Chapter 29 NURSING ASSESSMENT: haematological system
1. Outline the structures and functions of the haematological system.
2. Differentiate among the different types of blood cells and their functions.
3. Explain the process of haemostasis.
4. Describe the age-related changes in the haematological system and differences in haematological studies.
5. Outline the significant subjective and objective assessment data related to the haematological system that should be obtained from a patient.
6. Discuss the components of a physical assessment of the haematological system.
7. Differentiate normal from common abnormal findings of a physical assessment of the haematological system.
8. Outline the purpose, significance of results and nursing responsibilities related to diagnostic studies of the haematological system.
Haematology is the study of blood and blood-forming tissues. This includes the bone marrow, blood, spleen and lymph system. A basic knowledge of haematology is useful in clinical settings to evaluate the patient’s ability to transport oxygen and carbon dioxide, coagulate blood and combat infections. Assessment of the haematological system is based on the patient’s health history, physical examination and results of diagnostic studies.
Structures and functions of the haematological system
BONE MARROW
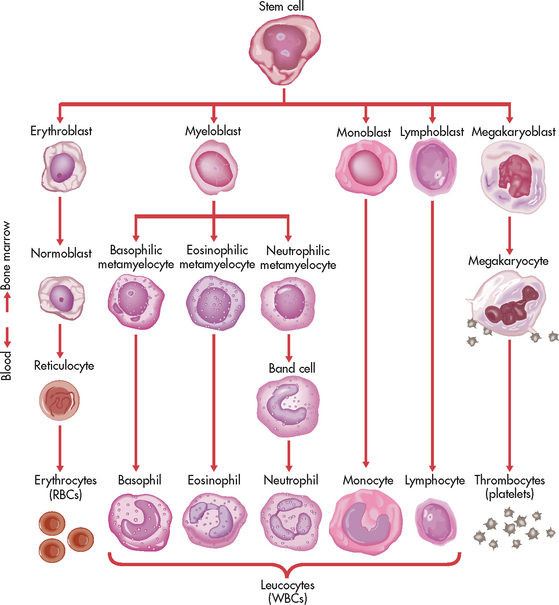
Blood cell production (haematopoiesis) occurs within the bone marrow. Bone marrow is the soft material that fills the central core of bones. Although there are two types of bone marrow (yellow [adipose] and red [haematopoietic]), it is the red marrow that actively produces blood cells. In the adult, the red marrow is found primarily in the flat and irregular bones, such as the ends of long bones, pelvic bones, vertebrae, sacrum, sternum, ribs, flat cranial bones and scapulae.
All three types of blood cells (red blood cells [RBCs], white blood cells [WBCs] and platelets) develop from a common haematopoietic stem cell within the bone marrow. The haematopoietic stem cell is best described as a non-differentiated immature blood cell found in the bone marrow. As the cells mature and differentiate, several different types of blood cells are formed (see Fig 29-1). The marrow is able to respond to increased demands for various types of blood cells by increasing production via a negative feedback system. The bone marrow is stimulated by various factors (e.g. erythropoietin), which cause the stem cells to differentiate into one of the committed haematopoietic cells (e.g. RBC).
BLOOD
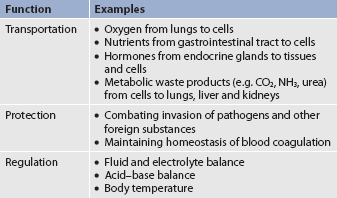
Blood is a type of connective tissue that performs three major functions: transportation, regulation and protection (see Table 29-1). Blood is responsible for the transportation of oxygen, nutrients, hormones and waste products around the body. Blood also plays a role in the regulation of fluids, electrolytes and the acid–base balance. Finally, the blood has a protective role in its ability to clot and combat infections. There are two major components to blood: plasma and blood cells.
Plasma
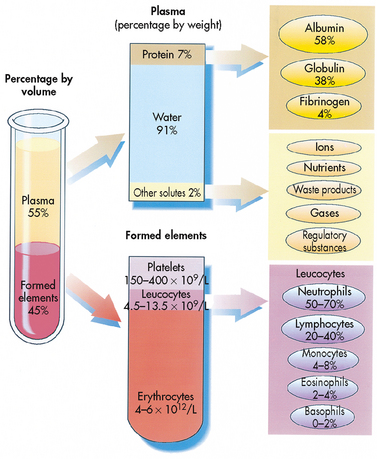
Approximately 55% of blood is plasma (see Fig 29-2). Plasma is composed primarily of water, but it also contains proteins, electrolytes, gases, nutrients and waste. The term serum refers to plasma minus its clotting factors.1 Plasma proteins include albumin, globulin and clotting factors, mostly fibrinogen.
Blood cells
About 45% of the blood (see Fig 29-2) is composed of formed elements or blood cells. There are three types of blood cells: erythrocytes (RBCs), leucocytes (WBCs) and thrombocytes (platelets). The primary function of erythrocytes is oxygen transportation, whereas leucocytes are involved in protection of the body from infection. Platelets function to promote blood coagulation.
Erythrocytes
The primary functions of RBCs include the transport of gases (both oxygen and carbon dioxide) and assistance in maintaining the acid–base balance. The composition and features of an erythrocyte are ideal for its gas transportation role. It is a flexible cell with a unique biconcave shape (see Fig 29-3). Flexibility enables the cell to alter its shape so that it can easily pass through tiny capillaries. The cell membrane is very thin to facilitate the diffusion of gases. Erythrocytes are primarily composed of a large molecule called haemoglobin. Haemoglobin, a complex protein–iron compound composed of haem (an iron compound) and globin (a simple protein), functions to bind with oxygen and carbon dioxide. As RBCs circulate through the capillaries surrounding alveoli within the lungs, oxygen attaches to the iron on the haemoglobin. The oxygen-bound haemoglobin is referred to as oxyhaemoglobin and is responsible for giving arterial blood its bright-red appearance. As RBCs flow to body tissues, oxygen detaches from the haemoglobin and diffuses from the capillaries into tissue cells. Carbon dioxide diffuses from tissue cells into the capillaries, attaches to the globin portion of haemoglobin and is transported to the lungs for removal. Haemoglobin also acts as a buffer and plays a role in maintaining the acid–base balance. This buffering function is described further in Chapter 16.
Erythropoiesis (the process of RBC production) is regulated by cellular oxygen requirements and general metabolic activity. Erythropoiesis is stimulated by hypoxia and controlled by erythropoietin, a glycoprotein growth factor synthesised and released by the kidneys. Erythropoietin stimulates the bone marrow to increase erythrocyte production. Normally the bone marrow releases 3 × 109 RBCs/kg body weight per day. Erythropoiesis is also influenced by the availability of nutrients. Many essential nutrients are necessary for erythropoiesis, including protein, iron, folate (folic acid), vitamin B12, riboflavin (vitamin B2) and vitamin B6.1 In addition, erythrocyte production is affected by endocrine hormones, such as thyroxine, corticosteroids and testosterone. For example, hypothyroidism can be the cause of a microcytic anaemia.2
Several distinct cell types evolve during erythrocyte maturation (see Fig 29-1). The reticulocyte is an immature erythrocyte. The reticulocyte count measures the rate at which new RBCs appear in the circulation. Reticulocytes can develop into mature RBCs within 48 hours of their release into the circulation. Therefore, assessing the number of reticulocytes is a useful means of evaluating the rate and adequacy of erythrocyte production.
Haemolysis (destruction of RBCs) by monocytes and macrophages removes abnormal, defective, damaged and old RBCs from circulation. Haemolysis normally occurs in the bone marrow, liver and spleen. Haemolysis of RBCs results in increased bilirubin to be processed by the body. When haemolysis occurs via normal mechanisms, the liver is able to conjugate and excrete all bilirubin that is released (see Fig 38-6). The normal life span of an erythrocyte is 120 days.
Leucocytes
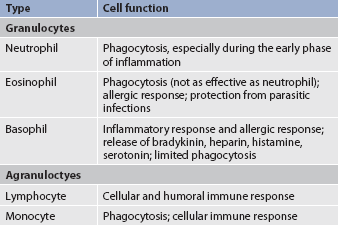
Leucocytes (WBCs) appear white when separated from blood. Like RBCs, leucocytes originate from stem cells within the bone marrow (see Fig 29-1). There are five different types of leucocyte, each of which has a different function. Leucocytes containing granules within the cytoplasm are called granulocytes (also known as polymorphonuclear leucocytes) and there are three types of granulocytes, namely neutrophils, basophils and eosinophils. Leucocytes that do not have granules within the cytoplasm are called agranulocytes and there are two types: lymphocytes and monocytes (see Table 29-2). Lymphocytes and monocytes are also referred to as mononuclear cells because they have only one discrete nucleus. Leucocytes have a widely variable life span. Granulocytes may live for only a few hours, whereas some T lymphocytes may live for years.
Granulocytes
The primary function of the granulocytes is phagocytosis, a process by which WBCs ingest or engulf any unwanted organism and then digest and kill it. The neutrophil is the most common type of granulocyte, accounting for 50–70% of all WBCs. Neutrophils are the primary phagocytic cells involved in acute inflammatory responses. A mature neutrophil is called a segmented neutrophil or ‘seg’ or ‘polysegmented neutrophil’ because the nucleus is segmented into two to five lobes connected by strands. An immature neutrophil is called a band (for the band appearance of the nucleus). Although band cells are sometimes found in the peripheral circulation of normal persons and are capable of phagocytosis, the mature neutrophil is much more effective.
Eosinophils account for only 2–4% of all WBCs. They have a similar but reduced role to neutrophils in phagocytosis. One of their primary functions is to engulf antigen–antibody complexes formed during an allergic response. They are also able to defend against parasitic infections.
Basophils make up less than 2% of all WBCs. They have a limited role in phagocytosis. These cells have cytoplasmic granules that contain heparin, serotonin and histamine. If a basophil is stimulated by an antigen or by tissue injury, it will respond by releasing substances within the granules. This is part of the response seen in allergic and inflammatory reactions.
Lymphocytes
Lymphocytes, which are agranular leucocytes, constitute 20–40% of the WBCs. The main function of lymphocytes is related to the immune response (see Ch 14). Lymphocytes form the basis of the cellular and humoral immune responses. Two lymphocyte subtypes are B cells and T cells. Although T cell precursors originate in the bone marrow, these cells migrate to the thymus gland for further differentiation into T cells. (Details of lymphocyte function are presented in Ch 13.)
Monocytes
Monocytes, the other type of agranular leucocytes, account for approximately 4–8% of the WBCs. Monocytes are potent phagocytic cells. They can ingest small or large masses of matter, such as bacteria, dead cells, tissue debris and old or defective RBCs. Monocytes are the second type of WBCs to arrive at the scene of an injury. These cells are present in the blood for only a short time before they migrate into the tissues and become macrophages (see Ch 12). In addition to macrophages that have differentiated from monocytes, resident macrophages can also be found in tissues. These resident macrophages are given special names (e.g. Kupffer cells in the liver, osteoclasts in the bone, alveolar macrophages in the lung). These macrophages protect the body from pathogens at these entry points and are more phagocytic than monocytes. Macrophages also interact with lymphocytes to facilitate the humoral and cellular immune responses (see Ch 13).
Thrombocytes
The primary function of thrombocytes, or platelets, is to initiate the clotting process by producing an initial platelet plug in the early phases of the clotting process. Platelets must be available in sufficient numbers and must be structurally and metabolically sound for blood clotting to occur. Platelets maintain capillary integrity by working as ‘plugs’ to close any openings in the capillary wall. At the site of any capillary damage, platelet activation is initiated. Increasing numbers of platelets accumulate to form an initial platelet plug that is stabilised with clotting factors. Platelets are also important in the process of clot shrinkage and retraction.
Platelets, like other blood cells, originate from stem cells within the bone marrow (see Fig 29-1). The stem cell undergoes differentiation by transforming into a megakaryocyte, which produces platelets. Platelet production is partly regulated by thrombopoietin, a growth factor acting on bone marrow to stimulate platelet production. Typically, platelets have a life span of only 5–9 days.
NORMAL IRON METABOLISM
Iron (Fe) is obtained from food and dietary supplements. Approximately 1 mg of every 10–20 mg of iron ingested is absorbed in the duodenum and upper jejunum. Therefore, only 5–10% of ingested iron is absorbed.
Iron is present in all RBCs as haem in haemoglobin and in a stored form. The haem in haemoglobin accounts for two-thirds of the body’s iron. The other one-third is stored as ferritin and haemosiderin (degraded form of ferritin) in the bone marrow, spleen, liver and macrophages (see Fig 29-4). When the stored iron is not replaced, haemoglobin production is reduced.
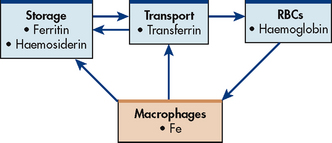
Figure 29-4 Normal iron (Fe) metabolism. Macrophages break down ingested red blood cells (RBCs). Iron is returned to blood bound to transferrin or stored as ferritin or haemosiderin.
Transferrin, which is synthesised in the liver, serves as a carrier plasma protein for iron. The degree to which transferrin is saturated with iron is a reliable indicator of the iron supply for developing RBCs.
As part of normal iron metabolism, iron is recycled after macrophages in the liver and spleen phagocytose, or ingest and destroy, old and damaged RBCs. Iron binds to transferrin in the plasma or is stored as ferritin or haemosiderin (see Fig 29-4).
NORMAL CLOTTING MECHANISMS
Haemostasis is the term used to describe the blood clotting process. This process is important in minimising blood loss when various body structures are injured. Four components contribute to normal haemostasis: the vascular response, platelet plug formation, the development of the fibrin clot on the platelet plug by plasma clotting factors and the ultimate lysis of the clot.
Vascular response
When a blood vessel is injured, an immediate local vasoconstrictive response occurs. Vasoconstriction reduces the leakage of blood from the vessel not only by restricting the vessel size but also by pressing the endothelial surfaces together. The latter reaction enhances vessel wall stickiness and maintains closure of the vessel even after the vasoconstriction subsides. Vascular spasm may last for 20–30 minutes. This allows time for the platelet response and plasma clotting factors to be activated, which are triggered by endothelial injury and the release of substances such as tissue factor (TF).3
Platelet plug formation
Platelets are activated when they are exposed to interstitial collagen from an injured blood vessel. Platelets stick to one another and form clumps. The stickiness is termed adhesiveness and the formation of clumps is termed aggregation or agglutination. When a blood vessel is injured, the circulating platelets are exposed to the collagen from the inner lining of the vessel (i.e. the endothelium). This interaction causes the platelets to release substances such as platelet factor 3 and serotonin, which facilitate coagulation. At the same time, platelets release adenosine diphosphate, which increases platelet adhesiveness and aggregation, thereby enhancing the formation of a platelet plug. Aspirin acts as a platelet aggregation inhibitor and is frequently prescribed for those with coronary heart disease.
In addition to their independent contribution to clotting, platelets also facilitate the reactions of the plasma clotting factors. As Figure 29-5 shows, platelet lipoproteins stimulate necessary conversions in the clotting process.
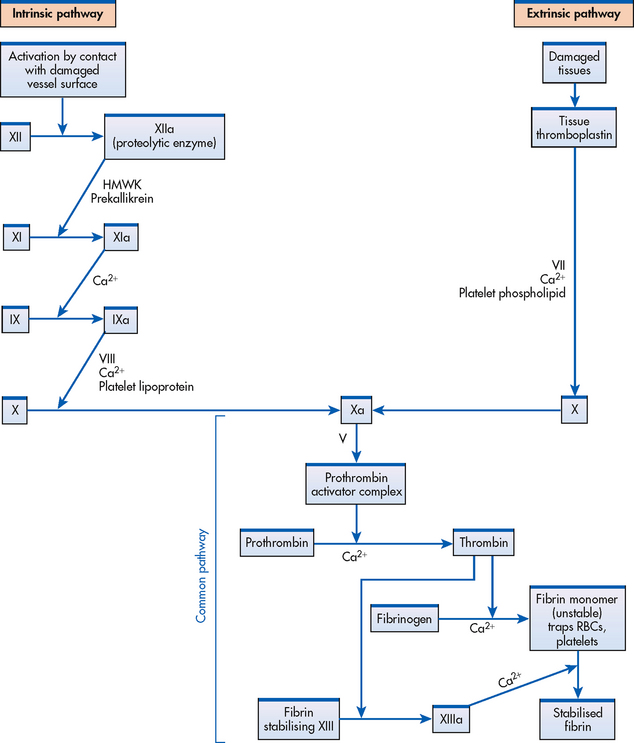
Plasma clotting factors
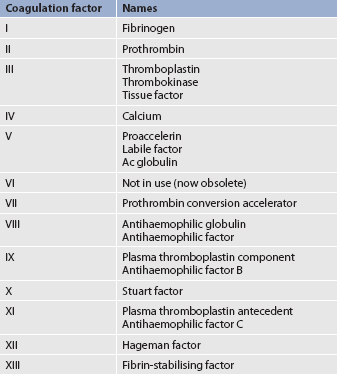
The formation of a visible fibrin clot on the platelet plug is the conclusion of a complex series of reactions involving different clotting factors. The plasma clotting factors are labelled with both names and Roman numerals (see Table 29-3). Plasma proteins circulate in inactive forms until stimulated to initiate clotting through one of two pathways: intrinsic or extrinsic. The intrinsic pathway is activated by collagen exposure from endothelial injury when the blood vessel is damaged. The extrinsic pathway is initiated when tissue factor or tissue thromboplastin is released extra-vascularly from injured tissues. In addition, von Willebrand factor (vWF) is important in forming an adhesive bridge between platelets and vascular subendothelial structures. It is synthesised in endothelial cells and megakaryocytes and acts as a carrier for factor VIII.4
Regardless of whether clotting is initiated by substances internal or external to the blood vessel, coagulation ultimately follows the same final common pathway of the clotting cascade. Thrombin, in the common pathway, is the most powerful enzyme in the coagulation process (see Fig 29-6). It converts fibrinogen to fibrin, which is an essential component of a blood clot.
Lysis of clots
Just as some blood elements foster coagulation (procoagulants), others interfere with clotting (anticoagulants). This counter-mechanism to blood clotting serves to keep blood in its fluid state. Anticoagulation may be achieved by two means: antithrombins and fibrinolysis. As the name implies, antithrombins keep blood in a fluid state by antagonising thrombin, a powerful coagulant. Endogenous heparin is an example of an anticoagulant. Other anticoagulants are protein C and protein S.
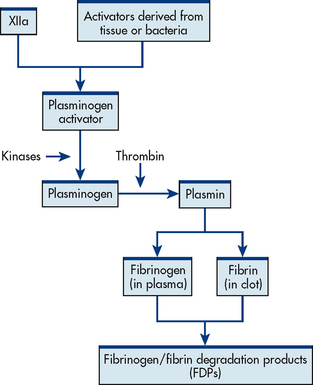
The second means of maintaining blood in its fluid form is fibrinolysis, a process resulting in the dissolution of the fibrin clot. The fibrinolytic system is initiated when plasminogen is activated to plasmin (see Fig 29-6). Thrombin is one of the substances that can activate the conversion of plasminogen to plasmin, thereby promoting fibrinolysis. The plasmin attacks either fibrin or fibrinogen by splitting the molecules into smaller elements known as fibrin split products (FSPs) or fibrin degradation products (FDPs). (More information about FSPs can be found in Table 29-9 and in the discussion of disseminated intravascular coagulation in Ch 30.)
| Study | Description and purpose | Normal values |
|---|---|---|
| Platelet count | Count of number of circulating platelets | 150–400 × 109/L |
| Prothrombin time (PT) | Assessment of extrinsic coagulation by measurement of factors I, II, V, VII, X | 12–15 s |
| International normalised ratio (INR) | Standardised system of reporting PT based on a reference calibration model and calculated by comparing the patient’s PT with a control value | 2-3* |
| Activated partial thromboplastin time (APTT) | Assessment of intrinsic coagulation by measuring factors I, II, V, VIII, IX, X, XI, XII; longer with use of heparin | 25–35 s |
| Automated coagulation time (ACT) | Evaluation of intrinsic coagulation status; more accurate than APTT; used during dialysis, coronary artery bypass procedures, arteriograms | 150–180 s |
| Thromboplastin generation test (TGT) | Reflection of generation of thromboplastin; if abnormal, second stage done to identify missing coagulation factor | <12 s (100%) |
| Bleeding time | Measurement of timed small skin incision bleeds; reflection of ability of small blood vessels to constrict | 1–6 min (usually <9 min) |
| Thrombin time | Reflection of adequacy of thrombin; prolonged thrombin time indicates that coagulation is inadequate secondary to decreased thrombin activity | 14–16 s |
| Fibrinogen | Reflection of level of fibrinogen; increase in fibrinogen is possible indication of enhanced fibrin formation, making patient hypercoagulable; decrease in fibrinogen indicates that patient possibly is predisposed to bleeding | (1.5–4 g/L) |
| Fibrin split products (FSPs)† | Reflection of degree of fibrinolysis and predisposition to bleed (if present); screening test for DIC. Elevated levels associated with DIC, advanced malignancy, severe inflammation | < 10μg/mL |
| D-dimer | Assay to measure a fragment of fibrin that is formed as a result of fibrin degradation and clot lysis; used in diagnosis of hypercoagulable conditions (e.g. DIC) | <250 μg/L |
| Antithrombin III (AT-III) | Naturally occurring protein synthesised by liver that inhibits coagulation through inactivation of thrombin and other factors. Is depleted in DIC | 210–300 mg/L or 85–115% of standard |
| Clot retraction | Reflection of clot shrinkage or retraction from sides of test tube after 24 h; used to confirm a platelet problem | 50–100% in 24 h |
| Capillary fragility test (tourniquet test, Rumpel-Leede test) | Reflection of capillary integrity when positive or negative pressure is applied to various areas of the body; positive test indicates thrombocytopenia, toxic vascular reactions | No petechiae or negative |
| Protamine sulfate tests | Reflection of presence of fibrin monomer (portion of fibrin remaining after elements that polymerise and stabilise clot detach); positive test indicates predisposition to bleed and possible presence of DIC | Negative |
DIC, disseminated intravascular coagulation.
*Desired level for anticoagulation regimens.
†also called fibrin degradation products (FDPs).
If fibrinolysis is excessive, the patient will be predisposed to bleeding. In such a situation, bleeding results from the destruction of fibrin in platelet plugs or from the anticoagulation effects of increased FSPs. Increased FSPs lead to impaired platelet aggregation, reduced prothrombin and an inability to stabilise fibrin.
SPLEEN
Another component of the haematological system is the spleen, which is located in the upper left quadrant of the abdomen. The functions of the spleen can be classified into four major functions: haematopoietic, filtration, immunological and storage. The haematopoietic function is manifested by the spleen’s ability to produce RBCs during fetal development. The filtration function is demonstrated by the spleen’s ability to remove old and defective RBCs from the circulation through the mononuclear phagocyte system. Filtration also involves the reuse of iron. The spleen is able to catabolise haemoglobin released by haemolysis and return the iron component of the haemoglobin to the bone marrow for reuse. The spleen also plays an important role in filtering circulating bacteria, especially encapsulated organisms such as Gram-positive cocci. The immunological function is demonstrated by the spleen’s rich supply of lymphocytes, monocytes and stored immunoglobulins. The storage function is reflected in its role as a storage site for RBCs and platelets. Approximately 30% of the platelet mass is stored in the spleen.
LYMPH SYSTEM
The lymph system, consisting of lymph fluid, lymphatic capillaries, ducts and lymph nodes, carries fluid from the interstitial spaces to the blood. It is by means of the lymph that proteins and fat from the gastrointestinal (GI) tract and certain hormones are able to return to the circulatory system. The lymph system also returns excess interstitial fluid to the blood, which is important in preventing the development of oedema.
Lymph fluid is pale yellow interstitial fluid that has diffused through lymphatic capillary walls. It circulates through a special vasculature, much as blood moves through blood vessels. The formation of lymph fluid increases when interstitial fluid increases, thereby forcing more fluid into the lymph system. When too much interstitial fluid develops or when something interferes with the reabsorption of lymph, lymphoedema develops. Lymphoedema that occurs as a complication of mastectomy or lumpectomy with dissection of auxiliary nodes is often caused by the obstruction of lymph flow due to the removal of lymph nodes.
The lymphatic capillaries are thin-walled vessels that have an irregular diameter. They are somewhat larger than blood capillaries and do not contain valves. Lymphatic capillaries unite to form lymphatic vessels (also called lymphatic channels) that carry all lymph fluid to either the right lymphatic duct or the thoracic duct. These large lymphatic ducts drain into subclavian veins in the neck (see Fig 29-7).
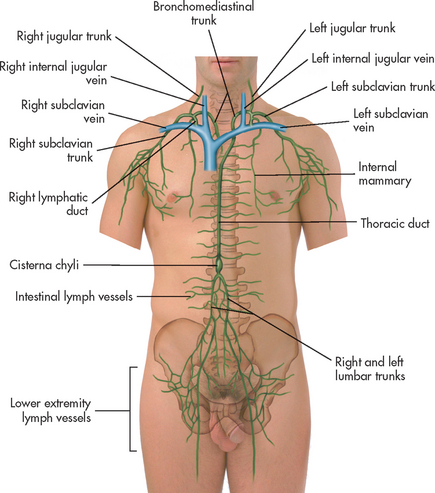
Figure 29-7 Lymphatic drainage. Lymph fluid drains from tiny lymph vessels. Lymph vessels drain into large lymph ducts. Lymph ducts merge into the venous system at the subclavian veins.
The lymph nodes are also a part of the lymphatic system. Structurally, the nodes are small clumps of lymphatic tissue and are found in groups along lymph vessels at various sites. There are more than 200 lymph nodes throughout the body, with the greatest predominance being in the abdomen surrounding the GI tract. Lymph nodes are situated both superficially and deep. The superficial nodes can be palpated, but evaluation of the deep nodes requires radiological examination.5 A primary function of lymph nodes is filtration of pathogens and foreign particles that are carried by lymph to the nodes.
LIVER
The liver functions as a filter. It also produces all the procoagulants that are essential to haemostasis and blood coagulation. Additionally, it stores iron that is in excess of tissue needs. Hepcidin, produced by the liver, is a key regulator of iron balance. The synthesis of hepcidin is stimulated by iron overload or inflammation.6 Other functions of the liver are described in Chapter 43.
Gerontological considerations: effects of ageing on the haematological system
Physiological ageing is a gradual process that involves cell loss and organ atrophy. The amount of red marrow and the number of stem cells decrease with ageing. However, there does not appear to be a complete depletion even in very old adults.7 The remaining stem cells maintain their functional capacity to divide, but they decrease in number because they are gradually replaced by non-functional fat cells. Although the older adult is still capable of maintaining adequate blood cell levels, the reserve capacity leaves the older adult more vulnerable to possible problems with clotting, oxygen transport and fighting infection, especially during periods of increased demand. This results in the diminished ability of the older adult to compensate for an acute or a chronic illness.7
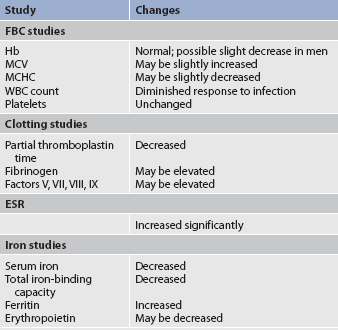
Haemoglobin levels begin to decrease in both men and women after middle age, with low normal levels seen in most older people. Estimates of the prevalence of anaemia in older people range from a low of 2% among the upper socioeconomic level of independently living elderly to a high of 40% among institutionalised older people.8 Although iron deficiency is usually responsible for the low haemoglobin levels, the cause of anaemia in many older patients is unknown. However, haematopoietic defects in the older adult during illness may in part be explained by impaired production of growth factors (e.g. erythropoietin, granulocyte–macrophage colony-stimulating factor [GM-CSF]) and thus a reduced response of haematopoiesis in illness.9
Iron absorption is not impaired in the older patient, but adequate nutritional intake of iron may be decreased. It is essential to assess for signs of disease processes such as GI bleeding before concluding that decreased haemoglobin levels are caused solely by ageing.
The osmotic fragility of RBCs is increased in the older person. This may account for a slight increase in mean corpuscular volume (MCV) and a slight decrease in mean corpuscular haemoglobin concentration (MCHC) of RBCs in some older individuals.
The total WBC count and differential are generally not affected by ageing.7 Leucocyte function is also well preserved. However, during an infection, the older adult may have only a minimal elevation in the total WBC count. These laboratory findings suggest a diminished bone marrow reserve of granulocytes in older adults and reflect the possible impaired stimulation of haematopoiesis. Platelets are unaffected by the ageing process. However, changes in vascular integrity from ageing can manifest as easy bruising.
The effects of ageing on haematological studies are listed in Table 29-4 and immune changes related to ageing in Chapter 13.
Assessment of the haematological system
Much of the evaluation of the haematological system is based on a thorough health history. Consequently, the nurse must be knowledgeable about what to include in the health history so that questions may be phrased in a manner eliciting the most information related to the haematological problem. Key questions to ask a patient with a haematological problem are presented in Table 29-5.
SUBJECTIVE DATA
Important health information
Past health history
It is important to learn whether the patient has had prior haematological problems. The nurse needs to conduct a comprehensive and systematic nursing assessment. Specifically, the nurse should ask about previous problems with anaemia, bleeding disorders and blood diseases such as leukaemia. Other related medical conditions—such as malabsorption, or liver (e.g. hepatitis, cirrhosis), kidney or spleen disorders—should also be documented. Patients may have received a kidney transplant, lost a spleen to traumatic injury or have a history of intravenous drug use that may affect their risk for haematological disorders. A history of recurrent infections or problems with blood clotting is also important to note.
Medications
A complete medication history of prescription and over-the-counter drugs is important in a haematological assessment. The use of vitamins, herbal products or dietary supplements should specifically be addressed as many patients may not consider these to be drugs. Many drugs may interfere with normal haematological function (see Table 29-6). Herbal therapy can interfere with clotting (see the Complementary & alternative therapies box in Ch 37). Antineoplastic agents used to treat malignant disorders (see Ch 15) and antiretroviral agents used to treat HIV infection (see Ch 14) may cause depression of the bone marrow (see Ch 15). A patient previously treated with chemotherapy agents, particularly alkylating agents, is at a higher risk of developing a secondary malignancy of leukaemia or lymphoma.
TABLE 29-6 Drugs affecting haematological function and laboratory values*
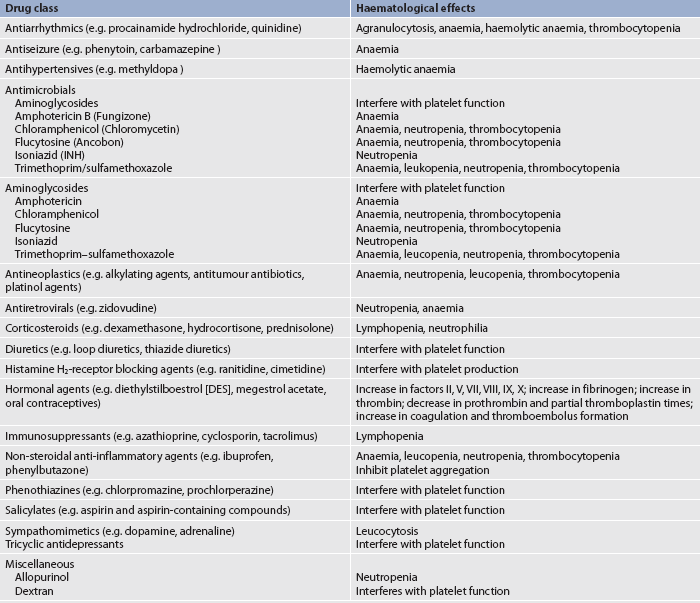
* This represents only a partial listing of drugs affecting the haematological system.
Surgery or other treatments
Specific past surgical procedures to ask the patient about include splenectomy, tumour removal, prosthetic heart valve placement, surgical excision of the duodenum (where iron absorption occurs), partial or total gastrectomy (which removes parietal cells, thus reducing intrinsic factor needed for the absorption of vitamin B12) and ileal (where vitamin B12 absorption takes place) resection. The nurse should ascertain how wound healing progressed postoperatively and if and when any bleeding problems occurred in relation to the surgery. Wound healing and bleeding should be discussed as responses to past injuries (including minor trauma) and to dental extractions. The number of previous blood transfusions and possible complications during administration should also be determined.
Functional health patterns
Health perception–health management pattern
The patient should be asked to describe the usual and present state of health. To assist the patient in maintaining optimal health, it is important to identify health perceptions, health practices and preventive practices.
Complete biographical data are needed, including age, sex, race and ethnic background. There are known genetic influences in certain haematological conditions, as well as in other blood diseases that follow familial patterns. For example, sickle cell disease occurs primarily in African Americans, and pernicious anaemia occurs most commonly in persons of Northern European descent. When a family health history is taken, the following health problems should be explored: jaundice, anaemia, malignancies, RBC disorders such as sickle cell disease and bleeding disorders such as haemophilia. The number of previous blood transfusions and possible complications during administration should be determined.
Risk factors that might disrupt the haematological system, such as alcohol and cigarette use, must be assessed. Alcohol use must be explored tactfully. Alcohol is a caustic agent to GI mucosa and damage to the GI tract secondary to alcohol can cause GI bleeding. Cigarette smoking increases low-density lipoprotein (LDL) cholesterol and levels of carbon dioxide, leading to hypoxia and altering the anticoagulant properties of the endothelium. Smoking increases platelet reactivity, plasma fibrinogen, haematocrit and blood viscosity.
Haematemesis (bright-red, brown or black vomitus) can be a symptom of this problem and should be investigated. Chronic alcohol abusers frequently have vitamin deficiencies. Alcohol also exerts a damaging effect on platelet function and the liver, where clotting factors are produced. Consequently, bleeding problems can develop and should be anticipated in cases of known alcohol abuse. Illicit drug use is important to document, as many of these drugs may affect haematopoiesis.
Nutritional–metabolic pattern
During the patient interview and assessment, the nurse obtains the patient’s weight and determines whether the patient has experienced anorexia, nausea, vomiting or oral discomfort. A dietary history may provide clues about the cause of anaemia. Iron, vitamin B12 and folic acid are necessary for the development of RBCs. Iron and folic acid deficiencies are associated with inadequate intake of foods such as liver, meat, eggs, wholegrain and enriched breads and cereals, potatoes, leafy green vegetables, dried fruits, legumes and citrus fruits. Folic acid deficiencies may be offset by a diet including foods that are also high in iron.10
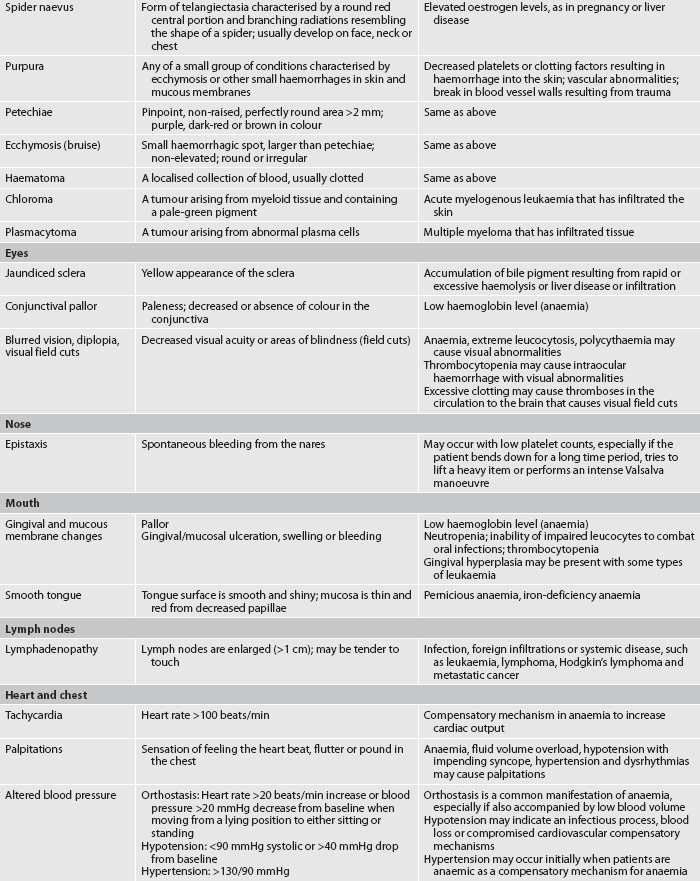
Any changes in the skin’s texture or colour should be explored. The patient should be asked about any bleeding of gum tissue. Any petechiae or ecchymotic areas on the skin should be noted. If present, the frequency, size and cause should be documented. The location of petechiae can indicate an accumulation of blood in the skin or mucous membranes. Small vessels leak under pressure and platelet numbers are insufficient to stop the bleeding. Petechiae are more likely to occur where clothing constricts the circulation.
The patient should also be questioned about any lumps or swelling in the neck, armpits or groin. Specifically the patient needs to be asked what the lumps feel like (i.e. hard or soft, tender or non-tender) and if they are mobile or fixed. Primary lymph tumours are usually not painful. A non-tender swollen lymph node may be a sign of Hodgkin’s lymphoma or non-Hodgkin’s lymphoma. Lymph nodes that are enlarged and tender are usually associated with an acute infection.11 Any incidents of fever should be explored thoroughly. It should be determined whether the patient currently has a fever, recurring fevers, chills or night sweats.
Patients should be asked if they have a history of cardiac or pulmonary diseases. Cardiovascular disorders such as valvular disease or hypertension may predispose patients to haemolysis. Many of the medications used to treat cardiovascular disease can also cause abnormalities in haematopoietic cell production or coagulation. Pulmonary disorders that lead to hypoxaemia may cause chronic stimulation of erythropoietin and result in polycythaemia (excessive RBCs).
Elimination pattern
The patient should be asked whether any blood has been noted in the urine or stools or whether black, tarry stools have occurred. Any decrease in urinary output or any diarrhoea should also be documented.
Activity–exercise pattern
Fatigue is a prominent symptom in many haematological disorders, so the patient should be asked about feelings of tiredness. Weakness and complaints of heavy extremities should also be determined. Symptoms of apathy, malaise, dyspnoea or palpitations should be documented. Any change in the patient’s ability to perform activities of daily living (ADLs) should be noted, especially as they relate to patient safety and a possible history of falling.
Sleep–rest pattern
The patient’s feeling of being rested after a night’s sleep should be determined. Fatigue secondary to a haematological problem often will not be resolved following sleep.
Cognitive–perceptual pattern
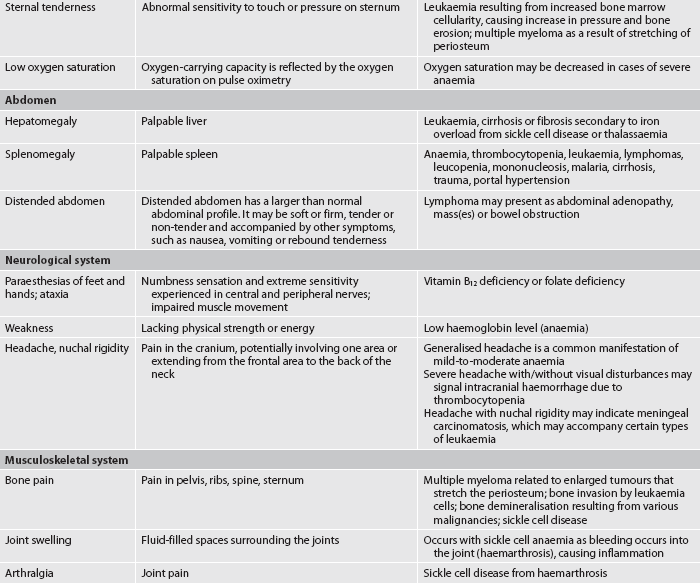
Arthralgia (joint pain) may be caused by a haematological problem and should be assessed. Pain in the joint may indicate an autoimmune disorder or may be caused by gout secondary to increased uric acid production as a result of a haematological malignancy or haemolytic anaemia. Aching bones may result from the pressure of expanding bone marrow with diseases such as leukaemia. Haemarthrosis (blood in a joint) occurs in the patient with bleeding disorders and can be painful.
Paraesthesias, numbness and tingling may be related to a haematological disorder and should be noted. Any changes in vision, hearing, taste or mental status should also be assessed carefully.
Self-perception–self-concept pattern
The effect of the health problem on the patient’s perception of self and personal abilities should be determined. The effect of certain problems, such as bruising, petechiae and lymph node swelling, on the patient’s personal appearance should also be assessed.
Role–relationship pattern
The patient should be questioned about any past or present occupational or household exposure to radiation or chemicals. If such exposure has occurred, the type, amount and duration of the exposure should be determined.
It is known that a person who has been exposed to radiation, as a treatment modality or by accident, has a higher incidence of certain haematological problems. The same is true of a person who has been exposed to chemicals (e.g. benzene, lead, naphthalene, phenylbutazone). These chemicals are commonly used by potters, dry-cleaners or individuals involved with occupations that use adhesives. The patient should also be questioned about a history in the military. Many Vietnam War veterans were exposed to a dioxin-containing defoliant (Agent Orange) that has been linked with leukaemia and lymphoma. It is important also to assess the effect of the present illness on the patient’s usual roles and responsibilities.
Sexuality–reproductive pattern
A careful menstrual history should be obtained from women, including the age at which menarche and menopause began, the duration and amount of bleeding, the incidence of clotting and cramping and any associated problems. Any intrapartum or postpartum bleeding problems should also be documented. Men should be asked if they have any problems related to impotence because this is not uncommon in men with haematological problems. The patient should also be questioned about sexual behaviour because human immunodeficiency virus (HIV) infection is potentially a concern, particularly among high-risk groups.12
Coping–stress tolerance pattern
The patient with a haematological problem often needs assistance with ADLs. The nurse should ask the patient if adequate support is available to meet daily needs. The patient’s usual methods of handling stress should also be determined. In the patient with platelet disorders or haemophilia, the potential for haemorrhage can be so frightening that usual life patterns may be drastically curtailed, affecting the person’s quality of life. It is important to explore the accuracy of the patient’s understanding of the problem.
Value–belief pattern
Some haematological problems are treated with blood transfusions or a bone marrow/stem cell transplant. Determine whether these types of treatments cause any conflicts with the patient’s value–belief system, including their cultural and religious beliefs related to blood and blood transfusions. If conflicts are identified, the healthcare provider should be notified.
OBJECTIVE DATA
Physical examination
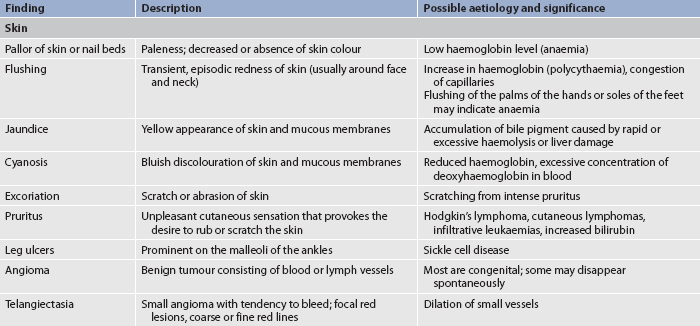
A complete physical examination is necessary to accurately examine all systems that affect or are affected by the haematological system (see Ch 3). The nurse must be aware that disorders of the haematological system can manifest in various ways; thus a patient’s presenting symptoms may not immediately point to a haematological problem (see Table 29-7).12 For example, paraesthesias of the lower extremities may not immediately appear to reflect a haematological problem, but when combined with other clinical findings or risk factors, nutritional anaemia may be suspected. Although a full examination should be performed on patients suspected of a haematological disorder, certain aspects of the physical examination are specifically relevant in haematological disorders. These include the lymph nodes, liver and spleen, and the skin. Examination of the skin is discussed in Chapter 23; liver and spleen examination is found in Chapter 38.
Lymph node assessment
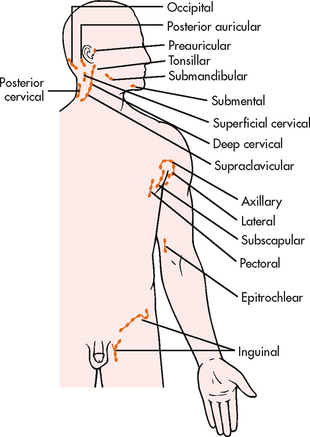
Lymph nodes are distributed throughout the body. Superficial lymph nodes can be evaluated by light palpation (see Fig 29-8). Deep lymph nodes cannot be palpated and are best evaluated by radiological examination. Lymph nodes should be assessed symmetrically with regard to location, size (in centimetres), degree of fixation (e.g. movable, fixed), tenderness and texture. To assess superficial lymph nodes, the nurse should lightly palpate the nodes using the pads of the fingers, gently rolling the skin over the area and concentrating on feeling for possible lymph node enlargement. Ordinarily, lymph nodes are not palpable in adults. If a node is palpable, it should be small (0.5–1 cm), mobile, firm and non-tender to be considered a normal finding. Abnormal findings, warranting further investigation, include any of the following: tender, hard, fixed or enlarged (regardless of whether they are tender or not). Tender nodes are usually a result of inflammation, whereas hard or fixed nodes suggest malignancy.11
It is important to develop a sequence when examining the lymph nodes. A convenient sequence for examination is to start at the head and neck. The preauricular, posterior auricular, occipital, tonsillar, submandibular, submental, superficial cervical, posterior cervical, deep cervical and supraclavicular nodes should be palpated first. Next, the axilliary lymph nodes and the pectoral, subscapular and lateral groups of nodes should be palpated. Then the epitrochlear nodes, located in the antecubital fossa between the biceps and triceps muscles, should be palpated, followed by the inguinal lymph nodes, found in the groin.
Palpation of the liver and spleen
The liver and spleen are normally not detectable by palpating the abdomen. When they are enlarged, they may be detectable by percussion or palpation. The degree of enlargement of the liver is measured by the number of finger breadths it extends below the rib border. The spleen may be more difficult to detect due to its deep location in the left abdomen. Specific techniques for palpating the liver and spleen are described in Chapter 38.
Skin assessment
In haematological disorders, assessment of the skin may be a valuable source of information about the haematological system. The skin should be examined over the entire body in a systematic manner (e.g. starting with the face and oral cavity and moving downwards over the body). In patients with RBC disorders the skin may be pale or have pasty skin tones or have a cyanotic tinge in severe anaemia. Erythrocytosis often produces small vessel occlusions causing a purple mottled appearance of the face, nose, fingers or toes. Clubbing of the fingers can be seen with chronic anaemia, such as in patients with sickle cell disease. Leucocyte disorders may cause infectious skin lesions or malignant nodular lesions. These may occur anywhere and have a variable distribution pattern. During the physical assessment of the skin, look carefully for petechiae (small purplish-red lesions), ecchymoses (bruising) or spider naevus (a form of telangiectasia) (see Table 29-7) as these can indicate bleeding disorders.
Diagnostic studies of the haematological system
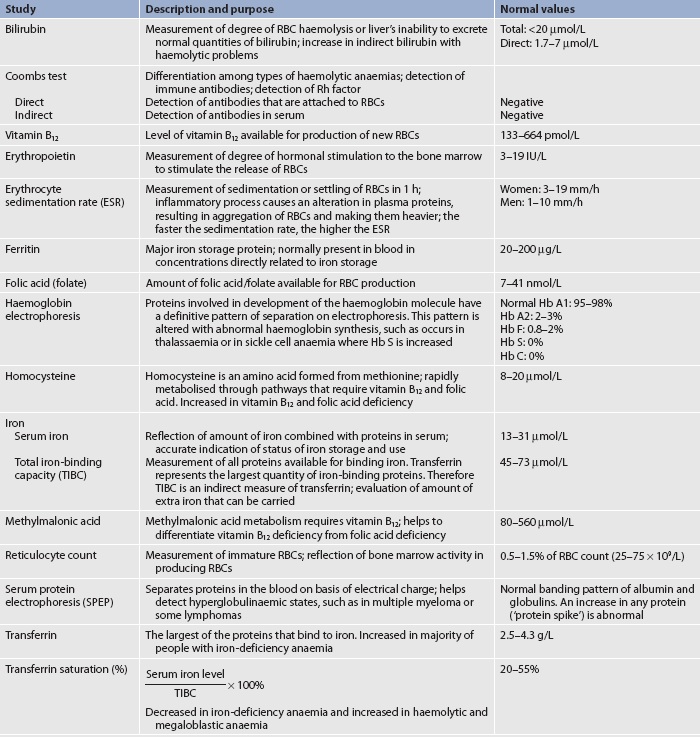
The most direct means of evaluating the haematological system is through laboratory analysis and other diagnostic studies. Repeated acquisition of blood specimens may be distressing for the patient. Some patients may become concerned that the amount of blood withdrawn for tests could lead to adverse effects. Although multiple blood studies may be uncomfortable, it is only in rare situations that diagnostic blood withdrawal predisposes the patient to significant loss of blood. For patients requiring frequent blood studies for several months, a central venous catheter may be recommended for venous access. Diagnostic tests of the haematological system are presented in Tables 29-8 to 29-12.
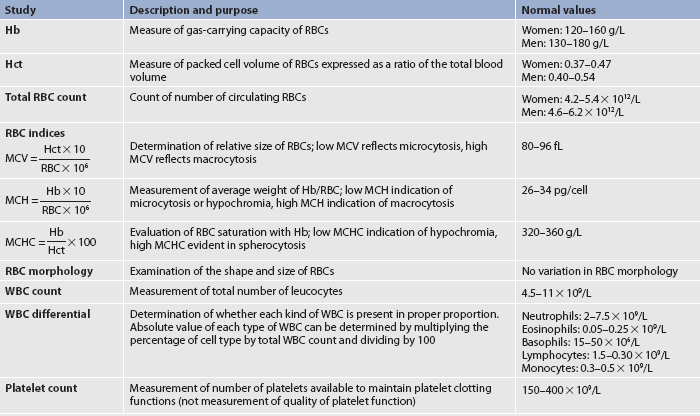
Hb, haemoglobin; Hct, haematocrit; MCH, mean corpuscular haemoglobin; MCHC, mean corpuscular haemoglobin concentration; MCV, mean corpuscular volume; RBC, red blood cell; WBC, white blood cell.
TABLE 29-10 ABO blood groups and compatibilities*
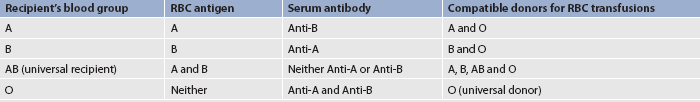
* ABO blood groups are named after the antigen found on the RBCs. Compatibility is based on the antibodies present in the serum.
LABORATORY STUDIES
Full blood count
The full blood count (FBC) involves several laboratory tests (see Table 29-8), each of which serves to assess the three major blood cell types formed in the bone marrow. Although the status of each cell type is important, the entire system may be disrupted by diseases, as well as by treatment of diseases. When the entire FBC is suppressed, a condition termed pancytopenia (marked decrease in the number of RBCs, WBCs and platelets) exists. In such cases the patient needs care directed towards the management of anaemia, infection and haemorrhage (see Ch 30). The effects of ageing on haematological studies are presented in Table 29-4.
Red blood cells
Normal values of some RBC tests are reported separately for men and for women because normal values are based on body mass and men usually have a larger body mass than women.
The haemoglobin (Hb) value is reduced in cases of anaemia, haemorrhage and states of haemodilution, such as those that occur when the fluid volume is excessive. Increases in haemoglobin are found in polycythaemia or in states of haemoconcentration, which can develop from volume depletion (dehydration).
The haematocrit (Hct) value is determined by spinning blood in a centrifuge, which causes RBCs and plasma to separate. RBCs, being the heavier elements, settle to the bottom. The haematocrit value represents the percentage of RBCs compared with the total blood volume. Reductions and elevations of haematocrit value are seen in the same conditions that raise and lower the haemoglobin value. The haematocrit value is generally three times the haemoglobin value.
The total RBC count is reported as RBC × 1012/L. However, total RBC count is not always reliable in determining the adequacy of RBC function. Consequently, other data, such as haemoglobin, haematocrit and RBC indices, must also be evaluated. The RBC count is altered by the same conditions that raise and lower the haemoglobin and haematocrit values.
RBC indices are special indicators that reflect RBC volume, colour and haemoglobin saturation (see Table 29-8). These parameters may provide insight into the cause of anaemia (the significance of these parameters is discussed further in Ch 30).
The shape and appearance of cells is called morphology. Cell morphology can provide clues to the presence of specific disease states. Examples of RBC morphologies that may be reported include Dohl bodies, Heinz bodies, anistocytes, schistocytes and sickled cells (see Ch 30).
White blood cells
The WBC count provides two different sets of information. The first is the total count of WBCs in 1 L of peripheral blood. Elevations in the WBC count over 13.5 × 109/L are associated with infection, inflammation, tissue injury or death and malignancies (e.g. leukaemia, lymphoma). Although the degree of WBC elevation does not necessarily predict the severity of illness, it can provide clues to the aetiology. Certain types of leukaemias are more likely to produce extremely high WBC counts (e.g. >25 × 109/L). A total WBC count less than 4.5 × 109/L (leucopenia) is associated with bone marrow depression, severe or chronic illness or some types of leukaemia.
The second aspect of the WBC count, the differential count, measures the percentage of each type of leucocyte. The information from the WBC differential provides valuable clues in determining the cause of illness. An important concept related to neutrophil counts is the shift to the left. When infections are severe, more granulocytes are released from the bone marrow as a compensatory mechanism. To meet the increased demand, many young, immature polymorphonuclear neutrophils (bands) are released into circulation. The usual laboratory procedure is to report the WBCs in order of maturity, with the less mature forms on the left side of the written report. Consequently, the existence of many immature cells is termed a ‘shift to the left’.
The WBC differential is of considerable significance because it is possible for the total WBC count to remain essentially normal despite a marked change in one type of leucocyte. For example, a patient may have a normal WBC count of 8 × 109/L while the differential count may show the relative proportion of lymphocytes to be reduced to 10%. This is an abnormal finding that warrants further investigation.
When there is a low lymphocyte count, an absolute lymphocyte count (ALC) may be tabulated. If the ALC is low, other diagnostic tests may be performed to investigate for an underlying reason.
When the bone marrow does not produce enough neutrophils, neutropenia occurs. Neutropenia is a condition associated with a neutrophil count (absolute neutrophil count [ANC]) less than 2 × 109/L; severe neutropenia is associated with an ANC of less than 0.5 × 109/L. The ANC is determined by multiplying the total WBC count by the percentage of neutrophils. Neutropenia results from a number of disease processes, such as leukaemia, or from bone marrow depression (see Ch 30) and is associated with a high risk of infection.
Platelet count
The platelet count is the number of platelets per litre of blood. Normal platelet counts are between 150 and 400 × 109/L; counts below 100 × 109/L signify a condition termed thrombocytopenia. Bleeding may occur with thrombocytopenia. Spontaneous haemorrhage is possible once platelet counts fall below 20 × 109/L.13 A more extensive description of clotting studies is presented in Table 29-9. Thrombocytosis is defined as excessive platelets, a disorder that occurs with inflammation and some malignant disorders (see Ch 30). The most likely complication related to thrombocytosis is excessive clotting.
Erythrocyte sedimentation rate
The erythrocyte sedimentation rate (ESR) measures the sedimentation or settling of RBCs and is used as a non-specific indicator of many diseases, especially inflammatory conditions. An increased ESR is common during acute and chronic inflammatory reactions when cell destruction is increased. The ESR is also increased in people with malignancy, myocardial infarction and end-stage renal disease. Although the ESR is a non-specific test, it is often used as a routine screening procedure.
Blood typing and Rh factor
Blood group antigens (A and B) are found only on RBC membranes and form the basis for the ABO blood typing system. The presence or absence of one or both of the two inherited antigens is the basis for the four blood groups: A, B, AB and O. Blood group A has A antigens, group B has B antigens, group AB has both antigens and group O has neither A nor B antigens. Each person has antibodies in the serum termed anti-A and anti-B that react with A or B antigens. These antibodies are found when the corresponding antigen is absent from the RBC surface. For example, B antibodies are found in the serum of persons with blood group A (see Table 29-10).
Blood reactions based on ABO incompatibilities result from intravascular haemolysis of the RBCs.13 RBCs agglutinate (or clump) when a serum antibody is present to react with the antigens on the RBC membrane. For example, agglutination would occur in the blood of a person with type A blood when blood is transfused from a person with B antigens (i.e. type B or AB) into the person with type A blood. The anti-B antibodies in the type A blood would react with the B antigens, thus initiating the process that results in RBC haemolysis.
The Rh system is based on a third antigen, D, which is also found on the RBC membrane. Rh-positive persons have the D antigen, whereas Rh-negative persons do not. As a result of transfusion therapy or during childbirth, an Rh-negative person may be exposed to Rh-positive blood. Such exposure results in the formation of an antibody, anti-D, which acts against Rh antigens. (Rh-positive persons normally have no anti-D.) The person is then sensitised to Rh-positive blood and a second exposure to Rh positive blood will cause a severe haemolytic reaction. A Coombs test can be used to evaluate the person’s Rh status (see Table 29-11).
Iron metabolism
The laboratory tests used in evaluating iron metabolism include serum iron, total iron-binding capacity (TIBC), serum ferritin and transferrin saturation. Additional tests for nutritional deficiencies leading to defective RBC production may also be done (see Table 29-11).14–16
Serum iron is a measurement of the amount of protein-bound iron circulating in the serum. The TIBC provides a measurement of all proteins that act to bind or transport iron between the tissues and bone marrow. Although this indirect measurement is a general reflection of the amount of transferrin present in the circulation, it overestimates transferrin levels by 16–20% because it also measures other proteins that can bind iron. These alternative proteins bind iron only when transferrin is more than half saturated. Also, the TIBC varies inversely with tissue iron stores; it is higher when iron stores are low and lower when iron stores are high.
Transferrin saturation is a better indicator of the availability of iron for erythropoiesis than serum iron because, unlike serum iron, the iron bound to transferrin is readily available for the body to use. Transferrin saturation is calculated by dividing serum iron by the TIBC and multiplying by 100. For example, a patient with a serum iron level of 13–31 μmol/L and a TIBC of 45–73 μmol/L would have a transferrin saturation of about 33%.
Under normal conditions, the serum ferritin concentration correlates closely with body iron stores. In normal patients, 1 ng/mL of ferritin corresponds to 8–10 mg of stored iron.
RADIOLOGICAL STUDIES
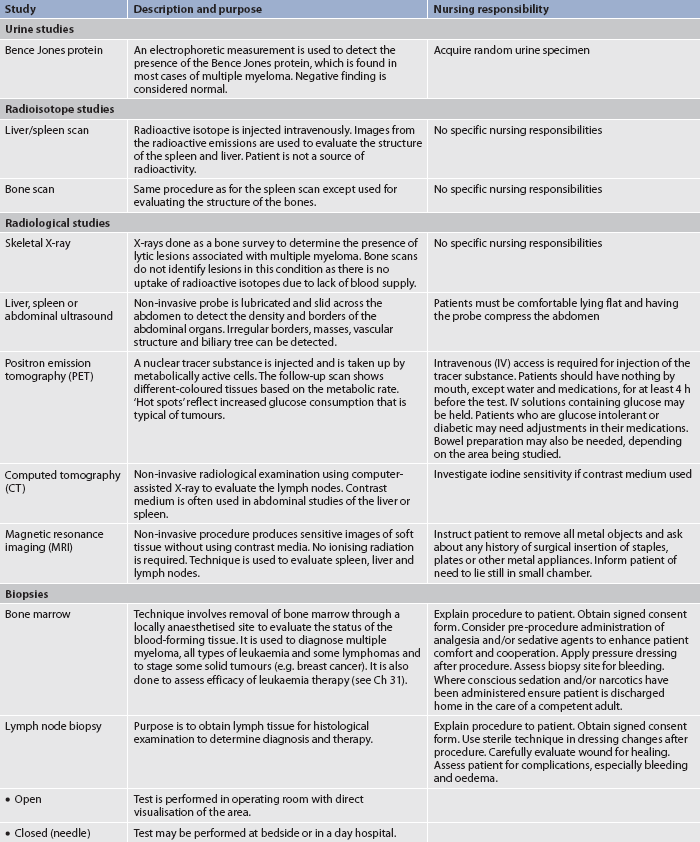
Radiological studies of the haematological system primarily involve the use of computed tomography (CT) or magnetic resonance imaging (MRI) for evaluating the spleen, liver and lymph nodes. In the past, lymphangiography with the use of contrast media was a common procedure used to evaluate deep lymph nodes. However, it has been replaced by the more sensitive CT and MRI tests. Spiral (helical) CT scans are used to evaluate lymph nodes.
Positron emission tomography (PET) scanning has become an invaluable diagnostic tool to detect active malignancy due to its ability to highlight areas with increased metabolism. This test has permitted more accurate diagnosis and monitoring of lymphoid malignant diseases. PET/CT scanning, with either fusion software (separate scans are taken and fused via the computer) or a combined PET/CT scanner, may also be used. Nursing responsibilities related to these studies are presented in Table 29-12.
BIOPSIES
Biopsy procedures specific to haematological assessment are bone marrow examination and lymph node biopsy. In general, these procedures are carried out because a peripheral blood smear is non-specific and usually a diagnosis cannot be established from a peripheral blood smear. Furthermore, a biopsy provides additional information about a haematological problem that is needed for diagnostic purposes, as well as planning treatment options.
Bone marrow examination
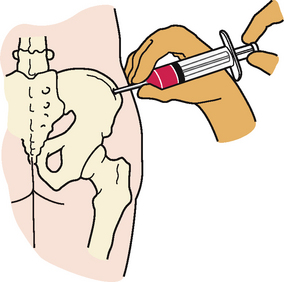
Bone marrow examination is important in the evaluation of many haematological disorders. The examination of the marrow may involve aspiration only or aspiration with biopsy. The benefits gained from bone marrow examination are: (1) a full evaluation of haematopoiesis; and (2) the ability to obtain specimens for cytopathology and chromosomal abnormalities.1
The preferred site for both aspiration and biopsy of bone marrow is the posterior iliac crest. The anterior iliac crest and sternum are alternative sites; however, the sternum is usually used only for aspiration. Bone marrow aspiration and biopsy are performed by a doctor or haematology nurse practitioner. Conscious sedation may be used to minimise anxiety and pain that the patient may experience. For bone marrow aspiration, the skin over the puncture site is cleansed with a bactericidal agent. The skin, subcutaneous tissue and periosteum are infiltrated with a local anaesthetic agent. The patient may be uncomfortable when the periosteum is penetrated. Once the area is anaesthetised, a bone marrow needle is inserted through the cortex of the bone. The stylet of the needle is then removed, the hub is attached to a 10-mL syringe and 0.2–0.5 mL of the fluid marrow is aspirated (see Fig 29-9). The patient experiences pain with aspiration. Although it lasts for only a few seconds, the pain may be quite uncomfortable. After the marrow aspiration, the needle is removed. Pressure is applied over the aspiration site to ensure haemostasis. If the patient is thrombocytopenic, pressure may be required for 5–10 minutes or longer.
If a bone biopsy is required, the preparatory procedure remains the same, but a different needle is used. The needle has a cutting blade that allows a specimen of the bone to be removed. When either a marrow aspirate or a biopsy specimen is acquired, a glass slide is carefully prepared with a thin film of the marrow.
Although complications of bone marrow aspiration are minimal, there is a possibility of damaging underlying structures. This hazard is greatest in aspiration procedures involving the sternum.17 Other complications include haemorrhage (particularly if the patient is thrombocytopenic) and infection (particularly if the patient is leucopenic).7 The needle aspiration or biopsy site is covered with a sterile pressure dressing. If bleeding is present, the patient should be advised to lie on the side for 30–60 minutes to maintain pressure on the site. If the bed is too soft, the patient can lie on a rolled towel to provide additional pressure. Where conscious sedation is used the patient should be discharged home in the care of a competent adult.
Lymph node biopsy
Lymph node biopsy involves obtaining lymph tissue for histological examination to determine the diagnosis and to help in planning therapy. This may be accomplished by either an open biopsy or a closed (needle) biopsy. In the open biopsy procedure, an incision is made and the lymph node and surrounding tissue are dissected (excised) whenever possible. Care must be taken because euplastic cells can be disseminated during the biopsy procedure if the scalpel passes through tissues containing cancerous cells. An open biopsy is performed in the operating room or procedure area using either local or general anaesthesia.
A closed (needle) biopsy or fine-needle aspiration is performed by a doctor or a haematology nurse practitioner at the bedside or in an outpatient area. Sterile technique is essential throughout the procedure. An extremely small needle is used to reduce the risk of tracking malignant cells through normal subcutaneous tissue. Nursing personnel must recognise the possibility of insidious bleeding and direct pressure should be applied to the area after the biopsy procedure to achieve haemostasis. Frequent observations of the site for bleeding and monitoring of vital signs should be done, especially if the platelet count is low. The sterile dressing should be changed as ordered and the wound should be inspected for healing and infection. It is important to recognise that if the results from a needle biopsy are negative, it may only indicate that the cancer cells were not part of the tissue in the biopsy specimen. However, a positive finding is sufficient evidence for confirming a diagnosis. This technique is rarely used to confirm an initial diagnosis because larger specimens are usually required in order to perform cytopathological tests, but it may be used to validate disease recurrence or a new site of disease.
Haematology nurse practitioners are becoming more common within haematology and oncology clinical areas. Their role is to perform some of the investigations previously performed by medical staff. Their expanded scope of practice also includes patient education and working with the multidisciplinary team in planning and implementing patient care.
MOLECULAR CYTOGENETICS AND GENE ANALYSIS
Testing for specific genetic or chromosomal variations in haematological conditions is often helpful in assisting in diagnosis and staging. These results also help to determine the treatment options and prognosis. If there are a large number of abnormal cells circulating in the blood, such as in acute leukaemia, these tests may be carried out by obtaining peripheral blood. However, testing is usually done on samples from bone marrow and lymph node biopsies. For example, fluorescent in situ hybridisation (FISH) can identify specific areas by attaching a probe to a targeted region of DNA. It may be used to illuminate an abnormal extra chromosome 8 that is common in certain leukaemias. Spectral karyotyping (SKY) allows each set of chromosomes to be painted different colours. It can be used to identify the chromosomal translocation of 22 to 9 in the Philadelphia chromosome of chronic myelogenous leukaemia.1 More information on genetics is given in Chapter 13.
1. An individual who lives at a high altitude may normally have an increased RBC count because:
2. Malignant disorders that arise from granulocytic cells in the bone marrow will have the primary effect of causing:
3. An anticoagulant, such as warfarin, that interferes with the production of prothrombin will alter the clotting mechanism during:
4. When reviewing laboratory results of an 83-year-old patient with an infection, the nurse would expect to find:
5. Significant information obtained from the patient’s health history that relates to the haematological system includes:
6. While assessing the lymph nodes, the nurse:
7. If a lymph node is palpated, which of the following is a normal finding?
8. Immediately following a bone marrow biopsy and aspiration, the nurse should instruct the patient to:
1 Craft J, Gordon C, Tiziani A. Understanding pathophysiology. Sydney: Elsevier, 2011.
2 Marks PW, Glader B. Approach to anemia in the adult and child. In Hoffman R, Benz EJ, Shattil SJ, et al, eds.: Hematology: basic principles and practice, 5th edn., Philadelphia: Elsevier, 2009.
3 Chu AJ. Tissue factor mediates inflammation. Arch Biochem Biophys. 2005;440(2):123–132.
4 Dahlback B. Blood coagulation and its regulation by anticoagulant pathways: genetic pathogenesis of bleeding and thrombotic diseases. J Intern Med. 2005;257(3):209–223.
5 Powell LD, Baum LG. Overview and compartmentalization of the immune system. In Hoffman R, Benz EJ, Shattil SJ, et al, eds.: Hematology: basic principles and practice, 5th edn., Philadelphia: Elsevier, 2009.
6 Andrews NC. Pathology of iron metabolism. In Hoffman R, Benz EJ, Shattil SJ, et al, eds.: Hematology: basic principles and practice, 5th edn., Philadelphia: Elsevier, 2009.
7 Lichtman MA, Williams WJ. Hematology in the aged. In Lichtman MA, Beutler E, Kipps TJ, et al, eds.: Williams hematology, 7th edn., New York: McGraw-Hill, 2006.
8 Kane RL, Ouslander JG, Abrass IB, Resnick B. Essentials of clinical geriatrics, 6th edn. New York: McGraw-Hill, 2009.
9 Rothstein G. Hematologic problems. In Cassel CK, ed.: Geriatric medicine: an evidence-based approach, 4th edn., New York: Springer-Verlag, 2003.
10 Grodner M, Long S, Walkingshaw BC. Foundations and clinical applications of nutrition: a nursing approach, 4th edn. St Louis: Mosby, 2007.
11 Wilson SF, Giddens JF. Health assessment for nursing practice, 4th edn. St Louis: Mosby, 2008.
12 Beutler E. Approach to the patient. In Lichtman MA, Beutler E, Kipps TJ, et al, eds.: Williams hematology, 7th edn., New York: McGraw-Hill, 2006.
13 Pagana KD, Pagana TJ. Mosby’s diagnostic and laboratory test reference, 7th edn. St Louis: Mosby, 2005.
14 Linker CA. Blood disorders. In Papadakis MA, McPhee SJ, eds.: Current medical diagnosis and treatment, 49th edn., New York: McGraw-Hill, 2010.
15 Antony AA. Megaloblastic anemias. In Hoffman R, Benz EJ, Shattil SJ, et al, eds.: Hematology: basic principles and practice, 5th edn., Philadelphia: Elsevier, 2009.
16 Van Leeuwen AM, Kranpitz TR, Smith LS. Davis’s comprehensive handbook of laboratory and diagnostic tests with nursing implications, 2nd edn. Philadelphia: FA Davis, 2006.
17 Ryan DH. Examination of the marrow. In Lichtman MA, Beutler E, Kipps TJ, et al, eds.: Williams hematology, 7th edn., New York: McGraw-Hill, 2006.