Initiating Opioid Therapy
Selecting an Analgesic and Route of Administration
Identifying the Need for an Opioid Dose Increase
Titration in Patients with Cancer Pain
Titration in Patients with Persistent Noncancer Pain
Titration in Patients with Severe Acute Pain
INITIATION of the opioid treatment plan is individualized to meet each patient’s unique characteristics and condition. After the opioid and route of administration are determined, multiple factors, including the patient’s age, previous exposure to opioids, and pain intensity are considered in selection of the initial dose. The initial dose is then titrated up or down based on the patient’s response (pain relief and adverse effects).
Selecting an Analgesic and Route of Administration
As a rule, treatment of moderate-to-severe cancer pain and persistent noncancer pain is started with just one opioid analgesic at a time by one route of administration. For example, the same drug and route are usually used for the short-acting opioid for breakthrough pain and for the around-the-clock (ATC) long-acting opioid. Starting with one drug and route at a time allows for simpler interpretation of adverse effects, if they occur, and presumably lowers the risk for additive toxicity. Notwithstanding those considerations, there are circumstances that may support the initiation of opioid therapy with more than one drug or route concurrently. For example, the emerging role of the oral transmucosal, rapid-onset fentanyl formulations for breakthrough pain was discussed in Chapter 14; these are often administered with transdermal or oral opioids for persistent pain. With close monitoring, the concomitant administration of a parenteral opioid for breakthrough pain may ensure very rapid onset of relief while a baseline drug is administered orally.
During planned long-term therapy, opioid selection is highly individualized, and treatment is usually initiated with a short-acting opioid, which can be titrated to effective analgesia more rapidly than a long-acting opioid (Fine, Mahajan, McPherson, 2009). As discussed in previous chapters, patients with persistent pain are commonly switched to a long-acting opioid to provide consistent pain relief and improve sleep and function. Initiating an opioid regimen in addition to an adjuvant analgesic is often warranted. For example, it may be necessary to use an opioid initially to control moderate to severe pain associated with a persistent noncancer pain syndrome for which the appropriate mainstay analgesic is an adjuvant that requires gradual titration over days or weeks before becoming effective (see Section V). In this case, it would be inhumane to expect the patient to endure severe pain while the adjuvant analgesic takes effect. As soon as the moderate to severe pain is resolved, the opioid can be tapered and discontinued if thought to be unnecessary to the treatment plan.
In contrast to the planned long-term management of persistent pain, the treatment of acute pain often requires beginning with more than one route and more than one analgesic. This is because there is not enough time to evaluate patients’ responses to one analgesic at a time. Therefore, decisions about postoperative analgesics are made on the basis of research findings and clinical experience. The intensity of pain is anticipated, and multimodal analgesic regimens are planned preoperatively whenever possible. For example, in addition to an ATC oral or intravenous (IV) nonsteroidal anti-inflammatory drug (NSAID) and acetaminophen for some major surgeries, an epidural catheter may be placed preoperatively for continuous infusion of opioid and local anesthetic to control anticipated severe postoperative pain.
Occasionally, two different routes of administration at the same time may be appropriate for postoperative pain. For example, continuous peripheral nerve blockade is often administered in conjunction with oral opioid analgesics or IV patient-controlled analgesia (PCA) (see Chapter 26 for continuous peripheral nerve blockade); however, the practice of using two different parenteral routes of administration at the same time or parenteral and intraspinal routes at the same time to administer opioids must be carried out with caution and appropriate monitoring. In particular, the administration of intramuscular (IM) opioids to opioid-naïve patients receiving IV or intraspinal analgesia can result in excessive sedation and clinically significant respiratory depression (see Chapter 19).
Selecting an Opioid Dose
Traditionally, age and weight have been used to determine opioid dose; however, studies have shown that there is no correlation between weight and analgesic requirements (Burns, Hodsman, McLintock, et al., 1989; Ginsberg, Cohen, Ossey, et al., 1989; Monk, Parker, White, 1990). A study comparing remifentanil pharmacokinetics in 12 obese and 12 lean patients undergoing surgery demonstrated no significant differences between the two groups (Egan, Huizinga, Gupta, et al., 1998). The researchers preferred dosing regimens based on ideal body weight, or lean body mass, as opposed to those based on total body weight.
Age, on the other hand, is a valid consideration (Hanks, Cherny, Fallon, 2004; Keita, Tubach, Maalouli, et al., 2008; Mercadante, Ferrera, Villari, et al., 2006). Starting doses should be adjusted for patients at the extremes of the age spectrum, such as neonates and infants, who have incomplete organ development; and older adults, who have increased sensitivity to drug effects. For both the very young and the very old, initial doses are adjusted downward and a longer interval between doses is anticipated. For example, a common recommendation is to lower the recommended starting dose for adults in older individuals (older than 70 years) by 25% to 50% (American Pain Society [APS], 2003). It is important to remember, however, that numerous factors, including genetics (Argoff, 2010; Chou, Wang, Liu, et al., 2006; Fillingim, 2005; Landau, 2006; Nielsen, Stubhaug, Price, et al., 2008; Pasternak, 2005, 2010); underlying pathology and medical condition (Hanks, Cherny, Fallon, 2004; Soares, Martins, Uchoa, 2003); surgical procedure and incision site (Chang, Dai, Ger, et al., 2006); opioid tolerance (Davis, Johnson, Egan, et al., 2003; Hanks, Cherny, Fallon, 2004; Patanwala, Jarzyna, Miller, et al., 2008; Rozen, DeGaetano, 2006); and pain intensity (Dahmani, Dupont, Mantz, et al., 2001), also contribute to wide variability in pain reports and dose requirements among patients.
Although pain intensity is obviously a very important factor to consider, selecting a dose based on a specific pain intensity can be dangerous and is strongly discouraged (Blumstein, Moore, 2003; Gordon, Dahl, Phillips et al., 2004; Lucas, Vlahos, Ledgerwood, 2007; Vila, Smith, Augustyniak, et al., 2005). The most important principle is to select a safe starting dose and be prepared to titrate to individualize the dose while monitoring patient response. No matter what method is used to predict analgesic requirements, the starting doses of opioid treatments are merely estimates. When starting doses are given, they are titrated up or down according to patient response (Fine, Mahajan, McPherson, 2009).
Using the Equianalgesic Chart
The term equianalgesia means approximately equal analgesia and is used when referring to the doses of various opioid analgesics that provide approximately the same amount of pain relief. Using the equianalgesic chart in Table 16-1 as an example, note that the chart provides a list of analgesics at doses, both oral and parenteral (with other routes such as rectal as appropriate), that are approximately equal to each other and theoretically interchangeable in their ability to provide pain relief in opioid-naïve patients. These doses are also referred to as equianalgesic dose units. Most of the doses in equianalgesic charts are made on the basis of single-dose studies, commonly conducted in surgical patients and using morphine, 10 mg IM, for comparison (Knotkova, Fine, Portenoy, et al., 2009). The parenteral doses listed are typical of IM doses given approximately every 3 to 4 hours. Equianalgesic dose calculation provides a basis for selecting the appropriate starting dose when changing from one opioid drug or route of administration to another. However, these calculations are just estimates and vary with repeated dosing and with opioid rotation (Knotkova, Fine, Portenoy, 2009; Shaheen, Walsh, Lasheen, et al., 2009). The optimal dose for the patient is always determined by titration (Fine, Portenoy, 2007).
Table 16-1
Equianalgesic Dose Chart
A Guide to Using Equianalgesic Dose Charts*

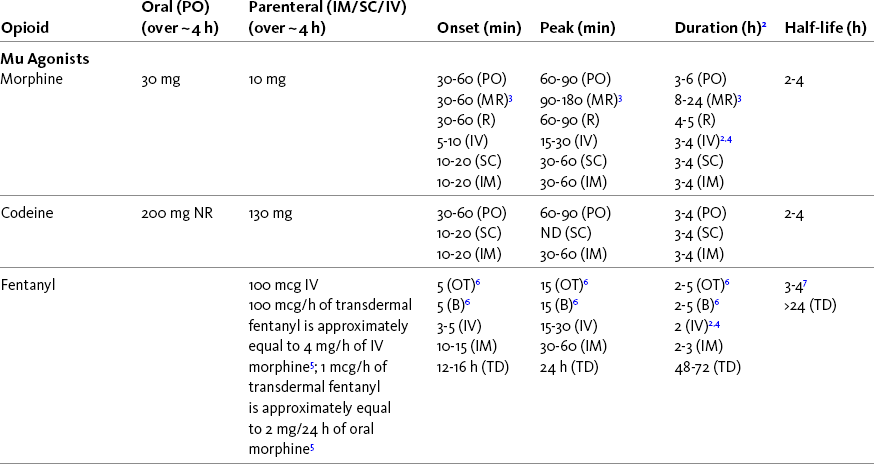
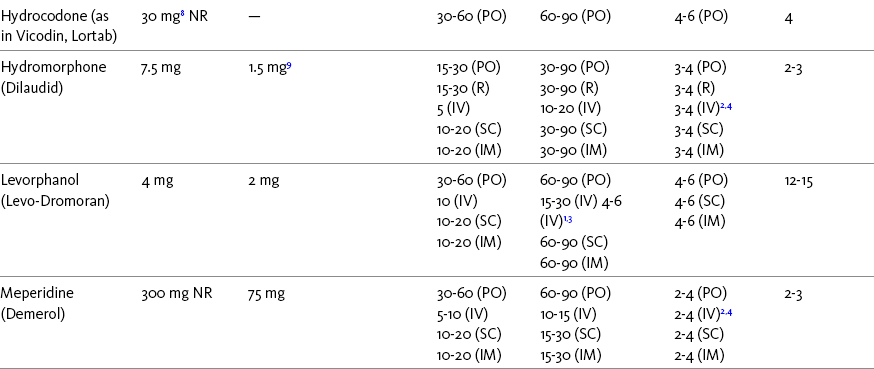
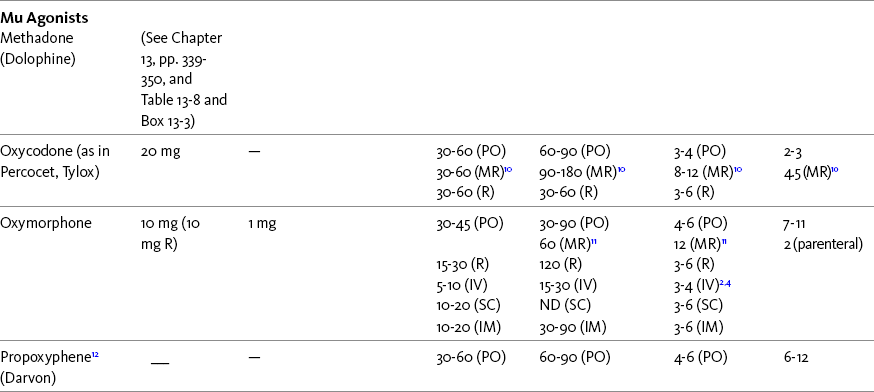
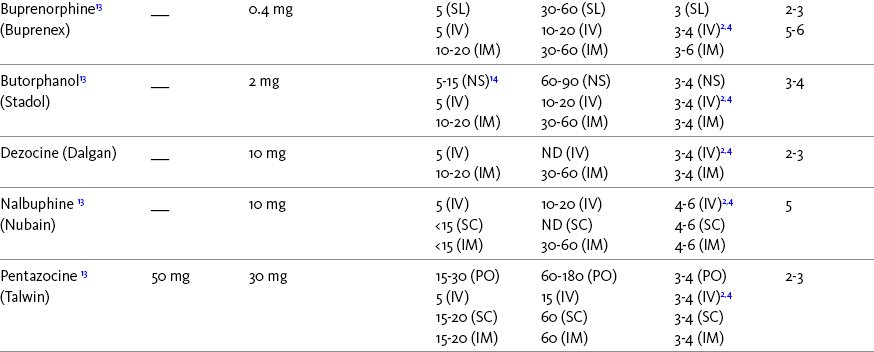
ATC, Around-the-clock; h, hour; IM, intramuscular; IV, intravenous; MR, oral modified-release; ND, no data; NR, not recommended; NS, nasal spray; OT, oral transmucosal; PO, oral; R, rectal; SC, subcutaneous; SL, sublingual; TD, transdermal.
*This table provides equianalgesic doses and pharmacokinetic information about selected opioid drugs. Characteristics and comments about selected mu opioid agonist drugs can be found in Table 13-1, pp. 326-327.
1An expert panel was convened for the purpose of establishing a new guideline for opioid rotation and recently proposed a two-step approach (Fine, Portenoy, Ad Hoc Expert Panel on Evidence Review and Guidelines for Opioid Rotation, 2009). The approach presented in the text for calculating the dose of a new opioid can be conceptualized as the panel’s Step One, which directs clinicians to calculate the equianalgesic dose of the new opioid based on the equianalgesic table. Step Two suggests that clinicians perform a second assessment of patients to evaluate the current pain severity (perhaps suggesting that the calculated dose be increased or decreased) and to develop strategies for assessing and titrating the dose as well as to determine the need for breakthrough doses and calculate those doses (see Box 16-2). The specific steps provided in the examples in the text reflect the panel’s two-step approach (see Fine, Portenoy, the Ad Hoc Expert Panel on Evidence Review and Guidelines for Opioid Rotation, 2009).
2Duration of analgesia is dose dependent; the higher the dose, usually the longer the duration.
3As in, e.g., MS Contin and Oramorph (8 to 12 hours) and Avinza and Kadian (12 to 24 hours).
4IV boluses may be used to produce analgesia that lasts nearly as long as IM or SC doses; however, of all routes of administration, IV produces the highest peak concentration of the drug, and the peak concentration is associated with the highest level of toxicity (e.g., sedation). To decrease the peak effect and lower the level of toxicity, IV boluses may be administered more slowly (e.g., 10 mg of morphine over a 15-min period); or smaller doses may be administered more often (e.g., 5 mg of morphine every 1 to 1.5 hours).
5This is the ratio that is used clinically.
6The delivery system for transmucosal fentanyl influences potency, e.g., buccal fentanyl is approximately twice as potent as oral transmucosal fentanyl (see Chapter 14).
7At steady state, slow release of fentanyl from storage in tissues can result in a prolonged half-life (e.g., 4 to 5 times longer).
8Equianalgesic data are not available.
9The recommendation that 1.5 mg of parenteral hydromorphone is approximately equal to 10 mg of parenteral morphine is based on single-dose studies. With repeated dosing of hydromorphone (as during PCA), it is more likely that 2 to 3 mg of parenteral hydromorphone is equal to 10 mg of parenteral morphine (see Chapter 13).
10As in, e.g., OxyContin.
11As in Opana ER.
1265 to 130 mg = approximately 1/6 of all doses listed in this chart.
13Used in combination with mu agonist opioids, this drug may reverse analgesia and precipitate withdrawal in opioid-dependent patients.
14In opioid-naive patients who are taking occasional mu agonist opioids, such as hydrocodone or oxycodone, the addition of butorphanol nasal spray may provide additive analgesia. However, in opioid-tolerant patients such as those receiving ATC morphine, the addition of butorphanol nasal spray should be avoided because it may reverse analgesia and precipitate withdrawal.
From Pasero, C., & McCaffery, M. Pain assessment and pharmacologic management, pp. 444-446, St. Louis, Mosby. Data from American Pain Society (APS). (2003). Principles of analgesic use in the treatment of acute pain and chronic cancer pain. Glenview, IL, APS; Breitbart, W., Chandler, S., Eagel, B., et al. (2000). An alternative algorithm for dosing transdermal fentanyl for cancer-related pain. Oncology, 14(5), 695-705. See discussion in same issue, pp. 705, 709-710, 712, 17; Coda, B. A., Tanaka, A., Jacobson, R. C., et al. (1997). Hydromorphone analgesia after intravenous bolus administration. Pain, 71(1), 41-48; Donner, B., Zenz, M., Tryba, M., et al. (1996). Direct conversion from oral morphine to transdermal fentanyl: A multicenter study in patients with cancer pain. Pain, 64(3), 527-534; Dunbar, P. J., Chapman, C. R., Buckley, F. P., et al. (1996). Clinical analgesic equivalence for morphine and hydromorphone with prolonged PCA. Pain, 68, 226-270; Fine, P. G., Portenoy, R. K., & Ad Hoc Expert Panel on Evidence Review and Guidelines for Opioid Rotation. (2009). Establishing best practices for opioid rotation: Conclusions of an expert panel. J Pain Symptom Manage, 38(3), 418-425; Gutstein, H. B., & Akil, H. (2006). Opioid analgesics. In L. L. Brunton, J. S. Lazo, & K. L. Parker (Eds.), Goodman & Gilman’s The pharmacological basis of therapeutics, ed 11, New York, McGraw-Hill; Hanks, G., Cherny, N. I., Fallon, M. (2004). Opioid analgesic therapy. In D. Doyle, G. Hanks, N. I. Cherny, et al (Eds.), Oxford textbook of palliative medicine, ed 3, New York, Oxford Press; Johnson, R. E., Fudala, P. J., & Payne, R. (2005). Buprenorphine: Considerations for pain management. J Pain Symptom Manage, 29(3), 297-326; Kaiko, R. F., Lacouture, P., Hopf, K., et al. (1996). Analgesic onset and potency of oral controlled release (CR) oxycodone CR and morphine. Clin Pharmacol Ther, 59(2), 130-133; Knotkova, H., Fine, P. G., & Portenoy, R. K. (2009). Opioid rotation: The science and limitations of the equianalgesic dose table. J Pain Symptom Manage, 38(3), 426-439; Lawlor, P., Turner, K., Hanson, J., et al. (1997). Dose ratio between morphine and hydromorphone in patients with cancer pain: A retrospective study. Pain, 72(1, 2), 79-85; Manfredi, P. L., Borsook, D., Chandler, S. W., et al. (1997). Intravenous methadone for cancer pain unrelieved by morphine and hydromorphone: Clinical observations. Pain, 70, 99-101; Portenoy, R. K. (1996). Opioid analgesics. In R. K. Portenoy, & R. M. Kanner (Eds.), Pain management: Theory and practice, Philadelphia, FA Davis; Sittl, R., Likar, R., & Nautrup, B. P. (2005). Equipotent doses of transdermal fentanyl and transdermal buprenorphine in patients with cancer and noncancer pain: Results of a retrospective cohort study. Clin Ther, 27(2), 225-237; Skaer, T. L. (2004). Practice guidelines for transdermal opioids in malignant pain. Drugs, 64(23), 2629-2638; Skaer, T. L. (2006). Transdermal opioids for cancer pain. Health Qual Life Outcomes, 4, 24; Vogelsang, J., & Hayes, S. R. (1991). Butorphanol tartrate (Stadol). A review. J Post Anesthes Nurs, 6(2), 129-135; Weinberg, D. S., Inturrisi, C. E., Reidenberg, B., et al. (1988). Sublingual absorption of selected opioid analgesics. Clin Pharmacol Ther, 44, 335-342; Wilson, J. M., Cohen, R. I., Kezer, E. A., et al. (1995). Single and multiple-dose pharmacokinetics of dezocine in patients with acute and chronic pain. J Clin Pharmacol, 35, 395-403. Pasero C, McCaffery M. May be duplicated for use in clinical practice.
A patient’s pain intensity (see previous discussion) and the equianalgesic chart are practical tools that can be used to determine an appropriate opioid dose for an opioid-naïve patient. To become familiar with the equianalgesic chart, note that it has several columns. The first column lists the common opioid analgesics. Morphine is listed first because it has been the standard for comparison, and the others follow in alphabetical order. The remaining doses are equianalgesic to the doses listed for morphine. The second column lists the equianalgesic doses of opioids by the oral route. The third column lists the equianalgesic doses of opioids by the parenteral (IM, subcutaneous [SC], and IV) routes. The last four columns provide pharmacokinetic information about specific opioids.
All of the opioid doses listed in the equianalgesic chart were developed in controlled trials that were conducted in opioid-naïve postoperative patients or in cancer patients with little or no prior exposure to opioids. For this reason, they may be considered a starting point for determining appropriate initial doses given about every 4 hours for opioid-naïve adults with severe pain. Percentages of these doses are used to determine the appropriate starting dose for moderate and mild pain, pain in patients at the extremes of age, and pain in patients who are medically frail or otherwise predisposed to the adverse effects of opioids. For example, the European Association for Palliative Care (EAPC) has recommended a starting dose of 5 mg of short-acting oral morphine every 4 hours in opioid-naïve patients and 10 mg in patients already being treated with “weak” opioids (Hanks, De Conno, Cherny, et al., 2001). This recommendation is more conservative than the dose that would be calculated from the equianalgesic dose table, but it recognizes the medical frailty of patients with advanced illness.
In contrast to the patient who is medically compromised, a healthy patient with severe pain who is to receive parenteral morphine might be prescribed as much as 10 mg of morphine parenterally every 4 hours. If, instead of severe pain, the patient has moderate pain, the starting dose would be 50% of 10 mg (5 mg) of morphine, and for mild pain, 25% of 10 mg (2.5 mg) (Box 16-1). When administered by the IV route, the total dose is given in smaller boluses over the 4-hour interval. Bolus doses over a 4-hour period are usually calculated by dividing the 4-hour dose by 4. In other words, the bolus dose is one-fourth of the 4-hour dose.
As discussed earlier in this section, mild pain may not require an opioid. A nonopioid, such as ibuprofen or acetaminophen, may be appropriate. If an opioid is indicated, mild pain can be treated with a low dose of any of the opioids or with an opioid-nonopioid combination, such as hydrocodone or oxycodone compounded with acetaminophen. In opioid-tolerant patients, these doses may be used as starting doses with the awareness that the dose will probably have to be titrated upward quickly.
As mentioned, the doses in the equianalgesic chart are made on the basis of a 4-hour dosing schedule. To determine the appropriate starting dose when the dosing schedule is other than every 4 hours, different calculations are necessary.
Continuous Infusions
In patients with cancer pain and in opioid-naive postoperative or trauma patients in the intensive care unit (ICU) who require parenteral opioids, a maintenance continuous infusion is initiated after pain is controlled by IV or SC boluses (Coyle, Cherny, Portenoy, 1995). As mentioned, additional boluses offered every 15 to 30 minutes may be prescribed for the management of breakthrough pain (see Breakthrough Doses, discussed next, and Chapter 17 for continuous infusions in opioid-naïve patients outside of the ICU).
Breakthrough Doses
The term breakthrough dose is used interchangeably with the terms supplemental dose or rescue dose. It is now conventional practice to offer all patients with pain related to active cancer or other serious illnesses who are receiving ATC opioid analgesics access to doses of a fast-acting mu agonist opioid analgesic to treat breakthrough pain (see Chapter 12 for a detailed discussion of breakthrough pain). The use of these short-acting supplemental doses for breakthrough pain should not be considered conventional practice in the large and heterogeneous population with persistent noncancer pain. In this population, the decision to provide such a treatment should be made after a careful risk-to-benefit analysis that assesses both the risk of pharmacologic adverse effects such as peak concentration sedation and the risk of problematic drug-related behaviors that may be consistent with abuse or addiction. Well-controlled research is needed to inform decisions on breakthrough pain in this diverse population (Devulder, Jacobs, Richarz, et al., 2009; Fine, Portenoy, 2007).
For patients taking oral opioids, the recommended amount of opioid for breakthrough doses is within a range of approximately 5% to 15% of the total daily dose of the ATC opioid analgesic; however, some clinicians prefer to use 10% to 15% unless there are circumstances that suggest a conservative calculation (5%) should be made, for example, in the case of a frail patient or a patient older than 70 years (Box 16-2). This calculation is used consistently, regardless of the total daily dose of opioid. For example, the range of a given breakthrough dose for a patient receiving a total daily dose of 8000 mg of modified-release morphine would be 800 mg (10%) to 1200 mg (15%).
The recommended breakthrough dose for patients receiving continuous parenteral opioid infusions is 25% to 50% of the hourly opioid dose (see Box 16-2). (Although 5% to 15% of the 24-hour dose can be used to calculate breakthrough doses for parenteral opioid treatment, hourly percentages are simpler when using continuous parenteral opioid infusions, and the results are essentially the same.) The total number of parenteral breakthrough doses given in 1 hour may be equal to but ordinarily do not exceed the hourly opioid dose. The need for breakthrough doses greater than the hourly opioid dose indicates a need to increase the hourly opioid dose. For example, an opioid-tolerant patient receiving 6 mg/h of IV morphine could be given a 3-mg breakthrough dose every 30 minutes but ordinarily should not repeatedly be given more than two 3-mg boluses in 1 hour. If more than two boluses are required, the continuous infusion should be increased.
Dose Titration
Titration of the opioid dose usually is required at the beginning of therapy and repeatedly during the course of treatment (Vascello, McQuillan, 2006). Whereas patients with cancer pain most often are titrated upward over time for progressive pain, patients with acute pain, particularly postoperative pain, are eventually titrated downward as pain resolves.
As discussed, considerable variation exists in the amount of opioid individuals require for comfort (APS, 2003). For example, research has established that as much as a tenfold difference exists among patients in opioid requirements during the postoperative period (Myles, 2004). Even greater differences are noted in patients with persistent pain (Hanks, Cherny, Fallon, 2004). This wide variability reinforces the need for prompt and individualized attention to unrelieved pain. At all times, inadequate pain relief is addressed by gradual escalation of the opioid dose until adequate analgesia is reported, or until intolerable and unmanageable adverse effects occur. The absolute opioid dose is unimportant as long as the balance between pain relief and adverse effects is favorable (Hanks, Cherny, Fallon, 2004). The goal of titration is to use the smallest dose that provides satisfactory pain relief with the fewest adverse effects. Clinicians new to titrating opioid doses in opioid-tolerant patients are often uncomfortable with the high doses sometimes required; seeking the assistance of a clinician experienced with providing opioids for opioid-tolerant patients may be helpful.
Identifying the Need for an Opioid Dose Increase
The first sign that an increase in opioid dose is needed is most commonly a decrease in the duration of analgesia for a given opioid dose. For example, patients receiving IV PCA may repeatedly attempt to self-administer PCA doses before the programmed lockout (delay) interval elapses (see Chapter 17), or patients taking a modified-release opioid may report breakthrough pain occurring invariably toward the end of the continuous analgesic dosing interval, such as in the eleventh hour of a 12-hour dosing schedule. Patients also may report the need for an increased number of breakthrough doses. As a rule of thumb, two or more breakthrough doses during a 12-hour period (four to six daily) should alert the clinician that the opioid regimen for cancer or persistent noncancer pain needs to be re-evaluated. Six doses a day approximates every-4-hour dosing and defeats the purpose of using modified-release formulations.
When an increase in the opioid dose is necessary, it can be done by percentages. When a slight improvement in analgesia is needed, a 25% increase in the total daily opioid dose may be sufficient; for a moderate effect, a 50% increase, and for a strong effect, such as for the treatment of severe pain, a 100% increase may be indicated. The time at which the dose should be increased is typically determined by considering the onset or peak effect of the opioid. For example, titration of IV opioid doses may occur as often as every 5 to 15 minutes (depending on the lipid solubility of the drug) (see the following patient example), whereas titration of oral modified-release opioids may occur every 24 to 48 hours. Increases in the ATC analgesia may need to be accompanied by proportional increases in the breakthrough dose, so that the size of breakthrough doses remains an effective percentage of the fixed dose. Whenever the dose of the baseline opioid is increased, the efficacy of the breakthrough pain dose should be re-evaluated and adjusted as needed (see Box 16-2).
Another method of increasing opioid doses is possible when patients are receiving ATC opioids and taking breakthrough doses. If the combination of the ATC dose and the breakthrough doses provide satisfactory pain relief, the ATC dose can be increased to the amount of opioid provided by the current ATC dose plus breakthrough doses. This may allow elimination or a considerable decrease in the number of breakthrough doses required (see the following patient examples).
Patients should be involved in the decision to increase the opioid dose. Valuable information is obtained by asking patients to describe the patterns of pain they are experiencing. For example, patients commonly take more breakthrough doses during the times when they are active than when they are resting. Patients with cancer pain or persistent noncancer pain who work or are particularly active frequently take more than 2 breakthrough doses during a 12-hour period. Many patients would prefer to administer additional doses during these periods of activity rather than risk increased sedation that can accompany an increase in the ATC opioid dose. Some postoperative patients receiving PCA prefer less than complete pain relief rather than risk nausea with an increased dose.
Inadequate Pain Relief During Analgesic Infusion Therapies
When patients report inadequate pain relief during analgesic infusion therapies, such as IV PCA or epidural analgesia, the entire infusion system, from the infusion pump to the IV site or epidural catheter site, should be checked. Inadequate pain relief may be caused by a number of mechanical and technical factors, including incorrect loading of the pump, disconnection of the catheter from the infusion pump tubing, an empty drug reservoir, a disconnected PCA button, or a malfunctioning pump or tubing. In patients receiving epidural analgesia, the epidural catheter may have been inadvertently pulled out.
When bolus doses and increases in the epidural analgesic dose do not yield satisfactory pain control or produce patchy (e.g., one-sided) analgesia, and the epidural catheter appears to be in place, the infusion line connected, and the infusion pump infusing correctly, the anesthesia provider or pain service is notified. The epidural catheter can be checked for optimal location by administering a concentrated dose of local anesthetic through the catheter. Optimal catheter placement would produce a bilateral sensory block of the desired dermatomes; lack of such a block would indicate that the catheter location is not optimal. If location is less than optimal, the epidural catheter should be removed and alternatives considered (see Chapter 15 for more on epidural analgesia).
Titration in Patients with Cancer Pain
As noted previously, the conventional approaches to titration of the opioid dose involve either an increase by percentages or an increase based on the usage of breakthrough pain medication during the past day or more. The typical percentage increase is 25% to 50% (Hanks, Cherny, Fallon, 2004), with the proviso that some patients with severe pain should be considered for a larger increase, as much as 100% if the pain is severe and the increment appears to be safe. The approach that involves summing the medication used for breakthrough pain and increasing the baseline dose by an equivalent amount has the advantage of a high likelihood that the increment is safe, given that the patient has taken a comparable amount prior to the change. The EAPC recommends a modification of the latter approach as the simplest method, suggesting that a short-acting opioid analgesic be administered every 4 hours and the same dose offered as needed for breakthrough pain as often as every hour (Hanks, De Conno, Chery, et al., 2001). The mainstay opioid dose can then be increased based on the amount of breakthrough medication the patient requires.
The provision of appropriate starting doses may influence the need for dose titration in some cancer patients. The EAPC recommends a starting dose of 5 mg of short-acting oral morphine every 4 hours in opioid-naïve patients and 10 mg in patients already being treated with “weak” opioids (Hanks, De Conno, Cherny, et al., 2001) (see Chapter 12, WHO Ladder). A multicenter study enrolled 159 consecutive patients with cancer pain and established that these dosing recommendations resulted in significant pain reduction and the need for minimal dose escalation (Ripamonti, Campa, Fagnoni, et al., 2009). The presence of neuropathic pain was associated with the need for higher opioid doses. Similarly, 40 cancer patients with uncontrolled pain were converted from nonopioid-opioid analgesics to oral short-acting morphine administered on a fixed schedule, and they achieved adequate pain control within 2.3 days; patients were then converted to an equivalent dose of modified-release morphine (Klepstad, Kaasa, Skauge, et al., 2000). The mean daily morphine dose during titration was 97 mg, adverse effects were essentially unaltered, and 82% were satisfied or very satisfied with treatment. A later study by these same researchers randomized 40 patients with uncontrolled cancer pain to titration of short-acting oral morphine given every 4 hours or titration of a modified-release morphine (Kapanol, Kadian) given once daily (Klepstad, Kaasa, Jystad, et al., 2003). The mean time to achieve adequate pain control was 2.1 days with short-acting morphine and 1.7 days with modified-release morphine. Those taking the latter reported feeling less tired at the end of titration. No other differences in adverse effects, health-related quality-of-life functions, or satisfaction with treatment were noted.
As discussed, the starting dose should be reduced in older patients. An interesting study of opioid titration in cancer patients found that although older patients required lower doses, opioid effects—as evaluated by dose escalation, number of opioids, or routes of administration to obtain a balance between analgesia and adverse effects—did not differ among age groups (Mercadante, Ferrera, Villari, et al., 2006). The researchers cautioned against labeling older patients as more responsive to opioids, either for analgesia or adverse effects, and emphasized the need for careful, individualized titration in this population, as is recommended in all patients.
Cancer Pain Crisis
The concept of pain emergency is poorly defined in the literature but is useful clinically. Some patients with cancer pain experience severe, and usually increasing, pain associated with high levels of distress and, in some cases, autonomic changes, such as tachycardia and hypertension. At some threshold, the treatment of these patients should be considered an emergency (Miaskowski, Cleary, Burney, et al., 2005; Soares, Martins, Uchoa, 2003). This designation may justify admission to the hospital and usually provides a rationale for a switch to an IV opioid. Although the level of pain can be managed with oral or SC opioid titration (Klepstad, Kaasa, Jystad, et al., 2003; Klepstad, Kaasa, Skauge, et al., 2000), IV opioids have advantages: much faster peak effects occur (e.g., 15 minutes for morphine); repeated doses can be given more frequently; and analgesia is achieved more rapidly (Miaskowski, Cleary, Burney, et al., 2005). In patients who are already taking an opioid, the APS recommends a dose that is 10% to 20% of the total daily dose of their current opioid regimens (Miaskowski, Cleary, Burney, et al., 2005) (see Chapter 18 for conversion from oral to IV route). If the patient is not taking an opioid, management of severe pain should begin with 2 to 5 mg of IV morphine or an equivalent short-acting opioid (again, less than the dose indicated on the equianalgesic dose table in recognition of the medical co-morbidities common in patients with cancer).
After the initial bolus, pain should be reassessed in 5 to 20 minutes and the same dose repeated. If repeated doses are necessary, and there appears to be no somnolence or other adverse effects, the size of the bolus can be increased, usually by 25% to 50%. Repeated boluses continue until pain is reduced by at least 50% or unmanageable and intolerable adverse effects occur.
The principle underlying this repeated-bolus approach to the management of severe pain is to repeat the dose after enough time has elapsed to observe the peak effect of the prior dose. If the oral route is used for rapid titration, the dose may be repeated in 60 to 90 minutes, and if the SC route is used, the interval typically is approximately 45 minutes.
When IV morphine is used to deliver repeated bolus injections, it is best to wait for 15 to 20 minutes between doses because this period is required to observe the peak effect. Shorter intervals can be used, but they require careful monitoring because of the risk for overshooting when additional injections are given prior to the peak effects of those that were administered earlier. IV morphine doses administered at 2-minute intervals achieved pain control in a mean time of 9.7 minutes in 45 patients admitted to an inpatient palliative care unit for severe cancer pain (Mercadante, Villari, Ferrera, et al., 2002). Patients’ effective doses were converted to oral morphine doses, which were initiated 4 hours after IV titration had been completed. This rapid conversion to oral morphine allowed early discharge back to the home setting.
For acute severe pain in patients with advanced cancer who are relatively opioid-naïve, Davis (2004) recommends IV treatment with 1 mg morphine (20 mcg fentanyl or 0.2 mg of hydromorphone may be substituted), repeated every 5 minutes until (1) the initial onset of analgesia; (2) opioid toxicity; or (3) a total of 30 mg of morphine (or the equivalent) over 45 minutes. This is followed by parenteral-maintenance morphine at one quarter of the titrated loading dose infused per hour; the breakthrough dose should equal the hourly dose. To calculate an oral morphine maintenance dose, the total effective titrated IV dose is calculated as the 4-hourly parenteral dose of morphine, then multiplied by 3. For example, if a 10-mg bolus morphine dose was necessary to achieve onset of analgesia, the conversion would be 30 mg of short-acting oral morphine every 4 hours or the equivalent dose in modified-release formulation (Davis, 2004).
In contrast to morphine, which is hydrophilic and requires many minutes to cross the blood-brain barrier and yield peak effects, more lipophilic opioids cross into the brain very quickly and produce peak effects almost immediately. IV fentanyl was used successfully to provide rapid analgesia for severe cancer pain in 18 patients admitted to the emergency department (ED) (Soares, Martins, Uchoa, 2003). The patients’ oral morphine doses were converted to IV morphine equivalents using a 3:1 ratio (average daily oral morphine dose was 276 mg). This amount was then converted to IV fentanyl using a ratio of 1:100. An IV fentanyl bolus dose equivalent to 10% of the total IV morphine daily dose taken in the previous 24 hours was administered and repeated every 5 minutes, with a 50% increase in dose if necessary to reduce patients’ pain intensity score to less than 4/10. Vital signs were monitored during titration and for 6 hours afterward. All 18 patients achieved pain control in an average of 11 minutes without significant adverse effects, although 5 patients experienced slight sedation. The mean dose of IV fentanyl required for pain control was 214 mcg.
Continuous IV infusions are also used to treat pain crises. This can be accomplished by converting the existing dose into the infusion rate and adding breakthrough doses equal to 50% to 200% of the hourly infusion rate available every 15 minutes as needed (Miaskowski, Cleary, Burney, et al., 2005).
These strategies for managing cancer pain crises require rapid decision making and sometimes very large opioid doses. If the clinician is not familiar with this approach, it may be advisable to contact another clinician or a prescriber who has had experience with these situations and can either demonstrate the strategy in person or be available by telephone to offer assurance and advice.
Fear of causing opioid-induced respiratory depression can pose a barrier to the use of aggressive titration, which led researchers to evaluate changes in respiratory parameters during IV opioid (morphine, hydromorphone, or fentanyl in various doses) titration in 25 opioid-tolerant and 5 opioid-naïve patients with severe uncontrolled cancer pain (Estfan, Mahmoud, Shaheen, et al., 2007). Several of the patients had compromised respiratory function resulting from a variety of sources, but none were receiving oxygen supplementation. Oxygen saturation remained above 92% during IV titration, and transcutaneous carbon dioxide levels never exceeded 50 mm Hg in any of the patients. Two opioid-naive patients experienced transient, self-resolving decreases in respiratory rate to 8 to 9 breaths/minute. No patient required arterial blood gas evaluation due to hypercapnia or hypoxia. Mean pain scores were reduced from 6.8 to 1.9.
After pain has been controlled by aggressive titration, a thorough evaluation of the underlying causes of acute or sudden worsening of pain is recommended (Davis, 2004). Such events may indicate complications, such as bone fractures, hemorrhage into tumor, infarction thrombosis, obstructed viscus, nerve compression, inflammation, or infection.
Titration in Patients with Persistent Noncancer Pain
There is a lack of research regarding opioid titration in patients with persistent noncancer pain. A retrospective study of 206 patients experiencing a variety of types of noncancer pain, including neuropathic pain, found that older patients, regardless of type of pain, demonstrated the need for increased opioid doses at significantly lower rates (two times) than younger patients over the 2-year study period (Buntin-Mushock, Phillip, Moriyana, et al., 2005). A slower rate of dose escalation was required in patients with neuropathic pain than in patients who had nociceptive pain. Portenoy and colleagues (2007) studied patients taking modified-release oxycodone for a variety of types of noncancer pain and reported a modest need for dose escalation during the 3-year study period.
Titration in Patients with Severe Acute Pain
Providing effective pain control while minimizing opioid-induced adverse effects presents special challenges for clinicians who work in outpatient surgery settings, postanesthesia care units (PACUs), and ICUs, because they must deal also with the additional central nervous system (CNS) depression caused by the sedative and anesthetic agents that are administered intraoperatively and sometimes throughout care in the ICU. Rapid analgesia must be provided to patients who are in a nonsteady state, and that adds to the complexity of titration (Berde, Brennan, Raja, 2003). Furthermore, many of these patients are opioid-naïve, which places them at greater risk for adverse opioid-induced effects, particularly excessive sedation and respiratory depression.
Less difficulty is experienced when a multimodality approach is used in patients with acute pain (Pasero, 2003; Pasero, McCaffery, 2007). As discussed earlier in this section, combinations of analgesics improve pain relief with lower analgesic doses; lower doses result in fewer adverse effects. If an NSAID was not given preoperatively and is not relatively contraindicated, it can be started as soon as the patient reaches the PACU or ICU; epidural opioids are usually combined with long-acting local anesthetics to reduce the opioid dose. Acetaminophen may be added to any treatment plan (see Section III).
Before a trauma or postoperative patient becomes oriented enough to provide self-reports of pain, the nurse should assume that pain is present by the fact that sufficient noxious stimuli are present (see Chapter 20 for discussion of opioid use and dose selection in critically ill patients who cannot report pain). As soon as it is safe to do so, an IV bolus of an opioid can be administered and then repeated as described previously to titrate rapidly against adverse effects (e.g., 2 to 3 mg morphine every 10 to 15 minutes [see subsequent material]). Patients must be observed closely for adverse effects, particularly sedation and respiratory depression (see Chapter 19) (Aubrun, Monsel, Langeron, et al., 2001; Lovovschi, Aubrun, Bonnet, et al., 2008). Epidural bolus doses may be given for uncontrolled pain in patients who are receiving epidural analgesia. The presence of other signs of pain, such as moaning, facial grimacing, or elevated vital signs, may be considered in the assessment of pain, but it is important that clinicians remember that their absence does not necessarily mean the absence of pain. Do not assume that sleeping means adequate pain control (see Section II).
Opioid Selection for Initial Titration in Patients with Acute Pain
The mu agonist opioids—morphine, hydromorphone, and fentanyl—are most commonly used for initial titration in patients with severe acute pain, but nurses often ask which one is the best choice. Important patient characteristics to consider when selecting an opioid for titration are discussed in detail in Chapter 13 and include, for example, previous exposure to and tolerance of opioids, current organ function, and hemodynamic stability. For example, fentanyl is favored in patients with any type of end-organ failure. It also produces minimal hemodynamic effects, which adds to its appeal for patients with unstable blood pressure.
In addition to patient characteristics, the pharmacokinetics of the opioids and the goals of treatment are considered when deciding which opioid is best for titration in particular patients. As discussed, morphine is hydrophilic and requires several minutes to cross the blood-brain barrier and yield peak effects after IV administration; the more lipophilic opioids such as fentanyl cross into the brain very quickly and produce peak effects almost immediately when given intravenously. Hydromorphone is less hydrophilic than morphine so has an intermediate effect. These pharmacokinetics help to explain why fentanyl is often selected when the goal is to control severe, rapidly escalating pain quickly (e.g., severe pain on admission to the ED or PACU) (see speed of injection, later in this chapter). Although IV fentanyl’s short duration is an advantage when short patient stays are expected, it can be a drawback when pain is expected to be continuous. For example, fentanyl tends to be a first-choice opioid for procedural pain and is a logical selection in an ambulatory surgery PACU where the goal is to transition patients quickly to the oral analgesic that the patients will take after discharge. However, research has shown that the use of fentanyl does not result in faster discharge times because patients require additional analgesia to control pain (Claxton, McGuire, Chung, et al., 1997). In addition, frequent dosing is necessary when fentanyl is used for ongoing continuous pain. For example, a short lockout (delay) interval (e.g., 5 minutes) and a basal rate are often prescribed when fentanyl is used for IV PCA; however, the lockout interval is 8 to 10 minutes when morphine is used for acute pain, and it is often administered without a basal rate (see Chapter 17).
For patients who have undergone major surgery, some PACU nurses like to administer a few doses of fentanyl, then follow with either hydromorphone or morphine for longer lasting analgesia. However, although it makes sense to use a fast-onset opioid such as fentanyl in patients presenting with severe, escalating pain, it may not be necessary and can complicate the assessment process in those with less severe pain; when opioids are combined and adverse effects occur, it is difficult to interpret which one might be the culprit. Therefore, a general principle of initial titration in patients with acute pain is to keep in mind the patients’ ongoing pain treatment plan. As an example, consider the patient who is admitted to the PACU and will have hydromorphone IV PCA for ongoing postoperative pain management. Unless the patient has severe, rapidly escalating pain on admission, it makes sense to begin titration with hydromorphone so that the effects (both pain relief and adverse effects) of the drug that will be used for the next day or so can be evaluated more easily. In all cases, the time it takes an opioid to reach its analgesic action site is always considered when determining dose and how often to administer it during titration; adequate time must be allowed to assess response to one dose before administering another (Loetsch, Dudziak, Freynhagen, et al., 2006).
Titration Protocols in the Emergency Department
Some studies have shown that standard practices related to titration in patients with acute pain may lead to undertreated pain. The use of adequate starting doses in the titration protocols appears to be critical. For example, although weight is not recommended for the calculation of opioid doses in adults, most guidelines for titration in patients with severe pain in the ED setting call for a starting dose based on weight; i.e., 0.1 mg/kg of IV morphine (approximately 7 mg in a 150-lb person) followed by titration until adequate pain relief is achieved (Ungar, Brandes Reinoehi, et al., 1999); however, a prospective study of 119 patients showed that this starting dose resulted in 67% of the patients’ experiencing less than 50% pain relief within 30 minutes; no patient required an opioid antagonist (Bijur, Kenny, Gallagher, 2005). A later randomized controlled study (N = 280) found that 0.15 mg/kg (approximately 10 mg in a 150-lb person) provided pain relief superior to 0.1 mg/kg in the ED (Birnbaum, Esses, Bijur, et al., 2007). This higher dose was supported in another study of 621 patients with severe pain in the ED; 3-mg increment doses of IV morphine (2 mg in older patients) were administered every 5 minutes to comfort or sleep and resulted in adequate pain relief in 82% of the patients (Lovovschi, Aubrun, Bonnet, et al., 2008). The mean morphine dose administered was 10.5 mg (0.16 mg/kg); the median time of titration was 15 minutes; and the median number of boluses was 3. Nausea and vomiting were the most common adverse effects (4.2%), and mild respiratory depression (slight decreases in respiratory rates and oxygen saturations) occurred in 2.6%. These researchers stressed the need to use flexible rather than fixed (mg/kg) dosing during titration. Although no serious adverse events were reported, it was unclear if sleep might have actually been excessive sedation in this study. It is important to remember that sedation can occur before pain is completely relieved and that sleep during opioid titration is usually not normal sleep but primarily the result of the sedative effects of the opioid (see Chapter 19). This type of rapid dosing always carries the risk for excessive sedation and respiratory depression; these parameters must be watched closely during titration and for at least 3 hours after the peak of the last dose administered (APS, 2003) (see Chapter 19).
A patient-driven titration procedure dubbed the 1 + 1 hydromorphone protocol may provide an alternative to traditional procedures. The protocol involved the administration of 1 mg of hydromorphone to 223 patients in the ED who had severe pain. That was followed by assessment and the offer of another 1 mg dose 15 minutes later (Chang, Bijur, Campbell, et al., 2009). This led to adequate analgesia in 95% of the patients. A follow-up study (N = 224) comparing this protocol to physician-driven management, which was described as being reflective of current practice and consisted of the administration of an IV opioid dose with no offer of additional analgesia, found that the 94% of the patients in the 1 + 1 group achieved adequate analgesia within 60 minutes of protocol initiation and had significantly greater decreases in pain than did the physician-driven group (Chang, Bijur, Davitt, et al., 2009). Just 10% of the patients in the physician-driven group were given a follow-up dose of analgesia. Adverse effects were similar in the groups, and no one required naloxone.
Another protocol, which called for the administration of 2 mg of IV hydromorphone over 2 to 3 minutes, relieved pain effectively and rapidly (within 5 minutes) in the ED but resulted in one or more periods of desaturation in 26% of the patients in 1 prospective study (Chang, Bijur, Napolitano, et al., 2009). The researchers appropriately concluded that 2 mg of IV hydromorphone is too much opioid to be given routinely to opioid-naïve patients as a single initial dose.
A common and recommended practice is to store a specified number of PCA pumps, drug reservoirs, and infusion tubings (number is dependent on the size of the institution) in the ED so that the therapy can be initiated without delay in patients who are admitted for treatment of severe pain crises. As discussed in Chapter 17, the clinician-administered bolus mode on most PCA pumps can be used to administer doses during titration.
Titration Protocols in the Postanesthesia Care Unit
Although opioid-induced adverse effects are dose related and have been identified as a limiting factor during titration in postoperative patients (Berde, Brennan, Raja, 2003), some suggest that the customary doses used to manage immediate postoperative pain are well tolerated and that an exaggerated fear of adverse effects may pose the greater threat (Larijani, Goldberg, 2004). A placebo-controlled study of 88 patients following abdominal hysterectomy or prostatectomy found that a single IV bolus dose of 7.5 mg of morphine did not cause any clinically significant cardiovascular (CV) or respiratory adverse effects but provided only slight relief of moderate to severe pain in the PACU (Larijani, Goldberg, Gratz, et al., 2004).
A large prospective, nonrandomized study illustrated the challenges of finding a balance between comfort and adverse effects in the immediate postoperative period by evaluating four different dosing regimens (Aubrun, Monsel, Langeron, et al., 2001). Each regimen consisted of administering 2 to 3 mg IV morphine boluses until patients experienced pain relief or adverse effects. SC morphine was given to all of the patients following IV bolus titration. The regimens were as follows. Group 1: boluses given every 10 minutes to a limit of five boluses (N = 400); group 2: boluses given every 5 minutes to a limit of five boluses (N = 400); group 3 and 4: boluses given in unlimited numbers every 5 minutes (N = 400 each group). Groups 1, 2, and 3 received SC morphine 4 hours after IV titration, and group 4 received SC morphine 2 hours after IV titration. Group 4 had the highest percentage of pain relief (73%) at the end of the PACU period, but sedation was dose-related and was highest in groups 3 (62%) and 4 (61%), both of which received an unlimited number of IV boluses compared with group 1 (27%), which received the more conservative regimen of IV boluses every 10 minutes up to a maximum of five boluses.
Titration in Older Adults with Severe Acute Pain
As discussed, age is an important consideration during opioid titration, and a common recommendation is to reduce the initial dose in older patients; however, one study (N = 224; 68% young, 32% old) showed that the dose of IV morphine required during postoperative titration to achieve adequate pain relief was not significantly different in older (0.14 mg/kg) compared with younger (0.15 mg/kg) patients (Aubrun, Bunge, Langeron, et al., 2003). Higher pain intensity was associated with higher morphine requirements; no patients required naloxone. It is important to note that the lack of difference noted in this research was assessed after normalizing for body weight, which was lower in the older patients. An earlier study (N = 875 young, 175 old) by these same researchers also found no differences in morphine requirements in age groups after doses were normalized for body weight (Aubrun, Monsel, Langeron, et al., 2002).
Other factors have been found to influence analgesic requirements. One study (N = 4317; 54% male, 46% female) observed that sex, but not age, was a predictor of severe pain and higher morphine requirements in postoperative patients (Aubrun, Salvi, Coriat, et al., 2005). Women experienced more severe postoperative pain than men and required higher opioid doses; however, an interesting finding in this study was that sex-related differences disappeared in the older patients. A prospective study of 149 surgical patients found that the patients’ initial pain-intensity ratings, rather than age, predicted morphine requirements in the PACU (Dahmani, Dupont, Mantz, et al., 2001). Nevertheless, conservative initial opioid doses, along with careful monitoring during titration, continue to be recommended in the older adult population; doses should be increased based on patients’ responses rather than specific age. An observational study (N = 418) demonstrated the safety of a postoperative protocol for patients older than 65 in whom IV morphine was started at a dose one third less than for younger patients (Keita, Tubach, Maalouli, et al., 2008). This method was found to be as safe and efficacious for the older patients at the lower dose (2 mg) as for younger patients at the higher dose (3 mg).
Dosing to a Specific Pain Intensity
Research has shown that the relationship between visual analog scale (VAS) pain intensity scores and dose requirement during and after titration in postoperative patients is not linear, suggesting that many factors influence pain and its relief and that there is no specific dose that will relieve pain of a specific intensity (Aubrun, Riou, 2004; Blumstein, Moore, 2003). Pain was assessed in a study of more than 3000 patients admitted consecutively to the PACU, and those with a VAS score above 30 were titrated with 3 mg of IV morphine every 5 minutes until their VAS scores were below 30 (Aubrun, Langeron, Quesnel, et al., 2003). The mean morphine requirement to obtain pain relief was 12 mg. A VAS score of 70 or higher was indicative of severe pain based on the need for a morphine dose of more than 0.15 mg/kg, which corresponds with the 10-mg morphine dose suggested by other research as being appropriate for some patients with severe pain. However, when VAS scores were analyzed, a sigmoid rather than linear relationship between morphine requirement and pain intensity was noted, as demonstrated by pain intensities that changed little with initial doses and then decreased rapidly with the final incremental dose. Although this study has noted limitations (Aubrun, Riou, 2004; Larijani, Goldberg, 2004; Myles, 2004), it underscores the importance of individualized selection of analgesic doses and systematic assessment of response during titration.
An interesting prospective, observational study evaluated patients’ desire for pain medication in 104 patients with acute pain and found that no single pain intensity can reliably predict a given patient’s analgesic requirements or desire for additional analgesia (Blumstein, Moore, 2003). As mentioned previously, dosing to a specific pain intensity can be dangerous and is strongly discouraged (Blumstein, Moore, 2003; Gordon, Dahl, Phillips et al., 2004; Lucas, Vlahos, Ledgerwood, 2007; Vila, Smith, Augustyniak, et al., 2005). Numerous other factors, such patient co-morbidities, previous opioid exposure, desire for pain medication, and the presence of adverse effects, particularly excessive sedation and respiratory depression, must be considered when selecting an opioid dose (see Chapter 19).
Transfer of Care: Hand-off Communication
Pain control should be included in the criteria for discharge from one area of care to another. Some EDs, short-stay units, outpatient surgery units, and PACUs establish a comfort-function goal of at least 4/10 before discharge (see Section II); however, the expectation that all patients must be discharged from these areas with pain ratings below an arbitrary number can lead to the unsafe administration of further opioid doses to patients who are excessively sedated (Blumstein, Moore, 2003; Lucas, Vlahos, Ledgerwood, 2007). Instead, achieving optimal pain relief is best viewed on a continuum, with the primary objective being to provide both effective and safe analgesia. Although it is not always possible to achieve a patient’s comfort-function goal within the short time the patient is in these areas, the comfort-function goal provides direction for ongoing care. Important information to give to the nurse assuming care of the patient is the patient’s comfort-function goal, how close the patient is to achieving it, what has been done thus far to achieve it (analgesics, doses, and times of administration), and how well the patient has tolerated the administration of analgesics (adverse effects) (see Chapter 19).
The transferring nurse should also alert staff on the receiving clinical unit of a patient’s risk factors for respiratory depression so that appropriate monitoring can be initiated (see Box 19-5 on p. 516). For example, opioid-naïve patients who require high opioid doses (e.g., more than 10 mg of IV morphine or its equivalent) during titration for acute pain are at higher risk for respiratory depression (Dahan, Aarts, Smith, 2010) and must be watched closely for at least 3 hours after the peak concentration of the last dose has passed (APS, 2003); the highest risk for respiratory depression is during the entire first 24 postoperative hours. Mechanical monitoring is warranted in patients with diagnosed or suspected obstructive sleep apnea or pulmonary disease. Providing the postoperative patient’s American Society of Anesthesiologists Patient Status Classification is recommended as a simple way to further communicate the patient’s risk for respiratory depression (see Chapter 19 for a detailed discussion and risk factors for sedation and respiratory depression).
Speed of Intravenous Injection
Too-rapid injection of IV medications can cause significant patient harm (Institute for Safe Medication Practices, 2003c). Chest wall rigidity and subsequent difficult ventilation is a potential complication of opioid administration and is most likely to occur with rapid IV administration of relatively high doses of lipophilic opioids such as the fentanils (Fukuda, 2005; Lalley, 2005). The injection time of opioid analgesics varies according to the opioid and the dose (i.e., the larger the dose, the longer the injection time); lower doses (e.g., 2 to 3 mg of morphine) may be given by steady injection over 2 or 3 minutes. Some clinicians dilute opioids with normal saline to reduce the likelihood of injecting too rapidly. It may be appropriate to administer large doses of opioids via piggyback infusion using an infusion device. During injection, the patient should be watched closely for effect, and injection must be stopped if adverse effects occur. Pharmacies are encouraged to make information readily available to clinicians about the maximum safe injection rate (mg/min) of the various drugs they administer (Institute for Safe Medication Practices, 2003c).
Clinician-Administered Boluses via the Infusion Pump
If IV PCA is used for pain control, the clinician-administered bolus mode on the infusion pump can be used in the PACU or ED to administer doses during the titration process and whenever they are needed on the clinical unit. This eliminates the time-consuming task of drawing up and administering opioid boluses from a separate syringe.
Supplemental clinician-administered epidural boluses also may be given via the analgesic infusion pump to patients receiving epidural analgesia (see the following patient example). This can help to avoid the need for IV opioid boluses and the sedation they can produce. Administration of epidural analgesic bolus doses is within the scope of practice for nurses in the United States. As discussed, the American Society for Pain Management Nursing (ASPMN) position paper endorses this practice as essential to registered nurses’ role in the management of pain by catheter techniques (Pasero, Eksterowicz, Primeau, et al., 2007) (see Chapter 19 for more about intraspinal analgesia).
Opioid Range Orders
Opioid range orders are medication orders in which the selected dose varies over a prescribed range according to the patient’s situation and status (Manworren, 2006). These orders have been used for decades to manage pain (Pasero, Manworren, McCaffery, 2007) and are considered essential to its effective management (Gordon, Dahl, Phillips, et al., 2004).
Support for Opioid Range Orders
The ASPMN and the APS developed a consensus statement advocating the use of range orders in 2004. It states that “a registered nurse who is competent in pain assessment and analgesic administration can safely interpret and implement properly written ‘as needed’ or PRN range orders for analgesic medications.” (p. 1, ASPMN, 2004). Similarly, The Joint Commission (TJC), an independent organization that accredits health care facilities in the United States, approves of the use of range orders, provided policies and procedures are in place and nurses are educated in their implementation (Manworren, 2006).
The ASPMN/APS task force that developed the consensus statement on range orders provided a list of considerations for writing and interpreting PRN opioid range orders in its publication of the statement (Gordon, Dahl, Phillips, et al., 2004). Among the considerations was the recommendation of a reasonable range, which was described as one with a maximum dose that is at least two times, but generally no larger than four times, the smallest dose in the range. Other important considerations are whether patients are opioid-naïve or opioid-tolerant; their previous responses to opioid analgesics; their ages, organ function, and co-morbidities; the severity of their pain; and the concomitant administration of sedating analgesics. The task force also emphasized the large inter- and intraindividual differences in responses to analgesics and that analgesics should be started at low doses and titrated gradually.
The lack of a predictable relationship between an opioid dose and pain relief underscores the danger of prescribing and administering predetermined doses based on specific pain intensities (Gordon, Dahl, Phillips, et al., 2004). For example, the practice of prescribing IV morphine boluses of 2 mg for mild pain, 4 mg for moderate pain, and 6 mg for severe pain should be avoided. As discussed, nurses must evaluate more than pain intensities when selecting opioid doses (Blumstein, Moore, 2003; Lucas, Vlahos, Ledgerwood, 2007; Taylor, Kirton, Staff, et al., 2005; Taylor, Voytovich, Kozol, 2003). Using the same example, it would be unsafe to administer a 6 mg IV morphine bolus to a patient with severe pain who is excessively sedated (see patient scenarios mentioned later).
Research conducted in two large academic settings to determine which factors nurses consider when implementing a range order revealed that there is wide variability in how nurses interpret such orders (Gordon, Pellino, Higgins, et al., 2008). The nurses in this study were asked to participate in a self-administered online survey that required them to read four vignettes about patients with acute postoperative pain and determine the appropriate dose and timing of dose administration. For example, the first vignette required the nurses to determine how soon a dose could be given to a patient with severe pain following an initial ineffective and safe 2-mg dose from a range order of 2 to 8 mg of IV morphine every 2 hours PRN. Although the majority (68%) responded appropriately, saying that they would administer a dose after 15 to 30 minutes (past peak time), 23% chose to make the patient wait the full 2-hour time period before giving another dose. Responses to another vignette revealed that only 25% of the nurses appeared to recognize that administration of more opioid to a patient experiencing excessive sedation following an initial dose is dangerous (see Chapter 19 for more information about sedation). Clearly, nursing education must precede the use of opioid-range orders in institutions.
Development of a Range Order Protocol
A protocol for implementing opioid range orders that is developed with input from nursing, medicine, and pharmacy may help to ensure more consistent interpretation of range orders and meet TJC requirements. Box 16-3
provides considerations for protocol development that are based on the principles of IV PCA. Fundamental to successful treatment by both IV PCA and range orders is an understanding that patients should be given access to as much analgesia as the prescription allows, administered in a way that maintains a balance between pain relief and adverse effects (Pasero, Manworren, McCaffery, 2007) (see the following patient examples).
Dose Frequency
One source of confusion regarding range order implementation is related to how often a nurse can administer doses within a range. Using IV PCA as a point of reference, consider that the recommended delay (lockout) interval for acute pain is 5 to 10 minutes (APS, 2003). Although a frequency of 5 to 10 minutes for nurse-administered IV doses may not be realistic in a busy clinical unit, it can be used as a framework for practice. That is, the protocol can state that unless the frequency is prescribed otherwise, nurses may administer subsequent IV doses within a range order as often as every 10 minutes. It may not always be possible to repeat a dose in 10 minutes, but such a protocol allows for those times when it is necessary, safe, and possible (Pasero, Manworren, McCaffery, 2007).
Total Time Interval
Another source of confusion is related to what is called the total time interval, which is the time in which the maximum dose in the range may be administered (Pasero, Manworren, McCaffery, 2007). There are at least two ways to define the total time interval: either according to the time the first dose was given; or according to a “rolling-clock” method whereby the total dose amount cannot be exceeded within a specified time interval, starting at the time of each individual dose, rather than the first dose. Consider a range order for 2 to 8 mg of IV morphine every 2 hours PRN. Clinicians who believe that the total time interval is defined by the time of the first dose would proceed as follows: if a dose is given at 0800, the patient may receive up to 8 mg between 0800 and 1000; the next time interval would begin at 1000 and the patient can be given another 8 mg between 1000 and 1200. Using this same range order example, clinicians who use the rolling-clock approach must be sure the dose about to be administered does not cumulatively exceed the maximum amount (8 mg) in the previous 2-hour period. There is no research or consensus about which method is best; therefore, it is recommended that each institution define the total time interval so that range orders in that institution are interpreted consistently, taking into consideration the potential for error and the time required to calculate the time interval prior to administering a dose (Pasero, Manworren, McCaffery, 2007). Regardless of how the total time interval is defined, it is essential to train nurses to select the appropriate doses and give them at safe intervals on the basis of patients’ status and the pharmacokinetics of the opioids. The use of opioid range orders is not recommended in institutions in which this level of training and the establishment of quality-improvement processes that monitor the safety of range order administrations are not possible. Following are two examples; one describes a scenario that applies the total time interval based on first dose; the second describes the same scenario but applies the total time interval based on the rolling clock, starting from each dose.
Conclusion
Selection of the initial opioid dose is based on many factors, including patient age, co-morbidities, previous exposure to opioids, and current pain status. Although pain intensity is an important consideration, selection of a dose based solely on a specific pain intensity is dangerous and strongly discouraged. No matter what method is used to predict analgesic requirements, the starting doses of opioid treatments are merely estimates. When starting doses are given, they are titrated up or down according to patient responses. The goal of titration is to use the smallest dose that provides satisfactory pain relief with the fewest adverse effects. It is clear that more research is needed to determine the best titration regimens and how aggressive titration affects the adverse-effect profiles of patients after discharge from areas like the ED and PACU. The best course of action is for clinicians and patients to view the achievement of optimal pain relief as occurring on a continuum, with the primary objective being to provide both effective and safe analgesia.