Chapter 62 NURSING MANAGEMENT: musculoskeletal trauma and orthopaedic surgery
1. Explain the aetiology, pathophysiology, clinical manifestations and multidisciplinary care of soft-tissue injuries, including strains, sprains, dislocations, subluxations, bursitis, repetitive strain injury, carpal tunnel syndrome, rotator cuff injury, meniscus injury and muscle spasms.
2. Describe the sequential events involved in fracture healing.
3. Differentiate between closed reduction, cast immobilisation, open reduction and traction in relation to purpose, complications and nursing management.
4. Describe the neurovascular assessment of an injured extremity.
5. Analyse the common complications associated with a fracture and fracture healing.
6. Describe the multidisciplinary care and nursing management of patients with specific fractures.
7. Describe the indications for, and the multidisciplinary care and nursing management of, the patient with an amputation.
8. Explore the types of joint replacement surgery associated with arthritis and connective tissue diseases.
9. Identify the preoperative and postoperative management of the patient having joint replacement surgery.
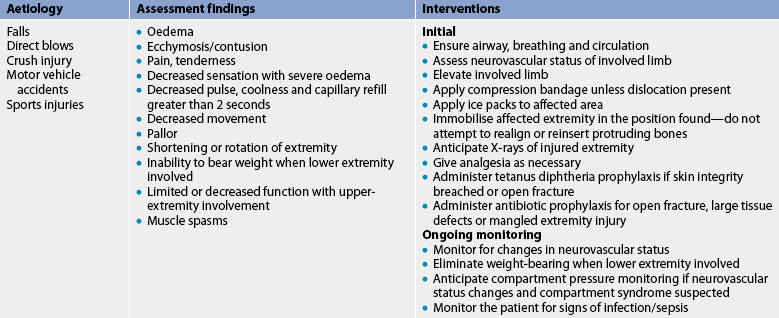
The most common cause of musculoskeletal injuries is a traumatic event resulting in fracture, dislocation and associated soft-tissue injuries. Although most of these injuries are not fatal, the cost in terms of pain, disability, medical expenses and lost wages is enormous. For all ages, accidents are exceeded only by heart disease, cancer and stroke as a cause of death.1,2
The nurse has an important role to play in educating the public about the basic principles of safety and accident prevention. The morbidity associated with accidents can be significantly reduced if people are aware of environmental hazards, use appropriate safety equipment and apply safety and traffic rules. In the occupational health and industrial setting, the nurse has the opportunity to teach employees and employers about the use of proper safety equipment and avoidance of hazardous working situations.
In home environments, falls account for many musculoskeletal injuries in adults 65 years and older. Research has shown that falls and fall-related injuries are a major cause of morbidity and mortality in older people. In response to the increasing problem, strategies for preventing falls continue to be developed in New Zealand and Australia.2,3 The evidence shows that for the older person, preventative education should be directed towards the importance of wearing shoes with functional and stable soles and heels, avoidance of wet or slippery surfaces, careful placement of carpets and removal of obstacles from the pathway of high-risk individuals, such as people with gait instability or visual or cognitive impairment. Ways to prevent common musculoskeletal problems in the older adult are listed in Box 62-1.
BOX 62-1 Prevention of musculoskeletal problems in the older adult
PATIENT & FAMILY TEACHING GUIDE
1. Use ramps in buildings and at street corners instead of steps to prevent falls.
2. Eliminate scatter rugs in the home.
3. Treat pain and discomfort from osteoarthritis.
4. Use a walker or cane to help with walking to prevent falls.
5. Eat the amount and kind of foods to prevent excess weight gain because obesity adds stress to joints, which may predispose to osteoarthritis.
6. Get regular and frequent exercise.
7. Use shoes with good support to provide safety and promote comfort.
8. Gradually initiate activities to promote optimal coordination. Rise slowly to a standing position to prevent dizziness, falls and fractures.
SOFT-TISSUE INJURIES
Soft-tissue injuries include sprains, strains, dislocations and subluxations. These common injuries are usually caused by trauma. The increase in the number of people who have committed themselves to a fitness program or who are participating in sports has contributed to the increased incidence of soft-tissue injuries and hospital admissions for sports injuries. Most sports injuries result from direct trauma, contusion or indirect sprain/strain injury and there is a growing body of literature focusing on the management of common sports injuries in Australia and New Zealand.4–6 Common sports-related injuries are summarised in Table 62-1.
TABLE 62-1 Common sports-related injuries
| Injury | Definition | Treatment |
|---|---|---|
| Impingement syndrome | Entrapment of soft-tissue structures under coracoacromial arch of the shoulder | NSAIDs; rest until symptoms decrease and then gradual ROM and strengthening exercises |
| Rotator cuff tear | Tear within muscle or tendinoligamentous structures around the shoulder | If minor tear, rest shoulder, NSAIDs and gradual mobilisation with ROm and strengthening exercises If major tear, surgical repair |
| Shin splints | Inflammation along anterior aspect of calf from periostitis caused by inappropriate shoes, overuse or running on hard pavement | Rest, ice, NSAIDs, proper shoes; gradual increase in activity; if pain persists, X-ray should be done to rule out stress fracture of tibia |
| Tendinitis | Inflammation of tendon as a result of overuse or incorrect use | Rest, ice, NSAIDs; gradual return to sporting activity; protective brace (orthosis) may be necessary if symptoms recur |
| Ligament injury | Tearing or stretching of ligament; usually occurs as a result of inversion, eversion, shearing or torque applied to a joint; characterised by sudden pain, swelling and instability | Rest, ice, NSAIDs; protection of affected extremity by use of brace; if symptoms persist, surgical repair may be necessary |
| Meniscus injury | Injury to fibrocartilage of the knee characterised by popping, clicking or tearing sensation, effusion and swelling | Rest, ice, NSAIDs; gradual return to regular activities; if symptoms persist, surgical arthroscopy may be necessary to diagnose and repair meniscus injury |
NSAIDs, non-steroidal anti-inflammatory drugs; ROm, range of motion.
Sprains and strains
Sprains and strains are the two most common types of injury affecting the musculoskeletal system. These injuries are usually associated with abnormal stretching or twisting forces that may occur during vigorous activities. These injuries tend to occur around joints and in the spinal musculature.
A sprain is an injury to tendinoligamentous structures surrounding a joint, usually caused by a wrenching or twisting motion. A sprain is classified according to the amount of ligament fibres torn. A first-degree (mild) sprain involves tears of only a few fibres resulting in mild tenderness and minimal swelling. A second-degree (moderate) sprain is partial disruption of the involved tissue with more swelling and tenderness. A third-degree (severe) sprain is a complete tearing of the ligament in association with moderate to severe swelling. A gap in the muscle may be apparent or palpated through the skin if the muscle is torn. Because areas around joints are rich in nerve endings, the injury can be extremely painful. The most common areas of sprains are the ankle and wrist.
A strain is an excessive stretching of a muscle and its fascial sheath. It often involves the tendon. Strains may also be classified as first-degree (mild or slightly pulled muscle), second-degree (moderate or moderately torn muscle) and third-degree (severely ruptured or torn muscles).7 The clinical manifestations of sprains and strains are similar and include pain, oedema, decrease in function and contusion. Pain aggravated by continued use is common. Oedema develops in the injured area because of tiny haemorrhages within the disrupted tissues and the ensuing local inflammatory response. Usually the patient will recount a history of traumatic injury, possibly of an inversion or twisting nature or recent exercise activity.
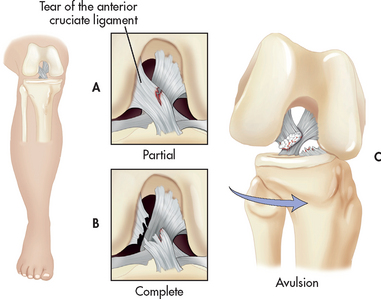
Mild sprains and strains are usually self-limiting, with full function returning within 3–6 weeks. A severe sprain can result in a concomitant avulsion fracture, in which the ligament pulls loose a fragment of bone. Alternatively, the joint structure may become unstable and result in subluxation or dislocation. At the time of injury, haemarthrosis (bleeding into a joint space or cavity) or disruption of the synovial lining may occur. An acute strain may involve partial or complete rupture of a muscle. Severe strains may require surgical suturing of the muscle and surrounding fascia.
X-rays of the affected part may be taken to rule out a fracture or widening of the joint structure. However, some healthcare providers use an assessment protocol known as the ‘Ottawa rules’ for the examination of an injured ankle or knee before ordering X-rays.8 These rules specify X-rays for a patient based on age, ability for flexion, location of tenderness and ability to bear weight immediately after the injury or when examined. Surgical repair may be necessary if the injury is significant enough to produce complete or severe disruption of ligamentous or muscle structures, fracture or dislocation.
 NURSING MANAGEMENT: SPRAINS AND STRAINS
NURSING MANAGEMENT: SPRAINS AND STRAINS
 Nursing implementation
Nursing implementation
 Health promotion
Health promotion
Stretching and warm-up prior to exercising and before vigorous activity significantly reduce sprains and strains.5 Preconditioning exercise protects an inherently weak joint because tissues tolerate slow stretching better than quick stretching. Warm-up exercises ‘prelengthen’ potentially strained tissues by avoiding the quick stretch often encountered in sports.9 Warm-up exercises also increase the temperature of muscle tissue, oxygen utilisation within muscle, cell metabolism and nerve impulse transmission. Stretching improves balance, coordination, flexibility and kinaesthetic awareness, thus lessening the chance of injury to muscle or joints.
Health impact of regular physical activity
HEALTH PROMOTION
• Helps prevent high blood pressure
• Increases lean muscle and decreases body fat
• Increases muscle strength, flexibility and endurance
• Appears to reduce symptoms of depression and anxiety
• Reduces the risk of heart disease, diabetes mellitus and colon cancer
• Lowers the death rate for adults of any age by preventative health
• Enhances psychological wellbeing and may reduce the risk of depression
Strengthening, balancing and endurance exercises are also important. Strengthening exercises involve lifting or pushing weights. These exercises build up muscle strength and bone density. Balance exercises, which may overlap with some strengthening exercises, help to prevent falls. Endurance exercises should start at a low level of effort and progress gradually to moderate activities (e.g. swimming, gardening, walking briskly). They should be sustained for 30 minutes.9
The use of elastic support bandages or adhesive tape wrapping before beginning a vigorous activity is thought to reduce the occurrence of sprains and is often used to support an injured joint post-injury while the athlete is competing and training. However, some healthcare providers do not believe in using elastic bandages or preventative wrapping or taping because they feel that it may predispose the athlete to injury.
 Acute intervention
Acute intervention
If an injury occurs, the immediate care focuses on: (1) stopping the activity and limiting movement; (2) applying ice compresses to the injured area; (3) compressing the involved extremity; (4) elevating the extremity; and (5) providing analgesia as necessary (see Table 62-2). RICE (rest, ice, compression, elevation) has been found to decrease local inflammation and pain for most musculoskeletal injuries.10 Movement should be restricted and the extremity rested as soon as pain is felt. Unless the injury is severe, prolonged rest is usually not necessary. Cold (cryotherapy) in several forms can be used to produce hypothermia to the involved part. Physiological changes that occur in soft tissue as a result of the use of cold include vasoconstriction and a reduction in the transmission and perception of nerve pain impulses. These changes result in analgesia and anaesthesia, reduction of muscle spasm without changes in muscular strength or endurance, reduction of local oedema and inflammation and reduction of local metabolic requirements. Cold is most useful when applied immediately after the injury has occurred. Ice applications should not exceed 20–30 minutes per application, allowing a ‘warm-up’ time of 10–15 minutes between applications.
Compression also helps limit swelling which, if left uncontrolled, could lengthen healing time. An elastic compression bandage can be wrapped around the injured part. The bandage is too tight if numbness is felt below the area of compression or there is additional pain or swelling beyond the edge of the bandage. The bandage can be left in place for 30 minutes and then removed for 15 minutes. However, some elastic bandages are left on during training, athletic and occupational activities.
The injured part should be elevated above the heart level to help mobilise excess fluid from the area and prevent further oedema. The injured part should be elevated even during sleep. Mild analgesics and non-steroidal anti-inflammatory drugs (NSAIDs) may be necessary to manage patient discomfort.
After the acute phase (usually lasting 24–48 hours), warm, moist heat may be applied to the affected part to reduce swelling and provide comfort. Heat applications should not exceed 20–30 minutes, allowing a ‘cool-down’ time between applications. NSAIDs may be recommended to decrease oedema and pain. The patient is encouraged to use the limb, provided that the joint is protected by means of casting, bracing, taping or splinting. Movement of the joint maintains nutrition to the cartilage, and muscle contraction improves circulation and resolution of the contusion and swelling.
 Ambulatory and home care
Ambulatory and home care
With the exception of treatment in the hospital emergency department following the injury, sprains and strains are treated in the outpatient setting. The patient should be instructed in the use of ice and elevation for 24–48 hours after the injury to reduce oedema. The use of mild analgesics to promote comfort should be encouraged. Use of an elastic bandage may provide additional support during activity. The patient should learn proper measures of strengthening and conditioning to prevent re-injury. The physiotherapist may help in providing pain relief by means of special techniques such as ultrasound. The physiotherapist may also teach the patient exercises to perform for flexibility and strength.
Dislocation and subluxation
A dislocation is a severe injury of the ligamentous structures that surround a joint. Dislocation results in the complete displacement or separation of the articular surfaces of the joint. A subluxation is a partial or an incomplete displacement of the joint surface. The clinical manifestations of a subluxation are similar to those of a dislocation but are less severe. Treatment of subluxation is similar to that of a dislocation, but subluxation may require less healing time.
Dislocations characteristically result from forces transmitted to the joint that cause a disruption of the soft-tissue support structures surrounding the joint. The joints most frequently dislocated in the upper extremity include the thumb, elbow and shoulder. In the lower extremity, the hip is vulnerable to dislocation occurring as a result of severe trauma, often associated with motor vehicle accidents (see Fig 62-1). The patella may dislocate because of instability of the tendons, ligaments and muscles surrounding the knee or from a severe twisting blow. Dislocations may also be the result of a congenital anomaly or of a pathological origin. Patellar dislocation has an increased incidence in females because the quadriceps muscles, particularly the vastus medialis, are not as toned and strong as in males. Overtraining and poor training techniques also contribute to injury.
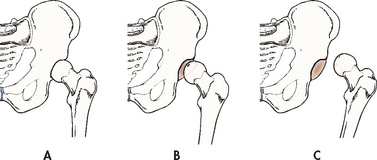
Figure 62-1 Soft-tissue injury of the hip. A, Normal. B, Subluxation (partial dislocation). C, Dislocation
The most obvious clinical manifestation of a dislocation is deformity. For example, if a hip is dislocated, the limb is shorter and often found externally rotated on the affected side. Additional manifestations include local pain, tenderness, loss of function of the injured part and swelling of the soft tissues in the region of the joint. The major complications of a dislocated joint are open joint injuries, intraarticular fractures, fracture dislocation, avascular necrosis (bone cell death as a result of inadequate blood supply) and damage to adjacent neurovascular tissue. Neurovascular assessment is critical.
X-ray studies are performed to determine the extent of displacement of the involved structures. The joint may also be aspirated to determine the presence of haemarthrosis or fat cells. Fat cells in the aspirate indicate a probable intraarticular fracture.
 NURSING AND COLLABORATIVE MANAGEMENT: DISLOCATION
NURSING AND COLLABORATIVE MANAGEMENT: DISLOCATION
A dislocation requires prompt attention and is considered an orthopaedic emergency. The longer the joint remains untreated, the greater the possibility of avascular necrosis. Compartment syndrome may also occur after dislocation and is associated with significant vascular injury. The hip joint is particularly susceptible to avascular necrosis. The first goal of management is to realign the dislocated portion of the joint in its original anatomical position. This can be accomplished by a closed reduction, which may be performed under local or general anaesthesia or intravenous (IV) conscious sedation. Anaesthesia is often necessary to produce muscle relaxation so that the bones can be manipulated. In some situations, surgical open reduction may be necessary. After reduction, the extremity is usually immobilised by bracing, splinting, taping or using a sling to allow the torn ligaments and capsular tissue time to heal.
Nursing management of subluxation or dislocation is directed towards relief of pain and support and protection of the injured joint. After the joint has been realigned and immobilised, motion is usually restricted. A carefully regulated rehabilitation program can prevent fracture instability and joint dysfunction. Gentle range of motion (ROM) may be started if the joint is stable and well supported. An exercise program slowly restores the joint to its original ROM without causing another dislocation. The patient should gradually return to normal activities.
A patient who has dislocated a joint may be at greater risk of repeated dislocations because shortened ligaments and scar tissue have weakened the joint. Activity restrictions of the affected joint may be imposed to decrease the risk of repeatedly dislocating the joint.
Repetitive strain injury
Repetitive strain injury (RSI) is a cumulative traumatic disorder resulting from prolonged, forceful or awkward movements. RSI is also known as repetitive trauma disorder, non-traumatic musculoskeletal injury, overuse syndrome (sports medicine), regional musculoskeletal disorder, work-related musculoskeletal disorder and ‘nintendinitis’ (from playing Nintendo games).11 Repeated movements strain the tendons, ligaments and muscles, causing tiny tears that become inflamed. If the tissues are not given time to heal properly, scarring can occur. Blood vessels of the arms and hands may become constricted, depriving tissues of vital nutrients and causing an accumulation of lactic acid. Without intervention, tendons and muscles can deteriorate and nerves can become hypersensitive. At this point even the slightest movement can cause pain.
In addition to the repetitive movements, other factors related to RSI include poor posture and positioning, poor work space ergonomics, a badly designed keyboard and repetitive lifting of heavy workloads without sufficient muscle rest. The result may cause inflammation, swelling and pain to the muscles, tendons and nerves of the neck, spine, shoulder, forearm and hand. Symptoms of RSI include pain, weakness, numbness or impairment of motor function. People who may be affected by RSI include musicians, dancers, butchers, factory workers, operators of vibratory tools and those who frequently use a computer mouse and keyboard.
Competitive athletes and poorly trained athletes may also develop RSI. Swimming, overhead throwing (e.g. bowlers), weightlifting, gymnastics, dancing, tennis, skiing, football (kicking sports) and horse-riding require repetitive motion and overtraining compounds the effects of RSI.
RSI can be prevented through education and ergonomics (consideration of the interaction of humans and their work environment). A few ergonomic considerations when typing include sitting with the hips and knees flexed to 90° with the feet flat, keeping the wrist straight to type, having the top of the computer screen even with the forehead and taking at least hourly stretch breaks. Once diagnosed, the treatment of RSI consists of identifying the precipitating activity, modifying equipment or the activity, managing pain (including heat/cold application), NSAIDs, rest, physiotherapy for strengthening and conditioning exercises, and lifestyle changes. There is some evidence to support the effectiveness of exercise therapy for patients with RSI.12
Carpal tunnel syndrome
Carpal tunnel syndrome (CTS) is a condition caused by compression of the median nerve, which enters the hand through the narrow confines of the carpal tunnel (see Fig 62-2). The carpal tunnel is formed by ligaments and bones. CTS is the most common compression neuropathy in the upper extremity. This condition is often caused by pressure from trauma or oedema caused by inflammation of a tendon (tenosynovitis), neoplasm, rheumatoid arthritis or soft-tissue masses such as ganglia. Symptoms of CTS are often seen during the premenstrual period, pregnancy and menopause, suggesting that hormones may be involved. People with diabetes mellitus and hypothyroidism also have a higher incidence of symptoms.13,14 This syndrome is associated with hobbies or occupations that require continuous wrist movement (e.g. butchers, tailors, musicians, painters, carpenters, computer operators, bowlers, knitters). Women are more likely than men to develop CTS, possibly due to a smaller carpal tunnel.15
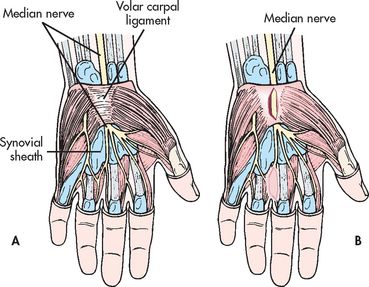
Figure 62-2 A, Wrist structures involved in carpal tunnel syndrome. B, Decompression of median nerve by incision through the transverse carpal ligament.
The clinical manifestations of CTS are weakness (especially of the thumb), burning pain (causalgia), numbness or impaired sensation in the distribution of the median nerve and clumsiness in performing fine hand movements. Numbness and tingling may awaken the patient at night. Holding the wrists for 60 seconds produces tingling and numbness over the distribution of the median nerve, the palmar surface of the thumb, index finger, middle finger and part of the ring finger (see Fig 62-3). This is known as a positive Phalen’s test.16 Tapping gently over the volar aspect of the wrist (area of the inflamed median nerve) may produce paraesthesia. This is known as a positive Tinel’s sign. In late stages there is atrophy of the thenar muscles around the base of the thumb, resulting in recurrent pain and eventual dysfunction of the hand.
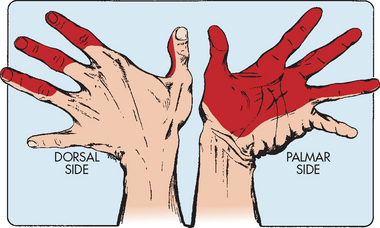
Figure 62-3 Median nerve distribution. Shaded areas depict the locations of pain in carpal tunnel syndrome.
Source: Pinnacle Systems.
 NURSING AND COLLABORATIVE MANAGEMENT: CARPAL TUNNEL SYNDROME
NURSING AND COLLABORATIVE MANAGEMENT: CARPAL TUNNEL SYNDROME
Prevention of CTS involves educating employees and employers to identify risk factors. Adaptive devices such as wrist splints may be worn to hold the wrist in a slight extension to relieve pressure on the median nerve. Special keyboard pads and mouses that help prevent repetitive pressure on the median nerve are available for computer users. Other ergonomic changes include workstation modifications, change in body positions and frequent breaks from work-related activities.
Multidisciplinary care of the patient with CTS is directed towards relieving the underlying cause of the nerve compression. The early symptoms associated with CTS can usually be relieved by stopping the aggravating movement and by placing the hand and wrist at rest by immobilising them in a hand splint. Injection of a corticosteroid drug directly into the carpal tunnel may provide short-term relief. As CTS may result in impaired sensation, the patient should be instructed to avoid hazards such as extremes in heat and cold because of the risk of thermal injury. The patient may be required to consider temporary occupational changes because of discomfort and sensory and functional changes.
If the problem continues, the median nerve may need to be surgically decompressed by longitudinal division of the transverse carpal ligament under local, regional or general anaesthesia (see Fig 62-2, B). This surgery is done on an outpatient basis or as day surgery. After surgery, the neurovascular status of the hand should be evaluated before discharge and the patient should be instructed in the appropriate assessments to perform at home. Endoscopic carpal tunnel release is a surgical procedure in which the decompression is performed through a small incision puncture site with the patient under local anaesthesia. Modified open carpal tunnel release procedure is another alternative surgical intervention. Rehabilitation can take up to 7 weeks.14
Rotator cuff injury
The rotator cuff is a complex of four muscles in the shoulder, including the supraspinatus, infraspinatus, teres minor and subscapularis muscles. These muscles act to stabilise the humeral head in the glenoid fossa while assisting with the ROM of the shoulder joint and rotation of the humerus. Degenerative changes of the rotator cuff may be associated with normal ageing.
A tear in the rotator cuff may occur as a gradual, degenerative process resulting from ageing, repetitive stress (especially overhead arm motions) or injury to the shoulder while falling.17 The rotator cuff can tear as a result of sudden adduction forces applied to the cuff while the arm is held in abduction. In sports, repetitive overhead motions, such as in swimming, racquet sports (tennis, squash) and cricket (especially pitching or bowling), are often activities that initiate injury. A fall to an outstretched hand, a blow to the upper arm, heavy lifting and repetitive work motions are also causative factors.
Manifestations of a rotator cuff injury include shoulder weakness and pain and decreased ROM. The patient usually experiences severe pain when the arm is abducted between 60 and 120° (the painful arc). The drop arm test, in which the arm falls suddenly after the patient is asked to slowly lower the arm to the side after it has been abducted 90°, is another sign. An X-ray alone is usually not beneficial in the diagnosis of a rotator cuff injury. A tear can be confirmed by arthrogram or magnetic resonance imaging (MRI).18
The goal of treatment emphasises maintaining passive ROM and the return of abduction strength. The patient with a partial tear or cuff inflammation may be treated conservatively with rest, ice and heat, NSAIDs, corticosteroid injections into the joint and physiotherapy. If the patient does not respond to conservative treatment or if a complete tear is present, surgical repair may be necessary.19 Surgical repair may be performed through an arthroscope. If an extensive tear is present, acromioplasty (surgical removal of part of the acromion to relieve compression of rotator cuff during movement) may be necessary. An immobilisation device such as a sling or, more commonly, a shoulder immobiliser may be used immediately after surgery. However, the shoulder should not be immobilised for too long because ‘frozen’ shoulder or arthrofibrosis may occur. Pendulum exercises and physiotherapy begin on the first postoperative day.
Meniscus injury
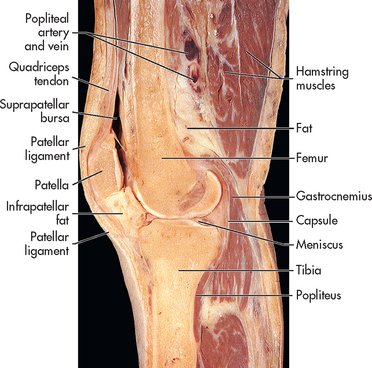
The menisci are crescent-shaped pieces of fibrocartilage in the knee. Menisci also line other joints. Meniscus injuries are closely associated with ligament sprains commonly occurring in athletes engaged in sports such as basketball, rugby, netball, soccer and hockey. These activities produce rotational stress when the knee is in varying degrees of flexion and the foot is planted or fixed. A blow to the knee can cause the meniscus to be sheared between the femoral condyles and the tibial plateau, resulting in a torn meniscus. (The knee joint is shown in Fig 62-4.) People who work in occupations that require squatting or kneeling may be at higher risk of meniscus injuries.
Meniscus injuries alone do not usually cause significant oedema because most of the cartilage is avascular. However, an acutely torn meniscus may be suspected when localised tenderness, pain and effusion are noted (see Fig 62-5). Pain is elicited by flexion, internal rotation and then extension of the knee (called the McMurray’s test). The usual clinical picture is the patient feeling that the knee is unstable and reporting that it may ‘click, pop, lock or give way’.20 Quadriceps atrophy may be evident if the injury has been present for some time. Traumatic arthritis may occur from repeated meniscus injury and chronic inflammation.
An arthrogram, arthroscopy or both can diagnose knee problems. MRI is beneficial in confirming the diagnosis before arthroscopy is used and it has also eliminated the use of an arthrogram as a diagnostic tool in many cases. Surgery may be indicated for a torn meniscus. The degree of knee pain and dysfunction, occupation, sport activities and age may affect the patient’s decision to have or postpone surgery.
 NURSING AND COLLABORATIVE MANAGEMENT: MENISCUS INJURY
NURSING AND COLLABORATIVE MANAGEMENT: MENISCUS INJURY
Injuries of the meniscus are commonly caused by sports-related activities, so athletes should be taught to do warm-up activities. Proper stretching may make the patient less prone to these kinds of injuries when falls or twisting occurs. Examination of the acutely injured knee should occur within 24 hours of injury. Initial care of this type of injury involves application of ice, immobilisation and partial weight-bearing with crutches. Most injuries of the meniscus are treated in an outpatient department. The patient should be allowed to ambulate as tolerated. Crutches may be necessary. Use of a knee brace or immobiliser during the first few days after the injury protects the knee and offers some pain relief.
After acute pain has decreased, physiotherapy can help with gradual increases in flexion and muscle strengthening to assist the patient to reach full functioning capacity. Surgical repair or excision of part of the meniscus (meniscectomy) may be necessary (see Fig 62-5).20,21 Meniscus surgery is performed by arthroscopy. Pain relief may include NSAIDs or other analgesics such as tramadol, or a mild combination of drugs such as paracetamol with codeine. Rehabilitation starts soon after surgery, including quadriceps and hamstring strengthening exercises and ROM. When the patient’s strength is back to its pre-injury level, normal activities may be resumed.
Bursitis
Bursae are closed sacs that are lined with synovial membrane and contain a small amount of synovial fluid. They are located at sites of friction, such as between tendons and bones and near the joints. Bursitis (inflammation of the bursa) results from repeated or excessive trauma or friction, gout, rheumatoid arthritis or infection. The primary clinical manifestations of bursitis are warmth, pain, swelling and limited ROM in the affected part. Sites at which bursitis commonly occurs include the hand, knee, greater trochanter of the hip, shoulder and elbow. Incorrect body mechanics, repetitive kneeling (carpet layers, coalminers and gardeners), jogging in worn-out shoes and prolonged sitting with crossed legs are common precipitating factors of injury.
Attempts should be made to determine and correct the cause of the bursitis. Rest is often the only treatment needed. Icing the area will decrease pain and may reduce local inflammation. The affected part may be immobilised in a compression dressing or splint. NSAIDs may be used to reduce inflammation and pain. Aspiration of the bursal fluid and intraarticular injection of a corticosteroid may be necessary.20 If the bursal wall has become thickened and continues to interfere with normal joint function, surgical excision (bursectomy) may be necessary. Septic bursae usually require surgical incision and drainage.
Muscle spasms
Local muscle spasms are a common condition often associated with sports and excessive everyday activities. Injury to a muscle results in inflammation and oedema, which irritates nerve endings, resulting in muscle spasm. The spasms produce additional pain, creating a repetitive cycle. The clinical manifestations of muscle spasm include pain; palpable, tense, firm muscle mass; diminished ROM if a joint is involved; and limitation of daily or occupational activities.
A careful history and physical examination should be performed to rule out central nervous system problems. Muscle spasms may be managed with medication or physiotherapy, or both. Drugs used for the treatment of local muscle spasms include mild analgesics, NSAIDs and skeletal muscle relaxants. A physiotherapy program may include the use of heat or ice, supervised exercise, massage, hydrotherapy, local heat-producing application, ultrasound (deep heat), manipulation and bracing.
FRACTURES
CLASSIFICATION
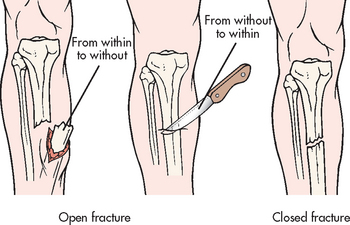
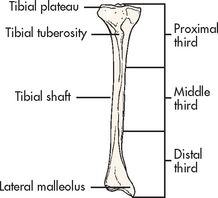
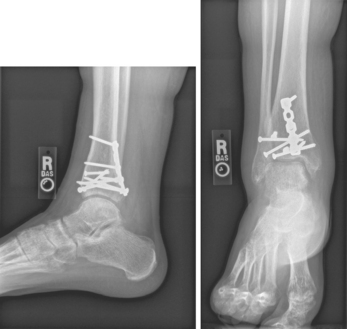
A fracture is a disruption or break in the continuity of the structure of bone. Traumatic injuries account for the majority of fractures, although some fractures are secondary to a disease process (pathological fractures from cancer or osteoporosis). Fractures are described and classified according to: (1) type (see Fig 62-6); (2) communication or non-communication with the external environment (see Fig 62-7); and (3) the location of the fracture on the involved bone (see Fig 62-8). Fractures are also described as stable or unstable. A stable fracture occurs when a piece of the periosteum is intact across the fracture and either external or internal fixation has rendered the fragments stationary. Stable fractures are usually transverse, spiral or greenstick. An unstable fracture is grossly displaced during injury and is a site of poor fixation. Unstable fractures are usually comminuted or oblique. A fracture can also be classified as closed (simple) or open. An open fracture (formerly called a compound fracture) involves communication of the fracture through the skin with the external environment (see Fig 62-7).
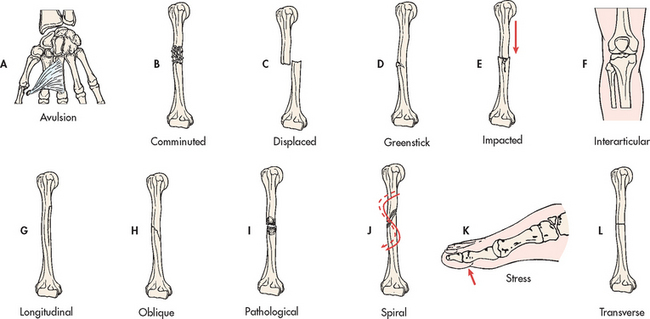
Figure 62-6 Types of fractures. A, Avulsion is a fracture of bone resulting from the strong pulling effect of tendons or ligaments at the bone attachment. B, Comminuted fracture is a fracture with more than two fragments. The smaller fragments appear to be floating. C, Displaced (overriding) fracture involves a displaced fracture fragment that is overriding the other bone fragment. The periosteum is disrupted on both sides. D, Greenstick fracture is an incomplete fracture with one side splintered and the other side bent. E, Impacted fracture is a comminuted fracture in which more than two fragments are driven into each other. F, Interarticular fracture is a fracture extending to the articular (joint) surface of the bone. G, Longitudinal fracture is an incomplete fracture in which the fracture line runs along the longitudinal axis of the bone. The periosteum is not torn away from the bone. H, Oblique fracture is a fracture in which the line of the fracture extends in an oblique direction. I, Pathological fracture is a spontaneous fracture at the site of a bone disease. J, Spiral fracture is a fracture in which the line of the fracture extends in a spiral direction along the shaft of the bone. K, Stress fracture is a fracture that occurs in normal or abnormal bone that is subject to repeated stress, such as from jogging or running. L, Transverse fracture is a fracture in which the line of the fracture extends across the bone shaft at a right angle to the longitudinal axis.
CLINICAL MANIFESTATIONS
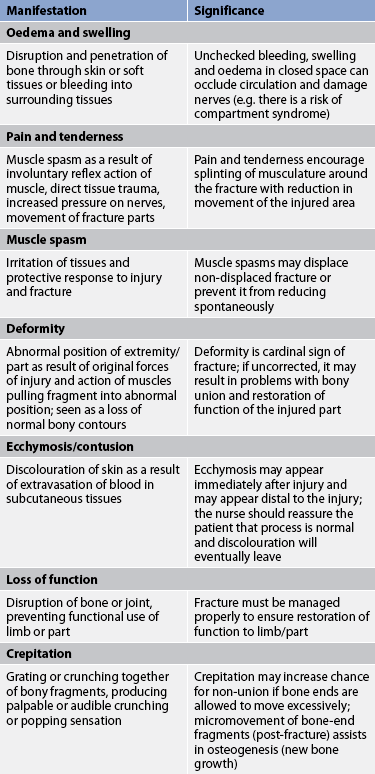
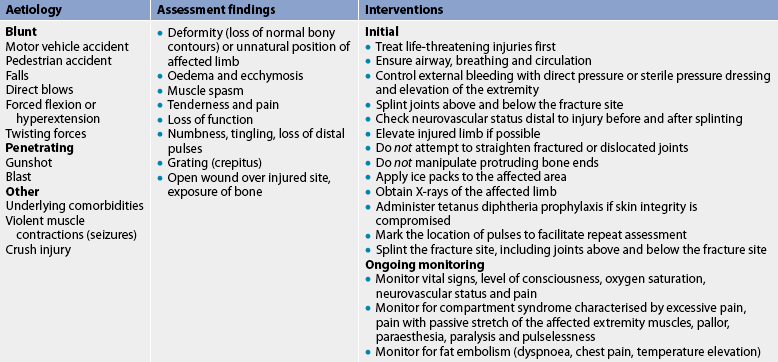
The patient’s history indicates a mechanism of injury associated with numerous signs and symptoms, including immediate localised pain, decreased function and inability to bear weight or use the affected part (see Table 62-3). The patient guards and protects the extremity against movement. The fracture may not be accompanied by obvious bone deformity. If a fracture is suspected, the extremity is immobilised in the position in which it is found. Unnecessary movement increases soft-tissue damage and may convert a closed fracture to an open fracture or create further injury to adjacent neurovascular structures.
FRACTURE HEALING
It is important to understand the principles of fracture healing (see Fig 62-9) to provide appropriate therapeutic interventions. Bone goes through a remarkable reparative process of self-healing (termed union) that occurs in the following stages:
1. Fracture haematoma. When a fracture occurs, bleeding creates a haematoma, which surrounds the ends of the fragments. The haematoma is extravasated blood that changes from a liquid to a semisolid clot. This occurs in the initial 72 hours after the injury.
2. Granulation tissue. During this stage, active phagocytosis absorbs the products of local necrosis. The haematoma converts to granulation tissue. Granulation tissue (consisting of new blood vessels, fibroblasts and osteoblasts) produces the basis for new bone substance called osteoid during days 3–14 post-injury.
3. Callus formation. As minerals (calcium, phosphorus and magnesium) and new bone matrix are deposited in the osteoid, an unorganised network of bone is formed that is woven about the fracture parts. Callus is primarily composed of cartilage, osteoblasts, calcium and phosphorus. It usually appears by the end of the second week after injury. Evidence of callus formation can be verified by X-ray.
4. Ossification. Ossification of the callus occurs from 3 weeks to 6 months after the fracture and continues until the fracture has healed. Callus ossification is sufficient to prevent movement at the fracture site when the bones are gently stressed. However, the fracture is still evident on X-ray. During this stage of clinical union the patient may be allowed limited mobility or the cast may be removed.
5. Consolidation. As the callus continues to develop, the distance between bone fragments diminishes and eventually closes. This stage is called consolidation and ossification continues. It can be equated with radiological union.
6. Remodelling. Excess bone tissue is reabsorbed in the final stage of bone healing and union is completed. Gradual return of the injured bone to its pre-injury structural strength and shape occurs. Bone remodels in response to physical loading stress or Wolff’s law.22 Initially, stress is provided through exercise. Weight-bearing is gradually introduced. New bone is deposited in sites subjected to stress and reabsorbed at areas where there is little stress.
7. Radiological union occurs when there is X-ray evidence of complete bony union. This phase can occur up to a year following injury.
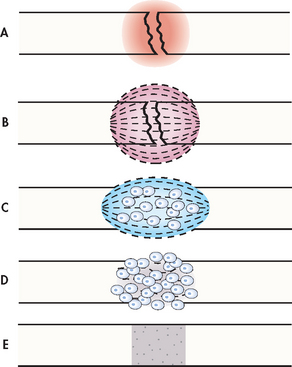
Figure 62-9 Bone healing (schematic representation). A, Bleeding at fractured ends of the bone with subsequent haematoma formation. B, Organisation of haematoma into fibrous network. C, Invasion of osteoblasts, lengthening of collagen strands and deposition of calcium. D, Callus formation: new bone is built up as osteoclasts destroy dead bone. E, Remodelling is accomplished as excess callus is reabsorbed and trabecular bone is laid down.
Many factors, such as age, initial displacement of the fracture, the site of the fracture, blood supply to the area, immobilisation, implants, infection and hormones influence the time required for fracture healing to be complete. Fracture healing may not occur in the expected time (delayed union) or may not occur at all (non-union). The ossification process is arrested by causes such as inadequate reduction and immobilisation, excessive movement of the fracture fragments, infection, poor nutrition and systemic disease. Healing time for fractures increases with age. For example, an uncomplicated midshaft fracture of the femur heals in 3 weeks in a newborn and in 20 weeks in an adult. Table 62-4 summarises complications of fracture healing.
TABLE 62-4 Complications of fracture healing
| Problem | Description |
|---|---|
| Delayed union | Fracture healing progresses more slowly than expected; healing eventually occurs |
| Non-union | Fracture fails to heal properly despite treatment, resulting in fibrous union or pseudoarthrosis |
| Malunion | Fracture heals in expected time but in unsatisfactory position, possibly resulting in deformity or dysfunction |
| Angulation | Fracture heals in abnormal position in relation to midline of structure (type of malunion) |
| Pseudoarthrosis | Type of non-union occurring at fracture site in which a false joint is formed on the shaft of long bones; it is a fracture site that failed to fuse; each bone end is covered with fibrous scar tissue |
| Refracture | New fracture occurs at original fracture site |
| Myositis ossificans | Deposition of calcium in muscle tissue at the site of significant blunt muscle trauma or repeated muscle injury |
Electrical stimulation and pulsed electromagnetic fields (PEMFs) can be used to stimulate bone healing in some situations of non-union or delayed union. The electric current acts by modifying cell mechanisms causing bone remodelling. The underlying mechanism for electrically induced bone-remodelling remains unknown. It is thought to be related to negative electrical fields attracting positive ions such as calcium. The electrodes are placed over the patient’s skin or cast and are used 10–12 hours each day, usually while sleeping.
MULTIDISCIPLINARY CARE
The overall goals of fracture treatment are: (1) anatomical realignment of bone fragments (reduction); (2) immobilisation to maintain realignment; and (3) restoration of normal or near-normal function of the injured part. Box 62-2 summarises the multidisciplinary care of fractures.
Fracture reduction
Closed reduction
Closed reduction is a non-surgical, manual realignment of bone fragments to their previous anatomical position. Traction and countertraction are manually applied to the bone fragments to restore position, length and alignment. Closed reduction is usually performed while the patient is under local or general anaesthesia. After reduction, the injured part is immobilised by traction, casting, external fixation, splints or orthoses (braces) to maintain alignment until healing occurs.
Open reduction
Open reduction is the correction of bone alignment through a surgical incision. It usually includes internal fixation of the fracture with the use of wires, screws, pins, plates, intramedullary rods or nails. The type and location of the fracture, age of patient and concurrent disease, as well as the result of attempted closed reduction by means of traction, may influence the decision to use open reduction. The chief disadvantages of this form of fracture management are the possibility of infection, the complications associated with anaesthesia and pre-morbid medical conditions (e.g. diabetes) in the patient.
If open reduction with internal fixation (ORIF) is used for intraarticular fractures (involving joint surfaces), early initiation of ROM of the joint is indicated. Machines that provide continuous passive motion (CPM) to various joints (e.g. knee, shoulder) are now available. Use of these machines can help prevent extraarticular and intraarticular adhesions and lead to faster reconstruction of the subchondral (beneath cartilage) bone plate, more rapid healing of the articular cartilage and possibly decreased incidence of later posttraumatic arthritis. ORIF facilitates early ambulation, which decreases the risk of complications related to prolonged immobility and promotes fracture healing with gradually increasing increments of stress placed on the affected joint and soft-tissue structures.
Traction
Traction is the application of a pulling force to an injured or a diseased part of the body or an extremity. The purpose of any traction is to: (1) prevent or reduce muscle spasm; (2) immobilise a joint or part of the body; (3) reduce a fracture or dislocation; and (4) treat a pathological joint condition. Traction is also indicated to: (1) provide immobilisation to prevent soft-tissue damage; (2) reduce muscle spasm associated with low back pain or cervical whiplash; (3) expand a joint space during arthroscopic procedures; and (4) expand a joint space before major joint reconstruction.23
Traction devices apply a pulling force to a fractured extremity to attain realignment while countertraction pulls in the opposite direction. The two most common types of traction are skin traction and skeletal traction. Skin traction is generally used for short-term treatment until skeletal traction or surgery is possible. Tape, boots or splints are applied directly to the skin to maintain alignment, assist in reduction and help diminish muscle spasms in the injured extremity. The traction weights are usually limited to between 2.3 and 4.5 kg. Pelvic or cervical skin traction may require heavier weights applied intermittently.
Skeletal traction (generally in place for longer periods than skin traction) is used to align injured bones and joints or to treat joint contractures and congenital hip dysplasia. It provides a long-term pull that keeps the injured bones and joints aligned. In order to apply skeletal traction the surgeon inserts a pin or wire into the bone, either partially or completely, to align and immobilise the injured body part. Weights for skeletal traction range from 2.3 to 20.4 kg. The use of too much weight can result in delayed union or non-union. The major disadvantages of skeletal traction are infection in the area of the bone where the skeletal pin has been inserted and the consequences of prolonged immobility.
When traction is used to treat fractures, the forces are usually exerted on the distal fragment to obtain alignment with the proximal fragment. Several types of traction can be used for this purpose. One of the types used is Buck’s traction (see Fig 62-10). Fracture alignment depends on correct positioning and alignment of the patient while the traction forces remain constant. For extremity traction to be effective, forces must be pulling in the opposite direction (countertraction) to prevent the patient from sliding to the end or side of the bed. Countertraction is commonly supplied by the patient’s body weight or may be augmented by elevating the end of the bed. It is imperative that the nurse maintains the traction constantly and does not alter the weight applied to the traction.
Fracture immobilisation
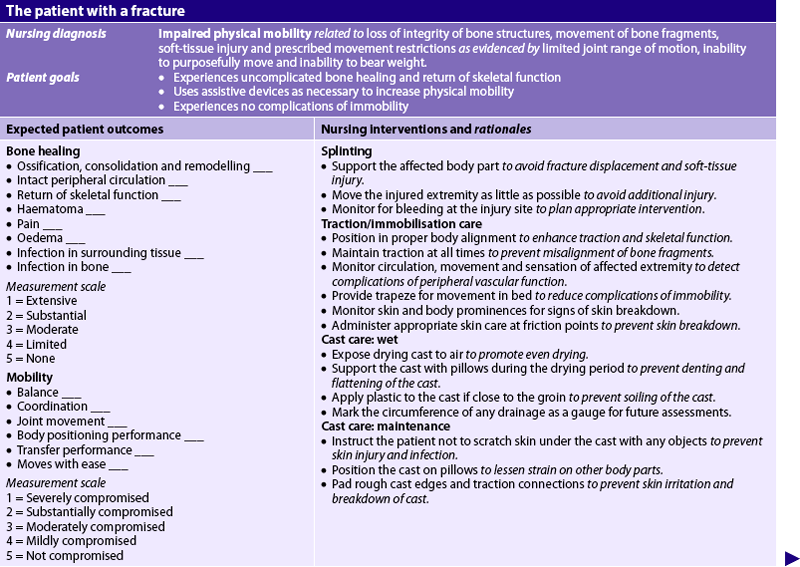
Casts
A cast is a temporary circumferential immobilisation device. Application of a cast is a common treatment following closed reduction. It allows the patient to perform many normal activities of daily living while providing sufficient immobilisation to ensure stability. Cast materials are natural (plaster of paris), synthetic acrylic, fibreglass-free, latex-free polymer or a hybrid of materials. Application of a cast generally incorporates the joint above and below a fracture. Immobilisation above and below a joint restricts tendinoligamentous movement, which assists with joint stabilisation while the fracture heals.
After immersion in water, plaster of paris is wrapped and moulded around the affected part after the bony prominences have been padded. The number of layers of plaster bandage and the technique of application determine the strength of the cast. After the cast is completely dry, it is strong and firm and can withstand stresses. The plaster sets within 15 minutes, depending on humidity, and the patient may move around without difficulty. It is not strong enough, however, for weight-bearing for about 24–72 hours.
A short time after application a new plaster cast can feel cold on the skin. It should never be covered with a blanket because air cannot circulate and heat builds up in the cast. During the drying period the cast should be kept dry and clean and direct pressure should be avoided. Once the cast is thoroughly dry, the edges may need to be petalled to avoid skin irritation from rough edges and to prevent plaster of paris debris from falling into the cast and causing irritation or pressure necrosis.
Synthetic casting materials (thermolabile plastic, thermoplastic resins, polyurethane and fibreglass) are moulded to fit the torso or extremity after being activated by submersion in cool or tepid water. Casts made of synthetic materials are being used more than plaster because they are lightweight, relatively waterproof and provide for immediate mobilisation.
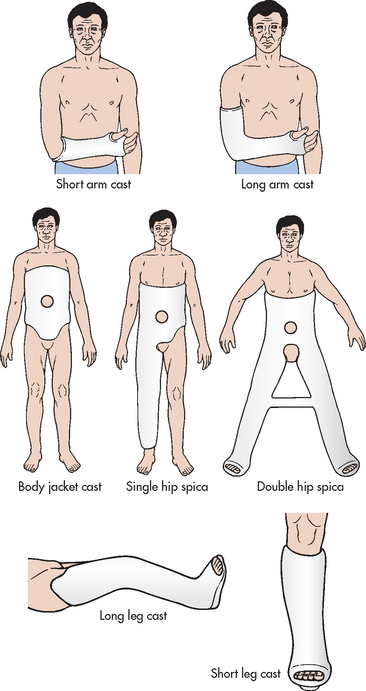
Types of casts
Immobilisation of an acute fracture or soft-tissue injury of the upper extremity is often accomplished by use of the sugar-tong splint, the posterior splint, the short arm cast and the long arm cast (see Fig 62-11). The sugar-tong splint is typically used for acute wrist injuries or injuries that may result in significant swelling. Plaster splints are applied over a well-padded forearm, beginning at the phalangeal joints of the hand, extending up the dorsal aspect of the forearm around the distal humerus and then extending down the volar aspect of the forearm to the distal palmar crease. The splinting material is wrapped with either elastic bandage or bias-cut stockinet. The sugar-tong posterior splint accommodates for swelling in the fractured extremity that occurs post-injury.
The short arm cast is often used for the treatment of stable wrist or metacarpal fractures. An aluminium finger splint can be fabricated into the short arm cast for concurrent treatment of phalangeal injuries. The short arm cast is a circular cast extending from the distal palmar area to the proximal forearm. This cast provides wrist immobilisation and permits unrestricted elbow motion.
The long arm cast is commonly used for stable forearm or elbow fractures and unstable wrist fractures. It is similar to the short arm cast but extends to the proximal humerus, restricting motion in the wrist and elbow. Nursing measures should be directed towards supporting the extremity and reducing the effects of oedema by maintaining extremity elevation with a sling. However, when a hanging arm cast is used for a proximal humerus fracture, elevation or a supportive sling is contraindicated because hanging provides traction and promotes fracture healing.
When a sling is used, the nurse must ensure that the axillary area is well padded to prevent skin excoriation and maceration associated with direct skin-to-skin contact. Placement of the sling should not put undue pressure on the posterior neck. Movement of the fingers (unless contraindicated) should be encouraged to enhance the pumping action of vascular and soft-tissue structures to decrease oedema. The nurse should also encourage the patient to actively move non-immobilised joints of the upper extremity to prevent stiffness and contractures.
The body jacket cast or brace is often used for immobilisation and support for stable spine injuries of the thoracic or lumbar spine. This cast is applied around the chest and abdomen and extends from above the nipple line to the pubis. After application of the cast, the nurse must assess the patient for the development of cast syndrome. This condition occurs if the body cast is applied too tightly and the cast compresses the superior mesenteric artery against the duodenum. The patient generally complains of abdominal pain, abdominal pressure, nausea and vomiting. The abdomen should be assessed for decreased bowel sounds (a window may be left over the umbilicus). Treatment includes gastric decompression with a nasogastric (NG) tube and suction. The cast may need to be removed or split. Nursing assessment also includes observation of respiratory status, bowel and bladder function and areas of pressure over the bony prominences, especially the iliac crest. During the time required for the cast to dry, the nurse should reposition the patient every 2–3 hours to promote even cast drying and to relieve pressure and discomfort.
The hip spica cast is used for treatment of femoral fractures. The purpose of the hip spica cast is to immobilise the affected extremity and the trunk securely. It includes two casts joined together: (1) the body jacket cast; and (2) the long leg cast. The location of the femoral fracture determines whether the thigh of the unaffected extremity will have to be immobilised to restrict rotation of the pelvis and possible hip motion on the side of the femur fracture. The hip spica cast extends from above the nipple line to the base of the foot (single hip spica) and may include the opposite extremity up to an area above the knee (hip spica and a half) or both extremities (double hip spica).
The nurse should assess the patient with a hip spica cast for the same problems that are associated with the body jacket cast. During the initial drying stage the patient should not be placed in the prone position because the cast may break. The patient should be turned to an oblique side position and supported with pillows. When the patient is repositioned, the support bar joining the thighs must never be used to assist in moving because the bar can break and cause cast disruption. After the cast has dried, the nurse (with assistance) can turn the patient to the prone position and provide pillow support under the chest and immobilised extremity. Skin care around the cast edges and the areas not encompassed by plaster is important to prevent any pressure sores. The nurse should instruct the patient in the positioning activities required to get on and off the bedpan. A slipper bedpan may be used to provide comfort and ease the movement of getting on and off the bedpan. After the hip spica cast has dried sufficiently, the patient may be instructed in ambulation techniques by the physiotherapist.
Injuries to the lower extremity
Injuries to the lower extremity are often immobilised by a long leg cast, a short leg cast, a cylinder cast, a Jones dressing or a prefabricated splint or immobiliser. The usual indications for applying a long leg cast are an unstable ankle fracture, soft-tissue injuries, a fractured tibia and knee injuries. The cast usually extends from the base of the toes to the groin and gluteal crease. The short leg cast can be used for a variety of conditions but is primarily used for stable ankle and foot injuries. A cylinder cast is used for knee injuries or fractures. The cast extends from the groin to the malleoli of the ankle. A Jones dressing is composed of bulky padding materials (absorption dressing and wellband), splints and an elastic bandage or bias-cut stockinet. After the application of a lower-extremity cast or dressing, the extremity should be elevated onto pillows above the heart level for the first 24 hours. After the initial phase, the extremity with the cast should not be placed in a dependent position because of the possibility of excessive oedema. Following cast application, the nurse should observe for signs of pressure, especially in the regions of the heel, anterior tibial border, fibular head and malleoli.
Prefabricated knee and ankle splints and immobilisers are being used in many settings. This type of immobilisation is easy to apply and remove and thus permits close observation of the affected joint for signs of swelling and skin breakdown. Depending on the injury, removal of the splint or immobiliser facilitates ROM of the affected joint and a faster return to function.
External fixation
An external fixator is a metallic device composed of metal pins. It can be used to apply traction or to compress fracture fragments and to immobilise reduced fragments when the use of a cast or other traction is not appropriate. The external device holds fracture fragments in place similar to a surgically implanted internal device. The external fixator is attached directly to the bones by percutaneous transfixing pins or wires (see Fig 62-12). External fixation is indicated in simple fractures (either open or closed), complex fractures with extensive soft-tissue damage, correction of bony defects (congenital), pseudoarthrosis, non-union or malunion and for limb lengthening.
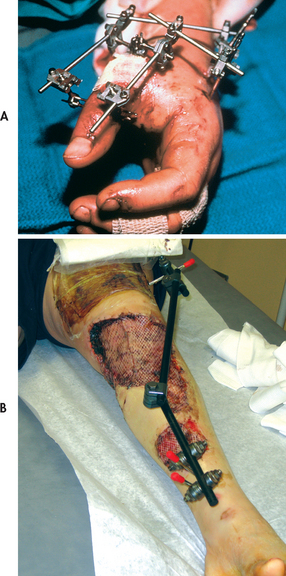
Figure 62-12 External fixators. A, Stabilisation of hand injury. B, Stabilisation of knee injury with pins in femur and tibia.
External fixation has many advantages over other fracture management strategies and is often employed in an attempt to salvage extremities that otherwise might require amputation. Because the use of an external device is a long-term process, ongoing assessment for pin loosening and infection is critical. Infection signalled by exudate, erythema, tenderness and pain may require removal of the device. The nurse should instruct the patient and family about meticulous pin care. Although each surgeon may have their own protocol for pin care cleaning, many different regimens have been used. These include regular cleaning with solutions such as half-strength hydrogen peroxide, normal saline or cooled boiled water.24,25
Internal fixation
Internal fixation devices (pins, plates, intramedullary rods and metal and bio-absorbable screws) are surgically inserted at the time of realignment (see Fig 62-13). Biologically inert metal devices such as stainless steel, Vitallium or titanium are used to realign and maintain bony fragments. Proper alignment is evaluated by X-ray studies at regular intervals.
Drug therapy
Patients with fractures experience varying degrees of pain associated with muscle spasms. Involuntary reflexes that result from oedema and nerve injury following muscle injury cause these spasms. Muscle relaxants, such as orphenadrine, may be prescribed for relief of pain associated with muscle spasms.
Common side effects associated with muscle relaxants are drowsiness, lassitude, headache, weakness, fatigue, blurred vision, ataxia and gastrointestinal upset.26 Hypersensitivity reactions may include skin rash or pruritus. Ingestion of large doses of muscle relaxants may cause hypotension, tachycardia or respiratory depression. The possible habituating effects associated with long-term use and the potential for abuse must be considered carefully.
In an open fracture the threat of tetanus can be reduced with tetanus diphtheria toxoid or tetanus immunoglobulin for the patient who has not been immunised previously. Bone-penetrating antibiotics, such as a cephalosporin, are used prophylactically.
Nutritional therapy
Proper nutrition is an essential component of the reparative process in injured tissue. An adequate energy source is needed to promote muscle strength and tone, build endurance and provide energy for ambulation and gait-training skills. The patient’s dietary requirements must include ample protein (e.g. 1 g per kg of body weight), vitamins (especially B, C and D) and calcium, phosphorus and magnesium to ensure optimal soft-tissue and bone healing.27 Low serum protein levels and vitamin C deficiencies interfere with tissue healing. Immobility and callus formation increase calcium needs. Three well-balanced meals a day will usually provide the necessary nutrients. These well-balanced meals should be supplemented by a fluid intake of 2–3 L per day to promote optimal bladder and bowel function. Adequate fluid and a high-fibre diet with fruits and vegetables will prevent constipation. If immobilised in bed with skeletal traction or in a body jacket or hip spica cast, the patient should be instructed to eat six small meals so as not to overeat and thus avoid abdominal pressure and cramping.
 NURSING MANAGEMENT: FRACTURES
NURSING MANAGEMENT: FRACTURES
 Nursing assessment
Nursing assessment
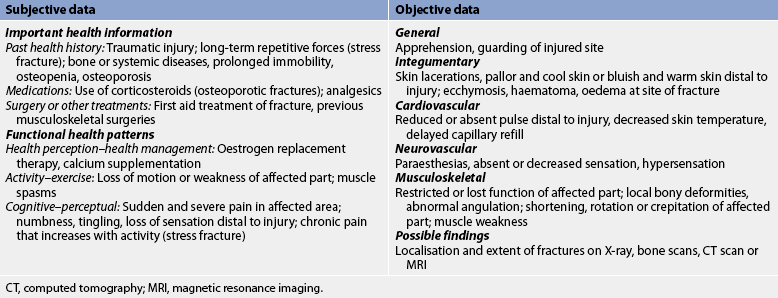
A brief history of the traumatic episode, mechanism of injury and the position in which the victim was found can be obtained from the patient or witnesses. As soon as possible, the patient should be transported to an emergency department where a thorough assessment and treatment can be initiated (see Table 62-5). Subjective and objective data that should be obtained from the patient with a fracture are presented in Table 62-6.
Special emphasis must be placed on the region distal to the site of injury. Clinical findings must be documented before fracture treatment is initiated to avoid doubts about whether a problem discovered later was missed during the original examination or was caused by the treatment.
 Neurovascular assessment
Neurovascular assessment
Musculoskeletal injuries have the potential for causing changes in the neurovascular status of an injured extremity. With musculoskeletal trauma, application of a cast or constrictive dressing, poor positioning and the physiological responses to the traumatic injury can cause nerve or vascular damage, usually distal to the injury. A thorough neurovascular assessment consists of a peripheral vascular assessment (colour, temperature, capillary refill, peripheral pulses and oedema) and a peripheral neurological assessment (sensation, motor function and pain). Throughout the neurovascular assessment, both extremities must be compared to obtain an accurate assessment.
Colour (pink, pale, cyanotic) and temperature (hot, warm, cool, cold) in the area of the affected extremity should be assessed. Pallor or a cool/cold extremity below the injury could indicate arterial insufficiency. A warm, cyanotic extremity could indicate poor venous return. Capillary refill (blanching of the nail bed) is assessed next. The standard for a compressed nail bed to return to its original colour is within 2 seconds.28 Accurate documentation and ongoing neurovascular assessments are the cornerstones of nursing care for the individual with a musculoskeletal injury.
Pulses on both the unaffected and the injured extremity are compared to identify differences in rate or quality. Pulses are described as strong, diminished, audible by Doppler or absent. A diminished or absent pulse distal to the injury can indicate vascular dysfunction and insufficiency. However, some adults do not have specific pulses, including an absent dorsalis pedis (17% of adults) and an absent posterior tibial (some people of African heritage).22 Peripheral oedema should also be assessed; pitting oedema may be present with severe injury.
Evaluating the ulnar, median and radial nerves assesses sensation and motor innervation in the upper extremity. Neurovascular status can be assessed by abduction and adduction of the fingers, opposition of the fingers and supination and pronation of the hand. In the lower extremity, dorsiflexion and plantar flexion assess motor function of the peroneal and tibial nerves. Sensory innervation is evaluated for the peroneal nerve on the dorsal part of the foot between the web space of the great and second toes. Tibial nerve assessment is performed by stroking the plantar surface (sole) of the foot. Contralateral evaluation is critical. The patient may report paraesthesia, decreased sensation, hypersensation/hyperaesthesia and partial or full loss of sensation (paresis/paralysis). Reduced motion or strength in an injured extremity alerts the nurse to potential limb-threatening complications or disability.
Pain is the final element of the neurovascular assessment. The nurse must carefully assess the location, quality and intensity of the pain (see Ch 8). Current nursing practice is to evaluate the patient’s level of pain on a scale of 0–10. Pain unrelieved by drugs and out of proportion to the level of injury is an indication of compartment syndrome (discussed later in the chapter).
Patients should be instructed to report any changes in their neurovascular status. Patients must verbalise and demonstrate a thorough understanding of all elements before being discharged from the treatment setting.
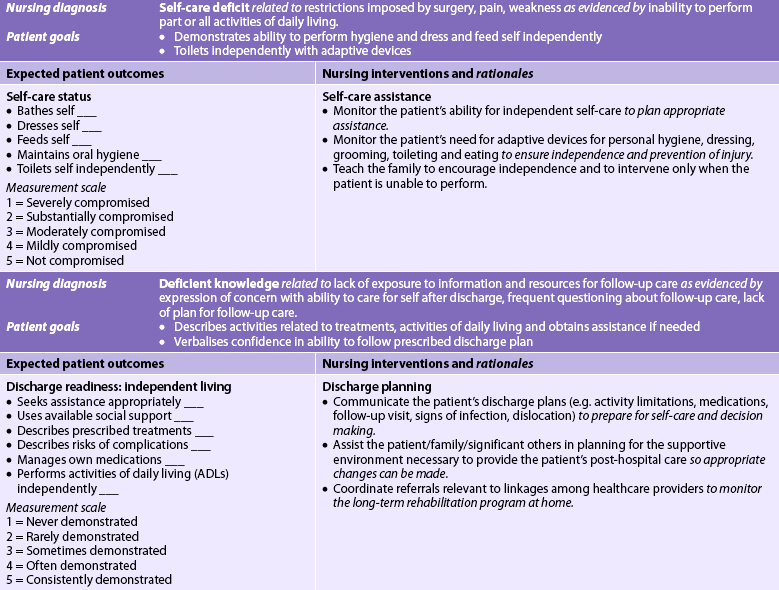
 Nursing diagnoses
Nursing diagnoses
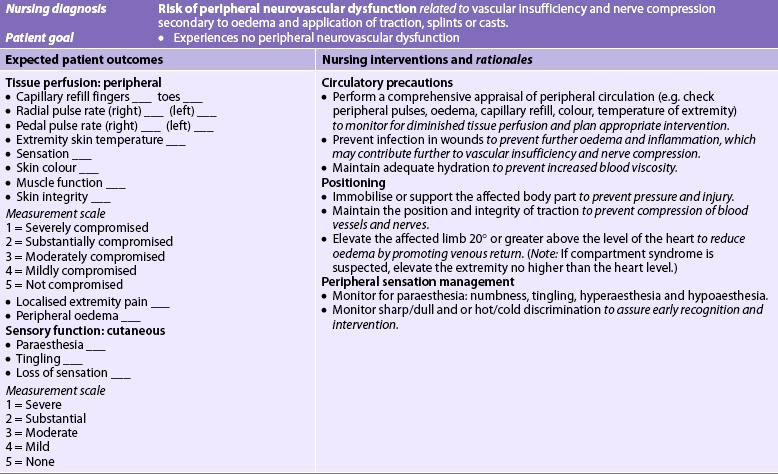
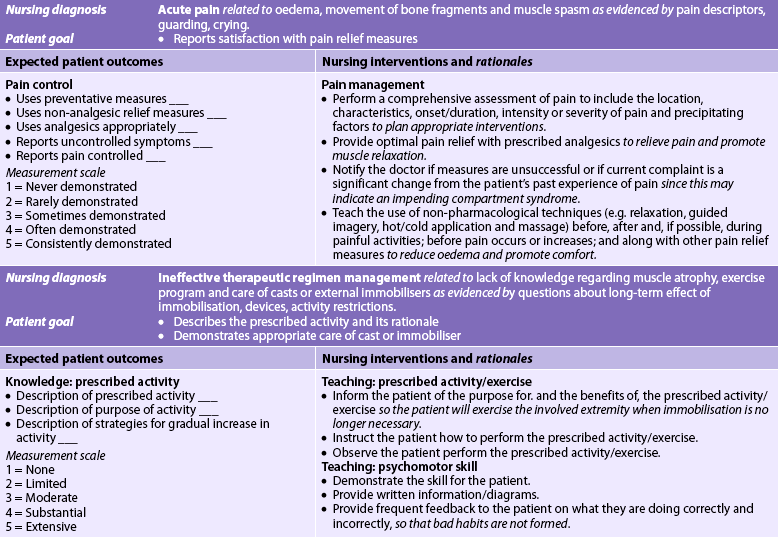
Nursing diagnoses for the patient with a fracture may include, but are not limited to, those presented in NCP 62-1.
 Planning
Planning
The overall goals are that the patient with a fracture will: (1) heal with no associated complications; (2) obtain satisfactory pain relief; and (3) achieve maximal functional rehabilitation.
 Nursing implementation
Nursing implementation
 Health promotion
Health promotion
The public should be taught to take appropriate safety precautions to prevent injuries while at home, at work, when driving or when participating in sports. Nurses should be staunch advocates for personal actions known to reduce injuries, such as regularly using seat belts, driving within speed limits, stretching and warming up muscles prior to exercise, using protective sports equipment (helmet; knee, wrist and elbow pads), using safety equipment at work and not combining drinking and driving.
Individuals (especially older adults) should be encouraged to participate in moderate exercise to aid in the maintenance of muscle strength and balance. To reduce falls, living environments should be examined so that loose carpets and rugs are removed, adequate lighting is maintained and paths to the bathroom are cleared for night-time use. The nurse should also stress the importance of adequate calcium and vitamin D intake. The Australian and New Zealand Bone and Mineral Society recommends a total daily calcium intake of 1000–1300 mg depending on age and gender, where possible through dietary intake of calcium rich foods.29
 Acute intervention
Acute intervention
Patients with fractures may be treated in an emergency department and released to home care or they may require hospitalisation for varying amounts of time. Specific nursing measures depend on the type of treatment used and the settings in which patients are placed.
 Preoperative management
Preoperative management
If surgical intervention is required to treat a fracture, patients will need preoperative preparation. In addition to the usual preoperative nursing measures (see Ch 17), the nurse should inform patients of the type of immobilisation and assistive devices that will be used and the expected activity limitations after surgery. Patients must be assured that their needs will be met by the nursing staff until they can again meet their own needs. Assurance that pain medication will be available if needed is often beneficial.
Proper skin preparation is an important part of preoperative preparation. The protocol for skin preparation varies among institutions and is usually the responsibility of the nurse. The aim of skin preparation is to assist in the cleansing of the skin and remove debris and hair to reduce the possibility of infection.30 Careful attention to this preoperative treatment can influence the postoperative course.
 Postoperative management
Postoperative management
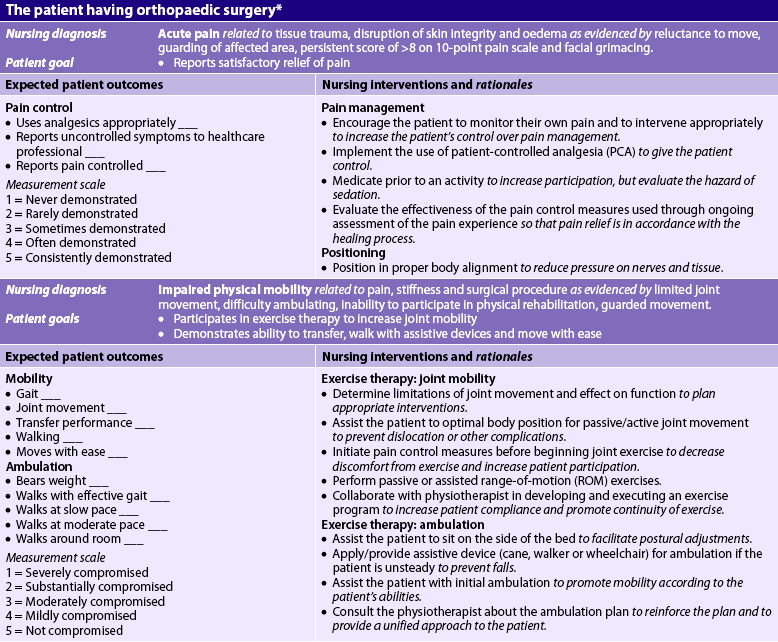
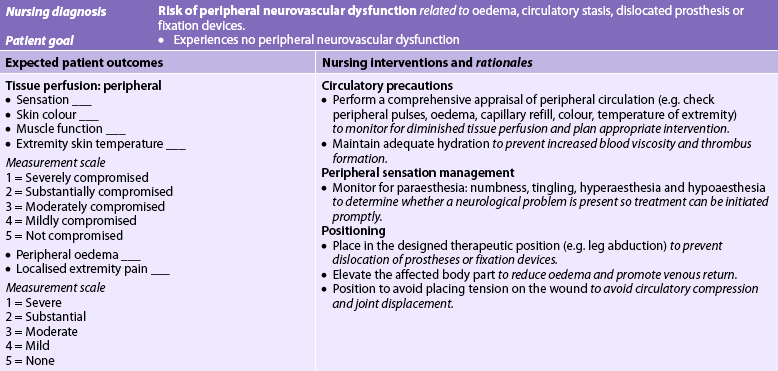
In general, postoperative nursing care and management are directed towards monitoring vital signs and applying the general principles of postoperative nursing care (see Ch 19). Frequent neurovascular assessments of the affected extremity are necessary to detect early or subtle neurovascular changes. Any limitations of movement or activity related to turning, positioning and extremity support should be monitored closely. Pain and discomfort can be minimised through proper alignment and positioning. Dressings or casts should be observed carefully for any overt signs of bleeding or drainage. A significant increase in size of the drainage area should be reported. If a wound drainage system is in place, the patency of the system and the volume of drainage should be routinely measured, assessed and documented. Whenever the contents of a drainage system are measured or emptied, nurses should use standard precautions to protect themselves and sterile technique to avoid patient contamination. Additional nursing responsibilities depend on the type of immobilisation used. A blood salvage and reinfusion system that allows for recovery and reinfusion of the patient’s own blood may be used. The blood is retrieved from a joint space or cavity and the patient receives this blood in the form of an autotransfusion.31 (Autotransfusion is discussed in Ch 30.) Additional nursing measures for the patient who has had orthopaedic surgery are discussed in NCP 62-2.
 Other measures
Other measures
Patients often have reduced mobility as a result of the fracture. The nurse must plan care to prevent the many complications associated with immobility. Constipation can be prevented by increased activity and maintenance of a high fluid intake (more than 2500 mL per day unless contraindicated by the patient’s health status) and a diet high in bulk and roughage (fresh fruit and vegetables). If these measures are not effective in maintaining the patient’s normal bowel pattern, warm fluids, stool softeners, laxatives or suppositories may be necessary. Maintaining a regular time for elimination aids in promoting regularity.
Renal calculi can develop as a result of bone demineralisation. The resulting hypercalcaemia causes a rise in urine pH and stone formation resulting from the precipitation of calcium. Unless contraindicated, a fluid intake of 2500 mL per day is recommended. Cranberry juice or ascorbic acid (500–1500 mg per day) may be recommended to acidify the urine and prevent calcium precipitation in the urine (renal calculi are discussed in Ch 45).
Rapid deconditioning of the cardiopulmonary system can occur as a result of prolonged bed rest, resulting in orthostatic hypotension and decreased lung capacity. Unless contraindicated, these effects can be diminished by permitting the patient to sit on the side of the bed, allowing the lower limbs to dangle over the bedside and allowing the patient to perform standing transfers. When the patient is allowed to increase activity, careful evaluation should be made to assess for orthostatic hypotension. Patients must also be assessed for deep vein thrombosis (DVT) and pulmonary emboli. (DVT is discussed in Ch 37.)
 Traction
Traction
When slings are used with traction, the nurse should inspect exposed skin areas as a matter of a routine. Pressure over a bony prominence created by the wrinkling of sheets or bedclothes may cause pressure necrosis. Persistent skin pressure may impair blood flow and cause injury to the peripheral neurovascular structures. Skeletal traction pin sites must be observed for signs of oozing or infection. Pin site care varies but usually includes regular removal of exudate with half-strength hydrogen peroxide, rinsing the site with sterile saline and drying the area with sterile gauze.32
External rotation of the hip can occur when skin traction is used on the lower extremity. The nurse can correct this by placing a pillow, sandbag or rolled-up draw sheet along the greater trochanteric region of the femur. Generally, the patient should be in the centre of the bed in a supine position. Incorrect alignment can result in increased pain, non-union or malunion.
To offset some of the problems associated with prolonged immobility, the nurse should discuss specific patient activity with the healthcare provider. If exercise is permitted, the nurse should encourage the patient to participate in a simple exercise regimen based on activity restrictions. Activities that the patient should participate in include frequent position changes, ROM exercises of unaffected joints, deep breathing exercises, isometric exercises and use of the trapeze bar (if permitted) to raise themselves off the bed for linen changes and use of the bedpan. These activities should be performed several times each day.
If allowed, active exercises that move uninvolved joints through the ROM are the preferred activity. Frequent exercise of the trunk and extremities is an excellent stimulus to deep breathing. Active, resistive exercise (isotonic) of uninvolved extremities helps reduce deconditioning from prolonged immobility.
 Ambulatory and home care
Ambulatory and home care
 Cast care
Cast care
Because many fractures are treated in an outpatient department, the patient often requires only a short hospitalisation or none at all. Regardless of the type of cast material, a cast can interfere with circulation and nerve function from being applied too tightly or because of excessive oedema that occurs after application. Thus frequent neurovascular assessments of the immobilised extremity are critical. The patient must be taught about signs of cast complications so that they can be reported promptly. Measures frequently used during the initial phase include elevation of the extremity above the level of the heart to promote venous return and applications of ice to control or prevent oedema. The nurse should instruct the patient to exercise joints above and below the cast. Pulling out the padding in the cast and scratching or placing foreign objects inside the cast is forbidden because it predisposes the patient to skin breakdown and infection.
Patient and family teaching is an important nursing responsibility to prevent complications. In addition to specific instructions for cast care and recognition of complications, the nurse should encourage the patient to contact the clinic or care provider should questions arise. Box 62-3 summarises patient and family instructions for cast care. The nurse should validate the patient’s and family’s understanding of these instructions before discharge from the hospital or other facility. Community nurse visits are warranted, especially with body or spica casts.
PATIENT & FAMILY TEACHING GUIDE
Do
1. Apply ice directly over the fracture site for the first 24 h (avoid getting the cast wet by keeping ice in a plastic bag and protecting the cast with cloth)
2. Check with the healthcare provider before getting the fibreglass cast wet
3. Dry the cast thoroughly after exposure to water:
4. Elevate the extremity above the level of the heart for first 48 h
5. Move joints above and below the cast regularly
6. Report signs of possible problems to the healthcare provider, including:
7. Keep the appointment to have the fracture and cast checked
*Some pain is normal, but if the pain does not respond to the pain killers that have been prescribed, or the pain is really severe and getting worse, you must contact your healthcare provider or return to the clinic or hospital as soon as possible.
When the cast is being removed patients often fear being cut by the oscillating blade of the plaster saw. The nurse should reassure the patient. More importantly, the nurse should educate the patient as to the possible alteration in the appearance of the skin that has been beneath the cast. Anxiety will also be present related to weight-bearing and continued follow-up care.
 Psychosocial problems
Psychosocial problems
Short-term rehabilitative goals are directed towards the transition from dependence to independence in performing simple activities of daily living and to preservation of, or increasing, strength and endurance. Long-term rehabilitative goals are aimed at preventing problems associated with musculoskeletal injury (see Table 62-7). An important part of nursing care during the rehabilitative phase is assisting the patient to adjust to any problems caused by the injury (e.g. separation from family, the financial impact of medical care, loss of income from inability to work, the potential for lifetime disability). The nurse must exhibit gentleness, support and encouragement and should actively listen to the patient’s and family’s fears.
TABLE 62-7 Problems associated with injury of the musculoskeletal system
| Problem | Description | Nursing considerations |
|---|---|---|
| Muscle atrophy | Decreased muscle mass normally occurs as a result of disuse following prolonged immobilisation. Loss of nerve innervation can precipitate muscle atrophy. | An isometric muscle-strengthening exercise regimen within the confines of the immobilisation device assists in reducing the amount of atrophy. Muscle atrophy interferes with and prolongs the rehabilitation process. |
| Contracture | Abnormal condition of joint characterised by flexion and fixation. Caused by atrophy and shortening of muscle fibres or by loss of normal elasticity of skin over a joint. | Can be prevented by frequent position change, correct body alignment and active–passive range-of-motion exercises several times a day. Intervention requires gradual and progressive stretching of the muscles or ligaments in the region of the joint. |
| Footdrop | Plantar-flexed position of the foot (footdrop) occurs when the Achilles tendon in the ankle shortens because it has been allowed to assume an unsupported position. Peroneal nerve palsy (a compression neuropathy) also causes footdrop. | Nursing management of the patient with long-term injuries must include preventative measures by supporting the foot in a neutral position. Once footdrop has developed, ambulation and gait training may be significantly hindered. The patient may require a splint to keep the foot/feet in a neutral position. |
| Pain | Frequently associated with fractures, oedema and muscle spasm; pain varies in intensity from mild to severe and is usually described as aching, dull, burning, throbbing, sharp or deep. | Causes of pain include incorrect positioning and alignment of the extremity, incorrect support of the extremity, sudden movement of the extremity and immobilisation device that is applied too tightly or in an incorrect position, constrictive dressings and motion occurring at the fracture site. Causes of pain should be determined so that corrective nursing action can be taken. |
| Muscle spasms | Caused by involuntary muscle contraction after fracture, muscle strain or nerve injury and may last as long as several weeks. Pain associated with muscle spasms is often intense and can last from several seconds to several minutes. | Nursing measures to reduce the intensity of the muscle spasms are similar to the corrective actions for pain control. Muscle spasms should not be massaged because massaging stimulates muscle tissue contraction that increases spasm and pain. Thermotherapy, especially heat, may reduce muscle spasm. |
 Ambulation
Ambulation
The nurse must know the overall goals of physiotherapy in relation to the patient’s abilities, needs and tolerance. Mobility training and instruction in the use of assistive aids (cane, crutches, walker) constitute major areas of responsibility of the physiotherapist. The nurse should reinforce these instructions to the patient. The patient with lower extremity dysfunction usually starts mobility training when able to sit in bed and dangle the feet over the side. This activity should be done two or three times daily for 10–15 minutes, with the nurse assisting as necessary. Collaboration between the nurse and the physiotherapist to coordinate pain management prior to a physiotherapy session will assist in patient participation at therapy sessions.
As endurance increases, the patient is instructed in the techniques of transferring from bed to chair. Progressive ambulation is usually started with parallel bars and progresses to ambulatory assistive devices. When the patient begins to ambulate, the nurse must know the patient’s weight-bearing status and the correct technique if the patient is using an assistive device. There are different degrees of weight-bearing ambulation: (1) non-weight-bearing (no weight borne) ambulation; (2) touch-down/toe-touch non-weight-bearing ambulation (contact with floor but no weight borne); (3) partial-weight-bearing ambulation (25–50% of patient’s weight borne); (4) weight-bearing as tolerated (dictated by the patient’s pain and tolerance); and (5) full-weight-bearing ambulation (no limitations).
Assistive devices
Devices for ambulation range from a cane, which can relieve up to 40% of the weight normally borne by a lower limb, to a walker or crutches, which may allow for complete non-weight-bearing ambulation. The decision about which device is appropriate for a patient is decided by the healthcare provider and involves balancing the need for maximum stability and safety against manoeuvrability (this is required in small spaces such as bathrooms and buses). Discussing with patients the requirements of their lifestyles and determining the device with which each patient feels most secure and independent is essential.
The technique for using assistive devices varies. The involved limb is usually advanced at the same time or immediately after the device is advanced. The uninvolved limb is advanced last. In almost all cases, canes are held in the hand opposite the involved extremity.
The common gait patterns with assistive devices are the two-point gait, the four-point gait, the swing-to gait and the swing-through gait:
• Two-point gait. Crutch on one side advances simultaneously with the opposite foot; this gait is also used with cane ambulation.
• Four-point gait. A slower version of the two-point gait, each ‘point’ is advanced separately.
• Swing-to gait. Both crutches are advanced together, followed by the lifting of both lower limbs to the same place; this gait is also used with walkers.
• Swing-through gait. This gait is similar to the swing-to gait, but the patient swings the body past the crutches. An alternate four-point/sweep-through gait for patients with concurrent visual and neuromuscular disability enables the crutches to ‘explore’ the upcoming terrain before they are placed in the traditional position.
A transfer belt should be placed around the patient’s waist to provide stability during the learning stages. The nurse should discourage the patient from reaching for furniture or relying on another person for support. When there is inadequate upper limb strength or poorly fitted crutches, the patient bears weight at the axilla rather than at the hands, endangering the neurovascular bundle that passes across the axilla. If verbal coaching does not correct the problem, the patient should be instructed in another form of ambulation until strength is adequate (e.g. platform crutches, walker).
Patients who must ambulate without weight-bearing require sufficient upper limb strength to lift their own weight at each step. Because the muscles of the shoulder girdle are not accustomed to this work, they require vigorous and diligent training in preparation for this task. Push-ups, pull-ups using the overhead trapeze bar and lifting weights develop the triceps and biceps. Straight-leg raises and quadriceps-setting exercises strengthen the quadriceps.
 Counselling and referrals
Counselling and referrals
During the rehabilitative process the patient’s family assumes an important role in the provision and follow-through of long-term care plans. The family must be instructed in the techniques of strength and endurance exercises, assistance with mobility training and promoting activities that enhance the quality of daily living. Sexual counselling should be included in discharge planning. Unless nurses have specific training for sexual health counselling, they should remember that wrong answers may be more harmful than no answers. For referral purposes, nurses must know whether weight-bearing status will affect sexual activity and whether any immobilisation or support devices are necessary.
Patients also need to be evaluated for posttraumatic stress disorder. This is especially important if significant injury to others or fatalities were associated with the patient’s injuries.
COMPLICATIONS OF FRACTURES
The majority of fractures heal without complications. If death occurs after a fracture, it is usually the result of damage to underlying organs and vascular structures or from complications of the fracture or immobility. Complications of fractures may be either direct or indirect. Direct complications include problems with bone infection, bone union and avascular necrosis. Indirect complications of fractures are associated with blood vessel and nerve damage resulting in conditions such as compartment syndrome, venous thrombosis, fat embolism and traumatic or hypovolaemic shock.33 Although most musculoskeletal injuries are not life-threatening, open fractures or fractures accompanied by severe blood loss and fractures that damage vital organs (such as the lungs, heart or bladder) are medical emergencies requiring immediate attention.
Infection
Open fractures and soft-tissue injuries have a high incidence of infection. An open fracture usually results from the impact of severe external forces. Massive or blunt soft-tissue injury often has more serious consequences than the fracture. Devitalised and contaminated tissue is an ideal medium for many common pathogens, including gas-forming (anaerobic) bacilli. Treatment of infection is costly in terms of extended nursing and medical care, time for treatment and loss of patient income. Osteomyelitis can become chronic (see Ch 63).
MULTIDISCIPLINARY CARE
Open fractures require aggressive surgical debridement.34 The wound is initially cleansed by jet pulsed lavage in the operating room. Gross contaminants are irrigated and mechanically removed. Contused, contaminated and devitalised tissue, such as muscle, subcutaneous fat, skin and fragments of bone, are surgically excised (debridement). The extent of the soft-tissue damage determines whether the wound will be closed at the time of surgery, whether closed suction drainage may be necessary and whether skin grafting will be needed. Depending on the location and extent of the fracture, reduction may be maintained by external fixation or traction. During surgery the open wound may be irrigated with antibiotic solution. Antibiotic impregnated beads may also be placed in the surgical site. During the postoperative phase the patient will have antibiotics administered intravenously for 3–7 days. Antibiotics, in conjunction with aggressive surgical management, have greatly reduced the occurrence of infection.
Compartment syndrome
Muscles are contained within non-elastic fascia (tissue) called compartments. There are thirty-eight compartments located in the upper and lower extremities of the body. Figure 62-14 shows the compartments of the lower leg. Compartment syndrome is a serious complication of fractures and is a condition in which elevated intracompartmental pressure within a confined myofascial compartment compromises the neurovascular function of tissues within that space. Compartment syndrome causes capillary perfusion to be reduced below the level necessary for tissue viability and is classified as acute, chronic/exertional or crush syndrome. There are two basic types of compartment syndrome: (1) decreased compartment size resulting from restrictive dressings, splints, casts, excessive traction or premature closure of fascia; and (2) increased compartment content related to bleeding, oedema, chemical response to snakebite or IV infiltration. Depending on the patient’s age and body mass index, the expected range of intracompartmental pressure readings is 0–15 mmHg. Readings of 30–50 mmHg indicate compartment syndrome.35
Oedema is a physiological response to soft-tissue injury in the general region of trauma and may elevate the compartment pressure. This can create sufficient pressure to obstruct circulation and cause venous occlusion, which increases oedema. Eventually arterial flow is compromised, resulting in inadequate arterial circulation (ischaemia) to the extremity. As ischaemia continues, muscle and nerve cells are destroyed over time and fibrotic tissue replaces the healthy tissue. Contracture, disability and loss of function can occur. Delay in diagnosis and treatment can result in irreversible muscle and nerve ischaemia, resulting in a functionally useless or severely impaired extremity.
Compartment syndrome is associated with trauma, fractures, extensive soft-tissue damage, crush injury, reperfusion syndrome, severe burns, venomous snakebite or following knee or leg surgery. Prolonged pressure on a muscle compartment may occur when someone is trapped under a heavy object or a person’s limb is trapped beneath the body because of an obtunded state, such as drug or alcohol overdose. It has even been known to occur as a result of a massive infiltration of IV fluids. Exertional compartment syndrome may occur after intensive exercise.36 The upper arm and lower leg are the most common sites of compartment syndrome. Fractures of the distal humerus and proximal tibia are the most common fractures associated with compartment syndrome. In the upper extremity this condition is referred to as Volkmann’s ischaemic contracture (see Fig 62-15) and in the lower extremity as anterior tibial compartment syndrome, although the underlying pathophysiological mechanism is similar.
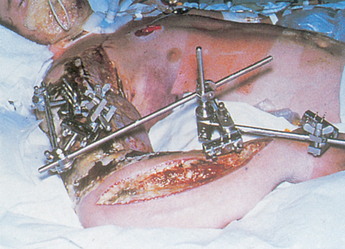
Figure 62-15 Volkmann’s ischaemic contracture of the forearm following acute compartment syndrome secondary to a supracondylar fracture of the humerus. Note the incision line of an unsuccessful fasciotomy.
CLINICAL MANIFESTATIONS
Early recognition and treatment of compartment syndrome are essential to avoid permanent damage to muscles and nerves. Ischaemia can occur within 4–12 hours after onset. Planned neurovascular assessments should be performed and documented for all patients with fractures, but especially those with injury of the distal humerus or proximal tibia or soft-tissue disruption in these areas. Compartment syndrome may occur initially from the physiological response of the body or may be delayed for several days from the original insult/injury.
The six Ps are characteristic of an impending compartment syndrome: (1) paraesthesia (numbness and tingling); (2) pain distal to the injury that is not relieved by opioid analgesics and pain on passive stretch of muscle travelling through the compartment; (3) pressure increases in the compartment; (4) pallor, coolness and loss of normal colour of the extremity; (5) paralysis or loss of function; and (6) pulselessness or diminished/absent peripheral pulses. The patient may present with one or all of the six Ps. Absence of a peripheral pulse and paralysis are late and ominous signs that indicate a severe disturbance of neurovascular status. The patient’s surgeon should be notified immediately of a patient’s changing condition.36
Because of the possibility of muscle damage, urine output must be assessed. Myoglobin released from damaged muscle cells precipitates as a gel-like substance causing obstruction in renal tubules because of its high molecular weight. Large amounts of myoglobinaemia may result in acute tubular necrosis, which causes acute kidney injury. Common signs of myoglobinuria are dark reddish-brown urine and clinical manifestations associated with acute kidney injury (see Ch 46).
MULTIDISCIPLINARY CARE
Prompt, accurate diagnosis of compartment syndrome is critical. Prevention or early recognition is the key. Elevation of the extremity may raise venous pressure and slow arterial perfusion; thus the extremity should not be elevated above heart level. Similarly, the application of cold compresses may result in vasoconstriction and exacerbate compartment syndrome. Elevation and ice should not be used in patients with suspected compartment syndrome. It may also be necessary to remove or loosen the bandage and bivalve the cast. A reduction in traction weight may also decrease external circumferential pressures.
Surgical decompression (e.g. fasciotomy) of the involved compartment may be necessary.35 The fasciotomy site is left open for several days to ensure adequate soft-tissue decompression. Infection resulting from delayed wound closure is a potential problem following a fasciotomy. Severe compartment syndrome may require amputation to decrease myoglobinaemia or to replace a functionally useless extremity with a prosthesis.
Venous thrombosis
The veins of the lower extremities and pelvis are highly susceptible to thrombus formation after fracture, especially hip fracture. Precipitating factors are venous stasis caused by incorrectly applied casts or traction, local pressure on a vein and immobility. Venous stasis is aggravated by inactivity of the muscles that normally assist in the pumping action of venous blood returning to the extremities. In addition to wearing compression gradient stockings (antiembolic stockings) and using sequential compression devices, the patient should be instructed to move (dorsiflex/plantarflex) the fingers or toes of the affected extremity against resistance and to perform ROM exercises on the unaffected lower extremities.
Because of the high risk of venous thrombosis in the patient with limited mobility, prophylactic anticoagulant drugs, such as aspirin, warfarin or heparin, may be ordered. Low-molecular-weight heparin (e.g. enoxaparin) is frequently used to prevent venous thrombosis. Because low-molecular-weight heparin has a predictable dose response, monitoring of prothrombin time is not necessary. A newer class of antithrombotic drugs (e.g. fondaparinux) works by inhibiting factor Xa, a key blood-clotting component. (Assessment and management of venous thrombosis are discussed in Ch 37.)
Fat embolism syndrome
Fat embolism syndrome (FES) is characterised by the presence of systemic fat globules that are distributed into tissues and organs after a traumatic skeletal injury.37 FES is a contributory factor in many deaths associated with fractures. The fractures that most often cause FES are those of the long bones, ribs, tibia and pelvis. FES has also been known to occur following total joint replacement, spinal fusion, liposuction, crush injuries and bone marrow transplantation. Two theories related to the origin of fat emboli include the mechanical theory and the biochemical theory. The mechanical theory suggests that fat is released from the marrow of injured bone. It is driven out by an increase in intramedullary pressure and enters the circulation through draining veins, travelling to pulmonary capillaries, where it lodges. The fat droplets traverse the capillary bed to enter the systemic circulation where they then embolise to other organs such as the brain. The biochemical theory postulates that catecholamines released at the time of trauma mobilise free fatty acids from the adipose tissue, causing the loss of chylomicron emulsion stability. The chylomicrons form large fat globules that eventually lodge in the lungs. This biochemical change initiated by injury sets up an inflammatory response secondary to destabilisation of free fatty acids, which causes a biochemical injury to the lung parenchyma. The tissues of the lungs, brain, heart, kidneys and skin are most often affected.
CLINICAL MANIFESTATIONS
Early recognition of FES is crucial in preventing a potentially lethal course. Initial manifestations usually occur 12–60 hours after injury.38 Severe forms have occurred within hours of injury. The fat globules transported to the lungs cause a haemorrhagic interstitial pneumonitis that produces signs and symptoms of acute respiratory distress syndrome (ARDS), such as chest pain, tachypnoea, cyanosis, dyspnoea, apprehension, tachycardia and decreased partial pressure of arterial oxygen (PaO2). All of these symptoms are caused by poor oxygen exchange. Because they are frequently the presenting symptoms, changes in mental status as a result of hypoxia are important to recognise. Memory loss, restlessness, confusion, elevated temperature and headache prompt further investigation so that central nervous system involvement is not mistaken for alcohol withdrawal or acute head injury. The continued change in level of consciousness and petechiae located around the neck, anterior chest wall, axilla, buccal membrane and conjunctiva of the eye help distinguish fat emboli from other problems. Petechiae result from intravascular thromboses caused by decreased oxygenation.
The clinical course of FES may be rapid and acute. Frequently the patient expresses a feeling of impending disaster. In a short time, skin colour changes from pallor to cyanosis and the patient may become comatosed. No specific laboratory examinations are available to aid in the diagnosis. However, certain diagnostic abnormalities may be present. These include fat cells in the blood, urine or sputum; a decrease of the PaO2 to less than 60 mmHg; ST segment changes on electrocardiogram; a decrease in the platelet count and haematocrit levels; and a prolonged prothrombin time resulting from haemorrhaging into the lungs. A chest X-ray may reveal areas of pulmonary infiltrate or multiple areas of consolidation. This is sometimes referred to as the white-out effect.
MULTIDISCIPLINARY CARE
Treatment for fat embolism is directed at prevention.39 Careful immobilisation of a long bone fracture is probably the most important factor in the prevention of fat embolism. Management of FES is essentially symptom related and supportive and includes fluid resuscitation to prevent hypovolaemic shock, correction of acidosis and replacement of blood loss. Coughing and deep breathing should be encouraged. The patient should be repositioned as little as possible before fracture immobilisation or stabilisation because of the danger of dislodging more fat droplets into the general circulation. Use of corticosteroids to prevent or treat fat embolism is controversial. Oxygen is administered to treat hypoxia. Intubation or intermittent positive pressure breathing may be considered if a satisfactory PaO2 cannot be obtained with supplementary oxygen alone. Some patients may develop pulmonary oedema, ARDS or both, leading to an increased mortality rate. Most people survive FES with few sequelae.
Colles’ fracture
A Colles’ fracture is a fracture of the distal radius and is one of the most common fractures in adults. The styloid process of the ulna may be involved as well. The injury usually occurs when the patient attempts to break a fall with an outstretched hand. This type of fracture most often occurs in women over the age of 50 whose bones are osteoporotic. The clinical manifestations of Colles’ fracture are pain in the immediate area of injury, pronounced swelling and dorsal displacement of the distal fragment (dinner-fork deformity). This will appear as an obvious deformity of the wrist. The major complication associated with a Colles’ fracture is vascular insufficiency as a result of oedema. Carpal tunnel syndrome can be a later complication.
A Colles’ fracture is usually managed by closed manipulation of the fracture and immobilisation by either a splint or a cast or, if displaced, by internal or external fixation. The elbow must be immobilised to prevent wrist supination and pronation. Nursing management should include measures to prevent or reduce oedema and frequent neurovascular assessments. Support and protection of the extremity should be provided, along with encouragement of active movement of the thumb and fingers. This type of movement helps reduce oedema and increases venous return. The patient should be instructed to perform active movements of the shoulder to prevent stiffness or contracture.
Fracture of the humerus
Fractures involving the shaft of the humerus are a common injury among young and middle-aged adults. The prominent clinical manifestations are an obvious displacement of the humerus shaft, a shortened extremity, abnormal mobility and pain (see Fig 62-16). The major complications associated with fracture of the humerus are radial nerve injury and vascular injury to the brachial artery as a result of laceration, transection or muscle spasm.
Figure 62-16 A, Supracondylar fracture of the humerus. This type of injury results in the formation of a large haematoma. B, Fracture of distal shaft of humerus.
The treatment for a fracture of the humerus depends on the location and displacement of the fracture. Non-operative treatment may include a hanging arm cast, a shoulder immobiliser or the sling and swathe, which is a type of immobilisation that prevents glenohumeral movement. The swathe encircles the trunk and humerus as an additional binder. It is often used for surgical repairs and shoulder dislocation.
When these devices are used, the head of the bed should be elevated to assist gravity in reducing the fracture. The arm should be allowed to hang freely when the patient is sitting and standing. Nursing care should include measures to protect the axilla and prevent skin maceration by placing lightly powdered absorbable dressing pads in the axilla and changing them twice daily or as needed. Skin or skeletal traction may be used for purposes of reduction and immobilisation.
During the rehabilitative phase an exercise program geared towards improving strength and motion of the injured extremity is extremely important. This should include assisted motion of the hand and fingers. The shoulder can also be exercised if the fracture is stable. This helps to prevent stiffness secondary to frozen shoulder or arthrofibrosis.
Fracture of the pelvis
Pelvic fractures range from benign to life-threatening depending on the mechanism of injury and associated vascular insult. High-speed vehicular or motorcycle crashes or skiing accidents can result in open book (anterior–posterior compression) fractures, leading to haemorrhagic life-threatening situations. An open book fracture is sustained when the external force pulls the pelvis apart, such as when struck or crushed from the front. A closed book fracture is sustained from lateral force compression, whereas a vertical shear injury is the result of a fall. Although only a small percentage of all fractures are pelvic fractures, this type of injury is associated with the highest mortality rate.40 Preoccupation with associated injuries at the time of a traumatic event may result in an oversight of pelvic injuries. Pelvic fractures may cause serious intraabdominal injury, such as paralytic ileus, haemorrhage and laceration of the urethra, bladder or colon. Patients may survive the initial pelvic injury, only to die from sepsis, FES or DVT complications.
Physical examination demonstrates local swelling, tenderness, deformity, unusual pelvic movement and ecchymosis on the abdomen. The neurovascular status of the lower extremities and manifestations of associated injuries should be assessed. Pelvic fractures are diagnosed by X-ray and computed tomography (CT) scan.
Treatment of a pelvic fracture depends on the severity of the injury. Stable, non-displaced fractures, such as those sustained in a fall, require limited intervention and early mobilisation. Bed rest for stable pelvic fractures is maintained from a few days to 6 weeks. More complex fractures may be treated with pelvic sling traction, skeletal traction, hip spica casts, external fixation, open reduction or a combination of these methods. Open reduction and internal fixation of a pelvic fracture may be necessary if the fracture is displaced.41 Extreme care in handling or moving the patient is important to prevent serious injury from a displaced fracture fragment. Because a pelvic fracture can damage other organs, assessment of bowel and urinary tract function and distal neurovascular status are important nursing measures.
The patient should be turned only when specifically ordered by the surgeon. Back care is provided while the patient is raised from the bed either by independent use of the trapeze or with adequate assistance. Weight-bearing on the affected side should be avoided until healing is complete. If the pelvic fracture is non-displaced, the patient is usually allowed to ambulate using a walker or crutches to distribute weight-bearing between the upper and lower extremities.
Fracture of the hip
Hip fractures are a common clinical problem in older adults in both New Zealand and Australia, with 20,000 hip fractures per year in Australia.42 It is estimated that by the age of 90, 33% of all women and 17% of all men will have sustained a hip fracture.43 Hip fracture numbers are predicted to double in Australia between 1996 and 2026 and rise to ‘epidemic proportions’ in New Zealand by 2011.44,45 In adults over the age of 65 in both countries, hip fractures occur more frequently in women than in men (because of osteoporosis). In New Zealand an estimated 84,000 osteoporotic fractures occur each year, 60% of them in women.42 Currently 2.2 million Australians are affected by osteoporosis—about 11% of men and 27% of women aged 60 years or more. The lifetime risk of osteoporotic fractures after age 50 is 42% in women and 27% in men. Hip fractures are also associated with a 1-year mortality rate of about 24%.46 Most patients who survive do not return to the level of mobility and independence they had before the fracture and many older adults develop disabilities necessitating long-term care.47 The total annual costs relating to osteoporosis are estimated to be NZ$1.15 billion in New Zealand and A$7.4 billion in Australia, of which A$1.9 billion are direct costs.42
A fracture of the hip (see Fig 62-17) refers to a fracture of the proximal third of the femur, which extends up to 5 cm below the lesser trochanter. Fractures that occur within the hip joint capsule are called intracapsular fractures. Intracapsular fractures (femoral neck) are further identified by their specific locations: (1) capital (fracture of the head of the femur); (2) subcapital (fractures just below the head of the femur); and (3) transcervical (fractures of the neck of the femur). These fractures are often associated with osteoporosis and minor trauma. Extracapsular fractures occur outside the joint capsule and are termed: (1) intertrochanteric if they occur in a region between the greater and lesser trochanter; or (2) subtrochanteric if they occur in the region below the lesser trochanter. Extracapsular fractures are usually caused by severe direct trauma or a fall.
CLINICAL MANIFESTATIONS
The clinical manifestations of hip fractures are external rotation, muscle spasm, shortening of the affected extremity and severe pain and tenderness in the region of the fracture site. Displaced femoral neck fractures cause serious disruption of the blood supply to the femoral head, which can result in avascular necrosis of the femoral head.
MULTIDISCIPLINARY CARE
Early surgical repair is the preferred method of managing intracapsular and extracapsular hip fractures (within 24–36 hours).44 Surgical treatment permits early mobilisation of the patient and decreases the risk of major complications. Buck’s traction (see Fig 62-10) may be temporarily used (maximum use 24–48 hours) to relieve painful muscle spasms and immobilise the affected extremity until the patient’s physical condition is stabilised and surgery can be performed.38
Intracapsular (femoral neck) fractures are usually repaired with the use of an endoprosthesis to replace the femoral head (hemiarthroplasty; see Fig 62-18, A). Extracapsular fractures are repaired using fixed nail plates, sliding nail plates, intramedullary devices and replacement prostheses (Fig 62-18, B). The principles of patient care for these procedures are similar.
 NURSING MANAGEMENT: HIP FRACTURE
NURSING MANAGEMENT: HIP FRACTURE
 Nursing implementation
Nursing implementation
 Preoperative management
Preoperative management
Because older adults are most prone to hip fractures, chronic health problems must often be considered when planning treatment. Diabetes mellitus, hypertension, cardiac decompensation, pulmonary disease and arthritis are chronic problems that may complicate clinical status. Surgery may be delayed for a brief time until the patient’s general health is stabilised.
Before surgery, severe muscle spasms can increase pain. Appropriate analgesics or muscle relaxants, comfortable positioning (unless contraindicated) and properly adjusted traction can help in managing the spasms.
Careful preoperative patient teaching can improve mobility.48 Teaching is often done in the emergency department because quick surgical intervention is the standard of care expected. Many patients will not have an overnight preoperative period in which to receive instructions or they may not have the cognitive abilities to retain this important patient education. When possible, the patient can be taught the method and frequency for exercising the unaffected leg and both arms. The patient should also be encouraged to use the overhead trapeze bar and the opposite side rail to assist in changing positions. A physiotherapist can begin to teach out-of-bed and chair transfers. The family must also be informed about the patient’s weight-bearing status after surgery. Plans for discharge begin as the patient enters the hospital because the length of stay postoperatively will be only a few days, although discharge will depend on the patient’s home situation and health status.
 Postoperative management
Postoperative management
The initial postoperative management of the patient following ORIF of a hip fracture is similar to that for any older surgical patient. The nurse must monitor vital signs, fluid intake and output; supervise respiratory activities, such as deep breathing and coughing; administer pain medication cautiously; and observe the dressing and later the incision for signs of bleeding and infection. A nursing care plan for the orthopaedic patient following surgery is presented in NCP 62-2.
In the early postoperative period there is a potential for neurovascular impairment. The nurse should assess the patient’s extremity for: (1) colour; (2) temperature; (3) capillary refill; (4) distal pulses; (5) oedema; (6) sensation; (7) motor function; and (8) pain. Oedema is alleviated by elevation of the leg whenever the patient is in a chair. The pain resulting from poor alignment of the affected extremity can be reduced by keeping pillows (or an abductor splint) between the knees when the patient is turning from side to side. Sandbags and pillows are also used to prevent external hip rotation. If an endoprosthesis was placed, the patient is at risk of hip dislocation. Hip precautions must be demonstrated and explained to the patient.
The physiotherapist usually supervises active assistance exercises for the affected extremity and ambulation when the surgeon permits it. Ambulation usually begins on the first or second postoperative day. The nurse, in collaboration with the physiotherapist, monitors the patient’s ambulation status for proper crutch walking or use of the walker. For the patient to be discharged home, the patient must be able to safely demonstrate use of crutches or a walker, the ability to transfer into and from a chair and bed and the ability to ascend and descend stairs.
Complications associated with femoral neck fracture include non-union, avascular necrosis, dislocation and degenerative arthritis. As a result of an intertrochanteric fracture, the affected leg may be shortened. A cane or built-up shoe may be required for safe ambulation.
If the hip fracture has been treated by insertion of a femoral head prosthesis, measures to prevent dislocation must always be used (see Box 62-4). The patient and family must be fully aware of positions and activities that predispose the patient to dislocation (greater than 90° of flexion, adduction or internal rotation). Many daily activities may reproduce these positions, including putting on shoes and socks, crossing the legs or feet while seated, assuming the side-lying position incorrectly, standing up or sitting down while the body is flexed greater than 90° relative to the chair and sitting on low seats, especially low toilet or car seats. Until the soft-tissue capsule surrounding the hip has healed sufficiently to stabilise the prosthesis, these activities must be avoided, usually for at least 6 weeks. Sudden severe pain, a lump in the buttock, limb shortening and external rotation indicate prosthesis dislocation. This requires a closed reduction with conscious sedation or an open reduction to realign the femoral head in the acetabulum.
BOX 62-4 Femoral head prosthesis
PATIENT & FAMILY TEACHING GUIDE
Do not
• Force hip into greater than 90° of flexion (e.g. sitting on low chairs or toilet seats)
• Force hip into internal rotation
• Put on own shoes or stockings until 8 weeks after surgery without adaptive device (e.g. long-handled shoehorn or stocking-helper)
• Sit on chairs without arms to aid rising to a standing position
Do
• Use toilet elevator on toilet seat
• Place chair inside shower or bath and remain seated while washing
• Use pillow between legs for first 8 weeks after surgery when lying on ‘good’ side or when supine
• Keep hip in neutral, straight position when sitting, walking or lying
• Notify surgeon if severe pain, deformity or loss of function occurs
• Inform dentist of presence of prosthesis before dental work so that prophylactic antibiotics can be given
In addition to teaching the patient and family how to prevent prosthesis dislocation, the nurse should: (1) place a large pillow between the patient’s legs when turning; (2) avoid extreme hip flexion; and (3) avoid turning the patient on the affected side until approved by the surgeon. In addition, some healthcare providers prefer the patient to keep leg abductor splints on except when bathing.
The patient may be out of bed on the first postoperative day. Weight-bearing on the involved extremity varies. Weight-bearing of especially fragile fractures may be restricted until X-ray examination indicates adequate healing, usually 6–12 weeks.
The nurse assists both the patient and the family in adjusting to the restrictions and dependence imposed by the hip fracture. Anxiety and depression can occur easily, but creative nursing care and awareness of the problem can do much to prevent it. The patient and family may need to be informed about community referral services that can assist in the post-discharge rehabilitation phase. Hospitalisation averages 3–4 days. Patients frequently require care in a subacute unit, at a skilled nursing facility or in a rehabilitation facility for a few weeks before returning home. Regular follow-up care after discharge, including community nursing, should be arranged.
Gerontological considerations: hip fracture
Factors that contribute to the occurrence of a hip fracture in older adults include a propensity to fall, an inability to correct a postural imbalance, the orientation of the fall, inadequacy of local tissue shock absorbers (e.g. fat, muscle bulk) and underlying skeletal strength. Several factors have been identified in older people that increase their risk of falling. These include reduced muscle strength, gait and balance problems, decreased vision and hearing, decreased reflexes, orthostatic hypotension and medication use. Leading hazards are loose rugs and slippery or uneven surfaces. Many falls are associated with getting into or out of a chair or bed. Falls to the side, the most common type in the frail elderly, are more likely to result in a hip fracture than a forward fall. External hip protectors have not been shown to be effective in reducing hip fractures in the elderly after a fall.49 A low level of patient compliance in wearing hip protectors, which are plastic shields or foam pads held in place with specially designed underwear, may be one reason for these results.
Two important factors influencing the amount of force imposed on the hip are the presence of energy-absorbing soft tissue over the greater trochanter and the state of leg muscle contraction at the time of the fall. Because many elderly people have poor muscle tone, these are important factors in the severity of a fall. Elderly women often have osteoporosis and accompanying low bone density, which increases the risk of hip fracture and other types of fractures.50
Targeted interventions to reduce hip fractures in the elderly include a variety of strategies. Calcium and vitamin D supplementation, oestrogen replacement and bisphosphonate drug therapy have been shown to decrease bone loss or increase bone density and decrease the likelihood of fracture. (Osteoporosis is discussed in Ch 64.) Some evidence also exists for the effectiveness of oral protein and energy feeds.51 Nurses must be vigilant in planning interventions for the elderly that are known to reduce the incidence of hip fracture.
 Evaluation
Evaluation
The expected outcomes for the patient with a fracture of the hip are presented in NCP 62-2.
Femoral shaft fracture
Femoral shaft fracture is a common injury occurring particularly in young adults. Severe direct force is required to produce this injury because the femur can bend slightly before actual fracture occurs. The force exerted to cause the fracture often causes damage to the adjacent soft-tissue structures. These injuries may be more serious than the bone injury. Displacement of the fracture fragments often results in open fracture and increased soft-tissue damage. This can result in considerable blood loss (1–1.5 L).
The clinical manifestations of a fracture of the femoral shaft are usually obvious. They include marked deformity and angulation, shortening of the extremity, inability to move either the hip or knee and pain. The common complications associated with fracture of the femoral shaft include fat embolism, nerve and vascular injury, and problems associated with bone union, open fracture and soft-tissue damage.
Initial management is directed towards stabilisation of the patient and immobilisation of the fracture. Treatment may consist of skeletal traction via a femoral or tibial pin and balanced suspension traction for 8–12 weeks. The nurse must encourage the patient to perform exercises and ROM activities for the uninvolved extremities and joints to discourage deconditioning. The treating doctor will determine when active exercise can be instituted on the affected extremity. When there is sufficient clinical evidence of bone union, a hip spica or long leg cast may be applied. Use of prolonged traction is uncommon as the current standard of care.
ORIF has become the preferred method to manage a femoral fracture. It is carried out with an intramedullary rod, compression plate and screws or side plate with an intercondylar nail. Internal fixation is often the preferred treatment because it reduces hospital stay and the complications associated with prolonged bed rest. Other indications for internal fixation are failure to obtain satisfactory reduction by non-surgical methods and multiple associated injuries. In some instances the surgically repaired femur may be supported by suspension traction for 3–4 days to prevent excessive movement of the extremity and to control rotation; non-weight-bearing gait training is then begun. Fractures associated with extensive soft-tissue injury may be treated with external fixation.
Promotion and maintenance of strength in the affected extremity usually include gluteal and quadriceps isometric exercises. It is important to ensure performance of ROM and strengthening exercises for all uninvolved extremities in preparation for ambulation. The patient may be immobilised in a hip spica cast and gradually progress to an articulating cast brace or may be allowed to begin non-weight-bearing activities with an ambulatory assistive device. Full weight-bearing is usually restricted until there is X-ray evidence of union of the fracture fragments.
Fracture of the tibia
Although the tibia is vulnerable to injury because it lacks a covering of anterior muscle, strong force is required to produce a fractured tibia. As a result, soft-tissue damage, devascularisation and open fracture are frequent. The tibia is one of the more common sites of a stress fracture. Complications associated with tibial fractures are compartment syndrome, fat embolism, problems associated with bony union and possible infection associated with open fracture. Amputation, although rare, may be required if adequate muscle and tissue coverage is not achieved following muscle and flap grafts.
The recommended management for closed tibial fracture is closed reduction followed by immobilisation in a long leg cast. ORIF either with intramedullary rods or compression plate or with external fixation is indicated for complex fractures and those with extensive soft-tissue damage. With either method of reduction, emphasis is placed on maintaining the strength of the quadriceps.
The neurovascular status of the affected extremity must be assessed at least every 2 hours during the first 48 hours. Patients are instructed to perform active ROM exercises with all uninvolved extremities, as well as exercises for the upper extremities, to build the strength required for crutch walking. When the healthcare provider has determined that the patient is ready for gait training, the patient is instructed in the principles of crutch walking. The patient may be on non-weight-bearing status for 6–12 weeks depending on healing. Patients with external fixation must be taught pin care and dressing changes if extensive tissue has been debrided. Community nursing visits can be initiated to augment outpatient appointments and monitor the patient’s progress.
Stable vertebral fractures
Stable fractures of the vertebral column are usually caused by motor vehicle accidents, falls or diving or sports injuries. A stable fracture is one in which the fracture or the fragment is not likely to move or cause spinal cord damage. This type of injury is frequently confined to the anterior element (vertebral body) of the spinal column in the lumbar region and involves the cervical and thoracic regions less frequently. The vertebral body is usually protected from displacement by the intact spinal ligaments.
Most patients with spinal fractures have stable fractures and experience only brief periods of disability. However, if the ligamentous structures are significantly disrupted, dislocation of the vertebral structures may occur, resulting in instability and injury to the spinal cord (unstable fracture). These injuries generally require surgery. The most serious complication of vertebral fractures is fracture displacement, which can cause damage to the spinal cord (see Ch 60). Although stable vertebral fractures are not associated with abnormal spinal cord pathological conditions, all spinal injuries should initially be considered unstable and potentially serious until diagnostic tests are performed and the fracture is determined to be stable.
The most common injury to the vertebral body is the compression type of fracture caused by excessive vertical axial loading, such as a severe fall on the buttocks from a height or injury resulting from sudden flexion that forces the spine beyond its normal ROM. The patient usually complains of pain and tenderness in the affected region of the spine. Sudden loss of function below the level of fracture indicates severe fracture with spinal cord impingement and paraplegia. Stable compression fractures are associated with a kyphotic deformity (flexion angulation of several vertebrae). This deformity may be noted during the physical examination. In patients with osteoporosis, several vertebral levels may be involved, as evidenced by a ‘dowager’s hump’ (abnormal curvature of thoracic spine). The cervical spine may also be involved. Bowel and bladder dysfunction may be an indication of an interruption of the autonomic nervous system nerves or injury to the spinal cord.
The overall goal in management of stable vertebral fractures is to keep the spine in good alignment until union has been accomplished. Many nursing interventions are aimed at assessing for the possibility of spinal cord trauma. Vital signs and bowel and bladder function should be evaluated routinely, as should the motor and sensory status of the peripheral nerves distal to the injured region. Any deterioration in the patient’s neurovascular status should be reported promptly.
Treatment includes support, heat and traction. The patient is usually placed in a standard hospital bed with firm support from the mattress or a bed board. The aim is to support the spinal column, relax muscles, decrease oedema and release potential compression on nerve roots. Heat and traction may be used to relieve muscle spasms resulting from the fracture. Traction may also be used to reduce and immobilise fracture fragments. A trapeze is not usually allowed because its use increases intrathoracic pressure. Both an upright position and turning of the torso are prohibited. When turning, the patient should be taught to keep the spine straight by turning the shoulders and pelvis together. Nursing assistance is necessary for the patient to learn the technique of ‘logrolling’.52 Several days after the initial injury, the healthcare provider may apply a specially constructed orthotic device (e.g. Milwaukee, Jewett or Taylor brace), a jacket cast or a removable corset if there is no evidence of neurological deficit.
Vertebral compression fractures, which are often due to osteoporosis, can be treated with two newer outpatient procedures: vertebroplasty and kyphoplasty. Vertebroplasty uses guided imaging to inject bone cement into the fractured area. The cement (when hardened) serves to stabilise and prevent further vertebral deterioration. Kyphoplasty initially involves inserting an inflated balloon to lift apart the injured vertebrae. The surgeon then fills the cavity with bone cement after the balloon has been deflated. Kyphoplasty results in a lower leakage of bone cement when compared to vertebroplasty. Many patients experience marked pain relief and improved function following these procedures.53,54
If the fracture is in the cervical spine, the patient may wear a cervical collar. Some cervical fractures are immobilised by use of a halo vest (see Fig 60-12). This consists of a plastic jacket or cast fitted about the chest and attached to a halo that is held in place by skeletal pins inserted into the cranium. These devices immobilise the spine in the fracture area but allow the patient to ambulate. The patient is discharged after: (1) regaining ambulation skills; (2) learning care of the cast or orthotic device; and (3) learning how to cope with interferences in safety and security imposed by the injury and treatment.
Facial fractures
Any bone of the face can be fractured as a result of trauma. Fractures can occur as a result of a collision with another person or object, fighting or blunt trauma. The primary concern after facial injury is to establish and maintain a patent airway and to provide adequate ventilation by removing foreign material and blood. Suctioning may be necessary. An alternative airway (tracheostomy) may be needed if a patent airway cannot be maintained. Pressure packing controls haemorrhage. Cervical spine injuries are common. All patients with facial injuries should be treated as though they have a cervical injury until proven otherwise by examination and imaging studies (e.g. CT scan, X-ray). Table 62-8 describes the clinical manifestations of common facial fractures.
TABLE 62-8 Clinical manifestations of facial fractures
| Fracture | Clinical manifestations |
|---|---|
| Frontal bone | Rapid oedema that may mask underlying fractures |
| Periorbital | Possible frontal sinus involvement, entrapment of ocular muscles |
| Nasal | Displacement of nasal bones, epistaxis |
| Zygomatic arch | Depression of zygomatic arch and entrapment of ocular muscles |
| Maxilla | Segmental motion of maxilla and alveolar fracture of teeth |
| Mandible | Tooth fractures, bleeding, limited motion of mandible |
Concurrent soft-tissue injury often makes assessment of a facial injury difficult. Oral and facial examinations should be performed after the patient has been stabilised and any life-threatening situations have been treated. Careful assessment is made of the ocular muscles and cranial nerve involvement (cranial nerves III, IV and VI). An X-ray documents the extent of the injury. CT imaging helps differentiate between bone and soft-tissue and gives a more specific view of the fracture.
Injury to the eye must be suspected when a facial injury occurs, particularly if the injury is near the orbit. If an eye-globe rupture is suspected, the examination is stopped and a protective shield is placed over the eye. Signs of globe rupture include the extrusion of vitreous humour or brown tissue (iris or ciliary body) on the surface of the globe or penetrating through a laceration with an eccentric or teardrop-shaped pupil. Specific treatment of a facial fracture depends on the site and extent of the fracture and the associated soft-tissue injury. Immobilisation or surgical stabilisation may be necessary.
The patient who sustains a facial fracture requires sensitive nursing care because any alteration in appearance after the trauma may be drastic. Oedema and discolouration subside with time, but concurrent soft-tissue injuries may result in permanent scarring. Attention to maintaining a patent airway and adequate nutrition are ongoing nursing concerns throughout the recovery period. Suction should always be available to maintain a patent airway for facial trauma patients.
Mandible fracture
A fracture of the mandible may result from trauma to the face or jaws. Maxillary fractures may also occur, but they are less common than mandibular fractures. The fracture may be simple, with no bone displacement, or it may involve loss of tissue and bone. The fracture may require immediate and sometimes long-term treatment to ensure survival and restore satisfactory appearance and function. Mandibular fracture may also be therapeutically performed to correct an underlying malocclusion problem that cannot be corrected by orthodontic procedures alone. In these conditions, the mandible is resected during surgery and manipulated forwards or backwards depending on the occlusion problem. For this patient, the procedure is performed on an elective basis.
Surgery consists of immobilisation, usually by wiring the jaws (intermaxillary fixation). Internal fixation may be accomplished with screws and plates. In a simple fracture with no loss of teeth, the lower jaw is wired to the upper jaw. Wires are placed around the teeth and then cross-wires or rubber bands are used to hold the lower jaw tight against the upper jaw (see Fig 62-19). Arch bars may be placed on the maxillary and mandibular arches of the teeth. Vertical wires are placed between the arch bars holding the jaws together. When teeth are missing or if there is bone displacement, other forms of fixation, such as metal arch bars in the mouth or insertion of a pin in the bone, may be needed. Bone grafting may also be required. Immobilisation is usually necessary for only 4–6 weeks because the fractures generally heal rapidly.
 NURSING MANAGEMENT: MANDIBULAR FRACTURE
NURSING MANAGEMENT: MANDIBULAR FRACTURE
 Nursing implementation
Nursing implementation
 Preoperative management
Preoperative management
The patient should be informed preoperatively about the surgical procedure, including what it involves, how the face will look and alterations caused by the surgery. The patient must be reassured that the immobility of the jaw will not affect the ability to breathe normally, speak and swallow liquids. Hospitalisation for respiratory monitoring is brief unless there are other injuries or problems.
 Postoperative management
Postoperative management
Postoperative care should focus on a patent airway, oral hygiene, communication, pain management and adequate nutrition. Two major potential problems in the immediate postoperative period are airway obstruction and aspiration of vomitus. Because the patient cannot open the jaw, measures to ensure an airway are essential. The nurse must observe for signs of respiratory distress (e.g. dyspnoea, alterations in rate, quality and depth of respirations). The patient should be placed on the side with the head slightly elevated immediately after surgery. A wire cutter or scissors (for rubber bands) must be taped to the head of the bed and sent with the patient on all appointments and examinations away from the bedside. These may be used to cut the wires or elastic bands in case of an emergency. The wires should be cut only as a last resort. Once the patient is awake the wires should be cut only in case of cardiac arrest or respiratory distress requiring access to the pharynx or lungs. The surgeon should explain, by using a picture, the appropriate wire or wires to cut and this should be included in the care plan. In some cases, cutting the wires may cause the entire facial and upper jaw structure to shift or collapse and worsen the problem. A tracheostomy tray or an endotracheal tray must always be available.
If the patient begins to vomit or choke, the nurse should try to clear the mouth and airway. Suctioning may be necessary and may be done by the nasopharyngeal or oral route, depending on the extent of injury and the type of repair. An NG tube may be used for decompression to remove fluids and gas from the stomach to help prevent aspiration. It also helps prevent vomiting. Antiemetics may be used. The NG tube can later be used as a feeding tube. The nurse should teach the patient to clear secretions and vomitus.
Oral hygiene is an extremely important part of the nursing care. The mouth should be rinsed frequently, particularly after meals and snacks, to remove food debris. Warm normal saline solution, water or alkaline mouthwashes may be used. A soft rubber catheter or a Water-Pik is effective for a thorough oral cleansing.55 The nurse should inspect the mouth several times a day to see that it is clean. A penlight torch is necessary and a tongue depressor is used to retract the cheeks. The lips and corners of the mouth, as well as the buccal mucosa, should be kept moist.
Communication may be a problem, particularly in the early postoperative period. An effective way of communication must be established preoperatively (e.g. picture board, pad and pencil, small chalkboard). Usually the patient can speak well enough to be understood, especially after the first few postoperative days.
Ingestion of sufficient nutrients poses a challenge because the diet must be liquid. The patient easily tires of sucking through a straw or laboriously using a spoon. The diet must be planned to include adequate kilojoules, protein and fluids. Liquid protein supplements may be helpful for improving nutritional status. The nurse should work with the dietician and the patient to ensure adequate nutrition. The low-bulk, high-carbohydrate diet and the intake of air through the straw can create a problem with constipation and flatus. Ambulation, prune juice and bulk-forming laxatives may help relieve these problems.
The patient is usually discharged with the wires in place. The nurse should encourage the patient to verbalise their feelings about their altered appearance. Discharge teaching should include oral care, techniques for handling secretions, diet, how and when to use wire cutters and when to seek medical assistance.
Amputation
During the past 20 years, major advances have been made in surgical amputation techniques, prosthetic design and rehabilitation programs.56 These advances enable people with amputation to return to productive and satisfying social roles. The middle and older age groups have the highest incidence of amputation because of the effects of peripheral vascular disease, atherosclerosis and vascular changes related to diabetes mellitus. Amputation in the young is usually secondary to trauma (e.g. motor vehicle accident or work-related injury).
CLINICAL INDICATIONS
The clinical indications for an amputation depend on the underlying disease or trauma. Amputation is required more often in people engaged in hazardous occupations, with a greater incidence in men. Common indications for amputation include circulatory impairment resulting from a peripheral vascular disorder, traumatic and thermal injuries, malignant tumours, uncontrolled or widespread infection of the extremity (e.g. gas gangrene, osteomyelitis) and congenital disorders. These conditions may manifest as loss of sensation, inadequate circulation, pallor and local or systemic manifestations of sepsis. Although pain is often present, it is not usually the primary reason for an amputation. The underlying problem dictates whether the amputation is performed as elective or emergency surgery. Consideration must also be given to the patient’s ability to use a prosthetic device successfully.
DIAGNOSTIC STUDIES
The types of diagnostic studies performed depend on the underlying problem that makes the amputation necessary (see Box 62-5). An elevated white blood cell (WBC) count with differential may indicate infection. Vascular tests such as arteriography, Doppler studies and venography provide information about the circulatory status of the extremity.
MULTIDISCIPLINARY CARE
The potential for revascularisation surgery rather than amputation can be assessed on the basis of vascular studies. If amputation is to be considered ‘elective’, the patient’s general health is assessed carefully. Chronic illnesses and the presence of infection are monitored closely. The patient and family should be assisted to understand the need for the amputation and be assured that rehabilitation can result in an active, useful life. If the amputation is performed on an emergency basis as a result of trauma, management of the patient is physically and emotionally more complicated.
The goal of amputation surgery is to preserve extremity length and function while removing all infected, damaged or ischaemic tissue. This improves the possibility of good prosthetic, cosmetic and functional satisfaction. (Levels of amputation of upper and lower extremities are illustrated in Fig 62-20.) The type of amputation depends on the reason for the surgery. A closed amputation is performed to create a weight-bearing residual limb (or stump). An anterior skin flap with dissected soft-tissue padding covers the bony part of the residual limb. The skin flap is sutured posteriorly so that it will not be positioned in a weight-bearing area. Special care is necessary to prevent the accumulation of drainage, which can produce pressure and harbour bacteria that may cause infection. Disarticulation is an amputation performed through a joint. A Syme’s amputation is a form of disarticulation at the ankle. An open amputation leaves a surface on the residual limb that is not covered with skin. This type of surgery is generally indicated for control of actual or potential infection. The wound is usually closed later by a second surgical procedure or by skin traction surrounding the residual limb. This type of amputation is often called a ‘guillotine amputation’.
 NURSING MANAGEMENT: AMPUTATION
NURSING MANAGEMENT: AMPUTATION
 Nursing assessment
Nursing assessment
Pre-existing illnesses must be adequately assessed because most amputations are performed as a result of vascular problems. Assessment of the vascular and neurological status is an important part of the assessment process (see Chs 31 and 55).
 Nursing diagnoses
Nursing diagnoses
Nursing diagnoses for the patient with an amputation may include, but are not limited to, the following:
 Planning
Planning
The overall goals are that the patient with an amputation will: (1) have adequate relief from the underlying health problem; (2) have satisfactory pain control; (3) reach maximum rehabilitation potential with the use of a prosthesis (if indicated); (4) cope with body image changes; and (5) make satisfying lifestyle adjustments.
 Nursing implementation
Nursing implementation
 Health promotion
Health promotion
Most lower-limb amputations result from peripheral vascular disease and most upper-limb amputations result from severe trauma. Knowing this, the nurse directs patient education towards prevention of amputation. Control of causative illnesses, such as peripheral vascular disease, diabetes mellitus, chronic osteomyelitis and pressure ulcers, can eliminate or delay the need for amputation. Patients with these problems should be taught to carefully examine their lower extremities daily for signs of potential problems. If the patient cannot assume this responsibility, a family member should be instructed in the procedure. Patients and their families should be instructed to report problems such as change in skin colour or skin temperature, decrease or absence of sensation in the feet and/or toes, tingling, burning pain or the presence of a lesion to the healthcare provider.
Instruction in proper safety precautions for recreational activities and in the performance of potentially hazardous work is an important nursing responsibility, especially for occupational health nurses. A mangled extremity with subsequent amputation is a serious consequence of trauma that can be avoided.
 Acute intervention
Acute intervention
The nurse must recognise the tremendous psychological and social implications of an amputation for the patient. The disruption in body image caused by an amputation often causes a patient to go through the psychological stages of the grieving process. Allowing the patient to go through the grieving process and recognising it as a normal consequence may do much to aid the patient’s acceptance of the amputation. The patient’s family must also be helped to work through the transitional process to arrive at a realistic and positive attitude about the future. The reasons for an amputation and the rehabilitation potential depend on age, diagnosis, occupation, personality, resources and support systems.
 Preoperative management
Preoperative management
Before surgery, the nurse should reinforce information that the patient and family have received about the reasons for the amputation, the proposed prosthesis and the mobility training program. In addition to the usual preoperative instructions, the patient undergoing an amputation has special education needs. To meet these needs, the nurse must know the level of amputation, the type of postsurgical dressings to be applied and the type of prosthesis to be utilised. The patient should receive instruction in the performance of upper-extremity exercises, such as push-ups in bed or the wheelchair, to promote arm strength. This instruction is essential later for crutch walking and gait training. General postoperative nursing care should be discussed, including positioning, support and residual limb care. If a compression bandage is to be used after surgery the patient should be instructed about its purpose and how it will be applied. If an immediate prosthesis is planned, the general ambulation expectations and program should be discussed.
The patient should be warned that they may feel that the amputated limb is still present after surgery. This phenomenon, termed phantom limb sensation, occurs in 80% of amputees and may cause patients grave concern unless they are forewarned. If pain was present in the affected limb preoperatively, the patient may also experience phantom limb pain postoperatively. The patient may complain of feelings of coldness and heaviness, cramping, and shooting, burning or crushing pain. Often, the patient may be extremely anxious about this pain because they know the limb is gone but still perceive pain in it. As recovery and ambulation progress, phantom limb sensation and pain usually subside, although the pain may not go altogether and can become chronic.57,58
 Postoperative management
Postoperative management
General postoperative care for the patient who has had an amputation depends largely on the patient’s general state of health, the reason for the amputation and the patient’s age. Nursing care must be individualised on the basis of these factors. For example, an older patient needs careful monitoring of respiratory status. A victim of a motor vehicle accident may need careful neurological monitoring. Individuals who undergo amputation as a result of a traumatic injury need to be monitored for posttraumatic stress disorder because they have had no time to prepare for or perhaps even participate in the decision to have a limb amputated.
Prevention and detection of complications are important nursing responsibilities during the postoperative period. Careful monitoring of the patient’s vital signs and dressings can alert the nurse to haemorrhage in the operative area. Careful attention to sterile technique during dressing changes reduces the potential for wound infection and subsequent interruption of the patient’s rehabilitation.
If an immediate postoperative prosthesis has been applied, the nurse must monitor vital signs. Careful surveillance of the surgical site is also required. A surgical tourniquet must always be available for emergency use. If excessive bleeding occurs, the surgeon should be notified immediately. Efforts to maintain haemostasis and to control the haemorrhage should begin at once.
The orthopaedic surgeon will decide the type of prosthetic fitting that will be used after surgery. An immediate prosthetic fitting is performed in the operating room after the amputation. A rigid, cast-like bandage is applied around the closed residual lower limb with a prosthetic pylon and an ankle–foot assembly. While the patient is still anaesthetised, the prosthetic pylon and ankle–foot assembly are aligned and adjusted to provide a smooth gait and to avoid excessive pressure on the residual limb area. A strap is placed on the proximal anterior surface of the rigid plaster bandage and attached to a waistband to prevent slippage. The main advantages of this device are reduction of oedema and the psychological benefit of early ambulation. A disadvantage in the application of an immediate postoperative device is the inability to directly visualise the surgical site.
The delayed prosthetic fitting may be the best choice for certain patients. Patients who have had amputations above the knee or below the elbow, older adults, debilitated individuals and those with infection usually have delayed prosthetic fittings (see Fig 62-21). The appropriate time to begin using a prosthesis depends on satisfactory healing of the residual limb, as well as on the general condition of the patient. A temporary prosthesis may be used for partial weight-bearing once the sutures are removed. Barring any problems, patients can bear full weight on permanent prostheses approximately 3 months after amputation.
Figure 62-21 Two types of prosthesis. A, Traditional fibreglass. B, New materials and techniques have made possible fabrication of prosthetic sockets that are light, soft, flexible and secure.
Not all patients are candidates for a prosthesis. It is important that the surgeon discuss ambulation possibilities frankly with the patient and family. The seriously ill or debilitated patient may not have the upper body strength and energy required to use a prosthesis. Mobility with a wheelchair may be the most realistic goal for patients who are not candidates for prosthesis.
Multidisciplinary care also includes the direction and coordination of the rehabilitation program for the amputee. Success depends on the physical and emotional health of the patient. Chronic illness and deconditioning complicate aggressive rehabilitation efforts. Both physiotherapy and occupational therapy must be an integral component of the patient’s overall plan of care.
Flexion contractures may delay the rehabilitation process. The most common and debilitating contracture is hip flexion. Hip adduction contracture is rare. Patients should avoid sitting in a chair for more than 1 hour with hips flexed or having pillows under the surgical extremity to prevent flexion contractures. Unless specifically contraindicated, patients should lie on their abdomen for 30 minutes 3–4 times each day and position the hip in extension while prone.
Proper residual limb bandaging fosters shaping and moulding for eventual prosthesis fitting (see Fig 62-22). The surgeon usually orders a compression bandage to be applied immediately after surgery to support the soft tissues, reduce oedema, hasten healing, minimise pain and promote residual limb shrinkage and maturation. This bandage may be an elastic roll applied to the residual limb or a residual limb shrinker, which is an elastic stocking that fits tightly over the residual limb and lower trunk area.59
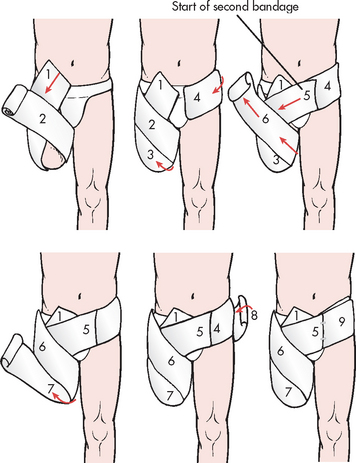
Figure 62-22 Bandaging for the above-the-knee amputation residual limb. Figure of eight style bandaging covers progressive areas of the residual limb. Two elastic bandages are required.
The compression bandage is initially worn at all times except during physiotherapy and bathing. The bandage is taken off and reapplied several times daily and care is taken so that it is applied snugly but not so tight as to interfere with circulation. Shrinker bandages should be washed and changed daily. It is recommended that the patient have two residual limb shrinker bandages so that one can be worn while the other is being washed. After healing has occurred, the residual limb is bandaged only when the patient is not wearing the prosthesis. The patient should be instructed to avoid dangling the residual limb over the bedside to minimise oedema formation.
As the patient’s overall condition improves, the nurse begins instruction in the principles and techniques of transferring from bed to chair and back again. Active exercises and conditioning are essential in developing ambulation skills. The exercise regimen is normally started under the supervision of the doctor and the physiotherapist. The nurse must have a clear understanding of the exercise regimen to reinforce it and ensure that the exercises are performed correctly. Active ROM exercises of all joints should be started as soon after surgery as the patient’s pain level and medical status permit. In preparation for mobility, the patient should increase triceps and shoulder strength and lower limb support and learn balance of the altered body. The loss of the weight from an amputated limb requires adaptation of the patient’s proprioceptive and coordination mechanisms to prevent falls and injury.
Crutch walking is started as soon as patients are physically able. If they have had immediate postsurgical fitting, orders related to weight-bearing must be carefully followed to avoid disruption of the skin flap and delay of the tissue healing process. Initial periods of ambulation should not exceed 5 minutes to prevent dependent oedema.
Before discharge, the patient and family need careful instruction related to residual limb care, ambulation, prevention of contractures, recognition of complications, exercise and follow-up care. Box 62-6 outlines patient and family teaching following an amputation.
BOX 62-6 Following an amputation
PATIENT & FAMILY TEACHING GUIDE
1. Inspect the residual limb daily for signs of skin irritation, especially erythema, excoriation and odour. Pay particular attention to areas prone to pressure.
2. Discontinue use of the prosthesis if an irritation develops. Have the area checked before resuming use of the prosthesis.
3. Wash the residual limb thoroughly each night with warm water and a bacteriostatic soap. Rinse thoroughly and dry gently. Expose the residual limb to air for 20 minutes.
4. Do not use any substance such as lotions, alcohol, powders or oil unless prescribed by the healthcare provider.
5. Wear only a residual limb sock that is in good condition and supplied by the prosthetist.
6. Change the residual limb sock daily. Wash in a mild soap, squeeze out and lay flat to dry.
7. Use prescribed pain management techniques.
8. Perform ROM exercises to all joints daily. Perform general strengthening exercises, including to the upper extremities, daily.
9. Do not elevate the residual limb on a pillow.
10. Lie prone with hip extension for 30 minutes three to four times a day.
 Ambulatory and home care
Ambulatory and home care
When the healing has occurred satisfactorily and the residual limb is well moulded, the patient is ready for fitting of the prosthesis. Walking with a below-the-knee prosthesis requires 40% additional energy and an above-the-knee prosthesis requires 60%. Matching the patient with a suitable prosthesis involves many factors, including age, general health, intelligence, motivation, occupation and finances. After the surgeon makes the recommendation, the patient is referred to a prosthetist, who initially makes a mould of the residual limb and measures landmarks for the fabrication of the prosthesis. The moulded limb socket allows the residual limb to fit snugly into the prosthesis. The limb is covered with a residual limb stocking to ensure good fit and prevent skin breakdown. The residual limb may continue to shrink, causing a loose fit, in which case a new socket has to be fabricated. The patient may need to have the prosthesis adjusted to prevent rubbing and friction between the residual limb and the socket. Excessive movement of a loose prosthesis can cause severe skin irritation and breakdown.
The prosthesis is fitted by the prosthetist, who may also train the patient to use it. It is important for the nurse to be familiar with the training program to encourage and assist the patient. Most often this learning occurs after the patient has been discharged from the inpatient setting. Learning to use a prosthesis is frustrating and the patient may easily become discouraged. The nurse must continually offer support until the patient is able to manage alone.
Artificial limbs become an integral part of the patient’s changed body image. Proper care ensures their long life and useful functioning. The patient should be instructed to clean the prosthesis socket daily with a mild soap and to rinse thoroughly to remove irritants. The leather and metal parts of the prosthesis should not get wet. The patient should be encouraged to have the prosthesis maintained regularly. The condition of the shoe also needs to be considered: a badly worn shoe alters the gait and may cause damage to the prosthesis.
Referral to a community health nurse can foster optimal physical and emotional adjustment. The family should be instructed on ambulation and transfer techniques and proper residual limb care.
 Special considerations in upper-limb amputation
Special considerations in upper-limb amputation
The emotional implications of an upper-limb amputation are often more devastating than those for lower-limb amputation. The enforced dependency brought about by one-handedness is both frustrating and humiliating for many patients. Because most upper-extremity amputations result from trauma, the patient may not have had the opportunity to adjust psychologically to an amputation or to participate in the decision-making process about amputation.
Both immediate and delayed prosthetic fittings are possible for the below-the-elbow amputee. Prosthetic fitting is delayed for the above-the-elbow amputee. The usual functional prosthesis is the arm and hook. A cosmetic hand is available but has limited functional value. As with the lower-limb prosthesis, patient motivation and endurance are major factors contributing to a satisfactory outcome.
 Evaluation
Evaluation
The expected outcomes are that the patient with an amputation will:
• accept the change in body image and integrate the necessary changes into their lifestyle
• have no evidence of skin breakdown
• have reduction or absence of pain
• become mobile within the limitations imposed by the amputation.
Gerontological considerations: amputation
If a lower-limb amputation has been performed on an older adult, the patient’s previous ability to ambulate may affect the extent of recovery. Use of a prosthesis requires significant strength and energy for ambulation. Older adults whose general health is altered and weakened by disorders such as cardiac or pulmonary dysfunction may not be candidates for prosthesis use. The patient’s ability to ambulate may be limited. If possible, this should be discussed with the patient and family before surgery so that realistic expectations can be set.
COMMON JOINT SURGICAL PROCEDURES
Surgery plays an important role in the treatment and rehabilitation of patients with various forms of arthritis, conditions related to trauma and other painful conditions resulting in functional disability. Joint replacement surgery is the most common orthopaedic operation performed on older adults and as a result there are increased risks of complication such as fat embolism.60,61 Australia and New Zealand both have National Joint Replacement Registries to provide quality demographic information on the practice of joint replacement surgery.62 Significant advances in the field of reconstructive surgery have resulted in improvements in prosthetic design, materials and surgical techniques that provide significant relief of pain and deformity and improve function and joint motion.
Indications for joint surgery
Surgery is aimed at relieving pain, improving joint motion, correcting deformity and malalignment and removing intraarticular causes of erosion. Debilitating joint pain is one of the primary reasons for arthroplasty. In addition to the effects of chronic pain on the physical and emotional health of the patient, any movement of the painful joint is often avoided. If this decreased functional ability is not corrected, contraction with permanent limitation of motion often occurs. Limitation of motion at any joint can be demonstrated on physical examination and by joint-space narrowing on radiological examination.
There may also be a slow loss of cartilage in affected joints, which may be related to loss of motion. Synovitis can cause tendon damage, resulting in rupture or subluxation of the joint and subsequent loss of function. Continuing disease activity may cause loss of cartilage and bony surface and result in mechanical barriers to movement requiring surgical intervention.
Indications for hip or knee arthroplasty include arthritis, connective tissue disease, failed prior procedures, sepsis, tumours, Paget’s disease, congenital hip dysplasia, severe varus or valgus deformity and spondyloarthropathies.
Types of joint surgery
SYNOVECTOMY
Synovectomy (removal of synovial membrane) is used as a prophylactic measure and as a palliative treatment of rheumatoid arthritis (RA). Removal of the synovial membrane, thought to be the location of the basic pathological changes in joint destruction, helps prevent further progression of joint damage. A synovectomy is best performed early in the disease process to prevent serious destruction of joint surfaces. Removal of the thickened synovium prevents extension of the inflammatory process into the adjacent cartilage, ligaments and tendons.
It is impossible to surgically remove all the synovium in a joint. The underlying disease process is still present and will again affect the regenerating synovium. However, the disease appears to be milder after synovectomy and definite improvements in pain, weight-bearing and ROM can be expected. Common sites for this surgery include the elbow, wrist and fingers. Synovectomy in the knee is done less frequently because knee joint replacement techniques are usually used.
OSTEOTOMY
An osteotomy is performed by removing or adding a wedge or slice of bone to change alignment (joint and vertebral) and to shift weight-bearing, thereby correcting deformity and relieving pain. Cervical osteotomy may be used to correct deformity in some patients with ankylosing spondylitis. A halo and body jacket is worn until fusion occurs (3 or 4 months). Subtrochanteric or femoral osteotomy may provide some relief of pain and improve motion in selected patients with hip osteoarthritis. Osteotomy has proven ineffective in patients with inflammatory joint disease. Osteotomy of the knee (tibia) provides relief of pain in selected patients, but advanced joint destruction is usually corrected by joint replacement surgery. The postoperative care is similar to the treatment of an internal fixation of a fracture at a comparable site. Internal wires, screws and plates, bone grafts or an external fixator usually fix the bone in place.
DEBRIDEMENT
Debridement is the removal of degenerative debris such as loose bodies, osteophytes, joint debris and degenerated menisci from a joint. This procedure is usually performed on the knee or the shoulder using a fibreoptic arthroscope. The procedure is generally done on an outpatient basis. A compression dressing is applied postoperatively. Weight-bearing is permitted following knee arthroscopy. Patient education includes monitoring for signs of infection, managing pain and restricting excessive activity for 24–48 hours.
ARTHROPLASTY
Arthroplasty is the reconstruction or replacement of a joint. This surgical procedure is performed to relieve pain, improve or maintain ROM and correct deformity. The most common uses of arthroplasty are for patients with osteoarthritis (OA), RA, avascular necrosis, congenital deformities or dislocations and other systemic problems. There are several types of arthroplasty, including replacement of part of a joint (hemiarthroplasty), surgical reshaping of the bones of the joints and total joint replacement. Replacement arthroplasty is available for the elbow, shoulder and phalangeal joints of the fingers, wrist, hip, knee, ankle and foot.
Hip arthroplasty
Total hip arthroplasty (THA) provides significant relief of pain and improvement of function for patients with OA and RA. Implants are often ‘cemented’ in place with polymethylmethacrylate, which bonds to the bone. With time, a significant number of femoral components loosen and require revision surgery. Because of this risk, cemented THAs are recommended for less active, older adults with compromised bone density. Younger individuals receive ‘cementless’ arthroplasties in an effort to prolong the lifetime of the prosthesis. Cementless THAs provide long-term implant stability by facilitating in-growth of new bone tissue into the porous surface coating of the prosthesis (see Fig 62-23). A patient with a high activity potential and a life expectancy of 25 years or more is an excellent candidate for a cementless prosthesis. Total hip replacements are shown in Figure 62-24.
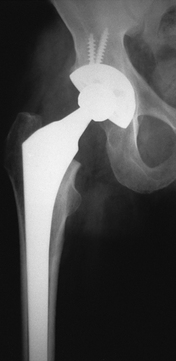
Figure 62-23 Total hip arthroplasty. Porous coated, non-cemented femoral prosthesis of metal alloy with a cemented high-density plastic acetabular socket.
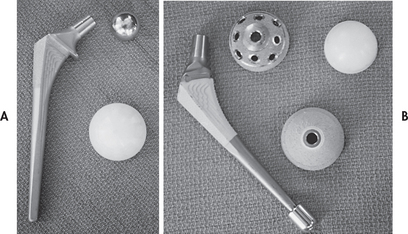
Figure 62-24 Total hip replacements. A, Coated cemented components. B, Porous cementless components.
In both types of arthroplasties, extremes of internal rotation, adduction and 90° flexion of the hip must be avoided for 4–6 weeks postoperatively. A foam abduction pillow is sometimes placed between the legs to prevent dislocation of the new joint (see Fig 62-25). Following surgery, flexion of the hip past 90° is contraindicated and elevated toilet seats and chair alterations at home are necessary. Bathing in a tub and driving a car are not allowed for 4–6 weeks. An occupational therapist may teach the patient to use assistive devices, such as reach bars (‘reachers’) to avoid bending over to pick something off the floor, long-handled shoehorns or sock pullers. The knees must be kept apart; the patient must never cross the legs or twist to reach behind. Physiotherapy is initiated on the first postoperative day with ambulation and weight-bearing with a walker for patients with a cemented prosthesis and weight-bearing on the operative side for those with a cementless prosthesis.
Exercises are designed to restore strength and muscle tone in the quadriceps and muscles about the hip essential to improved function and ROM. These include quadriceps setting (e.g. tightening the kneecap), gluteal muscle setting (e.g. tightening the buttocks), leg raises in supine and prone positions and abduction exercises (e.g. swinging the leg out but never crossing midline) from the supine and standing positions. The patient will continue these exercises for many months after discharge and the family should be well acquainted with the exercise program and offer encouragement at home.
Home care considerations include ongoing assessment of pain management, monitoring for infection and prevention of DVT. Not all patients will qualify for community nursing visits. The incision may be closed with metal staples, which are removed at the surgeon’s rooms. Because of the high risk of DVT, prothrombin times may be determined weekly and anticoagulation adjusted accordingly if warfarin is used. Enoxaparin, a low-molecular-weight heparin, is administered subcutaneously and can be given at home by the patient or family member. An advantage of enoxaparin is that it does not require monitoring of the patient’s coagulation status. The patient should be instructed to obtain prophylactic antibiotics prior to dental appointments and surgical procedures that might put them at risk of bacteraemia.
A physiotherapist will assess ROM, ambulation and compliance with the exercise regimen. The patient will gradually increase the number of repetitions of exercises, add weights to ankles, swim and may eventually use a stationary bicycle to tone quadriceps and improve cardiovascular fitness. High-impact exercises and sports, such as jogging and tennis, may loosen the implant and should be avoided. The elderly adult may require rehabilitation at a subacute or extended care facility until able to function independently.63
Knee arthroplasty
Unremitting pain and instability as a result of severe destructive deterioration of the knee joint is the main indication for total knee arthroplasty (TKA). The presence of osteoporosis may necessitate bone grafting to augment defects and to correct bone deficiencies. Either part of or the entire knee joint may be replaced with a metal and plastic prosthetic device. A compression dressing is used to immobilise the knee in extension immediately after the operation. This is removed before discharge and may be replaced with a knee immobiliser or posterior plastic shell, which maintains extension during ambulation and at rest for about 4 weeks.
Great emphasis is placed on postoperative physiotherapy and dislocation is not typical with TKA. Isometric quadriceps setting begins the first day after surgery. The patient progresses to straight-leg raises and gentle ROM to increase muscle strength and obtain 90° knee flexion. Active flexion exercises or passive flexion exercises through the use of a CPM machine postoperatively promote joint mobility. Full weight-bearing is begun before discharge. An active home exercise program involves progressive ROM with muscle strengthening and flexibility exercises.
Finger joint arthroplasty
A silicone rubber arthroplastic device is used to help restore function in the fingers of the patient with RA. The goal of hand surgery is primarily to restore function related to grasp, pinch, stability and strength rather than to correct cosmetic deformity. The metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints take longer to heal.56 Ulnar deviation is often present, which results in severe functional limitations of the hand. Before surgery the patient is instructed in hand exercises, including flexion, extension, abduction and adduction of the fingers. Postoperatively, the hand is kept elevated with a bulky dressing in place. Neurovascular assessment is conducted postoperatively and the nurse assesses for signs of infection. The success of the surgery depends largely on the postoperative treatment plan, which is often carried out under the direction of an occupational therapist. Once the dressing is removed, a guided splinting program is initiated. The patient is discharged with splints to use while sleeping and hand exercises to perform for 10–12 weeks at least 3–4 times a day. The patient is also instructed to avoid lifting heavy objects.
Elbow and shoulder arthroplasty
Although available, total replacement of elbow and shoulder joints is not as common as other forms of arthroplasty. Shoulder replacements are performed in patients with severe pain because of RA, OA, avascular necrosis or an old trauma. The shoulder replacement is usually considered if the patient has adequate surrounding muscle strength and bone stock. If joint replacement is necessary for both elbow and shoulder, the elbow is usually done first because a severely painful elbow interferes with the shoulder rehabilitation program.
Significant pain relief has been achieved following arthroplasty, with 90% of patients having no pain at rest or minimal pain with activity. Functional improvements have resulted in better hygiene and increased ability to perform activities of daily living in most patients. Rehabilitation is longer and more difficult than with other joint surgery.
Ankle arthroplasty
Total ankle arthroplasty (TAA) is indicated for RA, OA, trauma and avascular necrosis. Ankle fusion is often selected over arthroplasty because the result is more durable. However, the patient is left with a stiff foot and the inability to change heel height. TAA is advantageous because a more normal gait pattern can be achieved. Postoperatively, the patient may not bear weight for 6 weeks, must elevate the extremity to reduce and prevent oedema, be extremely careful to prevent postoperative infection and maintain immobilisation as directed by the surgeon. Although the use of TAA is not widespread, it is becoming a viable alternative to fusion for the treatment of severe ankle arthritis in selected patients.
ARTHRODESIS
Arthrodesis is the surgical fusion of a joint. This procedure is indicated only if articular surfaces are too severely damaged or infected to allow joint replacement or for reconstructive surgery failures. Arthrodesis relieves pain and provides a stable but immobile joint. The fusion is usually accomplished by removal of the articular hyaline cartilage and the addition of bone grafts across the joint surface. The affected joint must be immobilised until bone healing has occurred. Common areas of fusion are the wrist, ankle, cervical spine, lumbar spine and the metatarsophalangeal (MTP) joint of the great toe.
COMPLICATIONS OF JOINT SURGERY
Infection is a serious complication of joint surgery, particularly joint replacement surgery. The most common causative organisms are Gram-positive aerobic streptococci and staphylococci. Infection almost always leads to pain and loosening of the prosthesis, generally requiring extensive surgery. Efforts to reduce the incidence of infection include the use of specially designed self-contained operating suits, operating rooms with laminar airflow and prophylactic antibiotic administration.
DVT is another potentially serious complication after joint surgeries, particularly those involving the lower extremities.64 Prophylactic measures such as aspirin, warfarin, low-molecular-weight heparin and pneumatic compression of the legs are usually instituted. Patients may be followed postoperatively with venous Doppler ultrasound to detect DVT, the source of most pulmonary emboli. FES may also occur after total hip arthroplasty.
MULTIDISCIPLINARY CARE
Preoperative management
As surgical techniques and care improve, more patients with chronic diseases such as RA are being considered as surgical candidates. The primary goal of preoperative assessment is to identify risk factors associated with postoperative complications so that nursing strategies can be implemented to promote optimal positive outcomes. A careful history will include previous medical diagnoses and complications such as diabetes and thrombophlebitis, pain tolerance and management preferences, current functional status, expectations following surgery and level of social support and home care needs after discharge. The patient should be free from infection and acute joint inflammation.
If lower-extremity surgery is planned, upper-extremity muscle strength and joint function are assessed to determine the type of assistive devices needed postoperatively for ambulation and activities of daily living. Preoperative teaching informs the patient and family of the expected hospital course and postoperative management at home. In addition, it prepares them to maximise the usefulness and longevity of the prosthesis. Research has shown that preoperative education alone is not sufficient to ensure successful joint replacement; the patient must have a sense of self-efficacy for an optimal outcome to be achieved.65 Patients also need to realise that recovery is not going to happen ‘overnight’. Both patients and their families or significant others need to speak with individuals who have had a total joint arthroplasty to better understand the reality of dealing with a joint replacement.
Postoperative management
Postoperatively, neurovascular assessment is performed to assess nerve function and circulatory status. Anticoagulation therapy, analgesia and parenteral antibiotics are administered. In general, the affected joint is exercised and ambulation is encouraged as early as possible to prevent complications of immobility. Specific protocols vary according to the patient, type of prosthesis and surgeon preference. Pain management techniques used postoperatively may include epidural analgesia, patient-controlled analgesia, IV injections, oral narcotics or NSAIDs and the Q pump. The Q pump administers analgesics locally to an operative area.66
The hospital stay after arthroplasty is 3–5 days depending on the patient’s course and need for physiotherapy. Physiotherapy and ambulation enhance mobility, build muscle strength and reduce the risk of thrombus formation. If the patient is taking warfarin, therapy starts on the day of surgery and continues for 3 weeks with the prothrombin time measured on a regular basis. For those taking low-molecular-weight heparin (e.g. enoxaparin), therapy starts 24–36 hours after surgery and continues for 2 weeks postoperatively. Daily monitoring of the patient’s coagulation status is not necessary when the patient is taking low-molecular-weight heparin. The decision to use warfarin or low-molecular-weight heparin depends on many factors, including the patient’s age and overall state of health.
 NURSING MANAGEMENT: JOINT SURGERY
NURSING MANAGEMENT: JOINT SURGERY
The nursing management of the patient undergoing joint surgery begins with preoperative teaching and realistic goal setting. It is important that the patient understands and accepts the limitations of the proposed surgery and realises that it will not remove the underlying disease or inflammatory process. Postoperative procedures such as turning, deep breathing, use of a bedpan and bedside commode and use of abductor pillows should be explained and practice opportunities provided. The patient should be reassured that pain relief will be available. Patient-controlled analgesia can be helpful. A preoperative visit from a physiotherapist allows the patient to practise postoperative exercises and enables measurement for crutches or other assistive devices.
Current focus is on early mobilisation, so discharge planning begins immediately.67 The duration of the hospital stay and the expected postoperative events should be discussed because the patient and family must prepare ahead. The home environment must be assessed for safety (e.g. the presence of scatter rugs and electrical cords) and accessibility (e.g. are the bathroom and bedroom upstairs? Are doorframes wide enough to accommodate a walker?) Social support must also be assessed: Is a friend or family member available to assist the patient in the home? Will the patient require Meals on Wheels or other community support? The elderly patient may need the rehabilitation services of a subacute or extended care facility for a few weeks postoperatively to progressively develop independent living skills. Specific nursing interventions related to surgery for the patient having orthopaedic surgery are summarised in NCP 62-2.
Patient teaching includes instructions on reporting complications, including infection (e.g. fever, increased pain, drainage) and dislocation of the prosthesis (e.g. pain, loss of function, shortening or malalignment of an extremity). The home care nurse acts as the liaison between the patient and the general practitioner or surgeon, monitoring for postoperative complications, assessing comfort and ROM and facilitating improvements in functional performance.
The patient with a hip fracture
CASE STUDY
Patient profile
Bob Wells is an 82-year-old male admitted to the hospital through the emergency department. He tripped and fell on the pavement outside his home. It appears he may have sustained a fracture to his right hip. He has a history of type 2 diabetes mellitus and a smoking history of 20 cigarettes a day for the past 60 years that is now complicated by chronic obstructive pulmonary disease.
CRITICAL THINKING QUESTIONS
1. How do pre-existing medical conditions predispose this patient to postoperative complications?
2. What are the most likely postoperative complications for this patient?
3. What pre- and postoperative nursing interventions will be essential while taking care of this patient?
4. What are the priority nursing interventions for this patient?
5. Based on the data presented, write one or more appropriate nursing diagnoses.
6. What are the multidisciplinary care concerns for the elderly patient with a hip fracture?
1. The nurse suspects an ankle sprain when a patient at the casualty department:
2. The nurse explains to a patient with a distal tibial fracture returning for a 3-week check-up that healing is indicated by:
3. A patient with a comminuted fracture of the femur is to have an open reduction with internal fixation (ORIF) of the fracture. The nurse explains that ORIF is indicated when:
4. An indication of a neurovascular problem noted during assessment of the patient with a fracture is:
5. A patient with a stable, closed fracture of the humerus caused by trauma to the arm has a temporary splint with bulky padding applied with an elastic bandage. The nurse suspects compartment syndrome and notifies the doctor when the patient experiences:
6. A patient with symphysis pubis and pelvic rami fractures should be monitored for:
7. During the postoperative period, the patient with an above-the-knee amputation should be instructed that the residual limb should not be routinely elevated because:
8. A patient with rheumatoid arthritis is scheduled for an arthroplasty. The nurse explains that the purpose of this procedure is to:
9. The nurse teaches a patient scheduled for a total hip replacement that it is important after surgery to avoid:
1 Australian Bureau of Statistics (ABS). Causes of death, Australia, 2008. Cat. no. 3303.0. Canberra: ABS; 2008. Available at www.abs.gov.au accessed 10 October 2010.
2 Accident Compensation Corporation. Preventing injury from falls, 2005–2015/Te Arai i nga Aitua Hinga Te Rautaki a-Motu, 2005–2015. Accident Compensation Corporation; 2005. Available at www.acc.co.nz/PRD_EXT_CSMP/groups/external_ip/documents/guide/wcm2_020951.pdf accessed 7 January 2011.
3 Australian Commission on Safety and Quality in Health Care. Preventing falls and harm from falls in older people. Best practice guidelines. Commonwealth of Australia; 2009. Available at www.health.gov.au/internet/safety/publishing.nsf/content/com-pubs_FallsGuidelines/$File/30457-Guidelines-HOSP.PDF accessed 6 January 2011.
4 Andrew NE, Gabbe BJ, Wolfe R, Williamson OD, Richardson MD, Edwards ER, Cameron PA. Twelve-month outcomes of serious orthopaedic injuries admitted to level 1 trauma centres in Melbourne. Clin J Sports Med. 2008;18(5):387–393.
5 Orchard JW. Preventing sports injuries at the national level: time for other nations to follow New Zealand’s remarkable success. Br J Sports Med. 2008;42:392–393.
6 Bentley TA, Page SJ, Macky KA. Adventure tourism and adventure sports injury: the New Zealand experience. Applied Ergonomics. 2006;38(6):791–796.
7 Maher A. Trauma. In Taggart H, ed.: Core curriculum for orthopaedic nursing, 6th edn., Philadelphia: Lippincott Williams & Wilkins, 2008.
8 Jenkin M, Sitler M, Kelly J. Clinical usefulness of the Ottawa ankle rules for detecting fractures of the ankle and midfoot. J Athl Training. 2010;45(5):480–482.
9 National Institute of Aging, National Aeronautics and Space Administration. Exercise: guide from the National Institute of Aging and NASA. Available at www.weboflife.ksc.nasa.gov/exerciseandaging/home.html. accessed 18 October 2010.
10 Kneale J, Davis P, Powell M. Orthopaedic and trauma nursing, 2nd edn. Edinburgh: Churchill Livingstone, 2007.
11 Bagwell-Crum C. The shoulder. In Taggart H, ed.: Core curriculum for orthopaedic nursing, 6th edn., Philadelphia: Lippincott Williams & Wilkins, 2008.
12 Scanlon A, Maffei J. Carpal tunnel syndrome. J Neurosci Nurs. 2009;41:140.
13 Rapp SM. CTS surgery equally improves motor and sensory function in patients with diabetes. Orthop Today. 2008;28(12):55.
14 A helping hand for carpal tunnel syndrome. John Hopkins Health After. January 2006;50.
15 Wright J. Evidence-based orthopaedics: the best answers to clinical questions. Philadelphia: Saunders/Elsevier, 2009.
16 Kneale J, Davis P, Powell M. Orthopaedic and trauma nursing, 2nd edn. Edinburgh: Churchill Livingstone, 2007.
17 Atkinson-Smith M, Todd Smith W. Rotator cuff tears and overview. Orthop Nurs. 2010;29(5):319–325.
18 Orchard JW, Read JW, Anderson IJ. The use of diagnostic imaging in sports medicine. Med J Aust. 2005;183(9):482–486.
19 Coghlan JA, Buchbinder SG, Johnston RV, Bell SN. Surgery for rotator cuff disease. Cochrane Database Syst Rev. (1):2008. CD005619.
20 Abate J. Dislocations and soft-tissue injuries of the knee. In Browner BD, Levine RM, Trafton PG, eds.: Skeletal trauma, 4th edn., Philadelphia: Saunders, 2008.
21 Mutsaerts LE, van den Berkerom PJ, de Vries J, Bramer J, van Dijk CN, Kerkhoffs CN, Gino MMJ. Surgical interventions for treating meniscal injuries of the knee in adults. Cochrane Database Syst Rev. (6):2010. CD008543.
22 Kneale J, Davis P. Orthopaedic and trauma nursing. Edinburgh: Churchill Livingstone, 2004.
23 Parker MJ, Handoll HH. Pre-operative traction for fractures of the proximal femur in adults. Cochrane Database Syst Rev. (3):2006. CD000168.
24 Walker JA. Evidence for skeletal pin site care. Nurs Stand. 2007;21(45):70. 72, 74–76.
25 Mojtaba D. External and bone fixators & pins; pin site care. The Joanna Briggs Institute Report. Available at http://proquest.umi.com/pqdweb?did=1857336251&sid=1&Fmt=3&clientId=20928&RQT=309&VName=PQD, 15 August 2009. accessed 20 October 2010.
26 Australia New Zealand Nursing and Midwifery drug handbook. 4th edn. Philadelphia: Lippincott Williams & Wilkins; 2008.
27 Working Group of the Australian and New Zealand Bone and Mineral Society, Endocrine Society of Australia, Osteoporosis Australia. Vitamin D and adult bone health in Australia and New Zealand: a position statement. Med J Aust. 2005;182(6):281–285.
28 Judge NL. Neurovascular assessment. Nursing Standard. 2007;21(45):39–44.
29 Australian and New Zealand Bone and Mineral Society. Calcium supplements and vascular disease. Available at www.anzbms.org.au/resources/patient/Ca_supps.cfm. accessed 20 October 2010.
30 Edwards PS, Lipp A, Holmes A. Preoperative skin antiseptic for preventing surgical wound infections after clean surgery. Cochrane Database Syst Rev. (3):2004. CD003949. Last assessed as up to date July 2008.
31 Faber J, Hardin S. Outcomes of knee replacement patients using autotransfusion. Orthop Nurs. 2010;29(5):333–337.
32 Jayasekara R. External skeletal pin: site care. The Joanna Briggs Institute Report. Available at http://proquest.umi.com/pqdweb?did=1737369521&sid=1&Fmt=3&clientId=20928&RQT=309&VName=PQD, 25 February 2009. accessed 20 October 2010.
33 Shadgan B, Menon M, Sanders D, Berry G. Current thinking about acute compartment syndrome of the lower extremity. Can J Surg. 2010;53(5):329–335.
34 Buckley R, Panaro CD. General principles of fracture care. eMedicine from WebMD. Available at http://emedicine.medscape.com/article/1270717-overview, Updated January 2010. accessed 6 January 2011.
35 Paula R. Compartment syndrome, extremity. eMedicine from WebMD. Available at a http://emedicine.medscape.com/article/828456-overview, Updated December 2009. accessed 6 January 2011.
36 Malik A, Khan W, Chaudhry A, et al. Acute compartment syndrome—a life and limb threatening surgical emergency. J Periop Pract. 2009;19:137.
37 Galway U, Tetzlaff JE, Helfand R. Acute fatal fat embolism syndrome in bilateral total knee arthroplasty—a review of the fat embolism syndrome. Internet J Anesthesiol. 2009;19:1.
38 Curtis K, Ramsden C, Friendship J. Emergency & trauma nursing. Sydney: Elsevier, 2007.
39 Lee C, Porter K. The prehospital management of pelvic fractures. Emerg Med J. 2007;24:130–133.
40 Brauer C, Coca-Perraillon M, Cutler D, et al. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573.
41 Friese G, LaMay G. Emergency stabilization of unstable pelvic fractures. Emerg Med Serv. 2005;34(5):67–71.
42 International Osteoporosis Foundation. Facts and statistics. Available at http://iofbonehealth.org/facts-and-statistics.html. accessed 20 October 2010.
43 Bischoff-Ferrari HA, Willett WC, Wong JB, et al. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA. 2005;293(18):2257–2264.
44 Chilov MN, Cameron ID, March LM. Evidence-based guidelines for fixing broken hips: an update. Med J Aust. 2003;179(9):489–493.
45 Fielden JM, Purdie G, Horne G, et al. Hip fracture incidence in New Zealand, revisited. New Zealand Orthopaedic Association. J Bone Joint Surg. 2004;84-B(supp1 1):134.
46 Australian Institute of Health & Welfare, Department of Health and Ageing. A picture of osteoporosis in Australia. Available at www.aihw.gov.au/publications/phe/aposia/aposia.pdf, 2008. accessed 20 October 2010.
47 Rodts MF. Preventing hip fractures in our aging society. Orthop Nurs. 2006;25(4):234.
48 Kneale J, Davis P, Powell M. Orthopaedic and trauma nursing, 2nd edn. Edinburgh: Churchill Livingstone, 2007.
49 Parker MJ, Gillespie WJ, Gillespie LD. Hip protectors for preventing hip fractures in older people. Cochrane Database Syst Rev. (3):2005. CD001255. Assessed as up to date May 2010.
50 Martin JT, Coviak CP, Gendler P, et al. Female adolescents’ knowledge of bone health promotion behaviours and osteoporosis risk factors. Orthop Nurs. 2004;23(4):235–244.
51 Avenell A, Handoll HHG. Nutritional supplementation for hip fracture aftercare in older people. Cochrane Database Syst Rev. (4):2006. CD001880.
52 Pullen RL. Logrolling a patient. Nursing. 2004;34(2):22.
53 Ledlie J, Renfro M. Kyphoplasty treatment of vertebral fractures: 2-year outcomes show sustained benefits. Spine. 2006;31(1):57–64.
54 Hochmuth K, Proschek D, Schwarz W, et al. Percutaneous vertebroplasty in the therapy of osteoporotic vertebral compression fractures: a critical review. Eur Radiol. 2006;16(5):998–1004.
55 Barnes CM, Russell CM, Hlava GL, et al. Comparison of a waterpik dual-motor powered toothbrush and a manual toothbrush in affecting interproximal bleeding reduction and dental biofilm accumulation. J Clin Dent. 2003;14(3):49–52.
56 Esquenazi A. Amputation rehabilitation and prosthetic restoration. From surgery to community reintegration. Disabil Rehabil. 2004;26(14–15):831–836.
57 Siddle L. The challenge and management of phantom limb pain with amputation. Br J Nurs. 2004;13(11):664–667.
58 Woodhouse A. Phantom limb sensation. Clin Exp Pharmacol Physiol. 2005;32(1–2):132–134.
59 Hayes D. How to wrap an above the knee amputation stump. Nursing. 2003;33(1):70.
60 Schoen D. Hip and knee arthroplasties. Orthop Nurs. 2009;28(6):323–327.
61 Memtsoudis S, Rosenberger P, Matthias J. Critical care issues in the patient after major joint replacement. J Intensive Care Med. 2007;22(2):92–104.
62 Graves SE, Davidson D, Ingerson L, et al. Bone and joint disorders: prevention and control. The Australian Orthopaedic Association National Joint Replacement Registry. Med J Aust. 2004;180(5 suppl):S31–S34.
63 Childs SG. Hand and wrist problems. In Taggart H, ed.: Core curriculum for orthopaedic nursing, 6th edn., Philadelphia: Lippincott Williams & Wilkins, 2008.
64 O’Reilly RF, Burgess I, Zicat B. The prevalence of venous thromboembolism after hip and knee replacement surgery. Med J Aust. 2005;182(4):154–159.
65 Montin L, Johansson K, Kettunen J, Katajisto J, Leino-Kilpi H. Total joint arthroplasty patients’ perception of received knowledge of care. Orthop Nurs. 2010;29(4):246–253.
66 Altizer L. Patient education for total hip and knee replacement. Orthop Nurs. 2004;23(4):283–288.
67 Morris B, Benetti M, Marro H, Koch Rosenthal C. Clinical practice guidelines for early mobilization hours after surgery. Orthop Nurs 29(5):290–316.
Amputees Federation of New Zealand. www.amputee.co.nz
Amputees United of Australia. www.monash.edu.au/rehabtech/amputee/aua.htm
Arthritis Australia. www.arthritisaustralia.com.au
Arthritis New Zealand. www.arthritis.org.nz
Australian Institute of Sport. www.ausport.gov.au/ais
Australian and New Zealand Orthopaedic Nurses Association. www.anzona.net
Australian Orthopaedic Association. www.aoa.org.au
New Zealand Ministry of Health. www.health.govt.nz
New Zealand Orthopaedic Association. www.nzoa.org.nz