Intraspinal Analgesia (Epidural and Intrathecal)
Delivery of Intraspinal Analgesics
Percutaneous Intraspinal Catheterization
Intraspinal Analgesia for Persistent Cancer and Noncancer Pain
Stability and Compatibility of Agents for Analgesic Infusion Therapy
Methods for Administering Intraspinal Analgesia
Selected Analgesics Administered by the Intraspinal Routes
Complications Associated with the Intraspinal Routes of Administration
Dural Puncture and Postdural Puncture Headache
Direct Needle or Catheter Trauma
THE term intraspinal refers to the spaces or potential spaces surrounding the spinal cord or the nerve roots that constitute the cauda equina. Most often, the term is used when referring to the epidural and intrathecal spaces, each of which offers a route of administration for medications. The word neuraxial also is used to describe the group of spaces into which analgesic drugs can be administered. The word spinal is used interchangeably with the word intrathecal when referring to route of administration. It may also be used when referring generally to all of the routes of administration near or within the spinal meninges (Swarm, Karanikolas, Cousins, 2004). Intrathecal is often used synonymously with subarachnoid, but anatomically the intrathecal space includes the subdural space (Swarm, Karanikolas, Cousins, 2004) (see the following paragraphs on spinal anatomy). Table 15-1 shows some of the persistent misconceptions related to epidural analgesia. Box 15-1 presents patient selection guidelines and considerations for intraspinal analgesia.
Table 15-1
Misconceptions: Epidural Analgesia
| Misconception | Correction |
| Compared with opioid administration via IM injection and IV PCA, the incidence of respiratory depression is higher when opioids are administered by the epidural route. | The incidence of respiratory depression associated with the various pain control methods is not firmly established because of a lack of consensus on definitions and well-controlled research, but the incidence of respiratory depression with epidural analgesia is less than that of IM opioid injections and probably more consistent with that of IV PCA. A systematic review of the literature concluded that the mean reported incidence of opioid-induced respiratory depression varied between 0.8% and 37.0% for IM injection; 1.2% and 11.5% for IV PCA; and 1.1% and 15.0% for epidural analgesia (Cashman, Dolin, 2004). A study of the use of PCEA morphine with basal rate or IV PCA morphine with basal rate in 2696 patients after major surgery reported a higher incidence of respiratory depression with IV PCA (1.2%) than epidural analgesia (0.04%) (Flisberg, Rudin, Linner, et al., 2003). Clinically significant opioid-induced respiratory depression can be avoided in opioid-naïve patients by slow titration, careful nurse monitoring of sedation levels and respiratory status, and decreases in opioid dose when increased sedation is detected (see Chapter 19). |
| Patients receiving epidural analgesia must be cared for in intensive care settings where their respiratory status can be mechanically monitored. | Patients receiving epidural analgesia have been cared for safely outside of the intensive care setting for many years. Though mechanical monitoring is warranted in patients at high risk for respiratory complications (e.g., those with obstructive sleep apnea, chronic pulmonary disease), nurse assessment of sedation level and respiratory status is reliable and the most common method for monitoring most patients receiving epidural analgesia (see Chapter 19). |
| Epidural local anesthetics cause excessive and disabling sensory and motor blockade. | Local anesthetics are administered in low (subanesthetic) doses (e.g., 0.05% to 0.125% bupivacaine; 0.1% to 0.2% ropivacaine) for epidural analgesia. Higher doses are required to produce significant motor and sensory blockade (0.5% to 0.75% bupivacaine; 0.75 to 1.0% ropivacaine). Patients receiving epidural analgesia are able to ambulate and perform all the routine recovery activities expected of them to the extent their medical or surgical condition allows. The occasional occurrence of minor temporary numbness of lower extremities is resolved easily by decreasing the dose or removing the local anesthetic from the epidural analgesic solution. |
| Thoracic epidural catheter placement is technically more difficult and causes more damage than lumbar catheter placement. | The technique for placing a thoracic epidural catheter is quickly mastered by anesthesia providers. A review of 874 cases of high thoracic epidural analgesia provided over a 7-year period revealed no related neurologic complications (Royse, Soeding, Royse, 2007). |
IM, Intramuscular; IV, intravenous; PCA, patient-controlled analgesia.
In spite of widespread use, misconceptions related to epidural analgesia persist. This table corrects some of these misconceptions.
From Pasero, C., & McCaffery, M. Pain assessment and pharmacologic management, p. 405, St. Louis, Mosby. Data from American Society of Anesthesiologists Task Force on Neuraxial Opioids. (2009). Practice guidelines for the prevention, detection, and management of respiratory depression associated with neuraxial opioid administration. Anesthesiology, 110(2), 218-230; Brown, D. L. (2005). Spinal, epidural, and caudal anesthesia. In R. D. Miller (Ed.), Miller’s anesthesia, vol 2, ed 6, Philadelphia, Elsevier; Cashman, J. N., & Dolin, S. J. (2004). Respiratory and haemodynamic effects of acute postoperative pain management: Evidence from published data. Br J Anaesth, 93(2), 212-223; Cousins M. J., & Veering, B. T. (1998). Epidural neural blockade. In M. J. Cousins, & P. O. Bridenbaugh (Eds.), Neural blockade in clinical anesthesia and management of pain, Philadelphia, Lippincott-Raven; Dabu-Bondoc, S., Franco, S. A., & Sinatra, R. S. (2009). Neuraxial analgesia with hydromorphone, morphine, and fentanyl: Dosing and safety guidelines. In R. S. Sinatra, O. A. de Leon-Casasola, B. Ginsberg, et al. (Eds.), Acute pain management, Cambridge, NY, Cambridge University Press; Flisberg, P., Rudin, A., Linner, R., et al. (2003). Pain relief and safety after major surgery. A prospective study of epidural and intravenous analgesia in 2696 patients. Acta Anaesth Scand, 47(4), 457-465; Grape, S., & Schug, S. A. (2008). Epidural and spinal analgesia. In P. E. Macintyre, S. M. Walker, & D. J. Rowbotham (Eds.), Clinical pain management. Acute pain, ed 2, London, Hodder Arnold; Maalouf, D. B., & Liu, S. S. (2009). Clinical application of epidural analgesia. In R. S. Sinatra, O. A. de Leon-Casasola, B. Ginsberg, et al. (Eds.), Acute pain management, Cambridge, NY, Cambridge University Press; McCartney, C. J. L., & Niazi, A. (2006). Use of opioid analgesics in the perioperative period. In G. Shorten, D. B. Carr, D. Harmon, et al., (Eds.), Postoperative pain management: An evidence-based guide to practice, Philadelphia, Saunders; Royse, C. F., Soeding, P. F., & Royse, A. G. (2007). High thoracic epidural analgesia for cardiac surgery: An audit of 874 cases. Anaesth Intensive Care, 35(3), 374-377; Vascello, L., & McQuillan, R. J. (2006). Opioid analgesics and routes of administration. In O.A. de Leon-Casasola (Ed.), Cancer pain. Pharmacological, interventional and palliative care approaches, Philadelphia, Saunders. Pasero C, McCaffery M. May be duplicated for use in clinical practice.
Spinal Anatomy
The human spinal column consists of 33 individual vertebra referred to by their location: (1) 7 cervical, (2) 12 thoracic, (3) 5 lumbar, (4) 5 caudal or sacral (fused into one bone, the sacrum), and (5) 4 coccygeal (fused into one bone, the coccyx) (Figure 15-1).Vertebrae consist of an anterior body, the laminae that protect the lateral spinal cord, and spinous processes that project outwardly and posteriorly from the laminae. The vertebrae become larger as they descend in the vertebral column. The bones of the laminae are bound together by a number of ligaments (e.g., the dense ligamentum flavum) (Figure 15-2).
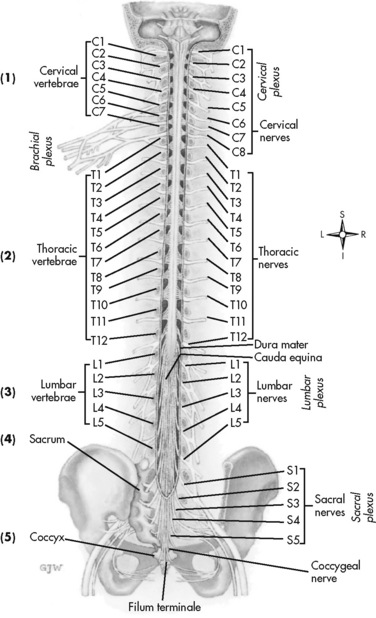
Figure 15-1 Vertebral column. The human spinal cord consists of 33 individual vertebra referred to by their location: (1) 7 cervical, (2) 12 thoracic, (3) 5 lumbar, (4) 5 caudal or sacral (fused into one bone, the sacrum), and (5) 4 coccygeal (fused into one bone, the coccyx). At each vertebral body level, nerve roots exit from the spinal cord bilaterally. Specific skin areas are innervated by a single spinal nerve or group of spinal nerves. From Thibodeau, G. A., & Patton, K. T. (1996). Anatomy & physiology, ed 3, St Louis, Mosby.
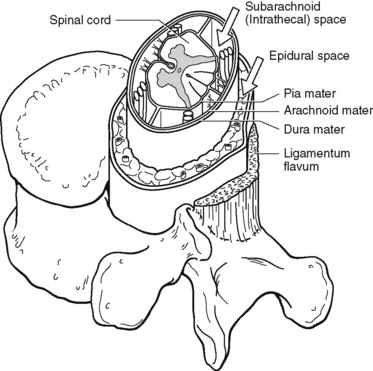
Figure 15-2 Spinal anatomy. The spinal cord is a continuous structure extending from the foramen magnum to approximately the first or second lumbar (L1-L2) vertebral interspace. The subarachnoid space (also called the intrathecal space in the caudal part of the spine) surrounds the spinal cord, separated by the pia mater. The subarachnoid space is filled with cerebrospinal fluid that continuously circulates and bathes the spinal cord. The dura is composed of the arachnoid and dura mater membranes and separates the epidural space from the subarachnoid space. The epidural space is a potential space filled with vasculature, fat, and a network of nerve extensions. From Salerno, E., & Willens, J. (1996). Pain management handbook, St Louis, Mosby.
The spinal cord is located within and protected by the bony vertebral column and connective tissue (meninges). It is a continuous structure extending from the foramen magnum to approximately the first or second lumbar (L1 to L2) vertebral interspace. Below the tip of the spinal cord, which is called the conus medullaris, are the nerve roots that exit the spine from below the L2 vertebra to the lower part of the sacrum. This tangle of roots is known as the cauda equina.
Moving from outside to inside the spine, the epidural space is first encountered. This is a potential space filled with vasculature, fat, and a network of nerve extensions. No fluid is in the epidural space; a true space is created when volume or air is injected into it (see Figure 15-2). The epidural space is outside of the dura, which is composed of the dura mater and the arachnoid membranes. The subarachnoid space (also called the intrathecal space in the caudal part of the spine) lies deep to the subarachnoid membrane, between this membrane and the spinal cord and cauda equina. The subarachnoid space is filled with clear, colorless cerebrospinal fluid (CSF) that continually circulates and bathes the spinal cord and nerve roots.
The fact that the epidural space is a potential space has clinical implications. Although injecting large amounts of air is not recommended, small amounts, such as tiny bubbles within the infusion tubing when therapy is initiated, are not considered dangerous. In addition, because the epidural catheter is in a space and not a blood vessel, a continuous epidural infusion may be stopped for hours and restarted without concern that the catheter has become occluded. However, crystallization of the saline within the epidural catheter can occur when catheters are unused for prolonged periods. In these cases, weekly or biweekly irrigation is recommended (DuPen, DuPen, 1998).
At each vertebral body level, nerve roots exit from the spinal cord bilaterally. A specific area of skin and subcutaneous tissue, known as a dermatome, is innervated by a single spinal nerve (Figure 15-3). The assessment of sensation in a dermatome is used to determine the integrity of the nerve root and subsequent pathway of innervation. Assessment of sensation in dermatomes is performed by anesthesia providers and others to determine the level of spinal anesthesia for surgical procedures and postoperative analgesia when epidural local anesthetics are used.
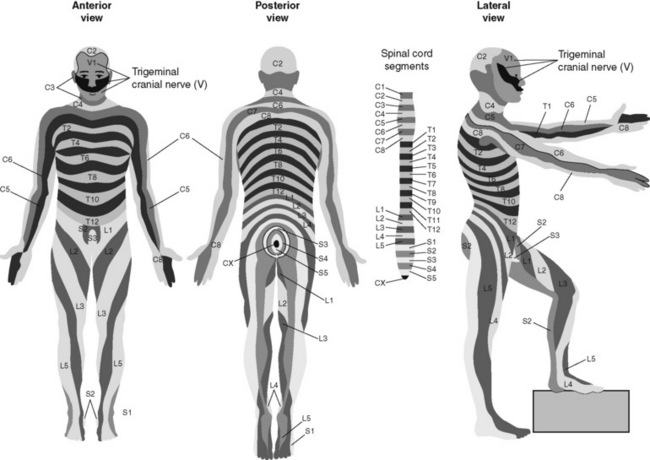
Figure 15-3 Dermatomes. Segmental dermatome distribution of spinal nerves to the front, back, and side of the body. C, Cervical segments; T, thoracic segments; L, lumbar segments; S, sacral segments; CX, Coccygeal segment. Dermatomes are specific skin areas innervated by a single spinal nerve or group of spinal nerves. Dermatome assessment is done to determine the level of spinal anesthesia for surgical procedures and postoperative analgesia when epidural local anesthetics are used. From Thibodeau, G. A., & Patton, K. T. (1996). Anatomy & physiology, ed 3, St Louis, Mosby.
Delivery of Intraspinal Analgesics
Delivery of analgesics by the intraspinal routes can be accomplished by inserting a needle into the subarachnoid space (for intrathecal analgesia) or the epidural space and injecting the analgesic, or threading a catheter through the needle and taping it in place temporarily for bolus dosing or continuous administration (Figures 15-4 to 15-6). Temporary catheters are used primarily for short-term acute pain management and are usually removed after 2 to 4 days. Intrathecal catheters for acute pain management are used more often for providing anesthesia and/or a single analgesic bolus dose.
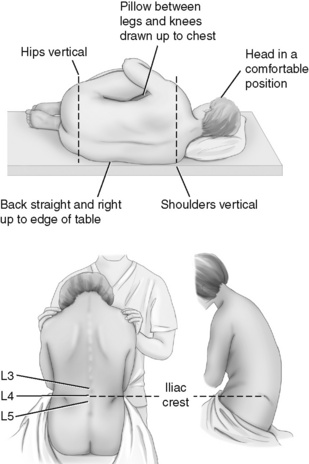
Figure 15-4 Patient positioned for catheter placement. This figure shows two positions patients can assume for the epidural catheter placement procedure. From Pasero C, McCaffery M: Pain assessment and pharmacologic management, p. 409, St. Louis, Mosby. May be duplicated for use in clinical practice.
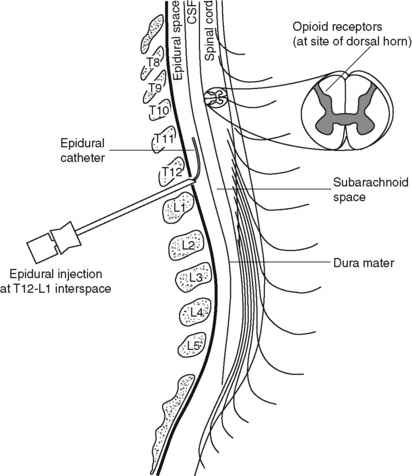
Figure 15-5 Epidural needle and catheter placement. Delivery of analgesia by the interstitial routes can be accomplished by inserting a needle into the epidural space (shown) for epidural analgesia or the subarachnoid space for intrathecal analgesia and injecting the analgesic or threading a catheter through the needle and taping it in place temporarily for bolus dosing or continuous administration. Modified from Sinatra, R. S., Hord, A. H., Ginsberg, B., et al. (Eds.). (1992). Acute pain mechanisms and management, St Louis, Mosby.
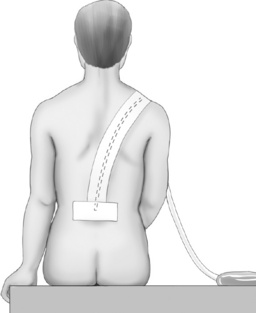
Figure 15-6 Epidural catheter taped in place. This figure shows the catheter taped in place for continuous epidural infusion, patient-controlled epidural analgesia, or intermittent epidural blousing. Courtesy Astra Pharmaceuticals. From Pasero C, McCaffery M. (2011). Pain assessment and pharmacologic management, p. 409, St. Louis, Mosby. May be duplicated for use in clinical practice.
For severe persistent cancer and noncancer pain, a catheter can be inserted then tunneled subcutaneously for intrathecal or epidural intermittent bolusing or continuous infusion or for patient-controlled epidural analgesia (PCEA) by an external ambulatory pump. The tunneling is done to decrease the incidence of infection and accidental displacement (Figure 15-7). These temporary tunneled catheters can be used for weeks to months to deliver analgesics. Temporary externalized intrathecal catheters are used less often than temporary epidural catheters primarily because of concerns about infection, although some clinicians report that such concerns may be unfounded (Vascello, McQuillan, 2006).
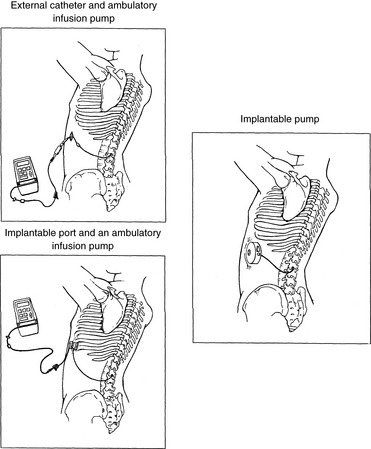
Figure 15-7 Intraspinal delivery systems for persistent pain. This figure shows three intraspinal opioid delivery systems for treatment of persistent pain. From St. Marie, B., & Williams, A. (1994). Management of cancer pain with epidural morphine (independent study module), St. Paul, MN, Sims Deltec Inc.
Although temporary tunneled epidural catheters continue to be useful for the management of intractable pain in some patients near end of life, totally implanted intrathecal infusion systems are preferred for long-term treatment of persistent pain (Deer, Krames, Hassenbusch, et al., 2007; Rathmell, Lake, Ramundo, 2006) (see Figure 15-7). Implanted catheters are less likely to dislodge and are associated with a lower infection rate than percutaneous catheters (Rathmell, Lake, Ramundo, 2006; Swarm, Karanikolas, Cousins, 2004) (see more on long-term intraspinal therapy later in the chapter).
The level of nociceptive input (e.g., surgical site, site of injury, tumor location), the characteristics of the opioid being administered, and the goals of care (e.g., reduced stress response) are most important in determining the vertebral level at which the catheter is placed (Maalouf, Liu, 2009). For example, long-term catheters for treatment of cancer pain associated with spinal lesions can be placed in a location that avoids the tumor while providing necessary analgesia (DuPen, DuPen, 1998). Temporary epidural catheters for acute pain management usually are placed at the lumbar or thoracic vertebral level depending on surgical site (see the section on dermatomal spread and catheter placement later in the chapter). For example, the high thoracic level is preferred by several clinicians for coronary artery bypass surgery because placement at this level improves coronary perfusion, decreases heart rate and endogenous stress response, and reduces the risk for myocardial ischemia (Kessler, Neidhart, Bremerich, et al., 2002; Paiste, Bjerke, Williams, et al., 2001; Royse, Royse, Soeding, et al., 2000).
Percutaneous Intraspinal Catheterization
Intraspinal needle and catheter insertion is performed usually by an anesthesiologist or certified registered nurse anesthetist (CRNA) or other advanced practice nurse. Nurses often assist with the procedure by preparing supplies and monitoring and supporting the patient during the procedure. Informed consent is obtained before the procedure.
The technique for placing a temporary percutaneous epidural catheter varies among practitioners; however, the points made in the Patient Example can be generalized to epidural catheter placement in all patients and may be helpful in reinforcing the anesthesia provider’s explanation of the procedure to patients. The same principles apply to intrathecal needle and catheter placement.
During intraspinal needle placement, most anesthesia providers are able to recognize when the point of the needle penetrates the dense ligamentum flavum (see Figure 15-2). In addition, entry into the epidural space exerts a negative pressure, which is registered by a loss of resistance in the syringe attached to the needle. Some anesthesia providers use the “hanging-drop” method whereby a drop of fluid at the needle hub is “sucked in” as soon as the needle tip passes the ligamentum flavum (Neruda, 2008); however, this method carries the risk of a small plug in the needle tip creating low or no negative pressure and is discouraged by some practitioners (Cousins, Veering, 1998). Once the ligamentum flavum is penetrated, the needle is not advanced if the epidural space is the desired location. If advanced further, the needle will penetrate the dura and enter the subarachnoid space. When in the subarachnoid space, free-flowing CSF can be aspirated. If a blood vessel is entered during placement, blood often can be aspirated.
Even when neither CSF nor blood is aspirated, epidural needle placement is often confirmed by injecting a test dose of lidocaine with epinephrine (if there is no contraindication to epinephrine; e.g., this approach is controversial in pregnant patients because of the potential difficulty in interpreting whether any variability in the woman’s heart rate is in response to epinephrine or to uterine blood flow and contractions; see Birnbach, Browne, 2005). If the needle is in a blood vessel, the epinephrine will cause the patient’s heart rate and blood pressure (BP) to increase suddenly and significantly; if in the subarachnoid space, the lidocaine will produce sensory anesthesia within 3 to 5 minutes (Covino, Wildsmith, 1998). If the patient exhibits neither of these changes, the needle is thought to be in the epidural space and the catheter is threaded through the needle.
Anesthesia providers turn the bevel of the intraspinal needle upward to facilitate threading the catheter 4 to 6 centimeters in a cephalad (toward the head) direction. Although rarely necessary for routine temporary intraspinal catheter placement, the only way to confirm conclusively the exact location of an intraspinal catheter is radiographically using contrast dye. When percutaneous catheters are to be used in the home setting, some clinicians recommend an epiduragram to confirm catheter position before patient discharge (DuPen, DuPen, 1998). (The reader is referred to a detailed explanation of epidural and intrathecal catheter placement techniques in the following two references: Brown, D. L. (2005). Spinal, epidural, and caudal anesthesia. In R. D. Miller (Ed.), Miller’s anesthesia, vol 2, ed 6, pp. 1653-1683, Philadelphia, Elsevier; and Cousins, M. J., & Veering, B. T. (1998). Epidural neural blockade. In M. J. Cousins, & P. O. Bridenbaugh (Eds.), Neural blockade in clinical anesthesia and management of pain, pp. 243-321, Philadelphia, Lippincott-Raven.
Intraspinal Analgesia for Persistent Cancer and Noncancer Pain
A systematic review of the literature in 2000 by a panel of experts revealed widespread acceptance of long-term intraspinal analgesia therapy despite a lack of scientific evidence to support it (Bennett, Serafini, Burchiel, et al., 2000). The need for more well-controlled research of this therapy continues today; most studies are retrospective and underpowered (Simpson, Jones, 2008). Another systematic review found reports of improvements in pain and function, but also remarked on methodologic problems with the studies in the review (Turner, Sears, Loeser, 2007). Another systematic review identified just 8 evaluable studies (177 patients) on long-term intraspinal analgesia, and all of the studies were described as low quality (Noble, Tregear, Treadwell, et al., 2008).
An early consensus guideline on long-term intrathecal analgesic drug delivery recommended morphine as the mainstay analgesic for long-term intrathecal pain management based on its long history of use, the panel’s extensive clinical experience with the drug, and responses to an online survey of physicians providing long-term intrathecal analgesia (Bennett, Burchiel, Buschser, et al., 2000). The survey revealed a usual starting morphine dose of 0.2 mg/day to 20 mg/day and an average maximum long-term infusion dose of 21.1 mg/day. Updated reviews of the literature and development of algorithms and dosing guidelines for the therapy were published in 2004 (Hassenbusch, Portenoy, Cousins, et al., 2004) and again in 2007 (Deer, Krames, Hassenbusch, et al., 2007). The 2007 recommendations list morphine, hydromorphone, and ziconotide as first-line options. Second-line choices included fentanyl alone, and combinations of morphine/hydromorphone plus ziconotide, or morphine/hydromorphone plus bupivacaine/clonidine. (See Section V for a detailed discussion of ziconotide and other agents administered for long-term intraspinal analgesia.) Table 15-2 provides concentrations and dosing recommendations from the most recent consensus guideline (Deer, Krames, Hassenbusch, et al., 2007).
Table 15-2
Concentrations and Doses of Intrathecal Agents Recommended by the Polyanalgesic Consensus Panelists, 2007
| Drug | Maximum Concentration | Maximum Dose/Day |
| Morphine | 20 mg/mL | 15 mg |
| Hydromorphone | 10 mg/mL | 4 mg |
| Fentanyl | 2 mg/mL | No known upper limit |
| Sufentanil | 50 mcg/mL (not available for compounding) | No known upper limit |
| Bupivacaine | 40 mg/mL | 30 mg |
| Clonidine | 2 mg/mL | 1.0 mg |
| Ziconotide | 100 mcg/mL | 19.2 mcg |
From Pasero, C., & McCaffery, M. Pain assessment and pharmacologic management, p. 411, St. Louis, Mosby. Data from Deer, T., Krames, E. S., Hassenbusch, S. J., et al. (2007). Polyanalgesic consensus conference: Recommendations for the management of pain by intrathecal (intraspinal) drug delivery; report of an interdisciplinary expert panel. Neuromodulation, 10(4), 300-328. Pasero C, McCaffery M. May be duplicated for use in clinical practice.
The above-mentioned online survey found that drug and dose adjustments were common and that one-half of patients receiving long-term intrathecal pain management who began on a single drug eventually received polytherapy, indicating a common need to adjust therapy to improve pain control or reduce adverse effects (Hassenbusch, Portenoy, Cousins, et al., 2004). A review of the literature found that 6.3% of patients withdrew from clinical trials of long-term intrathecal therapy because of adverse effects, and 10.5% withdrew because of insufficient pain relief (Noble, Tregear, Treadwell, et al., 2008).
Some publications provide insight into the pros and cons of the therapy. A summary of responses to a questionnaire administered to 36 patients with persistent low back pain receiving long-term intrathecal opioid treatment (mean 4.38 years) revealed significant improvements in pain after spinal implantation and a nonsignificant trend toward enhanced quality of life (Raphael, Southall, Gnanadurai, et al., 2002). The majority (88%) thought the therapy was quite or very worthwhile, and only 1 patient (3%) responded that it was not worthwhile. A systematic review of six articles on effectiveness and four others on complications associated with programmable intrathecal opioid delivery systems for persistent noncancer pain concluded that the therapy produced improvements in pain and functioning, but the typical opioid-induced adverse effects and device complications, such as mechanical failure and catheter migration, were relatively common (Turner, Sears, Loeser, 2007). A Cochrane Collaboration Review concluded that controlled research is lacking on neuraxial analgesia for cancer treatment, and although the therapy is often effective for cancer pain that is unresponsive to systemic analgesia, intraspinal catheter complications frequently occur (Ballantyne, Carwood, 2005).
An excellent review of the literature identified complications associated with programmable intrathecal opioid delivery systems, which include infection (e.g., wound infection, meningitis), hardware problems (e.g., mechanical failure, catheter occlusion), and opioid-related adverse effects (Turner, Sears, Loeser, 2007). Life-threatening complications were rare. The most common adverse effects were nausea (33%), pruritus (26%), and urinary retention (24%). Only two studies evaluated effects on sexual function and reported a variety, including amenorrhea and erectile dysfunction. One case of opioid withdrawal syndrome from catheter disconnection was reported. See later in this chapter for a detailed discussion of complications during intraspinal therapy.
Cost is a major consideration when implanted pumps are used in patients who are terminally ill; according to an early cost-benefit study, an implanted infusion pump was more favorable when survival times exceeded 3 months (Bedder, Burchiel, Larson, 1991). Some consider the intrathecal route to be more efficient, capable of providing a better distribution of medication, and less expensive for cancer pain (Vascello, McQuillan, 2006). An important aspect of care is helping the patient and the patient’s family weigh all of the risks and benefits of long-term intraspinal analgesia therapy prior to initiation.
Stability and Compatibility of Agents for Analgesic Infusion Therapy
Research has established the stability and compatibility of admixtures of many of the commonly used agents for intraspinal and other infusion therapies. (For a discussion of microbiologic research on solutions, see the research list following this paragraph.) The reader is also referred to the Polyanalgesic Consensus Conference 2004 publication (Hassenbusch, Portenoy, Cousins, et al., 2004) for discussion of stability and compatibility of intraspinal analgesics. See the 2007 Polyanalgesic Consensus Conference recommendations for a detailed review of the research on the various drugs used for long-term intrathecal analgesia (Deer, Krames, Hassenbusch, et al., 2007).
• Baclofen (Alvarez, de Mazancourt, Chartier-Kastler, et al., 2004; Goodwin, Kim, Zuniga, 2001)
• Bupivacaine (Allen, Stiles, Wang, 1993; Classen, Wimbish, Kupiec, 2004; Hildebrand, Elsberry, Deer, 2001b; Nitescu, Hultman, Appelgren, et al., 1992; Rudich, Peng, Dunn, et al., 2004; Tu, Stiles, Allen, 1990; Wulf, Gleim, Mignat, 1994)
• Buprenorphine (Nitescu, Hultman, Appelgren, et al., 1992)
• Clonidine (Alvarez, de Mazancourt, Chartier-Kastler, et al., 2004; Classen, Wimbish, Kupiec, 2004; Goodwin, Kim, Zuniga, 2001; Hildebrand, Elsberry, Anderson, 2001b; Vranken, van Kan, van der Vegt, 2006; Wulf, Gleim, Mignat, 1994)
• Dexamethasone with ketamine (Watson, Lin, Morton, et al., 2005)
• Fentanyl (Allen, Stiles, Tu, 1990; Allen, Stiles, Wang, 1993; Chapalain-Pargade, Laville, Paci, et al., 2006; Nitescu, Hultman, Appelgren, et al., 1992; Tu, Stiles, Allen, 1990)
• Hydromorphone (Hildebrand, Elsberry, Anderson, 2001b; Rudich, Peng, Dunn, et al., 2004; Walker, Law, DeAngelis, 2001)
• Ketamine (Schmid, Koren, Klein, et al., 2002; Walker, Law, DeAngelis, 2001; Watson, Lin, Morton, et al., 2005)
• Meperidine (Nitescu, Hultman, Appelgren, et al., 1992; Vranken, van Kan, van der Vegt, 2006)
• Morphine (Classen, Wimbish, Kupiec, 2004; Hildebrand, Elsberry, Hassenbusch, 2003; Nitescu, Hultman, Appelgren, et al., 1992; Schmid, Koren, Klein, et al., 2002; Trissel, Pham, 2002; Trissel, Xu, Pham, 2002; Vermiere, Remon, 1999; Wulf, Gleim, Mignat, 1994)
• Ropivacaine (Sanchez del Aguila, Jones, Vohra, 2003)
• Sufentanil (Boitquin, Hecq, Evrard, et al., 2004; Chapalain-Pargade, Laville, Paci, et al., 2006)
• Tramadol with halodroperidol (Negro, Martin, Azuara, et al., 2005)
Methods for Administering Intraspinal Analgesia
The three methods for administering intraspinal analgesia are: (1) bolus (administered by the clinician), (2) continuous infusion or basal rate (administered by a pump), and (3) PCEA (administered by the patient usually using a pump).
Clinician-Administered Bolus Method
Clinicians can provide analgesia by administering a single intrathecal or epidural bolus injection, or the catheter can be left in place for intermittent bolus injections. The duration of the patient’s pain usually determines which bolus method is used.
For some surgical procedures, a single intraspinal morphine bolus provides sufficient pain control for several hours. For example, an epidural or intrathecal bolus of morphine often is administered to manage pain that does not warrant placement of a catheter, such as after cesarean section and some gynecologic, orthopedic, and urologic procedures (Dabu-Bondoc, Franco, Sinatra, 2009). A single epidural morphine dose is capable of providing analgesia for up to 24 hours to 48 hours depending on the formulation used (see the paragraphs that follow). After this period of time, pain usually can be controlled with oral or IV analgesics. Single bolusing is also used when continuous epidural infusions are contraindicated such as in some patients who require anticoagulant therapy (Dabu-Bondoc, Franco, Sinatra, 2009).
When moderate to severe pain is expected to be constant for more than 24 hours, the epidural catheter can be left in place to provide intermittent analgesic bolus doses; however, this method is rarely used today with advances in infusion devices by which to administer therapy that is required for more than 24 hours. As mentioned, when the intrathecal route is used for acute pain, analgesia is administered most often by single bolus; however, implanted subcutaneous ports can be accessed to deliver intermittent boluses for long-term intraspinal pain management. When an intrathecal catheter is implanted for long-term pain control, analgesia usually is provided by continuous infusion.
The major drawback of the intermittent epidural bolus method is that a steady analgesic level is difficult to maintain, especially when bolus doses are administered PRN. Relatively large doses of the opioid are given, and a “peak and trough” effect occurs. Patients experience adverse effects at the peak (highest analgesic concentration level) and pain at the trough (lowest analgesic concentration level). Rather than a PRN approach to epidural dosing, it may be preferable to consider smaller scheduled around-the-clock (ATC) doses. A dosing frequency of less than every 6 hours is not recommended (DuPen, DuPen, 1998) (see Box 15-2 for guidelines for administering intermittent boluses through a temporary epidural catheter).
Continuous Infusion
The principle of providing continuous pain control with intraspinal analgesia can be accomplished by using an external (for acute pain and for persistent pain) or implanted (for persistent pain) infusion pump to deliver a continuous infusion (also called basal rate) of an analgesic solution. Supplemental bolus doses are prescribed for breakthrough pain and can be administered using the clinician-administered bolus mode available on most external infusion pumps or as outlined in Box 15-2. When implanted ports are used to deliver continuous infusion and/or intermittent boluses, meticulous aseptic precautions should be taken to protect the port from bacterial contamination (DuPen, DuPen, 1998; Holmfred, Vikerfors, Berggren, et al., 2006).
Continuous epidural analgesia has been shown to have more positive impact than IV PCA on some but not all patient outcomes following major surgery. One double-blind study randomized 60 patients undergoing radical prostatectomy to receive low thoracic (T10-T12) epidural ropivacaine (0.1%) plus fentanyl (2 mcg/mL) at 10 mL/h or IV PCA morphine (1 mg every 6 minutes) (Gupta, Fant, Axelsson, et al., 2006). Although there were no differences in hospital length of stay, those who received epidural analgesia had significantly better pain relief and expiratory muscle function than those who received IV PCA. Additionally, at 1 month, patients in the epidural group had better scores in emotional role, physical functioning, and general health. However, superior analgesia afforded by a continuous epidural infusion of bupivacaine and morphine in 60 older adults post–hip fracture surgery did not translate into improved rehabilitation in another randomized controlled study (Foss, Kristensen, Kristensen, et al., 2005). A prospective study showed similar results in 18 patients who received either epidural analgesia or IV PCA following mastectomy with immediate transverse rectus abdominis musculocutaneous (TRAM) flap breast reconstruction; continuous epidural analgesia produced better pain control and a 25-hour shorter hospital stay but no difference in time to first ambulation, first bowel sounds and flatus, tolerance of oral nutrition, and incidence of adverse effects (Correll, Viscusi, Grunwald, et al., 2001).
Patient-Controlled Epidural Analgesia (PCEA)
PCEA permits patients to treat their pain by self-administering doses of epidural analgesics to meet their individual analgesic requirements. A randomized controlled study compared fentanyl (4 mcg/mL) plus bupivacaine (0.125%) via PCEA (with basal rate) or continuous epidural infusion after colon resection and found that pain scores were similarly low but significantly fewer nurse/physician interventions for uncontrolled pain (e.g., epidural top-ups, systemic analgesia) were necessary, and patient satisfaction was significantly higher in those who received PCEA (Nightingale, Knight, Higgins, et al., 2007). Another randomized controlled study found that significantly less fentanyl-bupivacaine solution was consumed with PCEA (without basal rate) than with continuous epidural infusion following total knee arthroplasty (Silvasti, Pitkanen, 2001). Compared with nurse-administered PRN intermittent epidural bolus doses of meperidine (maximum of 50 mg/2 h), PCEA meperidine (25 mg PCEA dose with 10 minute lockout) resulted in better pain scores and a trend toward earlier return to activities of daily living and care for the newborn in women following cesarean section (Lim, Wilson, Katz, 2006). Although patient satisfaction was similar among the two groups, nurse satisfaction was higher with PCEA.
A retrospective review of the medical records of 245 patients who received PCEA (opioid plus local anesthetic) or IV PCA following lumbar spine surgery revealed that PCEA produced superior pain relief with less need for rescue analgesia (Cata, Noguera, Parke, et al., 2008). A randomized controlled study of older patients (N = 70) following major surgery observed better pain relief, higher patient satisfaction, and faster return of bowel function with PCEA (opioid plus local anesthetic) than with IV PCA (Mann, Pouzeratte, Boccara, et al., 2000). Although the incidence of postoperative delirium was similar among the two groups (24% to 26%), epidural analgesia was associated with improved mental status on the fourth and fifth day.
When PCEA is administered, a basal rate usually provides most of the patient’s analgesic requirement and the PCEA bolus doses are used to manage breakthrough pain. If a basal rate is not provided, it is especially important to remind patients to “stay on top of the pain,” to maintain a steady neuraxial analgesic level and self-administer bolus doses before pain is severe and out of control. Research has shown that this type of patient teaching is critical to successful therapy (Cywinski, Parker, Xu, et al., 2004). PCEA is safe and effective in older adults (Ishiyama, Iijima, Sugawara, et al., 2007; Mann, Pouzeratte, Boccara, et al., 2000), but the need for proper patient selection and frequent follow up to ensure appropriate PCEA use are emphasized (Silvasti, Pitkanen, 2001). See an example of a patient information brochure about PCEA on pp. 542-543 at the end of Section IV; see Chapter 12 for discussion of PCA principles and safeguards, such as patient-only use of PCA; and see Chapter 17 for discussion of PCA pump features and Table 17-2 on p. 469 for interventions for patients receiving PCEA.
Combined Spinal-Epidural Anesthesia/Analgesia (CSEA)
Although used less often than epidural analgesia, “combined spinal-epidural anesthesia/analgesia” (CSEA) is sometimes administered for labor and delivery and during and after cesarean section and other surgical procedures. CSEA involves placing an epidural needle (typically, 16- or 18-gauge Touhy) into the epidural space, and then passing a much smaller gauge and longer spinal needle (e.g., 29-gauge Quincke needle) through the epidural needle into the subarachnoid space. Subarachnoid placement is confirmed by aspiration of CSF. Opioid and/or local anesthetic is injected into the subarachnoid space, producing rapid and profound anesthesia/analgesia. The subarachnoid needle is removed, and an epidural catheter is inserted to administer supplemental doses as needed to prolong the block and provide ongoing analgesia (Cousins, Veering, 1998; Dabu-Bondoc, Franco, Sinatra, 2009).
The rationale for combining the routes is to minimize the shortcomings of both intrathecal and epidural analgesia while taking advantage of their benefits (Dabu-Bondoc, Franco, Sinatra, 2009; Teoh, Thomas, Tan, 2006). Intrathecal anesthesia has a rapid onset and produces dense neuraxial blockade, and epidural analgesia provides prolonged anesthesia and postoperative analgesia (Grape, Schug, 2008). For this reason, it has been suggested as an option for longer surgical procedures associated with significant postoperative pain, such as lower extremity surgery (Grape, Schug, 2008). CSEA improved intraoperative analgesia and reduced pain with cough better than an intermittent epidural bolus technique following major abdominal surgery in a prospective, randomized study (N = 160) (Stamenkovic, Geric, Slavkovic, et al., 2008) and produced faster motor recovery than single-shot spinal anesthesia following cesarean section in another prospective, randomized study (N = 62) (Lew, Yeo, Thomas, 2004). Because the CSEA technique uses the intrathecal route, lower doses of local anesthetic are possible for laboring patients, and less motor block is produced. The epidural route is used for low-dose supplemental analgesic boluses, and with appropriate assessment, patients have been able to safely and comfortably ambulate during labor while receiving CSEA (Brownridge, Cohen, Ward, 1998; Gautier, Debry, Fanard, et al., 1997).
Drug Bioavailability by the Intraspinal Routes
In contrast to drugs administered systemically, drugs administered intraspinally are more potent (i.e., small doses are effective) because distribution of the drug brings it close to the action site (opioid receptors in the dorsal horn of the spinal cord). This is particularly true when opioids are delivered by the intrathecal route where they are carried by the CSF to the dorsal horn. After epidural administration, drugs are distributed by three main pathways: (1) neural diffusion through the dura into the CSF then into the spinal cord directly to the receptors, (2) vascular uptake by the vessels in the epidural space into systemic circulation, and (3) uptake by the fat in the epidural space; a drug depot is created from which the drug enters the systemic circulation (Maalouf, Liu, 2009).
More direct delivery of opioids to the site of analgesic action explains why the dose of an opioid by the intraspinal routes is smaller than that required by the parenteral route to produce equal analgesia (i.e., the closer the opioid is delivered to the opioid receptors, the lower the required analgesic dose). For example, research has shown that epidural morphine provides superior analgesia at a lower dose compared with IV or IM morphine; the relative potency of epidural morphine compared with morphine by self-titrated IV PCA was 10:1 following orthopedic surgery (Maalouf, Liu, 2009). When converting opioid-tolerant patients from one route to another, the required dose of morphine is approximately three times less by the epidural route than by the IV route (ratio may vary for other opioids), and the dose required by the intrathecal route is approximately 10 times less than required by the epidural route to produce equal analgesia (DuPen, DuPen, 1998) (see Chapter 18 for switching to different routes of administration).
Drug Solubility
Drug solubility and the ability of the drug to traverse diffusion barriers (e.g., the dura mater) influence drug absorption and bioavailability by the intraspinal routes. The more lipid-soluble (readily dissolved in fatty tissue) the drug, the more readily it moves through membranes, resulting in faster absorption. For example, when administered by single epidural injection, lipid-soluble opioids, such as fentanyl, rapidly traverse the dura into the CSF, and then exit the aqueous CSF and easily penetrate the lipid-rich spinal tissue as well as surrounding vasculature (Maalouf, Liu, 2009; Dabu-Bondoc, Franco, Sinatra, 2009). This contributes to fentanyl’s fast onset of action (5 minutes) (Grape, Schug, 2008). In contrast, hydrophilic opioids (readily dissolved in aqueous solution), such as morphine and hydromorphone, have more difficulty traversing the dura to reach the aqueous CSF. By either the epidural or intrathecal route, once in the aqueous CSF, hydrophilic drugs prefer to remain there. Eventually, high enough concentrations of morphine are reached in the CSF, and the drug moves into the spinal cord to the opioid receptors. This helps to explain intraspinal morphine’s slow onset of action (30 to 60 minutes) (Dabu-Bondoc, Franco, Sinatra, 2009).
An opioid’s duration of action when administered by the intraspinal routes is determined in large part by the amount of the drug that remains in the CSF. Because morphine is hydrophilic, it tends to remain within the aqueous CSF. This ensures continued opioid receptor binding by replenishing molecules that dissociate and are cleared from the spinal action sites and helps to explain morphine’s large volume of distribution, high bioavailability, and exceptionally long duration of analgesia from a single intraspinal bolus dose (e.g., 12 to 24 hours). On the other hand, the highly lipid-soluble opioids such as fentanyl traverse membranes readily and are easily removed by vasculature or remain trapped within the fat of the epidural space (Dabu-Bondoc, Franco, Sinatra, 2009). This causes a rapid decline in drug concentration at action sites and results in a short duration of analgesia (2 hours). The highly lipid-soluble opioids are administered by continuous infusion to prolong their limited duration of activity if extended relief is desired. When steady state is reached by continuous infusion, the various opioids differ little in terms of duration.
Dermatomal Spread and Epidural Catheter Placement
An opioid drug deposited into the CSF, or diffusing into the CSF from the epidural space, distributes throughout the neuraxis with the movement of the CSF (Bernards, 2000; Maalouf, Liu, 2009). The extent to which the drug moves rostrally toward the brain, or caudally toward the lower end of the thecal sac, depends on the drug’s clearance rate (Bernards, 2000). Hydrophilic drugs such as morphine and hydromorphone tend to remain in the CSF and produce a broad spread of analgesia across many dermatomes (Dabu-Bondoc, Franco, Sinatra, 2009). The opposite is true of lipophilic opioids such as fentanyl and sufentanil, which are rapidly cleared from the CSF, tend to be transported for shorter distances, and produce what is called segmental analgesia. By rapidly leaving the CSF and redistributing into spinal cord tissue, epidural fat, and vasculature, these lipophilic opioids have little rostral spread (Dabu-Bondoc, Franco, Sinatra, 2009).
Particularly when using the lipophilic opioids, it is important to put the tip of the catheter at the spinal level where there is a high level of nociceptive input (Grape, Schug, 2008). Research has shown that placement of the catheter tip at the spinal level congruent with the dermatomes where the incision is performed provides superior analgesia, helps to reduce adverse effects, and is associated with decreased morbidity compared with placement at other spinal levels (Maalouf, Liu, 2009; Wu, 2005).
As noted, appropriate placement of the catheter is especially important if lipophilic drugs are used. Whereas hydrophilic opioids ascend in the CSF and are likely to cover the spinal segments receiving input from the incisional dermatomes irrespective of catheter placement, lipophilic opioids such as fentanyl and sufentanil may not ascend to the necessary spinal level, leading to a situation in which the analgesia is produced largely by systemic redistribution (movement of the dose from the CSF into the bloodstream and then back to sites of action in the brain and spinal cord) rather than by local action of the drug at the spinal cord level (Dabu-Bondoc, Franco, Sinatra, 2009; Wu, 2005). Some studies indicated that lumbar epidural fentanyl infusions are equivalent to IV fentanyl infusions, suggesting that spinal fentanyl may in fact produce most of its analgesia through this systemic redistribution (McCartney, Niazi, 2006). Even lumbar administration of dilute lipophilic solutions at high infusion rates may result in plasma concentration levels equal to parenterally administered opioids. Given the rapid redistribution of lipophilic opioids and the need for appropriate placement of catheters to obtain the intended segmental analgesic effects, it is recommended that the tip always be placed at the thoracic level (T10 or higher) for lipophilic drugs such as fentanyl so that the spinal cord is adjacent to the entry site of the drug (Dabu-Bondoc, Franco, Sinatra, 2009). For practitioners choosing lumbar placement of epidural catheters, especially to treat upper-abdominal and thoracic nociceptive input, morphine or hydromorphone may be the best choice of drug (McCartney, Niazi, 2006; Wu, 2005).
Thoracic Epidural Catheter Placement
There is a trend toward thoracic epidural catheter placement in general and particularly for major thoracoabdominal surgeries (Grape, Schug, 2008). For certain types of surgery, such as cardiac surgery, thoracic epidural anesthesia and analgesia clearly have more advantages than lumbar epidural anesthesia and analgesia (Bracco, Hemmerling, 2008). Thoracic epidural analgesia has been associated with improved dynamic pain relief, minimal lower extremity motor blockade, enhanced mobility and functional exercise capacity, better cardiac perfusion and tissue oxygen tension, and less urinary retention and hypotension (Bauer, Hentz, Ducrocq, et al., 2007; Brodner, Van Aken, Hertle, et al., 2001; Buggy, Doherty, Hart, et al., 2002; Carli, Mayo, Klubein, et al., 2002; Grape, Schug, 2008; Kabon, Fleischmann, Treschan, et al., 2003; Kessler, Neidhart, Brenerich, et al., 2002; Mayer, Boldt, Schellhaafs, et al., 2007; Paiste, Bjerke, Williams, et al., 2001; Priestley, Cope, Halliwell, et al., 2002; Wu, 2005). A meta-analysis of 33 randomized controlled trials (2366 patients) showed that thoracic epidural analgesia reduced the incidence of perioperative acute renal failure, the time on mechanical ventilation, and the composite endpoint mortality and myocardial infarction (MI) in patients undergoing cardiac surgery (Bignami, Landoni, Biondi-Zoccai, et al., 2010). Another meta-analysis revealed that compared with nonepidural analgesia, epidural analgesia resulted in a lower incidence of postoperative MI, and subgroup analysis showed that thoracic epidural placement was superior to lumbar placement in this regard (Beattie, Badner, Choi, 2001). A Cochrane Collaboration Review comparing systemic opioid analgesia with epidural analgesia following abdominal aortic surgery concluded that, regardless of regimen, thoracic epidural analgesia provided better pain relief, particularly during movement, for up to 3 postoperative days, reduced duration of mechanical ventilation and tracheal intubation time by 20%, and was associated with fewer cardiovascular (CV), gastrointestinal (GI), and renal complications (Nishimori, Ballantyne, Low, 2006). Others have found similar excellent results with epidural analgesia following this type of major surgery (Park, Thompson, Lee, et al., 2001). Whereas epidural analgesia appears to improve outcomes for cardiac surgical patients, a meta-analysis of 24 randomized controlled trials (1106 patients) concluded that spinal analgesia did not improve clinically relevant outcomes in patients undergoing cardiac surgery and discouraged further research on this method in these patients (Zangrillo, Bignami, Biondi-Zoccai, et al., 2009).
Thoracic epidural analgesia may provide solutions for the challenge of managing postoperative pain in individuals at risk for pulmonary complications. One study found that thoracic epidural analgesia with bupivacaine (0.25%) was safe and efficacious in patients with severe end-stage chronic obstructive pulmonary disease following thoracotomy (Gruber, Tschernko, Kritzinger, et al., 2001).
Some anesthesia providers may be reluctant to attempt thoracic catheter placement and prefer to insert lumbar catheters because the spinal cord becomes smaller as it progresses distally and the lumbar spinous processes are angulated posteriorly and farther apart making epidural catheter placement easier in the lumbar area. When placed below the spinal cord, the risk of trauma to the spinal cord is eliminated; however, it is a common misconception that thoracic epidural catheter placement is technically more difficult and causes more neurologic damage than lumbar catheter placement (Grape, Schug, 2008; Wu, 2005). It is a technique that is quickly mastered by anesthesia providers. A review of 874 cases of high thoracic epidural analgesia provided over a 7-year period revealed no related neurologic complications (Royse, Soeding, Royse, 2007).
Selected Analgesics Administered by the Intraspinal Routes
The two main types of drugs administered intraspinally to treat acute pain are opioids and local anesthetics. Other drugs used for the treatment of persistent pain are the calcium channel blocker ziconotide, the alpha2-adrenergic agonist clonidine, and less often, the N-methyl-d-aspartate (NMDA) antagonist ketamine. Intraspinal clonidine is also used for acute pain treatment. Baclofen (Lioresal), a muscle relaxant and antispastic agent, is administered intraspinally for treatment of spasticity (see Section V for a detailed discussion of all of these agents). These drugs can be given alone or in combination with each other. The rationale for combining drugs is that they work synergistically to provide better analgesia and fewer adverse effects at lower doses.
Opioids
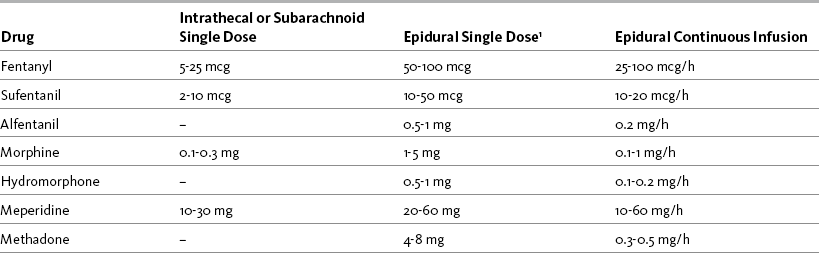
The mu agonist opioids morphine, fentanyl, and hydromorphone are the most common opioids administered by the intraspinal route; sufentanil, methadone, and meperidine (Demerol) are administered less often. Table 15-3 provides a summary of the characteristics of selected opioids administered intraspinally, and Table 15-4 provides dosing recommendations for the various neuraxial opioids. Following is a discussion of selected opioids.
Table 15-3
Summary of Characteristics of Selected Intraspinal Opioids
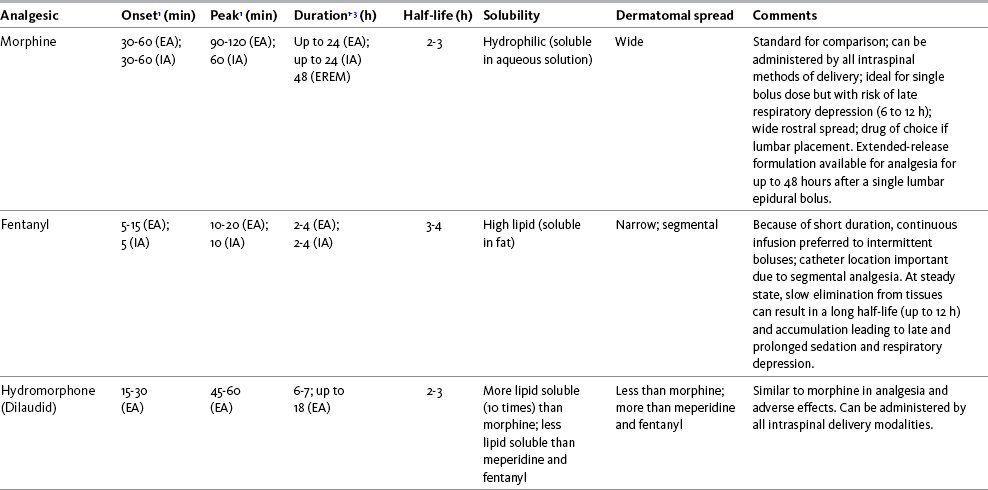
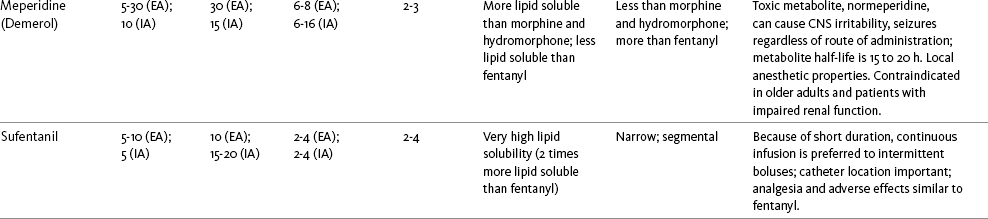
EA, Epidural analgesia (single bolus dose); EM, epidural morphine; ER, extended release; IA, intrathecal analgesia (single bolus dose).
1Onset, peak, and duration are based on single bolus administration.
2Duration of analgesia is dose dependent; the higher the dose, usually the longer the duration.
3When steady state is reached by continuous infusion, the various opioids differ little in terms of duration.
From Pasero, C., & McCaffery, M. Pain assessment and pharmacologic management, pp. 418-419, St. Louis, Mosby. Data from American Society of Anesthesiologists Task Force on Neuraxial Opioids. (2009). Practice guidelines for the prevention, detection, and management of respiratory depression associated with neuraxial opioid administration. Anesthesiology, 110(2), 218-230; Brown, D. L. (2005). Spinal, epidural, and caudal anesthesia. In R. D. Miller (Ed.), Miller’s anesthesia, vol 2, ed 6, Philadelphia, Elsevier; Cashman, J. N., & Dolin, S. J. (2004). Respiratory and haemodynamic effects of acute postoperative pain management: Evidence from published data. Br J Anaesth, 93(2), 212-223; Cousins, M. J., & Veering, B. T. (1998). Epidural neural blockade. In M. J. Cousins, & P. O. Bridenbaugh (Eds.), Neural blockade in clinical anesthesia and management of pain, Philadelphia, Lippincott-Raven; Dabu-Bondoc, S., Franco, S. A., & Sinatra, R. S. (2009). Neuraxial analgesia with hydromorphone, morphine, and fentanyl: Dosing and safety guidelines. In R. S. Sinatra, O. A. de Leon-Casasola, B. Ginsberg, et al. (Eds.), Acute pain management, Cambridge, NY, Cambridge University Press; Grape, S., & Schug, S. A. (2008). Epidural and spinal analgesia. In P. E. Macintyre, S. M. Walker, & D. J. Rowbotham (Eds.), Clinical pain management. Acute pain, ed 2, London, Hodder Arnold; Kedlaya, D., Reynolds, L., & Waldman, S. (2002). Epidural and intrathecal analgesia for cancer pain. Best Prac Res Clin Anaesth, 16(4), 651-665; Maalouf, D. B., & Liu, S. S. (2009). Clinical application of epidural analgesia. In R. S. Sinatra, O. A. de Leon-Casasola, B. Ginsberg, et al. (Eds.), Acute pain management, Cambridge, NY, Cambridge University Press; McCartney, C. J. L., & Niazi, A. (2006). Use of opioid analgesics in the perioperative period. In G. Shorten, D. B. Carr, D. Harmon, et al. (Eds.), Postoperative pain management: An evidence-based guide to practice, Philadelphia, Saunders; Vascello, L., & McQuillan, R. J. (2006). Opioid analgesics and routes of administration. In O. A. de Leon-Casasola (Ed.), Cancer pain. Pharmacological, interventional and palliative care approaches, Philadelphia, Saunders; Wu, C. L. (2005). Acute postoperative pain. In R. D. Miller (Ed.), Miller’s anesthesia, vol 2, ed 6, Philadelphia, Elsevier. Pasero C, McCaffery M. May be duplicated for use in clinical practice.
Morphine
Morphine was the first opioid to be administered intraspinally for the relief of pain in humans (Wang, Nauss, Thomas, 1979) and the first to receive FDA approval for intraspinal administration. It is an excellent choice of opioid for intraspinal analgesia because it produces spinal-mediated analgesia by the epidural and intrathecal routes and can be given by all of the intraspinal delivery methods. It is ideal for single-bolus dose intraspinal administration because it has a particularly long duration of action (up to 24 hours in the opioid-naïve patient; see the section on extended-release epidural morphine later in the chapter) (Dabu-Bondoc, Franco, Sinatra, 2009; Wu, 2005). Several studies have demonstrated superior postoperative analgesia with single-dose epidural morphine compared with parenteral opioids for a wide variety of surgical procedures (Dabu-Bondoc, Franco, Sinatra, 2009). As discussed, another advantage is that morphine spreads rostrally, which makes the vertebral location of intraspinal administration less critical than when administering lipophilic opioids for acute postoperative pain. For example, intraspinal morphine can be administered in the lumbar region to produce analgesia after thoracic surgery (Chaney, Furry, Fluder, et al., 1997; Wu, 2005) and in the thoracic region for oral and facial pain treatment (Sakuramoto, Kanai, Matoba, et al., 1996).
With bolus injection in the opioid-naïve patient, intraspinal morphine has a slow onset of analgesia (30 to 60 minutes) and a peak effect of approximately 90 minutes (Wu, 2005). Additional analgesia usually is required until morphine takes effect. For example, some clinicians administer an epidural dose of a faster-acting lipophilic opioid analgesic, such as fentanyl (onset 5 minutes), at the time of the single epidural morphine bolus or initiation of infusion therapy to provide analgesia until morphine takes effect. Table 15-5 provides recommended intraspinal morphine bolus doses for pain management following various surgical procedures. See also Table 15-4.
When continuous epidural morphine is administered to surgical patients, a loading epidural morphine bolus dose (2 to 5 mg), usually combined with ropivacaine (0.2%) or bupivacaine (0.25%), is given preincision followed by initiation of the epidural morphine infusion at the end of the surgical procedure (Dabu-Bondoc, Franco, Sinatra, 2009). Although a solution concentration of 60 mcg/mL is reported to produce more reliable analgesia, a 40 mcg/mL concentration at a rate of 4 to 10 mL/h is recommended to reduce adverse effects, such as nausea and pruritus (Dabu-Bondoc, Franco, Sinatra, 2009).
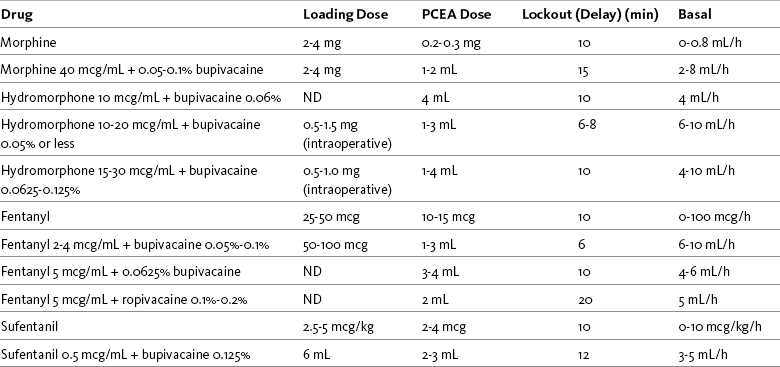
Morphine by PCEA has been used for many years in surgical patients (Pasero, Portenoy, McCaffery, 1999). It is less popular, however, than hydromorphone and fentanyl, which are easier to titrate and associated with fewer adverse effects by this modality (Dabu-Bondoc, Franco, Sinatra, 2009). Table 15-6 contains common PCEA prescription ranges for opioid-naïve patients.
Because of slow rostral spread in the CSF, large-volume bolus doses (more than 5 mg) of epidural morphine have been known to produce late respiratory depression (approximately 6 to 12 hours after lumbar injection, corresponding with the rate of CSF flow from the spinal level to the brainstem) (Angst, Ramaswamy, Riley, et al., 2000; McCartney, Niazi, 2006). Earlier respiratory depression at 5 to 10 minutes and before 2 hours also can occur due to vascular uptake of morphine. Monitoring the opioid-naïve patient’s level of sedation and respiratory status every hour for 12 hours after a clinician-administered intraspinal bolus of morphine is recommended (American Society of Anesthesiologists, 2009) (see Chapter 19 for more on sedation and respiratory depression).
Fear of late respiratory depression has caused some clinicians to avoid using epidural morphine in opioid-naïve patients; however, rostral spread and late respiratory depression are uncommon when epidural morphine is administered in smaller, more frequent bolus doses or by continuous infusion or PCEA. Continuous administration leads to less fluctuation of drug levels, which reduces the risk of peak concentration toxicity (Gianino, York, Paice, 1996). Continuous epidural morphine infusions with or without PCEA have been found to be highly effective and safe in opioid-naïve patients (Dabu-Bondoc, Franco, Sinatra, 2009).
As with more lipophilic drugs, when a continuous epidural infusion of morphine is discontinued, the concentration of opioid declines and adverse effects (e.g., sedation, respiratory depression) decrease, not increase. IV lines, if otherwise unnecessary, can be removed, and routine monitoring (e.g., every 4 to 8 hours in stable patients) of level of sedation and respiratory status is customary.
Consensus guidelines recommend morphine as the first-choice opioid for long-term intrathecal pain treatment (Deer, Krames, Hassenbusch, et al., 2007). When combined with bupivacaine for intrathecal administration with or without PCA capability, morphine has been shown to be highly effective for the management of refractory cancer pain (Vascello, McQuillan, 2006). Morphine and local anesthetics work synergistically, providing excellent pain relief with significantly smaller doses than are possible when administered epidurally. Advances in technology allow patients the benefits of neuraxial infusion therapy in the home setting (Vascello, McQuillan, 2006).
Extended-Release Epidural Morphine (EREM): EREM (Depodur™) is distinguished from conventional epidural morphine (e.g., Astramorph, Duramorph) by its unique delivery system called DepoFoam™, which consists of multiple microscopic, liposomal (fat-based) particles (Pasero, McCaffery, 2005b). The liposomes contain aqueous chambers that encapsulate preservative-free morphine (Carvalho, Riley, Cohen, et al., 2005). After epidural injection, the liposomes slowly release morphine over a period of 48 hours by erosion or reorganization of the lipid membranes (Heitz, Viscusi, 2009). It should be administered in the lumbar epidural space only. Primary advantages of this formulation are that it allows up to 48 hours of pain relief without the use of an indwelling catheter, which can pose a risk of infection, impede mobility, and raise concerns about postoperative anticoagulant therapy (Pasero, McCaffery, 2005b; Viscusi, Martin, Hartrick, et al., 2005) (see Table 15-8 on p. 439). Further, concerns regarding infusion device programming errors are eliminated with this approach (see later in chapter for more on operator errors).
An open-label study of 39 patients undergoing total hip arthroplasty compared a 5 mg dose of conventional epidural morphine with 10 to 30 mg doses of EREM and found that the median time to request for first analgesia was three to six times longer, supplemental analgesia consumption was less, and patient satisfaction was better in patients who received EREM (Viscusi, Kopacz, Hartrick, et al., 2006). Another study of patients post hip arthroplasty (Viscusi, Martin, Hartrick, et al., 2005) found similar positive results as did research following knee arthroplasty (Hartrick, Martin, Kantor, et al., 2006), cesarean section delivery (Carvalho, Riley, Cohen, et al., 2005), and lower abdominal surgery (Gambling, Hughes, Martin, et al., 2005). EREM produced lower pain scores on the first postoperative day but more nausea, vomiting, and pruritus than spinal anesthesia alone in patients undergoing total hip arthroplasty (Kahl, Parvizi, Viscusi, 2010).
Adverse effects associated with EREM are similar to conventional epidural morphine, with nausea and pruritus reported as most common (Hartrick, Hartrick, 2008; Pasero, McCaffery, 2005b). These appear to be at their worst during the first 24 hours after EREM administration (Gambling, Hughes, Martin, et al., 2005; Viscusi, Martin, Hartrick, et al., 2005). Administration of the lowest effective dose of EREM is critical; 10 to 15 mg is recommended (Heitz, Viscusi, 2009). This is facilitated when EREM is administered as part of a multimodal analgesic regimen, e.g., with an NSAID and acetaminophen (Hartrick, Hartrick, 2008). Higher doses (e.g., 20 to 25 mg) have been associated with clinically significant respiratory depression requiring naloxone administration (Hartrick, Hartrick, 2008; Pasero, McCaffery, 2005b). A meta-analysis of three randomized controlled trials concluded that, although EREM produced effective postoperative pain control for up to 48 hours, it was associated with a significantly higher risk of respiratory depression than IV PCA (Sumida, Lesley, Hanna, et al., 2009). See Box 15-3 for guidelines in the care of patients receiving EREM, and Chapter 19 for treatment of adverse effects.
Fentanyl
Epidural fentanyl has been used extensively for anesthesia and to provide postoperative analgesia (Finucane, Ganapathy, Carli, et al., 2001; Pasero, Portenoy, McCaffery, 1999; Wu, 2005). Single intraspinal doses of fentanyl provide analgesia for just 2 to 4 hours (Wu, 2005), making this method of administration appropriate only for very short-term pain control, such as following ambulatory surgery and when rapid analgesia is desired (onset is 5 to 15 minutes). Diluting the epidural dose (e.g., 50 to 100 mcg) in 10 mL of preservative-free normal saline helps to prolong analgesia and increase the initial spread and diffusion of the drug (Wu, 2005). Because lipid-soluble opioids such as fentanyl have such a short duration, administration by continuous infusion or PCEA, rather than intermittent bolus dosing, is preferred for extended pain control (Wu, 2005) (see Table 15-3).
As discussed, the concentration of fentanyl that can be measured in the blood after epidural delivery is very close to that attained from the same IV dose, suggesting that much of fentanyl’s action is the result of systemic uptake from the vasculature in the epidural space. Although some research shows better analgesia with epidural fentanyl compared with parenteral fentanyl, the advantages also have been reported to be marginal (Wu, 2005). This may help to explain why fentanyl is administered epidurally most often in combination with a local anesthetic, such as ropivacaine or bupivacaine; however, whereas hydrophilic opioids and local anesthetics work synergistically to provide improved analgesia, such a relationship between the lipophilic opioids and local anesthetics is less clear and the benefit of this combination compared with the administration of a local anesthetic alone has been questioned (McCartney, Niazi, 2006). Nevertheless, this approach continues to be widely used (Grape, Schug, 2008), and fentanyl is a primary drug for continuous infusion and PCEA following major surgery. As with other pain management therapies, a variety of factors influence fentanyl PCEA dose requirements. A 3-year prospective study of almost 2000 patients found that the type of surgical procedure had more influence on PCEA (fentanyl 1 mcg/mL plus 0.625% bupivacaine with basal rate) dose requirements than patient demographic variables (e.g., sex, height, weight); patients who had thoracic or abdominal surgery consumed higher doses than those who had lower-extremity surgery (Chang, Dai, Ger, et al., 2006) (see Table 15-4 and Table 15-6 for dosing recommendations).
After repetitive dosing or continuous infusion of fentanyl, a steady state is approached. In this condition, the terminal half-life of fentanyl depends on how much is taken into tissue for storage and how quickly it is released. Although fentanyl is reported to have a terminal half-life of approximately 3 to 4 hours, at steady state, slow removal of fentanyl from storage sites can result in a longer terminal half-life (up to 12 hours). This prolongation of half-life as the fat stores are saturated can lead to accumulation effects during the period of initial dosing and dose titration, as well as prolonged duration of sedation and respiratory depression. As a rule, however, early-onset respiratory depression is more common than delayed with epidural fentanyl. This reflects vascular uptake of the opioid and occurs most often within an hour of initial injection (McCartney, Niazi, 2006).
Hydromorphone
Hydromorphone has gained wide acceptance as a first-line opioid for intraspinal administration. Its lipid solubility is intermediate between morphine and fentanyl. Because it is 10 times more lipophilic than morphine, its onset of analgesia (15 to 30 minutes) is faster and its duration of action (6 to 7 hours) is shorter (Dabu-Bondoc, Franco, Sinatra, 2009) (see Table 15-3). This makes single bolus doses of the drug suitable for short-stay surgical patients who will be transitioned to oral analgesics within 5 to 12 hours after surgery (Dabu-Bondoc, Franco, Sinatra, 2009). Hydromorphone is capable of spreading rostrally and can produce delayed respiratory depression after large epidural bolus administration, but it is reported to produce less sedation, nausea, and pruritus than epidural morphine (Dabu-Bondoc, Franco, Sinatra, 2009; Rockford, DeRuyter, 2009).
Hydromorphone has metabolites, and although all of their effects have not been clearly defined, they are not believed to be clinically relevant during short-term epidural administration. Because hydromorphone has a short half-life (2 to 3 hours) and no clinically relevant metabolites by this route, it may be a better drug than morphine for patients with renal insufficiency. Hydromorphone also is a good alternative to morphine when high concentrations of drug are required, because epidural hydromorphone is more potent than epidural morphine; however, consensus guidelines recommend caution when converting patients from morphine to hydromorphone during intraspinal therapy because the exact potency ratio is unknown (Deer, Krames, Hassenbusch, et al., 2007). The switch to any new opioid should be done slowly with appropriate monitoring as described in Chapter 18 (Switching to Another Opioid).
Hydromorphone has been used for many years via continuous infusion and PCEA to provide effective analgesia following major surgery (Parker, White, 1992; Parker, Holtmann, White, 1997; Parker, Sawaki, White, 1992; Rapp, Egan, Ross, et al., 1996; Singh, Bossard, White, et al., 1997) (see Tables 15-4 and 15-6 for dosing recommendations). One early study showed that hydromorphone by PCEA provided satisfactory pain relief with three to four times less hydromorphone than when given by IV PCA (Parker, White, 1992). Others found similar results (Liu, Carpenter, Mulroy, et al., 1995), which prompted increased use of the drug (Dabu-Bondoc, Franco, Sinatra, 2009). Modifications in dosing protocols have evolved over the years to the current recommendations to infuse a lower concentration (e.g., from 50 to 30 mcg/mL previously to the current 10 to 20 mcg/mL) delivered at higher hourly infusion rates (e.g., from 2 to 5 mL/h previously to the current 10 to 12 mL/h). Local anesthetics (e.g., bupivacaine, ropivacaine) are often added to the infusion. Improved dosing regimens have produced greater efficacy and fewer adverse effects, and the drug has become the primary choice in many institutions for continuous infusion (Dabu-Bondoc, Franco, Sinatra, 2009). A detailed account of experience with thousands of patients at Yale-New Haven Hospital that includes dosing guidelines, drug preparation, assessment, and management of adverse effects and complications can be found in Dabu-Bondoc, S., Franco, S. A., & Sinatra, R. S. (2009). Neuraxial analgesia with hydromorphone, morphine, and fentanyl: Dosing and safety guidelines. In R. S. Sinatra, O. A. de Leon-Casasola, B. Ginsberg, et al (Eds.), Acute pain management, Cambridge, NY, Cambridge University Press.
There is limited research on the use of intrathecal hydromorphone for acute pain, and it is recommended for this type of pain only in patients who cannot tolerate intrathecal morphine (Dabu-Bondoc, Franco, Sinatra, 2009). Consensus guidelines for long-term intrathecal administration, however, recommend hydromorphone as a first-choice opioid with morphine and ziconotide (Deer, Krames, Hassenbusch, et al., 2007) (see Chapter 23 for a discussion on ziconotide).
Meperidine
Meperidine (Demerol) is administered by the epidural route less often than morphine, fentanyl, and hydromorphone in the United States and the United Kingdom; however, it is frequently used in Australia for treatment of post–cesarean section pain (Parris-Piper, 2008) (see Table 15-4 for dosing recommendations). Clinicians who prefer meperidine by this route cite the potential advantages of less vascular uptake than more lipophilic opioids such as fentanyl, a faster onset of analgesia (5 to 30 minutes) than less lipophilic opioids such as morphine, and an intermediate dermatomal spread that allows lumbar administration regardless of the site of nociceptive input (Slinger, Shennib, Wilson, 1995). Meperidine has been shown to produce local anesthetic effects (Armstrong, Morton, Nimmo, 1993), a characteristic that could have a favorable impact on analgesia and has not posed problems in terms of motor block when the drug is administered epidurally for pain control (Parris-Piper, 2008) (see Table 15-3).
Some experienced clinicians perceive that epidural meperidine produces fewer adverse effects than morphine (Parris-Piper, 2008), but there are very few comparative data. A randomized controlled trial (N = 37) found that subarachnoid morphine provided better pain relief than meperidine PCEA but with more nausea, pruritus, and sedation following cesarean section (Paech, Pavy, Orlikowski, et al., 2000). The addition of meperidine (10 mg) to intrathecal bupivacaine prolonged post–cesarean section analgesia but with a higher incidence of nausea and vomiting than without it (Yu, Ngan Kee, Kwan, 2002). Epidural meperidine and IV meperidine were found to produce similar satisfactory analgesia and adverse effects in patients following major abdominal surgery, but 33% less meperidine was required during the first 24 hours of therapy in patients who received the drug epidurally (Chen, Cheam, Ma, et al., 2001).
The primary potential disadvantage of using meperidine epidurally is its toxic metabolite normeperidine. Reports of normeperidine toxicity associated with epidural meperidine are rare, possibly because low doses are administered over a brief period of time; however, research shows that meperidine is more likely than other opioid drugs to cause delirium in postoperative patients of all ages (Fong, Sands, Leung, 2006). Meperidine plasma levels higher than 350 ng/mL and normeperidine plasma levels of more than 400 ng/mL are reported to cause CNS irritability (Kaiko, Foley, Grabinski, et al., 1983). In a case-control study (N = 91 with 1 to 2 controls), meperidine more than doubled the risk of delirium when given either epidurally or IV (Marcantonio, Juarez, Goldman, et al., 1994). In another early study comparing epidural with IV meperidine, plasma normeperidine levels were the same for both routes, despite the fact that the total meperidine dose was much less by the epidural route (Slinger, Shennib, Wilson, 1995). CNS irritability (shakiness and tremors) was noted when normeperidine plasma levels reached > 300 ng/mL, and the peak mean normeperidine plasma level after 72 hours of continuous epidural meperidine infusion was 573 ng/mL. No patients had seizures in this study, but 40% experienced CNS irritability. If meperidine is used epidurally, administering it by PCEA without a continuous infusion rather than by continuous infusion alone has been shown to reduce total meperidine consumption (Etches, Gammer, Cornish, 1996) and lower normeperidine plasma levels (Paech, Moore, Evans, 1994). The addition of bupivacaine also may allow a reduced dose and lower serum concentrations of meperidine (St. Onge, Fugere, Girard, 1997), but this combination may be associated with hypotension, oliguria, and excessive motor or sensory blockade (Etches, Gammer, Cornish, 1996). See Chapter 13 for more on meperidine and the assessment of normeperidine toxicity.
Intrathecal meperidine is rarely used. A study undertaken to determine if intrathecal meperidine would provide a long duration of anesthesia was discontinued after enrollment of 34 patients because of significant nausea and vomiting (Booth, Lindsay, Olufolabi, et al., 2000). Consensus guidelines for long-term intrathecal administration list meperidine as a fifth-line opioid because the data supporting its safety and efficacy are limited (Deer, Krames, Hassenbusch, et al., 2007).
Methadone
Methadone is a lipophilic opioid and, like other lipophilic opioids, produces less rostral spread than morphine when administered intraspinally. It has a fast onset of analgesic action (10 to 20 minutes), and because it is cleared rapidly from the CSF, it has a relatively short duration (4 to 8 hours) by the intraspinal routes (Kedlaya, Reynolds, Waldman, 2002). Methadone may be an option for patients with cancer pain who require continuous intraspinal pain treatment, but the concerns discussed in Chapter 13 about its long half-life (12 to 130 hours) and accumulation with repetitive dosing and continuous infusion apply. Consensus guidelines for long-term intrathecal administration describe methadone as a “promising alternative neuraxial agent for chronic pain”, but the data supporting its safety and efficacy are limited (Deer, Krames, Hassenbusch, et al., 2007). This suggests it should be at least a fourth-line option.
Although rarely used for postoperative pain management, some studies have demonstrated that epidural methadone can be effective and safe in this setting (see Table 15-4 for dosing recommendations). Ninety patients undergoing abdominal or lower limb surgery were randomized to receive methadone by continuous infusion (up to 12 mg over 24 hours) or intermittent boluses of 3 to 6 mg every 8 hours for 72 hours (Prieto-Alvarez, Tello-Galindo, Cuenca-Pena, et al., 2002). Pain relief was similar among the groups. No plasma accumulation was observed in either group, although plasma concentrations were higher in the bolus group. Miosis was also more frequent in this group. The drug also has been delivered via PCEA for postoperative pain. A randomized controlled trial compared methadone by IV PCA or PCEA in 30 patients following thoracic surgery (Parramon, Garcia, Gambus, et al., 2003). Patients were given an IV or epidural methadone loading dose of 0.05 mg/kg depending on the modality to which they were assigned. IV PCA or PCEA therapy was initiated with a basal rate of 0.5 mg/h, and patients could self-administer 0.5 mg every 10 minutes to a maximum of 4 doses/h. Pain relief was similar, but analgesia was achieved in less time and at a lower dose in patients receiving methadone by PCEA. Adverse effects were few and similar among the groups. Another study described the safe use of methadone plus bupivacaine (0.5%) via continuous epidural infusion in 136 patients after liver resection (Matot, Scheinin, Eid, et al., 2002).
Epidural methadone has also been used for the palliative treatment of dyspnea. Nine patients with emphysema-related dyspnea received a thoracic level epidural infusion of methadone (6 mg/24 h) and demonstrated significant improvement in their symptoms within one week (Juan, Ramon, Valia, et al., 2005). Continued improvements in respiratory function, exercise capacity, and health-related quality of life were noted after one month of treatment.
Sufentanil
Sufentanil is used less frequently than other opioids for epidural analgesia, most likely because it is more costly. It is two times more lipid-soluble than fentanyl. Pain relief by the epidural route is detected within 3 minutes of injection and duration (2 to 4 hours) is similar to fentanyl. Research shows that the quality of analgesia is similar to fentanyl as well (Lilker, Rofaeel, Balki, et al., 2009). When given epidurally, its analgesic effect is largely systemic rather than spinally mediated. This characteristic has led some to suggest its use is probably unwarranted (Maalouf, Liu, 2009). Another study found no clinically or statistically significant differences between morphine and sufentanil epidural analgesia in patients undergoing abdominal and urologic surgery (Delvecchio, Bettinelli, Klersy, et al., 2008).
Although there are no comparative data supporting its selection over other drugs, sufentanil has been included in intraspinal anesthesia protocols (Buyse, Stockman, Coumb, et al., 2007; Chen, Qian, Fu, et al., 2009; Kaya, Buyukkocak, Basar, et al., 2008; Parpaglioni, Baldassini, Barbati, et al., 2009). It is used occasionally for the intraspinal management of labor pain (Buyse, Stockman, Coumb, et al., 2007; Lilker, Rofaeel, Balki, et al., 2009); one study found epidural sufentanil to be superior to epidural fentanyl for this type of pain (Lilker, Rofaeel, Balki, et al., 2009). It has been administered both epidurally (Kaya, Buyukkocak, Basar, et al., 2008) and intrathecally (Chen, Qian, Fu, et al., 2009) for cesarean section delivery.
In combination with local anesthetics, sufentanil produces dose-sparing effects and enhances epidural analgesia (De Cosmo, Congedo, Lai, et al., 2008; Parpaglioni, Baldassini, Barbati, et al., 2009). For example, a randomized controlled study of 60 patients undergoing major abdominal surgery found that the addition of sufentanil to ropivacaine or bupivacaine improved pain relief during coughing and reduced local anesthetic requirements (Pouzeratte, Delay, Brunat, et al., 2001). There were no major adverse effects in any of the groups. A retrospective study of 171 patients after major abdominal or urological surgery compared 5 mL/h continuous epidural infusions of morphine (0.03 mg/mL) plus ropivacaine 0.2% to sufentanil (0.75 mcg/mL) plus ropivacaine 0.2% and reported comparable analgesia and use of PCEA but faster onset of analgesia with sufentanil (Delvecchio, Bettinelli, Klersy, et al., 2008). Nevertheless, the authors concluded that morphine should be the standard for neuraxial use.
Sufentanil also has been administered via PCEA. A prospective study of 58 patients following lumbar anterior-posterior fusion found that sufentanil (1 mcg/mL at 14 mL/h) plus ropivacaine (0.125%) via PCEA (14 mL/h basal rate, 5 mL bolus, 15 minute lockout) provided better pain relief at rest and during activity and greater patient satisfaction than IV PCA morphine (3 mg bolus, 15 minute lockout) (Schenk, Putzier, Kugler, et al., 2006); however, it is unknown if the addition of a basal rate and the use of smaller PCA bolus doses in patients receiving IV PCA might have improved pain control in that group. (See Tables 15-4 and 15-6 for dosing recommendations.)
Intraspinal Opioid Adverse Effects
Opioid adverse effects by the intraspinal routes are the same as by other routes of administration and include nausea, vomiting, pruritus, sedation, and respiratory depression, among others. The treatment of the various intraspinal opioid adverse effects is presented in Chapter 19.
Local Anesthetics
Low (“subanesthetic”) doses of local anesthetics are combined with intraspinal opioids for the treatment of acute or persistent pain because the two work synergistically to provide better analgesia at lower doses than would be possible with either drug alone (Jorgensen, Wetterslev, Moiniche, et al., 2001; Meek, 2004; Pouzeratte, Delay, Brunat, et al., 2001; Vascello, McQuillan, 2006). Local anesthetics are rarely used as a sole agent for postoperative pain management because a higher dose would be required to relieve pain, which would increase the likelihood of associated adverse effects, such as motor deficit and hypotension (Grape, Schug, 2008; Maalouf, Liu, 2009). Similarly, opioids alone are associated with an increase in adverse effects. However, in combination, the two agents allow a reduction in the doses of both the local anesthetic and the opioid, which results in a lower incidence of adverse effects of both agents (Ashburn, Caplan, Carr, et al., 2004; Doss, Ipe, Crimi, et al., 2001). In addition to improved analgesia and fewer adverse effects, adding local anesthetics to epidural opioids has been shown to improve GI function, suppress the stress response, and reduce CV, pulmonary, and infectious complications in postoperative patients (Basse, Hjort Jakobsen, Billesbolle, et al., 2000; Basse, Raskov, Hjort Jakobsen, et al., 2002; Jorgensen, Wetterslev, Moiniche, et al., 2001; Kehlet, 2005; Maalouf, Liu, 2009; Vadalouca, Mavromati, Goudas, et al., 2000). These benefits have the potential to reduce perioperative morbidity and mortality (Maalouf, Liu, 2009).
Low concentrations of the lipid-soluble, amide-type local anesthetics bupivacaine (Marcaine) (e.g., 0.05% to 0.125%) and ropivacaine (Naropin) (e.g., 0.05% to 0.2%) are used most often for epidural analgesia (see Table 15-6 for combinations used for PCEA). Levobupivacaine (0.1% to 0.125%) is used outside of the United States (Burlacu, Buggy, 2008; De Cosmo, Congedo, Lai, et al., 2008; Mendola, Ferrante, Oldani, et al., 2009). Ropivacaine in low concentrations may produce less motor blockade compared with low-dose bupivacaine (Maalouf, Liu, 2009), and the drug appears to have less CNS and cardiac toxicity than equipotent doses of bupivacaine (Covino, Wildsmith, 1998; Kedlaya, Reynolds, Waldman, 2002). Levobupivacaine may be less cardiotoxic than ropivacaine, but is used less often than ropivacaine, perhaps because ropivacaine is thought to produce less motor blockade (Grape, Schug, 2008). Compared with other local anesthetics, these are better able to block nerve fibers that transmit noxious stimuli with minimal effect on sensory and motor fibers (“differential sensory and motor blockade”) (Covino, Wildsmith, 1998; Gianino, York, Paice, 1996). Bupivacaine and ropivacaine are moderate to fast acting (onset within 5 to 20 minutes) and have a long duration of action (bupivacaine epidural block up to 12 hours; ropivacaine has a slightly shorter duration) (Covino, Wildsmith, 1998).
There appears to be no consensus on optimal drug, dose, concentration, and volume of local anesthetic used for postoperative epidural analgesia. Dose is an important factor in determining effectiveness of therapy (Kehlet, Dahl, 2002), but a wide variety of dosing regimens and local anesthetic concentrations are used, making it difficult to draw conclusions about the comparative efficacy of the various epidural local anesthetics (see Table 15-6). Concentrations of 0.05% and 0.01% of bupivacaine and ropivacaine via thoracic PCEA were found to be equipotent, producing equivalent analgesia and motor block in a randomized, double-blind study of 40 patients following abdominal surgery (Hodgson, Liu, 2001). Another randomized study found no differences in patient outcomes (pain, motor block, ability to walk, time to first flatus) among 60 women who received epidural 0.2% bupivacaine or 0.2% ropivacaine for abdominal hysterectomy except that those who received ropivacaine required significantly more ketorolac postoperatively (Jorgensen, Fomsgaard, Dirks, et al., 2000). A double-blind study randomized 60 patients undergoing major abdominal surgery to receive thoracic PCEA (all with a basal rate) of bupivacaine (0.125%) plus sufentanil (0.5 mcg/mL), ropivacaine (0.125%) plus sufentanil (0.5 mcg/mL), or ropivacaine (0.2%) alone and found that the addition of sufentanil improved pain relief during coughing and reduced local anesthetic requirements, but the bupivacaine combination produced more effective results than both concentrations of ropivacaine (Pouzeratte, Delay, Brunat, et al., 2001). There were no major adverse effects in any of the groups. Another study randomized 30 women undergoing major abdominal surgery to receive thoracic PCEA (all with a basal rate) bupivacaine (0.125%) plus sufentanil (0.5 mcg/mL) or ropivacaine (0.375%) plus sufentanil (0.5 mcg/mL) and observed comparable efficacy and incidence of adverse effects between the two groups (Gottschalk, Freitag, Burmeister, et al., 2002). Similarly, three different combinations of volumes and concentrations of levobupivacaine (0.5%, 0.25%, and 0.15%) combined with sufentanil (2.6 mcg/h) did not yield differences in sensory and motor block, pain scores, analgesic rescue dose requirements, patient satisfaction, or adverse effects in a randomized controlled trial of 150 patients postthoracotomy (Mendola, Ferrante, Oldani, et al., 2009).
An early study demonstrated that 0.2% ropivacaine plus fentanyl 4 mcg/mL produced a more intense motor block compared with ropivacaine 0.1% plus fentanyl 2 mcg/mL and ropivacaine 0.05 % plus fentanyl 1 mcg/mL, but the three treatments produced equivalent analgesia and similar mild adverse effects leading the authors to recommend the lower concentrations (0.05%, 0.1%) for low thoracic and lumbar PCEA (Liu, Moore, Luo, et al., 1999). Another randomized controlled study administered lumbar PCEA with concentrations of 0.05%, 0.075%, or 0.1% of ropivacaine, all combined with 4 mcg/mL fentanyl, to 312 women undergoing lower abdominal gynecologic surgery and found similar results (Iijima, Ishiyama, Kashimoto, et al., 2007). All three solutions produced comparable analgesia, motor blockade, GI motility, and mild adverse effects, which led the researchers to suggest the lowest ropivacaine concentration (0.05%) for lumbar PCEA.
Local anesthetics administered intrathecally (spinally) act faster than when administered epidurally because they are delivered in the immediate vicinity of the spinal cord and spinal nerve roots (action sites), and because such minute amounts are used when drugs are administered intrathecally, they also have a shorter duration of action (Covino, Wildsmith, 1998). Bupivacaine, lidocaine, and mepivacaine are administered most often for spinal anesthesia/analgesia. Bupivacaine and ropivacaine are the most common local anesthetics administered for long-term intrathecal therapy (Deer, Krames, Hassenbusch, et al., 2007). A small retrospective study reported the addition of intrathecal bupivacaine restored pain control and improved activity level, quality of life, and mental health in 17 patients with persistent noncancer pain (Kumar, Bodani, Bishop, et al., 2009).
Mechanism of Action
The sites of action of intraspinal local anesthetics are in the spinal cord and at the level of the spinal nerve roots, and they block both afferent and efferent signals to and from the spinal cord (Maalouf, Liu, 2009). As discussed in Sections I and V, local anesthetics are sodium blocking agents. They bind to sodium channels in nerve fibers and reduce action potential and subsequent nerve transmission of noxious stimuli (Maalouf, Liu, 2009; Strichartz, Berde, 2005). They also inhibit various potassium and calcium channels and other ion-gated channels, such as substance P receptors (Strichartz, 1998). Epidural local anesthetics are carried in the CSF to the dorsal root ganglion of the spinal nerve fibers immediately adjacent to their site of administration (see Figures 15-3 and 15-5). This results in segmental analgesia, which is influenced by the location of catheter placement as well as dose and volume of the local anesthetic.
The size of a nerve fiber influences its sensitivity to local anesthetics (Maalouf, Liu, 2009). As discussed in Section I, there are three categories of nerve fibers: (1) A fibers are myelinated somatic nerves, (2) B fibers are myelinated autonomic nerves, and (3) C fibers are unmyelinated nerves. A fibers are further divided into alpha, beta, gamma, and delta fibers. The thinnest of the A fibers are in the fast-conducting delta (δ) group. The smaller diameter A-δ and C nerve fibers carry pain impulses. This is fortuitous because local anesthetics block nerve conduction in small nerve fibers faster and at lower concentrations than in large fibers (Maalouf, Liu, 2009). Therefore it is possible to give very low doses intraspinally to block the impulses on the A-δ and C fibers without blocking the larger fibers that affect sensory and motor function (Gianino, York, Paice, 1996; Maalouf, Liu, 2009).
Lipid solubility of the local anesthetic determines its ability to cross membranes and access receptors (Lagan, McLure, 2004). Being lipid-soluble, bupivacaine and ropivacaine penetrate deeply into the spinal cord tissue (Strichartz, 1998). This characteristic also accounts for their rapid onset of action, especially by the intrathecal route (Covino, Wildsmith, 1998). The catheter is placed as close as possible to the dermatomes that, when blocked, will produce the most effective spread of analgesia for the site of nociceptive input (e.g., surgical site, site of injury, tumor location) (see Figure 15-3).
Adverse Reactions
Allergy to local anesthetics is uncommon, and the doses of local anesthetic used for intraspinal analgesia rarely result in blood concentrations sufficient to cause systemic effects. However, vascular uptake or injection or infusion of local anesthetic directly into the systemic circulation can result in adverse reactions related to high blood levels of local anesthetic, although there are reports of no adverse effects following accidental IV infusion of epidural doses of local anesthetics (Allegri, Baldi, Pitino, et al., 2009). CNS signs of systemic toxicity include ringing in ears, metallic taste, slow speech, irritability, twitching, and seizures. Signs of cardiotoxicity include circumoral tingling and numbness, bradycardia, cardiac dysrhythmias, acidosis, and CV collapse (Covino, Wildsmith, 1998).
Unwanted Effects
The goal of adding low-dose local anesthetics to epidural opioids for pain management is to provide analgesia, not to produce anesthesia. Patients should be able to ambulate if their condition allows, and epidural analgesia should not hamper this important recovery activity. However, many factors, including location of the epidural catheter, local anesthetic dose, and variability in patient response, can result in patients experiencing motor and sensory deficits and other unwanted local anesthetic effects. Because epidural local anesthetics produce a sympathetic blockade, vasodilation occurs, and minor hypotension, including orthostatic hypotension, is relatively common (Maalouf, Liu, 2009). The combination of opioid and local anesthetic may have an additive hypotensive effect. A randomized controlled trial of 155 patients undergoing colonic surgery compared ropivacaine 0.2% epidural infusions with and without fentanyl 2 mcg/mL (Finucane, Ganapathy, Carli, et al., 2001). Although the addition of fentanyl resulted in decreased infusion rates and better pain control, hypotension was more common, and time to discharge was approximately 1 day longer in the group receiving the opioid. This is not always the case, however. Other studies have shown that the addition of an opioid allows a lower dose of local anesthetic and less hypotension. For example, a minidose of bupivacaine (4 mg) with fentanyl (20 mcg) provided effective spinal anesthesia with less hypotension than 10 mg of bupivacaine alone and nearly eliminated the need for vasopressor support of BP during surgical repair of hip fracture in 20 patients ages 70 years and older (Ben-David, Frankel, Arzumonov, et al., 2000). CV adverse effects of spinal local anesthetics include hypotension as well as bradycardia, particularly when blockade is higher than T5 (Grape, Schug, 2009). Hypotension is much more common with the administration of spinal (33%) than with epidural (0.7% to 6%) local anesthetics (Grape, Schug, 2009) (see Chapter 19 for more on hypotension).
Intraspinal opioids are associated with a higher incidence of urinary retention than systemic opioids, and the addition of local anesthetics intraspinally can compound this adverse effect. However, urinary retention is seen less often in patients receiving thoracic than lumbar epidural analgesia, which indicates dermatomal level of the neuraxial blockade is a possible contributing factor (Dabu-Bondoc, Franco, Sinatra, 2009; Wu, 2005). (See Chapter 19 for further discussion of urinary retention during neuraxial analgesia.)
Studies show that thoracic placement of epidural catheters for administration of local anesthetics is associated with less sympathetic block of the lower extremities than lumbar-placed catheters and may reduce several postoperative adverse effects and complications, including urinary retention, postoperative ileus, orthostatic hypotension, and difficulty ambulating (Carli, Trudel, Belliveau, 2005; Wu, 2005). Thoracic rather than lumbar epidural administration of local anesthetics is recommended, especially when early postoperative ambulation is expected to be a priority.
Unwanted local anesthetic effects often can be corrected with simple treatment. For example, hypotension frequently is corrected with hydration, and a change in position may relieve temporary sensory loss in an extremity. Treatment of urinary retention and minor extremity weakness usually includes decreasing the epidural infusion rate slightly to reduce the local anesthetic dose. Patients are asked to remain in bed until muscle weakness resolves. A nonopioid can be given ATC to provide additional pain relief while the epidural analgesic dose is decreased. Sometimes removing the local anesthetic from the analgesic solution is necessary, such as when signs of local anesthetic toxicity are detected or simple treatment of hypotension, urinary retention, or motor and sensory deficits has been unsuccessful. In any case, care of the patient receiving epidural analgesia includes taking safety precautions and reporting unwanted effects of epidural local anesthetics to the anesthesia provider. Table 15-7 describes the assessment of some of the effects of epidural local anesthetics.
Local Anesthetic Neurotoxicity
Although controlled studies have not shown neurotoxicity to be a significant complication of intrathecal local anesthetic infusion (Kedlaya, Reynolds, Waldman, 2002), some clinicians have raised concerns (Chabbouh, Lentschener, Zuber, et al., 2005; Grape, Schug, 2008; Hodgson, Neal, Pollock, et al., 1999; Rohm, Boldt, 2006). Case reports describe the occurrence of transient neurologic symptoms, such as various paresthesias and unilateral or bilateral lower extremity pain, in 4% to more than 30% of patients 12 to 24 hours following spinal anesthesia and lasting 6 hours to 4 days, after which the symptoms resolve spontaneously (Grape, Schug, 2008).
A serious complication that has been linked to local anesthetic toxicity is cauda equina syndrome, which is the acute loss of neurologic function below the termination (conus) of the spinal cord as a result of damage to the spinal nerve roots. The cauda equina consists of nerves that are partially unmyelinated, and their increased surface area makes them prone to contact with neurotoxic agents (Neal, 2008). Local anesthetic toxicity is concentration-dependent and can occur at concentrations lower than those used in the clinical setting. Maldistribution and excessive local anesthetic dose concentration in the CSF are thought to be causes of cauda equina syndrome (Birnbach, Browne, 2005; Neal, 2008). Neal (2008) summarizes that neuraxial local anesthetics are remarkably safe in the majority of patients, but that a rare patient may be vulnerable to local anesthetic neurotoxicity even in “normal” clinical situations. See later in this chapter for more on complications of intraspinal analgesia.
Alpha2-Adrenergic Agonists (Clonidine and Dexmedetomidine)
The alpha2-adrenergic agonist clonidine is sometimes added in small doses to intraspinal analgesia to improve pain control, produce dose-sparing effects, and prolong analgesia (Andrieu, Roth, Ousmane, et al., 2009; Forster, Rosenberg, 2004; Huang, Lin, Huh, et al., 2007; Schug, Saunders, Kurowski, et al., 2006). A systematic review of 22 randomized trials concluded that clonidine prolonged the analgesic action and sensory and motor blockade of a variety of intrathecal local anesthetics in a dose-dependent manner, but was unable to establish the optimal dose of clonidine for these effects (Elia, Culebras, Mazza, et al., 2008) (see Chapter 22 for a detailed discussion of the underlying mechanisms and adverse effects of the alpha2-agonists). Although rarely used as a sole agent, clonidine administered alone epidurally produced significant improvements in pulmonary function in patients undergoing lung resection in one randomized controlled trial (Matot, Drenger, Weissman, et al., 2004).
Clonidine does not produce respiratory depression, nausea, pruritus, or urinary retention, which adds to its appeal as an adjuvant for intraspinal therapy (Grape, Schug, 2008); however, problems can occur with the tendency of alpha2-agonists to produce sedation and hypotension. In combination with opioids, clonidine may exacerbate these adverse effects. A prospective trial randomized 100 patients to receive general anesthesia supplemented by IV fentanyl or general anesthesia preceded by intrathecal clonidine (75 mcg), bupivacaine (15 mg), and morphine (0.2 mg) for radical prostatectomy (Brown, Hofer, Patterson, et al., 2004). Those who received intrathecal clonidine required more IV fluids and vasopressors intraoperatively but had lower pain scores, consumed less supplemental IV morphine postoperatively, and were discharged from the hospital significantly earlier (3 days compared with 5 days).
The timing of administration may be an important factor in determining efficacy of intraspinal clonidine. A single dose of epidural clonidine administered before abdominal surgery resulted in a greater opioid dose-sparing effect, better pain scores at 6 and 24 hours, and less sedation than epidural clonidine administered at the end of colorectal surgery (Persec, Persec, Bukovic, et al., 2007).
Another alpha2-adrenergic agonist, dexmedetomidine, given in low doses (3 mcg), prolonged bupivacaine spinal blockade in a similar fashion as clonidine (30 mcg) when administered for transurethral resection of prostate or bladder tumor (Kanazi, Aouad, Jabbour-Khoury, et al., 2006). Further research and clinical experience with dexmedetomidine in this setting is needed to define a role in epidural analgesia.
Prevention of Persistent Postsurgical Pain
Clonidine may have a role in preventing persistent postsurgical pain as well. A randomized controlled study found that intrathecal clonidine (300 mcg) given intraoperatively was more effective than intrathecal bupivacaine or placebo given intraoperatively in influencing several postoperative outcomes in patients undergoing colonic surgery (De Kock, Lavand’homme, Waterloos, 2005). For example, patients in the clonidine group had a longer time to first request for analgesia, lower pain scores and supplemental analgesic consumption, and a lower incidence of persistent postsurgical pain at 6 and 12 months compared with those in the other groups. No adverse events occurred in any of the groups. Intraoperative hemodynamic adverse effects, e.g., hypotension and bradycardia, occurred most often in the clonidine group but were corrected without incident. No one experienced excessive sedation. (See Section I and Section V for more on persistent postsurgical pain.)
Complications Associated with the Intraspinal Routes of Administration
Randomized controlled trials have demonstrated that intraspinal analgesia is associated with reduced morbidity and mortality (Rodgers, Walker, Schug, et al., 2000); however, the therapies are not without risk, and these must be considered when determining the appropriateness of therapy (Ballantyne, 2004; Choi, Bhandari, Scott, et al., 2003). Following is a discussion of the complications that can occur: postdural puncture headache; intraspinal catheter migration, and neurologic complications from trauma to neural tissue; injection or infusion of neurotoxic agents; infection; and hematoma.
Dural Puncture and Postdural Puncture Headache
Obviously the dura is punctured when intrathecal analgesia is administered. Microcatheters were used in the early 1990s for continuous spinal anesthesia, which helped to minimize dural puncture-related symptoms but were withdrawn from the market in the United States after multiple reports of cauda equina syndrome (Grape, Schug, 2008). Some practitioners use epidural catheters for continuous spinal anesthesia but with the disadvantage of producing a large dural leak and the risk of subsequent postdural puncture headache (PDPH); large needles (and catheters) produce larger dural punctures and carry a higher risk of PDPH (Turnbull, Shepherd, 2003).
Inadvertent dural puncture, often referred to as “wet tap,” can occur during placement of an epidural catheter. It is the most common complication from epidural catheter placement with an incidence between 0.32% and 3% (Grape, Schug, 2008; Maalouf, Liu, 2009). The incidence is influenced by the experience of the anesthesia provider as well as the technique used to place the needle, the size and orientation of the needle, and the thickness of the dura (Meek, 2004; Turnbull, Shepherd, 2003). A study of 547 women in labor demonstrated that the use of air alone with the loss of resistance technique for epidural needle placement (see earlier in this chapter) was associated with a higher incidence of dural punctures than when the technique was used with lidocaine alone or with lidocaine plus air (Evron, Sessler, Sadan, et al., 2004). However, rarely, more serious complications can occur from a dural puncture, such as infection (Turnbull, Shepherd, 2003) and pneumocephalus if air is used in the loss of resistance technique; the use of saline is recommended to prevent pneumocephalus (Maalouf, Liu, 2009; Nafiu, Bullough, 2007). Orientation of the needle bevel parallel to the dural fibers is reported to reduce dural puncture (Turnbull, Shepherd, 2003). Perforation in thick areas of the dura may be less likely to result in CSF leak, and the thickness of the dura varies among individuals (Turnbull, Shepherd, 2003). The anesthesia provider usually knows when a dural puncture has occurred and will attempt needle placement at a higher vertebral interspace.
The exact mechanism underlying the development of PDPH is not clear (Turnbull, Shepherd, 2003). Some patients experience no symptoms after a dural puncture, yet for others, the leakage of CSF through the hole created in the dura causes a dull, aching, or throbbing headache. The headache may be frontal, occipital, or diffuse in location. It usually is moderate to severe in intensity and may be accompanied by neck or back soreness or stiffness, photophobia, visual disturbances, nausea, and vomiting. The decrease in CSF pressure as a result of the dural puncture is thought to be the reason patients routinely report that the headache worsens when they move into a sitting or standing position and improves when they lie down (Grape, Schug, 2008).
After a dural puncture, the anesthesia provider usually alerts the nursing staff to assess the patient for headache. Sometimes the headache appears during epidural analgesia after a decrease in the epidural continuous infusion rate or when the epidural catheter is removed. Most commonly, the headache occurs 1 to 5 days after the dural puncture and persists for approximately 7 days. On rare occasions, a PDPH lasts for months and even years (Turnbull, Shepherd, 2003). The development of a headache between 5 and 14 days after the dural puncture should be reported promptly to the anesthesia provider as this may be a sign of more serious complications, including death (Turnbull, Shepherd, 2003).
Treatment of PDPH
Treatment of PDPH usually is symptomatic and conservative, consisting of administration of oral opioid and nonopioid analgesics and reassuring the patient that the headache will most likely resolve within a week. Nondrug interventions may be helpful. For example, concentrated CSF can worsen symptoms, so fluid intake is encouraged. Ice packs also may be helpful. Abdominal binders and bedrest have shown little value in reducing symptoms; patients should be told to sit and lie in positions that are most comfortable to them (Turnbull, Shepherd, 2003).
Although the effectiveness of caffeine in the treatment of PDPH is disputed (Halker, Demaerschalk, Wellik, et al., 2007), some clinicians use 300 to 500 mg of oral or IV caffeine, which is thought to relieve symptoms by producing cerebral vasoconstriction (Turnbull, Shepherd, 2003) (see Section III and Table 9-2, p. 242, for oral nonopioid analgesics that contain caffeine and Table 9-1, p. 241, for nutritional sources of caffeine). Sumatriptan, used for migraine headaches, has been administered as well but has not been shown to be particularly effective (Connelly, Parker, Rahimi, et al., 2000). Another novel treatment for PDPH is the IV infusion of adenocorticotropic hormone (ACTH). Patients with PDPH unresponsive to conservative treatment were given a single IV infusion of ACTH, 1.5 units/kg in 250 mL normal saline over a 30-minute period (Kshatri, Foster, 1997). Headache resolved within 2 to 6 hours. The exact mechanism of analgesic action of ACTH in patients with PDPH is unknown but is thought to result from its adrenal (release of hormones) or extra-adrenal (metabolic) physiologic actions.
Blood Patch: The observation that “bloody taps” resulted in a reduced incidence of headache led to the use of blood patches to treat PDPH (Turnbull, Shepherd, 2003). A blood patch consists of withdrawing the patient’s blood from the antecubital vein and injecting it into the epidural space. The blood is distributed both caudally and cephalad and forms a clot over the dural puncture site (Turnbull, Shephard, 2003). This has been described as similar to plugging a hole with a cork. There is no consensus on the ideal amount of blood to use for a blood patch, but 20 to 30 mL is reported to produce the best results; 2 to 3 mL is inadequate, and large volumes (e.g., 60 mL) have been associated with spontaneous intracranial hypotension (Turnbull, Shepherd, 2003). Patients are asked to lie still for 2 hours after the blood patch and then are allowed activity as tolerated.
A cohort study of 79 patients observed better success in relieving PDPH symptoms when the blood patch was performed at an early stage (i.e., at onset of severe headache) rather than waiting to see if the symptoms will subside (Vilming, Kloster, Sandvik, 2005). Clinical observations of effectiveness are often dramatic with rapid headache relief in many patients after blood patch; however, research is lacking. A Cochrane Collaboration Review could find only three randomized controlled trials (77 participants) comparing epidural blood patch and no blood patch in the prevention or treatment of PDPH and summarized that more well-controlled research is needed to draw conclusions about the efficacy of blood patch for PDPH (Sudlow, Warlow, 2001).
Complications from the blood patch procedure are very rare and include epidural infection and nerve root compression. Patients may report a minor backache after the injection of blood into the epidural space. Contraindications to the procedure are similar to those of epidural analgesia (see Box 15-1).
Dextran 40 in saline has also been injected epidurally for the treatment of PDPH. The rationale is that its high molecular weight and viscosity slows its removal and allows spontaneous closure of the puncture site, but there is no research to support the practice (Turnbull, Shepherd, 2003). In Sweden, a patient was treated with Dextran 40 when a PDPH persisted despite two consecutive blood patches (Reynvoet, Cosaert, Desmet, et al., 1997). Symptoms were relieved after 25 mL of Dextran 40 was injected into the epidural space and followed by a 3 mL/h infusion of Dextran 40 in saline through an epidural catheter. Informed consent is recommended because Dextran 40 in saline is not approved for epidural administration.
Surgical closure of the dural perforation has been performed for refractory PDPH. This is a last-resort treatment (Turnbull, Shepherd, 2003).
Catheter Displacement
Displacement of temporary catheters during epidural analgesia therapy is a relatively common occurrence (6%) and is often caused by patients accidentally pulling catheters out during activity (Heid, Piepho, Stengel, et al., 2009). Proper taping of the catheter and teaching patients to avoid tugging on the catheter helps to minimize the incidence of displacement. A variety of methods for dressing the epidural catheter sites are described in the literature, and some may be more effective than others in preventing catheter displacement and migration (Burns, Cowan, Barclay, et al., 2001). Catheters may also knot if they have been threaded too deep into the epidural space; a depth of 4 to 6 cm is recommended (Maalouf, Liu, 2009).
Catheter displacement can result in analgesic gaps and should be attended to promptly. When patients report inadequate pain relief during epidural analgesia continuous infusion or PCEA, the entire epidural line from the infusion pump to the epidural catheter site should be checked. Inadequate pain relief may be due to a number of mechanical and technical factors, including incorrect loading of the pump, a disconnection of the catheter from the infusion pump tubing, an empty drug reservoir, a disconnected PCEA button, a malfunctioning pump or tubing, or the epidural catheter may have been inadvertently pulled out.
When bolus doses and increases in the epidural analgesic dose do not yield satisfactory pain control or produce “patchy” analgesia (e.g., one-sided) and the epidural catheter appears to be in place, the infusion line connected, and the infusion pump infusing correctly, the anesthesia provider or the pain service is notified. The epidural catheter can be checked for optimal location by administering a concentrated dose of local anesthetic through the catheter. Optimal catheter placement would produce a bilateral sensory block of the desired dermatomes; lack of such a block would indicate that the catheter location is not optimal (see below and Box 15-4). If location is less than optimal, the epidural catheter should be removed and alternatives considered.
Catheter Migration
Catheter migration can occur at any time during epidural analgesia therapy and despite correct catheter placement. Epidural catheters can migrate out of the epidural space through the dura into the subarachnoid space or into the vascular system through an epidural blood vessel. The incidence of intrathecal migration of an epidural catheter during epidural analgesia is 0.15% to 0.18% and intravascular migration is 0.18% (Maalouf, Liu, 2009).
Early signs of intravascular or intrathecal epidural catheter migration during continuous infusion or PCEA are likely to be subtle and noted most often by a change in the patient’s pain control or adverse effects since the last assessment. Signs and symptoms of epidural catheter migration are more pronounced when analgesia is administered by the bolus method. Intrathecal injection of an epidural dose of local anesthetic or opioid can result in a high block and life-threatening respiratory depression requiring aggressive intervention and support. The epidural catheter can be tested with the administration of a small dose of epinephrine-containing local anesthetic before administration of an epidural bolus to rule out intravascular or intrathecal migration (Maalouf, Liu, 2009).
A case report described an intervertebral foraminal catheter migration during implanted intrathecal drug therapy (Ko, Ferrante, 2006). Three months after implantation, the patient reported new-onset radicular pain (low-back pain that radiated to the right lateral thigh and terminated in the right foot) without bowel or bladder symptoms. The migration was confirmed by MRI and CT scan and corrected surgically (right lumbar hemilaminectomy with dissection of epidural scar tissue). The patient recovered rapidly without sequelae. This case reinforces the importance of systematic assessment and attention to the patient’s response to therapy and particularly to any change in symptoms. See Box 15-4 and the Patient Example that follows.
Direct Needle or Catheter Trauma
Trauma to neural tissue from intraspinal needles and catheters is extremely rare (0.0005%). Case reports suggest that direct spinal cord trauma is most often the result of excessively caudad termination of the spinal cord or inaccurate determination of bony landmarks (Neal, 2008). Nerve root trauma is also rare and usually is indicated by patient reports of pain that is severe, sharp, and radiating along a nerve when the needle is placed.
Trauma from the indwelling intraspinal catheter is more common with long-term intraspinal analgesia treatment for persistent pain than with short-term intraspinal analgesia for acute pain management. For example, tissue fibrosis around the indwelling epidural catheter tip can occur with long-term use (Simpson, Jones, 2008). This can lead to spinal cord compression producing neurologic impairment of varying degrees. Tissue fibrosis rarely occurs with intrathecal catheters; factors limiting the occurrence include infusing solutions without additives, keeping drug solutions at pH 5, and using polyurethane or silicone catheters (Simpson, Jones, 2008).
Catheter Tip Granulomas
Catheter tip granulomas are one of the most serious adverse effects and risks of long-term intrathecal drug delivery (Deer, Krames, Hassenbusch, et al., 2007). Progressive motor and sensory deficits are usually permanent despite surgical intervention. A review of the literature revealed 41 cases between 1990 and 2000, but the researchers suggested that the condition may be underreported (Turner, Sears, Loeser, 2007). Granulomas have been reported with the use of all drugs except sufentanil and rarely with fentanyl (Deer, Krames, Hassenbusch, et al., 2007). Contributing factors are the dose and concentration of the drug, catheter position, and low CSF volume.
Injection or Infusion of Neurotoxic Agents
Generally local anesthetics and opioids administered in clinically-recommended doses are safe in the majority of patients; however, even under “normal” circumstances, some patients will be susceptible to neuraxial injury from neurotoxicity related to the administration of these agents (Neal, 2008). High concentrations of local anesthetics can cause neurotoxicity as can a number of preservative agents and antitoxins, including alcohol, phenol, formaldehyde, and sodium metabisulfite (Paice, Williams, 1995). Other agents that have resulted in significant neurologic damage after accidental epidural infusion include antibiotics, potassium chloride, and total parenteral nutrition. All agents and solutions injected or infused intraspinally must be sterile, preservative-free, and regarded safe for intraspinal administration. Epidural infusion lines that are color-coded and do not have injection ports should be used to prevent errors (Box 15-5).
Infection
Intraspinal infection, such as an epidural abscess, arachnoiditis, or bacterial meningitis, is a very rare but serious complication of neuraxial analgesia (Grape, Schug, 2008; Maalouf, Liu, 2009). A 6-year review of data from a hospital’s experience with 8100 patients who received epidural analgesia revealed just 6 cases of epidural abscess and 3 of meningitis (Christie, McCabe, 2007). Another review of the data of 8210 patients who received epidural analgesia over a 16-year period also revealed only 6 patients with epidural abscess (Cameron, Scott, McDonald, et al., 2007).
Intraspinal infection is thought to be more common when catheters are left in place for a prolonged time (Rathmell, Lake, Ramundo, 2006). Although data are lacking on the incidence of infection during long-term intraspinal therapy (Turner, Sears, Loeser, 2007), the longer the catheter is left in place, the greater the risk of infection, and the incidence of infection with externalized tunneled epidural systems is higher than with totally implanted drug delivery systems (Rathmell, Lake, Ramundo, 2006). The risk of infection in patients receiving epidural therapy for more than 70 days is 15%, but the incidence of infection extending to the epidural space (deep infection) is about 1% (Rathmell, Lake, Ramundo, 2006). An extensive review of postmarketing and medical device reporting data, meta-analyses, and other publications related to infection associated with implanted spinal cord stimulators and drug delivery systems revealed an overall infection rate of 5% (Follett, Boortz-Marx, Drake, et al., 2004) (see the section on symptoms and treatment later in the chapter).
Predisposing factors to intraspinal infection are immunocompromised state, diabetes, HIV infection, malignancy, steroid use, difficult insertion, and longer catheterization time (e.g., more than 3 days for short-term epidural analgesia) (Christie, McCabe, 2007; Grape, Schug, 2009; Horlocker, Wedel, 2006). Most clinicians recognize septicemia as a contraindication to intraspinal catheterization, although single-injection technique is sometimes used in patients with systemic infection (Grape, Schug, 2009). Some clinicians suggest that regional anesthesia may be acceptable if appropriate antibiotic therapy is initiated and the patient has shown response to antibiotic therapy before dural puncture (Wedel, Horlocker, 2006).
Causes of intraspinal infection include spontaneous infection, hematogenous spread during episodes of bacteremia, and infection as a result of poor aseptic technique. However, most epidural abscesses are thought not to be related to epidural catheter placement but to infections of the skin, soft tissue, spine, or hematogenous spread to the epidural space instead (Wedel, Horlocker, 2006). The importance of using aseptic techniques during the placement of epidural catheters is often stressed as an important preventive measure, but there are limited data on what practices are essential (Hebl, 2006). The recommendations for surgical hand washing should be applied to regional anesthesia techniques including intraspinal catheter placement; alcohol-based solutions containing 2% to 4% chlorhexidine appear to have the best extended antimicrobial activity; chlorhexidine is recommended as the first-choice antiseptic for regional anesthetic techniques (Hebl, 2006). Wearing a surgical mask during intraspinal needle and catheter placement to prevent droplet transmission of nasal and oropharyngeal flora is recommended (Institute for Safe Medication Practices, 2010). The reader is referred to Hebl’s (2006) excellent review of the research regarding aseptic technique and the variety of factors that may influence the incidence of infection during regional anesthesia, including the use of artificial nails; removal of jewelry; and donning of mask, cap, and gown.
Localized skin infection at the intraspinal needle or catheter entry site also can occur. The incidence of this may be influenced by level of insertion; one review reported an incidence of 2.8% and 0.8% in thoracic and lumbar level insertions, respectively, but the researchers pointed out that the thoracic catheters were in place longer than the lumbar catheters in this study (Cameron, Scott, McDonald, et al., 2007). Localized bacteria are known to track down the catheter from the skin entry site to the epidural or intrathecal space (Wheatley, Schug, Watson, 2001). Bacterial migration was found to be the most common route of epidural catheter colonization in one study of 105 patients who received postoperative epidural analgesia (Yuan, Zuo, Yu, et al., 2008). Contamination of the intrathecal or epidural system can occur during drug reservoir preparation, catheter placement, refilling implanted reservoirs, or administration of the analgesic. When an external infusion pump is used to deliver intraspinal analgesia, contamination can also occur while loading the pump, connecting the catheter to the infusion, or changing the drug reservoir. Even the infusion pumps are a potential source of bacteria. A one-month study of all reusable analgesia infusion pumps at one hospital found 45% of the PCA buttons (pendants) and 46% of the keypads grew bacteria with the most common organism being coagulase-negative staphylococcus; the simple intervention of cleaning the devices with 70% isopropyl alcohol reduced contamination by 6% and 4%, respectively (Rothwell, Pearson, Wright, et al., 2009). The implementation of a vigorous, systematic cleaning process is recommended.
Symptoms and Treatment of Intraspinal Infection
Early signs and symptoms of an intraspinal infection can be difficult to detect. Systematic assessment is essential. Skin site infection and fever are not always present (DuPen, DuPen, 1998). The cardinal signs of deep intraspinal infection are increasing diffuse back pain or tenderness or pain or paresthesia on intraspinal injection (radicular symptoms). These occur most often approximately 5 days after epidural catheterization (Grape, Schug, 2009), although late presentation is also reported (Bussink, Gramke, van Kleef, et al., 2005; Rohm, Boldt, 2006). Bowel or bladder dysfunction may be present. Any one of these signs should immediately arouse suspicion and further investigation. The risk of neurologic deficit from epidural abscess is nearly 50% because of late recognition (Grape, Schug, 2009; Wheatley, Schug, Watson, 2001). Formation of an epidural abscess can cause spinal cord compression or sepsis and, in extreme cases, paralysis, which occurs rapidly after the onset of motor weakness (Wedel, Horlocker, 2006). Intraspinal abscess is confirmed by magnetic resonance imaging (MRI) or computed tomography (CT), and a neurology or neurosurgery consultation is requested.
Although practice varies (Christie, McCabe, 2007), it is recommended that percutaneous epidural catheters be removed whenever there are signs of local infection (e.g., local erythema or discharge) (Wedel, Horlocker, 2006). Many clinicians culture the catheter tip; however, one study cultured the catheter tips of 1443 patients who had received short-term epidural analgesia and found at least one type of microorganism in 28.8%, but no epidural space infections were identified, which led the researchers to suggest that a routine culture of an epidural catheter tip is clinically irrelevant and not a good predictor of the presence of an epidural catheter infection (Simpson, Macintyre, Shaw, et al., 2000). To decrease the risk of hematogenous spread to the subarachnoid or epidural space, intraspinal catheters are also often removed when infection occurs outside the intrathecal or epidural space. Removal of implanted catheter systems sometimes can be avoided when superficial infections are treated early and aggressively with local wound care and antibiotics; however, deep infections warrant mandatory removal of the system (Rathmell, Lake, Ramundo, 2006).
Certain precautions to prevent intraspinal infection and regular assessment for signs of infection are essential nursing functions in caring for patients who receive any intraspinal analgesia technique (Pasero, Eksterowicz, Primeau, et al., 2007) (Box 15-6). Because the signs of intraspinal infection can occur after patients are released from the hospital and can occur even if the course of intraspinal analgesia was short and uneventful, it is imperative that discharge teaching include the signs and symptoms and to report them immediately if detected. Patients should know that it may be necessary to remind the primary care provider that they received intraspinal analgesia during hospitalization as this sometimes is overlooked as a possible cause of symptoms.
Epidural Catheter Disconnection
Occasionally epidural catheters become disconnected during therapy, which leaves the system open to the introduction of bacteria. The anesthesia provider must then determine whether or not to repair the line and continue therapy or remove the epidural catheter. Research is lacking regarding the correct action to take when this occurs, but one in vitro study demonstrated that 8 hours after contamination of an epidural catheter, no bacteria were detected more than 20 cm from the catheter hub, provided the fluid within the catheter remained static (i.e., no displacement of fluid toward the patient from the disconnected end) (Langevin, Gravenstein, Langevin, et al., 1996). If these conditions can be met (i.e., a recognized disconnect within 8 hours and a static fluid column), the anesthesia provider may immerse the catheter 10 inches from the disconnected end in povidone iodine for 3 minutes and allow it to dry completely. (Research has not been conducted on other antiseptic solutions, such as chlorhexidine, for this purpose.) The catheter should then be cut with a sterile instrument in the center of this area and reconnected with a sterile connector. However, if the disconnection was unwitnessed or the distal meniscus appears to have migrated more than 5 inches from the disconnected end (i.e., a nonstatic fluid state), the catheter should be removed (Hebl, 2006; Langevin, Gravenstein, Langevin, et al., 1996).
When a disconnection is detected, the nurse should wrap the end of the catheter with a sterile 4 × 4, taking care not to introduce any new bacteria, and contact the anesthesia provider immediately. The anesthesia provider makes the decision as to how to proceed in all cases. Repair of epidural catheter should be performed only by those who are trained in the procedure and supported by institutional policy and procedure.
Infusion Therapies: Solution and Tubing Changes
Although there are no guidelines to direct the frequency with which IV and percutaneous epidural analgesia infusion systems (solution and tubing) should be changed (also called hang time), the literature and clinical practice over the years support maintaining the integrity of the infusion system after therapy is initiated (Brooks, Pasero, Hubbard, et al., 1995; Dawson, Rosenfeld, Murphy, et al., 1991; Langevin, 2000; Sevarino, Pizarro, Sinatra, 2000; Strong, 1991; Waldman, 1991). This means that every effort should be made to minimize entry into the system to prevent inadvertent introduction of bacteria.
Because there are no guidelines, practice varies widely with regard to analgesic infusion hang time. Many institutions change main IV solutions and infusion tubing every 24 hours but extend the hang time for the IV PCA system; others change the solution but not the tubing every 24 hours, and still others maintain a closed system until the PCA reservoir is empty. Typically, epidural systems are not entered unless the reservoir is empty and a new one must be added. A survey of nurses who subscribed to the American Pain Society and American Society for Pain Management Nursing [ASPMN] e-mail list serve in 2007 yielded 48 responses to a question asking how often IV PCA solution and tubing were changed in their institutions (Pasero, unpublished data). Their responses reflected IV PCA hang times of 24 hours (12%), 48 hours (52%), and 72 hours (36%). Responses to the same question regarding epidural analgesia revealed hang times of 72 hours (88%), 96 hours (10%), and “whenever the drug reservoir runs dry” (2%). The difference in the hang times between the two therapies is not surprising given that most clinicians appreciate the need to keep the epidural infusion system closed to reduce inadvertent introduction of bacteria and the potential devastating consequences of an epidural abscess or meningitis.
Microbiologic research is lacking, but one early study established the safety of once-monthly filter and drug reservoir changes during long-term intrathecal analgesia infusion (Nitescu, Hultman, Appelgren, et al., 1992). Another study showed that fentanyl and sufentanil possess significant antimicrobial activity in solutions used for PCA delivery systems (Chapalain-Pargade, Laville, Paci, et al., 2006). For research on the stability and compatibility of various agents used for analgesic infusion therapies, see p. 412.
Bacterial Filters: There are no data to support the use of bacterial filters during short-term epidural or perineural catheter infusions (e.g., days) (Hebl, 2006). However, they are often used during therapy of a longer duration. A time-dependent study established a significant correlation between the incidence of catheter hub colonization and the frequency of epidural catheter filter changes during tunneled epidural therapy, prompting the researchers to recommend an extended interval of at least 60 days between filter changes during this long-term epidural therapy (De Cicco, Matovic, Castellani, et al., 1995).
Epidural Hematoma
Perhaps the most dreaded and serious adverse events of neuraxial analgesia are bleeding complications, such as an epidural hematoma, with resultant permanent spinal cord damage (Grape, Schug, 2009). Epidural hematomas occur as a result of epidural vessel puncture (Maalouf, Liu, 2009). The incidence of punctured vessels during needle and catheter placement is 3% to 12% of attempts, but these usually do not cause problems (Maalouf, Liu, 2009). The exact incidence of symptomatic epidural hematoma is unknown but thought to be extremely rare (Christie, McCabe, 2007; Grape, Schug, 2009). A review of the data of 8210 patients who received epidural analgesia over a 16-year period revealed only two spinal hematomas, and neither required surgical removal or resulted in adverse sequelae (Cameron, Scott, McDonald, et al., 2007). Concurrent anticoagulation therapy is a primary risk factor for spinal-epidural hematoma (Wilson, 2009). The American Society of Regional Anesthesia and Pain Medicine published a consensus guideline on neuraxial anesthesia and anticoagulation, and their recommendations are outlined in Table 15-8.
Like epidural infection, early detection of epidural hematoma is difficult because symptoms often are obscure. Patients may report inadequate or uneven (better on one side than the other) pain relief. The cardinal signs of epidural hematoma are increasing diffuse back pain or tenderness or pain or paresthesia on epidural injection (radicular symptoms). Bowel or bladder dysfunction may also be present. As the hematoma increases in size, sensory or motor deficit develop. Any one of these signs should immediately arouse suspicion and further investigation. Patient recovery without neurologic injury from a major bleeding complication related to neuraxial analgesia depends on early recognition and aggressive treatment (Butterworth, Douglas-Akinwande, 2007). Although paraplegia is extremely rare with intraspinal analgesia, when it does occur, it is most often caused by epidural hematoma, and it occurs rapidly after the onset of motor weakness (Wedel, Horlocker, 2006). Epidural hematoma is confirmed by MRI or CT, and a neurology or neurosurgery consultation is requested. Epidural hematomas usually are treated by immediate surgical removal of the hematoma.
Regular patient assessment of motor and sensory function and reporting to the anesthesia provider abnormal blood clotting studies and orders for anticoagulants before and during intraspinal analgesia may help to prevent epidural hematoma and are essential nursing functions (Pasero, Eksterowicz, Primeau, et al., 2007). Because the signs of epidural hematoma can occur after patients are released from the hospital and can occur even if the course of intraspinal analgesia was short and uneventful, it is imperative that discharge teaching include the signs and symptoms and to report them immediately if detected. Patients should know that it may be necessary to remind the primary care provider that they received intraspinal analgesia during hospitalization as this sometimes is overlooked as a possible cause of symptoms.
Operator Errors
Operator (human) errors, particularly incorrect loading and misprogramming of infusion pumps, have been identified as a major cause of significant patient injuries and deaths over the years (Hicks, Heath, Sikirica, et al., 2008; Institute for Safe Medication Practices, 2003a, 2003b, 2004b; Macintyre, Coldrey, 2008). The use of devices that are not approved for pain management is another potential source of patient injury. Both issues are discussed here.
Misprogramming Analgesic Infusion Pumps
The administration of the wrong dose as a result of incorrect pump programming was by far the most common error (38.9%) identified in an analysis of data submitted to MEDMARX and the USP Medication Errors Reporting Program between 1998 and 2003 (United States Pharmacopeia, 2004). To help prevent these types of errors, staff must be trained in the proper use of analgesic infusion devices, both through initial training and annual competency checks (Pasero, Eksterowicz, Primeau, et al., 2007). Institution policy and procedure should mandate that all analgesic infusion device programming be independently double-checked at specified times, such as before the initiation of analgesic infusion therapy, at the time of any adjustments in prescription, and during the nursing hand-off communication processes. An independent double-check consists of having another clinician (e.g., nurse, physician, pharmacist) compare the analgesic solution’s drug and concentration and the pump’s programmed prescription against the patient’s written prescription to ensure accuracy without prompting from the person administering the analgesic or anyone else. Distractions during programming and double-checks are identified as being factors contributing to errors (Hicks, Heath, Sikirica, et al., 2008), so clinicians must take steps to avoid being interrupted.
The concept of the 6 Rights (6 Rs) of medication safety (right patient, right drug, right dose, right time, right route, right documentation) forms the basis of safe analgesic therapies and is the responsibility of everyone on the health care team (Institute for Safe Medication Practices, 2004c). A concerted multidisciplinary effort that includes appropriate prescribing; the highest standard of drug-reservoir preparation, storage, and dispensation; uninterrupted attention during programming and double-checking; and careful patient monitoring during therapy are essential. Readers are encouraged to subscribe to the Institute for Safe Medication Practices newsletters, in which safety information on a variety of medications is regularly published (http://www.ismp.org). (See also Chapter 19 for transfer of care and hand-off communications.)
Use of Unapproved Infusion Devices for Analgesic Therapy
It goes without saying that the devices used to infuse analgesic therapies should be approved for that purpose. A surprisingly common practice, particularly in laboring patients, is to infuse epidural analgesia via an IV infusion pump that is not approved for epidural infusion. This practice has the potential for significant patient harm and is not recommended. Risk management personnel are encouraged to evaluate whether or not this practice exists in their institutions and facilitate the actions necessary to ensure approved devices are available for analgesic therapies in all clinical units.
Tapering and Cessation of Epidural Analgesia
For patients with acute pain who are receiving epidural analgesia, plans are necessary for smoothly weaning the patient as pain decreases or the patient is able to use a less invasive route of administration. Although most patients experience less pain as the days pass after surgery, it should not be assumed that all patients will follow this pattern. For example, the duration of postoperative pain tends to be longer in older patients (Melzack, Abbott, Zackon, et al., 1987). It is best to evaluate patients individually and taper analgesic doses on the basis of patients’ reports of pain and ability to perform recovery activities rather than a preconceived notion of when epidural analgesics should be discontinued.
In preparation for discharge, transition to oral analgesia should be started as soon as the patient is able to retain fluids and pain is well controlled. As function returns and pain lessens, the epidural opioid dose can be reduced by 25% once or twice daily. To make the transition from epidural analgesia to oral analgesia as smooth as possible, the characteristics of the epidural opioid are considered before discontinuing it. When patients are receiving lipophilic epidural opioids (short duration; e.g., fentanyl, sufentanil), the oral analgesic can be administered before discontinuing epidural analgesia so that patients remain comfortable during the transition. Because analgesia tends to last longer after discontinuing hydrophilic epidural opioids (e.g., morphine, hydromorphone), these patients can be informed of the availability of oral analgesia and reminded to ask for it as soon as they feel pain return and before it becomes severe. Many nurses encourage patients to take their oral analgesic before sleep on the evening that epidural analgesia is discontinued to prevent them from waking up in severe pain. In all cases, patients should be comfortable before epidural analgesia is discontinued. Frequent pain assessment (every 1 to 2 hours) during the transition provides an opportunity to evaluate and adjust the new analgesic regimen. (See Chapter 18 for patient examples that involve switching from the epidural route to the IV route of administration and from the oral route to the epidural route of administration in opioid tolerant patients.)
Epidural Catheter Removal
Removing short-term epidural catheters is within the scope of practice for registered nurses in most states in the United States. Most state boards of nursing have approved this activity by registered nurses who possess the knowledge and skill to do so and where institutional policy and procedure support it. The ASPMN issued a consensus paper defining and supporting the nurse’s role in the care of the patient receiving analgesia by catheter techniques, which includes the discontinuation and removal of catheters used to deliver analgesia (Pasero, Eksterowicz, Primeau, et al., 2007). The procedure for removing epidural catheters varies, but Box 15-7 lists important steps and considerations that can be generalized to all situations. See also Table 15-8 before performing intraspinal catheter removal.
Conclusion
Intraspinal analgesia is an extremely effective method for managing a variety of types of pain. It is a primary method for some types of postoperative pain and may provide relief for some patients with refractory persistent pain. This chapter discussed the many different agents administered intraspinally as well as their most common adverse effects and the complications of the therapy.