Assessing Medical Devices
The AP or PA chest x-ray is used to assess a number of medical devices in trauma patients. Common devices include endotracheal tubes, NG or OG tubes, central venous catheters, and thoracostomy tubes. A common misconception is that chest x-ray “confirms” device placement. The frontal chest x-ray provides information about the depth of insertion of devices but does not reveal the AP depth of these structures, as described earlier in the section on foreign bodies. While in some cases an abnormal position of a device may be readily apparent based on chest x-ray, in other cases a device can be catastrophically misplaced but appear in good position on chest x-ray. For example, AP chest x-ray can reveal the depth of insertion of an endotracheal tube, but a tube misplaced in the esophagus may not be recognized, because the esophagus lies posterior to the trachea. Consequently, other methods of assessment of device placement should always be used, in addition to assessment with x-ray.
Endotracheal Tubes
Chest x-ray allows assessment of the depth of insertion of an endotracheal tube. The tube has radiopaque markers and should be seen to terminate below the thoracic inlet and approximately 1 to 2 cm above the carina in adults (see Figures 6-25, 6-26, and 6-39). This position is optimal, because it prevents extubation or right main bronchus intubation resulting from changes in patient position. Chest x-ray can reveal a tube to be inserted too deeply, resulting in right main bronchus intubation. Associated findings can include right upper lobar collapse or left lung collapse. In other cases, the tube may be inserted to a very shallow depth, terminating far above the sternoclavicular joints. This places the patient at risk for accidental extubation—for example, if the neck is extended during patient repositioning. Remember that chest x-ray does not confirm the endotracheal tube to be correctly positioned within the trachea; a tube accidentally placed in the esophagus can have an identical midline position. Other methods of assessment of endotracheal tube positioning, including auscultation, end-tidal carbon dioxide measurement, and pulse oximetry, are essential additions to chest x-ray assessment.
Nasogastric and Orogastric Tubes
OG and NG tubes are visible on chest x-ray. X-ray allows assessment of depth of insertion but in some cases does not confirm correct placement in the stomach. X-ray can reveal a tube to be curled in the esophagus, but inadvertant placement into the trachea can have a similar appearance to correct esophageal placement. An NG or OG tube that deviates slightly from the midline in the thorax can suggest the presence of a mediastinal hematoma from aortic injury, resulting in deviation of the esophagus, as discussed earlier. An NG or OG tube that deviates substantially from the midline within the thorax may indicate accidental placement within a bronchus (see Figure 5-189), so great care should be taken to check the position before any materials are instilled into the tube. A gastric tube should normally be seen curling in the stomach; if the tube position overlies the thorax, diaphragm rupture with herniation of the stomach into the chest should be suspected (see Figure 6-49). Diaphragm injuries are discussed in more detail in Chapter 10.
Central Venous Catheters
Frontal chest x-ray should be rapidly performed following attempted internal jugular or subclavian central venous catheter placement—even following failed attempts. The primary purpose is to evaluate for accidental iatrogenic pneumothorax. Secondarily, chest x-ray reveals the depth of insertion. The central venous catheter should terminate in the superior vena cava, approximately at the level of the right tracheobronchial angle. Deeper insertion can result in irritation of the atrium or ventricle by the device, potentially increasing the risk for dysrhythmias and thrombus formation.35-38 Chest x-ray can reveal abnormal placement, including catheters that incorrectly traverse to the contralateral subclavian vein or ascend the internal jugular vein (see Figure 5-188). A common misconception is that chest x-ray confirms correct placement within the intended venous structure. Because the carotid artery and subclavian artery share similar positions to the internal jugular vein and subclavian vein, respectively, inadvertent arterial catheterization may not be evident on chest x-ray. In some cases, this incorrect placement may be evident if the catheter tip overlies the aorta. However, other means of assessment for arterial catheterization, including visual inspection for pulsatile blood flow, use of ultrasound guidance or postplacement ultrasound assessment, transducing pressure waveforms from the catheter to assess for venous or arterial waveforms, or blood gas analysis, should be considered.
Thoracostomy Tubes
Chest x-ray should be rapidly performed following placement of a thoracostomy tube. As with the other devices described earlier, chest x-ray can reveal grossly abnormal placement or can suggest correct placement, but other means of device assessment are essential. For example, a thoracostomy tube incorrectly placed into posterior subcutaneous tissues can appear to be correctly placed within the pleural space on frontal chest x-ray.
Several chest x-ray features should be assessed following thoracostomy tube placement. First, and critically, the tube should be placed to a sufficient depth that all fenestrations on the tube appear to overlie the pleural space (see Figs. 6-17, 6-26, 6-28, and 6-46). Fenestrations are marked as discontinuities in a radiopaque line along the length of the tube. If fenestrations are not within the pleural space (see Fig. 6-114), air may be entrained from outside of the patient, preventing evacuation of the pneumothorax. The tube should not intersect the mediastinum or cross the diaphragm. Second, the tube should not be kinked (see Figure 6-52) and should have an oblique position terminating near the apex of the lung on the side of insertion. These latter features are less important than tube function, and a properly functioning tube should not be removed solely because of suboptimal radiographic appearance.
If a pneumothorax was seen on chest x-ray before thoracostomy tube placement, it should be reduced in size following placement. A pneumothorax that remains large or increases in size following thoracostomy tube placement suggests one of several possibilities:
Further assessment of the function of the thoracostomy tube, including assessment for air leak, should be performed but is beyond the scope of this text.
Ultrasound
Ultrasound is useful in the evaluation of blunt and penetrating chest trauma, because it is portable, rapid to perform, uses no ionizing radiation, and adds little to the cost of patient care (see Table 6-1).
Bedside ultrasound has long been used to evaluate trauma patients for hemopericardium. Examination of the heart from a subdiaphragmatic location is a routine part of the focused assessment with sonography for trauma (FAST) exam. On ultrasound, fluid in the pericardial sac appears black (hypo- or anechoic) (Figures 6-136 and 6-137). In addition to determining the simple presence or absence of pericardial fluid, ultrasound can provide evidence of pericardial tamponade, as described later in the section on interpretation of ultrasound. Even a small traumatic pericardial effusion can compromise cardiac filling, resulting in pericardial tamponade. Ultrasound can be used to identify pneumothorax with high sensitivity,39-41 exceeding that of supine chest x-ray.42-44 However, as is the case for chest CT, it is not clear that detection of a pneumothorax by ultrasound when it is invisible on chest x-ray results in any clinical benefit to the patient. For example, a pneumothorax so small that it is undetectable by chest x-ray may not require thoracostomy even if it is detected by ultrasound. Arguably, detection of small pneumothoraces with ultrasound might be detrimental, because it might result in unnecessary procedures such as thoracostomy, extended clinical observation or admission, or repeat diagnostic imaging with increased costs and radiation exposures. The role of ultrasound in diagnosis of pneumothorax remains uncertain as a consequence of these concerns. Ultrasound also allows detection of fluid in the pleural space (compare x-ray Figure 6-139 and ultrasound Figure 6-140). Fluid in the pleural space appears black, and the lung may be seen floating or moving within the pleural fluid collection. Ultrasound to detect hemopneumothorax has been described as the “extended FAST exam.”
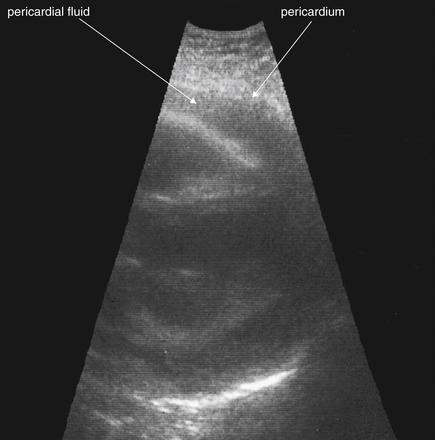
Figure 6-136 Pericardial effusion (hemopericardium).
Bedside echocardiogram shows hypoechoic fluid in the pericardial sac. In the setting of trauma, this is likely blood. Care should be taken to differentiate fluid from a pericardial fat pad (see text).
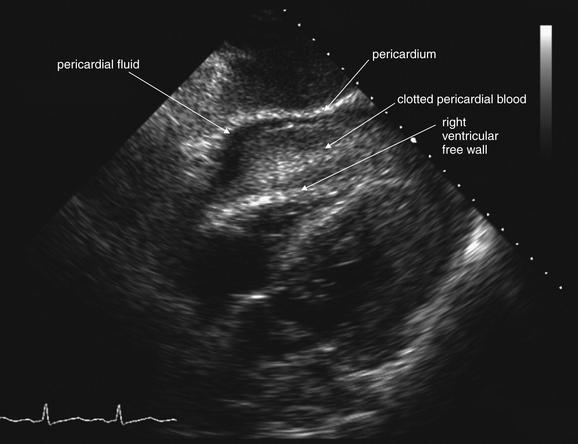
Figure 6-137 Pericardial effusion (hemopericardium).
This 57-year-old man struck a utility pole on a bicycle. He was alert but hypotensive on emergency department arrival. His bedside echocardiogram shows pericardial fluid and bowing of the right ventricular free wall, suggesting cardiac tamponade. Compare with the CT findings in Figure 6-138.
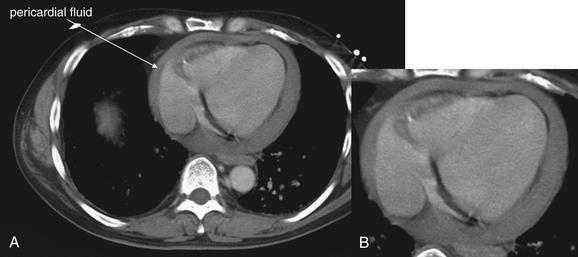
Figure 6-138 Pericardial effusion (hemopericardium), CT with IV contrast.
Same patient as in Figure 6-137. The patient remained stable and underwent CT for evaluation of other injuries. His CT also shows a large pericardial effusion, which was treated in the operating room with pericardial window. A, Axial CT slice, variation on soft-tissue window. B, Close-up from A.
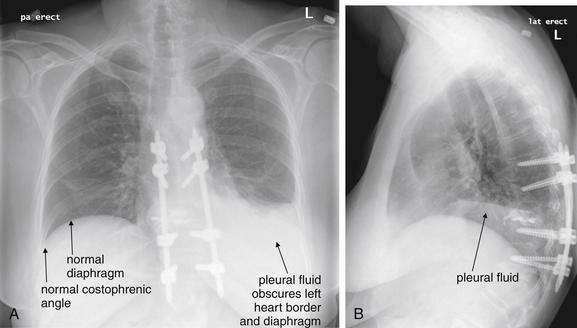
Figure 6-139 Pleural effusions.
Pleural fluid layers with gravity on upright chest x-ray. In the setting of trauma, fluid is assumed to represent blood. A, PA upright chest x-ray. B, Lateral upright chest x-ray. Compare these images with the thoracic ultrasound in Figure 6-140.
Acquisition and Interpretation of Ultrasound for Chest Trauma
Common views for cardiac ultrasound include subxiphoid, parasternal long axis, and apical four-chamber views. Any of these views can be used to assess for pericardial effusion, with patient body habitus dictating which view provides the best images. The subxiphoid view is obtained by placing the ultrasound probe below the xiphoid process in the upper abdomen, pointed cephalad toward the left shoulder. The liver is used as an acoustic window, and the inferior wall of the heart is usually seen, sometimes with views of all four cardiac chambers. The parasternal long axis view is obtained by placing the ultrasound probe left of the sternum in the third or fourth intercostal space, with its long axis parallel to that of the heart, pointed toward the left nipple. From this vantage, a portion of the right ventricle is seen in the proximal field. The left atrium and ventricle are seen in the far field of view, and a dependent pericardial effusion may be seen. The parasternal long-axis view is considered most accurate for detection of pericardial effusion, because the posterior pericardium is usually seen well, and fluid may preferentially accumulate in this dependent position.55-56 An apical four-chamber view is obtained by placing the ultrasound probe below the left nipple, angled toward the sternal notch. As the name indicates, all four cardiac chambers are seen, with the ventricles in the near field of view.
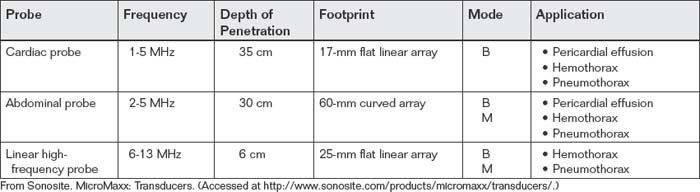
The appropriate ultrasound probe for examination of the chest depends on the intended application. For examination of the pericardium, a cardiac or an abdominal probe is appropriate. Both probes provide adequate depth of penetration and resolution for detection of pericardial fluid (Table 6-6). When a subxiphoid view or an apical four-chamber view is acquired, either probe is satisfactory. If a parasternal view is used, the cardiac probe has the advantage of a smaller footprint on the chest wall, allowing it to be used to image between ribs. The abdominal probe has a larger footprint, usually resulting in rib shadows obscuring portions of the image. A linear high-frequency transducer is usually not used for examination of the heart, as its depth of penetration is generally limited—around 6 cm. For assessment of pleural fluid or pneumothorax, any of the probes (cardiac, abdominal, or linear high frequency) may be used, because the pleural surface is usually within 6 cm of the skin surface. In obese patients, the linear high-frequency probe may have inadequate depth of penetration.
TABLE 6-6 Ultrasound Transducers (Probes) for Examination of the Thorax in Trauma111
Ultrasound Modes
Two ultrasound modes are useful in examination of the chest. Brightness (B) mode ultrasound refers to the common two-dimensional planar imaging mode familiar to most emergency physicians. Motion (M) mode provides a record of motion along a single line extending deep to the probe from the skin surface. In this mode, the user selects the line position on the two-dimensional B mode image, and then a graph of motion along this line is produced. In this chapter, all ultrasound applications refer to B mode imaging unless otherwise specified. M mode is specifically useful in detection of pneumothorax, as described later.
Ultrasound Findings of Pericardial Fluid (Hemopericardium)
Ultrasound has been used for diagnosis of pericardial fluid since the 1960s.45-51 Thourani et al.52 documented the replacement of pericardiocentesis with ultrasound for diagnosis of traumatic hemopericardium over a 22-year period in a large urban trauma center. In the setting of penetrating chest trauma, bedside ultrasound by trauma surgeons as been shown to be accurate (sensitivity = 100%, specificity = 96.9%) and to be associated with rapid time to operation.53 Immediate bedside echocardiography is associated with improved survival in patients with penetrating cardiac injury.54
The normal pericardium is a bright white line surrounding the ventricular and atrial chambers (see Figs. 6-136 and 6-137). In the presence of pericardial blood, a black stripe is seen within the confines of the pericardium, outside of the cardiac chambers. Even a relatively small pericardial effusion may interfere with ventricular filling, reducing cardiac output—if not causing frank cardiac tamponade. A pericardial fat pad may be present and may be hypo- or anechoic, simulating pericardial fluid.56
Pitfalls in Diagnosis of Pericardial Effusion by Ultrasound
Pericardial Fat Pad Simulating Pericardial Fluid
Blaivas, DeBehnke, and Phelan56 documented a significant rate of potential misdiagnosis of pericardial effusion. In a study of emergency medicine residents and faculty, participants were asked to evaluate short video clips of subxiphoid cardiac ultrasound examinations for the presence of effusion and tamponade. Pericardial fat pads and true effusions were frequently confused, with poor sensitivity (73%) and specificity (44%) for effusion. The authors suggest two alternative views to avoid this confusion:
Pericardial Laceration Decompressing Pericardial Fluid
Ball et al.57 described a series of patients with stab wounds to the chest, resulting in penetrating cardiac injury and laceration of the pericardial sac. All had negative cardiac ultrasound findings, which the authors attribute to decompression of the pericardial space through the pericardial laceration into the thoracic cavity.
Ultrasound Findings of Pericardial Tamponade
The presence of a pericardial effusion is necessary but not sufficient for the diagnosis of pericardial tamponade. Unfortunately, some small and localized but hemodynamically significant effusions may not be readily visible on transthoracic echocardiography. In the case of large pericardial effusions, the heart may swing like a pendulum within the pericardium, but this is not an indication of high pericardial pressures. As pericardial fluid pressure increases, it may exceed pressures in the right heart, resulting in collapse of the right atrium and ventricle. Right ventricular collapse during early diastole is insensitive (38% to 48%) but specific (84% to 100%) for pericardial tamponade. Right atrial collapse is too nonspecific (50% to 68%) and insensitive (55% to 60%) for clinical utility.58 Pericardial tamponade results in increased central venous pressures, which prevent the normal collapse of the IVC with sudden inspiration (the “sniff test”).56,58
If pericardial fluid is documented with ultrasound and cardiac tamponade develops, ultrasound-guided pericardiocentesis can reverse hemodynamic instability, at least as a temporizing measure. The procedure has a low complication rate, although pneumothorax, right ventricular laceration, intercostal vessel injury, and transient dysrhythmias may occur.59
Blunt myocardial injury (previously called myocardial contusion) may also be demonstrated by ultrasound, although controversy exists about the very definition. Pericardial fluid alone is not sufficient to make the diagnosis. Evidence of right ventricular dysfunction may be seen. No gold standard other than autopsy has been agreed upon, making sensitivity and specificity of ultrasound uncertain.60
Ultrasound Findings of Hemothorax
Fluid in the pleural space is readily seen on ultrasound. As with other ultrasound applications, fluid appears black (hypoechoic or anechoic). The lung parenchyma may be seen as a hyperechoic structure floating within pleural fluid. Because fluid layers with gravity, the dependent portions of the thorax should be inspected carefully. If the patient is able to be placed in an upright position, the sensitivity of ultrasound for detection of pleural fluid will be improved (Figure 6-140). Ma and Mateer61 prospectively compared ultrasound with chest x-ray for the detection of traumatic hemothorax in 240 patients, using a gold standard of CT. Both modalities showed 96.2% sensitivity, 100% specificity, and 99.6% overall accuracy. Brooks et al.62 showed similar performance of ultrasound (sensitivity = 92%, specificity = 100%) in a smaller cohort of 61 patients. In this study, four hemothoraces detected with ultrasound (25%) were not seen on initial chest x-ray. Whether small hemothoraces detected with ultrasound or CT alone require thoracostomy remains uncertain. Sharma, Hagler, and Oswanski63 reported that delayed development of hemothorax is rare after blunt trauma, occurring in only 5% of cases, but may occur in patients with rib fractures.
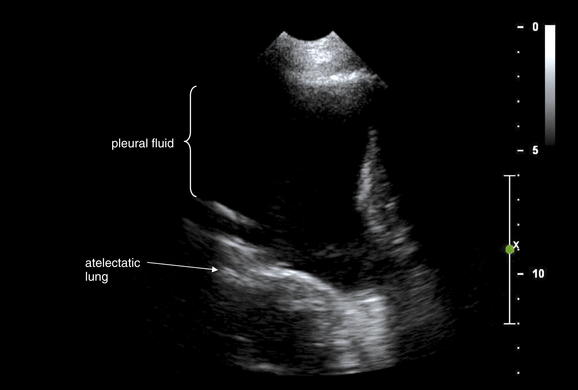
Figure 6-140 Pleural fluid or hemothorax: Ultrasound.
Same patient as in Figure 6-139. An ultrasound of the chest was performed. The large black region is pleural fluid. Lung tissue is seen deep to this and has echo characteristics similar to abdominal solid organs, because it is atelectatic resulting from the pleural fluid. In this location, 10 cm of pleural fluid separates the chest wall and lung.
Ultrasound Findings of Pneumothorax
As early as 1987, ultrasound was described as a method of detecting pneumothorax.64 Air in the pleural space (pneumothorax) can be detected using a cardiac, abdominal, or linear high-frequency probe. Even novice ultrasonographers have been shown to be capable of performing the ultrasound examination and recognizing abnormal findings with high sensitivity and specificity.65-66 The degree of pneumothorax can also be recognized by ultrasound.42,67 Wilkerson and Stone68 performed a systematic review and identified four prospective studies comparing ultrasound and x-ray with a gold standard of CT. The sensitivity of ultrasound ranged from 86% to 98%, and the specificity ranged from 97% to 100%. The sensitivity of supine AP chest radiographs for the detection of pneumothorax ranged from 28% to 75%, with a specificity of 100%.
Before describing the appearance of pneumothorax, we review the normal appearance of the lung and pleura using B mode ultrasound. We then describe the appearance of pneumothorax using B mode. Finally, we compare the ultrasound appearance of normal lung and pleura to that of pneumothorax using M mode ultrasound.69
B Mode Ultrasound Findings
Normal Lung and Pleura
Normally, in the absence of pneumothorax, the pleural surfaces of the chest wall (parietal pleura) and lung (visceral pleura) are in close contact, separated only by a thin film of fluid. This interface is called the pleural line (see Figure 6-32; see also Fig. 6-31 for chest x-ray findings in same patient). An ultrasound probe placed on the chest wall in a longitudinal orientation shows the pleural line as a bright white, thin, horizontal line. Bounding the pleural line cephalad and caudad are two adjacent ribs, each indicated by a bright white curve (the superficial cortex of the rib) with posterior acoustic shadowing. The superficial rib cortices are located about 0.5- to 1-cm superficial to the pleural line. The posterior acoustic shadows of ribs are created by the dense bone reflecting and absorbing the ultrasound beam, preventing through-transmission of sound. The pattern of a rib, followed by the pleural line, followed by an adjacent rib is sometimes described as the bat sign (see Figure 6-32) because of the resemblance to the wings and body of a flying bat. Deep to the pleural line, a series of horizontal lines is often seen—a repetition artifact called the A line. A lines are believed to arise from the interaction of the ultrasound beam with air; consequently, they are seen both with normal lung and in the presence of pneumothorax. On real-time ultrasound, lateral motion is seen at the pleural line as the parietal and visceral pleura slide relative to each other with respiration. This finding is sometimes called the sliding lung sign. Some ultrasound machines are equipped with automated software motion filters that can obscure this relatively subtle motion; these software filters should be turned off when assessing for this sign.
Comet tail artifacts (B lines) may be seen in normal cases (Table 6-7), and their absence can be a clue to the presence of pneumothorax. B lines must be distinguished from two other comet tail–like phenomena, which are described later. First, we define B lines, which have five mandatory features:
TABLE 6-7 Comet Tail Artifacts on Thoracic Ultrasound and Their Significance
From Lichtenstein DA, Meziere G, Lascols N, et al. Ultrasound diagnosis of occult pneumothorax. Crit Care Med33(6):1231-1238, 2005.
B lines are believed to arise from the ultrasound beam’s interaction with the visceral pleura; consequently, they are absent in cases of pneumothorax.
Two other comet tail–like findings may be seen, neither of which reliably confirm the presence or absence of pneumothorax. The first, Z lines, do not distinguish between normal lung and pneumothorax, because they may be seen with both. Z lines have four features that differ from those of B lines:
Emphysema (E) lines are comet tails arising superficial to the pleural line and preventing its visualization. The bat sign is therefore not seen. These lines extend deep to their origin and do not fade. The source of E lines is subcutaneous emphysema, which may accompany pneumothorax. However, because the pleural line itself is obscured by the E line comet tail, motion at the pleural line cannot be assessed.
Pneumothorax
In the case of pneumothorax, air separates the parietal and visceral pleural surfaces. As a consequence, the sliding lung sign is not seen. In addition, B lines, which are generated by the ultrasound beam’s interaction with the visceral pleura, are not seen resulting from the presence of air between the parietal and the visceral pleura. Therefore loss of the sliding lung sign and absence of B line comet tails are signs of pneumothorax.70 A lines may be seen despite pneumothorax, because they are an artifact arising from the air. The presence of A lines without any intersecting B lines is called the A line sign and is a highly sensitive but nonspecific indication of pneumothorax (sensitivity = 100%, specificity = 60%).70-71
M Mode Ultrasound Findings
As described earlier, M mode is an ultrasound setting that generates a graph of motion over time along a single line through the two-dimensional B mode image. Three important signs can distinguish between normal lung and pneumothorax using M mode.
In the absence of pneumothorax, tissues superficial to the pleural line do not move appreciably and thus generate a series of parallel horizontal lines on the M mode image, indicating no motion. Deep to the pleural line, moving lung tissue generates a homogeneous granular pattern. The combination of these findings is called the seashore sign resulting from its resemblance to wave fronts approaching a sandy beach (see Figure 6-33).
In the case of pneumothorax, air in the pleural space deep to the pleural line is also motionless, so the parallel horizontal lines seen in motionless superficial tissues continue throughout the M mode image. This appearance of parallel horizontal lines throughout the M mode image is called the stratosphere sign (see Figure 6-34). The seashore sign is absent in this case, because air in the pleural space prevents through-transmission of the ultrasound beam to the lung deeper in the chest.
Another M mode finding of pneumothorax is the lung point sign (see Figure 6-35). This finding is present when the seashore sign and the stratosphere sign alternate rhythmically in concert with the patient’s respiration. The finding is thought to arise as the region of pneumothorax shifts with variations in the degree of lung inflation. When ventilated lung is in contact with the parietal pleura or chest wall, the seashore sign is present. When air from the pneumothorax separates the lung surface from the parietal pleura or chest wall, the stratosphere sign is seen.70 The lung point sign has been reported to be highly specific though insensitive for pneumothorax (sensitivity = 66%, specificity = 100%).72
The reported sensitivity and specificity of various combinations of ultrasound findings are shown in Table 6-8. Chronic obstructive pulmonary disease has been reported to decrease the specificity of ultrasound.73
TABLE 6-8 Diagnostic Accuracy of Ultrasound Findings of Pneumothorax70,72,112
| Finding | Sensitivity | Specificity |
|---|---|---|
| Loss of sliding lung sign | 95%-100% | 78%-91% |
| Loss of sliding lung sign plus presence of A line sign | 95% | 94% |
| Lung point sign | 66%-79% | 100% |
From Lichtenstein DA, Menu Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill: Lung sliding. Chest 108(5):1345-1348, 1995; Lichtenstein D, Meziere G, Biderman P, et al. The “lung point”: An ultrasound sign specific to pneumothorax. Intensive Care Med 26(10):1434-1440, 2000; Lichtenstein DA, Meziere G, Lascols N, et al. Ultrasound diagnosis of occult pneumothorax. Crit Care Med 33(6):1231-1238, 2005.
Chest Computed Tomography
Chest CT with IV contrast is the most definitive diagnostic imaging test for a variety of blunt and penetrating chest injuries. Two important indications for chest CT are definitive diagnosis of aortic injury, for which chest CT is clearly superior to chest x-ray, and detection of thoracic spinal injury.20-21 As described in Chapter 3, multiplanar reformatted images derived from standard chest CT can also be used to evaluate for spinal trauma, with sensitivity far exceeding that of x-ray. Studies comparing chest CT to chest x-ray clearly demonstrate the higher sensitivity of chest CT for a variety of other injuries, including rib fractures, hemopneumothorax, subcutaneous air, diaphragmatic injuries, pneumopericardium, pneumomediastinum, and pneumoperitoneum. However, for many of these injuries, it is uncertain whether their detection with CT results in clinical benefit to patients. A classic example is the detection of a small pneumothorax on chest CT that was not visible on chest x-ray. A number of studies have examined the necessity for thoracostomy in this scenario, as described in detail at the end of this chapter. Thoracostomy does not appear to be routinely required for radiographically occult pneumothorax, even when the patient will be placed on positive pressure ventilation. For other injuries frequently detected on chest CT, including small hemothoraces, pulmonary contusions, rib fractures, transverse spinal process fractures, and pneumopericardium, no specific therapy is required. For example, regardless of whether rib fractures are confirmed with CT scan or simply suspected based on the patient’s complaint of chest pain with inspiration, management is usually supportive, using analgesics and incentive spirometry. Rarely, multiple rib fractures are managed more aggressively with epidural anesthesia. Pulmonary contusions detected on chest CT require no specific therapy; it is the patient’s clinical status, including hypoxia or increasing work of breathing, that determines the therapeutic intervention, not the radiographic appearance on CT scan.
Undoubtedly, the most important use of chest CT following trauma is in the detection of aortic injury. Before the clinical introduction of chest CT, suspected mediastinal injuries required formal angiography for evaluation. Angiography is resource intensive, requiring a team of specialized interventional radiologists; is time consuming; and has risks associated with femoral arterial catheterization. Modern chest CT is noninvasive, has excellent multiplanar spatial resolution, and is able to detect traumatic aortic injury with the level of detail sufficient for operative planning. Aortic branch vessel involvement can be readily detected. Modern studies suggest diagnostic sensitivity and specificity essentially equivalent to that of formal angiography. For patients with penetrating chest trauma, CT can sometimes provide critical information about the precise location of retained foreign bodies or about the path of transthoracic projectiles. This information sometimes dictates thoracotomy or eliminates concerns about mediastinal and cardiac injury (see Figures 6-115, 6-116, and 6-118 through 6-120).
Controversy exists about the need for chest CT in trauma patients with a normal chest x-ray. Studies suggest that a completely normal chest x-ray is only approximately 92% to 93% sensitive for detection of aortic injury.20,74,75 In addition, many findings suggestive of aortic injury are nonspecific, resulting in many normal CT scans following abnormal chest x-ray findings. If chest x-ray alone were used as the screening modality for aortic injury, as many as 1 in 12 patients with life-threatening aortic injury would be missed. However, this is an uncommon injury pattern overall, occurring in only 274 cases in 50 trauma centers over a 2.5-year period—a rate of only about 2 cases per year in major trauma centers.20 As a consequence, the negative predictive value of a normal chest x-ray is excellent in all but the most high-risk patients. In pediatric patients, if chest CT were used ubiquitously as the initial screening test in place of x-ray (or following a normal chest x-ray), the number needed to treat to find a single case of aortic injury has been estimated to be more than 200.76 Proponents of chest CT argue that the extreme morbidity and mortality (around 30% in those patients who survive to the emergency department) of aortic trauma necessitate definitive imaging with CT in all cases.20,74 Opponents of CT argue that the cost of CT, nephrotoxicity of contrast agents, and radiation exposure outweigh the benefits in low-risk trauma patients. The radiation exposure from chest CT is on the order of 400 times that of chest x-ray.77 Many trauma patients are relatively young, compounding concerns about carcinogenesis. New studies suggest cancer risks associated with chest CT, ranging from a lifetime risk around 1 in 400 at age 20 to 1 in 1000 at age 60.78-80 Trauma patients undergoing multiple CT scans have documented radiation exposures on the order of 40 mSv, the equivalent of more than 1000 chest x-rays.81 These and other controversies in the use of chest CT for trauma are discussed at the end of this chapter. Radiation risks of chest CT are discussed in more detail in Chapters 7 and 8.
Performance of Chest CT for Trauma
Chest CT for evaluation of thoracic trauma requires the use of injected contrast whenever possible. Parenteral contrast allows detection of vascular abnormalities, particularly injury to the thoracic aorta, the primary indication for chest CT. A rapid contrast bolus is performed, with CT image acquisition immediately following to ensure arterial phase imaging of the aorta. Automated bolus tracking software helps ensure that the CT is performed at the moment of optimal aortic enhancement, as described in Chapter 7.
What Diagnostic Procedure Should Be Performed in Patients with Contraindications to Intravenous Contrast Used for Computed Tomography?
In patients who have contraindications to the use of iodinated contrast, including renal insufficiency or contrast allergy, delays should generally not be allowed for premedication such as steroid prep or N-acetylcysteine therapy if traumatic aortic injury is legitimately suspected. This injury can lead to rapid death if not immediately detected and treated. Among initial survivors of this injury, 30% die within 6 hours if diagnosis is not made and treatment initiated.12 An individualized risk–benefit decision must be made for each patient. One option is immediate noncontrast chest CT. If this test is normal, with no evidence of mediastinal hematoma, the likelihood of aortic injury is very low; the American College of Radiology thus calls noncontrast CT useful to detect mediastinal hematoma when contrast is contraindicated.12 Depending on the level of pretest probability of aortic injury, other diagnostic imaging modalities can be employed, including CT with IV contrast after appropriate premedication, transthoracic or transesophageal echocardiography, or magnetic resonance imaging. If immediate noncontrast chest CT is performed and is abnormal (e.g., demonstrating a large mediastinal hematoma), the decision may be made to proceed with formal aortography, with therapeutic options such as placement of an endovascular graft. By proceeding with formal angiography rather than with chest CT, the total exposure to iodinated contrast may be minimized. Another strategy would be to perform CT with IV contrast following an abnormal noncontrast chest CT, confirming or excluding aortic injury. The disadvantage to this approach would be that the patient might require a second dose of iodinated contrast if CT findings then prompt formal aortography.
A second approach to the patient with contraindications to injected contrast material is to proceed with immediate IV contrast after consideration of risks. This decision should not be taken lightly, because renal failure requiring dialysis or CT severe anaphylaxis and death may occur. However, the late diagnosis of traumatic aortic injury may also have dire consequences, including death. The overall clinical scenario, including the patient’s hemodynamic stability, mechanism of the injury, complaints of chest pain, chest x-ray findings, and specific contraindications to injected contrast (e.g., severity of prior allergic reaction or degree of renal insufficiency), should be incorporated into this decision. When possible, informed consent from the patient or patient’s representatives should be obtained if a decision to give IV contrast despite significant contraindications is made.
Approach to Interpretation of Chest CT
Here, we describe a general approach to the interpretation of chest CT for evaluation of thoracic trauma. Chest CT can be rapidly interpreted by the emergency physician in a matter of minutes. Axial images alone are sufficient for detection and delineation of most important injuries, although we illustrate some of these injury patterns using additional coronal and sagittal images to assist you in visualizing the injury in three dimensions. We begin with a brief discussion of CT window settings.
CT windows are discussed in more detail in other chapters. In brief, windows allow the CT gray scale to be adjusted to accentuate detail of tissues of a particular density. A given CT image can be viewed on windows settings that accentuate soft tissue, lung, or bony detail. In general, the entire chest should be examined using first soft-tissue, then lung, and finally bone windows. Here, we review the findings that can be recognized on each of these three settings.
Soft-tissue windows
Soft-tissue windows (also called mediastinal, chest, vascular, or abdominal windows) are the key to detection of aortic injury. On this setting, the low-density lung parenchyma is invisible (black), and pneumothorax cannot be recognized readily. However, contrast-filled vascular structures including the aorta and its major branches are seen in detail (see Figure 6-4). Contrast appears white on this setting. Soft tissues including muscles and mediastinal soft tissues such as the heart or blood products (including mediastinal hematoma, or blood within the pleural space representing hemothorax) appear as an intermediate gray shade (see Figure 6-4). A pericardial effusion is also visible on this setting as an intermediate gray band surrounding the heart, whose chambers are generally filled with white contrast (see Figure 6-138).
On soft-tissue windows, inspect the entire aorta from the aortic root, cephalad through the arch, and caudad along the descending aorta to the diaphragm. Pay particular attention to the distal aortic arch and proximal descending aorta, the most common location of traumatic aortic injuries. A normal aorta has a well-defined, smooth margin. The ascending aorta and the descending aorta appear circular in axial cross section. The arch appears elliptic. The normal aorta should be uniformly filled with white vascular contrast, with no filling defects or visible intimal flaps. The branch vessels of the aorta, including the subclavian, brachiocephalic, and innominate arteries, should be inspected for these same qualities. The position of the medial clavicular heads relative to these vessels should be noted, because posterior dislocation of the clavicle with impingement on great vessels can occur (see Figures 6-131 through 6-133).
Mediastinal Hematoma
Mediastinal hemorrhage is a potentially serious finding in major trauma—with the most concerning source being aortic injury. Periaortic hematoma is a sensitive finding of aortic injury, occurring in 91% of cases.82-83 Other causes of mediastinal hematoma include venous hemorrhage and extension of prevertebral hematomas associated with cervical and thoracic spine injuries. When aortic injury is present, definitive therapy is essential, with endovascular grafting or open techniques applied depending on the location and type of injury. If no active bleeding or aortic injury is noted, most mediastinal hematomas require no specific therapy.
CT findings of hematoma include nonenhancing densities within the mediastinum, surrounding the aorta (see Figures 6-57 and 6-100). The density of hematoma is typically around 45 Hounsfield units, which appears an intermediate gray on CT soft-tissue windows. The hematoma may create mass effect, leading to a wide mediastinum and deviation of mediastinal structures including the trachea, endotracheal tube, and OG or NG tubes in the esophagus (see Figures 6-57).
Aortic Trauma
In aortic injury, irregularities in the normally smooth and circular aortic cross-sectional contour may be visible.84-85 Active extravasation of injected contrast indicating active hemorrhage may be visible as white contrast material outside the lumen of the aorta and is an indication for immediate thoracotomy.86 An intimal flap is visible in most cases—91% in one study.83 Pseudoaneurysm, a focal bulge or diffuse enlargement of the aortic diameter, occurs in 97% of cases at the site where the ligamentum arteriosus tethers the aorta, in the distal aortic arch. Pseudoaneurysms differ from true aneurysms in that true aneurysms refer to dilatation involving all three layers of the aortic wall. In a pseudoaneurysm, a tear in the muscularis layer occurs, through which the intimal layer may protrude. Although a patient with a pseudoaneurysm may be initially stable, the thin-walled pseudoaneurysm is at high risk for rupture, leading to sudden decompensation or death. Mediastinal or periaortic hematoma may be present as described earlier.83 Pericardial blood may be seen.84-85 Figures 6-56 through 6-108 demonstrate multiple aortic injuries with chest x-ray, CT, and aortogram images.
When contrast cannot be given, rapid assessment of the aorta for traumatic injury can be performed with noncontrast CT—though the sensitivity of this technique has not been rigorously tested.12 Because traumatic aortic injury differs from spontaneous aortic injury in the involvement of multiple layers of the aorta, mediastinal hematoma (which can be detected on noncontrast CT) usually accompanies this injury. Although details of the internal contour of the aorta cannot be recognized without contrast, the absence of mediastinal hematoma is strong evidence of the absence of traumatic aortic injury.12 Noncontrast CT is not routinely performed for chest trauma. As a result, information on the sensitivity of noncontrasted findings is based largely on studies of contrasted CT, extrapolated based on the likelihood that some findings would be seen without contrast. Periaortic hematoma at the level of the diaphragm on abdominal CT has a reported sensitivity of 70% and specificity of 94% for thoracic aortic injury.87 On chest CT, periaortic hematoma has a reported sensitivity of 91%, whereas pseudoaneurysm is reported to be 97% sensitive.83 Clearly, contrast should be used if possible, given the importance of accurate diagnosis of aortic injury and the limited evidence for noncontrasted CT in this setting.
CT with IV contrast for evaluation of aortic trauma has sensitivity and specificity described as exceeding 99% based on a combination of surgical findings, autopsy, and clinical follow-up.21,88-90 Some studies have suggested CT to be more sensitive that traditional aortography.91 As we have discussed in Chapter 7 with regard to chest CT for pulmonary embolism, the clinical outcome following negative chest CT may be more important than the reported test sensitivity. An outcome study of 278 consecutive trauma patients evaluated with contrast-enhanced chest CT suggested no mortality from aortic injury among those with negative CT.92
Diaphragm Injury
Gross diaphragm injuries with herniation of abdominal contents into the chest are readily seen using soft-tissue windows. Subtler injuries without overt visceral herniation are more difficult to recognize but are characterized by discontinuities of the diaphragm, which is visible as a thin, curved band on soft-tissue windows (see Figs. 6-49 through 6-55). These injuries are discussed in detail in Chapter 10.
Hemothorax
On soft-tissue windows, hemothorax is visible as a dependent region of intermediate gray opacity (around 45 Hounsfield units). Lung windows are needed to determine whether an associated pneumothorax is present, as normal lung parenchyma itself is invisible on soft-tissue windows (see Figs. 6-4, 6-5, and 6-10).
Pericardial Effusion
CT with IV contrast readily identifies pericardial effusions, provides accurate size information, and can differentiate hemorrhagic effusions from serous effusions (see Fig. 6-138).93 Blood has a density of approximately 45 Hounsfield units, depending on factors including hematocrit. Serous effusions have a density closer to that of water, 0 Hounsfield units. Whereas the size of a traumatic pericardial effusion (hemopericardium) can be assessed with CT, the hemodynamic effect is not as apparent—ultrasound is more definitive. Although CT can be used to generate electrocardiogram–gated movies of cardiac motion (see Chapter 8), typical trauma CT protocols do not use this capability, which results in a higher radiation exposure to the patient. Consequently, a static image of the heart is seen on trauma CT, and effects such as right ventricular collapse signifying pericardial tamponade are not evident. Occasionally, CT may reveal findings of cardiac tamponade, including right ventricular collapse, if image acquisition happens to coincide with this event.58
Foreign Bodies
Foreign bodies can be precisely localized using CT. In penetrating trauma, CT can identify injuries to structures such as the heart, affecting management decisions (see Figures 6-115, 6-116, and 6-118 through 6-120). Very dense foreign bodies can create metallic streak artifact, interfering with exact localization. Use of bone windows can minimize this effect. CT can identify some low-density foreign bodies that may be difficult to see on x-ray.
Lung Windows
Following review of soft-tissue windows for important injuries, review the entire chest again using lung windows to identify the injury types that follow.
Pneumothorax
Lung windows allow detection of even small pneumothoraces. As with soft-tissue windows, the entire thorax should be inspected to confirm or exclude an injury. On this window setting, all soft tissues and denser structures, including contrast-filled vessels and bones, appear bright white. Air appears nearly black on this window setting. The lung parenchyma is readily visible as a fine latticework of air-filled alveoli, which appears an intermediate gray because of the small size of air-filled structures alternating with a matrix of solid tissue (see Figures 6-18, 6-27, 6-29, and 6-30). As a consequence, air within the pleural space (nearly black) can be readily distinguished from air within the lung parenchyma. On this window setting, subcutaneous air or pneumomediastinum may also be easily recognized (see Figures 6-29, 6-36, and 6-112).
Pneumomediastinum
Pneumomediastinum is readily visible on CT scan using lung windows. Often, air is visible tracking along the superior mediastinum and following great vessels as they extend into the neck. The source of pneumomediastinum may be apparent from CT. In the case of an esophageal source, thickening of the esophagus at the site of injury may be visible (see Figures 6-36, 6-112, and 6-113). In the case of a significant tracheobronchial injury, a discontinuity in the bronchial wall may be seen (see Figures 6-27, 6-29, and 6-30). In other cases, the source of mediastinal air may not be evident—an associated pneumothorax may suggest the cause, or pneumomediastinum may be present in isolation without an obvious source.
Ironically, although CT is exquisitely sensitive for detection of pneumomediastinum, it may not be the best imaging modality to pursue a suspected esophageal source. Typically, a fluoroscopic Gastrografin swallow study, followed by a fluoroscopic barium swallow study, is performed to assess for mucosal or full-thickness esophageal tears. Chest CT is performed without orally ingested contrast, and CT does not offer good assessment of the undistended esophagus. Without oral contrast, CT assessment for esophageal tears relies on secondary signs of injury such as esophageal thickening or surrounding air.
In a patient presenting late after chest trauma, pneumomediastinum may also be a sign of gas-forming organisms within the mediastinum from mediastinitis. Therefore when fever and a mechanism for mediastinal infection are present, pneumomediastinum is an ominous finding. It is a normal finding immediately after mediastinal surgery.
Hemothorax
Hemothorax is visible on lung and on soft-tissue windows (see Figures 6-4, 6-5, 6-10, and 6-18). The typical Hounsfield density is around 45 Hounsfield units, which appears an intermediate gray on soft-tissue windows.
Pulmonary Contusion
Lung windows are also useful for identification of pulmonary contusion (see Figures 6-38, 6-42, and 6-44). Pulmonary contusion is characterized by hemorrhage into the alveolar space, which increases the density from the normal air density (−1000 Hounsfield units, nearly black on lung windows) to fluid density (around 0 Hounsfield units, white on lung windows). An area of pulmonary contusion is also visible on soft-tissue windows. Because contused lung parenchyma has a higher density than normal lung tissue (near that of other soft tissues), it stands out as an intermediate gray on soft-tissue windows, surrounded by normal lung tissue, which appears black on this window setting.
CT is more sensitive than chest x-ray for pulmonary contusion even on initial examination because of its fine ability to discriminate tissue densities. A parenchymal density seen on initial chest CT indicates alveolar fluid—usually blood and serous fluid in the case of blunt or penetrating trauma. However, aspiration occurring during the patient’s injury, during endotracheal intubation, or resulting from poor airway protection in an intoxicated or head-injured patient would have exactly the same appearance. Moreover, lung tissue may undergo compressive atelectasis resulting from adjacent hemopneumothorax, and this increases lung parenchymal density, giving the same radiographic appearance. The clinical context may differentiate contusion from these other conditions.
Atelectasis
Atelectasis is commonly seen in trauma patients. Usually, this appears as a bilaterally symmetrical band of increased density in the dependent lung fields (see Figures 6-10 and 6-48). Patients who have been immobilized for long periods on backboards or in bed before CT may have significant atelectasis seen on CT. This can usually be differentiated from pulmonary contusion by its dependent position and bilateral symmetry; pulmonary contusion, in contrast, may be present in any location and is often asymmetrical. Atelectasis, like pulmonary contusion, can be seen on soft-tissue or lung windows. The reported density of dependent atelectasis varies between −100 and +100 Hounsfield units.94 In contrast, hemothorax and pulmonary contusion always have a density greater than 0 Hounsfield units (water density), usually around 45 Hounsfield units.
Tracheobronchial Injuries
Tracheobronchial injuries can be identified on chest CT using lung windows (see Figures 6-27, 6-29, and 6-30). Typically, a large or even tension pneumothorax persists, regardless of the presence of thoracostomy tubes. Mediastinal shift may be present resulting from tension pneumothorax. A defect in the wall of major airways may be directly visible, connecting into the pleural space.8
Bone Windows
After reviewing soft-tissue and lung windows, inspect the chest a third time using bone windows. Bone windows allow detection of subtle, minimally displaced fractures. More gross and displaced fractures may be visible on lung or mediastinal windows, but the bright white appearance of bone on these window settings masks subtle injuries. In addition to the use of bone windows, special bone reconstruction algorithms are used to create thin sections of bones with minimal artifact. In many medical centers, these are routinely created from the CT dataset and are loaded into the digital picture archiving and communication system as an additional CT series. For most emergency purposes, use of bone windows from the body dataset is sufficient, and specific bone reconstruction views can be reviewed later to provide additional detail of detected injuries.
Although bony injuries are often less immediately dangerous than underlying soft-tissue chest injuries, some isolated bony injuries are quite important. Multiple adjacent rib fractures, particularly a flail segment, may compromise respiratory efficiency (see Figures 6-122 through 6-124). Fractures to the scapula, including intraarticular fractures involving the glenoid fossa, may be seen on bone windows. Thoracic spine fractures (discussed in detail in Chapter 3) can be seen using bone windows. Posterior dislocations of the sternoclavicular joints can threaten the great vessels and may be recognized on either bone or soft-tissue windows (see Figures 6-131 through 6-133).
Controversies in Thoracic Trauma Imaging
CT technology has become ubiquitous in the United States and much of the developed world, and a trend toward increasing use of CT for assessment of thoracic injury has occurred over the past decade. Questions remain about the clinical value to patients of this additional imaging, which carries with it both additional cost and radiation exposure with associated cancer risks.81 The high sensitivity of CT for thoracic spine fracture and aortic injury is not in dispute; when injuries to these regions are suspected, CT is advocated, because it far surpasses the sensitivity of x-ray. Important questions today relate to the indications for CT in stable patients with normal chest x-rays and the correct clinical action for subtle injuries detected by CT but not by chest x-ray. We explore some of these questions here.
Do Patients with Normal Chest X-ray Require CT to Assess for Aortic Injury? Does a Normal Chest X-ray Sufficiently Exclude Aortic Injury?
Historically, patients with no evidence of traumatic aortic injury on chest x-ray did not routinely undergo aortography to exclude aortic injury definitively. A normal chest x-ray is reported in only about 7% of aortic injuries, for a sensitivity of around 93%.20 Although this sensitivity is low for a life-threatening injury, aortic injury is relatively rare, occurring in only 274 patients in 2.5 years in a multicenter trial of 50 U.S. trauma centers—an average of only about 2 aortic injuries per trauma center per year.20 Ungar et al.16 reviewed 1096 consecutive blunt trauma patients at a major trauma center, all of whom had definitive imaging with CT, aortography, or both. The rate of traumatic aortic injury was only 2%. The authors derived a clinical decision rule for aortic injury based on chest x-ray and found that a chest x-ray without obscuration of the aortic knob, mediastinal widening, or displacement of the left paraspinous line had a sensitivity of 86% (95% CI = 65%-97%), a specificity of 77% (95% CI = 75%-80%), a positive predictive value of 7% (95% CI = 4%-11%), a negative predictive value of 99.6% (95% CI = 99.0%-99.9%), a positive likelihood ratio of 3.8 (95% CI = 1.1-12.9), and a negative likelihood ratio of 0.18 (95% CI = 0.05-0.61).16 Despite relative low sensitivity, the negative predictive value of a normal chest x-ray is very high resulting from the rarity of the injury. An individual patient might deserve definitive aortic imaging despite normal radiography if other evidence of substantial trauma is present, such as hemodynamic instability without a clear cause. Although as many as 14% of aortic injuries might be missed by chest x-ray in the study by Ungar et al.,16 the number needed to treat to detect 1 additional aortic injury using CT or aortography in patients with normal chest x-ray findings was high—nearly 300. Although the availability, speed, and ease of use of CT make it an attractive option to detect rare occult injuries, the cost and radiation exposure of CT may make the risk–benefit ratio of ubiquitous CT unfavorable.
Other Perspectives: Should Patients with a Normal Mediastinum on Chest X-ray but Other Evidence of Thoracic Trauma (e.g., Rib Fractures) Undergo CT? Should Patients with No Visible Evidence of Thoracic Injury Undergo Thoracic CT?
Other authors have argued that chest CT should be performed in every trauma patient regardless of the presence or absence of signs and symptoms of chest trauma. Exadaktylos et al.74 reported on injuries detected by CT but missed on initial chest x-ray in 93 consecutive patients with blunt trauma. They reported that 73.1% showed at least one pathologic sign on chest x-ray and 26.9% had normal chest x-ray. Of those with normal chest x-rays, 52% had injuries detected at CT, including two aortic injuries (8% of those with normal chest x-rays), three pleural effusions, and one pericardial effusion. The authors conclude that the rate of serious injuries missed by x-ray justifies routine use of thoracic CT. However, their reported rates of injury exceeded those from other studies of blunt aortic injury, such as the Multicenter Trial of the American Association for the Surgery of Trauma,20 strongly suggesting selection or spectrum bias. It is doubtful that the rate of injuries in trauma patients in community emergency departments would approach that in this study. Perhaps thoracic CT is routinely advisable in the high-risk patients seen in tertiary care trauma centers.
In a prospective study of 1000 patients, including 592 alert patients, Salim et al.95 reported a rate of “significant” thoracic injuries of 19.6% in patients “without visible signs of thoracic trauma.” Several criticisms of this study have been leveled, though it remains the basis for many trauma protocols incorporating CT. First, the researchers focused on “visible signs of thoracic trauma” but did not evaluate for the presence or absence of other readily available evidence of injury, including complaints of chest or dyspnea or tenderness to chest palpation. Thus it is possible that most injuries detected by CT would have been suspected based on simple history or examination techniques. Perhaps a normal physical examination and the absence of chest symptoms (pain or dyspnea) in an alert patient might rule out significant injury, eliminating the need for CT. Evidence to this effect exists from studies of the indications for chest x-ray.2-3
Second, the authors included patients with significant alteration of mental status in their study. Patients who cannot be clinically assessed as a consequence of head injury, instability, intoxication, or intubation constitute a distinct population from those who are stable, alert, and awake. The former patients are likely also at greater risk for concurrent chest injuries than are alert patients. Although the authors stratified patient results by status (“evaluable” or “unevaluable”), these two groups should not be combined in clinical decision-making.
Third, the authors classified as “important thoracic injuries” virtually every finding identified on CT, including rib fractures, pulmonary contusions, and small pneumothoraces and hemothoraces. Only 7.9% of 809 patients with normal chest x-rays in this study had abnormal CT findings: 27 with occult pneumothorax or small hemothorax (3.3%), 2 with suspicion for aortic injury (0.2%), 27 with pulmonary contusion (3.3%), and 30 with rib fractures (3.7%) [percentages do not sum to 7.9%, because some patients had more than one of the above injury types]. It is not clear that detection of these injuries resulted in clinically beneficial changes in management of patients, justifying the cost and radiation exposure of CT. The authors demonstrated a very low rate of indisputably important injuries such as aortic trauma: 4 of 1000 total patients (0.4%) and only 1 of 592 “evaluable” patients (0.2%). Thus the number needed to treat to identify 1 aortic injury by their methods would be nearly 600 in alert patients without visible chest trauma and 1 in 400 with normal chest x-ray.95
In their publication in Lancet, Strohm, Hauschild, and Sudkamp96 reported an association between use of pan-scan CT (CT of the head, spine, chest, abdomen, and pelvis) and reduced mortality, compared with patients undergoing more selective use of CT. This study (discussed in more detail in Chapter 10) is retrospective and can demonstrate only an association, not a causative relationship, between use of CT and survival. This study also lacks face validity in that many injuries detected by thoracic and abdominal CT (e.g., pulmonary contusions, rib fractures, and low-grade splenic and hepatic injuries) require only supportive management, so definitive diagnosis by CT would not necessarily be expected to alter management or outcome. Critics have pointed out numerous methodologic flaws in this study, including immortal time bias. This bias describes the fact that patients dying very early in their emergency department course would fail to undergo CT and would thus contribute to increased mortality in the selective CT group, whereas patients stable enough to survive through completion of a pan-scan would contribute to lower mortality in this group. A prospective, randomized, controlled trial is needed to examine the clinical value of pan-scan, and particularly thoracic CT, in more detail.96
When Injuries Are Detected by CT, What Interventions Are Appropriate? What Treatment Is Needed for Pneumothorax Detected Solely by CT?
CT is clearly more sensitive for multiple injury types than is chest x-ray, but less evident is the correct clinical action when subtle injuries are detected. Consider the case of small pneumothoraces detected by CT and not visible on chest x-ray. Is thoracostomy tube drainage necessary? Multiple small retrospective studies have examined this question, and collectively they suggest that thoracostomy tube drainage should not be routine. Even in the case of patients undergoing positive pressure ventilation, in whom a risk for tension pneumothorax might be assumed, routine thoracostomy drainage does not appear necessary. While a large prospective, randomized, controlled trial is needed to determine the value of thoracostomy tube for CT-only pneumothoraces, these studies call into question the benefit of CT detection of these injuries. It is possible that CT use simply results in the detection of more injuries requiring no specific management or that CT even results in patient harm by leading to unnecessary invasive procedures such as thoracostomy. We assess some of the studies on this topic here.
Ball et al.97 retrospectively studied 761 trauma patients, documenting 103 pneumothoraces on CT scan. Of these, 55% were not evident on initial chest x-ray. The authors noted that pneumothoraces were more often occult on chest x-ray when located anteriorly or in basal or apical locations, all potentially identifiable using ultrasound (though the authors did not attempt to identify these pneumothoraces with ultrasound). In a separate report on the same patients, they noted that 4 of 17 (24%) patients with occult pneumothoraces underwent uneventful positive pressure ventilation without thoracostomy tube placement.98
Neff et al.99 retrospectively reviewed the charts of 230 trauma patients with pneumothorax at admission and found that 126 (54.8%) were occult on chest x-ray, diagnosed instead by abdominal CT. The authors noted that 84 (66.7%) underwent thoracostomy tube placement and suggested that CT diagnosis of pneumothorax is beneficial. However, the necessity or benefit of this diagnosis is uncertain. It is possible that diagnoses of these occult pneumothoraces resulted in unnecessary thoracostomy with increased patient morbidity. Because of the retrospective methods of this study, it is not clear what percentage of patients with pneumothorax detected by CT alone had other signs or symptoms, such as hypoxia, which may have necessitated thoracostomy. Detection of occult pneumothorax may be beneficial in selected cases when patients require long intrahospital transportation or positive pressure ventilation that might result in progression to tension pneumothorax.
Brasel et al.100 prospectively randomized 39 patients with occult pneumothorax (detected on CT but not chest x-ray) to thoracostomy or observation. In each group, 9 patients received positive pressure ventilation, and no patient developed respiratory distress attributed to the occult pneumothorax or required emergent thoracostomy. The authors concluded that occult pneumothoraces do not require routine thoracostomy, even in patients undergoing positive pressure ventilation. This small study does not rule out the possibility of infrequent significant worsening of an occult pneumothorax when not treated with thoracostomy.
Enderson et al.101 performed a similar randomized controlled trial of 40 patients with occult pneumothorax. In this trial, 19 were randomized to tube thoracostomy, whereas 21 were observed without thoracostomy. While on positive pressure ventilation, 8 of 21 had progression of pneumothorax, with 3 developing tension pneumothorax. The authors concluded that patients with occult pneumothorax undergoing positive pressure ventilation should be treated routinely with thoracostomy.
Holmes et al.102 prospectively studied 538 children who underwent both chest x-ray and abdominal CT. Pneumothorax occurred in 20 patients (3.7%). Of these, 9 (45%) had pneumothorax identified on initial chest x-ray, whereas 11 patients (55%) had pneumothoraces not seen on chest x-ray. Of these 11 patients, 10 (91%) were managed successfully without thoracostomy tube, including 2 undergoing positive pressure ventilation.
Renton, Kincaid, and Ehrlich76 conducted a retrospective review and cost analysis to determine whether chest CT should replace chest x-ray as the initial imaging test in pediatric trauma. They noted that from 1996 to 2000, only 45 of 1638 pediatric trauma patients underwent CT at their institution, with 18 patients having injuries detected on CT that had not been recognized on x-ray. These included pulmonary contusions (12), hemothoraces (6), pneumothoraces (5), widened mediastinum (4), rib fractures (2), diaphragmatic injury (1), and aortic injury (1). They concluded that 8 patients (17.7% of the 45 undergoing CT) had clinical management changes as a result of CT findings: 5 with chest tube placement, 2 with aortography, and 1 with an operation performed. Based on an institutional cost of $200 per CT and a patient charge of $906 (compared with $96 for chest x-ray), they estimated that 200 thoracic CT scans would be required for each clinically significant change in management, at a patient cost of $180,000 and hospital cost of $40,000 per management change.
Which Patients Require CT to Evaluate Potential Thoracic Spine Trauma?
Imaging of thoracic spine injuries is discussed in detail in Chapter 3. Evaluation of thoracic spine injury is one of the major indications for chest CT today, because axial, sagittal, and coronal reconstructions of the spine can be performed from the data acquired from CT performed for evaluation of mediastinal and pulmonary injuries. CT has been shown to far surpass x-ray in sensitivity for these injuries. Indications for thoracic CT include spinal tenderness on exam; neurologic deficits potentially attributable to spinal trauma; obtundation, intoxication, or intubation preventing clinical assessment; thoracic spine abnormalities detected on imaging such as chest x-ray; and detection of cervical or lumbar fractures, because these injuries have been found to predict the presence of additional spinal injuries.
Can Imaging Detect Blunt Cardiac Injury?
Blunt cardiac injury (formerly called cardiac contusion) remains an area of controversy. Chest x-ray and CT scan offer no direct evidence of this diagnosis, although they may reveal associated pericardial hemorrhage or sternal fracture. Echocardiography provides the most clinically useful information regarding right and left ventricular ejection fracture, although formal echocardiography (rather than bedside echo as a part of the FAST examination) should not be routine in patients with blunt chest trauma. Indications for echocardiography are controversial but likely are restricted to patients with significant dysrhythmias or hemodynamic instability. Use of cardiac markers such as creatine kinase-MB isoenzyme and troponin I or T to detect blunt cardiac injury has fallen from favor resulting from a lack of supportive evidence.60,103-104
When Is Aortography Needed?
Aortography, the traditional gold standard for evaluation of blunt aortic injury, has been replaced almost entirely by chest CT for diagnosis. Modern CT provides sufficient anatomic detail to guide open surgical management and endovascular repair, which is used in some cases. Although some trauma surgeons have been vocal opponents of the use of CT, arguing for aortography, improvements in three-dimensional CT reconstructions have relegated aortography to a secondary role. It is most useful as a therapeutic modality allowing endovascular repair, or when CT is equivocal (see Chapter 16). Aortography has almost no role in primary diagnosis resulting from the availability of CT, which allows concurrent assessment of other important injuries, including intraabdominal and spinal trauma. In one retrospective study of 856 patients between 1997 and 2004, 24% of aortograms were preceded by CT; among 31 with confirmed aortic injury, 20 had preceding CT.105 Rarely, a patient with a chest x-ray highly suggestive of aortic injury may be taken directly to the operating room with a plan for intraoperative aortography, depending on stability, suspicion for other injuries, local institutional protocols, and resources. Hunink and Bos106 found CT before aortography to be more cost effective ($1468 per patient) than primary aortography ($2508 per patient) to pursue chest x-ray abnormalities suspicious for aortic injury in stable patients. For these reasons, in general, aortography is diminishing as an emergency department imaging study.
Can CT Guide Management of Penetrating Chest Trauma Such as Transmediastinal Gunshot Wounds and Stab Wounds to the Anterior Chest?
The speed of modern chest CT and its high spatial resolution have broadened its use for injury patterns that in the past were more commonly managed with surgery, angiography, bronchoscopy, esophagography, and esophagoscopy. Examples include transmediastinal gunshot wounds and stab wounds to the “cardiac box” (see Figs. 6-115, 6-116, and 6-118 through 6-120). Apparently stable patients with these injury mechanisms can have devastating injuries.107 In some cases, CT can exclude or confirm cardiac, vascular, tracheobronchial, and esophageal injuries, eliminating the need for other diagnostic studies and determining the need for operative intervention. Hanpeter et al.108 performed a prospective study of 24 stable patients with mediastinal gunshot wounds. One patient was taken for sternotomy and surgical removal of an embedded myocardial missile based on CT findings alone. Another 12 patients underwent additional imaging of the aorta or esophagus resulting from a missile track close to these structures; one had a bullet adjacent to the ascending aorta that was removed surgically. A further 11 patients had missile tracks not in proximity to the aorta, heart, or esophagus, and no further interventions were performed. Small studies such as this are just the starting point for more systematic research on the role of chest CT in penetrating thoracic trauma.
Summary
Chest trauma is a common indication for diagnostic imaging. Clinical decision rules can identify patients requiring no imaging. In those who require imaging, chest x-ray is the initial test of choice resulting from low cost and low radiation exposure with substantial clinical information obtained. Ultrasound is a sensitive bedside tool for detection of hemo- and pneumothorax, as well as pericardial effusion. Chest CT and formal echocardiography play important roles in selected patients. The indications for chest CT remain in debate and require further study. Aortography has been largely replaced by CT for diagnosis but retains a therapeutic role for aortic injuries.
1. American College of Surgeons Committee on Trauma. Advanced Trauma Life Support for Doctors, ed 7. Chicago: American College of Surgeons; 2004.
2. Bokhari F., Brakenridge S., Nagy K., et al. Prospective evaluation of the sensitivity of physical examination in chest trauma. J Trauma. 2002;53(6):1135-1138.
3. Holmes J.F., Sokolove P.E., Brant W.E., et al. A clinical decision rule for identifying children with thoracic injuries after blunt torso trauma. Ann Emerg Med. 2002;39(5):492-499.
4. Schwab C.W., Lawson R.B., Lind J.F., et al. Aortic injury: Comparison of supine and upright portable chest films to evaluate the widened mediastinum. Ann Emerg Med. 1984;13(10):896-899.
5. Rhea J.T., DeLuca S.A., Greene R.E. Determining the size of pneumothorax in the upright patient. Radiology. 1982;144(4):733-736.
6. Tack D., Defrance P., Delcour C., et al. The CT fallen-lung sign. Eur Radiol. 2000;10(5):719-721.
7. Ketai L., Brandt M.M., Schermer C. Nonaortic mediastinal injuries from blunt chest trauma. J Thorac Imaging. 2000;15(2):120-127.
8. Wan Y.L., Tsai K.T., Yeow K.M., et al. CT findings of bronchial transection. Am J Emerg Med. 1997;15(2):176-177.
9. Cohn S.M. Pulmonary contusion: Review of the clinical entity. J Trauma. 1997;42(5):973-979.
10. Shapiro M.J., Heiberg E., Durham R.M., et al. The unreliability of CT scans and initial chest radiographs in evaluating blunt trauma induced diaphragmatic rupture. Clin Radiol. 1996;51(1):27-30.
11. Gelman R., Mirvis S.E., Gens D. Diaphragmatic rupture resulting from blunt trauma: Sensitivity of plain chest radiographs. AJR Am J Roentgenol. 1991;156(1):51-57.
12. American College of Radiology. ACR Appropriateness Criteria: Blunt Chest Trauma—Suspected Aortic Injury. 2009. Available at: http://www.acr.org/SecondaryMainMenuCategories/quality_safety/app_criteria/pdf/Vascular/BluntChestTraumaSuspectedAorticInjuryDoc6.aspx. Accessed 3-15-2011.)
13. Savoca C.J., Austin J.H., Goldberg H.I. The right paratracheal stripe. Radiology. 1977;122(2):295-301.
14. Savoca C.J., Brasch R.C., Gooding C.A., et al. The right paratracheal stripe in children. Pediatr Radiol. 1978;6(4):203-207.
15. Woodring J.H., Pulmano C.M., Stevens R.K. The right paratracheal stripe in blunt chest trauma. Radiology. 1982;143(3):605-608.
16. Ungar T.C., Wolf S.J., Haukoos J.S., et al. Derivation of a clinical decision rule to exclude thoracic aortic imaging in patients with blunt chest trauma after motor vehicle collisions. J Trauma. 2006;61(5):1150-1155.
17. Lee J., Harris J.H.Jr., Duke J.H.Jr., et al. Noncorrelation between thoracic skeletal injuries and acute traumatic aortic tear. J Trauma. 1997;43(3):400-404.
18. Lee R.B., Bass S.M., Morris J.A.Jr., et al. Three or more rib fractures as an indicator for transfer to a level I trauma center: A population-based study. J Trauma. 1990;30(6):689-694.
19. Geusens E., Pans S., Prinsloo J., et al. The widened mediastinum in trauma patients. Eur J Emerg Med. 2005;12(4):179-184.
20. Fabian T.C., Richardson J.D., Croce M.A., et al. Prospective study of blunt aortic injury: Multicenter Trial of the American Association for the Surgery of Trauma. J Trauma. 1997;42(3):374-380. discussion 380-383
21. Wintermark M., Wicky S., Schnyder P. Imaging of acute traumatic injuries of the thoracic aorta. Eur Radiol. 2002;12(2):431-442.
22. Hamilton P., Rizoli S., McLellan B., et al. Significance of intraabdominal extraluminal air detected by CT scan in blunt abdominal trauma. J Trauma. 1995;39(2):331-333.
23. Kane N.M., Francis I.R., Burney R.E., et al. Traumatic pneumoperitoneum: Implications of computed tomography diagnosis. Invest Radiol. 1991;26(6):574-578.
24. Earls J.P., Dachman A.H., Colon E., et al. Prevalence and duration of postoperative pneumoperitoneum: Sensitivity of CT vs. left lateral decubitus radiography. AJR Am J Roentgenol. 1993;161(4):781-785.
25. Stapakis J.C., Thickman D. Diagnosis of pneumoperitoneum: Abdominal CT vs. upright chest film. J Comput Assist Tomogr. 1992;16(5):713-716.
26. American College of Radiology. ACR Appropriateness Criteria®: Blunt Chest Trauma — Rib Fractures. 2008. Available at: http://www.acr.org/SecondaryMainMenuCategories/quality_safety/app_criteria/pdf/ExpertPanelonThoracicImaging/RibFracturesDoc5.aspx. Accessed 3-16-2011.
27. Gunduz M., Unlugenc H., Ozalevli M., et al. A comparative study of continuous positive airway pressure (CPAP) and intermittent positive pressure ventilation (IPPV) in patients with flail chest. Emerg Med J. 2005;22(5):325-329.
28. Poole G.V. Fracture of the upper ribs and injury to the great vessels. Surg Gynecol Obstet. 1989;169(3):275-282.
29. Stawicki S.P., Grossman M.D., Hoey B.A., et al. Rib fractures in the elderly: A marker of injury severity. J Am Geriatr Soc. 2004;52(5):805-808.
30. Lee R.B., Morris J.A.Jr., Parker R.S. Presence of three or more rib fractures as an indicator of need for interhospital transfer. J Trauma. 1989;29(6):795-799. discussion 799-800
31. Kerr-Valentic M.A., Arthur M., Mullins R.J., et al. Rib fracture pain and disability: Can we do better? J Trauma. 2003;54(6):1058-1063. discussion 1063-1064
32. Restrepo C.S., Martinez S., Lemos D.F., et al. Imaging appearances of the sternum and sternoclavicular joints. Radiographics. 2009;29(3):839-859.
33. Ferrandez L., Yubero J., Usabiaga J., et al. Sternoclavicular dislocation: Treatment and complications. Ital J Orthop Traumatol. 1988;14(3):349-355.
34. Keats T.E., Pope T.L.Jr. The acromioclavicular joint: Normal variation and the diagnosis of dislocation. Skeletal Radiol. 1988;17(3):159-162.
35. Stuart R.K., Shikora S.A., Akerman P., et al. Incidence of arrhythmia with central venous catheter insertion and exchange. JPEN J Parenter Enteral Nutr. 1990;14(2):152-155.
36. Fuchs S., Pollak A., Gilon D. Central venous catheter mechanical irritation of the right atrial free wall: A cause for thrombus formation. Cardiology. 1999;91(3):169-172.
37. Oh W.K., Lee B.H., Sweitzer N.K. Nonmalignant diagnoses in patients: Case 3. Right atrial thrombus associated with a central venous catheter in a patient with metastatic adrenocortical carcinoma. J Clin Oncol. 2000;18(13):2638-2639.
38. Rizik D.G. Barman Andre Bouhasin NC, Bouhasin A: Right atrial thrombus attached to an indwelling central venous catheter. J Invasive Cardiol. 2003;15(12):736.
39. Dulchavsky S.A., Schwarz K.L., Kirkpatrick A.W., et al. Prospective evaluation of thoracic ultrasound in the detection of pneumothorax. J Trauma. 2001;50(2):201-205.
40. Goodman T.R., Traill Z.C., Phillips A.J., et al. Ultrasound detection of pneumothorax. Clin Radiol. 1999;54(11):736-739.
41. Knudtson J.L., Dort J.M., Helmer S.D., et al. Surgeon-performed ultrasound for pneumothorax in the trauma suite. J Trauma. 2004;56(3):527-530.
42. Blaivas M., Lyon M., Duggal S. A prospective comparison of supine chest radiography and bedside ultrasound for the diagnosis of traumatic pneumothorax. Acad Emerg Med. 2005;12(9):844-849.
43. Chung M.J., Goo J.M., Im J.G., et al. Value of high-resolution ultrasound in detecting a pneumothorax. Eur Radiol. 2005;15(5):930-935.
44. Garofalo G., Busso M., Perotto F., et al. Ultrasound diagnosis of pneumothorax. Radiol Med. 2006;111(4):516-525.
45. Feigenbaum H. Ultrasonic cardiology: Diagnostic ultrasound as an aid to the management of patients with pericardial effusion. Dis Chest. 1969;55(1):59-62.
46. Pridie R.B., Turnbull T.A. Diagnosis of pericardial effusion by ultrasound. BMJ. 1968;3(5614):356-357.
47. Klein J.J., Segal B.L. Pericardial effusion diagnosed by reflected ultrasound. Am J Cardiol. 1968;22(1):57-64.
48. Feigenbaum H., Zaky A., Waldhausen J.A. Use of reflected ultrasound in detecting pericardial effusion. Am J Cardiol. 1967;19(1):84-90.
49. Feigenbaum H., Zaky A., Waldhausen J.A. Use of ultrasound in the diagnosis of pericardial effusion. Ann Intern Med. 1966;65(3):443-452.
50. Moss A.J., Bruhn F. The echocardiogram. An ultrasound technic for the detection of pericardial effusion. N Engl J Med. 1966;274(7):380-384.
51. Feigenbaum H., Waldhausen J.A., Hyde L.P. Ultrasound diagnosis of pericardial effusion. JAMA. 1965;191:711-714.
52. Thourani V.H., Feliciano D.V., Cooper W.A., et al. Penetrating cardiac trauma at an urban trauma center: A 22-year perspective. Am Surg. 1999;65(9):811-816. discussion 817-8
53. Rozycki G.S., Feliciano D.V., Ochsner M.G., et al. The role of ultrasound in patients with possible penetrating cardiac wounds: A prospective multicenter study. J Trauma. 1999;46(4):543-551. discussion 551-52
54. Plummer D., Brunette D., Asinger R., et al. Emergency department echocardiography improves outcome in penetrating cardiac injury. Ann Emerg Med. 1992;21(6):709-712.
55. Levine M.J., Lorell B.H., Diver D.J., et al. Implications of echocardiographically assisted diagnosis of pericardial tamponade in contemporary medical patients: Detection before hemodynamic embarrassment. J Am Coll Cardiol. 1991;17(1):59-65.
56. Blaivas M., DeBehnke D., Phelan M.B. Potential errors in the diagnosis of pericardial effusion on trauma ultrasound for penetrating injuries. Acad Emerg Med. 2000;7(11):1261-1266.
57. Ball C.G., Williams B.H., Wyrzykowski A.D., et al. A caveat to the performance of pericardial ultrasound in patients with penetrating cardiac wounds. J Trauma. 2009;67(5):1123-1124.
58. Restrepo C.S., Lemos D.F., Lemos J.A., et al. Imaging findings in cardiac tamponade with emphasis on CT. Radiographics. 2007;27(6):1595-1610.
59. Tsang T.S., Freeman W.K., Barnes M.E., et al. Rescue echocardiographically guided pericardiocentesis for cardiac perforation complicating catheter-based procedures: The Mayo Clinic experience. J Am Coll Cardiol. 1998;32(5):1345-1350.
60. Plautz C.U., Perron A.D., Brady W.J. Electrocardiographic ST-segment elevation in the trauma patient: acute myocardial infarction vs. myocardial contusion. Am J Emerg Med. 2005;23(4):510-516.
61. Ma O.J., Mateer J.R. Trauma ultrasound examination versus chest radiography in the detection of hemothorax. Ann Emerg Med. 1997;29(3):312-315. discussion 315-6
62. Brooks A., Davies B., Smethhurst M., et al. Emergency ultrasound in the acute assessment of haemothorax. Emerg Med J. 2004;21(1):44-46.
63. Sharma O.P., Hagler S., Oswanski M.F. Prevalence of delayed hemothorax in blunt thoracic trauma. Am Surg. 2005;71(6):481-486.
64. Wernecke K., Galanski M., Peters P.E., et al. Pneumothorax: Evaluation by ultrasound: Preliminary results. J Thorac Imaging. 1987;2(2):76-78.
65. Monti J.D., Younggren B., Blankenship R. Ultrasound detection of pneumothorax with minimally trained sonographers: A preliminary study. J Spec Oper Med. 2009;9(1):43-46.
66. Noble V.E., Lamhaut L., Capp R., et al. Evaluation of a thoracic ultrasound training module for the detection of pneumothorax and pulmonary edema by prehospital physician care providers. BMC Med Educ. 2009;9:3.
67. Sargsyan A.E., Hamilton D.R., Nicolaou S., et al. Ultrasound evaluation of the magnitude of pneumothorax: A new concept. Am Surg. 2001;67(3):232-235. discussion 235-6
68. Wilkerson R.G., Stone M.B. Sensitivity of bedside ultrasound and supine anteroposterior chest radiographs for the identification of pneumothorax after blunt trauma. Acad Emerg Med. 2009;17(1):11-17.
69. Chan S.S. Emergency bedside ultrasound to detect pneumothorax. Acad Emerg Med. 2003;10(1):91-94.
70. Lichtenstein D.A., Meziere G., Lascols N., et al. Ultrasound diagnosis of occult pneumothorax. Crit Care Med. 2005;33(6):1231-1238.
71. Lichtenstein D., Meziere G., Biderman P., et al. The comet-tail artifact: An ultrasound sign ruling out pneumothorax. Intensive Care Med. 1999;25(4):383-388.
72. Lichtenstein D., Meziere G., Biderman P., et al. The “lung point”: An ultrasound sign specific to pneumothorax. Intensive Care Med. 2000;26(10):1434-1440.
73. Slater A., Goodwin M., Anderson K.E., et al. COPD can mimic the appearance of pneumothorax on thoracic ultrasound. Chest. 2006;129(3):545-550.
74. Exadaktylos A.K., Sclabas G., Schmid S.W., et al. Do we really need routine computed tomographic scanning in the primary evaluation of blunt chest trauma in patients with “normal” chest radiograph? J Trauma. 2001;51(6):1173-1176.
75. Woodring J.H. The normal mediastinum in blunt traumatic rupture of the thoracic aorta and brachiocephalic arteries. J Emerg Med. 1990;8(4):467-476.
76. Renton J., Kincaid S., Ehrlich P.F. Should helical CT scanning of the thoracic cavity replace the conventional chest x-ray as a primary assessment tool in pediatric trauma? An efficacy and cost analysis. J Pediatr Surg. 2003;38(5):793-797.
77. Brenner D.J., Hall E.J. Computed tomography: An increasing source of radiation exposure. N Engl J Med 29. 2007;357(22):2277-2284.
78. Einstein A.J., Henzlova M.J., Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA. 2007;298(3):317-323.
79. Berrington de Gonzalez A., Mahesh M., Kim K.P., et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077.
80. Smith-Bindman R., Lipson J., Marcus R., et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169(22):2078-2086.
81. Winslow J.E., Hinshaw J.W., Hughes M.J., et al. Quantitative assessment of diagnostic radiation doses in adult blunt trauma patients. Ann Emerg Med. 2008;52(2):93-97.
82. Wong Y.C., Wang L.J., Lim K.E., et al. Periaortic hematoma on helical CT of the chest: A criterion for predicting blunt traumatic aortic rupture. AJR Am J Roentgenol. 1998;170(6):1523-1525.
83. Cleverley J.R., Barrie J.R., Raymond G.S., et al. Direct findings of aortic injury on contrast-enhanced CT in surgically proven traumatic aortic injury: A multi-centre review. Clin Radiol. 2002;57(4):281-286.
84. Alkadhi H., Wildermuth S., Desbiolles L., et al. Vascular emergencies of the thorax after blunt and iatrogenic trauma: Multi-detector row CT and three-dimensional imaging. Radiographics. 2004;24(5):1239-1255.
85. Fishman J.E. Imaging of blunt aortic and great vessel trauma. J Thorac Imaging. 2000;15(2):97-103.
86. Shiau Y.W., Wong Y.C., Ng C.J., et al. Periaortic contrast medium extravasation on chest CT in traumatic aortic injury: A sign for immediate thoracotomy. Am J Emerg Med. 2001;19(3):229-231.
87. Wong H., Gotway M.B., Sasson A.D., et al. Periaortic hematoma at diaphragmatic crura at helical CT: Sign of blunt aortic injury in patients with mediastinal hematoma. Radiology. 2004;231(1):185-189.
88. Wicky S., Capasso P., Meuli R., et al. Spiral CT aortography: An efficient technique for the diagnosis of traumatic aortic injury. Eur Radiol. 1998;8(5):828-833.
89. Dyer D.S., Moore E.E., Ilke D.N., et al. Thoracic aortic injury: How predictive is mechanism and is chest computed tomography a reliable screening tool? A prospective study of 1561 patients. J Trauma. 2000;48(4):673-682. discussion 682-83
90. Dyer D.S., Moore E.E., Mestek M.F., et al. Can chest CT be used to exclude aortic injury? Radiology. 1999;213(1):195-202.
91. Gavant M.L., Menke P.G., Fabian T., et al. Blunt traumatic aortic rupture: Detection with helical CT of the chest. Radiology. 1995;197(1):125-133.
92. Ellis J.D., Mayo J.R. Computed tomography evaluation of traumatic rupture of the thoracic aorta: An outcome study. Can Assoc Radiol J. 2007;58(1):22-26.
93. Krejci C.S., Blackmore C.C., Nathens A. Hemopericardium: An emergent finding in a case of blunt cardiac injury. AJR Am J Roentgenol. 2000;175(1):250.
94. Lundquist H., Hedenstierna G., Strandberg A., et al. CT-assessment of dependent lung densities in man during general anaesthesia. Acta Radiol. 1995;36(6):626-632.
95. Salim A., Sangthong B., Martin M., et al. Whole body imaging in blunt multisystem trauma patients without obvious signs of injury: Results of a prospective study. Arch Surg. 2006;141(5):468-473. discussion 473-75
96. Strohm P.C., Hauschild O., Sudkamp N.P. Effect on survival of whole-body CT during trauma resuscitation. Lancet. 2009;374(9685):197-198. author reply 198-9
97. Ball C.G., Kirkpatrick A.W., Laupland K.B., et al. Factors related to the failure of radiographic recognition of occult posttraumatic pneumothoraces. Am J Surg. 2005;189(5):541-546. discussion 546
98. Ball C.G., Kirkpatrick A.W., Laupland K.B., et al. Incidence, risk factors, and outcomes for occult pneumothoraces in victims of major trauma. J Trauma. 2005;59(4):917-924. discussion 924-25
99. Neff M.A., Monk J.S.Jr., Peters K., et al. Detection of occult pneumothoraces on abdominal computed tomographic scans in trauma patients. J Trauma. 2000;49(2):281-285.
100. Brasel K.J., Stafford R.E., Weigelt J.A., et al. Treatment of occult pneumothoraces from blunt trauma. J Trauma. 1999;46(6):987-990. discussion 990-91
101. Enderson B.L., Abdalla R., Frame S.B., et al. Tube thoracostomy for occult pneumothorax: A prospective randomized study of its use. J Trauma. 1993;35(5):726-729. discussion 729-30
102. Holmes J.F., Brant W.E., Bogren H.G., et al. Prevalence and importance of pneumothoraces visualized on abdominal computed tomographic scan in children with blunt trauma. J Trauma. 2001;50(3):516-520.
103. Lindstaedt M., Germing A., Lawo T., et al. Acute and long-term clinical significance of myocardial contusion following blunt thoracic trauma: Results of a prospective study. J Trauma. 2002;52(3):479-485.
104. Maenza R.L., Seaberg D., D’Amico F. A meta-analysis of blunt cardiac trauma: Ending myocardial confusion. Am J Emerg Med. 1996;14(3):237-241.
105. Bruckner B.A., DiBardino D.J., Cumbie T.C., et al. Critical evaluation of chest computed tomography scans for blunt descending thoracic aortic injury. Ann Thorac Surg. 2006;81(4):1339-1346.
106. Hunink M.G., Bos J.J. Triage of patients to angiography for detection of aortic rupture after blunt chest trauma: Cost-effectiveness analysis of using CT. AJR Am J Roentgenol. 1995;165(1):27-36.
107. Nagy K.K., Roberts R.R., Smith R.F., et al. Trans-mediastinal gunshot wounds: Are “stable” patients really stable? World J Surg. 2002;26(10):1247-1250.
108. Hanpeter D.E., Demetriades D., Asensio J.A., et al. Helical computed tomographic scan in the evaluation of mediastinal gunshot wounds. J Trauma. 2000;49(4):689-694. discussion 694-95
109. Smithers B.M., O’Loughlin B., Strong R.W. Diagnosis of ruptured diaphragm following blunt trauma: Results from 85 cases. Aust N Z J Surg. 1991;61(10):737-741.
110. Crausman R.S. The ABCs of chest X-ray film interpretation. Chest. 1998;113(1):256-257.
111. Sonosite. MicroMaxx: Transducers. (Accessed at http://www.sonosite.com/products/micromaxx/transducers/.)
112. Lichtenstein D.A., Menu Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill: Lung sliding. Chest. 1995;108(5):1345-1348.