Intravenous Patient-Controlled Analgesia
Initial Intravenous Patient-Controlled Analgesia (PCA) Prescription
Bolus Dose and Lockout Interval
Continuous Infusion (Basal Rate)
PCA in Opioid-Tolerant Patients
Initiating PCA in the Postanaesthesia Care Unit and Patient Transfer to the Clinical Unit
Authorized Agent-Controlled Analgesia
Operator Errors: Misprogramming Analgesic Infusion Pumps
Infusion Solution and Tubing Changes
PATIENT-CONTROLLED analgesia (PCA) is an interactive method of pain management that permits patients to treat their pain by self-administering doses of analgesics. It has been used to manage all types of pain, most commonly acute pain and less often cancer pain because most cancer pain can be managed by oral or transdermal opioid analgesics. Although patients with pain often self-administer their oral analgesics, the term PCA is usually applied when opioids are administered by the intravenous (IV); subcutaneous (SC); continuous peripheral nerve block (patient-controlled regional analgesia [PCRA]; epidural; or intranasal routes of administration. The PCA approach recognizes that only the patient can feel the pain, and only the patient know how much analgesic will relieve it (Pasero, McCaffery, 1993; Pasero, Portenoy, McCaffery, 1999). By allowing patients to determine dosing, PCA addresses the significant variations in analgesic requirements between individuals (Grass, 2005; Lehmann, 2005).
PCA pumps are used to administer opioids by the SC, IV, and epidural routes. Their use requires prescribing a number of parameters (Box 17-1), many of which are safety features to help prevent overdosing; however, it is important for clinicians to appreciate that the safety of PCA depends on appropriate patient selection, initial and ongoing patient/family and staff teaching and goal setting, patient-only use, systematic assessment of responses, and adjustments in therapy as needed (Macintyre, Coldrey, 2009). The reader is referred to an in-depth discussion and comparison of the various analgesic infusion devices used to administer PCA in Sherman, B., Enu, I., & Sinatra, R. S. (2009). Patient-controlled analgesia devices and analgesic infusion pumps. In R. S. Sinatra, O. A. de Leon-Casasola, B. Ginsberg, et al. (Eds.), Acute pain management, Cambridge, NY, Cambridge University Press. Included are desirable pump features and general purchasing considerations.
The focus of this chapter is primarily on the clinical use of IV PCA. See Chapter 12 for the underlying principles and research on the efficacy of PCA, Chapter 15 for epidural patient-controlled analgesia (PCEA), and Section V for PCRA.
Initial Intravenous Patient-Controlled Analgesia (PCA)Prescription
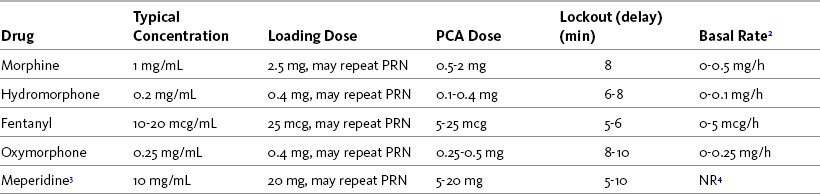
The starting prescription for IV PCA in an opioid-tolerant patient is based on the patient’s current total daily opioid dose. If the patient is switched from one opioid or route to another, this initial prescription is an estimate and must be adjusted according to the patient’s pain and adverse effect profile. The starting PCA prescription for an opioid-naive patient is also just an estimate of a patient’s opioid requirement and must be titrated on the basis of patient response. Table 17-1 provides guidelines for selecting an initial IV PCA prescription for opioid-naive patients and Form 17-1 provides an example of a PCA order set. See an example of a patient information brochure about IV PCA on pp. 544-545 at the end of Section IV.

Form 17-1 Example Order Set: Opioid-Naïve Adult IV PCA
CNS, Central nervous system; CV, cardiovascular; hs, at sleep; IV, intravenous; IVPB, IV piggyback; LOS, level of sedation; PO, oral; POSS, Pasero Opioid-Induced Sedation Scale; PR, per rectum; PRN, as needed; q, every. From Pasero, C., & McCaffery, M. Pain assessment and pharmacologic management, p. 464, St. Louis, Mosby. Pasero C, McCaffery M. May be duplicated for use in clinical practice.
Bolus Dose and Lockout Interval
To a great extent, the success of IV PCA depends on prescribing an adequate bolus dose that can be self-administered frequently enough for patients to manage their pain effectively. Small doses of analgesia (e.g., 1 mg of morphine or equivalent) and short lockout (delay) intervals (e.g., 5 to 10 minutes) are best for opioid-naïve patients so as to prevent excessive sedation at peaks (the highest blood levels of opioid) and to prevent breakthrough pain at troughs (the lowest blood levels of opioid); however, if the dose is too small, patients may have difficulty maintaining analgesia (Macintyre, Coldrey, 2008). Larger bolus doses at lockout intervals of 15 to 30 minutes are commonly required and are usually well tolerated by opioid-tolerant patients with cancer or persistent noncancer pain. The optimal dose should provide consistent, satisfactory analgesia without excessive or dangerous adverse effects (Macintyre, Coldrey, 2008).
There is surprisingly little research to guide selection of an optimal PCA bolus dose for an opioid-naïve patient. An early randomized controlled trial found comparable efficacy, morphine consumption, and adverse effects with PCA doses of 1 mg every 6 minutes, 1.5 mg every 9 minutes, and 2 mg every 12 minutes (Badner, Doyle, Smith, et al., 1996). PCA attempts, successful injections, missed injections, and dose adjustments were greatest in the group receiving 1 mg; however, one patient in the group receiving 1.5 mg and one in the group receiving 2 mg required naloxone for respiratory depression. The researchers concluded that 1 mg every 6 minutes represents appropriate titration but may result in lower patient satisfaction. Another study concluded similarly that 0.5 mg of IV morphine was not large enough to control pain, and 2 mg was associated with adverse effects, but 1 mg was an optimal bolus dose for postoperative pain in opioid-naïve patients (Owen, Plummer, Armstrong, et al., 1989). A randomized controlled study of IV fentanyl use during burn dressing changes showed that PCA bolus doses of 30 to 40 mcg of fentanyl with a lockout of 5 minutes produced better analgesia and demand/delivery ratios than did 10-mcg and 20-mcg bolus doses (Prakash, Fatima, Pawar, 2004). No research could be found on the optimal PCA bolus dose or lockout interval for hydromorphone, the other opioid commonly used for IV PCA; however, 0.1 to 0.2 mg of IV hydromorphone is considered roughly equal to 1 mg of IV morphine in this setting (Knotkova, Fine, Portenoy, 2009).
There is also very little research on the optimal lockout (delay) interval for IV PCA (Macintyre, Coldrey, 2008). The length of the lockout interval should allow for adequate analgesic coverage during times when patients need the most opioid. For example, postoperative patients should be able to activate PCA before and frequently during potentially painful activities, such as ambulation, self-care, and physical therapy or respiratory therapy treatments. The lockout interval also should be long enough for a patient to appreciate the effect of one bolus before self-administering another (Macintyre, Coldrey, 2008). The characteristics of the opioid, such as the onset and peak times, are primary determinants of the length of the lockout interval. The American Pain Society (APS, 2003) recommends a lockout interval of between 5 and 10 minutes for most of the IV opioids used for acute pain. For example, commonly used lockout intervals are 5 to 6 minutes for IV fentanyl and 6 to 8 minutes for IV PCA morphine and hydromorphone for acute pain management.
The customary adult starting dose should be reduced by 25% to 50% for opioid-naïve older patients because research has shown that analgesia requirements decrease with increasing age (Gagliese, Jackson, Ritvo, et al., 2000; Macintyre, Coldrey, 2009). In a review of more than 6000 patients aged 65 and older who had received IV PCA, bolus doses greater than 1 mg/dose and intraabdominal surgery were cited as risk factors for hypoxemia and respiratory depression during IV PCA (Sidebotham, Dijkhuizen, Schug, 1997). Also, the lockout interval is sometimes increased to 10 minutes in this population.
Hour Limit
The hour limit is the maximum amount of opioid a patient has access to in an hour-limit time period. Most PCA pumps can be programmed for a 1-hour or 4-hour limit, and some pumps allow this parameter to be bypassed altogether. If an hour limit has been programmed and the patient consumes the programmed amount before the time period has expired, the pump denies the patient any further PCA bolus doses until the next hour-limit time period begins. The programmed hour limit includes the number of PCA bolus doses and the basal rate (if one is programmed). Some PCA pumps also include clinician-administered bolus doses in the hour-limit amount.
There is no consensus about whether hour limits should be used. Those who choose not to use them do so because they think patients should have access to as much opioid as they need to manage their pain, restricted only by the amount of the dose and the length of the lockout interval. Those who choose to use an hour limit do so because they think it offers an additional safeguard against overdosing; however, this thinking may provide false reassurance; the only built-in safeguard of PCA is patient-only use (see discussion of PCA by proxy later in this chapter). A benefit of programming an hour limit is that it alerts caregivers that increased doses might be necessary. Hour limits seem to be used more commonly in opioid-naïve than in opioid-tolerant patients. If used, a 1-hour limit is preferable to a 4-hour limit for at least two reasons: (1) caregivers are alerted to the need for an increase in the opioid dose sooner (i.e., within 1 hour instead of within 4 hours); and (2) it eliminates a scenario in which an increase in dose is neglected in patients who use the 4-hour amount in less than 4 hours (i.e., a patient who uses the 4-hour amount in 2 hours and is left for 2 hours without analgesia). In an analysis of data submitted to MEDMARX and the United States Pharmacopeia (USP) Medication Errors Reporting Program between 1998 and 2003, the USP identified this scenario as a common PCA-related underdosing error (USP, 2004). It is essential that the hour limit be adjusted up or down as necessary on the basis of patient response. If used, the APS (2003) recommends that the hour limit be set at 3 to 5 times the projected hourly IV requirement for at least the first 24 hours.
Continuous Infusion (Basal Rate)
The purpose of a continuous infusion (basal rate) is to help maintain a stable analgesic level (American Society of Anesthesiologists [ASA], 2004; Flisberg, Rudin, Linner, et al., 2003). It has the advantage of letting the patient sleep without frequent interruptions by pain. If a continuous infusion is not added, patients must self-administer the PCA dose often enough to maintain a stable analgesic level. When IV PCA is used to manage cancer or persistent noncancer pain in opioid-tolerant patients, the continuous infusion usually provides the larger part of the patient’s total opioid requirement, with PCA doses being used as breakthrough doses (APS, 2003). Although continuous infusion is recommended and is commonly used for opioid-tolerant patients, the addition of continuous infusion to IV PCA bolus doses for opioid-naïve patients outside of a monitored setting such as the intensive care unit (ICU) is controversial (APS, 2003; Pasero, McCaffery, 2004). The primary safeguard in PCA therapy is that a patient must be awake to self-administer a PCA dose. Patients who are excessively sedated are likely to drop the PCA button, thereby preventing further sedation and clinically significant respiratory depression (Pasero, McCaffery, 2004). Herein lies the controversy. The patient has no control over the delivery of a continuous infusion, so the built-in safeguard is gone. The APS (2003) recommends extreme caution in using basal rates for acute pain management in opioid-naïve patients.
Studies have produced mixed findings in terms of the effectiveness and safety of adding continuous infusions to IV PCA in opioid-naïve patients. Early research showed that the addition of a continuous infusion did not improve analgesia, produced excessive adverse effects, and resulted in consumption of greater amounts of opioid drugs (Parker, Holtmann, White, 1991; Russell, Owen, Ilsley, et al., 1996; Smythe, Zak, O’Donnell, 1996). A more recent study of 35 patients following open heart surgery found no differences in pain intensity scores or adverse effects between patients who received PCA with or without a basal rate and concluded that no benefit could be found in adding a basal rate to PCA therapy (Dal, Canback, Cagler, et al., 2003). There was no excessive sedation or clinically significant respiratory depression. Another study of 60 patients after cardiac surgery reported that a basal rate with PCA increased morphine consumption but improved pain relief without increasing adverse effects (Guler, Unlugenc, Gundogan, et al., 2004). There were no episodes of respiratory depression or hypoxemia. Others have also found less fluctuation in sedation and better pain control and have recommended the addition of a basal rate to IV PCA for some patients (Hansen, Noyes, Lehman, 1991; Rayburn, Smith, Woods, 1989). A prospective study of more than 1000 patients receiving IV PCA morphine with basal rates after major surgery reported that respiratory depression occurred in 13 patients (1.2%) (Flisberg, Rudin, Linner, et al., 2003). Interestingly, a study of 178 postoperative patients who were monitored using continuous pulse oximetry (oxygen saturation) and capnography (end-tidal CO2) while receiving IV PCA demonstrated that despite consuming approximately two times more opioid, those who were receiving PCA with a continuous basal rate (1 mg/h morphine equivalent) had lower incidences of bradypnea (32% vs. 53%) and desaturation (8% vs. 17%) than did those who were receiving PCA bolus doses without a basal rate (Overdyk, Carter, Maddox, et al., 2007) (see Chapter 19 for discussion of mechanical monitoring).
An important finding in an early study by Parker and colleagues (1991) is noted in the percentages of patients in the various groups in the study who required changes in morphine PCA therapy or discontinuation of therapy entirely because of adverse effects. The lowest was 10% of the group receiving a 0.5 mg/h basal rate followed by 11% of those with no basal rate, 14% of those with a 1 mg/h basal rate, and 66% of those receiving a 2 mg/h basal rate (Parker, Holtmann, White, 1991). During the first 2 days of the study, the pain ratings were lowest in the group receiving the 0.5 mg/h basal rate. These findings reinforce the rationale that the adverse effects of opioids are dose related and that basal rates greater than 1 mg/h are not well tolerated and may be unsafe; however, a basal rate of 0.5 mg/h can be safe and may improve analgesia for some patients.
Clinical experience indicates that the addition of a continuous infusion to IV PCA can be done safely and may benefit some patients (ASA, 2004; Pasero, McCaffery, 2004). A cautious approach and one that is supported by research is to begin PCA without a basal rate, and then add one later if a patient has difficulty maintaining satisfactory pain control, especially after sleep (Pasero, Portenoy, McCaffery, 1999) (see the following patient example). Initiating therapy without a basal rate may be particularly appropriate in older patients. As mentioned, PCA requirements tend to decrease with increasing age (Macintyre, Coldrey, 2008; Sidebotham, Dijkhuizen, Schug, 1997); however, the decision to add a continuous infusion to IV PCA for older opioid-naïve patients should be made on the basis of patients’ responses rather than on a preconceived notion that they cannot tolerate continuous infusions.
Absolutely essential to the safe use of continuous infusions in opioid-naïve patients is close monitoring by nurses of sedation and respiratory status and prompt decreasing of the opioid dose if increased sedation is detected (Pasero, 2009b). The use of mechanical monitoring (e.g., pulse oximetry, capnography) may be appropriate in some patients receiving IV PCA (see Chapter 19 for more information about monitoring sedation and respiratory depression). If careful monitoring of sedation and respiratory status is not possible, the use of continuous opioid infusions in opioid-naive patients is not recommended (Pasero, McCaffery, 2004).
PCA in Opioid-Tolerant Patients
For patients with cancer or persistent noncancer pain who are receiving continuous opioid infusions, PCA offers an independent means of managing breakthrough pain (see the following patient example). In these opioid-tolerant patients, the continuous infusion provides most of the opioid requirements. The PCA bolus doses are larger, usually allowing patients to double the dose provided by the continuous infusion, and lockout intervals are longer than in opioid-naïve patients (APS, 2003). For example, a PCA bolus dose usually is 25% to 50% of the hourly continuous-infusion dose, and the lockout interval usually is set at 15 to 30 minutes.
Loading Dose
A key principle of PCA therapy is that it be initiated in a patient who has reasonably well-controlled pain. Before the PCA button is handed to the patient for self-management, loading doses are administered to establish analgesia (Cashman, 2006). A common and recommended practice is to store a specified number of PCA pumps, drug reservoirs, and infusion tubings (number is dependent on the size of the institution) in the emergency department [ED] so that the therapy can be initiated without delay in patients who are admitted for treatment of severe pain crises. Loading doses can be administered using the clinician-administered bolus mode on most PCA pumps (see Chapter 16 for titration in patients with severe acute pain and loading-dose recommendations in Table 17-1).
Initiating PCA in the Postanesthesia Care Unit and Patient Transfer to the Clinical Unit
When IV PCA is prescribed for postoperative patients, it should be initiated when the patients arrive in the postanesthesia care unit (PACU) (Krenzischek, Wilson, 2003). Initiating IV PCA in the PACU rather than in the nursing unit allows the health care team to evaluate patient responses to the therapy early in the postoperative period and prevents delays in analgesia (analgesic gaps) in the nursing unit. A particularly dangerous scenario that is to be avoided is that of patients receiving intramuscular (IM) injections of opioid in the clinical unit while waiting for IV PCA to be initiated (see Chapter 14 for more on the dangers of IM injections).
The PACU nurse can save time by using the PCA pump to administer loading doses and establish satisfactory analgesia. Then the PCA button should be given to patients as soon as they are awake and alert enough to manage their own pain. At that time, pain management plans can be reviewed with patients, including which actions to take when pain relief is inadequate. PACU nurses can reinforce information about the safety mechanisms of the PCA pump and about how to use the PCA button correctly, reminding patients that it is for their use only.
Some PACUs establish discharge goals that include acceptable pain ratings for patients, usually 4/10 or less (Pasero, McCaffery, 2003) (see Section II); however, the expectation that all patients must be discharged from the PACU with pain ratings below an arbitrary number can lead to the unsafe administration of further opioid doses to patients who are excessively sedated (Blumstein, Moore, 2003; Lucas, Vlahos, Ledgerwood, 2007). Instead, achieving optimal pain relief is best viewed on a continuum, with the primary objective being to provide both effective and safe analgesia. Although it is not always possible to achieve the patient’s comfort-function goal within the short time the patient is in an area like the PACU, the comfort-function goal provides direction for ongoing care. Important information to give to the nurse assuming care of the patient in the clinical unit is the patient’s comfort-function goal, how close the patient is to achieving it, what has been done thus far to achieve it (analgesics, doses, and times of administration), and how well the patient has tolerated the administration of analgesics (adverse effects) (see Chapter 19 for more on transfer of care and hand-off communication).
To save time and prevent errors, tables containing prescription ranges of PCAs commonly used in opioid-naïve patients can be developed in advance (see Table 17-1). These tables can be posted in areas where prescribers write orders for PCA. Preprinted order sets with the appropriate starting IV PCA prescriptions for the most commonly used opioids guide prescribers to select individualized doses on the basis of their patients’ unique characteristics (see Form 17-1).
Titration of IV PCA
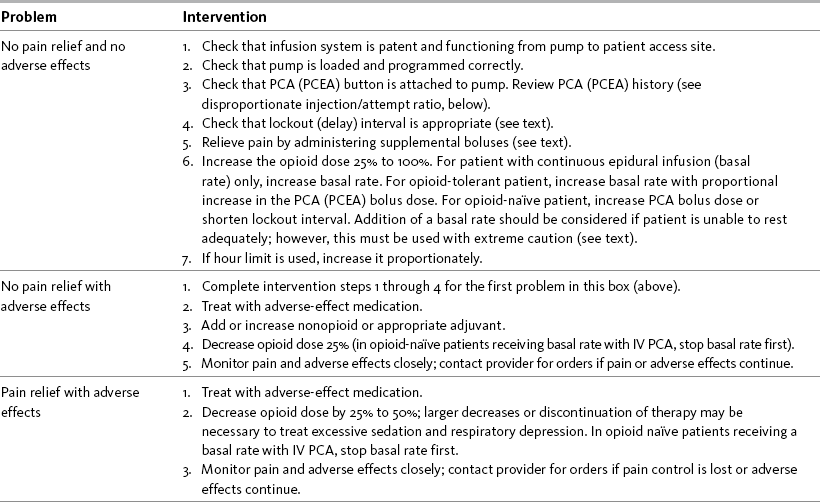
As discussed, initial PCA prescriptions are estimates of the amounts of opioid patients will require. It is crucial that patients be evaluated regularly and titrated when necessary to maintain adequate analgesia and tolerable and manageable adverse effects. Patients reporting inadequate pain relief with the use of PCA require prompt evaluation. The need for readjustment of the parameters of PCA is signaled by pain ratings above the identified comfort-function goal or by unmanageable and intolerable adverse effects. Pain ratings and the incidence of adverse effects, in addition to the patient’s account and the PCA history retained in the pump’s memory, provide valuable information about the pain and should be used to guide the various approaches to titration. For example, approaches differ between patients who are not activating PCA before painful activity (incident pain) and those who make multiple unsuccessful attempts to obtain a PCA dose. Table 17-2 provides interventions for patients receiving IV PCA or epidural analgesia.
The use of PCA does not absolve nurses from their roles as patients’ primary pain managers. Simply telling a patient to “press the PCA button” does not constitute acceptable pain management. Likewise, concluding that a patient is using too much opioid or pressing the PCA button too often (e.g., when a patient self-administers the maximum programmed amount [hour limit] in less than 1 hour) has no scientific basis and creates an adversarial relationship between patient and caregiver. More importantly, it presents a tremendous barrier to providing acceptable pain treatment and improving patient outcome.
PCA by Proxy
PCA by proxy is the unauthorized administration of a PCA dose by another person. This has the potential to produce significant patient harm because it circumvents an important safeguard of PCA; that is, the excessively sedated patient will drop the PCA button, thereby preventing further opioid administration and subsequent respiratory depression (Pasero, McCaffery, 2005a). Over the years, there have been reports of the dangers of PCA by proxy. One early report evaluated 3785 patients who received IV PCA and reported 14 critical events, 3 of which involved unauthorized family members pressing the PCA button (Ashburn, Love, Pace, 1994). A review of nearly 6000 patients who had received IV PCA with no basal rate identified unauthorized PCA delivery to sleeping patients by relatives as the cause of 2 of 14 cases of respiratory depression (Sidebotham, Dijkhuizen, Schug, 1997).
These types of reports prompted The Joint Commission (TJC), an independent accrediting body of health care facilities in the United States, to issue a “sentinel event alert” on unauthorized PCA administration. This alert identified 460 PCA-related adverse events over a 5-year period; 15 of these events were the result of unauthorized family or staff members pressing the PCA button (TJC, 2004). Others have echoed concerns regarding this phenomenon (Institute for Safe Medication Practices, 2003a, 2003b).
Given the risks associated with PCA by proxy, TJC now expects to see proof that institutions have taken steps to minimize the potential for this outcome. These include patient education about the use of PCA prior to initiation of therapy and the use of verbal and written instructions warning against individuals other than the patient pressing the PCA button (see Box 12-3 on p. 316). The observation that most PCA by proxy is initiated by well-intentioned family members who want to ensure that their loved one is comfortable underscores the importance of frequent assessment of patients during PCA therapy to identify those who are unable to manage their own pain effectively as well as telling family members to contact staff if they have concerns about the patient’s pain.
Authorized Agent-Controlled Analgesia
When patients are unable or unwilling to self-administer analgesics, another individual may be authorized to manage the patient’s pain using the PCA technology. For example, family-controlled analgesia (FCA) or caregiver-controlled analgesia (CCA) designates one person to be the patient’s primary pain manager, with the responsibility of pressing the PCA button (on the face of the pump or pendant attached to the pump) (Pasero, McCaffery, 1993; Pasero, Portenoy, McCaffery, 1999). With nurse-activated dosing (NAD) (also called nurse-controlled analgesia), the patient’s primary nurse has that responsibility. These methods have collectively been called “authorized agent-controlled analgesia” (AACA) (Wuhrman, Cooney, Dunwoody, et al., 2007) and have been safely and effectively used for many years in patients of all ages. AACA is supported by a position paper with clinical practice recommendations developed by the American Society for Pain Management Nursing (Wuhrman, Cooney, Dunwoody, et al., 2007) and endorsed by other nursing specialty organizations such as the Oncology Nursing Society and the Hospice and Palliative Care Nurses Association (see Chapter 12 for a more detailed discussion and Box 12-4 on p. 318 for guidelines for the use of AACA).
Operator Errors: Misprogramming Analgesic Infusion Pumps
Operator (human) errors, particularly incorrect loading and misprogramming of PCA pumps, have been identified as a major cause of significant patient injuries and deaths over the years (Hicks, Heath, Sikirica, et al., 2008; Institute for Safe Medication Practices, 2003a, 2003b, 2004b; Macintyre, Coldrey, 2008). Administration of the wrong dose as a result of incorrect pump programming was by far the most common error (38.9%) identified in an analysis of data submitted to MEDMARX and the USP Medication Errors Reporting Program between 1998 and 2003 (United States Pharmacopeia, 2004).
To help prevent these types of errors, staff must be trained in the proper use of analgesic infusion devices (initial training and annual competency checks) (Pasero, Eksterowicz, Primeau, et al., 2007). Institution policy and procedure should mandate that all analgesic infusion device programming be independently double-checked at specified times, such as prior to the initiation of analgesic infusion therapy, at the time of any adjustments in prescription, and during the nursing hand-off communication processes. An independent double-check consists of having another clinician (e.g., nurse, physician, or pharmacist) compare the analgesic solution’s drug and concentration and the pump’s programmed prescription against each patient’s written prescription to ensure accuracy without prompting from the person administering the analgesic or anyone else. Distractions during programming and double-checks are identified as being factors contributing to errors (Hicks, Heath, Sikirica, et al., 2008), so clinicians must take steps to avoid being interrupted.
The concept of the 6 Rights (6 Rs) of medication safety (right patient, right drug, right dose, right time, right route, right documentation) forms the basis of safe analgesic therapies and is the responsibility of everyone on the health care team (Institute for Safe Medication Practices, 2004c). A concerted multidisciplinary effort that includes appropriate prescribing; the highest standard of drug-reservoir preparation, storage, and dispensation; uninterrupted attention during programming and double-checking; and careful patient monitoring during therapy are essential. Readers are encouraged to subscribe to the Institute for Safe Medication Practices newsletters, which regularly publish safety information on a variety of medications (http://www.ismp.org). (See also Chapter 19 for transfer of care and hand-off communications.)
Infusion Solution and Tubing Changes
There are no national guidelines to direct the frequency with which IV PCA infusion systems (solution and tubing) should be changed (also called “hang time”), and microbiologic research is lacking on this therapy. As a result, practices vary widely with regard to this aspect of care. For a more in-depth discussion as well as research on the stability and compatibility of various agents used for analgesic infusion therapies, see Chapter 15.
Tapering and Cessation of Parenteral Analgesia
For patients with acute pain who are receiving parenteral analgesia, plans are necessary for smoothly weaning the patient as pain decreases or the patient is able to use a less invasive route of administration. Although most patients experience less pain as the days pass after surgery, it should not be assumed that all patients will follow this pattern. For example, the duration of postoperative pain tends to be longer in older patients (Melzack, Abbott, Zackon, et al., 1987). It is best to evaluate patients individually and taper analgesic doses on the basis of patients’ reports of pain and ability to perform recovery activities rather than a preconceived notion of when parenteral analgesics should be discontinued.
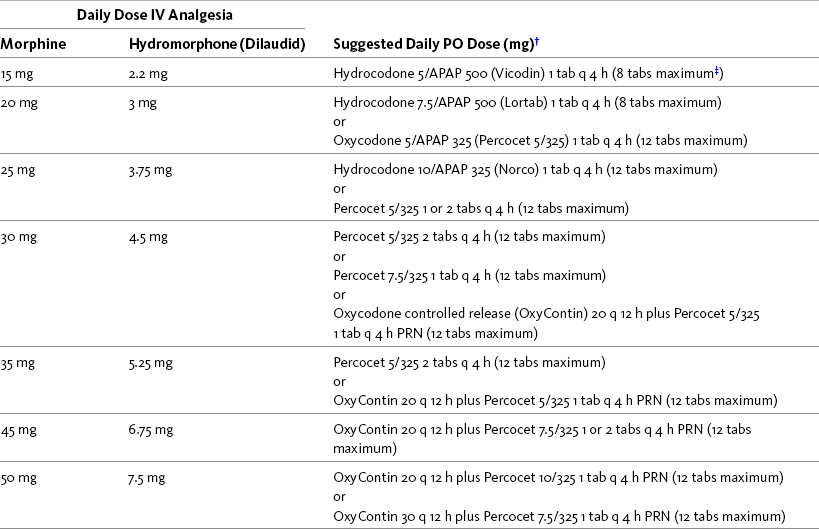
In preparation for discharge, transition to oral analgesia should be started as soon as the patient is able to retain fluids and pain is well controlled. As function returns and pain lessens, the parenteral opioid dose can be reduced 25% once or twice daily. Table 17-3 provides helpful dosing guidelines that can be used to make a smooth transition from IV to oral opioid analgesia using analgesics found on most hospital formularies. The table can be posted in clinical areas where analgesic orders are written so that clinicians may use it for a quick reference guide.
Conclusion
IV PCA is an effective, interactive method that allows patients to manage their own pain. A built-in safeguard of the therapy is patient-only activation of the PCA button. Close monitoring by nurses of sedation and respiratory status is critical to the safe use of a continuous infusion with IV PCA. Optimal PCA therapy is dependent on adequate initial prescriptions, the administration of loading doses to establish satisfactory analgesia prior to initiation of PCA, and PCA prescription adjustments based on patients’ responses to the therapy.