e. to taxpayers). The lists for drug use in the year 2008/09 are summarised in Table 1-4; note that only subsidised drugs are audited here, not those bought OTC or provided under private prescriptions.
Table 1-4 Australia’s top 10 drugs, 2008/09*
| Top 10 drugs counted by number of people taking the standard daily dose every day per thousand population | ||
| ORDER | DRUG (INDICATION) | DAILY DOSES PER THOUSAND PEOPLE |
| 1 | atorvastatin (lipid-lowering) | 77.7 |
| 2 | irbesartan (hypertension) | 36.6 |
| 3 | ramipril (hypertension) | 28.6 |
| 4 | perindopril (hypertension) | 27.5 |
| 5 | simvastatin (lipid-lowering) | 27.3 |
| 6 | paracetamol (analgesic) | 21.8 |
| 7 | candesartan (hypertension) | 21.4 |
| 8 | esomeprazole (oesophageal reflux) | 21.3 |
| 9 | aspirin (antiplatelet, anti-inflammatory) | 17.8 |
| 10 | frusemide (diuretic) | 17.5 |
| Top 10 drugs by prescription counts (in millions) | ||
| ORDER | DRUG (INDICATION) | MILLIONS OF PRESCRIPTIONS |
| 1 | atorvastatin (lipid-lowering) | 10.95 |
| 2 | esomeprazole (oesophageal reflux) | 5.89 |
| 3 | simvastatin (lipid-lowering) | 5.16 |
| 4 | paracetamol (analgesic) | 3.91 |
| 5 | perindopril (hypertension) | 3.89 |
| 6 | pantoprazole (oesophageal reflux) | 3.49 |
| 7 | atenolol (hypertension, angina, arrhythmias) | 3.22 |
| 8 | metformin (type II diabetes) | 3.20 |
| 9 | rosuvastatin (lipid-lowering) | 3.17 |
| 10 | irbesartan (hypertension) | 3.13 |
| Top 10 drugs by cost to government (in A$ millions) | ||
| ORDER | DRUG (INDICATION) | COST TO GOVERNMENT (A$ millions) |
| 1 | atorvastatin (lipid-lowering) | 621 |
| 2 | clopidogrel (thromboembolism) | 211 |
| 3 | esomeprazole (oesophageal reflux) | 205 |
| 4 | rosuvastatin (lipid-lowering) | 202 |
| 5 | simvastatin (lipid-lowering) | 171 |
| 6 | fluticasone with salmeterol (asthma) | 164 |
| 7 | olanzapine (schizophrenia, mania) | 159 |
| 8 | ranibizumab (ocular macular degeneration) | 155 |
| 9 | rituximab (rheumatoid arthritis) | 112 |
| 10 | venlafaxine (depression) | 111 |
DDD = defined daily dose.
* Note that the audit does not score drugs prescribed by private prescription or under PBS co-payment, or bought OTC. It is difficult to compare usage and costs between years, as the accounting periods are not for exactly the same number of weeks each time data are published.
Source: Australian Prescriber 2009; 32(6): 159, sourced from Drug Utilisation Sub-Committee database, September 2009.
Understandably, the government is concerned about the widespread use of the ‘statin’ drugs, used to lower blood cholesterol levels. As can be seen, in the list of drugs by cost, statins occupy three of the top five places. When these drugs were listed on the PBS there was a massive blow-out in their use, partly by people wishing to reduce their cardiovascular risk without the inconvenience of raising their exercise levels or decreasing their food intake! The top three statins together accounted for almost A$1 billion expenditure in the 2008/09 health budget. There are strict guidelines that must be met before these drugs can be prescribed on the PBS, including documentation of blood lipid levels and risk category (cardiovascular, diabetes, family history, age), and lipidlowering dietary therapy attempted before and during statin treatment. There is concern, however, that patients who are denied a prescription by one doctor may simply ‘shop around’ until they find a doctor who will decide that they meet the ‘high-risk’ criteria.
Comparing the lists of top 10 drugs by DDD/1000 population/day over the last few years, some interesting trends emerge:
New Zealand’s Pharmaceutical Management Agency (PHARMAC) also publishes lists of the most prescribed medications, as a ‘top 20’. Their top 10 in 2007 were as follows: paracetamol, aspirin, simvastatin, omeprazole, amoxycillin, amoxycillin + clavulanate, metoprolol, salbutamol, diclofenac, cilazapril.
The main differences appear to be the slightly different statins and ACE inhibitors used in New Zealand (cilazapril is not available in Australia), the inclusion of amoxycillin and diclofenac and absence of an angiotensin-receptor antagonist (like irbesartan) from the New Zealand list.
Drug information
Important drug information
The basic information important for a major drug includes its:
Contraindications are the medical conditions in which a drug should not be prescribed, e.g. a particular drug may be contraindicated in patients with kidney failure, or during pregnancy. Information as to potential toxic effects and treatment of poisoning may also be relevant, as well as safety of use in particular cohorts of patients, such as premature infants or the elderly. The Australian Drug Evaluation Committee’s Pregnancy Safety Category indicates the likely safety or risks with the use of a drug during pregnancy (see Chapter 9 and Table 9-1).
Drug information sources
Publication of data on new drugs and new information on old drugs is an ongoing process. Research papers in scientific journals, news releases, articles, patient information brochures, reference books and textbooks are written in an attempt to keep up with the new discoveries. Much information (some of it of dubious quality) finds its way onto the World Wide Web. Because no one reference is a complete source of drug data to meet the varied and specialised needs of clinical practice today, students need to be familiar with the primary drug reference sources available. It is always important to read critically and consider what credibility can be given to the author and the publication, particularly with information found on the Internet.
Official sources, pharmacopoeias and formularies
Official sources of drug information are published by governments and government bodies such as departments of health and hospitals, and by pharmaceutical societies and medical colleges, and contain legally accepted standards for drugs. Pharmacopoeias are reference texts containing a compendium or collected body of drug information relevant to a particular country. The pharmacopoeia usually contains information on all of the authorised drugs available within the country, including their descriptions, formulae, strengths, standards of purity and dosage forms.
Formularies are similar but may also include information on drug actions, adverse effects, general medical information, guidelines for pharmacists dispensing medicines, and the ‘recipes’ for formulation or production of different medicines, such as tablets, injections, ointments and eye-drops. A national formulary may also be used by the government to limit the drugs available or subsidised, in order to encourage rational, cost-efficient prescribing and enhance the quality use of medicine (QUM; see Chapter 2). Examples of official drug information sources are:
In New Zealand, there is no national formulary, but the BNF and APF are legal standards and are used for teaching. The Pharmaceutical Schedule from PHARMAC lists subsidised medicines and is updated every few months (see www.pharmac.govt.nz). The Medsafe website (www.medsafe.govt.nz/) is a great source of independent information for both health professionals and consumers (and students), with prescriber update articles, medicine data sheets, reporting of adverse reactions, ‘patient info leaflets’ and media releases, plus information about related topics including classification and regulation of medicines, medical devices, drug abuse, patient support groups, clinical trials and complementary medicines.
Semi-official sources
Semi-official sources of drug information may be published by government bodies or other groups, such as medical and pharmacology societies or independent publishers, and may include drug bulletins, reference books and updates, but no drug advertisements. While not official standards, they attempt to provide up-to-date, independent and unbiased information on drugs. Depending on the publication, information such as lists of food additives, patient support organisations, poisons information centres and prescribing guidelines may be included. Examples include the Australian Prescriber (a free bi-monthly independent review journal) which includes Medicines Safety Updates, the Therapeutic Guidelines series, the Paediatric Pharmacopoeia (Pharmacy Department, Melbourne Royal Children’s Hospital), National Prescribing Service newsletters, reference books such as the Australian Prescription Products Guide (known as the PP Guide), the Merck Index, Drug Interactions: Facts, and Drug Interactions: Analysis and Management, and journals such as Current Therapeutics and Drugs.
Some reference texts, e.g. the MIMS Annual, Mosby’s GenRx and the United States Pharmacopeial Convention’s Drug Information for the Health Care Professional (USP DI), provide actual photographs of drug formulations to assist in identifying an unknown tablet or capsule. In addition, manufacturers often place numbers with letters on their solid-dose formulations to aid in identification.
The Cochrane Collaboration is an international organisation that prepares systematic reviews of the effects of health-care interventions, such as clinical trials of drugs or other therapeutic techniques, with the aim of helping all people make well-informed decisions about health care. It aims to avoid duplication of studies, minimise bias and provide relevant, up-to-date easily accessible information. There are Cochrane databases of reviews, clinical trials, methodologies and economic evaluations, among others.
Consumer medicine information
Handing out consumer medicine information (CMI) pamphlets to patients is encouraged as an important way to improve people’s involvement with and understanding of the drugs they are prescribed. In Australia, all products have had CMI handouts since the end of 2002. They are particularly important when a drug is first provided, the dose or formulation changed or the information revised (see Aslani [2007]).
Drug or poisons information centres and pharmacists
Drug information centres, usually located in the pharmacy departments of major teaching hospitals, are set up to disseminate information about drugs, adverse reactions, drug interactions, treatment of drug overdoses and other related information, to maximise safety, efficacy and economy in drug use (see Appendix 6 and Australian Medicines Handbook, Appendix E). They are excellent sources of information for both the public and health professionals and for answering difficult pharmacological questions. In addition, pharmacists in hospitals and retail chemist shops are usually available and willing to provide drug information.
A new arm of the Community Quality Use of Medicines program was launched in Australia in January 2004, with pocket-sized ‘Medimate’ booklets distributed to doctors and pharmacists for patients. Each booklet contained general information about medicines, other therapies, CMI leaflets, use of OTC medicines, side effects and information sources, and questions that consumers should ask about their medicines, and provided a tear-off slip that could be filled in to summarise the person’s drug therapy. The Medimate website, run by the National Prescribing Service (http://www.nps.org.au/consumers/tools__and__tips/medimate), has similar information, as well as links to publications, videos and interviews, with information available in several languages.
Other drug information sources
An up-to-date pharmacology textbook is a valuable source of drug information for inclusion in the health-care professional’s library. Various ‘drug guides’ also exist, acting as quick reference sources of summarised information on drugs. Most of these have grown rather too large to fit in the pocket of a doctor’s, nurse’s or pharmacist’s uniform, but are useful on the desk or ward station. Examples are Havard’s Nursing Guide to Drugs and the MIMS bi-monthly drug reference guide.
Drug companies applying for registration of their products must supply to health authorities an enormous amount of information on all aspects of the drug, to prove safety, efficacy and cost-effectiveness. A summary of this information is available in publications such as the MIMS Annual, the PP Guide and in CMI sheets, advertisements and promotions. As this material is supplied by drug companies, it is inherently likely to be less objective than information in independent sources such as the Australian Medicines Handbook or Australian Prescriber. (Ethical aspects of drug advertising are discussed in Chapter 4.) It is important to consider the source of such information and beware of bias or selectivity of information.
The Internet
With the proliferation of medical sites on the Internet, many search engines and directories are available to provide both general and specialised drug information for everyone—health-care professionals and consumers/ patients. Some professional journals (medical, pharmacy and nursing), databases, indexes and abstracting services also provide current drug information on the Internet. It is essential to read Internet sites critically when seeking drug information because there is no screening system to determine the accuracy of Internet information, and erroneous, commercial or biased information may be posted.9 The best approach may be to consider the credibility, reputation and likely motive of the provider of the information. For example, does it come from reputable drug information centres; pharmacy, medical or nursing schools; professional journals; medical societies or colleges; government bodies; drug companies; or even individuals wanting to publicise or sell their own favourite remedies or products?
Many other sources of drug information are available. The criteria for using any particular source should be based on the information desired, the credibility of the provider and the currency and accuracy of the source.
Dosage measurements and calculations
Measurement systems
The main system of measurement in use for administering drugs is the metric system, based on SI units (Système International d’ Unités)—this is the most widely used and the most convenient, as units change in multiples of 10. The ‘household system’, utilising measures readily available in the home setting such as the teaspoon (about 5 mL), the tablespoon (15–20 mL) and cup (250 mL), is a less accurate system. The apothecary system, dating back hundreds of years and based on the English system of measures, was phased out in Australia in the 1960s, to the relief of all who had been required to learn its tables of measures.10 Useful conversion tables to convert between metric measures and imperial ones such as inches or pints are included in some reference books, or conversions can be done on-line (e.g. at http://www.onlineconversion.com/).
Metric system
Derived units
Other useful derived units are: for volume, the cubic metre (m3); for area, the square metre (m2); for temperature, degrees Celsius (°C); for mass, the gram (g, a little more than the weight of a small paper clip); and for radioactivity, the becquerel (Bq, in units s-1). Other accepted units are the minute (min), the litre (L, 1 L = 1 cubic decimetre = 1000 cm3; about 4 cups) and, for mass of atoms or molecules, the atomic mass unit (u), approximately equal to the mass of a hydrogen atom (also sometimes referred to as the dalton, Da).
The mole is the amount of any substance that contains Avogadro’s number (about 6.022 × 1023) of atoms or molecules of the substance, and is equivalent to the molecular weight expressed in grams. The mole is therefore a different weight depending on the substance; for example, one mole of sodium chloride (molecular weight 58.5) is present in 58.5 g pure NaCl and 1 mole of water in 18 g pure H2O. This unit is used mainly in laboratories and research situations, not for dosing drugs. A one molar solution (1 M = 1 mol/L) contains 1 mole of the particular solute dissolved in 1 L of the solvent (however the correct SI units for concentration are actually mol/m3 or mol/kg).
Metric prefixes
The metric system is a decimal system in which the basic units can be divided or multiplied by 10, 100 or 1000 to form a secondary unit. The names of the secondary units are formed by joining a Greek or Latin prefix to the name of the primary unit (Table 1-5); for example, the gram is the metric unit of weight commonly used in weighing chemicals and various pharmaceutical preparations. A gram is 1/1000 of a kilogram, and 1000 times greater than a milligram. Hence to change milligrams to grams, divide by 1000, and to change metres to centimetres, multiply by 100.
Table 1-5 Metric prefixes, meanings, and relations
| PREFIX | MEANING | POWER OF 10 |
| tera (T) | million millions | 1012 |
| giga (G) | billions | 109, 1 000 000 000 |
| mega (M) | millions | 106, 1 000 000 |
| kilo (k) | thousands | 103, 1 000 |
| hecto (h) | hundreds | 102, 100 |
| deca, deka (da) | tens | 101, 10 |
| deci (d) | tenths | 10−1, 1/10, 0.1 |
| centi (c) | hundredths | 10−2, 1/100, 0.01 |
| milli (m) | thousandths | 10−3, 1/1000, 0.001 |
| micro (μ or mc) | millionths | 10−6, 0.000001 |
| nano (n) | billionths | 10−9 |
| pico (p) | 10−12 | |
| femto (f) | 10−15 |
Note that the units hecto-, deca-, and centi- are not commonly used; that micro (μ) should be written out or mc used if there is possibility of confusion with ‘m’ (a mistake, e.g. dosing a patient with 250 mg digoxin instead of 250 μg, could be fatal); that the term billion may mean 109 or 1012 depending on local custom; and that a zero should be used before the decimal point if the number is <1, e.g. 0.5, not .5. There are other (less common) prefixes extending the range from 1024 to 10−24.
Conventional notations
The following is the style of notation as recommended for the International System of Units:
There are some situations in medicine in which SI units are not used. These include:
Dosage calculations
A thorough understanding of arithmetic (fractions, ratios and proportions) is necessary for correct calculations and safe drug administration. When in doubt, it is advisable to double-check all calculations with another health-care professional, especially with a pharmacist who will be highly trained in dose and concentration calculations. The examples of dose calculations at the end of this chapter are the types of problems that may be encountered. Each problem is solved in a step-wise manner and, where appropriate, helpful hints are included. (Note: for a review of arithmetic methods required in dose calculations, and more detailed explanations of methods used, see Gatford and Phillips [2006]).
Some helpful hints
Estimating can prevent mistakes!
In Example 9, the dose of pethidine required is 75 mg; your stock ampoules contain 100 mg in 2 mL, so you can estimate that there will be 75 mg in a bit less than 2 mL. If your answer comes out to 15 mL, or 0.0015 mL, you would realise that you had made a mistake (hopefully).
Rounding off
Don’t quote answers to several significant figures—calculators are very useful, but they can’t think for you! If the pethidine ampoules available in Example 9 had contained 120 mg pethidine per 2-mL ampoule, and the patient had been prescribed an 80-mg dose, you might have worked out that the 80-mg dose is contained in 2 mL ÷ 120 mg × 80 mg, and from your calculator this equals 1.3333333… mL, a volume that no syringe can measure exactly. It is usually sensible to round-off values to 2 or 3 significant figures; here, an answer of 1.3 mL is close enough.
Key points
 Pharmacology is the study of drugs, which are substances used for their beneficial effects on living systems. Useful drugs have the characteristics of potency, selectivity and specificity.
Pharmacology is the study of drugs, which are substances used for their beneficial effects on living systems. Useful drugs have the characteristics of potency, selectivity and specificity. Drugs may be solids (most commonly), liquids or gases. Most are organic (carbon-containing) chemicals. They may come from natural sources or be synthesised in laboratories.
Drugs may be solids (most commonly), liquids or gases. Most are organic (carbon-containing) chemicals. They may come from natural sources or be synthesised in laboratories. Pharmacologically active compounds derived from plants include alkaloids, glycosides, steroids, phenols and oils.
Pharmacologically active compounds derived from plants include alkaloids, glycosides, steroids, phenols and oils. People have searched for, been fascinated by, and used and abused drugs throughout recorded history; initially, natural compounds were discovered by trial and error, they were then studied for their medical actions and adverse effects.
People have searched for, been fascinated by, and used and abused drugs throughout recorded history; initially, natural compounds were discovered by trial and error, they were then studied for their medical actions and adverse effects. Now drugs and even receptors may be synthesised or genetically engineered and their mechanisms of action determined.
Now drugs and even receptors may be synthesised or genetically engineered and their mechanisms of action determined. Each drug is identified by three names: a chemical, generic (or approved) and proprietary (or trade) name. Generic prescribing is encouraged, and substitution of products considered bioequivalent is sometimes permitted.
Each drug is identified by three names: a chemical, generic (or approved) and proprietary (or trade) name. Generic prescribing is encouraged, and substitution of products considered bioequivalent is sometimes permitted. Drugs may be classified by many systems, including by chemical formula, by body system affected, by mechanism of action, by clinical use or by legal schedule.
Drugs may be classified by many systems, including by chemical formula, by body system affected, by mechanism of action, by clinical use or by legal schedule. Drug classifications help facilitate a student’s understanding of pharmacology by comparing the common characteristics of an example of a drug group or classification (the key, or prototype, drug) with those of other drugs in the same classification or category.
Drug classifications help facilitate a student’s understanding of pharmacology by comparing the common characteristics of an example of a drug group or classification (the key, or prototype, drug) with those of other drugs in the same classification or category.Review exercises
1 Calculation for oral administration of liquids
A child has been prescribed the antibiotic erythromycin 250 mg orally every 12 hours as prophylaxis for rheumatic fever. The stock liquid suspension contains 200 mg/5 mL. What volume of the mixture should be given every 12 hours?
Estimate: the child is going to need a bit more than 5 mL each dose.
1 Calculation for oral administration of tablets or capsules
The prescriber orders paracetamol 1 g orally every 6 hours for a patient with a high temperature. The label on the package states that each tablet contains 500 mg paracetamol. How many tablets do you administer to the patient every 6 hours?
1 Calculation of dosage based on body weight
A child has been prescribed the antibiotic ampicillin and the recommended dosage is 10 mg/kg every 6 hours. What will be the size of a single dose for a child weighing 30 kg?
Method 1 Calculation by formula:
The child should be given 300 mg every 6 hours.
Method 2 Calculation by simple proportion:
For 1 kg body weight, prescribe 10 mg dose.
So for 30 kg body weight, prescribe 10 mg/1 kg × 30 kg dose
1 Calculation for safe maximum dose of a local anaesthetic
The safe maximum dose (SMD) of lignocaine as a local anaesthetic in adults is set at 3 mg/kg body weight. You (a dentist) are provided with 5-mL ampoules of Lignocaine Hydrochlo ride Injection BP 2%. What is the maximum number of am poules you would expect to require for an average-sized adult patient before a dental extraction?
So the safe maximum dose will be present in 10.5 mL solution and, as the ampoules provided contain 5 mL, two ampoules (approximately 10 mL) will probably be adequate.
1 Calculation of divided doses
A child is to be given the antiprotozoal drug metronidazole to treat giardiasis. The recommended dosage is 30 mg/kg/day in three divided doses (i.e. 8-hourly). What is the size of a single dose if the child’s weight is 18 kg?
1 Conversion between weight units
An elderly patient is prescribed 0.125 mg digoxin once daily, which is available in your care facility pharmacy only as a 250 mcg tablet. How many tablets do you administer daily?
1 Calculation of dosage based on surface area
In some circumstances (e.g. critical-care situations, cancer chemo therapy), dosages of some drugs are calculated in terms of body surface area (see Chapter 9). These calculations usually involve a nomogram that relates height (or length), weight and surface area.
A woman is to be administered epirubicin for treatment of cancer. The prescribed dosage is 100 mg/m2 and her body surface area has been determined as 1.5 m2. The stock solution of epirubicin is 2 mg/mL. What volume should be drawn up for infusion?
The volume to be drawn up for injection is 75 mL. Note that this drug is available for injection in several pack sizes, ranging from 10 mg/5 mL to 50 mg/25 mL.
Helpful Hints for Example 6:
1 Calculation of drug dosage for intravenous infusion (by drip rate)
A patient has been ordered an infusion of sodium chloride and glucose, 500 mL over 24 hours. The IV infusion (giving) set delivers 20 drops/mL. At what rate (in drops/minute) should the giving set drip?
Method 1 Calculation by formula:
Step 1 Convert time to the same units, minutes:
Step 2 To calculate the drip rate, apply the formula:
The giving set should be adjusted to a drip rate of 7 drops/minute.
Method 2 Calculation by simple proportion:
The patient needs, in 1 day, 500 mL solution, i.e. in 1440 min needs 500 mL (as before), so in 1 min needs 500/1440 × 1 mL.
Each 1 mL contains 20 drops, so 500/1440 mL is contained in 20/1 × 500/1440 drops,
Therefore, the set should be adjusted to approximately 7 drops per minute.
1 Calculation of drug dosage for injection
A patient has been ordered 75 mg pethidine for pain relief. The ampoules available to you contain 100 mg in 2 mL. What volume is required for injection?
| Step 1 | Conversion is not required because in this example the units of weight are the same, mg. The strength of the solution in the ampoules is 100/2 mg/mL = 50 mg/mL. |
| Step 2 | To calculate the volume to be drawn up for injection, apply the formula: |
The volume to be drawn up for injection is 1.5 mL from the 2-mL ampoule.
1 Bolus doses from infusion pumps
A palliative care patient is self-administering patientcontrolled analgesia via an SC syringe pump, from a solution of fentanyl 300 mcg/30 mL normal saline, set for bolus doses of 1 mL. (a) What amount of fentanyl does she receive in each bolus dose? (b) If she administers 6 bolus doses in an hour, what is the fentanyl dose rate in mcg/minute? (c) If the pump is re-set to deliver 5 mL per hour, what dose rate will she receive? (d) At what rate should the pump be set to deliver an approximately equivalent dose to a 75-mcg/h patch?
Helpful Hints for Example 9:
1 Calculation of drug dosage for intravenous infusion (by infusion pump)
Example 11(a) For the same patient as in Example 8, an infusion pump becomes available. At what rate should the pump be set to deliver the sodium chloride/glucose solution?
| Step 1 | The calculation here is much simpler, and can be done by simple proportions, as we know that 500 mL need to be delivered over 24 hours. |
The infusion pump should be set to deliver approximately 21 mL/hour.
Example 11(b) The patient (a man weighing 80 kg) is then prescribed gentamicin 3 mg/kg/day in 3 doses/day by IV infusion, given every 8 hours over a 2-hour period. Each dose is to be diluted in 100 mL sterile normal saline. How should the infusion pump be set?
Method 1 Calculation by first principles:
Dose is to be diluted in 100 mL saline. Gentamicin Injection BP is provided as vials containing 80 mg/2 mL, so 1 vial contains 1 dose.
For each dose, contents of 1 vial are diluted to 100 mL in normal saline, giving 80 mg/100 mL, i.e. 0.8 mg/mL.
Solution is to be run in IV over 2 hours, i.e. 100 mL/2 h, so pump is set at an infusion rate of 50 mL/h.
Method 2 Calculation by formula:
From Method 1, each dose = 80 mg over 2 h = 40 mg/h, in a volume of 100 mL for a dose of 80 mg.
1 Calculation using strength of a solution
An ophthalmologist has prescribed timolol eye-drops 5 mg/mL, one drop in the affected eye twice daily. You (a pharmacist) have available timolol drops in 0.25% strength or 0.5% strength. What do you dispense?
Method 1 Check out the 0.25% drops:
| Step 1 | Convert the concentration of the stock solution to mg/mL: |
| Step 2 | To calculate mg/mL, apply the formula: |
| Step 3 | To calculate the number of drops required, apply the formula: |
The patient would have to instil two drops in the affected eye twice daily; however, note that two drops do not usually fit in the conjunctival sac (as discussed in Chapter 31), so check the 0.5% solution.
Method 2 Check out the 0.5% drops:
This is the strength prescribed by the doctor, so you dispense this strength and label the bottle as directed.
American Pharmacists Association. Handbook of Nonprescription Drugs: An Interactive Approach to Self-Care, 16th edn. Washington, DC: American Pharmacists Association; 2009.
Anonymous. Top 10 drugs. Australian Prescriber. 2009;32(6):159.
Aslani P. Consumer medicine information conundrums. Australian Prescriber. 2007;30(5):122-124.
Australian Medicines Handbook 2009. Adelaide: AMH, 2009.
Blainey G. Pharmacy 40, 000 Years Ago. Melbourne: Pharmaceutical Society of Victoria; 1977.
Bowman W.C., Rand M.J. Textbook of Pharmacology, 2nd edn. Oxford: Blackwell; 1980. [chs 7, 16]
British Medical Association, Royal Pharmaceutical Society of Great Britain. British National Formulary 58. London: BMJ Books; 2009.
British Pharmacopoeia Commission. British Pharmacopoeia 2010. London: Her Majesty’s Stationery Office; 2010. (5 volumes printed, also available as CD-ROM and in on-line format)
Chivian E., Bernstein A. Sustaining Life: How Human Health Depends on Biodiversity. USA: Oxford University Press; 2008.
Clauson K.A., Polen H.H., Kamel Boulos M.N., Dzenowagis J.H. Scope, completeness, and accuracy of drug information in Wikipedia. Annals of Pharmacotherapy. 2008;42(12):1814-1821.
Collins D.J., Culvenor C.C.J., Lamberton J.A., et al. Plants for Medicines: A Chemical and Pharmacological Survey of Plants in the Australian Region. Australia: CSIRO; 1990.
Cribb J.W. Australia’s medicinal plants. Medical Journal of Australia. 1985;143:574-577.
Duffin J. History of Medicine: A Scandalously Short Introduction. Toronto: University of Toronto Press; 2000.
Dunlop C. Atropt – Azopt substitution. Australian Prescriber. 2009;32(5):138-139.
European Directorate for the Quality of Medicines (Council of and Europe). European Pharmacopoeia, 6th edn. Strasbourg: EDQM; 2007.
Evans W.C. Trease and Evans’ Pharmacognosy, 15th edn. Edinburgh: Saunders; 2002. [ch 6]
Gatford J.D., Phillips N. Nursing Calculations, 7th edn. Edinburgh: Churchill Livingstone; 2006.
Gaut B. Defining moments in medicine: a golden age defined, fifty years of medical advances. Medical Journal of Australia. 2001;174(1):8.
Le Fanu J. The Rise and Fall of Modern Medicine. London: Little Brown; 1999.
Longmore M., Wilkinson I.B., Turmezei T., Cheung C.K., Smith E. Oxford Handbook of Clinical Medicine, 7th edn. Oxford: Oxford University Press; 2008.
McLachlan A.J., Ramzan I., Milne R.W. Frequently asked questions about generic medicines. Australian Prescriber. 2007;30(2):41-43.
Magner L.N. A History of Medicine. New York: Marcel Dekker; 1992.
Mann J. Murder, Magic and Medicine. Oxford: Oxford University Press; 1992.
O’Neil M.J., et al, editors. The Merck Index: An Encyclopedia of Chemicals, Drugs and Biologicals, 14th edn, Whitehouse Station, NJ: Merck, 2006.
Parker G.B. Would the pharmaceutical companies please mind their Ps and Qs, and their Xs, Ys and Zs. Medical Journal of Australia. 2000;173:662-663.
Pharmaceutical Management Agency (Pharmac), New Zealand. Annual Review 2007. Online. (http://www.pharmac.govt.nz/2007/11/21/PHARMAC_AR_2007.pdf)
Pharmaceutical Society of Australia. Australian Pharmaceutical Formulary and Handbook, 21st edn. Deakin, ACT: PSA; 2009.
Pharmacy Department Royal Children’s Hospital Melbourne. Paediatric Pharmacopoeia, 13th edn. Fitzroy: Arena Printing; 2002.
Smith A. It’s natural so it must be safe. Australian Prescriber. 2002;25(3):50-51.
Sweetman S.C., editor. Martindale: The Complete Drug Reference, 36th edn, London: Pharmaceutical Press, 2009.
Thomas J., editor. Australian Prescription Products Guide, 36th edn, Melbourne: Australian Pharmaceutical Publishing, 2007.
Tiziani A. Havard’s Nursing Guide to Drugs, 8th edn. Sydney: Mosby-Elsevier; 2009.
United States Pharmacopeial Convention. United States Pharmacopeia (USP), 33rd edn. Rockville, MD: USP Convention; 2010.
United States Pharmacopeial Convention. United States National Formulary, 28th edn. Rockville, MD: USP Convention; 2010.
Various authors. Articles on Australian industries in alkaloids, pyrethrums and marine-derived drugs. Chemistry in Australia. 1989;56(10):348-361.
World Health Organization. WHO Model List of Essential Medicines, 16th list. Geneva: WHO; 2009. March 2009
Adverse Drug Reactions Advisory Committee (ADRAC), Australian Adverse Drug Reactions Bulletin. Canberra: ADRAC: www.tga.gov.au/adr/aadrb.htm
Australian Pharmaceutical Formulary 21st edn, 2009 (APF): www.psa.org.au/site.php?id=1805
Australian Prescriber: www.australianprescriber.com/
British National Formulary: bnf.org/bnf/
British Pharmacopoeia: www.pharmacopoeia.co.uk/
Cane toads, toxicology and chemistry: www.raci.org.au/chemaust/docs/pdf/2009/CiA_May09p3.pdf
Cochrane Library: www.cochrane.org
European Pharmacopoeia: europa.eu/legislation_summaries/internal_market/single_market_for_goods/pharmaceutical_and_cosmetic_products/l21161_en.htm
Handbook of Nonprescription Drugs: www.otchandbook.com/16th_Edition_Home.aspx
International Non-Proprietary Names: www.who.int/medicines/services/inn/en/index.html
Martindale: The Complete Drug Reference: www.pharmpress.com/then search by keyword‘Martindale’
Medimate – NPS: www.nps.org.au/consumers/tools__and__tips/medimate
Medsafe (NZ): www.medsafe.govt.nz/
National Prescribing Service (NPS): www.nps.org.au/plus. Therapeutic Advice and Information Service for health.professionals at (ph) 1300 138 677 or (e-mail) tais@nps.org.au
NPS Medimate: www.nps.org.au/consumers/tools__and__tips/medimate
Pharmaceutical Management Agency, NZ (PHARMAC): www.pharmac.govt.nz/
Paediatric Pharmacopoeia of Royal Children’s Hospital, Melbourne: www.rch.org.au/pharmacy/dev/index.cfm?doc_id=11341
Therapeutic Guidelines: www.tg.org.au/
United States Pharmacopeia (USP): www.usp.org/aboutUSP/
WHO Essential Medicines List: www.who.int/medicines/publications/essentialmedicines/en/index.html
More weblinks at: evolve.elsevier.com/AU/Bryant/pharmacology/