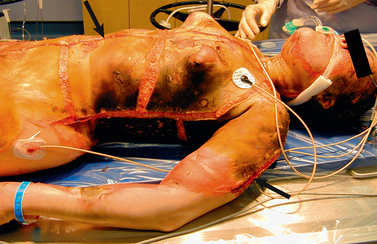
Chapter 24 NURSING MANAGEMENT: burns
1. Explain the causes and prevention of burn injuries.
2. Differentiate between partial-thickness and full-thickness burns.
3. Outline the parameters used to determine the severity of burns.
4. Compare the pathophysiology, clinical manifestations, complications and nursing and collaborative management of the three burn phases.
5. Compare fluid and electrolyte shifts during the emergent and acute burn phases.
6. Differentiate the nutritional therapy of the burn patient during the three burn phases.
7. Compare the various burn wound care techniques and surgical options for partial-thickness versus full-thickness burn wounds.
8. Prioritise nursing interventions in the management of the physiological and psychosocial needs of the burn patient throughout the three burn phases.
9. Examine the various physiological and psychosocial aspects of burn rehabilitation.
10. Design a plan of care to prepare the burn patient and carer for discharge.
A burn occurs when there is injury to the tissues of the body caused by heat, chemicals, electrical current or radiation. The resulting effects are influenced by the temperature of the burning agent, the duration of contact time and the type of tissue that is injured.
In Australia in 2008, total burn injury-related deaths accounted for 0.1% of all deaths, with males accounting for twice as many deaths as females,1 and in 2007–2008 the age-adjusted hospitalisation rate for injuries related to fire, burns and scalds was 27.6 cases per 100,000 population per year.2 During this period, burns and scalds were responsible for 5811 hospitalisations in public hospitals. The care of people with burn injuries and the provision of their ongoing care can be expensive for both the health facility and those with burn injuries. Approximately 50% of people hospitalised with burn injuries have some disability that may affect their daily living. In Australia, the major cause of burn injuries in adults is thermal injury, with flame burns being the most common burning agent. A major cause of fires in the home is carelessness with cigarettes. In children, scalding is the most common burning agent.3 Other causes of burns include hot water from water heaters set above 60°C, cooking, heaters, combustibles such as petrol and kerosene, steam from radiators and chemicals.
Health promotion
The majority of burn injuries are accidental and preventable. The public needs education on burn first aid and health workers need education on burn first aid and immediate care prior to transfer to hospital. Nurses advocate for scald and fire risk-reduction strategies in the home and provide public education on appropriate burn first aid and care of minor burn injuries at home. Home safety measures include the use of ionisation-type smoke alarms, fire blankets and fire extinguishers. Families should have fire drills, and each family member should know where to go and what to do in case of a fire. Local fire brigades can inform the public of regional fire regulations and perform home safety checks. Occupational health nurses provide educational strategies to reduce scald, chemical, electrical and thermal injuries in the work setting. Community fire awareness and prevention programs educate the public on the dangers of lighting fires, and the importance of clearing the garden of excess bushes, shrubs and leaves, especially in summer when the possibility of bushfires and house fires may be greater. People are made aware of the correct use of electrical appliances and the importance of fixing faulty appliances (see Boxes 24-1 and 24-2). School health promotion programs teach children about fire prevention and awareness, and the importance of sun protection during the hot summer.
BOX 24-2 Types of burn injury and risk reduction strategies
Flame or contact
• Never leave candles unattended or near open windows/curtains.
• Encourage use of ‘child-resistant’ lighters.
• Install smoke/carbon monoxide detectors.
• Encourage regular home fire-exit drills.
• Never use petrol or other flammable liquids as accelerants.
• Never leave hot oil unattended while cooking.
• Consider a flame-retardant smoking apron for elderly and/or ‘at-risk’ people.
Scald
• Lower hot water temperature to the ‘lowest point’ or 40°C.
• Use ‘anti-scald’ devices with showerhead or tap fixtures.
• Supervise bathing of small children, older adults or anyone with impaired physical movement/physical sensation/judgement.
• After running bath water, check temperature with back of hand or bath thermometer.
Types of burn injury
THERMAL BURNS
Thermal burns caused by flame, flash, scald, sunburn or contact with hot objects are the most common type of burn (see Box 24-2 and Fig 24-1).
CHEMICAL BURNS
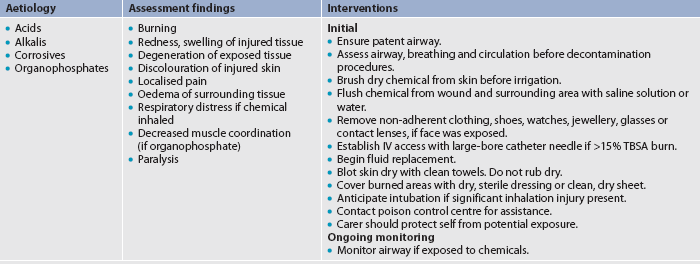
Chemical burns result from tissue injury and destruction from acids, alkalis and organic compounds. In addition to tissue damage, eyes can be injured if they are splashed with a chemical. Acids are found in many household cleaners and include hydrochloric, oxalic and hydrofluoric acid. Alkali burns can be more difficult to manage than acid burns since alkaline substances are not neutralised by tissue fluids as readily as acid substances. Alkalis adhere to tissue, causing protein hydrolysis and liquefaction. Alkalis are found in oven and drain cleaners, fertilisers and heavy industrial cleansers. Organic compounds, including phenols and petroleum products, produce contact burns and systemic toxicity. Phenols are found in chemical disinfectants; petroleum products include creosote and petrol.4
Chemicals can cause respiratory problems and other systemic manifestations, as well as skin and eye injuries. When chlorine gas is inhaled, it produces respiratory distress. By-products of burning substances (e.g. carbon) are toxic to the sensitive respiratory mucosa.
With chemical injuries, it is important to remove the person from the burning agent and begin to quickly remove the chemical from the skin. Dry chemical should be brushed from the skin, and then flushed with copious amounts of water to irrigate the affected area, anywhere from 20 minutes to 2 hours post-exposure. Irrigation should be with running water draining to the floor, avoiding contact with unaffected areas. Any clothing containing the chemical should be removed because the burning process will continue as long as the chemical is in contact with the skin. Tissue destruction may continue for up to 72 hours after a chemical injury.
SMOKE AND INHALATION INJURY
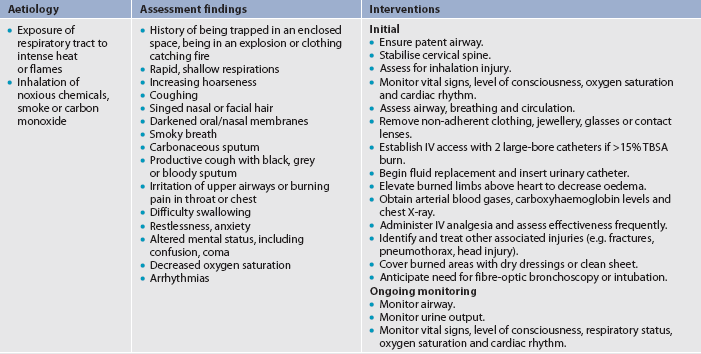
Smoke and inhalation injuries result from the inhalation of hot air or noxious chemicals and can cause damage to the tissues of the respiratory tract. Fortunately, gases are cooled to body temperature before they reach the lung tissue. Although damage to the respiratory mucosa can occur, it seldom happens because the vocal cords and glottis close as a protective mechanism. Redness and airway swelling (oedema) may result when damage occurs. Because smoke inhalation injuries are a major predictor of mortality in burn patients, a rapid assessment is critical.5
There are three types of smoke and inhalation injuries:
1. Carbon monoxide poisoning. Carbon monoxide (CO) poisoning and asphyxiation account for the majority of deaths at a fire scene. CO is produced by the incomplete combustion of burning materials. It is subsequently inhaled and displaces oxygen (O2) on the haemoglobin molecule, causing carboxyhaemoglobinaemia, hypoxia and ultimately death when the CO levels are greater than 20%. Skin colour is often described as ‘cherry red’ in appearance with severe CO poisoning. CO poisoning may occur in the absence of burn injury to the skin.
2. Inhalation injury above the glottis. In general, an inhalation injury above the glottis (upper airway injury) is thermally produced and may be caused by the inhalation of hot air, steam or smoke. Mucosal burns of the oropharynx and larynx are manifested by redness, blistering and oedema. Mechanical obstruction can occur quickly, presenting a true medical emergency. Clues that this injury is likely include the presence of facial burns, singed nasal hair, hoarseness, painful swallowing, darkened oral and nasal membranes, carbonaceous sputum, being burned in an enclosed space, and clothing burns around the chest and neck.
3. Inhalation injury below the glottis. An inhalation injury below the glottis (lower airway injury) is usually chemically produced. Tissue damage is related to the duration of exposure to smoke or toxic fumes. Clinical manifestations such as pulmonary oedema may not appear until 12–24 hours after the burn, and then they may manifest as acute respiratory distress syndrome (ARDS) (see Ch 67).
These patients must be observed closely for signs of respiratory distress or compromise and must be treated quickly and efficiently if they are to survive. Respiratory tract complications from burn injury are discussed in detail later in this chapter.
ELECTRICAL BURNS
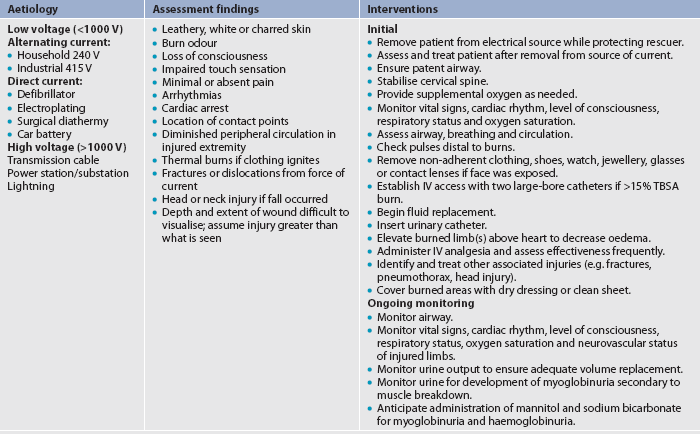
Electrical burns are the result of intense heat generated from an electric current (see Fig 24-2). Direct damage to nerves and vessels, causing tissue anoxia and death, can also occur. The severity of the electrical injury depends on the amount of voltage, tissue resistance, current pathways, the surface area in contact with the current and the length of time that the current flow was sustained. Tissue densities offer various amounts of resistance to electric current. For example, fat and bone offer the most resistance, whereas nerves and blood vessels offer the least resistance. Current that passes through vital organs (e.g. brain, heart, kidneys) will produce more life-threatening sequelae than that which passes through other tissues. In addition, electric sparks may ignite the patient’s clothing, causing a combination of thermal and electrical injuries.
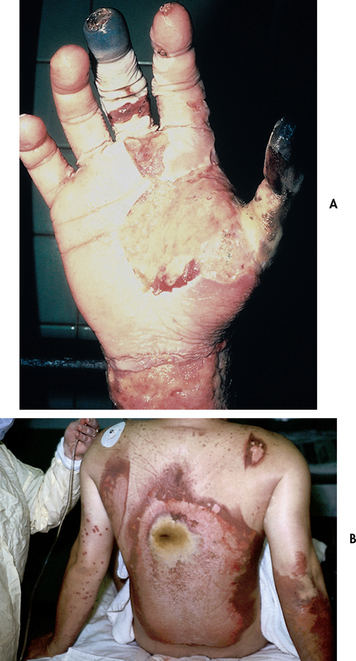
Figure 24-2 Electrical injury produces heat coagulation of blood supply and contact area as electrical current passes through the skin. A, Hand. B, Back.
As with inhalation injury, a rapid assessment of the patient with electrical injury must be performed. Transfer to a burns unit is indicated. The severity of an electrical injury can be difficult to determine as most of the damage is below the skin (known as the ‘iceberg effect’). Determination of electric current contact points and the history of the injury may help determine the probable path of the current and potential areas of injury. Contact with electric current can cause muscle contractions strong enough to fracture the long bones and vertebrae. Another reason to suspect long bone or spinal fractures is a fall resulting from the electrical injury. For this reason, all patients with electrical burns should be considered at risk for a potential cervical spine injury. Cervical spine immobilisation must be used during transport and subsequent diagnostic testing must be completed to rule out any injury.
Electrical injury puts the patient at risk for arrhythmias or cardiac arrest, severe metabolic acidosis and myoglobinuria, which can lead to acute tubular necrosis (ATN). The electric shock event can cause immediate cardiac standstill or ventricular fibrillation. Delayed cardiac arrhythmias or arrest may also occur without warning during the first 24 hours after injury. Myoglobin from injured muscle tissue and haemoglobin from damaged red blood cells (RBCs) are released into the circulation whenever massive muscle and blood vessel damage occurs. The released myoglobin pigments are transported to the kidneys, where they can mechanically block the renal tubules because of their large size. This process can result in ATN and eventual acute renal failure if not appropriately treated (see Ch 46).
Treatment consists of infusing Hartmann’s solution at a rate sufficient to maintain urinary output at 75–100 mL per hour until the urine sample analyses indicate that the myoglobin and haemoglobin have been flushed from the circulatory system. In addition, an osmotic diuretic (e.g. mannitol) may be given to maintain urine output, along with sodium bicarbonate to alkalinise the urine.
Classification of burn injuries
The treatment of burns is related to the severity of the injury. Severity is determined by: (1) the depth of the burn; (2) the extent of the burn, calculated as a percentage of total body surface area (TBSA); (3) the location of the burn; (4) patient risk factors; and (5) local response to the burn injury. The Australian and New Zealand Burn Association (ANZBA) has established referral criteria that recommend appropriate referrals to a burns unit for people with burn injuries (see Box 24-3).3 The ANZBA recommends that all patients with major burns be nursed in a burns unit and any patient suspected of sustaining an inhalational injury should be intubated prior to transfer. Burn injuries that fall outside of the referral criteria can be managed in the community or regional hospitals by non-burns unit personnel, on either an inpatient or an outpatient basis. The majority of minor burn-injured patients are seen in outpatient settings. Goals of care include wound healing, prevention of infection, pain management and rapid rehabilitation.
BOX 24-3 Burns unit referral criteria
Criteria for specialised burns treatment
• Burns >10% total body surface area (TBSA)
• Burns of special areas—face, hands, feet, genitalia, perineum and major joints
• Full-thickness burns >5% of TBSA
• Burns with an associated inhalation injury
• Circumferential burns of the limbs and chest
• Burns in the very young or very old
• Burns in people with pre-existing medical disorders that could complicate management, prolong recovery or increase mortality
Source: Australian and New Zealand Burn Association. EMSB manual, 2010. Available at www.anzba.org.au.
DEPTH OF BURN
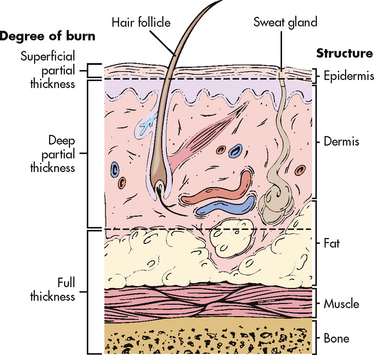
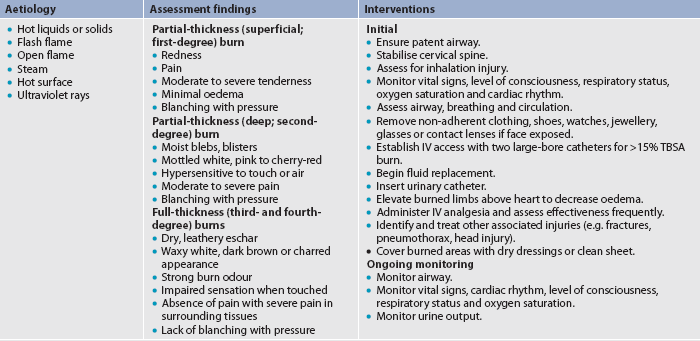
Burn injury involves the destruction of the integumentary system. The skin is divided into three layers: the epidermis, dermis and subcutaneous tissue (see Fig 24-3). The epidermis, or nonvascular outer layer of the skin, is approximately as thick as a sheet of paper. It is composed of many layers of non-living epithelial cells that provide a protective barrier to the skin, hold in fluids and electrolytes, help regulate body temperature and keep harmful agents in the external environment from injuring or invading the body. The dermis, which lies below the epidermis, is approximately 30–45 times thicker than the epidermis. The dermis contains connective tissues with blood vessels and highly specialised structures consisting of hair follicles, nerve endings, sweat glands and sebaceous glands. Under the dermis lies the subcutaneous tissue, which contains major vascular networks, fat, nerves and lymphatics. The subcutaneous tissue acts as a heat insulator for underlying structures, which include the muscles, tendons, bones and internal organs.
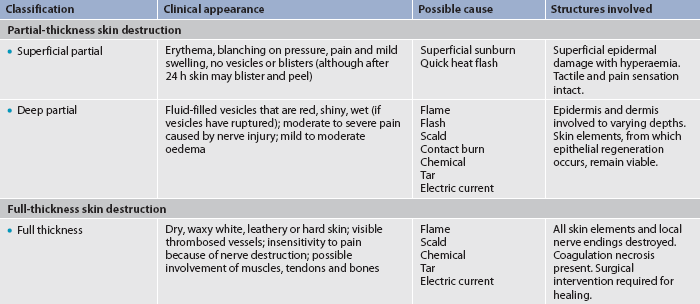
Burns are defined according to the depth of skin destruction: partial-thickness and full-thickness (see Fig 24-2). Table 24-1 shows a comparison of the depth of injury. Skin-reproducing (re-epithelialising) cells are located throughout the dermis and along the shafts of the hair follicles and sebaceous glands. If there is significant damage to the dermis (e.g. a full-thickness burn), there are not enough skin cells to regenerate new skin, and a permanent, alternative source of skin cover is needed.
EXTENT OF BURN
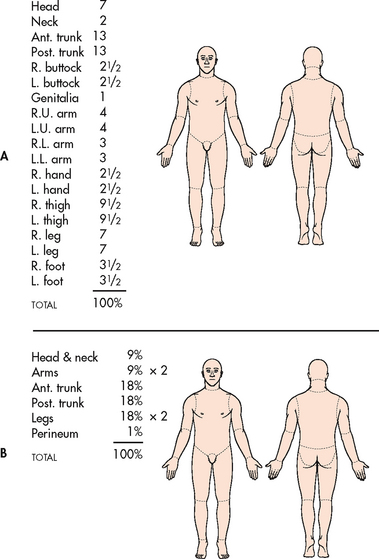
Two commonly used guides for determining the total body surface area affected or the extent of a burn wound are the Lund-Browder chart (see Fig 24-4, A) and the rule of nines chart (see Fig 24-4, B). (Superficial burns, equivalent to a sunburn, are not included when calculating TBSA.) The Lund-Browder chart is considered more accurate because the patient’s age, in proportion to relative body-area size, is taken into account. The rule of nines chart, which is easy to remember, is considered adequate for initial assessment of an adult burn patient. For irregular- or odd-shaped burns, the patient’s hand (including the fingers) is approximately 1% of the TBSA. The Sage burn diagram is a free, internet-based tool that is available for estimating the TBSA burned (see Resources on p 569). The extent of a burn is often revised after oedema has subsided and a demarcation of the zones of injury has occurred.
LOCATION OF BURN
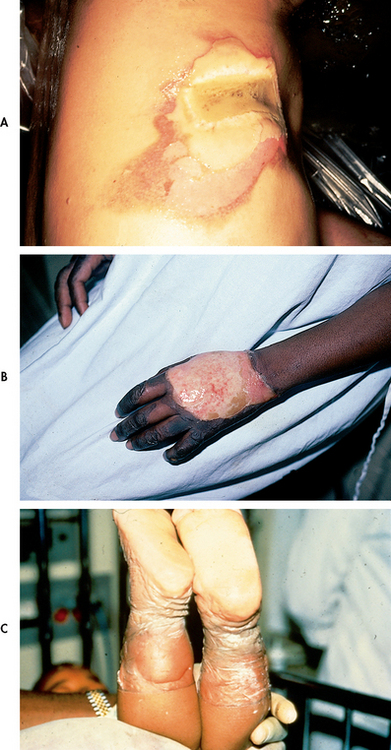
The severity of the burn injury is related to the location of the burn wound. Burns to the face and neck and circumferential burns to the chest/back may inhibit respiratory function due to mechanical obstruction secondary to oedema or leathery, devitalised tissue formation (eschar). These injuries may also indicate possible inhalation injury and respiratory mucosal damage.
Burns of the hands, feet, joints and eyes are of concern because they make self-care very difficult and may jeopardise future function. Burns of the hands and feet are challenging to manage because of superficial vascular and nerve supply systems and the need to maintain their function during healing.
Burns to the ears and nose are susceptible to infection because of poor blood supply to the cartilage. Burns to the buttocks or perineum are highly susceptible to infection. Circumferential burns to the extremities can cause circulatory compromise distal to the burn with subsequent neurological impairment of the affected extremity. Patients may also develop compartment syndrome (see Ch 62) from direct heat damage to the muscles and subsequent oedema and/or preburn vascular problems.
PATIENT RISK FACTORS
The older adult heals more slowly and usually experiences more difficulty with rehabilitation than a younger adult. Any patient with pre-existing cardiovascular, respiratory or renal disease has a poorer prognosis for recovery because of the tremendous demands placed on the body by a burn injury. The patient with diabetes mellitus or peripheral vascular disease is at high risk for poor healing and gangrene, especially with foot and leg burns. General physical debilitation from any chronic disease, including alcoholism, drug abuse and malnutrition, renders the patient less physiologically able to recover from a burn injury. In addition, the burn patient who has concurrently sustained fractures, head injuries or other trauma has a poorer prognosis for recovery.
LOCAL RESPONSE TO BURN INJURY
Local response to burn injury may be explained by Jackson’s burn wound model of three zones of injury. The ‘zone of coagulative necrosis’ is the central zone of tissue death. Tissue nearest to the heat source or other injuring agent is damaged immediately because heat cannot be conducted rapidly away, causing immediate coagulation of cellular proteins and rapid cell death. The ‘zone of stasis’ surrounding the zone of coagulative necrosis has less severe damage and cell death does not occur immediately. Skin and subcutaneous tissue in this area are compromised, circulation is sluggish and the zone may progress towards one of necrosis within 3 days of injury. This is caused by an outpouring of inflammatory mediators post injury into the tissue. The ‘zone of hyperaemia’ surrounds the zone of stasis. The vasculature is compromised and dilation of vessels occur. This zone will return to normal following the resolution of the hyperdynamic vascular response to the burn injury. The institution of immediate and adequate first aid and adequate resuscitation measures is vital to the survival of the tissues in the zone of stasis.3
PHASES OF BURN MANAGEMENT
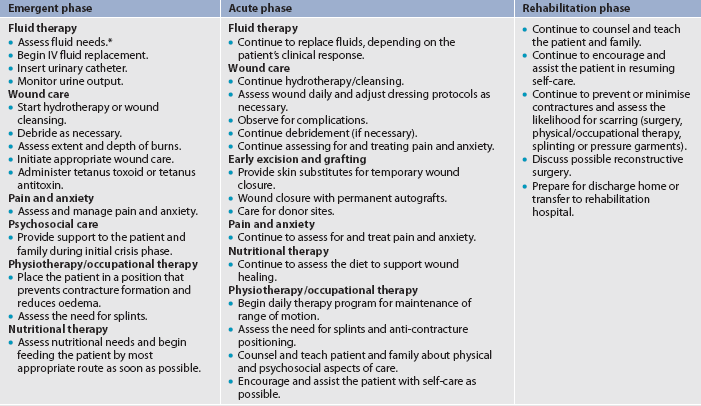
Historically, burn management has been organised chronologically into three phases that correspond to the key priority of each particular phase: emergent (resuscitative), acute (wound healing) and rehabilitative (restorative). An overlap in care exists from one phase to another. For example, although the emergent phase is seen as beginning in the emergency department, care often begins in the pre-hospital phase, depending on the skill level of the paramedics at the scene. Planning for rehabilitation begins on the day of the burn injury or admission to the burns unit. Formal rehabilitation begins as soon as functional assessments can be performed. Wound care is the primary focus of the acute phase, but it also takes place in both the emergent and the rehabilitative phases.
Pre-hospital care
At the scene of the injury, priority is given to removing the person from the source of the burn and stopping the burning process. Rescuers must also protect themselves from being injured. In the case of electrical injuries, initial management involves removing the patient from contact with the electric source.
Small thermal burns (≤10% TBSA) should be covered with a clean, cool, tap water–dampened towel for the patient’s comfort and protection until definitive medical care is instituted. Cooling of the injured area within 1–3 hours of injury helps minimise the depth of the injury. If the burn is large (>10% TBSA) or an electrical or inhalation burn is suspected, attention needs to be focused first on the ABCs:
Airway: check for patency, soot around nares/on the tongue, singed nasal hair, darkened oral or nasal membranes.
Breathing: check for adequacy of ventilation.
Circulation: check for the presence and regularity of pulses, and elevate the burned limb(s) above the heart to decrease pain and swelling.
To prevent hypothermia, large burns should be cooled for no more than 10 minutes. Do not immerse the burned body part in cool water since doing so might lead to extensive heat loss. Never cover a burn with ice as this can cause hypothermia and vasoconstriction of blood vessels, further reducing blood flow to the injury. Gently remove as much burned clothing as possible to prevent further tissue damage. Adherent clothing should be left in place until the patient is transferred to a hospital. The patient should then be wrapped in a dry, clean sheet or blanket to prevent further contamination of the wound and to provide warmth.
Chemical burns are best treated by quickly removing the solid particles from the skin. Any clothing containing the chemical must also be removed as the burning process continues while the chemical is in contact with the skin. The affected area should be flushed with copious amounts of water to irrigate the skin anywhere from 20 minutes to 2 hours postexposure.4 Tap water is acceptable for flushing eyes exposed to chemicals. Tissue destruction may continue for up to 72 hours after a chemical burn. (For information on handling specific agents, refer to a hazardous materials text.)
Patients with inhalation injuries must be observed closely for signs of respiratory distress or compromise. They need to be treated quickly and efficiently at the scene if they are to survive. If CO intoxication is suspected, the patient should be treated with 100% humidified O2. Patients who have both body burns and inhalation injury must be transferred to the nearest burns unit.
The patient may also have sustained other injuries that take priority over the burn wound. It is important for the individuals involved in the pre-hospital phase of burn care to adequately communicate the circumstances of the injury to the hospital-based healthcare providers. This is especially important when the patient’s injury involves entrapment in a closed space, hazardous chemicals, electricity or possible trauma (e.g. fall).
Pre-hospital and emergency management are presented in tables that describe chemical burns (see Table 24-2), inhalation injury (see Table 24-3), electrical burns (see Table 24-4) and thermal burns (see Table 24-5).
Emergent phase
The emergent (resuscitative) phase is the period of time required to resolve the immediate, life-threatening problems resulting from the burn injury. This phase usually lasts up to 72 hours from the time of the burn. The primary concerns are the onset of hypovolaemic shock and oedema formation. The phase ends when fluid mobilisation and diuresis begin.
PATHOPHYSIOLOGY
Fluid and electrolyte shifts
The greatest initial threat to a patient with a major burn is hypovolaemic shock (see Fig 24-5).6 It is caused by a massive shift of fluids out of the blood vessels as a result of increased capillary permeability and can begin as early as 20 minutes postburn. As the capillary walls become more permeable, water, sodium and later plasma proteins (especially albumin) move into interstitial spaces and other surrounding tissue. The colloidal osmotic pressure decreases with progressive loss of protein from the vascular space. This results in more fluid shifting out of the vascular space into the interstitial spaces. (Fluid accumulation in the interstitium is termed second spacing.) Fluid also moves to areas that normally have minimal to no fluid, a phenomenon termed third spacing. Examples of third spacing in burn injury are exudate and blister formation, as well as oedema in non-burned areas.
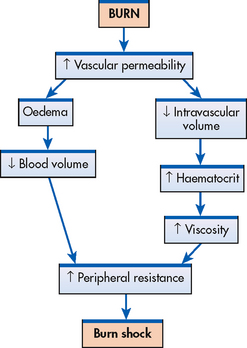
Figure 24-5 At the time of major burn injury, there is increased capillary permeability. All fluid components of the blood begin to leak into the interstitium, causing oedema and a decreased blood volume. Haematocrit increases and the blood becomes more viscous. The combination of decreased blood volume and increased viscosity produces increased peripheral resistance. Burn shock, a type of hypovolaemic shock, rapidly ensues and, if not corrected, death can result.
Other sources of fluid loss during this period are insensible losses by evaporation from large, denuded body surfaces and the respiratory system. The normal insensible loss of 30–50 mL per hour is increased in the severely burned patient. The net result of the fluid shifts and losses is intravascular volume depletion. Decreased blood pressure (BP), increased heart rate and other manifestations of hypovolaemic shock are clinically detectable signs (see Ch 66). If not corrected, irreversible shock and death may result.
The circulatory status is also impaired because of haemolysis of RBCs. The RBCs are haemolysed by circulating factors (e.g. oxygen free radicals) released at the time of the burn, as well as by the direct insult of the burn injury. Thrombosis in the capillaries of burned tissue causes an additional loss of circulating RBCs. An elevated haematocrit is commonly caused by haemoconcentration resulting from fluid loss. After fluid balance has been restored, lowered haematocrit levels are found secondary to dilution.
Major shifts in sodium and potassium also occur during this phase. Sodium rapidly shifts to the interstitial spaces and remains there until oedema formation ceases (see Fig 24-6). A potassium shift develops initially because injured cells and haemolysed RBCs release potassium into the circulation.
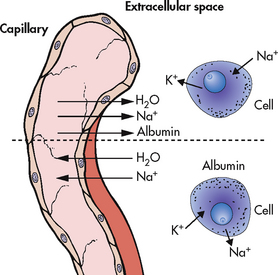
Figure 24-6 The effects of burn shock are shown above the dotted line. As the capillary seal is lost, interstitial oedema develops. The cellular integrity is also altered, with sodium (Na) moving into the cell in abnormal amounts and potassium (K) leaving the cell. The shifts after the resolution of burn shock are shown below the dotted line. The water and sodium move back into the circulating volume through the capillary. The albumin remains in the interstitium. Potassium is transported into the cell and sodium is transported out as the cellular integrity returns.
Towards the end of the emergent phase, capillary membrane permeability will be restored if fluid replacement is adequate. Fluid loss and oedema formation cease. Interstitial fluid gradually returns to the vascular space. Clinically, diuresis is noted with low urine specific gravities.
Inflammation and healing
Burn injury causes coagulation necrosis, whereby tissues and vessels are damaged or destroyed. Neutrophils and monocytes accumulate at the site of injury. Fibroblasts and newly formed collagen fibrils appear and begin wound repair within the first 6–12 hours after injury. (The inflammatory response is discussed in Ch 12.)
Immunological changes
Burn injury causes widespread impairment of the immune system. The skin barrier to invading organisms is destroyed, bone marrow depression occurs and circulating levels of immunoglobulins are decreased. Defects occur in the function of white blood cells (WBCs). The inflammatory cytokine cascade triggered by tissue damage impairs the function of lymphocytes, monocytes and neutrophils, which puts the patient at greater risk for infection.
CLINICAL MANIFESTATIONS
The burn patient is likely to be in shock from hypovolaemia. Frequently, areas of full-thickness and deep partial-thickness burns are initially anaesthetic because the nerve endings are destroyed. Superficial to moderate partial-thickness burns are painful. Blisters filled with fluid and protein may form in partial-thickness burns. Fluid is not actually lost from the body as much as it is sequestered in the interstitial spaces and third spaces. The patient with a larger burn may have signs of a paralytic ileus, such as absent or decreased bowel sounds, as a result of the body’s response to massive trauma and potassium shifts. Shivering may occur as a result of chilling that is caused by heat loss, anxiety or pain. Ongoing nursing assessment of the ABCs, vital signs, cardiac rhythm, oxygenation and level of consciousness are priorities in the emergent phase of burn care.
Most burn patients are quite alert and can provide answers to questions shortly after the injury or until they are intubated. They are often frightened and benefit from calm reassurances and simple explanations by all healthcare providers. Unconsciousness or altered mental status in a burn patient is usually not a result of the burn. The most common reason for unconsciousness or altered mental status is hypoxia associated with smoke inhalation. Other possibilities include head trauma, a history of substance abuse, or excessive amounts of sedation or pain medication.
COMPLICATIONS
The three major organ systems most susceptible to complications during the emergent phase of burn injury are the cardiovascular, respiratory and urinary systems.
Cardiovascular system
Cardiovascular system complications include arrhythmias and hypovolaemic shock, which may progress to irreversible shock. Circulation to the extremities can be severely impaired by deep circumferential burns and subsequent oedema formation. These processes occlude the blood supply by acting like a tourniquet. If untreated, ischaemia, paraesthesias, necrosis and eventually gangrene can occur. An escharotomy (a scalpel or diathermy incision through the full-thickness eschar) is frequently performed following transfer to a burns unit to restore circulation to compromised extremities (see Fig 24-7).
Initially, there is an increase in blood viscosity with burn injuries because of the fluid loss that occurs in the emergent period. Microcirculation is impaired because of the damage to skin structures that contain small capillary systems. These two events result in a phenomenon termed sludging. Sludging can be corrected by adequate fluid replacement.
Respiratory system
The respiratory system is especially vulnerable to two types of injury: (1) upper airway burns that cause oedema formation and obstruction of the airway; and (2) lower airway injury (see Box 24-4). Upper airway distress may occur with or without smoke inhalation, and airway injury at either level may occur in the absence of burn injury to the skin.
BOX 24-4 Manifestations of respiratory injury associated with burns
Upper airway injury
Upper airway injury results from direct heat injury or oedema formation and can lead to mechanical airway obstruction and asphyxia. The oedema associated with an upper respiratory tract burn injury can be massive and the onset insidious. Mechanical obstruction of the airway is not limited to the patient with flame burns to the upper airway. Swelling that accompanies scald burns to the face and neck can be lethal, as can pressure from the accumulated oedema compressing the airway externally. Flame burns to the neck and chest may contribute to respiratory difficulty because the inelastic eschar becomes tight and constricting due to the underlying oedema.
Lower airway injury
Lower airway (or inhalation) injury refers to a direct insult at the alveolar level secondary to the inhalation of toxic fumes or smoke. The result is interstitial oedema that prevents the diffusion of oxygen from the alveoli into the circulatory system.7 Fibre-optic bronchoscopy and carboxyhaemoglobin blood levels can be used to confirm a suspected inhalation injury. Another diagnostic indicator may be a history of prolonged exposure to smoke or fumes. Sputum that contains carbon may be present. The nurse must be especially sensitive to signs of impending respiratory distress such as increased agitation, restlessness or a change in the rate or character of respirations as the symptoms may not be present immediately. Generally, there is no correlation between the extent of TBSA burn and the severity of inhalation injury because inhalation injury is a factor of time exposure plus the type and density of the material inhaled. The initial chest X-ray may appear normal on admission, with changes noted over the next 24–48 hours. Arterial blood gas (ABG) values may also be within the normal range on admission and then change during hospitalisation.
Other cardiopulmonary problems
The patient with pre-existing heart disease (e.g. myocardial infarction) or lung disease (e.g. chronic obstructive pulmonary disease) is at risk for complications. If fluid replacement is too vigorous, the patient can develop heart failure or pulmonary oedema. Invasive measures (e.g. haemodynamic monitoring) may be necessary to monitor fluid resuscitation.
The patient with pre-existing respiratory problems is more likely to develop a respiratory infection. Pneumonia is a common complication of major burns and the leading cause of death in patients with an inhalation injury. Debilitation, abundant microbial flora and relative immobility predispose the patient to the development of pneumonia.
Burn patients are at risk for the development of venous thromboembolism (VTE). Risk for VTE increases if one or more of the following conditions are present: advanced age, morbid obesity, extensive or lower-extremity burns, concomitant lower-extremity trauma and/or prolonged immobility.
Urinary system
The most common complication of the urinary system in the emergent phase is ATN. If the patient becomes hypovolaemic, blood flow to the kidneys is decreased, causing renal ischaemia. If this continues, acute renal failure may develop.
With full-thickness and electrical burns, myoglobin (from muscle cell breakdown) and haemoglobin (from RBC breakdown) are released into the bloodstream and occlude renal tubules. Adequate fluid replacement can counteract myoglobin and haemoglobin obstruction of the tubules.
 NURSING AND COLLABORATIVE MANAGEMENT: EMERGENT PHASE
NURSING AND COLLABORATIVE MANAGEMENT: EMERGENT PHASE
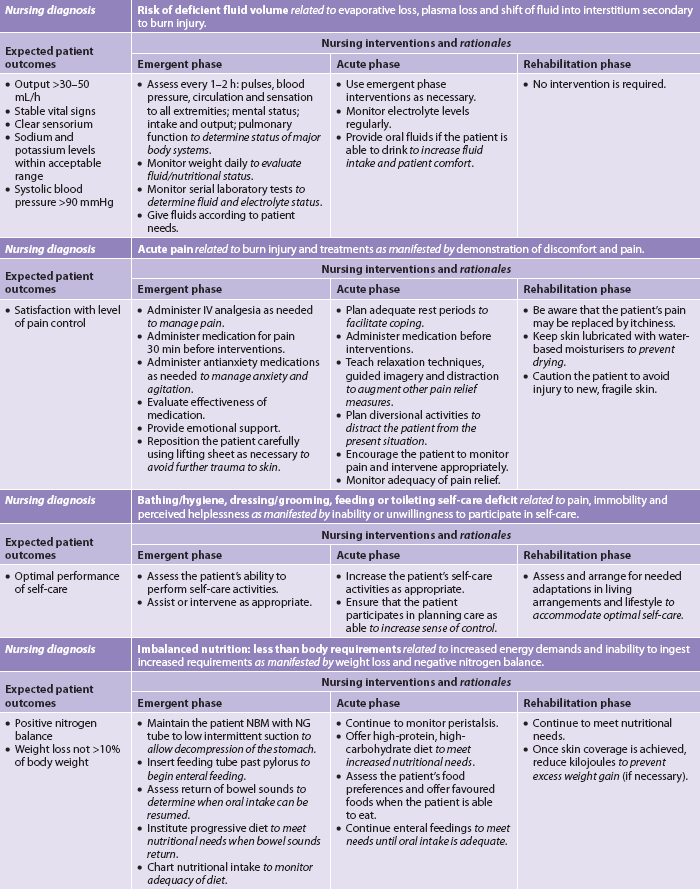
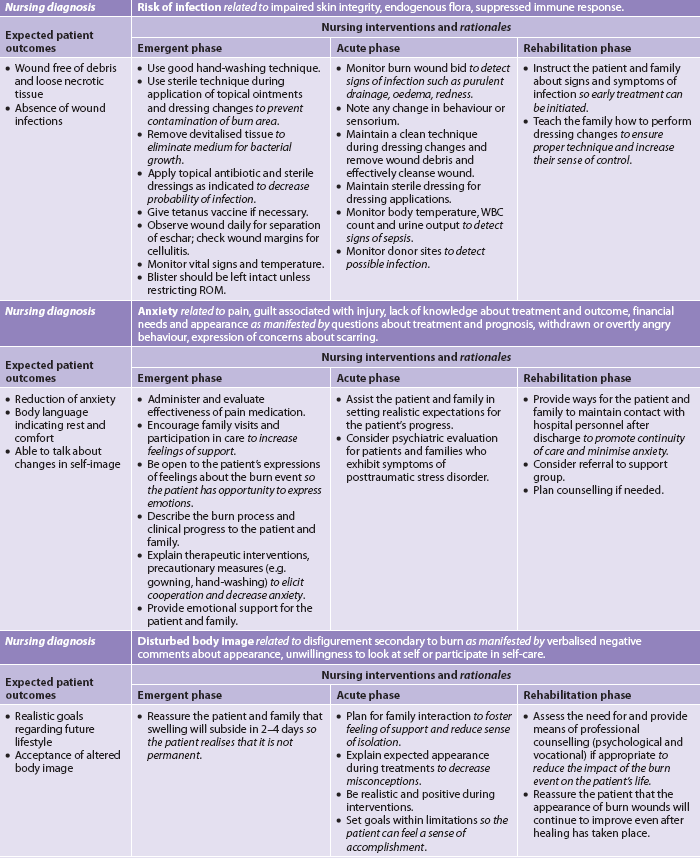
In the emergent phase, patient survival depends on rapid and thorough assessment and intervention.8 Usually the doctor and nurse make the initial assessment of depth and extent of the burn and coordinate the actions of the healthcare team. In a community hospital, decisions need to be made as to whether the patient requires inpatient or outpatient care and, in the case of inpatient care, whether the patient remains in that hospital or is transferred to the closest regional burns unit (see Box 24-3). From the onset of the burn event until the patient is stabilised, nursing and collaborative management predominantly consists of airway management, fluid therapy and wound care (see Table 24-6). Although the burn management can be chronologically categorised as emergent, acute and rehabilitative, the overall care requirements are not so easily classified. Depending on the acuity of the patient, the duration of time spent in each phase varies greatly, and conditions improve and worsen unpredictably on an almost daily basis. Care changes accordingly. Whereas physiotherapy and occupational therapy are a focus of the acute and rehabilitative phases, proper positioning and splinting begin at the time of admission. Support and teaching of patients and carers begin on admission and intensify in the rehabilitative phase (see NCP 24-1).
 Airway management
Airway management
Airway management frequently involves early endotracheal intubation. Early intubation eliminates the necessity for emergency tracheostomy after respiratory problems have become apparent. In general, the patient with major injuries involving burns to the face and neck requires intubation within 1–2 hours after burn injury. (Intubation is discussed in Ch 65.) After intubation, the patient is placed on ventilatory assistance, and the delivered oxygen concentration is determined by assessing ABG values. Extubation may be indicated when the oedema resolves, usually 3–6 days after burn injury, unless severe inhalation injury is involved. Escharotomies of the chest wall may be needed to relieve respiratory distress secondary to circumferential full-thickness burns of the neck and trunk.
Within 6–12 hours after injury in which smoke inhalation is suspected, a fibre-optic bronchoscopy should be performed to assess the lower airway. Significant findings include the appearance of carbonaceous material, mucosal oedema, vesicles, erythema, haemorrhage and ulceration.
When intubation is not performed, treatment of inhalation injury includes administration of 100% humidified O2 as needed. The patient should be placed in a high Fowler’s position, unless contraindicated (e.g. spinal injury), and encouraged to cough and breathe deeply every hour. The patient should be repositioned every 1–2 hours and provided with chest physiotherapy and suctioning as necessary. If respiratory failure develops, intubation and mechanical ventilation are initiated. Positive end-expiratory pressure (PEEP) may be used to prevent collapse of the alveoli and progressive respiratory failure (see Ch 65). Bronchodilators may be administered to treat severe bronchospasm.
Carbon monoxide poisoning is treated by administering 100% O2 until carboxyhaemoglobin levels return to normal. The use of hyperbaric oxygen therapy remains controversial.
 Fluid therapy
Fluid therapy
Establishing intravenous (IV) access is critical for fluid resuscitation and drug administration. At least two large-bore IV access routes must be obtained for burns greater than 15% of TBSA. It is critical to establish IV access that can accommodate large volumes of fluid. For burns greater than 20% of TBSA, a central line for fluid and drug administration, as well as blood sampling, should be considered. An arterial line should also be considered if frequent ABGs or invasive BP monitoring is needed.
The extent of the burn wound is assessed using a standardised chart (see Fig 24-4). This allows for the accurate estimation of fluid resuscitation requirements. The type of fluid replacement is determined by the size and depth of the burn, the age of the patient, and individual considerations, such as pre-existing chronic illness. Each burns unit has a preference for a replacement regimen. Fluid replacement is accomplished with crystalloid solutions (usually Hartmann’s solution, or 4% dextrose and 20% normal saline), colloids (albumin, dextran or other commercially prepared solutions), or a combination of the two. Paramedics generally give IV saline until arrival at hospital.
The Parkland (Baxter) formula for fluid replacement is the most common formula used, followed by the modified Brooke formula (see Box 24-5).9 It is important to remember that all formulas are estimates and must be titrated according to the patient’s physical response. For example, patients with an inhalation injury may have a greater than normal fluid requirement. Patients with inhalation injuries require 5.7 mL/kg/% TBSA burn as opposed to 3.98 mL/kg/% TBSA burn for non-inhalation burn-injured patients.8
BOX 24-5 Fluid resuscitation with the modified Parkland (Baxter) formula*
Example
For a 70 kg patient with a 50% TBSA burn:
4 mL × 70 kg × 50% TBSA burn = 14,000 mL = 14 L in 24 h
½ of total in first 8 h = 7000 mL (875 mL/h)
*Formulas are guidelines. Fluid is administered at a rate to produce 30–50 mL of urine output per hour.
TBSA, total body surface area.
Colloidal solutions (e.g. CSL, albumin) may be given. However, administration is recommended after the first 12–24 hours postburn when capillary permeability returns to normal or near normal. After this time, the plasma remains in the vascular space and expands the circulating volume. The replacement volume is calculated based on the patient’s body weight and TBSA burned (e.g. 0.3–0.5 mL/kg/% TBSA burn).
Assessment of the adequacy of fluid resuscitation is best made using clinical parameters. Urine output, the most commonly used parameter, and cardiac parameters are defined as follows:
1. Urine output: 0.5–1 mL/kg/h; 75–100 mL/h for electrical burn patient with evidence of haemoglobinuria/myoglobinuria.
2. Cardiac factors: Mean arterial pressure (MAP) greater than 65 mmHg, systolic BP greater than 90 mmHg, heart rate less than 120 beats per minute. MAP and BP are most appropriately measured by an arterial line. Peripheral measurement is often invalid because of vasoconstriction and oedema.
 Wound care
Wound care
Once a patent airway, adequate circulation and adequate fluid replacement have been established, priority is given to care of the burn wound. Partial-thickness wounds are pink to cherry-red and wet and shiny with serous exudate. These wounds may or may not have intact blisters and are painful when touched or exposed to air. Full-thickness wounds will be dry and waxy white to dark brown/black and will have only minor, localised sensation because nerve endings have been destroyed.
Cleansing and gentle debridement, using scissors and forceps, can occur in a mobile bath (see Fig 24-8), regular shower or patient bed. Releasing escharotomies and fasciotomies can be carried out in the emergent phase, either in the burns unit or in the operating room (see Fig 24-9). Care should be taken to accomplish these procedures as quickly and effectively as possible. During debridement, necrotic skin is removed.
Figure 24-8 Mobile bath. Showering presents an opportunity for physical therapy, as well as wound care.
Figure 24-9 Surgical debridement of full-thickness burns is necessary to prepare the wound for grafting.
Patients find the initial wound care to be both physically and psychologically demanding. The nurse’s emotional support is invaluable and assists in building an important sense of trust. Patients are showered using tap water, not exceeding 40°C. A once-daily shower and dressing change in the morning is a common routine in many burns units. Some of the newer antimicrobial dressings can be left in place from 3 to 14 days, thereby decreasing the frequency of the dressing changes.
Infection is the most serious threat to further tissue injury and possible sepsis.10 The source of infection in burn wounds is the patient’s own flora, predominantly from the skin (burned and unburned), respiratory tract and gastrointestinal (GI) tract. The prevention of cross-contamination from one patient to another is a priority for all members of the healthcare team.
Two approaches to burn wound treatment are the open method and the use of multiple dressing changes. In the open method, the patient’s burn is covered with a topical emollient or antimicrobial and has no dressing over the wound (usually for facial burns). In the multiple dressing change or closed method, sterile gauze dressings are impregnated with or laid over a topical antimicrobial. These dressings are changed every day or the frequency is dependent on the product used. Most burns units support the concept of moist wound healing and use dressings to cover the burned areas, with the exception of the burned face.
When the patient’s open burn wounds are exposed, the nurse must wear personal protective equipment (PPE) (e.g. disposable hats, masks, gowns, gloves). When removing contaminated dressings and washing the dirty wound, the nurse may use non-sterile, disposable gloves. Sterile gloves are used when applying ointments and sterile dressings. In addition, the room must be kept warm (approximately 29.4°C). All PPE should be removed and new PPE applied before treating another patient. This is necessary to avoid transmitting organisms from one patient to another, a significant risk especially when there is more than one patient to a room. Careful hand-washing and the use of an alcohol hand gel, both inside and outside each patient room, is also required to prevent cross-contamination. After the dressing change is completed, the equipment and immediate environment should be thoroughly cleaned and disinfected. The use of plastic liners on equipment is helpful in reducing the potential contamination of the equipment and facilitates cleaning.
Coverage is the primary goal for burn wounds.11 There is rarely enough unburned skin in the major (>50% TBSA) burn patient for immediate skin grafting. This necessitates the use of other temporary wound closure methods. Allograft (homograft) skin (usually from cadavers) is used, along with newer biosynthetic options, with varying frequency among burns units (see Table 24-7).
| Source | Graft name | Coverage |
|---|---|---|
| Patient’s own skin | Autograft | Permanent |
| Patient’s own skin and cell cultures | Cultured epithelial autograft (CEA) | Permanent |
| Bovine collagen and glycosaminoglycan bonded to silicone membrane | Integra | Permanent |
| Acellular dermal matrix derived from donated human skin | AlloDerm | Permanent |
| Porcine collagen bonded to silicone membrane | BioBrane | Temporary (10–21 days) |
| Porcine skin | Heterograft or xenograft (different species) | Temporary (3 days to 2 weeks) |
| Cadaveric skin | Homograft or allograft (same species) | Temporary (3 days to 2 weeks) |
 Other care measures
Other care measures
Certain parts of the body (e.g. face, eyes, ears, hands, arms, perineum) require particularly vigilant nursing care. The face is highly vascular and subject to a great amount of oedema. It is often covered with ointments and gauze but not wrapped to limit pressure on delicate facial structures. Eye care for corneal burns or oedema includes antibiotic ointments.12 An ophthalmology examination should occur soon after admission for all patients with facial burns. Periorbital oedema can prevent opening of the eyes and be frightening to the patient. The nurse should provide assurance that the swelling is not permanent. Instillation of methylcellulose drops or artificial tears into the eyes for moisture provides additional comfort.
Ears should be kept free of pressure because of their poor vascularisation and predisposition to infection. The patient with ear burns should not use pillows because pressure on the cartilage may cause chondritis and the ear may stick to the pillowcase, causing pain and bleeding. The patient’s head can be elevated using a rolled towel placed under the shoulders, being careful to avoid pressure necrosis. The same holds true for the patient with neck burns. Pillows should be removed and a rolled towel placed under the shoulders to hyperextend the neck and prevent neck wound contraction.
Hands and arms should be extended and elevated on pillows to minimise oedema. Splints may need to be applied to burned hands and feet to maintain them in positions of function.
The perineum must be kept as clean and dry as possible. In addition to providing hourly urine outputs, an indwelling catheter will prevent urine contamination of the perineal area. Regular, once- to twice-daily perineal and catheter care in the presence or absence of a perineal burn wound is essential.
Routine laboratory tests are performed to monitor fluid and electrolyte balance. ABGs are drawn to determine adequacy of ventilation and perfusion in all patients with suspected or confirmed inhalation or electrical injury.
Physiotherapy is begun immediately, sometimes during showering/dressing changes and before new dressings are applied. Early range-of-motion (ROM) exercises are necessary to facilitate mobilisation of the extravasated fluid back into the vascular bed. Exercise of body parts also maintains function, prevents contractures and reassures the patient that movement is still possible.
 Drug therapy
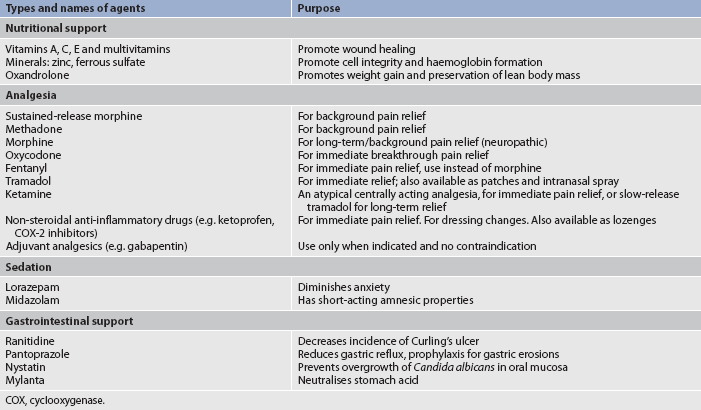
Drug therapy
 Analgesics and sedatives
Analgesics and sedatives
Analgesics are ordered to promote patient comfort. Early in the postburn period, IV pain medications should be given because (1) onset of action is fastest with this route; (2) GI function is slowed or impaired due to shock or paralytic ileus; and (3) intramuscular (IM) injections will not be absorbed adequately in burned or oedematous areas, causing pooling of medications in the tissues. When fluid mobilisation begins, the patient could be inadvertently overdosed from the interstitial accumulation of previous IM medications.
Common opioids used for pain control are listed in Table 24-8. The need for analgesia must be re-evaluated frequently as patients’ needs may change and tolerance to medications may develop over time. Initially, opioids are the drug of choice for pain control. When given appropriately, these drugs should provide adequate pain management. Sedative/hypnotics and antidepressant agents can also be given with analgesics to control the anxiety, insomnia and/or depression that patients may experience (see Table 24-8). Analgesic requirements can vary tremendously from one patient to another. The extent and depth of burn may not correlate with pain intensity. Hospital pharmacists, psychiatrists and multidisciplinary pain services are valuable resources for the more complex patient situations.
 Tetanus immunisation
Tetanus immunisation
Tetanus toxoid is given routinely to all burn patients because of the likelihood of anaerobic burn wound contamination. Adults should receive a booster dose of dT (dT; diphtheria-tetanus, adult formulation) if more than 5 years have elapsed since the last dose.13
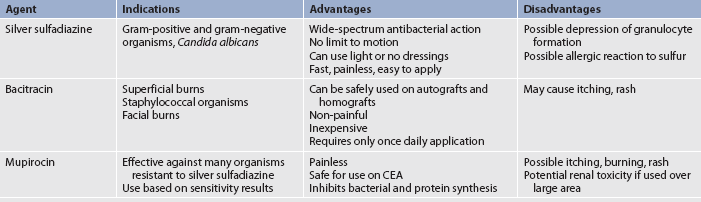
 Antimicrobial agents
Antimicrobial agents
After the wound is cleansed, topical antimicrobial agents are applied (see Fig 24-10 and Table 24-9) and covered with a light dressing. Systemic antibiotics are not routinely used to control burn wound flora because there is little or no blood supply to the burn eschar and, consequently, there is little delivery of the antibiotic to the wound. In addition, the routine use of systemic antibiotics increases the chance of developing multidrug-resistant organisms. Some topical burn agents penetrate the eschar, thereby inhibiting bacterial invasion of the wound. Silver-impregnated dressings (e.g. Acticoat, Silvercel, Aquacel AG) can be left in place anywhere from 1 to 14 days and are used in several burns units. Silver sulfadiazine (Flamazine) is also used.
Sepsis remains a leading cause of death in patients with major burns, which may lead to multiple organ dysfunction syndrome (see Ch 66). Systemic antibiotic therapy is initiated when the clinical diagnosis of invasive burn wound sepsis is made, or when some other source of infection is identified (e.g. pneumonia).
Fungal infections may develop in the patient’s mucous membranes (mouth and genitalia) as a result of systemic antibiotic therapy and low resistance in the host. The offending organism is usually Candida albicans. Oral infection is treated with nystatin mouthwash. When a normal diet is resumed, yoghurt or Lactobacillus may be given by mouth to reintroduce the normal intestinal flora that have been destroyed by antibiotic therapy.
 Venous thromboembolism prophylaxis
Venous thromboembolism prophylaxis
For burn patients who are also at risk for VTE (e.g. lower extremity burns, obesity) and if there are no contraindications, it is recommended that low-molecular-weight heparin or low-dose unfractionated heparin be started as soon as it is considered safe to do so. For burn patients who have a high bleeding risk, it is recommended that mechanical VTE prophylaxis with sequential compression devices and/or graduated compression stockings be used until the bleeding risk decreases and heparin can be started.14
 Nutritional therapy
Nutritional therapy
Once fluid replacement needs have been addressed, nutrition takes priority in the initial emergent phase. Early and aggressive nutritional support within several hours of the burn injury can decrease mortality risks and complications, optimise healing of the burn wound and minimise the negative effects of hypermetabolism and catabolism.15 Non-intubated patients with <20% TBSA burn will generally be able to eat enough to meet their nutritional requirements. Intubated patients and/or those with larger burns require additional support. Enteral feedings (gastric or intestinal) have almost entirely replaced the need for parenteral feeding. Early enteral feeding, usually with the use of smaller bore tubes, preserves GI function, increases intestinal blood flow and promotes optimal conditions for wound healing. The patient with large (>20% TBSA) burns can develop paralytic ileus within a few hours as a result of the body’s response to major trauma. If a large nasogastric tube is inserted on admission, gastric residuals should be checked frequently to rule out delayed gastric emptying. Bowel sounds should be assessed every 8 hours. In general, feeding can begin slowly at 20 to 40 mL per hour and can be increased to the goal rate within 24 to 48 hours.
A hypermetabolic state proportional to the size of the wound occurs after a major burn injury. Resting metabolic expenditure may be increased by 50–100% above normal in patients with major burns. Core temperature is elevated. Catecholamines, which stimulate catabolism and heat production, are increased. Massive catabolism can occur and is characterised by protein breakdown and increased gluconeogenesis. Failure to supply adequate kilojoules and protein leads to malnutrition and delayed healing. Kilojoule-containing nutritional supplements and milkshakes are often given because of the great need for kilojoules. Protein powder can also be added to food and liquids. Supplemental vitamins may be given as early as the emergent phase, with iron supplements often started in the acute phase.
Acute phase
The acute phase begins with the mobilisation of extracellular fluid and subsequent diuresis. This phase is concluded when the burned area is completely covered by skin grafts or when the wounds are healed. This may take weeks or many months.
PATHOPHYSIOLOGY
Burn injury involves pathophysiological changes in many body systems. Diuresis from fluid mobilisation occurs, and the patient is less oedematous. Areas that are full- or partial-thickness burns are more evident than in the emergent phase. Bowel sounds return. The patient may now become aware of the enormity of the situation and benefit from additional psychosocial support. Some healing begins as WBCs surround the burn wound and phagocytosis occurs. Necrotic tissue begins to slough. Fibroblasts lay down matrices of the collagen precursors that eventually form granulation tissue. A partial-thickness burn wound will heal from the edges and from the dermal bed below if kept free from infection and desiccation (dryness). However, full-thickness burn wounds, unless extremely small, must be covered by skin grafts. In some cases, healing time and length of hospitalisation are decreased by early excision and grafting.
Does silver sulfadiazine promote burn healing?
EVIDENCE-BASED PRACTICE
Clinical question
For patients with superficial and partial-thickness burns (P), does silver sulfadiazine (I) versus biosynthetic dressings (C) reduce healing time (O)?
Implications for nursing practice
• Silver sulfadiazine, the traditional covering for burns, is not the best choice for healing of superficial and partial-thickness burns.
• Burn dressing choice should be based on promotion of healing, ease of application and removal, dressing change requirements, cost and patient comfort.
P, patient population of interest; I, intervention or area of interest; C, comparison of interest or comparison group; O, outcome(s) of interest.
CLINICAL MANIFESTATIONS
Partial-thickness wounds form eschar, which begins separating fairly soon after injury. Once the eschar is removed, re-epithelialisation begins at the wound margins and appears as red or pink scar tissue. Epithelial buds from the dermal bed eventually close in the wound, which then heals spontaneously without surgical intervention, usually within 10 to 21 days.
Margins of full-thickness eschar take longer to separate. As a result, full-thickness wounds require surgical debridement and skin grafting for healing.
LABORATORY VALUES
Because the body is attempting to re-establish fluid and electrolyte homeostasis in the initial acute phase, it is important to follow serum electrolyte levels closely.
Sodium
Hyponatraemia can develop from excessive GI suction, diarrhoea and water intake. Manifestations of hyponatraemia include weakness, dizziness, muscle cramps, fatigue, headache, tachycardia and confusion. The burn patient may also develop a dilutional hyponatraemia called water intoxication. To avoid this condition, the patient should drink fluids other than water, such as juice, soft drinks or nutritional supplements.
Hypernatraemia may be seen following successful fluid resuscitation if copious amounts of hypertonic solutions were required. Other causes may be related to tube feeding therapy or inappropriate fluid administration. Manifestations of hypernatraemia include thirst; dried, furry tongue; lethargy; confusion; and possibly seizures.
Potassium
Hyperkalaemia is noted if the patient has renal failure, adrenocortical insufficiency or massive deep muscle injury (e.g. electrical burn) with large amounts of potassium released from damaged cells. Cardiac arrhythmias and ventricular failure can occur with elevated potassium levels. Muscle weakness and electrocardiographic (ECG) changes are observed clinically (see Ch 16).
Hypokalaemia occurs with vomiting, diarrhoea, prolonged GI suction and prolonged IV therapy without potassium supplementation. A constant potassium loss occurs through the burn wound. Manifestations of hypokalaemia include fatigue, muscle weakness, leg cramps, paraesthesias and decreased reflexes (see Ch 16).
COMPLICATIONS
Infection
The body’s first line of defence, the skin, has been destroyed by burn injury. Pathogens often proliferate before phagocytosis has adequately begun. The burn wound is now colonised with organisms. If the bacterial density at the junction of the eschar with underlying viable tissue rises to greater than 105/g of tissue, the patient has a burn wound infection.16 In the presence of an infection, localised inflammation, induration and sometimes suppuration can be seen at the burn wound margins. Partial-thickness burns can convert to full-thickness wounds when these organisms invade viable, adjacent, unburned tissue. Invasive wound infections may be treated with systemic antibiotics based on culture results.
Burn wound infection may progress to transient bacteraemia and sepsis as a result of burn wound manipulation (e.g. after showering and debridement; see Ch 66). Manifestations of sepsis include hypothermia or hyperthermia, increased heart and respiratory rate, decreased BP and decreased urine output. The patient may also have mild confusion, chills, malaise and loss of appetite. The WBC count will usually be 10–20 × 109/L. There are functional defects in the WBCs and the patient remains immunosuppressed for a period after the burn injury. The causative organisms of sepsis are usually gram-negative bacteria (e.g. Pseudomonas, Proteus organisms), putting the patient at further risk for septic shock.
When sepsis is suspected, cultures are immediately obtained from all possible sources, including the burn wound, blood, urine, sputum, oropharynx and perineal regions, and IV site. However, treatment should not be delayed pending results of the culture and sensitivity studies. Therapy should begin with antibiotics appropriate for the usual residual flora of the particular burns unit. The topical antibiotic in use may be continued or changed to another agent. At this stage the patient’s condition is critical, requiring close monitoring of vital signs. Collaboration with the infectious disease service is important to ensure appropriate antibiotic coverage.
Cardiovascular and respiratory systems
The same cardiovascular and respiratory systems complications present in the emergent phase may continue into the acute phase of care. In addition, new problems might arise, requiring timely intervention.
Neurological system
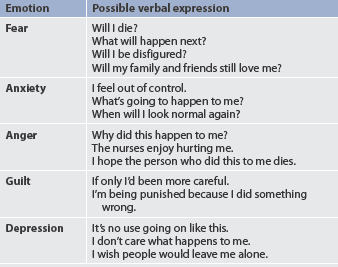
Neurologically, the patient usually has no physical symptoms, unless severe hypoxia from respiratory injuries or complications from electrical injuries occur. However, some patients may demonstrate certain behaviours that are not completely understood. The patient can become extremely disoriented, may withdraw or become combative and may have hallucinations and frequent nightmare-like episodes. Delirium is more acute at night and occurs more often in older patients. Consultation with psychiatric or geriatric services is helpful in quickly diagnosing and treating delirium or similar behaviours. The nurse can then focus on strategies to orient and reassure the confused or agitated patient. This is a transient state, lasting from 1 or 2 days to several weeks. Various causes have been considered, including electrolyte imbalance, stress, cerebral oedema, sepsis, sleep disturbances, and the use of analgesics and antianxiety drugs.
Musculoskeletal system
The musculoskeletal system is particularly prone to complications during the acute phase. As the burns begin to heal and scar tissue forms, the skin is less supple and pliant. Range of motion may be limited, and contractures can occur. Because of pain, the patient will prefer to assume a flexed position for comfort. The nurse should encourage the patient to stretch and move the burned body parts as much as possible. Splinting can be beneficial to prevent/reduce contracture formation.
Gastrointestinal system
The GI system may also exhibit complications during this phase. Paralytic ileus results from sepsis. Diarrhoea may be caused by the use of enteral feedings or antibiotics. Constipation can occur as a side effect of opioid analgesics, decreased mobility and a low-fibre diet. Curling’s ulcer is a type of gastroduodenal ulcer characterised by diffuse superficial lesions (including mucosal erosion). It is caused by a generalised stress response resulting in decreased production of mucus and increased gastric acid secretion. This condition is due to the decreased blood flow to the GI tract during the emergent phase. The best measure for preventing Curling’s ulcer is feeding the patient as soon as possible. Antacids, H2-histamine blockers and proton pump inhibitors (e.g. esomeprazole) are used prophylactically to neutralise stomach acids and inhibit histamine and the stimulation of hydrochloric acid (HCl) secretion (see Table 24-8). Patients with major burns may also have occult blood in their stools during the acute phase.
Endocrine system
An increase in blood glucose levels may be seen transiently because of stress-mediated cortisol and catecholamine release, resulting in the increased mobilisation of glycogen stores, gluconeogenesis and the subsequent production of glucose. There is also an increase in insulin production and release. However, insulin’s effectiveness is decreased because of relative insulin insensitivity, leading to an elevated blood glucose level. Later, hyperglycaemia can be caused by the increased kilojoule intake necessary to meet some patients’ metabolic requirements. When this occurs, the treatment is supplemental IV insulin, not decreased feeding. Serum glucose levels are checked frequently, and an appropriate amount of insulin is given if hyperglycaemia is present. Glucometers may be used to assess blood glucose at the bedside; serum glucose samples are more accurate than capillary blood analysis by glucometer. As the patient’s metabolic demands are met and less stress is placed on the entire system, this stress-induced condition is reversed.
 NURSING AND COLLABORATIVE MANAGEMENT: ACUTE PHASE
NURSING AND COLLABORATIVE MANAGEMENT: ACUTE PHASE
The predominant therapeutic interventions in the acute phase are: (1) wound care; (2) excision and grafting; (3) pain management; (4) physical and occupational therapy; (5) nutritional therapy and (6) psychosocial care.
 Wound care
Wound care
The goals of wound care are to: (1) prevent infection by cleansing and debriding the area of necrotic tissue that would promote bacterial growth; (2) promote wound re-epithelialisation and/or successful skin grafting; and (3) promote patient comfort.
Wound care consists of daily observation, assessment, cleansing, debridement and dressing reapplication. Non-surgical debridement, dressing changes, topical antimicrobial therapy, graft care and donor site care are performed as often as necessary, depending on the topical cream or dressing ordered. Enzymatic debriders such as a lactoperoxidase system/alginate gel may be used for the enzymatic debridement of burn wounds, which speeds up the removal of dead tissue from the healthy wound bed. Dressings that promote autolytic debridement such as a hydrocolloid dressing may be used. These dressing products either heal the wound (if the burn is not too deep) or prepare the wound bed (if the burn is deeper) for skin grafting. Wounds are cleansed with soap and tap water to gently remove the old antimicrobial agent and any loose necrotic tissue, scabs or dried blood, and a new dressing is applied. The wound is protected and dressed until it has re-epithelialised to a stage where it can be moisturised, or a retention tape is applied to protect the wound.
If grafting is necessary, the skin graft (which may be a meshed, split-thickness skin graft, discussed below) may be protected with the same greasy gauze dressings next to the graft, followed by middle and outer dressings. With facial grafts, the unmeshed sheet graft is left open, so it is possible for blebs (serosanguineous exudates) to form between the graft and the recipient bed. Blebs prevent the graft from permanently attaching to the wound bed. The evacuation of blebs is best performed by aspiration with a tuberculin syringe and only by those who have received instruction in this specialised skill.
 Excision and grafting
Excision and grafting
Current therapeutic management of full-thickness burn wounds involves early removal of the necrotic tissue followed by application of split-thickness autograft skin.17 This therapy has changed the management and mortality rate of burn patients. In the past, patients with major burns had low rates of survival because healing and wound coverage took so long that patients usually died of sepsis or malnutrition. As a result of current earlier intervention, mortality and morbidity rates have been greatly reduced. Many patients, especially those with major burns, are taken to the operating room for wound excision on day 1 or 2 (resuscitation phase). The wounds are covered with a biological dressing or allograft for temporary coverage until permanent grafting can occur (see Table 24-7).
During the procedure of excision and grafting, devitalised tissue (eschar) is excised down to the subcutaneous tissue or fascia, depending on the degree of injury. Surgical excision can result in massive blood loss, and blood conservation techniques are used to limit this complication. Topical application of adrenalin or thrombin, application of extremity tourniquets or application of new fibrin sealants all work to decrease surgical blood loss.18
Once haemostasis has been achieved, a graft is then placed on clean, viable tissue to achieve good adherence. Whenever possible, the freshly excised wound is covered with autograft (the patient’s own) skin (see Table 24-7). Recently, fibrin sealant has been used to attach skin grafts to the wound bed. Grafts can also be stapled or sutured into place. Temporary allograft can be used to test the suitability of the recipient site to accept a graft. The allograft is then removed several days later in the operating room and an autograft applied.
With early excision, function is restored and scar tissue formation is minimised. Clots between the graft and the wound keep the graft from adhering to the wound. Outer occlusive dressings apply just enough pressure to promote adherence of the graft to the wound bed and help control bleeding. Frequent observation for bleeding and circulation problems and appropriate nursing interventions can help identify and manage complications that would interfere with graft survival. Facial, neck and hand burns require skilful nursing care to identify and manage clots quickly for the best functional and aesthetic outcomes. Meshed skin grafts are often nursed closed and covered with paraffin gauze dressings followed by a wrung-out compress of 0.9% sodium chloride solution or a weak antiseptic solution such as povidone iodine 10%. These dressings are changed daily and special care is taken when removing the dressings due to possible adherence of the graft to the dressings.
Donor skin is taken from the patient for grafting by means of a dermatome, which removes a thin split-thickness layer of skin from an unburned site (see Fig 24-11). The donor skin can be meshed (usually a ratio of 1.5:1) to allow for greater wound coverage, or it may be applied as an unmeshed sheet graft for a better cosmetic result when grafting the face, neck and hands. The donor site now becomes a new open wound.
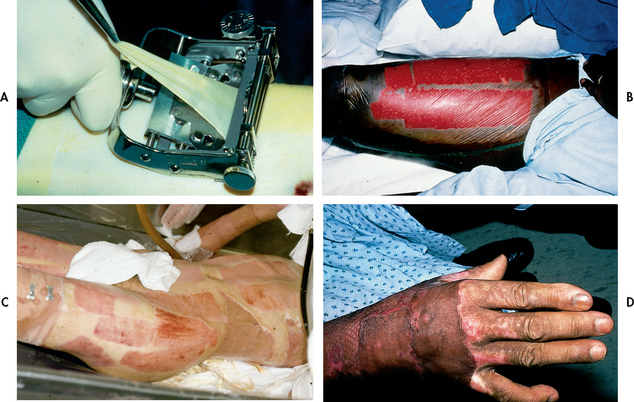
Figure 24-11 A, The surgeon harvests skin from a patient’s thigh using a dermatome. B, Appearance of donor site after harvesting split-thickness skin graft. Donor site is covered with a transparent occlusive dressing. C, Healed donor sites. D, Healed split-thickness sheet skin graft to the hand.
The goals of donor site care are to promote rapid, moist wound healing, decrease pain at the site and prevent infection. The choices of dressings vary among burns units and include calcium alginate and hydrophilic foam dressings. Nursing care of the donor site is specific to the dressing selected. Several of the newer dressing materials offer decreased healing time, which facilitates earlier reharvesting of skin at the same site. The average healing time for a donor site is 10 to 14 days.
 Cultured epithelial autografts
Cultured epithelial autografts
In the patient with large body surface area burns, only a limited amount of unburned skin may be available as donor sites for grafting, and some of that skin may be unsuitable for harvesting. Cultured epithelial autograft (CEA) is a method of wound closure with autologous skin for a person with limited available skin for harvesting. CEA is grown from biopsy specimens obtained from the patient’s own unburned skin.19 This procedure is performed in some burns units as soon as possible after admission on suitable patients. The specimens are sent to a commercial laboratory, where the biopsied keratinocytes are grown in a culture medium containing epidermal growth factor. After approximately 18–25 days, the keratinocytes have expanded up to 10,000 times and form confluent sheets that can be used as skin grafts. The cultured skin is returned to the burns unit, where it is placed on the patient’s excised burn wounds (see Fig 24-12). Because CEA grafts are made only of epidermal cells, meticulous care is required to prevent shearing injury or infection. CEA grafts generally form a seamless, smooth replacement skin tissue. Problems related to CEA include a poor graft take due to thin epidermal skin graft loss during healing, infection and contracture development.
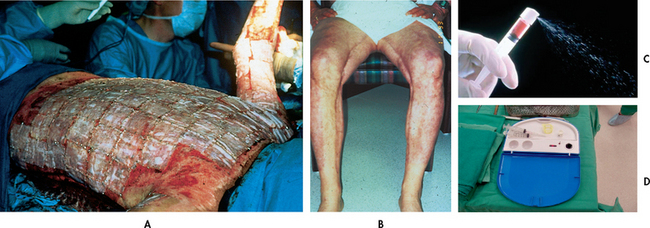
Figure 24-12 Patient with cultured epithelial autograft (CEA). A, Intraoperative application of CEA. B, Appearance of healed CEA. C, CEA spray. Cultured epithelial keratinocytes usually arrive from the skin bank in vials containing free-floating keratinocytes and growth medium. The suspension is drawn into a syringe with a wide-bore needle and a special nozzle, which will not damage the keratinocytes when the spray is applied. D, ReCell™ kit for instant preparation of CEA spray.
Source (C and D): Royal Perth Hospital, Burns Unit.
A new innovation is CEA delivered in the form of a spray of autologous keratinocytes. Instead of waiting 18 days for confluent CEA sheets, after 5–9 days pre-confluent keratinocytes in fluid suspension can be used to fill the interstices of the meshed split-thickness graft. Spraying with keratinocyte suspension enables the wound to heal rapidly, thus reducing granulation formation in the interstices, which results in a mesh pattern that is less obvious than using mesh alone.20
Currently, the ReCell™ kit is available to process the skin biopsy. A thin skin biopsy is processed into an immediate cell population for delivery onto the wound surface. The wound surface is used as the incubator for the keratinocytes.21 Dressings post-CEA application are usually Surfasoft™ dressings followed by two layers of povidone iodine 10% compresses, gauze padding and bandages. The Surfasoft™ dressing allows drainage from the wound without leakage of the keratinocytes.
 Dermal regeneration template
Dermal regeneration template
Any successful artificial skin must replace all functions of the skin and consist of both dermal and epidermal elements. The Integra artificial skin dermal regeneration template is an example of a successful skin replacement system available in burn care today. Its application requires a high degree of skill. As with CEA, it is indicated for use in the treatment of life-threatening, full-thickness or deep partial-thickness burn wounds when conventional autograft is not available or advisable, as in elderly or high-anaesthetic-risk patients. It has also been used successfully in reconstructive burn surgery procedures. As with CEA, it needs to be applied within a few days of admission for greatest success.
Integra artificial skin has a bilayer membrane composed of acellular dermis and silicone. The wound is debrided, the bilayer membrane is placed dermal layer down and the wound is wrapped with dressings in the operating room. The dermal layer functions as a biodegradable template that induces organised regeneration of new dermis by the body. The silicone layer remains intact for approximately 3 weeks as the dermal layer degrades and epidermal autografts become available. At this point, the silicone is removed during a second surgical procedure and replaced by the patient’s own epidermal autografts.22 In some situations, burns units use CEA as the source of epidermis.
Another currently available dermal replacement is AlloDerm, a cryopreserved allogenic dermis.23 Human allograft dermis, harvested from cadavers, is decellularised to render it immunogenic and then freeze-dried. Once thawed, AlloDerm is rehydrated with ultrathin epidermal autografts immediately before placement on a newly excised wound.
 Skin substitutes
Skin substitutes
Temporary skin substitutes applied after debridement provide temporary physiological wound closure when there is a lack of donor skin. Skin substitutes are human allograft, human amnion, xenografts and synthetic membrane (see Table 24-7).
 Pain management
Pain management
One of the most critical functions the nurse performs is individualised and ongoing pain assessment and management. Many aspects of burn care cause pain. However, patients experience moments of relative comfort if they receive adequate analgesia. A coordinated understanding of both the physiological and the psychological aspects of pain is essential if the nurse is to intervene with actions that are beneficial. (Pain management is discussed in Ch 9.) Burn patients experience two kinds of pain: (1) continuous, background pain that might be present throughout the day and night; and (2) treatment-induced pain associated with dressing changes, ambulation and rehabilitation activities. The first line of treatment is pharmacological (see Table 24-8). With background pain, a continuous IV infusion of an opioid will allow for a steady, therapeutic level of medication. If an IV infusion is not present, slow-release, twice-a-day opioid or opioid-like medications are indicated. Around-the-clock oral analgesics can also be used. Breakthrough doses of pain medication need to be available regardless of the regimen selected. Anxiolytics, which frequently potentiate analgesics, are also indicated and include lorazepam and midazolam.
For treatment-induced pain, premedication with an analgesic and an anxiolytic is required via the IV or oral route. For patients with an IV infusion, a potent, short-acting analgesic, such as ketamine or fentanyl, is useful. During treatment/activity, small doses should be given to keep the patient as comfortable as possible. Elimination of all pain is difficult to achieve and most patients indicate satisfaction with ‘tolerable’ levels of discomfort. Pain management is complex and ever-changing throughout the patient’s hospital stay and after discharge.
Pain can also be managed using non-pharmacological strategies. Mind–body interventions, such as relaxation, guided imagery, hypnosis, biofeedback and music therapy, are considered adjuncts to traditional pharmacological treatment of pain. They are not meant to be used exclusively to control pain but may help some patients to cope with the painful aspects of care, both in the hospital and after discharge (see Chs 7 and 8).
An important point to remember about pain management is that the more control the patient has in managing their pain, the more successful the chosen strategies. Patient-controlled analgesia (PCA) is used in selected circumstances in some burns units, with varying degrees of success. (PCA is discussed in Chs 9 and 19.) Active patient participation has also been found to be effective for some patients in anticipating and coping with treatment-induced pain.
 Physiotherapy and occupational therapy
Physiotherapy and occupational therapy
Rigorous physical therapy throughout burn recovery is imperative to maintain muscle strength and optimal joint function. A good time for exercise is during and after wound cleansing, when the skin is softer and bulky dressings are removed. Passive and active ROM should be performed on all joints. The patient with neck burns must sleep without pillows or with their head hanging slightly over the top of the mattress to encourage hyperextension. Custom-fitted splints are designed to keep joints in functional position. These must be re-examined frequently to ensure an optimal fit, with no undue pressure that might lead to skin breakdown or nerve damage.
 Nutritional therapy
Nutritional therapy
The goal of nutritional therapy during the acute burn phase is to provide adequate kilojoules and protein to promote healing. The burn patient is in a hypermetabolic and highly catabolic state as a result of the burn injury. Decreasing catecholamine release by minimising pain, fear, anxiety and cold can maximise patient comfort and conserve energy. Infection also increases the metabolic rate.
Meeting daily kilojoule requirements is crucial and should begin within the first 1–2 days postburn. The daily estimated kilojoule needs must be regularly calculated by a dietician and readjusted as the patient’s condition changes (e.g. wound healing, sepsis).
If the patient is on a mechanical ventilator or is unable to consume adequate kilojoules by mouth, a small-bore feeding tube is placed and enteral feedings are initiated. When the patient is extubated, a swallowing assessment should be performed by a speech pathologist before oral feeding is commenced. The alert patient should be encouraged to eat high-protein, high-carbohydrate foods to meet increased kilojoule needs. If carers wish to bring in favourite foods from home, this should be encouraged. Appetite is usually diminished, and constant encouragement may be necessary to achieve adequate intake. Ideally, weight loss should not be more than 10% of preburn weight. The nurse needs to record the patient’s daily kilojoule intake using kilojoule count sheets, which are monitored by the dietician. Patients are weighed routinely to evaluate progress.
 Psychosocial care
Psychosocial care
The patient and carer have many needs for psychosocial support during the often lengthy, unpredictable and complex course of care.24 The nurse and the social worker have important support and counselling roles to play. Pastoral care may also be appropriate for some patients and their carers. Support, information needs and degree of family involvement vary among cultures. At all times, the burn care team must remain culturally aware of and sensitive to individual patient and carer needs in the acute phase of care. (Patient and carer emotional needs are discussed later in this chapter and in Ch 6.)
Rehabilitation phase
The formal rehabilitation phase begins when the patient’s burn wounds have healed and the patient is able to resume a level of self-care activity. This can occur as early as 2 weeks or as long as 7–8 months after the burn injury. Goals for this period are to (1) assist the patient in resuming a functional role in society and (2) rehabilitate from functional and cosmetic reconstructive surgery. Rehabilitation-focused activities that have been taking place during the earlier emergent and acute phases begin in earnest once the patient’s wounds have healed.
PATHOPHYSIOLOGICAL CHANGES AND CLINICAL MANIFESTATIONS
Burn wounds heal either by primary intention or by grafting. Layers of epithelialisation begin rebuilding the tissue structure destroyed by the burn injury. Collagen fibres, present in the new scar tissue, assist with healing and add strength to weakened areas. The new skin appears flat and pink. In approximately 4–6 weeks, the area becomes raised and hyperemic. If adequate ROM is not instituted, the new tissue will shorten, causing a contracture. Mature healing is reached in about 12 months when suppleness has returned, and the pink or red colour has faded to a slightly lighter hue than the surrounding unburned tissue. It takes longer for more heavily pigmented skin to regain its dark colour because many of the melanocytes have been destroyed. Frequently, the skin does not regain its original colour. Paramedical cosmetic camouflage, the implantation of pigment within the skin, can help even out unequal skin tones and improve the patient’s overall appearance and self-image.
Scarring has two components: discolouration and contour. The discolouration of scars will fade somewhat with time. However, scar tissue tends to develop altered contours; that is, it is no longer flat or slightly raised but becomes elevated and enlarged above the original burned area. It is believed that pressure can help keep a scar flat. Gentle pressure can be maintained on the healed burn with custom-fitted pressure garments (e.g. Jobst or SecondSkin garments). They should never be worn over unhealed wounds and are removed only for short periods while bathing. These garments are worn up to 24 hours a day for as long as 12–18 months.
The patient typically experiences discomfort from itching where healing is occurring. Application of water-based moisturisers and use of oral antihistamines help reduce the itching. Massage oil, silicone gel sheeting, gabapentin and injectable steroids also may be helpful.25 As ‘old’ epithelium is replaced by new cells, flaking will occur. The newly formed skin is extremely sensitive to trauma. Blisters and skin tears are likely to develop from slight pressure or friction. Additionally, newly healed areas can be hypersensitive or hyposensitive to cold, heat and touch. Grafted areas are more likely to be hyposensitive until peripheral nerve regeneration occurs. Healed burn areas must be protected from direct sunlight for about 3 months to prevent hyperpigmentation and sunburn injury.
COMPLICATIONS
The most common complications during the rehabilitative phase are skin and joint contractures and hypertrophic scarring (see Fig 24-13). A contracture (an abnormal condition of a joint characterised by flexion and fixation) develops as a result of the shortening of scar tissue in the flexor tissues of the joint.26 Areas that are most susceptible to contracture formation include the anterior and lateral neck areas, axillae, antecubital fossae, fingers, groin area, popliteal fossae, knees and ankles. These areas encompass major joints. Not only does the skin over these areas develop contractures, but the underlying tissues, such as the ligaments and tendons, also have a tendency to shorten during the healing process.
Because of pain, the patient will prefer to assume a flexed position for comfort. This position predisposes wounds to contracture formation. Positioning, splinting and exercise should be instituted to minimise this complication. These procedures should be continued until the skin matures. Therapy is aimed at the extension of body parts because the flexors are stronger than the extensors. Burned legs may be wrapped with elastic bandages to assist with circulation to leg-graft and donor sites before ambulation. This additional pressure prevents blister formation, promotes venous return and decreases pain and itchiness. Once the skin is completely healed and less fragile, custom-fitted pressure garments replace the elastic bandages.
 NURSING AND COLLABORATIVE MANAGEMENT: REHABILITATION PHASE
NURSING AND COLLABORATIVE MANAGEMENT: REHABILITATION PHASE
During the rehabilitation phase, the patient and family are actively encouraged to participate in care. Since the patient may go home with small, unhealed wounds, education and ‘hands-on’ instruction will be needed in dressing changes and wound care. If needed, home care nursing services should be arranged to assist with care for the first few weeks after discharge. An emollient water-based cream (e.g. sorbolene, Vaseline) that penetrates into the dermis should be used routinely on healed areas to keep the skin supple and well moisturised, which will decrease itching and flaking. Oral antihistamines may be used if itching persists. Postburn reconstructive surgery is frequently required following a major burn. It is important for the patient to understand the need for, or possibility of, further surgery before leaving the hospital.
The continuous role of exercise and physiotherapy/occupational therapy cannot be overemphasised. Constant encouragement and reassurance are necessary to maintain the patient’s morale, particularly once the patient realises that recovery can be slow and rehabilitation may need to be a primary focus for at least the next 6–12 months.
Because of the tremendous psychological impact of a burn injury, healthcare providers should be particularly sensitive and attuned to the patient’s emotions and concerns. It is essential that patients be encouraged to discuss their fears regarding loss of their life as they once knew it, loss of function, temporary/permanent deformity and disfigurement, return to work and home life, and financial burdens resulting from a long and costly hospitalisation and rehabilitation.
Care should also be taken to address individual spiritual and cultural needs, as both these facets of a patient’s life play a role in recovery. Pastoral care and cultural groups may be helpful resources to the patient, carer and healthcare team. Patients can then be assisted towards a realistic and positive appraisal of their particular situation, emphasising what they can do instead of what they cannot do.
A person’s self-esteem may be adversely affected by a burn injury.27 In some individuals, an overwhelming fear may be the loss of relationships because of perceived or actual physical disfigurement. In a society that values physical beauty, alterations in body image can result in psychological distress. Encouraging appropriate independence, an eventual return to preburn activities and interactions with other burn survivors will involve the patient in familiar activities that may bring comfort and help to restore self-esteem. Counselling, which may have started in the acute phase of care, can be offered after discharge. Patients appreciate reassurance that their feelings during this period of adjustment are normal, and that their frustration is to be expected as they attempt to resume a normal lifestyle.
Gerontological considerations: burns
Older patients present many challenges for the burn team. The normal ageing process puts the patient at risk for injury because of the possibility of an unsteady gait, limited eyesight and diminished hearing. As people age, skin becomes drier, more wrinkled and looser. The dermal layer becomes thinner and there is a loss of elastic fibres, a reduction in subcutaneous adipose tissue and a decrease in vascularity. As a result, the thinner dermis, with reduced blood flow, sustains deeper burns with poorer rates of healing.28
Once injured, older adults can have more complications in the emergent and acute phases of burn resuscitation because of pre-existing medical conditions. For example, older patients with diabetes mellitus, heart failure or chronic obstructive pulmonary disease will have morbidity and mortality rates exceeding those of healthy, younger patients. In older patients, pneumonia is a frequent complication, burn wounds and donor sites take longer to heal and surgical procedures are less well tolerated. Weaning from a ventilator can be a challenge, and delirium from medication/anaesthesia may be a distressing, although usually self-limiting, outcome. It generally takes longer for these patients to rehabilitate to the point where they can safely return home. For some, a return home to independent living may not be possible. As the population ages, developing strategies to prevent burn injuries in older age groups is a priority.
Emotional needs of the patient and family
For the nurse to adequately manage the enormous range of emotional responses that the burn patient may exhibit, it is important to have an understanding of the circumstances of the burn, family relationships and previous coping experiences with stressful stimuli. At any time, the various emotions of fear, anxiety, anger, guilt and depression may be experienced (see Table 24-10).
A common emotional response is regression. The patient may revert to behaviour that helped in coping with stressful situations in the past. This response can be healthy and is usually short term in nature. Major emotional challenges confront patients and families throughout the burn patient’s recovery, and perhaps for years to come. As more and more independence is expected from the patient, new fears must be confronted: ‘Can I do it?’, ‘Am I a desirable partner/parent?’ Open and frequent communication among the patient, carer, close friends and burn team members is essential.
Burn survivors frequently experience thoughts and feelings that are frightening and disturbing, such as guilt about the burn incident, reliving the experience, fear of death, concern about future therapy and surgery, frustrations with ongoing discomfort and wound breakdown and, perhaps, hopelessness about the future. Family members may share some or all of these feelings. At times, they may feel helpless to assist their loved one. Continued support from trusted and familiar burn team members, particularly the nurse and social work staff, is essential. Assisting with aspects of care helps carers reconnect with their loved one and assists with the transition home. Many burn survivors and their families remark on the powerful learning experience of the burn and a renewed appreciation of life, despite the ongoing challenges of a prolonged and complex recovery. Nurses need to acknowledge that their feelings are real and common.
The stress of the burn injury occasionally precipitates a psychiatric/psychological crisis. Many patients realise that the experience is beyond their ability to cope. Assessment by a psychiatrist who can prescribe appropriate medication, if needed, and begin short-term counselling is frequently helpful. Early psychiatric intervention is essential if the patient has been previously treated for a psychiatric illness or if the injury resulted from a suicide attempt. The diagnosis of post-traumatic stress disorder is made in a number of burn patients.29 Treatment typically begins in the hospital, but links to community resources must be made before discharge to ensure continuity of psychological care. Once the patient is discharged, referral to a psychiatrist, psychologist, mental health counsellor, social worker or psychiatric clinical nurse specialist may be helpful if concerns are raised at burn clinic follow-up.
The difficult issue of sexuality must be met with honesty.30 Physical appearance will be altered in the patient who has sustained a major burn. Acceptance of any changes is difficult at first for the patient and significant other. The nature of skin injury in itself causes modifications in processing sexual stimuli. Touch is an important part of sexuality, and immature scar tissue may make the sensation of touch unpleasant or may dull it. This is usually transient, but the patient and their partner need to know that it is normal and receive anticipatory guidance from the healthcare team to avoid undue emotional strain.
Family and patient support groups may be beneficial in meeting the patient’s and family’s emotional needs at any phase of the recovery process. Speaking with others who have experienced burn trauma can be beneficial, both in terms of reaffirming that their feelings are normal and in allowing for the sharing of helpful advice. The Burn Foundation Australia (see Resources on p 568) offers support and resources to burns survivors, families and burn team personnel.
Special needs of the nursing staff
Warm, trusting, mutually satisfying relationships frequently develop between burn patients and nursing staff, not only during hospitalisation but also during the long-term rehabilitation. Sometimes the bond can be so strong that the patient has difficulty separating from the hospital and staff. The frequency and intensity of family contact can be rewarding as well as draining to the nurse. Nurses may find it difficult to cope with not only the deformities caused by burn injuries but also the odours, the unpleasant sight of the wound and the reality of the pain that accompanies a burn and its treatment.
In time, many nurses come to know that the care they provide makes a critical difference in helping patients not only to survive, but also to cope with and triumph over a severe and multifaceted injury. It is this belief that allows and inspires nurses to provide meaningful care to burn patients and their families.
Ongoing support services and critical incident stress debriefings for nursing staff led by a psychiatrist, psychologist, psychiatric clinical nurse specialist or social worker may be helpful at times. Peer support groups can serve a similar purpose by helping nurses to cope with difficult feelings they may experience when caring for burn patients. Because burn nursing is physically, psychologically and intellectually demanding, it has many challenges and inherent rewards. Attention to self-care is important for nurses to maintain a positive attitude and healthy work–life balance. Time with family and friends and rest and relaxation at home are essential parts of self-care and a life with purpose and meaning.
CASE STUDY
Patient profile
Sylvia Adams, a 54-year-old widow, was brought to the emergency department with extensive full-thickness burns to her upper body. She was rescued from her burning unit by the fire brigade. There was dense smoke in the unit when Sylvia was rescued. Her son and daughter live interstate and have been notified of her hospitalisation.
CRITICAL THINKING QUESTIONS
1. What are the first priorities for this patient in the prehospital environment? How should her airway be managed?
2. Why would this patient be considered at high risk of an inhalation injury? What interventions can be anticipated?
3. What intervention should the nurse anticipate in a patient with full-thickness circumferential burns to the extremities?
4. Describe the rationale for this patient’s lack of pain and her complaints of being cold. What drugs might be considered to promote her comfort?
5. What fluid and electrolyte disturbances would be expected in the first 48 hours of this patient’s hospitalisation? Explain the physiological bases for these changes.
6. What measures should be taken to support this patient’s family?
7. Based on the assessment data presented, write one or more appropriate nursing diagnoses. What are the collaborative problems?
Nursing research issues
1. What nursing interventions are most effective in preparing patients, families and community nurses for the early discharge and post-hospitalisation phase of burn care?
2. What pharmacological and non-pharmacological nursing interventions are most effective in the management of burn pain?
3. What nutritional supplements are best tolerated in the emergent and acute phases of burn recovery?
4. What are some expected psychological or cultural issues that may occur with burn patients?
1. In presenting a program on fire and burn prevention for parents, the nurse focuses on the most common cause of household fires as:
2. The injury that is least likely to result in a full-thickness burn is:
3. When assessing a partial-thickness burn, the nurse would expect to find:
4. The extent of burns is assessed by:
5. An 82-kg patient has a 45% TBSA burn. Using 3 mL/kg/% TBSA during the first 24 hours after a burn injury, the nurse would anticipate a fluid replacement of:
6. Fluid and electrolyte shifts that occur during the early emergent phase include:
7. To maintain a positive nitrogen balance in a major burn, the patient must:
8. Pain management for the burn patient is most effective when:
9. A therapeutic measure used to prevent hypertrophic scarring during the rehabilitative phase of burn recovery is:
1 Australian Bureau of Statistics. Causes of death. Available at www.abs.gov.au/ausstats/abs@.nsf/Latestproducts/BBE22D7978806FDBCA2576F600124132?opendocument, 2008. accessed 26 February 2011.
2 Australian Institute of Health & Welfare (AIHW). Australia’s health, 2010. Canberra: AIHW, 2010.
3 Australian and New Zealand Burn Association (ANZBA). Emergency management of severe burns: course manual, 14th edn. Brisbane: ANZBA, 2010.
4 Singer AJ, Dagum AB. Current management of acute cutaneous wounds. N Engl J Med. 2008;359:1037.
5 Mlcak RP, Suman OE, Herndon DN. Respiratory management of inhalation injury. Burns. 2007;33:2.
6 Saffle JR. Critical care management of the severely burned patient. In Parillo JE, Dellinger RP, eds.: Critical care medicine: principles of diagnosis and management in the adult, 3rd edn., Philadelphia: Saunders, 2008.
7 Nugent N, Herndon DN. Diagnosis and treatment of inhalation injury. In Herndon DN, ed.: Total burn care, 3rd edn., St Louis: Mosby, 2007.
8 Hackenschmidt A. Burn trauma priorities for a patient with 80% TBSA burns. J Emerg Nurs. 2007;33:405.
9 Pham TN, Cancio LC, Gibran NS. ABA practice guidelines: burn shock resuscitation. J Burn Care Res. 2008;29:257.
10 Greenhalgh DG, Saffle JR, Holmes JH, et al. ABA consensus conference to define sepsis and infection in burns. J Burn Care Res. 2007;28:776.
11 Makic MBF, Mann E. Burn injuries. In McQuillon KA, Makic MBF, Whalen E, eds.: Trauma nursing: from resuscitation through rehabilitation, 4th edn., St Louis: Mosby, 2009.
12 Friedman NJ, Kaiser PK. Cornea. In: Friedman NJ, Kaiser PK, eds. Essentials of ophthalmology. St Louis: Mosby, 2007.
13 The Australian immunisation handbook. 9th edn. Available at www.immunise.health.gov.au/internet/immunise/publishing.nsf/Content/Handbook-tetanus. accessed 26 February 2011.
14 Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines. 8th edn. Chest. 2008;133(suppl):S381.
15 Winkler MF, Malone AM. Medical nutrition therapy for metabolic stress: sepsis, trauma, burns and surgery. In: Mahan LK, Escott-Stump S, eds. Krause’s food and nutrition therapy. St Louis: Mosby, 2008.
16 Bauer GJ, Yurt RW. Burns. In Mandell GL, Bennett JE, Dolin R, eds.: Principles and practices of infectious diseases, 6th edn., St Louis: Mosby, 2005.
17 Mullen M, Gahankari D, Herndon DN. Operative wound management. In Herndon DN, ed.: Total burn care, 3rd edn., St Louis: Mosby, 2007.
18 Foster K, et al. Efficacy and safety of a fibrin sealant for adherence of autologous skin grafts to burn wounds: results of a phase 3 clinical study. J Burn Care Res. 2008;29:293.
19 Atiyeh BS, Costagliola M. Cultured epithelial autograft in burn treatment three decades later. Burns. 2007;33:405–413.
20 Wood FM, Kolybaba M, Allen P. The use of cultured epithelial autograft in the treatment of major burn wounds: eleven years of clinical experience. Burns. 2006;32:538–544.
21 Gravente G, DeFede M, Araco A, Grimaldi M, De Angelis B, et al. A randomised trial comparing recell system of rapid cell delivery versus classic skin grafts for the treatment of deep partial thickness burns. Burns. 2007;33:966–972.
22 Integra Life Science Corporation. Physician training module v3.0 for burns and reconstructive surgery: Integra™ dermal regeneration template. New Jersey: Integra Life Science Corporation, 2007.
23 Paul CN. Skin substitutes in burn care. Wounds. 2008;20:203.
24 Blakeney PE, Rosenberg L, Rosenberg M, et al. Psychosocial care of persons with severe burns. J Burn Care Res. 2008;34:433.
25 Brooks JA, Malic CC, Judkins KC. Scratching the surface—managing the itch associated with burns: a review of current knowledge. Burns. 2008;34:751.
26 Schneider JC, et al. Contractures in burn injury part II: investigating joints of the hand. J Burn Care Res. 2008;29:606.
27 Moi AL, Vindenes HA, Gjengedal E. The experience of life after burn injury: a new bodily awareness. J Adv Nurs. 2008;64:278.
28 Davidge K, Fish J. Older adults and burns. Geriatr Aging. 2008;11:270.
29 McKibben JBA, Bresnick MG, Wiechman ASA, et al. Acute stress disorder and post-traumatic stress disorder: a prospective study of prevalence, course and predictors in a sample with major burn injuries. J Burn Care Res. 2008;29:22.
30 Christians H, Fromhart B, Shanks T, et al. The impact of burn injury on intimacy is not adequately addressed in discharge education and after-care support. J Burn Care Res. 2008;29:S91.
Australian and New Zealand Burn Association. www.anzba.org.au
Australian Wound Management Association Inc. www.awma.com.au
Burn Foundation Australia. www.burnfoundation.org.au
Burnsurgery.org www.burnsurgery.org
Changing Faces. www.changingfaces.org.uk
Donor Tissue Bank of Victoria. www.vifm.org/n135.html
Integra LifeSciences Corporation. www.integra-ls.com
International Society for Burn Injuries. www.worldburn.org
Johnson & Johnson. www.jnj.com/home.htm
KIDS Foundation. www.kidsfoundation.org.au
McComb Research Foundation. mccomb.org.au
Research Centre for Injury Studies. www.nisu.flinders.edu.au
Sage burn diagram. www.sagediagram.com
Smith & Nephew. www.smith-nephew.com
WoundsWest. www.health.wa.gov.au/WoundsWest/education/Index.cfm